- Department of Dermatology, China-Japan Union Hospital of Jilin University, Changchun, Jilin, China
Background: Subungual amelanotic melanoma (SAM) poses significant diagnostic challenges due to its rarity and nonspecific clinical manifestations, such as nail dystrophy or indurated plaque.
Case presentation: We present the case of a 60-year-old woman with a three-year history of recurrent serous drainage and persistent pain in her left middle finger following an initial crush injury. Over a period of two years, she underwent three nail avulsion procedures, received systemic antibiotic therapy, and was treated with topical Chinese herbal therapies under a presumptive diagnosis of “chronic onychia following trauma” at a local hospital. Additionally, PET-CT imaging demonstrated localized inflammatory changes without evidence of neoplastic disease. Despite these interventions, the lesion remained refractory to treatment. A thorough reevaluation conducted by our department, incorporating histopathological and immunohistochemical analyses, ultimately confirmed the diagnosis of SAM.
Conclusions: This case underscores the importance of maintaining a high index of suspicion for SAM when evaluating atypical nail lesions. A low threshold for nail biopsy in cases of prolonged swelling and exudation of a single nail is advised. Additionally, prior trauma to the nail may contribute to the development of SAM through post-traumatic immunosuppression and persistent low-grade chronic inflammation. However, the exact role of trauma in the pathogenesis of melanoma remains unclear and requires further investigation.
Introduction
Amelanotic melanoma (AM) is a rare subtype of melanoma with little or no pigment on visual or histopathologic examination (1). Although AM represents 2% of all malignant melanoma cases, it presents with a greater Breslow depth, higher mitotic rate, more frequent ulceration, higher tumor stage, and lower survival rates than pigmented melanoma (2). Notably, About 25%-33% of subungual melanoma present as amelanotic lesions (3). Subungual amelanotic melanoma (SAM) manifests as a non-pigmented erythematous nodule arising from the nail bed, most commonly affecting the great toe and thumb (4). It often mimic conditions such as paronychias, pyogenic granulomas, hemangiomas, chronic infections, or squamous cell carcinomas (5). Established criteria for the clinical diagnosis of SAM are lacking, often leads to a delay in diagnosis. The combination of its rarity and nonspecific clinical manifestations contributes to an average diagnostic delay. Due to this delay, SAM is typically identified at an advanced stage, resulting in disease progression, and a poor prognosis with challenging treatment options (6).
We report the case of a 60-year-old Chinese woman with SAM on the left middle finger, who experienced a three-year history of recurrent serous drainage and persistent pain of her left middle finger following an initial crush injury. Over a period of two years, she underwent three nail avulsion procedures, received systemic antibiotic therapy, and was treated with topical Chinese herbal therapies under a presumptive diagnosis of “chronic onychia following trauma” at a local hospital. Additionally, PET-CT imaging demonstrated localized inflammatory changes without evidence of neoplastic disease. Despite these interventions, the lesion remained refractory to treatment. A thorough reevaluation conducted by our department, incorporating histopathological and immunohistochemical analyses, ultimately confirmed the diagnosis of SAM The patient’s history of trauma, along with the atypical clinical presentation significantly increase the likelihood of misdiagnosis, further underscoring the the diagnostic challenges inherent in SAM.
Case presentation
The patient was a 60-year-old Chinese female who presented to our dermatology department with a three-year history of recurrent swelling and exudation of the left middle finger. The condition originated three years earlier when the nail sustained trauma due to a door closure incident, after which it appeared normal initially. Approximately two and a half years prior, purulent discharge began to exude from the nail. The patient had attempted self-management using a topical Chinese herbal formulation known as Yunnan Baiyao for one month, but experienced no significant improvement. Subsequently, she sought care at the Hand and Foot Surgery Department of Hospital A and was diagnosed with chronic onychia following nail trauma. Over a period of two years, she underwent three nail avulsions, was treated with local red light therapy and received systemic antibiotic therapy. Specifically, following the first avulsion, she was prescribed cefuroxime axetil 500 mg twice daily for five days. After the second avulsion, she was treated with moxifloxacin 400 mg once daily for seven days. However, no significant clinical improvement was observed. Following the last avulsion, the nail failed to regrow, and the surgical wound did not heal properly, resulting in a persistent, painless wound on the nail bed of her left middle finger. Seeking further evaluation and treatment, the patient consulted the Hand and Foot Surgery Department at the China-Japan Union Hospital of Jilin University. Onychomycosis was suspected by the attending physician, and the patient was referred to our department for further assessment. During the disease course, the patient denied any systemic symptoms (e.g., fatigue, weight loss) and reported no history of infectious diseases, relevant family history, or personal history of cancer.
On physical examination, the left middle finger exhibited complete absence of the nail plate, accompanied by an irregularly swollen nail bed with serous exudation and crusting. And the margins of the skin lesion demonstrated signs of infiltration (Figure 1). Palpation revealed a moderately firm texture with mild tenderness. Black pigmentation of the adjacent nail fold, known as Hutchinson’s sign, is often considered a diagnostic indicator; however, in this patient, Hutchinson’s sign was negative. No abnormalities were noted in the remaining skin areas upon examination. There was no palpable enlarged superficial lymph nodes throughout the body.
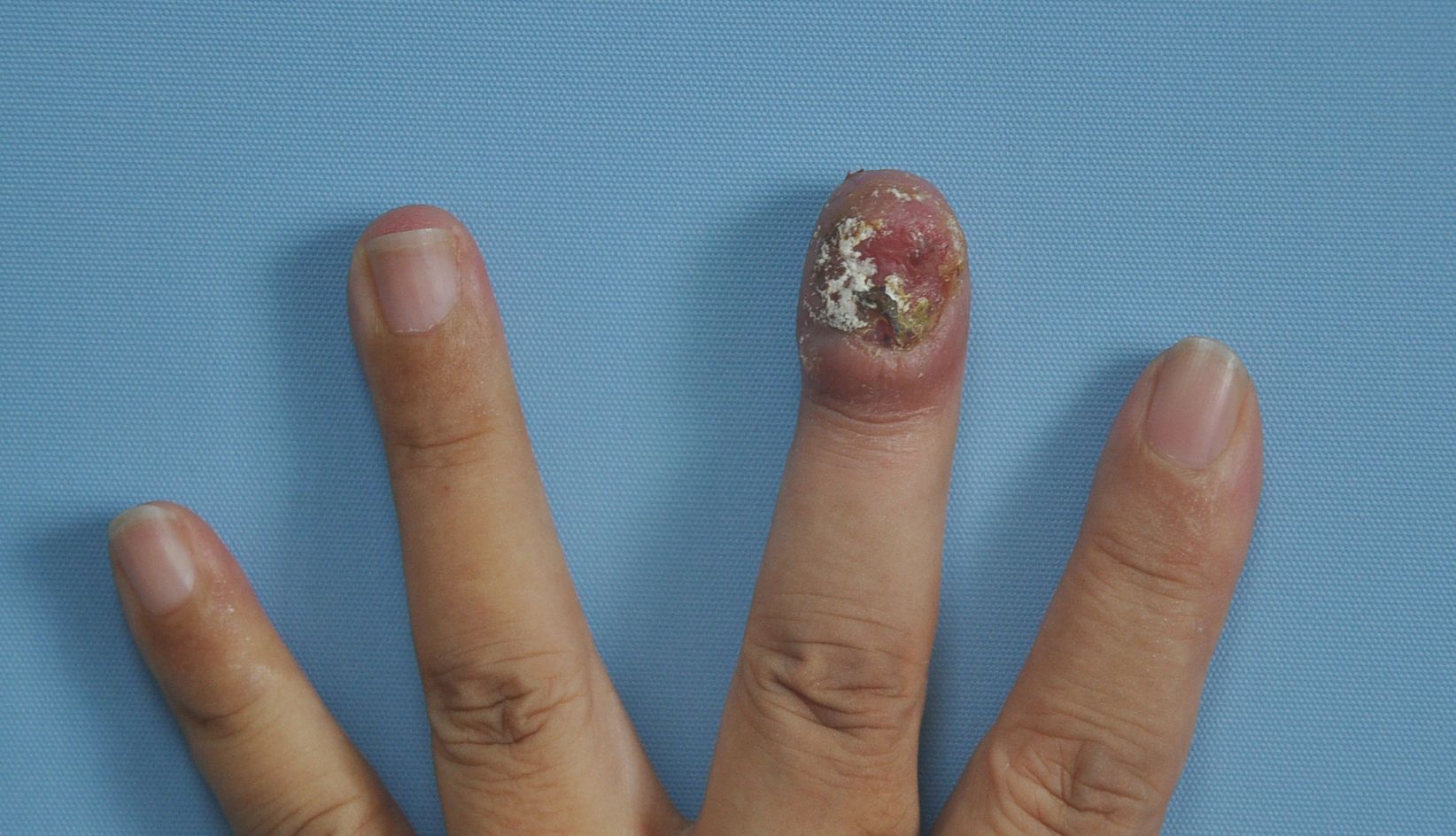
Figure 1. Images of the lesion on the left middle finger. It exhibited complete absence of the nail plate, accompanied by an irregularly swollen nail bed with serous exudation and crusting. And the margins of the skin lesion demonstrated signs of infiltration.
We conducted relevant laboratory and auxiliary tests. Fungal microscopy and culture were conducted, which came back negative. Given the potential for skin tumors, we advised the patient to undergo a histopathological examination, but the patient refused. Based on this, we alternatively proposed a Positron Emission Tomography-Computed Tomography (PET-CT) scan as a less invasive diagnostic approach. It demonstrated soft tissue thickening and mild glucose hypermetabolism (SUV max 2.23) around the distal phalanx of the left middle finger, indicative of inflammatory changes (Figure 2). However, local and systemic anti-inflammatory treatments have shown no improvement. Pathological examination was recommended to rule out neoplastic recurrence. Subsequently, a nail bed biopsy was performed and histopathological examination revealed nests of tumor cells at the dermoepidermal junction, exhibiting marked atypia, deep nuclear staining, and transparent cytoplasm. Additionally, Pagetoid spreading of tumor cells was observed within the epidermis (Figures 3A, B). Immunohistochemical analysis confirmed melanocytic differentiation (positive for S100, HMB45, and Melan-A) and excluded epithelial malignancy (negative for CK5/6, CK7, CEA, EMA and Her-2) (Figures 3C-J). The Ki-67 proliferation index was 20%, consistent with aggressive biological behavior (Figures 3K, L). Finally, the patient was definitively diagnosed as SAM.
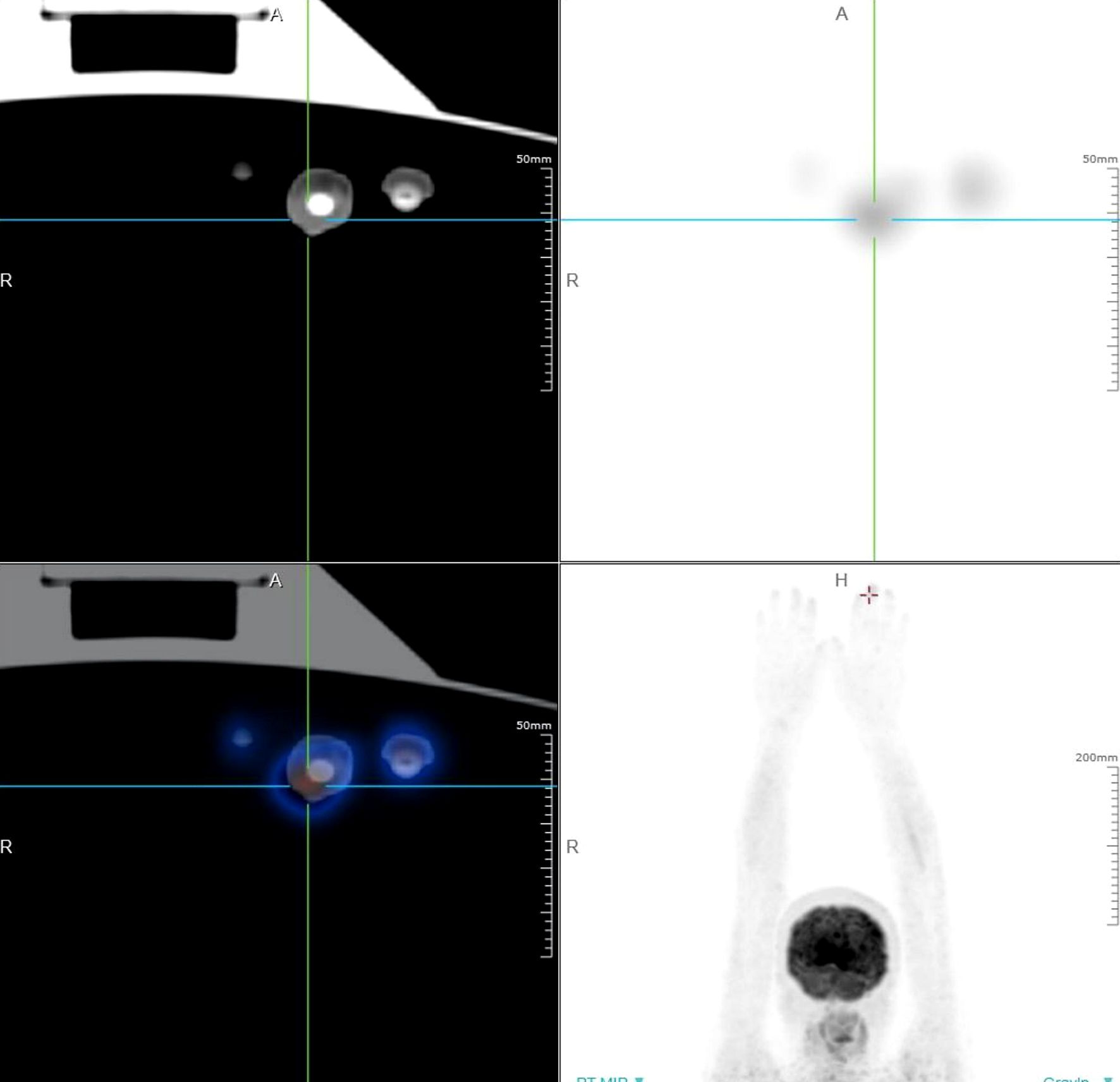
Figure 2. PET-CT scan of the patient with SAM. It demonstrated soft tissue thickening and mild glucose hypermetabolism (SUV max 2.23) around the distal phalanx of the left middle finger, indicative of inflammatory changes.

Figure 3. Histological and immunohistochemical examination of the lesion on the left middle finger. (A) Histopathological examination revealed the presence of tumor cell nests at the dermoepidermal junction, with no evidence of pigmentation. Additionally, pagetoid spread of tumor cells was identified within the epidermis. (B) The tumor cells exhibited marked cellular atypia, intense nuclear staining, and clear cytoplasm. (C-J) Immunohistochemical staining for HMB45 (diffuse +), S100 (diffuse +), Melan-A (+), and CK5/6 (–), CD7 (–), CEA (–), EMA (–) and Her-2 (–). (K, L) The Ki-67 proliferation index was 20%, consistent with aggressive biological behavior.
This case highlights the critical diagnostic challenges in SAM. The patient’s history of trauma, coupled with the presence of swelling and exudation, initially suggested a benign etiology. Importantly, the low clinical suspicion for SAM resulted in repeated misdiagnoses as nail infections and subsequent unnecessary nail avulsions—interventions that may potentially promote tumor dissemination. A definitive diagnosis was established based on histopathological and immunohistochemical analyses. Given the aggressive nature of the tumor and its digital location, which necessitated functional preservation, the patient was referred to the hand surgery department for definitive surgical management following multidisciplinary team evaluation. She subsequently underwent digit amputation. The clinical course of the patient is illustrated in Figure 3.
Discussion
Melanoma, a malignant proliferation of melanocytes, classically manifests as pigmented lesions with heterogeneous coloration such as black, blue, or brown (7). Amelanotic melanoma (AM), a rare type of melanoma that lacks pigmentation, accounts for approximately 2% of all melanoma cases and about 25%-33% is observed in the subungual region (2, 3). AM is typically diagnosed in patients over 50 years of age, which is significantly older than the typical age at diagnosis for pigmented melanoma (8). However, in contrast to adults, approximately 70% of melanomas diagnosed in children are amelanotic (9). Although in general, subungual melanoma is more common in black Africans and Asians, it has been suggested that subungual amelanotic melanoma (SAM) occurs mainly in in White individuals with a predilection for the female gender (10–12).
SAM presents nonspecifically as nonpigmented lesion that originates from the nail bed, most commonly affecting the great toe and thumb (4). It present as pink, red, or flesh-colored papules or may manifest with longitudinal erythronychia (13). Common associated clinical features include onycholysis, notching, splitting, bleeding, or ulceration (14). Black pigmentation of the adjacent nail fold, known as Hutchinson’s sign, is often considered a diagnostic indicator of subungual melanoma. However, Hutchinson’s sign was negative in SAM (6). The clinical presentation of SAM presents a considerable diagnostic challenge, even for experienced dermatologists. SAM requires differentiation from the infectious dermatoses, immune-mediated disorders, other neoplastic conditions (2). Therefore, a delay in the diagnosis of a subungual amelanotic melanoma can occur due to its morphologic resemblance to other benign and malignant nail conditions, such as chronic onychia, onychomycosis, lichen planus and squamous carcinoma (5). Consequently, this often results in delayed diagnosis and, therefore, a worse prognosis compared to other forms of melanoma (15).
For initial screening, noninvasive diagnostic techniques such as dermoscopy are recommended. Dermoscopy enhances the ability to distinguish between benign and malignant nail lesions (16). Characteristic dermoscopic features of amelanotic melanoma include polymorphous vascular structures, such as milky-red areas, hairpin vessels, dotted vessels, and linear-irregular vessels. In addition to these vascular patterns, other dermoscopic criteria include scar-like depigmentation, rims of pigmentary networks, ulceration, and white lines, which represent the most significant nonvascular features of amelanotic melanoma (17). Dermoscopy is particularly valuable in the detection of amelanotic melanoma due to its ability to identify characteristic vascular patterns that compensate for the lack of melanin (18). Modern imaging modalities, such as positron emission tomography-computed tomography (PET-CT), is considered superior to conventional CT and MRI in evaluating amelanotic melanoma, as it enable the detection of primary melanoma approximately six months earlier than traditional imaging methods (19).
Although clinical examination, dermoscopy and PET-CT are valuable tools for identifying suspicious nail abnormalities, a definitive diagnosis of SAM necessitates histopathological evaluation following biopsy (20). Histopathological analysis typically reveals characteristic features of melanoma, including dermal infiltration by atypical melanocytes arranged in cords or nests (5). However, AM demonstrates substantial histopathological and cytological heterogeneity, which necessitates the use of immunohistochemical markers to ensure accurate diagnosis. The most widely utilized immunohistochemical markers include S100, Melan-A, HMB-45, MITF and Ki-67. Among these, S100 exhibits the highest sensitivity, whereas HMB-45, Melan-A and MITF demonstrate greater specificity (21). Notably, the intensity of HMB-45 staining is closely correlated with melanin content, thereby enhancing its specificity (2). Ki-67 serves as a valuable adjunct in distinguishing benign melanocytic proliferations from malignant lesions (22).
In this case, the middle finger lesion was initially diagnosed as chronic onychia following nail trauma at Hospital A. Over a period of two years, she underwent three nail avulsions and received systemic antibiotic therapy. Following the last avulsion, the nail failed to regrow. A comprehensive clinical and histopathological reassessment in our hospital confirmed a diagnosis of SAM. This case highlights the critical diagnostic challenges in SAM. SAM is a rare condition, and delayed diagnosis is frequently observed. One contributing factor to this diagnostic delay is that both patients and some unexperienced clinicians commonly atribute the lesion to traumatic injury as benign lesion (23). Importantly, the low clinical suspicion for SAM resulted in repeated misdiagnoses as nail infections and subsequent unnecessary nail avulsions—interventions that may potentially promote tumor dissemination. Unlike cutaneous melanoma, SAM is not commonly associated with ultraviolet radiation exposure due to the density of the nail plate, which substantially restricts light penetration (8). It has been proposed that trauma may play a role in the development of nail unit melanoma (24). Both acute and chronic trauma have been implicated. Acute trauma includes isolated traumatic events, while chronic trauma encompasses activities such as extensive manual labor, field plowing, and other physically demanding tasks often performed by rural women. Studies have reported that between 23% and 44% of patients recall experiencing trauma prior to the onset of subungual melanoma (25).It remains unclear whether this association arises from post-traumatic immunosuppression contributing to carcinogenesis.
Interestingly, a recent study found trauma to an subungual melanoma to be a a considerable risk factor in the carcinogenesis (24). Following trauma, the immune system triggers a cascade of inflammatory processes at the injury site, which is subsequently followed by a phase of localized inflammation resolution that supports tissue repair and remodeling (26). This localized immune response involves intricate interactions among resident immune cells, including macrophages and dendritic cells, soluble signaling molecules such as cytokines and chemokines, as well as recruited immune cells such as neutrophils, monocytes, and mesenchymal stromal cells (27). When these initial immune responses are sufficiently pronounced, they can lead to systemic effects, resulting in a condition known as post-traumatic immunosuppression (26, 27). Sterile trauma induces alterations in post-traumatic immune responses, potentially leading to a state of immune compromise in affected patients. When trauma is accompanied by infection, it may further modify the immune status (28). Persistent post-traumatic low-grade chronic inflammation within the nail could create a pro-tumorigenic microenvironment: mechanical disruption of the nail bed matrix may recruit macrophages and neutrophils, leading to the release of reactive oxygen species that directly damage melanocyte DNA and participate in cell proliferation (29).
Preliminary data suggests other prognostic indicators for subungual melanoma are similar to cutaneous melanomas (30). Investigations involving larger case series suggest that individuals with amelanotic melanoma often face significantly higher risks of mortality and recurrence, as well as lower 5-year survival and overall survival rates, compared to those with pigmented melanoma (2, 8). However, Moreau et al. found no significant difference in survival between amelanotic melanoma and pigmented melanoma at regional or distant stages (31). Furthermore, a large study reported that amelanosis did not demonstrate any prognostic significance after adjusting for established risk factors, including tumor stage, Breslow thickness, level of invasion, mitotic rate, and ulceration (2). Crucially, population-based research indicates that the worse prognosis associated with amelanotic melanoma stems entirely from more advanced disease stages at diagnosis, rather than from amelanosis itself (8). Therefore, the poorer outcomes observed in amelanotic melanoma are likely attributable to its association with advanced tumor stages, rather than representing an intrinsic risk factor (2, 8).
Conclusion
In summary, SAM is often difficult to diagnose because it is rare and a great masquerader. This case underscores the importance of maintaining a high index of suspicion for SAM when evaluating atypical nail lesions. A low threshold for nail biopsy in cases of persistent prolonged swelling and exudation of a single nail is advised. Additionally, prior trauma to the nail may contribute to the development of SAM through post-traumatic immunosuppression and persistent low-grade chronic inflammation. However, the exact role of trauma in the pathogenesis of melanoma remains unclear and requires further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LA: Conceptualization, Investigation, Writing – original draft. ZL: Software, Formal analysis, Writing – review & editing. YJ: Resources, Visualization, Writing – review & editing. XC: Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The Jilin Provincial Science and Technology Development Program (20250602064RC) and the Bethune project of Jilin University (2024B41).
Acknowledgments
We would like to express our sincere gratitude to YJ for her invaluable contributions to the pathological diagnosis of this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1661698/full#supplementary-material
References
1. Pizzichetta MA, Talamini R, Stanganelli I, Puddu P, Bono R, Argenziano G, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol. (2004) 150:1117–24. doi: 10.1111/j.1365-2133.2004.05928.x
2. Gong HZ, Zheng HY, and Li J. Amelanotic melanoma. Melanoma Res. (2019) 29:221–30. doi: 10.1097/CMR.0000000000000571
3. Marti-Marti I, Pigem R, Narvaez MM, Alós L, and Puig S. Dermoscopy revealing an amelanotic subungual melanoma masked as contact dermatitis. Indian J Dermatol Venereol Leprol. (2021) 88:83–5. doi: 10.25259/IJDVL_1292_20
4. Fang X, Gao T, and Fu Y. Subungual amelanotic melanoma. JAMA Dermatol. (2025) 161:432–3. doi: 10.1001/jamadermatol.2024.6167
5. Cassalia F, Danese A, Cocchi E, Danese E, Ambrogio F, and Cazzato G. Misdiagnosis and clinical insights into acral amelanotic melanoma-A systematic review. J Pers Med. (2024) 14:518. doi: 10.3390/jpm14050518
6. Strazzulla LC, Li X, Zhu K, Okhovat JP, Lee SJ, and Kim CC. Clinicopathologic, misdiagnosis, and survival differences between clinically amelanotic melanomas and pigmented melanomas. J Am Acad Dermatol. (2019) 80:1292–8. doi: 10.1016/j.jaad.2019.01.012
7. Zhang Y, Ostrowski SM, and Fisher DE. Nevi and melanoma. Hematol Oncol Clin North Am. (2024) 38:939–52. doi: 10.1016/j.hoc.2024.05.005
8. Thomas NE, Kricker A, Waxweiler WT, Dillon PM, Busman KJ, From L, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. (2014) 150:1306–14. doi: 10.1001/jamadermatol.2014.1348
9. Cordoro KM, Gupta D, Frieden IJ, McCalmont T, and Kashani-Sabet M. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol. (2013) 68:913–25. doi: 10.1016/j.jaad.2012.12.953
10. Littleton TW, Murray PM, and Baratz ME. Subungual melanoma. Orthop Clin North Am. (2019) 50:357–66. doi: 10.1016/j.ocl.2019.03.003
11. Vernali S, Waxweiler WT, Dillon PM, Kanetsky PA, Orlow I, Luo L, et al. Association of incident amelanotic melanoma with phenotypic characteristics, MC1R status, and prior amelanotic melanoma. JAMA Dermatol. (2017) 153:1026–31. doi: 10.1001/jamadermatol.2017.2444
12. De Giorgi V, Gori A, Savarese I, D’Errico A, Papi F, and Grazzini M. Clinical and dermoscopic features of truly amelanotic plantar melanoma. Melanoma Res. (2017) 27:224–30. doi: 10.1097/CMR.0000000000000337
13. Arellano J, Goldman Y, Oyarzún A, and Espinoza A. Subungual amelanotic melanoma mimicking persistent onychomycosis. Ann Dermatol Venereol. (2025) 152:103333. doi: 10.1016/j.annder.2024.103333
14. Conway J, Bellet JS, Rubin AI, and Lipner SR. Adult and pediatric nail unit melanoma: epidemiology, diagnosis, and treatment. Cells. (2023) 12:964. doi: 10.3390/cells12060964
15. Jiang C, Jain NP, and Stewart CL. Amelanotic melanoma: Clinical presentation, diagnosis, and management. Clin Dermatol. (2025) 43:10–5. doi: 10.1016/j.clindermatol.2025.01.009
16. Lorier Roy E, Gourhant JY, Derancourt C, Jouan N, Dupuy A, Séi JF, et al. Clinicopathological, dermoscopic features and circumstances of diagnosis of amelanotic or hypomelanotic melanoma: A prospective multicentric study in the French private medical sector. Ann Dermatol Venereol. (2024) 151:103249. doi: 10.1016/j.annder.2024.103249
17. Paolino G, Pampena R, Di Ciaccio SM, Carugno A, Cantisani C, Di Nicola MR, et al. Thin amelanotic and hypomelanotic melanoma: clinicopathological and dermoscopic features. Med (Kaunas). (2024) 60:1239. doi: 10.3390/medicina60081239
18. Spadafora M, Megna A, Lippolis N, Cavicchi M, Borsari S, Piana S, et al. Dermoscopy and reflectance confocal microscopy of solitary flat pink lesions: A new combined score to diagnose amelanotic melanoma. J Eur Acad Dermatol Venereol. (2025) 39:109–16. doi: 10.1111/jdv.19991
19. Sultana M, Chatterjee RP, Kundu S, and Mahmud SA. Primary amelanotic Malignant melanoma of parotid and submandibular salivary gland: A rare case report. J Oral Maxillofac Pathol. (2022) 26:263–7. doi: 10.4103/jomfp.jomfp_183_21
20. Long GV, Swetter SM, Menzies AM, Gershenwald JE, and Scolyer RA. Cutaneous melanoma. Lancet. (2023) 402:485–502. doi: 10.1016/S0140-6736(23)00821-8
21. Ohsie SJ, Sarantopoulos GP, Cochran AJ, and Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. (2008) 35:433–44. doi: 10.1111/j.1600-0560.2007.00891.x
22. Kim JC, Choi JW, and Kim YC. Comparison of melanocyte-associated immunohistochemical markers in acral lentiginous melanoma and acral benign nevi. Am J Dermatopathol. (2023) 45:748–52. doi: 10.1097/DAD.0000000000002555
23. Van Demark R Jr, Richardson V, Beeler D, Tessendorf CD, VanBockern B, Durkin V, et al. Delayed diagnosis of subungual acral lentiginous melanoma. S D Med. (2025) 78:120–5.
24. Bormann G, Marsch WC, Haerting J, and Helmbold P. Concomitant traumas influence prognosis in melanomas of the nail apparatus. Br J Dermatol. (2006) 155:76–80. doi: 10.1111/j.1365-2133.2006.07235.x
25. Möhrle M and Häfner HM. Is subungual melanoma related to trauma? Dermatology. (2002) 204:259–61. doi: 10.1159/000063354
26. Islam MN, Bradley BA, and Ceredig R. Sterile post-traumatic immunosuppression. Clin Transl Immunol. (2016) 5:e77. doi: 10.1038/cti.2016.13
27. Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, and Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. (2010) 40:793–808. doi: 10.1007/s00595-010-4323-z
28. Johnson SB, Lissauer M, Bochicchio GV, Moore R, Cross AS, and Scalea TM. Gene expression profiles differentiate between sterile SIRS and early sepsis. Ann Surg. (2007) 245:611–21. doi: 10.1097/01.sla.0000251619.10648.32
29. Yu W, Tu Y, Long Z, Liu J, Kong D, Peng J, et al. Reactive oxygen species bridge the gap between chronic inflammation and tumor development. Oxid Med Cell Longev. (2022) 2022:2606928. doi: 10.1155/2022/2606928
30. O’Leary JA, Berend KR, Johnson JL, Levin LS, and Seigler HF. Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res. (2000) 378):206–12. doi: 10.1097/00003086-200009000-00031
Keywords: subungual amelanotic melanoma, trauma, post-traumatic immunosuppression, differential diagnosis, immunohistochemistry
Citation: An L, Liu Z, Chen X and Jia Y (2025) Rare subungual amelanotic melanoma presenting as prolonged swelling and exudation after trauma: case report and literature review. Front. Immunol. 16:1661698. doi: 10.3389/fimmu.2025.1661698
Received: 08 July 2025; Accepted: 11 September 2025;
Published: 26 September 2025.
Edited by:
Ravi Prakash Sahu, Wright State University, United StatesReviewed by:
Cristian Sorin Hariga, Carol Davila University of Medicine and Pharmacy, RomaniaAdriana Matter, Santa Casa de Curitiba, Brazil
Copyright © 2025 An, Liu, Chen and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangru Chen, Y3hyMTk5MUBqbHUuZWR1LmNu; Yuxi Jia, amlheXhAamx1LmVkdS5jbg==
 Lin An
Lin An Xiangru Chen
Xiangru Chen Yuxi Jia
Yuxi Jia