- 1Department of Surgery, Chung Shan Medical University Hospital, Taichung, Taiwan
- 2School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 3Department of Medical Research, Chung Shan Medical University, Taichung, Taiwan
- 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 5Division of Allergy, Immunology, Rheumatology, Changhua Christian Hospital, Changhua, Taiwan
- 6Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
- 7Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
- 8Office of Research and Development, Asia University, Taichung, Taiwan
1 Pathogenesis - blood–brain barrier dysfunction
Current evidence indicate that an intact blood–brain barrier (BBB) ordinarily prevents inflammatory cytokines and pathogenic autoantibodies from entering the central nervous system (CNS) (1). In SLE, compromised BBB integrity allowed autoantibodies and inflammatory cells to infiltrate the CNS, contributing to neuropsychiatric symptoms. However, as shown by Ho et al. (2), certain serum autoantibodies could compromise vascular integrity, cross the BBB, and bind to neural antigens, initiating in situ immune−complex formation. These immune complexes lay at the heart of SLE and NPSLE pathogenesis and often manifest clinically as elevated immunoglobulin index in cerebrospinal fluid (CSF), a potential diagnostic marker. The deposition of immune complexes on the endothelial surface activates the complement cascade, which in turn induces endothelial cytoskeletal reorganization, disassembly of tight-junction proteins (claudin-5, occludin), and increased barrier permeability (3).
Concurrent activation of the classical complement cascade by these immune complexes correlate with hypocomplementemia (low C3 and C4), an elevated CSF immunoglobulin index, and heightened neuropsychiatric disease activity, underscoring the pathogenic role of complement in NPSLE.
2 Pathogenesis – associated autoantibodies with NPSLE
Autoantibodies play a critical role in SLE, in early studies when antibodies to ribosomes were found, indicating association between anti-ribosomal P antibody and NPSLE. However, a previous study demonstrated low sensitivity and high specificity of anti-ribosomal P antibody in NPSLE, leading to a limitation in diagnosis (4). In recent study with functional magnetic resonance imaging (fMRI), increased amplitude of low-frequency fluctuations (ALFF) and degree centrality (DC) values were found in SLE patients with positive anti-ribosomal P antibody which suggested the early potential biomarker of brain injury (5).
Aquaporin 4-specific autoantibodies were studied in patients with NPSLE and found to cause severe demyelination and axonal damage in patients with concurrent neuromyelitis optica spectrum disorder (NMOSD). In demyelinating NPSLE, a previous study showed 27% patients have positive AQP-4 antibodies (6). In clinical practice, AQP4 antibodies were recommended to be investigated if SLE patients with optic neuritis.
Anti-N-methyl-D-aspartate receptor (NMDAR) antibodies have been investigated in relation to neuropsychiatric SLE (NPSLE) in recent studies, with a reported prevalence of approximately 30%. These autoantibodies constituted a subset of anti-double-stranded DNA (anti-dsDNA) antibodies that cross-reacted with the NR2A and NR2B subunits of the NMDAR (7). The correlation between anti–NR2 glutamate receptor antibodies in CSF was associated with diffuse NPSLE (8).
3 Early detection in patients with NPSLE
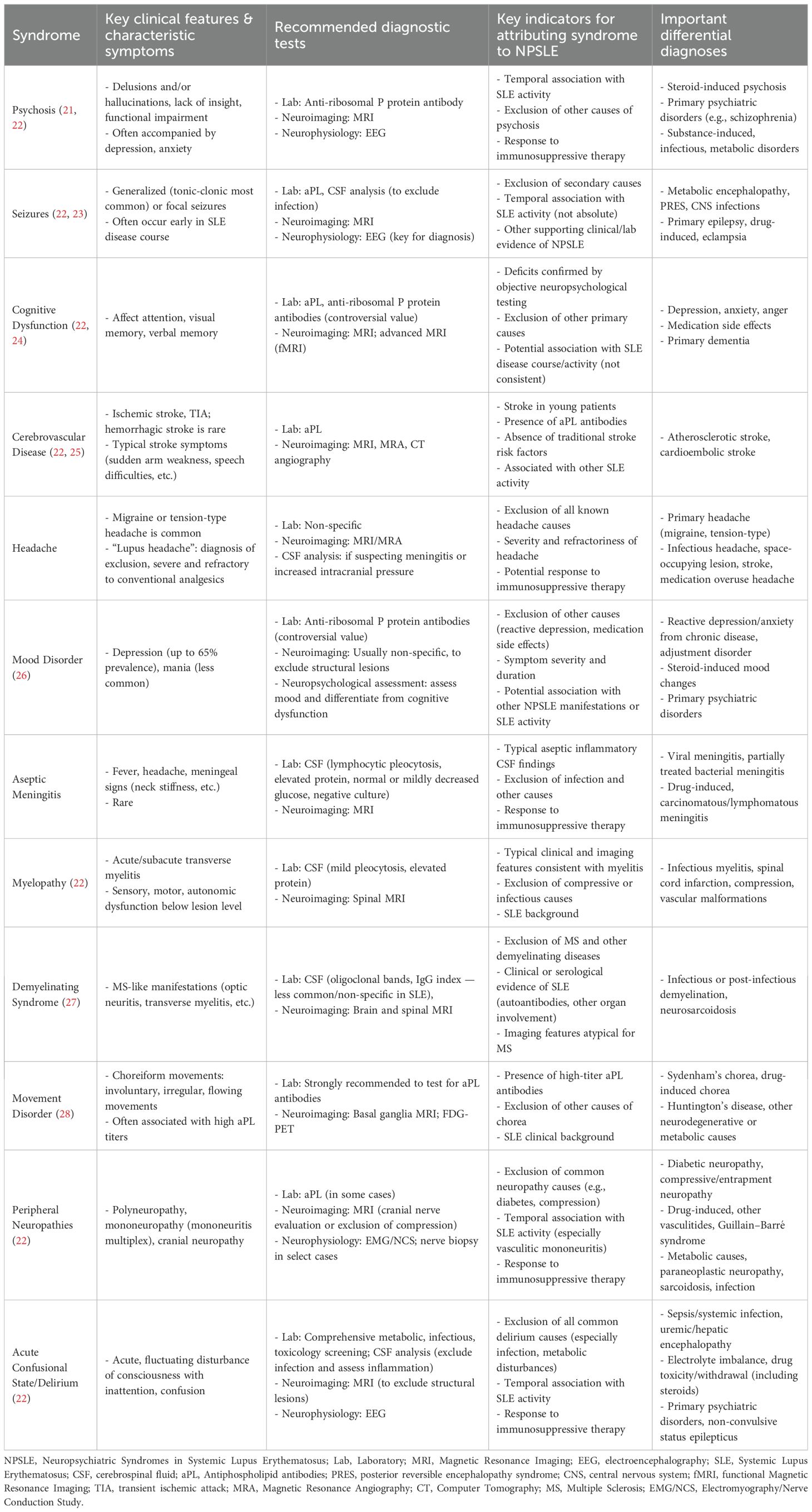
Despite the existing criteria for NPSLE, the unmet need in diagnostic uncertainty remained a major problem. Although cognitive impairment is categorized as one of the early diffuse manifestations of NPSLE, the symptoms remain debatable. Previous studies demonstrate no statistically significant differences in cognitive function between patients with non-NPSLE and those with NPSLE, although both groups performed worse than healthy controls (9). The symptom was not associated with disease activity but associated with anti-NMDAR antibodies (10). With MRI perfusion, significant reduction of cerebral blood flow was found in patients with NPSLE compared to non-NPSLE (11). MRI might be a effective tool in evaluating early NPSLE to assist the diagnosis. Table 1 summarized neuropsychiatric syndromes in systemic lupus erythematosus.
4 The application and effectiveness of immunosuppressive therapy: the potential of biologic agents in NPSLE
Treatment for NPSLE remain investigational, with limited clinical trials primarily exploring agents such as memantine, corticosteroids, and cyclophosphamide (12). “In routine clinical practice, available biologic therapies for SLE are also limited, primarily including belimumab, anifrolumab, and rituximab; however, evidence specifically addressing their efficacy in NPSLE remains limited “Belimumab has demonstrated efficacy in both non-renal SLE and lupus nephritis; however, high-level evidence supporting its use in NPSLE remains lacking A recent analysis of five clinical trials demonstrated the indeterminate nature of the protective or potentially predisposing role of belimumab (13).
There was no direct evidence in evaluation of neuropsychiatric symptoms in SLE patients treated with anifrolumab. However, a retrospective study including SLE patients who presented neuropsychiatric symptoms demonstrated that anifrolumab was effective in controlling overall disease activity (13). Additionally, data from a retrospective analysis using the LOOPS registry in Japan indicated that anifrolumab maintained its effectiveness even among patients diagnosed with NPSLE (14).
Rituximab demonstrated its effectiveness in controlling disease activity in SLE patients, especially in refractory patients. In a study with BILAG-BR registry, the results showed that rituximab could have been an effective treatment for NPSLE (15).
5 Future research directions and expectations
In NPSLE, increased interleukin (IL) – 6 was found in CSF in a previous study, and IL-6 also demonstrated the associated with incident psychosis (16, 17). Satralizumab demonstrated its effectiveness in NMOSD, which might have played a role in NPSLE via IL-6 axis.
The CD40–CD40L pathway played a pivotal role in systemic and renal inflammation in lupus nephritis (LN). CD40 signaling promoted B-cell activation, IgG class switching, plasma cell differentiation, and memory B-cell survival, perpetuating autoantibody production. In LN kidneys, CD40L was expressed by T cells and activated platelets, which stimulated CD40 on mesangial and endothelial cells, leading to proliferation, monocyte chemoattractant protein-1 (MCP-1) and transforming growth factor-β1 (TGF-β1) production, and enhanced leukocyte recruitment. This drove tubulointerstitial and glomerular inflammation and fibrosis. High renal CD40 expression correlated with severe pathology and poor prognosis, making CD40–CD40L blockade a compelling therapeutic strategy. Anti-CD40 and anti-CD40L therapies showed promise in reversing proteinuria, reducing autoantibodies, and prolonging survival in LN models (18).
Dapirolizumab pegol (DZP) was an Fc-free anti-CD40L agent, showed clinical benefit in the Phase 3 PHOENYCS GO trial by reducing disease activity and enabling corticosteroid tapering in SLE patients (19). Additionally, DZP plus standard of care improved fatigue outcomes, as measured by Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and Patient-Reported Outcome (FATIGUE-PRO) scales (20).
However, its clinical use was limited by thrombotic risks seen with earlier anti-CD40L agents, highlighting the need for safer, targeted strategies to modulate the CD40–CD40L pathway (18).
On the other hand, alternative routes of administration beyond intravenous therapy remain unresolved but crucial, given the limitations posed by the BBB on drug efficacy in NPSLE. Intrathecal, intraventricular, and intranasal routes were proposed as potentially feasible approaches to overcome BBB challenges.
6 Conclusion
NPSLE should focus on improving diagnostic tools, enhancing the understanding of its pathophysiology, and developing more effective, targeted therapies. A multidisciplinary approach is essential for accurate diagnosis, requiring collaboration among experts in neurology, psychiatry, vascular medicine, hematology, rheumatology, and neuroradiology to assess patient conditions. Future prospective studies can help optimize the management of NPSLE and improve quality of life for all patients.
Author contributions
S-TS: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. PK: Writing – original draft, Writing – review & editing, Supervision, Validation, Visualization. P-CS: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deijns SJ, Broen JC, Kruyt ND, Schubart CD, Andreoli L, Tincani A, et al. The immunologic etiology of psychiatric manifestations in systemic lupus erythematosus: a narrative review on the role of the blood brain barrier, antibodies, cytokines and chemokines. Autoimmun Rev. (2020) 19:102592. doi: 10.1016/j.autrev.2020.102592
2. Ho RC, Thiaghu C, Ong H, Lu Y, Ho CS, Tam WW, et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun Rev. (2016) 15:124–38. doi: 10.1016/j.autrev.2015.11.003
3. Shimizu F and Nakamori M. Blood–brain barrier disruption in neuroimmunological disease. Int J Mol Sci. (2024) 25:10625. doi: 10.3390/ijms251910625
4. Legge AC and Hanly JG. Recent advances in the diagnosis and management of neuropsychiatric lupus. Nat Rev Rheumatol. (2024) 20:712–28. doi: 10.1038/s41584-024-01175-3
5. Liang S, Maitiyaer M, Tan Q, Chen L, Chen X, Li Z, et al. Exploring immune-mediated brain function abnormalities in systemic lupus erythematosus: neuroimaging evidence of the impact of anti-ribosomal P protein antibodies. Acad Radiol. (2025) 32:2889–99. doi: 10.1016/j.acra.2025.01.005
6. Mader S, Jeganathan V, Arinuma Y, Fujieda Y, Dujmovic I, Drulovic J, et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorder: do they share common targets? Arthritis Rheumatol. (2018) 70:277–86. doi: 10.1002/art.40399
7. Brimberg L, Mader S, Fujieda Y, Arinuma Y, Kowal C, Volpe BT, et al. Antibodies as mediators of brain pathology. Trends Immunol. (2015) 36:709–24. doi: 10.1016/j.it.2015.09.003
8. Arinuma Y, Yanagida T, and Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. (2008) 58:1130–5. doi: 10.1002/art.23384
9. Langensee L, Mårtensson J, Jönsen A, Zervides K, Bengtsson A, Nystedt J, et al. Cognitive performance in systemic lupus erythematosus patients: a cross-sectional and longitudinal study. BMC Rheumatol. (2022) 6:22. doi: 10.1186/s41927-022-00257-5
10. Yue R, Gurung I, Long XX, Xian JY, and Peng XB. Prevalence, involved domains, and predictor of cognitive dysfunction in systemic lupus erythematosus. Lupus. (2020) 29:1743–51. doi: 10.1177/0961203320958642
11. Azizi N, Issaiy M, Jalali AH, Kolahi S, Naghibi H, Zarei D, et al. Perfusion-weighted MRI patterns in neuropsychiatric systemic lupus erythematosus: a systematic review and meta-analysis. Neuroradiology. (2025) 67:109–24. doi: 10.1007/s00234-024-03229-x
12. Patel V. The challenge of neuropsychiatric systemic lupus erythematosus: from symptoms to therapeutic strategies. Diagnostics. (2024) 14:1186. doi: 10.3390/diagnostics14111186
13. Palazzo L, Lindblom J, Cetrez N, Ala H, and Parodis I. Determinants of neuropsychiatric flares in patients with systemic lupus erythematosus: results from five phase III trials of belimumab. Rheumatology. (2024) 63:798–808. doi: 10.1093/rheumatology/kead470
14. Miyazaki Y, Funada M, Nakayamada S, Sonomoto K, Tanaka H, Hanami K, et al. Safety and efficacy of anifrolumab therapy in systemic lupus erythematosus in real-world clinical practice: LOOPS registry. Rheumatology. (2024) 63:2345–54. doi: 10.1093/rheumatology/keae129
15. Rodziewicz M, Dyball S, David T, Sutton E, Parker B, and Bruce I. OP0043 the effectiveness of rituximab in the real-world treatment of neuropsychiatric sle: results from the british iles lupus assessment group biologics register (BILAG-BR). Ann Rheum Dis. (2024) 83:137–8. doi: 10.1136/annrheumdis-2024-eular.1921
16. Shi J. POS1416 IL-6, IL-8, TNF-α are significantly increased in cerebrospinal fluid and associated with alterations of eye sign in patients with neuropsychiatric lupus erythematosus. Ann Rheum Dis. (2023) 82(Suppl 1):1062.1–1062. doi: 10.1136/annrheumdis-2023-eular.3567
17. Misiak B, Bartoli F, Carrà G, Stańczykiewicz B, Gładka A, Frydecka D, et al. Immune-inflammatory markers and psychosis risk: a systematic review and meta-analysis. Psychoneuroendocrinology. (2021) 127:105200. doi: 10.1016/j.psyneuen.2021.105200
18. Ramanujam M, Steffgen J, Visvanathane S, Mohan C, Fine JS, and Putterman C. Phoenix from the flames: Rediscovering the role of the CD40–CD40L pathway in systemic lupus erythematosus and lupus nephritis. Autoimmun Rev. (2020) 19:102668. doi: 10.1016/j.autrev.2020.102668
19. Clowse M, Isenberg D, Merrill J, Dörner T, Petri M, Vital E, et al. Dapirolizumab pegol demonstrated significant improvement in systemic lupus erythematosus disease activity: efficacy and safety results of a phase 3 trial. ACR Convergence. (2024) 84(Supplement 1):900–2. doi: 10.1136/annrheumdis-2025-eular.B909
20. Parodis I, Gordon C, Merrill J, Schneider M, Touma Z, Jimenez T, et al. Improvement of fatigue in patients with systemic lupus erythematosus treated with dapirolizumab pegol: 48-week results from a phase 3 trial. Ann Rheum Dis. (2025) 84(Supplement 1):1227–9. doi: 10.1136/annrheumdis-2025-eular.B909
21. Kang D and Mok CC. Management of psychosis in neuropsychiatric lupus. J Clin Rheumatol Immunol. (2019) 19:9–17. doi: 10.1142/S2661341719300015
22. Bertsias GK, Ioannidis JPA, Aringer M, Bollen E, Bombardieri S, Bruce IN, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. (2010) 69(12):2074–82. doi: 10.1136/ard.2010.130476
23. Appenzeller S, Cendes F, and Costallat LTL. Epileptic seizures in systemic lupus erythematosus. Neurology. (2004) 63:1808–12. doi: 10.1212/01.WNL.0000144178.32208.4F
24. Mak A, Ho RCM, and Lau CS. Clinical implications of neuropsychiatric systemic lupus erythematosus. Adv Psychiatr Treat. (2009) 15:451–8. doi: 10.1192/apt.bp.108.005785
25. Nikolopoulos D, Fanouriakis A, and Boumpas DT. Cerebrovascular events in systemic lupus erythematosus: diagnosis and management. Mediterr J Rheumatol. (2019) 30:7–15. doi: 10.31138/mjr.30.1.7
26. Schwartz N, Stock AD, and Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. (2019) 15:137–52. doi: 10.1038/s41584-018-0156-8
27. Nikolopoulos D, Kitsos D, Papathanasiou M, Kapsala N, Garantziotis P, Pieta A, et al. Demyelinating syndromes in systemic lupus erythematosus: data from the “Attikon” Lupus Cohort. Front Neurol. (2022) 13:889613. doi: 10.3389/fneur.2022.889613
Keywords: systemic lupus erythematosus, neuropsychiatric, NPSLE, cognitive, BBB
Citation: Su S‐T, Kuo P, Shih P-C and Wei JC-C (2025) The immunological mechanisms linking systemic lupus erythematosus and neuropsychiatric disorders. Front. Immunol. 16:1651874. doi: 10.3389/fimmu.2025.1651874
Received: 22 June 2025; Accepted: 15 September 2025;
Published: 30 September 2025.
Edited by:
Rosaria Talarico, University of Pisa, ItalyReviewed by:
Uma Sriram, Temple University, United StatesCopyright © 2025 Su, Kuo, Shih and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Cheng Shih, cm9iZXJ0cGNzaGloQGdtYWlsLmNvbQ==; James C. ‑ C. Wei, amNjd2VpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Shiuan‐Tzuen Su
Shiuan‐Tzuen Su Poi Kuo3
Poi Kuo3 Po-Cheng Shih
Po-Cheng Shih James C. - C. Wei
James C. - C. Wei