- 1Department of Nephrology, Hangzhou Traditional Chinese Medicine Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2The Second School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Department of Respiratory Internal Medicine, Lishui Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Lishui, Zhejiang, China
- 4Department of Nephrology, Lishui Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Lishui, Zhejiang, China
Primary membranous nephropathy (PMN) is a major cause of adult nephrotic syndrome and demonstrates considerable clinical heterogeneity. This review summarizes current evidence on the immunological mechanisms and clinical predictors underlying spontaneous remission (SR) in PMN. We discuss key factors including the dynamics of anti-PLA2R antibodies, proteinuria trends, renal function indicators, histopathological features, and emerging biomarkers. A staged immune modulation process is proposed, involving suppression of autoreactive responses and promotion of tissue repair. Integrating these insights, we also outline a personalized treatment approach based on dynamic risk stratification and longitudinal monitoring. Understanding the drivers of SR may help reduce unnecessary immunosuppression and guide precision management in PMN.
1 Introduction
Membranous nephropathy (MN) is a glomerular disease characterized by diffuse thickening of the glomerular basement membrane (GBM) (1, 2). It predominantly affects middle-aged and elderly individuals and represents one of the most common causes of nephrotic syndrome in adults (3, 4). The global incidence of MN is estimated at 8–10 cases per million population (3, 5). In China, MN accounts for 23.4% of biopsy-proven glomerular diseases, second only to IgA nephropathy, with a steadily increasing incidence over recent years (6). MN can be classified into primary and secondary forms, with primary MN (PMN) accounting for approximately 80% of cases (7). A major milestone in understanding MN pathogenesis was achieved in 2009, when Beck et al. identified the M-type phospholipase A2 receptor (PLA2R), located on podocyte membranes, as the predominant target antigen in idiopathic MN, along with its corresponding autoantibody (8). This discovery established MN as an autoimmune disease and significantly transformed both diagnostic and monitoring strategies. Subsequently, other target antigens, such as THSD7A, have also been identified (9, 10).
With growing insights into its pathogenesis, the diagnosis and treatment of MN have evolved toward more specific and effective approaches. Currently, immunosuppressive therapy, anti-CD20 monoclonal antibodies, and other novel therapies are the mainstay of clinical management (11). Notably, MN exhibits a highly heterogeneous clinical course, ranging from spontaneous remission (SR) to progression to end-stage kidney disease (ESKD). Approximately one-third of patients with PMN exhibit a benign or indolent disease course, with a SR rate of up to 30% (12). Another one-third of patients develop nephrotic syndrome while maintaining preserved renal function. Nonetheless, 15–50% of untreated patients progress to ESKD within 10 years (19, 20). SR is therefore an important clinical phenomenon in MN, with substantial implications for long-term prognosis and therapeutic decision-making. The occurrence of SR indicates that some patients may improve without the need for intensive immunosuppressive therapy, thereby avoiding the adverse effects associated with such treatments (13). However, due to the substantial proportion of patients who exhibit persistent disease activity or progression, the optimal timing to initiate immunosuppressive therapy remains controversial (14).
The KDIGO 2012 guideline took a conservative approach—it recommended up to 6 months of maximal supportive care before starting immunosuppressive therapy, in order to allow for potential SR. Immunosuppression was only advised for patients who remained at high risk of progression (15). This strategy, while avoiding unnecessary drug toxicity, had limited predictive accuracy—nearly half of patients meeting the high-risk criteria at 6 months may still go on to achieve SR without immunosuppressive therapy (16). In light of emerging prognostic markers, the KDIGO 2021 guideline introduced a more individualized, risk-stratified approach to managing primary MN (17). Patients are now categorized into low, moderate, high, or very high risk groups based on a combination of clinical and biomarker features rather than time-dependent proteinuria alone. Besides the degree of proteinuria and trend in renal function, the 2021 criteria incorporate serum albumin levels, PLA2R antibody titers, and even urinary biomarkers to refine risk assessment (18). According to the updated recommendations, patients in high-risk or very high-risk categories are advised to initiate immunosuppressive therapy promptly, rather than waiting a full six months, given their elevated risk of progressive kidney injury. In particular, individuals with persistently nephrotic-range proteinuria or with declining renal function are now considered for early intervention (18). This proactive strategy aims to prevent irreversible damage in those unlikely to undergo SR.
Crucially, even under the new paradigm, clinicians must recognize that some patients predicted to be “high risk” can still achieve SR. There are documented cases of very high-risk MN patients (with massive proteinuria and even complications like thrombosis) who have improved with conservative management alone (19). Such observations underscore the dynamic nature of MN: risk status at diagnosis is not a perfect predictor of disease trajectory. Therefore, current guidelines emphasize continual re-assessment and a personalized treatment approach. Careful monitoring of disease markers is recommended to guide if and when to escalate therapy. A thorough understanding of the mechanisms and determinants of SR in MN may help clinicians identify patients who are suitable for conservative management and those who require timely therapeutic intervention, ultimately facilitating personalized and precision medicine. This review summarizes the latest advances in the study of SR in MN, including mechanistic insights, clinical data and predictive factors, management strategies, and future research directions, aiming to provide a reference for clinical practice and further investigation.
2 Mechanisms of spontaneous remission
2.1 Immunological insights
The immunopathogenesis of PMN has been extensively elucidated. As the principal target cells in the immune response, podocytes serve as sites of injury by providing endogenous antigens or by creating a microenvironment that favors antigen deposition (20). In PMN, autoimmune injury is primarily mediated by autoantibodies that recognize target antigens on podocytes, leading to the formation of immune complexes beneath the foot processes and on the outer aspect of the GBM. These immune complexes activate the complement cascade and cause podocyte injury, resulting in increased GBM permeability and massive proteinuria, thereby initiating disease progression (21). However, not all MN patients exhibit progressive disease; a significant proportion experience spontaneous attenuation or cessation of the autoimmune response, leading to SR. Although the precise immunoregulatory mechanisms underlying SR remain unclear (22), clinical mechanistic data remain limited, as these low-risk patients have traditionally not been extensively studied. Nevertheless, biospecimen research in this population may yield valuable mechanistic insights into SR and help inform the development of more targeted and safer therapeutic strategies in MN. Several possible pathways have been proposed. Rosenzwajg et al. demonstrated the involvement of both B and T lymphocytes in the pathogenesis of MN (23). One hypothesis suggests that B lymphocytes possess an intrinsic self-limiting function. B cells differentiate into plasma cells, which produce anti-PLA2R antibodies targeting antigens on podocyte membranes (23). In some patients, the B cell clones responsible for anti-PLA2R antibody production may undergo exhaustion or be eliminated through immune surveillance over time, resulting in a spontaneous decline and eventual disappearance of antibody titers. A meta-analysis by Zhang J et al. further confirmed the predictive value of anti-PLA2R antibody levels for SR in idiopathic MN (IMN); lower baseline anti-PLA2R titers were associated with a higher likelihood of SR (24). In line with these findings, recent studies have shown that patients who achieve SR tend to have a less aggressive clone of autoreactive B cells and a more favorable immunoregulatory profile, including a recovery of regulatory B cells (Bregs) and regulatory T cells (Tregs) that are reduced during active disease but restored in remission (25). Restoration of immune tolerance in this context is thought to be mediated primarily through Tregs and potentially Bregs, which can suppress pathogenic immune responses and promote the re-establishment of immune homeostasis (26–28). In addition to the restoration of conventional CD4+ Tregs, recent experimental work has highlighted the contribution of CD8+ Tregs in MN immune regulation. Animal models of MN (Heymann nephritis) demonstrate a role for CD8+ T cells in down-regulating autoimmunity and attenuating disease. Classic adoptive-transfer studies identified antigen-specific OX8+ (CD8+) suppressor T cells that re-establish tolerance late in active Heymann’s nephritis (29). More recent work shows that CD8+ Tregs, induced by T-cell or peptide vaccination, limit autoantibody production and kidney injury by Qa-1 (HLA-E)–restricted elimination of autoreactive CD4+ T-follicular helper cells (30, 31). In humans, an analogous KIR+ CD8+ regulatory subset can delete pathogenic CD4+ T cells ex vivo across several autoimmune diseases (32), although its role in PMN remains to be defined. Together with Bregs and CD4+ Tregs, these CD8+ Tregs may provide an additional layer of immune regulation contributing to SR in MN.
Such immune tolerance mechanisms underscore the interplay between cellular and humoral immunity in MN, as T cells play a crucial role in supporting B cell-mediated antibody production. Enhanced activity of Tregs or suppression of pro-inflammatory T cells, such as follicular helper T cells (T_FH), could diminish B cell function. Clinical observations of disease remission following infections in some MN patients support the possibility of immune state reprogramming (33–35). It has been hypothesized that widespread immune activation induced by infection may paradoxically “reset” immune homeostasis and facilitate the disappearance of autoantibodies (23, 33). In addition, feedback regulation from the innate immune system may also contribute to SR. Ongoing immune complex deposition and podocyte injury might elicit negative feedback signals that attenuate immune responses. For example, anti-inflammatory cytokines secreted by macrophages during clearance of immune deposits or the upregulation of inhibitory co-stimulatory molecules such as PD-L1 on injured podocytes (36) may play roles in suppressing the autoimmune response. Nevertheless, these proposed mechanisms remain speculative and require further experimental validation.
2.2 Dynamics of anti-PLA2R antibodies
In PMN, approximately 70%–80% of patients have detectable circulating autoantibodies against the M-type PLA2R, predominantly of the IgG4 subclass (8). Serial monitoring of anti-PLA2R antibody levels has become an essential tool in disease assessment and offers important insights into the mechanisms of SR. Antibody titers closely correlate with disease activity, with elevated levels generally reflecting ongoing immune-mediated glomerular injury (37, 38). Clinically, a decline or seroconversion of anti-PLA2R antibody titers is often observed several months prior to the onset of SR (39–41). Studies on rituximab treatment in MN further support a temporal lag between antibody clearance and proteinuria resolution; whether antibody reduction is treatment-induced or occurs spontaneously, the improvement in proteinuria typically follows 3–6 months later (42). This temporal sequence suggests that a reduction in anti-PLA2R antibodies—whether through immunosuppressive treatment or intrinsic immune regulation—leads to a gradual cessation of new immune injury to the glomeruli. Subsequently, existing lesions enter a reparative phase, manifesting clinically as progressive reduction in proteinuria and eventual remission (42). In spontaneously remitting patients, the decline in antibody titers occurs endogenously.
Notably, baseline anti-PLA2R antibody levels are strongly predictive of disease course: patients with low titers are more likely to achieve SR, while those with high titers are less likely to do so. It has been reported that among patients with anti-PLA2R titers in the highest tertile, the probability of spontaneous immunologic remission (i.e., antibody clearance) under supportive care alone is as low as 4% (43–46). In contrast, patients with lower antibody levels may benefit from extended observation, allowing for the possibility of natural resolution of immune activity. In addition to PLA2R, anti-THSD7A antibodies are present in approximately 2.5%–5% of patients with primary MN and have similar pathogenic mechanisms (47). Although the SR rate of THSD7A-associated MN remains unclear, the frequent coexistence of malignancies in such cases suggests that resolution of the underlying tumor may contribute to disease remission (48). Beyond PLA2R and THSD7A, recent studies have identified other pathogenic podocyte antigens that together account for up to 20% of PMN cases, including neural epidermal growth factor-like 1 protein (NELL-1) (49), semaphorin-3B (SEMA3B) (50), protocadherin 7 (PCDH7) (51), and exostosin 1/2 (EXT1/2) (52). NELL-1, a secreted extracellular matrix–associated glycoprotein, accounts for ~5–10% of primary cases and is often detected in older adults, frequently in the context of malignancy. Histologically, NELL-1–associated MN is characterized by segmental or global subepithelial deposits with an IgG1-dominant pattern, and in paraneoplastic cases, tumor removal has been associated with disappearance of circulating antibodies and subsequent remission (49, 53). Although long-term data on SR in these non-PLA2R subtypes are limited, available evidence indicates that dynamic declines in their respective autoantibody levels—particularly following removal of an underlying trigger—may parallel the immunologic–clinical remission sequence observed in PLA2R-associated MN. Overall, dynamic monitoring of autoantibodies provides a critical window into the immune activity of MN. SR is often preceded by a natural decline in pathogenic antibody levels, likely reflecting a re-establishment of immune homeostasis.
2.3 Pathological repair processes
In MN, podocyte injury and subepithelial immune complex deposition are the primary causes of proteinuria. During SR, the attenuation of immune activity enables the glomeruli to initiate intrinsic repair processes. Ultrastructural studies have revealed a progression of pathological stages over time: early lesions are characterized by abundant subepithelial electron-dense deposits; in the intermediate phase, these deposits are enveloped by newly formed basement membrane material with characteristic “spikes” visible on silver staining; in later stages (Stage IV), deposits may be partially resorbed, dispersed, or calcified (54).
Renal biopsies from patients undergoing spontaneous or treatment-induced remission often show reduced or absent immune deposits, as well as residual “holes,” suggesting degradation or clearance of previously formed immune complexes and partial restoration of GBM integrity (54). This clearance is thought to be mediated by phagocytic activity of mesangial cells or infiltrating macrophages. Recent studies indicate that podocyte recovery during SR involves reassembly of slit diaphragm components such as nephrin and podocin, together with reorganization of the actin cytoskeleton (55). These molecular events are closely linked to the recovery of endothelial–podocyte crosstalk, which is critical for maintaining glomerular filtration integrity (56, 57). Furthermore, following resolution of injury, podocytes can partially recover their microvillus architecture, and foot process effacement may reverse, thereby restoring selective permeability of the filtration barrier. Importantly, SR is typically a gradual process. Even after cessation of new immune deposition, the clearance of existing subepithelial deposits and recovery of podocyte function may take months or even years (58). Clinically, this is reflected in a stepwise decline in proteinuria—from nephrotic-range levels to partial remission, and eventually to complete or near-complete remission (58). Restoration of the glomerular filtration barrier and nephron function is central to this process. It is worth noting that the capacity for SR also depends on the reversibility of the lesions and the compensatory function of remaining nephrons. In cases where irreversible basement membrane scarring or tubulointerstitial fibrosis has occurred, proteinuria may persist at a residual level despite immunologic quiescence. Therefore, the potential for SR is ultimately influenced by the extent of structural reversibility and renal reserve.
Therefore, most prevailing theories support that SR in MN is a process of gradually attenuating immune activity. This process is underpinned by a reduction or cessation in the production of pathogenic autoantibodies, clearance of immune deposits, restoration of podocyte structural and functional integrity, and the synergistic involvement of multi-level immunoregulatory mechanisms. These insights help explain why a subset of MN patients can achieve clinical improvement without the need for immunosuppressive therapy (Figure 1).
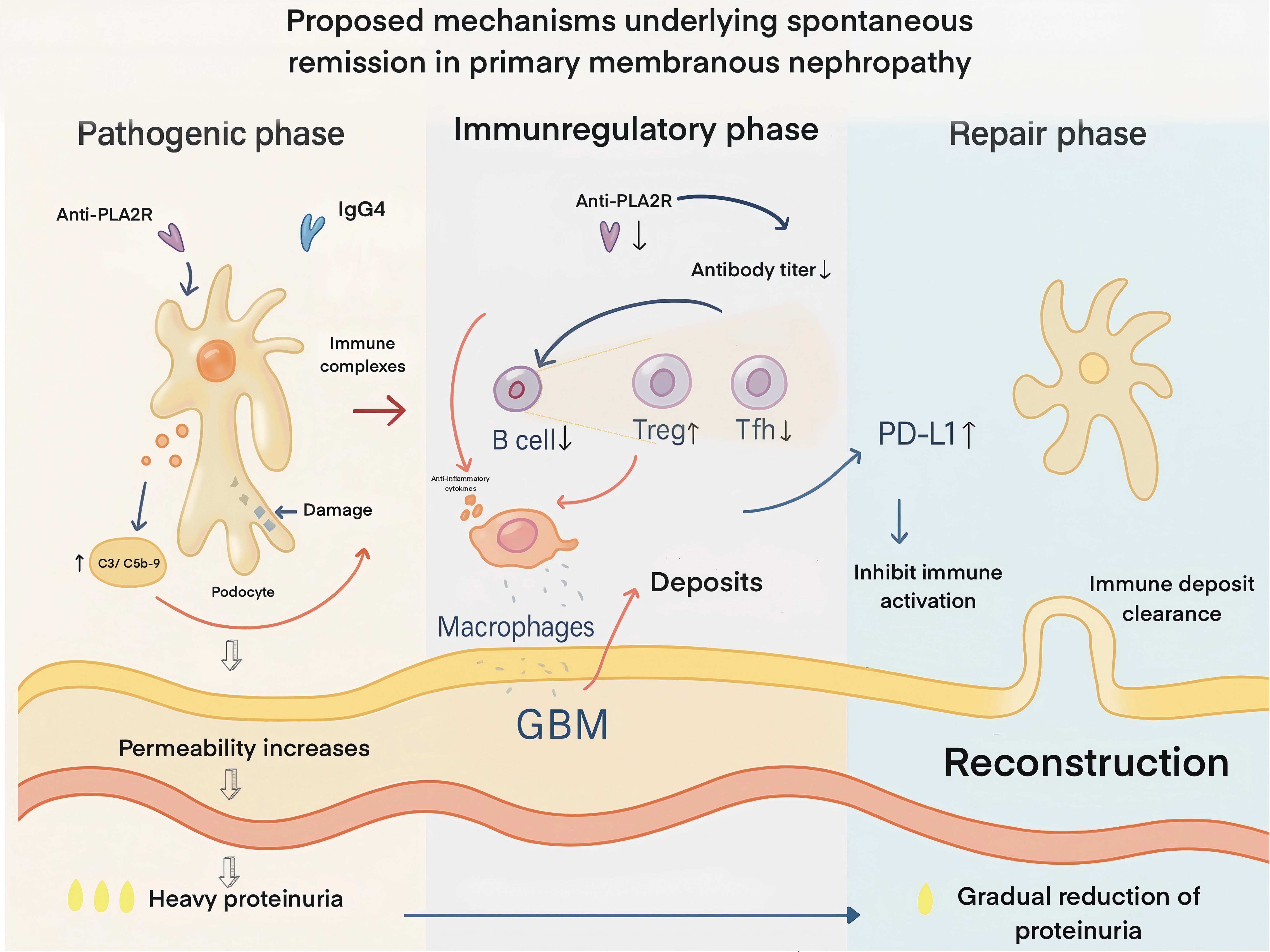
Figure 1. Proposed mechanisms underlying spontaneous remission in primary membranous nephropathy. In the pathogenic phase (left), IgG4 anti-PLA2R autoantibodies bind podocyte antigens, forming subepithelial immune complexes that activate complement (C3, C5b-9), injure podocytes, increase glomerular basement membrane (GBM) permeability, and cause heavy proteinuria. In the immunoregulatory phase (middle), autoantibody titers decline due to reduced pathogenic B-cell activity, enhanced regulatory T-cell (Treg) function, suppression of T follicular helper (T_FH) cells, and macrophage-mediated anti-inflammatory effects. Podocyte PD-L1 upregulation inhibits local immune activation, leading to cessation of new deposit formation. In the repair phase (right), immune deposits are progressively cleared, podocyte architecture and slit diaphragm integrity are restored, GBM structure is reconstructed, and proteinuria gradually resolves. PLA2R – phospholipase A2 receptor; GBM – glomerular basement membrane; Treg – regulatory T cell; T_FH – T follicular helper T cell; PD-1/PD-L1 – programmed death-1 and its ligand (an immune checkpoint pathway).
3 Predictors of spontaneous remission in PMN
Identifying factors that influence the likelihood of SR in MN has long been a key research focus. Based on cumulative evidence from previous studies, several predictors of SR have been proposed (Figure 2):
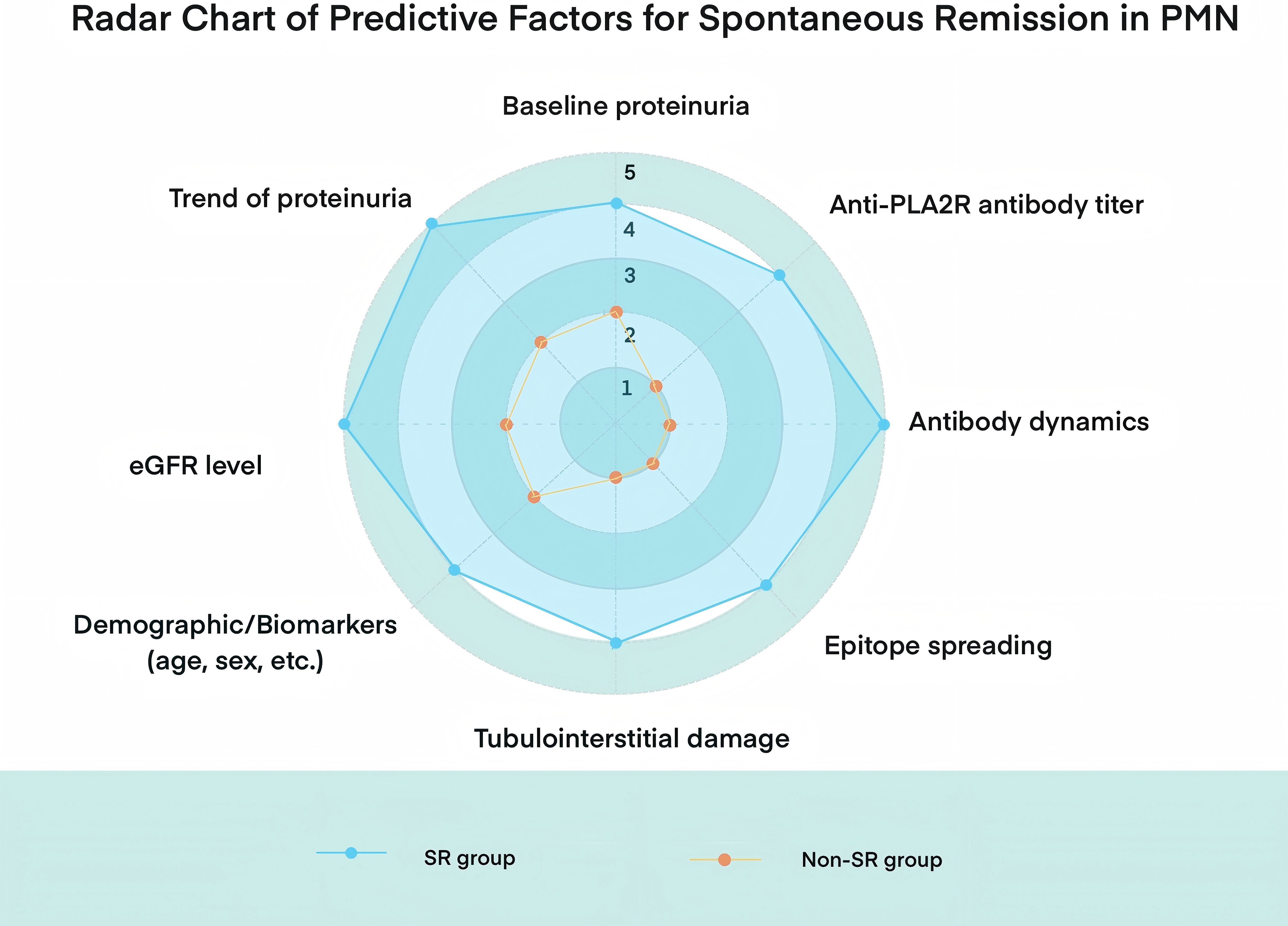
Figure 2. Radar chart comparing key clinical and immunological predictors between spontaneous remission (SR) and non-spontaneous remission (non-SR) groups in PMN.
3.1 Baseline proteinuria and its temporal trajectory
Proteinuria level is the most direct clinical indicator. Classic natural history studies have shown that higher baseline proteinuria and more severe nephrotic syndrome are associated with a lower likelihood of SR (59, 60), a finding that has been consistently validated in more recent investigations (61–64). Patients with subnephrotic proteinuria generally have an excellent prognosis, with 10-year renal survival rates approaching 100%. Many of these individuals can achieve SR even without immunosuppressive therapy (65). In general, the greater the baseline proteinuria, the lower the probability of SR. Although it is traditionally believed that patients with proteinuria >8 g/day rarely achieve SR (66, 67), recent large cohort studies have challenged this notion. These studies demonstrate that even among patients with very high proteinuria, a substantial proportion—nearly one-quarter—achieved remission following intensive supportive therapy. The same study also found that for every 1 g/day reduction in baseline proteinuria, the probability of remission increased accordingly (12). Thus, the overall trend remains: patients with lower baseline proteinuria are more likely to experience SR. As such, baseline proteinuria can serve as a basis for risk stratification.
Moreover, monitoring the trajectory of proteinuria over time is critical for distinguishing progressive disease from self-limited courses. If proteinuria shows a sustained decline under optimized supportive therapy, the patient is likely entering a remission pathway. In such cases, continued conservative management may allow SR to occur without immunosuppression. Conversely, the absence of improvement—or worsening proteinuria—within the first six months suggests ongoing disease activity and low likelihood of SR, warranting consideration of immunosuppressive therapy. According to the 2012 KDIGO guideline for idiopathic MN, initiation of immunosuppressive treatment is recommended when proteinuria exceeds 4 g/day, remains above 50% of baseline, and shows no progressive decline after six months of supportive care. Troyanov et al. proposed a practical rule: failure to achieve a ≥25% reduction in proteinuria at six months should be regarded as treatment failure and prompt a reassessment of therapeutic strategy (12). These dynamic criteria have been increasingly integrated into clinical decision-making to guide individualized treatment planning.
3.2 Anti-PLA2R antibody titers and their dynamic changes
Anti-PLA2R antibody titers represent one of the core biomarkers in prognostic evaluation of PMN. Recent meta-analyses suggest that baseline serum anti-PLA2R antibody levels are closely associated with the likelihood of clinical remission (68). Multiple studies have demonstrated that high antibody titers are associated with delayed or absent remission, whereas low titers predict a higher probability of SR (69–72). In a prospective cohort of 65 PMN patients, Jurubi-ă et al. used multivariate Cox regression and found that patients who were seronegative for anti-PLA2R at baseline had a significantly higher chance of achieving SR (hazard ratio ~3), indicating that serological status is an independent predictor (73). An updated systematic review that included 18 studies further confirmed that patients with high baseline anti-PLA2R titers had significantly lower clinical remission rates, with this association being particularly strong in Asian populations (24). Despite ongoing efforts to define an optimal anti-PLA2R threshold for predicting SR, there is currently no consensus regarding the ideal cutoff value, value range, or the most informative time points for measurement (39). Some earlier studies observed that patients with titers in the highest tertile or absolute levels >275 U/mL had only a ~20% chance of SR (71). More recently, a retrospective cohort study found that a titer <40 U/mL at diagnosis strongly predicted SR within the first 6 months (67). However, heterogeneity in immunologic and clinical practice standards between institutions makes it difficult to draw definitive conclusions regarding the predictive accuracy of any specific threshold (40, 74). It is important to note that antibody titers often correlate with the severity of proteinuria. Patients with higher titers tend to have more severe disease and are more likely to receive immunosuppressive therapy (75, 76), which may confound the evaluation of purely “spontaneous” remission.
Dynamic changes in anti-PLA2R titers are also important in predicting remission. Generally, a decrease or seroconversion of antibody titers precedes the onset of clinical proteinuria remission. Studies have shown that in nearly all patients, disappearance of anti-PLA2R antibodies precedes complete proteinuria remission by several weeks to months (42). A prospective study by Jatem-Escalante et al. demonstrated that a baseline titer ≤97.5 RU/mL and a ≥15% decline in anti-PLA2R titer within the first 3 months after diagnosis could predict a ≥50% reduction in proteinuria by 6 months (39). Thus, a sustained and significant decrease or seroconversion in antibody titers during observation strongly suggests that the patient is on a path toward SR. Conversely, persistently high anti-PLA2R titers indicate a low likelihood of SR.
3.3 Epitope spreading of the anti-PLA2R antibody response
Epitope spreading refers to the broadening of antibody reactivity to multiple epitopes of a given antigen. In PLA2R-associated MN, anti-PLA2R antibodies primarily target the N-terminal cysteine-rich (CysR) domain. However, in some patients, the antibody response “spreads” to include additional epitopes such as CTLD1 and CTLD7 domains. Some studies have suggested that patients with antibodies restricted to the CysR epitope are more likely to achieve SR, whereas those with epitope spreading exhibit more severe proteinuria and poorer outcomes (70). A French study involving 48 PLA2R-positive PMN patients reported that none of the patients with baseline epitope spreading achieved SR, in stark contrast to those without spreading (70). However, this concept has recently been challenged. Reinhard et al., in a long-term follow-up study of 150 PLA2R-positive patients, found that nearly all patients exhibited broad epitope reactivity at diagnosis. Their multivariate analysis showed that overall antibody titer levels, rather than epitope distribution, were more predictive of remission outcomes (77). Thus, the prognostic value of epitope spreading as an independent predictor remains inconclusive. In addition, a lack of standardized methods for epitope mapping poses challenges for routine clinical application. At present, dynamic quantification of anti-PLA2R antibody levels remains the primary approach for guiding clinical judgment.
3.4 Renal function indicators
Glomerular filtration rate (eGFR) and serum creatinine levels reflect the degree of renal parenchymal injury. In general, preserved renal function at diagnosis (i.e., normal or mildly reduced eGFR) is associated with a higher likelihood of SR. A retrospective study stratified by baseline eGFR found that patients with eGFR >60 mL/min/1.73 m² had significantly higher remission rates than those with lower eGFR (78). Data from Polanco et al. also demonstrated that lower baseline serum creatinine is an independent predictor of SR (12). Intact kidney function indicates the absence of substantial irreversible damage, suggesting a greater potential for functional recovery once immune deposits are cleared. It is noteworthy that some PMN patients may experience acute functional deterioration during the disease course, such as a sudden rise in serum creatinine, often due to severe hypoperfusion, renal vein thrombosis, or concurrent interstitial pathology. A recent study from China by Li et al. retrospectively reviewed 136 PMN patients and found that 24.1% had experienced acute kidney injury (AKI), with a significantly lower rate of complete SR compared to those without AKI (78). Renal biopsies in these patients often revealed acute tubular injury and extensive interstitial damage, suggesting that an episode of AKI may impose additional irreversible injury, thereby diminishing the likelihood of SR (79). In that study, the AKI group had slightly higher baseline proteinuria than the non-AKI group, but the difference was not statistically significant. Interestingly, anti-PLA2R antibody positivity was significantly lower in the AKI group, and neither proteinuria nor anti-PLA2R titer was identified as an independent predictor of AKI after multivariate adjustment. This suggests that the association between AKI and lower SR rates is not simply explained by higher proteinuria or antibody levels, and that AKI itself may be an independent marker of poor prognosis. Thus, a history of AKI may serve as a useful parameter in risk stratification: its presence indicates a poorer prognosis and warrants more proactive treatment rather than prolonged observation.
3.5 Histopathological findings
The degree of tubulointerstitial damage (TID) observed on renal biopsy is one of the most powerful histologic predictors of MN prognosis (80). In 2019, Maria J. Stangou et al. reported that glomerulosclerosis and tubulointerstitial injury in PMN patients were positively correlated with the severity of proteinuria and served as independent determinants of renal function impairment (81). More recent studies have further confirmed this association. Sun et al. found that PMN patients with greater degrees of chronic TID had significantly lower SR rates and a higher likelihood of progressing to renal insufficiency (79). Multivariate analysis indicated that TID was an independent risk factor for disease progression, and was strongly associated with higher proteinuria, serum creatinine, and anti-PLA2R titers (79, 82).
These findings suggest that once interstitial fibrosis and inflammatory infiltration occur, they often indicate cumulative and irreversible renal damage. The physiological substrate for SR—viable podocytes and nephron units—is therefore significantly compromised. This supports prior observations that the more prominent the chronic histological changes, the lower the chance of disease reversibility. Thus, if biopsy reports reveal extensive tubular atrophy or interstitial fibrosis, clinicians should be cautious: even in cases with immunologic remission, full clinical remission may not be achievable, with persistent proteinuria in this context more likely reflecting background glomerular scarring rather than ongoing immune-mediated injury; therefore, additional immunosuppression is unlikely to be beneficial and management should prioritize maximal conservative therapy (renin–angiotensin system blockade, optimal blood pressure control, dietary sodium restriction, and potentially SGLT2 inhibitors—recognizing that evidence in MN is limited).
3.6 Demographic characteristics
Age and sex have also been implicated as potential predictors of SR in some studies. Classic natural history data suggest that younger patients (<50 years) and females tend to have higher SR rates. This may be due to differences in immune response intensity or better responsiveness to immunosuppressive therapy. Conversely, older male patients are often considered a higher-risk group. However, it is worth noting that MN overall has a male predominance, with an approximate male-to-female ratio of 2:1 (83–85). Therefore, demographic factors may be interrelated with other clinical characteristics. The impact of race on SR in MN remains unclear. While some studies suggest a higher incidence in White populations, there is no definitive evidence indicating significant differences in SR rates among different ethnic groups (85).
3.7 Other biomarkers
Several studies have explored additional biomarkers as potential predictors of SR. These include urinary selectivity index and the urinary IgG-to-α1-microglobulin ratio. Poor selectivity (i.e., high IgG excretion) is considered a marker of severe glomerular barrier damage and has been associated with worse prognosis (65). Genetic susceptibility (e.g., HLA-DQA1 alleles), degree of comorbid hypertension, and other clinical factors may also influence disease progression (86).
3.8 Lifestyle and modifiable risk factors
Individual patient factors and lifestyle choices can also influence the likelihood of SR. Excess body weight has emerged as an important consideration: observational data from glomerular disease cohorts indicate that obesity is associated with a significantly lower probability of achieving complete remission of proteinuria. In adults with nephrotic syndrome (including PMN), obesity was linked to about a 20–30% reduction in the hazard of remission (87). This may be due to obesity-related hemodynamic stress and inflammation, which can sustain proteinuria and kidney injury (88–90). Similarly, smoking has been identified as a modifiable risk factor that adversely affects renal outcomes. Smoking is a known accelerator of chronic kidney disease progression in glomerular disorders (91). In IMN, one cohort study found that current smokers had a dramatically higher risk of a 30% decline in eGFR compared to non-smokers (adjusted hazard ratio ~7–8), although smoking was not significantly associated with attaining remission in that particular analysis (92). Overall, smoking’s deleterious effects on the vasculature and immune response likely impede recovery; therefore, smoking cessation is strongly recommended in all MN patients. Current clinical guidelines emphasize lifestyle modifications — including weight control, exercise, and smoking cessation — as a cornerstone of supportive therapy (93). Indeed, the KDIGO 2021 glomerular disease guideline advises that all patients receive counseling on diet (e.g. salt restriction), quitting smoking, and maintaining a healthy body mass index as part of initial management (93). These measures not only improve general health but may enhance the kidneys’ capacity to stabilize or recover, thereby increasing the chance of SR.
Other patient-specific factors may play more subtle roles. For instance, the medications a patient takes can influence disease course. Certain drugs are known to exacerbate MN or cause secondary forms of it – for example, prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs) has been associated with the development or worsening of MN and nephrotic syndrome (94). In patients with PMN, the avoidance of nephrotoxic medications (such as NSAIDs) and other potential triggers is advised to prevent undue additional injury. On the other hand, adherence to supportive medications that treat comorbid conditions (like rigorous blood pressure control with renin–angiotensin system blockers) can facilitate reductions in proteinuria and promote remission. Additionally, “living conditions” encompassing factors like socioeconomic status, diet, and exposure to environmental toxins may indirectly affect SR. While direct evidence is limited, patients in favorable living circumstances — with good access to healthcare, ability to maintain proper nutrition, and minimal exposure to pollutants or infections — are theoretically more likely to have better outcomes. In contrast, poor living conditions or high levels of chronic stress could hinder overall health and delay renal recovery. Clinicians should therefore adopt a holistic approach: beyond the biochemical and histologic risk markers, addressing lifestyle and environmental factors (encouraging weight loss, smoking cessation, balanced diet, and medication review) is an integral part of maximizing the likelihood of SR in PMN.
Multiple factors collectively influence the likelihood of SR in MN. In general, low-risk features—such as female sex, younger age, healthy lifestyle factors, moderate baseline proteinuria, preserved renal function, negative or low-titer anti-PLA2R antibodies, and favorable early treatment response to conservative therapy (e.g., ACE inhibitors or ARBs)—are associated with a higher probability of SR. In contrast, high-risk features—including older age, male sex, obesity, smoking, heavy proteinuria, impaired renal function, and high-titer anti-PLA2R antibodies—indicate a lower likelihood of SR and may necessitate more aggressive therapeutic intervention. It is essential to emphasize that these factors represent probabilistic associations rather than deterministic outcomes. Individual patients may deviate from these patterns. Therefore, clinical decision-making should be based on comprehensive assessment and repeated dynamic follow-up to accurately evaluate disease trajectory and guide optimal management.
4 Treatment and management strategies
4.1 Clinical management of patients under observation
Given that a considerable proportion of MN patients may achieve SR, a “watchful waiting” strategy is commonly adopted for low-risk individuals. This approach involves withholding immunosuppressive therapy while providing intensive supportive care and close clinical monitoring (95). International guidelines, such as the 2021 KDIGO Glomerular Diseases Guideline, recommend stratifying patients by risk (low, moderate, high) before determining therapeutic plans (96). Patients presenting with proteinuria <3.5 g/day, serum albumin >30 g/L, eGFR >60 mL/min/1.73 m², and no major complications (e.g., AKI, infections, or thromboembolic events) are typically suitable for observation and supportive care. These patients should be followed regularly with assessment of anti-PLA2R antibody titers and clinical parameters.
However, if any of the following high-risk features are present—persistent heavy proteinuria (>4 g/day for >6 months), serum albumin <25–30 g/L, progressive decline in eGFR, persistently high anti-PLA2R antibody titers (>50 RU/ml, as per KDIGO guidelines), or serious complications such as AKI, refractory edema, or thrombotic events—immunosuppressive therapy should be considered. The goal of this strategy is to avoid unnecessary toxicity in those likely to undergo SR while ensuring timely intervention in patients at risk of irreversible renal damage (97, 98) (Figure 3).
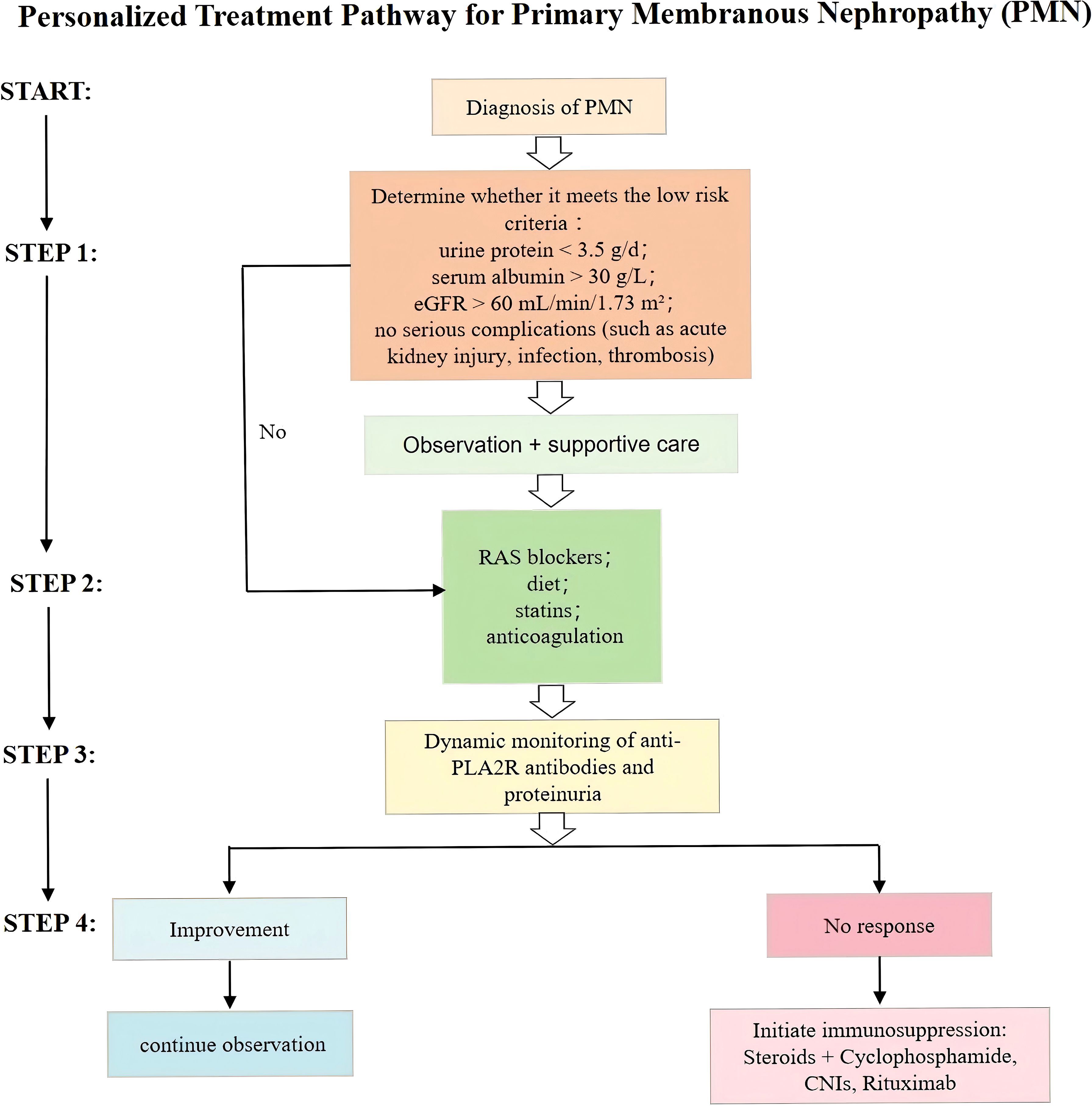
Figure 3. Personalized treatment pathway for primary membranous nephropathy (PMN) based on risk stratification, dynamic monitoring of anti-PLA2R antibodies and proteinuria, and stepwise therapeutic escalation.
Nevertheless, recent real-world data have raised concerns regarding prolonged conservative management in certain subgroups. A UK multicenter cohort study (99) reported that while low-risk patients achieved high SR rates (71%) with very low incidence of progressive CKD (9.5%) or ESKD (2.4%), moderate-risk patients with UPCR >600 — despite SR in approximately 30% (predominantly partial) — experienced substantial progression risk, with doubling of serum creatinine in 28% and ESKD in 16% over a median follow-up of 5 years. The mean eGFR decline of 7.7 mL/min/1.73 m² per year during watchful waiting highlights the challenge of balancing minimizing exposure/harm from immunosuppression (with hope of achieving SR) against loss of kidney function and progression to ESKD.
4.2 Role of non-immunosuppressive therapy in promoting remission
Supportive treatment forms the cornerstone of MN management and should be initiated promptly upon diagnosis to enhance the likelihood of SR and delay renal disease progression (7). Blood pressure control and anti-proteinuric strategies are central components of supportive therapy (100). Renin–angiotensin system (RAS) blockers, such as ACE inhibitors and ARBs, lower intraglomerular pressure and protein filtration, thereby providing established renoprotective effects in MN (101, 102). A multicenter study identified ACEI/ARB use as an independent favorable predictor of SR in MN (12).
In addition, sodium restriction and moderate protein intake (typically a high-quality, low-protein diet of ~0.8 g/kg/day) are recommended to reduce glomerular pressure and protein filtration burden, thus slowing renal function deterioration (103, 104). For patients with severe hypoalbuminemia and high thrombotic risk, individualized prophylactic anticoagulation may be considered based on bleeding risk assessment. Studies have shown that lower albumin levels are associated with a greater benefit-to-risk ratio for anticoagulation, particularly in patients with low bleeding risk. Decision-support tools incorporating both bleeding risk and albumin levels can help optimize anticoagulation strategies (105). Hyperlipidemia is commonly observed in MN, and statins may not only improve dyslipidemia but also offer potential benefits through anti-inflammatory effects and improvement of endothelial function (106, 107). Diuretics can be used to alleviate edema in MN patients; however, clinicians should be cautious of intravascular volume depletion due to excessive diuresis (108, 109).
Overall, through these comprehensive measures, supportive treatment alone can lead to a significant reduction in proteinuria, and some patients may even achieve partial remission without immunosuppressive therapy (12, 14). In practice, such “non-immunological” remission is often difficult to distinguish from true immunological SR, as the mechanisms of supportive therapy mainly involve improving hemodynamics and mitigating secondary injury (110), rather than eliminating immune complexes directly. Nevertheless, the adequacy of supportive therapy significantly influences disease progression, and it is critical to ensure that conservative management has been optimized before advancing to immunosuppressive treatment planning.
4.3 Monitoring during the observation period
For patients undergoing conservative management, close monitoring of parameters associated with disease activity and renal function is essential. Key clinical indicators include proteinuria, serum albumin, serum creatinine, and body weight (111–114). Dynamic changes in anti-PLA2R antibody titers offer important guidance for therapeutic decision-making. A progressive decline or seroconversion of anti-PLA2R antibodies during follow-up suggests attenuation of immunologic activity, indicating that the patient may be entering a phase of SR. In such cases, continued supportive care with vigilant follow-up is generally appropriate. Conversely, persistently elevated or rising anti-PLA2R titers reflect ongoing immunopathological processes and a lower likelihood of SR, warranting reconsideration of the treatment approach (4, 39).
4.4 Timing of immunosuppressive therapy
In MN patients who show no signs of improvement during the observation period or are classified as high risk, timely initiation of immunosuppressive therapy is essential to induce remission and prevent progression (115). The classical first-line regimen includes glucocorticoids in combination with alkylating agents (e.g., cyclophosphamide), which has been shown to significantly increase remission rates and improve renal outcomes (116, 117).
However, due to the potential toxicity associated with cyclophosphamide—including infection and malignancy—calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus, and anti-CD20 monoclonal antibodies such as rituximab, have increasingly been adopted as more tolerable alternatives (118). Several studies have reported that rituximab induces proteinuria remission in 60–80% of patients and is associated with a favorable safety and tolerability profile, making it an increasingly preferred first-line therapy in clinical practice (16, 17, 119). The need to initiate immunosuppressive treatment after a period of observation suggests that SR has not occurred or that the optimal window for intervention may have been missed. Therefore, immunosuppressive therapy should be initiated based on clearly defined clinical indications following an adequate observation period. The KDIGO 2021 guideline incorporates the concept of antibody-guided therapy and recommends shortening the observation period in patients with high anti-PLA2R antibody titers to optimize the timing of immunosuppressive intervention (18).
5 Future directions
5.1 Novel biomarkers
With advances in the molecular immunology of MN, the identification of novel biomarkers holds promise for improving the accuracy of predicting SR. First, new target antigens have been discovered in PLA2R- and THSD7A-negative MN cases, such as NELL-1, Sema3B, and EXT1/2 (120–122). To date, only anti-PLA2R and anti-THSD7A antibodies have been proven pathogenic in animal models. Clinically available assays are currently limited to anti-PLA2R ELISA (quantitative) and anti-THSD7A immunofluorescence (semi-quantitative). Antigen-specific MN subtypes may follow distinct clinical trajectories and remission patterns. For example, NELL-1-associated MN is characterized predominantly by IgG1 deposits and is occasionally associated with malignancies. Its disease course may differ from PLA2R-related MN (58). The development of clinically available assays for autoantibodies to novel antigens would facilitate prospective studies to correlate their levels with clinical outcomes, including SR, making them promising future biomarkers. Prognostic studies of these novel subtypes will help clarify their potential for SR and associated clinical behavior. In addition, detailed characterization of the autoantibody repertoire has garnered increasing attention. The epitope specificity of anti-PLA2R antibodies—i.e., the structural domains they recognize—has emerged as a key area of investigation (123). Studies have shown that the degree of epitope spreading at diagnosis is strongly associated with prognosis: patients with restricted epitope reactivity (e.g., only to the CysR domain) are more likely to achieve remission, while those with broader epitope recognition (e.g., CysR + CTLD domains) have poorer outcomes (41). Thus, epitope profiling of anti-PLA2R antibodies may become a valuable tool for risk stratification.
Other fluid-based biomarkers may also reflect disease activity. Recent research has identified PLA2R-rich migrasomes in the urine of MN patients, derived from podocytes. These may serve as natural urinary antigens for noninvasive monitoring, with potential utility in reflecting disease activity or remission status (124).
5.2 Predictive models for spontaneous remission
Multivariable predictive models represent a major direction for future research. Because single indicators are often insufficient to accurately predict individual outcomes, integrating clinical features with biological markers into comprehensive prediction tools may offer superior clinical utility. For example, one study developed a composite score incorporating both genetic risk and anti-PLA2R titers in PLA2R-related MN. The model outperformed clinical variables or antibody titers alone in predicting renal function decline, underscoring the value of multidimensional approaches (125). Another study involving 439 patients with idiopathic MN showed that a combination of age, eGFR, and proteinuria could be used to build a risk score with good predictive performance for renal function decline, end-stage kidney disease (ESKD), or death (126).
Future models may incorporate genetic polymorphisms (e.g., risk alleles in PLA2R1 and HLA-DQA1), as well as histopathological quantitative features such as the extent of glomerular immune complex deposition or IgG4 intensity (127, 128). With the application of machine learning techniques, it may be possible to develop scoring systems or risk calculators to predict SR probabilities, thereby offering clinicians objective and personalized tools for guiding patient management decisions. In line with this, findings from Hamilton et al. (99) highlight the urgent need for predictive tools capable of identifying patients at risk of significant renal decline during observation, particularly in moderate-risk groups. Such models, built on large datasets and validated across cohorts in different countries/populations, would be instrumental in optimizing the timing of immunosuppressive therapy and preventing the detrimental outcomes observed in this cohort.
6 Conclusion
Spontaneous remission represents a key clinical phenomenon in primary membranous nephropathy, offering a potential pathway to disease resolution without the risks associated with immunosuppressive therapy. Advances in our understanding of the immunological and structural underpinnings of SR—particularly the role of anti-PLA2R antibody kinetics, podocyte repair, and histologic reversibility—have refined our ability to identify patients likely to benefit from a conservative approach. Clinical predictors such as baseline proteinuria, renal function, and demographic characteristics, in conjunction with dynamic serological markers, can aid in individualizing therapy. As management strategies evolve, emphasis should be placed on optimizing supportive care and closely monitoring disease activity, reserving immunosuppression for those unlikely to remit spontaneously. Looking forward, the integration of emerging biomarkers, novel antigens, and predictive algorithms holds promise for enhancing prognostic accuracy and facilitating precision medicine in PMN.
Author contributions
MW: Writing – original draft. YC: Writing – original draft. ZH: Writing – original draft. YJ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by Grants-in-Aid from Zhejiang Province Traditional Chinese Medicine Science and Technology Project -Young Talent Support Program (project 2025ZR224).
Acknowledgments
The authors thank all colleagues and institutions involved in the development of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alok A and Yadav A. Membranous Nephropathy. Statpearls. Treasure Island (FL) ineligible companies. In: Disclosure: Anju Yadav declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2025. Treasure Island (FL): StatPearls Publishing LLC (2025).
2. Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. (2021) 7:69. doi: 10.1038/s41572-021-00303-z
3. Keri KC, Blumenthal S, Kulkarni V, Beck L, and Chongkrairatanakul T. Primary membranous nephropathy: comprehensive review and historical perspective. Postgraduate Med J. (2019) 95:23–31. doi: 10.1136/postgradmedj-2018-135729
4. Tesar V and Hruskova Z. Autoantibodies in the diagnosis, monitoring, and treatment of membranous nephropathy. Front Immunol. (2021) 12:593288. doi: 10.3389/fimmu.2021.593288
5. Vendemia F, Gesualdo L, Schena FP, and D’Amico G. Epidemiology of primary glomerulonephritis in the elderly. Rep Ital Registry Renal Biopsy. J Nephrol. (2001) 14:340–52.
6. Hou JH, Zhu HX, Zhou ML, Le WB, Zeng CH, Liang SS, et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis (Basel Switzerland). (2018) 4:10–9. doi: 10.1159/000484717
7. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrology: CJASN. (2017) 12:983–97. doi: 10.2215/cjn.11761116
8. Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New Engl J Med. (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
9. Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, et al. Autoantibodies against thrombospondin type 1 domain-containing 7a induce membranous nephropathy. J Clin Invest. (2016) 126:2519–32. doi: 10.1172/jci85265
10. Wang J, Cui Z, Lu J, Probst C, Zhang YM, Wang X, et al. Circulating antibodies against thrombospondin type-I domain-containing 7a in chinese patients with idiopathic membranous nephropathy. Clin J Am Soc Nephrology: CJASN. (2017) 12:1642–51. doi: 10.2215/cjn.01460217
11. Wang YN, Feng HY, Nie X, Zhang YM, Zou L, Li X, et al. Recent advances in clinical diagnosis and pharmacotherapy options of membranous nephropathy. Front Pharmacol. (2022) 13:907108. doi: 10.3389/fphar.2022.907108
12. Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrology: JASN. (2010) 21:697–704. doi: 10.1681/asn.2009080861
13. Wang M, Yang J, Fang X, Lin W, and Yang Y. Membranous nephropathy: pathogenesis and treatments. MedComm. (2024) 5:e614. doi: 10.1002/mco2.614
14. von Groote TC, Williams G, Au EH, Chen Y, Mathew AT, Hodson EM, et al. Immunosuppressive treatment for primary membranous nephropathy in adults with nephrotic syndrome. Cochrane Database systematic Rev. (2021) 11:Cd004293. doi: 10.1002/14651858.CD004293.pub4
15. Stai S, Lioulios G, Christodoulou M, Papagianni A, and Stangou M. From Kdigo 2012 Towards Kdigo 2021 in Idiopathic Membranous Nephropathy Guidelines: What Has Changed over the Last 10 years? J Nephrol. (2023) 36:551–61. doi: 10.1007/s40620-022-01493-9
16. Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrology: JASN. (2017) 28:348–58. doi: 10.1681/asn.2016040449
17. Teisseyre M, Cremoni M, Boyer-Suavet S, Ruetsch C, Graça D, Esnault VLM, et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol. (2022) 13:859419. doi: 10.3389/fimmu.2022.859419
18. Kdigo. Clinical practice guideline for the management of glomerular diseases. Kidney Int (2021). (2021) 100:S1–s276. doi: 10.1016/j.kint.2021.05.021
19. Bin Homam WM, Ayub F, Elkalashy A, Hasan MR, and Holthoff JH. I am the lucky third: spontaneous resolution of high-risk membranous nephropathy. . J Am Soc Nephrol. (2023) 34:286. doi: 10.1681/ASN.20233411S1286c
20. Ronco P and Debiec H. A podocyte view of membranous nephropathy: from heymann nephritis to the childhood human disease. Pflugers Archiv: Eur J Physiol. (2017) 469:997–1005. doi: 10.1007/s00424-017-2007-x
21. Miao H, Zhang Y, Yu X, Zou L, and Zhao Y. Membranous nephropathy: systems biology-based novel mechanism and traditional chinese medicine therapy. Front Pharmacol. (2022) 13:969930. doi: 10.3389/fphar.2022.969930
22. Tomas NM, Meyer-Schwesinger C, von Spiegel H, Kotb AM, Zahner G, Hoxha E, et al. A heterologous model of thrombospondin type 1 domain-containing 7a-associated membranous nephropathy. J Am Soc Nephrology: JASN. (2017) 28:3262–77. doi: 10.1681/asn.2017010030
23. Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. (2017) 92:227–37. doi: 10.1016/j.kint.2017.01.012
24. Zhang J, Fan Z, Wang P, and Zhang AH. Phospholipase A2 receptor antibodies and clinical prognosis in patients with idiopathic membranous nephropathy: an updated systematic review and meta-analysis. Kidney Blood Pressure Res. (2023) 48:102–13. doi: 10.1159/000529415
25. Ali A, Al-Mukhtar S, and Barghouthi H. Renal lesion in Iraqi body builders – a case series. In: ISN world congress of nephrology; 2020/03/26-2020/03/29; abu dhabi, United Arab Emirates: kidney international reports Philadelphia, PA: Elsevier Inc (2020). p. S1–S392.
26. Cantarelli C, Jarque M, Angeletti A, Manrique J, Hartzell S, O’Donnell T, et al. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int Rep. (2020) 5:1764–76. doi: 10.1016/j.ekir.2020.07.028
27. Ramachandran R, Kaundal U, Girimaji N, Rakha A, Rathi M, Gupta KL, et al. Regulatory B cells are reduced and correlate with disease activity in primary membranous nephropathy. Kidney Int Rep. (2020) 5:872–8. doi: 10.1016/j.ekir.2020.03.023
28. Ma DH, Yang XD, Hua QJ, Hou YL, Liu Y, Xu QY, et al. Changes and significance of treg and th17 in adult patients with primary membranous nephropathy. Clin Nephrol. (2021) 96:155–64. doi: 10.5414/cn110333
29. de Heer E, Daha MR, Burgers J, and van Es LA. Reestablishment of self tolerance by suppressor T-cells after active heymann’s nephritis. Cell Immunol. (1986) 98:28–33. doi: 10.1016/0008-8749(86)90264-9
30. Wang YM, Zhang GY, Hu M, Polhill T, Sawyer A, Zhou JJ, et al. Cd8+ Regulatory T cells induced by T cell vaccination protect against autoimmune nephritis. J Am Soc Nephrology: JASN. (2012) 23:1058–67. doi: 10.1681/asn.2011090914
31. Chung EYM, Wang YM, Shaw K, Ronning E, Wang Y, Zhang GY, et al. Cd8(+) regulatory T cells induced by peptide vaccination ameliorates experimental model of membranous nephropathy. Nephrol (Carlton Vic). (2025) 30:e70005. doi: 10.1111/nep.70005
32. Li J, Zaslavsky M, Su Y, Guo J, Sikora MJ, van Unen V, et al. Kir(+)Cd8(+) T cells suppress pathogenic T cells and are active in autoimmune diseases and covid-19. Sci (New York NY) (2022). (2022) 376:eabi9591. doi: 10.1126/science.abi9591
33. Takanohashi S, Sugiura T, Koyano A, Ueno T, Rachi H, Shiratori K, et al. Complete remission of primary membranous nephropathy following hepatitis E infection. CEN Case Rep. (2023) 12:384–9. doi: 10.1007/s13730-023-00780-z
34. Ye W, Wang Y, Wen Y, Li H, and Li X. Dramatic remission of nephrotic syndrome after unusual complication of mucormycosis in idiopathic membranous nephropathy. Int Urol Nephrol. (2014) 46:1247–51. doi: 10.1007/s11255-013-0628-3
35. Wen YK and Chen ML. Remission of nephrotic membranous glomerulonephritis after high-dose trimethoprim-sulfamethoxazole treatment for pneumocystis jiroveci pneumonia. Clin Nephrol. (2007) 68:99–103. doi: 10.5414/cnp68099
36. Chen R, Lin Q, Tang H, Dai X, Jiang L, Cui N, et al. Pd-1 immunology in the kidneys: A growing relationship. Front Immunol. (2024) 15:1458209. doi: 10.3389/fimmu.2024.1458209
37. Hoxha E, Harendza S, Pinnschmidt H, Panzer U, and Stahl RA. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrology: CJASN. (2014) 9:1883–90. doi: 10.2215/cjn.03850414
38. Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, and Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrology: JASN. (2014) 25:1357–66. doi: 10.1681/asn.2013040430
39. Jatem-Escalante E, Martín-Conde ML, Gràcia-Lavedan E, Benítez ID, Gonzalez J, Colás L, et al. Monitoring anti-pla2r antibody titres to predict the likelihood of spontaneous remission of membranous nephropathy. Clin Kidney J. (2021) 14:2556–62. doi: 10.1093/ckj/sfab116
40. Wei SY, Wang YX, Li JS, Zhao SL, Diao TT, Wang Y, et al. Serum anti-pla2r antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol. (2016) 43:129–40. doi: 10.1159/000445361
41. Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payré C, et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrology: JASN. (2018) 29:401–8. doi: 10.1681/asn.2017070734
42. Gauckler P, Shin JI, Alberici F, Audard V, Bruchfeld A, Busch M, et al. Rituximab in membranous nephropathy. Kidney Int Rep. (2021) 6:881–93. doi: 10.1016/j.ekir.2020.12.035
43. Hoxha E, Harendza S, Pinnschmidt H, Panzer U, and Stahl RA. Pla2r antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PloS One. (2014) 9:s:e110681. doi: 10.1371/journal.pone.0110681
44. Radice A, Trezzi B, Maggiore U, Pregnolato F, Stellato T, Napodano P, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (Pla2r) for monitoring disease activity in idiopathic membranous nephropathy (Imn). Autoimmun Rev. (2016) 15:146–54. doi: 10.1016/j.autrev.2015.10.004
45. Tran TH, JH G, Greenfeld C, and Pham JT. Overview of current and alternative therapies for idiopathic membranous nephropathy. Pharmacotherapy. (2015) 35:396–411. doi: 10.1002/phar.1575
46. van de Logt AE, Hofstra JM, and Wetzels JF. Pharmacological treatment of primary membranous nephropathy in 2016. Expert Rev Clin Pharmacol. (2016) 9:1463–78. doi: 10.1080/17512433.2016.1225497
47. Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7a in idiopathic membranous nephropathy. New Engl J Med. (2014) 371:2277–87. doi: 10.1056/NEJMoa1409354
48. Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al. A mechanism for cancer-associated membranous nephropathy. New Engl J Med. (2016) 374:1995–6. doi: 10.1056/NEJMc1511702
49. Andeen NK, Kung VL, and Avasare RS. Nell1 membranous nephropathy: clinical associations provide mechanistic clues. Front Nephrol. (2024) 4:1323432. doi: 10.3389/fneph.2024.1323432
50. Salvadori M and Tsalouchos A. New antigens involved in membranous nephropathy beyond phospholipase A2 receptor. World J Nephrol. (2022) 11:115–26. doi: 10.5527/wjn.v11.i4.115
51. Sethi S, Madden B, Debiec H, Morelle J, Charlesworth MC, Gross L, et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrology: JASN. (2021) 32:1249–61. doi: 10.1681/asn.2020081165
52. Bobart SA, Tehranian S, Sethi S, Alexander MP, Nasr SH, Moura Marta C, et al. A target antigen-based approach to the classification of membranous nephropathy. Mayo Clinic Proc. (2021) 96:577–91. doi: 10.1016/j.mayocp.2020.11.028
53. Hu X, Wang G, and Cheng H. Specific antigens in Malignancy-associated membranous nephropathy. Front Med. (2024) 11:1368457. doi: 10.3389/fmed.2024.1368457
54. Fogo AB, Lusco MA, Najafian B, and Alpers CE. Ajkd atlas of renal pathology: membranous nephropathy. Am J Kidney diseases: Off J Natl Kidney Foundation. (2015) 66:e15–7. doi: 10.1053/j.ajkd.2015.07.006
55. Blaine J and Dylewski J. Regulation of the actin cytoskeleton in podocytes. Cells. (2020) 9:1700. doi: 10.3390/cells9071700
56. Lee DH, Riquier AD, Yang LE, Leong PK, Maunsbach AB, and McDonough AA. Acute hypertension provokes acute trafficking of distal tubule na-cl cotransporter (Ncc) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol. (2009) 296:F810–18. doi: 10.1152/ajprenal.90606.2008
57. Ronco P and Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet (London England). (2015) 385:1983–92. doi: 10.1016/s0140-6736(15)60731-0
58. Alsharhan L and Beck LH Jr. Membranous nephropathy: core curriculum 2021. Am J Kidney diseases: Off J Natl Kidney Foundation. (2021) 77:440–53. doi: 10.1053/j.ajkd.2020.10.009
59. Laluck BJ Jr. and Cattran DC. Prognosis after a complete remission in adult patients with idiopathic membranous nephropathy. Am J Kidney diseases: Off J Natl Kidney Foundation. (1999) 33:1026–32. doi: 10.1016/s0272-6386(99)70138-1
60. Troyanov S, Wall CA, Miller JA, Scholey JW, and Cattran DC. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. (2004) 66:1199–205. doi: 10.1111/j.1523-1755.2004.00873.x
61. Yamaguchi M, Ando M, Katsuno T, Tsuboi N, and Maruyama S. Urinary protein and renal prognosis in idiopathic membranous nephropathy: A multicenter retrospective cohort study in Japan. Renal failure. (2018) 40:435–41. doi: 10.1080/0886022x.2018.1487864
62. Huh H, Lee H, Lee JP, Kim DK, Oh S, Oh YK, et al. Factors affecting the long-term outcomes of idiopathic membranous nephropathy. BMC Nephrol. (2017) 18:104. doi: 10.1186/s12882-017-0525-6
63. Cattran DC, Kim ED, Reich H, Hladunewich M, and Kim SJ. Membranous nephropathy: quantifying remission duration on outcome. J Am Soc Nephrology: JASN. (2017) 28:995–1003. doi: 10.1681/asn.2015111262
64. Joseph J, Subramanian T, Vellaisamy M, Nd S, Surendran S, Kaliaperumal T, et al. The association of lower levels of baseline proteinuria with earlier remission in primary membranous nephropathy. Cureus. (2024) 16:e61918. doi: 10.7759/cureus.61918
65. Lai KN. Membranous nephropathy: when and how to treat. Kidney Int. (2007) 71:841–3. doi: 10.1038/sj.ki.5002201
66. Wasserstein AG. Membranous glomerulonephritis. J Am Soc Nephrology: JASN. (1997) 8:664–74. doi: 10.1681/asn.V84664
67. Rodas LM, Matas-García A, Barros X, Blasco M, Viñas O, Llobell A, et al. Antiphospholipase 2 receptor antibody levels to predict complete spontaneous remission in primary membranous nephropathy. Clin Kidney J. (2019) 12:36–41. doi: 10.1093/ckj/sfy005
68. Rao SJ, Shen Q, Wang HM, Tang S, and Wang XY. The association of anti-pla2r with clinical manifestations and outcomes in idiopathic membranous nephropathy: A meta-analysis. Int Urol Nephrol. (2020) 52:2123–33. doi: 10.1007/s11255-020-02588-7
69. Kukuy OL, Cohen R, Gilburd B, Zeruya E, Weinstein T, Agur T, et al. The prognostic value of anti-pla2r antibodies levels in primary membranous nephropathy. Int J Mol Sci (2023) 24:9051. doi: 10.3390/ijms24109051
70. Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, et al. Epitope spreading of autoantibody response to pla2r associates with poor prognosis in membranous nephropathy. J Am Soc Nephrology: JASN. (2016) 27:1517–33. doi: 10.1681/asn.2014111061
71. Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrology: JASN. (2012) 23:1735–43. doi: 10.1681/asn.2012030242
72. Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, Damoiseaux JG, and van Paassen P. Anti-pla2r antibodies as a prognostic factor in pla2r-related membranous nephropathy. Am J Nephrol. (2015) 42:70–7. doi: 10.1159/000437236
73. Jurubi R, Obrica B, Sorohan B, Achim C, Micu GE, Mircescu G, et al. Clinical phenotypes and predictors of remission in primary membranous nephropathy. J Clin Med. (2021) 10:2624. doi: 10.3390/jcm10122624
74. Oh YJ, Yang SH, Kim DK, Kang SW, and Kim YS. Autoantibodies against phospholipase A2 receptor in korean patients with membranous nephropathy. PloS One. (2013) 8:e62151. doi: 10.1371/journal.pone.0062151
75. Kattah A, Ayalon R, Beck LH Jr., Sethi S, Sandor DG, Cosio FG, et al. Anti-phospholipase a2 Receptor antibodies in recurrent membranous nephropathy. Am J transplantation: Off J Am Soc Transplant Am Soc Transplant Surgeons. (2015) 15:1349–59. doi: 10.1111/ajt.13133
76. Leon J, Pérez-Sáez MJ, Batal I, Beck LH Jr., Rennke HG, Canaud G, et al. Membranous nephropathy posttransplantation: an update of the pathophysiology and management. Transplantation. (2019) 103:1990–2002. doi: 10.1097/tp.0000000000002758
77. Reinhard L, Zahner G, Menzel S, Koch-Nolte F, Stahl RAK, and Hoxha E. Clinical relevance of domain-specific phospholipase a(2) receptor 1 antibody levels in patients with membranous nephropathy. J Am Soc Nephrology: JASN. (2020) 31:197–207. doi: 10.1681/asn.2019030273
78. Li Z, Weng M, Lin L, Chen Y, Lin J, Cui J, et al. Acute kidney injury in patients with idiopathic membranous nephropathy: influencing factors and prognosis. Renal failure. (2023) 45:2194451. doi: 10.1080/0886022x.2023.2194451
79. Sun M, Li P, Dong J, Li Z, Li C, Zhang S, et al. Clinical characteristics and prognosis of patients with idiopathic membranous nephropathy with kidney tubulointerstitial damage. Renal failure. (2023) 45:2205951. doi: 10.1080/0886022x.2023.2205951
80. Liang J, Hao W, Xia F, Zhao Z, Wu Y, Yu F, et al. Clinicopathological features and outcome in elderly patients with idiopathic membranous nephropathy. Renal failure. (2023) 45:2212081. doi: 10.1080/0886022x.2023.2212081
81. Stangou MJ, Marinaki S, Papachristou E, Liapis G, Pateinakis P, Gakiopoulou H, et al. Histological grading in primary membranous nephropathy is essential for clinical management and predicts outcome of patients. Histopathology. (2019) 75:660–71. doi: 10.1111/his.13955
82. Zhang BO, Cheng M, Yang M, Han S, Zhang YH, Shi HG, et al. Analysis of the prognostic risk factors of idiopathic membranous nephropathy using a new surrogate end-point. Biomed Rep. (2016) 4:147–52. doi: 10.3892/br.2015.555
83. Cattran DC and Brenchley PE. Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int. (2017) 91:566–74. doi: 10.1016/j.kint.2016.09.048
84. Kumar V, Ramachandran R, Kumar A, Nada R, Suri D, Gupta A, et al. Antibodies to M-type phospholipase A2 receptor in children with idiopathic membranous nephropathy. Nephrol (Carlton Vic). (2015) 20:572–5. doi: 10.1111/nep.12478
85. De Vriese AS, Glassock RJ, Nath KA, Sethi S, and Fervenza FC. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrology: JASN. (2017) 28:421–30. doi: 10.1681/asn.2016070776
86. Diaz M, Agraz I, and Soler MJ. Anti-phospholipase A2 receptor antibody and spontaneous remission in membranous nephropathy. Clin Kidney J. (2019) 12:33–5. doi: 10.1093/ckj/sfy079
87. Shah PP, Brady TM, Meyers KEC, O’Shaughnessy MM, Gibson KL, Srivastava T, et al. Association of obesity with cardiovascular risk factors and kidney disease outcomes in primary proteinuric glomerulopathies. Nephron. (2021) 145:245–55. doi: 10.1159/000513869
88. Kovesdy CP, LF S, and Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Clin Kidney J. (2017) 10:1–8. doi: 10.1093/ckj/sfw139
89. Wickman C and Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. (2013) 33:14–22. doi: 10.1016/j.semnephrol.2012.12.006
90. Camici M, Galetta F, Abraham N, and Carpi A. Obesity-related glomerulopathy and podocyte injury: A mini review. Front bioscience (Elite edition). (2012) 4:1058–70. doi: 10.2741/e441
91. Floege J and Amann K. Primary glomerulonephritides. Lancet (London England). (2016) 387:2036–48. doi: 10.1016/s0140-6736(16)00272-5
92. Yamaguchi M, Ando M, Yamamoto R, Akiyama S, Kato S, Katsuno T, et al. Smoking is a risk factor for the progression of idiopathic membranous nephropathy. PloS One. (2014) 9:e100835. doi: 10.1371/journal.pone.0100835
93. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, and Chan TMKidney disease: improving global outcomes glomerular diseases work G. In: Kdigo 2021 clinical practice guideline for the management of glomerular diseases. Philadelphia, PA: Elsevier.
94. Drożdżal S, Lechowicz K, Szostak B, Rosik J, Kotfis K, Machoy-Mokrzyńska A, et al. Kidney damage from nonsteroidal anti-inflammatory drugs-myth or truth? Review of selected literature. Pharmacol Res Perspect. (2021) 9:e00817. doi: 10.1002/prp2.817
95. Hu X, Wang X, Yu X, Ni L, Gao C, Pan X, et al. The role of renal pla2r staining combined with serum pla2r antibody in membranous nephropathy risk stratification. J Clin Med. (2023) 13:68. doi: 10.3390/jcm13010068
96. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the kdigo 2021 guideline for the management of glomerular diseases. Kidney Int. (2021) 100:753–79. doi: 10.1016/j.kint.2021.05.015
97. Wang F, Xu J, Wang F, Yang X, Xia Y, Zhou H, et al. A dynamic online nomogram for predicting renal outcomes of idiopathic membranous nephropathy. BMC Med Inf decision making. (2024) 24:173. doi: 10.1186/s12911-024-02568-2
98. Saleem M, Shaikh S, Hu Z, Pozzi N, and Java A. Post-transplant thrombotic microangiopathy due to a pathogenic mutation in complement factor I in a patient with membranous nephropathy: case report and review of literature. Front Immunol. (2022) 13:909503. doi: 10.3389/fimmu.2022.909503
99. Hamilton P, Bate S, Ragy O, Hiremath M, Bukhari S, Rao A, et al. Impact of time-to-treatment on outcomes in autoimmune membranous nephropathy. Kidney Int Rep. (2025) 10:1907–16. doi: 10.1016/j.ekir.2025.04.005
100. Sarafidis P, Pella E, Kanbay M, and Papagianni A. Sglt-2 inhibitors and nephroprotection in patients with diabetic and non-diabetic chronic kidney disease. Curr medicinal Chem. (2023) 30:2039–60. doi: 10.2174/0929867329666220825121304
101. Bae E, Lee SW, Park S, Kim DK, Lee H, Huh H, et al. Treatment and clinical outcomes of elderly idiopathic membranous nephropathy: A multicenter cohort study in korea. Arch gerontology geriatrics. (2018) 76:175–81. doi: 10.1016/j.archger.2018.03.002
102. Yusei O, Nagasu H, Nakagawa N, Terawaki S, Moriwaki T, Itano S, et al. A case series of fabry diseases with ckd in Japan. Clin Exp Nephrol. (2024) 28:404–8. doi: 10.1007/s10157-023-02439-6
103. Xia Y and Coffman TM. Hold the salt for kidney regeneration. J Clin Invest. (2024) 134:e181397. doi: 10.1172/jci181397
104. Lin PC, Chou CL, Ou SH, Fang TC, and Chen JS. Systematic review of nutrition supplements in chronic kidney diseases: A grade approach. Nutrients. (2021) 13:469. doi: 10.3390/nu13020469
105. Lee T, Biddle AK, Lionaki S, Derebail VK, Barbour SJ, Tannous S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. (2014) 85:1412–20. doi: 10.1038/ki.2013.476
106. Guan M, Wu L, Cheng Y, Qi D, Chen J, Song H, et al. Defining the threshold: triglyceride to high-density lipoprotein cholesterol (Tg/hdl-C) ratio’s non-linear impact on tubular atrophy in primary membranous nephropathy. Front Endocrinol. (2024) 15:1322646. doi: 10.3389/fendo.2024.1322646
107. Zou P, Li H, Cai J, Chen Z, Li C, and Li X. Statins can benefit patients with primary membranous nephropathy on venous thromboembolism. Renal failure. (2021) 43:302–6. doi: 10.1080/0886022x.2021.1879853
108. Peritore L, Labbozzetta V, Maressa V, Casuscelli C, Conti G, Gembillo G, et al. How to choose the right treatment for membranous nephropathy. Medicina (Kaunas Lithuania). (2023) 59:1997. doi: 10.3390/medicina59111997
109. Meyrier A and Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. (2018) 94:861–9. doi: 10.1016/j.kint.2018.04.024
110. Boesen EI and Kakalij RM. Autoimmune-mediated renal disease and hypertension. Clin Sci (London England: 1979). (2021) 135:2165–96. doi: 10.1042/cs20200955
111. Ruggenenti P, Reinhard L, Ruggiero B, Perna A, Perico L, Peracchi T, et al. Anti-phospholipase A2 receptor 1 and anti-cysteine rich antibodies, domain recognition and rituximab efficacy in membranous nephropathy: A prospective cohort study. Am J Kidney diseases: Off J Natl Kidney Foundation. (2024) 83:588–600.e1. doi: 10.1053/j.ajkd.2023.10.013
112. Barbour SJ, Ronco P, Praga M, Fervenza FC, Induruwage D, Zhu B, et al. Predicting remission in anti-pla2r antibody-associated membranous nephropathy: A secondary analysis of the gemritux, mentor and starmen trials. Clin J Am Soc Nephrology: CJASN. (2025) 20:854–865. doi: 10.2215/cjn.0000000694
113. Ronco P, Plaisier E, and Debiec H. The role of pla2r antibody monitoring: what we know and what we do not know. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2023) 38:826–33. doi: 10.1093/ndt/gfab356
114. Chen X, Chen S, Li Z, Pan X, Jia Y, Hu Z, et al. Correlation of body mass index with clinicopathologic parameters in patients with idiopathic membranous nephropathy. Diabetes Metab syndrome obesity: Targets Ther. (2022) 15:1897–909. doi: 10.2147/dmso.S366100
115. Trujillo H, Caravaca-Fontán F, and Praga M. Ten tips on immunosuppression in primary membranous nephropathy. Clin Kidney J. (2024) 17:sfae129. doi: 10.1093/ckj/sfae129
116. Wang J, Bian D, and Sun J. Effect of cyclophosphamide combined with glucocorticoid therapy on idiopathic membranous nephropathy: A multicenter open-label randomized controlled trial. Clin Nephrol. (2024) 102:51–8. doi: 10.5414/cn111287
117. Ramachandran R, Kumar V, Bharati J, Rovin B, Nada R, Kumar V, et al. Long-term follow-up of cyclical cyclophosphamide and steroids versus tacrolimus and steroids in primary membranous nephropathy. Kidney Int Rep. (2021) 6:2653–60. doi: 10.1016/j.ekir.2021.07.028
118. Zonozi R, Laliberte K, Huizenga NR, Rosenthal JK, Jeyabalan A, Collins AB, et al. Combination of rituximab, low-dose cyclophosphamide, and prednisone for primary membranous nephropathy: A case series with extended follow up. Am J Kidney diseases: Off J Natl Kidney Foundation. (2021) 78:793–803. doi: 10.1053/j.ajkd.2021.04.014
119. Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. New Engl J Med. (2019) 381:36–46. doi: 10.1056/NEJMoa1814427
120. Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (Nell-1) associated membranous nephropathy. Kidney Int. (2020) 97:163–74. doi: 10.1016/j.kint.2019.09.014
121. Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, et al. Semaphorin 3b-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. (2020) 98:1253–64. doi: 10.1016/j.kint.2020.05.030
122. Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrology: JASN. (2019) 30:1123–36. doi: 10.1681/asn.2018080852
123. Qin Y, Wu Q, Sheng H, Li T, Liu X, Yang X, et al. Quantitative detection of anti-pla2r antibodies targeting different epitopes and its clinical application in primary membranous nephropathy. Clin Chem Lab Med. (2023) 61:251–9. doi: 10.1515/cclm-2022-0720
124. Yang R, Zhang H, Chen S, Lou K, Zhou M, Zhang M, et al. Quantification of urinary podocyte-derived migrasomes for the diagnosis of kidney disease. J extracellular vesicles. (2024) 13:e12460. doi: 10.1002/jev2.12460
125. Hu X, Xu J, Wang W, Liu L, Jing Y, Gao C, et al. Combined serologic and genetic risk score and prognostication of phospholipase A2 receptor-associated membranous nephropathy. Clin J Am Soc Nephrology: CJASN. (2024) 19:573–82. doi: 10.2215/cjn.0000000000000422
126. Xiaofan H, Jing X, Chenni G, Yifan W, Xialian Y, Li L, et al. New risk score for predicting progression of membranous nephropathy. J Trans Med. (2019) 17:41. doi: 10.1186/s12967-019-1792-8
127. Mariani LH, Martini S, Barisoni L, Canetta PA, Troost JP, Hodgin JB, et al. Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2018) 33:310–8. doi: 10.1093/ndt/gfw443
Keywords: primary membranous nephropathy, spontaneous remission, anti-PLA2R antibody, risk stratification, precision therapy
Citation: Wu M, Chen Y, He Z and Jin Y (2025) Spontaneous remission in primary membranous nephropathy: mechanisms, predictive factors, and implications for personalized management. Front. Immunol. 16:1651810. doi: 10.3389/fimmu.2025.1651810
Received: 22 June 2025; Accepted: 27 August 2025;
Published: 11 September 2025.
Edited by:
Andrea Angioi, G. Brotzu Hospital, ItalyReviewed by:
Edmund Chung, Children’s Hospital at Westmead, AustraliaOlimkhon Sharapov, Republican Specialized Scientific Practical Medical Center of Nephrology and Kidney Transplantation, Uzbekistan
Copyright © 2025 Wu, Chen, He and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youping Jin, empsc2NqaEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Mengqi Wu
Mengqi Wu Yanhao Chen
Yanhao Chen Zixin He3†
Zixin He3† Youping Jin
Youping Jin