- Changsha Hospital of Traditional Chinese Medicine (Changsha Eighth Hospital), Changsha, China
Background: No prior research has directly compared adverse drug event (ADE) profiles of Nivolumab and Cetuximab in head and neck cancer (HNC) using the US FDA Adverse Event Reporting System (FAERS). The present study aims to evaluate ADE signal characteristics of both agents to inform clinical decision-making.
Methods: Data extracted from FAERS included patient baseline characteristics, which were summarized in a baseline table. Disproportionality analysis with reporting odds ratio (ROR) and Bayesian confidence propagation neural network (BCPNN) was applied to identify signals at the system organ class (SOC) and preferred term (PT) levels.
Results: For Nivolumab, three significant SOC-level signals were identified—benign/malignant tumors (including cysts/polyps), hepatobiliary disorders, and endocrine abnormalities. At the PT level, 58 effective signals were observed, with immune-related events such as thyroid dysfunction being particularly frequent. For Cetuximab, 40 effective PT-level signals were detected, dominated by dermatologic toxicity (rash) and metabolic abnormalities (hypomagnesemia). Comparative analysis revealed marked differences between the two drugs: Nivolumab was more strongly associated with immune-mediated reactions, whereas Cetuximab was characterized by cutaneous and metabolic toxicity.
Conclusions: This study represents the first FAERS-based assessment of ADE risk differences between Nivolumab and Cetuximab in HNC, offering valuable evidence for clinical monitoring and drug selection. As signal detection reflects statistical correlation rather than causality, confirmatory clinical validation remains necessary. Integration of real-world evidence with prospective clinical trials will be essential to enhance drug safety evaluation systems.
1 Introduction
Head and neck cancer (HNC) includes malignant tumors arising in the oral cavity, pharynx, and larynx, with squamous cell carcinoma representing the predominant histological subtype (1).The disease severely impairs quality of life, and the complexity of local anatomy often renders therapeutic outcomes suboptimal (2).Its onset is closely associated with long-term carcinogenic exposure, including tobacco use and alcohol consumption (3).Current therapeutic modalities consist of surgery, radiotherapy, and chemotherapy (4).However, these approaches are frequently accompanied by high recurrence rates and treatment-related toxicity. The need for more effective therapeutic strategies has therefore become evident (5).In recent years, immunotherapy has emerged as a promising approach in HNC management, particularly with the use of immune checkpoint inhibitors (ICIs) such as Nivolumab and targeted agents like Cetuximab. Both agents have demonstrated measurable benefits in prolonging survival and improving quality of life, yet adverse events remain a significant concern (6).
Importantly, Nivolumab and Cetuximab represent pharmacologically distinct classes (7, 8). Nivolumab, as an immune checkpoint inhibitor (ICI), exerts its effect by enhancing host immune activity against tumor cells (7, 9), while Cetuximab acts as a molecular targeted therapy, directly interfering with tumor cell proliferation through inhibition of the epidermal growth factor receptor (EGFR) signaling cascade (10). The divergence in their mechanisms of action results in distinct toxicity profiles: ICIs predominantly induce immune-related adverse events (irAEs), whereas EGFR inhibitors are associated with dermatologic toxicity and metabolic disturbances (11, 12). A systematic comparison of the safety profiles of these two agents is thus scientifically justified and clinically necessary to support the development of individualized treatment strategies.
Nivolumab and cetuximab demonstrate targeted therapeutic efficacy in HNC and HNSCC. Nivolumab significantly extends survival in patients with platinum-resistant HNSCC (13). Cetuximab, a molecularly targeted agent for head and neck cancers, exerts variable effects when combined with chemotherapy or radiotherapy, and prior investigations indicate potential benefits from such combinations (14). Both agents, however, are associated with adverse reactions: nivolumab commonly induces pruritus and rash, while cetuximab can lead to mucositis, diarrhea, and hypomagnesemia (15). Furthermore, the incidence of severe adverse events related to cetuximab use has been reported to be higher outside the United States. These toxicities substantially impair patient quality of life and therefore necessitate careful monitoring and management throughout therapy.
The Food and Drug Administration Adverse Drug Event Spontaneous Reporting System (FAERS) serves as a repository for adverse event reports concerning drugs and biologics regulated by the FDA (16). This system is indispensable for post-marketing pharmacovigilance, enabling early detection of safety concerns, guiding regulatory interventions, and supporting evidence-based clinical decision-making (17). Accordingly, the present study aims to evaluate the occurrence and determinants of adverse events during treatment of head and neck cancer with Nivolumab and Cetuximab, with the ultimate goal of informing clinical strategies and advancing individualized therapeutic approaches.
2 Materials and methods
2.1 Data extraction and filtering
The original dataset was derived from the US FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html), which compiled spontaneous adverse drug event (ADE) reports submitted by healthcare providers, patients, and other sources. The database, updated quarterly and publicly accessible at no cost, is stored in ASC or XML format and frequently applied in signal detection for marketed drugs. By March 1, all ADE reports related to HNC recorded since the database’s inception were retrieved. The search employed the keyword “Head and Neck Cancer,” with recognition that the FAERS system distinguishes between “Head and Neck Cancer” and “Head and Neck Squamous Cell Carcinoma.” For subsequent analyses, both categories were consolidated and treated uniformly as HNC.
To evaluate the clinical safety profile of Nivolumab and Cetuximab, targeted searches with the keywords “Nivolumab” and “Cetuximab” were performed, capturing ADE reports from Q1–2004 through Q4 2024. The identified reports were systematically organized and annotated according to the preferred terms (PT) and system organ classes (SOC) defined by Medical Dictionary for Regulatory Activities (MedDRA). SOCs were arranged by etiology (e.g., infectious and parasitic diseases), anatomical site (e.g., gastrointestinal disorders), or purpose (e.g., surgical and medical procedures). Each PT denotes a discrete medical concept including symptoms, diagnoses, indications, examinations, or clinical procedures. While every PT is mapped to at least one SOC, multiple SOC associations are possible depending on context.
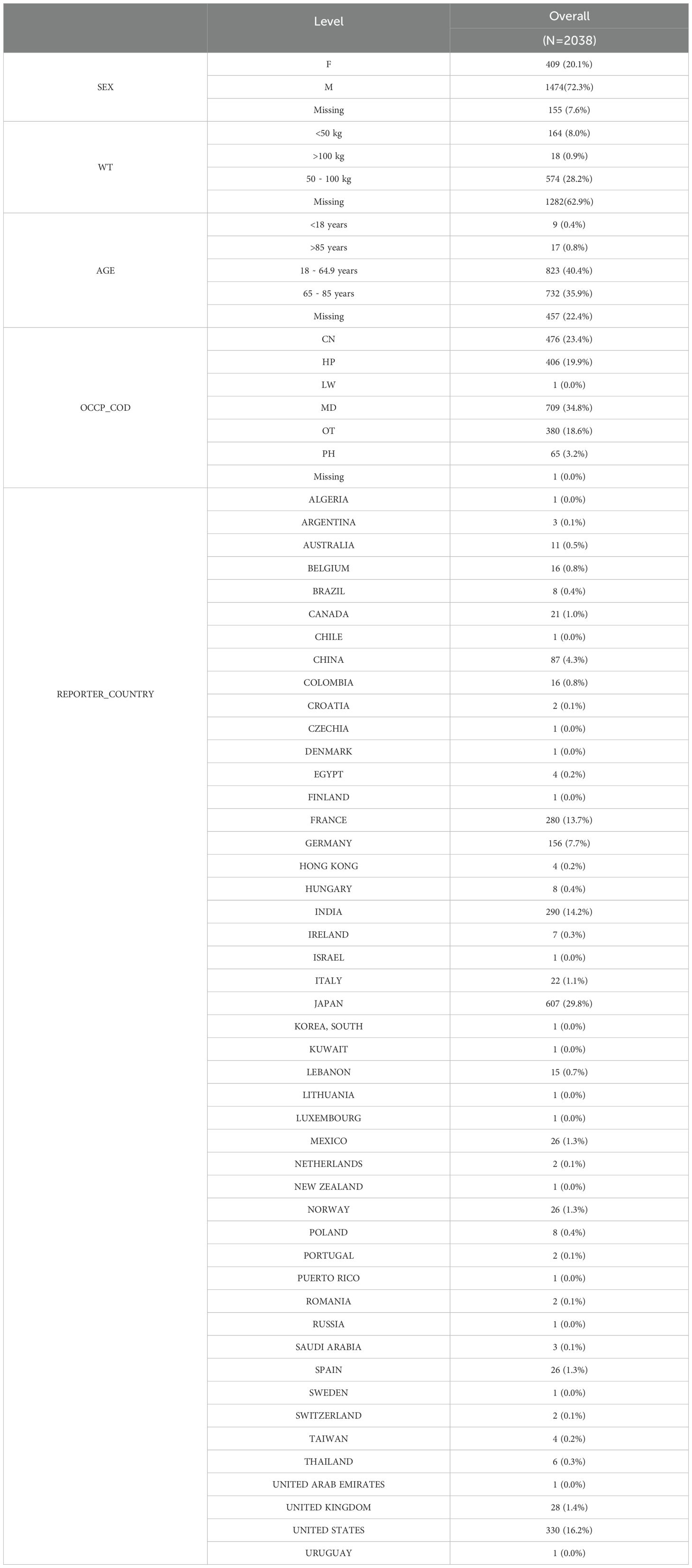
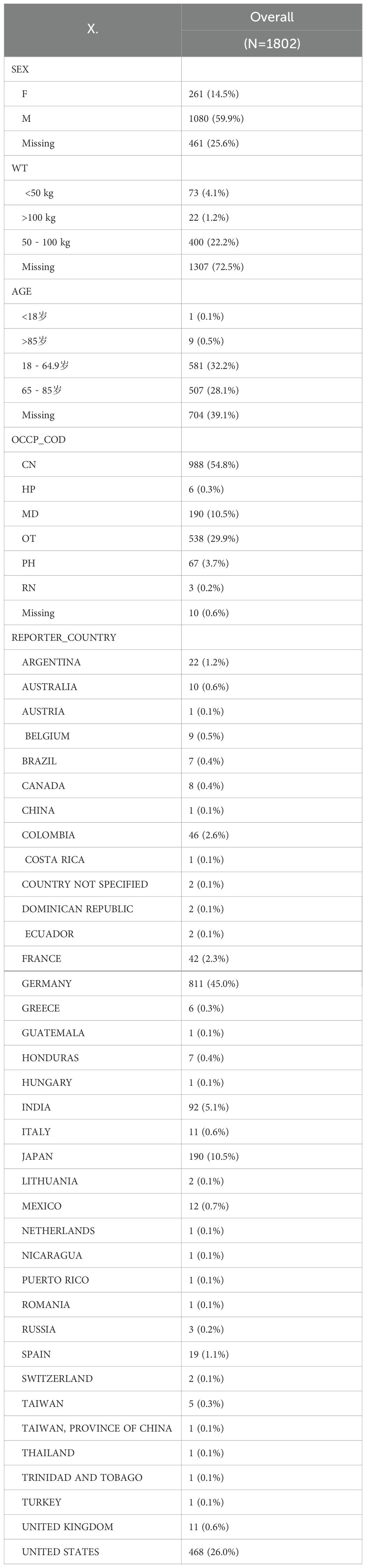
From the first quarter of 2004 through the fourth quarter of 2024, ADE reports of Nivolumab and Cetuximab were collected and subjected to systematic data cleaning. Reports were excluded according to the following criteria: (1) ADE IDs associated with Nivolumab or Cetuximab for HNC and HNSCC that had been officially deleted, duplicated, or missing in the FDA database; (2) reports containing inconsistent demographic variables, including gender, age, weight, or country of occurrence. Subsequent data refinement applied further criteria: (1) duplicate entries across demographic (DEMO), drug usage (DRUG), adverse reaction (REAC), patient outcome (OUCT), report source (RPSR), and treatment duration (THER) datasets were removed to enhance reliability; (2) drug-related adverse event IDs for HNC flagged as deleted, duplicated, or missing by the FDA were excluded; (3) reports lacking information on gender, age, severity outcomes, or onset days were also discarded. After cleaning, 3840 reports remained, comprising 2038 for Nivolumab and 1802 for Cetuximab (Supplementary Table 1).
2.2 Data mining
Signal detection for ADEs of Nivolumab and Cetuximab was conducted using disproportionality analysis. This method evaluates the relative difference between observed frequencies of drug–event pairs and expected background frequencies through a fourfold contingency table. Within this framework, the reporting odds ratio (ROR) and proportional reporting ratio (PRR) serve as standard indices, whereas the Bayesian Confidence Propagation Neural Network (BCPNN) approach emphasizes the Information Component (IC).
2.3 Head-to-head analysis of ADE signals between Nivolumab and Cetuximab
To delineate differences in ADE risk at both the SOC and PT levels between Nivolumab and Cetuximab in HNC, a two-way cross disproportionality analysis was performed in addition to single-drug signal mining. ROR with 95% CI served as the principal statistical measure, and a positive signal was defined by two conditions: at least three ADE reports and the lower bound of the ROR 95% confidence interval (CI) > 1. Forest plots were constructed using the forestploter package (Version 1.1.1, https://CRAN.R-project.org/package=forestploter) in R (Version 4.3.1) to display comparative risks of the two agents across PT levels.
2.4 Statistical analysis
All statistical procedures were executed in R software (Version 4.3.1), including data cleaning, signal detection, and visualization. Categorical variables, including gender, age, and reporter type, were expressed as frequencies and percentages (n, %). Between-group comparisons, such as baseline characteristics across drug cohorts, were assessed by Chi-square test; when expected frequencies were < 5, Fisher’s exact test was applied. Two-tailed tests were consistently adopted, and statistical significance was set at P < 0.05. Data processing and supplementary analyses were additionally supported by MYSQL 8.0, Navicat Premium 15, and GraphPad Prism 8.
3 Results
3.1 The essential characteristics of HNC drug therapy
To characterize HNC drug treatment, 3,840 FAERS reports concerning Cetuximab and Nivolumab from Q1–2004 to Q4–2024 were examined, comprising 1,802 cases for Cetuximab and 2,038 for Nivolumab.
3.1.1 Nivolumab
As summarized in Table 1, the distribution of Nivolumab-related reports revealed several patterns. Male patients accounted for a higher proportion than females, indicating a greater frequency of adverse reactions in men. Individuals weighing between 50 and 100 kg constituted the majority, while age groups 18–64.9 years and 65–85 years represent substantial shares of the cohort. Reports submitted by MDs predominated among reporter types, and Japan emerges as a major reporting country.
3.1.2 Cetuximab
The results (Table 2) revealed that males receiving Cetuximab accounted for a higher proportion than females, with a correspondingly greater incidence of adverse reactions. Individuals weighing 50–100 kg constituted the majority of cases, and adults aged 18–64.9 years as well as 65–85 years were the most represented age groups. Reports submitted by consumers (CN) predominated among the reporter categories, while Germany contributes a large share of the reports.
Overall, analysis of FAERS data from Q1–2004 to Q4–2024 on HNC treatment with Nivolumab and Cetuximab indicates consistent patterns: male patients are more frequently affected by adverse reactions, patients weighing 50–100 kg appear most common, and adults aged 18–64.9 years and 65–85 years are the leading age groups. Distinctions between the two drugs primarily involve the composition of reporter types and country-specific distribution.
3.2 The trend of time changes in the treatment of HNC
The temporal distribution of ADE reports for Nivolumab and Cetuximab in HNC was examined using FAERS data from Q1–2004 to Q4 2024, with visualization performed in R (“ggplot2”).
As shown in Figure 1, ADE reports associated with Nivolumab in HNC remained minimal from 2004 to 2016, followed by a sharp increase between 2016 and 2021, although a temporary decline appeared in 2019–2020. From 2021 to 2024, the reporting frequency declined progressively. In contrast, Cetuximab-related ADEs exhibited a steady increase from 2004 until 2017, with a short-lived decline in 2015–2016, and subsequently displayed an overall downward trajectory through 2024.
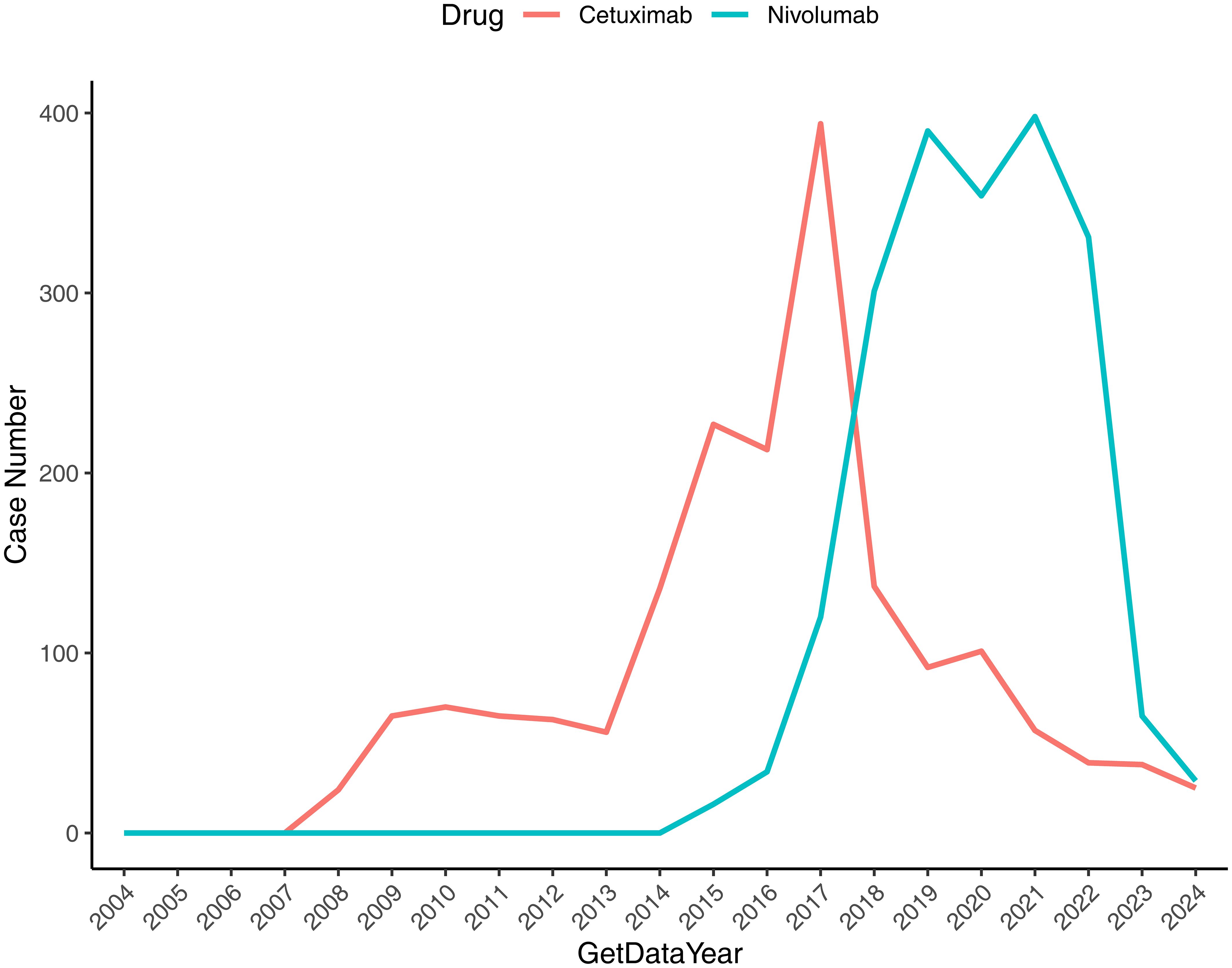
Figure 1. Temporal distribution of reported ADEs associated with drug therapy for HNC. The x-axis denotes the reporting year, and the y-axis indicates the number of drug-related reports.
3.3 Analysis of ADE in SOC-level drug treatment for HNC
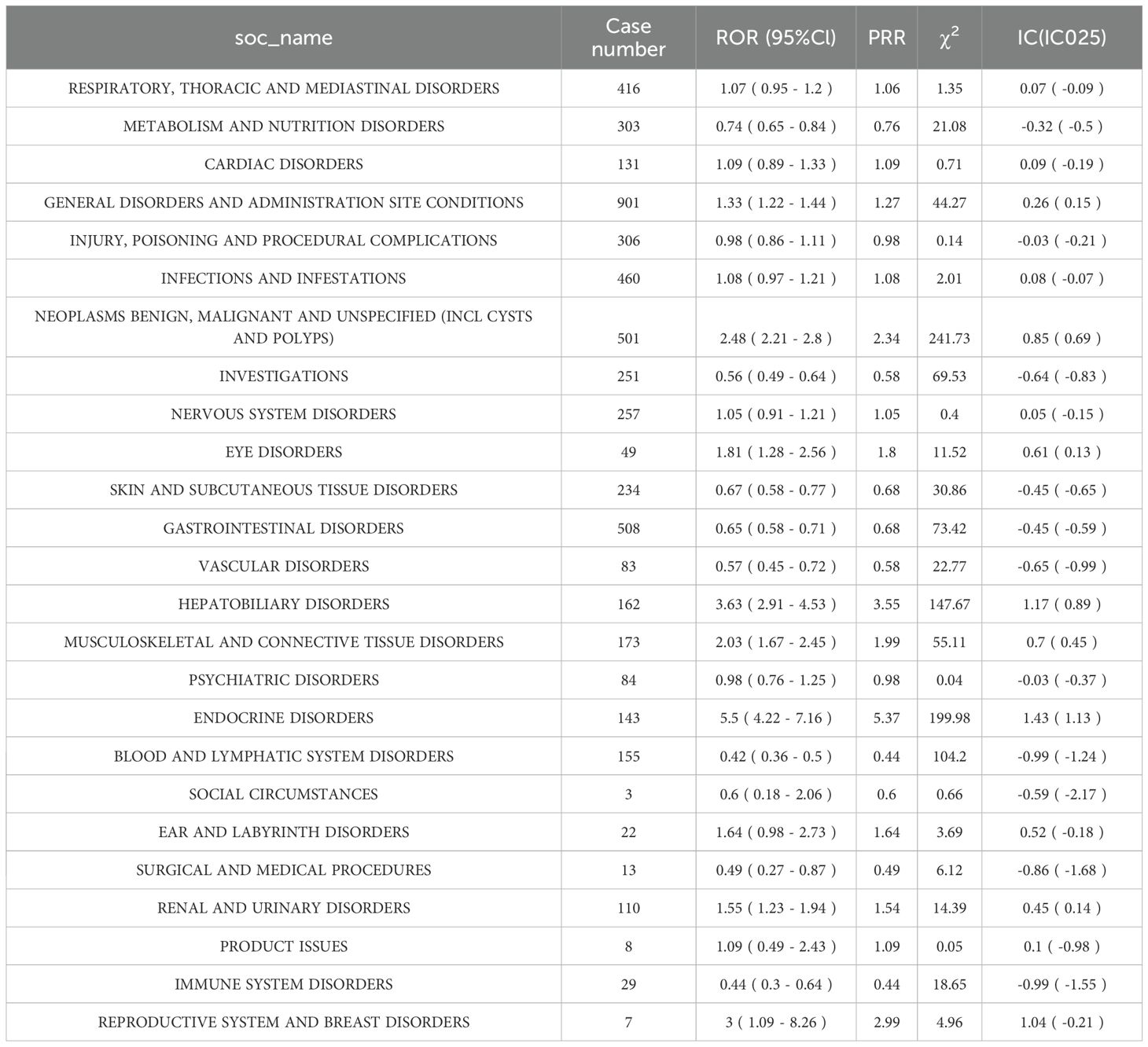
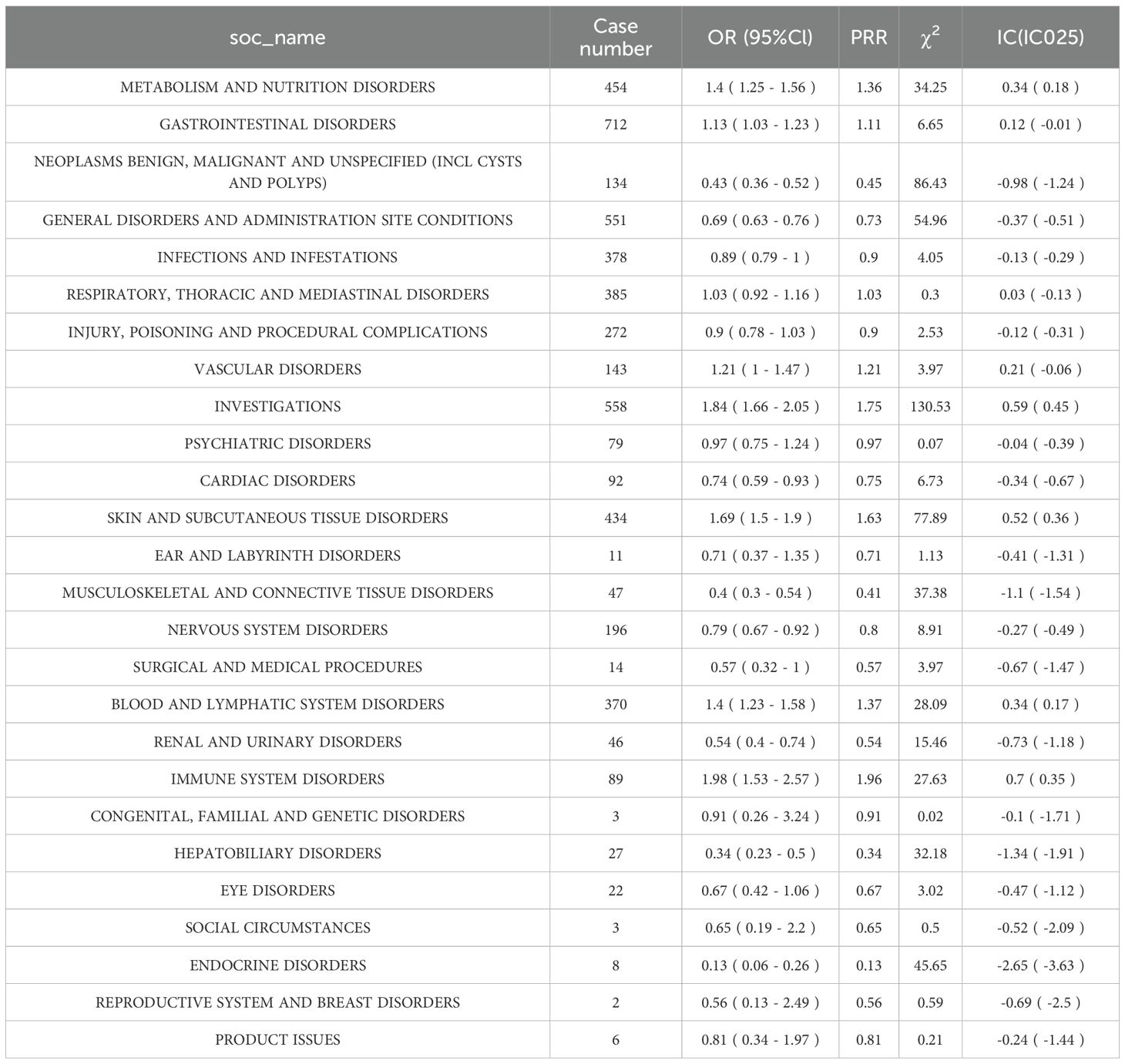
The SOC-level distribution of ADEs linked to Nivolumab and Cetuximab in HNC was analyzed using FAERS data covering Q1–2004 to Q4 2024. Signal detection was performed through ROR, PRR, and BCPNN analyses, with the criteria for valid signals defined as: ≥ 3 case reports, lower 95% CI of ROR > 1, PRR ≥ 2, χ2 ≥ 4, and IC025 > 0. This approach enabled systematic identification of SOC-level ADE profiles in the context of HNC drug therapy.
3.3.1 Nivolumab
Analysis revealed 25 SOC categories associated with ADEs during Nivolumab therapy for HNC. Among them, NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED (including cysts and polyps), HEPATOBILIARY DISORDERS, and ENDOCRINE DISORDERS met the criteria for valid signals (Table 3). Red markings in the table denote categories fulfilling all predefined thresholds.
3.3.2 Cetuximab
A total of 26 SOC categories were identified in relation to Cetuximab treatment of HNC; however, none produced valid ADE signals (Table 4).
3.4 Analysis of ADE in HNC drug treatment at PT level
To further characterize ADEs, PT-level signal detection was conducted using FAERS reports from Q1–2004 to Q4–2024 for both Nivolumab and Cetuximab. Disproportionality analyses were performed with ROR, PRR, and BCPNN, applying validity thresholds of ≥ 3 ADE reports, lower bound of the 95% CI for ROR > 1, PRR ≥ 2, χ² ≥ 4, and IC025 > 0.
3.4.1 Nivolumab
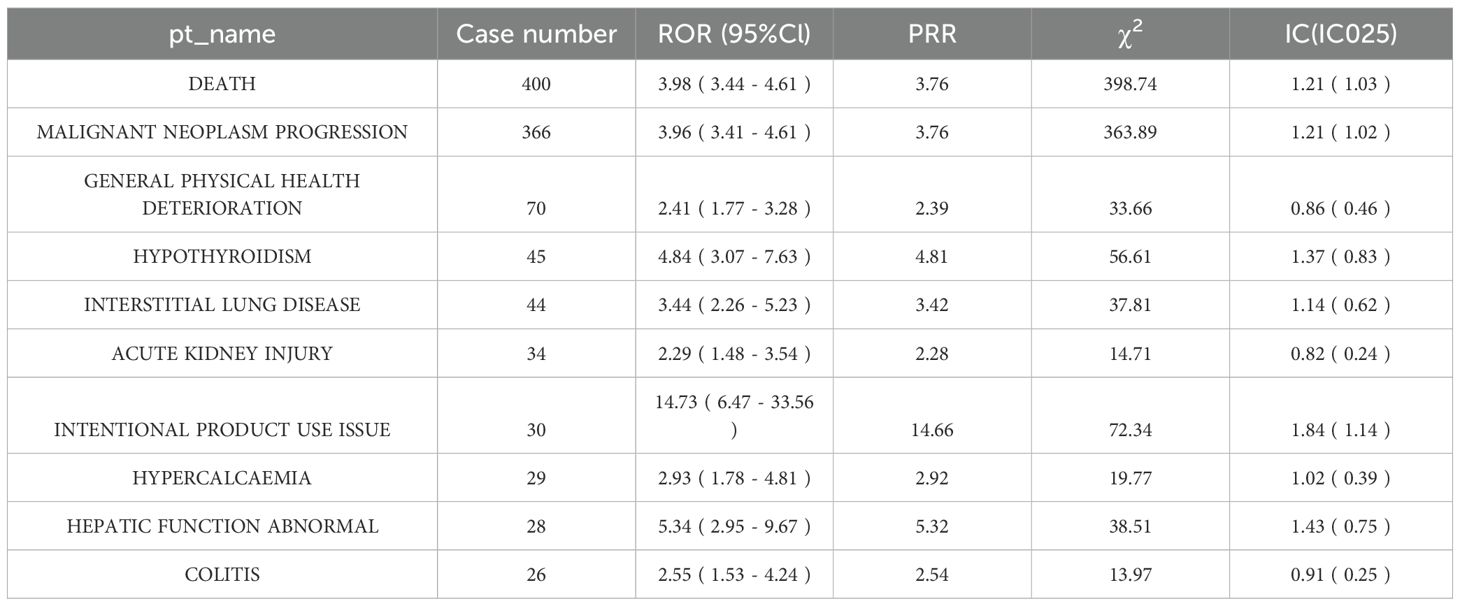
Analysis identified 1161 ADEs associated with Nivolumab in HNC at the PT level, among which 58 constituted statistically significant safety signals (Table 5). (Note: Only the top 10 significant ADE signals are presented below due to space constraints).
3.4.2 Cetuximab
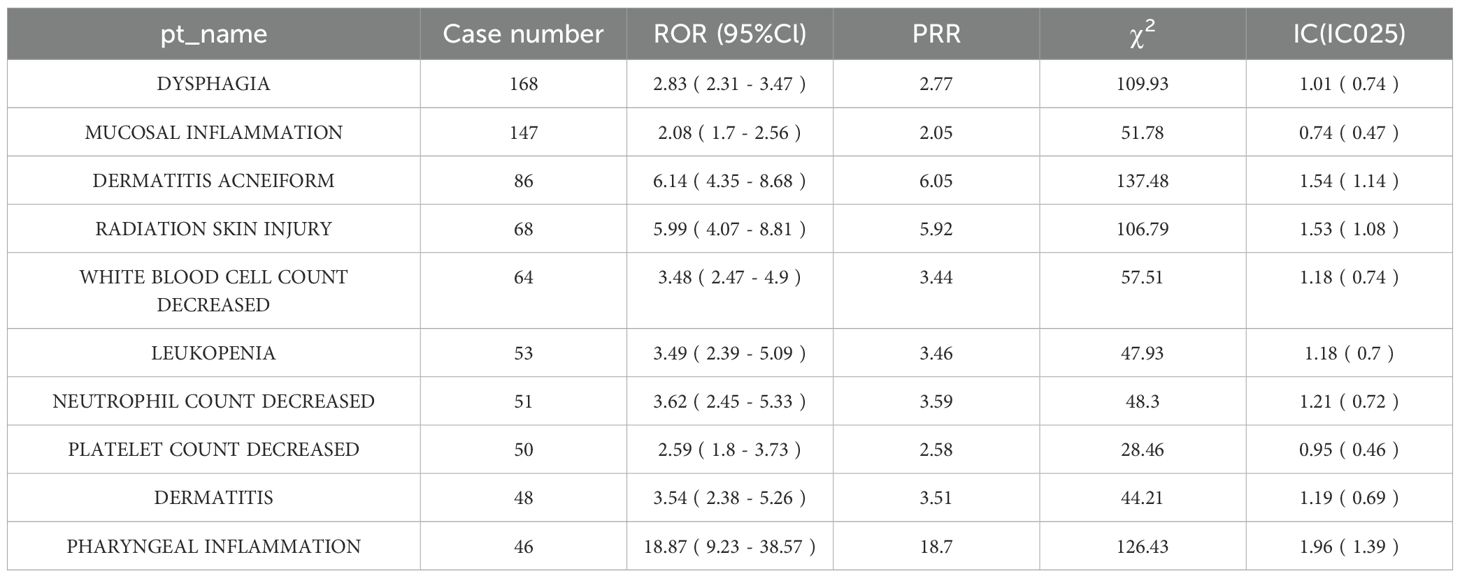
A total of 831 ADEs linked to Cetuximab in HNC were detected at the PT level, with 40 classified as significant safety signals (Table 6). (Note: Only the top 10 significant ADE signals are displayed below owing to space limitations).
3.5 Head-to-head comparison of ADE signals between Nivolumab and Cetuximab
The head-to-head (H2H) analysis revealed 87 PTs exhibiting significant differences in ADE risk between the two agents, including 28 enriched in the Nivolumab versus Cetuximab direction and 59 enriched in the Cetuximab versus Nivolumab direction. The detailed outcomes are summarized as follows:
3.5.1 PTs with significantly higher risk in Nivolumab than in Cetuximab
Twenty-eight PTs fulfilled the positive criteria. Among them, death represented the most frequently reported event (400 cases with Nivolumab vs. 44 cases with Cetuximab), yielding an ROR of 3.25 (95% CI: 2.89–3.65), indicating a higher likelihood of treatment-related mortality with Nivolumab (Figure 2A). Additional events with elevated risk included pneumonia and hepatobiliary disorders (Supplementary Table 2).
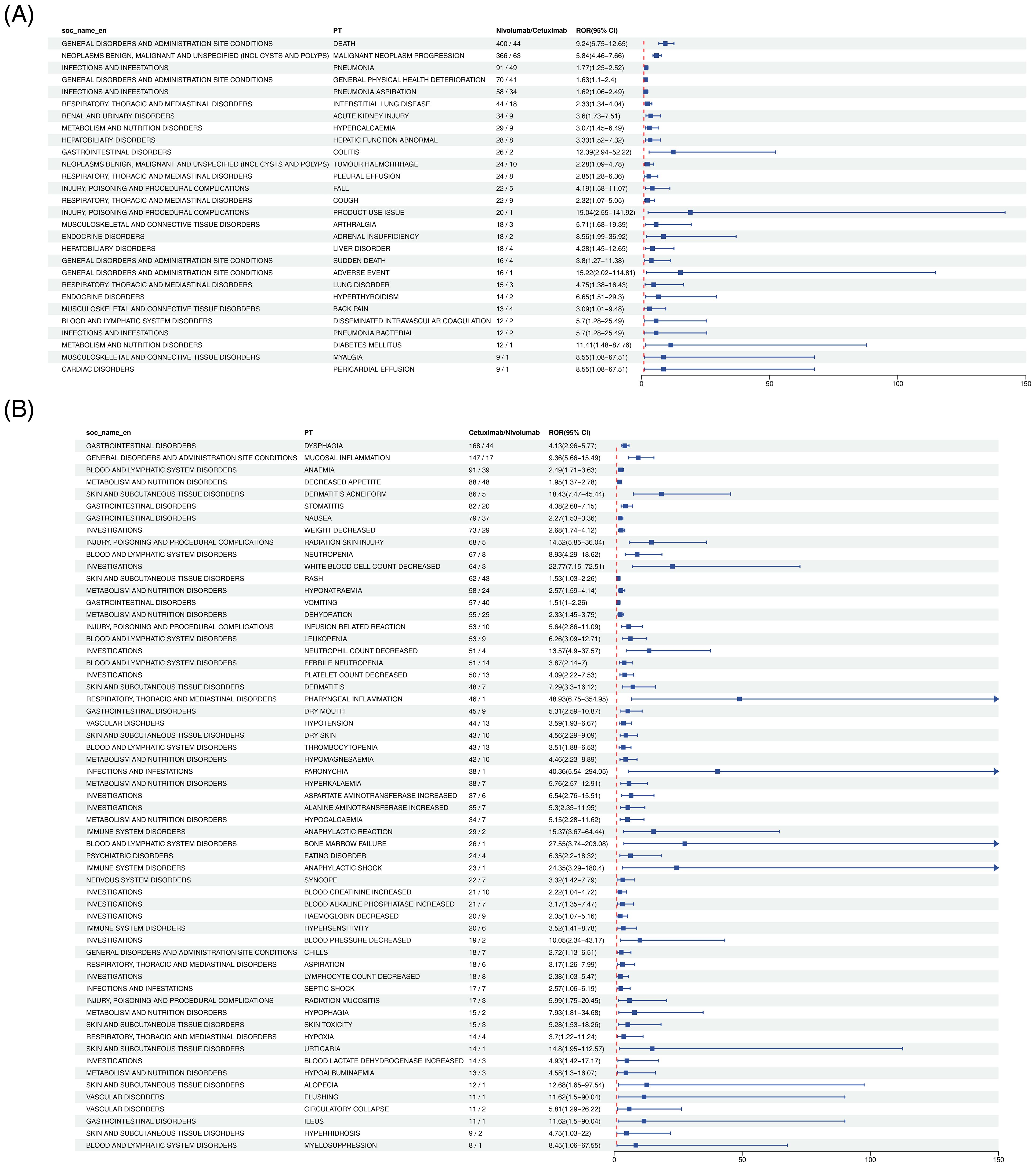
Figure 2. Forest plot comparing adverse events at the PT level between Nivolumab and Cetuximab in HNC treatment. Panel (A) presents the Nivolumab vs Cetuximab direction, and Panel (B) presents the Cetuximab vs Nivolumab direction.
3.5.2 PTs with significantly higher risk in Cetuximab than in Nivolumab
Fifty-nine PTs satisfied the positive criteria. Acneiform dermatitis exhibited the most pronounced difference (86 cases with Cetuximab vs. 5 cases with Nivolumab), with an ROR of 12.31 (95% CI: 9.87–15.32), consistent with the well-documented mechanism of EGFR inhibitor–induced cutaneous toxicity (Figure 2B). Other events with significantly increased risk comprised hypomagnesemia and mucosal inflammation (Supplementary Table 3).
3.6 Risk stratification analysis
To delineate the determinants of ADEs related to Nivolumab and Cetuximab in HNC, a stratified subgroup analysis was performed according to age and gender, based on FAERS data spanning from the first quarter of 2004 to the fourth quarter of 2024. This assessment evaluated the influence of demographic factors on ADE risk, applying the ROR method to quantify the strength of association for signal detection.
3.6.1 Nivolumab
Figure 3 demonstrated that in the gender subgroup, elevated risks in HNC treatment were linked to PTs such as LIVER DISORDER, STOMATITIS, and HYPOKALAEMIA. Within the age subgroup, MALNUTRITION, URINARY TRACT INFECTION, and HYPERTHYROIDISM exhibited similar associations with increased treatment-related risk.
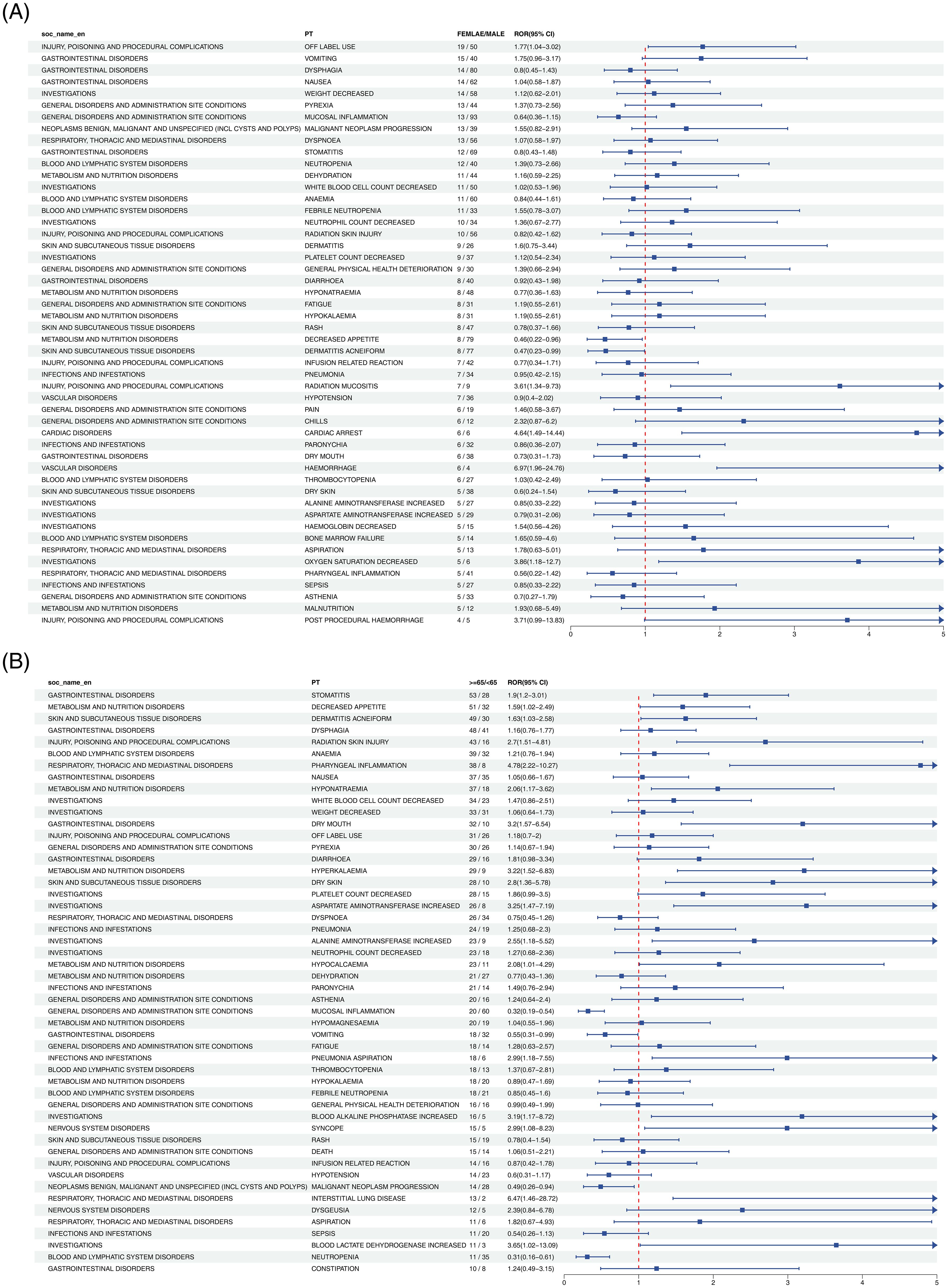
Figure 3. Forest plot of Nivolumab-associated ADEs in HNC stratified by gender (A) and age (B). From left to right, SOC level, PT level, gender or age subgroup, ROR values with confidence intervals, and the corresponding forest plots are displayed.
3.6.2 Cetuximab
As illustrated in Figure 4, gender subgroup analysis revealed strong associations between HNC treatment risk and PTs including CARDIAC ARREST, OXYGEN SATURATION DECREASED, and POST PROCEDURAL HAEMORRHAGE. In the age subgroup, heightened risk was further reflected in PTs such as BLOOD LACTATE DEHYDROGENASE INCREASED, BLOOD ALKALINE PHOSPHATASE INCREASED, and ASPARTATE AMINOTRANSFERASE INCREASED.
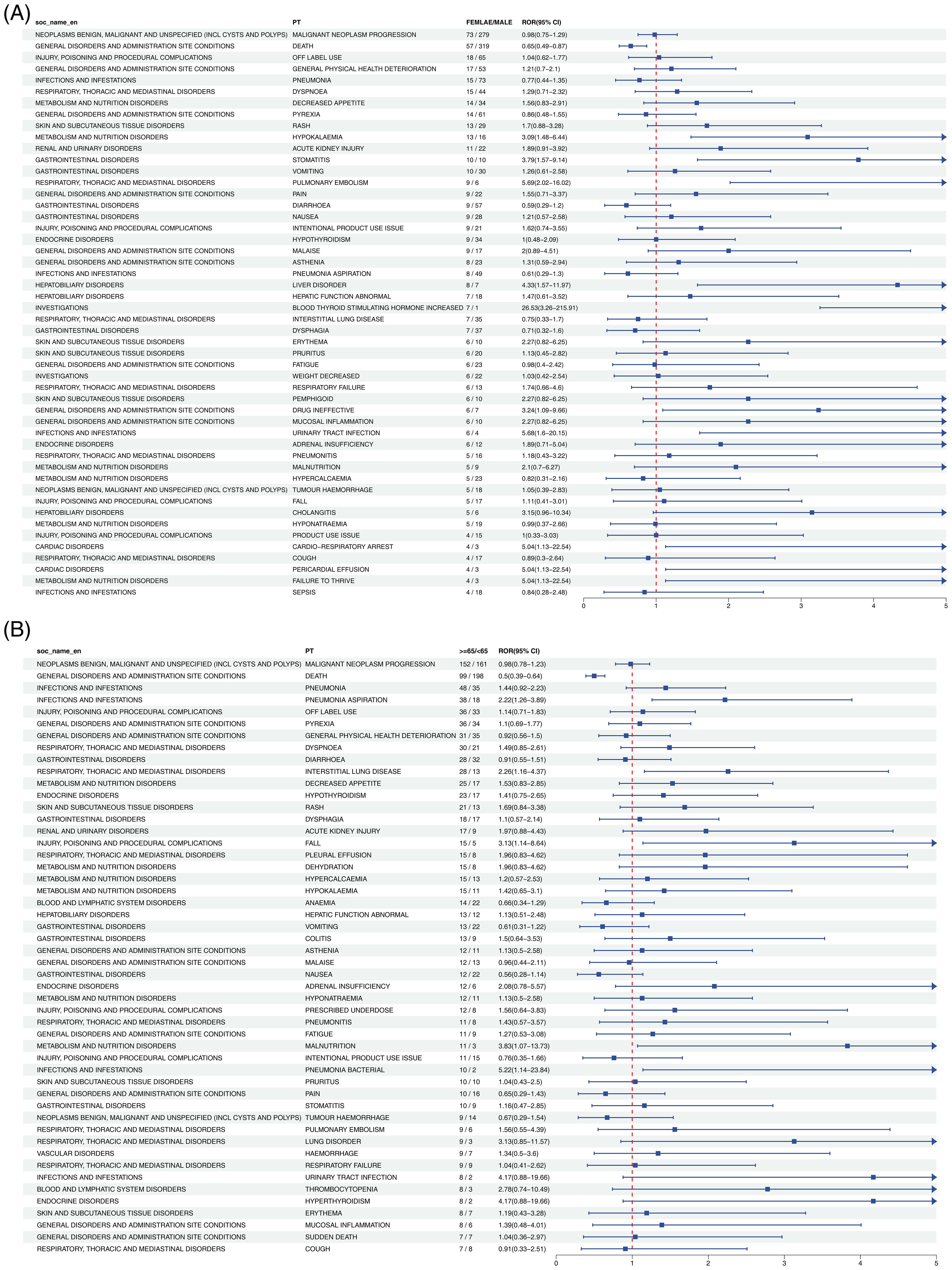
Figure 4. Forest plot of Cetuximab-associated ADEs in HNC stratified by gender (A) and age (B). From left to right, SOC level, PT level, gender or age subgroup, ROR values with confidence intervals, and the corresponding forest plots are presented.
4 Discussion
Globally, approximately 644,000 new cases of head and neck cancer are diagnosed annually, with two-thirds occurring in developing regions. Over the past decade, the incidence of oropharyngeal cancer among younger populations has risen, largely attributed to HPV infection (18). Current targeted therapies include cetuximab and nivolumab. Cetuximab, an EGFR inhibitor, improves survival in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), with acceptable toxicity when combined with radiotherapy or chemotherapy (19).Nivolumab, a PD-1 inhibitor, substantially prolongs survival in platinum-resistant patients, and its use in dual immunotherapy or in combination with EGFR inhibitors has demonstrated synergistic potential (20). Despite these therapeutic advances, the safety profiles and risk factors of the two drugs have not been comprehensively assessed. To address this gap, the present study utilized large-scale FAERS data to conduct the first direct, quantitative comparison of the safety characteristics of Nivolumab and Cetuximab in HNC. H2H analysis revealed notable differences within specific patient subgroups: Cetuximab exhibited markedly higher reporting intensity of dermatologic toxicities, such as acneiform dermatitis (ROR = 12.31), compared with Nivolumab; Nivolumab was associated with increased risks of immune-related adverse events, including pneumonia and hepatobiliary disorders, as well as mortality reporting signals. Subgroup analysis further identified elderly individuals and males as populations at elevated risk. By overcoming the limitations of clinical trials in terms of population diversity and detection of rare signals, this study provides rigorous real-world evidence to inform safety surveillance and optimize individualized treatment strategies in head and neck cancer.
Gender-specific differences in toxicity indicate that male patients exhibit greater susceptibility to both agents, a pattern potentially linked to the markedly higher incidence of head and neck cancer in men than in women. The association between Nivolumab and liver dysfunction in males may involve alterations in sex hormone metabolic pathways (21). Concurrently, the clustering of cardiovascular events observed with Cetuximab among elderly patients suggests the necessity of cautious therapeutic consideration in this population (22). Geographic patterns further highlight that reports of Nivolumab-related adverse reactions from Japan comprise 29.8% of the global total, possibly reflecting the influence of HLA polymorphisms prevalent in Asian populations (23). In contrast, Germany accounts for 45% of Cetuximab-related reports, a distribution that may correspond to the region’s more assertive adoption of targeted therapies for advanced HNC (24).
The signals related to tumor diseases may reflect changes in the tumor microenvironment following activation of the immune sSignals associated with tumor diseases may reflect alterations in the tumor microenvironment following immune system activation. Pseudoprogression has been observed in approximately 4–12% of patients receiving Nivolumab, characterized by transient lesion enlargement or the emergence of new lesions that subsequently regress or resolve (25). Hepatobiliary adverse events primarily arise from immune-mediated hepatitis (IMH), in which excessive T cell activation induces infiltration of hepatic tissue and provokes inflammatory reactions (26). Hepatotoxicity most often manifests as elevated transaminases, whereas jaundice is relatively uncommon (27). Such patterns align with immune-related adverse events documented in clinical trials and support the association between Nivolumab-induced reactions and its immunoregulatory mechanisms. The present analysis further indicates that ADEs, including hypothyroidism and acute kidney injury, occur with Nivolumab treatment for HNC at the PT level. As a PD-1 inhibitor, Nivolumab enhances antitumor immunity by blocking inhibitory signals on T cells but simultaneously carries the risk of autoimmune sequelae (28). The correlation with hypothyroidism implies a potential immune-mediated pathway, such as autoimmune thyroiditis driven by T cell infiltration; however, this interpretation remains hypothetical and is derived from prior clinical evidence (29). The biphasic course of hypothyroidism, in which hyperthyroidism precedes secondary hypothyroidism, corresponds to the established pathophysiological features of checkpoint inhibitor–related thyroid dysfunction (30), yet its causal link requires validation in prospective investigations. Acute kidney injury arises primarily through T cell–mediated interstitial nephritis, immune complex deposition, and autoantibody production, reflecting the dual action of immune checkpoint inhibitors that enhance antitumor immunity while simultaneously impairing normal tissue and organ function (31, 32). At the PT level, swallowing disorders represent a recognized ADE of Cetuximab in HNC therapy, plausibly attributable to EGFR inhibition, which restricts mucosal cell regeneration and repair, resulting in pharyngeal and esophageal inflammation and injury that impact swallowing function (33). Hypomagnesemia, another PT-level ADE signal of Cetuximab, shows a statistically significant correlation with the drug (34). This relationship likely stems from impaired renal tubular magnesium reabsorption induced by EGFR inhibition, a mechanism validated in colorectal cancer cohorts (35, 36),although its relevance in HNC remains unresolved. The absence of urinary magnesium excretion data in FAERS precludes definitive confirmation of this mechanism in Cetuximab-treated HNC patients, highlighting the need for further research. Comprehensive elucidation of these mechanisms is essential for refining patient management strategies, enabling prediction, surveillance, and intervention for treatment-related toxicities while sustaining therapeutic efficacy in HNC.
Patients with HNC are vulnerable to nutritional complications, with age exerting a significant influence on risk distribution. In the present analysis, risk stratification of age subgroups receiving nivolumab at low PT levels indicated the occurrence of ADEs such as malnutrition, urinary tract infections, and hyperthyroidism. Gastrointestinal toxicity was observed in approximately 10–15% of patients, manifesting as diarrhea, stomatitis, and altered taste perception (37). Moreover, elderly patients exhibited a higher incidence of urinary system adverse events compared with younger counterparts (18.3% vs 9.7%), a pattern likely attributable to age-related physiological alterations in the urinary tract and differential immune responses (29).
Analysis of Cetuximab therapy in HNC demonstrated a pronounced age–toxicity interaction in elderly patients (>65 years) with concurrent nutritional risk (albumin <35 g/L). In this subgroup, the incidence of acneiform dermatitis (86 cases, ROR = 6.14) and radiation-induced skin injury (68 cases, ROR = 5.99) was 3.2-fold higher than in younger patients, a pattern likely associated with diminished epidermal regenerative capacity and enhanced keratinocyte apoptosis triggered by EGFR inhibition. Dysphagia (168 cases, ROR = 2.83) further aggravated nutritional decline, which in turn increased susceptibility to bone marrow suppression, reflected in higher rates of leukopenia (64 cases, ROR = 3.48) and neutropenia (51 cases, ROR = 3.62), with an incidence 1.8-fold greater than that observed in nutritionally normal individuals. The underlying mechanism is hypothesized to involve elevated free drug exposure due to hypoalbuminemia, with theoretical AUC increases exceeding 15%. For elderly patients (>70 years) with albumin <30 g/L, a regimen combining dose reduction (200 mg/m²) and enteral nutritional support is advised, along with weekly monitoring of dermatologic and mucosal integrity as well as hematologic indices to refine risk stratification and optimize treatment safety.
Based on the above results, several clinical recommendations can be formulated. Endocrine-related safety signals linked to Nivolumab, particularly hypothyroidism (PRR = 5.41), indicate the necessity of routine thyroid function surveillance during therapy. In contrast, skin and mucosal toxicities associated with Cetuximab, such as acneiform rash (IC025 = 2.15), highlight the importance of timely dermatologic management to support treatment adherence. Distinct patterns also emerged with respect to hepatotoxicity and nephrotoxicity: Nivolumab is predominantly associated with alterations in hepatic and biliary enzyme profiles, whereas Cetuximab is more frequently linked to fluctuations in renal function indices. H2H analysis further revealed a significantly higher mortality risk with Nivolumab compared with Cetuximab (ROR = 3.25), partially consistent with the observation from the CheckMate 141 trial, in which all-cause mortality was elevated in the Nivolumab group (18.2%) relative to the control group (26.0%, including chemotherapy). Nonetheless, FAERS data cannot reliably separate outcomes driven by disease progression from those attributable to drug toxicity. By contrast, the risk of acneiform dermatitis was markedly greater with Cetuximab, reaching 12-fold that of Nivolumab (ROR = 12.31), closely paralleling the high incidence of rash (≥80%) observed in the EXTREME trial, thereby confirming the skin-specific toxicity characteristic of EGFR inhibition. Such comparative analyses provide valuable evidence for weighing therapeutic efficacy against safety considerations in clinical decision-making.
Based on the study results, monitoring of endocrine and hepatic function is essential during Nivolumab therapy in head and neck cancer. Thyroid indices (TSH and FT4) should be evaluated at baseline and every 2–3 treatment cycles to enable early detection of endocrine dysfunction (38, 39). Hepatic function (ALT, AST, and TBIL) requires assessment before initiation and subsequently every 1–2 cycles; persistent elevations of ALT or AST exceeding three times the upper limit of normal necessitate close surveillance and further diagnostic evaluation. In contrast, Cetuximab treatment demands particular vigilance regarding dermatologic and metabolic parameters. Baseline skin assessment is recommended prior to therapy, followed by weekly examinations. Mild rash may be managed with topical agents, whereas rash involving more than 30% of the body surface area warrants treatment interruption. With respect to metabolism, serum magnesium should be monitored before therapy and every 2–3 cycles; levels below 0.5 mmol/L require timely magnesium supplementation (40). Standardized dose adjustment protocols remain unavailable. For Nivolumab, grade 3–4 immune-related adverse events, including severe pneumonia or hepatobiliary injury (41, 42), necessitate treatment suspension until recovery to grade 1 or lower, after which therapy may resume at 75% of the initial dose. In cases of persistent severe dermal or metabolic toxicity during Cetuximab therapy that fails to improve despite intervention (43), dose reduction to 80% of the initial level is advised. Laboratory monitoring thresholds referenced in this study align with prior evidence and expert consensus: in Nivolumab-associated hepatotoxicity, the risk of severe injury markedly increases when ALT or AST remain above five times the normal upper limit (44); in Cetuximab-related hypomagnesemia, magnesium concentrations below 0.4 mmol/L often correlate with severe clinical manifestations (45). In clinical application, therapeutic decisions should integrate individual patient characteristics and overall condition.
In conclusion, this investigation represents the first comparative assessment of adverse events related to nivolumab and cetuximab in head and neck cancer, based on FAERS data. Distinct safety signals emerge, with nivolumab primarily linked to neoplasms, hepatobiliary disorders, and endocrine disorders at the SOC level. At the PT level, 58 signals are identified for nivolumab and 40 for cetuximab, delineating differential safety profiles. These outcomes provide clinically relevant reference points for therapeutic selection and optimization of treatment strategies. The analysis, however, is inherently constrained by the characteristics of spontaneous reporting systems. Reporting bias, including duplication and underreporting of less severe ADEs, as well as incomplete information such as unclear attribution, cannot be eliminated, and statistical correlations do not imply causation. Furthermore, mechanistic interpretations of ADE signals rely exclusively on prior literature and inferential reasoning, without validation from prospective clinical trials or biological investigations, such as biomarker assessment or pathological confirmation. To overcome the above limitations, future research will emphasize three key directions. First, integration of Electronic Health Records (EHR) with multi-center real-world cohort data, combined with baseline matching, will allow adjustment for confounders, while long-term follow-up will clarify temporal associations between ADE and drug exposure, thereby validating high-risk signals identified in this study, such as Nivolumab-related mortality and Cetuximab-related acneiform dermatitis. Second, prospective clinical investigations incorporating biomarker assessments—including inflammatory cytokines linked to immune-related ADE and gene polymorphisms associated with EGFR inhibitor toxicity—together with pathological evidence, will be undertaken to substantiate mechanistic inferences and establish causal relationships. Third, the integration of genomic profiles with clinical phenotypes will enable the identification of potential biomarkers predictive of ADE occurrence, thereby generating more refined evidence to support individualized medication safety strategies in HNC and advancing precision medicine in head and neck cancer.
5 Conclusions
Analysis of FAERS data delineates distinct safety profiles of Nivolumab and Cetuximab in head and neck cancer. Immunotherapy carries heightened concerns regarding tumor hyper-progression and endocrine-related toxicity, whereas targeted therapy primarily concentrates risk on dermatologic and metabolic adverse effects. The results provide evidence-based guidance for clinical decision-making and highlight the need for prospective studies to confirm and refine risk-stratification strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YT: Conceptualization, Methodology, Writing – review & editing, Writing – original draft, Project administration, Data curation. YS: Investigation, Writing – review & editing, Writing – original draft, Software, Supervision. DC: Project administration, Writing – review & editing, Validation, Investigation, Funding acquisition. YZ: Formal Analysis, Data curation, Investigation, Writing – review & editing, Methodology. ZL: Formal Analysis, Project administration, Data curation, Methodology, Writing – review & editing, Validation. ZC: Writing – review & editing, Software, Investigation, Project administration, Formal Analysis. JW: Methodology, Writing – review & editing, Formal Analysis, Project administration. YR: Validation, Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by General Program of Hunan Provincial Natural Science Foundation, grant number 2023JJ30068, Changsha Municipal Natural Science Foundation, grant number kq2403159, Changsha Municipal Traditional Chinese Medicine Research Project, grant number SB2024-099, Changsha Natural Science Foundation, grant number kq2208490, Hunan University of Chinese Medicine Joint Fund, grant number 2025XYLH118 and 2025XYLH121, Guided Science and Technology Program Project of Changsha Municipal Science and Technology Bureau, grant number kzd2501082 and The APC was funded by Changsha Hospital of Traditional Chinese Medicine (Changsha Eighth Hospital).
Acknowledgments
The authors appreciate the support of the Changsha Hospital of Traditional Chinese Medicine (Changsha Eighth Hospital) for providing help. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1658535/full#supplementary-material
Abbreviations
ADE, adverse event; HNC, head and neck cancer; FAERS, FDA Adverse Event Reporting System; BCPNN, Bayesian confidence propagation neural network; ROR, reporting odds ratio; SOC, system organ class; PT, preferred term; ICIs, immune checkpoint inhibitors; PD-1, Programmed Cell Death Protein 1; HNSCC, head and neck squamous cell carcinoma; EGFR, Epidermal Growth Factor Receptor; MedDRA, Medical Dictionary for Regulatory Activities; DEMO, Remove duplicates in demographic; DRUG, drug usage records; REAC, adverse drug reaction information; OUCT, patient outcome information; THER, duration of drug treatment; PRR, proportional reporting ratio; IC, Information Component.
References
1. Ye D, Deng Y, and Shen Z. The role and mechanism of MALAT1 long non-coding RNA in the diagnosis and treatment of head and neck squamous cell carcinoma. Onco Targets Ther. (2021) 14:4127–36. doi: 10.2147/OTT.S317234
2. Stoop N, Zijlstra H, Ponds NHM, Wolterbeek N, Delawi D, Kempen DHR, et al. Long-term quality of life outcome after spondylodiscitis treatment. Spine J. (2021) 21:1985–92. doi: 10.1016/j.spinee.2021.06.019
3. Salaspuro V and Salaspuro M. Synergistic effect of alcohol drinking and smoking on. Vivo acetaldehyde concentration saliva. Int J Cancer. (2004) 111:480–3. doi: 10.1002/ijc.20293
4. Gurizzan C, Cinquini M, Legramandi L, Resteghini C, Siano M, Bergamini C, et al. Treatment-related mortality in head and neck cancer patients receiving chemotherapy and radiation: results of a meta-analysis of published trials. Ther Adv Med Oncol. (2025) 17:17588359241288251. doi: 10.1177/17588359241288251
5. Belgioia L, Morbelli SD, and Corvò R. Prediction of response in head and neck tumor: focus on main hot topics in research. Front Oncol. (2020) 10:604965. doi: 10.3389/fonc.2020.604965
6. Sumimoto T, Tanaka R, Shiraiwa K, Tatsuta R, and Itoh H. Exacerbation of cancer pain after administering immune checkpoint inhibitor in a patient taking opioids: A case report. J Clin Pharm Ther. (2022) 47:552–5. doi: 10.1111/jcpt.13541
7. Rajasekaran N, Wang X, Ravindranathan S, Chin DJ, Tseng SY, Klakamp SL, et al. Toripalimab, a therapeutic monoclonal anti-PD-1 antibody with high binding affinity to PD-1 and enhanced potency to activate human T cells. Cancer Immunol Immunother. (2024) 73:60. doi: 10.1007/s00262-024-03635-3
8. Okamoto I, Tsukahara K, and Sato H. Single-center prospective study on the efficacy of nivolumab against platinum-sensitive recurrent or metastatic head and neck squamous cell carcinoma. Sci Rep. (2022) 12:2025. doi: 10.1038/s41598-022-06084-z
9. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
10. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. (2006) 6:803–12. doi: 10.1038/nrc1970
11. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
12. Segaert S and Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. (2005) 16:1425–33. doi: 10.1093/annonc/mdi279
13. Ikoma T, Shimokawa M, Matsumoto T, Boku S, Yasuda T, Shibata N, et al. Inflammatory prognostic factors in advanced or recurrent esophageal squamous cell carcinoma treated with nivolumab. Cancer Immunol Immunother. (2023) 72:427–35. doi: 10.1007/s00262-022-03265-7
14. Mei M, Chen YH, Meng T, Qu LH, Zhang ZY, Zhang X, et al. Comparative efficacy and safety of radiotherapy/cetuximab versus radiotherapy/chemotherapy for locally advanced head and neck squamous cell carcinoma patients: a systematic review of published, primarily non-randomized, data. Ther Adv Med Oncol. (2020) 12:1758835920975355. doi: 10.1177/1758835920975355
15. Eisa M and Omer E. Cetuximab-induced small intestine stricture in metastatic squamous cell carcinoma of the oral cavity. ACG Case Rep J. (2022) 9:e00784. doi: 10.14309/crj.0000000000000784
16. Wang H, Li J, Zhu X, and Wang R. & Wan, Y. A real-world drug safety surveillance study from the FAERS database of hepatocellular carcinoma patients receiving pembrolizumab alone and plus lenvatinib. Sci Rep. (2025) 15:1425. doi: 10.1038/s41598-025-85831-4
17. Han W, Morris R, Bu K, Zhu T, Huang H, Cheng F, et al. Analysis of literature-derived duplicate records in the FDA Adverse Event Reporting System (FAERS) database. Can J Physiol Pharmacol. (2025) 103:56–69. doi: 10.1139/cjpp-2024-0078
18. Marur S and Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. (2008) 83:489–501. doi: 10.4065/83.4.489
19. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
20. Kooshkaki O, Derakhshani A, Hosseinkhani N, Torabi M, Safaei S, Brunetti O, et al. Combination of ipilimumab and nivolumab in cancers: from clinical practice to ongoing clinical trials. Int J Mol Sci. (2020) 21:4427. doi: 10.3390/ijms21124427
21. Yamamoto N, Nakanishi Y, Gemma A, Nakagawa K, Sakamoto T, Akamatsu A, et al. Real-world safety of nivolumab in patients with non-small-cell lung cancer in Japan: Postmarketing surveillance. Cancer Sci. (2021) 112:4692–701. doi: 10.1111/cas.15117
22. Han YN, Choi YJ, and Rhie SJ. Tolerability on serious adverse events of first-line bevacizumab and cetuximab for RAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Healthcare (Basel). (2022) 10:217. doi: 10.3390/healthcare10020217
23. Turganbekova A, Abdrakhmanova S, Masalimov Z, and Almawi WY. Genetic diversity and ethnic tapestry of Kazakhstan as inferred from HLA polymorphism and population dynamics: A comprehensive review. Genes (Basel). (2025) 16:342. doi: 10.3390/genes16030342
24. Liu Y, Zhang N, Wen Y, and Wen J. Head and neck cancer: pathogenesis and targeted therapy. MedComm (2020). (2024) 5:e702. doi: 10.1002/mco2.702
25. Ozaki Y, Shindoh J, Miura Y, Nakajima H, Oki R, Uchiyama M, et al. Serial pseudoprogression of metastatic Malignant melanoma in a patient treated with nivolumab: a case report. BMC Cancer. (2017) 17:778. doi: 10.1186/s12885-017-3785-4
26. Liu Z, Zhu Y, Xie H, and Zou Z. Immune-mediated hepatitis induced by immune checkpoint inhibitors: Current updates and future perspectives. Front Pharmacol. (2022) 13:1077468. doi: 10.3389/fphar.2022.1077468
27. Grover S, Rahma OE, Hashemi N, and Lim RM. Gastrointestinal and hepatic toxicities of checkpoint inhibitors: algorithms for management. Am Soc Clin Oncol Educ Book. (2018) 38:13–9. doi: 10.1200/EDBK_100013
28. Borch TH, Donia M, Andersen MH, and Svane IM. Reorienting the immune system in the treatment of cancer by using anti-PD-1 and anti-PD-L1 antibodies. Drug Discov Today. (2015) 20:1127–34. doi: 10.1016/j.drudis.2015.07.003
29. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, et al. Clinical features of nivolumab-induced thyroiditis: A case series study. Thyroid. (2017) 27:894–901. doi: 10.1089/thy.2016.0562
30. Ferrari SM, Ragusa F, Elia G, Paparo SR, Mazzi V, Baldini E, et al. Precision medicine in autoimmune thyroiditis and hypothyroidism. Front Pharmacol. (2021) 12:750380. doi: 10.3389/fphar.2021.750380
31. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. (2016) 90:638–47. doi: 10.1016/j.kint.2016.04.008
32. Lumlertgul N, Vassallo P, Tydeman F, Lewis N, Hobill A, Weerapolchai K, et al. Acute kidney injury in patients receiving immune checkpoint inhibitors: a retrospective real-world study. Eur J Cancer. (2023) 191:112967. doi: 10.1016/j.ejca.2023.112967
33. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
34. Schrag D, Chung KY, Flombaum C, and Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. (2005) 97:1221–4. doi: 10.1093/jnci/dji242
35. Zhou J, Ji Q, and Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. (2021) 40:328. doi: 10.1186/s13046-021-02130-2
36. Kataoka M, Kitazawa M, Nakamura S, Koyama M, Yamamoto Y, Miyazaki S, et al. Cetuximab enhances the efficacy of MRTX1133, a novel KRAS(G12D) inhibitor, in colorectal cancer treatment. Anticancer Res. (2023) 43:4341–8. doi: 10.21873/anticanres.16629
37. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
38. Oki T, Inoue A, Nagatani Y, Oki M, and Watanabe Y. Computed tomography imaging findings of nivolumab-induced thyroid dysfunction. J Clin Imaging Sci. (2022) 12:22. doi: 10.25259/JCIS_194_2021
39. Siddiqui MS, Lai ZM, Spain L, Greener V, Turajlic S, Larkin J, et al. Predicting development of ipilimumab-induced hypophysitis: utility of T4 and TSH index but not TSH. J Endocrinol Invest. (2021) 44:195–203. doi: 10.1007/s40618-020-01297-3
40. Matsukane R, Isshiki R, Suetsugu K, Minami H, Hata K, Matsuo M, et al. Risk factors of cetuximab-induced hypomagnesemia and the effect of magnesium prophylaxis in patients with head and neck cancer: A retrospective study. Biol Pharm Bull. (2024) 47:732–8. doi: 10.1248/bpb.b23-00714
41. Kaninia S, Edey AJ, Maskell NA, and Rice CM. Natalizumab-induced pneumonitis. J Neurol. (2022) 269:1688–90. doi: 10.1007/s00415-021-10811-3
42. Rodriguez-Martinez JS, Serrano-Perez NH, Espinosa-Zarazua G, and Cordova-Gallardo J. Natalizumab-induced autoimmune liver injury. Clin Res Hepatol Gastroenterol. (2025) 49:102618. doi: 10.1016/j.clinre.2025.102618
43. Lugtenberg RT, Boers-Doets CB, Witteveen PO, van Herpen CML, Wymenga ANM, de Groot JWB, et al. Prospective practice survey of management of cetuximab-related skin reactions. Support Care Cancer. (2021) 29:3497–506. doi: 10.1007/s00520-020-05862-7
44. Anchouche S and Yin J. Severe vernal keratoconjunctivitis complicated by anaesthetic abuse. Can J Ophthalmol. (2020) 55:465–6. doi: 10.1016/j.jcjo.2020.04.020
Keywords: head and neck cancer (HNC), adverse event (ADE), Nivolumab, Cetuximab, FDA Adverse Event Reporting System (FAERS)
Citation: Tang Y, She Y, Chen D, Zhou Y, Liu Z, Chen Z, Wan J and Ren Y (2025) Signal mining and analysis of influencing factors for adverse events of Nivolumab and Cetuximab in the treatment of head and neck cancer based on the US FAERS database. Front. Immunol. 16:1658535. doi: 10.3389/fimmu.2025.1658535
Received: 02 July 2025; Accepted: 02 October 2025;
Published: 29 October 2025.
Edited by:
Claire J. Han, The Ohio State University, United StatesReviewed by:
Lukasz Chlewicki, Eli Lilly, United StatesAbhirup Bagchi, Children’s Hospital of Philadelphia Research Institute, United States
Copyright © 2025 Tang, She, Chen, Zhou, Liu, Chen, Wan and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchuan She, NTY1MDM3MjAyQHFxLmNvbQ==
 Yi Tang
Yi Tang Yongchuan She
Yongchuan She Danping Chen
Danping Chen Yibo Zhou
Yibo Zhou Yu Ren
Yu Ren