- 1Institute of Translational Medicine, Faculty of Medical Sciences, Private University in the Principality of Liechtenstein (UFL), Triesen, Liechtenstein
- 2Institute of Clinical Epidemiology, Public Health, Health Economics, Medical Statistics and Informatics, Medical University of Innsbruck, Innsbruck, Austria
Objectives: The aim of this systematic review with meta-analysis was to examine the association between the polymorphisms rs1801274 (FCGR2A H131R) and rs396991 (FCGR3A F158V) and susceptibility to autoimmune diseases (ADs), with a focus on the progress and novelty of studies published over the last two decades.
Methods: A meta-analysis systematically evaluated FCGR2A/3A gene variants in autoimmune diseases (ADs) using four genetic models: dominant, recessive, overdominant, and allelic contrast.
Results: The FCGR3A F158V polymorphism was significantly associated with immune thrombocytopenia in all four genetic models tested (dominant: OR = 2.67, 95% CI 1.94-3.67, for FV + VV vs. FF, recessive: OR = 2.38, 95% CI 1.78-3.19, for VV vs. FF + FV, overdominant: OR = 1.58, 95% CI 1.15-2.17, for FV vs. FF+VV, and allele comparison: OR = 1.97, 95% CI 1.70-2.29, for V vs. F, in the overall analyses). Statistically significant associations were also found between rheumatoid arthritis and FCGR3A F158V polymorphisms (recessive: OR = 1.36, 95% CI 1.09-1.69, for VV vs. FF + FV, and allele comparison: OR = 1.15, 95% CI 1.03-1.29, for V vs. F, in the overall analyses). Conversely, the overall analysis identified a negative association between the FCGR2A H131R polymorphism and rheumatoid arthritis in two genetic models (dominant: OR 0.83, 95% CI 0.69-1.00, for HR + RR vs. HH; allelic comparison: OR 0.86, 95% CI 0.76-0.97, for R vs. H).
Conclusion: This meta-analysis revealed an association between FCGR3A V158 and an increased risk of immune thrombocytopenia and rheumatoid arthritis. However, this polymorphism is likely to explain only part of the pathogenesis of both diseases. Conversely, a protective association was found between FCGR2A R131 and rheumatoid arthritis. Nevertheless, the quantification of the total genetic contribution of a single gene remains challenging.
1 Introduction
Autoimmune diseases (ADs) are characterized by intricate and multifactorial pathophysiological processes involving dysregulation of both innate and adaptive immune responses. The etiology and progression of ADs are influenced by a complex interplay of genetic predisposition and environmental triggers. Epidemiological studies estimate that the global prevalence and incidence of ADs affect approximately 7.6–9.4% of the population (1), posing substantial challenges for healthcare systems worldwide. Although advances in therapeutic interventions have significantly improved the outcomes and quality of life for patients, many of the molecular and immunological pathways underlying these disorders remain incompletely understood, necessitating continued research to approach the early on diagnosis and development of targeted therapies.
Recent advances in genetic research have begun to shed light on some of the molecular mechanisms underlying autoimmunity. Polymorphisms in genes such as HLA, CTLA4, and IL2RA are now recognized as major contributors to the onset and progression of various autoimmune diseases (2–4). Additionally, variants in highly polymorphic Fc gamma receptor (FcγR) genes, which mediate IgG antibody recognition, have been linked to several ADs (5). In particular, the H131R variant in FCGR2A (rs1801274) and the F158V variant in FCGR3A (rs396991) are among the most extensively studied single nucleotide variants (SNVs) in this context.
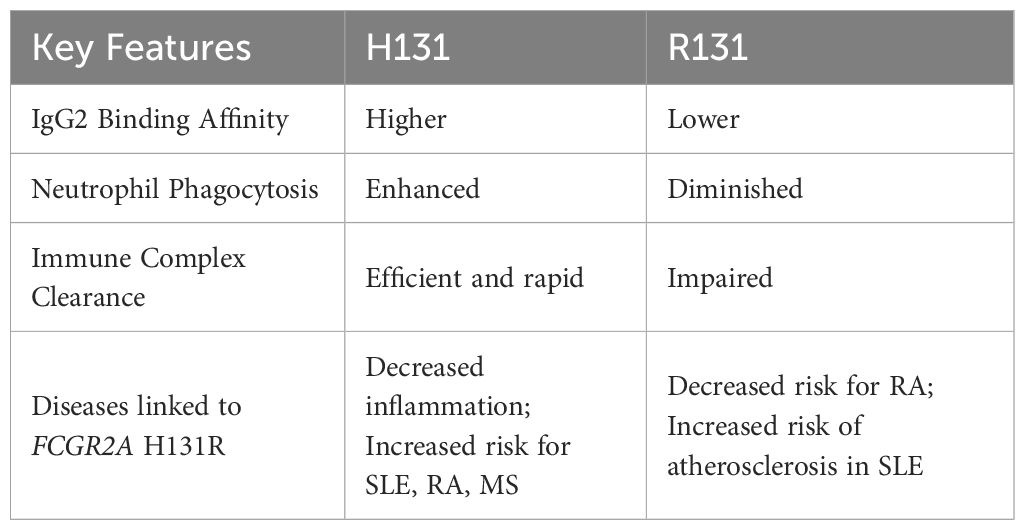
The FCGR2A gene is located on chromosome 1q23 and comprises 7 exons spanning ~15.58 kb (6). Its protein product, Fcγ receptor IIa (FcγRIIa), acts as a low-affinity receptor for monomeric IgG, but also forms interactions with larger immune complexes (7). FcγRIIa (CD32a) is an integral membrane protein with two extracellular Ig-like domains and a cytoplasmic tail containing an immunoreceptor tyrosine-based activation motif (ITAM) (8). This receptor is expressed by most leucocytes, including monocytes, dendritic cells, macrophages, natural killer cells, platelets and endothelial cells, and a subpopulation of T-cells. The H131R polymorphism substitutes histidine (H) with arginine (R) at position 131 within the second Ig-like domain of the FcγRIIa receptor, altering its ability to bind IgG2 antibodies (9) (Figure 1). While the H131 (‘wild-type’) allele enhances immune defense, it can also promote inflammation and tissue damage, contributing to autoimmune diseases such as rheumatoid arthritis (RA), Graves’ disease, ulcerative colitis, childhood immune thrombocytopenia (ITP), and Kawasaki disease (7). However, the R131 (‘risk’) allele’s reduced immune activation is linked to a higher risk of infections like sepsis (10) and is also associated with systemic lupus erythematosus (SLE) (11) due to less efficient clearance of immune complexes (Figure 1). Hence, both H and R alleles and their resulting phenotypes demonstrate the delicate effect of this FcγRIIa R/H131 polymorphism in regulating the immune responses, with each genotype predisposing individuals to different risks of inflammatory or infectious diseases.
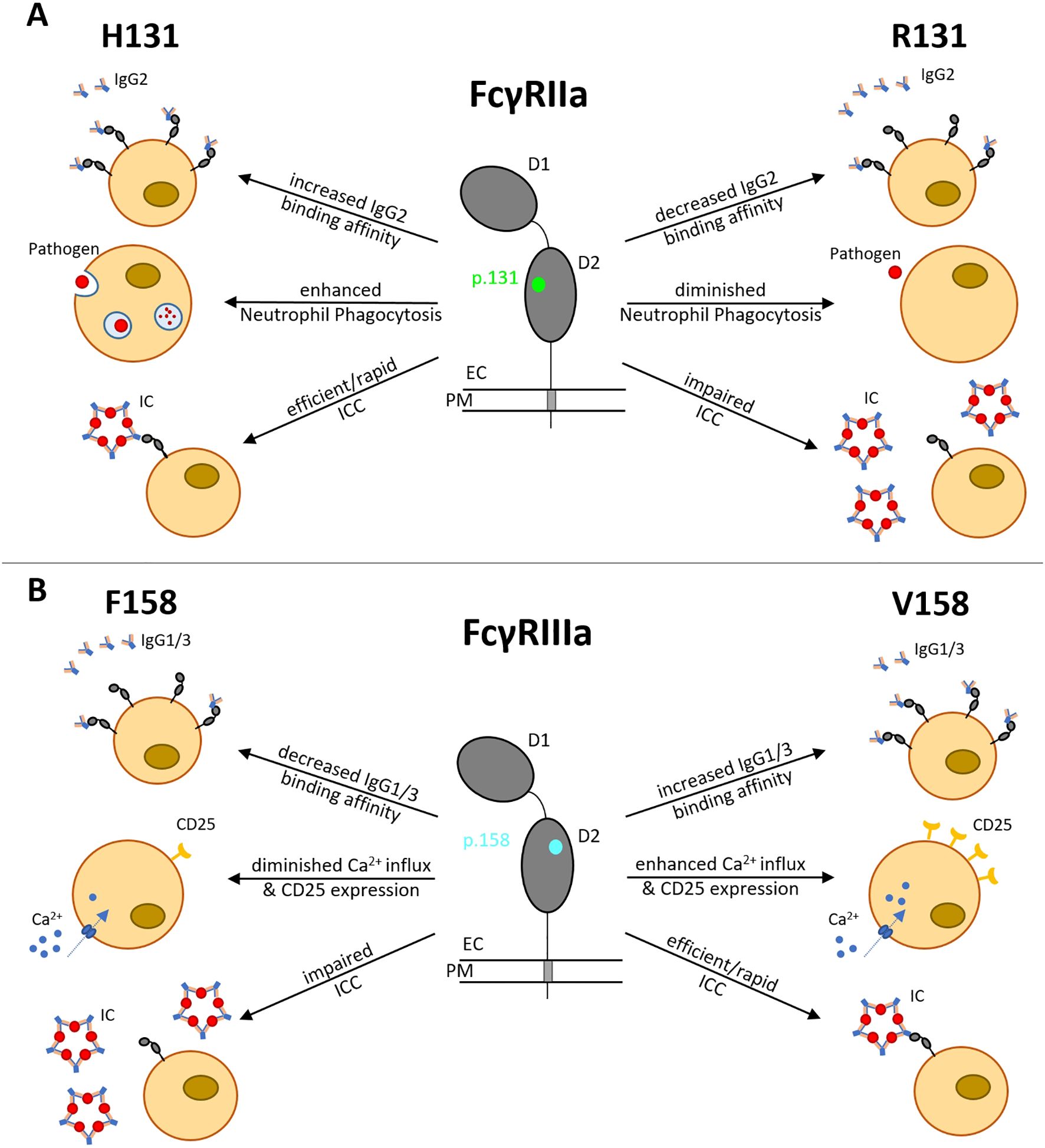
Figure 1. Functional consequences of FCGR2A H131R and FCGR3A F158V polymorphisms. (A) H131R (green) is located in the IgG-binding domain of FcγRIIa, influencing the binding affinity to IgG2, phagocytosis activity in neutrophils and ICC. (B) F158V (blue) is located in the IgG-binding domain of FcγRIIIa, influencing the binding affinity to IgG1/3, CD25 expression rate, Ca2+ influx and ICC. D1, Ig-like domain 1; D2, Ig-like domain 2; EC, extracellular; PM, plasma membrane; IC, immune complex; ICC, immune complex clearance.
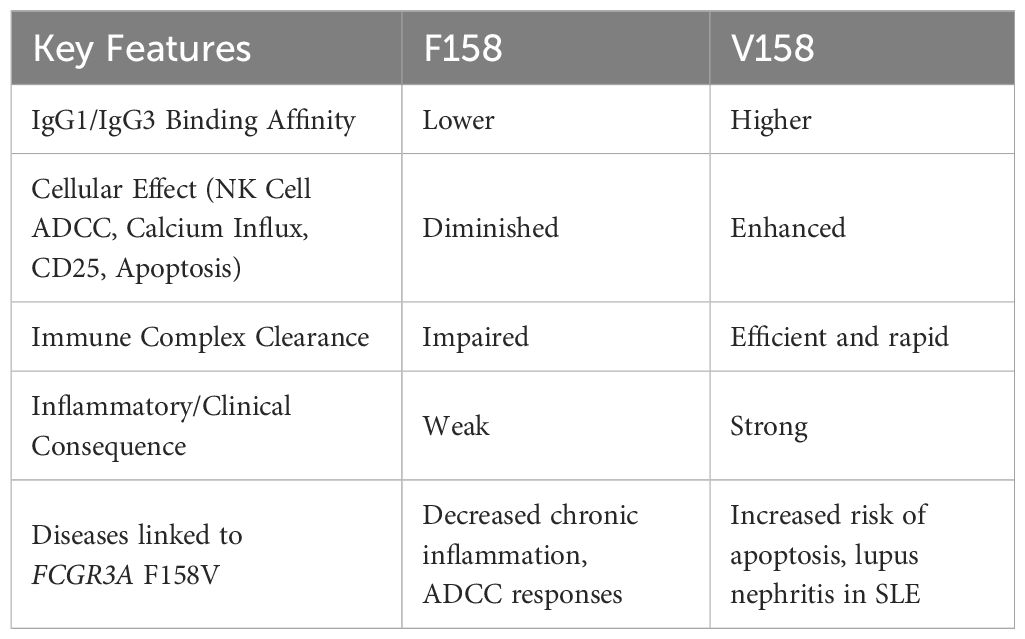
The FCGR3A gene is located on chromosome 1q23 and contains 7 exons spanning ~ 8.3 kb (12). It encodes the low-affinity Fcγ receptor IIIa (FcγRIIIa), which binds immunoglobulin G (IgG)-containing immune complexes (ICs) to mediate IC clearance (13), antibody-dependent cellular cytotoxicity (ADCC) and inflammatory cytokine release. The FCGR3A F158V polymorphism substitutes phenylalanine (F) with valine (V) at position 158 of FcγRIIIa (CD16a), also in the second IG-like domain, increasing receptor affinity for IgG1 and IgG3, and enhancing antibody-dependent cellular cytotoxicity (ADCC) by strengthening interactions with natural killer cells (NK) and macrophages, leading to hyperactive immune responses (14). The F158 (‘wild-type’, low-affinity) allele of the FCGR3A F158V polymorphism has a lower binding affinity of FcγRIIIa for IgG1 and IgG3. This leads to impaired immune complex (IC) clearance and dysregulated B - cell activation (15) (Figure 1). These pleiotropic effects therefore link the F158 variant to autoimmune pathogenesis, infectious disease susceptibility and variability in therapeutic outcomes (14), as well as reducing self-tolerance and predisposing individuals to SLE and lupus nephritis (LN) (7).
Higher-affinity (‘risk’) variant V158 enhances FcγRIIIa binding to IgG1 and IgG3, thereby improving IC clearance, but also increasing the risk of pathological immune overactivation.
Previous studies have either reported a single polymorphism across multiple autoimmune diseases (6) or several polymorphisms within a single disease (16–20), but comprehensive analyses examining multiple polymorphisms across multiple diseases are limited. Therefore, our systematic review with meta-analysis assesses and reports a structured, transparent summary of the current knowledge of FCGR2A (rs1801274) and FCGR3A (rs396991) polymorphisms in autoimmune diseases [immune thrombocytopenia (ITP), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Guillain-Barré syndrome (GBS), celiac disease (CD)]. We hypothesized that these functional FcγR gene variants influence disease susceptibility, with risk associations varying by ethnicity, disease subtype and age (in ITP).
2 Materials and methods
2.1 Literature search and inclusion criteria
A comprehensive literature search was performed to examine the association between FCGR2A rs1801274 and FCGR3A rs396991 polymorphisms and autoimmune diseases. The following terms were searched: ″Fcγ receptor″, ″Fc gamma receptor″, ″FCGR″, ″CD32″, ″CD16″, ″polymorphism″, ″variant″, ″mutation″, ″autoimmune disease″ in four source databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar (https://scholar.google.com/), Cochrane Library (https://www.cochranelibrary.com/) and Science.gov (https://www.science.gov/) between January 1st, 2004 and October 14th, 2024, and relevant articles were identified for further filtering.
In line with the research objective and the planned meta-analysis, the following criteria had to be met by the included studies: (a) case-control studies investigating FCGR polymorphism (FCGR2A H131R or FCGR3A F158V) in relation to ITP, SLE, RA, GBS or CD; (b) providing data for the calculation of odds ratios (ORs) and 95% confidence intervals (CIs); (c) the full text had to be available in English. Studies were excluded if they met any of the following criteria: (a) not related to FCGR2A/3A polymorphisms and autoimmune disease; (b) animal or cancer studies; (c) no control group included; (d) case reports or case series.
2.2 Data extraction and quality assessment
The following information was extracted from the eligible studies: (a) the first author’s name; (b) the year of publication; (c) the country and ethnicity of the participants; (d) the sample size; and (e) the genotypic distributions of FCGR2A/3A polymorphisms in cases and controls.
Hardy-Weinberg equilibrium (HWE) was assessed for each study using Chi-squared tests to determine whether observed genotype frequencies deviated significantly from expected frequencies. HWE P values were calculated using Meta Genyo (21), with P values ≤ 0.05 indicating statistically significant departure from equilibrium assumptions.
The Newcastle-Ottawa scale (NOS) was used to evaluate the quality of the eligible studies (22). The NOS has a score range of zero to nine, and studies achieving a score of more than seven were regarded as high-quality data. A flowchart illustrating the study selection process is given in Figure 2.
2.3 Statistical analyses
Statistical meta-analyses were performed using RStudio 4.4.3 with the ‘metafor’ package (version 4.8-0), supplemented by Meta Genyo (21) (https://metagenyo.genyo.es), and MetaAnalysisOnline (23) (https://metaanalysisonline.com) platforms. The association between FCGR2A rs1801274 and FCGR3A rs396991 polymorphisms and the onset of autoimmune diseases was evaluated using odds ratios (ORs) — a measure of association strength — and 95% confidence intervals (CIs), which reflect the precision of the estimates. Four genetic models were evaluated, including dominant (variant carrier vs. wild-type homozygote), recessive (variant homozygote vs. others), overdominant (heterozygote vs. combined homozygotes), and allelic (variant allele vs. wild-type allele) models.
The statistical significance of pooled ORs was determined using Z tests, with P values ≤ 0.05 considered statistically significant. Heterogeneity between studies was quantified using the I² statistic, where I² values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively (24). Given the expected clinical and methodological diversity across studies, random-effects models (DerSimonian and Laird method (25)) were employed for all analyses.
To explore potential sources of heterogeneity, subgroup analyses were conducted. The primary analyses were based on ethnicity (European, East Asian and North African populations) for each polymorphism in a specific disease. In the second analysis, several other populations were included in the studies’ pooled meta-analysis, which grouped each polymorphism with all diseases, as shown in Figures 3 and 4. For immune thrombocytopenia (ITP) studies, additional stratification by age (pediatric vs. adult) was performed in the primary analysis.
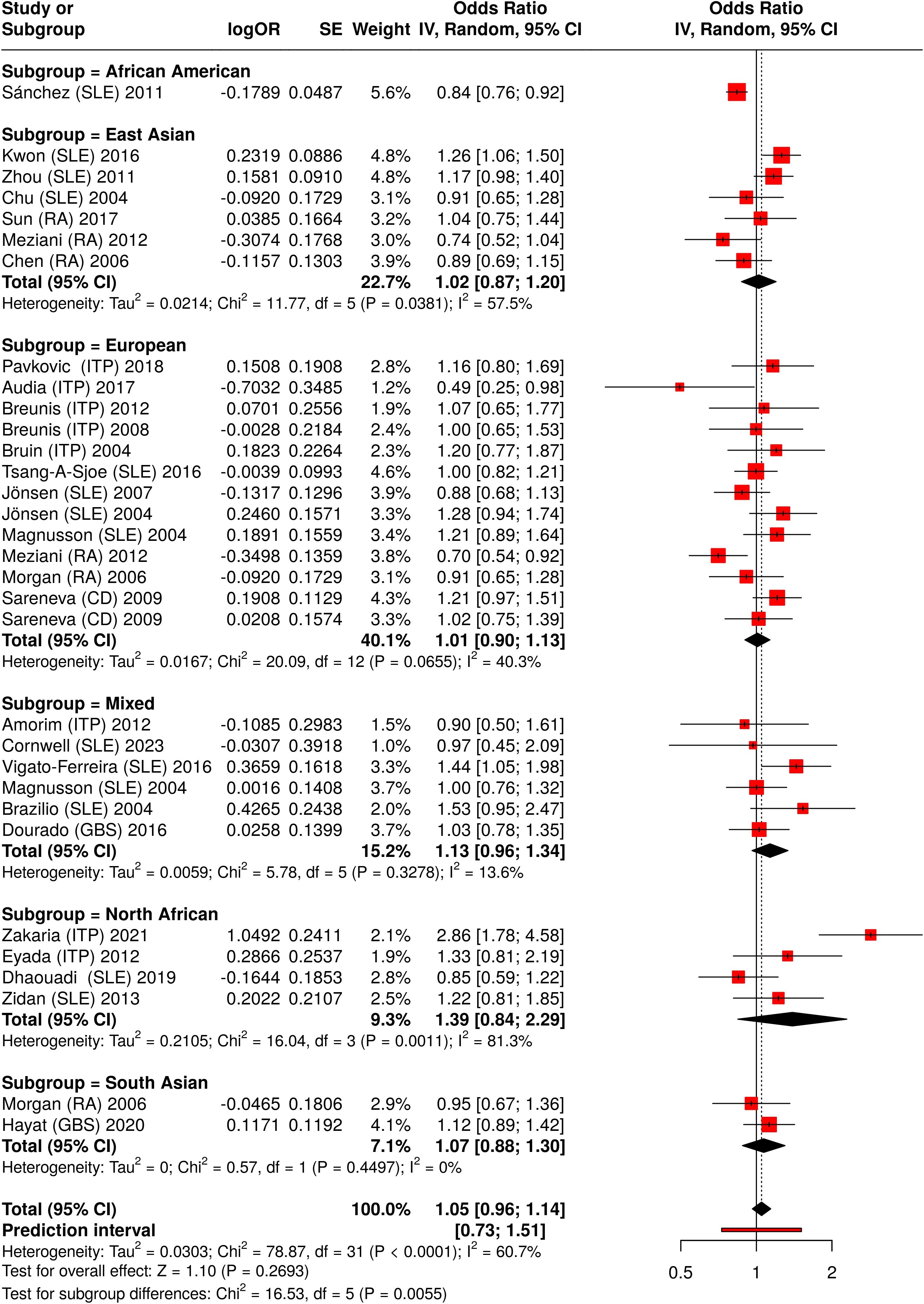
Figure 3. Meta-analysis of all pooled studies of FCGR2A rs1801274 polymorphisms by subset and summarized across all ADs.
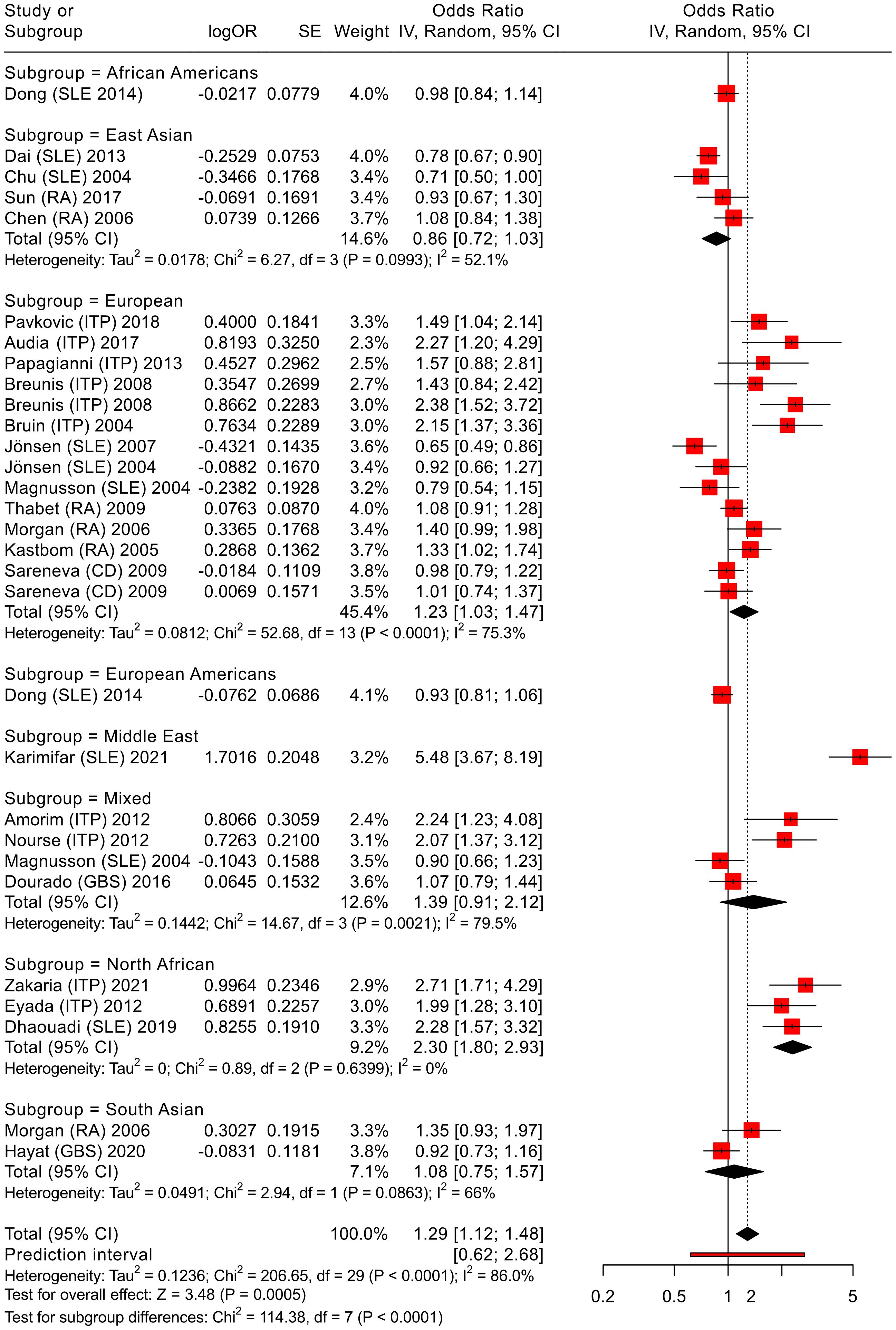
Figure 4. Meta-analysis of all pooled studies of FCGR3A rs396991 polymorphisms by subset and summarized across all ADs.
Sensitivity analyses were carried out by sequentially excluding individual studies to assess their impact on the overall effect magnitude.
Results were visualized using forest plots displaying individual and pooled effect sizes with corresponding 95% CIs. These forest plots illustrate the strength and direction of associations across individual studies, as well as the overall pooled population estimates. Publication bias was evaluated using Egger’s regression test (26). All statistical analyses were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27).
3 Results
3.1 Study selection
Our research strategy identified 781 potentially relevant articles from the four different databases PubMed, Google Scholar, Cochrane Library and Science.gov. A total of 648 records were retrieved after removing duplicates. After excluding irrelevant articles, 161 articles were retrieved for further evaluation. Upon reading the full text, 127 articles were subsequently excluded and a further 34 eligible studies (ITP - 9 studies (28–36), SLE - 16 studies (37–52), RA - 6 studies (53–58) Guillain-Barré syndrome (GBS) - 2 studies (56, 60) or celiac disease (CD) - 1 study (61)) were included for further quantitative analysis (Figure 2). All selected studies are given in Table 1.
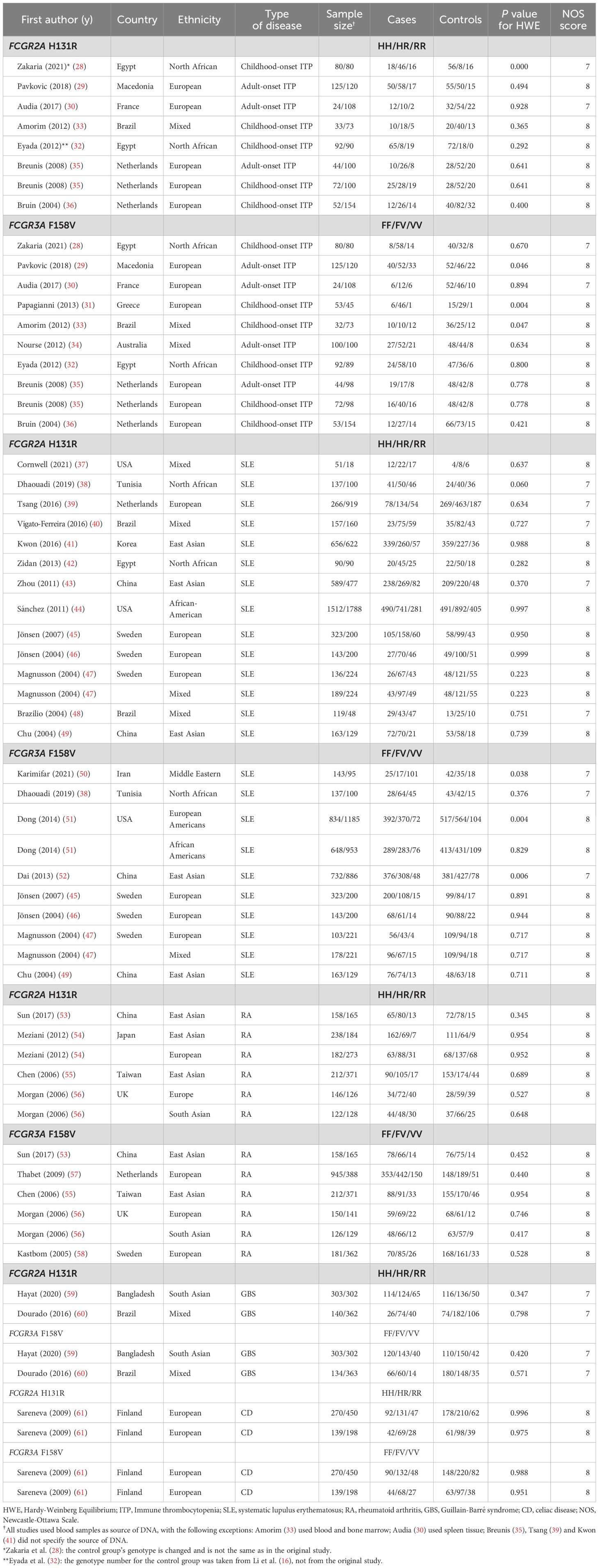
Table 1. The characteristics of included studies for FCGR polymorphism and various autoimmune diseases.
3.2 Study characteristics
Using the Newcastle-Ottawa scale (NOS), the quality of included studies ranged in between 7 and 8 and is shown in Table 1.
3.3 Overall and subgroup analyses for FCGR2A and FCGR3A
3.3.1 FCGR2A
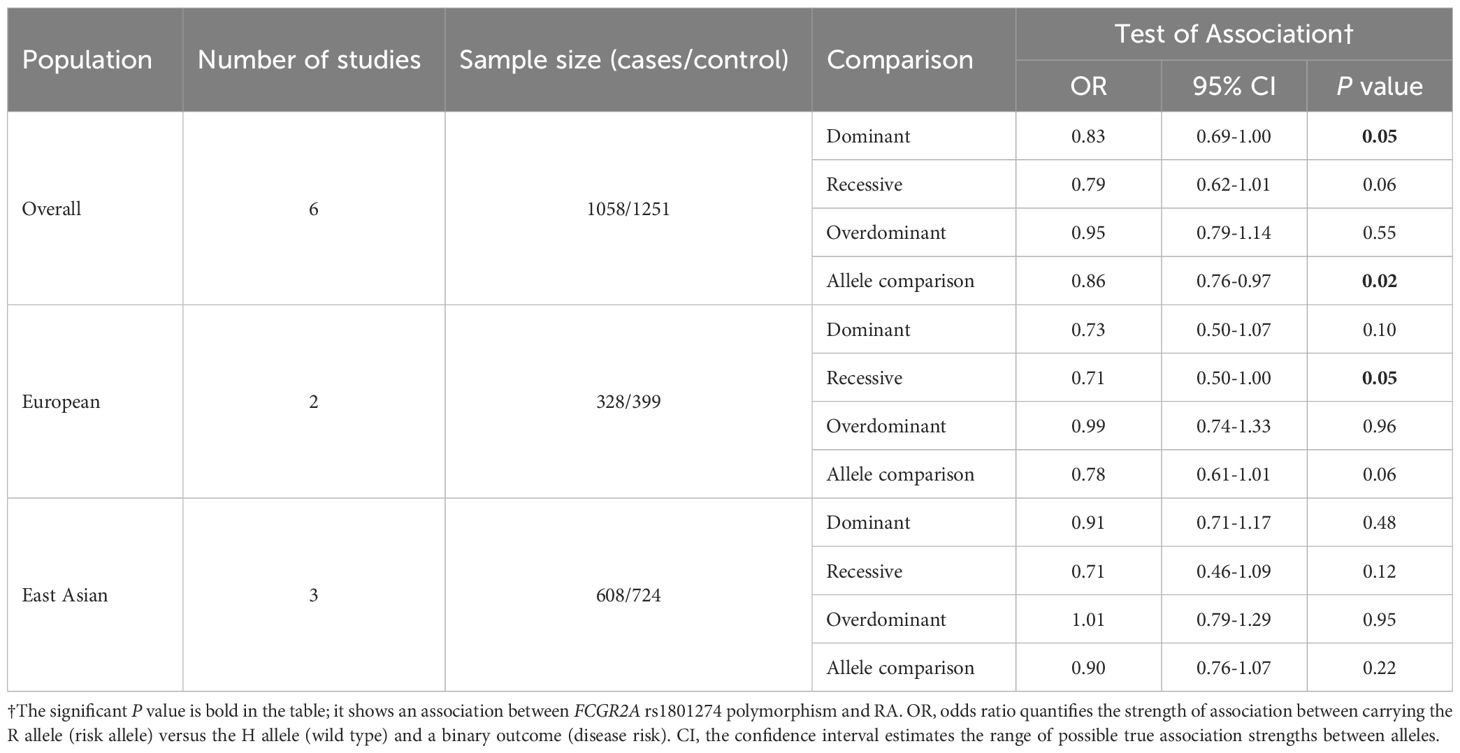
The data from the chosen studies, based on diverse autoimmune cohorts (ITP, SLE, RA, GBS, CD), revealed no statistically significant association between FCGR2A H131R and disease susceptibility across all genetic models tested (dominant, recessive, overdominant, allelic contrast) (Supplementary Tables S1-S4). However, marginal trends emerged in the dominant and allelic models in RA (Table 2), suggesting potential allele-specific effects.
The development of RA appears to be less likely in people with the FCGR2A R131 allele, according to the overall population analysis. According to the allelic model (R vs. H), the R131 allele has been found to be associated with a reduced risk of RA (OR 0.86, 95% CI 0.76-0.97, P = 0.02) (Table 2). Similarly, in the dominant model (HR + RR vs. HH), individuals homozygous for the H131 allele (HH) showed a higher risk compared to R131 allele carriers (OR 0.83, 95% CI 0.69-1.00, P = 0.05) (Table 2). Further analysis divided by population or disease category revealed no statistically significant associations, potentially due to insufficient statistical power or the presence of confounding factors (Table 2, Supplementary Tables S1-S4).
In the subgroup analyses, the East Asian group demonstrated a significant association in three genetic models (dominant, recessive, and allele comparison) between FCGR2A and SLE (see Supplementary Table S2). Conversely, the European and North African subgroups did not demonstrate any substantial associations between ADs and the FCGR2A H131R polymorphism (Supplementary Table S2).
3.3.2 FCGR3A
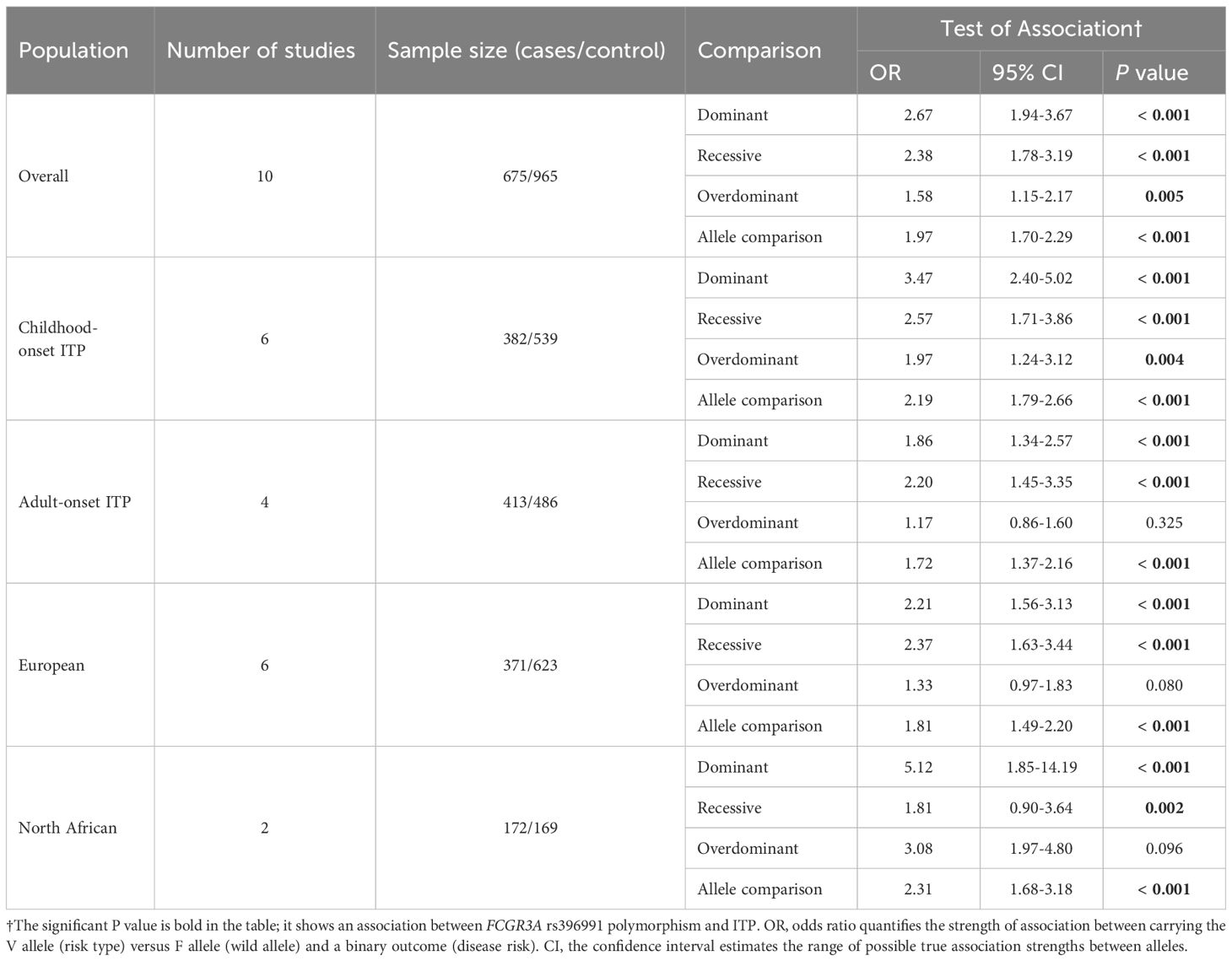
The FCGR3A F158V (rs396991) polymorphism demonstrated a statistically significant association with ITP susceptibility in the overall population analysis, across all four genetic models tested: dominant (OR = 2.67, 95% CI 1.94–3.67, P < 0.001, FV + VV vs. FF), recessive (OR = 2.38, 95% CI 1.78–3.19, P < 0.001, VV vs. FF + FV), overdominant (OR = 1.58, 95% CI 1.15-2.17, P = 0.005, FV vs. FF + VV). Furthermore, homozygotes for the V158 allele (VV) showed an increased risk compared to carriers of the F158 allele, in allele comparison (OR = 1.97, 95% CI 1.70-2.29, P < 0.001, V vs. F) (Table 3, Supplementary Table S5).
In the childhood-onset ITP subgroup, significant associations were also detected in all four genetic models, consistent with the overall analysis. In the European population subgroup, for adult-onset ITP we observed significant associations in all models except the overdominant model (Table 3, Supplementary Table S5).
We performed further comprehensive analyses of diverse autoimmune populations (ITP, SLE, RA, GBS and CD), identical to those performed for FCGR2A rs1801274 (A>G), as detailed in Supplementary Tables S5-S8.
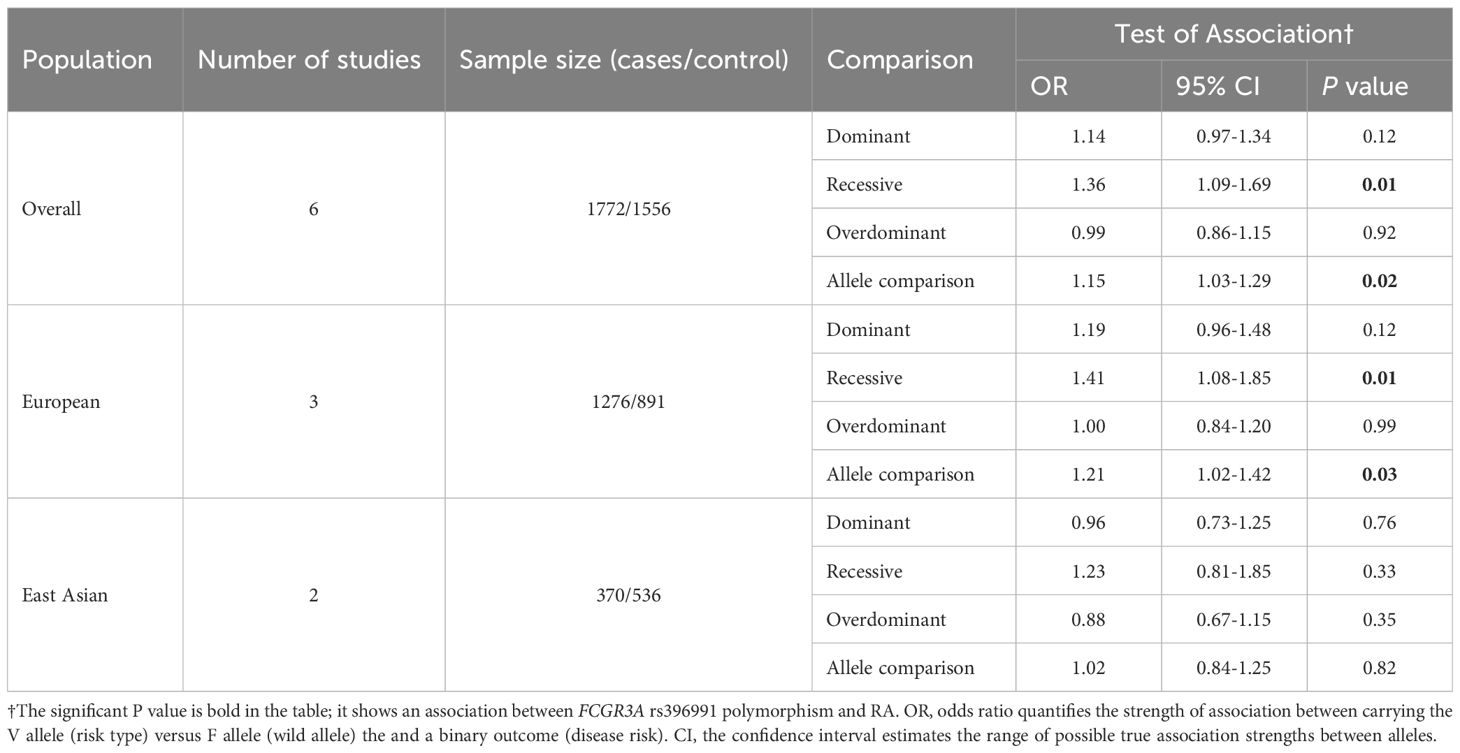
A statistically significant association between the FCGR3A F158V polymorphism and RA was identified in both the recessive model (OR = 1.36, 95% CI 1.09-1.69, P = 0.01, VV vs. FF + FV) and allele comparison (OR = 1.15, 95% CI 1.03-1.29, P = 0.02, V vs. F) (Table 4, Supplementary Table S7). Subgroup analyses by European ancestry yielded consistent positive results for both genetic models, in line with the overall analysis. No significant associations were found for the East Asian subgroup.
Across all analyses, a consistent relationship between the FCGR3A rs396991 polymorphism and the occurrence of both ITP and RA was observed (Tables 3, 4, Supplementary Tables S5, S7). This polymorphism appeared to afford protection against SLE in the European subgroup (Supplementary Table S6). The association between FCGR3A rs396991 and susceptibility to several autoimmune diseases was confirmed in an analysis of the European population (Tables 3, 4, Supplementary Tables S5, S8), but further studies with greater statistical power are needed to confirm these results.
3.4 Secondary analysis
3.4.1 FCGR2A
In the secondary analysis, we split all available studies on FCGR2A rs1801274 into subgroups based on their proband ethnicity. By aggregating data from multiple studies, the meta-analysis (Figure 3) provides a more robust estimate of the genetic effect of FCGR2A rs1801274 on autoimmune disease susceptibility.
The combined meta-analysis showed no overall significant association, but subgroup analyses identified significant associations between general autoimmune disease and the H131R polymorphism in North Africans (allelic comparisons, OR = 1.39, 95% CI 0.84-2.29, P < 0.01, R vs. H), and East Asians (allelic comparisons, OR = 1.02, 95% CI 0.87-1.20, P = 0.04, R vs. H) (Figure 3, Supplementary Table S9). Pooled odds ratios (ORs) from allelic comparisons were calculated using a random-effects meta-analysis model (25) for all diseases associated with FCGR2A (rs1801274).
3.4.2 FCGR3A
We applied an analogous analytical strategy to that used for FCGR2A polymorphism, as illustrated in Figure 4, to synthesize evidence from all studies. By combining findings from different populations, the analysis reduces the impact of insufficient statistical power in any one investigation and allows for a broader perspective on the role of FCGR3A in autoimmune disease susceptibility. The meta-analysis framework thus provides a more nuanced understanding of how this genetic F158V variant may influence disease risk across diverse autoimmune diseases.
The aim was to determine whether this genetic variant is linked to overall susceptibility to autoimmune diseases, or whether it is notable in a specific subgroup. The results show a significant association of allelic comparisons (OR = 1.29, 95% CI 1.12-1.48, P < 0.01, V vs. F, see Figure 4, Supplementary Table S10), supporting our initial hypothesis and confirming the findings from the above primary meta-analysis of ITP and RA cases. The pooled odds ratio was calculated using a random-effects model (25) based on allelic comparisons for all diseases associated with the FCGR3A rs396991 polymorphism. Notably, this association was particularly evident in the European subgroup (OR = 1.23, 95% CI 1.03-1.47, P < 0.01, V vs. F).
3.5 Sensitivity analysis
The pooled results remained unaltered in all comparisons, which suggests that our findings are statistically stable. The results were confirmed with three independent software applications.
3.6 Publication bias
The potential for publication bias was evaluated by Egger’s linear regression test, and a P value ≤ 0.05 was considered indicative of statistical publication bias (26). We cannot exclude the possibility of publication bias affecting our pooled estimates (see also Discussion and Limitations sections).
4 Discussion
The symptoms of ADs are wide-ranging, which can make diagnosis difficult, particularly in the early stages. In addition to environmental influences, genetic predisposition also plays an important role in the development and progression of diseases. Advances in genetic analysis have helped to identify genetic contributions, the genes themselves, and their polymorphisms, such as the polymorphisms in Fcγ receptors (FcγRs) examined in this study, in particular FCGR2A (rs1801274) and FCGR3A (rs396991). These genetic variants crucially modulate the handling of immune complexes (ICs) and inflammatory responses, thereby influencing susceptibility to autoimmune and inflammatory diseases.
In this systematic review with meta-analysis, we have analyzed two single-nucleotide polymorphisms (SNPs), FCGR2A (rs1801274) and FCGR3A (rs396991), which have been associated with ITP, SLE and RA in the past two decades. Due to the limited number of available studies on GBS and CD, no association was observed with those diseases in any of the four genetic models tested.
4.1 FCGR2A
The FCGR2A rs1801274 polymorphism is due to an A-to-G nucleotide exchange at coding nucleotide c.500 (NM_001136219.3), that encodes either histidine (H) or arginine (R) at amino acid position p.131 in the FcγRIIa receptor protein (62). This single amino acid substitution significantly influences the receptor’s binding affinity for IC handling: the H131 variant binds IgG2 and IgG3 with much higher affinity than R131 due to optimized electrostatic interactions with the Fc region (48). The reduced IgG2 affinity in R131 carriers specifically impairs neutrophil phagocytosis of IgG2-opsonized targets, while responses to IgG1/IgG3/IgG4 remain intact (63). This diminished IgG2 binding compromises the clearance of IgG2 immune complexes, allowing their deposition in tissues such as renal glomeruli and dermal vasculature (64, 65), which in turn promotes inflammation through FcγRIIa-mediated platelet activation and upregulation of endothelial adhesion molecules. These mechanisms contribute to thrombotic complications and accelerate atherosclerosis, particularly in SLE (66).
In addition, the R131 variant’s reduced IgG2 binding capacity appears to suppress neutrophil activation and matrix metalloproteinase (MMPs) release (67) which may help explain its association with reduced progression in RA despite its pro-inflammatory implications. This protective linkage was confirmed in our meta-analysis between the R131 polymorphism and RA in the overall population, suggesting a disease-specific influence on autoimmune pathogenesis.
The H131 variant demonstrates heightened binding affinity for IgG2 immune complexes (ICs) (68), facilitating efficient phagocytic clearance by neutrophils and macrophages. This increased efficiency not only enhances phagocytosis, neutrophil activation (69, 70), and IL-1β secretion (71), but also supports a more robust inflammatory response to IgG2 ICs. This enhanced clearance can trigger complement cascades and the production of pro-inflammatory cytokines, such as TNF-α and IL-6 (72). It can also promote sustained immune activation, driving chronic inflammation and the generation of autoantibodies — key pathogenic features of SLE, RA and multiple sclerosis (MS) (73). When this immune activation persists, the incomplete clearance of immune complexes (ICs) can lead to complications: these complexes may then accumulate in tissues such as the renal glomeruli and synovium (74), where complement-mediated lysis and cytokine-driven pathways can further exacerbate tissue injury and inflammation (75).
The higher prevalence of the H131 allele in Asian populations (76) as observed in our East Asian subgroup analysis by ethnicity, correlates with increased susceptibility to SLE (43) (Supplementary Table S2).
Therefore, the functional impact of the H131R polymorphism is context-dependent, modulating both immune defense and the risk of autoimmune tissue injury (Table 5).
4.2 FCGR3A
The F158V polymorphism arises from a T-to-G substitution at coding nucleotide c.526 (NM_000569.8), substituting phenylalanine (F158) with valine (V158) in the receptor’s extracellular domain (77). The V158 variant increases binding affinity for IgG1/IgG3 by ~5-fold compared to F158 (78), likely due to valine’s smaller side chain reducing steric hindrance and optimizing hydrophobic interactions with the Fc region. The high-affinity V158 variant enhances antibody-dependent cellular cytotoxicity (ADCC) by strengthening interactions between FcγRIIIa and the Fc region of IgG1/IgG3 (79, 80) which affects neutrophil activation and effector functions, including phagocytosis and reactive oxygen species production. Functionally, natural killer (NK) cells from individuals homozygous for FcγRIIIa 158V display increased calcium influx, elevated CD25 expression, and accelerated apoptosis relative to those from FcγRIIIa 158F homozygotes, reflecting a more robust activation profile. Concurrently, FcγRIIIa 158V enhances antibody-dependent cellular cytotoxicity (ADCC) in NK cells by stabilizing FcγRIIIa engagement with IgG1/IgG3-opsonized targets (81), driving perforin/granzyme polarization inducing apoptosis.
In SLE, the V158 variant’s enhanced IgG1/IgG3 binding is hypothesised to exacerbate neutrophil extracellular trap (NET) formation and renal IC deposition (82), accelerating lupus nephritis (LN) (83). Contradictorily, the same mechanism may improve clearance of apoptotic debris, reducing autoantigen persistence and dampening chronic inflammation. This duality underscores the context-dependent influence of FcγRIIIa polymorphisms. In clinical settings, the V158 variant’s heightened signalling capacity augments the efficacy of anti-CD20 therapies such as rituximab (84), as pronounced FcγRIIIa clustering on natural killer cells facilitates more effective B-cell depletion and enhances therapeutic outcomes (79, 80). Consequently, the V158 variant is associated with superior B-cell depletion and improved clinical responses to anti-CD20 treatment. Table 6 summarizes the functional implications of the F158V polymorphism.
4.3 FcγRIIa H131R and FcγRIIIa F158V: a putative synergistic interplay
Accumulating evidence from our study and others highlights both the H131R and F158V polymorphisms as key modulators of immune regulation, although their direct mechanistic roles in autoimmune pathogenesis remains incompletely resolved (81, 85). As outlined in the report, both FcγRIIa and FcγRIIIa are characterized by co-dominantly expressed allelic variants that modulate their ligand-binding affinities, thereby shaping the magnitude and quality of cellular responses to ICs (85, 86). Structural data show that both polymorphisms are located directly within the binding interface between the receptor ectodomains and the IgG Fc regions (87, 88) (Figure 5).
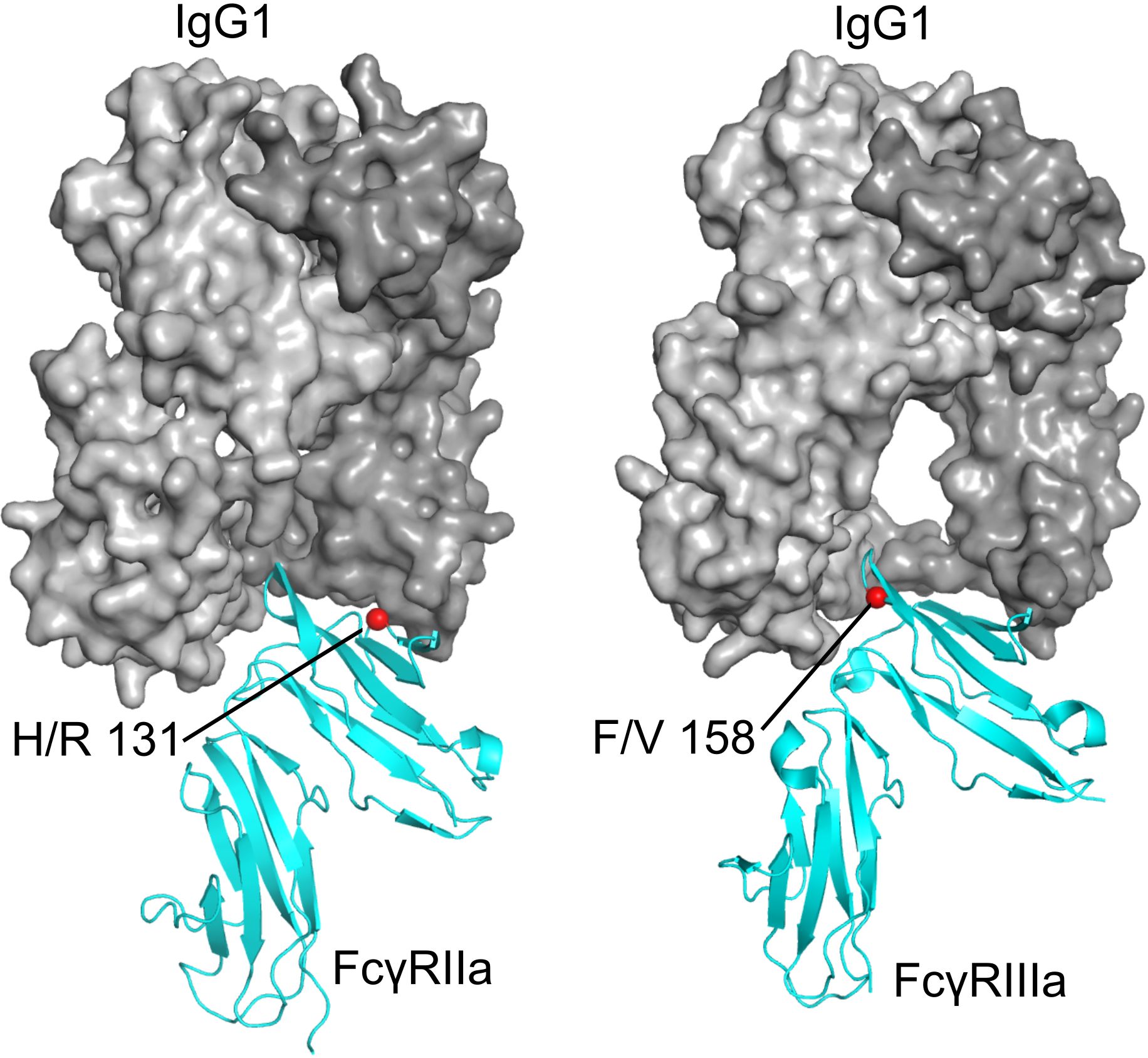
Figure 5. Positions of the FcγR polymorphisms investigated in this study. Left: FcγRIIa (cyan) bound to a human IgG1 Fc fragment (gray), with H/R 131 indicated by a red sphere (PDB ID 3RY4, 3RY6, PDB corresponding residue number 134) (87). Right: FcγRIIIa (cyan) bound to a human IgG1 Fc fragment (gray), with F/V 158 indicated by a red sphere (PDB ID 3SGJ) (88).
The presence of functionally distinct polymorphisms in these receptors can synergistically alter effector cell activation, cytokine production, and the balance between IC clearance and inflammation. The combined inheritance of FCGR2A H131R and FCGR3A F158V polymorphisms may establish a “dual-hit” scenario, wherein compromised IC clearance due to FcγRIIa dysfunction is compounded by augmented FcγRIIIa-mediated inflammatory signaling. This interplay could amplify chronic immune activation, thereby accelerating tissue injury and promoting the progression of autoimmune diseases such as SLE and RA (Table 7).
Reports on the pathogenic versus protective roles of these variants are conflicting. For instance, FCGR3A F158V has been linked to increased ITP and RA severity. In contrast, the FCGR2A H131R polymorphism has been identified as a risk factor for SLE. However, the present study only replicated this finding in the East Asian subgroup, and no clear effect on susceptibility for LN (89) was observed. The results suggest that the effect may vary between populations. The discrepancies highlight the complexity of Fcγ receptor biology and the influence of genetic background, environmental factors, and disease context.
Resolving these inconsistencies requires functional studies that elucidate how these polymorphisms collectively alter immune cell signalling networks, particularly within myeloid lineages such as neutrophils and macrophages. Future research should map the effects of FCGR2A and FCGR3A variants on IC handling, neutrophil activation, and B-cell tolerance. Such strategies, aligned with advances in genetics and immunology, will be essential to clarify these genetic associations and identify potential therapeutic targets in autoimmune disease.
4.4 Limitations
Several limitations of the present meta-analysis warrant consideration. The limited number of included studies for certain autoimmune diseases reduces the statistical power, increasing the risk of false-negative results. Variations in population characteristics, diagnostic criteria, reliability of the data, and genotyping methods may further limit comparability and applicability. Overall, heterogeneity was substantial, leading us to consistently apply random-effects models throughout our analyses. Random-effects models are the preferred method when heterogeneity is present between original studies, as they account for both within-study and between-study variance. Moreover, Egger’s linear regression test (P ≤ 0.05 threshold) identified publication bias across all comparisons, which may have influenced our outcome. Additionally, our analysis did not adjust for potential confounding variables such as sex, age, or environmental factors. These limitations indicate the requirement for future large-scale, well-controlled studies in diverse populations to confirm and extend our findings.
5 Conclusion
In summary, this meta-analysis found FCGR3A V158 to be associated with an increased susceptibility to two autoimmune diseases, namely immune thrombocytopenia (ITP) and rheumatoid arthritis (RA). However, the functional impact of the FCGR3A V158 polymorphism likely accounts for only a portion of the pathogenesis in both ITP and RA. Furthermore, our findings imply that the FCGR2A R131 allele may protect against RA, indicating a negative correlation with disease risk.
Additionally, the results suggest that both the FCGR2A (rs1801274) and FCGR3A (rs396991) polymorphisms may be associated with susceptibility to various autoimmune diseases in both European and East Asian populations. Future studies should use larger, well-designed case-control cohorts to improve statistical power and refine estimates of the effects of individual genes on the development of autoimmune diseases.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar (https://scholar.google.com/), Cochrane Library (https://www.cochranelibrary.com/), Science.gov (https://www.science.gov/).
Author contributions
ET: Visualization, Investigation, Writing – original draft, Conceptualization, Validation, Project administration, Writing – review & editing, Formal analysis. MB: Writing – review & editing, Investigation, Supervision, Visualization. MW: Writing – review & editing, Visualization, Supervision, Funding acquisition, Validation. CG: Funding acquisition, Writing – review & editing, Conceptualization, Supervision. HU: Conceptualization, Validation, Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, and/or publication of this article. We acknowledge the financial support provided to the Institute of Translational Medicine at the Private University in the Principality of Liechtenstein by the Hans Groeber-Stiftung (Vaduz, Principality of Liechtenstein) and the Tarom Foundation (Schaan, Principality of Liechtenstein) and funding of the project “Genetic Architecture of the FCGR2/3 Locus” by the Maiores Foundation (Vaduz, Principality of Liechtenstein).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1661502/full#supplementary-material
References
1. Gutierrez-Arcelus M, Rich SS, and Raychaudhuri S. Autoimmune diseases - connecting risk alleles with molecular traits of the immune system. Nat Rev Genet. (2016) 17:160–74. doi: 10.1038/nrg.2015.33
2. Chen L, Zhao J, and Meng Q. From genetic variants to therapeutic targets: insights into understanding rheumatoid arthritis. Front Immunol. (2025) 16:1556971. doi: 10.3389/fimmu.2025.1556971
3. Kailashiya V, Sharma HB, and Kailashiya J. Role of CTLA4 A49G polymorphism in systemic lupus erythematosus and its geographical distribution. J Clin Pathol. (2019) 72:659–62. doi: 10.1136/jclinpath-2019-206013
4. Garg G, Tyler JR, Yang JH, Cutler AJ, Downes K, Pekalski M, et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol. (2012) 188:4644–53. doi: 10.4049/jimmunol.1100272
5. Delpire B, Van Loon E, and Naesens M. The role of fc gamma receptors in antibody-mediated rejection of kidney transplants. Transpl Int. (2022) 35:10465. doi: 10.3389/ti.2022.10465
6. Zhang C, Wang W, Zhang H, Wei L, and Guo S. Association of FCGR2A rs1801274 polymorphism with susceptibility to autoimmune diseases: A meta-analysis. Oncotarget. (2016) 7:39436–43. doi: 10.18632/oncotarget.9831
7. Frampton S, Smith R, Ferson L, Gibson J, Hollox EJ, Cragg MS, et al. Fc gamma receptors: Their evolution, genomic architecture, genetic variation, and impact on human disease. Immunol Rev. (2024) 328:65–97. doi: 10.1111/imr.13401
8. Daëron M. Fc receptor biology. Annu Rev Immunol. (1997) 15:203–34. doi: 10.1146/annurev.immunol.15.1.203
9. Deng Y and Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. (2010) 6:683–92. doi: 10.1038/nrrheum.2010.176
10. Endeman H, Cornips MC, Grutters JC, van den Bosch JM, Ruven HJT, van Velzen-Blad H, et al. The Fcgamma receptor IIA-R/R131 genotype is associated with severe sepsis in community-acquired pneumonia. Clin Vaccine Immunol. (2009) 16:1087–90. doi: 10.1128/CVI.00037-09
11. Xu Y, Wei HT, Zou JJ, and Ma YR. Association of FcγRIIA-R/H131 polymorphism and systemic lupus erythematosus lupus nephritis risk: A meta-analysis. Int J Rheum Dis. (2020) 23:853–67. doi: 10.1111/1756-185X.13815
12. Amiah MA, Ouattara A, Okou DT, N’Guetta SA, and Yavo W. Polymorphisms in fc gamma receptors and susceptibility to malaria in an endemic population. Front Immunol. (2020) 11:561142. doi: 10.3389/fimmu.2020.561142
13. Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. (2002) 2:580–92. doi: 10.1038/nri856
14. Schiele P, Kolling S, Rosnev S, Junkuhn C, Walter AL, Einem JC, et al. Flow cytometric assessment of fcγRIIIa-V158F polymorphisms and NK cell mediated ADCC revealed reduced NK cell functionality in colorectal cancer patients. In Cells. (2024) 14:(1). doi: 10.3390/cells14010032
15. Kremer PG and Barb AW. The weaker-binding Fc γ receptor IIIa F158 allotype retains sensitivity to N-glycan composition and exhibits a destabilized antibody-binding interface. J Biol Chem. (2022) 298:102329. doi: 10.1016/j.jbc.2022.102329
16. Li G, Gao L, Ma R, Tian W, and Mingzhi L. Associations between FCGR polymorphisms and immune thrombocytopenia: A meta-analysis. Scand J Immunol. (2019) 89:e12758. doi: 10.1111/sji.12758
17. Uittenbogaard P, Netea SA, Tanck MWT, Geissler J, Buda P, Kowalczyk-Domagała M, et al. FCGR2/3 polymorphisms are associated with susceptibility to Kawasaki disease but do not predict intravenous immunoglobulin resistance and coronary artery aneurysms. Front Immunol. (2024) 15:132317. doi: 10.3389/fimmu.2024.323171
18. Niederer HA, Clatworthy MR, Willcocks LC, and Smith KG. FcgammaRIIB, FcgammaRIIIB, and systemic lupus erythematosus. Ann N Y Acad Sci. (2010) 1183:69–88. doi: 10.1111/j.1749-6632.2009.05132.x
19. Zhang L, Liu L, Li H, Guo L, Yu Q, and Teng J. Association of CD1 and FcγR gene polymorphisms with Guillain-Barré syndrome susceptibility: a meta-analysis. Neurol Sci. (2018) 39:2141–9. doi: 10.1007/s10072-018-3563-3
20. Lee YH, Bae SC, and Song GG. FCGR2A, FCGR3A, FCGR3B polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. (2015) 33:647–54.
21. Martorell-Marugan J, Toro-Dominguez D, Alarcon-Riquelme ME, and Carmona-Saez P. MetaGenyo: a web tool for meta-analysis of genetic association studies. BMC Bioinf. (2017) 18:563. doi: 10.1186/s12859-017-1990-4
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Fekete JT and Győrffy B. MetaAnalysisOnline.com: web-based tool for the rapid meta-analysis of clinical and epidemiological studies. J Med Internet Res. (2025) 27:e64016. doi: 10.2196/64016
24. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analysis. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. DerSimonian R and Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
26. Egger M, Davey SG, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Zakaria M, Al-Akhras A, Hassan T, Sherief L, Magdy W, and Raafat N. FcγRIIa and FcγRIIIa genes polymorphism in Egyptian children with primary immune thrombocytopenia. Hematol Transfus Cell Ther. (2023) 45:58–65. doi: 10.1016/j.htct.2021.05.007
29. Pavkovic M, Petlichkovski A, Karanfilski O, Cevreska L, and Stojanovic A. FC gamma receptor polymorphisms in patients with immune thrombocytopenia. Hematol (Amsterdam Netherlands). (2018) 23:163–8. doi: 10.1080/10245332.2017.1377902
30. Audia S, Santegoets K, Laarhoven AG, Vidarsson G, Facy O, Ortega-Deballon P, et al. Fcγ receptor expression on splenic macrophages in adult immune thrombocytopenia. Clin Exp Immunol. (2017) 188:275–82. doi: 10.1111/cei.12935
31. Papagianni A, Economou M, Tragiannidis A, Karatza E, Samarah F, Gombakis N, et al. FcγRIIa and FcγRIIIa polymorphisms in childhood primary immune thrombocytopenia: implications for disease pathogenesis and outcome. Blood Coagulation Fibrinol. (2013) 24:35–9. doi: 10.1097/MBC.0b013e328359bc3b
32. Eyada TK, Farawela HM, Khorshied MM, Shaheen IA, Selim NM, and Khalifa IAS. FcγRIIa and FcγRIIIa genetic polymorphisms in a group of pediatric immune thrombocytopenic purpura in Egypt. Blood Coagulation Fibrinol. (2012) 23:64–8. doi: 10.1097/MBC.0b013e32834ddf2f
33. Amorim DMMC and Silveira VSS. Fcg receptor gene polymorphisms in childhood immune thrombocytopenic purpura. J Pediatr Hematol/Oncol. (2012) 34:349–52. doi: 10.1097/MPH.0b013e3182580908
34. Nourse JP, Lea R, Crooks P, Wright G, Tran H, Catalano J, et al. The KIR2DS2/DL2 genotype is associated with adult persistent/chronic and relapsed immune thrombocytopenia independently of FCGR3a-158 polymorphisms. Blood Coagulation Fibrinol. (2012) 23:45–50. doi: 10.1097/MBC.0b013e32834d7ce3
35. Breunis WB, van Mirre E, Bruin M, Geissler J, Boer M, Peters M, et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. (2008) 111:1029–38. doi: 10.1182/blood-2007-03-079913
36. Bruin M, Bierings M, Uiterwaal C, Révész T, Bode L, Wiesman M-E, et al. Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: results of a prospective study. Br J Haematol. (2004) 127:561–7. doi: 10.1111/j.1365-2141.2004.05235.x
37. Cornwell MG, Bannoudi HE, Luttrell-Williams E, Engel A, Barrett TJ, Myndzar K, et al. Modeling of clinical phenotypes in systemic lupus erythematosus based on the platelet transcriptome and FCGR2a genotype. J Trans Med. (2023) 21:247. doi: 10.1186/s12967-023-04059-w
38. Dhaouadi T, Sfar I, Hassine LB, Turki S, Khalfallah N, and Gorgi TBAY. PTPN22, CTLA4, FcγRIIa, FcγRIIIa and FcγRIIIb polymorphisms in Tunisian patients with systemic lupus erythematosus. Int J Clin Rheumatol. (2019) 14:(1). doi: 10.4172/1758-4272.1000216
39. Tsang-A-Sjoe MWP, Nagelkerke SQ, Bultink IEM, Geissler J, Tanck MWT, Tacke CE, et al. Fc-gamma receptor polymorphisms differentially influence susceptibility to systemic lupus erythematosus and lupus nephritis. Rheumatol (Oxford England). (2016) 55:939–48. doi: 10.1093/rheumatology/kev433
40. Vigato-Ferreira ICC, Toller-Kawahisa JE, Pancoto JAT, Mendes-Junior CT, Martinez EZ, Donadi EA, et al. FcγRIIa and FcγRIIIb polymorphisms and associations with clinical manifestations in systemic lupus erythematosus patients. Autoimmunity. (2014) 47:451–8. doi: 10.3109/08916934.2014.921809
41. Kwon K-S, Cho H-Y, and Chung Y-J. Recapitulation of candidate systemic lupus erythematosus-associated variants in Koreans. Genomics Inf. (2016) 14:85–9. doi: 10.5808/GI.2016.14.3.85
42. Zidan HE, Sabbah NA, Hagrass HA, Tantawy EA, El-Shahawy EE, Nageeb GS, et al. Association of FcγRIIB and FcγRIIA R131H gene polymorphisms with renal involvement in Egyptian systemic lupus erythematosus patients. Mol Biol Rep. (2014) 41:733–9. doi: 10.1007/s11033-013-2912-9
43. Zhou XJ, Lv JC, Qin LX, Yang HZ, Yu F, Zhao MH, et al. Is FCGR2A a susceptibility gene to systemic lupus erythematosus in Chinese? Lupus. (2011) 20:1198–202. doi: 10.1177/0961203311409269
44. Sánchez E, Comeau ME, Freedman BI, Kelly JA, Kaufman KM, Langefeld CD, et al. Identification of novel genetic susceptibility loci in African American lupus patients in a candidate gene association study. Arthritis Rheum. (2011) 63:3493–501. doi: 10.1002/art.30563
45. Jönsen A, Gunnarsson I, Gullstrand B, Svenungsson E, Bengtsson AA, Nived O, et al. Association between SLE nephritis and polymorphic variants of the CRP and FcgammaRIIIa genes. Rheumatol (Oxford England). (2007) 46:1417–21. doi: 10.1093/rheumatology/kem167
46. Jönsen A, Bengtsson AA, Sturfelt G, and Truedsson L. Analysis of HLA DR, HLA DQ, C4A, FcγRIIa, FcγRIIIa, MBL, and IL-1Ra allelic variants in Caucasian systemic lupus erythematosus patients suggests an effect of the combined FcγRIIa R/R and IL-1Ra 2/2 genotypes on disease susceptibility. Arthritis Res Ther. (2004) 6:R557–62. doi: 10.1186/ar1224
47. Magnusson V, Johanneson B, Lima G, Odeberg J, Alarcón-Segovia D, and Alarcón-Riquelme ME. Both risk alleles for FcgammaRIIA and FcgammaRIIIA are susceptibility factors for SLE: a unifying hypothesis. Genes Immun. (2004) 5:130–7. doi: 10.1038/sj.gene.6364052
48. Bazilio AP, Viana VST, Toledo R, Woronik V, Bonfá E, and Monteiro RC. Fc gamma RIIa polymorphism: a susceptibility factor for immune complex-mediated lupus nephritis in Brazilian patients. Nephrol Dial Transplant. (2004) 19:1427–31. doi: 10.1093/ndt/gfh121
49. Chu ZT, Tsuchiya N, Kyogoku C, Ohashi J, Qian YP, Xu SB, et al. Association of Fcγ receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: a common susceptibility gene in the Asian populations. Tissue Antigens. (2004) 63:21–7. doi: 10.1111/j.1399-0039.2004.00142.x
50. Karimifar M, Akbari K, ArefNezhad R, Fathi F, Mousaei Ghasroldasht M, and Motedayyen H. Impacts of FcγRIIB and FcγRIIIA gene polymorphisms on systemic lupus erythematous disease activity index. BMC Res Notes. (2021) 14:455. doi: 10.1186/s13104-021-05868-2
51. Dong C, Ptacek TS, Redden DT, Zhang K, Brown EE, Edberg JC, et al. Fcγ receptor IIIa single-nucleotide polymorphisms and haplotypes affect human IgG binding and are associated with lupus nephritis in African Americans. Arthritis Rheumatol (Hoboken NJ). (2014) 66:1291–9. doi: 10.1002/art.38337
52. Dai M, Zhou Z, Wang X, Qian X, and Huang X. Association of FccRIIIa-158V/F with systemic lupus erythematosus in a Chinese population. Int J Rheum Dis. (2013) 16:685–91. doi: 10.1111/1756-185X.12176
53. Sun Y, Mo L, Feng X, Yang D, Tan T, Zeng L, et al. Association of Fcgamma receptor type 2A and 3A genotypes with rheumatoid arthritis in Chinese population. Pharmacogenomics. (2017) 18:255–64. doi: 10.2217/pgs-2016-0159
54. Meziani R, Yamada R, Takahashi M, Ohigashi K, Morinobu A, Terao C, et al. A trans-ethnic genetic study of rheumatoid arthritis identified FCGR2A as a candidate common risk factor in Japanese and European populations. Mod Rheumatol. (2012) 22:52–8. doi: 10.1007/s10165-011-0467-y
55. Chen J-Y, Wang C-M, Wu J-M, Ho H-H, and Luo S-F. Association of rheumatoid factor production with FcgammaRIIIa polymorphism in Taiwanese rheumatoid arthritis. Clin Exp Immunol. (2006) 144:10–6. doi: 10.1111/j.1365-2249.2006.03021.x
56. Morgan AW, Barrett JH, Griffiths B, Subramanian D, Robinson JI, Keyte VH, et al. Analysis of Fcgamma receptor haplotypes in rheumatoid arthritis: FCGR3A remains a major susceptibility gene at this locus, with an additional contribution from FCGR3B. Arthritis Res Ther. (2006) 8:R5. doi: 10.1186/ar1847
57. Thabet MM, Huizinga TWJ, Marques RB, Stoeken-Rijsbergen G, Bakker AM, Kurreeman FA, et al. Contribution of Fcgamma receptor IIIA gene 158V/F polymorphism and copy number variation to the risk of ACPA-positive rheumatoid arthritis. Ann Rheum Dis. (2009) 68:1775–80. doi: 10.1136/ard.2008.099309
58. Kastbom A, Ahmadi A, Söderkvist P, and Skogh T. The 158V polymorphism of Fc gamma receptor type IIIA in early rheumatoid arthritis: increased susceptibility and severity in male patients (the Swedish TIRA project). Rheumatol (Oxford England). (2005) 44:1294–8. doi: 10.1093/rheumatology/kei010
59. Hayat S, Babu G, Das A, Howlader ZH, Mahmud I, and Islam Z. Fc-gamma IIIa-V158F receptor polymorphism contributes to the severity of Guillain-Barré syndrome. Ann Clin Trans Neurol. (2020) 7:1040–9. doi: 10.1002/acn3.51072
60. Dourado ME, Ferreira LC, Freire-Neto FP, and Jeronimo SMB. No association between FCGR2A and FCGR3A polymorphisms in Guillain-Barré Syndrome in a Brazilian population. J Neuroimmunol. (2016) 298:160–4. doi: 10.1016/j.jneuroim.2016.07.020
61. Sareneva I, Koskinen LLE, Korponay-Szabo IR, Kaukinen K, Kurppa K, Ziberna F, et al. Linkage and association study of FcgammaR polymorphisms in celiac disease. Tissue Antigens. (2009) 73:54–8. doi: 10.1111/j.1399
62. Forthal DN and Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. (2009) 4:388–93. doi: 10.1097/COH.0b013e32832f0a89
63. Tebo AE, Kremsner PG, and Luty AJ. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin Exp Immunol. (2002) 130:300–6. doi: 10.1046/j.1365-2249.2002.01972.x
64. Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, et al. Endocytosis of soluble immune complexes leads to their clearance by FcγRIIIB but induces neutrophil extracellular traps via FcγRIIA in vivo. Blood. (2012) 120:4421–31. doi: 10.1182/blood-2011-12-401133
66. Clancy R, El Bannoudi H, Rasmussen SE, Bornkamp N, Allen N, Dann R, et al. Human low-affinity IgG receptor FcγRIIA polymorphism H131R associates with subclinical atherosclerosis and increased platelet activity in systemic lupus erythematosus. J Thromb Haemost. (2019) 17:532–7. doi: 10.1111/jth.14385
67. Gearing AJ, Thorpe SJ, Miller K, Mangan M, Varley PG, Dudeon T, et al. Selective cleavage of human IgG by the matrix metalloproteinases, matrilysin and stromelysin. Immunol Lett. (2002) 81:41–8. doi: 10.1016/s0165-2478(01)00333-9
68. Delidakis G, Kim JE, George K, and Georgiou G. Improving antibody therapeutics by manipulating the fc domain: immunological and structural considerations. Annu Rev BioMed Eng. (2022) 24:249–74. doi: 10.1146/annurev-bioeng-082721-024500
69. Bredius RG, Fijen CA, De Haas M, Kuijper EJ, Weening RS, Van de Winkel JG, et al. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology. (1994) 83:624–30.
70. Nicu EA, van der Velden U, Everts V, Van Winkelhoff AJ, Roos D, and Loos BG. Hyper-reactive PMNs in FcgammaRIIa 131 H/H genotype periodontitis patients. J Clin Periodontol. (2007) 34:938–45. doi: 10.1111/j.1600-051X.2007.01136.x
71. Yamamoto K, Kobayashi T, Sugita N, Tai H, and Yoshie H. The FcgammaRIIa polymorphism influences production of interleukin-1 by mononuclear cells. Int J Immunogenet. (2007) 34:369–72. doi: 10.1111/j.1744-313X.2007.00701.x
72. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
73. Bhol NK, Bhanjadeo MM, Singh AK, Dash UC, Ojha RR, Majhi S, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. BioMed Pharmacother. (2024) 178:117177. doi: 10.1016/j.biopha.2024.117177
74. Melki I, Allaeys I, Tessandier N, Mailhot B, Cloutier N, Campbell RA, et al. FcγRIIA expression accelerates nephritis and increases platelet activation in systemic lupus erythematosus. Blood. (2020) 136:2933–45. doi: 10.1182/blood.2020004974
75. Elbagir S, Sohrabian A, Elshafie AI, Elagib EM, Mohammed NEA, Nur MAM, et al. Accumulation of antinuclear associated antibodies in circulating immune complexes is more prominent in SLE patients from Sudan than Sweden. Sci Rep. (2020) 10:21126. doi: 10.1038/s41598-020-78213-5
76. Osborne JM, Chacko GW, Brandt JT, and Anderson CL. Ethnic variation in frequency of an allelic polymorphism of human Fc gamma RIIA determined with allele specific oligonucleotide probes. J Immunol Methods. (1994) 173:207–17. doi: 10.1016/0022-1759(94)90299-2
77. Abdel-Wahed MA, Sabbour GS, Hamed AI, E.l Kady MS, Mohammed SK, and Shaaban MAAM. FCGR3A V158F gene polymorphism and trastuzumab response in HER2-positive breast cancer patients. Sci Rep. (2024) 14:26037. doi: 10.1038/s41598-024-76024-6
78. Mahaweni NM, Olieslagers TI, Rivas IO, Molenbroeck SJJ, Groeneweg M, Bos GMJ, et al. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci Rep. (2018) 8:15983. doi: 10.1038/s41598-018-34258-1
79. Stewart R, Thom G, Levens M, Güler-Gane G, Holgate R, Rudd PM, et al. A variant human IgG1-Fc mediates improved ADCC. Protein Eng Des Sel. (2011) 24:671–8. doi: 10.1093/protein/gzr015
80. Vidarsson G, Dekkers G, and Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. (2014) 5:520. doi: 10.3389/fimmu.2014.00520
81. Li R, Peng H, Chen G-M, Feng C-C, Zhang Y-J, Wen P-F, et al. Association of FCGR2A-R/H131 polymorphism with susceptibility to systemic lupus erythematosus among Asian population: a meta-analysis of 20 studies. Arch Dermatol Res. (2014) 306:781–91. doi: 10.1007/s00403-014-1483-5
82. Yu Y and Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. (2013) 4:139. doi: 10.4172/2155-9899.1000139
83. Zhao Y, Wei W, and Liu ML. Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications. J Autoimmun. (2020) 115:102540. doi: 10.1016/j.jaut.2020.102540
84. Nath N, Godat B, Flemming R, and Urh MA. Homogeneous bioluminescent immunoassay for parallel characterization of binding between a panel of antibodies and a family of Fcγ receptors. Sci Rep. (2022) 12:12185. doi: 10.1038/s41598-022-15887-z
85. Bournazos S and Ravetch JV. Fcγ Receptor function and the design of vaccination strategies. Immunity. (2017) 47:224–33. doi: 10.1016/j.immuni.2017.07.009
86. Vedeler CA, Myhr KM, and Nyland H. Fc receptors for immunoglobulin G–a role in the pathogenesis of Guillain-Barré syndrome and multiple sclerosis. J Neuroimmunol. (2001) 118:187–93. doi: 10.1016/s0165-5728(01)00344-7
87. Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, et al. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol. (2011) 187:3208–17. doi: 10.4049/jimmunol.1101467
88. Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. (2011) 108:12669–74. doi: 10.1073/pnas.1108455108
Keywords: FCGR2A, FCGR3A, single nucleotide polymorphism, genetic variants, autoimmune diseases, meta-analysis, genetic association, Fc gamma receptor
Citation: Thaler E, Bublitz M, Wipplinger M, Gassner C and Ulmer H (2025) Association of FCGR2A rs1801274 and FCGR3A rs396991 polymorphisms with various autoimmune diseases: a meta-analysis. Front. Immunol. 16:1661502. doi: 10.3389/fimmu.2025.1661502
Received: 07 July 2025; Accepted: 11 August 2025;
Published: 10 September 2025.
Edited by:
Vita Golubovskaya, ProMab Biotechnologies, United StatesReviewed by:
Zheng Yuan, China Academy of Chinese Medical Sciences, ChinaLisie L. Patnayak, All India Institute of Medical Sciences Raipur, India
Copyright © 2025 Thaler, Bublitz, Wipplinger, Gassner and Ulmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Thaler, ZWxlbmEudGhhbGVyQHVmbC5saQ==
†These authors share senior authorship
 Elena Thaler
Elena Thaler Maike Bublitz
Maike Bublitz Martin Wipplinger
Martin Wipplinger Christoph Gassner
Christoph Gassner Hanno Ulmer
Hanno Ulmer