- 1Department of Gastrointestinal, Hernia and Enteric Fistula Surgery, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China
- 2Laboratory of General Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
Cell membrane-camouflaged nanoparticles (CNPs) have emerged as promising multifunctional platforms for colorectal cancer therapy, integrating drug delivery, immunomodulation, photothermal ablation, and anti-inflammatory effects. This review highlights recent advances in CNP-based strategies, emphasizing their unique capacity to enhance tumor-targeting specificity, potentiate immunotherapeutic efficacy, and overcome the limitations of conventional treatments. We summarize diverse approaches employing immune cell or tumor cell membrane coatings, as well as hybrid systems that combine CNPs with chemotherapy, metabolic modulation, or photothermal therapy. Accumulating evidence demonstrates that CNPs can effectively remodel the tumor immune microenvironment, increase the bioavailability of hydrophobic drugs, and promote synergistic therapeutic outcomes. Despite these encouraging results, clinical translation remains constrained by challenges in biodegradability, biosafety, large-scale manufacturing, and cost. Ongoing clinical trials are evaluating the safety and therapeutic potential of CNP-based nanomedicines. Overall, this review underscores the transformative role of CNPs as a next-generation platform for precision and personalized therapy in colorectal cancer.
Introduction
Colorectal cancer (CRC) is among the most prevalent and lethal malignancies globally (1). Its pathogenesis is closely linked to various genomic alterations, such as chromosomal instability, microsatellite instability, and defects in CpG island methylation (2). Patients with early-stage CRC can be treated well with surgery, chemotherapy, or combination therapy, but there is a lack of effective treatment for patients with advanced CRC, especially for recurrent and metastatic CRC (3). Therapeutic challenges include tumor heterogeneity, immune cell dysfunction, immunosuppressive tumor microenvironment, and systemic immunotoxicity (4).
The application of nanomaterials in medicine is developing rapidly, and cell membrane-encapsulated nanoparticles (CNPs) in particular have attracted much attention due to their unique biointerfacial properties. These nanoparticles can mimic the functions of natural cells, such as “self”-labeling, interaction with the immune system, biotargeting, and localization to specific regions, leading to improved biocompatibility, reduced immunogenicity, immune escape, prolonged circulation time, and enhanced tumor targeting (5, 6). Treatments for colorectal cancer include surgery, chemotherapy, and immunotherapy. Surgery is the main treatment for early-stage CRC; chemotherapy and targeted therapy are commonly used for patients with advanced CRC, including oxaliplatin, fluorouracil, and irinotecan, as well as angiogenesis inhibitors and epidermal growth factor receptor inhibitors etc. (7). Immunotherapy, particularly immune checkpoint blockade (ICB) therapy and CAR T-cell therapy, offers new promise for the treatment of CRC, although response rates are currently low (8).
CNPs provide distinct advantages in the therapeutic management of colorectal cancer. For instance, erythrocyte membrane-camouflaged nanoparticles can prolong systemic circulation and enhance tumor accumulation by reducing immune clearance and improving vascular retention. In contrast, platelet membrane-coated nanoparticles possess intrinsic affinity for subendothelial matrices, thrombotic sites, and activated endothelial cells, thereby enabling precise targeting across multiple stages of tumor progression (9). CNPs enhance therapeutic efficacy by mimicking the function of natural cells, improving the efficiency and targeting of drug delivery while reducing clearance by the immune system (10).
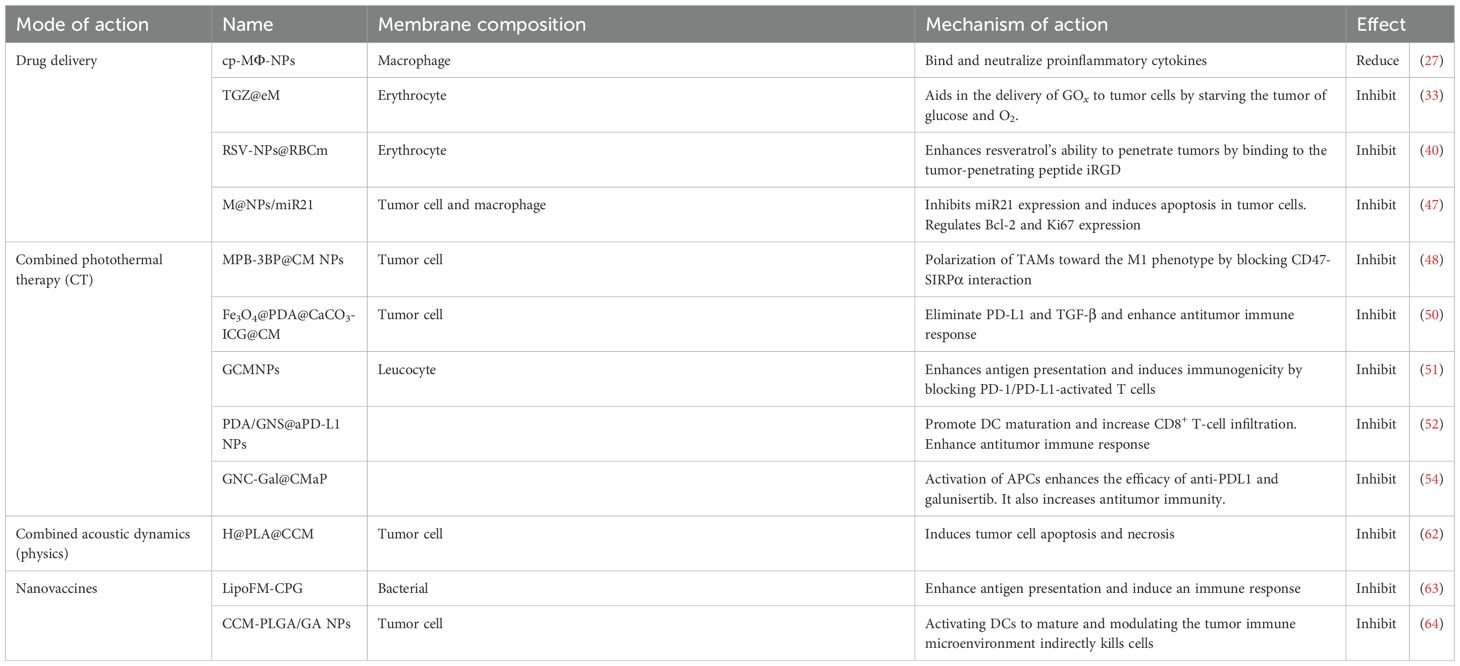
The treatment of colorectal cancer is gradually shifting from traditional surgery and chemotherapy to more precise immunotherapy and nanomedicine. This review explores the development of CNPs for the treatment of colorectal cancer, providing a comprehensive analysis of their applications in anticancer drug delivery, photothermal therapy, and immunotherapy. Future considerations for translating promising CNP platforms into the clinic are also discussed (Figure 1).
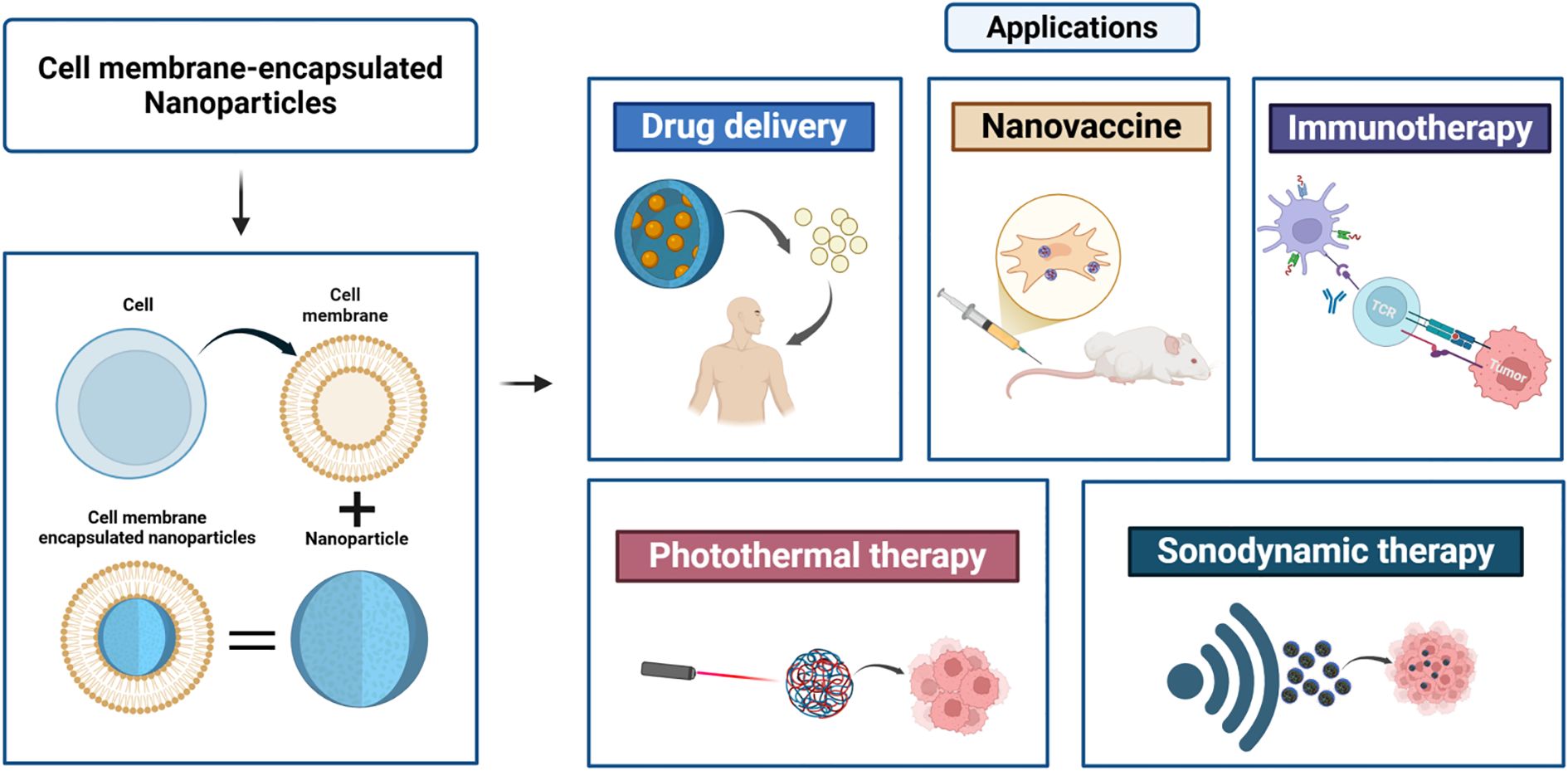
Figure 1. Preparation of cell membrane-encapsulated nanoparticles and their applications. Cell membrane-encapsulated nanoparticles are a cutting-edge biotechnology platform that forms a “bionic” structure by enveloping cell membranes around nanoparticles. This approach demonstrates broad application potential across multiple advanced therapeutic fields, including drug delivery, vaccine development, immunotherapy, and physical therapies (light, sound).
Status and classification of CNPs
CNPs, as an innovative nanocarrier, form a core–shell structure with cell-mimicking properties by covering natural cell membranes on a synthetic core (11). Due to their unique membrane structure and surface antigens, CNPs have significant advantages in drug delivery, photothermal therapy, and immunotherapy (12). The manufacturing process of CNPs involves the use of a variety of cell membrane materials, such as red blood cells, immune cells, and cancer cell membranes, each of which gives CNPs unique properties that enable them to play a key role in cancer therapy (9, 10, 13).
Red blood cell membrane-coated nanoparticles (RBCM) show significant advantages in tumor therapy due to their long-lasting properties, which prolong the circulation time in the body and thus increase the exposure to tumors (9). These nanoparticles play an important role in phototherapy, especially photothermal therapy (PTT) and photodynamic therapy (PDT), e.g., red blood cell membrane nanovesicles (RMNVs) conjugated with biomimetic black phosphorus quantum dots (BPQDs) effectively induce apoptosis of cancer cells and activate tumor-specific immune responses under near-infrared laser irradiation (14). In addition, RBCM has been used for efficient delivery of anticancer drugs (15). In immunotherapy, a novel immunotherapeutic system (Lmo@RBC) was constructed by wrapping erythrocyte membranes around bacteria, selectively deleting virulence factors. Lmo@RBC synergistically triggers tumor cell pyroptosis by upregulating the expression of the pore-forming protein GSDMC and activating ROS-induced activation of cysteine-containing aspartic acid protein hydrolase 8 (caspase-8), which is effective in inducing an antitumor immune response (16). The above demonstrates the potential of erythrocyte membrane-encapsulated nanoparticles to enhance the efficacy of tumor therapy and immune response.
Immune cell membrane-encapsulated nanomaterials play multiple roles in tumor therapy. They are able to present tumor antigens directly to the immune system to activate antitumor immune responses and to construct tumor vaccines to enhance the maturation and activation of dendritic cells to stimulate specific immune responses (17, 18). In addition, these nanomaterials can improve the tumor microenvironment, e.g., by enhancing antitumor immunity through reprogramming tumor-associated macrophages and alleviating tumor hypoxia (18). T lymphocyte membrane-encapsulated nanomaterials have also been used to activate T lymphocytes, further enhancing the efficacy of cancer immunotherapy (19). Macrophage membrane-coated nanoparticles, such as silica nanoparticles and near-infrared imaging probe-loaded gold nanoparticles, not only enhance drug delivery efficiency and anticancer effects but also increase tumor tissue accumulation under near-infrared laser irradiation, generate local heat to effectively inhibit tumor growth, and achieve selective ablation of cancer cells in the region of thermal irradiation (20). These applications demonstrate the potential and versatility of immune-cell membrane-encapsulated nanomaterials in tumor therapy.
Cancer cell membrane-encapsulated nanoparticles show potential for multifaceted applications in the field of tumor therapy. By co-loading photosensitizers and Toll-like receptor 7 agonists, they are able to enhance the efficacy of combined tumor immunotherapy by generating reactive oxygen species (ROS) to kill tumor cells in combination with photodynamic therapy and activating the host’s antitumor immune response to remove residual tumor cells (21). In addition, these nanoparticles enhance the bioavailability of poorly water-soluble drugs, increase selective drug accumulation in tumors, and improve therapeutic efficacy while reducing systemic toxicity (22). They also incorporate inducers of immunogenic cell death (ICD), enhance ICD in tumor cells, facilitate the release of tumor-associated antigens and damage-associated molecular patterns, thereby stimulating antitumor immune responses (23). In the construction of tumor vaccines, these nanoparticles enhance the maturation and activation of dendritic cells to stimulate specific antitumor immune responses and efficiently target and stimulate the maturation of DCs by modifying the TLR9 agonist, CpG oligonucleotides, and aptamers on tumor cell membrane vesicles to generate long-lasting antitumor immune responses (24).
The abovementioned powerful potential of these cell membrane-encapsulated nanoparticles to improve therapeutic efficacy and to activate and enhance the body’s antitumor immune response provides a new strategy for achieving personalized and precise tumor immunotherapy (Figure 2).
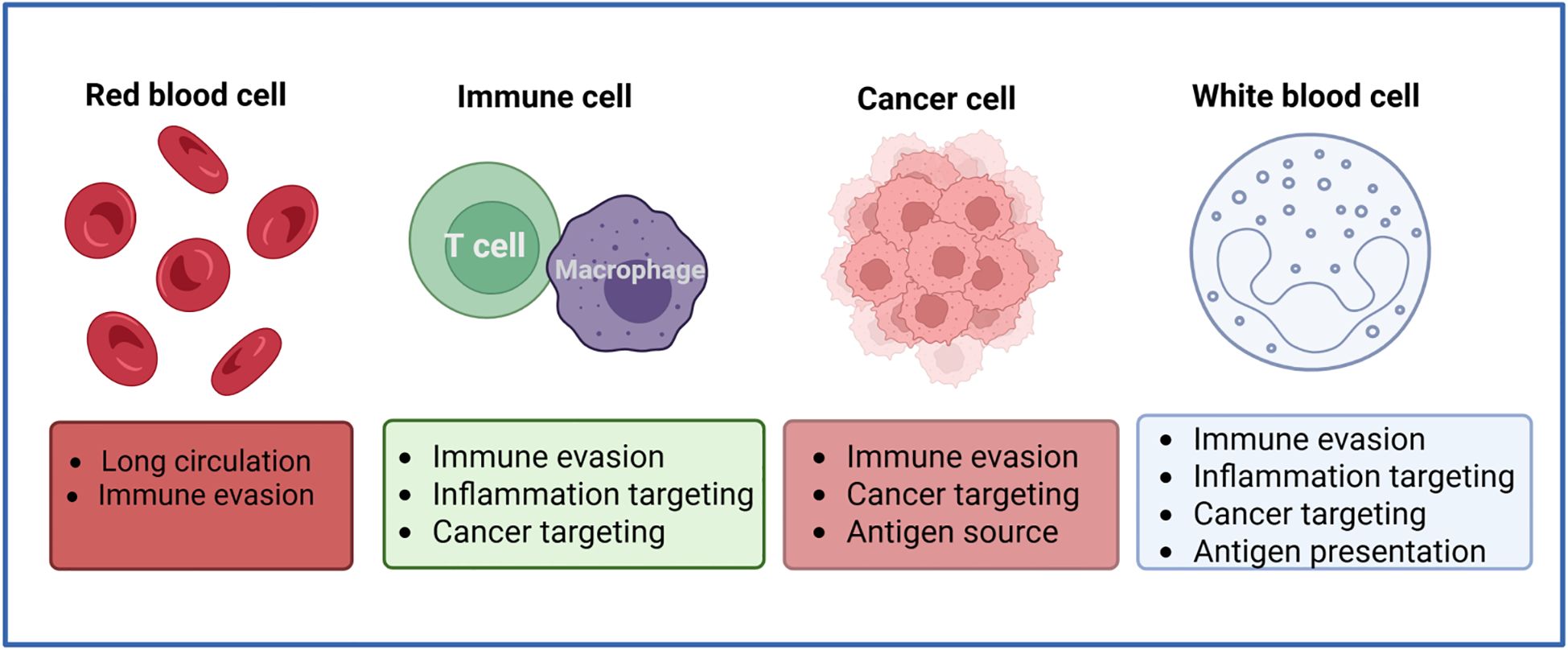
Figure 2. Sources of common cell membranes used for preparing cell membrane-encapsulated nanoparticles. Cell membrane-coated nanoparticles can be produced using cell membrane materials from red blood cells, immune cells, cancer cells, and leukocytes. Each type of membrane coating confers specific properties that can be used in anticancer applications.
CNPs in colorectal cancer treatment
Drug delivery
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract and is closely linked to an elevated risk of colorectal cancer. Research has demonstrated that individuals with IBD have a significantly higher risk of developing CRC (25). A team has developed a bionic vesicle (SLKs) that specifically targets leukocytes and used it to treat a mouse model of IBD. Experimental results showed that this treatment significantly reduced the inflammatory response and promoted the repair of intestinal epithelial tissue (26). Duan et al. developed an oral formulation of macrophage membrane-encapsulated nanoparticles (cp-MΦ-NPs) that bind and neutralize pro-inflammatory cytokines, significantly reducing the severity of IBD (27). Studies have shown that liposome-coated nanoparticles for the delivery of the inflammatory factor interleukin (IL)-12 (PLE-IL-12-NPs) and PLE-IL-12-NPs are able to release active IL-12 in vivo and trigger an immune response to inhibit the growth of colorectal cancer (28). In addition to interleukin inflammatory factor, tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) is also highly expressed in colorectal tumor cells, and studies have shown that the combination of the chemotherapeutic agent oxaliplatin with immunohybrid nanoparticles (OIHNPs) enables synergistic delivery of oxaliplatin and anti-TRAIL, leading to therapeutic inhibition of colorectal cancer tumor models (29). Studies have shown that the transient delivery of miR-200 via oral administration of miR-200-loaded lipid nanoparticles (LNPs) restores intestinal stem cells (ISCs) and enhances intestinal regeneration in mice following acute injury (30).
In recent times, metabolic reprogramming has emerged as a significant hallmark of cancerous cells, characterized by elevated glycolysis and augmented lactic acid fermentation. As a result, the inhibition of glycolysis is considered a promising therapeutic strategy for colorectal cancer. Glycolysis can be classified into two distinct categories: aerobic glycolysis and anaerobic glycolysis, which may or may not involve the use of oxygen. 2-Deoxy-d-glucose (2DG) is capable of eliminating the energy source of glucose glycolysis by inhibiting it, which ultimately leads to the death of cancer cells due to starvation. Wu et al. constructed a multifunctional nanoplatform containing a core of rare-earth doped nanoparticles (LnNPs) by combining mesoporous silica shells containing 2DG and the photosensitizer chlorinated e6 (Ce6) in mesoporous channels. In combination with photodynamic therapy, which disrupted mitochondrial function and enhanced the efficacy of 2DG by inhibiting the expression of hexokinase 2 (HK2) and lactate dehydrogenase, thereby inhibiting glucose metabolic reprogramming and increasing the efficiency of starvation therapy to inhibit colorectal cancer tumor growth (31). The inhibition of glucose supply using glucose oxidase (GOx) offers an alternative therapeutic strategy for tumor starvation (32). However, the in vivo application of GOx-based starvation therapy is significantly hindered by poor GOx delivery efficiency and self-limiting therapeutic effects. To address these challenges, Zhang et al. developed a nanoplatform that encapsulates GOx and tirazamine (TPZ) within erythrocyte-membrane-masked metal-organic framework (MOF) nanoparticles (TGZ@eM). This platform facilitates efficient GOx delivery to tumor cells, effectively depleting endogenous glucose and oxygen to induce tumor cell starvation. Notably, the hypoxia induced by this approach further activates the TPZ released from the nanoplatforms in the acidic lysosomal/endosomal environment, thereby enhancing the therapeutic efficacy against colon cancer (33). A study has developed a photosynthetic leaf-inspired abiotic/biotic nanolike vesicle (PLANT) system that employs its photosensitivity and capacity to catalyze the decomposition of hydrogen peroxide to enhance the oxygen levels of tumor cells and induce apoptosis, which in turn inhibits colorectal cancer cell growth (34). Hexokinase (HK) is the rate-limiting enzyme in the initial step of glycolysis. Benserazide, a selective inhibitor of HK2, has been shown to specifically bind to HK2 and markedly inhibit its enzymatic activity in vitro (35). In a study conducted by Li et al., benserazide nanoparticles were prepared and shown to demonstrate inhibition in a mouse model of colorectal cancer. Furthermore, the nanoparticles were demonstrated to be more efficacious than conventional treatment (36). The role of hydrogen peroxide (H2O2) in colorectal cancer has been the subject of numerous studies, which have revealed that it exerts a multifaceted influence on tumor cells. In addition to promoting the proliferation and metastasis of tumor cells, H2O2 has also been identified as a signaling molecule that induces apoptosis (37). In a single colorectal cancer Caco-2 cell, Wang et al. accurately measured the H2O2 gradient from extracellular to intracellular using highly sensitive platinum-functionalized nanomaterials in combination with noncontact hopping probe mode scanning ion conductivity microscopy (hPICM), a technique that allows for the measurement of ionic currents with high sensitivity and spatial resolution (38).
It has been demonstrated that resveratrol is an effective inhibitor of the mechanism of colon cancer cell growth through a ROS-dependent iron-concentration pathway (39). Zhang et al. developed an innovative biomimetic nanocarrier system, resveratrol (RSV)-NPs@RBCm, which achieves efficient encapsulation of RSV through the encapsulation of poly(ϵ-caprolactone)-poly(ethylene glycol) nanoparticles within erythrocyte membranes. The nanoparticles were prepared using poly(ϵ-caprolactone)-poly(ethylene glycol) (PCL-PEG) and erythrocyte membranes. When combined with the tumor-penetrating peptide, iRGD, RSV-NPs@RBCm demonstrated a significant enhancement in tumor penetration ability, thereby facilitating the treatment of colorectal cancer (40). Zeng et al. employed activated neutrophil membrane-encapsulated nanoparticles (aNEM NPs) as nanodecoys, which demonstrated a notable neutralizing impact and were capable of effectively inhibiting chemokine-mediated recruitment of neutrophils to the primary tumor, thereby mediating tumor growth (41). The hydrophobicity of certain pharmaceutical agents may present a challenge to their potential clinical utilization (42). The nanoprecipitation method, as employed by Zhang et al., was used to prepare ursolic acid-containing nanoparticles (UA-NPs). This resulted in enhanced apoptosis through the stronger inhibition of COX-2 and activation of cysteine-aspartic enzyme 3 when compared to free UA-NPs. Additionally, the nanoparticles demonstrated superior penetration of cell membranes (43). It has been demonstrated that the co-coating of apigenin and the chemotherapeutic drug 5-FU in multifunctional nanomaterials can result in a synergistic effect, thereby significantly enhancing the efficacy of chemotherapy in vivo in patients with CRC (44).
In conclusion, CNPs demonstrate considerable potential and significance in multiple pivotal domains. They can effectively modulate the inflammatory response, intervene against the glycolytic process in colorectal cancer, and achieve precise delivery of chemotherapeutic drugs. Additionally, they can reduce the hydrophobicity of drugs, enhance their bioavailability, and prolong the half-life of drugs in vivo. These advantages render the nanocarrier system a highly efficacious instrument for enhancing therapeutic outcomes and patients’ quality of life.
Nanomaterials combined with immunotherapy and PTT
Despite the extensive development of nanoparticle-based tumor therapeutic strategies, the therapeutic efficacy remains constrained by the inadequate accumulation of nanoparticles in tumor tissues and the suboptimal antitumor effect of single treatment modalities (45). The distinctive characteristics of nanomaterials and their collaborative effects with alternative therapeutic modalities have prompted the majority of researchers to develop treatments that are more efficacious than traditional therapies, thereby offering patients a more comprehensive and precise range of treatment options.
CD47 is markedly expressed in colorectal cancer tissues, and its elevated expression is correlated with a poor prognosis. It triggers immunosuppressive signaling pathways by binding to signal regulatory protein α (SIRPα) on macrophages, thereby enabling cancer cells to evade immune surveillance and clearance (46). Wang et al. constructed a dual-membrane-camouflaged miRNA21 antagonist delivery nanoplatform (M@NPs/miR21), which was self-assembled from ZnO/miRNA21 antagonist nanoparticles (NPs/miR21) and the membranes of MC38 tumor cells (M38) and macrophage cells (Ma) by ultrasonication. The delivery of miR21 antagonists to tumor tissues is effectively facilitated, resulting in the inhibition of miR21 expression, induction of tumor cell apoptosis, regulation of Bcl2 and Ki67 expression, and subsequent inhibition of colorectal tumor growth and metastasis (47). The nanoplatform retains the antitumor effect of ZnO nanoparticles while also exhibiting CD47 protein-mediated immune escape signaling and galectin-3 protein-mediated tumor cell aggregation. Professor Zhiyong Qian’s team developed the MPB-3BP@CM NPs cell membrane biomimetic nanomedicine platform, which employs microporous Prussian blue nanoparticles (MPB NPs) as a carrier for photothermal sensitizers and 3-bromopyruvic acid (3BP) encapsulated in cell membranes that express a high-affinity signal for protein variant SIRPα. The MPB-3BP@CM NPs could prolong the blood circulation time, effectively target colorectal cancer cells CD47, and inhibit colorectal cancer growth by blocking the CD47-SIRPα interaction and promoting the polarization of tumor-associated macrophages (TAMs) towards the M1 phenotype. Furthermore, the combination of MPB NPs-mediated photothermal therapy enhances the therapeutic efficacy against tumors (48). It has been demonstrated that red blood cells possess intrinsic characteristics that result in a prolonged cycle time (9). In order to obtain Cyp-MNC@RBC with significantly improved physiological stability in comparison to bare MNC, Wang et al. encapsulated superparamagnetic nanoclusters (MNC) with erythrocyte membranes. Furthermore, the researchers loaded the Cyp-MNC@RBC on near-infrared (NIR) carriers, which resulted in a significant increase in both NIR absorbance and photothermal conversion efficiency (49).
In the tumor microenvironment, the conventional mode of tumor cell death frequently lacks immunogenicity, which enables tumors to evade detection by the immune system. However, by inducing immunogenic cell death, tumor cells can release tumor-associated antigens and cytokines, which are molecular signals that recruit antigen-presenting cells and effector CD4+ and CD8+ T cells. This, in turn, activates both natural and adaptive immune responses, which enhances the effectiveness of antitumor immunotherapy (8). A team of researchers has developed a multifunctional biomimetic nanoplatform (Fe3O4@PDA@CaCO3-ICG@CM) based on magnetic dopamine (PDA) modified with CaCO3 containing indocyanine green (ICG). This nanomaterial has been shown to effectively scavenge programmed death ligand 1 (PD-L1) and transforming growth factor-beta (TGF-β), in addition to other functions. Furthermore, it induces apoptosis of CT26 cells by combining with photothermal therapy. This process can induce ICD, which activates the maturation of dendritic cells and subsequently promotes the activation of CD4+ and CD8+ T cells, thereby inhibiting the growth of colorectal cancer cells (50). In a novel approach, Li et al. developed a nanoplatform that employs leukocyte membranes encapsulated with glycyrrhizinic acids (GCMNPs) as an efficacious inducer of cellular immunogenic death. This design not only elicited a systemic immune response in CRC mice but also markedly reduced toxicity to the CRC (51).
Immune checkpoint blockade therapies targeting programmed death 1 (PD-1) or its primary ligand, PD-L1, have achieved remarkable success in the treatment of a wide range of tumors, including colorectal cancer. Nevertheless, the efficacy of PD-1/PD-L1 inhibitors is constrained in certain colorectal cancers characterized by an immunosuppressive tumor microenvironment, such as a low degree of immune cell infiltration (8). Xiao et al. developed anti-PD-L1 functionalized biomimetic dopamine-modified gold nanostar nanoparticles (PDA/Gold nanostar (GNS)@aPD-L1 NPs), which demonstrated considerable efficacy in inhibiting tumor growth and reducing the number of immunosuppressive cells. The maturation of dendritic cells is enhanced, and the infiltration of CD8+ T cells is increased, leading to prolonged overall survival. Additionally, PDA-GNS-mediated local photothermal ablation of tumors promotes the release of tumor-associated antigens, thereby activating the antitumor immune response. Simultaneously, photothermal therapy suppresses colorectal cancer growth by increasing tumor permeability and facilitating immune cell infiltration (52). Liu et al. designed a multifunctional nanomaterial by incorporating oxaliplatin (OXA) and ICG into hyaluronic acid (HA)-modified metal-organic framework MIL-100 nanoparticles, resulting in the development of multifunctional nanoparticles (OIMH NPs). These nanomaterials were shown to induce immunogenic cell death and enhance immune cell infiltration within the tumor microenvironment, thereby improving the efficacy of immune checkpoint blockade therapy (53). Wang et al. successfully constructed a novel nanocomposite, a hollow gold nanocage nanocomposite (GNC-Gal@CMaP), which demonstrated selective targeting of colon cancer cells and effective accumulation in the tumor microenvironment. The material effectively integrates PTT, a PD-L1 antibody, and a TGF-β inhibitor (galunisertib), subsequently enhancing the antitumor efficacy of an anti-PD-L1 antibody and galunisertib through the activation of antigen-presenting cells, which in turn trigger tumor-specific effector T cells (54).
The aforementioned findings indicate that multifunctional nanomaterials can effectively enhance the targeting and efficacy of photothermal therapy PTT, thereby potentiating the anticolorectal cancer tumor effect when employed in conjunction with immunotherapy.
Imaging technology
The suppressor of cytokine signaling 1 (SOCS-1) has been demonstrated to influence the development of colorectal cancer and the process of immune escape by regulating cytokine signaling (55). Guthula et al. have developed an innovative fiber optic nanoplasma biosensor that is sensitive to detecting human genomic DNA methylation levels of SOCS-1 in gastrointestinal tumors (56). By employing nanoimage printing technology, Gopal et al. successfully created netrin-1 nanodots with a specific distribution. The nanodots are capable of recruiting and aggregating the missing (dCC) receptor as well as F-actin in colorectal cancer, thereby providing new insights into the diagnosis and treatment of colorectal cancer (57). The use of carbon nanotubes in the diagnosis and treatment of colorectal cancer is a significant advancement due to their versatility. Abdolahad et al. successfully developed a novel electroendoscopic spectroscopic analysis tool utilizing vertically aligned carbon nanotubes (VACNTs) as the core component. This tool is capable of accurately distinguishing between the different cancerous stages of colorectal cancer by taking advantage of the electrical and optical properties of VACNTs (58). It has been established that membrane-bound TRAIL induces a more pronounced receptor aggregation and apoptosis than soluble TRAIL (59). In a recent study, Zakaria et al. demonstrated that nanovectorization of the tumor necrosis factor-associated apoptosis-inducing ligand TRAIL using single-walled carbon nanotubes significantly improves the efficacy of colorectal cancer tumor cell eradication (60). In addition to developing a novel tool for electroendoscopic spectroscopic analysis, Abdolahad has also fabricated a bioelectronic device based on silicon nanograss. This device is capable of detecting several human colon invasive cancer cells (SW48) in mixed cell cultures without any biochemical labeling of primary colon cancer cells (HT29). Furthermore, it is suitable for more accurate cancer staging (61). In a previous study, Wen et al. prepared a camouflaged ultrasound sensitizer by encapsulating hematoporphyrin molecules in a polylactic acid (PLA) matrix and subsequently coating them with cancer cell membranes (CCM) derived from colon tumor 26 (CT26) cells. This produced the H@PLA@CCM nanosystem. The biocompatible ultrasound nanosensitizer H@PLA@CCM, camouflaged with cancer cell membranes, has been shown to induce significant apoptosis and necrosis of tumor cells through effective sonodynamic therapy (SDT), as well as highly efficient acoustic kinetic ablation of colon tumors, thereby achieving tumor suppression (62). Wang et al. employed a combination of highly sensitive platinum-functionalized nanomaterials and noncontact hPICM to achieve precise measurement of H2O2 gradients from extracellular to intracellular compartments within individual colorectal cancer Caco-2 cells. This technique facilitates ion current measurements with high sensitivity and spatial resolution (38) (Figure 3).
Figure 3. Role of nanomaterials in imaging technology. In colorectal cancer imaging, nanoparticle-based targeted contrast agents integrate seamlessly with endoscopy, CT, and MRI to boost sensitivity down to submillimetric early lesions, sharpen resolution for accurate staging, and maintain exemplary biocompatibility to ensure patient safety.
In summary, the significance of nanomaterials in imaging technology is demonstrated by several factors, including the enhancement of imaging quality, the advancement of innovative imaging techniques, the expansion of imaging applications, and a deeper understanding of nanomaterial behavior in biological systems.
Nanovaccines
Nanovaccines have the potential to be used not only for the prevention of colorectal cancer but also for its treatment and diagnosis. The insertion of cholesterol-modified CpG oligodeoxynucleotides (Chol-CPG), a Toll-like receptor 9 agonist, into the nuclear membrane of autologously derived Fusobacterium nucleatum by Chen et al. represents a promising avenue of research in this field. Similarly, the development of a safe and efficient bacterial vaccine (LipoFM-CPG) by Chen L, Kang Z, Shen J et al. is a significant step forward. The bacterial vaccine was capable of selectively preventing F. nucleatum infection over the long term by enhancing antigen presentation and inducing an immune response. This resulted in an improvement in the therapeutic efficacy of chemotherapy and a reduction in cancer metastasis in F. nucleatum-infected CRCs (63). Huang et al. employed a two-step emulsification method to synthesize poly(lactic-co-glycolic acid)/GA nanoparticles (PLGA/GA NPs). They then utilized CT26 colon CCM to develop nanovaccines, designated as CCM-PLGA/GA NPs. These nanoparticles were shown to possess a dual capacity: directly targeting and killing tumors by enhancing the tumor-targeting ability of GA, and indirectly promoting tumor cell death by activating dendritic cell maturation, thereby modulating the tumor immune microenvironment (64). It was demonstrated that cellular nanodiscs manufactured from cancer cell membranes and combined with lipid-based adjuvants for antitumor vaccination were effective in inhibiting the growth of colorectal cancer in mice (65).
To sum up, nanovaccines can be used not only to specifically target and kill cancer cells but also to label and monitor pathogens (Figure 4).
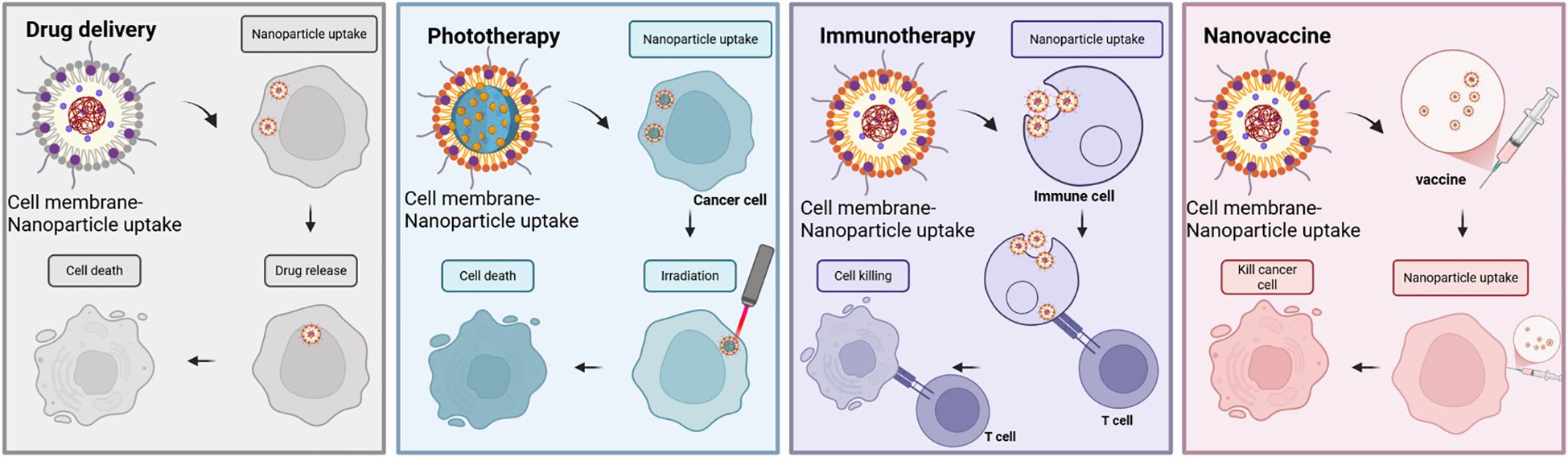
Figure 4. Anticancer applications of cell membrane-encapsulated nanoparticles. In cancer drug delivery, coatings derived from erythrocyte and cancer cell membranes enhance therapeutic efficacy by prolonging circulation and enabling targeted localization to tumors. Similarly, in phototherapy applications, cell membrane-encapsulated nanoparticles serve as excellent carriers for targeted delivery of photothermophiles and photosensitizers to tumors, thereby enhancing their effects under irradiation. In immunotherapy, cell membrane-encapsulated nanoparticles can deliver immunostimulants, either as a source of antigen or in direct contact with immune cells, to promote antitumor immunity. Taking advantage of their nanoscale size, nanovaccines have shown remarkable results against tumor cells. They play a unique role through efficient antigen delivery, precise targeting of tumor cells, and activation of a broad immune response.
Clinical research and challenges
In recent years, cell membrane-coated nanomaterials have been extensively investigated and have demonstrated remarkable targeting efficiency. However, their translation from laboratory research to clinical application remains challenging, primarily due to concerns regarding biodegradability, potential toxicity, and large-scale manufacturing costs. First, the biodegradability of CNPs is critical for clinical applications, and further research is needed to ensure that their degradation products are safe and do not pose long-term accumulation risks. Secondly, although CNPs exhibit low toxicity, their safety must be confirmed through long-term, large-scale clinical trials. One clinical trial (NCT06048367) is evaluating the safety and tolerability of intratumoral injections of carbon nanoparticle-loaded iron (CNSI-Fe[II]) in patients with advanced solid tumors.
The selection of appropriate nanomaterials is crucial, and U.S. Food and Drug Administration (FDA)-approved biodegradable nanoparticles, such as PLGA, should be prioritized to ensure the production of safe, biocompatible, and low-toxicity nanoparticles (66). Several FDA-approved nanomaterials are available for drug delivery platforms, and utilizing these materials can expedite the transition to clinical applications compared with unapproved alternatives (67).
The same consideration applies to the selection of animal models, which vary significantly in their ability to replicate human tumor physiology and immune responses. These differences can affect the clinical relevance of the findings and the feasibility of their translational application (68, 69). The human immune system is extremely complex, and no animal model can fully replicate all aspects of it. Therefore, the development and selection of appropriate models are crucial to ensure the successful clinical translation of research findings.
Although the transition from the laboratory to the clinic is challenging, several clinical trials are currently underway. As chemotherapeutic agents are highly toxic to both healthy and cancerous areas, effective targeting could provide substantial benefits for patients with advanced or metastatic tumors. For example, polymeric nanoparticles containing cetuximab, modified with growth inhibitor analogs, are being investigated for targeting colorectal cancer (NCT03774680). Clinical trials are evaluating the efficacy of oral nanoformulations of curcumin as an adjuvant therapy in the treatment of metastatic colorectal cancer with XELOX or FOLFOX regimens (IRCT20200408046990N7) (70). Clinicians are also investigating carbon nanoparticles as a lymph node tracer in laparoscopic colorectal surgery to improve lymph node sampling and detect micrometastases (NCT03350945) (71).
Conclusion
In this review, we provide a comprehensive overview of the development and application of CNPs in colorectal cancer, highlighting how the type of membrane encapsulation influences their functionality (Table 1). The multifunctionality of CNPs offers significant potential for a range of therapeutic applications, including drug delivery, anti-inflammatory effects, photothermal therapy, and immunotherapy. Despite the challenges that remain for clinical translation, it is clear that cell membrane-encapsulated nanomaterials hold considerable promise as a tool for cancer immunotherapy. This approach is expected to enhance the efficacy of oncology treatments and contribute to the advancement of precision, personalized medicine.
Author contributions
ZQ: Writing – original draft. WL: Writing – original draft. SX: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Guangxi Natural Science Foundation (No. 2025GXNSFAA069965; No. 2025GXNSFAA069108). The funding agency played no role in study design, data collection, analysis and interpretation, or manuscript writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Williams CJM, Peddle AM, Kasi PM, Seligmann JF, Roxburgh CS, Middleton GW, et al. Neoadjuvant immunotherapy for dMMR and pMMR colorectal cancers: therapeutic strategies and putative biomarkers of response. Nat Rev Clin Oncol. (2024) 21:839–51. doi: 10.1038/s41571-024-00943-6
3. Hilty Chu BK, Loria A, Dhimal T, Cai X, Gao S, Li Y, et al. Challenges of surveillance in implementing nonoperative management for rectal cancer. JAMA Netw Open. (2024) 7:e2448682.
4. Xie Q, Liu X, Liu R, Pan J, and Liang J. Cellular mechanisms of combining innate immunity activation with PD-1/PD-L1 blockade in treatment of colorectal cancer. Mol Cancer. (2024) 23:252. doi: 10.1186/s12943-024-02166-w
5. Wang W, Zou C, Liu X, He L, Cao Z, Zhu M, et al. Biomimetic dendritic cell-based nanovaccines for reprogramming the immune microenvironment to boost tumor immunotherapy. ACS Nano. (2024) 18(50):34063–76. doi: 10.1021/acsnano.4c09653
6. Zhang J, Wei Q, Piao Y, Shao S, Zhou Z, Tang J, et al. Synergistic combination of oral transcytotic nanomedicine and histone demethylase inhibitor for enhanced cancer chemoimmunotherapy. ACS Nano. (2024) 18(49):33729–42. doi: 10.1021/acsnano.4c14816
7. Lynch C, Pitroda SP, and Weichselbaum RR. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. (2024) 25:e352–62. doi: 10.1016/S1470-2045(24)00075-5
8. Li C and Li J. Dysregulation of systemic immunity in colorectal cancer and its clinical applications as biomarkers and therapeutics. Crit Rev Oncol Hematol. (2024) 204:104543. doi: 10.1016/j.critrevonc.2024.104543
9. Rao L, Bu LL, Xu JH, Ca B, Yu GT, Yu X, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. (2015) 11:6225–36. doi: 10.1002/smll.201502388
10. Harris JC, Scully MA, and Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers (Basel). (2019) 11(12):1836. doi: 10.3390/cancers11121836
11. Fang RH, Jiang Y, Fang JC, and Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. (2017) 128:69–83. doi: 10.1016/j.biomaterials.2017.02.041
12. Fang RH, Gao W, and Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. (2023) 20:33–48. doi: 10.1038/s41571-022-00699-x
13. Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. (2017) 29(47):10. doi: 10.1002/adma.201703969
14. Liang X, Ye X, Wang C, Xing C, Miao Q, Xie Z, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. (2019) 296:150–61. doi: 10.1016/j.jconrel.2019.01.027
15. Wang J, Zhu M, and Nie G. Biomembrane-based nanostructures for cancer targeting and therapy: From synthetic liposomes to natural biomembranes and membrane-vesicles. Adv Drug Delivery Rev. (2021) 178:113974. doi: 10.1016/j.addr.2021.113974
16. Liu Y, Lu Y, Ning B, Su X, Yang B, Dong H, et al. Intravenous delivery of living listeria monocytogenes elicits gasdmermin-dependent tumor pyroptosis and motivates anti-tumor immune response. ACS Nano. (2022) 16:4102–15. doi: 10.1021/acsnano.1c09818
17. Gong P, Wang Y, Zhang P, Yang Z, Deng W, Sun Z, et al. mmunocyte membrane-coated nanoparticles for cancer immunotherapy. I Cancers (Basel). (2020) 13(1):77.
18. Bagherifar R, Kiaie SH, Hatami Z, Ahmadi A, Sadeghnejad A, Baradaran B, et al. Nanoparticle-mediated synergistic chemoimmunotherapy for tailoring cancer therapy: recent advances and perspectives. J Nanobiotechnology. (2021) 19:110. doi: 10.1186/s12951-021-00861-0
19. Zhang Y, Zhang X, Li H, Liu J, Wei W, and Gao J. Membrane-coated biomimetic nanoparticles: A state-of-the-art multifunctional weapon for tumor immunotherapy. Membranes (Basel). (2022) 12(8):738. doi: 10.3390/membranes12080738
20. Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. (2018) 18:1908–15. doi: 10.1021/acs.nanolett.7b05263
21. Chen Y, Zhi S, Ou J, Gao J, Zheng L, Huang M, et al. Cancer cell membrane-coated nanoparticle co-loaded with photosensitizer and toll-like receptor 7 agonist for the enhancement of combined tumor immunotherapy. ACS Nano. (2023) 17:16620–32. doi: 10.1021/acsnano.3c02724
22. Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, et al. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv Healthc Mater. (2020) 9:e1901223. doi: 10.1002/adhm.201901223
23. Zhao C, Wang C, Shan W, Wang Z, Chen X, Deng H, et al. Nanomedicines for an enhanced immunogenic cell death-based in situ cancer vaccination response. Acc Chem Res. (2024) 57:905–18. doi: 10.1021/acs.accounts.3c00771
24. Zhang J, Fan B, Cao G, Huang W, Jia F, Nie G, et al. Direct presentation of tumor-associated antigens to induce adaptive immunity by personalized dendritic cell-mimicking nanovaccines. Adv Mater. (2022) 34:e2205950. doi: 10.1002/adma.202205950
25. Axelrad JE and Rubin DT. The management of colorectal neoplasia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2024) 22:1181–5. doi: 10.1016/j.cgh.2024.01.030
26. Corbo C, Cromer WE, Molinaro R, Toledano Furman NE, Hartman KA, De Rosa E, et al. Engineered biomimetic nanovesicles show intrinsic anti-inflammatory properties for the treatment of inflammatory bowel diseases. Nanoscale. (2017) 9:14581–91. doi: 10.1039/C7NR04734G
27. Duan Y, Zhang E, Fang RH, Gao W, and Zhang L. Capsulated cellular nanosponges for the treatment of experimental inflammatory bowel disease. ACS Nano. (2023) 17:15893–904. doi: 10.1021/acsnano.3c03959
28. Barberio AE, Smith SG, Correa S, Nguyen C, Nhan B, Melo M, et al. Cancer cell coating nanoparticles for optimal tumor-specific cytokine delivery. ACS Nano. (2020) 14:11238–53. doi: 10.1021/acsnano.0c03109
29. Tummala S, Gowthamarajan K, Satish Kumar MN, and Wadhwani A. Oxaliplatin immuno hybrid nanoparticles for active targeting: an approach for enhanced apoptotic activity and drug delivery to colorectal tumors. Drug Delivery. (2016) 23:1773–87. doi: 10.3109/10717544.2015.1084400
30. Hou M, Wei Y, Zhao Z, Han W, Zhou R, Zhou Y, et al. Immuno-engineered nanodecoys for the multi-target anti-inflammatory treatment of autoimmune diseases. Adv Mater. (2022) 34:e2108817. doi: 10.1002/adma.202108817
31. Wu X, Fan Y, Wang K, Miao Y, Chang Y, Ming J, et al. NIR-II imaging-guided precise photodynamic therapy for augmenting tumor-starvation therapy by glucose metabolism reprogramming interference. Sci Bull (Beijing). (2024) 69:1263–74. doi: 10.1016/j.scib.2024.02.008
32. Peng J, Zhou J, Liu X, Zhang X, Zhou X, Gong Z, et al. A biomimetic nanocarrier facilitates glucose consumption and reactive oxide species accumulation in enzyme therapy for colorectal cancer. J Control Release. (2024) 367:76–92. doi: 10.1016/j.jconrel.2024.01.041
33. Zhang L, Wang Z, Zhang Y, Cao F, Dong K, Ren J, et al. Erythrocyte membrane cloaked metal-organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. (2018) 12:10201–11. doi: 10.1021/acsnano.8b05200
34. Zheng D, Li B, Xu L, Zhang QL, Fan JX, Li CX, et al. Normalizing tumor microenvironment based on photosynthetic abiotic/biotic nanoparticles. ACS Nano. (2018) 12:6218–27. doi: 10.1021/acsnano.8b02977
35. Gu M, Zhou X, Sohn JH, Zhu L, Jie Z, Yang JY, et al. NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity. Nat Immunol. (2021) 22:193–204. doi: 10.1038/s41590-020-00829-6
36. Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. (2017) 36:58. doi: 10.1186/s13046-017-0530-4
37. Xu Y, Huang W, Duan H, and Xiao F. Bimetal-organic framework-integrated electrochemical sensor for on-chip detection of H(2)S and H(2)O(2) in cancer tissues. Biosens Bioelectron. (2024) 260:116463. doi: 10.1016/j.bios.2024.116463
38. Wang D, Woodcock E, Yang X, Nishikawa H, Sviderskaya EV, Oshima M, et al. Exploration of individual colorectal cancer cell responses to H(2)O(2) eustress using hopping probe scanning ion conductance microscopy. Sci Bull (Beijing). (2024) 69:1909–19. doi: 10.1016/j.scib.2024.04.004
39. Brockmueller A, Buhrmann C, Moravejolahkami AR, and Shakibaei M. Resveratrol and p53: How are they involved in CRC plasticity and apoptosis? J Adv Res. (2024) 66:181–95. doi: 10.1016/j.jare.2024.01.005
40. Zhang Z, Ji Y, Hu N, Yu Q, Zhang X, Li J, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. (2022) 17:751–66. doi: 10.1016/j.ajps.2022.07.006
41. Zeng W, Wang Y, Zhang Q, Hu C, Li J, Feng J, et al. Neutrophil nanodecoys inhibit tumor metastasis by blocking the interaction between tumor cells and neutrophils. ACS Nano. (2024) 18:7363–78. doi: 10.1021/acsnano.3c08946
42. Yingchoncharoen P, Kalinowski DS, and Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. (2016) 68:701–87. doi: 10.1124/pr.115.012070
43. Zhang H, Li X, Ding J, Xu H, Dai X, Hou Z, et al. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int J Pharm. (2013) 441:261–8. doi: 10.1016/j.ijpharm.2012.11.034
44. Sen K, Banerjee S, and Mandal M. Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf B Biointerfaces. (2019) 180:9–22. doi: 10.1016/j.colsurfb.2019.04.035
45. Nag S, Manna K, and Saha KD. Tannic acid-stabilized gold nano-particles are superior to native tannic acid in inducing ROS-dependent mitochondrial apoptosis in colorectal carcinoma cells via the p53/AKT axis. RSC Adv. (2019) 9:8025–38. doi: 10.1039/C9RA00808J
46. Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, et al. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics. (2020) 10:4056–72. doi: 10.7150/thno.40860
47. Shen T, Yang S, Qu X, Chen Z, Zeng L, Sun X, et al. A bionic “Trojan horse”-like gene delivery system hybridized with tumor and macrophage cell membrane for cancer therapy. J Control Release. (2023) 358:204–18. doi: 10.1016/j.jconrel.2023.04.046
48. Yang Y, Liu Q, Wang M, Li L, Yu Y, Pan M, et al. Genetically programmable cell membrane-camouflaged nanoparticles for targeted combination therapy of colorectal cancer. Signal Transduct Target Ther. (2024) 9:158. doi: 10.1038/s41392-024-01859-4
49. Wang S, Yin Y, Song W, Zhang Q, Yang Z, Dong Z, et al. Red-blood-cell-membrane-enveloped magnetic nanoclusters as a biomimetic theranostic nanoplatform for bimodal imaging-guided cancer photothermal therapy. J Mater Chem B. (2020) 8:803–12. doi: 10.1039/C9TB01829H
50. Wan X, Zhang Y, Wan Y, Xiong M, Xie A, Liang Y, et al. A multifunctional biomimetic nanoplatform for dual tumor targeting-assisted multimodal therapy of colon cancer. ACS Nano. (2024) 18:26666–89. doi: 10.1021/acsnano.4c05773
51. Li Q, Su R, Bao X, Cao K, Du Y, Wang N, et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. (2022) 144:109–20. doi: 10.1016/j.actbio.2022.03.030
52. Xiao Y, Zhu T, Zeng Q, Tan Q, Jiang G, Huang X, et al. Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy. Acta Biomater. (2023) 157:451–66. doi: 10.1016/j.actbio.2022.11.043
53. Liu H, Xu C, Meng M, Li S, Sheng S, Zhang S, et al. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. (2022) 144:132–41. doi: 10.1016/j.actbio.2022.03.023
54. Wang S, Song Y, Cao K, Zhang L, Fang X, Chen F, et al. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. (2021) 134:621–32. doi: 10.1016/j.actbio.2021.07.051
55. David M, Naudin C, Letourneur M, Polrot M, Renoir JM, Lazar V, et al. Suppressor of cytokine signaling 1 modulates invasion and metastatic potential of colorectal cancer cells. Mol Oncol. (2014) 8:942–55. doi: 10.1016/j.molonc.2014.03.014
56. Guthula LS, Yeh KT, Huang WL, Chen CH, Chen YL, Huang CJ, et al. Quantitative and amplification-free detection of SOCS-1 CpG methylation percentage analyses in gastric cancer by fiber optic nanoplasmonic biosensor. Biosens Bioelectron. (2022) 214:114540. doi: 10.1016/j.bios.2022.114540
57. Gopal AA, Ricoult SG, Harris SN, Juncker D, Kennedy TE, and Wiseman PW. Spatially selective dissection of signal transduction in neurons grown on netrin-1 printed nanoarrays via segmented fluorescence fluctuation analysis. ACS Nano. (2017) 11:8131–43. doi: 10.1021/acsnano.7b03004
58. Abdolahad M, Janmaleki M, Taghinejad M, Taghnejad H, Salehi F, and Mohajerzadeh S. Single-cell resolution diagnosis of cancer cells by carbon nanotube electrical spectroscopy. Nanoscale. (2013) 5:3421–7. doi: 10.1039/c3nr33430a
59. Tian X, Srinivasan PR, Tajiknia V, Sanchez Sevilla Uruchurtu AF, Seyhan AA, Carneiro BA, et al. Targeting apoptotic pathways for cancer therapy. J Clin Invest. (2024) 134(14):e179570. doi: 10.1172/JCI179570
60. Zakaria AB, Picaud F, Rattier T, Pudlo M, Dufour F, Saviot L, et al. Nanovectorization of TRAIL with single wall carbon nanotubes enhances tumor cell killing. Nano Lett. (2015) 15:891–5. doi: 10.1021/nl503565t
61. Abdolahad M, Shashaani H, Janmaleki M, and Mohajerzadeh S. Silicon nanograss based impedance biosensor for label free detection of rare metastatic cells among primary cancerous colon cells, suitable for more accurate cancer staging. Biosens Bioelectron. (2014) 59:151–9. doi: 10.1016/j.bios.2014.02.079
62. Wen M, Zhao Y, Qiu P, Ren Q, Tao C, Chen Z, et al. Efficient sonodynamic ablation of deep-seated tumors via cancer-cell-membrane camouflaged biocompatible nanosonosensitizers. J Colloid Interface Sci. (2023) 644:388–96. doi: 10.1016/j.jcis.2023.04.088
63. Chen L, Kang Z, Shen J, Zhao R, Miao Y, Zhang L, et al. An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria. Sci Bull (Beijing). (2024) 69:2565–79. doi: 10.1016/j.scib.2024.06.016
64. Huang F, Zhang Q, Xiao J, Zhang X, Han X, Shi X, et al. Cancer cell membrane-coated gambogic acid nanoparticles for effective anticancer vaccination by activating dendritic cells. Int J Nanomedicine. (2023) 18:2261–73. doi: 10.2147/IJN.S408521
65. Guo Z, Noh I, Zhu AT, Yu Y, Gao W, Fang RH, et al. Cancer cell membrane nanodiscs for antitumor vaccination. Nano Lett. (2023) 23:7941–9. doi: 10.1021/acs.nanolett.3c01775
66. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. (2012) 161:505–22. doi: 10.1016/j.jconrel.2012.01.043
67. Bulbake U, Doppalapudi S, Kommineni N, and Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. (2017) 9(2):12. doi: 10.3390/pharmaceutics9020012
68. Tao L and Reese TA. Making mouse models that reflect human immune responses. Trends Immunol. (2017) 38:181–93. doi: 10.1016/j.it.2016.12.007
69. Day CP, Merlino G, and Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. (2015) 163:39–53. doi: 10.1016/j.cell.2015.08.068
70. Ettrich TJ, Perkhofer L, Decker T, Hofheinz RD, Heinemann V, Hoffmann T, et al. Nintedanib plus mFOLFOX6 as second-line treatment of metastatic, chemorefractory colorectal cancer: The randomised, placebo-controlled, phase II TRICC-C study (AIO-KRK-0111). Int J Cancer. (2021) 148:1428–37. doi: 10.1002/ijc.33296
Keywords: CNPs, nanoparticles, immunity, colorectal cancer, immune microenvironment
Citation: Qiu Z, Liu W and Xu S (2025) Advances in the multifunctionality of cell membrane-encapsulated nanoparticles in the treatment of colorectal cancer. Front. Immunol. 16:1657722. doi: 10.3389/fimmu.2025.1657722
Received: 01 July 2025; Accepted: 16 October 2025;
Published: 31 October 2025.
Edited by:
Chun-Wai Mai, IMU University, MalaysiaReviewed by:
Abu Md Ashif Ikbal, Assam University, IndiaSobia Razzaq, University of Management and Technology, Pakistan
Copyright © 2025 Qiu, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Xu, Z3hxcm14czg2MzA2QDE2My5jb20=
†These authors share first authorship
 Zhengdong Qiu
Zhengdong Qiu Wenxuan Liu1,2†
Wenxuan Liu1,2† Sheng Xu
Sheng Xu