- 1Nephrology Unit, University of Brescia, ASST Spedali Civili, Brescia, Italy
- 2Renal Unit, Royal Berkshire Hospital, Reading, Berkshire, United Kingdom
- 3Department of Rheumatology and Clinical Immunology, University of Lübeck, Lübeck, Germany
- 4Molecular Immunity Unit, Department of Medicine, University of Cambridge, Cambridge, United Kingdom
- 5Cambridge University Hospitals NHS Foundation Trust, NIHR Cambridge Biomedical Research Centre, Cambridge, United Kingdom
- 6Rheumatology Unit, Department of Medicine (DiMed), University of Padua, Padua, Italy
- 7CSL Vifor, Glattbrugg, Switzerland
- 8Rheumatology Unit, Azienda USL-IRCCS di Reggio Emilia, Università di Modena e Reggio Emilia, Reggio Emilia, Italy
- 9Department of Rheumatology, Skåne University Hospital, Lund, Sweden
- 10Clinical Sciences, Rheumatology, Lund University, Lund, Sweden
- 11Department of Medicine, University of Cambridge, Cambridge, United Kingdom
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) has a relapsing-remitting course and, even with the availability of effective maintenance therapies such as rituximab, relapse rates remain high. Relapse is associated with the accrual of organ damage stemming from both the underlying disease and from the effects of AAV treatments; thus, early detection and proactive prevention are crucial. AAV study populations typically include mixed cohorts of patients with new-onset and relapsing disease. Although data specifically addressing re-induction of remission after relapse are limited, available evidence suggests high remission rates when rituximab is combined with glucocorticoids. However, the balance between effective disease control and the potential treatment-related side effects must be carefully considered, and new therapeutic options may help improve this tradeoff. The aim of this review is to explore what is known about relapse risk and relapse management while considering emerging pathogenic and therapeutic paradigms.
1 Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune necrotizing small vessel vasculitides that causes potentially life-threatening ischemic and inflammatory organ damage (1–3). The three subsets of AAV are granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Patients with GPA predominantly have ANCA directed against proteinase 3 (PR3), whereas, in MPA, 60 – 80% of patients have ANCA specific for myeloperoxidase (MPO) (4, 5). In EGPA, MPO-ANCA are detected in 30 – 40% of patients, whereas PR3-ANCA are rare. GPA is the most common AAV subtype with a global incidence of 9 per million person-years (6). Due to the distinct nature and genetic background of EGPA, it is often excluded from AAV clinical trials and will not be discussed in this paper.
Treatment of AAV with cytotoxic agents, immunosuppressive therapies, and biologics (particularly the monoclonal anti-CD20 antibody rituximab) have improved prognosis by effectively addressing active disease which, if untreated, can lead to impaired organ structure and/or function (organ damage) (7, 8). Although AAV is associated with premature mortality relative to the general population (9, 10), treatment-related improvements in survival together with a greater awareness of AAV leading to improved diagnosis have led to a rise in the overall prevalence of AAV (6, 11, 12).
These conditions, which were once fatal in nearly all patients, are now considered as chronic relapsing disorders with peaks and troughs in disease activity (13). Although relapses (defined as the return of active disease after remission) are common and can be clinically significant, it is not possible to reliably predict when they will occur. Re-establishing control of disease activity (re-induction) promptly in patients with relapse is important to prevent or minimize organ damage. However, the optimal treatment for AAV relapse remains to be determined (14).
This paper summarizes the risk factors for AAV relapse, evaluates the effectiveness of available and exploratory biomarkers for predicting relapse, explores factors that might help inform relapse prevention strategies, and assesses the available treatment options for re-inducing remission.
2 Pathophysiology of AAV
Immune dysfunction is fundamental to the development of characteristic inflammatory lesions in blood vessels and affected organs in AAV (2, 3). In patients with impaired immunological self-tolerance and a genetic predisposition, the production of ANCA plays a key role in AAV pathogenesis by promoting inflammatory responses (4, 15, 16). The binding of ANCA to MPO and PR3 exposed on neutrophils primed by complement fragment C5a and cytokines enhances leukocyte-endothelial interactions triggering neutrophil extracellular trap (NET) formation (17) as well as a strong inflammatory response. This process is part of a broader immune dysfunction which exacerbates endothelial injury, with C5a further amplifying inflammation by enhancing the generation of antibodies and the activation of phagocytic cells (18). Complement activation thus plays a key role in disease pathogenesis and therefore in the development of organ injury (19–22). Of note, pathophysiological studies in AAV have historically not distinguished between the mechanisms driving disease onset and those involved in relapse. Although these processes are likely to overlap, definitive evidence supporting this assumption is currently lacking.
3 AAV disease course
AAV can develop at any time, but incidence increases with age (23). This complex disease has heterogenous phenotypes resulting in different treatment priorities in different patients (Figure 1).
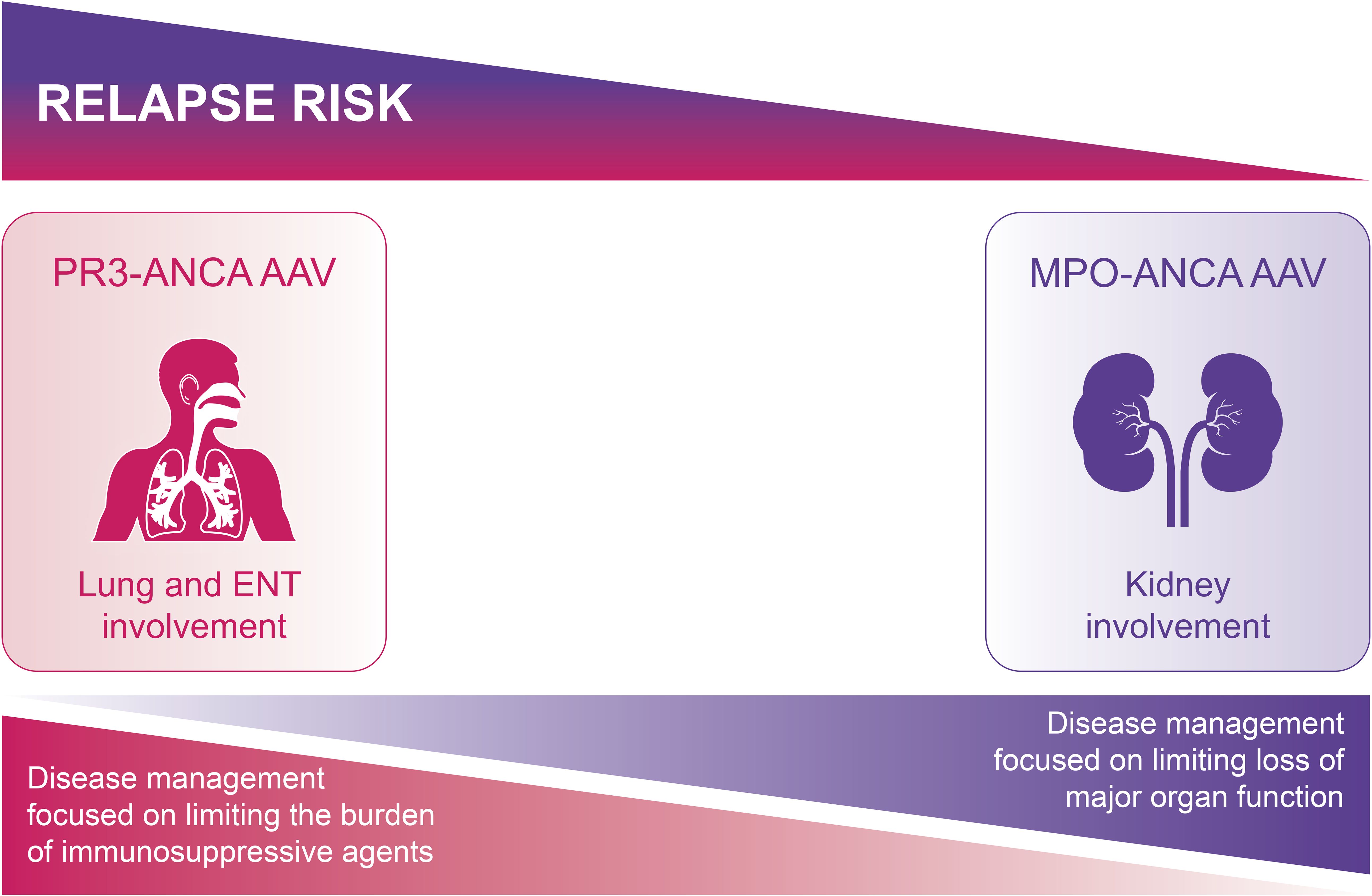
Figure 1. Aims of treatment differ according to AAV phenotype. For PR3-ANCA AAV, the relapse rate is higher, but the risk of major organ damage is lower, and so limiting the burden of re-treatment is the main aim of disease management. For MPO-ANCA AAV, although the relapse risk is lower, the higher chance of residual organ damage from the first relapse means there is higher risk of further progression of organ damage towards end-stage disease; the aim of disease management is thus to limit disease activity and reduce the risk of further loss of major organ function. AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; PR3, proteinase 3.
The early stages of AAV typically present with a range of non-specific symptoms suggestive of chronic inflammation, including fatigue, weight loss, fever, myalgia, and polyarthralgia (23). Patients with more advanced disease may show signs and symptoms of damage to the ears, nose and throat (ENT) (e.g., chronic rhinosinusitis, nasal bleeding and hearing loss (23, 24)), lung fibrosis (e.g., persistent cough and breathlessness (23)), and/or chronic kidney disease (CKD) (e.g., microscopic hematuria and reduced estimated glomerular filtration rate [eGFR] (23)). In addition, prolonged active disease can result in inflammation-related comorbidities, such as cardiovascular disease (25, 26). The longer AAV remains undiagnosed and untreated, the greater the risk of AAV-related comorbidities and irreversible organ damage.
AAV is a relapsing-remitting disease making increases in disease activity part of the natural disease course. However, there is considerable variability in the duration of remission between relapses (27, 28), with some patients never experiencing a relapse. These disparities may be partly explained by differences between patients in immune system characteristics and response to immunosuppressive treatment (29, 30).
Each relapse increases the risk of potentially fatal organ damage due to increased inflammatory disease activity and the use of higher-dose (more toxic) re-induction treatments (31). Mortality is also affected by treatment-related toxicities (in particular serious infections) and comorbidities, including cardiovascular disease (26). Although treatment effectively improves survival in most patients, survival rates are typically lower in older patients and in those with more severe kidney involvement and more active disease (32). Despite improvements in survival rates over recent decades, mortality remains approximately 2.7 times higher in patients with AAV than in the general population (26).
4 Treatment of AAV
The aim of AAV treatment is to control active disease whilst limiting treatment-related toxicities, thereby preventing or minimizing the risk of tissue damage. Treatment includes intensive induction therapy (typically high-dose glucocorticoids with rituximab or cyclophosphamide) to gain rapid disease control and, once this is achieved, less toxic maintenance strategies to sustain remission (14).
The choice of treatment is not straightforward and may be restricted by the presence of disease-induced organ damage and co-morbidities. Treatments should ideally be tailored according to disease activity, risk factors, age, and existing comorbidities, e.g., CKD and lung disease (14, 33). The risk of treatment-induced comorbidities, such as infertility, diabetes, osteoporosis and infections, must also be considered. Ongoing monitoring of risk factors and signs of relapse is recommended throughout remission, as relapse risk can fluctuate over time due to inherent factors and cumulative events. The ideal scenario would be to have reliable tools for predicting relapses so, where necessary, treatment can be intensified early.
Over the last 30 years, expanding AAV treatment options have led to a shift from high-dose glucocorticoids supplemented with cyclophosphamide to less toxic approaches (34–36). For example, cyclophosphamide is often replaced or supplemented with rituximab to reduce the cumulative dose (14, 33). The current consensus is that rituximab is the most effective treatment for maintaining remission (37–39), and a combination of rituximab and glucocorticoids is recommended for the treatment of relapsing disease in most settings (14, 33). According to treatment guidelines, once remission is established, glucocorticoids should be tapered to the lowest effective dose (typically ≤5 mg/day within 4 to 5 months) or completely withdrawn to limit treatment toxicity (14, 33).
Recent studies, including the phase 3 randomized controlled ADVOCATE trial (40), demonstrate that the complement 5a receptor 1 (C5aR1) antagonist, avacopan, can improve remission rates, sustain remission over time, and (in patients with ANCA-associated glomerulonephritis) improve kidney function in patients with new-onset or relapsing AAV treated with rituximab or cyclophosphamide and reduced glucocorticoid exposure (37, 39–44). The use of avacopan with a low-dose glucocorticoid regimen is associated with a lower incidence of toxicities, including serious infections, than standard (non-avacopan) treatment (45). Based on results from the ADVOCATE trial (40), EULAR and KDIGO guidelines recommend using avacopan to reduce glucocorticoid exposure in patients receiving standard treatment for GPA or MPA (14, 33).
5 Challenges in maintaining remission
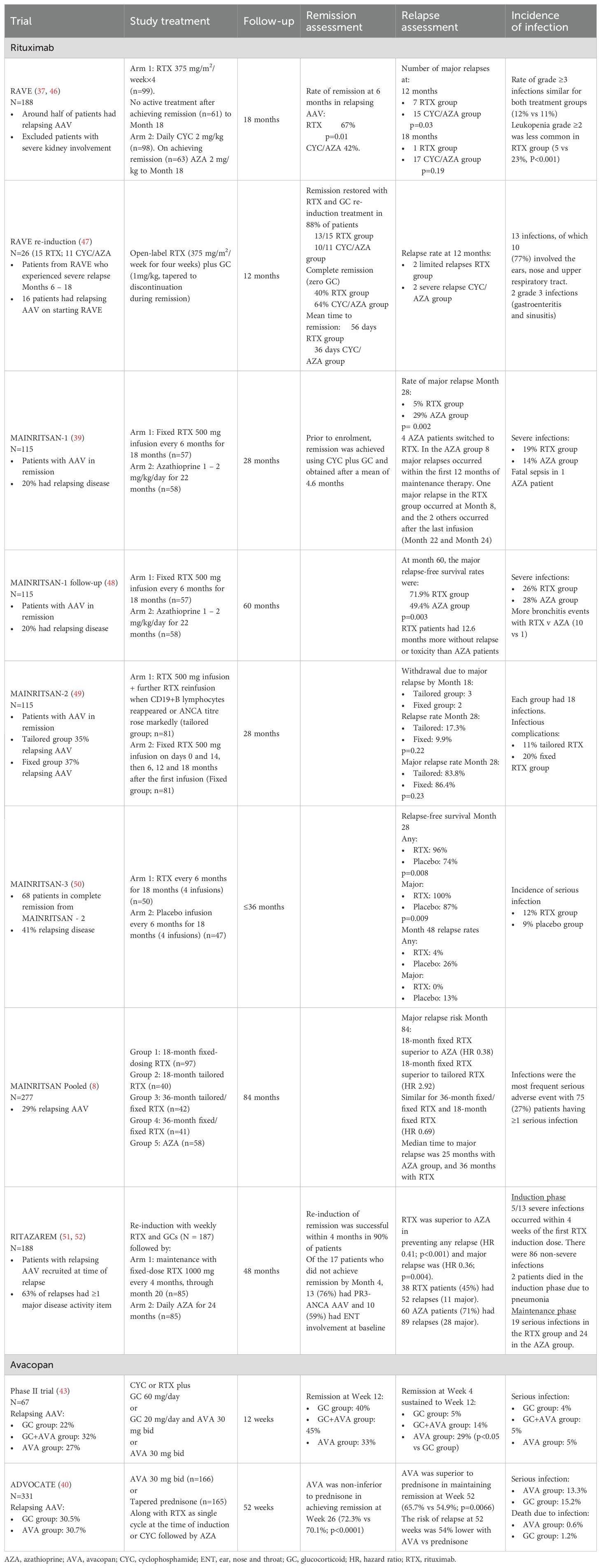
The optimal maintenance regimen has yet to be defined for patients achieving remission. Based on results from the MAINRITSAN trial (Table 1) (39), the recommended regimen includes pre-emptive rituximab doses of 500 mg every six months for 24 months (14, 53). However, the RITAZAREM trial found that a rituximab-based regimen of 1000 mg every 4 months was also effective in patients with relapsing disease (51) leading guidelines to suggest using the higher dose in patients who relapse on the 500 mg regimen (14).
Defining the optimal duration of rituximab treatment is particularly challenging due to the increased risk of adverse effects, including infection, hypogammaglobulinemia, and impaired vaccine response. The recommended duration of rituximab therapy varies between guidelines, with EULAR guidelines recommending continuing treatment for 24 to 48 months (14) and KDIGO guidelines recommending 18 to 48 months (33). Of note, the MAINRITSAN-3 trial demonstrated fewer relapses in patients receiving rituximab for 36 months versus 18 months (50). Although subsequent pooled analyses failed to show an improvement in relapse-free survival with extended dosing (8), results suggest that extending maintenance therapy may be beneficial in some contexts (54).
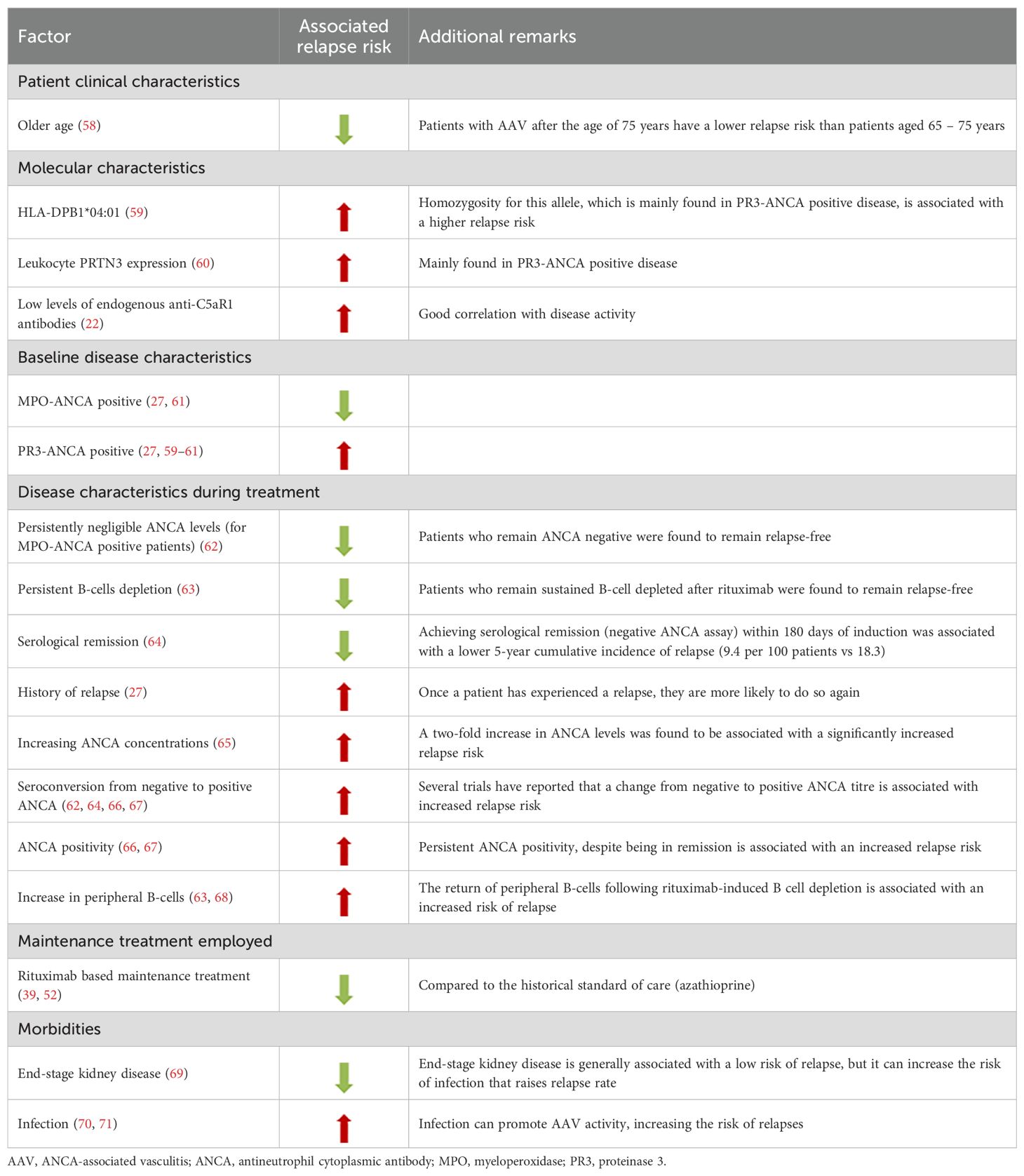
Rituximab maintenance treatment is generally effective in sustaining remission and is typically well-tolerated. However, this is not the case for all patients and several factors may limit its’ use. These include a reduced response to rituximab in some patients, which may be a consequence of high interpatient variability of serum rituximab levels due to genetic polymorphisms and/or (in patients with repeated rituximab exposure) neutralization of rituximab activity by anti-rituximab antibodies (55–57). Furthermore, the use of rituximab is associated with an increased risk of infection, as demonstrated by both the MAINRITSAN and RITAZAREM trials (Table 1) (39, 52). The observation that infection rates improved using an individually tailored approach with reduced rituximab exposure (49) raises the question of whether decisions abouts re-dosing and the duration of maintenance treatment should always be pre-emptive or whether they should be reduced in people with low relapse risk (e.g., those who remain MPO-ANCA negative or experience sustained B-cell depletion after rituximab treatment) (Table 2) (62, 63).
The risk of infection tends to increase in patients with hypogammaglobulinemia during the first year of rituximab induction. This is more prevalent among patients with low baseline IgG levels and is primarily linked to older age and glucocorticoid dose (72–75). Although hypogammaglobulinemia is not definitively linked to an increased risk of infection during maintenance treatment, patients receiving re-induction therapy after relapse may experience further decreases in IgG levels due to repeated rituximab administration (75, 76). Similarly, the diminished response to vaccines in patients undergoing B-cell depletion—a common phenomenon in rituximab-treated patients —may have important clinical implications, potentially heightening the risk of infection (77, 78).
The high risk of severe infections and other complications with glucocorticoids, even at low doses, means their use requires careful risk-benefit assessment, particularly in terms of co-morbidities (31). Whilst pre-rituximab data suggest that the extended use of glucocorticoids is associated with fewer relapses (79), a retrospective study in rituximab-treated patients found that extending the use of high-dose glucocorticoids for more than six months increased the risk of severe infection and other adverse effects (e.g., diabetes and cardiovascular complications) without reducing relapse risk (80). The long-term use of low-dose glucocorticoids for the prevention of relapse has not been specifically evaluated in randomized controlled trials. However, the TAPIR trial found that the risk of major relapse in rituximab-treated patients with GPA remained low irrespective of whether patients received low-dose or no glucocorticoids and that the benefits of low-dose glucocorticoids to prevent minor relapses were only observed among patients treated with non-rituximab-based regimens (81). This suggests that maintenance treatment with rituximab and little or no glucocorticoids may be possible in some patients.
6 Relapse in AAV
Relapse is classified according to the level of disease activity and the extent of organ involvement, assessed using the Birmingham Vasculitis Activity Score (BVAS) Version 3 (14, 82) or the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS-WG) (83). A relapse is typically deemed major if it affects key organs, such as the neurological system or the kidneys. Minor relapses are usually characterized by constitutional symptoms either in isolation or in association with non-life or non-organ-threatening manifestations. However, classification is often complicated by limitations in assessing disease activity. For example, kidney disease relapse does not have a generally accepted and shared definition, and there is no standardized modality for assessing kidney disease activity in patients with relapsing AAV.
ANCA testing is a central component of AAV diagnosis and is increasingly used to help define disease status, including remission and relapse. A negative serum ANCA assay characterizes serological remission while ANCA return suggests serological relapse. However, since ANCA titers do not necessarily reflect AAV activity, they are an imperfect indicator of relapse (78).
In the pre-rituximab era, the likelihood of relapse within the first five years was 40%–55% (82, 84). While the use of rituximab as maintenance therapy has significantly reduced this risk, relapse rates are not negligible and tend to increase significantly after maintenance treatment is withdrawn (8).
6.1 Relapse risk
Accurate risk assessment is essential for the prevention or early detection of relapse (31). Consequently, guidelines recommend continuously assessing patients throughout their journey (14, 33). Several factors determine the likelihood of relapse (Table 2). Generally, relapse is less common in patients with MPA (27), MPO-ANCA associated disease (27, 61), severe kidney disease (69), and older age (>75 years old) (58), while it is more common in those with GPA (27), PR3-ANCA associated disease (27, 59–61), and ENT involvement (66, 68, 70, 85–87). Other risk factors for relapse include infection (70, 71), relapse history (27), seroconversion to ANCA positivity and (possibly) rising ANCA titers during treatment (62, 64–67) (Table 2). In contrast, persistent ANCA negativity (especially in rituximab-treated patients with sustained B-cell depletion) is strongly predictive of a patient remaining relapse-free (62, 63, 68).
When assessing relapse risk in patients with AAV, the therapeutic regimen employed should also be taken into account. While robust evidence has established that a rituximab-based maintenance regimen is superior to azathioprine in preventing relapses (39, 52), emerging data suggest that the induction regimen may also influence relapse risk. A retrospective real-world study involving 101 patients with AAV demonstrated that an induction strategy combining rituximab and cyclophosphamide was associated with a lower relapse rate compared to rituximab monotherapy (88). Notably, this association was observed only when both major and minor relapses were considered together and was not confirmed for major relapses alone. Further evidence for the impact of combined induction with rituximab and cyclophosphamide compared to rituximab alone will be provided by the ongoing, randomized controlled ENDURRANCE trial (89).
The underlying mechanisms driving relapse are not fully understood; consequently, our understanding of the relevance of concomitant risk-modifying factors is limited. ENT damage, for example, is associated with a higher relapse risk but a lower risk of kidney disease and better survival rates (87). The high number of relapse risk factors ranging from inherent factors (e.g., ANCA specificity) to single patient “disease behavior” and comorbidities (e.g., previous relapse and infection) suggests a need for advanced modelling techniques that provide accurate estimates of future relapse risk.
6.2 The role of biomarkers to detect imminent relapse
Although ANCA levels, the timing of B-cell return after rituximab treatment, and (in some contexts) complement fluctuations may provide some indication of impending relapse (22, 62, 65, 68), the search for a reliable and reproducible biomarker continues (90). In addition to predicting relapse, biomarkers may provide information on factors that impact overall prognosis (e.g., subclinical disease activity) and help identify patients with an abnormal activation of fibrotic pathways that may contribute to organ damage (91). As our understating of AAV expands and reports emerge of several disease phenotypes (92), it is becoming increasingly likely that a reliable biomarker for patients with one phenotype may not necessarily be appropriate for patients with other characteristics.
6.2.1 Clinical biomarkers
Although increases in ANCA titers are typically associated with increased relapse risk, data from clinical trials and observational studies are often conflicting (8, 46, 49). In patients treated with rituximab, B-cell repopulation after complete peripheral depletion may help inform the risk of relapse and the optimal timing of rituximab administration within a tailored retreatment strategy (63, 66). This hypothesis has been tested in a prospective study, in which B-cell-driven rituximab maintenance therapy was more effective than ANCA-driven rituximab therapy for preventing relapse (78). Therefore, although the peripheral B-cell count monitored for personalized treatment does not necessarily correlate with tissue-resident B-cells, it may be a useful clinical indicator for relapse (93). Of note, characteristics of the B-cell compartment show significant interpatient variability at the time of repopulation and may provide an even more accurate indication of relapse risk (94). Indeed, a greater relapse risk was reported for repopulating B-cell compartments comprising a higher proportion of autoreactive PR3+ B-cells, switched memory B-cells or plasmablasts, and a lower proportion of naïve B-cells (94–96). This suggests that combining ANCA and B-cell monitoring (at least in rituximab-treated patients) may provide useful information on relapse risk in patients with a relative low relapse rate, such as those with MPO-ANCA MPA who exhibit persistent ANCA negativity and B-cell depletion.
Eventually, B-cell repopulation is likely to occur in rituximab-treated patients and further research should focus on determining how to optimize the depletion of the B-cells subsets that drive autoimmunity. It should also be noted that relapses may occur in patients without B-cell repopulation or increasing ANCA titers (48, 63, 66), and that some biomarkers may provide useful information on relapse risk in specific organs.
6.2.2 Exploratory biomarkers
Although exploratory biomarkers are not yet ready for use in clinical practice, they may provide useful insights into disease pathogenesis. Some of these biomarkers have been tested in randomized controlled studies (43).
A retrospective analysis of samples collected during the RAVE trial found high levels of interleukin-6 (IL-6) that positively correlated with ANCA levels in patients with PR3-ANCA but not MPO-ANCA (28).
In addition to IL-6, low levels of autoantibodies targeting C5aR1 show good correlation with both AAV disease activity and relapse risk (22). This suggests a physiologically antagonistic role for endogenous anti-C5aR1 antibodies as regulators of a C5aR1 immune checkpoint and provides support for the use of therapies such as avacopan, which target the complement system in patients with AAV.
Calprotectin is released by neutrophils and monocytes during inflammation and correlates with active AAV (97, 98). Increases in serum calprotectin may predict AAV relapse as a potential indicator of sub-clinical kidney inflammation (99, 100).
The potential role of NETs as biomarkers is supported by their central role in the pathogenesis of AAV (17). Notably, neutrophils expressing type II interferon (IFN) signature genes are increased in patients with MPA and are associated with persistent vasculitis symptoms (101). Furthermore, elevated IFN-γ levels at disease onset, a key cytokine driving the differentiation of mature neutrophils toward a type II IFN signature phenotype, are associated with an increased risk of disease relapse (101). These findings, derived from a Japanese cohort, warrant validation in other ethnic populations to assess their generalizability.
Finally, urinary biomarkers may inform on the status of kidney disease activity. MCP1 and CD163 have been associated with kidney vasculitis relapses (102, 103) and increases in urinary CD4+ T-cell (104, 105). Among the exploratory biomarkers, MCP-1 and CD163 appear to be the most advanced in terms of potential clinical application (40).
6.3 Treatment of relapse
Glucocorticoids are frequently included in treatment strategies for relapse with the dose varying according to relapse severity and treating physician experience (Figure 2). The risk of glucocorticoid-related toxicity is potentially higher in patients with previous relapses due to prior glucocorticoid exposure. The ideal form of management is therefore prevention and, since relapse frequently occurs after the cessation of maintenance therapy, extending the duration of maintenance treatment for patients with known risk factors (Table 2) could help reduce the likelihood of relapse (52, 66, 106).
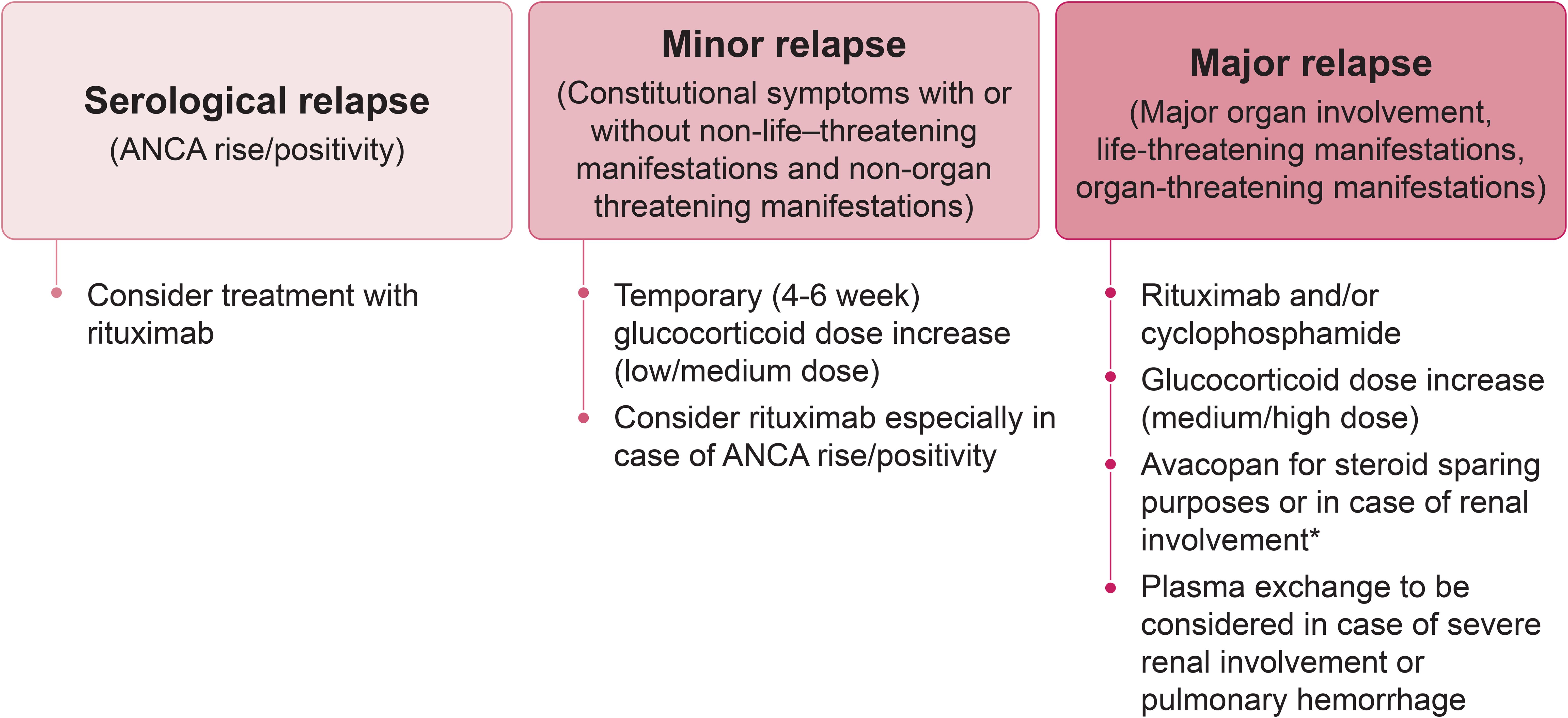
Figure 2. Flow-chart outlining the recommended approach for managing AAV relapse. * If avacopan is started, reduce/withdraw glucocorticoids in 4 – 6 weeks. ANCA, antineutrophil cytoplasmic antibody.
There is no “one-size-fits-all” solution, and decisions regarding re-induction treatment should be based on an individual assessment considering ANCA type, disease severity, comorbidities, affected organs, and other patient characteristics such as age (Figure 3). A major relapse requires re-initiation of induction treatment with rituximab or cyclophosphamide, as employed to achieve remission in newly diagnosed cases (14, 33) (Figure 2). In contrast, a minor relapse typically warrants only temporary treatment intensification, such as a short course of low-dose glucocorticoids (Figure 2). It should be noted, however, that minor relapses are often a prelude to a more serious event, especially if there is an accompanying rise in ANCA titer (Figure 2). Among the 44 patients who experienced a minor relapse during follow-up in the RAVE trial, 80% achieved remission with an increase in prednisone dose, but 70% relapsed within 6 months (27). Patients who were least likely to maintain prolonged remission tended to have GPA, be PR3-ANCA positive and have previous relapses. This suggests that adjusting the dose and/or duration of immunosuppression according to patient characteristics might help prevent the need for more intensive treatment in the future. In patients with frequent relapses, alternative strategies beyond a temporary increase in glucocorticoids are recommended (14). This often involves combining a low-dose glucocorticoid with an immunosuppressive agent, most commonly rituximab. However, as previously discussed, emerging evidence suggests that glucocorticoids might not be beneficial for all patients (81).
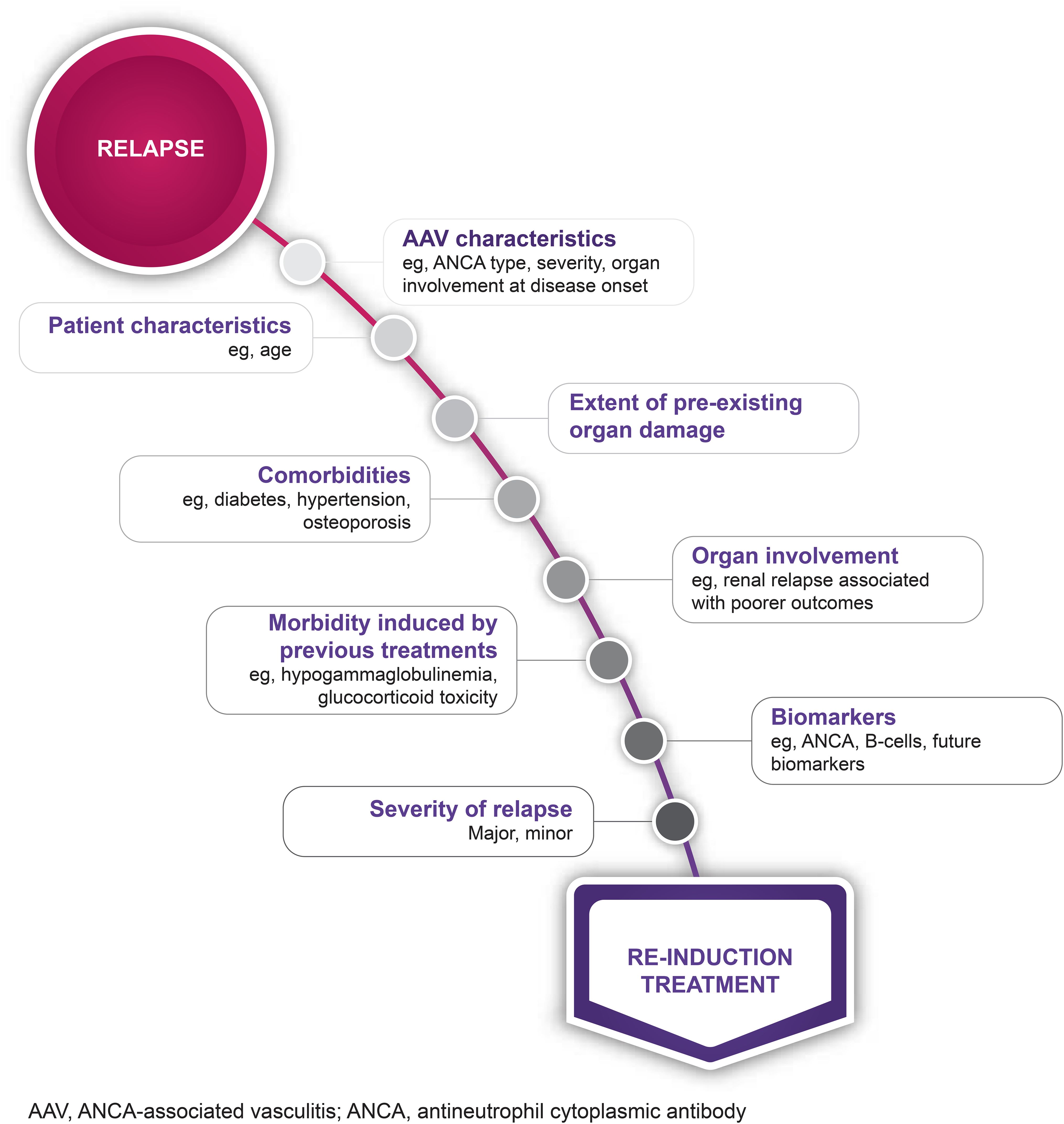
Figure 3. Considerations when choosing a re-induction treatment strategy. AAV, antineutrophil cytoplasmic antibody- (ANCA-) associated vasculitis.
Although data specifically pertaining to the treatment of relapse are scarce, many studies have included patients with a history of relapse (Table 1). Treatment of relapse typically includes rituximab and/or cyclophosphamide in addition to glucocorticoids (Figure 2). Rituximab has been shown to be as effective as cyclophosphamide in achieving remission and is more effective than azathioprine for maintaining remission (8, 37, 48, 107). A retrospective analysis of data from the RAVE trial found that 88% of patients with a major relapse six to 18 months after initial induction therapy achieved remission following re-induction with rituximab and glucocorticoids (47). Similarly, the RITAZAREM trial found that 90% of patients relapsing with GPA or MPA achieved remission within four months of starting re-induction therapy with rituximab and glucocorticoids (51). However, there is a minority of patients for whom AAV remission is not induced by rituximab (51). Reasons for this are unclear, but a single nucleotide polymorphism of the B-cell activating factor (BAFF) gene and high interpatient variability of serum rituximab levels have been proposed as potential causes (55, 56). Furthermore, data from the RITAZAREM trial confirmed findings from previous retrospective studies, in which higher glucocorticoid doses were associated with an increased risk of developing low IgG levels (48, 74). This supports the use of glucocorticoid-sparing treatment regimens for reducing the risk of infection.
Avacopan effectively enables a reduction in glucocorticoid exposure and glucocorticoid-related toxicity in patients receiving rituximab or cyclophosphamide for relapsing AAV (Table 1). The ADVOCATE trial demonstrated that avacopan was as effective as a prednisone taper as induction therapy in patients with new-onset or relapsing AAV treated with rituximab or cyclophosphamide, with avacopan-based treatment demonstrating a 54% estimated reduction in relapse risk compared to a prednisone taper (40). A supplementary analysis confirmed that avacopan effectively achieved remission in the subgroup of patients with relapsing disease (40). Importantly, patients who received the avacopan-based regimen received only a third of the cumulative dose of glucocorticoids (equating to a median of 2100 mg less total prednisone-equivalent over a year) compared with the prednisone taper group and experienced statistically significant and clinically meaningful improvements in health-related quality of life (40, 108). Results from the subset of patients who received rituximab echoed the main findings of the ADVOCATE trial, showing a 58% reduction in relapse risk in the avacopan group compared with prednisone (109).
Another benefit for avacopan is that it provides greater kidney function recovery than prednisone taper (40, 110, 111). The least-squares mean improvement in eGFR from baseline for patients with kidney involvement in the ADVOCATE study was significantly greater for those in the avacopan group than for those in the prednisone taper group at 52 weeks (7.3 vs 4.0 mL/min/1.73 m2; p=0.0259) (40, 112). An even greater improvement from baseline to week 52 (16.1 vs 7.7 mL/min/1.73 m2; p=0.003) was seen among patients with impaired kidney function at baseline (eGFR <20 mL/min/1.73 m2) (111). This is particularly relevant for patients requiring re-induction because (as previously discussed) each relapse increases the risk of organ damage due to increased inflammatory disease activity and the use of higher-dose (more toxic) re-induction treatments (31).
Evaluation of avacopan use in clinical practice, including patient categories not eligible for inclusion in the ADVOCATE trial, confirmed the high response rates and steroid-sparing effect of the avacopan regimen (113–115). Furthermore, a small study of Italian clinical practice revealed that avacopan was largely used in relapsing disease and was associated with a high sustained remission rate (116).
Overall, evidence supports the use of rituximab in patients at risk of relapse, and the inclusion of avacopan in the induction regimen, at least in patients who would otherwise be treated with prednisone and in those requiring full re-induction. As the therapeutic armamentarium expands, greater emphasis must be placed on personalizing treatment to achieve effective disease control while minimizing treatment-related toxicities (Figure 4).
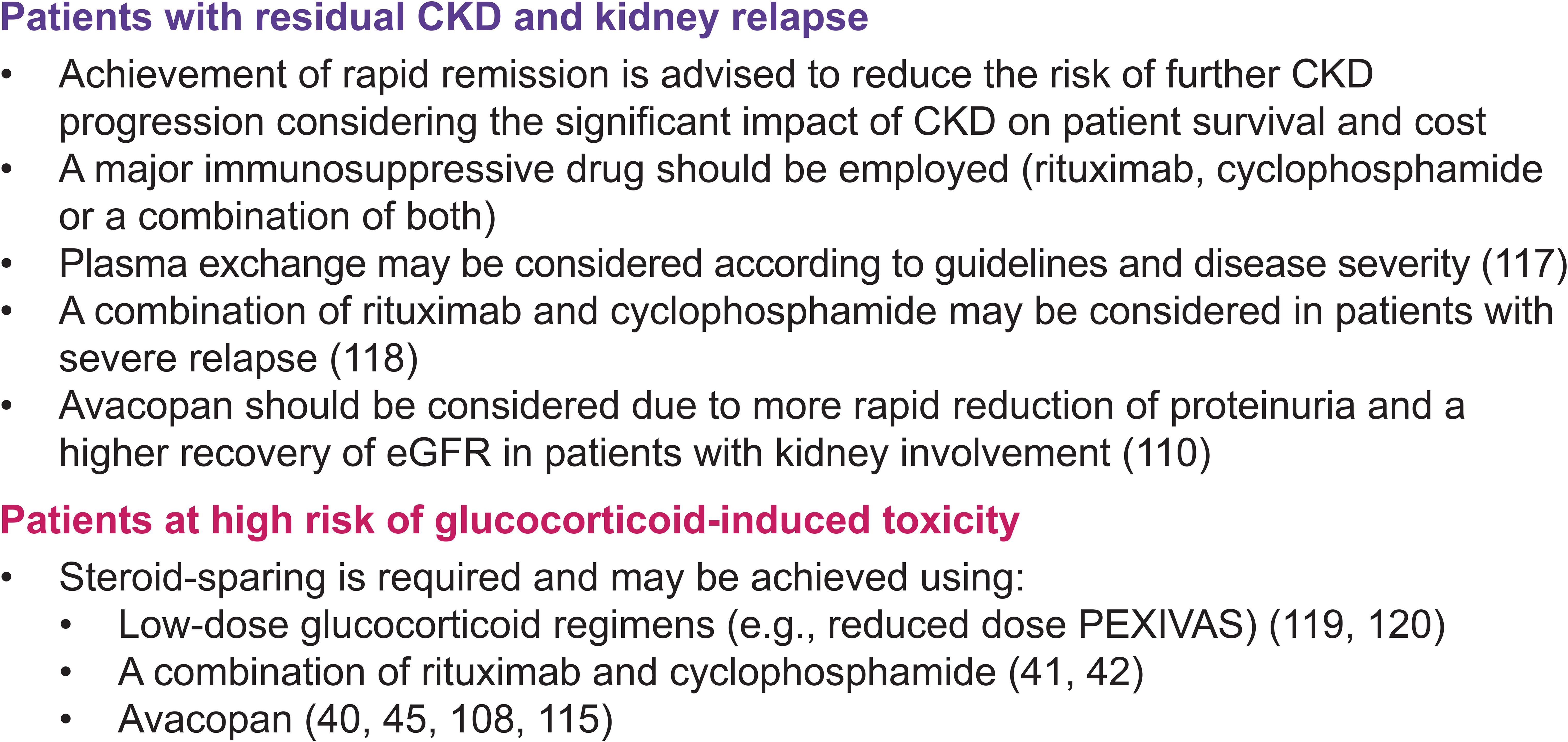
Figure 4. Scenarios illustrating wider treatment options in the management of AAV. AAV, antineutrophil cytoplasmic antibody- (ANCA-) associated vasculitis; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
7 Impact of patient perspectives on treatment decisions
Health-related quality of life (HR-QoL) is often lower in patients with AAV compared to the general population, with many patients reporting depression, anxiety, unemployment, fatigue and pain (26, 108, 121). As expected, patient-reported outcomes (PRO) tend to worsen during periods of active disease and improve with remission (121). A post-hoc analysis of PRO data from the ADVOCATE trial demonstrated improvements in HR-QoL from baseline to weeks 26 and 52 in patients with AAV treated with rituximab or cyclophosphamide alongside glucocorticoids (108). Improvements in 36-Item Short Form Health Survey (SF-36) summary scores were greater in patients receiving avacopan versus prednisone taper at weeks 26 and 52, while EuroQoL 5-Dimensions 5-Level Questionnaire (EQ-5D-5L) summary scores showed greater improvements for avacopan versus prednisone taper only at week 52 (108). Whether this is directly due to the effects of avacopan, the impact of lower cumulative glucocorticoid exposure, or a combination of both remains to be established in future studies. Either way, results suggest that patient-reported HR-QoL should be taken into account when choosing a treatment regimen.
EULAR guidelines for the treatment of AAV stress the importance of effective communication and shared decision-making between physicians and patients (14). However, results from an online survey completed by 170 healthcare professionals (HCP) and 69 patients suggest that HCPs and patients have different perspectives on treatment aims, with HCPs prioritizing treatment efficacy and clinical outcomes, while patients focus more on long-term outlook, mental health, and functional impact (122). Patient perspectives may also be shaped by individual experiences and disease history. For example, a survey of 470 patients with AAV from 13 countries found that patients with prior exposure to dialysis or plasma exchange were more likely to favor plasma exchange for relapse management than patients without prior dialysis or plasma exchange (123). This highlights the need for a more patient-centered approach to AAV management.
8 The way forward
Future studies are required to evaluate optimal dosing strategies and new combinations of immunosuppressants in patients with AAV. Ongoing studies include the COMBIVAS trial, in which patients with AAV are treated with rituximab in combination with the anti-BAFF agent, belimumab (55, 124, 125). The rationale for this study is that the increased BAFF levels reported in some patients with AAV may hinder the efficacy of rituximab. It is hoped that the distinct mechanisms of action for rituximab and belimumab will enhance B-cell targeting, especially as belimumab appears to mobilize CD27+ memory cells making them available to the cytotoxic effect of rituximab (126). Similarly, since activated CD4 T-cells are involved in the pathogenesis of AAV and are likely central in mediating kidney damage, a therapy that inhibits T-cell activation could help control disease activity (127, 128). However, the ABROGATE trial did not demonstrate reduced relapse rates in patients with GPA treated with abatacept (129). Data from patients with other indications suggest that obinutuzumab may achieve greater tissue B-cell depletion than rituximab (130). The OBIVAS trial is comparing the effects of obinutuzumab and rituximab on B-cell depletion and sustained remission in patients with AAV, with completion expected in late 2025 (131). Over time, the options for re-induction are likely to expand enabling further reductions in glucocorticoid use.
9 Concluding remarks
Effective relapse prevention and/or treatment is critical to minimizing tissue damage and glucocorticoid exposure in patients with AAV. Treatment choices are not straightforward due to interpatient variability in response and the high proportion of patients with significant comorbidities, many of which may be AAV-induced or treatment related. Balancing the benefits of treatment against individual risk factors, treatment toxicities, and organ damage is key to optimizing outcomes.
There is no definitive biomarker for predicting AAV relapse; however, monitoring ANCA titers and (in rituximab-treated patients) B-cell repopulation may help identify patients with a high risk of relapse, thereby guiding preventative dosing. When relapse prevention fails, early treatment re-introduction or intensification will help minimize the effects of active disease on tissue damage and optimize long-term outcomes. Rituximab effectively maintains remission and re-induces remission after relapse. Adjunctive therapy with avacopan can help minimize glucocorticoid exposure while improving kidney function and achieving lower relapse rates than a prednisone-based regimen aimed at corticosteroid withdrawal (40).
Further studies are required to investigate the effects of re-induction treatments specifically in patients with AAV relapse. Although specific data are lacking, it is likely that the pathogenetic mechanisms underlying new-onset AAV and AAV relapse are similar. Importantly, clinical evidence to date supports the comparable efficacy of the same therapeutic approaches in both settings. In both scenarios, reducing the risk of organ damage by minimizing disease activity whilst limiting treatment toxicity remains a top priority.
Author contributions
FA: Conceptualization, Writing – original draft, Writing – review & editing. OF: Conceptualization, Writing – original draft, Writing – review & editing. PL: Conceptualization, Writing – original draft, Writing – review & editing. KL: Conceptualization, Writing – original draft, Writing – review & editing. RP: Conceptualization, Writing – original draft, Writing – review & editing. TP: Conceptualization, Writing – original draft, Writing – review & editing. CS: Conceptualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Kate Bass and Jackie Read of Bright Red Fox Creative Ltd. for editorial support in the preparation of this manuscript.
Conflict of interest
FA: advisory board, consulting fees, honoraria for presentations or support for attending meetings from Vifor CSL, AstraZeneca, Bayer and Boehringer Lilly. OF: advisory board, consulting fees, honoraria for presentations or support for attending meetings from Vifor CSL, Consulting fees Boehringer Ingelheim. PL: research funding: BMBF, DFG, DGRh, John Grube Foundation, Vifor CSL; consulting fees: AbbVie, Astra Zeneca, GSK, Novartis, UCB, Vifor CSL; lecture fees: Astra Zeneca, Boehringer Ingelheim, GSK, Janssen, Novartis, Rheumaakademie, UCB, Vifor CSL. KL: advisory board and consulting fees: Boehringer Ingelheim, Vigor CSL; honoraria for presentations: Astra Zeneca; support for attending meetings: Novartis. AM: lecture and consultation fees from AMGEN, GSK, Roche Sweden, Vifor CSL, Abbvie and AstraZeneca; lecture fees from Eli Lilly Sweden and Boehringer Ingelheim. RP: consulting, advisory board and educational activities: GSK, CSL Vifor, Sanofi, AstraZeneca; speakers’ bureau: GSK, Sanofi, Vifor CSL; Grant and research support: Janssen. TP: employee Vifor CSL. CS: advisory board, consulting fees, honoraria for presentations or support for attending meetings from Vifor CSL.
The authors declare that this study received funding from Vifor Fresenius Medical Care Renal Pharma AG. The funder had the following involvement in the study: Vifor Fresenius Medical Care Renal Pharma AG sponsored the preparation of this paper and were involved in the decision to submit the article for publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, and Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol. (2018) 9:1166. doi: 10.3389/fneur.2018.01166
2. Arnold S, Kitching AR, Witko-Sarsat V, Wiech T, Specks U, Klapa S, et al. Myeloperoxidase-specific antineutrophil cytoplasmic antibody-associated vasculitis. Lancet Rheumatol. (2024) 6:e300–e13. doi: 10.1016/S2665-9913(24)00025-0
3. Falde SD, Fussner LA, Tazelaar HD, O’Brien EK, Lamprecht P, Konig MF, et al. Proteinase 3-specific antineutrophil cytoplasmic antibody-associated vasculitis. Lancet Rheumatol. (2024) 6:e314–e27. doi: 10.1016/S2665-9913(24)00035-3
4. Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. (2012) 367:214–23. doi: 10.1056/NEJMoa1108735
5. Jennette JC and Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. (2017) 12:1680–91. doi: 10.2215/CJN.02500317
6. Redondo-Rodriguez R, Mena-Vazquez N, Cabezas-Lucena AM, Manrique-Arija S, Mucientes A, and Fernandez-Nebro A. Systematic review and metaanalysis of worldwide incidence and prevalence of antineutrophil cytoplasmic antibody (ANCA) associated vasculitis. J Clin Med. (2022) 11:2573. doi: 10.3390/jcm11092573
7. Rhee RL, Hogan SL, Poulton CJ, McGregor JA, Landis JR, Falk RJ, et al. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody-associated vasculitis with renal disease. Arthritis Rheumatol. (2016) 68:1711–20. doi: 10.1002/art.39614
8. Delestre F, Charles P, Karras A, Pagnoux C, Neel A, Cohen P, et al. Rituximab as maintenance therapy for ANCA-associated vasculitides: pooled analysis and long-term outcome of 277 patients included in the MAINRITSAN trials. Ann Rheum Dis. (2024) 83:233–41. doi: 10.1136/ard-2023-224623
9. Heijl C, Mohammad AJ, Westman K, and Hoglund P. Long-term patient survival in a Swedish population-based cohort of patients with ANCA-associated vasculitis. RMD Open. (2017) 3:e000435. doi: 10.1136/rmdopen-2017-000435
10. Sanchez Alamo B, Moi L, Bajema I, Faurschou M, Flossmann O, Hauser T, et al. Long-term outcomes and prognostic factors for survival of patients with ANCA-associated vasculitis. Nephrol Dial Transplant. (2023) 38:1655–65. doi: 10.1093/ndt/gfac320
11. Watts RA, Hatemi G, Burns JC, and Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. (2022) 18:22–34. doi: 10.1038/s41584-021-00718-8
12. Rathmann J, Segelmark M, Englund M, and Mohammad AJ. Stable incidence but increase in prevalence of ANCA-associated vasculitis in southern Sweden: a 23-year study. RMD Open. (2023) 9:e002949. doi: 10.1136/rmdopen-2022-002949
13. Alberici F, Tedesco M, Popov T, Balcells-Oliver M, and Mescia F. Treatment goals in ANCA-associated vasculitis: defining success in a new era. Front Immunol. (2024) 15:1409129. doi: 10.3389/fimmu.2024.1409129
14. Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. (2024) 83:30–47. doi: 10.1136/ard-2022-223764
15. Merkel PA, Xie G, Monach PA, Ji X, Ciavatta DJ, Byun J, et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. (2017) 69:1054–66. doi: 10.1002/art.40034
16. Thiel J, Schmidt FM, Lorenzetti R, Troilo A, Janowska I, Niessen L, et al. Defects in B-lymphopoiesis and B-cell maturation underlie prolonged B-cell depletion in ANCA-associated vasculitis. Ann Rheum Dis. (2024) 83:1536–48. doi: 10.1136/ard-2024-225587
17. Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. (2009) 15:623–5. doi: 10.1038/nm.1959
18. Trouw LA, Pickering MC, and Blom AM. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol. (2017) 13:538–47. doi: 10.1038/nrrheum.2017.125
19. Gou SJ, Yuan J, Wang C, Zhao MH, and Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol. (2013) 8:1884–91. doi: 10.2215/CJN.02790313
20. Wu EY, McInnis EA, Boyer-Suavet S, Mendoza CE, Aybar LT, Kennedy KB, et al. Measuring circulating complement activation products in myeloperoxidase- and proteinase 3-antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. (2019) 71:1894–903. doi: 10.1002/art.41011
21. Moiseev S, Lee JM, Zykova A, Bulanov N, Novikov P, Gitel E, et al. The alternative complement pathway in ANCA-associated vasculitis: further evidence and a meta-analysis. Clin Exp Immunol. (2020) 202:394–402. doi: 10.1111/cei.13498
22. Klapa S, Muller A, Koch A, Kerstein-Stahle A, Kahler W, Heidecke H, et al. Low concentrations of C5a complement receptor antibodies are linked to disease activity and relapse in antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. (2023) 75:760–7. doi: 10.1002/art.42410
23. Hunter RW, Welsh N, Farrah TE, Gallacher PJ, and Dhaun N. ANCA associated vasculitis. BMJ. (2020) 369:m1070. doi: 10.1136/bmj.m1070
24. Padoan R, Campaniello D, Gatto M, Schiavon F, and Doria A. Current clinical and therapeutic approach to tumour-like mass lesions in granulomatosis with polyangiitis. Autoimmun Rev. (2022) 21:103018. doi: 10.1016/j.autrev.2021.103018
25. Kronbichler A, Leierer J, Gauckler P, and Shin JI. Comorbidities in ANCA-associated vasculitis. Rheumatol (Oxford). (2020) 59:iii79–83. doi: 10.1093/rheumatology/kez617
26. Mercuzot C, Letertre S, Daien CI, Zerkowski L, Guilpain P, Terrier B, et al. Comorbidities and health-related quality of life in Patients with Antineutrophil Cytoplasmic Antibody (ANCA) - associated vasculitis. Autoimmun Rev. (2021) 20:102708. doi: 10.1016/j.autrev.2020.102708
27. Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Outcomes of nonsevere relapses in antineutrophil cytoplasmic antibody-associated vasculitis treated with glucocorticoids. Arthritis Rheumatol. (2015) 67:1629–36. doi: 10.1002/art.39104
28. Berti A, Warner R, Johnson K, Cornec D, Schroeder DR, Kabat BF, et al. The association of serum interleukin-6 levels with clinical outcomes in antineutrophil cytoplasmic antibody-associated vasculitis. J Autoimmun. (2019) 105:102302. doi: 10.1016/j.jaut.2019.07.001
29. Gamerith G, Mildner F, Merkel PA, Harris K, Cooney L, Lim N, et al. Association of baseline soluble immune checkpoints with the risk of relapse in PR3-ANCA vasculitis following induction of remission. Ann Rheum Dis. (2023) 82:253–61. doi: 10.1136/ard-2022-222479
30. McKinney EF, Lee JC, Jayne DR, Lyons PA, and Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. (2015) 523:612–6. doi: 10.1038/nature14468
31. Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Hoglund P, et al. Damage in the ANCA-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. (2015) 74:177–84. doi: 10.1136/annrheumdis-2013-203927
32. Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. (2011) 70:488–94. doi: 10.1136/ard.2010.137778
33. Floege J, Jayne DRW, Sanders JF, Tesar V, Balk EM, Gordon CE, et al. Executive summary of the KDIGO 2024 clinical practice guideline for the management of ANCA-associated vasculitis. Kidney Int. (2024) 105:447–9. doi: 10.1016/j.kint.2023.10.009
34. Fauci AS, Haynes BF, Katz P, and Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. (1983) 98:76–85. doi: 10.7326/0003-4819-98-1-76
35. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. (2003) 349:36–44. doi: 10.1056/NEJMoa020286
36. de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. (2009) 150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004
37. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. (2010) 363:221–32. doi: 10.1056/NEJMoa0909905
38. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. (2010) 363:211–20. doi: 10.1056/NEJMoa0909169
39. Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. (2014) 371:1771–80. doi: 10.1056/NEJMoa1404231
40. Jayne DRW, Merkel PA, Schall TJ, Bekker P, and Group AS. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. (2021) 384:599–609. doi: 10.1056/NEJMoa2023386
41. Cortazar FB, Muhsin SA, Pendergraft WF 3rd, Wallace ZS, Dunbar C, Laliberte K, et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep. (2018) 3:394–402. doi: 10.1016/j.ekir.2017.11.004
42. Pepper RJ, McAdoo SP, Moran SM, Kelly D, Scott J, Hamour S, et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatol (Oxford). (2019) 58:260–8. doi: 10.1093/rheumatology/key288
43. Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. (2017) 28:2756–67. doi: 10.1681/ASN.2016111179
44. Merkel PA, Niles J, Jimenez R, Spiera RF, Rovin BH, Bomback A, et al. Adjunctive treatment with avacopan, an oral C5a receptor inhibitor, in patients with antineutrophil cytoplasmic antibody-associated vasculitis. ACR Open Rheumatol. (2020) 2:662–71. doi: 10.1002/acr2.11185
45. Merkel PA. Safety of avacopan for the treatment of ANCA-associated vasculitis: combined data from three clinical trials. ACR Open Rheumatol. (2025) 7(4):e70001. doi: 10.1002/acr2.70001
46. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. (2013) 369:417–27. doi: 10.1056/NEJMoa1213277
47. Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. (2014) 66:3151–9. doi: 10.1002/art.38788
48. Terrier B, Pagnoux C, Perrodeau E, Karras A, Khouatra C, Aumaitre O, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. (2018) 77:1150–6. doi: 10.1136/annrheumdis-2017-212768
49. Charles P, Terrier B, Perrodeau E, Cohen P, Faguer S, Huart A, et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann Rheum Dis. (2018) 77:1143–9. doi: 10.1136/annrheumdis-2017-212878
50. Charles P, Perrodeau E, Samson M, Bonnotte B, Neel A, Agard C, et al. Long-term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med. (2020) 173:179–87. doi: 10.7326/M19-3827
51. Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. (2020) 79:1243–9. doi: 10.1136/annrheumdis-2019-216863
52. Smith RM, Jones RB, Specks U, Bond S, Nodale M, Al-Jayyousi R, et al. Rituximab versus azathioprine for maintenance of remission for patients with ANCA-associated vasculitis and relapsing disease: an international randomised controlled trial. Ann Rheum Dis. (2023) 82:937–44. doi: 10.1136/ard-2022-223559
53. Treppo E, Binutti M, Agarinis R, De Vita S, and Quartuccio L. Rituximab induction and maintenance in ANCA-associated vasculitis: state of the art and future perspectives. J Clin Med. (2021) 10:3773. doi: 10.3390/jcm10173773
54. Tieu J, Smith R, Basu N, Brogan P, D’Cruz D, Dhaun N, et al. Rituximab for maintenance of remission in ANCA-associated vasculitis: expert consensus guidelines. Rheumatol (Oxford). (2020) 59:e24–32. doi: 10.1093/rheumatology/kez640
55. Alberici F, Smith RM, Fonseca M, Willcocks LC, Jones RB, Holle JU, et al. Association of a TNFSF13B (BAFF) regulatory region single nucleotide polymorphism with response to rituximab in antineutrophil cytoplasmic antibody-associated vasculitis. J Allergy Clin Immunol. (2017) 139:1684–7 e10. doi: 10.1016/j.jaci.2016.08.051
56. Khoudour N, Delestre F, Jabot-Hanin F, Jouinot A, Nectoux J, Letouneur F, et al. Association between plasma rituximab concentration and the risk of major relapse in antineutrophil cytoplasmic antibody-associated vasculitides during rituximab maintenance therapy. Arthritis Rheumatol. (2023) 75:2003–13. doi: 10.1002/art.42556
57. Faustini F, Dunn N, Kharlamova N, Ryner M, Bruchfeld A, Malmstrom V, et al. First exposure to rituximab is associated to high rate of anti-drug antibodies in systemic lupus erythematosus but not in ANCA-associated vasculitis. Arthritis Res Ther. (2021) 23:211. doi: 10.1186/s13075-021-02589-6
58. Thietart S, Beinse G, Smets P, Karras A, Philipponnet C, Augusto JF, et al. Patients of 75 years and over with ANCA-associated vasculitis have a lower relapse risk than younger patients: A multicentre cohort study. J Intern Med. (2022) 291:350–63. doi: 10.1111/joim.13417
59. Hilhorst M, Arndt F, Joseph Kemna M, Wieczorek S, Donner Y, Wilde B, et al. HLA-DPB1 as a risk factor for relapse in antineutrophil cytoplasmic antibody-associated vasculitis: A cohort study. Arthritis Rheumatol. (2016) 68:1721–30. doi: 10.1002/art.39620
60. Chen DP, Aiello CP, McCoy D, Stamey T, Yang J, Hogan SL, et al. PRTN3 variant correlates with increased autoantigen levels and relapse risk in PR3-ANCA versus MPO-ANCA disease. JCI Insight. (2023) 8:e166107. doi: 10.1172/jci.insight.166107
61. Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. (2013) 65:2441–9. doi: 10.1002/art.38044
62. Casal Moura M, Specks U, Tehranian S, Sethi S, Zubidat D, Nardelli L, et al. Maintenance of remission and risk of relapse in myeloperoxidase-positive ANCA-associated vasculitis with kidney involvement. Clin J Am Soc Nephrol. (2023) 18:47–59. doi: 10.2215/CJN.06460622
63. Mescia F, Salviani C, Tonoli M, Affatato S, Moratto D, Tedesco M, et al. Sustained post-rituximab B-cell depletion is common in ANCA-associated vasculitis and is affected by sex and renal function. Nephrol Dial Transplant. (2024) 39:683–93. doi: 10.1093/ndt/gfad197
64. McDermott G, Fu X, Cook C, Ahola C, Doliner B, Hanberg J, et al. The effect of achieving serological remission on subsequent risk of relapse, end-stage renal disease and mortality in ANCA-associated vasculitis: a target trial emulation study. Ann Rheum Dis. (2022) 81:1438–44. doi: 10.1136/annrheumdis-2022-222439
65. Schuster DD, Gillis D, Wong RCW, Kodituwakku A, Droney L, and Ranganathan D. Anti-neutrophil cytoplasmic antibody monitoring in vasculitis patients to predict relapse. Int J Rheum Dis. (2023) 26:1861–5. doi: 10.1111/1756-185X.14728
66. Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatol (Oxford). (2015) 54:1153–60. doi: 10.1093/rheumatology/keu452
67. McClure ME, Wason J, Gopaluni S, Tieu J, Smith RM, Jayne DR, et al. Evaluation of PR3-ANCA status after rituximab for ANCA-associated vasculitis. J Clin Rheumatol. (2019) 25:217–23. doi: 10.1097/RHU.0000000000001030
68. van Dam LS, Dirikgil E, Bredewold EW, Ray A, Bakker JA, van Kooten C, et al. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transplant. (2021) 36:1408–17. doi: 10.1093/ndt/gfaa066
69. Pope V, Sivashanmugathas V, Moodley D, Gunaratnam L, and Barra L. Outcomes in ANCA-associated vasculitis patients with end-stage kidney disease on renal replacement therapy-A meta-analysis. Semin Arthritis Rheum. (2023) 60:152189. doi: 10.1016/j.semarthrit.2023.152189
70. Salmela A, Rasmussen N, Tervaert JWC, Jayne DRW, Ekstrand A, and European Vasculitis Study G. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatol (Oxford). (2017) 56:965–72. doi: 10.1093/rheumatology/kex001
71. Theofilis P, Vordoni A, Koukoulaki M, Vlachopanos G, and Kalaitzidis RG. Overview of infections as an etiologic factor and complication in patients with vasculitides. Rheumatol Int. (2022) 42:759–70. doi: 10.1007/s00296-022-05100-9
72. Padoan R, Felicetti M, Gatto M, Polito P, Doria A, and Schiavon F. Rituximab-associated hypogammaglobulinaemia in ANCA-associated vasculitis and connective tissue diseases: a longitudinal observational study. Clin Exp Rheumatol. (2020) 38 Suppl 124:188–94.
73. Tieu J, Smith RM, Gopaluni S, Kumararatne DS, McClure M, Manson A, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol. (2021) 12:671503. doi: 10.3389/fimmu.2021.671503
74. Podesta MA, Mescia F, Ricchiuto A, Smith R, Tedesco M, Cassia MA, et al. Predictors of hypogammaglobulinemia in ANCA-associated vasculitis after a rituximab-based induction: a multicentre study. Rheumatol (Oxford). (2023) 62:2850–4. doi: 10.1093/rheumatology/keac716
75. Liberatore J, Nguyen Y, Hadjadj J, Cohen P, Mouthon L, Puechal X, et al. Risk factors for hypogammaglobulinemia and association with relapse and severe infections in ANCA-associated vasculitis: A cohort study. J Autoimmun. (2024) 142:103130. doi: 10.1016/j.jaut.2023.103130
76. Benavides-Villanueva F, Loricera J, Calvo-Rio V, Corrales-Selaya C, Castaneda S, and Blanco R. Intravenous immunoglobulin therapy in antineutrophil cytoplasmic antibody-associated vasculitis. Eur J Intern Med. (2023) 117:78–84. doi: 10.1016/j.ejim.2023.06.021
77. Salviani C, Scolari F, and Alberici F. Correspondence on ‘Immunogenicity and safety of anti-SARS-Cov-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort’. Ann Rheum Dis. (2021) 80:e158. doi: 10.1136/annrheumdis-2021-220496
78. Zonozi R, Cortazar FB, Jeyabalan A, Sauvage G, Nithagon P, Huizenga NR, et al. Maintenance of remission of ANCA vasculitis by rituximab based on B cell repopulation versus serological flare: a randomised trial. Ann Rheum Dis. (2024) 83:351–9. doi: 10.1136/ard-2023-224489
79. Walsh M, Merkel PA, Mahr A, and Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: A meta-analysis. Arthritis Care Res (Hoboken). (2010) 62:1166–73. doi: 10.1002/acr.20176
80. Speer C, Altenmuller-Walther C, Splitthoff J, Nusshag C, Kalble F, Reichel P, et al. Glucocorticoid maintenance therapy and severe infectious complications in ANCA-associated vasculitis: a retrospective analysis. Rheumatol Int. (2021) 41:431–8. doi: 10.1007/s00296-020-04752-9
81. Merkel P, Pagnoux C, Khalidi N, Specks U, Koenig C, and Langford C. A multicenter, randomized, controlled trial to evaluate the effects of low-dose glucocorticoids compared to stopping glucocorticoids to maintain remission of granulomatosis with polyangiitis: the TAPIR trial. Arthritis Rheumatol. (2024) 76(Suppl 9).
82. Junek ML, Merkel PA, Vilayur E, Wald R, Khalidi N, Jayne D, et al. Risk of relapse of antineutrophil cytoplasmic antibody-associated vasculitis in a randomized controlled trial of plasma exchange and glucocorticoids. Arthritis Rheumatol. (2024) 76:1431–8. doi: 10.1002/art.42843
83. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheumatol. (2021) 44:912–20.
84. Walsh M, Flossmann O, Berden A, Westman K, Hoglund P, Stegeman C, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. (2012) 64:542–8. doi: 10.1002/art.33361
85. Salmela A, Tornroth T, Poussa T, and Ekstrand A. Prognostic factors for survival and relapse in ANCA-associated vasculitis with renal involvement: A clinical long-term follow-up study. Int J Nephrol. (2018) 2018:6369814. doi: 10.1155/2018/6369814
86. Deshayes S, Martin Silva N, Khoy K, Yameogo S, Mariotte D, Lobbedez T, et al. Clinical impact of subgrouping ANCA-associated vasculitis according to antibody specificity beyond the clinicopathological classification. Rheumatol (Oxford). (2019) 58:1731–9. doi: 10.1093/rheumatology/kez016
87. Felicetti M, Cazzador D, Padoan R, Pendolino AL, Faccioli C, Nardello E, et al. Ear, nose and throat involvement in granulomatosis with polyangiitis: how it presents and how it determines disease severity and long-term outcomes. Clin Rheumatol. (2018) 37:1075–83. doi: 10.1007/s10067-018-4019-0
88. Gialouri CG, Chalkia A, Koutsianas C, Chavatza K, Argyriou E, Panagiotopoulos A, et al. Relapses and serious adverse events during rituximab maintenance therapy in ANCA-associated vasculitis: a multicentre retrospective study. Rheumatol (Oxford). (2025) 64:1989–98. doi: 10.1093/rheumatology/keae409
89. Dirikgil E, van Leeuwen JR, Bredewold OW, Ray A, Jonker JT, Soonawala D, et al. ExploriNg DUrable Remission with Rituximab in ANCA-associatEd vasculitis (ENDURRANCE trial): protocol for a randomised controlled trial. BMJ Open. (2022) 12:e061339. doi: 10.1136/bmjopen-2022-061339
90. Csernok E and Hellmich B. Usefulness of vasculitis biomarkers in the era of the personalized medicine. Autoimmun Rev. (2020) 19:102514. doi: 10.1016/j.autrev.2020.102514
91. Salama AD. Relapse in anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis. Kidney Int Rep. (2020) 5:7–12. doi: 10.1016/j.ekir.2019.10.005
92. Gisslander K, White A, Aslett L, Hruskova Z, Lamprecht P, Musial J, et al. Data-driven subclassification of ANCA-associated vasculitis: model-based clustering of a federated international cohort. Lancet Rheumatol. (2024) 6:e762–e70. doi: 10.1016/S2665-9913(24)00187-5
93. Ramponi G, Folci M, De Santis M, Damoiseaux J, Selmi C, and Brunetta E. The biology, pathogenetic role, clinical implications, and open issues of serum anti-neutrophil cytoplasmic antibodies. Autoimmun Rev. (2021) 20:102759. doi: 10.1016/j.autrev.2021.102759
94. Arnold J, Vital EM, Dass S, Aslam A, Rawstron AC, Savic S, et al. A personalized rituximab retreatment approach based on clinical and B-cell biomarkers in ANCA-associated vasculitis. Front Immunol. (2021) 12:803175. doi: 10.3389/fimmu.2021.803175
95. Elmer E, Smargianaki S, Pettersson A, Skattum L, Ohlsson S, Hellmark T, et al. Increased frequencies of switched memory B cells and plasmablasts in peripheral blood from patients with ANCA-associated vasculitis. J Immunol Res. (2020) 2020:8209737. doi: 10.1155/2020/8209737
96. Berti A, Hillion S, Konig MF, Moura MC, Hummel AM, Carmona E, et al. Autoreactive plasmablasts after B cell depletion with rituximab and relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. (2023) 75:736–47. doi: 10.1002/art.42388
97. Pepper RJ, Draibe JB, Caplin B, Fervenza FC, Hoffman GS, Kallenberg CG, et al. Association of serum calprotectin (S100A8/A9) level with disease relapse in proteinase 3-antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. (2017) 69:185–93. doi: 10.1002/art.39814
98. Romand X, Paclet MH, Chuong MV, Gaudin P, Pagnoux C, Guillevin L, et al. Serum calprotectin and renal function decline in ANCA-associated vasculitides: a post hoc analysis of MAINRITSAN trial. RMD Open. (2023) 9:e003477. doi: 10.1136/rmdopen-2023-003477
99. Anton-Pampols P, Martinez Valenzuela L, Fernandez Lorente L, Quero Ramos M, Gomez Preciado F, Martin Capon I, et al. Combining neutrophil and macrophage biomarkers to detect active disease in ANCA vasculitis: a combinatory model of calprotectin and urine CD163. Clin Kidney J. (2023) 16:693–700. doi: 10.1093/ckj/sfac257
100. Wester Trejo MAC, Flossmann O, Westman KW, Hoglund P, Hagen EC, Walsh M, et al. Renal relapse in antineutrophil cytoplasmic autoantibody-associated vasculitis: unpredictable, but predictive of renal outcome. Rheumatol (Oxford). (2019) 58:103–9. doi: 10.1093/rheumatology/key260
101. Nishide M, Nishimura K, Matsushita H, Kawada S, Shimagami H, Metsugi S, et al. Neutrophil single-cell analysis identifies a type II interferon-related subset for predicting relapse of autoimmune small vessel vasculitis. Nat Commun. (2025) 16:3581. doi: 10.1038/s41467-025-58550-7
102. Moran SM, Scott J, Clarkson MR, Conlon N, Dunne J, Griffin MD, et al. The clinical application of urine soluble CD163 in ANCA-associated vasculitis. J Am Soc Nephrol. (2021) 32:2920–32. doi: 10.1681/ASN.2021030382
103. Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, et al. Urinary monocyte chemoattractant protein-1 (MCP - 1) is a marker of active renal vasculitis. Nephrol Dial Transplant. (2004) 19:2761–8. doi: 10.1093/ndt/gfh487
104. Prskalo L, Skopnik CM, Goerlich N, Freund P, Wagner L, Grothgar E, et al. Urinary CD4 + T cells predict renal relapse in ANCA-associated vasculitis. J Am Soc Nephrol. (2024) 35:483–94. doi: 10.1681/ASN.0000000000000311
105. Sachez-Alamo B, Moi L, Bajema I, Berden A, Flossmann O, Hruskova Z, et al. Long-term outcome of kidney function in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. (2024) 39:1483–93. doi: 10.1093/ndt/gfae018
106. Karras A, Pagnoux C, Haubitz M, Groot K, Puechal X, Tervaert JWC, et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis. (2017) 76:1662–8. doi: 10.1136/annrheumdis-2017-211123
107. Geetha D, Specks U, Stone JH, Merkel PA, Seo P, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol. (2015) 26:976–85. doi: 10.1681/ASN.2014010046
108. Strand V, Jayne DRW, Horomanski A, Yue H, Bekker P, Merkel PA, et al. The impact of treatment with avacopan on health-related quality of life in antineutrophil cytoplasmic antibody-associated vasculitis: a post-hoc analysis of data from the ADVOCATE trial. Lancet Rheumatol. (2023) 5:e451–e60. doi: 10.1016/S2665-9913(23)00092-9
109. Geetha D, Dua A, Yue H, Springer J, Salvarani C, Jayne D, et al. Efficacy and safety of avacopan in patients with ANCA-associated vasculitis receiving rituximab in a randomised trial. Ann Rheum Dis. (2024) 83:223–32. doi: 10.1136/ard-2023-224816
110. Tanaka R, Toishi T, Masaki R, Aihara H, Sakamoto S, Ikeda M, et al. Effective management of necrotizing crescentic glomerulonephritis using an aggressive combination therapy including avacopan in a patient double-seropositive for anti-GBM antibodies and ANCA: a case report. CEN Case Rep. (2024) 14:183–7. doi: 10.1007/s13730-024-00929-4
111. Cortazar FB, Niles JL, Jayne DRW, Merkel PA, Bruchfeld A, Yue H, et al. Renal recovery for patients with ANCA-associated vasculitis and low eGFR in the ADVOCATE trial of avacopan. Kidney Int Rep. (2023) 8:860–70. doi: 10.1016/j.ekir.2023.01.039
112. Merkel. P, Jayne D, Yue H, Schall TJ, Kelleher C, and Bekker P. A Randomized, double-blind, active-controlled study of avacopan in anti-neutrophil cytoplasmic antibody (ANCA-) associated vasculitis. Ann Rheumatic Dis. (2020) 79:8.
113. Gabilan C, Pfirmann P, Ribes D, Rigothier C, Chauveau D, Casemayou A, et al. Avacopan as first-line treatment in antineutrophil cytoplasmic antibody-associated vasculitis: A steroid-sparing option. Kidney Int Rep. (2022) 7:1115–8. doi: 10.1016/j.ekir.2022.01.1065
114. Gabilan C, Belliere J, Moranne O, Pfirmann P, Samson M, Delattre V, et al. Avacopan for anti-neutrophil cytoplasm antibodies-associated vasculitis: a multicenter real-world study. Rheumatol (Oxford). (2024) 64(4):2214–9. doi: 10.1093/rheumatology/keae359
115. Zonozi R, Aqeel F, Le D, Cortazar FB, Thaker J, Zabala Ramirez MJ, et al. Real-world experience with avacopan in antineutrophil cytoplasmic autoantibody-associated vasculitis. Kidney Int Rep. (2024) 9:1783–91. doi: 10.1016/j.ekir.2024.03.022
116. Treppo E, Bello F, Galli E, Padoan R, Monti S, Moroni L, et al. Avacopan in the real-life practice: Evaluation of efficacy, safety, and impact on quality of life in the early stages of treat,ment. Ann Rheumatic Dis. (2023) 82:1606. doi: 10.1136/annrheumdis-2023-eular.4738
117. Casal Moura M, Gauckler P, Anders HJ, Bruchfeld A, Fernandez-Juarez GM, Floege J, et al. Management of antineutrophil cytoplasmic antibody-associated vasculitis with glomerulonephritis as proposed by the ACR 2021, EULAR 2022 and KDIGO 2021 guidelines/recommendations. Nephrol Dial Transplant. (2023) 38:2637–51. doi: 10.1093/ndt/gfad090
118. Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. (2015) 74:1178–82. doi: 10.1136/annrheumdis-2014-206404
119. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puechal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. (2020) 382:622–31. doi: 10.1056/NEJMoa1803537
120. De Vriese AS and Fervenza FC. PEXIVAS: the end of plasmapheresis for ANCA-associated vasculitis? Clin J Am Soc Nephrol. (2021) 16:307–9. doi: 10.2215//CJN.10550620
121. Tomasson G, Boers M, Walsh M, LaValley M, Cuthbertson D, Carette S, et al. Assessment of health-related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken). (2012) 64:273–9. doi: 10.1002/acr.20649
122. Padoan R, Mohammad AJ, Hellmich B, Anastasa Z, Popov T, Balcells M, et al. Care gaps in ANCA-associated vasculitis (AAV): Exploring unmet needs and misalignments between patients and physicians. Ann Rheumatic Dis. (2025) 84:1498. doi: 10.1016/j.ard.2025.06.851
123. Collister D, Farrar M, Farrar L, Brown P, Booth M, Firth T, et al. Plasma exchange for ANCA-associated vasculitis: an international survey of patient preferences. Kidney Med. (2023) 5:100595. doi: 10.1016/j.xkme.2022.100595
124. McClure ME, Gopaluni S, Wason J, Henderson RB, Van Maurik A, Savage CCO, et al. A randomised study of rituximab and belimumab sequential therapy in PR3 ANCA-associated vasculitis (COMBIVAS): design of the study protocol. Trials. (2023) 24:180. doi: 10.1186/s13063-023-07218-y
125. Jayne D, Blockmans D, Luqmani R, Moiseev S, Ji B, Green Y, et al. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized controlled study. Arthritis Rheumatol. (2019) 71:952–63. doi: 10.1002/art.40802
126. Arends EJ, Zlei M, Tipton CM, Cotic J, Osmani Z, de Bie FJ, et al. Disruption of memory B-cell trafficking by belimumab in patients with systemic lupus erythematosus. Rheumatol (Oxford). (2024) 63:2387–98. doi: 10.1093/rheumatology/keae286
127. Engesser J, Khatri R, Schaub DP, Zhao Y, Paust HJ, Sultana Z, et al. Immune profiling-based targeting of pathogenic T cells with ustekinumab in ANCA-associated glomerulonephritis. Nat Commun. (2024) 15:8220. doi: 10.1038/s41467-024-52525-w
128. Langford C, Khalidi N, Springer J, Friedman M, Hellmich B, and Pagnoux C. A Randomized, double-blind, placebo-controlled trial of Abatacept for the treatment of relapsing, non-severe granulomatosis with polyangiitis. Arthritis Rheumatol. (2024) 76:Abstract 0823.
129. Hung W, Cusnir I, Habib S, Smylie M, Solez K, and Yacyshyn E. Immune checkpoint inhibitor-induced granulomatosis with polyangiitis. Rheumatol (Oxford). (2021) 60:e190–e1. doi: 10.1093/rheumatology/keaa818
130. Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2022) 81:100–7. doi: 10.1136/annrheumdis-2021-220920
131. McGovern DP, McClure ME, Coates M, Bond S, Martinez Del Pero M, Mynard K, et al. Study protocol for a randomised, phase II, double-blind, experimental medicine study of obinutuzumab versus rituximab in ANCA-associated vasculitis: ObiVas. BMJ Open. (2024) 14:e083277. doi: 10.1136/bmjopen-2023-083277
Keywords: antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, AAV relapse, granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), remission re-induction
Citation: Alberici F, Flossmann O, Lamprecht P, Loudon KW, Padoan R, Popov T, Salvarani C and Mohammad AJ (2025) Antineutrophil cytoplasmic antibody-associated vasculitis: insights into relapse risk and future management directions. Front. Immunol. 16:1655326. doi: 10.3389/fimmu.2025.1655326
Received: 27 June 2025; Accepted: 18 August 2025;
Published: 18 September 2025.
Edited by:
Joshua Daniel Ooi, Monash University, AustraliaReviewed by:
Chrysoula Gialouri, National and Kapodistrian University of Athens, GreeceChaïmaâ Zeroual, Centre Hospitalier Universitaire Ibn Rochd, Morocco
Copyright © 2025 Alberici, Flossmann, Lamprecht, Loudon, Padoan, Popov, Salvarani and Mohammad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Alberici, ZmVkZXJpY28uYWxiZXJpY2lAZ21haWwuY29t
 Federico Alberici
Federico Alberici Oliver Flossmann
Oliver Flossmann Peter Lamprecht
Peter Lamprecht Kevin W. Loudon4,5
Kevin W. Loudon4,5 Roberto Padoan
Roberto Padoan Tamara Popov
Tamara Popov Carlo Salvarani
Carlo Salvarani Aladdin J. Mohammad
Aladdin J. Mohammad