- Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: Combined small-cell lung cancer is a rare subtype of SCLC, which is characterized by the coexistence of SCLC with any histological type of non-small cell lung cancer. There is limited clinical data. We aimed to explore the clinicopathological features and prognosis of C-SCLC patients who received anti-tumor therapy.
Methods: Eligible patients were histopathologically confirmed adult C-SCLC who received anti-tumor treatment at Sir Run Run Hospital. This analysis aimed to describe the clinicopathological characteristics and evaluate the tumor response rate (RR), disease control rate (DCR), and progression-free survival (PFS).
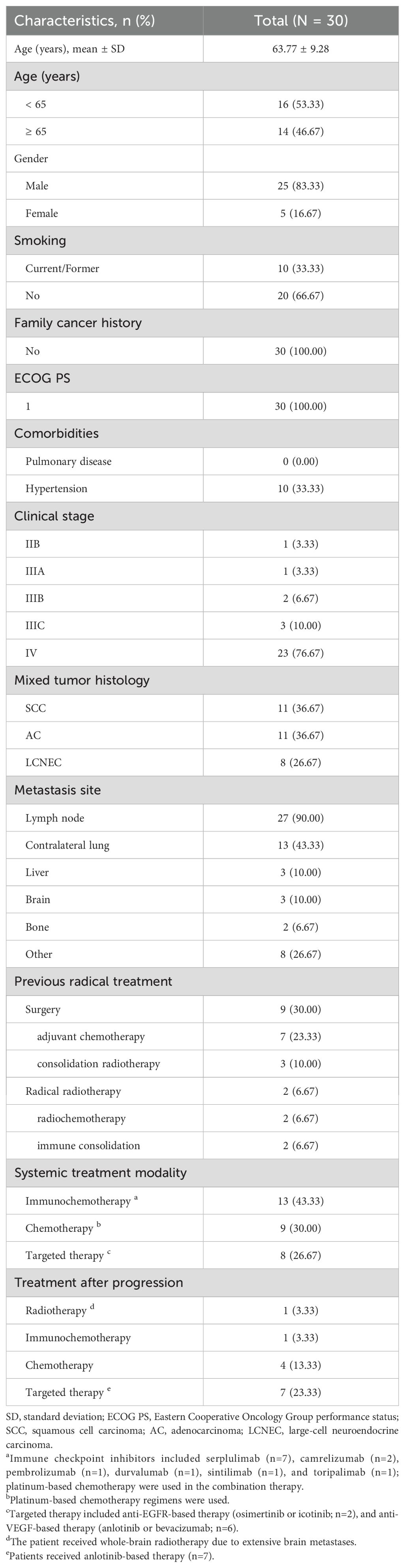
Results: Thirty patients were included. Eighty-three point three three percent were male, and sixty-six point six seven percent were non-smokers. Squamous cell carcinoma (SCC; 11/30) and adenocarcinoma (AC; 11/30) were the most frequently observed mixed components, followed by large-cell neuroendocrine carcinoma (LCNEC; 8/30). Patients received immunochemotherapy (13/30), platinum-based chemotherapy (9/30), or anti-EGFR-/anti-VEGF-based therapy (8/30). Most patients used the anti-PD-1 inhibitor Serplulimab (n=7). Among 27 patients with measurable disease, the RR and DCR were 51.85% (95% CI: 31.95 - 71.33%) and 85.19% (95% CI: 66.27 - 95.81%), respectively. The median PFS was 9.70 months (95% CI: 4.37 - 18.73). The median PFS of C-SCLC mixed with LCNEC was higher than that of those mixed with AC or SCC (10.62 vs. 9.70 vs. 4.17 months; P = 0.858); patients are more likely to benefit from immunotherapy than from chemotherapy and targeted therapy (9.70 vs. 5.27 vs. NR months; P = 0.685).
Conclusion: Our findings provide a basis for systematic treatment strategies in C-SCLC and suggest that patients may derive benefit from immunotherapy, although further studies are needed to confirm these observations.
1 Introduction
Small-cell lung cancer (SCLC), constituting approximately 15% of all lung cancer cases, is characterized by its aggressive nature and notoriously poor survival rates (1). This neoplasm is strongly associated with tobacco carcinogen exposure and is marked by an exceptionally high proliferative rate and a strong predilection for early metastasis.
Combined small-cell lung cancer (C-SCLC) is a rare yet increasingly recognized subtype of SCLC initially identified by the World Health Organization (WHO) in 1981, and SCLC was subsequently stratified into pure SCLC and C-SCLC in 1999 (2). C-SCLC is defined by the coexistence of SCLC with any histological type of non-small cell lung cancer (NSCLC), including adenocarcinoma (AC), squamous-cell carcinoma (SCC), large-cell carcinoma (LCC), and large-cell neuroendocrine carcinoma (LCNEC), among other less common variants such as spindle-cell carcinoma (SpCC) or giant cell carcinoma (GCC) (3). The current iteration of the National Comprehensive Cancer Network (NCCN) for SCLC establishes a revised diagnostic threshold for C-SCLC, requiring the presence of ≥ 10% LCNEC within the tumor architecture as a histopathological prerequisite for this composite neuroendocrine tumor designation (4). The prevalence of C-SCLC has seen a rise in recent years, representing 2% - 30.1% of all SCLC cases, mainly due to inconsistencies in the types of specimens used in different study centers (5–7).
From a molecular perspective, C-SCLC demonstrates conserved genomic aberrations similar to those of conventional SCLC, exhibiting chromosomal instability coupled with an exceptionally high tumor mutational burden (TMB). Functional inactivation of TP53 and RB1, common tumor suppressor genes in SCLC, remains a hallmark genomic feature in C-SCLC, with reported inactivation rates exceeding 50% in C-SCLC (8–10). Notably, the unique histological heterogeneity within C-SCLC raises critical questions regarding its distinctive tumor evolution trajectories, metastatic patterns, and resistance mechanisms to systemic therapies (11, 12). This biological complexity underscores the urgent need for clinicopathological studies to stratify patients for precisely tailored therapeutic interventions and optimize long-term oncologic outcomes. However, the optimal therapeutic approach for C-SCLC has not been fully established. It is often aligned with the conventional SCLC treatment guidelines and a multidisciplinary comprehensive approach. According to current evidence, C-SCLC patients typically receive multimodal therapy, including surgery, chemotherapy, and radiotherapy (13). Since the Food and Drug Administration’s (FDA) approval of atezolizumab for the first-line treatment of extensive-stage SCLC (ES-SCLC) within the landmark Impower133 study in 2019, the therapeutic landscape for SCLC has entered the immunotherapy era (14, 15). Immune checkpoint inhibitors (ICIs) combined with etoposide-platinum (EP) chemotherapy are recommended as the preferred approach for ES-SCLC patients. However, data on immunotherapy for C-SCLC patients are limited, with only a few case report studies (16, 17).
In this retrospective, real-world research, our objective was to describe the clinical and pathological characteristics of C-SCLC patients and investigate their treatment modalities and prognosis in real-world clinical practice. Furthermore, to contextualize our findings within the broader landscape of C-SCLC research, we conducted a systematic literature review summarizing existing evidence on C-SCLC epidemiology, molecular biology, treatment paradigms, and clinical outcomes. This integrated approach will provide a more comprehensive understanding of C-SCLC and inform future treatment strategies.
2 Patients and methods
2.1 Study design and study population
This observational retrospective study was conducted by utilizing the institutional electronic medical records system at Sir Run Run Hospital. We systematically reviewed the medical records of patients with a clinical diagnosis of lung cancer who presented at our hospital from January 2017 to December 2024, with the final follow-up completed by December 2024. Cases with histopathologically confirmed C-SCLC were included in this analysis. The diagnostic criteria of C-SCLC according to the NCCN guidelines for SCLC (version 2. 2022) were as follows (4): C-SCLC consists of both SCLC histology and NSCLC histology (squamous cell, adenocarcinoma, spindle/pleomorphic, and/or large cell carcinoma). No minimal percentage of NSCLC histologic elements is required for the classification of combined SCLC; if any elements are present along with SCLC, then this can be classified as combined SCLC. The exception is when SCLC is combined with LCNEC. At least 10% of the tumor should show LCNEC morphology to be classified as combined SCLC and LCNEC. The other specific inclusion criteria were patients aged 18 years and above who were receiving anti-tumor systemic treatment without particular limitations on the treatment regimen. Exclusion criteria were: (1) patients with insufficient data; (2) patients combined with other primary malignant tumors; (3) for patients who received immunotherapy, those with any active autoimmune disease, a history of autoimmune disease, or those who have been on long-term or high-dose use of corticosteroids or other immunomodulatory agents should be excluded.
This study protocol was reviewed and approved by the Ethics Committee of Sir Run Run Hospital (ethical approval number: 2025 - 0342), and written informed consent from patients was waived due to the retrospective study design. All patient data were anonymized and handled in compliance with the Declaration of Helsinki and reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary File).
2.2 Data and assessments
Two independent researchers (Liu Gong and Hongseng Li) conducted blinded medical record abstraction according to a standardized study protocol, and any inconsistencies in judgment were discussed and adjudicated by a third investigator (Jiawei Shou). Data collection encompassed both structured variables (such as age and gender) and unstructured textual data (such as diagnosis, medical history, general condition, and disease course records). Utilizing a pre-validated electronic case report form (eCRF), the researchers systematically extracted the demographic and clinicopathological characteristics at the initially diagnosed stage, including gender, age, family history of tumor, tobacco exposure, comorbidities, pathological diagnosis, clinical stage, metastasis status, Eastern Cooperative Oncology Group performance status (ECOG PS), and driver gene alteration status. The researchers also recorded the details of patients’ treatment, including surgery, radiotherapy, systemic treatment status (including perioperative and advanced disease treatment options), and progression/recurrence patterns.
The efficacy was assessed utilizing the progression-free survival (PFS), tumor response rate (RR), and disease control rate (DCR). PFS was defined as the time from the first dose of treatment to the first documented disease progression or death of any cause. The RR and DCR were assessed in patients with measurable disease at baseline and received at least one imaging evaluation after treatment. The tumor response was evaluated by investigators according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). RR is defined as the proportion of patients who achieve a complete response (CR) or partial response (PR); DCR is the proportion of patients who achieve CR, PR, or stable disease (SD).
2.3 Statistical analysis
The Shapiro-Wilk test was used to assess the normality of continuous variables. According to the normality test results, continuous variables were described as mean ± standard deviation (SD) or median (range). Categorical variables were summarized as counts and percentages. The 95% confidence intervals (CIs) for RR and DCR were calculated using the Clopper-Pearson method. The median PFS with corresponding 95% CI was calculated utilizing the Kaplan-Meier method. Subgroup survival analyses were stratified by different clinicopathological characteristics and treatment regimens, and hazard ratios with corresponding 95% CIs were displayed. Statistical analyses were performed with the R software (version 4.3.2), and a two-sided P-value < 0.05 was considered statistically significant.
3 Results
3.1 Clinicopathological characteristics
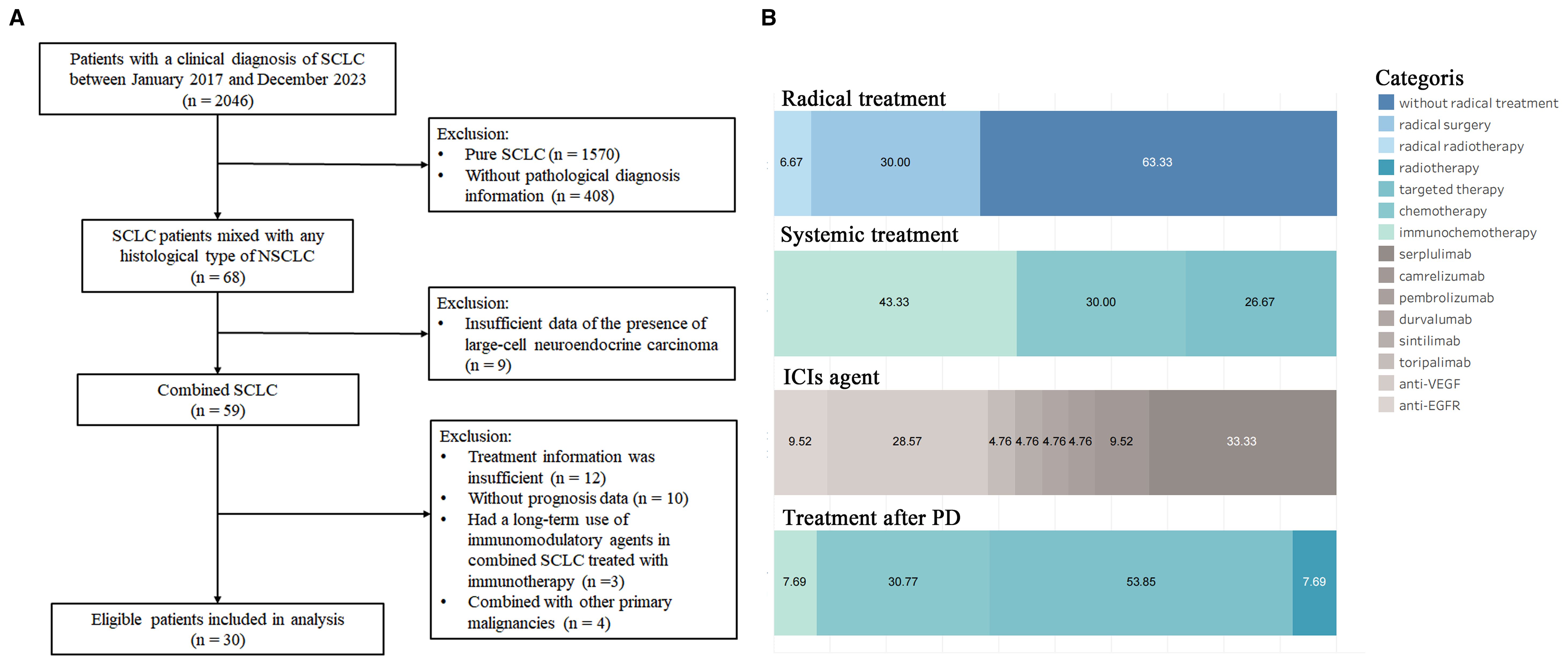
From January 2017 to December 2024, 59 patients with C-SCLC were included in the patient screening process. Based on predefined inclusion/exclusion criteria, 30 patients were ultimately enrolled (Figure 1A). Baseline clinicopathological characteristics are summarized in Table 1. The cohort had a mean age of 63.77 ± 9.28 years, with 14 patients (46.67%) aged 65 and above. Most participants were male (n=25, 83.33%), non-smokers (n=20, 66.67%), had stage IV disease (n=23, 76.67%), and exhibited an ECOG PS of 1 (n=30, 100.00%). No pulmonary comorbidities were reported, while hypertension was present in 10 patients (33.33%). Lymph node metastasis occurred in 27 cases (90.00%), contralateral lung metastasis in 13 patients (43.33%), and metastases to the liver (n=3, 10.00%), brain (n=3, 10.00%) and bone (n=2, 6.67%) were documented in a minority of cases. Histopathological analysis revealed the most common subtypes to be SCLC combined with AC (SCLC/AC; n=11, 36.67%) and SCC (SCLC/SCC; n=11, 36.67%), followed by SCLC combined with LCNEC (SCLC/LCNEC; n=8, 26.67%).
3.2 Treatment
The details of the treatment are displayed in Table 1 and Figure 1B. Among the 30 patients, 11 patients (36.67%) had received radical treatment, with nine receiving surgical resection and two receiving radical radiotherapy. Of the nine surgical patients, seven received adjuvant chemotherapy, and three underwent consolidation radiotherapy; two patients were treated with concurrent radiochemotherapy followed by immunotherapy consolidation. For systemic treatment strategies, 13 patients (43.33%) were treated with ICIs combined with platinum-based chemotherapy. The ICIs included serplulimab (n=7), camrelizumab (n=2), pembrolizumab (n=1), durvalumab (n=1), sintilimab (n=1), and toripalimab (n=1). Nine patients (30.00%) were treated with platinum-based chemotherapy alone, while the remaining cases (n=8, 26.67%) were managed with antiangiogenic therapies (anlotinib or bevacizumab; n=6) or anti-EGFR therapies (osimertinib or icotinib; n=2). Additionally, 13 out of 18 patients who experienced progressive disease received treatment after the disease progression. The treatments included anlotinib-based targeted therapy (n=7), platinum-based chemotherapy (n=4), immunochemotherapy (n=1), and one patient received whole-brain radiotherapy due to extensive brain metastases.
3.3 Prognosis
Among the 27 patients with measurable disease, two (7.41%) had CR, 12 (44.44%) had PR, nine (33.33%) had SD, and four (14.81%) had progressive disease following systemic treatment; two patients had target lesions disappear after receiving serplulimab-based immunochemotherapy. One was SCLC/SCC and the other was SCLC/LCNEC. The ORR was 51.85% (14/27; 95% CI: 31.95% - 71.33%), and DCR was 85.19% (23/27; 95% CI: 66.27% - 95.81%), respectively.
The median follow-up duration was 9.66 months. Overall, 18 patients (60.00%) experienced PFS events, with a median PFS of 9.70 months (95% CI: 4.37 - 18.73) and a 6-month PFS rate of 57.26% (95% CI: 40.69% - 80.57%) (Figure 2A). According to the subgroup analysis of different NSCLC histological types, a poor median PFS could be observed in SCLC/SCC patients (4.17 months; 95% CI: 4.03 - NR), compared with SCLC/AC (9.70 months; 95% CI: 4.37 - NR) and SCLC/LCNEC (10.62 months; 95% CI: 7.43 - NR), with a log-rank P-value of 0.858 (Figure 2B). In the subgroup analysis stratified by different treatment regimens (Figure 2C), the median PFS of patients who received immunochemotherapy (9.70 months; 95% CI: 7.43 - NR) was higher than that of patients treated with chemotherapy (5.27 months; 95% CI: 4.03 - NR) and targeted therapy (NR; 95% CI: 3.17 - NR), although the difference between groups was not statistically significant (log-rank P-value = 0.685). We further explored the associations between PFS and age, gender, smoking status, clinical stage, metastatic patterns, and prior radical treatment. While statistical significance was not reached, trends toward prolonged PFS were observed in the following subgroup: patients aged ≥ 65 years (9.70 vs. 7.43 months; P = 0.485), female (18.73 vs. 7.43 months; P = 0.641); non-smokers (9.70 vs. 4.37 months; P = 0.707), those without stage IV disease (13.80 vs. 5.27 months; P = 0.114), those with absence of lymph node metastasis (18.73 vs. 7.43 months; P = 0.351), those with absence of contralateral lung metastasis (12.20 vs. 4.37 months; P = 0.499), and those undergoing radical treatment (9.70 vs. 7.43 months; P = 0.619). See Table 2.
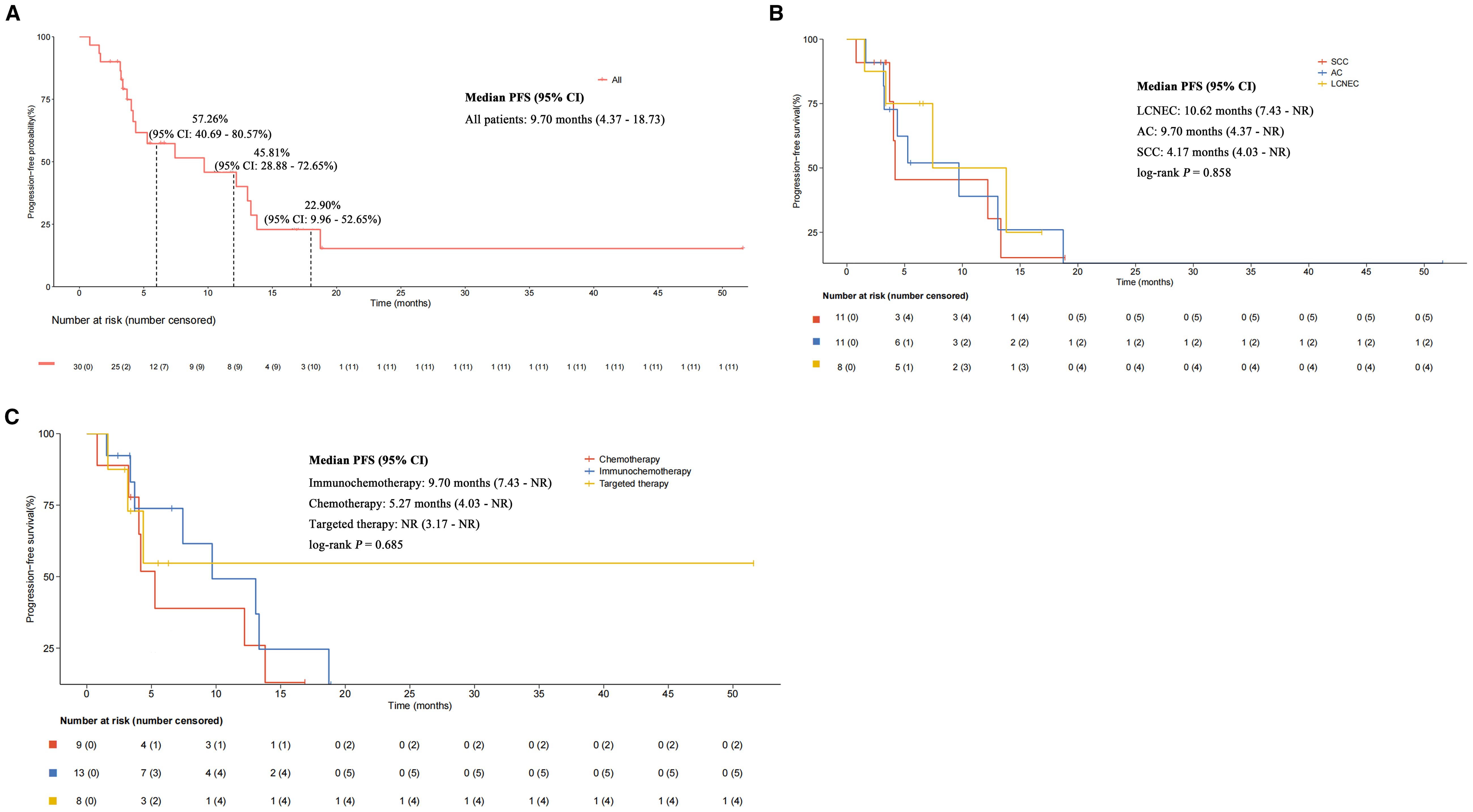
Figure 2. Kaplan-Meier estimates of progression-free survival. (A) All patients. (B) Subgroup analysis stratified by different mixed histological types. (C) Subgroup analysis stratified by different treatment strategies.

Table 2. Subgroup analysis of PFS according to different clinicopathological characteristics and treatments.
4 Discussion
The present study provides a single-center, retrospective analysis of clinicopathological characteristics, treatment patterns, and survival outcomes in 30 C-SCLC patients. Our findings highlight their distinct epidemiological profiles and prognostic heterogeneities among histological subtypes and treatment regimens, which could provide helpful stratifications for clinical decision-making of systemic treatment in C-SCLC.
Divergent specimen collection methodologies in various medical institutions have led to substantial prevalence fluctuations (2% - 30.1%) for C-SCLC [3 - 5]. As reported by Fushimi and his colleagues (18), the frequency of C-SCLC in the primary sites was statistically higher in autopsy specimens than in biopsy or cytology specimens (14.3% vs. 8.6%; P < 0.05). Furthermore, while advances in screening tools and diagnostic techniques have improved C-SCLC identification, the accurate subclassification of C-SCLC remains challenging in small biopsy specimens due to an increase in extrusion artefacts, making it necessary to rely on bronchoscopy and needle aspiration biopsy (19, 20). Contrary to the well-documented association between lung cancer and heavy smoking (21, 22), most patients in our cohort were without tobacco exposure. This observation may have two explanations. Firstly, this study was a single-center retrospective analysis, which is inherently limited by a relatively small sample size that may introduce potential biases. In addition, this discrepancy may reflect inherent biological differences in C-SCLC carcinogenesis, potentially involving alternative oncogenic pathways such as EGFR mutations or ALK rearrangements more prevalent in non-smokers and those with AC (23, 24). Historical data show that EGFR mutations are rare in pure SCLC, occurring in less than 5% of cases, but the prevalence increases to 15% - 20% in C-SCLC (25–27). Lei et al. (28) identified LCNEC as the predominant histologic subtype in C-SCLC; Men et al. (29) found SCC was the most frequent component; most SCLC patients in our study were mixed with AC or SCC. This inter-study heterogeneity may be attributed to various uncontrollable factors, such as the gender ratio, smoking history, and individual genetics.
The therapeutic landscape of C-SCLC remains challenging due to its intrinsic biological complexity. A central obstacle lies in the profound tumor heterogeneity of C-SCLC, where coexisting small cell and non-small cell components exhibit divergent molecular profiles and therapeutic vulnerabilities. For example, the SCLC component typically harbors bi-allelic inactivation of RB1/TP53 and demonstrates sensitivity to platinum-based chemotherapy (10, 30); the NSCLC components may retain oncogenic drivers such as EGFR mutations or ALK rearrangements (31, 32). This genomic bifurcation creates a therapeutic dilemma that conventional chemotherapy effectively targets the rapidly proliferating SCLC clones but exerts limited control over NSCLC subpopulations, while molecularly targeted agents, though validated in NSCLC, often fail against SCLC-dominant tumors due to intrinsic resistance mechanisms. Despite these challenges, the preferred treatment options remain unclear, and the common approach is to follow conventional SCLC treatment guidelines. Surgical therapy may be crucial for patients with early-stage disease, and immunotherapy, chemotherapy, and targeted therapy should be considered for advanced disease (13). In our study, most patients were treated with a combination of immunotherapy and chemotherapy, which demonstrated a numerically superior survival benefit compared with chemotherapy and targeted therapy (median PFS: 9.70 vs. 5.27 vs. NR months). This finding aligns with recent phase III trials demonstrating the survival benefits of ICIs combined with EP regimen compared with chemotherapy in patients with ES-SCLC (33, 34). However, the evidence of immunochemotherapy in C-SCLC patients is limited. Theoretically, C-SCLC is anticipated to be more susceptible to immunotherapy, given the highly unstable nature of the genome and chromosomes in SCLC. Liu et al. (35) reported that an SCLC patient with lung squamous cell carcinoma (LUSC) and high TMB achieved sustained clinical benefit from anti-PD-1 inhibitor therapy as a third-line treatment, with a PFS of 9.7 months. Qu et al. (36) documented a case initially diagnosed with SCLC that progressed to SCLC/AC after first-/second-line chemotherapy and radiotherapy. The patient showed stable lung lesions following third-line treatment with pembrolizumab, indicating a potential advantage of immunotherapy. In our cohort, six patients were treated with antiangiogenic therapies (anlotinib or bevacizumab) in the frontline, and seven patients received anlotinib after disease progression, which aligns with clinical practice guidelines. Anlotinib is recommended as a third-line treatment option for patients with SCLC in Chinese clinical practice, which can significantly prolong PFS by 3.4 months and reduce the risk of disease progression by 81% (37, 38). Recently, a phase III trial demonstrated that immunochemotherapy combined with anlotinib as first-line therapy could result in significant survival benefits for ES-SCLC compared to placebo plus chemotherapy (39). Furthermore, two patients with SCLC/AC patients received EGFR-TKIs (osimertinib and icotinib) but had a relatively limited prognosis, with a PFS of 1.63 and 3.17 months, respectively. Although EGFR-TKIs are widely used in NSCLC patients with EGFR mutations, the efficacy of EGFR-TKIs may vary in C-SCLC. Takagi et al. (40) reported a case with EGFR L861Q mutation in both SCLC and AC components, in which multiple brain metastases and enlarged mediastinal lymph nodes subsequently appeared after second-line erlotinib treatment. Another study reported a woman with SCLC/AC with an L858R mutation who achieved PR after gefitinib treatment (25). While EGFR-TKIs might be applied to C-SCLC harboring EGFR mutations, the limited data available makes it difficult to precisely determine their efficacy, which may also be less pronounced in SCLC or C-SCLC than in NSCLC.
Additionally, prognostic heterogeneity was found among histological subtypes in our study. The observed PFS gradient across subtypes (SCC/LCNEC: 10.62 vs. SCLC/AC: 9.70 vs. SCLC/SCC: 4.17 months) reveals clinically meaningful biological diversity. A retrospective analysis of 181 stage I-IIIa C-SCLC who received radical R0 surgery and platinum-based chemotherapy indicated that SCLC/LCNEC patients had a better prognosis compared with SCLC/AC and SCLC/SCC, with a median disease-free survival (DFS) of 44.1 vs. 20.4 months (P = 0.040) (28). The better prognosis in SCC/LCNEC may relate to preserved neuroendocrine differentiation and pathway genes of SCLC (TP53/RB1) and NSCLC (STK11/KEAP1/RAS), potentially enhancing sensitivity to platinum-based regimens (41). This finding suggests that molecular profiling in C-SCLC may guide personalized management for different patient groups. In addition, the prognosis of C-SCLC is modulated by multiple clinicopathological and treatment-related factors. In a population-based retrospective analysis of 784 C-SCLC cases identified from the SEER database between 2004 - 2016, researchers found that patients with poor differentiation and stage IV disease had worse survival (42). Another analysis of 114 cases with C-SCLC identified smoking, Karnofsky performance score (KPS) < 80, advanced TNM stage, no surgery, positive resection margin, positive lymph nodes ≥ 4, positive lymph node ratio > 10%, and non-multimodality treatment as risk factors for poor OS (29). Notably, the prognostic significance of emerging biomarkers, such as PD-L1 expression levels and TMB, remains undefined, highlighting a critical gap in precision prognostication for this heterogeneous malignancy.
Although our study provides novel insights into immunochemotherapy and targeted therapy in C-SCLC, this study has several limitations. Firstly, due to the lower incidence and difficulty in diagnosis, a small sample size was included in our study, which introduces potential selection bias and limits the generalizability of our findings to the broader C-SCLC population. While this study reveals distinct PFS patterns among C-SCLC histological subtypes, the subgroup comparisons were inherently limited by cohort size, preventing adjustment for clinically relevant confounders, including disease stage, performance status, and therapeutic heterogeneity. These unadjusted analyses must be interpreted with caution, requiring rigorous validation in future dedicated cohorts with sufficient power for robust statistical adjustment. Additionally, this single-center, retrospective study design limits the ability to establish causative relationships between treatment regimens and outcomes. Lastly, the limited genetic testing and lack of comprehensive analysis of driver genes and biomarkers prevent us from fully exploring the underlying mechanisms and identifying patients who may derive the greatest benefit from different therapy options.
5 Conclusion
In our cohort, SCLC/AC and SCLC/SCC were the most common subtypes of C-SCLC, but patients with SCLC/LCNEC showed longer survival than those with other mixed histopathological types. Immunochemotherapy was the primary treatment regimen for C-SCLC, and our data suggest that patients are more likely to benefit from this approach. Our findings suggest the need to further explore the relationship between different histopathological types and prognosis, and the investigation of biomarker-driven patient selection may facilitate the identification of optimal therapeutic strategies for patients with C-SCLC. To definitively validate these subtype-specific survival patterns and therapeutic implications, multi-institutional collaborative efforts are warranted to establish evidence-based precision frameworks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (ethical approval number:2025-0342). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study.
Author contributions
LG: Formal Analysis, Data curation, Conceptualization, Writing – original draft. HSL: Conceptualization, Data curation, Formal analysis, Writing – review & editing. JWS: Conceptualization, Data curation, Writing – review & editing, Formal analysis. JS: Investigation, Formal analysis, Writing – review & editing, Data curation. WJ: Writing – original draft, Data curation. HZL: Writing – review & editing, Conceptualization, Data curation. DL: Formal analysis, Data curation, Investigation, Writing – review & editing. HH: Data curation, Investigation, Writing – review & editing, Formal analysis. YF: Validation, Conceptualization, Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by the National Key Research and Development Program of China (Grant numbers 2021YFA1301100, 2021YFA1301104), Shenzhen Xihepu Biomedical Research Institute-Tumor Immunotherapy Project (CCHRPP-ZL-2023-Q-010), Zhejiang Provincial Natural Science Foundation ExplorationProject (LY24H160013), Zhejiang Provincial Administration of Traditional Chinese Medicine Project (2011ZB080), and CSCO Health Project (Grant numbers YQL2019-0316 and Y-MSD2020-0314).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1652803/full#supplementary-material
References
1. Gazdar AF, Bunn PA, and Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. (2017) 17:725–37. doi: 10.1038/nrc.2017.87
2. Beasley MB, Brambilla E, and Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. (2005) 40:90–7. doi: 10.1053/j.ro.2005.01.001
3. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
4. Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1441–64. doi: 10.6004/jnccn.2021.0058
5. Mangum MD, Greco FA, Hainsworth JD, Hande KR, and Johnson DH. Combined small-cell and non-small-cell lung cancer. J Clin Oncol. (1989) 7:607–12. doi: 10.1200/JCO.1989.7.5.607
6. Babakoohi S, Fu P, Yang M, Linden PA, and Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. (2013) 14:113–9. doi: 10.1016/j.cllc.2012.07.002
7. Zhang C, Yang H, Zhao H, Lang B, Yu X, Xiao P, et al. Clinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experience. J Thorac Dis. (2017) 9:151–8. doi: 10.21037/jtd.2017.01.07
8. Murase T, Takino H, Shimizu S, Inagaki H, Tateyama H, Takahashi E, et al. Clonality analysis of different histological components in combined small cell and non-small cell carcinoma of the lung. Hum Pathol. (2003) 34:1178–84. doi: 10.1053/j.humpath.2003.05.001
9. Gazdar AF, Savage TK, Johnson JE, Berns A, Sage J, Linnoila RI, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. (2015) 10:553–64. doi: 10.1097/JTO.0000000000000459
10. Zhang J, Zhang L, Luo J, Ge T, Fan P, Sun L, et al. Comprehensive genomic profiling of combined small cell lung cancer. Transl Lung Cancer Res. (2021) 10:636–50. doi: 10.21037/tlcr-20-1099
11. Lach KD, Sorin M, Huynh C, Alirezaie NS, Fiore A, Fiset B, et al. Combined small-cell lung carcinoma revealed to be an intratumoural metastasis by genetic analysis. Ann Oncol. (2021) 32:679–81. doi: 10.1016/j.annonc.2021.01.075
12. Jin Y, Chen Y, Qin Z, Hu L, Guo C, and Ji H. Understanding SCLC heterogeneity and plasticity in cancer metastasis and chemotherapy resistance. Acta Biochim Biophys Sin (Shanghai). (2023) 55:948–55. doi: 10.3724/abbs.2023080
13. Zeng C, Qiu G, Xie X, Liu T, Chen Z, Zhang X, et al. Combined small cell lung cancer: current progress and unmet needs. Am J Cancer Res. (2023) 13:3864–74.
14. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
15. Zhang S and Cheng Y. Immunotherapy for extensive-stage small-cell lung cancer: current landscape and future perspectives. Front Oncol. (2023) 13:1142081. doi: 10.3389/fonc.2023.1142081
16. Dong Y, Li Q, Li D, Fang Y, and Wang C. Whole-process treatment of combined small cell lung cancer initially diagnosed as "Lung squamous cell carcinoma": A case report and review of the literature. Front Immunol. (2022) 13:831698. doi: 10.3389/fimmu.2022.831698
17. Liu MH, Li YX, and Liu Z. Envafolimab combined with chemotherapy in the treatment of combined small cell lung cancer: A case report. World J Clin cases. (2023) 11:1115–21. doi: 10.12998/wjcc.v11.i5.1115
18. Fushimi H, Kikui M, Morino H, Yamamoto S, Tateishi R, Wada A, et al. Histologic changes in small cell lung carcinoma after treatment. Cancer. (1996) 77:278–83. doi: 10.1002/(SICI)1097-0142(19960115)77:2<278::AID-CNCR9>3.0.CO;2-I
19. Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. (2002) 26:1184–97. doi: 10.1097/00000478-200209000-00009
20. Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. (2012) 25 Suppl 1:S18–30. doi: 10.1038/modpathol.2011.150
21. Nooreldeen R and Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. (2021) 22(16):8661. doi: 10.3390/ijms22168661
22. Zhu M, Lv J, Huang Y, Ma H, Li N, Wei X, et al. Ethnic differences of genetic risk and smoking in lung cancer: two prospective cohort studies. Int J Epidemiol. (2023) 52:1815–25. doi: 10.1093/ije/dyad118
23. Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. (2016) 26:32–42. doi: 10.1007/s00330-015-3814-0
24. Vasudevan S, Krishna V, and Mehta A. Lung cancer in non-smokers: clinicopathological and survival differences from smokers. Cureus. (2022) 14:e32417. doi: 10.7759/cureus.32417
25. Tatematsu A, Shimizu J, Murakami Y, Horio Y, Nakamura S, Hida T, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res. (2008) 14:6092–6. doi: 10.1158/1078-0432.CCR-08-0332
26. Lu HY, Mao WM, Cheng QY, Chen B, Cai JF, Wang XJ, et al. Mutation status of epidermal growth factor receptor and clinical features of patients with combined small cell lung cancer who received surgical treatment. Oncol Lett. (2012) 3:1288–92. doi: 10.3892/ol.2012.666
27. Lu HY, Sun WY, Chen B, Zhang YP, Cai JF, Su D, et al. Epidermal growth factor receptor mutations in small cell lung cancer patients who received surgical resection in China. Neoplasma. (2012) 59:100–4. doi: 10.4149/neo_2012_013
28. Lei Y, Feng H, Qiang H, Shang Z, Chang Q, Qian J, et al. Clinical characteristics and prognostic factors of surgically resected combined small cell lung cancer: a retrospective study. Lung Cancer. (2020) 146:244–51. doi: 10.1016/j.lungcan.2020.06.021
29. Men Y, Hui Z, Liang J, Feng Q, Chen D, Zhang H, et al. Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res. (2016) 28:486–94. doi: 10.21147/j.issn.1000-9604.2016.05.03
30. George J, Maas L, Abedpour N, Cartolano M, Kaiser L, Fischer RN, et al. Evolutionary trajectories of small cell lung cancer under therapy. Nature. (2024) 627:880–9. doi: 10.1038/s41586-024-07177-7
31. Cooper AJ, Sequist LV, and Lin JJ. Author Correction: Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. (2022) 19:744. doi: 10.1038/s41571-022-00680-8
32. de Scordilli M, Michelotti A, Bertoli E, De Carlo E, Del Conte A, and Bearz A. Targeted therapy and immunotherapy in early-stage non-small cell lung cancer: current evidence and ongoing trials. Int J Mol Sci. (2022) 23(13):7222. doi: 10.3390/ijms23137222
33. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
34. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM–005 randomized clinical trial. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
35. Testori A, Ferraroli G, De Carlo C, Bossi P, Alloisio M, and Mangiameli G. Tracheal polypoid combined small cell lung cancer (C-SCLC): A case report. Thorac Cancer. (2021) 12:2035–8. doi: 10.1111/1759-7714.13992
36. Qu Z, Liu J, Luo F, Li L, Zhu L, and Zhou Q. MDT treatment of small cell lung cancer complicated with adenocarcinoma: A case report and literature review. Zhongguo Fei Ai Za Zhi. (2021) 24:808–14. doi: 10.3779/j.issn.1009-3419.2021.102.37
37. Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. (2021) 125:366–71. doi: 10.1038/s41416-021-01356-3
38. Oncology Society of Chinese Medical, A. Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer, (2024 edition). Zhonghua Zhong Liu Za Zhi. (2024) 46:805–43. doi: 10.3760/cma.j.cn112152-20240510-00189
39. Cheng Y, Chen J, Zhang W, Xie C, Hu Q, Zhou N, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med. (2024) 30:2967–76. doi: 10.1038/s41591-024-03132-1
40. Takagi Y, Nakahara Y, Hosomi Y, and Hishima T. Small-cell lung cancer with a rare epidermal growth factor receptor gene mutation showing "wax-and-wane" transformation. BMC Cancer. (2013) 13:529. doi: 10.1186/1471-2407-13-529
41. Derks JL, Leblay N, Lantuejoul S, Dingemans AC, Speel EM, and Fernandez-Cuesta L. New insights into the molecular characteristics of pulmonary carcinoids and large cell neuroendocrine carcinomas, and the impact on their clinical management. J Thorac Oncol. (2018) 13:752–66. doi: 10.1016/j.jtho.2018.02.002
Keywords: immunotherapy, chemotherapy, combined small-cell lung cancer, real-world study, treatment strategies
Citation: Gong L, Li H, Shou J, Sheng J, Jin W, Lou H, Li D, Hu H and Fang Y (2025) Clinicopathological characteristics and treatment patterns of combined small-cell lung cancer: a real-world single-center study with a mini review. Front. Immunol. 16:1652803. doi: 10.3389/fimmu.2025.1652803
Received: 24 June 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Yanqiang Li, Xi’an Jiaotong University, ChinaReviewed by:
Michael Shafique, Moffitt Cancer Center, United StatesChuan Jiang, Texas Tech University Health Sciences Center School of Medicine, United States
Copyright © 2025 Gong, Li, Shou, Sheng, Jin, Lou, Li, Hu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Fang, ZmFuZ3lvbmdAemp1LmVkdS5jbg==; ZmFuZ3lvbmdAc3Jyc2guY29t
 Liu Gong
Liu Gong Hongsen Li
Hongsen Li Jiawei Shou
Jiawei Shou Jin Sheng
Jin Sheng Da Li
Da Li Hong Hu
Hong Hu Yong Fang
Yong Fang