- 1Department of Hepatobiliary Surgery, People’s Hospital of Anshun City, Anshun, Guizhou, China
- 2Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Biliary tract cancer, which includes intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and gallbladder cancer, presents a significant clinical challenge because of its aggressive nature and limited therapeutic options. Although standard chemotherapy regimens, such as gemcitabine and cisplatin, are used, the prognosis for advanced biliary tract cancer patients remains poor due to the rapid development of resistance. Recently, advancements in immunotherapy, particularly immune checkpoint inhibitors, have shown promise. However, the response rate in patients with biliary tract cancer is still suboptimal primarily because of the highly immunosuppressive tumour microenvironment. This microenvironment includes a complex network of tumour-associated macrophages, regulatory T cells, and myeloid-derived suppressor cells, all of which contribute to immune evasion. In this review, we discuss the molecular mechanisms that drive biliary tract cancer, focusing on genetic alterations and the role of the TME in immune suppression. We also examine current combination strategies that integrate immune checkpoint inhibitors with chemotherapy and targeted therapies, which have demonstrated superior efficacy over monotherapy. Furthermore, we explore emerging therapeutic approaches, such as metabolic modulation, CAR-T-cell therapy, and mRNA vaccines, which are reshaping the treatment landscape. Finally, we highlight the need for personalized treatment strategies and the development of predictive biomarkers to guide therapy selection. Future research should focus on refining these combination therapies, optimizing patient selection, and validating biomarkers to improve clinical outcomes and survival in biliary tract cancer patients.
1 Introduction
Biliary tract cancers (BTCs) are malignant tumours that originate from the biliary system, including intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder cancer (GBC). Despite its relatively low incidence globally, BTC is associated with a poor prognosis and limited treatment options. The median overall survival of patients with advanced BTC is typically less than one year (1, 2). Although traditional chemotherapy regimens, such as gemcitabine combined with cisplatin (3, 4), offer short-term relief for some patients, their efficacy is limited, and most cancers rapidly develop resistance. Therefore, new therapeutic strategies are urgently needed to improve patient survival and quality of life.
In recent years, immunotherapy, particularly immune checkpoint inhibitors (ICIs), has become a standard treatment for various malignancies and has demonstrated significant efficacy in some tumour types. However, the response of BTC to immunotherapy remains poor, with only approximately 5% of patients benefiting from single-agent immune checkpoint inhibitors (5). This low response rate is primarily due to the unique tumour microenvironment (TME) of BTC, which is highly immunosuppressive and characterized by the accumulation of tumour-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs). These immunosuppressive cells play critical roles in tumour immune escape (6). As a result, single-agent immunotherapy has limited efficacy, necessitating the exploration of combination therapies to improve treatment outcomes.
Recent studies have proposed that reshaping the TME to reduce the number of immunosuppressive cells may significantly increase the efficacy of immunotherapy (7, 8). Compared with monotherapy, combination strategies that integrate immune checkpoint inhibitors with targeted therapies and chemotherapy have shown superior efficacy. Precision combination strategies, which combine molecular targeted therapy with immunotherapy, not only enhance immune responses but also overcome tumour resistance (9–11).
2 Molecular mechanisms and immune landscape of biliary tract cancer
2.1 Molecular heterogeneity in BTC
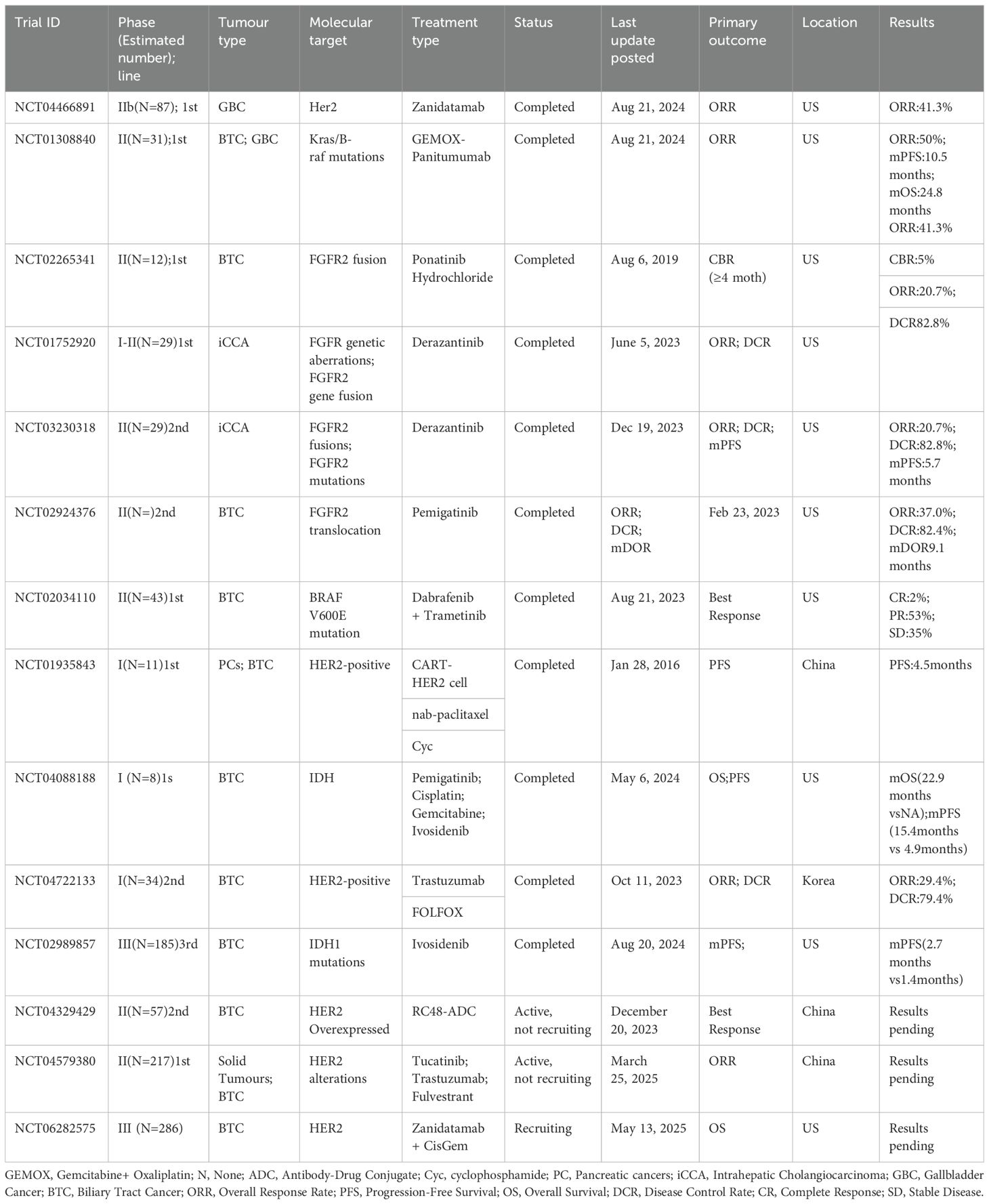
BTCs exhibit distinct genomic alterations that orchestrate oncogenic signalling and immune evasion. For example, FGFR2 fusions (≈15%) constitutively activate the MAPK/STAT3 pathways, upregulating PD-L1 via STAT3 binding to its promoter and recruiting immunosuppressive TAMs through CCL2 secretion, thereby establishing an immune-cold microenvironment (12). Similarly, IDH1/2 mutations (≈20%) drive the accumulation of (R)-2-hydroxyglutarate, which inhibits TET2-mediated demethylation and T-cell GAPDH activity, leading to impaired HLA-I antigen presentation and reduced IFN-γ production (13). In contrast, TP53 loss (35.5%) activates NF-κB-dependent IL-8 secretion, recruiting MDSCs that deplete arginine via ARG1 overexpression and deposit extracellular matrix (ECM) barriers to block T-cell infiltration (14–16). Moreover, KRAS mutations (≈27%) induce IL-8-mediated NETosis to physically trap T cells and compete for glutamine, suppressing mTOR-dependent T-cell function (17, 18). In GBC, HER2 amplification (27.2%) transfers HER2 to dendritic cells via tumour-derived exosomes, inhibiting DC maturation and antigen presentation (19), whereas SMAD4 inactivation in eCCA (11.3%) hyperactivates TGF-β signalling to directly promote Treg differentiation and collagen deposition by CAFs (20, 21). Notably, BRCA-deficient tumours (3.6%) presented elevated TMB (10.0 vs. 6.0 mut/Mb; P<0.001) and microsatellite instability (MSI-H: 17.9%), suggesting increased susceptibility to immune checkpoint blockade (22, 23). Overall, these driver mutations define molecular subtypes and offer therapeutic targets. Genomic heterogeneity stems from clonal evolution during chronic inflammation. In PSC-associated BTC specifically, TP53/KRAS mutations synergize with bile acid metabolic aberrations. This synergy drives malignancy (24, 25). Single-cell analyses revealed that ErbB pathway mutations in GBC promote tumour progression via T-cell exhaustion (26). Cellular origins further diversify ICC molecular profiles: Hepatocyte-derived iCCA frequently exhibits TERT promoter mutations, whereas cholangiocyte-derived tumours harbour BAP1 deletions (27–29). Epigenetic dysregulation (e.g., RBM10 splicing factor mutations) and homologous recombination defects (e.g., BRCA germline mutations) contribute to genomic instability (30, 31). In addition, spatial multiomics approaches, including single-cell sequencing and spatial transcriptomics, have revealed the significant intratumor heterogeneity present in BTCs. These technologies provide deeper insights into the complex cellular composition of tumours and their microenvironments, shedding light on how these heterogeneous regions influence treatment resistance (32, 33). For example, intratumor variation in immune cell infiltration and metabolic reprogramming has been linked to the development of resistance to immunotherapies and targeted therapies (33, 34). These findings underscore the importance of considering the spatial organization and functional diversity of tumours when therapeutic strategies are designed, as localized subpopulations within the tumour may exhibit differential responses to treatment (35, 36) (Figure 1).
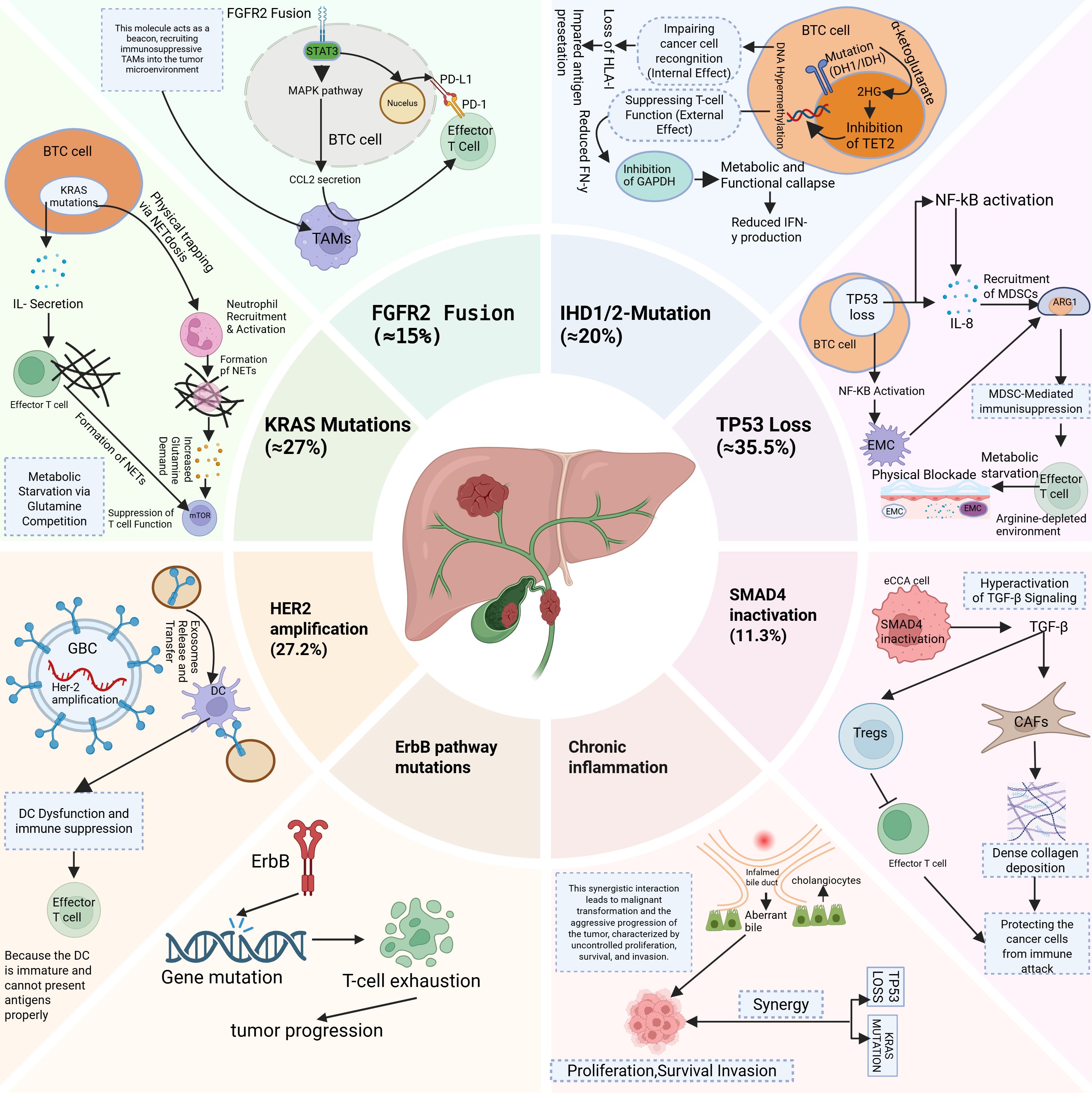
Figure 1. This diagram illustrates the genetic and molecular alterations in Biliary Tract Cancer (BTC) that contribute to immune evasion and tumour progression. BTC, Biliary tract cancer; TAMs, Tumour-associated macrophages; NETs, Neutrophil Extracellular Trap formation; MDSCs, Myeloid-derived suppressor cells; EMC, Extracellular matrix; GBC, Gallbladder cancer; DC, Dendritic cell; CAFs, Cancer-associated fibroblasts; Tregs, Regulatory T cells.
2.2 Epigenetics in BTC
In the development and progression of BTC, abnormal DNA methylation, histone modifications, and noncoding RNA regulation form a complex epigenetic network. Abnormal DNA methylation, through the collaborative action of UHRF1/DNMT1 [by recruiting the HELLS chromatin remodelling complex to facilitate the recognition of hemimethylated CpG (37)] and methylation recognition mechanisms mediated by MBD2, drives chemotherapy resistance (38) and presents genome-wide differential methylation features across anatomical subtypes (iCCA, eCCA, and GBC) (39). In clinical practice, methylation markers in bile [such as early cholangiocarcinoma (CCA) markers in PSC patients (40)], differentially methylated regions (DMRs/DHMRs) in plasma circulating free DNA (cfDNA) (41, 42), and the F12 gene CpG site (43) can serve as liquid biopsy tools, improving diagnostic accuracy for malignant biliary strictures. Targeted therapies for IDH1 mutation-associated methylation abnormalities (44) and DNMT3A overexpression [persistently present in the progression from cholelithiasis to gallbladder cancer (45, 46)] have shown potential for personalized treatment (47, 48). With respect to histone modifications, abnormal expression of the histone acetyltransferase KAT2B (49) and methyltransferase G9a [which promotes BTC invasion through the Hippo pathway LATS2/YAP regulation (50, 51)] drives tumour progression, whereas the heterochromatin protein HP1α regulates iCCA proliferation by interfering with the interferon pathway (52). Among noncoding RNAs, downregulation of circUGP2 (53) and abnormal expression of circACTN4 and cPKM [which promote chemotherapy resistance through the PKM2/β-catenin axis (38)] influence prognosis. LINC00511 (54) and miR-27a-3p [targeting the FOXO1/PI3K/AKT pathway (55, 56)] regulate tumour stem cell properties, whereas exosomal circRNAs [such as bile-derived CCA-circ1 (57)] and circNFIB [through miR-412-3p/PIK3R3 inhibition of metastasis (58)] can serve as molecular subtype biomarkers. These mechanisms, which are mediated by RNA splicing via RBM10 (59) and signalling pathways such as the PI3K/AKT pathway (60), lay the foundation for prognosis evaluation and targeted therapy development in BTC (Figure 2).
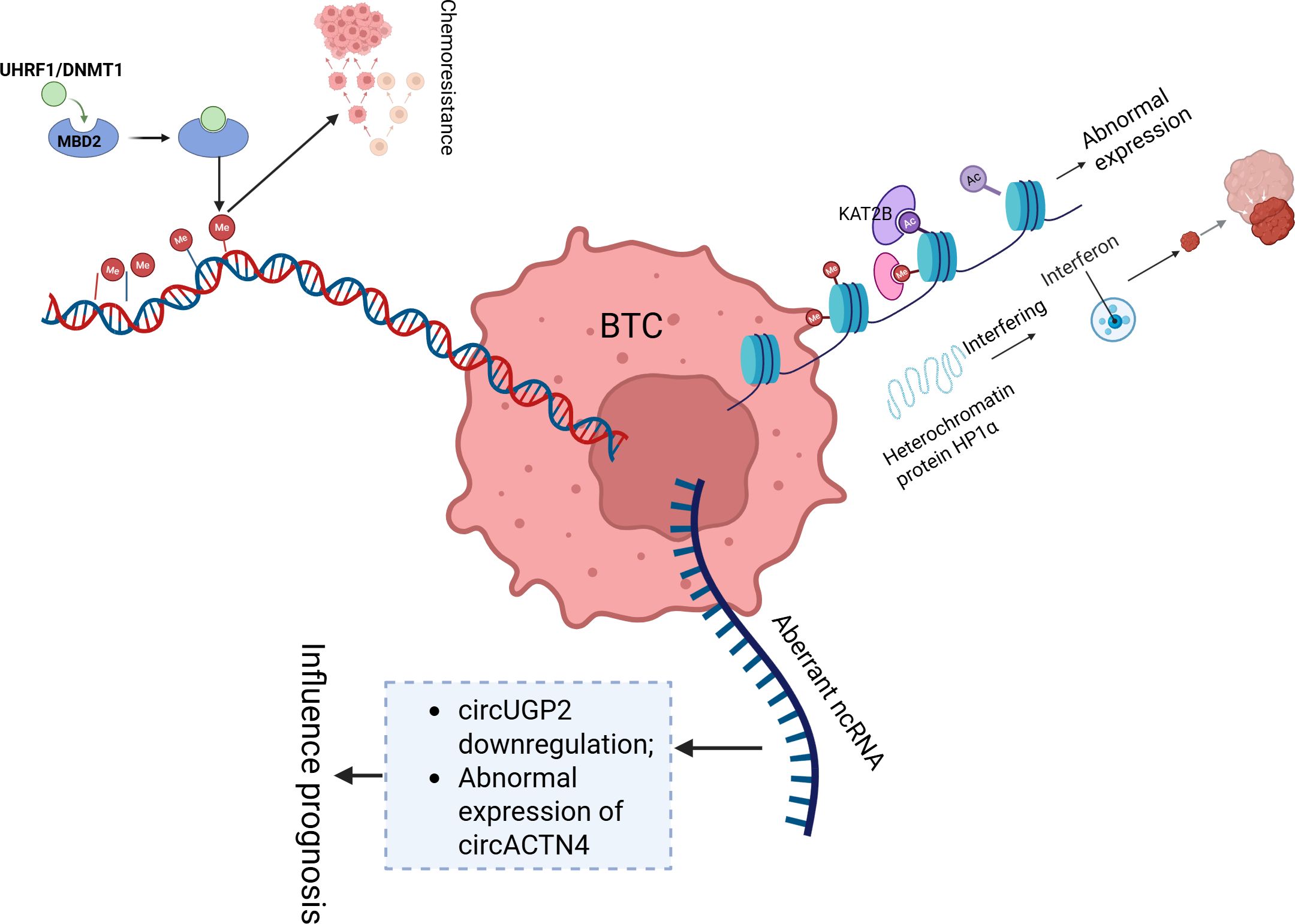
Figure 2. This diagram illustrates key epigenetic mechanisms involved in the development and progression of BTC. BTC, Biliary tract cancer; Me, Methylation; AC, Acetylation; ncRNA, Non-Coding RNA.
2.3 Changes in the tumour microenvironment
In iCCA, TAMs play a pivotal role in driving immunosuppression by upregulating PD-L1, which promotes T-cell exhaustion (61). This process is further enhanced by abnormally activated regulatory T cells (Tregs), which decrease antitumour immunity by suppressing effector T-cell function and the secretion of proinflammatory cytokines, such as IL-10 and TGF-β (62). The cross-talk between TAMs and Tregs creates a mutually reinforcing immunosuppressive loop in which TAMs secrete factors such as IL-6 and GM-CSF, which can promote Treg expansion and activation (63–65). IL-6, produced by both tumour and stromal cells, not only enhances cancer stem cell (CSC) proliferation but is also a key mediator of systemic inflammation, which is correlated with poor prognosis in CCA patients (66, 67). IDH1-mutant tumours exacerbate immune suppression through metabolic reprogramming, increasing the production of metabolites such as 2-HG, which inhibits the function of TET2 and reduces T-cell activation, contributing to resistance against ICIs (68–70). Furthermore, VEGF-C-driven lymphangiogenesis contributes to an immunosuppressive microenvironment by facilitating lymph node metastasis and further suppressing immune responses through the recruitment of immunosuppressive cells (71, 72).
Additionally, cancer-associated fibroblasts (CAFs), derived from TGF-β1-activated hepatic stellate cells (HSCs) that express α-SMA, play a critical role in remodelling the TME. CAFs secrete extracellular matrix (ECM) components that create mechanical barriers to promote invasiveness and chemoresistance in iCCA (73–75). CAFs not only increase tumour progression through the secretion of protumour factors, such as VEGF and IL-6, but also induce vasculogenic mimicry (VM), which accelerates metastasis in gallbladder cancer (67, 76, 77). Heterogeneous CAF subpopulations interact with myeloid-derived suppressor cells (MDSCs) and inhibit T-cell function by secreting immunosuppressive cytokines, such as IL-10 and TGF-β. This interaction is facilitated by the Notch1 signalling pathway, which not only amplifies tumour malignancy but also helps CAFs recruit MDSCs to the TME (78, 79). CAFs can transfer oncogenic molecules, such as miRNAs and proteins, via exosomes, further contributing to resistance in genomically distinct subtypes, including ERBB2-amplified tumours (80–82). These interactions between CAFs, TAMs, Tregs, and MDSCs create a complex immune-suppressive network that supports immune evasion and tumour progression in cholangiocarcinoma. These findings underscore the importance of targeting the functional diversity of these immune cells using combinatorial strategies, such as Notch inhibitors or immunotherapies, to overcome resistance and improve therapeutic outcomes (65, 83) (Figure 3).
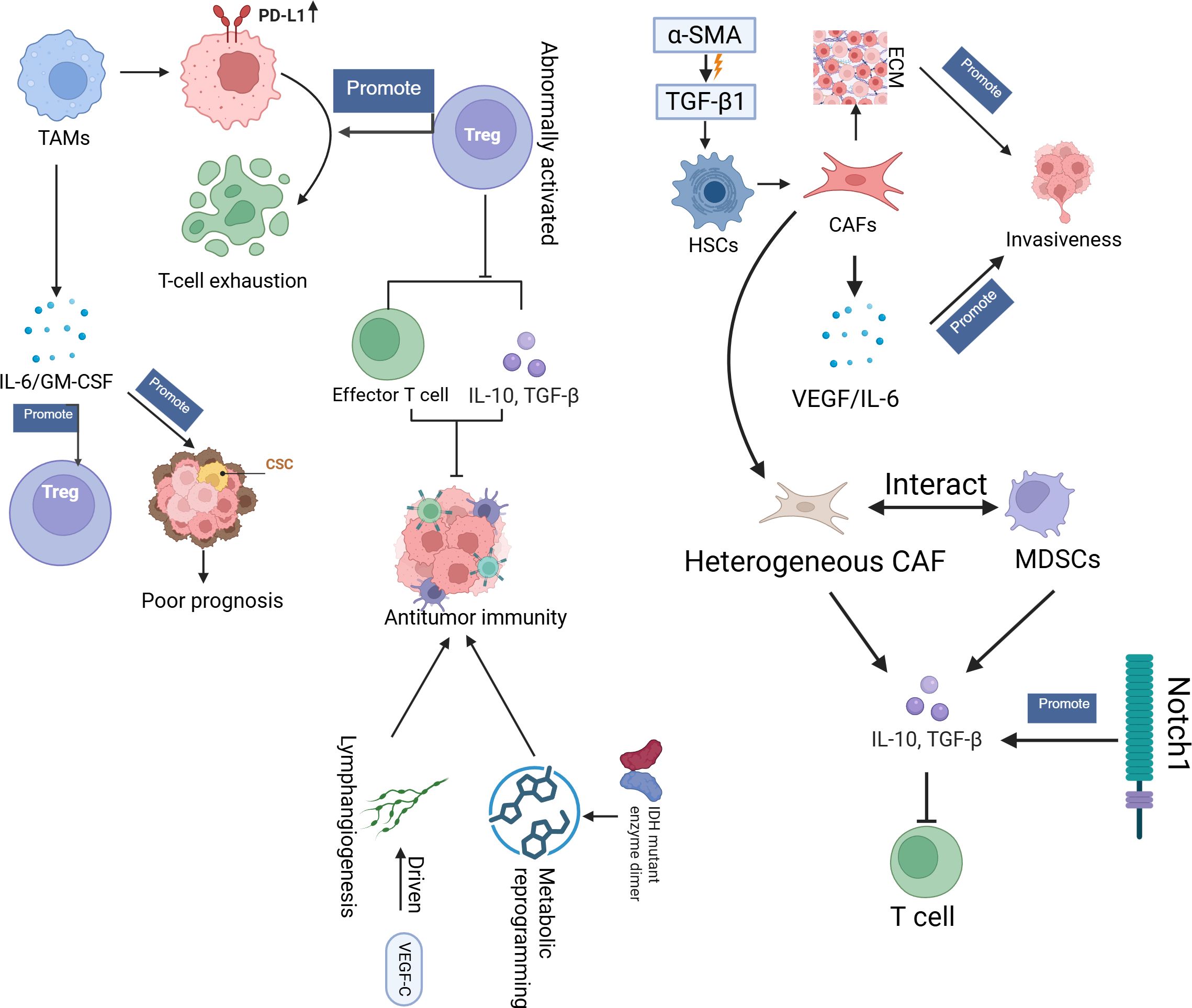
Figure 3. This diagram provides an overview of the TME in BTC, highlighting key cellular and molecular interactions that drive immune evasion and tumour progression. TME, tumour microenvironment; CAFs, Cancer-Associated Fibroblasts; TAMs, Tumour-Associated Macrophages, Tregs, Regulatory T-cells; MDSCs, Myeloid-Derived Suppressor Cells; EMC, Extracellular matrix; HSCs, Hepatic Stellate Cells; CSC, Cancer stem cells.
2.4 Tumour-induced immune evasion mechanisms
Multiomics remodelling has revealed profound metabolic heterogeneity in ICCA, including enhanced glycolysis, lipid dysregulation, and mitochondrial adaptation (84–86). Tumour cells prioritize endogenous glycogen over glucose as the primary glycolytic carbon source under hypoxia, with elevated glycogen phosphorylase activity fuelling tumour progression (84). Lipid metabolism alterations, such as increased synthesis and oxidation, and PGC-1α-mediated mitochondrial reprogramming sustain proliferation and represent therapeutic vulnerabilities (87). IDH1 mutations generate (R)-2-hydroxyglutarate, which inhibits α-ketoglutarate-dependent enzymes to induce epigenetic dysregulation and immune evasion (68). Cancer stem cells (CSCs) rely on mitochondrial metabolism, whereas TAMs exacerbate immunosuppression via glycolytic shifts (86, 87). These metabolic adaptations correlate with anatomical subtypes (e.g., ERBB2-amplified iCCA vs. PIK3CA-mutant extrahepatic tumours) and influence chemotherapy/targeted therapy efficacy (88–90). Multiomics analyses further revealed metabolic heterogeneity across subtypes: gallbladder cancer shows lipid-driven hypoxia adaptation, whereas extrahepatic tumours exhibit PI3KCAH1047R-driven transformation (90–92). Metabolic crosstalk within the TME involves CAFs that secrete lactate to promote immunosuppression and TAMs that enhance glycolysis to suppress immunity (53, 93, 94). Exosome-mediated signalling coordinates angiogenesis and fibrosis, whereas metabolic–immune network dysregulation (e.g., GPR109A pathway anomalies) underpins poor prognosis and therapy resistance (95–97). IDH1-mutant tumours resist ICIs via epigenetic–immune crosstalk, whereas CPS1-deficient tumours disrupt the urea cycle to alter the pH of the TME (98–100). Targeting metabolic nodes, such as glycolytic enzymes, mitochondrial pathways, or FGFR2/IDH1-related aberrations, may reverse immunosuppression and increase treatment sensitivity (18, 53, 94, 101). Overall, the bidirectional feedback between metabolic reprogramming and TME remodelling drives cholangiocarcinoma progression, necessitating precision strategies that integrate metabolic subtyping with immune and targeted therapies (86, 102, 103) (Figure 4).
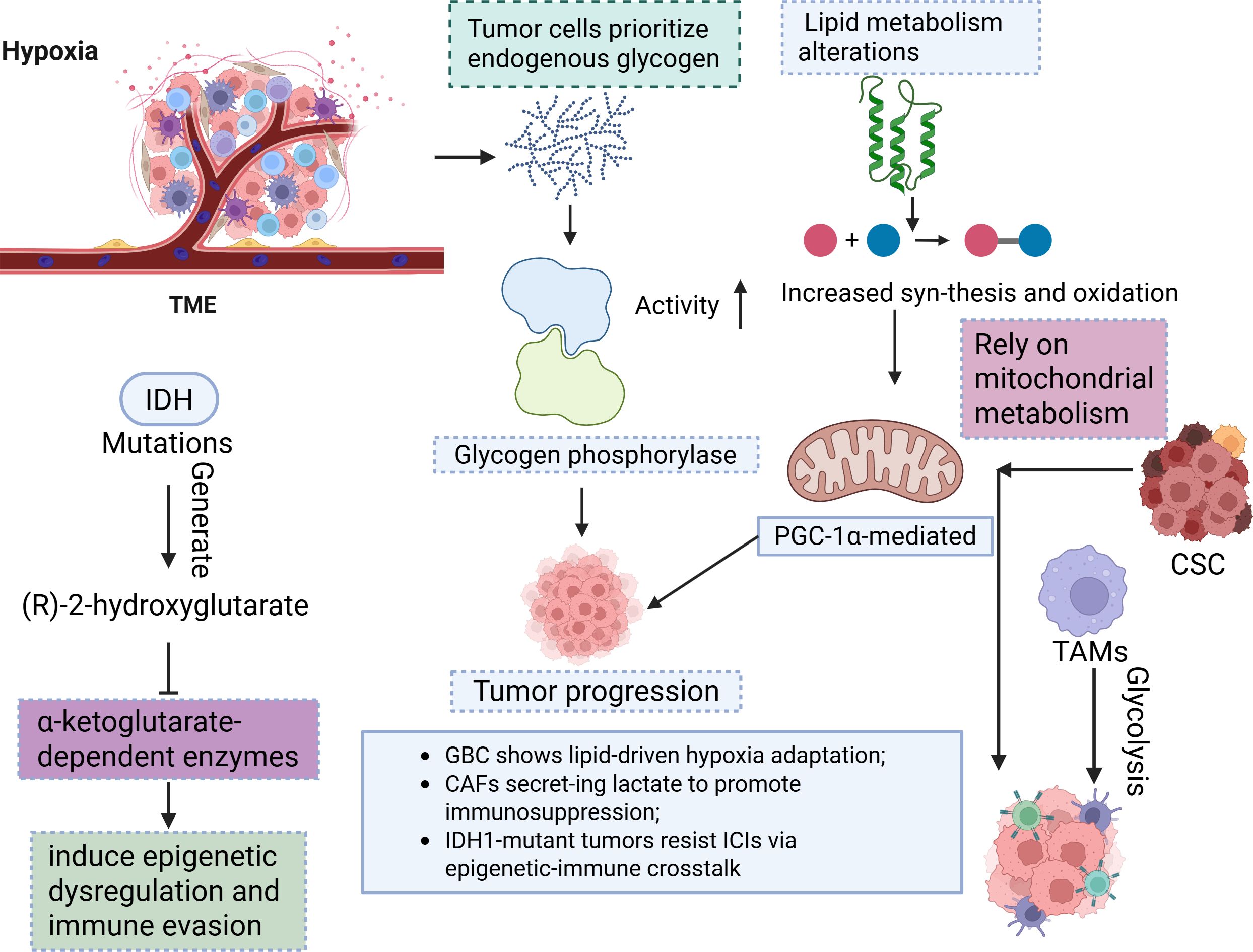
Figure 4. This diagram depicts the metabolic reprogramming in BTC driven by hypoxia and IDH mutations. TME, Tumour microenvironment; CSC, Cancer stem cells; TAM, Tumour-Associated Macrophages.
3 Current immunotherapy approaches for BTC
3.1 Monotherapy with immune checkpoint inhibitors
ICIs, particularly PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, are among the most successful immunotherapy strategies. PD-1/PD-L1 inhibitors work by disrupting immune suppression between tumour cells and immune cells, promoting the activation of T cells and enhancing antitumour immune responses (104–106). PD-1 inhibitors, such as nivolumab and pembrolizumab, have entered clinical trials for the treatment of BTC and have shown some efficacy (107, 108). This finding indicates the limitations of monotherapy in BTC and highlights the immune suppressive characteristics of the tumour microenvironment in this cancer.
3.2 Combination therapies
The combination of immune checkpoint inhibitors with chemotherapy has been increasingly investigated in BTC, with preliminary results suggesting that this combination can significantly improve clinical outcomes. For example, the combination of pembrolizumab with gemcitabine/cisplatin has improved progression-free survival (PFS) and overall survival (OS) in advanced BTC patients compared with chemotherapy alone (3). This combination approach harnesses the cytotoxic effects of chemotherapy while activating the immune response through immune checkpoint inhibitors, providing a more effective treatment option for BTC patients (109). Combining immunotherapy with targeted therapy is another promising strategy currently being explored for BTC. The combination of PD-1 inhibitors with FGFR2 inhibitors or IDH1 inhibitors aims to enhance immune responses and overcome tumour resistance mechanisms. For example, the combination of PD-1 inhibitors with FGFR2 inhibitors has shown potential in clinical trials, with some patients experiencing delayed disease progression (88, 110–113). These combination therapies aim to target both immune escape pathways and molecular drivers of tumour growth, offering a multipronged approach to improve BTC treatment outcomes.
These combination strategies offer several advantages in BTC treatment. First, they increase treatment efficacy by simultaneously targeting immune evasion pathways and molecular drivers of tumour growth, which improves clinical outcomes. Mechanistic studies support these clinical findings: Mechanistically, targeting TAMs and their PD-L1 upregulation can effectively alleviate immune suppression and promote T-cell-mediated tumour killing (114). Additionally, combining chemotherapy with immune checkpoint inhibitors synergistically amplifies the therapeutic response and improves PFS and OS (115). In addition, the combination of targeted therapies with immunotherapy helps overcome tumour resistance mechanisms, providing a more comprehensive treatment approach. Targeted drugs, such as FGFR2 inhibitors and IDH1 inhibitors, block tumour cell proliferation and signalling pathways, thus increasing the effectiveness of immunotherapy and restoring the ability of the immune system to recognize tumours (116, 117). This multifaceted strategy aims to address the complexity of BTC and may increase the sustainability of clinical responses.
However, combination therapy also faces several challenges. The combined use of chemotherapy and immune checkpoint inhibitors may increase toxicity and immune-related adverse events (irAEs), which may limit the tolerability of these treatments in some patient populations (115). Additionally, tumour heterogeneity in BTC is a significant challenge because the molecular characteristics of a patient may dictate the response to combination treatment. Mechanistic research suggests that molecular features, such as FGFR2 and IDH1 mutations, may influence immune evasion and resistance mechanisms (118, 119). Further research on the relationship between tumour molecular characteristics and treatment response is crucial to optimize combination therapies. Finally, reliable biomarkers to identify patients who will benefit the most from combination therapies are lacking, and the discovery of such biomarkers remains a significant challenge. Mechanistic studies have pointed to the potential of identifying biomarkers, such as key molecules in the tumour microenvironment, which may help optimize patient selection. Ongoing research is needed to identify suitable biomarkers for patient selection and refine these combination treatment strategies. Despite these challenges, combination therapy remains a promising avenue for improving BTC treatment outcomes (120), and further clinical trials and mechanistic studies are necessary to optimize these approaches.
4 Clinical trials and applications in BTC
Currently, clinical trials on immunotherapy in BTC are ongoing, and many are evaluating the effects of combining immunotherapy with chemotherapy and targeted therapies. As research advances, more immunotherapy regimens are expected to enter clinical practice, particularly in the areas of precision medicine and individualized treatment. Moreover, new immunotherapy targets and strategies that combine immunotherapy with metabolic modulation are being explored, and future research is expected to identify more treatment options for BTC patients (Table 1).
5 Reshaping the TME
5.1 TME Components and their impact on immunotherapy
Critically, TAMs are the predominant immune cells in the BTC TME. They play pivotal roles in promoting both tumour progression and immune suppression. TAMs secrete cytokines, such as IL-6 and TNF-α, that inhibit T-cell activation while promoting the proliferation of cancer stem cells. Moreover, TAMs upregulate immune checkpoints, such as PD-L1, which further suppresses CD8+ T-cell activity, thereby facilitating immune escape and promoting tumour growth (87, 121, 122). Tregs are another crucial cell type in the BTC TME that significantly contribute to immune suppression. Tregs secrete immunosuppressive cytokines, such as TGF-β and IL-10, to directly inhibit the function of effector T cells (e.g., CD8+ T cells). This action suppresses antitumour immunity. The accumulation of Tregs in the BTC TME is associated with poorer prognosis, indicating their role in immune evasion and resistance to treatment (123–125). MDSCs constitute another class of immune-suppressive cells that accumulate in the TME under the influence of tumour-secreted factors. MDSCs suppress T-cell activation and promote tumour progression by secreting immunosuppressive molecules, such as arginase-1 (Arg1) and inducible nitric oxide synthase (iNOS) (126). In BTC, the presence of MDSCs is correlated with immune suppression and disease progression (121, 127). CAFs are a key cell type in the TME and contribute to tumour progression through the secretion of ECM components (such as collagen and fibronectin) and protumourigenic factors (such as VEGF and IL-6). CAFs remodel the ECM, which can create physical barriers that impede immune cell infiltration. In addition, CAFs promote immune evasion by secreting lactate and other metabolic products, further suppressing immune responses (128).
5.2 Strategies to reshape the TME
One approach to reshaping the TME involves targeting immune-suppressive cells, such as TAMs, Tregs, and MDSCs. TAMs can be targeted using antibodies against CD40 or CSF1R, which reduce the number of TAMs and restore immune responses (129, 130). Targeting Tregs using anti-CTLA-4 antibodies or anti-CCR4 antibodies, which can deplete Tregs and promote effector T-cell function, has been proposed as another strategy to reshape the TME (131). Additionally, targeting MDSCs with anti-GM-CSF antibodies can reduce their accumulation and reverse immune suppression in the TME (130). The dense ECM in the TME often acts as a physical barrier that prevents immune cells from infiltrating tumour tissues. Strategies to target ECM components are being developed to increase immune cell infiltration. For example, the use of matrix metalloproteinase (MMP) inhibitors has been shown to degrade the ECM barrier, promoting immune cell entry and enhancing the effects of immunotherapy (132). Furthermore, targeting ECM components secreted by CAFs can help alleviate mechanical barriers in the TME and improve immune responses (133, 134). The metabolic reprogramming of both tumour and immune cells in the TME plays a crucial role in immune evasion. The acidic and hypoxic conditions in the TME, which are caused by altered metabolic pathways, suppress immune cell function. Targeting these metabolic pathways in tumour and immune cells, such as glycolysis and lipid metabolism, has been proposed as a strategy to restore immune function and increase the efficacy of immunotherapy (135, 136). For example, inhibiting lactate metabolism in tumour cells can reverse immune suppression and enhance the antitumour effects of immune cells (137).
5.3 Combination of immunotherapy and TME reshaping
Combining immunotherapy with strategies to reshape the TME has shown promising results in preclinical models. Targeting both immune-suppressive pathways in the TME and enhancing immune responses has synergistic effects (138). For example, combining PD-1 inhibitors with CAF-targeting therapies has resulted in increased antitumour effects in preclinical studies (139). This approach not only improves immune cell function but also suppresses tumour growth, providing a more effective treatment strategy for BTC.
6 Advancing precision combination strategies
6.1 Combination of immunotherapy with targeted therapy
FGFR2 gene fusions or amplifications are common in iCCA, with approximately 10–15% of iCCA patients harbouring FGFR2 alterations. FGFR2 inhibitors, such as pemigatinib and futibatinib, have shown significant efficacy in FGFR2 fusion-positive patients (93, 140). Combining FGFR2 inhibitors with PD-1 inhibitors (e.g., nivolumab) may enhance the effects of immunotherapy. This potential benefit arises because FGFR2 inhibitors inhibit tumour cell proliferation. Moreover, they enhance immune system responses (141). IDH1 mutations occur in a subset of BTC patients, particularly in iCCA patients. IDH1 inhibitors (e.g., ivosidenib) restore normal metabolic pathways, inhibiting tumour cell growth. In early clinical trials, the combination of IDH1 inhibitors with immunotherapy has shown potential for enhancing immune responses (142). HER2 overexpression or amplification is observed in some gallbladder cancers and iCCA. HER2-targeted therapies (e.g., trastuzumab) in combination with immunotherapy (e.g., PD-1 inhibitors) may enhance antitumour immune responses and overcome immune evasion (55, 143).
6.2 Combination of immunotherapy with chemotherapy
The combination of immune checkpoint inhibitors, such as nivolumab or pembrolizumab, with chemotherapy (e.g., gemcitabine and cisplatin) has shown superior efficacy in BTC treatment (3). Compared with chemotherapy alone, the combination of PD-1 inhibitors with chemotherapy significantly improves PFS and OS. Additionally, immunotherapy helps overcome chemotherapy resistance, enhancing immune responses against tumours (144). Chemotherapy induces tumour cell death, leading to the exposure of tumour antigens, which enhances immune system recognition of the tumour (145). Moreover, chemotherapy increases immune cell infiltration into the tumour, creating a more favourable environment for immunotherapy. The combination of chemotherapy and immune checkpoint inhibitors not only enhances immune responses but also provides complementary mechanisms to combat tumour growth, improving therapeutic efficacy (105, 107).
6.3 Combination of immunotherapy with metabolic modulation
Tumour cells enhance glycolysis and lipid metabolism to promote growth while suppressing immune cell function. Inhibiting tumour cell glycolysis or lipid biosynthesis restores immune cell antitumour functions, increasing the efficacy of immunotherapy. The combination of metabolic inhibitors with immunotherapy has demonstrated significant synergistic effects in preclinical models, particularly in BTC models (3, 146). Metabolic reprogramming in the TME creates an acidic and hypoxic environment that suppresses immune cell activity. Targeting these metabolic pathways and restoring normal metabolic states may reverse the immune-suppressive environment in the TME, thereby enhancing the effects of immunotherapy (147). For example, the use of glucose metabolism inhibitors or lactate removal agents has been shown to effectively restore antitumour responses of the immune system and improve immunotherapy response rates (148).
7 Challenges in clinical translation
Despite advancements in targeted and immunotherapies, several challenges hinder their clinical translation for BTC. The molecular heterogeneity of BTC, with varying genetic profiles across subtypes (iCCA, eCCA, and GBC), complicates the identification of universal therapeutic targets. The difficulty in obtaining sufficient tissue samples for molecular profiling is addressed by liquid biopsy technologies, particularly cfDNA, which offer a noninvasive method to monitor mutations and treatment responses in real time (149). The immunosuppressive tumour microenvironment, dominated by Tregs, MDSCs, and TAMs, limits the efficacy of ICIs. Overcoming this barrier requires strategies such as combining ICIs with immunomodulatory agents. Additionally, the development of predictive biomarkers, such as tumour mutational burden and specific genetic alterations, is essential for patient selection and optimizing treatment strategies.
Chemotherapy, targeted therapy, and immunotherapy each offer distinct advantages and limitations in the management of advanced CCA (150). Chemotherapy remains a cornerstone of treatment, particularly for advanced cases, but its effectiveness is often limited by systemic toxicity and a lack of durable responses (151). Targeted therapies, such as those targeting specific genetic alterations such as FGFR2 fusions or IDH mutations, have shown promising results in selected patients but are limited by the genetic heterogeneity of CCA and the development of resistance over time (152). Immunotherapy, particularly ICIs, offers a new approach by targeting immune evasion mechanisms within the tumour microenvironment (153). However, the immunosuppressive nature of the CCA microenvironment, which is dominated by regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and TAMs, limits the effectiveness of ICIs in many patients (154). Overcoming this challenge may require combining ICIs with immunomodulatory agents or other treatment strategies to enhance the immune response (155). Furthermore, predictive biomarkers, such as tumour mutational burden (TMB) and specific genetic alterations, are crucial for patient selection and optimizing the therapeutic approach, ensuring that patients most likely to benefit from each modality receive the appropriate treatment (156).
8 Overcoming clinical challenges in immunotherapy for BTC
8.1 Mechanisms of immunotherapy resistance
BTC tumour cells evade immune surveillance through several mechanisms. These processes primarily involve 1) the upregulation of immune checkpoints (such as PD-L1 and CTLA-4), 2) the accumulation of TAMs and regulatory Tregs, and 3) the reprogramming of tumour metabolic pathways. These mechanisms limit the immune response and contribute to tumour immune escape, thereby exacerbating resistance (157). For example, high PD-L1 expression in BTCs directly inhibits CD8+ T-cell-mediated antitumour effects (158). TAMs and Tregs, through the secretion of immunosuppressive cytokines such as IL-10 and TGF-β, further weaken immune responses (159). Metabolic reprogramming in the TME is another important factor leading to immune evasion. BTC cells promote glycolysis, lipid synthesis, and lactate secretion, creating an acidic, hypoxic, and nutrient-deprived microenvironment. This environment not only provides a growth advantage to tumour cells but also suppresses immune cell functions. For example, the accumulation of lactate in the TME impairs T-cell efficacy and suppresses immune responses (160).
8.2 Immune-related adverse events
Although ICIs have demonstrated efficacy in various cancers, immune-related adverse events (irAEs) associated with these treatments have become a significant clinical challenge. irAEs refer to autoimmune reactions triggered by the activation of the immune system, which can result in damage to various organs, including the skin, liver, lungs, and endocrine system (161, 162). In BTC patients, the incidence of irAEs due to ICIs is relatively high, and their management requires careful consideration of the patient’s immune status and comorbidities. This management is especially challenging in patients with advanced BTC or those with multiorgan metastasis, where irAEs complicate treatment. Therefore, balancing efficacy with the management of irAEs remains a major clinical challenge in BTC immunotherapy.
The management of irAEs in BTC patients involves a range of strategies aimed at controlling immune overactivation while maintaining the antitumour efficacy of ICIs. First-line treatment typically involves systemic corticosteroids, with additional immunosuppressive agents such as mycophenolate mofetil or infliximab for severe cases (163–165). Temporary interruption of ICIs may be recommended for mild irAEs, with reintroduction upon symptom resolution. Preventive measures focus on pretreatment screening to identify high-risk patients, particularly those with a history of autoimmune disorders. Low-dose corticosteroids before treatment and personalized monitoring using biomarkers predictive of immune activation are areas of active research. Although these strategies can reduce the severity of irAEs, further clinical validation is needed before they can be widely implemented.
Balancing efficacy with safety is a critical challenge in BTC immunotherapy, especially given the potential for severe irAEs. To optimize outcomes, clinicians must tightly monitor patients by assessing organ function and tracking symptoms regularly. An individualized approach is necessary, wherein the decision to continue or pause ICI therapy is based on the patient’s overall health, the severity of irAEs, and potential survival benefits (166). Furthermore, combining ICIs with other therapies, such as chemotherapy or targeted treatments, may help mitigate the immune activation seen with monotherapy, potentially reducing the risk of irAEs while retaining treatment efficacy (167, 168). Ongoing research into biomarkers to predict irAEs and methods to prevent their occurrence will be essential for improving the safety profile of ICIs in BTC and enhancing the clinical management of these patients (169).
8.3 Patient selection and the lack of biomarkers
Currently, the response rate of BTC patients to immunotherapy remains low, making patient selection crucial. However, accurately identifying patients who are most likely to benefit from immunotherapy is difficult due to the lack of effective predictive biomarkers. While some biomarkers, such as PD-L1 expression, microsatellite instability (MSI), and the TMB, have been shown to have predictive value in other cancers, their utility in BTC remains unvalidated (170, 171). Biomarker identification for immunotherapy response in BTC remains in its early stages. Emerging technologies, such as liquid biopsy, ctDNA, and genomic analysis, offer the potential for earlier and more accurate assessment of treatment efficacy and resistance. Furthermore, research suggests that the interaction between immunotherapy and the TME may affect the treatment response, highlighting the potential of TME components as predictive biomarkers (172).
8.4 Challenges in TME reshaping
Although reshaping the TME to increase immunotherapy efficacy has become a significant research focus, translating this strategy into clinical practice still faces multiple challenges. First, the complexity and heterogeneity of the TME hinder the complete elimination of immunosuppressive components (172, 173). Additionally, TME-reshaping drugs may interact with immunotherapies, potentially causing side effects. For example, targeting CAFs (cancer-associated fibroblasts) may lead to adaptive changes in tumour cells that result in therapy resistance (174). Therefore, precisely targeting immune-suppressive components within the TME while minimizing side effects and maximizing the effectiveness of immunotherapy remains a key focus for future research.
8.5 Limitations and challenges in clinical trials
Although numerous clinical trials related to immunotherapy are underway, some limitations still remain in the design of clinical trials for BTC. First, most clinical trials have focused on late-stage BTC patients, often overlooking patients with early-stage or locally advanced disease. Second, the heterogeneity of trial results is significant, due in part to individual patient differences, tumour heterogeneity, and variations in immune responses. Therefore, larger, more rigorously designed clinical trials are needed to validate the true effects and safety of immunotherapy in BTC (175) Table 2.
9 Future directions and emerging therapies
9.1 Next-generation immunotherapies
Chimeric antigen receptor T-cell (CAR-T) therapy is an emerging treatment strategy that involves genetically modifying a patient’s own T cells to recognize and attack tumour cells. Although CAR-T-cell therapy has achieved significant success in haematologic cancers, its application in solid tumours, including BTCs, faces challenges. Research indicates that the primary challenge for CAR-T-cell therapy in solid tumours is the ability to effectively penetrate the tumour microenvironment and enhance T-cell infiltration (176, 177). Current research focuses on optimizing CAR-T-cell therapy design, including targeting specific antigens found in BTCs, such as Mucin 1 and CEA, to improve efficacy and reduce side effects (178). Tumour vaccines, particularly mRNA-based vaccines, constitute another emerging strategy in immunotherapy. mRNA vaccines deliver specific tumour antigen genes to the patient’s body, inducing the immune system to recognize and attack tumour cells. mRNA vaccines that target BTCs are currently in the preclinical phase, and these vaccines have the potential to enhance antitumour immune responses (179). For example, mRNA vaccines that target KRAS mutations have shown therapeutic potential in other solid tumours and may be used for BTC treatment in the future (180).
9.2 Targeting novel immune checkpoints
T-cell immunoreceptor with Ig and ITIM domains (TIGIT) and lymphocyte-activation gene 3 (LAG-3) are newly identified immune checkpoint molecules that play critical roles in immune suppression in various cancers. Research indicates that the upregulation of TIGIT and LAG-3 is closely associated with immune evasion and tumour resistance (181–183). Currently, monoclonal antibodies that target TIGIT and LAG-3 are undergoing clinical trials. Combining PD-1/PD-L1 inhibitors with TIGIT or LAG-3 inhibitors may improve treatment responses in BTC patients (184). V-domain Ig suppressor of T-cell activation (VISTA) is another newly discovered immune checkpoint molecule that plays a key role in regulating T-cell function (185). VISTA upregulation in the TME has been shown to play a role in immune suppression, particularly in solid tumours. Antibodies that target VISTA are currently under development and may offer new treatment options for BTC patients when combined with existing immunotherapies (186).
9.3 Artificial intelligence and immunotherapy
AI and deep learning technologies can analyse patient genomic data, immune phenotypes, and clinical characteristics to identify biomarkers associated with immunotherapy response. These technologies can help clinicians select the patients most likely to benefit from immunotherapy and provide personalized treatment plans (187). AI algorithms can assist in optimizing immunotherapy regimens, including selecting the best immune checkpoint inhibitors, targeted therapies, and combination therapies. By simulating different treatment pathways and predicting treatment responses, AI can provide more precise therapeutic decisions for BTC patients (188). Furthermore, AI can integrate the analysis of the gut microbiome to further enhance personalized treatment strategies (189). Increasing evidence shows that the gut microbiome significantly influences immune therapy responses, with certain microbiome compositions enhancing immune responses and improving the efficacy of immunotherapy (190, 191). AI can analyse the microbiome data of patients to identify those whose microbiome features may affect immune therapy outcomes, thereby optimizing treatment plans (192). By integrating genomic data, immune phenotypes, clinical characteristics, and microbiome data, AI provides more comprehensive decision support for personalized treatment plans, helping to improve treatment efficacy and patient survival (193).
10 Future directions
As we move forward in the treatment of BTC, several key areas need further exploration and development to optimize patient outcomes. The identification of molecular subtypes in BTC, driven by specific genetic alterations such as FGFR2 fusion, IDH1 mutation, and BRAF mutation, will be essential for personalizing treatment strategies. These molecular profiles are critical for guiding targeted therapies and optimizing treatment plans. Ongoing clinical trials aim to integrate molecular profiling with precision medicine to identify patients who are most likely to benefit from specific therapies, and such efforts are expected to reshape the landscape of BTC treatment (93, 194).
The use of liquid biopsy technologies, particularly cfDNA-based assays, will play a pivotal role in the future of BTC treatment. Liquid biopsy is a noninvasive, real-time method for monitoring tumour evolution, assessing treatment responses, and detecting resistance mechanisms (42). The ability to track genetic alterations dynamically during treatment provides a powerful tool for adapting therapies and personalizing care. However, integrating these technologies into clinical practice poses challenges, including the standardization of assays, ensuring high sensitivity and specificity, and achieving clinical validation for broader use in routine diagnostics.
While monotherapies have demonstrated limited efficacy in BTC, combination therapies that involve targeted therapies, ICIs, and chemotherapy are being actively explored to improve survival outcomes. The development of reliable biomarkers to identify the patients who will benefit from these combination therapies remains a crucial research priority (110, 113, 146). The ability to accurately predict treatment response is key to enhancing patient outcomes and avoiding ineffective treatments. Additionally, the mechanisms of resistance to combination therapies need to be better understood to refine treatment protocols and prevent relapse.
Another significant development in BTC treatment is the growing use of artificial intelligence (AI) and machine learning (ML) technologies to analyse large datasets from clinical trials and patient registries. These tools are being leveraged to identify potential biomarkers, predict patient responses to different therapies, and optimize patient selection for clinical trials. AI-driven approaches are expected to improve clinical trial designs, helping to identify more appropriate patient populations and accelerating the development of new therapies for BTC (41, 195–197). However, challenges remain in integrating AI into clinical decision-making, particularly in ensuring the interpretability and clinical relevance of AI-generated insights.
11 Conclusion
Immunotherapy has shown great potential for treating BTC, particularly by enhancing antitumour immune responses and overcoming immune evasion mechanisms. However, the highly immunosuppressive TME in BTCs limits the efficacy of monotherapy. Combination strategies, such as immune checkpoint inhibitors with targeted therapies (e.g., FGFR2 and IDH1 inhibitors) and chemotherapy, have demonstrated promising results, significantly improving treatment outcomes. Additionally, combining immunotherapy with metabolic modulation offers new therapeutic possibilities. Although challenges such as resistance and immune-related adverse events persist, emerging therapies such as CAR-T-cell therapy, mRNA vaccines, and novel immune checkpoint inhibitors hold promise for more personalized and effective treatment options. Future research should focus on refining combination therapies, developing precise biomarkers, and conducting rigorous clinical trials to optimize immunotherapy in BTC, ultimately improving patient outcomes and quality of life.
Author contributions
JX: Conceptualization, Visualization, Writing – original draft. LZ: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. KZha: Visualization, Writing – original draft. KZho: Conceptualization, Supervision, Writing – review & editing. HZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Beijing Natural Science Foundation(L248022),National High Level Hospital Clinical Research Funding[2022-PUMCH-B-128], CAMS Innovation Fund for Medical Sciences (CIFMS) [2021-I2M-1-061] [2021-I2M-1–003], CSCO-Hengrui Cancer Research Fund [Y-HR2019-0239].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BTC, Biliary tract cancer; ctDNA, circulating tumour DNA; TLA, Three letter acronym; LD, Linear dichroism; iCCA, Intrahepatic cholangiocarcinoma; GBC, gallbladder cancer; eCCA, extrahepatic cholangiocarcinoma; cfDNA, circulating free DNA; CCA, Cholangiocarcinoma; TAMs, tumour-associated macrophages; Tregs, regulatory T cells; CAFs, Cancer-associated fibroblasts; HSCs, hepatic stellate cells; TME, tumour microenvironment; ECM, extracellular matrix; VM, vasculogenic mimicry; MDSCs, myeloid-derived suppressor cells; CSCs, Cancer stem cells; ICIs, immune checkpoint inhibitors; AI, Artificial intelligence.
References
1. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. (2021) 22:1290–300. doi: 10.1016/S1470-2045(21)00336-3
2. Vu QT, Nishimura Y, Harada K, Ito H, Higashionna T, Maruo A, et al. International trends in biliary tract cancer-related mortality, 2000–2022: an observational study of the World Health Organization Mortality Database. Hepatology. (2024) 10:1097. doi: 10.1097/HEP.0000000000001200
3. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
4. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. (2021) 22:690–701. doi: 10.1016/S1470-2045(21)00027-9
5. Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. (2020) 6:1405–9. doi: 10.1001/jamaoncol.2020.2814
6. Tang Y, Cui G, Liu H, Han Y, Cai C, Feng Z, et al. Converting “cold” to “hot”: epigenetics strategies to improve immune therapy effect by regulating tumor-associated immune suppressive cells. Cancer Commun. (2024) 44:601–36. doi: 10.1002/cac2.12546
7. Li J, Luo Y, and Pu K. Electromagnetic nanomedicines for combinational cancer immunotherapy. Angewandte Chemie Int Edition. (2021) 60:12682–705. doi: 10.1002/anie.202008386
8. Liu Y, Gong L, Feng J, Xiao C, Liu C, Chen B, et al. Co-delivery of axitinib and PD-L1 siRNA for the synergism of vascular normalization and immune checkpoint inhibition to boost anticancer immunity. J Nanobiotechnol. (2025) 23:194. doi: 10.1186/s12951-025-03170-y
9. Wang L, Bi S, Li Z, Liao A, Li Y, Yang L, et al. Napabucasin deactivates STAT3 and promotes mitoxantrone-mediated cGAS-STING activation for hepatocellular carcinoma chemo-immunotherapy. Biomaterials. (2025) 313:122766. doi: 10.1016/j.biomaterials.2024.122766
10. Dummer R, Ascierto PA, Nathan P, Robert C, and SChadendorf D. Rationale for immune checkpoint inhibitors plus targeted therapy in metastatic melanoma: a review. JAMA Oncol. (2020) 6:1957–66. doi: 10.1001/jamaoncol.2020.4401
11. Nam G-H, Kwon M, Jung H, Ko E, Kim SA, Choi Y, et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J ImmunoTherapy Cancer. (2021) 9:e002474. doi: 10.1136/jitc-2021-002474
12. Goyal L, Kongpetch S, Crolley VE, and Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. (2021) 95:102170. doi: 10.1016/j.ctrv.2021.102170
13. Makawita S, Lee S, Kong E, Kwong LN, Abouelfetouh Z, Danner De Armas A, et al. Comprehensive immunogenomic profiling of IDH1-/2-altered cholangiocarcinoma. JCO Precis Oncol. (2024) 8:e2300544. doi: 10.1200/PO.23.00544
14. Song Y, Li Y, Zhou J, Yu J, Hu Q, Yang F, et al. IL-8–NF-κB–ALDH1A1 loop promotes the progression of intrahepatic cholangiocarcinoma. Hepatol Commun. (2025) 9:e0664. doi: 10.1097/HC9.0000000000000664
15. Rattanasinchai C, Navasumrit P, and Ruchirawat M. Elevated ITGA2 expression promotes collagen type I-induced clonogenic growth of intrahepatic cholangiocarcinoma. Sci Rep. (2022) 12:22429. doi: 10.1038/s41598-022-26747-1
16. Liu D, Heij LR, Czigany Z, Dahl E, Lang SA, Ulmer TF, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. (2022) 41:127. doi: 10.1186/s13046-022-02340-2
17. Wu T, Gao R, Wang X, Guo D, Xie Y, Dong B, et al. Pancreatobiliary reflux increases macrophage-secreted IL-8 and activates the PI3K/NFκB pathway to promote cholangiocarcinoma progression. Trans Oncol. (2024) 45:101967. doi: 10.1016/j.tranon.2024.101967
18. Moffat GT, Hu ZI, Meric-Bernstam F, Kong EK, Pavlick D, Ross JS, et al. KRAS allelic variants in biliary tract cancers. JAMA Network Open. (2024) 7:e249840–e. doi: 10.1001/jamanetworkopen.2024.9840
19. Liu T, Cheng S, Peng B, Zang H, Zhu X, Wang X, et al. PD-L2 of tumor-derived exosomes mediates the immune escape of cancer cells via the impaired T cell function. Cell Death Disease. (2024) 15:800. doi: 10.1038/s41419-024-07191-7
20. Takayama H, Kobayashi S, Gotoh K, Sasaki K, Iwagami Y, Yamada D, et al. Prognostic value of functional SMAD4 localization in extrahepatic bile duct cancer. World J Surg Oncol. (2022) 20:291. doi: 10.1186/s12957-022-02747-3
21. Ni L, Xu J, Li Q, Ge X, Wang F, Deng X, et al. Focusing on the immune cells: recent advances in immunotherapy for biliary tract cancer. Cancer Manage Res. (2024) 16:941–63. doi: 10.2147/CMAR.S474348
22. Spizzo G, Puccini A, Xiu J, Goldberg RM, Grothey A, Shields AF, et al. Molecular profile of BRCA-mutated biliary tract cancers. Esmo Open. (2020) 5:e000682. doi: 10.1136/esmoopen-2020-000682
23. le Brun IC, Grapinet E, Hollebecque A, Aurillac V, Parent P, Girot P, et al. 297P Real-life efficacy of immune checkpoint inhibitors in microsatellite unstable/mismatch repair-deficient (dMMR/MSI-H) biliary tract cancer (BTC). Ann Oncol. (2024) 35:S125. doi: 10.1016/j.ejca.2023.04.012
24. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, and Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J hepatology. (2023) 78:614–26. doi: 10.1016/j.jhep.2022.11.030
25. Zhou Z-J, Ye Y-H, Hu Z-Q, Hou Y-R, Liu K-X, Sun R-Q, et al. Whole-exome sequencing reveals genomic landscape of intrahepatic cholangiocarcinoma and identifies SAV1 as a potential driver. Nat Commun. (2024) 15:9960. doi: 10.1038/s41467-024-54387-8
26. Nishida N and Kudo M. Genetic/epigenetic alteration and tumor immune microenvironment in intrahepatic cholangiocarcinoma: transforming the immune microenvironment with molecular-targeted agents. Liver cancer. (2024) 13:136–49. doi: 10.1159/000534443
27. Hu S, Molina L, Tao J, Liu S, Hassan M, Singh S, et al. NOTCH-YAP1/TEAD-DNMT1 axis drives hepatocyte reprogramming into intrahepatic cholangiocarcinoma. Gastroenterology. (2022) 163:449–65. doi: 10.1053/j.gastro.2022.05.007
28. Saborowski A, Vogel A, and Segatto O. Combination therapies for targeting FGFR2 fusions in cholangiocarcinoma. Trends Cancer. (2022) 8:83–6. doi: 10.1016/j.trecan.2021.11.001
29. Cho SY, Hwang H, Kim Y-H, Yoo BC, Han N, Kong S-Y, et al. Refining classification of cholangiocarcinoma subtypes via proteogenomic integration reveals new therapeutic prospects. Gastroenterology. (2023) 164:1293–309. doi: 10.1053/j.gastro.2023.02.045
30. Chang J, Zhang Y, Zhou T, Qiao Q, Shan J, Chen Y, et al. RBM10 C761Y mutation induced oncogenic ASPM isoforms and regulated β-catenin signaling in cholangiocarcinoma. J Exp Clin Cancer Res. (2024) 43:104. doi: 10.1186/s13046-024-03030-x
31. Lavacchi D, Caliman E, Rossi G, Buttitta E, Botteri C, Fancelli S, et al. Ivosidenib in IDH1-mutated cholangiocarcinoma: Clinical evaluation and future directions. Pharmacol Ther. (2022) 237:108170. doi: 10.1016/j.pharmthera.2022.108170
32. Zhu C, Ma J, Zhu K, Yu L, Zheng B, Rao D, et al. Spatial immunophenotypes predict clinical outcome in intrahepatic cholangiocarcinoma. JHEP Rep. (2023) 5:100762. doi: 10.1016/j.jhepr.2023.100762
33. Lan Z, Yang Y, Li L, Wang C, Sun Z, Wang Q, et al. Spatial omics technology potentially promotes the progress of tumor immunotherapy. Br J cancer. doi: 10.1038/s41416-025-03075-5
34. Chu Y-D, Chen C-W, Lai M-W, Lim S-N, and Lin W-R. Bioenergetic alteration in gastrointestinal cancers: the good, the bad and the ugly. World J Gastroenterology. (2023) 29:4499. doi: 10.3748/wjg.v29.i29.4499
35. Wu Y, Cheng Y, Wang X, Fan J, and Gao Q. Spatial omics: Navigating to the golden era of cancer research. Clin Trans Med. (2022) 12:e696. doi: 10.1002/ctm2.696
36. Zuo C, Zhu J, Zou J, and Chen L. Unravelling tumour spatiotemporal heterogeneity using spatial multimodal data. Clin Trans Med. (2025) 15:e70331. doi: 10.1002/ctm2.70331
37. Wassing IE, Nishiyama A, Shikimachi R, Jia Q, Kikuchi A, Hiruta M, et al. CDCA7 is an evolutionarily conserved hemimethylated DNA sensor in eukaryotes. Sci Adv. (2024) 10:eadp5753. doi: 10.1126/sciadv.adp5753
38. Wang D, Chen J, Wu G, Xiong F, Liu W, Wang Q, et al. MBD2 regulates the progression and chemoresistance of cholangiocarcinoma through interaction with WDR5. J Exp Clin Cancer Res. (2024) 43:272. doi: 10.1186/s13046-024-03188-4
39. Hlady RA, Zhao X, El Khoury LY, Wagner RT, Luna A, Pham K, et al. Epigenetic heterogeneity hotspots in human liver disease progression. Hepatology. (2025) 81:1197–210. doi: 10.1097/HEP.0000000000001023
40. Vedeld HM, Grimsrud MM, Andresen K, Pharo HD, von Seth E, Karlsen TH, et al. Early and accurate detection of cholangiocarcinoma in patients with primary sclerosing cholangitis by methylation markers in bile. Hepatology. (2022) 75:59–73. doi: 10.1002/hep.32125
41. Berchuck JE, Facchinetti F, DiToro DF, Baiev I, Majeed U, Reyes S, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol. (2022) 33:1269–83. doi: 10.1016/j.annonc.2022.09.150
42. Hwang S, Woo S, Kang B, Kang H, Kim JS, Lee SH, et al. Concordance of ctDNA and tissue genomic profiling in advanced biliary tract cancer. J Hepatology. (2025) 82:649–57. doi: 10.1016/j.jhep.2024.10.020
43. Cheishvili D, Wong C, Karim MM, Kibria MG, Jahan N, Das PC, et al. A high-throughput test enables specific detection of hepatocellular carcinoma. Nat Commun. (2023) 14:3306. doi: 10.1038/s41467-023-39055-7
44. Tiedemann RL, Hrit J, Du Q, Wiseman AK, Eden HE, Dickson BM, et al. UHRF1 ubiquitin ligase activity supports the maintenance of low-density CpG methylation. Nucleic Acids Res. (2024) 52:13733–56. doi: 10.1093/nar/gkae1105
45. Arora M, Bogenberger JM, Abdelrahman AM, Yonkus J, Alva-Ruiz R, Leiting JL, et al. Synergistic combination of cytotoxic chemotherapy and cyclin-dependent kinase 4/6 inhibitors in biliary tract cancers. Hepatology. (2022) 75:43–58. doi: 10.1002/hep.32102
46. Carotenuto P, Hedayat S, Fassan M, Cardinale V, Lampis A, Guzzardo V, et al. Modulation of biliary cancer chemo-resistance through microRNA-mediated rewiring of the expansion of CD133+ cells. Hepatology. (2020) 72:982–96. doi: 10.1002/hep.31094
47. Colyn L, Bárcena-Varela M, Álvarez-Sola G, Latasa MU, Uriarte I, Santamaría E, et al. Dual targeting of G9a and DNA methyltransferase-1 for the treatment of experimental cholangiocarcinoma. Hepatology. (2021) 73:2380–96. doi: 10.1002/hep.31642
48. Brägelmann J, Barahona Ponce C, Marcelain K, Roessler S, Goeppert B, Gallegos I, et al. Epigenome-wide analysis of methylation changes in the sequence of gallstone disease, dysplasia, and gallbladder cancer. Hepatology. (2021) 73:2293–310. doi: 10.1002/hep.31585
49. Ma W, Zhang J, Chen W, Liu N, and Wu T. The histone lysine acetyltransferase KAT2B inhibits cholangiocarcinoma growth: evidence for interaction with SP1 to regulate NF2-YAP signaling. J Exp Clin Cancer Res. (2024) 43:117. doi: 10.1186/s13046-024-03036-5
50. Nie J, Zhang S, Guo Y, Liu C, Shi J, Wu H, et al. Mapping of the T-cell landscape of biliary tract cancer unravels anatomic subtype-specific heterogeneity. Cancer Res. (2025) 85:704–22. doi: 10.1158/0008-5472.CAN-24-1173
51. Li J, Wang R, Jin J, Han M, Chen Z, Gao Y, et al. USP7 negatively controls global DNA methylation by attenuating ubiquitinated histone-dependent DNMT1 recruitment. Cell discovery. (2020) 6:58. doi: 10.1038/s41421-020-00188-4
52. Xiong F, Wang D, Xiong W, Wang X, Huang W-h, Wu G-h, et al. Unveiling the role of HP1α-HDAC1-STAT1 axis as a therapeutic target for HP1α-positive intrahepatic cholangiocarcinoma. J Exp Clin Cancer Res. (2024) 43:152. doi: 10.1186/s13046-024-03070-3
53. Scott AJ, Sharman R, and Shroff RT. Precision medicine in biliary tract cancer. J Clin Oncol. (2022) 40:2716–34. doi: 10.1200/JCO.21.02576
54. Guan C, Zou X, Gao X, Liu S, Gao J, Shi W, et al. Feedback loop LINC00511–YTHDF2–SOX2 regulatory network drives cholangiocarcinoma progression and stemness. MedComm. (2024) 5:e743. doi: 10.1002/mco2.743
55. Chen X, Wang D, Liu J, Qiu J, Zhou J, Ying J, et al. Genomic alterations in biliary tract cancer predict prognosis and immunotherapy outcomes. J Immunotherapy Cancer. (2021) 9:e003214. doi: 10.1136/jitc-2021-003214
56. Mondal P, Natesh J, Penta D, and Meeran SM. Progress and promises of epigenetic drugs and epigenetic diets in cancer prevention and therapy: A clinical update. Semin Cancer Biol. (2022) 83:503–22. doi: 10.1016/j.semcancer.2020.12.006
57. Shu L, Li X, Liu Z, Li K, Shi A, Tang Y, et al. Bile exosomal miR-182/183-5p increases cholangiocarcinoma stemness and progression by targeting HPGD and increasing PGE2 generation. Hepatology. (2024) 79:307–22. doi: 10.1097/HEP.0000000000000437
58. Peng W, Xie Y, Luo Z, Liu Y, Xu J, Li C, et al. UTX deletion promotes M2 macrophage polarization by epigenetically regulating endothelial cell-macrophage crosstalk after spinal cord injury. J Nanobiotechnology. (2023) 21:225. doi: 10.1186/s12951-023-01986-0
59. Machour FE R, Abu-Zhayia E, Kamar J, Barisaac AS, Simon I, and Ayoub N. Harnessing DNA replication stress to target RBM10 deficiency in lung adenocarcinoma. Nat Commun. (2024) 15:6417. doi: 10.1038/s41467-024-50882-0
60. Zhou C, Wei W, Ma J, Yang Y, Liang L, Zhang Y, et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol Ther. (2021) 29:1512–28. doi: 10.1016/j.ymthe.2020.12.034
61. Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology. (2022) 76:982–99. doi: 10.1002/hep.32387
62. Thepmalee C, Panya A, Junking M, Chieochansin T, and Yenchitsomanus P-t. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccines immunotherapeutics. (2018) 14:1423–31. doi: 10.1080/21645515.2018.1431598
63. Chen Z, Li H, Li Z, Chen S, Huang X, Zheng Z, et al. SHH/GLI2-TGF-β1 feedback loop between cancer cells and tumor-associated macrophages maintains epithelial-mesenchymal transition and endoplasmic reticulum homeostasis in cholangiocarcinoma. Pharmacol Res. (2023) 187:106564. doi: 10.1016/j.phrs.2022.106564
64. Tomić S, Joksimović B, Bekić M, Vasiljević M, Milanović M, Čolić M, et al. Prostaglanin-E2 potentiates the suppressive functions of human mononuclear myeloid-derived suppressor cells and increases their capacity to expand IL-10-producing regulatory T cell subsets. Front Immunol. (2019) 10:475. doi: 10.3389/fimmu.2019.00475
65. Zhang G, Zheng G, Zhang H, and Qiu L. MUC1 induces the accumulation of Foxp3+ Treg cells in the tumor microenvironment to promote the growth and metastasis of cholangiocarcinoma through the EGFR/PI3K/Akt signaling pathway. Int Immunopharmacol. (2023) 118:110091. doi: 10.1016/j.intimp.2023.110091
66. Nguyen MLT, Bui KC, Scholta T, Xing J, Bhuria V, Sipos B, et al. Targeting interleukin 6 signaling by monoclonal antibody siltuximab on cholangiocarcinoma. J Gastroenterol hepatology. (2021) 36:1334–45. doi: 10.1111/jgh.15307
67. Thongchot S, Vidoni C, Ferraresi A, Loilome W, Khuntikeo N, Sangkhamanon S, et al. Cancer-associated fibroblast-derived IL-6 determines unfavorable prognosis in cholangiocarcinoma by affecting autophagy-associated chemoresponse. Cancers. (2021) 13:2134. doi: 10.3390/cancers13092134
68. Wu M-J, Shi L, Dubrot J, Merritt J, Vijay V, Wei T-Y, et al. Mutant IDH inhibits IFNγ–TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer discovery. (2022) 12:812–35. doi: 10.1158/2159-8290.CD-21-1077
69. Delahousse J, Verlingue L, Broutin S, Legoupil C, Touat M, Doucet L, et al. Circulating oncometabolite D-2-hydroxyglutarate enantiomer is a surrogate marker of isocitrate dehydrogenase–mutated intrahepatic cholangiocarcinomas. Eur J Cancer. (2018) 90:83–91. doi: 10.1016/j.ejca.2017.11.024
70. Mu L, Long Y, Yang C, Jin L, Tao H, Ge H, et al. The IDH1 mutation-induced oncometabolite, 2-hydroxyglutarate, may affect DNA methylation and expression of PD-L1 in gliomas. Front Mol Neurosci. (2018) 11:82. doi: 10.3389/fnmol.2018.00082
71. Roy S, Kumaravel S, Banerjee P, White TK, O’Brien A, Seelig C, et al. Tumor lymphatic interactions induce CXCR2-CXCL5 axis and alter cellular metabolism and lymphangiogenic pathways to promote cholangiocarcinoma. Cells. (2021) 10:3093. doi: 10.3390/cells10113093
72. Jiang L, Liu M, Cai X, Xie L, She F, and Chen Y. Serum vascular endothelial growth factor−C levels predict lymph node metastasis and prognosis of patients with gallbladder cancer. Oncol letters. (2018) 16:6065–70. doi: 10.3892/ol.2018.9358
73. Mancarella S, Gigante I, Serino G, Pizzuto E, Dituri F, Valentini MF, et al. Crenigacestat blocking notch pathway reduces liver fibrosis in the surrounding ecosystem of intrahepatic CCA viaTGF-β inhibition. J Exp Clin Cancer Res. (2022) 41:331. doi: 10.1186/s13046-022-02536-6
74. Nicolás-Boluda A, Vaquero J, Laurent G, Renault G, Bazzi R, Donnadieu E, et al. Photothermal depletion of cancer-associated fibroblasts normalizes tumor stiffness in desmoplastic cholangiocarcinoma. ACS nano. (2020) 14:5738–53. doi: 10.1021/acsnano.0c00417
75. Sha M, Jeong S, Bj Q, Tong Y, Xia L, Xu N, et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. (2018) 7:4665–77. doi: 10.1002/cam4.1704
76. Thongchot S, Ferraresi A, Vidoni C, Loilome W, Yongvanit P, Namwat N, et al. Resveratrol interrupts the pro-invasive communication between cancer associated fibroblasts and cholangiocarcinoma cells. Cancer letters. (2018) 430:160–71. doi: 10.1016/j.canlet.2018.05.031
77. Pan M-S, Wang H, Ansari KH, Li X-P, Sun W, and Fan Y-Z. Gallbladder cancer-associated fibroblasts promote vasculogenic mimicry formation and tumor growth in gallbladder cancer via upregulating the expression of NOX4, a poor prognosis factor, through IL-6-JAK-STAT3 signal pathway. J Exp Clin Cancer Res. (2020) 39:1–21. doi: 10.1186/s13046-020-01742-4
78. Mancarella S, Serino G, Coletta S, Armentano R, Dituri F, Ardito F, et al. The tumor microenvironment drives intrahepatic cholangiocarcinoma progression. Int J Mol Sci. (2022) 23:4187. doi: 10.3390/ijms23084187
79. Cigliano A, Wang J, Chen X, and Calvisi DF. Role of the Notch signaling in cholangiocarcinoma. Expert Opin Ther targets. (2017) 21:471–83. doi: 10.1080/14728222.2017.1310842
80. Qin X, Lu M, Li G, Zhou Y, and Liu Z. Downregulation of tumor-derived exosomal miR-34c induces cancer-associated fibroblast activation to promote cholangiocarcinoma progress. Cancer Cell Int. (2021) 21:1–15. doi: 10.1186/s12935-020-01726-6
81. Utaijaratrasmi P, Vaeteewoottacharn K, Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C, et al. The microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated fibroblasts promotes migration of cancer cells. Mol Cancer. (2018) 17:1–14. doi: 10.1186/s12943-018-0760-x
82. Guo Q-r, Wang H, Yan Y-d, Liu Y, Su C-y, Chen H-b, et al. The role of exosomal microRNA in cancer drug resistance. Front Oncol. (2020) 10:472. doi: 10.3389/fonc.2020.00472
83. Tomassetti C, Insinga G, Gimigliano F, Morrione A, Giordano A, and Giurisato E. Insights into CSF-1R expression in the tumor microenvironment. Biomedicines. (2024) 12:2381. doi: 10.3390/biomedicines12102381
84. Pan Y, Zhou Y, Shen Y, Xu L, Liu H, Zhang N, et al. Hypoxia stimulates PYGB enzymatic activity to promote glycogen metabolism and cholangiocarcinoma progression. Cancer Res. (2024) 84:3803–17. doi: 10.1158/0008-5472.CAN-24-0088
85. Ruiz de Gauna M, Biancaniello F, González-Romero F, Rodrigues PM, Lapitz A, Gómez-Santos B, et al. Cholangiocarcinoma progression depends on the uptake and metabolization of extracellular lipids. Hepatology. (2022) 76:1617–33. doi: 10.1002/hep.32344
86. Raggi C, Taddei ML, Sacco E, Navari N, Correnti M, Piombanti B, et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J Hepatology. (2021) 74:1373–85. doi: 10.1016/j.jhep.2020.12.031
87. Xia T, Li K, Niu N, Shao Y, Ding D, Thomas DL, et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. J Hematol Oncol. (2022) 15:37. doi: 10.1186/s13045-022-01253-z
88. Merters J and Lamarca A. Integrating cytotoxic, targeted and immune therapies for cholangiocarcinoma. J hepatology. (2023) 78:652–7. doi: 10.1016/j.jhep.2022.11.005
89. Harding JJ, Fan J, Oh D-Y, Choi HJ, Kim JW, Chang H-M, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. (2023) 24:772–82. doi: 10.1016/S1470-2045(23)00242-5
90. Falcomatà C, Bärthel S, Ulrich A, Diersch S, Veltkamp C, Rad L, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer discovery. (2021) 11:3158–77. doi: 10.1158/2159-8290.CD-21-0209
91. Nepal C, Zhu B, O’Rourke CJ, Bhatt DK, Lee D, Song L, et al. Integrative molecular characterisation of gallbladder cancer reveals micro-environment-associated subtypes. J hepatology. (2021) 74:1132–44. doi: 10.1016/j.jhep.2020.11.033
92. McNamara MG, Lopes A, Wasan H, Malka D, Goldstein D, Shannon J, et al. Landmark survival analysis and impact of anatomic site of origin in prospective clinical trials of biliary tract cancer. J hepatology. (2020) 73:1109–17. doi: 10.1016/j.jhep.2020.05.014
93. Kam AE, Masood A, and Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol hepatology. (2021) 6:956–69. doi: 10.1016/S2468-1253(21)00171-0
94. Oh D-Y, Lee K-H, Lee D-W, Yoon J, Kim T-Y, Bang J-H, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol hepatology. (2022) 7:522–32. doi: 10.1016/S2468-1253(22)00043-7
95. Yang Y, Pei T, Liu C, Cao M, Hu X, Yuan J, et al. Glutamine metabolic competition drives immunosuppressive reprogramming of intratumour GPR109A+ myeloid cells to promote liver cancer progression. Gut. (2025) 74:255–69. doi: 10.1136/gutjnl-2024-332429
96. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis primers. (2021) 7:65. doi: 10.1038/s41572-021-00300-2
97. Goeppert B, Stichel D, Toth R, Fritzsche S, Loeffler MA, Schlitter AM, et al. Integrative analysis reveals early and distinct genetic and epigenetic changes in intraductal papillary and tubulopapillary cholangiocarcinogenesis. Gut. (2022) 71:391–401. doi: 10.1136/gutjnl-2020-322983
98. Zhu Y and Kwong LN. IDH1 inhibition reawakens the immune response against cholangiocarcinoma. Cancer discovery. (2022) 12:604–5. doi: 10.1158/2159-8290.CD-21-1643
99. Li Z, Liu J, Chen T, Sun R, Liu Z, Qiu B, et al. HMGA1-TRIP13 axis promotes stemness and epithelial mesenchymal transition of perihilar cholangiocarcinoma in a positive feedback loop dependent on c-Myc. J Exp Clin Cancer Res. (2021) 40:1–18. doi: 10.1186/s13046-021-01890-1
100. Sapisochin G, Ivanics T, and Heimbach J. Liver transplantation for intrahepatic cholangiocarcinoma: ready for prime time? Hepatology. (2022) 75:455–72. doi: 10.1002/hep.32258
101. Wang S, Wang Y, Zhu C, Liu K, Chao J, Zhang N, et al. Conversion surgery intervention versus continued systemic therapy in patients with a response after PD-1/PD-L1 inhibitor-based combination therapy for initially unresectable biliary tract cancer: a retrospective cohort study. Int J Surgery. (2024) 110:4608–16. doi: 10.1097/JS9.0000000000001540
102. Khorsandi SE, Dokal AD, Rajeeve V, Britton DJ, Illingworth MS, Heaton N, et al. Computational analysis of cholangiocarcinoma phosphoproteomes identifies patient-specific drug targets. Cancer Res. (2021) 81:5765–76. doi: 10.1158/0008-5472.CAN-21-0955
103. Zhu X-y, Liu W-t, Hou X-j, Zong C, Yu W, Shen Z-m, et al. CD34+ CLDN5+ tumor associated senescent endothelial cells through IGF2-IGF2R signaling increased cholangiocellular phenotype in hepatocellular carcinoma. J Advanced Res. (2024). doi: 10.1016/j.jare.2024.12.008
104. Liu Q, Guan Y, and Li S. Programmed death receptor (PD-) 1/PD-ligand (L) 1 in urological cancers: the “all-around warrior” in immunotherapy. Mol cancer. (2024) 23:183. doi: 10.1186/s12943-024-02095-8
105. Cai S, Chen Z, Wang Y, Wang M, Wu J, Tong Y, et al. Reducing PD-L1 expression with a self-assembled nanodrug: an alternative to PD-L1 antibody for enhanced chemo-immunotherapy. Theranostics. (2021) 11:1970–81. doi: 10.7150/thno.45777
106. Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L, et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res. (2021) 40:184. doi: 10.1186/s13046-021-01987-7
107. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. (2020) 6:888–94. doi: 10.1001/jamaoncol.2020.0930
108. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J cancer. (2020) 147:2190–8. doi: 10.1002/ijc.33013
109. Li X, Gao L, Wang B, Hu J, Yu Y, Gu B, et al. FXa-mediated PAR-2 promotes the efficacy of immunotherapy for hepatocellular carcinoma through immune escape and anoikis resistance by inducing PD-L1 transcription. J immunotherapy cancer. (2024) 12:e009565. doi: 10.1136/jitc-2024-009565
110. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J immunotherapy cancer. (2020) 8:e001240. doi: 10.1136/jitc-2020-001240
111. Macarulla T, Ren Z, Chon HJ, Park JO, Kim JW, Pressiani T, et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: clinical and biomarker data from the randomized phase II IMbrave151 trial. J Clin Oncol. (2025) 43:545–57. doi: 10.1200/JCO.24.00337
112. Benmebarek M-R, Oguz C, Seifert M, Ruf B, Myojin Y, Bauer KC, et al. Anti-vascular endothelial growth factor treatment potentiates immune checkpoint blockade through a BAFF-and IL-12-dependent reprogramming of the TME. Immunity. (2025) 58:926–45.e10. doi: 10.1016/j.immuni.2025.02.017
113. Yarchoan M, Cope L, Ruggieri AN, Anders RA, Noonan AM, Goff LW, et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J Clin Invest. (2021) 131. doi: 10.1172/JCI152670
114. Petty AJ, Dai R, Lapalombella R, Baiocchi RA, Benson DM, Li Z, et al. Hedgehog-induced PD-L1 on tumor-associated macrophages is critical for suppression of tumor-infiltrating CD8+ T cell function. JCI Insight. (2021) 6:e146707. doi: 10.1172/jci.insight.146707
115. Galluzzi L, Humeau J, Buqué A, Zitvogel L, and Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. (2020) 17:725–41. doi: 10.1038/s41571-020-0413-z
116. Zhang P, Yue L, Leng Q, Chang C, Gan C, Ye T, et al. Targeting FGFR for cancer therapy. J Hematol Oncol. (2024) 17:39. doi: 10.1186/s13045-024-01558-1
117. Baretti M, Shekhar S, Sahai V, Shu D, Howe K, Gunchick V, et al. Deep immune profiling of intrahepatic cholangiocarcinoma with CODEX multiplexed imaging. Hepatol Commun. (2025) 9:e0632. doi: 10.1097/HC9.0000000000000632
118. Goyal L, DiToro D, Facchinetti F, Martin E, Peng P, Baiev I, et al. A model for decoding resistance in precision oncology: acquired resistance to FGFR inhibitors in cholangiocarcinoma. Ann Oncol. (2024) 36:426–43. doi: 10.1016/j.annonc.2024.12.011
119. Cleary JM, Rouaisnel B, Daina A, Raghavan S, Roller LA, Huffman BM, et al. Secondary IDH1 resistance mutations and oncogenic IDH2 mutations cause acquired resistance to ivosidenib in cholangiocarcinoma. NPJ Precis Oncol. (2022) 6:61. doi: 10.1038/s41698-022-00304-5
120. Doleschal B, Taghizadeh H, Webersinke G, Piringer G, Schreil G, Decker J, et al. Real world evidence reveals improved survival outcomes in biliary tract cancer through molecular matched targeted treatment. Sci Rep. (2023) 13:15421. doi: 10.1038/s41598-023-42083-4
121. Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. (2020) 130:5380–96. doi: 10.1172/JCI137110
122. Wang L, Guo W, Guo Z, Yu J, Tan J, Simons DL, et al. PD-L1-expressing tumor-associated macrophages are immunostimulatory and associate with good clinical outcome in human breast cancer. Cell Rep Med. (2024) 5. doi: 10.1016/j.xcrm.2024.101420
123. Alvisi G, Termanini A, Soldani C, Portale F, Carriero R, Pilipow K, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J hepatology. (2022) 77:1359–72. doi: 10.1016/j.jhep.2022.05.043
124. Kang JH and Zappasodi R. Modulating Treg stability to improve cancer immunotherapy. Trends cancer. (2023) 9:911–27. doi: 10.1016/j.trecan.2023.07.015
125. Cui T, Sun L, Guo X, Cheng C, Zhang N, Zhou S, et al. Tumor-derived CD109 orchestrates reprogramming of tumor-associated macrophages to dampen immune response. J Hepatol. (2025). doi: 10.1016/j.jhep.2025.03.035
126. Lin H, Liu C, Hu A, Zhang D, Yang H, and Mao Y. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J Hematol Oncol. (2024) 17:31. doi: 10.1186/s13045-024-01544-7
127. Yan W, Li Y, Zou Y, Zhu R, Wu T, Sun X, et al. Breaking tumor immunosuppressive network by regulating multiple nodes with triadic drug delivery nanoparticles. ACS nano. (2023) 17:17826–44. doi: 10.1021/acsnano.3c03387
128. Li T, Xu D, Ruan Z, Zhou J, Sun W, Rao B, et al. Metabolism/immunity dual-regulation thermogels potentiating immunotherapy of glioblastoma through lactate-excretion inhibition and PD-1/PD-L1 blockade. Advanced science. (2024) 11:2310163. doi: 10.1002/advs.202310163
129. Liu P-S, Chen Y-T, Li X, Hsueh P-C, Tzeng S-F, Chen H, et al. CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions. Nat Immunol. (2023) 24:452–62. doi: 10.1038/s41590-023-01430-3
130. Ruffolo LI, Jackson KM, Kuhlers PC, Dale BS, Guilliani NMF, Ullman NA, et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut. (2022) 71:1386–98. doi: 10.1136/gutjnl-2021-324109
131. Semmrich M, Marchand J-B, Fend L, Rehn M, Remy C, Holmkvist P, et al. Vectorized Treg-depleting αCTLA-4 elicits antigen cross-presentation and CD8+ T cell immunity to reject ‘cold’tumors. J Immunotherapy Cancer. (2022) 10:e003488. doi: 10.1136/jitc-2021-003488
132. Maksymova L, Pilger YA, Nuhn L, and Van Ginderachter JA. Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines. Mol Cancer. (2025) 24:65. doi: 10.1186/s12943-025-02270-5
133. Sun K, Zhang X, Shi J, Huang J, Wang S, Li X, et al. Elevated protein lactylation promotes immunosuppressive microenvironment and therapeutic resistance in pancreatic ductal adenocarcinoma. J Clin Invest. (2025) 135. doi: 10.1172/JCI187024
134. Wan M, Pan S, Shan B, Diao H, Jin H, Wang Z, et al. Lipid metabolic reprograming: the unsung hero in breast cancer progression and tumor microenvironment. Mol Cancer. (2025) 24:61. doi: 10.1186/s12943-025-02258-1
135. Jin H-R, Wang J, Wang Z-J, Xi M-J, Xia B-H, Deng K, et al. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. (2023) 16:103. doi: 10.1186/s13045-023-01498-2
136. Shao N, Qiu H, Liu J, Xiao D, Zhao J, Chen C, et al. Targeting lipid metabolism of macrophages: A new strategy for tumor therapy. J Advanced Res. (2025) 68:99–114. doi: 10.1016/j.jare.2024.02.009
137. Han H, Wang S, Shahbazi M-A, Du Y, Zuhorn IS, Li J, et al. Local glycolysis-modulating hydrogel microspheres for a combined anti-tumor and anti-metastasis strategy through metabolic trapping strategy. J Controlled Release. (2025) 378:320–33. doi: 10.1016/j.jconrel.2024.12.025
138. Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. (2020) 72:965–81. doi: 10.1002/hep.31092
139. Belle JI, Sen D, Baer JM, Liu X, Lander VE, Ye J, et al. Senescence defines a distinct subset of myofibroblasts that orchestrates immunosuppression in pancreatic cancer. Cancer discovery. (2024) 14:1324–55. doi: 10.1158/2159-8290.CD-23-0428
140. Zhao L, Liu J, Li K, Zhang C, Chen T, Liu Z, et al. PTPN9 dephosphorylates FGFR2pY656/657 through interaction with ACAP1 and ameliorates pemigatinib effect in cholangiocarcinoma. Hepatology. (2024) 79:798–812. doi: 10.1097/HEP.0000000000000552
141. Baker H. Futibatinib for intrahepatic cholangiocarcinoma. 525 B STREET, STE 1900, SAN DIEGO, CA 92101–4495 USA: ELSEVIER INC (2023).
142. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. (2021) 7:1669–77. doi: 10.1001/jamaoncol.2021.3836
143. Yan D, Li X, Wang L, Cheng Y, and Liu S. Addition of PD-1 antibody camrelizumab overcame resistance to trastuzumab plus chemotherapy in a HER2-positive, metastatic gallbladder cancer patient. Cancer Res. (2022) 82:5517. doi: 10.1158/1538-7445.AM2022-5517
144. Kim DH, Im BN, Hwang HS, and Na K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials. (2018) 183:139–50. doi: 10.1016/j.biomaterials.2018.08.052
145. Fang T, Cao X, Shen B, Chen Z, and Chen G. Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment. Biomaterials. (2023) 300:122189. doi: 10.1016/j.biomaterials.2023.122189
146. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J, et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunotherapy Cancer. (2020) 8:e000367. doi: 10.1136/jitc-2019-000367
147. Shen W, Liu T, Pei P, Li J, Yang S, Zhang Y, et al. Metabolic homeostasis-regulated nanoparticles for antibody-independent cancer radio-immunotherapy. Advanced Materials. (2022) 34:2207343. doi: 10.1002/adma.202207343
148. Yang J, Su T, Wang Q, Shi R, Ding J, and Chen X. Glucose metabolism-targeted poly (amino acid) nanoformulation of oxaliplatin (IV)-aspirin prodrug for enhanced chemo-immunotherapy. Advanced Materials. (2025) 2419033. doi: 10.1002/adma.202419033
149. Pereira B, Chen CT, Goyal L, Walmsley C, Pinto CJ, Baiev I, et al. Cell-free DNA captures tumor heterogeneity and driver alterations in rapid autopsies with pre-treated metastatic cancer. Nat Commun. (2021) 12:3199. doi: 10.1038/s41467-021-23394-4
150. Du J, Lv X, Zhang Z, Huang Z, and Zhang E. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma. Front Immunol. (2023) 14:1142690. doi: 10.3389/fimmu.2023.1142690
151. Dickens A, Yuryev A, Catanzaro J, and Khan SS. Beyond chemotherapy: precision oncology and the shift toward non-toxic, adaptive cancer therapies. J Precis Biosciences. (2023) 5:1–9. doi: 10.25163/biosciences.515805
152. Katoh M, Loriot Y, Brandi G, Tavolari S, Wainberg ZA, and Katoh M. FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions. Nat Rev Clin Oncol. (2024) 21:312–29. doi: 10.1038/s41571-024-00869-z
153. Tang T, Huang X, Zhang G, Hong Z, Bai X, and Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal transduction targeted Ther. (2021) 6:72. doi: 10.1038/s41392-020-00449-4
154. Yang Y, Li S, To KK, Zhu S, Wang F, and Fu L. Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy. J Exp Clin Cancer Res. (2025) 44:145. doi: 10.1186/s13046-025-03377-9
155. Birnboim-Perach R and Benhar I. Using Combination therapy to overcome diverse challenges of Immune Checkpoint Inhibitors treatment. Int J Biol Sci. (2024) 20:3911. doi: 10.7150/ijbs.93697
156. Ahmed J, Das B, Shin S, and Chen A. Challenges and future directions in the management of tumor mutational burden-high (TMB-H) advanced solid Malignancies. Cancers. (2023) 15:5841. doi: 10.3390/cancers15245841
157. Shi R, Tang YQ, and Miao H. Metabolism in tumor microenvironment: Implications for cancer immunotherapy. MedComm. (2020) 1:47–68. doi: 10.1002/mco2.6
158. Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun. (2018) 9:741. doi: 10.1038/s41467-017-02696-6
159. Zhao F, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, et al. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity. (2018) 48:147–60.e7. doi: 10.1016/j.immuni.2017.12.004
160. Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. (2021) 12:440. doi: 10.1038/s41467-020-20723-x
161. Calabrese LH, Calabrese C, and Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. (2018) 14:569–79. doi: 10.1038/s41584-018-0074-9
162. Jing Y, Yang J, Johnson DB, Moslehi JJ, and Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. (2022) 19:269–80. doi: 10.1038/s41571-021-00597-8
163. Şen GA, Öztaş NŞ, Değerli E, Safarov S, Guliyev M, Bedir Ş, et al. Effects of immune related adverse events and corticosteroids on the outcome of patients treated with immune checkpoint inhibitors. Sci Rep. (2025) 15:6310. doi: 10.1038/s41598-025-91102-z
164. Ruf T, Kramer R, Forschner A, Leiter U, Meier F, Reinhardt L, et al. Second-line therapies for steroid-refractory immune-related adverse events in patients treated with immune checkpoint inhibitors. Eur J Cancer. (2024) 203:114028. doi: 10.1016/j.ejca.2024.114028
165. Alouani E, Laparra A, Perret A, Sakkal M, Messayke S, Danlos F-X, et al. Immunosuppressant mycophenolate mofetil for patients with steroid-refractory immune-related hepatitis induced by checkpoint inhibitors in oncology. Eur J Cancer. (2023) 193:113313. doi: 10.1016/j.ejca.2023.113313
166. Nagra D, Song K, Odia J, Wincup C, Mahto A, Rosa C, et al. The therapeutic potential for JAK inhibitors for immune-related adverse events from checkpoint inhibitors–a review of the literature. Rheumatology. (2025), keaf356. doi: 10.1136/annrheumdis-2025-eular.B133
167. Wang X, Niu X, An N, Sun Y, and Chen Z. Comparative efficacy and safety of immunotherapy alone and in combination with chemotherapy for advanced non-small cell lung cancer. Front Oncol. (2021) 11:611012. doi: 10.3389/fonc.2021.611012
168. Chen W, Shen L, Jiang J, Zhang L, Zhang Z, Pan J, et al. Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment. biomark Res. (2021) 9:59. doi: 10.1186/s40364-021-00312-w
169. Stephen B, Hajjar J, Sarda S, Duose DY, Conroy JM, Morrison C, et al. T-cell receptor beta variable gene polymorphism predicts immune-related adverse events during checkpoint blockade immunotherapy. J immunotherapy cancer. (2023) 11:e007236. doi: 10.1136/jitc-2023-007236
170. Luchini C, Bibeau F, Ligtenberg M, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30:1232–43. doi: 10.1093/annonc/mdz116
171. Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti–programmed cell death (PD)-1 and anti–programmed death-ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. (2018) 36:633–41. doi: 10.1200/JCO.2017.75.3384
172. Pei L, Liu Y, Liu L, Gao S, Gao X, Feng Y, et al. Roles of cancer-associated fibroblasts (CAFs) in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol cancer. (2023) 22:29. doi: 10.1186/s12943-023-01731-z
173. Kundu M, Butti R, Panda VK, Malhotra D, Das S, Mitra T, et al. Modulation of the tumor microenvironment and mechanism of immunotherapy-based drug resistance in breast cancer. Mol Cancer. (2024) 23:92. doi: 10.1186/s12943-024-01990-4
174. Hou L, Liu Q, Shen L, Liu Y, Zhang X, Chen F, et al. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics. (2018) 8:3781. doi: 10.7150/thno.24821
175. Rimassa L, Lamarca A, O’Kane GM, Edeline J, McNamara MG, Vogel A, et al. New systemic treatment paradigms in advanced biliary tract cancer and variations in patient access across Europe. Lancet Regional Health–Europe. (2025) 50. doi: 10.1016/j.lanepe.2024.101170
176. Luo Z, Yao X, Li M, Fang D, Fei Y, Cheng Z, et al. Modulating tumor physical microenvironment for fueling CAR-T cell therapy. Advanced Drug delivery Rev. (2022) 185:114301. doi: 10.1016/j.addr.2022.114301
177. Qiao Y, Chen J, Wang X, Yan S, Tan J, Xia B, et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun. (2023) 43:788–807. doi: 10.1002/cac2.12452
178. Li X-N, Wang F, Chen K, Wu Z, Zhang R, Xiao C, et al. XCL1-secreting CEA CAR-T cells enhance endogenous CD8+ T cell responses to tumor neoantigens to confer a long-term antitumor immunity. J Immunotherapy Cancer. (2025) 13:e010581. doi: 10.1136/jitc-2024-010581
179. Li Y-R, Lyu Z, Shen X, Fang Y, and Yang L. Boosting CAR-T cell therapy through vaccine synergy. Trends Pharmacol Sci. (2024) 46:180–99. doi: 10.1016/j.tips.2024.12.004
180. Huang X, Tang T, Zhang G, and Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol cancer. (2021) 20:1–17. doi: 10.1186/s12943-021-01342-6
181. Zaravinos A, Roufas C, Nagara M, de Lucas Moreno B, Oblovatskaya M, Efstathiades C, et al. Cytolytic activity correlates with the mutational burden and deregulated expression of immune checkpoints in colorectal cancer. J Exp Clin Cancer Res. (2019) 38:1–18. doi: 10.1186/s13046-019-1372-z
182. Lee WJ, Lee YJ, Choi ME, Yun KA, Won CH, Lee MW, et al. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J Am Acad Dermatol. (2019) 81:219–27. doi: 10.1016/j.jaad.2019.03.012
183. Cai L, Li Y, Tan J, Xu L, and Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. (2023) 16:101. doi: 10.1186/s13045-023-01499-1
184. Shaw G, Cavalcante L, Giles FJ, and Taylor A. Elraglusib (9-ING-41), a selective small-molecule inhibitor of glycogen synthase kinase-3 beta, reduces expression of immune checkpoint molecules PD-1, TIGIT and LAG-3 and enhances CD8+ T cell cytolytic killing of melanoma cells. J Hematol Oncol. (2022) 15:134. doi: 10.1186/s13045-022-01352-x
185. Ta HM, Roy D, Zhang K, Alban T, Juric I, Dong J, et al. LRIG1 engages ligand VISTA and impairs tumor-specific CD8+ T cell responses. Sci Immunol. (2024) 9:eadi7418. doi: 10.1126/sciimmunol.adi7418
186. Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, et al. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. (2020) 13:1–13. doi: 10.1186/s13045-020-00917-y
187. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Kim JW, et al. Durvalumab plus chemotherapy in advanced biliary tract cancer: 3-year overall survival update from the phase III TOPAZ-1 study. J Hepatol. (2025). doi: 10.1016/j.jhep.2025.05.003
188. Xu Z, Wang X, Zeng S, Ren X, Yan Y, and Gong Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm Sin B. (2021) 11:3393–405. doi: 10.1016/j.apsb.2021.02.007
189. Saxena R, Sharma V, Saxena AR, and Patel A. Harnessing AI and gut microbiome research for precision health. J Artif Intell Gen Sci (JAIGS). (2024) 3:74–88. doi: 10.60087/jaigs.v3i1.68
190. Choudhry H. The microbiome and its implications in cancer immunotherapy. Molecules. (2021) 26:206. doi: 10.3390/molecules26010206
191. Russo E, Nannini G, Dinu M, Pagliai G, Sofi F, and Amedei A. Exploring the food-gut axis in immunotherapy response of cancer patients. World J Gastroenterology. (2020) 26:4919. doi: 10.3748/wjg.v26.i33.4919
192. Cammarota G, Ianiro G, Ahern A, Carbone C, Temko A, Claesson MJ, et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat Rev Gastroenterol hepatology. (2020) 17:635–48. doi: 10.1038/s41575-020-0327-3
193. Koumpouros Y. Innovations in precision medicine and genomics: IGI global. (2025). doi: 10.4018/979-8-3693-5787-3
194. Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. (2020) 21:1234–43. doi: 10.1016/S1470-2045(20)30321-1
195. Yoon JG, Kim MH, Jang M, Kim H, Hwang HK, Kang CM, et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology. (2021) 74:1914–31. doi: 10.1002/hep.31862
196. Ren X, Huang M, Weng W, Xie Y, Wu Y, Zhu S, et al. Personalized drug screening in patient-derived organoids of biliary tract cancer and its clinical application. Cell Rep Med. (2023) 4. doi: 10.1016/j.xcrm.2023.101277
Keywords: biliary tract cancer, molecular mechanisms, immunotherapy, tumour microenvironment, combination therapy precision treatment strategies
Citation: Xue J, Zhang L, Zhang K, Zhou K and Zhao H (2025) Immunotherapy in biliary tract cancer: reshaping the tumour microenvironment and advancing precision combination strategies. Front. Immunol. 16:1651769. doi: 10.3389/fimmu.2025.1651769
Received: 22 June 2025; Accepted: 21 July 2025;
Published: 08 August 2025.
Edited by:
Ran Wei, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Taiyu Shang, Fudan University, ChinaJianan Jin, Zhejiang Chinese Medical University, China
Copyright © 2025 Xue, Zhang, Zhang, Zhou and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Zhao, emhhb2h0QHB1bWNoLmNu; Kai Zhou, Mzk2ODQzMTIyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jingnan Xue
Jingnan Xue Longhao Zhang
Longhao Zhang Kai Zhang
Kai Zhang Kai Zhou
Kai Zhou Haitao Zhao
Haitao Zhao