- 1Department of Immunology, Zunyi Medical University, Zhuhai, China
- 2Department of Pharmaceutics, Zunyi Medical University, Zhuhai, China
Introduction
In the immune system’s toolkit for identifying foreign antigens, antibodies serve as pivotal molecular agents (1). An antibody’s function in immune recognition depends on its variable regions. These regions contain the key complementarity-determining regions (CDRs), which together create the antigen-binding site (the paratope) (2). The paratope, in turn, is designed to bind a specific 3D structure on an antigen, known as the epitope (3, 4). This interaction is complex, which is why immunology teaching has often relied on simplifying analogies. For a long time, the “lock-and-key” model was the standard, portraying specificity as a matter of a rigid, perfect structural fit (5, 6). But that view is too simplistic. The model was necessarily updated to the “induced-fit” concept, which accounts for the conformational flexibility of both molecules—they actively adjust to each other upon binding (7, 8). This evolution in thought makes it clear that molecular recognition is not a static event but a dynamic process, one that hinges on the structural adaptability of both partners.
The “lock and key” model and the “induced-fit” model have undeniably formed valuable ground on which to introduce molecular complementarity. This logical evolution is, indeed, quite extraordinary. However, when used to explain the vast complexity of immune recognition, these two models reveal the limits of that binary logic on which they are based: structural complementarity. Whether the interaction is static or dynamic, the core evaluative question remains binary: do the molecules “fit” or not? This same assumption creates an ongoing pedagogical conundrum, one which cannot be resolved through mere refinements of the existing models. On the one side, students are taught that antibodies have “absolute specificity”, strongly binding only to that single, well-defined target molecule. On the other side, this scenario stands in stark contrast to established immunological phenomena such as cross-reactivity-given where a single antibody binds to structurally distinct antigens or where broad polyreactivity is observed in natural antibodies (9–12), for example, natural IgM (13–15). Within a framework governed by an all-or-nothing notion of molecular matching, these realities appear contradictory and difficult to reconcile.
This fundamental paradox gives rise to two immediate and significant consequences. First, at the pedagogical level, it imposes a considerable cognitive burden on students, who are expected to reconcile two seemingly contradictory concepts: “specificity” and “non-specificity” (polyreactivity). This often leads to confusion and fragmented understanding. Second, at the conceptual level, the binary framework promotes a tendency to dismiss nonspecific interactions as “meaningless”, “erroneous”, or merely “background noise”. This viewpoint neglects the fundamental physiology in which weak interactions participate in critical biological processes of immune surveillance, alteration of T-cell activation thresholds, and fine-tuning of signaling pathways (13–16). Hence, there is a compelling need for more integrative theoretical models that can reconcile the apparent contradictions between specificity and non-specificity as well as between high- and low-affinity interactions when teaching antigen-antibody binding.
Energy landscape theory: a unified physical framework of “specific” and “nonspecific” binding
A few refinements on old concepts alone will not help us to transcend the limits of classical structural models. What is called for is an entirely new conceptualization that draws upon existing insights but provides a greater explanatory foundation. At the microscopic scale of antigens and antibodies, these entities resemble dynamic particles whose movements and interactions can be understood in accordance with the physical science laws of nature, including thermodynamics and statistical mechanics. Thus, the energy landscape theory-imported from the field of physics-may be the grand unifying theory for molecular recognition in immunology. The binding of antibodies to antigens and unbinding are energy transitions on the energy landscape, where molecular conformations follow successive pathways toward thermodynamically favorable states. From a thermodynamic view point, Gibbs free energy change (ΔG) is the primary variable used to characterize the quantitative nature of molecular binding events (17, 18). At constant temperature and pressure, a negative ΔG indicates that the reaction is spontaneous with a tendency to occur, while a positive ΔG indicates that the reaction is nonspontaneous and may not occur (17, 18). The energy landscape itself can be thought of as a topological map quite intuitively, where “altitude” at a given point represents the free energy associated with a certain molecular conformation (Figure 1). Antigen-antibody binding under this scheme is an altogether different “fit”-it is a dynamic process in which the system is exploring the energetic terrain and stochastically settling into lower-energy regions, known as energy wells.
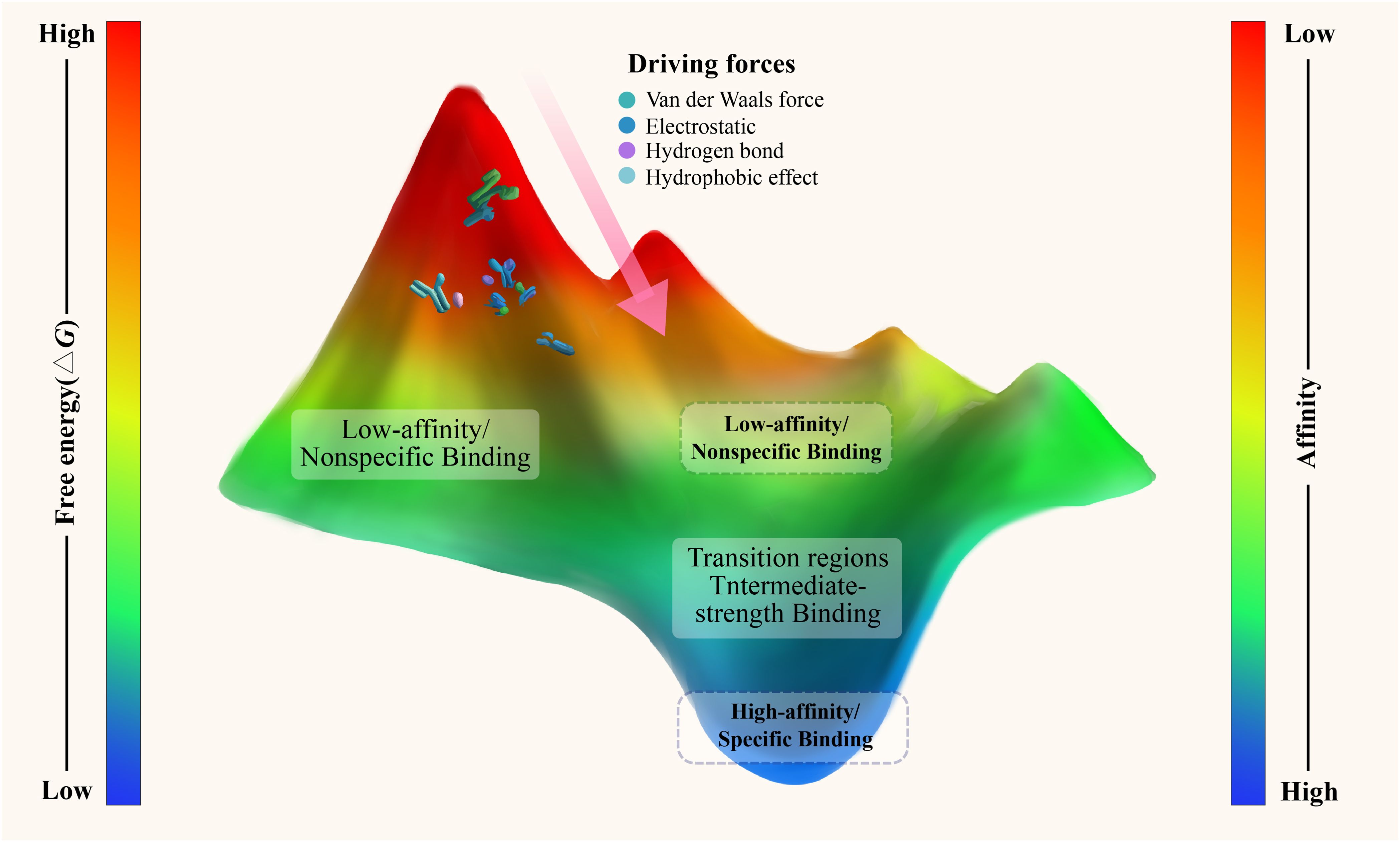
Figure 1. Energy landscape of antigen-antibody binding. This schematic represents the antigen-antibody binding process as a continuous energy landscape, where free energy (ΔG) varies from high (red) to low (blue) values, corresponding to binding affinities from weak to strong. Deep energy wells indicate high-affinity, specific interactions characterized by tight structural complementarity and strong noncovalent forces, including hydrophobic, electrostatic, hydrogen bonding, and van der Waals interactions. In contrast, shallow wells represent low-affinity, non-specific interactions formed through more generic molecular interfaces. The optimal binding process is depicted as a descent from higher to lower energy states. Affinity maturation is illustrated as the sculpting of deeper, more defined wells, enhancing specificity while preserving shallow interactions for broad-spectrum immune surveillance.
In this framework, high-affinity interactions are represented as deep and sharply defined energy wells. These binding events are typically characterized by a ΔG ranging from approximately -7 to -14 kcal/mol, which thermodynamically drives spontaneous molecular association (19, 20). This substantial free energy decrease arises from a delicate balance of enthalpic and entropic contributions, as captured in the thermodynamic equation ΔG = ΔH - TΔS (21, 22). A large negative enthalpy change (ΔH), often due to the precise geometric complementarity at the antibody-antigen interface (23), allows extensive non-covalent interactions such as hydrogen bonding, van der Waals forces, hydrophobic packing, and electrostatic interactions (24–27). At a molecular level, specific amino acid residues play key roles in this enthalpic drive. Tyrosine (Tyr) and tryptophan (Trp) contribute substantially through direct bonding and electrostatic interactions, while arginine (Arg) and aspartic acid (Asp) often form stabilizing salt bridges (28–31). A critical aspect of this process is the conformational preorganization of mature antibody binding sites (32, 33). These sites adopt a binding-competent conformation prior to antigen engagement (34), thereby minimizing the entropic penalty associated with structural ordering upon binding (i.e., reducing the unfavorable -TΔS term). In fact, affinity maturation in the immune system sculpts the energy landscape through somatic hypermutation, progressively refining the binding site to deepen and narrow the energy well (35–37). This consolidation of local minima into a single global minimum leads to significantly slower dissociation rates (k_off) and prolonged antigen-antibody residence times (38–40).
In stark contrast, lower-affinity or “non-specific” binding corresponds to the presence of broad, shallow energy basins on the molecular energy landscape. These interactions arise from more generic, less structurally refined molecular interfaces (31, 41). Importantly, such interactions are not errors or random noise; rather, they reflect a functional mode of recognition. In regions where precise geometric and chemical complementarity is lacking, fewer stabilizing interactions, such as hydrogen bonds and electrostatic contacts, are formed, leading to a less stable antigen-antibody complex (31, 41). Kinetically, this is reflected in the dynamic behavior of the antibody molecule itself. The CDR loops exhibit local conformational fluctuations on the picosecond-to-nanosecond timescale, while larger structural rearrangements occur over microseconds (42, 43). This continuous conformational sampling enables a single antibody to engage transiently with multiple, structurally diverse antigens. As a result, these interactions are characterized by rapid dissociation rates (k_off typically ranging from 10⁻¹ to 10¹ s⁻¹), yielding short residence times on the order of milliseconds to seconds (44, 45). Biologically, such transient interactions are far from inconsequential. Natural IgM exemplifies this polyspecific behavior: despite having relatively low affinity at individual binding sites, their pentameric structure provides strong overall avidity (46, 47). This architectural advantage allows the immune system to prioritize breadth over precision, facilitating rapid, high-throughput scanning of the molecular environment. In doing so, it establishes a critical first layer of immune surveillance, capable of detecting a wide array of potential threats with minimal prior information (13–15).
Therefore, the energy landscape theory redefines molecular binding as a probabilistic event, effectively bridging the perceived divide between “specific” and “non-specific” interactions by placing them along a continuous spectrum. These categories are no longer seen as fundamentally distinct, but rather as different outcomes governed by the same underlying physical principles. The distinction shifts from a binary, yes-or-no assessment to one based on probability and residence time. Specifically, the likelihood that an interaction will occur (determined by the depth of the energy well, ΔG), and the duration for which it persists (inversely related to the dissociation rate, k_off). Within this framework, interactions ranging from high-probability, long-residence “specific” bindings to low-probability, short-lived “cross-reactive” events are unified under a single conceptual model. This shift enables a more integrated understanding of antibody function, connecting molecular-scale physicochemical properties with immune system behavior at the systems level. It lays the groundwork for a comprehensive theoretical foundation that can better explain both the precision and flexibility of immune recognition.
Implications of the energy landscape theory for immunology education
Integrating energy landscape theory into immunology education is a pedagogical paradigm shift, not merely a conceptual update. It requires a transition from static or singularly dynamic structural descriptions to a broader understanding of dynamic processes, and from absolute specificity perception to relative interaction probability assessment. With this shift, students can have a more profound understanding of the essence of antibody recognition; new approaches can also be developed to resolve difficulties encountered in traditional teaching.
Energy landscape diagrams can be used to explain cross-reactivity and polyspecificity in a way that is inaccessible to both the lock-and-key and induced-fit models. They can be used to show differences in affinities when an antibody binds to different antigens by using diagrams that show deep wells for target antigens and shallower wells for low-affinity, cross-reactive antigens. They show that cross-reactive, low-affinity antigens only enter shallow wells with tertiary states only relevant to molecular structure and affinity differences. Using structural data, students can see examples of binding interfaces, where they can analyze adhesion in terms of the size of contact area, number of bonds, and hydrophobic interactions that enable affinity differences to be connected to xenobiotic molecular structures. They should also determine how cross-reactivity in immunity plays critical and essential roles, including broadly conferred antiviral immunity and maintenance of autoimmune tolerance. Polyreactive antibodies have unique characteristics that facilitate broad recognition because of the low-affinity and wide range of different binding capabilities that can be represented by shallower energy wells. Therefore, polyreactive antibodies may be produced quickly to respond to immune responses through surveillance mechanisms.
Furthermore, the energy landscape framework provides a powerful lens through which to understand the very first step of adaptive immunity: the selection of naive B cells for activation. Before affinity maturation can even begin, a naive B cell must be chosen from a vast repertoire based on its initial interaction with an antigen. This selection process can be conceptualized as a binding energy threshold. For a B cell to be activated, the binding of its B-cell receptor (BCR) to an antigen must be stable enough—that is, it must fall into an energy well of sufficient depth—to generate a sustained intracellular signal that surpasses this activation threshold. Interactions that are too transient (in very shallow energy wells) will fail to trigger a response. Thus, the energy landscape not only describes the subsequent optimization process within the germinal center but also governs the initial “go-or-no-go” decision that determines which B cells are deemed worthy of entering the affinity maturation pathway. This helps students connect the abstract concept of binding energy directly to the concrete biological outcome of cellular selection.
When explaining the mechanisms of affinity maturation, somatic hypermutation can be linked to energy landscape optimization. By showing students the antibody gene mutation selection process during affinity maturation, they can observe how antibodies gradually “adjust the shape of the key” or, more accurately, reshape their energy landscape to find the optimal conformation for antigen binding, ultimately “sliding down” to the lowest point of the energy landscape. Computational simulation tools can be used to demonstrate how antibody mutations alter the binding free energy, deepening and narrowing energy wells, consequently enhancing antibody affinity and specificity.
When explaining antibody effector functions, binding kinetics can be linked to functional regulation. The way by which the stability of antigen–antibody binding affects downstream effector functions (complement activation, antibody-dependent cell-mediated cytotoxicity) can be illustrated by introducing kinetic parameters such as k_off and residence time. Students can be guided to consider how to engineer antibodies to optimize their binding kinetics, thereby improving their therapeutic efficacy.
Ultimately, we should focus on the roles of nonspecific binding in the immune system (including, but not limited to, immune surveillance, immune cell migration, and regulation of the inflammatory response). We should also encourage students to explore the “dark matter” of nonspecific binding and determine whether they could possibly exploit nonspecific interactions in new immunotherapy applications. We should discuss polyreactive antibodies as a key aspect of nonspecific binding and their connection to these larger aspects of immune function.
In conclusion, the energy landscape theory should be incorporated into immunology education to help students develop a more advanced understanding of antibody recognition and enhance their scientific and creative thinking. By focusing on the full binding affinity spectrum of universality, more specifically including the functional importance of nonspecific interactions and polyreactivity, students can develop a more complete and deeper understanding of immune recognition.
Challenges of applying the energy landscape theory in immunology teaching and responses
The energy landscape theory is a new lens through which we can revisit antigen–antibody recognition and interactions between immune cells. It is based on the idea about a potential energy surface in physics; it also allows the visualization of molecular interactions as topographic maps of energetic changes, thereby clarifying features such as binding affinity, kinetics, and specificity. However, similar to other types of interdisciplinary teaching, adopting this theory in immunology classrooms has challenges, requiring attentiveness and responsivity to teaching practice.
The main hurdle is the cognitive barriers represented by the abstract nature of the concept. Energy landscapes, which are an abstract concept, depict principles from thermodynamics and statistical mechanics that can be difficult for students. Students may easily experience a disconnect between abstract energy curves and actual molecular interactions. Hence, teaching engineers and scientists should lower the theoretical wall by using understandable language, stimulating analogies, and linking abstract ideas as much as possible to everyday experiences. Importantly, if the visual teaching package is well developed such that students can experience and co-construct the notion of energy landscape for biologically relevant systems through computer simulation, animation, or interactive VR models, then students can learn these notions as dynamic and intuitive processes. Most of them will tend to implicitly understand model systems describing the fundamental concepts of binding affinity and the dynamic rates of association and dissociation of association.
Beyond in-class pedagogical strategies, a more structural approach at the curricular level could also be highly effective. For instance, institutions could consider offering an introductory short course or module in physical biochemistry as a prerequisite or co-requisite for the immunology course. Such a preparatory course would equip students with the foundational concepts of thermodynamics, kinetics, and the energy landscape itself, thereby significantly lowering the cognitive barrier when these ideas are applied to the complex context of immunology. This would create a smoother and more integrated learning experience for students.
Second, the energy landscape model can simplify complex biological systems; however, the pure and simple use of this model can cause students to become unaware of the intricate factors (e.g., solvent effects, molecular crowding, and glycosylation modifications) present in actual biological environments. As such, immunology education should not only describe the energy landscapes but also highlight the interaction events on antigen–antibody complexes that happen in real biological environments, where many factors influence these interactions. Teachers can use case study discussions to urge students to consider the factors that create challenges for antigen–antibody binding; thus, they help enhance the systemic thinking of students.
Another issue that needs attention is cognitive dissonance caused by conflicts with traditional teaching content. Energy landscape and traditional immunology theories, such as the “lock-and-key” and “induced-fit” models, have different descriptions of molecular recognition mechanisms, which may cause cognitive conflict among students. Therefore, teachers explaining energy landscapes should avoid directly negating traditional models; instead, they should adopt a comparative analysis approach to highlight the advantages and limitations of each theory. They should also emphasize that the energy landscape theory is a supplement to and an improvement of traditional models rather than a replacement. For example, they can encourage students to consider the advantages of the “lock-and-key” model in explaining high-specificity binding and the utility of the “induced-fit” model in describing the conformational flexibility required for many interactions. Then, they can demonstrate how the energy landscape provides a unifying physical basis that explains why and how these phenomena occur, while also accounting for cross-reactivity and polyreactivity, which the other models cannot. They should specifically address potential students’ misconceptions about “nonspecific” (low-affinity) binding and emphasize the clarification of the energy landscape perspective on its important roles through polyreactive antibodies.
Teachers should prevent students from falling into the trap of reductionism. When applying the energy landscape theory to analyze antigen–antibody interactions, teachers risk simplifying complex biological processes into a superposition of intermolecular forces while neglecting the regulatory role of the immune system as a whole. Therefore, teachers should highlight the holistic nature of the immune system and the complex relationships between antigen–antibody interactions and other immune cells, cytokines, and other factors. Thus, teachers can incorporate typical immune disease cases to guide students in the analysis of immune system dysregulation in disease states and the role of antigen–antibody interactions in the occurrence and development of diseases.
In conclusion, incorporating the energy landscape theory into instructions on immunology may help expand students’ thinking and understanding of the meaning of antigen–antibody interactions. However, in practice, instructors should be aware of the challenges; furthermore, they may use the role of the energy landscape theory more effectively in immunology education through innovation in teaching methods, focusing and developing content, and increasing the link between theory and practice.
Conclusion
The theory of energy landscapes will be proposed as a more fundamental physical model for resolving the classical teaching paradox in immunology-the impossibility of reconciling antibody specificity and cross-reactivity. The theory does not contest structural complementarity but relates it to a probabilistic and dynamic context, so that specific and non-specific interactions are taken under the continuum of energy and time. This should help students to construct a theoretical model of immune recognition that consists of affinity maturation, cross-reactivity, and the surveillance of immunity all in one phenomenon, ultimately cultivating a deeper, probabilistic scientific understanding.
Author contributions
ZW: Writing – original draft. JL: Writing – review & editing. MW: Writing – review & editing. YY: Writing – review & editing. YL: Writing – review & editing. QX: Writing – review & editing. PW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present research was supported by the Teaching Reform Project of Zunyi Medical University (nos. 2021Z07, ZH202113 and ZH202201B), and the Key Construction Discipline of Immunology and Pathogen biology in Zhuhai Campus of Zunyi Medical University (ZHGF2024-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nimmerjahn F and Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. (2010) 236:265–75. doi: 10.1111/j.1600-065X.2010.00910.x
2. Stanfield RL and Wilson IA. Antibody structure. Microbiol Spectr. (2014) 2. doi: 10.1128/microbiolspec.AID-0012-2013
3. Mariuzza RA and Poljak RJ. The basics of binding: mechanisms of antigen recognition and mimicry by antibodies. Curr Opin Immunol. (1993) 5:50–5. doi: 10.1016/0952-7915(93)90080-c
4. Sundberg EJ and Mariuzza RA. Molecular recognition in antibody-antigen complexes. Adv Protein Chem. (2002) 61:119–60. doi: 10.1016/s0065-3233(02)61004-6
5. Braden BC, Dall’Acqua W, Eisenstein E, Fields BA, Goldbaum FA, Malchiodi EL, et al. Protein motion and lock and key complementarity in antigen-antibody reactions. Pharm Acta Helv. (1995) 69:225–30. doi: 10.1016/0031-6865(94)00046-x
6. Kunz H. Emil Fischer–unequalled classicist, master of organic chemistry research, and inspired trailblazer of biological chemistry. Angew Chem Int Ed Engl. (2002) 41:4439–51. doi: 10.1002/1521-3773(20021202)41:23<4439::AID-ANIE4439>3.0.CO;2-6
7. Csermely P, Palotai R, and Nussinov R. Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem Sci. (2010) 35:539–46. doi: 10.1016/j.tibs.2010.04.009
8. Jimenez R, Salazar G, Baldridge KK, and Romesberg FE. Flexibility and molecular recognition in the immune system. Proc Natl Acad Sci USA. (2003) 100:92–7. doi: 10.1073/pnas.262411399
9. Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. (2004) 25:174–9. doi: 10.1016/j.it.2004.02.004
10. Borowska MT, Boughter CT, Bunker JJ, Guthmiller JJ, Wilson PC, Roux B, et al. Biochemical and biophysical characterization of natural polyreactivity in antibodies. Cell Rep. (2023) 42:113190. doi: 10.1016/j.celrep.2023
11. Gunti S and Notkins AL. Polyreactive antibodies: function and quantification. J Infect Dis. (2015) 212:S42–6. doi: 10.1093/infdis/jiu512
12. Dimitrov JD, Planchais C, Roumenina LT, Vassilev TL, Kaveri SV, and Lacroix-Desmazes S. Antibody polyreactivity in health and disease: statu variabilis. J Immunol. (2013) 191:993–9. doi: 10.4049/jimmunol.1300880
13. Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. (2000) 37:1141–9. doi: 10.1016/s0161-5890(01)00025-6
14. Ehrenstein MR and Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. (2010) 10:778–86. doi: 10.1038/nri2849
15. Lobo PI. Role of natural autoantibodies and natural IgM anti-leucocyte autoantibodies in health and disease. Front Immunol. (2016) 7:198. doi: 10.3389/fimmu.2016.00198
16. Sandeep, Shinde SH, and Pande AH. Polyspecificity: An emerging trend in the development of clinical antibodies. Mol Immunol. (2024) 29:103846. doi: 10.1016/j.drudis.2023.103846
17. Zhou HX and Gilson MK. Theory of free energy and entropy in noncovalent binding. Chem Rev. (2009) 109:4092–107. doi: 10.1021/cr800551w
18. Gohlke H and Klebe G. Approaches to the description and prediction of the binding affinity of small–molecule ligands to macromolecular receptors. Angew Chem Int Ed. (2002) 41:2644–76. doi: 10.1002/1521-3773(20020802)41:15<2644::AID-ANIE2644>3.0.CO;2-O
19. Novotný J, Bruccoleri R, and Saul FA. On the attribution of binding energy in antigen–antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry. (1989) 28:4735–49. doi: 10.1021/BI00437A034
20. Adams RM, Kinney JB, Walczak AM, and Mora T. Epistasis in a fitness landscape defined by antibody-antigen binding free energy. Cell Syst. (2019) 8:86–93. doi: 10.1016/j.cels.2018.12.004
21. Pan A, Kar T, Rakshit AK, and Moulik SP. Enthalpy-entropy compensation (EEC) effect: decisive role of free energy. J Phys Chem B. (2016) 120:10531–9. doi: 10.1021/acs.jpcb.6b05890
22. Mills EA and Plotkin SS. Protein transfer free energy obeys entropy-enthalpy compensation. J Phys Chem B. (2015) 119:14130–44. doi: 10.1021/acs.jpcb.5b09219
23. Braden BC and Poljak RJ. Structural features of the reactions between antibodies and protein antigens. FASEB J. (1995) 9:9–16. doi: 10.1096/fasebj.9.1.7821765
24. Persson BA, Jönsson B, and Lund M. Enhanced protein steering: cooperative electrostatic and van der Waals forces in antigen-antibody complexes. J Phys Chem B. (2009) 113:10459–64. doi: 10.1021/jp904541g
25. Bhat TN, Bentley GA, Boulot G, Greene MI, Tello D, Dall’Acqua W, et al. Bound water molecules and conformational stabilization help mediate an antigen-antibody association. Proc Natl Acad Sci USA. (1994) 91:1089–93. doi: 10.1073/pnas.91.3.1089
26. Sundberg EJ, Urrutia M, Braden BC, Isern J, Tsuchiya D, Fields BA, et al. Estimation of the hydrophobic effect in an antigen-antibody protein-protein interface. Biochemistry. (2000) 39:15375–87. doi: 10.1021/bi000704l
27. Tsutsui K, Koide N, Tomoda J, Hayashi H, Hatase O, and Oda T. Role of hydrophobic interaction in hapten-antibody binding. Acta Med Okayama. (1977) 31:289–94.
28. Fellouse FA, Wiesmann C, and Sidhu SS. Synthetic antibodies from a four-amino-acid code: a dominant role for tyrosine in antigen recognition. Proc Natl Acad Sci USA. (2004) 101:12467–72. doi: 10.1073/pnas.0401786101
29. Osajima T and Hoshino T. Roles of the respective loops at complementarity determining region on the antigen-antibody recognition. Comput Biol Chem. (2016) 64:368–83. doi: 10.1016/j.compbiolchem.2016.08.004
30. Hageman T, Wei H, Kuehne P, Fu J, Ludwig R, Tao L, et al. Impact of tryptophan oxidation in complementarity-determining regions of two monoclonal antibodies on structure-function characterized by hydrogen-deuterium exchange mass spectrometry and surface plasmon resonance. Pharm Res. (2018) 36:24. doi: 10.1007/s11095-018-2545-8
31. Karadag M, Arslan M, Kaleli NE, and Kalyoncu S. Physicochemical determinants of antibody-protein interactions. Adv Protein Chem Struct Biol. (2020) 121:85–114. doi: 10.1016/bs.apcsb.2019.08.011
32. Manivel V, Sahoo NC, Salunke DM, and Rao KV. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. (2000) 13:611–20. doi: 10.1016/s1074-7613(00)00061-3
33. Galanti M, Fanelli D, and Piazza F. Conformation-controlled binding kinetics of antibodies. Sci Rep. (2016) 6:18976. doi: 10.1038/srep18976
34. Liu C, Denzler LM, Hood OEC, and Martin ACR. Do antibody CDR loops change conformation upon binding? mAbs. (2024) 16:2322533. doi: 10.1080/19420862.2024.2322533
35. Kocks C and Rajewsky K. Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci USA. (1988) 85:8206–10. doi: 10.1073/pnas.85.21.8206
36. Chowdhury PS and Pastan I. Improving antibody affinity by mimicking somatic hypermutation. vitro. Nat Biotechnol. (1999) 17:568–72. doi: 10.1038/9872
37. Low NM, Holliger PH, and Winter G. Mimicking somatic hypermutation: Affinity maturation of antibodies displayed on bacteriophage using a bacterial mutator strain. J Mol Biol. (1996) 260:359–68. doi: 10.1006/jmbi.1996.0406
38. Drake AW, Myszka DG, and Klakamp SL. Characterizing high-affinity antigen/antibody complexes by kinetic- and equilibrium-based methods. Anal Biochem. (2004) 328:35–43. doi: 10.1016/j.ab.2003.12.025
39. Rispens T, Ooijevaar-de Heer P, Derksen NIL, Wolbink G, van Schouwenburg PA, Kruithof S, et al. Nanomolar to sub-picomolar affinity measurements of antibody-antigen interactions and protein multimerizations: fluorescence-assisted high-performance liquid chromatography. Anal Biochem. (2013) 437:118–22. doi: 10.1016/j.ab.2013.02.027
40. Canziani GA, Melero JA, and Lacy ER. Characterization of neutralizing affinity-matured human respiratory syncytial virus F binding antibodies in the sub-picomolar affinity range. J Mol Recognit. (2012) 25:136–46. doi: 10.1002/jmr.2149
41. Leckband DE, Kuhl TL, Wang HK, Müller W, Herron J, and Ringsdorf H. Force probe measurements of antibody-antigen interactions. Methods. (2000) 20:329–40. doi: 10.1006/meth.1999.0926
42. Tsuchiya Y and Mizuguchi K. The diversity of H3 loops determines the antigen-binding tendencies of antibody CDR loops. Protein Sci. (2016) 25:815–25. doi: 10.1002/pro.2874
43. Fernández-Quintero ML, Loeffler JR, Kraml J, Kahler U, Kamenik AS, and Liedl KR. Characterizing the diversity of the CDR-H3 loop conformational ensembles in relationship to antibody binding properties. Front Immunol. (2019) 9:3065. doi: 10.3389/fimmu.2018.03065
44. Ohlson S, Strandh M, and Nilshans H. Detection and characterization of weak affinity antibody antigen recognition with biomolecular interaction analysis. J Mol Recognit. (1997) 10:135–8. doi: 10.1002/(SICI)1099-1352(199705/06)10:3<135::AID-JMR355>3.0.CO;2-B
45. Strandh M, Persson B, Roos H, and Ohlson S. Studies of interactions with weak affinities and low-molecular-weight compounds using surface plasmon resonance technology. J Mol Recognit. (1998) 11:188–90. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<188::AID-JMR420>3.0.CO;2-O
46. Keyt BA, Baliga R, Sinclair AM, Carroll SF, and Peterson MS. Structure, function, and therapeutic use of IgM antibodies. Antibodies. (2020) 9:53. doi: 10.3390/antib9040053
Keywords: energy landscape, antigen, antibody, specificity, non-specificity
Citation: Wang Z, Li J, Wang M, Yu Y, Lu Y, Xia Q and Wei P (2025) Reconciling specificity and non-specificity in antibody binding: an energy landscape framework for immunology education. Front. Immunol. 16:1650722. doi: 10.3389/fimmu.2025.1650722
Received: 20 June 2025; Accepted: 25 August 2025;
Published: 04 September 2025.
Edited by:
Robert H. Carnahan, Vanderbilt University Medical Center, United StatesReviewed by:
Mahita Jarjapu, La Jolla Institute for Immunology (LJI), United StatesCopyright © 2025 Wang, Li, Wang, Yu, Lu, Xia and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Wang, d2FuZ3poaXlvbmdAem11emguZWR1LmNu; Pei Wei, d2VpcGVpQHptdXpoLmVkdS5jbg==
 Zhiyong Wang1*
Zhiyong Wang1* Yanxin Lu
Yanxin Lu Pei Wei
Pei Wei