- 1The First School of Clinical Medicine, Lanzhou University, Lanzhou, China
- 2Department of Gerontal Respiratory Medicine, The First Hospital of Lanzhou University, Lanzhou, China
Environmental pollution is a serious public health problem closely related to various chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD), bronchial asthma, and lung malignancies. Atmospheric particulate matter (PM) is an important component of environmental pollution, and its influence on COPD has been shown to be related to inflammation, oxidative stress, immune imbalance, abnormal cell death, and cell aging. A growing body of evidence has shown that an imbalance of the lung and intestinal microbiota, as well as changes in metabolites, is closely related to the occurrence and development of PM-induced COPD. PM exposure damages the respiratory system and alters the structure and activity of the gut microbiome. The metabolites produced by the gut microbiome, in turn, disrupt airway immunity and exacerbate respiratory inflammation. Therefore, the bidirectional influence of PM on the gut–lung axis has attracted widespread attention. This review explores the mechanisms by which PM causes oxidative stress damage to the lungs and intestines, as well as the characteristics of the resultant immune imbalance and changes in the microbiota and metabolite products. It also describes how PM disrupts barrier function through microecological imbalance and how it participates in the progression of COPD via the gut–lung axis. These mechanisms highlight the potential of targeting the microbial flora as a new approach for treating COPD caused by environmental pollution.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory condition characterized by chronic respiratory symptoms and persistent airflow limitation, which are primarily triggered by toxic particles and gases from tobacco smoke and air pollution. It is a preventable and treatable disease of the respiratory system (1). The global burden of COPD is increasing as a result of population aging and environmental factors, imposing a heavy economic burden on society (1–3). Atmospheric particulate matter (PM) exposure leads to increased oxidative stress in the lungs, yet this effect remains localized to the lungs (4). However, patients with COPD often have multiple systemic manifestations, such as gastrointestinal disorders, osteoporosis, anxiety, and depression (1), suggesting that COPD is not merely an isolated pulmonary disease but a complex systemic inflammatory disease. Studying the potential pathogenic mechanisms of COPD is crucial for slowing the progression of this disease and reducing mortality.
The structural and functional stability of respiratory microorganisms plays a major role in the pulmonary defense mechanism of the host. Microbes and their metabolites are involved in regulating the mucosal immunity of the respiratory system, maintaining the integrity of the respiratory epithelial barrier, and resisting the invasion of pathogenic bacteria (5). Evidence indicates that COPD patients exhibit dysbiosis in their respiratory microbiota (6, 7), manifesting as reduced microbial diversity, decreased colonization of bacteria, and replacement of some commensal bacteria by pathogenic bacteria such as Pseudomonas and Streptococcus (7–9). Patients with COPD also exhibit gut microbial dysbiosis, characterized by reduced richness and diversity, a depletion of beneficial metabolite-producing bacteria, and an enrichment of those producing harmful metabolites (10, 11). The gut–lung axis is characterized by close cross-communication between the gut and the lungs. This communication mainly relies on immune mediators and metabolic pathways, among which the gut microbiome and its metabolites play a central role. In the healthy state, the gut microbiota maintains the integrity of the intestinal barrier by producing beneficial metabolites (such as short-chain fatty acids [SCFAs]) and systematically regulates immune homeostasis, thereby providing protection to the distal lungs (12). Disorders of the intestinal microbiota in COPD may lead to impaired barrier function and the entry of inflammatory metabolites related to the intestine (such as lipopolysaccharide [LPS]) into the bloodstream, triggering systemic low-level inflammation and stimulating the pulmonary immune response (11). Conversely, co-infection with the influenza virus and Haemophilus influenzae leads to more severe infiltration of inflammatory mediators in the lung tissue of COPD mice, and the imbalance of the intestinal microbiota is also associated with the levels of inflammatory cytokines in the lungs. This suggests that changes in the intestinal flora are related to inflammatory infiltration in the lungs (13).
The harm of environmental pollution to human health has aroused widespread concern. PM in the environment consists of complex compounds and is an important environmental risk factor for COPD. It mainly comes from industrial, agricultural production, human life, and production, such as tobacco, automobile exhaust, tire wear, biofuel combustion, coal, decoration materials, and plastic bottles. PM exposure contributes to the incidence of COPD and elevates the risks of outpatient visits, acute exacerbation, hospitalization, and mortality rates in COPD patients (14–17). Emerging evidence indicates that PM exposure not only alters the structure and composition of the respiratory microbiota but also disrupts multiple pulmonary metabolic pathways (18–20). PM can also be directly transported to the intestine through swallowing, thereby disrupting the ecological balance of the intestinal flora (21). The gut–lung axis may constitute the core mechanism by which PM accelerates the progression of COPD. Our review explores the effects of PM on the lungs and the intestines, and explores the core mechanism of the gut–lung axis in the development and progression of COPD, with the aim of identifying new treatment directions to counter the influence of air pollutants on COPD.
2 Effects of PM on lungs
2.1 Mucosal barrier damage and oxidative stress
The muco-ciliary barrier system is the first physical barrier of the respiratory tract against pathogens, mainly including ciliary mucus clearance, epithelial barrier function, and the secretion of some proteins and peptides with antimicrobial activity. An in vitro simulation experiment found that diesel exhaust particles (DEPs) decreased the expression of cilia-related genes in human bronchial epithelial cells and epithelial tight junction protein, increased mucus secretion, resulting in the epithelial mucosal barrier function (22). PM triggers pulmonary oxidative stress by generating excess reactive oxygen species (ROS) through the activation of oxidases, metabolic enzymes, and mitochondrial impairment. This overwhelms the antioxidant defense system, evidenced by suppressed nuclear factor erythroid 2-related factor 2 (Nrf2), superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) expression. Culminating in oxidative/antioxidant imbalance, protein carbonylation, lipid peroxidation, and DNA damage (23, 24).
2.2 The macro impact of PM on immune cells
PM enters the respiratory tract with breathing. Depending on the size of the PM, PM can deposit in any area from the bronchi to the alveoli, which leads to infiltration of plenty of immune cells in the lung tissue (25, 26). PM can activate the immune system, leading to airway inflammation through the release of immune mediators (Figure 1). This chronic immune-inflammatory stimulation can cause alveoli to rupture, resulting in emphysema, thickening of the airway walls, and narrowing of the lumen, all of which jointly cause obstructive ventilatory dysfunction. Studies have shown that an increase in various immune cells in the airways of patients with COPD, such as macrophages, neutrophils, T lymphocytes, and B lymphocytes (27). PM stimulated the infiltration of neutrophils, macrophages, eosinophils, and lymphocytes in the bronchoalveolar lavage fluid (BALF) and airway submucosa of mice (28). These abnormal immune cell infiltrations are closely associated with the airway remodeling and chronic inflammation in COPD.
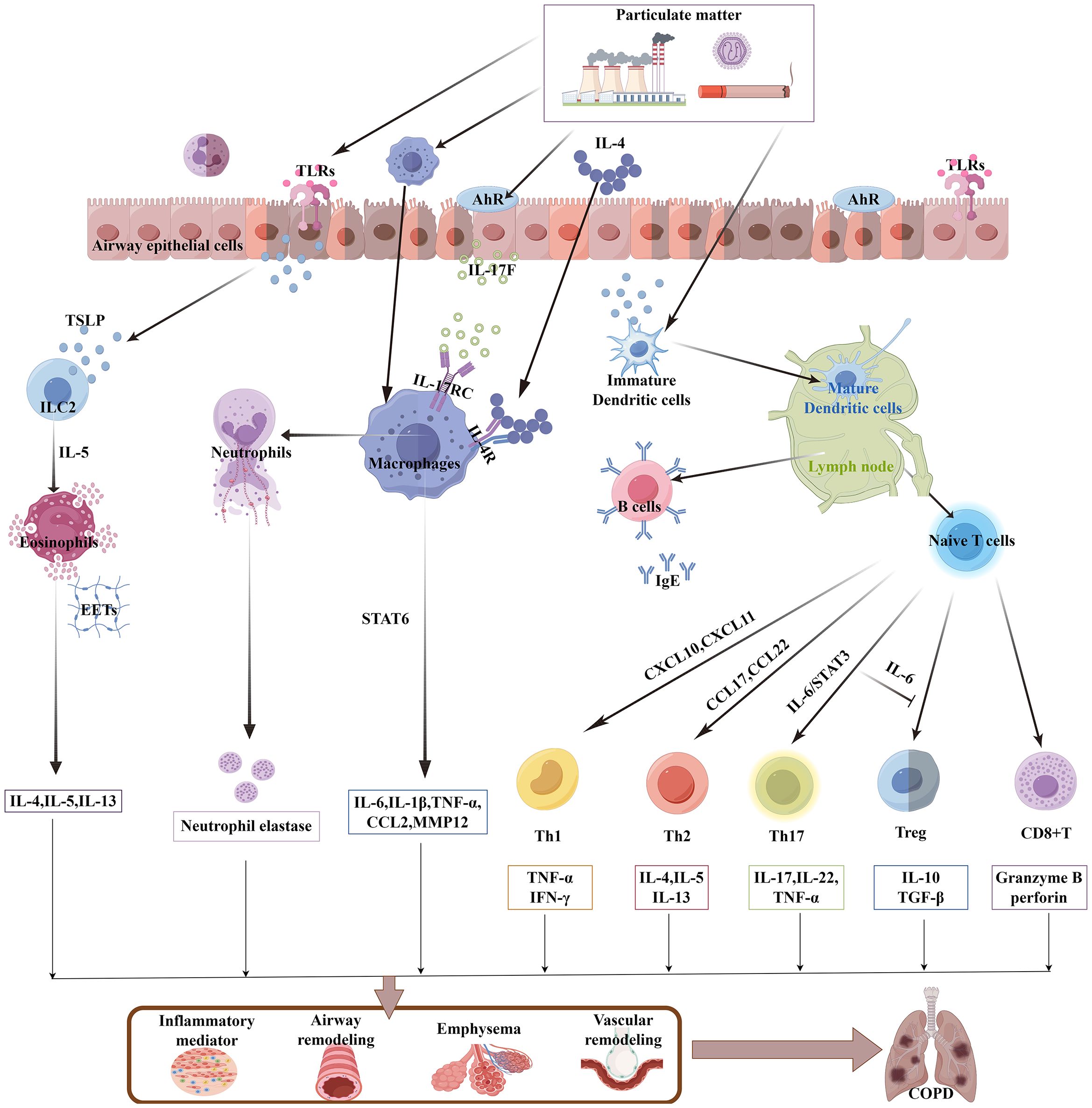
Figure 1. PM activates the lung immune system to participate in COPD. PM induces TSLP release from airway epithelial cells or directly activates DCs and alveolar macrophages, which in turn trigger additional innate immune responses (including eosinophils, neutrophils, and ILC2s). The activated DCs then migrate to lymph nodes, where they initiate adaptive immune responses. Naive lymphocytes are then activated and differentiate into T-cell subsets and mature B cells. A large number of proinflammatory cytokines, chemokines, and elastases contribute to the pathological development of COPD. PM, particulate matter; TSLP, thymic stromal lymphopoietin; DC, dendritic cell; ILC2, group 2 innate lymphoid cell; AhR, aryl hydrocarbon receptor; EETs, eosinophil extracellular traps.
2.3 PM on the microlevel of immune cells
Respiratory epithelial cells not only serve as the first line of defense for the airways but also possess various immune functions. The organic compounds and heavy metals in PM trigger cellular sensing mechanisms, such as interleukin (IL)-17A/IKKα and ROS/NF-kB through toll-like receptors (TLRs) and aryl hydrocarbon receptor (AhR), effectively stimulating the secretion of pleiotropic pro-inflammatory factor thymic stromal lymphopoietin (TSLP) by airway epithelial cells, to activate the innate immune response and the adaptive immune response successively (29, 30). In patients with COPD, TSLP can stimulate type 2 innate lymphoid cells (ILC2) to exert immune functions by producing IL-5, and promote the eosinophilic inflammatory response (31). Dendritic cells (DCs) are the most potent antigen-presenting cells and the key immune cells that connect innate immunity and adaptive immunity. PM can increase the number of DCs in the airways of patients with COPD (32). TSLP is also an activator for DCs (33), With the participation of DC, TSLP also recruits Th1 and Th2 cells through Th1 chemokines (CXCL10 and CXCL11) and Th2 chemokines (CCL17 and CCL22) (34), DCs can also be directly activated by PM, PM promotes the expression of CD80/major histocompatibility complex class 1 (MHC-I)/CD40 on bone marrow-derived DCs (BM-DCs) through multiple signaling pathways such as GATA3/MHC-R and TLR4-MYD88, homing to the lymph nodes and promote the development of lymphoid follicles, stimulate the initial lymphocytes to differentiate into T-cell subsets and mature B lymphocytes (35), they form a chronic inflammatory infiltration zone around the lung tissue and airways, which is known as “lymphoid follicles” (27), they are associated with airway remodeling. Furthermore, PM stimulates TLR2 on DCs, through the DNMT3a/c-Jun/AIF1 signaling pathway, induces the differentiation of Th17 cells (36, 37), further research has found that IL-6 can also reduce the inhibitory ability of regulatory T cells in suppressing T-cell proliferation (38), under their combined effect, it leads to the Th17/regulatory T-cell (Treg) imbalance in COPD.
Alveolar macrophages (AMs) are the core cells of the innate immune system. They have the functions of phagocytosing pathogenic bacteria and toxic particles, secreting cytokines, and chemotaxis (39). AMs can be activated by respiratory epithelial cells. Biomass smoke exposure stimulates the release of IL-17F by mouse lung epithelial cells, through paracrine mechanisms, which binds to the IL-17RC receptors on AMs, promoting the secretion of inflammatory factors such as IL-6 and CCL2 (40). AMs can engulf PM in diesel exhaust, as carbon particles can be observed deposited within the AMs (41). PM can directly act on AMs to exert different effects. Since the polarization of AMs plays a significant role in the progression of COPD, fine particulate matter, with aerodynamic diameter ≤ 2.5 µm (PM2.5) stimulated the high expression of IL-4 in mouse BALF, which a key inducer of M2 polarization, acting on the IL-4R on the surface of AM cells, inducing downstream STAT6 phosphorylation and nuclear translocation, binding to the Matrix Metalloproteinase -12 (MMP12) promoter region and promoting the expression of MMP12, causing excessive degradation of extracellular matrix (ECM), which is related to the destruction of lung tissue in COPD (42). Under continuous stimulation, AM shows decreased movement and phagocytic functions, unable to effectively remove apoptotic cells and inflammatory mediators. Instead, it continuously releases pro-inflammatory factors, maintaining the inflammatory state of COPD (39). In mice with COPD exposed to PM2.5, PM2.5 reduced NAD+/SIRT1 activity to increase the activity of histone acetyltransferases, resulting in elevated expression of pro-inflammatory genes in AM (43), releasing potent proinflammatory factors such as tumor necrosis factor (TNF)-α, IL-1β, and IL-8, causing a large influx of neutrophils from the circulation into the lung tissue (44). This is particularly evident during the acute exacerbation phase of COPD, as the persistent purulent sputum in the airways is an intuitive manifestation of excessive infiltration of neutrophils (45). Moreover, when neutrophils are clearing foreign substances, they release neutrophil elastase, which damages the elastic fibers of the alveoli and leads to emphysema (46).
CD8+ T cells are an important subgroup of T cells and are associated with tissue damage and the expansion of inflammatory responses. PM induces the aggregation of CD8+ T cells in the subepithelial layer of the airway and the alveolar septum of rats with COPD, to releases granzyme B and perforin, leading to an increase in cytotoxic T lymphocyte (CTL) response and resulting in lung tissue damage (47), It may be related to the fact that PM stimulates BM-DCs to highly express CD83/CCR7, thereby promoting the proliferation of CD8+ T cells (48). However, activated lymphocytes or inflammatory mediators reach the intestine, exposing the intestine to continuous inflammatory signals. Thus, the immune disorder in the lungs caused by PM exposure is the core of the vicious cycle of the gut–lung axis.
2.4 Changes in lung microorganisms and metabolites
Microorganisms have been confirmed to exist in the lungs, and their colonization occurs during the early stages of life, such as at birth, during the neonatal period, and even during the fetal stage (49, 50). Colonization of lung microorganisms in the early stages of life plays a crucial role in orchestrating alveolar development and pulmonary immune maturation (51). The lung microbiota plays multiple roles in the lung ecosystem, including the metabolism of nutrients, immune regulation, and maintenance of lung homeostasis. The composition of the lung microbiota and the levels of metabolites are influenced by various factors such as the environment, disease status, diet, and immune conditions (5).
PM affects the balance of lung microecology and alters the composition and metabolites of lung microorganisms. Both clinical and animal studies have demonstrated that exposure to PM2.5 alters the composition of oropharyngeal microflora. Specifically, it increases the overall bacterial load and diversity while reducing the population of beneficial bacteria like Lactobacillus. Conversely, PM2.5 promotes the growth of pathogenic bacteria, including Streptococcus and Staphylococcus. These shifts collectively impair the defensive function of the upper respiratory tract and elevate the risk of infection (52, 53). Epidemiological studies have revealed that PM alters airway microecology and affects lung function, and that forced expiratory volume in one second (FEV1) is significantly correlated with airway microbiota characteristics (20). At the phylum level, Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes are the major lung flora in healthy mice (54, 55); the pathogenic bacteria, inorganic salts, and organic compounds carried by PM are critical factors affecting the lung microenvironment and structure of the microbial flora (20, 56), and their effect is persistent (20). Moreover, the effects of PM on pulmonary microorganisms vary with exposure time, concentration, exposure mode, and regional differences in respiratory tract flora (57, 58).
The PM-induced changes in the lung microbiome are closely related to the occurrence of COPD and vary with the duration of PM exposure. In the early stages of exposure to moderate-to-high levels of PM2.5, the abundance and diversity of microorganisms in the airways increase, and the major flora in the lungs are Proteobacteria, Bacteroidetes, Cyanobacteria, and Firmicutes (18). Subchronic exposure to PM2.5 decreases the abundance of Firmicutes, Bacteroidetes, Actinobacteria, Chloroflexi, and Euryarchaeota in BALF at the phylum level, while the abundance of Proteobacteria, Tenericutes, Planctomycetes, and Acidobacteria increases (19). Epidemiological studies have shown that various respiratory diseases are characterized by a decrease in the abundance of Bacteroides and an increase in the abundance of Proteobacteria (59). Mechanistic insights from animal models reveal that the abundance of Bacteroidota was negatively correlated with the levels of IL-1β, TNF-α, and malonaldehyde (MDA) and positively correlated with the levels of CAT and GSH; these findings suggest that a decreased level of Bacteroidota is involved in the imbalance of inflammatory response and oxidative stress (60). This imbalance causes the pulmonary system to remain in a low-level but continuously activated inflammatory state, which is associated with airflow limitation and lung tissue damage in COPD (60). PM2.5 exposure can increase the abundance of Proteobacteria (60), and other animal studies have shown that exposure to PM2.5 in real environments increased the abundance of Alphaproteobacteria, Pseudomonadaceae, and Desulfovibrionaceae belonging to the phylum Proteobacteria; more importantly, the inflammatory response of lung tissue has been shown to be related to excessive proliferation of Proteobacteria (54). In patients with COPD, the number of Proteobacteria also increased and was higher during the acute exacerbation period than during the stable period (61). The increase in the abundance of Proteobacteria may be due to the fact that PM2.5 stimulates the secretion of Muc5ac in the airway, which Proteobacteria can metabolize. PM2.5 also promotes AMs to produce peroxynitrite, which provides nutrients for anaerobic respiration and selective growth of Proteobacteria. In addition, Proteobacteria are rich in lipopolysaccharides and flagellin, which are recognized by TLRs of AMs to cause oxidative stress damage to cells and release inflammatory mediators such as IL-1β, IL-6, TNF-α, and nuclear factor (NF)-κB, thereby recruiting more neutrophils and macrophages. Therefore, the increased abundance of Proteobacteria is closely related to respiratory tract pathogen infections, pulmonary oxidative stress, and inflammatory responses (60, 62, 63), which are closely associated with COPD. In comparison with healthy individuals, patients with COPD show significant differences in the airway microbiome, which is characterized by reduced microbial diversity, and the more severe the airflow limitation, the less diverse the microbial species in the sputum (64). An increase in pathogenic bacteria such as Streptococcus and Moraxella can release pathogen-associated molecular patterns (PAMPs), which, in turn, trigger acute exacerbation of COPD (8).
In addition, high concentrations of PM2.5 in the ambient air can accelerate the progression of COPD by increasing the abundance and variety of bacteria in the BALF of patients with stable COPD (65). The imbalance in the lung microbiota is significantly correlated with decreased lung function, as well as with the inflammatory factors IL-1β, IL-10, IL-17A, and interferon (IFN)-γ (66). Research has found that changes in the lung microbiota have been associated with the season of PM2.5 exposure and the severity of COPD. When patients with COPD exposed to PM2.5 were followed up by collecting their sputum samples every 3 months according to the season, the abundance in the lung microbiota was the highest in summer, with increased α-diversity and reduced abundance of Proteobacteria, Firmicutes, and Bacteroidetes. The influence of PM2.5 on the respiratory microbiome was more obvious in patients with moderate COPD and less pronounced in those with severe COPD (58). Notably, due to individual differences in respiratory microorganisms, microbial composition varies in different parts of the respiratory tract, and differences have been observed in individual immune statuses and susceptibility. Moreover, the composition of PM is also affected by climate, region, and source. These reasons can lead to different respiratory microbial changes under PM exposure in different studies. In addition, at the phylum level, the same bacterial phyla contain both probiotics and pathogenic bacteria; therefore, with regard to the changes in the main microorganisms in the lung, some studies showed an increase in the abundance of flora, while others showed the opposite results for phyla such as Firmicutes.
PM exposure alters the levels of pulmonary metabolites involved in metabolic pathways, disrupting various energy metabolism pathways, such as lipid, amino acid, and sugar metabolism. The molecules involved in these processes are key mediators of inflammatory signal transduction. Therefore, PM jointly promotes the pathological process of COPD through metabolic disorders of the lungs. Exposure to PM2.5 causes alterations in metabolites such as fumaric acid and the branched-chain amino acids valine and isoleucine, choline, creatine, betaine, citrate, pyruvate, and glutamine in mouse BALF, which are strongly associated with several typical lung microbiota. These metabolites are involved in various metabolic pathways, including the tricarboxylic acid cycle as well as pyruvate, purine, pyrimidine, and choline metabolism. PM leads to a decrease in the levels of fumaric acid, an important intermediate metabolite in the tricarboxylic acid cycle, and also causes a reduction in the levels of valine and isoleucine, which are essential branched-chain amino acids involved in energy metabolism (18, 19). A decrease in valine and isoleucine may also affect the antioxidant defense system (67), making lung tissue more susceptible to oxidative damage. And that fumaric acid inhibits the MAPK-dependent NF-kB signaling pathway, reduces the expression of TNF-α and CCR3, and alleviates the inflammatory response (68). All of these factors are related to the progression of COPD. Meanwhile, PM2.5 stimulates the metabolism of linoleic acid and upregulates the metabolism of arachidonic acid downstream; arachidonic acid and its metabolites play key roles in many inflammatory diseases (55). Therefore, the disruption of multiple energy metabolism pathways by PM2.5 is one of the causes of chronic inflammation in the lungs and lung function impairment in patients with COPD.
A clinical study revealed that in patients with COPD exposed to PM, the lung metabolic flora also has active metabolic ability, especially for the transport and metabolism of amino acids, inorganic ions, and carbohydrates. These active metabolic functions cause increased nutrient consumption, resulting in decreased immune function and increased risk of infection. The findings also suggested that PM promotes COPD progression in part through the altered abundance of airway microbes that accelerate airway repair and airway remodeling processes (65). Phosphatidylcholine (PC) is the most abundant phospholipid in mammalian cell membranes and subcellular organelles, and it plays an important role in maintaining cell membrane stability. In one animal study, PM2.5 was shown to damage alveolar type II cells of rats to reduce the secretion of the surfactant PC, which caused alveolar septa fracture and alveolar cavity fusion; these changes reduced the levels of the lung lipid metabolite lysophosphatidylcholine, which is associated with the prevention of airway inflammation (69). PM2.5 exposure also increased the accumulation of free fatty acids, acylcarnitines, lipid metabolites, and lipid toxicity associated with pathological damage (70). Impaired membrane stability promotes inflammatory factors and metabolites into the lymphatic and circulatory systems, and participates in the development of COPD through the gut–lung axis.
3 Effects of PM on the intestine
The harmful effects of PM on health extend beyond the respiratory system. Since both the lungs and intestines are closely connected to the external environment, PM may concurrently damage both organs (Table 1). PM-induced intestinal damage and dysfunction are caused by oxidative stress injury, immune imbalance, and microecological imbalances.
3.1 Oxidative stress and immune imbalance
Exposure to PM increases the risk of intestinal disease, such as inflammatory bowel disease and appendicitis (71). PM entering the intestine can directly or indirectly impair intestinal barrier function by destroying intestinal epithelial cells. Increasing animal studies have found that PM weakens intestinal barrier function by shortening jejunum and ileum villi and inhibiting intestinal epithelial mucus secretion in mice (19, 72). Subsequently, polycyclic aromatic hydrocarbons (PAHs), heavy metals, and trace elements in PM enter intestinal epithelial cells through passive transfer, diffusion, and active transport, causing intestinal epithelial injury through oxidative stress (72–74). PM2.5 induced excessive ROS production in mitochondria of intestinal epithelial cells, increased the lipid peroxide MDA, and decreased the antioxidant oxidase SODs, resulting in oxidation/antioxidant imbalance. Additionally, PM2.5 induced apoptosis of intestinal epithelial cells by lipid peroxidation and damaged the cytoskeleton microtubule structure (75, 76). Ultrafine PM upregulates the expression of lipid oxidation metabolites HETEs and HODEs in the intestine, causing an increase in the proinflammatory lipid PGD2 (72). PM2.5 induces oxidation-dependent nuclear NF-κB activation, which promotes the release of proinflammatory cytokines IL-6, IL-8, and TNF-α in the intestine; these factors contribute to intestinal inflammation (76, 77). In addition, PM inhibits the expression of the intercellular adhesion proteins E-cadherin and the tight junction protein ZO-1, resulting in increased intestinal permeability, allowing microbial metabolites and PM to enter the circulatory system through the damaged intestinal epithelial barrier (76, 78). These are regarded as the crucial pathological and physiological bases for the intestinal damage caused by PM exposure in COPD.
PM participates in inflammation by disrupting both the innate and adaptive immune systems of the intestine. Studies have found that PM causes a large number of macrophages and neutrophils to infiltrate the villi area of the intestine (72). PM causes an imbalance of the Th17/Treg ratio, in which the frequency of Th17 cells increases while the frequency of Treg cells decreases. PM also decreased the numbers of CD4+ T and B220+CD69+ cells in intestinal Peyer’s patches, reduced the secretion of the anti-inflammatory cytokine IL-10, and increased the proinflammatory cytokine TNF-α (79, 80). Moreover, innate immunity can interact with adaptive immunity; the regulatory dendritic cells (rDCs) in mesenteric lymph nodes can promote the differentiation of CD4+Foxp3+ T cells, exerting immune tolerance (81). These inflammatory cytokines and immune cells enter to mesenteric lymph nodes through the damaged intestinal barrier and reach the lungs by the gut–lung axis to damage lung tissue.
3.2 Effects of PM on intestinal microorganisms and metabolites
The intestinal microbiota consists of a complex microbial system that colonizes the intestinal tract. The balance of the intestinal microbiota is crucial for maintaining human health, enabling the intestines to perform their normal physical barrier function, shape a normal immune system, and promote the absorption of nutrients. The intestinal microbiota is also influenced by various factors, such as the environment, diet, and health status. PM in the oropharynx enters the gastrointestinal tract through swallowing and disturbs the intestinal microecology, manifesting as changes in the composition and diversity of the intestinal flora. The inorganic salts and organic matter in PM alter the intestinal microenvironment and influence the colonization of microorganisms in the intestine. PM that enters the intestine is metabolized into toxic substances by specific microbiota, which directly affect the survival of microorganisms and damage epithelial function (82). The impact of PM on the gastrointestinal microbiota extends throughout the gastrointestinal tract, with changes gradually becoming more pronounced from proximal to distal regions, and the most significant differences are observed in the fecal microbiota (83). This is because the bioaccessibility of PM is influenced by the particle size, the composition of the digestive fluid—including its pH—the levels of the digestive enzymes pepsin and trypsin, and the chemical properties of metal ions (74). Emerging evidence has shown that the intestinal microbial flora in patients with COPD is imbalanced (84, 85). Exposure to PM leads to an imbalance in the intestinal flora, which is associated with the onset of COPD. In a rat model of COPD exposed to environmental PM, a decrease in microbial richness and diversity was observed. This occurred concurrently with PM-induced pulmonary emphysema and the infiltration of macrophages and neutrophils, suggesting a possible relationship between the gut microbiota and COPD (11). An animal experiment employing fecal microbiota transplantation (FMT) confirmed that the gut microbiota affects pulmonary immune status and lung function in COPD. FMT alleviated emphysema in mice with COPD exposed to cigarette smoke (CS) and improved lung function and systemic inflammatory responses (10). Studies have also shown a reduction in the diversity of intestinal bacteria in coal miners, as well as a correlation between the composition of intestinal microorganisms and lung function impairment (86). These findings indicate that disorders of the intestinal flora are closely associated with pulmonary inflammation and decreased lung function in COPD.
PM damages the intestinal barrier by altering the intestinal flora. The intestinal barrier serves as the first line of defense against harmful substances entering the body from the intestine. The intestinal microbiota, as a complex ecosystem, not only constitutes a part of this defense system but also plays an important role as a regulator and maintainer of the system. In the early stages of PM exposure, dysbiosis of the intestinal microbiota first causes damage to the intestinal mucosal barrier (73). DEPs reduce the thickness of the acidic and neutral mucus layers in the colon, and mucus loss occurs before inflammatory infiltration (73). Another animal study found that short-term PM exposure did not cause colon damage or inflammation, while the intestinal microbial diversity was reduced and the total bacterial load was increased. An increase in Verruca microbacteria and Proteobacteria promoted the degradation of intestinal mucus, resulting in weakening of the intestinal mucus barrier. PM2.5 also inhibited the production of butyrate by decreasing the abundance of Lactobacillus, as butyrate is the main energy source for intestinal epithelial cells. These results indicate that PM2.5 exposure may begin to weaken the intestinal barrier by disrupting the intestinal microflora, which subsequently leads to intestinal villi destruction and an intestinal inflammatory response (87). Short-term PM exposure caused a brief increase in the abundance of gut Lactobacillus, which may represent a self-protective mechanism in the host (73). Under continuous exposure to concentrated ambient PM2.5 (CPM), the intestinal mucus of mice became thinner, the villi of the ileum became shorter, the tight junctions of the intestinal epithelium were damaged, and the abundance of Eubacterium rectale and Lactobacillus—both believed to have protective effects on the intestinal barrier—was altered (88). These findings provide a theoretical basis for the reverse entry of intestinal inflammatory mediators and the proinflammatory metabolite LPS into the lungs (11).
PM causes intestinal inflammation by disrupting the gut microbiota. Exposure to PM leads to a decrease in the abundance of Agathobacter and Romboutsia, which exhibit anti-inflammatory activity in the intestines, and an increase in the abundance of proinflammatory bacteria such as Klebsiella, Clostridium, Escherichia, and Haemophilus (86, 87). Klebsiella and Haemophilus are common pathogens in COPD, contributing to frequent acute exacerbations of the disease and accelerating the deterioration of lung function (64, 89). Thus, disruption of the intestinal flora can cause intestinal inflammation, which spreads to the lungs. Long-term exposure to PM increases the abundance of Akkermansia within the Bacteroidetes phylum, which can trigger intestinal inflammation. The levels of IL-6 and IL-10 were positively related to Parabacteroides, while the levels of IL-17A and IL-17F were positively correlated with Prevotella, which is believed to be associated with lung inflammation (90). These findings indicate that the PM-induced changes in intestinal microbiota and the resulting intestinal inflammation infiltration contribute to the reverse transfer of inflammation and the development of COPD.
PM disturbs the intestinal flora and various metabolites. The PM-induced imbalance in the intestinal flora leads to a decrease in the production of beneficial metabolites, such as SCFAs (91), and an increase in harmful metabolites, such as LPS (11). Patients with COPD also show decreased diversity of intestinal flora; for every 10-μg/m3 increase in PM2.5 concentration, the α-diversity of the intestinal flora decreases by 2.16%, the relative abundance of Bacteroidetes declines, and the fecal levels of beneficial SCFAs—including isobutyric acid and isovaleric acid levels—decrease with worsening disease (85, 92). Similar to the results of clinical studies, exposure to ambient PM for 2 months significantly reduced the abundance of intestinal microbiota in mice, reducing the abundance of Firmicutes and Actinomyces and increasing the abundance of Bacteroides and Cyanobacteria, which have low basal abundance (19). With prolonged exposure to PM, the diversity and abundance of intestinal flora in rats decreased. PM exposure also reduced the level of SCFAs in the colon contents; SCFAs function as a substrate for energy metabolism, improve intestinal barrier function, and reduce intestinal bacterial translocation. SCFAs also activate the Nrf2 signaling pathway to maintain the balance between oxidation/antioxidation, reducing the oxidative damage of PM to cells (11, 93). This may be related to the decreased abundance of several SCFA-producing bacteria, including Lactobacillus, in the gut (87). PM disrupts multiple metabolic pathways by altering the homeostasis of the bacterial community. The abundances of Enterococcus faecium and Bacteroides fragilis, which are involved in glycolysis, in the intestinal tract are closely associated with air pollutants. The NO2 level in air pollutants is negatively correlated with the serum levels of the metabolite melatonin, while the CO level is negatively correlated with the serum level of the metabolite C-8C1P, which is closely related with disturbances in lipid and fatty acid metabolism in patients with Acute Exacerbation of COPD (AECOPD) and plays a protective role in the inflammatory response (92). In an animal study, the diversity and abundance of the intestinal flora species decreased in Sprague–Dawley (SD) rats administered oral gavage of PM2.5, confirming that PM2.5 perturbs various metabolic pathways in serum by altering the composition of intestinal microorganisms. The most disturbed metabolic pathways are glycerophospholipid metabolism, which affects the ability to maintain cell membrane stability, transport triglycerides, and participate in inflammation, and linoleic acid metabolism, which is related to oxidative stress (94). Microbial functional analysis based on Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment showed that CPM-exposed mice had increased susceptibility to infectious diseases and viral infections. Moreover, basic metabolic processes, such as amino acid metabolism, lipid metabolism, and carbohydrate metabolism, were significantly attenuated in these mice (88). In addition, the intestinal microecological imbalance caused by PM varies depending on exposure time, dose, composition, and source (11, 95).
4 PM and the gut–lung axis in COPD
The close connection between the lungs and intestine plays a key role in the pathophysiology of PM-induced COPD. A large national clinical study showed that patients with COPD are more likely to develop intestinal disease, and the incidence increases as COPD worsens (96). Changes in the intestinal flora and metabolites can affect the progression of COPD. As shown in Figure 2, PM enters the body through two pathways and interacts with the gut and lungs of patients with COPD through the gut–lung axis. The joint participation of the circulatory and lymphatic systems creates a close network of remote interactions between the two major organs, the lungs and the intestines.
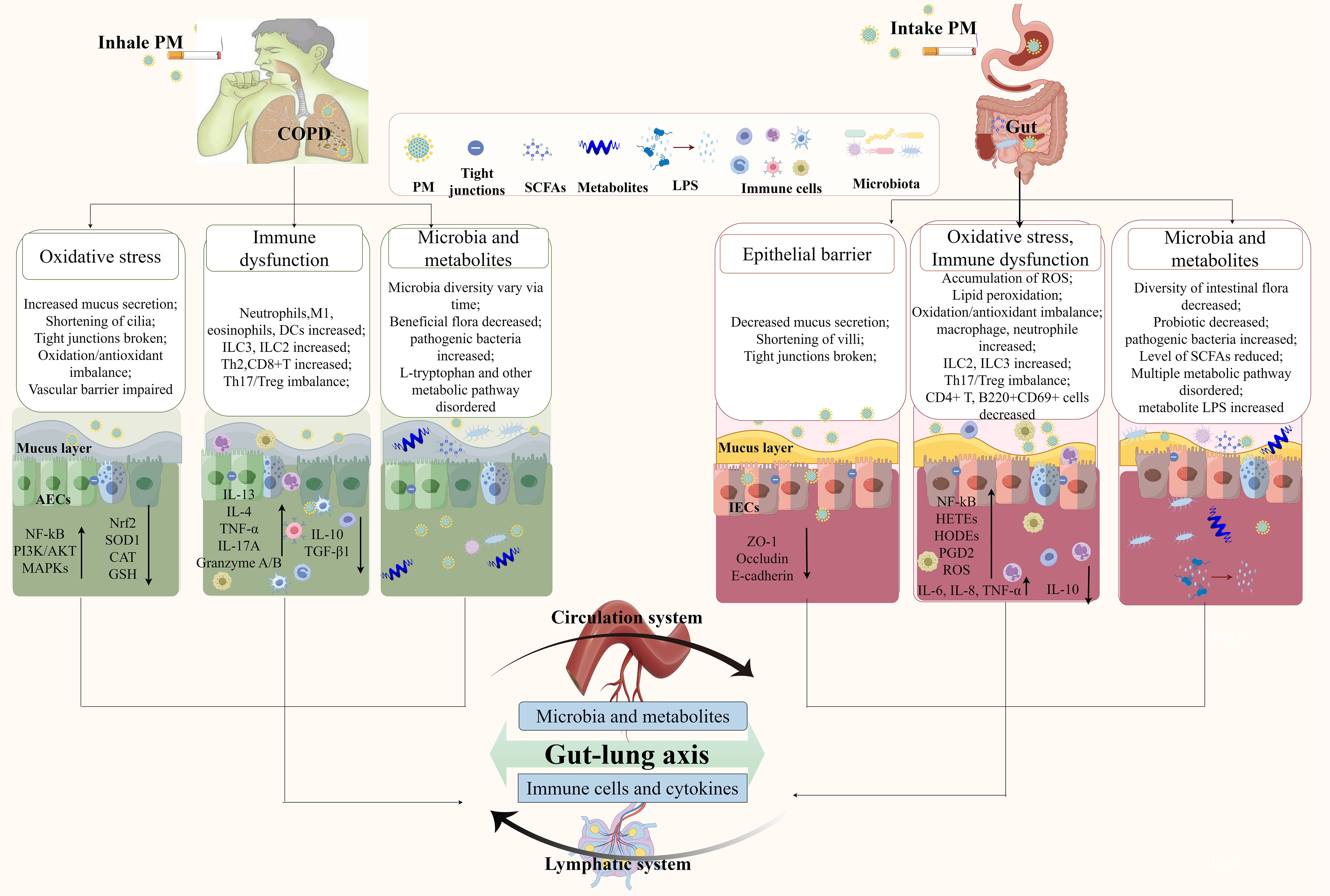
Figure 2. PM affects the gut–lung axis and is involved in COPD. Through the respiratory tract pathway, inhaled PM disrupts the lung epithelial barrier, immune homeostasis, lung microbiota, and metabolites, leading to the release of inflammatory mediators and abnormal metabolites that enter the bloodstream, disrupt the intestinal flora, and trigger intestinal inflammation and dysfunction. Through the digestive tract pathway, PM that enters the intestine via swallowing weakens the intestinal mucosal barrier, activates intestinal mucosal immunity, and disrupts the intestinal microecological balance. These abnormal metabolites and immune mediators reflux into the lungs, ultimately exacerbating the progression of COPD. AECs, alveolar epithelial cells; IECs, intestinal epithelial cells; PM, particulate matter; SCFAs, short-chain fatty acids.
The effects of PM on the gut–lung axis are mediated by immune-inflammatory mechanisms. Immune cells distributed on the mucosal surfaces of the body can communicate with each other across different mucosal tissues. Inflammatory cytokines are the common molecular basis for mucosal immunity in different parts and also serve as important immune mediators in the gut–lung axis. Activation of pulmonary immunity also increases the sensitivity of intestinal inflammation to environmental stimuli. An animal study showed that exposure to CS increased the release of IL-17A by Th17 cells in the lungs, and IL-17A increased the number of neutrophils in the circulation, activating the systemic inflammatory response. Neutrophil recruitment to the intestinal tissue is involved in intestinal pathological damage and is accompanied by increased secretion of IL-6 and IL-17A (97). A study using IL-17A gene knockout and anti-Ly6G depletion of neutrophils confirmed that the IL-17A–neutrophil axis was the indirect cause of the enhanced intestinal inflammatory response driven by CS exposure (97), illustrating the crucial role of pulmonary immune homeostasis in the tolerance of the lungs to environmental stimuli in the gut. The abnormal immune-inflammatory state in the lungs of patients with COPD also disrupts the intestinal microecological balance. Viral and bacterial infections are the main triggers of acute exacerbations of COPD. Infection with the influenza virus and Haemophilus influenzae leads to infiltration of neutrophils, natural killer cells, and macrophages in the lungs of COPD mice exposed to CS, as well as elevated levels of the inflammatory cytokines TNF-α, IL-1β, and IL-22. These inflammatory mediators enter the intestine through the circulatory system, causing a decrease in intestinal symbiotic bacteria and an increase in pathogenic bacteria, which is correlated with high levels of pulmonary inflammatory factors (13). Imbalance in the ratio of Firmicutes to Bacteroidetes in the intestinal microbiota can also trigger inflammatory signals (13), which may, in turn, exacerbate pulmonary inflammation. Furthermore, the activated intestinal immune system, as a remote inflammatory amplifier, actively releases proinflammatory signals that feed back to the lungs, exacerbating the pathological process of COPD. Under pathological conditions, ILC2s in the intestinal mucosa are activated and migrate from the intestine to the lungs through the lymphatic system and blood circulation. Inflammatory ILC2 (iILC2) entering the lung tissue then upregulate the expression of major histocompatibility complex class 2 (MHC-II) or secrete IL-13 and IL-4, providing a microenvironment for Th2 cell differentiation, promoting Th2-type immune responses, and transferring inflammation from the gut to the lungs, participating in the pathogenesis of COPD (98). The oral administration of traditional Chinese medicine formulas can maintain the Th17/Treg immune balance in the intestines of COPD mice exposed to PM, which can correct the Th17/Treg immune imbalance in the lungs, reduce inflammatory infiltration, and improve lung function (99). This bidirectional crosstalk involving immune-inflammatory mediators demonstrates the pathogenic mechanism of systemic inflammation in COPD caused by PM.
Furthermore, the interaction of the gut–lung axis is also realized through microbial translocation and changes in metabolites. Intestinal ecological disorders are closely correlated with changes in the composition of lung microbiota. A recent study showed that intratracheal instillation with PM2.5 changes the composition of the lung microbiota and triggers changes in lung metabolites, resulting in the upregulation of l-tryptophan metabolism, which is a key component and biomarker of the pulmonary cascade to the intestine; reduction of Helicobacter and Spirillum in the intestinal flora; and regulation of gastrointestinal motility and inflammation (55). PM causes proliferation of Bacteroidetes in both the lungs and intestines of mice (100), indicating possible microflora translocation. In mice, exposure to PM2.5 causes a decrease in Firmicutes and Actinomycetes in the lungs and intestines, and the high correlation between intestinal differential flora and serum metabolites and between pulmonary differential flora and BALF metabolites suggests that microbial flora and systemic metabolites may be involved in the pulmonary and intestinal interactions caused by PM2.5 (19). PM may directly disrupt the balance of the intestinal microbiota, and experimental studies on FMT have demonstrated that intestinal microbial imbalance plays a major role in the exacerbation of COPD. In a rat model of COPD exposed to PM, gut microbial diversity and abundance, as well as beneficial flora, decreased, although no significant changes were observed in lung microbes (11). The imbalance in the intestinal flora aggravates the deterioration of COPD. It might be the cause that PM disrupts the intestinal microbiota, activating immune cells in the intestine and causing them to migrate to the lungs, which are in an inflammatory state, and thereby exacerbating lung inflammation. An experiment employing intestinal microbiota transplantation confirmed this explanation. In this experiment, fecal flora isolated from COPD patients with GOLD stages I–II and III–IV disease were transplanted into mice that developed COPD after exposure to biomass smoke. The mice that received the intestinal microbiota of COPD patients showed worse lung function, which was accompanied by increased airway remodeling and greater mucus secretion, triggering higher expression of the inflammatory cytokines IL-1β and TNF-α in the serum, and increasing the number of neutrophils and T cells in the BALF (85).
The metabolic changes driven by the gut microbiota are important mediators of the gut–lung axis. PM disrupting the gut microbiota can also lead to abnormal purine metabolism (101), which results in the production of oxygen-free radicals, causing oxidative stress in the lungs. Moreover, purine metabolism disorders are associated with deterioration of lung function (102), which may explain the mechanism by which PM causes lung damage in COPD by disrupting gut microbiota. However, further interventional studies are needed to confirm this. Intestinal LPS is mainly released by Gram-negative gut bacteria, disrupting the intestinal epithelial barrier and activating inflammatory responses. PM induces the entry of LPS into the lung tissue through the circulatory system to mediate the inflammatory response and damage the lung tissue. Serum LPS levels are also significantly positively correlated with the mean linear intercept of the lung (72). The mechanism underlying this correlation may involve the leakage of LPS from intestinal microorganisms into the lungs, which then recognizes and activates the TLR4/NF-κB signaling pathway in lung epithelial cells to promote the inflammatory response in the lungs and participate in COPD (103). These findings indicate that imbalances in the intestinal flora aggravate lung inflammation and lung tissue damage by generating harmful metabolic products. SCFAs in the intestinal tract are mainly produced by the breakdown of dietary fiber by intestinal flora. Exposure to tobacco particles reduces SCFA levels in the feces of COPD mice. After administration of oral antibiotics to clear SCFAs from the intestines, emphysema and lung inflammation were exacerbated, demonstrating that SCFAs are involved in the inflammatory response and emphysema in COPD. Subsequently, in a mouse model of lung emphysema established using trypsin, the levels of SCFAs did not decrease. This finding indicates that the decrease in intestinal SCFA levels was not related to simple lung emphysema but was related to systemic inflammatory responses (84). This suggests that SCFAs a therapeutic approach for COPD hold significant potential.
5 Treatment
The heterogeneity of COPD may depend on the pulmonary microbiome and immune status of the host. In a clinical study that grouped patients with AECOPD based on differences in microbiota and airway inflammatory mediator levels, the abundance of Proteobacteria and the Proteobacteria/Firmicutes ratio in the pulmonary flora were higher in patients with elevated neutrophil counts and increased levels of inflammatory factors IL-1β, IL-6, and TNF-α. Similarly, in patients with eosinophilic infiltration and higher levels of the Th2 inflammatory mediators IL-5, IL-33, chemokine (C–C motif) ligand 13 (CCL13), and chemokine (C–C motif) ligand 17 (CCL17), α-diversity of the lung microbiota was high, with Bacteroidetes as the main flora. These patients also exhibited increased levels of type 1 inflammatory mediators such as chemokine ligand (CXCL)10, CXCL11, and IFN-γ, along with higher proportions of pulmonary bacteria, including Actinobacteria, Firmicutes, and Streptococcus. These findings provide a theoretical basis for personalized treatment of COPD based on the microbiome. The following outlines the treatment strategies (Table 2).
5.1 Microbiota-targeted interventions
Supplementation with probiotics can protect the respiratory system by maintaining the stability of intestinal flora, preserving epithelial barrier integrity, and supporting antioxidant and immunoregulatory functions. A single-center clinical study found that budesonide combined with Bifidobacterium and Lactobacillus effectively restored the balance of intestinal microbes and improved lung function by increasing the abundance of the beneficial gut bacteria Lactobacillus and Bifidobacterium, while decreasing the abundance of Enterobacteriaceae and Enterococcus (104). Probiotics can increase the Firmicutes/Bacteroidetes ratio and the abundance of beneficial bacteria in the intestine while reducing the abundance of proinflammatory bacteria, thereby mitigating alterations in intestinal flora caused by PM2.5 stimulation (105). Lactobacillus and Bifidobacterium are important probiotics in the gut, and oral administration of Lactococcus and Bifidobacteria can reduce the abundance of Proteobacteria, which produce proinflammatory factors such as TNF-α and IL-6 (106). Therefore, personalized intervention programs can be developed based on the microbial characteristics of patients with COPD.
In vitro studies have shown that Lactobacillus promotes the proliferation and migration of intestinal epithelial cells, regulates the expression of tight junction proteins claudin-1 and occludin, reduces paracellular permeability, and prevents the nuclear translocation of NF-κB p65 in intestinal epithelial cells, thereby inhibiting the release of proinflammatory cytokines TNF-α and IL-1β (107, 108). In the lungs of mice, Lactobacillus plantarum inhibits the recruitment of neutrophils and the production of proinflammatory cytokines IL-17 and TNF-α induced by air pollutants, exerting a protective effect on the respiratory system (109). By reducing the production of cellular ROS, Lactobacillus exerts an antioxidant effect, mitigating lung tissue damage caused by oxidative stress (110). In addition, probiotics demonstrate immunoregulatory activities by modulating the Th17/Treg balance to inhibit the release of the proinflammatory cytokine IL-17A, which recruits neutrophils via chemotaxis; enhance the release of anti-inflammatory factors, including transforming growth factor (TGF)-β and IL-10; and reduce the lung inflammatory damage induced by PM2.5 (80, 105).
FMT may represent another novel therapeutic approach for COPD (111). In CS-induced emphysema animal models, FMT from healthy donors significantly ameliorated emphysema and reduced levels of IL-6, γ-IFN, IL-1β, and TNF-α in lung tissue. More importantly, the gut microbiota in FMT-treated animals was restored toward a healthy state and produced higher levels of SCFAs, which were associated with improvements in alveolar destruction (112).
5.2 Dietary factors
Dietary modification strategies can improve COPD through the gut–lung axis, with SCFAs playing a key protective role. Daily intake of dietary fiber increases intestinal SCFA levels, alleviates emphysema, and reduces the release of the proinflammatory cytokines such as IL-6 and IFN-γ, thereby mitigating the symptoms of COPD (112). Dietary fiber intake not only affects the Firmicutes/Bacteroidetes ratio, altering the composition of intestinal and pulmonary microbiota, but also promotes the formation of macrophages and dendritic cell (DC) precursors, thereby modulating the pulmonary immune microenvironment (113). Clostridium in the intestine is also a source of SCFAs. In patients with AECOPD, the abundance of Clostridium intestinalis decreases, reducing butyrate production and thereby limiting its inhibitory effect on intestinal ILC2 activation to iILC2. Supplementation with butyrate can inhibit the activation and proliferation of intestinal innate lymphoid cells, reduce the release of proinflammatory cytokines, and attenuate inflammation in lung tissues (98).
5.3 Immunomodulators
Oral bacteria-derived immunomodulatory drugs and thymosin drugs can regulate respiratory immunity and reduce the risk of AECOPD by immunizing lymphocytes through the intestinal mucosa. Oral bacteria-derived immunomodulatory drugs include OM-85, Megloniella albus, Bistoid, and Ismigen. These drugs have similar mechanisms: after oral administration, they act as PAMPs and are recognized by pattern recognition receptors (PRRs) and TLRs on innate immune cells in intestinal mucosa-associated lymphoid tissue, inducing DC maturation and activation. This stimulates DCs to produce chemokines and B-cell-activating factor (BAFF) and activates natural killer cells and macrophages within the intestinal mucosa-associated lymphoid tissue. They induce the differentiation of naïve T lymphocytes into Th1 and Th17 cells, promoting the production of proinflammatory cytokines and chemokines, and stimulate the differentiation of Treg cells to maintain immune balance. Moreover, they inhibit Th2-type allergic reactions and promote the differentiation of B lymphocytes into immunoglobulin A (IgA). These activated immune cells, cytokines, and immunoglobulins enter the lung tissue via lymphatic homing and the circulatory system. Ultimately, they enhance respiratory mucosal immunity and exert anti-inflammatory effects (114–117).
5.4 Antibiotics
Macrolide antibiotics can regulate immunity and alter the composition of both lung and intestinal microbiota. A recent study showed that long-term erythromycin treatment could modulate lung and intestinal flora and correct metabolite disturbances in mice and patients with COPD, reduce the abundance of pathogenic bacteria, and increase the number of symbiotic bacteria (118). Azithromycin significantly reduces the frequency of acute exacerbations in patients with COPD (119). It not only interacts with tight junction proteins in epithelial cells to maintain the integrity of the physical barrier but also regulates DCs, neutrophils, macrophages, and the differentiation of T lymphocyte subgroups, thereby inhibiting the release of proinflammatory cytokines and chemokines to achieve immune regulation (120). However, azithromycin can alter the stability of the airway microbiota in healthy adults, reducing the airway’s resistance to outdoor PM2.5, indicating the importance of rational antibiotic use (121).
5.5 Traditional Chinese medicine
Multiple studies have indicated that traditional Chinese medicine (TCM) treatment can slow the progression of COPD (122, 123). Its mechanism is not limited to direct anti-inflammatory effects in the lungs, but also includes its ability to reduce intestinal permeability and decrease endotoxin translocation. By doing so, it cuts off the transmission of gut-derived inflammation to the lungs, reduces inflammatory cell infiltration in lung tissue, and lowers levels of IL-17, TNF-α, IL-8, and IL-6. These effects ultimately improve lung function and slow COPD progression (124).
TCM can also modulate immune responses in both the lungs and the gut. Qifeng Gubiao Granules not only correct the imbalance of M1/M2 macrophages in the lungs and spleen of COPD mice, but, more importantly, they significantly regulate M1 and M2 macrophages in the intestine. This finding indicates that Qifeng Gubiao Granules directly target intestinal immunity, subsequently transmit immunoregulatory signals to the lungs, and ultimately drive pulmonary macrophages to polarize toward the anti-inflammatory M2 phenotype. These results provide direct experimental evidence that TCM can ameliorate immune imbalance via the gut–lung axis (123). Furthermore, Qibai Pingfei Capsule has been shown to maintain the Th17/Treg immune balance in the lungs of COPD rats and improve pulmonary immune status. This effect is associated with alterations in both the lung and gut microbiota (122).
TCM regulates the gut microbiota of COPD mice. Specifically, since a decrease in the Firmicutes/Bacteroidetes (F/B) ratio in the gut is associated with reduced inflammation, Qifeng Gubiao Granules have been shown to decrease the abundance of p:Firmicutes and increase p:Bacteroidetes in the gut of COPD mice, thereby reducing inflammation (123). They also promote the production of beneficial metabolites, such as SCFAs, by the gut microbiota (124). These substances not only supply energy to the intestinal mucosa but also travel via the bloodstream to regulate lung immune cell function, thereby reducing airway inflammation and immune dysregulation in COPD. Furthermore, Qibai Pingfei Capsule exerts protective effects against COPD by mediating changes in the lung microbiota via the gut–lung axis (122).
6 Conclusions and prospects
PM affects the progression of COPD through the gut–lung axis, indicating a shift in understanding COPD from a focus on local lung tissue to a systemic perspective. This review emphasizes that immune cells, intestinal microbiota, and metabolites are central to the interaction within the gut–lung axis and elaborates on the mechanisms by which the gut–lung axis contributes to PM-induced COPD.
At the macroscopic level, epidemiological evidence has shown that exposure to PM increases the incidence, acute exacerbation rate, and mortality rate of COPD (14–17) and is also associated with a higher incidence of gastrointestinal diseases (71). Patients with COPD have an increased risk of developing inflammatory bowel disease (125), and conversely, inflammatory bowel disease increases the risk of death from COPD (126). This connection between the lungs and the intestines suggests a shared underlying link.
At the microlevel, PM exposure not only leads to the infiltration and activation of immune cells, such as macrophages, neutrophils, and lymphocytes, in the lungs and intestines (28, 72) but also causes significant disruptions in microbial communities in distant organs through immune cell homing and the leakage of immune mediators. More importantly, in the circulatory system (11), levels of inflammatory factors released into the blood and microbial metabolites increase simultaneously, providing direct evidence for bidirectional communication between the gut and the lungs.
The core of the interaction within the gut–lung axis lies in self-circulation. First, from the lungs to the intestines, PM entering the lungs activates the pulmonary immune system, releasing immune mediators such as TNF-α and IL-6 into the bloodstream. These mediators indirectly damage the intestines by disrupting the balance of intestinal microorganisms and impairing epithelial barrier function. Moreover, pathogenic bacteria in the lungs of COPD patients increase due to PM exposure; these bacteria can migrate to the intestines and cause further damage. Second, after PM reaches the intestine through swallowing or the circulatory system, it disrupts the intestinal flora and damages the intestinal epithelial barrier, increasing intestinal permeability. This, in turn, allows proinflammatory mediators to translocate into the circulatory system, triggering a systemic low-level inflammatory state. Undoubtedly, this exacerbates the inflammatory load in the lungs of COPD patients.
Based on the above mechanisms, targeting the gut–lung axis offers a new perspective for the treatment of COPD. Combining systemic therapies aimed at restoring homeostasis within the gut–lung axis with traditional anti-inflammatory and bronchodilator drugs provides a novel, comprehensive management strategy for COPD progression caused by PM exposure. However, the most fundamental approach remains the reduction of sources and exposure to environmental pollutants.
However, the effects of PM on the gut–lung axis in COPD are still at an early stage of investigation. First, as PM is a complex mixture, future studies should identify which specific components cause detailed changes in microorganisms. Second, longitudinal studies are needed to determine how protective mechanisms are activated in the early stages of long-term air pollution exposure and when the decompensation of these protective mechanisms begins. Moreover, although some studies have clarified the molecular mechanisms by which microbial metabolites, such as SCFAs and trimethylamine N-oxide (TMAO), act on cells, the molecular mechanisms by which PM contributes to COPD through microorganisms and immunity remain to be further explored. It is anticipated that the gut–lung axis will become a novel therapeutic target for COPD associated with PM exposure.
Author contributions
XP: Writing – review & editing, Writing – original draft. PH: Writing – review & editing. SH: Writing – original draft. XL: Supervision, Project administration, Conceptualization, Writing – original draft, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82260010) and the Gansu Province Key Research and Development Program (22YF7FA083).
Acknowledgments
We thank Figdraw for assistance with the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GOLD. Global Initiative for Chronic Obstructive Lung Disease. Gold 2025 Report: Global Strategy for Prevention, Diagnosis and Management of Copd: 2025 Report (2025). Available online at: https://Goldcopd.Org/2025-Gold-Report/ (Accessed February 5, 2025).
2. Samet J, Mathers CD, and Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PloS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
3. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, and Rudan I. Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (Copd) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir Med. (2022) 10:447–58. doi: 10.1016/s2213-2600(21)00511-7
4. Wong FH, AbuArish A, Matthes E, Turner MJ, Greene LE, Cloutier A, et al. Cigarette smoke activates cftr through ros-stimulated camp signaling in human bronchial epithelial cells. Am J Physiol Cell Physiol. (2018) 314:C118–C34. doi: 10.1152/ajpcell.00099.2017
5. Wypych TP, Wickramasinghe LC, and Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol. (2019) 20:1279–90. doi: 10.1038/s41590-019-0451-9
6. Garcia-Nuñez M, Millares L, Pomares X, Ferrari R, Pérez-Brocal V, Gallego M, et al. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol. (2014) 52:4217–23. doi: 10.1128/jcm.01967-14
7. Millares L, Pascual S, Montón C, García-Núñez M, Lalmolda C, Faner R, et al. Relationship between the respiratory microbiome and the severity of airflow limitation, history of exacerbations and circulating eosinophils in copd patients. BMC Pulm Med. (2019) 19(1):112. doi: 10.1186/s12890-019-0867-x
8. Lin Z, Jiang Y, Liu H, Yang J, Yang B, Zhang K, et al. Airway microbiota and immunity associated with chronic obstructive pulmonary disease severity. J Transl Med. (2025) 23:962. doi: 10.1186/s12967-025-06986-2
9. Rylance J, Kankwatira A, Nelson DE, Toh E, Day RB, Lin H, et al. Household air pollution and the lung microbiome of healthy adults in Malawi: A cross-sectional study. BMC Microbiol. (2016) 16:182. doi: 10.1186/s12866-016-0803-7
10. Budden KF, Shukla SD, Bowerman KL, Vaughan A, Gellatly SL, Wood DLA, et al. Faecal microbial transfer and complex carbohydrates mediate protection against copd. Gut. (2024) 73:751–69. doi: 10.1136/gutjnl-2023-330521
11. Li N, Yang Z, Liao B, Pan T, Pu J, Hao B, et al. Chronic exposure to ambient particulate matter induces gut microbial dysbiosis in a rat copd model. Respir Res. (2020) 21:271. doi: 10.1186/s12931-020-01529-3
12. Liu Q, Tian X, Maruyama D, Arjomandi M, and Prakash A. Lung immune tone via gut-lung axis: gut-derived lps and short-chain fatty acids’ Immunometabolic regulation of lung il-1β, ffar2, and ffar3 expression. Am J Physiology-Lung Cell Mol Physiol. (2021) 321:L65–78. doi: 10.1152/ajplung.00421.2020
13. Wu X, Li R-F, Lin Z-S, Xiao C, Liu B, Mai K-L, et al. Coinfection with influenza virus and non-typeable haemophilus influenzae aggregates inflammatory lung injury and alters gut microbiota in copd mice. Front Microbiol. (2023) 14:1137369. doi: 10.3389/fmicb.2023.1137369
14. Park J, Kim H-J, Lee C-H, Lee CH, and Lee HW. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Environ Res. (2021) 194:110703. doi: 10.1016/j.envres.2020.110703
15. Niu Y, Niu H, Meng X, Zhu Y, Ren X, He R, et al. Associations between air pollution and the onset of acute exacerbations of copd. Chest. (2024) 166:998–1009. doi: 10.1016/j.chest.2024.05.030
16. Delavar MA, Jahani MA, Sepidarkish M, Alidoost S, Mehdinezhad H, and Farhadi Z. Relationship between fine particulate matter (Pm2.5) concentration and risk of hospitalization due to chronic obstructive pulmonary disease: A systematic review and meta-analysis. BMC Public Health. (2023) 23:2229. doi: 10.1186/s12889-023-17093-6
17. Dong J, You J, Wang J, and Bao H. Association between short-term ambient air pollution and outpatient visits for acute exacerbation of chronic obstructive pulmonary disease in lanzhou, 2013–19. Environ Geochem Health. (2022) 45:2495–509. doi: 10.1007/s10653-022-01363-0
18. Li J, Hu Y, Liu L, Wang Q, Zeng J, and Chen C. Pm2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci Total Environ. (2020) 721:137432. doi: 10.1016/j.scitotenv.2020.137432
19. Ran Z, An Y, Zhou J, Yang J, Zhang Y, Yang J, et al. Subchronic exposure to concentrated ambient pm2.5 perturbs gut and lung microbiota as well as metabolic profiles in mice. Environ pollut. (2021) 272:115987. doi: 10.1016/j.envpol.2020.115987
20. Wang L, Cheng H, Wang D, Zhao B, Zhang J, Cheng L, et al. Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol Environ Saf. (2019) 167:269–77. doi: 10.1016/j.ecoenv.2018.09.079
21. Zhai Q, Li T, Yu L, Xiao Y, Feng S, Wu J, et al. Effects of subchronic oral toxic metal exposure on the intestinal microbiota of mice. Sci Bull. (2017) 62:831–40. doi: 10.1016/j.scib.2017.01.031
22. Park E, Kim B-Y, Lee S, Son KH, Bang J, Hong SH, et al. Diesel exhaust particle exposure exacerbates ciliary and epithelial barrier dysfunction in the multiciliated bronchial epithelium models. Ecotoxicol Environ Saf. (2024) 273:116090. doi: 10.1016/j.ecoenv.2024.116090
23. Wang Y, Liao S, Pan Z, Jiang S, Fan J, Yu S, et al. Hydrogen sulfide alleviates particulate matter-induced emphysema and airway inflammation by suppressing ferroptosis. Free Radical Biol Med. (2022) 186:1–16. doi: 10.1016/j.freeradbiomed.2022.04.014
24. Hou T, Zhu L, Wang Y, and Peng L. Oxidative stress is the pivot for pm2.5-induced lung injury. Food Chem Toxicol. (2024) 184:114362. doi: 10.1016/j.fct.2023.114362
25. Yang L, Li C, and Tang X. The impact of pm2.5 on the host defense of respiratory system. Front Cell Dev Biol. (2020) 8:91. doi: 10.3389/fcell.2020.00091
26. Arias-Pérez RD, Taborda NA, Gómez DM, Narvaez JF, Porras J, and Hernandez JC. Inflammatory effects of particulate matter air pollution. Environ Sci pollut Res Int. (2020) 27:42390–404. doi: 10.1007/s11356-020-10574-w
27. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. (2004) 350:2645–53. doi: 10.1056/NEJMoa032158
28. Jung SH, Bae CH, Kim JH, Park S-D, Shim J-J, and Lee J-L. Lactobacillus casei hy2782 and pueraria lobata root extract complex ameliorates particulate matter-induced airway inflammation in mice by inhibiting th2 and th17 immune responses. Prev Nutr Food Sci. (2022) 27:188–97. doi: 10.3746/pnf.2022.27.2.188
29. Glencross DA, Ho T-R, Camiña N, Hawrylowicz CM, and Pfeffer PE. Air pollution and its effects on the immune system. Free Radical Biol Med. (2020) 151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179
30. Anzalone G, Albano GD, Montalbano AM, Riccobono L, Bonanno A, Gagliardo R, et al. Il-17a-associated ikk-A Signaling induced tslp production in epithelial cells of copd patients. Exp Mol Med. (2018) 50:1–12. doi: 10.1038/s12276-018-0158-2
31. Królak-Nowak K, Wierzbińska M, Żal A, Antczak A, and Tworek D. Expression of epithelial alarmin receptor on innate lymphoid cells type 2 in eosinophilic chronic obstructive pulmonary disease. Adv Respir Med. (2024) 92:429–43. doi: 10.3390/arm92050039
32. Xiong J, Zhou L, Tian J, Yang X, Li Y, Jin R, et al. Cigarette smoke-induced lymphoid neogenesis in copd involves il-17/rankl pathway. Front Immunol. (2021) 11:588522. doi: 10.3389/fimmu.2020.588522
33. Cui X, Gao N, Me R, Xu J, and Yu F-SX. Tslp protects corneas frompseudomonas aeruginosainfection by regulating dendritic cells and il-23-il-17 pathway. Invest Opthalmology Visual Sci. (2018) 59:4228–37. doi: 10.1167/iovs.18-24672
34. Ying S, O'Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. (2008) 181:2790–8. doi: 10.4049/jimmunol.181.4.2790
35. Brusselle GG, Joos GF, and Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. (2011) 378:1015–26. doi: 10.1016/s0140-6736(11)60988-4
36. Huang D, Zhou B, Luo ZZ, Yu SC, and Tang B. Cigarette smoke extract promotes DNA methyltransferase 3a expression in dendritic cells, inducing th-17/treg imbalance via the C-jun/allograft inflammatory factor 1 axis. Kaohsiung J Med Sci. (2021) 37:594–603. doi: 10.1002/kjm2.12367
37. Pu J, Xu J, Chen L, Zhou H, Cao W, Hao B, et al. Exposure to biomass smoke induces pulmonary th17 cell differentiation by activating tlr2 on dendritic cells in a copd rat model. Toxicol Lett. (2021) 348:28–39. doi: 10.1016/j.toxlet.2021.05.010
38. Pasare C and Medzhitov R. Toll pathway-dependent blockade of cd4+Cd25+T cell-mediated suppression by dendritic cells. Science. (2003) 299:1033–6. doi: 10.1126/science.1078231
39. Akata K and van Eeden SF. Lung macrophage functional properties in chronic obstructive pulmonary disease. Int J Mol Sci. (2020) 21:853. doi: 10.3390/ijms21030853
40. He F, Yu X, Zhang J, Cui J, Tang L, Zou S, et al. Biomass-related pm2.5 induced inflammatory microenvironment via il-17f/il-17rc axis. Environ pollut. (2024) 342:123048. doi: 10.1016/j.envpol.2023.123048
41. Fang Z-F, Wang Z-N, Chen Z, Peng Y, Fu Y, Yang Y, et al. Fine particulate matter contributes to copd-like pathophysiology: experimental evidence from rats exposed to diesel exhaust particles. Respir Res. (2024) 25:14. doi: 10.1186/s12931-023-02623-y
42. Guo X, Yang S, Zhu H, Liu F, Li K, Li G, et al. Involvement of M2 macrophages polarization in pm2.5-induced copd by upregulating mmp12 via il4/stat6 pathway. Ecotoxicol Environ Saf. (2024) 283:116793. doi: 10.1016/j.ecoenv.2024.116793
43. Noh M, Sim JY, Kim J, Ahn JH, Min H-Y, Lee J-U, et al. Particulate matter-induced metabolic recoding of epigenetics in macrophages drives pathogenesis of chronic obstructive pulmonary disease. J Hazard Mater. (2024) 464:132932. doi: 10.1016/j.jhazmat.2023.132932
44. Murugan V and Peck MJ. Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp Lung Res. (2009) 35:439–85. doi: 10.1080/01902140902759290
45. Stockley RA. Assessment of airway neutrophils by sputum colour: correlation with airways inflammation. Thorax. (2001) 56:366–72. doi: 10.1136/thorax.56.5.366
46. Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, and Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. (2003) 163:2329–35. doi: 10.1016/s0002-9440(10)63589-4
47. He F, Wang N, Yu X, Zheng Y, Liu Q, Chen Q, et al. Gata3/long noncoding rna mhc-R regulates the immune activity of dendritic cells in chronic obstructive pulmonary disease induced by air pollution particulate matter. J Hazard Mater. (2022) 438:129459. doi: 10.1016/j.jhazmat.2022.129459
48. Pfeffer PE, Ho TR, Mann EH, Kelly FJ, Sehlstedt M, Pourazar J, et al. Urban particulate matter stimulation of human dendritic cells enhances priming of naive cd8 T lymphocytes. Immunology. (2017) 153:502–12. doi: 10.1111/imm.12852
49. Pattaroni C, Watzenboeck ML, Schneidegger S, Kieser S, Wong NC, Bernasconi E, et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe. (2018) 24:857–65.e4. doi: 10.1016/j.chom.2018.10.019
50. Al Alam D, Danopoulos S, Grubbs B, Ali NATBM, MacAogain M, Chotirmall SH, et al. Human fetal lungs harbor a microbiome signature. Am J Respir Crit Care Med. (2020) 201:1002–6. doi: 10.1164/rccm.201911-2127LE
51. Stankovic MM. Lung microbiota: from healthy lungs to development of chronic obstructive pulmonary disease. Int J Mol Sci. (2025) 26(4):1403. doi: 10.3390/ijms26041403
52. Qin T, Zhang F, Zhou H, Ren H, Du Y, Liang S, et al. High-level pm2.5/pm10 exposure is associated with alterations in the human pharyngeal microbiota composition. Front Microbiol. (2019) 10:54. doi: 10.3389/fmicb.2019.00054
53. Deng L, Ma M, Li S, Zhou L, Ye S, Wang J, et al. Protective effect and mechanism of baicalin on lung inflammatory injury in balb/cj mice induced by pm2.5. Ecotoxicol Environ Saf. (2022) 248:114329. doi: 10.1016/j.ecoenv.2022.114329
54. Wang J, Yan Y, Si H, Li J, Zhao Y, Gao T, et al. The effect of real-ambient pm2.5 exposure on the lung and gut microbiomes and the regulation of nrf2. Ecotoxicol Environ Saf. (2023) 254:114702. doi: 10.1016/j.ecoenv.2023.114702
55. Dai S, Wang Z, Cai M, Guo T, Mao S, and Yang Y. A multi-omics investigation of the lung injury induced by pm2.5 at environmental levels via the lung-gut axis. Sci Total Environ. (2024) 926:172027. doi: 10.1016/j.scitotenv.2024.172027
56. Chu X, Liu X-J, Qiu J-M, Zeng X-L, Bao H-R, and Shu J. Effects of astragalus and codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to pm2.5. Environ Toxicol Pharmacol. (2016) 48:76–84. doi: 10.1016/j.etap.2016.10.006
57. Lin L, Yi X, Liu H, Meng R, Li S, Liu X, et al. The airway microbiome mediates the interaction between environmental exposure and respiratory health in humans. Nat Med. (2023) 29:1750–9. doi: 10.1038/s41591-023-02424-2
58. Heo S-H, Choi B-Y, Kang J, Jung JY, Kim H-C, Lee S-J, et al. Dynamics of the airway microbiome in response to exposure to particulate matter 2.5 in patients with chronic obstructive pulmonary disease. Sci Total Environ. (2024) 956:177314. doi: 10.1016/j.scitotenv.2024.177314
59. Dickson RP, Erb-Downward JR, and Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. (2014) 7:245–57. doi: 10.1586/ers.13.24
60. Wang S, Zhou Q, Tian Y, and Hu X. The lung microbiota affects pulmonary inflammation and oxidative stress induced by pm2.5 exposure. Environ Sci Technol. (2022) 56:12368–79. doi: 10.1021/acs.est.1c08888
61. Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV, et al. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. (2014) 52:2813–23. doi: 10.1128/jcm.00035-14
62. Daniel S, Phillippi D, Schneider LJ, Nguyen KN, Mirpuri J, and Lund AK. Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57bl/6 wildtype mice on a high-fat diet. Part Fibre Toxicol. (2021) 18:3. doi: 10.1186/s12989-020-00393-9
63. Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol. (2018) 141:2027–36.e12. doi: 10.1016/j.jaci.2018.04.013
64. Bahetjan K, Yu X, Lin S, Aili N, Yang H, and Du S. Analysis of the bronchoalveolar lavage fluid microbial flora in copd patients at different lung function during acute exacerbation. Sci Rep. (2025) 15:13179. doi: 10.1038/s41598-025-96746-5
65. Che C, Sun X, Wu Y, Ma L, Hu Y, Yang W, et al. Effects of atmospheric fine particulate matter and its carrier microbes on pulmonary microecology in patients with copd. Int J Chron Obstruct Pulmon Dis. (2021) 16:2049–63. doi: 10.2147/copd.S314265
66. Laiman V, Chuang H-C, Lo Y-C, Yuan T-H, Chen Y-Y, Heriyanto DS, et al. Cigarette smoke-induced dysbiosis: comparative analysis of lung and intestinal microbiomes in copd mice and patients. Respir Res. (2024) 25:204. doi: 10.1186/s12931-024-02836-9
67. Da Silva MS, Bigo C, Barbier O, and Rudkowska I. Whey protein hydrolysate and branched-chain amino acids downregulate inflammation-related genes in vascular endothelial cells. Nutr Res. (2017) 38:43–51. doi: 10.1016/j.nutres.2017.01.005
68. Roh K-B, Jung E, Park D, and Lee J. Fumaric acid attenuates the eotaxin-1 expression in tnf-A-stimulated fibroblasts by suppressing P38 mapk-dependent nf-Kb signaling. Food Chem Toxicol. (2013) 58:423–31. doi: 10.1016/j.fct.2013.05.020
69. Chen W-L, Lin C-Y, Yan Y-H, Cheng KT, and Cheng T-J. Alterations in rat pulmonary phosphatidylcholines after chronic exposure to ambient fine particulate matter. Mol Biosyst. (2014) 10:3163–9. doi: 10.1039/c4mb00435c
70. Kurlawala Z, Singh P, Hill BG, and Haberzettl P. Fine particulate matter (Pm2.5)-induced pulmonary oxidative stress contributes to changes in the plasma lipidome and liver transcriptome in mice. Toxicol Sci. (2023) 192:209–22. doi: 10.1093/toxsci/kfad020
71. Feng J, Cavallero S, Hsiai T, and Li R. Impact of air pollution on intestinal redox lipidome and microbiome. Free Radical Biol Med. (2020) 151:99–110. doi: 10.1016/j.freeradbiomed.2019.12.044
72. Li R, Navab K, Hough G, Daher N, Zhang M, Mittelstein D, et al. Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ Health Perspect. (2015) 123:34–41. doi: 10.1289/ehp.1307036
73. Li X, Sun H, Li B, Zhang X, Cui J, Yun J, et al. Probiotics ameliorate colon epithelial injury induced by ambient ultrafine particles exposure. Adv Sci (Weinh). (2019) 6:1900972. doi: 10.1002/advs.201900972
74. Gao P, Guo H, Zhang Z, Ou C, Hang J, Fan Q, et al. Bioaccessibility and exposure assessment of trace metals from urban airborne particulate matter (Pm10 and pm2.5) in simulated digestive fluid. Environ pollut. (2018) 242:1669–77. doi: 10.1016/j.envpol.2018.07.109
75. Park M, Cho Y-L, Choi Y, Min J-K, Park Y-J, Yoon S-J, et al. Particulate matter induces ferroptosis by accumulating iron and dysregulating the antioxidant system. BMB Rep. (2023) 56:96–101. doi: 10.5483/BMBRep.2022-0139
76. Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. (2011) 8:19. doi: 10.1186/1743-8977-8-19
77. Dai S, Wang Z, Yang Y, Du P, and Li X. Pm2.5 induced weight loss of mice through altering the intestinal microenvironment: mucus barrier, gut microbiota, and metabolic profiling. J Hazard Mater. (2022) 431:128653. doi: 10.1016/j.jhazmat.2022.128653
78. Crawford MSS, Ulu A, Ramirez BM, Santos AN, Chatterjee P, Canale V, et al. Respiratory exposure to agricultural dust extract promotes increased intestinal tnfα Expression, gut barrier dysfunction, and endotoxemia in mice. Am J Physiol Gastrointest Liver Physiol. (2024) 326:G3–G15. doi: 10.1152/ajpgi.00297.2022
79. Lee Y-S, Park G-S, Ko S-H, Yang W-K, Seo H-J, Kim S-H, et al. Lactobacillus paracasei atg-E1 improves particulate matter 10 plus diesel exhaust particles (Pm10d)-induced airway inflammation by regulating immune responses. Front Microbiol. (2023) 14:1145546. doi: 10.3389/fmicb.2023.1145546
80. Xu J, Wang J, He Y, Chen R, and Meng QL. Acidophilus participates in intestinal inflammation induced by pm2.5 through affecting the treg/th17 balance. Environ pollut. (2024) 341:122977. doi: 10.1016/j.envpol.2023.122977
81. Kwon H-K, Lee C-G, So J-S, Chae C-S, Hwang J-S, Sahoo A, et al. Generation of regulatory dendritic cells and cd4 +Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U.S.A. (2010) 107:2159–64. doi: 10.1073/pnas.0904055107
82. Salim SY, Kaplan GG, and Madsen KL. Air pollution effects on the gut microbiota. Gut Microbes. (2013) 5:215–9. doi: 10.4161/gmic.27251
83. Mutlu EA, Comba IY, Cho T, Engen PA, Yazıcı C, Soberanes S, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ pollut. (2018) 240:817–30. doi: 10.1016/j.envpol.2018.04.130
84. Otake S, Chubachi S, Miyamoto J, Haneishi Y, Arai T, Iizuka H, et al. Impact of smoking on gut microbiota and short-chain fatty acids in human and mice: implications for copd. Mucosal Immunol. (2025) 18:353–65. doi: 10.1016/j.mucimm.2024.12.006
85. Li N, Dai Z, Wang Z, Deng Z, Zhang J, Pu J, et al. Gut microbiota dysbiosis contributes to the development of chronic obstructive pulmonary disease. Respir Res. (2021) 22:274. doi: 10.1186/s12931-021-01872-z
86. Yu X, Xiong T, Yu L, Liu G, Yang F, Li X, et al. Gut microbiome and metabolome profiling in coal workers’ Pneumoconiosis: potential links to pulmonary function. Microbiol Spectr. (2024) 12:e0004924. doi: 10.1128/spectrum.00049-24
87. Liu Y, Wang T, Si B, Du H, Liu Y, Waqas A, et al. Intratracheally instillated diesel pm2.5 significantly altered the structure and composition of indigenous murine gut microbiota. Ecotoxicol Environ Saf. (2021) 210:111903. doi: 10.1016/j.ecoenv.2021.111903
88. Ran Z, Yang J, Liu L, Wu S, An Y, Hou W, et al. Chronic pm2.5 exposure disrupts intestinal barrier integrity via microbial dysbiosis-triggered tlr2/5-myd88-nlrp3 inflammasome activation. Environ Res. (2024) 258:119415. doi: 10.1016/j.envres.2024.119415
89. Gopalakrishnan V, Sparklin B, Kim JH, Bouquet J, Kehl M, Kenny T, et al. Nthi killing activity is reduced in copd patients and is associated with a differential microbiome. Respir Res. (2025) 26:45. doi: 10.1186/s12931-025-03113-z
90. Xie S, Zhang C, Zhao J, Li D, and Chen J. Exposure to concentrated ambient pm2.5 (Capm) induces intestinal disturbance via inflammation and alternation of gut microbiome. Environ Int. (2022) 161:107138. doi: 10.1016/j.envint.2022.107138
91. Song X, Dou X, Chang J, Zeng X, Xu Q, and Xu C. The role and mechanism of gut-lung axis mediated bidirectional communication in the occurrence and development of chronic obstructive pulmonary disease. Gut Microbes. (2024) 16:2414805. doi: 10.1080/19490976.2024.2414805
92. Li H, Yang Y, Yang Y, Zhai C, Yao J, Liao W, et al. Multiomics was used to clarify the mechanism by which air pollutants affect chronic obstructive pulmonary disease: A human cohort study. Toxicology. (2024) 501:153709. doi: 10.1016/j.tox.2023.153709
93. Chen H, Qian Y, Jiang C, Tang L, Yu J, Zhang L, et al. Butyrate ameliorated ferroptosis in ulcerative colitis through modulating nrf2/gpx4 signal pathway and improving intestinal barrier. Biochim Biophys Acta Mol Basis Dis. (2024) 1870:166984. doi: 10.1016/j.bbadis.2023.166984
94. Zhang Y, Li M, Pu Z, Chi X, and Yang J. Multi-omics data reveals the disturbance of glycerophospholipid metabolism and linoleic acid metabolism caused by disordered gut microbiota in pm2.5 gastrointestinal exposed rats. Ecotoxicol Environ Saf. (2023) 262:115182. doi: 10.1016/j.ecoenv.2023.115182
95. Fouladi F, Bailey MJ, Patterson WB, Sioda M, Blakley IC, Fodor AA, et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ Int. (2020) 138:105604. doi: 10.1016/j.envint.2020.105604
96. Lee J, Im JP, Han K, Park S, Soh H, Choi K, et al. Risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease: A nationwide, population-based study. World J Gastroenterol. (2019) 25:6354–64. doi: 10.3748/wjg.v25.i42.6354
97. Kim M, Gu B, Madison MC, Song HW, Norwood K, Hill AA, et al. Cigarette smoke induces intestinal inflammation via a th17 cell-neutrophil axis. Front Immunol. (2019) 10:75. doi: 10.3389/fimmu.2019.00075
98. Jiang M, Li Z, Zhang F, Li Z, Xu D, Jing J, et al. Butyrate inhibits iilc2-mediated lung inflammation via lung-gut axis in chronic obstructive pulmonary disease (Copd). BMC Pulm Med. (2023) 23:163. doi: 10.1186/s12890-023-02438-z
99. Wang Y, Li N, Li Q, Liu Z, Li Y, Kong J, et al. Xuanbai chengqi decoction ameliorates pulmonary inflammation via reshaping gut microbiota and rectifying th17/treg imbalance in a murine model of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2021) 16:3317–35. doi: 10.2147/copd.S337181
100. Laiman V, Lo Y-C, Chen H-C, Yuan T-H, Hsiao T-C, Chen J-K, et al. Data on lung and intestinal microbiome after air pollution exposure in ageing rats. Data Brief. (2023) 47:109004. doi: 10.1016/j.dib.2023.109004
101. Zhou L, Song C, Lei Y, Zhao L, Han Y, Xu Y, et al. Health impacts of pm2.5 emissions from brake pad wear: A comprehensive study on pulmonary, metabolic, and microbiota alterations. Toxicology. (2025) 511:154055. doi: 10.1016/j.tox.2025.154055
102. Li L. An unexpected role for serum uric acid as a biomarker for severity of asthma exacerbation. Asian Pac J Allergy Immunol. (2013) 32:93–9. doi: 10.12932/ap0337.32.1.2014
103. Xu C, Xu H, Dai X, Gui S, and Chen J. Effects and mechanism of combination of platycodon grandiflorum polysaccharides and platycodon saponins in the treatment of chronic obstructive pulmonary disease rats through the gut-lung axis. J Ethnopharmacol. (2025) 341:119305. doi: 10.1016/j.jep.2024.119305
104. Liu Y, Ye F, and Ma L. The effect of budesonide combined with bifidobacteria and lactobacilli on lung function and gut microbiota of patients with chronic obstructive pulmonary disease. Pak J Med Sci. (2024) 40:695–700. doi: 10.12669/pjms.40.4.8514
105. Wu Y, Pei C, Wang X, Wang Y, Huang D, Shi S, et al. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying th17/treg imbalance in a rat model of pm2.5 induced lung injury. Ecotoxicol Environ Saf. (2022) 244:114060. doi: 10.1016/j.ecoenv.2022.114060
106. Shin Y-J, Ma X, Joo M-K, Baek J-S, and Kim D-H. Lactococcus lactis and bifidobacterium bifidum alleviate postmenopausal symptoms by suppressing nf-Kb signaling and microbiota dysbiosis. Sci Rep. (2024) 14:31675. doi: 10.1038/s41598-024-81500-0
107. Guo S, Gillingham T, Guo Y, Meng D, Zhu W, Walker WA, et al. Secretions of bifidobacterium infantis and lactobacillus acidophilus protect intestinal epithelial barrier function. J Pediatr Gastroenterol Nutr. (2017) 64:404–12. doi: 10.1097/mpg.0000000000001310
108. Luo S and Chen M. Systematic investigation of the effect of lactobacillus acidophilus tw01 on potential prevention of particulate matter (Pm)2.5-induced damage using a novel in vitro platform. Foods. (2023) 12:3278. doi: 10.3390/foods12173278
109. Park M-S, Kim Y-J, Shin H-J, Kwon YJ, Chu J, Lee I, et al. Protective effect of novel lactobacillus plantarum kc3 isolated from fermented kimchi on gut and respiratory disorders. Microorganisms. (2023) 11:967. doi: 10.3390/microorganisms11040967
110. Gupta N, Abd El-Gawaad NS, Osman Abdallah SA, and Al-Dossari M. Possible modulating functions of probiotic lactiplantibacillus plantarum in particulate matter-associated pulmonary inflammation. Front Cell Infect Microbiol. (2024) 13:1290914. doi: 10.3389/fcimb.2023.1290914
111. Ghani R, Chrysostomou D, Roberts LA, Pandiaraja M, Marchesi JR, and Mullish BH. Faecal (or intestinal) microbiota transplant: A tool for repairing the gut microbiome. Gut Microbes. (2024) 16:2423026. doi: 10.1080/19490976.2024.2423026
112. Jang YO, Lee SH, Choi JJ, Kim D-H, Choi J-M, Kang M-J, et al. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp Mol Med. (2020) 52:1128–39. doi: 10.1038/s12276-020-0469-y
113. Yuksel N, Gelmez B, and Yildiz-Pekoz A. Lung microbiota: its relationship to respiratory system diseases and approaches for lung-targeted probiotic bacteria delivery. Mol Pharm. (2023) 20:3320–37. doi: 10.1021/acs.molpharmaceut.3c00323
114. de Boer GM, Żółkiewicz J, Strzelec KP, Ruszczyński M, Hendriks RW, Braunstahl G-J, et al. Bacterial lysate therapy for the prevention of wheezing episodes and asthma exacerbations: A systematic review and meta-analysis. Eur Respir Rev. (2020) 29:190175. doi: 10.1183/16000617.0175-2019
115. Feleszko W, Rossi GA, Krenke R, Canonica GW, Van Gerven L, and Kalyuzhin O. Immunoactive preparations and regulatory responses in the respiratory tract: potential for clinical application in chronic inflammatory airway diseases. Expert Rev Respir Med. (2020) 14:603–19. doi: 10.1080/17476348.2020.1744436
116. Fraser A and Poole P. Immunostimulants versus placebo for preventing exacerbations in adults with chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2022) 2022:CD013343. doi: 10.1002/14651858.CD013343.pub2
117. Sun G, Hao W, Li Q, Ma Y, Sun F, Su Y, et al. Therapeutic and prophylactic effects of qipian on copd in mice: the role of lung and gut microbiota. Microbiol Spectr. (2025) 13:e0196924. doi: 10.1128/spectrum.01969-24
118. Pei G, Guo L, Liang S, Chen F, Ma N, Bai J, et al. Long-term erythromycin treatment alters the airway and gut microbiota: data from chronic obstructive pulmonary disease patients and mice with emphysema. Respiration. (2024) 103:461–79. doi: 10.1159/000538911
119. Uzun S, Djamin RS, Kluytmans JAJW, Mulder PGH, van't Veer NE, Ermens AAM, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (Columbus): A randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2014) 2:361–8. doi: 10.1016/s2213-2600(14)70019-0
120. Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, and Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. (2014) 143:225–45. doi: 10.1016/j.pharmthera.2014.03.003
121. Du S, Shang L, Zou X, Deng X, Sun A, Mu S, et al. Azithromycin exposure induces transient microbial composition shifts and decreases the airway microbiota resilience from outdoor pm 2.5 stress in healthy adults: A randomized, double-blind, placebo-controlled trial. Microbiol Spectr. (2023) 11:e0206622. doi: 10.1128/spectrum.02066-22
122. Jia Y, He T, Wu D, Tong J, Zhu J, Li Z, et al. The treatment of qibai pingfei capsule on chronic obstructive pulmonary disease may be mediated by th17/treg balance and gut-lung axis microbiota. J Transl Med. (2022) 20:281. doi: 10.1186/s12967-022-03481-w
123. Zheng M, Huang L, Yuan H, Li Z, Wang Y, Su Y, et al. Investigating the mechanism of qifenggubiao granules in copd treatment: an integrated exploration of ferroptosis and the gut–lung axis. Biomaterials Res. (2025) 29:263. doi: 10.34133/bmr.0263
124. Wang J, Ren C, Jin L, and Batu W. Seabuckthorn wuwei pulvis attenuates chronic obstructive pulmonary disease in rat through gut microbiota-short chain fatty acids axis. J Ethnopharmacol. (2023) 314:116591. doi: 10.1016/j.jep.2023.116591
125. Ekbom A, Brandt L, Granath F, Löfdahl C-G, and Egesten A. Increased risk of both ulcerative colitis and crohn’s disease in a population suffering from copd. Lung. (2008) 186:167–72. doi: 10.1007/s00408-008-9080-z
126. Vutcovici M, Bitton A, Ernst P, Kezouh A, Suissa S, and Brassard P. Inflammatory bowel disease and risk of mortality in copd. Eur Respir J. (2016) 47:1357–64. doi: 10.1183/13993003.01945-2015
127. Sun Y, Wang Y, Yang Z, Han X, Zhang Y, Chen L, et al. Neutral polysaccharide from platycodonis radix-ameliorated pm2.5-induced lung injury by inhibiting the tlr4/nf-Kb P65 pathway and regulating the lung and gut microbiome. J Agric Food Chem. (2024) 72:27923–38. doi: 10.1021/acs.jafc.4c07319
128. Li J, Chen Y, Shi Q, Sun J, Zhang C, and Liu L. Omega-3 polyunsaturated fatty acids ameliorate pm2.5 exposure induced lung injury in mice through remodeling the gut microbiota and modulating the lung metabolism. Environ Sci pollut Res Int. (2023) 30:40490–506. doi: 10.1007/s11356-022-25111-0
Keywords: particulate matter, chronic obstructive pulmonary disease, immune, microbiota, oxidative stress
Citation: Pang X, Huang P, Huang S and Liu X (2025) The gut–lung axis: a new perspective on the impact of atmospheric particulate matter exposure on chronic obstructive pulmonary disease. Front. Immunol. 16:1657675. doi: 10.3389/fimmu.2025.1657675
Received: 11 July 2025; Accepted: 20 October 2025;
Published: 04 November 2025.
Edited by:
Paris Salazar-Hamm, New Mexico Institute of Mining and Technology, United StatesCopyright © 2025 Pang, Huang, Huang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoju Liu, bGl1eGlhb2p1ODM1QDEyNi5jb20=
 Xichen Pang
Xichen Pang Ping Huang
Ping Huang Sha Huang
Sha Huang Xiaoju Liu
Xiaoju Liu