- Department of Pharmacy, Eastern Hepatobiliary Surgery Hospital, Naval Medical University, Shanghai, China
Background: The IMforte trial demonstrated that lurbinectedin combined with atezolizumab (LU-AT) as a first-line regimen offers clinical advantages over atezolizumab alone (AT) in patients with extensive-stage small-cell lung cancer (ES-SCLC). However, given the high costs of lurbinectedin and atezolizumab, the cost-effectiveness of LU-AT relative to AT remains uncertain. This study aims to assess the cost-effectiveness of LU-AT as a first-line treatment for ES-SCLC within the context of China’s and the United States’ healthcare system.
Methods: A partitioned survival analysis (PartSA) model was employed to assess the cost-effectiveness of LU-AT as a first-line treatment for ES-SCLC. Clinical efficacy data were sourced from the IMforte trial. Drug costs were based on national tender prices, while other costs and utility values were derived from the literature. Outcomes included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to assess the robustness of the model.
Results: The combination regimen of lurbinectedin plus atezolizumab yielded an additional 0.21 QALYs compared with atezolizumab monotherapy, leading to an ICER of $374,167.43 per QALY in China and $1,071,237.82 per QALY in the USA, both beyond the willing-to-pay threshold ($40,365.00/QALY in China and $150,000.00/QALY in the USA). The utility of progression-free survival (PFS), the cost of lurbinectedin, body surface area (BSA), and the cost of atezolizumab are the four most influential factors in both China and the United States. Across all sensitivity analyses, the outcomes generated by the models remained robust. At a willingness-to-pay threshold of $40,365 and $150,000 per QALY, the probability of LU-AT being cost-effective relative to AT was 0% in China and USA.
Conclusion: Within the framework of China’s and the United States’ healthcare system, LU-AT is unlikely to represent a cost-effective first-line treatment for ES-SCLC.
1 Introduction
Small-cell lung cancer (SCLC) is a highly aggressive malignancy, comprising approximately 15% of all lung cancer cases, with a 5-year survival rate of under 7% (1). Characterized by rapid cell proliferation and early metastasis, it is associated with a poor prognosis. Extensive-stage SCLC (ES-SCLC) accounts for 70% of all SCLC cases (2–4). For decades, etoposide-platinum chemotherapy has been the standard treatment for ES-SCLC. However, while initial response rates are high, most patients develop resistance to the therapy (1). In 2019, immune checkpoint inhibitors (ICIs) revolutionized the treatment landscape for ES-SCLC, with atezolizumab combined with carboplatin and etoposide emerging as the new first-line regimen (5). Despite the improved efficacy of first-line ICI plus platinum-based chemotherapy, most patients eventually relapse, and survival outcomes remain suboptimal.
Currently, the ICIs used for the treatment of extensive-stage small-cell lung cancer (ES-SCLC) mainly include Adebrelimab, benmelstobart, Serplulimab, Atezolizumab, and Durvalumab, among which Atezolizumab and Durvalumab have been recommended as first-line treatment regimens by the National Comprehensive Cancer Network (NCCN) guidelines in the United States and the Guidelines of Chinese society of clinical oncology (CSCO) (6, 7). With the widespread adoption of ICIs in the treatment of ES-SCLC, their economic value has become a global focus of attention. Although these agents can improve survival outcomes, their high pricing often results in incremental cost-effectiveness ratios (ICERs) that exceed local willingness-to-pay (WTP) thresholds. For instance, Atezolizumab and Durvalumab lack cost-effectiveness in both the United States and China (8–10). In addition, other ICIs such as Adebrelimab, benmelstobart, and Serplulimab have been shown to be cost-effective in the United States but not in China (2, 11–13). These discrepancies indicate that characteristics of healthcare systems—such as drug pricing mechanisms, medical insurance reimbursement policies, and clinical practice patterns—exert a significant influence on pharmacoeconomic conclusions.
Lurbinectedin, a synthetic alkylating agent, induces cancer cell death by inhibiting the binding of oncogenic transcription factors to their recognition sequences (14). In a Phase II clinical trial, lurbinectedin monotherapy demonstrated promising results (15). More recently, the Phase III IMforte trial assessed the efficacy and safety of lurbinectedin combined with atezolizumab as first-line maintenance therapy for ES-SCLC (16). The trial showed that the lurbinectedin-atezolizumab combination significantly improved median progression-free survival (PFS; 5.4 months vs. 2.1 months) and median overall survival (13.2 months vs. 10.6 months) compared to atezolizumab alone. These results suggest that lurbinectedin combined with atezolizumab could become a new first-line treatment for ES-SCLC.
Although the IMforte trial demonstrated that LU-AT significantly prolongs survival in patients with ES-SCLC compared to AT (16), its economic feasibility remains unclear, and this research gap urgently needs to be addressed. In current immunotherapy for ES-SCLC, existing regimens such as atezolizumab lack cost-effectiveness in both China and the United States due to high costs (8, 9), while the clinical promotion of new regimens must balance efficacy and economic efficiency. Both Chinese and American healthcare systems require cost evidence to support decision-making. Given that LU-AT has not yet been widely adopted, conducting this evaluation at this stage can prospectively provide a basis for clinical selection and healthcare insurance policy formulation, avoiding resource misallocation or patients missing out on treatment due to financial burdens, which holds important practical significance. Therefore, this study evaluates the cost-effectiveness of lurbinectedin combined with atezolizumab as a first-line treatment for ES-SCLC from the perspective of China’s and the United States’ healthcare system. The primary goal is to provide evidence for treatment decision-making in patients with ES-SCLC. Additionally, the study aims to inform national health insurance policies and support the rational allocation of healthcare resources.
2 Methods
2.1 Modeling
The Partitioned Survival Analysis (PartSA) model in TreeAge2022 software was employed to assess the cost-effectiveness of LU-AT (lurbinectedin combined with atezolizumab) versus AT (atezolizumab monotherapy) as a first-line treatment for ES-SCLC (Figure 1). The model incorporated three health states: PFS, disease progression (PD), and death. All patients initially entered the PFS state, with death as the absorbing state. The model’s cycle duration was set at 21 days, totaling 102 cycles over 8.5 years, with 99% of patients projected to die during this period. Model outcomes included total costs, QALYs, and ICERs. In accordance with the Chinese Pharmacoeconomic Evaluation Guidelines, the WTP threshold was set at three times the 2024 per capita GDP of China, amounting to US$40,365 per QALY (17). In contrast, the WTP threshold in the United States is $150,000 per QALY.A treatment strategy is deemed cost-effective if the ICER falls below this threshold.
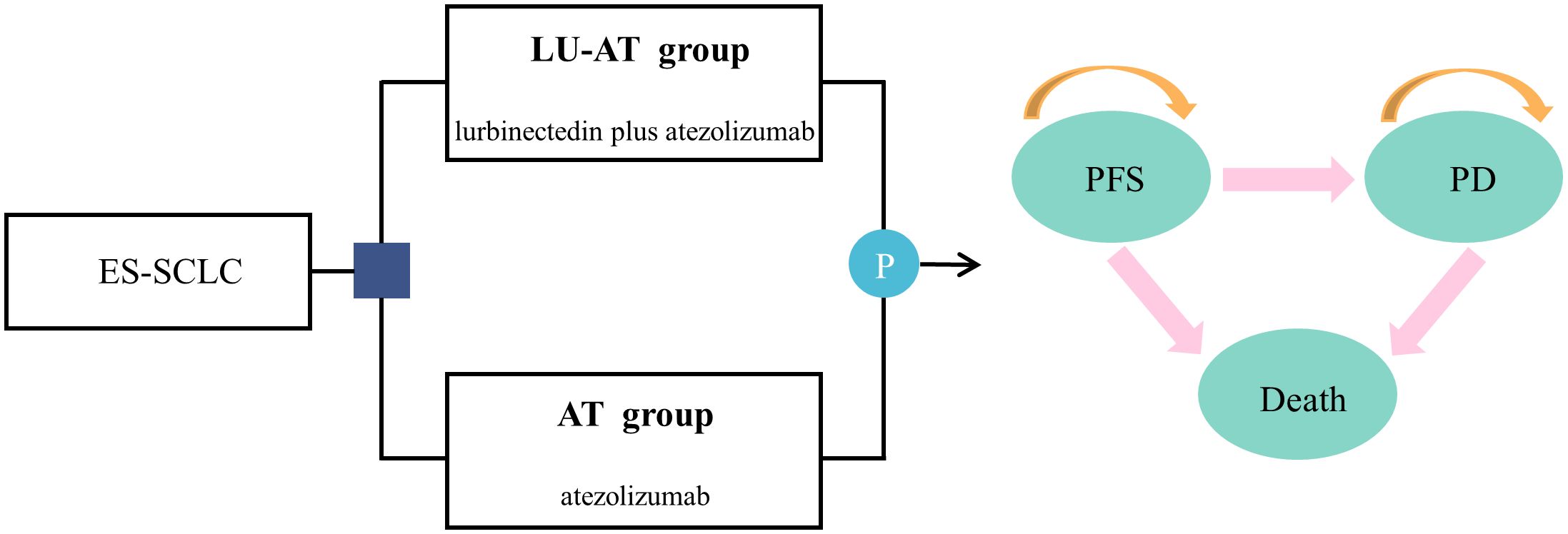
Figure 1. The partitioned survival model simulating outcomes for the IMforte trial. All patients started with PFS state and received treatment with LU-AT or AT. LU-AT, Lurbinectedin-Atezolizumab group; AT, Atezolizumab group; PFS, progression-free survival; PD, progressive disease.
2.2 Patient clinical treatment data
Clinical treatment data were derived from the IMforte trial, a randomized, multicenter, open-label, Phase 3 trial conducted across 96 hospitals and medical centers in 13 countries/regions (Belgium, Germany, Greece, Hungary, Italy, Mexico, Poland, South Korea, Spain, Taiwan, Türkiye, the UK, and the USA) (16). The trial compared lurbinectedin combined with atezolizumab to atezolizumab monotherapy as maintenance therapy for patients with ES-SCLC following standard first-line induction therapy with atezolizumab, carboplatin, and etoposide. Eligible patients were aged ≥ 18 years, had treatment-naive ES-SCLC, and received four cycles of 21-day induction therapy (atezolizumab, carboplatin, and etoposide). After induction, patients were randomized to receive either lurbinectedin (3.2 mg/m2) plus atezolizumab (1200 mg) or atezolizumab (1200 mg) intravenously every 3 weeks until PD (per RECIST v1.1), unacceptable toxicity, or withdrawal of consent. Treatment beyond PD was not permitted per the protocol. Unless contraindicated, patients receiving lurbinectedin plus atezolizumab also received prophylactic granulocyte colony-stimulating factor (G-CSF) and antiemetic premedication according to institutional guidelines. Between Nov 17, 2021, and Jan 11, 2024, 895 patients were screened for enrolment, of whom 660 (74%) were enrolled into the induction phase. Between May 24, 2022, and April 30, 2024, 483 (73%) of 660 patients entered the maintenance phase and were randomly assigned to lurbinectedin plus atezolizumab (n=242) or atezolizumab (n=241). According to the trial results, the median duration of treatment was 4.2 months for the LU-AT group and 2.1 months for the AT group, with a median of 7 doses for LU-AT and 4 doses for AT. Based on the second-line treatment data from the IMforte trial, we assumed that 37% of patients in the LU-AT group and 49% of patients in the AT group received chemotherapy after disease progression or the occurrence of unacceptable toxic reactions. In accordance with the NCCN and CSCO guidelines, topotecan-based chemotherapy is recommended as the standard second-line treatment for both groups (6, 7). Since the IMforte trial did not provide data on the duration of second-line treatment, we referred to the trial data by Song et al. and set the maintenance duration of second-line treatment at 1.73 months (18). The remaining patients all received best supportive care (BSC), including palliative radiotherapy, symptom control, nutritional support, and psychological support.
2.3 Survival transition probabilities
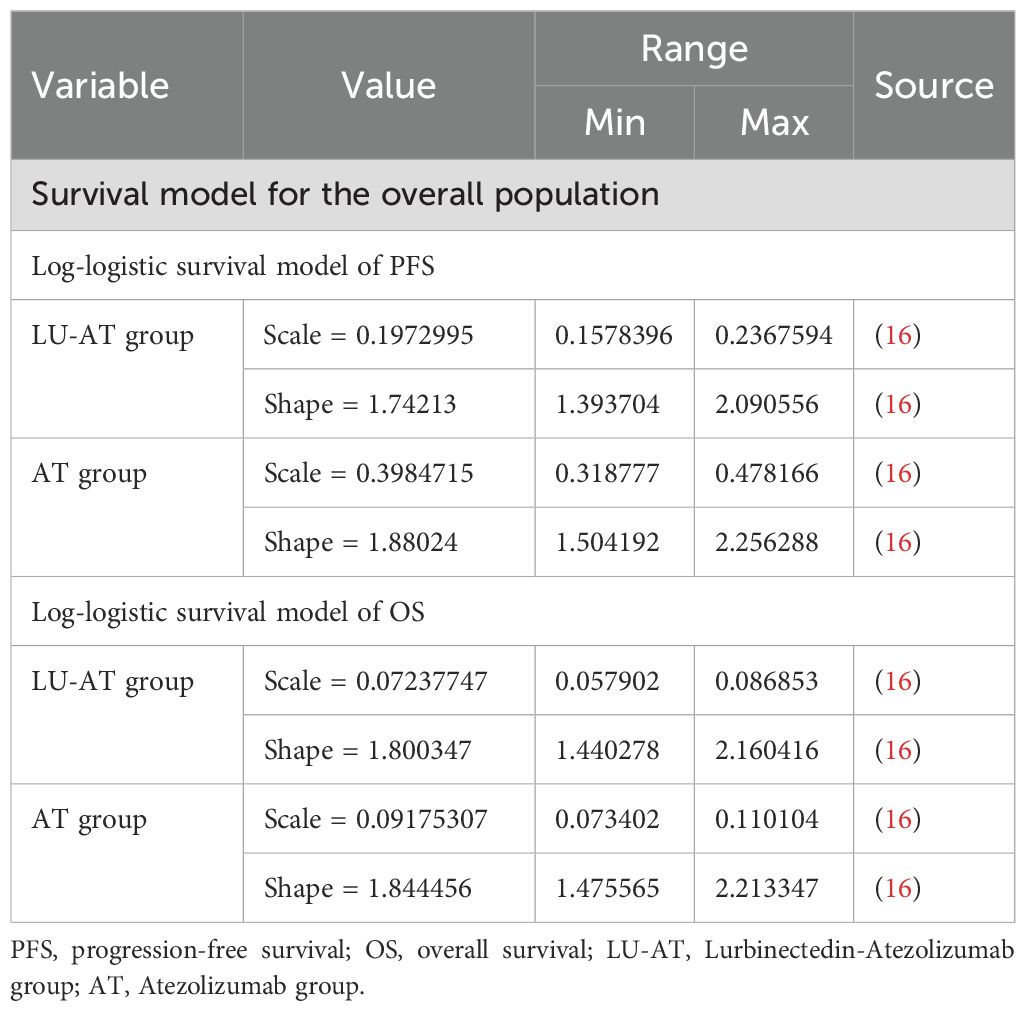
Kaplan-Meier curves from the IMforte trial were digitized using the GetData Graph Digitizer software. Various survival distributions were then fitted to the reconstructed individual patient data using R software to generate survival curves extending beyond the follow-up period reported in the trial (19). The distributions tested included exponential, gamma, generalized F, generalized gamma, Gompertz, Weibull, log-logistic, and log-normal models (Supplementary Table B) (20, 21). Based on the Akaike and Bayesian information criteria (22, 23), the log-logistic distribution was identified as the most suitable model for the original survival curves (Supplementary Figure 1; Table 1). This approach enabled the estimation of transition probabilities between different health states.
2.4 Cost and utility
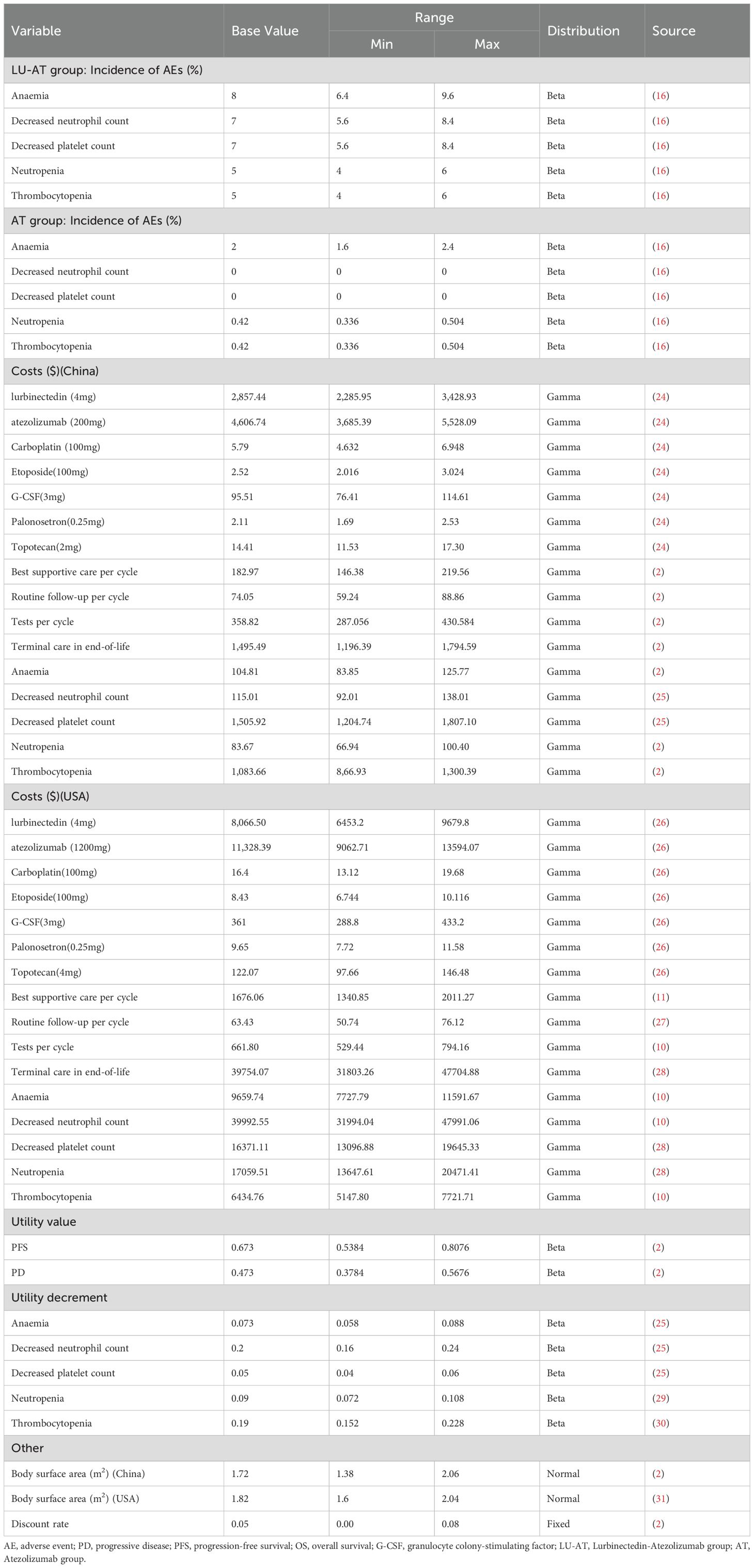
The study focused exclusively on direct medical costs, including those for medications, tests, routine follow-ups, BSC, management of grade ≥ 3 adverse events with an incidence exceeding 5%, and hospice care (Table 2). Drug costs were sourced from national tender prices, while other expenditures were derived from the literature and adjusted to 2024 values using the medical price index from the National Bureau of Statistics of China. The prices in the United States are sourced from Drugs.com (https://www.drugs.com/). All costs were expressed in US dollars, converted at the average 2024 exchange rate (1 USD = 7.12 CNY). As the IMforte trial did not provide quality-of-life data, utility values for PFS and PD were obtained from published studies (2). To mitigate potential bias from using identical utility values for both LU-AT and AT groups, the disutility of grade 3 or higher adverse events with an incidence exceeding 5% in each treatment arm was incorporated, improving the accuracy of health utility values for each group. In compliance with pharmacoeconomic guidelines, all costs and utility values were discounted at an annual rate of 5% (19).
2.5 Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis were performed to evaluate the robustness of the model. In the one-way sensitivity analysis, variables were adjusted within ranges reported in the literature; in the absence of data, variations of ± 20% from the base values were applied. The discount rate was varied from 0% to 8% (Table 2). The results were visualized using tornado diagrams. To assess the combined impact of parameter uncertainties, probabilistic sensitivity analysis was conducted through 1000 iterations of Monte Carlo simulations, with each parameter assigned a specific probability distribution (Table 2). The results were visualized as scatter plots.
3 Results
3.1 Basic analysis results
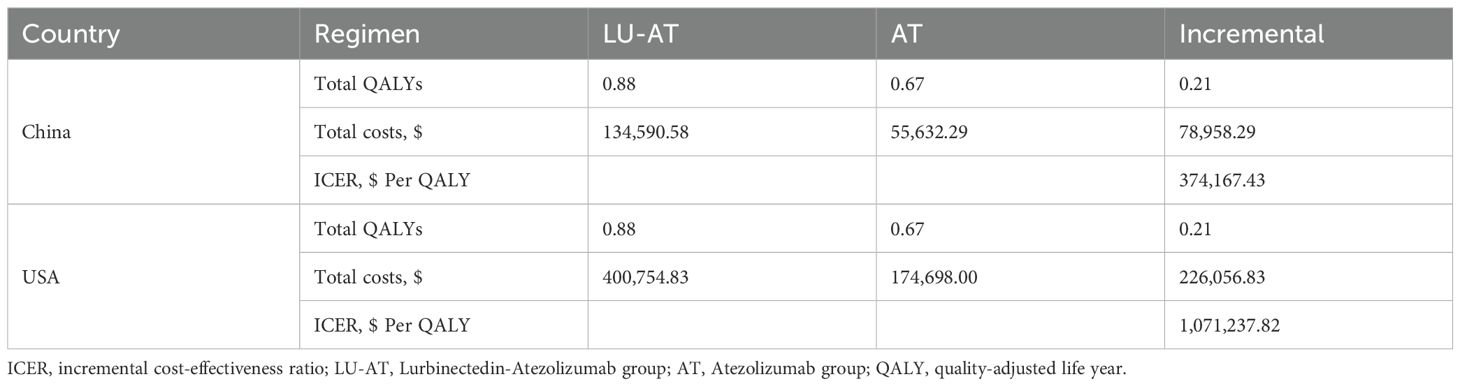
The results of this study are summarized in terms of total costs, QALYs, and ICERs (Table 3). The LU-AT group achieved 0.88 QALYs, compared with 0.67 QALYs in the AT group, resulting in an incremental gain of 0.21 QALYs. In China, the total cost was US$134,590.58 for the LU-AT group and US$55,632.29 for the AT group. This led to an incremental cost of US$78,958.29 and an ICER of US$374,167.43 per additional QALY gained. In the United States, total costs were US$400,754.83 and US$174,698.00 for LU-AT and AT, respectively, corresponding to an incremental cost of US$226,056.83 and an ICER of US$1,071,237.82 per incremental QALY. Both ICERs exceed the WTP thresholds, indicating that first-line LU- AT for ES-SCLC is unlikely to represent a cost-effective strategy from either the Chinese or the US perspective.
3.2 Sensitivity analysis
The results of the one-way sensitivity analysis are presented in the form of a tornado diagram (Figure 2). The most influential parameters on the model were the utility of PFS, the cost of lurbinectedin, BSA, and the cost of atezolizumab. When these parameters were allowed to vary within their specified ranges, the ICER consistently exceeded the predefined WTP threshold, suggesting that variations in input parameters did not significantly alter the model’s outcome and indicating the robustness of the results. The results of the probabilistic sensitivity analysis are shown as a scatter plot (China: 95% CI:270324.33–488936.48; USA: 95% CI:786393.67–1446803.88) (Figure 3). At the WTP threshold of $40,365 and $150,000 per QALY, the probability of LU-AT being cost-effective compared to AT was 0%. Even when the price of lurbinectedin was reduced to zero, LU-AT still did not prove to be cost-effective, likely due to the high costs associated with AT, which were exacerbated by the extended survival observed in the LU-AT group.
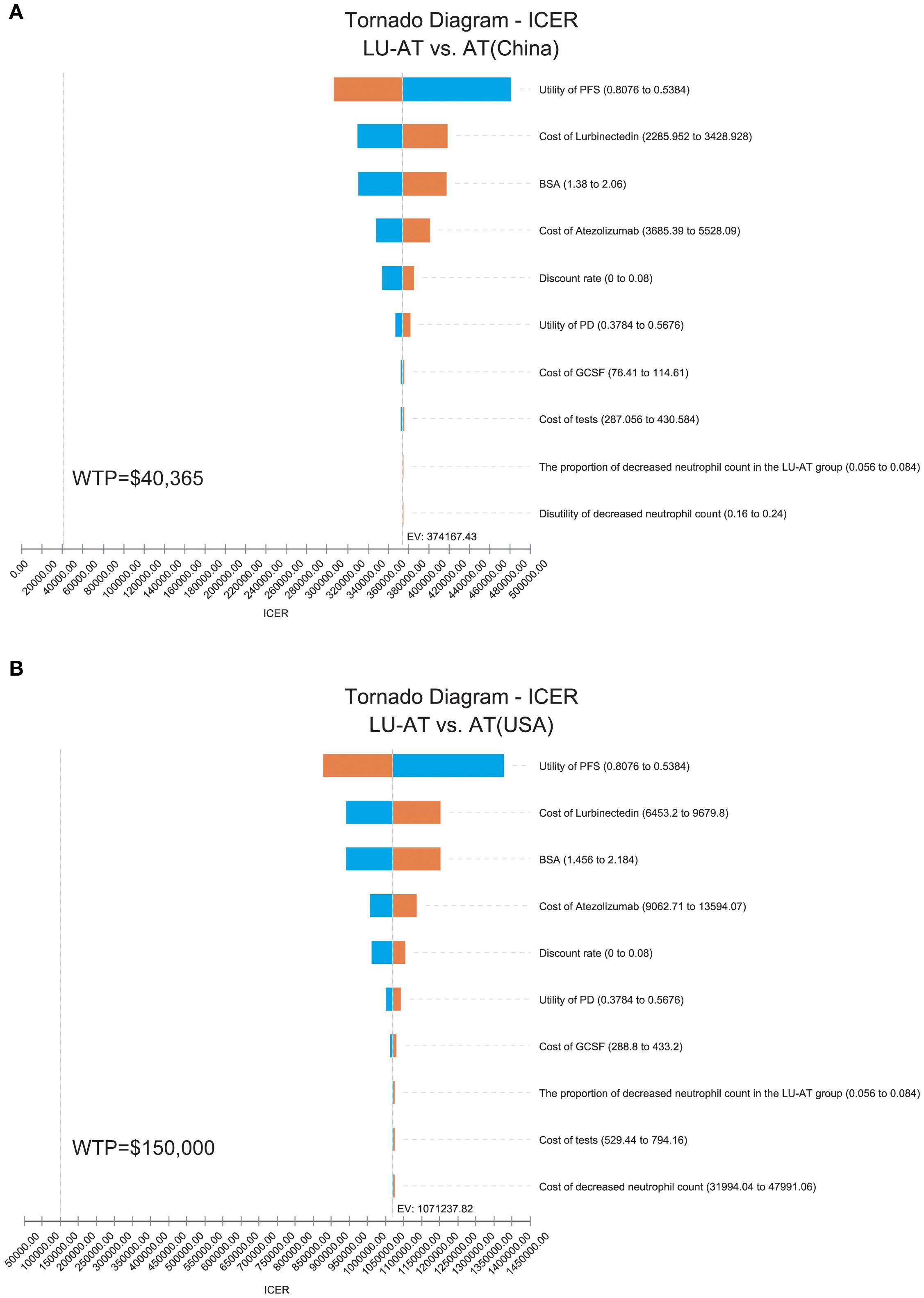
Figure 2. One-way sensitivity analyses in the overall population. The tornado diagram of one-way sensitivity analysis in China (A); The tornado diagram of one-way sensitivity analysis in the United States (B); ICER, incremental cost-effectiveness ratio; LU-AT, Lurbinectedin-Atezolizumab group; AT, Atezolizumab group; PFS, progression-free survival; PD, progressive disease; WTP, willingness-to-pay.
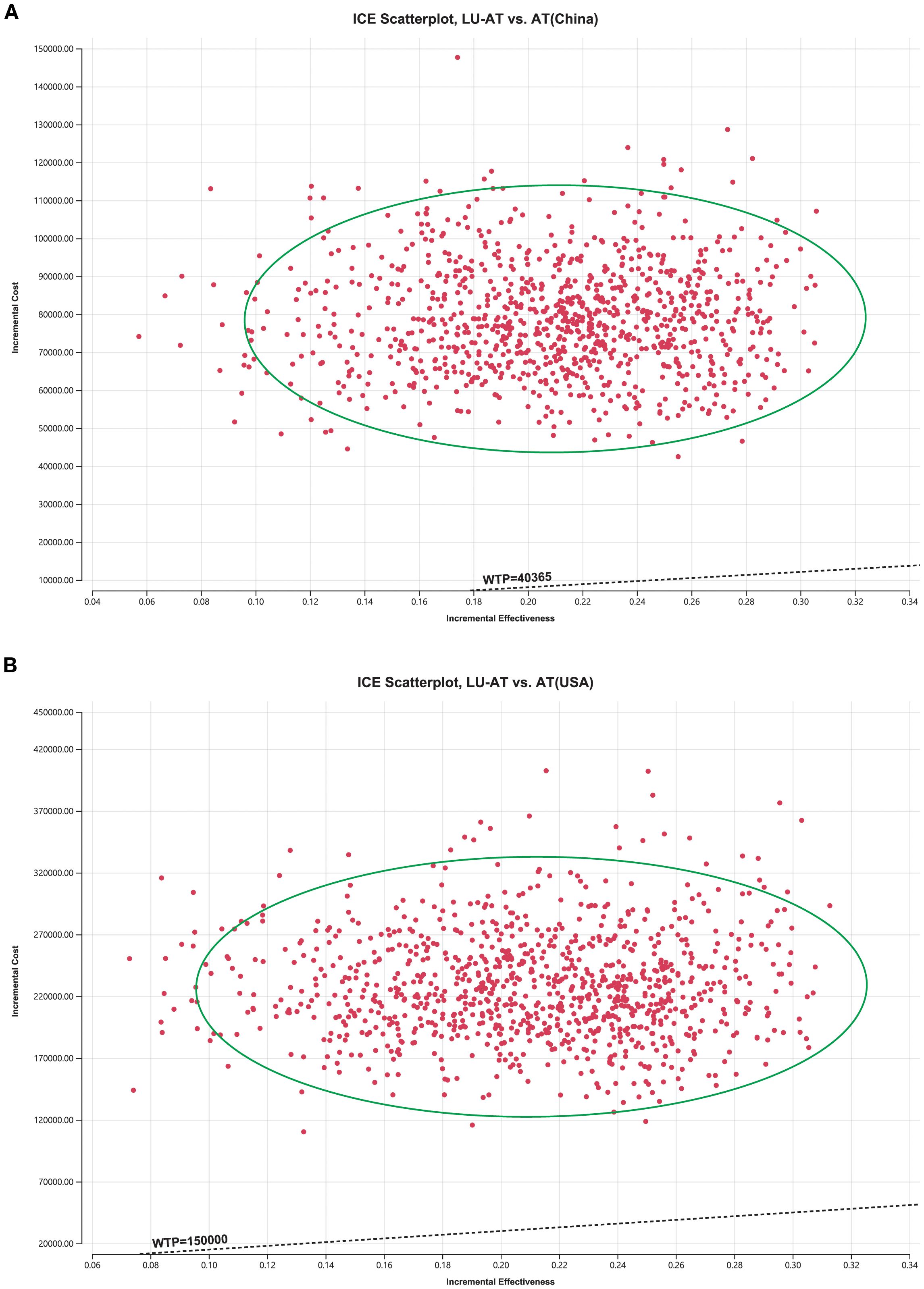
Figure 3. The cost-effectiveness probabilistic scatter plot in China (A); The cost-effectiveness probabilistic scatter plot in the United States (B); Ellipses are used to indicate 95% confidence intervals. Points that lie below the ICER threshold represent cost-effective simulations. LU-AT, Lurbinectedin-Atezolizumab group; AT, Atezolizumab group; PFS, progression-free survival; PD, progressive disease; WTP, willingness-to-pay.
3.3 Scenario analysis
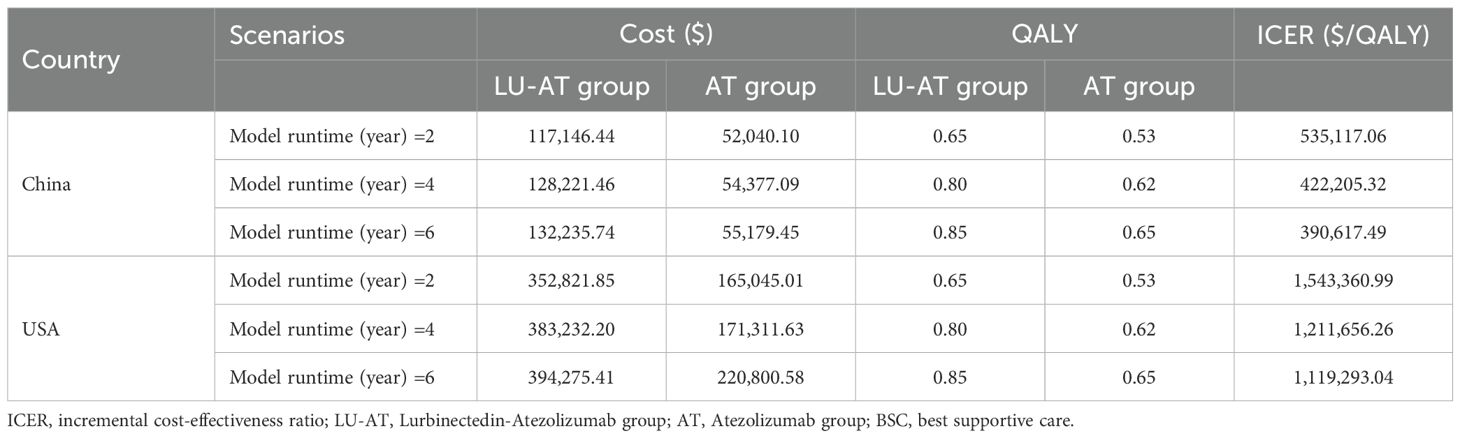
In Scenario 1, where the modeling duration was adjusted to 2, 4, and 6 years, the ICERs for LU-AT compared to AT were $528,658.82 per QALY, $417,303.22 per QALY, and $386,150.68 per QALY, respectively (Table 4). In Scenario 2, to eliminate the impact of ethnic factors, we conducted a sensitivity analysis on the survival curve parameters. The results showed that this did not alter the conclusion that LU-AT lacks cost-effectiveness.
4 Discussion
The IMforte trial evaluated the safety and efficacy of LU-AT versus AT as maintenance therapy for patients with ES-SCLC following standard first-line induction therapy with atezolizumab, carboplatin, and etoposide (16). The trial results demonstrated that LU-AT significantly prolonged median PFS (5.4 months vs. 2.1 months) and median overall survival (13.2 months vs. 10.6 months) compared to the AT group. These findings suggest that lurbinectedin combined with atezolizumab may serve as a novel first-line treatment option for patients with ES-SCLC. However, the high cost of LU-AT could limit its widespread adoption, particularly among patients with financial constraints. Therefore, the primary objective of this study was to assess the cost-effectiveness of LU-AT as a first-line treatment strategy for ES-SCLC within the Chinese and the United States’ healthcare system. The analytical results showed that in China, the incremental cost per QALY gained with LU-AT was $374,167.43, while in the United States, the incremental cost per QALY gained with LU-AT reached $1,071,237.82. The incremental costs in both China and the United States significantly exceeded the WTP thresholds. Therefore, LU-AT is deemed not cost-effective as a first-line treatment for ES-SCLC in both China and the United States.
The lack of cost-effectiveness of the LU-AT regimen is attributed to the requirement for long-term maintenance therapy with both lurbinectedin and atezolizumab, which substantially increases overall treatment costs without providing sufficient incremental survival benefits. However, these results should not be interpreted as a rationale to restrict the use of LU-AT, as this could potentially deprive patients of valuable therapeutic opportunities. One-way sensitivity analysis identified the cost of lurbinectedin as a key determinant of the model’s outcomes. Even when the cost of lurbinectedin was reduced to zero, the LU-AT regimen remained non-cost-effective. This was likely due to the prolonged administration of atezolizumab in the LU-AT group, which extended treatment duration without delivering proportional clinical benefits, despite modest survival gains. Meanwhile, scenario analysis—a methodology used to evaluate the cost-effectiveness of pharmaceuticals by incorporating various hypothetical conditions and uncertain factors to better reflect real-world circumstances—demonstrated that extending the treatment cycle could enhance the cost-effectiveness of LU-AT. This suggests that improving treatment adherence can optimize therapeutic value, which aligns with the interests of clinicians, patients, and their families, as well as broader ethical and social considerations.
Numerous anti-tumor drugs are deemed economically inefficient due to their inability to achieve favorable ICERs, such as benmelstobart combined with anlotinib (2) and adebrelimab combined with chemotherapy (2), which aligns with the findings of this study. Since the establishment of the National Health Commission of China in 2018, the country has initiated several rounds of drug price negotiations with pharmaceutical companies through national procurement strategies aimed at alleviating the financial burden on cancer individuals. Thus, the prices of many anti-cancer drugs have decreased by 30% to 70% (32). In tertiary hospitals, the reimbursement rate for insured patients’ medical expenses is approximately 70%, with primary healthcare institutions typically offering even higher reimbursement rates (33). With the progression of national medical insurance price negotiations in China, several treatment regimens have become cost-effective. For example, Yang et al. reported that Toripalimab combined with chemotherapy may represent a cost-effective first-line treatment for ES-SCLC (25), while Long et al. found that Tislelizumab plus chemotherapy could be the preferred option for patients with ES-SCLC (34). These improvements are likely attributed to reductions in drug costs following China’s national medical insurance volume-based procurement. The price of Toripalimab has dropped from $383.63 in 2021 to $261 in 2024 (25, 35), while Tislelizumab’s price has decreased from $675.84 in 2022 to $176.06 in 2025 (36). Meanwhile, the United States is also actively exploring measures to regulate drug prices. In 2025, the U.S. government took targeted actions to address the issue of exorbitant drug prices: on May 12 local time, President Donald Trump signed an executive order adopting the “most-favored-nation” principle (37). It mandates the U.S. Department of Health and Human Services to formulate an OECD-aligned “most-favored-nation price target” within 30 days, anchoring U.S. drug prices to the lowest levels among OECD member countries to tackle the prevalence of generally higher drug prices in the United States. This policy covers all prescription drugs and focuses on medications with high expenditure and significant price disparities, such as weight-loss drugs and chronic disease medications.
The findings of this study provide direct evidence to inform national price negotiations and potential healthcare insurance access decisions for LU-AT, covering perspectives from both China’s and the United States’ healthcare systems. In China, where cost-effectiveness is increasingly emphasized in the evaluation of the National Reimbursement Drug List (NRDL), this study shows that the current ICER of LU-AT ($374,167.43 per QALY) far exceeds the WTP threshold of $40,365 per QALY, indicating that its pricing is incompatible with the affordability of the healthcare system. This provides critical evidence for policymakers: substantial price reductions for lurbinectedin or atezolizumab through negotiations are necessary to bring the ICER below the threshold, following the successful examples of anti-tumor drugs such as toripalimab and tislelizumab (25, 35, 36), which improved cost-effectiveness and gained NRDL inclusion through negotiated price cuts. In the United States, based on the WTP threshold of $150,000 per QALY, LU-AT’s ICER ($1,071,237.82 per QALY) also significantly exceeds the threshold. This offers insights for U.S. reimbursement decision-making: at the current pricing, LU-AT is unlikely to secure healthcare insurance coverage support, and re-evaluation of its insurance eligibility through price adjustments or value demonstration is needed. For reimbursement decision-makers in both countries, adopting LU-AT at current prices may impose pressure on healthcare insurance funds due to its high incremental costs relative to modest QALY gains. The model in this study provides a baseline for re-evaluating cost-effectiveness post-price negotiations in both China and the United States, supporting evidence-based deliberations on its inclusion in insurance formularies after achieving optimized pricing. Ultimately, this study strengthens the link between clinical evidence and healthcare insurance policies in China and the United States, aiding in balancing therapeutic innovation, patient access, and the sustainable utilization of healthcare resources.
To the best of our knowledge, this is the first study to evaluate the efficacy of lurbinectedin combined with atezolizumab versus atezolizumab from the perspective of China’s and the United States’ healthcare system, providing up-to-date clinical evidence. This analysis offers significant reference value for China and the United States. While LU-AT was not deemed cost-effective compared to AT, it demonstrated a notable improvement in QALYs for patients with ES-SCLC (0.88 vs. 0.67 QALYs). However, several limitations of the study should be acknowledged. First, data limitations arose as long-term survival data beyond the clinical trial follow-up period were unavailable. Survival models were used to simulate data beyond the follow-up, potentially introducing bias compared to actual data. The cost-effectiveness analysis will be updated when long-term survival data become accessible. Second, the IMforte trial only included a small cohort of Asian populations such as patients from Taiwan, China, and South Korea. Due to potential ethnic differences across populations, this may have an impact on the study results. Third, Data on second-line treatment were derived solely from the IMforte trial and published literature, which may not fully reflect real-world clinical practices. Fourth, the model only accounted for grade 3 or higher adverse events with an incidence greater than 5%. However, sensitivity analysis indicated that variations in the probability of severe adverse events did not significantly affect the results. Despite these limitations, the study provides valuable insights for decision-makers considering lurbinectedin combined with atezolizumab as a first-line treatment for ES-SCLC in China and the United States.
5 Conclusion
This study is the first to assess the cost-effectiveness of LU-AT using recent clinical trial data from the perspective of China’s and the United States’ healthcare system. Our findings indicate that, as a first-line treatment for ES-SCLC, LU-AT is not cost-effective compared to AT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YH: Writing – original draft. ZX: Funding acquisition, Writing – review & editing. LB: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The current study was supported by the Postdoctoral Fellowship Program of CPSF (Number: GZC20242282), National Natural Science Foundation of China (Number: 82204379), Soaring Talent Development Program of Eastern Hepatobiliary Surgery Hospital (TF2024TJYB03), and Natural Science Foundation of Fujian Province (Grant number: 2023J011910).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1658740/full#supplementary-material.
References
1. Qin K, Gay CM, Byers LA, and Zhang J. The current and emerging immunotherapy paradigm in small-cell lung cancer. Nat Cancer. (2025) 6(6):954–66. doi: 10.1038/s43018-025-00992-5
2. You M, Luo L, Lu T, Chen S, and He Y. Cost-effectiveness analysis of benmelstobart, anlotinib, and chemotherapy in extensive-stage small-cell lung cancer. Front Immunol. (2024) 15:1477146. doi: 10.3389/fimmu.2024.1477146
3. Tariq S, Kim SY, Monteiro de Oliveira Novaes J, and Cheng H. Update 2021: management of small cell lung cancer. Lung. (2021) 199:579–87. doi: 10.1007/s00408-021-00486-y
4. van Meerbeeck JP, Fennell DA, and De Ruysscher DK. Small-cell lung cancer. Lancet. (2011) 378:1741–55. doi: 10.1016/S0140-6736(11)60165-7
5. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
6. Ganti A, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico T, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1441–64. doi: 10.6004/jnccn.2021.0058
7. Guidelines Working Committee of Chinese Society of Clinical Oncology. Guidelines of Chinese society of clinical oncology (CSCO) for small-cell lung cancer. Beijing: People’s Medical Publishing House (2024).
8. Wang Y, Rui M, Yang L, Wang X, Shang Y, Ma A, et al. Economic evaluation of first-line atezolizumab for extensive-stage small-cell lung cancer in the US. Front Public Health. (2021) 9:650392. doi: 10.3389/fpubh.2021.650392
9. Yi L, Zhou Z, Zeng X, Tan C, and Liu Q. First-line treatments for extensive-stage small-cell lung cancer with immune checkpoint inhibitors plus chemotherapy: a China-based cost-effectiveness analysis. Front Immunol. (2024) 15:1408928. doi: 10.3389/fimmu.2024.1408928
10. Ding D, Hu H, Li S, Zhu Y, Shi Y, Liao M, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw. (2021) 19:1141–7. doi: 10.6004/jnccn.2020.7796
11. Gan Y, Shi F, Zhu H, Han S, and Li D. Adebrelimab plus chemotherapy vs. chemotherapy for treatment of extensive-stage small-cell lung cancer from the US and Chinese healthcare sector perspectives: a cost-effectiveness analysis to inform drug pricing. Front Pharmacol. (2023) 14:1241130. doi: 10.3389/fphar.2023.1241130
12. Wan J, Xu Y, Wan B, and Ding H. Cost-effectiveness of benmelstobart-anlotinib-chemotherapy in extensive-stage small-cell lung cancer: A comparative analysis across United States and Chinese healthcare systems. Int J Clin Pharm. (2025). doi: 10.1007/s11096-025-01968-2
13. Liang X, Chen X, Li H, and Li Y. Cost-effectiveness analysis of first-line serplulimab combined with chemotherapy for extensive-stage small cell lung cancer. Front Public Health. (2023) 11:1156427. doi: 10.3389/fpubh.2023.1156427
14. Leal JF, Martínez-Díez M, García-Hernández V, Moneo V, Domingo A, Bueren-Calabuig JA, et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br J Pharmacol. (2010) 161:1099–110. doi: 10.1111/j.1476-5381.2010.00945.x
15. Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. (2020) 21:645–54. doi: 10.1016/S1470-2045(20)30068-1
16. Paz-Ares L, Borghaei H, Liu SV, Peters S, Herbst RS, Stencel K, et al. Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer (IMforte): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2025) 405:2129–43. doi: 10.1016/S0140-6736(25)01011-6
17. Yue X, Li Y, Wu J, and Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg Issues. (2021) 24:1–5. doi: 10.1016/j.vhri.2020.07.580
18. Song Z, Shao L, Lin B, and Zhang Y. Single-agent chemotherapy compared with combination chemotherapy as second-line treatment in extensive-stage small cell lung cancer: a retrospective analysis. Clin Transl Oncol. (2013) 15:843–8. doi: 10.1007/s12094-013-1013-5
19. Guyot P, Ades AE, Ouwens MJ, and Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
20. Su D, Wu B, and Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
21. Zhou Z, Yang Y, Chen S, and You M. Cost-effectiveness analysis of first-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma. Front Immunol. (2025) 16:1575627. doi: 10.3389/fimmu.2025.1575627
22. Ishak KJ, Kreif N, Benedict A, and Muszbek N. Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics. (2013) 31:663–75. doi: 10.1007/s40273-013-0064-3
23. Williams C, Lewsey JD, Mackay DF, and Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: A comparison of a multi-state modeling survival analysis approach with partitioned survival and markov decision-analytic modeling. Med Decis Making. (2017) 37:427–39. doi: 10.1177/0272989X16670617
24. Yao ZH. The big data service platform for China’s health industry: Information Query of Drug Bid Winning. Available online at: https://www.yaozh.com/ (Accessed June 15, 2025).
25. Yang J, Chen F, Li J, Zhou Y, Wang H, and Long Y. Cost-effectiveness analysis of Toripalimab regimen for extensive-stage small-cell lung cancer in China and America. Front Immunol. (2025) 16:1556100. doi: 10.3389/fimmu.2025.1556100
26. Available online at: https://www.drugs.com/ (Accessed August 16, 2025).
27. Zhang L, Hang Y, Liu M, Li N, and Cai H. First-line durvalumab plus platinum-etoposide versus platinum-etoposide for extensive-stage small-cell lung cancer: A cost-effectiveness analysis. Front Oncol. (2020) 10:602185. doi: 10.3389/fonc.2020.602185
28. Shao T, Zhao M, Liang L, and Tang W. Serplulimab plus chemotherapy vs chemotherapy for treatment of US and chinese patients with extensive-stage small-cell lung cancer: A cost-effectiveness analysis to inform drug pricing. BioDrugs. (2023) 37:421–32. doi: 10.1007/s40259-023-00586-6
29. Wu Q, Qin Y, and Li Q. Cost-effectiveness of atezolizumab versus chemotherapy in patients with non-small-cell lung cancer ineligible for platinum-based doublet chemotherapy. Front Public Health. (2025) 13:1349645. doi: 10.3389/fpubh.2025.1349645
30. Zheng Z, Zhu H, and Fang L. Tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for extensive-stage small cell lung cancer: A cost-effectiveness analysis. PloS One. (2025) 20:e0320189. doi: 10.1371/journal.pone.0320189
31. Lin S, Luo S, Gu D, Li M, Rao X, Wang C, et al. First-line durvalumab in addition to etoposide and platinum for extensive-stage small cell lung cancer: A U.S.-based cost-effectiveness analysis. Oncologist. (2021) 26:e2013–2013e2020. doi: 10.1002/onco.13954
32. Huang Y, You M, Wu Q, and Chen R. Cost-effectiveness analysis of zolbetuximab plus mFOLFOX6 as the first-line treatment for CLDN18.2-positive, HER2-negative advanced gastric or Gastroesophageal Adenocarcinoma. Front Pharmacol. (2023) 14:1238009. doi: 10.3389/fphar.2023.1238009
33. Qin Z, Liu S, Zhou M, Chen L, Huang W, and Shen L. Impacts of unifying urban and rural residents’ medical insurance on the hospitalisation expenses of rural patients in eastern China: an interrupted time series analysis. BMJ Open. (2023) 13:e067198. doi: 10.1136/bmjopen-2022-067198
34. Long R and Chen F. First-line chemotherapy with tislelizumab for patients with extensive-stage small cell lung cancer: a cost-effectiveness analysis. Sci Rep. (2024) 14:31958. doi: 10.1038/s41598-024-83509-x
35. Zhang M, Xu K, Lin Y, Zhou C, Bao Y, Zhang L, et al. Cost-effectiveness analysis of toripalimab plus chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer in China. Front Immunol. (2023) 14:1169752. doi: 10.3389/fimmu.2023.1169752
36. Zhou D, Luo X, Zhou Z, Zeng X, Wan X, Tan C, et al. Cost-effectiveness analysis of tislelizumab, nivolumab and docetaxel as second- and third-line for advanced or metastatic non-small cell lung cancer in China. Front Pharmacol. (2022) 13:880280. doi: 10.3389/fphar.2022.880280
37. Available online at: https://www.whitehouse.gov/fact-sheets/2025/04/fact-sheet-president-donald-j-trump-announces-actions-to-lower-prescription-drug-prices/ (Accessed August 25, 2025).
Keywords: cost-effectiveness, lurbinectedin, atezolizumab, extensive-stage small-cell lung cancer, partitioned survival model
Citation: Huang Y, Xu Z and Bao L (2025) Cost-effectiveness analysis of lurbinectedin plus atezolizumab as first-line treatment for extensive-stage small-cell lung cancer. Front. Immunol. 16:1658740. doi: 10.3389/fimmu.2025.1658740
Received: 03 July 2025; Accepted: 29 August 2025;
Published: 23 September 2025.
Edited by:
Wensi Tao, University of Miami Health System, United StatesReviewed by:
George Gourzoulidis, Health Through Evidence, GreeceXin Tong, Ragon Institute, United States
Daisuke Morinaga, Hokkaido University Hospital, Japan
Copyright © 2025 Huang, Xu and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leilei Bao, YW5uYWJhbzIxMkAxMjYuY29t
†These authors have contributed equally to this work
 Yufan Huang
Yufan Huang Zheqi Xu†
Zheqi Xu† Leilei Bao
Leilei Bao