- 1The Third Department of Medical Oncology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
- 2Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, China
- 3Department of Radiation Oncology, The Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia Autonomous Region, China
- 4Department of Pharmacy, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
- 5College of Clinical Medical, Ningxia Medical University, Yinchuan, Ningxia, China
Background: There is limited evidence concerning real-world efficacy of second-line (2L) treatment with immune checkpoint inhibitors (ICIs) in extensive-stage small-cell lung cancer (ES-SCLC). In this study, we evaluated the efficacy of 2L-ICIs therapy in patients with ES-SCLC.
Methods: In this retrospective study, we included patients with ES-SCLC who experienced disease progression following first-line (1L) therapy and received 2L treatment between March 2019 and December 2023. The primary endpoint of this study was progression-free survival (PFS), and the secondary endpoints included safety, the objective response rate (ORR), the disease control rate (DCR), and overall survival (OS). Survival analyses were conducted using Kaplan-Meier curves. One-to-one propensity score matching (PSM) was used to reduce confounding. Univariate and multivariate Cox regression analyses were conducted to identify factors associated with PFS and OS.
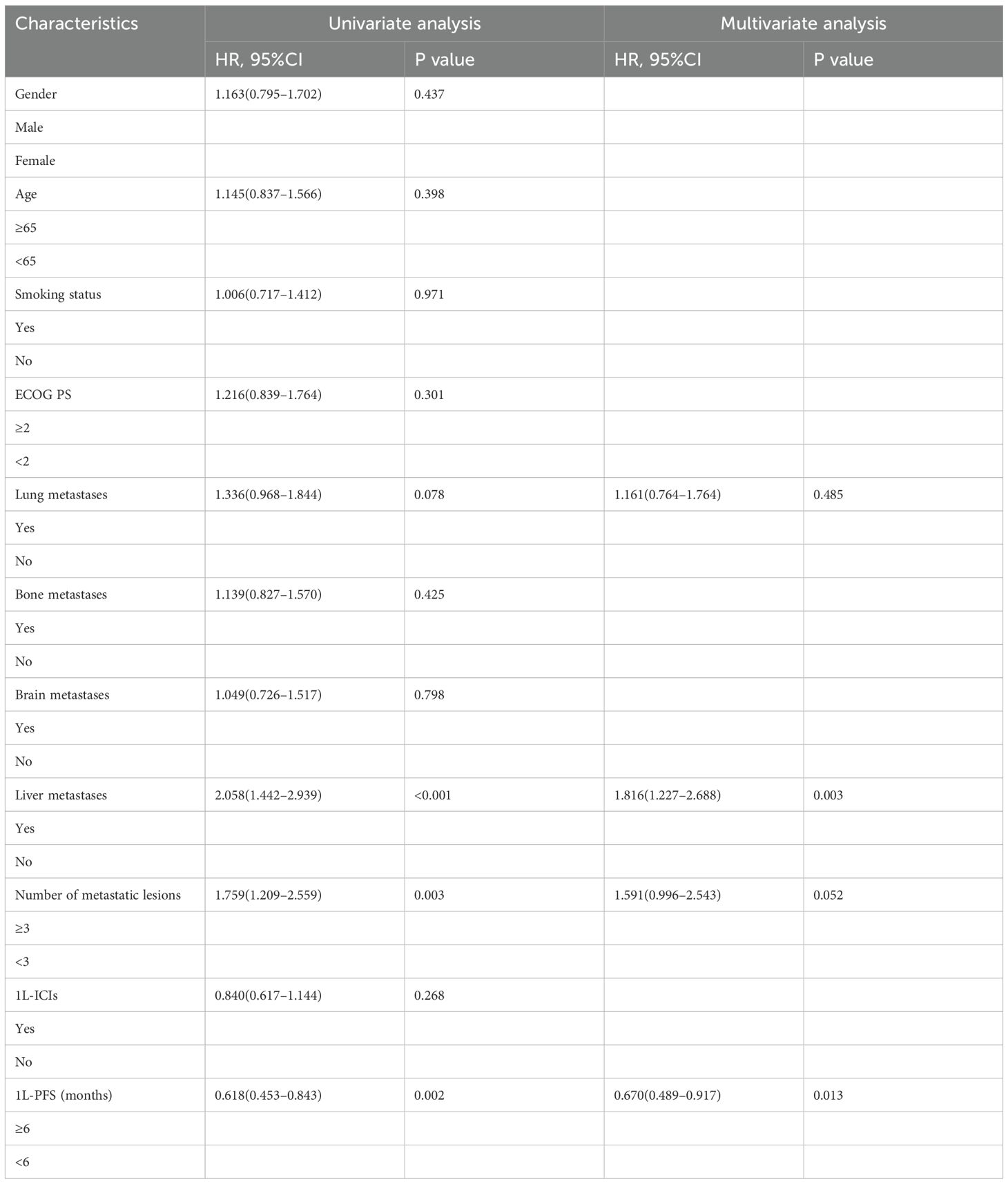
Results: We included 496 patients in this study; among them, 200 patients were in the 2L-ICIs group and 296 patients were in the 2L-non-ICIs group. The 2L-ICIs group demonstrated significantly longer PFS than the 2L-non-ICIs group (median PFS: 4.13 vs. 2.70 months; p < 0.001), and this benefit persisted after PSM (median PFS: 4.14 vs. 2.84 months; p < 0.001). The 2L-ICIs group also had a significantly higher ORR (ORR: 29.5% vs. 10.1%; p < 0.001) and DCR (DCR: 67.0% vs. 51.7%; p < 0.001). Treatment-related adverse events were comparable between the groups, with only one grade 3 rash reported in the 2L-ICIs group. Multivariate Cox regression identified liver metastases, the number of metastatic lesions, and the 1L-PFS as independent predictive factors for PFS.
Conclusion: In this study, 2L-ICIs demonstrate significant clinical benefits with acceptable toxicity in ES-SCLC patients who progressed after 1L therapy, supporting their use as a clinically actionable option.
1 Introduction
Lung cancer is the leading cause of cancer incidence and cancer-related mortality worldwide, accounting for an estimated 2.5 million new cases (12.4%) and 1.8 million deaths (18.7%) annually (1). However, significant variation in lung cancer incidence and mortality rates exists across different regions of the world, reflecting different patterns of tobacco smoking, exposure to environmental risk factors, and genetics (2). In this context, lung cancer poses a particularly severe public health challenge in China, accounting for a disproportionately high share of the global burden. Recent data from the Global Burden of Disease (GBD) study provided strong evidence for this occurrence. China accounts for 41.0% of new lung cancer cases and 40.4% of lung cancer-related deaths worldwide (3); these percentages are substantially greater than those reported in all other nations. Epidemiological trends in China diverge markedly from those in high-income countries. For example, the United States has experienced a decline in both incidence and mortality since the 1990s through multifaceted interventions, including comprehensive tobacco control, early screening implementation, and therapeutic advances (4). Small cell lung cancer (SCLC) is the most aggressive lung cancer subtype; it is strongly associated with tobacco exposure and accounts for about 15% of all new diagnoses (5, 6). About 70% of SCLC patients present with extensive-stage disease at initial diagnosis (7). The prognosis of extensive-stage SCLC (ES-SCLC) is extremely poor, with a median overall survival (OS) of only 6–10 months (8) and a five-year survival rate of less than 2% (9).
Progress concerning the treatments for SCLC over three decades has been limited (10). Platinum-based doublet chemotherapy is the standard first-line (1L) therapy for ES-SCLC (11). The recent integration of immune checkpoint inhibitors (ICIs) with platinum-based chemotherapy has become the new 1L standard, extending the median OS by about two months (12, 13). Subsequent phase III trials evaluating novel ICIs (e.g., serplulimab, adebrelimab, and tislelizumab) have demonstrated greater efficacy, achieving median OS ranging from 15.3 to 15.5 months (14–16). Despite high initial sensitivity to 1L chemotherapy, acquired resistance mediated by tumor evolution under selective therapeutic pressure leads to near-universal progression of disease (17). Consequently, the therapeutic landscape for second-line (2L) and subsequent treatments remains severely constrained. The 2L therapeutic agents currently used, including standard agents such as topotecan, the newer DNA-alkylating agent lurbinectedin (18), and the DLL3-targeted bispecific T-cell engager tarlatamab (19), are not easily accessible and have safety concerns (20).
The efficacy of ICIs in the 2L treatment of ES-SCLC remains controversial. Previous phase II/III trials evaluating ICI monotherapy or dual immunotherapy combinations have failed to demonstrate significant survival benefits (21–24). However, several studies have suggested that ICIs may provide clinical benefits in 2L therapy (25–28). Consequently, strong evidence is needed to support the efficacy of 2L ICIs after disease progression following 1L therapy in ES-SCLC patients in clinical decision-making. The safety issues associated with 2L ICIs also need to be investigated. In this multicenter study, we evaluated the efficacy and safety of 2L ICIs for treating ES-SCLC.
2 Materials and methods
2.1 Patients
Patients with ES-SCLC who received 2L therapy at Tianjin Medical University Cancer Institute and Hospital, General Hospital of Ningxia Medical University, and The Affiliated Hospital of Inner Mongolia Medical University between March 2019 and December 2023 were retrospectively included in this study. The inclusion criteria were as follows: (1) All patients must be ≥ 18 years old. (2) SCLC was confirmed via pathological or cytological examinations. (3) Patients presented with extensive-stage disease at initial diagnosis, defined by the NCCN criteria as extension beyond the ipsilateral hemithorax, including malignant pleural or pericardial effusion or hematogenous metastases. (4) Patients showed disease progression following 1L therapy. (5) Patients must have a baseline corticosteroid dose ≤10 mg/day of prednisone equivalent and adequate marrow and organ function. (6) Patients for whom complete clinical medical records were available. Exclusion criteria were as follows: (1) Histopathology showing combined SCLC with other cellular components (e.g., adenocarcinoma or large cell carcinoma). (2) Patients with a history of other concurrent malignancies. (3) Patients with active autoimmune disease, immune deficiency, hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, or active tuberculosis (TB). (4) Patients with symptomatic brain metastases.
Patients were stratified into 2L-ICIs and 2L-non-ICIs groups based on whether ICIs were added to 2L therapy.
2.2 Data collection
Baseline clinical characteristics at diagnosis, including gender, age, smoking status (never/former or current smoker), Eastern Cooperative Oncology Group (ECOG) performance status (PS), and metastatic sites, were extracted from electronic health records.
2.3 Outcomes and assessments
The primary outcome was progression-free survival (PFS), defined as the time from the initiation of 2L therapy to disease progression or death due to any cause. The secondary endpoints were safety, the objective response rate (ORR), the disease control rate (DCR), and OS. Tumor response was evaluated by conducting contrast-enhanced computed tomography of the chest and upper abdomen, as well as contrast-enhanced magnetic resonance imaging of the brain, initially at baseline and then after every second treatment cycle (6–8 weeks). Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1). The best overall response categories included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as the proportion of patients who achieved CR or PR. DCR was defined as the proportion of patients who achieved CR, PR, or SD. OS was defined as the time from the initiation of 2L therapy to the date of death due to any cause or the last day of follow-up. Treatment-related adverse events (TRAEs) were graded using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0). The last date of follow-up for the study was January 10, 2025. The follow-up time was defined from the initiation of 2L therapy until death or the last follow-up date, whichever occurred first.
2.4 Statistical analysis
Baseline characteristics for categorical variables are presented as frequencies and percentages. The Chi-square test or Fisher’s exact test was conducted to compare categorical variables between the two groups. To minimize potential confounding factors, one-to-one propensity score matching (PSM) was performed with a caliper width of 0.02. The Kaplan-Meier method was used to estimate PFS and OS, and differences between groups were assessed by conducting the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to identify factors associated with survival outcomes. Variables with P < 0.15 in the univariate analysis were included in the multivariate model. The hazard ratio (HR) was reported along with the 95% confidence interval (CI). While conducting subgroup analyses, we used an unstratified Cox proportional hazards model with 2L treatment as a covariate. The results were considered to be statistically significant at P < 0.05 (two-sided). All statistical analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
3 Results
3.1 Baseline characteristics
A total of 496 ES-SCLC patients who received 2L therapy were included in this study. The cohort had a median age of 62 years (range: 19–87), with predominant clinical characteristics, including male gender (79.6%), a smoking history (71.0%), and ECOG PS <2 (79.2%). Among the patients, lung (58.9%), bone (31.3%), brain (20.8%), and liver (20.2%) metastases were observed, with 72.0% of patients having ≥3 metastatic sites. ICIs were administered as a 1L treatment in 42.5% of the patients. After PSM, the baseline characteristics were balanced between the groups (Table 1).
3.2 Treatment
In the 2L-ICIs group (n = 200), all patients received ICI-based regimens, which included ICI-chemotherapy combinations (64.5%, n = 129), ICI-antiangiogenic therapy (22.5%, n = 45), triple therapy (ICI + chemotherapy + anti-angiogenic agent; 10.5%, n = 21), and ICI monotherapy (2.5%, n = 5). The ICI agents used were atezolizumab (52.5%, n = 105), durvalumab (27.0%, n = 54), serplulimab (12.5%, n = 25), tislelizumab (4.5%, n = 9), and adebrelimab (3.5%, n = 7). The median number of 2L ICIs therapy cycles was 3 (range: 1–16). In the 2L-non-ICIs group (n = 296), treatment regimens consisted of chemotherapy (82.1%, n = 243), including platinum-based combinations (e.g., etoposide/platinum, irinotecan/platinum), irinotecan monotherapy, taxanes, temozolomide, and the CAV regimen (cyclophosphamide, adriamycin, and vincristine), as well as anti-angiogenic therapy (17.9%, n = 53; anlotinib monotherapy or combination with chemotherapy). These patients received a median of two cycles of therapy (range 1–28).
3.3 Efficacy
In the whole population, the median follow-up time was 19.7 months, with median PFS and OS values of 3.12 and 7.90 months, respectively. In the 2L-ICIs group, the median PFS was 4.13 months (95% CI: 3.63–4.63), whereas it was 2.70 months (95% CI: 2.29–3.11) in the 2L-non-ICIs group (HR 0.62, 95% CI 0.51–0.75; p < 0.001; Figure 1A). The six-month PFS rates were 34.1% and 19.1%, and the one-year PFS rates were 18.3% and 6.2%, respectively. In terms of OS, although no significant difference was observed between the 2L-ICIs and 2L-non-ICIs groups, a slight improvement in OS was noted in the 2L-ICIs group. The median OS was 7.98 months (95% CI: 7.02–8.94) in the 2L-ICIs group versus 7.80 months (95% CI: 6.79–8.81) in the 2L-non-ICIs group (HR 0.83, 95% CI 0.68–1.00; p = 0.055; Figure 1B), with one-year OS rates of 37.8% and 28.4% and two-year OS rates of 9.5% and 6.3%, respectively.
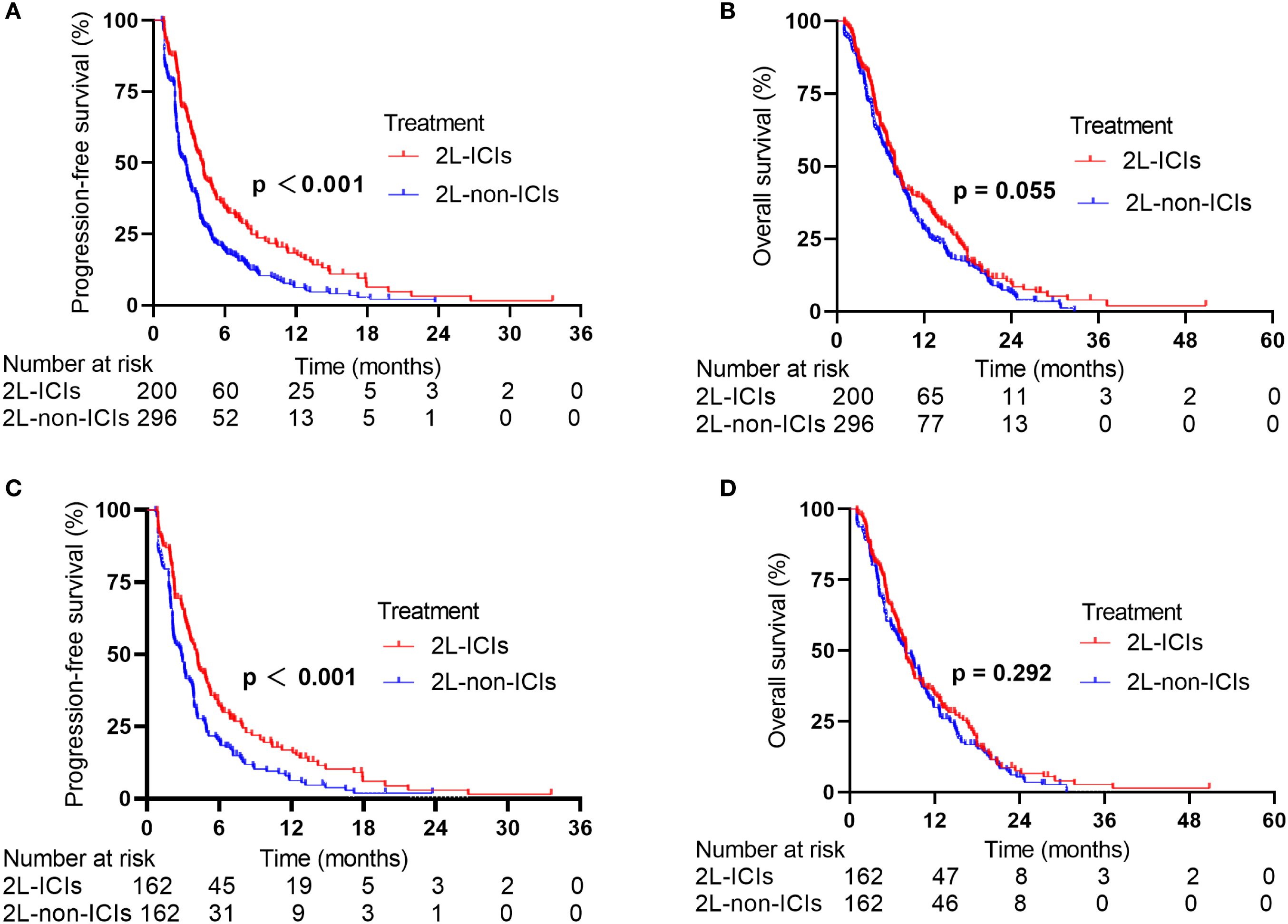
Figure 1. PFS and OS between the 2L-ICIs group and the 2L-non-ICIs group before and after PSM. (A) PFS before PSM, (B) OS before PSM, (C) PFS after PSM (D) OS after PSM.
After 1:1 PSM, the overall median PFS was 3.45 months, and the median OS was 7.95 months. The median PFS was 4.14 months (95% CI: 3.62–4.67) in the 2L-ICIs group versus 2.84 months (95% CI: 2.19–3.49) in the 2L-non-ICIs group (HR 0.65, 95% CI 0.51–0.82; p < 0.001; Figure 1C). The six-month PFS rates were 32.1% and 19.8%, and the one-year PFS rates were 16.9% and 6.3%, respectively. The median OS was 7.96 months (95% CI: 7.01–8.92) in the 2L-ICIs group and 7.87 months (95% CI: 6.13–9.61) in the 2L-non-ICIs group (HR: 0.88, 95% CI: 0.70–1.11; p = 0.292; Figure 1D). The one-year OS rates were 34.4% and 29.9%, and the two-year OS rates were 7.6% and 5.4%, respectively.
3.4 Response
The ORR and DCR for the entire cohort were 17.9% and 57.9%, respectively. In the 2L-ICIs group, the ORR was 29.5%, and the DCR was 67.0%, whereas in the 2L-non-ICIs group, the ORR and DCR were 10.1% and 51.7%, respectively. These differences were significant for both the ORR (p < 0.001) and DCR (p < 0.001). After PSM, the ORR was 29.6% in the 2L-ICIs group versus 8.0% in the 2L-non-ICIs group, and the DCR was 67.3% versus 51.9%, respectively, with continued significance for the ORR (p < 0.001) and DCR (p = 0.005) (Table 2).
3.5 Safety
The incidence of TRAEs was comparable between the two groups. In the 2L-ICIs group, the immune-related adverse events (irAEs) observed included hypothyroidism, rash, pneumonitis, diarrhea, and adrenal insufficiency. Only one case of grade 3 rash occurred in this group. Most adverse events were manageable and generally reversible with standard clinical interventions (Table 3).
3.6 Cox regression analysis for PFS and OS
For the 2L-ICIs population, we further investigated the risk factors affecting PFS. The results of the multivariate Cox regression analysis revealed that baseline liver metastases and the number of metastatic lesions were independent factors associated with worse PFS, and that 1L-PFS was an independent factor associated with favorable PFS (Table 4).
For OS, baseline liver metastases was identified as an independent factor associated with worse OS. In contrast, 1L-PFS was identified as an independent factor associated with favorable OS in the multivariate analysis (Table 5).
3.7 Subgroup analysis
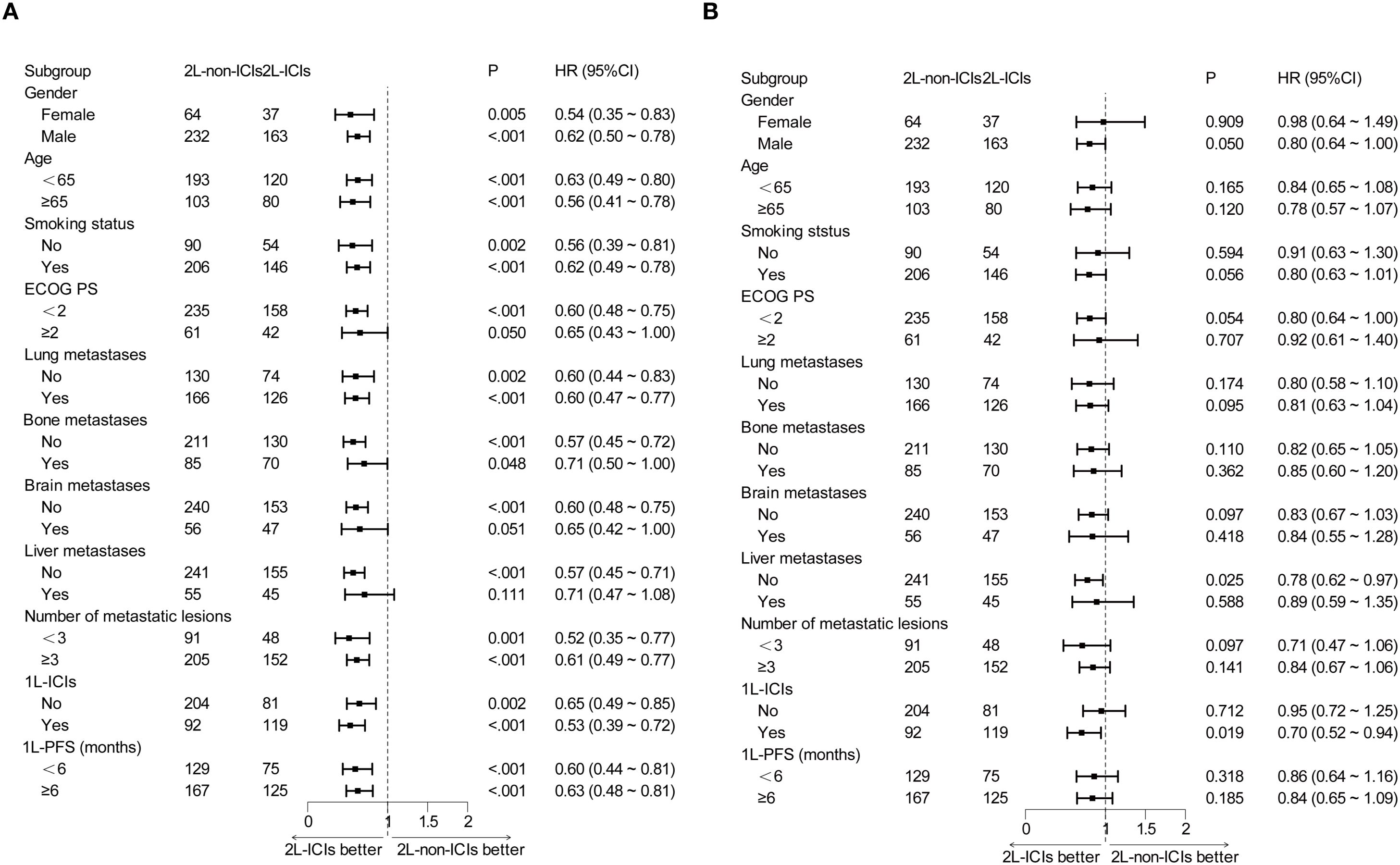
Prespecified subgroup analyses stratified by baseline characteristics were performed. In most subgroups, 2L-ICIs improved PFS (Figure 2A). However, the improvement in OS with 2L-ICIs was observed only in the subgroup without liver metastases (HR 0.78, 95% CI 0.62–0.97; p = 0.025) and in those where the subgroups received 1L ICIs (HR 0.70, 95% CI 0.52–0.94; p = 0.019) (Figure 2B).
Given the poor prognosis of patients with baseline liver metastases, we focused on patients with baseline liver metastases. In the 2L-ICIs cohort, patients with baseline liver metastases had shorter survival outcomes than those without baseline liver metastases. The median PFS was 2.74 months (95% CI: 2.03–3.45) versus 4.46 months (95% CI: 3.73–5.19) (p < 0.001, Figure 3A), with six-month PFS rates of 18.3% versus 38.8% and one-year PFS rates of 3.4% versus 22.5%. The difference was more pronounced in OS, where patients with liver metastases had a median OS of 5.32 months (95% CI: 4.54–6.10) compared to the median OS of 8.98 months (95% CI: 6.14–11.83) in patients without liver metastases (p < 0.001, Figure 3B). The one-year OS rates were 19.5% and 43.1%, and the two-year OS rates were 2.8% and 11.4%, respectively.
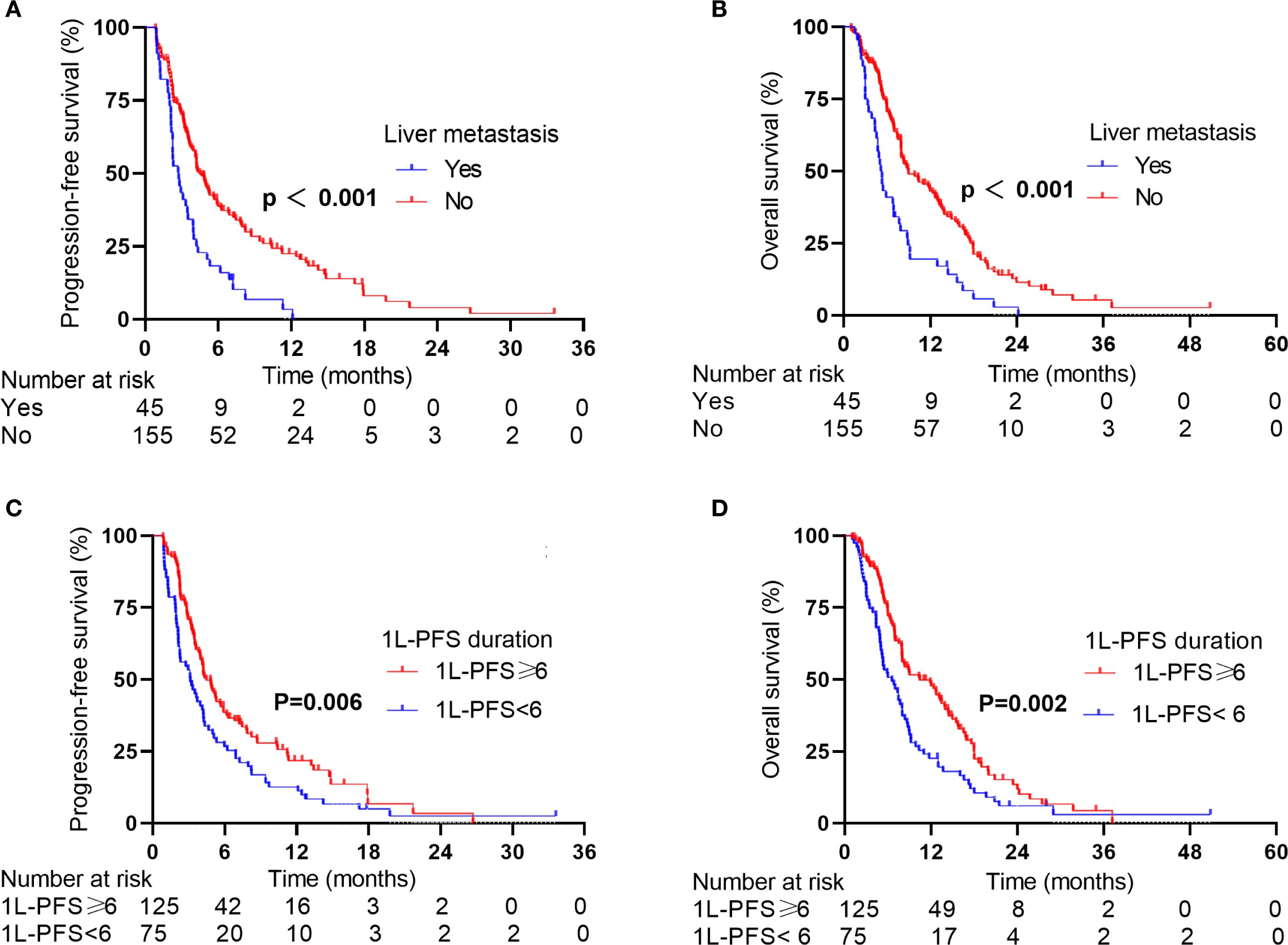
Figure 3. PFS and OS of patients in the 2L-ICIs group according to stratification factors. (A) PFS according to liver metastases status. (B) OS according to liver metastases status. (C) PFS according to the 1L-PFS duration. (D) OS according to the 1L-PFS duration.
Considering 1L-PFS as an additional independent prognostic factor for survival, we further stratified patients by 1L-PFS to analyze survival differences. In the 2L-ICIs group, patients with a 1L-PFS of ≥6 months had a median PFS of 4.46 months (95% CI: 3.56–5.36) versus 3.13 months (95% CI: 1.90–4.36) in those with a 1L-PFS of <6 months (p = 0.006, Figure 3C), with corresponding 6-month PFS rates of 54.6% versus 27.2% and one-year PFS rates of 19.5% versus 17.2%. The survival benefit was evident in OS. Patients with a 1L-PFS of ≥6 months demonstrated a median OS of 10.43 months (95% CI: 6.57–14.29) compared to 6.54 months (95% CI: 4.73–8.35) in those with a 1L-PFS of <6 months (p = 0.002, Figure 3D). The one-year OS rates were 60.0% and 30.5%, and the two-year OS rates were 15.0% and 7.4%, respectively.
4 Discussion
Extensive-stage small-cell lung cancer is an aggressive malignancy with a poor prognosis, and treatment options after disease progression following 1L therapy remain limited. Currently available 2L treatments for ES-SCLC have minimal clinical benefit. The clinical evidence supporting ICIs efficacy in 2L therapy is insufficient. The cohort investigated in this study represents the largest real-world cohort evaluating the efficacy of 2L ICIs in ES-SCLC patients. We found that compared to 2L-non-ICIs therapy, 2L-ICIs therapy significantly improved PFS, ORR, and DCR while maintaining a favorable safety profile. The benefit of 2L-ICIs therapy persisted after PSM, a robust methodology minimizing confounding biases, confirming that our findings were reliable. This consistency in results highlights that ICIs can serve as a viable 2L treatment option for patients with ES-SCLC.
A study on the combination of camrelizumab and apatinib in ES-SCLC reported an ORR of 30%, a DCR of 70%, and a median PFS of 4.9 months (25). In our study, we found similar improvements in ORR and DCR with 2L ICIs, reinforcing the therapeutic benefit of ICIs for patients with ES-SCLC. Another phase II study examined the combination of sintilimab, anlotinib, and chemotherapy in patients with relapsed ES-SCLC and reported an ORR of 60%, a DCR of 76%, a median PFS of 6.0 months, and a median OS of 13.4 months (26). This study demonstrated considerably better clinical outcomes than our findings, probably because it exclusively enrolled patients who received the intensive triplet regimen of ICIs, anlotinib, and chemotherapy. The NCT02551432 trial, which analyzed the combination of pembrolizumab and paclitaxel, achieved a DCR of 80.7%, with a median PFS of 5.0 months and a median OS of 9.1 months (29). Our study similarly revealed that 2L ICIs significantly prolonged median PFS compared to 2L-non-ICIs, further supporting the efficacy of ICIs. Complementary real-world evidence confirmed these observations. A multicenter study reported that the combination of PD-1/PD-L1 inhibitors with anlotinib significantly outperformed paclitaxel monotherapy, with a higher DCR (80.5% vs. 54.5%; p = 0.005), longer median PFS (3.40 vs. 2.83 months; p = 0.02), and improved median OS (8.20 vs. 5.87 months; p = 0.048) (30). In another cohort of 103 patients, the administration of 2L ICIs significantly prolonged median PFS (4.4 months vs. 3.9 months, HR = 0.45, p = 0.001) and OS (10.0 months vs. 6.9 months, HR = 0.56, p = 0.015) compared to 2L-non-ICIs (31). Although a significant benefit of OS was absent in our cohort, clinically meaningful improvements in PFS, ORR, and DCR were observed, which is consistent with these findings. Our findings provide real-world evidence supporting the efficacy of treatment using 2L ICIs in clinically diverse ES-SCLC patients.
Potential explanations for the observed clinical benefits of 2L ICIs differ based on prior exposure to ICIs. For patients without prior exposure to IL ICIs, 2L ICIs block immune checkpoint pathways, abrogating suppressive signals in tumor-infiltrating T lymphocytes (TILs), thus identifying and destroying cancer cells (32). In contrast, for patients previously exposed to 1L ICIs, the sustained benefit from continued ICIs in 2L therapy is potentially attributable to the modification of the therapeutic regimen at this stage, such as alterations in the chemotherapy regimen or combinations of anti-angiogenic agents. These strategic therapeutic adaptations can contribute to the remodeling of the tumor immune microenvironment, thereby sustaining the efficacy of ICIs. However, the mechanisms underlying this observed benefit need to be further elucidated through dedicated mechanistic studies. Additionally, while our study demonstrated a significant improvement in PFS with 2L-ICIs compared to 2L-non-ICIs regimens, the absolute difference of 1.3 months is of limited clinical relevance. Consequently, the assessment of more effective and less toxic treatment strategies to optimize clinical outcomes remains a key focus of current research.
In this study, while 2L ICIs failed to significantly improve OS compared to 2L-non-ICIs, prespecified subgroup analyses confirmed a significant improvement in OS in patients without liver metastases in the 2L-ICIs group. This finding corroborates existing evidence indicating that cancer patients with liver metastases respond significantly more poorly to anti-PD-1 immunotherapy than those without liver metastases (24, 33–37). Consistent with these findings, our results demonstrated considerably poorer outcomes for patients with liver metastases. Both the median PFS (2.74 vs. 4.46 months) and OS (5.32 vs. 8.98 months) were about half the median PFS reported in patients without liver metastases (p < 0.001 for both). This survival disparity became more pronounced over time. The one-year PFS rate was only 3.4% in patients with liver metastases versus 22.5% in those without liver metastases, whereas a nearly fourfold difference was observed in the two-year OS rate (2.8% vs. 11.4%). Mechanistic insights from clinical and preclinical studies support these findings. Lee et al. proposed that liver tumors not only compromise intrahepatic immunity but also impair systemic antitumor immunity, potentially explaining the reduced efficacy of systemic anti-PD-1 treatment observed clinically (38). Supporting this mechanistic insight, Yu et al. reported that in preclinical models, liver metastases induce systemic loss of tumor-specific CD8+ T cells and abrogate the efficacy of immunotherapy, a phenomenon that mirrors systemic T-cell dysfunction and decreases the treatment response observed in patients with liver metastases (39). These results highlight the importance of performing routine assessment for liver metastases before initiating 2L ICIs therapy. For patients with liver metastases, 2L regimens combining ICIs with tumor microenvironment (TME)-modulating agents represent a strategic approach aimed at counteracting the underlying systemic immunosuppression. Such combinatorial strategies need to be validated by conducting prospective studies.
The results of subgroup analyses revealed significant improvements in OS with 2L ICIs in patients who received 1L ICIs. These findings are consistent with published results. Zhang et al. reported that continuing ICIs after progression provides a survival benefit for ES-SCLC patients without significantly increasing additional treatment-related toxicity. Their study revealed a longer median PFS (4.4 months vs 3.9 months, HR = 0.45, p = 0.001) and median OS (10.0 months vs 6.9 months, HR = 0.56, p = 0.015) in the 2L-ICIs group than in the 2L-non-ICIs group (31). Similarly, Shi et al. reported that atezolizumab continuation therapy had promising efficacy and manageable safety in ES-SCLC patients who progressed after 1L therapy, with a median PFS of 4.07 months (95% CI: 1.15–6.98) and a considerably longer median OS of 18.87 months (95% CI: 15.28–22.45) (40). Consequently, the optimal implementation of 2L ICIs requires precise patient selection to identify those patients who will respond well to ICIs therapy. However, the underlying mechanisms driving this phenomenon need to be elucidated.
In the 2L-ICIs cohort in this study, multivariate Cox regression analysis identified baseline liver metastases as an independent prognostic factor for both PFS and OS. These results align with previous findings that patients with liver metastases are typically associated with a poor prognosis (9, 24, 41, 42). Additionally, multivariate analysis confirmed that 1L-PFS was an independent prognostic factor for both PFS and OS in this cohort. Patients who achieved a 1L PFS of ≥6 months derived greater clinical benefit from subsequent 2L ICIs, a result confirmed by real-world evidence from Zhang et al. (31). This enhanced benefit might be attributed to the fact that patients with a 1L PFS of ≥6 months have a less aggressive tumor phenotype. Additionally, extending 1L-PFS enables critical immune reconstitution, thereby establishing favorable immunological findings for subsequent ICIs therapy. Collectively, these findings identify a 1L-PFS ≥6 months as a clinically actionable biomarker for selecting patients for 2L ICIs therapy. Prospective validation across different cohorts is needed to confirm its utility in guiding stratified treatment strategies, and further research is needed to determine its integration into clinical practice. Therefore, optimizing therapeutic decisions requires the consideration of multiple factors, including disease characteristics, duration of response to 1L therapy, patient preferences, and socioeconomic factors.
The absence of reliable molecular biomarkers for predicting the efficacy of ICIs treatment in ES-SCLC patients is widely recognized. Although PD-L1 expression has emerged as a potential biomarker in other types of cancer (43), its utility in ES-SCLC remains limited. This is largely due to the lower and less consistent expression of PD-L1 in SCLC than in non-small cell lung cancer (NSCLC), making it a less reliable predictor of response to ICIs (44). The variability in PD-L1 expression and the heterogeneity of SCLC tumors further complicate the interpretation of PD-L1 expression in this setting. Emerging biomarkers, such as DLL3 expression, T-cell-inflamed gene expression profiles, and blood tumor mutational burden, show promise in preliminary studies but require validation in prospective ES-SCLC cohorts (45). Predictive biomarker exploration was beyond the scope of this study; however, future prospective research—featuring molecularly characterized cohorts and validated biomarker panels—is needed to optimize immunotherapeutic strategies for patients with ES-SCLC.
The safety analysis revealed comparable tolerability profiles for 2L-ICIs and 2L-non-ICIs when assessing TRAEs excluding irAEs. The 2L ICIs cohort exhibited a favorable safety profile, with only a single case of grade 3 cutaneous toxicity (rash) reported. These findings suggest a reduced susceptibility to severe dose-limiting toxicities in this patient population. This favorable safety profile is clinically significant, given the established links between effective adverse event management and both patient quality of life and treatment adherence. The observed low incidence of severe irAE further indicates that careful patient selection and monitoring can mitigate immunotherapy-related safety concerns. This phenomenon can be explained by two key factors. First, the shorter ICIs treatment duration in the 2L therapy (median of three cycles) may be insufficient to trigger irAEs compared to that in the 1L ICIs group. Second, given the retrospective nature of the study, low-grade irAEs may be under-reported due to inconsistent documentation practices inherent in real-world data.
Although our study provided valuable insights into the efficacy and safety of 2L ICIs therapy in ES-SCLC, it had several limitations. First, the retrospective design and reliance on real-world data may have introduced selection biases and confounding factors, potentially influencing the results. Second, heterogeneous treatment regimens, including various ICIs, chemotherapeutic agents, and combination approaches, introduce significant complexity with potential implications for the interpretation of outcomes. Consequently, future studies should use consistent combined treatment strategies to reduce confounding. Third, we did not perform stratified analyses based on 1L therapy. Further studies are needed to determine the efficacy of cross-line ICIs therapy in subgroups receiving 1L ICIs. Additionally, the lack of effective predictive molecular biomarkers is a significant constraint. Despite these limitations, our findings provided strong evidence for the use of ICIs in ES-SCLC patients after administering 1L therapy. In the future, prospective studies with larger cohorts, standardized treatment protocols, and comprehensive biomarker analyses are needed to validate our findings and identify predictive biomarkers for treatment response. These findings may facilitate precise patient stratification while administering 2L ICIs treatment to patients with ES-SCLC and foster biomarker-driven therapeutic innovations, ultimately improving survival outcomes and quality of life.
5 Conclusions
In conclusion, we found that 2L ICIs enhance survival and are safe for use in ES-SCLC patients; therefore, this modality is a viable 2L therapeutic option.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Cancer Institute and Hospital of Tianjin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. J-XG: Writing – original draft, Data curation, Formal analysis. PL: Writing – review & editing, Data curation, Formal analysis. X-YJ: Formal analysis, Writing – review & editing, Data curation. YW: Supervision, Conceptualization, Funding acquisition, Writing – review & editing. L-JZ: Funding acquisition, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The editor ZW declared a shared affiliation with the author LZ to the handling editor at the time of review.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1708889.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Leiter A, Veluswamy RR, and Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
3. Wu X, Zhu B, Xu S, Liu Y, Bi Y, and Zhou B. A comparison of the burden of lung cancer attributable to tobacco exposure in China and the USA. Ann Transl Med. (2020) 8:1412. doi: 10.21037/atm-20-996
4. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Rudin CM, Brambilla E, Faivre-Finn C, and Sage J. Small-cell lung cancer. Nat Rev Dis Primers. (2021) 7:3. doi: 10.1038/s41572-020-00235-0
6. Cittolin-Santos GF, Knapp B, Ganesh B, Gao F, Waqar S, Stinchcombe TE, et al. The changing landscape of small cell lung cancer. Cancer. (2024) 130:2453–61. doi: 10.1002/cncr.35281
7. Zhu L and Qin J. Predictive biomarkers for immunotherapy response in extensive-stage SCLC. J Cancer Res Clin Oncol. (2024) 150:22. doi: 10.1007/s00432-023-05544-x
8. Zhang S, Li S, Cui Y, Zhao P, Sun X, and Cheng Y. Consideration of surrogate endpoints for overall survival associated with first-line immunotherapy in extensive-stage small cell lung cancer. Front Oncol. (2021) 11:696010. doi: 10.3389/fonc.2021.696010
9. Arriola E, Trigo JM, Sánchez-Gastaldo A, Navarro A, Perez C, Crama L, et al. Prognostic value of clinical staging according to TNM in patients with SCLC: A real-world surveillance epidemiology and end-results database analysis. JTO Clin Res Rep. (2022) 3:100266. doi: 10.1016/j.jtocrr.2021.100266
10. Koinis F, Kotsakis A, and Georgoulias V. Small cell lung cancer (SCLC): no treatment advances in recent years. Transl Lung Cancer Res. (2016) 5:39–50. doi: 10.3978/j.issn.2218-6751.2016.01.03
11. Armstrong SA and Liu SV. Dashing decades of defeat: long anticipated advances in the first-line treatment of extensive-stage small cell lung cancer. Curr Oncol Rep. (2020) 22:20. doi: 10.1007/s11912-020-0887-y
12. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. (2021) 39:619–30. doi: 10.1200/JCO.20.01055
13. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2021) 22:51–65. doi: 10.1016/S1470-2045(20)30539-8
14. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
15. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:739–47. doi: 10.1016/S1470-2045(22)00224-8
16. Cheng Y, Fan Y, Zhao Y, Huang D, Li X, Zhang P, et al. Tislelizumab plus platinum and etoposide versus placebo plus platinum and etoposide as first-line treatment for extensive-stage SCLC (RATIONALE-312): A multicenter, double-blind, placebo-controlled, randomized, phase 3 clinical trial. J Thorac Oncol. (2024) 19:1073–85. doi: 10.1016/j.jtho.2024.03.008
17. Shi Y and Sun Y. Medical management of lung cancer: Experience in China. Thorac Cancer. (2015) 6:10–6. doi: 10.1111/1759-7714.12168
18. Singh S, Jaigirdar AA, Mulkey F, Cheng J, Hamed SS, Li Y, et al. FDA approval summary: lurbinectedin for the treatment of metastatic small cell lung cancer. Clin Cancer Res. (2021) 27:2378–82. doi: 10.1158/1078-0432.CCR-20-3901
19. Ahn MJ, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. (2023) 389:2063–75. doi: 10.1056/NEJMoa2307980
20. Cheng Y, Wu C, Wu L, Zhao J, Zhao Y, Chen L, et al. A pivotal bridging study of lurbinectedin as second-line therapy in Chinese patients with small cell lung cancer. Sci Rep. (2024) 14:3598. doi: 10.1038/s41598-024-54223-5
21. Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. (2020) 15:618–27. doi: 10.1016/j.jtho.2019.12.109
22. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
23. Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol. (2019) 14:903–13. doi: 10.1016/j.jtho.2019.01.008
24. Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331☆. Ann Oncol. (2021) 32:631–41. doi: 10.1016/j.annonc.2021.01.071
25. Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase 2 trial. J Thorac Oncol. (2021) 16:299–309. doi: 10.1016/j.jtho.2020.10.002
26. Han X, Guo J, Li L, Huang Y, Meng X, Wang L, et al. Sintilimab combined with anlotinib and chemotherapy as second-line or later therapy in extensive-stage small cell lung cancer: a phase II clinical trial. Signal Transduct Target Ther. (2024) 9:241. doi: 10.1038/s41392-024-01957-3
27. Blanco AC, Mendivil AFN, de Spéville BD, Colomé EÁ, Luken MJDM, Alvarez RM, et al. 1989MO Lurbinectedin (LUR) in combination with pembrolizumab (PBL) in relapsed small cell lung cancer (SCLC): The phase I/II LUPER study. Ann Oncol. (2023) 34:S1060–1. doi: 10.1016/j.annonc.2023.09.1220
28. Cheng Y, Shen L, Yu X, Chen Z, Song L, Zhang Y, et al. 157P Surufatinib plus toripalimab in patients with advanced small cell lung cancer (SCLC) after failure of first-line systemic chemotherapy. Ann Oncol. (2021) 32:S1448–9. doi: 10.1016/j.annonc.2021.10.176
29. Kim YJ, Keam B, Ock CY, Song S, Kim M, Kim SH, et al. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer. (2019) 136:122–8. doi: 10.1016/j.lungcan.2019.08.031
30. Yu L, Xu J, Qiao R, Han B, Zhong H, and Zhong R. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer Med. (2023) 12:5372–83. doi: 10.1002/cam4.5360
31. Zhang B, Bao H, Li Z, Chen J, Yu H, Li M, et al. Continuing immune checkpoint inhibitors after progression: Real-world patterns of care and outcomes in second-line treatment for extensive-stage small-cell lung cancer. Lung Cancer. (2025) 199:108021. doi: 10.1016/j.lungcan.2024.108021
32. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
33. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. (2017) 5:417–24. doi: 10.1158/2326-6066.CIR-16-0325
34. Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S, et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother. (2018) 67:1825–32. doi: 10.1007/s00262-018-2239-4
35. Bilen MA, Shabto JM, Martini DJ, Liu Y, Lewis C, Collins H, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. (2019) 19:857. doi: 10.1186/s12885-019-6073-7
36. Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. (2019) 22:793–802. doi: 10.1007/s10120-018-00922-8
37. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187
38. Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. (2020) 5:eaba0759. doi: 10.1126/sciimmunol.aba0759
39. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. (2021) 27:152–64. doi: 10.1038/s41591-020-1131-x
40. Shi W, Bao X, Xiong J, Wu Y, Sun J, Xu Z, et al. Efficacy and safety analysis of atezolizumab continuation beyond progression in extensive-stage small cell lung cancer. Clin Exp Med. (2025) 25:71. doi: 10.1007/s10238-025-01606-1
41. Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. (2012) 4:617–20. doi: 10.3892/ol.2012.792
42. Li J, Zhu H, Sun L, Xu W, and Wang X. Prognostic value of site-specific metastases in lung cancer: A population based study. J Cancer. (2019) 10:3079–86. doi: 10.7150/jca.30463
43. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
44. Schultheis AM, Scheel AH, Ozretić L, George J, Thomas RK, Hagemann T, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. (2015) 51:421–6. doi: 10.1016/j.ejca.2014.12.006
Keywords: extensive-stage small cell lung cancer, immune checkpoint inhibitors, second-line therapy, survival, prognosis
Citation: Zhang J, Guo J-x, Li P, Jin X-y, Wang Y and Zhao L-j (2025) Real-world outcomes of immune checkpoint inhibitors as second-line therapy for extensive-stage small-cell lung cancer: a multicenter retrospective analysis. Front. Immunol. 16:1658017. doi: 10.3389/fimmu.2025.1658017
Received: 02 July 2025; Accepted: 05 September 2025;
Published: 19 September 2025; Corrected: 08 October 2025.
Edited by:
Cleber Machado-Souza, Pelé Pequeno Príncipe Research Institute, BrazilReviewed by:
Dechao Feng, University College London, United KingdomLuigi Liguori, University of Salerno, Italy
Copyright © 2025 Zhang, Guo, Li, Jin, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-jun Zhao, emhhb2x1anVuQHRqbXVjaC5jb20=; Yan Wang, bWd3eTE5NzRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jiao Zhang
Jiao Zhang Jia-xing Guo
Jia-xing Guo Ping Li4
Ping Li4 Lu-jun Zhao
Lu-jun Zhao