- 1Department of General Surgery, Wusheng County People’s Hospital (Wusheng Hospital Affiliated Hospital of North Sichuan Medical College), Guangan, China
- 2Department of Clinical Medicine, North Sichuan Medical College, Nanchong, China
- 3Department of General Surgery, Nanbu County People’s Hospital, Nanchong, China
- 4Department of Orthopedics, Shenzhen Children’s Hospital, Shenzhen, China
- 5Department of Anatomy, North Sichuan Medical College, Nanchong, China
- 6Department of Thyroid and Breast Surgery, Gaoping District People’s Hospital of Nanchong City (Affiliated Hospital of China West Normal University), Nanchong, China
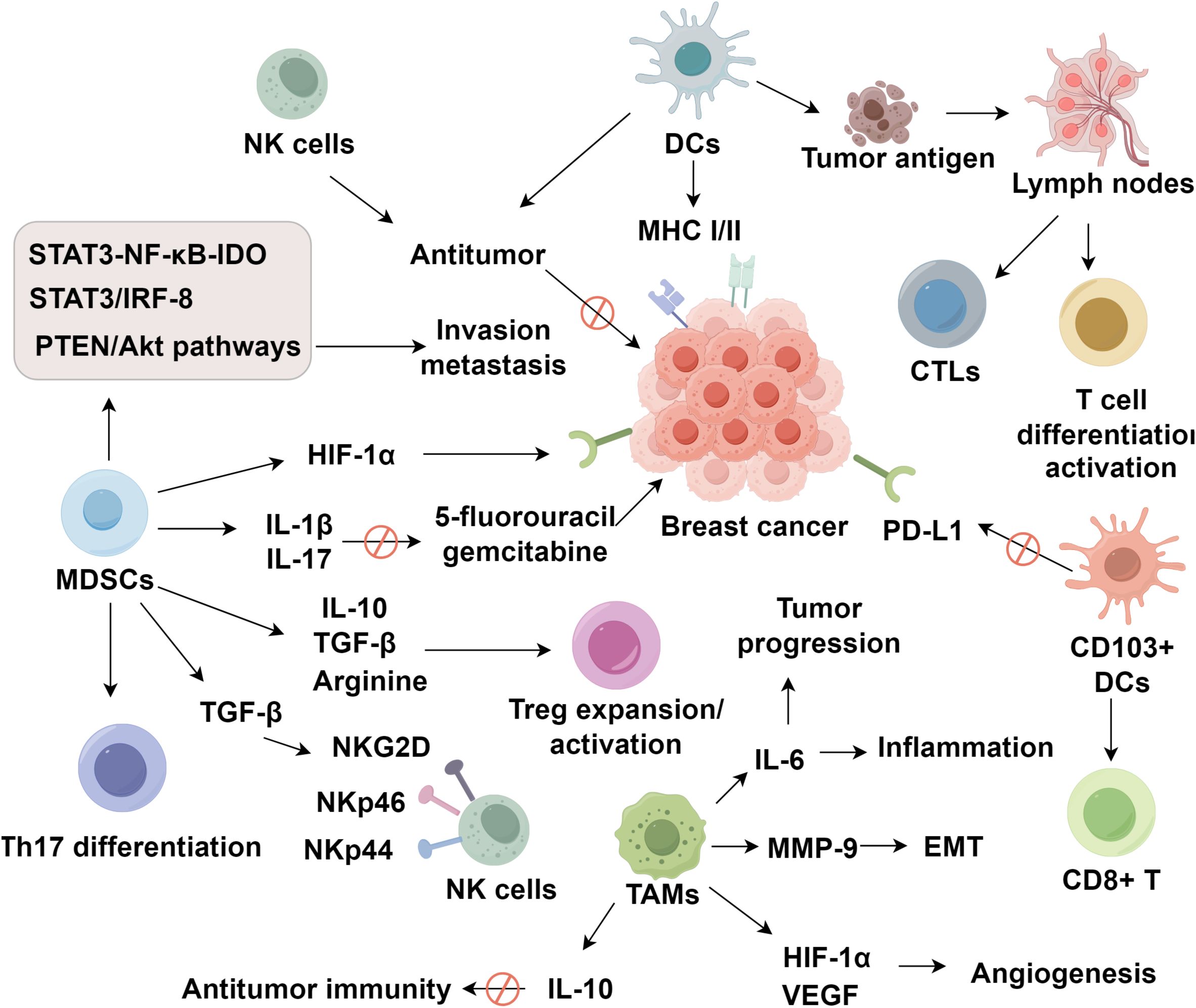
Breast cancer remains the most frequently diagnosed malignancy and a leading cause of cancer-related mortality among women worldwide. Increasing evidence underscores the pivotal yet paradoxical roles of innate immune cells and their associated cytokines in orchestrating the dynamic landscape of the breast tumor immune microenvironment (TIME). Innate immune effectors, including tumor-associated macrophages (TAMs) and natural killer (NK) cells, exert dual functions by either initiating robust antitumor responses or facilitating immune evasion, metastatic dissemination, and therapeutic resistance. For instance, MDSCs suppress T and NK cell activity via STAT3/NF-κB signaling and modulate the cytokine milieu through IL-10 and TGF-β. Similarly, M2-polarized TAMs promote angiogenesis, epithelial–mesenchymal transition, and chemoresistance via IL-10/STAT3/Bcl-2 pathways. In contrast, NK cells and CD103+ DCs mediate tumor cell cytolysis and prime antigen-specific immunity, though their activity is frequently compromised in advanced disease. Moreover, key cytokines and chemokines, including IL-6, IL-10, IL-8, TNF-α, TGF-β, and CCL2/5, demonstrate subtype-specific and context-dependent effects, acting as both tumor-promoting and tumor-suppressing agents through complex signaling networks. This review highlights the dualistic nature of innate immune components in breast cancer, discusses their prognostic and therapeutic implications, and proposes novel intervention strategies, such as TAM repolarization, and cytokine modulation, to reprogram the TIME and restore effective immune surveillance, particularly in aggressive subtypes like triple-negative breast cancer.
1 Introduction
Breast cancer is the most prevalent malignancy and primary cause of cancer-related death in women worldwide (1, 2). Recent advances in immunotherapy have shown significant potential in improving treatment outcomes and survival rates (3, 4). A comprehensive analysis of the tumor immune microenvironment could optimize immunotherapeutic approaches for breast cancer (5). Studies indicate that immune cells and mediators within this microenvironment not only combat tumors but also promote immune evasion, facilitating cancer progression (6, 7). Shared signaling pathways between immune and oncogenic processes regulate cell proliferation, apoptosis, and angiogenesis (8–11). Early in tumor development, malignant cells manipulate immune components to avoid detection, while advanced tumors establish an immunosuppressive niche, resisting immune-mediated destruction (12).
Effector T cells play a critical role in antitumor immunity, yet their function is often suppressed in breast cancer (13). Meanwhile, innate immune cells including macrophages, natural killer (NK) cells, and myeloid-derived suppressor cells (MDSCs), and mediators exhibit remarkable functional plasticity, exerting either tumoricidal or tumor-promoting effects depending on microenvironmental cues (14–16). The diversity of these immune components varies by tumor subtype and stage, offering diagnostic and prognostic value (17, 18). This review explores the mechanisms of innate immune cells and mediators in breast cancer progression, highlighting their clinical implications.
2 Innate immune cells in the breast cancer immune microenvironment
2.1 NK cells mediate direct antitumor cytotoxicity
NK cells are glycolipid-reactive lymphocytes with intrinsic cytotoxic capacity against tumor cells. Studies have demonstrated that activation of NK cells enhances antitumor immunity and survival in murine models of postoperative metastatic breast cancer (19, 20). Chemotherapeutic agents such as gemcitabine and cyclophosphamide may facilitate NK cell recruitment to the primary tumor site, and in combination with NK cell activation, significantly improve antitumor efficacy and reduce recurrence rates (21–23). In HER2-positive patients receiving adjuvant chemotherapy, the tumor microenvironment exhibited increased infiltration of NK cells and regulatory T cells, with this population showing reduced chemotherapy-related pathological responses (24–26). The increase in regulatory T cells may be associated with NK cell-mediated inhibition of tumor stem cell proliferation, reversal of MDSC-induced immunosuppression, and restoration of T cell proliferation (27, 28). Moreover, distinct NK cell subsets are associated with different stages of breast cancer progression. For instance, CD56brightCD16+ and CD56dimCD16− NK cell populations are significantly elevated in the peripheral blood of patients with progressive invasive breast cancer (29). Intratumoral CD56+ NK cell density is positively correlated with tumor grade and stage, and although a lower level of CD56+ NK cells is generally indicative of favorable prognosis, no clear association has been established with overall survival (30). However, the cytotoxic activity of NK cells is often impaired in advanced-stage breast cancer due to multiple tumor-induced immunosuppressive mechanisms (31). Notably, transforming growth factor-beta (TGF-β), abundantly present in the tumor microenvironment, downregulates the expression of key NK cell–activating receptors such as NKG2D and NKp30, thereby compromising tumor cell recognition and cytolytic function (32–36). Additionally, MDSCs inhibit NK cell cytotoxicity by producing reactive oxygen species, particularly hydrogen peroxide, and immunosuppressive cytokines like TGF-β, which further dampen NK cell activation and IFN-γ production (37, 38). These suppressive pathways collectively lead to NK cell exhaustion, reduced granzyme B/perforin secretion, and impaired tumor control (39, 40). Understanding the mechanisms behind their functional impairment, particularly receptor downregulation and MDSC-mediated suppression, may yield valuable insights for diagnostic and therapeutic innovation.
2.2 Dendritic cells present tumor antigens to activate antigen-specific T cells
DCs, as pivotal antigen-presenting cells in adaptive immunity, play a central role in antitumor responses by promoting the expression of both exogenous and endogenous major histocompatibility complex (MHC) class I and II molecules (41, 42). They facilitate tumor antigen trafficking to draining lymph nodes, cross-present antigens to activate cytotoxic T lymphocytes (CTLs), and orchestrate T cell differentiation and activation (43–45). Among DC subsets, CD103+ conventional type 1 dendritic cells (cDC1s) are uniquely equipped for antigen cross-presentation, a process by which exogenous tumor-derived antigens are processed and presented on MHC class I molecules (46, 47). This activation relies on key components such as the Sec22b vesicle trafficking protein, the BATF3 transcription factor, and cross-priming signals via the STING and type I interferon pathways (48–50). Upon migration to lymph nodes, CD103+ DCs engage CD8+ T cells through MHC-I–peptide complexes and co-stimulatory molecules such as CD80/CD86, ultimately inducing tumor-specific cytotoxic responses (51). However, the frequency and functional competence of CD103+ DCs are often reduced in advanced breast cancer, leading to impaired priming of effector CD8+ T cells (52, 53). Tumor-derived suppressive cytokines such as IL-10, and TGF-β, as well as hypoxic conditions, inhibit CD103+ DC differentiation and antigen-presenting capacity (54, 55). Additionally, elevated expression of PD-L1 on dysfunctional DCs can further suppress T cell activation. These alterations in CD103+ DC function contribute to ineffective antitumor immunity, enhanced immune evasion, and poor therapeutic outcomes. Recent studies have shown that CD103+ DCs are capable of delivering intact tumor antigens to peripheral lymph nodes, thereby priming tumor-specific CD8+ T cells and locally suppressing PD-L1 activity (52, 56). However, current research on DCs in the breast cancer context remains limited, and further mechanistic studies are warranted.
2.3 MDSCs promote breast cancer progression and affect prognosis through multiple signaling pathways and immunomodulatory factors
MDSCs comprise a heterogeneous population of myeloid progenitor cells, including immature granulocytic (G-MDSC) and monocytic (M-MDSC) subsets (57, 58). Clinical data indicate a close association between MDSC levels and breast cancer stage, tumor burden in metastatic disease, and chemotherapy efficacy (59). Elevated MDSC levels are linked to increased risk of postoperative recurrence and metastasis, whereas patients with lower MDSC counts demonstrate higher rates of pathological complete response (60, 61). In stage IV breast cancer patients, high levels of HLA-DRneg/low, CD33+, CD11b+ MDSCs are associated with significantly reduced survival (62). Furthermore, MDSCs can promote the production of IL-1β and IL-17, reducing the efficacy of chemotherapeutic agents such as 5-fluorouracil and gemcitabine, thereby adversely affecting prognosis (63, 64). Mechanistically, MDSCs in the breast tumor microenvironment promote invasion and metastasis via pathways such as STAT3-NF-κB-IDO, STAT3/IRF-8, and PTEN/Akt (65), involving both inhibitory and stimulatory cytokines. These pathways drive MDSC expansion and lead to downstream functional consequences that impair antitumor immunity. For instance, activation of STAT3 induces expression of arginase-1 and iNOS, resulting in depletion of L-arginine and accumulation of reactive oxygen species (ROS), which in turn inhibit CD8+ T cell receptor ζ-chain expression and induce T cell anergy (66–68). Concurrently, IL-2 production suppression further impairs T cell proliferation and effector function (69). The PTEN/AKT axis supports MDSC resistance to apoptosis and enhances their immunosuppressive capacity through sustained IL-10 and TGF-β secretion (65). Collectively, these mechanisms contribute to immune evasion, tumor progression, and treatment resistance.
On one hand, cytokines such as TGF-β and Flt3L induce CD11b+ MDSC differentiation, while IL-6 and IL-18 promote CD33+ MDSC proliferation (70). Chemokines including CXCL5/CXCR2 are essential for MDSC recruitment in 4T1 BALB/c murine tumor models, while CCL1, CCL2, CCL5, GM-CSF, and G-CSF facilitate MDSC expansion and aggregation in the tumor milieu (71–73). On the other hand, MDSCs suppress antitumor immune responses by modulating the cytokine environment and cellular interactions. For example, MDSCs induce Th17 differentiation, mediate crosstalk between macrophages and tumor cells, and reshape the local microenvironment to favor tumor cell growth and metastasis (74). They also secrete IL-10 and TGF-β to promote regulatory T cell expansion, and enhance Treg activation through arginine metabolism and TGF-β–mediated pathways, contributing to immune suppression (75, 76). Additionally, MDSCs downregulate NK cell activation by producing TGF-β and hydrogen peroxide, which suppress the expression of NK cell-activating receptors such as NKG2D, NKp46, and NKp44 (76). Some MDSC subsets, particularly under hypoxic conditions, upregulate PD-L1 expression via HIF-1α activation; however, this phenomenon is not universal across all MDSC populations (77). In summary, MDSC accumulation in the breast cancer microenvironment may compromise surgical and chemotherapeutic efficacy. Targeting MDSC recruitment and immunosuppressive functions via multiple regulatory pathways holds promise for enhancing therapeutic outcomes.
2.4 TAMs mediate broad immunosuppressive effects through multiple mechanisms
Tumor-associated macrophages (TAMs) originate from circulating monocytes that infiltrate the tumor microenvironment and subsequently undergo polarization into either classically activated M1 or alternatively activated M2 phenotypes (78). The recruitment of TAMs is driven by chemokines such as CCL2 and cytokines like CSF-1 and VEGF, which establish a permissive environment for macrophage infiltration (79–81). Once recruited, macrophage polarization is largely dictated by local signals. Hypoxic conditions, IL-4, IL-10, and TGF-β collectively promote the differentiation of macrophages into the M2 phenotype, which is closely associated with immunosuppressive and tumor-promoting functions (82–85). M2-polarized TAMs play key roles in tumor angiogenesis, epithelial–mesenchymal transition (EMT), metastasis, and tissue remodeling (86). The S1PR1 gene in TAMs inhibits pulmonary metastasis and lymphangiogenesis in murine breast cancer models by downregulating inflammatory component NLRP3 (87). COX2+ TAMs induce MMP-9 expression and promote EMT in the breast tumor microenvironment. The COX2/PGE2 axis also enhances IL-6 secretion from macrophages, exacerbating inflammation and further promoting tumor progression (88). Under hypoxic conditions, TAMs upregulate VEGF and HIF-1α expression to stimulate tumor angiogenesis (89). In triple-negative breast cancer, TAMs are recruited from peripheral circulation and, upon classical or alternative activation, contribute to tumor progression by suppressing cytokine production, impairing TILs function, promoting Treg expansion, and modulating PD-1 expression in the tumor milieu (90). Regarding chemoresistance, paclitaxel efficacy has been linked to M2 TAM depletion (91). Moreover, TAM-induced resistance is mediated by increased expression of Bcl-2 and STAT3, enhancing IL-10 secretion via the IL-10/STAT3/Bcl-2 signaling cascade to suppress antitumor immunity (85). In conclusion, TAMs exert multifaceted immunosuppressive effects within the breast cancer microenvironment by promoting angiogenesis, metastasis, immune evasion, and therapeutic resistance (Figure 1).
3 Innate immune factors in the tumor immune microenvironment of breast cancer
3.1 Dual roles of interleukins in breast cancer
Interleukins comprise a diverse family of lymphokines with pleiotropic biological activities. Their roles in breast cancer are highly context-dependent and vary by subtype. IL-6, for instance, is upregulated in over half of breast cancer patients, with elevated levels particularly noted in early-stage or high-grade tumors (92). In estrogen receptor (ER) positive breast cancer cell lines, IL-6 generally exhibits tumor-suppressive properties, while no significant effect has been observed in ER-negative cell lines (93, 94). Mechanistically, IL-6 promotes phosphorylation of JAK/STAT3 via interaction with its homodimeric or heterodimeric receptor complexes, thereby activating transcriptional programs. The feedback loop further enhances IL-6 expression via activated STAT3, rendering IL-6 less effective in breast cancer cells with low STAT3 expression (95). IL-8, a proinflammatory chemokine-like cytokine, is associated with poor survival in ER-negative patients (96). It promotes lymph node metastasis and is elevated in advanced-stage tumors. Nonetheless, some studies suggest its involvement in immune activation under certain conditions, illustrating its duality (97–99). IL-10 is generally considered an immunosuppressive cytokine and is associated with poor prognosis in breast cancer. Its expression is regulated primarily through the STAT3 and SOCS3 pathways, where STAT3 silencing markedly reduces IL-10 levels, while SOCS3 silencing enhances its expression (100). The IL-10/STAT3/Bcl-2 axis plays a pivotal role in mediating TAM-induced breast cancer cell survival and paclitaxel resistance. Inhibition of IL-10 receptor signaling enhances CD8+ T cell responses and upregulates IL-12 and intratumoral dendritic cells, thereby improving chemotherapy efficacy (101).
IL-11 exerts its effects through binding to IL-11 receptor alpha (IL-11Ra) and gp130, activating JAK kinases and downstream STAT3 and SOCS3. These pathways regulate tumor cell proliferation, survival, motility, and invasion (102). In breast cancer patients with bone metastases, elevated IL-11 mRNA and increased expression of p38, p-c-Jun, and p-STAT3 have been observed, highlighting its predictive value for bone metastatic potential (103). IL-15 primarily exerts indirect antitumor effects in the TIME. Through activation of PI3K signaling, IL-15 selectively stimulates T lymphocytes and enhances vaccine-like antitumor responses in combination with MEK inhibitors (104). It also potentiates NK cell-mediated cytotoxicity against CD44+CD24- breast cancer stem-like cells and augments cetuximab efficacy (105). In a study by Gillgrass et al. (106), C57BL/6 mice receiving IL-15 via intravenous injection or harboring IL-15 transgenes exhibited a tenfold reduction in breast cancer metastasis compared to controls, likely due to enhanced NK cell cytotoxicity. Collectively, ILs exert tumor-promoting or tumor-suppressing effects in breast cancer primarily through engagement with specific receptors and activation of downstream signaling cascades. Deciphering their mechanistic roles may offer prognostic biomarkers and therapeutic targets (Supplementary Table S1).
3.2 Chemokines promote breast cancer cell invasion and metastasis
A growing body of clinical evidence supports the pivotal role of chemokines in breast cancer metastatic dissemination, and prognosis (107, 108). CCL2 and CCL5 are among the most extensively studied chemokines in the breast cancer microenvironment. In estrogen-rich conditions, both enhance tumor cell dissemination (109). Notably, levels of CCL2 and CCL5 are significantly elevated in the blood of breast cancer patients, particularly those with ER-positive tumors, and positively correlate with TAM infiltration (110). Persistent expression of CCL2 by mammary epithelial cells promotes chronic low-grade inflammation, increases glandular density, and elevates cancer risk (111). CCL2 may also modulate monocyte–macrophage crosstalk within the tumor niche (112). ELISA results indicate genotype-dependent differences in CCL2 expression across breast cancer cell suspensions, with an inverse correlation to ER and PR status. Kaplan–Meier analysis further associates low CCL2 levels with favorable prognosis (112). CCL5 enhances GLUT1 expression on tumor cells, promoting glucose uptake and metabolic reprogramming to support proliferation (113). In CCL5-deficient mice, both primary tumor burden and pulmonary metastases are markedly reduced. This may be attributed to CCR3 activation, Gfi1 expression, and Th2 polarization, which collectively establish a pre-metastatic niche conducive to myeloid cell recruitment (114). CCL18, CCL20, and CCL25 similarly contribute to prognosis prediction and promote TAM infiltration, angiogenesis, and metastatic progression (115–117). Serum CCL18 levels are significantly higher in breast cancer patients than in those with benign tumors or healthy controls, correlating with advanced clinical stage and poor survival (118). CCL20 facilitates tumor invasion and MMP-2/9 secretion in basal-like TNBC, with high CCL20 expression predicting reduced metastasis-free and overall survival (119). CCL25 promotes EMT via the CCL25/CCR9 axis, enhancing invasiveness and metastatic potential (120). Besides, CXCL1 expression in the tumor stroma is associated with tumor grade and recurrence, likely due to its negative regulation by TGF-β (121). CXCL13 transcription correlates with pathological complete response rates and favorable immune responses in breast cancer, possibly through activation of TFH13 cells and differentiation of germinal center memory B cells, thereby shifting from regulatory T cell–mediated suppression toward effective humoral immunity (122, 123).
3.3 TNF-α: a double-edged sword in breast cancer progression
TNF-α, a proinflammatory cytokine, orchestrates tissue homeostasis by regulating cytokine production, cell survival, and apoptosis (124). On one hand, TNF-α induces cell cycle arrest in ER-positive breast cancer cells at the G0/G1 phase, impeding DNA synthesis and exerting tumor-suppressive effects. On the other hand, it activates the NF-κB pathway and facilitates RIP1 ubiquitination, thereby stimulating JNK/ROS signaling and promoting tumor cell proliferation, enhancing the cytotoxic effects of chemotherapy and radiotherapy both in vitro and in vivo (125). Clinical data suggest that TNF-α levels are negative associated with breast cancer progression risk (126). However, TNF-α can also promote tumor growth, migration, and invasion, potentially through activation of the Wnt pathway and establishment of a tumor-permissive niche (127). Notably, the interpretation of TNF-α’s dual roles is limited by heterogeneity in immune status, inflammation levels, and disease stage across studies, necessitating further investigation (128).
3.4 TGF-β promotes breast cancer cell proliferation and metastasis
TGF-β, primarily synthesized by platelets, monocytes/macrophages, lymphocytes, fibroblasts, and epithelial cells, plays a central role in tumor progression, with TGF-β1 as the predominant isoform (129). TGF-β1 stimulates angiogenesis and enhances tumor cell affinity, invasiveness, and adhesion, while inhibiting normal mammary epithelial cell proliferation (130). Current research implicates the Smad signaling pathway as a major mediator of TGF-β-induced distant metastasis in breast cancer (131). TGF-β also suppresses IL-2 production and impairs T cell antitumor activity (132, 133). Additionally, it upregulates local cytokine expression, activates infiltrating immune cells, inhibits granzyme and perforin expression, downregulates MHC class I on tumor cells, and diminishes NK cell–mediated cytotoxicity (134). Importantly, TGF-β signaling has been shown to facilitate EMT and endow breast cancer cells with stem cell–like properties (135, 136). This dual role not only promotes tumor invasion and metastasis but also contributes to immune evasion through induction of an immunosuppressive microenvironment. Mechanistically, TGF-β activates EMT through canonical Smad-mediated transcriptional reprogramming and MAPK signaling pathways (137). Recent studies demonstrate that FAP/VCAN enhances the expression of EMT-associated transcription factors, further driving mesenchymal transition and increasing tumor cell plasticity (138). Moreover, TGF-β–induced PI3K/Akt activation promotes the expression of stemness markers like ALDH1 and CD44^high/CD24^low, enabling tumor-initiating capacity and resistance to chemotherapy (139). This signaling crosstalk between PI3K/Akt and Smad pathways orchestrates both immune suppression and cellular reprogramming, allowing breast cancer cells to evade immune surveillance while acquiring aggressive phenotypes. Through PI3K/Akt signaling, TGF-β can further induce EMT, thereby enhancing tumor growth and dissemination (140, 141).
4 Conclusion
The breast cancer immune microenvironment is profoundly shaped by the dual roles of innate immune cells and cytokines, which can either support antitumor immunity or promote immune evasion and disease progression. MDSCs and TAMs are central mediators of immunosuppression through pathways such as STAT3/NF-κB and IL-10/STAT3/Bcl-2, while NK cells and dendritic cells retain critical, yet often impaired, antitumor functions. Additionally, cytokines such as IL-6, IL-8, IL-10, TNF-α, and TGF-β demonstrate context-dependent activities, intricately regulating immune responses, tumor growth, and metastasis. The functional plasticity of these innate components highlights both the complexity and therapeutic potential of targeting the innate immune axis in breast cancer. Particularly in subtypes like triple-negative breast cancer, which lack effective targeted therapies, strategies aimed at reprogramming innate immune cells, blocking suppressive cytokines, or restoring cytotoxic activity could significantly enhance clinical outcomes. A deeper mechanistic understanding of innate immunity will not only advance prognostic biomarker development but also enable the design of rational combination therapies that synergize immunomodulation with conventional and emerging treatment modalities.
Author contributions
CZ: Writing – original draft. WL: Writing – original draft. PY: Writing – original draft. RL: Writing – original draft. LP: Writing – original draft, Writing – review & editing. HZ: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Research Project of Sichuan Provincial Research Center for the Development of Primary Health Care (No: SWFZ24-W-20) and Nanchong Science and Technology Project (Grant No.22YYJCYJ0088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1654947/full#supplementary-material
References
1. Oliveira FP, Nogueira ML, Galvão AFC, Dias RB, and Bezerra DP. Translational drugs targeting cancer stem cells in triple-negative breast cancer. Mol Ther Oncol. (2025) 33:201008. doi: 10.1016/j.omton.2025.201008
2. Xie H, Xi X, Lei T, Liu H, and Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
3. Yan J, Ye G, Jin Y, Miao M, Li Q, and Zhou H. Identification of novel prognostic circRNA biomarkers in circRNA-miRNA-mRNA regulatory network in gastric cancer and immune infiltration analysis. BMC Genomics. (2023) 24:323. doi: 10.1186/s12864-023-09421-2
4. Zhang Y, Qin N, Wang X, Liang R, Liu Q, Geng R, et al. Glycogen metabolism-mediated intercellular communication in the tumor microenvironment influences liver cancer prognosis. Oncol Res. (2024) 32:563–76. doi: 10.32604/or.2023.029697
5. Wan M, Mei J, Cai Y, Zhou J, Xue N, Jiang Y, et al. Targeting IGF1R overcomes armored and cold tumor microenvironment and boosts immune checkpoint blockade in triple-negative breast cancer. Adv Sci (Weinh). (2025) 2025:e01341. doi: 10.1002/advs.202501341
6. Tsutsumi E, Macy AM, LoBello J, Hastings KT, and Kim S. Tumor immune microenvironment permissive to metastatic progression of ING4-deficient breast cancer. PloS One. (2024) 19:e0304194. doi: 10.1371/journal.pone.0304194
7. Wang X, Tan B, Liu J, Wang J, Chen M, Yang Q, et al. EChinacoside inhibits tumor immune evasion by downregulating inducible PD-L1 and reshaping tumor immune landscape in breast and colorectal cancer. Phytomedicine. (2024) 135:156188. doi: 10.1016/j.phymed.2024.156188
8. Wang X, Jian Q, Zhang Z, Gu J, Wang X, and Wang Y. Effect of tumor-derived extracellular vesicle-shuttled lncRNA MALAT1 on proliferation, invasion and metastasis of triple-negative breast cancer by regulating macrophage M2 polarization via the POSTN/Hippo/YAP axis. Transl Oncol. (2024) 49:102076. doi: 10.1016/j.tranon.2024.102076
9. Vallega KA, Bosco DB, Ren Y, and Sang QA. Macrophage-conditioned media promotes adipocyte cancer association, which in turn stimulates breast cancer proliferation and migration. Biomolecules. (2022) 12:1757. doi: 10.3390/biom12121757
10. Zhang Y, Yang H, Jiang Y, Jiang Y, and Mao R. Angiogenesis and immune microenvironment in triple-negative breast cancer: Targeted therapy. Biochim Biophys Acta Mol Basis Dis. (2025) 1871:167880. doi: 10.1016/j.bbadis.2025.167880
11. Mazloumi Z, Rafat A, Dizaji Asl K, and Nozad Charoudeh H. A combination of telomerase inhibition and NK cell therapy increased breast cancer cell line apoptosis. Biochem Biophys Res Commun. (2023) 640:50–5. doi: 10.1016/j.bbrc.2022.11.090
12. Imani S, Farghadani R, Roozitalab G, Maghsoudloo M, Emadi M, Moradi A, et al. Reprogramming the breast tumor immune microenvironment: cold-to-hot transition for enhanced immunotherapy. J Exp Clin Cancer Res. (2025) 44:131. doi: 10.1186/s13046-025-03394-8
13. Miller KD, O’Connor S, Pniewski KA, Kannan T, Acosta R, Mirji G, et al. Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Nat Cancer. (2023) 4:1491–507. doi: 10.1038/s43018-023-00636-6
14. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, and Li X. : Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
15. Tommasi C, Pellegrino B, Diana A, Palafox Sancez M, Orditura M, Scartozzi M, et al. The innate immune microenvironment in metastatic breast cancer. J Clin Med. (2022) 11:5986. doi: 10.3390/jcm11205986
16. Mehta AK, Kadel S, Townsend MG, Oliwa M, and Guerriero JL. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol. (2021) 12:643771. doi: 10.3389/fimmu.2021.643771
17. Ye Y, Xu C, Chen F, Liu Q, and Cheng N. Targeting innate immunity in breast cancer therapy: A narrative review. Front Immunol. (2021) 12:771201. doi: 10.3389/fimmu.2021.771201
18. Harris MA, Savas P, Virassamy B, O’Malley MMR, Kay J, Mueller SN, et al. Towards targeting the breast cancer immune microenvironment. Nat Rev Cancer. (2024) 24:554–77. doi: 10.1038/s41568-024-00714-6
19. Ben-Shmuel A, Gruper Y, Halperin C, Levi-Galibov O, Rosenberg-Fogler H, Barki D, et al. Cancer-associated fibroblasts serve as decoys to suppress NK cell anticancer cytotoxicity in breast cancer. Cancer Discov. (2025) 15:1247–69. doi: 10.1158/2159-8290.CD-24-0131
20. Bushnell GG, Sharma D, Wilmot HC, Zheng M, Fashina TD, Hutchens CM, et al. Natural killer cell regulation of breast cancer stem cells mediates metastatic dormancy. Cancer Res. (2024) 84:3337–53. doi: 10.1158/0008-5472.CAN-24-0030
21. Noh KM, Jangid AK, Park J, Kim S, and Kim K. Membrane-immobilized gemcitabine for cancer-targetable NK cell surface engineering. J Mater Chem B. (2024) 12:12087–102. doi: 10.1039/D4TB01639D
22. Van der Meer JMR, de Jonge P, van der Waart AB, Geerlings AC, Moonen JP, Brummelman J, et al. CD34(+) progenitor-derived NK cell and gemcitabine combination therapy increases killing of ovarian cancer cells in NOD/SCID/IL2Rg(null) mice. Oncoimmunology. (2021) 10:1981049. doi: 10.1080/2162402X.2021.1981049
23. Gebremeskel S, Lobert L, Tanner K, Walker B, Oliphant T, Clarke LE, et al. Natural killer T-cell immunotherapy in combination with chemotherapy-induced immunogenic cell death targets metastatic breast cancer. Cancer Immunol Res. (2017) 5:1086–97. doi: 10.1158/2326-6066.CIR-17-0229
24. Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S, et al. Improved Natural Killer cell activity and retained anti-tumor CD8(+) T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med. (2015) 13:204. doi: 10.1186/s12967-015-0567-0
25. Thomas N, Foukakis T, and Willard-Gallo K. The interplay between the immune response and neoadjuvant therapy in breast cancer. Front Oncol. (2025) 15:1469982. doi: 10.3389/fonc.2025.1469982
26. Luque M, Sanz-Álvarez M, Morales-Gallego M, Madoz-Gúrpide J, Zazo S, Domínguez C, et al. Tumor-infiltrating lymphocytes and immune response in HER2-positive breast cancer. Cancers (Basel). (2022) 14:6034. doi: 10.3390/cancers14246034
27. Bushnell GG, Sharma D, Wilmot HC, Zheng M, Fashina TD, Hutchens CM, et al. Natural killer cell regulation of breast cancer stem cells mediates metastatic dormancy. bioRxiv. (2023) 84:3337–53. doi: 10.1101/2023.10.02.560493
28. Neo SY, Tong L, Chong J, Liu Y, Jing X, Oliveira MMS, et al. Tumor-associated NK cells drive MDSC-mediated tumor immune tolerance through the IL-6/STAT3 axis. Sci Transl Med. (2024) 16:eadi2952. doi: 10.1126/scitranslmed.adi2952
29. Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. (2013) 190:2424–36. doi: 10.4049/jimmunol.1200140
30. Rathore AS, Goel MM, Makker A, Kumar S, and Srivastava AN. Is the tumor infiltrating natural killer cell (NK-TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac J Cancer Prev. (2014) 15:3757–61. doi: 10.7314/apjcp.2014.15.8.3757
31. Kos K, Aslam MA, van de Ven R, Wellenstein MD, Pieters W, van Weverwijk A, et al. Tumor-educated T(regs) drive organ-specific metastasis in breast cancer by impairing NK cells in the lymph node niche. Cell Rep. (2022) 38:110447. doi: 10.1016/j.celrep.2022.110447
32. Arianfar E, Khandoozi SR, Mohammadi S, and Memarian A. Suppression of CD56(bright) NK cells in breast cancer patients is associated with the PD-1 and TGF-βRII expression. Clin Transl Oncol. (2023) 25:841–51. doi: 10.1007/s12094-022-02997-3
33. Wang J, Liu K, Xiao T, Liu P, Prinz RA, and Xu X. Uric acid accumulation in DNA-damaged tumor cells induces NKG2D ligand expression and antitumor immunity by activating TGF-β-activated kinase 1. Oncoimmunology. (2022) 11:2016159. doi: 10.1080/2162402X.2021.2016159
34. Lee YS, Choi H, Cho HR, Son WC, Park YS, Kang CD, et al. Downregulation of NKG2DLs by TGF-β in human lung cancer cells. BMC Immunol. (2021) 22:44. doi: 10.1186/s12865-021-00434-8
35. Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U.S.A. (2003) 100:4120–5. doi: 10.1073/pnas.0730640100
36. Hawke LG, Mitchell BZ, and Ormiston ML. TGF-β and IL-15 synergize through MAPK pathways to drive the conversion of human NK cells to an innate lymphoid cell 1-like phenotype. J Immunol. (2020) 204:3171–81. doi: 10.4049/jimmunol.1900866
37. Park SI, Soki FN, and McCauley LK. Roles of bone marrow cells in skeletal metastases: no longer bystanders. Cancer Microenviron. (2011) 4:237–46. doi: 10.1007/s12307-011-0081-8
38. Ohl K and Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. (2018) 9:2499. doi: 10.3389/fimmu.2018.02499
39. Zhang H, Wang J, and Li F. Modulation of natural killer cell exhaustion in the lungs: the key components from lung microenvironment and lung tumor microenvironment. Front Immunol. (2023) 14:1286986. doi: 10.3389/fimmu.2023.1286986
40. Sun H, Huang Q, Huang M, Wen H, Lin R, Zheng M, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. (2019) 70:168–83. doi: 10.1002/hep.30347
41. Manoury B, Maisonneuve L, and Podsypanina K. The role of endoplasmic reticulum stress in the MHC class I antigen presentation pathway of dendritic cells. Mol Immunol. (2022) 144:44–8. doi: 10.1016/j.molimm.2022.02.007
42. Chatterjee F and Spranger S. MHC-dressing on dendritic cells: Boosting anti-tumor immunity via unconventional tumor antigen presentation. Semin Immunol. (2023) 66:101710. doi: 10.1016/j.smim.2023.101710
43. Shevchenko JA, Khristin AA, Kurilin VV, Kuznetsova MS, Blinova DD, Starostina NM, et al. Autologous dendritic cells and activated cytotoxic T−cells as combination therapy for breast cancer. Oncol Rep. (2020) 43:671–80. doi: 10.3892/or.2019.7435
44. Luangwattananun P, Chiraphapphaiboon W, Thuwajit C, Junking M, and Yenchitsomanus PT. Activation of cytotoxic T lymphocytes by self-differentiated myeloid-derived dendritic cells for killing breast cancer cells expressing folate receptor alpha protein. Bioengineered. (2022) 13:14188–203. doi: 10.1080/21655979.2022.2084262
45. Safaei S, Alipour S, Bahojb Mahdavi SZ, Shalmashi H, Shahgoli VK, Shanehbandi D, et al. Triple-negative breast cancer-derived exosomes change the immunological features of human monocyte-derived dendritic cells and influence T-cell responses. Mol Biol Rep. (2024) 51:1058. doi: 10.1007/s11033-024-10007-8
46. de Mingo Pulido Á, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, et al. TIM-3 regulates CD103(+) dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell. (2018) 33:60–74.e66. doi: 10.1016/j.ccell.2017.11.019
47. Borges TJ, Murakami N, MaChado FD, Murshid A, Lang BJ, Lopes RL, et al. March1-dependent modulation of donor MHC II on CD103(+) dendritic cells mitigates alloimmunity. Nat Commun. (2018) 9:3482. doi: 10.1038/s41467-018-05572-z
48. Montealegre S and van Endert P. MHC class I cross-presentation: stage lights on sec22b. Trends Immunol. (2017) 38:618–21. doi: 10.1016/j.it.2017.07.002
49. Patel R and Sad S. Transcription factor Batf3 is important for development of CD8+ T-cell response against a phagosomal bacterium regardless of the location of antigen. Immunol Cell Biol. (2016) 94:378–87. doi: 10.1038/icb.2015.98
50. Vatner RE and Janssen EM. STING, DCs and the link between innate and adaptive tumor immunity. Mol Immunol. (2019) 110:13–23. doi: 10.1016/j.molimm.2017.12.001
51. Zhou Y, Slone N, Chrisikos TT, Kyrysyuk O, Babcock RL, Medik YB, et al. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103(+) conventional dendritic cells. J Immunother Cancer. (2020) 8:e000474. doi: 10.1136/jitc-2019-000474
52. Hong Y, Kim YK, Kim GB, Nam GH, Kim SA, Park Y, et al. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103(+) dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J Extracell Vesicles. (2019) 8:1670893. doi: 10.1080/20013078.2019.1670893
53. Wu TC, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, et al. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. (2014) 2:487–500. doi: 10.1158/2326-6066.CIR-13-0217
54. Chrisikos TT, Zhou Y, Li HS, Babcock RL, Wan X, Patel B, et al. STAT3 inhibits CD103(+) cDC1 vaccine efficacy in murine breast cancer. Cancers (Basel). (2020) 12:128. doi: 10.3390/cancers12010128
55. Shigehiro T, Ueno M, Kijihira M, Takahashi R, Umemura C, Taha EA, et al. Immune state conversion of the mesenteric lymph node in a mouse breast cancer model. Int J Mol Sci. (2022) 23:11035. doi: 10.3390/ijms231911035
56. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. (2016) 44:924–38. doi: 10.1016/j.immuni.2016.03.012
57. Wesolowski R, Duggan MC, Stiff A, Markowitz J, Trikha P, Levine KM, et al. 3rd: Circulating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Immunol Immunother. (2017) 66:1437–47. doi: 10.1007/s00262-017-2038-3
58. Zhang R, Dong M, Tu J, Li F, Deng Q, Xu J, et al. PMN-MDSCs modulated by CCL20 from cancer cells promoted breast cancer cell stemness through CXCL2-CXCR2 pathway. Signal Transduct Target Ther. (2023) 8:97. doi: 10.1038/s41392-023-01337-3
59. Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, et al. Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PloS One. (2015) 10:e0127028. doi: 10.1371/journal.pone.0127028
60. Ornstein MC, Diaz-Montero CM, Rayman P, Elson P, Haywood S, Finke JH, et al. Myeloid-derived suppressors cells (MDSC) correlate with clinicopathologic factors and pathologic complete response (pCR) in patients with urothelial carcinoma (UC) undergoing cystectomy. Urol Oncol. (2018) 36:405–12. doi: 10.1016/j.urolonc.2018.02.018
61. Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. (2012) 132:215–23. doi: 10.1007/s10549-011-1889-0
62. Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. (2011) 118:2254–65. doi: 10.1182/blood-2010-12-325753
63. Liu H, Wang Z, Zhou Y, and Yang Y. MDSCs in breast cancer: an important enabler of tumor progression and an emerging therapeutic target. Front Immunol. (2023) 14:1199273. doi: 10.3389/fimmu.2023.1199273
64. Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. (2013) 19:57–64. doi: 10.1038/nm.2999
65. Shou D, Wen L, Song Z, Yin J, Sun Q, and Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. (2016) 7:64505–11. doi: 10.18632/oncotarget.11352
66. Mao D, Feng L, and Gong H. The antitumor and immunomodulatory effect of yanghe decoction in breast cancer is related to the modulation of the JAK/STAT signaling pathway. Evid Based Complement Alternat Med. (2018) 2018:8460526. doi: 10.1155/2018/8460526
67. Dar AA, Patil RS, Pradhan TN, Chaukar DA, D’Cruz AK, and Chiplunkar SV. Myeloid-derived suppressor cells impede T cell functionality and promote Th17 differentiation in oral squamous cell carcinoma. Cancer Immunol Immunother. (2020) 69:1071–86. doi: 10.1007/s00262-020-02523-w
68. Ma Z, Zhen Y, Hu C, and Yi H. Myeloid-derived suppressor cell-derived arginase-1 oppositely modulates IL-17A and IL-17F through the ESR/STAT3 pathway during colitis in mice. Front Immunol. (2020) 11:687. doi: 10.3389/fimmu.2020.00687
69. Li X, Lu P, Li B, Zhang W, Yang R, Chu Y, et al. Interleukin 2 and interleukin 10 function synergistically to promote CD8(+) T cell cytotoxicity, which is suppressed by regulatory T cells in breast cancer. Int J Biochem Cell Biol. (2017) 87:1–7. doi: 10.1016/j.biocel.2017.03.003
70. Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. (2011) 9:90. doi: 10.1186/1479-5876-9-90
71. Parker KH, Beury DW, and Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. (2015) 128:95–139. doi: 10.1016/bs.acr.2015.04.002
72. Xu W, Jiang T, Shen K, Zhao D, Zhang M, Zhu W, et al. GADD45B regulates the carcinogenesis process of chronic atrophic gastritis and the metabolic pathways of gastric cancer. Front Endocrinol (Lausanne). (2023) 14:1224832. doi: 10.3389/fendo.2023.1224832
73. Oo MW, Kawai H, Takabatake K, Tomida S, Eguchi T, Ono K, et al. Resident stroma-secreted chemokine CCL2 governs myeloid-derived suppressor cells in the tumor microenvironment. JCI Insight. (2022) 7:e148960. doi: 10.1172/jci.insight.148960
74. Umansky V, Blattner C, Gebhardt C, and Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel). (2016) 4:36. doi: 10.3390/vaccines4040036
75. Tomić S, Joksimović B, Bekić M, Vasiljević M, Milanović M, Čolić M, et al. Prostaglanin-E2 potentiates the suppressive functions of human mononuclear myeloid-derived suppressor cells and increases their capacity to expand IL-10-producing regulatory T cell subsets. Front Immunol. (2019) 10:475. doi: 10.3389/fimmu.2019.00475
76. Najafi M, Farhood B, and Mortezaee K. Contribution of regulatory T cells to cancer: A review. J Cell Physiol. (2019) 234:7983–93. doi: 10.1002/jcp.27553
77. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. (2014) 211:781–90. doi: 10.1084/jem.20131916
78. Tharp KM, Kersten K, Maller O, Timblin GA, Stashko C, Canale FP, et al. Tumor-associated macrophages restrict CD8(+) T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat Cancer. (2024) 5:1045–62. doi: 10.1038/s43018-024-00775-4
79. Chen X, Yang M, Yin J, Li P, Zeng S, Zheng G, et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. (2022) 20:92. doi: 10.1186/s12964-022-00888-2
80. Duran CL, Surve CR, Ye X, Chen X, Lin Y, Harney AS, et al. Targeting CSF-1 signaling between tumor cells and macrophages at TMEM doorways inhibits breast cancer dissemination. Oncogene. (2025). doi: 10.1038/s41388-025-03485-y
81. Wang L, Zhang L, Zhao L, Shao S, Ning Q, Jing X, et al. VEGFA/NRP-1/GAPVD1 axis promotes progression and cancer stemness of triple-negative breast cancer by enhancing tumor cell-macrophage crosstalk. Int J Biol Sci. (2024) 20:446–63. doi: 10.7150/ijbs.86085
82. Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. (2019) 21:498–510. doi: 10.1038/s41556-019-0299-0
83. Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. (2018) 100:1034–43. doi: 10.1016/j.ijrobp.2017.11.043
84. Zhang J, Dong Y, Yu S, Hu K, Zhang L, Xiong M, et al. IL-4/IL-4R axis signaling drives resistance to immunotherapy by inducing the upregulation of Fcγ receptor IIB in M2 macrophages. Cell Death Dis. (2024) 15:500. doi: 10.1038/s41419-024-06875-4
85. Yang C, He L, He P, Liu Y, Wang W, He Y, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol. (2015) 32:352. doi: 10.1007/s12032-014-0352-6
86. Melwani PK, Checker R, Balla MMS, and Pandey BN. Crosstalk between macrophages and breast cancer cells: networking within tumors. Results Probl Cell Differ. (2024) 74:213–38. doi: 10.1007/978-3-031-65944-7_8
87. Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J Exp Med. (2017) 214:2695–713. doi: 10.1084/jem.20160392
88. Gan L, Qiu Z, Huang J, Li Y, Huang H, Xiang T, et al. Cyclooxygenase-2 in tumor-associated macrophages promotes metastatic potential of breast cancer cells through Akt pathway. Int J Biol Sci. (2016) 12:1533–43. doi: 10.7150/ijbs.15943
89. Mu G, Zhu Y, Dong Z, Shi L, Deng Y, and Li H. Calmodulin 2 facilitates angiogenesis and metastasis of gastric cancer via STAT3/HIF-1A/VEGF-A mediated macrophage polarization. Front Oncol. (2021) 11:727306. doi: 10.3389/fonc.2021.727306
90. Meng Z, Zhang R, Wu X, Zhang M, and Jin T. PD−L1 mediates triple−negative breast cancer evolution via the regulation of TAM/M2 polarization. Int J Oncol. (2022) 61:150. doi: 10.3892/ijo.2022.5440
91. Deswal B, Bagchi U, and Kapoor S. Curcumin suppresses M2 macrophage-derived paclitaxel chemoresistance through inhibition of PI3K-AKT/STAT3 signaling. Anticancer Agents Med Chem. (2024) 24:146–56. doi: 10.2174/0118715206275259231105184959
92. Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH, and Xu J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med. (2017) 26:421–6. doi: 10.17219/acem/62120
93. Xing J, Li J, Fu L, Gai J, Guan J, and Li Q. SIRT4 enhances the sensitivity of ER-positive breast cancer to tamoxifen by inhibiting the IL-6/STAT3 signal pathway. Cancer Med. (2019) 8:7086–97. doi: 10.1002/cam4.2557
94. Dethlefsen C, Højfeldt G, and Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. (2013) 138:657–64. doi: 10.1007/s10549-013-2488-z
95. Manore SG, Doheny DL, Wong GL, and Lo HW. IL-6/JAK/STAT3 signaling in breast cancer metastasis: biology and treatment. Front Oncol. (2022) 12:866014. doi: 10.3389/fonc.2022.866014
96. Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. (2003) 22:256–65. doi: 10.1038/sj.onc.1206113
97. Liao H, Li H, Song J, Chen H, Si H, Dong J, et al. Expression of the prognostic marker IL-8 correlates with the immune signature and epithelial-mesenchymal transition in breast cancer. J Clin Lab Anal. (2023) 37:e24797. doi: 10.1002/jcla.24797
98. Dominguez C, McCampbell KK, David JM, and Palena C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight. (2017) 2:e94296. doi: 10.1172/jci.insight.94296
99. Foldi J, Blenman KRM, Marczyk M, Gunasekharan V, Polanska A, Gee R, et al. Peripheral blood immune parameters, response, and adverse events after neoadjuvant chemotherapy plus durvalumab in early-stage triple-negative breast cancer. Breast Cancer Res Treat. (2024) 208:369–77. doi: 10.1007/s10549-024-07426-3
100. Lee EB, Kim A, Kang K, Kim H, and Lim JS. NDRG2-mediated modulation of SOCS3 and STAT3 activity inhibits IL-10 production. Immune Netw. (2010) 10:219–29. doi: 10.4110/in.2010.10.6.219
101. Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. (2014) 26:623–37. doi: 10.1016/j.ccell.2014.09.006
102. Ren L, Wang X, Dong Z, Liu J, and Zhang S. Bone metastasis from breast cancer involves elevated IL-11 expression and the gp130/STAT3 pathway. Med Oncol. (2013) 30:634. doi: 10.1007/s12032-013-0634-4
103. Johnstone CN, Chand A, Putoczki TL, and Ernst M. Emerging roles for IL-11 signaling in cancer development and progression: Focus on breast cancer. Cytokine Growth Factor Rev. (2015) 26:489–98. doi: 10.1016/j.cytogfr.2015.07.015
104. Allegrezza MJ, Rutkowski MR, Stephen TL, Svoronos N, Tesone AJ, Perales-Puchalt A, et al. IL15 agonists overcome the immunosuppressive effects of MEK inhibitors. Cancer Res. (2016) 76:2561–72. doi: 10.1158/0008-5472.CAN-15-2808
105. Roberti MP, Rocca YS, Amat M, Pampena MB, Loza J, Coló F, et al. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. (2012) 136:659–71. doi: 10.1007/s10549-012-2287-y
106. Gillgrass A, Gill N, Babian A, and Ashkar AA. The absence or overexpression of IL-15 drastically alters breast cancer metastasis via effects on NK cells, CD4 T cells, and macrophages. J Immunol. (2014) 193:6184–91. doi: 10.4049/jimmunol.1303175
107. Yoshimura T, Li C, Wang Y, and Matsukawa A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell Mol Immunol. (2023) 20:714–38. doi: 10.1038/s41423-023-01013-0
108. Valdivia-Silva J and Chinney-Herrera A. Chemokine receptors and their ligands in breast cancer: The key roles in progression and metastasis. Int Rev Cell Mol Biol. (2024) 388:124–61. doi: 10.1016/bs.ircmb.2024.07.002
109. Li X, Wang M, Gong T, Lei X, Hu T, Tian M, et al. A S100A14-CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics. (2020) 10:5687–703. doi: 10.7150/thno.42087
110. Svensson S, Abrahamsson A, Rodriguez GV, Olsson AK, Jensen L, Cao Y, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer. Clin Cancer Res. (2015) 21:3794–805. doi: 10.1158/1078-0432.CCR-15-0204
111. Sun X, Glynn DJ, Hodson LJ, Huo C, Britt K, Thompson EW, et al. CCL2-driven inflammation increases mammary gland stromal density and cancer susceptibility in a transgenic mouse model. Breast Cancer Res. (2017) 19:4. doi: 10.1186/s13058-016-0796-z
112. Mandal PK, Biswas S, Mandal G, Purohit S, Gupta A, Majumdar Giri A, et al. CCL2 conditionally determines CCL22-dependent Th2-accumulation during TGF-β-induced breast cancer progression. Immunobiology. (2018) 223:151–61. doi: 10.1016/j.imbio.2017.10.031
113. Gao D, Rahbar R, and Fish EN. CCL5 activation of CCR5 regulates cell metabolism to enhance proliferation of breast cancer cells. Open Biol. (2016) 6:160122. doi: 10.1098/rsob.160122
114. Zhang Q, Qin J, Zhong L, Gong L, Zhang B, Zhang Y, et al. CCL5-mediated th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res. (2015) 75:4312–21. doi: 10.1158/0008-5472.CAN-14-3590
115. Zhao C, Zheng S, Yan Z, Deng Z, Wang R, and Zhang B. CCL18 promotes the invasion and metastasis of breast cancer through Annexin A2. Oncol Rep. (2020) 43:571–80. doi: 10.3892/or.2019.7426
116. Kwantwi LB, Wang S, Sheng Y, and Wu Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): mechanisms and communication networks in breast cancer progression. Bioengineered. (2021) 12:6923–34. doi: 10.1080/21655979.2021.1974765
117. Chen H, Cong X, Wu C, Wu X, Wang J, Mao K, et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9(+) T cells. Sci Adv. (2020) 6:eaax4690. doi: 10.1126/sciadv.aax4690
118. Sun JH, Fan N, and Zhang Y. Correlation between serum level of chemokine (C-C motif) ligand 18 and poor prognosis in breast cancer. Genet Mol Res. (2016) 15. doi: 10.4238/gmr.15038632
119. Lee SK, Park KK, Kim HJ, Park J, Son SH, Kim KR, et al. Human antigen R-regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep. (2017) 7:9610. doi: 10.1038/s41598-017-09040-4
120. Zhang Z, Sun T, Chen Y, Gong S, Sun X, Zou F, et al. CCL25/CCR9 signal promotes migration and invasion in hepatocellular and breast cancer cell lines. DNA Cell Biol. (2016) 35:348–57. doi: 10.1089/dna.2015.3104
121. Zou A, Lambert D, Yeh H, Yasukawa K, Behbod F, Fan F, et al. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins. BMC Cancer. (2014) 14:781. doi: 10.1186/1471-2407-14-781
122. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. (2017) 2:e91487. doi: 10.1172/jci.insight.91487
123. Bedognetti D, Wang E, and Marincola FM. Meta-analysis and metagenes: CXCL-13-driven signature as a robust marker of intratumoral immune response and predictor of breast cancer chemotherapeutic outcome. Oncoimmunology. (2014) 3:e28727. doi: 10.4161/onci.28727
124. Cruceriu D, Baldasici O, Balacescu O, and Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). (2020) 43:1–18. doi: 10.1007/s13402-019-00489-1
125. Shen WH, Zhou JH, Broussard SR, Freund GG, Dantzer R, and Kelley KW. Proinflammatory cytokines block growth of breast cancer cells by impairing signals from a growth factor receptor. Cancer Res. (2002) 62:4746–56.
126. Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, and Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. (2016) 279:541–62. doi: 10.1111/joim.12470
127. Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. (2019) 378:41–50. doi: 10.1016/j.yexcr.2019.03.005
128. Eftekhari R, Esmaeili R, Mirzaei R, Bidad K, de Lima S, Ajami M, et al. Study of the tumor microenvironment during breast cancer progression. Cancer Cell Int. (2017) 17:123. doi: 10.1186/s12935-017-0492-9
129. Honda CK, Kurozumi S, Fujii T, Pourquier D, Khellaf L, Boissiere F, et al. Cancer-associated fibroblast spatial heterogeneity and EMILIN1 expression in the tumor microenvironment modulate TGF-β activity and CD8(+) T-cell infiltration in breast cancer. Theranostics. (2024) 14:1873–85. doi: 10.7150/thno.90627
130. Zhang Z, Lin M, Wang J, Yang F, Yang P, Liu Y, et al. Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT via BATF/TGF-β1. Aging (Albany NY). (2021) 13:16009–23. doi: 10.18632/aging.203093
131. Luo D, Zeng X, Zhang S, Li D, Cheng Z, Wang Y, et al. Pirfenidone suppressed triple-negative breast cancer metastasis by inhibiting the activity of the TGF-β/SMAD pathway. J Cell Mol Med. (2023) 27:456–69. doi: 10.1111/jcmm.17673
132. Meulmeester E and Ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. (2011) 223:205–18. doi: 10.1002/path.2785
133. Zhao Z, Luo Q, Liu Y, Jiang K, Zhou L, Dai R, et al. Multi-level integrative analysis of the roles of lncRNAs and differential mRNAs in the progression of chronic pancreatitis to pancreatic ductal adenocarcinoma. BMC Genomics. (2023) 24:101. doi: 10.1186/s12864-023-09209-4
134. Ma D and Niederkorn JY. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology. (1995) 86:263–9.
135. Luo W, Shi Q, Han M, Zhang Z, Reiter RJ, Ashrafizadeh M, et al. TGF-β-driven EMT in cancer progression and drug resistance. Cytokine Growth Factor Rev. (2025). doi: 10.1016/j.cytogfr.2025.05.004
136. Li ZX, Chen JX, Zheng ZJ, Cai WJ, Yang XB, Huang YY, et al. TGF-β1 promotes human breast cancer angiogenesis and Malignant behavior by regulating endothelial-mesenchymal transition. Front Oncol. (2022) 12:1051148. doi: 10.3389/fonc.2022.1051148
137. Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY, et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin. (2020) 41:670–7. doi: 10.1038/s41401-019-0324-7
138. Ping Q, Wang C, Cheng X, Zhong Y, Yan R, Yang M, et al. TGF-β1 dominates stromal fibroblast-mediated EMT via the FAP/VCAN axis in bladder cancer cells. J Transl Med. (2023) 21:475. doi: 10.1186/s12967-023-04303-3
139. Konge J, Leteurtre F, Goislard M, Biard D, Morel-Altmeyer S, Vaurijoux A, et al. Breast cancer stem cell-like cells generated during TGFβ-induced EMT are radioresistant. Oncotarget. (2018) 9:23519–31. doi: 10.18632/oncotarget.25240
140. Lee JC, Lee KM, Kim DW, and Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. (2004) 172:7335–40. doi: 10.4049/jimmunol.172.12.7335
Keywords: breast cancer, tumor immune microenvironment, innate immune cells, immunomodulatory factors, immune surveillance, prognostic biomarkers
Citation: Zhang C, Liu W, Yang P, Lin R, Pu L and Zhang H (2025) Dual roles of innate immune cells and cytokines in shaping the breast cancer microenvironment. Front. Immunol. 16:1654947. doi: 10.3389/fimmu.2025.1654947
Received: 27 June 2025; Accepted: 28 July 2025;
Published: 20 August 2025.
Edited by:
Jin Bin, Shandong University, ChinaReviewed by:
Qi Wang, Jiangsu University, ChinaCopyright © 2025 Zhang, Liu, Yang, Lin, Pu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongying Zhang, Wmhhbmdob25neWluZ3poeTEyQDE2My5jb20=; Lulan Pu, MTI3MzI3NjM3OEBxcS5jb20=
†These authors have contributed equally to this work
 Chen Zhang1,2†
Chen Zhang1,2† Hongying Zhang
Hongying Zhang