- Department of Neurology, Peking University First Hospital, Beijing, China
Recently, more and more studies have begun to focus on the role of gut microbiota in neurological diseases, especially immune-mediated disorders including multiple sclerosis (MS). The bidirectional communication between the gut microbiome and the central nervous system (CNS) is known as the gut-brain axis, which includes two key barriers, namely blood-brain barrier (BBB) and the gut barrier, and has become a crucial framework for understanding the pathophysiological mechanisms of various neurological disorders. Gut microbes co-evolved with humans and play important roles in maintaining steady state via various pathways, including immune regulation. An altered gut microbiota, referred to as dysbiosis, not only induces increased intestinal permeability locally, but also promotes systemic immune responses in the CNS. Increased BBB permeability has been considered the core mechanism for MS, and a “leaky” gut has also been reported in MS as well as its animal models. Therefore, the gut-brain axis is increasingly being considered as playing a crucial role in the pathogenesis of MS, with a major focus on specific gut microbiota alterations associated with the disease. Here, we review how the possible dysfunction of the gut-brain axis might impact MS, with particular emphasis on the barrier function.
1 Introduction
In recent years, a growing body of evidence has indicated the significant role of gut microbiota in neurological diseases, particularly multiple sclerosis (MS). The bidirectional communication between the gut microbiome and the central nervous system (CNS) is known as the gut-brain axis, a concept that has become a crucial framework for understanding the pathophysiological mechanisms of various neurological disorders. With the advancement of metagenomics and other omics technologies, the brain-gut axis is increasingly being considered as playing a central role in the pathogenesis of MS. The gut barrier and the blood-brain barrier (BBB), as two key components of the brain-gut axis, are important gateways for communication between the CNS and the gut, ensuring the selective and secure exchange of information and substances. Furthermore, increased BBB permeability has been considered the core mechanism for MS, and a “leaky” gut has also been reported in MS as well as its animal models. Studies have shown that the gut microbiota and its metabolites may play crucial roles in maintaining the integrity of these barriers via various mechanisms. And growing evidence suggests that the interaction between gut microbiota and the host immune system is also a key factor in regulating brain-gut communication. In this review, we describe the complex, multidirectional interactions between the brain and the gut microbiome in MS, mainly focusing on the impact of BBB and intestinal barrier.
2 Epidemiology and pathophysiology of MS
MS is a complex multifactorial disorder of the CNS that affects approximately 2 million individuals globally. It is recognized as the leading cause of neurological disability among young adults (1, 2). Characterized as a chronic neurodegenerative and neuroinflammatory condition, MS involves an aberrant immune response targeting the CNS. It is now understood that the interplay between genetic predispositions as well as environmental factors culminates in the development of demyelinating plaques in MS. Environmental risk factors include Epstein–Barr virus (EBV) infection, vitamin D3 deficiency, low UV radiation exposure, cigarette smoking, obesity, and dietary habits (3, 4). All these environmental factors would have the ability of changing the proportion of different cell subsets, leading to aberrant immune processes in the disease. The Human Leukocyte Antigen (HLA) gene complex, has been identified as a significant genetic risk factor for MS (5, 6). Therefore, among individuals with genetic predispositions, the intricate interactions between environmental triggers can surpass systemic and CNS immune tolerance mechanisms, thereby facilitating the onset of chronic inflammation and the pathogenic processes.
A recently proposed environmental risk factor for MS is the gut microbiome, a complex ecosystem comprising approximately 100 trillion microbes. Notably, the gastrointestinal (GI) tract is also recognized as the largest immune organ within the body, housing a diversity of immune cell types closely related to the gut microbiota (7). Research into autoimmune and inflammatory conditions, particularly MS and its animal model, experimental autoimmune encephalomyelitis (EAE), was among the pioneering studies in the microbiome research. Consequently, the commensal gut microbiota is now acknowledged to play a crucial role in regulating the development, homeostasis, and function of host immune systems and the CNS, particularly in MS (8, 9).
3 Gut microbiota and MS
3.1 Gut microbiota and inflammation
The human gastrointestinal tract is inhabited by a vast array of microorganisms, including viruses, bacteria, and fungi, collectively referred to as the gut microbiota (GM) (10). This microbiome establishes a symbiotic relationship with the host, wherein the host supplies nutrients and habitat necessary for microbial survival and proliferation, while the microbes contribute to the host’s health by facilitating various physiological processes (11, 12). Recent research has highlighted the capability of gut microbiome to engage in bidirectional communication with the CNS (13, 14). Consequently, the concept of the microbiota-gut-brain (MGB) axis is increasingly popular in the fields of neurobiology, medicine, and immunology.
GM significantly influences various physiological functions within the human body, including immunomodulation (15, 16). The interplay between the GM and gut immunity is pivotal in determining the occurrence and propagation of inflammation. When microbial debris and its metabolites translocate to subepithelial sites, the resultant immune response intensifies and disseminates into the systemic circulation (17). The pathogenesis of systemic inflammation associated with GM is multifaceted, and several key factors may facilitate this process, including: (1) intestinal barrier disruption, which is influenced by the equilibrium between gut mucosal immunity and luminal microorganisms (18); (2) gut dysbiosis induced by dietary habits or aging, can modify T cell activity within pro-inflammatory environment (19); (3) metabolites derived from the GM or components of gut, including small molecules and microbial components (17, 18, 20); and (4) epitope spreading and molecular mimicry mechanisms (21). Immune responses to microbial antigens may lead to tissue damage and release of self-antigens; the subsequent presentation of both microbial and self-antigens may result in the autoimmunity through a process known as epitope spreading. Molecular mimicry occurs when microbial molecules resemble host tissues.
3.2 Microbiota and MS
3.2.1 Microbiota in MS
In fact, early evidence suggesting the involvement of the GM in autoimmune diseases can be traced back to studies on EAE. Germ-free (GF) mice, which are bred and maintained in isolators to prevent exposure to and colonization by microbiota, exhibit immunological immaturity. The mice show a marked reduction in proinflammatory Th17 cells and a skewing toward Th2 responses (22). Furthermore, GF mice demonstrate reduced severity of EAE, corresponding with lower levels of proinflammatory cytokines in the intestine and spinal cord, alongside an increase in regulatory T cells (Tregs) (23, 24). Perhaps most importantly, GM from patients with MS (PwMS), when transplanted into GF transgenic mice expressing myelin-reactive T cells, leads to an increased incidence of spontaneous EAE, which underscores the sufficiency of GM changes in MS for driving CNS autoimmunity (25).
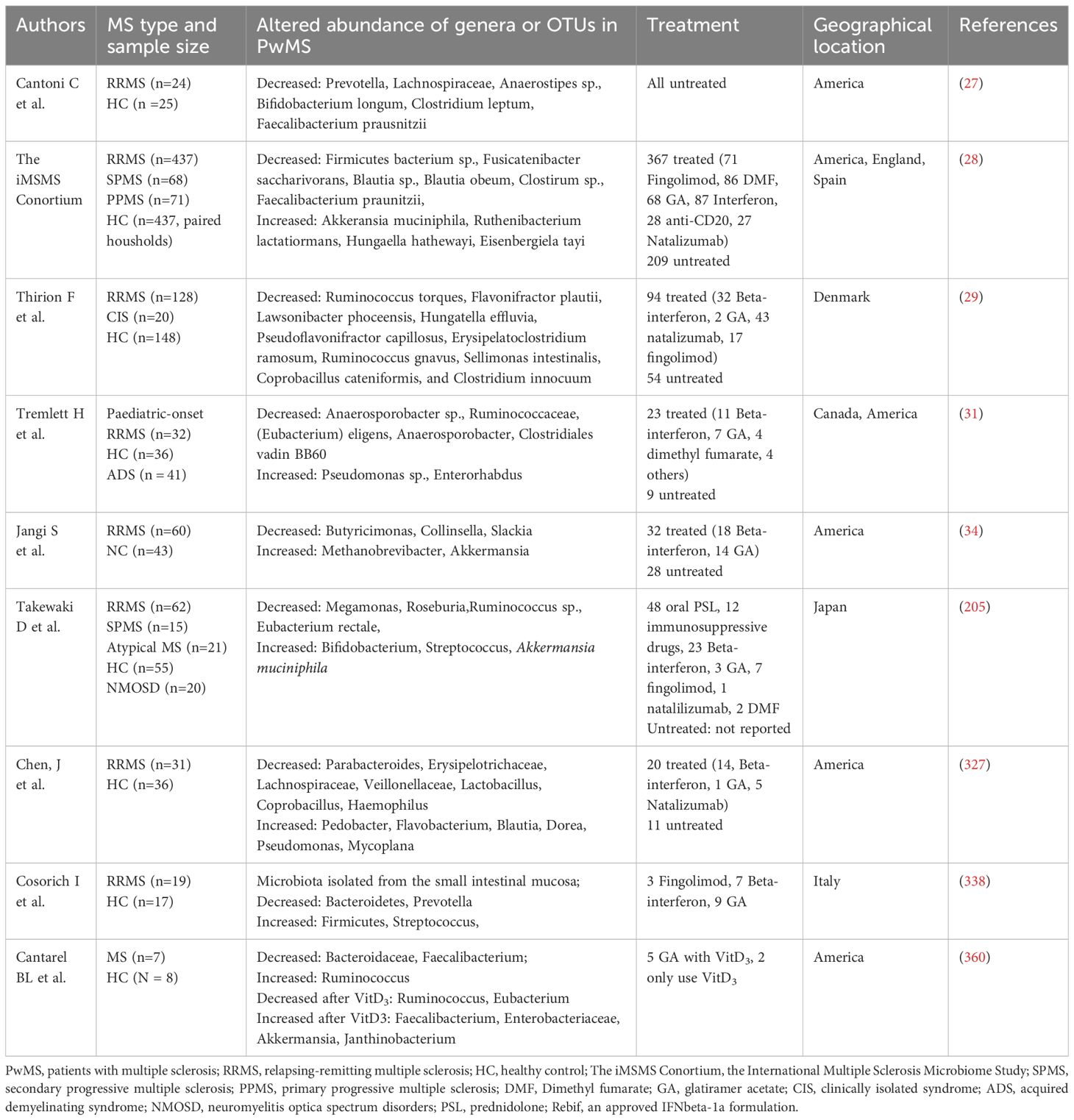
Numerous studies have documented that PwMS experience gut dysbiosis (26–31) as summarized in Table 1. Specifically, PwMS exhibit an enrichment of bacterial genera such as Ruminococcus, Blautia, Dorea, Bifidobacterium, Bilophila, Sutterella, Pedobacteria, Flavobacterium, Pseudomonas, Acinetobacter, Eggerthella, and Akkermansia. Conversely, there is a reduced abundance of genera including Clostridium, Faecalibacterium, Eubacterium, Parabacteroides, Haemophilus, Adlercreutzia, Ruminococcus, Butyricimonas, Bacteroides, Coprobacillus, Lactobacillus, and Prevotella in PwMS. Furthermore, PwMS have been observed to possess a distinct mycobiome (fungus) (29, 32).
Studies also indicate the correlation between gut microbiota species richness and MS phenotypes. Patients who were clinically non-active exhibited an increased abundance of Faecalibacterium prausnitzii, Gordonibacter urolithinfaciens, Anaerostipes hadrus, Gemmiger formicilis, and Roseburia inulinivorans (28). Specific microbial taxa were also found to be linked with a reduced risk of MS relapse, such as Butyricicoccus desmolans, Odoribacter splanchnicus, Lachnospiraceae NK4A136, and Ruminococcaceae, whereas Blautia, Lachnoclostridium, Lachnospiraceae_UCG-004, and Coriobacteriales were associated with an increased risk (33). In both relapsing-remitting MS (RRMS) and progressive MS, Clostridium bolteae, Ruthenibacterium lactatiformans, and Akkermansia was observed, while a decrease in Blautia wexlerae, Dorea formicigenerans, and Erysipelotrichaceae CCM was noted. The administration of disease-modifying therapies (DMTs) also impacts the microbiota composition. For instance, reduced levels of Prevotella and Sutterella have been observed in patients with untreated MS (34).
3.2.2 How microbiota influence MS
The altered gut microbiome observed in MS has fueled intense research interest in elucidating the factors that shape this microbial community and the mechanisms through which these microbes may influence MS pathogenesis. Microbiota dysbiosis can initiate a cascade of events, including the proliferation of pathogenic bacteria and the release of harmful toxins, resulting in a proinflammatory environment and a compromised gut barrier (35, 36). The leaky gut syndrome (LGS) is further characterized by increased intestinal permeability, which facilitates bacterial translocation and the growth and colonization of pathobionts, thereby triggering systemic inflammation. Numerous studies have demonstrated that EAE mice exhibit increased gut permeability (35, 37). Thus, LGS is linked to MS and can play an important role. We will discuss the specific relationship among between LGS, MS, and gut microbiota dysbiosis in Section 3.1.3.
Furthermore, certain bacteria can directly modulate the immune system in MS, influencing the development and behavior of immune cells, such as CD4 T cells, B cells, DCs, and macrophages, which are the main culprits in the pathophysiology of MS (38). MS is predominantly mediated by myelin-specific CD4+ T helper cells, with the Th17 cell lineage being particularly implicated. Th17 cells are known to produce the pro-inflammatory cytokine IL-17 and migrate to the CNS during active disease phase (39, 40). Concurrently, PwMS display dysregulation in CD4+ Tregs, characterized by a marked reduction in their suppressive ability (41, 42). GF mice demonstrate resistance to EAE and a lack of Th17 cells (24). However, mono-colonization with segmented filamentous bacteria (SFB) is sufficient to restore susceptibility to EAE disease and to induce the expansion of Th17 cells in the CNS (24). These findings underscore the necessity of gut bacteria in the EAE pathology. Interestingly, certain bacterial species, such as Akkermansia muciniphila and Acinetobacter calcoaceticus, are also capable of activating intestinal Th17 cells and promoting inflammation in the spinal cords of EAE mice (7, 43–45). These bacteria are found in increased abundance in the small intestine of PwMS, potentially enhancing the pathogenicity of CNS-autoreactive T cells within the intestine. The microbiota can also influence the expansion and maintenance of Tregs (32). Kasper et al. showed that the Bacteroides fragilis can enhance Treg numbers in cervical lymph nodes, which leads to amelioration of EAE (46). Moreover, alterations in gut microbiota composition may indirectly influence the capacity of Tregs to control autoimmunity by inducing of Th1 and Th17 cells or by modulating the T cell microenvironment (47). Besides well-established T cell, recent research has elucidated a gut microbiota-dependent, anti-inflammatory function of B cells in MS. Rojas et al. found a marked reduction in immunoglobulin A (IgA)+ plasma cells within the gut during EAE (48, 49). A subsequent study revealed that IgA+ B cells migrate across the BBB during active MS and exhibit specificity for MS-associated immunostimulatory bacterial strains.
Microorganisms also influence the innate immune response. Toll-like receptors (TLRs), widely distributed on immune cells and nonimmune cells including intestinal epithelial cells, neurons, and glial cells, are pattern recognition receptors (PRRs) capable of detecting exogenous and endogenous pathogenic molecules (50–52). They can be activated by microbe-related antigens like peptidoglycan, lipoteichoic acid, and LPS in the intestine, thereby initiating downstream reactions by recruiting signaling molecules through the myeloid differentiation factor 88 (MyD88) pathway or MyD88-independent signal transduction (53). MyD88 signaling results in the activation of transcription factors including the nuclear factor kB (NF-κB) and activating protein-1 (AP-1), which activate the expression of a variety of genes encoding proinflammatory cytokines and chemokines, as well as molecules important in antigen presentation. This could facilitate the reactivation of myelin-reactive T cells in the target tissue in EAE and MS (54, 55). MyD88 knockout mice are resistant to the development of active EAE, further supporting for a role of MyD88-dependent signaling in disease development (56). The composition of the intestinal flora also impacts TLRs expression, thereby influencing intestinal barrier integrity and immune homeosis (57). Microbiota is also a source of signaling molecules, immune mediators, and gut hormones, which have been shown to be involved in TLR signaling (51, 58, 59).
GMs co-evolved with humans to provide essential enzymes for digesting complex fibers, transforming humans into holobionts dependent on gut bacteria for functions such as vitamin production, nutrient digestion, and immune regulation (60–62). Dysbiosis can result in alterations in the metabolites, contributing to a proinflammatory environment (63). Recent advancements in metabolomics technology have revealed significant changes in microbial metabolites like short-chain fatty acid (SCFAs), tryptophan metabolites, bile acids, and phytoestrogens in MS. Furthermore, various classes of bacterial compounds like LPS, have been shown to penetrate systemic circulation, even reaching the CNS. We will discuss these topics in Section 3.3.
4 Gut microbiota and biological barrier in MS
There are two natural barriers within the BGM axis: the intestinal barrier and the BBB. Gut microbes, stress, and inflammation can alter the permeability of both structures. In this section, we will explore the roles of the two biological barriers in MS and their interactions with gut microbiota. Given the higher susceptibility of women to MS and the recent emphasis on the important role of gut microbiota in the female reproductive system and its mucosal immunity, we also briefly discuss related content.
4.1 Gut microbiota and BBB
BBB breakdown is an early pathological event in MS, occurring before lesion formation and in normal-appearing white matter, alongside pathogenic immune cell infiltration. Thus, limiting proinflammatory immune cells from crossing the BBB into the CNS could be an effective treatment strategy. Research indicates that gut microbiota disorders are related to BBB damage. Here, we summarize BBB components and their dysregulation in the context of gut-induced inflammation in MS.
4.1.1 The composition and function of the BBB and neuron-vascular unit
The BBB refers to the barrier between plasma and brain cells, formed by brain endothelial cells (BECs), the perivascular foot processes of astrocytes, a basement membrane (BM), and pericytes (PCs). In July 2001, the National Institute of Neurological Disorders and Stroke introduced the neurovascular unit (NVU) concept to highlight the dynamic interactions between the BBB, neurons, extracellular matrix, and microglia, which collectively regulate BBB structure and function (64, 65).
BECs exhibit low penetrance to intravascular materials due to a thick luminal glycocalyx layer, specialized tight junction (TJ) structures, lack of fenestration, and selective transporters, which underpin the trans-endothelial permeability and guarantee metabolic and immunological homeostasis for normal brain functions (66). BECs also actively recruit inflammatory cells into the CNS to suppress local inflammation at BBB (67). NVU astrocytes have versatile roles, supporting vascular endothelium, responding to immune stimuli, forming endfeet and the glia limitans, and regulating intracerebral fluid flow. Astrocyte endfeet, together with secreted basal material, form the glia limitans, an immune barrier preventing T cells entry into the parenchyma (68). Connexin 43 in astrocytes helps maintain the BBB, and its loss causes continuous immune cell recruitment (69).Pericytes ensure endothelial integrity, regulating astrocytic endfeet, leukocyte trafficking, and vascular immune homeostasis and vasomotor (69, 70). Pericytes can regulate astrocytic endfeet and BBB endothelium formation. They also limit lymphocyte and monocyte transmigration into the brain (71, 72).
The BM of the BBB is a multilayered extracellular matrix composed of laminin, collagen IV, nidogen, and proteoglycan, formed by the interplay between astrocytes, BECs, and pericytes (66, 73). It allows fluid and soluble molecule passage while blocking leukocyte infiltration and binding growth factors (73). BM laminins affect T lymphocyte extravasation and migration into the brain. The perivascular space (PVS), situated between the BM secreted by BECs and astrocytes, is a key component of the highly organized glymphatic system, which include meningeal lymphatic vessels (MLVs) (69, 74). This system, characterized by astrocyte endfeet expressing polarized aquaporin-4 (AQP4) water channels, shares key functions with the peripheral lymphatic vessels and aids in CSF-interstitial fluid (ISF) exchange, waste removal, and immune cells trafficking (75, 76). The CSF exchanges with ISF through the PVS of the penetrating arteries and is ultimately drained by arachnoid granulations and MLVs, aiding nutrient delivery and metabolic waste clearance within the brain parenchyma (77, 78). Macromolecules in the subarachnoid space are transported to deep cervical lymph nodes (dCLNs) and superficial cervical lymph nodes via MLVs (79). MLVs also work with CNS immune cells, including microglial, to regulate immuno-lymphatic interface and enhance neurotrophic signaling (79, 80). Studies have shown that AQP4 serves as a crucial regulator of fluid dynamics within the brain (81).
Unlike the BECs, the choroid plexus vascular barrier (PVB) is fenestrated, allowing small molecules, water, and solutes to pass, which is crucial for CSF production (82). Animal models have demonstrated that the PVB remains permissive under normal physiological conditions but can close in response to intestinal and systemic inflammation (82, 83).
Oligodendrocytes and microglia are also crucial for BBB integrity, although they do not directly form the BBB. Seo et al. revealed oligodendrocytes can enhance TJs via TGF-β signaling (84). Studies indicate that BBB leakage initially causes astrocyte damage, followed by alterations in oligodendrocytes. Multiple mechanisms, such as imbalances in protein synthesis and degradation (85, 86), the impact of aquaporin-1 and AQP4 (87), and ionic equilibrium (88), have been suggested to explain this phenomenon.
4.1.2 BBB disruption in neuroinflammation and MS
To function as an exquisite machine with highly regulatable dynamics, the BBB or NVU is crucial for brain health. Maintaining brain homeostasis requires the NVU coupling to work synergistically among BECs, pericytes, astrocytes, microglia, neurons as well as cerebral lymphatic system.
BECs, with a thick glycocalyx layer on the luminal surface as mentioned above, block macromolecule leakage and leukocyte adhesion. Hypoxia, inflammation, and TNF-α can disrupt the glycocalyx. Therefore, attenuated glycocalyx coats are involved in the early pathogenesis of neuroinflammation and brain aging. Microglia, central players in neuroinflammation, can transform into a phagocytic state and remodel neuronal connectivity, damaging the BBB by engulfing astrocyte endfeet AQP4 when peripheral inflammation breaches microvessels (89). Activated microglia also stimulate astrocytes to release TNF and glutamate (90), interacting with BECs and neurons to produce chemokines to recruit leukocytes into the CNS (91). Importantly, they also communicate with infiltrating lymphocytes and other immune cells, potentially worsening CNS inflammation (75). The cerebral lymphatic system affects MS progression by influencing immune cell movement, inflammatory responses, and oligodendrocytes function. In acute MS lesions, glial cells retraction and astrocyte damage occur, leading to reduced diffusivity along the PVS, which correlates with increased disability and longer disease duration in MS (92, 93). Impaired lymphatic fluid flow results in the accumulation of inflammatory cells and neurotoxic elements, impairing the clearance of toxic molecules and metabolites from the ventricles and deep gray matter, as well as the clearance of inflammatory microglia, thereby exacerbating cortical demyelination and gray matter pathology (94–96). MLVs facilitate meningeal T cells migration to dCLNs, and their ablation attenuates CD4+ T cell infiltration and spinal cord demyelination, improving EAE prognosis (97, 98). However, MLVs may also exert neuroprotective effects in MS by modulating the function of oligodendrocytes and astrocytes (99).
Chemokines, cytokines, and immune cells, may also influence BBB during systemic or local inflammation. Pro-inflammatory cytokines such as IL-1, TNF-α, and IL-6 have been linked to neuroinflammation in the CNS and peripheral nervous diseases including MS, Parkinson’s disease (PD), Alzheimer’s disease (AD) and diabetic neuropathy (100, 101). They activate signaling pathways like NF-κB and JAK/STAT, leading to neuroinflammation and BBB disruption (102–104). This disruption allows more immune cells and cytokines into the inflammation lesions, worsening inflammation in MS and other conditions (105, 106). Persistent cytokines activity leads to neuronal loss, demyelination, and chronic activation of microglia, astrocytes, and peripheral immune cells. These cytokines initiate and sustain a neuroinflammatory feedback loop, culminating in BBB breakdown, increased oxidative stress, synaptic dysfunction, and neuronal death. The persistence of this inflammatory environment is a significant element in the genesis and progression of neurological disorders, including MS (107–109). Many other cytokines like IL-22, and IFN-γ also damage the BBB by modulating TJs and increasing the expression of transmigratory molecules expression on BECs (110–112). Circulating cytokines also enhance inflammasome activation like NLRP3, which could downregulate TJ proteins and increase BBB permeability (113).
Chemokines are crucial for BBB integrity, lymphocyte chemotaxis, CNS immunosurveillance, and neural regulation (114). Studies have found the chemokines levels, such as CXCL13, CXCL9 and CCL2, are significantly increased in MS (115, 116), potentially activating the p38 mitogen-activated protein kinase (MAPK) pathway and compromising the BBB (117). The NVU controls peripheral leukocytes entry into the CNS, a process that can be disrupted by cytokines. IL-17, in particular, impairs NVU function by attracting circulating neutrophils and downregulating TJs like occludin and ZO-1 (118). Human BECs express low levels of IL-17R normally but increase expression near active MS lesions. It also facilitates CD4+T cell transmigration and enhances the ICAM-1-dependent monocyte adhesion through the BBB (119).
MS has long been seen as a T-cell-mediated disease, especially involving CD4 myelin-reactive T cells including Th1 cells and Th17 cells. Th1 cells primarily secret IFN-γ and TNF-α, which play a crucial role in activating local glial cells and antigen-presenting cells (APCs). Th17 cells can secrete matrix metalloproteinases 3 (MMP-3) and MMP-9, which can degrade the BM and facilitate peripheral leukocyte migration through the BBB (111, 120). Furthermore, Th17 lymphocytes highly express granzyme B, which subsequently kills neurons and recruits more CD4+ lymphocytes (119). Activated Th cells interact with various autoantigens like CNS resident cells, leading to a rapid clonal expansion and an amplified immune response. This ultimately triggers a cascade of inflammatory, demyelinating, and neurodegenerative events, thereby further affecting BBB permeability in MS (121). Recent studies have demonstrated that plasma cells originating from the gut, which secrete IgA, play a role in mitigating neuroinflammation in the CNS through the production of IL-10 (49). In contrast, the accumulation of IgA-producing cells that are reactive to gut bacterial strains associated with MS has been correlated with acute inflammatory episodes in MS (122).
NVU coupling relies heavily on the canonical Wnt/β-catenin, Sonic Hedgehog (SHH), PDGF-β, and TGF-β signaling pathways (123, 124). Additionally, inflammatory mediators, including mitochondrial reactive oxygen species (ROS), can stimulate proinflammatory signaling pathways (Jak-STAT, NF-κB, and NLRs) in BECs, pericytes, and astrocytes, potentially damaging the BBB and interfering with the morphogen signaling (Wnt/β-catenin and SHH) as well as transcriptional program of the BECs, leading to NVU breakage.
The BBB disruption will finally leads to transcytosis, cerebral ion metabolism imbalance, brain perfusion abnormalities, and influx of erythrocytes, cytotoxic iron, as well as fibrinogen, thrombin, and immunoglobulins, which might further drive pathology of MS. The plasminogen cascade activation is associated with MMP activity and BBB disruption in acute MS lesions (125, 126). In progressive MS, postmortem brain tissue shows increased fibrin and fibrinogen deposition in the motor cortex (127). Plasma-derived extracellular vesicles in RRMS are also enriched in fibrinogen (128). Studies reported that fibrin and fibrinogen deposition likely follow BBB breakdown, with blood-derived thrombin mediating further BBB breakdown, eliciting a Ca2+ influx, nitric oxide and ROS production, stress fibers formation, and TJ disruption in MS (129). In the NVU, BECs regulate ion transport through modulating TJ, receptors, and ion channel expression (130). In MS, the BBB disruption might impair selective ion exchange and lead to the neurotoxicity. Iron buildup has been associated with increased ROS, lipid peroxidation, decreased antioxidants, and neurodegeneration in these patients (126). Lastly, modern approaches to BBB disruption in MS also focus on the vascular changes at the NVU, where BBB function and cerebral perfusion are closely interconnected. The NVU harmoniously couples cerebral blood flow with neural activity in different regions of the brain through vascular activity, which has been reported to play a central role in MS pathology (125, 130, 131). Under MS pathology, increased NVU permeability is secondary to BECs dysfunction. Additionally, pericytes contract and undergo apoptosis, leading to capillary constriction and increased BBB damage. Global hypoperfusion in both the white and gray matter is associated with active MS with cognitive dysfunction (132). Therefore, the involvement of the NVU in MS highlights the importance of cerebral hypoperfusion in MS pathology and could represent a potential treatment target.
4.1.3 The impact of gut microbiota on the BBB
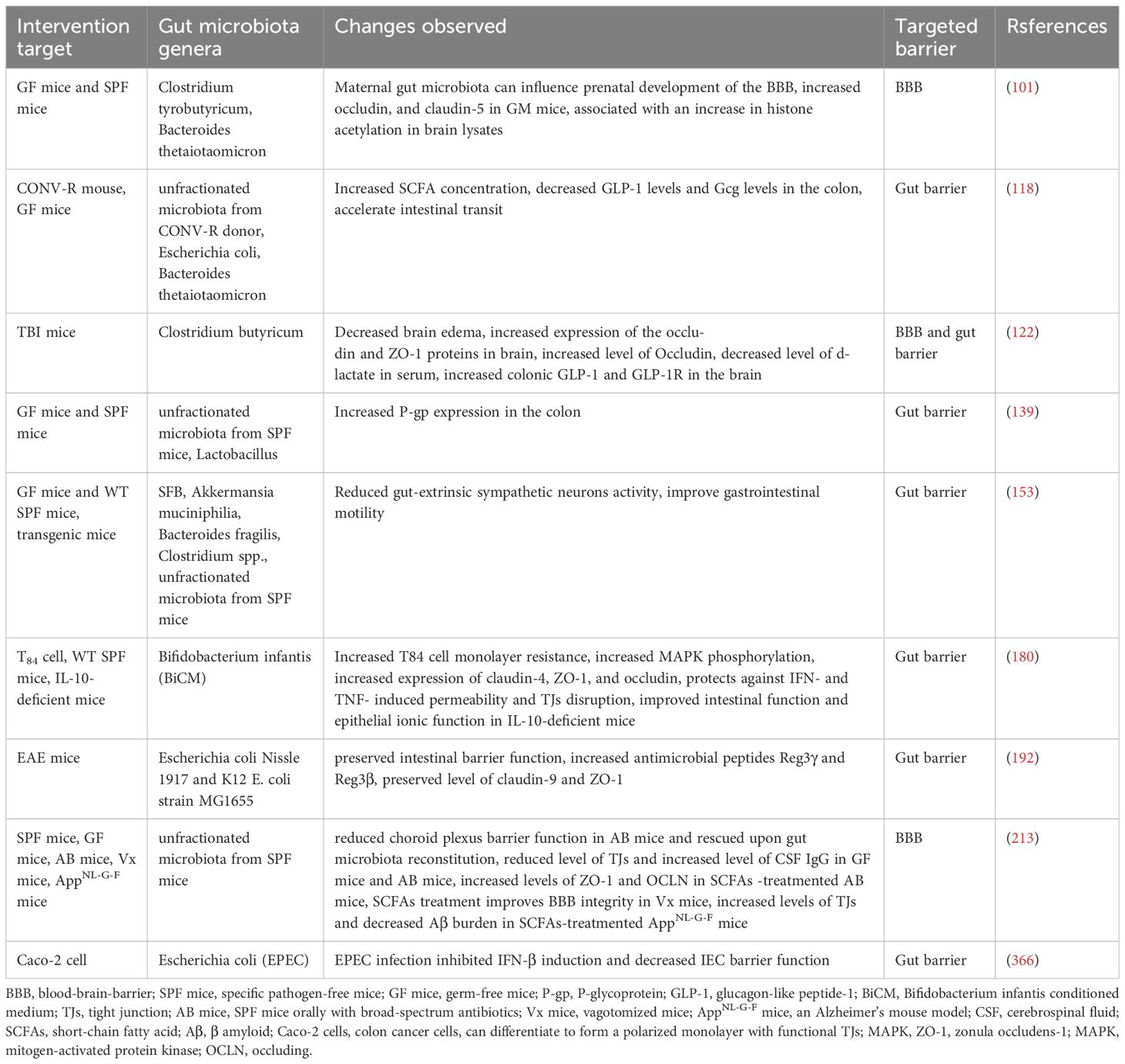
Studies increasingly show that gut microbes significantly impact BBB integrity. In 2014, Braniste et al. found that GF mice exhibited higher BBB permeability in various brain regions compared to pathogen-free (PF) mice, which was linked to reduced occludin and claudin-5 expression. After fecal transplantation from PF mice or administration of SCFA-producing bacteria to GF mice restored BBB integrity by increasing TJ expression. Current research suggests that the gut microbiota regulate the BBB through a variety of pathways, including the vagus and sympathetic nerves (133), the immune system (134), the endocrine systems (135), and microbial metabolites such as SCFAs, microbial structural components such as LPS and peptidoglycans (8), and microbial membrane vesicles (136). We will discuss this part in the following section.
4.1.3.1 Microbial metabolites as signaling molecules
The GM converts dietary components into various metabolites, which play crucial roles in metabolism and signaling functions, affecting host homeostasis, including BBB integrity and brain function. Recognizing the significance of structural components derived from bacterial cell walls, such as LPS and bacterial membrane vesicles, is also crucial due to their effect on host physiology. These components, often called microorganism-associated molecular patterns (MAMPs), which can play crucial roles that extend beyond innate immunity (137). In Section 4, we will discuss in detail the effects of gut microbial metabolites and their components on the biological barrier.
4.1.3.2 Vagus nerve
The vagus nerve is a key channel for the communication between the intestinal microbiota and the brain. GM influence the intestinal neurons and the CNS by modifying the vagus signals to trigger anti-inflammatory reflexes, releasing mediators like acetylcholine (Ach), and interacting with immune cells (133). Vagus nerve stimulation decreases the co-localization of neutrophils and ICAM-1 induced by LPS stimulation, decreasing gene expression of hypothalamic inflammatory mediators and brain inflammatory responses (138). In a rat model of ischemic stroke, non-invasive vagus nerve stimulation was observed to reduce BBB leakage, improve TJ levels, and reduce MMP-2/9 expression, thereby protecting the BBB integrity (139).
Intestinal microbes are also bale to secrete neurotransmitters like Gamma-aminobutyric acid (GABA), 5-HT, catecholamine, and histamine (140). While being transported to the brain via circulation and neural channels, they can also activate the vagal nerve chemoreceptors by paracrine signaling, and ultimately stimulate anti-inflammatory reflexes (141, 142). They also regulate information transmission between periphery and the CNS by controlling BBB function (143, 144).
4.1.3.3 Endocrine pathway
Neuroendocrine hypothalamic-pituitary-adrenal axis. The endocrine pathway allows the transfer of humoral factors to mediate bidirectional activity between the gut microbiota and the brain (145, 146). Changes in the gut microbiota structure lead to the increased intestinal barrier permeability; therefore, LPS crosses the barrier into circulation and activates the HPA axis (147, 148). As a result, mast cells are activated and corticotropin-releasing hormone (CRH) is released, resulting in increased permeability of the BBB. CRH and adrenocorticotropic hormone can also directly activate microglia to release neuroinflammatory mediators and promote the brain inflammation (149).
Enterogenous hormones. GM influence enteroendocrine cells (EECs) and production hormones, such as leptin, ghrelin, and glucagon-like peptide 1 (GLP-1), through local stimulation and production of metabolites (150, 151). GLP-1, secreted by intestinal L cells, can participate in the regulation of a variety of CNS functions including BBB integrity (152). Clostridium butyricum (Cb) boosts butyrate production in the intestinal tract, stimulating the production of gastrointestinal hormones in the colon (153). In traumatic brain injury mice, Cb supplementation reduces inflammatory reactions and intestinal permeability, thereby improving the neurological dysfunction and BBB injury, likely due to increased GLP-1 secretion (154).
4.1.3.4 Immune pathway
Under normal conditions, the GM and host coexist symbiotically. When disrupted, the microorganisms and their metabolites may interact with the host immune system (155, 156). Changes in GM composition increase the intestinal permeability and trigger an immune response. Activated immune cells and the signaling molecules then reach the BBB via the blood circulation, causing systemic inflammation and elevated levels of circulating cytokines that upregulate adhesion molecules, chemokines, and MMPs in the BBB (157, 158), while downregulating TJs to increase the permeability of the BEC layer (159). The compromised BBB permits fibrin entry, which is deposited as insoluble fibrin and activates further immune response (160). Solutes and toxins entering the brain increase inflammation and attract immune cells (157), which stimulate the inflammatory signaling of the NVU (161). Thus, intracerebral inflammation and neurodegeneration are exacerbated via a vicious cycle.
As discussed above, GM can influence the BBB, brain neurons, and the endocrine and immune systems to guard against the CNS pathology associated with ageing and inflammation.
4.2 Gut microbiota and intestinal barrier
The human gastrointestinal tract features physical and biological barriers whose function is not only to isolate the internal host’s milieu from the outside, but also to regulate the immune system, nutrients absorption, and to limit the microorganism access. Hence, the intestinal mucosa operates in a dynamic manner to maintain intestinal integrity and immune homeostasis. Disruption in these barriers is linked not only to digestive system diseases but also to autoimmune disorders outside the gut like MS, in both experimental models and humans. In this section, we examine the multiple lines of evidence linking the intestinal barrier function and MS pathophysiology.
4.2.1 The composition and function of the intestinal barrier
The intestinal barrier consists of mucus layer, epithelial barrier, and gut vascular barrier, behaving as a coordinated and multilayered network that protects host physiology from external insults and regulates several gut functions. Intestinal immune compartments are divided into inductive sites, like mesenteric lymph nodes and gut-associated lymphoid tissue (GALT), where adaptive immune cells are primed and differentiated, and effector sites, such as the intestinal lamina propria and epithelium, where these cells localize to support barrier integrity and immunity (162).
The gut epithelial barrier, made up of columnar epithelial cells (enterocytes) and specialized secretory cells (Paneth and goblet cells), is underpinned by intestinal stem cells in mucosal crypts (163, 164). The mucus layer, secreted by goblet cells, shields gut epithelial cells from harmful substances and provides a habitat for microbiota, facilitating beneficial interactions while preventing pathogen entry (165, 166). Components such as TJs, antimicrobial peptides (AMPs), secretory IgA, and glycosylated proteins contribute to this protective mechanism (167–169). Mucosal surfaces also serve as an immune barrier acting as part of the innate immune response against microbial pathogens (170). Beneath the mucus layer, the gut epithelial lining acts as a semipermeable barrier, maintaining a balance between microbial-host interactions. Enterocytes are connected by junctional complexes that regulate paracellular transport and maintain intestinal permeability (166, 171). The gut barrier also features ATP-binding cassette transporters that prevent toxin accumulation and inflammation, influenced by gut microbiota (172, 173). Enteric glial cells, similar to astrocytes in regulating BBB, can also impact gut epithelial barrier function (130, 174, 175).
The immunological layer of the intestinal barrier, following the mucus and the epithelial lining includes innate lymphoid cells and intraepithelial lymphocytes that protect against pathogens and modulate immune responses (176–178). The GALT consists of multifollicular lymphoid tissues, including Peyer’s patches, isolated lymphoid follicles, the appendix, cecal and colonic patches and rectal lymphoid tissues, with all dependent on the microbiota (179, 180). These tissues house diverse immune cells including CD4+ Th cells, Tregs, CD8+ T cytotoxic cells, DCs, macrophages, and innate lymphoid cells (ILCs) that initiate and propagate immune responses, with the IgA+ Marginal Zone B cells (MBCs) being particularly prominent. IgA+ B cells from GALT are vital for the gut-meningeal immune axis and protect the CNS from gut-derived infections (179, 181). GALT that drains mucosal surfaces will constantly encounters foreign structures from commensal microbiota, infectious pathogens and antigens, which also establish tolerance to autoantigens with the changes in autoreactive T cells phenotypes, including peptides from the CNS (182). The gut vascular barrier, with fenestrated endothelium and TJs, prevents microbial entry into circulation and controls the access of dietary compounds (183). This barrier is crucial for gut-brain axis communication, with disruptions linked to the closure of PVB in mice (83). This finding suggests a functional linkage between barriers along the BGM axis, potentially underlying the frequent comorbidity of neurological and gastrointestinal symptoms (184).
The enteric nervous system (ENS), an autonomous division of the autonomic nervous system (ANS), autonomously regulates gastrointestinal functions through its submucosal and myenteric plexuses (185). It contains neurons, glia, and immune cells, forming intrinsic circuits for gastrointestinal motility, secretion, immunity, and tissue repair (186). ENS neurons can release many neurotransmitters and are closely related to vagus efferent input, forming intrinsic sensorimotor circuits (187, 188). Enteric glial cells interact with neurons, EECs, immune cells and epithelial cells, thereby modulating barrier function (175, 189, 190). Studies of human Peyer’s patches also reveal peptidergic innervation including Substance P, Vasoactive Intestinal Peptide (VIP) and Calcitonin gene-related peptide (CGRP) immunoreactivity in cells within the GALT (191).Microbiota and enterochromaffin cell (ECC)-derived 5-HT further influence glial homeostasis. Interstitial cells, including interstitial cells of Cajal (ICCs) and platelet-derived growth factor receptor alpha-positive (PDGFRα+) cells, can facilitate gut motility via electrical coupling with smooth muscle (192, 193). Macrophages, the most abundant immune cells in the GI tract, can modulate barrier homeostasis, with their activation influencing ENS integrity and being linked to microbiota dysbiosis (194–197).
4.2.2 Intestinal barrier homeostasis, the microbiome and neuroinflammation
The intricate interplay between gut microorganisms and the immune system is regulated at the gut barriers through multifaceted mechanisms. Intestinal microorganisms and their metabolites impact both immune system and intestinal epithelial barriers (IEBs), while intestinal layers reciprocally shape microbial composition and immune activity. This microbiome-mediated barrier homeostasis is pivotal in regulating of neuroinflammation.
The mucus layer critically governs gut microbial community and immune interactions. Adhesion to host epithelial cells and mucus is a key property for gut bacteria colonization, which can be modulated by host-specific mucin glycosylation (198–200). Dysregulated mucin glycosylation is correlated with increased inflammation and microbial translocation by regulating mucin degradation (201–203). The transmembrane mucins also enhance the intestinal immune functions (204). The IEB impairment is a crucial mechanism for several inflammatory and immune-mediated disorders (205). As result of gut barrier imbalances, some microorganisms, bacterial products, and toxins may translocate across the epithelium uncontrollably, leading to both the local and systemic inflammation (34, 206–209). Paracellular translocation, often linked to the impairment of TJs, has been associated with direct damage to enterocytes and their supporting structures, along with significant changes in intestinal TJs gene expression and downregulation of both ZO-1 and occludin (210, 211).
The interplay between intestinal epithelial cells (IECs) and mucosal immune components, such as intraepithelial lymphocytes and lamina propria immune cells, sustains intestinal immune homeostasis. IECs detect antigens, secrete antimicrobials, and modulate immune responses, while immune cells regulate IEC-derived cytokines (212, 213). Interactions between DCs and IECs maintain anti-inflammatory environments under steady-state conditions (214). Commensals reinforce IEB integrity via TJ regulation and IECs proliferation (215). IECs-associated inflammasomes like NLRP6 are critical for mucosal homeostasis and infection defense (216). Inflammasome-deficient mice exhibit microbiota dysbiosis, amplifying inflammatory responses and IBD susceptibility (217, 218). NLRP6 deficiency also disrupts goblet cell mucus secretion and the production of epithelial IL-18 and AMPs, impairing bacterial control (219, 220), and leading to AMPs imbalance, dysbiosis, and autoimmunity.
Additionally, as previously mentioned, gut microbiota can regulate the expression and phenotype of inflammatory cells both locally and at distant sites. Studies indicate that the microbiota in the GALT remotely influences T cell development in the thymus via soluble factors (221). In the EAE model, MOG-specific T cells proliferate substantially in the GALT under SPF conditions, but less so in germ-free environments, suggesting microbiota-induced T cells stimulation (221). Recent findings emphasize the crucial role of IgA antibody-secreting cells (ASCs) in the CNS, acting as a “brain firewall” to protect the BBB and maintain intestinal homeostasis (222). In both mice and humans, meninges contain gut-derived, commensal-specific IgA ASCs, which help prevent pathogens from entering the CNS (223). Gut bacteria stimulate secretory IgA production, which compartmentalizes commensal bacteria away from the host epithelium and modulates chemotaxis and TLRs signaling (224, 225). In mice, non-invasive bacteria residing gut are coated with IgA, promoting the production of diverse, species-specific IgA in mice (226). In an adaptation to this specific microenvironment, intestinal plasma cells (PCs) might have a distinct metabolic profile. IgA ASCs can utilize diet- and gut microbiota-derived SCFAs as one carbon source to maintain metabolism (227). Inflammatory responses induced by environmental factors or intestinal dysbiosis might dramatically change oxygenation and the metabolic profile of the PC niches in the gut.
Recently identified ILCs are key regulators of intestinal immune responses and have also been implicated in CNS autoimmunity. Among ILCs, ILC3s are notable for their similarities to Th17 cells, which are crucial in CNS inflammation and can be modulated by many cues from the gut microbiota (228, 229). ILC3s are critical for the generation of the organized lymphoid tissue in the intestinal wall and regulating microbiota content and the integrity of the intestinal barrier (230, 231). Found in different GALT compartments, ILC3 interact with immune cells including Th1 cells, Th17 cells, and Tregs, efficiently controlling effector T cells and promoting a Treg balance (232–234). ILC3s produce IL-17 to attract neutrophils to the intestine during bacterial and fungal infections (235, 236), which can also induce AMPs and TJs production (237). They are also a key source of IL-22, crucial for maintaining the intestinal barrier (238). IL-22 production is stimulated by a glial-derived neurotrophic factor from enteric glial cells in response to TLR ligands (239), and is also enhanced by SCFAs that act through AhR and FFAR, respectively (240–243).
An altered microbiome also affects bacteria-associated products that influence neuroimmune responses. Besides microbial metabolites, structural components derived from bacterial cell walls and membrane vesicles also significantly impact host physiology and gut permeability, see Section 3.3.
Overall, barrier permeability is dynamic and must be carefully orchestrated and constantly adapted to maintain homeostasis, with gut microorganisms playing a major part in achieving this goal.
4.2.3 The intestinal barrier in MS: consequences of a leaky gut
The topic of intestinal permeability (IP) in neuroinflammation is actively being studied, with several lines of investigation exploring the plausible relationships between gut barrier disruption and MS, as well as on translational implications based on IP.
In a study of 12 jejunal biopsies from MS patients, Lange and Shiner observed subtle histological changes, including villous atrophy and intestinal inflammatory cell infiltration (244). The latest study used the lactulose/mannitol test to evaluate intestinal permeability in MS patients and found that 73% of cases presented with abnormal permeability (245). Elevated serum zonulin levels in both RRMS and SPMS further confirm diminished intestinal barrier function in MS, as zonulin can rapidly increase both intestinal and BBB permeability in vitro. Similar findings were also described in the EAE model, with increased intestinal permeability, reduced submucosal thickness, and altered TJ expression in IECs, which have been associated with a mucosal imbalance between Th1/Th17 and Treg cell subsets in intestinal lamina propria, Peyer’s patches, and mesenteric lymph nodes (41, 246). They also found that treatment with probiotic Escherichia coli strain Nissle 1917 preserved TJs and decreased intestinal permeability, leading to reduced EAE severity and decreased pro-inflammatory cytokines (41).
The above studies indicate that PwMS indeed experience an alteration in the intestinal barrier due to an altered intestinal immune response and microbial dysbiosis (247). The leaky gut may be involved in the pathophysiological process of MS via the following mechanisms. Firstly, intestinal barrier dysfunction has been associated with susceptibility to systemic infections, which are common complications in MS patients (247, 248). Furthermore, the interaction between intestinal barrier and commensal microbiota could modulate the immune response pathologically, shaping the development of immune cells such as CD4+ T cells, B cells, DCs and macrophages. Additionally, changes in intestinal permeability could exacerbate neuroimmune dysregulation by allowing transmucosal passage of injurious or immunogenic antigens. Interestingly, recent work suggests a connection between the IP changes (IPC) and MS risk factors. For example, Vitamin D deficiency may reduce intestinal calcium absorption, causing gut stasis and subsequent IPC, which would allow gut microbiota to transfer more endotoxins into the blood and trigger inflammatory cytokines production within the CNS (249).
Alterations in the gut homeostasis in MS could increase translocation of bacterial and their toxic products through an impaired intestinal barrier. A recent study found higher plasma levels of endotoxin LPS in MS, linked to in vivo IL-6 production and in vitro Th17-like responses (195). In another study, investigators also found increased LPS-binding protein levels in the serum of MS patients (196). Besides LPS, MAMPs such as bacterial lipoproteins and double-stranded RNA can enter the bloodstream and modulate the immune system through TLRs, which are present in microglia and to modulate the initiation and severity of EAE models (197). Dysbiosis may alter gut bacteria metabolites, reducing health-promoting ones like SCFAs and dietary tryptophan, which may further contribute to increased gut barrier permeability and pro-inflammatory setting. Additionally, microbiota dysbiosis disrupts IgA synthesis and AMPs production, which act as anti-inflammatory mediators beyond the gut.
Increased intestinal permeability, alterations in TJs functioning, and modifications in intestinal morphology occurred along with the changes in the immune cells including T cells, IgA ASCs and ILCs, as well as gut microbiota dysbiosis in GALT, thus indicating that disruption of intestinal homeostasis was dependent on the immune response at the initiation of EAE. Thus, the combination of LPS- and MAMPs-induced inflammation, leaky gut, metabolic imbalance, and immune activation creates a perfect storm for dysregulated immune activation that can fuel chronic disease in MS.
4.3 Sex and microbiota-gut-reproductive tract axis in MS
Autoimmune diseases, including MS, are more common in females, who also exhibit stronger immune responses and higher relapse rates than males with RRMS, while males face a greater risk of long-term disability progression (250–252). Mechanisms involved may include gene-environment interactions or epigenetic factors. Additionally, sex chromosome as well as sex hormone effects on peripheral and the CNS autoimmunity and neurodegeneration have been shown in MS preclinical models (253, 254).
Studies have found a relationship between microbiota and sex hormones (255). The gut microbiome influences sex hormone levels through its metabolites, the immune system, chronic inflammation, and neuroendocrine axes, including the gut-brain axis. The microbiome can metabolize estrogens via β-glucuronidase, allowing estrogen to enter the bloodstream and act on its receptors, impacting reproductive health, cardiovascular risk, metabolism, bone health, and the CNS (256). GM can also impact the function of the hypothalamic-pituitary-gonadal (HPG) axis by modulating key reproductive hormones (257). Microbiota and their metabolites, like SCFA and LPS, can impact female health by colonizing the vaginal tract. SCFAs link reproductive hormone regulation with gut microbial activity via metabolic and immune mechanisms, reducing inflammation and modulating gonadotrophin-releasing hormone (GnRH) secretion. SCFAs can suppress NF-κB activity, regulate cytokine profiles, and promote regulatory Treg activity (258), which helps establish immune tolerance at the maternal–fetal interface. Gut microorganisms influence neurotransmitter production, such as serotonin and GABA, adding a layer of neuroendocrine control over fertility by affecting GnRH pulsatility and hypothalamic communication (16, 17). This links gut health to reproductive hormone regulation. Changes in cytokine levels, like IL-6 and TNF-α, can impact endometrial receptivity and ovulation, further connecting microbial balance to reproductive outcomes (259). Sex hormones and stress affect gut motility, sensitivity, and microbiota by interacting with brain-gut axis receptors in a reciprocal manner (260). This interplay leads to the concept of microbiota-gut-reproductive tract axis (261).
The gut microbiota plays a crucial role in regulating extra-intestinal mucosal and barrier homeostasis. Key bacteria, such as Bifidobacterium, Lactobacillus, and others, are common in both the gut and vaginal tract (262). The gut microbiota also influences reproductive health by maintaining intestinal barrier integrity, which, if compromised, can lead to chronic low-grade inflammation and disrupt critical reproductive processes (257). In the reproductive system, especially the vaginal tract, microbiota protect against harmful bacteria by strengthening the mucosal barrier and producing antimicrobial substances (263). Cervical mucus acts as a barrier by trapping pathogens and enabling immune responses (264). Vaginal dysbiosis bacteria can disrupt the epithelial barrier through oxidative stress and miRNA changes, leading to cell cycle arrest, apoptosis, and necrosis, while also secreting harmful metabolites that cause immune disorders and contain factors such as IgG, IgA, and lactoferrin (265). Gut dysbiosis can trigger abnormal systemic and mucosal immune responses, increasing pro-inflammatory cytokines and cytokines and impairing embryo implantation and placental development, which is linked to infertility and repeated implantation failure (266).
Sex hormones play a role in the peripheral and central immune regulation of MS. Gut microbiota regulate the appropriate effects of sex hormones through multiple mechanisms, including metabolism, chronic inflammation, and neuroendocrine functions. However, the dysregulation of gut microbiota in MS may affect this process. Additionally, microbiota are involved in maintaining local and systemic barrier homeostasis and inflammatory processes, which play an important role in maintaining reproductive health.
4.4 The impact of metabolites and structural components of microbiota on biological barriers
As detailed above, compounds produced by gut microbes act locally on immune, epithelial, and EECs to affect barrier integrity, systemic immune responses, and hormone secretion (8). There are obviously many thousands of different microbiota-derived molecules that could potentially circulate to reach and penetrate the CNS. The effects of structural components derived from bacterial cell walls and of bacterial membrane vesicles on host physiology are also important extend beyond innate immunity, frequently termed MAMPs as stated above. We summarize the content covered in this article in Table 2 and further discuss it in the following chapters.
4.4.1 Diet-related metabolites and microbiota in MS
Produced by microbiota fermenting dietary fiber and resistant starch in the intestines, SCFAs (acetate, propionate, and butyrate) provide energy for both the host and the gut microbiota, and can enter host circulation and cross the BBB, enabling a role in maintaining barrier integrity (60, 267–269). Lower levels of SCFAs have been observed in MS patients (270–273). Furthermore, diminished SCFAs have been correlated with increased intestinal permeability and worsening EDSS in MS (270, 271, 273). Moreover, known SCFAs-producing gut microbiota are reduced in MS, including Butyricimonas, Bacteroides, Lachnospira, and Eubacterium (272, 273). GF mice, naturally lacking SCFAs, show a compromised BBB, while introducing butyrate or butyrate-producing bacteria like Clostridium tyrobutyricum can improve BBB dysfunction in these mice (141). The mechanism by which SCFAs influence barrier function is not fully understood. SCFAs bind G protein-coupled receptors (GPCRs) (141) and the free fatty acid receptors (FFAR2 or FFAR3) on intestinal epithelial cells and brain ECs, protecting the barrier from oxidative stress (274–278). Knox et al. also found that butyrate and propionate promote remodeling of actin cytoskeleton and TJs in an BBB model (279). In GF mice, SCFAs can improve barrier function and TJs expression at the choroid plexus in antibiotic-treated mice (280). SCFAs are also known to support mitochondrial function (281, 282), as they protect against mitochondrial disruption in brain endothelial cell treated with LPS (279). For intestinal homeostasis, SCFAs can mediate sodium transport, energize intestinal epithelial cells, and influence gene transcription that supports colon homeostasis by inhibiting histone deacetylase activity (283–285). SCFAs also reduce T cell proliferation and cytokine production in the gut, partly by inhibiting the activation of NF-κB pathway in immune cells and intestinal epithelial cells (286–288).
Bile acids (BAs), derived from cholesterol metabolites in the liver and modified in the gall bladder, become primary bile acids conjugated with glycine or taurine. Primary Bas, cholic acid and chenodeoxycholic acid (CDCA), can be further metabolized by gut microorganisms into secondary bile acids (2BAs), such as Deoxycholic acid (DCA), chenodeoxycholic acid (CDCA) and lithocholic acid (LCA) (289), which can enter systemic circulation and affect the CNS (290, 291). DCA and CDCA have been shown to have disruptive effects on the gut barrier (292, 293), whereas LCA seems to have a protective role (294). CDCA and DCA have also shown disruptive effects on the BBB in animal models, which may suggest common mechanisms of disruption across barriers (295). BAs can interact with many receptors such as Farnesoid X receptor (FXR), the VDR, PXR and Takeda G protein-coupled receptor 5 (TGR5), to exert various functions (296–298). Without these receptors, the intestinal barrier weakens, allowing the translocation of bacteria (299). Moreover, FXR modulates gut immune responses driven by microbes during inflammation, potentially linking them to BA metabolism dysregulation (300). Gut microbes can activate TGR5, affecting the expression of EECs involved in immune regulation (301). This, in turn, directly influences macrophage polarization and the subsequent inflammatory response. Once TGR5 is activated, BAs may suppress the production of inflammatory cytokines such as IL-1, IL-6, and TNF-α (302).
Tryptophan is acquired through digestion of dietary protein in the small intestine (303–305). This essential amino acid is crucial for protein synthesis and the production of serotonin (5-HT) and kynurenine (155, 306, 307). Studies have noted reduced levels of tryptophan and its metabolites in PwMS, also correlating with EDSS scores (308–310). Dietary tryptophan restriction in EAE models can abolish BBB disruption, leukocyte infiltration, and CNS demyelination, likely by inhibiting Th1/Th17 skewing and impairing migratory capacity (311). This effect is partially lost in GF mice, suggesting a microbiota-dependent mechanism. However, tryptophan and its metabolites can also exert protective effects, which are partially mediated by binding to the aryl hydrocarbon receptor (AhR). AhR regulates astrocyte and microglial crosstalk in the CNS, which controls inflammation and neurodegeneration (312, 313). Furthermore, tryptamine-mediated EAE suppression relies on AhR and modifies the gut microbiome composition to increase butyrate-producing microbiota (310).
Microbial fermentation can also produce compounds like methylamines, indoleacetate, phenylacetate, and phenolic compounds (314), as well as branched-chain amino acids (BCAAs) such as 2-methylbutyrate, isovalerate, and isobutyrate (314). BCAAs may play a role in autism spectrum disorder pathophysiology and barrier modulation (315). Gut microbes convert dietary methylamines dylcholine into trimethylamine (TMA), which is subsequently rapidly converted into TMA N-oxide (TMAO) in the liver and circulates systemically (314). TMAO can enhance BBB function through annexin A1 signaling (307, 311). Bacterial fermentation of dietary tyrosine and phenylalanine into p-cresol (314), whose metabolite, p-cresol glucuronide, protect human BECs line hCMEC/D3 upon LPS challenge (316). Kynurenine has been shown to protect barrier function in a colitis mouse model (317) and, along with tryptophan, crosses the BBB via the amino acid transporter SLC7A5 or L-type amino acid transporter 1, affecting neurotransmitter production (307).
In summary, the gut microbiome significantly influences how diet-related metabolites affect health and disease. A better understanding of how these diet- related metabolites alter the composition and function of gut bacteria could pave the way for improved treatments for PwMS.
4.4.2 Microbial structural components and microbial membrane vesicles in MS
Recognizing the significance of structural components derived from bacterial cell walls and bacterial membrane vesicles is also crucial due to their effects on host physiology. Microbial structures, such as LPS and bacterial membrane vesicles, have previously been discussed as regulators of gut barrier function as well as BBB through various signals at the micro-gut-brain axis (193, 289, 318).
LPS, a component of Gram-negative bacteria cell wall, is recognized for its association with compromised gut barrier function and activation of immune system (194, 279, 280). Gut microbiota disorders can increase LPS release, leading to higher intestinal permeability and activation of gastrointestinal immune cells to release inflammatory cytokines (319, 320). In MS, elevated levels of LPS have been detected in the bloodstream (321). The same study also reported increased levels of LPS in the brain, spinal cord, and blood of EAE model (321). LPS activates TLR4 on microglia, leading to the release of inflammatory cytokines and chemokines (322), and promotes neuronal apoptosis and endothelial cells damage (323, 324). And Singh et al. showed that LPS also interacted with lipoteichoic acid on the cell wall of G+ bacteria, reduced mRNA levels of ZO-1, occludin, and JAMs, while increasing levels of TNF-α and IL-1β at the border of NVU. Additionally, LPS also affects adhesion proteins, membrane transporters, the basal lamina, and the extracellular matrix in the BBB (324). Therefore, LPS affects the integrity of BBB and NVU through a variety of mechanisms, providing a potential target for the treatment of related diseases.
Peptidoglycans, found in the cell walls of G+ and, to a lesser degree, G- bacteria, play key roles in host physiology. Bacterial membrane vesicles are lipid bilayer capsules released from the outer membranes of both Gram-negative and Gram-positive bacteria. They can transport and protect various cargoes, including proteins, DNA, RNA, metabolites, enzymes, peptidoglycans, polysaccharides, and toxins (325, 326). Gut microbial membrane vesicles can traverse the intestinal barrier, enter the bloodstream, and cross the BBB, constituting a key component of the BGM axis (200, 327). Notably, these vesicles influence gut barrier function by modulating mucosal innate immune cells such as macrophages and DCs (328). Thus, LPS and other MAMPs could constitute another pathway through which compromised barrier function impacts neuroimmune responses in MS.
4.5 The synergistic effect of other risk factors with microbiota dysbiosis in MS
Recent studies highlight the crucial role of the immune system’s interaction with gut microbiota as a link through which environmental factors such as Vitamin D deficiency, EBV, smoking, and obesity impact MS (83).These factors commonly disrupt immune regulation and gut microbiota, promoting MS development. This underscores the need to view MS through a comprehensive lens that considers both individual risk factors and its underlying pathogenic processes.
EBV infects over 90% of the global population and is linked to a 2–3 fold higher risk of MS after infectious mononucleosis (IM) (329, 330). MS patients show elevated EBV-specific immune responses correlate with disease activity (331–333). EBV interacts with the main genetic risk factor for MS, HLA-DRB1*1501, leading to higher Epstein-Barr Nuclear Antigen 1 (EBNA1)-specific antibody levels in carriers (334). Molecular mimicry is a key mechanism in EBV-MS immune response, with EBV proteins BamHI Rightward Reading Frame 2 (BRRF2), BamHI Fragment Rightward Open Reading Frame 3 (BFRF3), and EBNA1 exhibiting cross-reactivity with CNS autoantigens like myelin basic protein (MBP) and glial cell adhesion protein (GlialCAM) (335–337). This cross-reactive contributes to the formation of oligoclonal bands, produced by clonal B cell-derived plasma cells in the CNS (335, 338). Some findings locate this B cell response for the cross-reactive within the GALT. EBV infection induces the expression of the integrins α4β7 and CX3CR on memory B cells, which subsequently migrate to GALT, interact with the microbiota, and engage with CD4+ T cells (339). CXCR3+EBV-infected memory B cells may reactive viral antigen specific and autoimmune T cell responses in intestinal and CNS lymphoid tissues including meninges and brain parenchyma during MS, potentially stimulating CD8+ T cells and contributing to CNS inflammation (340–342). In gut lymphoid tissues, microbiota composition influences autoimmune T cell and B cell stimulation through cross-reactivity with bacteria, EBV and autoantigens (343). EBV infection also generates a large pool of antigen-presenting B cells, with latent EBV infection transforming B cells into potent antigen-presenters and inducing mutations in B cell receptors (BCRs) and co-stimulatory molecules, facilitating antigen uptake and presentation to CNS specific CD4+ T cells (344–346). EBV can infect human intestinal epithelial cells via cell contact, establishing latent infections (347, 348). This triggers immune responses that activate inflammatory pathways like NF-ĸB pathways, potential damaging the normal intestinal immune environment (349, 350). EBV latency type I genes, such as EBNA1 and LMP2A, downregulate the miR-200 family and reduce E-cadherin expression, compromising epithelial tissues integrity (351). In intestinal inflammatory diseases and gastrointestinal tumors, microbiota, especially H. pylori and its interaction with the EBV are significant. EBV latent proteins and the H.pylori Cytotoxin-Associated Gene A (CagA) synergistically enhance inflammatory signaling and oncogenic pathways, such as NF-kB and MAPKs, potentially causing gastric epithelium transformation and increased pro-inflammatory cytokines (352, 353). There has been reported that H. pylori infection is more frequent in MS, with recent data indicating its immunomodulatory proteins in MS experimental model (354, 355), suggesting a possible role of H. pylori in the disease. Colonization by H. pylori and/or EBV is linked with extra-gastric diseases and neuroinflammatory pathways, potentially affecting the gut–brain axis and leading to neurological disorders (356). Lastly, studies described the effect of virus infection, such as HIV and SARS-CoV-2, can alter the composition of the gut microbiome and metabolites (357–360). Therefore, EBV may be involved in the brain-gut-microbiota axis communication in MS through various mechanisms, including affecting gut microbiota composition and metabolites, inducing an inflammatory microenvironment in the gut, damaging the intestinal barrier and influencing the phenotypes of immune cells in the gut and CNS by cooperating and antagonizing with the bacteria.
Low VitD levels, along with insufficient ultraviolet B (UVB) exposure, increase the risk of MS. VitD acts as a steroid hormone, crucial for calcium and phosphate metabolism, immune balance, and brain function (361). Different studies demonstrated a decrease of around 41% in MS risk with increased serum Vit D level (362). VitD receptor elements (VDREs), regulated by VitD, are present in more than 80% of MS-associated genes (363). Moreover, VDR and CYP27B1, are found in the neurons and astrocytes, suggesting these cells might be involved in Vit D regulation (364). VitD also regulates immune cell epigenetics, promoting immunological tolerance in T cells, and reducing the inflammatory response, both of which contribute to MS pathogenesis (361). It also helps protect against CNS inflammation by regulating microglial and astrocytic activation and maintaining BBB integrity by reducing endothelial cell apoptosis and inhibiting TJ loss (365–367). Interestingly, reduced serum levels of EBNA-1 antibodies have been reported in vitamin D-supplemented MS patients (368, 369). VitD and its receptor help maintain intestinal balance by boosting bacterial diversity, reducing inflammation, and improving barrier function (370). Vitamin D3 can positively influence microbiota, fostering the growth of microorganisms that produce anti-inflammatory compounds beneficial to overall health (371). Vitamin D3 administration in MS increased the prevalence of the mucosal-integrity-promoting species such as Akkermansia, together with Fecalibacterium and Coprococcus (372). Studies conducted on mice found that the number of Bacteroidetes was higher in groups with VDR gene deletions or those on a low VitD diet (373). Meanwhile, microbiota-derived metabolites may modulate immune cell activity, enhancing vitamin D-mediated anti-inflammatory effects (371, 374, 375). GF mice exhibited hypocalcemia and decreased levels of 1,25-dihydroxyvitamin D and 24,25-dihydroxyvitamin D, in contrast with conventional mice, which showed elevated levels of FGF-23, an essential regulator of VitD metabolism (144). The gut microbiota may hinder the vitamin’s activity through secondary bile acids, particularly lithocholic acid, which interferes with vitamin D binding to and stimulating the VDR. Metabolic byproducts of bacteria, particularly SCFA-like butyrate, enhance intestinal expression of VDR by mitigating inflammation (376). The connection between the immune system and microbiome is clear, with vitamin D as a crucial intermediary. The interactions between vitamin D, the gut microbiota, and the immune system may also be among the determining mechanisms in the pathogenesis of MS.
5 Microbiota-brain-gut axis
The BGM system describes the complex, bidirectional interactions between the brain, the gut connectome, the gut-associated immune system, and the gut microbiome (377). This system involves intricate signaling pathways, including neuronal (378), hormonal (379), immune (380), and microbial factors (381) to maintain homeostasis and influence various physiological processes. Alterations in these interactions are implicated involved not only in the classic functional gastrointestinal disorders, but also in a growing list of psychiatric and neurologic pathologies including MS (382–386). Here, we discuss the mechanisms of BGM axis and its role in MS, as shown in Figure 1.
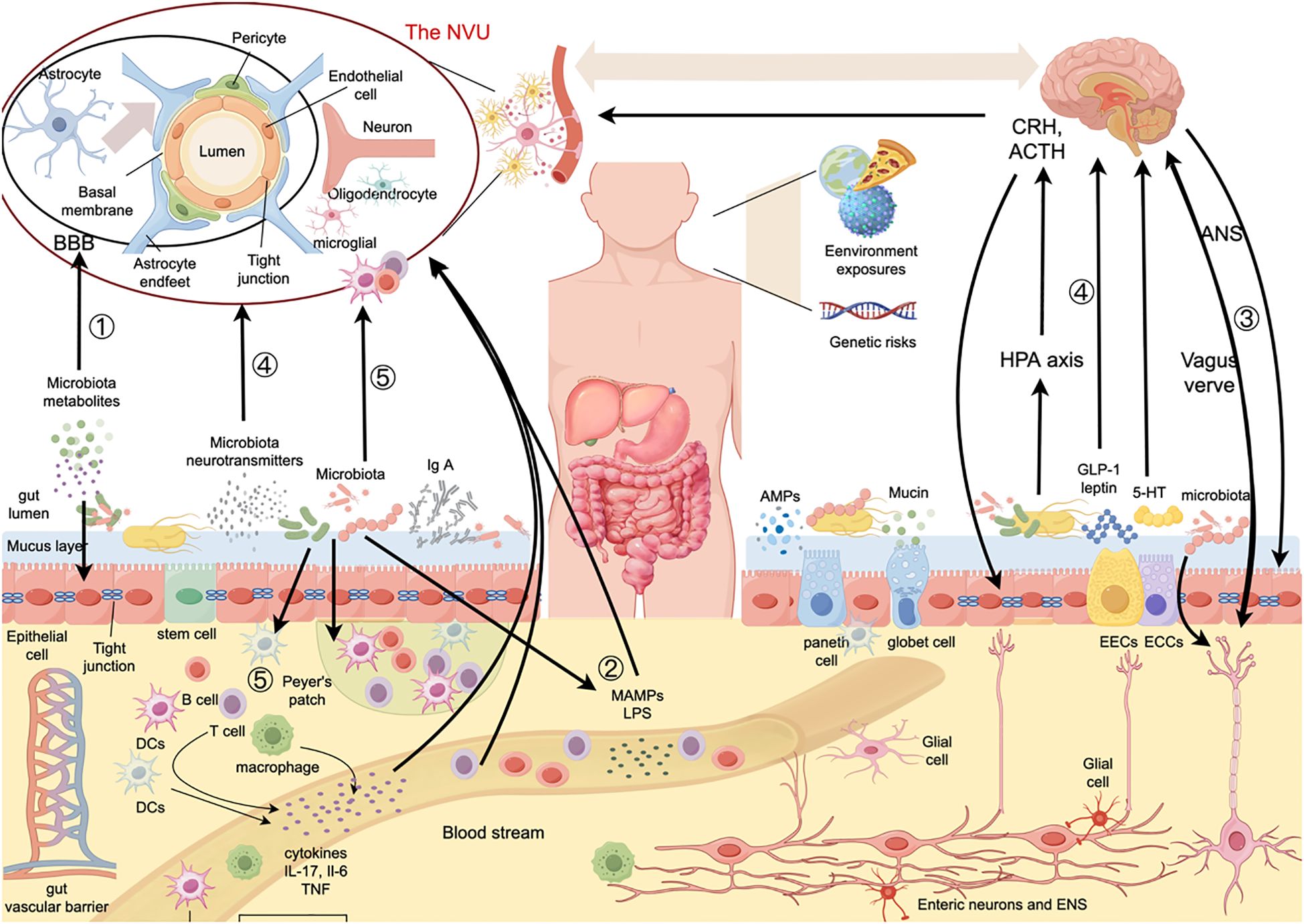
Figure 1. Pathways of the effects of gut microbiota on the BBB and intestinal barrier in MS. The gut microbiota can affect the structure and function of the BBB and gut barrier as well as BGM axis communications through various pathways, such as (1) microbial metabolites, which act on both the BBB and the intestinal barrier, (2) Intestinal microbiota structures, such as LPS and microbial membrane vesicles, enter the bloodstream through a “leaky gut”, act on the BBB, and enter the CNS, causing immune dysregulation, (3) The ANS regulate gut functions and influence microbiota composition and activity, among which vagal fibers can also activate enteric neurons. Additionally, the vagus nerve can sense signals from gut microbiota, enteric neurons, hormones, and peptides and transmit them to the CNS, (4) Neuroendocrine system can directly interact with microbiota via release of signaling molecules, like GLP-1, 5-HT, dynorphin, from neurons and ECCs. HPA axis, the main humoral component of the gut-brain axis, can modulate micoribiota composition and gut function by releasing glucocorticoids. The gut microbiota can also secrete neurotransmitters, such as GABA and 5-HT, which can further modulate CNS activity and the HPA axis. and (5) Gut microbiota can directly and indirectly influence immune cells of the CNS through a variety of pathways. The lymphoid tissues in the gut regulate immune cells within the gut and in the systemic circulation in conjunction with the gut microbiota. BBB, blood-brain-barrier; NVU, neurovascular unit; IgA, immunoglobulin A; MAMPs, microbe-associated molecular patterns; LPS, lipopolysaccharide; AMPs, antimicrobial peptides; HPA axis, hypothalamic-pituitary-adrenal axis; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; ANS, autonomic nervous system; GLP-1, glucagon-like peptide-1; EECs, enterochromaffin cells; ECCs, enteroendocrine cells; ENS, enteric nervous system; 5-HT, 5-hydroxytryptamine; DCs, dendritic cells; IL-17, interleukin-17; IL-6, interleukin-6; TNF, tumor necrosis factor.
Current evidence indicates that bottom-up modulation of the CNS by the microbiome occurs primarily through neuroimmune and neuroendocrine pathways, often involving the vagus nerve (153, 387, 388). This communication is mediated by several microbially derived molecules, including SCFAs (388–392), 2BAs and tryptophan metabolites (392, 393), which not only enter the systemic circulation but also interact with gut EECs, ECCs and the mucosal immune system locally (394–396). The microbiota can also independently produce various neuroactive molecules, such as GABA (397), 5-HT (398), norepinephrine (398, 399), and dopamine (398, 399). On the other hand, the CNS exerts regulatory control over intestinal microorganisms through multiple mechanisms, including the ANS efferent pathways and transmitter release. Furthermore, signals originating from the CNS can directly influence intestinal motility, intestinal barrier integrity, intestinal cell functions, and the living environment of intestinal microbiota, thereby impacting overall intestinal health and function.
5.1 Signaling mechanisms from the gut microbiota to the brain
SCFAs, BAs, and other metabolites have been implicated as signaling molecules mediating host-microbe communication via EECs and ECCs by acting on the corresponding receptors, which can regulate many CNS activities, including energy and glucose metabolism as well as HPA activity (400–404). 5-HT and its precursor, tryptophan, both play important roles in the BGM axis (401). 5-HT is mainly produced by the ECCs and is affected by gut microbiota for CNS synthesis, as the host is unable to produce tryptophan (405). The EAE model has identified direct neuroimmune regulatory roles for gut microbiota, as they can regulate immune cell trafficking and influence the development and function of the CNS-resident immune cells, particularly microglia (406–408). Relative to SPF mice, GF mice have compromised microglial maturation and morphology, resulting in weaker responses to pathogen exposure (408). Additionally, antibiotic treatment in SPF adult mice causes microglia to revert to an immature state, which can be normalized by recolonization with complex microbiota, indicating the necessity of microbial signaling throughout adulthood to preserve microglial maturation (408). Intestinal immune cells like IFN-producing meningeal NK cells and some IgA-secreting plasma cells, can directly influence neuroimmune responses, which are also regulated by gut microbiome (49, 409). Lastly, vagal receptors can detect regulatory gut peptides, inflammatory molecules, dietary elements, and bacterial metabolites to relay signals to the CNS via direct neural signaling (410), but there is also some evidence for direct activation of neurons by the gut microbiota. L. rhamnosus (JB-1), B. fragilis, and its isolated polysaccharide A all have been shown to activate intestinal afferent neurons ex vivo (411). Microbial metabolites are also candidates mediating direct activation of neurons, including microbially derived SCFAs.
5.2 Signaling from the brain to the gut microbiota
The ANS regulate gut functions including regional motility, secretion of gastric acid, mucus, bicarbonate, gut peptides, antimicrobial peptides, epithelial fluid maintenance, intestinal permeability, and mucosal immune response. These changes influence the microbial habitat, thereby modulating microbiota composition and activity. Vagal efferent fibers also influence immune responses and cytokine production, and they can also activate enteric neurons by synapsing with the ENS in the myenteric plexus (412–414). The sympathetic nervous system affects intestinal immune activity, while the HPA axis, the main humoral component of the gut-brain axis, responds to environmental stress or intestinal inflammation by releasing glucocorticoids and then restoring homeostasis or causing GI dysfunction by modulating enteric immune cells, gut function, and microbial composition (415).
MS can cause a variety of GI symptoms, including constipation and gastroparesis (416–419). GI function tests can also show delayed colonic transit time in MS (420). Regional intestinal transit times influence water content, nutrient availability, and microbial richness and composition (421, 422). The CNS can influence intestinal motility through multiple mechanisms, such as efferent vagus nerves, ENS, and neurotransmitters like 5-HT. Stress and inflammation can cause epithelial barrier defects by directly modulating epithelial permeability and altering the intestinal mucosal properties (420). The ANS influences mucus secretion by intestinal goblet cells, impacting intestinal mucus layer thickness and quality. Stress through catecholamine signaling can reduce mucus protective capacity and alter its composition and size (423). Changes in the intestinal barrier will further induce gut microbiota alterations.
Besides CNS-induced changes in the intestinal microbial environment, the neuroendocrine system can directly interact with microbiota via release of signaling molecules, like catecholamines, 5-HT, dynorphin, and cytokines, from neurons, immune cells, and ECCs (424, 425). Epinephrine and norepinephrine have been shown to enhance the virulence of certain enteric microbes by activating native quorum-sensing mechanisms (424, 426, 427). These findings support the notion that host neuroendocrine system can directly influence microbiota composition and function.
Therefore, pathology-associated barrier disruption may occur at several levels along the BGM axis, compromising its bidirectional communication due to the high molecular and cellular similarities. Changes in gut microbiota and microbial-derived products could contribute to damaged barriers in both gut and brain. Dysfunctional gut barriers allow these products, which could in turn reach and potentially alter brain barriers. Furthermore, gut microbiota dysbiosis could further influence barrier function by modulating neuroimmune signals. Additionally, signals from the brain, especially via the sympathetic and parasympathetic nervous systems and the ENS, can trigger intestinal inflammation and increased barrier permeability following CNS injury (428). This, in turn, will lead to gastrointestinal dysfunction, immune cell activation in the gut, gut dysbiosis, and finally escalate CNS inflammation.
5.3 Gut-brain communication in MS
As discussed above, gastrointestinal manifestations are common in MS. In the EAE model, gut dysbiosis causes increased intestinal permeability that precedes the CNS immune changes and induces symptoms of neuroinflammation, which suggests that gut dysbiosis promotes humoral signaling of inflammatory factors across the BGM axis (429).
Chronological age is the most significant factor influencing the clinical course of MS (430). PwMS with later onset often experience faster disability progression and poorer treatment response, possibly due to the immune system and CNS. Aging also raises the risks of age-related comorbidities like vascular and metabolic issues (431). Importantly, aging-related cognitive decline is associated with a chronic, low-grade enteric and central inflammatory state, including an increase in microglia, T cells, and border-associated macrophages in the CNS, as well as altered gut microbiota (432, 433). Aging-associated B cells also invade the meninges from the periphery and differentiate into IgM-producing plasma cells (434).
Diet-induced inflammation constitutes another important trigger in MS. It has been reported that unhealthy diet can induce cellular and neurobehavioral changes through the BGM axis (435). A Western diet, high in saturated fat and sugar, can change the expression of intestinal barrier markers, decrease EEC-derived GLP1, and induce hypothalamic inflammation through proinflammatory cytokines released by microglia (435–437). This inflammation is lessened in the absence of gut microbiome. Furthermore, transplantation of fecal content from high-fat diet–treated mice to naïve mice leads to behavioral abnormalities, indicating that diet-induced gut microbiome dysbiosis may contribute to CNS phenotypes (435). Besides diet-induced inflammation, environmental factors including obesity, viruses, smoking, tobacco use and VitD deficiency, are also considered risk factors for MS, potentially altering the microbiome and causing a leaky gut observed in PwMS and EAE animals (438).
Migration of intestinal immune cells to the CNS may also contribute to the pathogenesis of neurological and neurodegenerative diseases. In MS, the gut microbiota promotes the development of myelin-reactive Th1 and Th17 cells as well as Treg cells in the intestines, which then migrate to the CNS to promote or suppress inflammation, respectively (439). Although bacterial metabolites such as polysaccharide A and SCFAs can induce Treg cells, specific microbial components that activate proinflammatory T cells remain unclear (439).
As discussed previously, gut-derived metabolites and EECs are also crucial in the BGM axis. Metabolites from diet fibers such as SCFAs play key roles in the host’s metabolism and immune system. Metabolites like tryptophan, 2Bas have also been found to be dysregulated in both MS patients and EAE model. Changes in these gut derived metabolites and related microorganisms can exert different effects through the BGM axis. Neurotransmitters such as 5-HT, dopamine and GABA, secreted by the gut microbiome, impact the body’s defense system via immune cell receptors. A preclinical study on EAE-induced mice showed increased 5-HT levels within the neural region, enhanced 5-HT innervation of the spinal cord, and decreased EAE severity after treatment with a monoamine oxidase inhibitor (440). Therefore, the connection between these neurotransmitters and microbial regulation highlights their potent role in gut-brain interactions in neuroinflammation and MS.
In summary, the mechanism underlying the BGM communication is intricate and dynamic. Recent discoveries in the field have highlighted the significance of gut bacteria in this neuroimmunoendocrine process. This bidirectional communication not only plays a crucial role in maintaining the health of the two systems but also offers new avenues for therapy.
6 Therapeutic implications for barrier function in MS
Considering the impact of gut microbiota on immune modulation and barrier function, targeted interventions to normalize the gut microbiota could be a promising treatment for MS. Various approaches are currently being explored, as detailed in the following sections.
6.1 Probiotics
Using probiotics to replenish health-promoting gut bacteria has been suggested to maintain gut integrity and prevent pathological alterations. Probiotics are live organisms that, when administered in adequate amounts, can confer a health benefit (441). In one study, administration of probiotics increased certain taxonomic groups, such as Lactobacillus species which were depleted in MS, and decreased others that have been associated with MS, including Akkermansia and Blautia species (442). Research in laboratories and animal models has suggested multiple mechanisms by which probiotics could mediate beneficial effects, including induction of antimicrobial peptides, release of antimicrobial factors, suppression of immune cell proliferation, and enhancement of gut barrier function (443, 444). Animal models also suggest that probiotics can mitigate EAE by boosting IL-10 and TGFβ production from immune cells, amplifying Treg cell populations in gut-associated lymphoid organs and the CNS, and reducing levels of TNF, IFNγ and IL-17 (445–448).
6.2 Prebiotics
Prebiotics are non-digestible short-chain carbohydrates that promote some beneficial colonic bacteria production when selectively consumed (441). Their health benefits stem from their resistance to hydrolysis in the upper gastrointestinal tract and from fermentation in the large intestine by mainly anaerobic bacteria (449). Common prebiotics include disaccharides (lactulose), oligosaccharides (fructooligosaccharides, FOS; galactooligosaccharides, GOS) and polysaccharides (inulin) (450). Other sources of prebiotics include resistant starches, pectin, whole grains, and polyphenols (451). Animal studies have shown that prebiotics can influence brain function (452, 453) and modulate mood as well as stress responses by affecting the HPA axis. In healthy mice, supplementation with FOS or GOS can decrease anxiety and increase social behavior by promoting the growth of beneficial species such as Bifidobacteria (452, 454). However, human studies investigating the benefits of prebiotics on the brain are limited and inconclusive. Synbiotics, a combination of prebiotics and probiotics, have been found to improve age-related memory impairment in rats and are currently being tested in humans to promote intestinal health (455–457).
6.3 Fecal microbiota transplantation
FMT involves transferring fecal contents from a healthy donor into a patient, typically after broad-spectrum antibiotics, to correct disease-induced dysbiosis. It is gaining attention for treating neurodegenerative diseases by modulating gut microbiota and restoring homeostasis in the gut–brain axis primarily. Research highlights its potential in managing conditions such as PD, AD, and MS (330). In PD, FMT has been shown to alleviate motor dysfunction, reduce neuroinflammation, and regulate microbial populations by suppressing pro-inflammatory signaling pathways, including TLR4, MyD88 and NF-kB, while boosting dopamine and serotonin levels within the substantia nigra (458, 459). FMT has demonstrated benefits in reducing amyloid-β plaques, enhancing cognitive performance, and modulating neuroinflammation in AD. Furthermore, It also alters the gut metabolome, increasing SCFAs and reducing inflammatory cytokines (460). In MS, FMT has proven promising in preclinical studies by restoring gut microbial balance, reducing microglial activation, strengthening BBB integrity, and alleviating axonal damage (461). Some case–control studies have reported encouraging outcomes, showing that FMT can improve neurological symptoms in PwMS for 10 to 15 years, proving to be safe and tolerable (462, 463).
Reproducibility, scalability, and safety concerns may limit fecal FMT practice. Donor fecal material heterogeneity can cause outcome variability, and the risk of transmitting pathogens persists despite testing. A new method involves delivering defined bacterial communities instead of undefined fecal contents to colonize the recipient’s gut and restore a healthy microbiome (429). This approach is more compatible with standard manufacturing practices, addressing FMT’s limitations. Future efforts should refine FMT through personalized strategies like microbiota profiling, dietary interventions, and engineered probiotics development to enhance therapeutic results and address stability and safety issues. Understanding underlying mechanisms and advancing controlled clinical trials are critical steps toward establishing FMT as a dependable intervention for neurological conditions (330).
6.4 Diet and DMT
In MS, several dietary interventions have been suggested to reduce inflammation and promote clinical improvement, with some beneficial effects attributable to their impact on the gut microbiota. These interventions include the ketogenic diet, the palaeolithic diet (along with modified versions), and intermittent fasting, among others (464–467). In summary, modifying dietary patterns may be a viable, feasible, and cost-effective intervention with potential benefits in MS. Nonetheless, dietary interventions are notoriously difficult to enforce, and limited RCTs have been conducted to date. Therefore, there is a pressing need for larger, more rigorous clinical studies.
Current interventional therapies for MS, known as DMTs, include dimethyl fumarate (468), fingolimod (469), natalizumab (470), ocrelizumab (471) and others. Many DMTs have been found to alter gut microbiota composition and actively act on the barrier function, including both the BBB and intestinal barrier (372, 472–476). Glucocorticosteroids are still prescribed for acute MS relapses, which may improve BBB function by enhancing TJs and adherens junctions, and downregulate inflammation-induced endothelial CAMs in vitro. The first approved DMT, IFNβ, has shown stabilizing properties in biological barriers (such as the intestinal, BBB and blood–lung barriers), partly by upregulation of TJ proteins in endothelial cell layers (476). IFNβ also reduces trans-endothelial migration of proinflammatory CD4+ Th1 cells in RRMS patients (476). The commensal microbiota also can boost DCs to produce IFNβ, increasing Treg proliferation in the intestine (477). Natalizumab, a monoclonal antibody against α4β1 integrin, the cognate ligand of VCAM-1, directly disrupts the migration of immune cells, thereby reducing further damage and inflammation of the BBB. Natalizumab can also affect on integrins and lymphocyte trafficking in the gut, potentially modulating gut inflammatory in MS (477). These findings suggest that therapeutic properties of DMT in MS might rely, at least in part, on communication within the BMG axis.
As stated, probiotics and SCFAs metabolites play a colossal part in MS. Recently, the anti-inflammatory compound butyrate and its derivative, 4-phenyl butyrate, approved by the US FDA, have been shown to enhance BBB integrity and ameliorate EAE course (478). Sodium butyrate is a potent HDAC inhibitor that mainly interferes with the activity of class I HDAC enzymes. Sodium butyrate and other HDAC inhibitors, such as belinostat, exert anti-inflammatory and neuroprotective effects, which are also advantageous in acute EAE (479, 480). Besides mechanisms mediated by viable bacteria, host responses to conserved bacterial structures are crucial for intestinal homeostasis (481). Thus, immune responses can be beneficially modulated by directly sensing such bacterial structures. For instance, oral administration of commensal-derived cell wall components has been shown to promote immune tolerance in mouse models of IBD (482, 483) via specific interactions with host TLRs. These findings suggest that using either artificial ligands that mimic bacterial structures and their associated signaling responses, or isolated cell wall components from beneficial gut microbes, could support microbiome manipulation for therapy. However, care must be taken, as molecules like LPS can cross the intestinal barrier and trigger harmful immune responses. Additionally, the success of the regimen depends on delivering bacterial cell wall structures to the right place.
7 Conclusion
Significant advancements have been achieved over the past decade in characterizing the interaction between the gut microbiome and the CNS in various CNS inflammatory disorders. The disruption of the intricate ecosystem of the intestinal microbiota is now implicated in numerous conditions affecting both the intestine and the brain. Some pathways through which microbes influence the gut-brain axis are beginning to be elucidated. Consequently, manipulating the microbiota is garnering attention as a promising strategy for the prevention or treatment of various extra-intestinal diseases. The beneficial effects of an ‘optimized’ gut microbiota include the immune and epithelial homeostasis, enteric nervous system regulation, and optimal digestion and metabolism.
The study of the BGM system is dynamically and rapidly advancing due to ever-more-powerful biological techniques, such as metagenomics and metatranscriptomics, combined with novel bioinformatic and computational methods that enable multi-omic integration of microbial and host data via machine learning approaches. While some initial evidence suggests changes in the BGM axis in MS and EAE, the putative role of the gut microbiota and different barriers in the pathogenesis of brain autoimmunity has yet to be fully investigated. Furthermore, the causal link between an altered enteric barrier and CNS pathology has yet to be established. An appropriate test for assessing intestinal barrier impairments still needs to be established. Human studies on prodromal and untreated patients are essential to determine whether intestinal barrier changes occur early in CNS diseases or result from systemic immune and inflammatory conditions. Lastly, it is also unclear whether these intestinal barrier abnormalities are consistent across different CNS conditions. The primary trigger for the dysfunctional gut-brain axis in MS remains within a chicken-and-egg causality dilemma, with an apparent self-perpetuating loop of brain-gut inflammation.
Research on the BGM system also tends towards therapeutic transformation. Current strategies involve modifying gut microbial by altering nutrient availability to boost specific bacteria (prebiotics), introducing or expanding ‘beneficial’ species (probiotics), or transplanting entire communities or portions thereof from other intestinal donors (FMT and more selective stool transplants). Although these methods have demonstrated encouraging results in small-scale studies involving MS patients and EAE models, the absence of large-scale prospective randomized controlled trials means that these findings still suffer from limited reproducibility and challenges in generalizability across diverse populations. Furthermore, given that exogenous interventions in the gut microbiota may disrupt the intrinsic gut environment and lead to secondary complications, the aforementioned treatments possess inherent limitations. Consequently, significant progress is required before these methods can be effectively applied in the clinical management of patients with MS and other neurological diseases.
The BGM system presents several emerging research areas and unanswered questions, including the investigation of microbial metabolites and their specific effects on the host, the roles of viral and fungal components within the gut ecosystem, and the impact of environmental factors (the exposome) on the system. Further research should also focus on the role of the BGM system in different phases of the lifespan, in particular in neurodevelopmental and neurodegenerative disorders, the gut microbiota-immune system interaction, the underexplored contributions of the virome and its interactions with the gut bacteria and the interaction of the gut microbiota with the female reproductive system in maintaining homeostasis. Furthermore, integrating machine learning and artificial intelligence with multi-omics imaging and microbiome datasets has the potential to significantly enhance our understanding of the BGM system at a systems biology level. Lastly, large-scale, highly controlled, longitudinal human studies are urgently needed to identify the causes and sequelae of dysbiotic gut states and explain interindividual differences in susceptibility to BGM-related diseases.
All in all, these and other improvements in our understanding of microbe–host interactions at the gut and brain barriers will hopefully accelerate research into the use of microbiota-modulating therapies to prevent and treat neurological and neurodegenerative diseases.
Author contributions
JR: Conceptualization, Writing – original draft, Visualization. ZN: Writing – original draft, Visualization. JW: Writing – original draft, Visualization. JG: Methodology, Writing – original draft. HH: Writing – review & editing, Methodology. FG: Methodology, Writing – review & editing. RL: Writing – review & editing, Conceptualization. ZW: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We are grateful to all the members for participating in this study. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ascherio A and Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin Neurol. (2016) 36:103–14. doi: 10.1055/s-0036-1579693
2. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. (2020) 26:1816–21. doi: 10.1177/1352458520970841
3. Sospedra M and Martin R. Immunology of multiple sclerosis. Semin Neurol. (2016) 36:115–27. doi: 10.1055/s-0036-1579739
4. Laschinger M and Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. (2000) 102:32–43. doi: 10.1016/s0165-5728(99)00156-3
5. McKenna PG and Yasseen AA. Increased sensitivity to cell killing and mutagenesis by chemical mutagens in thymidine-kinase-deficient subclones of a Friend murine leukaemia cell line. Genet Res. (1982) 40:207–12. doi: 10.1017/s0016672300019078
6. Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. (2005) 175:4320–30. doi: 10.4049/jimmunol.175.7.4320
7. Sinha S, Boyden AW, Itani FR, Crawford MP, and Karandikar NJ. CD8(+) T-cells as immune regulators of multiple sclerosis. Front Immunol. (2015) 6:619. doi: 10.3389/fimmu.2015.00619
8. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
9. Clemente JC, Ursell LK, Parfrey LW, and Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. (2012) 148:1258–70. doi: 10.1016/j.cell.2012.01.035
10. Gensollen T, Iyer SS, Kasper DL, and Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378
11. Rosenbaum M, Knight R, and Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. (2015) 26:493–501. doi: 10.1016/j.tem.2015.07.002
12. Shreiner AB, Kao JY, and Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. (2015) 31:69–75. doi: 10.1097/MOG.0000000000000139
13. Valdes AM, Walter J, Segal E, and Spector TD. Role of the gut microbiota in nutrition and health. BMJ. (2018) 361:k2179. doi: 10.1136/bmj.k2179
14. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, and Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
15. Geng ZH, Zhu Y, Li QL, Zhao C, and Zhou PH. Enteric nervous system: the bridge between the gut microbiota and neurological disorders. Front Aging Neurosci. (2022) 14:810483. doi: 10.3389/fnagi.2022.810483
16. Mayer EA, Nance K, and Chen S. The gut-brain axis. Annu Rev Med. (2022) 73:439–53. doi: 10.1146/annurev-med-042320-014032
17. Rustenhoven J and Kipnis J. Brain borders at the central stage of neuroimmunology. Nature. (2022) 612:417–29. doi: 10.1038/s41586-022-05474-7
18. Al Bander Z, Nitert MD, Mousa A, and Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. (2020) 17(20):7618. doi: 10.3390/ijerph17207618
19. Shkoporov AN, Clooney AG, Sutton TDS, Ryan FJ, Daly KM, Nolan JA, et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. (2019) 26:527–41 e5. doi: 10.1016/j.chom.2019.09.009
20. Mohammad S and Thiemermann C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front Immunol. (2020) 11:594150. doi: 10.3389/fimmu.2020.594150
21. Kurita N, Yamashiro K, Kuroki T, Tanaka R, Urabe T, Ueno Y, et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J Cereb Blood Flow Metab. (2020) 40:2505–20. doi: 10.1177/0271678X19899577
22. Antonini M, Lo Conte M, Sorini C, and Falcone M. How the interplay between the commensal microbiota, gut barrier integrity, and mucosal immunity regulates brain autoimmunity. Front Immunol. (2019) 10:1937. doi: 10.3389/fimmu.2019.01937
23. Niess JH, Leithauser F, Adler G, and Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. (2008) 180:559–68. doi: 10.4049/jimmunol.180.1.559
24. Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. (2011) 479:538–41. doi: 10.1038/nature10554
25. Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. (2009) 183:6041–50. doi: 10.4049/jimmunol.0900747
26. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. (2015) 23:141–7. doi: 10.1016/j.mib.2014.11.013
27. Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine. (2022) 76:103798. doi: 10.1016/j.ebiom.2021.103798
28. i MCEasbue, i MC. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. (2022) 185:3467–86 e16. doi: 10.1016/j.cell.2022.08.021
29. Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. (2023) 15:1. doi: 10.1186/s13073-022-01148-1
30. Yadav M, Ali S, Shrode RL, Shahi SK, Jensen SN, Hoang J, et al. Multiple sclerosis patients have an altered gut mycobiome and increased fungal to bacterial richness. PloS One. (2022) 17:e0264556. doi: 10.1371/journal.pone.0264556
31. Tremlett H, Zhu F, Arnold D, Bar-Or A, Bernstein CN, Bonner C, et al. The gut microbiota in pediatric multiple sclerosis and demyelinating syndromes. Ann Clin Transl Neurol. (2021) 8:2252–69. doi: 10.1002/acn3.51476
32. Hoffman K, Brownell Z, Doyle WJ, and Ochoa-Reparaz J. The immunomodulatory roles of the gut microbiome in autoimmune diseases of the central nervous system: Multiple sclerosis as a model. J Autoimmun. (2023) 137:102957. doi: 10.1016/j.jaut.2022.102957
33. Lee YK, Menezes JS, Umesaki Y, and Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. (2011) 108 Suppl 1:4615–22. doi: 10.1073/pnas.1000082107
34. Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. (2016) 7:12015. doi: 10.1038/ncomms12015
35. Inczefi O, Bacsur P, Resal T, Keresztes C, and Molnar T. The influence of nutrition on intestinal permeability and the microbiome in health and disease. Front Nutr. (2022) 9:718710. doi: 10.3389/fnut.2022.718710
36. Fawkner-Corbett D, Simmons A, and Parikh K. Microbiome, pattern recognition receptor function in health and inflammation. Best Pract Res Clin Gastroenterol. (2017) 31:683–91. doi: 10.1016/j.bpg.2017.11.001
37. Shahi SK, Yadav M, Ghimire S, and Mangalam AK. Role of the gut microbiome in multiple sclerosis: From etiology to therapeutics. Int Rev Neurobiol. (2022) 167:185–215. doi: 10.1016/bs.irn.2022.06.001
38. Rudzki L and Maes M. The microbiota-gut-immune-glia (MGIG) axis in major depression. Mol Neurobiol. (2020) 57:4269–95. doi: 10.1007/s12035-020-01961-y
39. Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, et al. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav Immun. (2018) 69:1–8. doi: 10.1016/j.bbi.2017.08.026
40. Martel J, Chang SH, Ko YF, Hwang TL, Young JD, and Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab. (2022) 33:247–65. doi: 10.1016/j.tem.2022.01.002
41. Nouri M, Bredberg A, Westrom B, and Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PloS One. (2014) 9:e106335. doi: 10.1371/journal.pone.0106335
42. Gauthier AE, Rotjan RD, and Kagan JC. Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biol. (2022) 12:220146. doi: 10.1098/rsob.220146
43. Viglietta V, Baecher-Allan C, Weiner HL, and Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. (2004) 199:971–9. doi: 10.1084/jem.20031579
44. Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. (2005) 35:3343–52. doi: 10.1002/eji.200526065
45. Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, et al. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun. (2011) 36:115–24. doi: 10.1016/j.jaut.2010.12.003
46. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. (2017) 114:10713–8. doi: 10.1073/pnas.1711235114
47. He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol. (2019) 10:385. doi: 10.3389/fimmu.2019.00385
48. MaChado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. (2018) 141:2066–82. doi: 10.1093/brain/awy151
49. Rojas OL, Probstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, et al. Recirculating intestinal igA-producing cells regulate neuroinflammation via IL-10. Cell. (2019) 176:610–24 e18. doi: 10.1016/j.cell.2018.11.035
50. Behzadi P, Kim CH, Pawlak EA, and Algammal A. Editorial: The innate and adaptive immune system in human urinary system. Front Immunol. (2023) 14:1294869. doi: 10.3389/fimmu.2023.1294869
51. Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. (2013) 145:1323–33. doi: 10.1053/j.gastro.2013.08.047
52. Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, et al. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. (2015) 68:24–35. doi: 10.1016/j.mcn.2015.03.018
53. Brown B, Ojha V, Fricke I, Al-Sheboul SA, Imarogbe C, Gravier T, et al. Innate and adaptive immunity during SARS-coV-2 infection: biomolecular cellular markers and mechanisms. Vaccines (Basel). (2023) 11(2):408. doi: 10.3390/vaccines11020408
54. Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. (2001) 167:5887–94. doi: 10.4049/jimmunol.167.10.5887
55. Visser L, Jan de Heer H, Boven LA, van Riel D, van Meurs M, Melief MJ, et al. Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol. (2005) 174:808–16. doi: 10.4049/jimmunol.174.2.808
56. Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. (2006) 116:456–64. doi: 10.1172/JCI26078
57. Kuugbee ED, Shang X, Gamallat Y, Bamba D, Awadasseid A, Suliman MA, et al. Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig Dis Sci. (2016) 61:2908–20. doi: 10.1007/s10620-016-4238-7
58. Latorre E, Layunta E, Grasa L, Castro M, Pardo J, Gomollon F, et al. Intestinal serotonin transporter inhibition by toll-like receptor 2 activation. A feedback modulation. PloS One. (2016) 11:e0169303. doi: 10.1371/journal.pone.0169303
59. Caputi V, Marsilio I, Cerantola S, Roozfarakh M, Lante I, Galuppini F, et al. Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front Pharmacol. (2017) 8:350. doi: 10.3389/fphar.2017.00350
60. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. (2010) 107:14691–6. doi: 10.1073/pnas.1005963107
61. Ehlers S and Kaufmann SH. Participants of the 99 Dahlem C. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. (2010) 31:184–90. doi: 10.1016/j.it.2010.02.003
62. van de Guchte M, Blottiere HM, and Dore J. Humans as holobionts: implications for prevention and therapy. Microbiome. (2018) 6:81. doi: 10.1186/s40168-018-0466-8
63. Montgomery TL, Peipert D, and Krementsov DN. Modulation of multiple sclerosis risk and pathogenesis by the gut microbiota: Complex interactions between host genetics, bacterial metabolism, and diet. Immunol Rev. (2024) 325:131–51. doi: 10.1111/imr.13343
64. Obermeier B, Daneman R, and Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. (2013) 19:1584–96. doi: 10.1038/nm.3407
65. Hawkins BT and Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. (2005) 57:173–85. doi: 10.1124/pr.57.2.4
66. Thomsen MS, Routhe LJ, and Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. (2017) 37:3300–17. doi: 10.1177/0271678X17722436
67. Li P, Wang L, Zhou Y, Gan Y, Zhu W, Xia Y, et al. C-C chemokine receptor type 5 (CCR5)-mediated docking of transferred tregs protects against early blood-brain barrier disruption after stroke. J Am Heart Assoc. (2017) 6(8):e006387. doi: 10.1161/JAHA.117.006387
68. Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, et al. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest. (2017) 127:3136–51. doi: 10.1172/JCI91301
69. Boulay AC, Cisternino S, and Cohen-Salmon M. Immunoregulation at the gliovascular unit in the healthy brain: A focus on Connexin 43. Brain Behav Immun. (2016) 56:1–9. doi: 10.1016/j.bbi.2015.11.017
70. Sweeney MD, Ayyadurai S, and Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. (2016) 19:771–83. doi: 10.1038/nn.4288
71. Villasenor R, Kuennecke B, Ozmen L, Ammann M, Kugler C, Gruninger F, et al. Region-specific permeability of the blood-brain barrier upon pericyte loss. J Cereb Blood Flow Metab. (2017) 37:3683–94. doi: 10.1177/0271678X17697340
72. Persidsky Y, Hill J, Zhang M, Dykstra H, Winfield M, Reichenbach NL, et al. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab. (2016) 36:794–807. doi: 10.1177/0271678X15606149
73. Torok O, Schreiner B, Schaffenrath J, Tsai HC, Maheshwari U, Stifter SA, et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc Natl Acad Sci U.S.A. (2021) 118(10):e2016587118. doi: 10.1073/pnas.2016587118
74. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Corrigendum: Structural and functional features of central nervous system lymphatic vessels. Nature. (2016) 533:278. doi: 10.1038/nature16999
75. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. (2020) 16:137–53. doi: 10.1038/s41582-020-0312-z
76. Nedergaard M and Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. (2020) 370:50–6. doi: 10.1126/science.abb8739
77. Hannocks MJ, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, et al. Molecular characterization of perivascular drainage pathways in the murine brain. J Cereb Blood Flow Metab. (2018) 38:669–86. doi: 10.1177/0271678X17749689
78. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. (2014) 76:845–61. doi: 10.1002/ana.24271
79. Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. (2020) 30:229–43. doi: 10.1038/s41422-020-0287-8
80. Mogensen FL, Delle C, and Nedergaard M. The glymphatic system (En)during inflammation. Int J Mol Sci. (2021) 22(14):7491. doi: 10.3390/ijms22147491
81. Palazzo C, Buccoliero C, Mola MG, Abbrescia P, Nicchia GP, Trojano M, et al. AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathol Commun. (2019) 7:51. doi: 10.1186/s40478-019-0707-5
82. Carloni S, Bertocchi A, Mancinelli S, Bellini M, Erreni M, Borreca A, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. (2021) 374:439–48. doi: 10.1126/science.abc6108
83. Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. (2021) 184:3056–74 e21. doi: 10.1016/j.cell.2021.04.003
84. Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PloS One. (2014) 9:e103174. doi: 10.1371/journal.pone.0103174
85. Gankam Kengne F, Nicaise C, Soupart A, Boom A, Schiettecatte J, Pochet R, et al. Astrocytes are an early target in osmotic demyelination syndrome. J Am Soc Nephrol. (2011) 22:1834–45. doi: 10.1681/ASN.2010111127
86. Gankam-Kengne F, Couturier BS, Soupart A, Brion JP, and Decaux G. Osmotic stress-induced defective glial proteostasis contributes to brain demyelination after hyponatremia treatment. J Am Soc Nephrol. (2017) 28:1802–13. doi: 10.1681/ASN.2016050509
87. Popescu BF, Bunyan RF, Guo Y, Parisi JE, Lennon VA, and Lucchinetti CF. Evidence of aquaporin involvement in human central pontine myelinolysis. Acta Neuropathol Commun. (2013) 1:40. doi: 10.1186/2051-5960-1-40
88. Gankam Kengne F, Soupart A, Pochet R, Brion JP, and Decaux G. Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int. (2009) 76:614–21. doi: 10.1038/ki.2009.254
89. Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. (2019) 10:5816. doi: 10.1038/s41467-019-13812-z
90. Canedo T, Portugal CC, Socodato R, Almeida TO, Terceiro AF, Bravo J, et al. Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine. Neuropsychopharmacology. (2021) 46:2358–70. doi: 10.1038/s41386-021-01139-7
91. de Haas AH, van Weering HR, de Jong EK, Boddeke HW, and Biber KP. Neuronal chemokines: versatile messengers in central nervous system cell interaction. Mol Neurobiol. (2007) 36:137–51. doi: 10.1007/s12035-007-0036-8
92. Brosnan CF and Raine CS. The astrocyte in multiple sclerosis revisited. Glia. (2013) 61:453–65. doi: 10.1002/glia.22443
93. Bateman GA and Bateman AR. The dilated veins surrounding the cord in multiple sclerosis suggest elevated pressure and obstruction of the glymphatic system. Neuroimage. (2024) 286:120517. doi: 10.1016/j.neuroimage.2024.120517
94. Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, and Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain. (2022) 145:2785–95. doi: 10.1093/brain/awab454
95. Yang T, Tang Y, Liu X, Gong S, and Yao E. Microglia synchronizes with the circadian rhythm of the glymphatic system and modulates glymphatic system function. IUBMB Life. (2024) 76:1209–22. doi: 10.1002/iub.2903
96. De Meo E, Storelli L, Moiola L, Ghezzi A, Veggiotti P, Filippi M, et al. In vivo gradients of thalamic damage in paediatric multiple sclerosis: a window into pathology. Brain. (2021) 144:186–97. doi: 10.1093/brain/awaa379
97. Louveau A. Meningeal immunity, drainage, and tertiary lymphoid structure formation. Methods Mol Biol. (2018) 1845:31–45. doi: 10.1007/978-1-4939-8709-2_3
98. Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. (2019) 10:229. doi: 10.1038/s41467-018-08163-0
99. das Neves SP, Delivanoglou N, Ren Y, Cucuzza CS, Makuch M, Almeida F, et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity. (2024) 57:2328–43 e8. doi: 10.1016/j.immuni.2024.08.004
100. Zhang W, Xiao D, Mao Q, and Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. (2023) 8:267. doi: 10.1038/s41392-023-01486-5
101. Yang Y, Zhao B, Wang Y, Lan H, Liu X, Hu Y, et al. Diabetic neuropathy: cutting-edge research and future directions. Signal Transduct Target Ther. (2025) 10:132. doi: 10.1038/s41392-025-02175-1
102. Yan M, Sun Z, Zhang S, Yang G, Jiang X, Wang G, et al. SOCS modulates JAK-STAT pathway as a novel target to mediate the occurrence of neuroinflammation: Molecular details and treatment options. Brain Res Bull. (2024) 213:110988. doi: 10.1016/j.brainresbull.2024.110988
103. Hu X, Li J, Fu M, Zhao X, and Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. (2021) 6:402. doi: 10.1038/s41392-021-00791-1
104. Ageeva T, Rizvanov A, and Mukhamedshina Y. NF-kappaB and JAK/STAT signaling pathways as crucial regulators of neuroinflammation and astrocyte modulation in spinal cord injury. Cells. (2024) 13(7):581. doi: 10.3390/cells13070581
105. Amoriello R, Memo C, Ballerini L, and Ballerini C. The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology. Mol Brain. (2024) 17:4. doi: 10.1186/s13041-024-01077-7
106. Zierfuss B, Larochelle C, and Prat A. Blood-brain barrier dysfunction in multiple sclerosis: causes, consequences, and potential effects of therapies. Lancet Neurol. (2024) 23:95–109. doi: 10.1016/S1474-4422(23)00377-0
107. Wang WY, Tan MS, Yu JT, and Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. (2015) 3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49
108. Malkiewicz MA, Szarmach A, Sabisz A, Cubala WJ, Szurowska E, and Winklewski PJ. Blood-brain barrier permeability and physical exercise. J Neuroinflammation. (2019) 16:15. doi: 10.1186/s12974-019-1403-x
109. Dias-Carvalho A, Sa SI, Carvalho F, Fernandes E, and Costa VM. Inflammation as common link to progressive neurological diseases. Arch Toxicol. (2024) 98:95–119. doi: 10.1007/s00204-023-03628-8
110. Sadowska GB, Chen X, Zhang J, Lim YP, Cummings EE, Makeyev O, et al. Interleukin-1beta transfer across the blood-brain barrier in the ovine fetus. J Cereb Blood Flow Metab. (2015) 35:1388–95. doi: 10.1038/jcbfm.2015.134
111. Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, et al. IFN-gamma, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. (2018) 507:274–9. doi: 10.1016/j.bbrc.2018.11.021
112. Sonar SA, Shaikh S, Joshi N, Atre AN, and Lal G. IFN-gamma promotes transendothelial migration of CD4(+) T cells across the blood-brain barrier. Immunol Cell Biol. (2017) 95:843–53. doi: 10.1038/icb.2017.56
113. Nyul-Toth A, Kozma M, Nagyoszi P, Nagy K, Fazakas C, Hasko J, et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav Immun. (2017) 64:220–31. doi: 10.1016/j.bbi.2017.04.010
114. Williams JL, Holman DW, and Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. (2014) 8:154. doi: 10.3389/fncel.2014.00154
115. Lepennetier G, Hracsko Z, Unger M, Van Griensven M, Grummel V, Krumbholz M, et al. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflammation. (2019) 16:219. doi: 10.1186/s12974-019-1601-6
116. Mirhafez SR, Pasdar A, Avan A, Esmaily H, Moezzi A, Mohebati M, et al. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br J Nutr. (2015) 113:1911–9. doi: 10.1017/S0007114515001038
117. Guo F, Xu D, Lin Y, Wang G, Wang F, Gao Q, et al. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. (2020) 34:1872–84. doi: 10.1096/fj.201902203RR
118. Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. (2010) 24:1023–34. doi: 10.1096/fj.09-141978
119. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. (2007) 13:1173–5. doi: 10.1038/nm1651
120. Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. (2015) 10:1040–54. doi: 10.1016/j.celrep.2015.01.037
121. Sayed A, Bahbah EI, Kamel S, Barreto GE, Ashraf GM, and Elfil M. The neutrophil-to-lymphocyte ratio in Alzheimer’s disease: Current understanding and potential applications. J Neuroimmunol. (2020) 349:577398. doi: 10.1016/j.jneuroim.2020.577398
122. Probstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. (2020) 5(53):eabc7191. doi: 10.1126/sciimmunol.abc7191
123. Pan W, Kastin AJ, Bell RL, and Olson RD. Upregulation of tumor necrosis factor alpha transport across the blood-brain barrier after acute compressive spinal cord injury. J Neurosci. (1999) 19:3649–55. doi: 10.1523/JNEUROSCI.19-09-03649.1999
124. Engelhardt B and Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. (2014) 355:687–99. doi: 10.1007/s00441-014-1811-2
125. Spencer JI, Bell JS, and DeLuca GC. Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. J Neurol Neurosurg Psychiatry. (2018) 89:42–52. doi: 10.1136/jnnp-2017-316011
126. Yong HYF and Yong VW. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol. (2022) 18:40–55. doi: 10.1038/s41582-021-00581-x
127. Yates RL, Esiri MM, Palace J, Jacobs B, Perera R, and DeLuca GC. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann Neurol. (2017) 82:259–70. doi: 10.1002/ana.24997
128. Willis CM, Nicaise AM, Menoret A, Ryu JK, Mendiola AS, Jellison ER, et al. Extracellular vesicle fibrinogen induces encephalitogenic CD8+ T cells in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. (2019) 116:10488–93. doi: 10.1073/pnas.1816911116
129. Brailoiu E, Shipsky MM, Yan G, Abood ME, and Brailoiu GC. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. (2017) 1657:167–75. doi: 10.1016/j.brainres.2016.12.011
130. Sweeney MD, Zhao Z, Montagne A, Nelson AR, and Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. (2019) 99:21–78. doi: 10.1152/physrev.00050.2017
131. Langen UH, Ayloo S, and Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol. (2019) 35:591–613. doi: 10.1146/annurev-cellbio-100617-062608
132. D’Haeseleer M, Cambron M, Vanopdenbosch L, and De Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurol. (2011) 10:657–66. doi: 10.1016/S1474-4422(11)70105-3
133. Forsythe P, Bienenstock J, and Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. (2014) 817:115–33. doi: 10.1007/978-1-4939-0897-4_5
134. Powell N, Walker MM, and Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. (2017) 14:143–59. doi: 10.1038/nrgastro.2016.191
135. Cani PD and Knauf C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol Metab. (2016) 5:743–52. doi: 10.1016/j.molmet.2016.05.011
136. Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. (2021) 10:e12159. doi: 10.1002/jev2.12159
137. Gonzalez-Santana A and Diaz Heijtz R. Bacterial peptidoglycans from microbiota in neurodevelopment and behavior. Trends Mol Med. (2020) 26:729–43. doi: 10.1016/j.molmed.2020.05.003
138. Schweighofer H, Rummel C, Roth J, and Rosengarten B. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Med Exp. (2016) 4:19. doi: 10.1186/s40635-016-0091-4
139. Yang Y, Yang LY, Orban L, Cuylear D, Thompson J, Simon B, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. (2018) 11:689–98. doi: 10.1016/j.brs.2018.01.034
140. RoshChina VV. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv Exp Med Biol. (2016) 874:25–77. doi: 10.1007/978-3-319-20215-0_2
141. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
142. Zhang S, Cheng S, Jiang X, Zhang J, Bai L, Qin X, et al. Gut-brain communication in hyperfunction of 5-hydroxytryptamine induced by oral zinc oxide nanoparticles exposure in young mice. Food Chem Toxicol. (2020) 135:110906. doi: 10.1016/j.fct.2019.110906
143. Holzer P. Neuropeptides, microbiota, and behavior. Int Rev Neurobiol. (2016) 131:67–89. doi: 10.1016/bs.irn.2016.08.005
144. Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, and Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. (2014) 817:221–39. doi: 10.1007/978-1-4939-0897-4_10
145. Clark A and Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. (2016) 13:43. doi: 10.1186/s12970-016-0155-6
146. Farzi A, Frohlich EE, and Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. (2018) 15:5–22. doi: 10.1007/s13311-017-0600-5
147. Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. (2002) 303:1061–6. doi: 10.1124/jpet.102.038497
148. de Punder K and Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. (2015) 6:223. doi: 10.3389/fimmu.2015.00223
149. Karagkouni A, Alevizos M, and Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. (2013) 12:947–53. doi: 10.1016/j.autrev.2013.02.006
150. Wichmann A, Allahyar A, Greiner TU, Plovier H, Lunden GO, Larsson T, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. (2013) 14:582–90. doi: 10.1016/j.chom.2013.09.012
151. Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, and Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. (2018) 315:G53–65. doi: 10.1152/ajpgi.00346.2017
152. Salcedo I, Tweedie D, Li Y, and Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. (2012) 166:1586–99. doi: 10.1111/j.1476-5381.2012.01971.x
153. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. (2012) 61:364–71. doi: 10.2337/db11-1019
154. Li H, Sun J, Du J, Wang F, Fang R, Yu C, et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. (2018) 30:e13260. doi: 10.1111/nmo.13260
155. O’Mahony SM, Clarke G, Borre YE, Dinan TG, and Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
156. Dinan TG and Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. (2017) 595:489–503. doi: 10.1113/JP273106
157. Thomsen MS, Birkelund S, Burkhart A, Stensballe A, and Moos T. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J Neurochem. (2017) 140:741–54. doi: 10.1111/jnc.13747
158. Skelly DT, Hennessy E, Dansereau MA, and Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PloS One. (2013) 8:e69123. doi: 10.1371/journal.pone.0069123
159. Mark KS and Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. (1999) 64:1941–53. doi: 10.1016/s0024-3205(99)00139-3
160. Davalos D and Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
161. Perry VH. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun. (2007) 21:45–6. doi: 10.1016/j.bbi.2006.08.004
162. Mowat AM and Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. (2014) 14:667–85. doi: 10.1038/nri3738
163. Peterson LW and Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. (2014) 14:141–53. doi: 10.1038/nri3608
164. Smith RJ, Rao-Bhatia A, and Kim TH. Signaling and epigenetic mechanisms of intestinal stem cells and progenitors: insight into crypt homeostasis, plasticity, and niches. Wiley Interdiscip Rev Dev Biol. (2017) 6(5). doi: 10.1002/wdev.281
165. McCallum G and Tropini C. The gut microbiota and its biogeography. Nat Rev Microbiol. (2024) 22:105–18. doi: 10.1038/s41579-023-00969-0
166. Schroder K and Bosch TC. The origin of mucosal immunity: lessons from the holobiont hydra. mBio. (2016) 7(6):e01184-16. doi: 10.1128/mBio.01184-16
167. Bevins CL and Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. (2011) 9:356–68. doi: 10.1038/nrmicro2546
168. Chen K, Magri G, Grasset EK, and Cerutti A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat Rev Immunol. (2020) 20:427–41. doi: 10.1038/s41577-019-0261-1
169. Gustafsson JK and Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. (2022) 19:785–803. doi: 10.1038/s41575-022-00675-x
170. Dupont A, Heinbockel L, Brandenburg K, and Hornef MW. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes. (2014) 5:761–5. doi: 10.4161/19490976.2014.972238
171. Kanaya T, Williams IR, and Ohno H. Intestinal M cells: Tireless samplers of enteric microbiota. Traffic. (2020) 21:34–44. doi: 10.1111/tra.12707
172. Foley SE, Tuohy C, Dunford M, Grey MJ, De Luca H, Cawley C, et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome. (2021) 9:183. doi: 10.1186/s40168-021-01137-3
173. Foley SE, Dente MJ, Lei X, Sallis BF, Loew EB, Meza-Segura M, et al. Microbial metabolites orchestrate a distinct multi-tiered regulatory network in the intestinal epithelium that directs P-glycoprotein expression. mBio. (2022) 13:e0199322. doi: 10.1128/mbio.01993-22
174. Yu YB and Li YQ. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol. (2014) 20:11273–80. doi: 10.3748/wjg.v20.i32.11273
175. Seguella L and Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. (2021) 18:571–87. doi: 10.1038/s41575-021-00423-7
176. Bostick JW and Zhou L. Innate lymphoid cells in intestinal immunity and inflammation. Cell Mol Life Sci. (2016) 73:237–52. doi: 10.1007/s00018-015-2055-3
177. Cheroutre H, Lambolez F, and Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. (2011) 11:445–56. doi: 10.1038/nri3007
178. Olivares-Villagomez D and Van Kaer L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. (2018) 39:264–75. doi: 10.1016/j.it.2017.11.003
179. Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. (2020) 587:472–6. doi: 10.1038/s41586-020-2886-4
180. Morbe UM, Jorgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. (2021) 14:793–802. doi: 10.1038/s41385-021-00389-4
181. Di Marco Barros R, Fitzpatrick Z, and Clatworthy MR. The gut-meningeal immune axis: Priming brain defense against the most likely invaders. J Exp Med. (2022) 219(3):e20211520. doi: 10.1084/jem.20211520
182. Bergqvist P, Gardby E, Stensson A, Bemark M, and Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. (2006) 177:7772–83. doi: 10.4049/jimmunol.177.11.7772
183. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. (2015) 350:830–4. doi: 10.1126/science.aad0135
184. Spadoni I, Fornasa G, and Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. (2017) 17:761–73. doi: 10.1038/nri.2017.100
185. Giuffre M, Moretti R, Campisciano G, da Silveira ABM, Monda VM, Comar M, et al. You talking to me? Says the enteric nervous system (ENS) to the microbe. How intestinal microbes interact with the ENS. J Clin Med. (2020) 9(11):3705. doi: 10.3390/jcm9113705
186. Marklund U. Diversity, development and immunoregulation of enteric neurons. Nat Rev Gastroenterol Hepatol. (2022) 19:85–6. doi: 10.1038/s41575-021-00553-y
187. Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, and Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. (2018) 39:677–96. doi: 10.1016/j.it.2018.04.002
188. Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. (2020) 583:441–6. doi: 10.1038/s41586-020-2474-7
189. Rosenberg HJ and Rao M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience. (2021) 24:102863. doi: 10.1016/j.isci.2021.102863
190. Ahmadzai MM, Seguella L, and Gulbransen BD. Circuit-specific enteric glia regulate intestinal motor neurocircuits. Proc Natl Acad Sci U.S.A. (2021) 118(40):e2025938118. doi: 10.1073/pnas.2025938118
191. Vulchanova L, Casey MA, Crabb GW, Kennedy WR, and Brown DR. Anatomical evidence for enteric neuroimmune interactions in Peyer’s patches. J Neuroimmunol. (2007) 185:64–74. doi: 10.1016/j.jneuroim.2007.01.014
192. Sanders KM, Kito Y, Hwang SJ, and Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiol (Bethesda). (2016) 31:316–26. doi: 10.1152/physiol.00006.2016
193. Popescu LM and Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. (2010) 14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x
194. Yip JLK, Balasuriya GK, Spencer SJ, and Hill-Yardin EL. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol Gastroenterol Hepatol. (2021) 12:1701–18. doi: 10.1016/j.jcmgh.2021.08.021
195. Ji S, Traini C, Mischopoulou M, Gibbons SJ, Ligresti G, Faussone-Pellegrini MS, et al. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRalpha-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil. (2021) 33:e13993. doi: 10.1111/nmo.13993
196. Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, and Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. (2018) 67:827–36. doi: 10.1136/gutjnl-2016-312940
197. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. (2014) 63:938–48. doi: 10.1136/gutjnl-2013-304676
198. McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AL, and Foster KR. Host selection of microbiota via differential adhesion. Cell Host Microbe. (2016) 19:550–9. doi: 10.1016/j.chom.2016.02.021
199. Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. (2008) 320:1647–51. doi: 10.1126/science.1155725
200. Rawls JF, Mahowald MA, Ley RE, and Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. (2006) 127:423–33. doi: 10.1016/j.cell.2006.08.043
201. An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. (2007) 204:1417–29. doi: 10.1084/jem.20061929
202. Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. (2011) 121:1657–66. doi: 10.1172/JCI45538
203. Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflammation Bowel Dis. (2011) 17:2299–307. doi: 10.1002/ibd.21625
204. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. (2002) 295:1726–9. doi: 10.1126/science.1069094
205. Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. (1995) 3:149–54. doi: 10.1016/s0966-842x(00)88906-4
206. Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Luciano R, et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci U S A. (2019) 116:15140–9. doi: 10.1073/pnas.1814558116
207. DeMeo MT, Mutlu EA, Keshavarzian A, and Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. (2002) 34:385–96. doi: 10.1097/00004836-200204000-00003
208. Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, and Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. (2007) 22:464–71. doi: 10.1111/j.1440-1746.2007.04933.x
209. Slyepchenko A, Maes M, MaChado-Vieira R, Anderson G, Solmi M, Sanz Y, et al. Intestinal dysbiosis, gut hyperpermeability and bacterial translocation: missing links between depression, obesity and type 2 diabetes. Curr Pharm Des. (2016) 22:6087–106. doi: 10.2174/1381612822666160922165706
210. Wells CL, van de Westerlo EM, Jechorek RP, and Erlandsen SL. Exposure of the lateral enterocyte membrane by dissociation of calcium-dependent junctional complex augments endocytosis of enteric bacteria. Shock. (1995) 4:204–10. doi: 10.1097/00024382-199509000-00009
211. Joly Condette C, Khorsi-Cauet H, Morliere P, Zabijak L, Reygner J, Bach V, et al. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PloS One. (2014) 9:e102217. doi: 10.1371/journal.pone.0102217
212. Goto Y and Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol Rev. (2012) 245:147–63. doi: 10.1111/j.1600-065X.2011.01078.x
213. Regoli M, Man A, Gicheva N, Dumont A, Ivory K, Pacini A, et al. Morphological and functional characterization of IL-12Rbeta2 chain on intestinal epithelial cells: implications for local and systemic immunoregulation. Front Immunol. (2018) 9:1177. doi: 10.3389/fimmu.2018.01177
214. Ivanov II and Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. (2011) 14:106–14. doi: 10.1016/j.mib.2010.12.003
215. Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. (2008) 295:G1025–34. doi: 10.1152/ajpgi.90227.2008
216. Zaki MH, Lamkanfi M, and Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. (2011) 32:171–9. doi: 10.1016/j.it.2011.02.002
217. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. (2011) 145:745–57. doi: 10.1016/j.cell.2011.04.022
218. Elinav E, Thaiss CA, and Flavell RA. Analysis of microbiota alterations in inflammasome-deficient mice. Methods Mol Biol. (2013) 1040:185–94. doi: 10.1007/978-1-62703-523-1_14
219. Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. (2014) 156:1045–59. doi: 10.1016/j.cell.2014.01.026
220. Levy M, Shapiro H, Thaiss CA, and Elinav E. NLRP6: A multifaceted innate immune sensor. Trends Immunol. (2017) 38:248–60. doi: 10.1016/j.it.2017.01.001
221. Kawakami N and Wekerle H. Life history of a brain autoreactive T cell: From thymus through intestine to blood-brain barrier and brain lesion. Neurotherapeutics. (2024) 21:e00442. doi: 10.1016/j.neurot.2024.e00442
222. Hepworth MR, Greenhalgh AD, and Cook PC. B cells on the brain: meningeal IgA and a novel gut-brain firewall. Immunol Cell Biol. (2021) 99:17–20. doi: 10.1111/imcb.12412
223. Rojas OL, Probstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, et al. Recirculating intestinal igA-producing cells regulate neuroinflammation via IL-10. Cell. (2019) 177:492–3. doi: 10.1016/j.cell.2019.03.037
224. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. (2011) 334:255–8. doi: 10.1126/science.1209791
225. Gallo RL and Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. (2012) 12:503–16. doi: 10.1038/nri3228
226. Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, et al. BALB/c and C57BL/6 mice differ in polyreactive igA abundance, which impacts the generation of antigen-specific igA and microbiota diversity. Immunity. (2015) 43:527–40. doi: 10.1016/j.immuni.2015.08.011
227. Kim M, Qie Y, Park J, and Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. (2016) 20:202–14. doi: 10.1016/j.chom.2016.07.001
228. Ercolano G, Wyss T, Salome B, Romero P, Trabanelli S, and Jandus C. Distinct and shared gene expression for human innate versus adaptive helper lymphoid cells. J Leukoc Biol. (2020) 108:723–37. doi: 10.1002/JLB.5MA0120-209R
229. Domingues RG and Hepworth MR. Immunoregulatory sensory circuits in group 3 innate lymphoid cell (ILC3) function and tissue homeostasis. Front Immunol. (2020) 11:116. doi: 10.3389/fimmu.2020.00116
230. Kim CH, Hashimoto-Hill S, and Kim M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. (2016) 37:68–79. doi: 10.1016/j.it.2015.11.003
231. Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. (2011) 334:1561–5. doi: 10.1126/science.1214914
232. Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. (2013) 498:113–7. doi: 10.1038/nature12240
233. Klose CSN and Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. (2020) 30:475–91. doi: 10.1038/s41422-020-0323-8
234. Zhou L, Chu C, Teng F, Bessman NJ, Goc J, Santosa EK, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. (2019) 568:405–9. doi: 10.1038/s41586-019-1082-x
235. Nakagawa T, Mori N, Kajiwara C, Kimura S, Akasaka Y, Ishii Y, et al. Endogenous IL-17 as a factor determining the severity of Clostridium difficile infection in mice. J Med Microbiol. (2016) 65:821–7. doi: 10.1099/jmm.0.000273
236. Gladiator A, Wangler N, Trautwein-Weidner K, and LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. (2013) 190:521–5. doi: 10.4049/jimmunol.1202924
237. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. (2006) 203:2271–9. doi: 10.1084/jem.20061308
238. Deng T, Suo C, Chang J, Yang R, Li J, Cai T, et al. ILC3-derived OX40L is essential for homeostasis of intestinal Tregs in immunodeficient mice. Cell Mol Immunol. (2020) 17:163–77. doi: 10.1038/s41423-019-0200-x
239. Ibiza S, Garcia-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. (2016) 535:440–3. doi: 10.1038/nature18644
240. Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. (2012) 36:92–104. doi: 10.1016/j.immuni.2011.11.011
241. Fachi JL, Secca C, Rodrigues PB, Mato FCP, Di Luccia B, Felipe JS, et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J Exp Med. (2020) 217(3):jem.20190489. doi: 10.1084/jem.20190489
242. Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. (2020) 11:4457. doi: 10.1038/s41467-020-18262-6
243. Sadeghi Hassanabadi N, Broux B, Marinovic S, and Gotthardt D. Innate lymphoid cells - neglected players in multiple sclerosis. Front Immunol. (2022) 13:909275. doi: 10.3389/fimmu.2022.909275
244. Lange LS and Shiner M. Small-bowel abnormalities in multiple sclerosis. Lancet. (1976) 2:1319–22. doi: 10.1016/s0140-6736(76)91972-3
245. Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. (2017) 23:442–6. doi: 10.1177/1352458516652498
246. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. (2011) 91:151–75. doi: 10.1152/physrev.00003.2008
247. Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, et al. Oral administration of the probiotic strain escherichia coli nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol. (2017) 8:1096. doi: 10.3389/fimmu.2017.01096
248. Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. (2016) 7:e196. doi: 10.1038/ctg.2016.54
249. Ghareghani M, Reiter RJ, Zibara K, and Farhadi N. Latitude, vitamin D, melatonin, and gut microbiota act in concert to initiate multiple sclerosis: A new mechanistic pathway. Front Immunol. (2018) 9:2484. doi: 10.3389/fimmu.2018.02484
250. Pelfrey CM, Cotleur AC, Lee JC, and Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J Neuroimmunol. (2002) 130:211–23. doi: 10.1016/s0165-5728(02)00224-2
251. Kalincik T, Vivek V, Jokubaitis V, Lechner-Scott J, Trojano M, Izquierdo G, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. (2013) 136:3609–17. doi: 10.1093/brain/awt281
252. Voskuhl RR and Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. (2012) 8:255–63. doi: 10.1038/nrneurol.2012.43
253. Du S, Itoh N, Askarinam S, Hill H, Arnold AP, and Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. (2014) 111:2806–11. doi: 10.1073/pnas.1307091111
254. Kim RY, Mangu D, Hoffman AS, Kavosh R, Jung E, Itoh N, et al. Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis. Brain. (2018) 141:132–47. doi: 10.1093/brain/awx315
255. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. (2013) 339:1084–8. doi: 10.1126/science.1233521
256. Baker JM, Al-Nakkash L, and Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
257. Escorcia Mora P, Valbuena D, and Diez-Juan A. The role of the gut microbiota in female reproductive and gynecological health: insights into endometrial signaling pathways. Life (Basel). (2025) 15(5):762. doi: 10.3390/life15050762
258. Mirzaei R, Kavyani B, Nabizadeh E, Kadkhoda H, Asghari Ozma M, and Abdi M. Microbiota metabolites in the female reproductive system: Focused on the short-chain fatty acids. Heliyon. (2023) 9:e14562. doi: 10.1016/j.heliyon.2023.e14562
259. Hu S, Ding Q, Zhang W, Kang M, Ma J, and Zhao L. Gut microbial beta-glucuronidase: a vital regulator in female estrogen metabolism. Gut Microbes. (2023) 15:2236749. doi: 10.1080/19490976.2023.2236749
260. Jiang Y, Greenwood-Van Meerveld B, Johnson AC, and Travagli RA. Role of estrogen and stress on the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. (2019) 317:G203–G9. doi: 10.1152/ajpgi.00144.2019
261. Li B, Xiong Y, Guo D, Deng G, and Wu H. The gut-reproductive axis: Bridging microbiota balances to reproductive health and fetal development. Int Immunopharmacol. (2025) 144:113627. doi: 10.1016/j.intimp.2024.113627
262. Qi X, Yun C, Pang Y, and Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. (2021) 13:1–21. doi: 10.1080/19490976.2021.1894070
263. Smith SB and Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. (2017) 595:451–63. doi: 10.1113/JP271694
264. Boukari H, Brichacek B, Stratton P, Mahoney SF, Lifson JD, Margolis L, et al. Movements of HIV-virions in human cervical mucus. Biomacromolecules. (2009) 10:2482–8. doi: 10.1021/bm900344q
265. Dong M, Dong Y, Bai J, Li H, Ma X, Li B, et al. Interactions between microbiota and cervical epithelial, immune, and mucus barrier. Front Cell Infect Microbiol. (2023) 13:1124591. doi: 10.3389/fcimb.2023.1124591
266. Ebrahimi F, Omidvar-Mehrabadi A, Shahbazi M, and Mohammadnia-Afrouzi M. Innate and adaptive immune dysregulation in women with recurrent implantation failure. J Reprod Immunol. (2024) 164:104262. doi: 10.1016/j.jri.2024.104262
267. Macfarlane S and Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. (2003) 62:67–72. doi: 10.1079/PNS2002207
268. Koppel N, Maini Rekdal V, and Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. (2017) 356(6344):eaag2770. doi: 10.1126/science.aag2770
269. Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. (2000) 66:1654–61. doi: 10.1128/AEM.66.4.1654-1661.2000
270. Park J, Wang Q, Wu Q, Mao-Draayer Y, and Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep. (2019) 9:8837. doi: 10.1038/s41598-019-45311-y
271. Olsson A, Gustavsen S, Nguyen TD, Nyman M, Langkilde AR, Hansen TH, et al. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol. (2021) 12:661493. doi: 10.3389/fimmu.2021.661493
272. Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. (2020) 11:1390. doi: 10.3389/fimmu.2020.01390
273. Takewaki D, Suda W, Sato W, Takayasu L, Kumar N, Kimura K, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci U S A. (2020) 117:22402–12. doi: 10.1073/pnas.2011703117
274. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. (2003) 278:11312–9. doi: 10.1074/jbc.M211609200
275. Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, et al. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. (2018) 6:55. doi: 10.1186/s40168-018-0439-y
276. Cole GM, Ma QL, and Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. (2010) 68 Suppl 2:S102–11. doi: 10.1111/j.1753-4887.2010.00345.x
277. Ho L, Ono K, Tsuji M, Mazzola P, Singh R, and Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. (2018) 18:83–90. doi: 10.1080/14737175.2018.1400909
278. Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease beta-amyloid oligomerization. Mol Nutr Food Res. (2015) 59:1025–40. doi: 10.1002/mnfr.201400544
279. Knox EG, Aburto MR, Tessier C, Nagpal J, Clarke G, O’Driscoll CM, et al. Microbial-derived metabolites induce actin cytoskeletal rearrangement and protect blood-brain barrier function. iScience. (2022) 25:105648. doi: 10.1016/j.isci.2022.105648
280. Xie J, Bruggeman A, De Nolf C, Vandendriessche C, Van Imschoot G, Van Wonterghem E, et al. Gut microbiota regulates blood-cerebrospinal fluid barrier function and Abeta pathology. EMBO J. (2023) 42:e111515. doi: 10.15252/embj.2022111515
281. Schonfeld P and Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. (2016) 57:943–54. doi: 10.1194/jlr.R067629
282. Rose S, Bennuri SC, Davis JE, Wynne R, Slattery JC, Tippett M, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry. (2018) 8:42. doi: 10.1038/s41398-017-0089-z
283. Kiela PR and Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. (2016) 30:145–59. doi: 10.1016/j.bpg.2016.02.007
284. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, and Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. (2012) 48:612–26. doi: 10.1016/j.molcel.2012.08.033
285. Sivaprakasam S, Gurav A, Paschall AV, Coe GL, Chaudhary K, Cai Y, et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. (2016) 5:e238. doi: 10.1038/oncsis.2016.38
286. D’Souza WN, Douangpanya J, Mu S, Jaeckel P, Zhang M, Maxwell JR, et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PloS One. (2017) 12:e0180190. doi: 10.1371/journal.pone.0180190
287. Wan Saudi WS and Sjoblom M. Short-chain fatty acids augment rat duodenal mucosal barrier function. Exp Physiol. (2017) 102:791–803. doi: 10.1113/EP086110
288. Kellow NJ, Coughlan MT, and Reid CM. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr. (2014) 111:1147–61. doi: 10.1017/S0007114513003607
289. Collins SL, Stine JG, Bisanz JE, Okafor CD, and Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. (2023) 21:236–47. doi: 10.1038/s41579-022-00805-x
290. Staley C, Weingarden AR, Khoruts A, and Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. (2017) 101:47–64. doi: 10.1007/s00253-016-8006-6
291. Wahlstrom A, Sayin SI, Marschall HU, and Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
292. Stenman LK, Holma R, Eggert A, and Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol. (2013) 304:G227–34. doi: 10.1152/ajpgi.00267.2012
293. Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G906–13. doi: 10.1152/ajpgi.00043.2007
294. Sarathy J, Detloff SJ, Ao M, Khan N, French S, Sirajuddin H, et al. The Yin and Yang of bile acid action on tight junctions in a model colonic epithelium. Physiol Rep. (2017) 5:e13294. doi: 10.14814/phy2.13294
295. Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, and DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. (2014) 46:527–34. doi: 10.1016/j.dld.2014.01.159
296. Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. (2015) 163:1428–43. doi: 10.1016/j.cell.2015.10.048
297. Fiorucci S, Biagioli M, Zampella A, and Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. (2018) 9:1853. doi: 10.3389/fimmu.2018.01853
298. Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PloS One. (2011) 6:e25637. doi: 10.1371/journal.pone.0025637
299. Yang M, Gu Y, Li L, Liu T, Song X, Sun Y, et al. Bile acid-gut microbiota axis in inflammatory bowel disease: from bench to bedside. Nutrients. (2021) 13(9):3143. doi: 10.3390/nu13093143
300. Chavez-Talavera O, Tailleux A, Lefebvre P, and Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. (2017) 152:1679–94 e3. doi: 10.1053/j.gastro.2017.01.055
301. Jia W, Xie G, and Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. (2018) 15:111–28. doi: 10.1038/nrgastro.2017.119
302. Jia ET, Liu ZY, Pan M, Lu JF, and Ge QY. Regulation of bile acid metabolism-related signaling pathways by gut microbiota in diseases. J Zhejiang Univ Sci B. (2019) 20:781–92. doi: 10.1631/jzus.B1900073
303. Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, and Bjorklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. (2019) 59:72–88. doi: 10.1080/10408398.2017.1357534
304. Mu C, Yang Y, Yu K, Yu M, Zhang C, Su Y, et al. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids. (2017) 49:771–81. doi: 10.1007/s00726-017-2379-4
305. Yu M, Mu C, Yang Y, Zhang C, Su Y, Huang Z, et al. Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets. Amino Acids. (2017) 49:1587–99. doi: 10.1007/s00726-017-2451-0
306. Le Floc’h N, Otten W, and Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. (2011) 41:1195–205. doi: 10.1007/s00726-010-0752-7
307. Agus A, Planchais J, and Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
308. Yang F, Wu SC, Ling ZX, Chao S, Zhang LJ, Yan XM, et al. Altered plasma metabolic profiles in chinese patients with multiple sclerosis. Front Immunol. (2021) 12:792711. doi: 10.3389/fimmu.2021.792711
309. Fitzgerald KC, Smith MD, Kim S, Sotirchos ES, Kornberg MD, Douglas M, et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep Med. (2021) 2:100424. doi: 10.1016/j.xcrm.2021.100424
310. Gaetani L, Boscaro F, Pieraccini G, Calabresi P, Romani L, Di Filippo M, et al. Host and microbial tryptophan metabolic profiling in multiple sclerosis. Front Immunol. (2020) 11:157. doi: 10.3389/fimmu.2020.00157
311. Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun. (2019) 10:4877. doi: 10.1038/s41467-019-12776-4
312. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
313. Rothhammer V, Borucki DM, Kenison JE, Hewson P, Wang Z, Bakshi R, et al. Detection of aryl hydrocarbon receptor agonists in human samples. Sci Rep. (2018) 8:4970. doi: 10.1038/s41598-018-23323-4
314. Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. (2020) 586:281–6. doi: 10.1038/s41586-020-2745-3
315. Smith AM, King JJ, West PR, Ludwig MA, Donley ELR, Burrier RE, et al. Amino acid dysregulation metabotypes: potential biomarkers for diagnosis and individualized treatment for subtypes of autism spectrum disorder. Biol Psychiatry. (2019) 85:345–54. doi: 10.1016/j.biopsych.2018.08.016
316. Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, et al. Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. (2021) 9:235. doi: 10.1186/s40168-021-01181-z
317. Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. (2017) 10:1133–44. doi: 10.1038/mi.2016.133
318. Louis P, Young P, Holtrop G, and Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. (2010) 12:304–14. doi: 10.1111/j.1462-2920.2009.02066.x
319. Kim SJ and Kim HM. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Rep. (2017) 50:55–7. doi: 10.5483/bmbrep.2017.50.2.011
320. Bian Y, Dong Y, Sun J, Sun M, Hou Q, Lai Y, et al. Protective effect of kaempferol on LPS-induced inflammation and barrier dysfunction in a coculture model of intestinal epithelial cells and intestinal microvascular endothelial cells. J Agric Food Chem. (2020) 68:160–7. doi: 10.1021/acs.jafc.9b06294
321. Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. (2016) 165:842–53. doi: 10.1016/j.cell.2016.04.007
322. Harry GJ. Microglia during development and aging. Pharmacol Ther. (2013) 139:313–26. doi: 10.1016/j.pharmthera.2013.04.013
323. Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, et al. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. (2011) 286:43855–70. doi: 10.1074/jbc.M111.299248
324. Lee S, Lee WH, Lee MS, Mori K, and Suk K. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res. (2012) 90:540–50. doi: 10.1002/jnr.22779
325. Xie J, Haesebrouck F, Van Hoecke L, and Vandenbroucke RE. Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends Microbiol. (2023) 31:1206–24. doi: 10.1016/j.tim.2023.05.010
326. Haas-Neill S and Forsythe P. A budding relationship: bacterial extracellular vesicles in the microbiota-gut-brain axis. Int J Mol Sci. (2020) 21(23):8899. doi: 10.3390/ijms21238899
327. Liang X, Dai N, Sheng K, Lu H, Wang J, Chen L, et al. Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes. (2022) 14:2134689. doi: 10.1080/19490976.2022.2134689
328. Martin CR, Osadchiy V, Kalani A, and Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. (2018) 6:133–48. doi: 10.1016/j.jcmgh.2018.04.003
329. Aloisi F, Giovannoni G, and Salvetti M. Epstein-Barr virus as a cause of multiple sclerosis: opportunities for prevention and therapy. Lancet Neurol. (2023) 22:338–49. doi: 10.1016/S1474-4422(22)00471-9
330. Eslami M, Adampour Z, Fadaee Dowlat B, Yaghmayee S, Motallebi Tabaei F, Oksenych V, et al. A novel frontier in gut-brain axis research: the transplantation of fecal microbiota in neurodegenerative disorders. Biomedicines. (2025) 13(4):915. doi: 10.3390/biomedicines13040915
331. Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A, et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. (2008) 131:1712–21. doi: 10.1093/brain/awn108
332. Farrell RA, Antony D, Wall GR, Clark DA, Fisniku L, Swanton J, et al. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology. (2009) 73:32–8. doi: 10.1212/WNL.0b013e3181aa29fe
333. Ascherio A, Munger KL, and Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. (2012) 8:602–12. doi: 10.1038/nrneurol.2012.198
334. Waubant E, Mowry EM, Krupp L, Chitnis T, Yeh EA, Kuntz N, et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology. (2011) 76:1989–95. doi: 10.1212/WNL.0b013e31821e552a
335. Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. (2022) 603:321–7. doi: 10.1038/s41586-022-04432-7
336. Tengvall K, Huang J, Hellstrom C, Kammer P, Bistrom M, Ayoglu B, et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc Natl Acad Sci U S A. (2019) 116:16955–60. doi: 10.1073/pnas.1902623116
337. Lindsey JW. Antibodies to the Epstein-Barr virus proteins BFRF3 and BRRF2 cross-react with human proteins. J Neuroimmunol. (2017) 310:131–4. doi: 10.1016/j.jneuroim.2017.07.013
338. Wang Z, Kennedy PG, Dupree C, Wang M, Lee C, Pointon T, et al. Antibodies from multiple sclerosis brain identified epstein-barr virus nuclear antigen 1 & 2 epitopes which are recognized by oligoclonal bands. J Neuroimmune Pharmacol. (2021) 16:567–80. doi: 10.1007/s11481-020-09948-1
339. Leffler J, Trend S, Hart PH, and French MA. Epstein-Barr virus infection, B-cell dysfunction and other risk factors converge in gut-associated lymphoid tissue to drive the immunopathogenesis of multiple sclerosis: a hypothesis. Clin Transl Immunol. (2022) 11:e1418. doi: 10.1002/cti2.1418
340. Bjornevik K, Munz C, Cohen JI, and Ascherio A. Epstein-Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat Rev Neurol. (2023) 19:160–71. doi: 10.1038/s41582-023-00775-5
341. van Langelaar J, Wierenga-Wolf AF, Samijn JPA, Luijks CJM, Siepman TA, van Doorn PA, et al. The association of Epstein-Barr virus infection with CXCR3(+) B-cell development in multiple sclerosis: impact of immunotherapies. Eur J Immunol. (2021) 51:626–33. doi: 10.1002/eji.202048739
342. Soldan SS, Su C, Lamontagne RJ, Grams N, Lu F, Zhang Y, et al. Epigenetic plasticity enables CNS-trafficking of EBV-infected B lymphocytes. PloS Pathog. (2021) 17:e1009618. doi: 10.1371/journal.ppat.1009618
343. Wang J, Jelcic I, Muhlenbruch L, Haunerdinger V, Toussaint NC, Zhao Y, et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell. (2020) 183:1264–81 e20. doi: 10.1016/j.cell.2020.09.054
344. He B, Raab-Traub N, Casali P, and Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. (2003) 171:5215–24. doi: 10.4049/jimmunol.171.10.5215
345. Jog NR, McClain MT, Heinlen LD, Gross T, Towner R, Guthridge JM, et al. Epstein Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J Autoimmun. (2020) 106:102332. doi: 10.1016/j.jaut.2019.102332
346. Choi IK, Wang Z, Ke Q, Hong M, Qian Y, Zhao X, et al. Signaling by the Epstein-Barr virus LMP1 protein induces potent cytotoxic CD4(+) and CD8(+) T cell responses. Proc Natl Acad Sci U S A. (2018) 115:E686–E95. doi: 10.1073/pnas.1713607115
347. Xiao J, Palefsky JM, Herrera R, Berline J, and Tugizov SM. The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology. (2008) 370:430–42. doi: 10.1016/j.virol.2007.09.012
348. Shannon-Lowe C and Rowe M. Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PloS Pathog. (2011) 7:e1001338. doi: 10.1371/journal.ppat.1001338
349. Looi CK, Hii LW, Chung FF, Mai CW, Lim WM, and Leong CO. Roles of inflammasomes in epstein-barr virus-associated nasopharyngeal cancer. Cancers (Basel). (2021) 13(8):1786. doi: 10.3390/cancers13081786
350. Zebardast A, Tehrani SS, Latifi T, and Sadeghi F. Critical review of Epstein-Barr virus microRNAs relation with EBV-associated gastric cancer. J Cell Physiol. (2021) 236:6136–53. doi: 10.1002/jcp.30297
351. Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, et al. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. (2010) 70:4719–27. doi: 10.1158/0008-5472.CAN-09-4620
352. Cardenas-Mondragon MG, Carreon-Talavera R, Camorlinga-Ponce M, Gomez-Delgado A, Torres J, and Fuentes-Panana EM. Epstein Barr virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PloS One. (2013) 8:e62850. doi: 10.1371/journal.pone.0062850
353. Polakovicova I, Jerez S, Wichmann IA, Sandoval-Borquez A, Carrasco-Veliz N, and Corvalan AH. Role of microRNAs and Exosomes in Helicobacter pylori and Epstein-Barr Virus Associated Gastric Cancers. Front Microbiol. (2018) 9:636. doi: 10.3389/fmicb.2018.00636
354. Wang F, Yao Z, Jin T, Mao B, Shao S, and Shao C. Research progress on Helicobacter pylori infection related neurological diseases. Ageing Res Rev. (2024) 99:102399. doi: 10.1016/j.arr.2024.102399
355. Du J, Shen W, Zhou Z, Wu Q, and Ai Z. Association of Helicobacter pylori infection with the risk of neurodegenerative disorders: a systematic review and meta-analysis. Front Med (Lausanne). (2025) 12:1573299. doi: 10.3389/fmed.2025.1573299
356. Kandpal M, Indari O, Baral B, Jakhmola S, Tiwari D, Bhandari V, et al. Dysbiosis of gut microbiota from the perspective of the gut-brain axis: role in the provocation of neurological disorders. Metabolites. (2022) 12(11):1064. doi: 10.3390/metabo12111064
357. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PloS Pathog. (2014) 10:e1003829. doi: 10.1371/journal.ppat.1003829
358. Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. (2014) 7:983–94. doi: 10.1038/mi.2013.116
359. Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. (2021) 70:698–706. doi: 10.1136/gutjnl-2020-323020
360. Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. (2020) 581:465–9. doi: 10.1038/s41586-020-2196-x
361. Carlberg C and Mycko MP. Linking mechanisms of vitamin D signaling with multiple sclerosis. Cells. (2023) 12(19):2391. doi: 10.3390/cells12192391
362. Sintzel MB, Rametta M, and Reder AT. Vitamin D and multiple sclerosis: A comprehensive review. Neurol Ther. (2018) 7:59–85. doi: 10.1007/s40120-017-0086-4
363. Ramagopalan SV, Dyment DA, Cader MZ, Morrison KM, Disanto G, Morahan JM, et al. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol. (2011) 70:881–6. doi: 10.1002/ana.22678
364. Dumont D, Noben JP, Raus J, Stinissen P, and Robben J. Proteomic analysis of cerebrospinal fluid from multiple sclerosis patients. Proteomics. (2004) 4:2117–24. doi: 10.1002/pmic.200300715
365. Galoppin M, Kari S, Soldati S, Pal A, Rival M, Engelhardt B, et al. Full spectrum of vitamin D immunomodulation in multiple sclerosis: mechanisms and therapeutic implications. Brain Commun. (2022) 4:fcac171. doi: 10.1093/braincomms/fcac171
366. Dehghani L, Meamar R, Etemadifar M, Sheshde ZD, Shaygannejad V, Sharifkhah M, et al. Can vitamin d suppress endothelial cells apoptosis in multiple sclerosis patients? Int J Prev Med. (2013) 4:S211–5.
367. Sadeghian N, Shadman J, Moradi A, Ghasem Golmohammadi M, and Panahpour H. Calcitriol protects the Blood-Brain Barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res Bull. (2019) 150:281–9. doi: 10.1016/j.brainresbull.2019.06.010
368. Najafipoor A, Roghanian R, Zarkesh-Esfahani SH, Bouzari M, and Etemadifar M. The beneficial effects of vitamin D3 on reducing antibody titers against Epstein-Barr virus in multiple sclerosis patients. Cell Immunol. (2015) 294:9–12. doi: 10.1016/j.cellimm.2015.01.009
369. Rolf L, Muris AH, Mathias A, Du Pasquier R, Koneczny I, Disanto G, et al. Exploring the effect of vitamin D(3) supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult Scler. (2018) 24:1280–7. doi: 10.1177/1352458517722646
370. Zhu W, Yan J, Zhi C, Zhou Q, and Yuan X. 1,25(OH)(2)D(3) deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. (2019) 11:8. doi: 10.1186/s13099-019-0291-z
371. Aggeletopoulou I, Tsounis EP, Mouzaki A, and Triantos C. Exploring the role of vitamin D and the vitamin D receptor in the composition of the gut microbiota. Front Biosci (Landmark Ed). (2023) 28:116. doi: 10.31083/j.fbl2806116
372. Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. (2015) 63:729–34. doi: 10.1097/JIM.0000000000000192
373. Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, et al. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. (2019) 58:2895–910. doi: 10.1007/s00394-018-1842-7
374. Wang J, Mei L, Hao Y, Xu Y, Yang Q, Dai Z, et al. Contemporary perspectives on the role of vitamin D in enhancing gut health and its implications for preventing and managing intestinal diseases. Nutrients. (2024) 16(19):2391. doi: 10.3390/nu16142352
375. Sun J and Zhang YG. Vitamin D receptor influences intestinal barriers in health and disease. Cells. (2022) 11(7):1129. doi: 10.3390/cells11071129
376. Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. (2016) 12:1057–8. doi: 10.1080/15548627.2015.1072670
377. Carabotti M, Scirocco A, Maselli MA, and Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
378. Wachsmuth HR, Weninger SN, and Duca FA. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. (2022) 54:377–92. doi: 10.1038/s12276-021-00677-w
379. Foster JA, Baker GB, and Dursun SM. The relationship between the gut microbiome-immune system-brain axis and major depressive disorder. Front Neurol. (2021) 12:721126. doi: 10.3389/fneur.2021.721126
380. Rutsch A, Kantsjo JB, and Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. (2020) 11:604179. doi: 10.3389/fimmu.2020.604179
381. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. (2011) 12:453–66. doi: 10.1038/nrn3071
382. Rhee SH, Pothoulakis C, and Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
383. Cryan JF and Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
384. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. (2016) 167:1469–80 e12. doi: 10.1016/j.cell.2016.11.018
385. Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A. (2008) 105:2193–7. doi: 10.1073/pnas.0711891105
386. Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. (2014) 5:4432. doi: 10.1038/ncomms5432
387. Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. (2016) 36:7428–40. doi: 10.1523/JNEUROSCI.1114-16.2016
388. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
389. Trend S, Leffler J, Jones AP, Cha L, Gorman S, Brown DA, et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci Rep. (2021) 11:5244. doi: 10.1038/s41598-021-84881-8
390. Perez-Perez S, Dominguez-Mozo MI, Alonso-Gomez A, Medina S, Villarrubia N, Fernandez-Velasco JI, et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ. (2020) 8:e10220. doi: 10.7717/peerj.10220
391. Dominguez-Mozo MI, Perez-Perez S, Villarrubia N, Costa-Frossard L, Fernandez-Velasco JI, Ortega-Madueno I, et al. Herpesvirus antibodies, vitamin D and short-chain fatty acids: their correlation with cell subsets in multiple sclerosis patients and healthy controls. Cells. (2021) 10(1):119. doi: 10.3390/cells10010119
392. Cuello JP, Martinez Gines ML, Garcia Dominguez JM, Tejeda-Velarde A, Lozano Ros A, Higueras Y, et al. Short-chain fatty acids during pregnancy in multiple sclerosis: A prospective cohort study. Eur J Neurol. (2022) 29:895–900. doi: 10.1111/ene.15150
393. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. (2008) 105:16767–72. doi: 10.1073/pnas.0808567105
394. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. (2009) 106:3698–703. doi: 10.1073/pnas.0812874106
395. Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. (2015) 43:817–29. doi: 10.1016/j.immuni.2015.09.007
396. Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, and Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. (2012) 113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x
397. Minuk GY. Gamma-aminobutyric acid (GABA) production by eight common bacterial pathogens. Scand J Infect Dis. (1986) 18:465–7. doi: 10.3109/00365548609032366
398. Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. (2012) 303:G1288–95. doi: 10.1152/ajpgi.00341.2012
399. Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. (2009) 90:1236–43. doi: 10.3945/ajcn.2009.28095
400. Hsuchou H, Pan W, and Kastin AJ. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS. (2013) 10:32. doi: 10.1186/2045-8118-10-32
401. Marcelin G, Jo YH, Li X, Schwartz GJ, Zhang Y, Dun NJ, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. (2014) 3:19–28. doi: 10.1016/j.molmet.2013.10.002
402. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. (2002) 143:1741–7. doi: 10.1210/endo.143.5.8850
403. Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. (2004) 145:2594–603. doi: 10.1210/en.2003-1671
404. Perry RJ, Lee S, Ma L, Zhang D, Schlessinger J, and Shulman GI. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun. (2015) 6:6980. doi: 10.1038/ncomms7980
405. Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, and Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. (2006) 8:1–27. doi: 10.1017/S1462399406000068
406. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. (2016) 22:516–23. doi: 10.1038/nm.4068
407. Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. (2016) 47:1354–63. doi: 10.1161/STROKEAHA.115.011800
408. Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
409. Sanmarco LM, Wheeler MA, Gutierrez-Vazquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, et al. Gut-licensed IFNgamma(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. (2021) 590:473–9. doi: 10.1038/s41586-020-03116-4
410. de Lartigue G, de la Serre CB, and Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. (2011) 105:100–5. doi: 10.1016/j.physbeh.2011.02.040
411. Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, and Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun. (2013) 4:1465. doi: 10.1038/ncomms2478
412. Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. (2011) 334:98–101. doi: 10.1126/science.1209985
413. Goverse G, Stakenborg M, and Matteoli G. The intestinal cholinergic anti-inflammatory pathway. J Physiol. (2016) 594:5771–80. doi: 10.1113/JP271537
414. Bonaz B, Bazin T, and Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. (2018) 12:49. doi: 10.3389/fnins.2018.00049
415. Straub RH, Wiest R, Strauch UG, Harle P, and Scholmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut. (2006) 55:1640–9. doi: 10.1136/gut.2006.091322
416. Levinthal DJ, Rahman A, Nusrat S, O’Leary M, Heyman R, and Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int. (2013) 2013:319201. doi: 10.1155/2013/319201
417. Blackburn KM, Kubiliun M, Harris S, and Vernino S. Neurological autoimmune disorders with prominent gastrointestinal manifestations: A review of presentation, evaluation, and treatment. Neurogastroenterol Motil. (2019) 31:e13611. doi: 10.1111/nmo.13611
418. Ahmed K and Lal Y. Unusual case of gastroparesis leading to severe gastromegaly with demyelinating disease as a rare cause. S D Med. (2013) 66:467–9.
419. Preziosi G, Raptis DA, Raeburn A, Panicker J, and Emmanuel A. Autonomic rectal dysfunction in patients with multiple sclerosis and bowel symptoms is secondary to spinal cord disease. Dis Colon Rectum. (2014) 57:514–21. doi: 10.1097/DCR.0000000000000048
420. Chiaro G, Fratila C, Martig F, Zecca C, and Gobbi C. Relapsing paralytic ileus in multiple sclerosis requiring surgery: a video case report. Clin Auton Res. (2019) 29:349–51. doi: 10.1007/s10286-018-0573-4
421. Saad RJ, Rao SS, Koch KL, Kuo B, Parkman HP, McCallum RW, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. (2010) 105:403–11. doi: 10.1038/ajg.2009.612
422. Tottey W, Feria-Gervasio D, Gaci N, Laillet B, Pujos E, Martin JF, et al. Colonic transit time is a driven force of the gut microbiota composition and metabolism: in vitro evidence. J Neurogastroenterol Motil. (2017) 23:124–34. doi: 10.5056/jnm16042
423. Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. (2002) 123:1099–108. doi: 10.1053/gast.2002.36019
424. Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. (1993) 137:343–5. doi: 10.1677/joe.0.1370343
425. Mayer EA, Savidge T, and Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. (2014) 146:1500–12. doi: 10.1053/j.gastro.2014.02.037
426. Clarke MB, Hughes DT, Zhu C, Boedeker EC, and Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. (2006) 103:10420–5. doi: 10.1073/pnas.0604343103
427. Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, et al. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. (2000) 232:480–9. doi: 10.1097/00000658-200010000-00003
428. Gunterberg V, Simren M, Ohman L, Friberg P, Jones MP, Van Oudenhove L, et al. Autonomic nervous system function predicts the inflammatory response over three years in newly diagnosed ulcerative colitis patients. Neurogastroenterol Motil. (2016) 28:1655–62. doi: 10.1111/nmo.12865
429. Stein RR, Tanoue T, Szabady RL, Bhattarai SK, Olle B, Norman JM, et al. Computer-guided design of optimal microbial consortia for immune system modulation. Elife. (2018) 7:e30916. doi: 10.7554/eLife.30916
430. Newbould RD, Nicholas R, Thomas CL, Quest R, Lee JS, Honeyfield L, et al. Age independently affects myelin integrity as detected by magnetization transfer magnetic resonance imaging in multiple sclerosis. NeuroImage Clin. (2014) 4:641–8. doi: 10.1016/j.nicl.2014.02.004
431. Neto LO, Ruiz JA, and Gromisch ES. Perceived health- related quality of life in persons with multiple sclerosis with and without a vascular comorbidity. Qual Life Res. (2024) 33:573–81. doi: 10.1007/s11136-023-03546-3
432. Golomb SM, Guldner IH, Zhao A, Wang Q, Palakurthi B, Aleksandrovic EA, et al. Multi-modal single-cell analysis reveals brain immune landscape plasticity during aging and gut microbiota dysbiosis. Cell Rep. (2020) 33:108438. doi: 10.1016/j.celrep.2020.108438
433. Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. (2018) 48:380–95 e6. doi: 10.1016/j.immuni.2018.01.011
434. Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. (2021) 373(6553):eabf9277. doi: 10.1126/science.abf9277
435. Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. (2015) 77:607–15. doi: 10.1016/j.biopsych.2014.07.012
436. Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, and Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. (2014) 9:2124–38. doi: 10.1016/j.celrep.2014.11.018
437. Heiss CN, Manneras-Holm L, Lee YS, Serrano-Lobo J, Hakansson Gladh A, Seeley RJ, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. (2021) 35:109163. doi: 10.1016/j.celrep.2021.109163
438. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. (2016) 6:28484. doi: 10.1038/srep28484
439. Kadowaki A and Quintana FJ. The gut-CNS axis in multiple sclerosis. Trends Neurosci. (2020) 43:622–34. doi: 10.1016/j.tins.2020.06.002
440. Benson CA, Wong G, Tenorio G, Baker GB, and Kerr BJ. The MAO inhibitor phenelzine can improve functional outcomes in mice with established clinical signs in experimental autoimmune encephalomyelitis (EAE). Behav Brain Res. (2013) 252:302–11. doi: 10.1016/j.bbr.2013.06.019
441. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
442. Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. (2018) 83:1147–61. doi: 10.1002/ana.25244
443. Klingensmith NJ and Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. (2016) 32:203–12. doi: 10.1016/j.ccc.2015.11.004
444. Khailova L, Petrie B, Baird CH, Dominguez Rieg JA, and Wischmeyer PE. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PloS One. (2014) 9:e97861. doi: 10.1371/journal.pone.0097861
445. Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS One. (2010) 5:e9009. doi: 10.1371/journal.pone.0009009
446. Ezendam J and van Loveren H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. Br J Nutr. (2008) 99:83–90. doi: 10.1017/S0007114507803412
447. Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, et al. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PloS One. (2011) 6:e27644. doi: 10.1371/journal.pone.0027644
448. Rezende RM, Oliveira RP, Medeiros SR, Gomes-Santos AC, Alves AC, Loli FG, et al. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J Autoimmun. (2013) 40:45–57. doi: 10.1016/j.jaut.2012.07.012
449. Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. (2017) 3:e1700492. doi: 10.1126/sciadv.1700492
450. Chen HQ, Wang XJ, Jin ZY, Xu XM, Zhao JW, and Xie ZJ. Protective effect of isoflavones from Trifolium pratense on dopaminergic neurons. Neurosci Res. (2008) 62:123–30. doi: 10.1016/j.neures.2008.07.001
451. Ellestad KK, Tsutsui S, Noorbakhsh F, Warren KG, Yong VW, Pittman QJ, et al. Early life exposure to lipopolysaccharide suppresses experimental autoimmune encephalomyelitis by promoting tolerogenic dendritic cells and regulatory T cells. J Immunol. (2009) 183:298–309. doi: 10.4049/jimmunol.0803576
452. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
453. Severance EG, Prandovszky E, Castiglione J, and Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. (2015) 17:27. doi: 10.1007/s11920-015-0574-0
454. Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells. (2021) 10(11):3164. doi: 10.3390/cells10113164
455. Saiyasit N, Chunchai T, Apaijai N, Pratchayasakul W, Sripetchwandee J, Chattipakorn N, et al. Chronic high-fat diet consumption induces an alteration in plasma/brain neurotensin signaling, metabolic disturbance, systemic inflammation/oxidative stress, brain apoptosis, and dendritic spine loss. Neuropeptides. (2020) 82:102047. doi: 10.1016/j.npep.2020.102047
456. Diestel A, Aktas O, Hackel D, Hake I, Meier S, Raine CS, et al. Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J Exp Med. (2003) 198:1729–40. doi: 10.1084/jem.20030975
457. Teunissen CE, Dijkstra CD, Polman CH, Hoogervorst EL, von Bergmann K, and Lutjohann D. Decreased levels of the brain specific 24S-hydroxycholesterol and cholesterol precursors in serum of multiple sclerosis patients. Neurosci Lett. (2003) 347:159–62. doi: 10.1016/s0304-3940(03)00667-0
458. Scheperjans F, Levo R, Bosch B, Laaperi M, Pereira PAB, Smolander OP, et al. Fecal microbiota transplantation for treatment of parkinson disease: A randomized clinical trial. JAMA Neurol. (2024) 81:925–38. doi: 10.1001/jamaneurol.2024.2305
459. Zhang X, Tang B, and Guo J. Parkinson’s disease and gut microbiota: from clinical to mechanistic and therapeutic studies. Transl Neurodegener. (2023) 12:59. doi: 10.1186/s40035-023-00392-8
460. Quraishi MN, Shaheen W, Oo YH, and Iqbal TH. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin Exp Immunol. (2020) 199:24–38. doi: 10.1111/cei.13397
461. Xu D, Ren L, Zhang W, Wu S, Yu M, He X, et al. Therapeutic effects and mechanisms of fecal microbiota transplantation on EAE partly through HPA axis-mediated neuroendocrine regulation. Heliyon. (2024) 10:e33214. doi: 10.1016/j.heliyon.2024.e33214
462. Al KF, Craven LJ, Gibbons S, Parvathy SN, Wing AC, Graf C, et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult Scler J Exp Transl Clin. (2022) 8:20552173221086662. doi: 10.1177/20552173221086662
463. Kociolek LK and Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol. (2016) 13:150–60. doi: 10.1038/nrgastro.2015.220
464. Storoni M and Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. (2015) 2015:681289. doi: 10.1155/2015/681289
465. Wahls TL, Titcomb TJ, Bisht B, Eyck PT, Rubenstein LM, Carr LJ, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin. (2021) 7:20552173211035399. doi: 10.1177/20552173211035399
466. Mousavi-Shirazi-Fard Z, Mazloom Z, Izadi S, and Fararouei M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing-remitting multiple sclerosis patients: a randomized clinical trial. Int J Neurosci. (2021) 131:657–65. doi: 10.1080/00207454.2020.1750398
467. Frank J, Gupta A, Osadchiy V, and Mayer EA. Brain-gut-microbiome interactions and intermittent fasting in obesity. Nutrients. (2021) 13(2):584. doi: 10.3390/nu13020584
468. Luckel C, Picard F, Raifer H, Campos Carrascosa L, Guralnik A, Zhang Y, et al. IL-17(+) CD8(+) T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat Commun. (2019) 10:5722. doi: 10.1038/s41467-019-13731-z
469. Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. (2017) 16:271–81. doi: 10.1016/S1474-4422(17)30007-8
470. Cassotta A, Mikol V, Bertrand T, Pouzieux S, Le Parc J, Ferrari P, et al. A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients. Nat Med. (2019) 25:1402–7. doi: 10.1038/s41591-019-0568-2
471. Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. (2017) 376:221–34. doi: 10.1056/NEJMoa1601277
472. Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. (2010) 120:4445–52. doi: 10.1172/JCI43796
473. Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, and Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. (2007) 178:2458–68. doi: 10.4049/jimmunol.178.4.2458
474. Rumah KR, Vartanian TK, and Fischetti VA. Oral Multiple Sclerosis Drugs Inhibit the In vitro Growth of Epsilon Toxin Producing Gut Bacterium, Clostridium perfringens. Front Cell Infect Microbiol. (2017) 7:11. doi: 10.3389/fcimb.2017.00011
475. Ma N, Wu Y, Xie F, Du K, Wang Y, Shi L, et al. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget. (2017) 8:44625–38. doi: 10.18632/oncotarget.17886
476. Long TM, Nisa S, Donnenberg MS, and Hassel BA. Enteropathogenic Escherichia coli inhibits type I interferon- and RNase L-mediated host defense to disrupt intestinal epithelial cell barrier function. Infect Immun. (2014) 82:2802–14. doi: 10.1128/IAI.00105-14
477. Nakahashi-Oda C, Udayanga KG, Nakamura Y, Nakazawa Y, Totsuka N, Miki H, et al. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat Immunol. (2016) 17:441–50. doi: 10.1038/ni.3345
478. Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. (2012) 2:86. doi: 10.3389/fcimb.2012.00086
479. Dai Y, Wei T, Shen Z, Bei Y, Lin H, and Dai H. Classical HDACs in the regulation of neuroinflammation. Neurochem Int. (2021) 150:105182. doi: 10.1016/j.neuint.2021.105182
480. Shen Y, Yang R, Zhao J, Chen M, Chen S, Ji B, et al. The histone deacetylase inhibitor belinostat ameliorates experimental autoimmune encephalomyelitis in mice by inhibiting TLR2/MyD88 and HDAC3/NF-kappaB p65-mediated neuroinflammation. Pharmacol Res. (2022) 176:105969. doi: 10.1016/j.phrs.2021.105969
481. Birchenough GM, Nystrom EE, Johansson ME, and Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. (2016) 352:1535–42. doi: 10.1126/science.aaf7419
482. Steimle A, Michaelis L, Di Lorenzo F, Kliem T, Munzner T, Maerz JK, et al. Weak agonistic LPS restores intestinal immune homeostasis. Mol Ther. (2019) 27:1974–91. doi: 10.1016/j.ymthe.2019.07.007
Keywords: MS, gut microbiota, blood-brain barrier, brain-gut axis, leaky gut, DMTs
Citation: Ren J, Niu Z, Wang J, Guo J, Hao H, Gao F, Liu R and Wang Z (2025) The link between gut microbiota and multiple sclerosis from the perspective of barrier function. Front. Immunol. 16:1652796. doi: 10.3389/fimmu.2025.1652796
Received: 24 June 2025; Accepted: 25 September 2025;
Published: 15 October 2025.
Edited by:
Ana Raquel Santiago, University of Coimbra, PortugalReviewed by:
María Dominguez-Mozo, Health Research Institute of the Hospital Clínico San Carlos (IdISSC), SpainRamtin Naderian, Semnan University of Medical Sciences, Iran
Copyright © 2025 Ren, Niu, Wang, Guo, Hao, Gao, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Liu, ZHl5eHlzQDEyNi5jb20=; Zhaoxia Wang, ZHJ3YW5nenhAMTYzLmNvbQ==
 Jingru Ren
Jingru Ren Zhenyu Niu
Zhenyu Niu Jianchun Wang
Jianchun Wang Hongjun Hao
Hongjun Hao Feng Gao
Feng Gao Ran Liu
Ran Liu Zhaoxia Wang
Zhaoxia Wang