- 1Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, VIC, Australia
- 2Victorian Infectious Diseases Service, Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, VIC, Australia
- 3Monash Infectious Diseases, Monash Health, Clayton, VIC, Australia
- 4Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, VIC, Australia
The intestinal immune compartment plays a central role in HIV pathogenesis, serving as an early site for viral replication and a significant reservoir for latent infection. Despite the success of antiretroviral therapy (ART) in suppressing plasma viremia, HIV persists indefinitely in latently infected cells, commonly found in the intestinal tract due to its unique immunological and structural environment. Targeting HIV-infected cells that persist in the intestinal tract is an important consideration for therapeutic strategies and is also important when considering an HIV cure. This review describes the therapeutic approaches aimed at addressing HIV persistence in the intestinal tract, or gut. We provide a brief overview of mechanisms underlying reservoir formation and maintenance, discuss the challenges posed by gut-specific factors, and examine emerging strategies, including latency reversal agents, immune modulation, gut-targeted ART, and novel delivery systems. This review will focus on contemporary advances in knowledge in this space, gaps in the literature and areas for future research focus.
1 Introduction
While antiretroviral therapy (ART) effectively suppresses plasma viral levels, it fails to eliminate latent reservoirs of HIV, particularly in gut associated lymphoid tissue (GALT) (1). The gut serves as a major anatomical reservoir due to the high density of activated, HIV-susceptible CD4+ T cells expressing the major co-receptors for HIV, CCR5 and CXCR4 (2–4), unique lymphocyte trafficking patterns, variable antiretroviral (ARV) penetration (5–8), and an immunoregulatory environment. The gut contains over 85% of lymphoid tissue and more than 90% of all lymphocytes (9), making it a critical compartment in HIV pathogenesis and persistence.
The intestinal immune system comprises inductive sites (e.g., mesenteric lymph nodes and Peyer’s patches), where adaptive immune cells (CD4+ T cells, CD8+ T cells and B cells) are initially activated and differentiate; and effector sites (e.g., lamina propria and epithelium), where differentiated immune cells mount mucosal defence (10, 11).
A critical component within this compartment, GALT, plays a key role in antigen sampling and comprises multi-follicular structures like Peyer’s patches in the small intestine, isolated lymphoid follicles (ILFs) that are dispersed throughout the small and large intestines (10, 11), as well as sites such as the appendix (12) and rectal lymphoid tissue (13, 14). The abundance of activated CD4+ T cells, with a predominantly memory (CD45RO+) phenotype that also express the HIV co-receptors CCR5/CXCR4 (2–4) coupled with the extensive mucosal surface area at this site, renders GALT especially susceptible to HIV infection (4). Early studies revealed a profound depletion of CCR5+ memory CD4+ T cells following acquisition of HIV (15), particularly in effector sites of the lamina propria (16, 17), where CCR5-expressing memory CD4+ T cells were rapidly lost (18). As shown in both SIV and HIV infection, there is a preferential depletion of CD4+ T cell in mucosal-associated lymphoid tissue compared with peripheral blood that is more severe in mucosal tissues than in peripheral blood, and disproportionately affects Th17, Th22 and other immune-regulatory subsets essential for maintaining mucosal barrier function (18–25). Notably, other contemporaneous studies suggested that the virus spares long-lived naïve and central memory CD4+ T cells, which may replenish depleted effector cells (26, 27). Countering the prevailing hypothesis of direct viral cytopathicity (28), these studies suggested that chronic immune activation may be the primary driver of progressive CD4+ T cell loss (26, 27). Nonetheless, HIV persistence in the intestinal immune compartment poses a formidable barrier to achieving remission or eradication of HIV (10–14). In this review, we examine the mechanisms underpinning HIV persistence in the gut and explore emerging therapeutic strategies tailored to this complex immune compartment.
2 Mechanisms of HIV reservoir persistence in the gut
2.1 Structural and cellular factors
HIV disrupts the gut’s three key barriers: the microbial barrier (commensal bacteria) (29), the mechanical barrier (tight junctions between epithelial and endothelial cells) (30, 31), and the immunologic barrier (mucosal lymphocytes, mesenteric lymph nodes, and cytokines) (17, 32, 33). These disruptions drive chronic immune activation and facilitate viral persistence (Figure 1).
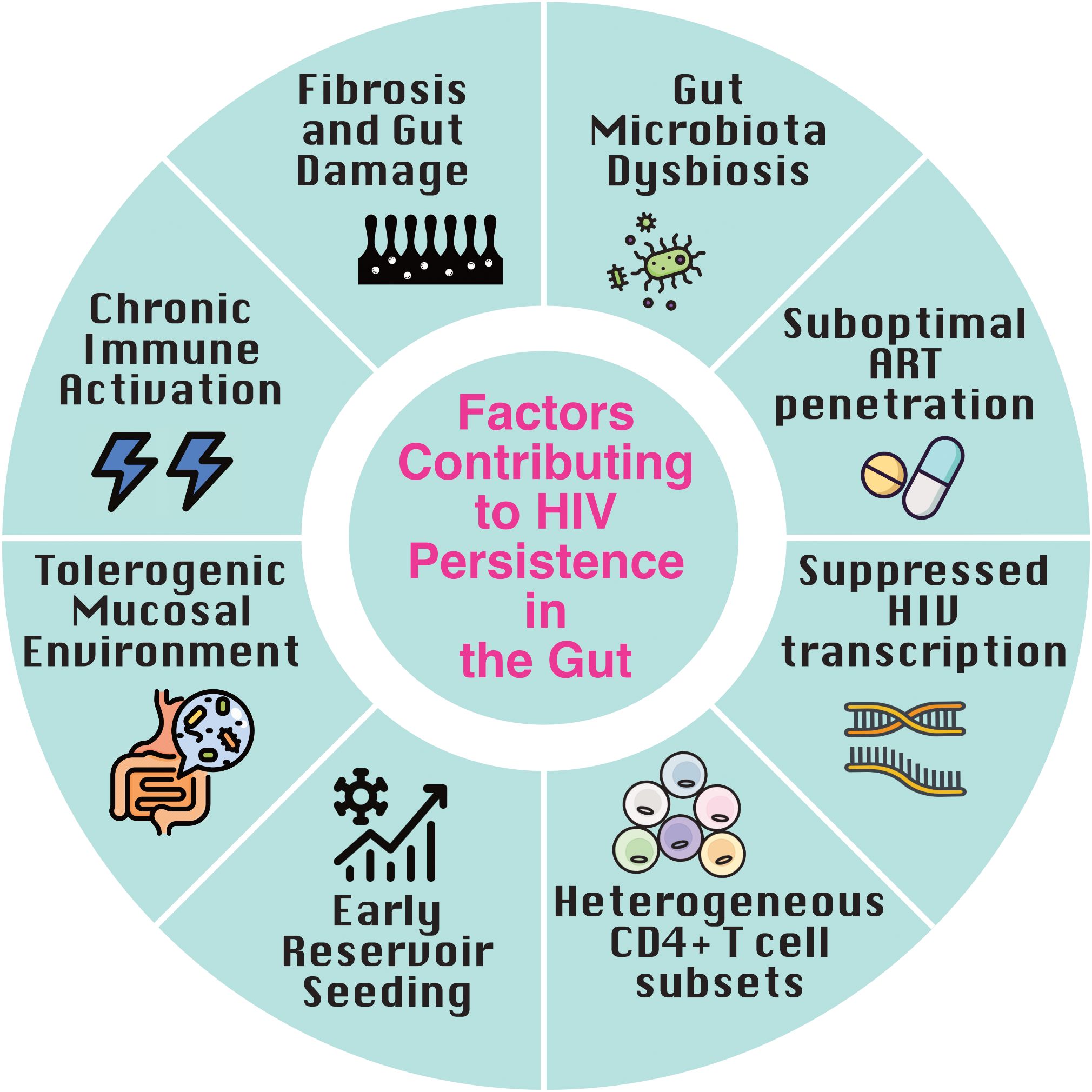
Figure 1. Factors contributing to HIV persistence in the gut. Multiple factors converge in the gut to promote HIV latency and persistence despite antiretroviral therapy (ART). Early reservoir seeding during acute infection, combined with a tolerogenic mucosal environment and immune-evasive tissue-resident memory (TRM) cells, establishes a durable reservoir. Chronic immune activation driven by microbial translocation, along with gut-associated lymphoid tissue (GALT) fibrosis, impairs immune clearance. Heterogeneous CD4+ T cell subsets, suppressed transcriptional activity, suboptimal ART penetration, and gut microbiota dysbiosis further reinforce viral persistence and immune evasion within this immunologically distinct tissue.
Anatomical sites vary in immune cell composition (34), tolerogenic features (35), and the transcriptional landscape of the HIV reservoir (36), resulting in differential viral burden (37, 38) and tissue-specific responses to latency-targeting interventions (39). Microbial sensing by gut macrophages leads to IL-1 production and downstream secretion of regulatory cytokines (retinoic acid, IL-10, TGF-β), which foster a tolerogenic environment (40, 41). The gut, in particular, is a key site of immune tolerance and a primary reservoir for HIV, where constant exposure to microbiota-derived signals drives the differentiation and maintenance of HIV-susceptible CD4+ T cell subsets, including regulatory T cells (Tregs), Th1, Th2, Th17, and Th22 cells (42–45). Many of these cells express high levels of HIV co-receptor CCR5 (43), rendering them highly susceptible to infection during acute and chronic phases. Th17 and Th22 subsets, which are enriched in the lamina propria and play critical roles in maintaining mucosal barrier integrity and microbial defense, are particularly vulnerable targets (25, 46, 47). Recent findings suggest that CCR6-expressing CD4+ T cell subsets, including Th17, Th1Th17, and CCR6+CCR4-CXCR3- cells, represent a substantial and preferentially infected reservoir in the gut, enriched for replication-competent HIV due to their mucosal localization, memory phenotype, and high susceptibility to infection (47–49).
Another pivotal cellular reservoir in the gut are tissue-resident memory T cells (TRM), which differ from circulating memory T cells in both phenotype and function. TRM are long-lived, non-recirculating cells that localize to mucosal barrier sites and are poised for rapid immune responses upon local antigen re-encounter (50). In the gut, regionalized signaling within the intestinal microenvironment supports the maintenance of two phenotypically distinct TRM states: terminally differentiated TRM cells localized to the upper villus, and progenitor-like TRM residing in the lower villus (51). CD8+ TRMs acquire CD103 expression under the influence of local TGF-β and IL-10 (52, 53), while most CD4+ TRMs lack CD103 (54). Despite this, both gut-resident and circulating CD103+ CD4+ T cells share a gene expression profile enriched for HIV DNA but exhibit low levels of RNA transcription per provirus, consistent with latent infection (55). The shared molecular mechanisms, including reduced expression of ribosomal proteins and components of mRNA processing and transcriptional machinery, suggest common mechanisms of proviral silencing (55). Functionally, TRMs express elevated levels of inflammatory and cytotoxic genes (54, 56, 57), enabling rapid effector responses. However, they also upregulate inhibitory markers such as PD-1 and CD101, which constrain proliferation and IL-2 production (54, 58). These dual attributes, activation readiness and functional suppression, highlight their role as both sentinels of mucosal immunity and long-lived HIV reservoirs that may be less accessible to immune clearance or ART penetration (54, 55). These features may contribute to immune evasion and present barriers to latency reversal strategies, underscoring the need for targeted approaches that can overcome the unique functional and transcriptional constraints of the gut-resident reservoir.
2.2 Persistent immune activation and inflammation fuel HIV persistence
Chronic immune activation is a hallmark of HIV infection and a key driver of reservoir persistence (Figure 1). Persistent infection is characterized by a dynamic equilibrium in which ongoing immune activation coexists with regulatory mechanisms that limit immunopathology but may also permit viral persistence (43). Elevated levels of pro-inflammatory markers such as IFN-γ, IL-6, IP-10, and indoleamine 2,3-dioxygenase promote CD4+ T cell susceptibility and sustain inflammatory cycles (59–62). A higher proportion of activated and proliferating T cells are found in the gut compared to peripheral blood (22, 63), contributing to reservoir maintenance. This activation is largely triggered by HIV-mediated damage to the intestinal epithelium, leading to microbial translocation- the leakage of bacterial products like lipopolysaccharide (LPS) into circulation- which fuels systemic immune activation in both people with HIV (PWH) and SIV-infected macaques, and strongly predicts disease progression (29, 64–66). Fungal translocation, particularly of (1→3)-β-D-glucan, further amplifies inflammation via pattern recognition receptor signaling, and remains elevated despite ART, contributing to gut damage and disease progression (67, 68).
Although ART effectively suppresses plasma viremia, it does not fully restore gut epithelial integrity or microbiome diversity (22, 67, 69, 70). Consequently, immune activation and microbial translocation markers persist, undermining immune homeostasis and facilitating continued HIV persistence (67, 71–73). Ongoing inflammation also recruits new target cells for infection, reinforcing the reservoir despite viral suppression (74). Together, these features create a uniquely permissive environment in the gut for HIV latency and immune evasion, even under sustained ART.
2.3 Gut microbiota, dysbiosis and microbial translocation
Recent findings suggest that gut microbiota composition may modulate reservoir size and immune control. A recent germ-free humanised mouse model demonstrated the role the microbiome plays in HIV persistence, with lower levels of HIV replication in plasma and tissues of germ-free mice, depleted of their resident microbiota, compared with conventional humanised mice (75). In a study of HIVconsv immunogen (conserved regions of HIV-1 Gag, Pol, Vif, and Env) and the histone deacetylase inhibitor (HDACi) romidepsin, a latency reversing agent (LRA); individuals with higher baseline gut Bacteroidales: Clostridiales ratios showed smaller HIV reservoirs and more sustained control of viremia (76). Bacteroidales species, known producers of immunomodulatory metabolites like short-chain fatty acids, may influence T cell function and mucosal immunity (77), implicating microbial dysbiosis as both a consequence and modulator of HIV persistence. Finally, a pilot randomised controlled trial of repeated faecal microbiota transplantation in PWH found no differences in biomarkers of inflammation and bacterial translocation between treatment and control groups, but no comparisons of HIV reservoirs were conducted in this study (78).
The presence and mechanism of a causal link between gut barrier dysfunction, microbial translocation, systemic inflammation and HIV persistence is not well understood (79, 80) and warrants further study.
2.4 ARV penetration in the gut: barriers to reservoir elimination
Reduced antiretroviral drug penetration in the gut may pose a challenge to the elimination of the HIV reservoir in this compartment. Studies have shown that mucosal tissue penetrance varies with antiviral agents; due to a range of intrinsic and extrinsic factors (protein binding, molecular size, lipophilicity, ionization, and blood perfusion), physical barriers, as well as efflux and uptake transporter expression (5); leading to levels of some ARVs, such as the integrase strand transfer inhibitor (INSTI) dolutegravir and non-nucleoside reverse transcriptase inhibitor rilpivirine, falling below therapeutic thresholds in gut tissues compared to peripheral blood (5, 81). Consistent with these findings, individuals with lower tissue drug concentrations exhibit higher HIV transcription at these sites despite systemic viral suppression (82, 83). Some studies have even suggested that the HIV reservoir is constantly replenished by low-level virus replication in lymphoid tissue despite undetectable viral RNA in plasma (81, 84), although this remains controversial and has been strongly challenged by others (85, 86). Nonetheless, recent pharmacokinetic studies of newer, long-acting (LA) injectable drug formulations also demonstrate lower gut tissue penetrance (6), despite their long half-life and superiority over combination tenofovir disoproxil fumarate/emtricitabine in the setting of HIV pre-exposure prophylaxis (87, 88). In a Phase I study of the INSTI cabotegravir-LA, rectal concentrations of the drug were <8% of the corresponding plasma concentration (6). In contrast, drug concentrations of LA injectable rilpivirine in rectal tissue were found to exceed plasma levels in vivo and showed a dose-dependent antiviral effect ex vivo (7), suggestive of more durable mucosal protection. A recent study of LA cabotegravir plus rilpivirine found that some individuals continued to shed HIV-1 RNA in rectal secretions despite plasma suppression, and rectal rilpivirine levels, though above the protein-adjusted EC90, did not correlate with viral shedding, suggesting that drug exposure alone may not fully suppress HIV transcription or replication in the gut (8). Other factors including ongoing immune activation, suboptimal immune control and high burden of latently infected cells in the gut (89) likely also contribute to observed persistent transcription and compartmentalized viral replication. Notably, while genetically intact HIV DNA can be detected in tissues including the ileum, colon, and rectosigmoid, the presence of markers of transcription completion and protein production (polyadenylated and multiply-spliced HIV transcripts) are infrequently detected in gut and female genital tract tissues in virally-suppressed PWH (36, 38). These findings underscore the complexity of achieving complete viral suppression in different mucosal compartments and the need to consider both drug distribution and local tissue factors when evaluating the efficacy of long-acting ART formulations.
3 Emerging therapeutic approaches
3.1 Latency modulating agents
Latency reversal agents (LRAs) aim to reactivate latent HIV to increase virus transcription, protein expression and virion production (Figure 2), thereby making infected cells visible to the immune system for immune-mediated clearance (90). A diverse array of transcription activating LRAs have been assessed both in vitro, ex vivo and in vivo for their ability to reactivate HIV transcription with varying levels of success- a topic that has been comprehensively reviewed elsewhere (90–93).
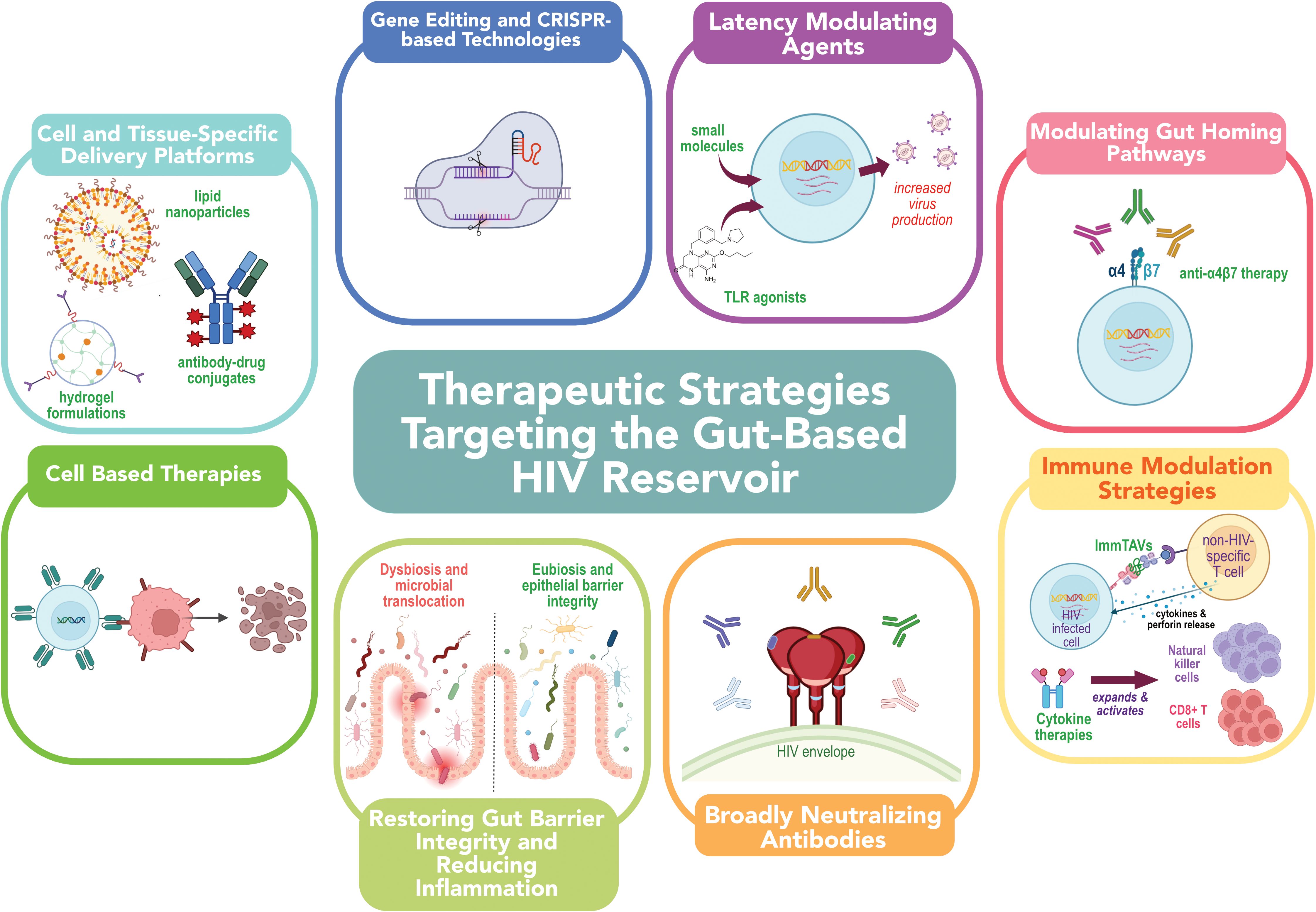
Figure 2. Emerging therapeutic strategies for targeting the HIV reservoir in the gut. Strategies include: (1) latency-modulating agents to induce viral gene expression; (2) gut-homing pathway modulation via integrin-targeting antibodies; (3) immune-based therapies to enhance clearance of infected cells; (4) broadly neutralizing antibodies to block infection and mediate cytotoxicity; (5) interventions to restore gut barrier integrity and reduce inflammation; (6) cell-based therapies, (7) gene-editing and CRISPR-based approaches; and (8) targeted delivery platforms such as nanoparticles and antibody-drug conjugates to improve localization and efficacy of therapeutics within the gastrointestinal tract. Together, these approaches aim to overcome anatomical and immunological barriers to HIV cure.
Ex vivo analysis of LRAs demonstrate that agents that can induce HIV transcription in peripheral blood may not exert the same magnitude of effect in gut tissues (39). Notably, even when HIV transcription is induced, the translation of viral proteins or production of virions- crucial for immune recognition- may be limited, raising questions about the functional efficacy of many LRAs, particularly in tissue-resident cells. As such, the distinct immune milieu of the gut (Figure 1) may necessitate the development of gut-specific LRAs or combination strategies that improve both HIV reactivation and immune-mediated clearance in this compartment.
Emerging immunomodulatory LRAs, such as Toll-like receptor (TLR) agonists, TLR-1/2, TLR7, and TLR9 (94–97), have demonstrated potential in both reactivating latent virus and modulating immune responses in humans and non-human primates. TLR agonist stimulation induces an activated plasmacytoid dendritic cell phenotype, with increased expression of TNF-α, IFN-α, interferon regulatory genes and restriction factors that contribute to an increased HIV-specific T cell response (98). Treatment with the TLR9 agonist lefitolimod results in induction of an interferon stimulated gene signature consistent with potent IFN-α induction but without concomitant excessive inflammation in the gut mucosa (95, 99), suggesting this treatment could exert beneficial effects in the gut. The combination of TLR7 agonist vesatolimod (GS-9620) and broadly neutralizing antibody PGT121 led to lower levels of HIV DNA in lymph nodes of treated SHIV-SF162P3-infected rhesus monkeys at week 120 (100) and decreases in intact proviral DNA in peripheral CD4+ T cells during ART coupled with a delay in viral rebound during ART interruption in a phase 1b clinical trial (101). More recently, dual TLR7/8 agonists have demonstrated enhanced latency reversal and immune activation compared to single agonists, resulting in greater reductions in the inducible HIV reservoir and improved control of viral rebound in preclinical (ex vivo PBMC/cell line) models (102).
While considerable progress has been made in the development of latency reversal strategies, there is limited evidence supporting the efficacy of LRAs in the gut. Latency reversing effects in colonic and rectal tissue have been demonstrated in clinical trials of the HDACis panobinostat and vorinostat (103, 104), and the TLR9-agonist lefitolimod (99), however in the case of vorinostat, this effect less than that noted in peripheral blood cells. Notably, few clinical trials investigating LRAs have routinely conducted biopsies of gastrointestinal tissue.
An alternative latency-modulating approach under investigation, the “block and lock” strategy, seeks to drive HIV into a long-lived, transcriptionally silent state. This has been studied with agents such as bromodomain-containing protein 4 (BRD4) modulators (105), Heat shock protein 90 (HSP90) inhibitors (106–108), LEDGINs (109, 110), Jak-STAT inhibitors (111, 112), HIV-1 Tat inhibitor didehydro-cortistatin A (113). Notably, didehydro-cortistatin A has a favorable pharmacokinetic profile, stability, activity in the absence of ART, and the ability to cross the blood–brain barrier, making it a particularly attractive candidate (113, 114). Given the gut’s role as a major site of persistent HIV transcription, strategies that can durably suppress proviral expression in tissue-resident immune cells could be critical for achieving long-term remission. The distinct immunologic landscape of the gut underscores a critical gap in current HIV cure strategies, highlighting the need for more trials to evaluate the impact of interventions on gut reservoirs and to develop gut-targeted or combination approaches that both induce HIV transcription and enhance immune-mediated clearance.
3.2 Modulating gut-homing pathways through antibody-based interventions
CD4+ T cells migrate into gastrointestinal tissues by engaging α4β7 integrin, expressed on their surface, with MAdCAM-1, a key adhesion molecule found on the gut endothelium (115, 116). HIV gp120 binds to this gut-homing integrin (117), enhancing the susceptibility of α4β7+CD4+ T cells to HIV infection (118). Therefore, targeting α4β7, which facilitates the trafficking of HIV-infected cells to the gut (118–121), represents a novel strategy to disrupt reservoir formation and persistence.
Treatment with anti-α4β7 therapy [vedolizumab (VDZ)] in PWH on ART with concomitant inflammatory bowel disease (IBD) has demonstrated promise in attenuating the formation of lymphoid aggregates within the gut (122), which are known to serve as key sanctuary sites for maintaining viral reservoirs (123–126). While VDZ resulted in sustained virologic control in one study of macaques infected with an attenuated strain of SIV, SIVmac239 (127), this finding triggered some controversy as the SIV strain used in the study had a stop codon in the Nef coding region (128). Subsequent studies in macaques infected with SIVmac251 (129) and in PWH on ART (130), could not replicate this observation. Nonetheless, a recent clinical trial suggested that the level of α4β7 blockade may inversely correlate with HIV DNA levels (131), highlighting a potential role for integrin-targeting strategies in reducing viral reservoirs, though further research is needed to explore their utility in combination cure approaches.
In addition to α4β7, other trafficking molecules such as CCR9, which is important for gut homing particularly in Th17 cells during HIV infection (132) and MadCAM-1 are being explored as potential therapeutic targets in reducing inflammation (133) that could provide gut-selective options that avoid systemic immunosuppression.
3.3 Immune modulation strategies to enhance HIV reservoir clearance
Immune modulation strategies aim to restore antiviral immunity, reduce chronic inflammation, and enhance the immune system’s capacity to recognize and eliminate infected cells within mucosal tissues (134). Checkpoint blockade targeting inhibitory receptors (e.g., PD-1, CTLA-4, LAG-3, TIGIT) has shown promise in reversing T cell exhaustion and enhancing HIV-specific CD8+ T cell responses in preclinical studies [reviewed elsewhere (134)]. These approaches may synergise with LRAs (135), but systemic administration risks immune-related adverse events (136), highlighting the need for gut-targeted strategies.
Cytokine-based therapies, such as IL-15 superagonists may enhance mucosal effector responses (137, 138). The IL-15 superagonist N-803 (Figure 2) modestly reduced inducible HIV in peripheral blood mononuclear cells (PBMC) in a Phase I trial, alongside natural killer (NK) cell expansion (137). In SIV-infected macaques, N-803 increased CD8+ T cell and NK cell activation and trafficking to lymphoid and mucosal tissues (139, 140), highlighting its potential to bolster immune clearance mechanisms in mucosal reservoirs. However, the effects of N-803 in the human gut remain largely uncharacterized, underscoring the need for dedicated studies to evaluate its impact on the gut HIV reservoir.
Emerging bispecific platforms like ImmTAVs- soluble, engineered T-cell receptors (TCRs) fused to anti-CD3, redirect polyclonal CD8+ T cells to eliminate HIV-infected CD4+ T cells presenting low levels of HIV antigen (141). Given the low-level HIV Gag expression detectable in gut tissues of ART-suppressed individuals (142, 143), ImmTAVs may offer a potent strategy for mucosal reservoir clearance. Although current constructs are HLA-restricted and untested in gut tissue, their efficacy in solid tumours (144) supports their translational potential. Advantages of this strategy include: (i) targeting cells expressing very low antigen levels; (ii) bypassing exhausted HIV-specific T cells; and (iii) compatibility with combination therapies.
Immune-based approaches must carefully balance antiviral activity with the preservation of mucosal barrier function and limitation of inflammation-induced damage. Refinement of these strategies is ongoing, aiming to enhance antiviral responses while preserving mucosal integrity. For instance, targeted delivery mechanisms, such as nanoparticle formulations and antibody-drug conjugates (Section 2.7) are being explored to localize immune modulation to mucosal tissues and reduce systemic toxicity. Combinatorial approaches, such as pairing cytokines like IL-15 or IL-21 with checkpoint blockade (145) or probiotic therapy (146), are being optimized to enhance effector cell function without inducing excessive inflammation. Additionally, advances in cancer immunotherapy, such as checkpoint blockade targeting myeloid-derived suppressor cells (MDSCs) to overcome their immunosuppressive effects and enhance the efficacy of immune checkpoint inhibitors (ICIs) and adoptive cell therapies (147), may inform combinatorial strategies for targeting the HIV reservoir. These emerging approaches reflect a broader shift toward precision immunotherapies that are tailored to the distinct immunologic and structural characteristics of mucosal tissues- an important step toward more effective strategies for targeting and eliminating HIV reservoirs in these challenging anatomical sites.
3.4 Broadly neutralizing antibodies
Broadly neutralizing antibodies (bNAbs) can induce direct viral neutralization and immune responses through antibody-dependent cellular cytotoxicity (ADCC) (148–151). Despite some promising data showing that the combination of bNAbs 3BNC-117 and 10–1074 can significantly delay viral rebound following ART interruption (152), concomitant decreases in the size of the viral reservoir have not been demonstrated. A recent proof-of-concept study using a 10–1074 formulated for topical vaginal application demonstrated that mucosal delivery of potent bNAbs provided protection against repeated cell-associated SHIV162P3 vaginal challenge in non-human primates (153). Clinical trials are currently ongoing to assess the effect of long-acting bNAbs on the tissue-resident viral reservoirs (154).
3.5 Restoring gut barrier integrity and reducing inflammation
A wide array of therapeutic strategies has been explored to target the gut microbiome in PWH, aiming to reduce persistent inflammation and immune dysfunction despite effective ART (Figure 2).
Antibiotics, administered experimentally, have shown mixed outcomes, with some studies in nonhuman primates (NHPs) suggesting reduced gut inflammation and altered susceptibility to SIV infection, however concerns remain about long-term dysbiosis and resistance (155) that may compromise gut barrier integrity. In human trials examining antibiotics as a possible modality to ameliorate persistent immune dysfunction in ART-suppressed PWH, neither rifaximin nor cotrimoxazole treatment altered bacterial translocation (156, 157). Although antibiotics can influence the gut microbiome in PWH (155), their use warrants caution due to broad microbial disruptions and the risk of antimicrobial resistance.
Prebiotics and probiotics have demonstrated modest benefits on immune markers in some exploratory trials (158–160), but larger controlled studies in children infected with HIV, and ART naive adults with HIV failed to show consistent improvements in gut dysbiosis, immune recovery or reduction in inflammatory biomarkers (161–163). Despite some reported benefits, including potential improved gut barrier integrity (164), current prebiotic and probiotic formulations lack sufficient evidence and regulatory oversight to support their use in PWH (155), highlighting the need for more targeted, next-generation approaches.
More recently, attention has shifted to postbiotics and live biotherapeutic products (LBPs) driven by progress in the treatment of Clostridium difficile (165, 166). Approaches include delivery of targeted bacterial consortia, designed to restore or improve gut microbiota composition, and microbial metabolites like butyrate (167), that could have anti-inflammatory or immune-modulating effects (159, 168) and enhance epithelial barrier function (169), however their immunological efficacy in the setting of HIV remains under investigation. Faecal microbiota transplantation (FMT) is under investigation as a strategy to reverse HIV-associated gut dysbiosis, with early-phase trials demonstrating transient donor engraftment (170), enhanced microbial diversity (171), and indications of reduced gut epithelial damage (172). While these findings highlight FMT’s potential to modulate the gut microbiota in PWH, evidence for its impact on systemic inflammation and HIV persistence remains inconclusive, underscoring the need for further research into microbiota-targeted interventions to address HIV-driven immune dysfunction.
Therapies aimed at reducing systemic inflammation or restoring gut barrier integrity in PWH target key drivers such as microbial translocation, residual viral replication, and immune dysregulation. Investigational approaches include anti-inflammatory agents (e.g., statins (173–176)); immunomodulators like IL-1β blockers (177, 178), IL-6 blockers (177, 178), tumour necrosis factor a (TNFa) blockers (179, 180), toll-like receptor 4 (TLR4) antagonists (181), PPAR agonists (182, 183), or Janus kinase (JAK) inhibitors (179, 180); farnesoid X nuclear receptor (FXR) agonists (184) sulfonamide drugs (185); and gut-tropic agents such as GLP-2 analogue teduglutide (Clinical Trial: NCT02431325). Apolipoprotein A-I (apoA-I) mimetic peptides bind bioactive lipids and endotoxin (LPS) to exert an anti-inflammatory effect (186). Previously investigated as a treatment modality for cardiovascular disease and cancer (187, 188), recent work in humanized mouse models of HIV infection suggest that these peptides can reduce levels of proinflammatory proteins, such as ADAM17, that contribute to both systemic and gut inflammation (189).
While several of these strategies have demonstrated reductions in biomarkers of inflammation, their capacity to meaningfully improve immune function or reduce clinical comorbidities in PWH has yet to be definitively established.
Together, these findings underscore the complexity of therapeutically targeting the gut in PWH and highlight a critical need for rigorously designed, mechanistically informed studies to identify microbiota-directed or gut-specific interventions that can durably reduce inflammation, restore mucosal integrity, and ultimately contribute to HIV remission or cure strategies.
3.6 Therapeutic vaccines to restore gut immunity and reduce mucosal inflammation
Therapeutic vaccines represent a promising avenue for enhancing gut immunity in PWH. By targeting the gut mucosa, these strategies aim to restore immune function, reduce inflammation, and improve overall health outcomes in PWH. Several approaches are under investigation, including intranasal or mucosal vaccines adjuvanted with IL-13Rα2 blockers, which have been shown to enhance mucosal CD8+ T cell responses in gut-associated lymphoid tissues (190). Other strategies involve dendritic cell-targeted vaccines designed to induce durable HIV-specific immunity at mucosal sites (191, 192), oral vaccines using recombinant Lactococcus lactis expressing HIV antigens which have demonstrated the ability to elicit mucosal immune responses (193), and mRNA-based vaccines that promote polyfunctional T cell responses within the gastrointestinal tract.
While prophylactic mRNA HIV vaccines are progressing (e.g., NCT05001371, NCT05414786, NCT05217641), therapeutic HIV vaccine development remains limited (194). Unlike prophylactic strategies that focus on eliciting envelope-specific neutralizing antibodies (195), therapeutic vaccines must induce strong, Gag-specific polyfunctional CD8+ T cell responses (196–198). To date, only a limited number of therapeutic vaccine candidates have progressed beyond preclinical evaluation in mouse and non-human primate models (199–201). Therapeutic vaccines, such as ALVAC-HIV/Lipo-6T/IL-2 (202), Vacc-4x (203), and HIVACAT T-cell immunogen-based vaccines (204) have shown promise in enhancing viral control in the absence of ART, and may even help overcome the impact of gut microbiota depletion on IFNγ-producing T-cell responses (205). However, their standalone efficacy has been limited, therefore combination strategies may be necessary to achieve sustained viral remission and counteract immune dysfunction originating in the gut. A major gap remains in understanding how to direct antigen-specific immune responses to the gut and how to measure functional improvements in gut immune health after therapeutic vaccination. While clinical translation is ongoing, these approaches represent promising adjuncts to antiretroviral therapy by addressing the immunologic damage and inflammation that persist in the gastrointestinal mucosa.
3.7 Gene and cell-based therapeutic strategies
Gene editing technologies, particularly CRISPR-based approaches, have emerged as promising tools for targeting persistent HIV reservoirs. These strategies are especially relevant to GALT, where therapeutic interventions must be able to access, persist, and function effectively.
Viral-directed approaches aim to excise integrated provirus (206), durably silence transcription (207) or activate latent proviruses to enhance clearance (208). Recent efforts emphasize the need for delivery systems that achieve effective biodistribution within lymphoid tissues, where the majority of the reservoir resides (209, 210). The CRISPR-based therapy EBT-001, delivered by adeno-associated virus (AAV), achieved broad biodistribution in lymphoid tissues and demonstrated evidence of proviral cutting in preclinical simian immunodeficiency virus (SIV) models (206). Its HIV counterpart, EBT-101, was recently shown to be safe in a first-in-human clinical trial, although viral rebound occurred following analytical treatment interruption, highlighting the need for further refinement (211).
Host-directed approaches aim to render target cells resistant to infection or to enhance antiviral immunity in mucosal compartments (212), CCR5 remains a leading gene-editing target, with multiple studies demonstrating disruption of CCR5 using zinc finger nucleases (ZFNs), TALENs, and CRISPR is feasible in vitro, ex vivo, and in vivo (212–215). These approaches provide proof-of-concept for durable resistance to HIV infection and, importantly, could protect gut-homing memory CD4+ T cells from reinfection. and engineering of host cells resistant to infection (212).
Complementary to gene editing, cell-based therapies are advancing in parallel. In nonhuman primate models, stem-cell-derived CAR T cells demonstrated superior persistence, tissue trafficking, and antiviral activity, reinforcing their potential in mucosal immune compartments (216). Notably, a macaque study demonstrated that hematopoietic stem cell (HSC)-derived CAR T cells engraft and persist within tissue-associated HIV reservoirs, including GALT, where they maintained proliferative capacity and antiviral activity (216). Similarly, CAR/CXCR5 T cells showed modest presence in gut tissues (ileum, rectum) alongside sustained reductions in viral RNA within lymphoid follicles, underscoring both the potential and current limitations of tissue penetration (217). Early-phase clinical trials, including CAR T-cell therapies targeting gp120 (218), are underway, though mapping gut homing and durability of responses remain critical next steps.
Together, these gene- and cell-editing strategies underscore the potential to overcome the unique barriers posed by gut reservoirs, where persistence, immune evasion, and tissue accessibility converge, positioning the gut as a critical testing ground for next-generation HIV cure interventions.
3.8 Cell and tissue-specific delivery platforms
Targeting therapeutic agents directly to gut-associated lymphoid tissue represents a major challenge and opportunity in HIV cure research. The anatomical and immunological complexity of the gut (9, 10), coupled with its unique barriers to drug penetration and immune accessibility (89), necessitates the development of innovative delivery systems designed to enhance the localization, uptake, and activity of targeted therapeutics (219, 220). Nanoparticle-based delivery systems have emerged as promising platforms for gut-specific targeting (220). These include lipid nanoparticles, polymer-based carriers, and biodegradable vesicles engineered to protect therapeutic cargo from enzymatic degradation in the gastrointestinal tract and promote uptake by mucosal immune cells (220).
Nano-drug delivery systems (NDDs) can be engineered to enhance mucosal adhesion (221), cellular uptake (222), and targeted delivery of antiretroviral agents or latency-reversing therapeutics (223) directly to infected cells in the gut. By bypassing efflux mechanisms and enabling sustained drug release (224), NDDs may achieve higher local drug concentrations and more effective suppression or elimination of HIV within tissue reservoirs. Incorporating targeting moieties, such as antibodies or ligands specific to infected cells (223) or the gut epithelium further enhances specificity and likely minimises off-target effects. Thus, NDDs represent a novel and rational approach to overcoming a key barrier in HIV cure strategies. Hydrogel-based (225) and mucoadhesive formulations (226, 227) offer additional avenues for localized delivery. These systems can be designed to release drugs in a sustained manner and enhance adhesion to the intestinal epithelium or Peyer’s patches, improving exposure to target cells while minimizing systemic absorption (228).
Antibody-drug conjugates (ADCs) are a class of precision therapeutics that employ monoclonal antibodies to selectively bind cell surface antigens, enabling targeted delivery of potent cytotoxic agents to tumour cells (229), including those in gastrointestinal cancers (230). Advancements in this field have led to the development of next-generation ADCs, such as bispecific ADCs, Probody-drug conjugates, immunostimulatory ADCs, degrader-antibody conjugates, and dual-payload ADCs (229). A variety of HIV-targeted (e.g., Env, Tat, Vif) and host-directed (e.g., CD25, CD4, CCR5, CXCR4, IL-2R) ADCs have been investigated, employing diverse payloads such as toxins, siRNAs, radionuclides, small molecule inhibitors, photosensitisers, and lipids (231). While clinical experience with HIV-specific ADCs remains limited, the field stands to benefit from advances in cancer immunotherapy (231). Targeting HIV-infected cells in the gut using ADCs represents a promising yet underexplored strategy, particularly given the tissue’s enrichment for latent reservoir cells and parallels with gastrointestinal cancer targeting.
Beyond anatomical targeting, delivery systems are being optimized to home to specific cellular reservoirs that represent major sources of persistent HIV in the gut (232). Ligand-conjugated nanoparticles are being engineered to exploit surface markers (e.g., integrins, chemokine receptors) expressed preferentially by target populations (233). These precision-targeting approaches aim to increase therapeutic efficacy while limiting off-target effects and could be applied in the context of HIV. As latency reversal and immunomodulation strategies progress toward clinical application, the integration of advanced delivery technologies will be critical to achieving therapeutic concentrations in gut tissues and enhancing the safety and specificity of HIV cure interventions.
4 Discussion
The gut constitutes one of the most formidable barriers to HIV eradication. As the largest immune organ in the body, it contains the vast majority of lymphoid tissue and CD4+ T cells (9), rendering it both a primary target for HIV infection and a long-lived viral reservoir. Despite the efficacy of ART in suppressing plasma viremia, HIV persists within GALT due to a convergence of structural, microbial, immunological, and pharmacological barriers. Cure strategies must therefore address not only systemic viral suppression but also the unique features of the gut reservoir, including its size, immune environment, and pharmacologic challenges.
Eliminating HIV reservoirs in the gut will likely require a multipronged therapeutic approach. Latency-reversing agents (LRAs) have shown partial activity in gut-derived cells, but their efficacy is limited by poor tissue penetration and lack of potency in reversing deep latency. Immune-based interventions, such as broadly neutralizing antibodies and immune checkpoint inhibitors, hold promise for enhancing reservoir clearance, but their ability to reach and act within GALT remains to be demonstrated. Drug delivery innovations, including nanoparticle formulations and tissue-targeted vectors, may help overcome the pharmacologic barriers posed by mucosal tissues, yet require rigorous evaluation in both preclinical and clinical settings.
In parallel, gene- and cell-editing approaches are emerging as transformative strategies for targeting gut HIV reservoirs. CRISPR-based interventions, such as EBT-101, and SIV-directed precursors have demonstrated broad biodistribution to lymphoid tissues, including the gut, with preclinical evidence of proviral excision. Although early clinical studies highlight the need for greater efficacy, these findings establish proof-of-concept that gene editing can indeed reach and act within GALT. Similarly, cell-based therapies, including CCR5-edited T cells and CAR-T platforms, offer the potential to repopulate the gut with resistant or effector cells capable of directly suppressing local HIV replication. The ability of engineered cells to traffic to and persist within mucosal tissues will be a critical determinant of their long-term success.
Strategies aimed at restoring gut barrier integrity and reducing inflammation, such as FMT, statins, anti-cytokine therapies, and gut-tropic agents, may act synergistically to suppress the drivers of HIV persistence. Therapeutic vaccines capable of eliciting robust mucosal CD8+ T cell responses are another key area under development, although translating these approaches into durable immune control remains a significant hurdle.
To overcome the anatomical and pharmacologic challenges of targeting the gut reservoir, innovative drug delivery systems, including ligand-targeted nanoparticles and mucoadhesive formulations, are under active investigation. These platforms may improve tissue penetration, increase drug stability, and allow for targeted delivery to infected cells within the mucosal environment.
Together, these efforts underscore the importance of integrating genetic engineering strategies with gut-specific delivery systems, immune modulation, and barrier-restoring interventions. The next phase of HIV cure research will require assessing not only the safety and durability of gene- and cell-editing therapies but also their functional impact on gut reservoirs. As such, trials should incorporate tissue-based endpoints, including gut biopsies and molecular reservoir profiling, to determine whether systemic interventions translate into meaningful reductions in mucosal reservoirs.
Looking ahead, several key knowledge gaps remain. The field would benefit from validated biomarkers of gut reservoir size and activity to assess therapeutic efficacy. Furthermore, most clinical trials do not include tissue-based endpoints, limiting our understanding of how interventions affect HIV persistence outside the peripheral blood. Longitudinal studies incorporating tissue pharmacokinetics, host immune responses, and microbiome dynamics are critical to informing rational therapeutic design. Ultimately, integration of multi-modal strategies targeting latency, inflammation, immune dysfunction, and mucosal damage will likely be necessary to achieve durable reductions in the gut HIV reservoir.
In conclusion, the gut represents a uniquely challenging and significant reservoir for latent HIV despite the clinical effectiveness of ART. Rational combination therapies, guided by mechanistic insights and empowered by advanced delivery platforms, offer a promising path forward in the endeavour to eliminate HIV reservoirs in the gut. However, success will depend on continued investment in tissue-based research and the development of clinical tools to measure and target HIV persistence at this critical site.
Author contributions
JL: Writing – review & editing, Writing – original draft, Conceptualization. SL: Resources, Writing – review & editing, Conceptualization. ST: Resources, Funding acquisition, Writing – review & editing, Conceptualization, Writing – original draft, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Doherty Institute for Infection and Immunity Locarnini Fellowship in Virology (S.T.), University of Melbourne Department of Infectious Diseases Research Support Package (S.T.), and Gilead Australia Fellowship (S.T.). J.L is supported by an NHMRC Emerging Leadership Grant (APP2034489). S.R.L. is supported by a National Institute of Allergy and Infectious Disease of the National Institutes of Health award (UM1AI164560) and grants from the National Health and Medical Research Council (NHMRC) including a Program Grant (APP1149990), Practitioner Fellowship (APP1135851) and Investigator Grant (APP2026490).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. (2008) 197:714–20. doi: 10.1086/527324
2. Olsson J, Poles M, Spetz AL, Elliott J, Hultin L, Giorgi J, et al. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J Infect Dis. (2000) 182:1625–35. doi: 10.1086/317625
3. Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. (2000) 14:1761–5. doi: 10.1097/00002030-200008180-00011
4. Poles MA, Elliott J, Taing P, Anton PA, and Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol. (2001) 75:8390–9. doi: 10.1128/JVI.75.18.8390-8399.2001
5. Thompson CG, Cohen MS, and Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. (2013) 63 Suppl 2:S240–7. doi: 10.1097/QAI.0b013e3182986ff8
6. Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. (2014) 67:481–6. doi: 10.1097/QAI.0000000000000301
7. McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV. (2016) 3:e569–e78. doi: 10.1016/S2352-3018(16)30113-8
8. Masia M, Fernandez-Gonzalez M, Ledesma C, Losada-Echeberria M, Gonzalo-Jimenez N, Mascarell P, et al. Impact of switching to long-acting injectable cabotegravir plus rilpivirine on rectal HIV-1 RNA shedding and implications for transmission risk. J Infect Dis. (2025) 231:e792–802. doi: 10.1093/infdis/jiaf117
9. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. (2003) 3:331–41. doi: 10.1038/nri1057
10. Mowat AM and Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. (2014) 14:667–85. doi: 10.1038/nri3738
11. Morbe UM, Jorgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. (2021) 14:793–802. doi: 10.1038/s41385-021-00389-4
12. Kooij IA, Sahami S, Meijer SL, Buskens CJ, and Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. (2016) 186:1–9. doi: 10.1111/cei.12821
13. Farris AB, Lauwers GY, Ferry JA, and Zukerberg LR. The rectal tonsil: a reactive lymphoid proliferation that may mimic lymphoma. Am J Surg Pathol. (2008) 32:1075–9. doi: 10.1097/PAS.0b013e318162c3ec
14. Hong JB, Kim HW, Kang DH, Choi CW, Park SB, Kim DJ, et al. Rectal tonsil: a case report and literature review. World J Gastroenterol. (2015) 21:2563–7. doi: 10.3748/wjg.v21.i8.2563
15. Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, and Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group Gut. (1995) 37:524–9. doi: 10.1136/gut.37.4.524
16. Clayton F, Snow G, Reka S, and Kotler DP. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. (1997) 107:288–92. doi: 10.1111/j.1365-2249.1997.236-ce1111.x
17. Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. (2004) 200:761–70. doi: 10.1084/jem.20041196
18. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. (2004) 200:749–59. doi: 10.1084/jem.20040874
19. Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, and Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. (1998) 72:6646–56. doi: 10.1128/JVI.72.8.6646-6656.1998
20. Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, et al. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. (1999) 116:1115–23. doi: 10.1016/S0016-5085(99)70014-4
21. Vajdy M, Veazey R, Tham I, deBakker C, Westmoreland S, Neutra M, et al. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J Infect Dis. (2001) 184:1007–14. doi: 10.1086/323615
22. Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. (2003) 77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003
23. Veazey RS, Marx PA, and Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. (2003) 187:769–76. doi: 10.1086/368386
24. Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PloS Pathog. (2014) 10:e1004543. doi: 10.1371/journal.ppat.1004543
25. Xu H, Wang X, and Veazey RS. Th17 cells coordinate with th22 cells in maintaining homeostasis of intestinal tissues and both are depleted in SIV-infected macaques. J AIDS Clin Res. (2014) 5(5):302. doi: 10.4172/2155-6113.1000302
26. Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. (1999) 179:859–70. doi: 10.1086/314660
27. Grossman Z, Meier-Schellersheim M, Paul WE, and Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. (2006) 12:289–95. doi: 10.1038/nm1380
28. Ameisen JC and Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. (1991) 12:102–5. doi: 10.1016/0167-5699(91)90092-8
29. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. (2006) 12:1365–71. doi: 10.1038/nm1511
30. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PloS Pathog. (2010) 6:e1000852. doi: 10.1371/journal.ppat.1000852
31. Assimakopoulos SF, Dimitropoulou D, Marangos M, and Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection. (2014) 42:951–9. doi: 10.1007/s15010-014-0666-5
32. Fenton TM, Jorgensen PB, Niss K, Rubin SJS, Morbe UM, Riis LB, et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity. (2020) 52:557–70 e6. doi: 10.1016/j.immuni.2020.02.001
33. Senda T, Dogra P, Granot T, Furuhashi K, Snyder ME, Carpenter DJ, et al. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. (2019) 12:378–89. doi: 10.1038/s41385-018-0110-8
34. Yukl SA, Shergill AK, Girling V, Li Q, Killian M, Epling L, et al. Site-specific differences in T cell frequencies and phenotypes in the blood and gut of HIV-uninfected and ART-treated HIV+ adults. PloS One. (2015) 10:e0121290. doi: 10.1371/journal.pone.0121290
35. Xie G, Moron-Lopez S, Siegel DA, Yin K, Polos A, Cohen J, et al. Common and divergent features of T cells from blood, gut, and genital tract of antiretroviral therapy-treated HIV(+) women. J Immunol. (2022) 208:1790–801. doi: 10.4049/jimmunol.2101102
36. Telwatte S, Lee S, Somsouk M, Hatano H, Baker C, Kaiser P, et al. Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PloS Pathog. (2018) 14:e1007357. doi: 10.1371/journal.ppat.1007357
37. Yukl SA, Sinclair E, Somsouk M, Hunt PW, Epling L, Killian M, et al. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS. (2014) 28:439–42. doi: 10.1097/QAD.0000000000000166
38. Moron-Lopez S, Xie G, Kim P, Siegel DA, Lee S, Wong JK, et al. Tissue-specific differences in HIV DNA levels and mechanisms that govern HIV transcription in blood, gut, genital tract and liver in ART-treated women. J Int AIDS Soc. (2021) 24:e25738. doi: 10.1002/jia2.25738
39. Telwatte S, Kim P, Chen TH, Milush JM, Somsouk M, Deeks SG, et al. Mechanistic differences underlying HIV latency in the gut and blood contribute to differential responses to latency-reversing agents. AIDS. (2020) 34:2013–24. doi: 10.1097/QAD.0000000000002684
40. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. (2014) 343:1249288. doi: 10.1126/science.1249288
41. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. (2011) 331:337–41. doi: 10.1126/science.1198469
42. Shao T, Hsu R, Rafizadeh DL, Wang L, Bowlus CL, Kumar N, et al. The gut ecosystem and immune tolerance. J Autoimmun. (2023) 141:103114. doi: 10.1016/j.jaut.2023.103114
43. Khan S, Telwatte S, Trapecar M, Yukl S, and Sanjabi S. Differentiating immune cell targets in gut-associated lymphoid tissue for HIV cure. AIDS Res Hum Retroviruses. (2017) 33:S40–58. doi: 10.1089/aid.2017.0153
44. Nayrac M, Requena M, Loiseau C, Cazabat M, Suc B, Carrere N, et al. Th22 cells are efficiently recruited in the gut by CCL28 as an alternative to CCL20 but do not compensate for the loss of Th17 cells in treated HIV-1-infected individuals. Mucosal Immunol. (2021) 14:219–28. doi: 10.1038/s41385-020-0286-6
45. Wiche Salinas TR, Zhang Y, Sarnello D, Zhyvoloup A, Marchand LR, Fert A, et al. Th17 cell master transcription factor RORC2 regulates HIV-1 gene expression and viral outgrowth. Proc Natl Acad Sci U S A. (2021) 118(48):e2105927118. doi: 10.1073/pnas.2105927118
46. Sonnenberg GF, Fouser LA, and Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. (2011) 12:383–90. doi: 10.1038/ni.2025
47. Anderson JL, Khoury G, Fromentin R, Solomon A, Chomont N, Sinclair E, et al. Human immunodeficiency virus (HIV)-infected CCR6+ Rectal CD4+ T cells and HIV persistence on antiretroviral therapy. J Infect Dis. (2020) 221:744–55. doi: 10.1093/infdis/jiz509
48. Wacleche VS, Goulet JP, Gosselin A, Monteiro P, Soudeyns H, Fromentin R, et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology. (2016) 13:59. doi: 10.1186/s12977-016-0293-6
49. Maek ANW, Buranapraditkun S, Klaewsongkram J, and Ruxrungtham K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. (2007) 20:66–75. doi: 10.1089/vim.2006.0063
50. Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. (2010) 207:553–64. doi: 10.1084/jem.20090858
51. Reina-Campos M, Monell A, Ferry A, Luna V, Cheung KP, Galletti G, et al. Tissue-resident memory CD8 T cell diversity is spatiotemporally imprinted. Nature. (2025) 639:483–92. doi: 10.1038/s41586-024-08466-x
52. Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. (1994) 372:190–3. doi: 10.1038/372190a0
53. Zhang N and Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. (2013) 39:687–96. doi: 10.1016/j.immuni.2013.08.019
54. Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. (2017) 20:2921–34. doi: 10.1016/j.celrep.2017.08.078
55. Yukl SA, Khan S, Chen TH, Trapecar M, Wu F, Xie G, et al. Shared mechanisms govern HIV transcriptional suppression in circulating CD103(+) and gut CD4(+) T cells. J Virol. (2020) 95(2):e01331–20. doi: 10.1128/JVI.01331-20
56. Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. (2016) 352:459–63. doi: 10.1126/science.aad2035
57. Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. (2017) 46:287–300. doi: 10.1016/j.immuni.2017.01.009
58. Soares LR, Tsavaler L, Rivas A, and Engleman EG. V7 (CD101) ligation inhibits TCR/CD3-induced IL-2 production by blocking Ca2+ flux and nuclear factor of activated T cell nuclear translocation. J Immunol. (1998) 161:209–17. doi: 10.4049/jimmunol.161.1.209
59. Fan J, Bass HZ, and Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. (1993) 151:5031–40. doi: 10.4049/jimmunol.151.9.5031
60. Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. (1990) 144:480–4. doi: 10.4049/jimmunol.144.2.480
61. Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. (2010) 2:32ra6. doi: 10.1126/scitranslmed.3000632
62. Noel N, Boufassa F, Lecuroux C, Saez-Cirion A, Bourgeois C, Dunyach-Remy C, et al. Elevated IP10 levels are associated with immune activation and low CD4(+) T-cell counts in HIV controller patients. AIDS. (2014) 28:467–76. doi: 10.1097/QAD.0000000000000174
63. Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. (2007) 81:599–612. doi: 10.1128/JVI.01739-06
64. Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. (2011) 25:1385–94. doi: 10.1097/QAD.0b013e3283471d10
65. Tudesq JJ, Dunyach-Remy C, Combescure C, Doncesco R, Laureillard D, Lavigne JP, et al. Microbial translocation is correlated with HIV evolution in HIV-HCV co-infected patients. PloS One. (2017) 12:e0183372. doi: 10.1371/journal.pone.0183372
66. Epeldegui M, Magpantay L, Guo Y, Halec G, Cumberland WG, Yen PK, et al. A prospective study of serum microbial translocation biomarkers and risk of AIDS-related non-Hodgkin lymphoma. AIDS. (2018) 32:945–54. doi: 10.1097/QAD.0000000000001771
67. Mehraj V, Ramendra R, Isnard S, Dupuy FP, Ponte R, Chen J, et al. Circulating (1–>3)-beta-D-glucan is associated with immune activation during human immunodeficiency virus infection. Clin Infect Dis. (2020) 70:232–41. doi: 10.1093/cid/ciz212
68. Kang X, Kirui A, Muszynski A, Widanage MCD, Chen A, Azadi P, et al. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat Commun. (2018) 9:2747. doi: 10.1038/s41467-018-05199-0
69. Pinto-Cardoso S, Lozupone C, Briceno O, Alva-Hernandez S, Tellez N, Adriana A, et al. Fecal Bacterial Communities in treated HIV infected individuals on two antiretroviral regimens. Sci Rep. (2017) 7:43741. doi: 10.1038/srep43741
70. Isnard S, Ramendra R, Dupuy FP, Lin J, Fombuena B, Kokinov N, et al. Plasma levels of C-type lectin REG3alpha and gut damage in people with human immunodeficiency virus. J Infect Dis. (2020) 221:110–21. doi: 10.1093/infdis/jiz423
71. Cassol E, Misra V, Holman A, Kamat A, Morgello S, and Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. (2013) 13:203. doi: 10.1186/1471-2334-13-203
72. Hoenigl M, de Oliveira MF, Perez-Santiago J, Zhang Y, Woods SP, Finkelman M, et al. Correlation of (1–>3)-beta-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis. (2015) 3:Doc3. doi: 10.3205/id000018
73. Ramendra R, Isnard S, Lin J, Fombuena B, Ouyang J, Mehraj V, et al. Cytomegalovirus seropositivity is associated with increased microbial translocation in people living with human immunodeficiency virus and uninfected controls. Clin Infect Dis. (2020) 71:1438–46. doi: 10.1093/cid/ciz1001
74. Paiardini M and Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. (2013) 254:78–101. doi: 10.1111/imr.12079
75. Wahl A, Yao W, Liao B, Chateau M, Richardson C, Ling L, et al. A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection. Nat Biotechnol. (2024) 42:905–15. doi: 10.1038/s41587-023-01906-5
76. Borgognone A, Noguera-Julian M, Oriol B, Noel-Romas L, Ruiz-Riol M, Guillen Y, et al. Gut microbiome signatures linked to HIV-1 reservoir size and viremia control. Microbiome. (2022) 10:59. doi: 10.1186/s40168-022-01247-6
77. Marin-Sanchez N, Paredes R, and Borgognone A. Exploring potential associations between the human microbiota and reservoir of latent HIV. Retrovirology. (2024) 21:21. doi: 10.1186/s12977-024-00655-w
78. Serrano-Villar S, Talavera-Rodriguez A, Gosalbes MJ, Madrid N, Perez-Molina JA, Elliott RJ, et al. Fecal microbiota transplantation in HIV: A pilot placebo-controlled study. Nat Commun. (2021) 12:1139. doi: 10.1038/s41467-021-21472-1
79. Tincati C, Douek DC, and Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. (2016) 13:19. doi: 10.1186/s12981-016-0103-1
80. Mudd JC and Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J Infect Dis. (2016) 214 Suppl 2:S58–66. doi: 10.1093/infdis/jiw258
81. Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. (2014) 111:2307–12. doi: 10.1073/pnas.1318249111
82. Lee SA, Telwatte S, Hatano H, Kashuba ADM, Cottrell ML, Hoh R, et al. Antiretroviral therapy concentrations differ in gut vs. Lymph node tissues and are associated with HIV viral transcription by a novel RT-ddPCR assay. J Acquir Immune Defic Syndr. (2020) 83:530–7. doi: 10.1097/QAI.0000000000002287
83. Fletcher CV, Kroon E, Schacker T, Pinyakorn S, Chomont N, Chottanapund S, et al. Persistent HIV transcription and variable antiretroviral drug penetration in lymph nodes during plasma viral suppression. AIDS. (2022) 36:985–90. doi: 10.1097/QAD.0000000000003201
84. Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Pond SLK, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. (2016) 530:51–6. doi: 10.1038/nature16933
85. Bozzi G, Simonetti FR, Watters SA, Anderson EM, Gouzoulis M, Kearney MF, et al. No evidence of ongoing HIV replication or compartmentalization in tissues during combination antiretroviral therapy: Implications for HIV eradication. Sci Adv. (2019) 5:eaav2045. doi: 10.1126/sciadv.aav2045
86. McManus WR, Bale MJ, Spindler J, Wiegand A, Musick A, Patro SC, et al. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J Clin Invest. (2019) 129:4629–42. doi: 10.1172/JCI126714
87. Landovitz RJ, Donnell D, Clement ME, Hanscom B, Cottle L, Coelho L, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. (2021) 385:595–608. doi: 10.1056/NEJMoa2101016
88. Delany-Moretlwe S, Hughes JP, Bock P, Ouma SG, Hunidzarira P, Kalonji D, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. (2022) 399:1779–89. doi: 10.1016/S0140-6736(22)00538-4
89. Thompson CG, Gay CL, and Kashuba ADM. HIV persistence in gut-associated lymphoid tissues: pharmacological challenges and opportunities. AIDS Res Hum Retroviruses. (2017) 33:513–23. doi: 10.1089/aid.2016.0253
90. Kim Y, Anderson JL, and Lewin SR. Getting the “Kill” into “Shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe. (2018) 23:14–26. doi: 10.1016/j.chom.2017.12.004
91. Tanaka K, Kim Y, Roche M, and Lewin SR. The role of latency reversal in HIV cure strategies. J Med Primatol. (2022) 51:278–83. doi: 10.1111/jmp.12613
92. Zerbato JM, Purves HV, Lewin SR, and Rasmussen TA. Between a shock and a hard place: challenges and developments in HIV latency reversal. Curr Opin Virol. (2019) 38:1–9. doi: 10.1016/j.coviro.2019.03.004
93. Ait-Ammar A, Kula A, Darcis G, Verdikt R, De Wit S, Gautier V, et al. Current status of latency reversing agents facing the heterogeneity of HIV-1 cellular and tissue reservoirs. Front Microbiol. (2019) 10:3060. doi: 10.3389/fmicb.2019.03060
94. Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. (2018) 10(439):eaao4521. doi: 10.1126/scitranslmed.aao4521
95. Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, et al. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis. (2017) 64:1686–95. doi: 10.1093/cid/cix201
96. Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, et al. Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology. (2013) 10:119. doi: 10.1186/1742-4690-10-119
97. Duan S, Xu X, Wang J, Huang L, Peng J, Yu T, et al. TLR1/2 agonist enhances reversal of HIV-1 latency and promotes NK cell-induced suppression of HIV-1-infected autologous CD4(+) T cells. J Virol. (2021) 95:e0081621. doi: 10.1128/JVI.00816-21
98. Jimenez-Leon MR, Gasca-Capote C, Tarancon-Diez L, Dominguez-Molina B, Lopez-Verdugo M, Ritraj R, et al. Toll-like receptor agonists enhance HIV-specific T cell response mediated by plasmacytoid dendritic cells in diverse HIV-1 disease progression phenotypes. EBioMedicine. (2023) 91:104549. doi: 10.1016/j.ebiom.2023.104549
99. Krarup AR, Abdel-Mohsen M, Schleimann MH, Vibholm L, Engen PA, Dige A, et al. The TLR9 agonist MGN1703 triggers a potent type I interferon response in the sigmoid colon. Mucosal Immunol. (2018) 11:449–61. doi: 10.1038/mi.2017.59
100. Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. (2018) 563:360–4. doi: 10.1038/s41586-018-0600-6
101. SenGupta D, Brinson C, DeJesus E, Mills A, Shalit P, Guo S, et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci Transl Med. (2021) 13(599):eabg3071. doi: 10.1126/scitranslmed.abg3071
102. Li Y, Wang Z, Hou Y, Liu X, Hong J, Shi X, et al. Novel TLR7/8 agonists promote activation of HIV-1 latent reservoirs and human T and NK cells. Front Microbiol. (2023) 14:1033448. doi: 10.3389/fmicb.2023.1033448
103. Bjerg Christensen A, Dige A, Vad-Nielsen J, Brinkmann CR, Bendix M, Ostergaard L, et al. Administration of panobinostat is associated with increased IL-17A mRNA in the intestinal epithelium of HIV-1 patients. Mediators Inflamm. (2015) 2015:120605. doi: 10.1155/2015/120605
104. Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PloS Pathog. (2014) 10:e1004473. doi: 10.1371/journal.ppat.1004473
105. Niu Q, Liu Z, Alamer E, Fan X, Chen H, Endsley J, et al. Structure-guided drug design identifies a BRD4-selective small molecule that suppresses HIV. J Clin Invest. (2019) 129:3361–73. doi: 10.1172/JCI120633
106. Joshi P and Stoddart CA. Impaired infectivity of ritonavir-resistant HIV is rescued by heat shock protein 90AB1. J Biol Chem. (2011) 286:24581–92. doi: 10.1074/jbc.M111.248021
107. Anderson I, Low JS, Weston S, Weinberger M, Zhyvoloup A, Labokha AA, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. (2014) 111:E1528–37. doi: 10.1073/pnas.1320178111
108. Kim H, Choi MS, Inn KS, and Kim BJ. Inhibition of HIV-1 reactivation by a telomerase-derived peptide in a HSP90-dependent manner. Sci Rep. (2016) 6:28896. doi: 10.1038/srep28896
109. Christ F, Voet A, Marchand A, Nicolet S, Desimmie BA, Marchand D, et al. Rational design of small-molecule inhibitors of the LEDGF/p75-integrase interaction and HIV replication. Nat Chem Biol. (2010) 6:442–8. doi: 10.1038/nchembio.370
110. Christ F, Shaw S, Demeulemeester J, Desimmie BA, Marchand A, Butler S, et al. Small-molecule inhibitors of the LEDGF/p75 binding site of integrase block HIV replication and modulate integrase multimerization. Antimicrob Agents Chemother. (2012) 56:4365–74. doi: 10.1128/AAC.00717-12
111. Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, and SChinazi RF. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob Agents Chemother. (2014) 58:1977–86. doi: 10.1128/AAC.02496-13
112. Gavegnano C, Brehm JH, Dupuy FP, Talla A, Ribeiro SP, Kulpa DA, et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PloS Pathog. (2017) 13:e1006740. doi: 10.1371/journal.ppat.1006740
113. Li C, Mousseau G, and Valente ST. Tat inhibition by didehydro-Cortistatin A promotes heterochromatin formation at the HIV-1 long terminal repeat. Epigenet Chromatin. (2019) 12:23. doi: 10.1186/s13072-019-0267-8
114. Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “Block-and-lock” Strategy for HIV-1 treatment. Cell Rep. (2017) 21:600–11. doi: 10.1016/j.celrep.2017.09.080
115. Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. (1997) 151:97–110.
116. Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, and Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. (1994) 153:517–28. doi: 10.4049/jimmunol.153.2.517
117. Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. (2008) 9:301–9. doi: 10.1038/ni1566
118. Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. (2009) 106:20877–82. doi: 10.1073/pnas.0911796106
119. Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. (2009) 2:439–49. doi: 10.1038/mi.2009.90
120. Wang X, Xu H, Gill AF, Pahar B, Kempf D, Rasmussen T, et al. Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. (2009) 2:518–26. doi: 10.1038/mi.2009.104
121. Martinelli E, Veglia F, Goode D, Guerra-Perez N, Aravantinou M, Arthos J, et al. The frequency of alpha(4)beta(7)(high) memory CD4(+) T cells correlates with susceptibility to rectal simian immunodeficiency virus infection. J Acquir Immune Defic Syndr. (2013) 64:325–31. doi: 10.1097/QAI.0b013e31829f6e1a
122. Uzzan M, Tokuyama M, Rosenstein AK, Tomescu C, SahBandar IN, Ko HM, et al. Anti-alpha4beta7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci Transl Med. (2018) 10(461):eaau4711. doi: 10.1126/scitranslmed.aau4711
123. Tenner-Racz K, Stellbrink HJ, van Lunzen J, Schneider C, Jacobs JP, Raschdorff B, et al. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med. (1998) 187:949–59. doi: 10.1084/jem.187.6.949
124. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. (2012) 122:3281–94. doi: 10.1172/JCI63039
125. Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. (1996) 274:985–9. doi: 10.1126/science.274.5289.985
126. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. (2015) 21:132–9. doi: 10.1038/nm.3781
127. Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, et al. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science. (2016) 354:197–202. doi: 10.1126/science.aag1276
128. Berg J. Editorial expression of concern. Science. (2019) 363:1406. doi: 10.1126/science.aax2933
129. Abbink P, Mercado NB, Nkolola JP, Peterson RL, Tuyishime H, McMahan K, et al. Lack of therapeutic efficacy of an antibody to alpha(4)beta(7) in SIVmac251-infected rhesus macaques. Science. (2019) 365:1029–33. doi: 10.1126/science.aaw8562
130. Sneller MC, Clarridge KE, Seamon C, Shi V, Zorawski MD, Justement JS, et al. An open-label phase 1 clinical trial of the anti-alpha(4)beta(7) monoclonal antibody vedolizumab in HIV-infected individuals. Sci Transl Med. (2019) 11(509):eaax3447. doi: 10.1126/scitranslmed.aax3447
131. Jimenez-Leon MR, Gasca-Capote C, Roca-Oporto C, Espinosa N, Sobrino S, Fontillon-Alberdi M, et al. Vedolizumab and ART in recent HIV-1 infection unveil the role of alpha4beta7 in reservoir size. JCI Insight. (2024) 9(16):e182312. doi: 10.1172/jci.insight.182312
132. Mavigner M, Cazabat M, Dubois M, L’Faqihi FE, Requena M, Pasquier C, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. (2012) 122:62–9. doi: 10.1172/JCI59011
133. Lamb CA, O’Byrne S, Keir ME, and Butcher EC. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J Crohns Colitis. (2018) 12(suppl_2):S653–S68. doi: 10.1093/ecco-jcc/jjy060
134. Gubser C, Chiu C, Lewin SR, and Rasmussen TA. Immune checkpoint blockade in HIV. EBioMedicine. (2022) 76:103840. doi: 10.1016/j.ebiom.2022.103840
135. Van der Sluis RM, Kumar NA, Pascoe RD, Zerbato JM, Evans VA, Dantanarayana AI, et al. Combination immune checkpoint blockade to reverse HIV latency. J Immunol. (2020) 204:1242–54. doi: 10.4049/jimmunol.1901191
136. Gay CL, Bosch RJ, McKhann A, Moseley KF, Wimbish CL, Hendrickx SM, et al. Suspected immune-related adverse events with an anti-PD-1 inhibitor in otherwise healthy people with HIV. J Acquir Immune Defic Syndr. (2021) 87:e234–e6. doi: 10.1097/QAI.0000000000002716
137. Miller JS, Davis ZB, Helgeson E, Reilly C, Thorkelson A, Anderson J, et al. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat Med. (2022) 28:392–400. doi: 10.1038/s41591-021-01651-9
138. Garrido C, Abad-Fernandez M, Tuyishime M, Pollara JJ, Ferrari G, Soriano-Sarabia N, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. (2018) 92(12):e00235-18. doi: 10.1128/JVI.00235-18
139. Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv. (2018) 2:76–84. doi: 10.1182/bloodadvances.2017012971
140. Webb GM, Molden J, Busman-Sahay K, Abdulhaqq S, Wu HL, Weber WC, et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PloS Pathog. (2020) 16:e1008339. doi: 10.1371/journal.ppat.1008339
141. Wallace Z, Singh PK, and Dorrell L. Combination strategies to durably suppress HIV-1: Soluble T cell receptors. J Virus Erad. (2022) 8:100082. doi: 10.1016/j.jve.2022.100082
142. Graf EH, Pace MJ, Peterson BA, Lynch LJ, Chukwulebe SB, Mexas AM, et al. Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance in vitro and can be detected in vivo [corrected. PloS One. (2013) 8:e71879. doi: 10.1371/journal.pone.0071879
143. Wu G, Zuck P, Goh SL, Milush JM, Vohra P, Wong JK, et al. Gag p24 is a marker of human immunodeficiency virus expression in tissues and correlates with immune response. J Infect Dis. (2021) 224:1593–8. doi: 10.1093/infdis/jiab121
144. Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. (2021) 385:1196–206. doi: 10.1056/NEJMoa2103485
145. Hoang TN and Paiardini M. Role of cytokine agonists and immune checkpoint inhibitors toward HIV remission. Curr Opin HIV AIDS. (2019) 14:121–8. doi: 10.1097/COH.0000000000000528
146. Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. (2016) 9:458–67. doi: 10.1038/mi.2015.75
147. Ibrahim A, Mohamady Farouk Abdalsalam N, Liang Z, Kashaf Tariq H, Li R, L OA, et al. MDSC checkpoint blockade therapy: a new breakthrough point overcoming immunosuppression in cancer immunotherapy. Cancer Gene Ther. (2025) 32:371–92. doi: 10.1038/s41417-025-00886-9
148. Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. (2014) 158:989–99. doi: 10.1016/j.cell.2014.07.043
149. Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1. vivo Sci. (2016) 352:1001–4. doi: 10.1126/science.aaf1279
150. Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. (2017) 543:559–63. doi: 10.1038/nature21435
151. Julg B, Stephenson KE, Wagh K, Tan SC, Zash R, Walsh S, et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat Med. (2022) 28:1288–96. doi: 10.1038/s41591-022-01815-1
152. Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. (2018) 561:479–84. doi: 10.1038/s41586-018-0531-2
153. Suphaphiphat K, Desjardins D, Lorin V, Dimant N, Bouchemal K, Bossevot L, et al. Mucosal application of the broadly neutralizing antibody 10–1074 protects macaques from cell-associated SHIV vaginal exposure. Nat Commun. (2023) 14:6224. doi: 10.1038/s41467-023-41966-4
154. Lee MJ, Collins S, Babalis D, Johnson N, Falaschetti E, Prevost AT, et al. The RIO trial: rationale, design, and the role of community involvement in a randomised placebo-controlled trial of antiretroviral therapy plus dual long-acting HIV-specific broadly neutralising antibodies (bNAbs) in participants diagnosed with recent HIV infection-study protocol for a two-stage randomised phase II trial. Trials. (2022) 23:263. doi: 10.1186/s13063-022-06151-w
155. Brenchley JM and Serrano-Villar S. From dysbiosis to defense: harnessing the gut microbiome in HIV/SIV therapy. Microbiome. (2024) 12:113. doi: 10.1186/s40168-024-01825-w
156. Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, et al. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. (2015) 211:780–90. doi: 10.1093/infdis/jiu515
157. Kyosiimire-Lugemwa J, Anywaine Z, Abaasa A, Levin J, Gombe B, Musinguzi K, et al. Effect of stopping cotrimoxazole preventive therapy on microbial translocation and inflammatory markers among human immunodeficiency virus-infected Ugandan adults on antiretroviral therapy: the COSTOP trial immunology substudy. J Infect Dis. (2020) 222:381–90. doi: 10.1093/infdis/jiz494
158. Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. (2015) 68:256–63. doi: 10.1097/QAI.0000000000000468
159. Serrano-Villar S, Vazquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando-Martinez S, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. (2017) 10:1279–93. doi: 10.1038/mi.2016.122
160. Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. (2011) 4:554–63. doi: 10.1038/mi.2011.15
161. Serrano-Villar S, de Lagarde M, Vazquez-Castellanos J, Vallejo A, Bernadino JI, Madrid N, et al. Effects of immunonutrition in advanced human immunodeficiency virus disease: A randomized placebo-controlled clinical trial (Promaltia study). Clin Infect Dis. (2019) 68:120–30. doi: 10.1093/cid/ciy414
162. Sainz T, Gosalbes MJ, Talavera-Rodriguez A, Jimenez-Hernandez N, Prieto L, Escosa L, et al. Effect of a nutritional intervention on the intestinal microbiota of vertically HIV-infected children: the pediabiota study. Nutrients. (2020) 12(7):2112. doi: 10.3390/nu12072112
163. Sainz T, Diaz L, Rojo D, Clemente MI, Barbas C, Gosalbes MJ, et al. Targeting the gut microbiota of vertically HIV-infected children to decrease inflammation and immunoactivation: A pilot clinical trial. Nutrients. (2022) 14(5):992. doi: 10.3390/nu14050992
164. Dempsey E and Corr SC. Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front Immunol. (2022) 13:840245. doi: 10.3389/fimmu.2022.840245
165. Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. (2022) 386:220–9. doi: 10.1056/NEJMoa2106516
166. Orenstein R, Dubberke ER, Khanna S, Lee CH, Yoho D, Johnson S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. (2022) 22:245. doi: 10.1186/s12879-022-07256-y
167. Missailidis C, Sorensen N, Ashenafi S, Amogne W, Kassa E, Bekele A, et al. Vitamin D and phenylbutyrate supplementation does not modulate gut derived immune activation in HIV-1. Nutrients. (2019) 11(7):1675. doi: 10.3390/nu11071675
168. Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS. (2017) 31:511–21. doi: 10.1097/QAD.0000000000001366
169. Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol. (2017) 199:2976–84. doi: 10.4049/jimmunol.1700105
170. Vujkovic-Cvijin I, Rutishauser RL, Pao M, Hunt PW, Lynch SV, McCune JM, et al. Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut Microbes. (2017) 8:440–50. doi: 10.1080/19490976.2017.1334034
171. Utay NS, Monczor AN, Somasunderam A, Lupo S, Jiang ZD, Alexander AS, et al. Evaluation of six weekly oral fecal microbiota transplants in people with HIV. Pathog Immun. (2020) 5:364–81. doi: 10.20411/pai.v5i1.388
172. Diaz-Garcia C, Moreno E, Talavera-Rodriguez A, Martin-Fernandez L, Gonzalez-Bodi S, Martin-Pedraza L, et al. Fecal microbiota transplantation alters the proteomic landscape of inflammation in HIV: identifying bacterial drivers. Microbiome. (2024) 12:214. doi: 10.1186/s40168-024-01919-5
173. Hussain SK, Golozar A, Widney DP, Rappocciolo G, Penugonda S, Bream JH, et al. Effect of statin use on inflammation and immune activation biomarkers in HIV-infected persons on effective antiretroviral therapy. AIDS Res Hum Retroviruses. (2021) 37:357–67. doi: 10.1089/aid.2020.0127
174. Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. (2011) 203:756–64. doi: 10.1093/infdis/jiq115
175. Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. (2015) 68:396–404. doi: 10.1097/QAI.0000000000000478
176. Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. (2017) 31:797–806. doi: 10.1097/QAD.0000000000001427
177. Soare AY, Durham ND, Gopal R, Tweel B, Hoffman KW, Brown JA, et al. P2X antagonists inhibit HIV-1 productive infection and inflammatory cytokines interleukin-10 (IL-10) and IL-1beta in a human tonsil explant model. J Virol. (2019) 93(1):e01186-18. doi: 10.1128/JVI.01186-18
178. Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, et al. IL-1beta inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. (2018) 72:2809–11. doi: 10.1016/j.jacc.2018.09.038
179. Beltran B, Nos P, Bastida G, Iborra M, Hoyos M, and Ponce J. Safe and effective application of anti-TNF-alpha in a patient infected with HIV and concomitant Crohn’s disease. Gut. (2006) 55:1670–1. doi: 10.1136/gut.2006.101386
180. Gallitano SM, McDermott L, Brar K, and Lowenstein E. Use of tumor necrosis factor (TNF) inhibitors in patients with HIV/AIDS. J Am Acad Dermatol. (2016) 74:974–80. doi: 10.1016/j.jaad.2015.11.043
181. Funderburg NT, Shive CL, Chen Z, Tatsuoka C, Bowman ER, Longenecker CT, et al. Interleukin 6 blockade with tocilizumab diminishes indices of inflammation that are linked to mortality in treated human immunodeficiency virus infection. Clin Infect Dis. (2023) 77:272–9. doi: 10.1093/cid/ciad199
182. Sarnelli G, Seguella L, Pesce M, Lu J, Gigli S, Bruzzese E, et al. HIV-1 Tat-induced diarrhea is improved by the PPARalpha agonist, palmitoylethanolamide, by suppressing the activation of enteric glia. J Neuroinflamm. (2018) 15:94. doi: 10.1186/s12974-018-1126-4
183. Boisvert M, Cote S, Vargas A, Pasvanis S, Bounou S, Barbeau B, et al. PGJ2 antagonizes NF-kappaB-induced HIV-1 LTR activation in colonic epithelial cells. Virology. (2008) 380:1–11. doi: 10.1016/j.virol.2008.07.023
184. Zhang DY, Zhu L, Liu HN, Tseng YJ, Weng SQ, Liu TT, et al. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease. Drug Des Devel Ther. (2019) 13:2249–70. doi: 10.2147/DDDT.S207277
185. Bahraoui E, Briant L, and Chazal N. E5564 inhibits immunosuppressive cytokine IL-10 induction promoted by HIV-1 Tat protein. Virol J. (2014) 11:214. doi: 10.1186/s12985-014-0214-z
186. Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, and Fogelman AM. Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol. (2010) 30:164–8. doi: 10.1161/ATVBAHA.109.187518
187. Chattopadhyay A, Grijalva V, Hough G, Su F, Mukherjee P, Farias-Eisner R, et al. Efficacy of tomato concentrates in mouse models of dyslipidemia and cancer. Pharmacol Res Perspect. (2015) 3:e00154. doi: 10.1002/prp2.154
188. Chattopadhyay A, Yang X, Mukherjee P, Sulaiman D, Fogelman HR, Grijalva V, et al. Treating the intestine with oral apoA-I mimetic tg6F reduces tumor burden in mouse models of metastatic lung cancer. Sci Rep. (2018) 8:9032. doi: 10.1038/s41598-018-26755-0
189. Daskou M, Mu W, Sharma M, Vasilopoulos H, Heymans R, Ritou E, et al. ApoA-I mimetics reduce systemic and gut inflammation in chronic treated HIV. PloS Pathog. (2022) 18:e1010160. doi: 10.1371/journal.ppat.1010160
190. Ranasinghe C, Trivedi S, Stambas J, and Jackson RJ. Unique IL-13Ralpha2-based HIV-1 vaccine strategy to enhance mucosal immunity, CD8(+) T-cell avidity and protective immunity. Mucosal Immunol. (2013) 6:1068–80. doi: 10.1038/mi.2013.1
191. Ruane D, Do Y, Brane L, Garg A, Bozzacco L, Kraus T, et al. A dendritic cell targeted vaccine induces long-term HIV-specific immunity within the gastrointestinal tract. Mucosal Immunol. (2016) 9:1340–52. doi: 10.1038/mi.2015.133
192. Cohn L and Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. (2014) 5:255. doi: 10.3389/fimmu.2014.00255
193. Chamcha V, Jones A, Quigley BR, Scott JR, and Amara RR. Oral immunization with a recombinant lactococcus lactis-expressing HIV-1 antigen on group A streptococcus pilus induces strong mucosal immunity in the gut. J Immunol. (2015) 195:5025–34. doi: 10.4049/jimmunol.1501243
194. D’Haese S, den Roover S, Verbeke R, Aernout I, Meulewater S, Cosyns J, et al. The role of mRNA-galsomes and LNPs in enhancing HIV-specific T cell responses across various lymphoid organs. Mol Ther Nucleic Acids. (2024) 35(4):102372. doi: 10.1016/j.omtn.2024.102372
195. Willis JR, Prabhakaran M, Muthui M, Naidoo A, Sincomb T, Wu W, et al. Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science. (2025) 2025(6759):eadr8382. doi: 10.1126/science.adr8382
196. Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines. (2021) 6:50. doi: 10.1038/s41541-021-00307-6
197. Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. (2007) 13:46–53. doi: 10.1038/nm1520
198. Mu Z, Wiehe K, Saunders KO, Henderson R, Cain DW, Parks R, et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. (2022) 38:110514. doi: 10.1016/j.celrep.2022.110514
199. Moyo N, Vogel AB, Buus S, Erbar S, Wee EG, Sahin U, et al. Efficient Induction of T Cells against Conserved HIV-1 Regions by Mosaic Vaccines Delivered as Self-Amplifying mRNA. Mol Ther Methods Clin Dev. (2019) 12:32–46. doi: 10.1016/j.omtm.2018.10.010
200. Moyo N, Wee EG, Korber B, Bahl K, Falcone S, Himansu S, et al. Tetravalent immunogen assembled from conserved regions of HIV-1 and delivered as mRNA demonstrates potent preclinical T-cell immunogenicity and breadth. Vaccines (Basel). (2020) 8(3):360. doi: 10.3390/vaccines8030360
201. Valentin A, Bergamaschi C, Rosati M, Angel M, Burns R, Agarwal M, et al. Comparative immunogenicity of an mRNA/LNP and a DNA vaccine targeting HIV gag conserved elements in macaques. Front Immunol. (2022) 13:945706. doi: 10.3389/fimmu.2022.945706
202. Levy Y, Gahery-Segard H, Durier C, Lascaux AS, Goujard C, Meiffredy V, et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. AIDS. (2005) 19:279–86.
203. Jones T. Vacc-4x, a therapeutic vaccine comprised of four engineered peptides for the potential treatment of HIV infection. Curr Opin Investig Drugs. (2010) 11:964–70.
204. Bailon L, Molto J, Curran A, Cadinanos J, Carlos Lopez Bernaldo de Quiros J, de Los Santos I, et al. Safety, immunogenicity and effect on viral rebound of HTI vaccines combined with a TLR7 agonist in early-treated HIV-1 infection: a randomized, placebo-controlled phase 2a trial. Nat Commun. (2025) 16:2146. doi: 10.1038/s41467-025-57284-w
205. Elizalde-Torrent A, Borgognone A, Casadella M, Romero-Martin L, Escriba T, Parera M, et al. Vaccination with an HIV T-cell immunogen (HTI) using DNA primes followed by a chAdOx1-MVA boost is immunogenic in gut microbiota-depleted mice despite low IL-22 serum levels. Vaccines (Basel). (2023) 11(11):1663. doi: 10.3390/vaccines11111663
206. Burdo TH, Chen C, Kaminski R, Sariyer IK, Mancuso P, Donadoni M, et al. Preclinical safety and biodistribution of CRISPR targeting SIV in non-human primates. Gene Ther. (2024) 31:224–33. doi: 10.1038/s41434-023-00410-4
207. da Costa LC, Bomfim LM, Dittz UVT, Velozo CA, da Cunha RD, and Tanuri A. Repression of HIV-1 reactivation mediated by CRISPR/dCas9-KRAB in lymphoid and myeloid cell models. Retrovirology. (2022) 19:12. doi: 10.1186/s12977-022-00600-9
208. Cevaal PM, Kan S, Fisher BM, Moso MA, Tan A, Liu H, et al. Efficient mRNA delivery to resting T cells to reverse HIV latency. Nat Commun. (2025) 16:4979. doi: 10.1038/s41467-025-60001-2
209. Deleage C, Wietgrefe SW, Del Prete G, Morcock DR, Hao XP, Piatak M Jr., et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun. (2016) 1:68–106. doi: 10.20411/pai.v1i1.100
210. Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med. (2017) 23:1271–6. doi: 10.1038/nm.4411
211. Kennedy W. Study of EBT-101 in Aviremic HIV-1 Infected Adults on Stable ART (2025). Available online at: https://clinicaltrials.gov/study/NCT05144386 (Accessed 14 August 2025).
212. Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. (2014) 370:901–10. doi: 10.1056/NEJMoa1300662
213. Li C, Guan X, Du T, Jin W, Wu B, Liu Y, et al. Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J Gen Virol. (2015) 96:2381–93. doi: 10.1099/vir.0.000139
214. Mock U, Machowicz R, Hauber I, Horn S, Abramowski P, Berdien B, et al. mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res. (2015) 43:5560–71. doi: 10.1093/nar/gkv469
215. Li S, Holguin L, and Burnett JC. CRISPR-Cas9-mediated gene disruption of HIV-1 co-receptors confers broad resistance to infection in human T cells and humanized mice. Mol Ther Methods Clin Dev. (2022) 24:321–31. doi: 10.1016/j.omtm.2022.01.012
216. Barber-Axthelm IM, Barber-Axthelm V, Sze KY, Zhen A, Suryawanshi GW, Chen IS, et al. Stem cell-derived CAR T cells traffic to HIV reservoirs in macaques. JCI Insight. (2021) 6(1):e141502. doi: 10.1172/jci.insight.141502
217. Pampusch MS, Abdelaal HM, Cartwright EK, Molden JS, Davey BC, Sauve JD, et al. CAR/CXCR5-T cell immunotherapy is safe and potentially efficacious in promoting sustained remission of SIV infection. PloS Pathog. (2022) 18:e1009831. doi: 10.1371/journal.ppat.1009831
218. Carvalho T. First two patients receive CAR T cell therapy for HIV. Nat Med. (2023) 29:1290–1. doi: 10.1038/d41591-023-00042-6
219. Bachhav SS, Dighe VD, Kotak D, and Devarajan PV. Rifampicin Lipid-Polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int J Pharm. (2017) 532:612–22. doi: 10.1016/j.ijpharm.2017.09.040
220. Garbati P, Picco C, Magrassi R, Signorello P, Cacopardo L, Dalla Serra M, et al. Targeting the gut: A systematic review of specific drug nanocarriers. Pharmaceutics. (2024) 16(3):431. doi: 10.3390/pharmaceutics16030431
221. Kim K, Kim K, Ryu JH, and Lee H. Chitosan-catechol: a polymer with long-lasting mucoadhesive properties. Biomaterials. (2015) 52:161–70. doi: 10.1016/j.biomaterials.2015.02.010
222. Shreya AB, Raut SY, Managuli RS, Udupa N, and Mutalik S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: recent advances. AAPS PharmSciTech. (2018) 20:15. doi: 10.1208/s12249-018-1262-2
223. Asl FD, Mousazadeh M, Taji S, Bahmani A, Khashayar P, Azimzadeh M, et al. Nano drug-delivery systems for management of AIDS: liposomes, dendrimers, gold and silver nanoparticles. Nanomed (Lond). (2023) 18:279–302. doi: 10.2217/nnm-2022-0248
224. Hasan AM, Cavalu S, Kira AY, Hamad RS, Abdel-Reheim MA, Elmorsy EA, et al. Localized drug delivery in different gastrointestinal cancers: navigating challenges and advancing nanotechnological solutions. Int J Nanomed. (2025) 20:741–70. doi: 10.2147/IJN.S502833
225. Monroe MK, Wang H, Anderson CF, Qin M, Thio CL, Flexner C, et al. Antiviral supramolecular polymeric hydrogels by self-assembly of tenofovir-bearing peptide amphiphiles. Biomater Sci. (2023) 11:489–98. doi: 10.1039/D2BM01649D
226. Sarparanta MP, Bimbo LM, Makila EM, Salonen JJ, Laaksonen PH, Helariutta AM, et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials. (2012) 33:3353–62. doi: 10.1016/j.biomaterials.2012.01.029
227. Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, and Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. (2011) 3:89–100. doi: 10.4103/0975-7406.76478
228. Bachhav SS, Dighe VD, and Devarajan PV. Exploring peyer’s patch uptake as a strategy for targeted lung delivery of polymeric rifampicin nanoparticles. Mol Pharm. (2018) 15:4434–45. doi: 10.1021/acs.molpharmaceut.8b00382
229. Long R, Zuo H, Tang G, Zhang C, Yue X, Yang J, et al. Antibody-drug conjugates in cancer therapy: applications and future advances. Front Immunol. (2025) 16:1516419. doi: 10.3389/fimmu.2025.1516419
230. Singh D, Dheer D, Samykutty A, and Shankar R. Antibody drug conjugates in gastrointestinal cancer: From lab to clinical development. J Control Release. (2021) 340:1–34. doi: 10.1016/j.jconrel.2021.10.006
231. Umotoy JC and de Taeye SW. Antibody conjugates for targeted therapy against HIV-1 as an emerging tool for HIV-1 cure. Front Immunol. (2021) 12:708806. doi: 10.3389/fimmu.2021.708806
232. Jaimalai T, Meeroekyai S, Suree N, and Prangkio P. Drug delivery system targeting CD4(+) T cells for HIV-1 latency reactivation towards the viral eradication. J Pharm Sci. (2020) 109:3013–20. doi: 10.1016/j.xphs.2020.06.019
Keywords: HIV reservoir, gut-associated lymphoid tissue (GALT), HIV persistence, mucosal immunity, latency reversal, gene and cell therapies, HIV cure strategies, antiretroviral penetration
Citation: Lau JSY, Lewin SR and Telwatte S (2025) HIV and the gut: implications for HIV persistence, immune dysfunction and cure strategies. Front. Immunol. 16:1650852. doi: 10.3389/fimmu.2025.1650852
Received: 20 June 2025; Accepted: 03 September 2025;
Published: 18 September 2025.
Edited by:
Gabriella d’Ettorre, Sapienza University of Rome, ItalyReviewed by:
Letizia Santinelli, Sapienza University of Rome, ItalyDaan Pieren, Wilhelmina Children’s Hospital, Netherlands
Copyright © 2025 Lau, Lewin and Telwatte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sushama Telwatte, c3VzaGFtYS50ZWx3YXR0ZUB1bmltZWxiLmVkdS5hdQ==
 Jillian S. Y. Lau1,2,3,4
Jillian S. Y. Lau1,2,3,4 Sharon R. Lewin
Sharon R. Lewin Sushama Telwatte
Sushama Telwatte