- 1Biotechnology Center, Federal University of Paraíba, João Pessoa, Paraíba, Brazil
- 2Laboratory of Pharmacology and Immunity, Institute of Biological Sciences and Health, Federal University of Alagoas, Maceió, Brazil
- 3Medical Sciences and Nursing Complex, Federal University of Alagoas, Arapiraca, Alagoas, Brazil
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults, characterized by rapid proliferation, diffuse infiltration, and resistance to conventional therapies. Despite advances in surgery, radiotherapy, and chemotherapy, the prognosis remains dismal, with median survival rarely exceeding 15 months. The immunosuppressive and heterogeneous tumor microenvironment (TME), along with profound tumor-intrinsic resistance mechanisms, contributes significantly to treatment failure. Cardiotonic steroids (CTS), such as ouabain, have recently gained attention for their pleiotropic effects beyond Na+/K+-ATPase inhibition, including modulation of intracellular signaling, induction of cell death, and immune regulation. In GBM, ouabain has been shown to reduce tumor cell viability, impair migration, disrupt angiogenesis, and alter different signaling pathways. Although direct evidence of ouabain’s effects on the GBM immune microenvironment is limited, findings from other models suggest that it can modulate both innate and adaptive immune responses, affecting T cells, regulatory T cells, dendritic cells, monocytes, and NK cells. While previous reviews have explored the anticancer and pharmacological aspects of cardiotonic steroids, the immunological dimension of ouabain’s activity remains underrepresented. This review integrates current evidence on ouabain’s dual actions in tumor biology and immune regulation, emphasizing its emerging therapeutic potential and the need for deeper investigation within high-grade glioma models.
1 Introduction
Glioblastoma (GBM) is the most aggressive primary brain tumor, characterized by rapid proliferation, diffuse invasion into surrounding brain tissue, and resistance to conventional therapies (1, 2). Standard treatment typically involves maximal surgical resection followed by radiotherapy and chemotherapy [i.e., temozolomide (TMZ)] (3). However, despite their widespread use, the success rate of treatment remains low due to intrinsic and acquired resistance mechanisms. Tumor cells often develop resistance through O6-methylguanine-DNA methyltransferase (MGMT) expression, DNA repair pathways, and metabolic adaptations that allow survival even with cytotoxic drugs (4–6). As a result, median survival for GBM patients remains approximately 12–15 months (7, 8), underscoring the urgent need for novel therapeutic approaches.
The highly heterogeneous and immunosuppressive tumor microenvironment (TME) in GBM contributes to tumor progression and therapeutic resistance by impairing anti-tumor immune responses and promoting tumor-supportive interactions (9, 10). Non-immunological components such as the vasculature, cancer stem cells (CSCs), astrocytes and neurons actively contribute to sustaining the tumor microenvironment, providing structural support, modulating metabolic exchanges, and influencing tumor plasticity (11–14). Among the main immune components in the GBM TME are tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), all of which actively suppress tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells (15, 16), thereby facilitating immune evasion and challenging the use of immunotherapies (17, 18).
One potential approach for therapeutic intervention involves targeting ion homeostasis in both cancer and immune cells (19–21). Ouabain, a cardiotonic steroid known for its ability to inhibit the ubiquitous ion pump Na+/K+-ATPase at higher concentrations, has emerged as a potential modulator of cellular signaling beyond its classical role in cardiac function (22, 23). At lower concentrations, ouabain induces calcium oscillations leading to the activation of important signaling pathways involved in cellular homeostasis and function (23–26). Different studies in tumor models suggest that ouabain can influence tumor cell proliferation, apoptosis, and migration, including in GBM cell lines (26–32). Additionally, ouabain can potentially modulate immune cell activity in the TME, shifting the balance from an immunosuppressive to an anti-tumor immune response (33, 34).
Given ouabain’s dual role in cancer and immune cell modulation, understanding its effects in the GBM TME could offer new insights into its therapeutic potential. Although few comprehensive reviews have examined CTS as anticancer agents, most have centered on their cytotoxicity, structure–activity relationships, or general pharmacology across multiple malignancies (35–37). In contrast, the present review provides a focused analysis of ouabain, emphasizing not only its established effects on GBM cell survival and signaling but also its underexplored immunomodulatory properties. Given the absence of studies directly assessing ouabain in the GBM TME, we draw on findings from other tumor and immune models to infer how ouabain might reshape immune–tumor dynamics in GBM. This integrative perspective bridges tumor-intrinsic and immune-related mechanisms, offering a framework to understand ouabain’s multifaceted therapeutic potential in high-grade gliomas.
2 GBM architecture: from the core to the neighborhood
Glioblastoma is characterized by profound cellular heterogeneity and a complex genomic landscape, with frequent alterations in pathways regulating proliferation, apoptosis, metabolism, and DNA repair, all of which contribute to therapeutic resistance and complicate effective disease management (38). Beyond its intrinsic resistance to treatment, GBM’s malignant behavior is also shaped by a complex TME that includes both tumor-intrinsic and tumor-extrinsic components (39). A comprehensive understanding of this integrated tumor microenvironmental network is essential for identifying mechanisms of GBM progression and uncovering novel therapeutic targets.
At the tumor core, GBM cells exhibit substantial genetic and phenotypic heterogeneity, often driven by mutations in critical regulators such as IDH1/2, EGFR, TP53, and PTEN (40). The presence of glioma stem-like cells (GSCs), typically marked by CD133 expression, contributes significantly to tumor persistence and therapeutic failure, as these cells exhibit enhanced capacities for self-renewal, resistance to apoptosis, and evasion of immune surveillance (41). GSCs also actively remodel the microenvironment by secreting factors such as CSF-1 and CCL2, which recruit monocytes and promote their polarization into tumor-supportive macrophages (42, 43).
The TME of GBM exhibits distinct features compared to other solid tumors, owing primarily to its localization within the CNS and its unique immune and stromal composition. It is broadly categorized into immunological and non-immunological compartments, both of which interact extensively with tumor cells to support disease progression (11). Non-immunological components include a highly disorganized and permeable vasculature, a rigid and biochemically active extracellular matrix (ECM), reactive astrocytes, and neurons that undergo functional reprogramming in response to tumor-derived signals (12, 44–47). The abnormal vasculature contributes to elevated interstitial fluid pressure and regional hypoxia, which in turn stabilizes HIF-1α, an important transcriptional regulator that promotes angiogenesis, metabolic reprogramming, and cellular adaptation to oxygen deprivation, including a shift toward aerobic glycolysis consistent with the Warburg effect (48, 49).
Neurons and astrocytes, once considered passive bystanders, are now recognized as active contributors to tumor progression (13). Neuronal activity has been shown to directly influence tumor growth through the activity-dependent release of neuroligin-3 (NLGN3), which promotes GBM cell proliferation via activation of the PI3K-mTOR signaling pathway (50, 51). Astrocytes similarly contribute to tumor maintenance by secreting a variety of mitogenic and trophic factors, including cytokines and growth factors that enhance glioma cell survival, invasion, and therapy resistance (14, 52).
The immunological compartment of the GBM TME is dominated by immunosuppressive mechanisms that hinder effective antitumor responses (9). TAMs, which may arise from resident microglia or peripheral monocytes, are the most abundant immune cells in GBM (53). These cells are often polarized to an M2-like phenotype, characterized by high expression of IL-10, TGF-β, and MMPs, contributing to tissue remodeling, angiogenesis, and immunosuppression. Notably, bone marrow–derived TAMs constitute approximately 85% of the macrophage population in GBM and exhibit particularly tumor-promoting features (54).
T cell infiltration in GBM is often sparse and functionally compromised (55). The T cell compartment is enriched in Tregs, which suppress effector responses, while cytotoxic CD8+ T cells frequently exhibit an exhausted phenotype, marked by the upregulation of multiple immune checkpoint receptors, including PD-1, TIM-3, LAG-3, and CTLA-4 (56–58). This dysfunctional state is associated with reduced proliferative capacity, diminished cytokine production, and impaired cytotoxic function (59). The extent and composition of T cell infiltration appear to be influenced by IDH mutation status. IDH-wild-type tumors tend to display greater immune cell infiltration and elevated immune checkpoint expression, whereas IDH-mutant GBMs are typically characterized by a more immunologically quiescent microenvironment with limited lymphocyte presence and reduced immunogenicity (60).
Beyond the limited and dysfunctional T cell compartment, innate immune cells constitute a substantial portion of the GBM TME and play diverse, often immunosuppressive roles. NK cells can recognize and eliminate GSCs, and their presence has been associated with favorable outcomes in specific glioma subtypes (61–63). However, in this scenario, NK cell function is frequently impaired due to chronic activation, exposure to immunosuppressive cytokines, and the expression of inhibitory ligands such as B7-H6 on tumor cells, which contribute to NK cell exhaustion (64, 65). Moreover, NK cells interact with DCs through chemokines such as XCL1 and FLT3L, promoting the recruitment of conventional type 1 dendritic cells (cDC1) that are critical for cross-presentation of tumor antigens (66, 67). Despite this crosstalk, DCs in high-grade gliomas often exhibit functional deficiencies, particularly in the context of IDH-mutant tumors, where impaired antigen presentation further compromises antitumor immunity (68).
Additional myeloid populations, including neutrophils and MDSCs, further contribute to immune evasion and tumor progression. Neutrophils can exert protumor effects through the secretion of pro-inflammatory and pro-angiogenic mediators such as S100A4 and IL-8, and by forming neutrophil extracellular traps (NETs) that activate NF-κB signaling in glioma cells (69–71). Their role in GBM is highly context-dependent, as they exhibit considerable phenotypic plasticity, shifting between tumor-promoting (N2-like) and potentially tumor-inhibiting (N1-like) states depending on microenvironmental cues (72). Neutrophils actively migrate into the GBM TME primarily from the skull and vertebral bone marrow, utilizing specialized cranial bone channels and intracranial lymphatic vessels. Once within the TME, tumor-associated neutrophils (TANs) preferentially localize in necrotic tumor cores and undergo functional reprogramming, sustaining tumor progression through the release of NETs and immunosuppressive mediators (72, 73). MDSCs, which are commonly expanded in GBM, suppress T cell proliferation and cytokine production through arginase-1 activity, nitric oxide production, and the release of immunosuppressive cytokines, thereby reinforcing the profoundly suppressive immune landscape characteristic of this malignancy (74–76).
Altogether, the TME of GBM is a tightly regulated network in which tumor cells, immune components, and neural elements interact to create a profoundly immunosuppressive and tumor-supportive niche. This complexity not only contributes to therapeutic resistance but also poses a significant challenge for the development of effective immunotherapies. Targeting this microenvironment, whether by reprogramming TAMs, enhancing NK and T cell function, or disrupting tumor-neuron crosstalk, represents a promising frontier in GBM research.
3 GBM modulation by ouabain
Over the past decade, there has been increasing attention on CTS for their emerging antitumor properties (35). Originally recognized for their role in cardiovascular therapy by inhibiting the Na+/K+-ATPase pump, CTS have demonstrated pleiotropic effects in cancer models, including the modulation of cell proliferation, apoptosis, angiogenesis, and immune responses (36). Among these compounds, ouabain stands out because of its well-characterized molecular targets and its capacity to influence intracellular signaling pathways in a concentration-dependent manner (23, 77). Although the antitumor potential of CTS has been investigated in several malignancies (37, 78), evidence specifically linking ouabain to GBM is beginning to emerge.
Different studies have explored the multifaceted effects of ouabain in GBM cells. By interacting with the Na+/K+-ATPase, ouabain influences intracellular ion balance; however, its biological impact extends well beyond this canonical function, affecting key signaling pathways and cellular processes that are central to GBM pathophysiology.
One of the most consistent findings across in vitro studies is the capacity of ouabain to impair GBM cell viability. In both TMZ-sensitive and TMZ-resistant glioma cell lines, ouabain reduces proliferation and promotes cell death through mechanisms that involve apoptosis, necrosis, or a hybrid mechanism (30, 79), as well as suppressing tumor growth in vivo (80). This cytotoxic effect appears to be mediated, at least in part, by mitochondrial pathways, with activation of pro-apoptotic proteins such as Bak and an increase in reactive oxygen species (ROS) (30). Notably, the study by Yan et al. (2015) revealed that ouabain-induced ROS production is regulated via the ERK-p66Shc pathway, suggesting a specific molecular cascade through which oxidative stress is triggered (32). This ROS generation contributes to mitochondrial dysfunction and ultimately to apoptotic cell death (81), providing a mechanistic explanation for the antitumor activity observed.
In addition to its pro-apoptotic properties, ouabain has been shown to impair GBM cell migration and invasion, likely through the disruption of the Akt/mTOR signaling axis, as observed in U-87MG cells (31). This pathway is crucial not only for cell movement but also for metabolic adaptation and resistance to stress (82, 83), implying that ouabain may impair GBM cells’ ability to survive in its hostile microenvironment (84). Interestingly, while most studies report a downregulation of Akt signaling following ouabain exposure, Weidemann et al. (2023) demonstrated a concentration-dependent modulation in TMZ-resistant T98G cells, with a marked upregulation of phosphorylated Akt at 0.1 µM and a significant downregulation of pan-Akt at 1 µM (30). These divergent responses suggest a context- and dose-dependent effect of ouabain, potentially reflecting adaptive signaling mechanisms in resistant GBM phenotypes.
Supporting this, Hsu et al. (2015) demonstrated that ouabain induces cytosolic acidification and downregulates phosphorylated Akt in GBM cells, further promoting mitochondrial apoptosis through Bak activation. Although less potent than Epi-reevesioside F, a cardiac glycoside evaluated in the study, ouabain exhibited similar mechanisms of action, reinforcing the role of Na+/K+-ATPase inhibition in disrupting metabolic homeostasis and triggering cell death in GBM (85).
The interaction of ouabain with angiogenesis also adds another dimension to its therapeutic potential. GBM relies heavily on the formation of abnormal vasculature to sustain its rapid growth, largely driven by hypoxia-inducible factors such as HIF-1α. Ouabain has been shown to inhibit VEGF-A–induced angiogenesis in vitro, with submicromolar potency in HUVEC spheroids, and to suppress HIF-1α expression, which could limit the tumor’s capacity to establish and maintain blood supply (30). This anti-angiogenic effect aligns with ouabain’s ability to interfere with pro-survival and pro-growth pathways and reinforces its potential role in targeting the GBM microenvironment.
On a broader scale, ouabain treatment in GBM culture has also been associated with changes in Na+/K+-ATPase subunit expression, as shown in early studies demonstrating marked upregulation of α1 and α3 isoforms following exposure (86). These alterations may represent compensatory responses but also underscore the role of Na+/K+-ATPase as more than a passive ion transporter, serving instead as a signaling platform that interacts with oncogenic networks.
This understanding of ouabain’s molecular targets has prompted interest in identifying tumor-specific markers of sensitivity. Recent pan-cancer data also explore the potential selectivity of ouabain in glioma. Zhang et al. (2024) identified a negative correlation between PLAT expression, a venous thromboembolism-associated gene upregulated in gliomas, and ouabain sensitivity, suggesting that tumors with high PLAT levels may be more susceptible to ouabain’s cytotoxic effects (87). Although not experimentally validated in GBM models, these findings offer a rationale for further exploring ouabain responsiveness in molecularly stratified glioma subtypes.
In addition to its direct cytotoxic and signaling-modulatory effects on GBM cells, ouabain has also been implicated in regulating immune cell activity across multiple biological systems. Although tumor-intrinsic effects of ouabain are increasingly documented, its impact on the tumor immune microenvironment (TIME) in GBM remains poorly defined. Despite direct evidence of ouabain’s immunomodulatory effects within the GBM microenvironment is limited, drawing on findings from other tumor models and immune studies provides a preliminary framework to hypothesize how ouabain might influence glioma-associated immune cells.
CTS can modulate antitumor immunity beyond their canonical cytotoxic roles. These compounds can induce immunogenic cell death (ICD) characterized by calreticulin exposure, ATP and HMGB1 release, and secretion of HSP70/90, thereby promoting dendritic cell activation and antigen presentation. Mechanistically, ICD triggered by CTS has been linked to activation of the PERK/eIF2α/ATF4/CHOP pathway, connecting endoplasmic reticulum stress to adaptive immune priming (88).
In addition, these molecules can reshape the TIME by modulating checkpoint and cytokine signaling pathways. Ouabain and related compounds can influence the expression of PD-L1 and other immunoregulatory molecules, as well as enhance antigen-presentation machinery, suggesting a context-dependent dual role in immune evasion and sensitization to checkpoint blockade (34). It has also been demonstrated that digoxin alters myeloid cell composition and promotes early inflammatory remodeling, while Na+/K+-ATPase inhibition by CTS can activate the NLRP3 inflammasome and IL-1β release, fostering local immune activation (89, 90). NLRP3 inflammasome activation is well known to play a dual role in cancer immunity, promoting both inflammatory anti-tumor responses and, in some cases, tumor progression depending on the microenvironment (91, 92). These findings indicate that cardiac glycosides may not only act as direct cytotoxins but also as immunomodulatory adjuvants capable of converting immunologically “cold” tumors into more inflamed, immune-responsive phenotypes.
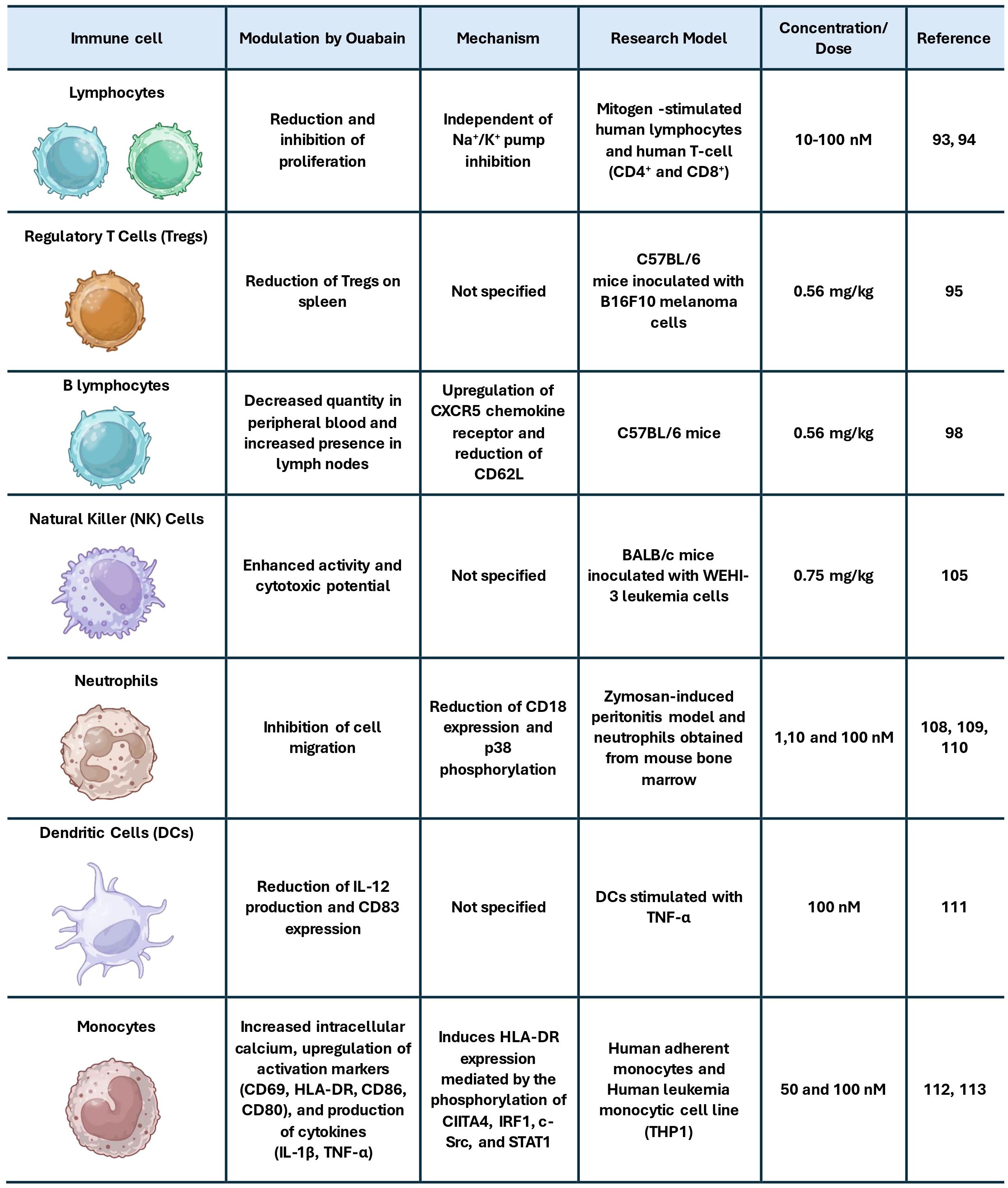
Although specific data on ouabain in TIME is limited, its effects on different contexts provide insights into its broader immunomodulatory capacity. One of the first described immunomodulatory effects of ouabain was its ability to inhibit lymphocyte proliferation induced by the mitogens phytohemagglutinin and concanavalin A (93). Subsequent studies demonstrated that ouabain reduces the proliferation of CD4+ and CD8+ T lymphocytes at concentrations that do not diminish NKA activity, indicating that this immunomodulatory mechanism occurs independently of pump inhibition (94). Complementing these effects, it was demonstrated that pre-treatment of mice with ouabain, both injected and non-injected with melanoma, reduced the number of Tregs in their spleens, an effect associated with increased survival in these animals (95). Given the well-established role of Tregs in suppressing cytotoxic responses in the GBM microenvironment (58), this effect raises the possibility that ouabain could alleviate immune suppression in gliomas by modulating Treg homeostasis, as observed with other approaches (96, 97).
Ouabain also influences B lymphocyte behavior, decreasing the quantity of mature B lymphocytes in peripheral blood while increasing their presence in lymph nodes (95, 98). Although B cells are less prominent in the GBM immune landscape, tertiary lymphoid structures and activated B cell subsets have been implicated in both contexts of promoting tumor progression (99, 100) and shaping antitumor immunity (101–103), warranting further exploration of ouabain’s influence on humoral responses in glioma.
Regarding innate immunity, ouabain appears to exert dose-dependent effects on NK cells. While early in vitro studies suggested that NK cell cytotoxicity is largely resistant to ouabain at low concentrations (104), more recent in vivo data indicate enhanced NK cell activity following ouabain treatment, evidenced by increased cytotoxic potential in NK cells isolated from mice administered 0.75 mg/kg of ouabain (105). This is particularly relevant given the capacity of NK cells in targeting glioma stem-like cells (106, 107) and their association with improved outcomes in certain GBM subtypes (61, 62). In addition, neutrophil infiltration promotes glioma cell proliferation, alters cellular organization, enhances NF-κB–dependent signaling, and correlates with poor prognosis (72, 78). Thus, given the prominent role of neutrophil migration in promoting glioblastoma progression, the ability of ouabain to inhibit neutrophil infiltration (108–110) may represent a beneficial immunomodulatory effect within the GBM tumor microenvironment.
Beyond its effects previously mentioned, ouabain has also been shown to reduce IL-2 production and CD83 expression in DCs stimulated with TNF-α (111). It also promotes an increase in intracellular calcium in monocytes, along with higher expression of activation surface markers like CD69, HLA-DR, CD86, and CD80, and an increased production of cytokines such as IL-1β and TNF-α (112). Corroborating this, a recent study demonstrated that ouabain induces HLA-DR expression in monocytes, mediated by the phosphorylation of CIITA4, IRF1, c-Src, and STAT1 (113). Considering that monocyte-derived cells are the predominant myeloid population in the GBM microenvironment and that defective antigen presentation is a major barrier to effective antitumor immunity (114–116), these findings suggest that ouabain may have the potential to modulate myeloid cells toward a more immunostimulatory phenotype in gliomas. Figure 1 summarizes and integrates the main effects of ouabain on immune cells.
Despite the reported preclinical data, several challenges still limit the translational potential of ouabain in GBM therapy. A major obstacle is its poor permeability through the blood–brain barrier (BBB), which may restrict its therapeutic concentrations within the tumor parenchyma (117, 118). Strategies such as nanoencapsulation or chemical modification to improve BBB penetration could help overcome this limitation (119). Another critical aspect is ouabain’s narrow therapeutic index. As a cardiotonic steroid, its systemic use carries the risk of cardiac and metabolic toxicity, emphasizing the need for precise dose control and the development of targeted delivery systems capable of minimizing off-target effects (35, 120). Furthermore, the biological responses elicited by ouabain are highly dose-dependent, ranging from cytotoxic to immunomodulatory (30, 121), underscoring the importance of determining concentration-specific effects in glioma models.
From a therapeutic standpoint, the pleiotropic mechanisms of ouabain suggest that it could act synergistically with current GBM treatments. Its ability to inhibit proliferative and prosurvival signaling pathways may potentiate temozolomide or radiotherapy efficacy, while its immunomodulatory properties could complement immune checkpoint inhibitors by alleviating local immune suppression (30, 122, 123). Future studies should focus on integrating these mechanistic insights into combinatorial approaches, while addressing pharmacological limitations such as BBB permeability and toxicity.
Collectively, these findings explore how ouabain exhibits extensive immunomodulatory action directly or indirectly affects GBM biology. The integration of these mechanisms reinforces ouabain’s relevance as a candidate for therapeutic repurposing. To date, there are no clinical or clinical-stage studies evaluating ouabain or other cardiotonic steroids as therapeutic agents for GBM. All available evidence remains preclinical. This highlights the current insufficiency of translational data and reinforces the need for future studies to address key issues such as efficacy in immunocompetent models, blood–brain barrier permeability, and potential systemic toxicity before any clinical application can be considered.
4 Final considerations
Glioblastoma remains one of the most difficult challenges in oncology due to its intrinsic resistance mechanisms and profoundly immunosuppressive microenvironment. Ouabain, a classical cardiotonic steroid, has demonstrated promising antitumor activity in GBM models (Figure 2), primarily through the modulation of intracellular signaling pathways, induction of cell death, and inhibition of tumor-promoting processes such as migration and angiogenesis. While direct evidence of its effects on the GBM tumor immune microenvironment remains limited, findings from other biological systems suggest that ouabain may exert broad immunomodulatory effects on both innate and adaptive immunity. These preliminary insights position ouabain as a candidate for therapeutic repurposing in GBM; however, further studies, particularly in vivo and within immunocompetent models, are essential to validate its efficacy, define optimal dosing, and understand its impact on immune-tumor dynamics. Future research should also address pharmacological challenges, such as brain barrier permeability and potential systemic toxicity, to enable safe and effective clinical translation.
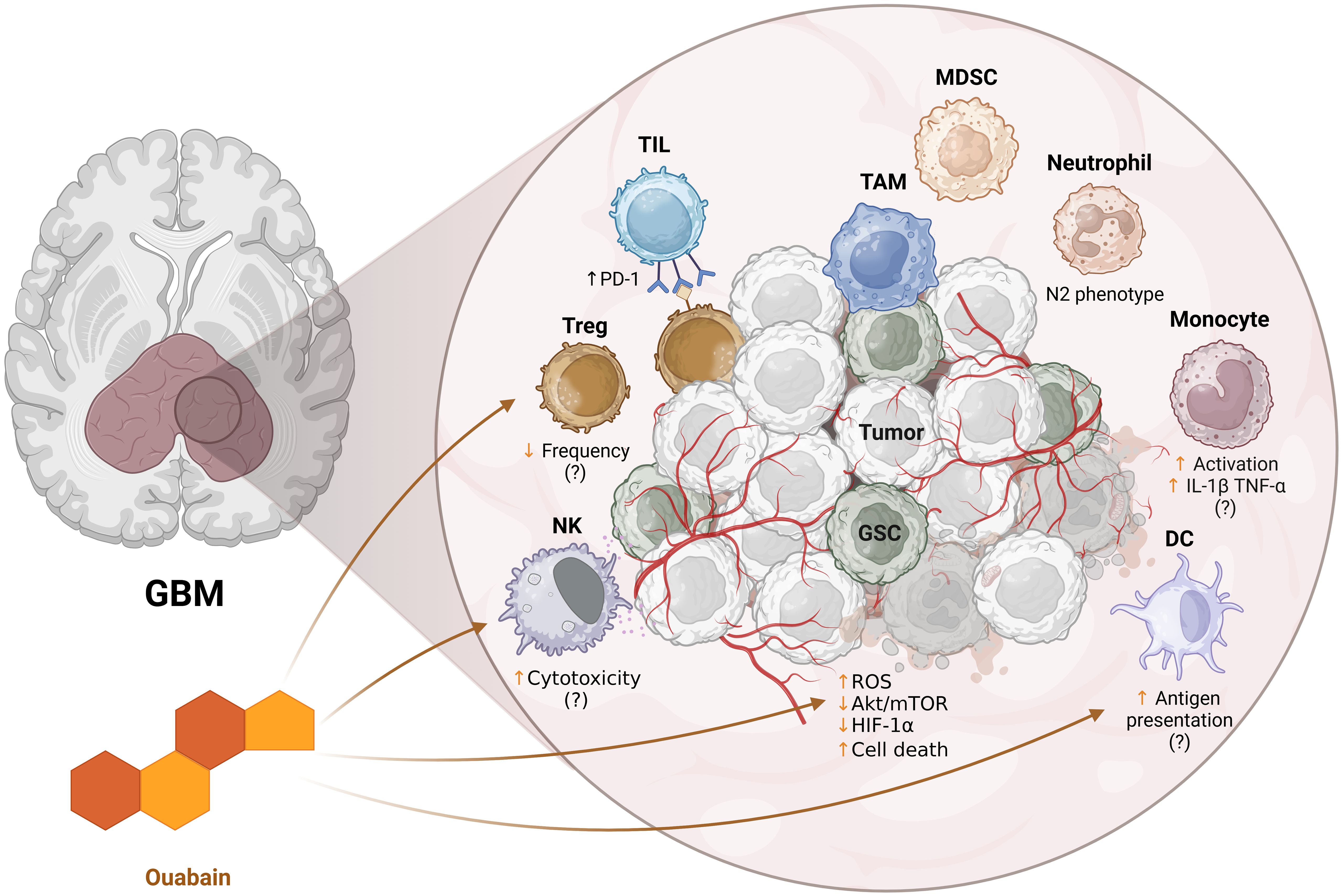
Figure 2. Schematic representation of the potential immunomodulatory effects of ouabain on the tumor microenvironment of glioblastoma (GBM). The enlarged panel on the right details the tumor microenvironment, composed of tumor cells (Tumor), glioma stem cells (GSC), immunosuppressive cells such as TAMs (tumor-associated macrophages), MDSCs (myeloid suppressor cells), Tregs (regulatory T lymphocytes) and neutrophils (e.g.: N2 phenotype), as well as effector cells such as tumor infiltrating lymphocytes (TILs), NK cells and dendritic cells (DCs). Ouabain influences intracellular pathways associated with oxidative stress (ROS), Akt/mTOR pathway, HIF-1α, and tumor cell death. In addition, it appears to modulate different cellular components: NK cell cytotoxicity, Treg frequency, antigen presentation by DCs, and monocyte activation. The suggested effects still lack confirmation (indicated by “?”) and reflect hypotheses based on evidence in other models. ↑ - increase, ↓ - decrease. Created in https://BioRender.com.
Author contributions
AA: Writing – review & editing, Writing – original draft. DC: Writing – review & editing, Writing – original draft. DM: Writing – original draft, Writing – review & editing. AQ: Visualization, Writing – review & editing. MA-M: Supervision, Writing – review & editing, Resources. SR-M: Writing – review & editing, Supervision, Resources. LC-S: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors are grateful for the financial support of National Council for Scientific and Technological Development (CNPq) through PQ fellow–MCTI/CNPq 09/2022 (grant number: 303765/2022-4, 310843/2022-7) and FINEP (“Financiadora de Estudos e Projetos”, Brazil, grant number: 1640/22).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo X, Gu L, Li Y, Zheng Z, Chen W, Wang Y, et al. Histological and molecular glioblastoma, IDH-wildtype: a real-world landscape using the 2021 WHO classification of central nervous system tumors. Front Oncol. (2023) 13:1200815/BIBTEX. doi: 10.3389/FONC.2023.1200815/BIBTEX
2. Park YW, Vollmuth P, Foltyn-Dumitru M, Sahm F, Ahn SS, Chang JH, et al. The 2021 WHO classification for gliomas and implications on imaging diagnosis: part 1—Key points of the fifth edition and summary of imaging findings on adult-type diffuse gliomas. J Magnetic Resonance Imaging. (2023) 58:677–89. doi: 10.1002/JMRI.28743
3. Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, et al. Glioblastoma Multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res. (2021) 171:105780. doi: 10.1016/J.PHRS.2021.105780
4. Singh N, Miner A, Hennis L, and Mittal S. Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review. Cancer Drug Resist. (2021) 4:17–43. doi: 10.20517/CDR.2020.79
5. Habashy KJ, Mansour R, Moussalem C, Sawaya R, and Massaad MJ. Challenges in glioblastoma immunotherapy: mechanisms of resistance and therapeutic approaches to overcome them. Br J Cancer. (2022) 127:976–87. doi: 10.1038/s41416-022-01864-w
6. Zhou W and Wahl DR. Metabolic abnormalities in glioblastoma and metabolic strategies to overcome treatment resistance. Cancers. (2019) 11:1231. doi: 10.3390/CANCERS11091231
7. Brown NF, Ottaviani D, Tazare J, Gregson J, Kitchen N, Brandner S, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers. (2022) 14:1–10. doi: 10.3390/cancers14133161
8. Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, Mcdermott MW, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J Neurosurg. (2012) 117:1032–8. doi: 10.3171/2012.9.JNS12504
9. Sharma P, Aaroe A, Liang J, and Puduvalli VK. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol Adv. (2023) 5:1–16. doi: 10.1093/noajnl/vdad009
10. Lin H, Liu C, Hu A, Zhang D, Yang H, and Mao Y. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J Hematol Oncol. (2024) 17:1–81. doi: 10.1186/S13045-024-01544-7
11. Tomaszewski W, Sanchez-Perez L, Gajewski TF, and Sampson JH. Brain tumor microenvironment and host state: implications for immunotherapy. Clin Cancer Res. (2019) 25:4202–10. doi: 10.1158/1078-0432.CCR-18-1627
12. Pacheco C, Martins C, Monteiro J, Baltazar F, Costa BM, and Sarmento B. Glioblastoma vasculature: from its critical role in tumor survival to relevant in vitro modelling. Front Drug Delivery. (2022) 2:823412. doi: 10.3389/FDDEV.2022.823412
13. De Silva MI, Stringer BW, and Bardy C. Neuronal and tumourigenic boundaries of glioblastoma plasticity. Trends Cancer. (2023) 9:223–36. doi: 10.1016/J.TRECAN.2022.10.010/ASSET/C0D81B82-9B6E-4237-B593-7DEAE86A4E87/MAIN.ASSETS/GR4.JPG
14. Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. (2019) 10:1–12. doi: 10.1038/s41467-019-10493-6
15. Chen Z and Hambardzumyan D. Immune microenvironment in glioblastoma subtypes. Front Immunol. (2018) 9:1004. doi: 10.3389/FIMMU.2018.01004
16. Antunes ARP, Scheyltjens I, Duerinck J, Neyns B, Movahedi K, and Van Ginderachter JA. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife. (2020) 9:1–16. doi: 10.7554/ELIFE.52176
17. Brown NF, Carter TJ, Ottaviani D, and Mulholland P. Harnessing the immune system in glioblastoma. Br J Cancer. (2018) 119:1171–81. doi: 10.1038/s41416-018-0258-8
18. Liu Y, Zhou F, Ali H, Lathia JD, and Chen P. Immunotherapy for glioblastoma: current state, challenges, and future perspectives. Cell Mol Immunol. (2024) 21:1354–75. doi: 10.1038/s41423-024-01226-x
19. Leanza L, Managò A, Zoratti M, Gulbins E, and Szabo I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochim Biophys Acta (BBA) - Mol Cell Res. (2016) 1863:1385–97. doi: 10.1016/J.BBAMCR.2015.11.032
20. Bose T, Cieslar-Pobuda A, and Wiechec E. Role of ion channels in regulating Ca2+ homeostasis during the interplay between immune and cancer cells. Cell Death Dis. (2015) 6:e1648–8. doi: 10.1038/cddis.2015.23
21. Abed T, Ganser K, Eckert F, Stransky N, and Huber SM. Ion channels as molecular targets of glioblastoma electrotherapy. Front Cell Neurosci. (2023) 17:1133984/PDF. doi: 10.3389/FNCEL.2023.1133984/PDF
22. Liu J, Tian J, Haas M, Shapiro JI, Askari A, and Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. (2000) 275:27838–44. doi: 10.1074/jbc.M002950200
23. Edwards A and Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol. (2007) 293:F1518–F1532. doi: 10.1152/AJPRENAL.00251.2007
24. Lopachev AV, Lopacheva OM, Osipova EA, Vladychenskaya EA, Smolyaninova LV, Fedorova TN, et al. Ouabain-induced changes in MAP kinase phosphorylation in primary culture of rat cerebellar cells. Cell Biochem Funct. (2016) 34:367–77. doi: 10.1002/CBF.3199
25. Hiyoshi H, Abdelhady S, Segerström L, Sveinbjörnsson B, Nuriya M, Lundgren TK, et al. Quiescence and γH2AX in neuroblastoma are regulated by ouabain/Na,K-ATPase. Br J Cancer. (2012) 106:1807–15. doi: 10.1038/bjc.2012.159
26. Panizza E, Zhang L, Fontana JM, Hamada K, Svensson D, Akkuratov EE, et al. Ouabain-regulated phosphoproteome reveals molecular mechanisms for Na+, K+-ATPase control of cell adhesion, proliferation, and survival. FASEB J. (2019) 33:10193–206. doi: 10.1096/FJ.201900445R
27. Xiao Y, Meng C, Lin J, Huang C, Zhang X, Long Y, et al. Ouabain targets the Na+/K+-ATPase α3 isoform to inhibit cancer cell proliferation and induce apoptosis. Oncol Lett. (2017) 14:6678–84. doi: 10.3892/OL.2017.7070
28. Pongrakhananon V, Chunhacha P, and Chanvorachote P. Ouabain suppresses the migratory behavior of lung cancer cells. PloS One. (2013) 8:e68623. doi: 10.1371/JOURNAL.PONE.0068623
29. Wang L, Cai W, Han B, Zhang J, Yu B, and Chen M. Ouabain exhibited strong anticancer effects in melanoma cells via induction of apoptosis, g2/m phase arrest, and migration inhibition. Onco Targets Ther. (2021) 14:1261–73. doi: 10.2147/OTT.S283548
30. Weidemann H, Feger D, Ehlert JE, Menger MM, and Krempien RC. Markedly divergent effects of Ouabain on a Temozolomide-resistant (T98G) vs. a Temozolomide-sensitive (LN229) Glioblastoma cell line. Discover Oncol. (2023) 14:1–16. doi: 10.1007/S12672-023-00633-2
31. Yang XS, Xu ZW, Yi TL, Xu RC, Li J, Bin Zhang W, et al. Ouabain suppresses the growth and migration abilities of glioma U-87MG cells through inhibiting the Akt/mTOR signaling pathway and downregulating the expression of HIF-1α. Mol Med Rep. (2018) 17:5595. doi: 10.3892/MMR.2018.8587
32. Yan X, Liang FL, Li D, and Zheng J. Ouabain elicits human glioblastoma cells apoptosis by generating reactive oxygen species in ERK-p66SHC-dependent pathway. Mol Cell Biochem. (2015) 398:95–104. doi: 10.1007/S11010-014-2208-Y/FIGURES/5
33. Harich OO, Gavriliuc OI, Ordodi VL, Tirziu A, Paunescu V, Panaitescu C, et al. In vitro study of the multimodal effect of na+/K+ ATPase blocker ouabain on the tumor microenvironment and Malignant cells. Biomedicines. (2023) 11:2205. doi: 10.3390/BIOMEDICINES11082205/S1
34. Yang K, Li Z, Chen Y, Yin F, Ji X, Zhou J, et al. Na, K-ATPase α1 cooperates with its endogenous ligand to reprogram immune microenvironment of lung carcinoma and promotes immune escape. Sci Adv. (2023) 9:1–21. doi: 10.1126/SCIADV.ADE5393/SUPPL_FILE/SCIADV.ADE5393_DATA_FILES_S1_TO_S3.ZIP
35. Ren J, Gao X, Guo X, Wang N, and Wang X. Research progress in pharmacological activities and applications of cardiotonic steroids. Front Pharmacol. (2022) 13:902459/XML/NLM. doi: 10.3389/FPHAR.2022.902459/XML/NLM
36. Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, and Kiss R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta (BBA) - Rev Cancer. (2007) 1776:32–57. doi: 10.1016/J.BBCAN.2007.06.002
37. Matozzo FH, Votto APS, Rodrigues-Mascarenhas S, Cavalcante-Silva LHA, Valente RC, and Rumjanek VM. Ouabain as an anti-cancer agent? Curr Top Biochem Res. (2020) 21:25–40.
38. Dymova MA, Kuligina EV, and Richter VA. Molecular mechanisms of drug resistance in glioblastoma. Int J Mol Sci. (2021) 22:6385. doi: 10.3390/IJMS22126385
39. Da Ros M, De Gregorio V, Iorio AL, Giunti L, Guidi M, de Martino M, et al. Glioblastoma chemoresistance: the double play by microenvironment and blood-brain barrier. Int J Mol Sci. (2018) 19:2879. doi: 10.3390/IJMS19102879
40. Degl’Innocenti DA, di Leo N, and Ciofani PG. Genetic hallmarks and heterogeneity of glioblastoma in the single-cell omics era. Adv Ther (Weinh). (2019) 3:1900152. doi: 10.1002/ADTP.201900152
41. Gimple RC, Bhargava S, Dixit D, and Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. (2019) 33:591. doi: 10.1101/GAD.324301.119
42. Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. (2010) 12:1113–25. doi: 10.1093/NEUONC/NOQ082
43. Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, et al. Glioma-initiating cells: A predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. (2011) 232:75–82. doi: 10.1016/j.jneuroim.2010.10.011
44. Marino S, Menna G, Di Bonaventura R, Lisi L, Mattogno P, Figà F, et al. The extracellular matrix in glioblastomas: A glance at its structural modifications in shaping the tumoral microenvironment—A systematic review. Cancers (Basel). (2023) 15:1879. doi: 10.3390/CANCERS15061879
45. Brandao M, Simon T, Critchley G, and Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia. (2019) 67:779–90. doi: 10.1002/GLIA.23520
46. Faust Akl C, Andersen BM, Li Z, Giovannoni F, Diebold M, Sanmarco LM, et al. Glioblastoma-instructed astrocytes suppress tumour-specific T cell immunity. Nature. (2025) 2025:1–11. doi: 10.1038/s41586-025-08997-x
47. Tetzlaff SK, Reyhan E, Layer N, Bengtson CP, Heuer A, Schroers J, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell. (2025) 188:390–411.e36. doi: 10.1016/J.CELL.2024.11.002
48. Lo Dico A, Martelli C, Diceglie C, Lucignani G, and Ottobrini L. Hypoxia-inducible factor-1α activity as a switch for glioblastoma responsiveness to temozolomide. Front Oncol. (2018) 8:249/FULL. doi: 10.3389/FONC.2018.00249/FULL
49. Gabriely G, Wheeler MA, Takenaka MC, and Quintana FJ. Role of AHR and HIF-1α in glioblastoma metabolism. Trends Endocrinol Metab. (2017) 28:428. doi: 10.1016/J.TEM.2017.02.009
50. Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. (2015) 161:803. doi: 10.1016/J.CELL.2015.04.012
51. Li Z, Gao W, Fei Y, Gao P, Xie Q, Xie J, et al. NLGN3 promotes neuroblastoma cell proliferation and growth through activating PI3K/AKT pathway. Eur J Pharmacol. (2019) 857:1–7. doi: 10.1016/j.ejphar.2019.172423
52. Perelroizen R, Philosof B, Budick-Harmelin N, Chernobylsky T, Ron A, Katzir R, et al. Astrocyte immunometabolic regulation of the tumour microenvironment drives glioblastoma pathogenicity. Brain. (2022) 145:3288. doi: 10.1093/BRAIN/AWAC222
53. Wang G, Zhong K, Wang Z, Zhang Z, Tang X, Tong A, et al. Tumor-associated microglia and macrophages in glioblastoma: From basic insights to therapeutic opportunities. Front Immunol. (2022) 13:964898/XML/NLM. doi: 10.3389/FIMMU.2022.964898/XML/NLM
54. Wang S, Wang J, Chen Z, Luo J, Guo W, Sun L, et al. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis Oncol. (2024) 8:1–19. doi: 10.1038/s41698-024-00522-z
55. Losurdo A, Di Muzio A, Cianciotti BC, Dipasquale A, Persico P, Barigazzi C, et al. T cell features in glioblastoma may guide therapeutic strategies to overcome microenvironment immunosuppression. Cancers (Basel). (2024) 16:603. doi: 10.3390/CANCERS16030603
56. Lowther DE, Goods BA, Lucca LE, Lerner BA, Raddassi K, van Dijk D, et al. PD-1 marks dysfunctional regulatory T cells in Malignant gliomas. JCI Insight. (2016) 1:e85935. doi: 10.1172/JCI.INSIGHT.85935
57. DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, et al. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology. (2018) 7:e1448329. doi: 10.1080/2162402X.2018.1448329
58. Humphries W, Wei J, Sampson JH, and Heimberger AB. The role of tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg Clin N Am. (2010) 21:125. doi: 10.1016/J.NEC.2009.08.012
59. Ravi VM, Neidert N, Will P, Joseph K, Maier JP, Kückelhaus J, et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat Commun. (2022) 13:1–16. doi: 10.1038/s41467-022-28523-1
60. Asioli S, Gatto L, Vardy U, Agostinelli C, Di Nunno V, Righi S, et al. Immunophenotypic profile of adult glioblastoma IDH-wildtype microenvironment: A cohort study. Cancers (Basel). (2024) 16:3859. doi: 10.3390/CANCERS16223859
61. Zhang S, Liu W, Hu B, Wang P, Lv X, Chen S, et al. Prognostic significance of tumor-infiltrating natural killer cells in solid tumors: A systematic review and meta-analysis. Front Immunol. (2020) 11:1242/XML/NLM. doi: 10.3389/FIMMU.2020.01242/XML/NLM
62. Sun F, Lv H, Feng B, Sun J, Zhang L, and Dong B. Identification of natural killer cell-related characteristics to predict the clinical prognosis and immune microenvironment of patients with low-grade glioma. Aging (Albany NY). (2023) 15:6264. doi: 10.18632/AGING.204850
63. Breznik B, Ko MW, Tse C, Chen PC, Senjor E, Majc B, et al. Infiltrating natural killer cells bind, lyse and increase chemotherapy efficacy in glioblastoma stem-like tumorospheres. Commun Biol. (2022) 5:1–15. doi: 10.1038/s42003-022-03402-z
64. Jia H, Yang H, Xiong H, and Luo KQ. NK cell exhaustion in the tumor microenvironment. Front Immunol. (2023) 14:1303605/XML/NLM. doi: 10.3389/FIMMU.2023.1303605/XML/NLM
65. Wang M, Zhou Z, Wang X, Zhang C, and Jiang X. Natural killer cell awakening: unleash cancer-immunity cycle against glioblastoma. Cell Death Dis. (2022) 13:588. doi: 10.1038/S41419-022-05041-Y
66. Régnier P, Vetillard M, Bansard A, Pierre E, Li X, Cagnard N, et al. FLT3L-dependent dendritic cells control tumor immunity by modulating Treg and NK cell homeostasis. Cell Rep Med. (2023) 4:1–20. doi: 10.1016/j.xcrm.2023.101256
67. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. (2018) 172:1022. doi: 10.1016/J.CELL.2018.01.004
68. Friedrich M, Hahn M, Michel J, Sankowski R, Kilian M, Kehl N, et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. (2023) 25:263–76. doi: 10.1093/NEUONC/NOAC138
69. Massara M, Persico P, Bonavita O, Poeta VM, Locati M, Simonelli M, et al. Neutrophils in gliomas. Front Immunol. (2017) 8:1349/BIBTEX. doi: 10.3389/FIMMU.2017.01349/BIBTEX
70. Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N, et al. Neutrophils promote the Malignant glioma phenotype through S100A4. Clin Cancer Res. (2014) 20:187–98. doi: 10.1158/1078-0432.CCR-13-1279
71. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. (2020) 17:154. doi: 10.20892/J.ISSN.2095-3941.2019.0353
72. Sun C, Wang S, Ma Z, Zhou J, Ding Z, Yuan G, et al. Neutrophils in glioma microenvironment: from immune function to immunotherapy. Front Immunol. (2024) 15:1393173/XML/NLM. doi: 10.3389/FIMMU.2024.1393173/XML/NLM
73. Rubenich DS, de Souza PO, Omizzollo N, Aubin MR, Basso PJ, Silva LM, et al. Tumor-neutrophil crosstalk promotes in vitro and in vivo glioblastoma progression. Front Immunol. (2023) 14:1183465/PDF. doi: 10.3389/FIMMU.2023.1183465/PDF
74. Jackson C, Cherry C, Bom S, Dykema AG, Wang R, Thompson E, et al. Distinct myeloid-derived suppressor cell populations in human glioblastoma. Science. (2025) 387:eabm5214. doi: 10.1126/SCIENCE.ABM5214/SUPPL_FILE/SCIENCE.ABM5214_MDAR_REPRODUCIBILITY_CHECKLIST.PDF
75. Chai E, Zhang L, and Li C. LOX-1+ PMN-MDSC enhances immune suppression which promotes glioblastoma multiforme progression. Cancer Manag Res. (2019) 11:7307–15. doi: 10.2147/CMAR.S210545
76. Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Bossman SAJFH, Ter Laan M, et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol. (2016) 18:1253. doi: 10.1093/NEUONC/NOW034
77. Harwood S and Yaqoob MM. Ouabain-induced cell signaling. Front Biosci. (2005) 10:2011–7. doi: 10.2741/1676
78. Du J, Jiang L, Chen F, Hu H, and Zhou M. Cardiac glycoside ouabain exerts anticancer activity via downregulation of STAT3. Front Oncol. (2021) 11:684316/PDF. doi: 10.3389/FONC.2021.684316/PDF
79. Chen D, Song M, Mohamad O, and Yu SP. Inhibition of Na+/K+-ATPase induces hybrid cell death and enhanced sensitivity to chemotherapy in human glioblastoma cells. BMC Cancer. (2014) 141–15. doi: 10.1186/1471-2407-14-716
80. Ono Y, Chiba S, Yano H, Nakayama N, Saio M, Tsuruma K, et al. Glycoprotein nonmetastatic melanoma protein B (GPNMB) promotes the progression of brain glioblastoma via Na+/K+-ATPase. Biochem Biophys Res Commun. (2016) 481:7–12. doi: 10.1016/j.bbrc.2016.11.034
81. An X, Yu W, Liu J, Tang D, Yang L, and Chen X. Oxidative cell death in cancer: mechanisms and therapeutic opportunities. Cell Death Dis 2024 15:8. (2024) 15:1–20. doi: 10.1038/s41419-024-06939-5
82. Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. (2016) 7:33440. doi: 10.18632/ONCOTARGET.7961
83. Langhans J, Schneele L, Trenkler N, Von Bandemer H, Nonnenmacher L, Karpel-Massler G, et al. The effects of PI3K-mediated signalling on glioblastoma cell behaviour. Oncogenesis. (2017) 6:1–9. doi: 10.1038/S41389-017-0004-8;SUBJMETA=631,67,80,84;KWRD=CANCER,CELL+MIGRATION
84. Hashemi M, Etemad S, Rezaei S, Ziaolhagh S, Rajabi R, Rahmanian P, et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomedicine Pharmacotherapy. (2023) 158:114204. doi: 10.1016/J.BIOPHA.2022.114204
85. Hsu JL, Liu FL, Hsu LC, Chang HS, Leu WJ, Yu CC, et al. Epi-reevesioside F inhibits Na+/K+-ATPase, causing cytosolic acidification, Bak activation and apoptosis in glioblastoma. Oncotarget. (2015) 6:24032. doi: 10.18632/ONCOTARGET.4429
86. Huang X, Lei Z, Li XP, and El-Mallakh RS. Response of sodium pump to ouabain challenge in human glioblastoma cells in culture. World J Biol Psychiatry. (2009) 10:884–92. doi: 10.1080/15622970902995620
87. Zhang J, Zhao Q, Du Y, Wang W, and Liu C. Pan-cancer analysis identifies venous thromboembolism-related genes F3, PLAT, and C1S as potential prognostic biomarkers for glioblastoma and lower grade glioma. Mol Biomedicine. (2024) 5:1–15. doi: 10.1186/S43556-024-00197-9
88. Li X, Zheng J, Chen S, dong Meng F, Ning J, and lan Sun S. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. (2021) 12:1–15. doi: 10.1038/S41419-021-03605-Y;TECHMETA
89. Kobayashi M, Usui-Kawanishi F, Karasawa T, Kimura H, Watanabe S, Mise N, et al. The cardiac glycoside ouabain activates NLRP3 inflammasomes and promotes cardiac inflammation and dysfunction. PloS One. (2017) 12:e0176676. doi: 10.1371/JOURNAL.PONE.0176676
90. Sanderson SM, Xiao Z, Wisdom AJ, Bose S, Liberti MV, Reid MA, et al. Digoxin targets central carbon metabolism and remodels the tumor microenvironment. BioRxiv. (2020) 2020:3. doi: 10.1101/2020.03.31.018739
91. Lee HE, Lee JY, Yang G, Kang HC, Cho YY, Lee HS, et al. Inhibition of NLRP3 inflammasome in tumor microenvironment leads to suppression of metastatic potential of cancer cells. Sci Rep. (2019) 9. doi: 10.1038/S41598-019-48794-X
92. Shadab A, Mahjoor M, Abbasi-Kolli M, Afkhami H, Moeinian P, and Safdarian AR. Divergent functions of NLRP3 inflammasomes in cancer: a review. Cell Commun Signal. (2023) 21:232. doi: 10.1186/S12964-023-01235-9
93. Dornand J and Kaplan JG. Persistent effects of ouabain treatment on human lymphocytes: synthesis of DNA, RNA and protein in stimulated and unstimulated cells. Can J Biochem. (1976) 54:280–6. doi: 10.1139/O76-041
94. Brodie C, Tordai A, Saloga J, Domenico J, and Gelfand EW. Ouabain induces inhibition of the progression phase in human T-cell proliferation. J Cell Physiol. (1995) 165:246–53. doi: 10.1002/JCP.1041650205
95. da Silva JMC, Campos MLA, Teixeira MP, da Silva Faustino R, Aleixo RC, Cavalcante FJP, et al. Ouabain pre-treatment modulates B and T lymphocytes and improves survival of melanoma-bearing animals. Int Immunopharmacol. (2020) 86:1–9. doi: 10.1016/j.intimp.2020.106772
96. Wainwright DA, Dey M, Chang A, and Lesniak MS. Targeting tregs in Malignant brain cancer: Overcoming IDO. Front Immunol. (2013) 4:116/XML/NLM. doi: 10.3389/FIMMU.2013.00116/XML/NLM
97. Amoozgar Z, Kloepper J, Ren J, Tay RE, Kazer SW, Kiner E, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun. (2021) 12:1–16. doi: 10.1038/s41467-021-22885-8
98. da Silva JMC, das Neves Azevedo A, dos Santos Barbosa RP, Vianna TAG, Fittipaldi J, Teixeira MP, et al. Dynamics of murine B lymphocytes is modulated by in vivo treatment with steroid ouabain. Immunobiology. (2016) 221:368–76. doi: 10.1016/J.IMBIO.2015.09.020
99. Gao J, Gu D, Yang K, Zhang J, Lin Q, Yuan W, et al. Infiltrating plasma cells maintain glioblastoma stem cells through IgG-Tumor binding. Cancer Cell. (2025) 43:122–143.e8. doi: 10.1016/J.CCELL.2024.12.006
100. Zhang C, Li J, Wang H, Song SW, Zhang C, Li J, et al. Identification of a five B cell-associated gene prognostic and predictive signature for advanced glioma patients harboring immunosuppressive subtype preference. Oncotarget. (2016) 7:73971–83. doi: 10.18632/ONCOTARGET.12605
101. Lauss M, Donia M, Svane IM, Jönsson G, and Cells B. and tertiary lymphoid structures: friends or foes in cancer immunotherapy? Clin Cancer Res. (2022) 28:1751–8. doi: 10.1158/1078-0432.CCR-21-1130
102. Cakmak P, Lun JH, Singh A, Macas J, Schupp J, Köhler M, et al. Glioma-associated tertiary lymphoid structures are sites of lymphocyte clonal expansion and plasma cell formation. BioRxiv. (2024) 2024:7.04.602038. doi: 10.1101/2024.07.04.602038
103. Li H, Zhang MJ, Zhang B, Lin WP, Li SJ, Xiong D, et al. Mature tertiary lymphoid structures evoke intra-tumoral T and B cell responses via progenitor exhausted CD4+ T cells in head and neck cancer. Nat Commun. (2025) 16:1–20. doi: 10.1038/s41467-025-59341-w
104. Moraes VLG, Olej B, Rocque L, and Rumjanek VM. Lack of sensitivity to ouabain in natural killer activity. FASEB J. (1989) 3:2425–9. doi: 10.1096/FASEBJ.3.12.2477295
105. Shih YL, Shang HS, Chen YL, Hsueh SC, Chou HM, Lu HF, et al. Ouabain promotes immune responses in WEHI-3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol. (2019) 34:659–65. doi: 10.1002/TOX.22732
106. Poorva P, Mast J, Cao B, Shah MV, Pollok KE, and Shen J. Killing the killers: Natural killer cell therapy targeting glioma stem cells in high-grade glioma. Mol Ther. (2025) 33:24262478 . doi: 10.1016/j.ymthe.2025.02.043
107. Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. (2009) 182:3530–9. doi: 10.4049/JIMMUNOL.0802845
108. Cavalcante-Silva LHA, de É, Lima A, Carvalho DCM, and Rodrigues-Mascarenhas S. Ouabain reduces the expression of the adhesion molecule CD18 in neutrophils, I. nflammopharmacology. (2020) 28:787–93. doi: 10.1007/S10787-019-00602-8
109. Cavalcante Silva LHA, Carvalho DCM, de Almeida Lima É, and Rodrigues Mascarenhas S. Ouabain inhibits p38 activation in mice neutrophils. Inflammopharmacology. (2021) 29:1829–33. doi: 10.1007/S10787-021-00882-Z
110. Leite JA, De Abreu Alves AK, Galvão JGM, Teixeira MP, Cavalcante-Silva LHA, Scavone C, et al. Ouabain modulates zymosan-induced peritonitis in mice. Mediators Inflammation. (2015) 2015:1–12. doi: 10.1155/2015/265798
111. Nascimento CR, Valente RC, Echevarria-Lima J, Fontes CFL, De Araujo-Martins L, Araujo EG, et al. The influence of Ouabain on human dendritic cells maturation. Mediators Inflammation. (2014) 2014:1–15. doi: 10.1155/2014/494956
112. Teixeira MP and Rumjanek VM. Ouabain affects the expression of activation markers, cytokine production, and endocytosis of human monocytes. Mediators Inflammation. (2014) 2014:1–10. doi: 10.1155/2014/760368
113. Chen L, Zhang M, Wang X, Liu Y, Bian J, Yan D, et al. Cardiac steroid ouabain transcriptionally increases human leukocyte antigen DR expression on monocytes. Steroids. (2021) 175:1–11. doi: 10.1016/j.steroids.2021.108915
114. Ricard C, Tchoghandjian A, Luche H, Grenot P, Figarella-Branger D, Rougon G, et al. Phenotypic dynamics of microglial and monocyte-derived cells in glioblastoma-bearing mice. Sci Rep. (2016) 6:1–15. doi: 10.1038/srep26381
115. Kushchayev SV, Kushchayeva YS, Wiener PC, Scheck AC, Badie B, and Preul MC. Monocyte-derived cells of the brain and Malignant gliomas: the double face of janus. World Neurosurg. (2014) 82:1171–86. doi: 10.1016/J.WNEU.2012.11.059
116. Waibl Polania J, Hoyt-Miggelbrink A, Tomaszewski WH, Wachsmuth LP, Lorrey SJ, Wilkinson DS, et al. Antigen presentation by tumor-associated macrophages drives T cells from a progenitor exhaustion state to terminal exhaustion. Immunity. (2024) 58:232–246. doi: 10.1016/j.immuni.2024.11.026
117. Abaimov DA, Kazanskaya RB, Ageldinov RA, Nesterov MS, Timoshina YA, Platova AI, et al. Evaluation of ouabain’s tissue distribution in C57/black mice following intraperitoneal injection, using chromatography and mass spectrometry. Int J Mol Sci. (2024) 25:1–14. doi: 10.3390/IJMS25084318
118. Wu D, Chen Q, Chen X, Han F, Chen Z, and Wang Y. The blood–brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. (2023) 8:1–27. doi: 10.1038/S41392-023-01481-W;SUBJMETA
119. Liu J, Yang F, Hu J, and Zhang X. Nanoparticles for efficient drug delivery and drug resistance in glioma: New perspectives. CNS Neurosci Ther. (2024) 30:e14715. doi: 10.1111/CNS.14715
120. Omole JG, Udom GJ, Aturamu A, Agbana RD, Aziakpono OM, Oritsemuelebi B, et al. Cardiac glycosides: Looking beyond heart failure and atrial fibrillation. Indian J Pharmacol. (2025) 57:33. doi: 10.4103/IJP.IJP_934_24
121. Shandell MA, Capatina AL, Lawrence SM, Brackenbury WJ, and Lagos D. Inhibition of the Na+/K+-ATPase by cardiac glycosides suppresses expression of the IDO1 immune checkpoint in cancer cells by reducing STAT1 activation. J Biol Chem. (2022) 298:101707. doi: 10.1016/J.JBC.2022.101707
122. Valluri A, Lawrence L, Denning KL, Cuda J, and Zhu GZ. Cardiac glycosides increase temozolomide anticancer activity in therapy resistant glioblastoma cells. Int J Trans Med. (2022) 2:148–55. doi: 10.3390/IJTM2020012
Keywords: cancer, cardiotonic steroid, tumor microenvironment, GBM, cytotoxicity
Citation: Andrade AGd, Carvalho DCM, Magalhaes DWA, de Queiroz AC, Alexandre-Moreira MS, Rodrigues-Mascarenas S and Cavalcante-Silva LHA (2025) From hormonal immunomodulation to glioblastoma therapy: the emerging role of Ouabain. Front. Immunol. 16:1657671. doi: 10.3389/fimmu.2025.1657671
Received: 01 July 2025; Accepted: 23 October 2025;
Published: 07 November 2025.
Edited by:
Kaige Chen, Wake Forest University, United StatesReviewed by:
Zhi Li, University of Arizona, United StatesCopyright © 2025 Andrade, Carvalho, Magalhaes, de Queiroz, Alexandre-Moreira, Rodrigues-Mascarenas and Cavalcante-Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luiz Henrique Agra Cavalcante-Silva, bHVpei5hZ3JhQGFyYXBpcmFjYS51ZmFsLmJy
 Arthur Gomes de Andrade
Arthur Gomes de Andrade Deyse Cristina Madruga Carvalho
Deyse Cristina Madruga Carvalho Daniel Wilson Arruda Magalhaes1
Daniel Wilson Arruda Magalhaes1 Aline Cavalcanti de Queiroz
Aline Cavalcanti de Queiroz Magna Suzana Alexandre-Moreira
Magna Suzana Alexandre-Moreira Sandra Rodrigues-Mascarenas
Sandra Rodrigues-Mascarenas Luiz Henrique Agra Cavalcante-Silva
Luiz Henrique Agra Cavalcante-Silva