- 1Department of Nephrology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Nephrology, Jinan, China
- 2Department of Nephrology, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 3Department of Nephrology, Shandong Second Provincial General Hospital, Jinan, China
- 4Department of Emergency Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Steroid-resistant nephrotic syndrome (SRNS) in children is associated with various histopathological subtypes;however, the prevalence of these subtypes remains insufficiently defined. These subtypes often demonstrate variable responses to identical therapeutic regimens and are indicative of distinct clinical outcomes. This systematic review and meta-analysis seeks to comprehensively elucidate the prevalence of the most common histopathological subtypes in pediatric SRNS, offering valuable insights that may inform future therapeutic strategies and improve prognostic predictions for affected patients.
Methods: We conducted a comprehensive literature search across four major databases: PubMed, Embase, Web of Science, and Cochrane, with coverage extending from the inception of these databases up to December 2023. Two independent researchers screened the identified studies, selecting only cross-sectional and longitudinal studies that reported the prevalence of histopathological subtypes in pediatric SRNS. The quality of the studies was assessed using the Joanna Briggs Institute Critical Appraisal Checklist, and only those meeting the required quality criteria were included. Data were extracted from the selected studies using a standardized data extraction form. Meta-analysis was performed with Stata software to estimate the prevalence of different histopathological subtypes in pediatric SRNS. Subgroup analyses were conducted to explore potential sources of heterogeneity. Publication bias was assessed using funnel plots and the Begg test, and sensitivity analysis was also conducted.
Results: The most commonly reported histopathological subtypes associated with SRNS in children are focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), minimal change disease (MCD), mesangioproliferative glomerulonephritis (MesPGN), and membranoproliferative glomerulonephritis (MPGN). Among these, the subtype with the highest average prevalence is FSGS, at 39%, followed by MCD (27%), MesPGN (23%), MPGN (18%), and MN (4%).
Conclusion: FSGS exhibits a notably high prevalence in SRNS and remains the most frequently observed histopathological lesion associated with this condition.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251000869, identifier CRD420251000869.
1 Introduction
The prevalence of common histopathological subtypes associated with steroid-resistant nephrotic syndrome (SRNS) in children appears to be undergoing global changes, and different histopathological subtypes often exhibit divergent responses to the same therapeutic regimens (1). Nephrotic syndrome (NS) is characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema. Many pediatric kidney diseases manifest as nephrotic syndrome, and for the majority of patients who respond to steroid treatment, the primary therapeutic approach involves the use of prednisone. However, a certain proportion of patients fail to respond to steroid treatment, a phenomenon known as steroid resistance. And frequent relapses may occur during treatment, necessitating the use of alternative immunosuppressive agents. Steroid resistance is a critical factor in the progression of pediatric nephrotic syndrome to chronic kidney disease or end-stage renal disease. NS can be classified based on response to steroid therapy, relapse patterns, histopathological findings, or genetic mutations. The simplest classification is based on clinical response to steroid treatment, distinguishing between steroid-sensitive (SS) and steroid-resistant (SR) forms. SRNS is defined as the failure to achieve complete remission after four weeks of treatment with prednisone at standard doses (60 mg/m²/day or 2 mg/kg/day) in nephrotic syndrome patients.
SRNS is a clinically diagnosed disease characterized by histological features identified through kidney biopsy. The treatment of SRNS remains a significant challenge for nephrologists. Although renal biopsy is often indicated in children with SRNS, few large-scale studies have evaluated the role of histopathology in the contemporary era (2), where genetic diagnoses are feasible (3), or in the presence of obstacles such as contraindications to biopsy, lack of nephropathology expertise, or unavailability of electron microscopy. Minimal change nephrotic syndrome (MCNS) typically shows no significant abnormalities on light microscopy, whereas electron microscopy reveals diffuse foot process effacement of podocytes. This subtype is most commonly observed in children aged 2–5 years. Approximately 80-90% of MCNS cases respond to steroid treatment and are classified as steroid-sensitive nephrotic syndrome (SSNS).In contrast, SRNS patients often exhibit FSGS on kidney biopsy. Light microscopy reveals segmental obstruction of the glomerular capillaries due to extracellular matrix expansion, causing encapsulation and sclerosis of plasma proteins. The electron microscopy findings in FSGS typically resemble those of MCNS, with diffuse effacement of podocyte foot processes.
Steroids are the most commonly used treatment for nephrotic syndrome in clinical practice. However, different histopathological subtypes often predict varying outcomes and responses to steroid therapy. Some patients with idiopathic nephrotic syndrome may undergo a series of potentially harmful treatments without clinical benefit (1). Previous study have indicated a high prevalence of focal segmental glomerulosclerosis(FSGS) in children with nephrotic syndrome in sub-Saharan Africa (4), making it the most common histopathological subtype of SRNS worldwide. However, due to the limited number of studies included in that review, a meta-analysis has not yet been performed. To the best of our knowledge, no meta-analysis has been published on the prevalence of pathological subtypes in children with SRNS. In this study, we conducted a systematic literature search to extract data on the prevalence of histopathological subtypes and performed a meta-analysis to determine the prevalence of each subtype. Our findings will provide valuable insights for guiding clinical treatment decisions and predicting outcomes in children with SRNS.
2 Methods
A comprehensive literature search was conducted in PubMed, Embase, Web of Science, and Cochrane databases from their inception to December 2023, adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart to identify relevant studies for this systematic review. The search was systematically performed using Boolean operators (OR and AND) to combine key terms. The search strategy was based on three primary concepts: “steroid,” “nephrotic syndrome,” and “drug resistance.”
This review was conducted in accordance with the guidelines of the PRISMA statement for systematic reviews and meta-analyses. Additionally, this study has been registered with PROSPERO, registration number: CRD420251000869.
2.1 Search strategy
2.1.1 Databases searched
We conducted a comprehensive literature search across PubMed, Embase, Web of Science, and the Cochrane Library, covering the period from database inception through December 2023, to identify studies relevant to the objectives of this systematic review. All retrieved records were manually screened for eligibility.
2.1.2 Search terms and search strategy
A systematic search was conducted using Boolean operators (OR and AND) to combine key terms. The search strategy was based on three primary concepts: “steroid,” “nephrotic syndrome,” and “drug resistance.” The search strategy was developed according to the PICOs principle:(1)P (Population): Children with SRNS.(2)I (Intervention) and C (Comparison): Not applicable to this study.(3)O (Outcome): Prevalence of histopathological subtypes in SRNS children or studies reporting the number of SRNS histopathological subtypes to facilitate prevalence calculation.(4)S (Study design): This review includes cross-sectional and longitudinal studies. Non-SRNS populations, qualitative studies, and secondary analyses were excluded. For studies involving both adults and children, only data specific to pediatric SRNS histopathological subtypes were retained. Among multiple articles using the same dataset, the study with the most comprehensive data or the largest sample size was selected.
2.1.3 Inclusion and exclusion criteria
2.1.3.1 Inclusion criteria
①SRNS: Defined as the failure to achieve complete remission after 4 weeks of prednisone treatment at standard doses (60 mg/m²/day or 2 mg/kg/day) (5).②Kidney Biopsy Results: Studies must report the kidney biopsy findings of the patients.③Age Range: Only studies involving children aged 3 months to 18 years will be considered (6).
2.1.3.2 Exclusion criteria
①Case reports, letters to the editor, and review articles were excluded.②Studies that did not report kidney biopsy results.③Studies involving children with steroid-dependent nephrotic syndrome (SDNS) or frequently relapsing nephrotic syndrome (FRNS) were excluded.
2.2 Study screening
The search results were imported into EndNote X7, and duplicates were removed. Two researchers(Sen lin and Wanying Dong) independently screened the titles and abstracts of the identified studies using EndNote X7. At least one researcher reviewed the full text of studies that were potentially eligible for inclusion. The eligibility of each study was assessed by both researchers, and in cases of disagreement, a third researcher(Yangyang Tian) was consulted. Additionally, the reference lists of included studies were manually screened to identify any additional relevant studies that met the inclusion criteria.
2.3 Data extraction
Two researchers (Sen lin and Wanying Dong)independently extracted data using a pre-tested data extraction form. Any discrepancies in data extraction were resolved through discussion or by consulting the original publications. The information collected included the title, first author, publication year, study period, country of study, study design, sample size, participant age range, mean age, histopathological subtypes, and their prevalence. Countries were categorized according to the World Bank (WB) classification as high-income, low- and middle-income countries.
2.4 Quality assessment
The quality of the included studies was assessed using the Joanna Briggs Institute Critical Appraisal Checklist. Studies meeting the quality criteria were included. Two researchers(Sen lin and Wanying Dong)independently reviewed all included studies, and a third researcher(Yangyang Tian) was involved when necessary to reach a consensus.
2.5 Meta-analysis
Data analysis was performed using Stata versions 14.0, 15.0, and 17.0, along with the meta and metafor software packages. The overall prevalence of histopathological subtypes associated with pediatric SRNS was calculated. Subgroup analysis was conducted based on the income level of the country where the study was conducted. Due to high heterogeneity, a random-effects model (DerSimonian-Laird method) was applied in the meta-analysis to determine the overall prevalence of histopathological subtypes related to pediatric SRNS. Forest plots were generated to visually summarize the details of individual studies and estimate the common effect size, as well as the degree of heterogeneity. Cochran’s Q test was used to assess heterogeneity, and the I² statistic was employe for quantification. I² values of 25%, 50%, and 75% were considered indicative of low, moderate, and high heterogeneity, respectively. Funnel plots were used for a qualitative visual assessment of publication bias, and the Begg rank correlation test was employed for quantitative assessment. Sensitivity analysis was conducted for each histopathological subtype to evaluate the influence of individual studies and assess whether any study had an inordinate effect on the overall results.
3 Results
The articles included in this review comprised prospective studies, retrospective studies, and randomized controlled trials, focusing on children and adolescents aged 3 months to 18 years. A total of 3,219 articles were initially retrieved, with 1,250 duplicates removed. After reviewing the titles and abstracts of the remaining 1,969 articles, 283 were selected for full-text review. Of these 283 articles, 9 did not have the original text available, and 274 articles were successfully retrieved in full. Upon full-text review, 247 articles were excluded, including those the absence or incompleteness of renal biopsy data(144 articles), case reports (4 articles), reviews (9 articles), studies involving adult SRNS (42 articles), studies unrelated to steroid resistance (38 articles), letters (8 articles), case series (14 articles). Ultimately, 15 studies were included. Figure 1 shows the PRISMA flowchart of the selection process.
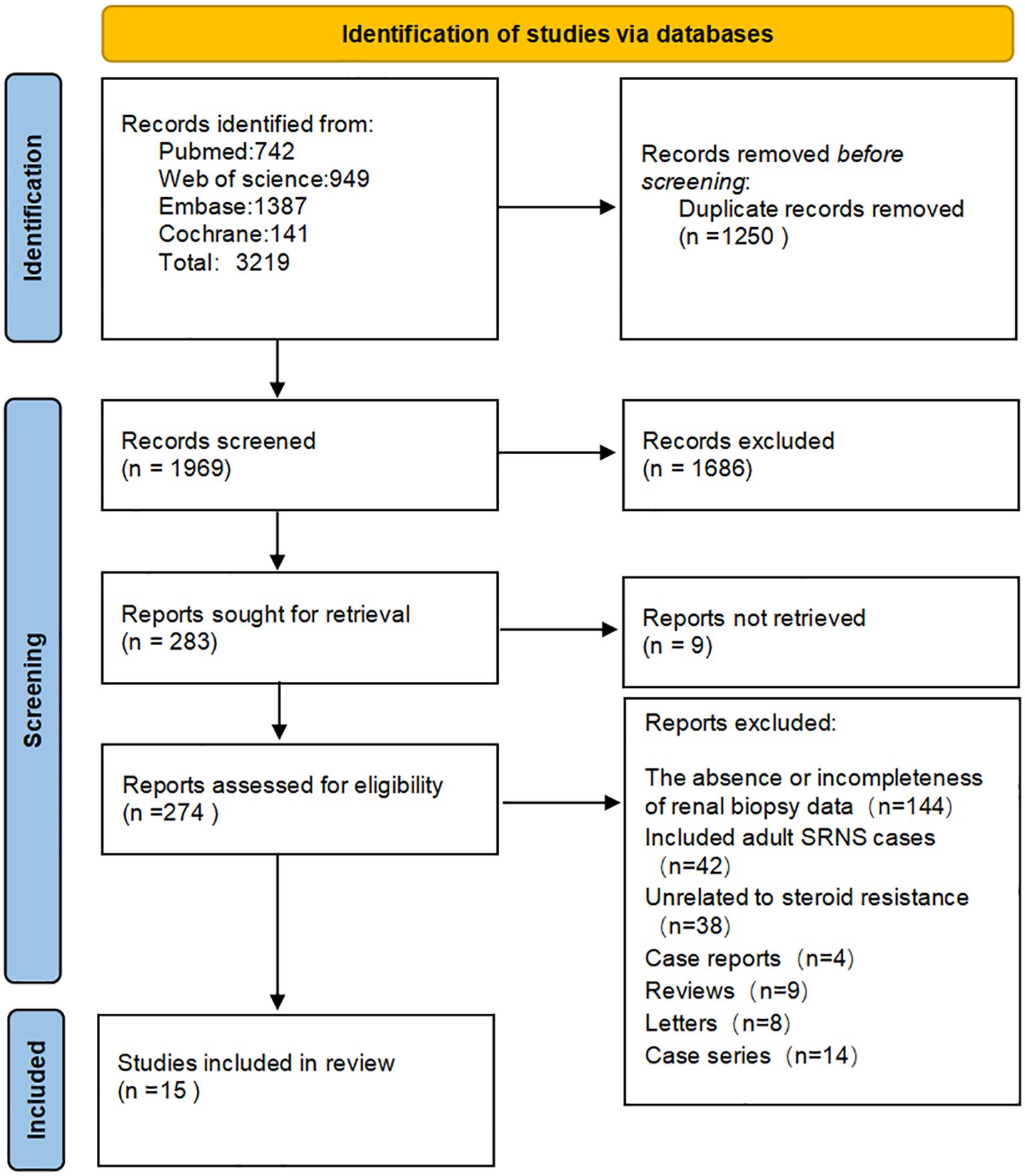
Figure 1. Preferred reporting items for systematic review and meta‐analysis protocols flow diagram. EMBASE, Excerpta Medica database; Cochrane, Cochrane Central Register of Controlled Trials.
3.1 Description of included studies
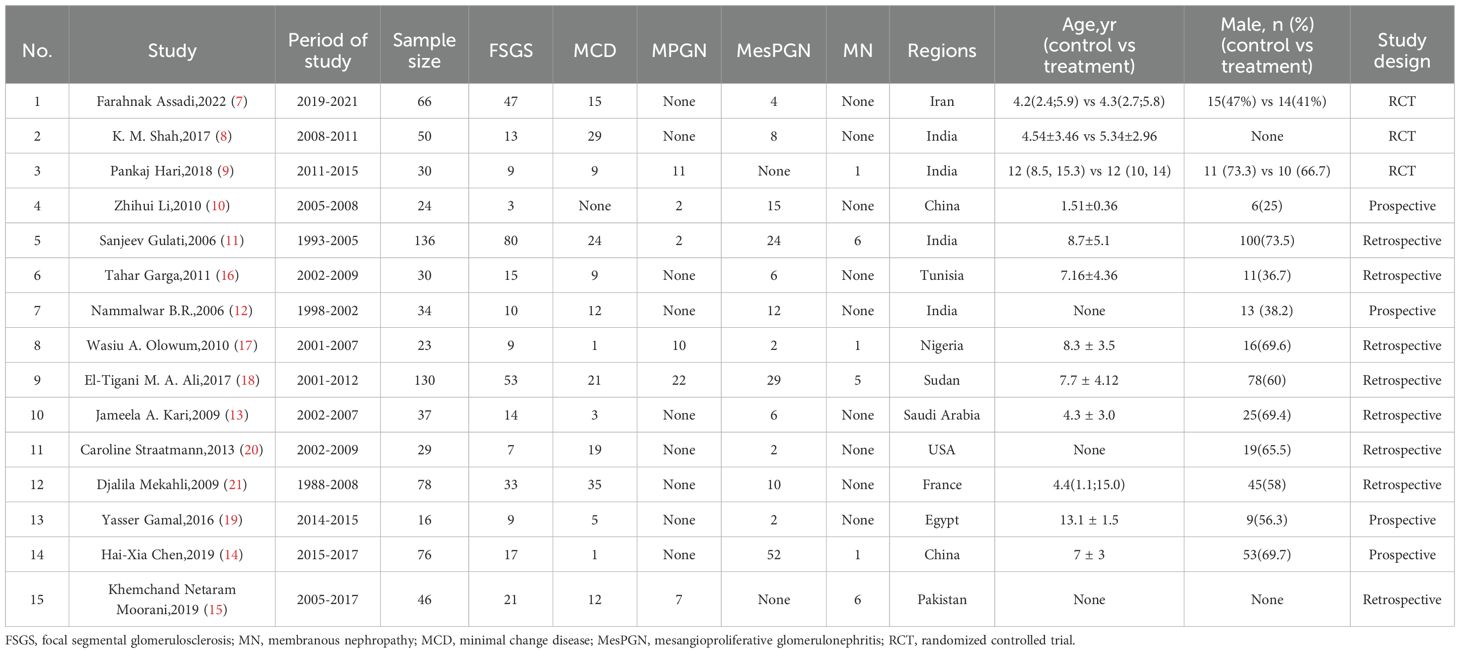
Table 1 summarizes the characteristics of the studies included in this review. The studies were conducted in 11 different countries or regions, distributed across Asia [9 studies (7–15)], Africa [4 studies (16–19)] and Europe/North America [2 studies (20, 21)]. The participant distribution across the included countries was: Iran (66), India (250), China (100), Kingdom of Saudi Arabia (37), Pakistan (46), Tunisia (30), Nigeria (23), Sudan (130), United States (29), France (78), and Egypt (16)(Table 1, Figure 2). The majority of studies were observational (n = 12), while the remaining three studies (7–9) were randomized controlled trials (RCTs). All studies focused on children aged 3 months to 18 years diagnosed with SRNS. Each study reported the prevalence of various histopathological subtypes of SRNS, with five subtypes included in the analysis: FSGS, membranous nephropathy (MN), minimal change disease (MCD), mesangioproliferative glomerulonephritis (MesPGN), and membranoproliferative glomerulonephritis (MPGN). The most commonly reported subtypes were FSGS (n = 15), MCD (n = 14), and MesPGN (n = 14). Eleven studies (7–10, 14–20) were published after 2010. All studies reported prevalence data for both boys and girls. The quality score for each included study was at least 6 points, with the detailed quality assessment provided in the Supplementary Table 1. When patient ages followed a normal distribution, they were presented as mean ± standard deviation; otherwise, the median and interquartile range were reported.
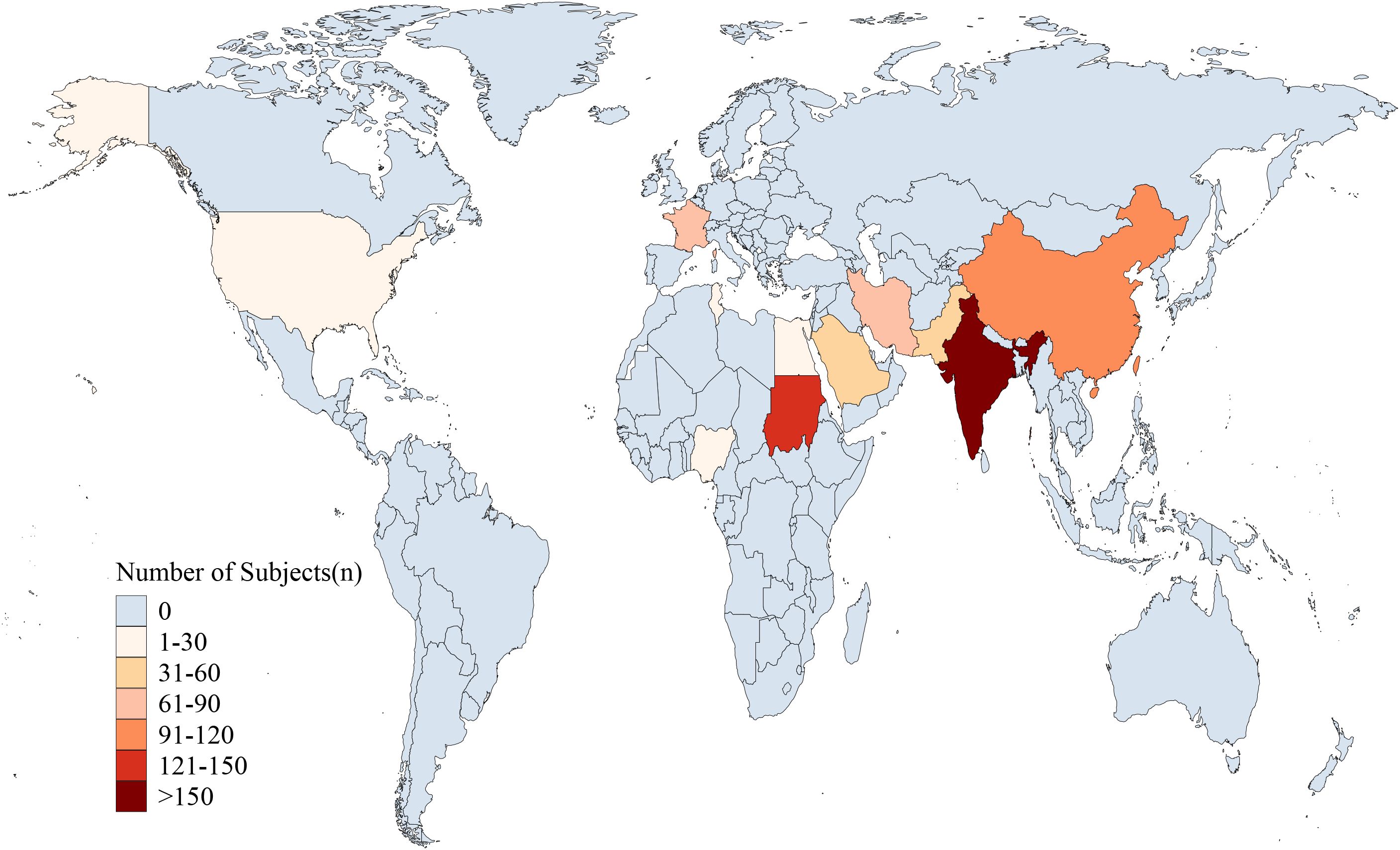
Figure 2. Geographical distribution of study participants. (The participant distribution across the included countries was: Iran (66), India (250), China (100), Kingdom of Saudi Arabia (37), Pakistan (46), Tunisia (30), Nigeria (23), Sudan (130), United States (29), France (78), and Egypt (16).
3.2 Quality assessment
Supplementary Table 1 presents the quality assessment results for the included studies. The review performed well on questions 1, 4, 5, 6, 7, and 8, which address the appropriateness of the sample frame, detailed description of study subjects and setting, thorough data analysis, accurate diagnosis, standardization of measurement methods, and proper statistical analysis. However, the review performed poorly on questions 2, 3, and 9, which address the appropriateness of the sampling method, sample size adequacy, and follow-up rate.
3.3 Prevalence of histopathological subtypes in SRNS
The estimated prevalence of histopathological subtypes associated with SRNS is as follows: FSGS: 39% (95% CI, 31-48; I² = 85.4%; p < 0.000) (Figure 3A); MN: 4% (95% CI, 1-6; I² = 25.4%; p = 0.244) (Figure 3D); MCD: 27% (95% CI, 17-36; I² = 94.0%; p < 0.000) (Figure 3B); MesPGN: 23% (95% CI, 14-32; I² = 91.6%; p < 0.000) (Figure 3C); MPGN: 18% (95% CI, 7-29; I² = 90.9%; p < 0.000) (Figure 3E). The I² statistic reveals high heterogeneity for most SRNS histopathological subtypes, except for MN, which exhibited low heterogeneity (I² < 50%).
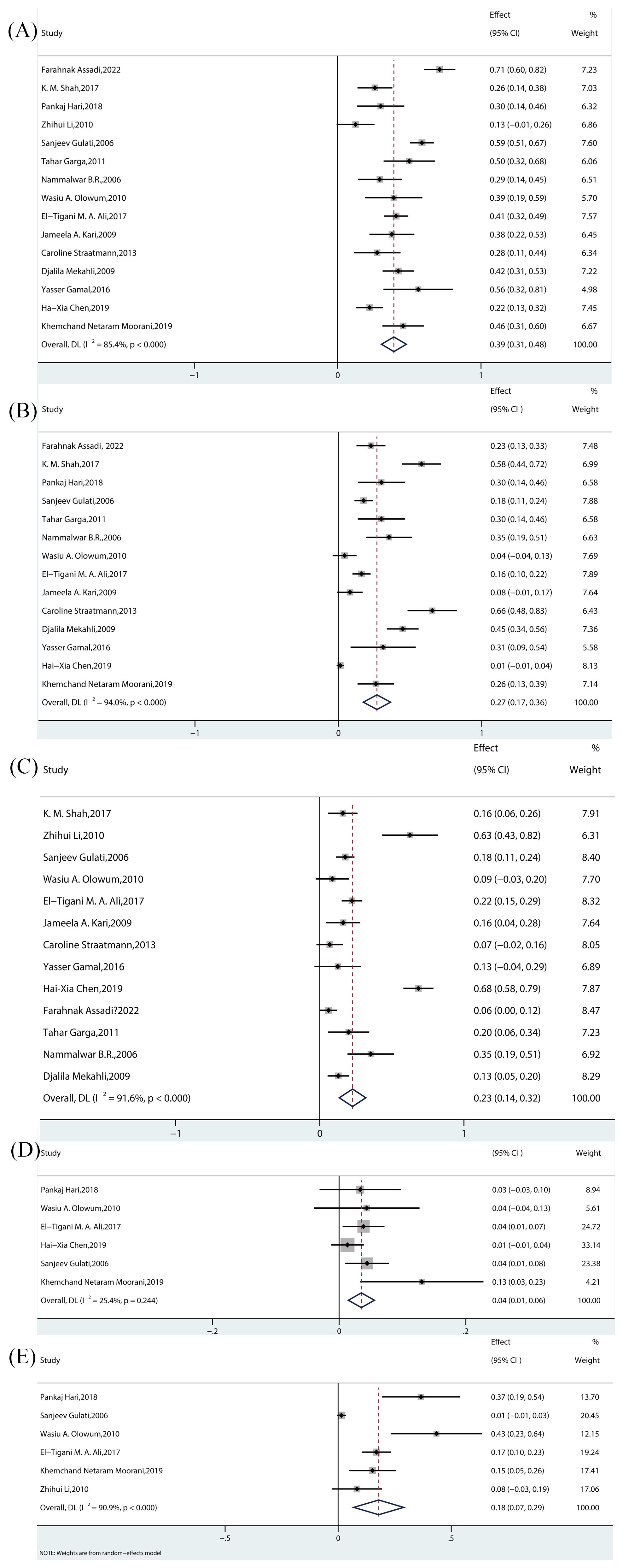
Figure 3. Meta-analyses of the prevalence rates across pathological subtypes. (A) Prevalence of FSGS in children. (B) Prevalence of MCD in children. (C) Prevalence of MesPGN in children. (D) Prevalence of MN in children. (E) Prevalence of MPGN in children. FSGS, focal segmental glomerulosclerosis; MCD, minimal Change Disease; MesPGN, mesangial proliferative glomerulonephritis; MN, membranous nephropathy;MPGN, membrano-proliferative glomerulonephritis.
3.3.1 FSGS
FSGS was the most commonly reported histopathological subtype, with all 15 studies reporting its prevalence. Among these, 3 experimental studies (7–9)(all randomized controlled trials [RCTs]) reported prevalence rates ranging from 26% to 71.21%. The remaining 12 observational studies (10–21)(including prospective, retrospective, and cross-sectional studies) reported prevalence rates ranging from 12.50% to 58.8%. The pooled effect size was statistically significant [Rate = 39%, 95% CI (31–48), p = 0.00].
3.3.2 MCD
MCD was the second most frequently reported histopathological subtype, with 14 studies including it. Among these, 3 experimental studies (7–9)(all RCTs) reported prevalence rates between 22.7% and 58.00%, while 11 observational studies (11–21)(prospective, retrospective, and cross-sectional) reported prevalence rates ranging from 1.32% to 65.5%. The pooled effect size was statistically significant [Rate = 27%, 95% CI (17–36), p = 0.00].
3.3.3 MesPGN
A total of 13 studies reported the prevalence of MesPGN. Of these, 2 experimental studies (7, 8) (RCTs) reported prevalence rates between 6.1% and 16%, while 11 observational studies (10–14, 16–21) (prospective, retrospective, and cross-sectional) reported rates ranging from 8.7% to 68.42%. The pooled effect size was statistically significant [Rate = 23%, 95% CI (14–32), p = 0.00].
3.3.4 MN
Six studies reported the prevalence of MN. Among these, 1 experimental study (9) (RCT) reported a prevalence rate of 9.68%, and 5 observational studies (11, 14, 15, 17, 18) (prospective, retrospective, and cross-sectional) reported rates between 1.3% and 13%. The pooled effect size was statistically significant [Rate = 4%, 95% CI (1–6), p = 0.244].
3.3.5 MPGN
Six studies reported the prevalence of MPGN. Of these, 1 experimental study (9) (RCT) reported a prevalence rate of 36.6%, while 5 observational studies (10, 11, 15, 17, 18) (prospective, retrospective, and cross-sectional) reported rates between 1.5% and 43.48%. The pooled effect size was statistically significant [Rate = 18%, 95% CI (7-29), p = 0.00].
3.4 Subgroup analysis of SRNS histopathological subtypes
From the forest plots of each histopathological subtype, it is evident that most of the subtypes exhibit high heterogeneity. Therefore, we performed subgroup meta-analyses to identify potential sources of heterogeneity. Typically, each subgroup should contain at least three studies. However, it was not possible to group studies by the world health organization(WHO) regional standards, as only two studies (20, 21) belonged to the Europe/North America region, and subgroup analysis could not be conducted for this group. Subgroup analyses by study type showed that heterogeneity remained substantial both before and after subgrouping.
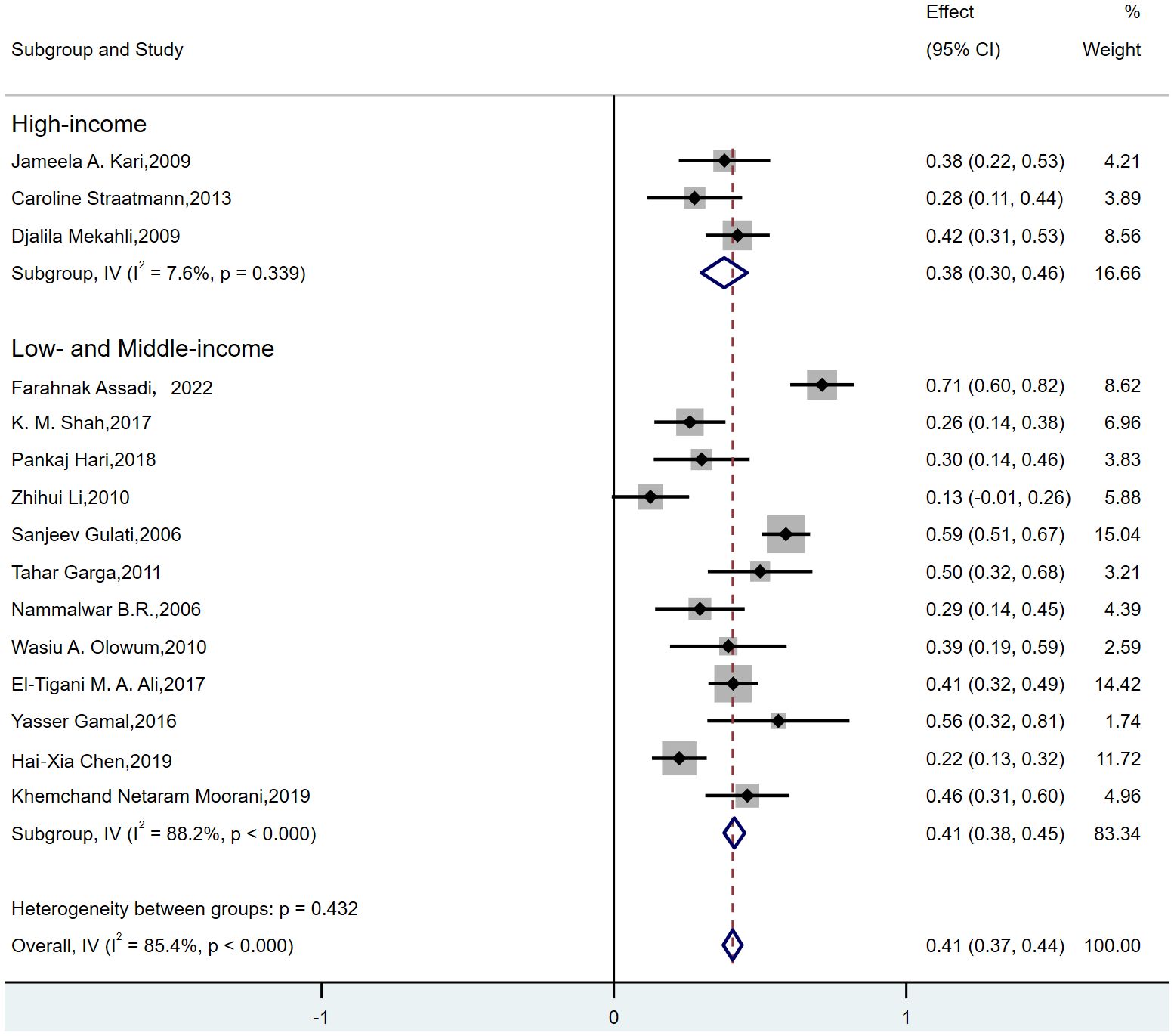
For FSGS, MCD, and MesPGN, subgroup analyses were conducted based on the income level of the country where the study was conducted (high-income, low-income, and middle-income countries). Since MN exhibited low heterogeneity, no subgroup analysis was conducted for this subtype. Additionally, no studies from high-income countries were available for MPGN, so subgroup analysis was not performed for these two histopathological subtypes. The results showed that when grouping by the income level of the country where the study was conducted, the following observations were made:for FSGS(Figure 4), the “low- and middle-income” group (7–12, 14–19) exhibited high heterogeneity (I² = 88.2%, p < 0.000), while the “high-income” group (13, 20, 21) showed low heterogeneity (I² = 7.6%, p = 0.339). Therefore, the heterogeneity in FSGS could be attributed to the “low- and middle-income” populations. For MesPGN (Supplementary Figure 10), the “low- and middle-income” group (7, 8, 10–12, 14, 16–19) showed high heterogeneity (I² = 93.2%, p < 0.000), while the “high-income” group showed very low heterogeneity (I² = 0.0%, p = 0.431). This suggests that the heterogeneity in MesPGN might also stem from the “low- and middle-income” populations. For MCD(Supplementary Figure 11), heterogeneity remained high both before and after subgrouping based on “country income level”.
3.5 Sensitivity analysis
As shown in Supplementary Figures 5-9, removing any single study for each histopathological subtype did not significantly affect the final pooled effect size, indicating that the results of this study are relatively stable and the findings are highly credible.
3.6 Publication bias
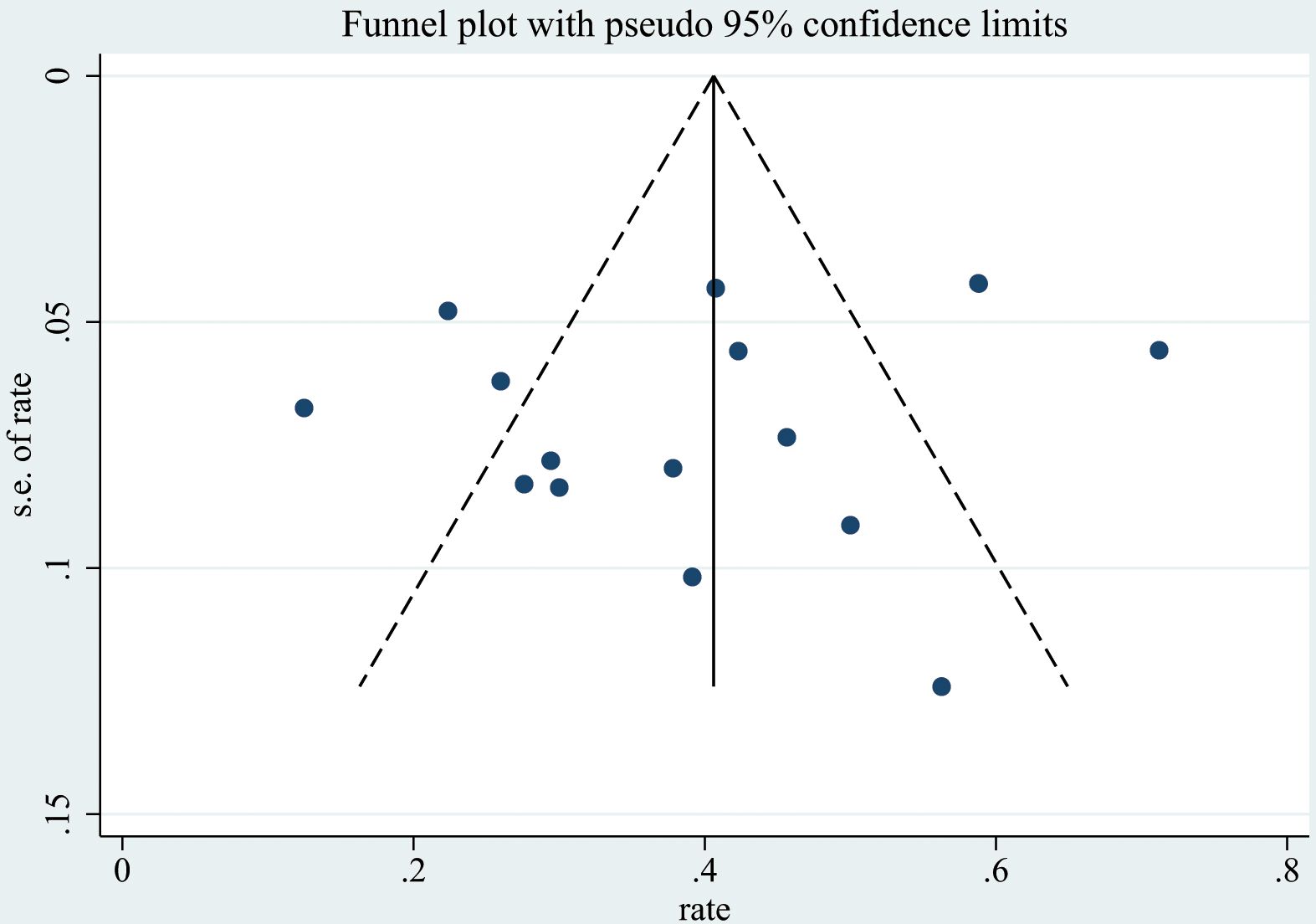
Publication bias was qualitatively assessed using funnel plots(Figure 5, Supplementary Figures 1-4). However, assessing the symmetry of funnel plots can be highly subjective. Therefore, we also used the Begg rank correlation test (Table 2) for a quantitative assessment. The results indicated that the p-values for the Begg test for FSGS, MesPGN, MN, MCD, and MPGN were all greater than 0.05, suggesting that there is minimal publication bias, and the results are therefore reliable.
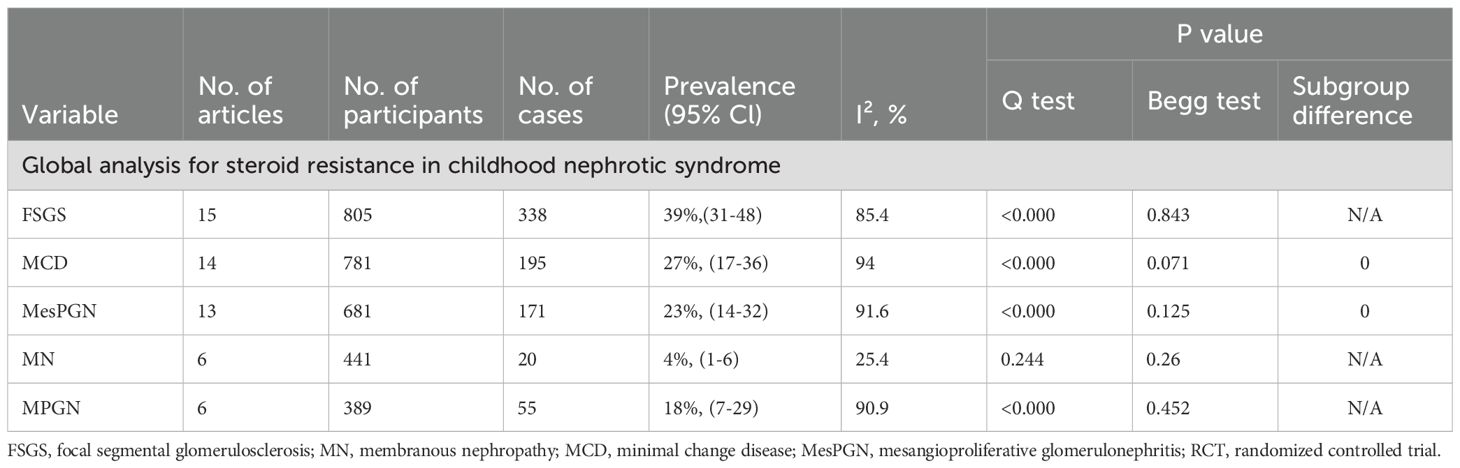
Table 2. Prevalence rates of histopathologic subtypes SRNS Using Random-Effects Meta-analysis and Subgroup Meta-analysis.
4 Discussion
Historically, pediatric NS was considered a benign condition with a favorable response to steroid treatment. Morphologically, most cases presented as minimal change glomerulonephritis, which typically had a good long-term prognosis. Over 85% of children and adolescents with NS experience complete remission of proteinuria after steroid therapy (2, 22). A positive response to steroids has long been considered an important prognostic indicator for preserving kidney function. Although many patients with SSNS often experience relapses or develop steroid resistance, their long-term renal prognosis is generally favorable. The main challenge for these patients is the side effects of prolonged corticosteroid therapy and the use of other immunosuppressive agents.
However, for those who do not respond to steroids, a diagnosis of SRNS is made. SRNS is typically diagnosed when there is no complete remission after 4 weeks of prednisone treatment at standard doses (60 mg/m²/day or 2 mg/kg/day). SRNS is considered a significant risk factor for progression to end-stage renal disease (ESRD). Steroid resistance most commonly occurs during the initial period of prednisone treatment (initial resistance), but it may also develop during relapse treatment in patients who previously responded to steroids or second-line therapies (late resistance).
Renal biopsy remains a critical tool for both diagnosis and prognostic classification in nephrology (23). It allows for the assessment of tissue characteristics through optical, immunofluorescent, and electron microscopy techniques. An adequate biopsy typically includes approximately 25 glomeruli, especially when evaluating focal or segmental lesions (22). The pioneering International Study of Kidney Disease in Children (ISKDC), conducted between 1967 and 1976 with patients recruited across three continents, first reported that MCNS was the most common histological finding in kidney biopsies from children with idiopathic NS (24, 25). In contrast, FSGS is a rare cause of childhood NS, with a biopsy positivity rate of only 5%-7% (24, 25). Subsequent studies (22) have reported that in patients with SRNS, the prevalence of FSGS, MCD and MesPGN ranges from 35-55%, 25-40%, and 10-15%, respectively, which aligns closely with the conclusions of our meta-analysis. Our meta-analysis included 15 studies, all of which incorporated FSGS. Seven of these studies consistently identified FSGS as the most prevalent histological subtype associated with SRNS in children. In pediatric nephrology, steroid resistance is often considered a hallmark of FSGS, as clinicians typically resort to renal biopsy to clarify the etiology after corticosteroid treatment failure. Moreover, compared to other histological subtypes, the global prevalence of FSGS appears to be on the rise (43% vs 62% vs 86%; P = 0.03) (26) particularly in Western countries and Asia (27–32). However, these reports have not systematically assessed patients’ steroid responsiveness; therefore, it remains unclear whether the histological changes are concomitant with sustained steroid resistance and long-term prognosis variations. A study conducted in Nigeria indicates that the most common cause of nephrotic syndrome has shifted from quinine-induced nephropathy (QMN) in the 1960s, to MPGN in the 1980s, and now to FSGS (33). A recent review highlighted this trend, suggesting that Nigeria may be undergoing a transition from QMN to MPGN, and now to FSGS (34). The reasons behind the increasing prevalence of FSGS remain unclear. It may be attributed to advancements in diagnostic methods or could reflect an absolute increase in incidence, potentially driven by obesity and chronic inflammation. For instance, the improvement in renal biopsy expertise may contribute to the rising prevalence, as FSGS might be easily overlooked if only cortical glomeruli are biopsied, without sampling medullary glomeruli to capture a more comprehensive picture.
Recently, FSGS has been classified into six forms (35) primary or idiopathic FSGS, adaptive FSGS (the two most common forms), high-mutation genetic FSGS, virus-mediated FSGS, drug-induced FSGS (three less common forms), and the newly identified APOL1-related FSGS. Emerging evidence indicates that FSGS associated with the APOL1 gene is more likely to progress to ESKD. A morphologic classification (36) was previously proposed based on the Columbia classification, which defines 5 variants: collapsing, tip, cellular, perihilar, and not otherwise specified(NOS).FSGS (NOS) represents the most prevalent “classic” variant (36), which is a diagnosis of exclusion established after ruling out other specific subtypes. Its prognosis correlates primarily with the severity of proteinuria and serum creatinine levels at clinical presentation, with nephrotic-range proteinuria portending a poorer outcome. In pediatric populations, this variant is frequently accompanied by diffuse mesangial hypercellularity; however, this finding is generally regarded as an early disease manifestation and does not appear to influence long-term prognosis. The perihilar variant is defined by the presence of perihilar sclerosis and hyalinosis involving greater than 50% of segmentally sclerotic glomeruli. The perihilar variant is strongly associated with secondary forms of FSGS (37), such as those related to obesity or renal hypoplasia, and clinical management should prioritize addressing the underlying etiology. The cellular variant is characterized by segmental endocapillary hypercellularity and typically presents with severe nephrotic syndrome of acute onset. This variant is thought to represent an early, active stage of FSGS, and due to its dynamic histologic features, may demonstrate a more favorable response to immunosuppressive therapy, including corticosteroids, compared to other subtypes. The tip variant of FSGS is defined by the presence of at least one glomerulus with a segmental lesion involving the tip domain (i.e., the peripheral 25% of the glomerular tuft next to the origin of the proximal tubule).The relationship of tip lesion to minimal change disease and FSGS has been hotly debated. Regarding the tip variant, a substantial proportion of studies indicate that affected patients exhibit higher rates of response to corticosteroid treatment and maintain good long-term renal survival, resembling outcomes seen in minimal change disease. Nonetheless, some cases may still progress to classic FSGS. The designation of collapsing variant (also known as collapsing glomerulopathy) is applied to cases of FSGS in which at least one glomerulus displays segmental or global obliteration of the glomerular capillary lumina by wrinkling and collapse of glomerular basement membranes(GBMs) associated with podocyte hypertrophy and hyperplasia. When compared with patients with FSGS (NOS), patients with idiopathic collapsing FSGS are more likely to be black (38) and to present with more severe markers of nephrotic syndrome, including more severe proteinuria, hypoalbuminemia, and hypercholesterolemia. The collapsing variant is typically resistant to corticosteroid therapy. Although the included studies lacked further pathological subtyping of FSGS, we hypothesize, based on existing literature and clinical observations, that the higher prevalence of the collapsing variant may explain the greater propensity for steroid resistance in children with FSGS. This insight offers a promising avenue for future mechanistic investigations. In addition, a considerable proportion of steroid-resistant patients present with MCD, with a prevalence of 27% in our study cohort, second only to FSGS. This finding aligns with previous reports suggesting that histological minimal changes do not always correlate with steroid responsiveness (39) and sometimes progress to FSGS in follow-up biopsies (40, 41).Some studies propose that MCD and idiopathic FSGS are two manifestations of the same disease progression. FSGS is also a consistent feature of massive podocyte loss in animal models and is a common trait of nearly all progressive glomerular diseases. In clinical trials, idiopathic FSGS should be regarded as an advanced stage of disease progression, with a reduced likelihood of response to treatment when compared to the earlier stages of the disease, typically characterized by MCD (42).A study (43) involving repeated biopsies of 171 patients revealed that in 47 cases, 26 (55%) had their diagnosis changed from minimal change disease to FSGS, while 16 of 33 (48%) had their diagnosis changed from mesangioproliferative glomerulonephritis to FSGS.
Through subgroup analysis, we observed no significant difference in the prevalence of FSGS between high-income and middle-low-income countries. However, the prevalence of MCD was notably higher in high-income countries compared to middle-low-income countries, while the prevalence of MesPGN was significantly lower in high-income countries. It has been previously suggested that race or ethnicity may serve as risk factors for chronic kidney disease (CKD) (e.g., higher risks in African Americans, Indigenous populations in Australia, Canada, and the United States, South Asians, and East Asians). However, these associations may be influenced by socioeconomic factors (44, 45). Some studies have also indicated a link between socioeconomic status and systemic diseases that may involve the kidneys, such as systemic lupus erythematosus (SLE) (46). It is essential to clarify the relationship between socioeconomic status and glomerular diseases for various reasons. For instance, economically disadvantaged populations are more vulnerable to other chronic conditions, which may complicate the management of glomerular diseases (47). Low socioeconomic status can also undermine access to routine care, quality of life, and medication adherence, leading to a vicious cycle (48). Studies (49) have confirmed a negative correlation between socioeconomic status and FSGS. Combined with our subgroup analysis results, this may explain the heterogeneity in FSGS pathology subtypes, which may be attributed to the “national income level.”
The prevalence of other pathological subtypes, including MesPGN, MN, and MPGN, was 23%, 4%, and 18%, respectively. Due to the limited number of studies on C1q nephropathy and IgA nephropathy (both with only two studies), a meta-analysis was not conducted for these conditions, and therefore, these two studies were excluded.
This study, through meta-analysis, provides a comprehensive distribution of pathological subtypes in pediatric SRNS, offering valuable insights for clinical diagnosis and treatment. We have elucidated the distribution of different pathological subtypes in children with SRNS. On one hand, this serves as a valuable addition to epidemiological research on SRNS, allowing for informed assumptions regarding the pathological patterns in these patients, which may enhance nephrologists’ understanding of the disease. On the other hand, these findings, combined with guideline recommendations, can support the empirical selection of appropriate therapies, thereby helping to prevent delays in treatment and reduce the risk of adverse drug reactions. However, it is important to note that our study focused on children with SRNS who have already been diagnosed and undergone renal biopsy, mainly examining the distribution of their pathological subtypes. In clinical practice, there are still cases where a renal biopsy is performed without prior treatment. For these patients diagnosed with a particular pathological subtype of nephrotic syndrome, the likelihood of steroid resistance remains uncertain. Therefore, more reliable evidence from clinical studies is needed to guide the diagnosis and treatment of these patients.
5 Strengths and limitations
Previous reviews have primarily focused on the distribution of pathological subtypes in pediatric SRNS within specific regions (e.g., sub-Saharan Africa). To the best of our knowledge, this represents the first meta-analysis to elucidate the distribution of pathological subtypes in pediatric patients with SRNS. However, there are several limitations in this study. Although our search encompassed studies on children with SRNS worldwide, the final inclusion of only 15 studies—spanning 3 continents and 11 different countries (Figure 2)—was hampered by obstacles such as limited healthcare resources, lack of renal pathology data, and generally low quality of the available literature. Given the current body of literature, relevant data from many countries or regions remain difficult to obtain or are incomplete. Consequently, the geographical coverage of this meta-analysis is limited and insufficient to reflect the global prevalence of pathological subtypes among children with SRNS. Thus, the findings of this study should be regarded as a snapshot of the currently available evidence. Future epidemiological research should prioritize investigating “dark sites”—regions from which no data on this issue are currently available. Among the 15 studies included, only three were RCTs, with the remainder being observational studies. This may introduce potential biases due to non-random sampling methods and insufficient sample sizes in some of the studies. Another limitation is the lack of an exploration into the distribution of pathological subtypes according to gender and age groups. Most of the included studies did not correlate pathological types with age and gender, thus preventing subgroup analyses based on these variables. Furthermore, a notable heterogeneity was observed in the random-effects models for most pathological types in the meta-analysis. Except for the MN studies, which exhibited low heterogeneity, the I² values for most SRNS pathological subtypes were greater than 75%. This level of heterogeneity is commonly observed in meta-analyses of single-arm studies, such as those on disease prevalence by pathological type.
6 Conclusion
The most commonly reported pathological subtypes associated with SRNS in children include FSGS, MN, MCD, MesPGN, and MPGN. Among these, FSGS had the highest prevalence, with an average rate of 39%, followed by MCD (27%), MesPGN (23%), MPGN (18%), and MN (4%). This systematic review and meta-analysis is the first to provide a comprehensive description of the global prevalence of common pathological subtypes associated with steroid resistance in pediatric nephrotic syndrome. The findings of this study will serve as a valuable reference for guiding future pharmacological interventions and predicting outcomes in children with SRNS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing, Funding acquisition, Resources, Visualization. SZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft. WD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft. YT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – review & editing. HL: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. XG: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Conceptualization, Supervision. FQ: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Software. YL: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Conceptualization, Project administration, Validation, Writing – review & editing. CM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82170742) and Young Taishan Scholars Program(tsqn202408366).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used artificial intelligence-assisted technology (ChatAI-ChatGPT4o) to polish this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1647608/full#supplementary-material
References
1. Campbell RE and Thurman JM. The immune system and idiopathic nephrotic syndrome. Clin J Am Soc Nephrol. (2022) 17:1823–34. doi: 10.2215/cjn.07180622
2. Barnett HL and Edelmann CM. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the international study of kidney disease in children. J Pediatr. (1981) 98:561–4. doi: 10.1016/s0022-3476(81)80760-3
3. Kamei K, Nozu K, Horinouchi T, Sakakibara N, Nishi K, Fujita N, et al. Relationship between clinical and pathologic findings and the presence of genetic variants in patients with steroid-resistant nephrotic syndrome. Pediatr Nephrol. (2025) 40:3111–20. doi: 10.1007/s00467-025-06842-x
4. Uwaezuoke SN, Ndu IK, and Mbanefo NR. Prevalence rates of histopathologic subtypes associated with steroid resistance in childhood nephrotic syndrome in sub-saharan africa: A systematic review. Int J Nephrol Renovascular Dis. (2019) 12:167–76. doi: 10.2147/ijnrd.S207372
5. Kidney Disease: Improving Global Outcomes Nephrotic Syndrome In Children Work G, Floege J, Gibson KL, Vivarelli M, Liew A, Radhakrishnan J, et al. Kdigo 2025 clinical practice guideline for the management of nephrotic syndrome in children. Kidney Int. (2025) 107:S241–S89. doi: 10.1016/j.kint.2024.11.007
6. Habashy D, Hodson EM, and Craig JC. Interventions for steroid-resistant nephrotic syndrome: A systematic review. Pediatr Nephrol. (2003) 18:906–12. doi: 10.1007/s00467-003-1207-0
7. Assadi F, Mazaheri M, and Sadeghi-Bodj S. Randomized controlled trial to compare safety and efficacy of mycophenolate vs. Cyclosporine after rituximab in children with steroid-resistant nephrotic syndrome. Pharmacotherapy. (2022) 42:690–6. doi: 10.1002/phar.2721
8. Shah KM, Ohri AJ, and Ali US. A randomized controlled trial of intravenous versus oral cyclophosphamide in steroid-resistant nephrotic syndrome in children. Indian J Nephrol. (2017) 27:430–4. doi: 10.4103/ijn.IJN_201_16
9. Hari P, Khandelwal P, Satpathy A, Hari S, Thergaonkar R, Lakshmy R, et al. Effect of atorvastatin on dyslipidemia and carotid intima-media thickness in children with refractory nephrotic syndrome: A randomized controlled trial. Pediatr Nephrol. (2018) 33:2299–309. doi: 10.1007/s00467-018-4036-x
10. Li Z, Duan C, He J, Wu T, Xun M, Zhang Y, et al. Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. (2010) 25:883–8. doi: 10.1007/s00467-009-1375-7
11. Gulati S, Sengupta D, Sharma RK, Sharma A, Gupta RK, Singh U, et al. Steroid resistant nephrotic syndrome: role of histopathology. Indian pediatrics. (2006) 43:55–60.
12. Nammalwar BR, Vijaykumar M, Prahlad N, and Jain DV. Steroid resistant nephrotic syndrome is sustained remission attainable. Indian pediatrics. (2006) 43:39–43.
13. Kari JA, Halawani M Fau - Mokhtar G, Mokhtar G Fau - Jalalah SM, Jalalah Sm Fau - Anshasi W, and Anshasi W. Pattern of steroid resistant nephrotic syndrome in children living in the kingdom of Saudi Arabia: A single center study. Saudi J Kidney Dis Transpl. (2009) 20:854–7.
14. Chen HX, Cheng Q, Li F, He QN, Cao Y, Yi ZW, et al. Efficacy and safety of tacrolimus and low-dose prednisone in chinese children with steroid-resistant nephrotic syndrome. World J Pediatr. (2020) 16:159–67. doi: 10.1007/s12519-019-00257-z
15. Moorani KN, Hotchandani HM, Zubair AM, Lohana NC, and Veerwani NR. Immunosuppressive therapy in children with primary nephrotic syndrome: single center experience, karachi, Pakistan. BMC Nephrol. (2019) 20:239. doi: 10.1186/s12882-019-1347-5
16. Gargah T, Labassi A, Goucha-Louzir R, Ben Moussa F, and Lakhoua MR. Histopathological spectrum of childhood idiopathic steroid-Resistant nephrotic syndrome in Tunisia. LA TUNISIE MEDICALE - 20. (2011) 89:258–61.
17. Olowu WA, Adelusola KA, and Adefehinti O. Childhood idiopathic steroid resistant nephrotic syndrome in southwestern Nigeria. Saudi J Kidney Dis Transpl. (2010) 21:979–90.
18. Ali E-TM, Makki HFK, Abdelraheem MB, Makke SO, and Allidir RA. Childhood idiopathic steroid-resistant nephrotic syndrome at a single center in khartoum. Saudi J Kidney Dis Transpl. (2017) 28:851–9.
19. Gamal Y, Badawy A, Swelam S, Tawfeek MS, and Gad EF. Glomerular glucocorticoid receptors expression and clinicopathological types of childhood nephrotic syndrome. Fetal Pediatr Pathol. (2017) 36:16–26. doi: 10.1080/15513815.2016.1225872
20. Straatmann C, Ayoob R, Gbadegesin R, Gibson K, Rheault MN, Srivastava T, et al. Treatment outcome of late steroid-resistant nephrotic syndrome: A study by the midwest pediatric nephrology consortium. Pediatr Nephrol. (2013) 28:1235–41. doi: 10.1007/s00467-013-2483-y
21. Mekahli D, Liutkus A, Ranchin B, Yu A, Bessenay L, Girardin E, et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: A multicenter study. Pediatr Nephrol. (2009) 24:1525–32. doi: 10.1007/s00467-009-1138-5
22. Tullus K, Webb H, and Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. (2018) 2:880–90. doi: 10.1016/s2352-4642(18)30283-9
23. Li Z, Lu Y, and Yang L. Imaging and spatial omics of kidney injury: significance, challenges, advances and perspectives. Med Rev. (2023) 3:514–20. doi: 10.1515/mr-2023-0046
24. Churg J, Habib R, and White RH. Pathology of the nephrotic syndrome in children: A report for the international study of kidney disease in children. Lancet. (1970) 760:1299–302. doi: 10.1016/s0140-6736(70)91905-7
25. Barnett HL. Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the international study of kidney disease in children. Kidney Int. (1978) 13:159–65. doi: 10.1038/ki.1978.23
26. Boyer O, Moulder JK, and Somers MJG. Focal and segmental glomerulosclerosis in children: A longitudinal assessment. Pediatr Nephrol. (2007) 22:1159–66. doi: 10.1007/s00467-007-0493-3
27. Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. (1999) 55:1885–90. doi: 10.1046/j.1523-1755.1999.00408.x
28. Srivastava T, Simon SD, and Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. (1999) 13:13–8. doi: 10.1007/s004670050555
29. Filler G, Young E, Geier P, Carpenter B, Drukker A, and Feber J. Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis. (2003) 42:1107–13. doi: 10.1053/j.ajkd.2003.08.010
30. Haas M, Spargo BH, and Coventry S. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: A 20-year renal biopsy study. Am J Kidney Dis. (1995) 26:740–50. doi: 10.1016/0272-6386(95)90437-9
31. Haas M, Meehan SM, Karrison TG, and Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976–1979 and 1995-1997. Am J Kidney Dis. (1997) 30:621–31. doi: 10.1016/s0272-6386(97)90485-6
32. Kitiyakara C, Kopp JB, and Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. (2003) 23:172–82. doi: 10.1053/snep.2003.50025
33. Asinobi AO, Ademola AD, Okolo CA, and Yaria JO. Trends in the histopathology of childhood nephrotic syndrome in ibadan Nigeria: preponderance of idiopathic focal segmental glomerulosclerosis. BMC Nephrol. (2015) 16:213. doi: 10.1186/s12882-015-0208-0
34. Anigilaje EA and Olutola A. Prospects of genetic testing for steroid-resistant nephrotic syndrome in Nigerian children: A narrative review of challenges and opportunities. Int J Nephrol Renovascular Dis. (2019) 12:119–36. doi: 10.2147/ijnrd.S193874
35. Ingulli E and Tejani A. Racial differences in the incidence and renal outcome of idiopathic focal segmental glomerulosclerosis in children. Pediatr Nephrol. (1991) 5:393–7. doi: 10.1007/BF01453661
36. D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. (2003) 23:117–34. doi: 10.1053/snep.2003.50012
37. Chia-Gil A, Floege J, Stamellou E, and Moeller MJ. Perihilar fsgs lesions originate from flat parietal epithelial cells. J Nephrol. (2024) 37:1405–9. doi: 10.1007/s40620-024-01886-y
38. Smith KD and Akilesh S. Collapsing glomerulopathy: unraveling varied pathogeneses. Curr Opin Nephrol Hypertension. (2023) 32:213–22. doi: 10.1097/mnh.0000000000000873
39. Barnett HL. Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the international study of kidney disease in children. J Pediatr. (1982) 101:514–8. doi: 10.1016/s0022-3476(82)80692-6
40. HOGG RJ. Focal segmental glomerulosclerosis in children with idiopathic nephrotic syndrome. A report of the southwest pediatric nephrology study group. Kidney Int. (1985) 27:442–9. doi: 10.1038/ki.1985.29
41. Lichtig C, Ben-Izhak O, On A, Levy J, and Allon U. Childhood minimal change disease and focal segmental glomerulosclerosis: A continuous spectrum of disease?: pathologic study of 33 cases with long-term follow-up. Am J Nephrol. (2008) 11:325–31. doi: 10.1159/000168331%
42. Maas RJ, Deegens JK, Smeets B, Moeller MJ, and Wetzels JF. Minimal change disease and idiopathic fsgs: manifestations of the same disease. Nat Rev Nephrol. (2016) 12:768–76. doi: 10.1038/nrneph.2016.147
43. Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children. Clin J Am Soc Nephrol. (2015) 10:592–600. doi: 10.2215/cjn.06260614
44. Romagnani P, Agarwal R, Chan JCN, Levin A, Kalyesubula R, Karam S, et al. Chronic kidney disease. Nat Rev Dis Primers. (2025) 11:8. doi: 10.1038/s41572-024-00589-9
45. Zeng X, Liu J, Tao S, Hong HG, Li Y, and Fu P. Associations between socioeconomic status and chronic kidney disease: A meta-analysis. J Epidemiol Community Health. (2018) 72:270–9. doi: 10.1136/jech-2017-209815
46. Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among us adults with medicaid coverage, 2000–2004. Arthritis Rheumatism. (2013) 65:753–63. doi: 10.1002/art.37795
47. Yu S, Yang H, Wang B, Guo X, Li G, and Sun Y. Nomogram for predicting risk of mild renal dysfunction among general residents from rural northeast China. J Transl Int Med. (2024) 12:244–52. doi: 10.2478/jtim-2023-0003
48. Barbour S, Lo C, Espino-Hernandez G, Sajjadi S, Feehally J, Klarenbach S, et al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrol Dialysis Transplant. (2018) 33:626–34. doi: 10.1093/ndt/gfx185
Keywords: steroid-resistant nephrotic syndrome in children, histopathologic subtypes, focal segmental glomerulosclerosis (FSGS), prevalence, immune-mediated
Citation: Lin S, Zheng S, Dong W, Tian Y, Li H, Gao X, Qin F, Ma C and Liu Y (2025) Prevalence of histopathological subtypes associated with steroid-resistant nephrotic syndrome in children: a systematic review and meta-analysis. Front. Immunol. 16:1647608. doi: 10.3389/fimmu.2025.1647608
Received: 16 June 2025; Accepted: 03 November 2025;
Published: 20 November 2025.
Edited by:
Agnieszka Swiatecka-Urban, University of Virginia, United StatesReviewed by:
Robert P. Woroniecki, Stony Brook Children’s Hospital, United StatesQiu Li, Chongqing Medical University, China
Ayah Elmaghrabi, University of Virginia, United States
Hilary Hotchkiss, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2025 Lin, Zheng, Dong, Tian, Li, Gao, Qin, Ma and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yipeng Liu, eXBsaXVAZW1haWwuc2RmbXUuZWR1LmNu; Chaoqun Ma, NDE0MzA5ODc4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Sen Lin1
Sen Lin1 Shanshan Zheng
Shanshan Zheng Yipeng Liu
Yipeng Liu