- 1Department of Anorectal, Kunming Municipal Hospital of Traditional Chinese Medicine, The Third Affiliated Hospital of Yunnan University of Chinese Medicine, Kunming, Yunnan, China
- 2Department of Orthopedics, Kunming Municipal Hospital of Traditional Chinese Medicine, The Third Affiliated Hospital of Yunnan University of Chinese Medicine, Kunming, Yunnan, China
Gastrointestinal (GI) cancers remain a leading cause of global cancer morbidity and mortality, demanding novel therapeutic strategies that overcome existing limitations. Nanomedicine has recently emerged as a transformative approach, offering the potential to significantly enhance immunotherapy outcomes through precision targeting and modulation of tumour immune microenvironments. This review discusses the principal categories of precision-engineered nanoparticles—including lipid-based carriers, polymeric systems, protein-derived formulations, and metallic-hybrid composites—emphasising their capacity for targeted immune modulation and improved pharmacokinetic profiles. These nanoparticle platforms strategically intervene across multiple stages of the cancer-immunity cycle, facilitating antigen presentation, T-cell activation, and cytotoxic lymphocyte infiltration, and augmenting immune checkpoint blockade efficacy. Clinically approved nanoformulations such as Abraxane, Doxil, Onivyde, and emerging mRNA-based nanovaccines highlight promising translational outcomes in GI malignancies, demonstrating improved therapeutic indices and reduced systemic toxicity. Nonetheless, clinical implementation remains challenged by nanoparticle complexity, heterogeneous tumour biology, clearance mechanisms, and toxicity concerns. Future success will depend on integrated strategies combining advanced nanoparticle engineering, precise administration routes, rigorous translational validation, and rational therapeutic combinations to realise the full potential of nanomedicine-based immunotherapies in gastrointestinal oncology.
Introduction
Worldwide, gastrointestinal (GI) tract cancers accounted for 26.0% of all new cancer diagnoses and 35.0% of cancer-related deaths in 2018 (1). By 2040, the global incidence of GI cancers is projected to rise to approximately 7.5 million cases annually, resulting in an estimated 5.6 million deaths (2). In 2020, the lifetime probability of developing and dying from a GI malignancy was 8.20% (95% CI 8.18–8.21) and 6.17% (95% CI 6.16–6.18), respectively. Colorectal cancer constitutes the largest share of this burden, accounting for 38.5% of lifelong incidence and 28.2% of GI cancer mortality, followed by cancers of the stomach, liver, oesophagus, pancreas, and gallbladder (3).
Despite therapeutic advances, cancer continues to impose a substantial global health burden, compelling ongoing efforts to develop novel preventive, therapeutic, and supportive strategies to enhance patient outcomes (4). Immunotherapy has transformed the oncology landscape by leveraging endogenous immune mechanisms to generate potent and targeted antitumour responses, often demonstrating reduced toxicity compared to conventional chemotherapy (5, 6). These biologic or synthetic immunotherapeutic agents stimulate innate and adaptive immune pathways to counteract immune-evasive mechanisms exploited by tumours. As such, immunotherapies hold promise for achieving durable remission and, in some cases, complete tumour eradication. However, precise immune regulation remains challenging, as these therapies may induce significant immune-related adverse events, including autoimmunity and systemic inflammation (7).
Nonetheless, clinical outcomes from immunotherapy remain constrained by suboptimal response rates, limited therapeutic efficacy, and safety concerns (8, 9). Many immunotherapeutics suffer from unfavourable pharmacokinetic profiles, characterised by poor solubility, inadequate stability, and rapid clearance, thus limiting sustained therapeutic effectiveness (10). Moreover, severe adverse reactions, such as hypersensitivity and inflammatory responses, occasionally result in serious or fatal outcomes (11). The immunosuppressive tumour microenvironment (iTME) further restricts the effective delivery and activation of immune agents, while the inherent low immunogenicity of tumours, alongside the accumulation of regulatory T cells, myeloid-derived suppressor cells, and suppressive cytokines within the iTME, collectively undermine immunotherapy’s efficacy (12, 13).
GI malignancies exhibit distinct biological characteristics that influence their immunotherapeutic responses. Colorectal, gastric, pancreatic, and oesophageal cancers generally present immunologically “cold” tumour microenvironments (TME), characterised by low immune cell infiltration, enriched immunosuppressive populations such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumour-associated macrophages, along with dense fibrotic stroma. These collectively impede effective immune cell infiltration and activation, significantly limiting anti-tumour immunity (14). Current immunotherapy, largely based on programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) blockade, has achieved notable but moderate successes in GI cancers. Phase 3 clinical trials have validated PD-1 inhibitors such as nivolumab for advanced gastric cancer and pembrolizumab, nivolumab, tislelizumab, and camrelizumab for advanced oesophageal cancer, yet objective response rates (ORR) hover around only 15% (15). Particularly in metastatic colorectal cancer (mCRC), the therapeutic benefit of anti-PD-1 therapy is restricted predominantly to microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) subsets, whereas patients with microsatellite stable (MSS) or proficient mismatch repair (pMMR) status, comprising approximately 95% of the mCRC population, exhibit response rates as low as 0–2% (16). Such modest clinical outcomes highlight the necessity for novel strategies to overcome intrinsic biological and therapeutic barriers in GI malignancies.
Nano-enabled immunotherapy offers a complementary solution by improving pharmacokinetics and biodistribution, co-packaging synergistic immunomodulators, and delivering them with spatial and temporal precision to remodel the GI tumour microenvironment. Rationally designed nanoparticles can enhance antigen preservation and cross-presentation, boost T-cell priming and intratumoural trafficking, induce or amplify immunogenic cell death, and activate innate immune pathways (e.g., STING/TLR) while limiting systemic toxicity (17–19). In parallel, theranostic nanoparticle platforms integrate radiosensitisation and real-time molecular/immunologic imaging, enabling response-adapted combinations and dosing in GI cancers (20–22). In particular, Li et al. have comprehensively reviewed how nanoparticles can potentiate radio-immunotherapy by amplifying radiation-induced immunogenic cell death, enhancing dendritic-cell cross-presentation of tumour antigens, and remodeling the tumour immune microenvironment—thereby fostering robust abscopal effects and durable antitumour immunity (23). Complementarily, biomarker-driven molecular imaging probes—such as PET tracers for PD-L1 expression and CD8+ T-cell infiltration—have been developed to noninvasively monitor immune activation and radiation response in vivo, supporting patient stratification and real-time optimisation of combined radio-immunotherapy regimens (21).
Nanotechnology-based immunotherapies have emerged as a promising approach, strategically intervening at distinct stages of the cancer–immunity cycle through four conceptual modules (1): tumour antigen release and presentation, where engineered nanoparticles such as mesoporous silica nanoparticles protect endogenous tumour antigens from clearance and enhance lymphoid tissue delivery, as exemplified by the work of Qian et al. (24); (2) T-cell priming and activation, with artificial antigen-presenting cells (aAPCs) optimised at the nanoscale for efficient lymph-node targeting and costimulatory activation—though particle size critically influences efficacy, as demonstrated by Hickey et al. (25); (3) cytotoxic T-lymphocyte (CTL) trafficking and infiltration, where nanocarriers encoding gene therapies like chemokine “traps” modulate chemotactic signals to enhance T-cell infiltration, as illustrated by Goodwin et al. (26) Moreover, in orthotopic colorectal models, STING-activating nanosystems reduce tumour hypoxia and upregulate endothelial ICAM-1/VCAM-1 expression, resulting in a twofold increase in CD8+ T-cell tumour infiltration and improved survival (27); and (4) CTL recognition and tumour cell killing, involving nanoparticles engineered for enhanced checkpoint inhibitor avidity and retention, such as generation-7 PAMAM dendrimer conjugates reported by Bu et al. (28) Additionally, combinatorial nano-immunotherapies integrate complementary agents acting across multiple immunologic stages, addressing the limitations of monotherapy and enabling synergistic antitumour effects.
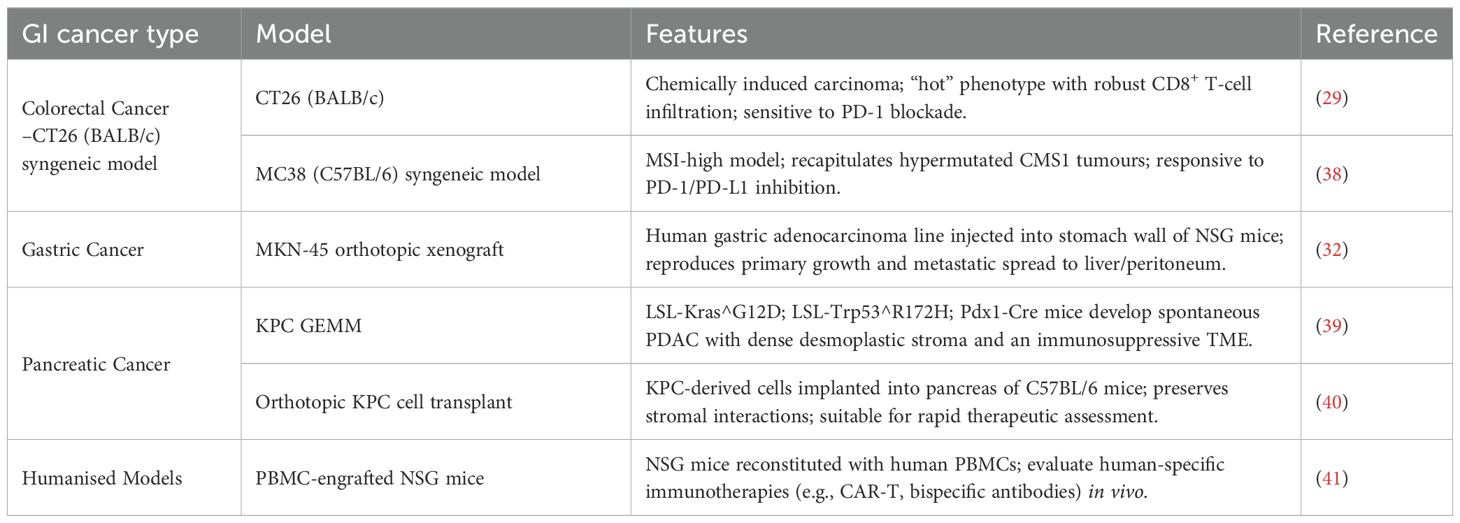
Representative GI cancer models and platforms
To evaluate nano-enabled immunotherapies in relevant settings, we summarise here the most widely used in vivo systems for each major GI cancer subtype (Table 1):
Colorectal Cancer (CRC): CT26 (BALB/c) and MC38 (C57BL/6) syngeneic models, modeling MSI-high and “hot” CRC phenotypes, respectively, and routinely used to assess PD-1/PD-L1 blockade efficacy (29, 30).
Gastric cancer (GC): Orthotopic engraftment of human MKN-45 or NUGC-4 cells into NSG mice reproduces primary tumour growth, metastatic spread, and immune exclusion, providing clinically relevant platforms (31, 32).
Pancreatic ductal adenocarcinoma (PDAC): The KPC GEMM (LSL-Kras^G12D; LSL-Trp53^R172H; Pdx1-Cre) develops spontaneous, desmoplastic PDAC with immunosuppressive stroma. Orthotopic transplantation of KPC-derived tumour cells into syngeneic hosts offers rapid evaluation of therapeutic interventions (33, 34).
Humanised platforms: PBMC-engrafted NSG and HLA-transgenic NSG mice permit in vivo testing of human-specific immunotherapies (e.g., CAR-T cells, bispecific antibodies), enabling translational assessment of nano-enabled strategies (35, 36). For example, PLGA nanoparticles encapsulating STING agonists (e.g., ONP-302) in PBMC-engrafted NSG mice elicit IL-15–dependent activation of human NK cells and CD8+ T cells, demonstrating significant tumour growth suppression and translational relevance (37).
Nanomaterial-facilitated immunostimulation in gastrointestinal malignancies
Originally designed to enhance pharmacokinetic profiles and improve tumour specificity, several clinically approved nanomedicines have found renewed relevance as immunomodulatory tools in GI oncology (42, 43). Recent studies have demonstrated that various nanomaterial platforms can significantly enhance immunostimulatory effects within GI tumour models. For instance, mesoporous silica nanoparticles and metal-based nanoparticles have shown potent induction of immunogenic cell death (ICD), characterised by calreticulin (CRT) exposure, extracellular ATP release, and HMGB1 secretion, collectively enhancing dendritic cell maturation and subsequent T-cell priming (44). Herein, we outline principal categories of nanomaterials (Figure 1), briefly noting their approved indications within GI cancers, before examining how these platforms have been re-engineered to engage both innate and adaptive immune responses within the tumour microenvironment.
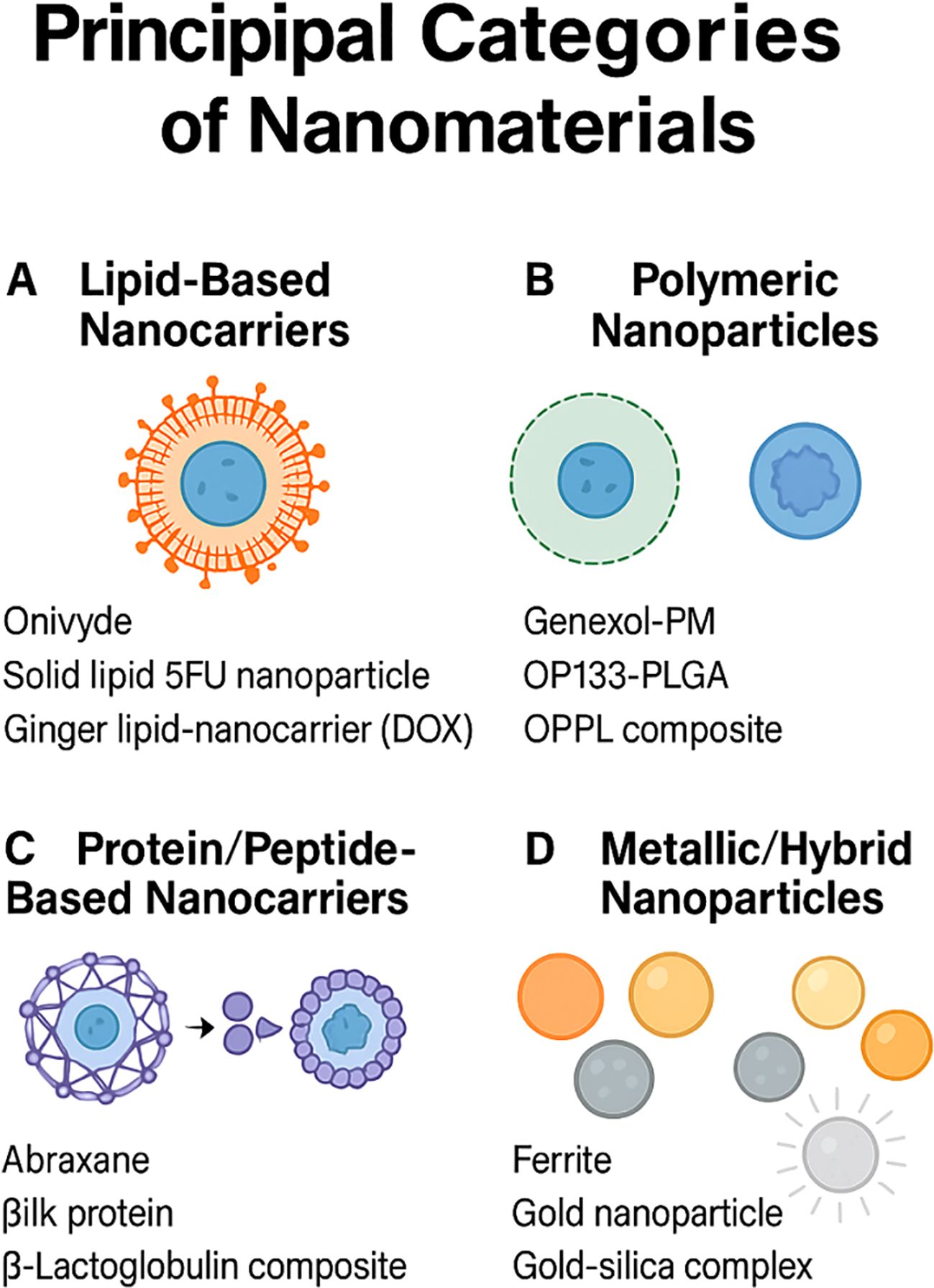
Figure 1. Principal categories of nanomaterials for drug delivery. (A) Lipid‐based nanocarriers such as liposomes and solid‐lipid particles (Onivyde; solid‐lipid 5-FU nanoparticle; ginger lipid carrier loaded with doxorubicin). (B) Polymeric nanoparticles including Genexol-PM, OP133-PLGA, and OPPL composites. (C) Protein/peptide assemblies exemplified by Abraxane, silk protein, and β-lactoglobulin composites. (D) Metallic and hybrid nanoparticles such as ferrite, gold, and gold–silica complexes.
Lipid-based nanocarriers
Lipid-based systems, particularly liposomes and lipid nanoparticles (LNPs), represent the most clinically pervasive nanotechnologies in oncology, attributable to their favourable safety profiles, modular drug-loading capacities, and scalability in production (45). Among GI indications, the PEGylated liposomal formulation of irinotecan (Onivyde), licensed for metastatic pancreatic cancer, exemplifies this class (46). By promoting tumour-specific accumulation and extended plasma half-life, it not only augments cytotoxicity but also triggers immunogenic cell death, facilitating the liberation of neoantigens for dendritic cell engagement and cross-priming.
The clinical utility of LNPs was cemented by the 2018 FDA approval of ONPATTRO, the first liver-targeting nanoparticle for RNA delivery (47). More compact liposomal LNP–mRNA formulations rich in bilayer lipids—egg sphingomyelin and cholesterol—achieve >90% encapsulation, extended circulation, and efficient extrahepatic transfection via pH-triggered core release (48).
Investigational liposomal oxaliplatin formulations under evaluation in colorectal cancer similarly exploit these immunogenic properties, inducing calreticulin exposure and ATP release (49, 50). Juang and colleagues further engineered pH-sensitive, peptide-functionalised LNPs co-loaded with irinotecan and miR-200, a microRNA known to inhibit metastatic progression (51). Given its widespread use in colorectal cancer, 5-fluorouracil (5-FU) remains a key chemotherapeutic agent, despite its short half-life and systemic toxicity. To address these pharmacological limitations, Patel et al encapsulated 5-FU within solid lipid nanoparticles, demonstrating enhanced cytotoxicity in Caco-2 cells in a dose-dependent manner (52).
Doxorubicin (DOX), though potent, is hindered by its cardiotoxicity and poor tumour selectivity. To overcome these drawbacks, Zhang et al utilised nanovectors derived from ginger lipids (GDNVs) to encapsulate and deliver DOX efficiently to tumour sites, improving therapeutic precision and limiting off-target effects (53).
Polymeric nanoparticles
Polymeric nanostructures, including nanospheres and nanocapsules, offer a highly customisable platform for drug delivery, enabling responsive release profiles, environmental stability, and surface functionalisation for targeted therapy (54, 55). These systems can exploit pathophysiological features of the tumour milieu—such as acidic pH and elevated enzymatic activity—to trigger site-specific drug liberation (56–58).
Genexol-PM, a paclitaxel-loaded polymeric micelle approved for breast and pancreatic malignancies, exemplifies how prolonged drug exposure may elicit immunogenic cell death in GI models (59). In colon cancer models, chitosan-coated PLGA nanoparticles encapsulating 5-FU markedly suppressed HT-29 cell viability compared to free drug or uncoated formulations (60). Further innovation has yielded a multifunctional nanocomposite—OPPL—integrating oleic acid-modified superparamagnetic iron oxide with an amphiphilic polymer for the co-delivery of a platinum prodrug and lauric acid, aiming to synergistically amplify colorectal tumour cytotoxicity and disrupt microbial biofilms (61).
In recent preclinical work, polyguanidine-derived nanoinhibitors have been engineered to localise within hepatocyte lysosomes and inhibit V-ATPase activity, thereby activating AMPK signalling to reduce fatty acid synthesis and enhance lipolysis in models of liver lipid overload (62). Equally promising are enzyme-responsive branched glycopolymer nanoassemblies that, upon cathepsin B–mediated cleavage in gastric cancer cells, co-release paclitaxel and an Akt inhibitor to achieve potent synergistic tumour kill with mitigated systemic toxicity (63).
Protein- and peptide-based nanocarriers
Nanoparticles derived from natural proteins—such as albumin, silk fibroin, gelatin, and lipoproteins—are increasingly explored for cancer nanomedicine owing to their intrinsic biodegradability, low immunogenicity, and high drug-loading capacity (18, 64).
Albumin-bound paclitaxel (Abraxane), while approved for various solid tumours, has demonstrated promise in GI cancers when co-administered with immune checkpoint inhibitors, fostering dendritic cell maturation and macrophage repolarisation via receptor-mediated endocytosis (65). Recent advances include the development of folate-conjugated sericin nanoparticles (FA-SND), engineered for pH-responsive doxorubicin release and selective accumulation in tumour tissues (66). Another innovative construct, based on a GE11-HGFI fusion protein, enabled the targeted delivery of curcumin to EGFR-overexpressing colorectal cancer cells, yielding enhanced intratumoural drug retention and pronounced anticancer activity (67). β-Lactoglobulin nanoparticles co-loaded with 5-FU and sodium butyrate, and functionalised with folic acid, were found to selectively home to folate receptor–expressing colorectal tumours, offering a strategy for combined chemotherapeutic and epigenetic modulation (68).
Metallic and hybrid nanoparticles
While iron oxide–based agents such as NanoTherm are approved for thermotherapy in glioma and prostate cancer (69), their application has been adapted for GI tumours, particularly colorectal cancer. In preclinical settings, iron oxide nanoparticles co-administered with TLR agonists generated reactive oxygen species and heightened dendritic cell activation, contributing to local immune potentiation (70). Recent studies have extended these approaches: artemisinin−protected magnetic iron−oxide nanoparticles hyperactivate autophagy to overcome hyperthermia resistance in gastric cancer, while glucose−coated Fe₃O₄ nanoparticles conjugated with safranal selectively induce apoptosis and G₂/M cell−cycle arrest in hepatocellular carcinoma models (71, 72).
Hybrid nanoparticles—featuring gold cores for photothermal ablation and polymeric coatings harbouring STING agonists—have yielded synergistic effects in orthotopic pancreatic cancer models, enhancing both innate immune engagement and antigen release (73). In MC38-bearing mice, dual-STING agonist nanoparticles (STANs) have been shown to elevate intratumoural IFN-β by more than threefold and increase CD86+ dendritic cell frequencies, correlating with enhanced CD8+ T-cell infiltration and durable antitumour responses (74). Variations in gold nanoparticle systems, including PEGylated AuS, have exhibited substantial photothermal efficiency and anti-tumour effects in gastric cancer (75, 76). Nanosystems such as FAL-ICG-HAuNS not only exert photothermal cytotoxicity but also elicit robust immune responses in vivo (77). Moreover, composite constructs integrating gold nanorods with multi-walled carbon nanotubes enable enhanced imaging in gastric tumours (78), while folate-targeted silica-encapsulated gold nanoclusters demonstrate both diagnostic and therapeutic utility, offering precise tumour localisation and treatment monitoring (79). Beyond these, multifunctional metallic−hybrid platforms—such as pH−responsive Fe−doped mesoporous polydopamine co−loaded with sorafenib for synergistic ferroptosis and photothermal therapy, ultrathin 2D FeS nanosheets delivering CRISPR/Cas9 to downregulate heat−shock proteins, and self−assembled copper−based nanoplatforms enabling chemodynamic, photodynamic, and antiangiogenic tritherapy—have shown potent multimodal cytotoxicity across diverse GI malignancy models (80–82).
Nanomaterial-enhanced immunotherapeutic strategies in gastrointestinal cancers: mechanisms and clinical translation
Nanotechnology offers transformative potential for GI cancer immunotherapy by integrating enhanced permeability and retention effects with active targeting, deep tissue penetration, and controlled release kinetics. Functionalisation with antibodies or ligands allows for precise delivery of immunotherapeutics—ranging from checkpoint inhibitors to cytokines and neoantigens—enabling reprogramming of the tumour immune microenvironment while limiting systemic toxicity (18). Here, we highlight four key nanotechnology-enabled strategies (Figure 2): precision checkpoint blockade, adoptive cell therapy enhancement, nanovaccines, and cytokine delivery.
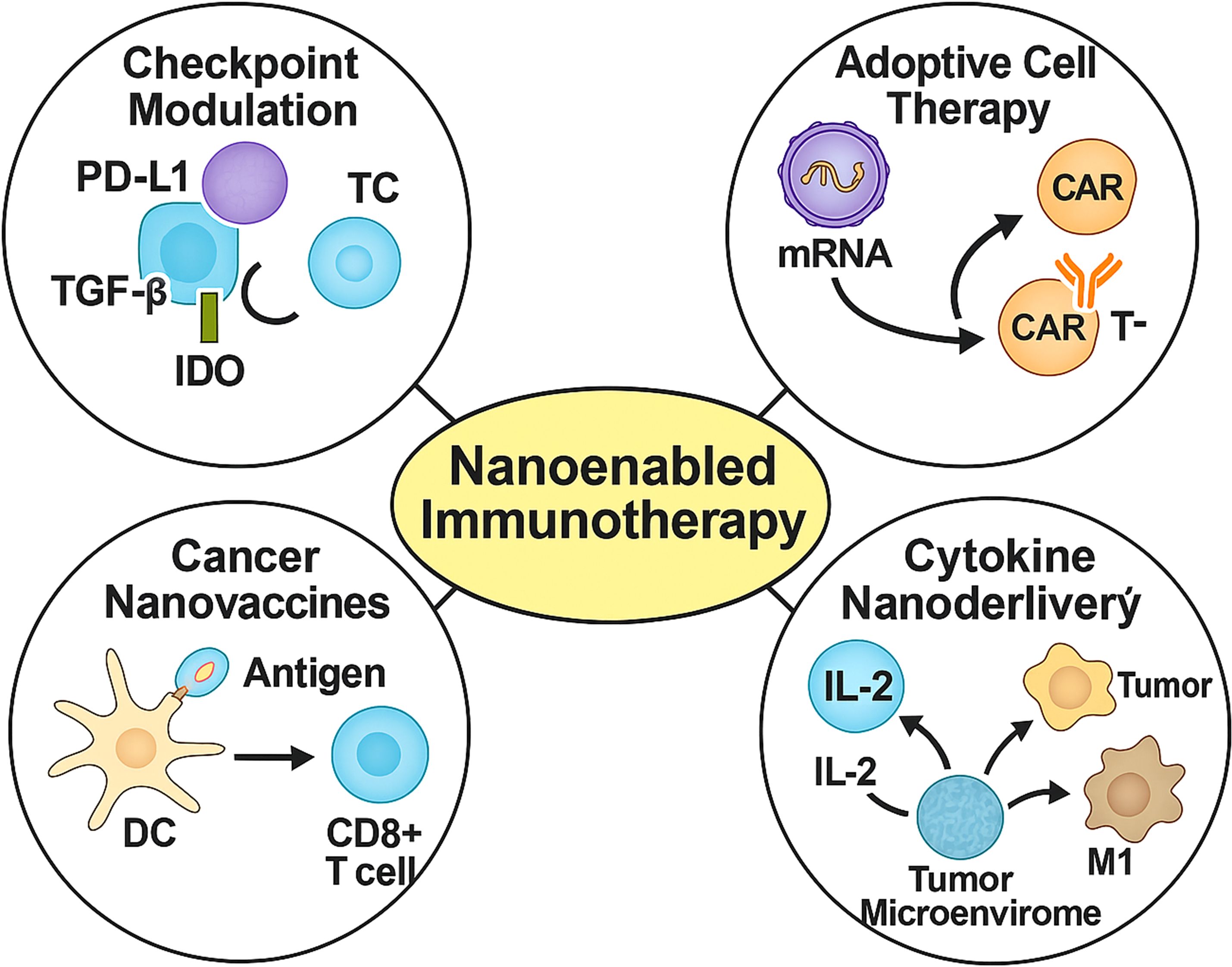
Figure 2. Four representative nanomedicine-enabled immunotherapeutic strategies for gastrointestinal cancers. Nanoparticle platforms facilitate checkpoint inhibition (e.g., PD-L1, TGF-β, IDO), in vivo programming of CAR-T or NK cells, dendritic cell-mediated vaccination, and targeted cytokine delivery (e.g., IL-2, IL-12), enhancing tumour immune infiltration, cytotoxicity, and therapeutic selectivity.
Precision immune checkpoint modulation
Nanoparticles can be tailored to modulate immune checkpoints on tumour or immune cells, thus promoting robust innate and adaptive immune responses (Figure 3). One such system involves BSA-based nanocapsules functionalised with glucose-modified PMPC polymers and IgG, achieving precise immune regulation (83). In another study, polydopamine nanoparticles co-loaded with gemcitabine and the IDO inhibitor NLG919 suppressed intratumoural IDO activity by 85%, doubled intratumoural granzyme B+ CD8+ T-cell density, and induced durable responses in 60% of KPC mice, outperforming gemcitabine alone (10%) (84).
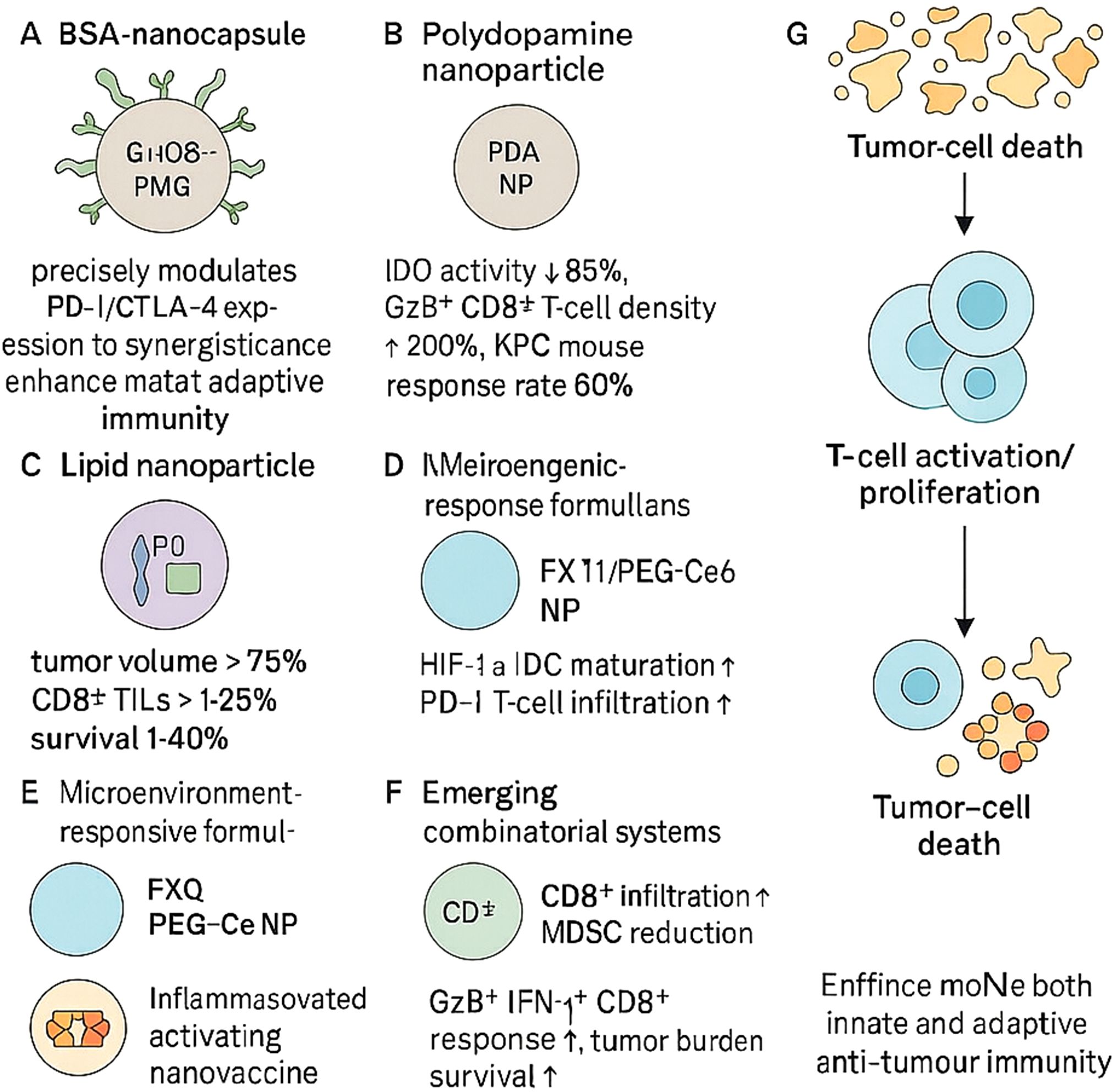
Figure 3. Nanoparticle-based precision immune checkpoint modulation enhances antitumour immunity. Engineered nanoparticles modulate immune checkpoints to overcome tumour-induced immunosuppression and promote effective immune responses. (A) BSA nanocapsules enhance PD-1/CTLA-4 regulation and adaptive immunity. (B) Polydopamine nanoparticles reduce IDO activity and increase CD8+ T-cell infiltration, improving response rates in pancreatic models. (C) Lipid nanoparticles restore CD8+ TILs and reduce tumour burden. (D, E) Microenvironment-responsive formulations alleviate hypoxia or acidity to enhance dendritic cell and T-cell function. (F) Combinatorial systems further boost CD8+ T-cell responses and reduce MDSCs. (G) Together, these strategies promote tumour cell death through enhanced innate and adaptive immunity.
Lipid-based nanoparticles co-encapsulating PD-L1 siRNA and a TGF-β receptor antagonist reduced tumour volumes by over 75% in pancreatic cancer models and restored CD8+ T-cell infiltration to >25% of tumour-infiltrating lymphocytes, prolonging survival by 40% compared with free drugs (85). Chemotherapy-induced tumour RNA nanoparticles synergised with PD-1 blockade to suppress colorectal tumour progression and prolong survival (86). Similarly, oxaliplatin-loaded liposomes combined with anti-PD-1 achieved 90% tumour growth inhibition in CT26 models—substantially surpassing monotherapies (30–50%) via enhanced immunogenic cell death and antigen cross-presentation (85).
Recent microenvironment-responsive formulations have further refined checkpoint modulation. In gastric cancer, FX-11@PEG-Ce6 nanoparticles alleviated TME acidity, promoted dendritic cell maturation and CD8+ T-cell cytokine secretion, and markedly enhanced the efficacy of α-PD-1 therapy in vivo (87). Hypoxia-activated TH-302 encapsulated in mPEG-PLGA NPs selectively released under low-oxygen conditions, reduced HIF-1α and PD-L1 expression, and, in combination with PD-1 blockade, significantly increased CD8+ T-cell infiltration and pro-inflammatory cytokine production in gastric tumour models (88). In hepatocellular carcinoma, pH-sensitive nanocarriers co-delivering anti-PD-1 and MDK-siRNA reprogrammed TAMs and MDSCs towards an immunostimulatory phenotype, overcoming ICB resistance (89). Lastly, AEAA-targeted PLGA nanoparticles co-encapsulating mitoxantrone and the STAT3 inhibitor napabucasin activated cGAS-STING signalling in HCC cells, synergised with anti-PD-1, and achieved durable tumour regression (90).
A nanoparticle system carrying siRNAs against ZDHHC9 enhanced CD8+ T-cell infiltration and reduced MDSC presence, converting the immunosuppressive tumour microenvironment into a pro-inflammatory one when combined with PD-L1 blockade (91). Inflammasome-activating nanovaccines also promoted GzB+IFN-γ+ CD8+ T-cell responses and synergised with CTLA-4/PD-L1 inhibitors to reduce tumour burden and extend survival across various murine cancer models (92).
Nano-enabled adoptive cell therapies
Nanomaterials have been harnessed to augment the expansion, homing, and persistence of engineered lymphocytes within fibrotic GI tumours. One approach used antibody-decorated ionisable lipid nanoparticles to deliver CD19-CAR mRNA systemically, inducing ~35% CAR expression in circulating T cells, with functional persistence and >90% target B-cell depletion in mice (93). Toll-like receptor 7/8 agonist-loaded nanoparticles targeting dendritic cells upregulated IL-12 and IL-18 by four-fold, amplified NK cell activity, and achieved complete tumour control in 70% of pancreatic models (Figure 4) (94).An injectable alginate-based nanogel releasing CCL21 chemokine enhanced CD8+ T-cell and dendritic cell infiltration five-fold and reduced metastatic spread in colorectal models (95).
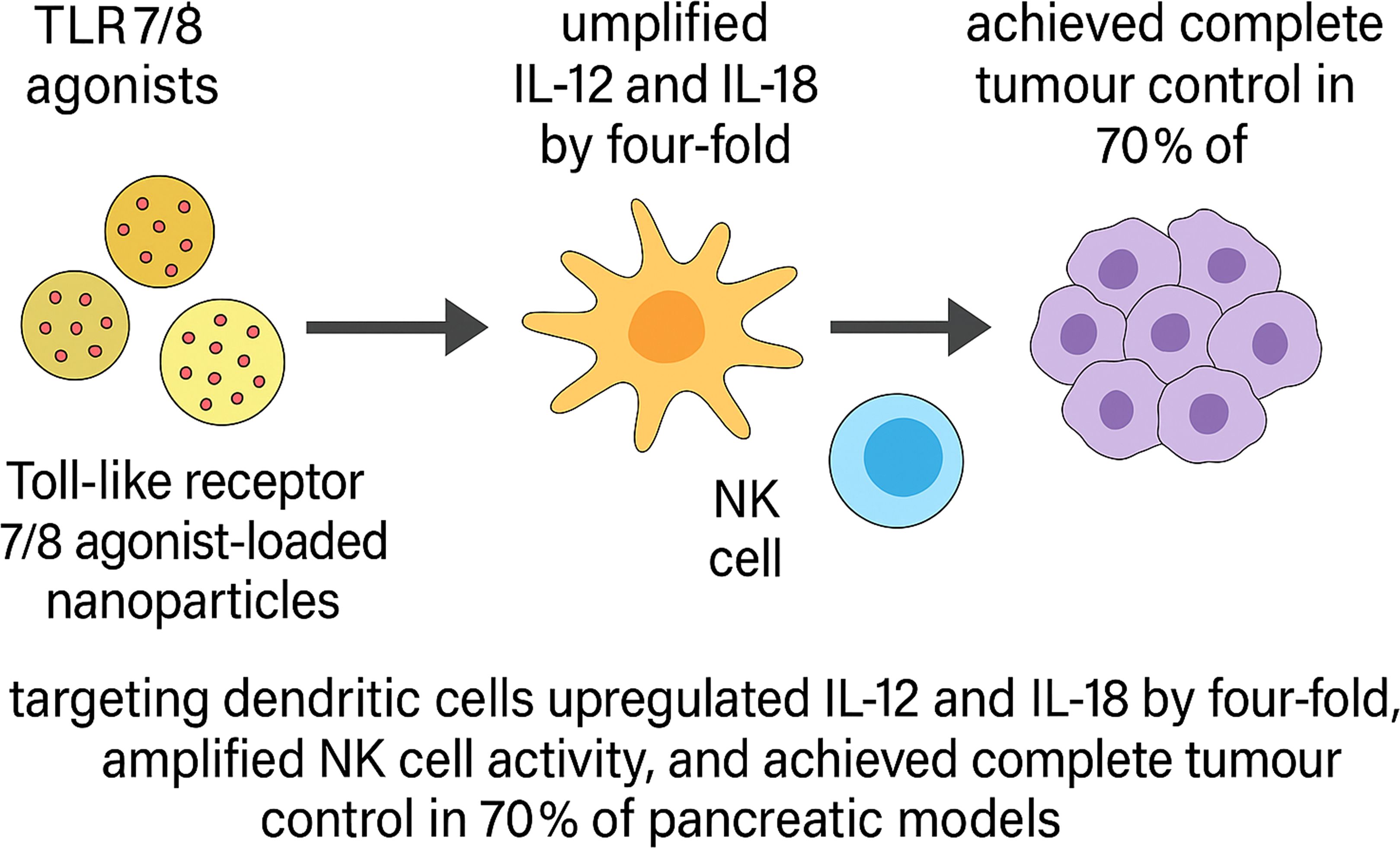
Figure 4. Schematic illustration of TLR7/8 agonist-loaded nanoparticle–mediated innate immune activation and tumour control in pancreatic cancer models. Toll-like receptor 7/8 (TLR7/8) agonist-loaded nanoparticles selectively target dendritic cells, leading to a four-fold upregulation of proinflammatory cytokines IL-12 and IL-18. These cytokines promote natural killer (NK) cell activation, resulting in enhanced cytotoxic activity. In preclinical pancreatic tumour models, this strategy achieved complete tumour regression in 70% of treated animals.
Building on this foundation, a STAR-based in vivo selection yielded a CEA-specific nanobody (VHHB30); consequently, third-generation VHHB30–CAR T cells eradicated colorectal and gastric xenografts and outperformed second-generation constructs (96). In parallel, LNP platforms have emerged as versatile tools to transiently program immune cells with high efficiency and low cytotoxicity: in HCC models, a bespoke lipid blend directed CAR mRNA–loaded LNPs to liver-associated myeloid cells, thereby enhancing local delivery (97); moreover, Billingsley’s top-performing C14-4 ionizable LNP enabled robust CAR mRNA transfection of primary human T cells (98), and antibody-decorated LNPs (anti-CD3 Ab-LNPs) further improved T-cell targeting (99).
Nanovaccines in GI oncology
Nanovaccine platforms enable precise antigen presentation with improved stability, enhanced lymph node trafficking, and durable immunological memory (Figure 5) (100–102). Antigen payloads may include peptides, tumour lysates, or mRNA, aiming to broaden the T-cell repertoire and overcome immunosuppression (103, 104).
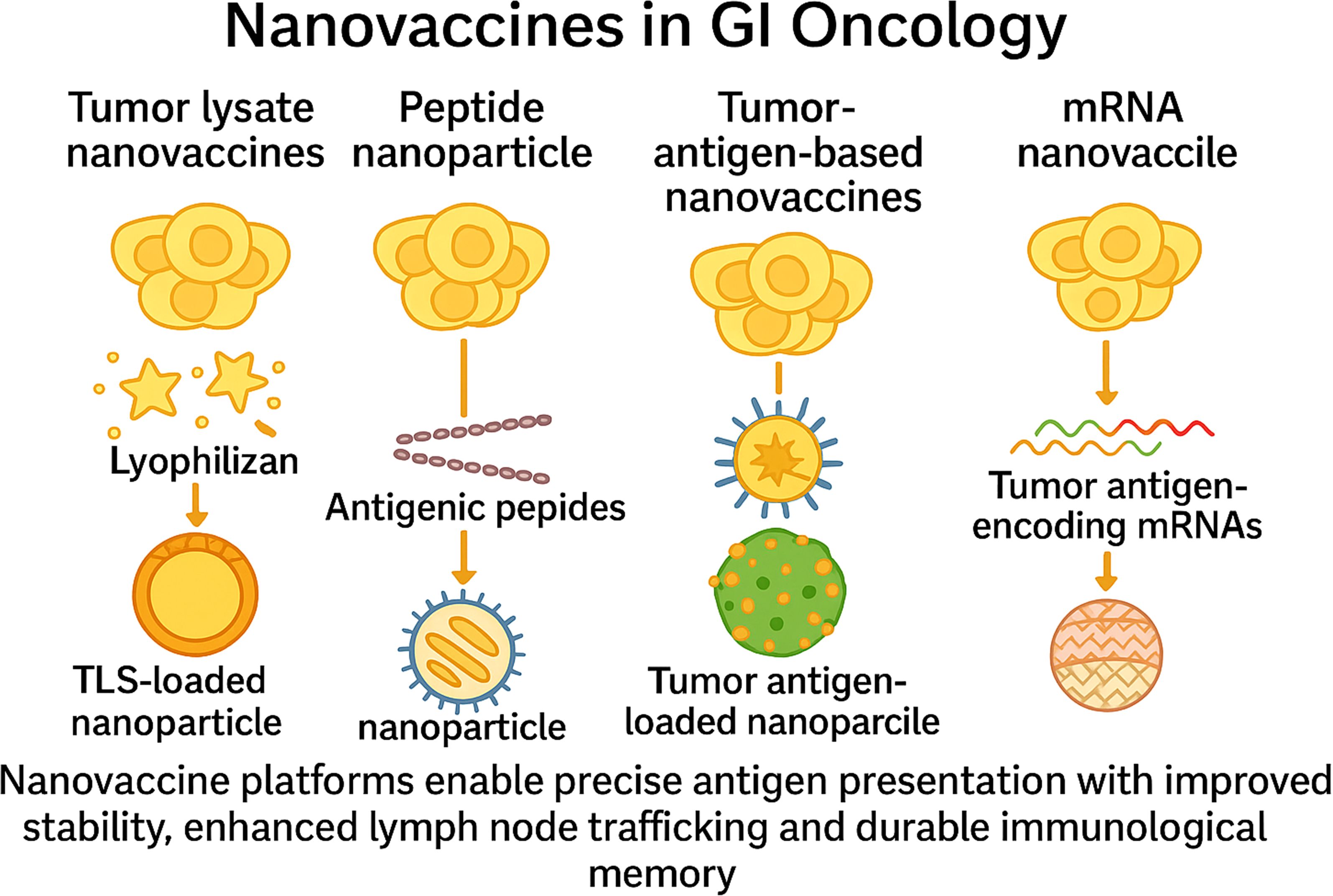
Figure 5. Nanovaccine platforms in gastrointestinal oncology. This figure depicts four nanovaccine strategies used in GI cancers. Tumour lysate nanovaccines deliver whole-cell antigens via nanoparticle encapsulation. Peptide nanovaccines incorporate defined antigenic peptides to elicit specific T-cell responses. Tumour antigen-based nanovaccines co-deliver tumour antigens and adjuvants to enhance dendritic cell activation. mRNA nanovaccines encode tumour antigens for in situ expression. All platforms aim to enhance antigen stability, lymph node trafficking, and durable immune memory.
In hepatocellular carcinoma, a phase I/II study of the DNA vaccine GNOS-PV02 plus pembrolizumab achieved an objective response rate >30%, exceeding historical anti-PD-1 monotherapy benchmarks (14–17%) and underscoring the need for larger confirmatory trials (105). A smart, tumour-microenvironment–responsive Fe/Mn nanovaccine that synchronously releases sorafenib, Fe³+ and Mn²+ triggered pyroptosis-mediated immunogenic cell death and cGAS–STING activation, driving dendritic-cell maturation and potent antitumour immunity (106). Complementarily, an in situ layered double hydroxide–cGAMP nanovaccine deployed after radiofrequency ablation adsorbed tumour-associated antigens, boosted type I interferon signalling, increased CTL/DC infiltration, and markedly enhanced αPD-L1 efficacy in poorly immunogenic liver tumours (107).
A lipopolyplex-based mRNA vaccine encoding CT26 neoantigens, co-delivered with CpG-ODN, induced IFN-γ responses in >80% of neoantigen-specific CD8+ T cells and achieved complete tumour regression in 80% of mice, with 90% protected against rechallenge (108). Similarly, biodegradable polymeric nanoparticles encoding KRAS^G12D mRNA eradicated MC38 and CT26 tumours while inducing long-term memory responses (109).
Engineered Salmonella coated with tumour lysate–adsorbed PDA nanoparticles (EnS@PDA@CL) homed to hypoxic tumour regions and activated dendritic cells and cytotoxic T cells, delaying pancreatic tumour growth (110). In another example, autologous membrane antigen–laden LNPs (C5) derived from surgically resected colorectal tumours exhibited potent antitumour activity and, when combined with PD-1 blockade, significantly increased remission rates (111).
A liposomal nanovaccine carrying the CD155 gene and modified with Lycium barbarum polysaccharides (LBP-CD155L NVs) improved both prophylactic and therapeutic efficacy in CRC models (112). PLGA-based nanovaccines co-loaded with STING agonists and tumour antigens enhanced lymph node targeting, dendritic cell maturation, and CD8+ T-cell activation, suppressing colorectal tumour growth (113). SeaMac nanoparticles—engineered to self-adjuvant antigen presentation—further improved antitumour responses (114). Peptide-based supramolecular nanocarriers enabled tumour-microenvironment-restricted STING agonist release, driving systemic immunity (115).
Gold and silica-based inorganic nanovaccines provide an alternative platform. AuNP-based vaccines promoted photothermal and immunostimulatory activity, leading to CD8+ T-cell activation and improved survival in CRC models (116). Silica nanoparticle–based vaccines enhanced T-cell infiltration and tumour control in preclinical colorectal settings (117).
Cytokine nanodelivery
Nanocarriers permit localised cytokine administration with reduced systemic toxicity (Figure 6). IL-2-loaded porous nanoparticles (BALLkine-2) prolonged circulation and reduced toxicity versus free IL-2 (118). Cyclodextrin nanoplexes co-delivering 5-FU and IL-2 demonstrated superior anticancer efficacy in CRC models (119). Another strategy used boron–nitrogen coordinated nanoparticles to improve IL-2 pharmacokinetics and potentiate immune responses in colon cancer, particularly when paired with CDK4/6 inhibitors (120).
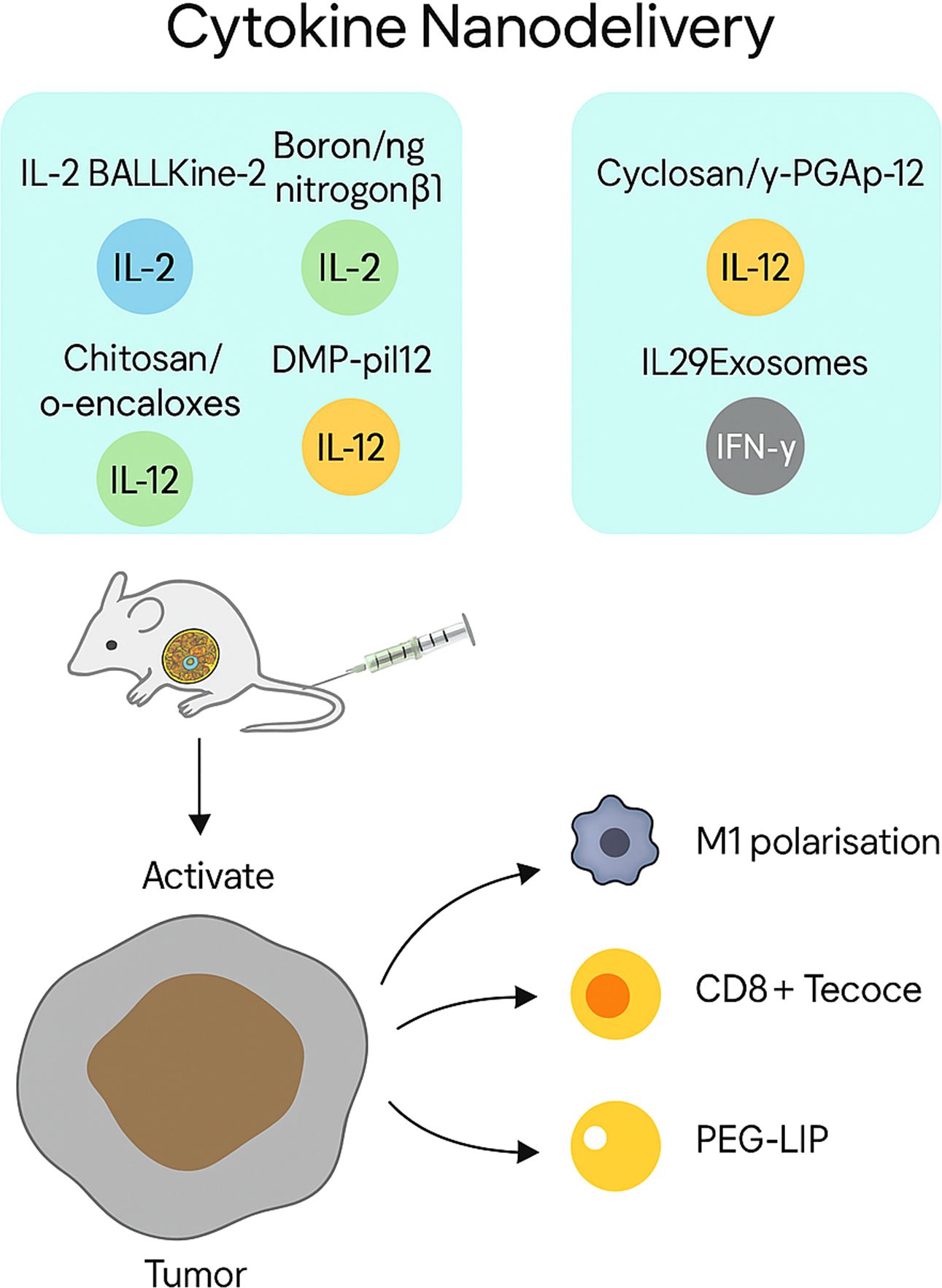
Figure 6. Cytokine nanodelivery for tumour-targeted immune activation. The figure illustrates representative nanocarriers used to deliver cytokines—including IL-2, IL-12, IL-29, and IFN-γ—directly to tumour sites. Platforms such as porous nanoparticles (BALLKine-2), boron–nitrogen nanoparticles, and PEGylated liposomes enable prolonged circulation, controlled release, and reduced systemic toxicity. These systems activate CD8+ T cells and dendritic cells, and reprogramme macrophages towards an M1 phenotype, collectively enhancing antitumour immunity.
IL-12 encapsulation via nanoparticles led to enhanced tumour accumulation and reduced off-target toxicity (121). Extending this concept, a self-stabilising chitosan/poly-(glutamic acid) nanoplatform co-loading doxorubicin and IL-12 achieved prolonged circulation, preferential intratumoural release, and macrophage re-education toward an M1 phenotype, resulting in marked suppression of H22 hepatocellular carcinoma with minimal systemic toxicity (122). The DMP-pIL12 gene delivery system significantly inhibited tumour growth by inducing apoptosis, reducing angiogenesis, and halting proliferation in subcutaneous and peritoneal CRC models (123). A folate-targeted lipoplex (F-PLP/pIL12) delivering IL-12 plasmid DNA resulted in 56.6% tumour inhibition and reduced malignant ascites and tumour burden (124).
IL-29-loaded exosomes showed sustained cytokine release and effective tumour targeting in vitro (125). IFN-γ–loaded PEGylated liposomes enhanced macrophage M1 polarisation and accumulated efficiently in colon tumours, suppressing growth in murine models (126).
Clinical translation of nanomedicine-based therapies in gastrointestinal malignancies
The integration of nanomedicine with immunotherapy has yielded promising antitumour activity in gastrointestinal malignancies, with some agents already entering clinical evaluation. Notably, Abraxane, a nanoparticle albumin-bound formulation of paclitaxel, has been tested in combination with PD-1 blockade across multiple oesophageal and pancreatic cancer trials, consistently demonstrating encouraging response rates and prolonged survival outcomes. Similarly, mRNA nanovaccines—delivered via lipid nanoparticles—have shown robust immunogenicity in early-phase studies in PDAC and metastatic gastrointestinal cancers, offering a platform for personalised immune activation in the adjuvant setting. (Table 2)
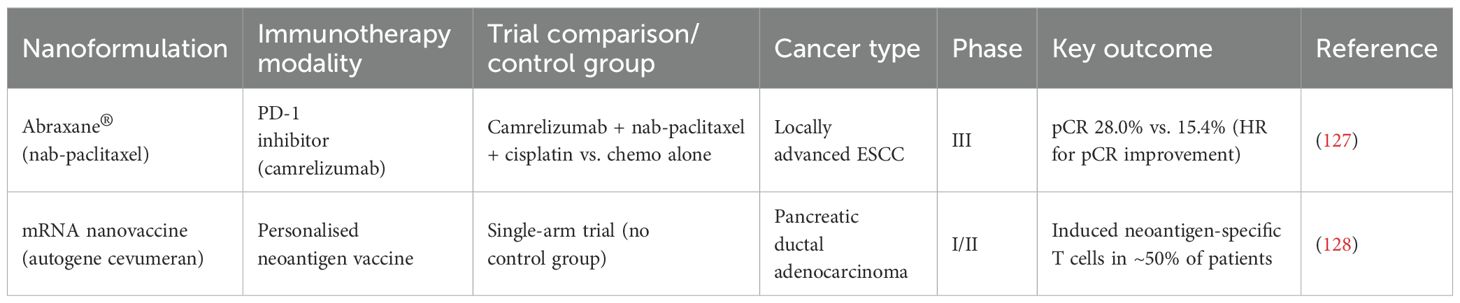
Table 2. Representative clinical trials of nanomedicine-enabled immunotherapy in gastrointestinal cancers.
Several other nanotherapeutics, including Doxil and Onivyde, have demonstrated meaningful cytotoxic efficacy in gastric and pancreatic cancers, respectively. However, these formulations were primarily optimised for chemotherapeutic delivery rather than immunomodulation, and clinical data supporting their synergy with immune checkpoint inhibitors or cellular therapies remain limited. Their inclusion here underscores the therapeutic value of nanomedicine in GI oncology, while highlighting opportunities for further development in immune-based strategies.
Abraxane
Abraxane, a nanoparticle albumin-bound (nab) formulation of paclitaxel, offers enhanced drug solubility, tumour penetration, and reduced reliance on toxic solvents (129). In advanced oesophageal squamous cell carcinoma (ESCC), combination regimens incorporating Abraxane and PD-1 blockade have demonstrated promising clinical outcomes. Specifically, camrelizumab combined with Abraxane and carboplatin achieved a complete response (CR) rate of 9.8% in patients with advanced ESCC (130), and was associated with 2-year recurrence-free survival (RFS) and overall survival (OS) rates of 67.9% and 78.1%, respectively (131). In another study, intensive induction therapy using camrelizumab with Abraxane and capecitabine resulted in CR and major pathological response (MPR) rates of 33.3% and 64.3%, respectively (132). A phase 2 trial evaluating tislelizumab, a PD-1 inhibitor, in combination with carboplatin and Abraxane, reported MPR and pathological complete response (pCR) rates of 57.5% and 40% in patients with resectable ESCC (133).
In pancreatic cancer, multimodal strategies incorporating Abraxane have also been explored. A combination of CAR T cells targeting EGFR, Abraxane, and cyclophosphamide was deemed safe and induced disease stabilisation and expansion of central memory T cells in patients with metastatic pancreatic cancer, with a median OS of 4.9 months (134). In a phase 1/2 trial, dendritic cell vaccination combined with gemcitabine and Abraxane yielded a median progression-free survival (PFS) of 8.1 months and OS of 15.1 months, without severe adverse events (135). Another phase 1b/2 study assessed a CD40 agonist (sotigalimab) in combination with gemcitabine, Abraxane, and nivolumab, reporting an objective response rate (ORR) of 58% in pancreatic adenocarcinoma (136).
Beyond pancreatic and oesophageal cancers, Abraxane has been investigated in biliary tract cancers (BTCs). When added to the standard gemcitabine–cisplatin regimen, nab-paclitaxel improved outcomes, yielding a median PFS of 11.8 months and OS of 19.2 months, with a disease control rate (DCR) of 84% and ORR of 45% (137). A separate study evaluating nab-paclitaxel plus capecitabine as first-line therapy for advanced or metastatic BTCs reported an ORR of 23.3% and a DCR of 69.8% (138).
However, efficacy in colorectal malignancies appears limited. In a single-arm phase II study, nab-paclitaxel monotherapy demonstrated minimal activity in heavily pretreated metastatic colorectal cancer (mCRC) (139). Similarly, a phase II trial of nab-paclitaxel in CIMP-high mCRC and refractory small bowel adenocarcinoma reported no significant clinical benefit (140).
mRNA nanovaccines
Lipid nanoparticles (LNPs), composed of a homogeneous lipid core, have emerged as leading non-viral vectors for nucleic acid delivery. Their clinical success in mRNA-based COVID-19 vaccines has accelerated interest in applying this platform to cancer immunotherapy (141, 142). The appeal of mRNA nanovaccines lies in their ability to elicit transient yet potent antigen expression, making them suitable not only for infectious disease prophylaxis but also for applications in cancer vaccination, protein replacement, and gene editing. However, the intrinsic instability of naked mRNA—due to susceptibility to enzymatic degradation and hydrolysis—necessitates protection via encapsulation. LNPs safeguard mRNA from extracellular RNases while facilitating intracellular delivery and endosomal escape (143).
A recent study employed tumour-infiltrating lymphocyte analysis to identify immunogenic mutations in metastatic gastrointestinal cancers. By concatenating validated neoantigens, predicted epitopes, and driver mutations into a single mRNA construct, researchers generated personalised cancer vaccines. The vaccine was well tolerated and induced neoantigen-specific T cell responses, including T cell receptors targeting KRAS^G12D. However, no objective tumour responses were observed in the four patients treated (144). These findings suggest that although mRNA vaccines are immunogenic, combinatorial strategies involving checkpoint inhibition or adoptive T cell transfer may be required to achieve therapeutic benefit.
In a separate phase I study, a personalised uridine mRNA–lipoplex vaccine (autogene cevumeran) was synthesised in real time from resected pancreatic ductal adenocarcinoma (PDAC) tissue. Vaccination expanded neoantigen-specific T cell populations in 50% of treated patients, demonstrating feasibility and immunogenicity in the adjuvant setting (128).
Doxil
Doxil, a PEGylated liposomal formulation of doxorubicin, was the first nanomedicine approved for oncological use and remains a benchmark in nanoparticle-based chemotherapy (145). Although extensively validated for tumour cytoreduction, it was not originally engineered for immunomodulatory synergy, and its role in immune-based strategies remains to be fully elucidated. By prolonging systemic circulation and enhancing tumour accumulation via the enhanced permeability and retention (EPR) effect, Doxil offers a means to reduce cardiotoxicity while preserving antitumour efficacy.
In a phase II randomised trial comparing two regimens for advanced gastric cancer, a triplet combination of 5-fluorouracil (5-FU), cisplatin, and Doxil (arm A) was evaluated against a standard regimen containing 5-FU, cisplatin, and mitomycin-C (arm B). Arm A achieved a significantly higher overall response rate (ORR) of 64.1% versus 38.5% in arm B (P=0.041). Median time to tumour progression (TTP) was 7.93 vs 5.14 months (P=0.04), and overall survival (OS) was 12.1 vs 8.3 months (P=0.02), favouring the Doxil-containing regimen (146). Another phase II study investigated a four-drug combination including pegylated liposomal doxorubicin, mitomycin C, infusional 5-FU, and sodium folinic acid in patients with advanced gastric cancer. This regimen demonstrated an ORR of 47%, despite the cohort comprising predominantly elderly patients at moderate to high baseline risk (147).
In a phase I trial involving patients with upper gastrointestinal malignancies, preliminary antitumour activity was observed with the same four-drug regimen. Notably, responses were recorded in pretreated pancreatic cancer and untreated gastric cancer, supporting further investigation of Doxil-based combinations in this setting (148).
Onivyde
Irinotecan, a topoisomerase I inhibitor, is an integral component of the FOLFIRINOX regimen, currently established as first-line therapy for pancreatic ductal adenocarcinoma (PDAC) in multiple international guidelines. However, its efficacy in second-line settings remains limited, and few randomised trials have conclusively defined its value beyond frontline therapy. The liposomal formulation of irinotecan (nal-IRI) exemplifies nanotechnology’s potential to enhance therapeutic efficacy without exacerbating systemic toxicity (149).
In the phase 3 PAN-HEROIC-1 trial, irinotecan hydrochloride liposome (HR070803) combined with 5-fluorouracil and leucovorin significantly improved median overall survival in patients with locally advanced or metastatic PDAC following gemcitabine failure, compared with placebo (150). In a phase 1/2 trial of nal-IRI plus S-1 in gemcitabine-refractory metastatic PDAC, the median overall survival reached 10.3 months, and a confirmed partial response was achieved in 20.4% of patients (151). In Japan, multiple studies have evaluated the safety and efficacy of nal-IRI-based regimens in post-gemcitabine PDAC. One phase 2 trial confirmed the tolerability of nal-IRI with fluorouracil and leucovorin in Japanese patients with unresectable disease (152). Another randomised phase 2 trial comparing nal-IRI plus 5-FU/LV versus 5-FU/LV alone showed improved objective response rate (18% vs 0%) and a median overall survival of 6.3 months in the experimental arm, although OS in the control arm was not reached at the time of analysis (153).
Beyond pancreatic cancer, nal-IRI is being explored in other gastrointestinal malignancies. In RAS wild-type metastatic colorectal cancer, a phase II study (CRACK trial, cohort B) investigated a combination of cetuximab retreatment, camrelizumab, and liposomal irinotecan. This regimen yielded an objective response rate of 25%, a disease control rate of 75%, and median progression-free and overall survival times of 6.9 and 15.1 months, respectively (154). In locally advanced rectal cancer, liposomal irinotecan is being incorporated into multimodal neoadjuvant strategies. A recent phase 2 trial examined nal-IRI in combination with 5-FU, leucovorin, and oxaliplatin, followed by chemoradiotherapy in a watch-and-wait programme. Among patients achieving clinical complete response (cCR), the median disease-free survival (DFS) was not reached after 32 months of follow-up, with 1-, 2-, and 3-year DFS rates of 90.0%, 80.0%, and 80.0%, respectively (155).
Future perspectives and conclusion
Nanoformulations hold great promise for onco-immunotherapy, owing to their biocompatibility, stability, and capacity for precise tumour targeting (156). Yet, their translation into the clinic faces several key hurdles. First, nanoparticles are inherently more complex than small molecules: parameters such as size, composition, surface chemistry, and shape all influence in vivo behaviour (157), and these properties can shift upon protein corona formation, complicating PK–PD relationships. Second, the rapid clearance of nanoparticles by the reticuloendothelial system (RES) limits tumour bioavailability and risks off-target toxicity in the liver and spleen; although PEGylation and size minimisation can mitigate this (158), complete RES evasion remains elusive. Third, heterogeneous tumour architectures and variable EPR effects mean that neither active nor passive targeting guarantees uniform delivery (159); responsive nanocarriers that release payloads under specific TME cues (e.g., pH, enzyme activity) offer a way forward but require precise tuning of size and charge to optimise penetration and retention (160). Finally, nanotoxicology—encompassing genotoxicity, immunotoxicity, and organ‐specific adverse effects—remains under-characterised; systematic, standardised assays in physiologically relevant models are essential before first-in-human studies (161).
In addition, the practical feasibility and commercial viability of nanomedicines depend upon reproducible synthesis methods, cost-effective manufacturing, stringent quality control, and scalable production capabilities (162, 163). The inherent complexity of nanoformulations often renders large-scale production challenging, necessitating simplified yet robust synthetic approaches that maintain consistency between batches (164). Rigorous examination of toxicity profiles, biosafety risks, and nanocarrier–tissue interactions is paramount, as discrepancies between in vitro and in vivo outcomes frequently occur during preclinical development (165–167). Furthermore, robustly designed clinical trials remain critical, demanding comprehensive evaluation not only of efficacy but also mechanisms of action, toxicity, survival outcomes, and clinically relevant adverse events (168).
Beyond these considerations, nano-immunotherapeutics must also navigate stringent regulatory and good-manufacturing-practice (GMP) complexities. Regulatory guidelines from the US FDA (Drug Products, Including Biological Products, that Contain Nanomaterials) and EMA (Reflection Paper on nanotechnology-based medicinal products) emphasise early identification and stringent control of critical quality attributes (CQAs), including particle size, polydispersity, surface charge, encapsulation efficiency, residual solvents, endotoxin limits, sterility, and in vitro release characteristics, aligned with quality-by-design frameworks (ICH Q8–Q10) (169). Continuous-flow or microfluidic manufacturing processes, while enabling precise control of particle characteristics, introduce GMP-specific challenges related to cleaning validation, in-line monitoring, and batch consistency (170, 171). Real-world cases of approved nanomedicines underscore these complexities: PEGylated liposomal doxorubicin (Doxil®) faced significant manufacturing deviations necessitating revalidation and bioequivalence studies (172, 173); patisiran (Onpattro®), the first siRNA LNP therapeutic, leveraged GMP-compliant microfluidic platforms for consistent production (170); and the rapid deployment of mRNA–LNP COVID-19 vaccines highlighted additional challenges in RNA stability, cold-chain logistics, and impurity control (174, 175). Consequently, systematic toxicological assessment and rigorous biosafety evaluations remain prerequisites for the successful translation of nanomedicine into clinical practice.
Emerging clinical and preclinical data support the synchronised use of nano-formulated immunotherapeutics with checkpoint inhibitors, adoptive cell therapies, or TLR/STING agonists to overcome tumour immunosuppression. Neoadjuvant trials in colorectal cancer—such as AVANA, combining chemoradiation with pembrolizumab—have achieved pathological complete response rates up to 23% and major pathological response rates of 61.5% (176), highlighting the feasibility of early‐stage intervention and on-treatment biomarker assessment. Incorporating nanomedicines into these settings may further amplify immune activation, improve margin clearance, and allow rapid pharmacodynamic readouts to refine dosing and sequence.
While intravenous lipid nanoparticles have dominated the field, alternative administration routes better aligned with tumour biology warrant vigorous exploration. Oral or intraluminal delivery of nanoparticles—designed to withstand gastrointestinal barriers—can directly target colorectal lesions, as reviewed by Pavitra et al. in orally administrable formulations achieving enhanced mucosal uptake and reduced systemic toxicity (177). Similarly, intraperitoneal delivery may increase regional exposure in peritoneal metastases of gastric or ovarian origin. Development of selective‐organ‐targeting LNPs, which exploit lipid composition to redirect payloads to specific organs, further exemplifies how chemical tuning can achieve precision biodistribution.
The complexity of nanoparticle disposition—shaped by protein corona formation, RES clearance, and tumour penetration—demands embedded PK–PD and immune readouts in early clinical trials. High‐resolution imaging (e.g., PET‐labeled nanoparticles), single‐cell RNA sequencing of serial biopsies, and circulating immune profiling will enable correlations between formulation parameters and therapeutic response. Machine‐learning models trained on these multimodal datasets can then predict optimal nanoparticle designs and dosing regimens, closing the loop between experimental insight and clinical application (178).
In concert, these strategies—rational combination regimens, tailored delivery approaches, and rigorous translational science—will be essential to overcome current barriers and deliver precision‐engineered nanotherapies with durable efficacy and safety in gastrointestinal malignancies.
Author contributions
CC: Conceptualization, Writing – original draft, Writing – review & editing. JL: Methodology, Writing – original draft. XH: Methodology, Writing – original draft. TD: Methodology, Writing – original draft. ZZ: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. (2020) 159:335–49.e15. doi: 10.1053/j.gastro.2020.02.068
3. Wang S, Zheng R, Li J, Zeng H, Li L, Chen R, et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol. (2024) 9:229–37. doi: 10.1016/S2468-1253(23)00366-7
4. Kalager M, Adami HO, Lagergren P, Steindorf K, and Dickman PW. Cancer outcomes research-a European challenge: measures of the cancer burden. Mol Oncol. (2021) 15:3225–41. doi: 10.1002/1878-0261.13012
5. Ahmed S and Rai KR. Interferon in the treatment of hairy-cell leukemia. Best Pract Res Clin Haematol. (2003) 16:69–81. doi: 10.1016/S1521-6926(02)00084-1
6. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. (2014) 192:5451–8. doi: 10.4049/jimmunol.1490019
7. Riley RS, June CH, Langer R, and Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. (2019) 18:175–96. doi: 10.1038/s41573-018-0006-z
8. Jiang W, Wang Y, Wargo JA, Lang FF, and Kim BYS. Considerations for designing preclinical cancer immune nanomedicine studies. Nat Nanotechnol. (2021) 16:6–15. doi: 10.1038/s41565-020-00817-9
9. Cao J, Huang D, and Peppas NA. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv Drug Delivery Rev. (2020) 167:170–88. doi: 10.1016/j.addr.2020.06.030
10. Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10. doi: 10.1101/cshperspect.a028472
11. Yong SB, Chung JY, Song Y, Kim J, Ra S, and Kim YH. Non-viral nano-immunotherapeutics targeting tumor microenvironmental immune cells. Biomaterials. (2019) 219:119401. doi: 10.1016/j.biomaterials.2019.119401
12. Chen Z, Wang Z, and Gu Z. Bioinspired and biomimetic nanomedicines. Acc Chem Res. (2019) 52:1255–64. doi: 10.1021/acs.accounts.9b00079
13. Vasan N, Baselga J, and Hyman DM. A view on drug resistance in cancer. Nature. (2019) 575:299–309. doi: 10.1038/s41586-019-1730-1
14. Chong X, Madeti Y, Cai J, Li W, Cong L, Lu J, et al. Recent developments in immunotherapy for gastrointestinal tract cancers. J Hematol Oncol. (2024) 17:65. doi: 10.1186/s13045-024-01578-x
15. Wang ZX, Pan YQ, Li X, Tsubata T, and Xu RH. Immunotherapy in gastrointestinal cancers: advances, challenges, and countermeasures. Sci Bull (Beijing). (2023) 68:763–6. doi: 10.1016/j.scib.2023.03.036
16. Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. (2022) 15:95. doi: 10.1186/s13045-022-01294-4
17. Ngo TH, Menon S, and Rivero-Müller A. Nano-immunotherapy: Merging immunotherapy precision with nanomaterial delivery. iScience. (2025) 28:112319. doi: 10.1016/j.isci.2025.112319
18. Wang M, Yu F, and Zhang Y. Present and future of cancer nano-immunotherapy: opportunities, obstacles and challenges. Mol Cancer. (2025) 24:26. doi: 10.1186/s12943-024-02214-5
19. Zhang M, Liu C, Tu J, Tang M, Ashrafizadeh M, Nabavi N, et al. Advances in cancer immunotherapy: historical perspectives, current developments, and future directions. Mol Cancer. (2025) 24:136. doi: 10.1186/s12943-025-02305-x
20. Kim EH, Choi J, Jang H, Kim Y, Lee JW, Ryu Y, et al. Targeted delivery of anti-miRNA21 sensitizes PD-L1(high) tumor to immunotherapy by promoting immunogenic cell death. Theranostics. (2024) 14:3777–92. doi: 10.7150/thno.97755
21. Li H, Gong Q, and Luo K. Biomarker-driven molecular imaging probes in radiotherapy. Theranostics. (2024) 14:4127–46. doi: 10.7150/thno.97768
22. Muradova Z, Carmès L, Brown N, Rossetti F, Guthier R, Yasmin-Karim S, et al. Targeted-theranostic nanoparticles induce anti-tumor immune response in lung cancer. J Nanobiotechnology. (2025) 23:466. doi: 10.1186/s12951-025-03542-4
23. Li H, Luo Q, Zhang H, Ma X, Gu Z, Gong Q, et al. Nanomedicine embraces cancer radio-immunotherapy: mechanism, design, recent advances, and clinical translation. Chem Soc Rev. (2023) 52:47–96. doi: 10.1039/D2CS00437B
24. Qian M, Chen L, Du Y, Jiang H, Huo T, Yang Y, et al. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. (2019) 19:8409–17. doi: 10.1021/acs.nanolett.9b02448
25. Hickey JW, Vicente FP, Howard GP, Mao HQ, and Schneck JP. Biologically inspired design of nanoparticle artificial antigen-presenting cells for immunomodulation. Nano Lett. (2017) 17:7045–54. doi: 10.1021/acs.nanolett.7b03734
26. Goodwin TJ, Zhou Y, Musetti SN, Liu R, and Huang L. Local and transient gene expression primes the liver to resist cancer metastasis. Sci Transl Med. (2016) 8:364ra153. doi: 10.1126/scitranslmed.aag2306
27. Zeng Q, Liu M, Wang Z, Zhou R, and Ai K. Enhancing radiotherapy-induced anti-tumor immunity via nanoparticle-mediated STING agonist synergy. Mol Cancer. (2025) 24:176. doi: 10.1186/s12943-025-02366-y
28. Bu J, Nair A, Iida M, Jeong WJ, Poellmann MJ, Mudd K, et al. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. (2020) 20:4901–9. doi: 10.1021/acs.nanolett.0c00953
29. Thomas EM, Wright JA, Blake SJ, Page AJ, Worthley DL, and Woods SL. Advancing translational research for colorectal immuno-oncology. Br J Cancer. (2023) 129:1442–50. doi: 10.1038/s41416-023-02392-x
30. Greenlee JD and King MR. A syngeneic MC38 orthotopic mouse model of colorectal cancer metastasis. Biol Methods Protoc. (2022) 7:bpac024. doi: 10.1093/biomethods/bpac024
31. Awasthi N, Schwarz MA, Kaurich Q, Zhang C, Hilberg F, and Schwarz RE. Enhancing gastric cancer conventional chemotherapy effects by triple angiokinase inhibitor nintedanib in preclinical models. Front Oncol. (2023) 13:1145999. doi: 10.3389/fonc.2023.1145999
32. Wang C, Xie GM, Zhang LP, Yan S, Xu JL, Han YL, et al. High engraftment and metastatic rates in orthotopic xenograft models of gastric cancer via direct implantation of tumor cell suspensions. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16040759
33. Ferrari DP, Ramos-Gomes F, Alves F, and Markus MA. KPC-luciferase-expressing cells elicit an anti-tumor immune response in a mouse model of pancreatic cancer. Sci Rep. (2024) 14:13602. doi: 10.1038/s41598-024-64053-0
34. Dennaoui R, Shrestha H, and Wagner KU. Models of pancreatic ductal adenocarcinoma. Cancer Metastasis Rev. (2021) 40:803–18. doi: 10.1007/s10555-021-09989-9
35. Bang HJ, Lee KH, Park MS, Sun EG, Cho SH, Chung IJ, et al. Dynamic changes in immune cells in humanized liver metastasis and subcutaneous xenograft mouse models. Sci Rep. (2024) 14:20338. doi: 10.1038/s41598-024-69988-y
36. Chuprin J, Buettner H, Seedhom MO, Greiner DL, Keck JG, Ishikawa F, et al. Humanized mouse models for immuno-oncology research. Nat Rev Clin Oncol. (2023) 20:192–206. doi: 10.1038/s41571-022-00721-2
37. Podojil JR, Cogswell AC, Chiang MY, Eaton V, Ifergan I, Neef T, et al. Biodegradable nanoparticles induce cGAS/STING-dependent reprogramming of myeloid cells to promote tumor immunotherapy. Front Immunol. (2022) 13:887649. doi: 10.3389/fimmu.2022.887649
38. Shields NJ, Peyroux EM, Ferguson AL, Steain M, Neumann S, and Young SL. Late-stage MC38 tumours recapitulate features of human colorectal cancer - implications for appropriate timepoint selection in preclinical studies. Front Immunol. (2023) 14:1152035. doi: 10.3389/fimmu.2023.1152035
39. Xiao Z, Todd L, Huang L, Noguera-Ortega E, Lu Z, Huang L, et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat Commun. (2023) 14:5110. doi: 10.1038/s41467-023-40850-5
40. Ju Y, Xu D, Liao MM, Sun Y, Bao WD, Yao F, et al. Barriers and opportunities in pancreatic cancer immunotherapy. NPJ Precis Oncol. (2024) 8:199. doi: 10.1038/s41698-024-00681-z
41. Mosely SI, Prime JE, Sainson RC, Koopmann JO, Wang DY, Greenawalt DM, et al. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol Res. (2017) 5:29–41. doi: 10.1158/2326-6066.CIR-16-0114
42. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, and Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. (2021) 20:101–24. doi: 10.1038/s41573-020-0090-8
43. He B, Sui X, Yu B, Wang S, Shen Y, and Cong H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. (2020) 27:1474–90. doi: 10.1080/10717544.2020.1831106
44. Ma C, Cheng Z, Tan H, Wang Y, Sun S, Zhang M, et al. Nanomaterials: leading immunogenic cell death-based cancer therapies. Front Immunol. (2024) 15:1447817. doi: 10.3389/fimmu.2024.1447817
45. Dhayalan M, Wang W, Riyaz SUM, Dinesh RA, Shanmugam J, Irudayaraj SS, et al. Advances in functional lipid nanoparticles: from drug delivery platforms to clinical applications. 3 Biotech. (2024) 14:57. doi: 10.1007/s13205-023-03901-8
46. Lamb YN and Scott LJ. Liposomal irinotecan: A review in metastatic pancreatic adenocarcinoma. Drugs. (2017) 77:785–92. doi: 10.1007/s40265-017-0741-1
47. Böttger R, Pauli G, Chao PH, Al Fayez N, Hohenwarter L, and Li SD. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Delivery Rev. (2020) 154-155:79–101. doi: 10.1016/j.addr.2020.06.017
48. Cheng MHY, Zhang Y, Fox K, Leung J, Strong C, Kang E, et al. Liposomal lipid nanoparticles for extrahepatic delivery of mRNA. Nat Commun. (2025) 16:4135. doi: 10.1038/s41467-025-58523-w
49. Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. (2010) 29:482–91. doi: 10.1038/onc.2009.356
50. Yu H, Liu S, Yuan Z, Huang H, Yan P, and Zhu W. Targeted co-delivery of rapamycin and oxaliplatin by liposomes suppresses tumor growth and metastasis of colorectal cancer. BioMed Pharmacother. (2024) 178:117192. doi: 10.1016/j.biopha.2024.117192
51. Juang V, Chang CH, Wang CS, Wang HE, and Lo YL. pH-responsive PEG-shedding and targeting peptide-modified nanoparticles for dual-delivery of irinotecan and microRNA to enhance tumor-specific therapy. Small. (2019) 15:e1903296. doi: 10.1002/smll.201903296
52. Patel MN, Lakkadwala S, Majrad MS, Injeti ER, Gollmer SM, Shah ZA, et al. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS PharmSciTech. (2014) 15:1498–508. doi: 10.1208/s12249-014-0168-x
53. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. (2016) 24:1783–96. doi: 10.1038/mt.2016.159
54. Soppimath KS, Aminabhavi TM, Kulkarni AR, and Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. (2001) 70:1–20. doi: 10.1016/S0168-3659(00)00339-4
55. Owens DE 3rd and Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. (2006) 307:93–102. doi: 10.1016/j.ijpharm.2005.10.010
56. Łukasiewicz S, Szczepanowicz K, Podgórna K, Błasiak E, Majeed N, Ogren S, et al. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf B Biointerfaces. (2016) 140:342–52. doi: 10.1016/j.colsurfb.2015.12.044
57. Mainini F and Eccles MR. Lipid and polymer-based nanoparticle siRNA delivery systems for cancer therapy. Molecules. (2020) 25. doi: 10.3390/molecules25112692
58. DeFrates K, Markiewicz T, Gallo P, Rack A, Weyhmiller A, Jarmusik B, et al. Protein polymer-based nanoparticles: fabrication and medical applications. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19061717
59. Mu W, Chu Q, Liu Y, and Zhang N. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nanomicro Lett. (2020) 12:142. doi: 10.1007/s40820-020-00482-6
60. Badran MM, Mady MM, Ghannam MM, and Shakeel F. Preparation and characterization of polymeric nanoparticles surface modified with chitosan for target treatment of colorectal cancer. Int J Biol Macromol. (2017) 95:643–9. doi: 10.1016/j.ijbiomac.2016.11.098
61. Li X, Niu J, Deng L, Yu Y, Zhang L, Chen Q, et al. Amphiphilic polymeric nanodrug integrated with superparamagnetic iron oxide nanoparticles for synergistic antibacterial and antitumor therapy of colorectal cancer. Acta Biomater. (2024) 173:432–41. doi: 10.1016/j.actbio.2023.11.019
62. Zhao Y, Hu K, Wang F, Zhao L, Su Y, Chen J, et al. Guanidine-derived polymeric nanoinhibitors target the lysosomal V-ATPase and activate AMPK pathway to ameliorate liver lipid accumulation. Adv Sci (Weinh). (2025) 12:e2408906. doi: 10.1002/advs.202408906
63. Song X, Cai H, Shi Z, Li Z, Zheng X, Yang K, et al. Enzyme-responsive branched glycopolymer-based nanoassembly for co-delivery of paclitaxel and akt inhibitor toward synergistic therapy of gastric cancer. Adv Sci (Weinh). (2024) 11:e2306230. doi: 10.1002/advs.202306230
64. Hong S, Choi DW, Kim HN, Park CG, Lee W, and Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. (2020) 12. doi: 10.3390/pharmaceutics12070604
65. Rodríguez F, Caruana P, de la Fuente N, Español P, Gámez M, Balart J, et al. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges. Biomolecules. (2022) 12. doi: 10.3390/biom12060784
66. Huang L, Tao K, Liu J, Qi C, Xu L, Chang P, et al. Design and fabrication of multifunctional sericin nanoparticles for tumor targeting and pH-responsive subcellular delivery of cancer chemotherapy drugs. ACS Appl Mater Interfaces. (2016) 8:6577–85. doi: 10.1021/acsami.5b11617
67. Han Z, Song B, Yang J, Wang B, Ma Z, Yu L, et al. Curcumin-encapsulated fusion protein-based nanocarrier demonstrated highly efficient epidermal growth factor receptor-targeted treatment of colorectal cancer. J Agric Food Chem. (2022) 70:15464–73. doi: 10.1021/acs.jafc.2c04668
68. Heydarian R, Divsalar A, Kouchesfehani HM, and Rasouli M. Folic acid-targeted β-lactoglobulin nanocarriers for enhanced delivery of 5-fluorouracil and sodium butyrate in colorectal cancer treatment. Int J Pharm. (2025) 671:125262. doi: 10.1016/j.ijpharm.2025.125262
69. Rivera Gil P, Hühn D, del Mercato LL, Sasse D, and Parak WJ. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. (2010) 62:115–25. doi: 10.1016/j.phrs.2010.01.009
70. Nascimento CS, Alves É AR, de Melo CP, Corrêa-Oliveira R, and Calzavara-Silva CE. Immunotherapy for cancer: effects of iron oxide nanoparticles on polarization of tumor-associated macrophages. Nanomedicine (Lond). (2021) 16:2633–50. doi: 10.2217/nnm-2021-0255
71. Attri K, Chudasama B, Mahajan RL, and Choudhury D. Perturbation of hyperthermia resistance in gastric cancer by hyperstimulation of autophagy using artemisinin-protected iron-oxide nanoparticles. RSC Adv. (2024) 14:34565–77. doi: 10.1039/D4RA05611F
72. Mikaeili Ghezeljeh S, Salehzadeh A, and Ataei EJS. Iron oxide nanoparticles coated with Glucose and conjugated with Safranal (Fe(3)O(4)@Glu-Safranal NPs) inducing apoptosis in liver cancer cell line (HepG2). BMC Chem. (2024) 18:33. doi: 10.1186/s13065-024-01142-1
73. Savchuk V, Wang R, Small L, and Pinchuk A. Synergistic effect in hybrid plasmonic conjugates for photothermal applications. ACS Omega. (2024) 9:47436–41. doi: 10.1021/acsomega.4c05068
74. Wang J, Wang X, Xiong Q, Gao S, Wang S, Zhu S, et al. A dual-STING-activating nanosystem expands cancer immunotherapeutic temporal window. Cell Rep Med. (2024) 5:101797. doi: 10.1016/j.xcrm.2024.101797
75. Pakravan A, Salehi R, and Mahkam M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagnosis Photodyn Ther. (2021) 33:102144. doi: 10.1016/j.pdpdt.2020.102144
76. Xin J, Fu L, Wang J, Wang S, Zhang L, Zhang Z, et al. Influence of parameters on the death pathway of gastric cells induced by gold nanosphere mediated phototherapy. Nanomaterials (Basel). (2022) 12. doi: 10.3390/nano12040646
77. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. (2019) 10:3349. doi: 10.1038/s41467-019-11269-8
78. Wang C, Bao C, Liang S, Fu H, Wang K, Deng M, et al. RGD-conjugated silica-coated gold nanorods on the surface of carbon nanotubes for targeted photoacoustic imaging of gastric cancer. Nanoscale Res Lett. (2014) 9:264. doi: 10.1186/1556-276X-9-264
79. Zhou Z, Zhang C, Qian Q, Ma J, Huang P, Zhang X, et al. Folic acid-conjugated silica capped gold nanoclusters for targeted fluorescence/X-ray computed tomography imaging. J Nanobiotechnology. (2013) 11:17. doi: 10.1186/1477-3155-11-17
80. Liu S, Liu Y, Chang Q, Celia C, Deng X, and Xie Y. pH-responsive sorafenib/iron-co-loaded mesoporous polydopamine nanoparticles for synergistic ferroptosis and photothermal therapy. Biomacromolecules. (2024) 25:522–31. doi: 10.1021/acs.biomac.3c01173
81. Liu H, Yang Y, Zhang N, Hou Y, Zhang Z, Yu X, et al. Overcoming photothermal resistance of gastric cancer by bionic 2D iron-based nanoplatforms with precise CRISPR/cas9 delivery. ACS Nano. (2025) 19:18188–202. doi: 10.1021/acsnano.4c16846
82. Wang Y, Zhang X, Ma Y, Zhou X, Xu W, Qin S, et al. Self-assembled copper-based nanoparticles for enzyme catalysis-enhanced chemodynamic/photodynamic/antiangiogenic tritherapy against hepatocellular carcinoma. J Nanobiotechnology. (2024) 22:375. doi: 10.1186/s12951-024-02626-x
83. Zheng C, Wang Q, Wang Y, Zhao X, Gao K, Liu Q, et al. In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv Mater. (2019) 31:e1902542. doi: 10.1002/adma.201902542
84. Sun J, Wan Z, Xu J, Luo Z, Ren P, Zhang B, et al. Tumor size-dependent abscopal effect of polydopamine-coated all-in-one nanoparticles for immunochemo-photothermal therapy of early- and late-stage metastatic cancer. Biomaterials. (2021) 269:120629. doi: 10.1016/j.biomaterials.2020.120629
85. Liu H, Du Y, Zhan D, Yu W, Li Y, Wang A, et al. Oxaliplatin lipidated prodrug synergistically enhances the anti-colorectal cancer effect of IL12 mRNA. Drug Delivery Transl Res. (2024) 14:3186–99. doi: 10.1007/s13346-024-01540-x
86. Su L, Pan W, Li X, Zhou X, Ma X, and Min Y. Utilizing chemotherapy-induced tumor RNA nanoparticles to improve cancer chemoimmunotherapy. Acta Biomater. (2023) 158:698–707. doi: 10.1016/j.actbio.2022.12.039
87. Zhu M, Wang Z, He Y, Zhang B, Wu L, Liu C, et al. Acidic tumor microenvironment-modulated nanoparticle potentiates gastric cancer photoimmunotherapy. J Adv Res. (2025). doi: 10.1016/j.jare.2025.06.008
88. Wang Z, Zhu M, Dong R, Cao D, Li Y, Chen Z, et al. TH-302-loaded nanodrug reshapes the hypoxic tumour microenvironment and enhances PD-1 blockade efficacy in gastric cancer. J Nanobiotechnology. (2023) 21:440. doi: 10.1186/s12951-023-02203-8
89. Xu H, Li S, Liu Y, Sung YY, Zhou Y, and Wu H. A novel pH-sensitive nanoparticles encapsulating anti-PD-1 antibody and MDK-siRNA overcome immune checkpoint blockade resistance in HCC via reshaping immunosuppressive TME. J Exp Clin Cancer Res. (2025) 44:148. doi: 10.1186/s13046-025-03396-6
90. Wang L, Bi S, Li Z, Liao A, Li Y, Yang L, et al. Napabucasin deactivates STAT3 and promotes mitoxantrone-mediated cGAS-STING activation for hepatocellular carcinoma chemo-immunotherapy. Biomaterials. (2025) 313:122766. doi: 10.1016/j.biomaterials.2024.122766
91. Lin Z, Huang K, Guo H, Jia M, Sun Q, Chen X, et al. Targeting ZDHHC9 potentiates anti-programmed death-ligand 1 immunotherapy of pancreatic cancer by modifying the tumor microenvironment. BioMed Pharmacother. (2023) 161:114567. doi: 10.1016/j.biopha.2023.114567
92. Manna S, Maiti S, Shen J, Weiss A, Mulder E, Du W, et al. Nanovaccine that activates the NLRP3 inflammasome enhances tumor specific activation of anti-cancer immunity. Biomaterials. (2023) 296:122062. doi: 10.1016/j.biomaterials.2023.122062
93. Billingsley MM, Gong N, Mukalel AJ, Thatte AS, El-Mayta R, Patel SK, et al. In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism. Small. (2024) 20:e2304378. doi: 10.1002/smll.202304378
94. Kim H, Khanna V, Kucaba TA, Zhang W, Sehgal D, Ferguson DM, et al. TLR7/8 agonist-loaded nanoparticles augment NK cell-mediated antibody-based cancer immunotherapy. Mol Pharm. (2020) 17:2109–24. doi: 10.1021/acs.molpharmaceut.0c00271
95. Poelaert BJ, Romanova S, Knoche SM, Olson MT, Sliker BH, Smits K, et al. Nanoformulation of CCL21 greatly increases its effectiveness as an immunotherapy for neuroblastoma. J Control Release. (2020) 327:266–83. doi: 10.1016/j.jconrel.2020.07.024
96. Feng Z, Zhang X, Peng Z, Aghamajidi A, Wu Y, and Hua X. Nanobody-directed CEA-targeting CAR T cells eliminate gastrointestinal cancer xenografts. Cancer Immunol Res. (2025) 13(8):1160–71. doi: 10.1158/2326-6066.c.7960390
97. Yang Z, Liu Y, Zhao K, Jing W, Gao L, Dong X, et al. Dual mRNA co-delivery for in situ generation of phagocytosis-enhanced CAR macrophages augments hepatocellular carcinoma immunotherapy. J Control Release. (2023) 360:718–33. doi: 10.1016/j.jconrel.2023.07.021
98. Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, and Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. (2020) 20:1578–89. doi: 10.1021/acs.nanolett.9b04246
99. Kheirolomoom A, Kare AJ, Ingham ES, Paulmurugan R, Robinson ER, Baikoghli M, et al. In situ T-cell transfection by anti-CD3-conjugated lipid nanoparticles leads to T-cell activation, migration, and phenotypic shift. Biomaterials. (2022) 281:121339. doi: 10.1016/j.biomaterials.2021.121339
100. Zhang Y, Lin S, Wang XY, and Zhu G. Nanovaccines for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2019) 11:e1559. doi: 10.1002/wnan.1559
101. Aikins ME, Xu C, and Moon JJ. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc Chem Res. (2020) 53:2094–105. doi: 10.1021/acs.accounts.0c00456
102. Diao L and Liu M. Rethinking antigen source: cancer vaccines based on whole tumor cell/tissue lysate or whole tumor cell. Adv Sci (Weinh). (2023) 10:e2300121. doi: 10.1002/advs.202300121
103. Hernandez R and Malek TR. Fueling cancer vaccines to improve T cell-mediated antitumor immunity. Front Oncol. (2022) 12:878377. doi: 10.3389/fonc.2022.878377
104. Kaiser J. Personalized tumor vaccines keep cancer in check. Science. (2017) 356:122. doi: 10.1126/science.356.6334.122
105. Llovet JM, Pinyol R, Yarchoan M, Singal AG, Marron TU, Schwartz M, et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. (2024) 21:294–311. doi: 10.1038/s41571-024-00868-0
106. Du Q, Luo Y, Xu L, Du C, Zhang W, Xu J, et al. Smart responsive Fe/Mn nanovaccine triggers liver cancer immunotherapy via pyroptosis and pyroptosis-boosted cGAS-STING activation. J Nanobiotechnology. (2024) 22:95. doi: 10.1186/s12951-024-02354-2
107. Tian Z, Hu Q, Sun Z, Wang N, He H, Tang Z, et al. A booster for radiofrequency ablation: advanced adjuvant therapy via in situ nanovaccine synergized with anti-programmed death ligand 1 immunotherapy for systemically constraining hepatocellular carcinoma. ACS Nano. (2023) 17:19441–58. doi: 10.1021/acsnano.3c08064
108. Fan T, Xu C, Wu J, Cai Y, Cao W, Shen H, et al. Lipopolyplex-formulated mRNA cancer vaccine elicits strong neoantigen-specific T cell responses and antitumor activity. Sci Adv. (2024) 10:eadn9961. doi: 10.1126/sciadv.adn9961
109. Ben-Akiva E, Karlsson J, Hemmati S, Yu H, Tzeng SY, Pardoll DM, et al. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc Natl Acad Sci U S A. (2023) 120:e2301606120. doi: 10.1073/pnas.2301606120
110. Wu C, He W, Chen Y, Cai J, Zeng F, Lu Z, et al. Personalized bacteria loaded with autoantigens for the enhancement of tumor immunotherapy. Adv Healthc Mater. (2023) 12:e2203026. doi: 10.1002/adhm.202203026
111. Shi G, Xu Y, Qiu H, Cao F, Xiao ZX, Zhang C, et al. Personalized membrane protein vaccine based on a lipid nanoparticle delivery system prevents postoperative recurrence in colorectal cancer models. Acta Biomater. (2025) 192:315–27. doi: 10.1016/j.actbio.2024.12.003
112. Yan Y, Duan T, Xue X, Yang X, Liu M, Ma B, et al. LBP-CD155 liposome nanovaccine efficiently resist colorectal cancer and enhance ICB therapy. Int J Nanomedicine. (2025) 20:1047–63. doi: 10.2147/IJN.S492734
113. Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. (2017) 12:648–54. doi: 10.1038/nnano.2017.52
114. Luo Z, He T, Liu P, Yi Z, Zhu S, Liang X, et al. Self-adjuvanted molecular activator (SeaMac) nanovaccines promote cancer immunotherapy. Adv Healthc Mater. (2021) 10:e2002080. doi: 10.1002/adhm.202002080
115. Ghazizadeh Y, Salehi Shadkami H, Madani F, Niknam S, and Adabi M. Advances in cancer nanovaccines: a focus on colorectal cancer. Nanomedicine (Lond). (2025) 20:1029–41. doi: 10.1080/17435889.2025.2486930
116. Li J, Huang D, Cheng R, Figueiredo P, Fontana F, Correia A, et al. Multifunctional biomimetic nanovaccines based on photothermal and weak-immunostimulatory nanoparticulate cores for the immunotherapy of solid tumors. Adv Mater. (2022) 34:e2108012. doi: 10.1002/adma.202108012
117. Mai J, Li Z, Xia X, Zhang J, Li J, Liu H, et al. Synergistic activation of antitumor immunity by a particulate therapeutic vaccine. Adv Sci (Weinh). (2021) 8:2100166. doi: 10.1002/advs.202100166
118. Kim J, Kang S, Kim KW, Heo MG, Park DI, Lee JH, et al. Nanoparticle delivery of recombinant IL-2 (BALLkine-2) achieves durable tumor control with less systemic adverse effects in cancer immunotherapy. Biomaterials. (2022) 280:121257. doi: 10.1016/j.biomaterials.2021.121257
119. Akkın S, Varan G, Işık A, Gökşen S, Karakoç E, Malanga M, et al. Synergistic antitumor potency of a self-assembling cyclodextrin nanoplex for the co-delivery of 5-fluorouracil and interleukin-2 in the treatment of colorectal cancer. Pharmaceutics. (2023) 15. doi: 10.3390/pharmaceutics15020314
120. Wang D, Wang X, Zhang Y, Yu L, An J, Wang X, et al. The combination of IL-2 nanoparticles and Palbociclib enhances the anti-tumor immune response for colon cancer therapy. Front Immunol. (2024) 15:1309509. doi: 10.3389/fimmu.2024.1309509
121. Barberio AE, Smith SG, Pires IS, Iyer S, Reinhardt F, Melo MB, et al. Layer-by-layer interleukin-12 nanoparticles drive a safe and effective response in ovarian tumors. Bioeng Transl Med. (2023) 8:e10453. doi: 10.1002/btm2.10453
122. Li T, Liu Z, Fu X, Chen Y, Zhu S, and Zhang J. Co-delivery of Interleukin-12 and doxorubicin loaded Nano-delivery system for enhanced immunotherapy with polarization toward M1-type Macrophages. Eur J Pharm Biopharm. (2022) 177:175–83. doi: 10.1016/j.ejpb.2022.07.002
123. Liu X, Gao X, Zheng S, Wang B, Li Y, Zhao C, et al. Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomedicine. (2017) 13:1993–2004. doi: 10.1016/j.nano.2017.04.006
124. Luo M, Liang X, Luo ST, Wei XW, Liu T, Ren J, et al. Folate-modified lipoplexes delivering the interleukin-12 gene for targeting colon cancer immunogene therapy. J BioMed Nanotechnol. (2015) 11:2011–23. doi: 10.1166/jbn.2015.2136
125. Fujimura NA, Fatima SE, Ahmed N, Akram M, Tahir S, Khan MA, et al. Evaluation of exosomes encapsulated recombinant Interleukin-29 for its in vitro anticancer studies. J Biotechnol. (2023) 373:24–33. doi: 10.1016/j.jbiotec.2023.06.008
126. Kateh Shamshiri M, Jaafari MR, and Badiee A. Preparation of liposomes containing IFN-gamma and their potentials in cancer immunotherapy: In vitro and in vivo studies in a colon cancer mouse model. Life Sci. (2021) 264:118605. doi: 10.1016/j.lfs.2020.118605
127. Qin J, Xue L, Hao A, Guo X, Jiang T, Ni Y, et al. Neoadjuvant chemotherapy with or without camrelizumab in resectable esophageal squamous cell carcinoma: the randomized phase 3 ESCORT-NEO/NCCES01 trial. Nat Med. (2024) 30:2549–57. doi: 10.1038/s41591-024-03064-w
128. Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. (2023) 618:144–50. doi: 10.1038/s41586-023-06063-y
129. Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. (2006) 17:1263–8. doi: 10.1093/annonc/mdl104
130. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-004291
131. Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, et al. Two-year outcomes of clinical N2–3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Surg. (2024) 167:838–47.e1. doi: 10.1016/j.jtcvs.2023.08.056
132. Yang G, Su X, Huang Y, Luo G, Wang Z, Cai P, et al. Intensive cycles of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma: a single-arm, phase II trial. J Transl Med. (2023) 21:411. doi: 10.1186/s12967-023-04273-6
133. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
134. Liu Y, Guo Y, Wu Z, Feng K, Tong C, Wang Y, et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: A phase I clinical trial. Cytotherapy. (2020) 22:573–80. doi: 10.1016/j.jcyt.2020.04.088
135. Ota S, Miyashita M, Yamagishi Y, and Ogasawara M. Baseline immunity predicts prognosis of pancreatic cancer patients treated with WT1 and/or MUC1 peptide-loaded dendritic cell vaccination and a standard chemotherapy. Hum Vaccin Immunother. (2021) 17:5563–72. doi: 10.1080/21645515.2021.2003645
136. O'Hara MH, O'Reilly EM, Varadhachary G, Wolff RA, Wainberg ZA, Ko AH, et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. (2021) 22:118–31. doi: 10.1016/S1470-2045(20)30532-5
137. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol. (2019) 5:824–30. doi: 10.1001/jamaoncol.2019.0270
138. Xu LX, Yuan JJ, Xue R, and Zhou J. Nab-paclitaxel plus capecitabine as first-line treatment for advanced biliary tract cancers: An open-label, non-randomized, phase II clinical trial. World J Gastroenterol. (2024) 30:3564–73. doi: 10.3748/wjg.v30.i30.3564
139. Ducreux M, Bennouna J, Adenis A, Conroy T, Lièvre A, Portales F, et al. Efficacy and safety of nab-paclitaxel in patients with previously treated metastatic colorectal cancer: a phase II COLO-001 trial. Cancer Chemother Pharmacol. (2017) 79:9–16. doi: 10.1007/s00280-016-3193-5
140. Overman MJ, Adam L, Raghav K, Wang J, Kee B, Fogelman D, et al. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol. (2018) 29:139–44. doi: 10.1093/annonc/mdx688
141. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
142. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-coV-2 vaccine. N Engl J Med. (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
143. Eygeris Y, Gupta M, Kim J, and Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res. (2022) 55:2–12. doi: 10.1021/acs.accounts.1c00544
144. Cafri G, Gartner JJ, Zaks T, Hopson K, Levin N, Paria BC, et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. (2020) 130:5976–88. doi: 10.1172/JCI134915
145. Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. (2012) 160:117–34. doi: 10.1016/j.jconrel.2012.03.020
146. Cascinu S, Galizia E, Labianca R, Ferraù F, Pucci F, Silva RR, et al. Pegylated liposomal doxorubicin, 5-fluorouracil and cisplatin versus mitomycin-C, 5-fluorouracil and cisplatin for advanced gastric cancer: a randomized phase II trial. Cancer Chemother Pharmacol. (2011) 68:37–43. doi: 10.1007/s00280-010-1424-8
147. Gnad-Vogt SU, Hofheinz RD, Saussele S, Kreil S, Willer A, Willeke F, et al. Pegylated liposomal doxorubicin and mitomycin C in combination with infusional 5-fluorouracil and sodium folinic acid in the treatment of advanced gastric cancer: results of a phase II trial. Anticancer Drugs. (2005) 16:435–40. doi: 10.1097/00001813-200504000-00010
148. Hofheinz RD, Willer A, Weisser A, Gnad U, Saussele S, Kreil S, et al. Pegylated liposomal doxorubicin in combination with mitomycin C, infusional 5-fluorouracil and sodium folinic acid. A phase-I-study in patients with upper gastrointestinal cancer. Br J Cancer. (2004) 90:1893–7. doi: 10.1038/sj.bjc.6601786
149. Milano G, Innocenti F, and Minami H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. (2022) 113:2224–31. doi: 10.1111/cas.15377
150. Cui J, Qin S, Zhou Y, Zhang S, Sun X, Zhang M, et al. Irinotecan hydrochloride liposome HR070803 in combination with 5-fluorouracil and leucovorin in locally advanced or metastatic pancreatic ductal adenocarcinoma following prior gemcitabine-based therapy (PAN-HEROIC-1): a phase 3 trial. Signal Transduct Target Ther. (2024) 9:248. doi: 10.1038/s41392-024-01948-4
151. Imaoka H, Ikeda M, Ozaka M, Oshima K, Okano N, Shimizu S, et al. Phase 1/2 study of liposomal irinotecan plus S-1 for metastatic pancreatic cancer refractory to gemcitabine-based treatment. Eur J Cancer. (2025) 222:115424. doi: 10.1016/j.ejca.2025.115424
152. Furuse J, Ueno M, Ikeda M, Okusaka T, Teng Z, Furuya M, et al. Liposomal irinotecan with fluorouracil and leucovorin after gemcitabine-based therapy in Japanese patients with metastatic pancreatic cancer: additional safety analysis of a randomized phase 2 trial. Jpn J Clin Oncol. (2023) 53:130–7. doi: 10.1093/jjco/hyac177
153. Ueno M, Nakamori S, Sugimori K, Kanai M, Ikeda M, Ozaka M, et al. nal-IRI+5-FU/LV versus 5-FU/LV in post-gemcitabine metastatic pancreatic cancer: Randomized phase 2 trial in Japanese patients. Cancer Med. (2020) 9:9396–408. doi: 10.1002/cam4.3558
154. Quan M, Chen J, Chen Z, Hai Y, Zhou Y, Chao Q, et al. China special issue on gastrointestinal tumors-Cetuximab retreatment plus camrelizumab and liposomal irinotecan in patients with RAS wild-type metastatic colorectal cancer: Cohort B of the phase II CRACK study. Int J Cancer. (2023) 153:1877–84. doi: 10.1002/ijc.34531
155. Muñoz C, Riesco Martinez MC, Ugidos L, García-Alfonso P, Alvarez-Gallego R, Peinado P, et al. Phase 2, multicenter, open-label, nonrandomized study of neoadjuvant chemotherapy liposomal irinotecan with 5-fluorouracil, leucovorin, and oxaliplatin, followed by chemoradiotherapy in patients with rectal cancer in a watch-and-wait program. Am J Clin Oncol. (2025) 48:142–7. doi: 10.1097/COC.0000000000001157
156. Gavas S, Quazi S, and Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett. (2021) 16:173. doi: 10.1186/s11671-021-03628-6
157. Lane LA, Qian X, Smith AM, and Nie S. Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Annu Rev Phys Chem. (2015) 66:521–47. doi: 10.1146/annurev-physchem-040513-103718
158. Tang Y, Wang X, Li J, Nie Y, Liao G, Yu Y, et al. Overcoming the reticuloendothelial system barrier to drug delivery with a "Don't-eat-us" Strategy. ACS Nano. (2019) 13:13015–26. doi: 10.1021/acsnano.9b05679
159. Danhier F, Feron O, and Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. (2010) 148:135–46. doi: 10.1016/j.jconrel.2010.08.027
160. Niculescu AG and Grumezescu AM. Novel tumor-targeting nanoparticles for cancer treatment-A review. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23095253
161. Yang W, Wang L, Mettenbrink EM, DeAngelis PL, and Wilhelm S. Nanoparticle toxicology. Annu Rev Pharmacol Toxicol. (2021) 61:269–89. doi: 10.1146/annurev-pharmtox-032320-110338
162. Metselaar JM and Lammers T. Challenges in nanomedicine clinical translation. Drug Delivery Transl Res. (2020) 10:721–5. doi: 10.1007/s13346-020-00740-5
163. Abdullah KM, Sharma G, Singh AP, and Siddiqui JA. Nanomedicine in cancer therapeutics: current perspectives from bench to bedside. Mol Cancer. (2025) 24:169. doi: 10.1186/s12943-025-02368-w
164. He H, Liu L, Morin EE, Liu M, and Schwendeman A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc Chem Res. (2019) 52:2445–61. doi: 10.1021/acs.accounts.9b00228
165. Shi J, Kantoff PW, Wooster R, and Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. (2017) 17:20–37. doi: 10.1038/nrc.2016.108
166. Younis MA, Tawfeek HM, Abdellatif AAH, Abdel-Aleem JA, and Harashima H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv Drug Delivery Rev. (2022) 181:114083. doi: 10.1016/j.addr.2021.114083
167. Szebeni J, Simberg D, González-Fernández Á, Barenholz Y, and Dobrovolskaia MA. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat Nanotechnol. (2018) 13:1100–8. doi: 10.1038/s41565-018-0273-1
168. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. (2018) 11:156–64. doi: 10.1016/j.conctc.2018.08.001
169. de Vlieger JSB, Crommelin DJA, Tyner K, Drummond DC, Jiang W, McNeil SE, et al. Report of the AAPS guidance forum on the FDA draft guidance for industry: "Drug products, including biological products, that contain nanomaterials. AAPS J. (2019) 21:56. doi: 10.1208/s12248-019-0329-7
170. Roces CB, Lou G, Jain N, Abraham S, Thomas A, Halbert GW, et al. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. (2020) 12. doi: 10.3390/pharmaceutics12111095
171. Webb C, Forbes N, Roces CB, Anderluzzi G, Lou G, Abraham S, et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int J Pharm. (2020) 582:119266. doi: 10.1016/j.ijpharm.2020.119266
172. Gabizon AA, Gabizon-Peretz S, Modaresahmadi S, and La-Beck NM. Thirty years from FDA approval of pegylated liposomal doxorubicin (Doxil/Caelyx): an updated analysis and future perspective. BMJ Oncol. (2025) 4:e000573. doi: 10.1136/bmjonc-2024-000573
173. Berger JL, Smith A, Zorn KK, Sukumvanich P, Olawaiye AB, Kelley J, et al. Outcomes analysis of an alternative formulation of PEGylated liposomal doxorubicin in recurrent epithelial ovarian carcinoma during the drug shortage era. Onco Targets Ther. (2014) 7:1409–13. doi: 10.2147/OTT.S62881
174. Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. (2021) 601:120586. doi: 10.1016/j.ijpharm.2021.120586
175. Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, and Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. (2021) 110:997–1001. doi: 10.1016/j.xphs.2020.12.006
176. Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou Y, et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol. (2022) 13:795972. doi: 10.3389/fimmu.2022.795972
177. Ying K, Bai B, Gao X, Xu Y, Wang H, and Xie B. Orally administrable therapeutic nanoparticles for the treatment of colorectal cancer. Front Bioeng Biotechnol. (2021) 9:670124. doi: 10.3389/fbioe.2021.670124
Keywords: gastrointestinal cancers, nanomedicine, tumour microenvironment, precision oncology, immunotherapy
Citation: Chen C, Li J, Hua X, Deng T and Zhang Z (2025) Nano-enabled strategies for targeted immunotherapy in gastrointestinal cancers. Front. Immunol. 16:1653829. doi: 10.3389/fimmu.2025.1653829
Received: 25 June 2025; Accepted: 31 July 2025;
Published: 14 August 2025.
Edited by:
Afza Ahmad, Babu Banarasi Das University, IndiaReviewed by:
Haonan Li, University of Electronic Science and Technology of China, ChinaJaimin R. Shah, University of California, San Diego, United States
Copyright © 2025 Chen, Li, Hua, Deng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyun Zhang, amlhbmNoaWRhb2RpXzIwMTBAMTYzLmNvbQ==
 Chaofan Chen
Chaofan Chen Jinlei Li2
Jinlei Li2