- 1Department of Respiratory Medicine, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated with Shanxi Medical University, Taiyuan, China
- 2Department of Respiratory Medicine, University Hospital, Ludwig Maximilians University, Munich, Germany
- 3School of Pharmacy, Shanxi Medical University, Taiyuan, China
- 4Medicine Imaging Department, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated with Shanxi Medical University, Taiyuan, China
- 5Department of Medicine, Xiamen Spacegen Co., Ltd., Xiamen, China
- 6College of Pulmonary and Critical Care Medicine, The Eighth Medical Center, Chinese PLA General Hospital, Beijing, China
- 7Department of Thoracic Oncology, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated with Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated with Shanxi Medical University, Taiyuan, China
- 8Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
In recent years, the introduction of immune checkpoint inhibitors (ICIs) has revolutionized the treatment landscape for malignant tumors, markedly improving survival outcomes across various cancers, such as lung cancer, esophageal cancer, and melanoma. Consequently, ICIs have become a cornerstone of first-line therapy for numerous malignancies. However, while ICIs effectively modulate immune responses to combat tumor cells, they may also trigger excessive immune activation and T-cell dysfunction, thereby leading to a spectrum of immune-related adverse events (irAEs). The organs most frequently affected by these irAEs include the skin, gastrointestinal tract, endocrine system, and lungs. Among these adverse events, the development of severe immune checkpoint inhibitor-related pneumonitis (CIP) may result in significant disability, permanent discontinuation of ICIs, and even death, with real-world incidence rates exceeding those reported in clinical trials. Early detection, precise diagnosis, and timely intervention are critical for optimizing patient outcomes. However, diagnosing CIP remains challenging because it relies heavily on high-resolution chest CT imaging and a detailed treatment history. The radiological features of CIP are often nonspecific, complicating its identification. This complexity is further exacerbated in patients receiving consolidative immunotherapy following concurrent or sequential chemoradiotherapy for stage III unresectable non-small cell lung cancer, where distinguishing between radiation pneumonitis and CIP becomes particularly difficult. To address these challenges, an increasing number of imaging experts are investigating the potential of radiomics and machine learning techniques in predicting the occurrence and assessing the prognosis of CIP. This article comprehensively reviews the pathogenesis of CIP, the predictive value of radiomics in identifying this condition and recent advancements in treatment strategies, with the aim of providing novel insights for future research and clinical management of CIP.
1 Introduction
In recent years, immune checkpoint inhibitors (ICIs), which target cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death 1 (PD-1) and programmed cell death ligand-1 (PD-L1), have shown significant efficacy in treating various malignant tumors. ICIs meticulously modulate the activity of CD8-positive cytotoxic T lymphocytes (CD8+ T cells), CD4-positive helper T lymphocytes (CD4+ T cells), and regulatory T cells (Tregs), ultimately enhancing the antitumor immune response (1, 2). However, excessive activation of T-cell responses can disrupt the delicate balance among T-lymphocyte subsets, leading to deregulated expression of inflammatory mediators and triggering an overly aggressive immune response, potentially culminating in a spectrum of immune-related adverse events (irAEs), including but not limited to various degrees of rashes, pneumonia, hepatitis, colitis and endocrinopathies (3–5). Among these irAEs, checkpoint inhibitor-related pneumonitis(CIP) is of particular concern because of its potentially life-threatening nature. CIP manifests as the onset of respiratory system-related symptoms such as dyspnea, shortness of breath, and cough with sputum in patients receiving ICI therapy. Crucially, these symptoms are accompanied by new pulmonary infiltrates on imaging studies while excluding tumor progression, new pulmonary infections, or other conditions that could cause pulmonary changes (6). This distinctive combination of clinical and radiological features makes CIP a critical focus in the management of patients receiving ICIs.
2 Epidemics and risk factors
2.1 CIP epidemic
CIP is the most common fatal adverse reaction to PD-1/PD-L1 inhibitors (7). Compared with patients with other cancers, patients with lung cancer have a greater incidence and severity of CIP (8). Compared with monotherapy, combination immunotherapy results in more frequent CIP (9, 10). Clinical studies have reported a 3%-5% overall incidence of CIP with 1% grade≥3 cases (9, 11, 12), whereas real-world data indicate higher rates of 13%-19% (13–15).
Moey et al. (16) conducted an analysis of the WHO drug safety database and reported that the mortality rate associated with CIP was significantly higher than that reported in clinical trials, with 20.4% in lung cancer patients, potentially due to diagnostic challenges distinguishing CIP from tumor progression or infections (17). CIP may occur anytime post-ICI therapy, with a median onset of 2.8 months (range: 9 days–19.2 months) (18), rarely extending to 36 months (19). Onset occurs earlier in non-small cell lung cancer (NSCLC) patients (1.1 vs 3.1 months). Severe CIP is associated with a 61.5% mortality rate, with a median survival of 104 days (20). Consequently, for patients with a history of ICI treatment, CIP should be prioritized as a differential diagnosis if they exhibit respiratory-related symptoms or abnormal chest imaging findings.
2.2 Risk factors for CIP
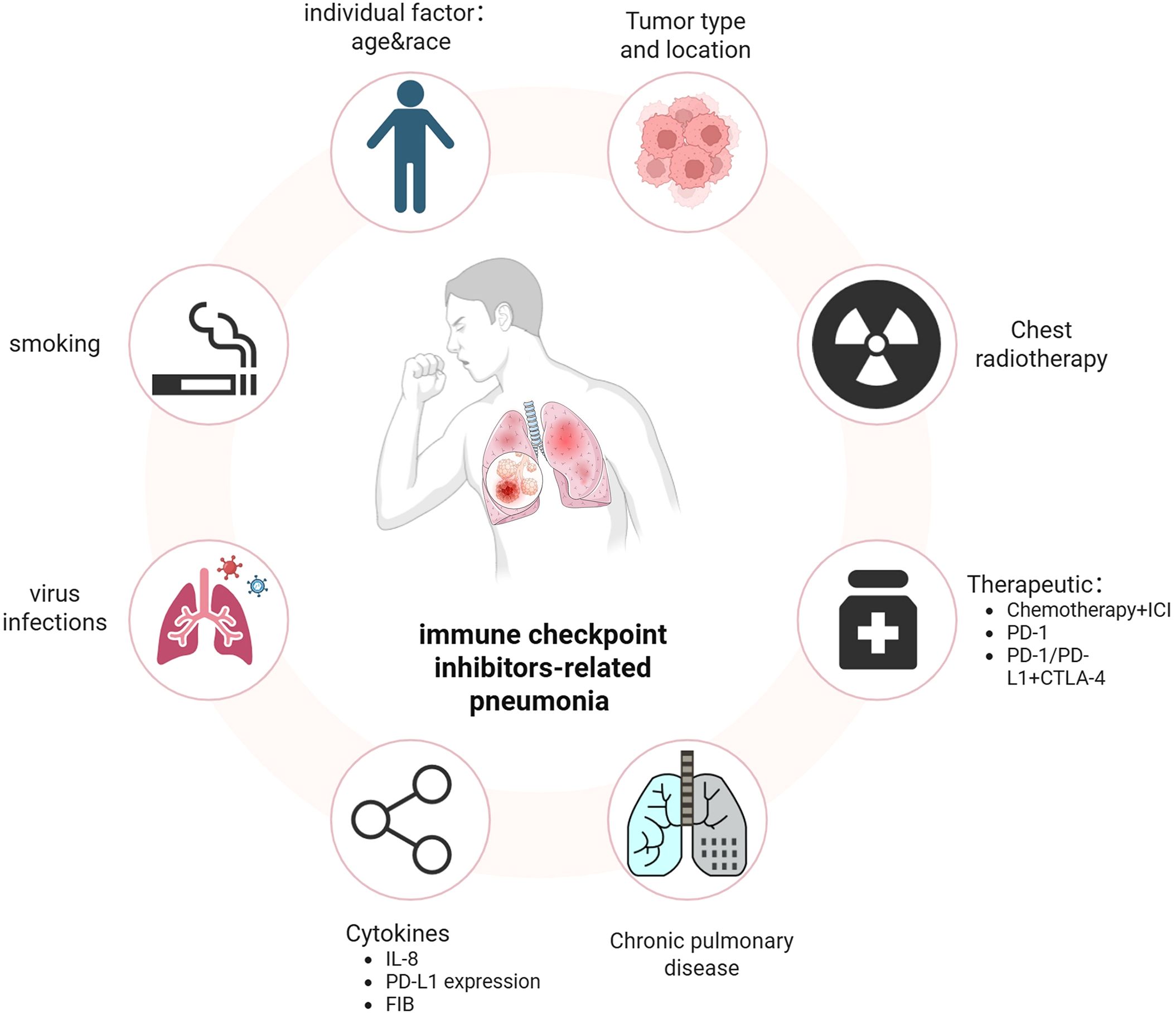
Identifying the risk factors associated with CIP is crucial for facilitating early diagnosis and effective management of high-risk patients. Previous retrospective studies have reported that a high incidence of immune checkpoint inhibitor-related pneumonia (CIP) is associated with various antitumor treatments, tumor pathological types, chronic underlying lung diseases, age, smoking history, radiotherapy history, levels of certain inflammatory factors, and genetic susceptibility (Figure 1).
2.2.1 Antineoplastic drug regimen
A meta-analysis assessing ICIs in stage III NSCLC patients revealed a significantly greater incidence of grade 2 pneumonia with anti-PD-1 therapy than with anti-PD-L1 therapy (22.7% vs 14.9%; OR=2.10, P<0.01) (21). Consistent findings were corroborated in additional studies (21). A meta-analysis revealed that the incidence of CIP was 1.6% with anti-PD-1 monotherapy vs. 6.6% with the anti-PD-1/CTLA-4 combination, indicating a greater CIP risk with dual vs. single ICI therapy (9). With the increasing prominence of perioperative neoadjuvant immunotherapy in the treatment of non-small cell lung cancer, emerging evidence indicates that the incidence of CIP among patients receiving neoadjuvant immunotherapy ranges from 3% to 25% (22). Pathological partial response (PCR) positivity rate among patients in the CIP group is significantly lower than that among patients in the non-CIP group (20.8% vs 26%) (23), while surgical intervention itself constitutes a traumatic stimulus factor. In a retrospective study, the incidence of CIP among patients who received sequential targeted drug therapy within 8 weeks following immune checkpoint inhibitor (ICI) treatment was notably greater (18.8%) than that among those who received cytotoxic chemotherapy (7.4%) and those who did not undergo any chemotherapy (5.1%) (24). Patients receiving postimmunotherapy targeted drugs developed CIP earlier than those receiving chemotherapy did (35 vs 62 days) (24). All cases of CIP were graded as having a severity of ≥3, and the condition was associated with a relatively high mortality rate (26.1%) (24). Another cohort study revealed that the median overall survival (mOS) of patients with NSCLC who received ICI therapy, such as epidermal growth factor receptor (EGFR)-TKI therapy, following tyrosine kinase inhibitor (TKI) treatment was significantly shorter than that of patients who did not receive TKIs (7.6 months vs. 18.5 months). This observed disparity may be attributed to an increased risk of immune-related adverse events (irAEs), including chemotherapy-induced pneumonitis (CIP), as well as alterations in the tumor microenvironment associated with drug resistance (25).
2.2.2 Chronic lung diseases
Studies (26) have shown that, compared with noncomorbid cases, chronic obstructive pulmonary disease (COPD)/asthma in NSCLC patients is associated with an increased incidence of CIP (2.3%). Chronic interstitial lung disease (ILD) patients have a threefold greater CIP risk. UIP-type ILD patients receiving ICIs face independent acute ILD exacerbation risks (27). Conversely, isolated pleuroparenchymal fibroelastosis (PPFE) patients exhibit significantly lower CIP rates (p=0.024) and longer survival, potentially due to the lack of acute ILD exacerbation associated with PPFE, which primarily causes chronic respiratory failure (28–30).
2.2.3 Site and histological type of the primary tumor
Cui et al. demonstrated a significant association between squamous cell carcinoma and the occurrence of CIP. Furthermore, obstructive pneumonia may contribute to an increased risk of CIP, as squamous cell carcinomas are predominantly central lung cancers that frequently result in obstructive pneumonia (31).
2.2.4 Baseline indicators of population characteristics
A meta-analysis was conducted by Zhang et al (32). demonstrated that both race and age significantly influence the incidence of CIP. Specifically, the incidence of pneumonia is greater in Asian populations than in Western populations. Additionally, studies with a greater proportion of elderly participants revealed an increased incidence of CIP, particularly among individuals aged 65 years or older. Furthermore, a history of smoking has been identified as an independent risk factor for the development of CIP (33).
2.2.5 History of radiotherapy
The KEYNOTE-001 trial revealed that the incidence of CIP was 13% in NSCLC patients receiving thoracic radiotherapy before ICI therapy versus 1% in nonradiated patients (34). Studies combining ICIs with chemoradiotherapy for locally advanced NSCLC have shown that ≥grade 2 CIP occurs in ~10% of cases (34, 35), which suggests that patients with a history of thoracic radiotherapy are at increased risk of developing CIP. Barron et al. proposed that radiotherapy-induced DNA damage, immune cell infiltration, cytokine upregulation, and collagen deposition alter the lung immune microenvironment. Thoracic radiation injury combined with hyperactivated T cells during ICI therapy may drive CIP development (36).
2.2.6 Other factors
According to a study by Chao et al. (37), patients with a PD-L1 expression level of ≥50% and an IL-8 concentration of < 9.0 pg/mL presented a significantly elevated risk of developing CIP. Mao et al. (38) revealed that when the fibrinogen (FIB) level reached 3.955 g/L, the mortality rate increased by 22%, suggesting that fibrinogen, which serves as a marker of the inflammatory process, is associated with an increased risk of CIP due to its elevated levels. A retrospective study revealed that patients who tested positive for HLA-B*35 and HLA-DRB1*11 molecules presented a significantly elevated risk of developing CIP (39). It is currently recognized that in autoimmune diseases, the expression of HLA-B*35 is linked to an increased risk of severe primary pulmonary hypertension in scleroderma patients, as well as to nephritis accompanied by marked leukocytosis and high-risk juvenile idiopathic arthritis. Furthermore, HLA-DRB1*11 expression has been associated with the development of systemic sclerosis (40–42). The human leukocyte antigen (HLA) complex is crucial for the efficacy and regulation of T-cell-mediated immune responses. It specifically functions by presenting peptides derived from tumor antigens to T-cell receptors on effector T cells (43). The proinflammatory effects and endoplasmic reticulum stress (ERS) induced by the HLA-B*35 allele, combined with the Th2-promoting capability of DRB1*11, may explain the increased incidence of immune-related pneumonitis (IRP) in cancer patients receiving PD-1/PD-L1 immune checkpoint inhibitor therapy (42, 44). A previous study (45) reported that the incidence of CIP follows a seasonal pattern, with a higher prevalence observed during the winter months. These findings suggest a link between ICI treatment, viral infections, and CIP, indicating that seasonal viruses may contribute to CIP development. Additionally, the literature suggests a potential association between cytomegalovirus (CMV) infection and the development of CIP. In this study, serological samples from 29 patients diagnosed with grade 3–4 CIP were analyzed, and CMV pp65 antigen was positive in 28 of these patients (46).
3 Progress on the mechanisms of CIP
The molecular mechanism of CIP involves a complex interplay of multiple factors. Although the precise molecular mechanisms underlying this condition remain incompletely understood, several key processes are believed to contribute to the onset of pneumonitis during ICI treatment (Figure 2). Emerging scientific evidence suggests the involvement of diverse pathways in the pathogenesis of CIP, which are delineated below.
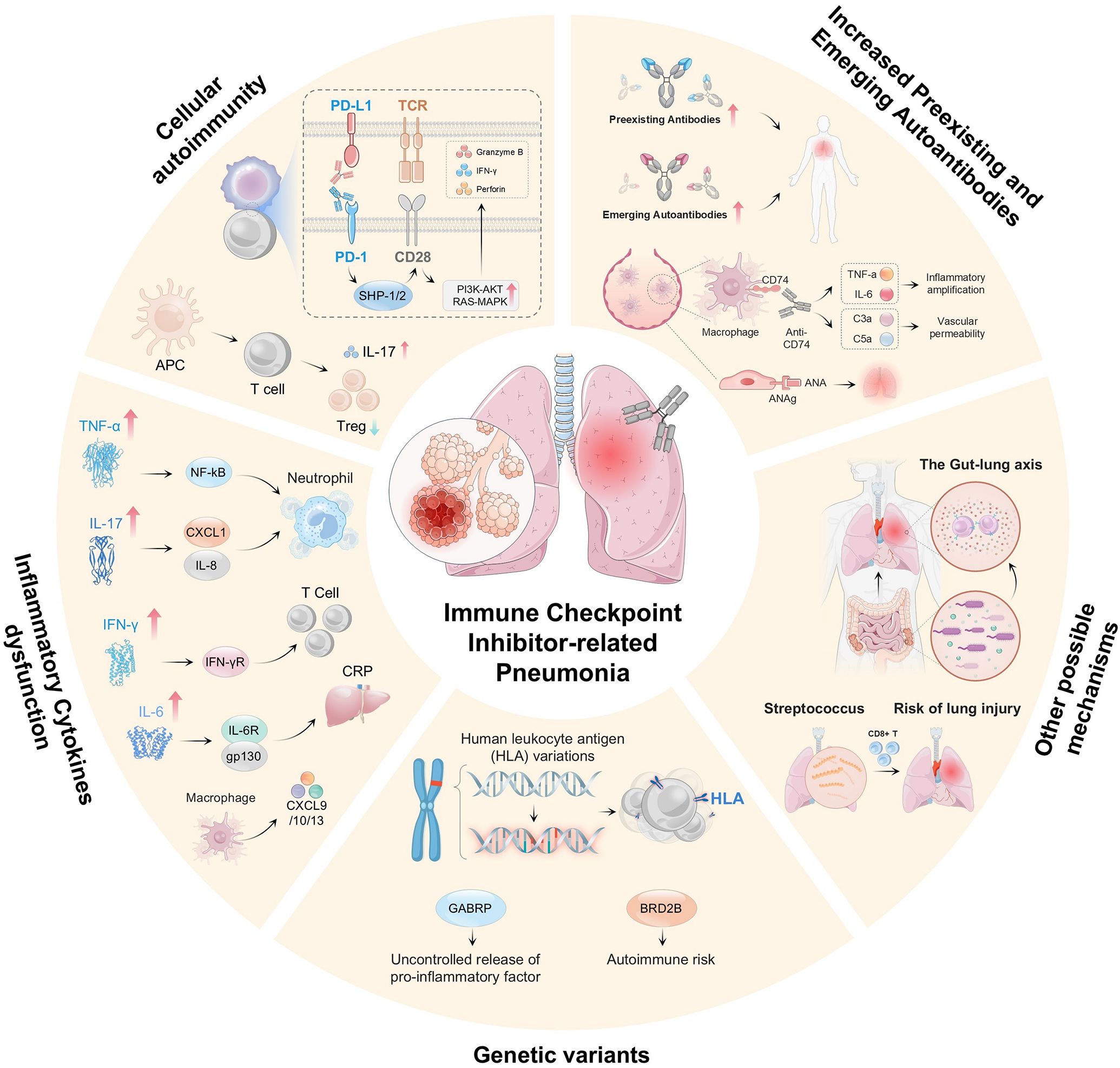
Figure 2. Shows that CIP development involves multiple mechanisms and factors. These include dysregulation of the PD-1/PD-L1 and CTLA-4 pathways, which causes autoimmune dysfunction; excessive T-cell activation and cytotoxicity with impaired Treg function; imbalance in cytokine networks (e.g., elevated TNF-α, IL-6, IL-17, and IFN-γ) and proinflammatory mediators (e.g., CXCL1, IL-8, and CXCL9/10/13), which activate neutrophils and T cells; genetic variations, which affect immune responses (e.g., HLA polymorphisms, GABRP, and BRD2B); autoantibodies, which amplify inflammation via complement activation (e.g., C3a/C5a); gut–lung axis imbalance (e.g., altered microbiota), which increases the risk of lung injury; and infections (e.g., streptococcal), which trigger autoimmune reactions.
3.1 T-cell dysfunction
PD-1/PD-L1 inhibitors can augment the antitumor efficacy of T cells (47, 48). Accumulating evidence indicates that the upregulation of T cells may play a role in the pathogenesis of CIP (4, 49). The increased activity of these targeted T cells can lead to the attack of cross-reactive antigens shared between tumors and normal lung tissue, resulting in off-target toxicity.
Hiroyuki and colleagues reported the presence of T-cell-predominant lymphocytosis in the BALF of patients with CIP (50). Notably, the proportions of CD8+ T cells expressing immune checkpoint proteins, a hallmark of tumor-infiltrating T lymphocytes (TILs), are greater in patients with CIP than in those with other immune-related adverse events (irAEs) (51–53). Naidoo et al. proposed increased infiltration of highly proliferative CD8+ T cells in the lung biopsy tissue of NSCLC patients who developed chronic bronchiolitis obliterans organizing pneumonia subsequent to nivolumab therapy (54). Subudhi et al. demonstrated a significant correlation between the abundance of CD8+ T-cell clones in the peripheral blood and the incidence of irAEs (P=0.01), particularly grade 2–3 irAEs (P<0.0001), among patients treated with ipilimumab, a CTLA-4 inhibitor (55, 56). Suresh and colleagues reported a significant increase in CD4+ T cells in the BAL samples of CIP patients, primarily those with NSCLC, following treatment with anti-PD-1/PD-L1 inhibitors (57). These findings support a lymphocyte-driven hyperimmune response.
In addition, the expression levels of CTLA-4 and PD-1 on Treg cells in CIP patients were significantly decreased, suggesting an attenuated Treg suppressive phenotype (57, 58). When regulated by ICIs, CTLA-4, a key molecule in Treg cell function, can alter the suppressive capabilities of Treg cells within the tumor immune microenvironment. This alteration may lead to the development of CIP by relieving the immunosuppressive effects of Treg cells (2, 59). Moreover, deficiency of CTLA-4, in addition to PD-1, may further compromise the ability of Treg cells to control the proinflammatory responses of conventional T cells and macrophages, thereby exacerbating the unchecked immune dysregulation observed in CIP (57).
T-cell activation and infiltration within the lung tissue of CIP patients signify enhanced antitumor immunity. However, this heightened immune activity poses a risk of normal tissue damage due to overly aggressive immune reactions (60, 61), which support the hypothesis that ICI-related pneumonitis may be induced when ICI-activated T cells recognize self-peptides or epitopes that are shared between the tumor and the host (62). This hyperresponsive immune state, triggered by T-cell activation and infiltration, may lead to the misidentification of self-antigens in lung tissue, thereby triggering an autoimmune reaction (63).
3.2 Increased preexisting and emerging autoantibodies
An increasing number of studies indicate that the occurrence of CIP may be linked to elevated levels of both preexisting and newly emerging autoantibodies within the human immune system (58, 64). These autoantibodies, which may be present at low concentrations before the initiation of ICI therapy or generated de novo during treatment, are believed to contribute to the pathogenesis of certain nonpulmonary irAEs (58).
PD-1/PD-L1-targeted therapy has been shown to induce Treg dysfunction and foster the generation of pathological autoantibodies, as evidenced in both PD-1-knockout mice and patients undergoing such treatment (65, 66). Adverse effects such as pneumonitis, arthralgia, vitiligo, and hypothyroidism are frequently observed in patients receiving PD-1/PD-L1 inhibitor treatment (67, 68). Additionally, Toi et al. demonstrated that NSCLC patients have preexisting autoantibodies, such as rheumatoid factor (69).
Klocke et al. investigated the role of CTLA-4 by ablating CTLA-4 expression in adult mice and compared the resulting autoimmunity with that observed in congenital CTLA-4 deficiency. They identified autoimmune disease phenotypes, including pneumonitis, accompanied by organ-specific autoantibodies (70). In a retrospective study of 22 patients with CTLA-4 insufficiency and COVID-19, Ochoa et al. examined autoantibodies against type 1 IFNs at baseline. They reported that most patients with CTLA-4 insufficiency and COVID-19 had nonsevere disease and lacked autoantibodies against type 1 Interferons (71). These findings suggest a potential explanation for the risk of developing autoimmune complications in cancer patients during treatment with the CTLA4-blocking checkpoint inhibitor ipilimumab (72).
Emerging evidence implicates multiple distinct autoantibodies in the development of CIP. Notably, CD74, an autoantibody-active protein, functions as an intracellular chaperone for major histocompatibility complex class II (MHC-II) and can stimulate the release of inflammatory mediators. Although primarily intracellular, CD74 is also expressed on the cell membrane of immune cells, including macrophages (73). It serves as a high-affinity receptor for macrophage inhibitory factors, thereby inducing inflammatory mediator release and cell proliferation (74, 75). In normal human lung tissue, CD74 is expressed at modest levels; however, its expression is dramatically increased in lung tissues affected by ICI-induced pneumonitis (76). A large-scale screening study of ICI-treated patients revealed that serum levels of anti-CD74 antibodies were elevated in patients with CIP compared with their pretreatment levels (77). Salahaldin A. Tahir et al. conducted a high-throughput serological analysis of recombinant cDNA expression to investigate autoantibodies in patients receiving ICIs. They reported a significant median 1.34-fold increase in anti-CD74 antibody levels post-ICI treatment in patients with CIP, whereas no significant changes were detected in a comparison group of 20 patients without pneumonitis, suggesting a pathogenic role for CD74 autoantibodies in the development of pneumonitis (78). These findings indicate the pathogenic involvement of CD74 and its autoantibodies in the development of CIP.
3.3 Inflammatory cytokine imbalance
The administration of ICIs can activate T cells, resulting in excessive release of cytokines and potent proinflammatory responses, which in turn promote the development of various adverse events, including CIP. Elevated levels of various cytokines have been observed in patients experiencing irAEs following ICI treatment, highlighting significant changes in cytokine profiles (79). Moreover, certain cytokines have shown promise as predictive biomarkers for irAEs, offering promising avenues for early detection and management strategies.
Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interferon-γ (IFN-γ) collectively contribute to tissue damage and exacerbate the immune-mediated lung injury characteristic of CIP (63, 80, 81). Dysregulated cytokine production disrupts immune homeostasis by initiating inflammatory cascades, which recruit neutrophils and macrophages to injury sites and suppress anti-inflammatory counterregulation, ultimately worsening CIP severity (82–84). Mechanistically, Lin et al. demonstrated that the infiltration of Th2 cells in the BALF of CIP patients drives the overproduction of interleukin clusters (IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13), thereby creating a self-sustaining inflammatory loop (85).
Similarly, other studies have also identified specific cytokines associated with the development of irAEs, including pneumonitis. Lim’s team, who studied melanoma patients receiving ICIs, identified 11 plasma cytokines (including granulocyte–colony-stimulating factor [G-CSF], granulocyte–macrophage colony–stimulating factor [GM–CSF], and IL–13) that dynamically increase before or during the onset of high-grade irAEs (86). Notably, NSCLC studies have revealed both similarities and divergences in cytokine patterns: while elevations in IL-1β and IL-8 align with findings in melanoma, decreased G-CSF has emerged as a unique predictor in lung cancer cohorts (87). This discrepancy underscores microenvironment-driven variations in the cytokine network. Furthermore, a study of 204 NSCLC patients with or without PD-L1 inhibitor monotherapy (43 of whom developed irAEs) revealed a proinflammatory increase in IL-1β and elevated levels of IL-5, IL-8, IL-10, IL-12p70, and granzyme A, along with decreased G-CSF, as predictors of irAEs, including pneumonitis (86). Receptors for IL-8 are present on neutrophils, Tregs, monocytes, and NK cells, indicating their potential involvement in the complex biology of CIP (49, 88). However, the role of IL-5 and IL-10 in lung injury associated with pneumonitis remains uncertain.
IL-6 has garnered significant attention in CIP research because of its potential role in its pathogenesis. One study reported elevated IL-6 levels in CIP patients compared with those at baseline, whereas another examination of BALF cytokines in 12 CIP patients revealed significantly higher IL-6 levels than in controls (89, 90). However, it is important to note that IL-6 elevation is not universal in the BALF of CIP patients (57). Despite this variability, tocilizumab, an IL-6 inhibitor, has shown efficacy in treating steroid-refractory CIP in a single-center study (91). C-reactive protein (CRP), an acute-phase protein secreted by liver cells in response to inflammatory cytokines such as IL-6 and TNF-α, is also relevant. Given the observed elevated IL-6 levels in some CIP patients, it is unsurprising that CRP levels are increased in NSCLC patients who develop CIP postatezolizumab treatment compared with baseline (92, 93). These findings suggest that CIP development may be attributed to excessive immune system activation induced by both CRP and IL-6, along with their potent proinflammatory properties (81).
IL-17, a cytokine with diverse functions, plays crucial roles in autoimmune diseases and inflammation. Its abnormal expression has been implicated in the pathology of various lung diseases, including asthma, pneumonia, and pulmonary fibrosis (94). Lou et al. demonstrated a significant increase in serum IL-17 levels in NSCLC patients who developed CIP following ICI treatment (95). This phenomenon may be attributed to the disruption of immune tolerance and enhanced T-cell activation resulting from PD-1 and PD-L1 blockade (96, 97). A separate analysis of serum and BALF from 13 NSCLC patients with CIP post-PD-1/PD-L1 therapy revealed elevated levels of both IL-17A and IL-35 in these compartments. Furthermore, serum IL-17A levels were found to be positively correlated with the Th17 cell subtype (98). Given that IL-17A is implicated in other autoimmune disorders, acute lung injury, and lung fibrosis, it is plausible that it also contributes to the pathogenesis of CIP (49, 99, 100).
C-X-C chemokines (CXCLs) are pivotal in regulating the differentiation of primary T cells into Th1 cells and facilitating the migration of immune cells to tumor sites through interactions with molecules such as CXCL9, CXCL10, CXCL11, and CXCL13 (101, 102). By assessing 40 cytokines in plasma, Shaheen Khan and colleagues reported significant upregulation of various cytokines, particularly CXCL9, 10, 11, and 13, following ICI treatment, which was closely linked to the onset of irAEs (103).
Despite the variability in underlying tumor histology, host factors, and disease profiles among patients, emerging evidence suggests that cytokine dysregulation is involved in the pathogenesis of CIP. This dysregulation leads to the release of proinflammatory cytokines and the infiltration of immune cells into the lung parenchyma, thereby exacerbating tissue damage and contributing to the development of pneumonitis (81).
3.4 Genetic variants
Given the widespread belief that the development of irAEs is linked to autoimmunity, genetic variations have emerged as a potential contributing factor to this phenomenon. Notably, human leukocyte antigen (HLA) variations, which are crucial at the immune cell interface, have garnered significant attention in recent research. In a cohort of 256 patients undergoing PD-1/PD-L1 treatment, including 29 CIP patients, HLA typing revealed a strong correlation between the incidence of CIP and the germline expression of HLA-B allele 35 and HLA-DRB1 allele 11 (39). These alleles have also been implicated in other autoimmune disorders, further underscoring the potential significance of genetic factors in the pathogenesis of CIP (104). Furthermore, specific genetic polymorphisms have been associated with susceptibility to CIP. These include polymorphisms in genes related to immune regulation and inflammation, such as those encoding gamma-aminobutyric acid type A receptor subunit pi (GABRP), desmocollin, and bromodomain adjacent to zinc finger domain 2B (BRD2B). However, further research is necessary to elucidate their precise roles in the development of CIP (82, 105). Collectively, these findings suggest that genetic variations are likely to contribute to the dysregulation underlying CIP.
3.5 Other possible mechanisms
Hakozaki et al. highlighted disparities in the gut microbiome between advanced NSCLC patients experiencing low- and high-grade irAEs (106). This has prompted a growing focus on the ‘gut–lung axis’, a concept describing the interaction between the gut and respiratory microbiomes and its potential impact on immune responses (107). Although research on respiratory microbiomes in the context of cancer immunotherapy is relatively less extensive than that on gut microbiomes, studies have indicated that anti-PD-1 treatment can modulate the diversity and abundance of specific microbiome populations (108, 109). Notably, Zhang et al. discovered that certain respiratory microbes, particularly enriched Streptococcus, might potentiate antitumor immune responses by enhancing antigen presentation and effector T-cell function, thereby potentially increasing the incidence of irAEs, including CIP (110).
Hsu et al. demonstrated that PD-1-expressing NK cells can mediate immunosuppression in tumor microenvironments by interacting with PD-L1-positive tumor cells. PD-1/PD-L1 blockade can release the cytotoxic potential of NK cells against tumors by increasing PD-1-induced immune inhibition. However, when ICI treatment triggers inflammation in nonmalignant lung tissue via diverse non-NK cell-mediated pathways, activated NK cells can directly eliminate infected cells and secrete proinflammatory cytokines. Consequently, NK cell activation mediated by PD-1/PD-L1 blockade might exacerbate inflammation and contribute to damage in normal lung tissues (111).
4 Imaging manifestations of CIP
In a previous retrospective study, Park et al. (112) initially identified common imaging features of CIP, including ground-glass opacity (GGO), consolidation, reticular density, and traction bronchiectasis. In Pozzessere’s study (113), CIP was categorized into eight distinct types: organizing pneumonia (OP), nonspecific interstitial pneumonia (NSIP), hypersensitivity pneumonia (HP), acute interstitial pneumonia/diffuse alveolar damage (AIP/DAD), bronchiolitis, nodular or mass-like lesions, the unclassifiable type, and sarcoidosis-like manifestations. The predominant distribution pattern of lung injury induced by ICIs is bilateral, involving multiple lobes and segments. However, unilateral or single-lobe lesions may also occur. The lower lobes are most frequently affected. In recurrent cases, the imaging distribution may remain consistent or exhibit migratory characteristics (45, 114).
4.1 Classical imaging manifestations of CIP
The 2021 Fleischner guidelines categorized the CT imaging patterns associated with drug-induced pneumonia into five distinct types (115), four of which can be observed in patients receiving immunotherapy.
4.1.1 Organizing pneumonia (OP)
OP is characterized by multiple patchy areas of increased density, which are typically distributed around bronchovascular bundles and/or in the peripheral lung fields, and may be associated with a reverse halo sign (Figure 3A). Histologically, it is characterized by granulation tissue filling the alveolar ducts and surrounding alveolar spaces, accompanied by inflammatory infiltration of the lung parenchyma (116). Bronchoalveolar lavage revealed a decreased CD4/CD8 T-cell ratio, with an increase of 20% to 40% in activated T lymphocytes (117).

Figure 3. Classic CT imaging of CIP. (A) Organizing pneumonia (OP); (B) Nonspecific interstitial pneumonia (NSIP); (C) Hypersensitivity pneumonia (HP); (D) Acute interstitial pneumonia/diffuse alveolar damage (AIP/DAD).
4.1.2 Nonspecific interstitial pneumonia (NSIP)
NSIP presents with imaging features ranging from patchy ground–glass opacities to irregular reticular shadows, accompanied by destruction of the lung lobe structure and traction bronchiectasis, with or without consolidation (Figure 3B). Lesions typically exhibit bilateral and symmetrical distributions, predominantly involving the lower lobes of the lungs. A distinguishing feature of NSIP compared with OP is the relative sparing of subpleural regions (116). Histologically, NSIP is characterized by lymphocytic and plasma cell infiltration, as well as uniform thickening of the alveolar walls (118).
4.1.3 Hypersensitivity pneumonia (HP)
The HP pattern comprises indistinct centrilobular nodules, ground-glass opacities in both lungs, and large patchy or lobar regions exhibiting mosaic perfusion (Figure 3C). The CTCAE grade for the HP pattern tends to be relatively low, typically ranging from grade 1 to 2 (114). Histologically, it is distinguished by diffuse lymphocytic and plasmacytic infiltration surrounding the bronchi, as well as loose, nonnecrotizing granuloma formation (119).
4.1.4 Diffuse alveolar damage (DAD)
DAD, also referred to as AIP or acute respiratory distress syndrome (ARDS), is characterized by extensive ground–glass opacities with consolidation in both lungs during the exudative phase. The organizing and fibrotic stages are characterized by traction bronchiectasis, whereas in the late stage, there is a notable reduction in lung volume (Figure 3D). Additionally, DAD may present as thickening of the interlobular and intralobular septa, forming a “crazy paving” pattern (120). The clinical manifestations and pulmonary injury associated with the AIP pattern tend to be severe, typically corresponding to a CTCAE grade of 3 or higher (114). Histologically, DAD is characterized by diffuse alveolar damage and pulmonary edema (120).
4.2 Radiation recall pneumonitis (RRP)
In addition to the classic manifestations of CIP, radiation recall pneumonitis (RRP) has attracted increasing interest from oncologists and radiation therapists in recent years. The incidence of RRP has increased since immunotherapy, which is used as a consolidation treatment following chemoradiotherapy for locally advanced NSCLC, was promoted clinically. Some studies report that the incidence of RRP can reach 18.8%, with a median onset time of 450 days post-radiotherapy (116). RRP is characterized by CT-detected pneumonia outside the typical radiation pneumonitis window (4–12 weeks post-RT) and is localized to the irradiated area (Figures 4A, B). Imaging features include patchy ground-glass opacity/consolidation (120). Pathologically, it is characterized by mucosal congestion, leukocyte infiltration, alveolitis, type II alveolar cell hyperplasia, and fibrosis (121). ICI-induced RRP results in radiation field-aligned consolidation/ground-glass opacity with clear lesion-normal tissue borders. ICI-induced RRP mechanisms may involve overactivation of T lymphocytes post-ICI binding to irradiated lung tissue, triggering hypersensitivity; radiation-induced endothelial damage, increasing vascular permeability and localized ICI accumulation in irradiated areas; and synergistic damage to alveolar epithelial cells caused by both therapies (122, 123).
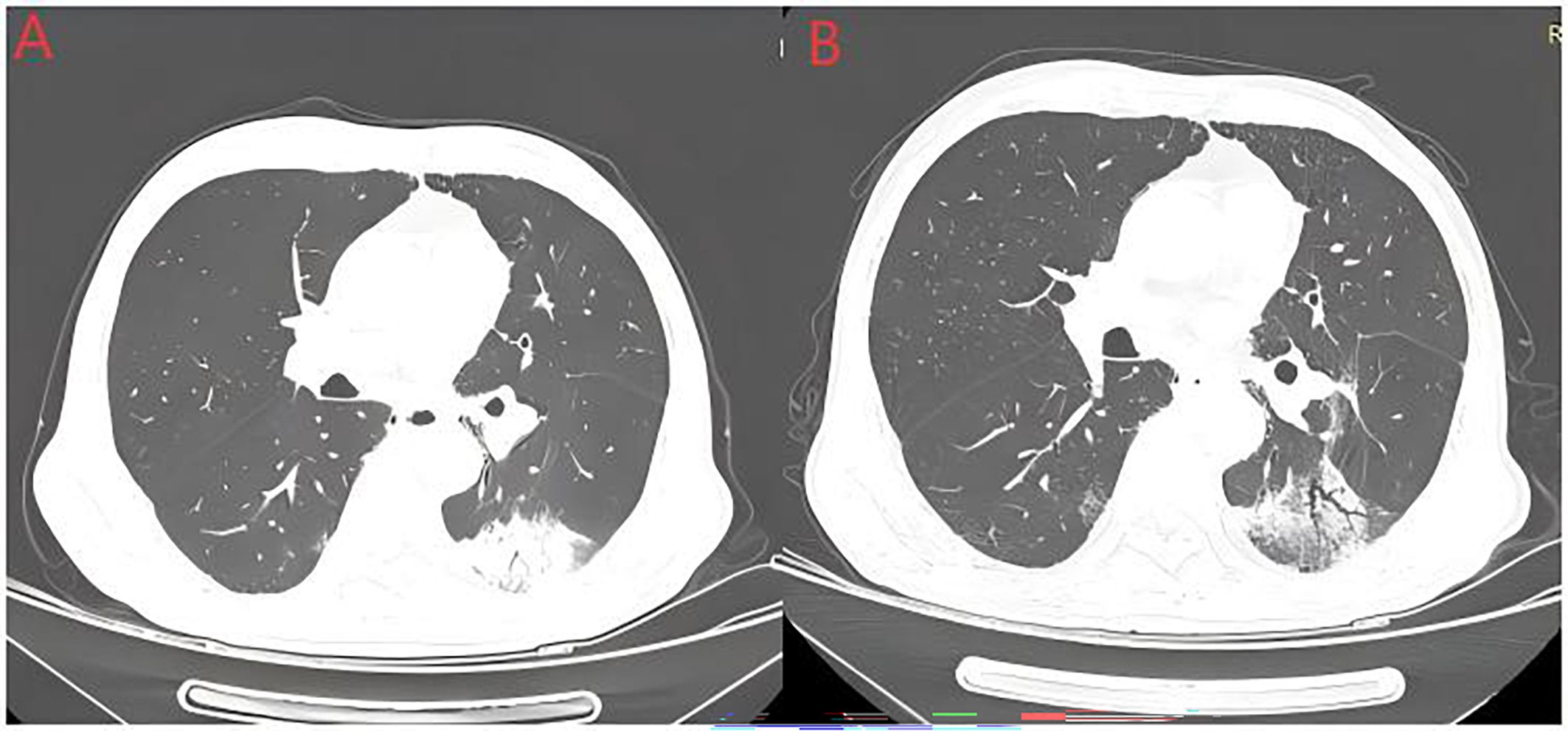
Figure 4. Radiation recall pneumonitis (RRP). The above image shows an elderly man with a history of smoking who was diagnosed with stage IIIB squamous cell carcinoma. He received 4 cycles of albumin-bound paclitaxel combined with carboplatin treatment, followed by radical radiotherapy. Image (A) shows the occurrence of pneumonia after one cycle of tislelizumab treatment following sequential chemoradiotherapy. Image (B) shows partial absorption of pneumonia in the left lower lobe after 2 weeks of methylprednisolone therapy.
4.3 Other rare imaging modalities
Bronchiolitis has been reported exclusively in case studies. Histologically, bronchiolitis is obliterative and characterized by centripetal fibrosis of the submucosa and peribronchial tissues of the terminal and respiratory bronchioles (124). CT findings include thickened bronchial walls and diffuse tree-in-bud opacities in the peripheral lung zones, often accompanied by bronchiectasis or sharply demarcated mosaic attenuation patterns of the lung parenchyma, which are more pronounced during expiration (125). SLR represents a drug-induced multisystem granulomatous reaction that is histologically characterized by noncaseating epithelioid granulomas with multinucleated giant cells at the center and surrounding scattered lymphocytes (126). It occurs in approximately 5% to 7% of patients receiving nivolumab therapy (127). Typical HRCT features include miliary nodules predominantly distributed in the upper and middle lung zones along the lymphatic vessels, which are associated with mediastinal and bilateral hilar lymphadenopathy (128). Most SLR cases require no treatment owing to minimal symptoms. ICI-induced AEP is rare and presents as bilateral ground–glass opacities, consolidation, septal thickening, and pleural effusion on CT. Diagnosis requires elevated eosinophils in the blood and bronchoalveolar fluid. Monitoring blood eosinophils during ICI therapy aids early detection of CIP (129, 130).
Imaging exams are crucial for diagnosing CIP, with manifestations linked to disease progression, hormone therapy response, and prognosis. Studies have shown that organizing pneumonia (OP) is the most common CT pattern in CIP patients (114). Clinically, the severity of CIP follows the order of DAD/AIP > NSIP/HP > OP (131). Capaccione et al. (132) demonstrated through multimodal imaging analysis that the imaging characteristics of CIP patients are significantly associated with the type and duration of immunotherapy. Specifically, CIP linked to anti-PD-1/PD-L1 therapy predominantly exhibits an OP pattern, whereas CIP associated with anti-CTLA-4 therapy typically manifests as an NSIP pattern. Research indicates that individuals in CIP stages G1–2 have higher survival rates than those in G3–4. Hormone therapy benefits HP/OP-like patients, whereas DAD-like patterns show rapid progression and poor treatment response (33, 115).
4.4 CIP with infection
Clinically, patients with CIP often exhibit no specific symptoms, and approximately one-third of them may remain asymptomatic. Therefore, differential diagnosis is essential to exclude other potential conditions, such as pulmonary infection, tumor pseudoprogression, radiation-induced lung injury, and pulmonary edema. Among them, opportunistic pulmonary infections, including tuberculosis (TB), aspergillosis, cytomegalovirus pneumonia (CMVP), and Pneumocystis jirovecii pneumonia (PJP), present significant diagnostic challenges and constitute important differential diagnoses (133–135). A meta-analysis revealed that patients treated with PD-1/PD-L1 inhibitors are not only at risk of developing various types of immune-related pneumonitis but also exhibit an increased incidence of infectious pneumonia compared with patients in the chemotherapy or placebo groups (136). The presence of symptoms such as fever, expectoration, and elevated blood counts may indicate infection in patients. Obstructive pneumonia represents a common type of pulmonary infection among lung cancer patients, with bacterial pathogens being the most prevalent cause. P. jirovecii infection may result in bilateral ground–glass opacities and hypoxemia, whereas viral infections can also cause diffuse pulmonary involvement. Furthermore, several case reports have documented fungal infections, fungal airway disease, and active pulmonary tuberculosis in patients receiving ICI therapy (137–139). Pulmonary infection and CIP may present with overlapping imaging features, making differential diagnosis challenging. Therefore, it is essential to integrate findings from sputum microbiology and serum biomarkers, including Legionella antibodies, Mycoplasma antibodies, (1, 3)-β-D-glucans, Aspergillus galactomannan, Cryptococcus capsular polysaccharide antigen, and interferon-γ release assays, to facilitate accurate differentiation (140). In patients who develop immune checkpoint inhibitor-associated pneumonitis (CIP), particularly those with grade 2 or higher CIP, bronchoscopy and/or bronchoalveolar lavage (BAL) are recommended as initial diagnostic procedures to exclude infectious etiologies. When concurrent infection is suspected, prompt initiation of empirical anti-infective therapy is also warranted (120). In cases where CIP and infectious pneumonia cannot be definitively differentiated, occur concurrently, or are followed by secondary infection, empirical antibiotic therapy should be initiated following appropriate diagnostic evaluation, while simultaneous efforts are made to identify the etiological agent. Moreover, throughout the management of CIP, continuous monitoring is needed for opportunistic infections that may arise as a consequence of immunosuppressive therapy (141).
5 Radiomics in CIP: from basic imaging features to advanced predictive modeling
Early studies focused on the basic imaging features of CIP. With the development of radiomics, subsequent studies explored the links among imaging findings, histopathology, treatment response, and the potential of radiomics for assessing CIP risk, diagnosis, and prognosis. Radiomics extracts high-throughput data from medical images via computational algorithms, transforming qualitative metrics into quantitative metrics. It includes traditional and deep learning-based types. Common feature selection methods in traditional radiomics include least absolute shrinkage and selection operator (LASSO) (142), principal component analysis (PCA) (143), and minimum redundancy maximum relevance (MRMR) (144). However, traditional radiomic approaches may overlook higher-level features during feature extraction. Deep learning-based radiomics uses CNNs and transformers for forward propagation to generate predictive outcomes through data transformation. Backpropagation optimizes network parameters via gradient calculations from prediction errors. This interaction enables automatic feature extraction and advanced radiomic analysis. The CNN remains the classical architecture widely applied in medical imaging, with GoogLeNet/Inception, ResNet, VGGNet, and DenseNet being prominent models in lung cancer radiomics (145).
5.1 Radiomics-driven CIP risk stratification
In 2019, Colen et al. (146) pioneered a radiomic model for predicting CIP in 32 patients. Using 3D Slicer for CT image segmentation, they extracted 1,860 texture features on the basis of histograms and the GLCM. The mRMR method selected predictive features, achieving 100% accuracy (p = 0.0033). However, the small sample size limits the generalizability of the results. Building on Cole’s work, Zhang et al. (147) increased the robustness of the NSCLC prediction model by integrating greyscale-dependent matrix radiomic features. Features were selected via combined MRMR and RFE methods, with added model evaluation techniques. The ICC consistency assessment ensured reliability. Ultimately, the model constructed using the RS, clinical, and SF features demonstrated excellent performance in terms of ROC curves, calibration curves, and DCA. This advancement enables physicians to predict the likelihood of CIP in NSCLC patients with greater speed and accuracy.
Tan et al. (148) developed a multimodal deep learning model using a 3D ResNet18 architecture to predict chemotherapy-induced pneumonia in lung cancer patients. The model integrated nine clinical and radiomic features through 18 convolutional layers and fully connected layers. Compared with two-stage transfer learning with contrastive learning, it achieves an AUC of 0.918 via fivefold cross-validation. Similarly, Cheng et al. (149) developed a multimodal nomogram integrating clinical and deep imaging radiomics to predict CIP risk in 141 lung cancer patients. They optimized ResNet-50-V2 with a feature pyramid network (FPN), showing that the multimodal model outperformed the radiomic-only and clinical-only models, achieving AUCs of 0.910 vs 0.871 vs 0.778 (training set) and 0.900 vs 0.856 vs 0.869 (test set), respectively.
Chen et al. (150) constructed a radiomic prediction vector for lung cancer immunotherapy (LCI-RPV) on the basis of radiomic tumor features from enhanced chest CT scans, CD274 counts in NSCLC patients treated with anti-PD-1/PD-L1 therapy, and RNA expression levels of the PD-L1 protein-coding gene as the response variable. Subgroup analysis revealed that the LCI-RPV could predict CIP in PD-L1 inhibitor patients, with an AUC of 0.74 (95% CI: 0.53–0.95). Thomas et al. pioneered functional lung radiomics in locally advanced NSCLC, assessing its role in pneumonia risk stratification for combined radiation and ICI therapy. Baseline COPD significantly increased pneumonia risk (HR 4.59). COPD was the primary predictor, with a c-index of 0.69 (0.59–0.80) (151).
5.2 Identification of CIPs
The incidence of RP and CIP is greater than that of other types of treatment-related pneumonitis in NSCLC patients. RP is dose dependent with radiation field confinement, whereas CIP presents bilateral multilobe involvement without dose correlation (120, 152, 153). Concurrent RT-ICI-induced pneumonia combines the features of CIP and RP (11, 154) rather than simple superposition. In the PEMBRO-RT trial and PACIFIC study, the incidence of pneumonia in NSCLC patients receiving radiotherapy (RT) and ICI was reported to be as high as 26% to 33.9% (95, 155). Differentiating CIP from RP is crucial for clinical management, guiding steroid dosage and ICI rechallenge or discontinuation (156).
Chen et al. (157) analyzed CT data from 82 NSCLC patients with pneumonia (immunotherapy/radiotherapy/combined therapy). They built a radiomic model using LASSO-selected features and grid search optimization, achieving a training set AUC of 0.76 for distinguishing ICI- and RT-induced pneumonia. Cheng et al. (158) analyzed CT images and clinical data from 73 patients with pneumonia related to ICIs, radiotherapy or combined therapy. Three CT texture features (intensity histogram, GLCM-based, and Bow) were extracted, with the Bow showing the best cross-validation performance (AUC 0.937). Logistic regression, random forest, and linear SVM models underwent 10-fold validation in ICI/radiotherapy-only patients. Testing in combined therapy patients yielded AUCs of 0.765, 0.848, and 0.937, respectively. The optimal model achieved an AUC of 0.896 in the combined treatment cohort, outperforming prior studies. QIU et al. (159) developed a CT radiomic model that integrates radiomic, clinical, and radiological factors to differentiate CIP from RP, with excellent performance (AUC 0.953 and 0.947). Bilateral CT changes were more likely in patients with ICI-induced pneumonia than in those with RP (p<0.001). CIP patients had less well-defined boundaries (p=0.001), which aligns with other studies (157). The radiomic nomogram outperformed the Rad-score in distinguishing CIP from RP.
Wang et al. (160) developed a CT-based radiomic model for RP and CIP patients. They built two models (random forest and linear discriminant classifiers), achieving test cohort AUCs of 0.851 and 0.842, respectively, with 83% accuracy. Additionally, this study identified four highly discriminative features, including one first-order feature, one gray-level run-length matrix (GLRLM)-based feature, one gray-level co-occurrence matrix (GLCM)-based feature, and one neighborhood gray-tone difference matrix (NGTDM)-based feature. Previous studies (161, 162) have shown that multifrequency CT decomposition effectively decodes the underlying phenotypic differences between CIP and RP in patients undergoing RT and ICI therapy.
The De Ruysscher team published a study in 2021 at the European Society for Medical Oncology (ESMO), which distinguished CIP from other causes of pneumonia (163). They extracted 837 radiomic features and used recursive feature elimination (RFE) to select the 42 most correlated features, and the areas under the ROC curves of the clinical, radiomic, and combined models for predicting ICI-R pneumonitis were 0.99, 0.65, and 0.99, respectively.
Early identification and diagnosis of CIP are crucial but complicated by its broad onset window and clinical and radiological heterogeneity. The imaging features of CIP frequently overlap with those of infectious pneumonia. Since the emergence of the COVID-19 pandemic, this diagnostic challenge has become even more significant. Sumeet Hindocha et al (164) developed a rigorously validated machine learning tool capable of differentiating CIP from radiation pneumonitis (RP), COVID-19, and other infectious pneumonia. Compared with radiologists, the models for distinguishing RP from COVID-19, CIP from COVID-19, and CIP from non-COVID-19 interstitial pneumonia (IP) demonstrated superior performance, as evidenced by test set AUCs of 0.92 versus 0.8 and 0.8; 0.68 versus 0.43 and 0.4; and 0.71 versus 0.55 and 0.63, respectively.
Recent research has expanded the application scope of radiomics in the treatment of CIP and improved its value in immunotherapy monitoring. The application of radiomics in the treatment of CIP has gradually expanded from initial risk stratification to automatic identification, differential diagnosis, and prognosis prediction.
6 Management of CIP
On the basis of the Toxicity Management Guidelines for Immune Checkpoint Inhibitor Pneumonitis (CIP) published by the National Comprehensive Cancer Network (NCCN) (165), CIP is categorized into four grades: Grade 1, asymptomatic, lesions are limited to a single lobe or < 25% of the lung parenchyma; Grade 2, new symptoms or worsening of existing symptoms, including shortness of breath, cough, fever, chest pain, and hypoxia; lesions involve multiple lobes or 25% to 50% of the lung parenchyma, affecting daily life, requiring pharmacological intervention; Grade 3, severe new symptoms, lesions involve all lobes or >50% of the lung parenchyma, self-care ability is limited, oxygen is needed, hospitalization is needed for treatment; and Grade 4, life-threatening respiratory distress syndrome, acute respiratory distress syndrome. Current guidelines recommend the management of CIP on the basis of severity grading (166).
6.1 Graded treatment of CIP
For Grade 1 CIP, ICI discontinuation is advised with self-monitoring of symptoms and weekly physical examination and oxygen saturation monitoring during the first 3 weeks. If symptoms occur, chest CT should be implemented in advance. Without clinical progress, a follow-up chest CT after 3–4 weeks should be performed. If imaging shows improvement, resumption of ICI therapy with close follow-up is recommended. In cases of no radiographic improvement, treatment should be escalated to Grade 2, and ICI therapy should be paused.
The recommended approaches for Grade 2 CIP include baseline assessment, such as blood tests (complete blood count, liver and kidney function, electrolytes), and pulmonary function analysis. ICI therapy should be paused until the condition improves to Grade 1 or lower. Intravenous methylprednisolone at 1–2 mg/(kg·d) is advised for 48–72 hours. If symptoms improve, the steroid dose should be tapered over 4–6 weeks and reduced by 5–10 mg weekly. If there is no improvement, treatment should be escalated to Grade 3–4 protocols. If infection cannot be ruled out, empirical antibiotics should be considered. Bronchoscopy with bronchoalveolar lavage is recommended for atypical lesions.
Generally, for Grade 3–4 CIP, ICI therapy should be permanently discontinued. Intravenous methylprednisolone at 2 mg/(kg·d) is recommended. If clinical improvement is observed within 48 hours, continue steroid therapy until symptoms improve to Grade 1 or lower and then taper over 4–6 weeks. Bronchoscopy with bronchoalveolar lavage is advised for atypical lesions, and biopsy may be considered. Empirical antibiotic therapy should be initiated if infection cannot be ruled out. If there is no significant improvement with steroids, alternative therapies such as tocilizumab (8 mg/kg, repeatable after 14 days), infliximab (5 mg/kg, repeatable after 14 days), mycophenolate mofetil (1–1.5 g twice daily), or intravenous immunoglobulin (IVIG) should be considered.
Antimicrobial agents constitute a critical component of the pharmacological management of CIP. Patients who have received antitumor immunotherapy, chemotherapy, radiotherapy, as well as steroid hormones and immunosuppressive therapies for CIP are frequently immunocompromised, rendering them highly susceptible to a range of infectious diseases, including opportunistic infections. Respiratory infections can potentially trigger or worsen CIP, and a subset of patients may concurrently present with both CIP and pulmonary infectious diseases, thereby complicating the clinical management of CIP. In cases of coexisting infection, an individualized antibiotic regimen should be formulated on the basis of the clinical and radiological characteristics of CIP, etiological findings, and the patient’s baseline therapeutic strategy (140). Some experts propose that during the treatment of CIP with steroid hormones and/or immunosuppressants, prophylactic sulfonamide therapy can be administered. In the absence of contraindications, a prophylactic dose of sulfonamide should be routinely administered orally. Common high-risk factors are poorly controlled diabetes mellitus, long-term use of hormones (prednisone equivalent ≥ 20 mg/d) and/or immunosuppressants, bone marrow suppression following oncological treatment, a history of Pneumocystis jirovecii infection, and a peripheral blood CD4+ T-cell count of less than 400 cells/μl (167–170).
6.2 Corticosteroid therapy and escalation strategies for CIP
Corticosteroids are generally recommended as the initial treatment for symptomatic checkpoint inhibitor pneumonia (CIP) patients. Retrospective data indicate that over 90% of Grade 1–2 CPI events can improve or resolve with either the cessation of the drug or the use of corticosteroids, with or without additional interventions. Furthermore, 60%-86% of patients who experience Grade 3 or more severe CIP can achieve remission or cure through corticosteroids or supplementary immunosuppressive therapy (11, 153). Steroid-refractory CIP is defined as the absence of clinical improvement following high-dose corticosteroids administered for 48 hours, which necessitates the introduction of additional immunosuppressive treatments (131).
6.2.1 The occurrence and prognosis of steroid-refractory CIP
According to reported data from relatively large cohorts of CIP patients, the proportion of patients with steroid-refractory CIP ranged from 11.6% (11) to 18.5% (131). KL-6 may serve as a viable screening biomarker for CIP, particularly in NSCLC patients, and could predict steroid responsiveness (171). Immune toxicity has been associated with improved efficacy of immunotherapies. In an advanced NSCLC cohort (171), patients with severe CIP presented the highest overall response rate (ORR) to immune checkpoint inhibitors (ICIs), followed by those with grade 1–2 CIP, compared with non-CIP patients (44.44%, 35.3%, and 28.35%, respectively). However, the prognosis for patients with steroid-refractory CIP is suboptimal. A retrospective cohort study involving 26 patients with steroid-refractory CIP reported a mortality rate of 23% due to pneumonitis and 12% attributed to infections, which may have been associated with immunosuppression (172). Furthermore, the mortality attributable to CIP and/or associated infectious complications was 75% (8/12) in another cohort (131). Compared with steroid-responsive CIP, steroid-refractory CIP is associated with a higher 1-year mortality rate, with a hazard ratio (HR) of 15.1 (95% CI: 3.9–57.8, P < 0.0001) (173).
6.2.2 Advances in the treatment of steroid-refractory CIP
For steroid-refractory CIP, there is currently no unified standardized treatment strategy. Several immunosuppressants, including infliximab, mycophenolate mofetil, intravenous immunoglobulin (IVIG), and cyclophosphamide, are recommended primarily on the basis of their efficacy in treating other steroid-refractory immune-related adverse events (irAEs). However, the efficacy and safety data for these therapies specifically targeting CIP are limited and exhibit significant variability across retrospective studies. Jason Beattie and colleagues reported that among 26 patients receiving additional immunomodulators (tumor necrosis factor-alpha inhibitors and/or mycophenolate) for steroid-refractory CIP, 38% (10/26) achieved durable improvement (172). In another study, Aanika Balaji and colleagues examined 65 patients with CIP, of whom 12 were steroid refractory. Among these patients, those treated with infliximab, with or without IVIG, experienced 100% mortality (5/5 patients), whereas those treated with IVIG alone had a mortality rate of 42.9% (3/7 patients) (131). An earlier study reported improvement in CIP with infliximab in 4 out of 9 patients within a small, retrospective cohort, where bronchoscopy was performed in 67% of cases (172). Given the challenges in differentiating infection from pneumonitis, particularly in severe cases, the mixed efficacy of the aforementioned therapies may be partially attributed to variations in the utility of bronchoscopy.
Agents targeting IL-6R, such as tocilizumab, have been shown to be effective approaches for treating several types of steroid-refractory immune-related adverse events (irAEs) (91), including one case of CIP (174). Clinical trials are currently ongoing to evaluate the safety and efficacy of tocilizumab in combination with ICIs (NCT04940299, NCT03999749). Other reported therapies for steroid-refractory CIP patients include pulse corticosteroids (175), cyclophosphamide (176), and cyclosporine (177).
Given the high mortality associated with severe CIP and the lack of reliable treatments for steroid-refractory cases, there is a pressing need to explore various treatment approaches for these patients in prospective studies. Conducting such trials may be challenging owing to the relatively low incidence of this condition (NCT04438382). Currently, prospective studies are evaluating the role of concurrent immunosuppressants with ICIs in preventing irAEs (NCT04940299, NCT03999749). Notably, the addition of early short-course corticosteroids to ICI therapy has been shown to reduce the incidence of irAEs that lead to ICI discontinuation in patients with NSCLC (5.8% vs. 15.7%, OR = 0.34; 95% CI, 0.12–0.85; p = 0.013) (178). For patients requiring long-term corticosteroid therapy to manage irAEs, prophylaxis against certain opportunistic infections is advisable, even though the incidence of Pneumocystis jirovecii pneumonia (PJP) has been reported to be low (169).
6.3 Advances in the treatment of chronic CIP
Some researchers propose dividing the clinical phenotypes of CIP into acute, subacute, and chronic phases and dividing the 2 pathological processes of CIP into inflammatory, profibrotic, and fibrotic stages (179, 180). Early-onset CIP often occurs within 6 weeks of patients receiving ICI treatment, and the symptoms are generally severe with a poor prognosis. Late-onset CIP typically appears after 6 weeks of ICI treatment, with fewer symptoms and a better prognosis. Chronic CIP is defined as CIP that persists or worsens after the reduction of steroid treatment, and such patients require more than 12 weeks of immunosuppressive therapy after the discontinuation of ICIs (181). During the chronic CIP phase, fibrotic interstitial lung diseases (such as complete damage to normal lung structure, thickening and obstruction of blood vessels) are histologically dominant, which may impair the efficiency of blood–gas exchange in the lungs (182, 183). Typical sequelae may include persistent pulmonary interstitial fibrosis and reduced pulmonary function caused by severe CIP (54). These concepts provide new directions for the treatment of CIP beyond immunosuppressive therapy, namely, antifibrotic treatment. Anti-fibrosis agents such as pirfenidone have demonstrated efficacy (184). In a retrospective study (185), compared with the glucocorticoid-only group, the glucocorticoid-pirfenidone group demonstrated significant improvements in forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), 6-minute walk distance (6MWD), and oxygen saturation without supplemental oxygen (P < 0.05). Among patients with grade 2 immune checkpoint inhibitor-related pneumonia (CIP), those receiving combination therapy exhibited a significantly shorter duration of symptom improvement than those treated with glucocorticoids alone did. Furthermore, following the resumption of immunotherapy, the incidence of CIP recurrence was lower in the glucocorticoid-pirfenidone group than in the glucocorticoid-only group. Nintedanib is a small molecule tyrosine kinase inhibitor that has been approved for the antifibrotic treatment of patients with idiopathic pulmonary fibrosis (IPF) and chronic interstitial lung disease (ILD) (186). Additionally, nintedanib has demonstrated efficacy against solid tumors in multiple clinical trials. For patients with advanced squamous carcinoma, the LUME-Lung 3 study reported that with first-line combination treatment of nintedanib plus cisplatin plus gemcitabine, the disease control rate (DCR) was 81.3%, the median progression-free survival (mPFS) was 4.2 months, and the median overall survival (mOS) was 6.7 months (187). In lung adenocarcinoma, the combination treatment of nintedanib and docetaxel has been approved by the European Union as a first-line chemotherapy regimen (https://www.ema.europa.eu/en/medicines/human/EPAR/vargatef). Several case reports have documented the use of nintedanib for salvage treatment in refractory and chronic CIP patients, with clinical efficacy (186, 188).
6.4 ICI rechallenge in resolved CIP: strategies and long-term vigilance
When grade 1–2 CIP events resolve or revert to grade 1, patients may continue their previous ICI regimen. The recurrence of CIP should be closely monitored, particularly for early-onset irAEs (189). Delaunay et al. reported that among 10 patients with non-small cell lung cancer (NSCLC) who were rechallenged with ICIs after initially managing grade 1–2 CIP with ICI withdrawal or corticosteroids, 3 patients (30%) experienced recurrent interstitial lung disease (ILD) (153). A larger retrospective cohort study revealed that 9 patients (20.0%) developed recurrent pneumonitis, and 11 patients (24.4%) experienced new irAEs among 45 CIP patients who underwent ICI rechallenge (190). A more recent analysis (191) revealed that while ICI rechallenge beyond disease progression did not introduce new safety concerns, it did not improve clinical outcomes in patients with locally advanced or metastatic NSCLC. However, patients who demonstrate a favorable response to initial ICI treatment still benefit from subsequent ICI rechallenge therapy. Similarly, systematic analysis revealed that the incidence rates of all-grade and high-grade (grade 3 or 4) irAEs were not significantly different between initial and readministered ICIs, and no significant difference in overall survival (OS) was observed between the ICI rechallenge and discontinuation cohorts (192). For patients who have recovered from grade 2 pneumonitis, cautious reintroduction of ICIs is advised, particularly for those with a positive response to initial ICIs and without feasible alternative therapies. This resumption may be accompanied by concomitant prednisone treatment, typically at a dosage of 20–30 mg/day, and should be conducted under close monitoring with chest CT (189). Although reintroduction of ICIs has been proven safe in rare and carefully selected cases (193), it is not recommended for patients who have recovered from grade 3 CIP events. For patients with grade 4 CIP, the use of ICIs should be permanently avoided.
Overall, most cases of CIP can be effectively managed through the withholding of ICIs and the administration of corticosteroids. Although severe cases are relatively uncommon, they are associated with poor prognosis and high mortality, underscoring the need for prompt recognition and intervention. Currently, there are no prospective trials evaluating the efficacy of treatments for severe cases; thus, no universal guidelines for optimal therapy beyond corticosteroids exist. For patients who have recovered from CIPs, immunotherapy rechallenge may be feasible, particularly for those who experienced low-grade CIPs and benefit from initial ICI treatment; however, the recurrence of CIP or the emergence of new irAEs should be closely monitored.
7 Conclusion and future points
With the expanding application of immune checkpoint inhibitors (ICIs) in oncology, the real-world incidence of immune checkpoint inhibitor-related pneumonia (CIP) has been progressively increasing. Despite advances in understanding its pathogenesis, imaging features, and therapeutic strategies, the prediction and early diagnosis of CIP remain challenging in clinical practice. The lack of specific imaging patterns and validated biomarkers often leads to delayed diagnosis and treatment, particularly contributing to higher mortality rates among patients with steroid-refractory pneumonia. Emerging studies investigating the underlying mechanisms and potential predictive biomarkers of CIP have identified associations between its occurrence, severity, and response to steroid therapy and various inflammatory mediators, chemokines, autoantibodies, and certain genetic predispositions. Additionally, evidence suggests that the baseline neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are correlated with the risk of immune-related adverse events (irAEs) in patients with solid tumors receiving ICIs (147), whereas the baseline lymphocyte and eosinophil counts have also been linked to irAE development (194). Nevertheless, these biomarkers have not yet been widely adopted in clinical settings, and their diagnostic sensitivity and specificity require further validation through large-scale prospective cohort studies.
Radiomics and machine learning, as critical branches of artificial intelligence in medical imaging, enable the extraction of many quantitative features from high-resolution CT scans. These technologies support the clinical risk stratification of immune checkpoint inhibitor-related pneumonia (CIP) and facilitate its differential diagnosis from other types of pneumonia, such as radiation pneumonitis and infectious pneumonia. Recently, various convolutional neural network (CNN)-based deep learning models and multimodal models that integrate clinical, imaging, and pathological data have demonstrated outstanding classification performance, with area under the curve (AUC) values generally exceeding 0.9 (158, 164), significantly surpassing traditional radiological evaluation methods. For example, Wang et al. utilized deep learning visualization techniques and reported that regions beyond the tumor—such as pleural retraction, pleural effusion, and vascular abnormalities—are crucial for predicting the risk of CIP during immunotherapy (195).
Despite their promising applications in CIP diagnosis and risk prediction, several challenges remain: a lack of standardized protocols for image acquisition; insufficient repeatability and stability in image segmentation, leading to variability in region of interest (ROI) delineation across different software platforms, drawing methods, and observers; and most current studies on CIP are single-center retrospective analyses with limited sample sizes. To validate these findings more robustly, large-scale multicenter prospective studies along with external dataset validation and training are needed. To date, research on CIP has focused primarily on predictive modeling and differential diagnosis, with relatively limited exploration of disease prognosis and therapeutic response. This gap highlights an important direction for future investigations. In model development, the acquisition of high-quality images is fundamental to ensuring model robustness. During radiomic feature extraction, standardization of the slice thickness, imaging protocols, and ROI delineation procedures is essential. Integrating radiomic features with patients’ clinical profiles, cytokine levels, autoantibody panels, and genetic predispositions, followed by advanced bioinformatics analysis, may yield models with enhanced accuracy in diagnosis, prognosis, and outcome prediction (196).
In conclusion, the prediction, diagnosis, and management of immune checkpoint inhibitor-related pneumonitis (CIP) are progressively advancing toward the integration of multiomics biomarkers and imaging artificial intelligence technologies. Future efforts should focus on strengthening interdisciplinary collaboration, validating the clinical utility of novel models through prospective, multicenter studies, and facilitating the broad implementation of these cutting-edge tools in routine clinical practice. Only through such systematic approaches can the mortality and treatment discontinuation risks associated with CIP be effectively mitigated, thereby fully harnessing the therapeutic potential of immunotherapy.
Author contributions
SL: Formal Analysis, Writing – original draft, Investigation. ZG: Formal Analysis, Writing – original draft, Investigation. SAH: Formal Analysis, Writing – original draft, Investigation. JZ: Conceptualization, Writing – review & editing, Investigation. YY: Resources, Writing – original draft, Visualization. QW: Writing – review & editing. XXZ: Writing – review & editing, Validation. XFZ: Investigation, Formal Analysis, Writing – review & editing. RH: Project administration, Writing – review & editing. SYH: Funding acquisition, Conceptualization, Writing – review & editing. JW: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Shanxi Province Science and Technology Department major research and development plan (202202130501016), Fundamental Research Program of Shanxi Province (202303021222152). Shengshu Li acknowledges the support of the China Scholarship Council programme (202508080232).
Acknowledgments
The authors are grateful to the directors (Songyan Han and Jie Wang) and team members of the Department of Respiratory Medicine, The Tumor Hospital of Shanxi Medical University, for the guidance and wonderful mentoring that led to the writing of this paper.
Conflict of interest
Author QW was employed by Xiamen Spacegen Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang H, Zhao Y, Zhang X, Si X, Song P, Xiao Y, et al. Clinical characteristics and management of immune checkpoint inhibitor-related pneumonitis: A single-institution retrospective study. Cancer Med. (2021) 10:188–98. doi: 10.1002/cam4.3600
2. Gong B, Li Y, Guo Y, Wang J, Liu W, Zhou G, et al. The impact of pulmonary artery to ascending aorta diameter ratio progression on the prognosis of NSCLC patients treated with immune checkpoint inhibitors. Front Immunol. (2024) 15:1302233. doi: 10.3389/fimmu.2024.1302233
3. Friedman CF, Proverbs-Singh TA, and Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. (2016) 2:1346–53. doi: 10.1001/jamaoncol.2016.1051
4. Franken A, Van Mol P, Vanmassenhove S, Donders E, Schepers R, Van Brussel T, et al. Single-cell transcriptomics identifies pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in immune checkpoint inhibitor-related pneumonitis. J Immunother Cancer. (2022) 10:e005323. doi: 10.1136/jitc-2022-005323
5. Ma S, Ming Y, Wu J, and Cui G. Cellular metabolism regulates the differentiation and function of T-cell subsets. Cell Mol Immunol. (2024) 21:419–35. doi: 10.1038/s41423-024-01148-8
6. Suresh K, Naidoo J, Lin CT, and Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. (2018) 154:1416–23. doi: 10.1016/j.chest.2018.08.1048
7. Perdigoto AL, Kluger H, and Herold KC. Adverse events induced by immune checkpoint inhibitors. Curr Opin Immunol. (2021) 69:29–38. doi: 10.1016/j.coi.2021.02.002
8. Li M, Spakowicz D, Zhao S, Patel SH, Johns A, Grogan M, et al. Brief report: inhaled corticosteroid use and the risk of checkpoint inhibitor pneumonitis in patients with advanced cancer. Cancer Immunol Immunother. (2020) 69:2403–8. doi: 10.1007/s00262-020-02674-w
9. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, and Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
10. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced nonancedramaon lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. (2019) 37:2518–27. doi: 10.1200/JCO.19.00934
11. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
12. Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, et al. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Med. (2021) 10:1222–39. doi: 10.1002/cam4.3718
13. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. (2019) 10:2006–12. doi: 10.1111/1759-7714.13187
14. Poulose V. Incidence of immune checkpoint inhibitor-related pneumonitis in lung cancer. Chest. (2022) 161:e196–196e197. doi: 10.1016/j.chest.2021.10.041
15. Tiu BC, Zubiri L, Iheke J, Pahalyants V, Theodosakis N, Ugwu-Dike P, et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J Immunother Cancer. (2022) 10:e004670. doi: 10.1136/jitc-2022-004670
16. Moey M, Gougis P, Goldschmidt V, Johnson DB, Lebrun-Vignes B, Moslehi J, et al. Increased reporting of fatal pneumonitis associated with immune checkpoint inhibitors: a WHO pharmacovigilance database analysis. Eur Respir J. (2020) 55:2000038. doi: 10.1183/13993003.00038-2020
17. Gomatou G, Tzilas V, Kotteas E, Syrigos K, and Bouros D. Immune checkpoint inhibitor-related pneumonitis. Respiration. (2020) 99:932–42. doi: 10.1159/000509941
18. Kim S and Lim JU. Immune checkpoint inhibitor-related interstitial lung disease in patients with advanced non-small cell lung cancer: systematic review of characteristics, incidence, risk factors, and management. J Thorac Dis. (2022) 14:1684–95. doi: 10.21037/jtd-22-93
19. Allen E, Umoru G, Ajewole V, and Bernicker E. Incidence and outcome of immune checkpoint-induced pneumonitis in oncology patients with history of pulmonary disease. Front Oncol. (2023) 13:1283360. doi: 10.3389/fonc.2023.1283360
20. Isobe K, Nakamura Y, Sakamoto S, Tomii K, Takimoto T, Miyazaki Y, et al. Immune checkpoint inhibitors in patients with lung cancer having chronic interstitial pneumonia. ERJ Open Res. (2024) 10:00981–2023. doi: 10.1183/23120541.00981-2023
21. Balasubramanian A, Onggo J, Gunjur A, John T, and Parakh S. Immune checkpoint inhibition with chemoradiotherapy in stage III non-small-cell lung cancer: A systematic review and meta-analysis of safety results. Clin Lung Cancer. (2021) 22:74–82. doi: 10.1016/j.cllc.2020.10.023
22. Le T, Minna JD, and Gerber DE. Checkpoint inhibitor pneumonitis: too clinically serious for benefit. J Thorac Oncol. (2019) 14:332–5. doi: 10.1016/j.jtho.2018.12.017
23. Jiang L, Jiang S, Miao W, Shen Y, Bolotina L, Zhu H, et al. Clinical characteristics and management of checkpoint inhibitor pneumonitis in non-small-cell lung cancer patients after neoadjuvant immunotherapy. Clin Lung Cancer. (2025) 26:e91–91e98. doi: 10.1016/j.cllc.2024.10.012
24. Jung J, Kim HY, Kim DG, Park SY, Ko AR, Han JY, et al. Sequential treatment with an immune checkpoint inhibitor followed by a small-molecule targeted agent increases drug-induced pneumonitis. Cancer Res Treat. (2021) 53:77–86. doi: 10.4143/crt.2020.543
25. Sayer MR, Mambetsariev I, Lu KH, Wong CW, Duche A, Beuttler R, et al. Predicting survival of NSCLC patients treated with immune checkpoint inhibitors: Impact and timing of immune-related adverse events and prior tyrosine kinase inhibitor therapy. Front Oncol. (2023) 13:1064169. doi: 10.3389/fonc.2023.1064169
26. Zeng Z, Qu J, Yao Y, Xu F, Lu S, Zhang P, et al. Clinical outcomes and risk factor of immune checkpoint inhibitors-related pneumonitis in non-small cell lung cancer patients with chronic obstructive pulmonary disease. BMC Pulm Med. (2022) 22:458. doi: 10.1186/s12890-022-02190-w
27. Takahara Y, Tanaka T, Ishige Y, Shionoya I, Yamamura K, Sakuma T, et al. Risk factors for acute exacerbation in lung cancer complicated by interstitial lung disease with slight reticular shadows. Thorac Cancer. (2021) 12:2758–66. doi: 10.1111/1759-7714.14121
28. Enomoto Y, Nakamura Y, Colby TV, Johkoh T, Sumikawa H, Nishimoto K, et al. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PloS One. (2017) 12:e0180283. doi: 10.1371/journal.pone.0180283
29. Namba M, Masuda T, Takao S, Terada H, Yamaguchi K, Sakamoto S, et al. Extent of pulmonary fibrosis on high-resolution computed tomography is a prognostic factor in patients with pleuroparenchymal fibroelastosis. Respir Investig. (2020) 58:465–72. doi: 10.1016/j.resinv.2020.05.009
30. Osaki M, Arai T, Sumikawa H, Takimoto T, Takeuchi N, Tamiya A, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer patients with interstitial lung disease: significance of radiological pleuroparenchymal fibroelastosis. Oncology. (2023) 101:303–12. doi: 10.1159/000529204
31. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: A case-control study. Cancer Med. (2018) 7:4115–20. doi: 10.1002/cam4.1579
32. Li Y, Zhang Y, Jia X, Jiang P, Mao Z, Liang T, et al. Effect of immune-related adverse events and pneumonitis on prognosis in advanced non-small cell lung cancer: A comprehensive systematic review and meta-analysis. Clin Lung Cancer. (2021) 22:e889–889e900. doi: 10.1016/j.cllc.2021.05.004
33. Pang L, Xie M, Ma X, Huang A, Song J, Yao J, et al. Clinical characteristics and therapeutic effects of checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. BMC Cancer. (2023) 23:203. doi: 10.1186/s12885-023-10649-0
34. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017) 18:895–903. doi: 10.1016/S1470-2045(17)30380-7
35. Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. (2020) 15:248–57. doi: 10.1016/j.jtho.2019.10.024
36. Barrar F, Sarrar R, Arroyo-Herntho M, Blanco C, Zatarain-Barro ZL, Catalai R, et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol. (2020) 10:570233. doi: 10.3389/fonc.2020.570233
37. Chao Y, Zhou J, Hsu S, Ding N, Li J, Zhang Y, et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl Lung Cancer Res. (2022) 11:295–306. doi: 10.21037/tlcr-22-72
38. Mao Z, Pang G, Huang X, Chen X, Wu J, Xu X, et al. Risk factors of immune checkpoint inhibitor-related pneumonitis after neoadjuvant immunochemotherapy for resectable NSCLC. BMC Pulm Med. (2024) 24:253. doi: 10.1186/s12890-024-03041-6
39. Correale P, Saladino RE, Giannarelli D, Sergi A, Mazzei MA, Bianco G, et al. HLA expression correlates to the risk of immune checkpoint inhibitor-induced pneumonitis. Cells. (2020) 9:1964. doi: 10.3390/cells9091964
40. Eisenbud L. Unresolved issues in hospital dental practice–subject matter for a long debate. J Hosp Dent Pract. (1979) 13:73–6.
41. Kuwana M, Kaburaki J, Arnett FC, Howard RF, Medsger TA Jr, and Wright TM. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheumatol. (1999) 42:465–74. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y
42. Parker B, Urowitz MB, Gladman DD, Lunt M, Donn R, Bae SC, et al. Impact of early disease factors on metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. (2015) 74:1530–6. doi: 10.1136/annrheumdis-2013-203933
43. Correale P, Saladino RE, Giannarelli D, Giannicola R, Agostino R, Staropoli N, et al. Distinctive germline expression of class I human leukocyte antigen (HLA) alleles and DRB1 heterozygosis predict the outcome of patients with non-small cell lung cancer receiving PD-1/PD-L1 immune checkpoint blockade. J Immunother Cancer. (2020) 8:e000733. doi: 10.1136/jitc-2020-000733
44. Silva J MF, Ladomenou F, Carpenter B, Chandra S, Sedlacek P, Formankova R, et al. Allogeneic hematopoietic stem cell transplantation for severe, refractory juvenile idiopathic arthritis. Blood Adv. (2018) 2:777–86. doi: 10.1182/bloodadvances.2017014449
45. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. (2018) 13:1930–9. doi: 10.1016/j.jtho.2018.08.2035
46. Lin X, Lu T, Li S, Xie X, Chen X, Jiang J, et al. Cytomegalovirus infection as an underestimated trigger for checkpoint inhibitor-related pneumonitis in lung cancer: a retrospective study. Clin Transl Oncol. (2021) 23:389–96. doi: 10.1007/s12094-020-02432-5
47. Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. (2022) 13:964442. doi: 10.3389/fimmu.2022.964442
48. Li T, Niu M, Zhou J, Wu K, and Yi M. The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling. Cell Commun Signal. (2024) 22:179. doi: 10.1186/s12964-024-01562-5
49. Ghanbar MI and Suresh K. Pulmonary toxicity of immune checkpoint immunotherapy. J Clin Invest. (2024) 134:e170503. doi: 10.1172/JCI170503
50. Suzuki K, Yanagihara T, Matsumoto K, Kusaba H, Yamauchi T, Ikematsu Y, et al. Immune-checkpoint profiles for T cells in bronchoalveolar lavage fluid of patients with immune-checkpoint inhibitor-related interstitial lung disease. Int Immunol. (2020) 32:547–57. doi: 10.1093/intimm/dxaa022
51. Ohue Y, Kurose K, Nozawa R, Isobe M, Nishio Y, Tanaka T, et al. Survival of lung adenocarcinoma patients predicted from expression of PD-L1, galectin-9, and XAGE1 (GAGED2a) on tumor cells and tumor-infiltrating T cells. Cancer Immunol Res. (2016) 4:1049–60. doi: 10.1158/2326-6066.CIR-15-0266
52. LaFleur MW, Nguyen TH, Coxe MA, Miller BC, Yates KB, Gillis JE, et al. PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat Immunol. (2019) 20:1335–47. doi: 10.1038/s41590-019-0480-4
53. Lin MX, Zang D, Liu CG, Han X, and Chen J. Immune checkpoint inhibitor-related pneumonitis: research advances in prediction and management. Front Immunol. (2024) 15:1266850. doi: 10.3389/fimmu.2024.1266850
54. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer. (2020) 8:e000840. doi: 10.1136/jitc-2020-000840
55. Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. (2016) 113:11919–24. doi: 10.1073/pnas.1611421113
56. Hu X, Ren J, Xue Q, Luan R, Ding D, Tan J, et al. AntiHu3/pnas.16 and antiHu3/pna associated checkpoint inhibitor pneumonitis in nonumonit cell lung cancer: Occurrence, pathogenesis and risk factors (Review). Int J Oncol. (2023) 63:122. doi: 10.3892/ijo.2023.5570
57. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. (2019) 129:4305–15. doi: 10.1172/JCI128654
58. Feng X, Li G, and Li C. Recent advances in the study of immune checkpoint inhibitor-associated pneumonia. Crit Rev Oncol Hematol. (2025) 206:104591. doi: 10.1016/j.critrevonc.2024.104591
59. Ding R, Yu X, Hu Z, Dong Y, Huang H, Zhang Y, et al. Lactate modulates RNA splicing to promote CTLA-4 expression in tumor-infiltrating regulatory T cells. Immunity. (2024) 57:528–40.e6. doi: 10.1016/j.immuni.2024.01.019
60. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. (2020) 26:842–4. doi: 10.1038/s41591-020-0901-9
61. Zhu Y, Yu J, Ren Q, Wu X, Xu H, Tian T, et al. Clinical characteristics and biomarkers of severe immune checkpoint inhibitor-related pneumonitis triggered by immunotherapy followed by radiation: a case report. Front Immunol. (2024) 15:1454114. doi: 10.3389/fimmu.2024.1454114
62. Guo C, Zhang Q, Zhou P, Cheng Y, Nie L, and Wang G. The application of bronchoscopy in the assessment of immune checkpoint inhibitor-related pneumonitis severity and recurrence. Sci Rep. (2024) 14:17137. doi: 10.1038/s41598-024-66768-6
63. Sakowska J, Arcimowicz Łr, Jankowiak M, Papak I, Markiewicz A, Dziubek K, et al. Autoimmunity and cancer-two sides of the same coin. Front Immunol. (2022) 13:793234. doi: 10.3389/fimmu.2022.793234
64. Pan S, Xie H, Wang L, Wang Y, Zou M, Xu Y, et al. Case report: Checkpoint inhibitor pneumonitis with positive anti-melanoma differentiation-associated gene 5 antibodies in a patient with lung cancer. Front Immunol. (2023) 14:1309531. doi: 10.3389/fimmu.2023.1309531
65. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. (2003) 9:1477–83. doi: 10.1038/nm955
66. Cai J, Wang D, Zhang G, and Guo X. The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther. (2019) 12:8437–45. doi: 10.2147/OTT.S221340
67. Khoja L, Day D, Wei-Wu Chen T, Siu LL, and Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
68. Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, and Manne A. Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
69. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860
70. Klocke K, Sakaguchi S, Holmdahl R, and Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. (2016) 113:E2383–92. doi: 10.1073/pnas.1603892113
71. Ochoa S, Abers MS, Rosen LB, Rump A, Howe K, Lieberman JA, et al. Management and outcome of COVID-19 in CTLA-4 insufficiency. Blood Adv. (2023) 7:5743–51. doi: 10.1182/bloodadvances.2023010105
72. Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. (2020) 26:1218–23. doi: 10.1038/s41591-020-0979-0
73. Bonnin E, Rodrigo Riestra M, Marziali F, Mena Osuna R, Denizeau J, Maurin M, et al. CD74 supports accumulation and function of regulatory T cells in tumors. Nat Commun. (2024) 15:3749. doi: 10.1038/s41467-024-47981-3
74. Su H, Na N, Zhang X, and Zhao Y. The biological function and significance of CD74 in immune diseases. Inflammation Res. (2017) 66:209–16. doi: 10.1007/s00011-016-0995-1
75. Zhang L, Woltering I, Holzner M, Brandhofer M, Schaefer CC, Bushati G, et al. CD74 is a functional MIF receptor on activated CD4(+) T cells. Cell Mol Life Sci. (2024) 81:296. doi: 10.1007/s00018-024-05338-5
76. Ando H, Suzuki K, and Yanagihara T. Insights into potential pathogenesis and treatment options for immune-checkpoint inhibitor-related pneumonitis. Biomedicines. (2021) 9:1484. doi: 10.3390/biomedicines9101484
77. Punt C, Huiskens J, van Gulik T, and Engelbrecht M. Pseudoprogression on bevacizumab treatment: tumor-dynamics in the modern era of systemic treatment for metastatic colorectal cancer. Acta Oncol. (2018) 57:681–2. doi: 10.1080/0284186X.2017.1374556
78. Tahir SA, Gao J, Miura Y, Blando J, Tidwell R, Zhao H, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. (2019) 116:22246–51. doi: 10.1073/pnas.1908079116
79. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br J Cancer. (2019) 120:63–8. doi: 10.1038/s41416-018-0155-1
80. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
81. Yin J, Wu Y, Yang X, Gan L, and Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front Immunol. (2022) 13:830631. doi: 10.3389/fimmu.2022.830631
82. Riondino S, Rosenfeld R, Formica V, Morelli C, Parisi G, Torino F, et al. Effectiveness of immunotherapy in non-small cell lung cancer patients with a diagnosis of COPD: is this a hidden prognosticator for survival and a risk factor for immune-related adverse events. Cancers (Basel). (2024) 16:1251. doi: 10.3390/cancers16071251
83. Riyaz Tramboo S, Elkhalifa A, Quibtiya S, Ali SI, Nazir Shah N, Taifa S, et al. The critical impacts of cytokine storms in respiratory disorders. Heliyon. (2024) 10:e29769. doi: 10.1016/j.heliyon.2024.e29769
84. Zheng J, Li Y, Kong X, and Guo J. Exploring immune-related pathogenesis in lung injury: Providing new insights Into ALI/ARDS. BioMed Pharmacother. (2024) 175:116773. doi: 10.1016/j.biopha.2024.116773
85. Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. (2021) 11:698832. doi: 10.3389/fonc.2021.698832
86. Schindler H, Lusky F, Daniello L, Elshiaty M, Gaissmaier L, Benesova K, et al. Serum cytokines predict efficacy and toxicity, but are not useful for disease monitoring in lung cancer treated with PD-(L)1 inhibitors. Front Oncol. (2022) 12:1010660. doi: 10.3389/fonc.2022.1010660
87. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795
88. Matsushima K, Yang D, and Oppenheim JJ. Interleukin-8: An evolving chemokine. Cytokine. (2022) 153:155828. doi: 10.1016/j.cyto.2022.155828
89. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, and Walker PR. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol. (2018) 57:705–8. doi: 10.1080/0284186X.2017.1406668
90. Kowalski B, Valaperti A, Bezel P, Steiner UC, Scholtze D, Wieser S, et al. Analysis of cytokines in serum and bronchoalveolar lavage fluid in patients with immune-checkpoint inhibitor-associated pneumonitis: a cross-sectional case-control study. J Cancer Res Clin Oncol. (2022) 148:1711–20. doi: 10.1007/s00432-021-03750-z
91. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. (2019) 25:551–7. doi: 10.1177/1078155217745144
92. Del Giudice M and Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. (2018) 70:61–75. doi: 10.1016/j.bbi.2018.02.013
93. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020) 75:1730–41. doi: 10.1111/all.14238
94. Iwanaga N and Kolls JK. Updates on T helper type 17 immunity in respiratory disease. Immunology. (2019) 156:3–8. doi: 10.1111/imm.13006
95. Luo W, Wang Z, Tian P, and Li W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. (2018) 144:1851–9. doi: 10.1007/s00432-018-2707-4
96. Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J Exp Med. (2021) 218:e20182232. doi: 10.1084/jem.20182232
97. Liu R, Li HF, and Li S. PD-1-mediated inhibition of T cell activation: Mechanisms and strategies for cancer combination immunotherapy. Cell Insight. (2024) 3:100146. doi: 10.1016/j.cellin.2024.100146
98. Wang YN, Lou DF, Li DY, Jiang W, Dong JY, Gao W, et al. Elevated levels of IL-17A and IL-35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Oncol Lett. (2020) 20:611–22. doi: 10.3892/ol.2020.11618
99. Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. (2011) 187:3003–14. doi: 10.4049/jimmunol.1004081
100. Xiao H, Peng L, Jiang D, Liu Y, Zhu L, Li Z, et al. IL-17A promotes lung fibrosis through impairing mitochondrial homeostasis in type II alveolar epithelial cells. J Cell Mol Med. (2022) 26:5728–41. doi: 10.1111/jcmm.17600
101. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. (2018) 63:40–7. doi: 10.1016/j.ctrv.2017.11.007
102. Zhou C, Gao Y, Ding P, Wu T, and Ji G. The role of CXCL family members in different diseases. Cell Death Discov. (2023) 9:212. doi: 10.1038/s41420-023-01524-9
103. Deckers J, Anbergen T, Hokke AM, de Dreu A, Schrijver DP, de Bruin K, et al. Engineering cytokine therapeutics. Nat Rev Bioeng. (2023) 1:286–303. doi: 10.1038/s44222-023-00030-y
104. Kohansal Vajari M, Ehsan M, Ghiasi S, Fooladi S, Karami N, Hassanshahi G, et al. Human leukocyte antigen alleles (HLA-A, HLA-B, and HLA-DRB1) are associated with acute lymphoblastic leukemia (ALL): A case-control study in a sample of Iranian population. Asian Pac J Cancer Prev. (2024) 25:1507–13. doi: 10.31557/APJCP.2024.25.5.1507
105. Abdel-Wahab N, Diab A, Yu RK, Futreal A, Criswell LA, Tayar JH, et al. Genetic determinants of immune-related adverse events in patients with melanoma receiving immune checkpoint inhibitors. Cancer Immunol Immunother. (2021) 70:1939–49. doi: 10.1007/s00262-020-02797-0
106. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. (2020) 8:1243–50. doi: 10.1158/2326-6066.CIR-20-0196
107. Zhang YB, Liu SJ, Hu ZD, Zhou JX, Wang YZ, Fang B, et al. Increased Th17 activation and gut microbiota diversity are associated with pembrolizumab-triggered tuberculosis. Cancer Immunol Immunother. (2020) 69:2665–71. doi: 10.1007/s00262-020-02687-5
108. Dora D, Ligeti B, Kovacs T, Revisnyei P, Galffy G, Dulka E, et al. Non-small cell lung cancer patients treated with Anti-PD1 immunotherapy show distinct microbial signatures and metabolic pathways according to progression-free survival and PD-L1 status. Oncoimmunology. (2023) 12:2204746. doi: 10.1080/2162402X.2023.2204746
109. Li B, Wang D, Zhang C, Wang Y, Huang Z, Yang L, et al. Role of respiratory system microbiota in development of lung cancer and clinical application. Imeta. (2024) 3:e232. doi: 10.1002/imt2.232
110. Zhang C, Wang J, Sun Z, Cao Y, Mu Z, and Ji X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. (2021) 112:3005–17. doi: 10.1111/cas.14979
111. Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. (2018) 128:4654–68. doi: 10.1172/JCI99317
112. Park H, Sholl LM, Hatabu H, Awad MM, and Nishino M. Imaging of precision therapy for lung cancer: current state of the art. Radiology. (2019) 293:15–29. doi: 10.1148/radiol.2019190173
113. Pozzessere C, Lazor R, Jumeau R, Peters S, Prior JO, and Beigelman-Aubry C. Imaging features of pulmonary immune-related adverse events. J Thorac Oncol. (2021) 16:1449–60. doi: 10.1016/j.jtho.2021.05.017
114. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. (2016) 22:6051–60. doi: 10.1158/1078-0432.CCR-16-1320
115. Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the fleischner society. Chest. (2021) 159:1107–25. doi: 10.1016/j.chest.2020.11.027
116. Oliveira DS, Araive Filho JA, Paiva A, Ikari ES, Chate RC, and Nomura CH. Idiopathic interstitial pneumonias: review of the latest American Thoracic Society/European Respiratory Society classification. Radiol Bras. (2018) 51:321–7. doi: 10.1590/0100-3984.2016.0134
117. Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S, and Koumarianou A. A case of organizing pneumonia (OP) associated with pembrolizumab. Drug Target Insights. (2016) 10:9–12. doi: 10.4137/DTI.S31565
118. Chinese Thoracic Society CMA and Chinese Association of Chest Physician CMDA. Chinese expert consensus on multidisciplinary discussion of interstitial lung disease. Zhonghua Jie He He Hu Xi Za Zhi. (2023) 46:1176–88. doi: 10.3760/cma.j.cn112147-20230726-00030
119. Liu S. and Wang X. G. Interpretation and prospect of the guidelines for the diagnosis of hypersensitivity pneumonitis in adults by the ATS, The JRS and the LATS in 2020. Zhonghua Jie He He Hu Xi Za Zhi. (2020) 43:1011–4. doi: 10.3760/cma.j.cn112147-20200727-00852
120. Kalisz KR, Ramaiya NH, Laukamp KR, and Gupta A. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. (2019) 39:1923–37. doi: 10.1148/rg.2019190036
121. McGovern K, Ghaly M, Esposito M, Barnaby K, and Seetharamu N. Radiation recall pneumonitis in the setting of immunotherapy and radiation: a focused review. Future Sci OA. (2019) 5:FSO378. doi: 10.2144/fsoa-2018-0123
122. Kitani H, Kosaka T, Fujihara T, Lindquist K, and Elkind MM. The "recall effect" in radiotherapy: is subeffective, reparable damage involved. Int J Radiat Oncol Biol Phys. (1990) 18:689–95. doi: 10.1016/0360-3016(90)90078-x
123. Teng F, Li M, and Yu J. Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications. BMC Med. (2020) 18:275. doi: 10.1186/s12916-020-01718-3
124. Balagani A, Arain M, and Sheshadri A. Bronchiolitis obliterans after combination immunotherapy with pembrolizumab and ipilimumab. J Immunotherapy Precis Oncol. (2018) 1:49. doi: 10.4103/JIPO.JIPO_8_18
125. Domblides M, Geier M, Decroisette C, and Descourt R. Durvalumab-induced lesions of bronchiolitis and fully reversible bronchiectasis in a patient with non-small cell lung cancer: A case report. Thorac Cancer. (2021) 12:1240–3. doi: 10.1111/1759-7714.13862
126. Gkiozos I, Kopitopoulou A, Kalkanis A, Vamvakaris IN, Judson MA, and Syrigos KN. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. (2018) 13:1076–82. doi: 10.1016/j.jtho.2018.04.031
127. Montaudia H, Pradelli J, Passeron T, Lacour JP, and Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. (2017) 176:1060–3. doi: 10.1111/bjd.14808
128. Chorti E, Kanaki T, Zimmer L, Hadaschik E, Ugurel S, Gratsias E, et al. Drug-induced sarcoidosis-like reaction in adjuvant immunotherapy: Increased rate and mimicker of metastasis. Eur J Cancer. (2020) 131:18–26. doi: 10.1016/j.ejca.2020.02.024
129. Jodai T, Yoshida C, Sato R, Kakiuchi Y, Sato N, Iyama S, et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-PD-1 immune checkpoint antibody in a lung cancer patient. Immun Inflammation Dis. (2019) 7:3–6. doi: 10.1002/iid3.238
130. Hara K, Yamasaki K, Tahara M, Kimuro R, Yamaguchi Y, Suzuki Y, et al. Immune checkpoint inhibitors-induced eosinophilic pneumonia: A case report. Thorac Cancer. (2021) 12:720–4. doi: 10.1111/1759-7714.13848
131. Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. (2021) 9:e001731. doi: 10.1136/jitc-2020-001731
132. Capaccione KM, Valiplackal JP, Huang A, Roa T, Fruauff A, Liou C, et al. Checkpoint inhibitor immune-related adverse events: A multimodality pictorial review. Acad Radiol. (2022) 29:1869–84. doi: 10.1016/j.acra.2022.03.007
133. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–119iv142. doi: 10.1093/annonc/mdx225
134. Picchi H, Mateus C, Chouaid C, Besse B, Marabelle A, Michot JM, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect. (2018) 24:216–8. doi: 10.1016/j.cmi.2017.12.003
135. Inthasot V, Bruyneel M, Muylle I, and Ninane V. Severe pulmonary infections complicating nivolumab treatment for lung cancer: a report of two cases. Acta Clin Belg. (2020) 75:308–10. doi: 10.1080/17843286.2019.1629078
136. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front Immunol. (2019) 10:108. doi: 10.3389/fimmu.2019.00108
137. Kyi C, Hellmann MD, Wolchok JD, Chapman PB, and Postow MA. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer. (2014) 2:19. doi: 10.1186/2051-1426-2-19
138. Fujita K, Terashima T, and Mio T. Anti-PD1 antibody treatment and the development of acute pulmonary tuberculosis. J Thorac Oncol. (2016) 11:2238–40. doi: 10.1016/j.jtho.2016.07.006
139. Uchida N, Fujita K, Nakatani K, and Mio T. Acute progression of aspergillosis in a patient with lung cancer receiving nivolumab. Respirol Case Rep. (2018) 6:e00289. doi: 10.1002/rcr2.289
140. Chinese Thoracic Society CMA, Association ORDCoCA, and Association BCPaTR. Chinese experts consensus on the diagnosis and management of checkpoint inhibitor pneumonitis(2025). Zhonghua Jie He He Hu Xi Za Zhi. (2025) 48:329–43. doi: 10.3760/cma.j.cn112147-20241121-00694
141. Wang H, Guo X, Zhou J, Duan L, Si X, Zhang L, et al. Clinical diagnosis and treatment recommendations for the pneumonitis associated with immune checkpoint inhibitor. Zhongguo Fei Ai Za Zhi. (2019) 22:621–6. doi: 10.3779/j.issn.1009-3419.2019.10.03
142. Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics signature: A potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology. (2016) 281:947–57. doi: 10.1148/radiol.2016152234
143. Zhang Y, Oikonomou A, Wong A, Haider MA, and Khalvati F. Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep. (2017) 7:46349. doi: 10.1038/srep46349
144. Yang F, Zhang J, Zhou L, Xia W, Zhang R, Wei H, et al. CT-based radiomics signatures can predict the tumor response of non-small cell lung cancer patients treated with first-line chemotherapy and targeted therapy. Eur Radiol. (2022) 32:1538–47. doi: 10.1007/s00330-021-08277-y
145. Zhou Y, Xu X, Song L, Wang C, Guo J, Yi Z, et al. The application of artificial intelligence and radiomics in lung cancer. Precis Clin Med. (2020) 3:214–27. doi: 10.1093/pcmedi/pbaa028
146. Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs. (2018) 36:601–7. doi: 10.1007/s10637-017-0524-2
147. Zhang GY, Du XZ, Xu R, Chen T, Wu Y, Wu XJ, et al. Development and validation of a machine learning-based model using CT radiomics for predicting immune checkpoint inhibitor-related pneumonitis in patients with NSCLC receiving anti-PD1 immunotherapy: A multicenter retrospective caseControl study. Acad Radiol. (2024) 31:2128–43. doi: 10.1016/j.acra.2023.10.039
148. Tan P, Huang W, Wang L, Deng G, Yuan Y, Qiu S, et al. Deep learning predicts immune checkpoint inhibitor-related pneumonitis from pretreatment computed tomography images. Front Physiol. (2022) 13:978222. doi: 10.3389/fphys.2022.978222
149. Cheng M, Lin R, Bai N, Zhang Y, Wang H, Guo M, et al. Deep learning for predicting the risk of immune checkpoint inhibitor-related pneumonitis in lung cancer. Clin Radiol. (2023) 78:e377–377e385. doi: 10.1016/j.crad.2022.12.013
150. Chen M, Lu H, Copley SJ, Han Y, Logan A, Viola P, et al. A novel radiogenomics biomarker for predicting treatment response and pneumotoxicity from programmed cell death protein or ligand-1 inhibition immunotherapy in NSCLC. J Thorac Oncol. (2023) 18:718–30. doi: 10.1016/j.jtho.2023.01.089
151. Thomas H, Hippe DS, Forouzannezhad P, Sasidharan BK, Kinahan PE, Miyaoka RS, et al. Radiation and immune checkpoint inhibitor-mediated pneumonitis risk stratification in patients with locally advanced non-small cell lung cancer: role of functional lung radiomics. Discov Oncol. (2022) 13:85. doi: 10.1007/s12672-022-00548-4
152. Tsujino K, Hashimoto T, Shimada T, Yoden E, Fujii O, Ota Y, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol. (2014) 9:983–90. doi: 10.1097/JTO.0000000000000187
153. Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. (2017) 50:1700050. doi: 10.1183/13993003.00050-2017
154. Tsoutsou PG and Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. (2006) 66:1281–93. doi: 10.1016/j.ijrobp.2006.08.058
155. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
156. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
157. Chen X, Sheikh K, Nakajima E, Lin CT, Lee J, Hu C, et al. Radiation versus immune checkpoint inhibitor associated pneumonitis: distinct radiologic morphologies. Oncologist. (2021) 26:e1822–1822e1832. doi: 10.1002/onco.13900
158. Cheng J, Pan Y, Huang W, Huang K, Cui Y, Hong W, et al. Differentiation between immune checkpoint inhibitor-related and radiation pneumonitis in lung cancer by CT radiomics and machine learning. Med Phys. (2022) 49:1547–58. doi: 10.1002/mp.15451
159. Qiu Q, Xing L, Wang Y, Feng A, and Wen Q. Development and validation of a radiomics nomogram using computed tomography for differentiating immune checkpoint inhibitor-related pneumonitis from radiation pneumonitis for patients with non-small cell lung cancer. Front Immunol. (2022) 13:870842. doi: 10.3389/fimmu.2022.870842
160. Peiliang Wang MD, Yikun Li MM, Mengyu Zhao MM, Jinming Yu MD, and Feifei Teng MD. Distinguishing immune checkpoint inhibitor-related pneumonitis from radiation pneumonitis by CT radiomics features in non-small cell lung cancer. Int Immunopharmacol. (2024) 128:111489. doi: 10.1016/j.intimp.2024.111489
161. Ha S. Perspectives in radiomics for personalized medicine and theranostics. Nucl Med Mol Imaging. (2019) 53:164–6. doi: 10.1007/s13139-019-00578-x
162. Espinasse M, Pitre-Champagnat S, Charmettant B, Bidault F, Volk A, Balleyguier C, et al. CT texture analysis challenges: influence of acquisition and reconstruction parameters: A comprehensive review. Diagnostics (Basel). (2020) 10:258. doi: 10.3390/diagnostics10050258
163. Tohidinezhad F, Traverso A, Zhovannik I, Romita A, Hendriks L, Dingemans A, et al. 1283P Predicting the differential diagnosis of pneumonitis in stage IV non-small cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs) by combining clinical and radiomic features. Ann OF Oncol. (2021) 32:S997. doi: 10.1016/j.annonc.2021.08.1885
164. Hindocha S, Hunter B, Linton-Reid K, George Charlton T, Chen M, Logan A, et al. Validated machine learning tools to distinguish immune checkpoint inhibitor, radiotherapy, COVID-19 and other infective pneumonitis. Radiother Oncol. (2024) 195:110266. doi: 10.1016/j.radonc.2024.110266
165. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:387–405. doi: 10.6004/jnccn.2022.0020
166. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
167. Chang HL, Wei PJ, Wu KL, Huang HL, and Yang CJ. Checkpoint inhibitor pneumonitis mimicking COVID-19 infection during the COVID-19 pandemic. Lung Cancer. (2020) 146:376–7. doi: 10.1016/j.lungcan.2020.06.013
168. Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA, et al. Treatment of community-acquired pneumonia in immunocompromised adults: A consensus statement regarding initial strategies. Chest. (2020) 158:1896–911. doi: 10.1016/j.chest.2020.05.598
169. Shah NJ, Cook MR, Wu T, Lev-Ari S, Blackburn MJ, Serzan MT, et al. The risk of opportunistic infections and the role of antibiotic prophylaxis in patients on checkpoint inhibitors requiring steroids. J Natl Compr Canc Netw. (2022) 20:800–7.e1. doi: 10.6004/jnccn.2022.7020
170. Fan B, Sun X, Han W, Zou Y, Chen F, Lan F, et al. Immune checkpoint inhibitor increased mortality in lung cancer patients with Pneumocystis jirovecii pneumonia: a comparative retrospective cohort study. Front Oncol. (2024) 14:1398357. doi: 10.3389/fonc.2024.1398357
171. Yang J, Lyu M, Feng X, Liu F, Zeng R, Sun X, et al. The predict factors and clinical prognosis value of immune-related pneumonia of receiving PD-1 inhibitor in advanced non-small cell lung cancer: A retrospective study. Int Immunopharmacol. (2024) 142:113140. doi: 10.1016/j.intimp.2024.113140
172. Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin IH, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. (2021) 9:e001884. doi: 10.1136/jitc-2020-001884
173. Soto-Lanza F, Glick L, Chan C, Zhong L, Wilson N, Faiz S, et al. Long-term clinical, radiological, and mortality outcomes following pneumonitis in nonsmall cell lung cancer patients receiving immune checkpoint inhibitors: A retrospective analysis. Clin Lung Cancer. (2024) 25:624–33.e2. doi: 10.1016/j.cllc.2024.07.017
174. Fa'ak F, Buni M, Falohun A, Lu H, Song J, Johnson DH, et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J Immunother Cancer. (2023) 11:e006814. doi: 10.1136/jitc-2023-006814
175. Lai KC, Hsiao YH, and Chen SC. Pulse corticosteroid therapy in the treatment of steroid-refractory immune checkpoint inhibitor-related pneumonitis: Case report and review. Front Immunol. (2022) 13:994064. doi: 10.3389/fimmu.2022.994064
176. Camard M, Besse B, Cariou PL, Massayke S, Laparra A, Noel N, et al. Prevalence and outcome of steroid-resistant/refractory pneumonitis induced by immune checkpoint inhibitors. Respir Med Res. (2022) 82:100969. doi: 10.1016/j.resmer.2022.100969
177. Deng J, Guan W, Hu M, Deng H, Mo W, Li R, et al. Cyclosporine successfully treats steroid-resistant checkpoint inhibitor-related pneumonitis: a case report. BMC Pulm Med. (2024) 24:577. doi: 10.1186/s12890-024-03258-5
178. Huang DD, Liao BC, Hsu WH, Yang CY, Lin YT, Wu SG, et al. Effects of early short-course corticosteroids on immune-related adverse events in non-small cell lung cancer patients receiving immune checkpoint inhibitors. Oncology. (2024) 102:318–26. doi: 10.1159/000534350
179. Imran S, Golden A, Feinstein M, Plodkowski A, Bodd F, Rekhtman N, et al. Immune check-point inhibitor-related pneumonitis: acute lung injury with rapid progression and organising pneumonia with less severe clinical disease. Histopathology. (2022) 81:724–31. doi: 10.1111/his.14704
180. Zhou C, Yang Y, Lin X, Fang N, Chen L, Jiang J, et al. Proposed clinical phases for the improvement of personalized treatment of checkpoint inhibitor-related pneumonitis. Front Immunol. (2022) 13:935779. doi: 10.3389/fimmu.2022.935779
181. Huang A, Xu Y, Zang X, Wu C, Gao J, Sun X, et al. Radiographic features and prognosis of early- and late-onset non-small cell lung cancer immune checkpoint inhibitor-related pneumonitis. BMC Cancer. (2021) 21:634. doi: 10.1186/s12885-021-08353-y
182. Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med. (2020) 17:599–611. doi: 10.20892/j.issn.2095-3941.2020.0102
183. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in north carolina. Chest. (2021) 160:731–42. doi: 10.1016/j.chest.2021.02.032
184. Miao K, Xu Y, Xu W, Zhang Y, Xu Y, Tian X, et al. Treatment of steroid-resistant checkpoint inhibitor pneumonitis with pirfenidone: A case report. Thorac Cancer. (2021) 12:2214–6. doi: 10.1111/1759-7714.13921
185. Li Y, Huang H, Ye X, Zeng B, Huang F, and Chen L. A retrospective study of combination therapy with glucocorticoids and pirfenidone for PD-1 inhibitor-related immune pneumonitis. Med (Baltimore). (2024) 103:e37808. doi: 10.1097/MD.0000000000037808
186. Pan L, Meng F, Wang W, Wang XH, Shen H, Bao P, et al. Nintedanib in an elderly non-small-cell lung cancer patient with severe steroid-refractory checkpoint inhibitor-related pneumonitis: A case report and literature review. Front Immunol. (2022) 13:1072612. doi: 10.3389/fimmu.2022.1072612
187. Forster M, Hackshaw A, De Pas T, Cobo M, Garrido P, Summers Y, et al. A phase I study of nintedanib combined with cisplatin/gemcitabine as first-line therapy for advanced squamous non-small cell lung cancer (LUME-Lung 3). Lung Cancer. (2018) 120:27–33. doi: 10.1016/j.lungcan.2018.03.007
188. Xie XH, Deng HY, Lin XQ, Wu JH, Liu M, Xie ZH, et al. Case report: nintedanib for pembrolizumab-related pneumonitis in a patient with non-small cell lung cancer. Front Oncol. (2021) 11:673877. doi: 10.3389/fonc.2021.673877
189. Velimirovic M, Brignola M, Chheng E, Smith M, and Hassan KA. Management of pulmonary toxicities associated with systemic therapy in non small cell lung cancer. Curr Treat Options Oncol. (2024) 25:1297–311. doi: 10.1007/s11864-024-01257-6
190. Lin X, Deng H, Chu T, Chen L, Yang Y, Qiu G, et al. Safety and efficacy of immunotherapy rechallenge following checkpoint inhibitor-related pneumonitis in advanced lung cancer patients: a retrospective multi-center cohort study. Transl Lung Cancer Res. (2022) 11:2289–305. doi: 10.21037/tlcr-22-732
191. Yan X, Zhao L, Wu F, Shen B, Zhou G, Feng J, et al. Efficacy and safety analysis of immune checkpoint inhibitor rechallenge therapy in locally advanced and advanced non-small cell lung cancer: a retrospective study. J Thorac Dis. (2024) 16:1787–803. doi: 10.21037/jtd-23-1767
192. Xu H, Yang Y, Yan Y, Li M, Wu S, Cao L, et al. Safety and efficacy of rechallenge with immune checkpoint inhibitors in advanced solid tumor: A systematic review and meta-analysis. Cancer Med. (2024) 13:e70324. doi: 10.1002/cam4.70324
193. Nair DR and Trehan R. Pembrolizumab induced remission of recurrent and metastatic sinonasal squamous cell carcinoma after overcoming checkpoint-inhibitor pneumonitis: A case report and literature review. Cancer Rep (Hoboken). (2023) 6:e1778. doi: 10.1002/cnr2.1778
194. Chu X, Zhao J, Zhou J, Zhou F, Jiang T, Jiang S, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer. (2020) 150:76–82. doi: 10.1016/j.lungcan.2020.08.015
195. Wang N, Zhao Z, Duan Z, and Xie F. Predicting immune checkpoint inhibitor-related pneumonitis via computed tomography and whole-lung analysis deep learning. Curr Med Imaging. (2024) 20:e15734056314192. doi: 10.2174/0115734056314192241002075034
Keywords: immune checkpoint inhibitor-related pneumonitis (CIP), imaging, radiomics, prediction, steroid-refractory pneumonia
Citation: Li S, Geng Z, Hong S, Zhang J, Yang Y, Wei Q, Zhang X, Zhuang X, Huo R, Han S and Wang J (2025) Advances in the mechanisms, imaging characteristics and management strategies for immune checkpoint inhibitor-related pneumonitis. Front. Immunol. 16:1656063. doi: 10.3389/fimmu.2025.1656063
Received: 29 June 2025; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Qiang Feng, University of Texas Southwestern Medical Center, United StatesReviewed by:
Wei Zhang, Second Military Medical University, ChinaJunhong Jiang, Suzhou Dushu Lake Hospital, China
Aijun Shen, Lingang Laboratory, China
Copyright © 2025 Li, Geng, Hong, Zhang, Yang, Wei, Zhang, Zhuang, Huo, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songyan Han, aGFuc29uZ3lAeWVhaC5uZXQ=; Jie Wang, emxodXhpQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Shengshu Li1,2†
Shengshu Li1,2† Xinxin Zhang
Xinxin Zhang Xiaofei Zhuang
Xiaofei Zhuang Rujie Huo
Rujie Huo Songyan Han
Songyan Han Jie Wang
Jie Wang