- 1Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Republic of Korea
- 2Department of Genomic Medicine, Seoul National University Hospital, Seoul, Republic of Korea
- 3Division of Rheumatology, Department of Internal Medicine, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Gyeonggi, Republic of Korea
- 4Laboratory of Autoimmunity and Inflammation (LAI), Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Republic of Korea
- 5Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Republic of Korea
Background: Cryopyrin-associated periodic syndrome (CAPS) is an autoinflammatory disease caused by a gain-of-function mutation in the NLRP3 gene, which regulates inflammasome-mediated interleukin-1β (IL-1β) production. This leads to recurrent episodes of fever, rash, and arthritis, typically beginning in childhood.
Objective: To demonstrate the role of a missense mutation, c.386A>G, in NLRP3 in adult-onset CAPS in a previously healthy middle-aged woman.
Methods: Whole-exome sequencing was performed. Serum levels of IL-1β, interleukin-1 receptor antagonist (IL-1RA), and tumor necrosis factor α (TNF-α) were measured. CD14-positive monocytes, isolated from the patient before and during IL-1 inhibition therapy, were stimulated with lipopolysaccharide (LPS), and cytokine production was assessed.
Results: A 47-year-old woman presented with recurrent periorbital swelling and inflammatory symptoms, along with elevated IL-1RA and IL-6 levels. Genetic analysis revealed a heterozygous missense mutation in the NLRP3 gene (NM_001243133, c.386A>G, p.Lys129Arg). Serum IL-1RA levels were significantly elevated during active disease. Monocytes from the patient produced high levels of IL-1β and TNF-α upon LPS stimulation. Treatment with anakinra ameliorated all symptoms and normalized inflammatory cytokine overproduction in the monocytes.
Conclusion: We report a case of adult-onset CAPS in a previously healthy woman, caused by a missense mutation (c.386A>G) in the NLRP3 gene, who exhibited a remarkable response to anakinra treatment. Autoinflammatory diseases should be considered in patients presenting with fever, skin rashes, and systemic symptoms, regardless of age.
Introduction
Autoinflammatory diseases are characterized by abnormal activation of inflammatory pathways in the absence of antigen-driven autoimmunity (1). Dysregulated inflammasome activation leads to excessive production of interleukin-1β (IL-1β), resulting in recurrent sterile inflammation that manifests as episodic fever, rashes, and arthritis, among other symptoms (2). Gain-of-function (GOF) variants in the NLRP3 gene cause excessive activation of the NLRP3 inflammasome, leading to IL-1β overproduction (3). The excellent clinical response to IL-1β-targeted therapies underscores its critical role in disease pathogenesis (4).
Heterozygous missense mutations in NLRP3 cause autosomal dominant cryopyrin-associated periodic syndromes (CAPS) (5). The three major subtypes of CAPS—familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disorder (NOMID)—typically present early in life. In contrast, somatic NLRP3 mutations with mosaicism, restricted to myeloid cell lines, have been associated with adult-onset CAPS (6). However, reports of adult-onset CAPS due to a heterozygous germline mutation are rare (7). Mutations in NLRP3 can be clinically insignificant or pathogenic, with variable penetrance depending on the specific mutation site (8). With the increasing availability of genetic testing, many variants previously classified as “variants of uncertain significance” are now being reclassified as pathogenic based on emerging clinical and functional evidence (9).
In this study, we identified a case of adult-onset CAPS associated with a germline missense mutation at c.386A>G (p.Lys129Arg) in the NLRP3 (NM_001243133) gene in a previously healthy middle-aged Korean woman who presented with chronic, recurrent episodes of periorbital swelling, fever, rashes, and arthritis. The patient exhibited a dramatic response to anti–IL-1 treatment. This finding supports the pathogenicity of this variant in the Asian population and suggests that it may be an underrecognized cause of recurrent febrile illness.
Methods
Study subjects
Blood samples were collected from the patient and healthy controls without autoimmune diseases after written informed consent was obtained. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-2306-123-1440).
Genomic analysis
Genomic DNA was extracted from the patient’s peripheral blood and fragmented using a Covaris E220 focused ultrasonicator (Covaris, Woburn, MA, USA). Whole-exome capture and library preparation were performed using the Agilent SureSelect All Exon V8 kit and the SureSelectXT Target Enrichment Protocol (Agilent Technologies, Santa Clara, CA, USA). Whole-exome sequencing (WES) was conducted on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) using 150 base pair (bp) paired-end sequencing.
Sequencing reads were aligned to the reference human genome (GRCh37/hg19) using Burrows–Wheeler Aligner (BWA v0.7.17). Variant calling followed the GATK Best Practices pipeline (v4.0.2.1). Single-nucleotide variants (SNVs) and small insertions/deletions (indels) were identified using an in-house pipeline incorporating SNVer (v0.5.3) and LoFreq (v2.1.2). Variants were functionally annotated using SnpEff (v5.0e).
Human monocyte stimulation and cytokine analysis
Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation with Ficoll-Separating Solution (BIOCHROM Inc., Cambridge, UK). Monocytes were subsequently isolated from PBMCs using anti-CD14 magnetic beads (Miltenyi Biotec Inc., Auburn, CA, USA).
Isolated monocytes were stimulated with lipopolysaccharide (LPS) at a concentration of 10 ng/mL for 24 h. Cytokine levels—including interleukin-1 receptor antagonist (IL-1RA), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α)—were quantified from plasma or culture supernatants using commercial human enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific, Waltham, MA, USA). Optical density measurements were obtained using an Infinite M200 microplate reader (Tecan, Männedorf, Switzerland).
Results
Case presentation
A 47-year-old Korean woman with hypothyroidism awoke with swelling in her left eyelid, which was red, warm, itchy, and tender to the touch. She also experienced a severe headache located around the eye and the left side of her head (Figure 1A). There was no known family history of autoimmune or autoinflammatory diseases, including recurrent febrile illnesses. A computed tomography (CT) scan of the head and neck revealed soft tissue infiltration in the preseptal region, lacrimal gland, and left orbit, along with enlarged cervical lymph nodes (Figure 1B). A biopsy of the affected eyelid showed multifocal lymphoplasmacytic infiltration with a mixed population of CD3+ T cells and CD20+ B cells, with a normal Ki-67 proliferation index. IgG4-producing cells were scarce (Figure 1C).
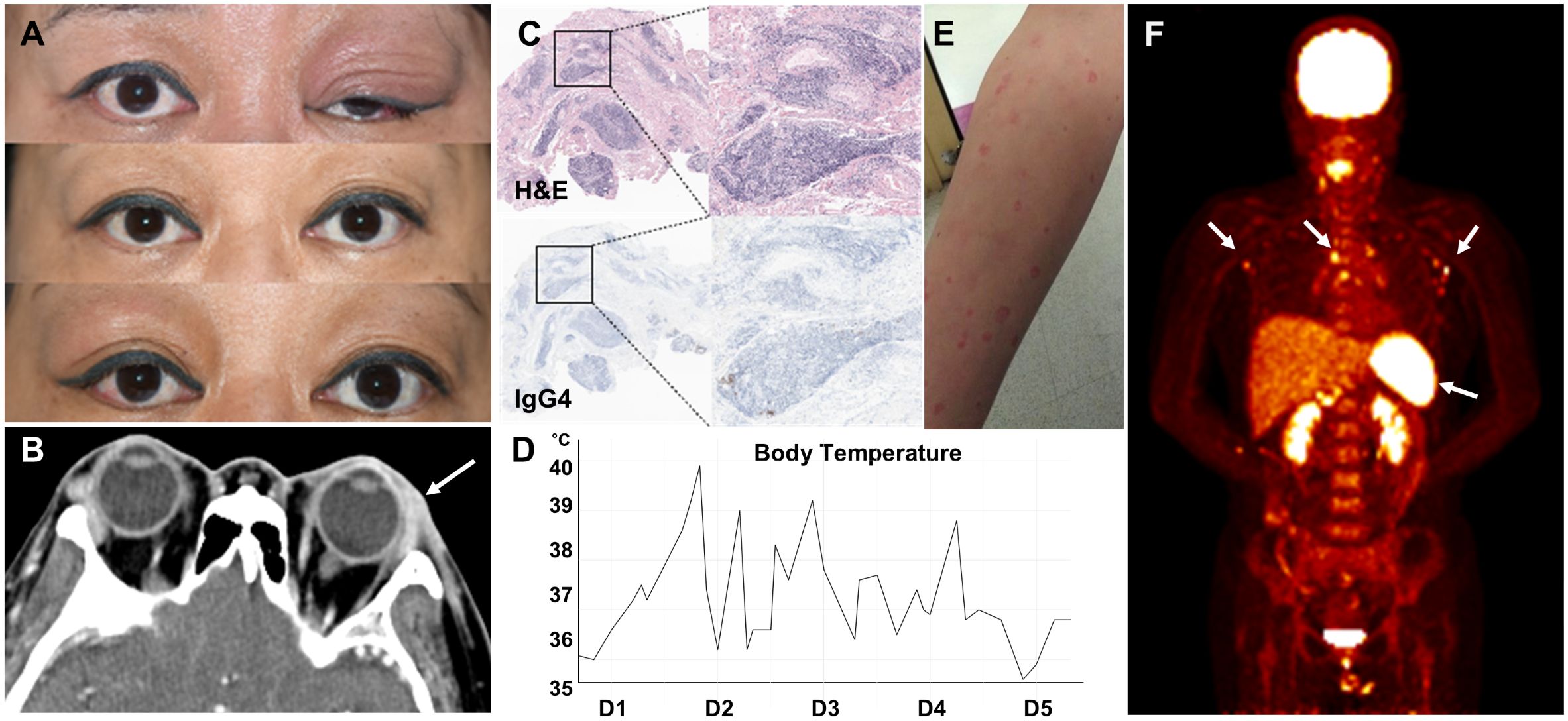
Figure 1. Recurrent periorbital swelling. (A) Recurrent and alternating swelling of the patient’s eyelids. (B) CT image of the orbital area showing soft tissue infiltration in the preseptal region, lacrimal gland, and left orbit (arrow). (C) Lacrimal gland biopsy showing multifocal lymphoplasmacytic infiltration (upper panel) and a few IgG4-positive cells (lower panel). (D) Temperature graph showing the patient’s fever pattern during the first 5 days of admission. (E) Maculopapular eruption. (F) PET scan showing increased metabolic activity in lymph nodes and spleen (arrows).
Anti-thyroid peroxidase and anti-thyroglobulin antibody levels were >4,000 IU/mL and 253 U/mL, respectively. Thyroid function test results were within normal limits. Suspecting an autoimmune disease such as thyroid-associated ophthalmopathy, she was treated with weekly methylprednisolone pulse therapy (250–500 mg) for 2 months, resulting in partial improvement. However, periorbital swelling recurred, alternating between the left and right eye over subsequent months.
Following her third dose of the COVID-19 vaccine, she developed fever with temperatures up to 40°C, cervical lymphadenopathy, skin rash, abdominal pain, myalgia, and arthralgia. The fever was intermittent (Figure 1D). The skin rashes were generalized, erythematous, confluent, maculopapular, and urticarial, measuring 1–2 cm in diameter. They were mildly pruritic and painful (Figure 1E). Laboratory results revealed a white blood cell (WBC) count of 9,230/µL, C-reactive protein (CRP) level of 9.20 mg/dL (normal <0.5 mg/dL), and erythrocyte sedimentation rate (ESR) of 91 mm/h. Her ferritin level was 2,341.4 ng/mL (normal: 21.8–274.7 ng/mL). Both serum and urine protein electrophoresis (PEP) showed no evidence of monoclonal gammopathy. Rheumatoid factor, antinuclear antibody (ANA), and anti-neutrophil cytoplasmic antibody (ANCA) were negative. An extensive infectious workup, including blood and urine cultures, was also negative.
CT imaging of the neck, chest, and abdomen showed diffuse lymphadenopathy and mild splenomegaly. A positron emission tomography (PET) scan revealed increased metabolic activity in the lymph nodes, bone marrow, and spleen (Figure 1F). A cervical lymph node biopsy demonstrated lymphoid follicular hyperplasia without evidence of malignancy. A bone marrow biopsy revealed reactive normocellular marrow without malignant cells.
The fever persisted, with spikes up to 40°C. CRP and ferritin levels rose to 13.5 mg/dL and 16,567.19 ng/mL, respectively. Based on the constellation of fever, skin rash, diffuse lymphadenopathy, splenomegaly, and markedly elevated ferritin levels, adult-onset Still’s disease (AOSD) was suspected. Prednisolone 30 mg/day was initiated, with partial response. However, the patient continued to experience fever, fatigue, and recurrent periorbital inflammation, although CRP and ferritin levels declined to 2.82 mg/dL and 86.6 ng/mL, respectively. Fever returned when the prednisolone dose was tapered below 20 mg/day. Tocilizumab, methotrexate, and later tacrolimus provided little to no clinical improvement (Figure 2A).
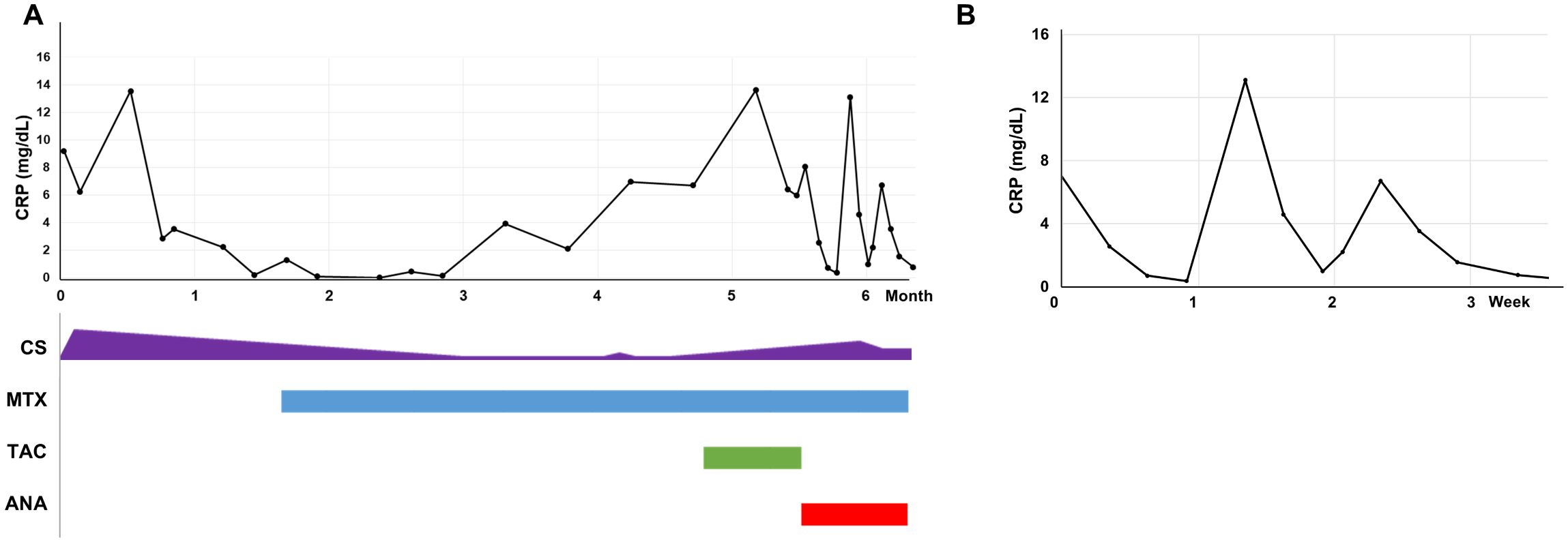
Figure 2. C-reactive protein levels in response to treatment. (A) C-reactive protein (CRP) levels over the first 6 months of treatment. (B) CRP changes with and without anakinra. ANA, anakinra; CRP, C-reactive protein; CS, corticosteroids; MTX, methotrexate; TAC, tacrolimus.
An acquired autoinflammatory disease was suspected. A daily subcutaneous dose of anakinra (100 mg) was initiated. Remarkably, fever, constitutional symptoms, and periorbital edema resolved within hours of the first injection. For the first time since disease onset, the patient reported feeling completely well. CRP levels normalized to 0.35 mg/dL (normal <0.5 mg/dL) after 6 days of anakinra treatment. However, when anakinra was interrupted, both symptoms and CRP levels worsened immediately (Figure 2B).
Genomic analysis
To identify the underlying genetic cause of the disease, next-generation sequencing-based whole-exome sequencing (WES) was performed. Among the autoinflammation-related genes, a heterozygous non-synonymous single-nucleotide variant (SNV) in the NLRP3 gene (NM_001243133, c.386A>G, p.Lys129Arg) was identified and suspected to be the causative pathogenic change. Of the 90 total sequencing reads at this position, 41 (45.6%) carried the variant allele, consistent with a heterozygous germline mutation (Figure 3).
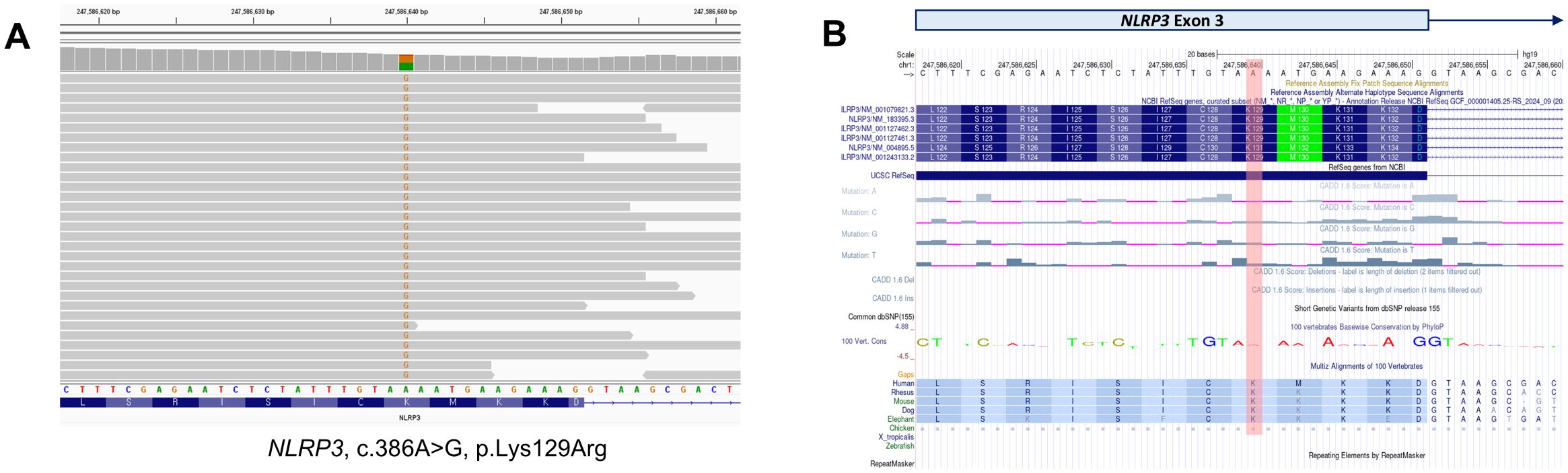
Figure 3. Genetic analysis. (A) Whole-exome sequencing identified a non-synonymous single-nucleotide variant in the NLRP3 gene. The variant was detected in 41 sequencing reads, with a variant allele frequency of 45.6%. (B) The NLRP3 variant results in the substitution of the 129th amino acid from lysine (K) to arginine (R) in exon 3 of transcript NM_001243133. The UCSC Genome Browser (https://genome.ucsc.edu/) was used to visualize the variant.
Plasma cytokines analysis
The patient’s plasma interleukin-1 receptor antagonist (IL-1RA) level was markedly elevated at 5,933 pg/mL, compared with 155.9 ± 8.9 pg/mL in three healthy controls (Figure 4A). IL-1β levels were low in both patient and controls, while IL-6 level was slightly elevated in the patient. TNF-αlevels were comparable to those in controls. Following treatment with anakinra, plasma levels of IL-1RA, IL-1β, and IL-6 became undetectable.
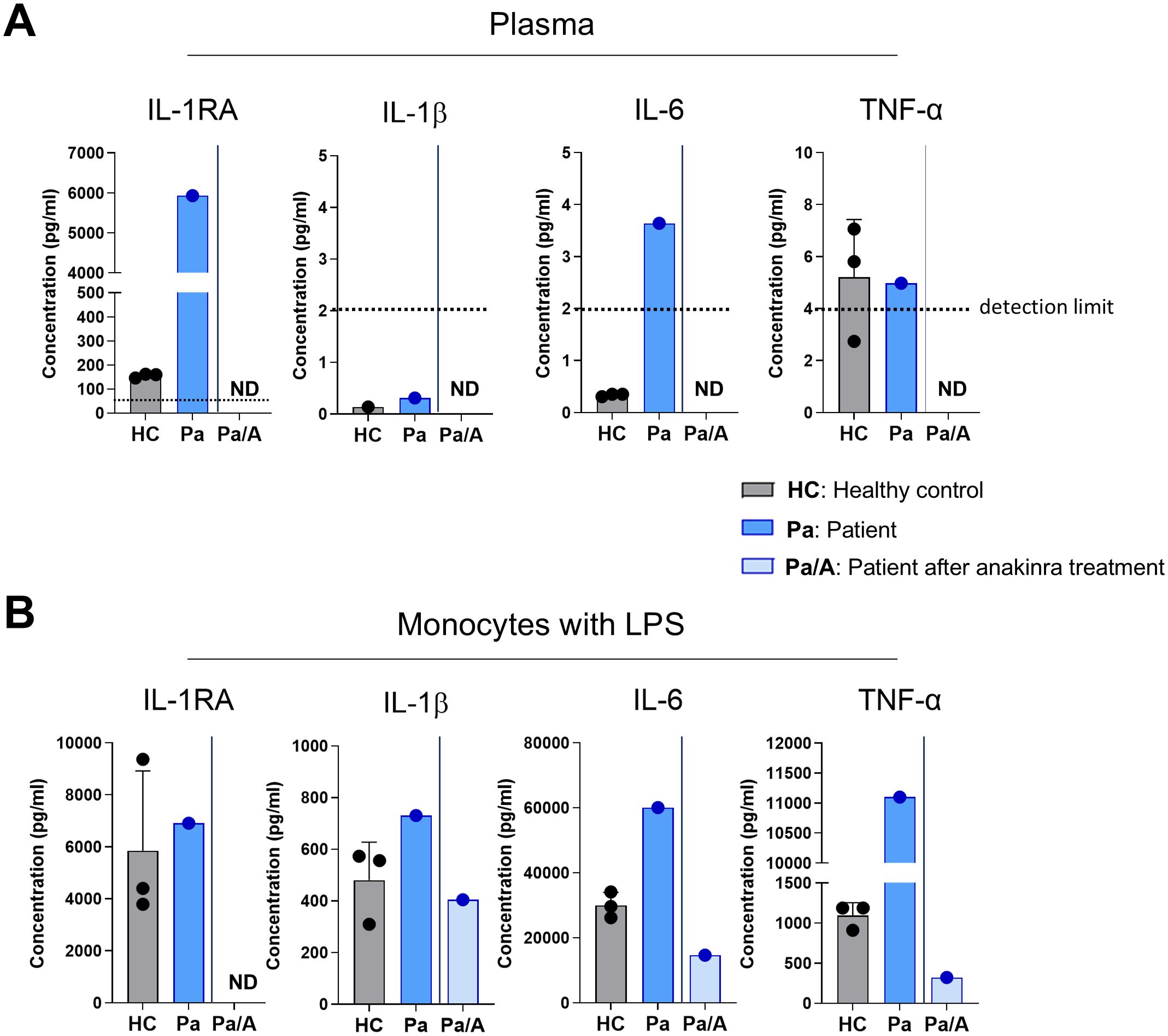
Figure 4. Elevated proinflammatory cytokine levels and hyperinflammatory response upon stimulation. (A) Cytokine concentrations in the plasma of healthy controls (n = 3) and the patient, before and after anakinra treatment, measured using ELISA. The dotted line represents the detection limit. (B) Purified CD14+ monocytes were stimulated with lipopolysaccharide (LPS, 10 ng/mL) for 24 h, and cytokine levels in the supernatant were quantified by ELISA. LPS, lipopolysaccharide; ND, not detected; Pt, patient.
Cytokine production upon stimulation
Upon LPS stimulation, CD14+ monocytes from the patient produced IL-1RA levels comparable to controls (6,914 pg/mL vs. 5,856 ± 3,060 pg/mL). However, the patient’s monocytes secreted higher levels of IL-1β (730.6 pg/mL vs. 479.7 ± 147.7 pg/mL) and IL-6 (60,195 pg/mL vs. 30,029 ± 3,973 pg/mL) than healthy controls. TNF-α production was approximately 10-fold higher in the patient’s monocytes (11,102 pg/mL vs. 1,095 ± 160 pg/mL). Monocytes isolated after anakinra treatment produced lower levels of IL-1RA, IL-1β, and IL-6—even compared to controls (Figure 4B).
Discussion
In this report, we describe a case of adult-onset CAPS in a previously healthy middle-aged Korean woman who developed recurrent high fevers, skin rashes, eyelid swelling, myalgia, and arthralgia, accompanied by elevated plasma IL-1RA and IL-6 levels. Genetic analysis revealed a heterozygous missense mutation in the NLRP3 gene, and in vitro studies demonstrated increased production of the proinflammatory cytokines IL-1β and IL-6 upon stimulation. The patient responded remarkably to treatment with an IL-1β inhibitor.
The constellation of recurrent fevers, urticarial rash, arthritis, and myalgia raised suspicion for an autoinflammatory disease, including CAPS (1). CAPS results from gain-of-function mutations in NLRP3, leading to aberrant inflammasome activation and excessive IL-1β production (4). CAPS typically manifests early in life, often within the first few years. Therefore, given the new onset of febrile disease in this middle-aged patient, alternative diagnoses such as adult-onset Still’s disease (AOSD), acquired autoinflammatory conditions like VEXAS syndrome, or myelodysplastic syndrome with secondary autoimmunity were initially considered (10, 11). However, the patient’s suboptimal response to glucocorticoids and IL-6 inhibitors, along with a negative cancer workup, made AOSD and hematologic malignancies less likely (12). These unusual findings, including the episodic nature of inflammation, led to suspicion of an autoinflammatory disease. The diagnosis of adult-onset CAPS was confirmed by identifying a heterozygous missense mutation in NLRP3 (rs188623199).
The patient’s initial presentation with eyelid swelling—fluctuating in severity and occurring in the absence of systemic inflammatory signs—led to a misdiagnosis of thyroid-associated ophthalmopathy, for which corticosteroid therapy provided only partial improvement. However, the subsequent emergence of systemic features, such as episodic fever and rash, prompted consideration of an autoinflammatory etiology. The rapid resolution of all symptoms, including eyelid swelling, following IL-1 blockade further supported this possibility and led to genetic testing for autoinflammatory diseases. This highlights how atypical features of adult-onset CAPS can delay diagnosis.
The identified NLRP3 variant (NM001243133, c.386A>G, p.Lys129Arg), rs188623199, was classified as a variant of uncertain significance in the ClinVar database (variant ID: 234287). According to the Genome Aggregation Database (gnomAD), its allele frequency (AF) was 0.003%, with a higher AF of 0.1% in the East Asian population. Although several in silico prediction tools classify this variant as likely benign (CADD score: 13.7; REVEL: 0.0480; SpliceAI: 0.00; phyloP: 0.669), it has been reported in patients with NLRP3-associated autoinflammatory diseases, particularly among Chinese and Japanese populations (13–15).
More specifically, the p.Lys129Arg variant was recently reported as potentially pathogenic in a cohort of 16 Chinese patients with adult-onset NLRP3-associated autoinflammatory disease (13). The patient carrying this variant presented with fever, myalgia, and lymphadenopathy, similar to our case, except for the presence of periorbital swelling. Both patients lacked sensorineural hearing loss, a common feature of classic CAPS (16).
Feng et al. analyzed 534 NLRP3 variants, including many previously classified as variants of uncertain significance, using data from INFEVERS and ClinVar (17). Their findings suggest that certain amino acid substitutions may stabilize the active NLRP3 conformation, disrupt its autoinhibited state, and promote oligomerization, contributing to variable inflammasome activation. This may explain the wide range of clinical phenotypes associated with NLRP3 variants.
Our findings support that p.Lys129Arg may be a pathogenic driver and should be considered likely pathogenic, particularly in East Asian populations. This case highlights the disconnect between in silico predictions and clinical outcomes and underscores the importance of interpreting rare variants within the appropriate clinical context.
Adult-onset autoinflammatory diseases are rare and exhibit variable clinical manifestations, influenced by both regulatory mechanisms and the mutation-specific effects of NLRP3 variants (18, 19). This patient initially presented with localized periorbital inflammation, which progressed to systemic disease following her third dose of the COVID-19 vaccine—a known trigger for dysregulated NLRP3 activation (20). NLRP3 variants with reduced penetrance may require an additional inflammatory trigger, or “second hit,” such as a viral infection or vaccination, to precipitate overt clinical disease (21).
Before treatment with an IL-1 inhibitor, plasma levels of IL-1RA and IL-6 were elevated. Treatment with the IL-1 inhibitor anakinra normalized these levels, consistent with previous reports (22). Interestingly, IL-1β and tumor necrosis factor α (TNF-α) levels were not elevated. It remains unclear whether IL-1β production is locally elevated within inflamed tissues. Alternatively, excess IL-1RA may have neutralized circulating IL-1β, rendering it undetectable in serum. Furthermore, the limited sensitivity of ELISA for IL-1β in plasma may also account for the low measured levels.
Notably, monocytes isolated from the patient during active disease exhibited increased production of IL-1β, IL-6, and TNF-α upon stimulation compared with healthy controls. IL-1β is known to potentiate IL-6 and TNF-α production via NF-κB activation, which may have contributed to the heightened inflammatory response (23). Following anakinra treatment, the hyperinflammatory response was normalized. Further studies are needed to confirm whether the observed NLRP3 variant directly enhances inflammasome activity in vivo.
The remarkable response to IL-1β inhibition observed in this patient is characteristic of CAPS. In contrast, her poor response to IL-6 inhibitors and the suboptimal efficacy of corticosteroids is consistent with reports indicating that CAPS is primarily an IL-1β–driven disease (24). This underscores IL-1β as the central pathogenic cytokine, while IL-6 elevation may be secondary. In patients with suspected adult-onset Still’s disease (AOSD), an inadequate response to IL-6 inhibition should prompt further evaluation for inflammasome-mediated disease.
Conclusions
In conclusion, we report a case of adult-onset CAPS in a previously healthy woman, driven by a missense mutation in the NLRP3 gene (c.386A>G, p.Lys129Arg). The patient demonstrated a dramatic response to anakinra, highlighting the importance of genetic analysis in the diagnosis of autoinflammatory diseases. This case underscores the need to consider monogenic autoinflammatory diseases in patients presenting with fevers, skin rashes, and systemic symptoms—particularly when conventional therapies fail.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board (IRB) of Seoul National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing. JK: Writing – review & editing. J-WK: Writing – original draft. SP: Writing – original draft. W-WL: Writing – original draft. JP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by a grant (Grant no: RS-2022-NR070765) from the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (MSIT), Republic of Korea.
Acknowledgments
We thank all the study participants for their support of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. An J, Marwaha A, and Laxer RM. Autoinflammatory diseases: A review. J Rheumatol. (2024) 51:848–61. doi: 10.3899/jrheum.2023-1209
2. Levy R, Gerard L, Kuemmerle-Deschner J, Lachmann HJ, Kone-Paut I, Cantarini L, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. (2015) 74:2043–9. doi: 10.1136/annrheumdis-2013-204991
3. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, and Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. (2004) 20:319–25. doi: 10.1016/S1074-7613(04)00046-9
4. Kuemmerle-Deschner JB. CAPS–pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. (2015) 37:377–85. doi: 10.1007/s00281-015-0491-7
5. Hoffman HM and Simon A. Recurrent febrile syndromes: what a rheumatologist needs to know. Nat Rev Rheumatol. (2009) 5:249–56. doi: 10.1038/nrrheum.2009.40
6. Mensa-Vilaro A, Teresa Bosque M, Magri G, Honda Y, Martinez-Banaclocha H, Casorran-Berges M, et al. Brief report: late-onset cryopyrin-associated periodic syndrome due to myeloid-restricted somatic NLRP3 mosaicism. Arthritis Rheumatol. (2016) 68:3035–41. doi: 10.1002/art.39770
7. Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Soderkvist P, and Sarndahl E. Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum. (2008) 58:888–94. doi: 10.1002/art.23286
8. Kuemmerle-Deschner JB, Verma D, Endres T, Broderick L, de Jesus AA, Hofer F, et al. Clinical and molecular phenotypes of low-penetrance variants of NLRP3: diagnostic and therapeutic challenges. Arthritis Rheumatol. (2017) 69:2233–40. doi: 10.1002/art.40208
9. Chen E, Facio FM, Aradhya KW, Rojahn S, Hatchell KE, Aguilar S, et al. Rates and classification of variants of uncertain significance in hereditary disease genetic testing. JAMA Netw Open. (2023) 6:e2339571. doi: 10.1001/jamanetworkopen.2023.39571
10. Beck DB, Ferrada MA, Sikora KA, Ombrello AK, Collins JC, Pei W, et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med. (2020) 383:2628–38. doi: 10.1056/NEJMoa2026834
11. Lee SJ, Park JK, Lee EY, Joo SH, Jung KC, Lee EB, et al. Certain autoimmune manifestations are associated with distinctive karyotypes and outcomes in patients with myelodysplastic syndrome: A retrospective cohort study. Med (Baltimore). (2016) 95:e3091. doi: 10.1097/MD.0000000000003091
12. Fautrel B, Mitrovic S, De Matteis A, Bindoli S, Anton J, Belot A, et al. EULAR/PReS recommendations for the diagnosis and management of Still’s disease, comprising systemic juvenile idiopathic arthritis and adult-onset Still’s disease. Ann Rheum Dis. (2024) 83:1614–27. doi: 10.1136/ard-2024-225851
13. Wu N, Wu D, Miao J, Zhao M, Wang Y, Yu W, et al. The phenotype and genotype of Chinese adult patients with NLRP3-associated autoinflammatory disease. Clin Rheumatol. (2023) 42:2841–8. doi: 10.1007/s10067-023-06679-5
14. Hua Y, Wu D, Shen M, Yu K, Zhang W, and Zeng X. Phenotypes and genotypes of Chinese adult patients with systemic autoinflammatory diseases. Semin Arthritis Rheumatol. (2019) 49:446–52. doi: 10.1016/j.semarthrit.2019.05.002
15. Nakayama M, Oda H, Nakagawa K, Yasumi T, Kawai T, Izawa K, et al. Accurate clinical genetic testing for autoinflammatory diseases using the next-generation sequencing platform MiSeq. Biochem Biophys Rep. (2017) 9:146–52. doi: 10.1016/j.bbrep.2016.12.002
16. Maksimovic L, Stirnemann J, Caux F, Ravet N, Rouaghe S, Cuisset L, et al. New CIAS1 mutation and anakinra efficacy in overlapping of Muckle-Wells and familial cold autoinflammatory syndromes. Rheumatol (Oxford). (2008) 47:309–10. doi: 10.1093/rheumatology/kem318
17. Feng S, Wierzbowski MC, Hrovat-Schaale K, Dumortier A, Zhang Y, Zyulina M, et al. Mechanisms of NLRP3 activation and inhibition elucidated by functional analysis of disease-associated variants. Nat Immunol. (2025) 26:511–23. doi: 10.1038/s41590-025-02088-9
18. Fidan S, Seferli S, Durak S, Konca Seferoglu C, Okatan IE, Cebi AH, et al. Comparison of clinical and genetic characteristics of familial mediterranean fever patients among adult age groups. Turk J Gastroenterol. (2024) 35:618–24. doi: 10.5152/tjg.2024.23662
19. Silva GD, Mahler JV, da Silva Junior SRP, Mendonca LO, de Sa Barreto Lima PLG, Nobrega PR, et al. Identifying high-risk neurological phenotypes in adult-onset classic monogenic autoinflammatory diseases: when should neurologists consider testing? BMC Neurol. (2024) 24:130. doi: 10.1186/s12883-024-03621-3
20. Amin S, Aktar S, Rahman MM, and Chowdhury MMH. NLRP3 inflammasome activation in COVID-19: an interlink between risk factors and disease severity. Microbes Infect. (2022) 24:104913. doi: 10.1016/j.micinf.2021.104913
21. Cosson C, Riou R, Patoli D, Niu T, Rey A, Groslambert M, et al. Functional diversity of NLRP3 gain-of-function mutants associated with CAPS autoinflammation. J Exp Med. (2024) 221:e20231200. doi: 10.1084/jem.20231200
22. Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. (2009) 206:1029–36. doi: 10.1084/jem.20082481
23. Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis. (2017) 76:1191–8. doi: 10.1136/annrheumdis-2016-210021
24. Kuemmerle-Deschner JB, Gautam R, George AT, Raza S, Lomax KG, and Hur P. Systematic literature review of efficacy/effectiveness and safety of current therapies for the treatment of cryopyrin-associated periodic syndrome, hyperimmunoglobulin D syndrome and tumour necrosis factor receptor-associated periodic syndrome. RMD Open. (2020) 6(2):e001227. doi: 10.1136/rmdopen-2020-001227
Keywords: autoinflammatory diseases, cryopyrin-associated periodic syndrome (CAPS), anakinra, adult onset, mutation, IL-1β
Citation: Cho SI, Kim S, Kim JY, Kim J-W, Pyeon S, Lee W-W and Park JK (2025) Adult onset cryopyrin-associated periodic syndrome due to germline missense mutation in NLRP3 in a previously healthy middle-aged woman. Front. Immunol. 16:1652177. doi: 10.3389/fimmu.2025.1652177
Received: 23 June 2025; Accepted: 24 July 2025;
Published: 25 August 2025.
Edited by:
Emanuele Bizzi, Vita-Salute San Raffaele University, ItalyReviewed by:
Antonio Gidaro, ASST Fatebenefratelli Sacco, ItalyCyrielle Hou, University of Lausanne, Switzerland
Copyright © 2025 Cho, Kim, Kim, Kim, Pyeon, Lee and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Kyun Park, amlua3l1bnBhcmtAc251LmFjLmty; Won-Woo Lee, d29ud29vbGVlQHNudS5hYy5rcg==
†These authors have contributed equally to this work
 Sung Ik Cho
Sung Ik Cho Sheehyun Kim
Sheehyun Kim Ju Yeon Kim3
Ju Yeon Kim3 Won-Woo Lee
Won-Woo Lee Jin Kyun Park
Jin Kyun Park