- 1Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou, China
- 2Intensive Care Unit, Northern Jiangsu People’s Hospital, Yangzhou, China
- 3Binhai County People’s Hospital, Yancheng, Jiangsu, China
- 4The Yangzhou Clinical College of Xuzhou Medical University, Xuzhou, Jiangsu Province, China
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection, exhibiting high global morbidity and mortality. Accumulating evidence indicates that post-translational modifications (PTMs), as pivotal epigenetic mechanisms, play a crucial role in regulating diverse biological processes. The significance of PTMs in sepsis is increasingly recognized, as they may influence disease progression by modulating protein stability, activity, and localization. In recent years, advances in mass spectrometry have elucidated a series of novel PTMs, including succinylation (Ksucc), S-palmitoylation, lactylation (Kla), crotonylation (Kcr), 2-hydroxyisobutyrylation (Khib), β-hydroxybutyrylation (Kbhb), and malonylation (Kmal). This review presents the first comprehensive analysis of the characteristics, functions, and implications of these seven lysine acylation modifications in the pathogenesis and progression of sepsis, aiming to provide valuable insights for diagnosis and therapeutic intervention.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). In recent years, with the timely application of fluid resuscitation, antibiotic therapy, and organ function support, the mortality rate of sepsis has gradually declined. However, its high treatment costs remain a significant global public health concern (2, 3). The pathophysiology of sepsis is complex and frequently involves multiple organ dysfunctions, such as septic cardiomyopathy, acute liver injury, acute kidney injury, acute lung injury, coagulopathy, and endothelial dysfunction (4), Currently, effective diagnostic and therapeutic strategies to improve the prognosis of sepsis and reduce associated treatment costs remain lacking.
Epigenetics is a fundamental area of biology, encompassing DNA methylation, post-translational modifications, and non-coding RNAs, all of which contribute to heritable changes in biological phenotypes without altering the underlying genetic sequence (5, 6). Post-translational modifications (PTMs) are a central mechanism of epigenetic regulation and have attracted widespread attention in recent years due to their strong association with the pathogenesis and progression of various diseases, as well as their potential as therapeutic targets (7). PTMs refer to the chemical alterations of specific amino acid residues through covalent attachment of functional groups, which regulate protein structure and function. These modifications are ubiquitous in mammalian cells and play pivotal roles in modulating cellular molecular functions (8, 9). To date, over 400 types of PTMs have been identified to influence protein functionality. Among these, metabolic PTMs, particularly various newly discovered acylation modifications of histones or non-histone proteins, have been demonstrated to significantly impact the pathogenesis and progression of multiple diseases, including inflammatory, cancer, cardiovascular diseases, kidney diseases, and metabolic syndromes (7, 10). Acetylation was the first identified homogenous lysine modification linked to multiple cellular functions. In recent years, advancements in mass spectrometry, proteomics, and bioinformatics have facilitated the discovery of novel lysine modifications, including succinylation (Ksucc), S-palmitoylation, lactylation (Kla), crotonylation (Kcr), 2-hydroxyisobutyrylation (Khib), β-hydroxybutyrylation (Kbhb), and malonylation (Kmal). These modifications have emerged as significant areas of research in the field (6). The formation of certain novel PTMs requires specific energy metabolism intermediates as acyl donors (11). Accumulating evidence has revealed that acylation modifications constitute a dynamic and diverse network of metabolic modifications (10), whose dysregulation is closely associated with the pathogenesis of sepsis and its associated multi-organ dysfunction.
This review aims to summarize recent advancements in the study of novel acylation modifications in sepsis, with an emphasis on their molecular mechanisms and contributions to the pathogenesis of sepsis (Figure 1, Table 1). Through comprehensive analyses of Ksucc, S-palmitoylation, Kla, Kcr, Khib, Kbhb, and Kmal, we seek to offer new insights for future basic research and clinical applications, ultimately contributing to the development of diagnostic and therapeutic strategies for sepsis and its associated multi-organ dysfunction.
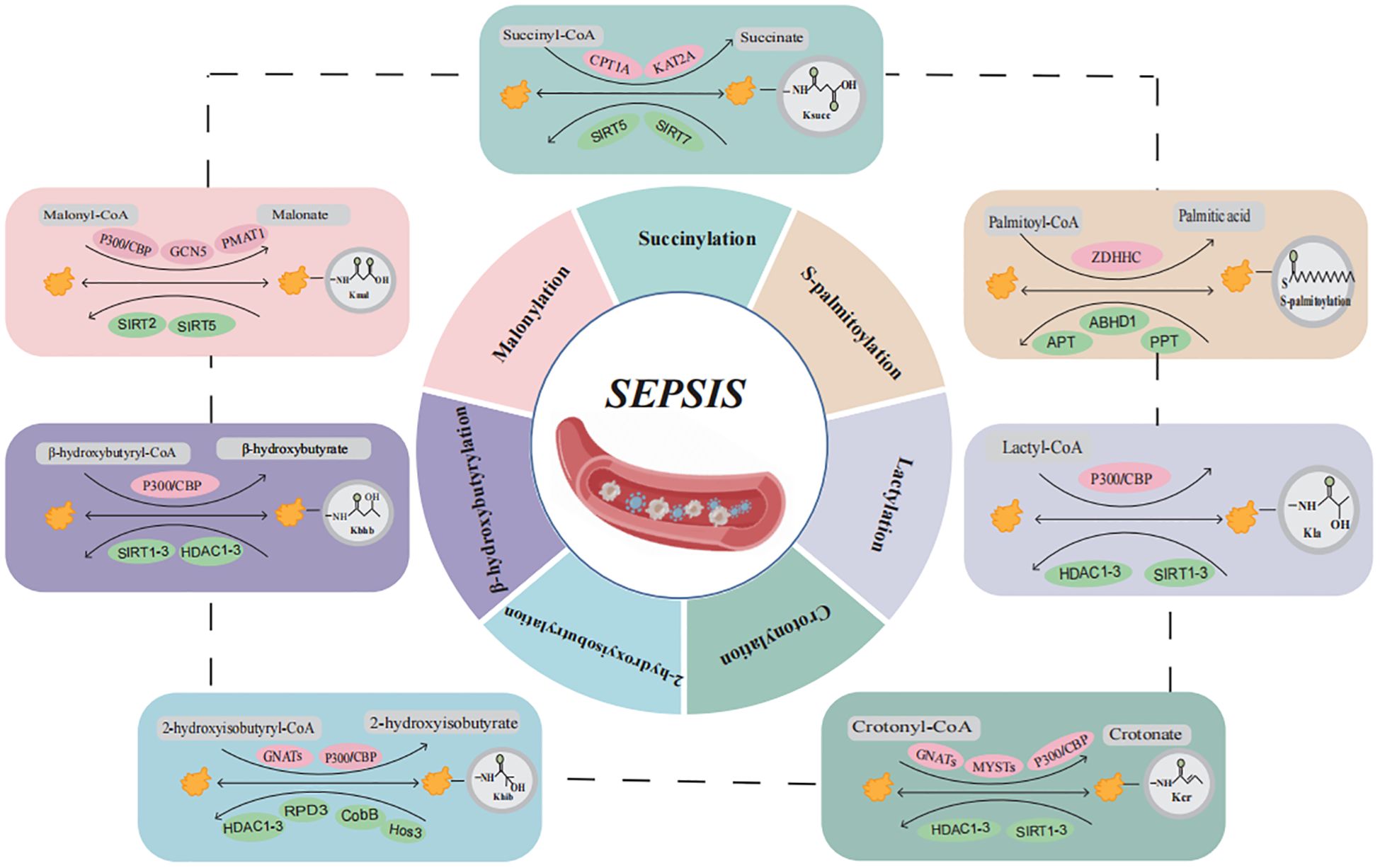
Figure 1. Overview of the composition and regulatory mechanisms of protein acylation in sepsis. Sepsis and multiple organ dysfunction are caused by protein acylation after translation for succinylation, S-palmitoylation, lactylation, crotonylation, 2-hydroxyisobutyrylation, β-hydroxybutyrylation, and malonylation may participate in the occurrence of sepsis development but have not yet been confirmed. (Created with BioGDP.com). CoA, coenzyme A; CPT1A, carnitine palmitoyl transferase 1A; KAT2A, lysine acetyltransferase 2A; SIRT5, sirtuin 5; SIRT7, sirtuin 7; ZDHHC, zinc finger Asp-His-His-Cys motif-containing; APT, acyl-protein thioesterase; PPT, Palmitoyl protein thioesterase; CBP, CREB binding protein; HDAC, histone deacetylases; SIRT1-3, sirtuin 1-3; GNATs, GCN5-related nacetyltrans-ferases; PMAT1, Plasma membrane monoamine transporter 1.
Succinylation (Ksucc)
Ksucc is a PTM involved in the partial transfer of succinyl coenzyme A (CoA) to lysine residues (ϵ-amino), and can be produced by elevated levels of succinic acid. This post-translational modification involves the enzymatic addition of succinyl groups to specific amino acid residues in proteins, playing a critical role in regulating innate immunity and inflammatory responses (Figure 1, Table 1) (12, 13). Acylation reactions are catalyzed through both enzymatic and non-enzymatic mechanisms. In enzymatic processes, acyltransferases and deacylases play a key role in the transfer and removal of acyl groups (6). Dysregulation of succinylation modification can lead to alterations in the activity and function of proteins involved in energy metabolism and downstream epigenetic modifications, which are closely associated with the pathogenesis and progression of various diseases, including inflammation and cancer (14, 15). Succinyl-CoA, derived from the tricarboxylic acid (TCA) cycle, serves as the primary substrate for Ksucc (16). Ksucc represents a reversible, dynamic, abundant, and evolutionarily conserved histone modification in eukaryotic cells, with identified modification sites present on histones H2A, H2B, H3, and H4 (17). The Ksucc modification is dynamically regulated by both succinyltransferases, such as carnitine palmitoyltransferase 1A (CPT1A) and lysine acetyltransferase 2A (KAT2A), as well as desuccinylases, including sirtuin 5 (SIRT5) and sirtuin 7 (SIRT7) (18, 19). KAT2A (also known as GCN5) was originally identified as a histone acetyltransferase. Research has indicated that chicoric acid inhibits the KAT2A/α-tubulin complex, leading to reduced acetylation of α-tubulin and inactivation of the NLRP3 inflammasome. This mechanism contributes to the amelioration of acute kidney injury (AKI) and myocardial injury induced by sepsis (20, 21). However, recent studies have demonstrated that KAT2A also possesses succinyltransferase activity and exhibits higher affinity for succinyl-CoA than for acetyl-CoA (22, 23). However, it is regrettable that no studies have yet elucidated how KAT2A-mediated Ksucc specifically influences the pathogenesis and progression of sepsis. SIRT5 is the most extensively studied desuccinylase, primarily localized in mitochondria. It exhibits NAD+-dependent desuccinylation activity, which reduces the succinylation levels of mitochondrial proteins, thereby modulating the target activity of substrate proteins to maintain metabolic homeostasis (15, 24–26). SIRT7 is predominantly found in the nucleus, where it performs essential functions such as stimulating ribosomal RNA expression, facilitating DNA damage repair, and regulating chromatin compaction (27, 28). Studies have demonstrated that pyruvate kinase M2 (PKM2) serves as a critical target of Ksucc in macrophages. SIRT5-mediated desuccinylation activates PKM2, thereby suppressing lipopolysaccharide (LPS)-induced interleukin-1β (IL-1β) production and preventing dextran sulfate sodium (DSS)-induced colitis in mice (29). Wang et al. established an in vitro model of septic lung injury by stimulating lung epithelial cells with LPS. Their study demonstrated that SIRT5-mediated desuccinylation of Homeobox A5 (HOXA5) mitigated ferroptosis by activating ferroptosis suppressor protein 1 (FSP1), ultimately reducing LPS-induced lung injury (30). As a central kinase, TBK1 plays a pivotal role in both NF-κB and interferon regulatory factor (IRF) signaling pathways (13). The expression of pro-inflammatory cytokines and chemokines, including Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), and interferon-β (IFN-β), is contingent upon the activation of NF-kB and IRF3 (31). Research indicates that in endotoxemia and sepsis models, a reduction in macrophage SIRT5 levels leads to increased succinylation of TBK1, thereby impairing its interaction with IRF3 and TRAF2, which consequently suppresses the inflammatory response (13). Additionally, glutamine has been shown to enhance the activity of pyruvate dehydrogenase (PDH) in macrophages. This effect occurs through the inhibition of SIRT5-mediated desuccinylation of pyruvate dehydrogenase (PDHA1), leading to the restoration of PDH activity. This process promotes M2 polarization of macrophages and ameliorates burn sepsis in murine models (32). In summary, succinylation has been demonstrated to be a naturally occurring lysine modification that participates in nearly all biological processes within organisms. It plays a critical role in the initiation and progression of sepsis, as well as the associated organ dysfunction, suggesting that the regulation of Ksucc could serve as a promising therapeutic target for sepsis.
S-palmitoylation
Palmitoylation is a PTM in which palmitic acid or palmitoyl-CoA acts as the acyl donor. This modification involves the covalent binding of palmitic acid to the amino acid residues of lysine, serine, glycine, threonine, and cysteine (Figure 1, Table 1) (33, 34). Based on the chemical linkage between fatty acids and proteins, palmitoylation modifications can be classified into S-palmitoylation, N-palmitoylation, and O-palmitoylation. Among these, S-palmitoylation has been the most extensively studied (10). Palmitoylation occurs through both enzymatic and non-enzymatic mechanisms, with the enzymatic process being the dominant form of catalysis (6). Palmitoylation is catalyzed by palmitoyltransferases (PATs) that contain the zinc finger Asp-His-His-Cys (ZDHHC) motif, whereas depalmitoylation is mediated by acyl-protein thioesterases (APTs) (35–37). S-Palmitoylation, predominantly observed in eukaryotic cells, modulates the membrane affinity of substrate proteins, thereby influencing their stability, subcellular localization, and interactions with other proteins. This post-translational modification has been implicated in various pathological conditions, including cancer, inflammation, and cardiovascular diseases (38–41). Studies have demonstrated that under inflammatory conditions, both palmitoylation and DHHC21 activity are significantly upregulated (42, 43). Palmitoylation serves as a novel regulatory factor for the formation and function of extracellular vesicles (EVs) during sepsis. EVs isolated from wild-type septic mice significantly promoted neutrophil adhesion, migration, and the formation of neutrophil extracellular traps (NETs). However, DHHC21 deficiency inhibited this process by blocking palmitoylation, thereby reducing neutrophil infiltration and improving survival rates in mice (44). In addition, the NOD-like receptor family pyrin domain-containing 3 (NLRP3) plays a critical role in the pathogenesis of sepsis, with inflammatory pathways being tightly regulated through dynamic palmitoylation of NLRP3. Multiple studies have demonstrated that inhibition of the NLRP3 inflammasome can ameliorate sepsis-induced inflammation and associated organ dysfunction (45–48). Studies have demonstrated that vaccarin inactivates the NLRP3 inflammasome by promoting its palmitoylation via zDHHC12, thereby ameliorating LPS-induced cardiomyopathy (49). Furthermore, zDHHC12-mediated palmitoylation facilitates NLRP3 degradation via the chaperone-mediated autophagy pathway. The absence of zDHHC12 exacerbates inflammatory symptoms and increases mortality in LPS-induced endotoxic shock (50, 51). The NLRP3 inflammasome triggers and amplifies inflammatory responses by inducing pyroptosis and the secretion of inflammatory cytokines, a process that is dependent on gasdermin D (GSDMD) (52). Numerous studies have demonstrated that upon LPS stimulation of macrophages, GSDMD undergoes palmitoylation modification at Cys191/192. Moreover, the upregulation of LPS-induced DHHC7 autopalmitoylation has been shown to play a critical role in GSDMD palmitoylation (53–56). Therefore, LPS may induce GSDMD palmitoylation by upregulating the expression levels of DHHC palmitoyltransferases and their autopalmitoylation activity (57), Inhibition of inflammasome-stimulated GSDMD palmitoylation significantly suppresses GSDMD-dependent pyroptosis and IL-1β release, mitigates organ injury, and improves survival rates in septic mice (55). Palmitoylation of GSDMD-NT may serve as a novel therapeutic target for sepsis. In addition to the aforementioned mechanisms, palmitoylation serves as a crucial post-translational modification in the regulation of the STING pathway (58). In sepsis, stimulator of interferon genes (STING) is hyperactivated and phosphorylated, leading to excessive production of inflammatory cytokines such as IL-6, IFN-β, and TNF-α (59). Moreover, the activation of STING in platelets serves as a critical driver of sepsis-induced pathological alterations (60). Studies have demonstrated that fatty acid synthase (FASN) suppresses STING palmitoylation via malonyl-CoA, thereby alleviating sepsis-induced liver injury (61). In summary, S-palmitoylation is essential in the pathogenesis of sepsis and its induced multi-organ dysfunction. A deeper understanding of the molecular mechanisms underlying S-palmitoylation will provide novel perspectives on the pathophysiological processes of sepsis and identify more accurate therapeutic targets for future interventions.
Lactylation (Kla)
Lactate, the end product of glycolysis, was historically regarded as a metabolic waste product until 2019, when the research team led by Yingming Zhao discovered that lactate serves as the precursor for histone lysine lactylation (Kla) (Figure 1, Table 1) (62). Current evidence indicates that lactate has emerged as an epigenetic modification substrate capable of inducing lactylation on lysine residues of both histones and non-histone proteins, thereby playing a regulatory role in gene transcription and protein function (63–65). Lactylation modification is closely associated with inflammation and plays a pivotal role in regulating inflammatory responses (66). The lactylation of histones H3 and H4 is a p53-dependent process mediated by the acetyltransferase p300, which influences transcriptional regulation of genes (62). Clinical study results demonstrated that compared with the healthy control group, septic shock patients exhibited significantly elevated levels of lactosylation and H3K18la expression. Furthermore, H3K18la expression was positively correlated with IL-6, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) score (67, 68). Lactate Promotes LPS-Induced NF-κB Signaling Pathway Activation in Bovine Mammary Epithelial Cells via p300/CBP-Mediated H3K18 Lactylation (69). Moreover, lactate can upregulate heparanase in pulmonary microvascular endothelial cells through histone H3K18 lactylation, thereby promoting degradation of the pulmonary endothelial glycocalyx and exacerbating sepsis-associated acute lung injury (70). Moreover, histone lactylation plays a pivotal role in macrophage polarization. The gradual accumulation of lactate in macrophages results in a significant increase in histone lactylation, thereby promoting the transition of macrophages from the M1 to the M2 phenotype (62, 71). Subsequent studies have revealed that lactylation modification is not restricted to histones but also occurs on non-histone proteins. High mobility group box 1 (HMGB1), a highly conserved and ubiquitously expressed protein, is released by activated macrophages and plays a critical role in inducing innate immune responses and amplifying proinflammatory signaling pathways during sepsis (72). The regulation of HMGB1 lactylation by p300/CBP plays a crucial role in the progression of sepsis. In polymicrobial sepsis, the acetyltransferase p300/CBP acts as a key enzyme that promotes HMGB1 lactylation in macrophages. Lactylated/acetylated HMGB1 is secreted through exosomes, where it disrupts endothelial cell integrity and impairs endothelial barrier function (73). Moreover, HMGB1 lactylation induced by lactate accumulation can activate the HMGB1-NETs signaling pathway, thereby contributing to and worsening mouse SAKI (74, 75). These findings provide strong support for HMGB1 as a therapeutic target in the treatment of sepsis and its associated organ dysfunction. Elevated serum lactate concentration is closely associated with increased mortality in patients with sepsis (66). Research indicates that both endogenous and exogenous increases in lactate levels exacerbate SAKI by mediating the lactylation of fission 1 (Fis1). Conversely, reducing lactate levels and Fis1 lactylation can alleviate AKI (76).This study uncovers a novel mechanism connecting lactate levels to organ damage in sepsis, emphasizing the critical importance of therapeutic strategies focused on reducing serum lactate levels in sepsis patients. Overall, lactate and its associated lactylation modifications play a crucial role in the pathogenesis and progression of sepsis. Further investigation into the molecular mechanisms underlying lactylation will offer new perspectives for understanding the pathological processes of sepsis and provide more precise therapeutic targets. As scientific research continues to advance, we anticipate uncovering more complex interactions between lactylation modifications and sepsis.
Crotonylation (Kcr)
Crotonylation (Kcr) is a short-chain lysine acylation modification, in which a crotonyl group is transferred to a lysine residue under the action of crotonyltransferases using crotonyl-CoA as the substrate (Figure 1, Table 1) (77). While many regulatory factors and sites overlap between Kcr and Kac, the distinctive planar structure and four-carbon chain length of Kcr differentiate it from Kac (22). Crotonyltransferases and de-crotonylases dynamically regulate histone crotonylation. Additionally, various factors, including the levels of crotonyl-CoA and the activities of positive regulators [acyl-CoA oxidase 1 (ACOX1), acyl-CoA oxidase 3 (ACOX3), acyl-CoA dehydrogenase short-chain (ACADS), and acyl-CoA synthetase short-chain family member 2 (ACSS2)] and negative regulators [chromodomain Y-like (CDYL) and short-chain enoyl-CoA hydratase 1 (ECHS1)], govern the Kcr modification of proteins (77, 78). Lysine crotonylation plays a crucial role in bacterial infections. A study found that following methicillin-resistant Staphylococcus aureus (MRSA) infection in THP1 cells, the crotonylation modification profile was altered, with 1,384 downregulated crotonylation sites across 899 proteins and 193 upregulated crotonylation sites across 160 proteins (79). Research has demonstrated that supplementation with crotonyl-CoA in macrophages results in a substantial increase in histone H3K18Cr. Additionally, knockdown of ACSS2, an enzyme involved in crotonyl-CoA synthesis, reduced histone H3K18Cr levels and the expression of inflammatory genes in LPS-stimulated RAW264.7 cells. These findings indicate a strong link between crotonylation and sepsis, suggesting that modulation of crotonylation could regulate the progression of inflammation (80). Furthermore, short-chain fatty acids (SCFAs) can modulate sepsis progression by regulating lysine crotonylation modifications through the inhibition of histone deacetylase (HDAC). Therefore, elucidating the mechanistic roles of both histone and non-histone Kcr modification targets in sepsis pathogenesis is critical for developing effective therapeutic strategies (80). However, our understanding of the underlying mechanisms by which Kcr influences sepsis progression remains incomplete. Future studies should focus on elucidating the specific mechanisms through which Kcr modulates sepsis progression and its functional role in sepsis pathogenesis.
2-hydroxyisobutyrylation (Khib)
Lysine 2-hydroxyisobutyrylation (Khib), a novel acylation modification first identified in 2014, utilizes 2-hydroxyisobutyric acid and its coenzyme-acylated form, 2-hydroxyisobutyryl-CoA, as substrates (Figure 1, Table 1). This modification is conserved in both eukaryotic and prokaryotic cells and is involved in a variety of biological processes (81–83). The hallmark of Khib is the addition of a hydroxyl group to lysine residues (7). These PTMs not only affect histones but also regulate non-histone proteins, participating in diverse physiological processes including glycolysis, gluconeogenesis, and the TCA cycle. Khib plays a crucial role in modulating both normal physiological functions and pathological developments in mammals (81, 84). The acetyltransferase Tip60 has been identified as a “writer” of Khib in mammalian cells, demonstrating catalytic activity for Khib modification both ex vivo and in vivo (85). Furthermore, the p300 protein has been shown to catalyze Khib, while HDAC1-3 and sirtuin 3 (SIRT3) exhibit de-2-hydroxyisobutyrylase activity (83, 84). Currently, research on the relationship between Khib and sepsis is limited. However, it is noteworthy that 2-hydroxyisobutyric acid, a SCFA, is significantly upregulated in various inflammatory diseases. This suggests that alterations in its levels, along with Khib, are closely associated with the onset, progression, and regulation of inflammation (80). Research has indicated that in the skin tissues of psoriasis patients, proteins encoded by the S100A9, FUBP1, and SERPINB2 genes exhibit significant Khib modifications, which are associated with proteins in the PI3K-Akt signaling pathway (86). Notably, S100A9 is a potential therapeutic target for sepsis, though further investigation is required to determine whether its Khib modification plays a role in the onset and progression of sepsis (87). Although there is currently a lack of direct evidence linking Khib to sepsis, Khib is fundamentally involved in the regulation of various inflammation-related diseases. This underscores the need for further investigation into the role of Khib modifications in histones and non-histones in the onset and progression of sepsis, to identify potential new therapeutic targets for sepsis. In conclusion, as a novel epigenetic modification, Khib research is still in its early stages; however, its significant role in epigenetic regulation offers promising new directions for the early diagnosis and treatment of sepsis.
β-hydroxybutyrylation (Kbhb)
Lysine β-hydroxybutyrylation (Kbhb) is a novel protein acylation modification mediated by β-hydroxybutyrate (Figure 1, Table 1) (88, 89). To date, Kbhb has been detected in Drosophila, yeast, murine, and human cells, with 46 identified Kbhb sites distributed across the four core histones (H2A, H2B, H3, and H4) and the linker histone H1 (90, 91). P300/CBP are well-known acyltransferases that catalyze the covalent attachment of β-hydroxybutyryl-CoA to lysine residues, resulting in the formation of Kbhb (91). HDAC1-3 and SIRT1-3 have been identified as de-β-hydroxybutyrylases, which remove Kbhb modifications, thus maintaining the homeostasis of intracellular modification levels (90, 92). Notably, in contrast to HDAC1-3, SIRT3 exhibits class-selective histone de-β-hydroxybutyrylase activity (91, 93, 94). Additionally, 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) and β-hydroxybutyrate dehydrogenase 1 (BDH1), key enzymes involved in ketone body metabolism, can indirectly modulate histone Kbhb modifications (90). Moreover, β-hydroxybutyrate, as a critical substrate, influences Kbhb in a concentration-dependent manner. β-Hydroxybutyrate (BHB) is the most abundant ketone body in human blood, accounting for approximately 70% of total ketones (95). Early clinical studies demonstrated that serum BHB levels were significantly higher in sepsis survivors compared to non-survivors (96). Mechanistically, β-HB suppresses the activation of NF-κB and the NLRP3 inflammasome, thereby exerting anti-inflammatory, anti-apoptotic, and mitochondrial dysfunction-antagonizing effects (97, 98). Additionally, it functions as a histone deacetylase inhibitor, contributing to antioxidative properties and the reduction of pro-inflammatory macrophage activation (99, 100). The ketogenic diet (KD), a high-fat, low-carbohydrate, moderate-protein dietary regimen, has been utilized clinically since the 1920s (101). KD decreases glucose utilization and promotes the conversion of free fatty acids in the liver into ketone bodies (primarily BHB). A study in COVID-19 patients demonstrated that KD significantly reduced IL-6 levels in these individuals (102). Furthermore, a single-center study evaluating the efficacy and safety of the ketogenic diet in sepsis patients showed that ventilation-free, vasopressor-free, dialysis-free, and intensive care unit–free days were higher in the ketogenic group (103). Unfortunately, these studies did not examine the impact of BHB on Kbhb, which complicates the understanding of how β-hydroxybutyrate affects sepsis through Kbhb modifications. Mass spectrometry analysis has revealed that Kbhb modifications also occur in non-histone proteins, especially in transcription factors and key metabolic enzymes. Furthermore, β-hydroxybutyrate may influence disease progression by modulating non-histone Kbhb (98, 104). It is noteworthy that elevated ketone body levels in septic patients may increase the risk of fatal metabolic acidosis due to impaired energy metabolism and mitochondrial dysfunction. A dose-exploration study of ketone salts demonstrated that a dosage of 40 mmol/kg/day approaches the toxicity threshold. In fact, a 20% daily dose escalation, due to excessive Na+ intake, exacerbates organ damage and increases mortality (105, 106). Therefore, further research is required to establish the safety of elevating ketone body levels in the treatment of septic patients. This indicates that regulating the Kbhb levels of key metabolic enzymes and critical genes (via ketogenic diets, fasting, or direct supplementation of β-hydroxybutyrate) could potentially slow the progression of sepsis. In conclusion, Kbhb connects metabolic reprogramming with epigenetic regulation, highlighting its significant biological functions. This provides new avenues for exploring chromatin regulation and the roles of β-hydroxybutyrate in both physiological and pathological processes. Consequently, Kbhb represents a promising clinical target with vast research potential, especially in sepsis and associated organ dysfunction, and is poised to become a new focal point in research.
Malonylation (Kmal)
Lysine malonylation (Kmal) is a novel acylation modification based on malonyl-CoA that was first identified in bacteria and mammals in 2011 and is involved in various pathophysiological processes (Figure 1, Table 1) (107). Kmal is regulated by the concentration of malonyl-CoA, which is modulated by acetyl-CoA carboxylase (ACC), propionyl-CoA carboxylase (PCC), fatty acid synthase (FASN), and malonyl-CoA decarboxylase (MCD) (6). Additionally, similar to other acylation modifications, Kmal is controlled by acyltransferases and demodification enzymes. Studies have demonstrated that acetyltransferase 2A (KAT2A) can function as a lysine malonyltransferase. In animal models, knockdown of KAT2A specifically reduces histone Kmal levels (108), while SIRT5 exhibits significant demalonylase activity (109). Kmal is highly prevalent in mitochondrial proteins involved in regulating metabolic pathways, including glycolysis, fatty acid oxidation, and inflammation, particularly in endothelial cells and macrophages. Macrophages typically undergo two polarized states—classical activation (M1 type) and alternative activation (M2 type)—each serving distinct functions in inflammatory responses and tissue homeostasis. LPS stimulation can influence the Kmal levels of various proteins (110). Studies indicate that in LPS-stimulated macrophages, elevated malonyl-CoA levels upregulate Kmal, thereby modulating inflammatory signaling and enhancing the production of pro-inflammatory factors. The mechanism involves Kmal modification of lysine at position 213 in GAPDH, resulting in increased enzyme activity and dissociation from TNF-α mRNA, which subsequently promotes TNF-α translation (110). Additionally, in LPS-induced RAW264.7 cells, Atractylodin has been shown to inhibit GAPDH Kmal, leading to a reduction in TNF-α levels (111). In conclusion, Kmal modification plays a pivotal role in the regulation of sepsis. Future research should focus on identifying other “write-erase” enzymes involved in this modification, pinpointing their specific or non-specific sites, and assessing the Kmal levels of histones and non-histones relevant to sepsis. Ultimately, further investigation into Kmal will provide novel insights and approaches for the diagnosis and treatment of sepsis.
Crosstalk between metabolism and novel protein acylation in sepsis
PTMs have emerged as pivotal regulators in shaping protein functions, providing novel insights into the intricate interplay between metabolic and phenotypic regulation in sepsis. Metabolites can modulate protein acylation by serving as acyl group donors or by altering the activity of acyltransferases and deacylases. Conversely, protein acylation participates in critical cellular processes associated with both physiology and pathology, including protein stability, subcellular localization, enzymatic activity, transcriptional regulation, protein-protein interactions, and protein-DNA interactions (112). Acyl-CoA acts as an acyl group donor in protein acylation modifications, primarily sourced from metabolic products of glucose, fatty acids, and amino acids (Figure 2). For example, lactate, a product of glycolysis and a key energy source, plays a crucial role in lactylation modification. Additionally, lipid β-oxidation increases the abundance of crotonyl-CoA, β-hydroxybutyryl-CoA, and palmitoyl-CoA, thereby facilitating the corresponding modifications. In addition, amino acid catabolism significantly contributes to acetylation and crotonylation via acetyl-CoA and crotonyl-CoA. The levels of protein acylation are closely associated with intracellular acyl-CoA concentrations and are thus dynamically regulated by metabolic status (113). In sepsis, the activation of inflammatory factors such as IL-1, IL-6, and TNF-α can directly or indirectly mediate metabolic alterations (114). Additionally, during the acute phase of sepsis, extensive immune system activation drives a shift in energy metabolism from oxidative phosphorylation and fatty acid β-oxidation to glycolysis, a process commonly referred to as the Warburg effect (115). PKM2, a critical enzyme in glycolysis, has been shown to reduce lactate production and cytokine release both in vitro and in vivo upon knockdown, thereby protecting mice from fatal endotoxemia and sepsis-related damage (116). During sepsis, significant disruptions occur not only in glucose metabolism but also in lipid and amino acid metabolism, resulting in changes to cellular metabolite levels and activity. The modification of these metabolites can influence the pathophysiological processes of sepsis by modulating enzyme activity or altering the structure and interactions of proteins (73). Notably, acylation modifications can take place in histones, affecting gene expression regulation, or in non-histones, impacting their function or protein-protein interactions (117, 118). Thus, alterations in metabolite levels during sepsis can modulate inflammation and immune responses by regulating the levels of acylation modifications. In summary, nearly all types of acylation modifications correspond to metabolic changes. The metabolic reprogramming and shifts in the external metabolic microenvironment during sepsis can regulate protein acylation. Conversely, protein acylation modifications can also reprogram and influence the metabolic state (112).In summary, future research should further explore metabolic reprogramming and protein acylation modifications, offering deeper insights into the interrelationship between metabolic reprogramming, acylation modifications, and sepsis.
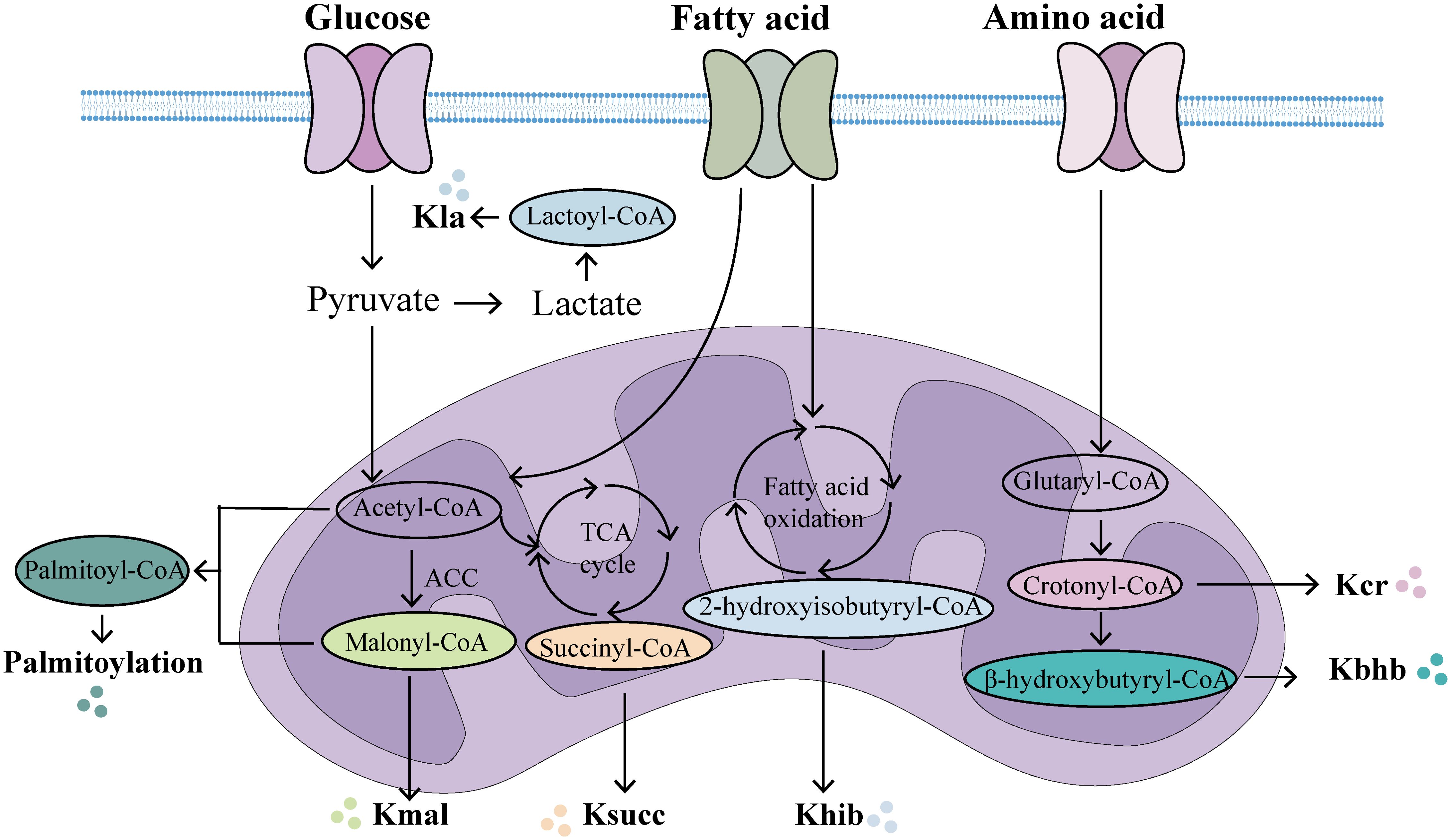
Figure 2. The main metabolic origins of novel acylations. In the mitochondria, multiple metabolic pathways generate acyl-CoA, which provides acylation groups to covalently modify proteins. (Created with BioGDP.com). Kla, lactylation; Kcr, crotonylation; Kbhb, β-hydroxybutyrylation; Khib, 2-hydroxyisobutyrylation; Ksucc, succinylation; Kmal, malonylation.
Summary and perspectives
Sepsis is a life-threatening condition triggered by infection, leading to a systemic inflammatory response syndrome and often resulting in multiple organ dysfunction (119, 120). Current treatment strategies emphasize comprehensive interventions, including antibiotic therapy, fluid resuscitation, hemodynamic support, immune modulation, and organ support (121, 122). However, due to the complex pathophysiology and high heterogeneity of sepsis, no single treatment has been proven effective in improving patient survival. As a result, the exploration of new therapeutic approaches is essential. Recently, novel acylation modifications have attracted significant attention as promising therapeutic targets. This review highlights recent advances in protein acylation research, focusing on the properties and functions of seven novel acylation modifications and their potential relevance in sepsis and sepsis-induced multiple organ dysfunction.
Currently, several studies have extensively investigated novel pharmacological agents targeting sepsis-induced acylation modifications. Research has demonstrated that myeloid differentiation primary response 88 (MYD88) serves as the most prevalent adaptor molecule within the Toll-like receptor (TLR) family. Inhibition of MYD88 S-palmitoylation has been shown to suppress TLR-mediated inflammatory responses (123). 2-bromopalmitic acid (2BP) is a commonly used irreversible inhibitor of palmitoylation that suppresses TLR2 S-palmitoylation, resulting in reduced TLR2 expression on the plasma membrane and attenuated inflammatory responses (124). However, due to its toxicity and off-target effects, including interference with fatty acid metabolism, its clinical application is not feasible (125). Furthermore, selectively manipulating the palmitoylation status of specific target proteins may offer a novel approach to mitigate adverse effects (126). The clinical application of the depalmitoylation inhibitor Palm-B is also limited due to its inhibitory effect on the activity of a series of serine hydrolases (127). A feasible strategy for drug development involves screening compound libraries. In a recent study, screening of a covalent compound library containing 565 compounds identified NU6300 as capable of covalently reacting with C191 of GSDMD. This interaction blocks GSDMD cleavage and palmitoylation, thereby inhibiting GSDMD-N membrane translocation and subsequent oligomerization. The compound effectively suppressed pyroptosis both in vivo and ex vivo, while mitigating inflammatory responses (128). Metabolic reprogramming represents one of the hallmark characteristics of sepsis. Metabolic intermediates can directly drive novel acylation modifications, modulating chromatin structure and gene transcription, thereby forming intricate and dynamic feedback loops with epigenetic regulation. These metabolism-driven PTMs not only demonstrate the direct substrate role of metabolites but also highlight the bidirectional regulatory relationship between metabolism and epigenetic processes. Therefore, alongside pharmacological treatments, dietary strategies such as ketogenic diets and restrictions on glucose or long-chain fatty acid intake may prevent or treat sepsis by modulating protein acylation through metabolic intermediates. However, the optimal concentration of BHB that can be employed without inducing lethal acidosis remains unclear. Carefully designed clinical and preclinical studies are required to achieve a comprehensive understanding of the molecular basis of BHB in epigenetics and other biological processes, particularly considering its interplay with other acylation modifications and potential side effects induced by ketosis-promoting therapeutic interventions. With advancing understanding of novel acylation modifications in sepsis, it is warranted to anticipate that further studies will be conducted to explore effective therapeutic strategies for sepsis management.
Although PTMs hold significant potential as novel therapeutic targets for sepsis, they currently face several challenges. Firstly, despite rapid advancements in mass spectrometry technology, detection sensitivity may remain insufficient for low-abundance proteins and amino acid residues with low modification stoichiometry (10). Additionally, certain detection methods may exhibit specificity issues, potentially leading to false-positive or false-negative identification of PTMs. Secondly, when multiple modifications occur on the same amino acid residue, potential interactions between different PTMs may arise, thereby increasing the complexity of PTM networks (129). Furthermore, PTMs typically occur rapidly and are difficult to quantify, making the investigation of their crosstalk particularly challenging (130). Thirdly, as PTMs predominantly occur intracellularly, direct targeting of specific modifications through external drug administration remains technically demanding. Even if intracellular drug delivery is achieved, ensuring precise targeting of the intended PTMs presents a significant pharmacological challenge (131). In conclusion, continuous development and refinement of detection technologies are required to overcome these limitations, thereby enabling a more comprehensive understanding of protein functions and mechanisms of action. Once these challenges are addressed, acylation modifications are expected to play a more significant role in the diagnosis and treatment of sepsis and its associated multiple organ dysfunction syndrome.
This review offering new insights into novel acylation modifications in the pathogenesis of the sepsis. Through a comprehensive investigation of the signaling pathways and molecular mechanisms that regulate PTMs, we aim to clarify their specific involvement in sepsis-induced organ dysfunction, providing a stronger theoretical basis for the development of targeted drugs and therapeutic strategies. This study has several limitations. First, a considerable proportion of existing studies have employed LPS-induced inflammatory models, either in vitro or in vivo, to investigate the functions of acylation modifications. While LPS stimulation effectively mimics the early hyperinflammatory phase of sepsis by activating TLR4-mediated signaling cascades, it fails to recapitulate the complex immunological dynamics observed in clinical sepsis. Second, the temporal and spatial dynamics of acylation modifications during the progression of sepsis remain largely unexplored. Large-scale clinical studies are needed to evaluate the diagnostic and prognostic value of these novel acylation modifications in sepsis.
Author contributions
JW: Software, Funding acquisition, Data curation, Writing – original draft. AH: Writing – review & editing, Methodology. LS: Software, Writing – review & editing. WJ: Writing – review & editing, Investigation. LX: Writing – review & editing. RZ: Funding acquisition, Writing – review & editing, Supervision. JY: Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX25_2403). National key clinical specialty, Financial Appropriations of National No.176 (2022); Jiangsu Provincial Medical Key Discipline Cultivation Unit (JSDW20221); Yancheng Science and Technology Bureau (YCBE202365); Jiangsu Vocational College of Medicine’s School-Local Collaborative Innovation Research Project (202491001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801. doi: 10.1001/jama.2016.0287
2. Gavelli F, Castello LM, and Avanzi GC. Management of sepsis and septic shock in the emergency department. Internal Emergency Med. (2021) 16:1649–61. doi: 10.1007/s11739-021-02735-7
3. Liu D, Huang S-Y, Sun J-H, Zhang H-C, Cai Q-L, Gao C, et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Military Med Res. (2022) 9:56. doi: 10.1186/s40779-022-00422-y
4. Song L, Jiang W, Lin H, Yu J, Liu K, and Zheng R. Post-translational modifications in sepsis-induced organ dysfunction: Mechanisms and implications. Front Immunol. (2024) 15:1461051. doi: 10.3389/fimmu.2024.1461051
5. Ling C and Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. (2019) 29:1028–44. doi: 10.1016/j.cmet.2019.03.009
6. Shi H, Cui W, Qin Y, Chen L, Yu T, and Lv J. A glimpse into novel acylations and their emerging role in regulating cancer metastasis. Cell Mol Life Sci. (2024) 81:76. doi: 10.1007/s00018-023-05104-z
7. Yao W, Hu X, and Wang X. Crossing epigenetic frontiers: The intersection of novel histone modifications and diseases. Sig Transduct Target Ther. (2024) 9:232. doi: 10.1038/s41392-024-01918-w
8. Fang J, Wu S, Zhao H, Zhou C, Xue L, Lei Z, et al. New types of post-translational modification of proteins in cardiovascular diseases. J Cardiovasc Transl Res. (2025) 18:634–49. doi: 10.1007/s12265-025-10600-7
9. Cheng X, Wang K, Zhao Y, and Wang K. Research progress on post-translational modification of proteins and cardiovascular diseases. Cell Death Discov. (2023) 9:275. doi: 10.1038/s41420-023-01560-5
10. Li X, Yu T, Li X, He X, Zhang B, and Yang Y. Role of novel protein acylation modifications in immunity and its related diseases. Immunology. (2024) 173:53–75. doi: 10.1111/imm.13822
11. Li Z, Chen J, Huang H, Zhan Q, Wang F, Chen Z, et al. Post-translational modifications in diabetic cardiomyopathy. J Cell Mol Medi. (2024) 28:e18158. doi: 10.1111/jcmm.18158
12. Zhang Z, Tan M, Xie Z, Dai L, Chen Y, and Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. (2011) 7:58–63. doi: 10.1038/nchembio.495
13. Zhang X, Ling C, Xiong Z, Gong T, Luo S, Liu X, et al. Desuccinylation of TBK1 by SIRT5 regulates inflammatory response of macrophages in sepsis. Cell Rep. (2024) 43:115060. doi: 10.1016/j.celrep.2024.115060
14. Zhang Z, Chen Y, Fang L, Zhao J, and Deng S. The involvement of high succinylation modification in the development of prostate cancer. Front Oncol. (2022) 12:1034605. doi: 10.3389/fonc.2022.1034605
15. Chinopoulos C. The mystery of extramitochondrial proteins lysine succinylation. IJMS. (2021) 22:6085. doi: 10.3390/ijms22116085
16. Wagner GR and Payne RM. Widespread and enzyme-independent nepsilon-acetylation and nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. (2013) 288:29036–45. doi: 10.1074/jbc.M113.486753
17. Li D, Zhang L, He Y, Zhou T, Cheng X, Huang W, et al. Novel histone post-translational modifications in diabetes and complications of diabetes: The underlying mechanisms and implications. Biomedicine Pharmacotherapy. (2022) 156:113984. doi: 10.1016/j.biopha.2022.113984
18. Sun L, Zhang H, and Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. (2022) 13:877–919. doi: 10.1007/s13238-021-00846-7
19. Kurmi K, Hitosugi S, Wiese EK, Boakye-Agyeman F, Gonsalves WI, Lou Z, et al. Carnitine palmitoyltransferase 1A has a lysine succinyl transferase activity. Cell Rep. (2018) 22:1365–73. doi: 10.1016/j.celrep.2018.01.030
20. Zhu X-X, Zheng G-L, Lu Q-B, Su J-B, Liu Y, Wang M, et al. Cichoric acid ameliorates sepsis-induced acute kidney injury by inhibiting M1 macrophage polarization. Eur J Pharmacol. (2024) 976:176696. doi: 10.1016/j.ejphar.2024.176696
21. Sun H-J, Zheng G-L, Wang Z-C, Liu Y, Bao N, Xiao P-X, et al. Chicoric acid ameliorates sepsis-induced cardiomyopathy via regulating macrophage metabolism reprogramming. Phytomedicine. (2024) 123:155175. doi: 10.1016/j.phymed.2023.155175
22. Wang Z, Liu Z, Lv M, Luan Z, Li T, and Hu J. Novel histone modifications and liver cancer: Emerging frontiers in epigenetic regulation. Clin Epigenet. (2025) 17:30. doi: 10.1186/s13148-025-01838-8
23. Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyl transferase. Nature. (2017) 552:273–7. doi: 10.1038/nature25003
24. Green SR and Storey KB. Skeletal muscle of torpid Richardson’s ground squirrels (Urocitellus richardsonii) exhibits a less active form of citrate synthase associated with lowered lysine succinylation. Cryobiology. (2021) 101:28–37. doi: 10.1016/j.cryobiol.2021.06.006
25. Zhang Y and Goetzman E. The enzyme activity of mitochondrial trifunctional protein is not altered by lysine acetylation or lysine succinylation. PloS One. (2021) 16:e0256619. doi: 10.1371/journal.pone.0256619
26. Lukey MJ, Greene KS, and Cerione RA. Lysine succinylation and SIRT5 couple nutritional status to glutamine catabolism. Mol Cell Oncol. (2020) 7:1735284. doi: 10.1080/23723556.2020.1735284
27. Lagunas-Rangel FA. The dark side of SIRT7. Mol Cell Biochem. (2024) 479:2843–61. doi: 10.1007/s11010-023-04869-y
28. Bai W, Cheng L, Xiong L, Wang M, Liu H, Yu K, et al. Protein succinylation associated with the progress of hepatocellular carcinoma. J Cell Mol Med. (2022) 26:5702–12. doi: 10.1111/jcmm.17507
29. Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. (2017) 19:2331–44. doi: 10.1016/j.celrep.2017.05.065
30. Wang L, Fan H, Sun M, and Ye J. SIRT5-mediated HOXA5 desuccinylation inhibits ferroptosis to alleviate sepsis induced-lung injury. Kaohsiung J Med Scie. (2025) 41:e12921. doi: 10.1002/kjm2.12921
31. Wu J and Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. (2014) 32:461–88. doi: 10.1146/annurev-immunol-032713-120156
32. Zhu Y, Chen X, Lu Y, Xia L, Fan S, Huang Q, et al. Glutamine mitigates murine burn sepsis by supporting macrophage M2 polarization through repressing the SIRT5-mediated desuccinylation of pyruvate dehydrogenase. Burns Trauma. (2022) 10:tkac041. doi: 10.1093/burnst/tkac041
33. Yang H-Q, Martinez-Ortiz W, Hwang J, Fan X, Cardozo TJ, and Coetzee WA. Palmitoylation of the KATP channel Kir6.2 subunit promotes channel opening by regulating PIP2 sensitivity. Proc Natl Acad Sci U.S.A. (2020) 117:10593–602. doi: 10.1073/pnas.1918088117
34. Huang K-Y, Lee T-Y, Kao H-J, Ma C-T, Lee C-C, Lin T-H, et al. dbPTM in 2019: Exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. (2019) 47:D298–308. doi: 10.1093/nar/gky1074
35. Chang Y, Zhu J, Li X, Deng Y, Lai B, Ma Y, et al. Palmitoylation regulates myelination by modulating the ZDHHC3-Cadm4 axis in the central nervous system. Sig Transduct Target Ther. (2024) 9:254. doi: 10.1038/s41392-024-01971-5
36. Abazari D, Wild AR, Qiu T, Dickinson BC, and Bamji SX. Activity-dependent post-translational regulation of palmitoylating and depalmitoylating enzymes in the hippocampus. J Cell Sci. (2023) 136:jcs260629. doi: 10.1242/jcs.260629
37. Dong G, Adak S, Spyropoulos G, Zhang Q, Feng C, Yin L, et al. Palmitoylation couples insulin hypersecretion with β cell failure in diabetes. Cell Metab. (2023) 35:332–344.e7. doi: 10.1016/j.cmet.2022.12.012
38. Zhang Y, Qin Z, Sun W, Chu F, and Zhou F. Function of protein S-palmitoylation in immunity and immune-related diseases. Front Immunol. (2021) 12:661202. doi: 10.3389/fimmu.2021.661202
39. Zhang M, Zhou L, Xu Y, Yang M, Xu Y, Komaniecki GP, et al. A STAT3 palmitoylation cycle promotes TH17 differentiation and colitis. Nature. (2020) 586:434–9. doi: 10.1038/s41586-020-2799-2
40. Chen S, Han C, Miao X, Li X, Yin C, Zou J, et al. Targeting MC1R depalmitoylation to prevent melanomagenesis in redheads. Nat Commun. (2019) 10:877. doi: 10.1038/s41467-019-08691-3
41. Wei X, Adak S, Zayed M, Yin L, Feng C, Speck SL, et al. Endothelial palmitoylation cycling coordinates vessel remodeling in peripheral artery disease. Circ Res. (2020) 127:249–65. doi: 10.1161/CIRCRESAHA.120.316752
42. Beard RS, Yang X, Meegan JE, Overstreet JW, Yang CGY, Elliott JA, et al. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat Commun. (2016) 7:12823. doi: 10.1038/ncomms12823
43. Yang X, Zheng E, Ma Y, Chatterjee V, Villalba N, Breslin JW, et al. DHHC21 deficiency attenuates renal dysfunction during septic injury. Sci Rep. (2021) 11:11146. doi: 10.1038/s41598-021-89983-x
44. Yang X, Zheng E, Chatterjee V, Ma Y, Reynolds A, Villalba N, et al. Protein palmitoylation regulates extracellular vesicle production and function in sepsis. J Extracellular Biol. (2022) 1:7. doi: 10.1002/jex2.50
45. Zhong C, Yang J, Deng K, Lang X, Zhang J, Li M, et al. Tiliroside attenuates NLRP3 inflammasome activation in macrophages and protects against acute lung injury in mice. Molecules. (2023) 28:7527. doi: 10.3390/molecules28227527
46. Gao N, Chen J, Li Y, Ding Y, Han Z, Xu H, et al. The CYP2E1 inhibitor Q11 ameliorates LPS-induced sepsis in mice by suppressing oxidative stress and NLRP3 activation. Biochem Pharmacol. (2023) 214:115638. doi: 10.1016/j.bcp.2023.115638
47. Khodir AE, Samra YA, and Said E. A novel role of nifuroxazide in attenuation of sepsis-associated acute lung and myocardial injuries; role of TLR4/NLPR3/IL-1β signaling interruption. Life Sci. (2020) 256:117907. doi: 10.1016/j.lfs.2020.117907
48. Li J, Wan T, Liu C, Liu H, Ke D, and Li L. ANGPTL2 aggravates LPS-induced septic cardiomyopathy via NLRP3-mediated inflammasome in a DUSP1-dependent pathway. Int Immunopharmacology. (2023) 123:110701. doi: 10.1016/j.intimp.2023.110701
49. Zhu X-X, Meng X-Y, Zhang A, Zhao C-Y, Chang C, Chen T-X, et al. Vaccarin alleviates septic cardiomyopathy by potentiating NLRP3 palmitoylation and inactivation. Phytomedicine. (2024) 131:155771. doi: 10.1016/j.phymed.2024.155771
50. Wang L and Cui J. Palmitoylation promotes chaperone-mediated autophagic degradation of NLRP3 to modulate inflammation. Autophagy. (2023) 19:2821–3. doi: 10.1080/15548627.2023.2187957
51. Wang L, Cai J, Zhao X, Ma L, Zeng P, Zhou L, et al. Palmitoylation prevents sustained inflammation by limiting NLRP3 inflammasome activation through chaperone-mediated autophagy. Mol Cell. (2023) 83:281–297.e10. doi: 10.1016/j.molcel.2022.12.002
52. Huang Y, Xu W, and Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. (2021) 18:2114–27. doi: 10.1038/s41423-021-00740-6
53. Du G, Healy LB, David L, Walker C, El-Baba TJ, Lutomski CA, et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature. (2024) 630:437–46. doi: 10.1038/s41586-024-07373-5
54. Zhang N, Zhang J, Yang Y, Shan H, Hou S, Fang H, et al. A palmitoylation–depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis. Nat Cell Biol. (2024) 26:757–69. doi: 10.1038/s41556-024-01397-9
55. Balasubramanian A, Hsu AY, Ghimire L, Tahir M, Devant P, Fontana P, et al. The palmitoylation of gasdermin D directs its membrane translocation and pore formation during pyroptosis. Sci Immunol. (2024) 9:94. doi: 10.1126/sciimmunol.adn1452
56. Liu Z, Li S, Wang C, Vidmar KJ, Bracey S, Li L, et al. Palmitoylation at a conserved cysteine residue facilitates gasdermin D-mediated pyroptosis and cytokine release. Proc Natl Acad Sci United States America. (2024) 121:29. doi: 10.1073/pnas.2400883121
57. Zhang N, Yang Y, and Xu D. Emerging roles of palmitoylation in pyroptosis. Trends Cell Biol. (2024) 35(6):500–14. doi: 10.1016/j.tcb.2024.10.005
58. Hansen AL, Mukai K, Schopfer FJ, Taguchi T, and Holm CK. STING palmitoylation as a therapeutic target. Cell Mol Immunol. (2019) 16:236–41. doi: 10.1038/s41423-019-0205-5
59. Hopfner K-P and Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signaling. Nat Rev Mol Cell Biol. (2020) 21:501–21. doi: 10.1038/s41580-020-0244-x
60. Yang M, Jiang H, Ding C, Zhang L, Ding N, Li G, et al. STING activation in platelets aggravates septic thrombosis by enhancing platelet activation and granule secretion. Immunity. (2023) 56:1013–1026.e6. doi: 10.1016/j.immuni.2023.02.015
61. Kang J, Wu J, Liu Q, Jiang H, Li W, Li Y, et al. FASN regulates STING palmitoylation via malonyl-CoA in macrophages to alleviate sepsis-induced liver injury. Biochim Biophys Acta (BBA) - Mol Basis Disease. (2024) 1870:167299. doi: 10.1016/j.bbadis.2024.167299
62. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1
63. Wang Y, Chen L, Zhang M, Li X, Yang X, Huang T, et al. Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the ereg/MAPK signaling pathway. Atherosclerosis. (2023) 375:45–58. doi: 10.1016/j.atherosclerosis.2023.05.009
64. Du S, Zhang X, Jia Y, Peng P, Kong Q, Jiang S, et al. Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis-mediated HMGB1 lactylation and secretion of hepatocytes. Theranostics. (2023) 13:3856–71. doi: 10.7150/thno.82607
65. Jia M, Yue X, Sun W, Zhou Q, Chang C, Gong W, et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci Adv. (2023) 9:22. doi: 10.1126/sciadv.adg4993
66. Jiang X, Yang Y, Li X, Li T, Yu T, and Fu X. Lactylation: An innovative approach to disease control. Aging Dis. (2024) 16(4):2130–50. doi: 10.14336/AD.2024.0918
67. Chu X, Di C, Chang P, Li L, Feng Z, Xiao S, et al. Lactylated histone H3K18 as a potential biomarker for the diagnosis and predicting the severity of septic shock. Front Immunol. (2022) 12. doi: 10.3389/fimmu.2021.786666
68. Li X, Shang Y, Zhang J, Mu G, Duan Y, Lu Z, et al. Predictive value of H3K18 lactylation for early detection and prognosis of sepsis-related ARDS: A prospective observational clinical study. Shock. (2025) 64:154–60. doi: 10.1097/SHK.0000000000002601
69. Ma N, Wang L, Meng M, Wang Y, Huo R, Chang G, et al. D-sodium lactate promotes the activation of NF-κB signaling pathway induced by lipopolysaccharide via histone lactylation in bovine mammary epithelial cells. Microbial Pathogenesis. (2025) 199:107198. doi: 10.1016/j.micpath.2024.107198
70. Lu Z, Fang P, Li S, Xia D, Zhang J, Wu X, et al. Lactylation of histone H3k18 and Egr1 promotes endothelial glycocalyx degradation in sepsis-induced acute lung injury. Advanced Science. (2025) 12:2407064. doi: 10.1002/advs.202407064
71. Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, and Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci United States America. (2020) 117:30628–38. doi: 10.1073/pnas.2009778117
72. Wei S, Dai Z, Wu L, Xiang Z, Yang X, Jiang L, et al. Lactate-induced macrophage HMGB1 lactylation promotes neutrophil extracellular trap formation in sepsis-associated acute kidney injury. Cell Biol Toxicol. (2025) 41:78. doi: 10.1007/s10565-025-10026-6
73. Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. (2022) 29:133–46. doi: 10.1038/s41418-021-00841-9
74. Zhu L, Zheng Q, Liu X, Ding H, Ma M, Bao J, et al. HMGB1 lactylation drives neutrophil extracellular trap formation in lactate-induced acute kidney injury. Front Immunol. (2025) 15:1475543. doi: 10.3389/fimmu.2024.1475543
75. Tadie J-M, Bae H-B, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with toll-like receptor 4. Am J Physiology-Lung Cell Mol Physiol. (2013) 304:L342–9. doi: 10.1152/ajplung.00151.2012
76. An S, Yao Y, Hu H, Wu J, Li J, Li L, et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. (2023) 14:457. doi: 10.1038/s41419-023-05952-4
77. Yang P, Qin Y, Zeng L, He Y, Xie Y, Cheng X, et al. Crotonylation and disease: Current progress and future perspectives. Biomedicine Pharmacotherapy. (2023) 165:115108. doi: 10.1016/j.biopha.2023.115108
78. Zhao H, Han Y, Zhou P, Guan H, and Gao S. Protein lysine crotonylation in cellular processions and disease associations. Genes Diseases. (2024) 11:101060. doi: 10.1016/j.gendis.2023.06.029
79. Zhang H, Ma W, Liu H, Tang W, Shu J, Zhou J, et al. Systematic analysis of lysine crotonylation in human macrophages responding to MRSA infection. Front Cell Infection Microbiol. (2023) 13. doi: 10.3389/fcimb.2023.1126350
80. Zhang L, Shi X, Qiu H, Liu S, Yang T, Li X, et al. Protein modification by short-chain fatty acid metabolites in sepsis: A comprehensive review. Front Immunol. (2023) 14:1171834. doi: 10.3389/fimmu.2023.1171834
81. Liu Z, Yang J, Du M, and Xin W. Functioning and mechanisms of PTMs in renal diseases. Front Pharmacol. (2023) 14:1238706. doi: 10.3389/fphar.2023.1238706
82. Huang H, Luo Z, Qi S, Huang J, Xu P, Wang X, et al. Landscape of the regulatory elements for lysine 2-hydroxyisobutyrylation pathway. Cell Res. (2018) 28:111–25. doi: 10.1038/cr.2017.149
83. Wang N, Jiang Y, Peng P, Liu G, Qi S, Liu K, et al. Quantitative proteomics reveals the role of lysine 2-hydroxyisobutyrylation pathway mediated by Tip60. Oxid Med Cell Longevity. (2022) 2022:1–13. doi: 10.1155/2022/4571319
84. Huang H, Tang S, Ji M, Tang Z, Shimada M, Liu X, et al. p300-mediated lysine 2-hydroxyisobutyrylation regulates glycolysis. Mol Cell. (2018) 70:663–678.e6. doi: 10.1016/j.molcel.2018.04.011
85. Lu Y, Li X, Zhao K, Qiu P, Deng Z, Yao W, et al. Global landscape of 2-hydroxyisobutyrylation in human pancreatic cancer. Front Oncol. (2022) 12:1001807. doi: 10.3389/fonc.2022.1001807
86. Ge H, Li B, Chen W, Xu Q, Chen S, Zhang H, et al. Differential occurrence of lysine 2-hydroxyisobutyrylation in psoriasis skin lesions. J Proteomics. (2019) 205:103420. doi: 10.1016/j.jprot.2019.103420
87. Zhao B, Lu R, Chen J, Xie M, Zhao X, and Kong L. S100A9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway. Respir Res. (2021) 22:45. doi: 10.1186/s12931-021-01641-y
88. Dong H, Zhai G, Chen C, Bai X, Tian S, Hu D, et al. Protein lysine de-2-hydroxyisobutyrylation by CobB in prokaryotes. Sci Adv. (2019) 5:eaaw6703. doi: 10.1126/sciadv.aaw6703
89. Xie Z, Zhang D, Chung D, Tang Z, Huang H, Dai L, et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol Cell. (2016) 62:194–206. doi: 10.1016/j.molcel.2016.03.036
90. Zhou T, Cheng X, He Y, Xie Y, Xu F, Xu Y, et al. Function and mechanism of histone β-hydroxybutyrylation in health and disease. Front Immunol. (2022) 13:981285. doi: 10.3389/fimmu.2022.981285
91. Huang H, Zhang D, Weng Y, Delaney K, Tang Z, Yan C, et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci Adv. (2021) 7:eabe2771. doi: 10.1126/sciadv.abe2771
92. Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, and Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. (2019) 9:742. doi: 10.1038/s41598-018-36941-9
93. Liu K, Li F, Sun Q, Lin N, Han H, You K, et al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. (2019) 10:243. doi: 10.1038/s41419-019-1463-y
94. Zhang X, Cao R, Niu J, Yang S, Ma H, Zhao S, et al. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. (2019) 5:35. doi: 10.1038/s41421-019-0103-0
95. Takahara S, Soni S, Maayah ZH, Ferdaoussi M, and Dyck JRB. Ketone therapy for heart failure: Current evidence for clinical use. Cardiovasc Res. (2022) 118:977–87. doi: 10.1093/cvr/cvab068
96. Acar R. Association between beta-hydroxybutyrate levels and survival in sepsis patients. EJMI. (2021) 5(1):39–44. doi: 10.14744/ejmi.2021.15575
97. Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, et al. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A. (2019) 116:13533–42. doi: 10.1073/pnas.1820282116
98. Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. (2021) 128:232–45. doi: 10.1161/CIRCRESAHA.120.317933
99. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. (2013) 339:211–4. doi: 10.1126/science.1227166
100. Zhang L, Shi J, Du D, Niu N, Liu S, Yang X, et al. Ketogenesis acts as an endogenous protective program to restrain inflammatory macrophage activation during acute pancreatitis. EBioMedicine. (2022) 78:103959. doi: 10.1016/j.ebiom.2022.103959
101. Meeusen H, Romagnolo A, Holsink SAC, van den Broek TJM, van Helvoort A, Gorter JA, et al. A novel hepatocyte ketone production assay to help the selection of nutrients for the ketogenic diet treatment of epilepsy. Sci Rep. (2024) 14:11940. doi: 10.1038/s41598-024-62723-7
102. Sukkar SG, Cogorno L, Pisciotta L, Pasta A, Vena A, Gradaschi R, et al. Clinical efficacy of eucaloric ketogenic nutrition in the COVID-19 cytokine storm: A retrospective analysis of mortality and intensive care unit admission. Nutrition. (2021) 89:111236. doi: 10.1016/j.nut.2021.111236
103. Rahmel T, Effinger D, Bracht T, Griep L, Koos B, Sitek B, et al. An open-label, randomized controlled trial to assess a ketogenic diet in critically ill patients with sepsis. Sci Transl Med. (2024) 16:eadn9285. doi: 10.1126/scitranslmed.adn9285
104. Luo W, He M, Luo Q, and Li Y. Proteome-wide analysis of lysine β-hydroxybutyrylation in the myocardium of diabetic rat model with cardiomyopathy. Front Cardiovasc Med. (2022) 9:1066822. doi: 10.3389/fcvm.2022.1066822
105. Weckx R, Goossens C, Derde S, Pauwels L, Vander Perre S, Van Den Bergh G, et al. Identification of the toxic threshold of 3-hydroxybutyrate-sodium supplementation in septic mice. BMC Pharmacol Toxicol. (2021) 22:50. doi: 10.1186/s40360-021-00517-7
106. Weckx R, Goossens C, Derde S, Pauwels L, Vander Perre S, Van Den Berghe G, et al. Efficacy and safety of ketone ester infusion to prevent muscle weakness in a mouse model of sepsis-induced critical illness. Sci Rep. (2022) 12:10591. doi: 10.1038/s41598-022-14961-w
107. Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. (2011) 10:M111.012658. doi: 10.1074/mcp.M111.012658
108. Zhang R, Bons J, Scheidemantle G, Liu X, Bielska O, Carrico C, et al. Histone malonylation is regulated by SIRT5 and KAT2A. iScience. (2023) 26:106193. doi: 10.1016/j.isci.2023.106193
109. Xu H, Wu M, Ma X, Huang W, and Xu Y. Function and mechanism of novel histone posttranslational modifications in health and disease. BioMed Res Int. (2021) 2021:6635225. doi: 10.1155/2021/6635225
110. Galván-Peña S, Carroll RG, Newman C, Hinchy EC, Palsson-McDermott E, Robinson EK, et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat Commun. (2019) 10:338. doi: 10.1038/s41467-018-08187-6
111. Qu L, Lin X, Liu C, Ke C, Zhou Z, Xu K, et al. Atractylodin attenuates dextran sulfate sodium-induced colitis by alleviating gut microbiota dysbiosis and inhibiting inflammatory response through the MAPK pathway. Front Pharmacol. (2021) 12:665376. doi: 10.3389/fphar.2021.665376
112. Shang S, Liu J, and Hua F. Protein acylation: Mechanisms, biological functions and therapeutic targets. Signal Transduct Target Ther. (2022) 7:396. doi: 10.1038/s41392-022-01245-y
113. Xu Y, Shi Z, and Bao L. An expanding repertoire of protein acylations. Mol Cell Proteomics. (2022) 21:100193. doi: 10.1016/j.mcpro.2022.100193
114. Englert JA and Rogers AJ. Metabolism, metabolomics, and nutritional support of patients with sepsis. Clin Chest Med. (2016) 37:321–31. doi: 10.1016/j.ccm.2016.01.011
115. Cheng S-C, Scicluna BP, Arts RJW, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol. (2016) 17:406–13. doi: 10.1038/ni.3398
116. Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the warburg effect and promotes HMGB1 release in sepsis. Nat Commun. (2014) 5:4436. doi: 10.1038/ncomms5436
117. Millán-Zambrano G, Burton A, Bannister AJ, and Schneider R. Histone post-translational modifications - cause and consequence of genome function. Nat Rev Genet. (2022) 23:563–80. doi: 10.1038/s41576-022-00468-7
118. Lee JM, Hammarén HM, Savitski MM, and Baek SH. Control of protein stability by post-translational modifications. Nat Commun. (2023) 14:201. doi: 10.1038/s41467-023-35795-8
119. Vincent J-L. Current sepsis therapeutics. EBioMedicine. (2022) 86:104318. doi: 10.1016/j.ebiom.2022.104318
120. Srzić I, Adam VN, and Pejak DT. Sepsis definition: What’s new in the treatment guidelines. Acta clinica Croatica. (2022) 61:67–72. doi: 10.20471/acc.2022.61.s1.11
121. Backer DD, Deutschman CS, Hellman J, Myatra SN, Ostermann M, Prescott HC, et al. Surviving sepsis campaign research priorities 2023. Crit Care Med. (2024) 52:268–96. doi: 10.1097/CCM.0000000000006135
122. Zampieri FG, Bagshaw SM, and Semler MW. Fluid therapy for critically ill adults with sepsis: A review. JAMA. (2023) 329:1967. doi: 10.1001/jama.2023.7560
123. Kim Y-C, Lee SE, Kim SK, Jang H-D, Hwang I, Jin S, et al. Toll-like receptor mediated inflammation requires FASN-dependent MYD88 palmitoylation. Nat Chem Biol. (2019) 15:907–16. doi: 10.1038/s41589-019-0344-0
124. Guns J, Vanherle S, Hendriks JJA, and Bogie JFJ. Protein lipidation by palmitate controls macrophage function. Cells. (2022) 11:565. doi: 10.3390/cells11030565
125. Davda D, Azzouny MAE, Tom CTMB, Hernandez JL, Majmudar JD, Kennedy RT, et al. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem Biol. (2013) 8:1912–7. doi: 10.1021/cb400380s
126. Fraser NJ, Howie J, Wypijewski KJ, and Fuller W. Therapeutic targeting of protein S-acylation for the treatment of disease. Biochem Soc Trans. (2020) 48:281–90. doi: 10.1042/BST20190707
127. Lin DTS and Conibear E. ABHD17 proteins are novel protein depalmitoylases that regulate N-ras palmitate turnover and subcellular localization. eLife. (2015) 4:e11306. doi: 10.7554/eLife.11306.017
128. Jiang X, Zhang X, Cai X, Li N, Zheng H, Tang M, et al. NU6300 covalently reacts with cysteine-191 of gasdermin D to block its cleavage and palmitoylation. Sci Adv. (2024) 10(6):eadi9284. doi: 10.1126/sciadv.adi9284
129. Diskin C, Ryan TAJ, and O’Neill LAJ. Modification of proteins by metabolites in immunity. Immunity. (2021) 54:19–31. doi: 10.1016/j.immuni.2020.09.014
130. Ji F, Zhou M, Zhu H, Jiang Z, Li Q, Ouyang X, et al. Integrative proteomic analysis of multiple posttranslational modifications in inflammatory response. Genomics Proteomics Bioinf. (2022) 20:163–76. doi: 10.1016/j.gpb.2020.11.004
Keywords: sepsis, epigenetics, acylation, organ dysfunction, inflammation
Citation: Wang J, He A, Song L, Jiang W, Xu L, Zheng R and Yu J (2025) Role of novel protein acylation modifications in sepsis. Front. Immunol. 16:1657194. doi: 10.3389/fimmu.2025.1657194
Received: 01 July 2025; Accepted: 18 September 2025;
Published: 02 October 2025.
Edited by:
Fangchen Gong, Shanghai Jiao Tong University, ChinaReviewed by:
Wolfgang Vivas, University Hospital Jena, GermanyDa Gu, Dushu Lake Hospital Affiliated to Soochow University, China
Copyright © 2025 Wang, He, Song, Jiang, Xu, Zheng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiqiang Zheng, emhlbmdydWlxaWFuZzIwMjFAMTYzLmNvbQ==; Jiangquan Yu, eXVqaWFuZ3F1YW4yMDIxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jing Wang
Jing Wang Aifeng He
Aifeng He Lin Song
Lin Song Wei Jiang1,2
Wei Jiang1,2 Ruiqiang Zheng
Ruiqiang Zheng Jiangquan Yu
Jiangquan Yu