- 1Dermatology Department, Zhuzhou 331 Hospital, Zhuzhou, China
- 2Science and Education Department, Zhuzhou 331 Hospital, Zhuzhou, China
Background: Psoriasis is a chronic, relapsing, immune-mediated skin disease with a complex pathogenesis and significant individual variability in treatment response. It is characterized by a high relapse rate. In recent years, the critical role of natural killer (NK) cells in the onset and relapse of psoriasis has increasingly attracted attention, and related immune intervention strategies have become a research hotspot.
Objective: This study aims to systematically review the research progress in the field of “NK cells and psoriasis” using bibliometric methods. It identifies research hotspots and key trends, constructs the knowledge structure of this field, and provides data support for related mechanistic studies and clinical translation.
Methods: Based on the Web of Science Core Collection and PubMed databases, we retrieved relevant English-language literature from 2000 to 2025. Using tools such as CiteSpace and VOSviewer, we conducted keyword clustering, author and institutional collaboration analysis, journal co-citation analysis, and highly cited literature mining to create knowledge maps.
Results: A total of 432 publications were included, with a sustained increase in the number of publications over time. The United States, China, and Germany were the main contributing countries, while Rockefeller University and Karolinska Institute were core institutions. The journal Frontiers in Immunology was identified as an important publication venue. Current research focuses on three hotspots: (1) the role of tissue-resident NK cells in “trained immunity” during psoriasis relapse; (2) metabolic reprogramming of NK cells in lesions, with a close correlation between their metabolic status and pro-inflammatory functions; and (3) the development of NK cell-targeted therapeutic strategies, such as dimethyl fumarate (DMF) and IL - 23 inhibitors, which show promising prospects.
Conclusion: This study constructed a comprehensive knowledge map of “NK cells–psoriasis” research, clarifying the hotspots and structural characteristics. Future research will focus on two trends: (1) exploring the pivotal role of NK cells in multi-systemic immune comorbidities and (2) developing specific intervention strategies for different stages of disease progression to advance the development of precision immunotherapy for psoriasis.
1 Introduction
Psoriasis is a common chronic immune-mediated skin disease with a strong propensity for recurrence, severely impacting patients’ quality of life. Globally, the prevalence of psoriasis is on the rise, with incidence rates exceeding 2% in some countries and regions (1). Although a variety of biologics and targeted drugs have been developed and achieved certain therapeutic effects in clinical practice, the highly heterogeneous pathogenesis and complex immune regulatory network of psoriasis result in significant individual variability in treatment responses, with a persistently high relapse rate. Achieving clinical remission remains a formidable challenge (2).
In recent years, the pivotal role of the innate immune system in the pathogenesis of psoriasis has been increasingly recognized, particularly the core functions of natural killer (NK) cells in initiating immune responses, amplifying inflammation, and maintaining tissue homeostasis (3). NK cells are involved in the inflammatory processes and relapse reactions of psoriasis through multiple mechanisms, including KIR-MHC recognition, spontaneous release of cytokines (such as IFN-γ and IL - 17), and regulation of the Th17 axis (4, 5). Against this backdrop, immune intervention strategies targeting NK cell functions have garnered increasing attention and have gradually entered the stages of basic research and early clinical validation, offering new insights for addressing the refractory and recurrent nature of psoriasis.
With the development of multi-omics technologies, the functions, spatial localization of NK cells, and their immune network connections with keratinocytes and T cells have been more deeply characterized. However, the field currently lacks a systematic review of the literature, particularly in terms of the overall grasp of literature distribution, research hotspots, core teams, interdisciplinary collaboration, and the application of cutting-edge technologies (6). In this context, the application of bibliometric and visualization analysis techniques can help researchers systematically evaluate the developmental trajectory, research foci, and evolutionary trends of this field. It can also identify key authors, institutions, and collaborative patterns, and provide quantitative decision-making support for future basic and translational research (7).
Therefore, based on the Web of Science Core Collection, this study systematically collected and analyzed English-language literature on “NK cells and psoriasis” from 2000 to 2025. Using bibliometric methods such as keyword clustering, author networks, collaboration maps, and citation analysis, we comprehensively revealed the developmental dynamics, research hotspots, and knowledge structure of this field, aiming to provide theoretical support and a data foundation for further exploring the potential of NK cells in psoriasis treatment.
2 Materials and methods
2.1 Data source and literature search
The literature data was obtained from the Web of Science Core Collection (WoSCC) database and PubMed database, and the search time was from 1 January 2000 to May 31, 2025. The search formula used was(TS = (psoriasis) AND TS = (“natural killer cell*” OR “NK cell*” OR “CD56+ lymphocyte*” OR “innate lymphoid cell*”).
2.2 Data screening
2.2.1 Inclusion criteria
(1) Literature related to natural killer (NK) cells and psoriasis;(2) Literature published in English; (3) Literature types include clinical trial studies, in vitro experimental studies, in vivo experimental studies, public database analysis studies, reviews, etc.; (4) Literature with complete bibliographic information (including title, country, author, keywords, source).
2.2.2 Exclusion criteria
(1) Conference papers, newspapers, patents, achievements, health and popular science literature, etc.; (2) Duplicate publications;(3) The literature cannot be fully obtained.
The inclusion and exclusion process is independently conducted by two reviewers. If the inclusion and exclusion results are inconsistent, the third reviewer will participate in the work.
2.2.3 Data standardization
After screening, the literature was exported in Refworks and plain text formats. Special characters and redundant spaces were removed. all keywords were standardized by merging synonymous or semantically similar terms into unified categories. For instance, terms such as “2DL1”, “2DS1”, and “killer immunoglobulin-like receptor” were grouped under the unified term “KIR receptors”, reflecting common NK cell recognition molecules. Similarly, variations related to “interleukin” including “IL-17”, “IL-22”, and “IL-23” were unified under the umbrella term “IL cytokines” to highlight key inflammatory mediators. Disease-specific keywords such as “psoriasis arthritis”, “psoriatic lesion”, and “plaque psoriasis” were all merged under “psoriasis” for thematic coherence. Terms referring to immune cell types such as “ILC”, “NK-like cells”, and “innate lymphoid cells” were collectively categorized as “innate lymphoid cells (ILCs)”. Additionally, methodological terms such as “3D imaging”, “mass spectrometry”, and “single-cell RNA-seq” were respectively standardized as “imaging techniques” and “omics technologies”. This standardization process ensured that frequency and co-occurrence analyses accurately reflected research hotspots without terminological redundancy. Country/Region names were standardized for consistency in bibliometric analysis. For example, “Hong Kong”, “Macau”, and “Taiwan” were categorized under “China”, while “Scotland”, “Wales”, and “England” were grouped under “United Kingdom”. Subsequently, the Data Import/Export function in CiteSpace software was used to convert and process the retrieved literature, ensuring the uniformity of metadata for further analysis.
2.2.4 Keyword analysis
The keyword co-occurrence network was constructed by selecting the top N nodes (N = 30) and applying Pruning: Pathfinder + Pruning sliced networks to reduce noise. The thematic density (Density) and centrality (Centrality) were calculated to identify the key nodes in this field. Keyword burst detection was employed to reveal shifts in research hotspots. A temporal evolution map of keywords was generated to illustrate the dynamics of hotspot migration. Furthermore, high-frequency keywords related to NK cells and psoriasis were extracted and subjected to LLR clustering analysis, enabling the identification of major research domains.
2.2.5 Data analysis
2.2.5.1 Data extraction
The normalized text data will be incorporated into a structured form designed by two researchers, who will then extract relevant data. The extracted data includes the following parts: publication information, encompassing the year of publication, country/region, issuing organization, issuing journal, authors, cited literature, and keywords.
2.2.5.2 Analysis methods
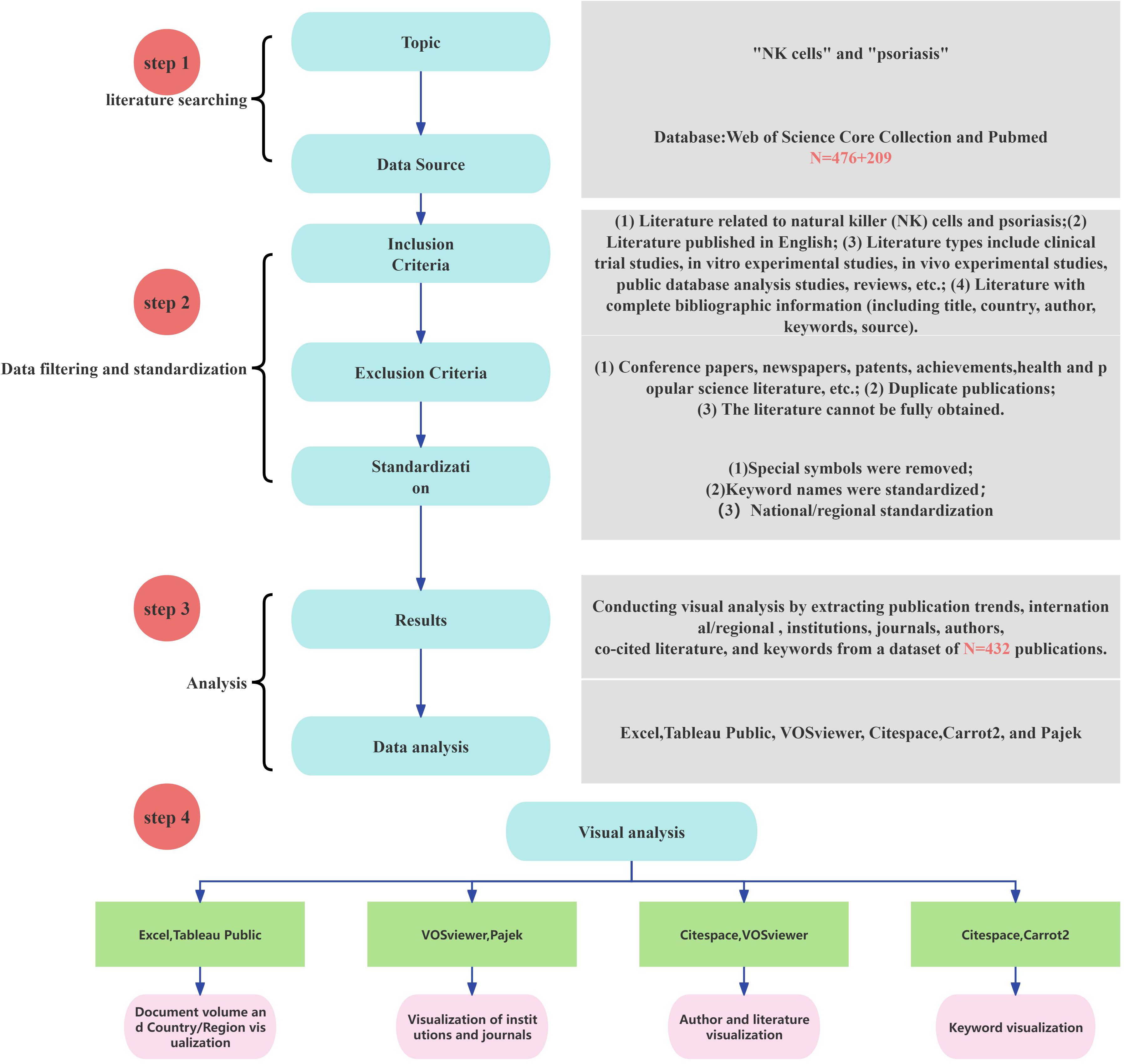
In this study, we employed bibliometric visualization analysis to comprehensively organize and uncover valuable hidden information in the field. The deduplication and merging of TXT files retrieved from the Web of Science Core Collection (WOS) and PubMed databases were carried out using Endnote. For the visualization of publication output, the textual data were converted to Excel via Citespace’s data processing tool, and a fitting curve was further plotted to accurately predict future publication trends. The visualization analysis of countries/regions was realized by converting the textual data to Excel using VOSviewer and subsequently visualizing it with Tableau Public. The visualization of publishing institutions was jointly achieved using VOSviewer and Pajek. The data analysis of journals and authors was conducted by exporting the textual data to CSV files via VOSviewer and then organizing them. The keyword visualization analysis involved converting the textual data to XML files using Citespace and subsequently generating bubble charts with Carrot 2. The process of data acquisition and analysis is illustrated in Figure 1.
3 Results
3.1 Publication output
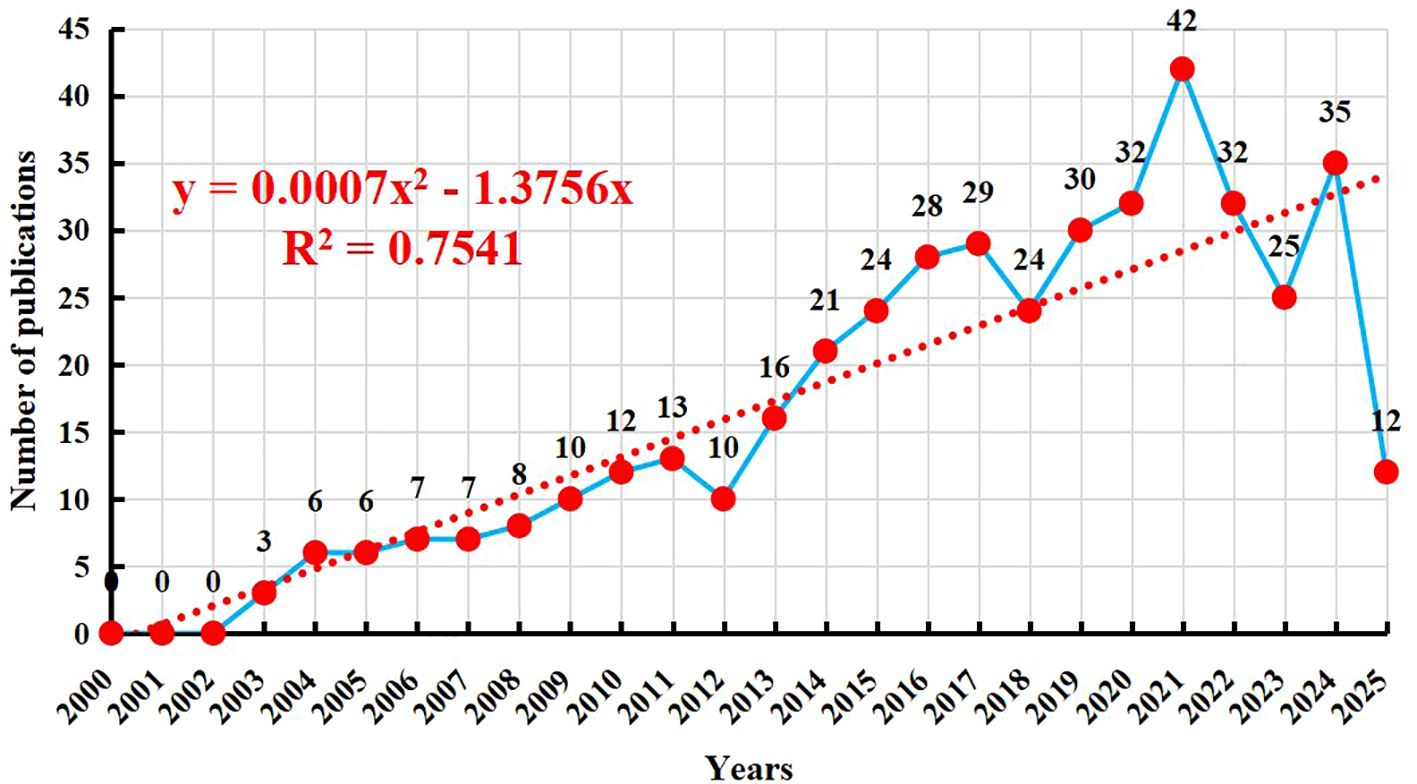
Figure 2 presents the publication trends over the past 20 years. A total of 432 publications were identified in this period. No relevant studies were published between 2000 and 2002. The first sporadic publications appeared in 2003, and the number of publications gradually increased thereafter. From 2003 to 2010, the number of publications slowly rose from 3 to 12. The growth rate accelerated between 2011 and 2015, with the annual publication output increasing from 13 to 24. The period from 2016 to 2021 witnessed a sustained increase, with the annual publication count rising from 28 to a peak of 42, indicating a significant surge in research interest in “NK cells and psoriasis.” There was a slight decline in 2022 (32 publications) and a further decrease in 2023 to 25 publications. However, the number rebounded to 35 in 2024, suggesting that this research direction remains highly relevant. As of the search date in 2025, 12 related publications had already been released, and it is anticipated that the annual publication output will remain at a considerable level for the entire year. Overall, the field has demonstrated a consistent upward trend over the past two decades, particularly in the last decade, which has been a period of active research. To more accurately predict future trends, a polynomial fitting curve was plotted, as indicated by the red dashed line in Figure 2. This curve suggests that publications in this field will continue to grow in the future. The coefficient of determination (R² = 0.7541) indicates that the model can explain 75.41% of the data variability, suggesting that this trend is highly informative. The increasing publication output highlights that NK cell immunotherapy in psoriasis is currently a focal point of research and holds promising prospects for future development.
3.2 Countries/Regions
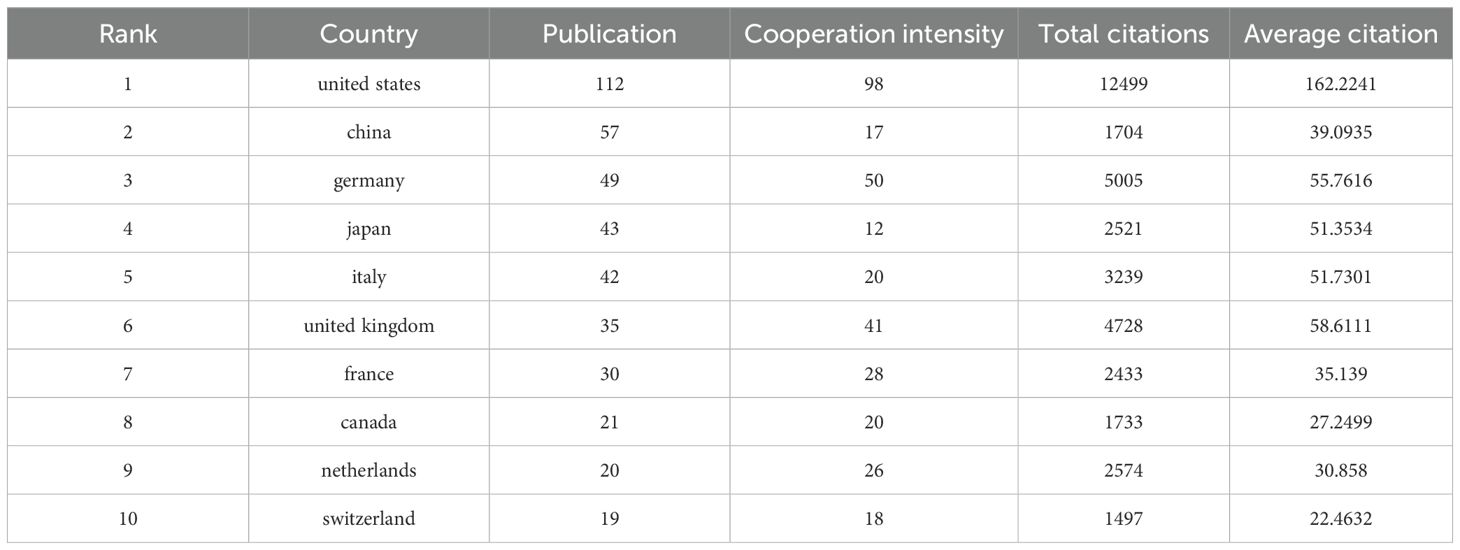
Over the past 20 years, scholars from 60 countries/regions worldwide have contributed to publications related to NK cells and psoriasis. As shown in Figure 3, the geographical distribution of publications is highly concentrated, primarily in developed countries in Europe, America, and East Asia. The United States has the highest number of publications (n = 112), followed by China (n = 57), Germany (n = 49), Japan (n = 43), Italy (n = 42), and the United Kingdom (n = 35). In terms of international collaboration, the United States holds a dominant position (cooperation intensity = 98), followed by Germany (n = 50) and the United Kingdom (n = 41), indicating that European and American countries have established stable collaborative research networks in this field.
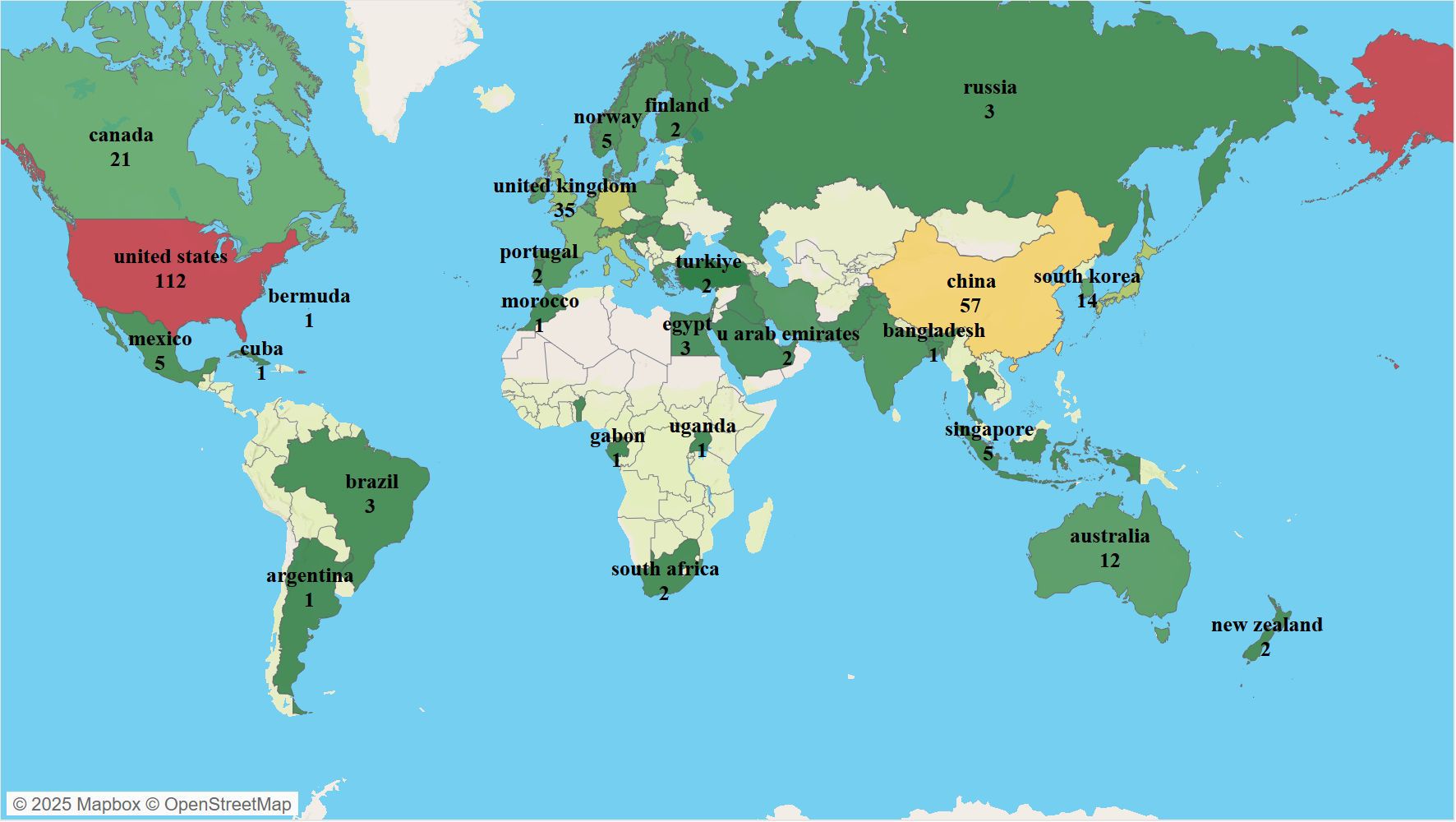
Figure 3. Visualization of Countries/Regions in NK Cell Therapy Research for Psoriasis; colors and numbers represent the number of publications from each country in this field.
Table 1 provides a detailed overview of the top 10 countries in terms of publication output, cooperation intensity, total citations, and average citations per publication. The United States not only has the highest total citations (n = 12,499) but also the highest average citations per publication (162.22), demonstrating its absolute advantage in producing high-impact research outcomes. Although China ranks second in publication output, its average citation count is 39.09, significantly lower than that of Germany (55.76), the United Kingdom (58.61), and Italy (51.73), indicating that there is room for improvement in research impact.
In summary, research on NK cells and psoriasis has formed a global pattern, with the United States and European countries as the academic core. While Asian countries have shown rapid growth in publication numbers, they still need to enhance international collaboration and accumulate high-quality research outcomes to strengthen their academic impact.
3.3 Institution and author
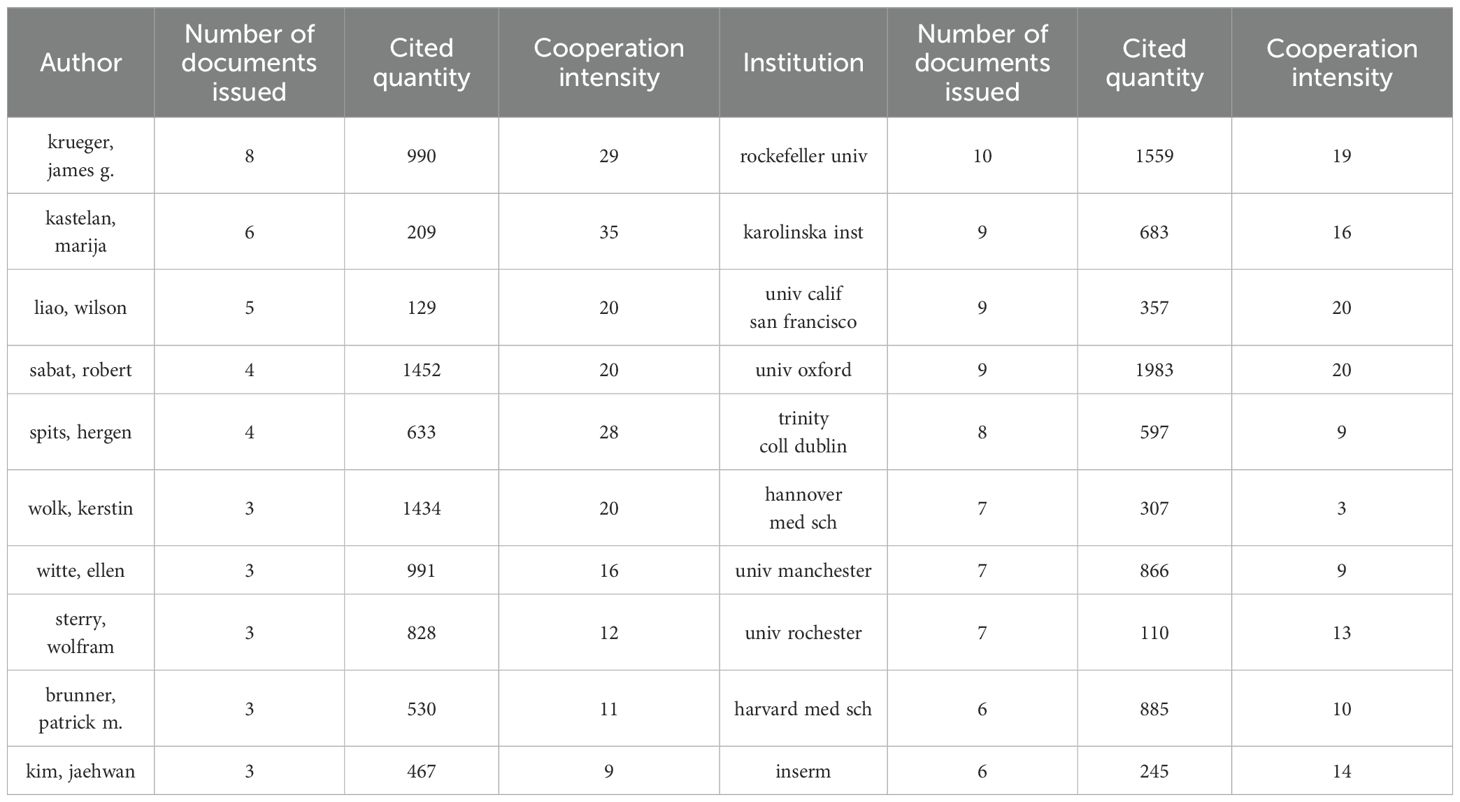
Over the past 20 years, a total of 2,558 authors from 806 institutions worldwide have published their research findings on NK cells in the field of psoriasis. Figure 4A presents the author collaboration network, in which Krueger, James G. (N = 8) is the most prolific author, while Sabat, Robert (C = 1,452) is the most cited author. During this period, five key teams centered around Krueger, James G., Bissonnette, Robert, Eyerich, Kilian, Brunner, Patrick M., and Christophe, Thierry have collectively propelled the development of this field, as shown in Figure 4B. Figure 4C illustrates the institutional collaboration network, with Rockefeller Univ (N = 10), Karolinska Inst (N = 9), Univ Calif San Francisco (N = 9), Univ Oxford (N = 9), and Trinity Coll Dublin (N = 8) being the most productive institutions in terms of publication output. The Univ Oxford (C = 1,983) is the most cited institution, and Univ Calif San Francisco and Univ Oxford have the highest cooperation intensity. The collective efforts of these authors and institutions have driven the rapid development of this field. The specific information of the authors and institutions with major contributions is shown in Table 2.
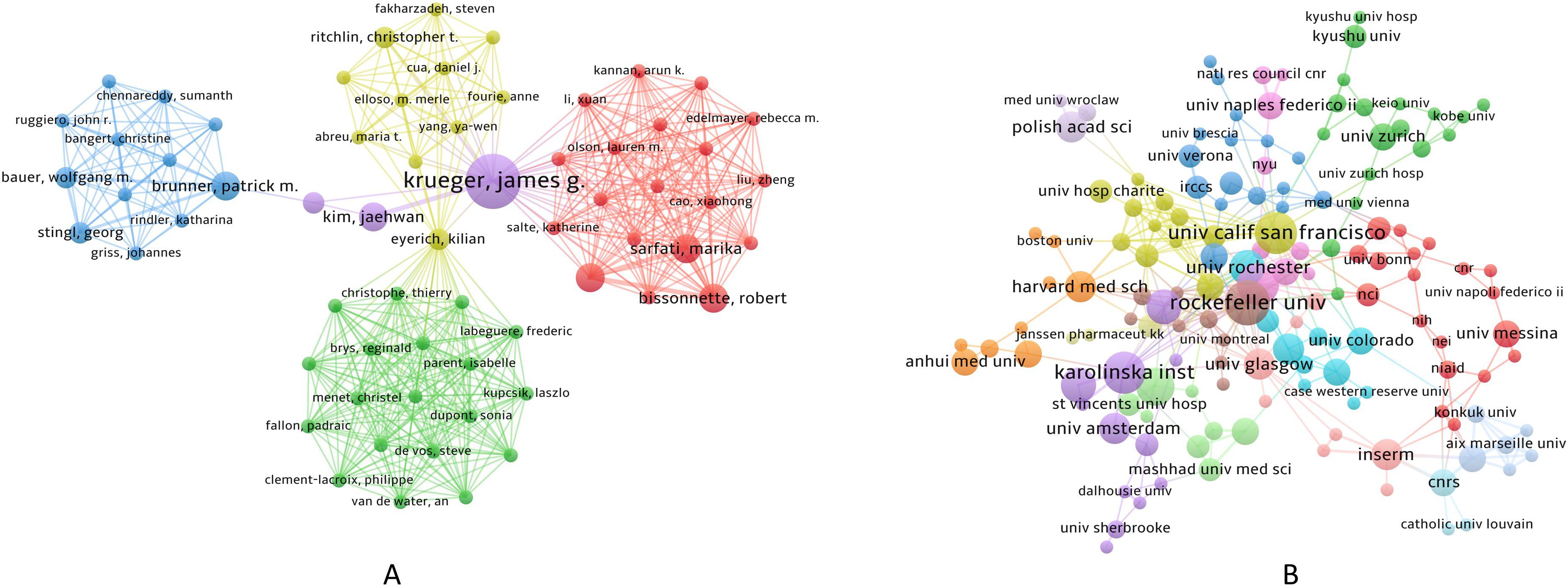
Figure 4. Visualization of Authors and Institutions; (A) Author collaboration network; (B) Institutional collaboration network; In panel (A) the circle size represents the publication frequency of each author, with larger circles indicating higher output; in panel (B) the circle size represents the number of publications from each institution, with larger circles indicating higher output.
3.4 Journals
A total of 217 journals have published research findings on NK cell immunotherapy in psoriasis. Figure 5A displays journals with citation counts greater than or equal to 3. Among them, Frontiers in Immunology (N = 32), International Journal of Molecular Sciences (N = 23), Journal of Investigative Dermatology (N = 16), Journal of Allergy and Clinical Immunology (N = 14), and British Journal of Dermatology (N = 12) are the most prolific journals in terms of publication output. The journals with the highest citation counts are Frontiers in Immunology (C = 2,151), Nature (C = 1,508), Journal of Allergy and Clinical Immunology (C = 1,419), European Journal of Immunology (C = 1,253), and International Journal of Molecular Sciences (C = 1,182). Figure 5B presents the co-citation network of journals, with J. Immunol (CC = 2,479), J. Invest. Dermatol. (CC = 1,620), J. Exp. Med. (CC = 1,359), Immunity (CC = 1,247), and Nat. Immunol. (CC = 1,112) being the most co-cited journals. The dual-map overlay of journals (Figure 5C) illustrates the citation relationships between citing and cited journals. The left side represents the clustering of citing journals, indicating the distribution of knowledge frontiers in the field, while the right side represents the clustering of cited journals, highlighting the important knowledge bases of the field. The orange path in Figure 5C indicates that journals from the fields of Molecular, Biology, and Genetics are most likely to be cited by journals related to Molecular, Biology, and Immunology, suggesting that the latest research involves substantial interdisciplinary studies. The green path indicates that journals from Molecular, Biology, and Genetics are most likely to be cited by journals related to Medicine, Medicinal, and Clinical, highlighting the multidisciplinary integration and convergence in this field.
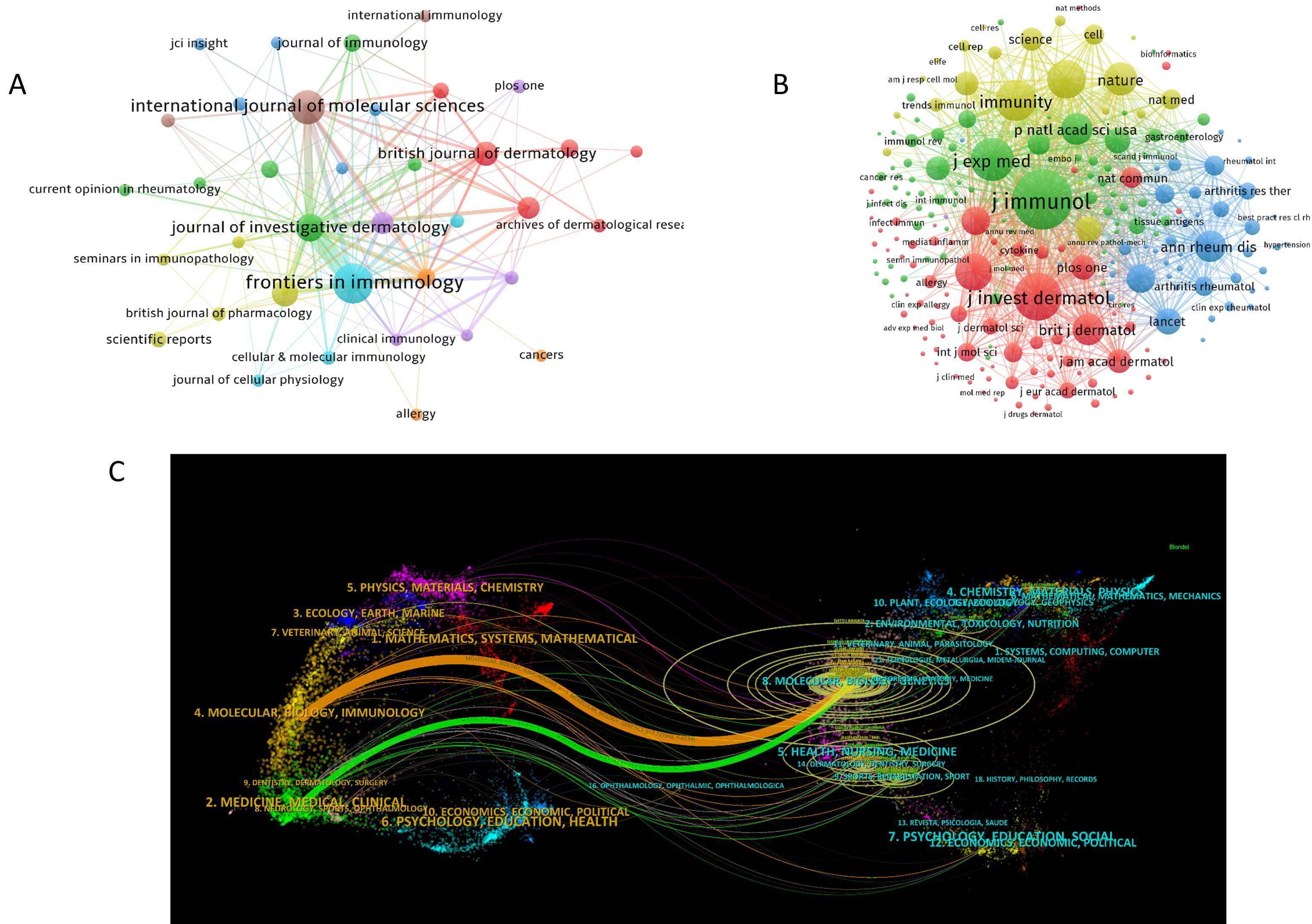
Figure 5. Visualization of Journals in NK Cell Immunotherapy Research for Psoriasis; (A). Network of publication output for journals with citation counts ≥ 3;(B). Co-citation network of journals; (C). Dual-map overlay of journals; In panel (A) the circle size represents the citation frequency of journals, with larger circles indicating higher citations; in panel (B) the circle size represents the co-citation frequency of journals, with larger circles indicating higher co-citations; in panel (C) the left side shows clusters of citing journals and the right side shows clusters of cited journals, with ring size representing citation frequency, while orange and green curves indicate citation paths.
3.5 Keywords
Through the visualization analysis of keywords, we obtained key information in the current field. Figure 6A presents a bubble chart of keywords, with modules such as inflammation, atopic dermatitis, psoriatic arthritis, autoimmunity, skin, natural killer cells, innate lymphoid cells, IL - 17, innate immunity, and cytokines being the focal points of research. To further analyze the developmental trends of this field over time, Figure 6B shows a timeline of key clusters, indicating that 16 main clusters were formed in the field of NK cell immunotherapy and psoriasis research between 2018 and 2025.
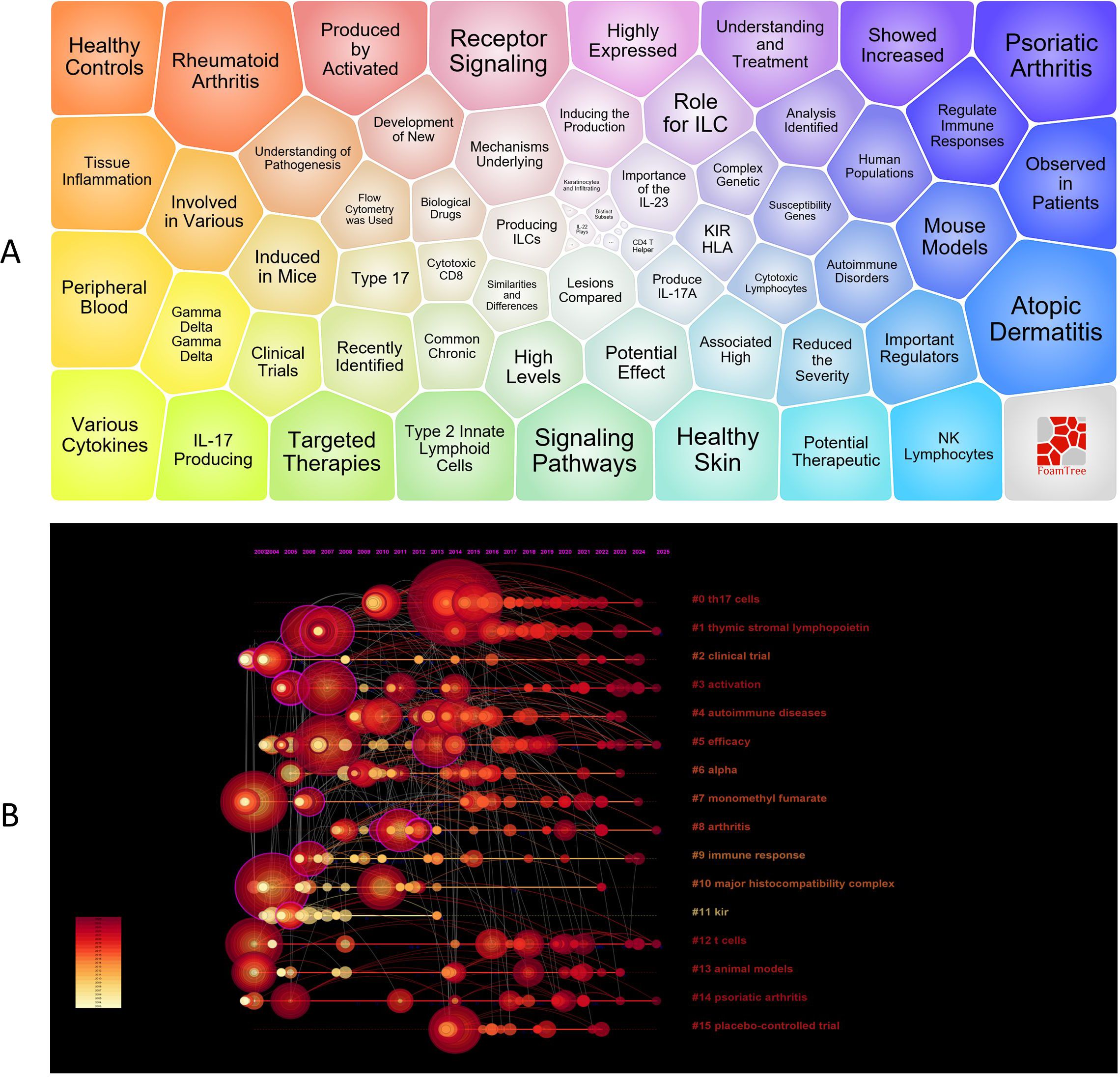
Figure 6. Visualization of Keywords; (A) Keyword bubble chart analysis; (B) Timeline of keyword clusters; In panel (A) larger bubbles represent higher occurrence frequency of keywords. In panel (B) the circle size indicates the frequency of keyword occurrence, with larger circles denoting higher frequency. A greater number of circles within the same cluster reflects a higher concentration of research in that area.
Through keyword clustering analysis, we identified three key themes in the field of NK cell immunotherapy and psoriasis research. These themes not only cover the main threads of basic research and clinical translation but also reflect the research progress in immune regulation mechanisms and therapeutic target exploration.
Firstly, the autoimmune pathogenesis of psoriasis (core keywords: #4 autoimmune diseases, #9 immune response, #12 T cells, #14 psoriatic arthritis, #13 animal models) forms the theoretical foundation of the research. As a typical immune-mediated inflammatory disease, psoriasis is closely related to the dysfunction of the adaptive immune system, especially the abnormal activation of the Th17/IL-23 axis mediated by T cells. In-depth studies of T cell functions, IL - 17 production, and keratinocyte responses in animal models have provided an important basis for elucidating the regulatory role of NK cells in the immune network.
Secondly, the activation mechanisms of NK cells and innate immune signaling pathways (core keywords: #1 thymic stromal lymphopoietin, #3 activation, #10 major histocompatibility complex, #11 KIR, #0 Th17 cells) are important research directions in this field. Studies have shown that NK cells not only recognize target cells through KIR-MHC interactions but are also activated by cytokines such as TSLP and IL - 12, inducing Th17 responses and releasing IFN-γ, thereby amplifying and maintaining psoriatic inflammation. These studies provide theoretical support for revealing the cross-regulation mechanisms between innate and adaptive immunity.
Lastly, preclinical and clinical explorations of NK cell-targeted immunotherapy (core keywords: #2 clinical trial, #5 efficacy, #6 alpha, #7 monomethyl fumarate, #15 placebo-controlled trial, #8 arthritis) constitute the translational frontier of this research. In recent years, small molecule drugs (such as monomethyl fumarate) and biologics (such as IL - 17/IL-23 monoclonal antibodies) targeting NK cell functions have shown promising efficacy and safety in improving skin and joint lesions in psoriasis and psoriatic arthritis through multiple randomized controlled clinical trials, laying the foundation for the development of personalized immune intervention strategies.
In summary, the three key themes—from the basic research of psoriasis immune mechanisms to the inflammation amplification pathways mediated by NK cells and the clinical evaluation of immunotherapy—constitute the core knowledge framework of the field of “NK cell immunotherapy and psoriasis,” reflecting a systematic developmental trend from mechanism elucidation to clinical application.
3.6 References
By conducting an in-depth analysis of highly cited literature, we can further explore the developmental trends and key turning points of NK cell immune regulation in psoriasis research. Figure 7 presents a density map of articles cited more than 10 times, highlighting the important positions of some core articles in the co-citation network.
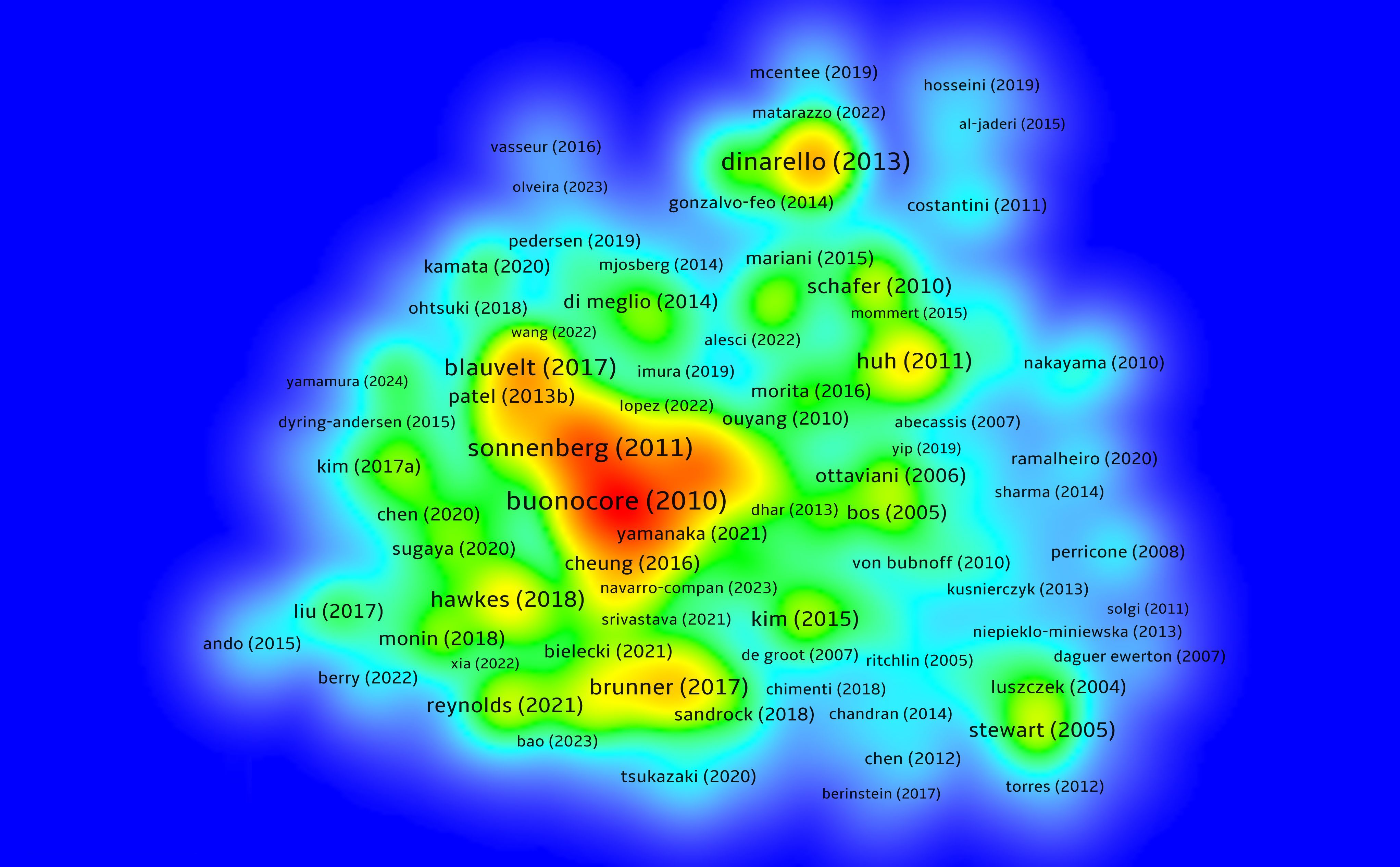
Figure 7. Density map analysis of highly cited articles; The central orange area represents a high-density region, indicating that the literature in this area has been cited more frequently.
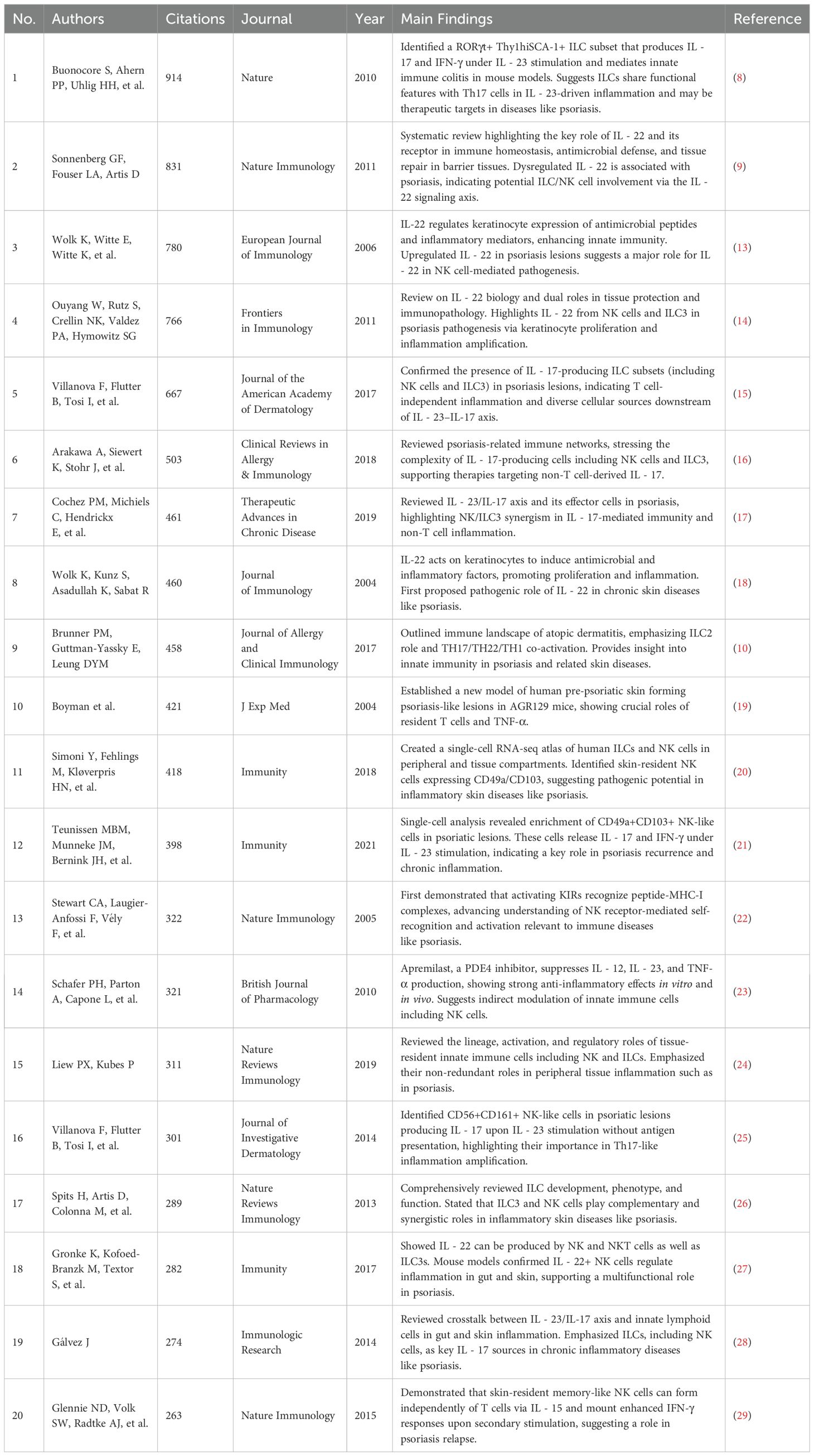
Buonocore et al. (2010) published a study in Nature (8) that first revealed that RORγt+, IL - 23R+ innate lymphoid cells (ILCs) can independently induce IL - 17 and IFN-γ expression in a T cell-deficient Rag– mouse model, driving the occurrence of intestinal inflammation in IL - 23-mediated inflammatory responses. This study clarified the pathogenic potential of ILCs in tissue-specific inflammation and provided a solid experimental basis for IL - 23 pathway-related autoimmune diseases such as psoriasis, occupying a core node in the co-citation network.
Gregory F. Sonnenberg et al. (2011) (9) systematically reviewed the functions of IL - 22 and its receptor pathway in immune regulation of barrier tissues, pointing out that IL - 22 plays a key role in maintaining immune homeostasis, antimicrobial defense, and tissue repair in the skin, gut, and respiratory tract. The study emphasized that the imbalance of IL - 22 expression is closely related to diseases such as psoriasis and inflammatory bowel disease, suggesting that the ILC family (including NK-like cells) participates in chronic inflammation and tissue homeostasis imbalance through the IL - 22 signaling axis. Their theoretical contributions are of great significance for NK cell function research and the development of targeted therapies.
Brunner et al. (2017) (10) reviewed the immune landscape of atopic dermatitis in the Journal of Allergy and Clinical Immunology, emphasizing the central role of ILC2 in TH2-dominated inflammatory responses. The article pointed out that AD is not only characterized by TH2 skewing but also involves TH17, TH22, and partial TH1 responses. ILC2 is significantly enriched in lesions, releasing cytokines such as IL - 5 and IL - 13, leading to barrier disruption and persistent inflammation. This review clearly supports the effectiveness of targeting the IL - 4/IL-13 pathway (e.g., dupilumab) and provides theoretical support for understanding the role of innate immune cells in chronic skin inflammation, including psoriasis.
Villani et al. (2018) (11) constructed a high-resolution atlas of human peripheral immune cells through single-cell RNA sequencing, identifying multiple functionally heterogeneous innate immune cell subsets, including different types of dendritic cells, monocytes, and NK cells. The study revealed the dynamic expression characteristics of ILC family cells in different inflammatory microenvironments, providing foundational resources for further exploring the functional differentiation and pathogenesis of NK cells in complex immune diseases such as psoriasis.
Teunissen et al. (2021) (12) used single-cell multi-omics strategies to analyze the immune features of memory T cells (TRM) and innate lymphoid cells (including ILCs and NK-like cells) in human skin tissue. The study found that psoriatic lesions contain a large number of CD49a+CD103+ NK-like cells that rapidly produce IL - 17 and IFN-γ in response to IL - 23, driving typical Th17-type inflammatory responses. This study proposed that “innate memory” cells may play a central role in psoriasis relapse mechanisms, offering new strategies for targeting TRM and ILCs to intervene in psoriasis relapse. The main findings of the top 20 highly cited articles are further summarized in Table 3.
4 Discussion
4.1 Research hotspots
4.1.1 In vivo studies: the role of NK cells in psoriasis relapse
Recent studies have consistently highlighted the central immunological functions of NK cells in psoriasis relapse (30). Beyond their early role in disease onset by secreting IL - 17 and IFN-γ to drive inflammation, specific subsets of tissue-resident NK cells (trNK) exhibit “trained immunity” features. These cells can persist in the skin during remission and, upon re-exposure to inflammatory cytokines such as IL - 23 and IL - 15, are rapidly reactivated to reinitiate Th17-like inflammatory pathways (30).
Another study using a chronic skin inflammation model emphasized that trNK cells display strong chemotaxis and metabolic adaptability, forming a positive feedback inflammatory network with keratinocytes and dermal dendritic cells, thereby sustaining chronic lesions (3). Since their activation is independent of antigen presentation, trNK cells are often refractory to conventional T cell–targeted therapies, suggesting that they may serve as “hidden drivers” of disease recurrence.
4.1.2 In vitro studies: functional phenotypes and inflammatory mechanisms
In vitro experiments simulating the psoriatic microenvironment have shown that NK cells significantly upregulate pro-inflammatory cytokine expression and activate STAT3 and NF-κB pathways under IL - 23 and IL - 15 stimulation. This pro-inflammatory phenotype, when co-cultured with keratinocytes, creates an amplified inflammatory loop, underscoring their potential role in sustaining psoriasis inflammation. Evidence further indicates that CD49a+CD103+ NK-like cells enriched in psoriatic lesions rapidly release IL - 17 and IFN-γ in response to IL - 23, serving as a key source for maintaining and amplifying local inflammation (12). Moreover, these cells establish a stable tissue-resident state and respond to external stimuli via critical signaling axes such as STAT3 and T-bet, endowing them with rapid effector functions. Additional experiments revealed that such innate lymphoid cells can independently drive inflammation even in the absence of T cells (8), highlighting their autonomous pathogenic potential in psoriasis relapse.
4.1.3 Metabolomics: NK cell metabolic reprogramming and pathological activation
Similar to T cells, NK cell activation is accompanied by profound metabolic reprogramming, including enhanced glucose uptake, upregulated oxidative phosphorylation, and activation of the mTOR pathway. Such metabolic rewiring not only shapes their effector functions but also determines their persistence and amplification capacity within psoriatic lesions (31). Studies have shown that local lactate accumulation and hypoxic microenvironments in psoriatic lesions skew NK cells toward an IL - 17-producing phenotype, thereby enhancing their pathogenicity (31).
Furthermore, in chronic inflammatory settings, NK cells display increased reliance on glycolysis, with the secretion of IL - 17 and GM-CSF tightly associated with glycolytic metabolite accumulation, reflecting their dynamic and plastic metabolic states. Recent evidence also demonstrates that oxidative stress and mitochondrial dysfunction are critical drivers of pathological NK cell activation (32). Persistent ROS burden in chronic psoriatic inflammation activates NF-κB and STAT3 pathways, promoting continuous pro-inflammatory cytokine release; concurrently, elevated mTORC1 activity and impaired AMPK signaling serve as pivotal bridges between metabolism and effector function (33). Targeting these metabolic pathways (e.g., with AMPK agonists or mTORC1 inhibitors) may partially reverse the pro-inflammatory phenotype of NK cells, providing novel strategies for immunometabolic interventions in psoriasis.
4.1.4 Targeting NK cells in clinical strategies
Current treatments for psoriasis primarily focus on T cell-related pathways. However, with the deepening understanding of innate immune mechanisms, NK cells have gradually emerged as one of the potential therapeutic targets. Studies have shown that dimethyl fumarate (DMF) and its metabolite monomethyl fumarate (MMF) can significantly regulate the number and function of NK cells. After DMF treatment, the proportion of CD56bright NK cells in peripheral blood significantly increases. This subset, mainly characterized by immune regulatory functions, can clear activated autoreactive T cells through the release of perforin, granzyme B, and granzyme K. Further in vitro experiments have found that both DMF and MMF can enhance the degranulation capacity of NK cells and increase their cytotoxic activity against activated T cells, potentially playing a role in limiting adaptive immune-mediated inflammation (34). This mechanism suggests that the anti-inflammatory effects of DMF-class drugs are not limited to T/B cell regulation but may also be achieved by enhancing the immune suppressive functions of NK cells.
Further research has shown that IL - 23 plays a key role in maintaining the pathogenic phenotype of NK-like cells in psoriatic tissues. IL - 23 not only promotes the secretion of IL - 17A and GM-CSF by NK cells but also helps stabilize the inflammatory microenvironment (35). In psoriasis models, blocking the IL - 23 signaling pathway not only reduces T cell-mediated inflammatory responses but also significantly inhibits the expression of pro-inflammatory cytokines by NK-like cells, demonstrating a dual immune regulatory effect on “non-classical effector cells.” Moreover, GM-CSF, identified as a key downstream molecule of NK cells, plays an important role in inducing keratinocyte stress responses and immune cell infiltration, providing a basis for developing new therapeutic strategies targeting GM-CSF.
In summary, future efforts should build on existing treatment systems to further construct multi-target immune intervention strategies, incorporating innate immune-related pathways such as IL - 23, GM-CSF, and TSLP into an integrated targeting system. Such strategies are expected to enhance the fine-tuning of complex immune networks, especially for refractory psoriasis patients with poor traditional treatment responses, frequent disease recurrence, or persistent tissue inflammation. They hold significant translational application prospects.
4.2 Research trends
4.2.1 Exploration of immune comorbidities
Psoriasis is not only a localized inflammatory skin disease but also a phenotype of systemic immune dysregulation, with significantly increased risks of comorbidities with various immune-mediated diseases. Recent studies have found that common immune comorbidities in psoriasis patients include ulcerative colitis, ankylosing spondylitis, rheumatoid arthritis, Hashimoto’s thyroiditis, etc. Shared genetic susceptibility loci and immune regulatory pathways may underlie these associations (36). Particularly, the IL - 23/IL-17 axis, TSLP, and IFN signaling collectively participate in the initiation and maintenance of chronic inflammation in different organ systems.
Research also indicates that NK cells may serve as “common effector cells” in multiple comorbid diseases, exhibiting tissue-specific inflammatory profiles in different tissues. In models of psoriatic arthritis and inflammatory bowel disease, NK cells show high expression of IL - 17A and IFN-γ, suggesting their role as a key immune hub connecting the skin-joint-gut axis. This understanding prompts a shift from a “skin-centric” model to a “systemic immune imbalance” model, emphasizing the integrative role of innate lymphoid cells, including NK cells, in systemic disease spectra (37). Therefore, identifying the functional states and migration patterns of NK cells in multi-system immune comorbidities will not only help reveal the systemic inflammatory mechanisms of psoriasis but also provide a theoretical basis for combined intervention strategies, promoting a transition from organ-specific treatments to multi-system immune modulation.
4.2.2 Stage-specific intervention strategies
The progression of psoriasis encompasses distinct stages—including initial activation, chronic maintenance, and recurrence susceptibility—each characterized by differences in immune cell subsets, activation states, and inflammatory mechanisms (38). Therefore, designing targeted and precisely timed, stage-specific intervention strategies is essential for enhancing therapeutic efficacy and delaying disease relapse.
In the acute phase, natural killer (NK) cells upregulate activating receptors such as NKG2D and CD69, leading to robust secretion of IFN-γ and TNF-α. These cytokines stimulate keratinocytes to produce chemokines and act synergistically with T cells to amplify the inflammatory response (39). Therapeutic interventions at this stage should prioritize the blockade of NK–T cell interaction pathways to rapidly mitigate the inflammatory cascade.
During the chronic phase, the therapeutic focus shifts toward maintaining local immune homeostasis. Prolonged exposure of NK cells to bacterial metabolites and signals from epidermal damage may induce a sub-exhausted or pathologically activated state, thereby sustaining a low-grade chronic inflammatory milieu. Intervention strategies in this phase should aim to modulate cellular metabolism and reprogram NK cell function to stabilize the immune microenvironment.
In the remission phase, tissue-resident NK cells (CD49a+CD103+) exhibiting features of “trained immunity” can be rapidly reactivated upon re-exposure to stimuli, triggering disease recurrence. Recent studies have demonstrated that bioactive compounds from natural medicines—such as paeoniflorin—can ameliorate psoriatic pathology by suppressing NK cell activation pathways and alleviating oxidative stress in skin tissues, offering a promising approach for remission-phase intervention (40).
In summary, implementing precisely targeted immunomodulatory strategies tailored to each immunological stage may disrupt the “relapse–recurrence” cycle, prolong remission duration, and ultimately improve long-term disease control in psoriasis.
5 Conclusion
The role of NK cells in psoriasis has emerged as a cutting-edge focus in immunological research, with ongoing in-depth exploration of their immune functions, metabolic states, and therapeutic potential. Current research is concentrated on three main hotspots: First, tissue-resident NK cells have been confirmed to possess “trained immunity” characteristics with significant pathogenic potential in psoriasis recurrence, particularly their rapid reactivation capability during disease remission. Second, metabolic reprogramming during NK cell activation is decisive for their pro-inflammatory functions, with enhanced glycolysis, hypoxia adaptation, and ROS signaling collectively shaping their pathological state. Third, clinical strategies targeting NK cells are gradually taking shape, with drugs such as DMF and IL - 23 inhibitors demonstrating therapeutic potential through direct or indirect modulation of NK cells, offering new pathways for controlling inflammation beyond T cell - dominated mechanisms.
In terms of future development trends, research is progressively expanding from a focus on localized inflammation to a systemic perspective of immune comorbidities. NK cells may serve as a crucial link between psoriasis and other autoimmune diseases. Meanwhile, the concept of devising stage-specific intervention strategies based on the disease course of psoriasis is emerging, emphasizing precise modulation of NK cell activity at different stages to potentially break the cycle of recurrent disease episodes and improve long-term therapeutic outcomes. Overall, a systematic dissection of the functional spectrum and dynamic features of NK cells in psoriasis will provide a solid foundation for disease mechanism studies and personalized treatments, propelling the field into a new era of more precise and efficient immune interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LiC: Formal analysis, Validation, Supervision, Project administration, Data curation, Conceptualization, Writing – review & editing, Methodology, Resources, Writing – original draft, Investigation, Software, Visualization, Funding acquisition. LinC: Writing – review & editing, Funding acquisition, Investigation, Conceptualization, Writing – original draft, Resources, Supervision, Project administration, Validation, Formal analysis, Software, Methodology, Visualization, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wadhavane S, Rithvik A, and Rasool M. Psoriasis relapse: exploring the role of epigenetics, metabolic reprogramming, and inflammatory memory. Immunol Invest. (2025), 1–27. doi: 10.1080/08820139.2025.2519666
2. Wang S, Zhuang D, Xu Q, Hou D, Feng T, Guo J, et al. Biomaterial-enhanced delivery of stem cell-derived exosomes for skin inflammatory diseases: Mechanisms and therapeutic advances. Int J Biol Macromol. (2025) 318:145213. doi: 10.1016/j.ijbiomac.2025.145213
3. Kucuksezer UC, Aktas Cetin E, Esen F, Tahrali I, Akdeniz N, Gelmez MY, et al. The role of natural killer cells in autoimmune diseases. Front Immunol. (2021) 12:622306. doi: 10.3389/fimmu.2021.622306
4. Dizaji Asl K, Velaei K, Rafat A, Tayefi Nasrabadi H, Movassaghpour AA, Mahdavi M, et al. The role of KIR positive NK cells in diseases and its importance in clinical intervention. Int Immunopharmacol. (2021) 92:107361. doi: 10.1016/j.intimp.2020.107361
5. Chen S, Liu J, Xia K, Gao Y, and Zeng Y. Research hotspots and trends of NK cell immunotherapy for acute myeloid leukemia: A bibliometric analysis from 2000 to 2023. Cancer Control. (2024) 31:1369238311. doi: 10.1177/10732748241310937
6. Hojjatipour T, Aslani S, Salimifard S, Mikaeili H, Hemmatzadeh M, Gholizadeh Navashenaq J, et al. NK cells - Dr. Jekyll and Mr. Hyde in autoimmune rheumatic diseases. Int Immunopharmacol. (2022) 107:108682. doi: 10.1016/j.intimp.2022.108682
7. Chen S, Liu J, He G, Tang N, and Zeng Y. Research hotspots and trends in global cancer immunometabolism:A bibliometric analysis from 2000 to 2023. J Multidiscip Healthc. (2024) 17:5117–5137. doi: 10.2147/JMDH.S495330
8. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. (2010) 464:1371–5. doi: 10.1038/nature08949
9. Sonnenberg GF, Fouser LA, and Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. (2011) 12:383–90. doi: 10.1038/ni.2025
10. Brunner PM, Guttman-Yassky E, and Leung DYM. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. (2017) 139:S65–76. doi: 10.1016/j.jaci.2017.01.011
11. de la Torre-Ubieta L, Stein JL, Won H, Opland CK, Liang D, Lu D, et al. The dynamic landscape of open chromatin during human cortical neurogenesis. Cell. (2018) 172:289–304. doi: 10.1016/j.cell.2017.12.014
12. Matteoli M and Pozzi D. Feeling depressed? Keep calm, and watch microglia. Immunity. (2021) 54:191–3. doi: 10.1016/j.immuni.2021.01.010
13. Navarro G, Gómez-Autet M, Morales P, Rebassa JB, Llinas Del Torrent C, Jagerovic N, et al. Homodimerization of CB(2) cannabinoid receptor triggered by a bivalent ligand enhances cellular signaling. Pharmacol Res. (2024) 208:107363. doi: 10.1016/j.phrs.2024.107363
14. Dinarello CA, Novick D, Kim S, and Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. (2013) 4:289. doi: 10.3389/fimmu.2013.00289
15. Scher RK and Lipner SR. Pediatric melanomas often mimic benign skin lesions. J Am Acad Dermatol. (2017) 76:e131. doi: 10.1016/j.jaad.2016.10.046
16. Blauvelt A and Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. (2018) 55:379–90. doi: 10.1007/s12016-018-8702-3
17. Sabat R, Ouyang W, and Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. (2014) 13:21–38. doi: 10.1038/nrd4176
18. Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. (2011) 472:486–90. doi: 10.1038/nature09978
19. Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO, et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. (2004) 199:731–6. doi: 10.1084/jem.20031482
20. Hawkes JE, Yan BY, Chan TC, and Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. (2018) 201:1605–13. doi: 10.4049/jimmunol.1800013
21. Veale DJ and Fearon U. The pathogenesis of psoriatic arthritis. Lancet. (2018) 391:2273–84. doi: 10.1016/S0140-6736(18)30830-4
22. Stewart CA, Laugier-Anfossi F, Vély F, Saulquin X, Riedmuller J, Tisserant A, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. (2005) 102:13224–9. doi: 10.1073/pnas.0503594102
23. Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. (2010) 159:842–55. doi: 10.1111/j.1476-5381.2009.00559.x
24. Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. (2021) 371(6527):eaba6500. doi: 10.1126/science.aba6500
25. Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. (2014) 134:984–91. doi: 10.1038/jid.2013.477
26. Baliwag J, Barnes DH, and Johnston A. Cytokines in psoriasis. Cytokine. (2015) 73:342–50. doi: 10.1016/j.cyto.2014.12.014
27. Isailovic N, Daigo K, Mantovani A, and Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. (2015) 60:1–11. doi: 10.1016/j.jaut.2015.04.006
28. Ebbo M, Crinier A, Vély F, and Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. (2017) 17:665–78. doi: 10.1038/nri.2017.86
29. Zenewicz LA and Flavell RA. Recent advances in IL-22 biology. Int Immunol. (2011) 23:159–63. doi: 10.1093/intimm/dxr001
30. Hashemi E and Malarkannan S. Tissue-resident NK cells: development, maturation, and clinical relevance. Cancers (Basel). (2020) 12(6):1553. doi: 10.3390/cancers12061553
31. Chen C, Yi X, Liu P, Li J, Yan B, Zhang D, et al. CD147 facilitates the pathogenesis of psoriasis through glycolysis and H3K9me3 modification in keratinocytes. Res (Wash D C). (2023) 6:167. doi: 10.34133/research.0167
32. Sarandi E, Krueger-Krasagakis S, Tsoukalas D, Sidiropoulou P, Evangelou G, Sifaki M, et al. Psoriasis immunometabolism: progress on metabolic biomarkers and targeted therapy. Front Mol Biosci. (2023) 10:1201912. doi: 10.3389/fmolb.2023.1201912
33. Li JH, Zhou A, Lee CD, Shah SN, Ji JH, Senthilkumar V, et al. MEF2C regulates NK cell effector functions through control of lipid metabolism. Nat Immunol. (2024) 25:778–89. doi: 10.1038/s41590-024-01811-2
34. Smith MD, Calabresi PA, and Bhargava P. Dimethyl fumarate treatment alters NK cell function in multiple sclerosis. Eur J Immunol. (2018) 48:380–3. doi: 10.1002/eji.201747277
35. Dhamija B, Marathe S, Sawant V, Basu M, Attrish D, Mukherjee D, et al. IL-17A orchestrates reactive oxygen species/HIF1α-mediated metabolic reprogramming in psoriasis. J Immunol. (2024) 212:302–16. doi: 10.4049/jimmunol.2300319
36. Stull CM, Rakita U, Wallis L, and Krunic A. Successful treatment of acquired vulvar lymphangiectasia with 1% Polidocanol sclerotherapy. Acta Derm Venereol. (2021) 101:adv520. doi: 10.2340/00015555-3876
37. Petrovic A, Samuelsen VM, Davies R, Aarebrot AK, Holmes T, Sarkar I, et al. Immune cell activity during anti-TNF treatment in patients with psoriasis and psoriatic arthritis. Clin Exp Immunol. (2024) 218:329–40. doi: 10.1093/cei/uxae070
38. Patel F, Marusina AI, Duong C, Adamopoulos IE, and Maverakis E. NKG2C, HLA-E and their association with psoriasis. Exp Dermatol. (2013) 22:797–9. doi: 10.1111/exd.12280
39. Lu J, Wang Y, Li Y, Zhong X, Gong Y, Ding Y, et al. Based on gene expression analysis: low-density neutrophil expression is a characteristic of the fast responders treated with guselkumab for psoriasis. Front Immunol. (2022) 13:865875. doi: 10.3389/fimmu.2022.865875
Keywords: psoriasis, natural killer cells, trained immunity, metabolic reprogramming, immunotherapy, bibliometrics
Citation: Chen L and Cheng L (2025) New hope for the treatment of recurrent and refractory psoriasis: NK cell immunotherapy—A scientometric analysis. Front. Immunol. 16:1656398. doi: 10.3389/fimmu.2025.1656398
Received: 30 June 2025; Accepted: 25 August 2025;
Published: 23 October 2025.
Edited by:
Alina Maria Holban, University of Bucharest, RomaniaReviewed by:
Lukasz Chlewicki, Eli Lilly, United StatesYulin Zou, University Medical Center Göttingen, Germany
Copyright © 2025 Chen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Cheng, NTMyMjQ1OTYzQHFxLmNvbQ==
 Li Chen
Li Chen Lin Cheng2*
Lin Cheng2*