- 1Associazione Italiana Ricercare per Curare ODV ETS (AIRpC), Lecco, Italy
- 2Nephrology Unit, Azienda Sociosanitaria Liguria 5, La Spezia, Italy
- 3Nephrology and Dialysis Unit, IRCCS Humanitas Research Hospital, Rozzano, Italy
- 4Nephrology Unit, Parma University Hospital, Parma, Italy
- 5Nephrology, Dialysis, Transplantation, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy
- 6Nephrology, Dialysis and Kidney Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 7Nephrology, Dialysis and Transplantation Unit, Department of Biomedical Sciences, Foggia, Italy
- 8Nephrology and Dialysis Unit, Maria Santissima Addolorata Hospital, Eboli, Italy
- 9UO Nefrologia, Dialisi e Trapianto, IRCCS Policlinico San Martino, Genova, Italy
- 10Nephrology and Dialysis Unit, Ospedale Degli Infermi, Rimini, Italy
- 11Nephrology and Dialysis Unit, ASL Viterbo, Viterbo, Italy
- 12Nephrology and Dialysis Unit, Department of Clinical and Experimental Medicine, AOU “G. Martino”, Messina, Italy
- 13Nephrology and Dialysis Unit, ASST Fatebenefratelli-Sacco, Milano, Italy
- 14Nephrology, Dialysis and Transplantation Unit, Department of Emergency and Organ Transplantation, University of Bari Aldo Moro, Bari, Italy
- 15Bocconi University, Milan, Italy
- 16SCDU Nefrologia e Dialisi, AOU “SS Antonio e Biagio e Cesare Arrigo”, Alessandria, Italy
- 17Nephrology and Dialysis Unit, “Casa Sollievo Della Sofferenza” Foundation, Scientific Institut for Research and Health Care, San Giovanni Rotondo, Italy
- 18Nephrology and Dialysis Unit, Ospedale Santa Maria delle Croci, Ravenna, Italy
- 19Nephrology and Dialysis Unit, Regina Margherita, Torino, Italy
- 20Nephrology and Dialysis Unit, Ospedale A. Perrino, Brindisi, Italy
- 21Nephrology and Dialysis Unit, Morgagni-Pierantoni Hospital, Forlì, Italy
- 22Nephrology and Dialysis Unit, Policlinico Gemelli, Roma, Italy
- 23Nephrology and Dialysis Unit, S.Maria della Misericordia Hospital, Perugia, Italy
- 24Nephrology and Dialysis Unit, Umberto Parini Hospital, Aosta, Italy
- 25UOSD Nefrologia e Dialisi Pediatrica, Ospedale Giovanni XXIII, Bari, Italy
- 26Nephrology and Dialysis Unit, Santa Marta and Santa Venera Hospital, Acireale, ASP 3 Catania, Acireale, Italy
- 27Nephrology and Dialysis Unit, Ospedale San Giacomo Apostolo, Castelfranco Veneto, Italy
- 28Nephrology and Dialysis Unit Sant’Andrea Hospital, La Spezia, Italy
- 29Nephrology, Dialysis and Kidney Transplant Unit, Azienda Ospedaliero Universitaria di Modena, Modena, Italy
Objective: The role of complement in the long-term renal survival of patients with lupus nephritis (LN) remains poorly understood. Recent studies suggest its potential impact; however, long-term data are lacking.
Methods: This multicenter, observational, retrospective study aimed at investigating the influence of complement levels on long-term renal outcomes in LN patients. We evaluated whether isolated C3 hypocomplementemia (i-LowC3), defined as serum low C3 (≤80 mg/dL) and normal C4 (>10 mg/dL) six months after kidney biopsy is associated with subsequent risk of chronic kidney disease (CKD), End Stage Kidney Disease (ESKD) or death.
Results: 445 patients with LN were studied (median follow-up 4.9 years). Based on six-month C3/C4 levels, patients were categorized into i-LowC3 (91 patients) and controls (354 patients). Over the first six months, serum C3 and C4 levels increased by a median of 20 mg/dL and 5 mg/dL, respectively. i-LowC3 was significantly associated with twice the risk of a poor outcome, including CKD, ESKD, composite outcome of CKD or death and ESKD or death, with lower survival rates for all these outcomes compared to controls (P < 0.001). Multivariate Cox regression analysis revealed a lower risk of CKD and CKD or death with increases in C3 levels during the first six months, while i-LowC3 was associated with an independent higher risk for these outcomes.
Conclusion: The trajectory of serum C3 levels within the first six months appears to predict long-term renal prognosis of LN patients. These findings support the use of i-LowC3 as a low-cost, readily available biomarker to guide early treatment of LN patients.
Introduction
Lupus nephritis (LN) is a major complication of systemic lupus erythematosus (SLE), affecting approximately 30 – 40% of SLE patients. Its onset is typically characterized by proteinuria, alterations in urinary sediment, and, in some cases, acute renal failure (1).
Numerous studies have sought to identify prognostic predictors of End-Stage Kidney Disease (ESKD) in patients with LN to optimize the administration of immunosuppressive therapy according to individual risk profiles. Established prognostic factors include baseline estimated glomerular filtration rate (eGFR), a high National Institutes of Health Chronicity Index, non-Caucasian ethnicity, duration of SLE prior to LN onset, age at diagnosis, and the presence of hypertension (2, 3), which primarily reflect pre-existing, non-specific renal damage rather than identifying a distinct high-risk phenotype. Conversely, an early predictor of favorable long-term renal outcomes in contemporary cohorts is low proteinuria at 12 months (4–6).
Complement proteins are among the earliest and most extensively studied pathogenic mediators in SLE (7–13). Whether complement activation can reliably predict the development of chronic kidney disease (CKD) in LN patients has been a subject of ongoing debate (14–16). Conflicting data on this topic arise partly because complement activation in SLE has dual roles. While overactivation may contribute to tissue injury, the complement classical pathway (cCP) has protective functions, including the clearance of immune complexes, cellular debris, and apoptotic bodies. Low copy number and/or genetic deficiencies in the cCP are well-documented predisposing factors for the development of SLE (17).
Conversely, dysregulation of the complement alternative pathway (cAP) can exacerbate renal injury. Excessive production of potent anaphylatoxins, such as C5a, drives neutrophil recruitment and sustains inflammation during acute renal flares. Persistent overactivation of the cAP further contributes to endothelial damage, promoting the progression from acute kidney injury to CKD (18).
Unlike atypical hemolytic uremic syndrome, which is often characterized by a genetically determined dysfunction of cAP regulatory proteins, no similar genetic pattern has been identified in SLE or LN. However, recent findings suggest that cAP overactivation in LN may be attributed to the presence of circulating anti-C3 antibodies. These autoantibodies, detected in approximately one-third of LN patients, appear to inhibit cAP regulatory factors such as factor H and complement receptor 1, thereby stabilizing the cAP C3 convertase (18, 19).
Moreover, anti-C3 antibody titers are inversely correlated with persistent serum C3 consumption and are significantly associated with inflammatory renal damage in LN patients. These findings provide a potential explanation for the dual role of complement in LN pathogenesis. While the physiological activation of the cCP exerts both inflammatory and protective effects, cAP dysregulation leads to progressive endothelial damage, culminating in thrombotic microangiopathy (20).
In a previous retrospective study, a persistent hyperactivation of the cAP—identified in patients with persistently low serum C3 levels six months after initiating immunosuppressive therapy for a first episode of proliferative LN—was associated with the progression of renal damage and the development of ESKD over the long term (18).
Our study aimed to validate these findings in a large independent Italian cohort of the Italian Registry of Renal Biopsy.
Materials and methods
Patient selection
The Italian study centers involved in this observational multi-center study are detailed in the Appendix. As an observational study, patient enrollment criteria were not subject to modification. All consecutive patients undergoing native kidney biopsy during the active recruitment period, with a primary histological diagnosis of LN, were deemed eligible for inclusion.
Exclusion criteria included patients with LN who had a follow-up period of less than three months, those without available C3 and C4 determinations prior to native kidney biopsy, and patients with previously diagnosed LN who underwent repeat kidney biopsy. The latter exclusion aimed at minimizing biases related to prior exposure to immunosuppressive therapies.
Data collection was centralized and conducted through a purpose-built web-based database (http://www.statgate.it) integrated with the Italian Registry of Renal Biopsy (IRRB) (http://www.irrb.net). All patients provided written informed consent, and the study protocol received approval from the Ethics Committee of Bari University. The study adhered to the principles of the Declaration of Helsinki and was conducted as an independent initiative without external sponsorship. It was registered at ClinicalTrials.gov (No. NCT04948593).
Study groups
The primary objective of this study was to evaluate the long-term renal survival of patients with a primary diagnosis of LN, focusing on the potential independent predictive value of complement C3 and C4 levels at the six-month follow-up after native kidney biopsy. Following a similar approach to our previous study (18), the cohort was divided into two groups: patients with isolated C3 hypocomplementemia (i-LowC3), defined as low serum C3 levels (≤80 mg/dL) and normal serum C4 levels (>10 mg/dL), at six months after the initiation of immunosuppressive therapy, and a control group.
Study outcomes
Four key outcomes were assessed: (i) the development of CKD, defined as an eGFR) ≤60 mL/min/1.73 m², as calculated using the CKD-EPI equation (21); (ii) the composite outcome of CKD or death; (iii) the development of ESKD; (iv) the composite outcome of ESKD or death.
Additionally, the trajectory of serum C3 levels within the first six months, following immunosuppressive therapy, was tested to predict renal prognosis in patients with LN. The study also evaluated possible confounding variables, including baseline kidney function, age, gender, proteinuria levels before biopsy, proteinuria reduction during the first six months of follow-up, and the number and type of immunosuppressive drugs used.
Variables
Relevant patient-related covariates recorded included age, gender, eGFR (calculated using the CKD-EPI equation (21)), 24-hour proteinuria magnitude, and its reduction over the first six months of follow-up, and the LN histological class according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification (22).
Statistical analysis
Quantitative variables were summarized using medians and the 10th and 90th percentiles as measures of central tendency and variability, respectively. The Mann-Whitney U test was employed to compare continuous variables between the two groups defined by C3/C4 levels at six months. Categorical variables were reported as absolute numbers and percentages, and comparisons were conducted using the Chi-squared test.
Kaplan-Meier survival curves were generated for each outcome, stratified by six-month C3 and C4 levels using cut-off values of 80 mg/dL and 10 mg/dL, respectively. Differences in survival between groups were assessed using the log-rank test.
For inferential analysis, multivariate Cox regression was performed for the defined outcomes. Predictor variables were parametrized according to their categorical, discrete, or continuous nature, with indicator dummy variables used for categorical predictors (1 for present, 0 for absent). A stepwise iterative approach was applied, beginning with a full model, followed by a backward elimination strategy using the Likelihood Ratio (LR) test to determine the final model. Inclusion and exclusion criteria for covariates were based on thresholds of Pin = 0.1 and Pout = 0.05. The missing data excluded the corresponding records from the analysis. A P-value ≤0.05 was considered statistically significant. For each selected covariate, beta coefficients, exponential beta coefficients (as estimates of Relative Risk, RR), and their 95% confidence intervals were reported.
All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS for Windows, version 23.0).
Results
This study involved 26 Italian centers (detailed in the Appendix) and included 609 patients with a histological diagnosis of LN, enrolled between June 15, 1980, and September 11, 2023. After excluding patients with a follow-up period of less than three months (56 patients, 9.2%), those without C3 and C4 measurements before kidney biopsy (17 patients, 2.8%), and patients with repeated native kidney biopsies (91 patients, 14.9%), a final cohort of 445 patients (73%) was selected for analysis. This final cohort constituted the study group for the present report.
Patient characteristics
The main characteristics of the analyzed patients are presented in Table 1. The median patient age was 37.3 years, with a median proteinuria of 2.8 g/day and a median eGFR of 87.5 mL/min/1.73 m². Females predominated (82%), with ISN/RPS Class III/III+V in 20.7% of cases and Class IV/IV+V in 49.2%, without statistically significant differences between the two groups (Table 2).
Table 1. Baseline patient characteristics at the time of native kidney biopsy grouped by isolated C3 serum levels at 6 month (i-LowC3) follow-up (quantitative variables).
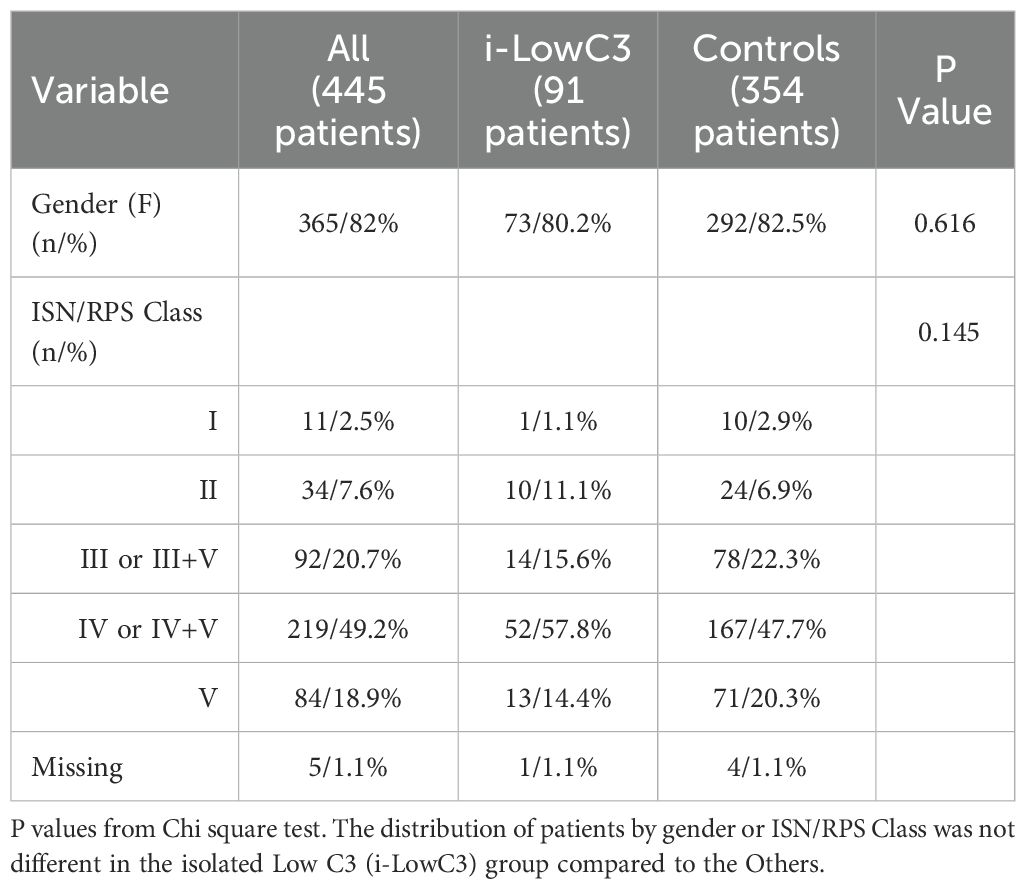
Table 2. Patient characteristics grouped by isolate Low C3 at 6 months follow-up (categorical variables).
As expected, the median pre-biopsy levels of C3 and C4 in the study population were low (65 mg/dL and 10.7 mg/dL, respectively) (Table 1). Among the cohort, 91 patients (20.4%) were identified as belonging to the i-LowC3 group. Age (Table 1) and gender (Table 2) distributions were similar between the i-LowC3 and control groups.
C3 serum levels before biopsy were significantly lower in the i-LowC3 group compared with the control group (56.0 mg/dL vs. 69.0 mg/dL, P = 0.001). Additionally, the i-LowC3 group compared with the control group demonstrated significantly reduced renal function at baseline (median eGFR of 76.1 vs. 90.1 mL/min/1.73 m², P = 0.014, Table 1). The i-LowC3 group, at baseline shows higher diastolic blood pressure values (P = 0.028) compared with the control group (Table 1).
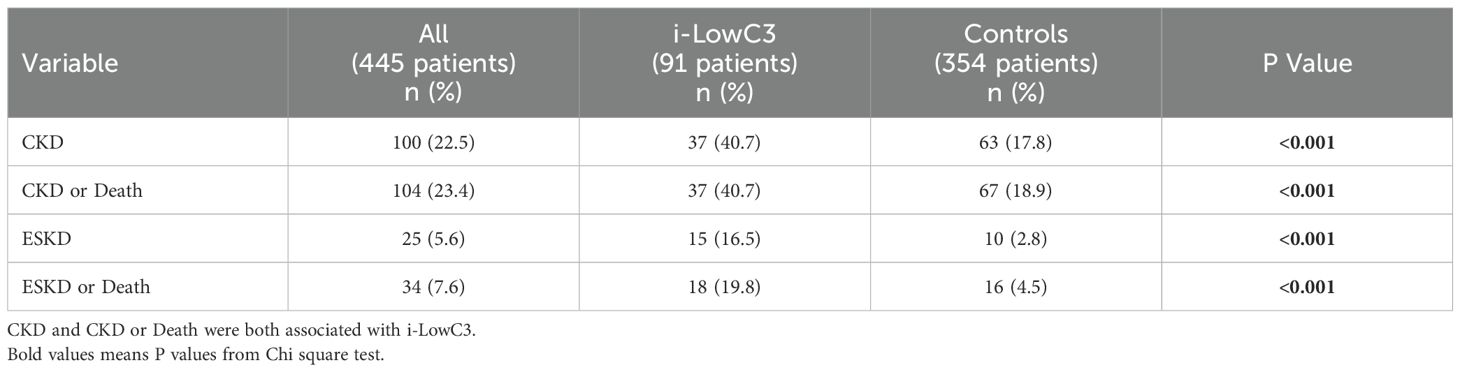
Immunosuppressive treatments
The use of immunosuppressive agents prescribed after renal biopsy is detailed in Table 3. Mycophenolate mofetil (MMF) (59.6%) and cyclophosphamide (31.2%) were the most frequently administered drugs, in addition to corticosteroids and Hydroxychloroquine. However, the distribution of LN treatments differed significantly between the two groups; immunosuppressive agents, particularly MMF, were less frequently used in the i-LowC3 group. MMF use was also less frequent in non-proliferative classes 1 and 2 compared to proliferative classes 3 and 4 (55.0% vs. 61.7%), though this difference was not statistically significant (P = 0.192). A trend toward reduced Hydroxychloroquine use in the i-LowC3 group was also noted (Table 3).
Serum complement levels trends
After the first six months of follow-up, a median increase of 20 mg/dL in C3 levels was observed, accompanied by a similar pattern for C4, with a median increase of 5 mg/dL. Correlation coefficients for C3 and C4 levels were 0.72 at baseline and 0.63 at six months, both statistically different from the zero value (P < 0.001).
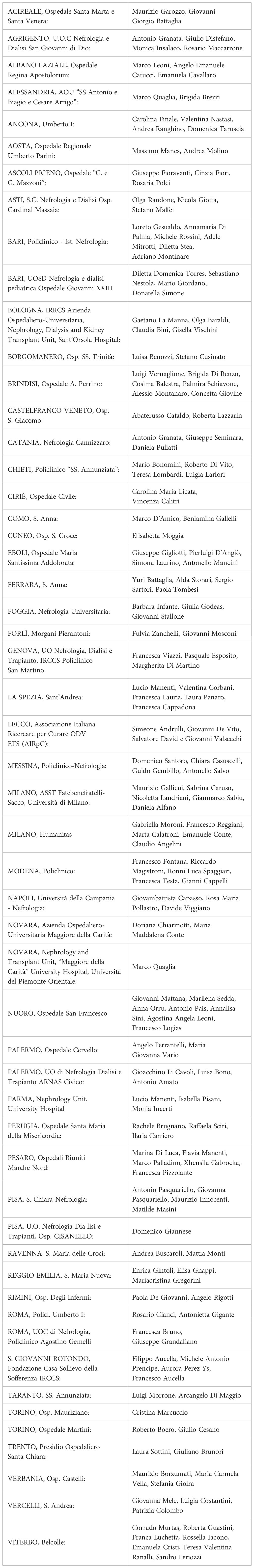
Outcome results
The study outcomes, grouped by i-LowC3 and normal C4 levels at the six-month follow-up, are presented in Table 4. CKD, the composite outcome of CKD or death, ESKD, and the composite outcome of ESKD or death were all significantly directly associated with i-LowC3 (P < 0.001). The risk of CKD in patients with i-LowC3 compared with the control group was more than double, increasing from 17.8% to 40.7% (228%).
Univariate survival analysis
Kaplan-Meier long-term survival curves for CKD Panel A), the composite outcome of CKD or death (Panel B), ESKD (Panel C) and the composite outcome ESKD or death (Panel D) are shown in Figure 1. In all cases, survival was significantly lower in the i-LowC3 group at the six-month follow-up compared to the control group (Log-rank test, P < 0.001 for all Panels).
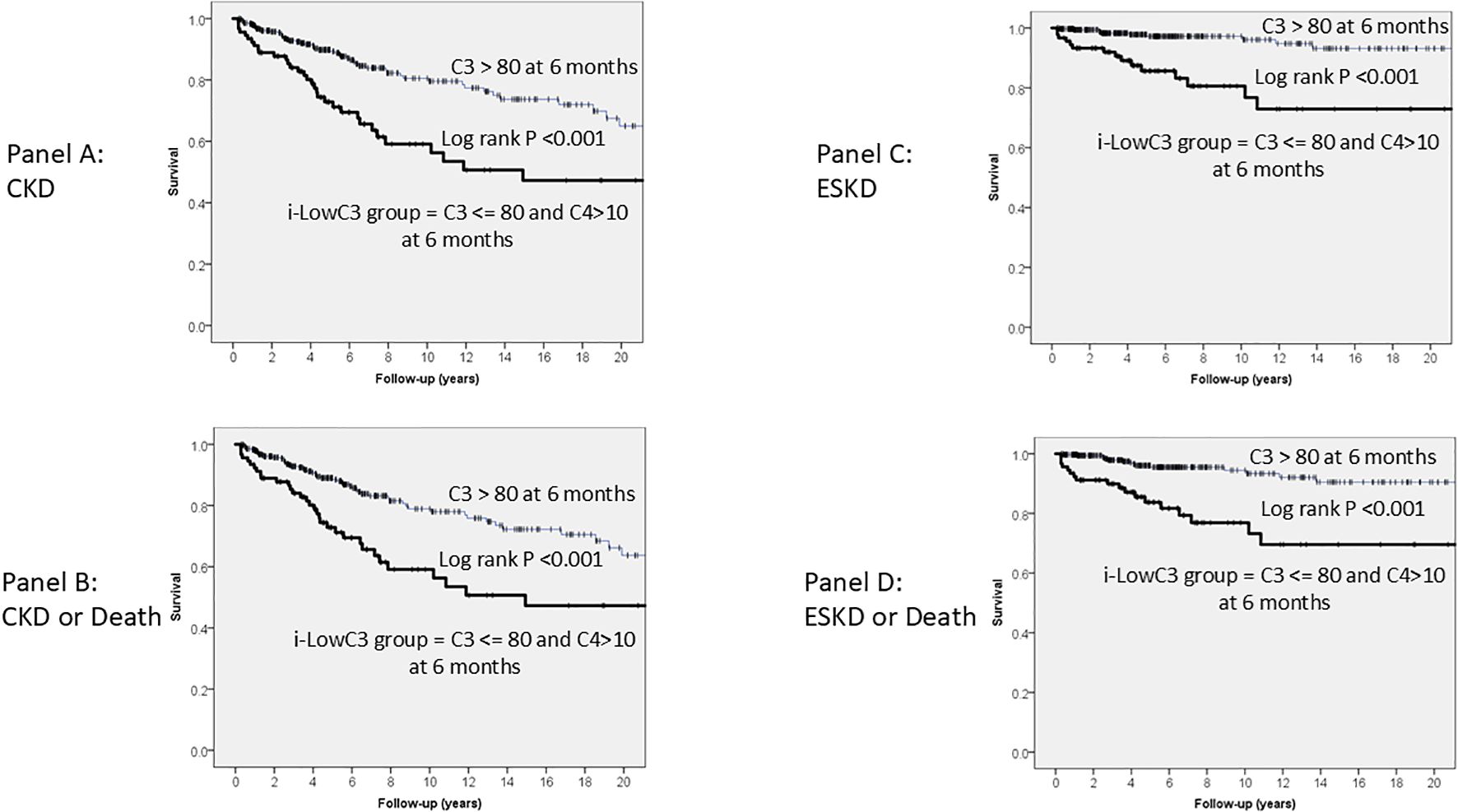
Figure 1. Kaplan-Meier long-term survival curves for CKD (A), CKD or Death (B), ESKD (C) and ESKD or Death (D), grouped, at six month follow up, by levels of C3 <= 80 mg/dL and C4 > 10 mg/dL (i-LowC3 group). In all cases, survival was lower in the i-LowC3 group compared with Controls.
Multivariate Cox regression analysis
Multivariate Cox regression analyses of predictors associated with CKD (Panel A) and the composite outcome of CKD or death (Panel B) are shown in Table 5A. A lower risk of CKD and CKD or death was significantly associated with: (i) higher eGFR before biopsy; (ii) a decrease in proteinuria at the six-month follow-up, and (iii) an increase in C3 levels at the six-month follow-up compared with their baseline values at the time of biopsy. Conversely, a higher risk of CKD and CKD or death was significantly associated with: (i) i-LowC3 group, (ii) higher proteinuria before biopsy, (iii) older age at biopsy, and (iiii) MMF treatment.
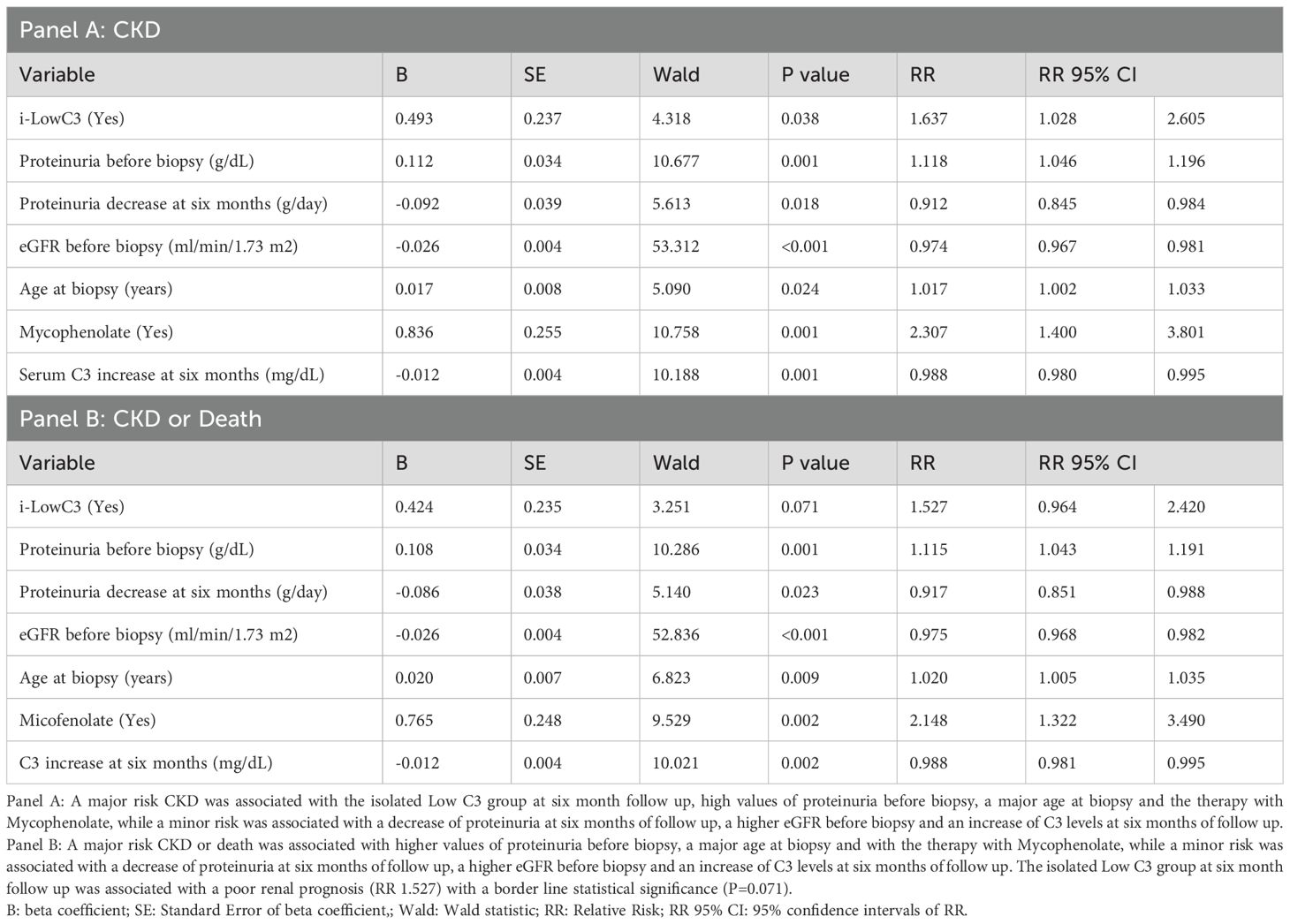
Table 5a. Multivariate Cox regression analysis of the predictors associated with CKD (Panel A) and CKD or Death (Panel B).
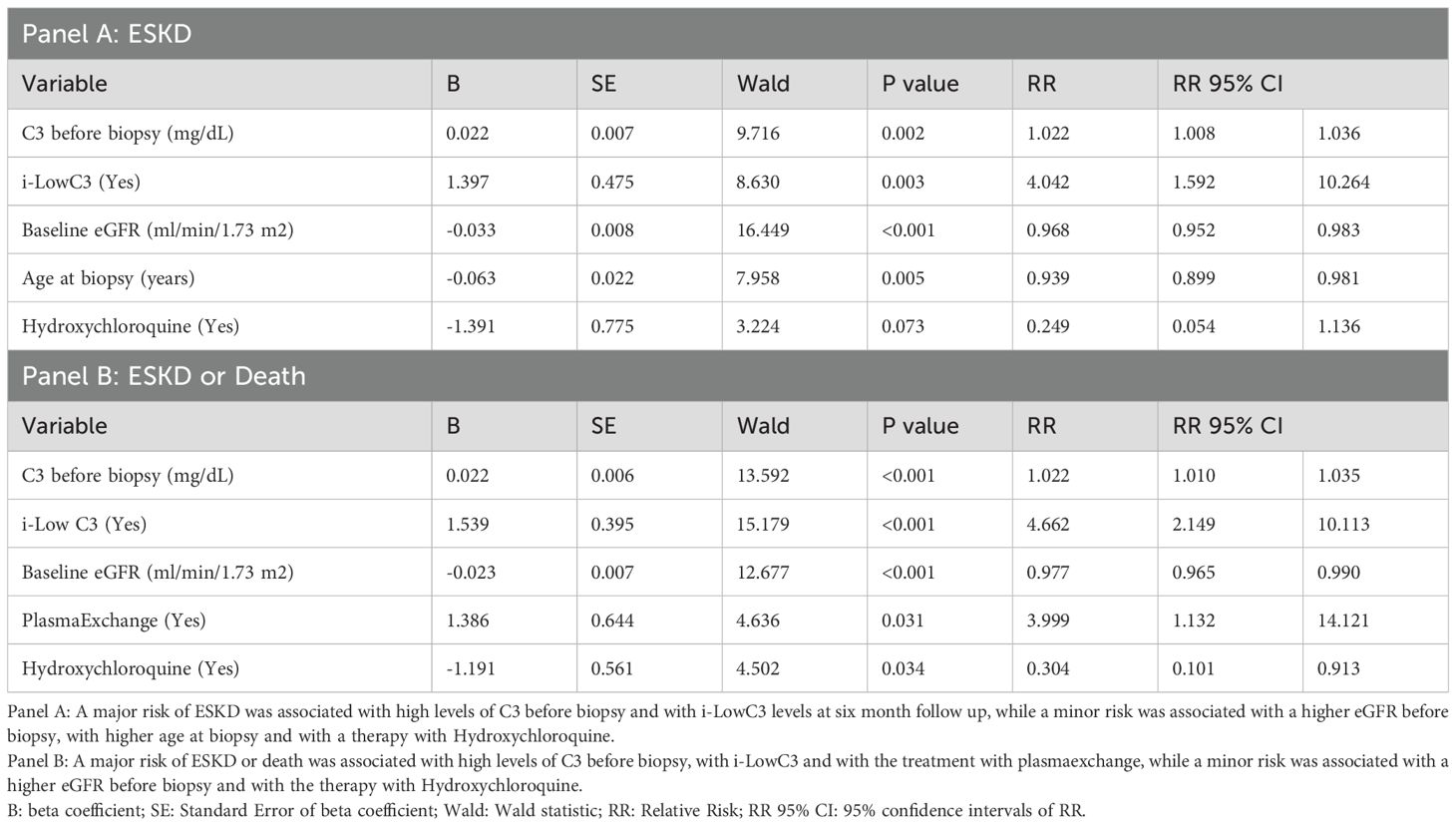
Table 5b. Multivariate Cox regression analysis of the predictors associated with ESKD (Panel A) and ESKD or death (Panel .
Predictors associated with ESKD (Panel A) and with the composite outcome of ESKD or death (Panel B) are shown in Table 5B. A major risk of ESKD was associated with high levels of C3 before biopsy and with i-LowC3 levels at six month follow up, while a minor risk was associated with a higher eGFR before biopsy, with higher age at biopsy and with a therapy with Hydroxychloroquine (Panel A). A major risk of ESKD or death was associated with high levels of C3 before biopsy and i-LowC3 at six month follow up and with the treatment with plasmaexchange, while a minor risk was associated with a higher eGFR before biopsy and with the therapy with Hydroxychloroquine (Panel B).
The proliferative histological classes (Class 3 and Class 4) compared to non-proliferative classes (Class 1 and Class 2) were not significantly associated with the risk of CKD (P = 0.585) or CKD or death (P = 0.804). Additionally, the interaction of MMF therapy with proliferative (Classes 3 and 4) versus non-proliferative (Classes 1, 2 and 5) histological classes was not significant for CKD (P = 0.377) or CKD or death (P = 0.356). This indicates that the increased risk of CKD and CKD or death associated with MMF exposure was present in both proliferative and non-proliferative histological classes.
Discussion
This large, retrospective, multicenter study from the Italian Registry of Renal Biopsy (IRRB), conducted in patients with a newly established histological diagnosis of LN, provides robust evidence that i-LowC3, a low-cost and readily available biomarker, is strongly associated with an increased doubled risk of CKD, ESKD or death. This predictive effect persisted after adjusting for initial disparities in eGFR between groups, as well as other confounding variables, including 24-hour proteinuria and induction treatment regimens (Tables 5A, B). The survival curves demonstrate the worse prognosis of the i-LowC3 group, across all considered endpoints. Furthermore, our results suggest that an increase in serum C3 levels at six months confers additional significant protection against the development of CKD (1% lower risk for each mg increase of C3, Table 5A).
The findings of this study align with and extend the results of a recent retrospective investigation (18), which assessed the renal prognosis of an international cohort of patients with LN. That study stratified patients based on the presence of persistent i-LowC3, hypothesizing that the persistent activation of the cAP - reflected by persistently reduced serum C3 levels (following adequate induction treatment) - represents a failure to suppress complement-mediated kidney injury. This earlier investigation demonstrated that patients with LN and persistent i-LowC3 exhibited a markedly increased risk of CKD, even when controlling for established prognostic factors such as age, sex, ethnicity and baseline eGFR.
Similarly, our study analyzed complement dynamics over six months following the first renal biopsy in a large cohort of LN patients, encompassing all histological classes. These results further support the hypothesis that serum C3 and C4 levels post-immunosuppressive therapy function as independent predictors of renal outcomes. This interpretation is consistent with experimental findings demonstrating the critical role of complement factor H (CFH), a key regulator of the cAP, in mitigating kidney injury. For instance, lupus-prone mice deficient in CFH exhibit unregulated cAP activation, hypocomplementemia, severe proteinuria, accelerated kidney failure, and early mortality compared to CFH-intact counterparts (23). Conversely, experimental deletion of other essential cAP components, such as complement factor B (24) or factor D (25), significantly reduces renal injury severity in murine lupus models.
Despite these experimental insights, translating these findings to clinical practice has proven challenging. While the association between low serum C3 levels and LN (26) and between complement levels normalization and favorable renal outcomes in LN (27) are well-established, and transient reductions in serum C3 and/or C4 levels are frequently associated with renal flares (15), these observations have not been sufficient to reliably identify individual patients at high risk for CKD. Furthermore, prior studies often assessed low serum C3 levels without concurrently analyzing fluctuations in C4 levels, thereby conflating the pathological effects of persistent cAP dysregulation (as reflected by i-LowC3) with the physiological activation of the cCP due to circulating immune complexes (reflected by reductions in both C3 and C4). This conflation has obscured the specific contribution of cAP dysregulation to LN-related kidney damage.
Recent studies are beginning to clarify these complex interactions. For instance, Song et al. demonstrated that serum Bb, a cAP activation product derived from complement factor B, is elevated during LN flares and is a significant predictor of poor renal outcomes (20). Furthermore, other investigations have identified anti-C3 autoantibodies that stabilize the cAP C3 convertase, thereby perpetuating cAP dysregulation and resulting in persistent C3 consumption (19). These findings provide a mechanistic basis for the observed association between persistent low serum C3 levels and adverse renal outcomes.
In addition, this study underscores the clinical utility of monitoring trends in serum complement levels, particularly i-LowC3, as an independent predictor of CKD and ESKD in LN. These results contribute to the growing body of evidence supporting the role of complement in LN pathogenesis and highlight the potential of complement-targeted therapies to address the unmet needs of this high-risk patient subgroup.
The clearer delineation of the roles of complement cascade activation pathways in LN provides critical insights, particularly regarding therapeutic considerations at the end of the initial period of immunosuppressive therapy. Persistent activation of the cAP appears to be a key driver of poor renal prognosis. While standard LN therapies, such as cyclophosphamide or MMF, may effectively control the proliferative inflammatory component, failure to rapidly correct cAP dysregulation could promote progressive endothelial damage, ultimately leading to CKD and ESKD. It is interesting to note that a similar pathophysiological role of a defective complement regulation has been suggested also in active and progressive cases of immunoglobulin A nephropathy (28–30).
In light of the findings by Vasilev et al. (19), therapies that achieve profound and sustained suppression of antibody production may facilitate a more rapid restoration of cAP balance, taking also into account that likely antiC3-autoantibodies and C3 Nephritic factor have different pathway actions. It is noteworthy that studies evaluating the addition of anti-CD20 therapies (rituximab, obinutuzumab) to standard LN care demonstrated faster normalization of serum C3 levels in treated patients compared with the controls. However, the follow-up durations in these studies were insufficient to confirm whether normalizing serum C3 translates into renal protection and prevention of ESKD (31–35). Preliminary data suggest that obinutuzumab, in particular, may more effectively rebalance the cAP and mitigate the risk of ESKD (36).
Our study also analyzed follow-up data on proteinuria and immunosuppressive treatments. In alignment with prior studies, our results reaffirm that 24-hour proteinuria serves as a key predictor of renal outcomes: higher proteinuria at baseline predicted worse outcomes, while a significant reduction after six months of induction therapy was associated with favorable renal prognosis. Additionally, our findings underscore the nephroprotective role of Hydroxychloroquine.
Limitations of the study
The major limitations of this study include the retrospective design and the related difficulties in the interpretations of some results. Notably, patients in the i-LowC3 group received significantly less immunosuppressive therapy and particularly less cyclophosphamide (Table 3), a finding that is challenging to interpret given their significantly lower eGFR at the time of biopsy—a negative prognostic marker that would typically warrant more aggressive therapy. In addition, treatment differences could be due to center-specific and time-varying protocols, toxicity concerns or delayed diagnosis, that are unavailable in this study.
Despite this limitation, the strong association between i-LowC3 and CKD risk remained significant even after adjusting for baseline eGFR and prescribed therapies (Tables 5A, B). Another surprising aspect comes from the analysis of used immunosuppressive therapies. In particular, we observed an elevated risk of CKD and ESKD for patients exposed to Mycophenolate (MMF) treatment. We know that the fixed MMF dosing used in clinical practice and trials may not provide adequate levels of the active MMF metabolite mycophenolic acid (MPA). Up to tenfold variation in the MPA area under the plasma concentration-time curve has been observed with fixed MMF dosing (37). This variability could consequently have led to a negative effect on the renal outcome over longer follow-up times than in conventional clinical trials. It is interesting to note that a systematic review evaluating the risk of ESKD in LN patients in published studies from 1971 to 2015 identified an increase of the ESKD risk probably due to the progressive substitution of cyclophosphamide with MMF (38). Our study seems to confirm that MMF is related to CKD/ESKD risk, however, due to the retrospective design of the study, it was not possible to account for all confounding factors influencing treatment decisions during the induction phase. For instance, patients treated with MMF could be negatively selected, when their clinical evaluation suggested poor renal prognosis and selection treatment bias of sicker patients preferentially receiving MMF.
Conclusions
This study suggests that, six months after the renal biopsy: 1) The combination of serum C3 levels below normal with normal C4 levels, once inflammatory activity is controlled with immunosuppression, identifies LN patients at high risk of developing CKD and ESKD, and 2) Linear increases in C3 levels reduce independently the same risk. Isolated low C3 at 6 month follow up was associated with twice the risk of CKD, ESKD or death, highlighting the plausible critical role of cAP activation as a driver of kidney damage. These findings underscore the need for further investigations into complement-targeted treatments in LN, particularly in patients with i-LowC3.
Data availability statement
Data supporting this study may be shared upon reasonable request to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Bari University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. FR: Investigation, Writing – original draft, Writing – review & editing. IP: Investigation, Writing – original draft, Writing – review & editing. DG: Investigation, Writing – original draft, Writing – review & editing. GVi: Investigation, Writing – original draft, Writing – review & editing. GVa: Conceptualization, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. GGo: Investigation, Writing – original draft, Writing – review & editing. GGi: Investigation, Writing – original draft, Writing – review & editing. PE: Investigation, Writing – original draft, Writing – review & editing. PDG: Investigation, Writing – original draft, Writing – review & editing. CM: Investigation, Writing – original draft, Writing – review & editing. CC: Investigation, Writing – original draft, Writing – review & editing. SC: Investigation, Writing – original draft, Writing – review & editing. MR: Investigation, Writing – original draft, Writing – review & editing. GA: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. MQ: Investigation, Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing. AB: Investigation, Writing – original draft, Writing – review & editing. GR: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. FM: Investigation, Writing – original draft, Writing – review & editing. BD: Investigation, Writing – original draft, Writing – review & editing. FZ: Investigation, Writing – original draft, Writing – review & editing. FB: Investigation, Writing – original draft, Writing – review & editing. RS: Investigation, Writing – original draft, Writing – review & editing. MM: Investigation, Writing – original draft, Writing – review & editing. DT: Investigation, Writing – original draft, Writing – review & editing. MG: Investigation, Writing – original draft, Writing – review & editing. RL: Investigation, Writing – original draft, Writing – review & editing. VC: Investigation, Writing – original draft, Writing – review & editing. FF: Investigation, Writing – original draft, Writing – review & editing. MC: Investigation, Writing – original draft, Writing – review & editing. MI: Investigation, Writing – original draft, Writing – review & editing. CB: Investigation, Writing – original draft, Writing – review & editing. BI: Investigation, Writing – original draft, Writing – review & editing. PD’A: Investigation, Writing – original draft, Writing – review & editing. MD: Investigation, Writing – original draft, Writing – review & editing. AR: Investigation, Writing – original draft, Writing – review & editing. LG: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication of this article was assured by the financial support provided by the Associazione Italiana Ricercare per Curare (AIRpC).
Acknowledgments
The authors extend their gratitude to the Società Italiana di Nefrologia (SIN), the Associazione Italiana Ricercare per Curare (AIRpC) and the Fondazione Italiana del Rene (FIR) for their support in the development of the web-based database used for this study.
Conflict of interest
LM received consulting fees from Vifor CSL, GSK, Alexion and payment or honoraria for lectures and presentations from Vifor CSL, GSK, Alexion and Astra Zeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gasparotto M, Gatto M, Binda V, Doria A, and Moroni G. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatol (Oxford England). (2020) 59:v39–51. doi: 10.1093/rheumatology/keaa381
2. Adler M, Chambers S, Edwards C, Neild G, and Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatol (Oxford England). (2006) 45:1144–7. doi: 10.1093/rheumatology/kel039
3. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine. (2003) 82:299–308. doi: 10.1097/01.md.0000091181.93122.55
4. Moroni G, Gatto M, Tamborini F, Quaglini S, Radice F, Saccon F, et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis. Ann Rheumatic Dis. (2020) 79:1077–83. doi: 10.1136/annrheumdis-2020-216965
5. Tamirou F, Lauwerys BR, Dall’Era M, Mackay M, Rovin B, Cervera R, et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med. (2015) 2:e000123. doi: 10.1136/lupus-2015-000123
6. Dall’Era M, Cisternas MG, Smilek DE, Straub L, Houssiau FA, Cervera R, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol (Hoboken N.J.). (2015) 67:1305–13. doi: 10.1002/art.39026
7. Wallace DJ, Hahn B, and Dubois EL. Dubois’ lupus erythematosus and related syndromes. 8th ed. Philadelphia, PA: Elsevier/Saunders (2013).
8. Bao L, Cunningham PN, and Quigg RJ. Complement in lupus nephritis: new perspectives. Kidney Dis (Basel Switzerland). (2015) 1:91–9. doi: 10.1159/000431278
9. Ho A, Barr SG, Magder LS, and Petri M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheumatism. (2001) 44:2350–7. doi: 10.1002/1529-0131(200110)44:10<2350::AID-ART398>3.0.CO;2-A
10. Gandino IJ, Scolnik M, Bertiller E, Scaglioni V, Catoggio LJ, and Soriano ER. Complement levels and risk of organ involvement in patients with systemic lupus erythematosus. Lupus Sci Med. (2017) 4:e000209. doi: 10.1136/lupus-2017-000209
11. Swaak AJ, Groenwold J, and Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheumatic Dis. (1986) 45:359–66. doi: 10.1136/ard.45.5.359
12. Sturfelt G and Truedsson L. Complement and its breakdown products in SLE. Rheumatol (Oxford England). (2005) 44:1227–32. doi: 10.1093/rheumatology/keh719
13. Senaldi G, Makinde VA, Vergani D, and Isenberg DA. Correlation of the activation of the fourth component of complement (C4) with disease activity in systemic lupus erythematosus. Ann Rheumatic Dis. (1988) 47:913–7. doi: 10.1136/ard.47.11.913
14. Bao L and Quigg RJ. Complement in lupus nephritis: the good, the bad, and the unknown. Semin Nephrol. (2007) 27:69–80. doi: 10.1016/j.semnephrol.2006.09.009
15. Birmingham DJ, Irshaid F, Nagaraja HN, Zou X, Tsao BP, Wu H, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus. (2010) 19:1272–80. doi: 10.1177/0961203310371154
16. Li NL, Birmingham DJ, and Rovin BH. Expanding the role of complement therapies: the case for lupus nephritis. J Clin Med. (2021) 10:626. doi: 10.3390/jcm10040626
17. Truedsson L, Bengtsson AA, and Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. (2007) 40:560–6. doi: 10.1080/08916930701510673
18. Rossi GM, Maggiore U, Peyronel F, Fenaroli P, Delsante M, Benigno GD, et al. Persistent isolated C3 hypocomplementemia as a strong predictor of end-stage kidney disease in lupus nephritis. Kidney Int Rep. (2022) 7:2647–56. doi: 10.1016/j.ekir.2022.09.012
19. Vasilev V, Artero MR, Petkova M, Mihaylova G, Dragon-Durey MA, Radanova M, et al. Clinical relevance of anti-C3 and anti-C4 autoantibodies in lupus nephritis. Kidney Int Rep. (2024) 9:1429–40. doi: 10.1016/j.ekir.2024.01.052
20. Song D, Guo W-Y, Wang F-M, Li YZ, Song Y, Yu F, et al. Complement alternative pathway's activation in patients with lupus nephritis. Am J Med Sci. (2017) 353:247–57. doi: 10.1016/j.amjms.2017.01.005
21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
22. Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. (2004) 65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x
23. Bao L, Haas M, and Quigg RJ. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrology: JASN. (2011) 22:285–95. doi: 10.1681/ASN.2010060647
24. Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol (Baltimore Md.: 1950). (2000) 164:786–94. doi: 10.4049/jimmunol.164.2.786
25. Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, and Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. (2004) 65:129–38. doi: 10.1111/j.1523-1755.2004.00371.x
26. Durcan L and Petri M. The clinical and serological associations of hypocomplementemia in a longitudinal sle cohort. Semin Arthritis Rheumatism. (2020) 50:1081–6. doi: 10.1016/j.semarthrit.2020.06.009
27. Appel AE, Sablay LB, Golden RA, Barland P, Grayzel AI, and Bank N. The effect of normalization of serum complement and anti-DNA antibody on the course of lupus nephritis: a two year prospective study. Am J Med. (1978) 64:274–83. doi: 10.1016/0002-9343(78)90056-6
28. Rossi GM, Ricco F, Pisani I, Delsante M, Maggiore U, Fiaccadori E, et al. C3 hypocomplementemia predicts the progression of CKD towards end-stage kidney disease in igA nephropathy, irrespective of histological evidence of thrombotic microangiopathy. J Clin Med. (2024) 13:2594. doi: 10.3390/jcm13092594
29. Tringali E, Vetrano D, Tondolo F, Maritati F, Fabbrizio B, Pasquinelli G, et al. Role of serum complement C3 and C4 on kidney outcomes in IgA nephropathy. Sci Rep. (2024) 14:16224. doi: 10.1038/s41598-024-65857-w
30. Trimarchi H and Coppo R. Glomerular endothelial activation, C4d deposits and microangiopathy in immunoglobulin A nephropathy. Nephrol Dial Transplant. (2021) 36:581–6. doi: 10.1093/ndt/gfz241
31. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheumatism. (2012) 64:1215–26. doi: 10.1002/art.34359
32. Weidenbusch M, Römmele C, Schröttle A, and Anders H-J. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dialysis Transplant. (2013) 28:106–11. doi: 10.1093/ndt/gfs285
33. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall'Era M, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrology: CJASN. (2018) 13:1502. doi: 10.2215/CJN.01070118
34. Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheumatic Dis. (2022) 81:100–7. doi: 10.1136/annrheumdis-2021-220920
35. Rovin BH, Furie RA, Ross Terres JA, Giang S, Schindler T, Turchetta A, et al. Kidney outcomes and preservation of kidney function with obinutuzumab in patients with lupus nephritis: A post hoc analysis of the NOBILITY trial. Arthritis Rheumatol (Hoboken N.J.). (2024) 76:247–54. doi: 10.1002/art.42734
36. Arnold J, Dass S, Twigg S, Jones CH, Rhodes B, Hewins P, et al. Efficacy and safety of obinutuzumab in systemic lupus erythematosus patients with secondary non-response to rituximab. Rheumatol (Oxford England). (2022) 61:4905–9. doi: 10.1093/rheumatology/keac150
37. Zahr N, Arnaud L, Marquet P, Haroche J, Costedoat-Chalumeau N, Hulot JS, et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patientstreated with mycophenolate mofetil. Arthritis Rheumatol. (2010) 62:2047–54. doi: 10.1002/art.27495
38. Tektonidou MG, Dasgupta A, and Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971 - 2015: A systematic review and bayesian meta-analysis. Arthritis Rheumatol. (2016) 68:1432–41. doi: 10.1002/art.39594
Appendix: Cities, affiliations and names of collaborative authors of the ITA-KID-BIOPSY Group.
Keywords: CKD, ESKD, proteinuria, complement, lupus, immunosuppression
Citation: Andrulli S, Manenti L, Reggiani F, Pisani I, Giannese D, Vischini G, Valsecchi G, Godeas G, Gigliotti G, Esposito P, De Giovanni P, Murtas C, Casuscelli C, Caruso S, Rossini M, Andrulli G, Quaglia M, Aucella F, Buscaroli A, Rossi GM, Mattozzi F, Di Renzo B, Zanchelli F, Bruno F, Sciri R, Manes M, Torres DD, Garozzo M, Lazzarin R, Corbani V, Fontana F, Calatroni M, Incerti M, Bini C, Infante B, D’Angio’ P, Di Martino M, Rigotti A and Gesualdo L (2025) Isolated C3 hypocomplementemia as an early predictor of chronic kidney disease in lupus nephritis. Front. Immunol. 16:1655825. doi: 10.3389/fimmu.2025.1655825
Received: 28 June 2025; Accepted: 18 August 2025;
Published: 12 September 2025.
Edited by:
Marzena Olesinska, National Institute of Geriatrics, Rheumatology and Rehabilitation, PolandReviewed by:
Emanuele Bizzi, Vita-Salute San Raffaele University, ItalyTheo De Malmanche, New South Wales Health Pathology, Regional and Rural, Orange Hospital, Australia
Edoardo Tringali, ASL TO4, Italy
Copyright © 2025 Andrulli, Manenti, Reggiani, Pisani, Giannese, Vischini, Valsecchi, Godeas, Gigliotti, Esposito, De Giovanni, Murtas, Casuscelli, Caruso, Rossini, Andrulli, Quaglia, Aucella, Buscaroli, Rossi, Mattozzi, Di Renzo, Zanchelli, Bruno, Sciri, Manes, Torres, Garozzo, Lazzarin, Corbani, Fontana, Calatroni, Incerti, Bini, Infante, D’Angio’, Di Martino, Rigotti and Gesualdo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simeone Andrulli, c2ltZW9uZS5hbmRydWxsaUBhbGljZS5pdA==
 Simeone Andrulli
Simeone Andrulli Lucio Manenti
Lucio Manenti Francesco Reggiani3
Francesco Reggiani3 Pasquale Esposito
Pasquale Esposito Paola De Giovanni
Paola De Giovanni Corrado Murtas
Corrado Murtas Marco Quaglia
Marco Quaglia Filippo Aucella
Filippo Aucella Giovanni Maria Rossi
Giovanni Maria Rossi Massimo Manes
Massimo Manes Marta Calatroni
Marta Calatroni