- Department of Hematology,The First Hospital of Jilin University, Changchun, China
Diffuse large B-cell lymphoma and follicular lymphoma exhibit complex metabolic and immune microenvironments that influence disease progression and treatment response. Metabolic reprogramming, including glycolysis, amino acid, and lipid metabolism, supports tumor growth while suppressing anti-tumor immunity. Immune components such as tumor-infiltrating lymphocytes and checkpoint molecules (PD-L1, LAG-3, TIM-3) further modulate prognosis. Elevated tumor metabolic volume and glycolytic activity correlate with aggressive disease and poor outcomes. Conversely, high TIL density often predicts better responses. Integrating metabolic and immune biomarkers enhances risk stratification and therapeutic strategies, highlighting the potential for combined metabolic inhibitors and immunotherapies to improve precision medicine in lymphoma.
1 Introduction
Diffuse Large B-Cell Lymphoma (DLBCL) and Follicular Lymphoma (FL) are the two most common subtypes of non-Hodgkin lymphoma (NHL) (1–3). Despite significant differences in their biological behavior, clinical features, and treatment strategies, both exhibit high heterogeneity and complex tumor microenvironments (TME) (1–3). From a cellular origin perspective, DLBCL primarily arises from germinal center B cells or activated B cells, with tumor cells typically extensively involving lymph nodes and extranodal organs (e.g., gastrointestinal tract, central nervous system) (4, 5). FL originates from germinal center B cells, with tumor cells proliferating mainly in lymph nodes and the spleen to form follicular structures (6, 7).
Currently, the standard first-line treatment for DLBCL is the R-CHOP regimen (combination chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (8, 9). Treatment strategies for FL are more diverse, determined by disease grading and staging, and include watchful waiting, rituximab monotherapy or in combination with chemotherapy, as well as emerging immunomodulators and targeted therapies (10, 11). Although R-CHOP achieves cure in some DLBCL patients, 30–40% still face recurrence or refractory disease (12, 13). FL poses long-term management challenges due to its high recurrence rate and risk of transforming into more aggressive DLBCL (14, 15). The heterogeneity of these diseases and the diversity of treatment responses suggest that, beyond traditional clinical and pathological features, metabolic and immune factors within the TME may play a critical role in disease progression and prognosis.
Recent studies on tumor metabolic reprogramming and the immune microenvironment have provided new insights into the biological behavior of DLBCL and FL (16). Through metabolic reprogramming, tumor cells not only meet their rapid proliferation energy demands but may also reshape the immune microenvironment via metabolic byproducts, thereby suppressing antitumor immune responses (17, 18). For instance, Tumor Metabolic Volume (TMV) and glycolytic activity have been demonstrated to correlate with disease aggressiveness and prognosis in both DLBCL and FL (19–21). Concurrently, tumor-infiltrating lymphocytes (TILs), programmed death-ligand 1 (PD-L1) expression levels, and the distribution of immune checkpoint molecules within the immune microenvironment also play crucial roles in regulating antitumor immune responses (22, 23). High TIL density is typically associated with favorable treatment response and prognosis (24), whereas infiltration of immunosuppressive cells (e.g., regulatory T cells (Tregs), M2 macrophages) may promote immune escape and disease progression (25, 26). Given the critical importance of tumor-microenvironment interactions, therapeutic strategies targeting these crosstalk pathways—such as using immune checkpoint inhibitors to reverse T cell exhaustion or employing BTK inhibitors to modulate BCR signaling and the microenvironment—have emerged as novel research focuses.
Therefore, exploring the roles of metabolic parameters (e.g., tumor microenvironment volume, glycolytic activity) and immune parameters (e.g., TIL density, PD-L1 expression) in DLBCL and FL not only helps elucidate the biological mechanisms of the disease but may also provide novel biomarkers and therapeutic targets for risk stratification, prognostic prediction, and personalized treatment.
This review aims to systematically summarize research advances on the metabolic and immune microenvironments in DLBCL and FL, explore their relationship with disease risk and prognosis, and discuss their potential applications in clinical translation. By integrating metabolic and immune parameters, we hope to provide novel insights for improving treatment strategies in DLBCL and FL, thereby advancing precision medicine in this field.
2 Interplay between metabolism and immunity
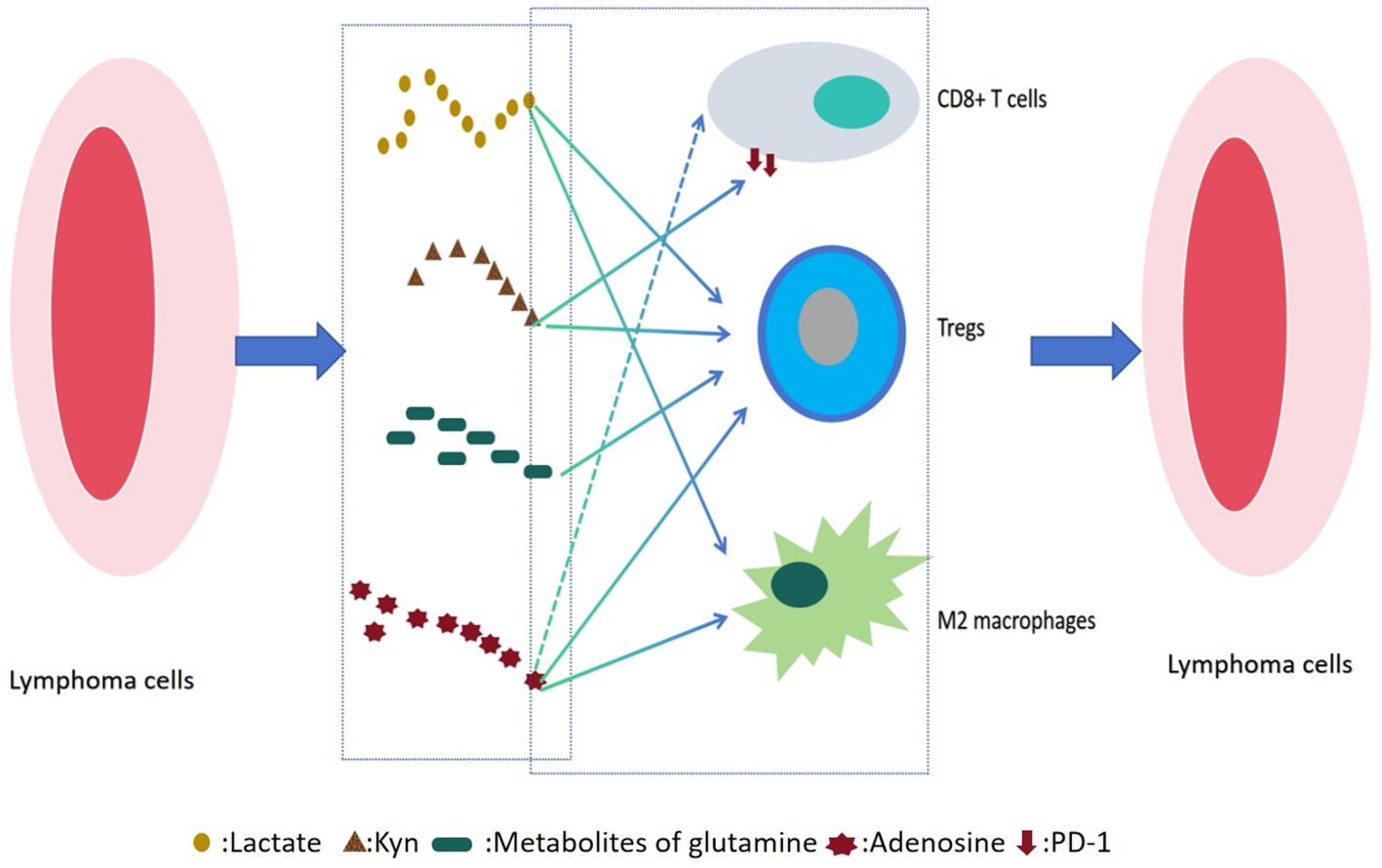
In DLBCL and FL, the metabolic reprogramming of the TME forms a dynamic and reciprocal regulatory network with immune cell function, playing a pivotal role in lymphoma progression(as shown in Figure 1). Tumor cells enhance glycolysis and amino acid metabolism not only to fuel rapid proliferation and biosynthesis but also to reshape the local microenvironment through the release of metabolic byproducts such as lactate, glutamine, and adenosine. These metabolites can directly suppress the cytotoxic functions of effector immune cells such as CD8+ T cells and natural killer (NK) cells, while promoting the expansion of immunosuppressive populations like Tregs and M2-polarized macrophages.
Importantly, immune cells are not merely passive responders. Activated T cells can secrete interferon-gamma (IFN-γ) to disrupt tumor metabolic pathways, and the polarization state of macrophages (M1 versus M2) can in turn modulate tumor glycolysis and mitochondrial metabolism. This bidirectional metabolic–immune crosstalk profoundly influences lymphoma heterogeneity, therapeutic resistance, and patient prognosis, providing a strong rationale for the development of combined metabolic and immune-targeted treatment strategies.
2.1 Effects of metabolic reprogramming on immune cells
Metabolic reprogramming in tumor cells has emerged as a central hallmark in cancer biology, playing a pivotal role in tumor initiation and progression. Recent advances in TME research have highlighted that metabolic alterations—particularly enhanced glycolysis and amino acid metabolism—not only fulfill the energetic and biosynthetic demands of rapidly proliferating tumor cells but also profoundly reshape the immune milieu, suppressing anti-tumor immune responses and facilitating immune evasion and disease progression.
2.1.1 Immunomodulatory effects of glycolysis and lactate metabolism
Glycolysis and lactate accumulation play central roles in tumor-associated immune suppression. Tumor cells exhibit a distinctive metabolic phenotype known as the Warburg effect, in which they preferentially utilize glycolysis for energy production even under normoxic conditions. This leads to substantial lactate accumulation and acidification of the TME (27). Lactate is extruded from tumor cells through monocarboxylate transporters (MCTs, primarily MCT4) (28–30). Subsequently, it enters the tumor microenvironment via MCT1, which is mainly expressed on immune cells, thus decreasing the extracellular pH value (28–30). Notably, lactate dehydrogenase (LDH), a key enzyme in lactate metabolism, is markedly overexpressed in DLBCL and other tumors. The lactate produced by LDH impairs the cytotoxic functions of CD8+ T cells and NK cells, thereby weakening the host’s anti-tumor immunity (31–33). In addition, lactate promotes the expansion of Tregs and the polarization of macrophages toward an M2 immunosuppressive phenotype (34–37). Moreover, lactate inhibits the differentiation and antigen-presenting capacity of dendritic cells, further disrupting T cell activation and facilitating immune escape (31, 37, 38). According to the Warburg effect, lactic acidosis can occur when lactate homeostasis is disrupted due to overproduction and/or reduced utilization. Lactic acidosis is classified into two types: type A, caused by tissue hypoxia, and type B, which occurs under normoxic conditions due to non-hypoxic factors such as drugs or toxins. Type B lactic acidosis is associated with altered glycolysis and redox imbalance (39). It is frequently observed in human malignancies—particularly lymphomas—and is associated with poor prognosis if not treated promptly (40–42). Another significant cause of lactic acidosis is thiamine deficiency, which is a distinct feature associated with type B lactic acidosis (43, 44). This mechanism has important clinical significance, as thiamine is a key coenzyme in the pyruvate dehydrogenase complex, responsible for converting the glycolytic end product pyruvate into acetyl CoA, which enters the tricarboxylic acid cycle (43). When thiamine is deficient, pyruvate cannot be oxidized normally, resulting in a large amount of conversion to lactate under the action of LDH, leading to severe type B lactic acidosis (43). In lymphoma patients, thiamine deficiency may be caused by high tumor consumption, loss of appetite, or treatment side effects (45). Identifying and correcting this condition is crucial for managing such tumor emergencies and improving patient prognosis (45). Clinically, elevated serum LDH levels are significantly associated with poor prognosis in DLBCL and have been incorporated into the International Prognostic Index (IPI). Similarly, LDH has prognostic value in FL and is a component of the Follicular Lymphoma International Prognostic Index (FLIPI). As a direct marker of tumor metabolic activity, elevated LDH reflects increased tumor burden and the formation of a lactate-mediated immunosuppressive microenvironment.
2.1.2 Immunoregulatory effects of amino acid metabolism
Abnormal amino acid metabolism is another crucial mechanism underlying tumor immune evasion. In tryptophan metabolism, CD11c+ myeloid dendritic cells (mDCs) within the FL microenvironment exhibit high expression of indoleamine 2,3-dioxygenase 1 (IDO1), which depletes tryptophan and leads to the accumulation of kynurenine (Kyn) (46). This process promotes immune tolerance through two mechanisms: first, Kyn activates the aryl hydrocarbon receptor (AhR) pathway, inducing Treg differentiation and suppressing effector T cell function (47, 48); second, Kyn-AhR signaling enhances PD-1 expression on CD8+T cells via intercellular signaling within the TME, resulting in a PD-1+ exhausted T cell phenotype (49). Quantification of Kyn and tryptophan levels has shown promise as a prognostic biomarker in both DLBCL and FL (46, 50, 51). Furthermore, inhibitors targeting key enzymes in tryptophan metabolism (e.g.,IDO1/TDO) have demonstrated therapeutic potential in preclinical models, providing a new avenue for precision therapy in lymphoma (52–54). Serine metabolism also supports tumor growth, survival, and adaptation to hostile microenvironments through multiple pathways and has emerged as a promising therapeutic target (55). The rate-limiting enzyme in the serine synthesis pathway (SSP), phosphoglycerate dehydrogenase (PHGDH), is frequently overexpressed or amplified in various cancers, making it a critical vulnerability for therapeutic intervention (56). In DLBCL, PHGDH overexpression is strongly associated with MYC activation, particularly in the germinal center B-cell-like (GCB) subtype. This may drive chemotherapy resistance through enhanced serine flux, reducing the efficacy of R-CHOP and shortening overall survival (57). In addition, serine metabolic enzymes interact with immunosuppressive cells in the TME—such as M2 macrophages—potentially modulating responses to immunotherapies like PD-1 inhibitors (58, 59). Future studies should investigate the therapeutic potential of PHGDH inhibitors in lymphoma, with the aim of overcoming resistance and improving efficacy through combination strategies. It is also essential to identify predictive biomarkers to select patient subgroups most likely to benefit from such approaches.
2.1.3 Immunomodulatory role of glutamine metabolism
As a central node in tumor energy metabolism, glutamine metabolism plays a critical role in both DLBCL and FL. This pathway begins with the active uptake of glutamine via transporters such as ASCT2/SLC1A5, followed by its conversion into glutamate by glutaminase (GLS), and subsequently into α-ketoglutarate (α-KG), which enters the tricarboxylic acid (TCA) cycle to provide energy and biosynthetic precursors for tumor cells (60, 61). Notably, glutamine metabolism also modulates the tumor immune microenvironment through multiple mechanisms: it competes with effector T cells for nutrients, thereby limiting their metabolic fitness (62), supports the survival and function of immunosuppressive cells such as Tregs and myeloid-derived suppressor cells (MDSCs) (63), and is involved in the regulation of immune checkpoint molecules such as PD-L1 (64). Metabolomic profiling reveals elevated levels of glutamine catabolites in the plasma of lymphoma patients (65), and all tested DLBCL cell lines express GLS1 regardless of subtype classification (66). GLS1 inhibition using CB-839, in combination with the BCL2 inhibitor ABT-199, not only induces significant reactive oxygen species (ROS) production but also exerts synergistic cytotoxicity—suggesting that co-targeting GLS1 and BCL2 could be a promising therapeutic strategy in DLBCL (66). Furthermore, recent studies have uncovered a key mechanism of glutamine metabolic reprogramming in DLBCL involving the mitochondrial pyruvate carrier (MPC) and glutamate-pyruvate transaminase 2 (GPT2) pathway (67). Studies have found that DLBCL can be further classified according to cell origin and molecular characteristics. OXPHOS DLBCL (oxidative phosphorylation subtype) is mainly manifested in the activation of oxidative phosphorylation metabolic pathway and the enhancement of mitochondrial function, while BCR-DLBCL (B cell receptor subtype) is highly dependent on B cell receptor signaling pathway and its downstream glycolysis process (68, 69). Because of this difference in metabolic dependence, the sensitivity of the two subtypes to targeted treatment strategies is also different: OXPHOS DLBCL may be more sensitive to inhibitors targeting oxidative phosphorylation related pathways such as glutamine metabolism, while BCR-DLBCL may be more responsive to drugs targeting BCR signaling pathways such as Btk inhibitors. It is worth noting that although these two subtypes exhibit distinct metabolic characteristics, they are highly dependent on glutamine metabolism to maintain the activity of the TCA cycle (66, 70). GPT2-mediated α-KG production requires mitochondrial pyruvate input, which is dependent on MPC activity. Under extracellular matrix (ECM)-mimicking conditions that better simulate solid tumor environments, MPC inhibition significantly reduces α-KG production and suppresses DLBCL proliferation—an effect not observed in conventional suspension culture, highlighting the critical influence of the microenvironment on metabolic behavior (67). Moreover, MPC inhibition increases DLBCL sensitivity to ammonia, due to impaired ammonia detoxification via glutamate dehydrogenase (GDH) under ECM conditions (67). These findings challenge traditional views of DLBCL metabolism and suggest therapeutic potential for targeting the MPC–GPT2 axis. Future studies should investigate the feasibility of GPT2-specific inhibitors and explore how microenvironment-driven metabolic changes influence treatment resistance.
2.1.4 Immunomodulatory role of lipid metabolism
Lipid metabolism plays a dual role in the development of DLBCL and FL: on one hand, it directly supports tumor cell proliferation and survival, and on the other, it shapes the tumor immune microenvironment to facilitate immune escape. In B-cell malignancies, constitutive activation of the BCR-PI3K-AKT signaling pathway leads to mTORC1 hyperactivation, which upregulates anabolic processes including lipid and cholesterol biosynthesis (71). Cholesterol synthesis driven by this pathway may form a positive feedback loop that sustains BCR signaling, further promoting lymphoma progression. Cholesterol, a key component of membrane homeostasis, is essential for tumor cell proliferation. Lymphoma cells utilize cholesterol via BCR signaling to maintain proliferative and pro-survival pathways. Excess free cholesterol is rapidly esterified by Acetyl coenzyme A acetyltransferase (ACAT) or exported via transporters such as scavenger receptor class B type I (SR-BI) and ATP-binding cassette protein A1—processes particularly evident in pathological conditions like macrophage foam cell formation (72). Targeting cholesterol metabolism has emerged as a novel therapeutic strategy in lymphoma. SR-BI inhibitors have shown anti-lymphoma activity by disrupting cholesterol homeostasis (72), and metformin has been reported to improve prognosis in DLBCL patients with type 2 diabetes, potentially by modulating cholesterol metabolism (73). Additionally, lipid metabolism-related gene signatures have been validated as independent prognostic factors in DLBCL, enhancing predictive accuracy when combined with the IPI (74, 75). Clinical observations suggest that statins, when used alongside standard chemoimmunotherapy in the rituximab era, do not compromise treatment efficacy in DLBCL/FL, although their potential benefit in FL remains to be further confirmed (76). Within the TME, lipids represent a double-edged sword—they can both support and suppress anti-tumor immunity. For instance, enhanced fatty acid oxidation (FAO) promotes the expansion of tumor-reactive CD8+ T cells and improves response to PD-1 blockade (77, 78); Conversely, lipid uptake by CD8+ T cells via CD36 leads to lipid peroxidation and ferroptosis, impairing their effector function and weakening anti-tumor immunity (79, 80). Therefore, metabolic interventions must carefully balance the dynamic demands of both tumor and immune cells. Future strategies should integrate metabolomics with immune profiling to develop precise approaches that overcome the “double-edged sword” nature of metabolic reprogramming, ultimately facilitating more effective clinical translation.
2.1.5 Immunomodulatory role of adenosine metabolism
Adenosine metabolism plays a key immunosuppressive role in the tumor immune microenvironment. The main pathway of extracellular ATP production is the enzymatic cascade reaction of CD39 and CD73 (ATP→ADP→AMP→adenosine), while intracellular AMP can also catalyze the production of adenosine under energy stress (81–84). In DLBCL and FL, high expression of CD39—particularly in the non-GCB subtype of DLBCL—is strongly associated with poor prognosis. This effect is mediated by adenosine binding to A2A receptors (A2AR), triggering a broad spectrum of immunosuppressive responses, including inhibition of CD8+ T and NK cell activity, expansion of Tregs, polarization of macrophages toward the M2 phenotype, and impairment of dendritic cell antigen presentation (85–87). Hypoxic conditions in the TME further induce CD39/CD73 expression via HIF-1α, leading to increased adenosine accumulation. This process synergizes with other immunosuppressive pathways such as lactate metabolism and IDO1-mediated tryptophan catabolism, forming a tightly integrated immunosuppressive network (88–91). Current therapeutic approaches focus on inhibitors targeting CD39/CD73 and A2AR antagonists. Preclinical studies suggest that combining these agents with PD-1 inhibitors can reverse immune suppression and restore anti-tumor responses (92, 93). Future directions include the development of precision stratification methods based on CD39/CD73 expression and investigation of combination strategies targeting the hypoxia–CD39/CD73–A2AR axis to overcome immune evasion in lymphoma.
2.2 Immune cell-mediated regulation of tumor metabolism
Within the TME, immune cells and tumor cells engage in a complex and dynamic bidirectional interaction that profoundly influences tumor initiation, progression, and response to therapy. Immune cells are not merely passive victims of tumor metabolic reprogramming; they actively modulate the metabolic status of tumor cells through cytokine secretion and direct cell–cell contact. This reciprocal regulation is of significant theoretical and clinical importance, particularly in the context of lymphomas.
2.2.1 Tumor-infiltrating regulatory T cells and tumor metabolism
Tumor-infiltrating Tregs exert potent immunosuppressive effects in the TME through metabolic mechanisms, thereby promoting immune evasion (94–96). The key Treg transcription factor FOXP3 suppresses glycolysis while enhancing OXPHOS and NAD+ oxidation, enabling Tregs to survive and function under hypoglycemic and hypoxic conditions commonly found in the TME (97, 98). This adaptation relies on activation of the LKB1/AMPK pathway, where LKB1 phosphorylates AMPK to enhance mitochondrial metabolic efficiency while inhibiting mTORC1 signaling—collectively preserving Treg stability under metabolic stress (99, 100). Tregs contribute to immunosuppression not only through secretion of cytokines such as TGF-β, IL-10, and IL-35 but also by competitively depleting key nutrients such as glucose, glutamine, and tryptophan—thereby impairing effector T cell function. Specifically, TGF-β suppresses the cytotoxic activity of NK cells and cytotoxic T lymphocytes (CTLs) and promotes the transdifferentiation of Th17 cells into Tregs, exacerbating immune suppression (101, 102). IL-10 directly inhibits effector T cell cytotoxicity, while IL-35 promotes T cell exhaustion (103, 104). Notably, under nutrient-deprived conditions, Tregs can utilize abundant metabolic byproducts in the TME—such as lactate and fatty acids—to maintain their function (105, 106). Additionally, through the CD39/CD73–adenosine axis and IDO–kynurenine pathway, Tregs secrete immunosuppressive metabolites (e.g., adenosine and kynurenine), which further inhibit effector T cells and reinforce tumor glycolytic reprogramming—forming a self-reinforcing immunosuppressive loop (48, 107, 108). This metabolic advantage correlates with adverse clinical outcomes. High Treg infiltration in DLBCL and FL is associated with poor treatment response, increased risk of disease progression, and shorter progression-free survival (PFS) (109–111). Moreover, LKB1 has been shown to promote DLBCL immune evasion by enhancing Treg metabolic stability and suppressive activity (112). Therefore, targeting Treg-specific metabolic pathways—such as CD39/CD73, IDO, or fatty acid oxidation—represents a promising approach to reversing the immunosuppressive microenvironment and boosting anti-tumor immunity.
2.2.2 Interplay between CD8+ T cells and tumor metabolism
CD8+ T cells are the principal effectors of adaptive anti-tumor immunity (113, 114). Studies have shown that CD8+ TILs secrete IFN-γ, which acts on tumor cells through the JAK–STAT pathway to suppress glycolysis (i.e., reversal of Warburg effect) and mitochondrial oxidative phosphorylation—thereby limiting tumor energy metabolism and biosynthetic capacity (114–117). However, lymphoma cells can evade immune surveillance through multiple mechanisms. IFN-γ induces tumor cells to upregulate PD-L1, which binds to PD-1 on CD8+ T cells, leading to their inactivation, exhaustion, and reduced proliferation—ultimately establishing an immunosuppressive feedback loop (118–120). Additionally, lymphoma cells reprogram metabolism (e.g., increasing lactate production, competitively consuming glucose and amino acids in the TME) to further suppress CD8+ T cell function and facilitate immune evasion (121).
In DLBCL, both the density and functional status of CD8+ TILs are significantly associated with treatment response to R-CHOP (122). High CD8+ T cell infiltration predicts better complete response rates and prolonged PFS, suggesting that CD8+T cell-mediated immunometabolic regulation may influence chemosensitivity (123, 124). A similar relationship has been observed in FL, where CD8+ TILs correlate with lower progression risk and improved immunochemotherapy outcomes (125, 126).
In recent years, immunotherapy for CD8+T cell metabolism has made progress in relapsed/refractory DLBCL and FL, but the problem of drug resistance needs to be solved urgently (127–130). Recent studies have found that the abnormally activated fibroblast activation protein (FAP) - positive fibroblast reticular cells (FRCS) in DLBCL can inhibit the function of CD8+T cells, and the combination of FAP targeted drugs and glofitamab can significantly enhance the anti-tumor activity of TIL (131). This suggests that simultaneously targeting T cell metabolism and tumor microenvironment may be a new direction to overcome drug resistance. FAP is a type II transmembrane serine protease, which is characterized by high expression on the surface of cancer associated fibroblasts (CAF), but is highly restricted in most normal adult tissues. This unique expression pattern makes it a potential therapeutic target in the tumor microenvironment (132–134).
2.2.3 Impact of macrophage polarization on tumor metabolism
Macrophages, as abundant and functionally diverse immune cells within the TME, exert significant influence on tumor metabolism depending on their polarization status (135, 136). Owing to their plasticity, macrophages can differentiate into distinct functional phenotypes in response to environmental cues, primarily the classically activated M1 phenotype and the alternatively activated M2 phenotype (135, 136). These polarization states exhibit opposing roles in tumor metabolic regulation and thus critically shape tumor progression and therapeutic responses (135, 136).
M1 macrophages, considered anti-tumor “guardians,” are typically induced by proinflammatory stimuli such as IFN-γ and lipopolysaccharide (LPS) (137–139). They exert cytotoxic effects through secretion of tumor necrosis factor-α (TNF-α), nitric oxide (NO), and ROS, which not only directly kill tumor cells but also suppress their metabolic activity (140). Functionally, M1 macrophages undergo metabolic reprogramming characterized by a shift from oxidative phosphorylation (OXPHOS) to aerobic glycolysis (Warburg effect), enabling rapid ATP generation and provision of metabolic intermediates to sustain proinflammatory responses (141, 142). High expression of inducible nitric oxide synthase (iNOS) in M1 macrophages promotes arginine catabolism to NO, which damages mitochondrial function and induces apoptosis in tumor cells (143). Conversely, M2 macrophages are induced by anti-inflammatory cytokines such as IL-4, IL-10, and IL-13, and generally exhibit tumor-promoting properties (140). M2 macrophages rely primarily on OXPHOS for energy production, which allows them to adapt to the hypoxic and nutrient-depleted TME (142). This may seem contradictory, but it actually stems from its metabolic flexibility. M2 macrophages maintain energy homeostasis and perform tumor promoting functions under hypoxic conditions by enhancing mitochondrial efficiency, using alternative substrates (such as fatty acids) for oxidative phosphorylation, and coordinating HIF-1 α and AMPK signaling pathways (142, 144). In FL—an indolent B-cell malignancy that may transform into aggressive DLBCL—macrophage polarization is closely linked to tumor metabolism and disease progression (145). A high infiltration of M2 macrophages in FL patients correlates with increased risk of relapse and poorer prognosis (146). Mechanistically, M2 macrophages promote FL progression via multiple pathways. First, cytokines such as IL-10 and TGF-β activate tumor-intrinsic PI3K-AKT-mTOR and STAT3 signaling pathways, enhancing glycolysis and lipid biosynthesis in lymphoma cells (147, 148); Second, M2 macrophages suppress anti-tumor immune responses, fostering an immune-privileged niche for tumor survival (149). Collectively, these effects enhance tumor proliferation, drug resistance, and transformation risk in FL.
In conclusion, the polarization state of macrophages plays a key role in the regulation of tumor metabolism. Intervention strategies for macrophage polarization (such as promoting M1 polarization or inhibiting M2 polarization) may become a new direction to improve tumor microenvironment and enhance anti-tumor efficacy (150–153). Especially in lymphoma such as DLBCL and FL, the treatment method targeting macrophage polarization is expected to provide a new breakthrough for inhibiting disease progression (154–156).
2.2.4 Cytokine-mediated regulation of tumor metabolism
Cytokines serve as pivotal mediators of immune cell communication and play key roles in shaping tumor metabolic phenotypes (157). In both DLBCL and FL, cytokines modulate metabolic reprogramming to promote tumor progression (158). IL-6 and IL-10, for example, activate the STAT3 pathway to enhance glycolysis and glutathione metabolism, supplying energy and biosynthetic precursors for rapid tumor cell proliferation (159, 160). Although IL-16 was previously linked to cutaneous T-cell lymphoma (161), recent studies have revealed its role in DLBCL (162). IL-16 recruits CD4+ monocytes into the TME, promoting macrophage infiltration, angiogenesis, and upregulation of tumor-promoting cytokines such as IL-6 and IL-10 (162). Notably, IL-16 also suppresses T cell infiltration, collectively facilitating tumor progression (162).
Cytokines and their metabolic effects are not only mechanistic drivers but also valuable prognostic markers. Elevated serum levels of IL-6 and IL-10 are consistently associated with poor prognosis, higher tumor aggressiveness, and shorter survival in patients with DLBCL and FL (163–166). LDH, a key enzyme reflecting cytokine-driven glycolysis, remains a classic independent prognostic factor in both the DLBCL IPI and FL risk models. Moreover, the abundance of immunosuppressive cells such as M2 tumor-associated macrophages, induced by cytokines like IL-10, has been linked to adverse outcomes and higher risk of histologic transformation—particularly in FL (167, 168).
Understanding how specific cytokines drive metabolic programs provides a biological rationale for risk stratification and therapy development. Although agents such as the IL-6R monoclonal antibody tocilizumab have demonstrated potential in reversing cytokine-induced metabolic dependency, challenges remain due to cytokine network redundancy and microenvironmental heterogeneity (169). Future research should focus on identifying dominant cytokine-metabolism axes in different lymphoma subtypes and investigating their downstream metabolites as dynamic biomarkers to inform personalized treatment strategies.
2.2.5 Immune checkpoint molecules and tumor metabolism
The PD-1/PD-L1 axis, a central immune checkpoint pathway, not only suppresses T cell anti-tumor activity but also directly reshapes the metabolic landscape of the TME through metabolic reprogramming (170–172). n B-cell lymphomas, PD-1 activation downregulates the PI3K/Akt/mTOR pathway and its downstream effector MYC, resulting in suppressed glycolysis and enhanced fatty acid β-oxidation (172, 173). This metabolic shift contributes to T cell exhaustion and reinforces PD-1 expression—forming a feedforward loop—while also remodeling the TME to favor immune evasion and tumor progression (174). Interestingly, PD-1/PD-L1 interactions may also activate oncogenic signaling in tumor cells. Dong et al. demonstrated that PD-1 binding to PD-L1 directly activates the AKT/mTOR pathway in DLBCL cells (175),echoing findings from Lastwika et al. in NSCLC, where AKT/mTOR activation upregulates PD-L1 expression to facilitate immune escape (176), These results suggest a positive feedback loop between PD-1/PD-L1 and PI3K/AKT/mTOR signaling that may enhance DLBCL aggressiveness. Hence, combined blockade of PD-1/PD-L1 and AKT/mTOR signaling may represent a promising therapeutic strategy for specific DLBCL subsets.
Emerging checkpoints such as LAG-3 and TIM-3 have attracted attention for their immunomodulatory roles in DLBCL and FL, although their involvement in metabolic regulation remains to be fully elucidated. LAG-3 co-expresses with PD-1 and suppresses T cell function, possibly through similar metabolic mechanisms (177, 178). LAG-3 is also a surface marker of Tregs and may facilitate their suppressive function (179). Notably, Tregs suppress effector T cells through metabolic competition—such as glucose depletion—suggesting that LAG-3 may participate in shaping T cell metabolic reprogramming (180). TIM-3 may impair mitochondrial function and oxidative phosphorylation via the HMGB1/galectin-9 axis, thereby promoting immune suppression and altering TME metabolism (181–183). Co-expression of LAG-3 and TIM-3 with PD-1 may amplify these metabolic effects (184). Preclinical studies indicate that dual blockade of LAG-3 and PD-1 reduces tumor progression and improves anti-tumor T cell responses, underscoring the potential role of metabolic reprogramming as a convergent downstream effect of multi-checkpoint inhibition (185).
However, direct evidence of LAG-3 and TIM-3 regulating metabolism in B-cell lymphomas remains limited. Integrative single-cell metabolomics and immune checkpoint profiling are needed to elucidate whether these molecules contribute to immune escape through metabolic reshaping, and to inform the design of rational combination therapies targeting both immune checkpoints and tumor metabolism.
3 Clinical relevance of metabolic parameters
3.1 Definition and measurement of tumor metabolic volume and glycolytic activity
TMV and glycolytic activity are critical parameters reflecting the metabolic state of tumors, commonly assessed by positron emission tomography/computed tomography (PET/CT) combined with radiolabeled glucose analogs such as 18F-fluorodeoxyglucose (18F-FDG) (186, 187). TMV represents the volumetric extent of FDG uptake within tumor tissue, thereby illustrating the spatial distribution of metabolically active tumor cells (188). Glycolytic activity is quantified by standardized uptake values (SUV), indicating the intensity of glucose uptake and metabolism by tumor cells (189). These parameters not only provide a direct visualization of tumor metabolic activity but also enable dynamic assessment of intratumoral heterogeneity. With advancements in imaging technologies, PET/CT-derived metabolic parameters have become indispensable tools for diagnosis, staging, and therapeutic response evaluation in DLBCL and FL (190).
3.2 Correlation of metabolic parameters with disease risk and prognosis
The prognostic value of metabolic parameters such as TMV and glycolytic activity in DLBCL and FL has been extensively investigated and closely associates with disease risk and clinical outcomes. In DLBCL, elevated TMV and increased glycolytic activity typically denote a more aggressive disease phenotype and correlate significantly with adverse prognostic factors, including higher IPI scores and advanced disease stages, as well as inferior treatment responses (191). Moreover, enhanced metabolic activity may promote tumor immune evasion by lactate-mediated suppression of T cell function, further accelerating disease progression. Similarly, in the indolent yet potentially transformative FL, elevated metabolic activity, as evidenced by increased FDG uptake, associates with higher histologic grades, greater risk of disease progression and transformation, and shortened PFS (192–195). Metabolic parameters have emerged as robust prognostic indicators. Baseline TMV and total lesion glycolysis (TLG) serve as independent predictors of poor PFS and overall survival (OS) in DLBCL (196, 197), whereas metabolic remission status assessed by interim PET/CT effectively predicts therapeutic response and relapse risk in FL, correlating with long-term survival outcomes (198). These findings underscore the utility of metabolic parameters in risk stratification and individualized treatment decision-making. For instance, DLBCL patients exhibiting high metabolic activity might benefit from intensified therapeutic regimens or agents targeting metabolic pathways, while FL patients with low metabolic activity may be candidates for watchful waiting strategies.
Although metabolic parameters show great prognostic potential, their clinical application still faces challenges. The most critical point is that there are differences in pet/ct scanners, imaging protocols and image analysis software used by different medical institutions, which may lead to the lack of direct comparability of the measured values of SUVmax, TMV, TLG and other parameters (199). This standardization problem limits the wide applicability of these indicators in cross center research and clinical practice to a certain extent. In the future, it is necessary to solve this problem by establishing unified imaging guidelines, calibration standards and automated analysis processes.
4 Clinical relevance of immune parameters
4.1 Definition and measurement of TIL density and immune checkpoint molecule expression
TILs density and expression of immune checkpoint molecules such as LAG-3 and TIM-3 are key metrics for evaluating the tumor immune microenvironment. TIL density refers to the quantity and spatial distribution of lymphocytes infiltrating tumor tissue—primarily CD8+ and CD4+ T cells—quantified by immunohistochemistry (IHC) or multiplex immunofluorescence techniques. The expression levels of immune checkpoints PD-L1, LAG-3, and TIM-3 are typically assessed by IHC or flow cytometry. PD-L1 expression on tumor cell surfaces binds to PD-1 on T cells, suppressing their activation and function to facilitate immune evasion. Similarly, LAG-3 (lymphocyte activation gene-3) and TIM-3 (T cell immunoglobulin and mucin domain-containing protein-3) are inhibitory receptors on T cells; their overexpression also dampens anti-tumor T cell activity, exacerbating immune escape. The synergistic action of these molecules creates a multifaceted immunosuppressive milieu, impacting the efficacy of immunotherapies.
4.2 Association of immune parameters with disease risk and prognosis
As discussed in the previous section (2.2.5), immune checkpoint molecules such as PD-1/PD-L1 can mediate immunosuppression through metabolic reprogramming, and their expression levels also have important prognostic value in clinic. In both DLBCL and FL, TIL density and the expression levels of PD-1/PD-L1 and emerging immune checkpoints LAG-3 and TIM-3 correlate strongly with disease risk and prognosis. In DLBCL, higher TIL density—particularly CD8+ T cell infiltration—is generally associated with favorable outcomes, including prolonged PFS and OS (200–202). However, the presence of immunosuppressive cells such as Tregs and M2 macrophages may attenuate CD8+ T cell anti-tumor efficacy and affect therapeutic responses (112, 202). PD-L1 expression in DLBCL has a dual role: high expression may reflect tumor immune evasion via checkpoint pathways (203), but also predicts enhanced sensitivity to PD-1/PD-L1 blockade therapies (204). Emerging immune checkpoints LAG-3 and TIM-3 are increasingly recognized for their immunoregulatory functions in DLBCL. LAG-3 is highly expressed on exhausted T cells, contributing to T cell dysfunction and immune escape (205), with its expression linked to shorter disease-free survival (DFS) (206). Similarly, TIM-3 expression correlates with T cell exhaustion and portends poorer prognosis (207). Notably, co-expression of PD-1, TIM-3, and LAG-3 is associated with inferior PFS and OS, suggesting that combinatorial blockade of these checkpoints could be a promising therapeutic avenue (208).
The immune microenvironment of FL is typically characterized by profound immunosuppression, where diverse immune cell populations collectively influence disease progression and transformation risk. Elevated CD68+ macrophage counts, diffuse infiltration of FOXP3+ Tregs, and high PD-L1 expression are all linked to shorter time to transformation (209). Remarkably, pre-treatment PD-1 expression in tumor tissue predicts subsequent FL transformation into DLBCL (210). In addition to PD-1, other potential predictive markers have also attracted much attention, such as high-frequency gene mutations (such as EZH2, TP53), the gene map of circulating tumor DNA (ctDNA), and the distribution characteristics of M2 macrophages or specific T cell subsets in the microenvironment (211–214). Integrating multi omics markers may build a more accurate transformation prediction model.LAG-3 and TIM-3 also play critical roles in FL immune escape. The FL microenvironment often features exhausted T cells, with high LAG-3 and TIM-3 expression further amplifying immunosuppression. Increased LAG-3+ TILs associate with disease progression and poor prognosis (215), whereas elevated TIM-3 expression may reduce immunotherapy responsiveness (216). These findings highlight the potential of targeting LAG-3 and TIM-3, particularly in combination with PD-1/PD-L1 inhibitors, to develop novel treatments for FL.
5 Conclusion and perspectives
The interaction between metabolism and immune microenvironment opens up a new way for the treatment of DLBCL and FL. Studies have shown that metabolic reprogramming and immunosuppressive microenvironment characteristics are closely related to disease progression, treatment response and prognosis, which provides a theoretical basis for the development of innovative therapies (217, 218). In terms of clinical transformation, targeting key metabolic pathways such as IDHA inhibitors to regulate glycolysis, IDO1 inhibitors to interfere with tryptophan metabolism, and CD73 inhibitors to block adenosine signaling can effectively reverse the immunosuppressive state and enhance the antitumor activity of T cells and NK cells (219–221). In the field of immunotherapy optimization, for the limited efficacy of PD-1/PD-L1 inhibitors, the combination of metabolic regulators such as metformin or novel immune checkpoint inhibitors such as LAG-3/Tim-3 blockers is expected to improve the treatment response (222, 223). At the same time, the strategies of regulating macrophage polarization to promote M1 transformation and inhibiting Treg function by targeting CD39/adenosine axis also showed good application prospects (26, 224). In terms of prognosis evaluation, the integration of metabolic parameters such as TMV and glycolytic activity, as well as immune characteristics such as TILs density and PD-L1/LAG-3 expression can significantly improve the prediction accuracy of existing prognosis models such as IPI and FLIPI (225, 226), while dynamic monitoring technologies such as PET/CT combined with liquid biopsy provide new ideas for early efficacy evaluation and recurrence warning (227, 228).
Looking forward to the future, multi omics integration research including single-cell sequencing, spatial transcriptome and metabonomics will deeply reveal the cell-specific mechanism of metabolism immune interaction, especially focusing on the key drivers of FL to DLBCL transformation (145, 229). In terms of treatment strategy development, it is very important to design a joint scheme targeting the dual pathways of metabolism and immunity, such as PHGDH inhibitor combined with PD-1 inhibitor, and verify its efficacy through clinical trials. The combination of strategies targeting the metabolism immune axis and emerging immunotherapies (such as CAR-T cells and bispecific antibodies) has great potential (92, 230). For example, the use of IDHA inhibitors or A2AR antagonists to improve the immunosuppressive status of TME may reverse the depletion of CAR-T cells in vivo and enhance their persistence and anti-tumor efficacy (230, 231). Similarly, during bispecific antibody therapy, simultaneous intervention of adenosine or tryptophan metabolic pathways is expected to relieve the inhibition of endogenous T cells and produce synergistic anti-tumor effects (92). These joint strategies will become an important direction of clinical transformation research in the next step. At the same time, microenvironment remodeling strategies such as regulating cytokines such as IL-6/IL-10 or intervening in nutritional competition may significantly enhance treatment sensitivity (166, 232, 233). In terms of technological innovation, the development of non-invasive monitoring technologies such as imageomics virtual biopsy, and the establishment of PDX model and organ like platform will greatly promote the process of drug research and development and clinical transformation. Finally, an individualized treatment system based on patient specific metabolic and immune characteristics such as TME typing, such as the design of IDHA inhibitors combined with immunotherapy for patients with high lactic acid microenvironment, will promote lymphoma treatment into a new era of precision medicine.
Author contributions
CC: Conceptualization, Writing – original draft. WG: Visualization, Writing – original draft. HW: Writing – original draft. LC: Writing – original draft. OB: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Department of Finance of Jilin Province(JLSWSRCZX2023-8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KY declared a past co-authorship with the authors WG, HW, LC, and OB to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yamauchi N and Maruyama D. Current treatment approach and future perspectives in B cell lymphoma. Int J Hematol. (2025) 121:342–55. doi: 10.1007/s12185-024-03879-w
2. Brauge B, Dessauge E, Creusat F, and Tarte K. Modeling the crosstalk between Malignant B cells and their microenvironment in B-cell lymphomas: challenges and opportunities. Front Immunol. (2023) 14:1288110. doi: 10.3389/fimmu.2023.1288110
3. Carreras J, Ikoma H, Kikuti YY, Miyaoka M, Hiraiwa S, Tomita S, et al. Mutational, immune microenvironment, and clinicopathological profiles of diffuse large B-cell lymphoma and follicular lymphoma with bcl6 rearrangement. Virchows Arch. (2024) 484:657–76. doi: 10.1007/s00428-024-03774-z
4. Nastoupil LJ and Bartlett NL. Navigating the evolving treatment landscape of diffuse large B-cell lymphoma. J Clin Oncol. (2023) 41:903–13. doi: 10.1200/jco.22.01848
5. Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, and Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. (2013) 87:146–71. doi: 10.1016/j.critrevonc.2012.12.009
6. Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, López-Guillermo A, et al. Follicular lymphoma. Nat Rev Dis Primers. (2019) 5:83. doi: 10.1038/s41572-019-0132-x
7. Chung C. A promising future for precision epigenetic therapy for follicular and diffuse large B-cell lymphoma? Blood Lymphat Cancer. (2022) 12:99–106. doi: 10.2147/blctt.S282247
8. Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, et al. Chop-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the mabthera international trial (Mint) group. Lancet Oncol. (2011) 12:1013–22. doi: 10.1016/s1470-2045(11)70235-2
9. Iacoboni G, Zucca E, Ghielmini M, and Stathis A. Methodology of clinical trials evaluating the incorporation of new drugs in the first-line treatment of patients with diffuse large B-cell lymphoma (Dlbcl): A critical review. Ann Oncol. (2018) 29:1120–9. doi: 10.1093/annonc/mdy113
10. Cheng S and Liu Y. Advances in personalized treatment and prognostic factors of follicular lymphoma. Curr Treat Options Oncol. (2025) 26:313–30. doi: 10.1007/s11864-025-01297-6
11. Hanel W and Epperla N. Evolving therapeutic landscape in follicular lymphoma: A look at emerging and investigational therapies. J Hematol Oncol. (2021) 14:104. doi: 10.1186/s13045-021-01113-2
12. Hilton LK, Ngu HS, Collinge B, Dreval K, Ben-Neriah S, Rushton CK, et al. Relapse timing is associated with distinct evolutionary dynamics in diffuse large B-cell lymphoma. J Clin Oncol. (2023) 41:4164–77. doi: 10.1200/jco.23.00570
13. Bock AM and Epperla N. Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: recent advances and emerging therapies. J Hematol Oncol. (2025) 18:68. doi: 10.1186/s13045-025-01702-5
14. Vaughn JL and Epperla N. Survival of patients with transformed follicular lymphoma in the United States: A multiple cohort study. biomark Res. (2023) 11:84. doi: 10.1186/s40364-023-00525-1
15. Li ZH, Zhang MY, Federico M, Civallero M, Manni M, Alonso-Alvarez S, et al. Early histological transformation of follicular lymphoma to diffuse large B-cell lymphoma indicating adverse survival: A population-based analysis and validation. Cancer. (2024) 130:3321–32. doi: 10.1002/cncr.35378
16. Serganova I, Chakraborty S, Yamshon S, Isshiki Y, Bucktrout R, Melnick A, et al. Epigenetic, metabolic, and immune crosstalk in germinal-center-derived B-cell lymphomas: unveiling new vulnerabilities for rational combination therapies. Front Cell Dev Biol. (2021) 9:805195. doi: 10.3389/fcell.2021.805195
17. Ohshima K and Morii E. Metabolic reprogramming of cancer cells during tumor progression and metastasis. Metabolites. (2021) 11(1). doi: 10.3390/metabo11010028
18. Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP, et al. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond). (2023) 43:525–61. doi: 10.1002/cac2.12416
19. Choi JW, Dean EA, Lu H, Thompson Z, Qi J, Krivenko G, et al. Repeatability of metabolic tumor burden and lesion glycolysis between clinical readers. Front Immunol. (2023) 14:994520. doi: 10.3389/fimmu.2023.994520
20. So H, Lee H, Hyun SH, Cho YS, Moon SH, Choi JY, et al. Metabolic bulk volume from fdg pet as an independent predictor of progression-free survival in follicular lymphoma. Front Oncol. (2023) 13:1283582. doi: 10.3389/fonc.2023.1283582
21. Wang X, Chen Y, and Huang Y. Prognostic value of 18f-fdg pet/ct parameters at baseline and end of treatment in follicular lymphoma: A systematic review and meta-analysis. Acad Radiol. (2025). doi: 10.1016/j.acra.2025.08.003
22. Autio M, Leivonen SK, Brück O, Karjalainen-Lindsberg ML, Pellinen T, and Leppä S. Clinical impact of immune cells and their spatial interactions in diffuse large B-cell lymphoma microenvironment. Clin Cancer Res. (2022) 28:781–92. doi: 10.1158/1078-0432.Ccr-21-3140
23. Yu T, Xu-Monette ZY, Lagoo A, Shuai W, Wang B, Neff J, et al. Flow cytometry quantification of tumor-infiltrating lymphocytes to predict the survival of patients with diffuse large B-cell lymphoma. Front Immunol. (2024) 15:1335689. doi: 10.3389/fimmu.2024.1335689
24. Cho Y, Lee J, Han B, Yoon SE, Kim SJ, Kim WS, et al. Tumor-infiltrating T lymphocytes evaluated using digital image analysis predict the prognosis of patients with diffuse large B-cell lymphoma. J Pathol Transl Med. (2024) 58:12–21. doi: 10.4132/jptm.2023.11.02
25. Maharaj K, Uriepero A, Sahakian E, and Pinilla-Ibarz J. Regulatory T cells (Tregs) in lymphoid Malignancies and the impact of novel therapies. Front Immunol. (2022) 13:943354. doi: 10.3389/fimmu.2022.943354
26. Xiong X, Xie X, Wang Z, Zhang Y, and Wang L. Tumor-associated macrophages in lymphoma: from mechanisms to therapy. Int Immunopharmacol. (2022) 112:109235. doi: 10.1016/j.intimp.2022.109235
27. Koppenol WH, Bounds PL, and Dang CV. Otto warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. (2011) 11:325–37. doi: 10.1038/nrc3038
28. Payen VL, Mina E, Van Hée VF, Porporato PE, and Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. (2020) 33:48–66. doi: 10.1016/j.molmet.2019.07.006
29. Singh M, Afonso J, Sharma D, Gupta R, Kumar V, Rani R, et al. Targeting monocarboxylate transporters (Mcts) in cancer: how close are we to the clinics? Semin Cancer Biol. (2023) 90:1–14. doi: 10.1016/j.semcancer.2023.01.007
30. Chen J, Huang Z, Chen Y, Tian H, Chai P, Shen Y, et al. Lactate and lactylation in cancer. Signal Transduct Target Ther. (2025) 10:38. doi: 10.1038/s41392-024-02082-x
31. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. Ldha-associated lactic acid production blunts tumor immunosurveillance by T and nk cells. Cell Metab. (2016) 24:657–71. doi: 10.1016/j.cmet.2016.08.011
32. Scott KE and Cleveland JL. Lactate wreaks havoc on tumor-infiltrating T and nk cells. Cell Metab. (2016) 24:649–50. doi: 10.1016/j.cmet.2016.10.015
33. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. (2007) 109:3812–9. doi: 10.1182/blood-2006-07-035972
34. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
35. Zhou J, Gu J, Qian Q, Zhang Y, Huang T, Li X, et al. Lactate supports treg function and immune balance via mgat1 effects on N-glycosylation in the mitochondria. J Clin Invest. (2024) 134(20). doi: 10.1172/jci175897
36. Jedlička M, Feglarová T, Janstová L, Hortová-Kohoutková M, and Frič J. Lactate from the tumor microenvironment - a key obstacle in nk cell-based immunotherapies. Front Immunol. (2022) 13:932055. doi: 10.3389/fimmu.2022.932055
37. Dai E, Wang W, Li Y, Ye D, and Li Y. Lactate and lactylation: behind the development of tumors. Cancer Lett. (2024) 591:216896. doi: 10.1016/j.canlet.2024.216896
38. Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. (2006) 107:2013–21. doi: 10.1182/blood-2005-05-1795
39. Kundu R, Chatterjee A, Nathani A, Shrivastava G, Bhavsar S, and Chaisson N. Type a versus type B lactic acidosis in the context of undiagnosed B-cell lymphoma. BMJ Case Rep. (2025) 18(6). doi: 10.1136/bcr-2024-264218
40. Wang C, Lv Z, and Zhang Y. Type B lactic acidosis associated with diffuse large B-cell lymphoma and the warburg effect. J Int Med Res. (2022) 50:3000605211067749. doi: 10.1177/03000605211067749
41. Sanivarapu R, Upadrista PK, Otero-Colon J, Shah K, Cadet B, Tao Q, et al. An oncological emergency: severe type B lactic acidosis from warburg effect in diffuse large B-cell lymphoma. Cureus. (2022) 14:e26557. doi: 10.7759/cureus.26557
42. Atamna B, Rozental A, Haj Yahia M, Itchaki G, Gurion R, Yeshurun M, et al. Tumor-associated lactic acidosis and early death in patients with lymphoma. Cancer Med. (2025) 14:e70824. doi: 10.1002/cam4.70824
43. Dean RK, Subedi R, Gill D, and Nat A. Consideration of alternative causes of lactic acidosis: thiamine deficiency in Malignancy. Am J Emerg Med. (2017) 35:1214.e5–.e6. doi: 10.1016/j.ajem.2017.05.016
44. Masood U, Sharma A, Nijjar S, and Sitaraman K. B-cell lymphoma, thiamine deficiency, and lactic acidosis. Proc (Bayl Univ Med Cent). (2017) 30:69–70. doi: 10.1080/08998280.2017.11929534
45. Pacei F, Tesone A, Laudi N, Laudi E, Cretti A, Pnini S, et al. The relevance of thiamine evaluation in a practical setting. Nutrients. (2020) 12(9). doi: 10.3390/nu12092810
46. Masaki A, Ishida T, Maeda Y, Ito A, Suzuki S, Narita T, et al. Clinical significance of tryptophan catabolism in follicular lymphoma. Hematol Oncol. (2020) 38:742–53. doi: 10.1002/hon.2804
47. Solvay M, Holfelder P, Klaessens S, Pilotte L, Stroobant V, Lamy J, et al. Tryptophan depletion sensitizes the ahr pathway by increasing ahr expression and gcn2/lat1-mediated kynurenine uptake, and potentiates induction of regulatory T lymphocytes. J Immunother Cancer. (2023) 11(6). doi: 10.1136/jitc-2023-006728
48. Campesato LF, Budhu S, Tchaicha J, Weng CH, Gigoux M, Cohen IJ, et al. Blockade of the ahr restricts a treg-macrophage suppressive axis induced by L-kynurenine. Nat Commun. (2020) 11:4011. doi: 10.1038/s41467-020-17750-z
49. Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce pd-1 expression in cd8(+) T cells by transferring kynurenine and ahr activation. Cancer Cell. (2018) 33:480–94.e7. doi: 10.1016/j.ccell.2018.02.005
50. Masaki A, Ishida T, Maeda Y, Suzuki S, Ito A, Takino H, et al. Prognostic significance of tryptophan catabolism in adult T-cell leukemia/lymphoma. Clin Cancer Res. (2015) 21:2830–9. doi: 10.1158/1078-0432.Ccr-14-2275
51. Guo D, Wang Y, Wu X, Gao Y, Wang A, Zhang Z, et al. Expression of tryptophan metabolism enzymes in patients with diffuse large B-cell lymphoma and nk/T-cell lymphoma. Cancer Med. (2023) 12:12139–48. doi: 10.1002/cam4.5903
52. Nakamura N, Hara T, Shimizu M, Mabuchi R, Nagano J, Ohno T, et al. Effects of indoleamine 2,3-dioxygenase inhibitor in non-hodgkin lymphoma model mice. Int J Hematol. (2015) 102:327–34. doi: 10.1007/s12185-015-1835-8
53. Suwa S, Kasubata A, Kato M, Iida M, Watanabe K, Miura O, et al. The tryptophan derivative, tranilast, and conditioned medium with indoleamine 2,3-dioxygenase-expressing cells inhibit the proliferation of lymphoid Malignancies. Int J Oncol. (2015) 46:1369–76. doi: 10.3892/ijo.2015.2825
54. Zhang X, Yang Y, Zhang L, Lu Y, Zhang Q, Fan D, et al. Mesenchymal stromal cells as vehicles of tetravalent bispecific tandab (Cd3/cd19) for the treatment of B cell lymphoma combined with ido pathway inhibitor D-1-methyl-tryptophan. J Hematol Oncol. (2017) 10:56. doi: 10.1186/s13045-017-0397-z
55. Yang M and Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. (2016) 16:650–62. doi: 10.1038/nrc.2016.81
56. Lee CM, Hwang Y, Kim M, Park YC, Kim H, and Fang S. Phgdh: A novel therapeutic target in cancer. Exp Mol Med. (2024) 56:1513–22. doi: 10.1038/s12276-024-01268-1
57. D’Avola A, Legrave N, Tajan M, Chakravarty P, Shearer RL, King HW, et al. Phgdh is required for germinal center formation and is a therapeutic target in myc-driven lymphoma. J Clin Invest. (2022) 132(9). doi: 10.1172/jci153436
58. Cai Z, Li W, Hager S, Wilson JL, Afjehi-Sadat L, Heiss EH, et al. Targeting phgdh reverses the immunosuppressive phenotype of tumor-associated macrophages through A-ketoglutarate and mtorc1 signaling. Cell Mol Immunol. (2024) 21:448–65. doi: 10.1038/s41423-024-01134-0
59. Wilson JL, Nägele T, Linke M, Demel F, Fritsch SD, Mayr HK, et al. Inverse data-driven modeling and multiomics analysis reveals phgdh as a metabolic checkpoint of macrophage polarization and proliferation. Cell Rep. (2020) 30:1542–52.e7. doi: 10.1016/j.celrep.2020.01.011
60. Yoo HC, Yu YC, Sung Y, and Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. (2020) 52:1496–516. doi: 10.1038/s12276-020-00504-8
61. Bernfeld E and Foster DA. Glutamine as an essential amino acid for kras-driven cancer cells. Trends Endocrinol Metab. (2019) 30:357–68. doi: 10.1016/j.tem.2019.03.003
62. Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, et al. Glutamine uptake and metabolism are coordinately regulated by erk/mapk during T lymphocyte activation. J Immunol. (2010) 185:1037–44. doi: 10.4049/jimmunol.0903586
63. Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. (2020) 130:3865–84. doi: 10.1172/jci131859
64. Byun JK, Park M, Lee S, Yun JW, Lee J, Kim JS, et al. Inhibition of glutamine utilization synergizes with immune checkpoint inhibitor to promote antitumor immunity. Mol Cell. (2020) 80:592–606.e8. doi: 10.1016/j.molcel.2020.10.015
65. Barberini L, Noto A, Fattuoni C, Satta G, Zucca M, Cabras MG, et al. The metabolomic profile of lymphoma subtypes: A pilot study. Molecules. (2019) 24(13). doi: 10.3390/molecules24132367
66. Gomez Solsona B, Horn H, Schmitt A, Xu W, Bucher P, Heinrich A, et al. Inhibition of glutaminase-1 in dlbcl potentiates venetoclax-induced antitumor activity by promoting oxidative stress. Blood Adv. (2023) 7:7433–44. doi: 10.1182/bloodadvances.2023010964
67. Wei P, Bott AJ, Cluntun AA, Morgan JT, Cunningham CN, Schell JC, et al. Mitochondrial pyruvate supports lymphoma proliferation by fueling a glutamate pyruvate transaminase 2-dependent glutaminolysis pathway. Sci Adv. (2022) 8:eabq0117. doi: 10.1126/sciadv.abq0117
68. Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. (2012) 22:547–60. doi: 10.1016/j.ccr.2012.08.014
69. Norberg E, Lako A, Chen PH, Stanley IA, Zhou F, Ficarro SB, et al. Differential contribution of the mitochondrial translation pathway to the survival of diffuse large B-cell lymphoma subsets. Cell Death Differ. (2017) 24:251–62. doi: 10.1038/cdd.2016.116
70. Li M, Teater MR, Hong JY, Park NR, Duy C, Shen H, et al. Translational activation of atf4 through mitochondrial anaplerotic metabolic pathways is required for dlbcl growth and survival. Blood Cancer Discov. (2022) 3:50–65. doi: 10.1158/2643-3230.Bcd-20-0183
71. Lee J, Mani A, Shin MJ, and Krauss RM. Leveraging altered lipid metabolism in treating B cell Malignancies. Prog Lipid Res. (2024) 95:101288. doi: 10.1016/j.plipres.2024.101288
72. Yano H, Fujiwara Y, Horlad H, Pan C, Kai K, Niino D, et al. Blocking cholesterol efflux mechanism is a potential target for antilymphoma therapy. Cancer Sci. (2022) 113:2129–43. doi: 10.1111/cas.15349
73. Jiang XN, Zhang Y, Wang WG, Sheng D, Zhou XY, and Li XQ. Alteration of cholesterol metabolism by metformin is associated with improved outcome in type ii diabetic patients with diffuse large B-cell lymphoma. Front Oncol. (2021) 11:608238. doi: 10.3389/fonc.2021.608238
74. Zhang Z, Zhao C, Yang S, Lu W, and Shi J. A novel lipid metabolism-based risk model associated with immunosuppressive mechanisms in diffuse large B-cell lymphoma. Lipids Health Dis. (2024) 23:20. doi: 10.1186/s12944-024-02017-z
75. Wang C, Zhang R, Zhang H, Gao H, Zhu Y, Jiao L, et al. Lipid metabolism-related gene signature predicts prognosis and unveils novel anti-tumor drugs in specific type of diffuse large B cell lymphoma. Mol Med. (2024) 30:210. doi: 10.1186/s10020-024-00988-4
76. Nowakowski GS, Maurer MJ, Habermann TM, Ansell SM, Macon WR, Ristow KM, et al. Statin use and prognosis in patients with diffuse large B-cell lymphoma and follicular lymphoma in the rituximab era. J Clin Oncol. (2010) 28:412–7. doi: 10.1200/jco.2009.23.4245
77. Chowdhury PS, Chamoto K, Kumar A, and Honjo T. Ppar-induced fatty acid oxidation in T cells increases the number of tumor-reactive cd8(+) T cells and facilitates anti-pd-1 therapy. Cancer Immunol Res. (2018) 6:1375–87. doi: 10.1158/2326-6066.Cir-18-0095
78. Feng M, Liu X, Hao X, Ren Y, Dong G, Tian J, et al. Fatty acids support the fitness and functionality of tumor-resident cd8+ T cells by maintaining scml4 expression. Cancer Res. (2023) 83:3368–84. doi: 10.1158/0008-5472.Can-23-0287
79. Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. Cd36-mediated ferroptosis dampens intratumoral cd8(+) T cell effector function and impairs their antitumor ability. Cell Metab. (2021) 33:1001–12.e5. doi: 10.1016/j.cmet.2021.02.015
80. Xu S, Chaudhary O, Rodríguez-Morales P, Sun X, Chen D, Zappasodi R, et al. Uptake of oxidized lipids by the scavenger receptor cd36 promotes lipid peroxidation and dysfunction in cd8(+) T cells in tumors. Immunity. (2021) 54:1561–77.e7. doi: 10.1016/j.immuni.2021.05.003
81. Allard B, Allard D, Buisseret L, and Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. (2020) 17:611–29. doi: 10.1038/s41571-020-0382-2
82. Vijayan D, Young A, Teng MWL, and Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. (2017) 17:709–24. doi: 10.1038/nrc.2017.86
83. Allard D, Allard B, and Stagg J. On the mechanism of anti-cd39 immune checkpoint therapy. J Immunother Cancer. (2020) 8(1). doi: 10.1136/jitc-2019-000186
84. Yegutkin GG, Henttinen T, Samburski SS, Spychala J, and Jalkanen S. The evidence for two opposite, atp-generating and atp-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem J. (2002) 367:121–8. doi: 10.1042/bj20020439
85. Casey M, Segawa K, Law SC, Sabdia MB, Nowlan B, Salik B, et al. Inhibition of cd39 unleashes macrophage antibody-dependent cellular phagocytosis against B-cell lymphoma. Leukemia. (2023) 37:379–87. doi: 10.1038/s41375-022-01794-9
86. Nakamura K, Casey M, Oey H, Vari F, Stagg J, Gandhi MK, et al. Targeting an adenosine-mediated “Don’t eat me signal” Augments anti-lymphoma immunity by anti-cd20 monoclonal antibody. Leukemia. (2020) 34:2708–21. doi: 10.1038/s41375-020-0811-3
87. Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, et al. Human follicular lymphoma cd39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. (2009) 183:6157–66. doi: 10.4049/jimmunol.0900475
88. Vaupel P and Multhoff G. Hypoxia-/hif-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting Malignant progression. Adv Exp Med Biol. (2018) 1072:171–5. doi: 10.1007/978-3-319-91287-5_27
89. Vaupel P and Multhoff G. Fatal alliance of hypoxia-/hif-1α-driven microenvironmental traits promoting cancer progression. Adv Exp Med Biol. (2020) 1232:169–76. doi: 10.1007/978-3-030-34461-0_21
90. Hatfield SM and Sitkovsky M. A2a adenosine receptor antagonists to weaken the hypoxia-hif-1α Driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. (2016) 29:90–6. doi: 10.1016/j.coph.2016.06.009
91. Hatfield SM, Kjaergaard J, Lukashev D, Belikoff B, Schreiber TH, Sethumadhavan S, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl). (2014) 92:1283–92. doi: 10.1007/s00109-014-1189-3
92. Kolbe C, Kauer J, Brinkmann B, Dreger P, Huber W, Müller-Tidow C, et al. Blocking the cd39/cd73 pathway synergizes with anti-cd20 bispecific antibody in nodal B-cell lymphoma. J Immunother Cancer. (2025) 13(1). doi: 10.1136/jitc-2024-009245
93. Zhang T, Liu H, Jiao L, Zhang Z, He J, Li L, et al. Genetic characteristics involving the pd-1/pd-L1/L2 and cd73/A2ar axes and the immunosuppressive microenvironment in dlbcl. J Immunother Cancer. (2022) 10(4). doi: 10.1136/jitc-2021-004114
94. Kang JH and Zappasodi R. Modulating treg stability to improve cancer immunotherapy. Trends Cancer. (2023) 9:911–27. doi: 10.1016/j.trecan.2023.07.015
95. Shan F, Somasundaram A, Bruno TC, Workman CJ, and Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. (2022) 8:944–61. doi: 10.1016/j.trecan.2022.06.008
96. Nishikawa H and Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. (2021) 9(7). doi: 10.1136/jitc-2021-002591
97. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. (2017) 25:1282–93.e7. doi: 10.1016/j.cmet.2016.12.018
98. Grzes KM, Field CS, and Pearce EJ. Treg cells survive and thrive in inhospitable environments. Cell Metab. (2017) 25:1213–5. doi: 10.1016/j.cmet.2017.05.012
99. Yang K, Blanco DB, Neale G, Vogel P, Avila J, Clish CB, et al. Homeostatic control of metabolic and functional fitness of T(Reg) cells by lkb1 signalling. Nature. (2017) 548:602–6. doi: 10.1038/nature23665
100. He N, Fan W, Henriquez B, Yu RT, Atkins AR, Liddle C, et al. Metabolic control of regulatory T cell (Treg) survival and function by lkb1. Proc Natl Acad Sci U.S.A. (2017) 114:12542–7. doi: 10.1073/pnas.1715363114
101. Flavell RA, Sanjabi S, Wrzesinski SH, and Licona-Limón P. The polarization of immune cells in the tumour environment by tgfbeta. Nat Rev Immunol. (2010) 10:554–67. doi: 10.1038/nri2808
102. Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector nk cells into type 1 innate lymphoid cells. Nat Immunol. (2017) 18:1004–15. doi: 10.1038/ni.3800
103. Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of il-10(+) and il-35(+) T(Reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. (2019) 20:724–35. doi: 10.1038/s41590-019-0346-9
104. Turnis ME, Sawant DV, Szymczak-Workman AL, Andrews LP, Delgoffe GM, Yano H, et al. Interleukin-35 limits anti-tumor immunity. Immunity. (2016) 44:316–29. doi: 10.1016/j.immuni.2016.01.013
105. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2
106. Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of T(Reg) cells in tumours. Nature. (2021) 591:306–11. doi: 10.1038/s41586-021-03235-6
107. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, and Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. (2010) 185:3190–8. doi: 10.4049/jimmunol.0903670
108. Churov A and Zhulai G. Targeting adenosine and regulatory T cells in cancer immunotherapy. Hum Immunol. (2021) 82:270–8. doi: 10.1016/j.humimm.2020.12.005
109. Spasevska I, Sharma A, Steen CB, Josefsson SE, Blaker YN, Kolstad A, et al. Diversity of intratumoral regulatory T cells in B-cell non-hodgkin lymphoma. Blood Adv. (2023) 7:7216–30. doi: 10.1182/bloodadvances.2023010158
110. Chang C, Wu SY, Kang YW, Lin KP, Chen TY, Medeiros LJ, et al. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am J Clin Pathol. (2015) 144:935–44. doi: 10.1309/ajcpujgmvv6zf4gg
111. Li X, Liu M, Shi Q, Fang Y, Fu D, Shen ZX, et al. Elevated serum il-13 level is associated with increased treg cells in tumor microenvironment and disease progression of diffuse large B-cell lymphoma. Hematol Oncol. (2023) 41:230–8. doi: 10.1002/hon.2993
112. Su X, Sun T, Li M, Xia Y, Li M, Wang D, et al. Lkb1 aggravates diffuse large B-cell lymphoma by promoting the function of treg cells and immune escape. J Transl Med. (2022) 20:378. doi: 10.1186/s12967-022-03588-0
113. Zhang L and Romero P. Metabolic control of cd8(+) T cell fate decisions and antitumor immunity. Trends Mol Med. (2018) 24:30–48. doi: 10.1016/j.molmed.2017.11.005
114. St Paul M and Ohashi PS. The roles of cd8(+) T cell subsets in antitumor immunity. Trends Cell Biol. (2020) 30:695–704. doi: 10.1016/j.tcb.2020.06.003
115. Nishida M, Yamashita N, Ogawa T, Koseki K, Warabi E, Ohue T, et al. Mitochondrial reactive oxygen species trigger metformin-dependent antitumor immunity via activation of nrf2/mtorc1/P62 axis in tumor-infiltrating cd8t lymphocytes. J Immunother Cancer. (2021) 9(9). doi: 10.1136/jitc-2021-002954
116. Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, and Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. (2016) 354:481–4. doi: 10.1126/science.aaf6284
117. Jorgovanovic D, Song M, Wang L, and Zhang Y. Roles of ifn-Γ in tumor progression and regression: A review. biomark Res. (2020) 8:49. doi: 10.1186/s40364-020-00228-x
118. Ok CY and Young KH. Targeting the programmed death-1 pathway in lymphoid neoplasms. Cancer Treat Rev. (2017) 54:99–109. doi: 10.1016/j.ctrv.2017.01.009
119. Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, et al. Interferon-Γ-induced activation of jak1 and jak2 suppresses tumor cell susceptibility to nk cells through upregulation of pd-L1 expression. Oncoimmunology. (2015) 4:e1008824. doi: 10.1080/2162402x.2015.1008824
120. Xue W, Li W, Zhang T, Li Z, Wang Y, Qiu Y, et al. Anti-pd1 up-regulates pd-L1 expression and inhibits T-cell lymphoma progression: possible involvement of an ifn-Γ-associated jak-stat pathway. Onco Targets Ther. (2019) 12:2079–88. doi: 10.2147/ott.S187280
121. Liang P, Li Z, Chen Z, Chen Z, Jin T, He F, et al. Metabolic reprogramming of glycolysis, lipids, and amino acids in tumors: impact on cd8+ T cell function and targeted therapeutic strategies. FASEB J. (2025) 39:e70520. doi: 10.1096/fj.202403019R
122. Hou H, Luo Y, Tang G, Zhang B, Ouyang R, Wang T, et al. Dynamic changes in peripheral blood lymphocyte subset counts and functions in patients with diffuse large B cell lymphoma during chemotherapy. Cancer Cell Int. (2021) 21:282. doi: 10.1186/s12935-021-01978-w
123. Song JY, Nwangwu M, He TF, Zhang W, Meawad H, Bedell V, et al. Low T-cell proportion in the tumor microenvironment is associated with immune escape and poor survival in diffuse large B-cell lymphoma. Haematologica. (2023) 108:2167–77. doi: 10.3324/haematol.2022.282265
124. Zhu Q, Yang Y, Zeng Y, Chen K, Zhang Q, Wang L, et al. The significance of cd8(+) tumor-infiltrating lymphocytes exhaustion heterogeneity and its underlying mechanism in diffuse large B-cell lymphoma. Int Immunopharmacol. (2024) 137:112447. doi: 10.1016/j.intimp.2024.112447
125. Wahlin BE, Sundström C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clin Cancer Res. (2011) 17:4136–44. doi: 10.1158/1078-0432.Ccr-11-0264
126. Hagos YB, Akarca AU, Ramsay A, Rossi RL, Pomplun S, Ngai V, et al. High inter-follicular spatial co-localization of cd8+Foxp3+ with cd4+Cd8+ Cells predicts favorable outcome in follicular lymphoma. Hematol Oncol. (2022) 40:541–53. doi: 10.1002/hon.3003
127. Park J, Hsueh PC, Li Z, and Ho PC. Microenvironment-driven metabolic adaptations guiding cd8(+) T cell anti-tumor immunity. Immunity. (2023) 56:32–42. doi: 10.1016/j.immuni.2022.12.008
128. Hickman A, Koetsier J, Kurtanich T, Nielsen MC, Winn G, Wang Y, et al. Lfa-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with ctla-4 blockade. J Clin Invest. (2022) 132(28). doi: 10.1172/jci154152
129. Yan ZX, Dong Y, Qiao N, Zhang YL, Wu W, Zhu Y, et al. Cholesterol efflux from C1qb-expressing macrophages is associated with resistance to chimeric antigen receptor T cell therapy in primary refractory diffuse large B cell lymphoma. Nat Commun. (2024) 15:5183. doi: 10.1038/s41467-024-49495-4
130. Wu H, Tang X, Kim HJ, Jalali S, Pritchett JC, Villasboas JC, et al. Expression of klrg1 and cd127 defines distinct cd8(+) subsets that differentially impact patient outcome in follicular lymphoma. J Immunother Cancer. (2021) 9(7). doi: 10.1136/jitc-2021-002662
131. Apollonio B, Spada F, Petrov N, Cozzetto D, Papazoglou D, Jarvis P, et al. Tumor-activated lymph node fibroblasts suppress T cell function in diffuse large B cell lymphoma. J Clin Invest. (2023) 133(13). doi: 10.1172/jci166070
132. Li L, Wu J, Abdi R, Jewell CM, and Bromberg JS. Lymph node fibroblastic reticular cells steer immune responses. Trends Immunol. (2021) 42:723–34. doi: 10.1016/j.it.2021.06.006
133. Panocha D, Roet JEG, Kuipers JE, de Winde CM, and Mebius RE. Lymph node fibroblast-produced extracellular matrix shapes immune function. Trends Immunol. (2025) 46:229–43. doi: 10.1016/j.it.2025.02.002
134. Wei R, Song J, Liu C, Zhao Z, Liu X, Yamamoto M, et al. Fap upregulates pd-L1 expression in cancer-associated fibroblasts to exacerbate T cells dysfunction and suppress anti-tumor immunity. Cancer Lett. (2025) 612:217475. doi: 10.1016/j.canlet.2025.217475
135. Li M, Yang Y, Xiong L, Jiang P, Wang J, and Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. (2023) 16:80. doi: 10.1186/s13045-023-01478-6
136. Jin R, Neufeld L, and McGaha TL. Linking macrophage metabolism to function in the tumor microenvironment. Nat Cancer. (2025) 6:239–52. doi: 10.1038/s43018-025-00909-2
137. Basak U, Sarkar T, Mukherjee S, Chakraborty S, Dutta A, Dutta S, et al. Tumor-associated macrophages: an effective player of the tumor microenvironment. Front Immunol. (2023) 14:1295257. doi: 10.3389/fimmu.2023.1295257
138. Wang S, Liu R, Yu Q, Dong L, Bi Y, and Liu G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. (2019) 452:14–22. doi: 10.1016/j.canlet.2019.03.015
139. Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, et al. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). (2023) 137:1067–93. doi: 10.1042/cs20220531
140. Wang H, Wang X, Zhang X, and Xu W. The promising role of tumor-associated macrophages in the treatment of cancer. Drug Resist Update. (2024) 73:101041. doi: 10.1016/j.drup.2023.101041
141. Ye L, Jiang Y, and Zhang M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. (2022) 68:81–92. doi: 10.1016/j.cytogfr.2022.11.001
142. Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. biomark Res. (2021) 9:1. doi: 10.1186/s40364-020-00251-y
143. Kashfi K, Kannikal J, and Nath N. Macrophage reprogramming and cancer therapeutics: role of inos-derived no. Cells. (2021) 10(11). doi: 10.3390/cells10113194
144. Wang T, Liu H, Lian G, Zhang SY, Wang X, and Jiang C. Hif1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflammation. (2017) 2017:9029327. doi: 10.1155/2017/9029327
145. Sarkozy C, Wu S, Takata K, Aoki T, Neriah SB, Milne K, et al. Integrated single cell analysis reveals co-evolution of Malignant B cells and tumor micro-environment in transformed follicular lymphoma. Cancer Cell. (2024) 42:1003–17.e6. doi: 10.1016/j.ccell.2024.05.011
146. Gouni S, Marques-Piubelli ML, and Strati P. Follicular lymphoma and macrophages: impact of approved and novel therapies. Blood Adv. (2021) 5:4303–12. doi: 10.1182/bloodadvances.2021005722
147. Deng Y, Ma J, Zhao S, Yang M, Sun Y, and Zhang Q. Expression of glucose transporter-1 in follicular lymphoma affected tumor-infiltrating immunocytes and was related to progression of disease within 24 months. Transl Oncol. (2023) 28:101614. doi: 10.1016/j.tranon.2022.101614
148. Bai B, Horlad H, Saito Y, Ohnishi K, Fujiwara Y, Takeya M, et al. Role of stat3 activation in cell-cell interaction between B-cell lymphoma and macrophages: the in vitro study. J Clin Exp Hematop. (2013) 53:127–33. doi: 10.3960/jslrt.53.127
149. Pan Y, Yu Y, Wang X, and Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
150. Mantovani A, Allavena P, Marchesi F, and Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. (2022) 21:799–820. doi: 10.1038/s41573-022-00520-5
151. DeNardo DG and Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. (2019) 19:369–82. doi: 10.1038/s41577-019-0127-6
152. Cassetta L and Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. (2018) 17:887–904. doi: 10.1038/nrd.2018.169
153. Poh AR and Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. (2018) 8:49. doi: 10.3389/fonc.2018.00049
154. An R, Zhang Z, Zhang D, Li Y, Lin Y, Sun H, et al. A novel ezh1/2 dual inhibitor inhibits gcb dlbcl through cell cycle regulation and M2 tumor-associated macrophage polarization. J Biol Chem. (2024) 300:107788. doi: 10.1016/j.jbc.2024.107788
155. Valero JG, Matas-Céspedes A, Arenas F, Rodriguez V, Carreras J, Serrat N, et al. The receptor of the colony-stimulating factor-1 (Csf-1r) is a novel prognostic factor and therapeutic target in follicular lymphoma. Leukemia. (2021) 35:2635–49. doi: 10.1038/s41375-021-01201-9
156. Lou X, Fu J, Zhao X, Zhuansun X, Rong C, Sun M, et al. Mir-7e-5p downregulation promotes transformation of low-grade follicular lymphoma to aggressive lymphoma by modulating an immunosuppressive stroma through the upregulation of fasl in M1 macrophages. J Exp Clin Cancer Res. (2020) 39:237. doi: 10.1186/s13046-020-01747-z
157. Kureshi CT and Dougan SK. Cytokines in cancer. Cancer Cell. (2025) 43:15–35. doi: 10.1016/j.ccell.2024.11.011
158. Atallah-Yunes SA and Robertson MJ. Cytokine based immunotherapy for cancer and lymphoma: biology, challenges and future perspectives. Front Immunol. (2022) 13:872010. doi: 10.3389/fimmu.2022.872010
159. Lu L, Zhu F, Zhang M, Li Y, Drennan AC, Kimpara S, et al. Gene regulation and suppression of type I interferon signaling by stat3 in diffuse large B cell lymphoma. Proc Natl Acad Sci U.S.A. (2018) 115:E498–e505. doi: 10.1073/pnas.1715118115
160. Thuya WL, Cao Y, Ho PC, Wong AL, Wang L, Zhou J, et al. Insights into il-6/jak/stat3 signaling in the tumor microenvironment: implications for cancer therapy. Cytokine Growth Factor Rev. (2025) 85: 26–42. doi: 10.1016/j.cytogfr.2025.01.003
161. Richmond J, Tuzova M, Cruikshank W, and Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J Cell Physiol. (2014) 229:139–47. doi: 10.1002/jcp.24441
162. Guan X, Wang Y, Fang T, Wang J, Li R, Hao M, et al. Lymphoma cell-driven il-16 is expressed in activated B-cell-like diffuse large B-cell lymphomas and regulates the pro-tumor microenvironment. Haematologica. (2025) 110:425–38. doi: 10.3324/haematol.2024.285304
163. Li G and Li D. Relationship between il-10 gene polymorphisms and the risk of non-hodgkin lymphoma: A meta-analysis. Hum Immunol. (2016) 77:418–25. doi: 10.1016/j.humimm.2016.03.006
164. Mozas P, Rivas-Delgado A, Rivero A, Dlouhy I, Nadeu F, Balagué O, et al. High serum levels of il-2r, il-6, and tnf-A Are associated with higher tumor burden and poorer outcome of follicular lymphoma patients in the rituximab era. Leuk Res. (2020) 94:106371. doi: 10.1016/j.leukres.2020.106371
165. Bao C, Gu J, Huang X, You L, Zhou Z, and Jin J. Cytokine profiles in patients with newly diagnosed diffuse large B-cell lymphoma: il-6 and il-10 levels are associated with adverse clinical features and poor outcomes. Cytokine. (2023) 169:156289. doi: 10.1016/j.cyto.2023.156289
166. Castellanos SV, Castellanos MPR, Avendaño MCG, Asprilla MCA, Leal MSG, and Tirado G. Impact of the tumor microenvironment on progression and treatment response in lymphoma and chronic lymphocytic leukemia: A systematic review of the literature. Crit Rev Oncol Hematol. (2025) 213:104782. doi: 10.1016/j.critrevonc.2025.104782
167. Liu L, Li Y, and Li B. Interactions between cancer cells and tumor-associated macrophages in tumor microenvironment. Biochim Biophys Acta Rev Cancer. (2025) 1880:189344. doi: 10.1016/j.bbcan.2025.189344
168. Enemark MH, Jensen ML, Andersen MD, Plesner TL, Hamilton-Dutoit S, and Ludvigsen M. Impact of the immune landscape in follicular lymphoma: insights into histological transformation in the rituximab era. Cancers (Basel). (2024) 16:20. doi: 10.3390/cancers16203553
169. Hashwah H, Bertram K, Stirm K, Stelling A, Wu CT, Kasser S, et al. The il-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol Med. (2019) 11:e10576. doi: 10.15252/emmm.201910576
170. Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong W, et al. Regulatory mechanisms of pd-1/pd-L1 in cancers. Mol Cancer. (2024) 23:108. doi: 10.1186/s12943-024-02023-w
171. Guo D, Tong Y, Jiang X, Meng Y, Jiang H, Du L, et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of Iκbα. Cell Metab. (2022) 34:1312–24.e6. doi: 10.1016/j.cmet.2022.08.002
172. Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. Pd-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. (2015) 6:6692. doi: 10.1038/ncomms7692
173. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. Ctla-4 and pd-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. (2005) 25:9543–53. doi: 10.1128/mcb.25.21.9543-9553.2005
174. Beielstein AC and Pallasch CP. Tumor metabolism as a regulator of tumor-host interactions in the B-cell lymphoma microenvironment-fueling progression and novel brakes for therapy. Int J Mol Sci. (2019) 20(17). doi: 10.3390/ijms20174158
175. Dong L, Lv H, Li W, Song Z, Li L, Zhou S, et al. Co-expression of pd-L1 and P-akt is associated with poor prognosis in diffuse large B-cell lymphoma via pd-1/pd-L1 axis activating intracellular akt/mtor pathway in tumor cells. Oncotarget. (2016) 7:33350–62. doi: 10.18632/oncotarget.9061
176. Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of pd-L1 expression by oncogenic activation of the akt-mtor pathway in non-small cell lung cancer. Cancer Res. (2016) 76:227–38. doi: 10.1158/0008-5472.Can-14-3362
177. Andrews LP, Butler SC, Cui J, Cillo AR, Cardello C, Liu C, et al. Lag-3 and pd-1 synergize on cd8(+) T cells to drive T cell exhaustion and hinder autocrine ifn-Γ-dependent anti-tumor immunity. Cell. (2024) 187:4355–72.e22. doi: 10.1016/j.cell.2024.07.016
178. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules lag-3 and pd-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. (2012) 72:917–27. doi: 10.1158/0008-5472.Can-11-1620
179. Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of lag-3 in regulatory T cells. Immunity. (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
180. Vilbois S, Xu Y, and Ho PC. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer. (2024) 10:242–55. doi: 10.1016/j.trecan.2023.11.007
181. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating dcs suppress nucleic acid-mediated innate immune responses through interactions between the receptor tim-3 and the alarmin hmgb1. Nat Immunol. (2012) 13:832–42. doi: 10.1038/ni.2376
182. Zhu Q, Yang Y, Chen K, Zhang Q, Huang Y, and Jian S. Diffuse large B-cell lymphoma: the significance of cd8(+) tumor-infiltrating lymphocytes exhaustion mediated by tim3/galectin-9 pathway. J Transl Med. (2024) 22:174. doi: 10.1186/s12967-024-05002-3
183. Das M, Zhu C, and Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. (2017) 276:97–111. doi: 10.1111/imr.12520
184. Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. (2018) 558:454–9. doi: 10.1038/s41586-018-0206-z
185. Acharya N, Sabatos-Peyton C, and Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. (2020) 8(1). doi: 10.1136/jitc-2020-000911
186. Knaup H, Weindler J, van Heek L, Voltin CA, Fuchs M, Borchmann P, et al. Pet/ct reconstruction and its impact on [Measures of] metabolic tumor volume. Acad Radiol. (2024) 31:3020–5. doi: 10.1016/j.acra.2023.12.016
187. Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using pet-fdg imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. (1999) 2:159–71. doi: 10.1016/s1095-0397(99)00016-3
188. Im HJ, Bradshaw T, Solaiyappan M, and Cho SY. Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging. (2018) 52:5–15. doi: 10.1007/s13139-017-0493-6
189. Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, and Subramaniam RM. 18f-fdg metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. (2012) 53:709–15. doi: 10.2967/jnumed.111.099531
190. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on Malignant lymphomas imaging working group. J Clin Oncol. (2014) 32:3048–58. doi: 10.1200/jco.2013.53.5229
191. Zhang H, Xu Z, Zhou W, Chen J, Wei Y, Wu H, et al. Metabolic Tumor Volume from Baseline [18 f]Fdg Pet/Ct at Diagnosis Improves the Ipi Stratification in Patients with Diffuse Large B-Cell Lymphoma. Ann Hematol. (2024) 103:5891–902. doi: 10.1007/s00277-024-05717-9
192. Xie M, Wang L, Jiang Q, Luo X, Zhao X, Li X, et al. Significance of initial, interim and end-of-therapy (18)F-fdg pet/ct for predicting transformation risk in follicular lymphoma. Cancer Cell Int. (2021) 21:394. doi: 10.1186/s12935-021-02094-5
193. Jiang C, Qian C, Jiang Q, Zhou H, Jiang Z, Teng Y, et al. Virtual biopsy for non-invasive identification of follicular lymphoma histologic transformation using radiomics-based imaging biomarker from pet/ct. BMC Med. (2025) 23:49. doi: 10.1186/s12916-025-03893-7
194. Cottereau AS, Rebaud L, Trotman J, Feugier P, Nastoupil LJ, Bachy E, et al. Metabolic tumor volume predicts outcome in patients with advanced stage follicular lymphoma from the relevance trial. Ann Oncol. (2024) 35:130–7. doi: 10.1016/j.annonc.2023.10.121
195. Rossi C, Tosolini M, Gravelle P, Pericart S, Kanoun S, Evrard S, et al. Baseline suvmax is related to tumor cell proliferation and patient outcome in follicular lymphoma. Haematologica. (2022) 107:221–30. doi: 10.3324/haematol.2020.263194
196. Guo B, Tan X, Ke Q, and Cen H. Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: A meta-analysis. PloS One. (2019) 14:e0210224. doi: 10.1371/journal.pone.0210224
197. Kostakoglu L, Mattiello F, Martelli M, Sehn LH, Belada D, Ghiggi C, et al. Total metabolic tumor volume as a survival predictor for patients with diffuse large B-cell lymphoma in the goya study. Haematologica. (2022) 107:1633–42. doi: 10.3324/haematol.2021.278663
198. Liang JH, Zhang YP, Xia J, Ding CY, Wu W, Wang L, et al. Prognostic value of baseline and interim total metabolic tumor volume and total lesion glycolysis measured on 18f-fdg pet-ct in patients with follicular lymphoma. Cancer Res Treat. (2019) 51:1479–87. doi: 10.4143/crt.2018.649
199. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. Fdg pet/ct: eanm procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. (2015) 42:328–54. doi: 10.1007/s00259-014-2961-x
200. Xu-Monette ZY, Li Y, Snyder T, Yu T, Lu T, Tzankov A, et al. Tumor-infiltrating normal B cells revealed by immunoglobulin repertoire clonotype analysis are highly prognostic and crucial for antitumor immune responses in dlbcl. Clin Cancer Res. (2023) 29:4808–21. doi: 10.1158/1078-0432.Ccr-23-1554
201. Shi Y, Deng L, Song Y, Lin D, Lai Y, Zhou L, et al. Cd3+/cd8+ T-cell density and tumoral pd-L1 predict survival irrespective of rituximab treatment in chinese diffuse large B-cell lymphoma patients. Int J Hematol. (2018) 108:254–66. doi: 10.1007/s12185-018-2466-7
202. Autio M, Brück O, Pollari M, Karjalainen-Lindsberg ML, Beiske K, Jørgensen JM, et al. Characterization of tumor microenvironment and cell interaction patterns in testicular and diffuse large B-cell lymphomas. Haematologica. (2025) 110:1339–50. doi: 10.3324/haematol.2024.286267
203. Cheng Z, Dai Y, Wang J, Shi J, Ke X, and Fu L. High pd-L1 expression predicts poor prognosis in diffuse large B-cell lymphoma. Ann Hematol. (2018) 97:1085–8. doi: 10.1007/s00277-018-3266-0
204. Xie W, Medeiros LJ, Li S, Yin CC, Khoury JD, and Xu J. Pd-1/pd-L1 pathway and its blockade in patients with classic hodgkin lymphoma and non-hodgkin large-cell lymphomas. Curr Hematol Malig Rep. (2020) 15:372–81. doi: 10.1007/s11899-020-00589-y
205. Ma J, Yan S, Zhao Y, Yan H, Zhang Q, and Li X. Blockade of pd-1 and lag-3 expression on cd8+ T cells promotes the tumoricidal effects of cd8+ T cells. Front Immunol. (2023) 14:1265255. doi: 10.3389/fimmu.2023.1265255
206. William MI, Tantawy DA, Elsergany AR, El-Hawary AK, and Yussif SM. Immunohistochemical expression of pd1, lag3, and ctla4 in diffuse large B cell lymphoma, clinicopathological correlation, and prognostic value. J Egypt Natl Canc Inst. (2025) 37:47. doi: 10.1186/s43046-025-00303-0
207. Zhang L, Du H, Xiao TW, Liu JZ, Liu GZ, Wang JX, et al. Prognostic value of pd-1 and tim-3 on cd3+ T cells from diffuse large B-cell lymphoma. BioMed Pharmacother. (2015) 75:83–7. doi: 10.1016/j.biopha.2015.08.037
208. Chen BJ, Dashnamoorthy R, Galera P, Makarenko V, Chang H, Ghosh S, et al. The immune checkpoint molecules pd-1, pd-L1, tim-3 and lag-3 in diffuse large B-cell lymphoma. Oncotarget. (2019) 10:2030–40. doi: 10.18632/oncotarget.26771
209. Xie M, Jiang Q, Zhao S, Zhao J, Ye X, and Qian W. Prognostic value of tissue-infiltrating immune cells in tumor microenvironment of follicular lymphoma: A meta-analysis. Int Immunopharmacol. (2020) 85:106684. doi: 10.1016/j.intimp.2020.106684
210. Beck Enemark M, Monrad I, Madsen C, Lystlund Lauridsen K, Honoré B, Plesner TL, et al. Pd-1 expression in pre-treatment follicular lymphoma predicts the risk of subsequent high-grade transformation. Onco Targets Ther. (2021) 14:481–9. doi: 10.2147/ott.S289337
211. Huet S, Xerri L, Tesson B, Mareschal S, Taix S, Mescam-Mancini L, et al. Ezh2 alterations in follicular lymphoma: biological and clinical correlations. Blood Cancer J. (2017) 7:e555. doi: 10.1038/bcj.2017.32
212. Yoon SE, Shin SH, Nam DK, Cho J, Kim WS, and Kim SJ. Feasibility of circulating tumor DNA analysis in patients with follicular lymphoma. Cancer Res Treat. (2024) 56:920–35. doi: 10.4143/crt.2023.869
213. Kridel R, Chan FC, Mottok A, Boyle M, Farinha P, Tan K, et al. Histological transformation and progression in follicular lymphoma: A clonal evolution study. PloS Med. (2016) 13:e1002197. doi: 10.1371/journal.pmed.1002197
214. Araujo-Ayala F and Béguelin W. Biology as vulnerability in follicular lymphoma: genetics, epigenetics, and immunogenetics. Blood. (2025) 16:blood.2024026020. doi: 10.1182/blood.2024026020
215. Yang ZZ, Kim HJ, Villasboas JC, Chen YP, Price-Troska T, Jalali S, et al. Expression of lag-3 defines exhaustion of intratumoral pd-1(+) T cells and correlates with poor outcome in follicular lymphoma. Oncotarget. (2017) 8:61425–39. doi: 10.18632/oncotarget.18251
216. Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, et al. Il-12 upregulates tim-3 expression and induces T cell exhaustion in patients with follicular B cell non-hodgkin lymphoma. J Clin Invest. (2012) 122:1271–82. doi: 10.1172/jci59806
217. Pi M, Kuang H, Yue C, Yang Q, Wu A, Li Y, et al. Targeting metabolism to overcome cancer drug resistance: A promising therapeutic strategy for diffuse large B cell lymphoma. Drug Resist Update. (2022) 61:100822. doi: 10.1016/j.drup.2022.100822
218. Taylor JG and Gribben JG. Microenvironment abnormalities and lymphomagenesis: immunological aspects. Semin Cancer Biol. (2015) 34:36–45. doi: 10.1016/j.semcancer.2015.07.004
219. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U.S.A. (2010) 107:2037–42. doi: 10.1073/pnas.0914433107
220. Prendergast GC, Malachowski WP, DuHadaway JB, and Muller AJ. Discovery of ido1 inhibitors: from bench to bedside. Cancer Res. (2017) 77:6795–811. doi: 10.1158/0008-5472.Can-17-2285
221. Xia C, Yin S, To KKW, and Fu L. Cd39/cd73/A2ar pathway and cancer immunotherapy. Mol Cancer. (2023) 22:44. doi: 10.1186/s12943-023-01733-x
222. Park SH, Lee J, Yun HJ, Kim SH, and Lee JH. Metformin suppresses both pd-L1 expression in cancer cells and cancer-induced pd-1 expression in immune cells to promote antitumor immunity. Ann Lab Med. (2024) 44:426–36. doi: 10.3343/alm.2023.0443
223. Cai L, Li Y, Tan J, Xu L, and Li Y. Targeting lag-3, tim-3, and tigit for cancer immunotherapy. J Hematol Oncol. (2023) 16:101. doi: 10.1186/s13045-023-01499-1
224. Ishida T and Ueda R. Immunopathogenesis of lymphoma: focus on ccr4. Cancer Sci. (2011) 102:44–50. doi: 10.1111/j.1349-7006.2010.01767.x
225. Yang T, Sun Z, Shi Y, Teng Y, Cheng L, Zhu R, et al. Development and validation of prognostic models based on (18)F-fdg pet radiomics, metabolic parameters, and clinical factors for elderly dlbcl patients. Ann Hematol. (2024) 103:5583–98. doi: 10.1007/s00277-024-06071-6
226. Zheng R, Li Y, Shi K, Pan Y, Liu K, Song J, et al. New horizons in B-cell lymphoma immunotherapy: from immune checkpoints to precision medicine. Neoplasia. (2025) 67:101206. doi: 10.1016/j.neo.2025.101206
227. Jiménez-Ubieto A, Poza M, Martin-Muñoz A, Ruiz-Heredia Y, Dorado S, Figaredo G, et al. Real-life disease monitoring in follicular lymphoma patients using liquid biopsy ultra-deep sequencing and pet/ct. Leukemia. (2023) 37:659–69. doi: 10.1038/s41375-022-01803-x
228. Alcoceba M, Stewart JP, García-Álvarez M, Díaz LG, Jiménez C, Medina A, et al. Liquid biopsy for molecular characterization of diffuse large B-cell lymphoma and early assessment of minimal residual disease. Br J Haematol. (2024) 205:109–21. doi: 10.1111/bjh.19458
229. Amin R and Braza MS. The follicular lymphoma epigenome regulates its microenvironment. J Exp Clin Cancer Res. (2022) 41:21. doi: 10.1186/s13046-021-02234-9
230. Li N, Tang N, Cheng C, Hu T, Wei X, Han W, et al. Improving the anti-solid tumor efficacy of car-T cells by inhibiting adenosine signaling pathway. Oncoimmunology. (2020) 9:1824643. doi: 10.1080/2162402x.2020.1824643
231. Giuffrida L, Sek K, Henderson MA, Lai J, Chen AXY, Meyran D, et al. Crispr/cas9 mediated deletion of the adenosine A2a receptor enhances car T cell efficacy. Nat Commun. (2021) 12:3236. doi: 10.1038/s41467-021-23331-5
232. Garcia-Lacarte M, Grijalba SC, Melchor J, Pascual M, Goñi E, Clemente-Larramendi I, et al. Il-10 from tumoral B cells modulates the diffuse large B-cell lymphoma microenvironment and response to immunotherapy. Blood. (2025) 145:2746–61. doi: 10.1182/blood.2024025755
Keywords: tumor metabolism, immune microenvironment, metabolic-immune axis, lymphoma, targeted therapy
Citation: Chen C, Guo W, Wang H, Cao L and Bai O (2025) Metabolic-immune axis in the tumor microenvironment: a new strategy for prognostic assessment and precision therapy in DLBCL and FL. Front. Immunol. 16:1659011. doi: 10.3389/fimmu.2025.1659011
Received: 03 July 2025; Accepted: 15 September 2025;
Published: 01 October 2025.
Edited by:
Martin Böttcher, Otto-von-Guericke University Magdeburg, GermanyReviewed by:
Laura Gardano, Université Sorbonne Paris Nord, FranceKen Young, Duke University, United States
Copyright © 2025 Chen, Guo, Wang, Cao and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ou Bai, YmFpb3VAamx1LmVkdS5jbg==
 Chengqian Chen
Chengqian Chen Wei Guo
Wei Guo Haotian Wang
Haotian Wang Luming Cao
Luming Cao Ou Bai
Ou Bai