- 1School of Forensic Medicine, Xinxiang Medical University, Xinxiang, Henan, China
- 2School of Basic Medical Sciences, Xinxiang Medical University, Xinxiang, Henan, China
- 3School of Junji College, Xinxiang Medical University, Xinxiang, Henan, China
- 4Xinxiang Engineering Technology Research Center of immune checkpoint drug for Liver-Intestinal Tumors, Xinxiang Medical University, Xinxiang, Henan, China
B lymphocytes exhibit a multifaceted and context-dependent role in tumor biology, acting as both promoters and suppressors of malignancy through dynamic interactions within the tumor microenvironment (TME). This review synthesizes current evidence on the dual functions of B cells in tumor immunity, highlighting their capacity to orchestrate antitumor responses via antigen presentation, antibody-dependent cytotoxicity, and tertiary lymphoid structure (TLS)-mediated T cell activation, while paradoxically driving immunosuppression through regulatory B cells (Bregs), pro-angiogenic signaling, and immune checkpoint modulation. Key mechanisms include TLS formation, which enhances cytotoxic T cell priming and correlates with improved immunotherapy outcomes, and Breg-mediated secretion of IL-10/TGF-β, which fosters T cell exhaustion and myeloid-derived suppressor cell recruitment. Tumor-type specificity is evident: TLS-rich malignancies like melanoma and Non-Small Cell Lung Cancer (NSCLC) show B cell-driven immune activation, whereas pancreatic and hepatocellular carcinomas demonstrate B cell functional plasticity influenced by metabolic and epigenetic reprogramming. Therapeutically, B cell-targeted strategies—including CD20 antibodies, CAR-T cells, and B cell epitope vaccines—demonstrate efficacy in hematologic and solid tumors, yet face challenges due to subset heterogeneity and sex-specific response disparities. Emerging approaches combine immune checkpoint inhibitors (ICBs) with TLS-inducing agents or exploit B cell-derived biomarkers for personalized therapy. Future directions emphasize deciphering B cell metabolic-niche crosstalk, optimizing combinatorial regimens, and leveraging spatial multiomics to resolve functional heterogeneity. By bridging mechanistic insights with clinical translation, this work underscores B cells as pivotal regulators of tumor immunity and advocates for precision strategies to harness their antitumor potential while mitigating pro-tumor plasticity.
1 Introduction
Tumorigenesis represents a multifaceted biological process involving dynamic interactions between malignant cells and host immunity. While T lymphocytes have dominated tumor immunology research, emerging evidence underscores the critical yet underappreciated role of B lymphocytes within the TME (1). As integral components of adaptive immunity, B cells undergo a tightly regulated differentiation process: upon antigen encounter, naïve B cells can proliferate and differentiate into memory B cells(MBCs) or antibody-secreting plasma cells(PCs), mediating long-term humoral immunity. Beyond their canonical role in antibody production, B cells also function as antigen-presenting cells and cytokine secretors, thereby modulating both innate and adaptive immune responses (2).
Under physiological conditions, this differentiation is critically shaped by signals from the microenvironment, including cytokines, T cell help, and antigen affinity. However, in the tumor context, these precisely regulated processes are often co-opted or dysregulated. Recent advances have elucidated novel mechanisms by which B cells shape anti-tumor immunity, revealing their potential as diagnostic biomarkers and therapeutic targets (3). This evolving paradigm highlights the urgency to decipher B cell biology in malignancy, which may catalyze the development of innovative immunotherapies and prognostic tools.
2 Classification and functional overview of B cells
Single-cell transcriptome profiling has revolutionized B cell classification, refining it into numerous phenotypically and functionally distinct subsets beyond traditional lineages, particularly in the context of human cancers. Naïve B cells, marked by genes like TCL1A, FCER2, and IGHD, serve as primary reservoirs for antigen recognition (4). Activated B cell subsets, such as EGR1+ACB1, NR4A2+ACB2, and CCR7+ACB3, are characterized by CD69 and CD83 expression, reflecting early activation states (5). Germinal center (GC) B cells had relatively high expression of MKI67, which mediate somatic hypermutation(SHM) and class-switch recombination for high-affinity antibody production (6). Key newly identified subsets include atypical MBCs, expressing markers like ITGAX and FCRL5, with an exhausted phenotype and progenitor potential for extrafollicular-derived antibody-secreting cells (ASCs); tumor-associated atypical B cells, which interact with CD4+ T cells and predict favorable prognosis; and heterogeneous MBCs (1, 5). ASCs, including plasmablasts and multiple plasma cell subclusters, exhibit distinct tissue preferences and functional specialization in antibody secretion, with isotype shifts (e.g., IgG bias in tumors vs. IgA-dominance in adjacent tissues) driven by the microenvironment (5).
Clonal expansion characteristics, inferred from B cell receptor (BCR) repertoire and single-cell RNA sequencing (scRNA-seq) dynamics, further distinguish B cell subsets and underpin their functional roles. GC-derived ASCs, enriched in some cancers (e.g., colon adenocarcinoma), show high clonal diversity, extensive SHM, and preferential class-switch recombination to IGHA1/2, reflecting antigen-driven affinity maturation (5). In contrast, EF-derived ASCs, dominant in others (e.g., liver hepatocellular carcinoma), exhibit oligoclonal expansion with lower SHM, limited class-switch recombination, and enrichment of IGHM/IGHG4, linked to polyreactive/autoantibody production (5). Atypical MBCs display moderate clonal overlap with EF-derived ASCs, independent of GC pathways, and their expansion correlates with immunosuppressive microenvironments. tumor-associated atypical B cells and IgG+ ASCs undergo robust clonal expansion in tumors, indicative of antigen-driven activation (1). GC B cells show frequent clonal sharing, supporting iterative differentiation into MBCs and ASCs with accumulated SHM to enhance antibody affinity. These clonal dynamics, coupled with subset-specific interactions (e.g., tumor-associated atypical B cells with CD4+ T cells via MHC-II), shape B cells’dual roles in humoral immunity and T cell regulation, with implications for antitumor immunity, immune evasion, and immunotherapy response (1).
3 Basic characteristics of B cells in the tumor microenvironment
While the classification of B cell subsets provides a foundational understanding of their functional diversity, their spatial distribution and organizational states within the TME further dictate their roles in tumor immunity.
The TME, a multicellular ecosystem comprising malignant cells, stromal fibroblasts, vascular networks, and immune populations, exhibits significant spatial heterogeneity in B lymphocyte distribution (7). B cell infiltration patterns demonstrate tumor-type specificity and stage-dependent variation: while malignancies such as melanoma and triple-negative breast cancer display dense B cell infiltrates that frequently organize into TLSs– organized lymphoid aggregates supporting coordinated antitumor immunity – other tumors like pancreatic adenocarcinoma show minimal B cell recruitment (8). This spatial heterogeneity stems from tripartite regulatory mechanisms: tumor-intrinsic features (e.g., mutational burden, chemokine secretion profiles); microenvironmental constraints (hypoxia, extracellular matrix remodeling), and host immunological status (peripheral B cell repertoire diversity, pre-existing antitumor memory) (9). Notably, TLS formation correlates with enhanced cytotoxic T cell priming and improved clinical outcomes, highlighting the functional implications of B cell spatial organization in the TME (10–13).
4 The dual role of B cells in tumor immunity
4.1 Antitumor mechanisms
4.1.1 Antigen presentation and coordinated T cell activation
B lymphocytes orchestrate antitumor responses through multifaceted mechanisms, most notably via professional antigen presentation (7). By internalizing tumor-associated antigens (TAAs) and processing them through MHC-II pathways, B cells present immunogenic peptides to CD4+ T cells via TCR engagement, crucially licensing dendritic cells for subsequent CD8+ T cell priming through CD40-CD40L costimulatory interactions (14). Mechanistically, this antigen cross-presentation cascade amplifies cytotoxic T lymphocyte activation, proliferation, and tumor infiltration while promoting immunological memory formation (14). Experimental evidence from B cell-deficient murine models reveals profound impairment of T cell effector functions and tumor control, underscoring the essential role of B cells in coordinating adaptive immunity (15). Furthermore, B cell-derived cytokines including IL-12 and IFN-γ enhance Th1 polarization and macrophage tumoricidal activity, creating a self-reinforcing antitumor immune loop (16) (Figure 1A).
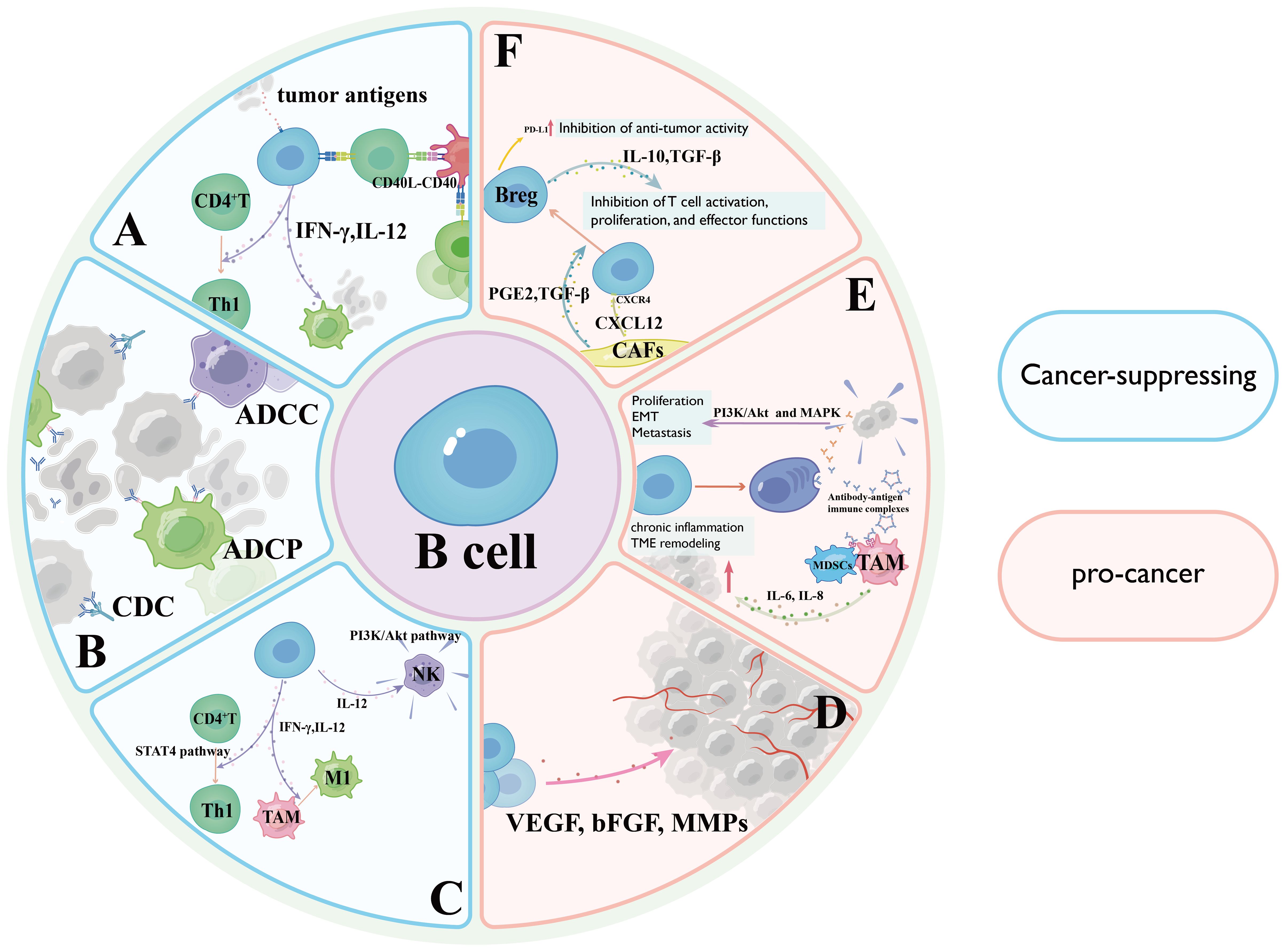
Figure 1. Diagram illustrating the dual roles of B cells in cancer, highlighting cancer-suppressing mechanisms in light blue and pro-cancer mechanisms in pink. (A) Antigen Presentation and Coordinated T Cell Activation Mechanisms; (B) Antibody-Dependent Antitumor Effector Mechanisms; (C) Cytokine Network and Tumor Microenvironment Reprogramming; (D) Secretion of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and matrix metalloproteinases (MMPs) by activated B cells stimulates endothelial cell proliferation and migration through VEGF receptor 2 (VEGFR2) signaling; (E) tumor-infiltrating B cells may paradoxically promote oncogenesis through antibody-dependent pathways; (F) Regulatory B cells (Bregs) exert immunosuppressive effects through the secretion of potent immunosuppressive cytokines.
4.1.2 Antibody-dependent antitumor effector mechanisms
Upon antigen-specific activation, tumor-reactive B cells differentiate into antibody-secreting PCs that generate high-affinity immunoglobulins targeting tumor-associated surface markers (17). These antibodies execute multifaceted antitumor effects through three principal mechanisms: Antibody-dependent cellular cytotoxicity mediated by Fcγ receptor activation on natural killer cells and macrophages; Complement-dependent cytotoxicity initiated through C1q binding and membrane attack complex formation; Opsonization-enhanced phagocytosis via Fc receptor engagement on myeloid cells (18). Clinically, this paradigm is exemplified by CD20-targeting rituximab in B cell lymphomas, where antibody-mediated B cell depletion achieves durable remissions (19). Furthermore, certain antibodies disrupt oncogenic signaling by competitively inhibiting growth factor receptors (20) (Figure 1B).
4.1.3 Cytokine network and tumor microenvironment reprogramming
Within the TME, B lymphocytes orchestrate coordinated antitumor immunity through cytokine-mediated crosstalk (21). Secretion of interleukin-12 and interferon-γ enables B cells to potentiate T cell cytotoxicity by driving Th1 polarization through signal transducer and activator of transcription4-dependent transcriptional programming, while simultaneously enhancing natural killer cell degranulation capacity via PI3K/Akt pathway activation (21). Furthermore, interferon-γ reprograms tumor-associated macrophages (TAMs) toward an immunostimulatory M1 phenotype characterized by heightened phagocytic activity and inducible nitric oxide synthase expression, effectively reversing immunosuppressive TME conditions (22). Clinical correlative studies demonstrate that B cell-derived interleukin-12/interferon-γ levels correlate with improved cytotoxic lymphocyte infiltration and survival outcomes in melanoma and colorectal carcinoma (23) (Figure 1C).
A Antigen Presentation and Coordinated T Cell Activation Mechanisms; B Antibody-Dependent Antitumor Effector Mechanisms; C Cytokine Network and Tumor Microenvironment Reprogramming; D Secretion of vascular endothelial growth factor(VEGF), basic fibroblast growth factor, and matrix metalloproteinases by activated B cells stimulates endothelial cell proliferation and migration through VEGF receptor 2 signaling; E tumor-infiltrating B cells (TIL-Bs) may paradoxically promote oncogenesis through antibody-dependent pathways; F Bregs exert immunosuppressive effects through the secretion of potent immunosuppressive cytokines.
4.1.4 The links between TLS maturity and B cell function
The maturity of TLSs directly influences the efficiency of B cell activation, antibody secretion, and T cell helper functions. The specific mechanisms are as follows:
4.1.4.1 Immature TLS and B cell activation
Immature TLS (such as primary follicles) typically lack GCs, resulting in distinct B cell differentiation trajectories compared to mature TLS. In this context, B cell activation relies on direct antigen stimulation; however, the affinity is relatively low, and the main antibody secreted is IgM (24, 25). Additionally, B cells in immature TLS tend to differentiate into Bregs. These Bregs inhibit immune responses by secreting cytokines like IL-10, thereby establishing an immune-tolerant environment (24, 25).
4.1.4.2 Mature TLS and B cell activation
Mature TLS, such as secondary follicles, possess active GCs where B cells undergo class switching (e.g., from IgM to IgG) and affinity maturation. Here, the two-signal activation model plays a dominant role: the first signal is triggered when the BCR specifically binds to antigenic epitopes, and the second signal is provided by T cells—via the binding of CD40L to CD40 on B cells—along with T cells secreting cytokines like IL-4, which together drive B cell proliferation and differentiation into PCs and memory cells (24).
4.1.4.3 Mature TLS and antibody secretion
In mature TLS, plasma cell differentiation is more efficient, and the primary antibodies produced are of the IgG class. These antibodies clear pathogens or TAAs through mechanisms such as neutralization and opsonization. Simultaneously, B cells enhance antibody affinity via SHM, contributing to the formation of immunological memory (25).
4.1.4.4 Aberrant TLS and immune imbalance
Aberrantly immature TLS may contribute to autoimmune diseases (e.g., systemic lupus erythematosus) due to enhanced BCR signaling and the lack of effective co-stimulatory regulation. Conversely, excessively mature TLS might trigger tumor immune evasion. For instance, in solid tumors like breast cancer, IgG antibodies produced by high-density PCs may facilitate the masking of TAAs (24, 26).
4.1.5 TLS-mediated T cell activation
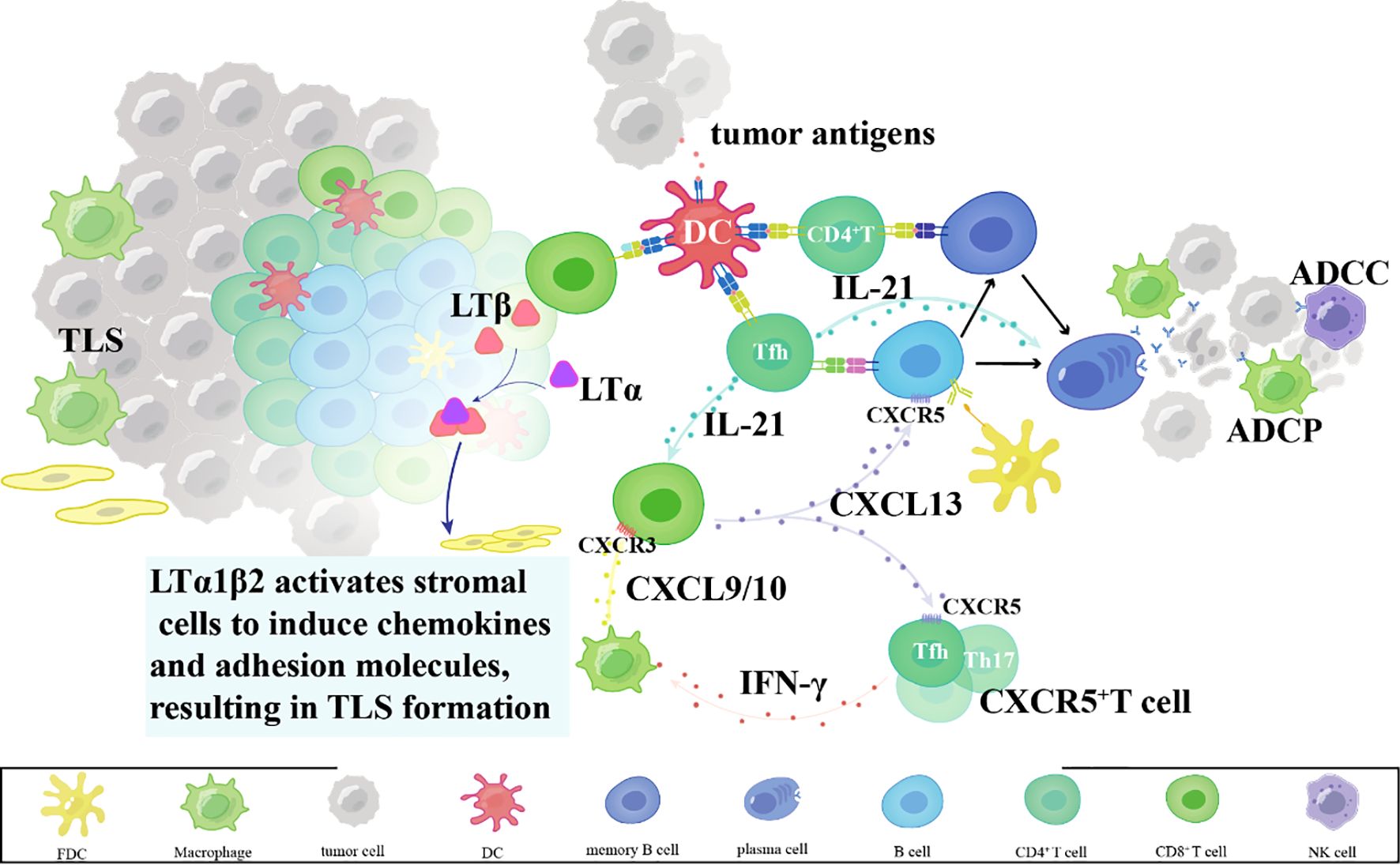
Within TLSs, B cells function as professional antigen-presenting cells, internalizing and processing tumor-derived antigens for presentation via MHC class II molecules to CD4+ T cells (27). Concurrently, B cell-derived chemokines such as CXCL13 establish chemotactic gradients that recruit CXCR5+ T cell subsets – particularly follicular helper T cells and Th17 cells – into TLS niches (28–30). These recruited T cells amplify antitumor responses through dual mechanisms: IFN-γ secretion to enhance CTL tumor infiltration via CXCL9/10 induction, and IL-21 production to support B cell antibody affinity maturation (31–33). Spatial proximity within TLS thus creates an immunologically active hub for coordinated T-B cell collaboration (34, 35) (Figure 2).
4.1.6 TLS-dependent dendritic cell regulation
B cells also modulate dendritic cell (DC) activity within TLS via cytokine-mediated crosstalk (36). Mature DCs reciprocally enhance TLS function by cross-presenting tumor antigens via MHC class I to activate CD8+ T cells and producing IL-12 to sustain Th1 polarization (37). This bidirectional synergy establishes a self-reinforcing loop: DC-primed T cells secrete lymphotoxin-β to maintain TLS stromal architecture, while TLS-resident DCs acquire enhanced capacity for tumor antigen uptake through Fc gamma receptor-mediated immune complex internalization (38). Such coordinated interactions transform TLS into functional equivalents of secondary lymphoid organs, enabling sustained adaptive immunity against progressing malignancies (38) (Figure 2).
Tertiary lymphoid structures in tumors; mechanisms of tertiary lymphoid structure formation.
While B cells can orchestrate potent antitumor immune responses through the mechanisms described above, it is increasingly evident that certain B cell subsets also contribute to immunosuppression and tumor progression. The following section discusses these paradoxical pro-tumor roles, highlighting the context-dependent nature of B cell functions in cancer.
4.2 Pro-tumor mechanisms
Certain B cell subsets, particularly Bregs, exert immunosuppressive effects through the secretion of potent immunosuppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (39, 40). These cytokines directly inhibit T cell activation, proliferation, and effector functions, facilitating tumor progression and immune evasion (40). Preclinical studies across multiple tumor models demonstrate a strong correlation between Breg infiltration, accelerated tumor growth, and poor clinical outcomes (41) (Figure 1F).
A subset of TIL-Bs may paradoxically promote oncogenesis through antibody-dependent pathways (5). Pathogenic autoantibodies targeting tumor-associated surface antigens can activate pro-survival signaling cascades (e.g., PI3K/Akt and MAPK pathways), thereby enhancing tumor cell proliferation, epithelial-mesenchymal transition, and metastatic dissemination (42). Furthermore, antibody-antigen immune complexes engage Fcγ receptors on myeloid-derived suppressor cells and TAMs, amplifying immunosuppressive cytokine networks (e.g., IL-6, IL-8) while triggering chronic inflammation that fosters tumor niche establishment (43) (Figure 1E).
B cells also contribute to tumor neovascularization through paracrine interactions within the TME (44). Secretion of VEGF, basic fibroblast growth factor, and matrix metalloproteinases by activated B cells stimulates endothelial cell proliferation and migration through VEGF receptor 2 signaling, ultimately establishing pro-angiogenic networks that sustain tumor metabolic demands and facilitate hematogenous metastasis (45) (Figure 1D).
Emerging evidence indicates bidirectional crosstalk between Bregs and stromal components, particularly cancer-associated fibroblasts (46). CAFs recruit B cell precursors via CXCL12/CXCR4 axis activation and induce Breg polarization through dual secretion of Transforming Growth Factor-beta(TGF-β) and prostaglandin E2 (47).
4.3 Molecular mechanisms governing B cell functional plasticity
The functional plasticity of B cells in the TME is principally governed by dynamic transcriptional networks. Key transcription factors play decisive roles in fate determination: B-cell lymphoma 6 maintains GC B cell identity and prevents premature differentiation, whereas B lymphocyte-induced maturation protein-1 promotes plasma cell maturation and antibody production (48, 49). Conversely, the differentiation of Bregs is driven by STAT3 activation, often induced by cytokines such as IL-10 and IL-35 within the TME (50, 51). BTB and CNC homology 2 acts as a critical repressor that preserves B cell plasticity by restraining terminal differentiation and maintaining a reversible functional state (52).
Beyond transcriptional control, epigenetic and metabolic mechanisms stabilize B cell phenotypes and enforce functional commitment. Tumor-derived signals—including hypoxia, cytokines, and nutrient scarcity—reshape the epigenetic landscape through DNA methylation, histone modifications, and non-coding RNAs, thereby locking B cells into specific transcriptional programs (53). Concurrently, metabolic reprogramming modulates B cell function; hypoxia-inducible factor-1α activation under low oxygen tension alters glycolytic flux and oxidative phosphorylation, influencing survival, proliferation, and immunoglobulin class switching.
The integration of extrinsic signals through specific receptors ultimately dictates B cell function via coordinated molecular cascades. For instance, Transforming Growth Factor-beta plus IL-21 signaling through cytokine receptors promotes Breg generation via STAT pathways, while CD40L and IL-4 engagement activates NF-κB and PI3K signaling to foster GC responses (50). These signaling networks converge to regulate the transcriptional, epigenetic, and metabolic networks described above, forming a coherent “signal–mechanism–phenotype” axis that explicates how microenvironmental cues steer B cells toward either pro-tumor or anti-tumor identities.
Given the context-dependent nature of B cell functions, it is imperative to examine how these mechanisms manifest across different cancer types, which we explore in the following section.
5 B cell interactions within the tumor microenvironment: crosstalk with immune and cancer cells
5.1 Interactions between B cells and T cells
B cells and T cells engage in bidirectional functional cooperation during antitumor immune responses (2, 54–56). As professional antigen-presenting cells, B cells prime CD4+ T cell activation through MHC-II-mediated tumor antigen presentation (57). This antigen-specific stimulation, complemented by co-stimulatory signals such as CD80/CD86, ensures full T cell activation.
Reciprocally, activated T cells secrete cytokines such as IL-21, which drives B cell proliferation, plasma cell differentiation, and enhanced antibody production through STAT3-dependent transcriptional activation (58, 59). Tfh cells are crucial instructors of B cell fate and function (60–62). Through direct cell contact mediated by CD40L binding to CD40 on B cells, and through the secretion of cytokines—most notably IL-21—Tfh cells provide essential signals that guide B cell differentiation. IL-21 signaling activates the JAK-STAT3 pathway in B cells, leading to transcriptional upregulation of genes that promote proliferation, isotype switching, and ultimately, their differentiation into antibody-secreting PCs or GC B cells (63). This high-level cooperation is spatially coordinated within tumor-associated TLSs, which serve as organized hubs for the generation of high-affinity, tumor-specific antibodies and MBCs (64) (Figure 3A).
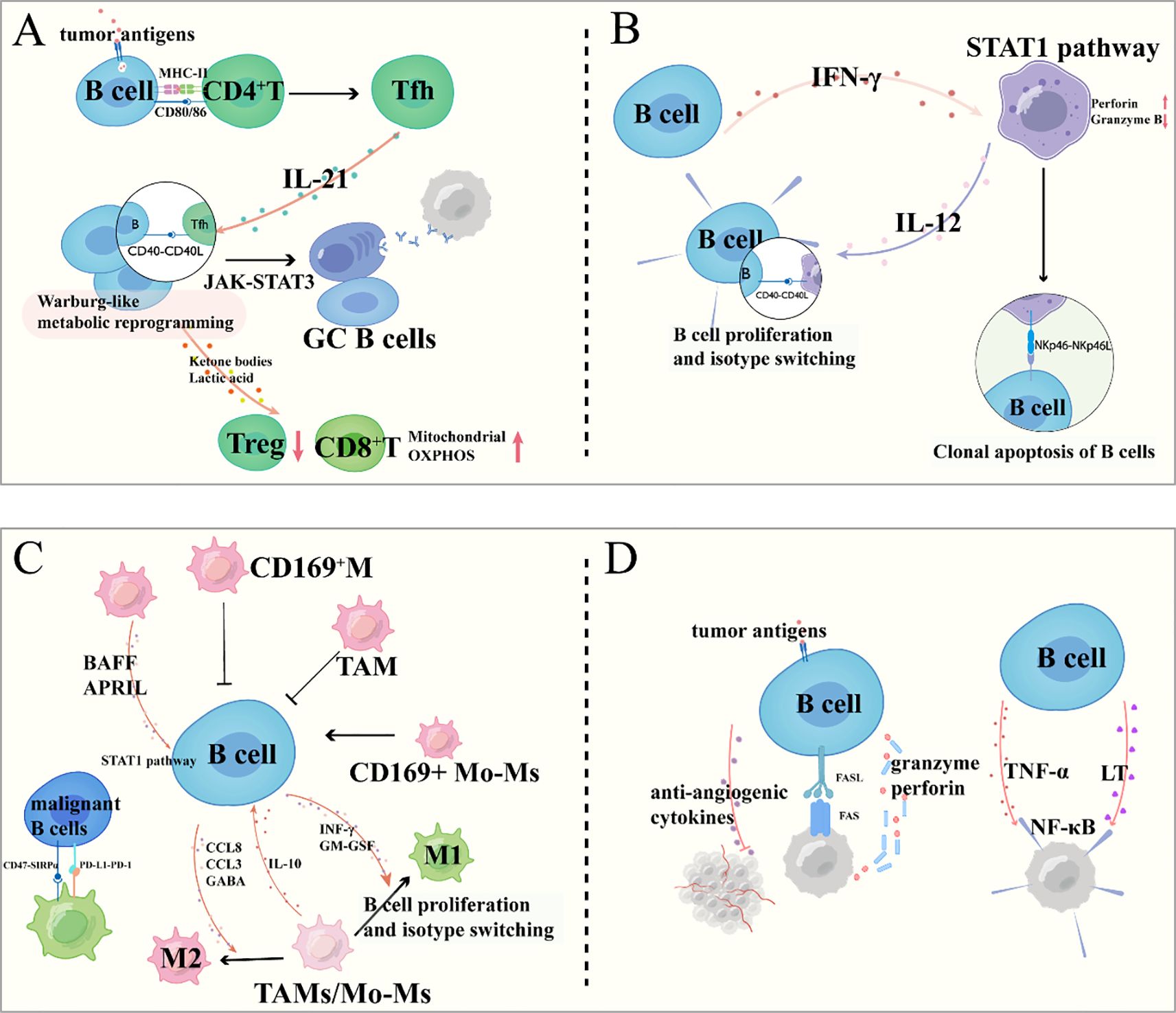
Figure 3. B cell interactions within the tumor microenvironment: crosstalk with immune and cancer cells. (A) Interactions between B Cells and T Cells; (B) Regulatory Dynamics between B Cells and NK Cells; (C) Bidirectional Crosstalk between B Cells and Tumor-Associated Macrophages; (D) Direct and Indirect Interactions between B Cells and Cancer Cells.
Conversely, B cells significantly influence the function and differentiation of T cells, particularly Tfh cells. By presenting antigen, B cells help sustain Tfh cell survival and functional maturation within TLS (64). Furthermore, activated B cells undergo metabolic reprogramming, increasing their glycolytic flux and glutamine metabolism. The resulting metabolic byproducts, such as lactate, can enhance mitochondrial oxidative phosphorylation in neighboring effector T cells, thereby boosting their antitumor activity, while simultaneously inhibiting the expansion of immunosuppressive regulatory T cells (65, 66).
In summary, the interaction between B and T cells is a dynamic, reciprocal relationship encompassing antigen presentation, co-stimulatory signaling, cytokine communication, and metabolic cross-talk. This coordinated network is vital for directing both cellular and humoral arms of the immune system against tumors.
5.2 Regulatory dynamics between B cells and natural killer cells
The interplay between B cells and NK cells exhibits dual regulatory roles in tumor immunity (7, 67). B cell-derived interferon-γ primes NK cell activation through STAT1-mediated transcriptional upregulation of perforin and granzyme B, thereby enhancing NK-mediated tumor cell lysis. Conversely, NK cells regulate B cell responses through both contact-dependent and cytokine-mediated mechanisms (68). NK cell-derived IL-12 and membrane-bound CD40L directly promote B cell proliferation and immunoglobulin class switching, while NKp46 engagement with B cell surface ligands induces apoptosis of malignant or dysfunctional B cell clones—a quality control mechanism maintaining immune homeostasis (69) (Figure 3B).
5.3 Bidirectional crosstalk between B cells and tumor-associated macrophages
The functional interplay between B cells and TAMs critically shapes the immunomodulatory landscape of the TME (70). For instance, in melanoma (71)and breast cancer (72), subcapsular sinus macrophages, particularly CD169+ subsets, act as tumor suppressors by inhibiting B cell activation, possibly through forming a physical barrier that restricts B cell activity (71, 73, 74). However, in breast cancer, this regulation is subtype-specific: while Subcapsular sinus macrophages (72)and CD169+ TAMs (75) suppress B cell activation, CD169+ monocyte-derived macrophages (75) promote B cell activation, highlighting the functional diversity of macrophages in TME. Additionally, macrophage-derived factors like BAFF and APRIL (76–80), which drive B cell proliferation via NF-κB signaling, may contribute to the expansion of malignant B cells in lymphomas (81).
Conversely, B cells actively shape macrophage functions to favor tumor progression through multiple mechanisms. In diffuse large B-cell lymphoma, B cells secrete CCL8, which binds to CCR1/2/3/5 on macrophages, inducing M2 polarization and limiting anti-tumor immunity (82). In mantle cell lymphoma, B cell-derived CCL3 forms a positive feedback loop with M2 macrophages: CCL3 promotes M2 polarization, and M2 macrophages secrete IL-10 to further stimulate CCL3 secretion by MCL cells, accelerating tumor growth (83). B cells also evade macrophage-mediated phagocytosis via pathways like CD47/SIRPα (84) and PD-L1/PD-1 (85), where CD47 on malignant B cells binds SIRPα on macrophages, and PD-L1 interacts with PD-1 on macrophages, both inhibiting phagocytic activity. Furthermore, B cell-derived GABA induces monocyte differentiation into M2 macrophages, which secrete IL-10 to support tumor survival (86). Besides, B cell-derived cytokines, including interferon-γ and granulocyte-macrophage colony-stimulating factor, drive macrophage polarization toward an immunostimulatory M1 phenotype characterized by enhanced tumoricidal activity through nitric oxide production and pro-inflammatory cytokine secretion (87) (Figure 3C).
5.4 Direct and indirect interactions between B cells and cancer cells
B cells engage in both direct and indirect crosstalk with tumor cells, significantly influencing tumor progression and immune surveillance. A key direct mechanism involves BCR-mediated recognition of TAAs, which can initiate specific activation, proliferation, and antibody production. This antigen-specific engagement may also trigger direct cytotoxic effects against tumor cells via Fas-FasL and granzyme-perforin pathways under certain conditions (88–90) (Figure 3D).
Indirectly, B cells modulate tumor behavior through cytokine networks. For instance, B cell-derived lymphotoxin and TNF-α can promote cancer cell survival and invasion by activating NF-κB signaling in malignant cells (91) (Figure 3D). Conversely, certain B cell subsets produce anti-angiogenic cytokines that inhibit tumor vascularization (88).
6 The role of B cells in tumor immunobiology across cancer types
The dual roles of B cells are not uniformly exhibited across all malignancies; rather, they are shaped by tumor-intrinsic factors, microenvironmental cues, and host immune status. The following section examines how these mechanisms manifest in a cancer-type-specific manner, highlighting both common themes and context-dependent variations.
6.1 Hematologic malignancies
In B cell-derived lymphomas, malignant transformation arises from dysregulated proliferation, differentiation, and apoptotic pathways intrinsic to B cell development (92). Within the lymphoma microenvironment, bidirectional crosstalk occurs between neoplastic B cells and residual normal B lymphocytes (93). Tumor cells subvert neighboring B cells through IL-6/STAT3 signaling and CD40L-mediated activation, reprogramming them into pro-tumor effectors that secrete survival factors (94).
Clinically, B cell-targeted therapies demonstrate remarkable efficacy: anti-CD20 monoclonal antibodies (e.g., rituximab) induce complement-dependent lysis in CD20+ lymphomas, while B-cell maturation antigen -directed CAR-T cells and bispecific antibodies achieve deep responses in refractory myeloma by eradicating malignant plasma cell clones (95, 96).
6.2 Solid tumors
6.2.1 Melanoma
B cells exhibit dual roles in the immune regulation of melanoma, with their functions critically dependent on subset heterogeneity and microenvironmental features (67, 97–100). On one hand, B cells can enhance antitumor immunity by forming TLSs in collaboration with T cells (97). These structures are enriched with MBCs and activated T cells, significantly improving the efficacy of immune checkpoint blockade (ICB) and correlating with better patient survival (97, 98). Concurrently, clonal expansion, antibody diversity, and affinity maturation of TIL-Bs suggest their involvement in localized antitumor responses, particularly through IgG subclass antibodies that may suppress tumor progression (97). On the other hand, specific B cell subsets exert immunosuppressive effects by secreting IL-10 or upregulating PD-L1, thereby promoting tumor growth (67, 101). Notably, PD-L1+ naïve-like B cells directly inhibit T cell function, and their abundance is positively associated with advanced melanoma bone metastasis and poor prognosis (102). Additionally, peripheral blood B-cell levels serve as a predictive biomarker for anti-PD-1 therapy response, with lower B-cell levels correlating with longer survival (103). This functional heterogeneity is closely linked to the balance of B-cell subsets, spatial organization (e.g., infiltrative patterns of activated B-cell follicles in metastatic lymph nodes), and autoimmune-like features, highlighting that targeting specific B-cell subsets or disrupting their immunosuppressive signaling may represent novel therapeutic strategies for melanoma (100).
6.2.2 NSCLC
B cells play a critical immunomodulatory role in NSCLC through the formation of TLSs and functional heterogeneity (104). TLS, serving as hubs for adaptive immune responses, coordinate B and T cell interactions, and their presence significantly enhances the efficacy of PD-L1 inhibitors (e.g., atezolizumab), correlating with prolonged patient survival independently of CD8+ T cell signals (104). B cell subsets exhibit dynamic functional divergence: naïve-like B cells suppress tumor growth in early stages by secreting inhibitory factors but diminish in advanced NSCLC, correlating with poor prognosis, while plasma-like B cells exert antitumor activity in early stages but may promote tumor progression in advanced disease via secretion of specific immunoglobulins (e.g., IgG subclasses) or microenvironmental interactions (104, 105). Additionally, B cells generate antitumor effects through antibodies targeting endogenous retroviruses, which are amplified during ICB therapy and enhance therapeutic responses via CXCL13-dependent TLS formation (106). The heterogeneity of B and PCs is influenced by smoking status and TME, with their functional phenotypes (e.g., immunosuppressive plasma cell profiles) predicting postoperative outcomes and ICB efficacy (105). Collectively, strategies targeting TLS formation (e.g., CXCL13 therapy), balancing B cell subsets, or modulating antibody responses (e.g., enhancing anti-Endogenous retrovirus activity) may represent novel therapeutic avenues for NSCLC immunotherapy (106, 107).
6.2.3 Breast cancer
B cells play multiple critical roles in the immune regulation of breast cancer, with their diverse functional subsets and dynamic changes significantly influencing antitumor immune responses and therapeutic outcomes (108). Studies demonstrate that B cells aggregate via TLSs and interact with T follicular helper cells to promote antibody production and T cell activation, constituting a core mechanism underlying responses to ICBs (6). Single-cell sequencing reveals substantial heterogeneity in TIL-Bs, where follicular B cell subsets and chemotherapy-induced inducible T-cell costimulator ligand (ICOSL)+ B cell subsets are closely associated with immunotherapy and chemotherapy efficacy, respectively (6). Additionally, B cell infiltration exhibits a synergistic relationship with tumor-associated neutrophils, particularly in TLS-low groups, highlighting the complexity of microenvironmental interactions. Mechanistically, B cell function is regulated by complement signaling (e.g., complement receptor 2/CD55 pathways), as chemotherapy-induced immunogenic cell death drives ICOSL+ B cell differentiation via complement-complement receptor 2 signaling, thereby enhancing the effector-to-regulatory T cell ratio to potentiate antitumor immunity (109). These findings not only underscore the therapeutic potential of targeting B cells (e.g., CD23 as a TLS biomarker) but also provide a theoretical foundation for combinatorial immunotherapeutic strategies (e.g., targeting ICOSL or complement pathways).
6.2.4 Renal cell carcinoma
B cells exhibit complex and dynamic dual roles in the immune regulation of renal cell carcinoma (110). On one hand, intratumoral TLSs host B cells that differentiate into antibody-secreting PCs (e.g., IgG/IgA-producing subtypes), mediating antibody-dependent antitumor effects and correlating with improved clinical responses and survival in patients treated with ICBs (111). Spatial transcriptomics reveals that B cells within TLS undergo clonal diversification, expansion, and migration, with their maturation status and localization (e.g., tumor-proximal mature TLS containing CD23+ GCs) significantly influencing prognosis. Mature TLS are associated with favorable survival outcomes, while immature tumor-distal TLS are enriched with immunosuppressive cells (e.g., PD-L1+ macrophages and regulatory T cells), reflecting microenvironmental heterogeneity (111). On the other hand, dynamic shifts in B cell subsets are linked to treatment efficacy and toxicity: combined ICB therapy promotes differentiation of circulating B cells into memory phenotypes (e.g., increased switched MBCs correlate with therapeutic efficacy), whereas plasmablast expansion is associated with severe immune-related adverse events (e.g., hypophysitis) (112). Notably, high TIL-Bs may recruit M2 macrophages and Tregs, exacerbate T-cell exhaustion (marked by upregulation of PD-1/CTLA-4/TIM-3), and diminish the efficacy of combination therapies (e.g., anti-PD-1 inhibitors combined with axitinib) (113). These findings underscore the dual nature of B cells in RCC—balancing antitumor potential and immunosuppressive risks—with their functional polarization shaped by spatial distribution, maturation status, and therapeutic interventions, offering critical insights for precision immunotherapy strategies.
6.2.5 Hepatocellular carcinoma
The role of B cells in HCC is dualistic and complex, encompassing both antitumor immune regulation and tumor-promoting mechanisms through specific subsets or signaling pathways (114–116). Studies indicate that B cells collaborate with T cells to suppress HCC progression, such as by forming TLSs to enhance T cell memory function or via B-cell-related gene models that predict immunophenotypes and prognosis, highlighting their antitumor potential (117). However, B cells can also drive immunosuppression through distinct mechanisms, such as IL-21 receptor signaling inducing immunosuppressive IgA+ B cells to inhibit CD8+ T cell activity, or Ten-eleven translocation methylcytosine dioxygenase 2-mediated IL-10+ regulatory B cells (Breg) promoting tumor immune evasion (118). Additionally, interactions between B cells and innate lymphoid cells, exemplified by ICOSL signaling, exacerbate inflammatory microenvironments and accelerate HCC progression (115). Spatial dynamics analysis further reveals that colocalization patterns of B and T cells (e.g., TLS or lymphoplasmacytic microenvironments) significantly influence clinical outcomes and immunotherapy responses (119). Molecular regulatory mechanisms within the TME, such as Pre-mRNA processing factor 19-mediated degradation of DEAD-box helicase 5 suppressing B cell recruitment or dysregulation of the CXCL12/CXCR4 axis, alongside epigenetic modifications (e.g., Ten-eleven translocation methylcytosine dioxygenase 2-dependent IL-10 expression), reshape B cell functionality and emerge as potential therapeutic targets (120, 121). In summary, B cells exhibit functional heterogeneity in HCC, with their pro- or antitumor effects determined by subset characteristics, spatial distribution, and microenvironmental signaling networks. Targeting B cell-related pathways (e.g., ICOS, IL-21R, Ten-eleven translocation methylcytosine dioxygenase 2) or combining therapies with ICBs may offer novel strategies for personalized immunotherapy in HCC.
6.2.6 Colorectal cancer
B cells in CRC exhibit functional diversity and microenvironment dependency, contributing to both antitumor immune regulation and pro-tumor mechanisms (122–124). Studies show that B cell subsets such as TLS-associated CD20+ B cells and IgG PCs collaborate with CXCL13+ CD8+ T cells to promote TLS formation, enhance antigen presentation, and correlate positively with high microsatellite instability, high tumor mutation burden, and immunotherapy response, indicating their antitumor potential (122). However, specific B cell subsets, such as leucine-tRNA synthase 2-expressing B cells, suppress antitumor immunity through TGF-β1-dominant regulatory features (123). These cells are driven by leucine metabolism and rely on mitochondrial nicotinamide adenine dinucleotide+ regeneration and sirtuin 1 signaling, promoting CRC immune evasion (123). Spatial heterogeneity analysis reveals distinct B cell developmental trajectories and CD20+ B cell abundance between right- and left-sided CRC, with CD20+ B cell enrichment in right-sided CRC predicting favorable prognosis, while their depletion impairs anti-PD-1 therapy efficacy (124). Additionally, microbiota-immune interactions regulate B cell function: specific bacteria like Helicobacter hepaticus induce follicular helper T cells to promote TLS maturation and antitumor immunity, whereas Alcaligenes faecalis suppresses IgA+ B cell homing and disrupts the intestinal barrier via acetate-mediated vinculin acetylation, driving the inflammation-to-cancer transition (11, 125). Notably, activated B cells inhibit CRC liver metastasis via the SDF-1-CXCR4 axis, yet their depletion in metastatic sites correlates with increased metastasis, while immature plasma cell subsets are linked to metastasis progression (126). In summary, B cells in CRC exhibit a dual role, with their function determined by subset characteristics, metabolic states, spatial localization, and dynamic interactions with microbiota and T cells. Targeted modulation of B cell subsets—such as inhibiting leucine-tRNA synthase 2-expressing B cells, enhancing CD20+ B cells, or promoting follicular helper T-B cell collaboration—may provide novel strategies for CRC immunotherapy.
6.3 Analysis of similarities and differences in B cell function across tumor types
In the same tumor, the dual antitumor and pro-tumor roles of B cells arise from the integration of multiple interconnected mechanisms. Firstly, B cell subset heterogeneity is foundational: antitumor subsets, such as GC B cells and PCs, exert protective effects by producing high-affinity antibodies and forming TLSs to recruit and activate T cells (127). In contrast, pro-tumor subsets like Bregs secrete immunosuppressive cytokines (e.g., IL-10, TGF-β) to inhibit effector T/NK cells and promote Treg differentiation, while exhausted or aged B cells impair antigen presentation and express high levels of immune checkpoints (e.g., PD-1, TIM-1) to exhaust T cells (128).
Secondly, the TME actively shapes this duality through metabolic and signaling regulation (129). Metabolic stress, including nutrient competition with tumor cells and hypoxia, induces hypoxia-inducible factor-1α and impairs B cell effector functions (e.g., reduced antibody production) (129). Meanwhile, TME-derived signals selectively suppress antitumor B cells while recruiting or activating Bregs (130).
Thirdly, heterogeneous expression of immune checkpoint molecules contributes: pro-tumor subsets (Bregs, exhausted B cells) upregulate PD-L1, CTLA-4, etc., to transmit inhibitory signals, whereas antitumor subsets retain low checkpoint expression to maintain functionality (131).
Lastly, clonal origin and antigen specificity differences underpin functional divergence. B cell clones recognizing TAAs or neoantigens differentiate into antitumor PCs, while those targeting self-antigens or non-specific inflammatory signals are prone to becoming Bregs (132). These clones compete for resources and signals in the TME, further amplifying functional heterogeneity.
Collectively, these mechanisms—subset diversity, TME regulation, checkpoint heterogeneity, and clonal specificity—synergistically drive the coexistence of B cells’ contradictory roles within the same tumor.
The functional heterogeneity of B cells across different cancer types underscores the necessity of understanding their roles in a context-specific manner. This knowledge not only enhances our comprehension of tumor immunobiology but also informs the development of tailored therapeutic strategies. In the following section, we explore how B cells influence—and can be harnessed to improve—current cancer treatments, including ICBs, cancer vaccines, and chemotherapy.
7 Comparative analysis of B cell functions in human and murine systems
B cells in both humans and mice play dual and multifaceted roles in tumor immunity, contributing to both anti-tumor and pro-tumor responses through conserved mechanisms. In both species, B cells are involved in antigen presentation, antibody production, and the formation of TLSs, which support T-cell activation and adaptive immunity. For example, in human cancers such as breast and lung adenocarcinoma, as well as in mouse models like 4T1 and CT26, B cells enhance antitumor immunity by producing antibodies, facilitating antigen cross-presentation, and promoting cytotoxic T-cell responses (2). Bregs also exist in both systems and suppress immune activity through cytokines such as IL-10 and TGF-β, highlighting a shared functional dichotomy in B cell responses (133, 134).
However, important species-specific differences are evident. In humans, Bregs are commonly identified by the phenotype CD19+CD24hiCD38hi (135), particularly in gastric and pancreatic cancers, whereas murine Bregs are often characterized as CD19+CD5+CD1Dhi (136). Additionally, therapeutic outcomes can diverge; anti-CD20 therapy effectively depletes B cells in some human cancers but may exacerbate tumor growth in mice due to the expansion of CD20low Breg subsets (137, 138). Human B cells also display greater heterogeneity and spatial complexity within tumors, frequently including distinctive subsets such as double-negative (CD27−IgD−) MBCs and dense plasma cell infiltrates, whose roles are not fully paralleled in mouse models (139). These differences underscore the necessity of cautiously translating murine findings to human applications and combining insights from both systems to advance cancer immunotherapy.
8 The impact of B cells on tumor immunotherapy and chemotherapy
Building upon the mechanistic insights into B cell functions in tumor immunity, we now turn to their clinical implications, particularly in the context of immunotherapy and chemotherapy, where B cells serve as both mediators of response and potential therapeutic targets.
8.1 Immune checkpoint inhibitor therapy
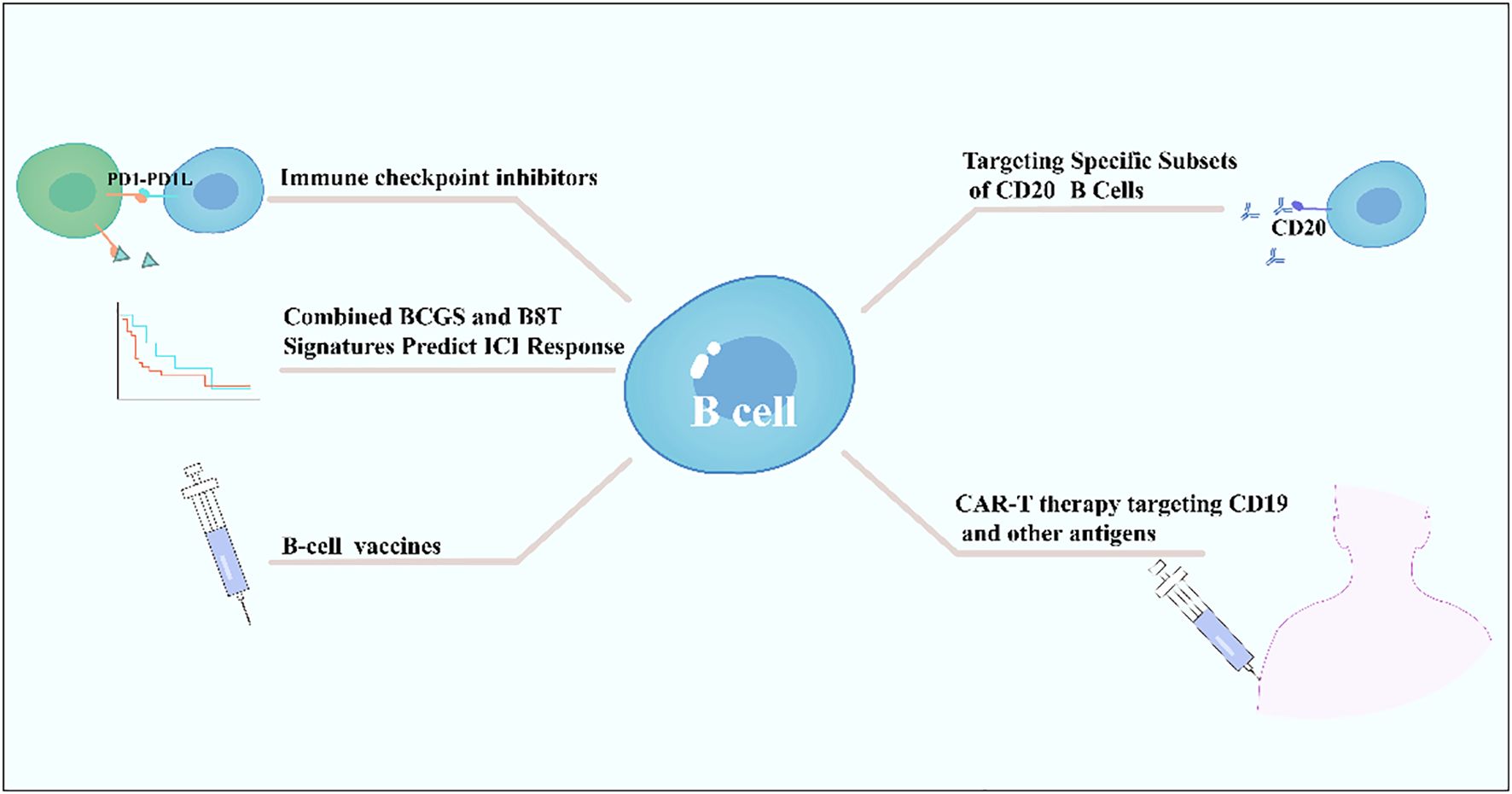
B cells exhibit dual roles in immune checkpoint inhibitor therapy, with their functions and predictive value varying by tumor type and immune microenvironment. In solid tumors such as bladder cancer, a high intratumoral B-cell gene signature combined with CD8+ T-cell signals significantly predicts ICB efficacy, correlating with prolonged overall survival particularly in male patients, while no such association is observed in females, suggesting sex-specific immune regulatory mechanisms (140). In B-cell lymphomas, HLA-dependent antigen presentation defects—such as class II-associated invariant chain peptide retention caused by HLA-DM deficiency—constitute a key immune escape mechanism, impairing tumor antigen presentation and potentially diminishing ICB efficacy. Notably, abnormal HLA expression patterns occur in 62-88% of cases in classic Hodgkin lymphoma and diffuse large B-cell lymphoma (141). Additionally, combination strategies targeting B-cell surface molecules (e.g., CD20-targeting antibodies) with ICBs have emerged as a research focus. Bibliometric analyses highlight CAR-T therapy, next-generation CD20 antibodies, and PD-1/PD-L1 inhibitors as emerging directions, potentially overcoming treatment barriers in B-cell lymphomas by modulating B-cell functions or enhancing T-cell activity (142). In summary, B cells serve not only as potential predictive biomarkers for ICB efficacy (e.g., B8T signature) but also as critical targets for therapeutic optimization (e.g., CD20-targeted combinations) and immune escape mechanism analysis (e.g., HLA abnormalities). Their multifaceted roles necessitate comprehensive evaluation based on tumor type, sex, and molecular characteristics (140–142) (Figure 4).
8.2 Cancer vaccines
B cells play a critical role in cancer vaccine development as key effector cells, enhancing antitumor responses through the activation of humoral immunity and synergistic action with adaptive immunity (143). B-cell epitope vaccines, such as HER-vaxx, target linear epitopes of TAAs (e.g., Her-2/neu) to induce polyclonal, high-affinity antibodies (144). Their efficacy rivals that of monoclonal antibodies (e.g., trastuzumab) while offering advantages such as lower cost, reduced toxicity, and controllable half-lives (145). For instance, a CD19-targeted fusion protein (scFv-Her2D4) combined with PD-1 antibodies reverses T-cell exhaustion, reduces MDSC/Treg infiltration, and promotes complete tumor regression (146) (Figure 4).
To enhance immunogenicity, vaccine design employs multivalent antigen strategies (e.g., bivalent antigens), which promote GC B cell differentiation and long-lived plasma cell generation through B-cell receptor (BCR) crosslinking, significantly boosting antibody levels (147). Antigen delivery systems targeting DC surface markers (e.g., CD11c, Xcr1) or MHC II molecules further optimize antigen-presenting cell (APC) presentation efficiency (147).
Synergy between B-cell and T-cell vaccines is achieved through coordinated mechanisms: CD8+ T cells directly lyse tumors via pMHC recognition, while B cells, with CD4+ T-cell assistance, secrete antibodies mediating antibody-dependent cellular cytotoxicity or blocking immunosuppressive signals (e.g., PD-1/PD-L1) (148). Additionally, active immunization strategies using immune checkpoint mimotopes (e.g., PD-1 mimotopes) induce endogenous antibodies to block inhibitory pathways (143). When combined with tumor-specific vaccines (e.g., Her-2), this approach significantly enhances efficacy, as demonstrated in preclinical models by reduced tumor proliferation and induced apoptosis (143) (Figure 4).
Novel vaccine platforms integrate chemical modifications (e.g., fluorinated cyclic dinucleotides to enhance STING activation), nanocarriers (e.g., black phosphorus nanosheets), and dynamic APC behavior regulation (e.g., DNA hydrogels) to achieve multimodal immune activation (148). Future directions focus on optimizing multivalent antigen design, APC-targeted delivery systems, and B/T cell dual pathway synergy to overcome clinical translation barriers, advancing cancer immunotherapy toward high-efficiency, precision-driven multidimensional strategies (147).
8.3 B cell dynamics during chemotherapy
Chemotherapy exerts profound and selective effects on B cell subsets, altering their proportions, differentiation trajectories, and functional phenotypes in ways that impact antitumor immunity. Notably, chemotherapy often increases the proportion of naive B cells (e.g., IgD+CD27- subsets) while depleting MBCs, with incomplete recovery of MBCs even years post-treatment (149). Transitional B cells rebound rapidly after chemotherapy, exceeding baseline levels temporarily, whereas follicular and marginal zone B cells show prolonged depletion, particularly marginal zone B cells, which are critical for rapid antibody responses to T-independent antigens (149–152). PCs exhibit relative resistance to chemotherapy-induced depletion compared to other B cell subsets, with preserved or enhanced antibody secretion—including IgG targeting TAAs—facilitated by chemotherapy-induced antigen release and immune complex formation (153). Bregs are selectively reduced by certain chemotherapeutics, with decreased IL-10 secretion and increased apoptosis, potentially alleviating immunosuppression and enhancing treatment efficacy (154).
These dynamic changes in B cell subsets are tightly linked to treatment responses. For instance, post-chemotherapy increases in ICOSL+ B cells correlate with elevated effector T cell/Treg ratios and improved survival in breast cancer (109), while class-switched MBCs with upregulated CD86 predict better responses to platinum-based therapy in ovarian cancer (155). Clonal expansion patterns also shift: chemotherapy drives naive B cell differentiation into antigen-specific MBCs and PCs, with clonal diversity linked to robust antitumor antibody responses (149). Additionally, chemotherapy modulates B cell-T cell crosstalk—via CD86 upregulation on B cells or ICOSL-ICOS interactions—to enhance T cell activation, further shaping treatment outcomes (156). Together, these dynamics highlight B cells as key regulators of chemotherapy efficacy, with subset-specific changes offering potential biomarkers and therapeutic targets.
8.4 Clinically approved B cell-targeted therapies in solid tumor
In solid tumor therapy, several B cell-targeted strategies have been applied in clinical practice or clinical trials. Monoclonal antibodies (mAbs) represent a key approach. Rituximab, an anti-CD20 mAb, has been used to deplete B cells in certain solid tumors; for example, it inhibits cross-talk between B cells and TAMs in pancreatic ductal adenocarcinoma, showing potential in preclinical and early clinical settings (157). Ibrutinib, a Bruton’s tyrosine kinase inhibitor, has also been explored for B cell depletion in pancreatic ductal adenocarcinoma, aiming to reduce the pro-tumorigenic effects of Bregs (157). Additionally, anti-CD20 mAbs have been tested in melanoma, with studies indicating that depleting tumor-associated B cells may improve outcomes in patients at high risk of recurrence (158, 159).
Vaccines and adjuvants targeting B cells to enhance anti-tumor immunity are another active area. HER-2/neu-based vaccines, such as those using GM-CSF as an adjuvant, have been evaluated in breast cancer to boost anti-HER2/neu antibody responses (160). A Phase Ib trial of the B cell epitope vaccine IMU-131/HER-Vaxx in HER-2+ gastroesophageal adenocarcinoma demonstrated dose-dependent increases in HER2/neu-specific IgG levels (161). Adjuvants like CpG oligodeoxynucleotides and monophosphoryl lipid A have been combined with tumor antigens in vaccines for melanoma, non-small cell lung cancer, and prostate cancer, enhancing cytotoxic antibody functions and inducing IgG production (162, 163).
9 Future perspectives
While significant progress has been made in unraveling the roles of B cells in tumor biology, critical challenges and knowledge gaps remain. Future studies should prioritize elucidating the functional heterogeneity of B-cell subsets within the TME, delineating their precise mechanisms in tumorigenesis, therapeutic response, and immune regulation. This includes deciphering complex crosstalk between B cells and other immune components (e.g., T cells, DCs) and identifying microenvironmental factors (e.g., cytokines, metabolic cues) that modulate these interactions. Concurrently, advancing targeted B-cell therapies requires optimizing specificity to suppress pro-tumor subsets while preserving antitumor functions, alongside developing combination strategies with checkpoint inhibitors or chemotherapy to minimize toxicity and enhance therapeutic efficacy. Translational efforts must focus on identifying robust biomarkers—such as B-cell receptor diversity or TLS signatures—to predict treatment outcomes and guide personalized immunotherapy. Additionally, emerging mechanisms involving non-coding RNAs, metabolic reprogramming, and exosome-mediated communication between B cells and tumors warrant deeper exploration for their diagnostic and therapeutic potential. By addressing these priorities, a deeper understanding of B-cell biology in oncology will pave the way for innovative immunotherapies, ultimately improving survival and quality of life for cancer patients.
Author contributions
ZL: Writing – original draft. RY: Formal analysis, Writing – original draft. KZ: Writing – original draft, Formal analysis. RW: Writing – original draft, Formal analysis. XS: Writing – original draft, Resources. JW: Resources, Writing – original draft. LL: Resources, Writing – original draft. JJ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Henan province (232300421189), the Startup Foundation for Doctor of Xinxiang Medical University (XYBSKYZZ202002, XYBSKYZZ202116).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang Y, Chen X, Pan J, Ning H, Zhang Y, Bo Y, et al. Pan-cancer single-cell dissection reveals phenotypically distinct B cell subtypes. Cell. (2024) 187:4790–811 e22. doi: 10.1016/j.cell.2024.06.038
2. Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and cd4 T follicular helper cell collaboration promotes anti-tumor cd8 T cell responses. Cell. (2021) 184:6101–18 e13. doi: 10.1016/j.cell.2021.11.007
3. Li X, Wang R, Wang S, Wang L, and Yu J. Construction of a B cell-related gene pairs signature for predicting prognosis and immunotherapeutic response in non-small cell lung cancer. Front Immunol. (2022) 13:989968. doi: 10.3389/fimmu.2022.989968
4. Fitzsimons E, Qian D, Enica A, Thakkar K, Augustine M, Gamble S, et al. A pan-cancer single-cell rna-seq atlas of intratumoral B cells. Cancer Cell. (2024) 42:1784–97 e4. doi: 10.1016/j.ccell.2024.09.011
5. Ma J, Wu Y, Ma L, Yang X, Zhang T, Song G, et al. A blueprint for tumor-infiltrating B cells across human cancers. Science. (2024) 384:eadj4857. doi: 10.1126/science.adj4857
6. Wang Q, Sun K, Liu R, Song Y, Lv Y, Bi P, et al. Single-cell transcriptome sequencing of B-cell heterogeneity and tertiary lymphoid structure predicts breast cancer prognosis and neoadjuvant therapy efficacy. Clin Transl Med. (2023) 13:e1346. doi: 10.1002/ctm2.1346
7. Laumont CM and Nelson BH. B cells in the tumor microenvironment: multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell. (2023) 41:466–89. doi: 10.1016/j.ccell.2023.02.017
8. Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut. (2023) 72:1927–41. doi: 10.1136/gutjnl-2022-328697
9. Quek C, Pratapa A, Bai X, Al-Eryani G, Pires da Silva I, Mayer A, et al. Single-cell spatial multiomics reveals tumor microenvironment vulnerabilities in cancer resistance to immunotherapy. Cell Rep. (2024) 43:114392. doi: 10.1016/j.celrep.2024.114392
10. Singh S, Lee N, Pedroza DA, Bado IL, Hamor C, Zhang L, et al. Chemotherapy coupled to macrophage inhibition induces T-cell and B-cell infiltration and durable regression in triple-negative breast cancer. Cancer Res. (2022) 82:2281–97. doi: 10.1158/0008-5472.CAN-21-3714
11. Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. (2021) 54:2812–24 e4. doi: 10.1016/j.immuni.2021.11.003
12. Cui C, Craft J, and Joshi NS. T follicular helper cells in cancer, tertiary lymphoid structures, and beyond. Semin Immunol. (2023) 69:101797. doi: 10.1016/j.smim.2023.101797
13. Wei F, Xu X, Wang J, Mei SW, Zhao FQ, Huang F, et al. Intratumoural cd8(+) cxcr5(+) follicular cytotoxic T cells have prognostic value and are associated with cd19(+) cd38(+) B cells and tertiary lymphoid structures in colorectal cancer. Cancer Immunol Immunother. (2024) 74:36. doi: 10.1007/s00262-024-03887-z
14. Li C, Ke F, Mao S, Montemayor Z, Traore MDM, Balsa AD, et al. Sars-cov-2 B epitope-guided neoantigen nanovaccines enhance tumor-specific cd4/cd8 T cell immunity through B cell antigen presentation. ACS Nano. (2025) 19:7038–54. doi: 10.1021/acsnano.4c15113
15. Yu X, Li T, Shen Z, Jing H, Xie X, Zhou X, et al. The establishment of B cell-deficient igh-J ko mouse model by gene editing and efficacy evaluation. Int Immunopharmacol. (2023) 116:109704. doi: 10.1016/j.intimp.2023.109704
16. Yarchoan M, Ho WJ, Mohan A, Shah Y, Vithayathil T, Leatherman J, et al. Effects of B cell-activating factor on tumor immunity. JCI Insight. (2020) 5(10):e136417. doi: 10.1172/jci.insight.136417
17. Basso K. Biology of germinal center B cells relating to lymphomagenesis. Hemasphere. (2021) 5:e582. doi: 10.1097/HS9.0000000000000582
18. Park JE, Kim SE, Keam B, Park HR, Kim S, Kim M, et al. Anti-tumor effects of nk cells and anti-pd-L1 antibody with antibody-dependent cellular cytotoxicity in pd-L1-positive cancer cell lines. J Immunother Cancer. (2020) 8(2):e000873. doi: 10.1136/jitc-2020-000873
19. Grzelak L, Roesch F, Vaysse A, Biton A, Legendre R, Porrot F, et al. Irf8 regulates efficacy of therapeutic anti-cd20 monoclonal antibodies. Eur J Immunol. (2022) 52:1648–61. doi: 10.1002/eji.202250037
20. Barreyro L, Sampson AM, Ishikawa C, Hueneman KM, Choi K, Pujato MA, et al. Blocking ube2n abrogates oncogenic immune signaling in acute myeloid leukemia. Sci Transl Med. (2022) 14:eabb7695. doi: 10.1126/scitranslmed.abb7695
21. Kinker GS, Vitiello GAF, Ferreira WAS, Chaves AS, Cordeiro de Lima VC, and Medina TDS. B cell orchestration of anti-tumor immune responses: A matter of cell localization and communication. Front Cell Dev Biol. (2021) 9:678127. doi: 10.3389/fcell.2021.678127
22. Kashfi K, Kannikal J, and Nath N. Macrophage reprogramming and cancer therapeutics: role of inos-derived no. Cells. (2021) 10(11):3194. doi: 10.3390/cells10113194
23. de Wit J, Jorritsma T, Makuch M, Remmerswaal EBM, Klaasse Bos H, Souwer Y, et al. Human B cells promote T-cell plasticity to optimize antibody response by inducing coexpression of T(H)1/T(Fh) signatures. J Allergy Clin Immunol. (2015) 135:1053–60. doi: 10.1016/j.jaci.2014.08.012
24. Tang Z, Bai Y, Fang Q, Yuan Y, Zeng Q, Chen S, et al. Spatial transcriptomics reveals tryptophan metabolism restricting maturation of intratumoral tertiary lymphoid structures. Cancer Cell. (2025) 43:1025–44 e14. doi: 10.1016/j.ccell.2025.03.011
25. Schumacher TN and Thommen DS. Tertiary lymphoid structures in cancer. Science. (2022) 375:eabf9419. doi: 10.1126/science.abf9419
26. Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, and Sautes-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. (2022) 19:441–57. doi: 10.1038/s41571-022-00619-z
27. Chandnani N, Gupta I, and Mandal A. Sarkar K. Participation of B cell in immunotherapy of cancer. Pathol Res Pract. (2024) 255:155169. doi: 10.1016/j.prp.2024.155169
28. Lin J, Jiang S, Chen B, Du Y, Qin C, Song Y, et al. Tertiary lymphoid structures are linked to enhanced antitumor immunity and better prognosis in muscle-invasive bladder cancer. Adv Sci (Weinh). (2025) 12:e2410998. doi: 10.1002/advs.202410998
29. Ding L, Sun L, Bu MT, Zhang Y, Scott LN, Prins RM, et al. Antigen presentation by clonally diverse cxcr5+ B cells to cd4 and cd8 T cells is associated with durable response to immune checkpoint inhibitors. Front Immunol. (2023) 14:1176994. doi: 10.3389/fimmu.2023.1176994
30. Hoellwerth M, Koelblinger P, Lang R, and Harrer A. Revisiting the role of the cxcl13/cxcr5-associated immune axis in melanoma: potential implications for anti-pd-1-related biomarker research. Life (Basel). (2023) 13(2):553. doi: 10.3390/life13020553
31. Kacher J, Manches O, Aspord C, Sartelet H, and Chaperot L. Impaired antitumor immune response in mycn-amplified neuroblastoma is associated with lack of ccl2 secretion and poor dendritic cell recruitment. Cancer Res Commun. (2022) 2:577–89. doi: 10.1158/2767-9764.CRC-21-0134
32. Chen Z, Cui Y, Yao Y, Liu B, Yunis J, Gao X, et al. Heparan sulfate regulates il-21 bioavailability and signal strength that control germinal center B cell selection and differentiation. Sci Immunol. (2023) 8:eadd1728. doi: 10.1126/sciimmunol.add1728
33. Calvanese AL, Cecconi V, Staheli S, Schnepf D, Nater M, Pereira P, et al. Sustained innate interferon is an essential inducer of tertiary lymphoid structures. Eur J Immunol. (2024) 54:e2451207. doi: 10.1002/eji.202451207
34. Hu C, You W, Kong D, Huang Y, Lu J, Zhao M, et al. Tertiary lymphoid structure-associated B cells enhance cxcl13(+)Cd103(+)Cd8(+) tissue-resident memory T-cell response to programmed cell death protein 1 blockade in cancer immunotherapy. Gastroenterology. (2024) 166:1069–84. doi: 10.1053/j.gastro.2023.10.022
35. Teillaud JL, Houel A, Panouillot M, Riffard C, and Dieu-Nosjean MC. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer. (2024) 24:629–46. doi: 10.1038/s41568-024-00728-0
36. Gulubova MV, Valkanov SP, Ignatova MMK, and Minkov GA. Tertiary lymphoid structures in colorectal cancer - organization and immune cell interactions. Am J Clin Exp Immunol. (2024) 13:236–45. doi: 10.62347/GRYY2849
37. Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoid structures and are associated with poor clinical outcome in nsclc. Commun Biol. (2022) 5:1416. doi: 10.1038/s42003-022-04356-y
38. An D, Chen G, Cheng WY, Mohrs K, Adler C, Gupta NT, et al. Ltbetar agonism promotes antitumor immune responses via modulation of the tumor microenvironment. Cancer Res. (2024) 84:3984–4001. doi: 10.1158/0008-5472.CAN-23-2716
39. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, and van de Veen W. Regulatory B cells, a to Z. Allergy. (2021) 76:2699–715. doi: 10.1111/all.14763
40. Horii M and Matsushita T. Regulatory B cells and T cell regulation in cancer. J Mol Biol. (2021) 433:166685. doi: 10.1016/j.jmb.2020.10.019
41. Dang M, Yu J, Galant-Swafford J, and Karam SD. The dichotomy of regulatory B cells in cancer versus allergic disease. Mol Carcinog. (2024) 63:11–21. doi: 10.1002/mc.23633
42. Ben-Smith A, Dove SK, Martin A, Wakelam MJ, and Savage CO. Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: comparison with conventional fcgamma receptor ligation. Blood. (2001) 98:1448–55. doi: 10.1182/blood.v98.5.1448
43. Wang L, Niu Z, Wang X, Li Z, Liu Y, Luo F, et al. Phd2 exerts anti-cancer and anti-inflammatory effects in colon cancer xenografts mice via attenuating nf-kappab activity. Life Sci. (2020) 242:117167. doi: 10.1016/j.lfs.2019.117167
44. Blonska M, Agarwal NK, and Vega F. Shaping of the tumor microenvironment: stromal cells and vessels. Semin Cancer Biol. (2015) 34:3–13. doi: 10.1016/j.semcancer.2015.03.002
45. Pegahi R, Poyer F, Legrand E, Cazin L, Vannier JP, and Lamacz M. Spontaneous and cytokine-evoked production of matrix metalloproteinases by bone marrow and peripheral blood pre-B cells in childhood acute lymphoblastic leukaemia. Eur Cytokine Netw. (2005) 16:223–32.
46. Ng WL, Ansell SM, and Mondello P. Insights into the tumor microenvironment of B cell lymphoma. J Exp Clin Cancer Res. (2022) 41:362. doi: 10.1186/s13046-022-02579-9
47. Sati S, Huang J, Kersh AE, Jones P, Ahart O, Murphy C, et al. Recruitment of cxcr4+ Type 1 innate lymphoid cells distinguishes sarcoidosis from other skin granulomatous diseases. J Clin Invest. (2024) 134(17):e178711. doi: 10.1172/JCI178711
48. Huang C and Melnick A. Mechanisms of action of bcl6 during germinal center B cell development. Sci China Life Sci. (2015) 58:1226–32. doi: 10.1007/s11427-015-4919-z
49. Tang TF, Chan YT, Cheong HC, Cheok YY, Anuar NA, Looi CY, et al. Regulatory network of blimp1, irf4, and xbp1 triad in plasmacytic differentiation and multiple myeloma pathogenesis. Cell Immunol. (2022) 380:104594. doi: 10.1016/j.cellimm.2022.104594
50. Catalan D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillon JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
51. Zhao KL, Yang XJ, Jin HZ, Zhao L, Hu JL, and Qin WJ. Double-edge role of B cells in tumor immunity: potential molecular mechanism. Curr Med Sci. (2019) 39:685–9. doi: 10.1007/s11596-019-2092-5
52. Liu G and Liu F. Bach2: A key regulator in th2-related immune cells and th2 immune response. J Immunol Res. (2022) 2022:2814510. doi: 10.1155/2022/2814510
53. Mittelstaedt NN, Becker AL, de Freitas DN, Zanin RF, Stein RT, and Duarte de Souza AP. DNA methylation and immune memory response. Cells. (2021) 10(11):2943. doi: 10.3390/cells10112943
54. Naderi W, Schreiner D, and King CG. T-cell-B-cell collaboration in the lung. Curr Opin Immunol. (2023) 81:102284. doi: 10.1016/j.coi.2023.102284
55. Rodriguez-Zhurbenko N and Hernandez AM. The role of B-1 cells in cancer progression and anti-tumor immunity. Front Immunol. (2024) 15:1363176. doi: 10.3389/fimmu.2024.1363176
56. Jo A, Jeong D, Eum HH, Kim N, Na M, Kang H, et al. Ctla-4 inhibition facilitates follicular T and B cell interaction and the production of tumor-specific antibodies. Int J Cancer. (2023) 152:1964–76. doi: 10.1002/ijc.34438
57. Van Meerhaeghe T, Neel A, Brouard S, and Degauque N. Regulation of cd8 T cell by B-cells: A narrative review. Front Immunol. (2023) 14:1125605. doi: 10.3389/fimmu.2023.1125605
58. Xu Y, Huang X, Li F, Liu T, Yang T, Chen F, et al. Il-21 enhances stat3/blimp-1 signaling pathway in B cells and contributes to plasma cell differentiation in newly diagnosed patients with myasthenia gravis. Immunol Res. (2021) 69:59–70. doi: 10.1007/s12026-020-09164-2
59. Kim Y, Manara F, Grassmann S, Belcheva KT, Reyes K, Kim H, et al. Il-21 shapes the B cell response in a context-dependent manner. Cell Rep. (2025) 44:115190. doi: 10.1016/j.celrep.2024.115190
60. Faliti CE, Mesina M, Choi J, Belanger S, Marshall MA, Tipton CM, et al. Interleukin-2-secreting T helper cells promote extra-follicular B cell maturation via intrinsic regulation of a B cell mtor-akt-blimp-1 axis. Immunity. (2024) 57:2772–89 e8. doi: 10.1016/j.immuni.2024.11.006
61. Sainz TP, Sahu V, Gomez JA, Dcunha NJ, Basi AV, Kettlun C, et al. Role of the crosstalk B:Neoplastic T follicular helper cells in the pathobiology of nodal T follicular helper cell lymphomas. Lab Invest. (2024) 104:102147. doi: 10.1016/j.labinv.2024.102147
62. Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T follicular helper cells and germinal center B cells are required for cgvhd and bronchiolitis obliterans. Blood. (2014) 123:3988–98. doi: 10.1182/blood-2014-03-562231
63. Wu L, Chai Y, Gao A, Lin Y, Han J, Li L, et al. Il-21 signaling promotes igm(+) B cell proliferation and antibody production via jak/stat3 and akt pathways in early vertebrates. Dev Comp Immunol. (2025) 164:105325. doi: 10.1016/j.dci.2025.105325
64. Li H, Zhang MJ, Zhang B, Lin WP, Li SJ, Xiong D, et al. Mature tertiary lymphoid structures evoke intra-tumoral T and B cell responses via progenitor exhausted cd4(+) T cells in head and neck cancer. Nat Commun. (2025) 16:4228. doi: 10.1038/s41467-025-59341-w
65. Stephenson S and Doody GM. Metabolic reprogramming during B-cell differentiation. Methods Mol Biol. (2023) 2675:271–83. doi: 10.1007/978-1-0716-3247-5_20
66. Chakraborty S, Khamaru P, and Bhattacharyya A. Regulation of immune cell metabolism in health and disease: special focus on T and B cell subsets. Cell Biol Int. (2022) 46:1729–46. doi: 10.1002/cbin.11867
67. Bod L, Kye YC, Shi J, Torlai Triglia E, Schnell A, Fessler J, et al. B-cell-specific checkpoint molecules that regulate anti-tumour immunity. Nature. (2023) 619:348–56. doi: 10.1038/s41586-023-06231-0
68. Kayaoglu B, Kasap N, Yilmaz NS, Charbonnier LM, Geckin B, Akcay A, et al. Stepwise reversal of immune dysregulation due to stat1 gain-of-function mutation following ruxolitinib bridge therapy and transplantation. J Clin Immunol. (2021) 41:769–79. doi: 10.1007/s10875-020-00943-y
69. Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, et al. Bacillus calmette guerin triggers the il-12/ifn-gamma axis by an irak-4- and nemo-dependent, non-cognate interaction between monocytes, nk, and T lymphocytes. Eur J Immunol. (2004) 34:3276–84. doi: 10.1002/eji.200425221
70. Chen Z, Zhang G, Ren X, Yao Z, Zhou Q, Ren X, et al. Cross-talk between myeloid and B cells shapes the distinct microenvironments of primary and secondary liver cancer. Cancer Res. (2023) 83:3544–61. doi: 10.1158/0008-5472.CAN-23-0193
71. Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. Scs macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. (2016) 352:242–6. doi: 10.1126/science.aaf1328
72. Tacconi C, Commerford CD, Dieterich LC, Schwager S, He Y, Ikenberg K, et al. Cd169(+) lymph node macrophages have protective functions in mouse breast cancer metastasis. Cell Rep. (2021) 35:108993. doi: 10.1016/j.celrep.2021.108993
73. Louie DAP, Oo D, Leung G, Lin Y, Stephens M, Alrashed O, et al. Tumor-draining lymph node reconstruction promotes B cell activation during E0771 mouse breast cancer growth. Front Pharmacol. (2022) 13:825287. doi: 10.3389/fphar.2022.825287
74. Pellin D, Claudio N, Guo Z, Ziglari T, and Pucci F. Gene expression profiling of lymph node sub-capsular sinus macrophages in cancer. Front Immunol. (2021) 12:672123. doi: 10.3389/fimmu.2021.672123
75. Gunnarsdottir FB, Briem O, Lindgren AY, Kallberg E, Andersen C, Grenthe R, et al. Breast cancer associated cd169(+) macrophages possess broad immunosuppressive functions but enhance antibody secretion by activated B cells. Front Immunol. (2023) 14:1180209. doi: 10.3389/fimmu.2023.1180209
76. Craxton A, Magaletti D, Ryan EJ, and Clark EA. Macrophage- and dendritic cell–dependent regulation of human B-cell proliferation requires the tnf family ligand baff. Blood. (2003) 101:4464–71. doi: 10.1182/blood-2002-10-3123
77. Li Q, Tan S, Xu K, Fu X, Yu J, Yang H, et al. Curcumin attenuates lupus nephritis in mrl/lpr mice by suppressing macrophage-secreted B cell activating factor (Baff). Int J Clin Exp Pathol. (2019) 12:2075–83.
78. Burbano C, Villar-Vesga J, Vasquez G, Munoz-Vahos C, Rojas M, and Castano D. Proinflammatory differentiation of macrophages through microparticles that form immune complexes leads to T- and B-cell activation in systemic autoimmune diseases. Front Immunol. (2019) 10:2058. doi: 10.3389/fimmu.2019.02058
79. Hunegnaw R, Helmold Hait S, Enyindah-Asonye G, Rahman MA, Ko EJ, Hogge CJ, et al. A mucosal adenovirus prime/systemic envelope boost vaccine regimen elicits responses in cervicovaginal and alveolar macrophages of rhesus macaques associated with delayed siv acquisition and B cell help. Front Immunol. (2020) 11:571804. doi: 10.3389/fimmu.2020.571804
80. Szymula A, Samayoa-Reyes G, Ogolla S, Liu B, Li S, George A, et al. Macrophages drive kshv B cell latency. Cell Rep. (2023) 42:112767. doi: 10.1016/j.celrep.2023.112767
81. Chiu A, Xu W, He B, Dillon SR, Gross JA, Sievers E, et al. Hodgkin lymphoma cells express taci and bcma receptors and generate survival and proliferation signals in response to baff and april. Blood. (2007) 109:729–39. doi: 10.1182/blood-2006-04-015958
82. Lou X, Zhao K, Xu J, Shuai L, Niu H, Cao Z, et al. Ccl8 as a promising prognostic factor in diffuse large B-cell lymphoma via M2 macrophage interactions: A bioinformatic analysis of the tumor microenvironment. Front Immunol. (2022) 13:950213. doi: 10.3389/fimmu.2022.950213
83. Le K, Sun J, Ghaemmaghami J, Smith MR, Ip WKE, Phillips T, et al. Blockade of ccr1 induces a phenotypic shift in macrophages and triggers a favorable antilymphoma activity. Blood Adv. (2023) 7:3952–67. doi: 10.1182/bloodadvances.2022008722
84. Narla RK, Modi H, Bauer D, Abbasian M, Leisten J, Piccotti JR, et al. Modulation of cd47-sirpalpha innate immune checkpoint axis with fc-function detuned anti-cd47 therapeutic antibody. Cancer Immunol Immunother. (2022) 71:473–89. doi: 10.1007/s00262-021-03010-6
85. Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S, et al. Immune evasion via pd-1/pd-L1 on nk cells and monocyte/macrophages is more prominent in hodgkin lymphoma than dlbcl. Blood. (2018) 131:1809–19. doi: 10.1182/blood-2017-07-796342
86. Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, et al. B cell-derived gaba elicits il-10(+) macrophages to limit anti-tumour immunity. Nature. (2021) 599:471–6. doi: 10.1038/s41586-021-04082-1
87. Bingaman AW, Pearson TC, and Larsen CP. The role of cd40l in T cell-dependent nitric oxide production by murine macrophages. Transpl Immunol. (2000) 8:195–202. doi: 10.1016/s0966-3274(00)00026-5
88. Downs-Canner SM, Meier J, Vincent BG, and Serody JS. B cell function in the tumor microenvironment. Annu Rev Immunol. (2022) 40:169–93. doi: 10.1146/annurev-immunol-101220-015603
89. Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, et al. Antitumor effector B cells directly kill tumor cells via the fas/fasl pathway and are regulated by il-10. Eur J Immunol. (2015) 45:999–1009. doi: 10.1002/eji.201444625
90. Xia Y, Tao H, Hu Y, Chen Q, Chen X, Xia L, et al. Il-2 augments the therapeutic efficacy of adoptively transferred B cells which directly kill tumor cells via the cxcr4/cxcl12 and perforin pathways. Oncotarget. (2016) 7:60461–74. doi: 10.18632/oncotarget.11124
91. Ammirante M, Luo JL, Grivennikov S, Nedospasov S, and Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. (2010) 464:302–5. doi: 10.1038/nature08782
92. Pereira JL, Ferreira F, and Dos Santos NR. Antibody targeting of surface P-selectin glycoprotein ligand 1 leads to lymphoma apoptosis and tumorigenesis inhibition. Hematol Oncol. (2024) 42:e3257. doi: 10.1002/hon.3257
93. Zhou T, Karrs J, Ho T, Doverte A, Kochenderfer JN, Shah NN, et al. Circulating cd22+/cd19-/cd24- progenitors and cd22+/cd19+/cd24- mature B cells: diagnostic pitfalls for minimal residual disease detection in B-lymphoblastic leukemia. Cytometry B Clin Cytom. (2023) 104:294–303. doi: 10.1002/cyto.b.22104
94. Chen X, Wang C, Sun N, Pan S, Li R, Li X, et al. Aurka loss in cd19(+) B cells promotes megakaryocytopoiesis via il-6/stat3 signaling-mediated thrombopoietin production. Theranostics. (2021) 11:4655–71. doi: 10.7150/thno.49007
95. Avivi I, Stroopinsky D, and Katz T. Anti-cd20 monoclonal antibodies: beyond B-cells. Blood Rev. (2013) 27:217–23. doi: 10.1016/j.blre.2013.07.002
96. Qin C, Dong MH, Zhou LQ, Wang W, Cai SB, You YF, et al. Single-cell analysis of refractory anti-srp necrotizing myopathy treated with anti-bcma car-T cell therapy. Proc Natl Acad Sci U.S.A. (2024) 121:e2315990121. doi: 10.1073/pnas.2315990121
97. Rodgers CB, Mustard CJ, McLean RT, Hutchison S, and Pritchard AL. A B-cell or a key player? The different roles of B-cells and antibodies in melanoma. Pigment Cell Melanoma Res. (2022) 35:303–19. doi: 10.1111/pcmr.13031
98. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
99. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
100. Therien AD, Beasley GM, Rhodin KE, Farrow NE, Tyler DS, Boczkowski D, et al. Spatial biology analysis reveals B cell follicles in secondary lymphoid structures may regulate anti-tumor responses at initial melanoma diagnosis. Front Immunol. (2022) 13:952220. doi: 10.3389/fimmu.2022.952220
101. Zhong Q, Hao H, Li S, Ning Y, Li H, Hu X, et al. B cell C-maf signaling promotes tumor progression in animal models of pancreatic cancer and melanoma. J Immunother Cancer. (2024) 12(11):e009861. doi: 10.1136/jitc-2024-009861
102. Wu H, Xia L, Jia D, Zou H, Jin G, Qian W, et al. Pd-L1(+) regulatory B cells act as a T cell suppressor in a pd-L1-dependent manner in melanoma patients with bone metastasis. Mol Immunol. (2020) 119:83–91. doi: 10.1016/j.molimm.2020.01.008
103. Liu X, Liu S, Jiang Z, Yang C, Yang X, Li J, et al. Peripheral B-cell levels predict efficacy and overall survival in advanced melanoma patients under pd-1 immunotherapy. Immunotherapy. (2024) 16:223–34. doi: 10.2217/imt-2023-0105
104. Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to pd-L1 blockade in non-small cell lung cancer. Cancer Cell. (2022) 40:289–300 e4. doi: 10.1016/j.ccell.2022.02.002
105. Patel AJ, Khan N, Richter A, Naidu B, Drayson MT, and Middleton GW. Deep immune B and plasma cell repertoire in non-small cell lung cancer. Front Immunol. (2023) 14:1198665. doi: 10.3389/fimmu.2023.1198665
106. Ng KW, Boumelha J, Enfield KSS, Almagro J, Cha H, Pich O, et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature. (2023) 616:563–73. doi: 10.1038/s41586-023-05771-9
107. Jiang S, Zhu D, and Wang Y. Tumor-infiltrating B cells in non-small cell lung cancer: current insights and future directions. Cancer Cell Int. (2025) 25:68. doi: 10.1186/s12935-025-03668-3
108. Sammut SJ, Galson JD, Minter R, Sun B, Chin SF, De Mattos-Arruda L, et al. Predictability of B cell clonal persistence and immunosurveillance in breast cancer. Nat Immunol. (2024) 25:916–24. doi: 10.1038/s41590-024-01821-0
109. Lu Y, Zhao Q, Liao JY, Song E, Xia Q, Pan J, et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell. (2020) 180:1081–97 e24. doi: 10.1016/j.cell.2020.02.015
110. Xu W, Lu J, Liu WR, Anwaier A, Wu Y, Tian X, et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J Immunother Cancer. (2023) 11(12):e006667. doi: 10.1136/jitc-2023-006667
111. Meylan M, Petitprez F, Becht E, Bougouin A, Pupier G, Calvez A, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. (2022) 55:527–41 e5. doi: 10.1016/j.immuni.2022.02.001
112. Uehara K, Tanoue K, Yamaguchi K, Ohmura H, Ito M, Matsushita Y, et al. Preferential B cell differentiation by combined immune checkpoint blockade for renal cell carcinoma is associated with clinical response and autoimmune reactions. Cancer Immunol Immunother. (2023) 72:3543–58. doi: 10.1007/s00262-023-03505-4
113. Lin Z, Xiao S, Qi Y, Guo J, and Lu L. Tumor infiltrating B lymphocytes (Tibs) associate with poor clinical outcomes, unfavorable therapeutic benefit and immunosuppressive context in metastatic clear cell renal cell carcinoma (Mccrcc) patients treated with anti-pd-1 antibody plus axitinib. J Cancer Res Clin Oncol. (2024) 150:262. doi: 10.1007/s00432-024-05803-5
114. Xu KQ, Gong Z, Yang JL, Xia CQ, Zhao JY, and Chen X. B-cell-specific signatures reveal novel immunophenotyping and therapeutic targets for hepatocellular carcinoma. World J Gastroenterol. (2024) 30:3894–925. doi: 10.3748/wjg.v30.i34.3894
115. He Y, Luo J, Zhang G, Jin Y, Wang N, Lu J, et al. Single-cell profiling of human cd127(+) innate lymphoid cells reveals diverse immune phenotypes in hepatocellular carcinoma. Hepatology. (2022) 76:1013–29. doi: 10.1002/hep.32444
116. Xie S, Yan R, Zheng A, Shi M, Tang L, Li X, et al. T cell receptor and B cell receptor exhibit unique signatures in tumor and adjacent non-tumor tissues of hepatocellular carcinoma. Front Immunol. (2023) 14:1161417. doi: 10.3389/fimmu.2023.1161417
117. Shu DH, Ho WJ, Kagohara LT, Girgis A, Shin SM, Danilova L, et al. Immunotherapy response induces divergent tertiary lymphoid structure morphologies in hepatocellular carcinoma. Nat Immunol. (2024) 25:2110–23. doi: 10.1038/s41590-024-01992-w
118. Xie Y, Huang Y, Li ZY, Jiang W, Shi NX, Lu Y, et al. Interleukin-21 receptor signaling promotes metabolic dysfunction-associated steatohepatitis-driven hepatocellular carcinoma by inducing immunosuppressive iga(+) B cells. Mol Cancer. (2024) 23:95. doi: 10.1186/s12943-024-02001-2
119. Kurebayashi Y, Sugimoto K, Tsujikawa H, Matsuda K, Nomura R, Ueno A, et al. Spatial dynamics of T- and B-cell responses predicts clinical outcome of resectable and unresectable hepatocellular carcinoma. Clin Cancer Res. (2024) 30:5666–80. doi: 10.1158/1078-0432.CCR-24-0479
120. Lu Z, Liu R, Wang Y, Jiao M, Li Z, Wang Z, et al. Ten-eleven translocation-2 inactivation restrains il-10-producing regulatory B cells to enable antitumor immunity in hepatocellular carcinoma. Hepatology. (2023) 77:745–59. doi: 10.1002/hep.32442
121. Liu Z, Lin X, Zhang D, Guo D, Tang W, Yu X, et al. Increased prp19 in hepatocyte impedes B cell function to promote hepatocarcinogenesis. Adv Sci (Weinh). (2024) 11:e2407517. doi: 10.1002/advs.202407517
122. Xia J, Xie Z, Niu G, Lu Z, Wang Z, Xing Y, et al. Single-cell landscape and clinical outcomes of infiltrating B cells in colorectal cancer. Immunology. (2023) 168:135–51. doi: 10.1111/imm.13568
123. Wang Z, Lu Z, Lin S, Xia J, Zhong Z, Xie Z, et al. Leucine-trna-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity. (2022) 55:1748. doi: 10.1016/j.immuni.2022.07.017
124. Ji L, Fu G, Huang M, Kao X, Zhu J, Dai Z, et al. Scrna-seq of colorectal cancer shows regional immune atlas with the function of cd20(+) B cells. Cancer Lett. (2024) 584:216664. doi: 10.1016/j.canlet.2024.216664
125. Zheng J, Zhou C, Li Z, Jin X, Zou Y, Bai S, et al. Alcaligenes faecalis promotes colitis to colorectal cancer transition through iga+ B cell suppression and vinculin acetylation. Gut Microbes. (2025) 17:2473511. doi: 10.1080/19490976.2025.2473511
126. Xu Y, Wei Z, Feng M, Zhu D, Mei S, Wu Z, et al. Tumor-infiltrated activated B cells suppress liver metastasis of colorectal cancers. Cell Rep. (2022) 40:111295. doi: 10.1016/j.celrep.2022.111295
127. Fridman WH, Meylan M, Pupier G, Calvez A, Hernandez I, and Sautes-Fridman C. Tertiary lymphoid structures and B cells: an intratumoral immunity cycle. Immunity. (2023) 56:2254–69. doi: 10.1016/j.immuni.2023.08.009
128. Asashima H, Akao S, and Matsumoto I. Emerging roles of checkpoint molecules on B cells. Immunol Med. (2025) 48(3):171–82. doi: 10.1080/25785826.2025.2454045
129. Pruitt L and Abbott RK. Hypoxia-adenosinergic regulation of B cell responses. Front Immunol. (2024) 15:1478506. doi: 10.3389/fimmu.2024.1478506
130. Xue D, Hu S, Zheng R, Luo H, and Ren X. Tumor-infiltrating B cells: their dual mechanistic roles in the tumor microenvironment. BioMed Pharmacother. (2024) 179:117436. doi: 10.1016/j.biopha.2024.117436
131. Shi X, Cheng X, Jiang A, Shi W, Zhu L, Mou W, et al. Immune checkpoints in B cells: unlocking new potentials in cancer treatment. Adv Sci (Weinh). (2024) 11:e2403423. doi: 10.1002/advs.202403423
132. Boucher A, Anderson C, Hinman R, Kindschuh M, Fung J, Wang T, et al. Engineered human B cells targeting tumor-associated antigens exhibit antigen presentation and antibody-mediated functions. Front Immunol. (2025) 16:1621222. doi: 10.3389/fimmu.2025.1621222
133. Michaud D, Steward CR, Mirlekar B, and Pylayeva-Gupta Y. Regulatory B cells in cancer. Immunol Rev. (2021) 299:74–92. doi: 10.1111/imr.12939
134. Mirlekar B, Michaud D, Lee SJ, Kren NP, Harris C, Greene K, et al. B cell-derived il35 drives stat3-dependent cd8(+) T-cell exclusion in pancreatic cancer. Cancer Immunol Res. (2020) 8:292–308. doi: 10.1158/2326-6066.CIR-19-0349
135. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. Cd19(+)Cd24(Hi)Cd38(Hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. (2010) 32:129–40. doi: 10.1016/j.immuni.2009.11.009
136. Das S and Bar-Sagi D. Btk signaling drives cd1d(Hi)Cd5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene. (2019) 38:3316–24. doi: 10.1038/s41388-018-0668-3
137. Horikawa M, Minard-Colin V, Matsushita T, and Tedder TF. Regulatory B cell production of il-10 inhibits lymphoma depletion during cd20 immunotherapy in mice. J Clin Invest. (2011) 121:4268–80. doi: 10.1172/JCI59266
138. Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-cd20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of cd20 and cd137l. Cancer Res. (2013) 73:2127–38. doi: 10.1158/0008-5472.CAN-12-4184
139. Jenks SA, Cashman KS, Woodruff MC, Lee FE, and Sanz I. Extrafollicular responses in humans and sle. Immunol Rev. (2019) 288:136–48. doi: 10.1111/imr.12741
140. Aragaki AK, Jing Y, Hoffman-Censits J, Choi W, Hahn NM, Trock BJ, et al. Gender-specific stratification of survival following immune checkpoint inhibitor therapy based on intratumoral expression of a B cell gene signature. Eur Urol Oncol. (2022) 5:338–46. doi: 10.1016/j.euo.2021.07.003
141. Nijland M, Veenstra RN, Visser L, Xu C, Kushekhar K, van Imhoff GW, et al. Hla dependent immune escape mechanisms in B-cell lymphomas: implications for immune checkpoint inhibitor therapy? Oncoimmunology. (2017) 6:e1295202. doi: 10.1080/2162402X.2017.1295202
142. Wu X, Sun X, Deng W, Xu R, and Zhao Q. Combination therapy of targeting cd20 antibody and immune checkpoint inhibitor may be a breakthrough in the treatment of B-cell lymphoma. Heliyon. (2024) 10:e34068. doi: 10.1016/j.heliyon.2024.e34068
143. Kaumaya PT. B-cell epitope peptide cancer vaccines: A new paradigm for combination immunotherapies with novel checkpoint peptide vaccine. Future Oncol. (2020) 16:1767–91. doi: 10.2217/fon-2020-0224
144. Ede NJ, Good AJ, Tobias J, Garner-Spitzer E, Zielinski CC, and Wiedermann U. Development of the B cell cancer vaccine her-vaxx for the treatment of her-2 expressing cancers. Front Oncol. (2022) 12:939356. doi: 10.3389/fonc.2022.939356
145. Tobias J, Battin C, De Sousa Linhares A, Lebens M, Baier K, Ambroz K, et al. A new strategy toward B cell-based cancer vaccines by active immunization with mimotopes of immune checkpoint inhibitors. Front Immunol. (2020) 11:895. doi: 10.3389/fimmu.2020.00895
146. Lv Z, Zhang P, Li D, Qin M, Nie L, Wang X, et al. Cd19-targeting fusion protein combined with pd1 antibody enhances anti-tumor immunity in mouse models. Oncoimmunology. (2020) 9:1747688. doi: 10.1080/2162402X.2020.1747688
147. Hinke DM, Andersen TK, Gopalakrishnan RP, Skullerud LM, Werninghaus IC, Grodeland G, et al. Antigen bivalency of antigen-presenting cell-targeted vaccines increases B cell responses. Cell Rep. (2022) 39:110901. doi: 10.1016/j.celrep.2022.110901
148. Li WH, Su JY, and Li YM. Rational design of T-cell- and B-cell-based therapeutic cancer vaccines. Acc Chem Res. (2022) 55:2660–71. doi: 10.1021/acs.accounts.2c00360
149. Verma R, Foster RE, Horgan K, Mounsey K, Nixon H, Smalle N, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. (2016) 18:10. doi: 10.1186/s13058-015-0669-x
150. Irie E, Shirota Y, Suzuki C, Tajima Y, Ishizawa K, Kameoka J, et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol. (2010) 91:501–8. doi: 10.1007/s12185-010-0528-6
151. Goswami M, Prince G, Biancotto A, Moir S, Kardava L, Santich BH, et al. Impaired B cell immunity in acute myeloid leukemia patients after chemotherapy. J Transl Med. (2017) 15:155. doi: 10.1186/s12967-017-1252-2
152. van Tilburg CM, van der Velden VH, Sanders EA, Wolfs TF, Gaiser JF, de Haas V, et al. Reduced versus intensive chemotherapy for childhood acute lymphoblastic leukemia: impact on lymphocyte compartment composition. Leuk Res. (2011) 35:484–91. doi: 10.1016/j.leukres.2010.10.005
153. Henriksen JR, Nederby L, Donskov F, Waldstrom M, Adimi P, Jakobsen A, et al. Prognostic significance of baseline T cells, B cells and neutrophil-lymphocyte ratio (Nlr) in recurrent ovarian cancer treated with chemotherapy. J Ovarian Res. (2020) 13:59. doi: 10.1186/s13048-020-00661-4
154. Yang J, Li W, Luo F, Zhao N, Zhang W, Zhang D, et al. Low percentage of cd24hicd27(+)Cd19(+) B cells decelerates gastric cancer progression in xelox-treated patients. Int Immunopharmacol. (2015) 26:322–7. doi: 10.1016/j.intimp.2015.04.011
155. Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Bohm S, et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin Cancer Res. (2017) 23:250–62. doi: 10.1158/1078-0432.CCR-16-0081
156. Lv J, Wei Y, Yin JH, Chen YP, Zhou GQ, Wei C, et al. The tumor immune microenvironment of nasopharyngeal carcinoma after gemcitabine plus cisplatin treatment. Nat Med. (2023) 29:1424–36. doi: 10.1038/s41591-023-02369-6
157. Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. (2016) 6:270–85. doi: 10.1158/2159-8290.CD-15-0827
158. Winkler JK, Schiller M, Bender C, Enk AH, and Hassel JC. Rituximab as a therapeutic option for patients with advanced melanoma. Cancer Immunol Immunother. (2018) 67:917–24. doi: 10.1007/s00262-018-2145-9
159. Pinc A, Somasundaram R, Wagner C, Hormann M, Karanikas G, Jalili A, et al. Targeting cd20 in melanoma patients at high risk of disease recurrence. Mol Ther. (2012) 20:1056–62. doi: 10.1038/mt.2012.27
160. Zhou Y. Her2/neu-based vaccination with li-key hybrid, gm-csf immunoadjuvant and trastuzumab as a potent triple-negative breast cancer treatment. J Cancer Res Clin Oncol. (2023) 149:6711–8. doi: 10.1007/s00432-023-04574-9
161. Wiedermann U, Garner-Spitzer E, Chao Y, Maglakelidze M, Bulat I, Dechaphunkul A, et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with her2/neu-overexpressing advanced gastric cancer-results from phase ib trial imu.Acs.001. Clin Cancer Res. (2021) 27:3649–60. doi: 10.1158/1078-0432.CCR-20-3742
162. Kanzler H, Barrat FJ, Hessel EM, and Coffman RL. Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat Med. (2007) 13:552–9. doi: 10.1038/nm1589
Keywords: B cells, tumor microenvironment, immunotherapy, tumor immunity, tertiary lymphoid structure
Citation: Lv Z, Yang R, Zhang K, Wang R, Shi X, Wu J, Liu L and Jiao J (2025) The dual immunomodulatory role of B cells in tumorigenesis: mechanisms, microenvironment crosstalk, and therapeutic implications. Front. Immunol. 16:1649812. doi: 10.3389/fimmu.2025.1649812
Received: 19 June 2025; Accepted: 15 October 2025;
Published: 30 October 2025.
Edited by:
Sudhir Paul, University of Texas Health Science Center at Houston, United StatesReviewed by:
Ma Jia Qiang, Chinese Academy of Sciences (CAS), ChinaCopyright © 2025 Lv, Yang, Zhang, Wang, Shi, Wu, Liu and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junna Jiao, amlhb25hamRAMTYzLmNvbQ==
 Zhuangwei Lv
Zhuangwei Lv Ruohao Yang2
Ruohao Yang2 Kai Zhang
Kai Zhang Junna Jiao
Junna Jiao