- 1Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Center for Cellular Immunotherapies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 3Brigham & Women’s Hospital, Mass General Brigham, Boston, MA, United States
- 4Gastroenterology, MiNK Therapeutics, Lexington, MA, United States
- 5Life Sciences, Imperial College, London, United Kingdom
Over the past 30 years, work of immunologists worldwide has phenotypically and functionally defined “Natural Killer T cells” (NKT) and their subsets, including “invariant Natural Killer T cells” (iNKT). NKT cells make up a substantial fraction of T cells that express NK cell markers and have TCRs restricted to either conventional MHC molecules or the monomorphic CD1d molecule. Among these, iNKT cells are CD1d-restricted and more common within NKT cells than T cells without NK markers. While the definition of NKT cells, whether based on phenotype, function, or both, remains a topic of debate, iNKT cells represent a distinct T cell population characterized by a recurrent, conserved TCR rearrangement (TRAV10–TRAJ18 in humans) paired with a limited Vβ repertoire (mostly encoded by TRBV25-1 in humans). iNKT cells are restricted by CD1d, which, unlike CD1a-c molecules, is expressed not only on professional antigen-presenting cells and thymocytes but also on certain non-hematopoietic somatic tissues, both normal and neoplastic. Like all CD1 family members, CD1d presents various lipid antigens by accommodating their long hydrophobic tails in deep binding pockets, in contrast to the shallow peptide grooves of conventional MHC molecules. However, the ligand repertoire of CD1d is distinct from that of CD1a-c. This review focuses on CD1d-restricted iNKT cells. Activation of iNKT cells via their semi-invariant TCR, often in synergy with NK receptors and other co-stimulatory molecules, triggers a rapid, polyfunctional response. Unlike conventional MHC-restricted T cells, individual iNKT cells can simultaneously produce both Th1- and Th2-type cytokines and exert cytotoxic activity in an immune synapse-directed fashion. Through this combination of direct cytotoxicity and cytokine-mediated immunomodulation, iNKTs can eliminate target cells while activating myeloid and other lymphoid populations to amplify immune responses. Their versatility has fueled growing interest in harnessing iNKT cells across inflammatory, infectious, and oncological diseases, where early-phase studies have demonstrated their safety and preliminary efficacy. Moreover, because they are restricted by the non-polymorphic CD1d molecule and possess immune-regulatory properties, iNKT cells lack graft-versus-host potential, making them ideal candidates for allogeneic, off-the-shelf therapies. This review summarizes how iNKT cells are being reimagined as innovative tools for immune intervention across a range of clinical settings.
1 Introduction: iNKT cells act as hybrid immune powerhouses
Invariant Natural Killer T (iNKT) cells are a unique, rare subset of T cells (0.01%-0.2% on average) (1–3) that play crucial roles in immune regulation, homeostasis and defense against pathogens and cancers (4–10). They are termed “invariant” because they express a highly conserved T cell receptor (TCR), characterized by an α-chain with a nearly constant junction across individuals (TRAV10–TRAJ18 in humans), paired with a β-chain that uses a limited set of Vβ genes (mostly TRBV25-1 in humans), combined with diverse D and J gene segments. While the TCRα chains are formally oligoclonal rather than monoclonal, they typically encode an identical amino acid sequence through different codon combinations, and are highly conserved across species, from mice to humans (11).
The story of iNKTs began in the late 1980s, when researchers discovered unusual lymphoid cells with a hybrid T and NK cell phenotype in mice (12). These cells predominantly expressed αβ TCRs along with CD161, then known as NK1.1 in mice and NKR-P1A in humans, an antigen previously thought to be exclusive to NK cells. This hybrid identity led to their initial designation as “NK T cells” in 1995 (12). In parallel, a population with an almost clonal TCRα and a limited set of TCRβ chains was described in both humans and mice (5, 6). Bendelac and colleagues showed that these cells were restricted to the monomorphic, MHC-related molecule CD1d, linking them to a subset of the previously described “NK T” cells in mice, and revealing these cells to represent one and the same population rather than two separate ones (12, 13). Their human counterparts were reported two years later by Exley and colleagues (14).
What truly sets iNKTs apart from conventional T cells is their invariant TCR α-chain, in contrast to the diverse TCRs expressed by the latter (15). This discovery led to the introduction of the term “invariant NKT cells” or “iNKT cells” by S. Brian Wilson and his team in 2001 (16). Researchers also realized that activation and development of these cells required β2-microglobulin, even though the majority do not express the CD8 co-receptor and were either CD4+ or ‘double-negative’ (DN) for both CD4 and CD8 (4, 17–21). This finding helped establish iNKT cells as a unique subset of T cells with specific mechanisms of function and a distinct, specialized role in the immune system.
The identification of CD1d as the restriction element for iNKT cells was a major functional breakthrough (13). Of note, while mice and rats only have one or two CD1d genes (6, 17), multiple isoforms, namely, CD1a, CD1b, CD1c, and CD1e, were identified in humans, with CD1e remaining intracellular. Soon after, it became clear that CD1d, like the other CD1 isoforms, presents lipid antigens derived from both endogenous and microbial sources (22–25). CD1d is found on antigen-presenting cells (APCs), including B lymphocytes, monocytes, dendritic cells (DCs) and macrophages, as well as thymocytes and certain non-hematopoietic cells such as epithelial, parenchymal and vascular smooth muscle cells (26–28). Because of the invariant TCRα and its peculiar binding properties, iNKTs are uniquely adept at stereospecific recognition of α-galactosylceramide (αGalCer) in complex with CD1d (29) (Figure 1), which drives potent expansion of human iNKTs (30). The development of CD1d tetramers loaded with αGalCer enabled direct detection of these cells and solidified their classification as a highly specialized T cell subset (31, 32).
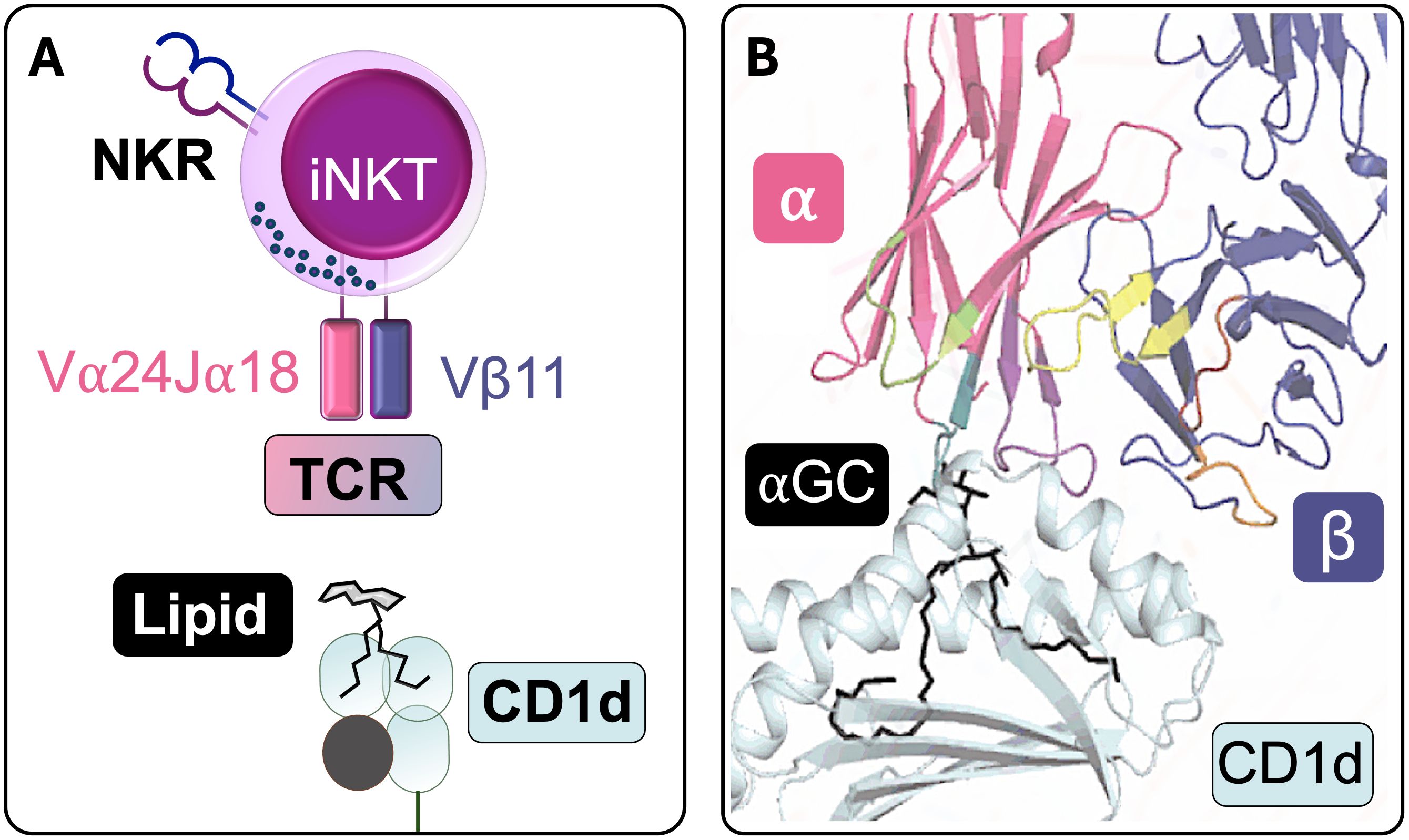
Figure 1. iNKTCR recognition of CD1d-lipid. (A) Schematic representation of a human iNKT cell recognizing a CD1d-lipid antigen complex (NKR: NK receptor; TCR: T cell receptor). (B) Structural depiction of stereospecific recognition of α-galactosylceramide (αGC) in complex with CD1d. The lipid tails are buried within CD1d’s hydrophobic pocket, whereas the polar headgroup protrudes from CD1d to interact with the TCR. The TCRα chain makes contact with αGC, whereas the β chain primarily interacts with CD1d, contributing to the modulation of overall TCR affinity (Adapted from Rossjohn J., et al, 2015 (141)).
Today, iNKTs are recognized for their unique biological features, which hold great promise for developing novel cellular therapies to treat challenging conditions such as cancer, infections, and inflammatory diseases, conditions that are often difficult or unsuitable to address with conventional MHC-restricted T and NK cells.
In this review, we discuss the latest advances in translating iNKT therapeutic potential into clinical practice.
2 iNKT cells have wide therapeutic potential across individuals and disease settings
iNKT cells are a rare yet strikingly heterogeneous T cell subset, comprising functionally specialized subpopulations. Historically, CD4- and CD4+ subsets have been used to distinguish human iNKT populations with enhanced cytotoxic potential versus those with greater regulatory capacity respectively (33). Subsequent studies have revealed broader human iNKT diversity by combining expression of canonical surface markers of T and NK activation, differentiation and homing (e.g., CD28, CD27, CD62L, NKG2D, CD161, KLRs, CCR7, CCR5) (14, 21, 34, 35), with human iNKT-specific cytokine patterns and transcriptional programs (36,37). Unlike their mouse counterparts, human iNKT subsets are less clearly defined, consistent with their marked polyfunctionality. Furthermore, although they share classical memory and effector molecules with MHC-restricted T and NK cells, human iNKTs cannot be readily classified into conventional naïve, memory, and effector states (38). Still, their ability to proliferate, survive, and function varies both within and across individuals, correlating with CD62L+ phenotypic patterns (34) and with exposure to distinct cytokine milieus (39, 40), differences that may lead to divergent functional outcomes in therapeutic settings. While a detailed description of iNKT subset biology is beyond the scope of this review, these insights underscore that the therapeutic potential of human iNKT cells lies not only in their unique biology but also in their heterogeneity. Decoding iNKT diversity is an active area of research, critical to understanding how best to deploy iNKT cells in the clinic, for instance, to predict treatment responses or to design tailored strategies targeting specific tissues or modulating the duration and nature of immune responses (e.g., cytotoxic, immunoadjuvant, or tolerogenic). This is especially relevant for the development of allogeneic and off-the-shelf iNKT-based cell therapies, where pre-manufactured products could be customized to match desired immune outcomes.
Owing to their ability to both modulate immune responses and act directly as effector cells, iNKTs are emerging as powerful players in the immune system, showing protective roles in cancer (41), infections (42, 43), and immune-mediated conditions (44). Further, they combine features of both innate and adaptive immunity, which makes them ‘hybrid effectors’ with a primed ‘ready-to-act’ state (45). Much like classical innate immune cells, iNKTs respond rapidly to danger signals, pro-inflammatory cytokines, and CD1d-presented lipid antigen stimuli within minutes or hours. At the same time, like conventional MHC-restricted T cells, they use their TCR to initiate specific, CD1d-restricted responses, including cytotoxicity and immune modulation (46–49). Though relatively rare, iNKT cells compare favorably with antigen-specific T cells in effector and immunomodulatory functions. This along with their innate-like reactivity allows iNKTs to both act faster than T cells and produce unusually large amounts of cytokines per cell that orchestrate both innate and adaptive immune responses (Figure 2,left). As a result, iNKTs have significant therapeutic potential that extends beyond cancer immunotherapy, offering hope for treating severe infections and chronic inflammatory conditions.
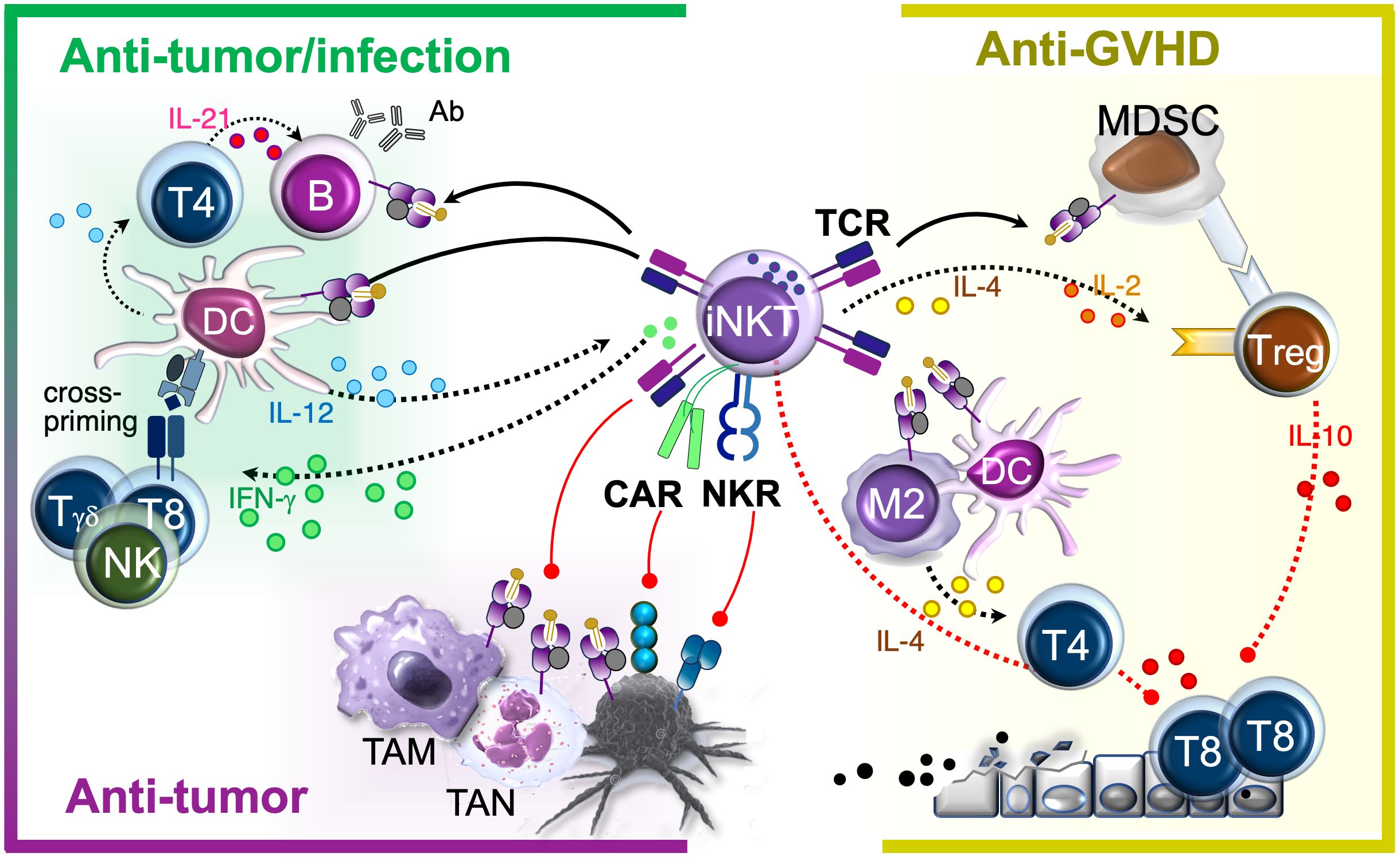
Figure 2. iNKT therapeutic effects. iNKTs naturally home to infection and tumor sites where they activate APCs and recruit endogenous immune cells, playing a critical role in reprogramming the microenvironment to promote antimicrobial and tumor-directed immunoadjuvant responses. In parallel, they exert direct effector functions by targeting both immunosuppressive cells within the microenvironment and infected or malignant cells, contributing to disease eradication. These immune-activating functions coexist with the selective suppression of alloreactive cells in allogeneic settings. In immunocompetent murine models, such effect was attributed to the induction of immunomodulators (MDSC, Treg, Th2 cells). In human xenograft models, allosuppression was also mediated by the direct elimination of APCs capable of activating alloreactive T cells. Black arrows: activation. Red vectors: inhibition. Solid lines: contact-mediated effects. Dotted lines: cytokine-mediated effects. TAM, Tumor-Associated Macrophage; TAN, Tumor-Associated Neutrophil; M2, M2 Macrophage; T4, CD4+ T cell; T8, CD8+ T cells.
One of the main iNKT advantages for therapy is their ability to be transferred from donor to recipient without causing graft-versus-host disease (GVHD) (50–52). This property stems from their invariant TCRα chain and restriction to the monomorphic CD1d molecule, and is in stark contrast to classical MHC-restricted T cells, eliminating the need for endogenous TCR ablation required for allogeneic conventional T cells. As a result, banks of allogeneic iNKT cells can be generated for off-the-shelf cellular therapy, circumventing common T cell therapy challenges related to T cell fitness, manufacturing costs and time needed to generate autologous T cell products from patient apheresis or heavily gene edited allogeneic products from healthy donors. In addition to avoiding GVHD, iNKT cells can suppress alloreactive donor T cells that cause GVHD, as part of their specialized ability to orchestrate immune responses (53–59) (Figure 2, right). This property is particularly valuable in allogeneic hematopoietic stem cell transplantation (HSCT), where iNKT cells can help prevent and control potentially lethal immune responses while simultaneously promoting protective immunity through graft-versus-tumor (GVT) effects (53), and anti-microbial defense (60). These functions are especially critical for HSCT recipients, who are typically immunosuppressed due to their underlying disease, conditioning regimens and prolonged anti-GVHD therapies. Additionally, by suppressing inflammatory triggers known to impair hematopoietic stem and progenitor cell engraftment (61), iNKTs can support durable donor chimerism without requiring complete elimination of host immune cells (61). This whole-in-one functionality has the potential to extend graft persistence (61) while minimizing alloreactive flare-ups (55, 56), infectious complications (60) and disease recurrence (53) thereby enhancing overall transplant success. Altogether, these seemingly paradoxical actions, i.e., suppressing harmful immune responses while enhancing protective ones, make context-reactive iNKT cells valuable for a wide range of applications in transplantation and allogeneic cell therapies, both as unmodified (50, 51, 62) and engineered products for enhanced therapeutic benefit (52).
Lastly, iNKTs exhibit pronounced tissue tropism and home to specific tissues more effectively than circulating conventional MHC-restricted T cells, displaying tissue-resident-like behaviors, driven by distinct chemokine and homing receptors expression patterns (63, 64). In mice, the anatomical distribution of iNKTs is well correlated with functional specialization and is amenable to pharmacologic modulation (65, 66). For instance, intravenous administration of αGalCer activates hepatic and splenic perivascular iNKTs, inducing robust systemic IFN-γ responses, whereas oral delivery primarily engages iNKTs in mesenteric lymph nodes, triggering localized IL-4-mediated tolerogenic effects (66). Similarly, in humans, emerging evidence points to tissue-specific functional programs that could be leveraged to shape therapeutic responses in cancer, vaccine, and transplant settings, modulating the type (immunoadjuvant or tolerogenic), extent (systemic or local) and kinetics (rapid or delayed) of iNKT-cell based immunotherapies. Importantly, tissue homing capacities have been linked to increased trafficking and function at tumor sites (67–69), where iNKTs can play a critical role in switching the tumor microenvironment (TME) from immunosuppressive to pro-inflammatory and anti-tumor. In fact, by targeting immune-suppressive myeloid cells via CD1d, recruiting cytotoxic T and NK cells, and activating APCs, iNKTs can create the conditions necessary for tumor eradication in solid malignancies and lymphoproliferative solid-like neoplasms (64, 67, 69–71). Peripheral blood-derived iNKTs are practical to isolate and broadly reflect features of their tissue-resident counterparts, supporting their use as versatile effectors capable of trafficking to and functioning within diverse tissues, including immune-privileged sites such as the brain. However, certain iNKT subsets may exhibit superior trafficking or persistence in specific anatomical niches. Disease-specific applications could therefore benefit from the enrichment or use of tissue-derived iNKTs, analogous to tumor-infiltrating lymphocyte (TIL) therapies, to enhance site-specific activity. Accordingly, therapeutic strategies may need to account for tissue-specific differences when designing persistence-tracking approaches, including consideration of tumor biopsies or bone marrow sampling, depending on the disease context.
In conclusion, this collection of features establishes iNKTs as a highly versatile T cell subset with broad therapeutic potential. Their ability to simultaneously exert effector functions, modulate immune responses, facilitate donor cell/tissue engraftment, and home to specific sites, combined with a safe therapeutic profile, makes them a promising tool, not just for cancer immunotherapy, but also for treating severe infections, systemic inflammatory diseases, and enhancing outcomes in hemopoietic and organ/cell transplantation. Their role in tissue immune responses and homeostasis also opens up new opportunities to address challenges that conventional T and NK cell-based therapies still face (72, 73).
3 iNKT cells can induce complete remissions in solid cancer patients
Tumors arising from certain CD1d+ cell types, such as some B cell lymphomas, commonly retain CD1d expression, though levels may vary with tumor stage, for example, declining during progression in multiple myeloma (74). However, in cancer patients, iNKT frequency and/or function are often diminished (75–81). This reduction in both the number of circulating iNKTs and their ability to function effectively is associated with poorer clinical outcomes (reviewed in (82, 83)). Likewise, both basally and therapeutically increased iNKT numbers have been linked to better prognoses and improved patient outcomes (80, 84). Collectively, these observations suggest that patient’s own iNKT cells play a meaningful role in anti-tumor immunity, supporting strategies that aim to restore the number and function of endogenous iNKT cells.
Importantly, patient-derived iNKT cells can be expanded ex vivo, and show repaired functionality under optimal culture conditions, reversing the dysfunction seen in cancer patients (75, 78–80, 85). Expanded autologous iNKTs retain their natural ability to home to tumors and mitigate tumor-associated immune suppression and heterogeneity through direct anti-TME activity and NK-like cytotoxicity, alongside immunoadjuvant effects (see below). These findings support the use of iNKT-based therapies, including patient-derived expanded iNKTs, to enhance endogenous iNKT numbers and function. Whether through endogenous activation or adoptive transfer, autologous iNKT cells offer a valuable platform to restore anti-tumor immunity and provide a novel therapeutic strategy for cancer patients (Table 1).
Early clinical trials in patients with lung cancer and melanoma explored the feasibility of expanding autologous (patient-derived) iNKTs ex vivo and re-infusing them into the same patients to restore iNKT’s normal frequency and functionality. In a study of six patients with non-small cell lung cancer (NSCLC), autologous peripheral blood mononuclear cells (PBMCs) were enriched for iNKTs over a 3-week expansion period and administered in two infusions at doses of 1 to 5 × 107 iNKTs/m2/infusion (purity min – max: 0.01 - 25%). While the treatment did not induce tumor regression, it stabilized the disease in two patients for 9 and 12 months, with all participants showing signs of immune activation (86). A trial in melanoma patients employed an improved manufacturing protocol, entailing iNKT enrichment with a monoclonal antibody to the iNKT cell receptor (iNKTCR) prior to ex vivo expansion, resulting in higher purity of the autologous iNKT products (13- 87%, median 66%). Three out of nine patients remained progression-free for over four years (53-65 months), and the overall time to progression was approximately one and a half years, even including one patient with actively progressing disease at the time of treatment. Collectively, this pioneering experience provided encouraging data supporting the exploration of optimized iNKT strategies to further enhance clinical efficacy (87).
One promising approach to improve therapeutic success of iNKT cells involves genetic engineering with chimeric antigen receptors (CARs), recombinant TCRs (rTCRs), chimeric TCRs, and other synthetic modifications. In preclinical studies, CAR-iNKTs and rTCR-iNKTs were more effective than unmodified iNKTs in killing target cells, while retaining their innate trafficking and immunomodulatory capabilities alongside functionality of their endogenous TCR and NK receptors, required for maximal activity against the TME as well as malignant cells (34, 67, 88–97). In a landmark clinical trial (NCT03294954), twelve children and young adults with neuroblastoma unresponsive to the standard of care were treated with autologous iNKTs engineered to express a GD2-specific CAR and IL-15 (98, 99). This CAR-iNKT cell therapy proved safe and clinically effective, yielding one complete and two partial responses, alongside evidence of in vivo expansion and tumor homing (98). Of note, neuroblastoma patients treated at the same institution with third-generation GD2-specific CAR T cells, which are designed to be more potent than commonly used second-generation versions by including two costimulatory domains instead of one to enhance anti-tumor responses, did not achieve an objective response (100). Collectively, these results underscore a promising improvement in iNKT therapeutic activity that was enhanced via synthetic engineering. Another two ongoing trials (NCT06182735 and NCT06728189) are evaluating autologous CD70-targeted CAR-iNKT in patients with metastatic renal cell carcinoma (mRCC) and advanced solid tumors. Interim results from six mRCC patients revealed significant tumor reductions in two of four evaluable cases, with responses lasting at least nine months despite low CD70 expression (101). CAR-iNKTs persisted in circulation for five months, with no evidence of severe cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS). These early data further corroborate the feasibility of genetically redirected iNKTs for the treatment of as-yet incurable solid cancers with promising signs of therapeutic activity. Further refinements are underway, and some are already being tested in the clinic, including innovative combinatorial strategies to optimize iNKT cell expansion, persistence, and anti-tumor potency in patients with solid cancers (see NCT03294954 below and Table 1). Additional pre-clinical efforts in partnerships with biotech companies are actively focusing on developing CAR-iNKT therapeutic products with desirable traits, such as less differentiated (CD62L+) (98), less exhausted (BTG1-knockdown) (98), and Th1-polarized subsets (40, 102), to enhance durability and functionality. Specifically, there is robust data supporting that CD62L expression marks a central memory-like population characterized by sustained anti-tumor effects in preclinical in vivo models (34) and more durable responses in patients (98, 99). This subset is especially attractive for therapeutic development, offering the potential for long-term immune surveillance and tumor control.
Taken together, these advancements and the promising clinical activity of CAR-iNKTs in challenging solid tumor patients mark a significant milestone in the cell therapy field. Beyond establishing the feasibility of this versatile cellular platform, these findings demonstrate that CAR-iNKTs can drive meaningful clinical responses, warranting further investigation for treating incurable diseases where existing therapies remain largely ineffective.
4 Allogeneic, off-the-shelf iNKT therapies are feasible in refractory cancer patients
A second major advancement in iNKT therapy is the shift toward allogeneic, rather than autologous, approaches to exploit the full functionality of healthy iNKT cells that were never exposed to the suppression by tumors and the toxicity of cancer treatments (Table 2). Unlike conventional T cells, iNKTs and CAR-iNKTs do not cause GVHD and can actively suppress alloreactive responses. Moreover, despite their rare frequency in the circulation, iNKT and their engineered products exhibit a unique potential for substantial and sustained antigen-specific proliferative responses (62, 103), making them ideal for streamlining manufacturing and enhancing feasibility of off-the-shelf cellular therapies (Figure 3).
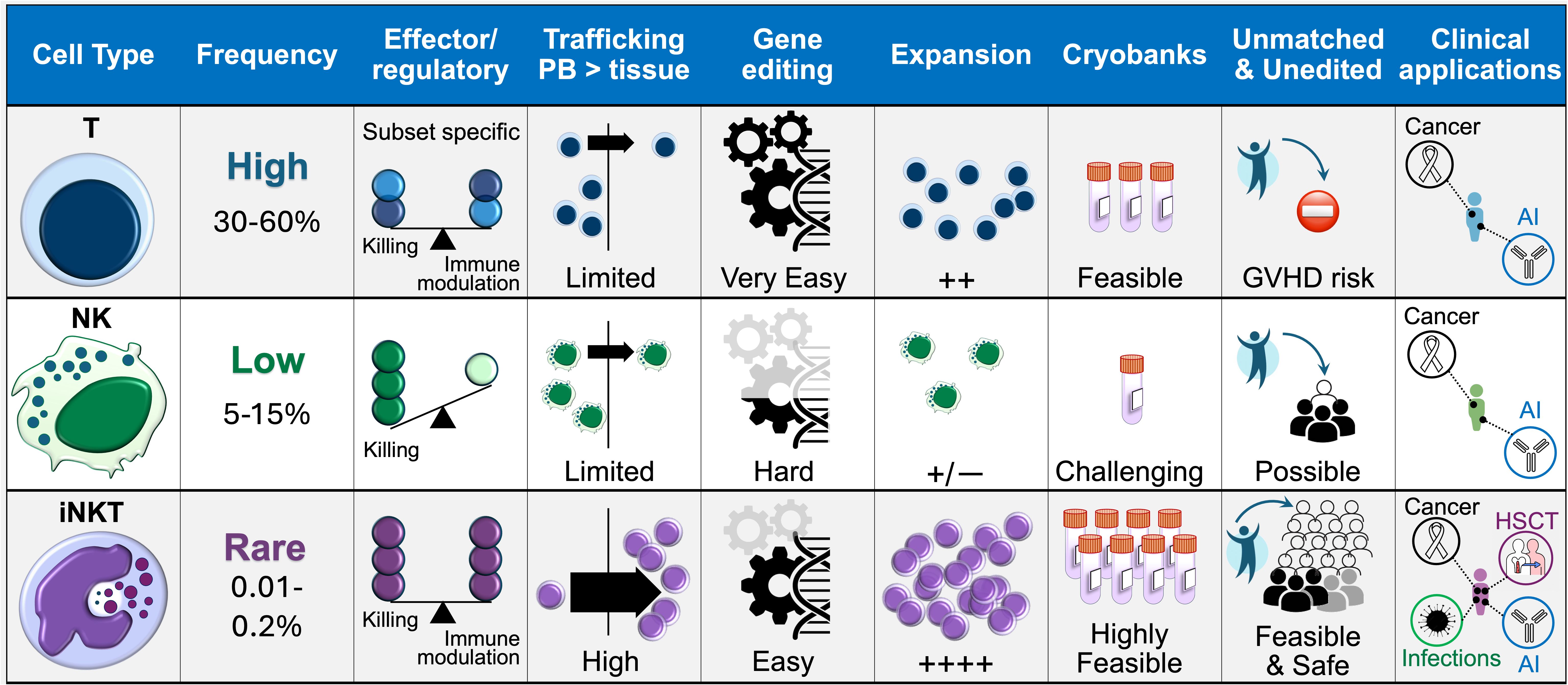
Figure 3. Comparison between T, NK and iNKT cell platforms. The biological properties of iNKT cells, along with their amenability to CAR engineering, in vitro expansion, and off-the-shelf use, lend themselves to clinical application in any patient regardless of MHC match and across a range of pathological settings, potentially enhancing the feasibility and accessibility of adoptive cell therapies. AI, autoimmune conditions.
In trial NCT04754100, donor-derived, pure, unedited iNKT cell products, manufactured and cryopreserved ahead of demand, were administered to unrelated and non-lymphodepleted patients with relapsed/refractory multiple myeloma, known to express CD1d (104). Despite relatively high cell doses (up to total 1×109), no GVHD, CRS, or ICANS was observed. Stable disease was reported in two of eight treated patients, including one with a sustained reduction of over 50% in bone marrow malignant cells despite prior refractoriness to six lines of treatment, including anti-CD38, PD-1, and SLAMF7 monoclonal antibodies (104).
Allogeneic iNKTs are now being explored in engineered settings. In trial NCT00840853, CD19-CAR-iNKT were co-engineered with IL-15 and shRNAs targeting β2-microglobulin and CD74, established strategies to downmodulate MHC class I and II molecules and promote immune evasion, and administered alongside a standard lymphodepleting regimen. Preliminary data showed that as low as 1 ×107 CD19-CAR-iNKTs/m2 induced complete remission in patients with refractory B cell malignancies, which included one acute lymphoblastic leukemia and three non-Hodgkin lymphoma (NHL) patients respectively (52). Donor iNKTs remained detectable for five weeks, a preliminary observation that raises as-yet unresolved questions about the role of iNKT cells in immune rejection, and the potential for their immune-tolerizing capabilities to counteract elimination of donor-derived CAR-iNKTs themselves. To date, this aspect of iNKT biology remains largely uncharted. It is also unclear whether long-term functional persistence of iNKTs is required to sustain remission, or if transient restoration of anti-tumor immunity may be sufficient in some patients. Results from this and future clinical trials using engineered allogeneic iNKTs will provide critical insights into these important questions.
To further advance off-the-shelf iNKT therapies, researchers are also exploring induced pluripotent stem cell (iPS)- and hematopoietic stem cell (HS)-derived iNKTs (105). In a first-in-human trial (jRCT2033200116), iPS-iNKTs are being administered into tumor-feeding arteries of patients with advanced recurrent/refractory head and neck squamous cell carcinoma (HNSCC) (106). The primary endpoint is to determine the safety profile and dose-limiting toxicity with infusions of up to 1×108 iPS-NKT cells/m2 without prior lymphodepletion (106), laying the foundation for clinical development of enhanced CAR iPS-NKTs (107, 108). Similarly, CAR HS-iNKTs are in preclinical development, with studies indicating that they retain the innate invariant NKT cell’s TCR, NK-like, and CAR effector functions of PBMC-derived iNKTs (109, 110). Both PBMC-derived and stem-cell-induced iNKTs boast exceptional expandability, enabling the production of many billions of cells in a single manufacturing run. However, a side-by-side comparison suggests that HS-iNKTs are skewed towards NK-like differentiation with more prominent direct cytotoxicity, and are phenotypically less immunogenic, which could enhance resistance to host immune rejection (109).
Altogether, these studies represent a stepping-stone towards the development of allo-resistant, off-the-shelf cells. While iNKTs’ ability to prevent their own rejection remains hypothetical, clinical and preclinical studies with allogeneic donor cells demonstrate their versatility and scalability for off-the-shelf cancer immunotherapy. The achievement of complete remissions in refractory B malignancies marks a major milestone (52). With iPS-NKT therapies already in the clinic and HS-iNKT therapies on the horizon, the field is rapidly advancing. These next-generation approaches promise more accessible, potentially more effective treatments, positioning the iNKT platform at the forefront of cancer immunotherapy with growing clinical evidence of their benefit.
5 Donor iNKTs show life-saving potential in acute inflammatory and infectious diseases
iNKTs play multiple, pivotal roles in the rapid clearance of pathogens, and they are critical in acute viral infections, where low iNKT numbers often correlate with severe outcomes (42, 46). While CD1d and glycolipid agonists have been extensively explored as vaccine adjuvants and potential treatments for chronic viral infections, direct administration of donor iNKTs could offer a more powerful strategy in inducing rapid remission in critically ill patients, overcoming limitations of patient iNKT’s low numbers and dysfunctions. Additionally, off-the-shelf availability without the need for prior chemotherapy or donor-recipient MHC matching, combined with iNKT’s innate-like, fast-acting function, make them particularly well suited for emergency interventions.
In trial NCT04582201, a cryobank of unmodified, allogeneic iNKT cells expanded from healthy donors enabled the treatment of twenty-one critically ill patients with acute respiratory distress syndrome (ARDS), including those on mechanical ventilation (50). A single infusion of donor-derived iNKTs led to a significant survival benefit (70% compared to 10-39% in controls), with some patients able to be extubated within 24 hours from iNKT infusion. The treatment also markedly reduced secondary infections, with no treatment-related toxicity observed even at doses as high as 109 cells. These impressive outcomes likely stemmed from iNKTs’ dual antiviral and immunomodulatory effects, effectively reversing immune exhaustion, mitigating cytokine storms, and restoring immune homeostasis. Further underscoring their clinical potential, a recent case report described a dramatic recovery in an immunosuppressed kidney transplant patient who developed severe COVID-19, progressing to respiratory failure requiring mechanical ventilation and extracorporeal membrane oxygenation (ECMO) (60). Complications included severe disseminated intravascular coagulation (DIC), life-threatening gastrointestinal and airway bleeding necessitating transfusions, and renal failure requiring continuous renal replacement therapy. Remarkably, a single infusion of 1 × 109 iNKT cells, infused under emergency authorization (IND 30029), led to rapid and complete recovery: the patient was weaned off oxygen, dialysis, and returned to baseline renal function with full independence in daily activities.
Collectively, these findings represent a milestone in off-the-shelf allogeneic cell therapy, demonstrating the feasibility, safety, and efficacy of unedited iNKTs in acute infectious settings where conventional MHC-restricted T cell therapies are neither indicated nor feasible. The potential for redosing iNKTs (possibly optimally from unrelated donors) without sustained alloantibody responses (50) further supports their suitability for repeated administration in patients facing recurrent infections, paving the way for broader clinical applications in critical care medicine.
6 iNKTs cells pave the way for allo-transplantation without immunosuppressive agents
Both donor and recipient iNKTs can play a critical role in suppressing alloreactive T cells responsible for GVHD. For example, clinical studies and experimental murine models of acute GVHD demonstrated that total lymphoid irradiation (TLI) preconditioning may promote the relative expansion of endogenous radio-resistant, Th2-polarized iNKTs, leading to the suppression of donor alloreactive T cells and activation of regulatory T cells (Tregs) (57–59). Similarly, donor iNKTs were shown to alleviate experimental GVHD in models of human alloreactivity and murine allogeneic transplantation (56, 111). As such, there is increasing interest in iNKT-based therapies for GVHD treatment.
Beyond their suppressive effects, iNKTs also play an active preventive role in GVHD, as demonstrated by findings in allo-HSCT recipients, where a lower risk of acute GVHD correlated with higher numbers of CD4- iNKT cells in donor grafts (56, 57), as well as faster and more robust post-transplant recovery of circulating iNKT cells (112–114). In pediatric haploidentical allo-HSCT, higher iNKT cell counts were associated not only with GVHD protection, but also with a reduced risk of leukemia relapse (115), further supporting the use of iNKTs as an anti-GVHD prophylaxis after allo-HSCT. The ability of iNKT to control pathogen infections (42, 46) provide additional rationale for their use in HSCT, where acute viral, bacterial and fungal infections and reactivation of latent virus infections, are frequent and at high risk of preventing engraftment as well as being fatal.
A Phase 1 study (NCT03802695) is evaluating the safety, tolerability, and efficacy of engineered donor grafts, termed Orca-Q grafts, in which CD34+ hematopoietic stem cell products are enriched in donor iNKTs, along with other T cell subsets prior to infusion (116–119). Among thirty-three recipients of haploidentical HSCT who underwent myeloablative conditioning and received only minimal GVHD prophylaxis (standalone tacrolimus without post-transplant cyclophosphamide), no cases of grade 4 acute GVHD were observed (120). Grade 2–3 acute GVHD occurred in only five patients, and no moderate-to-severe chronic GVHD was reported at ~1-year median follow-up compared to a historical control cohort with a 24–33% incidence of moderate-to-severe chronic GVHD. Additionally, one-year relapse-free survival reached 82%, highlighting the therapeutic promise of iNKT-enriched donor grafts (76). In an expansion arm of the same study, Orca-Q was administered without any GVHD prophylaxis (121, 122). This cohort differed from the previous one in that patients and donors were HLA-identical, and myeloablative conditioning was either busulfan/fludarabine/thiotepa (BFT; eleven patients) or total body irradiation-based (TBI; three patients). In the BTF group, only two had acute GVHD (grade 2), none developed chronic GVHD, and only one patient experienced relapse. At one year, overall survival (OS), relapse-free survival (RFS), and GVHD/relapse-free survival (GRFS) were all 90%. In the TBI group, one case of grade 3 acute GVHD and one of mild chronic GVHD were observed, leading to an 85% OS and RFS, and a 77% GRFS. These results highlight the potential of iNKT-enriched donor grafts to act as a bridge to faster immune reconstitution, reducing the need for anti-GVHD immune suppression while preserving GVT effect. Outcomes could be further improved in combination with iNKT-tailored preconditioning regimens.
Building on this momentum, a clinical trial at University Hospital Pilsen, Czech Republic, set to begin in 2026, will evaluate the feasibility and safety of third-party iNKT products as a part of GVHD prophylaxis in allo-HSCT recipients (123). Unlike prior studies in the transplant setting that have relied on donor at least partially matched iNKT cells, this trial will investigate the prophylactic infusion of third-party iNKT cells, further highlighting their unique versatility beyond conventional therapeutic applications. Similarly, MiNK Therapeutics, in collaboration with the University of Wisconsin, is developing an allo-iNKT platform for the prevention (as well as the treatment) of GVHD (124, 144). Preclinical studies in mice support this approach (53), demonstrating that third-party iNKTs can provide GVHD protection. By eliminating the requirement for donor-matched iNKTs, this strategy could significantly expand the feasibility and availability of iNKT therapies for both GVHD prophylaxis and broader applications in allo-HSCT.
7 Rational combinations with iNKT cells
Historically, the first trials exploring combinatorial strategies involving iNKT therapy employed DCs pulsed with KRN7000, a synthetic αGalCer derivative and potent iNKT agonist that is effectively presented by DCs and induces production of both Th1 and Th2 cytokines (UMIN000000722 (125), UMIN000000852 (126). Overall, eight out of eighteen patients with recurrent/refractory HNSCC showed significant tumor shrinkage within four to five weeks following infusion of iNKTs via tumor-feeding arteries in combination with one or two submucosal doses of KRN7000-pulsed DCs. Nine patients maintained stable disease, and all but three exhibited systemic immune responses and tumor-infiltrating lymphocyte (TIL) expansion, with no severe side effects. These studies demonstrated the feasibility of iNKT combinations to achieve superior activation, while preserving a safe profile. Variations in the lipid chemistry and formulation as well as the choice of specific APCs may in future enable tailored approaches to distinct clinical settings. For example, unlike aqueous KRN7000, liposomal formulations preferentially target B cells and skew iNKT responses toward allotolerizing cytokines, raising the possibility that they could be leveraged to promote both persistence of allogeneic iNKTs and CAR-iNKTs and optimal activation (62, 127, 128). Pharmacologic immune modulation in addition to KRN7000 has also been proposed, e.g., aimed at upregulating CD1d transcriptionally and epigenetically on CAR-iNKT targets to enhance killing, or co-administering low-dose lenalidomide to augment iNKT immunoadjuvant responses (129).
Checkpoint inhibitors have also been explored in combination with adoptively transferred iNKTs from healthy donors in relapsed or refractory solid tumors (NCT05108623) (130). A patient with refractory gastric cancer who had previously failed anti-PD-1 therapy demonstrated significant tumor regression after receiving a single iNKT cell infusion in combination with anti-PD1, with evidence of local immune activation and CD8+ T-mediated adjuvant effects (51). These clinical observations support the potential of iNKT-based therapies to overcome immune exhaustion in the TME. Additionally, they provide the rationale for using unmodified iNKT and checkpoint inhibitor combinations, even in CD1d-negative cancers, as an adjuvant strategy to overcome T and NK cell exhaustion and transform the TME into an immunologically “hot” ecosystem that supports continued tumor recognition and killing by endogenous cytotoxic cells. A single-arm phase 2 trial at Memorial Sloan Kettering (NCT06251793) is evaluating allo-iNKTs in combination with anti-CTLA4 (botensilimab), anti-PD1 (balstilimab), anti-VEGF (ramucirumab), and paclitaxel in patients with advanced refractory gastro-esophageal adenocarcinoma. Early results from fifteen patients suggest promising synergy between allo-iNKTs, checkpoint inhibitors, and sequential chemotherapy (131). Systemic increase in interferon-gamma correlated with immune expansion and T cell memory response in the circulation, and T cell infiltration and cross-presentation at the tumor site, all of which are known biomarkers of clinical efficacy and could predict durable clinical responses in otherwise unresponsive solid tumors. Notably, paclitaxel was administered after iNKTs and checkpoint inhibitors, preserving iNKT-mediated immune priming: a benefit that would have been likely lost with pre-transfer lymphodepletion.
Other monoclonal antibodies have been proposed in combination with CAR-iNKTs. In NCT03294954, GD2-CAR-iNKTs are infused with etanercept, an anti-TNF antibody, based on the observation that TNF promoted tumor progression while inhibition of TNF signaling improved outcomes in murine xenograft models of neuroblastoma (132). Additionally, anti-GD2 monoclonal antibodies are being explored in combination with iPS-derived NKTs to leverage their antibody dependent cellular cytotoxicity (ADCC) against neuroblastoma cells and enhance cytotoxicity through CD16-mediated degranulation and cytokine production (133). Collectively, these studies demonstrate the growing potential of rational combinations not only to enhance iNKT therapies but also overcome barriers to the clinical success of current treatment options in cancer and transplant settings (Figure 4).
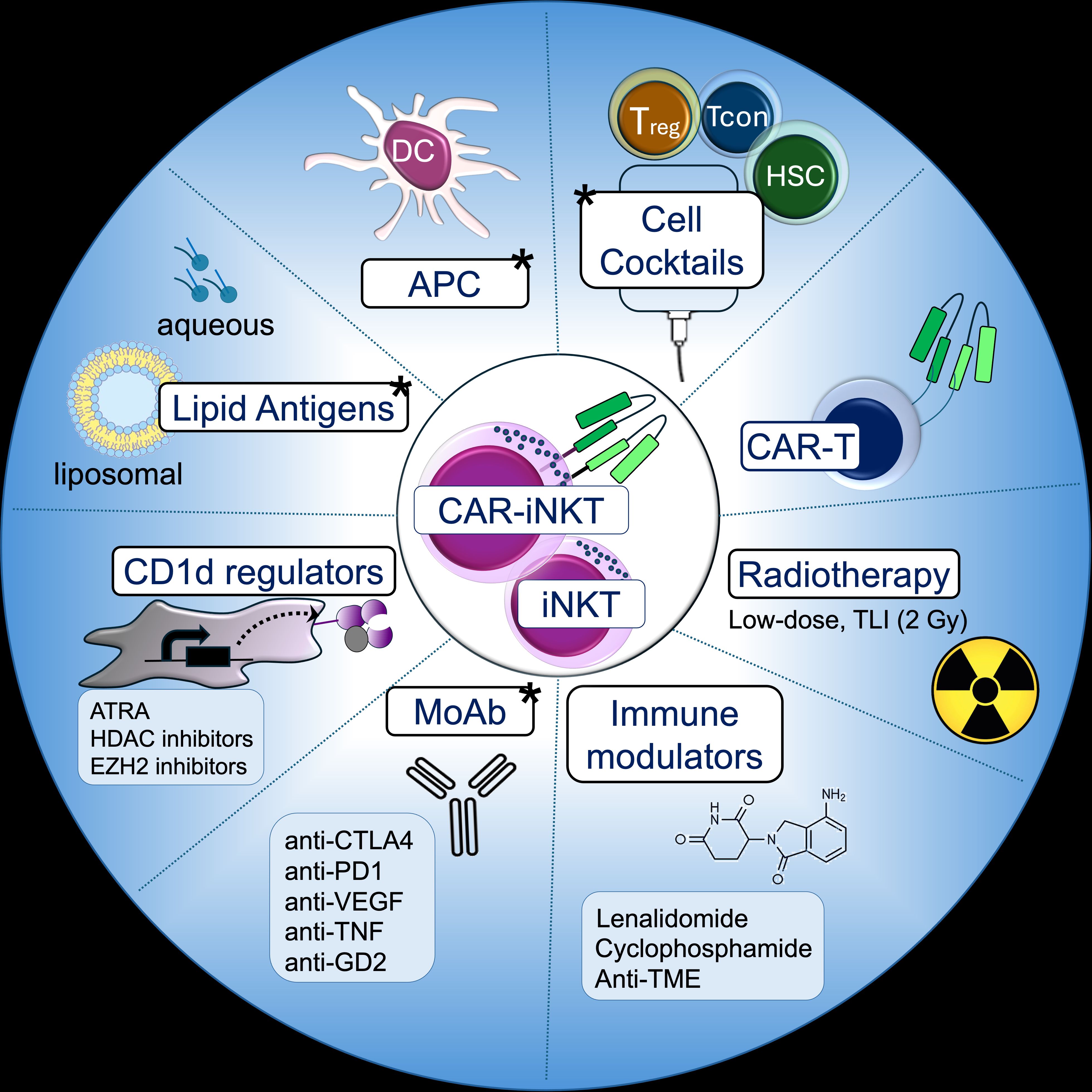
Figure 4. iNKT-based combinations. Strategies involving iNKT and CAR-iNKT cells that have been proposed and clinically tested (marked with a star).
8 Conclusions and prospects
These are transformative times for iNKT cell-based therapies. Since their discovery about 40 years ago, these unique cells have emerged as a powerful tool against a range of currently incurable diseases. Around 200 patients treated with iNKT cell-related therapies have been reported to date, including 129 patients with cancer and severe inflammatory conditions who received autologous or allogeneic iNKT cells, while the remainder were treated with either αGalCer-based approaches or iNKT-enriched cell products. Strong evidence supports that iNKT-based approaches are practical, and that large-scale iNKT cell manufacturing is feasible with promising signs of deep and durable remissions. Some patients with refractory solid tumors achieved complete remissions, while others with acute infection-related life-threatening inflammation were cured. Clinical success was attained with both autologous and allogeneic products, and while further optimizations will be instrumental in realizing their full curative potential, off-the-shelf iNKT therapies appear to be a feasible goal in the next few years.
As clinical trials progress, we see fundamental questions being addressed with important takeaways that will inform future cell therapy studies:
1. How to best harness iNKT cell products and their multiple beneficial activities (anti-pathogen, immunomodulatory, promotion of engraftment and GvHD suppression)? Should persistence be prioritized, or is their ability to restore immune homeostasis sufficient for durable responses? While acute infections and immune-mediated complications may resolve without long-lived donor cells (NCT04582201, IND 30029), the potential of iNKT cells to reverse immune exhaustion and ignite endogenous anti-tumor responses raises the possibility of lasting remissions, even without continuous patrolling by donor-derived iNKTs (NCT05108623). Ongoing and future trials in cancer patients will provide valuable insights into the role of long-lasting immunoadjuvant effects over iNKT persistence and direct immunosurveillance in durable remissions and possibly cures.
2. Given the feasibility of off-the-shelf iNKT products, should customized products, i.e., tailored to different disease settings and patient needs, be employed? Ongoing molecular studies are shedding light on human iNKT heterogeneity within and across individuals, refining our understanding of distinct subsets and their therapeutic roles (NCT03294954 and (62, 98, 134–136). Donor-specific iNKT biomarkers of survival and immune fitness were found to correlate with better clinical outcomes (NCT03294954 and (98, 134, 135), suggesting an intriguing potential for predicting optimal cellular products to ensure maximal therapeutic effect. With the increasing use of single-cell sequencing technologies, novel biomarkers of function could soon guide both donor selection and the customization of cellular products. Additionally, trials in canine cancer patients have emerged as a valuable preclinical platform to refine iNKT cell therapies in a parallel patient population with tumors and immune system that closely mirror those of humans (62). These studies serve as a critical bridge between mouse and human, enabling the selective advancement of the most promising iNKT therapies to the clinic, with designs best suited to patient needs and disease contexts.
3. Regarding the need of bespoke approaches, should iNKT-guided schedules be designed and administered using ad hoc preconditioning regimens based on iNKTs’ unique mechanisms? Thus far, PBMC-derived allogeneic CAR-iNKTs have followed conventional CAR-T cell paradigms, including MHC editing and lymphodepletion typically required to counteract T cell rejection (NCT00840853, and (52, 137)). However, some allogeneic iNKT and iPS-iNKT products have been administered without prior MHC editing or intensive preconditioning, capitalizing on iNKT-specific mode of action (jRCT2033200116, NCT04754100, NCT05108623, NCT06251793). Trials and correlative studies in COVID-19 patients with severe ARDS (50) and parallel studies in canines (62) support a path toward such administration strategy, informed by iNKTs’ intrinsic properties rather than conventional T-cell approaches.
4. Last, iNKT safety profile makes them ideal for combinatorial strategies, integrating with pharmacologic agents, biologics, and even other cell therapies. Learning from the success of polytherapy regimens that improved clinical outcomes of cancer patients compared to standalone monotherapies (138–140), will the optimal iNKT therapy be a multimodal platform incorporating synergistic compounds, biological agents and other cell types? The emerging evidence that iNKTs can mediate durable donor cell engraftment with simultaneous anti-GVHD and pro-GVT effects (121) may offer unprecedented opportunities to combine iNKT cells with multiple allogeneic cell types: bulk T, selected αβ or yð T, NK, macrophages and other APCs, and even non-hematopoietic stem cell and progenitors, whether unmodified or engineered with CARs, TCRs and other molecules, and from the same or different donors. The projected clinical impact is far-reaching, with the potential to expand the versatility, feasibility, and efficacy of cellular therapies by harnessing the complementary therapeutic profiles of distinct cell populations across a range of diseases while minimizing the risk of severe toxicities.
While future trials are anticipated to address these points, iNKT present two significant advantages over conventional T cell therapies, supporting their broad clinical implementation. Firstly, both allogeneic and stem cell-derived iNKTs offer exceptional scalability, with evidence of over a million-fold expansion in less than one month of ex-vivo culture, far surpassing the capabilities of conventional T cell-based products (62, 88). One production round can yield thousands of billions of cells (> 1000 doses), enabling the creation of cost-efficient, universal cell banks. This could revolutionize accessibility, providing ready-to-use, off-the-shelf therapies that transcend geographical and logistical barriers. Secondly, iNKT cells represent a uniquely versatile platform, with applications spanning cancer, infections, and immune-mediated disorders associated with promising clinical evidence of efficacy. Unlike conventional adoptive T and NK cell therapies, iNKT cells act rapidly, exert both effector and immunomodulatory functions, and may not require chemotherapy preconditioning, vastly expanding their therapeutic reach.
With these strengths, iNKT cell therapies are poised to gain significant traction in the coming years, offering an excellent opportunity to reshape treatment landscapes and deliver breakthrough therapies for patients who currently have no options. As the field advances, the next decade may mark the tipping point where iNKT cells transition from a promising innovation to a cornerstone of cellular immunotherapy.
Author contributions
AR: Project administration, Writing – review & editing, Data curation, Visualization, Conceptualization, Writing – original draft. NM: Writing – review & editing, Funding acquisition, Resources, Project administration. ME: Project administration, Writing – review & editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Institute of Health U01CA272270-03.
Acknowledgments
AR and NM are supported by the National Institute of Health (U01CA272270-03), a Sebastian Strong Foundation Discovery Science Award, the University of Pennsylvania Sarcoma Research Program and the University of Pennsylvania Research Foundation (AR). ME is the Chief Scientific Officer at LIfT BioSciences, and MiNK Therapeutics Senior Advisor & SAB Member.
Conflict of interest
Author ME is a Senior Advisor & SAB Member at Mink Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. (2001) 98:3294–8. doi: 10.1073/pnas.051604498
2. Gumperz JE, Miyake S, Yamamura T, and Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. (2002) 195:625–36. doi: 10.1084/jem.20011786
3. Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. (2007) 122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x
4. Bendelac A, Killeen N, Littman D, and Schwartz R. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. (1994) 263:1774.
5. Dellabona P, Padovan E, Casorati G, Brockhaus M, and Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med. (1994) 180:1171–6. doi: 10.1084/jem.180.3.1171
6. Lantz O and Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. (1994) 180:1097–106. doi: 10.1084/jem.180.3.1097
7. Matsuda JL, Gapin L, Fazilleau N, Warren K, Naidenko OV, and Kronenberg M. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor beta repertoire and small clone size. Proc Natl Acad Sci USA. (2001) 2001:98:12636–12641. doi: 10.1073/pnas.221445298
8. Bendelac A, Savage PB, and Teyton L. The biology of NKT cells. Annu Rev Immunol. (2006) 25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711
9. Kronenberg M and Gapin L. The unconventional lifestyle of NKT cells [10. 1038/nri854]. Nat Rev Immunol. (2002) 2:557–68. doi: 10.1038/nri854
10. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, and Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. (2004) 4:231–7. doi: 10.1038/nri1309. PMID 15039760
11. Greenaway HY, Ng B, Price DA, Douek DC, Davenport MP, and Venturi V. NKT and MAIT invariant TCRα sequences can be produced efficiently by VJ gene recombination. Immunobiology. (2013) 218:213–24. doi: 10.1016/j.imbio.2012.04.003
12. Makino Y, Kanno R, Ito T, Higashino K, and Taniguchi M. Predominant expression of invariant V[alpha]14+ TCR [alpha] chain in NK1.1+ T cell populations. Int Immunol. (1995) 7:1157–61. doi: 10.1093/intimm/7.7.1157
13. Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, and Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. (1995) 268:863–5. doi: 10.1126/science.7538697
14. Exley M, Garcia J, Balk SP, and Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. (1997) 186:109–20. doi: 10.1084/jem.186.1.109
15. Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, and Taniguchi M. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc Natl Acad Sci U S A. (1986) 83:8708–12.
16. Naumov YN, Bahjat KS, Gausling R, Abraham R, Exley MA, Koezuka Y, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci U S A. (2001) 98:13838–43. doi: 10.1073/pnas.251531798
17. Ohteki T and MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4 + 8- and CD4-8- subsets of natural killer 1.1+ T cell receptor-[alpha]/[beta]+ cells in the liver of mice. J Exp Med. (1994) 180:699–704. doi: 10.1084/jem.180.2.699
18. Bix M, Coles M, and Raulet D. Positive selection of V beta 8+ CD4-8- thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. (1993) 178:901–8. doi: 10.1084/jem.178.3.901
19. Coles MC and Raulet DH. Class I dependence of the development of CD4+ CD8- NK1.1+ thymocytes. J Exp Med. (1994) 180:395–9. doi: 10.1084/jem.180.1.395
20. Adachi Y, Koseki H, Zijlstra M, and Taniguchi M. Positive selection of invariant V alpha 14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow-derived cells. Proc Natl Acad Sci U S A. (1995) 92:1200–4. doi: 10.1073/pnas.92.4.1200
21. Porcelli S, Yockey CE, Brenner MB, and Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. (1993) 178:1–16. doi: 10.1084/jem.178.1.1
22. Porcelli S, Morita CT, and Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. (1992) 360:593–7. doi: 10.1038/360593a0
23. Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, and Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. (1997) 277:339–45. doi: 10.1126/science.277.5324.339
24. Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. (1998) 279:1541–4. doi: 10.1126/science.279.5356.1541
25. Kawano T. CD1d-restricted and TCR-mediated activation of V[alpha]14 NKT cells by glycosylceramides. Science. (1997) 278:1626–9. doi: 10.1126/science.278.5343.1626
26. Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. (2000) 100:37–47.
27. Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, and Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. (1993) 80:561–5.
28. Brigl M and Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. (2004) 22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608
29. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. (1997) 278:1626–9. doi: 10.1126/science.278.5343.1626
30. Webb TJ, Bieler JG, Schneck JP, and Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods. (2009) 346:38–44. doi: 10.1016/j.jim.2009.05.003
31. Benlagha K, Weiss A, Beavis A, Teyton L, and Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. (2000) 191:1895–903. doi: 10.1084/jem.191.11.1895
32. Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. (2000) 192:741–54. doi: 10.1084/jem.192.5.741
33. Lee PT, Benlagha K, Teyton L, and Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. (2002) 195:637–41. doi: 10.1084/jem.20011908
34. Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. (2016) 126:2341–55. doi: 10.1172/JCI83476
35. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. (2011) 41:1913–23. doi: 10.1002/eji.200940278
36. Jayasinghe RG, Hollingsworth D, Schedler NC, Landy E, Boonchalermvichian C, Gupta B, et al. Single-cell transcriptomic profiling reveals diversity in human iNKT cells across hematologic tissues. Cell Rep. (2025) 44. doi: 10.1016/j.celrep.2025.115587
37. Maas-Bauer K, Köhler N, Stell AV, Zwick M, Acharya S, Rensing-Ehl A, et al. Single-cell transcriptomics reveal different maturation stages and sublineage commitment of human thymic invariant natural killer T cells. J Leukoc Biol. (2024) 115:401–9. doi: 10.1093/jleuko/qiad113
38. Eger KA, Sundrud MS, Motsinger AA, Tseng M, Van Kaer L, and Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PloS One. (2006) 1:e50. doi: 10.1371/journal.pone.0000050
39. Ngai H, Tian G, Courtney AN, Ravari SB, Guo L, Liu B, et al. IL-21 selectively protects CD62L(+) NKT cells and enhances their effector functions for adoptive immunotherapy. J Immunol. (2018) 201:2141–53. doi: 10.4049/jimmunol.1800429
40. Landoni E, Woodcock MG, Barragan G, Casirati G, Cinella V, Stucchi S, et al. IL-12 reprograms CAR-expressing natural killer T cells to long-lived Th1-polarized cells with potent antitumor activity. Nat Commun. (2024) 15:89. doi: 10.1038/s41467-023-44310-y
41. Vivier E, Ugolini S, Blaise D, Chabannon C, and Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. (2012) 12:239–52. doi: 10.1038/nri3174
42. Brigl M and Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. (2010) 22:79–86. doi: 10.1016/j.smim.2009.10.006
43. Tupin E, Kinjo Y, and Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. (2007) 5:405–17. doi: 10.1038/nrmicro1657
44. Novak J and Lehuen A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine. (2011) 53:263–70. doi: 10.1016/j.cyto.2010.11.001
45. Stetson DB. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. (2003) 198:1069–76. doi: 10.1084/jem.20030630
46. Brigl M, Bry L, Kent SC, Gumperz JE, and Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. (2003) 4:1230–7. doi: 10.1038/ni1002
47. Nagarajan NA and Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. (2007) 178:2706–13. doi: 10.4049/jimmunol.178.5.2706
48. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. (2007) 27:597–609. doi: 10.1016/j.immuni.2007.08.017
49. Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. (2007) 104:20490–5. doi: 10.1073/pnas.0710145104
50. Hammond TC, Purbhoo MA, Kadel S, Ritz J, Nikiforow S, Daley H, et al. A phase 1/2 clinical trial of invariant natural killer T cell therapy in moderate-severe acute respiratory distress syndrome. Nat Commun. (2024) 15:974. doi: 10.1038/s41467-024-44905-z
51. Hadfield MJ, Safran H, Purbhoo MA, Grossman JE, Buell JS, and Carneiro BA. Overcoming resistance to programmed cell death protein 1 (PD-1) blockade with allogeneic invariant natural killer T-cells (iNKT). Oncogene. (2024). doi: 10.1038/s41388-024-02948-y
52. Ramos CA, Courtney AN, Lulla PD, Hill LC, Kamble RT, Carrum G, et al. Off-the-shelf CD19-specific CAR-NKT cells in patients with relapsed or refractory B-cell Malignancies. Transplant Cell Ther. (2024) 30:S41–2. doi: 10.1016/j.jtct.2023.12.072
53. Schneidawind D, Baker J, Pierini A, Buechele C, Luong RH, Meyer EH, et al. Third-party CD4+ invariant natural killer T cells protect from murine GVHD lethality. Blood. (2015) 125:3491–500. doi: 10.1182/blood-2014-11-612762
54. Schneidawind D, Pierini A, Alvarez M, Pan Y, Baker J, Buechele C, et al. CD4+ invariant natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells. Blood. (2014) 124:3320–8. doi: 10.1182/blood-2014-05-576017
55. Coman T, Rossignol J, D’Aveni M, Fabiani B, Dussiot M, Rignault R, et al. Human CD4- invariant NKT lymphocytes regulate graft versus host disease. Oncoimmunology. (2018) 7:e1470735. doi: 10.1080/2162402x.2018.1470735
56. Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. (2012). doi: 10.1182/blood-2011-11-389304
57. Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. (2005) 353:1321–31. doi: 10.1056/NEJMoa050642
58. Yao Z, Liu Y, Jones J, and Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. (2009) 39:763–75. doi: 10.1002/eji.200838657
59. Kohrt HE, Pillai AB, Lowsky R, and Strober S. NKT cells, Treg, and their interactions in bone marrow transplantation. Eur J Immunol. (2010) 40:1862–9. doi: 10.1002/eji.201040394
60. Hammond TC, Channick C, Favano T, Purbhoo MA, Exley M, Vandiver MS, et al. Persistent SARS-coV-2, COVID-19 associated acute respiratory distress syndrome, severe coagulopathy and massive hemoptysis in a renal transplant patient on veno-venous extracorporeal membrane oxygenation. Am J Respir Crit Care Med. (2024) 2024:2024. doi: 10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A5563
61. Hess NJ, SB N, Bobeck EA, McDougal CE, Ma S, Sauer JD, et al. iNKT cells coordinate immune pathways to enable engraftment in nonconditioned hosts. Life Sci Alliance. (2021) 4. doi: 10.26508/lsa.202000999
62. Rotolo A, Whelan EC, Atherton MJ, Kulikovskaya I, Jarocha D, Fraietta JA, et al. Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients. Cell Rep Med. (2023) 4. doi: 10.1016/j.xcrm.2023.101241
63. Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. (2004) 199:1213–21. doi: 10.1084/jem.20031462
64. Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. (2012) 122:2221–33. doi: 10.1172/jci59535
65. Crosby CM and Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. (2018) 18:559–74. doi: 10.1038/s41577-018-0034-2
66. Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, and Hogquist KA. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity. (2015) 43:566–78. doi: 10.1016/j.immuni.2015.06.025
67. Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. (2014) 124:2824–33. doi: 10.1182/blood-2013-11-541235
68. Rotolo A, Chaidos A, and Karadimitris A. The role of invariant NKT cells in immunity. Encyclopedia Immunobiology. (2016). doi: 10.1016/B978-0-12-374279-7.03010-1
69. Delfanti G, Cortesi F, Perini A, Antonini G, Azzimonti L, de Lalla C, et al. TCR-engineered iNKT cells induce robust antitumor response by dual targeting cancer and suppressive myeloid cells. Sci Immunol. (2022) 7:eabn6563. doi: 10.1126/sciimmunol.abn6563
70. Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. (2011) 140:119–29. doi: 10.1016/j.clim.2010.10.005
71. Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. (2009) 119:1524–36. doi: 10.1172/jci37869
72. Bald T, Krummel MF, Smyth MJ, and Barry KC. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol. (2020) 21:835–47. doi: 10.1038/s41590-020-0728-z
73. D’Avanzo C, Blaeschke F, Lysandrou M, Ingelfinger F, and Zeiser R. Advances in cell therapy: progress and challenges in hematological and solid tumors. Trends Pharmacol Sci. (2024) 45:1119–34. doi: 10.1016/j.tips.2024.10.016
74. Spanoudakis E, Hu M, Naresh K, Terpos E, Melo V, Reid A, et al. Regulation of multiple myeloma survival and progression by CD1d. Blood. (2009) 113:2498–507. doi: 10.1182/blood-2008-06-161281
75. Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. (2001) 167:4046–50.
76. Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. (2005) 116:87–93. doi: 10.1002/ijc.20998
77. Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. (2007) 25:862–8. doi: 10.1200/jco.2006.08.5787
78. Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to Malignant multiple myeloma. J Exp Med. (2003) 197:1667–76. doi: 10.1084/jem.20021650
79. Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, and Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. (2003) 122:617–22. doi: 10.1046/j.1365-2141.2003.04465.x
80. Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. (2002) 8:3702–9.
81. Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, and Nicol AJ. Modulation of human Valpha24(+)Vbeta11(+) NKT cells by age, Malignancy and conventional anticancer therapies. Br J Cancer. (2004) 91:1880–6. doi: 10.1038/sj.bjc.6602218
82. Exley MA, Lynch L, Varghese B, Nowak M, Alatrakchi N, and Balk SP. Developing understanding of the roles of CD1d-restricted T cell subsets in cancer: reversing tumor-induced defects. Clin Immunol. (2011) 140:184–95. doi: 10.1016/j.clim.2011.04.017
83. Terabe M and Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. (2008) 101:277–348. doi: 10.1016/s0065-230x(08)00408-9
84. Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. (2005) 11:7322–7. doi: 10.1158/1078-0432.Ccr-05-0877
85. Yanagisawa K, K-i S, Ishikawa Y, Nozue M, Todoroki T, and Fukao K. Impaired proliferative response of Vα24 NKT cells from cancer patients against α-galactosylceramide1. J Immunol. (2002) 168:6494–9. doi: 10.4049/jimmunol.168.12.6494
86. Motohashi S, Okamoto Y, Yoshino I, and Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. (2011) 140:167–76. doi: 10.1016/j.clim.2011.01.009
87. Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: A phase I clinical trial. Clin Cancer Res. (2017) 23:3510–9. doi: 10.1158/1078-0432.CCR-16-0600
88. Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, et al. Enhanced anti-lymphoma activity of CAR19-iNKT cells underpinned by dual CD19 and CD1d targeting. Cancer Cell. (2018) 34:596–610.e11. doi: 10.1016/j.ccell.2018.08.017
89. Poels R, Drent E, Lameris R, Katsarou A, Themeli M, van der Vliet HJ, et al. Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int J Mol Sci. (2021) 22:1096. doi: 10.3390/ijms22031096
90. Simon B, Wiesinger M, März J, Wistuba-Hamprecht K, Weide B, Schuler-Thurner B, et al. The generation of CAR-transfected natural killer T cells for the immunotherapy of melanoma [Article. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19082365
91. Rotolo A, Whelan EC, Atherton MJ, Kulikovskaya I, Jarocha D, Fraietta JA, et al. Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients. Cell Rep Med. (2023). doi: 10.1016/j.xcrm.2023.101241
92. Jiang W, Gu G, Zhang Y, Song Y, Shi M, Wang G, et al. Novel mesothelin-targeted chimeric antigen receptor-modified UNKT cells are highly effective in inhibiting tumor progression [Article. Pharmacol Res. (2023) 197. doi: 10.1016/j.phrs.2023.106942
93. Liu Y, Dang Y, Zhang C, Liu L, Cai W, Li L, et al. IL-21-armored B7H3 CAR-iNKT cells exert potent antitumor effects [Article. iScience. (2024) 27. doi: 10.1016/j.isci.2023.108597
94. Xu X, Huang W, Heczey A, Liu D, Guo L, Wood M, et al. NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin Cancer Res. (2019) 25:7126–38. doi: 10.1158/1078-0432.Ccr-19-0421
95. Rowan AG, Ponnusamy K, Ren H, Taylor GP, Cook LBM, and Karadimitris A. CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma. Front Immunol. (2023) 14:1118681. doi: 10.3389/fimmu.2023.1118681
96. Simonetta F, Lohmeyer JK, Hirai T, Maas-Bauer K, Alvarez M, Wenokur AS, et al. Allogeneic CAR-invariant natural killer T cells exert potent antitumor effects through host CD8 T cell cross-priming. Clin Cancer Res. (2021). doi: 10.1158/1078-0432.CCR-21-1329
97. O’Neal J, Cooper ML, Ritchey JK, Gladney S, Niswonger J, González LS, et al. Anti-myeloma efficacy of CAR-iNKT is enhanced with a long-acting IL-7, rhIL-7-hyFc. Blood advances. (2023) 7:6009–22. doi: 10.1182/bloodadvances.2023010032
98. Heczey A, Xu X, Courtney AN, Tian G, Barragan GA, Guo L, et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med. (2023) 29:1379–88. doi: 10.1038/s41591-023-02363-y
99. Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. (2020) 26:1686–90. doi: 10.1038/s41591-020-1074-2
100. Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. (2017) 25:2214–24. doi: 10.1016/j.ymthe.2017.05.012
101. Zhang J, Shen W, Kuang M, Guo F, Qu Y, Zhang H, et al. Interim safety and efficacy of anti-CD70 CAR-NKT (CGC729) for patients with advanced renal cell carcinoma. In: 27th Annual Meeting of the American Society for Gene & Cell Therapy; 2024 April. Mol Therapy;. (2024) 2024:881. Available online at: https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(24)00237-5. (Accessed July 25, 2025).
102. Arovella. Research agreement with university of north carolina . Available online at: https://www.arovella.com/news-presentations/vojj6rxl10pjhsai2j2kj6q5f9r1g52025. (Accessed July 25, 2025).
103. Chantzoura E, Sharoni EA, Masakayan R, Popis M, Moskowitz D, Ibbett P, et al. 322 MiNK-413: a next generation armored allogenic BCMA CAR iNKT product. J ImmunoTherapy Cancer. (2022) 10:A338–8. doi: 10.1136/jitc-2022-SITC2022.0322
104. Stevens D, Mo C, Garmezy B, Hamm J, Carneiro B, Wilky B, et al. 647 Phase I studies of AgenT-797, a novel allogeneic invariant natural killer T (iNKT) cell therapy, for the treatment of patients with solid tumors or multiple myeloma. J ImmunoTherapy Cancer. (2022) 10:A678–8. doi: 10.1136/jitc-2022-SITC2022.0647
105. Aoki T, Motohashi S, and Koseki H. Regeneration of invariant natural killer T (iNKT) cells: application of iPSC technology for iNKT cell-targeted tumor immunotherapy. Inflammation Regeneration. (2023) 43:27. doi: 10.1186/s41232-023-00275-5
106. Motohashi S. The role of regenerative invariant NKT cells in cancer immunotherapy for head and neck cancer. Personalized Med Universe. 2022. (2022) 11):6. doi: 10.46459/pmu.2022004
107. Shioya K, Matsumura T, Urakami A, Shigeura T, Naito A, Lin Y, et al. iPSC-derived HER2 CAR-iNKT cells enhance the activity of immune cells against cancer cells. In: American Association for Cancer Research Annual Meeting 2025; 2025 2025 Apr 25-30;. Cancer Res;. (2025).
108. Urakami A, Shigeura T, Watanabe N, Shiraishi M, Lin Y, Shioya K, et al. Generation of Functional BCMA CAR-iNKT Cells from Clinical-Grade iPSCs via a GMP-Compliant Manufacturing Process with Capacity for Linear Scale-Up ASGCT 28th Annual Meeting; 2025 May 13, 2025; New Orleans. Mol Therapy;. (2025) 33. Available online at: https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(25)00302-8 (Accessed July 25, 2025).
109. Zhu Y, Smith DJ, Zhou Y, Li YR, Yu J, Lee D, et al. Development of hematopoietic stem cell-engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell. (2019) 25:542–557.e9. doi: 10.1016/j.stem.2019.08.004
110. Li Y-R, Fang Y, Niu S, Zhu Y, Chen Y, Lyu Z, et al. Allogeneic CD33-directed CAR-NKT cells for the treatment of bone marrow-resident myeloid Malignancies. Nat Commun. (2025) 16:1248. doi: 10.1038/s41467-025-56270-6
111. Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism [Research Support, N.I.H., Extramural. Blood. (2011) 117:3220–9. doi: 10.1182/blood-2010-08-303008
112. Komanduri KV. GVHD protection? ThiNK iNKT cells. Blood. (2012) 120:1972–3. doi: 10.1182/blood-2012-07-439612
113. De Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. (2011) 41:602–10. doi: 10.1002/eji.201041211
114. Rubio MT, Moreira-Teixeira L, Bachy E, Bouillie M, Milpied P, Coman T, et al. Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival [Research Support, Non-U.S. Gov’t]. Blood. (2012) 120:2144–54. doi: 10.1182/blood-2012-01-404673
115. De Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state [Research Support, Non-U.S. Gov’t]. J Immunol. (2011) 186:4490–9. doi: 10.4049/jimmunol.1003748
116. Srour SA, Salhotra A, Lowsky R, Hoeg RT, Waller EK, Pavlova A, et al. Safety and efficacy of orca-Q with haploidentical donors for the treatment of advanced hematologic Malignancies without the use of post-transplant cyclophosphamide. Blood. (2023) 142:773–3. doi: 10.1182/blood-2023-189534
117. Srour SA, Salhotra A, Lowsky R, Hoeg RT, Saad A, Pavlova A, et al. 33 - orca-Q demonstrates favorable gvHD-and-relapse-free survival with haploidentical donors without post-transplant cyclophosphamide. Transplant Cell Ther. (2023) 29:S28–9. doi: 10.1016/S2666-6367(23)00102-1
118. Salhotra A, Srour SA, Hoeg RT, Saad A, Meyer EH, Pavlova A, et al. Orca-Q demonstrates favorable gvHD-and-relapse-free survival in haploidentical transplants without post-transplant cyclophosphamide. Blood. (2022) 140:1865–6. doi: 10.1182/blood-2022-170459
119. Salhotra A, Srour SA, Hoeg RT, Saad A, Meyer EH, Pavlova A, et al. Safety and Efficacy of Orca-Q with Haploidentical Donors for the Treatment of Advanced Hematologic Malignancies without the Use of Post-Transplant Cyclophosphamide. ASH. (2023) 2022. Available online at: https://orcabio.com/wp-content/uploads/2023/12/ASH-2023_Safety-and-Efficacy-of-Orca-Q-with-Haploidentical-Donors-for-the-Treatment-of-Advanced-Hematologic-Malignancies-without-the-Use-of-Post-Transplant-Cyclophosphamide.pdf (Accessed July 25, 2025).
120. Srour SA, Salhotra A, Lowsky R, Hoeg RT, Waller EK, Pavlova A, et al. Safety and Efficacy of Orca-Q with Haploidentical Donors for the Treatment of Advanced Hematologic Malignancies without the Use of Post-Transplant Cyclophosphamide(2023). Available online at: https://orcabio.com/wp-content/uploads/2023/12/ASH-2023_Safety-and-Efficacy-of-Orca-Q-with-Haploidentical-Donors-for-the-Treatment-of-Advanced-Hematologic-Malignancies-without-the-Use-of-Post-Transplant-Cyclophosphamide.pdf. (Accessed July 25, 2025).
121. Abedi M, Srour SA, Salhotra A, Hoeg RT, Waller EK, Choe H, et al. Preliminary safety and efficacy of myeloablative orca-Q with no gvHD prophylaxis for treatment of advanced hematologic Malignancies. Blood. (2024) 144:382–2. doi: 10.1182/blood-2024-204256
122. Salhotra A, Abedi M, Srour SA, Hoeg RT, Waller EK, Choe HK, et al. Preliminary safety and efficacy of myeloablative orca-Q with no gvHD prophylaxis for treatment of advanced hematologic Malignancies. Transplant Cell Ther. (2025) 31:S107. doi: 10.1016/j.jtct.2025.01.169
123. Kříž T, Dekojová T, Gmucová H, Klieber R, Lysák D, Jindra P, et al. Reconstitution of Invariant Natural Killer T Cells Post-Allogeneic Stem Cell Transplantation: Pilot data for a Phase I Clinical Trial. EHA. (2024) 2024:4114. Available online at: https://onlinelibrary.wiley.com/toc/25729241/2024/8/S1. (Accessed July 25, 2025).
124. MiNK Therapeutics. MiNK therapeutics awarded prestigious NIAID grant to advance allo-iNKT cell therapy for prevention of gvHD in stem cell transplant patients. (2025). Available online at: https://investor.minktherapeutics.com/news-releases/news-release-details/mink-therapeutics-awarded-prestigious-niaid-grant-advance-allo.
125. Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and α-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. (2009) 100:1092–8. doi: 10.1111/j.1349-7006.2009.01135.x
126. Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. (2011) 138:255–65. doi: 10.1016/j.clim.2010.11.014
127. Rotolo A, Whelan EC, Atherton MJ, Kulikovskaya I, Jarocha D, Fraietta JA, et al. Allogeneic chimeric antigen receptor invariant NKT cells for the treatment of canine and human solid tumors. World Veterinary Cancer Congress Tokyo. (2024) 2024.
128. Whelan EC, Sussman J, Swain JJ, Srivastava S, Fu Y, Sun Y, et al. (2025). Deveolpment of CAR-B7H3 iNKT cells for the treatment of solid tumors, in: CiRT 2025 Meeting, Prague, (Prague: Consortium for iNKT Research and Therapy (CiRT)) February 14-15, 2025.
129. Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. (2013) 121:423–30. doi: 10.1182/blood-2012-06-435503
130. Carneiro B, Garmezy B, Hamm JT, Sanborn RE, Wise-Draper T, Khoueiry A--, et al. Abstract CT275: Phase 1 clinical update of allogeneic invariant natural killer T cells (iNKTs), agenT-797, alone or in combination with pembrolizumab or nivolumab in patients with advanced solid tumors. Cancer Res. (2023) 83:CT275–5. doi: 10.1158/1538-7445.Am2023-ct275
131. Cytryn SL, Evans P, Song J, Hieronymi S, Segal M, Tsai C, et al. Biomarker Analysis From a Phase II Study of AgenT-797 (invariant natural killer T cells) with Botensilimab (Fc-enhanced CTLA-4 inhibitor) and Balstilimab (anti-PD-1) in PD-1 Refractory Gastroesophageal Cancer. AACR IO. (2025). Available online at: https://minktherapeutics.com/wp-content/uploads/2025/02/Cytryn_AACR-IO-2025-proffed-paper_.pdf (Accessed July 25, 2025).
132. Tomolonis JA, Xu X, Dholakia KH, Zhang C, Guo L, Courtney AN, et al. Interaction between tumor cell TNFR2 and monocyte membrane-bound TNF-α triggers tumorigenic inflammation in neuroblastoma. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2022-005478
133. Nishimura K, Aoki T, Kobayashi M, Takami M, Ozaki K, Ogawa K, et al. Antibody-Dependent Cellular Cytotoxicity of iPS Cell-Derived Natural Killer T Cells by Anti-GD2 mAb for Neuroblastoma. Cancer Sci. (2025) 116(4):884–96. doi: 10.1111/cas.70008
134. Jayasinghe RG, Hollingsworth D, Boonchalermvichian C, Gupta B, Yan H, Baker J, et al. Single cell resolution analysis of multi-tissue derived human iNKT cells reveals novel transcriptional paradigms. bioRxiv. (2024) 2024:3.22.583992. doi: 10.1101/2024.03.22.583992
135. Ferguson S, Riel M, Baiu D, Bharadwaj N, Johannsen E, and Gumperz J. Analysis of iNKT-DC adjuvancy mechanisms through RNAseq. In: CiRT 2025 meeting, vol. 2025. . Prague: Consortium for iNKT Research and Therapy (CiRT) (2025). Available online at: https://meeting2025.cirt-net.org/program.html (Accessed July 25, 2025).
136. Li YR, Zhou Y, Kim YJ, Zhu Y, Ma F, Yu J, et al. Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Rep Med. (2021) 2:100449. doi: 10.1016/j.xcrm.2021.100449
137. Ramos CA, Courtney AN, Robinson SN, Dakhova O, Lulla PD, Kamble R, et al. Allogeneic NKT cells expressing a CD19-specific CAR in patients with relapsed or refractory B-cell Malignancies: an interim analysis. Blood. (2021) 138:2819–9. doi: 10.1182/blood-2021-149712
138. Ott PA, Hodi FS, Kaufman HL, Wigginton JM, and Wolchok JD. Combination immunotherapy: a road map. J ImmunoTherapy Cancer. (2017) 5:16. doi: 10.1186/s40425-017-0218-5
139. Rallis KS, Lai Yau TH, and Sideris M. Chemoradiotherapy in cancer treatment: rationale and clinical applications. Anticancer Res. (2021) 41:1–7. doi: 10.21873/anticanres.14746
140. Chatterjee N and Bivona TG. Polytherapy and targeted cancer drug resistance. Trends Cancer. (2019) 5:170–82. doi: 10.1016/j.trecan.2019.02.003
141. Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, and McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. (2015) 33:169–200. doi: 10.1146/annurev-immunol-032414-112334
142. Biotherapeutics B. Topline Results of Phase I Clinical Trial of iPS-NKT in Patients with Relapsed or Refractory Head and Neck Cancer. Tokyo, Japan: BrightPath Biotherapeutics (2024). Available online at: https://www.brightpathbio.com/english/news/20240304_release_en.pdf (Accessed July 25, 2025).
143. Biotherapeutics B. Corporate presentation (2024). Available online at: https://www.brightpathbio.com/english/img/BrightPath_CorpratePres052024.pdf. (Accessed July 25, 2025).
144. NIH. Developing allogeneic iNKT cell adoptive therapy to improve outcomes for HSCT indications. Research Portfolio Online Reporting Tools (RePORT) (2025). Available online at: https://reporter.nih.gov/search/nd4UHUKbqkyO34OJaTyGuA/project-details/11185953.
Keywords: CD1, CD1d, invariant natural killer T (iNKT) cell, NKT cell, allogeneic cells, off-the-shelf cells, graft versus host disease (GVHD), COVID-19
Citation: Rotolo A, Mason NJ and Exley MA (2025) Innate iNKT cells: from biological insight to clinical impact. Front. Immunol. 16:1653183. doi: 10.3389/fimmu.2025.1653183
Received: 24 June 2025; Accepted: 08 August 2025;
Published: 10 September 2025.
Edited by:
Luc Van Kaer, Vanderbilt University Medical Center, United StatesReviewed by:
Giulia Casorati, San Raffaele Hospital (IRCCS), ItalyLeonid Metelitsa, Baylor College of Medicine, United States
Copyright © 2025 Rotolo, Mason and Exley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonia Rotolo, YW50b25pYS5yb3RvbG9AcGVubm1lZGljaW5lLnVwZW5uLmVkdQ==; Mark A. Exley, bWFyay5leGxleUBtaU5LVGhlcmFwZXV0aWNzLmNvbQ==
 Antonia Rotolo
Antonia Rotolo Nicola J. Mason
Nicola J. Mason Mark A. Exley
Mark A. Exley