- 1Department of Dermatology, Peking University Shenzhen Hospital, Institute of Dermatology, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, China
- 2Shenzhen Key Laboratory for Translational Medicine of Dermatology, Institute of Dermatology, Shenzhen Peking University - the Hong Kong University of Science and Technology Medical Center, Shenzhen, Guangdong, China
- 3Biomedical Research Institute, Shenzhen Peking University - the Hong Kong University of Science and Technology Medical Center, Shenzhen, Guangdong, China
Introduction: Prurigo nodularis (PN) is a chronic inflammatory dermatosis characterized by robust pruritus and highly keratinizing nodules sympathetically distributed on the trunk and extensor sides of the limbs with an impact on quality of life and socioeconomic burden. To investigate the pathogenesis and develop novel therapeutic approaches, a preclinical animal model recapitulating the PN phenotypes is needed.
Methods: We constructed a novel PN-like mouse model by applying repetitive mechanical scratching in the context of type 2 inflammation. We utilized 6-8-week-old C57BL/6 mice for a 28 - day modeling study. The modeling protocol consisted of daily MC903 treatment plus 100 cell scraper scratches for the first 14 days, followed by alternate-day MC903 with continued daily scratching for the subsequent 14 days, with bandaging applied to prevent spontaneous scratching.
Results: Histological analysis of lesions revealed aberrant epidermal hyperplasia and differentiation, dermal fibrosis, inflammatory infiltration, angiogenesis, increased intraepidermal nerve fiber density, and enhanced nerve fiber sprouting. Immunological characterization revealed a mixed Th1/Th2/Th17/Th22 profile with a skewing toward Th17/Th22 polarization.
Conclusion: This optimized model faithfully recapitulates the key clinicopathological features of PN patients, providing a robust preclinical platform for investigating disease mechanisms and evaluating potential therapeutics.
1 Introduction
Prurigo nodularis (PN) is a chronic, intensely pruritic skin disorder characterized by symmetrically distributed hyperkeratotic nodules primarily affecting the trunk and extensor surfaces of the limbs (1). The disease predominantly affects African Americans and Asians and imposes a substantial burden on patients’ quality of life and socioeconomic well-being (2–4). Among chronic pruritic dermatoses, PN is associated with the greatest intensity and frequency of pruritus (5, 6). It often coexists with other inflammatory skin diseases, particularly atopic dermatitis (AD), a prototypical type 2 inflammatory disease, which is the most common cutaneous comorbidity observed in PN patients (7).
The characteristic nodular lesions of PN are thought to arise from repetitive mechanical trauma driven by persistent “itch-scratch” cycles in the context of neuroimmune dysregulation (8). Histopathologically, PN nodules exhibit pronounced hyperkeratosis, pseudoepitheliomatous hyperplasia, dermal fibrosis with thickened collagen, increased papillary capillaries, dense perivascular and interstitial inflammatory cell infiltration, and neural structural abnormalities (9–11). These changes are underpinned by a complex immune dysregulation involving Th1, Th2, Th17, and Th22 cells and their associated cytokine networks (7).
Although novel targeted therapies have emerged in recent years, their efficacy in resolving established PN nodules remains limited (8, 12–14). The lack of representative animal models that faithfully recapitulate the clinical and immunopathological features of PN has been a major obstacle to mechanistic studies and therapeutic development. Therefore, establishing a reliable PN-like mouse model is critical for advancing our understanding of the disease and preclinical evaluation of novel interventions.
In this study, we established a murine model with PN-like lesions by applying repetitive mechanical scratching under a type 2 - skewed inflammatory milieu. The resulting lesions closely resembled human PN nodules in histological alterations, inflammatory cell infiltration, and immune cell composition. This model offers a valuable experimental platform for elucidating the pathogenesis of PN and for exploring innovative therapeutic approaches.
2 Materials and methods
2.1 Mouse models
This model utilized C57BL/6 mice aged 6–8 weeks, with a modeling duration of 28 days. Topical MC903, known as calcipotriol, was widely used to induce AD-like inflammation (15). During the first 14 days, the shaved dorsal skin of mice was treated daily with 2 nmol MC903 diluted in 20 μL ethanol or 20 μL ethanol alone. After the skin was slightly dry, mechanical stimulation mimicking PN was induced by scratching the skin 100 times with a cell scraper. In the subsequent 14 days, 20 μL ethanol or 20 μL 2 nmol MC903 was applied every other day, while the mice scratched their backs 100 times each day. Throughout the experimental period, the shaved dorsal skin (approximately 2 cm × 3.5 cm area) of mice was subjected to daily standardized mechanical stimulation: an experienced technician used a 1.7 cm-wide cell scraper to scratch the defined area, with one complete back-and-forth motion counted as a single scratch, repeated 100 times daily at an intensity carefully controlled to reach just below the threshold of causing bleeding. Following each scratching session, the damaged skin was immediately wrapped with a bandage to prevent additional scratching by the mice.
On day 29, mice were humanely euthanized for harvesting and analysis of back skin. Four experimental groups were established: (1) EtOH group: ethanol application without scratching throughout the modeling period; (2) scratching (SC) group: daily ethanol application with daily scratching for the first 14 days, followed by ethanol application every other day with daily scratching for the remaining 14 days; (3) AD-like group: MC903 application without scratching throughout the modeling period; (4) PN-like group: daily MC903 application with daily scratching for the first 14 days, followed by MC903 application every other day with daily scratching for the remaining 14 days.
2.2 Histopathological assessment of skin
Skin specimens were fixed in 10% formalin (Phygene, Fuzhou, China) and embedded in paraffin. Slides of 5-μm-thick sections were prepared and stained with hematoxylin and eosin (H&E). Masson’s trichrome (MT) staining was using Masson’s Trichrome Stain Kit (Solarbio, Beijing, China) according to the manufacturer’s instructions. The eosinophil infiltration was evaluated by Chromotropic acid 2R staining using the Eosinophil Staining Kit (Solarbio, Beijing, China), and mast cells (MCs) were stained by toluidine blue staining (Solarbio, Beijing, China). Slides were then digitally scanned by Aperio CS2 scanner (Leica, Germany) and analyzed using software QuPath (v0.5.1) (16). The measurements of epidermal and dermal thickness in H&E staining and positively stained cells and area were evaluated from at least six randomly selected fields of each sample using the software ImageJ.
2.3 Immunohistochemistry analysis
Following deparaffinization, antigen retrieval and blocking the nonspecific binding, slides were incubated with primary antibodies against Cytokeratin 5/K5 (ab64081), Cytokeratin 10/K10 (ab76318), Loricrin (ab85679), CD31 (ab28364), PGP9.5 (ab8189) obtained from Abcam (Cambridge, UK); primary antibody against F4/80 obtained from Cell Signaling Technology (70076S, Danvers, USA); primary antibody against Cytokeratin 16/K16 obtained from Proteintech (17265-1-AP, Chicago, USA). Biotinylated secondary antibodies for immunohistochemistry (IHC) staining were from Beyotime Biotechnology (A0208, Shanghai, China). The signaling detection of IHC slides was performed by the DAB kit (ZSGB-BIO, Beijing, China). Slides were then digitally scanned by Aperio CS2 scanner (Leica, Germany). The percentage of positively stained area (CD31 and F4/80) was calculated at least six fields per each section by the ImageJ software.
2.4 Intraepidermal nerve fiber density quantification
PGP9.5 positive nerve fibers were used for the following analysis. Only single intraepidermal nerve fibers (IENFs) that cross the papillary dermis-epidermal junction were considered while secondary branching or fragments were not considered (17). IENF density (IENFD) was counted by the the total number of IENFs per site divided by the total length of the epidermis (fibers/mm) (18). Each section was calculated by at least two examiners who were researchers by the QuPath (v0.5.1) software at three fields.
2.5 RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from skin tissues using TRIzol (Invitrogen, USA) according to manual instruction, which was qualified and quantified using an Agilent 2100 Bioanalyzer (Agilent, CA, USA). cDNA was generated by PrimeScript™ RT kit (Takara, Beijing, China). The expression of transcripts was analyzed for various genes using the CFX96 (Bio-rad) thermocycler. The relative expression was calculated by the 2-ΔΔCT method and β-actin was used to normalize. All primers are shown in Supplementary Table S1.
2.6 RNA sequencing and data analysis
Library preparation is performed using Optimal Dual-mode mRNA Library Prep Kit (BGI-Shenzhen, China). Sequencing was performed on DNBSEQ platform with PE150. Clean data using the SOAPnuke (19) were obtained by raw data that filtered out low-quality reads, adapter contaminants, etc. Software HISAT (20) was used for reads alignment to a reference genome (GRCm39.v2201) and Bowtie2(v2.3.4.3) (21) was used to map gene sequence. Gene expression quantification was performed using RSEM (v1.3.1) (22). DESeq2 (v1.4.5) (23) was employed for differentially expressed genes (DEGs), with criteria set as false discovery rate (FDR) <0.05 and |log2 fold change| > 1.
2.7 Protein-protein interaction network analysis and identification of hub genes
The Protein-protein interaction (PPI) network was constructed with STRING database (https://cn.string-db.org/) (24) to predict the internal connections among the selected DEGs, with a confidence score threshold set to 0.4. Network visualization was performed using Cytoscape software (Version 3.10.4) (25). The CytoHubba plugin was used to rank the nodes in the target network based on Maximal Clique Centrality (MCC), and the top 15 genes were selected as hub genes.
2.8 Statistical analysis
Statistical analyses were performed by Graphpad Prism (v10.1.0) software. One-way ANOVA followed by Tukey’s multiple comparison test, or Kruskal-Wallis test followed by Dunn’s multiple comparison test was performed among multiple groups relying on whether the data were normally distributed. The gene sets defined for gene set variation analysis (GSVA) were derived from the website Metascape (26), with detailed gene sets provided in Supplementary Table S2. GSVA was conducted by “GSVA” R package with R (v4.4.2). The number of samples used in each experiment was indicated in the figure legends. * P < 0.05 was considered statistically significant. The significance levels of the data were defined as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3 Results
3.1 Significant thickening of epidermis and dermis in PN-like mouse model
We successfully established a PN-like murine model through the combined application of topical MC903 and mechanical scratching stimulation (Figure 1A). As shown in Figure 1B, both the MC903-induced AD-like model and the PN-like model exhibited gross signs of inflammation, with the PN-like model showing more prominent raised plaques. However, nodular lesions were not evident in the PN-like model, necessitating histological analysis for further characterization. Histological examination revealed that the skin lesions in PN-like mice partially mirrored those observed in PN patients (27), including hyperkeratosis, pseudoepitheliomatous hyperplasia with hypergranulosis, thickened collagen, dilated and thinned blood vessels in the superficial dermis (Figure 1C). Among the four experimental groups, the PN-like mice exhibited the most significant increase in both epidermal and dermal thickness. Compared to the AD-like group, the SC group mice showed thicker dermal layers but thinner epidermal layers (Figure 1D).
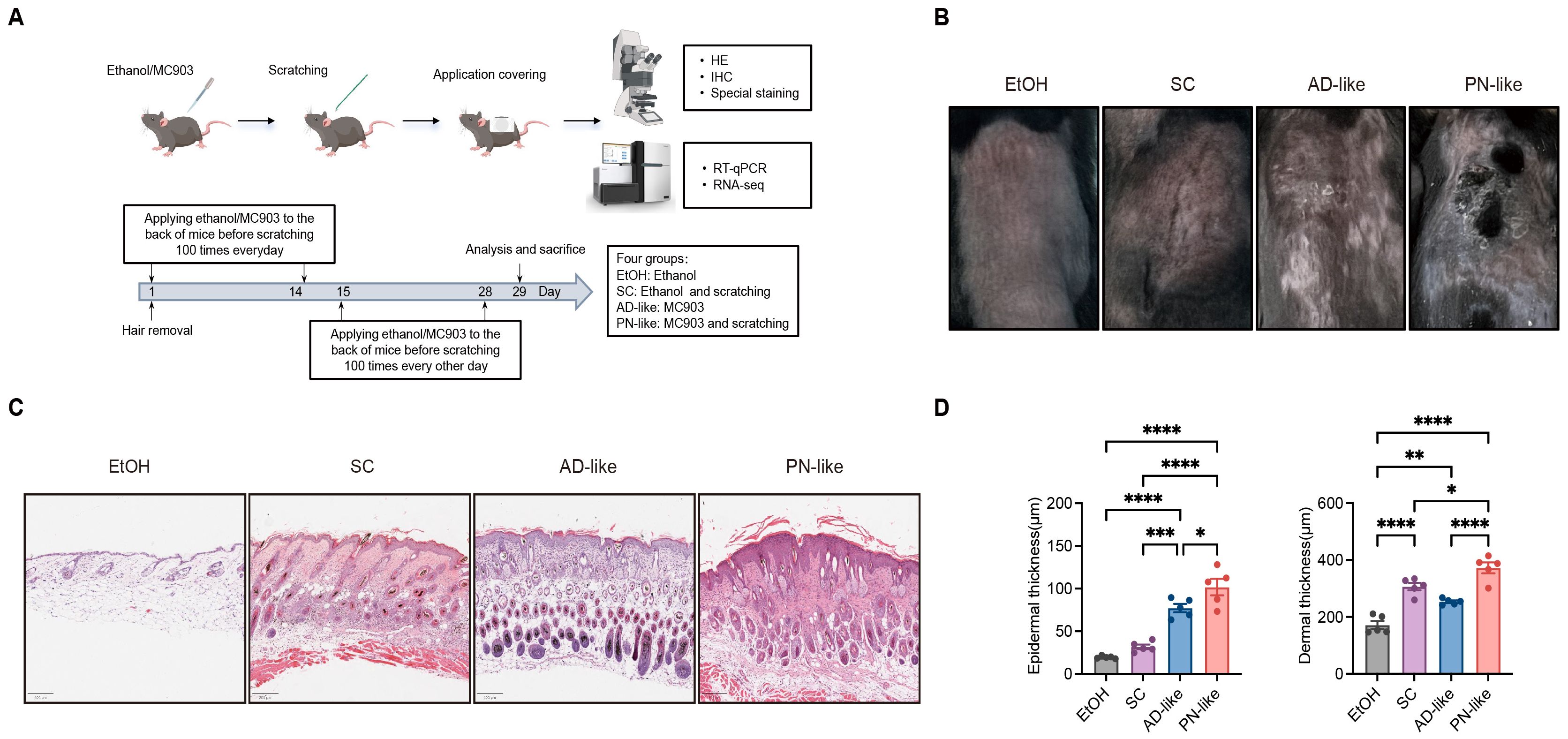
Figure 1. Phenotypic characteristics of PN-like mouse models. (A) Schematic diagram illustrating the modeling process for the four experimental groups. N = 5 mice per group. (B) Representative gross appearance of the back lesions of four murine groups. (C) On day 29, skin tissues were collected from all groups. Representative H&E-stained images of murine skin tissues are shown. Scale bar: 200 μm. (D) Epidermal and dermal thickness were measured from scanned H&E images using QuPath software (v0.5.1). Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; H&E, hematoxylin and eosin. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.2 Aberrant keratin expression and excessive collagen deposition in PN-like mice
Abnormal keratins expression was present in PN lesional skin (28). To investigate whether the expression pattern of keratins in PN-like mouse skin recapitulates that of PN patients, IHC was conducted to assess the distribution of key keratin markers, including keratin 5 (K5), keratin 10 (K10), keratin 16 (K16), and loricrin (LOR) in the skin lesions of four murine groups. In all groups, K5 and K16 were predominantly localized in the basal layer and lower suprabasal layers, while K10 and LOR were mainly expressed in the granular layer and upper suprabasal layers (Figure 2A). The expression levels of Lor and Krt16 were significantly upregulated, while Ivl expression was markedly downregulated in the PN-like mouse models (Figure 2B).
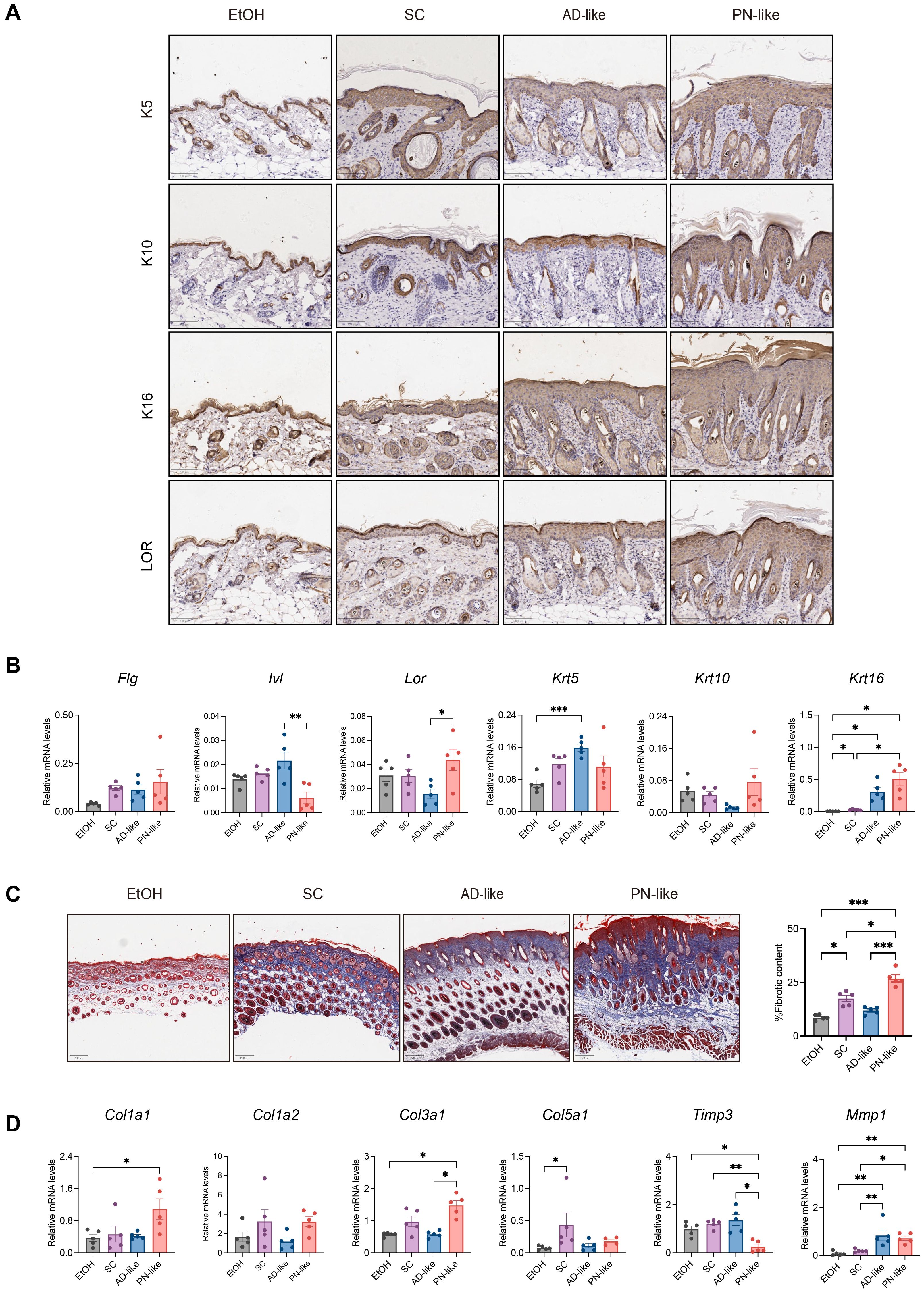
Figure 2. Dysregulated keratin expression and prominent collagen accumulation in PN-like lesions. (A) Representative IHC images of keratin markers (K5, K10, K16) and LOR across experimental groups; scale bar: 100 μm. (B) Quantitative RT-PCR analysis of epidermal differentiation-related genes. (C) Representative MT images for collagen fibers (left panel) and statistical analysis of collagen fibers-positive areas (right panel); scale bar: 200 μm; (D) Quantitative RT-PCR analysis of collagen-related genes. Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; MT, Masson’s trichrome; IHC, immunohistochemistry. *P < 0.05, **P < 0.01, ***P < 0.001.
Dermal fibrosis is a well-known histological feature of PN (29). MT staining revealed significant collagen fiber proliferation in PN-like mice compared to the other three groups (Figure 2C). The expression of fibrosis-related genes, including Col1a1, Col3a1 and Mmp1 was markedly upregulated, while Timp3 expression was downregulated in PN-like mice (Figure 2D).
3.3 Hyperactive inflammatory responses in PN-like mice
The inflammatory response, characterized by immune cell infiltration and the release of inflammatory mediators, plays a central role in the pathogenesis of PN (10). Our investigation focused on the density and distribution patterns of MCs, macrophages, and eosinophils in skin tissues. As demonstrated in Figure 3A, MCs in AD-like mice were predominantly localized in the papillary dermis, whereas in SC mice, MCs were mainly distributed in the deep dermis and subcutaneous layer. Notably, PN-like mice exhibited a diffuse distribution of MCs throughout the entire dermis and subcutaneous layer with significantly increased MC numbers, accompanied by similarly elevated macrophage and eosinophil counts in both AD-like and PN-like mice compared to controls. The distribution pattern of macrophages in mouse skin closely resembled that of mast cells. The number of macrophages and eosinophils was notably increased in both AD-like and PN-like mice.
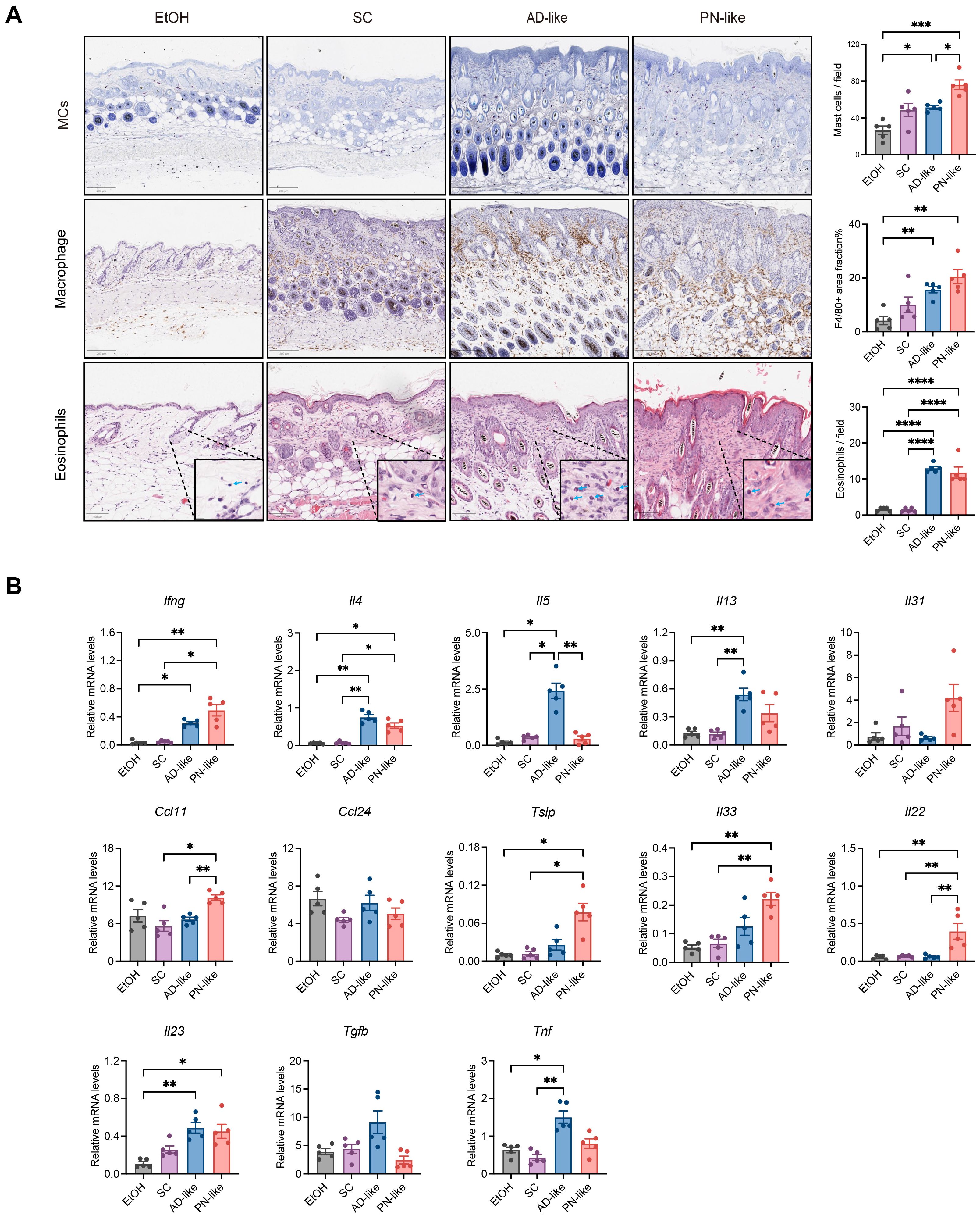
Figure 3. PN-like mice show significantly enhanced inflammatory activity. (A) Left panel: representative IHC images of MCs, macrophages and eosinophils. Right panel: statistical analysis of positively stained cells. Scale bar: 200 μm (MCs and macrophages) or 100 μm (eosinophils); (B) mRNA expression levels of inflammatory cytokines measured by quantitative RT-PCR. Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; MC, mast cells. *P < 0.05, **P < 0.01, ***P < 0.001.
Additionally, we analyzed the expression levels of inflammatory cytokines. Cytokine analysis demonstrated that PN-like lesions showed upregulated expression of Th1-related (Ifng), Th17-related gene (Il23), and Th17/Th22-related gene (Il22) genes. The characteristic Th2-related cytokines (Il4, Il5, Il13) were markedly increased in AD-like lesions, while Il4 and Ccl11 expression was elevated to various extent in PN-like lesions (Figure 3B) appeared to be associated with dermal eosinophilia. The epithelial cell–derived cytokines Tslp and Il33 were significantly elevated in PN-like lesions (Figure 3B).
3.4 IENFD and blood vessels in PN-like mice
We assessed the distribution of nerve fibers in the skin of PN-like mice using the IENFD method. The results showed that PN-like mice exhibited the highest IENFD, followed by AD-like mice, with both PN-like and AD-like mice showing prominent nerve fiber branching in the epidermis (Supplementary Figure S1A). This finding contrasts with the reduced IENFD observed in the skin lesions of PN patients (30), which may be attributed to differences in the timing and extent of skin structural damage. Additionally, we examined the number and morphology of blood vessels in the superficial dermis. Additionally, we quantified vascular density in the superficial dermis. We detected the vascular endothelial marker CD31 in lesions of PN-like mice by IHC and found that PN-like mice exhibited the highest vascular density among all experimental groups (Supplementary Figure S1B).
3.5 PN-like mice demonstrate a distinct pattern of transcriptome
In Figure 4A, skin transcriptome profiles were performed in four murine models: the ethanol EtOH group (n = 5), the SC group (n = 4), the AD-like group (n = 5), and the PN-like group (n = 5). Principal component analysis effectively distinguished the PN-like group from the EtOH and AD-like groups, but not from the SC group (Figure 4B), indicating a similar transcriptional pattern between the PN-like and SC groups with a |log2 fold change| > 1 and FDR < 0.05 (Figures 4C-E). We performed an intersection analysis of DEGs between the PN-like group and the other three groups of mice and identified 144 DEGs that were unique to the PN-like group (Figure 4F). The full lists of DEGs for all comparisons were provided in Supplementary Table S3.
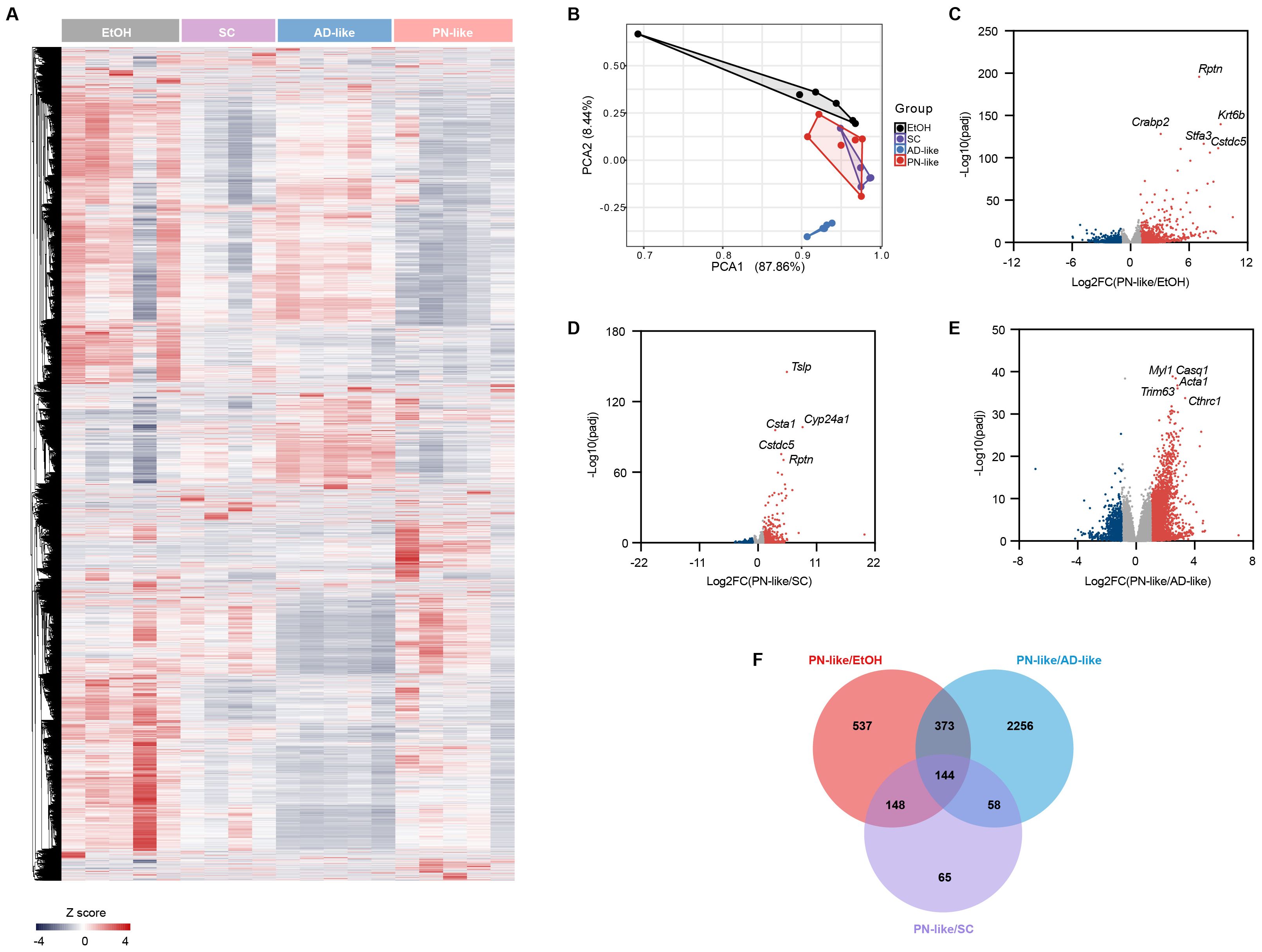
Figure 4. Distinct transcriptomic profile of the PN-like murine model. (A)Heatmap of all genes analyzed by RNA-seq in the skin of groups of EtOH(n=5), SC(n=4), AD-like (n=5), and PN-like (n=5) groups. The color scale represents the row z scores. (B) PCA of skin transcriptome profiles in the four murine models. Each point represents an individual sample. (c-e) Volcano plot comparing gene expression between (C) PN-like and EtOH skin, (D) PN-like and SC groups, and (E) PN-like and AD-like groups. Red points denote significantly upregulated DEGs (log2 FC > 1, FDR < 0.05), while blue points represent significantly downregulated DEGs (log2 FC < -1, FDR < 0.05). (F) Venn diagram showing the overlap of DEGs among the PN-like, EtOH, SC, and AD-like groups. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; PCA, principal component analysis; DEG, differentially expressed genes; FC, fold change; FDR, false discovery rate.
3.6 The functional enrichment of DEGs in the PN-like model
Based on the functional annotations in Gene Ontology, Kyoto Encyclopedia of Genes and Genomes and Reactome Gene Sets, we further analyzed the biological function of 144 specific DEGs in the PN-like model (Figure 5A). Our analysis revealed that DEGs were mainly enriched in the pathways related to protein metabolism, cytokine-chemokine interactions, regulation of myeloid dendritic cell chemotaxis, IL-36 pathway, negative regulation of leukocyte adhesion to vascular endothelial cell, and keratinocyte differentiation (Figure 5B). Notably, previous studies have reported significant upregulation of IL-36 family genes in lesions of PN patients (31). To further explore this observation, GSVA of the IL-36 pathway across the four groups revealed marked upregulation in PN-like mice and moderate upregulation in AD-like mice relative to the EtOH group, while PN-like mice also exhibited substantial upregulation of the keratinocyte differentiation pathway (Figure 5C). To further identify hub genes among the 144 DEGs specifically expressed in the PN-like murine model, we combined PPI network analysis with the MCC algorithm from the CytoHubba plugin and identified 15 hub genes: Itgax, Calm4, Cd8a, Ccl22, Rptn, Il7r, Cxcl9, Flg, Serpinb3a, Il1f5, Krt16, Krt6b, Serpinb6c, Havcr2 and Lce1f (Figure 5D).
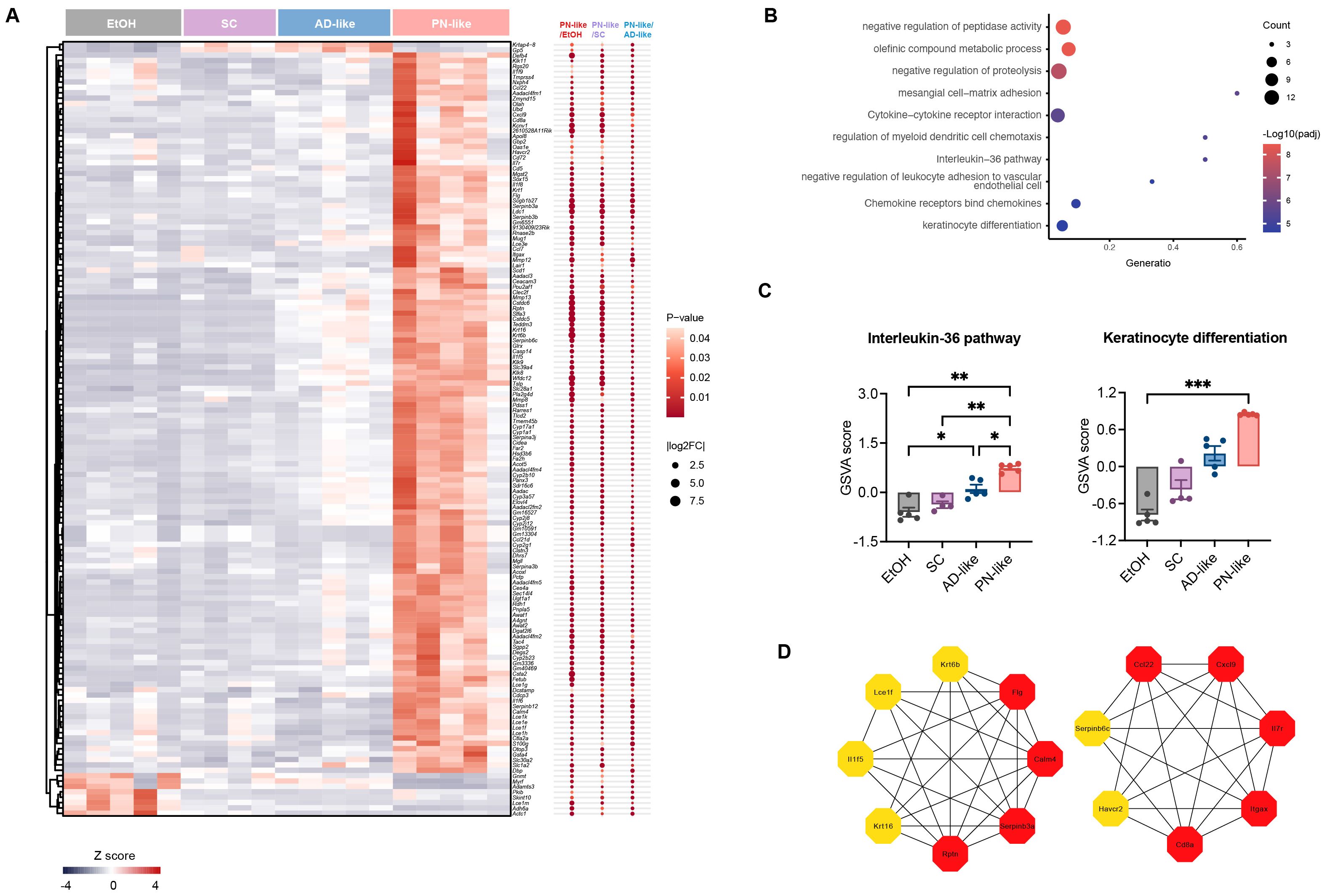
Figure 5. Functional enrichment analysis of DEGs in the PN-like murine model. (A) Heatmap displaying the expression patterns of 144 overlapping DEGs across three comparison groups: PN-like vs. EtOH, PN-like vs. SC, and PN-like vs. AD-like. The color scale represents row z-scores, indicating standardized gene expression levels. (B) Functional enrichment pathways of the 144 DEGs. (C) GSVA results illustrating the activity scores of the IL-36 signaling pathway and keratinocyte differentiation pathway across the four murine models. (D) The top 15 genes ranked by the MCC algorithm among the 144 DEGs were identified as hub genes. Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; DEG, differentially expressed genes; FC, fold change; GSVA, gene set variation analysis; IL, interleukin; MCC, maximal clique centrality. *P < 0.05, **P < 0.01, ***P < 0.001.
A notable proportion of PN patients have a history of atopy or current atopic comorbidity (12), such as AD. This clinical observation aligns with our PN-like mouse model, which was established on an AD-like background. To delineate the molecular distinctions between PN-like and AD-like, we analyzed the 2256 unique DEGs identified from the PN-like vs. AD-like comparison (Figure 4F) through functional enrichment analysis. These DEGs were predominantly enriched in pathways related to keratinization, inflammatory response, cell migration, extracellular matrix organization (ECM), and blood vessel development (Supplementary Figure S2A). PN-like mice and SC mice showed higher GSVA scores of ECM compared to AD-like mice (Supplementary Figure S2B).
3.7 Robust transcriptional signaling of Th17/Th22 skewing and fibrotic responses in PN-like mice
To characterize the immune phenotypes in PN-like mice and their resemblance to human PN lesions, we analyzed the transcriptional expression of marker genes associated with distinct CD4+ T cell subsets (32). RNA-seq revealed significant upregulation of key immune markers in PN-like mice compared to the other three groups, including Cxcl9 (Th1-associated gene), Ccl22 and Tslp (Th2-associated genes), Defb4 (Th17-associated gene), and Calm4, Serpinb3a/3b (Th22-associated genes). Furthermore, PN-like mice exhibited pronounced increases in Th17/IL17-associated genes (Ccl20, Cxcl2/3, Lcn2), Th17/Th22-associated genes (S100a8/a9) and Th22/IL22-associated genes (Il1b, Calm5, Ccl7, Cxcl5, Sepinb1a/1b/1c) compared to the EtOH and/or AD-like groups (Figure 6A). To quantify these observations, GSVA was performed to compare the expression of Th1, Th2, Th17, and Th22 gene markers. The analysis demonstrated robust upregulation of Th22-related genes in PN-like mice relative to all other groups, significant elevation of Th17 markers compared to the EtOH and AD-like groups, and modest increases in Th1 and Th2 markers compared to the EtOH group (Figure 6B). These findings collectively indicate that the immune phenotype in PN-like mice closely recapitulates the Th17/Th22-skewed immune signature observed in human PN lesions.
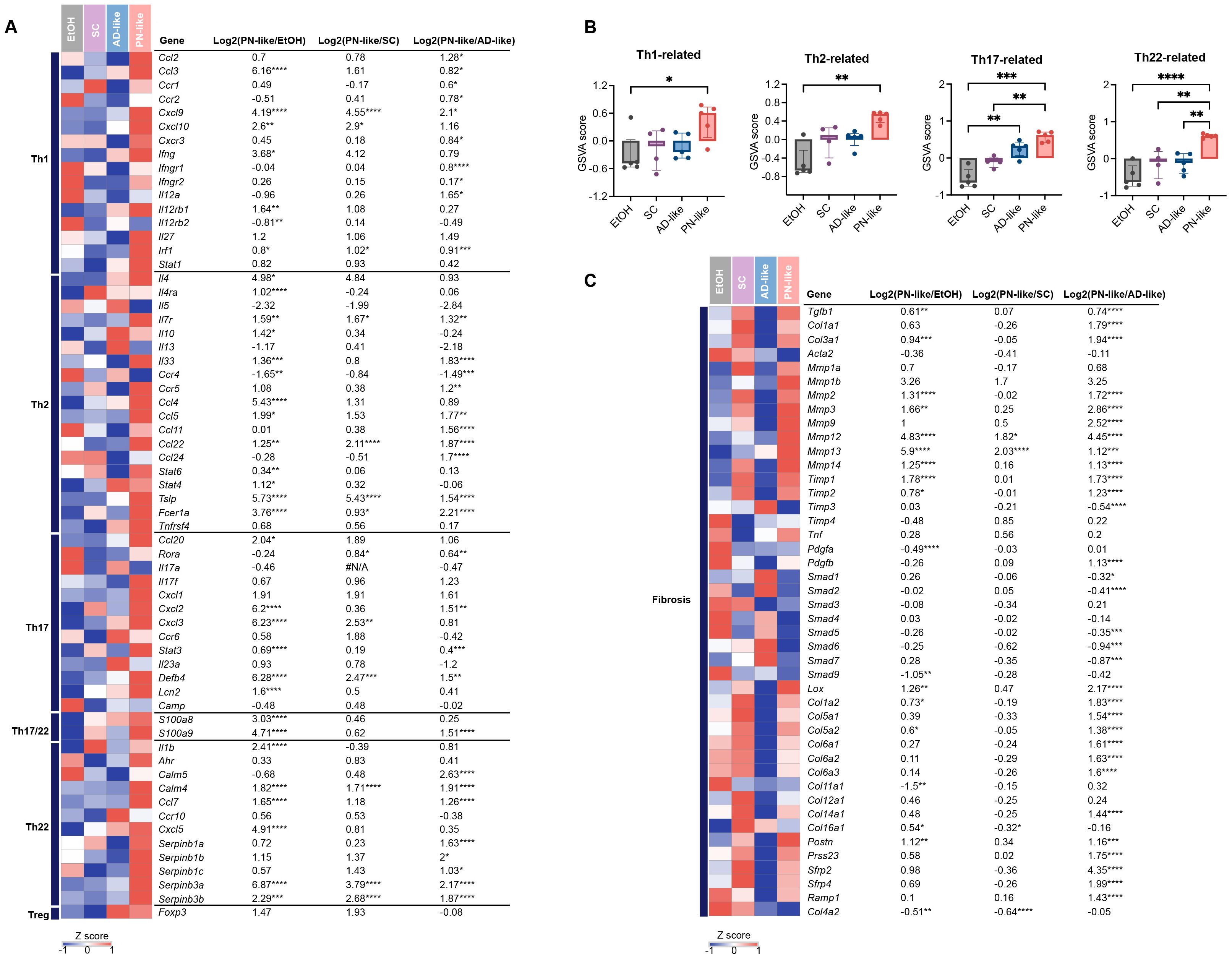
Figure 6. Distinct immune and fibrotic signatures in the PN-like murine model revealed by cutaneous gene expression profiling. (A) Heatmap depicting the average gene expression of T cell subsets across the four murine models. The color code represents the row z scores. (B) GSVA score comparison of T cell subset-related genes (Th1, Th2, Th17, and Th22) in the skin of the four murine models. (C) Heatmap illustrating the average expression of fibrosis-related genes across the four murine models. Data are presented as median ± interquartile range. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; GSVA, gene set variation analysis; Th, T helper. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Previous studies have demonstrated a distinct mesenchymal dysregulation in the dermal fibrosis of PN, which is marked by enhanced angiogenesis, fibrosis, and extracellular matrix production (29). In PN-like mice, there was a notable upregulation of collagen-encoding genes (Col1a1/a2, Col3a1, etc.) and matrix metalloproteinase-associated genes (Mmp2/3/9/12, etc.) to varying extent. Additionally, recently identified fibroblast marker genes in PN skin (33, 34), including Postn, Prss23, Srfp2/4, Ramp, showed a significant increase in PN-like mice (Figure 6C). Furthermore, genes related to vessel function, such as Angpt1/2, Vcam1, Nos3 and Il6, were enhanced in PN-like mice (Supplementary Figure S3).
Moreover, the mRNA expression of skin barrier proteins, including keratins (Krt1/10/16), filaggrin (Flg), loricrin (Lor), kallikreins (Klk9/11), as well as neural remodeling proteins like nerve growth factor (Ngf) and substance P (Tac1), was elevated to varying extents in PN mice (Supplementary Figure S3).
To further evaluate the similarity between the PN-like murine model and PN patients, we compared the 144 DEGs with those from human PN skin lesions (32). The results demonstrate that while the PN-like mouse model effectively recapitulates the upregulated gene expression profile observed in human PN, its capacity to replicate downregulated gene signatures remains limited (Supplementary Table S4).
4 Discussion
The lack of representative animal models and the limited efficacy of current targeted therapies in nodular lesions underscore the urgent need for reliable experimental models of PN (8, 12–14). In this study, we established a PN-like mouse model driven by type 2 inflammation and mechanical scratching that recapitulates key pathological features observed in PN patients, including aberrant epidermal proliferation and differentiation, pronounced dermal fibrosis, mixed T helper cell - driven immune activation (Th1/Th2/Th17/Th22), and heightened cutaneous inflammatory responses.
Notably, the PN-like lesions in our model exhibited significant thickening of the epidermis, consistent with observations in human PN nodules. IHC and transcription analysis further revealed aberrant expression of markers involved in keratinocyte proliferation and differentiation, such as K16, LOR and IVL, indicating disrupted keratinocyte homeostasis. Keratins play critical roles in epidermal structural integrity and barrier homeostasis, as well as in wound repair and inflammation (35–37). In particular, K16 is a well-established stress-responsive keratin that function as an early alarmin in damaged skin (38), whose expression is strongly induced by scratching-induced keratinocyte stress and mechanical damage to subsequently modulate keratinocyte proliferation and inflammatory responses (31, 39). Epidermal K16 expression was upregulated at both the transcriptional and translational levels in our PN-like mouse model, which is consistent with the enhanced K16 expression in the lesional skin of PN patients (28). The skin barrier protein LOR showed granular layer localization in both AD-like and PN-like mouse models, consistent with its expression pattern in human AD and PN lesions. While lesional skin from AD and PN patients exhibited increased LOR and involucrin (IVL) but decreased filaggrin (FLG) at the protein level (40), our results revealed elevated Lor but reduced Ivl expression at the transcription level in PN-like models compared to AD-like mice, with unchanged Flg levels. This differential expression pattern suggests compensatory mechanisms among barrier proteins that ultimately fail to restore skin barrier homeostasis under pathological conditions (40).
Dermal fibrosis is a well-established histopathological hallmark of PN (29). Transcriptomic data of PN patients have consistently demonstrated profound dysregulation in the interstitial compartment compared to AD, characterized by fibroblast activation, excessive ECM deposition, and fibrotic remodeling, as evidenced by prior studies (33, 41, 42). In our PN-like murine model, both H&E and MT staining confirmed excessive collagen accumulation within the superficial dermis, recapitulating the fibrotic features observed in PN lesions. Transcriptional analysis in a PN-like mouse model revealed upregulation of collagen-encoding genes (e.g., Col1a1, Col3a1), while ECM regulatory genes exhibited opposing trends - specifically, Mmp1 expression was elevated, and its negative regulator Timp3 was downregulated. This paradoxical expression pattern, where increased Mmp1 and decreased Timp3 coincide with heightened collagen deposition, contrasts with the expected ECM degradation phenotype. Notably, this dysregulation paralleled the expression profile in PN patients, showing elevated MMP1 and reduced TIMP3 expression (32), yet the mechanism remains poorly understood. Intriguingly, in AD lesions, MMP1 expression is also elevated in both the epidermis and dermis (43), and serum MMP-1 levels correlate with the severity of epidermal barrier dysfunction (44–46). The upregulation of Mmp1 in both AD-like and PN-like mouse models suggests that MMP1 could be involved not only in collagen remodeling but also in epidermal dysfunction. These findings demonstrate that the dermal fibrosis in PN-like mice closely mirrors the structural and molecular alterations seen in PN nodular lesions, validating the model’s relevance for mechanistic and therapeutic studies.
Mixed infiltration of immune cells and subsequent robust inflammatory responses in the dermis are central to the pathogenesis of PN (7). PN lesions demonstrated significantly enhanced type 2 inflammatory responses relative to healthy controls, their intensity remained attenuated compared to AD (29). Our study revealed that canonical Th2 cytokines (Il4, Il5, Il13) demonstrate the highest expression levels in AD-like mice consistent with findings from 14-day AD-like models (47), and followed by PN-like models, while type 2-associated immune cells displayed differential infiltration patterns - macrophages reached peak levels in PN-like mice, with eosinophil accumulation second only to AD-like specimens. GSVA suggested PN-like mice exhibit the most prominent Th2 signatures. PN-like mice exhibited the highest transcriptional expression of Ifng and the greatest Th1 response GSVA score, aligning with the Th1-dominant immune phenotype seen in PN patient lesional skin - likely driven by scratching-induced keratinocyte stress responses (29). Notably, the characteristic enrichment of IL-17, IL-22 and IL-36 in PN (versus AD) may underlie its clinical overlap with psoriasis (31, 32), a mechanism validated in our PN-like model through GSVA demonstrating robust Th17/Th22 responses and IL-36 pathway activation in lesional skin.
In PN patients, lesions showed decreased IENFD but increased nerve fiber branching in the epidermis, likely due to chronic scratching-induced fragmentation and subsequent sprouting of nerve fibers (39). Interestingly, our PN-like models exhibited increased IENFD, suggesting this structural preservation may relate to differences in scratching intensity/duration or different species. The study by Yamaoka et al. describes a transient increase in IENFD following a single episode of skin scratching, which resolves within 2–3 weeks (48). In contrast, our model employs repeated, chronic scratching for 28 days. We find that the inflammatory background appears to be a key driver of this sustained neuroproliferation as evidenced by enhanced nerve fiber branching in both AD-like and PN-like models. We hypothesize that chronic inflammation, in synergy with mechanical injury, drives a persistent and potent “sprouting phase”, which appears to be supported by the elevated expression of the branching-associated gene NGF in both PN patients (39) and our PN-like models. Intriguingly, the Hashimoto prurigo-like model showed more pronounced nerve fiber sprouting and NGF expression at peripheral lesions versus central lesions (49). Unfortunately, we didn’t compare lesions with adjacent non-lesional skin. The alterations in IENFD and branching patterns are likely independent, and only through two- or three-dimensional assessment of both parameters can a comprehensive neuroanatomical profile be established (30). Our preliminary investigations have identified dermal nerve fiber bundles in PN-like murine models that demonstrate morphological similarities to PN patient lesions, although the current lack of standardized quantification protocols for dermal nerve fibers precludes definitive comparative analysis.
CD31 IHC revealed a significant increase in superficial dermal vascular density in the PN-like group compared to the EtOH group, consistent with the characteristic vascular hyperplasia observed in lesional skin of PN patients (50). Notably, epidermal thickness showed strong correlation with vascular density (50), and our data demonstrated that PN-like mice exhibited both the thickest epidermis and highest vascular counts among all groups. Furthermore, GSVA identified significant enrichment of vessel development-related gene signatures in PN-like mice. Collectively, these findings indicate robust neovascularization in the superficial dermis of PN-like murine lesions.
In summary, the PN-like mouse model we established under a type 2 inflammatory background effectively recapitulates the nodular skin lesions of PN patients in terms of histological alterations, inflammatory cell infiltration, and immune cell composition. However, several limitations should be acknowledged: First, the current method failed to recapitulate characteristic nodular lesions. Second, the model inadequately addresses disease comorbidities, focusing solely on type 2 inflammation background. Given that PN patients frequently present with type 2 diabetes, chronic kidney disease, etc (51), it is dispensable to elucidate pathogenic mechanisms across different comorbidity backgrounds. Third, our PN-like model shows deficiencies in recapitulating neural remodeling including species-divergent IENFD changes. Our future investigations will implement the following strategic modifications: (1) extending the duration of mechanical stimulation to simulate long-term consequences of chronic scratching; (2) incorporating pruritogen-induced spontaneous scratching behavior to establish a more authentic, stimulus-independent itch-scratch cycle; and (3) combining exogenous administration of key cytokines (e.g., IL-31) to synergistically drive both pruritic and pro-fibrotic pathways.
Data availability statement
The original contributions presented in the study are publicly available. The data have been deposited in the Mendeley Data repository under accession number [10.17632/my59z5zsvh.1](https://data.mendeley.com/datasets/my59z5zsvh/1).
Ethics statement
The animal study was approved by Committee for the Ethics of Animal Experiments, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JW: Formal Analysis, Writing – original draft, Conceptualization, Visualization. XL: Writing – review & editing, Visualization, Data curation. SH: Formal Analysis, Writing – review & editing, Resources. ST: Writing – review & editing, Validation, Resources. KZ: Methodology, Investigation, Writing – review & editing. JY: Writing – review & editing, Visualization, Investigation. XC: Writing – review & editing, Supervision, Methodology, Conceptualization. XD: Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Shenzhen High-level Hospital Construction Fund, and Peking University Shenzhen Hospital Scientific Research Fund (KYQD2024401) and Shenzhen Natural Sciences Foundation (JCYJ20210324105411030).
Acknowledgments
We thank Shenzhen Public Service Platform for Molecular Diagnosis of Dermatology for the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1648830/full#supplementary-material
Supplementary Figure 1 | Increased IENFD and blood vessels in PN-like mice. (A) Representative IHC images for PGP9.5 (left panel) and statistical analysis of IENFD (right panel) in the four experimental groups; scale bar: 20 μm. (B) Representative IHC images for CD31 (left panel) and statistical analysis of CD31-positive areas (right panel) in the four experimental groups; scale bar: 100 μm. Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; IHC, immunohistochemistry. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 2 | Functional enrichment analysis of DEGs in PN-like versus AD-like murine models. (A) Functional enrichment pathways of the 2,256 unique DEGs identified from the PN-like vs. AD-like comparison. (B) Comparison of GSVA scores for the 2,256 unique DEGs across the four murine models. Data are presented as mean ± SEM. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; DEG, differentially expressed genes; GSVA, gene set variation analysis; ECM, extracellular matrix organization. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Supplementary Figure 3 | Differential expression of vessel function, skin barrier, and neural remodeling-related genes in PN-like mice. Heatmap illustrating the average expression of genes associated with vessel function, skin barrier and neural remodeling across the four murine models. The color code represents the row z scores. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Supplementary Table 1 | RT-qPCR primers. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis.
Supplementary Table 2 | Gene sets for GSVA. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis.
Supplementary Table 3 | DEGs list of PN-like versus the other three groups. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis.
Supplementary Table 4 | The overlapping genes between the PN-like murine model and human PN. The data in the table are displayed as log2FC along with adjusted p-values. EtOH, ethanol; SC, scratching; AD, atopic dermatitis; PN, prurigo nodularis; FC, fold change. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
References
1. Huang AH, Williams KA, and Kwatra SG. Prurigo nodularis: Epidemiology and clinical features. J Am Acad Dermatol. (2020) 83:1559–65. doi: 10.1016/j.jaad.2020.04.183
3. Whang KA, Kang S, and Kwatra SG. Inpatient burden of prurigo nodularis in the United States. Medicines (Basel). (2019) 6. doi: 10.3390/medicines6030088
4. Elmariah S, Kim B, Berger T, Chisolm S, Kwatra SG, Mollanazar N, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. (2021) 84:747–60. doi: 10.1016/j.jaad.2020.07.025
5. Zeidler C, Pereira MP, Dugas M, Augustin M, Storck M, Weyer-Elberich V, et al. The burden in chronic prurigo: patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skin: A comparative, retrospective, explorative statistical analysis of 4,484 patients in a real-world cohort. J Eur Acad Dermatol Venereol. (2021) 35:738–43. doi: 10.1111/jdv.16929
6. Whang KA, Le TK, Khanna R, Williams KA, Roh YS, Sutaria N, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. (2022) 86:573–80. doi: 10.1016/j.jaad.2021.05.036
7. Liao V, Cornman HL, Ma E, and Kwatra SG. Prurigo Nodularis: New insights into pathogenesis and novel therapeutics. Br J Dermatol. (2024) 190:798–810. doi: 10.1093/bjd/ljae052
8. Kwatra SG, Yosipovitch G, Legat FJ, Reich A, Paul C, Simon D, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. (2023) 389:1579–89. doi: 10.1056/NEJMoa2301333
9. Zeidler C and Stander S. The pathogenesis of Prurigo nodularis–’Super-Itch’ in exploration. Eur J Pain. (2016) 20:37–40. doi: 10.1002/ejp.767
10. Williams KA, Huang AH, Belzberg M, and Kwatra SG. Prurigo nodularis: Pathogenesis and management. J Am Acad Dermatol. (2020) 83:1567–75. doi: 10.1016/j.jaad.2020.04.182
11. Johansson O, Liang Y, and Emtestam L. Increased nerve growth factor- and tyrosine kinase A-like immunoreactivities in prurigo nodularis skin – an exploration of the cause of neurohyperplasia. Arch Dermatol Res. (2002) 293:614–9. doi: 10.1007/s00403-001-0285-8
12. Yosipovitch G, Mollanazar N, Stander S, Kwatra SG, Kim BS, Laws E, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. (2023) 29:1180–90. doi: 10.1038/s41591-023-02320-9
13. Weisshaar E, Szepietowski JC, Bernhard JD, Hait H, Legat FJ, Nattkemper L, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. (2022) 36:453–61. doi: 10.1111/jdv.17816
14. Pezzolo E, Narcisi A, Gargiulo L, Di Lernia V, Napolitano M, Patruno C, et al. Effective response to upadacitinib in patients affected by prurigo nodularis and by atopic dermatitis with a predominant prurigo nodularis pattern: A multicenter case series study. J Am Acad Dermatol. (2024) 91:e147–e50. doi: 10.1016/j.jaad.2024.06.094
15. Sakamoto K and Nagao K. Mouse models for atopic dermatitis. Curr Protoc. (2023) 3:e709. doi: 10.1002/cpz1.709
16. Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. (2017) 7:16878. doi: 10.1038/s41598-017-17204-5
17. Pereira MP, Muhl S, Pogatzki-Zahn EM, Agelopoulos K, and Stander S. Intraepidermal nerve fiber density: diagnostic and therapeutic relevance in the management of chronic pruritus: a review. Dermatol Ther (Heidelb). (2016) 6:509–17. doi: 10.1007/s13555-016-0146-1
18. Burgess J, Marshall A, Rapteas L, Hamill KJ, Marshall A, Malik RA, et al. Automated immunohistochemistry of intra-epidermal nerve fibres in skin biopsies: A proof-of-concept study. J Peripher Nerv Syst. (2024) 29:329–38. doi: 10.1111/jns.12650
19. Chen Y, Chen Y, Shi C, Huang Z, Zhang Y, Li S, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. (2018) 7:1–6. doi: 10.1093/gigascience/gix120
20. Kim D, Langmead B, and Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. (2015) 12:357–60. doi: 10.1038/nmeth.3317
21. Langmead B and Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. (2012) 9:357–9. doi: 10.1038/nmeth.1923
22. Li B and Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. (2011) 12:323. doi: 10.1186/1471-2105-12-323
23. Love MI, Huber W, and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. (2014) 15:550. doi: 10.1186/s13059-014-0550-8
24. Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. (2023) 51:D638–D46. doi: 10.1093/nar/gkac1000
25. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) 13:2498–504. doi: 10.1101/gr.1239303
26. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. (2019) 10:1523. doi: 10.1038/s41467-019-09234-6
27. Weigelt N, Metze D, and Stander S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. (2010) 37:578–86. doi: 10.1111/j.1600-0560.2009.01484.x
28. Yang LL, Jiang B, Chen SH, Liu HY, Chen TT, Huang LH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. (2022) 2:e75. doi: 10.1002/ski2.75
29. Conrad C and Schlapbach C. Prurigo nodularis forecast: Light type 2 inflammation with high chances of fibrosis. J Allergy Clin Immunol. (2024) 153:93–4. doi: 10.1016/j.jaci.2023.11.003
30. Agelopoulos K and Stander S. Response to neuroanatomic signatures in brachioradial pruritus, chronic prurigo, and atopic dermatitis. J Invest Dermatol. (2023) 143:1844–5. doi: 10.1016/j.jid.2023.03.1675
31. Tsoi LC, Hacini-Rachinel F, Fogel P, Rousseau F, Xing X, Patrick MT, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol. (2022) 149:1329–39. doi: 10.1016/j.jaci.2021.10.004
32. Belzberg M, Alphonse MP, Brown I, Williams KA, Khanna R, Ho B, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. (2021) 141:2208–18 e14. doi: 10.1016/j.jid.2021.02.749
33. Ma F, Gharaee-Kermani M, Tsoi LC, Plazyo O, Chaskar P, Harms P, et al. Single-cell profiling of prurigo nodularis demonstrates immune-stromal crosstalk driving profibrotic responses and reversal with nemolizumab. J Allergy Clin Immunol. (2024) 153:146–60. doi: 10.1016/j.jaci.2023.07.005
34. Patel JR, Joel MZ, Lee KK, Kambala A, Cornman H, Oladipo O, et al. Single-cell RNA sequencing reveals dysregulated POSTN+WNT5A+ Fibroblast subclusters in prurigo nodularis. J Invest Dermatol. (2024) 144:1568–78 e5. doi: 10.1016/j.jid.2023.12.021
35. Hatano Y and Elias PM. Outside-to-inside,” “inside-to-outside,” and “intrinsic” endogenous pathogenic mechanisms in atopic dermatitis: keratinocytes as the key functional cells involved in both permeability barrier dysfunction and immunological alterations. Front Immunol. (2023) 14:1239251. doi: 10.3389/fimmu.2023.1239251
36. Romashin DD, Tolstova TV, Varshaver AM, Kozhin PM, Rusanov AL, and Luzgina NG. Keratins 6, 16, and 17 in health and disease: A summary of recent findings. Curr Issues Mol Biol. (2024) 46:8627–41. doi: 10.3390/cimb46080508
37. Pondeljak N, Lugovic-Mihic L, Tomic L, Parac E, Pedic L, and Lazic-Mosler E. Key factors in the complex and coordinated network of skin keratinization: their significance and involvement in common skin conditions. Int J Mol Sci. (2023) 25. doi: 10.3390/ijms25010236
38. Zhang X, Yin M, and Zhang LJ. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells. (2019) 8. doi: 10.3390/cells8080807
39. Agelopoulos K, Renkhold L, Wiegmann H, Dugas M, Suer A, Zeidler C, et al. Transcriptomic, epigenomic, and neuroanatomic signatures differ in chronic prurigo, atopic dermatitis, and brachioradial pruritus. J Invest Dermatol. (2023) 143:264–72 e3. doi: 10.1016/j.jid.2022.08.042
40. Folster-Holst R, Reimer R, Neumann C, Proksch E, Rodriguez E, Weidinger S, et al. Comparison of epidermal barrier integrity in adults with classic atopic dermatitis, atopic prurigo and non-atopic prurigo nodularis. Biol (Basel). (2021) 10. doi: 10.3390/biology10101008
41. Deng J, Parthasarathy V, Marani M, Bordeaux Z, Lee K, Trinh C, et al. Extracellular matrix and dermal nerve growth factor dysregulation in prurigo nodularis compared to atopic dermatitis. Front Med (Lausanne). (2022) 9:1022889. doi: 10.3389/fmed.2022.1022889
42. Alkon N, Assen FP, Arnoldner T, Bauer WM, Medjimorec MA, Shaw LE, et al. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J Allergy Clin Immunol. (2023) 152:420–35. doi: 10.1016/j.jaci.2023.04.019
43. Li C, Lu Y, and Han X. Identification of effective diagnostic biomarkers and immune cell infiltration in atopic dermatitis by comprehensive bioinformatics analysis. Front Mol Biosci. (2022) 9:917077. doi: 10.3389/fmolb.2022.917077
44. Harper JI, Godwin H, Green A, Wilkes LE, Holden NJ, Moffatt M, et al. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br J Dermatol. (2010) 162:397–403. doi: 10.1111/j.1365-2133.2009.09467.x
45. Basalygo M, Sliwinska J, Zbikowska-Gotz M, Lis K, Socha E, Bartuzi Z, et al. Assessment of serum concentrations of matrix metalloproteinase 1, matrix metalloproteinase 2 and tissue inhibitors of metalloproteinases 1 in atopic dermatitis in correlation with disease severity and epidermal barrier parameters. Postepy Dermatol Alergol. (2021) 38:773–9. doi: 10.5114/ada.2021.110043
46. Weidinger S and Novak N. Atopic dermatitis. Lancet. (2016) 387:1109–22. doi: 10.1016/S0140-6736(15)00149-X
47. Zhang S, Fang X, Xu B, Zhou Y, Li F, Gao Y, et al. Comprehensive analysis of phenotypes and transcriptome characteristics reveal the best atopic dermatitis mouse model induced by MC903. J Dermatol Sci. (2024) 114:104–14. doi: 10.1016/j.jdermsci.2024.05.003
48. Yamaoka J, Di ZH, Sun W, and Kawana S. Erratum to “changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci. (2007) 47:172–82. doi: 10.1016/j.jdermsci.2006.12.012
49. Hashimoto T, Satoh T, and Yokozeki H. Protective role of STAT6 in basophil-dependent prurigo-like allergic skin inflammation. J Immunol. (2015) 194:4631–40. doi: 10.4049/jimmunol.1401032
50. Krull C, Schoepke N, Ohanyan T, Brachaczek M, Maurer M, Lange-Asschenfeldt B, et al. Increased angiogenesis and VEGF expression correlates with disease severity in prurigo patients. J Eur Acad Dermatol Venereol. (2016) 30:1357–61. doi: 10.1111/jdv.13406
Keywords: prurigo nodularis, preclinical model, type 2 inflammation, Th17/Th22 skewing, dermal fibrosis
Citation: Wu J, Li X, Huang S, Tang S, Zhang K, Yan J, Chen X and Dou X (2025) A murine model of prurigo nodularis-like skin lesions induced by persistent scratching under type 2 inflammatory conditions. Front. Immunol. 16:1648830. doi: 10.3389/fimmu.2025.1648830
Received: 17 June 2025; Accepted: 13 October 2025;
Published: 28 October 2025.
Edited by:
Jie Li, Central South University, ChinaReviewed by:
Simone Garcovich, Agostino Gemelli University Polyclinic (IRCCS), ItalyHui Tang, Fudan University, China
Copyright © 2025 Wu, Li, Huang, Tang, Zhang, Yan, Chen and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofan Chen, bGl0dGxlY2FudmFAMTYzLmNvbQ==; Xia Dou, ZHJkb3V4aWFAMTYzLmNvbQ==
 Jiali Wu1,2
Jiali Wu1,2 Siyi Tang
Siyi Tang Xiaofan Chen
Xiaofan Chen Xia Dou
Xia Dou