- 1The Third Clinical College, Shanxi University of Chinese Medicine, Taiyuan, China
- 2Department of Plastic and Aesthetic Surgery, Shanxi Provincial People’s Hospital Affiliated to Shanxi Medical University, Taiyuan, China
Cutaneous squamous cell carcinoma (cSCC) is a common cutaneous malignant tumor, with its development and progression closely linked to immune dysregulation within the tumor microenvironment (TME). This review highlights cSCC-specific TME features—such as UV-induced mutational burden and the immunosuppressive effects observed in transplant recipients—and systematically outlines the composition and functional roles of tumor cells, immune cells (Tregs, MDSCs, TAMs), and stromal cells (CAFs) within the TME. The immunosuppressive mechanisms mediated by these cellular components are clarified, particularly through pathways including PD-L1/PD-1 and TGF-β/Smad. Building on this foundation, the potential clinical value of immune checkpoint inhibitors (cemiplimab, pembrolizumab) in treating advanced cSCC is summarized based on data from relevant clinical trials. Additionally, the impact of gender differences on cSCC incidence and therapeutic outcomes is discussed. This review is distinguished from general tumor immunotherapy reviews by offering dedicated references for cSCC precision immunotherapy. In addition, priority is emphasized for future investigations into combination therapy regimens and the development of personalized tumor vaccines.
1 Preamble
1.1 Clinical epidemiological characteristics of cSCC
Squamous cell carcinoma of the skin (cSCC) accounts for 20%–30% of cutaneous malignant tumors, and its incidence has been increasing steadily over the years. Although the fatality rate of squamous cell carcinoma of the skin is significantly lower than that of melanoma—with a 5-year survival rate of approximately 60% for melanoma and around 30%–40% for advanced cSCC (1)—it remains higher than that of basal cell carcinoma, which has a 5-year survival rate exceeding 95% and rarely metastasizes. Therefore, effective treatment of cSCC is crucial for reducing mortality associated with skin cancer. The primary etiological factor of cSCC is exposure to ultraviolet (UV) radiation. Prolonged UV exposure can cause DNA damage in skin cells, leading to gene mutations—such as those in TP53—which in turn promote tumor development (2).
1.2 cSCC morbidity and prognostic gender differences
A higher incidence of cSCC has been observed in males, with incidence rates approximately 2–3 times greater than those in females. This disparity may be attributed to prolonged outdoor exposure to ultraviolet radiation and lower awareness of skin protection among men (3). Regarding tumor progression, male patients with cSCC tend to exhibit greater tumor aggressiveness and higher rates of lymph node metastasis. These differences are underpinned by immunological and biological mechanisms rather than solely by environmental exposure. In a cohort of 1,178 primary cSCC cases, males demonstrated significantly higher risks of high-grade tumors, thick-walled lesions, lymph node involvement, and long-term recurrence compared to females (P < 0.001). Analysis of the tumor microenvironment showed that the densities of intralesional CD8+/CD4+ T cells and M1 macrophages in males were 40%–60% lower than those in females, indicating that insufficient immune infiltration may contribute to a more aggressive tumor phenotype (4). Currently, no dedicated studies have investigated gender differences in the efficacy of immune checkpoint inhibitors (ICI) for cSCC, and thus, it remains unclear whether objective response rates differ between males and females. It is recommended that future clinical trials systematically incorporate sex-based analyses to better elucidate this issue.
1.3 Association between the tumor microenvironment and immunotherapy in cSCC
The TME of cSCC constitutes a complex network of cellular and molecular components, with its immunosuppressive state playing a pivotal role in immune surveillance evasion and the emergence of therapeutic resistance. In recent years, significant advances have been achieved with immune checkpoint inhibitors in the treatment of advanced cSCC. However, a proportion of patients still experience either primary or acquired resistance, which is closely linked to the accumulation of MDSCs and functional dysregulation of Tregs within the TME (5, 6). Therefore, a comprehensive investigation into the composition and regulatory mechanisms of the TME in cSCC is essential for optimizing immunotherapeutic strategies and enhancing clinical outcomes.
2 The role of the tumor microenvironment in cSCC
2.1 Composition of the tumor microenvironment
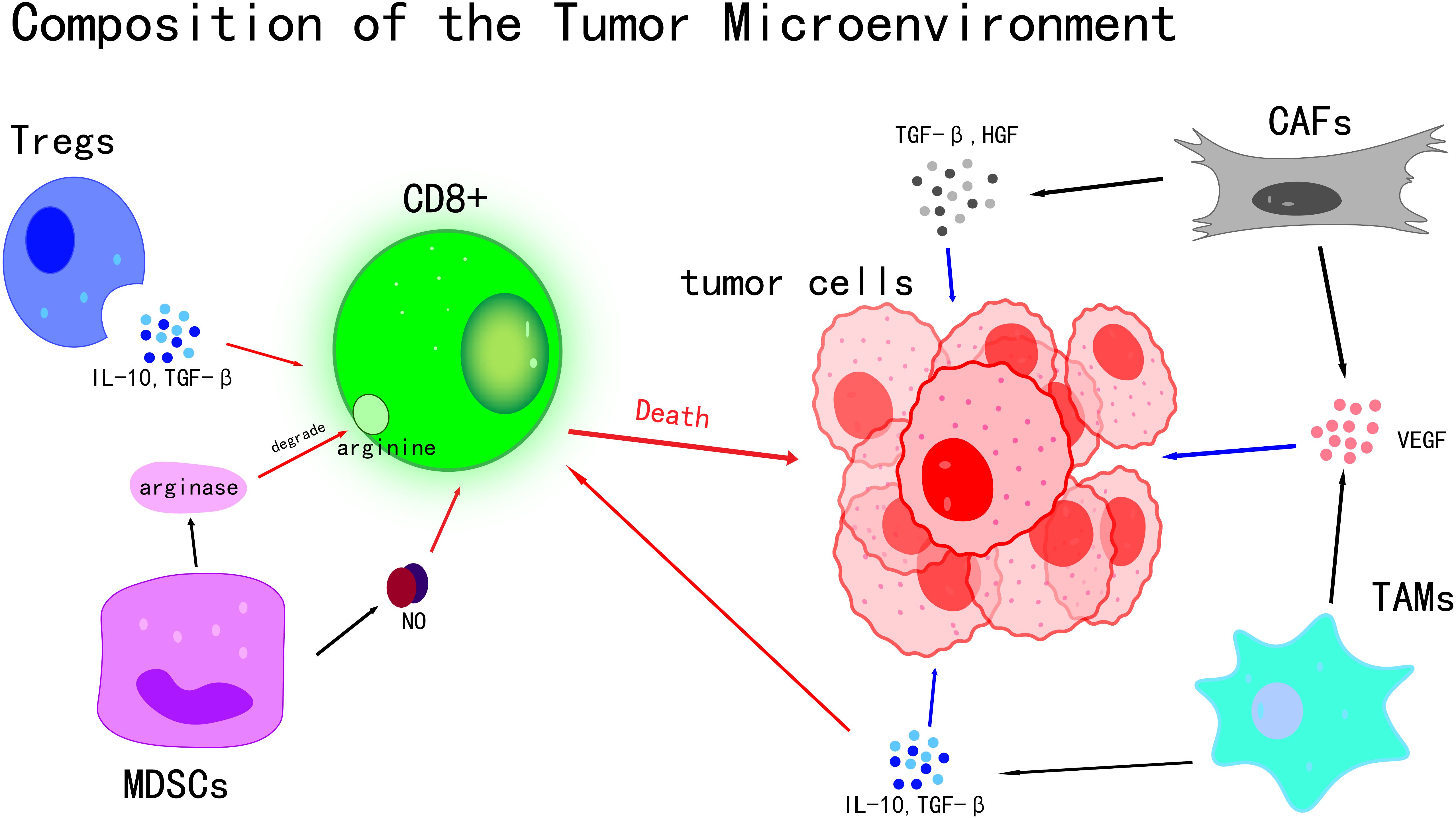
The tumor microenvironment (TME) is a complex and dynamic system that plays a pivotal role in tumor initiation, progression, and metastasis (7). As the central component of the TME, Tumor Cells exhibit a range of distinctive biological characteristics that allow them to bypass normal physiological regulatory mechanisms, leading to uncontrolled proliferation, invasion, and metastatic potential (8). Rather than existing in isolation, Tumor Cells actively secrete various Cytokines and Chemokines, which significantly influence the behavior of surrounding cells. Through these interactions, a microenvironment favorable to tumor growth and Immune Evasion is established (9) (Figure 1). A high Mutational Burden induced by UV has been observed in cSCC Tumor Cells, with an average mutation frequency of approximately 50 mutations per Megabase, which is markedly higher than that found in most other Solid Tumors. This elevated mutational load can result in the production of more Tumor Antigens, such as mutant TP53 protein. However, immune evasion is also facilitated through mechanisms including the downregulation of Major Histocompatibility Complex (MHC) class I molecules and the upregulation of PD-L1 (10).The link between mutational burden and immune evasion is especially evident in areas such as the face and scalp, which are chronically exposed to UV radiation, leading to a higher propensity for local tumor progression.
immune cells exhibit highly complex roles within the TME, characterized by dual biological functions (11). On one hand, they can recognize and attack Tumor Cells, thereby suppressing tumor growth (11, 12); on the other hand, under certain conditions, immune cells may be subverted by Tumor Cells, ultimately facilitating tumor progression (12). Among the various subsets of immune cells, T cells are recognized as pivotal players in the anti-tumor immune response. Effector T cells, especially CD8+ T cells, function as precise “killers” capable of specifically identifying antigens presented on the surface of Tumor Cells. Upon activation, they initiate a cascade of cytotoxic responses that directly eliminate Tumor Cells (13).In contrast, regulatory T cells (Tregs) play an opposing role in the Tumor Microenvironment. The activity of effector T cells is suppressed by Tregs through the secretion of various inhibitory Cytokines, including IL-10 and TGF-β (14). These inhibitory Cytokines disrupt the proliferation, activation, and cytotoxic functions of effector T cells (15, 16), thereby impairing the body’s Immune Surveillance and antitumor response, and facilitating the Immune Evasion of Tumor Cells.
Myeloid-derived suppressor cells (MDSCs) are a population of myeloid-origin cells with potent immunosuppressive properties (17). Their immunosuppressive effects are mediated through multiple mechanisms, with the secretion of inhibitory molecules such as arginase and nitric oxide (NO) being one of the primary pathways (18).Arginine, an amino acid essential for T cell proliferation and activation, is degraded by arginase, resulting in impaired T cell function (19, 20). Nitric oxide further suppresses T cell proliferation and activity by disrupting normal physiological functions through mechanisms such as oxidative stress (18, 21), thereby weakening the body’s anti-tumor immune response.
High plasticity has also been observed in macrophages within the TME, with their phenotype and function dynamically altered in response to various signaling molecules present in the microenvironment (22). In the Tumor Microenvironment, these macrophages can differentiate into pro-tumor M2-type macrophages, also known as tumor-associated macrophages (TAMs).Various Cytokines, such as IL-10 (23) and TGF-β (24), are secreted by TAMs. Acting synergistically, IL-10 and TGF-β establish an immunosuppressive microenvironment that inhibits anti-tumor immune responses. This environment not only suppresses T cell activity but also promotes the proliferation and invasion of Tumor Cells, thereby directly contributing to tumor progression (25, 26).Additionally, growth factors secreted by TAMs, including vascular endothelial growth factor (VEGF) (27), stimulate tumor angiogenesis (28). This neovascularization supplies Tumor Cells with ample nutrients and oxygen, thereby supporting sustained tumor growth and metastasis (29).
Stromal cells are also essential components of the TME, primarily comprising cancer-associated fibroblasts (CAFs) and endothelial cells. Multiple roles have been attributed to CAFs in tumor initiation and progression. By secreting a variety of Cytokines, such as TGF-β and HGF, along with growth factors like VEGF, significant influences are exerted on the biological behavior of Tumor Cells (30). These factors have been shown to promote the proliferation of Tumor Cells, facilitating their rapid growth and expansion. Additionally, they enhance the migratory capacity of Tumor Cells, thereby contributing to tumor invasion and metastasis (30, 31).
Endothelial cells are primarily involved in tumor angiogenesis (32).In the early stages of tumor development, a series of angiogenic factors, such as VEGF, are secreted by Tumor Cells, which attract endothelial cells to migrate toward the tumor site and promote their proliferation and lumen formation (33). The role of VEGF in the Tumor Microenvironment extends beyond angiogenesis. It also modulates intercellular interactions within the Tumor Microenvironment, including the regulation of pericyte proliferation and migration, as well as the mediation of interactions between tumor-associated macrophages and carcinoma cells. These interactions contribute to PDL-1-mediated immunosuppression and Nrf2-driven epithelial-mesenchymal transition (EMT) (34). The newly formed tumor vasculature not only ensures an adequate blood supply to meet the high metabolic demands of Tumor Cells for nutrients and oxygen, but also facilitates their metastasis by providing a direct route for dissemination through the bloodstream to distant sites, where secondary metastatic lesions can form.
The extracellular matrix (ECM), as a crucial structural and functional component of the TME, has been shown to significantly influence the invasion and metastasis of Tumor Cells through alterations in its composition and architecture (35). It is primarily composed of collagen, fibronectin, and laminin (36). In terms of compositional changes, increased collagen deposition has been observed in the Tumor Microenvironment, particularly with upregulated expression of collagen types I and III (37). This accumulation contributes to tissue stiffening, thereby facilitating the invasive behavior of Tumor Cells (35). Elevated levels of hyaluronic acid (HA) have also been detected in various tumors, especially low-molecular-weight HA oligosaccharides, which interact with CD44 receptors to activate pro-tumor signaling pathways and enhance cell migration and invasion (37).Alterations in the expression and deposition of fibronectin have been observed, which affect cell adhesion and migration (35). Structurally, increased cross-linking of collagen fibers results in greater stiffness and rigidity of the extracellular matrix (ECM). This enhanced stiffness activates intracellular mechanotransduction pathways, including the integrin and focal adhesion kinase (FAK) signaling cascades, thereby promoting the invasion of Tumor Cells. In the Tumor Microenvironment, collagen fibers become more orderly aligned, forming channels that facilitate the migration of Tumor Cells (37).
Within the TME, Cytokines and signaling molecules act as messengers, establishing a complex and efficient communication network between Tumor Cells and surrounding cells, and playing a pivotal regulatory role (38).Cytokines are small-molecule proteins secreted by various types of cells, including interleukins (IL), interferons (IFN), and tumor necrosis factors (TNF). They exert dual functions within the TME, both promoting tumor growth, metastasis, and Immune Evasion, and activating immune cells to suppress tumor progression (39). For instance, Cytokines such as IL-6 and TGF-β, secreted by Tumor Cells, have been shown to inhibit the activity of immune cells, thereby facilitating Immune Evasion. This is achieved through the suppression of immune cells proliferation, differentiation, and Cytokines production, ultimately weakening the body’s Immune Surveillance and cytotoxic response against tumors. As a result, Tumor Cells are able to survive and proliferate within a relatively “safe” immune environment, enabling the realization of Immune Evasion (39).immune cells (such as tumor-associated macrophages TAMs) have been shown to secrete Cytokines (such as IL-1β and IL-18), which promote the proliferation and metastasis of Tumor Cells (38).
Moreover, metabolic byproducts within the TME have been found to play a significant role in tumor progression. Glutamine metabolism is critically involved in the function of Tumor Cells (40). A high dependency on glutamine is exhibited by Tumor Cells, as it is utilized through the glutamine fermentation pathway to generate energy necessary for their rapid growth and proliferation. Glutamine serves as a nitrogen source for the biosynthesis of amino acids and nucleotides, and also as a carbon source to replenish the tricarboxylic acid (TCA) cycle, thereby sustaining accelerated cellular proliferation (41).Meanwhile, the acid-base balance of the TME has been shown to be altered by Tumor Cells through metabolic byproducts such as lactate (42). The accumulation of lactate results in the acidification of the TME, creating an acidic microenvironment that suppresses immune cells by diminishing their activity and function. This immunosuppressive environment further promotes the immune evasion of Tumor Cells and creates more favorable conditions for their invasion and metastasis (42, 43).
Pyroptosis, a form of programmed cell death, contributes to the remodeling of the TME by promoting the release of pro-inflammatory cytokines and sustaining chronic inflammation, thereby affecting processes such as Immune Evasion and angiogenesis. However, its dual role in both cancer progression and treatment-related complications—such as cytokine release syndrome—poses significant challenges for its therapeutic application in oncology (44).
Key cellular components and their interactions within the Tumor Microenvironment are illustrated. The death of Tumor Cells is induced by CD8+ T cells through direct cytotoxic mechanisms. The activity of CD8+ T cells is suppressed by Tregs via the secretion of IL-10 and TGF-β. T cell function is inhibited by MDSCs through the production of arginase and nitric oxide (NO). CAFs and TAMs contribute to tumor growth and angiogenesis by releasing TGF-β, HGF, and VEGF, thereby fostering an immunosuppressive microenvironment. Collectively, these cellular interactions shape tumor progression and influence the response to therapy.
2.2 Immunoregulatory role of the tumor microenvironment
The TME has been identified as a central target for elucidating the mechanisms underlying tumor initiation and progression, as well as for addressing therapeutic challenges. As a dynamic and complex multicellular ecosystem, the TME encompasses not only Tumor Cells but also immune cells, stromal cells, and components of the extracellular matrix. It plays a pivotal role in tumor Immune Evasion and progression through tightly regulated cellular and molecular pathways. Clarifying the immunoregulatory mechanisms of the TME, especially the activation patterns of immunosuppressive pathways and potential strategies for their modulation, is of great clinical significance for the development of effective anti-tumor therapies.
2.2.1 Core pathways of immunosuppressive mechanisms
Multiple core pathways have been identified in the TME of cSCC that contribute to the formation of an immunosuppressive barrier. Among them, the PD-L1/PD-1 and TGF-β/Smad pathways represent key molecular mechanisms driving Immune Evasion. These pathways impair anti-tumor immune responses by respectively inhibiting the activation signals and proliferative capacity of effector T cells.
The PD-L1/PD-1 pathway, a principal target in current immunotherapy, displays distinct cell-type specificity during its activation. In the TME of cSCC, high levels of PD-L1 expression have been observed in both Tumor Cells and TAMs (45). Upon binding of PD-L1 to PD-1 on effector T cells, immunosuppressive signaling cascades such as the PI3K/Akt pathway are activated within the T cells (46).Activation of this signaling pathway has been shown to directly suppress the proliferative capacity of T cells and reduce the secretion of cytotoxic molecules such as perforin and granzymes. As a result, effector T cells are unable to effectively recognize and eliminate Tumor Cells, thereby promoting tumor Immune Evasion (47). Clinical studies have demonstrated that immune checkpoint inhibitors targeting this pathway (e.g., Cosibelimab) can restore the antitumor activity of T cells by blocking the interaction between PD-L1 and PD-1, leading to significant survival benefits in patients with cSCC (48).
The TGF-β/Smad pathway primarily relies on TGF-β secreted by Tregs and cancer-associated CAFs. Upon binding of TGF-β to its receptor on the surface of effector T cells, a downstream Smad signaling cascade is initiated. This involves the phosphorylation of Smad2/3 transcription factors, which then form a heterotrimeric complex with Smad4. The resulting complex translocates into the nucleus, where it directly regulates the expression of target genes. The primary effect of this process is the downregulation of the co-stimulatory molecule CD28 on the surface of T cells, accompanied by a decrease in IL-2 secretion. CD28, a critical “second signal” molecule required for T cell activation, when downregulated, results in impaired activation of T cells (49, 50). Concurrently, IL-2, a key Cytokines essential for T cell proliferation, when secreted in reduced amounts, directly suppresses the clonal expansion of T cells. Sustained activation of this pathway maintains effector T cells in the TME in a functionally suppressed state, thereby hindering the development of an effective antitumor immune response.
It is important to note that immunosuppression within the TME is not driven by a single pathway, but rather arises from the synergistic effects of multiple signaling cascades, including the PD-L1/PD-1 and TGF-β/Smad pathways. For example, the activation of the Smad pathway by Tregs through TGF-β secretion is accompanied by the release of IL-10, which further amplifies their immunosuppressive effects (51). In parallel, the overexpression of PD-L1 on Tumor Cells enables its interaction with PD-L1 on the surface of TAMs, forming a “dual barrier” that intensifies the suppression of T cell activity (52). This synergistic immunosuppressive network involving multiple pathways is a key factor contributing to the limited effectiveness of conventional immunotherapy.
2.2.2 Association between TME cellular and molecular pathways and tumor progression
Cellular and molecular pathways within the TME have been shown to not only regulate immune responses but also directly contribute to malignant behaviors such as invasion and migration of Tumor Cells. Notably, the EMT pathway mediated by CAFs and the STAT3 pathway associated with MDSCs represent critical hubs that link immunosuppression to tumor progression. By modulating the phenotype of Tumor Cells and the function of immune cells, these pathways collectively drive the malignant advancement of cSCC.
The EMT pathway mediated by CAFs is recognized as a central mechanism facilitating the invasion and metastasis of cSCC. As the predominant type of stromal cells within the TME, CAFs secrete TGF-β, which activates the TGF-β/Smad signaling cascade in Tumor Cells, thereby inducing EMT (53). Specifically, TGF-β secreted by CAFs binds to the TGF-β receptor complex (TGF-β R1/R2) on the surface of Tumor Cells, initiating conformational changes that activate downstream Smad2/3 proteins (54). Once phosphorylated, Smad2/3 forms a transcriptional complex with Smad4, which translocates into the nucleus. This complex simultaneously downregulates the epithelial marker E-cadherin—whose loss disrupts intercellular adhesion among Tumor Cells and reduces cell cohesion (55)—and upregulates mesenchymal markers such as N-cadherin and vimentin, thereby enhancing the motility and stromal invasiveness of Tumor Cells (56).Multiple clinical studies have demonstrated a significant association between the activation level of this pathway and the depth of invasion in cSCC, underscoring its critical role in tumor progression (57, 58).
The STAT3 pathway in MDSCs has been shown to promote both Immune Evasion and tumor proliferation through a dual mechanism involving immunosuppression and metabolic deprivation. MDSCs, a key immunosuppressive cell population within the TME, rely heavily on the sustained activation of signal transduction and the STAT3 pathway for their functional maintenance (59). During the initiation and progression of cSCC, UV irradiation serves as a major contributing factor, markedly increasing IL-6 secretion within the TME (60). IL-6 binds to its receptor on the surface of MDSCs, leading to the activation of Janus kinase (JAK), which in turn phosphorylates STAT3 (61). Once activated, STAT3 translocates into the nucleus, where it directly regulates the expression of arginase-1 (Arg-1) and inducible nitric oxide synthase (iNOS) (62).Arg-1 depletes arginine, an amino acid essential for T cell activation in the TME, thereby impairing T cell proliferation due to “metabolic starvation” (63). Meanwhile, iNOS produces large amounts of nitric oxide (NO), which damages T cell DNA and suppresses their cytotoxic function, resulting in a dual immunosuppressive effect of “metabolic deprivation + toxic injury” (64). In animal studies, treatment with STAT3 inhibitors (such as WP1066) has been shown to significantly reduce the accumulation of MDSCs in murine tumor models, while restoring T cell proliferation and cytotoxic activity (65), supporting the potential of this pathway as a therapeutic target.
Beyond these two core pathways, the Cytokines network within the TME also contributes to tumor progression through a mechanism of cross-regulation. For example, activation of the MDSCs STAT3 pathway by the pro-inflammatory Cytokines IL-6 has been identified as a key signaling event. In addition, IL-6 promotes STAT3 activation in Tumor Cells, thereby enhancing their resistance to apoptosis and increasing their proliferation rate (66). Meanwhile, the immunosuppressive Cytokines TGF-β not only inhibits T cell function but also upregulates the expression of matrix metalloproteinases (MMPs) on the surface of Tumor Cells, enhancing their capacity to degrade the extracellular matrix and further facilitating invasive metastasis (67). This intercellular signaling crosstalk between immune and tumor cells establishes a self-perpetuating malignant cycle within the TME, thereby accelerating tumor progression and metastasis.
2.2.3 cSCC TME immunosuppressive mechanisms and corresponding regulatory strategies
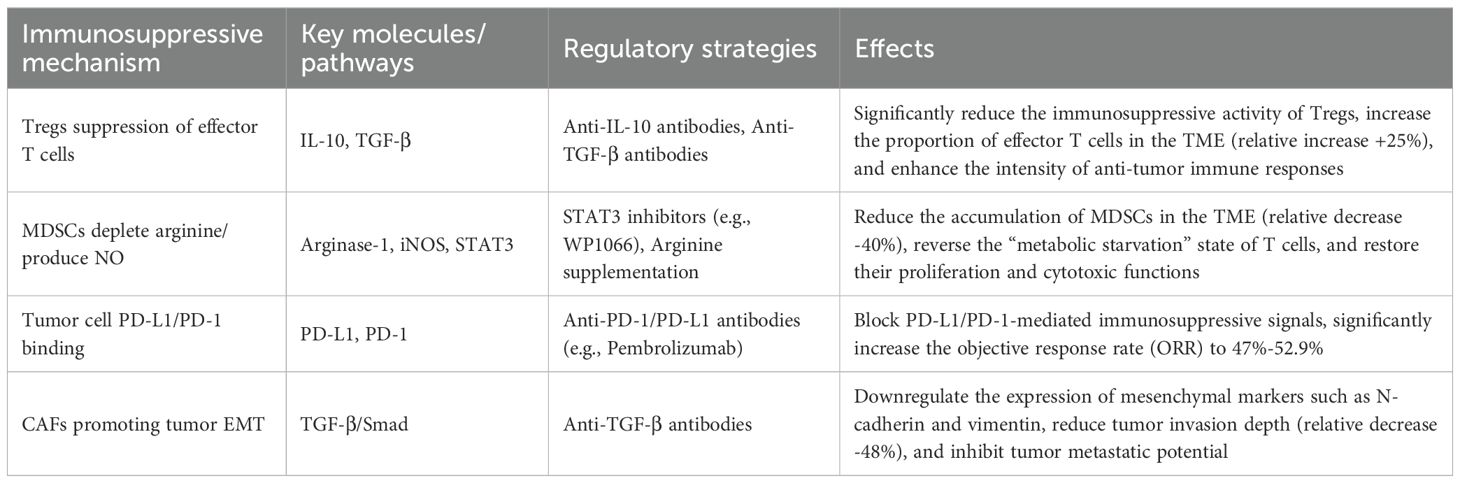
From a clinical translational perspective, targeting a single pathway has often proven insufficient to overcome the immunosuppressive network of the TME, making combination therapy strategies a growing focus of research. For instance, the co-administration of anti-PD-1 antibodies with STAT3 inhibitors has been shown to simultaneously disrupt PD-L1/PD-1-mediated immunosuppression and the functional maintenance pathways of MDSCs, thereby significantly enhancing T cell activation efficiency (68). Similarly, the combination of anti-TGF-β and anti-PD-L1 antibodies has been demonstrated to suppress tumor EMT progression while improving the efficacy of immune checkpoint inhibitors (69) (Table 1). Looking ahead, molecular subtyping based on key pathways within the cSCC TME is expected to guide the development of individualized combination therapy regimens, representing a promising direction for enhancing the therapeutic outcomes of cSCC.
3 Justification for the priority of immunotherapy in cSCC
3.1 Comparison with surgical treatment
Surgical resection, owing to its advantage of directly eliminating lesions, is considered the first-line curative approach for early-stage cSCC (characterized by localized tumors without lymph node or distant metastasis), achieving a 5-year cure rate of over 90% (70). However, its primary limitation lies in spatial dependence. In advanced-stage (III–IV) cSCC, where tumors invade major blood vessels in the head and neck (e.g., the carotid artery), cranial nerves, or are accompanied by multiple metastases to organs such as the lungs or liver, complete surgical removal becomes challenging, with a curative rate of less than 10%. Moreover, surgery may result in serious complications, including major intraoperative bleeding and postoperative swallowing dysfunction, and could even accelerate tumor dissemination—surgical trauma may activate platelet-derived growth factors within the TME, thereby promoting Tumor Cells migration (71, 72). Immunotherapy has addressed this limitation by “remodeling the TME immune balance.” In a phase II clinical trials (NCT02760498) evaluating the first-line agent cemiplimab for advanced cSCC, 59 patients with metastatic cSCC were enrolled. An objective response rate (ORR) of 47% was observed, and the median overall survival (OS) was not reached; at a median follow-up of 7.9 months, 82% of responders maintained their response (73). In comparison, conventional treatment—surgery combined with postoperative chemotherapy—has been associated with poorer outcomes. Analysis of the SEER database revealed that patients with advanced cSCC, particularly those with regional lymph node metastases or locally advanced disease, who underwent surgery and adjuvant radiotherapy, had a median OS often below 12 months, indicating a significant disparity (74). More critically, “tumor downstaging conversion” can be achieved through immunotherapy by inhibiting the PD-L1/PD-1 pathway within the TME and reducing the accumulation of MDSCs (75). Clinical data indicate that, following neoadjuvant immunoradiotherapy, 90% of patients with locally advanced head and neck squamous cell carcinoma (HNSCC) experienced clinical-to-pathological downstaging, and 67% achieved a pathological complete response (pCR) (76).
3.2 Comparison with radiotherapy
Radiotherapy induces DNA damage in Tumor Cells via localized ionizing radiation and is applicable to locally advanced cSCC (e.g., tumors infiltrating the deep dermis without distant metastasis), with a five-year local control rate of approximately 65%. However, its limitations include a restricted treatment scope and damage to the TME. On one hand, radiotherapy is ineffective against distant metastases, with a distant metastasis rate of 15%–20% observed in patients with advanced cSCC receiving this treatment (77). On the other hand, radiotherapy induces local skin fibrosis, characterized by a 30% increase in collagen fiber deposition, and leads to depletion of immune cells within the TME. Following radiotherapy, PD-1 expression on T cells increases by 40%, and the proportion of M2-type TAMs rises by 20%, thereby further aggravating immunosuppression (78).
The characteristic of “systemic immune activation” in immunotherapy effectively addresses this limitation. First, a significant reduction in the rate of distant metastasis in advanced cSCC has been observed with immunotherapy, primarily through the activation of circulating effector T cells that recognize and eliminate metastatic lesions (79).Secondly, Immunotherapy also contributes to the restoration of the radiation-damaged TME. Tumor antigens released by radiotherapy, such as mutant TP53 protein, can be presented by dendritic cells. Concurrently, immune checkpoint inhibitors (e.g., pembrolizumab) can block the PD-L1/PD-1 signaling pathway, thereby preventing effector T cell exhaustion and promoting a synergistic effect between radiotherapy-induced antigen release and immune activation (80). A combined trial demonstrated that the use of radiotherapy in conjunction with pembrolizumab in patients with locally advanced cSCC resulted in a higher response rate, with a complete response (CR) rate reaching up to 50%, and was well tolerated (79). Furthermore, the combination of radiotherapy and immunotherapy has been shown to enhance the production of antitumor Cytokines such as IFN-γ, while reducing the infiltration of immunosuppressive cells, including M2-type TAMs. This mechanism has been validated in multiple Solid Tumors, such as non-small cell lung cancer (80).In addition, immunotherapy has been shown to reduce adverse reactions associated with radiotherapy. Multiple studies have demonstrated that the safety profile of immunotherapy combined with radiotherapy is generally manageable, with immune-related adverse events (such as skin reactions) typically being mild to moderate. For instance, in the KEYNOTE-629 trial, the incidence of grade ≥3 treatment-related adverse events with pembrolizumab monotherapy was reported to be 5.7% (81).
3.3 Comparison with targeted therapy
Targeted therapy for cSCC primarily relies on EGFR inhibitors (such as cetuximab), which act by blocking EGFR-mediated Tumor Cells proliferation signaling. However, three major limitations have been identified: rapid development of resistance, limited applicability, and lack of improvement in the TME. First, the median time to resistance with cetuximab is only 6–9 months (82), and this resistance is associated with changes in the TME—following resistance, increased secretion of HGF by CAFs within the TME activates the MET bypass pathway, thereby counteracting the inhibitory effects of EGFR (83). Second, the efficacy of cetuximab is restricted to patients with high EGFR expression (IHC score ≥2+), yet only 60% of cSCC patients exhibit such expression levels (84), with an objective response rate (ORR) of merely 20%–30% (85). Finally, targeted therapy does not modulate the immunosuppressive state of the TME, and even after resistance develops, the proportion of Tregs within the TME remains above 15%, thereby failing to elicit a sustained anti-tumor immune response (86).
Immunotherapy has demonstrated advantages through its “broad-spectrum efficacy” and “TME remodeling.” In terms of applicability, immune checkpoint inhibitors have shown effectiveness in both PD-L1-positive (TPS ≥ 1%) and -negative patients (87, 88). Regarding the durability of response, multiple trials have reported that the median duration of response (DOR) has not been reached; for example, in the KEYNOTE-001 trial, the median DOR was 12.5 months, with some patients experiencing responses lasting over two years. A notably high proportion of patients in KEYNOTE-001 maintained a response for ≥1 year (87), which is believed to be associated with the induction of immune memory. Following treatment, the proportion of memory T cells (CD45RO+) in the TME increases, enabling long-term surveillance of tumor recurrence (89).Regarding adverse events, the incidence of grade 3 rash with targeted therapy is approximately 6.1%–13% (90), and diarrhea is also relatively common. In contrast, the incidence of grade 3 adverse events associated with immunotherapy—such as thyroid dysfunction and rash—ranges from about 9.5% to 29.6%, most of which can be effectively managed with hormonal intervention (87, 91, 92).More importantly, the TME can be fundamentally improved through immunotherapy. Treatment with anti-PD-1 has been shown to reduce the expression of MDSCs functional markers, such as arginase-1, while enhancing the infiltration of effector T cells. For instance, preclinical studies have demonstrated that PD-1 blockade decreases the presence of immunosuppressive cells within the TME (93), enabling some patients to regain responsiveness to treatment—an effect on TME modulation that cannot be achieved by targeted therapies.
4 Application of immunotherapy in cSCC
4.1 Immune checkpoint inhibitors
The emergence of immune checkpoint inhibitors (ICIs) in recent years has revolutionized tumor therapy, offering renewed hope to countless cancer patients. By precisely blocking the signaling pathways of immune checkpoint molecules, ICIs effectively restore the antitumor activity of effector T cells and markedly enhance the immune response (94), thereby ushering in a new era of cancer immunotherapy.
Immune checkpoint molecules serve as critical regulatory components of the immune system, functioning under normal physiological conditions to prevent excessive immune activation that could harm healthy tissues. However, Tumor Cells have evolved to exploit these molecules, converting them into mechanisms that suppress T cell activity. This enables them to evade immune surveillance and establish conditions conducive to their growth and metastasis. Currently, among the various immune checkpoint molecules, CTLA-4, PD-1, and PD-L1 have been the most extensively studied and hold significant clinical relevance. CTLA-4 plays a critical role during the early activation phase of T cells. By binding to members of the B7 family (95, 96), it modulates T cell activation or suppression. Inhibitors of CTLA-4, such as ipilimumab (97), function by precisely blocking the interaction between CTLA-4 and its ligands, thereby lifting the inhibitory signal. This facilitates enhanced activation and proliferation of T cells (96), effectively acting as a potent “vanguard” for the ensuing anti-tumor immune response.
In contrast, PD-1 is primarily involved during the effector phase of T cell responses. Upon binding of PD-1 to its ligand PD-L1, an inhibitory signal is transmitted to T cells, resulting in the suppression of their activity and impairing their ability to effectively recognize and eliminate Tumor Cells (98). By precisely blocking this signaling pathway, PD-1/PD-L1 inhibitors—such as pembrolizumab (99) and cemiplimab (100)—have been shown to successfully restore the antitumor function of T cells, enabling them to regain activity and mount effective attacks against Tumor Cells (101).
immune checkpoint inhibitors have demonstrated impressive therapeutic efficacy across a range of tumors, standing out like brilliant pearls in the vast landscape of cancer treatment.
4.1.1 Key clinical trials results
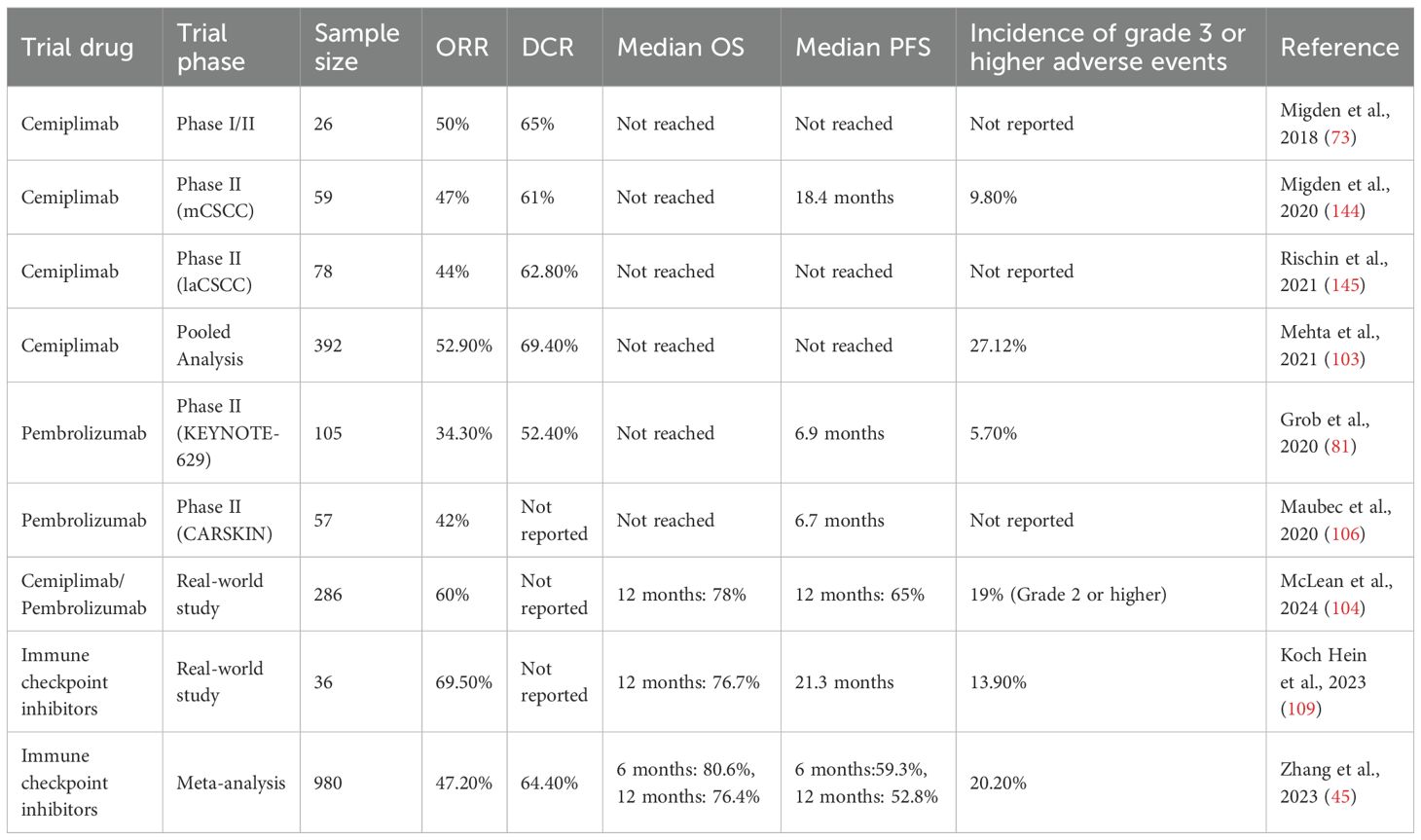
cSCC (CSCC) is the second most common type of skin cancer after basal cell carcinoma. While most CSCC cases can be cured surgically, recurrence occurs in approximately 15% to 28% of patients following excision. For those with advanced CSCC who are ineligible for curative surgery or radiotherapy, treatment options remain limited and the prognosis is generally poor. In recent years, immune checkpoint inhibitors (ICI) have offered new therapeutic possibilities for advanced CSCC. Extensive efforts have been made by research teams to investigate the role of CPI in cSCC treatment through systematic reviews, meta-analyses, and cohort studies. These reviews have consistently demonstrated that CPI therapy yields favorable clinical outcomes in advanced cSCC, with manageable toxicity profiles (102–104) (Table 2).
Among them, cemiplimab (105) and pembrolizumab (106–108) have distinguished themselves with outstanding therapeutic efficacy. Demonstrated in clinical trials, their strong antitumor activity and relatively favorable safety profiles have garnered significant attention from the global medical community. Based on this robust clinical evidence, the U.S. Food and Drug Administration (FDA) approved cemiplimab for the treatment of advanced cSCC, marking a milestone that effectively addressed the lack of treatment options for advanced cSCC. Cemiplimab, a PD-1 inhibitor, achieved an overall response rate (ORR) of 47% in a phase I/II clinical trials for CSCC, making it the first PD-1 antibody approved by the FDA for the treatment of advanced CSCC (102).In addition, a pooled analysis demonstrated that the objective response rate (ORR) of Cemiplimab in treating advanced CSCC was 52.9%, with a disease control rate (DCR) of 69.4%. The median overall survival (OS) was not reached, suggesting a survival duration exceeding 6.3 months. Another pooled analysis of 392 patients showed that immune checkpoint inhibitors (including Cemiplimab) achieved an ORR of 42.43% and a DCR of 58.05%, with 92% of patients maintaining a response at the data cut-off (103). Pembrolizumab, another PD-1 inhibitor, was assessed in two phase II trials (KEYNOTE-629 and the CARSKIN trial), which reported an ORR ranging from 34.3% to 41% for advanced CSCC, with a median progression-free survival (PFS) of 6.7 to 6.9 months (102).
A systematic review and meta-analysis that included 13 studies and a total of 980 patients reported that immune checkpoint inhibitors achieved an ORR of 47.2% and a DCR of 64.4%. The 6-month and 12-month progression-free survival rates were 59.3% and 52.8%, respectively, while the 6-month and 12-month overall survival rates were 80.6% and 76.4%, respectively (102). A retrospective real-world cohort study conducted in Australia included 286 patients, reporting an objective response rate (ORR) of 60%, a 12-month overall survival rate of 78%, and a progression-free survival rate of 65% (104). In another single-center retrospective cohort study from Canada involving 36 patients, a partial response rate of 41.7% and a complete response rate of 27.8% were observed. The 1-year progression-free survival rate reached 58.1%, with a median progression-free survival of 21.3 months (109). Regarding drug safety, among the 392 patients included in the pooled analysis, grade 3 or higher adverse events were observed in only 27.12% of cases. In the Australian study, 19% of patients experienced grade 2 or higher immune-related adverse events, with no treatment-related deaths reported. In a Canadian study, grade 3–4 immune-related adverse events were reported in 13.9% of patients.
In the field of melanoma, nivolumab as a first-line treatment has offered new hope for therapy-naive melanoma patients (110). Significant survival benefits have been demonstrated in clinical studies, with notable extensions in overall survival and improvements in quality of life. In the treatment of non-small cell lung cancer, the superiority of PD-1/PD-L1 inhibitors in first-line therapy has been consistently supported by multiple studies. Compared with conventional chemotherapy, these inhibitors not only significantly increase survival rates but also improve the overall treatment experience, presenting a promising therapeutic alternative for patients with non-small cell lung cancer (111).
The objective response rate (ORR) of cemiplimab in patients with advanced cSCC has been reported at 47%–52.9%, which is slightly lower than that observed in other Solid Tumors, such as melanoma, where the ORR exceeds 50% in some studies. Nevertheless, given that a substantial proportion of cSCC patients are elderly (approximately 70% aged ≥65 years), have impaired immune function, and often present with chronic skin conditions (e.g., chronic eczema), this level of efficacy reflects meaningful clinical benefit. Furthermore, the incidence of grade ≥3 adverse events associated with immune checkpoint inhibitors in cSCC patients ranges from 19.0% to 27.12%, which is lower than the rates reported in melanoma (exceeding 30% in some studies), suggesting better overall tolerability in this population.
4.1.2 Table of clinical impact and pharmacokinetic characteristics of common immunotherapies for cSCC
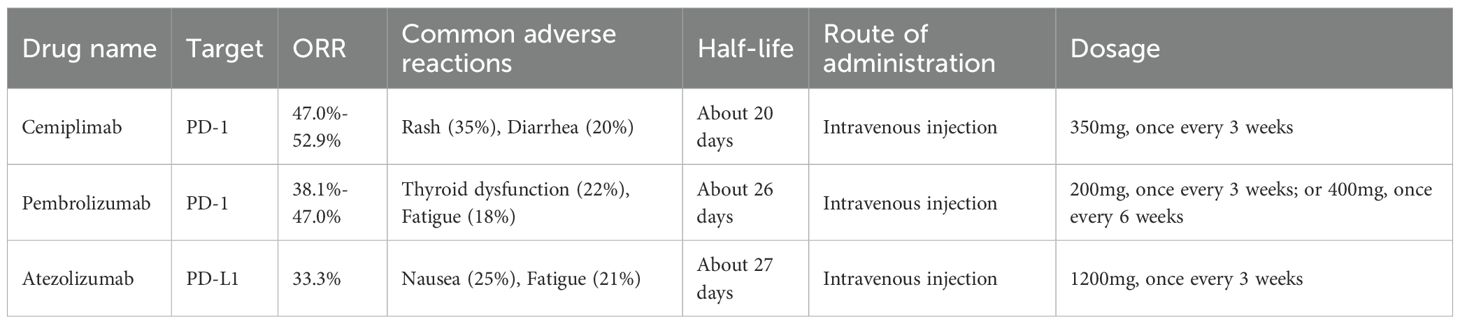
In the immunotherapy of advanced cSCC (cSCC), both cemiplimab (cemiplimab) and pembrolizumab (pembrolizumab) have shown notable clinical value, although differences exist in their pharmacokinetic profiles and predictive markers of efficacy (Table 3). Over the past five years, multiple trials have reported that the median overall survival (OS) for cemiplimab has not yet been reached, with the longest follow-up period being approximately 16.6 months. The 24-month OS rate has been estimated at around 62%, indicating a sustained survival benefit (112). Furthermore, the expression level of PD-L1 (TPS ≥1%) has been identified as a potential predictive biomarker for treatment response. Patients with PD-L1-positive tumors have demonstrated significantly higher objective response rates (ORR) compared to those with PD-L1-negative tumors. While data from other cancer types suggest ORRs of 58% versus 32%, a similar trend has been observed in cSCC studies, despite some variation in exact values (73, 113).In terms of pharmacokinetics, an increase in the clearance of pembrolizumab has been observed with increasing body weight—approximately an 8% rise in clearance for every 10 kg gain—necessitating weight-based dose adjustments (e.g., 2 mg/kg) to prevent subtherapeutic exposure that could compromise efficacy. In contrast, the half-life of cemiplimab remains stable (19–22 days) and is unaffected by age, sex, body weight, or mild hepatic or renal impairment, making a fixed-dose regimen (350 mg every 3 weeks) more suitable for standardization. Taken together, cemiplimab offers notable efficacy and convenient administration in advanced cSCC, with PD-L1 expression serving as a predictive biomarker of response, whereas pembrolizumab requires individualized dosing to optimize therapeutic outcomes.
However, the use of immune checkpoint inhibitors is not without risks, as some patients may develop immune-related adverse events (irAEs).These adverse reactions are primarily attributed to the “friendly fire” triggered by excessive activation of the immune system (114). Skin toxicity is among the most frequently observed side effects across multiple systems. Symptoms such as rash and pruritus are generally mild to moderate in severity, yet they can still cause considerable discomfort and negatively impact patients’ quality of life. Endocrine toxicity also warrants attention. Conditions like hypothyroidism and hypophysitis may insidiously disrupt hormonal balance, requiring regular monitoring and prompt medical intervention. Gastrointestinal toxicity remains a significant clinical concern. Diarrhea and colitis can severely compromise digestive function and, in severe cases, may pose life-threatening risks. In addition, hepatotoxicity may occur, resulting in abnormal liver function. This necessitates close monitoring of liver function parameters during the administration of immune checkpoint inhibitors to promptly detect and manage potential risks of liver injury.
In recent years, a series of encouraging breakthroughs have been reported in the field of immune checkpoint inhibitors, marking significant progress and continuously expanding the boundaries of our understanding of this therapeutic strategy. In the search for novel targets, emerging molecules such as LAG-3, TIM-3, and TIGIT have gained increasing attention, bringing new momentum to the development of immune checkpoint inhibitors (115–117).
Similarly, substantial progress has been made in the research of biomarkers. Biomarker studies have focused on PD-L1 expression levels, tumor mutational burden (TMB), and microsatellite instability (MSI). Assessment of PD-L1 expression serves as a critical reference for identifying patients who are likely to benefit from immune checkpoint inhibitors therapy (118). High TMB in Tumor Cells has been associated with the release of a greater number of Tumor Antigens, which can enhance immune activation and improve the therapeutic response to immune checkpoint inhibitors (119). This insight provides a novel perspective and valuable tool for predicting immunotherapy outcomes. Similarly, microsatellite instability (MSI) plays a key role in forecasting the efficacy of immune checkpoint inhibitors treatment (120).
4.2 Potential of bispecific antibody in the treatment of cSCC
Bispecific antibodies are a class of antibody drugs designed to simultaneously bind two distinct antigens. Their primary advantage is the targeted recruitment of immune cells, such as T cells, to the tumor site, thereby enhancing the local anti-tumor immune response while minimizing the systemic toxicity associated with off-target T cell activation often observed in conventional immunotherapy. In the treatment of cSCC, research has predominantly focused on EGFR/CD3 bispecific antibodys, with additional investigations exploring other targets such as PD-L1/CD3 and EpCAM/CD3. The following section presents a detailed analysis of EGFR/CD3 bispecific antibodies.
4.2.1 Core mechanism of action
High expression of EGFR has been observed on the surface of cSCC Tumor Cells (121), while CD3 serves as a critical component of the TCR-CD3 complex on T cells, mediating the transmission of activation signals (122). The EGFR/CD3 bispecific antibody functions via a “dual-antigen bridging” mechanism. Specifically, its “tumor-targeting arm”—an anti-EGFR single-chain antibody fragment—selectively binds to EGFR on cSCC Tumor Cells, effectively anchoring T cells in close proximity to the Tumor Cells and minimizing off-target activation in non-tumor tissues (123). Concurrently, the “immune-activating arm”—an anti-CD3 single-chain antibody fragment—binds to the CD3ϵ chain on T cells, triggering intracellular signaling cascades such as the ZAP-70 and ERK1/2 pathways through cross-linking of the TCR-CD3 complex. This activation induces T cells to release cytotoxic molecules, including perforin and granzyme B, which directly eliminate the bound Tumor Cells (124). In addition, activated T cells secrete Cytokines such as IFN-γ and TNF-α, which further recruit additional effector T cells (e.g., CD8+ T cells) and natural killer (NK) cells into the Tumor Microenvironment, contributing to the establishment of long-lasting anti-tumor immune memory (124). Compared with conventional anti-EGFR monoclonal antibodies (such as cetuximab), EGFR/CD3 bispecific antibodies not only block EGFR-mediated tumor proliferation signaling but also actively recruit T cells to exert cytotoxic effects. This dual mechanism makes them particularly suitable for patients with cSCC characterized by immune-desert microenvironments, where T-cell infiltration is minimal (123).
4.2.2 Preclinical evidence
Although no EGFR/CD3 bispecific antibodies targeting cSCC have yet entered the clinical trials stage, multiple preclinical studies have demonstrated their potent antitumor activity and favorable tolerability in various EGFR-overexpressing Solid Tumors models.
While specific investigations on cSCC remain limited, several studies employing cutaneous squamous cell carcinoma cell lines (e.g., A431) have confirmed the efficacy of EGFR/CD3 bispecific antibodies. In A431 cells, representing a model of cutaneous squamous cell carcinoma, these bispecific antibodies (such as the ATTACK format) have shown strong binding affinity and effectively inhibited downstream EGFR signaling pathways and cell proliferation (124).In the A431 xenograft model, significant inhibition of tumor growth was achieved by the EGFR/CD3 bispecific antibody through activation of T cell-mediated Tumor Cells killing (125).
Preclinical data from tumors with high EGFR expression, such as head and neck squamous cell carcinoma (HNSCC) and colorectal carcinoma, further support the potential application of the EGFR/CD3 bispecific antibody in cSCC. In HNSCC models (e.g., HNSCC cell lines), T cell-dependent lysis of Tumor Cells was induced by the EGFR/CD3 bispecific antibody (126). In colorectal carcinoma models, Probody-engineered EGFR/CD3 bispecific antibodies (e.g., CI107) demonstrated a marked reduction in toxicity while maintaining therapeutic efficacy (127).
4.2.3 Current challenges
Despite encouraging preclinical findings, three key challenges must be addressed for the clinical translation of EGFR/CD3 bispecific antibodies in cSCC:
Cytokine release syndrome (CRS): Activation of T cells by bispecific antibodies may provoke an excessive immune response, resulting in the massive release of cytokines such as IL-6 and IL-1β, which can manifest as fever, hypotension, and organ dysfunction (128).In preclinical studies of EGFR/CD3bispecific antibody, the severity of CRS has been found to correlate with the extent of T-cell activation. However, this can be mitigated through engineering approaches, such as reducing CD3 binding affinity, which lowers Cytokines release while preserving antitumor efficacy (129).
Heterogeneous EGFR expression has been observed: approximately 10%–20% of cSCC patients exhibit low EGFR expression (IHC score 1+) or are negative in tumor tissues, potentially rendering them unresponsive to EGFR/CD3 bispecific antibodies (130). Higher EGFR expression levels have been reported in cSCC lesions exposed to greater UV radiation (e.g., face, scalp) compared to those on the trunk (mean IHC score 3+ vs. 2+), indicating the importance of biomarker-based patient selection (131).
off-target toxicity remains a concern, as normal epidermal keratinocytes also express low levels of EGFR (IHC score 1+). The bispecific antibody may bind to these normal skin cells and activate local T cells, potentially resulting in dermatologic toxicities such as rash, pruritus, and epidermal detachment (132). In preclinical mouse models, administration of high-dose EGFR/CD3 bispecific antibody (20 mg/kg) resulted in diffuse erythema on the skin, with histopathological analysis revealing lymphocytic infiltration in the epidermis. To mitigate binding to normal cells, optimization through antibody engineering is required (133).
4.3 Combination immunotherapy
At the forefront of tumor treatment, combination immunotherapy has garnered significant attention for its distinctive advantages. By strategically integrating multiple therapeutic approaches, it generates synergistic effects that enhance the efficacy of immunotherapy, offering novel perspectives and strategies for overcoming the challenges posed by tumors.
Chemotherapeutic agents play a pivotal role in combination immunotherapy. By inducing immunogenic cell death (ICD) of Tumor Cells, they facilitate the release of Tumor Antigens and activate the immune system, thereby augmenting the effectiveness of immune checkpoint inhibitors. The potential of this combined approach has been strongly supported by results from clinical trials (108). For instance, in patients with advanced cSCC (cSCC), the combination of pembrolizumab and chemotherapy significantly increased the objective response rate (ORR), leading to more substantial therapeutic benefits. This achievement highlights the synergistic benefits of combining chemotherapy with immune checkpoint inhibitors, offering new therapeutic avenues for the treatment of advanced cSCC.
The combination of targeted therapies with immune checkpoint inhibitors has also shown considerable therapeutic promise. Agents such as EGFR inhibitors (134) and BRAF inhibitors (135) effectively block critical signaling pathways in Tumor Cells, thereby suppressing their growth and survival. This suppression further enhances the antitumor efficacy of immune checkpoint inhibitors. In patients with EGFR-mutated cSCC, a treatment regimen combining pembrolizumab with cetuximab, an EGFR inhibitor, has demonstrated marked clinical efficacy. The overall safety of pembrolizumab in combination with cetuximab has been found to be manageable. The most common grade 3–4 adverse event reported is stomatitis, with no treatment-related deaths observed (136). These findings suggest that the combination therapy regimens are generally well tolerated in patients with advanced CSCC. The therapeutic mechanism of this combination involves the targeted agent impairing the defense mechanisms of Tumor Cells, thereby enabling immune checkpoint inhibitors to more effectively activate the immune system for a coordinated antitumor response. This synergistic interaction offers a more tailored treatment option for patients whose tumors carry specific genetic mutations.
Encouraging outcomes have also been achieved in clinical studies investigating the combination of radiotherapy with immune checkpoint inhibitors. High-energy radiation has been shown to destroy the DNA of Tumor Cells, thereby inducing immunogenic cell death (ICD) (137). During this process, a substantial amount of Tumor Antigens is released by Tumor Cells, which activates the immune system and enhances the anti-tumor efficacy of immune checkpoint inhibitors. According to clinical trials, the combination of radiotherapy and pembrolizumab significantly improves the objective response rate (ORR) in patients with locally advanced cSCC (79). These findings demonstrate that the synergistic use of radiotherapy and immune checkpoint inhibitors enables a multifaceted attack on tumors, thereby enhancing therapeutic outcomes and offering new hope for patients with locally advanced cSCC. Moreover, ICD induced by radiotherapy not only strengthens the local immune response against tumors but also converts “cold” tumors (characterized by a lack of lymphocytic infiltration) into “hot” tumors (responsive to immunotherapy), thereby improving the overall effectiveness of immunotherapeutic strategies (138). This combined therapeutic strategy, known as radioimmunotherapy, has demonstrated promising potential in the treatment of various cancers (139). In one study, a liposomal nanomedicine named C/J-LipoRGD was developed by co-encapsulating a biological enzyme and a BRD4 inhibitor, enabling tumor-targeted delivery and modulation of the TIME. This formulation improved the oxygenation status of the Tumor Microenvironment, reduced the expression of PD-L1, reversed T cell exhaustion, significantly suppressed tumor growth, and induced ICD, thereby activating a T cell-mediated antitumor immune response (140).
immune checkpoint inhibitors can also be combined with other innovative therapeutic approaches, such as oncolytic viruses (141) and radionuclide therapy (142). Oncolytic viruses represent a novel therapeutic strategy characterized by a unique antitumor mechanism. They selectively infect and lyse Tumor Cells, while simultaneously releasing a substantial amount of Tumor Antigens within the Tumor Microenvironment. This process further stimulates the immune system and triggers a new inflammatory response aimed at eliminating Tumor Cells (141). In parallel, radionuclide therapy employs the localized radiation effects of radioactive nuclides to precisely target Tumor Cells (143), while also activating immune responses and enhancing the efficacy of immune checkpoint inhibitors. These combination therapies offer more diversified treatment options and are anticipated to play an increasingly important role in future clinical applications.
In summary, combination immunotherapies markedly amplify antitumor immune responses through the synergistic action of multiple mechanisms, offering renewed hope for cancer patients. With ongoing research advancements and the integration of new technologies, combination immunotherapies are expected to be continually optimized and refined. Emerging therapeutic strategies will be developed, existing regimens will become more precise and efficient, and personalized treatment approaches will see broader implementation. These advancements are anticipated to further enhance patient survival rates and quality of life, driving cancer treatment toward greater precision, efficacy, and individualization.
5 Conclusion
A critical role has been established for the TME of cSCC in tumor initiation, progression, and immune evasion. The Tumor Microenvironment constitutes a complex ecological system comprising immune cells, stromal cells, Cytokines, and the extracellular matrix (ECM). Through intricate interactions, these components collectively modulate tumor growth, invasion, and metastasis. Comprehensive exploration of the immunoregulatory mechanisms within the Tumor Microenvironment—including the recruitment and activation of immune cells, the establishment of an immunosuppressive milieu, Immune Evasion by Tumor Cells, and the regulation of Cytokines networks—has laid a robust theoretical foundation for the development of innovative immunotherapeutic strategies.
Immunotherapy, particularly the use of immune checkpoint inhibitors and approaches targeting the immunosuppressive microenvironment, has shown marked efficacy in the treatment of cSCC, offering promising prospects for enhancing patient survival and quality of life. However, immunotherapy may also lead to immune-related adverse events, such as skin and endocrine toxicities, which require careful attention and effective management in clinical settings.
Notably, this review differs from general discussions on tumor immunotherapy by highlighting the unique association between UV-induced mutations and Immune Evasion within the context of cSCC and the Tumor Microenvironment. In addition, the therapeutic advantages of immune checkpoint inhibitors in this disease are clarified. This focused investigation addresses a critical gap in understanding the specific mechanisms of immunotherapy for cSCC, thereby offering more precise theoretical support for clinical application.
To further improve the effectiveness of immunotherapy, ongoing research is actively investigating novel targets, combination treatment strategies, and potential biomarkers. Future research should place greater emphasis on regulating the immunosuppressive microenvironment, with particular focus on disrupting its protective role in tumor progression and enhancing the immune system’s capacity to eliminate tumor cells. Priority should also be given to investigating the application of bispecific antibodies in combination with immune checkpoint inhibitors in cSCC, while considering the influence of gender differences on treatment selection to further promote the development of personalized therapies for cSCC.
Optimizing combination immunotherapy represents another critical direction for future studies. By integrating immune checkpoint inhibitors with chemotherapy, radiotherapy, targeted therapy, and other treatment strategies, tumors can be targeted through multiple mechanisms, thereby improving overall therapeutic outcomes. In addition, the development of personalized treatment strategies remains a key focus of future research. By tailoring precise therapeutic approaches to individual patient characteristics and tumor-specific features, treatment efficacy can be maximized while minimizing adverse effects.
The integration of tumor metabolism and immunotherapy has also emerged as a prominent area of investigation. Clarifying how tumor metabolism affects immune responses, and how its modulation can enhance the effectiveness of immunotherapy, is expected to provide new insights for the treatment of cSCC. At the same time, the application of advanced technologies such as mass cytometry, single-cell transcriptomics, and epigenetics will enable a deeper understanding of the complex interactions between the Tumor Microenvironment and immune responses, thereby offering scientific evidence and novel strategies for optimizing immunotherapeutic approaches. By fostering multidisciplinary collaboration and innovation, ongoing progress in precision and personalized treatment for cSCC is expected to offer patients more effective therapeutic options and renewed hope, ultimately leading to improved prognosis and enhanced quality of life for those affected by cSCC.
Author contributions
QD: Writing – review & editing, Writing – original draft. ZZ: Writing – review & editing. SL: Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by: Shanxi Provincial Department of Science and Technology (Grant No:202303021211236) AND Science and Technology Bureau of Luliang (grant numbers: 2023RC-2-4)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Use AI translation.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kaufhold M, Asadi S, Ghoreishi Y, Brekner A, Grabbe S, Stege H, et al. Cutaneous squamous cell carcinoma risk factors: are current criteria still valid? A retrospective, monocenter analysis. Life (Basel). (2025) 15. doi: 10.3390/life15081257
2. Zheng S, Yu H, Zhang J, Lau WC, Chen M, Cheng H, et al. Prediction of relative survival trends in patients with cutaneous squamous cell carcinoma using a model-based period analysis: a retrospective analysis of the surveillance, epidemiology, and end results database. BMJ Open. (2024) 14:e086488. doi: 10.1136/bmjopen-2024-086488
3. Mrotzek P, Hofmann SC, Kreuter A, Appelbaum S, Wesselmann U, Wehry UP, et al. Cutaneous squamous cell carcinoma in the post-COVID-19 era: dramatic increase of new cases in 2023 in two German skin tumour centers. Acta Derm Venereol. (2025) 105:adv43609. doi: 10.2340/actadv.v105.43609
4. Budden T, Gaudy-Marqueste C, Craig S, Hu Y, Earnshaw CH, Gurung S, et al. Female immunity protects from cutaneous squamous cell carcinoma. Clin Cancer Res. (2021) 27:3215–23. doi: 10.1158/1078-0432.CCR-20-4261
5. Mousa AM, Enk AH, Hassel JC, and Reschke R. Immune checkpoints and cellular landscape of the tumor microenvironment in non-melanoma skin cancer (NMSC). Cells. (2024) 13. doi: 10.3390/cells13191615
6. Shi H, Li K, Ni Y, Liang X, and Zhao X. Myeloid-derived suppressor cells: implications in the resistance of Malignant tumors to T cell-based immunotherapy. Front Cell Dev Biol. (2021) 9:707198. doi: 10.3389/fcell.2021.707198
7. Li Z, Li J, Bai X, Huang X, and Wang Q. Tumor microenvironment as a complex milieu driving cancer progression: a mini review. Clin Transl Oncol. (2025) 27:1943–52. doi: 10.1007/s12094-024-03697-w
8. Xiao Y, Hassani M, Moghaddam MB, Fazilat A, Ojarudi M, and Valilo M. Contribution of tumor microenvironment (TME) to tumor apoptosis, angiogenesis, metastasis, and drug resistance. Med Oncol. (2025) 42:108. doi: 10.1007/s12032-025-02675-8
9. Anderson NM and Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r925. doi: 10.1016/j.cub.2020.06.081
10. Bottomley MJ, Thomson J, Harwood C, and Leigh I. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20082009
11. Peña-Romero AC and Orenes-Piñero E. Dual effect of immune cells within tumour microenvironment: pro- and anti-tumour effects and their triggers. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14071681
12. Zamarron BF and Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. (2011) 7:651–8. doi: 10.7150/ijbs.7.651
13. Thompson ED, Enriquez HL, Fu YX, and Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. (2010) 207:1791–804. doi: 10.1084/jem.20092454
14. Goldmann O, Nwofor OV, Chen Q, and Medina E. Mechanisms underlying immunosuppression by regulatory cells. Front Immunol. (2024) 15:1328193. doi: 10.3389/fimmu.2024.1328193
15. Zhang A, Fan T, Liu Y, Yu G, Li C, and Jiang Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol Cancer. (2024) 23:251. doi: 10.1186/s12943-024-02156-y
16. Schmidt A, Oberle N, and Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. (2012) 3:51. doi: 10.3389/fimmu.2012.00051
17. Yang Y, Li C, Liu T, Dai X, and Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to antigen specificity and microenvironmental regulation. Front Immunol. (2020) 11:1371. doi: 10.3389/fimmu.2020.01371
18. Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. (2014) 134:2853–64. doi: 10.1002/ijc.28622
19. Zhu X, Pribis JP, Rodriguez PC, Morris SM Jr, Vodovotz Y, Billiar TR, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. (2014) 259:171–8. doi: 10.1097/SLA.0b013e31828611f8
20. Werner A, Koschke M, Leuchtner N, Luckner-Minden C, Habermeier A, Rupp J, et al. Reconstitution of T Cell Proliferation under Arginine Limitation: Activated Human T Cells Take Up Citrulline via L-Type Amino Acid Transporter 1 and Use It to Regenerate Arginine after Induction of Argininosuccinate Synthase Expression. Front Immunol. (2017) 8:864. doi: 10.3389/fimmu.2017.00864
21. Navasardyan I and Bonavida B. Regulation of T cells in cancer by nitric oxide. Cells. (2021) 10. doi: 10.3390/cells10102655
22. Kuo WT, Chang JM, Chen CC, Tsao N, and Chang CP. Autophagy drives plasticity and functional polarization of tumor-associated macrophages. IUBMB Life. (2022) 74:157–69. doi: 10.1002/iub.2543
23. Sun J, Corradini S, Azab F, Shokeen M, Muz B, Miari KE, et al. IL-10R inhibition reprograms tumor-associated macrophages and reverses drug resistance in multiple myeloma. Leukemia. (2024) 38:2355–65. doi: 10.1038/s41375-024-02391-8
24. Liu C, Zhang W, Wang J, Si T, and Xing W. Tumor-associated macrophage-derived transforming growth factor-β promotes colorectal cancer progression through HIF1-TRIB3 signaling. Cancer Sci. (2021) 112:4198–207. doi: 10.1111/cas.15101
25. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. (2020) 11:1731. doi: 10.3389/fimmu.2020.01731
26. Zhao W, Zhang Z, Xie M, Ding F, Zheng X, Sun S, et al. Exploring tumor-associated macrophages in glioblastoma: from diversity to therapy. NPJ Precis Oncol. (2025) 9:126. doi: 10.1038/s41698-025-00920-x
27. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, and Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. (2020) 353:104119. doi: 10.1016/j.cellimm.2020.104119
28. Wu H, Xu JB, He YL, Peng JJ, Zhang XH, Chen CQ, et al. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. (2012) 106:462–8. doi: 10.1002/jso.23110
29. Lee WS, Yang H, Chon HJ, and Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. (2020) 52:1475–85. doi: 10.1038/s12276-020-00500-y
30. Jia H, Chen X, Zhang L, and Chen M. Cancer associated fibroblasts in cancer development and therapy. J Hematol Oncol. (2025) 18:36. doi: 10.1186/s13045-025-01688-0
31. Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, et al. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. (2018) 9:867. doi: 10.1038/s41419-018-0922-1
32. Hida K, Maishi N, Torii C, and Hida Y. Tumor angiogenesis–characteristics of tumor endothelial cells. Int J Clin Oncol. (2016) 21:206–12. doi: 10.1007/s10147-016-0957-1
33. Shi Z, Kuai M, Li B, Akowuah CF, Wang Z, Pan Y, et al. The role of VEGF in Cancer angiogenesis and tumorigenesis: Insights for anti-VEGF therapy. Cytokine. (2025) 189:156908. doi: 10.1016/j.cyto.2025.156908
34. Shaw P, Dwivedi SKD, Bhattacharya R, Mukherjee P, and Rao G. VEGF signaling: Role in angiogenesis and beyond. Biochim Biophys Acta Rev Cancer. (2024) 1879:189079. doi: 10.1016/j.bbcan.2024.189079
35. Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. (2021) 6:153. doi: 10.1038/s41392-021-00544-0
36. Eble JA and Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. (2019) 36:171–98. doi: 10.1007/s10585-019-09966-1
37. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, and Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. (2020) 11:5120. doi: 10.1038/s41467-020-18794-x
38. Wang H, Wang T, Yan S, Tang J, Zhang Y, Wang L, et al. Crosstalk of pyroptosis and cytokine in the tumor microenvironment: from mechanisms to clinical implication. Mol Cancer. (2024) 23:268. doi: 10.1186/s12943-024-02183-9
39. Pradhan R, Kundu A, and Kundu CN. The cytokines in tumor microenvironment: from cancer initiation-elongation-progression to metastatic outgrowth. Crit Rev Oncol Hematol. (2024) 196:104311. doi: 10.1016/j.critrevonc.2024.104311
40. Nan D, Yao W, Huang L, Liu R, Chen X, Xia W, et al. Glutamine and cancer: metabolism, immune microenvironment, and therapeutic targets. Cell Commun Signal. (2025) 23:45. doi: 10.1186/s12964-024-02018-6
41. Jin J, Byun J-K, Choi Y-K, and Park K-G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp Mol Med. (2023) 55:706–15. doi: 10.1038/s12276-023-00971-9
42. Hosonuma M and Yoshimura K. Association between pH regulation of the tumor microenvironment and immunological state. Front Oncol. (2023) 13:1175563. doi: 10.3389/fonc.2023.1175563
43. Rahman MA, Yadab MK, and Ali MM. Emerging role of extracellular pH in tumor microenvironment as a therapeutic target for cancer immunotherapy. Cells. (2024) 13. doi: 10.3390/cells13221924
44. Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. (2021) 11:e492. doi: 10.1002/ctm2.492
45. Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. (2023) 22:58. doi: 10.1186/s12943-023-01725-x
46. Quan Z, Yang Y, Zheng H, Zhan Y, Luo J, Ning Y, et al. Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J Cancer. (2022) 13:3434–43. doi: 10.7150/jca.77619
47. Wang Q, Xie B, Liu S, Shi Y, Tao Y, Xiao D, et al. What happens to the immune microenvironment after PD-1 inhibitor therapy? Front Immunol. (2021) 12:773168. doi: 10.3389/fimmu.2021.773168
48. Idris OA, Westgate D, Saadaie Jahromi B, Shebrain A, Zhang T, and Ashour HM. PD-L1 inhibitor cosibelimab for cutaneous squamous cell carcinoma: comprehensive evaluation of efficacy, mechanism, and clinical trial insights. Biomedicines. (2025) 13. doi: 10.3390/biomedicines13040889
49. Ghahremanifard P, Chanda A, Bonni S, and Bose P. TGF-β Mediated immune evasion in cancer-spotlight on cancer-associated fibroblasts. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12123650
50. Zhang Y, Chen Q, Chen D, and Yang G. Role of the TGF-β/Smad3 pathway in pancreatic cancer cell growth and stem cell characteristics. Discov Oncol. (2025) 16:1480. doi: 10.1007/s12672-025-03220-9
51. Cui H, Wang N, Li H, Bian Y, Wen W, Kong X, et al. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun Signal. (2024) 22:99. doi: 10.1186/s12964-024-01505-0
52. Xu J, Ding L, Mei J, Hu Y, Kong X, Dai S, et al. Dual roles and therapeutic targeting of tumor-associated macrophages in tumor microenvironments. Signal Transduct Target Ther. (2025) 10:268. doi: 10.1038/s41392-025-02325-5
53. Li Y, Liu Q, Jing X, Wang Y, Jia X, Yang X, et al. Cancer-associated fibroblasts: heterogeneity, cancer pathogenesis, and therapeutic targets. MedComm (2020). (2025) 6:e70292. doi: 10.1002/mco2.70292
54. Yang W, Zhang S, Li T, Zhou Z, and Pan J. Single-cell analysis reveals that cancer-associated fibroblasts stimulate oral squamous cell carcinoma invasion via the TGF-β/Smad pathway. Acta Biochim Biophys Sin (Shanghai). (2022) 55:262–73. doi: 10.3724/abbs.2022132
55. Miyazawa K, Itoh Y, Fu H, and Miyazono K. Receptor-activated transcription factors and beyond: multiple modes of Smad2/3-dependent transmission of TGF-β signaling. J Biol Chem. (2024) 300:107256. doi: 10.1016/j.jbc.2024.107256
56. Lien HE, Berg HF, Halle MK, Trovik J, Haldorsen IS, Akslen LA, et al. Single-cell profiling of low-stage endometrial cancers identifies low epithelial vimentin expression as a marker of recurrent disease. EBioMedicine. (2023) 92:104595. doi: 10.1016/j.ebiom.2023.104595
57. Lv T, Huang J, Wu M, Wang H, Zeng Q, and Wang X. Halofuginone enhances the anti-tumor effect of ALA-PDT by suppressing NRF2 signaling in cSCC. Photodiagnosis Photodyn Ther. (2022) 37:102572. doi: 10.1016/j.pdpdt.2021.102572
58. Asahina R, Egawa G, Nakamizo S, and Kabashima K. 001 Maintenance of CD4+ tissue-resident memory T cells via perivascular clusters with CD301b+ dermal dendritic cells in a mouse model of allergic dermatitis. J Invest Dermatol. (2021) 141:S149. doi: 10.1016/j.jid.2021.08.002
59. Zhao Y, Du J, and Shen X. Targeting myeloid-derived suppressor cells in tumor immunotherapy: Current, future and beyond. Front Immunol. (2023) 14:1157537. doi: 10.3389/fimmu.2023.1157537
60. He Y, Tian T, Li Y, Zeng Y, Wang X, Qian L, et al. From neglect to necessity: the role of innate immunity in cutaneous squamous cell carcinoma therapy. Front Immunol. (2025) 16:1570032. doi: 10.3389/fimmu.2025.1570032
61. Jiang M, Chen J, Zhang W, Zhang R, Ye Y, Liu P, et al. Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front Immunol. (2017) 8:1840. doi: 10.3389/fimmu.2017.01840
62. Stayer K, Pathan S, Biswas A, Li H, Zhu Y, Lam FW, et al. Exogenous arginine differentially regulates inflammatory cytokine and inducible nitric oxide synthase expression in macrophages. Immunohorizons. (2025) 9. doi: 10.1093/immhor/vlaf028
63. Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. (2004) 64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465
64. Parker KH, Beury DW, and Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. (2015) 128:95–139. doi: 10.1016/bs.acr.2015.04.002
65. Shrestha P, Shrestha R, Zhou Y, Zielinski R, Priebe W, and Kleinerman ES. STAT3 inhibition in combination with CD47 blockade inhibits osteosarcoma lung metastasis. Front Immunol. (2025) 16:1608375. doi: 10.3389/fimmu.2025.1608375
66. Supsrisunjai C, Hsu C-K, Michael M, Duval C, Lee JYW, Yang H-S, et al. Coagulation factor XIII-A subunit missense mutation in the pathobiology of autosomal dominant multiple dermatofibromas. J Invest Dermatol. (2020) 140:624–635.e7. doi: 10.1016/j.jid.2019.08.441
67. Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li C, et al. TGF-β signaling in health, disease and therapeutics. Signal Transduct Target Ther. (2024) 9:61. doi: 10.1038/s41392-024-01764-w
68. Li X, Zhong J, Deng X, Guo X, Lu Y, Lin J, et al. Targeting myeloid-derived suppressor cells to enhance the antitumor efficacy of immune checkpoint blockade therapy. Front Immunol. (2021) 12:754196. doi: 10.3389/fimmu.2021.754196
69. Castiglioni A, Yang Y, Williams K, Gogineni A, Lane RS, Wang AW, et al. Combined PD-L1/TGFβ blockade allows expansion and differentiation of stem cell-like CD8 T cells in immune excluded tumors. Nat Commun. (2023) 14:4703. doi: 10.1038/s41467-023-40398-4
70. Sciubba JJ and Larian B. Oral squamous cell carcinoma: early detection and improved 5-year survival in 102 patients. Gen Dentistry. (2018) 66 6:e11–6.
71. Solares CA, Lee K, Parmar P, O’Rourke P, and Panizza B. Epidemiology of clinical perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. (2012) 146:746–51. doi: 10.1177/0194599811434897
72. Sullivan CB, Andresen NS, Kendell N, Al-Qurayshi Z, and Pagedar NA. Survival outcomes for advanced cutaneous squamous cell carcinoma of the head and neck. Ann Otology Rhinol Laryngol. (2019) 128:949–55. doi: 10.1177/0003489419848786
73. Migden MR, Rischin D, Schmults C, Guminski A, Hauschild A, Lewis K, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. New Engl J Med. (2018) 379:341–51. doi: 10.1056/NEJMoa1805131
74. Harris BN, Pipkorn P, Nguyen KNB, Jackson RS, Rao S, Moore MG, et al. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. (2019) 145:153–8. doi: 10.1001/jamaoto.2018.3650
75. Topalian SL, Taube JM, and Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. (2020) 367:eaax0182. doi: 10.1126/science.aax0182
76. Leidner R, Crittenden M, Young K, Xiao H, Wu Y, Couey MA, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002485
77. Barker HE, Paget JT, Khan AA, and Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15:409–25. doi: 10.1038/nrc3958
78. Seifert L, Werba G, Tiwari S, Giao Ly NN, Nguy S, Alothman S, et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology. (2016) 150:1659–1672.e5. doi: 10.1053/j.gastro.2016.02.070
79. Lavaud J, Blom A, Longvert C, Fort M, Funck-Brentano E, and Saiag P. Pembrolizumab and concurrent hypo-fractionated radiotherapy for advanced non-resectable cutaneous squamous cell carcinoma. Eur J Dermatol. (2019) 29:636–40. doi: 10.1684/ejd.2019.3671
80. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124:687–95. doi: 10.1172/JCI67313
81. Grob JJ, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629). J Clin Oncol. (2020) 38:2916–25. doi: 10.1200/JCO.19.03054
82. Galbiati D, Cavalieri S, Alfieri S, Resteghini C, Bergamini C, Orlandi E, et al. Activity of platinum and cetuximab in cutaneous squamous cell cancer not amenable to curative treatment. Drugs Context. (2019) 8:212611. doi: 10.7573/dic.212611
83. Novoplansky O, Fury M, Prasad M, Yegodayev K, Zorea J, Cohen L, et al. MET activation confers resistance to cetuximab, and prevents HER2 and HER3 upregulation in head and neck cancer. Int J Cancer. (2019) 145:748–62. doi: 10.1002/ijc.32170
84. Burns C, Kubicki S, Nguyen QB, Aboul-Fettouh N, Wilmas KM, Chen OM, et al. Advances in cutaneous squamous cell carcinoma management. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14153653
85. Dereure O, Missan H, Girard C, Costes V, and Guillot B. Efficacy and tolerance of cetuximab alone or combined with chemotherapy in locally advanced or metastatic cutaneous squamous cell carcinoma: an open study of 14 patients. Dermatology. (2017) 232:721–30. doi: 10.1159/000461578
86. Hartmann S, Bhola NE, and Grandis JR. HGF/met signaling in head and neck cancer: impact on the tumor microenvironment. Clin Cancer Res. (2016) 22:4005–13. doi: 10.1158/1078-0432.CCR-16-0951
87. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
88. Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. (2016) 34:3838–45. doi: 10.1200/JCO.2016.68.1478
89. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
90. Hanna N, Lilenbaum R, Ansari R, Lynch T, Govindan R, Jänne PA, et al. Phase II trial of cetuximab in patients with previously treated non–small-cell lung cancer. J Clin Oncol. (2006) 24:5253–8. doi: 10.1200/jco.2006.08.2263
91. Ott PA, Elez E, Hiret S, Kim D-W, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. (2017) 35:3823–9. doi: 10.1200/JCO.2017.72.5069
92. Hsu C, Lee S-H, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. (2017) 35:4050–6. doi: 10.1200/JCO.2017.73.3675
93. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, and Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U.S.A. (2002) 99:12293–7. doi: 10.1073/pnas.192461099
94. Yang W, Lei C, Song S, Jing W, Jin C, Gong S, et al. Immune checkpoint blockade in the treatment of Malignant tumor: current statue and future strategies. Cancer Cell Int. (2021) 21:589. doi: 10.1186/s12935-021-02299-8
95. Han Y, Liu D, and Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10:727–42.
96. Babamohamadi M, Mohammadi N, Faryadi E, Haddadi M, Merati A, Ghobadinezhad F, et al. Anti-CTLA-4 nanobody as a promising approach in cancer immunotherapy. Cell Death Dis. (2024) 15:17. doi: 10.1038/s41419-023-06391-x
97. Saad P and Kasi A. Ipilimumab. In: StatPearls. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC, Treasure Island (FL (2025).
98. Jiang Y, Chen M, Nie H, and Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. (2019) 15:1111–22. doi: 10.1080/21645515.2019.1571892
99. Kwok G, Yau TC, Chiu JW, Tse E, and Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. (2016) 12:2777–89. doi: 10.1080/21645515.2016.1199310
100. Liu C, Seeram NP, and Ma H. Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: a review. Cancer Cell Int. (2021) 21:239. doi: 10.1186/s12935-021-01946-4
101. Liu J, Chen Z, Li Y, Zhao W, Wu J, and Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. (2021) 12:731798. doi: 10.3389/fphar.2021.731798
102. Zhang H, Zhong A, and Chen J. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: A systemic review and meta-analysis. Skin Res Technol. (2023) 29:e13229. doi: 10.1111/srt.13229
103. Mehta NK, Li AR, Nguyen SA, Kaczmar JM, Neskey DM, and Day TA. Immune checkpoint inhibitors for advanced cutaneous squamous cell carcinoma: A systematic review with meta-analysis. Target Oncol. (2021) 16:743–52. doi: 10.1007/s11523-021-00844-z
104. McLean LS, Lim AM, Bressel M, Lee J, Ladwa R, Guminski AD, et al. Immune checkpoint inhibitor therapy for advanced cutaneous squamous cell carcinoma in Australia: a retrospective real world cohort study. Med J Aust. (2024) 220:80–90. doi: 10.5694/mja2.52199
105. Patel R and Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. (2019) 20:477–82. doi: 10.1007/s40257-019-00426-w
106. Maubec E, Boubaya M, Petrow P, Beylot-Barry M, Basset-Seguin N, Deschamps L, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. (2020) 38:3051–61. doi: 10.1200/JCO.19.03357
107. Hughes BGM, Munoz-Couselo E, Mortier L, Bratland Å, Gutzmer R, Roshdy O, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. (2021) 32:1276–85. doi: 10.1016/j.annonc.2021.07.008
108. Stewart M and Geiger JL. Pembrolizumab for the treatment of locally advanced cutaneous squamous cell carcinoma: patient selection and special considerations. Cancer Manag Res. (2025) 17:211–7. doi: 10.2147/CMAR.S379963
109. Koch Hein EC, Vilbert M, Hirsch I, Fernando Ribeiro M, Muniz TP, Fournier C, et al. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: real-world experience from a canadian comprehensive cancer centre. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15174312
110. Rendon A and Rayi A. Nivolumab. In: StatPearls. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC, Treasure Island (FL (2025).
111. Papaporfyriou A, Bartziokas K, Apessos I, Mueller J, Leivaditis V, Koletsis E, et al. Comparative efficacy and safety of neoadjuvant immunotherapy with nivolumab vs. pembrolizumab in resectable non-small cell lung cancer: A systematic review. Curr Oncol. (2024) 31:6289–99. doi: 10.3390/curroncol31100469
112. Ge W, Wu N, Chen CI, Inocencio TJ, LaFontaine PR, Seebach F, et al. Real-world treatment patterns and outcomes of cemiplimab in patients with advanced cutaneous squamous cell carcinoma treated in US oncology practices. Cancer Manag Res. (2024) 16:841–54. doi: 10.2147/CMAR.S445910
113. Zhu J, Armstrong AJ, Friedlander TW, Kim W, Pal SK, George DJ, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J ImmunoTher Cancer. (2018) 6:4. doi: 10.1186/s40425-018-0314-1
114. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
115. Aggarwal V, Workman CJ, and Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. (2023) 24:1415–22. doi: 10.1038/s41590-023-01569-z
116. Zhao L, Cheng S, Fan L, Zhang B, and Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol. (2021) 99:107933. doi: 10.1016/j.intimp.2021.107933
117. Chauvin JM and Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-000957
118. Chen Y, Wang P, Lian R, Yuan M, Yu P, He H, et al. Comprehensive characterization of PD-L1 expression and immunotherapy-related genomic biomarkers in early- versus advanced-stage non-small cell lung cancer. BMC Pulmonary Med. (2025) 25:219. doi: 10.1186/s12890-025-03687-w
119. Wang X and Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. (2019) 20:4. doi: 10.1186/s12865-018-0285-5
120. Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, et al. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. (2021) 112:1105–13. doi: 10.1111/cas.14798
121. Liang D and Zhang Z. MicroRNA-27b-3p inhibits the proliferation and invasion of cutaneous squamous cell carcinoma by targeting EGFR and MMP-13. Oncol Lett. (2021) 22:729. doi: 10.3892/ol.2021.12990
122. Aboul-Fettouh N, Morse D, Patel J, and Migden MR. Immunotherapy and systemic treatment of cutaneous squamous cell carcinoma. Dermatol Pract Concept. (2021) 11:e2021169S. doi: 10.5826/dpc.11S2a169S
123. Harwood SL, Alvarez-Cienfuegos A, Nuñez-Prado N, Compte M, Hernández-Pérez S, Merino N, et al. ATTACK, a novel bispecific T cell-recruiting antibody with trivalent EGFR binding and monovalent CD3 binding for cancer immunotherapy. Oncoimmunology. (2017) 7:e1377874. doi: 10.1080/2162402x.2017.1377874
124. Zhong L, Shi W, Gan L, Liu X, Huo Y, Wu P, et al. Human endoglin-CD3 bispecific T cell engager antibody induces anti-tumor effect in vivo. Theranostics. (2021) 11:6393–406. doi: 10.7150/thno.53121
125. Reusch U, Olson S, Davis J, Davol P, Sundarum M, Liu P, et al. T-cell based cancer immunotherapy with a bispecific antibody directed at CD3 and EGFR. J Clin Oncol. (2005) 23:2552–2. doi: 10.1200/jco.2005.23.16_suppl.2552
126. Kühl L, Schäfer AK, Kraft S, Aschmoneit N, Kontermann RE, and Seifert O. eIg-based bispecific T-cell engagers targeting EGFR: Format matters. MAbs. (2023) 15:2183540. doi: 10.1080/19420862.2023.2183540
127. Boustany LM, LaPorte SL, Wong L, White C, Vinod V, Shen J, et al. A probody T cell-engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity. Cancer Res. (2022) 82:4288–98. doi: 10.1158/0008-5472.CAN-21-2483
128. Li J, Piskol R, Ybarra R, Chen YJJ, Li J, Slaga D, et al. CD3 bispecific antibody–induced cytokine release is dispensable for cytotoxic T cell activity. Sci Trans Med. (2019) 11:eaax8861. doi: 10.1126/scitranslmed.aax8861
129. Dang K, Castello G, Clarke SC, Li Y, Balasubramani A, Boudreau A, et al. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002488
130. Mauerer A, Herschberger E, Dietmaier W, Landthaler M, and Hafner C. Low incidence of EGFR and HRAS mutations in cutaneous squamous cell carcinomas of a German cohort. Exp Dermatol. (2011) 20:848–50. doi: 10.1111/j.1600-0625.2011.01334.x
131. Wang Y, Deng X, Dai Y, Niu X, and Zhou M. miR-27a downregulation promotes cutaneous squamous cell carcinoma progression via targeting EGFR. Front Oncol. (2020) 9. doi: 10.3389/fonc.2019.01565
132. Kubo A, Hashimoto H, Takahashi N, and Yamada Y. Biomarkers of skin toxicity induced by anti-epidermal growth factor receptor antibody treatment in colorectal cancer. World J Gastroenterol. (2016) 22:887–94. doi: 10.3748/wjg.v22.i2.887
133. Reusch U, Sundaram M, Davol PA, Olson SD, Davis JB, Demel K, et al. Anti-CD3 × Anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res. (2006) 12:183–90. doi: 10.1158/1078-0432.CCR-05-1855
134. Abourehab MAS, Alqahtani AM, Youssif BGM, and Gouda AM. Globally approved EGFR inhibitors: insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules. (2021) 26. doi: 10.3390/molecules26216677
135. Hanrahan AJ, Chen Z, Rosen N, and Solit DB. BRAF — a tumour-agnostic drug target with lineage-specific dependencies. Nat Rev Clin Oncol. (2024) 21:224–47. doi: 10.1038/s41571-023-00852-0
136. Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. (2021) 22:883–92. doi: 10.1016/S1470-2045(21)00136-4
137. Vaes RDW, Hendriks LEL, Vooijs M, and De Ruysscher D. Biomarkers of radiotherapy-induced immunogenic cell death. Cells. (2021) 10. doi: 10.3390/cells10040930
138. Zhu S, Wang Y, Tang J, and Cao M. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front Immunol. (2022) 13:1074477. doi: 10.3389/fimmu.2022.1074477
139. Liu T, Pei P, Shen W, Hu L, and Yang K. Radiation-induced immunogenic cell death for cancer radioimmunotherapy. Small Methods. (2023) 7:e2201401. doi: 10.1002/smtd.202201401
140. Liu Y, Zhang Y, Yang X, Lang S, Zhu Y, Song J, et al. Reprogramming of radiation-deteriorated TME by liposomal nanomedicine to potentiate radio-immunotherapy. J Control Release. (2025) 383:113792. doi: 10.1016/j.jconrel.2025.113792
141. Santos Apolonio J, Lima de Souza Gonçalves V, Cordeiro Santos ML, Silva Luz M, Silva Souza JV, Rocha Pinheiro SL, et al. Oncolytic virus therapy in cancer: A current review. World J Virol. (2021) 10:229–55. doi: 10.5501/wjv.v10.i5.229
142. Palot Manzil FF and Kaur H. Radioactive iodine therapy for thyroid Malignancies. In: StatPearls. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC, Treasure Island (FL (2025).
143. Burkhardt C, Bühler L, Viertl D, and Stora T. New isotopes for the treatment of pancreatic cancer in collaboration with CERN: A mini review. Front Med (Lausanne). (2021) 8:674656. doi: 10.3389/fmed.2021.674656
144. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. (2020) 21:294–305. doi: 10.1016/S1470-2045(19)30728-4
145. Rischin D, Khushalani NI, Schmults CD, Guminski A, Chang ALS, Lewis KD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. (2021) 9:e002757. doi: 10.1136/jitc-2021-002757
Keywords: cutaneous squamous cell carcinoma, tumor microenvironment, immunotherapy, immunosuppression, immune checkpoint inhibitors
Citation: Dong Q, Zhang Z, Li S and Liang L (2025) Mechanisms of immunotherapy in cutaneous squamous cell carcinoma in the tumor microenvironment. Front. Immunol. 16:1660272. doi: 10.3389/fimmu.2025.1660272
Received: 05 July 2025; Accepted: 30 October 2025;
Published: 17 November 2025.
Edited by:
Vincenzo Flati, University of L’Aquila, ItalyReviewed by:
Elisabetta Palazzo, University of Modena and Reggio Emilia, ItalyKevinn Eddy, Eurofins PSS, United States
Copyright © 2025 Dong, Zhang, Li and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Liang, YW15bGlhbmdsaUAxNjMuY29t
 Qinyi Dong
Qinyi Dong Zijian Zhang1
Zijian Zhang1 Lili Liang
Lili Liang