- 1Department of Microbiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Virology, University Hospital of Essen, Essen, Germany
- 3Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 5Mucosal Immunity Research Group, State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, China
A Correction on
Pre-activation of toll-like receptor 2 enhances CD8+ T-cell responses and accelerates hepatitis B virus clearance in the mouse models
By Lin Y, Huang X, Wu J, Liu J, Chen M, Ma Z, Zhang E, Liu Y, Huang S, Li Q, Zhang X, Hou J, Yang D, Lu M and Xu Y (2018) Front. Immunol. 9:1495. doi: 10.3389/fimmu.2018.01495
In the published article, there was an error in Figure 2 as published. Panel D of Figure 2 appears in the published article by mistake. These errors have been corrected, and the updated panel D has been incorporated into the figure. The corrected Figure 2 and its caption, appear below.
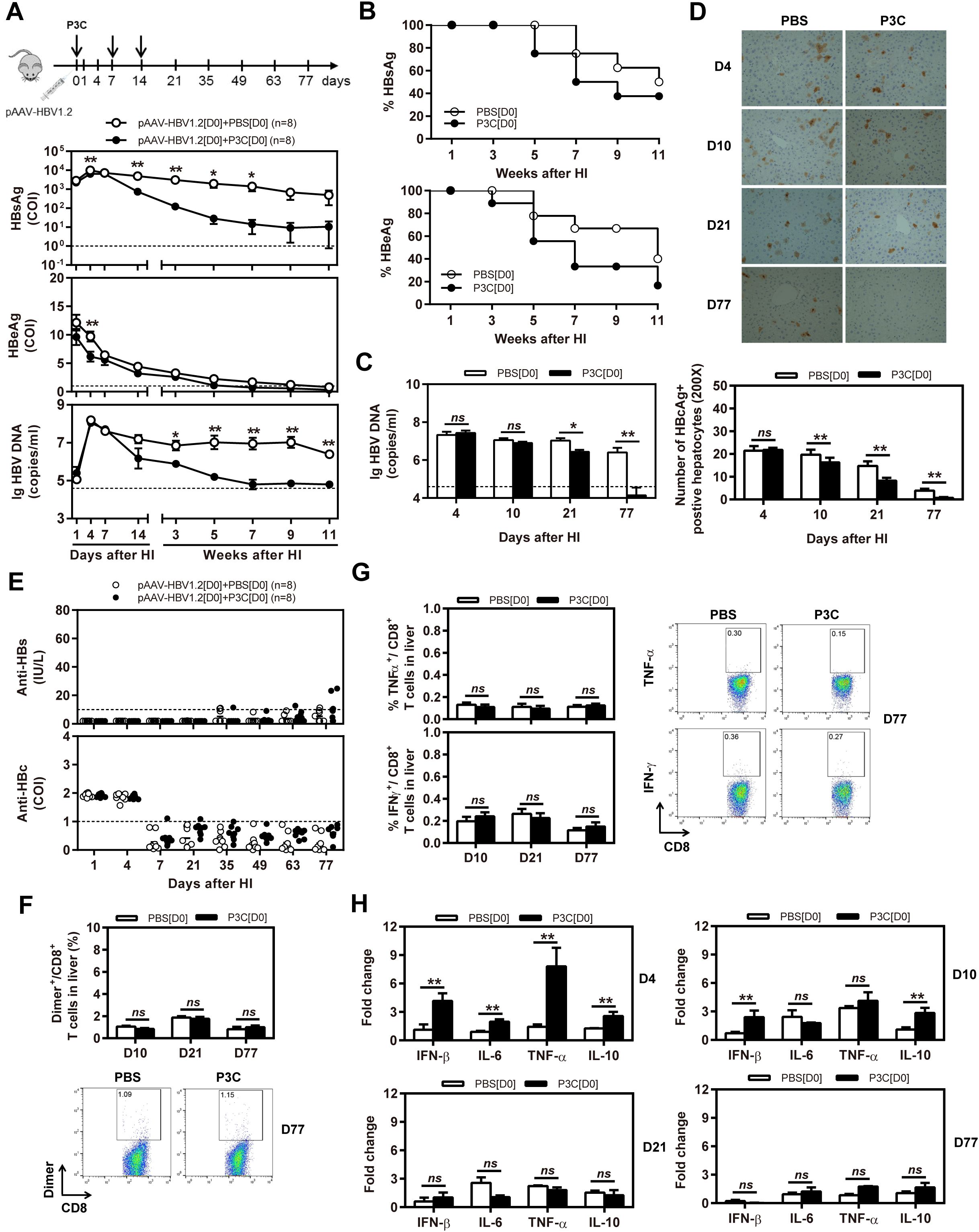
Figure 2. Early application of TLR2 ligand P3C with pAAV-HBV1.2 by HI inhibits HBV replication without promoting HBV-specific immune response in the mouse model for persistent HBV replication. C57BL/6 mice received hydrodynamic injection (HI) with plasmid pAAV-HBV1.2. The mice were treated three times with 50 μg of P3C or PBS administered by subcutaneous (SC) injection at day 0, 7, and 14 (therefore designated as group D0). (A) Serological markers of HBV infection HBsAg, HBeAg, and HBV DNA were assayed at the indicated time points by ECLIA (Roche). The cut-off value of the HBsAg and HBeAg assays was set at cut-off index (COI) of 1.0. The cut-off value of the HBV DNA real-time PCR was 4.0 × 104 copies/ml. (B) Positivity for HBsAg or HBeAg was defined as ≥1. (C) HBV DNA levels in the liver were measured by quantitative real-time PCR. (D) Liver tissue sections were stained with anti-HBc antibodies (magnification, ×200). The number of HBcAg positive hepatocytes was counted. (E) The serum levels of anti-HBs and anti-HBc antibodies were detected at the indicated time points by ECLIA. The cut-off value of anti-HBs antibody assay was 10 IU/L. The cut-off value of anti-HBc antibody assay was 1.0 COI (<1.0 COI indicates a positive reaction). (F, G) Lymphocytes were isolated from the mouse liver at day 10, 21, and 77 after HI. (F) The specific CD8+ T cells against HBcAg Cor93–100 epitope were detected by Cor93–100 peptide-loaded dimer staining. (G) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining after ex vivo stimulation with peptide Cor93–100 for 5 h. (H) Liver tissues were collected from the mouse liver at day 4, 10, 21, and 77 after HI. The mRNA expression levels of cytokines in the liver were determined by real-time RT-PCR. Beta-actin was used as an internal reference. Eight mice were analysed per group, and the experiments were repeated at least once. Data were analysed using an unpaired Student’s t test. Statistically significant differences between the groups are indicated as *P < 0.05 and **P < 0.01.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: toll-like receptor 2, hepatitis B virus, mouse model, proinflammatory cytokines, T-cell immunity
Citation: Lin Y, Huang X, Wu J, Liu J, Chen M, Ma Z, Zhang E, Liu Y, Huang S, Li Q, Zhang X, Hou J, Yang D, Lu M and Xu Y (2025) Correction: Pre-activation of toll-like receptor 2 enhances CD8+ T-cell responses and accelerates hepatitis B virus clearance in the mouse models. Front. Immunol. 16:1650574. doi: 10.3389/fimmu.2025.1650574
Received: 20 June 2025; Accepted: 14 July 2025;
Published: 06 August 2025.
Edited and Reviewed by:
Luwen Zhang, University of Nebraska-Lincoln, United StatesCopyright © 2025 Lin, Huang, Wu, Liu, Chen, Ma, Zhang, Liu, Huang, Li, Zhang, Hou, Yang, Lu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengji Lu, bWVuZ2ppLmx1QHVuaS1kdWUuZGU=; Yang Xu, b3JhbmdleHV5YW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Yong Lin
Yong Lin Xuan Huang
Xuan Huang Jun Wu
Jun Wu Jia Liu
Jia Liu Mingfa Chen3
Mingfa Chen3 Zhiyong Ma
Zhiyong Ma Ejuan Zhang
Ejuan Zhang Qian Li
Qian Li Xiaoyong Zhang
Xiaoyong Zhang Jinlin Hou
Jinlin Hou Dongliang Yang
Dongliang Yang Mengji Lu
Mengji Lu Yang Xu
Yang Xu