- Day Surgery Center, Honghui Hospital Affiliated to Xi'an Jiaotong University, Xi’an, China
Rotator cuff injuries frequently result in poor tendon-to-bone healing due to the failure to regenerate the native fibrocartilaginous enthesis and the persistence of a dysregulated immune microenvironment. Mesenchymal stem cells (MSCs) have emerged as promising therapeutic agents, not only for their multilineage differentiation potential but also for their potent immunomodulatory functions. Emerging evidence highlights that MSCs engage in bidirectional crosstalk with immune cells such as macrophages, T cells, and NK cells through both paracrine factors and direct cell–cell contact, critically shaping the reparative versus fibrotic outcome of tendon-to-bone healing. This review summarizes the biological mechanisms underlying MSC-mediated tendon-to-bone healing, with a focus on immune modulation. We discuss recent advances in cell-free approaches, biomaterial-assisted delivery systems, and strategies to enhance the local immune milieu. Current challenges—including MSC heterogeneity, variable patient immune responses, and translational barriers—are also addressed. Finally, we highlight future directions such as personalized immunomodulatory therapies, 3D humanized testing models, and AI-based prediction tools aimed at improving clinical outcomes. Specifically, AI algorithms that integrate patient-specific immune profiles—such as single-cell transcriptomics and cytokine signatures—may enable responder stratification and guide individualized MSC-based interventions. Understanding and leveraging the MSC–immune interaction is key to unlocking the full potential of regenerative therapies in rotator cuff repair.
1 Introduction
Rotator cuff tears are one of the most common causes of shoulder dysfunction and chronic pain, affecting millions of people worldwide. In the United States alone, over 460,000 rotator cuff repair surgeries are performed annually, underscoring the significant clinical and socioeconomic burden of this condition (1). Despite advances in surgical techniques and materials, postoperative tendon-to-bone healing remains suboptimal, with reported retear rates ranging from 29.5% to as high as 94% in some populations (2, 3). Such high failure rates significantly compromise patient outcomes and long-term quality of life.
A key biological obstacle lies in the inability to regenerate the native fibrocartilaginous enthesis, a highly specialized transition zone between tendon and bone. Instead, surgical repair commonly results in fibrous scar tissue formation at the tendon-bone junction, which lacks the zonal organization and mechanical strength of the native interface, making the repair site susceptible to mechanical failure (4, 5).
Moreover, emerging evidence suggests that dysregulation of the local immune microenvironment and persistent inflammation also play a critical role in impairing the quality of tendon-to-bone healing (6). Following injury and surgical repair, the recruitment and activation of immune cells such as macrophages, T cells, and neutrophils initiate an inflammatory cascade that may either support or hinder regeneration, depending on the balance between pro-inflammatory and pro-regenerative signals (7).
In recent years, cell-based therapies—particularly those involving mesenchymal stem cells (MSCs)—have gained attention for their multifaceted role in musculoskeletal regeneration. MSCs are multipotent stromal cells capable of differentiating into osteogenic, chondrogenic, and tenogenic lineages. Beyond differentiation, MSCs exert profound immunomodulatory and paracrine effects by secreting anti-inflammatory cytokines and regenerative growth factors such as TGF-β, VEGF, and IL-10 (8, 9). These properties enable MSCs to reshape the immune microenvironment, reduce excessive inflammation, and facilitate the formation of a more functional enthesis-like structure.
Furthermore, MSC-derived extracellular vesicles (EVs), conditioned media, and scaffold-based delivery systems have been explored as cell-free strategies to harness the regenerative potential of MSCs while minimizing the risks associated with direct cell transplantation (10, 11). In parallel, immunomodulatory strategies—such as promoting M2 macrophage polarization or inhibiting excessive M1 activation—have shown promise in improving tendon-to-bone healing outcomes (12).
This review aims to provide a comprehensive overview of the current understanding of MSCs and their interaction with the immune microenvironment in the context of rotator cuff tendon-to-bone healing. We focus on the biological mechanisms underlying their crosstalk, highlight recent advances in preclinical and translational research, and discuss future directions for biomaterial-assisted, immune-responsive, stem cell-based therapies.
2 Biological basis of tendon-to-bone healing in rotator cuff repair
2.1 Native enthesis structure and healing response
2.1.1 Four-zone interface: tendon, fibrocartilage, mineralized fibrocartilage, and bone
The tendon-to-bone interface of the rotator cuff, or enthesis, is a specialized structure that facilitates load transfer between tendon and bone. As Figure 1 depicts, it comprises four distinct zones: (1) tendon, (2) unmineralized fibrocartilage, (3) mineralized fibrocartilage, and (4) bone (13–15).

Figure 1. A schematic representation of the tendon-to-bone interface, depicting the histological characteristics of its four transitional zones.
Each zone features unique cells, matrix components, and mechanical roles:
Zone 1 – Tendon: Rich in aligned type I collagen and tenocytes, this region offers strong tensile resistance (13).
Zone 2 – Unmineralized Fibrocartilage: Contains type II collagen, proteoglycans, and chondrocyte-like cells, helping to absorb and spread mechanical stress (13, 16).
Zone 3 – Mineralized Fibrocartilage: Shows gradual collagen mineralization with type II and X collagen and hypertrophic chondrocytes, forming a transitional zone for stress transfer (14, 15).
Zone 4 – Bone: Composed of mineralized matrix and osteocytes, providing structural anchoring (14).
This four-zone architecture develops after birth, with collagen and cell phenotypes gradually establishing a spatial gradient along the interface (14, 15). This organization ensures efficient load transmission and mechanical durability.
Following injury and repair, this native structure is rarely restored. The interface is often replaced by disorganized fibrous scar tissue lacking zonal features, with disrupted collagen alignment, reduced chondrocyte presence, and impaired mechanical strength (13, 17, 18). Animal studies show that the regenerated interface typically resembles a single fibrous layer with limited function (15, 17, 18).
Recent tissue engineering strategies have attempted to replicate the zonal structure using growth factors, scaffolds, and stem cells. Some animal models have achieved partial regeneration of multilayered tissue with improved outcomes, but clinical success remains limited (14, 16, 19).
The absence of native enthesis architecture remains a major barrier to durable repair.
2.1.2 Inadequate reformation after injury leading to biomechanical weakness
Following rotator cuff injury and surgical repair, the restoration of the native tendon-to-bone interface (enthesis) is rarely achieved. Instead, healing is typically characterized by the formation of disorganized fibrovascular scar tissue, which lacks the highly specialized zonal architecture and mineralized fibrocartilage of the native enthesis (20, 21). This aberrant repair tissue exhibits inferior mechanical properties, including reduced load-to-failure and decreased stiffness, predisposing the repair site to retear under physiological loading (1, 15).
Histological and ultrastructural analyses reveal that the repaired interface fails to recapitulate the gradation of collagen types, mineral content, and cellular morphology seen in the normal enthesis (16, 17). The absence of these gradients results in a biomechanical mismatch between tendon and bone, further compromising the integrity of the repair (17, 21). Animal studies consistently demonstrate that the newly formed interface is mechanically weaker than the native insertion, with lower ultimate tensile strength and increased susceptibility to failure (22, 23).
Current repair techniques, including suture and anchor-based methods, do not adequately address the biological requirements for enthesis regeneration, often resulting in persistent biomechanical deficits (1, 22). While scaffold augmentation and biologic strategies such as growth factor delivery and stem cell incorporation have shown promise in preclinical models for enhancing fibrocartilage formation and improving mechanical strength, translation to consistent clinical benefit remains limited (3, 24). Thus, inadequate reformation of the enthesis after injury remains a principal cause of biomechanical weakness and high retear rates following rotator cuff repair (15, 17).
2.2 Phases of tendon-to-bone healing
Tendon-to-bone healing after rotator cuff repair is classically divided into three overlapping phases (Figure 2): the inflammatory phase, the proliferation phase, and the remodeling phase (20). Each phase is characterized by distinct cellular and molecular events that ultimately determine the quality of the repair tissue and its biomechanical properties (25, 26).
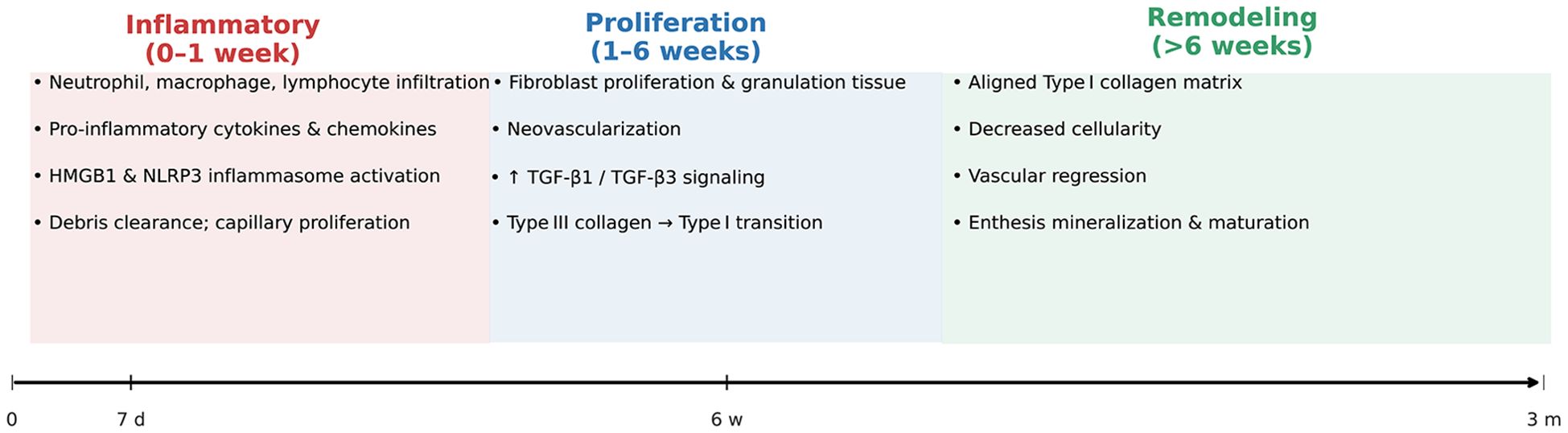
Figure 2. Temporal Phases of Tendon-to-Bone Healing After Rotator Cuff Repair. This schematic timeline illustrates the sequential inflammatory (0–1 week), proliferative (1–6 weeks), and remodeling (>6 weeks) phases of rotator cuff tendon-to-bone healing. Key cellular activities (e.g., immune-cell infiltration, fibroblast proliferation, collagen remodeling) and principal molecular signals (HMGB1/NLRP3, TGF-β1/β3) are annotated within each phase, highlighting the transition from an early inflammatory milieu to organized, mineralizing enthesis tissue.
2.2.1 Inflammatory phase
Immediately after tendon reattachment, the healing site undergoes an acute inflammatory response. Neutrophils, macrophages, and lymphocytes infiltrate the repair interface, releasing pro-inflammatory cytokines and chemokines that initiate debris clearance and recruit reparative cells (27). Upregulation of HMGB1 and activation of the NLRP3 inflammasome have been implicated in amplifying inflammation and extracellular matrix (ECM) disorganization during this period. Capillary proliferation and increased cellularity are observed within the first week, peaking around 7–10 days post-repair. This early inflammatory milieu is essential for subsequent healing, but excessive or prolonged inflammation can impair matrix organization and enthesis regeneration (28, 29).
2.2.2 Proliferation phase
The proliferative phase is marked by robust fibroblast proliferation, neovascularization, and synthesis of new ECM, predominantly type III collagen. Growth factors such as TGF-β1 and TGF-β3 are upregulated, promoting cell proliferation and matrix deposition. This phase typically spans from the end of the first week to several weeks post-injury (27, 30). The repair tissue remains highly cellular and disorganized, with ongoing angiogenesis and granulation tissue formation. The transition from type III to type I collagen synthesis begins during this phase, setting the stage for subsequent tissue maturation (27, 31).
2.2.3 Remodeling phase
During the remodeling phase, which can extend from several weeks to months, the initially disorganized collagen matrix is gradually replaced by more organized, aligned type I collagen fibers. Cellular density decreases, and the vascular network regresses (20, 27). The enthesis undergoes slow maturation, with partial restoration of zonal architecture and mineralization, although the regenerated interface rarely achieves the structural and biomechanical properties of the native enthesis. The ratio of type I to type III collagen increases, and the tissue becomes more resistant to mechanical loading, but persistent differences in organization and strength compared to uninjured tendon-bone insertions remain (30, 31).
Overall, the incomplete recapitulation of the native enthesis structure and the persistence of scar-mediated healing contribute to the biomechanical weakness and high retear rates observed after rotator cuff repair (27, 31).
2.3 Role of immune cells in tendon-to-bone healing
Below, we provide a detailed overview of how both innate (neutrophils, macrophages) and adaptive (T and B lymphocytes, dendritic cells) immune cells coordinate the inflammatory, proliferative, and remodeling phases of tendon-to-bone healing; their dynamic interplay is schematically summarized in Figure 3.
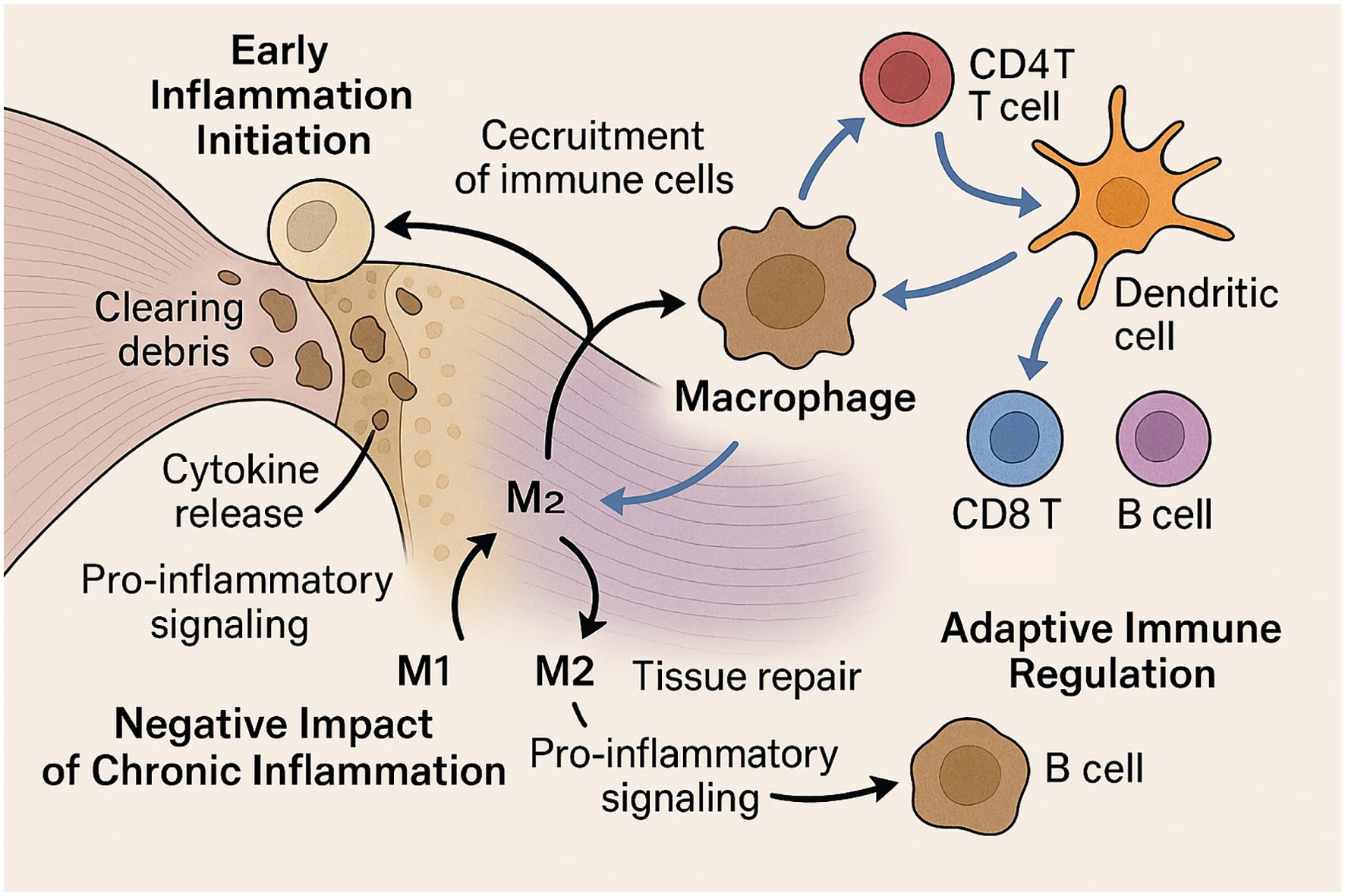
Figure 3. Immune Cell Dynamics in Rotator Cuff Tendon-to-Bone Healing. This schematic illustration depicts the roles of immune cells in rotator cuff tendon-to-bone healing across different stages. In the early phase, neutrophils are the first responders, clearing debris and releasing cytokines to recruit additional immune cells. Macrophages follow, mediating phagocytosis and orchestrating the inflammatory response. Chronic inflammation, driven by sustained M1 macrophage activity, leads to excessive scarring and impaired matrix organization. In contrast, M2 macrophage polarization promotes tissue repair. During later phases, adaptive immune cells such as CD4+ and CD8+ T cells, B cells, and dendritic cells accumulate at the healing site and coordinate with macrophages to modulate inflammation and facilitate tissue remodeling. This coordinated immune regulation is critical for optimal tendon-to-bone integration.
2.3.1 Neutrophils and macrophages initiate inflammation
Following rotator cuff injury and repair, neutrophils are among the first immune cells to infiltrate the tendon-to-bone interface, rapidly clearing debris and releasing cytokines that recruit additional immune cells (32). Macrophages follow, performing phagocytosis, secreting growth factors, and orchestrating the early inflammatory response (33). Both tendon-resident and circulating macrophages are involved, with chemokine receptor CCR2 playing a critical role in their recruitment and function (34). These early innate immune responses are essential for debris clearance and the initiation of tissue repair, but their magnitude and duration must be tightly regulated (35).
2.3.2 Chronic inflammation impairs interface regeneration
While acute inflammation is necessary for healing, persistent or dysregulated inflammation impairs tendon-to-bone interface regeneration (36, 37). Prolonged macrophage activation and sustained pro-inflammatory signaling can lead to excessive scar formation, extracellular matrix disorganization, and biomechanical weakness at the repair site (38). The balance between pro-inflammatory (M1) and anti-inflammatory (M2) macrophage phenotypes is particularly important; a shift toward M2 polarization is associated with improved healing outcomes, while persistent M1 activity is linked to fibrosis and poor integration (39). Biologic adjuvants and stem cell-derived exosomes have been shown to promote M2 polarization and attenuate chronic inflammation, thereby enhancing tendon-to-bone healing (40).
2.3.3 T cells and dendritic cells in adaptive immune modulation
Adaptive immune cells, including T cells and dendritic cells, accumulate at the repair site and in draining lymph nodes during the subacute and remodeling phases (33, 34). CD4+ T cells and dendritic cells coordinate the immune response, influence macrophage polarization, and regulate the transition from inflammation to tissue remodeling (36, 37). B cells and CD8+ T cells also increase over time, suggesting a sustained adaptive response that may impact long-term healing quality. The coordinated actions of these immune cell populations are essential for optimal tendon-to-bone healing, and dysregulation at any stage can compromise repair integrity (40).
3 Mesenchymal stem cells in tendon-to-bone healing
3.1 Sources and properties of MSCs
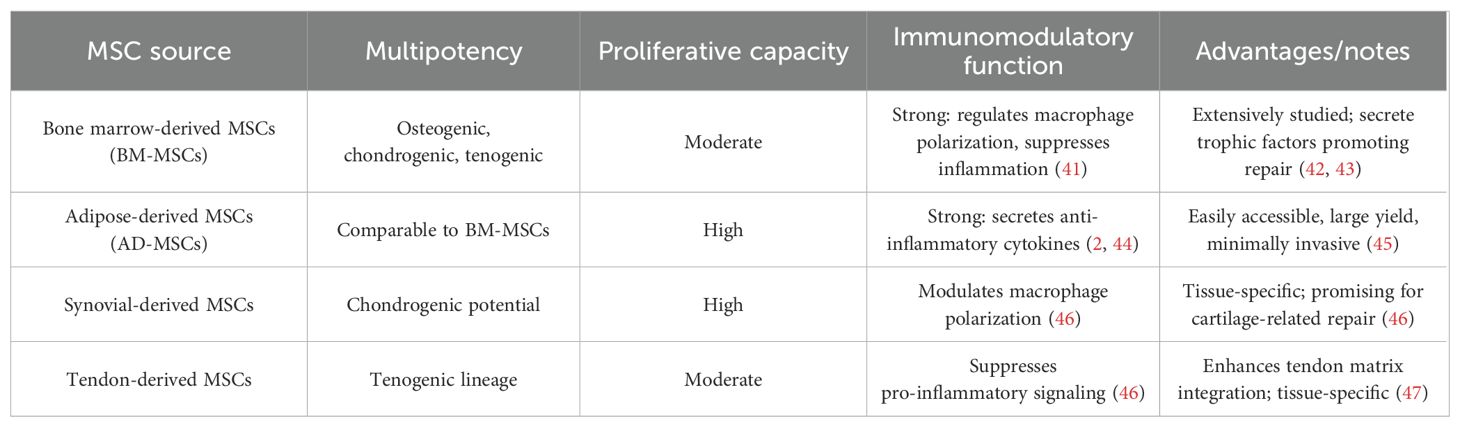
The common types of MSCs, including those derived from bone marrow, adipose tissue, synovium, and tendon, along with key features are summarized in Table 1.
3.1.1 Bone marrow-derived MSCs
BM−MSCs are most used; they show tri−lineage differentiation and trophic, proangiogenic, and immunoregulatory (anti−inflammatory, macrophage−polarizing) effects that support tendon–bone healing (42, 43, 48).
3.1.2 Adipose-derived MSCs
AD−MSCs are high−yield and minimally invasive (43), retain BM−MSC−like multipotency but greater proliferation and anti−inflammatory paracrine activity (2), aiding inflammation resolution, matrix remodeling, and interface regeneration (44).
3.1.3 Synovial and tendon-derived MSCs
Synovial MSCs are highly proliferative/chondrogenic, and tendon−derived MSCs are tenogenic; both modulate macrophage polarization and curb pro−inflammatory signaling, favoring targeted tendon/enthesis repair (46).
3.1.4 Characteristics: multipotency, trophic effects, immune regulation
Across sources, MSCs are multipotent, yielding tendon− and bone−relevant lineages. Through trophic factor/EV secretion they recruit cells, promote angiogenesis and ECM remodeling, and via immunomodulation (↓ pro−inflammatory, ↑ anti−inflammatory, immune−phenotype control) they establish a regenerative milieu that improves tendon–bone healing (44).
MSCs show source−dependent phenotypes and functions that shape regeneration. AD−MSCs are strongly immunomodulatory and tolerate inflammatory niches (49–51), whereas BM-MSCs have superior osteogenic capacity, supporting bone and enthesis repair (52, 53). Synovial MSCs are chondrogenic, while tendon−derived MSCs are tenogenic, aligning with cartilage and tendon repair, respectively (54–57). These traits likely drive outcome differences in preclinical models and support source–tissue–context matching.
3.2 MSC mechanisms of action
Mesenchymal stem cells (MSCs) have emerged as a promising therapeutic modality for enhancing tendon-to-bone healing, a process that remains clinically challenging due to the complex structure and limited regenerative capacity of the enthesis. The mechanisms by which MSCs facilitate this repair are multifactorial and involve both direct and indirect actions (44, 47, 48, 58).
3.2.1 Differentiation into tenocytes, chondrocytes, osteoblasts
MSCs possess multilineage differentiation potential, enabling them to give rise to tenocytes, chondrocytes, and osteoblasts under appropriate microenvironmental cues (45, 58). This plasticity is critical for reconstructing the transitional zones of the tendon-bone interface, which require the regeneration of fibrocartilaginous and osseous tissues (59). In vivo and in vitro studies have demonstrated that MSCs can integrate into injured sites and differentiate along these lineages, contributing to the restoration of native tissue architecture and biomechanical properties (59, 60).
3.2.2 Secretion of bioactive factors: TGF-β, VEGF, IGF-1, IL-6
Beyond differentiation, MSCs exert significant paracrine effects by secreting a repertoire of bioactive molecules, including transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and interleukin-6 (IL-6) (58). These factors modulate the local microenvironment by promoting cell proliferation, suppressing inflammation, and enhancing extracellular matrix (ECM) synthesis. The MSC secretome, including exosomes, has been shown to influence tenocyte migration, angiogenesis, and matrix remodeling, further supporting tissue repair (8, 61).
3.2.3 Promotion of angiogenesis and matrix remodeling
MSCs facilitate neovascularization at the repair site, primarily through the secretion of angiogenic factors such as VEGF. Enhanced vascularity is essential for nutrient delivery and waste removal, supporting the metabolic demands of regenerating tissues (46, 62). Additionally, MSCs promote ECM remodeling by upregulating the synthesis of type I collagen and other matrix proteins, and by modulating the balance between matrix metalloproteinases and their inhibitors, thereby improving the quality and organization of the repair tissue (8, 61).
3.2.4 Recruitment and activation of endogenous progenitor cells
MSCs can recruit and activate endogenous progenitor cells through the release of chemotactic and immunomodulatory factors. This recruitment amplifies the regenerative response by increasing the pool of reparative cells at the injury site (46, 58). Furthermore, MSCs modulate the inflammatory milieu, creating a pro-regenerative environment that supports the survival and function of both transplanted and resident progenitor cells (59, 61).
Collectively, these mechanisms underscore the therapeutic potential of MSCs in tendon-to-bone healing. However, challenges remain regarding the optimization of cell source, delivery methods, and standardization of protocols to maximize clinical efficacy (47, 60, 63).
3.3 Preclinical and clinical evidence
3.3.1 Overview of in vivo studies using MSCs in rotator cuff models
Recent preclinical studies have demonstrated that MSCs and their derivatives can significantly enhance tendon-to-bone healing in rotator cuff injury models. In rat and rabbit models, local delivery of MSCs—whether as cell suspensions, exosomes, or cell sheets—has been shown to improve fibrocartilage formation, collagen maturation, and biomechanical strength at the enthesis (64, 65). For example, exosomes derived from kartogenin-preconditioned MSCs or loaded with kartogenin have been shown to promote chondrogenesis, collagen organization, and superior biomechanical properties compared to controls (66). Menstrual blood-derived MSCs encapsulated in platelet-rich gel facilitated new bone and fibrocartilage formation, with improved mechanical properties in a rabbit chronic tear model (65). Similarly, umbilical cord MSC-derived extracellular vesicles and cryopreserved adipose-derived stem cell sheets have demonstrated efficacy in large animal and rabbit models, respectively, supporting the translational potential of off-the-shelf or cell-free approaches (7, 67).
Genetic modification of MSCs, such as Msx1 overexpression, has also been explored to enhance proliferation and migration, resulting in improved tendon-bone integration and mechanical strength in rat models (11). The use of biomaterial scaffolds, such as 3D-printed polycaprolactone loaded with basic fibroblast growth factor and MSCs, further augments osteogenesis and immunomodulation, leading to improved enthesis healing (68). Conditioned medium from human bone marrow-derived MSCs has been shown to promote tendon-bone healing via immunomodulation, particularly through macrophage polarization (8). Collectively, these studies provide strong mechanistic and functional evidence for the role of MSCs in enhancing tendon-to-bone healing in preclinical models.
3.3.2 Clinical trials and limitations
Clinical translation of MSC-based therapies for rotator cuff repair remains in early stages, with limited but growing evidence from human studies. Early-phase clinical trials and case series suggest that MSC augmentation may reduce retear rates and improve structural outcomes after rotator cuff repair, but results are heterogeneous and often limited by small sample sizes, lack of standardized protocols, and short-term follow-up (2). Safety profiles are generally favorable, with no major adverse events reported in the available literature (2).
Key limitations include variability in MSC source (bone marrow, adipose, umbilical cord, menstrual blood), cell processing methods, delivery vehicles, and dosing regimens. Clinical heterogeneity in MSC-based therapies arises from several factors beyond cell source and delivery. Variations in surgical technique—such as single- vs. double-row repair and open vs. arthroscopic approaches—can affect MSC retention and local biomechanics, influencing treatment outcomes (69, 70). Patient-related variables including age, diabetes, smoking, and baseline inflammation also impact MSC responsiveness, with evidence that aging and metabolic disorders reduce MSC efficacy (71, 72). Differences in processing—such as cryopreservation, passage number, and preconditioning—as well as culture conditions contribute to product variability and functional inconsistency (72–74). These factors are rarely standardized across studies, complicating data comparison and contributing to inconsistent results. Harmonizing protocols and improving reporting practices are essential for enhancing reproducibility in MSC research.
Furthermore, the optimal timing, route of administration, and patient selection criteria remain to be defined. The lack of large, multicenter randomized controlled trials with standardized outcome measures and long-term follow-up precludes definitive conclusions regarding efficacy (64). Regulatory, ethical, and logistical challenges also hinder widespread clinical adoption. Ongoing research is focused on optimizing cell-free approaches (e.g., exosomes, conditioned medium), biomaterial scaffolds, and strategies to recruit endogenous MSCs, which may address some of these barriers and facilitate broader clinical translation (7, 8).
In summary, robust preclinical evidence supports the potential of MSC-based therapies to enhance tendon-to-bone healing in rotator cuff repair, but clinical evidence remains preliminary. Further high-quality, standardized clinical trials are needed to establish efficacy, safety, and best practices for MSC application in this context.
4 The immune microenvironment and its impact on MSC function
The immune microenvironment at the tendon-to-bone interface following rotator cuff injury is a dynamic and complex milieu that critically influences the reparative capacity of mesenchymal stem cells (Figure 4). The interplay between immune cells, cytokines, and MSCs determines the balance between regeneration and fibrosis, ultimately impacting clinical outcomes after rotator cuff repair (2, 8, 38, 39).
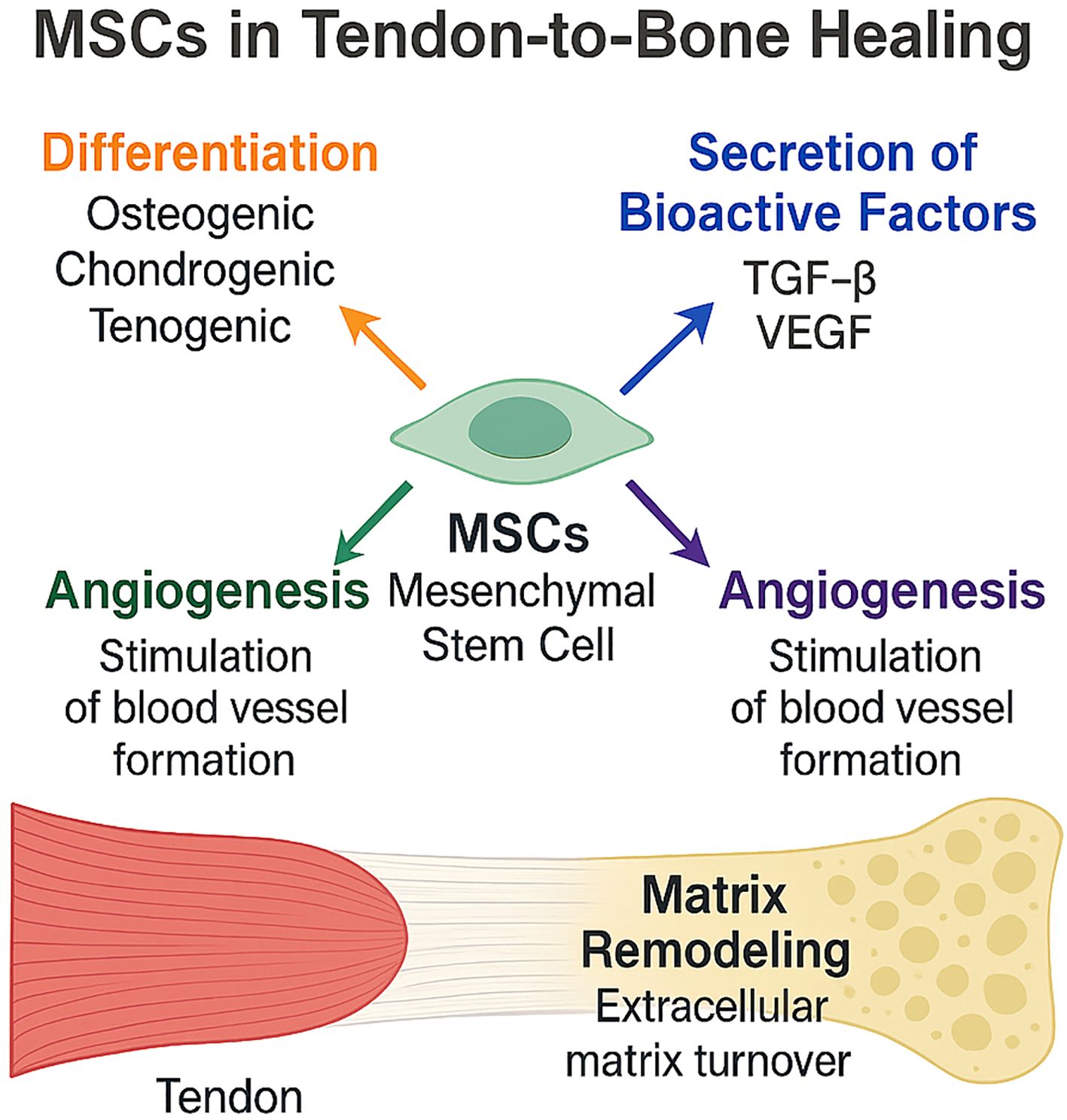
Figure 4. Mechanisms of Mesenchymal Stem Cell (MSC)-Mediated Tendon-to-Bone Healing. This schematic illustrates the multifactorial contributions of MSCs to rotator-cuff tendon-to-bone repair. MSCs differentiate toward osteogenic, chondrogenic, and tenogenic lineages; secrete bioactive factors such as TGF-β and VEGF; stimulate angiogenesis; and drive extracellular-matrix remodeling at the enthesis, collectively promoting robust tendon-bone integration.
4.1 The local immune landscape after rotator cuff injury
4.1.1 Acute vs. chronic inflammation
Acute inflammation is characterized by a rapid influx of neutrophils and pro-inflammatory macrophages, which are essential for debris clearance and the initiation of tissue repair. However, a timely resolution of this response is necessary to prevent chronic inflammation, which is associated with persistent infiltration of inflammatory cells, elevated levels of pro-inflammatory cytokines, and impaired tendon-to-bone healing (37, 39). Chronic inflammation, often observed in aged or degenerative rotator cuff tears, leads to a fibrotic microenvironment that impairs MSC function and promotes scar tissue formation rather than regeneration (1, 38, 39).
4.1.2 M1 vs. M2 macrophage polarization
Macrophages are central regulators of the immune microenvironment, exhibiting plasticity between the pro-inflammatory M1 phenotype and the anti-inflammatory, pro-regenerative M2 phenotype. M1 macrophages secrete high levels of tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), promoting inflammation and tissue degradation. In contrast, M2 macrophages produce interleukin-10 (IL-10) and transforming growth factor beta (TGF-β), supporting resolution of inflammation, matrix remodeling, and tissue regeneration (8, 38, 42). The polarization state of macrophages at the tendon-bone interface is a key determinant of MSC fate and function. Recent studies demonstrate that MSC-derived secretomes and exosomes can shift macrophage polarization from M1 to M2, thereby creating a more favorable environment for tendon-to-bone healing (38, 39, 75).
4.1.3 Pro-inflammatory (TNF-α, IL-1β) vs. anti-inflammatory (IL-10, TGF-β) cytokine profiles
The cytokine milieu at the injury site is a major modulator of both immune cell and MSC behavior. Elevated levels of TNF-α and IL-1β are hallmarks of the pro-inflammatory phase and are associated with impaired healing and increased fibrosis (38, 75). Conversely, anti-inflammatory cytokines such as IL-10 and TGF-β promote the resolution of inflammation, enhance MSC-mediated tissue repair, and support the transition to a regenerative microenvironment (8, 38, 39). The balance between these cytokine profiles is dynamic and can be therapeutically modulated. For example, administration of MSC-conditioned medium or exosomes has been shown to suppress pro-inflammatory cytokines and upregulate anti-inflammatory mediators, thereby improving tendon-to-bone healing in preclinical models (1, 8, 38, 75).
In summary, the immune microenvironment after rotator cuff injury is a critical determinant of MSC function and tendon-to-bone healing. Acute inflammation is necessary for initiating repair, but chronic inflammation impairs regeneration. The polarization of macrophages and the balance of cytokine profiles are central to this process, and therapeutic strategies that modulate these factors—such as MSC-derived secretomes—hold promise for improving clinical outcomes (1, 8, 38).
4.2 Crosstalk between MSCs and immune cells
The crosstalk between mesenchymal stem cells (MSCs) and immune cells is central to tendon-to-bone healing after rotator cuff injury (Figure 5) (8, 76). MSCs exert immunomodulatory effects through both direct cell-cell contact and paracrine signaling, influencing the phenotype and function of innate and adaptive immune cells, while the inflammatory microenvironment reciprocally modulates MSC behavior and differentiation (77, 78).
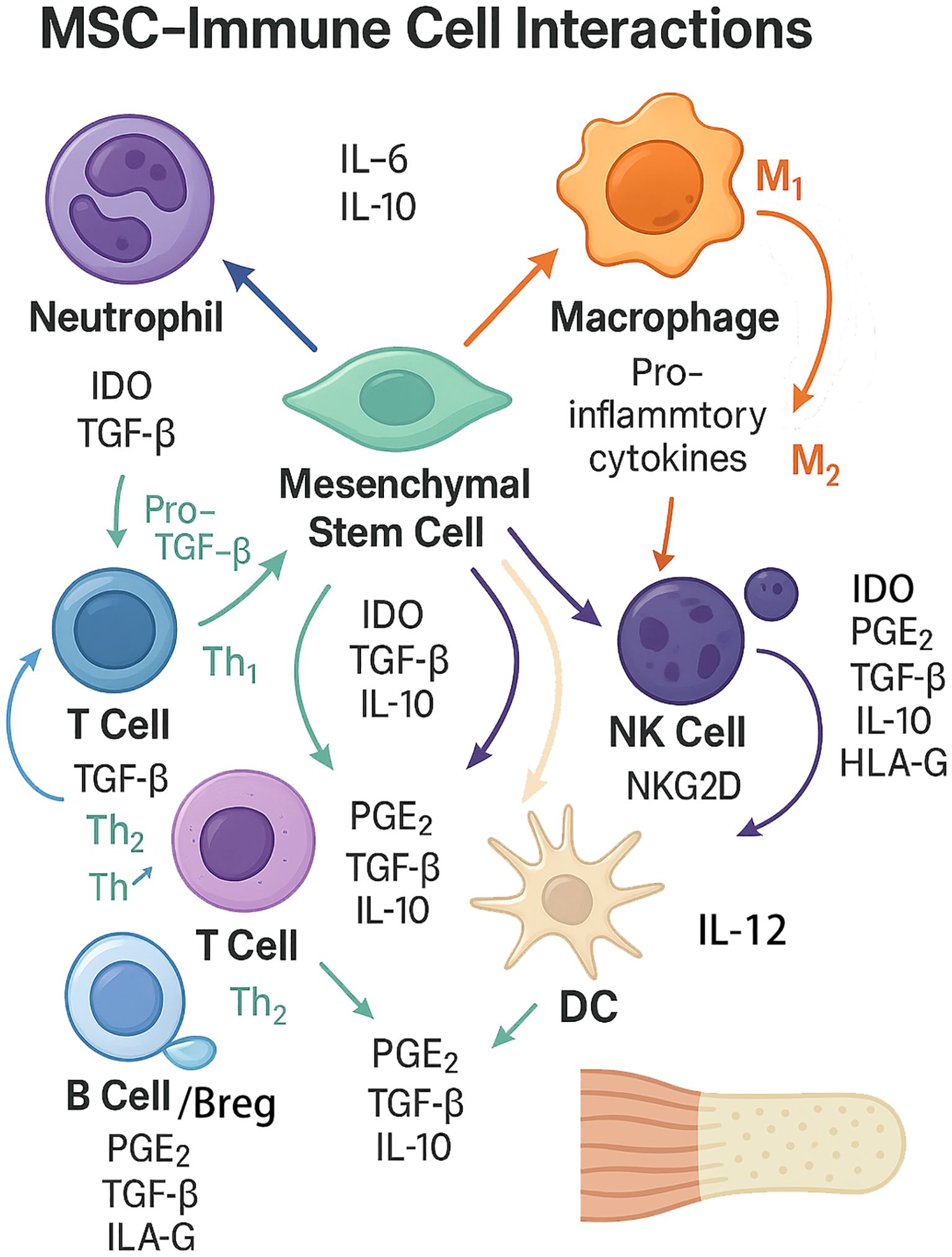
Figure 5. The complex bidirectional interactions between mesenchymal stem cells (MSCs) and key immune cell types involved in tendon-to-bone healing. The diagram summarizes bidirectional interactions between mesenchymal stem cells (MSCs) and innate/adaptive immune cells. MSCs suppress neutrophil−driven inflammation and modulate macrophage polarization toward an M2, pro−repair phenotype through PGE2, TGF−β, IL−10, and IDO, with auxiliary signals HLA−G and TSG−6; MSC extracellular vesicles (EVs) further reinforce anti−inflammatory signaling. MSCs inhibit effector T cells while favoring Treg/Th2 responses (IDO–kynurenine pathway, NO, TGF−β, PGE2, HLA−G, TSG−6), attenuate NK cell activation and cytotoxicity (PGE2, TGF−β, IL−10, IDO, HLA−G; NKG2D pathway), restrain dendritic cell maturation and IL−12/co−stimulation to promote tolerogenic DCs, and limit B−cell proliferation/differentiation while inducing IL−10+ Breg cells. Conversely, inflammatory cues from immune cells—especially TNF−α and IFN−γ from macrophages/T cells—can “license” MSCs or, when excessive, inhibit MSC survival and osteo/tenogenic differentiation. This coordinated immunoregulation mitigates excessive inflammation and supports matrix remodeling and enthesis integration.
4.2.1 MSC-induced M2 macrophage polarization
MSC-induced M2 macrophage polarization is a key mechanism by which MSCs promote tissue repair. MSCs secrete soluble factors such as prostaglandin E2 (PGE2), TGF-β, and IL-10, which drive macrophage polarization toward the anti-inflammatory M2 phenotype (8, 76, 78). M2 macrophages, in turn, secrete cytokines and growth factors that support matrix remodeling and regeneration, while reducing pro-inflammatory mediators. This polarization is further enhanced by MSC-derived extracellular vesicles, which can transfer regulatory microRNAs and proteins to macrophages (39, 78).
4.2.2 MSC-mediated suppression of T cell activation and proliferation
MSC-mediated suppression of T cell activation and proliferation occurs via the release of immunomodulatory molecules including indoleamine 2,3-dioxygenase (IDO), nitric oxide, and TGF-β, as well as through direct cell-cell interactions (76, 77). These mechanisms reduce effector T cell responses and promote the expansion of regulatory T cells, thereby dampening local inflammation and preventing immune-mediated tissue damage (1, 42).
4.2.3 Bidirectional effects: inflammatory milieu can inhibit or enhance MSC differentiation
Bidirectional effects of the inflammatory milieu are evident, as acute inflammation can “license” MSCs, enhancing their immunosuppressive and regenerative functions, while chronic or excessive inflammation impairs MSC viability and differentiation potential (76). Pro-inflammatory cytokines such as TNF-α and IFN-γ can inhibit MSC osteogenic and tenogenic differentiation, whereas anti-inflammatory conditions or M2 macrophage presence promote MSC-mediated tissue integration (1, 39).
4.2.4 Secreted factors: PGE2, IDO, TSG-6, HLA-G
Secreted factors including PGE2, IDO, TSG-6, and HLA-G are central to MSC-immune crosstalk. PGE2 and IDO are critical for macrophage polarization and T cell suppression, while TSG-6 and HLA-G contribute to the anti-inflammatory and immunosuppressive milieu that supports tissue regeneration (8, 76, 77). The coordinated action of these factors underpins the therapeutic potential of MSCs in modulating the immune microenvironment for optimal tendon-to-bone healing.
Brief priming with IFN−γ—alone or combined with TNF−α—elevates IL−10, IDO, PGE2 and CXCR4/CCR2, thereby strengthening T−cell suppression, Treg/M2 skewing and homing to inflamed sites (79, 80). Inflammation−responsive IL−10 transgenes give a similar context−specific anti−inflammatory boost without constitutive expression (80). These immunoengineering tactics overcome immune barriers in rotator−cuff repair while preserving MSC osteo− and tenogenic potential (81).
4.3 Immune-modulatory potentials of MSC-derived extracellular vesicles
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have emerged as critical mediators of immune modulation and tissue regeneration, offering a promising cell-free alternative to traditional MSC-based therapies. Their ability to influence the immune microenvironment is particularly relevant in the context of rotator cuff tendon-to-bone healing, where immune regulation is essential for optimal tissue integration and repair (82, 83).
4.3.1 EVs as cell-free therapeutic tools
MSC-EVs are lipid bilayer-enclosed particles that encapsulate a diverse cargo of proteins, lipids, and nucleic acids, mirroring the therapeutic properties of their parent cells while minimizing risks associated with cell transplantation, such as immune rejection and tumorigenicity (84, 85). Preclinical studies have demonstrated that MSC-EVs can modulate both innate and adaptive immune responses, suppressing pro-inflammatory cytokine production, promoting regulatory T cell expansion, and attenuating macrophage activation (83, 86, 87). These properties are particularly advantageous in musculoskeletal repair, where excessive inflammation impedes tendon-to-bone healing. Additionally, MSC-EVs exhibit favorable pharmacokinetics, low immunogenicity, and the capacity to cross biological barriers, further supporting their translational potential (85, 86, 88). Despite these advantages, challenges remain regarding large-scale production, standardization, and precise targeting of EVs in clinical applications.
4.3.2 Immunomodulatory miRNAs and proteins within EVs
The immunomodulatory effects of MSC-EVs are largely attributed to their cargo of microRNAs (miRNAs) and proteins, which orchestrate complex regulatory networks within recipient immune cells (83, 85, 89). Key miRNAs, such as miR-21, miR-146a, and miR-155, have been shown to downregulate inflammatory signaling pathways (e.g., NF-κB, STAT3), inhibit pro-inflammatory cytokine release, and promote the polarization of macrophages toward an anti-inflammatory (M2) phenotype (86, 87). In addition, MSC-EVs are enriched in immunoregulatory proteins, including TGF-β, HGF, and galectins, which further suppress immune activation and foster a regenerative microenvironment (85, 86). These molecular mediators collectively contribute to the attenuation of local inflammation, enhancement of tissue repair, and prevention of fibrosis in tendon-to-bone healing models (83, 89).
Precisely engineered MSC−derived extracellular vesicles (MSC−EVs) are emerging acellular immunomodulators. Hypoxia, IFN−γ, TNF−α or kartogenin preconditioning enrich cargo (miR−21/146a/155, TGF−β, HGF, galectins), amplifying M2 macrophage polarization, T−reg expansion and NF−κB/STAT3 suppression (90–92). Surface RGD/mannose ligands or SPIO labelling further improve homing to the tendon–bone interface (82, 93). These modifications boost efficacy, cut batch variability and favor scalable “off−the−shelf” translation for rotator−cuff repair (93).
In summary, MSC-EVs represent a versatile and potent cell-free therapeutic platform with robust immunomodulatory capabilities, mediated by their unique repertoire of miRNAs and proteins. Ongoing research is focused on optimizing their production, characterization, and delivery to maximize their clinical utility in musculoskeletal and other regenerative applications.
5 Modulation of the immune microenvironment to enhance MSC therapy
Modulation of the immune microenvironment is increasingly recognized as a critical determinant of mesenchymal stem cell (MSC) efficacy in rotator cuff tendon-to-bone healing (Figure 6). The interplay between MSCs and immune cells, particularly macrophages, orchestrates the inflammatory milieu and subsequent tissue regeneration. Recent studies highlight that targeted immunoengineering strategies can enhance MSC-mediated repair by promoting a pro-regenerative environment and mitigating deleterious inflammation (1, 8, 94).

Figure 6. Schematic Overview of Immunoengineering Approaches to Optimize MSC Therapy in Tendon-to-Bone Healing. This diagram illustrates strategies to enhance mesenchymal stem cell (MSC) efficacy by modulating immune interactions, including cytokine co-delivery (e.g., IL-4 to shift macrophages from pro-inflammatory M1 to repair-promoting M2 phenotypes), pharmacological suppression of inflammation via immune modulators (e.g., corticosteroids, statins), and genetic engineering of MSCs to boost anti-inflammatory factor expression. These approaches synergistically improve the immune microenvironment, fostering bidirectional interactions between MSCs and immune cells to accelerate tissue repair and optimize therapeutic outcomes in tendon-to-bone healing.
5.1 Immunoengineering strategies
Immunoengineering approaches aim to optimize the local immune context to support MSC survival, engraftment, and paracrine activity. These strategies include the co-delivery of immunomodulatory cytokines, use of pharmacologic agents, and genetic modification of MSCs to augment their anti-inflammatory properties (47, 78, 94, 95).
5.1.1 Co-delivery of cytokines (e.g., IL-4 to promote M2 macrophages)
The co-delivery of cytokines, particularly interleukin-4 (IL-4), has been shown to shift macrophage polarization toward the M2 phenotype, which is associated with anti-inflammatory and tissue-reparative functions. In preclinical models, MSC-derived secretomes and conditioned media have demonstrated the capacity to inhibit pro-inflammatory M1 macrophages and promote M2 polarization, thereby enhancing tendon-bone healing and reducing fibrotic scar formation. The beneficial effects of MSCs in this context are mediated, at least in part, by the regulation of macrophage phenotype via paracrine signaling pathways, including Smad2/3 activation (8, 39, 78).
5.1.2 Use of immune modulators (e.g., corticosteroids, statins)
Pharmacologic agents such as corticosteroids and statins have been investigated for their ability to modulate the immune microenvironment and synergize with MSC therapy (1). These agents can suppress excessive inflammation and promote a milieu conducive to MSC-mediated repair (39). While corticosteroids are well-established anti-inflammatory agents, their use must be balanced against potential inhibitory effects on tissue regeneration (95). Statins, in addition to their lipid-lowering properties, exhibit pleiotropic immunomodulatory effects that may enhance MSC survival and function in the context of tendon healing (47). However, the optimal dosing and timing of these agents in combination with MSCs require further investigation in translational models (96).
5.1.3 Genetic modification of MSCs to enhance anti-inflammatory potential
Genetic engineering of MSCs represents a promising strategy to augment their immunomodulatory and regenerative properties. Approaches include overexpression of anti-inflammatory cytokines, enhancement of paracrine factor secretion, and modification of surface molecules to improve immune evasion and homing (94). Preclinical studies have demonstrated that genetically modified MSCs can more effectively suppress local inflammation, promote M2 macrophage polarization, and facilitate tendon-bone integration (47, 96). The use of exosome-based delivery systems derived from engineered MSCs further amplifies these effects by providing a cell-free platform for targeted immune modulation (96).
Engineering MSCs to express IL−10, TSG−6, HLA−G, HGF or to up−regulate CXCR4/CCR2 augments M2 skewing, T−cell inhibition, angiogenesis and targeted enthesis homing (97, 98)[2−3]. Short IFN−γ priming further elevates IDO activity without safety loss (99, 100)[5−6]. These approaches consistently depress TNF−α/IL−1β, boost IL−10/TGF−β and enhance fibrocartilage strength and load−to−failure outcomes (97, 98)[2−3].
Collectively, these immunoengineering strategies highlight the critical role of modulating the immune microenvironment to fully harness the therapeutic potential of MSCs in rotator cuff tendon-to-bone healing. Continued research is essential to optimize these approaches and facilitate their clinical translation.
5.2 Biomaterial-assisted delivery systems
Biomaterial-assisted delivery systems have emerged as a cornerstone in enhancing the therapeutic efficacy of mesenchymal stem cells (MSCs) and immunomodulatory agents for rotator cuff tendon-to-bone healing (101). These platforms address key challenges such as poor cell retention, limited engraftment, and suboptimal control of the local immune microenvironment, which are critical determinants of successful tissue regeneration (102–104).
5.2.1 Hydrogels, scaffolds, microspheres for controlled MSC and cytokine release
Hydrogels, particularly those based on natural or synthetic macromolecules, provide a three-dimensional, extracellular matrix-mimetic environment that supports MSC viability, proliferation, and differentiation while enabling localized, sustained release of bioactive factors (102, 105, 106). Decellularized extracellular matrix (dECM) hydrogels further enhance MSC retention and integration by closely recapitulating native tissue architecture and minimizing immunogenicity (106). Hybrid hydrogels combining dECM with proteins or polysaccharides can further optimize mechanical and biological properties for tendon-to-bone interface repair (106).
Microsphere-containing hydrogels and composite scaffolds allow for precise spatial and temporal control of MSC and cytokine delivery. For example, polylactic-glycolic acid (PLGA) microspheres embedded in hydrogels can sequentially release chemotactic and immunomodulatory peptides, first recruiting endogenous MSCs and then modulating macrophage polarization to favor tissue regeneration (107, 108). Such systems have demonstrated enhanced osteogenesis and improved immune response regulation in preclinical models (107, 108). Electrospun scaffolds, especially when integrated with biological polymers, further improve MSC adhesion, paracrine signaling, and engraftment, supporting robust tissue remodeling (109).
Injectable micro-fragmented nanofiber-hydrogel composites (mfNHCs) have shown promise as minimally invasive carriers for MSC delivery, promoting host macrophage infiltration, pro-regenerative polarization, and angiogenesis in both small and large animal models (110). These advances collectively underscore the importance of biomaterial design in controlling the local microenvironment and optimizing MSC-based therapies for tendon-to-bone healing (101, 102, 108, 109).
5.2.2 Immune-responsive biomaterials to regulate local inflammation
The immune response to implanted biomaterials is a critical determinant of healing outcomes. Recent strategies focus on engineering immune-responsive biomaterials that actively modulate the local inflammatory milieu to promote constructive tissue remodeling (103–105). Hydrogels and scaffolds can be functionalized with immunomodulatory agents or designed to present specific physical and chemical cues that direct macrophage polarization toward a pro-regenerative (M2) phenotype, thereby reducing chronic inflammation and fibrosis (107, 108, 111).
Sequential release systems, such as those delivering LL37 and W9 peptides, exemplify how biomaterials can orchestrate the transition from an initial pro-inflammatory response (necessary for debris clearance and cell recruitment) to a subsequent anti-inflammatory, regenerative phase (107). The physicochemical properties of hydrogels—including crosslinking density, degradation rate, and surface chemistry—can be tuned to modulate immune cell infiltration and phenotype (101, 103, 105, 111). Standardized in vitro pathways for evaluating immune responses to biomaterials are being developed to ensure safety and reproducibility in regenerative applications (103, 104).
Engineered MSCs and cargo−enriched EVs—achieved through IL−10/TSG−6/HLA−G or CXCR4/CCR2 overexpression, brief IFN−γ priming, and miRNA−boosting preconditioning—further reinforce M2/T−reg immunity and fibrocartilage formation, but evidence is still largely preclinical and awaits protocol standardization (83, 93).
Overall, incorporating immunomodulatory design principles into biomaterial platforms is crucial for optimizing MSC–immune interactions and improving tendon-to-bone healing outcomes.
5.3 Combination therapies
Emerging evidence indicates that combination therapies leveraging mesenchymal stem cells (MSCs) with adjunctive modalities can potentiate tendon-to-bone healing by modulating the immune microenvironment and enhancing tissue regeneration (97, 112, 113). These strategies address the limitations of MSC monotherapy, such as poor engraftment, low survival, and variable efficacy.
5.3.1 MSCs with growth factors or gene therapy
The co-administration of MSCs with growth factors, or genetic modification of MSCs to overexpress trophic factors (e.g., hepatocyte growth factor [HGF]), has been shown to improve cell survival, paracrine signaling, and regenerative capacity in preclinical models (112, 114, 115). HGF gene-modified MSCs, for example, demonstrate enhanced anti-inflammatory and pro-angiogenic effects, which are critical for tendon-bone interface healing (114). Preconditioning MSCs with bioactive substances or optimizing culture conditions further augments their therapeutic potential (97, 115). These approaches are under active investigation for musculoskeletal repair, including tendon injuries (42, 112).
5.3.2 MSCs with regulatory T cells for immune tolerance induction
Combining MSCs with regulatory T cells (Tregs) has shown synergistic immunomodulatory effects, leading to superior attenuation of inflammation compared to either cell type alone (116, 117). In models of traumatic injury, MSC+Treg therapy more effectively suppresses neuroinflammation and modulates systemic immune responses, suggesting potential for improved tendon-to-bone healing by promoting immune tolerance and reducing chronic inflammation (116, 118). This strategy is particularly relevant in settings where immune-mediated tissue damage impedes regeneration.
5.3.3 Synergy with physical rehabilitation protocols
Integrating MSC therapy with structured physical rehabilitation protocols—termed “regenerative rehabilitation”—can enhance functional recovery by promoting graft integration, neural plasticity, and tissue remodeling (112, 119). Rehabilitation modalities may optimize the local microenvironment, facilitating MSC engraftment and paracrine activity (112). Preclinical studies in musculoskeletal and spinal cord injury models support the use of combinatorial regimens to maximize anatomical and functional outcomes (120, 121).
Engineered MSCs combined with regulatory T cells enhance immune tolerance and curb chronic inflammation, but evidence remains limited to animal and early−phase studies (122). Designer EVs enriched with IL−10 or miR−146a suppress inflammation, drive M2/Treg responses, and, when paired with rehabilitation or bFGF−loaded scaffolds, boost angiogenesis and graded mineralization (87, 123). Translation is hampered by dose, delivery, standardization, and safety challenges (93). Advancing MSC-based therapies for rotator cuff repair increasingly relies on integrated strategies, which hold significant promise but require further investigation to establish effective clinical protocols and pathways for translation.
6 Current challenges and future directions
6.1 Immune and cellular heterogeneity
The principal biological bottleneck is the two−way variability between therapeutic MSCs and the patient’s immune landscape. Source− and donor−dependent heterogeneity (age, sex, metabolic status) produces wide ranges in transcriptomic profile, trophic factor output and immunoregulatory strength (124, 125). Single−cell RNA−seq and proteomics expose discrete MSC sub−clusters with divergent proliferative and anti−inflammatory capacities, explaining batch−to−batch inconsistency (126, 127). Donor−to−donor variation persists even after pooling or pre−treatment strategies (128). On the host side, ageing, diabetes and chronic low−grade inflammation tilt macrophages toward an M1 bias, deplete regulatory T cells and impair enthesis remodelling (129–132). Senescent tendon−stem cells in elderly rotator−cuff tears create a positive pro−inflammatory feedback loop with macrophages that can be broken only by exogenous, rejuvenated EVs38. These intertwined variabilities demand precise MSC characterization, selection of functional sub−populations and immune−responsive licensing—approaches highlighted in Section5 (e.g., IFN−γ priming; IL−10/TSG−6/HLA−G over−expression; cargo−enriched EVs) (79–81, 97–100).
6.2 Technical and translational barriers
Regulation & manufacturing. In the UnitedStates, only minimally manipulated same−day autologous MSCs are allowed outside IND trials; expanded products must meet stringent GMP criteria, yet lack universal potency assays (133–135). Variability in cell yield, viability and purity across tissue sources, donors and processing sites hampers multi−center trials and large−scale deployment (1, 134).
Dose, delivery & long−term safety. Optimal cell/EV dose, timing and route remain undefined. Pulmonary first−pass trapping and rapid systemic clearance reduce bioavailability, whereas ectopic bone or fibrotic nodules—though uncommon—raise safety concerns (73, 125, 129). Smart hydrogels with ROS− or MMP−cleavable linkers (Section5.2) prolong local retention, while CXCR4/CCR2 up−regulation enhances homing to inflamed enthesis (101, 107). Long−term monitoring is still needed to rule out tumourigenicity or aberrant differentiation (125, 136).
Protocol & endpoint standardization. Heterogeneous isolation, expansion and storage methods, as well as disparate imaging (MRI vs ultrasound) and biomechanical read−outs, impede meta−analysis (95, 134, 137). Quantitative MRI−T2* and circulating EV signatures are being explored as early, reproducible surrogate markers of tendon−to−bone healing (138–140).
6.3 Strategic innovations
6.3.1 Personalized immunomodulatory MSC therapy
Advances in single−cell transcriptomics and multiplex cytokine profiling enable patient immune stratification and MSC sub−typing (74, 141). Matching low−inflammatory MSC subsets to hyper−inflammatory patients—or licensing cells exvivo with IFN−γ to normalize IL−10/IDO output—could minimize heterogeneity and maximize benefit (79–81).
6.3.2 Organoid & 3D−printed enthesis models
Humanized organoids and bioprinted constructs now recreate tendon–bone zonal ECM, permitting high−throughput screening of MSC–immune–biomaterial interactions (142–144). However, current 3D−bioprinted enthesis models still fail to reproduce gradient mineralization, zonal load transfer and anisotropic tendon−bone integration (145–147). Fabricating multiphasic scaffolds with precise spatial control over ECM, stiffness and mineral composition remains technically difficult, impeding functional replication of native enthesis and slowing clinical translation (142, 148).
Consequently, these unresolved biomechanical gaps underscore that present 3D-bioprinted enthesis constructs continue to face substantial mechanical constraints. These include inadequate reproduction of gradient mineralization, impaired zonal load transfer, and insufficient anisotropic integration between tendon and bone tissues (145–147). Moreover, fabricating multiphasic scaffolds with precise spatial organization of ECM, stiffness, and mineral content remains technically difficult (142, 148). These challenges continue to hinder the functional replication of native enthesis and represent major barriers to the clinical translation of 3Dbioprinting in tendon−to−bone repair.
6.3.3 Systems biology & AI−guided prediction tools
Integrating single−cell transcriptomes, cytokine arrays and proteomics with machine−learning enables prediction of MSC potency, identification of immune subtypes linked to poor healing and optimization of donor selection and licensing parameters (149, 150). These AI−derived signatures promise to reduce therapeutic variability and guide personalized immuno−engineering strategies, advancing data −driven precision therapy (151, 152).
Artificial−intelligence tools offer promising means to integrate high−dimensional immune data—such as single−cell transcriptomics, cytokine profiles, and proteomics—for predictive modeling and personalized MSC therapy optimization (152, 153). By uncovering complex patterns across omics datasets, AI algorithms can stratify patients, predict MSC potency, and identify immune subtypes linked to poor healing (154, 155). These approaches also guide donor selection, cell processing, and immunomodulatory strategies, helping to reduce therapeutic variability and enhance clinical outcomes in tendon−to−bone repair.
Together, these emerging strategies tackle cellular−immune heterogeneity and translational barriers, complementing the immuno−engineering and biomaterial approaches detailed earlier. Their convergence is expected to deliver reproducible, patient−tailored MSC/EV therapies for rotator−cuff tendon−to−bone repair.
7 Conclusion
Tendon-to-bone healing after rotator cuff repair remains a major clinical challenge due to the limited regenerative capacity of the enthesis and a complex immune microenvironment. MSCs offer promising therapeutic potential through their regenerative and immunomodulatory functions. However, their efficacy is highly dependent on immune interactions at the repair site. Advancements in biomaterial delivery, immunoengineering, and cell-free approaches have improved outcomes in preclinical models. Future strategies that incorporate personalized immune profiling, humanized testing platforms, and AI-driven optimization are expected to further enhance the clinical translation and effectiveness of MSC-based therapies.
Author contributions
DL: Writing – original draft, Conceptualization, Data curation, Writing – review & editing, Formal analysis. YZo: Investigation, Writing – review & editing, Visualization. YZh: Project administration, Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used exclusively for language polishing and grammatical refinement, with no alterations to scientific content, data interpretation, or conceptual frameworks. All core ideas, analyses, and conclusions remain solely the work of the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Durtschi MS, Kim S, Li J, Kim C, Chu C, Cheung E, et al. Optimizing tissue engineering for clinical relevance in rotator cuff repair. Tissue Eng Part B Rev. (2024) 30:559–69. doi: 10.1089/ten.teb.2023.0320
2. Hooper N, Marathe A, Jain NB, and Jayaram P. Cell-based therapies for rotator cuff injuries: an updated review of the literature. Int J Mol Sci. (2024) 25:3139. doi: 10.3390/ijms25063139
3. Kim YS, Sung CH, Chung SH, Kwak SJ, and Koh YG. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. (2017) 45:2010–8. doi: 10.1177/0363546517702863
4. Thangarajah T, Sanghani-Kerai A, Henshaw F, Lambert SM, Pendegrass CJ, and Blunn GW. Application of a demineralized cortical bone matrix and bone marrow-derived mesenchymal stem cells in a model of chronic rotator cuff degeneration. Am J Sports Med. (2018) 46:98–108. doi: 10.1177/0363546517727512
5. Liu Q, Yu Y, Reisdorf RL, Qi J, Lu C-K, Berglund LJ, et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. (2019) 192:189–98. doi: 10.1016/j.biomaterials.2018.10.037
6. Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. (2020) 11:496. doi: 10.1186/s13287-020-02005-x
7. Jenner F, Wagner A, Gerner I, Ludewig E, Trujanovic R, Rohde E, et al. Evaluation of the potential of umbilical cord mesenchymal stromal cell-derived small extracellular vesicles to improve rotator cuff healing: A pilot ovine study. Am J Sports Med. (2023) 51:331–42. doi: 10.1177/03635465221145958
8. Chen W, Sun Y, Gu X, Cai J, Liu X, Zhang X, et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. (2021) 271:120714. doi: 10.1016/j.biomaterials.2021.120714
9. Zhang C, Jin J-L, Zhou C-H, Ruan C-X, Lei P-F, and Cai Y-Z. Magnetic seeding of SPIO-BMSCs into a biphasic scaffold can promote tendon-bone healing after rotator cuff repair. Am J Sports Med. (2024) 52:1707–18. doi: 10.1177/03635465241247288
10. Yea J-H, Park J-K, Kim IJ, Sym G, Bae T-S, and Jo CH. Regeneration of a full-thickness defect of rotator cuff tendon with freshly thawed umbilical cord-derived mesenchymal stem cells in a rat model. Stem Cell Res Ther. (2020) 11:387. doi: 10.1186/s13287-020-01906-1
11. Liu K, Fu X-W, and Wang Z-M. Msx1-modified rat bone marrow mesenchymal stem cell therapy for rotator cuff repair: A comprehensive analysis of tendon-bone healing and cellular mechanisms. J Orthop Res Off Publ Orthop Res Soc. (2025) 43:859–69. doi: 10.1002/jor.26039
12. Baker KC, Fleischer M, Newton MD, Galasso L, Cavinatto L, Weisz KM, et al. Pharmacologic mobilization and chemokine-directed recruitment of mesenchymal stromal cells to the surgically repaired rotator cuff. Am J Sports Med. (2025) 53:3635465251341439. doi: 10.1177/03635465251341439
13. Jensen PT, Lambertsen KL, and Frich LH. Assembly, maturation, and degradation of the supraspinatus enthesis. J shoulder Elb Surg. (2018) 27:739–50. doi: 10.1016/j.jse.2017.10.030
14. Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, and Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res Off Publ Orthop Res Soc. (2007) 25:1621–8. doi: 10.1002/jor.v25:12
15. Kanazawa T, Gotoh M, Ohta K, Honda H, Ohzono H, Shimokobe H, et al. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. Sci Rep. (2016) 6:33800. doi: 10.1038/srep33800
16. Bai L, Han Q, Meng Z, Chen B, Qu X, Xu M, et al. Bioprinted living tissue constructs with layer-specific, growth factor-loaded microspheres for improved enthesis healing of a rotator cuff. Acta Biomater. (2022) 154:275–89. doi: 10.1016/j.actbio.2022.10.058
17. Villarreal-Espinosa JB, Berreta RS, Boden SA, Khan ZA, Carter AJ, Cole BJ, et al. Inlay scaffold augmentation of rotator cuff repairs enhances histologic resemblance to native enthesis in animal studies: A systematic review. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc North Am Int Arthrosc Assoc. (2025) 41:2048–60. doi: 10.1016/j.arthro.2024.06.048
18. Campbell TM, Gao L, Laneuville O, Louati H, Uhthoff HK, and Trudel G. Rotator cuff anchor repair: Histological changes associated with the recovering mechanical properties in a rabbit model. J Tissue Eng Regen Med. (2021) 15:567–76. doi: 10.1002/term.v15.6
19. Liu X, Hao M, Chen Z, Zhang T, Huang J, Dai J, et al. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials. (2021) 272:120771. doi: 10.1016/j.biomaterials.2021.120771
20. Luzzi AJ, Ferrer X, Fang F, Golman M, Song L, Marshall BP, et al. Hedgehog activation for enhanced rotator cuff tendon-to-bone healing. Am J Sports Med. (2023) 51:3825–34. doi: 10.1177/03635465231203210
21. Patel S, Gualtieri AP, Lu HH, and Levine WN. Advances in biologic augmentation for rotator cuff repair. Ann N Y Acad Sci. (2016) 1383:97–114. doi: 10.1111/nyas.2016.1383.issue-1
22. Du C, Chen W, Fang J, Zhang Y, Yan W, Dai W, et al. Comparison of 3 different surgical techniques for rotator cuff repair in a rabbit model: direct suture, inlay suture, and polyether ether ketone (PEEK) suture anchor. Am J Sports Med. (2024) 52:1428–38. doi: 10.1177/03635465241240140
23. Kennedy CS, Núñez CNV, Poli A, Vega-Soto E, Arruda EM, and Larkin LM. Engineered tissue graft for repair of injured infraspinatus rotator cuff tendon. Tissue Eng Part A. (2023) 29:471–80. doi: 10.1089/ten.tea.2022.0196
24. Kovacevic D, Suriani RJJ, Levine WN, and Thomopoulos S. Augmentation of rotator cuff healing with orthobiologics. J Am Acad Orthop Surg. (2022) 30:e508–16. doi: 10.5435/JAAOS-D-20-01011
25. Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res Off Publ Orthop Res Soc. (2006) 24:541–50. doi: 10.1002/jor.20067
26. Thankam FG, Roesch ZK, Dilisio MF, Radwan MM, Kovilam A, Gross RM, et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci Rep. (2018) 8:1–14. doi: 10.1038/s41598-018-27250-2
27. Wang H, Luo C, Xu H, Guo Y, Chen Q, Gong Y, et al. Anatomical and interpositional bursa preservation showed similar improved tendon to bone healing compared with the bursa removal in a rat rotator cuff tear model. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc North Am Int Arthrosc Assoc. (2023) 39:1141–9. doi: 10.1016/j.arthro.2022.11.022
28. Lorbach O, Baums MH, Kostuj T, Pauly S, Scheibel M, Carr A, et al. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. (2015) 23:530–41. doi: 10.1007/s00167-014-3487-2
29. You X, Shen Y, Yu W, and He Y. Enhancement of tendon−bone healing following rotator cuff repair using hydroxyapatite with TGFβ1. Mol Med Rep. (2018) 17:4981–8. doi: 10.3892/mmr.2018.8499
30. Bertrand J, Kräft T, Gronau T, Sherwood J, Rutsch F, Lioté F, et al. BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a. Ann Rheumatol Dis. (2020) 79:975–84. doi: 10.1136/annrheumdis-2019-216648
31. Jabara J, Kiani S, Youn A, and Feeley B. Orthobiologic augmentation to improve rotator cuff repair outcomes: current and future strategies. J Am Acad Orthop Surg. (2025) 33:e731–6. doi: 10.5435/JAAOS-D-25-00069
32. Muscat S, Nichols AEC, Gira E, and Loiselle AE. CCR2 is expressed by tendon resident macrophage and T cells, while CCR2 deficiency impairs tendon healing via blunted involvement of tendon-resident and circulating monocytes/macrophages. FASEB J Off Publ Fed Am Soc Exp Biol. (2022) 36:e22607. doi: 10.1096/fj.202201162R
33. Noah AC, Li TM, Martinez LM, Wada S, Swanson JB, Disser NP, et al. Adaptive and innate immune cell responses in tendons and lymph nodes after tendon injury and repair. J Appl Physiol. (2020) 128:473–82. doi: 10.1152/japplphysiol.00682.2019
34. Crosio G and Huang AH. Innate and adaptive immune system cells implicated in tendon healing and disease. Eur Cell Mater. (2022) 43:39–52. doi: 10.22203/eCM.v043a05
35. Eliasberg CD, Carballo CB, Piacentini A, Caughey S, Havasy J, Khan M, et al. Effect of CCR2 knockout on tendon biomechanical properties in a mouse model of delayed rotator cuff repair. J Bone Joint Surg Am. (2023) 105:779–88. doi: 10.2106/JBJS.22.01160
36. Chisari E, Rehak L, Khan WS, and Maffulli N. The role of the immune system in tendon healing: a systematic review. Br Med Bull. (2020) 133:49–64. doi: 10.1093/bmb/ldz040
37. Jiang F, Zhao H, Zhang P, Bi Y, Zhang H, Sun S, et al. Challenges in tendon-bone healing: emphasizing inflammatory modulation mechanisms and treatment. Front Endocrinol (Lausanne). (2024) 15:1485876. doi: 10.3389/fendo.2024.1485876
38. Zhang X, Song W, Liu Y, Han K, Wu Y, Cho E, et al. Healthy tendon stem cell-derived exosomes promote tendon-to-bone healing of aged chronic rotator cuff tears by breaking the positive-feedback cross-talk between senescent tendon stem cells and macrophages through the modulation of macrophage polarization. Small. (2024) 20:1–19. doi: 10.1002/smll.202311033
39. Russo V, El Khatib M, Prencipe G, Citeroni MR, Faydaver M, Mauro A, et al. Tendon immune regeneration: insights on the synergetic role of stem and immune cells during tendon regeneration. Cells. (2022) 11:434. doi: 10.3390/cells11030434
40. Zhou W, Zhang Y, Dong Y, Zou X, Alhaskawi A, Yi F, et al. Recent progress in macrophage-mediated tendon injury and healing. Histol Histopathol. (2025), 18945. doi: 10.14670/HH-18-945
41. Zou J, Yang W, Cui W, Li C, Ma C, Ji X, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnology. (2023) 21:14. doi: 10.1186/s12951-023-01778-6
42. Jiang L, Lu J, Chen Y, Lyu K, Long L, Wang X, et al. Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review). Int J Mol Med. (2023) 52:70. doi: 10.3892/ijmm.2023.5273
43. Costa-Almeida R, Calejo I, and Gomes ME. Mesenchymal stem cells empowering tendon regenerative therapies. Int J Mol Sci. (2019) 20:3002. doi: 10.3390/ijms20123002
44. Yuan Z, Yu H, Long H, Dai Y, Shi L, Zhao J, et al. Stem cell applications and tenogenic differentiation strategies for tendon repair. Stem Cells Int. (2023) 2023:3656498. doi: 10.1155/2023/3656498
45. Ahmad Z, MRCS MBBS, Wardale J, Brooks R, Henson F, Noorani A, et al. Exploring the application of stem cells in tendon repair and regeneration. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc North Am Int Arthrosc Assoc. (2012) 28:1018–29. doi: 10.1016/j.arthro.2011.12.009
46. Wu Y, Wang X, Zhao D, Lin R, Zhang X, and Lin X. Tendon-bone healing: synergistic role of platelets and mesenchymal stem cells in tissue engineering. Tissue Eng Part B Rev. (2025). doi: 10.1089/ten.teb.2024.0333
47. Migliorini F, Tingart M, and Maffulli N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. (2020) 20:1373–9. doi: 10.1080/14712598.2020.1786532
48. Zou J, Yang W, Cui W, Li C, Ma C, Ji X, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing. J Nanobiotechnology. (2023) 21:1–24. doi: 10.1186/s12951-023-01778-6
49. Ménard C, Dulong J, Roulois D, Hébraud B, Verdière L, Pangault C, et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells. (2020) 38:146–59. doi: 10.1002/stem.3077
50. Ganguly A, Swaminathan G, Garcia-Marques F, Regmi S, Yarani R, Primavera R, et al. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Rep. (2023) 18:190–204. doi: 10.1016/j.stemcr.2022.11.006
51. Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. (2019) 47:1722–33. doi: 10.1177/0363546519848678
52. Yen BL, Liu K-J, Sytwu H-K, and Yen M-L. Clinical implications of differential functional capacity between tissue-specific human mesenchymal stromal/stem cells. FEBS J. (2023) 290:2833–44. doi: 10.1111/febs.v290.11
53. Costela-Ruiz VJ, Melguizo-Rodríguez L, Bellotti C, Illescas-Montes R, Stanco D, Arciola CR, et al. Different sources of mesenchymal stem cells for tissue regeneration: A guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. (2022) 23:6356. doi: 10.3390/ijms23116356
54. Hou W, Duan L, Huang C, Li X, Xu X, Qin P, et al. Cross-tissue characterization of heterogeneities of mesenchymal stem cells and their differentiation potentials. Front Cell Dev Biol. (2021) 9:781021. doi: 10.3389/fcell.2021.781021
55. Sangeetha KN, Vennila R, Secunda R, Sakthivel S, Pathak S, Jeswanth S, et al. Functional variations between Mesenchymal Stem Cells of different tissue origins: A comparative gene expression profiling. Biotechnol Lett. (2020) 42:1287–304. doi: 10.1007/s10529-020-02898-x
56. Tang Q, Li J, Wang Y, and Sun Q. Identification and verification of hub genes associated with ferroptosis in ischemia and reperfusion injury during renal transplantation. Int Immunopharmacol. (2023) 120:110393. doi: 10.1016/j.intimp.2023.110393
57. Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, and Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. (2019) 76:3323–48. doi: 10.1007/s00018-019-03125-1
58. Leong DJ and Sun HB. Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges. Ann N Y Acad Sci. (2016) 1383:88–96. doi: 10.1111/nyas.2016.1383.issue-1
59. Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon I-H, et al. Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med. (2020) 48:3347–58. doi: 10.1177/0363546520964445
60. Tang Y, Chen C, Liu F, Xie S, Qu J, Li M, et al. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials. (2020) 241:119837. doi: 10.1016/j.biomaterials.2020.119837
61. Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. (2020) 106:328–41. doi: 10.1016/j.actbio.2020.01.051
62. Wolint P, Miescher I, Mechakra A, Jäger P, Rieber J, Calcagni M, et al. Therapeutic potential of mesenchymal stem cell and tenocyte secretomes for tendon repair: proteomic profiling and functional characterization In Vitro and In Ovo. Int J Mol Sci. (2025) 26:3622. doi: 10.3390/ijms26083622
63. Donderwinkel I, Tuan RS, Cameron NR, and Frith JE. Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells. Acta Biomater. (2022) 145:25–42. doi: 10.1016/j.actbio.2022.04.028
64. Wang Y, Qin J-Z, Xie C-Y, Peng X-Z, Wang J-H, and Wang S-J. Kartogenin-loaded exosomes derived from bone marrow mesenchymal stem cells enhance chondrogenesis and expedite tendon enthesis healing in a rat model of rotator cuff injury. Am J Sports Med. (2024) 52:3520–35. doi: 10.1177/03635465241296141
65. Song Y, Li P, Xu Y, Lin Z, Deng Z, and Chen C. Menstrual blood-derived mesenchymal stem cells encapsulated in autologous platelet-rich gel facilitate rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. (2023) 51:1872–85. doi: 10.1177/03635465231168104
66. Cai J, Xu J, Ye Z, Wang L, Zheng T, Zhang T, et al. Exosomes derived from kartogenin-preconditioned mesenchymal stem cells promote cartilage formation and collagen maturation for enthesis regeneration in a rat model of chronic rotator cuff tear. Am J Sports Med. (2023) 51:1267–76. doi: 10.1177/03635465231155927
67. Song W, Zhang D, Wu D, Zhong L, Zhu Q, Bai Z, et al. Cryopreserved adipose-derived stem cell sheets: an off-the-shelf scaffold for augmenting tendon-to-bone healing in a rabbit model of chronic rotator cuff tear. Am J Sports Med. (2023) 51:2005–17. doi: 10.1177/03635465231171682
68. Ni Y, Tian B, Lv J, Li D, Zhang M, Li Y, et al. 3D-printed PCL scaffolds loaded with bFGF and BMSCs enhance tendon-bone healing in rat rotator cuff tears by immunomodulation and osteogenesis promotion. ACS Biomater Sci Eng. (2025) 11:1123–39. doi: 10.1021/acsbiomaterials.4c02340
69. Park Y-B. Editorial commentary: stem cell therapy for the knee: heterogeneity in cell sources, delivery methods, and concomitant surgery needs to be considered. Arthroscopy: J arthroscopic related surgery: Off Publ Arthroscopy Assoc North America Int Arthroscopy Assoc. (2021) 37:379–80. doi: 10.1016/j.arthro.2020.07.035
70. Ossendorff R, Walter SG, Schildberg FA, Khoury M, and Salzmann GM. Controversies in regenerative medicine: should knee joint osteoarthritis be treated with mesenchymal stromal cells? Eur Cell Mater. (2022) 43:98–111. doi: 10.22203/eCM.v043a09
71. Przywara D, Petniak A, and Gil-Kulik P. Optimizing mesenchymal stem cells for regenerative medicine: influence of diabetes, obesity, autoimmune, and inflammatory conditions on therapeutic efficacy: A review. Med Sci Monit Int Med J Exp Clin Res. (2024) 30:e945331. doi: 10.12659/MSM.945331
72. Beane OS, Fonseca VC, Cooper LL, Koren G, and Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PloS One. (2014) 9:e115963. doi: 10.1371/journal.pone.0115963
73. Calcat-I-Cervera S, Rendra E, Scaccia E, Amadeo F, Hanson V, Wilm B, et al. Harmonised culture procedures minimize but do not eliminate mesenchymal stromal cell donor and tissue variability in a decentralised multicentre manufacturing approach. Stem Cell Res Ther. (2023) 14:120. doi: 10.1186/s13287-023-03352-1
74. Pharoun J, Berro J, Sobh J, Abou-Younes M-M, Nasr L, Majed A, et al. Mesenchymal stem cells biological and biotechnological advances: Implications for clinical applications. Eur J Pharmacol. (2024) 977:176719. doi: 10.1016/j.ejphar.2024.176719
75. Zhu M, Cao L, Melino S, Candi E, Wang Y, Shao C, et al. Orchestration of mesenchymal stem/stromal cells and inflammation during wound healing. Stem Cells Transl Med. (2023) 12:576–87. doi: 10.1093/stcltm/szad043
76. Qi K, Li N, Zhang Z, and Melino G. Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cell Immunol. (2018) 326:86–93. doi: 10.1016/j.cellimm.2017.11.010
77. Mahajan A and Bhattacharyya S. Immunomodulation by mesenchymal stem cells during osteogenic differentiation: Clinical implications during bone regeneration. Mol Immunol. (2023) 164:143–52. doi: 10.1016/j.molimm.2023.11.006
78. Ning J, Sah RK, and Wang J. Coculture of mesenchymal stem cells and macrophage: A narrative review. J Pharmacol Exp Ther. (2025) 392:103531. doi: 10.1016/j.jpet.2025.103531
79. Wang J, Zhou Y, Donohoe E, Canning A, Moosavizadeh S, Ryan AE, et al. Immunomodulatory potential of cytokine-licensed human bone marrow-derived mesenchymal stromal cells correlates with potency marker expression profile. Stem Cells. (2024) 42:1040–54. doi: 10.1093/stmcls/sxae053
80. Rossello-Gelabert M, Igartua M, Santos-Vizcaino E, and Hernandez RM. Fine-tuning licensing strategies to boost MSC-based immunomodulatory secretome. Stem Cell Res Ther. (2025) 16:183. doi: 10.1186/s13287-025-04315-4
81. Li W, Liu Q, Shi J, Xu X, and Xu J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front Immunol. (2023) 14:1074863. doi: 10.3389/fimmu.2023.1074863
82. Williams T, Salmanian G, Burns M, Maldonado V, Smith E, Porter RM, et al. Versatility of mesenchymal stem cell-derived extracellular vesicles in tissue repair and regenerative applications. Biochimie. (2023) 207:33–48. doi: 10.1016/j.biochi.2022.11.011
83. van Griensven M and Balmayor ER. Extracellular vesicles are key players in mesenchymal stem cells’ dual potential to regenerate and modulate the immune system. Adv Drug Deliv Rev. (2024) 207:115203. doi: 10.1016/j.addr.2024.115203
84. Padinharayil H, Varghese J, Wilson C, and George A. Mesenchymal stem cell-derived exosomes: Characteristics and applications in disease pathology and management. Life Sci. (2024) 342:122542. doi: 10.1016/j.lfs.2024.122542
85. Wu T, Liu Y, Wang S, and Shi C. MSC-derived extracellular vesicles: roles and molecular mechanisms for tissue repair. Int J Nanomedicine. (2025) 20:7953–74. doi: 10.2147/IJN.S525394
86. Wang J, Guo X, Kang Z, Qi L, Yang Y, Wang J, et al. Roles of exosomes from mesenchymal stem cells in treating osteoarthritis. Cell Reprogram. (2020) 22:1–11. doi: 10.1089/cell.2019.0098
87. Ma M, Cui G, Liu Y, Tang Y, Lu X, Yue C, et al. Mesenchymal stem cell-derived extracellular vesicles, osteoimmunology and orthopedic diseases. PeerJ. (2023) 11:e14677. doi: 10.7717/peerj.14677
88. Turano E, Scambi I, Virla F, Bonetti B, and Mariotti R. Extracellular vesicles from mesenchymal stem cells: towards novel therapeutic strategies for neurodegenerative diseases. Int J Mol Sci. (2023) 24:2917. doi: 10.3390/ijms24032917
89. Teo KYW, Tan R, Wong KL, Hey DHW, Hui JHP, and Toh WS. Small extracellular vesicles from mesenchymal stromal cells: the next therapeutic paradigm for musculoskeletal disorders. Cytotherapy. (2023) 25:837–46. doi: 10.1016/j.jcyt.2023.04.011
90. Chen S, Sun F, Qian H, Xu W, and Jiang J. Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells Int. (2022) 2022:1779346. doi: 10.1155/2022/1779346
91. Gómez-Ferrer M, Villanueva-Badenas E, Sánchez-Sánchez R, Sánchez-López CM, Baquero MC, Sepúlveda P, et al. HIF-1α and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int J Mol Sci. (2021) 22:3416. doi: 10.3390/ijms22073416
92. Kim H, Lee MJ, Bae E-H, Ryu JS, Kaur G, Kim HJ, et al. Comprehensive molecular profiles of functionally effective MSC-derived extracellular vesicles in immunomodulation. Mol Ther. (2020) 28:1628–44. doi: 10.1016/j.ymthe.2020.04.020
93. Varderidou-Minasian S and Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. (2020) 10:5979–97. doi: 10.7150/thno.40122
94. Chen J, Wang Z, Yi M, Yang Y, Tian M, Liu Y, et al. Regenerative properties of bone marrow mesenchymal stem cell derived exosomes in rotator cuff tears. J Transl Med. (2025) 23:47. doi: 10.1186/s12967-024-06029-2
95. Bianco ST, Moser HL, Galatz LM, and Huang AH. Biologics and stem cell-based therapies for rotator cuff repair. Ann N Y Acad Sci. (2019) 1442:35–47. doi: 10.1111/nyas.2019.1442.issue-1
96. Seo Y, Kang M-J, and Kim H-S. Strategies to potentiate paracrine therapeutic efficacy of mesenchymal stem cells in inflammatory diseases. Int J Mol Sci. (2021) 22:3397. doi: 10.3390/ijms22073397
97. Lee B-C and Kang K-S. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther. (2020) 11:397. doi: 10.1186/s13287-020-01920-3
98. Sarsenova M, Kim Y, Raziyeva K, Kazybay B, Ogay V, and Saparov A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol. (2022) 13:1010399. doi: 10.3389/fimmu.2022.1010399
99. García JR, Quirós M, Han WM, O’Leary MN, Cox GN, Nusrat A, et al. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials. (2019) 220:119403. doi: 10.1016/j.biomaterials.2019.119403
100. López-García L and Castro-Manrreza ME. TNF-α and IFN-γ Participate in improving the immunoregulatory capacity of mesenchymal stem/stromal cells: importance of cell-cell contact and extracellular vesicles. Int J Mol Sci. (2021) 22:9531. doi: 10.3390/ijms22179531
101. Tian B, Liu J, Guo S, Li A, and Wan J-B. Macromolecule-based hydrogels nanoarchitectonics with mesenchymal stem cells for regenerative medicine: A review. Int J Biol Macromol. (2023) 243:125161. doi: 10.1016/j.ijbiomac.2023.125161
102. Li X and Wu X. The microspheres/hydrogels scaffolds based on the proteins, nucleic acids, or polysaccharides composite as carriers for tissue repair: A review. Int J Biol Macromol. (2023) 253:126611. doi: 10.1016/j.ijbiomac.2023.126611
103. Salthouse D, Novakovic K, Hilkens CMU, and Ferreira AM. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. (2023) 155:1–18. doi: 10.1016/j.actbio.2022.11.003
104. Est-Witte S, Jain M, Ben-Akiva E, Yang J, Warren T, Tzeng SY, et al. Immunomodulatory delivery materials for tissue repair. Adv Healthc Mater. (2025), e2501400. doi: 10.1002/adhm.202501400
105. Zhang Z, He C, and Chen X. Designing hydrogels for immunomodulation in cancer therapy and regenerative medicine. Adv Mater. (2024) 36:e2308894. doi: 10.1002/adma.202308894
106. Soltanmohammadi F, Mahmoudi Gharehbaba A, Alizadeh E, and Javadzadeh Y. Innovative approaches to tissue engineering: Utilizing decellularized extracellular matrix hydrogels for mesenchymal stem cell transport. Int J Biol Macromol. (2025) 290:138893. doi: 10.1016/j.ijbiomac.2024.138893
107. Ma S, Wang C, Dong Y, Jing W, Wei P, Peng C, et al. Microsphere-gel composite system with mesenchymal stem cell recruitment, antibacterial, and immunomodulatory properties promote bone regeneration via sequential release of LL37 and W9 peptides. ACS Appl Mater Interfaces. (2022) 14:38525–40. doi: 10.1021/acsami.2c10242
108. Zhang S, Lin A, Tao Z, Fu Y, Xiao L, Ruan G, et al. Microsphere-containing hydrogel scaffolds for tissue engineering. Chem - Asian J. (2022) 17:e202200630. doi: 10.1002/asia.202200630
109. Barcena AJR, Mishra A, Bolinas DKM, Martin BM, and Melancon MP. Integration of electrospun scaffolds and biological polymers for enhancing the delivery and efficacy of mesenchymal stem/stromal cell therapies. Front Biosci (Landmark Ed. (2024) 29:228. doi: 10.31083/j.fbl2906228
110. Yao Z-C, Yang Y-H, Kong J, Zhu Y, Li L, Chang C, et al. Biostimulatory micro-fragmented nanofiber-hydrogel composite improves mesenchymal stem cell delivery and soft tissue remodeling. Small. (2022) 18:e2202309. doi: 10.1002/smll.202202309
111. Fu M, Yang C, and Sun G. Recent advances in immunomodulatory hydrogels biomaterials for bone tissue regeneration. Mol Immunol. (2023) 163:48–62. doi: 10.1016/j.molimm.2023.09.010
112. Pang Q-M, Deng K-Q, Zhang M, Wu X-C, Yang R-L, Fu S-P, et al. Multiple strategies enhance the efficacy of MSCs transplantation for spinal cord injury. Biomed Pharmacother. (2023) 157:114011. doi: 10.1016/j.biopha.2022.114011
113. Trapana J, Weinerman J, Lee D, Sedani A, Constantinescu D, Best TM, et al. Cell-based therapy in the treatment of musculoskeletal diseases. Stem Cells Transl Med. (2024) 13:959–78. doi: 10.1093/stcltm/szae049
114. Meng H-F, Jin J, Wang H, Wang L-S, and Wu C-T. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J Cell Mol Med. (2022) 26:4745–55. doi: 10.1111/jcmm.v26.18
115. Alagesan S, Brady J, Byrnes D, Fandiño J, Masterson C, McCarthy S, et al. Enhancement strategies for mesenchymal stem cells and related therapies. Stem Cell Res Ther. (2022) 13:75. doi: 10.1186/s13287-022-02747-w
116. Caplan HW, Prabhakara KS, Toledano Furman NE, Zorofchian S, Kumar A, Martin C, et al. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cells. (2021) 39:358–70. doi: 10.1002/stem.3320
117. Negi N and Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells. (2020) 38:596–605. doi: 10.1002/stem.3151
118. Piekarska K, Urban-Wójciuk Z, Kurkowiak M, Pelikant-Małecka I, Schumacher A, Sakowska J, et al. Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat Commun. (2022) 13:856. doi: 10.1038/s41467-022-28338-0
119. Tashiro S, Nakamura M, and Okano H. Regenerative rehabilitation and stem cell therapy targeting chronic spinal cord injury: A review of preclinical studies. Cells. (2022) 11:685. doi: 10.3390/cells11040685
120. Kurihara K, Sasaki M, Nagahama H, Obara H, Fukushi R, Hirota R, et al. Repeated intravenous infusion of mesenchymal stem cells enhances recovery of motor function in a rat model with chronic spinal cord injury. Brain Res. (2023) 1817:148484. doi: 10.1016/j.brainres.2023.148484
121. Yamashita T, Sasaki M, Sasaki Y, Nagahama H, Oka S, Kataoka-Sasaki Y, et al. Rehabilitation facilitates functional improvement following intravenous infusion of mesenchymal stem cells in the chronic phase of cerebral ischemia in rats. Brain Res. (2024) 1825:148709. doi: 10.1016/j.brainres.2023.148709
122. Dabrowska S, Andrzejewska A, Janowski M, and Lukomska B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. (2020) 11:591065. doi: 10.3389/fimmu.2020.591065
123. Brennan MÁ, Layrolle P, and Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. (2020) 30:1909125. doi: 10.1002/adfm.201909125
124. Ouzin M and Kogler G. Mesenchymal stromal cells: heterogeneity and therapeutical applications. Cells. (2023) 12:2039. doi: 10.3390/cells12162039
125. Česnik AB and Švajger U. The issue of heterogeneity of MSC-based advanced therapy medicinal products-a review. Front Cell Dev Biol. (2024) 12:1400347. doi: 10.3389/fcell.2024.1400347
126. Medrano-Trochez C, Chatterjee P, Pradhan P, Stevens HY, Ogle ME, Botchwey EA, et al. Single-cell RNA-seq of out-of-thaw mesenchymal stromal cells shows tissue-of-origin differences and inter-donor cell-cycle variations. Stem Cell Res Ther. (2021) 12:565. doi: 10.1186/s13287-021-02627-9
127. Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. (2021) 78:447–67. doi: 10.1007/s00018-020-03600-0
128. Zhang C, Han X, Liu J, Chen L, Lei Y, Chen K, et al. Single-cell transcriptomic analysis reveals the cellular heterogeneity of mesenchymal stem cells. Genomics Proteomics Bioinf. (2022) 20:70–86. doi: 10.1016/j.gpb.2022.01.005
129. Jones S, Tai M, Ayushman M, Peasah A, Johannsen J, and Yang F. Donor variability and 3D culture models influence human mesenchymal stem cell differentiation. Tissue Eng Part A. (2025). doi: 10.1089/ten.tea.2025.0028
130. He J, Ping S, Yu F, Yuan X, Wang J, and Qi J. Mesenchymal stem cell-derived exosomes: therapeutic implications for rotator cuff injury. Regen Med. (2021) 16:803–15. doi: 10.2217/rme-2020-0183
131. Wei G, Wang Z, Liu R, Zhou C, Li E, Shen T, et al. A combination of hybrid polydopamine-human keratinocyte growth factor nanoparticles and sodium hyaluronate for the efficient prevention of postoperative abdominal adhesion formation. Acta Biomater. (2022) 138:155–67. doi: 10.1016/j.actbio.2021.10.015
132. Carp DM and Liang Y. Universal or personalized mesenchymal stem cell therapies: impact of age, sex, and biological source. Cells. (2022) 11:2077. doi: 10.3390/cells11132077
133. Weber SC. Editorial commentary: stem cells in rotator cuff surgery: in search of the holy grail. Arthroscopy: J arthroscopic related surgery: Off Publ Arthroscopy Assoc North America Int Arthroscopy Assoc. (2020) 36:981–2. doi: 10.1016/j.arthro.2020.01.011
134. Di Matteo B, Ranieri R, Manca A, Cappato S, Marcacci M, Kon E, et al. Cell-based therapies for the treatment of shoulder and elbow tendinopathies: A scoping review. Stem Cells Int. (2021) 2021:5558040. doi: 10.1155/2021/5558040
135. Wang D, Zhang X, Huang S, Liu Y, Fu BS, Mak KK, et al. Engineering multi-tissue units for regenerative Medicine: Bone-tendon-muscle units of the rotator cuff. Biomaterials. (2021) 272:120789. doi: 10.1016/j.biomaterials.2021.120789
136. Lyu Z, Xin M, Oyston DR, Xue T, Kang H, Wang X, et al. Cause and consequence of heterogeneity in human mesenchymal stem cells: Challenges in clinical application. Pathol Res Pract. (2024) 260:155354. doi: 10.1016/j.prp.2024.155354
137. Utsunomiya H, Sekiya I, and Uchida S. Editorial commentary: are we ready to apply stem cell therapy in rotator cuff tear surgery? Arthroscopy: J arthroscopic related surgery: Off Publ Arthroscopy Assoc North America Int Arthroscopy Assoc. (2020) 36:86–7. doi: 10.1016/j.arthro.2019.09.001
138. Nocera NL, Burke CJ, Gyftopoulos S, and Adler RS. Ultrasound-MRI correlation for healing of rotator cuff repairs using power doppler, sonographic shear wave elastography and MR signal characteristics: A pilot study. J ultrasound Med Off J Am Inst Ultrasound Med. (2021) 40:2055–68. doi: 10.1002/jum.v40.10
139. Xu J, Zhang Y, Liu Y, You Y, Li F, Chen Y, et al. Vitality-enhanced dual-modal tracking system reveals the dynamic fate of mesenchymal stem cells for stroke therapy. Small. (2022) 18:e2203431. doi: 10.1002/smll.202203431
140. Shalaby N, Kelly JJ, Sehl OC, Gevaert JJ, Fox MS, Qi Q, et al. Complementary early-phase magnetic particle imaging and late-phase positron emission tomography reporter imaging of mesenchymal stem cells. vivo Nanoscale. (2023) 15:3408–18. doi: 10.1039/D2NR03684C
141. Dunn CM, Kameishi S, Grainger DW, and Okano T. Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater. (2021) 133:114–25. doi: 10.1016/j.actbio.2021.03.069
142. Zhang X, Song W, Han K, Fang Z, Cho E, Huangfu X, et al. Three-dimensional bioprinting of a structure-, composition-, and mechanics-graded biomimetic scaffold coated with specific decellularized extracellular matrix to improve the tendon-to-bone healing. ACS Appl Mater Interfaces. (2023) 15:28964–80. doi: 10.1021/acsami.3c03793
143. Monteiro RF, Bakht SM, Gomez-Florit M, Stievani FC, Alves ALG, Reis RL, et al. Writing 3D in vitro models of human tendon within a biomimetic fibrillar support platform. ACS Appl Mater Interfaces. (2023) 15:50598–50611. doi: 10.1021/acsami.2c22371
144. Du L, Qin C, Zhang H, Han F, Xue J, Wang Y, et al. Multicellular bioprinting of biomimetic inks for tendon-to-bone regeneration. Adv Sci (Weinheim Baden-Wurttemberg Ger. (2023) 10:e2301309. doi: 10.1002/advs.202301309
145. Chen Y, Hao M, Bousso I, Thomopoulos S, and Xia Y. Reliable fabrication of mineral-graded scaffolds by spin-coating and laser machining for use in tendon-to-bone insertion repair. Adv Healthc Mater. (2024) 13:e2402531. doi: 10.1002/adhm.202402531
146. Altunbek M, Afghah F, Caliskan OS, Yoo JJ, and Koc B. Design and bioprinting for tissue interfaces. Biofabrication. (2023) 15. doi: 10.1088/1758-5090/acb73d
147. Potyondy T, Uquillas JA, Tebon PJ, Byambaa B, Hasan A, Tavafoghi M, et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication. (2021) 13. doi: 10.1088/1758-5090/abc8de
148. Khalak FA-H, Decuyper JM, Khalak KA-H, Alonso SR, Saenz-Del-Burgo L, and Pedraz Muñoz JL. 3D bioprinting approaches for musculoskeletal interfaces in tissue engineering. Int J Pharm. (2025) 682:125939. doi: 10.1016/j.ijpharm.2025.125939
149. Goyal P and Malviya R. Developments in stem cell therapy by utilizing artificial intelligence. Curr Pharm Des. (2023) 29:2223–8. doi: 10.2174/0113816128266696230926094423
150. Srinivasan A, Sathiyanathan P, Yin L, Liu TM, Lam A, Ravikumar M, et al. Strategies to enhance immunomodulatory properties and reduce heterogeneity in mesenchymal stromal cells during ex vivo expansion. Cytotherapy. (2022) 24:456–72. doi: 10.1016/j.jcyt.2021.11.009
151. Yan R, Fan C, Yin Z, Wang T, and Chen X. Potential applications of deep learning in single-cell RNA sequencing analysis for cell therapy and regenerative medicine. Stem Cells. (2021) 39:511–21. doi: 10.1002/stem.3336
152. Asadi Sarabi P, Shabanpouremam M, Eghtedari AR, Barat M, Moshiri B, Zarrabi A, et al. AI-Based solutions for current challenges in regenerative medicine. Eur J Pharmacol. (2024) 984:177067. doi: 10.1016/j.ejphar.2024.177067
153. Zhang C, Zhou L, Wang Z, Gao W, Chen W, Zhang H, et al. Eradication of specific donor-dependent variations of mesenchymal stem cells in immunomodulation to enhance therapeutic values. Cell Death Dis. (2021) 12:357. doi: 10.1038/s41419-021-03644-5
154. Ong WK, Chakraborty S, and Sugii S. Adipose tissue: understanding the heterogeneity of stem cells for regenerative medicine. Biomolecules. (2021) 11:918. doi: 10.3390/biom11070918
Keywords: rotator cuff tear, tendon-to-bone healing, mesenchymal stem cells, immune microenvironment, immunomodulation, tissue regeneration
Citation: Li D, Zou Y and Zhao Y (2025) The interplay between mesenchymal stem cells and the immune microenvironment in rotator cuff tendon-to-bone healing: current progress and future directions. Front. Immunol. 16:1661340. doi: 10.3389/fimmu.2025.1661340
Received: 07 July 2025; Accepted: 11 August 2025;
Published: 05 September 2025.
Edited by:
Thiago DeSouza-Vieira, Oswaldo Cruz Foundation (Fiocruz), BrazilCopyright © 2025 Li, Zou and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanting Zhao, ZHJ4eHkwMjAzQDE2My5jb20=
 Dong Li
Dong Li Yuanting Zhao
Yuanting Zhao