- 1Department of Biochemistry, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 2Nutrition and Food Sciences Department, National Research Centre, Giza, Egypt
- 3Molecular Cell Biology Unit, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
- 4Natural and Medical Sciences Research Center, University of Nizwa, Nizwa, Oman
- 5Department of Genetics, University of the Free State, Bloemfontein, South Africa
- 6Department of Home Economics, Specific Education Faculty, Port Said University, Port Said, Egypt
- 7Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 8Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 9Department of Mathematics and Statistics, Faculty of Science, Imam Mohammed Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
- 10Biology Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia
- 11Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
Dietary polyphenols, particularly flavonoids, have been extensively recognized for their role as a source of bioactive molecules that contribute to the prevention of various diseases, including cancer. This review aims to provide a comprehensive overview of dietary polyphenols by examining their sources, classification, mechanisms of action, and biological effects, with a particular emphasis on their nutritional and immunological roles. It also highlights the need for ongoing research into preventive strategies and the development of improved therapeutic options. Despite their broad spectrum of antioxidant, anti-inflammatory, neuroprotective, antimicrobial, anti-diabetic, and anti-cancer activities, the therapeutic application of polyphenols is significantly hindered by their inherently poor bioavailability. This limitation poses a substantial challenge, as it prevents polyphenols from achieving the systemic concentration necessary to elicit a therapeutic effect. This review critically evaluates current strategies, including nano- and liposomal-based delivery systems. Liposomal systems play a crucial role in enhancing the bioavailability of polyphenols by encapsulating these compounds in lipid bilayers. This encapsulation improves the solubility and stability of polyphenols, protects them from environmental degradation and rapid metabolism, and facilitates their controlled release and absorption in the body. Liposomes enable polyphenols to better traverse biological membranes and protect them from unfavorable conditions in the gastrointestinal tract, resulting in greater systemic availability and improved therapeutic efficacy compared to non-encapsulated forms. The current review also explores the modulatory impact of polyphenols on the immune system, their influence on gut microbiota, and their implications across various life stages, from infancy to aging, as well as in athletic performance and dermatological health. Future directions are proposed to optimize their clinical utility, including standardized dosing, improved delivery technologies, and targeted nutritional interventions. Ultimately, integrating polyphenols into daily dietary practices may offer promising avenues for enhancing immune resilience and preventing chronic diseases.
1 Introduction
Life expectancy in developing nations is increasing in tandem with socioeconomic progress. As a result of this shifting lifestyle, age-related illnesses, such as cancer, diabetes, cardiovascular disease, metabolic disorders, hepatitis, and neurological conditions, are on the rise (1). The absence of early detection technologies or effective treatments has prompted researchers to focus on preventive measures (1). In this context, attention has turned to dietary and nutritional strategies, such as the Mediterranean diet. These dietary habits may mitigate the risk of age-related disorders associated with lifestyle changes (1). Predominantly based on plant-derived foods such as vegetables, fruits, legumes, and herbs, the Mediterranean diet highlights the potential role of natural polyphenols, plant-based bioactive compounds, in preventing disease and aging while promoting overall health and well-being (2).
Polyphenols are naturally occurring, water-soluble compounds derived from plants, with molecular weights ranging from 500 to 4000 Da. They are abundant in plant-based foods, including fruits, vegetables, cereals, and beverages, and comprise a complex group of over 8000 known compounds (3). These compounds are classified as secondary metabolites (4), which are produced to defend against biotic stressors (e.g., bacteria, fungi, and insects) and abiotic stressors (e.g., environmental stress, free radicals, and metabolic disorders) (5, 6).
Based on the number of phenolic rings and structural linkages, they are commonly categorized into five main classes: tannins, lignans, phenolic acids, flavonoids, and stilbenes (7). They exhibit a wide range of biological activities, including anti-inflammatory, anti-cancer, antimicrobial, and anti-aging effects, due to their structural properties and biological interactions (8, 9). Consequently, they have shown great potential in the management of various diseases, including cancers and neurological, cardiovascular, and metabolic conditions (8, 10).
This review highlights the major classes of polyphenols, evaluated as secondary metabolites, along with methods used for their extraction and characterization. It also outlines their bioavailability and diverse health benefits, as reported in previous studies. In addition, the advantages of polyphenol consumption across different population groups, including athletes, mothers, infants, children, adults, and the elderly, are discussed.
2 Types of polyphenols
The basic phenolic structure of polyphenols is exemplified by these naturally occurring compounds, which are classified according to their chemical composition, particularly the number of aromatic rings, the substituent groups on these rings, and the structural linkages between them (7).
Figure 1 depicts the chemical structures of essential polyphenol subclasses (lignans, phenolic acids, flavonoids, tannins, coumarins, and stilbenes) along with their respective plant-based food sources, highlighting dietary consumption sources.
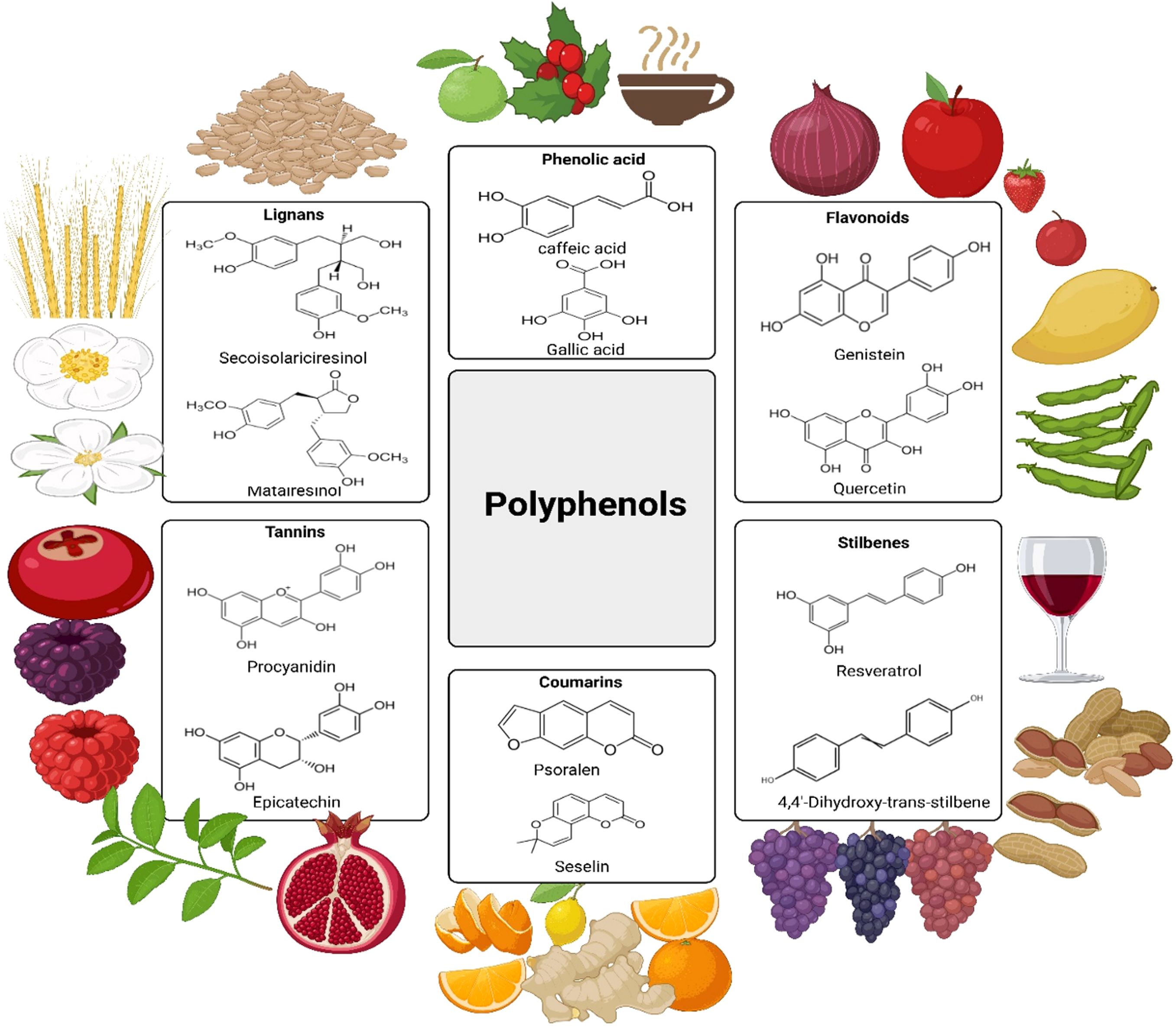
Figure 1. Chemical structures of principal polyphenol subclasses (lignans, phenolic acids, flavonoids, tannins, coumarins, and stilbenes) alongside their respective plant-based food sources exemplifying dietary consumption.
2.1 Phenolic acids and flavonoids
Phenolic acids differ from other acids in that they contain a single phenolic ring, characterized by the presence of one carboxylic acid group and one or more hydroxyl groups (11). Phenolic acids are structurally similar to other phenolic compounds. As a result, phenolic acids are commonly associated with esters, amides, and glycosides (11).
They are generally divided into two major subgroups: hydroxybenzoic acids and hydroxycinnamic acids. Hydroxybenzoic acid, derived from benzoic acid, possesses a C6-C1 carbon structure, while hydroxycinnamic acids, derived from cinnamic acid, typically occur in plants as simple esters with quinic acid or glucose (12). Phenolic acids are widely distributed in various foods, particularly cereals, fruits, legumes, vegetables, herbs, and beverages (13).
Flavonoids are the most well-known and extensively studied class of polyphenols (13). Their basic structure consists of two aromatic rings connected by a three-carbon bridge, forming an oxygen-containing heterocyclic ring. Based on the degree of oxidation of the central carbon ring, flavonoids are categorized into six major subclasses: flavonols, flavanones, flavones, flavanols, isoflavones, and anthocyanidins (13, 14). Flavonoids, identified as secondary metabolites in specific plant structures such as seeds and fruits, play a crucial role in contributing to the color, flavor, and aroma of plants. This structurally diverse group of polyphenols that exists in various forms is among the most thoroughly investigated in plant science (13).
Phenolic acids could donate hydrogen atoms, suggesting their antioxidant properties (13). Additionally, they are notable for their therapeutic properties in managing several chronic conditions, including diabetes, cardiovascular diseases, cancer, and neurodegenerative conditions (15, 16). Their fundamental structural features, the aromatic rings, hydroxyl groups (-OH) at specific positions, and the unsaturated side chains, contribute to a range of biological activities, with particular emphasis on anti-cancer effects (17).
On the other hand, flavonoids contribute to activating defense responses by modulating the production of reactive oxygen species (ROS) under stress conditions (18, 19). Accordingly, flavonoids demonstrate a wide range of bioactive properties beneficial to human health, including anti-inflammatory, antioxidant, cardioprotective, neuroprotective, anti-cancer, and anti-aging effects (20, 21).
2.2 Stilbenes
This class of polyphenols represents a distinct group of non-flavonoid phytochemicals characterized by two aromatic rings linked by a methylene bridge (13). Stilbenes are structurally defined by a 1,2-diphenylethylene core, distinguishing them as a subclass of phenylpropanoids. Resveratrol, the most prominent stilbene, is naturally found in peanuts and grapes and is present in high concentrations in red wine (14, 22).
The abundance of resveratrol in red wine has contributed to hypotheses regarding its potential role in preventing chronic diseases (23). Additionally, resveratrol has been reported to possess anti-inflammatory and antioxidant properties (14, 24). It has also been suggested to contribute to wine preservation (25). Previous studies reported the development of a wine quality index based on the concentration and composition of stilbenes (26).
2.3 Lignans
Lignans are another class of polyphenols that share structural similarities with phenolic acids (14). Their diphenolic structure includes a carbon-carbon bond formed between two phenylpropane units, which polymerize to produce compounds commonly found in plant seeds, roots, and leaves (27). Lignans are classified into eight subgroups based on their cyclization pattern, the incorporation of oxygen atoms, and carbon skeleton structures. These subclasses include furofurans, furans, aryltetralins, arylnaphthalenes, dibenzylbutyrolactones, dibenzylbutanes, dibenzybutyrolactols, and dibenzocyclooctadienes (27). Li et al. (27) reported that the position of the oxygen atom also plays a key role in the classification of lignans.
Lignans are primarily found in vegetables, cereals, and legumes. Diets rich in lignans have been associated with various health-promoting effects. Notably, lignans exhibit anti-cancer activity through multiple regulatory pathways (28, 29). Furthermore, they possess anti-inflammatory, antioxidant, and anti-menopausal properties, providing protective effects against cardiovascular and bone diseases, as well as antimicrobial effects (30, 31).
3 Factors affecting the appropriate extraction methods for phenolic compounds
Polyphenols comprise a broad array of chemical structures, resulting in varied chemical and physical properties (32). This structural heterogeneity necessitates the application of extraction techniques specifically designed for the distinct properties of each compound and the nature of the sample matrix (32). The selection of an efficient extraction method depends on various aspects, including the chemical structure of the target polyphenols, the sample’s particle size, and the existence of other coexisting chemicals that may interfere or interact during the extraction process (33).
Furthermore, extraction efficiency is highly sensitive to operational parameters such as pH, type of solvent employed, solvent-to-sample ratio, and duration of the extraction process (34). Despite considerable technological progress in extraction techniques, significant challenges remain in precisely identifying and quantifying polyphenols. Obtaining accurate and reproducible data on the composition and concentration of polyphenols is critical for substantiating their health-promoting properties and ensuring the reliability of related scientific assessments (35).
3.1 Extraction methods of phenolic compounds
3.1.1 Ultrasound-assisted extraction method
Ultrasound-assisted extraction is a widely used and efficient technique for isolating phenolic compounds, offering high yields in a relatively short time (36). Ultrasonic radiation with frequencies above 20 kHz enhances the extraction of inorganic and organic substances using liquid solvents. This method is considered environmentally sustainable as it reduces extraction time, solvent consumption, and energy requirements (37).
The process relies on acoustic cavitation, wherein ultrasonic waves disrupt plant cell walls by inducing rapid expansion and contraction of solid surfaces (38), increasing cell wall permeability, facilitating solvent penetration, and promoting the release of water-soluble compounds from the plant matrix (37). In recent years, ultrasound-assisted extraction has been applied to extract polyphenols from various plant parts, including pecan nutshells, Randia monantha, mango seed kernels, olive pomace, and pine needles (36, 39). Studies have focused on optimizing extraction conditions and evaluating the antioxidant and antifungal properties of the resulting polyphenol-rich extracts (36, 37, 39).
Ultrasound-assisted extraction provides notable advantages for the extraction of polyphenols. This technique significantly enhances yield and efficiency compared to conventional methods, allowing for higher concentrations of bioactive compounds in a much shorter extraction time (40). Ultrasound-assisted extraction reduces both solvent and energy consumption, making the process more environmentally friendly and cost-effective. The operation at lower temperatures also helps preserve the structural integrity of heat-sensitive polyphenols, minimizing their thermal degradation during extraction (40). These benefits are reflected in various studies, which demonstrate that the ultrasound-assisted extraction delivers superior extraction performance while aligning with green chemistry principles (40).
However, the ultrasound-assisted extraction also has its limitations (41). Excessive ultrasound intensity or prolonged application can lead to the generation of free radicals and high local temperatures, potentially causing the degradation or modification of certain sensitive polyphenolic compounds (41). The method’s efficacy is highly dependent on the careful optimization of operating parameters, such as ultrasound power, extraction time, solvent type, and temperature, as suboptimal conditions may reduce extraction yields or lead to inconsistent results (41). Additionally, scaling up the ultrasound-assisted extraction from laboratory to industrial production remains challenging due to equipment limitations and the need to replicate the cavitation effects that drive the process consistently (41).
3.1.2 Microwave-assisted extraction method
Microwave-assisted extraction is an environmentally friendly technique used to isolate polyphenols from plants, herbs, and plant-based products (42). Water is commonly used as the solvent due to its efficiency, cost-effectiveness, and reliability compared to other extraction media. Optimizing operational parameters is essential, as the heat generated during microwave exposure influences the release of targeted polyphenols (42). Extraction efficiency depends on factors such as solvent type and ratio, microwave power, and extraction time (43). These conditions must be maintained and optimized to obtain the highest yield. Depending on the plant sample, the solvent can be water, ethanol, or a combination (43).
Microwave-assisted extraction is frequently employed to recover polyphenols from agricultural by-products and processed wastes, such as pomace, leaves, and peels (44). Extracted polyphenols by the microwave-assisted extraction method have demonstrated various biological activities, including antimicrobial, antioxidant, and anti-cancer activities (44, 45). These bioactive compounds have potential applications in pharmaceuticals and nutraceuticals (43, 46, 47).
This technique significantly reduces extraction time and energy consumption compared to conventional methods. Microwave-assisted extraction enhances extraction efficiency by rapidly heating the sample and solvent, which disrupts plant cell walls and allows better solvent penetration, leading to higher yields of polyphenolic compounds (41). The method also offers improved selectivity and precise control of temperature, helping preserve the integrity of thermosensitive compounds (41).
Nonetheless, microwave-assisted extraction possesses limitations. Excessive microwave power or prolonged exposure can lead to the degradation of heat-sensitive or volatile phenolics, thus reducing the quality and quantity of the extracted compounds (48). Additionally, optimization is required for each plant matrix and polyphenol type, as extraction parameters such as microwave power, temperature, solvent composition, and sample-to-solvent ratio can significantly influence outcomes (48). Some specialized equipment and careful calibration are needed to ensure reproducibility and scalability for industrial applications (48).
3.1.3 Microwave-assisted ultrasound extraction method
Microwave-assisted ultrasound extraction presents notable advantages for extracting polyphenols (49). Microwave-assisted ultrasound extraction is a hybrid method that combines microwave and ultrasonic treatments to enhance the yield of phenolic compounds, reduce extraction time, and minimize solvent usage compared to ultrasound-assisted extraction and microwave-assisted extraction alone (49). This method employs microwave heating to extract compounds through dielectric heating, while ultrasound enhances cell wall permeability and facilitates solvent penetration. A comparative study reported that microwave-assisted extraction yielded higher polyphenol content and antioxidant activity than ultrasound-assisted extraction (50).
While microwave-assisted extraction required less extraction time, ultrasound-assisted extraction demonstrated greater energy efficiency and environmental sustainability (51). When both techniques were combined, the resulting method improved extraction efficiency and polyphenol yield. For instance, a study comparing enzyme-assisted ultrasound extraction and ultrasound-microwave-assisted extraction from mangosteen peels found that the enzyme-assisted ultrasound method produced a higher polyphenol yield (51). Nonetheless, both extraction approaches yielded polyphenol-rich extracts with promising applications as functional food additives and in pharmaceutical formulations (51).
By combining microwave and ultrasound energies, microwave-assisted ultrasound extraction disrupts plant cell walls more efficiently, allowing improved release of polyphenols with reduced solvent consumption and lower energy usage (51). Additionally, the process can preserve the antioxidant activities of the extracted compounds and is considered both cost-effective and environmentally friendly (48).
However, there are some limitations. Precise control of operational parameters—such as power, temperature, and extraction duration—is essential, as excessive energy input or prolonged treatment can degrade sensitive polyphenolic structures, potentially lowering yield or altering compound profiles (52). Variability in sample characteristics and the risk of free radical generation during ultrasound application can also influence extraction efficiency and product quality (52). Despite these challenges, when carefully optimized, microwave-assisted ultrasound extraction remains a powerful, green technology for extracting high-value polyphenols from complex plant matrices (52).
3.1.4 Supercritical fluid extraction method
Supercritical fluid extraction is an alternative two-step technique. First, soluble phenolic compounds are extracted from the herbal cell matrix using a supercritical fluid, followed by depressurization to separate the bioactive components, converting the supercritical fluid into a gas phase (53, 54). Supercritical fluids are generated when pressure (10–35 MPa) and temperature (40–80 °C) exceed critical values. This method enhances safety by using less hazardous solvents, such as methyl tert-butyl ether, methanol, hexane, and dichloromethane (55).
Supercritical fluid extraction is considered a green technology, frequently employing gases like CO2, CH3, C2H6, C2H6O, C3H8, C6H6, and NH3 during depressurization (56). Additionally, compared to conventional methods, supercritical fluid extraction protects bioactive compounds from air and light, reducing degradation and minimizing contamination risk from impure solvents (57). This technique has recently been applied to extract polyphenols from sources such as chestnut shells, Ailanthus excelsa, and Dunaliella salina (54, 55, 58).
Supercritical fluid extraction, particularly with supercritical carbon dioxide (CO2), offers several advantages for the extraction of polyphenols (59). It is an environmentally friendly “green” technology that uses non-toxic, non-flammable CO2, resulting in solvent-free extracts that are safe for food, pharmaceutical, and cosmetic uses. This method operates at moderate temperatures, which helps preserve the structural integrity and bioactivity of heat-sensitive polyphenols (59). Supercritical fluid extraction also features tunable selectivity; by adjusting pressure, temperature, and the use of co-solvents such as ethanol or water, it can be optimized for higher purity and targeted extraction of diverse phenolic compounds (59). Additionally, supercritical fluid extraction minimizes solvent residue and maintains high extract quality, thus supporting the production of high-purity polyphenols (59).
However, supercritical fluid extraction also has limitations. The high initial cost and technical complexity of the required equipment are significant barriers to large-scale industrial application (60). Extraction efficiency can be lower for highly polar compounds unless co-solvents are used to enhance solubility (60). The method typically requires longer extraction times compared to some alternative techniques, and optimizing operational parameters such as pressure, temperature, and co-solvent composition can be challenging. Furthermore, scaling up the process for industrial throughput poses logistical and operational hurdles, and energy consumption is relatively high due to the need to maintain supercritical conditions (60).
3.1.5 Subcritical water extraction method
Also known as hot liquid or superheated water extraction (61). In subcritical water extraction, water remains in a liquid state at temperatures between 100°C and 347°C under pressures up to 220 bar (62). Under these subcritical conditions, hydrogen bonding between water molecules is reduced, lowering the dielectric constant. Consequently, changes in temperature and pressure influence both the dielectric constant and extraction efficiency (62). Compared to supercritical fluid extraction, subcritical water extraction is potentially more economical as it utilizes water rather than organic solvents (63). Subcritical water extraction also produces rapid extraction, high efficiency, and environmental sustainability (64). It has been successfully applied to extract phenolic and natural compounds from materials such as cocoa bean husks and saffron tepals (62, 64, 65).
Subcritical water extraction offers green, efficient technology for polyphenol extraction, but has several limitations. A primary drawback of subcritical water extraction is the requirement for high temperatures, typically between 100°C and 374°C, which can cause thermal degradation of heat-sensitive polyphenolic compounds, thereby reducing their yield and bioactivity (66).
Moreover, at elevated temperatures, subcritical water extraction tends to be less selective, extracting a wider range of plant matrix components, which can complicate downstream purification (66). The use of water as a solvent under subcritical conditions also necessitates additional steps, such as evaporation or dehydration, to remove water from the extracts, increasing processing complexity (66).
Subcritical water extraction equipment requires more rigorous maintenance and corrosion prevention due to the high reactivity and corrosiveness of water at elevated temperature and pressure (66). Lastly, optimization of variables such as temperature, extraction time, pressure, and solvent-to-solid ratio is critical yet challenging, as these parameters profoundly influence extraction efficiency and compound stability (66). Thus, while subcritical water extraction is promising and eco-friendly, its limitations in compound stability, selectivity, and process complexity require careful management to maximize polyphenol recovery and bioactivity (66).
3.1.6 Pulsed electric field method
A nonthermal method that employs high-voltage pulses between two electrodes arranged in a sandwich configuration. Pulsed electric field is classified into batch (100–300 V/cm) and continuous (20–80 kV/cm) systems, depending on pulse frequency. The electric field induces a transmembrane potential in plant cells, increasing membrane permeability and facilitating the excretion of phenolic compounds (67). Pulsed electric field effectiveness depends on the extent, the surrounding medium, and the physicochemical properties of plant tissues (68, 69). This method has been used to extract polyphenols from green tea, laurel leaves, cannabis, and Phyllanthus emblica, with extracts showing anti-inflammatory and antioxidant activity (70, 71).
Pulsed electric field technology, while promising as a non-thermal and efficient method for extracting polyphenols, has several limitations that should be considered. The effectiveness of pulsed electric field extraction depends on various factors, including the electric field strength, treatment time, and the specific properties of the plant tissue, such as cell size, shape, and membrane composition (72). One key limitation is the challenge of achieving a uniform electric field distribution throughout the sample, which can result in inconsistent cell permeabilization and variable extraction yields. Additionally, pulsed electric field treatment may cause only reversible electroporation in some cells, limiting the release of intracellular compounds (72).
Another constraint is related to the physical and chemical characteristics of the extraction matrix; factors such as solvent type, solvent conductivity, and polarity significantly influence extraction efficiency and can complicate optimization (73). Furthermore, pulsed electric field is typically better suited for liquid or semi-liquid matrices and may be less effective for solid or highly fibrous plant materials without prior size reduction or pretreatment (73). Although considered a non-thermal process, extended treatment times or high pulse numbers can lead to a rise in temperature, risking the degradation of sensitive phenolic compounds (73).
3.1.7 Pressurized liquid extraction method
Also referred to as accelerated solvent extraction (74). It typically employs organic solvents in the presence of nitrogen to extract phenolic compounds from solid or semi-solid samples. Operating at high temperatures and pressures, accelerated solvent extraction enhances solvent penetration without altering compound structure, thereby improving phenolic yield (56). This green extraction method minimizes solvent and energy use while increasing extraction efficiency. Automation enhances process reproducibility with minimal manual intervention (75). Accelerated solvent extraction has been used to extract phenolics from strawberry and onion peels, with applications focused on evaluating their antimicrobial and antibiofilm activities (75, 76).
Pressurized liquid extraction offers efficient recovery of polyphenols from various plant matrices; however, it also presents certain limitations. One key challenge is the high operational cost associated with the specialized equipment and maintenance requirements (77). Additionally, pressurized liquid extraction involves the application of elevated temperatures and pressures, which can potentially lead to the degradation of thermolabile polyphenolic compounds, thereby reducing the yield and altering the composition of the extracts (77).
The choice of solvent is critical, as water, often used for its green credentials, may be inefficient in extracting less polar phenolics, resulting in lower overall extraction efficiency compared to organic solvents like ethanol (77). Optimization of operational parameters such as temperature, solvent composition, solvent-to-feed ratio, and extraction time is essential but can be complex and sample-specific, especially when dealing with complex matrices like propolis (77). Moreover, while pressurized liquid extraction reduces solvent use and extraction time compared to traditional methods, incomplete extraction of certain compounds can still occur, necessitating complementary techniques or further refinement (77). Finally, the process demands careful balancing between maximizing extraction efficiency and preventing compound degradation, which remains a key limitation in fully harnessing pressurized liquid extraction for polyphenol extraction (77).
3.2 Common methods for polyphenol quantification
3.2.1 Spectrophotometric methods
Spectrophotometry is a simple and widely used technique for identifying phenolic compounds in plants (78). Total phenolic content is commonly assessed using the Folin–Denis and Folin–Ciocalteu methods. These techniques have recently been applied to evaluate the phenolic content, antioxidant activity, and total phenolics in broken-bone twigs (79, 80). Both methods rely on chemical reduction, typically involving reagents such as molybdenum and tungsten (81). Additionally, colorimetric assays are used to quantify total flavonoids, condensed tannins, and phenolics by forming complexes with AlCl3, with absorbance measured in the 410–423 nm range (82).
Anthocyanins, another important group of phenolics, can be quantified spectrophotometrically under mildly acidic conditions, with absorbance measured between 490 and 550 nm (83). These colorimetric assays are user-friendly and cost-effective; however, they do not allow for the quantification of individual compounds and provide only approximate estimates of total phenolics above a certain threshold (61). Despite this limitation, spectrophotometric methods remain valuable for the rapid and economical screening of a wide range of plant-derived bioactive compounds. For instance, red poppy extracts have recently been used as colorimetric sensors to detect anthocyanins (84), and similar analyses have been conducted on grape juice and elderberries (85, 86).
3.2.2 Gas chromatography method
Gas chromatography is widely used to identify and quantify polyphenols, including flavonoids, phenolic acids, and tannins (87). This technique involves the movement of analytes through a column using carrier gas such as nitrogen (N2), helium (He), or hydrogen (H2). Gas chromatography operates based on gas-liquid partitioning or gas-solid adsorption, utilizing a nonvolatile liquid as the stationary phase and typically employing a flame ionization detector. Commonly, silica capillary columns are used, typically 30 m in length, with a 0.25 µm film thickness and an inner diameter of 25–32 µm (56).
The integration of gas chromatography with mass spectrometry (GC-MS) has gained attention owing to its improved sensitivity and selectivity (88). This combination is crucial for analyzing the degradation patterns of plant-derived bioactive compounds and for identifying their chemical structures by correlating chromatographic and mass spectral data (89). Gas chromatography analysis was used to evaluate the antimicrobial properties and polyphenol content of Sonneratia caseolaris fruits, as well as to determine the bioactive compound composition of fast-growing plant leaves (90).
3.2.3 High-performance liquid chromatography method
High-performance liquid chromatography (HPLC) remains one of the most widely used analytical methods for the identification of phenolic compounds. Generally, following the purification of phenolics, the samples are analyzed using a C18 column as the stationary phase (91). This technique uses acidified polar organic solvents as the mobile phase and utilizes photodiode array detectors for compound detection. With technological advancements, rapid and refined methods such as chromatographic fingerprint analysis have been developed for the characterization of herbal medicines (56). These fingerprint profiles enable species-specific identification and differentiation from related species, as they accurately reflect the chemical composition of the plant material (56).
Several factors affect the sensitivity or effectiveness of HPLC, including phenolic purification steps, mobile phase composition, column selection, and pre-concentration procedures (35). The pH of the mobile phase is particularly critical, as improper pH levels may lead to the ionization of phenolic compounds, affecting detection accuracy (35). Column selection is based on polarity and particle size, with various phenolic classes requiring different specifications. More sophisticated HPLC systems employ novel column types with varying particle sizes to optimize separation (35).
HPLC run times typically range from 10 to 150 min. For longer analyses, maintaining a constant temperature is essential to ensure reproducibility and stability of results (92). Recent studies using HPLC have successfully characterized the antioxidant and antimicrobial properties, metabolomic profiles, and phenolic components of samples such as apple pomace, grape juice, Lysimachia nummularia, and Acacia species (93, 94).
3.2.4 Other methods for polyphenol quantification
In addition to widely used techniques, several other methods are employed to identify plant-derived bioactive compounds, including capillary electrophoresis, paper chromatography, supercritical fluid chromatography, spectrophotometric assays, HPLC, and gas chromatography (78). Among these, paper chromatography is a simple and effective method, particularly for identifying bioactive compounds in tea leaves (78). Ashraf et al. (95) demonstrated the application of high-performance thin-layer chromatography in analyzing caffeine content in green tea leaves. Paper chromatography has also been applied to assess the biological activities of medicinal herbal extracts, such as anti-inflammatory, antimicrobial, and antioxidant properties linked to compounds like flavonoids and fatty acids (96).
However, paper chromatography is used less frequently than HPLC and gas chromatography due to its limited sensitivity and specificity (56). Capillary electrophoresis is a high-efficiency technique that utilizes thin capillary columns filled with ionic solutions to separate charged bioactive compounds and low-to-medium-molecular-weight plant constituents. It requires minimal sample and reagent volumes and offers rapid and effective analysis (56). Capillary electrophoresis techniques include micellar electrokinetic chromatography, capillary electrochromatography, capillary zone electrophoresis with ultraviolet detection, and capillary zone electrophoresis coupled with mass spectrometry (56). Recent applications of capillary electrophoresis include the quantification of free sulfur dioxide in wine and cider and the chemical profiling of tobacco samples (97, 98). Additionally, indirect UV detection with capillary zone electrophoresis has been used to investigate cassines and spectalines in Senna spectabilis (99).
Supercritical fluid chromatography is an advanced method increasingly used for the analysis of complex plant materials (100). Compared to HPLC and gas chromatography, supercritical fluid chromatography exhibits higher efficiency, faster analysis times, environmentally friendly operation, and superior resolution (56). Its distinguishing feature lies in column design, which incorporates fully porous particles smaller than 2 µm or superficially porous particles under 3 µm (101). Recent studies have applied this technique to successfully characterize isomeric urolithin glucuronides and lignans derived from softwood species (102, 103).
4 Bioavailability of polyphenols
Bioavailability refers to the proportion of polyphenol-derived nutrients that are consumed, absorbed, and metabolized (104, 105). Several factors influence the bioavailability of polyphenols, including gut microbiota, nutritional matrix, molecule size, sex, previous dietary habits, transmembrane transport capacity, and chemical structure (106, 107). Additionally, polyphenols interact with gut microbial strains, which can alter their molecular states and affect their subsequent bioactivity (106).
Polyphenols are also subject to various denaturing conditions (103), such as heat, light, oxygen, pH variations, and enzymatic degradation, which reduce their bioavailability and limit their efficacy as bioactive compounds (108). Their bioavailability varies depending on their chemical forms, such as esters, glycosides, or polymers (104). Gao et al. (86) reported that after digestion, the bioavailability of phenolic compounds in Cannabis sativa L. seeds was 142.39%, whereas that of flavonoid compounds was 29.47%.
Sánchez-Velázquez et al. (105) revealed that phenolic compounds from wild blackberries might exhibit greater bioactivity and bioavailability in the human body than those from commercial varieties. Similarly, Frazzini et al. (109) examined the effect of in vitro gastrointestinal digestion on the bioavailability and stability of polyphenols in commercial and wild Mexican blackberries. Other studies have demonstrated that polyphenols are more stable in organic solvents and water than in cell culture media, where they degrade more rapidly (110). This suggests that polyphenols are prone to degradation in biological systems, potentially reducing their bioavailability and biological efficacy (110–112).
Generally, most dietary polyphenols undergo hydrolysis by colonic bacteria and are then methylated and conjugated into glucuronide and sulfate metabolites by the hepatic and other tissues (106). An increase in plasma antioxidant capacity following the intake of polyphenol-rich foods, such as apples, tea, blackcurrants, and red wine, indicates that polyphenols can cross the intestinal barrier and exert systemic effects (113). Bioavailability has also been directly assessed by measuring polyphenol concentrations in plasma and urine after ingestion of purified compounds or polyphenol-rich foods (114). However, despite their health benefits, the low absorption rate of polyphenols (approximately 5–10% via the small intestine) and their rapid metabolism and excretion significantly limit their ability to reach target tissues (114). Kou et al. (115) reported that purified blueberry polyphenol extract exhibited higher antioxidant activity in different in vitro assays, whereas the crude blueberry extract demonstrated greater antioxidant effectiveness in in vivo models.
To improve the bioavailability of polyphenols, an investigation (116) was conducted to evaluate their stability in sports nutritional products incorporating both plant polyphenols and milk proteins. A study by van de Langerijt (116) examined the potential of integrating these components into sports supplements to preserve polyphenol content and enhance bioavailability during digestion (116). It showed that anthocyanins remained stable during in vitro digestion, with enhanced bioavailability observed in milk-blackberry mixtures, particularly those made with full-cream milk (116). Another study evaluated the bioavailability of total polyphenols from coffee silver skin extract using simulated gastrointestinal digestion and colonic fermentation (117). The findings suggested that fermentation enhanced antioxidant activity and enabled delivery to target sites, supporting potential health benefits (117).
Heat treatment has also been shown to improve polyphenol stability and bioactivity. Franková et al. (118) reported that heat processing of sweet potatoes enhanced both their antioxidant capacity and phenolic content. These findings indicate that thermal processing enhances the bioavailability of polyphenols in sweet potatoes and may guide advancements in food processing technologies. Furthermore, nanoencapsulation techniques, such as incorporating polyphenols into nanoparticles (NPs) or liposomes, can further improve their bioavailability and biological activity (108).
Despite significant progress in understanding polyphenol bioavailability, several gaps and future research priorities remain. One major limitation is the incomplete knowledge of the metabolic pathways and transformations that polyphenols undergo after ingestion, particularly due to interactions with the gut microbiome and the formation of diverse metabolites whose biological activities are not well characterized (119). Additionally, the influence of food processing, individual genetic variability, and the complex interactions between polyphenols and other dietary or environmental components on their absorption and bioactivity requires further exploration (120).
There is also a critical need for well-designed long-term safety studies addressing the potential side effects of chronic polyphenol supplementation, as current data are mainly limited to short-term animal experiments or isolated compounds, and results from these do not always translate directly to humans (121). While some polyphenol-rich extracts, such as grape seed extract, have shown high tolerability in animal and short-term human studies, the safety of long-term, high-dose intake across a broad population spectrum remains to be confirmed (122, 123). Special attention should be given to possible interactions with medications, effects on nutrient absorption (such as iron), and risks to sensitive populations (121, 124, 125).
Clinical research on polyphenol bioavailability is advancing, but large-scale intervention trials remain scarce. More chronic, placebo-controlled human studies are required to evaluate not only the bioavailability and efficacy of various polyphenol formulations and delivery systems but also to establish standardized dosages, monitor potential side effects, and assess inter-individual differences in responses due to genetics and gut microbiota composition (126, 127). The development and validation of robust biomarkers for polyphenol intake and metabolism are also needed to improve accuracy in such studies (127).
Future studies should focus on enhancing the understanding of the metabolic pathways of polyphenols and the bioactivity of their metabolites. It is necessary to expand extensive chronic clinical trials to evaluate the long-term safety, efficacy, and optimal dosing of polyphenols, examine gene-diet and microbiota-polyphenol interactions to elucidate inter-individual variability, develop innovative delivery systems to improve bioavailability and facilitate clinical translation, and clarify potential drug interactions and safety in vulnerable populations.
4.1 Bioavailability of polyphenols encapsulated in liposomes or NPs and their functional impact
Encapsulation is a delivery mechanism that incorporates bioactive compounds, such as drugs or food ingredients, into carrier systems (108). This approach protects the active substances from degradation during processing and storage while increasing their bioactivity by facilitating targeted delivery to specific organs or tissues (108). Despite their potential, polyphenols remain underutilized in functional foods and dietary supplements due to several physicochemical properties, including low epithelial permeability, poor solubility in gastrointestinal fluids, structural modifications during digestion, and limited oral bio-accessibility (128, 129).
To overcome these challenges, various technologies have been developed to improve the bioavailability of polyphenols, with nanoencapsulation and liposomal encapsulation considered the most effective strategies. Effective delivery of bioactive compounds to target sites requires a reduction in particle size (130). Nanoencapsulation, typically within a diameter range of 10 to 1000 nm, enhances bioavailability, protects against degradation, and enables precise delivery of polyphenols to targeted sites (130). Liposomal encapsulation is an advanced method designed to stabilize sensitive bioactive compounds (131). It supports the encapsulation of both hydrophobic and hydrophilic molecules, thereby optimizing nutrient absorption and biological efficacy. Lipid- and water-based vesicles enhance solubility and membrane permeability, facilitating accurate delivery to the target tissues (131). Furthermore, lipophilic complexes facilitate intestinal absorption while shielding polyphenols from adverse interactions or breakdown during the digestive process (131).
These encapsulation technologies have shown promising potential in improving the bioavailability and biological activity of polyphenols. Ali et al. (132) demonstrated that grape seed extract encapsulated in liposomes exhibited anti-aging, skin-brightening, and moisturizing effects in human skin cells. These findings advocate the development of more soluble and aesthetically desirable formulations for a broad range of skincare products (133). Altan et al. (134) conducted a study to promote bone wound healing in a rat model using a liposomal formulation of gallic acid. The study included four groups of rats. The group treated with gallic acid liposomes showed the greatest improvement and the lowest infection rate, whereas the negative control group exhibited the least improvement and the highest infection rate (134). These findings indicate that liposomal encapsulation improves the bioavailability and bioactivity of gallic acid polyphenols (134).
Previous research on polyphenols from various plant sources encapsulated in nanoliposomes has shown that increased bioavailability correlates with enhanced antimicrobial activity (135, 136). For instance, Nateghi et al. (135) assessed the antimicrobial activity of phenolic compounds from Achillea millefolium encapsulated in nanoliposomes against Campylobacter jejuni infection in mice. The study demonstrated that nanoencapsulated polyphenols significantly enhanced antioxidant levels, hepatic function, and food consumption compared to nonencapsulated treatments (135). Furthermore, the proliferation of C. jejuni was markedly reduced in mice receiving nano-encapsulated polyphenols, supporting the potential use of polyphenol-loaded nanoliposomes as phytobiotics against this infection (135).
In a similar study, Hassirian et al. (136) investigated the dietary phytobiotic effects of the phenolic-rich fraction of Alcea rosea against Escherichia coli infection in mice. The study aimed to assess the antimicrobial and potential health-promoting properties of phenolic-rich nanoliposomes, which showed greater efficacy compared to unencapsulated polyphenols at the same dosage (136). Furthermore, Shamansoori et al. (137) demonstrated that an extract of Rheum ribes encapsulated in nanoliposomes acted as a novel phytogenic antibiotic, effectively protecting mice from E. coli infection. Encapsulated polyphenols (10 mg TPC/kg) significantly improved health markers in mice compared to non-encapsulated forms (137).
Similarly, Mehdizadeh et al. (138) reported comparable results using Artemisia aucheri phenolic compounds encapsulated in nanoliposomes to treat C. jejuni infection in mice. In vivo studies also demonstrated the protective effects of liposome-encapsulated ferulic acid against oxidative liver damage (139). Encapsulated ferulic acid exhibited antioxidant properties by reducing CCl4-induced cytotoxicity in vitro and significantly alleviated hepatotoxicity, ROS production, and tissue damage in rat liver following intravenous administration (139).
Another animal study reported that liposome-encapsulated p-coumaric acid (CA) inhibited osteoclast formation and bone resorption in a rat model of rheumatoid arthritis, suggesting its potential to prevent bone degradation and calcium loss (140). A study on broiler breeder roosters investigated the effect of ellagic acid-loaded liposomes on post-thaw sperm quality (141). Results indicated that 1 mM ellagic acid liposomes significantly improved sperm antioxidant levels and overall quality after thawing. Furthermore, research on green tea polyphenols in photodynamic cancer therapy demonstrated that NPs of these polyphenols induced higher apoptotic rates and more potent inhibition of cancer cell proliferation than non-NP forms (142). This underscores the role of nanomedicine in enhancing the ‘anti-tumor’ bioactivity and bioavailability of green tea polyphenols (142). Additionally, the anti-cancer effects of silk fibroin NPs encapsulating rosmarinic acid (RA), a polyphenol with antimicrobial, antioxidant, and other bioactivities, were investigated in HeLa and MCF-7 cell lines. The study concluded that NPs improve the solubility and bioavailability of RA, thereby augmenting its anti-cancer efficacy (143).
Zhu et al. (144) enhanced the anti-cancer efficacy of curcumin NPs. Curcumin, a potent phenolic compound, exhibits various physiological effects, including anti-inflammatory, antioxidant, and ‘anti-tumor’ properties. However, its application is limited by volatility and poor buccal bioavailability. Moreover, curcumin was encapsulated into pea protein using a pH-driven NP method. This method yielded curcumin-loaded pea protein NPs with significantly higher loading efficiency and improved water solubility (144). In a separate study, potent antioxidative and ‘anti-tumor’ NPs were synthesized from tea polyphenols using an amino acid-induced ultrafast method (145). Epigallocatechin gallate (EGCG), a primary antioxidant in green tea, was used to prepare a therapeutic nano-agent via a rapid process involving five amino acids: lysine, arginine, leucine, glycine, and glutamic acid. The study found that lysine and arginine were depleted within 50 seconds of induction. The resulting NPs displayed tenfold greater antioxidant activity than conventional NPs and demonstrated therapeutic efficacy against cancer in both in vitro and in vivo models (145). Another study utilized Punica granatum (pomegranate) extract for the green synthesis of silver NPs (146). Silver NPs synthesized from a polyphenol-rich fraction exhibited antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, and Sarcina lutea (146).
These findings suggest that encapsulation enhances the bioactivity, solubility, and permeability of polyphenols by increasing their bioavailability. However, further studies are required to demonstrate that encapsulation improves the biological efficacy of polyphenols conclusively.
5 Health benefits of polyphenols
The inclusion of polyphenol-rich foods and beverages, including tea, herbs, fruits, and wine, in the diet is an effective approach to harness their health-promoting properties (147, 148). Polyphenols exhibit a wide range of biological activities, including anti-inflammatory, anti-diabetic, antimicrobial, antioxidant, anti-aging, anti-cancer, and cytotoxic properties (149, 150). These properties contribute to the prevention of chronic diseases and support therapeutic strategies. Furthermore, polyphenols have demonstrated positive effects on cardiovascular health and cognitive function, potentially through the prevention of neurodegenerative disorders (151).
Figure 2 highlights the diverse health advantages of polyphenols: a visual depiction of their functions in enhancing antioxidant capacity, mitigating alcohol-related hepatic damage, obstructing carcinogenic effects, retarding the aging process, regulating gut microbiota, facilitating weight management and obesity prevention, reducing blood glucose levels, augmenting nutritional value, and substituting preservatives by inhibiting pathogenic bacterial proliferation.
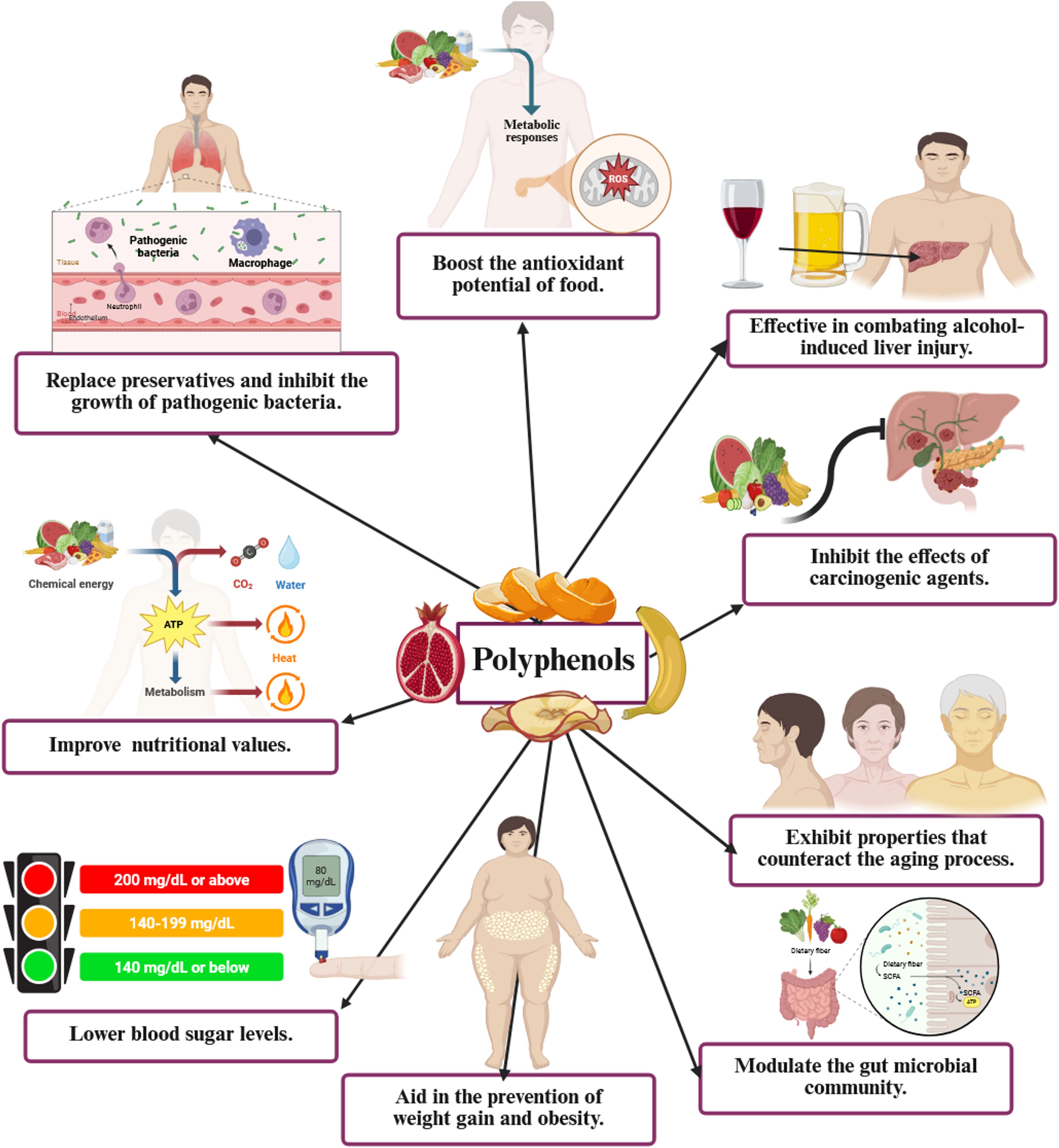
Figure 2. Comprehensive health advantages of polyphenols: A visual depiction of their functions in enhancing antioxidant capacity, mitigating alcohol-related liver damage, suppressing carcinogenic effects, countering aging, regulating gut microbiota, facilitating weight management and obesity prevention, reducing blood glucose levels, augmenting nutritional value, and substituting preservatives by inhibiting pathogenic bacterial proliferation. ROS, reactive oxygen species.
5.1 Antioxidant activity
One of the most extensively studied properties is their antioxidant activity. A key function of polyphenols is their ability to reduce or prevent ROS, which are harmful to human health (152, 153). By neutralizing ROS, polyphenols exert protective effects against oxidative stress and skin degradation (154, 155). Polyphenols interact with ROS primarily through three mechanisms governed by their molecular structure: single electron transfer, hydrogen atom transfer, and transition metal chelation (156, 157). In the hydrogen atom transfer mechanism, polyphenols donate a hydrogen atom from their phenolic hydroxyl group, producing free radicals that neutralize ROS (156). The efficiency of this reaction is associated with the bond dissociation enthalpy of the O–H bond; lower bond dissociation enthalpy corresponds to higher reactivity. For instance, in the reaction R + ArOH → ArO + RH, a lower bond dissociation enthalpy facilitates hydrogen donation (158, 159).
In the single electron transfer mechanism, antioxidant capacity is related to ionization potential; molecules with low ionization potential values act as efficient electron donors in the reaction R + ArOH → R– + ArOH+ → RH + ArO (158). In the transition metal chelation mechanism, polyphenol anions chelate heavy metals through the deprotonation of hydroxyl groups, forming metal complexes and releasing a proton (ArOH → ArO– + H+) (160). These three pathways collectively evaluate the antioxidant potential of polyphenols in protecting human health against oxidative damage (157).
Different polyphenols exert distinct effects on antioxidant activity (161, 162). For instance, quercetin has demonstrated potent antioxidant properties (163). The antioxidant efficacy of polyphenols has been extensively investigated in both in vivo and in vitro studies (164, 165), confirming their preventive role against various diseases (166, 167). The pharmacological potential of Rhododendron tomentosum has been linked to its polyphenolic composition, including chlorogenic acid, caffeic acid, rutin, and quercetin, as identified by high-performance thin-layer chromatography (168).
The antioxidant activities of these compounds were confirmed using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay. Similarly, the DPPH assay was used to evaluate the antioxidant activity of Zhourat plants (169). Following the quantification of total phenolic acids, different solvent extractions were assessed for free radical scavenging capacity. Water/ethanol extracts often exhibit superior antioxidant activity compared to pure water or ethanol-only extracts, with two exceptions (169). Bashmil et al. (170) showed that unripe bananas possessed higher free radical scavenging ability than ripe ones, highlighting the influence of polyphenol type, structure, and phenolic ring count on antioxidant efficacy. Janarny et al. (171) examined the antioxidant capacity of edible flowers from the family Fabaceae (171). In Chamanerion angustifolium L. (fireweed) leaves, antioxidant activity varied with fermentation conditions. Notably, activity decreased after 24 h of fermentation under both aerobic and anaerobic conditions; however, it increased after 48 h compared to unfermented leaves (171).
Bobkova et al. (172) evaluated the antioxidant potential of coffee using free radical-scavenging methods, revealing that antioxidant capacity varied with geographical origin due to differences in polyphenol content (172). Alsaud et al. (173) reported that Leptospermum scoparium (Manuka) leaves exhibited significant ferric-reducing antioxidant power (FRAP assay) and free radical scavenging activity (DPPH assay) (173). The ethanolic extract outperformed most deep eutectic solvent extracts, though some deep eutectic solvent extracts exhibited higher ferric-reducing antioxidant power values. Overall, polyphenols exhibit antioxidant properties through various pathways, including free radical scavenging and the augmentation of endogenous antioxidant enzyme activity (174, 175).
Table 1 illustrates the sources, classifications, antioxidant efficacy, and modes of action of polyphenols. Figure 3 illustrates the antioxidant properties of natural compounds, specifically resveratrol from red wine and curcumin from turmeric, as therapeutic interventions for oxidative stress-induced chronic obstructive pulmonary disease caused by exposure to harmful particles, smoking, and infections.
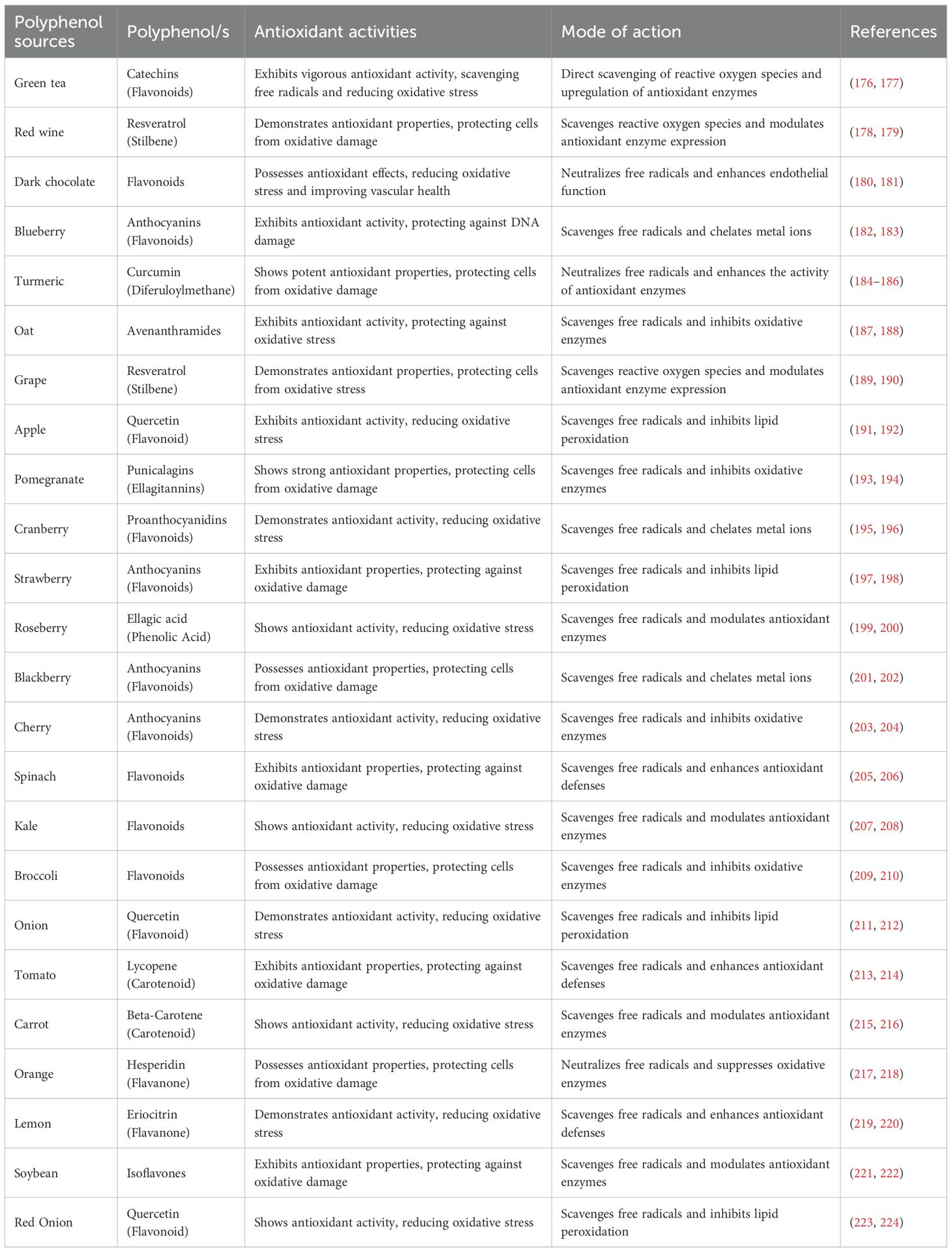
Table 1. Diverse sources and types of polyphenols, their antioxidant properties, and modes of action.
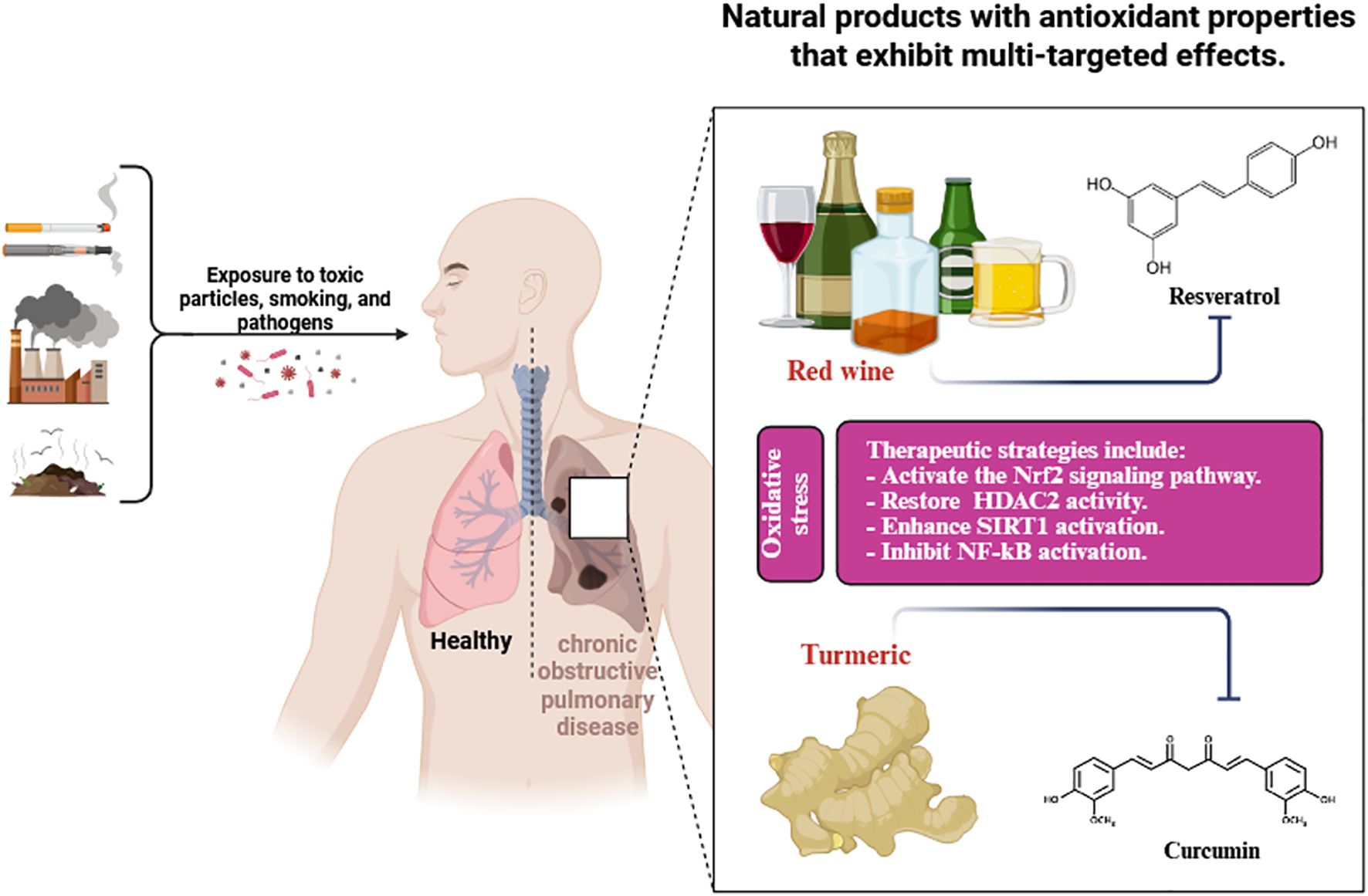
Figure 3. Antioxidant properties of natural compounds (resveratrol from red wine and curcumin from turmeric) as therapeutic approaches to combat oxidative stress-induced chronic obstructive pulmonary disease caused by exposure to harmful particles, smoking, and infections.
5.2 Anti-inflammatory activity
The hydroxyl groups and unique aromatic ring structures of polyphenols allow them to exert regulatory effects on various inflammatory pathways (225, 226). Polyphenols can suppress the expression and activity of key pro-inflammatory mediators, such as nuclear factor-κB (NF-κB), a transcription factor vital to the regulation of the inflammatory response, as shown in Table 2 (269, 270).
By inhibiting the activation of NF-κB, polyphenols reduce the expression of pro-inflammatory genes and the synthesis of inflammatory cytokines and enzymes (271, 272). Additionally, polyphenols may modulate the biosynthesis of pro-inflammatory lipid mediators by affecting the enzymatic activities involved in inflammation, thereby contributing to their anti-inflammatory action (270, 273).
Polyphenols also regulate immune cell function by modulating the activity of dendritic cells, lymphocytes, and macrophages (274, 275). Moreover, they influence immune recruitment and migration by altering the synthesis of chemokines and adhesion molecules (271, 276). Synergistic interactions among various polyphenolic compounds may further enhance their anti-inflammatory effects (277). A comparative analysis of polyphenols extracted from celery, coriander, and parsley revealed that celery had the highest total polyphenol content, followed by coriander and parsley (278). However, parsley polyphenols demonstrated the most potent nitric oxide scavenging activity, which is essential in inflammation due to the overproduction of nitric oxide. When tested for their ability to prevent the protein denaturation effect, parsley extract again showed superior activity (278). Similarly, in membrane stabilization assays, used to assess the protection of erythrocyte membranes under inflammatory stress, parsley extract demonstrated superior activity (278). Polyphenolic compounds in berries have also been extensively studied for their anti-inflammatory properties (279, 280).
Kim et al. (281) reported that polyphenols from black raspberry roots significantly inhibited the production of nitric oxide and prostaglandin E2 in lipopolysaccharide (LPS)-activated RAW264.7 macrophages in a dose-dependent manner. These root polyphenols were more effective than those from unripe fruits in reducing the levels of pro-inflammatory cytokines and downregulating the mRNA expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (281).
Furthermore, these polyphenols exhibited strong antimicrobial activity against methicillin-resistant Bacillus anthracis, S. aureus (MRSA), and carbapenem-resistant Acinetobacter baumannii. Peng et al. (282) demonstrated that polyphenol-rich extracts from rice wine significantly downregulated inducible nitric oxide synthase expression and reduced nitric oxide production. The extracts also suppressed the expression of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) (282). These effects were associated with the inhibition of NF-κB nuclear translocation and reduced phosphorylation of κB and mitogen-activated protein kinases, including p38, extracellular signal-regulated kinases 1 and 2 (Erk 1/2), and c-Jun N-terminal kinase (282). Zhang et al. (283) found that polyphenols inhibited nitric oxide production and reduced the expression of IL-1β, IL-6, TNF-α, and nitric oxide synthase in LPS-activated macrophages. These compounds also suppressed NF-κB activation and mitogen-activated protein kinases phosphorylation (extracellular signal-regulated kinases 1 and 2 (Erk 1/2), and c-Jun N-terminal kinase).
A study by de Araújo (284) involved the determination of minimum inhibitory concentration (MIC) and agar well-diffusion assays against methicillin-resistant strains of S. aureus, Salmonella enteritidis, E. coli, Enterococcus faecalis, and Staphylococcus epidermidis (284). Polyphenols from Psidium guajava exhibited the largest zones of inhibition in the agar diffusion test. Notably, the polyphenol extracts were more effective against Gram-positive bacteria and ineffective against Gram-negative strains (284).
Anti-inflammatory activity was further evaluated using the carrageenan-induced peritonitis model in mice. Administration of plant extracts significantly reduced the inflammatory response induced by carrageenan (284). However, in acetic acid-induced writhing and analgesic tests, the extracts did not exhibit significant pain-relieving effects, suggesting selective anti-inflammatory rather than analgesic activity (284). These findings support the potential of these plant-derived polyphenols in managing inflammatory conditions (283, 284).
Fermentation plays a significant role in modifying the bioactivity of polyphenol-rich plant materials (284, 285). Recent research by Sim et al. (286) showed that complex fermentation of Pyrus montana and Maclura tricuspidata using lactic acid bacteria, yeast, and Aspergillus shirousamii enhanced their phenolic content and anti-inflammatory activity. Fermented extracts exhibited increased DPPH and ABTS radical-scavenging capacities and significantly reduced nitric oxide production from day six of fermentation. Western blot analysis revealed suppression of TNF-α, COX-2, and nitric oxide synthase protein expression, indicating effective inhibition of inflammation-related signaling pathways. Overall, polyphenols from various plants, algae, and natural sources possess notable anti-inflammatory potential and contribute to the prevention and management of chronic inflammatory diseases (287, 288). They also offer protective effects against metabolic disorders through their ability to regulate inflammatory signaling pathways (289).
Table 2 illustrates various sources and types of polyphenols, along with their anti-inflammatory properties and modes of action.
5.3 Antimicrobial activity
Antimicrobial activity refers to the ability of a substance to inhibit or reduce the growth of microorganisms, including bacteria, viruses, parasites, and fungi (290, 291). Antimicrobial agents are widely used in medicine, agriculture, and the food industry to combat microbial infections (292, 293). As shown in Table 3, the antibacterial properties of plant extracts are attributed mainly to their phenolic compounds (337, 338).
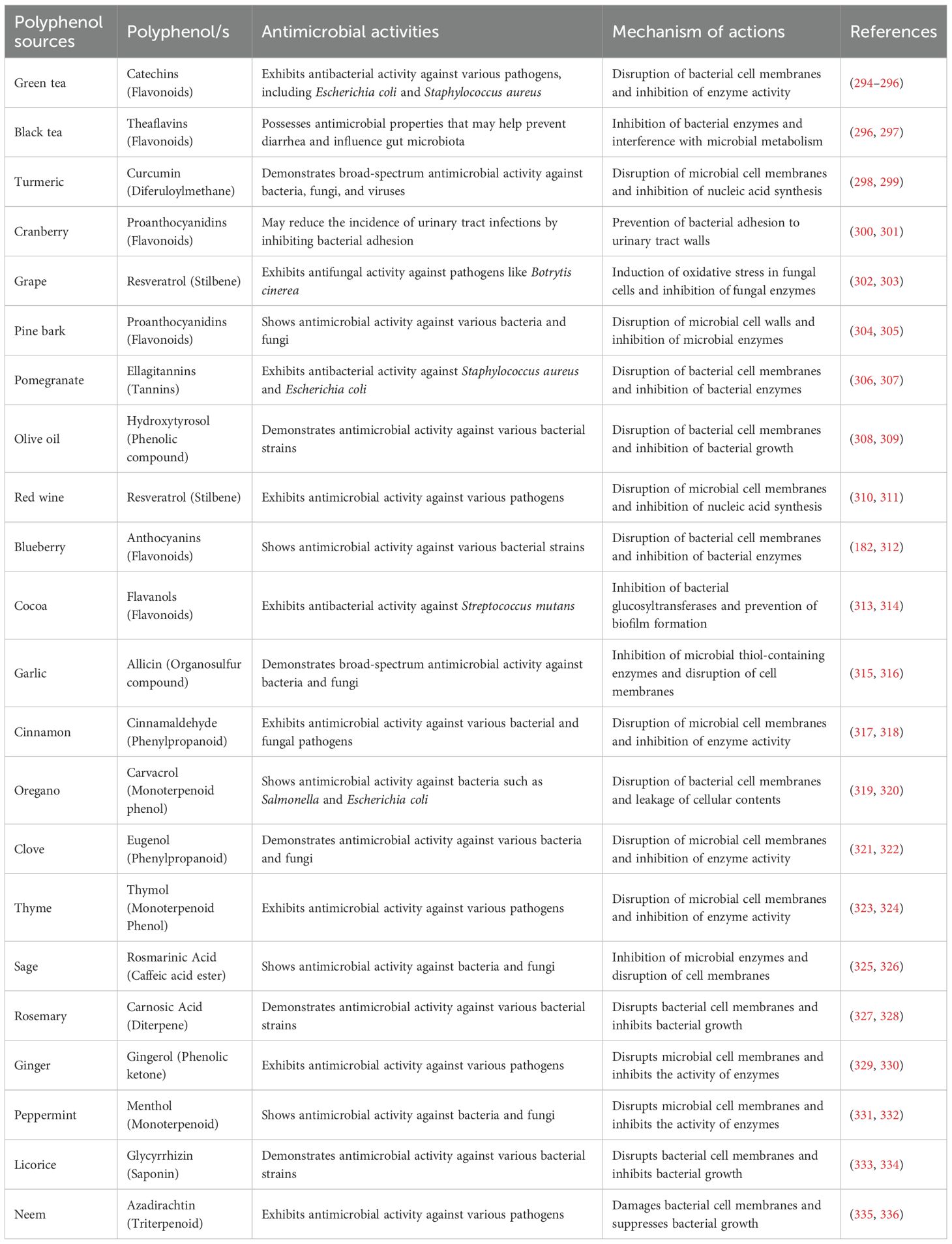
Table 3. Numerous polyphenol sources, categories, antimicrobial properties, and mechanisms of action.
Numerous polyphenols exhibit antimicrobial properties by disrupting cell structures and membranes and interfering with essential enzymatic cellular functions (14, 339). Key determinants of their antimicrobial activity include the presence of carboxyl groups and the arrangement of functional subgroups on the benzene ring (340). Menhem et al. (169) assessed the antimicrobial activity of Zhourat Shamia herbal tea (mixture of herbs and dried flowers) using a disk diffusion assay against foodborne pathogens, including two Gram-positive bacteria (S. aureus and B. cereus) and two Gram-negative bacteria (E. coli and Pseudomonas aeruginosa). The phenolic compounds in Zhourat extracts exhibited antimicrobial activity, though efficacy varied depending on the extract and microbial species (169).
A separate study by Gutiérrez-Venegas et al. (341) indicated that rutin, quercetin, and morin had antimicrobial action against Actinomyces naeslundii and Actinomyces viscosus. While each flavonoid has antimicrobial capabilities against some strains, no antimicrobial impacts have been observed against Streptococcus oralis and Streptococcus sanguinis (341). The number and type of hydroxyl, carboxyl, and ester groups also play a crucial role by facilitating interactions between polyphenols and microbial cells, thereby inhibiting microbial growth (342, 343). Additionally, polyphenols can interfere with intracellular processes by impairing the activity of enzymes necessary for microbial survival, leading to reduced proliferation (344–346).
De Angelis et al. (347) reported that combinations of polyphenols and micronutrients (A5+) exert antiviral effects against influenza A and SARS-CoV-2. In this study, resveratrol demonstrated antiviral efficacy against respiratory viruses, while polydatin was used as its precursor. Treatment with A5+ and resveratrol significantly reduced SARS-CoV-2 replication. Furthermore, both agents suppressed the expression of essential viral replication proteins and IL-6 in influenza A virus-infected cells. Singh et al. (348) evaluated polyphenols as natural antiviral agents against SARS-CoV-2 using in silico analysis, targeting the RNA-dependent RNA polymerase (RdRp) responsible for viral RNA replication. The study found that eight different polyphenols demonstrated favorable binding kinetics, suggesting their potential to inactivate SARS-CoV-2 RdRp (348).
Therefore, polyphenols are considered promising antiviral agents. Musarra-Pizzo et al. (349) conducted antiviral and antimicrobial assays using Prunus dulcis L. against S. aureus and herpes simplex virus type 1. The antibacterial activity of almonds was inhibited entirely by polyphenols at a concentration of 0.62 mg/mL. Furthermore, antiviral assays revealed that 0.4 mg/mL of almond polyphenols reduced both the expression of viral proteins and the accumulation of viral DNA (349). Park et al. (350) demonstrated that the ethanolic extract of Aronia melanocarpa, rich in polyphenols and flavonoids, exhibits antiviral activity. A 0.0625 mg sample of the extract significantly inhibited viral surface proteins in 70% of tested influenza strains, including H1 and H3 subtypes. Pagliarulo et al. (351) evaluated the antimicrobial activity of Punica granatum against S. aureus and E. coli. Pomegranate juice was extracted and then subjected to ethanolic polyphenol extraction of pomegranate using a 50% ethanol/water (v/v) solution. The juice, particularly rich in anthocyanins, was tested in quantities of 1, 2, 4, 8, 10, and 20 mg per disk. The result demonstrated that the extracts inhibited the growth and survival of the tested bacterial strains (351).
Certain extracts exhibited no efficacy against several bacteria, while others exhibited selective antimicrobial effects. Nibir et al. (352) analyzed the total phenolic and flavonoid levels, as well as the antioxidant and antimicrobial properties, of four Chinese tea varieties: broken orange pekoe, black tea, red dust, and green tea. The green tea variety had the highest phenolic and flavonoid content and demonstrated superior antioxidant and antimicrobial activity. The antimicrobial potential of these teas was tested against Shigella dysenteriae, Shigella boydii, Vibrio cholerae, Salmonella paratyphi, Salmonella typhi, Klebsiella pneumoniae, and E. coli employing agar well-diffusion and MIC assays. These findings confirm that green tea has greater antimicrobial efficacy than the other types (352).
Notably, the antimicrobial activity of polyphenols can be influenced by the extraction procedure and the solvent used (339, 353). Chaudhry et al. (354) examined the effects of extraction methods and solvent systems on yield. Traditional maceration- and ultrasound-assisted extraction techniques were compared using methanol, ethanol, and acetone at 25%, 50%, 75%, and 100% concentrations. Among these, ultrasound-assisted extraction yielded the highest polyphenol content from banana peels (354). Ethanol proved to be the most effective solvent compared to the alternatives. Solvent concentration significantly influenced the yield of polyphenols. Ethanol-based extracts demonstrated superior antioxidant activity, as indicated by the DPPH radical scavenging assay. In contrast, banana peel extracts at various concentrations were tested against E. coli, P. aeruginosa, S. aureus, and Saccharomyces cerevisiae using the agar disk diffusion method. Measurement of the inhibition zones revealed that ethanol-containing extracts exerted more substantial antimicrobial effects than those obtained with other solvents (354).
In the gut, polyphenols linked to indigestible fibers can contribute to health benefits by releasing bioactive phenolic compounds through microbial fermentation. Thus, incorporating fermentable fiber into the diet may support the growth of beneficial gut microbiota and exert prebiotic effects (355). Although the antimicrobial properties of phenolic compounds are well established, these effects may be modified during gastric digestion (356).
Caponio et al. (357) reported that digestive processes may influence the free radical-scavenging ability of phenolic compounds. Antimicrobial activity was assessed based on effects on the probiotic and pathogenic strains, specifically Lactiplantibacillus plantarum, Bacillus megaterium, E. coli, and Listeria monocytogenes. These findings indicated that grape pomace-derived polyphenols promoted probiotic growth while inhibiting pathogenic bacteria (357). Similarly, a study on the antimicrobial and digestive behavior of polyphenols from Hibiscus sabdariffa showed that these compounds were rapidly released and metabolized in the human digestive tract (358). Polyphenols have demonstrated antimicrobial efficacy against pathogenic bacteria, including L. monocytogenes and S. aureus (359, 360), making them promising candidates for use as antimicrobial agents (361, 362).
Several in vivo studies have confirmed the stability and efficacy of polyphenols following gastrointestinal digestion. For example, dietary supplementation of polyphenol-rich extracts in animal models, such as grape seed extract in broiler chickens and pigs, has been shown to increase the concentration of antioxidant markers like vitamin E in plasma and tissues, suggesting not only the bioavailability but also the effective physiological action of polyphenols after digestion (363). In another study, grape pomace-supplemented feed improved the ratio of polyunsaturated to saturated fatty acids and enhanced the oxidative stability of animal products, indicating that a considerable portion of polyphenols retained their bioactivity after digestive processes (357). Similarly, research has demonstrated that polyphenolic compounds maintain significant antioxidant effects in vivo, as evidenced by enhanced plasma antioxidant capacity and reduced markers of oxidative stress in animals supplemented with polyphenols (364).
The findings indicate that, despite specific degradation during digestion, a significant proportion of polyphenols and their metabolites remain sufficiently stable for absorption, hence facilitating their potential health-promoting effects in living organisms post-absorption.
Table 3 shows the various polyphenol sources, kinds, antimicrobial properties, and their mechanisms of action. Figure 4 illustrates the antimicrobial mechanisms of polyphenols, illustrating the disruption of microbial cell structures (lipopolysaccharide cell wall, peptidoglycan cell wall, phospholipid bilayers, cell membrane proteins) and the impairment of essential cellular functions (inhibition of DNA gyrase and RNA synthesis, pore formation causing leakage, damage to membrane lipid bilayers, inhibition of enzyme activity, disruption of cell wall biosynthesis, and inactivation of lipopolysaccharide) in bacteria such as S. aureus, E. coli, and P. aeruginosa by specific polyphenol compounds (catechin, quercetin, EGCG, myricetin, ferulic acid, gallic acid, proanthocyanidins, tannin, and kaempferol-3-rutinoside).
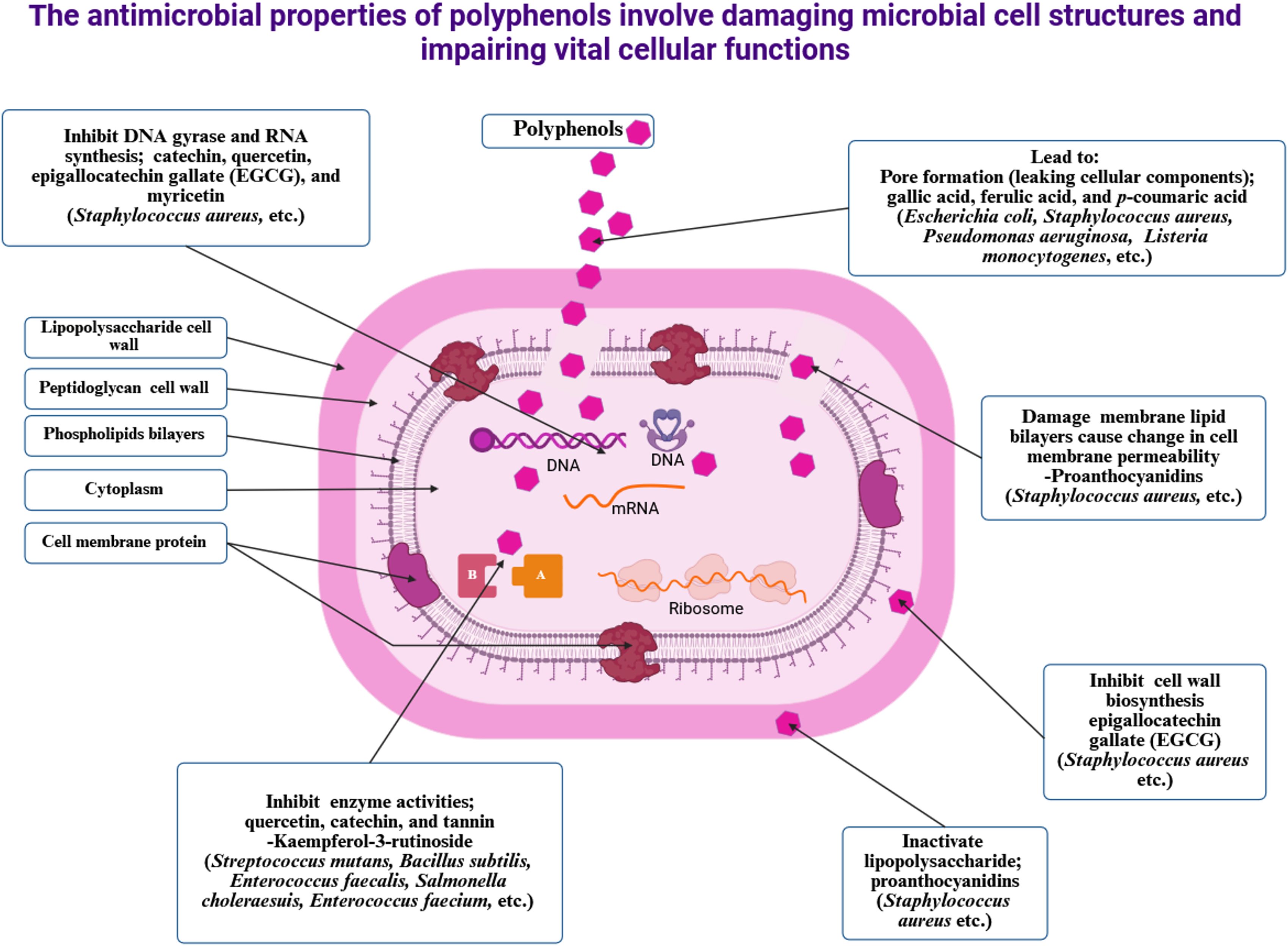
Figure 4. The antimicrobial mechanisms of polyphenols involve the disruption of microbial cell structures, including lipopolysaccharide cell walls, peptidoglycan cell walls, phospholipid bilayers, and cell membrane proteins, as well as the impairment of essential cellular functions such as inhibition of DNA gyrase and RNA synthesis, pore formation causing leakage, damage to membrane lipid bilayers, inhibition of enzyme activity, suppression of cell wall biosynthesis, and inactivation of lipopolysaccharides in bacteria like Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa by specific polyphenol compounds, including catechin, quercetin, epigallocatechin gallate, myricetin, ferulic acid, gallic acid, proanthocyanidins, tannin, and kaempferol-3-rutinoside.
5.4 Anti-diabetic activity
Natural products play a significant role in promoting human health (365, 366). Plants have long been used in various cultures to treat diseases and disorders (367, 368). Accordingly, research continues to explore plant-derived compounds for managing type 2 diabetes mellitus, a metabolic disorder increasingly prevalent due to modern lifestyle changes (369, 370). Type 2 diabetes mellitus is characterized by chronic hyperglycemia resulting from insulin resistance, amyloid deposition, pancreatic β-cell dysfunction, and impaired glucose regulation (371, 372).
Current studies indicate that insulin regulation involves several mechanisms, including pancreatic cell protection, modulation of cell proliferation and apoptosis, oxidative stress reduction, insulin signaling activation, increased insulin secretion, inhibition of glucose uptake, gut microbiome regulation, and attenuation of inflammatory responses (16, 373). Therefore, dietary polyphenols hold the potential for managing type 2 diabetes mellitus (374, 375). Additionally, compounds such as resveratrol, curcumin, and quercetin have shown that they can lower oxidative stress and inflammation by modulating key insulin-related signaling pathways (14, 376). Numerous studies have reported the anti-diabetic effects of tea polyphenols in experimental diabetes models, demonstrating their ability to lower blood glucose levels, improve insulin sensitivity, and reduce oxidative stress and inflammation associated with type 2 diabetes mellitus (377, 378).
Sabu et al. (377) found that administration of 500 mg/kg green tea polyphenols significantly inhibited the increase in serum glucose levels at 60 min. Similarly, polyphenols extracted from spicate eugenia (Syzygium zeylanicum L.) exhibited anti-diabetic influences in 2.5–3-month-old diabetic zebrafish subjected to overfeeding and hyperglycemic conditions. The findings suggest these polyphenols may regulate genes involved in lipid and glucose metabolism and influence glucose absorption and utilization, contributing to the normalization of fasting blood glucose levels (379). Animal studies have also demonstrated the anti-diabetic effects of flax (Linum usitatissimum) in 8–12-week-old female rats, with consistent reductions in blood glucose levels and body weight (380). Histological analyses revealed partial improvement in pancreatic, hepatic, and renal tissues following treatment with the plant extract (380).
Zuo et al. (381) investigated the anti-diabetic properties of Phaseolus vulgaris L. in 5–6-week-old male rats. In this study, type 2 diabetes mellitus rats were fed either a high-fat diet or a standard diet with detailed macronutrient compositions. The results showed that P. vulgaris L. could regulate blood glucose and cholesterol levels, reduce insulin resistance, and increase gut short-chain fatty acid production, thereby mitigating pancreatic and hepatic damage and restoring intestinal microbiota balance (381). Another study assessed the anti-diabetic properties of yellow and green papaya (Carica papaya), revealing lipid-lowering activity and enhanced hepatic glucose metabolism, suggesting its therapeutic potential in diabetes management (382).
Similarly, Pieczykolan et al. (383) revealed that Aerva lanata L. has been shown to possess anti-diabetic, antioxidant, and anti-inflammatory properties via inhibition of α-amylase and α-glucosidase, enzymes associated with glucose metabolism. Further investigations have explored the anti-diabetic potential of ethanolic propolis extracts under in vitro and in vivo conditions (384). In one experiment, diabetic rats were administered a 0.5 mL/100 g dose of either 15% or 30% propolis extract for 4 weeks, resulting in significant blood glucose reduction (384). A separate study investigated the therapeutic effects of vinegar extract from Zhenjiang aromatic vinegar in diabetic mice. The extract improved body weight, lowered blood glucose, enhanced glucose and insulin tolerance, and reduced liver inflammation. These effects were partly attributed to the modulation of the gut microbiota and short-chain fatty acid levels, indicating a potential role in diabetes therapy (385).
Vaithiyalingam et al. (386) investigated the pharmacokinetic characteristics of curcumin from Curcuma longa, highlighting its strong ligand-binding interactions with key protein targets, including α-amylase, α-glucosidase, DPP-4, PPAR, and SGLT-2. These findings position curcumin as a promising candidate for diabetes treatment. Jahan et al. (387) examined the functional potential of haustoria from coconut (Cocos nucifera) and palmyra palm (Borassus flabellifer) for anti-diabetic applications. B. flabellifer demonstrated superior antioxidant capacity through DPPH and H2O2 scavenging and lipid peroxidation inhibition compared to C. nucifera. Additionally, α-amylase and α-glucosidase inhibition assays showed greater enzyme inhibitory activity in B. flabellifer (387).
The anti-diabetic efficacy of diverse polyphenols has been demonstrated in both in vivo and in vitro studies, supporting their potential as therapeutic agents (388, 389). However, despite promising findings, current research remains insufficient, and further investigations are needed to validate these compounds for future clinical applications.
Figure 5 illustrates the antidiabetic mechanism of polyphenols in the human body, showing deconjugation in the gastrointestinal tract, absorption, hepatic portal circulation, reconjugation in the liver, biliary excretion, renal excretion, and microbial deconjugation in the colon, ultimately leading to fecal excretion.
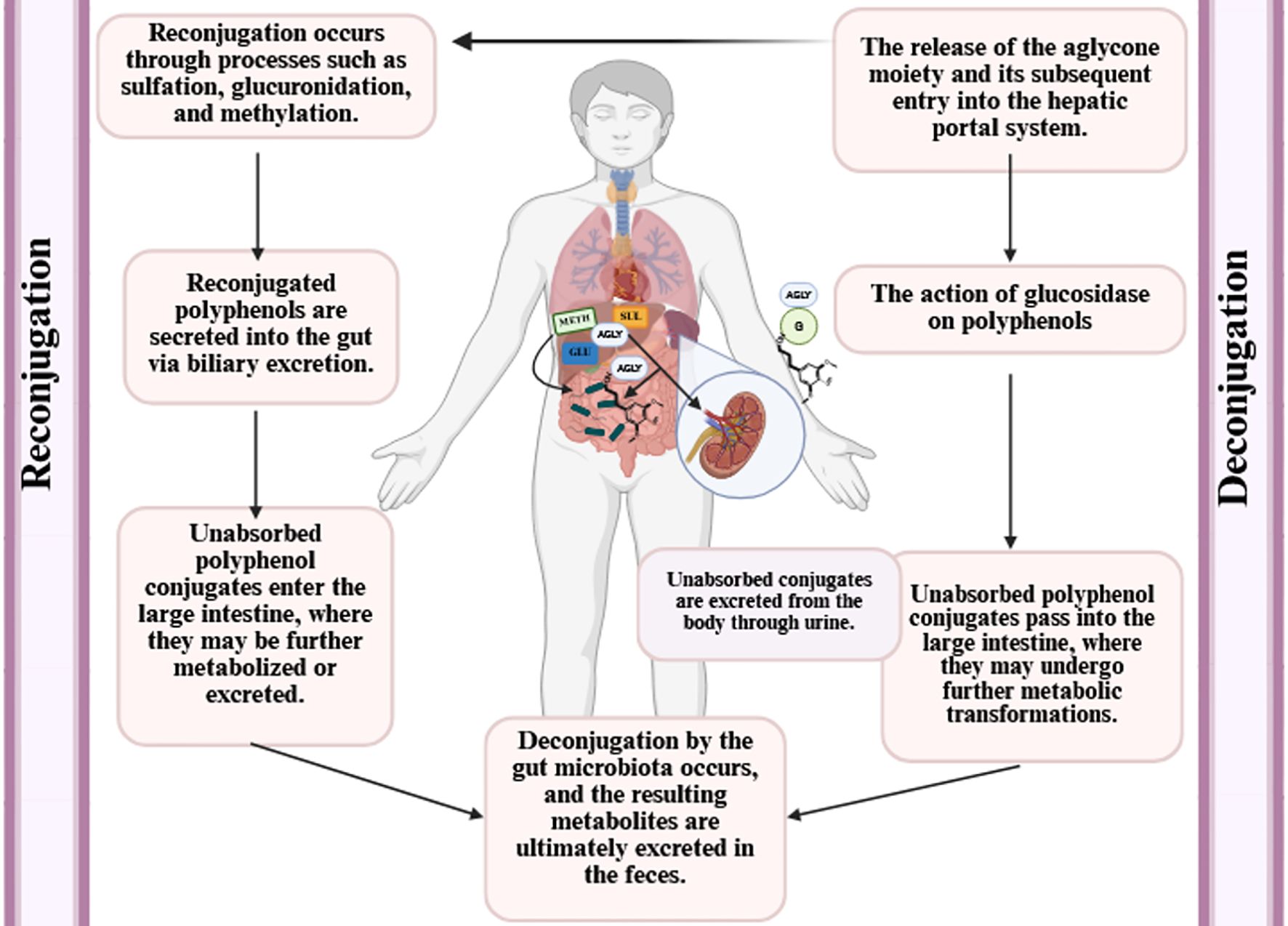
Figure 5. Antidiabetic mechanism of polyphenols in the human body, illustrating deconjugation in the gastrointestinal tract, absorption, hepatic portal circulation, reconjugation in the liver, biliary excretion, renal excretion, and microbial deconjugation in the colon, resulting in fecal excretion.
5.5 Application in skin and hair health
The skin, the body’s largest organ, acts as a dynamic interface with the environment, playing essential roles in protection against UV radiation, pathogens, and extreme temperatures (390, 391). Its constant exposure to environmental stressors, combined with its complex functions, contributes to the development of various dermatological conditions (392). Similarly, hair follicles—extensions of the epidermis—are influenced by both internal and external factors. Hair follows a cyclical growth pattern encompassing the anagen, catagen, and telogen phases. Disruptions in this cycle can lead to hair thinning or loss, adversely affecting an individual’s psychosocial well-being, self-esteem, and mental health, often leading to social anxiety and depression (392).
A range of factors—genetic predisposition, hormonal imbalances, infections, stress, and psychological disorders—contribute to both skin and hair disorders. While conventional pharmaceutical and treatments are available, many synthetic drugs pose limitations or adverse effects, fueling growing interest in natural alternatives (391). Among these, plant-derived polyphenols have garnered attention for their broad therapeutic potential, including anti-inflammatory, antioxidant, anti-aging, anti-carcinogenic, antimicrobial, and depigmenting properties (393).
Several studies have highlighted the potential of polyphenols in dermatological applications. For example, grape seed extract, applied to normal human melanocyte and dermal fibroblast cells, was shown to enhance skin youthfulness by stimulating collagen and elastin production (133). It also reduced UVB-induced inflammation and DNA damage, while promoting skin hydration and reducing melanin production—key factors in diminishing wrinkle formation (133).
Similarly, Caralluma europaea extracts demonstrated both anti-tumor and wound-healing effects. These extracts inhibited leukemia and hepatocellular carcinoma cell lines in vitro, while topical application in rats accelerated wound healing (394). Ethanolic extracts of Acacia nilotica showed potent free radical scavenging activity due to their hydroxyl group content, indicating their potential as natural antioxidants (393).
In a clinical trial by Montenegro et al. (395), the topical application of resveratrol-loaded lipid nanocarriers significantly improved skin hydration, emphasizing the potential of lipid-based delivery systems in skincare. Other studies showed that combining polyphenols with sunscreen components provided synergistic UV protection (377). Notably, naringenin-loaded NPs exhibited superior antioxidant activity and sustained skin retention compared to the native compounds (395).
Curcumin-loaded nanocubosomal hydrogels were found to reduce signs of skin irritation (e.g., erythema and edema) in rats and displayed enhanced antibacterial activity against E. coli (396). Likewise, a hydrophilic extract of Rhus coriaria promoted collagen production, accelerated wound healing, and showed antimicrobial activity against several pathogenic bacteria (397). A liposome-based extract from Hibiscus sabdariffa L. calyx showed no irritation in rabbit skin models and was effective as an anti-aging skincare product due to its antioxidative properties (398). Polyphenols from Malpighia emarginata DC also demonstrated skin-lightening effects by reducing UVB-induced pigmentation and melanin synthesis (399). Similarly, strawberry extracts protected against UVA-induced skin damage by reducing ROS and inflammatory markers (400).
Other findings revealed that Penthorum chinense extracts possess anti-aging and moisturizing properties (383), and green tea polyphenols exhibit anti-inflammatory effects against acne vulgaris (401). In another study, Coffee arabica L. hydrogels—especially those from green beans—promoted skin regeneration and reduced oxidative stress in wound areas (402). Overall, polyphenols offer significant potential in promoting skin and hair health (402). However, more comprehensive studies are needed to optimize their application and explore their full therapeutic potential in dermatology.
5.6 Neuroprotective effect
Polyphenols are increasingly recognized for their neuroprotective properties, primarily attributed to their potent antioxidant and anti-inflammatory activities (377, 403). These compounds can neutralize free radicals and reduce oxidative stress, mechanisms implicated in the pathogenesis of several neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease (404, 405).
Curcumin, a polyphenol derived from C. longa, has demonstrated anti-inflammatory, antioxidant, and anti-amyloid effects in various Alzheimer’s disease models (388). For instance, curcumin-loaded lipid-core nanocapsules mitigated Aβ-induced behavioral changes and synaptotoxicity in rats. The purified polyphenols from pomace significantly reduced paralysis in Caenorhabditis elegans Alzheimer’s disease and showed antioxidant effects compared to non-purified forms (388).
In Alzheimer’s disease, the accumulation of β-amyloid (Aβ) peptides and tau protein aggregates disrupts neuronal function (406). Blends of polyphenols—including resveratrol and grape juice—have been shown to reduce amyloid neuropathology and improve cognitive deficits in animal models (406). Resveratrol, in particular, activates the Sirt1 gene, enhances glutathione and superoxide dismutase levels, and reduces oxidative stress (407). Grape leaf polyphenols also exhibited neurotrophic, anti-inflammatory, and antioxidant effects in aluminum chloride-induced Alzheimer’s disease rat models, suggesting a potential therapeutic role (408).
Parkinson’s disease, characterized by the degeneration of dopaminergic neurons and the aggregation of α-synuclein, polyphenols again show promise (409). EGCG from green tea has been shown to inhibit α-synuclein aggregation and prevent mitochondrial dysfunction (410, 411). A unique polyphenol-micronutrient blend, A5+, was found to block apoptotic pathways, reduce oxidative stress, and suppress pro-inflammatory cytokines in Parkinson’s disease models (387). In addition, nanosheet polyphenolic fractions from propolis demonstrated enhanced antioxidant effects in vitro and in vivo (412). Olive-derived polyphenols improved locomotor ability and lifespan in C. elegans Parkinson’s disease models (405). In Huntington’s disease, caused by polyglutamine expansions in the Huntingtin protein, curcumin reduced photoreceptor degradation and motor impairment in Drosophila models (413).
Despite promising evidence, challenges remain (414, 415). The mechanisms underlying the neuroprotective effects of polyphenols are not fully understood, and issues with their bioavailability persist (416, 417). Future research is essential to elucidate these mechanisms, improve delivery systems, and determine effective therapeutic dosages for preventing and managing neurodegenerative diseases.
5.7 Anti-tumor and anti-cancer activity
Researchers have long been interested in exploring the anti-tumor and anti-cancer potential of polyphenols (418, 419). These natural chemicals exhibit chemo-preventive benefits against multiple cancer types, as shown in Table 4 (465, 466). Research suggests that polyphenols may significantly limit tumor growth and prevent cancer formation due to their anti-inflammatory, antioxidant, and antiproliferative effects (467, 468). Multiple methodologies exist to evaluate the antiproliferative, and anti-cancer properties of polyphenols (469, 470). One strategy entails performing in vitro research using cancer cell lines (471, 472). Researchers can examine the effects of polyphenols derived from various sources or using different quantities on tumor cells, and their effects on cellular growth and proliferation (473, 474).
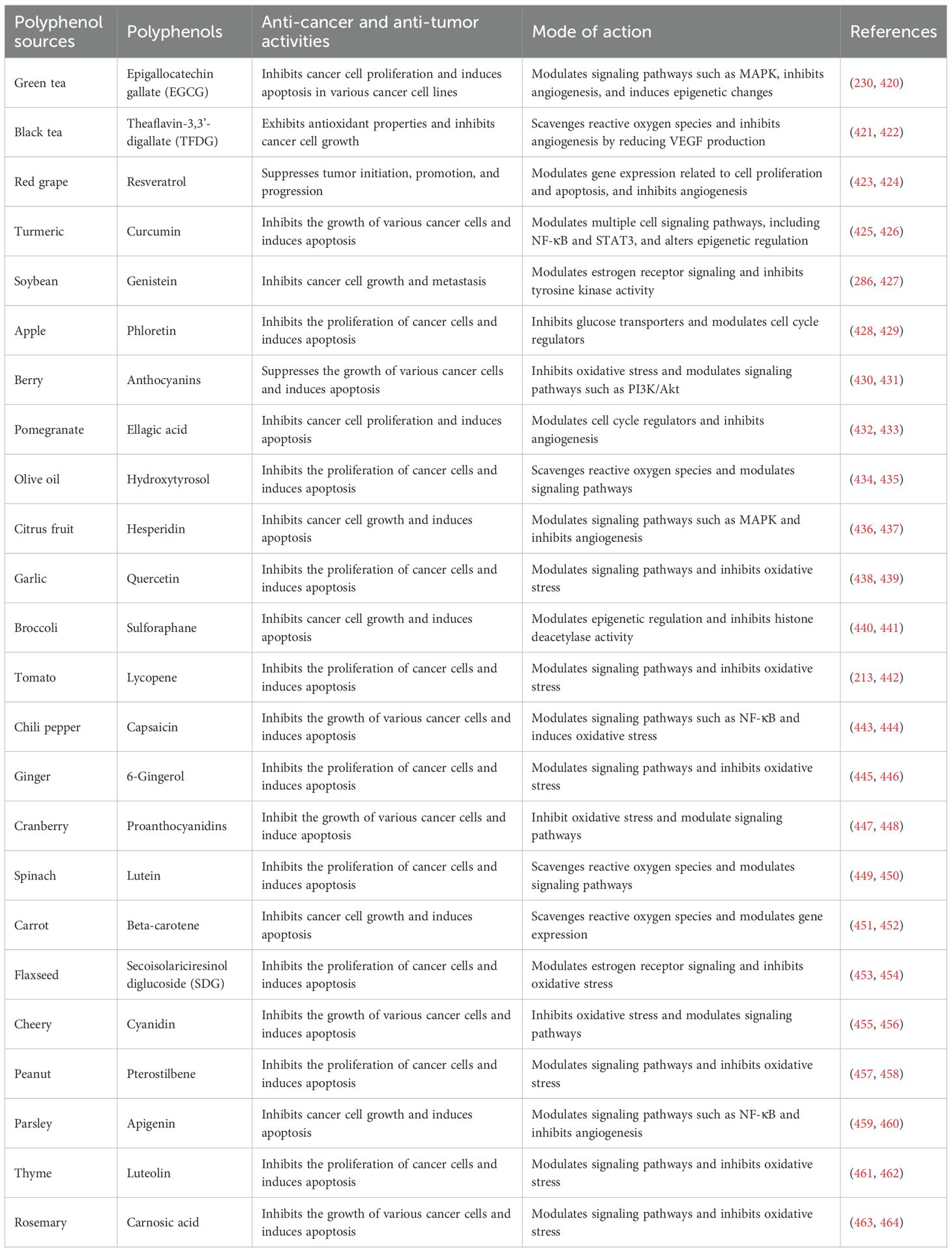
Table 4. Sources, types, anti-cancer and antitumor properties, and the mechanism of action of polyphenols.
Zhang et al. (456) indicated that Cerasus humilis fruit, recognized for its high polyphenol content, demonstrated considerable inhibitory effects on hepatic, colon, and stomach tumor cells. A modern experiment demonstrated that the phenolic component of Cerasus europaea extracts displayed ‘anti-tumor’ potential versus human leukemia (K562 and HL60) and hepatic tumor (Huh-7) cell lines (394). Yi et al. (475) showed that the purified polyphenols possess antiproliferative properties on distinct cancer cell lines in the human colon tumor stem cell line (LOVO cell line), the pure polyphenols acquired in this research may be utilized to manufacture functional meals.
Furthermore, Yi et al. (476) conducted a study demonstrating that isolated polyphenols from Pinus koraiensis pinecones have an anti-cancer impact on colon cancer cells via stimulating death via caspase activation. Huang et al. (477) indicated that the extracted polyphenols from the bark of P. koraiensis have a significant inhibitory effect on colon cancer cells via augmenting the quantity of apoptotic cells (477). The investigation examined the use of several polyphenols to mitigate the side impacts of tumor, beside their direct impacts on tumor cells (478–480).
Another method to examine these impacts is by performing in vivo experiments with animal models (481, 482). These investigations often entail observing cancer progression in affected animals via the supply of polyphenols using diverse approaches (482, 483). Extensive research may include both in vitro and in vivo trials (484, 485).
These findings clarify the molecular mechanisms influenced by polyphenols (472, 486, 487). The fundamental purpose of these investigations is to study the impacts of polyphenols on apoptosis, angiogenesis, cell cycle regulation, and metastasis (488, 489). Wu et al. (488) revealed a notable reduction in cell viability corresponding to elevated dosages of polyphenols derived from Hippophae rhamnoides labeled as HPs60, showing an inactivation impact of HPs60 on tumor cell proliferation. The modification of miRNA expression patterns resulting from HPs60 therapy influenced the alterations in cell viability through modulating cell cycle progression as well as apoptosis (488). In vivo investigations on mice indicated no discernible poisonousness throughout HPs60 therapy, as demonstrated by the lack of substantial changes in body weight across the groups (488). In contrast, there was a notable decrease in cancer volume following HPs60 therapy relative to the control, demonstrating its anti-tumor efficacy in inhibiting cancer growth in vivo. Moreover, HPs60 therapy was demonstrated to influence the expression of microRNAs (miRNAs) in cancer-bearing animals (488).
Moreover, the group receiving the blueberry anthocyanin and crude polyphenol extract demonstrates the most significant cancer suppressor impacts, presumably due to synergistic interactions between the components (490, 491). Furthermore, the extract improved the overall health of mice by augmenting cellular immunological action, enhancing antioxidant enzymatic activities, and diminishing lipid peroxidation (492, 493). To conclude, various studies indicate that polyphenols might substantially affect cancer prevention, influencing disease progression and perhaps improving treatment methods such as radiotherapy and chemotherapy (483, 492, 493). Table 4 delineates the sources, classifications, anti-cancer and antitumor characteristics, as well as the mechanisms of action of polyphenols.
5.8 Other effects
The influence of polyphenols on health encompasses multiple aspects (494, 495). A study approved by de Jesús Romero‐Prado et al. (496) indicates a significant reduction in both systolic as well as diastolic blood pressure, as well as decreases in whole cholesterol, LDL cholesterol, as well as triglyceride levels after the inclusion of dietary flavonoids. The incorporation of flavonoids into pharmacological antihypertensive treatment demonstrates supplementary advantages for blood pressure, lipid profile, leptin levels, obesity, and inflammation (496). Polyphenols contribute to reducing obesity through a variety of interrelated mechanisms. They can inhibit adipogenesis by regulating key signaling pathways and transcription factors such as PPARγ and C/EBPα, thereby limiting the formation and differentiation of new adipocytes (497). Polyphenols also promote the browning of white adipose tissue and enhance thermogenesis, which increases energy expenditure and fat burning (497). Additionally, these compounds stimulate β-oxidation of fatty acids, promote lipolysis, and suppress lipogenesis, collectively improving lipid metabolism and reducing fat accumulation (498).
Moreover, a notable reduction in levels of C-reactive protein was seen, suggesting a possible role in reducing the hazard of cardiovascular disorders. Bogolitsyn et al. (276) observed that polyphenols elevated the quantity of sticky leukocytes in the bloodstream of both leukemia cases and healthy people. Moreover, leukocytes from leukemia cases exhibited a reduced propensity to attach to surfaces relative to those from healthy people, suggesting that algal polyphenols regulated the adhesive activities of leukocytes in a dose-reliant methods. Moreover, polyphenols augmented the adhesion and contact capabilities of cells by stimulating defensive mechanisms against malignant cells (276).
Figure 6 outlines the various health benefits of polyphenols within the human body, highlighting their antioxidant, anticancer, antibacterial, dermatological, neuroprotective, anti-inflammatory, and anti-diabetic properties, as well as their modes of action at both cellular and systemic levels.
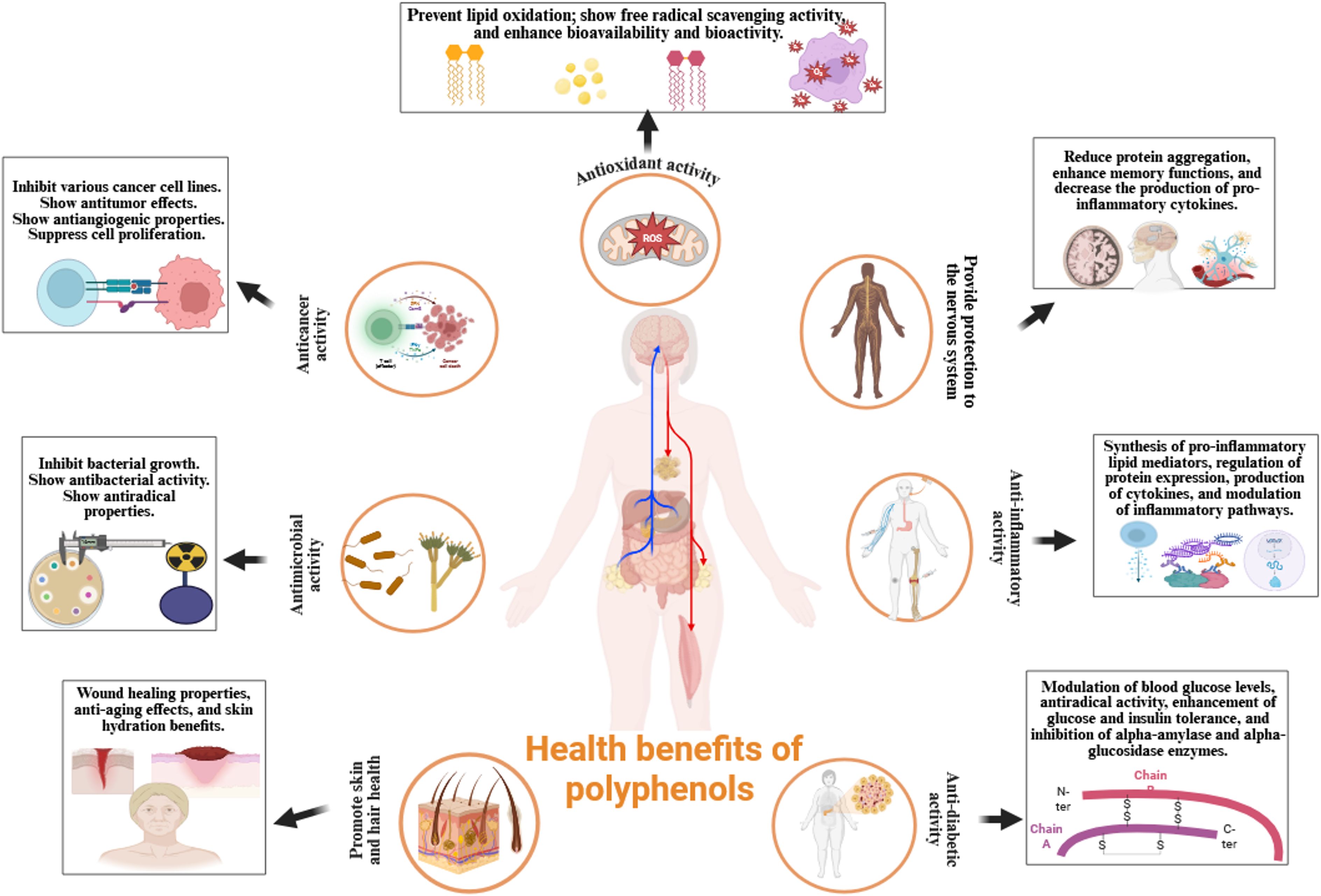
Figure 6. The numerous health advantages of polyphenols in the human body demonstrate their antioxidant, anti-cancer, antibacterial, dermatological, neuroprotective, anti-inflammatory, and anti-diabetic properties, as well as their modes of action at both cellular and systemic levels.
6 Polyphenols and nutritional aspects
Research indicates that the intake of polyphenol-rich foods can promote health, chiefly owing to their antioxidant, anti-inflammatory, anticarcinogenic, and several other qualities (499, 500). Moreover, these qualities are believed to support intestinal health by fostering the proliferation of good bacteria (495). These attributes promote the intake of foods abundant in polyphenols (501), yielding numerous beneficial consequences (502, 503).
Figure 7 illustrates the impact of malnutrition (high-fat, high-sugar diet) in contrast to a nutritious diet (fruits and vegetables) on metabolism, gut microbiota composition (dysbiosis vs. eubiosis), intestinal barrier integrity, systemic inflammation, insulin resistance, dyslipidemia, adipose tissue accumulation, and immune cell responses (TLR4, TLR2, Treg, Th1, Th2, Th17), culminating in adverse obesity rather than a healthy metabolic condition.
Figure 7. The effects of malnutrition (high-fat, high-sugar diet) compared to a healthy diet (fruits and vegetables) on metabolism, gut microbiota composition (dysbiosis vs. eubiosis), intestinal barrier integrity, systemic inflammation, insulin resistance, dyslipidemia, adipose tissue accumulation, and immune cell responses (TLR4, TLR2, Treg, Th1, Th2, Th17) resulting in detrimental obesity as opposed to a healthy metabolic state.
6.1 Role of nutrition and gut microbiota in maternal and infant health
The early stage of life is crucial for the growth of the infant’s gastrointestinal microbiome. The maturation of the gastrointestinal microbiome throughout infancy and early childhood can affect health and the likelihood of disorders in later life (504). Alterations in the gut microbiota throughout this time precipitate the onset of chronic disorders such as asthma, allergies, and obesity in both adulthood and childhood (505, 506). Studies indicate that human milk plays a crucial role in establishing an infant’s gut microbiome and serves as a significant source. Consequently, the mother’s breastfeeding practices safeguard the infant versus gastrointestinal as well as respiratory pathogens, while also mitigating the dangers of inflammatory deterioration (507).
Maternal microbial components are believed to be transmitted to the newborn via human milk, along with the transfer of non-microbial molecules (508). Consequently, research elucidating the connection between nutrition and gastrointestinal microbiome in adults has sought to demonstrate a correlation with postpartum mothers (508, 509). The dietary habits of postpartum mothers, modifications to these habits, and the variety of food types ingested influence the mother’s microbiota, thus altering the human milk microbiota. This may subsequently influence the gut flora of the newborns (508).
Polyphenols, chemicals essential for plant defense, are prevalent in individual foods. Polyphenols obtained from diverse food sources exert advantageous impacts on various metabolic problems, cognitive decline, and offer protection against conditions such as tumors and aging (510). Among these, beneficial effects such as antioxidant and anti-inflammatory qualities, as well as modulation of hormonal and mitochondrial functions, polyphenols may enhance mother milk production, as well as nursing efficacy (510). The nature and composition of the mother’s food are crucial for the infant’s health, both throughout pregnancy and throughout the breastfeeding period, including the time before and after this phase (510). Consequently, a Mediterranean diet-style regimen, abundant in polyphenols and fiber, enhances the mother’s nutritional profile and is beneficial for maternal and newborn health (511). Limited research with dairy animals, like goats and cows, have shown that polyphenols from fenugreek (Trigonella foenum-graecum L.) enhance milk output and improve the quality and content of milk lipid (511, 512). Fenugreek is predominantly utilized to enhance the quality of milk production in postpartum mothers (513).
Fenugreek encompass several polyphenols, including quercetin, isovitexin, rutin, vitexin, diosgenin, and saponins (514). Additionally, other research indicates that fenugreek significantly enhances milk flow, yield, oxytocin expression, and lipid level in pregnant rats (515, 516). Scholars have shown that Moringa oleifera, which contains various flavonoids such as kaempferol, myricetin, quercetin, and phenolic acid, influences milk level and enhances the macronutrient content, involving protein and lipid, in dairy animals (517, 518). In contrast, Olvera-Aguirre et al. (519) indicated no impact on milk supply or quality on dairy animals utilizing the same herb.
In vitro studies suggest that extracts or leaves of the M. oleifera can diminish ROS, and enhance glutathione levels and casein gene expression in bovine mammary cells. M. oleifera exhibits a preventive function versus induced ROS in vitro (520, 521). Additionally, various herbal formulations, such as fenugreek, Sauropus androgynus, and M. oleifera, were evaluated for their lactation-enhancing effects on lactating rats (522, 523). The study’s findings indicated an increase in milk output. Furthermore, several animal experiments, including female rats and Balb/c mice, demonstrated the lactation hormone-stimulating effect of milk thistle and S. androgynus (524, 525). These research findings indicate that the prolactin hormone’s expression, linked to enhanced breast milk production in postpartum women, and the oxytocin hormone, recognized as the milk-ejecting hormone, were elevated (526, 527).
Sani et al. (528) investigated the impact of the polyphenol resveratrol from the Launaea taraxacifolia on milk yield and serum levels of oxytocin and prolactin in rats. The study’s results demonstrate that resveratrol may enhance milk production and elevate prolactin and serum oxytocin levels (528). One study indicated that quercetin polyphenols enhance prolactin formation in the pituitary gland, while another study showed that the same polyphenol from the Ligustrum lucidum could diminish the inflammation of the mammary gland (529, 530). Zhao et al. (531) showed that orange peel extract increases milk output among dairy animals, and Ceballos-Sánchez et al. (511) demonstrated that this fiber and polyphenol-rich diet supplemented exerted a trophic impact on both the pregnant rats and their newborns. Nevertheless, additional research is required to clarify the mechanisms (511).
6.2 Role of polyphenols from childhood to the elderly
The intake of polyphenol-rich foods is essential for people across all age groups, including children, adults, and the elderly. Integrating polyphenol-rich meals can improve growth and development in children and adolescents (532). Moreover, polyphenols may be ingested to improve cognitive action and overall health, especially in adults and the elderly (533, 534).
Moreover, incorporating polyphenols into the diet of adults and the elderly can enhance overall health, diminish the hazard of chronic disorders, and promote cardiovascular well-being (535, 536). Ziauddeen et al. (532) analyzed documents from the National Diet and Nutrition Survey Rolling Programme (NDNS RP) 2008–2014 to evaluate polyphenol consumption among the UK population. The study results suggested that polyphenol consumption escalated with age, with a more pronounced increase observed in male subjects. In children, the principal sources of polyphenols were potatoes, legumes, fruit juice, and tea. The primary sources of polyphenols for adults include chocolate, tea, fruits, wine, and vegetables (532).
Corbo et al. (537) indicated that sweet cherry polyphenol extracts suppressed spontaneous osteoclastogenesis in obese youngsters by diminishing the development of multinucleated TRAP+ osteoclasts in peripheral blood mononuclear cell cultures (537). Moreover, the polyphenol extracts diminished the capacity of peripheral blood mononuclear cells to create extensive resorption zones on calcium phosphate film-coated Millenium slides, consequently impeding the bone resorption functions of osteoclasts and reducing TNFα mRNA levels (537). Conversely, the evaluation of polyphenol extracts on cell viability in peripheral blood mononuclear cell cultures, conducted via the MTT assay, revealed that these extracts were non-toxic and promoted the preservation of healthy cells (537). The research findings indicate that sweet cherry extracts abundant in polyphenols can aid in the prevention and/or enhancement of bone health issues related to obesity (537).
A separate study by Whyte and Williams (538) noted that blueberry anthocyanins positively influenced some memory actions in youngsters, however, this impact did not encompass all cognitive domains. Moreover, participants who ingested the blueberry beverage exhibited superior performance relative to people who took a placebo, especially in long-delay recall tasks of children in 10-year-olds (538). A study including 400 children aged 4 to 12 aimed to examine the correlation between dietary polyphenol intake and the risk of attention deficit hyperactivity disorder (539). Polyphenols may offer protection against attention deficit hyperactivity disorder by altering membrane fluidity and adrenergic receptors, demonstrating antioxidant properties, inducing vasodilation, and regulating catecholamine metabolism (539).
Mengfa et al. (540) examined the correlation between polyphenol consumption and the incidence of type 2 diabetes. The results indicated that a higher consumption of polyphenols correlated with a diminished risk of type 2 diabetes. Guo et al. (541) indicated that polyphenol consumption may decrease obesity risk in elderly adults with elevated cardiovascular risk. Guglielmetti et al. (542) indicated that a diet high in polyphenols positively influenced gut permeability in ageing, leading to reduced serum zonulin levels. Decreases were noted in inflammatory markers like IL-6, C-reactive protein, and TNF-α, ROS indicators involving DNA injury, and measures of vascular action. Moreover, polyphenol-rich diets help preserve metabolomic profiles and microbiome equilibrium in the elderly (542).
6.3 Role of polyphenols on athlete health
One advantageous outcome of addressing the athlete’s nutritional requirements is enhancing athletic performance. Environmental, endocrine, muscular fiber relationships, athletic objectives, dietary, and genetic factors create individual variances that may also influence athletic performance (543). Genetic and dietary combinations can influence nutritional availability and bodily systems associated with athletic performance (544). The amount and composition of macronutrients, lipids, carbohydrates, and proteins in a person’s dietary regimen significantly influence athletes’ muscular functions and performance (544). Recent evidence indicates that the kind and amount of protein are essential for muscle hypertrophy and athletic performance, with individual differences in protein consumption and amino acid absorption-metabolism associated with both protein quantity and quality, as well as genetic variances between persons (545).
Genetic differences might change the quantity of bioactive peptides obtained from protein resources, hence influencing muscle function and development (546). Consequently, daily nutritional guidance includes tailored nutritional suggestions for each athlete throughout training and pre-, intra-, and post-competition periods (546). Alongside these macronutrients, it is advisable to daily ingest foods abundant in manganese, butyrate, omega-3, and polyphenols, and to contemplate incorporating supplements such as antioxidants and anti-inflammatories (546). Moreover, nourishment supplies energy to the body and helps maintain physiological equilibrium. Furthermore, diet is crucial in enhancing the body’s reaction to exercise-induced stress (547).
Consequently, an athlete must regulate the homeostasis of oxidative stress while training. Oxidative stress, resulting from the generation of ROS, can lead to inflammation and cellular destruction and impede muscle recovery if it coincides with training adaptations (548). Antioxidant supplementation during exercise influences athletic performance, but mitochondrial adenosine triphosphate generation is not entirely effective, forming superoxide radicals. The increased oxygen consumption leads to a higher generation of superoxide radicals that require neutralization (548). Muscle injury results in excessive free radical production, hindering recovery, and the body's intrinsic systems for eliminating these radical species are inadequate (548).
Plant-based diets are garnering interest in contemporary sports nutrition because of their substantial nutrients of bioactive compounds (549). Polyphenols offer several benefits for athletes, including anti-inflammatory, antioxidant, and antimicrobial characteristics, promoting overall wellness (550). These advantages have linked some polyphenols, such as resveratrol, quercetin, and curcumin, to muscle health (551). Numerous studies on sports nutrition and polyphenols are now underway. Many of these studies encompass the significant impacts of polyphenolic materials on post-exercise muscle destruction as well as their influence on enhancing physical performance (552). Polyphenol compounds have been investigated under various situations employing diverse supplementation regimens for differing periods and dosages (503). Polyphenols, extensively researched for their numerous beneficial effects. Consequently, diets rich in polyphenols were examined to mitigate oxidative stress induced by physical performance (553, 554).
Additionally, the impact of quercetin polyphenol supply on athletic performance was examined. Quercetin, a flavonoid polyphenol, plays a crucial function in muscle remodeling by inhibiting muscle loss by controlling protein catabolism and promoting muscular anabolism via increased phosphorylation (555). A study of top cyclists revealed enhancements in aerobic performance among athletes consuming 1200 mg of the supplement daily for six weeks (556). Sgrò et al. (557) indicated that the group administered 1 g of quercetin daily for two weeks exhibited reduced plasma markers of eccentric muscle injury relative to the placebo group. This indicates that quercetin facilitates the regeneration of muscle injury (557). A study by Martin-Rincon et al. (558), including 24 female and 33 male active athletes, attempted to assess their conditions following long-distance running performances of five and ten kilometers (558). The results indicated that the blend of Zynamite and quercetin mitigates muscle discomfort and injury while expediting the therapy of muscular performance. The advantages of quercetin supply are believed to be enhanced with elevated levels (558). Additionally, polyphenols such as resveratrol, which are predominantly found in red wine and grape skin, can stimulate anabolic muscle metabolism by augmenting signaling pathway components (545). de Sousa et al. (559) indicated that the grape juice supplement enhanced the athletes’ endurance times.
A separate study by de Lima Tavares Toscano et al. (560) observed that one dose of purple grape juice demonstrated an ergogenic impact in recreational runners via prolonging duration to exhaustion throughout running and enhancing antioxidant (560). In addition to this, animal research are also incorporated in the literature (561, 562). Nonetheless, the limited sample sizes in resveratrol studies and the application of numerous unspecified supplement dosages hinder the establishment of a definitive safety and efficacy range for this supplement in athletes; thus, further research is required (562).
Besides the advantages of resveratrol administration for athletes, it may also regulate glucose and insulin sensitivity (563). For athletes, it is crucial that the body utilizes insulin with optimal efficiency throughout a physical change. A study investigating the impact of resveratrol on glucose revealed that resveratrol can enhance glucose regulation and insulin sensitivity in diabetic rats (564).
Consequently, many findings indicate that resveratrol may serve as a potent bioactive agent for athletes experiencing hyperglycemic swings and insulin resistance. Moreover, curcumin, a principal bioactive polyphenol found in the spice turmeric, exhibits notable antioxidant and anti-inflammatory activities. Due to its antioxidant properties, it effectively mitigates oxidative stress and promotes muscle regeneration through enhanced myofibrillar proliferation, hence decreasing muscle loss in an animal model of induced muscular atrophy (545). In human studies, curcumin supply resulted in a decrease in muscle damage and inflammatory biomarkers, with an approximate dosage of 150–1500 mg/day administered pre-, post-, and during exercise, potentially enhancing athletic performance and muscle repair by mitigating exercise-induced muscle injury and modulating the inflammatory reaction (565, 566).
Nevertheless, further investigation is required regarding the potential effects of curcumin supply on the molecular pathways that regulate muscle growth caused by resistance training. Furthermore, the advantages of curcumin are associated with its interaction with the gut bacteria. In animal studies, curcumin and resveratrol have anti-carcinogenic and anti-inflammatory properties on microbiota by altering the Firmicutes/Bacteroidetes ratio (567, 568). Curcumin enhances beneficial microbiome, involving lactobacilli, bifidobacteria, and butyrate-forming bacteria, while promoting intestinal barrier integrity through immunomodulatory effects (569, 570).
In a single-blind parallel-design clinical trial, Atan et al. (571) found that hardaliye ingestion increased total serum antioxidant capacity and decreased oxidative stress index and nitric oxide levels compared to the placebo group (571). The intake of hardaliye among young soccer players shows antioxidative properties (571). A distinct investigation comprising two sub-studies investigated the effects of sugar-polyphenol-rich diluted hazy apple juice on the intestinal barrier of ultra-marathon runners (572). The study findings indicated substantial impacts on indicators of intestinal inflammation and permeability in the serum of participants who took the test drink after exercise, yielding positive outcomes compared to those who ingested the placebo drink (572). Diluted apple juice was recognized for its rehydration properties post-exercise and can also positively influence the intestinal barrier and immunity following physical activities (572). Mengfan et al. (540) also indicate that polyphenolic substances from Lonicera caerulea may alleviate swimming fatigue at ambient and low temperatures. Moreover, the buildup of metabolites, energy metabolism, and the downregulation of inflammatory factor production were enhanced (540).
Tropospheric ozone, an element of urban air contamination, is generated via photochemical processes that involve nitrogen oxides, hydrocarbons, and volatile organic substances. Ozone exposure impacts the central nervous system, leading to neurological illnesses including Alzheimer’s and Parkinson’s diseases, cognitive deficits, and neuroinflammation (573). Both human and animal research demonstrate the neurotoxic consequences of ozone in this setting. These impacts encompass the diminution of dopaminergic neurons, the buildup of pathogenic proteins, and similar phenomena (573).
The hippocampus, a specific brain area, is susceptible to ozone exposure for multiple reasons. This area contains brain-derived neurotrophic factors and other elements important in neural growth, differentiation, memory, as well as learning (573). Research on brain-derived neurotrophic factors in humans and animals has demonstrated that short episodes of exercise enhance neuronal function, brain vascularization, and neuronal synthesis by increasing levels of derived neurotrophic factors, hence fostering improved mood and enhanced cognition (573). Nevertheless, exercising in contaminated air was demonstrated to suppress acute exercise-induced brain-derived neurotrophic factor release (573). Consequently, contact with contaminated air is believed to impede cognitive health and the repair of the central nervous system. A trial on high-intensity bikers showed that polyphenol supply elevated contaminated levels in those exercising in contaminated air (573).
Figure 8 illustrates the fate of dietary polyphenols within the human digestive system, beginning with their natural occurrence in fruits and vegetables as aglycones, glycosides, or bound forms. Mechanical processing in the stomach liberates these compounds, facilitating absorption in the small intestine, while unabsorbed fractions reach the large intestine for microbial metabolism, generating bioactive metabolites that subsequently enter systemic circulation. This metabolic journey has a direct impact on athletes’ health, as polyphenols exert antioxidant, anti-inflammatory, and regulatory effects on muscle metabolism, oxidative stress, and recovery. Thus, effective digestion and biotransformation of polyphenols underpins their capacity to enhance performance, endurance, and post-exercise repair.
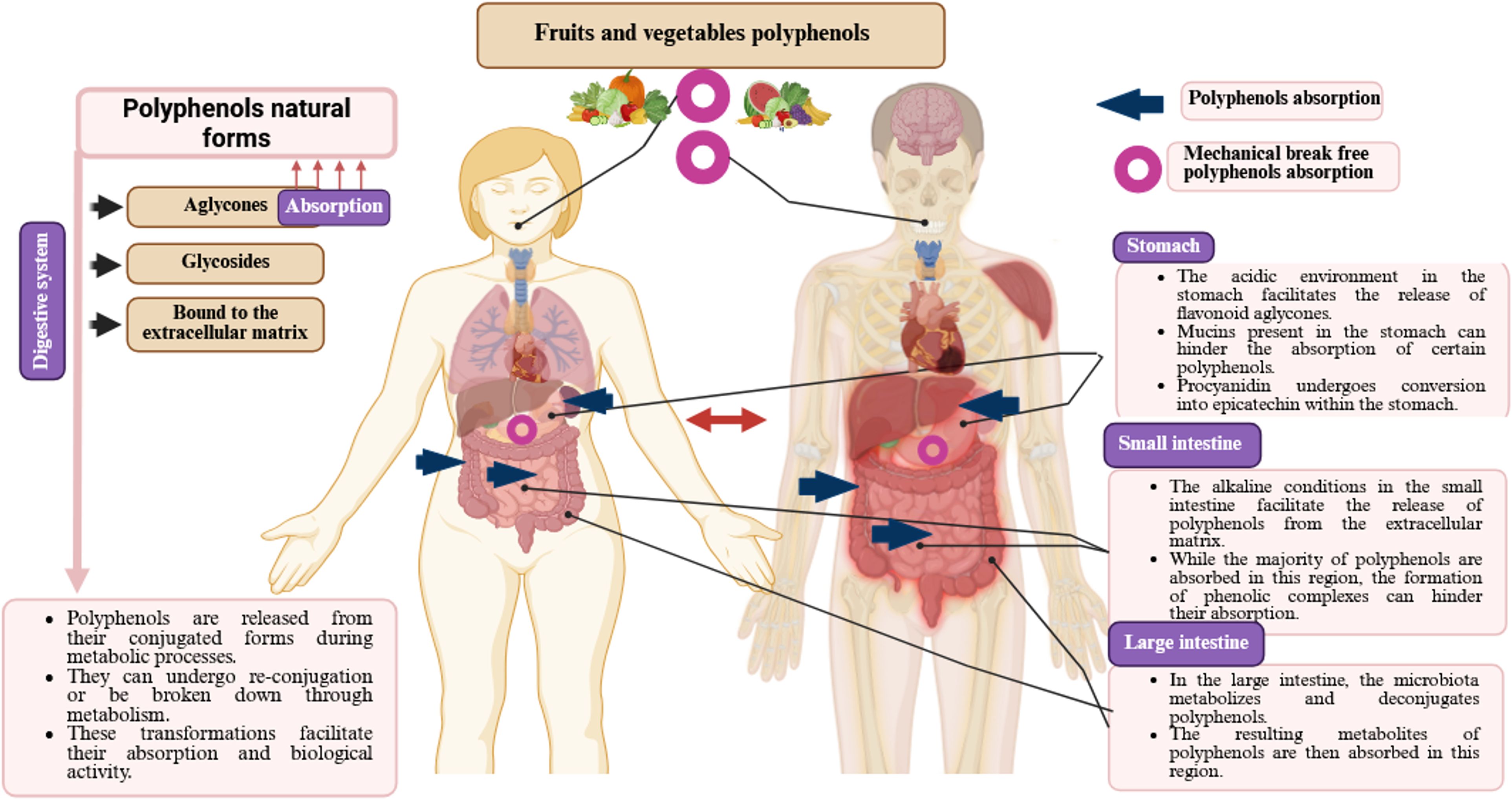
Figure 8. Polyphenol digestion and absorption in the human digestive system, depicting the natural forms of polyphenols (aglycones, glycosides, bound to extracellular matrix) found in fruits and vegetables, their mechanical breakdown and release in the stomach, absorption in the small intestine, microbial metabolism and metabolite absorption in the large intestine, and subsequent systemic circulation.
6.4 Heart diseases and polyphenols
Globally, cardiovascular diseases are the leading cause of mortality, according to data from the World Health Organization (WHO). About 20 million fatalities annually, or 31% of all deaths, were attributed to cardiovascular diseases (574). Heart attacks and strokes account for around 85% of the fatalities listed above. Approximately 75% of worldwide cardiovascular disease-related mortality occurs in low- and middle-income nations (574). In accordance with the most current heart disease and stroke statistics published by the American Heart Association, it is estimated that more than 100 million people in the USA, which is equivalent to more than 50% of all people over the age of 18, have hypertension. Numerous health advantages and cardiovascular disease have been linked to polyphenols found in numerous dietary sources, for instance, apples, coffee, tea, and cocoa (575, 576).
Epidemiological research strongly suggests consuming polyphenols because it is unmistakably associated with a lower incidence of cardiovascular diseases (577, 578). Researchers now think that polyphenolic substances work at the molecular level to improve endothelial function and lower platelet aggregation because they can stop blood clots, reduce inflammation, and stop platelets from sticking together (401). Thus, polyphenolic substances are significant in the prevention and treatment of cardiovascular disease. According to some research, those who consume more flavonoids in their diets than those who consume the least have a 47% increased risk of cardiovascular disease (579).
Research has shown that consuming flavan-3-ol from various food sources may have positive effects on cardiometabolic outcomes and reduce the risk of diabetes and cardiovascular-related outcomes (such as blood pressure, cholesterol, and myocardial infarction). Flavan-3-ols, a well-recognized polyphenol, are found in significant concentrations in a number of frequently eaten foods, including tea, almonds, cocoa (chocolate), grapes, and legumes (580–582). Red and blue fruits and vegetables, including blueberries, raspberries, strawberries, bilberries, red grapes, and cherries, are rich sources of anthocyanins, a kind of flavonoid (583). Like other polyphenols, anthocyanins that are consumed through diet are metabolized by the microbiome and the host to create active metabolites that have anti-inflammatory properties, improve vascular outcomes, reduce the risk of myocardial infarction in both men and women, and have additional positive effects on cardiovascular risk factors (583, 584).
Stilbene is mainly found in berries, red wine, and grapes. In addition to its antioxidant and anti-inflammatory properties, it also stimulates sirtuins, which slow down the aging process (407). Resveratrol supplements are said to significantly reduce fasting blood sugar, total cholesterol, C-reactive protein, and both systolic and diastolic blood pressure (585). Apple flavonol quercetin has been shown to lower systolic blood pressure, improve endothelial function, and lessen the risk of cardiovascular disease (585–587).
6.5 Polyphenols and Alzheimer’s disease
Alzheimer’s disease is a catastrophic neurodegenerative condition that affects elderly people worldwide (588). Damage to neuron structure and function is the primary cause, which ultimately results in the death of nerve cells in the human brain (588). WHO has disclosed that over 50 million people globally suffer from dementias, including Alzheimer’s disease, and that it is expected to rise to over 152 million by the year 2050. Approximately 60% of dementia patients globally originate from low- or middle-income nations (589).
Alzheimer’s disease is thought to be at risk due to both genetic and environmental factors (590). Free radicals are very reactive chemical groups that arise from both physiological and pathological processes. They have an odd number of electrons. At normal concentrations, ROS participate in many cellular and signaling pathways, including phagocytosis, enzyme activation, and cell cycle control (591). However, excessive ROS generation may lead to harmful consequences, such as damage to proteins, lipids, and DNA (591). Cell damage may result from an imbalance in the status of oxidants and antioxidants. It has been proposed that oxidative damage, a consequence of ROS, plays a role in the pathogenesis—the formation, process, and advancement of neurodegenerative diseases and disorders, cancer, diabetes, and aging (592).
Extensive scholarly research has shown that nitric oxide, hydrogen peroxide, hydroxyl radicals, and superoxide anion are essential components of oxidative stress, which ultimately results in Alzheimer’s disease (593). However, the defensive systems known as enzymatic and non-enzymatic antioxidants eliminate ROS. Polyphenolic chemicals have antioxidant properties and are primarily involved in neuroprotection. Pomegranate juice, dates, and figs are all high in polyphenols and should be added to the diet to help with behavioral problems and brain damage by keeping the balance between oxidants and antioxidants in transgenic APPs w/T g 2576 animals (594). Additionally, researchers found that extract from walnuts, whose polyphenols are the most effective among all nuts, has a remarkable ability to shield PC12 cells from oxidative stress caused by amyloid beta peptide (594).
6.6 Polyphenols’ anti-cariogenic properties
Tooth decay, or dental caries, affects 60–90% of children and most adults worldwide and is one of the most common and serious oral health issues (595). Teeth, oral flora, and nutritional factors all have an impact on dental caries disease. Dental plaque absorbs dietary carbohydrates like sucrose or sugars, which bacteria (found in dental plaque on the outside of teeth) then convert into organic acids like lactic acid (595). Demineralization, or the net loss of mineral structure on the tooth’s surface, is the result of the acid produced gradually removing calcium and phosphate from the tooth’s surface (595).
Polyphenols, which are present in tea, coffee, red grape seeds, and cocoa, have antibacterial properties that may help prevent cariogenic processes. They may slow down the growth of bacteria, protect the tooth surface, and inhibit the activity of enzymes like glucosyltransferase and amylase. Flavonoids are also effective anti-cariogenic compounds (596).
Two categories can delineate the anti-cariogenic properties of phenolic compounds: (I) plant extracts rich in polyphenols without recognized constituents; and (II) antibacterial polyphenolic agents. It has been shown that extracts derived from unfermented cocoa, green tea, and red grape seeds that include a high polyphenol concentration are effective against Streptococcus mutants and periodontal disorders. A flavonoid called quercetin-3-O-α-L-arabinose-pyranoside (guaijaverin) stops the growth of S. mutants, which is likely an anti-plaque effect (597, 598).
7 Possible negative impact of polyphenols
People use polyphenols, an essential component of plant-derived food with numerous health benefits, to prevent and cure multiple diseases (599). Tragically, like any chemical substance, polyphenols may be harmful depending on dosage, circumstances, and environmental interactions. One of the important negative consequences of polyphenols is their ability to block iron uptake in the human body (599). Despite being a trace element that is necessary for human survival, iron deficiency is a widespread ailment that affects people all over the globe (599). Polyphenols have the capacity to bind to transition metal ions such as iron and copper. This stops free radicals from being produced by the Fenton and Haber-Weiss reactions (600). In addition to the polyphenolic compound’s structure, the pH or ion form (Fe2+ and Fe3+) influences both binding strength and total ion concentration. Anemia occurs when an individual consumes a diet rich in polyphenols or takes supplements containing these compounds (600). Polyphenols bind to iron in the gastrointestinal tract, inhibiting its absorption. Additionally, they may influence the regulation of iron homeostasis (599, 600).
Flavonoids can form complexes of proteins by both nonspecific mechanisms such as hydrogen bonding and hydrophobic effects, as well as with covalent bond formation. Polyphenols form complexes with proteins, which may be either soluble or insoluble. These complexes alter the structure, isoelectric point, hydrophobicity, solubility, and susceptibility of the proteins to enzymes (601). Polyphenols may have detrimental effects on the digestive system’s function by impacting the composition of the intestinal flora and inhibiting digestive enzymes (602).
8 Conclusions and future perspective
Polyphenols, a diverse class of natural compounds, exhibit a wide range of biological activities largely determined by the number and position of their hydroxyl groups. Abundant in herbs and prevalent in traditional Asian and Mediterranean diets, these compounds have attracted significant scientific interest for their potential health benefits. Despite extensive research highlighting their neuroprotective, antioxidant, anti-inflammatory, antibacterial, dermatological, antitumor, and antidiabetic properties, the underlying mechanisms remain only partially understood.
A key limitation in translating these benefits into clinical practice lies in the low bioavailability of polyphenols. The practical improvement of daily polyphenol intake can be achieved through several strategies. Consuming a diverse range of polyphenol-rich foods, such as fruits, vegetables, whole grains, nuts, tea, coffee, and certain herbs across meals throughout the day, ensures both variety and a steady supply of these bioactive compounds. Choosing minimally processed foods and combining different sources may enhance overall intake and synergistic effects. Additionally, adopting preparation methods that preserve polyphenol content, such as steaming instead of boiling, and consuming polyphenol-rich foods with healthy fats may improve absorption.
Additionally, polyphenols that benefit most from nano-delivery systems are those with inherently poor water solubility, instability in the gastrointestinal tract, or rapid metabolism, factors that limit their absorption and therapeutic potential. Notably, curcumin, quercetin, resveratrol, tea polyphenols such as EGCG, and catechins have shown significant improvements in bioavailability, biological activity, and stability when encapsulated in nanoscale carriers. Nano-delivery approaches also enhance the performance of lignans and tannic acid, as well as complex polyphenolic extracts from sources like grape seed and propolis. By protecting these compounds from degradation and promoting controlled, targeted release, nano-delivery systems make these polyphenols more effective for use in health and disease management applications.
Many are rapidly metabolized or degraded before reaching their target tissues, reducing their therapeutic potential. To address this, advanced drug delivery systems such as liposomes and nanocarriers have been widely investigated. However, a universally effective delivery method applicable across different polyphenol classes is yet to be established, highlighting the need for further targeted research. Polyphenols continue to be an important focus in nutritional science, with growing evidence supporting their role in health maintenance across various populations, including athletes. Yet, current literature lacks consensus on optimal intake levels, and comprehensive studies encompassing all polyphenol subclasses are still limited.
Looking forward, enhancing the bioavailability and targeted delivery of polyphenols could open new avenues in drug development for metabolic disorders, dermatological applications, and the formation of functional foods aimed at improving physical performance and overall well-being. Bridging current knowledge gaps and integrating polyphenols into daily dietary practices may contribute significantly to promoting healthier lifestyles and improving performance outcomes, particularly among future generations.
Author contributions
AS: Writing – original draft, Writing – review & editing, Formal analysis. DM: Writing – original draft, Methodology, Formal analysis, Writing – review & editing. SSA: Formal analysis, Writing – review & editing, Writing – original draft, Software, Methodology. SG: Formal analysis, Writing – review & editing, Resources, Writing – original draft. SN: Resources, Writing – review & editing, Writing – original draft, Project administration, Methodology. HMS: Writing – review & editing, Methodology, Software, Writing – original draft. MF: Writing – review & editing, Writing – original draft, Software, Resources. HES: Resources, Software, Writing – review & editing, Writing – original draft. EI: Writing – review & editing, Software, Methodology, Resources, Writing – original draft. SFA: Resources, Methodology, Writing – review & editing, Writing – original draft, Funding acquisition, Supervision. KE-T: Funding acquisition, Investigation, Writing – original draft, Resources, Validation, Writing – review & editing, Supervision. ME-S: Resources, Writing – original draft, Software, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was supported by Khalifa Center for Genetic Engineering and Biotechnology-UAEU (Grant number: 12R255), and the UAEU program of Advanced Research (Grant number: 12S169). The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (R. G. P. 2/396/46).
Acknowledgments
The authors gratefully acknowledge Khalifa Center for Genetic Engineering and Biotechnology-UAEU (Grant number: 12R255), and the UAEU program of Advanced Research (Grant number: 12S169) for supporting this work. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (R. G. P. 2/396/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leri M, Scuto M, Ontario ML, Calabrese V, Calabrese EJ, Bucciantini M, et al. Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci. (2020) 21:1250. doi: 10.3390/ijms21041250
2. Benkhouili FZ, Moutawalli A, Ouchari L, El Fahime E, Benzeid H, Doukkali A, et al. Evaluation of the content of polyphenols, flavonoids and tannins, the antioxidant capacity, and the antimicrobial activity of different organic and aqueous fractions of stems of. Retama monosperma. Plant Sci Today. (2024) 11:401–11. doi: 10.14719/pst.2024.11
3. Quideau S, Deffieux D, Douat-Casassus C, and Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed. (2011) 50:586–621. doi: 10.1002/anie.201000044
4. Swallah MS, Sun H, Affoh R, Fu H, and Yu H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int J Food Sci. (2020) 2020:9081686. doi: 10.1155/2020/9081686
5. Karav S, Arikal AO, and Eksi A. Effect of cold storage of various pomegranate cultivars fruit juices on health promoting compounds and their activities. J Food Nutr Res. (2015) 3:593–8. doi: 10.12691/jfnr-3-9-6
6. Stefani M and Rigacci S. Beneficial properties of natural phenols: highlight on protection against pathological conditions associated with amyloid aggregation. BioFactors. (2014) 40:482–93. doi: 10.1002/biof.1171
7. Liu Y, Shi Y, Zhang M, Han F, Liao W, and Duan X. Natural polyphenols for drug delivery and tissue engineering construction: a review. Eur J Med Chem. (2024) 266:116141. doi: 10.1016/j.ejmech.2022.116141
8. Ganesan K and Xu B. A critical review on polyphenols and health benefits of black soybeans. Nutrients. (2017) 9:455. doi: 10.3390/nu9050455
9. Karav S, Arikal AO, and Eksi A. Apple peel is a promising source of natural bioactive compounds that promote human health. J Food Nutr Res. (2015) 3:624–8. doi: 10.12691/jfnr-3-10-1
10. Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, and Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. (2013) 18:1818–92. doi: 10.1089/ars.2012.4581
11. Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem. (2003) 51:2866–87. doi: 10.1021/jf026182t
12. Tsimogiannis DO and Oreopoulou V. Classification of phenolic compounds in plants. In: Watson RR, editor. Polyphenols in Plants. Academic Press, Cambridge, MA, USA (2019). pp.263–84. doi: 10.1016/B978-0-12-813768-0.00026-8
13. Galanakis CM. Food Bioactives and Health. Springer, Cham, Switzerland (2021). 378 p. doi: 10.1007/978-3-030-57469-7
14. Verma C. Science and Engineering of Polyphenols: Fundamentals and Industrial Scale Applications. Hoboken, NJ, USA: John Wiley and Sons (2024). 624 p.
15. Abotaleb M, Liskova A, Kubatka P, and Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. (2020) 10:221. doi: 10.3390/biom10020221
16. Sun XM, Ye HQ, Liu JB, Wu L, Lin DB, Yu YL, et al. Assessment of anti-diabetic activity of peanut shell polyphenol extracts. J Zhejiang Univ Sci B. (2018) 19:764. doi: 10.1631/jzus.B1700401
17. Anantharaju PG, Gowda PC, Vimalambike MG, and Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. (2016) 15:99. doi: 10.1186/s12937-016-0217-2
18. Di Ferdinando M, Brunetti C, Fini A, and Tattini M. Flavonoids as antioxidants in plants under abiotic stresses. In: Ahmad P and Prasad M, editors. Abiotic Stress Responses in Plants. Springer, NY, USA (2012). pp.159–79. doi: 10.1007/978-1-4614-0634-1_9
19. Dias MC, Pinto DC, and Silva AM. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:5377. doi: 10.3390/molecules26175377
20. Bondonno CP, Bondonno NP, Dalgaard F, Murray K, Gardener SL, Martins RN, et al. Flavonoid intake and incident dementia in the Danish diet, cancer, and health cohort. Alzheimer's Dement (TRCI). (2021) 7:e12175. doi: 10.1002/trc2.12175
21. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, and Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. (2016) 2016:7432797. doi: 10.1155/2016/7432797
22. Chong J, Poutaraud A, and Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. (2009) 177:143–55. doi: 10.1016/j.plantsci.2009.05.012
23. Wahab A, Gao K, Jia C, Zhang F, Tian G, Murtaza G, et al. Significance of resveratrol in clinical management of chronic diseases. Molecules. (2017) 22:1329. doi: 10.3390/molecules22081329
24. El Tannir H, Houhou D, Debs E, Koubaa M, Jammoul A, Azakir B, et al. Optimization of aqueous extraction of polyphenols from Cuminum cyminum seeds using response surface methodology and assessment of biological activity. BioTech. (2024) 13:7. doi: 10.3390/biotech13010007
25. Raposo R, Chinnici F, Ruiz-Moreno MJ, Puertas B, Cuevas FJ, Carbú M, et al. Sulfur free red wines through the use of grapevine shoots: Impact on the wine quality. Food Chem. (2018) 243:453–60. doi: 10.1016/j.foodchem.2017.09.111
26. Guerrero RF, Valls-Fonayet J, Richard T, and Cantos-Villar E. A rapid quantification of stilbene content in wine by ultra-high pressure liquid chromatography–mass spectrometry. Food Control. (2020) 108:106821. doi: 10.1016/j.foodcont.2019.106821
27. Li W, Chen H, Xu B, Wang Y, Zhang C, Cao Y, et al. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J Future Foods. (2023) 3:289–305. doi: 10.1016/j.jfutfo.2023.03.001
28. Chen J and Thompson LU. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat. (2003) 80:163–70. doi: 10.1023/A:1024513815374
29. Bayar İ, Yavuz SÇ, and Akkoç S. In vitro and in silico studies on lignan secoisolariciresinol diglucoside. J Fac Pharm Ankara Univ. (2023) 48:127–37. doi: 10.33483/jfpau.1368474
30. Jang WY, Kim MY, and Cho JY. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int J Mol Sci. (2022) 23:15482. doi: 10.3390/ijms232415482
31. Chen DF, Zhang SX, Chen KE, Zhou BN, Wang P, Cosentino LM, et al. Two new lignans, interiotherins A and B, as anti-HIV principles from Kadsura interior. J Nat Prod. (1996) 59:1066–8. doi: 10.1021/np9601667
32. Hernández-Ruiz RG, Olivares-Ochoa XC, Salinas-Varela Y, Guajardo-Espinoza D, Roldán-Flores LG, Rivera-Leon EA, et al. Phenolic compounds and anthocyanins in legumes and their impact on inflammation, oxidative stress, and metabolism: Comprehensive review. Molecules. (2025) 30:174. doi: 10.3390/molecules30010174
33. Nigar S, Shimul IM, Hossain MS, Sultana R, Asha S, and Huq AO. Comparative analysis on phytonutrient properties of different fig varieties (Ficus spp.). Food Chem Adv. (2025) 6:100878. doi: 10.1016/j.focha.2024.100878
34. Pham TN, Lam TD, Nguyen MT, Le XT, Vo DVN, Toan TQ, et al. (2019). Effect of various factors on extraction efficiency of total anthocyanins from Butterfly pea (Clitoria ternatea L. flowers) in Southern Vietnam. IOP Conference Series: Materials Science and Engineering, Volume 544, 2nd International Conference on Mechanical Engineering and Applied Composite Materials 7–8 December 2018 pp. 544, Harbin, China. doi: 10.1088/1757-899X/544/1/012013
35. Chiriac ER, Chiţescu CL, Geană EI, Gird CE, Socoteanu RP, and Boscencu R. Advanced analytical approaches for the analysis of polyphenols in plant matrices—A review. Separations. (2021) 8:65. doi: 10.3390/separations8050065
36. Soufi O, Medouni-Haroune L, Bachirbey M, Medouni-Adrar S, Idir F, Heddad T, et al. Statistical optimization of ultrasound-assisted extraction of polyphenols from olive pomace. Sustain Chem Pharm. (2023) 36:101260. doi: 10.1016/j.scp.2023.101260
37. Zhang S, Xie H, Huang J, Chen Q, Li X, Chen X, et al. Ultrasound-assisted extraction of polyphenols from pine needles (Pinus elliottii): Comprehensive insights from RSM optimization, antioxidant activity, UHPLC-Q-exactive orbitrap MS/MS analysis and kinetic model. Ultrason Sonochem. (2024) 102:106742. doi: 10.1016/j.ultsonch.2023.106742
38. Vilchis-Gómez DS, Calderón-Santoyo M, Barros-Castillo JC, Zamora-Gasga VM, and Ragazzo-Sánchez JA. Ultrasound-assisted extraction of polyphenols from Randia monantha: Optimization, characterization, and antifungal activity. Ind Crops Prod. (2024) 209:117932. doi: 10.1016/j.indcrop.2023.117932
39. Riaz T, Hayat Z, Saleem K, Akram K, Rehman HU, Rehman SU, et al. Optimization of an ultrasound-assisted extraction method to obtain gallic acid-rich extracts from mango seed kernels. Food Sci Nutr. (2024) 12:4038–48. doi: 10.1002/fsn3.4060
40. Bouaoudia-Madi N, Boulekbache-Makhlouf L, Madani K, Silva AMS, Dairi S, Oukhmanou-Bensidhoum S, et al. Optimization of ultrasound-assisted extraction of polyphenols from Myrtus communis L. pericarp. Antioxidants. (2019) 8:205. doi: 10.3390/antiox8070205
41. Carreira-Casais A, Otero P, Garcia-Perez P, Garcia-Oliveira P, Pereira AG, Carpena M, et al. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int J Environ Res Public Health. (2021) 18:9153. doi: 10.3390/ijerph18179153
42. Tomasi IT, Santos SC, Boaventura RA, and Botelho CM. Optimization of microwave-assisted extraction of phenolic compounds from chestnut processing waste using response surface methodology. J Clean Prod. (2023) 395:136452. doi: 10.1016/j.jclepro.2023.136452
43. Sai-Ut S, Kingwascharapong P, Mazumder MAR, and Rawdkuen S. Optimization of polyphenolic compounds from Gossampinus malabarica flowers by microwave-assisted extraction technology. Future Foods. (2023) 8:100271. doi: 10.1016/j.fufo.2023.100271
44. Murugesan S, Maran P, Venkatesan M, and Alexander RA. Microwave assisted extraction of polyphenols from Pithecellobium dulce Benth fruit peels and evaluation of its anti-cancer and antioxidant activity. Waste Biomass Valorization. (2024) 15:841–55. doi: 10.1007/s12649-023-02183-8
45. García-Martín JF, Feng CH, Domínguez-Fernández NM, and Álvarez-Mateos P. Microwave-assisted extraction of polyphenols from bitter orange industrial waste and identification of the main compounds. Life. (2023) 13:1864. doi: 10.3390/life13091864
46. Marđokić A, Maldonado AE, Klosz K, Molnár MA, Vatai G, and Bánvölgyi S. Optimization of conditions for microwave-assisted extraction of polyphenols from olive pomace of Žutica variety: waste valorization approach. Antioxidants. (2023) 12:1175. doi: 10.3390/antiox12061175
47. Duke K, Syeunda C, Brantsen JF, Nindawat S, and Awika JM. Polyphenol recovery from sorghum bran waste by microwave assisted extraction: Structural transformations as affected by grain phenolic profile. Food Chem. (2024) 444:138645. doi: 10.1016/j.foodchem.2024.138645
48. Mobasheri F, Khajeh M, Ghaffari-Moghaddam M, Piri J, and Bohlooli M. Machine learning optimization of microwave-assisted extraction of phenolics and tannins from pomegranate peel. Sci Rep. (2025) 15:19439. doi: 10.1038/s41598-025-04798-4
49. Rostagno MA, D’Arrigo M, and Martínez JA. Combinatory and hyphenated sample preparation for the determination of bioactive compounds in foods. TrAC - Trends Anal Chem. (2010) 29:553–61. doi: 10.1016/j.trac.2010.02.015
50. Bouchez A, Vauchel P, Périno S, and Dimitrov K. Multi-criteria optimization including environmental impacts of a microwave-assisted extraction of polyphenols and comparison with an ultrasound-assisted extraction process. Foods. (2023) 12:1750. doi: 10.3390/foods12091750
51. Ma NB, Ton NMN, and Le NL. Co-optimization of polysaccharides and polyphenols extraction from mangosteen peels using ultrasound-microwave assisted extraction (UMAE) and enzyme-ultrasound assisted extraction (EUAE) and their characterization. J Food Meas Charact. (2024) 18:6379–93. doi: 10.1007/s11694-024-02656-x
52. Biswas R, Sarkar A, Alam M, Roy M, and Mahdi Hasan MM. Microwave and ultrasound-assisted extraction of bioactive compounds from Papaya: A sustainable green process. Ultrason Sonochem. (2023) 101:106677. doi: 10.1016/j.ultsonch.2023.106677
53. Ahangari H, King JW, Ehsani A, and Yousefi M. Supercritical fluid extraction of seed oils–A short review of current trends. Trends Food Sci Technol. (2021) 111:249–60. doi: 10.1016/j.tifs.2021.02.066
54. Amaresh YS, Kavyasri M, Ashwathanarayana DS, Raghavendra BT, and Hiregoudar S. Chemical characterization of supercritical fluid extract (SFE) of Ailanthus excelsa and estimation of total phenols and flavonoids in SFE. Int J Plant Sci. (2024) 36:81–8. doi: 10.9734/ijpss/2024/v36i44456
55. Amador-Luna VM, Herrero M, Domínguez-Rodríguez G, Ibáñez E, and Montero L. Enhancing the bioactivity of Dunaliella salina extracts through ultra-high pressure supercritical fluid extraction (UHP-SFE). Innov Food Sci Emerg Technol. (2024) 95:103697. doi: 10.1016/j.ifset.2024.103697
56. Khoddami A, Wilkes MA, and Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. (2013) 18:2328–75. doi: 10.3390/molecules18022328
57. Ignat I, Volf I, and Popa VI. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. (2011) 126:1821–35. doi: 10.1016/j.foodchem.2010.12.026
58. Pinto D, López-Yerena A, Lamuela-Raventós R, Vallverdú-Queralt A, Delerue-Matos C, and Rodrigues F. Predicting the effects of in vitro digestion in the bioactivity and bioaccessibility of antioxidant compounds extracted from chestnut shells by supercritical fluid extraction – a metabolomic approach. Food Chem. (2024) 435:137581. doi: 10.1016/j.foodchem.2023.137581
59. Herzyk F, Piłakowska-Pietras D, and Korzeniowska M. Supercritical extraction techniques for obtaining biologically active substances from a variety of plant byproducts. Foods. (2024) 13:1713. doi: 10.3390/foods13111713
60. Tomas M, Wen Y, Liao W, Zhang L, Zhao C, McClements DJ, et al. Recent progress in promoting the bioavailability of polyphenols in plant-based foods. Crit Rev Food Sci Nutr. (2025) 65:2343–64. doi: 10.1080/10408398.2024.2336051
61. Kronholm J, Hartonen K, and Riekkola ML. Analytical extractions with water at elevated temperatures and pressures. TrAC-Trends Anal Chem. (2007) 26:396–412. doi: 10.1016/j.trac.2007.03.004
62. Žagar T, Frlan R, and Kočevar Glavač N. Using subcritical water to obtain polyphenol-rich extracts with antimicrobial properties. Antibiotics. (2024) 13:334. doi: 10.3390/antibiotics13040334
63. Herrero M, Cifuentes A, and Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. (2006) 98:136–48. doi: 10.1016/j.foodchem.2005.05.058
64. Anoraga SB, Shamsudin R, Hamzah MH, Sharif S, Saputro AD, and Basri MSM. Optimization of subcritical water extraction for pectin extraction from cocoa pod husks using the response surface methodology. Food Chem. (2024) 459:140355. doi: 10.1016/j.foodchem.2024.140355
65. Vardakas A, Vassilev K, Nenov N, Passon M, Shikov V, Schieber A, et al. Combining enzymatic and subcritical water treatments for green extraction of polyphenolic co-pigments from saffron tepals. Waste Biomass Valor. (2024) 15:207–17. doi: 10.1007/s12649-023-02189-2
66. Somat HA, Thani NM, Mustapha WA, Lim SJ, Seng NS, Rahman HA, et al. Subcritical water extraction of bioactive compounds from plant materials: recent advances. J Fut Foods. (2025) In press. doi: 10.1016/j.jfutfo.2024.10.005
67. Hegde S, Sivamani Y, Muthuraman A, and Elayaperumal S. Pulsed electric field extraction. In: Sarkar T and Pati S, editors. Bioactive Extraction and Application in Food and Nutraceutical IndustriesMethods and Protocols in Food Sci. Springer, NY, USA (2024). pp.223–53. doi: 10.1007/978-1-0716-3601-5_10
68. Poojary MM, Lund MN, and Barba FJ. Pulsed electric field (PEF) as an efficient technology for food additives and nutraceuticals development. In: Barba FJ, Parniakov O, and Wiktor A, editors. Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow. Academic Press, Cambridge, Massachusetts, USA (2020). pp.65–99. doi: 10.1016/B978-0-12-816402-0.00004-5
69. Teh SS, Niven BE, Bekhit AEDA, Carne A, and Birch EJ. Microwave and pulsed electric field assisted extractions of polyphenols from defatted canola seed cake. Int J Food Sci Technol. (2015) 50:1109–15. doi: 10.1111/ijfs.12749
70. Jara-Quijada E, Pérez-Won M, Tabilo-Munizaga G, Lemus-Mondaca R, González-Cavieres L, Palma-Acevedo A, et al. Liposomes loaded with green tea polyphenols—optimization, characterization, and release kinetics under conventional heating and pulsed electric fields. Food Bioprocess Technol. (2024) 17:396–408. doi: 10.1007/s11947-023-03136-8
71. Ye L, Luo W, Nie Y, Chen M, Wu Q, Yan P, et al. Phyllanthus emblica polyphenols: Optimization of high-voltage pulsed electric field assisted extraction, an antioxidant and anti-inflammatory effects in vitro. J Dermatol Sci Cosmet Technol. (2024) 1:100038. doi: 10.1016/j.jdsct.2024.100038
72. Ranjha MMAN, Kanwal R, Shafique B, Arshad RN, Irfan S, Kieliszek M, et al. A critical review on pulsed electric field: a novel technology for the extraction of phytoconstituents. Molecules. (2021) 26:4893. doi: 10.3390/molecules26164893
73. Carpentieri S and Ferrari G and Pataro G. Optimization of pulsed electric fields-assisted extraction of phenolic compounds from white grape pomace using response surface methodology. Front Sustain Food Syst. (2022) 6:854968. doi: 10.3389/fsufs.2022.854968
74. De Aguiar Saldanha Pinheiro AC, Martí-Quijal FJ, Barba FJ, Benítez-González AM, Meléndez-Martínez AJ, Castagnini JM, et al. Pulsed electric fields (PEF) and accelerated solvent extraction (ASE) for valorization of red (Aristeus antennatus) and camarote (Melicertus kerathurus) shrimp side streams: antioxidant and HPLC evaluation of the carotenoid astaxanthin recovery. Antioxidants. (2023) 12:406. doi: 10.3390/antiox12020406
75. Dhowlaghar N, Dhanani T, Pillai SS, and Patil BS. Accelerated solvent extraction of red onion peel extract and its antimicrobial, antibiofilm, and quorum-sensing inhibition activities against Listeria monocytogenes and Chromobacterium violaceum. Food Biosci. (2023) 53:102649. doi: 10.1016/j.fbio.2023.102649
76. Wójciak M, Mazurek B, Wójciak W, Kostrzewa D, Żuk M, Chmiel M, et al. Optimizing the extraction of the polyphenolic fraction from defatted strawberry seeds for tiliroside isolation using accelerated solvent extraction combined with a Box–Behnken design. Molecules. (2024) 29:3051. doi: 10.3390/molecules29133051
77. Sanches Contieri L, Mendes de Souza Mesquita L, Ferreira VC, Moreno JA, Pizani RS, Forster Carneiro T, et al. Sustainable and innovative method for real-time extraction and analysis of polyphenols from green propolis by pressurized liquid extraction coupled inline with photodiode array detection (PLE-PDA). ACS Sustain Chem Eng. (2024) 12:18735–47. doi: 10.1021/acssuschemeng.4c08602
78. Naczk M and Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm BioMed Anal. (2006) 41:1523–42. doi: 10.1016/j.jpba.2006.04.002
79. Hordiei K, Gontova T, Trumbeckaite S, Yaremenko M, and Raudone L. Phenolic composition and antioxidant activity of Tanacetum parthenium cultivated in different regions of Ukraine: insights into the flavonoids and hydroxycinnamic acids profile. Plants. (2023) 12:2940. doi: 10.3390/plants12162940
80. Zulfisa Z, Fika R, Agusfina M, Yonrizon Y, and Muhsanah A. Determination of total phenolic content of ethanol extract of broken bone twigs (Euphorbia tirucalli Linn.) by Folin-Ciocalteu method spectrophotometrically. J Eduhealth. (2023) 14:1326–31. doi: 10.54209/jurnaleduhealth.v14i3.2603
81. Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. (2007) 30:3268–95. doi: 10.1002/jssc.200700261
82. Fernandes AJD, Ferreira MRA, Randau KP, de Souza TP, and Soares LAL. Total flavonoids content in the raw material and aqueous extractives from Bauhinia monandra Kurz (Caesalpiniaceae). Sci World J. (2012) 2012:923462. doi: 10.1100/2012/923462
83. Welch CR, Wu Q, and Simon JE. Recent advances in anthocyanin analysis and characterization. Curr Anal Chem. (2008) 4:75–101. doi: 10.2174/157341108784587795
84. Tavassoli M, Khezerlou A, Firoozy S, Ehsani A, and Punia Bangar S. Chitosan-based film incorporated with anthocyanins of red poppy (Papaver rhoeas L.) as a colorimetric sensor for the detection of shrimp freshness. Int J Food Sci Technol. (2023) 58:3050–7. doi: 10.1111/ijfs.16432
85. Česlová L, Kalendová P, Dubnová L, Pernica M, and Fischer J. The effect of sample pretreatment on the anthocyanin content in Czech wild elderberry (Sambucus nigra L.). Molecules. (2023) 28:6690. doi: 10.3390/molecules28186690
86. Gao Z, Yang G, Zhao X, Jiao L, Wen X, Liu Y, et al. Rapid measurement of anthocyanin content in grape and grape juice: Raman spectroscopy provides non-destructive, rapid methods. Comput Electron Agric. (2024) 222:109048. doi: 10.1016/j.compag.2024.109048
87. Proestos C, Boziaris IS, Nychas GJ, and Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. (2006) 95:664–71. doi: 10.1016/j.foodchem.2005.01.049
88. Bié J, Sepodes B, Fernandes PC, and Ribeiro MH. Polyphenols in health and disease: gut microbiota, bioaccessibility, and bioavailability. Compounds. (2023) 3:40–72. doi: 10.3390/compounds3010005
89. Arruda TR, Junior BRDC, Marques CS, Bernardes PC, Magalhães CG, and Pinheiro PF. Emerging techniques for extraction and characterization of natural compounds. In: Prakash B and de São José JFB, editors. Green Products in Food Safety. Elsevier, Amsterdam, The Netherlands (2023). pp.29–79. doi: 10.1016/B978-0-323-95590-4.00009-6
90. Palma A, Ruiz-Montoya M, Díaz MJ, Giráldez I, and Morales E. Optimization of bioactive compounds by ultrasound extraction and gas chromatography-mass spectrometry in fast-growing leaves. Microchem J. (2023) 193:109231. doi: 10.1016/j.microc.2023.109231
91. Sabir AM, Moloy M, and Bhasin PS. HPLC method development and validation: A review. Int Res J Pharm. (2013) 4:39–46. doi: 10.7897/2230-8407.04407
92. Roggero JP, Archier P, and Coen S. Chromatography of phenolics in wine. ACS Symposium Series. (1997) 661:6–11. doi: 10.1021/bk-1997-0661.ch002
93. Al-Askar AA, Bashir S, Mohamed AE, Sharaf OA, Nabil R, Su Y, et al. Antimicrobial efficacy and HPLC analysis of polyphenolic compounds in a whole-plant extract of. Eryngium campestre. Separations. (2023) 10:362. doi: 10.3390/separations10060362
94. Pereira-Coelho M, da Silva Haas IC, Vitali L, and dos Santos Madureira LA. Dispersive pipette extraction and HPLC-DAD for the determination of polyphenols in grape juice. Food Anal Methods. (2024) 17:269–83. doi: 10.1007/s12161-023-02565-7
95. Ashraf GJ, Das P, Sahu R, Nandi G, Paul P, and Dua TK. Impact of ultrasound-assisted extraction of polyphenols and caffeine from green tea leaves using high-performance thin-layer chromatography. BioMed Chromatogr. (2023) 37:e5698. doi: 10.1002/bmc.5698
96. Jović MD, Agatonovic-Kustrin SM, Ristivojević PM, Trifković JĐ, and Morton DW. Bioassay-guided assessment of antioxidative, anti-inflammatory and antimicrobial activities of extracts from medicinal plants via high-performance thin-layer chromatography. Molecules. (2023) 28:7346. doi: 10.3390/molecules28217346
97. Ashmore PL, Valdez F, Harbertson JF, Boulton RB, and Collins TS. Rapid determination of free sulfur dioxide in wine and cider by capillary electrophoresis. J Chromatogr A. (2023) 1695:463936. doi: 10.1016/j.chroma.2023.463936
98. Li Z, Wu Q, Zhang X, and Chen G. Advances in the applications of capillary electrophoresis to tobacco analysis. Curr Anal Chem. (2023) 19:77–99. doi: 10.2174/1573411018666220927094137
99. Barbosa MF, Pivatto M, Cardoso AA, and da Silveira Petruci JF. Analysis of cassine and spectaline in the Senna spectabilis ethanolic extracts by capillary zone electrophoresis with indirect UV detection. Phytochem Anal. (2024) 35:1688–94. doi: 10.1002/pca.3411
100. Bamba T. Application of supercritical fluid chromatography to the analysis of hydrophobic metabolites. J Sep Sci. (2008) 31:1274–8. doi: 10.1002/jssc.200700499
101. West C, Lemasson E, Bertin S, Hennig P, and Lesellier E. An improved classification of stationary phases for ultra-high performance supercritical fluid chromatography. J Chromatogr A. (2016) 1440:212–28. doi: 10.1016/j.chroma.2016.02.052
102. Ul'yanovskii NV, OnuChina AA, Ovchinnikov DV, Faleva AV, Gorbova NS, and Kosyakov DS. Analytical and preparative separation of softwood lignans by supercritical fluid chromatography. Separations. (2023) 10:449. doi: 10.3390/separations10080449
103. Ares AM, Toribio L, García-Villalba R, Villalgordo JM, Althobaiti Y, Tomás-Barberán FA, et al. Separation of isomeric forms of urolithin glucuronides using supercritical fluid chromatography. J Agric Food Chem. (2023) 71:3033–9. doi: 10.1021/acs.jafc.2c07145
104. Scalbert A and Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. (2000) 130:2073S–85S. doi: 10.1093/jn/130.8.2073S
105. Sánchez-Velázquez OA, Mulero M, Cuevas-Rodríguez EO, Mondor M, Arcand Y, and Hernández-Álvarez AJ. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. (2021) 12:7358–78. doi: 10.1039/D1FO00986A
106. Cassidy A and Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. (2017) 105:10–22. doi: 10.3945/ajcn.116.136051
107. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, and Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. (2013) 24:1415–22. doi: 10.1016/j.jnutbio.2013.05.001
108. Ozkan G, Ceyhan T, Çatalkaya G, Rajan L, Ullah H, Daglia M, et al. Encapsulated phenolic compounds: Clinical efficacy of a novel delivery method. Phytochem Rev. (2024) 23:781–819. doi: 10.1007/s11101-023-09909-5
109. Frazzini S, Torresani MC, Roda G, Dell’Anno M, Ruffo G, and Rossi L. Chemical and functional characterization of the main bioactive molecules contained in hulled Cannabis sativa L. seeds for use as functional ingredients. J Agric Food Res. (2024) 16:101084. doi: 10.1016/j.jafr.2024.101084
110. Xiao JB and Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr Med Chem. (2015) 22:23–38. doi: 10.2174/0929867321666140706130807
111. Aatif M. Current understanding of polyphenols to enhance bioavailability for better therapies. Biomedicines. (2023) 11:2078. doi: 10.3390/biomedicines11072078
112. Rudrapal M, Rakshit G, Singh RP, Garse S, Khan J, and Chakraborty S. Dietary polyphenols: review on chemistry/sources, bioavailability/metabolism, antioxidant effects, and their role in disease management. Antioxidants. (2024) 13:429. doi: 10.3390/antiox13040429
113. Kashi DS, Shabir A, Da Boit M, Bailey SJ, and Higgins MF. The efficacy of administering fruit-derived polyphenols to improve health biomarkers, exercise performance and related physiological responses. Nutrients. (2019) 11:2389. doi: 10.3390/nu11102389
114. D’Angelo S. Polyphenols: Potential beneficial effects of these phytochemicals in athletes. Curr Sports Med Rep. (2020) 19:260–5. doi: 10.1249/JSR.0000000000000729
115. Kou X, Han L, Li X, Xue Z, and Zhou F. Antioxidant and ‘anti-tumor’ effects and immunomodulatory activities of crude and purified polyphenol extract from blueberries. Front Chem Sci Eng. (2016) 10:108–19. doi: 10.1007/s11705-016-1553-7
116. van de Langerijt TM, O'Callaghan YC, Tzima K, Lucey A, O'Brien NM, O'Mahony JA, et al. The influence of milk with different compositions on the bioavailability of blackberry polyphenols in model sports nutrition beverages. Int J Dairy Technol. (2023) 76:828–43. doi: 10.1111/1471-0307.12987
117. Castaldo L, Narváez A, Izzo L, Graziani G, and Ritieni A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules. (2020) 25:2132. doi: 10.3390/molecules25092132
118. Franková H, Musilová J, Árvay J, Šnirc M, Jančo I, Lidiková J, et al. Changes in antioxidant properties and phenolics in sweet potatoes (Ipomoea batatas L.) due to heat treatments. Molecules. (2022) 27:1884. doi: 10.3390/molecules27061884
119. Scott MB, Styring AK, and McCullagh JSO. Polyphenols: bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens. (2022) 11:770. doi: 10.3390/pathogens11070770
120. Marino M, Del Bo' C, Martini D, Porrini M, and Riso P. A review of registered clinical trials on dietary (poly)phenols: past efforts and possible future directions. Foods. (2020) 9:1606. doi: 10.3390/foods9111606
121. Duda-Chodak A and Tarko T. Possible side effects of polyphenols and their interactions with medicines. Molecules. (2023) 28:2536. doi: 10.3390/molecules28062536
122. Nagasako-Akazome Y. Safety of high and long-term intake of polyphenols. In: Watson RR, Preedy VR, and Zibadi S, editors. Polyphenols In Human Health and Disease. Academic Press, Cambridge, Massachusetts, USA (2014). pp.747–56. doi: 10.1016/B978-0-12-398456-2.00058-X
123. Ofosu FK, Daliri EB-M, Elahi F, Chelliah R, Lee B-H, and Oh D-H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front Sustain Food Syst. (2020) 4:525810. doi: 10.3389/fsufs.2020.525810
124. Hossain MS, Wazed MA, Asha S, Hossen MA, Fime SNM, Teeya ST, et al. Flavor and well-being: a comprehensive review of food choices, nutrition, and health interactions. Food Sci Nutr. (2025) 13:e70276. doi: 10.1002/fsn3.70276
125. Hossain MS, Wazed MA, Asha S, Amin MR, and Shimul IM. Dietary phytochemicals in health and disease: mechanisms, clinical evidence, and applications-a comprehensive review. Food Sci Nutr. (2025) 13:e70101. doi: 10.1002/fsn3.70101
126. Ed Nignpense B, Francis N, Blanchard C, and Santhakumar A. The bioavailability of polyphenols following acute consumption of pigmented barley and wheat. Food Funct. (2024) 15:9330–42. doi: 10.1039/d4fo01162g
127. Quesada-Vázquez S, Eseberri I, Les F, Pérez-Matute P, Herranz-López M, Atgié C, et al. Polyphenols and metabolism: from present knowledge to future challenges. J Physiol Biochem. (2024) 80:603–25. doi: 10.1007/s13105-024-01046-7
128. Actis-Goretta L, Leveques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. Intestinal absorption, metabolism, and excretion of (–)-epicatechin in healthy humans assessed by using an intestinal perfusion technique. Am J Clin Nutr. (2013) 98:924–33. doi: 10.3945/ajcn.113.065789
129. Pouton CW and Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Delivery Rev. (2008) 60:625–37. doi: 10.1016/j.addr.2007.10.010
130. Suganya V and Anuradha V. Microencapsulation and nanoencapsulation: A review. Int J Pharm Clin Res. (2017) 9:233–9. doi: 10.25258/ijpcr.v9i3.8324
131. Subramani T and Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J Food Sci Technol. (2020) 57:3545–55. doi: 10.1007/s13197-020-04360-2
132. Ali A, Oon CC, Chua BL, Figiel A, Chong CH, Wojdylo A, et al. Volatile and polyphenol composition, anti-oxidant, anti-diabetic and anti-aging properties, and drying kinetics as affected by convective and hybrid vacuum microwave drying of Rosmarinus officinalis L. Ind Crops Prod. (2020) 151:112463. doi: 10.1016/j.indcrop.2020.112463
133. Tao K, Guo L, Hu X, Fitzgerald C, Rouzard K, Healy J, et al. Encapsulated activated grape seed extract: a novel formulation with anti-aging, skin-brightening, and hydration properties. Cosmetics. (2021) 9:4. doi: 10.3390/cosmetics9010004
134. Altan A, Yuce HB, Karataş Ő, Taşkan MM, Gevrek F, Çolak S, et al. Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats. Asian Pac J Trop Biomed. (2020) 10:156–63. doi: 10.4103/2221-1691.280297
135. Nateghi N, Karimi E, and Oskoueian E. Nanoliposome-encapsulated and non-encapsulated phenolics from Achillea millefolium and their biological function in mice challenged by Campylobacter jejuni: a comparative study. Front Mol Biosci. (2022) 8:832022. doi: 10.3389/fmolb.2021.832022
136. Hassirian N, Karimi E, and Oskoueian E. Nanoliposome-encapsulated phenolic-rich fraction from Alcea rosea as a dietary phytobiotic in mice challenged by Escherichia coli. Ann Microbiol. (2022) 72:6. doi: 10.1186/s13213-022-01665-9
137. Shamansoori MT, Karimi E, and Oskoueian E. Rheum ribes extract-loaded nanoliposome as a novel phytogenic antibiotic alternative in mice challenged by Escherichia coli (O157:H7). Biotechnol Appl Biochem. (2022) 69:2540–9. doi: 10.1002/bab.2303
138. Mehdizadeh A, Karimi E, and Oskoueian E. Nano-liposomal encapsulation of Artemisia aucheri phenolics as a potential phytobiotic against Campylobacter jejuni infection in mice. Food Sci Nutr. (2022) 10:3314–22. doi: 10.1002/fsn3.2921
139. Purushothaman JR, Rizwanullah M, and Purushothaman R. Ferulic Acid: A comprehensive review. Cureus. (2024) 16:e68063. doi: 10.7759/cureus.68063
140. Neog MK and Rasool M. Targeted delivery of p-coumaric acid encapsulated mannosylated liposomes to the synovial macrophages inhibits osteoclast formation and bone resorption in the rheumatoid arthritis animal model. Eur J Pharm Biopharm. (2018) 133:162–75. doi: 10.1016/j.ejpb.2018.10.010
141. Najafi A, Taheri RA, Mehdipour M, Martínez-Pastor F, Rouhollahi AA, and Nourani MR. Improvement of post-thawed sperm quality in broiler breeder roosters by ellagic acid-loaded liposomes. Poult Sci. (2019) 98:440–6. doi: 10.3382/ps/pey353
142. Chen X, Yi Z, Chen G, Ma X, Su W, Deng Z, et al. Carrier-enhanced photodynamic cancer therapy of self-assembled green tea polyphenol-based nanoformulations. ACS Sustain Chem Eng. (2020) 8:16372–84. doi: 10.1021/acssuschemeng.0c06645
143. Fuster MG, Carissimi G, Montalbán MG, and Víllora G. ‘Anti-tumor’ activity of rosmarinic acid-loaded silk fibroin nanoparticles on HeLa and MCF-7 cells. Polymers. (2021) 13:3169. doi: 10.3390/polym13183169
144. Zhu J, Li Q, Wu Z, Xu Y, and Jiang R. Curcumin for treating breast cancer: A review of molecular mechanisms, combinations with anti-cancer drugs, and nanosystems. Pharmaceutics. (2024) 16:79. doi: 10.3390/pharmaceutics16010079
145. Yi Z, Chen G, Chen X, Ma X, Cui X, Sun Z, et al. Preparation of strong antioxidative, therapeutic nanoparticles based on amino acid-induced ultrafast assembly of tea polyphenols. ACS Appl Mater Interfaces. (2020) 12:33550–63. doi: 10.1021/acsami.0c10282
146. Swilam N and Nematallah KA. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci Rep. (2020) 10:14851. doi: 10.1038/s41598-020-71847-5
147. Santos SC, Fortes GA, Camargo LT, Camargo AJ, and Ferri PH. Antioxidant effects of polyphenolic compounds and structure-activity relationship predicted by multivariate regression tree. LWT-Food Sci Technol. (2021) 137:110366. doi: 10.1016/j.lwt.2020.110366
148. Ozen IT, Karav S, and Eksi A. Variability of sorbitol/xylitol content in pomegranate (Punica Granatum) juice as affected by processing conditions. Int J Food Nutr Sci. (2014) 3:4–7.
149. Jovanović AA, Djordjević VB, Petrović PM, Pljevljakušić DS, Zdunić GM, Šavikin KP, et al. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J Appl Res Med Aromat Plants. (2021) 25:100328. doi: 10.1016/j.jarmap.2021.100328
150. Zhang Y, Lan M, Lü JP, Li JF, Zhang KY, Zhi H, et al. Antioxidant, anti-inflammatory and cytotoxic activities of polyphenols extracted from. Chroogomphus rutilus. Chem Biodivers. (2020) 17:e1900479. doi: 10.1002/cbdv.201900479
151. Im YR, Kim I, and Lee J. Phenolic composition and antioxidant activity of purple sweet potato (Ipomoea batatas (L.) Lam.): Varietal comparisons and physical distribution. Antioxidants. (2021) 10:462. doi: 10.3390/antiox10030462
152. Bautista-Hernández I, Aranda-Ledesma NE, Rojas R, Tafolla-Arellano JC, and Martínez-Ávila GC. Antioxidant activity of polyphenolic compounds obtained from Euphorbia antisyphilitica by-products. Heliyon. (2021) 7:e06734. doi: 10.1016/j.heliyon.2021.e06734
153. Nguyen NQ, Nguyen MT, Nguyen VT, Le VM, Trieu LH, Le XT, et al. The effects of different extraction conditions on the polyphenol, flavonoids components and antioxidant activity of Polyscias fruticosa roots. IOP Conf Series: Materials Sci Engineering. (2020) 736:22067. doi: 10.1088/1757-899X/736/2/022067
154. Tan S, Lan X, Chen S, Zhong X, and Li W. Physical character, total polyphenols, anthocyanin profile and antioxidant activity of red cabbage as affected by five processing methods. Food Res Int. (2023) 169:112929. doi: 10.1016/j.foodres.2023.112929
155. Abdullah SS and Mazlan AN. Quantification of polyphenols and antioxidant activity in several herbal and green tea products in Malaysia. Mater Today Proc. (2020) 31:A106–13. doi: 10.1016/j.matpr.2020.12.1083
156. Mansoori A, Singh N, Dubey SK, Thakur TK, Alkan N, Das SN, et al. Phytochemical characterization and assessment of crude extracts from Lantana camara L. for antioxidant and antimicrobial activity. Front Agron. (2020) 2:582268. doi: 10.3389/fagro.2020.582268
157. Lang Y, Gao N, Zang Z, Meng X, Lin Y, Yang S, et al. Classification and antioxidant assays of polyphenols: A review. J Future Foods. (2024) 4:193–204. doi: 10.1016/j.jfutfo.2023.07.002
158. Xue Y, Zheng Y, An L, Dou Y, and Liu Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. (2014) 151:198–206. doi: 10.1016/j.foodchem.2013.11.064
159. Olszowy M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem. (2019) 144:135–43. doi: 10.1016/j.plaphy.2019.09.039
160. Rajan VK and Muraleedharan K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, gallic acid. Food Chem. (2017) 220:93–9. doi: 10.1016/j.foodchem.2016.09.178
161. Huang D, Li C, Chen Q, Xie X, Fu X, Chen C, et al. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. (2022) 377:131922. doi: 10.1016/j.foodchem.2021.131922
162. House NC, Puthenparampil D, Malayil D, and Narayanankutty A. Variation in the polyphenol composition, antioxidant, and anti-cancer activity among different Amaranthus species. S Afr J Bot. (2020) 135:408–12. doi: 10.1016/j.sajb.2020.09.026
163. Tiji S, Benayad O, Berrabah M, El Mounsi I, and Mimouni M. Phytochemical profile and antioxidant activity of Nigella sativa L growing in Morocco. Sci World J. (2021) 2021:6623609. doi: 10.1155/2021/6623609
164. Mehta D, Yadav K, Chaturvedi K, Shivhare US, and Yadav SK. Impact of cold plasma on extraction of polyphenol from de-oiled rice and corn bran: Improvement in extraction efficiency, in vitro digestibility, antioxidant activity, cytotoxicity and anti-inflammatory responses. Food Bioproc Technol. (2022) 15:1142–56. doi: 10.1007/s11947-022-02801-8
165. Li W, Zhang X, He Z, Chen Y, Li Z, Meng T, et al. In vitro and in vivo antioxidant activity of eucalyptus leaf polyphenols extract and its effect on chicken meat quality and cecum microbiota. Food Res Int. (2020) 136:109302. doi: 10.1016/j.foodres.2020.109302
166. Hossain MN, Sarker U, Raihan MS, Al-Huqail AA, Siddiqui MH, and Oba S. Influence of salinity stress on color parameters, leaf pigmentation, polyphenol and flavonoid contents, and antioxidant activity of Amaranthus lividus leafy vegetables. Molecules. (2022) 27:1821. doi: 10.3390/molecules27061821
167. Wojdyło A and Oszmiański J. Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants. (2020) 9:567. doi: 10.3390/antiox9070567
168. Kukhtenko H, Bevz N, Konechnyi Y, Kukhtenko O, and Jasicka-Misiak I. Spectrophotometric and chromatographic assessment of total polyphenol and flavonoid content in Rhododendron tomentosum extracts and their antioxidant and antimicrobial activity. Molecules. (2024) 29:1095. doi: 10.3390/molecules29051095
169. Menhem C, Mattar J, Carrillo C, and Serhan M. Determination of polyphenols, antioxidant activity, and antimicrobial properties of Zhourat using different extraction conditions. Appl Food Res. (2021) 1:100021. doi: 10.1016/j.afres.2021.100021
170. Bashmil YM, Ali A, Bk A, Dunshea FR, and Suleria HA. Screening and characterization of phenolic compounds from Australian grown bananas and their antioxidant capacity. Antioxidants. (2021) 10:1521. doi: 10.3390/antiox10101521
171. Janarny G, Ranaweera KKDS, and Gunathilake KDPP. Digestive recovery of polyphenols, antioxidant activity, and anti-inflammatory activity of selected edible flowers from the family Fabaceae. J Food Biochem. (2022) 46:e14052. doi: 10.1111/jfbc.14052
172. Bobková A, Hudáček M, Jakabová S, Belej Ľ, Capcarová M, Čurlej J, et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J Environ Sci Health Part B. (2020) 55:495–500. doi: 10.1080/03601234.2020.1724660
173. Alsaud N, Shahbaz K, and Farid M. Application of deep eutectic solvents in the extraction of polyphenolic antioxidants from New Zealand Manuka leaves (Leptospermum scoparium): Optimization and antioxidant activity. J Mol Liq. (2021) 337:116385. doi: 10.1016/j.molliq.2021.116385
174. Kiselova-Kaneva Y, Galunska B, Nikolova M, Dincheva I, and Badjakov I. High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chem. (2022) 367:130759. doi: 10.1016/j.foodchem.2021.130759
175. Yammine S, Delsart C, Vitrac X, Peuchot MM, and Ghidossi R. Characterisation of polyphenols and antioxidant potential of red and white pomace by-product extracts using subcritical water extraction. Oeno One. (2020) 54:263–78.
176. Forester SC and Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res. (2011) 55:844–54. doi: 10.1002/mnfr.201000641
177. Atak M, Kutlu EY, Çavuş D, and Çomoğlu M. Handmade green tea: Antioxidant content and activity. Appl Food Res. (2024) 4:100626. doi: 10.1016/j.afres.2024.100626
178. Micallef M, Lexis L, and Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr J. (2007) 6:1–8. doi: 10.1186/1475-2891-6-27
179. Lombardo M, Feraco A, Camajani E, Caprio M, and Armani A. Health effects of red wine consumption: a narrative review of an issue that still deserves debate. Nutrients. (2023) 15:1921. doi: 10.3390/nu15081921
180. Topka P, Rudzińska M, Poliński S, Szydłowska-Czerniak A, and Tańska M. Enhancing antioxidant activity and nutritional profile of dark chocolate through enrichment with plant sterols: A study on phytosterol concentrations and functional properties. Foods. (2024) 13:3578. doi: 10.3390/foods13223578
181. Behzadi M, Bideshki MV, Ahmadi-Khorram M, Zarezadeh M, and Hatami A. Effect of dark chocolate/cocoa consumption on oxidative stress and inflammation in adults: a GRADE-assessed systematic review and dose-response meta-analysis of controlled trials. Complement Ther Med. (2024) 84:103061. doi: 10.1016/j.ctim.2024.103061
182. Ashique S, Mukherjee T, Mohanty S, Garg A, Mishra N, Kaushik M, et al. Blueberries in focus: Exploring the phytochemical potentials and therapeutic applications. J Agric Food Res. (2024) 18:101300. doi: 10.1016/j.jafr.2024.101300
183. Kalt W, Cassidy A, Howard LR, Krikorian R, Stull AJ, Tremblay F, et al. Recent research on the health benefits of blueberries and their anthocyanins. Adv Nutr. (2020) 11:224–36. doi: 10.1093/advances/nmz065
184. Dehzad MJ, Ghalandari H, Nouri M, and Askarpour M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Cytokine. (2023) 164:156144. doi: 10.1016/j.cyto.2023.156144
185. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. (2020) 11:1021. doi: 10.3389/fphar.2020.01021
186. Kaur K, Al-Khazaleh AK, Bhuyan DJ, Li F, and Li CG. A review of recent curcumin analogues and their antioxidant, anti-inflammatory, and anti-cancer activities. Antioxidants. (2024) 13:1092. doi: 10.3390/antiox13091092
187. Xie X, Lin M, Xiao G, Liu H, Wang F, Liu D, et al. Phenolic amides (avenanthramides) in oats–an update review. Bioengineered. (2024) 15:2305029. doi: 10.1080/21655979.2024.2305029
188. Lee SH, Kye SB, Lim ST, and Oh SJ. Antioxidant and in vitro cosmeceutical activities of germinated oats. Cereal Chem. (2024) 101:771–84. doi: 10.1002/cche.10779
189. de Almeida Sousa Cruz MA, de Barros Elias M, Calina D, Sharifi-Rad J, and Teodoro AJ. Insights into grape-derived health benefits: A comprehensive overview. Food Prod Process Nutr. (2024) 6:1–23. doi: 10.1186/s43014-024-00267-z
190. Sabra A, Netticadan T, and Wijekoon C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem X. (2021) 12:100149. doi: 10.1016/j.fochx.2021.100149
191. Angeli L, Populin F, Morozova K, Ding Y, Asma U, Bolchini S, et al. Comparative analysis of antioxidant activity and capacity in apple varieties: Insights from stopped flow DPPH• kinetics, mass spectrometry and electrochemistry. Food Biosci. (2024) 58:103729. doi: 10.1016/j.fbio.2024.103729
192. Boyer J and Liu RH. Apple phytochemicals and their health benefits. Nutr J. (2004) 3:1–15. doi: 10.1186/1475-2891-3-5
193. Lorzadeh E, Heidary Z, Mohammadi M, Nadjarzadeh A, Ramezani-Jolfaie N, and Salehi-Abargouei A. Does pomegranate consumption improve oxidative stress? A systematic review and meta-analysis of randomized controlled clinical trials. Clin Nutr ESPEN. (2022) 47:117–27. doi: 10.1016/j.clnesp.2021.11.017
194. Malkawi R, Jarwan B, Waleed R, and Younis R. Pharmaceutical prospects of pomegranate antioxidants in combating microbial infections. Int J Food Prop. (2024) 27:768–82. doi: 10.1080/10942912.2024.2360985
195. Nemzer BV, Al-Taher F, Yashin A, Revelsky I, and Yashin Y. Cranberry: chemical composition, antioxidant activity and impact on human health: overview. Molecules. (2022) 27:1503. doi: 10.3390/molecules27051503
196. Wang J, Yuan ZY, Wang XY, Zhu JX, Huang WF, Xu GH, et al. Anthocyanins-rich cranberry extract attenuates DSS-induced IBD in an intestinal flora independent manner. Curr Res Food Sci. (2024) 9:100815. doi: 10.1016/j.crfs.2024.100815
197. Čakar U, Čolović M, Milenković D, Pagnacco M, Maksimović J, Krstić D, et al. Strawberry and drupe fruit wines antioxidant activity and protective effect against induced oxidative stress in rat synaptosomes. Antioxidants. (2025) 14:155. doi: 10.3390/antiox14020155
198. Martínez-Ferri E, Forbes-Hernandez TY, Cervantes L, Soria C, Battino M, and Ariza MT. Relation between strawberry fruit redness and bioactivity: Deciphering the role of anthocyanins as health promoting compounds. Foods. (2023) 13:110. doi: 10.3390/foods13010110
199. Sanchez-Ballesta MT, Balderas C, Escribano MI, Merodio C, and Romero I. Metabolic and antioxidant variations in “Regina” Raspberries: A comparative analysis of early and late harvests. Plants. (2025) 14:888. doi: 10.3390/plants14060888
200. Gomathi R, Umamaheswari TN, Prethipa R, Gomathi R, and Umamaheswari TN. Evaluation of antioxidant, anti-inflammatory, and antimicrobial activities of raspberry fruit extract: an in vitro study. Cureus. (2024) 16:e54045. doi: 10.7759/cureus.54045
201. Tripathi A, Pandey VK, Mishra H, Dar AH, Singh G, Rustagi S, et al. Enforcing the antioxidant properties of blackberries against breast cancer by activating different cell signaling mechanisms: An updated review. Food Biosci. (2024) 62:105266. doi: 10.1016/j.fbio.2024.105266
202. Roppolo P, Buzzanca C, D’Amico A, Culmone A, Tinebra I, Passafiume R, et al. Improvement of antioxidant activity and sensory properties of functional cookies by fortification with ultrasound-assisted hot-air-drying blackberry powders. Foods. (2024) 13:2402. doi: 10.3390/foods13152402
203. López-Parra MM, Barraso C, Martín-Mateos MJ, Curbelo P, Ortiz A, León L, et al. Use of cherry as a natural antioxidant and its influence on the physicochemical, technological and sensory properties of lamb burgers. Measurement: Food. (2024) 13:100143. doi: 10.1016/j.meafoo.2024.100143
204. Carrión-Antolí A, Badiche-El Hilali F, Lorente-Mento JM, Díaz-Mula HM, Serrano M, and Valero D. Antioxidant systems and quality in sweet cherries are improved by preharvest GABA treatments leading to delay postharvest senescence. Int J Mol Sci. (2023) 25:260. doi: 10.3390/ijms25010260
205. Murcia MA, Jiménez-Monreal AM, Gonzalez J, Martínez-Tomé M. Spinach. In: González-Molina JT, Jiménez-Monreal AM, and Martínez-Tomé M eds. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables. London, UK: Academic Press (2020) pp.181–95.
206. Neelab, Zeb A, and Jamil M. Spinach ameliorates dietary oxidized tallow-induced NAFLD by regulating inflammatory cytokines, peroxisome proliferator-activated receptors, and antioxidant systems. Phytomedicine Plus. (2025) 5:100722. doi: 10.1016/j.phyplu.2024.100722
207. Podsędek A, Frąszczak B, Kajszczak D, and Sosnowska D. Evaluation of bioactive compounds and antioxidant activity of green and red kale (Brassica oleracea L. var. acephala) microgreens grown under white, red, and blue LED combinations. Agronomy. (2024) 14:2454. doi: 10.3390/agronomy14112454
208. Michalak-Tomczyk M, Rymuszka A, Kukula-Koch W, Szwajgier D, Baranowska-Wójcik E, Jachuła J, et al. Studies on the effects of fermentation on the phenolic profile and biological activity of three cultivars of kale. Molecules. (2024) 29:1727. doi: 10.3390/molecules29081727
209. Uvaraj D, Alharbi NS, Kadaikunnan S, Thiruvengadam M, and Venkidasamy B. Comprehensive study on the differential extraction and comparison of bioactive health potential of the broccoli (Brassica oleracea). Int J Med Sci. (2024) 21:593. doi: 10.7150/ijms.92456
210. Zhao Y, Zhang Y, Yang H, Xu Z, Li Z, Zhang Z, et al. A comparative metabolomics analysis of phytochemicals and antioxidant activity between broccoli floret and by-products (leaves and stalks). Food Chem. (2024) 443:138517. doi: 10.1016/j.foodchem.2024.138517
211. Joković N, Matejić J, Zvezdanović J, Stojanović-Radić Z, Stanković N, Mihajilov-Krstev T, et al. Onion peel as a potential source of antioxidants and antimicrobial agents. Agronomy. (2024) 14:453. doi: 10.3390/agronomy14030453
212. Samota MK, Sharma M, Kaur K, Sarita, Yadav DK, Pandey AK, et al. Onion anthocyanins: Extraction, stability, bioavailability, dietary effect, and health implications. Front Nutr. (2022) 9:917617. doi: 10.3389/fnut.2022.917617
213. Collins EJ, Bowyer C, Tsouza A, and Chopra M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology. (2022) 11:239. doi: 10.3390/biology11020239
214. Hanson PM, Yang RY, Wu J, Chen JT, Ledesma D, Tsou SC, et al. Variation for antioxidant activity and antioxidants in tomato. J Am Soc Hortic Sci. (2004) 129:704–11. doi: 10.21273/JASHS.129.5.704
215. Akter J, Hassan J, Rahman MM, Biswas MS, Khan HI, Rajib MMR, et al. Colour, nutritional composition and antioxidant properties of dehydrated carrot (Daucus carota var. sativus) using solar drying techniques and pretreatments. Heliyon. (2024) 10:e24165. doi: 10.1016/j.heliyon.2024.e24165
216. Sabahi S, Abbasi A, and Mortazavi SA. Phenolic components from carrot (Daucus carota L.) pomace: Optimizing the extraction and assessing its potential antioxidant and antimicrobial activities. Heliyon. (2024) 10:e36971. doi: 10.1016/j.heliyon.2024.e36971
217. García-Infante M, Moreno-Rojas JM, Ordoñez-Díaz JL, Hervalejo A, Romero-Rodríguez E, and Arenas-Arenas FJ. Evaluation of antioxidant properties of thirteen orange genotypes [Citrus sinensis L. Osbeck] with juice industry interest. J Agric Food Res. (2024) 18:101491. doi: 10.1016/j.jafr.2024.101491
218. Modica G, Legua P, La Malfa S, Gentile A, and Continella A. Qualitative traits and antioxidant properties of blood oranges are affected by the genotype and the climatic conditions. Foods. (2024) 13:3137. doi: 10.3390/foods13193137
219. Athanasiadis V, Chatzimitakos T, Mantiniotou M, Bozinou E, and Lalas SI. Exploring the antioxidant properties of Citrus limon (lemon) peel ultrasound extract after the cloud point extraction method. Biomass. (2024) 4:202–16. doi: 10.3390/biomass4010010
220. Liu Z, Wang P, Liu C, and Tang X. Flavonoid profiles in the pulp of different lemon cultivars and their antioxidant activity based on UPLC–Q–TOF–MS. Molecules. (2024) 29:3464. doi: 10.3390/molecules29153464
221. Zhang Y, Liu Y, Li W, and Han W. Antioxidant activity of soybean peptides and Keap1 protein: A combined in vitro and in silico analysis. LWT-Food Sci Technol. (2024) 212:117019. doi: 10.1016/j.lwt.2024.117019
222. Spréa RM, Finimundy TC, Calhelha RC, Pires TC, Prieto MA, Amaral JS, et al. Comprehensive analysis of soybean (Glycine max L.) by-products: Nutritional value, phenolic composition, and bioactive properties. Food Biosci. (2024) 62:105382. doi: 10.1016/j.fbio.2024.105382
223. Kim Y, Kim YJ, and Shin Y. Comparative analysis of polyphenol content and antioxidant activity of different parts of five onion cultivars harvested in Korea. Antioxidants. (2024) 13:197. doi: 10.3390/antiox13020197
224. Gorrepati K, Kumar A, Ahammed Shabeer TP, Khan Z, Satpute P, Anandhan S, et al. Characterization and evaluation of antioxidant potential of onion peel extract of eight differentially pigmented short-day onion (Allium cepa L.) varieties. Front Sustain Food Syst. (2024) 8:1469635. doi: 10.3389/fsufs.2024.1469635
225. Usha T, Middha SK, Bhattacharya M, Lokesh P, and Goyal AK. Rosmarinic acid, a new polyphenol from Baccaurea ramiflora Lour. leaf: a probable compound for its anti-inflammatory activity. Antioxidants. (2014) 3:830–42. doi: 10.3390/antiox3040830
226. Siraj MA, Shilpi JA, Hossain MG, Uddin SJ, Islam MK, Jahan IA, et al. Anti-inflammatory and antioxidant activity of Acalypha hispida leaf and analysis of its major bioactive polyphenols by HPLC. Adv Pharm Bull. (2016) 6:275. doi: 10.15171/apb.2016.039
227. Asadirad A, Nashibi R, Khodadadi A, Ghadiri AA, Sadeghi M, Aminian A, et al. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res. (2022) 36:1023–31. doi: 10.1002/ptr.7375
228. Nair HB, Sung B, Yadav VR, Kannappan R, Chaturvedi MM, and Aggarwal BB. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol. (2010) 80:1833–43. doi: 10.1016/j.bcp.2010.07.021
229. Ayusso LL, Girol AP, Souza HR, Yoshikawa AH, de Azevedo LR, Carlos CP, et al. The anti-inflammatory properties of green tea extract protect against gentamicin-induced kidney injury. BioMed Pharmacother. (2024) 179:117267. doi: 10.1016/j.biopha.2024.117267
230. Radeva-Ilieva M, Stoeva S, Hvarchanova N, and Georgiev KD. Green Tea: Current knowledge and issues. Foods. (2025) 14:745. doi: 10.3390/foods14050745
231. Kim MJ, Yang YJ, Min GY, Heo JW, Son JD, You YZ, et al. Anti-inflammatory and antioxidant properties of Camellia sinensis L. extract as a potential therapeutic for atopic dermatitis through NF-κB pathway inhibition. Sci Rep. (2025) 15:2371. doi: 10.1038/s41598-025-86678-5
232. Sheng Y, Meng G, Li G, and Wang J. Red wine alleviates atherosclerosis-related inflammatory markers in healthy subjects rather than in high cardiovascular risk subjects: A systematic review and meta-analysis. Medicine. (2024) 103:e38229. doi: 10.1097/MD.0000000000038229
233. Kim SJ, Jung CW, Anh NH, Kim SW, Park S, Kwon SW, et al. Effects of oats (Avena sativa L.) on inflammation: A systematic review and meta-analysis of randomized controlled trials. Front Nutr. (2021) 8:722866. doi: 10.3389/fnut.2021.722866
234. Cortijo-Alfonso ME, Romero MP, Macià A, Yuste S, Moralejo M, Rubió-Piqué L, et al. Effect of barley and oat consumption on immune system, inflammation and gut microbiota: a systematic review of randomized controlled trials. Curr Nutr Rep. (2024) 13:582–97. doi: 10.1007/s13668-024-00543-x
235. Studzińska-Sroka E, Paczkowska-Walendowska M, Woźna Z, Plech T, Szulc P, and Cielecka-Piontek J. Elderberry leaves with antioxidant and anti-inflammatory properties as a valuable plant material for wound healing. Pharmaceuticals. (2024) 17:618. doi: 10.3390/ph17050618
236. Ferreira SS, Martins-Gomes C, Nunes FM, and Silva AM. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem X. (2022) 15:100437. doi: 10.1016/j.fochx.2022.100437
237. Samanta S, Sarkar T, Chakraborty R, Rebezov M, Shariati MA, Thiruvengadam M, et al. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr Res Food Sci. (2022) 5:1916–43. doi: 10.1016/j.crfs.2022.10.017
238. Sacconi R, Pezzella M, Ribarich N, Menean M, Servillo A, Bandello F, et al. Benefits of dark chocolate intake on retinal vessels functionality: a randomized, blind, crossover clinical trial. Sci Rep. (2024) 14:20203. doi: 10.1038/s41598-024-70289-7
239. Serreli G, Boronat A, de la Torre R, Rodriguez-Moratò J, and Deiana M. Cardiovascular and metabolic benefits of extra virgin olive oil phenolic compounds: Mechanistic insights from in vivo studies. Cells. (2024) 13:1555. doi: 10.3390/cells13181555
240. Tamburini B, Di Liberto D, Pratelli G, Rizzo C, Barbera LL, Lauricella M, et al. Extra virgin olive oil polyphenol-enriched extracts exert antioxidant and anti-inflammatory effects on peripheral blood mononuclear cells from rheumatoid arthritis patients. Antioxidants. (2025) 14:171. doi: 10.3390/antiox14020171
241. Dehghani F, Soltani S, Kolahdouz-Mohammadi R, Clark CT, and Abdollahi S. The effects of grape/grape products on inflammatory and oxidative stress markers: Systematic review and meta-analysis of randomized controlled trials. J Food Biochem. (2024) 2024:5086541. doi: 10.1155/2024/5086541
242. Pop RM, Boarescu PM, Bocsan CI, Gherman ML, Chedea VS, Jianu EM, et al. Anti-Inflammatory and antioxidant effects of white grape pomace polyphenols on isoproterenol-induced myocardial infarction. Int J Mol Sci. (2025) 26:2035. doi: 10.3390/ijms26052035
243. Mohammadi N, Franchin M, Pressete CG, Antunes LM, and Granato D. Green recovery and application of berry anthocyanins in functional gummies: Stability study, plasma and cellular antioxidant and anti-inflammatory activity. Food Res Int. (2024) 196:115128. doi: 10.1016/j.foodres.2024.115128
244. Joseph SV, Edirisinghe I, and Burton-Freeman BM. Berries: Anti-inflammatory effects in humans. J Agric Food Chem. (2014) 62:3886–903. doi: 10.1021/jf4044056
245. Widjaja G, Rudiansyah M, Sultan MQ, Ansari MJ, Izzat SE, Al Jaber MS, et al. Effect of tomato consumption on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin Nutr ESPEN. (2022) 50:93–100. doi: 10.1016/j.clnesp.2022.04.019
246. Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L, et al. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. (2015) 2015:401630. doi: 10.1155/2015/401630
247. Kang MS, Jang HJ, Kim JM, Jo HJ, Park KM, Chung YH, et al. Evaluation of anti-inflammatory activity of garlic extracts in 3D bioprinted skin equivalents. Adv NanoBiomed Res. (2024) 4:2400007. doi: 10.1002/anbr.202400007
248. Balogun O, Brownmiller CR, Lee SO, and Kang HW. Onion peel extract prevents intestinal inflammation via AMK-activated protein kinase activation in Caco-2/HT-29 cells. Nutrients. (2024) 16:3609. doi: 10.3390/nu16213609
249. Stoica F, Rațu RN, Veleșcu ID, Stănciuc N, and Râpeanu G. A comprehensive review on bioactive compounds, health benefits, and potential food applications of onion (Allium cepa L.) skin waste. Trends Food Sci Technol. (2023) 141:104173. doi: 10.1016/j.tifs.2023.104173
250. Syed RU, Moni SS, Break MKB, Khojali WM, Jafar M, Alshammari MD, et al. Broccoli: A multi-faceted vegetable for health: An in-depth review of its nutritional attributes, antimicrobial abilities, and anti-inflammatory properties. Antibiotics. (2023) 12:1157. doi: 10.3390/antibiotics12071157
251. Uthaman SK, Kang WS, Park JY, Kim S, Le DD, Oh SJ, et al. Endogenous extraction yielded high quality sulforaphane from broccoli sprouts unveils potent antioxidant and anti-alzheimer's activities. Heliyon. (2025) 11:e42673. doi: 10.1016/j.heliyon.2025.e42673
252. Kim GY, Kim SA, Kong SY, Seong H, Bae JH, and Han NS. Synergistic antioxidant and anti-inflammatory activities of kale juice fermented with Limosilactobacillus reuteri EFEL6901 or Limosilactobacillus fermentum EFEL6800. Antioxidants. (2023) 12:1850. doi: 10.3390/antiox12101850
253. Chatupos V, Neelawatanasook S, Sangutai T, Khanutwong A, Srichairatanakool P, Tipsuwan W, et al. Comparison of analgesic and anti-inflammatory effects of kale extract versus ibuprofen after impacted mandibular third molar surgery: A randomized, double-blind split-mouth clinical trial. Nutrients. (2024) 16:3821. doi: 10.3390/nu16223821
254. Islam S. Sweetpotatoes [Ipomoea batatas (L.) Lam]: The super food of the next century? An intensive review on their potential as a sustainable and versatile food source for future generations. CyTA-J Food. (2024) 22:2397553. doi: 10.1080/19476337.2024.2397553
255. Xi-You L, Rong-Jiao L, Xin-Yu M, Yun L, Xi Z, and Wei-Xi L. Comparison of nutrients and antioxidant activities in sweet potatoes. J Food Biochem. (2024) 2024:6645155. doi: 10.1155/2024/6645155
256. Kim SO, Choi A, Lee HH, Lee JY, Park SJ, and Kim BH. Purple corn extract improves benign prostatic hyperplasia by inhibiting 5 alpha-reductase type 2 and inflammation in testosterone propionate-induced rats. Front Pharmacol. (2025) 15:1485072. doi: 10.3389/fphar.2024.1485072
257. Lao F, Sigurdson GT, and Giusti MM. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr Rev Food Sci Food Saf. (2017) 16:234–46. doi: 10.1111/1541-4337.12249
258. Singh A, Singh J, Kaur S, Gunjal M, Kaur J, Nanda V, et al. Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: A review. Food Chem X. (2024) 23:101527. doi: 10.1016/j.fochx.2024.101527
259. Lone JK, Pandey R, and Gayacharan. Microgreens on the rise: Expanding our horizons from farm to fork. Heliyon. (2024) 10:e25870. doi: 10.1016/j.heliyon.2024.e25870
260. Maharjan A, Vasamsetti BMK, and Park J-H. A comprehensive review of capsaicin: Biosynthesis, industrial productions, processing to applications, and clinical uses. Heliyon. (2024) 10:e39721. doi: 10.1016/j.heliyon.2024.e39721
261. Hazekawa M, Hideshima Y, Ono K, Nishinakagawa T, Kawakubo-Yasukochi T, Takatani-Nakase T, et al. Anti-inflammatory effects of water extract from bell pepper (Capsicum annuum L. var. grossum) leaves in vitro. Exp Ther Med. (2017) 14:4349–55. doi: 10.3892/etm.2017.5106
262. Mizuno M and Minato KI. Anti-inflammatory and immunomodulatory properties of polysaccharides in mushrooms. Curr Opin Biotechnol. (2024) 86:103076. doi: 10.1016/j.copbio.2024.103076
263. Yin Z, Zhang J, Qin J, Guo L, Guo Q, Kang W, et al. Anti-inflammatory properties of polysaccharides from edible fungi on health-promotion: A review. Front Pharmacol. (2024) 15:1447677. doi: 10.3389/fphar.2024.1447677
264. Vieira EF, Ramirez P, Correia A, and Delerue-Matos C. Chayote (Sechium edule) peel extracts: A source of bioactive compounds for cosmeceutical design. Biol Life Sci Forum. (2025) 40:40. doi: 10.3390/blsf2024040040
265. Marra A, Manousakis V, Zervas GP, Koutis N, Finos MA, Adamantidi T, et al. Avocado and its by-products as natural sources of valuable anti-inflammatory and antioxidant bioactives for functional foods and cosmetics with health-promoting properties. Appl Sci. (2024) 14:5978. doi: 10.3390/app14145978
266. Onyedikachi UB, Nkwocha CC, Ejiofor E, and Nnanna CC. Investigation of chemical constituents, antioxidant, anti-inflammatory, and nutritional properties of oil of Persea americana (Avocado) seeds. Food Chem Adv. (2024) 5:100770. doi: 10.1016/j.focha.2024.100770
267. Ismail J, Shebaby WN, Daher J, Boulos JC, Taleb R, Daher CF, et al. The wild carrot (Daucus carota): A phytochemical and pharmacological review. Plants. (2023) 13:93. doi: 10.3390/plants13010093
268. Saini A, Shams R, Dash KK, and Kovács B. Anthocyanin extraction from black carrot: Health-promoting properties and potential applications. J Agric Food Res. (2025) 19:101533. doi: 10.1016/j.jafr.2024.101533
269. Mattioli R, Francioso A, d'Erme M, Trovato M, Mancini P, Piacentini L, et al. Anti-inflammatory activity of a polyphenolic extract from Arabidopsis thaliana in in vitro and in vivo models of Alzheimer’s disease. Int J Mol Sci. (2019) 20:708. doi: 10.3390/ijms20030708
270. Costa V, Costa M, Videira RA, Andrade PB, and Paiva-Martins F. Anti-inflammatory activity of olive oil polyphenols—the role of oleacein and its metabolites. Biomedicines. (2022) 10:2990. doi: 10.3390/biomedicines10112990
271. Grigore A, Colceru-Mihul S, Litescu S, Panteli M, and Rasit I. Correlation between polyphenol content and anti-inflammatory activity of Verbascum phlomoides (mullein). Pharm Biol. (2013) 51:925–9. doi: 10.3109/13880209.2013.767361
272. García-Lafuente A, Moro C, Manchón N, Gonzalo-Ruiz A, Villares A, Guillamón E, et al. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. (2014) 161:216–23. doi: 10.1016/j.foodchem.2014.04.004
273. Chen GL, Munyao Mutie F, Xu YB, Saleri FD, Hu GW, and Guo MQ. Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals. (2020) 13:55. doi: 10.3390/ph13040055
274. Rupasinghe HV, Boehm MM, Sekhon-Loodu S, Parmar I, Bors B, and Jamieson AR. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules. (2015) 5:1079–98. doi: 10.3390/biom5021079
275. Hooshmand S, Kumar A, Zhang JY, Johnson SA, Chai SC, and Arjmandi BH. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. (2015) 6:1719–25. doi: 10.1039/C5FO00173K
276. Bogolitsyn K, Dobrodeeva L, Samodova A, and Parshina A. In vitro immunostimulant activity of the polyphenolic extract from the arctic brown algae Fucus vesiculosus. Plant Foods Hum Nutr. (2024) 79:511–7. doi: 10.1007/s11130-024-01174-x
277. Ben Salem M, Affes H, Athmouni K, Ksouda K, Dhouibi R, Sahnoun Z, et al. Chemical compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid Based Complement Altern Med. (2017) 2017:4951937. doi: 10.1155/2017/4951937
278. Derouich M, Bouhlali EDT, Hmidani A, Bammou M, Bourkhis B, Sellam K, et al. Assessment of total polyphenols, flavonoids and anti-inflammatory potential of three Apiaceae species grown in the Southeast of Morocco. Sci Afr. (2020) 9:e00507. doi: 10.1016/j.sciaf.2020.e00507
279. Barfoot KL, Istas G, Feliciano RP, Lamport DJ, Riddell P, Rodriguez-Mateos A, et al. Effects of daily consumption of wild blueberry on cognition and urinary metabolites in school-aged children: A pilot study. Eur J Nutr. (2021) 60:4263–78. doi: 10.1007/s00394-021-02588-y
280. Su X, Zhang J, Wang H, Xu J, He J, Liu L, et al. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules. (2017) 22:312. doi: 10.3390/molecules22020312
281. Kim SK, Kim H, Kim SA, Park HK, and Kim W. Anti-inflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. Korean J Physiol Pharmacol. (2013) 17:73. doi: 10.4196/kjpp.2013.17.1.73
282. Peng L, Ai-Lati A, Ji Z, Chen S, and Mao J. Polyphenols extracted from huangjiu have anti-inflammatory activity in lipopolysaccharide stimulated RAW264.7 cells. RSC Adv. (2019) 9:5295–301. doi: 10.1039/C8RA09671F
283. Zhang TT, Hu T, Jiang JG, Zhao JW, and Zhu W. Antioxidant and anti-inflammatory effects of polyphenols extracted from Ilex latifolia Thunb. RSC Adv. (2018) 8:7134–41. doi: 10.1039/C7RA13569F
284. de Araújo AA, Soares LAL, Ferreira MRA, de Souza Neto MA, da Silva GR, de Araújo RF Jr., et al. Quantification of polyphenols and evaluation of antimicrobial, analgesic and anti-inflammatory activities of aqueous and acetone–water extracts of Libidibia ferrea, Parapiptadenia rigida and Psidium guajava. J Ethnopharmacol. (2014) 156:88–96. doi: 10.1016/j.jep.2014.07.031
285. Sarıtaş S, Duman H, and Karav S. Nutritional and functional aspects of fermented algae. Int J Food Sci Technol. (2024) 59:5270–84. doi: 10.1111/ijfs.17297
286. Sim SH, KyoungChoi H, Na SC, Hwang DI, Oh HB, Lim YT, et al. Changes in physiologically active ingredients and anti-inflammatory properties of underutilized wild vegetables by complex fermentation using beneficial microorganisms. Food Sci Preserv. (2024) 31:287–97. doi: 10.11002/fsp.2024.31.2.287
287. Labhar A, Ahidar N, El-Mernissi Y, Benamari O, Ait Alla K, Jahjah S, et al. Phytochemical, antioxidant, and anti-inflammatory properties of Tetraclinis articulata (Vahl) Masters extracts from Al Hoceima province. E3S Web Conf. (2024) 527:1016. doi: 10.1051/e3sconf/202452701016
288. Golua L, Shakulashvili N, Tskokilauri A, Kotorashvili T, Pazouki N, Rodrigues K, et al. Polyphenol content, antioxidant and anti-inflammatory activities of Georgian wine and green tea. World J Biopharm Sci. (2024) 18:246–50. doi: 10.30574/wjbphs.2024.18.2.0276
289. Khare P, Maurya R, Bhatia R, Mangal P, Singh J, Podili K, et al. Polyphenol rich extracts of finger millet and kodo millet ameliorate high fat diet-induced metabolic alterations. Food Funct. (2020) 11:9833–47. doi: 10.1039/D0FO01643H
290. Nichitoi MM, Josceanu AM, Isopescu RD, Isopencu GO, Geana EI, Ciucure CT, et al. Polyphenolics profile effects upon the antioxidant and antimicrobial activity of propolis extracts. Sci Rep. (2021) 11:20113. doi: 10.1038/s41598-021-97130-9
291. Afshari M, Rahimmalek M, and Miroliaei M. Variation in polyphenolic profiles, antioxidant and antimicrobial activity of different Achillea species as natural sources of antiglycative compounds. Chem Biodivers. (2018) 15:e1800075. doi: 10.1002/cbdv.201800075
292. Sun S, Huang S, Shi Y, Shao Y, Qiu J, Sedjoah RCAA, et al. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. (2021) 351:129232. doi: 10.1016/j.foodchem.2021.129232
293. Fyfe L, Okoro P, Paterson E, Coyle S, and McDougall GJ. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT-Food Sci Technol. (2017) 79:52–9. doi: 10.1016/j.lwt.2017.01.023
294. Alkufeidy RM, Altuwijri LA, Aldosari NS, Alsakabi N, and Dawoud TM. Antimicrobial and synergistic properties of green tea catechins against microbial pathogens. J King Saud Univ Sci. (2024) 36:103277. doi: 10.1016/j.jksus.2024.103277
295. Gopal J, Muthu M, Paul D, Kim DH, and Chun S. Bactericidal activity of green tea extracts: The importance of catechin-containing nanoparticles. Sci Rep. (2016) 6:19710. doi: 10.1038/srep19710
296. Harfoush A, Swaidan A, Khazaal S, Sokhn ES, Grimi N, Debs E, et al. From spent black and green tea to potential health boosters: Optimization of polyphenol extraction and assessment of their antioxidant and antibacterial activities. Antioxidants. (2024) 13:1588. doi: 10.3390/antiox13121588
297. Huang Z, Zhang L, Xuan J, Zhao T, and Peng W. Antibacterial and antiallergic effects of three tea extracts on histamine-induced dermatitis. Pharmaceuticals. (2024) 17:1181. doi: 10.3390/ph17091181
298. Roytrakul S, Charoenlappanit S, Kittisenachai S, Siangpro N, Sichaem J, Chuakrut S, et al. Antimicrobial and antioxidant activities of peptide derived from turmeric plant (Curcuma longa L). PloS One. (2024) 19:e0314482. doi: 10.1371/journal.pone.0314482
299. Odo EO, Ikwuegbu JA, Obeagu EI, Chibueze SA, and Ochiaka RE. Analysis of the antibacterial effects of turmeric on particular bacteria. Medicine. (2023) 102:e36492. doi: 10.1097/MD.0000000000036492
300. Prasad S, Patel B, Kumar P, Mitra P, and Lall R. Cranberry: A promising natural product for animal health and performance. Curr Issues Mol Biol. (2025) 47:80. doi: 10.3390/cimb47020080
301. Karim N, Rashwan AK, Liu S, Tangpong J, Lin T, and Chen W. An updated review on chemical compositions, biological capabilities, and clinical benefits of cranberries. Food Biosci. (2023) 54:102877. doi: 10.1016/j.fbio.2023.102877
302. Radulescu C, Buruleanu LC, Nicolescu CM, Olteanu RL, Bumbac M, Holban GC, et al. Phytochemical profiles, antioxidant and antibacterial activities of grape (Vitis vinifera L.) seeds and skin from organic and conventional vineyards. Plants. (2020) 9:1470. doi: 10.3390/plants9111470
303. Grosu AC, Diguță FC, Pristavu MC, Popa A, Badea F, Dragoi Cudalbeanu M, et al. Exploring the phytochemical profiles, and antioxidant and antimicrobial activities of the hydroethanolic grape pomace extracts from two Romanian indigenous varieties. Fermentation. (2024) 10:470. doi: 10.3390/fermentation10090470
304. Sánchez-Moya T, López-Nicolás R, Peso-Echarri P, González-Bermúdez CA, and Frontela-Saseta C. Effect of pine bark extract and its phenolic compounds on selected pathogenic and probiotic bacterial strains. Front Nutr. (2024) 11:1381125. doi: 10.3389/fnut.2024.1381125
305. Mehmood S, Abbasi AM, Hussain H, Khan SA, Almutairi HH, Ismail AM, et al. Phytochemical profile, antioxidant and antibacterial activities analysis of crude extract and essential oil of Pinus roxburghii and Pinus wallichiana: In vitro and in silico analyses. Cogent Food Agric. (2024) 10:2403648. doi: 10.1080/23311932.2024.2403648
306. Scaglione E, Sateriale D, Mantova G, Di Rosario M, Continisio L, Vitiello M, et al. Antimicrobial efficacy of Punica granatum Lythraceae peel extract against pathogens belonging to the ESKAPE group. Front Microbiol. (2024) 15:1383027. doi: 10.3389/fmicb.2024.1383027
307. Abu-Niaaj LF, Al-Daghistani HI, Katampe I, Abu-Irmaileh B, and Bustanji YK. Pomegranate peel: Bioactivities as antimicrobial and cytotoxic agents. Food Sci Nutr. (2024) 12:2818–32. doi: 10.1002/fsn3.3963
308. Fancello F, Multineddu C, Santona M, Molinu MG, Zara G, Dettori S, et al. Antimicrobial activities of virgin olive oils in vitro and on lettuce from pathogen-inoculated commercial quick salad bags. Food Control. (2022) 133:108657. doi: 10.1016/j.foodcont.2021.108657
309. Nazzaro F, Fratianni F, Cozzolino R, Martignetti A, Malorni L, De Feo V, et al. Antibacterial activity of three extra virgin olive oils of the Campania region, southern Italy, related to their polyphenol content and composition. Microorganisms. (2019) 7:321. doi: 10.3390/microorganisms7090321
310. Chala D, Sabadashka M, Morozovych A, Krychowiak-Maśnicka M, Królicka A, and Sybirna N. Immunomodulatory and antibacterial effect of red wine concentrate rich in a natural complex of polyphenols under diabetes mellitus. BioMed Pharmacother. (2024) 170:116023. doi: 10.1016/j.biopha.2023.116023
311. Sánchez MC, Ribeiro-Vidal H, Esteban-Fernández A, Bartolomé B, Figuero E, Moreno-Arribas MV, et al. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement Altern Med. (2019) 19:1–12. doi: 10.1186/s12906-019-2533-5
312. Nathan VB, Eckrote S, Li S, and Reddivari L. Crude Blueberry phenolic extracts improve gut barrier integrity and exert anti-inflammatory and antimicrobial activity in an in vitro weaning stress model. Antioxidants. (2024) 13:1044. doi: 10.3390/antiox13091044
313. Tušek K, Valinger D, Jurina T, Sokač Cvetnić T, Gajdoš Kljusurić J, and Benković M. Bioactives in cocoa: Novel findings, health benefits, and extraction techniques. Separations. (2024) 11:128. doi: 10.3390/separations11040128
314. Fideles SOM, Ortiz ADC, Reis CHB, Buchaim DV, and Buchaim RL. Biological properties and antimicrobial potential of cocoa and its effects on systemic and oral health. Nutrients. (2023) 15:3927. doi: 10.3390/nu15183927
315. Nakamoto M, Kunimura K, Suzuki JI, and Kodera Y. Antimicrobial properties of hydrophobic compounds in garlic: allicin, vinyldithiin, ajoene, and diallyl polysulfides. Exp Ther Med. (2020) 19:1550–3. doi: 10.3892/etm.2019.8388
316. Li G, Ma X, Deng L, Zhao X, Wei Y, Gao Z, et al. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J Microbiol. (2015) 8:e14814. doi: 10.5812/jjm.14814
317. Shu C, Ge L, Li Z, Chen B, Liao S, Lu L, et al. Antibacterial activity of cinnamon essential oil and its main component of cinnamaldehyde and the underlying mechanism. Front Pharmacol. (2024) 15:1378434. doi: 10.3389/fphar.2024.1378434
318. Iseppi R, Truzzi E, Sabia C, and Messi P. Efficacy and synergistic potential of cinnamon (Cinnamomum zeylanicum) and clove (Syzygium aromaticum L. Merr. and Perry) essential oils to control food-borne pathogens in fresh-cut fruits. Antibiotics. (2024) 13:319. doi: 10.3390/antibiotics13040319
319. Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, and Heredia JB. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules. (2017) 22:989. doi: 10.3390/molecules22060989
320. Walasek-Janusz M, Grzegorczyk A, Malm A, Nurzyńska-Wierdak R, and Zalewski D. Chemical composition, and antioxidant and antimicrobial activity of oregano essential oil. Molecules. (2024) 29:435. doi: 10.3390/molecules29020435
321. Pandey VK, Srivastava S, Dash KK, Singh R, Dar AH, Singh T, et al. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: a comprehensive review. Heliyon. (2024) 10:e22437. doi: 10.1016/j.heliyon.2023.e22437
322. Maggini V, Semenzato G, Gallo E, Nunziata A, Fani R, and Firenzuoli F. Antimicrobial activity of Syzygium aromaticum essential oil in human health treatment. Molecules. (2024) 29:999. doi: 10.3390/molecules29050999
323. Vassiliou E, Awoleye O, Davis A, and Mishra S. Anti-inflammatory and antimicrobial properties of thyme oil and its main constituents. Int J Mol Sci. (2023) 24:6936. doi: 10.3390/ijms24086936
324. Wirtu SF, Ramaswamy K, Maitra R, Chopra S, Mishra AK, and Jule LT. Isolation, characterization and antimicrobial activity study of Thymus vulgaris. Sci Rep. (2024) 14:21573. doi: 10.1038/s41598-024-71012-2
325. Akacha BB, Kačániová M, Mekinić IG, Kukula-Koch W, Koch W, Orhan IE, et al. Sage (Salvia officinalis L.): A botanical marvel with versatile pharmacological properties and sustainable applications in functional foods. S Afr J Bot. (2024) 169:361–82. doi: 10.1016/j.sajb.2024.04.044
326. Mokhtari R, Kazemi Fard M, Rezaei M, Moftakharzadeh SA, and Mohseni A. Antioxidant, antimicrobial activities, and characterization of phenolic compounds of thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and thyme–sage mixture extracts. J Food Qual. (2023) 2023:2602454. doi: 10.1155/2023/2602454
327. Magtalas TJ and Anapi GR. Evaluation of bacteriostatic effect of rosemary and oregano essential oils against a non-pathogenic surrogate of Salmonella spp. (E. coli ATCC 9637). Biol Life Sci Forum. (2025) 40:7. doi: 10.3390/blsf2024040007
328. Nieto G, Ros G, and Castillo J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis L.): A review. Medicines. (2018) 5:98. doi: 10.3390/medicines5030098
329. Rahmani AH and Aly SM. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol. (2014) 6:125.
330. Sulieman AME, Ibrahim SM, Alshammari M, Abdulaziz F, Idriss H, Alanazi NAH, et al. Zingiber officinale uncovered: Integrating experimental and computational approaches to antibacterial and phytochemical profiling. Pharmaceuticals. (2024) 17:1551. doi: 10.3390/ph17111551
331. Jabbar M, Baboo I, Majeed H, Farooq Z, and Palangi V. Characterization and antibacterial application of peppermint essential oil nanoemulsions in broiler. Poultry Sci. (2024) 103:104432. doi: 10.1016/j.psj.2024.104432
332. McKay DL and Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother Res. (2006) 20:619–33. doi: 10.1002/ptr.1936
333. Wang L, Yang R, Yuan B, Liu Y, and Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. (2015) 5:310–5. doi: 10.1016/j.apsb.2015.05.005
334. Chen RY, Shi JJ, Liu YJ, Yu J, Li CY, Tao F, et al. The state-of-the-art antibacterial activities of glycyrrhizin: A comprehensive review. Microorganisms. (2024) 12:1155. doi: 10.3390/microorganisms12061155
335. Wylie MR and Merrell DS. The antimicrobial potential of the neem tree Azadirachta indica. Front Pharmacol. (2022) 13:891535. doi: 10.3389/fphar.2022.891535
336. Mahmoud GAE, Rashed NM, El-Ganainy SM, and Salem SH. Unveiling the neem (Azadirachta indica) effects on biofilm formation of food-borne bacteria and the potential mechanism using a molecular docking approach. Plants. (2024) 13:2669. doi: 10.3390/plants13182669
337. Papastavropoulou K, Oz E, Oz F, and Proestos C. Polyphenols from plants: Phytochemical characterization, antioxidant capacity, and antimicrobial activity of some plants from different sites of Greece. Separations. (2022) 9:186. doi: 10.3390/separations9080186
338. Staszowska-Karkut M and Materska M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients. (2020) 12:463. doi: 10.3390/nu12020463
339. Guo L, Sun Q, Gong S, Bi X, Jiang W, Xue W, et al. Antimicrobial activity and action approach of the olive oil polyphenol extract against Listeria monocytogenes. Front Microbiol. (2019) 10:1586. doi: 10.3389/fmicb.2019.01586
340. Alves MJ, Ferreira IC, Froufe HJ, Abreu RM, Martins A, and Pintado M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J Appl Microbiol. (2013) 115:346–57. doi: 10.1111/jam.12196
341. Gutiérrez-Venegas G, Gómez-Mora JA, Meraz-Rodríguez MA, Flores-Sánchez MA, and Ortiz-Miranda LF. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon. (2019) 5:e03013. doi: 10.1016/j.heliyon.2019.e03013
342. Kamboj A, Saluja AK, Kumar M, and Atri P. Antiviral activity of plant polyphenols. J Pharm Res. (2012) 5:2402–12.
343. Dziedzinski M, Kobus-Cisowska J, Szymanowska D, Stuper-Szablewska K, and Baranowska M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules. (2020) 25:3527. doi: 10.3390/molecules25153527
344. Fei P, Ali MA, Gong S, Sun Q, Bi X, Liu S, et al. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control. (2018) 94:289–94. doi: 10.1016/j.foodcont.2018.07.022
345. Prabakaran M, Kim SH, Sasireka A, Chandrasekaran M, and Chung IM. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. (2018) 26:23–9. doi: 10.1016/j.fbio.2018.09.003
346. Silva V, Igrejas G, Falco V, Santos TP, Torres C, Oliveira AM, et al. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control. (2018) 92:516–22. doi: 10.1016/j.foodcont.2018.05.031
347. De Angelis M, Della-Morte D, Buttinelli G, Di Martino A, Pacifici F, Checconi P, et al. Protective role of combined polyphenols and micronutrients against influenza A virus and SARS-CoV-2 infection in vitro. Biomedicines. (2021) 9:1721. doi: 10.3390/biomedicines9111721
348. Singh S, Sk MF, Sonawane A, Kar P, and Sadhukhan S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: an in silico analysis. J Biomol Struct Dyn. (2021) 39:6249–64. doi: 10.1080/07391102.2020.1796810
349. Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, and Mandalari G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients. (2019) 11:2355. doi: 10.3390/nu11102355
350. Park S, Kim JI, Lee I, Lee S, Hwang MW, Bae JY, et al. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem Biophys Res Commun. (2013) 440:14–9. doi: 10.1016/j.bbrc.2013.08.090
351. Pagliarulo C, De Vito V, Picariello G, Colicchio R, Pastore G, Salvatore P, et al. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. (2016) 190:824–31. doi: 10.1016/j.foodchem.2015.06.028
352. Nibir YM, Sumit AF, Akhand AA, Ahsan N, and Hossain MS. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac J Trop Biomed. (2017) 7:352–7. doi: 10.1016/j.apjtb.2017.01.005
353. Prasad P, Mansoori A, Prajapati N, Tripathi J, Sharma K, Kumar A, et al. Phytochemical screening and antimicrobial activities of Guizotia abyssinica L. leaf and flower extracts. J Nat Pestic Res. (2024) 9:100083. doi: 10.1016/j.napere.2024.100083
354. Chaudhry F, Ahmad ML, Hayat Z, Ranjha MMAN, Chaudhry K, Elboughdiri N, et al. Extraction and evaluation of the antimicrobial activity of polyphenols from banana peels employing different extraction techniques. Separations. (2022) 9:165. doi: 10.3390/separations9070165
355. Thomson C, Garcia AL, and Edwards CA. Interactions between dietary fiber and the gut microbiota. Proc Nutr Soc. (2021) 80:398–408. doi: 10.1017/S0029665121002834
356. Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, et al. Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure–activity relationship) models. Front Microbiol. (2019) 10:829. doi: 10.3389/fmicb.2019.00829
357. Caponio GR, Noviello M, Calabrese FM, Gambacorta G, Giannelli G, and De Angelis M. Effects of grape pomace polyphenols and in vitro gastrointestinal digestion on antimicrobial activity: Recovery of bioactive compounds. Antioxidants. (2022) 11:567. doi: 10.3390/antiox11030567
358. Majdoub YOE, Ginestra G, Mandalari G, Dugo P, Mondello L, and Cacciola F. The digestibility of Hibiscus sabdariffa L. polyphenols using an in vitro human digestion model and evaluation of their antimicrobial activity. Nutrients. (2021) 13:2360. doi: 10.3390/nu13072360
359. Szewczyk K, Zidorn C, Biernasiuk A, Komsta Ł, and Granica S. Polyphenols from Impatiens spp. (Balsaminaceae) and their antioxidant and antimicrobial activities. Ind Crops Prod. (2016) 86:262–72. doi: 10.1016/j.indcrop.2016.03.053
360. Todorovic V, Milenkovic M, Vidovic B, Todorovic Z, and Sobajic S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J Food Sci. (2017) 82:1020–7. doi: 10.1111/1750-3841.13672
361. Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, et al. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci Technol. (2016) 65:1025–30. doi: 10.1016/j.lwt.2015.09.038
362. Benbelaïd F, Khadir A, Bendahou M, Zenati F, Bellahsene C, Muselli A, et al. Antimicrobial activity of Rosmarinus eriocalyx essential oil and polyphenols: An endemic medicinal plant from Algeria. J Coast Life Med. (2016) 4:39–44. doi: 10.12980/jclm.4.2016j5-221
363. Serra V, Salvatori G, and Pastorelli G. Dietary polyphenol supplementation in food producing animals: effects on the quality of derived products. Animals. (2021) 11:401. doi: 10.3390/ani11020401
364. Waqas M, Salman M, and Sharif M. Application of polyphenolic compounds in animal nutrition and their promising effects. J Anim Feed Sci. (2023) 32:233–56. doi: 10.22358/jafs/159718/2023
365. Liu Z, Zhai J, Han N, and Yin J. Assessment of anti-diabetic activity of the aqueous extract of leaves of Astilboides tabularis. J Ethnopharmacol. (2016) 194:635–41. doi: 10.1016/j.jep.2016.10.003
366. Díaz-de-Cerio E, Verardo V, Gómez-Caravaca AM, Fernández-Gutiérrez A, and Segura-Carretero A. Exploratory characterization of phenolic compounds with demonstrated anti-diabetic activity in guava leaves at different oxidation states. Int J Mol Sci. (2016) 17:699. doi: 10.3390/ijms17050699
367. Guo S, Ren X, He K, Chen X, Zhang S, Roller M, et al. The anti-diabetic effect of eight Lagerstroemia speciosa leaf extracts based on the contents of ellagitannins and ellagic acid derivatives. Food Funct. (2020) 11:1560–71. doi: 10.1039/C9FO03091C
368. Krishnakumar IM, Issac A, Johannah NM, Ninan E, Maliakel B, and Kuttan R. Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. (2014) 5:2208–20. doi: 10.1039/C4FO00130C
369. Ouahabi S, Loukili EH, Daoudi NE, Chebaibi M, Ramdani M, Rahhou I, et al. Study of the phytochemical composition, antioxidant properties, and in vitro anti-diabetic efficacy of Gracilaria bursa-pastoris extracts. Mar Drugs. (2023) 21:372. doi: 10.3390/md21070372
370. Hartung NM, Fischer J, Ostermann AI, Willenberg I, Rund KM, Schebb NH, et al. Impact of food polyphenols on oxylipin biosynthesis in human neutrophils. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:1536–44. doi: 10.1016/j.bbalip.2019.05.002
371. Fatima N, Hafizur RM, Hameed A, Ahmed S, Nisar M, and Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur J Nutr. (2017) 56:591–601. doi: 10.1007/s00394-015-1103-y
372. Nie T and Cooper GJ. Mechanisms underlying the anti-diabetic activities of polyphenolic compounds: A review. Front Pharmacol. (2021) 12:798329. doi: 10.3389/fphar.2021.798329
373. Mezni F, Stiti B, Fkiri S, Ayari F, Slimane LB, Ksouri R, et al. Phenolic profile and in vitro anti-diabetic activity of acorn from four African Quercus species (Q. suber, Q. canariensis, Q. coccifera and Q. ilex). S Afr J Bot. (2022) 146:771–5. doi: 10.1016/j.sajb.2021.12.023
374. Naz R, Saqib F, Awadallah S, Wahid M, Latif MF, Iqbal I, et al. Food polyphenols and type II diabetes mellitus: pharmacology and mechanisms. Molecules. (2023) 28:3996. doi: 10.3390/molecules28103996
375. Sun C, Zhao C, Guven EC, Paoli P, Simal-Gandara J, Ramkumar KM, et al. Dietary polyphenols as anti-diabetic agents: Advances and opportunities. Food Front. (2020) 1:18–44. doi: 10.1002/fft2.15
376. Ojo AB and Adanlawo IG. Antioxidant, anti-diabetic, and anti-inflammatory activities of flavonoid-rich fractions of Solanum anguivi Lam. fruit: In vitro and ex vivo studies. Heliyon. (2024) 10:e31895. doi: 10.1016/j.heliyon.2024.e31895
377. Sabu MC, Smitha K, and Ramadasan K. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. (2002) 83:109–16. doi: 10.1016/S0378-8741(02)00217-9
378. Tang W, Li S, Liu Y, Huang MT, and Ho CT. Anti-diabetic activity of chemically profiled green tea and black tea extracts in a type 2 diabetes mice model via different mechanisms. J Funct Foods. (2013) 5:1784–93. doi: 10.1016/j.jff.2013.08.007
379. Nguyen MT, Pham VH, Tran MD, and Nguyen QV. Syzygium zeylanicum (L.) DC. polyphenols exhibit anti-diabetic activity by modulation of ACC1, SGLT1, and GLP-1 genes and restoration of gut microbiota in overfeeding and high glucose exposure-induced diabetic zebrafish. J Funct Foods. (2024) 112:105921. doi: 10.1016/j.jff.2023.105921
380. Draganescu D, Andritoiu C, Hritcu D, Dodi G, and Popa MI. Flaxseed lignans and polyphenols enhanced activity in streptozotocin-induced diabetic rats. Biology. (2021) 10:43. doi: 10.3390/biology10010043
381. Zuo Z, Pang W, Sun W, Lu B, Zou L, Zhang D, et al. Metallothionein–kidney bean polyphenol complexes showed anti-diabetic activity in type 2 diabetic rats by improving insulin resistance and regulating gut microbiota. Foods. (2023) 12:3139. doi: 10.3390/foods12163139
382. Li H, Beg OU, Rafie AR, Kanwal S, Ovalle-Cisneros A, Faison MO, et al. Characterization of green and yellow papaya (Carica papaya) for anti-diabetic activity in liver and myoblast cells and wound-healing activity in fibroblast cells. Nutrients. (2023) 15:1929. doi: 10.3390/nu15081929
383. Pieczykolan A, Pietrzak W, Gawlik-Dziki U, and Nowak R. Antioxidant, anti-inflammatory, and anti-diabetic activity of phenolic acids fractions obtained from Aerva lanata (L.) Juss. Molecules. (2021) 26:3486. doi: 10.3390/molecules26123486
384. El Adaouia Taleb R, Djebli N, Chenini H, Sahin H, and Kolayli S. In vivo and in vitro anti-diabetic activity of ethanolic propolis extract. J Food Biochem. (2020) 44:e13267. doi: 10.1111/jfbc.13267
385. Xia T, Zhang Z, Zhao Y, Kang C, Zhang X, Tian Y, et al. The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. (2022) 393:133443. doi: 10.1016/j.foodchem.2022.133443
386. Vaithiyalingam M, Sumathi DL, and Sabarathinam S. Isolation and in silico study of curcumin from Curcuma longa and its anti-diabetic activity. Appl Biochem Biotechnol. (2023) 195:947–57. doi: 10.1007/s12010-022-04173-3
387. Jahan NI, Job JT, Alfarhan A, Rajagopal R, Kavungal V, Oprea E, et al. Elemental composition, antioxidant, anti-inflammatory and anti-genotoxic properties of Nitophyllum punctatum. J King Saud Univ Sci. (2024) 36:103311. doi: 10.1016/j.jksus.2024.103311
388. Moloto MR, Phan ADT, Shai JL, Sultanbawa Y, and Sivakumar D. Comparison of phenolic compounds, carotenoids, amino acid composition, in vitro antioxidant and anti-diabetic activities in the leaves of seven cowpea (Vigna unguiculata) cultivars. Foods. (2020) 9:1285. doi: 10.3390/foods9091285
389. Paul BM, Jagadeesan G, Kannan G, Raj FJ, Annadurai Y, Piramanayagam S, et al. Exploring the hypoglycaemic efficacy of bio-accessed antioxidative polyphenolics in thermally processed Cucumis dipsaceus fruits – an in vitro and in silico study. Food Chem. (2024) 435:137577. doi: 10.1016/j.foodchem.2023.137577
390. Csekes E and Račková L. Skin aging, cellular senescence and natural polyphenols. Int J Mol Sci. (2021) 22:12641. doi: 10.3390/ijms222312641
391. Sun M, Deng Y, Cao X, Xiao L, Ding Q, Luo F, et al. Effects of natural polyphenols on skin and hair health: a review. Molecules. (2022) 27:7832. doi: 10.3390/molecules27227832
392. Yamasaki T, Takeshita S, Tomiya C, and Koikeda T. Effect of polyphenols from water chestnut pericarp on hair-related quality of life in healthy middle-aged, and older subjects: A randomized, placebo-controlled, double-blinded, parallel-group, comparison study. Glycative Stress Res. (2024) 11:21–32. doi: 10.24659/gsr.11.1_21
393. Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, and Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. (2010) 2:1106–31. doi: 10.3390/nu2111106
394. Amrati FEZ, Chebaibi M, Galvao de Azevedo R, Conte R, Slighoua M, Mssillou I, et al. Phenolic composition, wound healing, antinociceptive, and anti-cancer effects of Caralluma europaea extracts. Molecules. (2023) 28:1780. doi: 10.3390/molecules28041780
395. Montenegro L, Parenti C, Turnaturi R, and Pasquinucci L. Resveratrol-loaded lipid nanocarriers: Correlation between in vitro occlusion factor and in vivo skin hydrating effect. Pharmaceutics. (2017) 9:58. doi: 10.3390/pharmaceutics9040058
396. Archana A, Vijayasri K, Madhurim M, and Kumar C. Curcumin loaded nano cubosomal hydrogel: preparation, in vitro characterization and antibacterial activity. Chem Sci Trans. (2015) 4:75–80. doi: 10.7598/cst2015.913
397. Gabr SA and Alghadir AH. Evaluation of the biological effects of lyophilized hydrophilic extract of Rhus coriaria on myeloperoxidase (MPO) activity, wound healing, and microbial infections of skin wound tissues. Evid Based Complement Alternat Med. (2019) 2019:5861537. doi: 10.1155/2019/5861537
398. Pinsuwan S, Amnuaikit T, Ungphaiboon S, and Itharat A. Liposome-containing Hibiscus sabdariffa calyx extract formulations with increased antioxidant activity, improved dermal penetration and reduced dermal toxicity. J Med Assoc Thai. (2010) 93:S216–26.
399. Hanamura T, Uchida E, and Aoki H. Skin-lightening effect of a polyphenol extract from acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci Biotechnol Biochem. (2008) 72:3211–8. doi: 10.1271/bbb.80421
400. Gasparrini M, Forbes-Hernandez TY, Afrin S, Reboredo-Rodriguez P, Cianciosi D, Mezzetti B, et al. Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. Nutrients. (2017) 9:605. doi: 10.3390/nu9060605
401. Lu PH and Hsu CH. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement Ther Med. (2016) 25:159–63. doi: 10.1016/j.ctim.2016.03.004
402. Affonso RCL, Voytena APL, Fanan S, Pitz H, Coelho DS, Horstmann AL, et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxid Med Cell Longev. (2016) 2016:1923754. doi: 10.1155/2016/1923754
403. Tan A, Morton KR, Lee JW, Hartman R, and Lee G. Adverse childhood experiences and depressive symptoms: protective effects of dietary flavonoids. J Psychosom Res. (2020) 131:109957. doi: 10.1016/j.jpsychores.2020.109957
404. Pacifici F, Salimei C, Pastore D, Malatesta G, Ricordi C, Donadel G, et al. The protective effect of a unique mix of polyphenols and micronutrients against neurodegeneration induced by an in vitro model of Parkinson’s disease. Int J Mol Sci. (2022) 23:3110. doi: 10.3390/ijms23063110
405. Gan J, Zhang X, Ma C, Sun L, Feng Y, He Z, et al. Purification of polyphenols from Phyllanthus emblica L. pomace using macroporous resins: antioxidant activity and potential anti-Alzheimer's effects. J Food Sci. (2022) 87:1244–56. doi: 10.1111/1750-3841.16028
406. Wang J, Bi W, Cheng A, Freire D, Vempati P, Zhao W, et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer's disease—experimental approach and therapeutic implications. Front Aging Neurosci. (2014) 6:42. doi: 10.3389/fnagi.2014.00042
407. Ma X, Sun Z, Han X, Li S, Jiang X, Chen S, et al. Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front Neurosci. (2020) 13:1400. doi: 10.3389/fnins.2019.01400
408. Qi Y, Shang L, Liao Z, Su H, Jing H, Wu B, et al. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metab Brain Dis. (2019) 34:257–66. doi: 10.1007/s11011-018-0348-6
409. Malar DS, Prasanth MI, Brimson JM, Sharika R, Sivamaruthi BS, Chaiyasut C, et al. Neuroprotective properties of green tea (Camellia sinensis) in Parkinson’s disease: A review. Molecules. (2020) 25:3926. doi: 10.3390/molecules25173926
410. Zhao J, Liang Q, Sun Q, Chen C, Xu L, Ding Y, et al. (–)-Epigallocatechin-3-gallate (EGCG) inhibits fibrillation, disaggregates amyloid fibrils of α-synuclein, and protects PC12 cells against α-synuclein-induced toxicity. Rsc Advances. (2017) 7:32508–17. doi: 10.1039/C7RA03752J
411. Xu Y, Zhang Y, Quan Z, Wong W, Guo J, Zhang R, et al. Epigallocatechin gallate (EGCG) inhibits alpha-synuclein aggregation: A potential agent for Parkinson’s disease. Neurochem Res. (2016) 41:2788–96. doi: 10.1007/s11064-016-1995-9
412. Mamashli F, Meratan AA, Ghasemi A, Obeidi N, Salmani B, Atarod D, et al. Neuroprotective effect of propolis polyphenol-based nanosheets in cellular and animal models of rotenone-induced Parkinson’s disease. ACS Chem Neurosci. (2023) 14:851–63. doi: 10.1021/acschemneuro.2c00605
413. Chongtham A and Agrawal N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci Rep. (2016) 6:18736. doi: 10.1038/srep18736
414. Elifani F, Amico E, Pepe G, Capocci L, Castaldo S, Rosa P, et al. Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington’s disease. Hum Mol Genet. (2019) 28:4012–21. doi: 10.1093/hmg/ddz247
415. Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease. Mol Neurodegener. (2012) 7:12. doi: 10.1186/1750-1326-7-12
416. Abdolahi M, Jafarieh A, Sarraf P, Sedighiyan M, Yousefi A, Tafakhori A, et al. The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/ iNOS network in migraines: a clinical trial study from gene expression to clinical symptoms. Endocr Metab Immune Disord Drug Targets. (2019) 19:874–84. doi: 10.2174/1871530319666190212170140
417. Abdolahi M, Karimi E, Sarraf P, Tafakhori A, Siri G, Salehinia F, et al. The omega-3 and Nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial. BMC Res Notes. (2021) 14:283. doi: 10.1186/s13104-021-05700-x
418. Prieto K, Cao Y, Mohamed E, Trillo-Tinoco J, Sierra RA, Urueña C, et al. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. (2019) 5:134. doi: 10.1038/s41420-019-0214-2
419. Gao Y, Wang S, Dang S, Han S, Yun C, Wang W, et al. Optimized ultrasound-assisted extraction of total polyphenols from Empetrum nigrum and its bioactivities. J Chromatogr B. (2021) 1173:122699. doi: 10.1016/j.jchromb.2021.122699
420. Cheng Z, Zhang Z, Han Y, Wang J, Wang Y, Chen X, et al. A review on anti-cancer effect of green tea catechins. J Funct Foods. (2020) 74:104172. doi: 10.1016/j.jff.2020.104172
421. Deng H, Liu J, Xiao Y, Wu JL, and Jiao R. Possible mechanisms of dark tea in cancer prevention and management: A comprehensive review. Nutrients. (2023) 15:3903. doi: 10.3390/nu15183903
422. O’Neill EJ, Termini D, Albano A, and Tsiani E. Anti-cancer properties of theaflavins. Molecules. (2021) 26:987. doi: 10.3390/molecules26040987
423. Zhou K and Raffoul JJ. Potential anti-cancer properties of grape antioxidants. J Oncol. (2012) 2012:803294. doi: 10.1155/2012/803294
424. Kaur M, Agarwal C, and Agarwal R. Anti-cancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. (2009) 139:1806S–12S. doi: 10.3945/jn.109.106864
425. Cozmin M, Lungu II, Gutu C, Stefanache A, Duceac LD, Șoltuzu BD, et al. Turmeric: From spice to cure. A review of the anti-cancer, radioprotective and anti-inflammatory effects of turmeric sourced compounds. Front Nutr. (2024) 11:1399888. doi: 10.3389/fnut.2024.1399888
426. Zoi V, Kyritsis AP, Galani V, Lazari D, Sioka C, Voulgaris S, et al. The role of curcumin in cancer: a focus on the PI3K/Akt pathway. Cancers. (2024) 16:1554. doi: 10.3390/cancers16081554
427. Nurkolis F, Qhabibi FR, Yusuf VM, Bulain S, Praditya GN, Lailossa DG, et al. Anti-cancer properties of soy-based tempe: A proposed opinion for future meal. Front Oncol. (2022) 12:1054399. doi: 10.3389/fonc.2022.1054399
428. Nezbedova L, McGhie T, Christensen M, Heyes J, Nasef NA, and Mehta S. Onco-preventive and chemo-protective effects of apple bioactive compounds. Nutrients. (2021) 13:4025. doi: 10.3390/nu13114025
429. Tu SH, Chen LC, and Ho YS. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. J Food Drug Anal. (2017) 25:119–24. doi: 10.1016/j.jfda.2016.10.016
430. Kristo AS, Klimis-Zacas D, and Sikalidis AK. Protective role of dietary berries in cancer. Antioxidants. (2016) 5:37. doi: 10.3390/antiox5040037
431. Lamenza FF, Upadhaya P, Roth P, Shrestha S, Jagadeesha S, Horn N, et al. Berries vs. disease: revenge of the phytochemicals. Pharmaceuticals. (2024) 17:84. doi: 10.3390/ph17010084
432. Esmaeli M, Dehabadi MD, and Ghanbari A. Anti-cancer effects of pomegranate extract on breast cancer cell lines: a systematic review of the literature. Discover Med. (2025) 2:1–10. doi: 10.1007/s44337-025-00193-0
433. Rahmani AH, Alsahli MA, and Almatroodi SA. Potential ‘anti-tumor’ effects of pomegranates and its ingredients. Pharmacognosy Rev. (2017) 11:136. doi: 10.4103/phrev.phrev_25_17
434. Markellos C, Ourailidou ME, Gavriatopoulou M, Halvatsiotis P, Sergentanis TN, and Psaltopoulou T. Olive oil intake and cancer risk: A systematic review and meta-analysis. PloS One. (2022) 17:e0261649. doi: 10.1371/journal.pone.0261649
435. Bonfiglio C, Reddavide R, Cisternino AM, Campanella A, Fontana L, and Giannelli G. Protective effect of extra virgin olive oil on cancers, gastrointestinal cancers, and all-cause mortality: A competing risk analysis in a Southern Italian cohort. Cancers. (2024) 16:3575. doi: 10.3390/cancers16213575
436. Cirmi S, Maugeri A, Ferlazzo N, Gangemi S, Calapai G, Schumacher U, et al. Anti-cancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front Pharmacol. (2017) 8:420. doi: 10.3389/fphar.2017.00420
437. Li J, Zhao X, and Jiao B. Research progress on anti-cancer components in citrus and their action mechanisms. Food Sci. (2024) 45:328–40. doi: 10.7506/spkx1002-6630-20230728-301
438. Pandey P, Khan F, Alshammari N, Saeed A, Aqil F, and Saeed M. Updates on the anti-cancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front Pharmacol. (2023) 14:1154034. doi: 10.3389/fphar.2023.1154034
439. Talib WH, Atawneh S, Shakhatreh AN, Shakhatreh GAN, Hamed RA, and Al-Yasari IH. Anti-cancer potential of garlic bioactive constituents: Allicin, Z-ajoene, and organosulfur compounds. Pharmacia. (2024) 71:1–23. doi: 10.3897/pharmacia.71.e114556
440. Baladia E, Moñino M, Pleguezuelos E, Russolillo G, and Garnacho-Castaño MV. Broccoli consumption and risk of cancer: An updated systematic review and meta-analysis of observational studies. Nutrients. (2024) 16:1583. doi: 10.3390/nu16111583
441. Kaiser AE, Baniasadi M, Giansiracusa D, Giansiracusa M, Garcia M, Fryda Z, et al. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers. (2021) 13:4796. doi: 10.3390/cancers13194796
442. Kapała A, Szlendak M, and Motacka E. The anti-cancer activity of lycopene: a systematic review of human and animal studies. Nutrients. (2022) 14:5152. doi: 10.3390/nu14235152
443. Chapa-Oliver AM and Mejía-Teniente L. Capsaicin: From plants to a cancer-suppressing agent. Molecules. (2016) 21:931. doi: 10.3390/molecules21080931
444. Zhang W, Zhang Y, Fan J, Feng Z, and Song X. Pharmacological activity of capsaicin: Mechanisms and controversies. Mol Med Rep. (2024) 29:38. doi: 10.3892/mmr.2024.13162
445. Prasad S and Tyagi AK. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. (2015) 2015:142979. doi: 10.1155/2015/142979
446. Habib SHM, Makpol S, Hamid NAA, Das S, Ngah WZW, and Yusof YAM. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics. (2008) 63:807–13. doi: 10.1590/S1807-59322008000600017
447. Weh KM, Clarke J, and Kresty LA. Cranberries and cancer: An update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants. (2016) 5:27. doi: 10.3390/antiox5030027
448. Singh AP, Singh RK, Kim KK, Satyan KS, Nussbaum R, Torres M, et al. Cranberry proanthocyanidins are cytotoxic to human cancer cells and sensitize platinum-resistant ovarian cancer cells to paraplatin. Phytother Res. (2009) 23:1066–74. doi: 10.1002/ptr.2667
449. Abdelgawad SM, Hetta MH, Ibrahim MA, Balachandran P, Zhang J, Wang M, et al. Phytochemical investigation of Egyptian spinach leaves, a potential source for antileukemic metabolites: In vitro and in silico study. Rev Bras Farmacogn. (2022) 32:774–85. doi: 10.1007/s43450-022-00307-0
450. Jermnak U, Supsavhad W, Kunakornsawat S, Jaroensong T, Watcharasit P, Visitnonthachai D, et al. Anti-cancer potentials of Gynura procumbens leaves extract against two canine mammary cancer cell lines. Vet Med Sci. (2022) 8:69–84. doi: 10.1002/vms3.684
451. Mandrich L, Esposito AV, Costa S, and Caputo E. Chemical composition, functional and anti-cancer properties of carrot. Molecules. (2023) 28:7161. doi: 10.3390/molecules28207161
452. Pan Y, Lee YJ, Sin SI, Park SH, and Park KY. Anti-cancer activity of mineral-supplemented organically cultivated carrot on HT-29 cells and its anti-inflammatory effect on mice splenocytes. Appl Sci. (2023) 13:9209. doi: 10.3390/app13169209
453. Merkher Y, Kontareva E, Alexandrova A, Javaraiah R, Pustovalova M, and Leonov S. Anti-cancer properties of flaxseed proteome. Proteomes. (2023) 11:37. doi: 10.3390/proteomes11040037
454. Stepień AE, Trojniak J, and Tabarkiewicz J. Antioxidant and anti-cancer properties of flaxseed. Int J Mol Sci. (2025) 26:1226. doi: 10.3390/ijms26031226
455. Fonseca LR, Silva GR, Luís Â, Cardoso HJ, Correia S, Vaz CV, et al. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules. (2021) 26:2941. doi: 10.3390/molecules26102941
456. Zhang Y, Gu J, Wang Y, Zhao Z, Wang Z, and Li W. The anti-tumor activity and critical active compounds of polyphenols from Chinese dwarf cherry (Cerasus humilis). J Berry Res. (2023) 13:211–25. doi: 10.3233/JBR-230005
457. Hassan MK. Isolation and characterization of peanut (Arachis hypogaea) lectin and study of its anti-cancer properties. (2013) MSc thesis. National Institute of Technology, Rourkela, Odisha, India.
458. Cordeiro-Massironi KA, Soares-Freitas RAM, Sampaio GR, Pinaffi-Langley ACDC, Bridi R, de Camargo AC, et al. In vitro digestion of peanut skin releases bioactive compounds and increases cancer cell toxicity. Antioxidants. (2023) 12:1356. doi: 10.3390/antiox12071356
459. Fejes SZ, Blazovics A, Lemberkovics E, Petri G, Szöke E, and Kery A. Free radical scavenging and membrane protective effects of methanol extracts from Anthriscus cerefolium L. (Hoffm.) and Petroselinum crispum (Mill.) Nym. ex AW Hill. Phytotherapy Res. (2000) 14:362–5. doi: 10.1002/1099-1573(200008)14:5<362::AID-PTR554>3.0.CO;2-G
460. Thiviya P, Gunawardena N, Gamage A, Madhujith T, and Merah O. Apiaceae family as a valuable source of biocidal components and their potential uses in agriculture. Horticulturae. (2022) 8:614. doi: 10.3390/horticulturae8070614
461. Miraj S and Kiani S. Study of pharmacological effect of Thymus vulgaris: A review. Der Pharmacia Lettre. (2016) 8:315–20.
462. Ali A. Chemical composition, α-glucosidase inhibitory and anti-cancer activity of essential oil of Thymus vulgaris leaves. J Essent Oil-Bear Plants. (2021) 24:695–703. doi: 10.1080/0972060X.2021.1973575
463. Jaglanian A and Tsiani E. Inhibition of prostate cancer cell proliferation and survival by rosemary extract. FASEB J. (2019) 33:652.10. doi: 10.1096/fasebj.2019.33.1_supplement.652.10
464. Veenstra JP and Johnson JJ. Rosemary (Salvia rosmarinus): Health-promoting benefits and food preservative properties. Int J Nutr. (2021) 6:1–10. doi: 10.14302/issn.2379-7835.ijn-21-3874
465. Wang B, Wang J, and Zhao XH. Bioactivity of two polyphenols quercetin and fisetin against human gastric adenocarcinoma AGS cells as affected by two coexisting proteins. Molecules. (2022) 27:2877. doi: 10.3390/molecules27092877
466. Prakash MD, Stojanovska L, Feehan J, Nurgali K, Donald EL, Plebanski M, et al. Anti-cancer effects of polyphenol-rich sugarcane extract. PloS One. (2021) 16:e0247492. doi: 10.1371/journal.pone.0247492
467. Erden Tayhan S, Bilgin S, Yıldırım A, and Koç E. Biological screening of polyphenol derivatives for anti-proliferative, anti-apoptotic and anti-migrative activities in human breast cancer cell lines MCF-7. Chem Biodivers. (2023) 20:e202200872. doi: 10.1002/cbdv.202200872
468. Wu W, Xiang F, and He F. Polyphenols from Artemisia argyi leaves: Environmentally friendly extraction under high hydrostatic pressure and biological activities. Ind Crops Prod. (2021) 171:113951. doi: 10.1016/j.indcrop.2021.113951
469. Mechchate H, Costa de Oliveira R, Es-Safi I, Vasconcelos Mourao EM, Bouhrim M, Kyrylchuk A, et al. Antileukemic activity and molecular docking study of a polyphenolic extract from coriander seeds. Pharmaceutics. (2021) 14:770. doi: 10.3390/ph14080770
470. Girardelo JR, Munari EL, Dallorsoleta JC, Cechinel G, Goetten AL, Sales LR, et al. Bioactive compounds, antioxidant capacity and ‘anti-tumor’ al activity of ethanolic extracts from fruits and seeds of Eugenia involucrata DC. Food Res Int. (2020) 137:109615. doi: 10.1016/j.foodres.2020.109615
471. Wu Z, Gao R, Li H, Wang Y, Luo Y, Zou J, et al. New insight into the joint significance of dietary jujube polysaccharides and 6-gingerol in antioxidant and ‘anti-tumor’ activities. RSC Adv. (2021) 11:33219–34. doi: 10.1039/D1RA03640H
472. Chen BH, Hsieh CH, Tsai SY, Wang CY, and Wang CC. Anti-cancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci Rep. (2020) 10:5163. doi: 10.1038/s41598-020-62136-2
473. Kato K, Nagane M, Aihara N, Kamiie J, Miyanabe M, Hiraki S, et al. Lipid-soluble polyphenols from sweet potato exert ‘anti-tumor’ activity and enhance chemosensitivity in breast cancer. J Clin Biochem Nutr. (2021) 68:193–200. doi: 10.3164/jcbn.20-73
474. Gomez-Cadena A, Urueña C, Prieto K, Martinez-Usatorre A, Donda A, Barreto A, et al. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. (2016) 7:e2243–3. doi: 10.1038/cddis.2016.134
475. Yi J, Wang Z, Bai H, Yu X, Jing J, and Zuo L. Optimization of purification, identification and evaluation of the in vitro ‘anti-tumor’ activity of polyphenols from Pinus koraiensis pinecones. Molecules. (2015) 20:10450–67. doi: 10.3390/molecules200610450
476. Yi J, Wang Z, Bai H, Li L, Zhao H, Cheng C, et al. Polyphenols from pinecones of Pinus koraiensis induce apoptosis in colon cancer cells through the activation of caspase in vitro. RSC Adv. (2016) 6:5278–87. doi: 10.1039/C5RA24913A
477. Huang Y, Zhu X, Zhu Y, and Wang Z. Pinus koraiensis polyphenols: Structural identification, in vitro antioxidant activity, immune function and inhibition of cancer cell proliferation. Food Funct. (2021) 12:4176–98. doi: 10.1039/D0FO03347B
478. Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y, et al. Green tea polyphenol EGCG attenuates MDSCs-mediated immunosuppression through canonical and non-canonical pathways in a 4T1 murine breast cancer model. Nutrients. (2020) 12:1042. doi: 10.3390/nu12041042
479. Luz JRDD, López JA, Ferreira MP, de Sousa RM, Silva SVE, Almeida MDG, et al. In vitro antithrombotic, ‘anti-tumor’ and antiangiogenic activities of green tea polyphenols and its main constituent epigallocatechin-3-gallate. Processes. (2022) 11:76. doi: 10.3390/pr11010076
480. Yang CY, Ha W, Lin Y, Jiang K, Yang JL, and Shi YP. Polyphenols isolated from Xanthoceras sorbifolia husks and their anti-tumor and radical-scavenging activities. Molecules. (2016) 21:1694. doi: 10.3390/molecules21121694
481. Delgado-Roche L, González K, Mesta F, Couder B, Tavarez Z, Zavala R, et al. Polyphenolic fraction obtained from Thalassia testudinum marine plant and thalassiolin B exert cytotoxic effects in colorectal cancer cells and arrest tumor progression in a xenograft mouse model. Front Pharmacol. (2020) 11:592985. doi: 10.3389/fphar.2020.592985
482. Fahmi AA, El Raey MA, Ibrahim AY, Abdelfattah MA, Abdelmageed AM, and Sobeh M. A sulfated polyphenols-rich extract from Sabal yapa exhibits ‘anti-tumor’ activities in Ehrlich ascites carcinoma. Saudi J Biol Sci. (2021) 28:3117–25. doi: 10.1016/j.sjbs.2021.02.056
483. Wu X, Xue L, Tata A, Song M, Neto CC, and Xiao H. Bioactive components of polyphenol-rich and non-polyphenol-rich cranberry fruit extracts and their chemopreventive effects on colitis-associated colon cancer. J Agric Food Chem. (2020) 68:6845–53. doi: 10.1021/acs.jafc.0c02604
484. Liu G, Shi A, Wang N, Li M, He X, Yin C, et al. Polyphenolic proanthocyanidin-B2 suppresses proliferation of liver cancer cells and hepatocellular carcinogenesis through directly binding and inhibiting AKT activity. Redox Biol. (2020) 37:101701. doi: 10.1016/j.redox.2020.101701
485. Ferrario A, Luna M, Rucker N, Wong S, and Gomer CJ. Pro-apoptotic and anti-inflammatory properties of the green tea constituent epigallocatechin gallate increase photodynamic therapy responsiveness. Lasers Surg Med. (2011) 43:644–50. doi: 10.1002/lsm.21081
486. Bona NP, Pedra NS, Azambuja JH, Soares MS, Spohr L, Gelsleichter NE, et al. Tannic acid elicits selective ‘anti-tumor’ al activity in vitro and inhibits cancer cell growth in a preclinical model of glioblastoma multiforme. Metab Brain Dis. (2020) 35:283–93. doi: 10.1007/s11011-019-00519-9
487. Chatterjee K, Mukherjee S, Vanmanen J, Banerjee P, and Fata JE. Dietary polyphenols, resveratrol and pterostilbene exhibit ‘anti-tumor’ activity on an HPV E6-positive cervical cancer model: an in vitro and in vivo analysis. Front Oncol. (2019) 9:352. doi: 10.3389/fonc.2019.00352
488. Wu H, Li C, Cui M, Guo H, Chen S, Du JE, et al. Polyphenols from Hippophae rhamnoides suppressed colon cancer growth by regulating miRNA-mediated cell cycle arrest and apoptosis in vitro and in vivo. J Funct Foods. (2021) 87:104780. doi: 10.1016/j.jff.2021.104780
489. Juli G, Oliverio M, Bellizzi D, Gallo Cantafio ME, Grillone K, Passarino G, et al. Anti-tumor activity and epigenetic impact of the polyphenol oleacein in multiple myeloma. Cancers. (2019) 11:990. doi: 10.3390/cancers11070990
490. Yang S, Wang C, Li X, Wu C, Liu C, Xue Z, et al. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J Food Sci. (2021) 86:614–27. doi: 10.1111/1750-3841.15598
491. Yang R, Shan S, Zhang C, Shi J, Li H, and Li Z. Inhibitory effects of bound polyphenol from foxtail millet bran on colitis-associated carcinogenesis by the restoration of gut microbiota in a mice model. J Agric Food Chem. (2020) 68:3506–17. doi: 10.1021/acs.jafc.0c00370
492. Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. (2012) 12:163–76. doi: 10.2174/156652412798889063
493. Gao L, Gou N, Yuan E, and Ren J. Bioactivity-oriented purification of polyphenols from Cinnamomum cassia Presl. with anti-proliferation effects on colorectal cancer cells. Plant Foods Hum Nutr. (2020) 75:561–8. doi: 10.1007/s11130-020-00846-8
494. Alshuniaber MA, Krishnamoorthy R, and AlQhtani WH. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J Biol Sci. (2021) 28:459–64. doi: 10.1016/j.sjbs.2020.10.029
495. Charoensiddhi S, Chanput WP, and Sae-Tan S. Gut microbiota modulation, anti-diabetic and anti-inflammatory properties of polyphenol extract from mung bean seed coat (Vigna radiata L.). Nutrients. (2022) 14:2275. doi: 10.3390/nu14112275
496. de Jesús Romero-Prado MM, Curiel-Beltrán JA, Miramontes-Espino MV, Cardona-Muñoz EG, Rios-Arellano A, and Balam-Salazar LB. Dietary flavonoids added to pharmacological antihypertensive therapy are effective in improving blood pressure. Basic Clin Pharmacol Toxicol. (2015) 117:57–64. doi: 10.1111/bcpt.12360
497. He L, Su Z, and Wang S. The anti-obesity effects of polyphenols: a comprehensive review of molecular mechanisms and signal pathways in regulating adipocytes. Front Nutr. (2024) 11:1393575. doi: 10.3389/fnut.2024.1393575
498. Aloo SO, Ofosu FK, Kim NH, Kilonzi SM, and Oh DH. Insights on dietary polyphenols as agents against metabolic disorders: obesity as a target disease. Antioxidants. (2023) 12:416. doi: 10.3390/antiox12020416
499. Sabadashka M, Hertsyk D, Strugała-Danak P, Dudek A, Kanyuka O, Kucharska AZ, et al. Anti-diabetic and antioxidant activities of red wine concentrate enriched with polyphenol compounds under experimental diabetes in rats. Antioxidants. (2021) 10:1399. doi: 10.3390/antiox10091399
500. Wang T, Li X, Zhou B, Li H, Zeng J, and Gao W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J Funct Foods. (2015) 13:276–88. doi: 10.1016/j.jff.2014.12.049
501. Kumar Y, Singhal S, Tarafdar A, Pharande A, Ganesan M, and Badgujar PC. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. (2020) 52:102114. doi: 10.1016/j.algal.2020.102114
502. Parigi SM, Eldh M, Larssen P, Gabrielsson S, and Villablanca EJ. Breast milk and solid food shaping intestinal immunity. Front Immunol. (2015) 6:415. doi: 10.3389/fimmu.2015.00415
503. D'Angelo S. Polyphenols and athletic performance: A review on human data. In: Soto-Hernández M, García-Mateos R, and Palma-Tenango M, editors. Plant Physiological Aspects of Phenolic Compounds. IntechOpen, London, UK (2019). pp.1–15. doi: 10.5772/intechopen.85031
504. Tanaka M and Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. (2017) 66:515–22. doi: 10.1016/j.alit.2017.07.010
505. Gomez-Gallego C, Garcia-Mantrana I, Salminen S, and Collado MC. The human milk microbiome and factors influencing its composition and activity. Semin Fetal Neonatal Med. (2016) 21:400–5. doi: 10.1016/j.siny.2016.05.003
506. Stinson LF. Establishment of the early-life microbiome: a DOHaD perspective. J Dev Orig Health Dis. (2020) 11:201–10. doi: 10.1017/S2040174419000588
507. Xu L, Lochhead P, Ko Y, Claggett B, Leong RW, and Ananthakrishnan AN. Systematic review with meta-analysis: breastfeeding and the risk of Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. (2017) 46:780–9. doi: 10.1111/apt.14291
508. Asnicar F, Manara S, Zolfo M, Truong DT, Scholz M, Armanini F, et al. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems. (2017) 2:e00164–16. doi: 10.1128/mSystems.00164-16
509. Jiang Z, Liu Y, Zhu Y, Yang J, Sun L, Chai X, et al. Characteristic chromatographic fingerprint study of short-chain fatty acids in human milk, infant formula, pure milk and fermented milk by gas chromatography–mass spectrometry. Int J Food Sci Nutr. (2016) 67:632–40. doi: 10.1080/09637486.2016.1195798
510. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. (2004) 23:588S–95S. doi: 10.1080/07315724.2004.10719428
511. Ceballos-Sánchez D, Casanova-Crespo S, Rodríguez-Lagunas MJ, Castell M, Massot-Cladera M, and Pérez-Cano FJ. A maternal diet enriched in fiber and polyphenols during pregestation, gestation, and lactation has an intestinal trophic effect in both the dam and the offspring. Biol Life Sci Forum. (2023) 29:2. doi: 10.3390/IECN2023-15970
512. Al-Shaikh MA, Al-Mufarrej SI, and Mogawer HH. Effect of fenugreek seeds (Trigonella foenumgraecum L.) on lactational performance of dairy goat. J Appl Anim Res. (1999) 16:177–83. doi: 10.1080/09712119.1999.9706279
513. Kwan SH and Abdul-Rahman PS. Clinical study on plant Galactagogue worldwide in promoting women’s lactation: A scoping review. Plant Foods Hum Nutr. (2021) 76:257–69. doi: 10.1007/s11130-021-00901-y
514. Wani SA and Kumar P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. (2018) 17:97–106. doi: 10.1016/j.jssas.2016.01.007
515. Sevrin T, Alexandre-Gouabau MC, Castellano B, Aguesse A, Ouguerram K, Ngyuen P, et al. Impact of fenugreek on milk production in rodent models of lactation challenge. Nutrients. (2019) 11:2571. doi: 10.3390/nu11112571
516. Sevrin T, Boquien CY, Gandon A, Grit I, de Coppet P, Darmaun D, et al. Fenugreek stimulates the expression of genes involved in milk synthesis and milk flow through modulation of insulin/GH/IGF-1 axis and oxytocin secretion. Genes. (2020) 11:1208. doi: 10.3390/genes11101208
517. Afzal A, Hussain T, Hameed A, Shahzad M, Mazhar MU, and Yang G. Dietary Moringa oleifera alters periparturient plasma and milk biochemical indicators and promotes productive performance in goats. Front Vet Sci. (2022) 8:787719. doi: 10.3389/fvets.2021.787719
518. Kekana TW, Marume U, and Nherera-Chokuda FV. Prepartum supplementation of Moringa oleifera leaf meal: Effects on health of the dam, colostrum quality, and acquisition of immunity in the calf. J Dairy Sci. (2022) 105:5813–21. doi: 10.3168/jds.2021-21535
519. Olvera-Aguirre G, Mendoza-Taco MM, Arcos-Álvarez DN, Piñeiro-Vázquez AT, Moo-Huchin VM, Canul-Solís JR, et al. Effect of feeding lactating ewes with Moringa oleifera leaf extract on milk yield, milk composition and preweaning performance of ewe/lamb pair. Animals. (2020) 10:1117. doi: 10.3390/ani10071117
520. Cheng WN, Jeong CH, Seo HG, and Han SG. Moringa extract attenuates inflammatory responses and increases gene expression of casein in bovine mammary epithelial cells. Animals. (2019) 9:391. doi: 10.3390/ani9070391
521. Liu J, Ma G, Wang Y, and Zhang Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod Domest Anim. (2020) 55:711–9. doi: 10.1111/rda.13670
522. Mustofa Y, Yuliani FS, Purwono S, Sadewa AH, Damayanti E, and Heriyanto DS. Polyherbal formula (ASILACT®) induces milk production in lactating rats through upregulation of α-lactalbumin and aquaporin expression. BMC Complement Med Ther. (2020) 20:1–8. doi: 10.1186/s12906-020-03152-7
523. Liu H, Hua Y, Luo H, Shen Z, Tao X, and Zhu X. An herbal galactagogue mixture increases milk production and aquaporin protein expression in the mammary glands of lactating rats. Evid Based Complement Altern Med. (2015) 2015:760585. doi: 10.1155/2015/760585
524. Capasso R, Aviello G, Capasso F, Savino F, Izzo AA, Lembo F, et al. Silymarin BIO-C®, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine. (2009) 16:839–44. doi: 10.1016/j.phymed.2009.02.007
525. Soka S, Alam H, Boenjamin N, Agustina TW, and Suhartono MT. Effect of Sauropus androgynus leaf extracts on the expression of prolactin and oxytocin genes in lactating BALB/C mice. Lifestyle Genom. (2010) 3:31–6. doi: 10.1159/000319710
526. Tyson JE, Khojandi M, Illig K, Huth J, and Andreassen B. The influence of prolactin secretion on human lactation. J Clin Endocrinol Metab. (1975) 40:764–73. doi: 10.1210/jcem-40-5-764
527. Faraz A, Waheed A, Nazir MM, Hameed A, Tauqir NA, Mirza RH, et al. Impact of oxytocin administration on milk quality, reproductive performance and residual effects in dairy animals—A review. Punjab Univ J Zool. (2020) 35:61–7. doi: 10.17582/journal.pujz/2020.35.1.61.67
528. Sani NIA, Kawu MU, and Bako IG. Effects of Launaea taraxacifolia and resveratrol on milk yield and serum prolactin and oxytocin levels: a lactogenic study. Int J Vet Sci Med. (2019) 7:71–7. doi: 10.1080/23144599.2019.1694307
529. Lin M, Wang N, Yao B, Zhong Y, Lin Y, and You T. Quercetin improves postpartum hypogalactia in milk-deficient mice via stimulating prolactin production in pituitary gland. Phytother Res. (2018) 32:1511–20. doi: 10.1002/ptr.6079
530. Cao L, Wang T, Mi X, Ji P, Zhao X, and Zhang Y. Exploring the action mechanism of the active ingredient of quercetin in Ligustrum lucidum on the mouse mastitis model based on network pharmacology and molecular biology validation. Evid Based Complement Altern Med. (2022) 2022:4236222. doi: 10.1155/2022/4236222
531. Zhao Y, Yu S, Zhao H, Li L, Li Y, Tu Y, et al. Lipidomic profiling using GC and LC-MS/MS revealed the improved milk quality and lipid composition in dairy cows supplemented with citrus peel extract. Food Res Int. (2022) 161:111767. doi: 10.1016/j.foodres.2022.111767
532. Ziauddeen N, Rosi A, Del Rio D, Amoutzopoulos B, Nicholson S, Page P, et al. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014). Eur J Nutr. (2019) 58:3183–98. doi: 10.1007/s00394-018-1862-3
533. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remon A, Quintana M, Corella D, Pinto X, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. (2012) 29:773–82. doi: 10.3233/JAD-2012-1117
534. Goñi I and Hernández-Galiot A. Intake of nutrient and non-nutrient dietary antioxidants: Contribution of macromolecular antioxidant polyphenols in an elderly Mediterranean population. Nutrients. (2019) 11:2165. doi: 10.3390/nu11092165
535. Miranda AM, Steluti J, Fisberg RM, and Marchioni DM. Dietary intake and food contributors of polyphenols in adults and elderly adults of Sao Paulo: A population-based study. Br J Nutr. (2016) 115:1061–70. doi: 10.1017/S0007114515005061
536. Kim HY, Kim OH, and Sung MK. Effects of phenol-depleted and phenol-rich diets on blood markers of oxidative stress, and urinary excretion of quercetin and kaempferol in healthy volunteers. J Am Coll Nutr. (2003) 22:217–23. doi: 10.1080/07315724.2003.10719296
537. Corbo F, Brunetti G, Crupi P, Bortolotti S, Storlino G, Piacente L, et al. Effects of sweet cherry polyphenols on enhanced osteoclastogenesis associated with childhood obesity. Front Immunol. (2019) 10:1001. doi: 10.3389/fimmu.2019.01001
538. Whyte AR and Williams CM. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition. (2015) 31:531–4. doi: 10.1016/j.nut.2014.09.013
539. Darzi M, Abbasi K, Ghiasvand R, Akhavan Tabib M, and Rouhani MH. The association between dietary polyphenol intake and attention-deficit hyperactivity disorder: A case-control study. BMC Pediatr. (2022) 22:700. doi: 10.1186/s12887-022-03768-3
540. Mengfan F, Zhang C, Liu B, Liu S, Shi D, Liu J, et al. Effect of polyphenol compound formula on relieving exercise fatigue in mice. Food Machinery. (2024) 39:145–52. doi: 10.13652/j.spjx.1003.5788.2023.80342
541. Guo X, Tresserra-Rimbau A, Estruch R, Martínez-González MA, Medina-Remón A, Fitó M, et al. Polyphenol levels are inversely correlated with body weight and obesity in an elderly population after 5 years of follow up (the randomized PREDIMED study). Nutrients. (2017) 9:452. doi: 10.3390/nu9050452
542. Guglielmetti S, Bernardi S, Del Bo’ C, Cherubini A, Porrini M, Gargari G, et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: Study protocol of the MaPLE randomised controlled trial. BMC Geriatr. (2020) 20:1–10. doi: 10.1186/s12877-020-1472-9
543. Joyner MJ. Genetic approaches for sports performance: how far away are we? Sports Med. (2019) 49:199–204. doi: 10.1007/s40279-019-01164-z
544. Kaufman M, Nguyen C, Shetty M, Oppezzo M, Barrack M, and Fredericson M. Popular dietary trends’ impact on athletic performance: a critical analysis review. Nutrients. (2023) 15:3511. doi: 10.3390/nu15163511
545. Nikawa T, Ulla A, and Sakakibara I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules. (2021) 26:4887. doi: 10.3390/molecules26164887
546. Sorrenti V, Fortinguerra S, Caudullo G, and Buriani A. Deciphering the role of polyphenols in sports performance: From nutritional genomics to the gut microbiota toward phytonutritional epigenomics. Nutrients. (2020) 12:1265. doi: 10.3390/nu12051265
547. Vargas-Mendoza N, Angeles-Valencia M, Morales-González Á, Madrigal-Santillán EO, Morales-Martínez M, Madrigal-Bujaidar E, et al. Oxidative stress, mitochondrial function and adaptation to exercise: new perspectives in nutrition. Life. (2021) 11:1269. doi: 10.3390/life11111269
548. Myburgh KH. Polyphenol supplementation: benefits for exercise performance or oxidative stress? Sports Med. (2014) 44:57–70. doi: 10.1007/s40279-014-0151-4
549. Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, et al. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PloS One. (2013) 8:e72215. doi: 10.1371/journal.pone.0072215
550. Clemente-Suárez VJ, Bustamante-Sanchez Á, Mielgo-Ayuso J, Martínez-Guardado I, Martín-Rodríguez A, and Tornero-Aguilera JF. Antioxidants and sports performance. Nutrients. (2023) 15:2371. doi: 10.3390/nu15102371
551. Paoli A, Cerullo G, Bianco A, Neri M, Gennaro F, Charrier D, et al. Not only protein: Dietary supplements to optimize the skeletal muscle growth response to resistance training: The current state of knowledge. J Hum Kinet. (2024) 91:225. doi: 10.5114/jhk/18666
552. Malaguti M, Angeloni C, and Hrelia S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid Med Cell Longev. (2013) 2013:825928. doi: 10.1155/2013/825928
553. Sellami M, Slimeni O, Pokrywka A, Kuvačić G, Hayes LD, Milic M, et al. Herbal medicine for sports: a review. J Int Soc Sports Nutr. (2018) 15:14. doi: 10.1186/s12970-018-0218-y
554. Keli SO, Hertog MG, Feskens EJ, and Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. (1996) 156:637–42. doi: 10.1001/archinte.1996.00440060059007
555. Otsuka Y, Egawa K, Kanzaki N, Izumo T, Rogi T, and Shibata H. Quercetin glycosides prevent dexamethasone-induced muscle atrophy in mice. Biochem Biophys Rep. (2019) 18:100618. doi: 10.1016/j.bbrep.2019.100618
556. MacRae HS and Mefferd KM. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int J Sport Nutr Exerc Metab. (2006) 16:405–19. doi: 10.1123/ijsnem.16.4.405
557. Sgrò P, Ceci R, Lista M, Patrizio F, Sabatini S, Felici F, et al. Quercetin modulates IGF-I and IGF-II levels after eccentric exercise-induced muscle-damage: A placebo-controlled study. Front Endocrinol. (2021) 12:745959. doi: 10.3389/fendo.2021.745959
558. Martin-Rincon M, Gelabert-Rebato M, Galvan-Alvarez V, Gallego-Selles A, Martinez-Canton M, Lopez-Rios L, et al. Supplementation with a mango leaf extract (Zynamite®) in combination with quercetin attenuates muscle damage and pain and accelerates recovery after strenuous damaging exercise. Nutrients. (2020) 12:614. doi: 10.3390/nu12030614
559. de Sousa BRV, de Lima Tavares Toscano L, de Almeida Filho EJB, Sena KF, Costa MS, de Souza Cunha RC, et al. Purple grape juice improves performance of recreational runners, but the effect is genotype dependent: a double blind, randomized, controlled trial. Genes Nutr. (2022) 17:9. doi: 10.1186/s12263-022-00710-1
560. de Lima Tavares Toscano L, Silva AS, de França ACL, de Sousa BRV, de Almeida Filho EJB, da Silveira Costa M, et al. A single dose of purple grape juice improves physical performance and antioxidant activity in runners: a randomized, crossover, double-blind, placebo study. Eur J Nutr. (2020) 59:2997–3007. doi: 10.1007/s00394-019-02139-6
561. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. (2012) 15:675–90. doi: 10.1016/j.cmet.2012.04.003
562. Hart N, Sarga L, Csende Z, Koltai E, Koch LG, Britton SL, et al. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem Toxicol. (2013) 61:53–9. doi: 10.1016/j.fct.2013.01.051
563. Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, et al. Health benefits of resveratrol: Evidence from clinical studies. Med Res Rev. (2019) 39:1851–91. doi: 10.1002/med.21565
564. Liu K, Zhou R, Wang B, and Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. (2014) 99:1510–9. doi: 10.3945/ajcn.113.082024
565. Tanabe Y, Chino K, Ohnishi T, Ozawa H, Sagayama H, Maeda S, et al. Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand J Med Sci Sports. (2019) 29:524–34. doi: 10.1111/sms.13373
566. McFarlin BK, Venable AS, Henning AL, Sampson JNB, Pennel K, Vingren JL, et al. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. (2016) 5:72–8. doi: 10.1016/j.bbacli.2016.02.003
567. Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GG, and Hu J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients. (2017) 9:1146. doi: 10.3390/nu9101146
568. Qiao Y, Sun J, Xia S, Tang X, Shi Y, and Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. (2014) 5:1241–9. doi: 10.1039/C3FO60630A
569. Di Meo F, Margarucci S, Galderisi U, Crispi S, and Peluso G. Curcumin, gut microbiota, and neuroprotection. Nutrients. (2019) 11:2426. doi: 10.3390/nu11102426
570. Dey P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. (2019) 147:104367. doi: 10.1016/j.phrs.2019.104367
571. Atan RM, Ersoy G, and Çakıcı Ç. Effects of Hardaliye, a fermented grape drink, on oxidative stress, lipid profile, and blood pressure in young soccer players: A randomized controlled trial. J Am Nutr Assoc. (2024) 43:356–64. doi: 10.1080/27697061.2023.2291789
572. Valder S, Staltner R, Bizjak DA, Esatbeyoglu T, Herdegen V, Köpsel M, et al. Effect of sugar- and polyphenol-rich, diluted cloudy apple juice on the intestinal barrier after moderate endurance exercise and in ultra-marathon runners. Nutrients. (2024) 16:1353. doi: 10.3390/nu16091353
573. Morton L, Paton C, and Braakhuis A. The effects of polyphenol supplementation on BDNF, cytokines and cognition in trained male cyclists following acute ozone exposure during high-intensity cycling. Nutrients. (2024) 16:233. doi: 10.3390/nu16020233
574. Poppas A. Cardiovascular disease risk: A focus on women. JACC Case Rep. (2020) 2:168–70. doi: 10.1016/j.jaccas.2019.12.013
575. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American heart association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2017 Update: A report from the American heart association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
576. Liu X, Du X, Han G, and Gao W. Association between tea consumption and risk of cognitive disorders: A dose-response meta-analysis of observational studies. Oncotarget. (2017) 8:43306–21. doi: 10.18632/oncotarget.17429
577. Huang H, Chen G, Liao D, Zhu Y, and Xue X. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. (2016) 6:23625. doi: 10.1038/srep23625
578. Williamson G. The role of polyphenols in modern nutrition. Nutr Bull. (2017) 42:226–35. doi: 10.1111/nbu.12278
579. Gormaz JG, Valls N, Sotomayor C, Turner T, and Rodrigo R. Potential role of polyphenols in the prevention of cardiovascular diseases: Molecular bases. Curr Med Chem. (2016) 23:115–28. doi: 10.2174/0929867323666151127201732
580. Mendonça RD, Carvalho NC, Martin-Moreno JM, Pimenta AM, Lopes ACS, Gea A, et al. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr Metab Cardiovasc Dis. (2019) 29:69–78. doi: 10.1016/j.numecd.2018.09.012
581. Murillo GA and Fernandez ML. The relevance of dietary polyphenols in cardiovascular protection. Curr Pharm Des. (2017) 23:2444–52. doi: 10.2174/1381612823666170329144307
582. Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, and Schmitz HH. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem. (2006) 54:1571–6. doi: 10.1021/jf0525941
583. Lazarus SA, Hammerstone JF, Adamson GE, and Schmitz HH. High-performance liquid chromatography/mass spectrometry analysis of proanthocyanidins in food and beverages. Methods Enzymol. (2001) 335:46–57. doi: 10.1016/S0076-6879(01)35230-8
584. Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem. (2003) 51:7513–21. doi: 10.1021/jf034815d
585. Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol Aspects Med. (2018) 61:76–82. doi: 10.1016/j.mam.2017.05.002
586. McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, and Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. (2012) 95:454–64. doi: 10.3945/ajcn.111.016634
587. Cassidy A, Bertoia M, Chiuve S, Flint A, Forman J, and Rimm EB. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am J Clin Nutr. (2016) 104:587–94. doi: 10.3945/ajcn.116.133132
588. Uttara B, Singh AV, Zamboni P, and Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. (2009) 7:65–74. doi: 10.2174/157015909787602823
589. Butterfield DA and Boyd-Kimball D. Amyloid β-Peptide (1-42) Contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. (2004) 14:426–32. doi: 10.1111/j.1750-3639.2004.tb00087.x
590. Mertaş B and Boşgelmez İİ. The role of genetic, environmental, and dietary factors in Alzheimer’s disease: A narrative review. Int J Mol Sci. (2025) 26:1222. doi: 10.3390/ijms26031222
591. Pulliam JF, Jennings CD, Kryscio RJ, Davis DG, Wilson D, Montine TJ, et al. Association of HFE mutations with neurodegeneration and oxidative stress in Alzheimer's disease and correlation with APOE. Am J Med Genet B Neuropsychiatr Genet. (2003) 119:48–53. doi: 10.1002/ajmg.b.10069
592. Bird TD. Genetic aspects of Alzheimer disease. Genet Med. (2008) 10:231–9. doi: 10.1097/GIM.0b013e31816b64dc
593. Verbon EH, Post JA, and Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. (2012) 511:1–6. doi: 10.1016/j.gene.2012.08.038
594. Son Y, Kim S, Chung HT, and Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. (2013) 528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1
595. World Health Organization. Global oral health status report: towards universal health coverage for oral health by 2030 Vol. 1. World Health Organization (2022) Geneva, Switzerland. Available online at: https://www.who.int/publications/i/item/9789240061484 (Accessed October 7, 2025).
596. Łuczaj W and Skrzydlewska E. Antioxidative properties of black tea. Prev Med. (2005) 40:910–8. doi: 10.1016/j.ypmed.2004.10.014
597. Milgrom P, Riedy CA, Weinstein P, Tanner ACR, Manibusan L, and Bruss J. Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6-to 36-month-old children. Community Dent Oral Epidemiol. (2000) 28:295–306. doi: 10.1034/j.1600-0528.2000.280408.x
598. Matsumoto M, Minami T, Sasaki H, Sobue S, Hamada S, and Ooshima T. Inhibitory effects of oolong tea extract on caries–inducing properties of mutans Streptococci. Caries Res. (1999) 33:441–5. doi: 10.1159/000016549
599. Fernandez MT, Mira ML, Florêncio MH, and Jennings KR. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J Inorg Biochem. (2002) 92:105–11. doi: 10.1016/S0162-0134(02)00511-1
600. Mladěnka P, Macáková K, Filipský T, Zatloukalová L, Jahodář L, Bovicelli P, et al. In vitro analysis of iron chelating activity of flavonoids. J Inorg Biochem. (2011) 105:693–701. doi: 10.1016/j.jinorgbio.2011.02.003
601. Sęczyk Ł, Świeca M, Kapusta I, and Gawlik-Dziki U. Protein–phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules. (2019) 24:408. doi: 10.3390/molecules24030408
Keywords: antioxidant, bioavailability, biological properties, immunological properties, polyphenols
Citation: Saad AM, Mohammed DM, Alkafaas SS, Ghosh S, Negm SH, Salem HM, Fahmy MA, Semary HE, Ibrahim EH, AbuQamar SF, El-Tarabily KA and El-Saadony MT (2025) Dietary polyphenols and human health: sources, biological activities, nutritional and immunological aspects, and bioavailability– a comprehensive review. Front. Immunol. 16:1653378. doi: 10.3389/fimmu.2025.1653378
Received: 24 June 2025; Accepted: 29 September 2025;
Published: 03 November 2025.
Edited by:
Jing Gao, National Engineering Research Center for Oil Tea, ChinaReviewed by:
Jhon Carlos Castaño, University of Quindío, ColombiaMd. Sakhawot Hossain, Jashore University of Science and Technology, Bangladesh
Copyright © 2025 Saad, Mohammed, Alkafaas, Ghosh, Negm, Salem, Fahmy, Semary, Ibrahim, AbuQamar, El-Tarabily and El-Saadony. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Synan F. AbuQamar, c2FidXFhbWFyQHVhZXUuYWMuYWU=; Khaled A. El-Tarabily, a3RhcmFiaWx5QHVhZXUuYWMuYWU=
 Ahmed M. Saad
Ahmed M. Saad Dina Mostafa Mohammed
Dina Mostafa Mohammed Samar Sami Alkafaas
Samar Sami Alkafaas Soumya Ghosh
Soumya Ghosh Shaimaa H. Negm
Shaimaa H. Negm Heba M. Salem
Heba M. Salem Mohamed A. Fahmy
Mohamed A. Fahmy Hatem E. Semary9
Hatem E. Semary9 Essam H. Ibrahim
Essam H. Ibrahim Synan F. AbuQamar
Synan F. AbuQamar Khaled A. El-Tarabily
Khaled A. El-Tarabily Mohamed T. El-Saadony
Mohamed T. El-Saadony