- Department of Bacterial Molecular Genetics, Faculty of Biology, University of Gdańsk, Gdańsk, Poland
In the era of increasing bacterial antibiotic resistance, finding new ways of combating pathogens is especially important. An attractive possibility is targeting bacterial survival strategies that microorganisms employ either to evade the host immune-responses or to adapt to the hostile environment encountered once inside the host. An example of the latter is the stringent response, mediated by guanosine penta- and tetra-phosphate, collectively referred to as (p)ppGpp. These molecules (alarmones) are responsible for switching bacterial gene expression and metabolism to allow survival under various stresses, such as nutritional deprivation and oxidative stress. (p)ppGpp turnover is mediated by various enzymes belonging to the RSH (RelA-SpoT homolog) family, some of which are capable of both, (p)ppGpp synthesis and hydrolysis, while others can perform only one of these functions. In this minireview, we discuss strategies that aim to disrupt or modulate the stringent response either by inhibiting these enzymes or on the contrary – enhancing their activities, as that goal can be achieved by several ways, i.e. blocking (p)ppGpp synthesis, inducing its synthesis or blocking its hydrolysis.
1 Introduction
The process of colonization of the host organism by pathogenic bacteria is a complex and multi-stage phenomenon. To effectively colonize the host, pathogens must overcome numerous protective barriers, including physical structures, chemical factors and highly specialized mechanisms of the immune response. In addition, the most common obstacles include nutrient deficiency - the host limits, e.g. amino acids (1), carbon availability (2) and essential metal ions (e.g. iron) (3). Other challenges that pathogens have to face is oxidative (4) and nitrosative stress (5). In some locations (e.g. the digestive or urinary tract), bacteria must also adapt to changing osmotic conditions, rapid pH fluctuations and the action of lytic enzymes and antimicrobial peptides (6–8). In response, bacteria have evolved a variety of adaptive strategies that allow them to successfully survive and proliferate in the hostile host environment. Many of these strategies are controlled by the stringent response, mediated by the guanosine penta- and tetra-phosphate, collectively referred to as (p)ppGpp, which alert the cell to a multitude of stress factors – including nutrient starvation (i.e. amino acid, carbon, nitrogen, phosphate, iron and lipid limitation) and physico-chemical stresses (such as osmotic, oxidative, and acid stress) (9, 10). Synthesis of (p)ppGpp is carried out from GTP (in case of pppGpp) or GDP (ppGpp) and ATP which is the donor of the pyrophosphate group (transferred from ATP to 3′ position of GTP or GDP) (9).
At its core, the stringent response aims to inhibit growth and activate survival mechanisms at the same time. In particular, this response has been reported to be involved at various stages of bacterial infection, such as adherence, invasion, immune evasion, bacterial cell dissemination, biofilm formation, sporulation, persistence and antibiotic tolerance, including but not limited to production of specific enzymes (e.g. catalases that protect pathogens from oxidative stress) and structures (such as fimbriae), as well as activation of pathogenicity-related gene expression (10, 11). Although (p)ppGpp affects a multitude of pathways, the impact on bacterial virulence is typically regulated through transcriptional changes – either by its direct binding to the RNA polymerase (e.g. in proteobacteria) or by causing a decrease in GTP levels (as in Bacillota, Actinobacteria, and Deinococcus-Thermus) (10, 12, 13).
The (p)ppGpp turnover is mediated by various enzymes, most notably those belonging to the RSH (RelA-SpoT homolog) superfamily. Many of these enzymes possess both, the (p)ppGpp synthetase and hydrolase domains along with regulatory domains (the so-called long RSH enzymes), while others possess short catalytic domains only (i.e. small alarmone synthetases (SASs) and small alarmone hydrolases (SAHs)) (14). In addition to bifunctional long RSH enzymes (e.g. Rel in Bacillus subtilis or SpoT in Escherichia coli) present in almost all bacteria, most β- and γ-proteobacteria also possess a synthetase-only long RSH (e.g. RelA in E. coli) (13, 15). In that case, each long RSH enzyme can respond to a different environmental stress, e.g. E. coli RelA responds to amino acid starvation while E. coli SpoT responds to all other stresses (9). In contrast to examples described above, bacteria from the Planctomycetes, Verrucomicrobia, and Chlamydiae phyla (the PVC superphylum) do not possess a long RSH enzyme at all (15).
It should be noted, that the long and short RSH enzymes can coexist in various combinations, for example in addition to Rel, B. subtilis contains two SASs – SAS1 (also known as RelQ) and SAS2 (RelP) (16). On the other hand, while all mycobacteria encode a bifunctional long RSH protein (e.g. RelMtb in Mycobacterium tuberculosis and RelMsm in Mycolicibacterium smegmatis), M. smegmatis also possesses a SAS protein – RelZ (which is unusual among SASs as it features an additional RNase HII domain), while M. tuberculosis encodes its non-functional ortholog – Rv1366 (17).
RSH enzymes are subject to diverse regulatory mechanisms, including transcriptional control; ligand-mediated regulation through substrates, products, or atypical ligands such as ssRNA; heterologous protein interactions; as well as indirect regulation via crosstalk with other secondary messenger nucleotides (18). Moreover, oligomerization may play an important role in regulation of RSH enzymes activity - in E. coli, RelA forms dimers via C-terminal domain (CTD) interactions that lower its synthetase activity, while RelMtb forms less active trimers that dissociate upon substrate or product binding. However, this seems not to be limited to long RSH enzymes, as even though lacking the CTD, activity of SAS1 from B. subtilis is dependent on tetramerization (18).
In addition to the enzymes described above, there is a number of (p)ppGpp metabolizing enzymes that do not belong to the RSH superfamily, such as GppA phosphatase found in γ- and some δ-proteobacteria (19) or NuDiX hydrolases, e.g. NahA from B. subtilis or MutT from E. coli, that can cleave (p)ppGpp into such derivatives as pGpp or pGp, respectively (10, 20, 21).
2 Approaches aimed at targeting the stringent response
2.1 (p)ppGpp synthesis and hydrolysis pathways as direct molecular targets in combating different bacteria
Since (p)ppGpp is the mediator of the stringent response and is required for turning on the survival strategies upon various stresses, including nutrient starvation, as well as for activation of pathogenicity-related gene expression, the most straightforward approach to combat these effects seems to be limitation of (p)ppGpp production. Indeed, most of the antimicrobial strategies based on altering the stringent response focus on that aspect of (p)ppGpp metabolism (Figure 1). In that case, (p)ppGpp synthetases are generally the target.
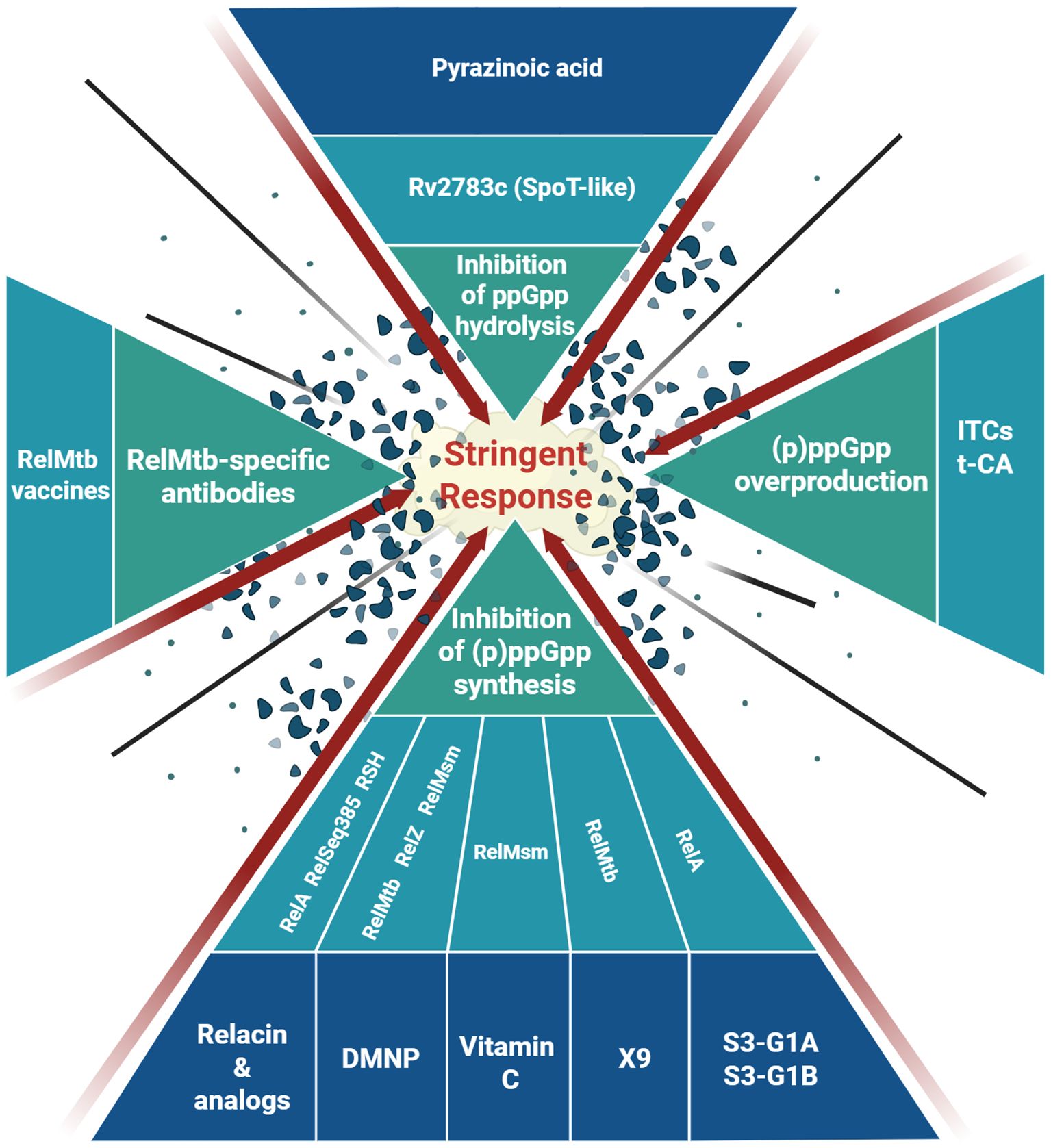
Figure 1. Summary of the current approaches applied to target the bacterial stringent response. See text for details. Created in BioRender. Gąsior, F. (2025) https://BioRender.com/1f62hnc.
In the first report exploring this idea, several ppGpp analogues were tested, and the one containing bisphosphonate groups at the 5′ and 3′ positions instead of the natural pyrophosphates displayed the most inhibitory effects (22). In vitro tests had demonstrated it to cause partial inhibition of RelA from E. coli and truncated RelSeq enzyme from Streptococcus dysgalactiae subsp. equisimilis (RelSeq1-385). This analogue most likely competes with GDP and GTP for binding at the active site of these enzymes. However, the use of this compound was not tested in vivo (22).
In a subsequent study, another ppGpp analogue, called relacin, was found to be much more efficient at inhibiting (p)ppGpp synthetases than the previous versions. Two synthetases were tested in vitro, representing Gram(-) (E. coli RelA) and Gram(+) (Deinococcus radiodurans RSH) species. Relacin was obtained by replacing ppGpp’s phosphate groups by glycyl-glycine dipeptides and adding an isobutyryl group at the C - 2 position of the guanine base; similarly to the compound used in the previous study, it was designed to bind to the tested synthetases’ active site (23). As with the other ppGpp analogues, relacin had no effect on E. coli cells, however, a promising in vivo effect was observed for Gram(+) species (B. subtilis, B. anthracis, D. radiodurans and Group A Streptococcus) where it was shown to limit (p)ppGpp production (tested in B. subtilis), impede entrance into stationary phase (all four species), inhibit biofilm formation (B. subtilis) and sporulation (B. subtilis and B. anthracis). The lack of effect on E. coli cells is thought to be due to relacin’s inability to cross the Gram(-) bacteria’s membranes and enter the cell (23). In addition to these studies, it was shown that relacin inhibited RSHCd (RSH enzyme from Clostridioides difficile) activity in vitro, but was efficient in vivo against the epidemic strain of C. difficile only in combination with metronidazole or clindamycin (24).
Most other studies that followed the ones described above and which focused on targeting (p)ppGpp synthetases were concerned almost exclusively with mycobacteria. For example, ppGpp analogues created by replacing the ppGpp’s 5′ and 3′ phosphate groups by acetyl groups, adding the acetyl group at the 2′ ribose and adding either the acetyl or isobutyryl group at the C - 2 position of the guanine base (the AC and AB compounds, respectively) were shown to be effective at inhibiting RelMsm (M. smegmatis Rel) in vitro, with AB being twice as efficient (25). In addition, both compounds reduced (p)ppGpp level and limited bacterial survival under stress conditions (M. smegmatis), as well as reduced biofilm formation by M. smegmatis and M. tuberculosis (25). Importantly, MTT tests on a human lung cancer cell line (H460) and hemolysis tests showed no cytotoxicity and no damage to erythrocyte membranes, respectively, suggesting these compounds could be used in treating human infections (25).
Other studies on targeting the stringent response in mycobacteria were conducted using a truncated RelMtb protein (RelMtb53-446), which contains the (p)ppGpp synthetase and hydrolase domains. Two million compounds from the GlaxoSmithKline (GSK) compound library were screened, among which compound X9 showed a strong in vitro activity against RelMtb and was effective in vivo against M. tuberculosis wild type strains, while it had no effect on the ΔrelMtb mutant strain. X9 also enhanced the activity of isoniazid (INH) which is a standard drug used against mycobacteria (26).
Another example of a successful library screen was reported by (27) where 4 million commercially available compounds from the University of California, San Francisco (UCSF) ZINC database were first screened in silico by molecular docking to E. coli RelA. Two of them, S3-G1A and S3-G1B, exhibited better parameters than relacin and were shown to be effective in RelA inhibition in vitro and in vivo (27).
Natural compounds or their synthetic analogues, unrelated to (p)ppGpp structure, were also tested as (p)ppGpp synthetase inhibitors. An intriguing example is the well-known Vitamin C, which was shown to bind to RelMsm (28). In vivo, this natural compound prevents biofilm formation by mycobacteria which may be related to its ability to lower cellular (p)ppGpp levels (28). On the other hand, DMNP (4-(4,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)pentanoic acid) – a synthetic analogue of naturally occurring erogorgiaene (a metabolite found in soft coral Antillogorgia elisabethae), was shown to reduce the (p)ppGpp synthetase activity of mycobacterial RelMsm and RelZ (29, 30), as well as RelMtb (31).
An interesting molecular target related to the stringent response is the M. tuberculosis Rv2783c protein, which resembles E. coli SpoT in its function, but mainly acts as a ppGpp hydrolase. It has been shown that pyrazinoic acid (POA), which is a metabolite of the M. tuberculosis directed drug - pyrazinamide (PZA), binds to Rv2783c and blocks ppGpp hydrolysis. This suggests that the effectiveness of PZA against mycobacteria relies on disruption of the stringent response metabolism (32). So far, this is the only promising example of an attempt to directly target (p)ppGpp hydrolysis and not synthesis.
Initially promising results were also reported for peptide 1018 (IDR (innate defense regulator) -1018) which was thought to inhibit the development of biofilm produced by Pseudomonas aeruginosa by promoting faster ppGpp degradation (33, 34). However, this was later challenged by a report which showed that a similar inhibitory effect by 1018 was observed for P. aeruginosa unable to produce ppGpp (35).
2.2 Indirect modulators of the stringent response - a promising strategy against bacterial pathogens
Over the past decade, various reports have also focused on the activity of natural compounds that can indirectly modulate bacterial stringent response by inducing specific cellular stresses. Isothiocyanates (ITC), a group of natural antimicrobial compounds derived from the Brassicaceae plant family, was demonstrated to directly induce the stringent response. All ITCs tested were shown to increase (p)ppGpp cellular level to varying degree, with phenethyl ITC being the most active (36). This process was dependent on the presence of relA, which suggested induction of the amino acid deprivation-dependent pathway of the stringent response. In enterohemorrhagic E. coli (EHEC) ITC led to bacterial growth inhibition and suppression of the Shiga toxin gene expression, whose activation is associated with lambdoid prophage induction (36, 37). This finding is especially important because use of the standard antibiotics alone is discouraged in the treatment of EHEC infections, since here inhibition of bacterial growth is usually accompanied by Shiga toxin production, which can lead to serious health complications (38).
The antibacterial potential of ITC has been also demonstrated against another bacterial pathogen, i.e. Vibrio cholerae. Two well-studied ITCs, sulforaphane and phenethyl isothiocyanate, were shown to exert their antibacterial potential via the same route as for EHEC – by the induction of the stringent response (39). Studies conducted on V. cholerae demonstrated inhibition of biofilm formation and bacterial growth, as well as a reduction in toxin production by ITCs (39). Interestingly, although ITCs inhibit growth of various bacterial pathogens, their mechanism of action not always involves the stringent response – e.g. in B. subtilis the ITC treatment has not led to (p)ppGpp accumulation and its antibacterial effect is due to bacterial membrane integrity disruption (37).
On the other hand, recent research on t-cinnamaldehyde (t-CA) revealed that it disrupts pyruvate metabolism and limits lysine biosynthesis, which in turn leads to metabolic stress that induces E. coli RelA activity and consequently indirectly stimulates (p)ppGpp production. This results in EHEC growth inhibition and decreased Shiga toxin production which in turn reduces bacterial toxicity, as observed in vivo with the use of the Galleria mellonella model (40). Moreover, combining this natural compound with azithromycin enhances its antimicrobial effect against EHEC, indicating potential optimization strategies for antimicrobial therapy (41).
2.3 Vaccines against stringent response related proteins
One of the latest approaches in combating pathogenic bacteria by disrupting the stringent response involves the use of DNA vaccines, as exemplified by a vaccine containing four stringent response-related genes from M. tuberculosis—relMtb, sigE, ppk2, and ppx. The sigE, ppk2 and ppx gene products were implicated earlier in the signaling network involving RelMtb (42). Tested in mouse models, this intramuscularly introduced vaccine induced production of RelMtb specific IgG antibodies and activation of CD4+ T cells producing IFN-γ and TNF-α (43). Further studies had shown that exposure of M. tuberculosis-infected macrophages to isoniazid (a typically employed mycobacterial drug) strongly upregulated relMtb expression, which led to the development of a DNA vaccine targeting this gene-product alone (44). In addition, this vaccine increased bacterial susceptibility to isoniazid and significantly limited M. tuberculosis replication after the end of antibiotic treatment (44), thus confirming the choice of RelMtb as an appropriate immunological target to support effective therapy.
3 Discussion
Although the approaches described above and summarized in Figure 1 seem promising and the first molecule specifically inhibiting (p)ppGpp synthesis had been obtained over a decade ago, to the best of our knowledge none of the compounds described above have so far reached the clinical trial phase focused exclusively on targeting the stringent response. However, new light can be shed on already known compounds, e.g. thanks to extensive in silico analysis. Recently, a known antituberculosis peptide drug (Pantocin wh-1) was shown by molecular docking to bind to the Staphylococcus aureus RelP which may be a promising approach in targeting methicillin-resistant staphylococcal strains (45).
On the other hand, although novel compounds targeting the bacterial stringent response are continually being developed, the high concentrations that are required to demonstrate their activity in vitro present a substantial challenge. This is illustrated by the high half-maximal inhibitory concentration (IC50) values of relacin (~840 µM; 46) and DMNP (~195–303 µM; 30), highlighting how difficult it would be to apply these compounds in the living organisms.
Another challenge is that (p)ppGpp synthetases are widespread and highly conserved among different bacterial species, including those that are part of the natural human microbiota. The use of drugs targeting these enzymes would thus be the same as using non-discriminatory antibiotics, although their ability to penetrate through the Gram(-) and Gram(+) cellular membranes might be a means of affording some specificity. An interesting alternative would be targeting the RSH enzymes regulatory domains instead of their catalytic centers. For example, it has been shown that regulatory domains of RSH enzymes encoded by the pathogenic strains of P. aeruginosa, Klebsiella pneumoniae, Shigella flexneri, and Listeria monocytogenes display greater interspecies differences than their highly conserved catalytic domains (47).
Also, an important question to consider is whether it is better to target (p)ppGpp synthesis (either through inhibition or induction) or hydrolysis. All of these approaches may bring beneficial results, however they each have their drawbacks as well. For instance, although (p)ppGpp synthesis inhibition may limit virulence gene expression, it is well known that E. coli cells devoid of (p)ppGpp often give rise to RNA polymerase mutants that in the absence of (p)ppGpp behave as if this alarmone was still there (48, 49); this would diminish the expected therapeutic effects in bacteria where RNA polymerase is (p)ppGpp’s direct target. In case of drugs inducing (p)ppGpp production, it can be imagined that if high cellular alarmone levels were induced, this would lead to growth inhibition; however, if these levels were not high enough, a possibly contrary effect would be obtained – virulence and pathogenicity pathways would be turned on and infection would proceed. Additionally, it has been shown recently that increased (p)ppGpp levels drive persister formation in bacteria, i.e. bacteria that are phenotypically resistant to antibiotics although genotypically they should be sensitive (50). Similar outcomes could be imagined for (p)ppGpp hydrolysis disruption, although if hydrolysis was to be fully inhibited, this approach might be the most promising since lack of an enzyme able to remove (p)ppGpp would be lethal (e.g. it is well known that E. coli relA+ ΔspoT mutants are nonviable (9)).
All in all, although targeting the stringent response to combat pathogens seems very attractive, many challenges remain. Nevertheless, continued research and a deeper understanding of this complex mechanism will be key to unlocking its full therapeutic potential.
Author contributions
FG: Conceptualization, Writing – original draft, Writing – review & editing. KB: Writing – original draft, Writing – review & editing. WK: Writing – original draft, Writing – review & editing. KP: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research in KP laboratory is funded by the National Science Centre (Poland) (grant number UMO-2021/43/B/NZ2/00855) and by statutory funds of the University of Gdańsk, Poland.
Acknowledgments
We would like to thank Prof. Agnieszka Szalewska-Pałasz for critical reading of this manuscript. We also acknowledge support from our colleagues at the Department of Bacterial Molecular Genetics, Faculty of Biology, University of Gdańsk.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang YJ and Rubin EJ. Feast or famine: The host-pathogen battle over amino acids. Cell Microbiol. (2013) 15:1079–1087. doi: 10.1111/cmi.12140
2. Eisenreich W, Dandekar T, Heesemann J, and Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol. (2010) 8:401–412. doi: 10.1038/nrmicro2351
3. Hood MI and Skaar EP. Nutritional immunity: Transition metals at the pathogen-host interface. Nat Rev Microbiol. (2012) 10:525–537. doi: 10.1038/nrmicro2836
4. Imlay JA. Where in the world do bacteria experience oxidative stress? Environ Microbiol. (2019) 21:521–530. doi: 10.1111/1462-2920.14445
5. Poole RK. Nitric oxide and nitrosative stress tolerance in bacteria. Biochem Soc Trans. (2005) 33:176–180. doi: 10.1042/BST0330176
6. Ali ASM, Townes CL, Hall J, and Pickard RS. Maintaining a sterile urinary tract: The role of antimicrobial peptides. J Urol. (2009) 182:21–28. doi: 10.1016/j.juro.2009.02.124
7. Smith JL. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J Food Prot. (2003) 66:1292–1303. doi: 10.4315/0362-028x-66.7.1292
8. Horn N and Bhunia AK. Food-associated stress primes foodborne pathogens for the gastrointestinal phase of infection. Front Microbiol. (2018) 9:1962. doi: 10.3389/fmicb.2018.01962
9. Potrykus K and Cashel M. (p)ppGpp: still magical?*. Annu Rev Microbiol. (2008) 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903
10. Irving SE, Choudhury NR, and Corrigan RM. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat Rev Microbiol. (2021) 19:256–271. doi: 10.1038/s41579-020-00470-y
11. Dalebroux ZD, Svensson SL, Gaynor EC, and Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Reviews : MMBR. (2010) 74:171. doi: 10.1128/MMBR.00046-09
12. Liu K, Bittner AN, and Wang JD. Diversity in (p)ppGpp metabolism and effectors. Curr Opin Microbiol. (2015) 24:72. doi: 10.1016/j.mib.2015.01.012
13. Bange G, Brodersen DE, Liuzzi A, and Steinchen W. Two P or not two P: understanding regulation by the bacterial second messengers (p)ppGpp. Annu Rev Microbiol. (2021) 75:383–406. doi: 10.1146/annurev-micro-042621-122343
14. Atkinson GC, Tenson T, and Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PloS One. (2011) 6:e23479. doi: 10.1371/journal.pone.0023479
15. Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, and Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. (2015) 13:298–309. doi: 10.1038/nrmicro3448
16. Steinchen W, Vogt MS, Altegoer F, Giammarinaro PI, Horvatek P, Wolz C, et al. Structural and mechanistic divergence of the small (p) ppGpp synthetases RelP and RelQ. Sci Rep. (2018) 8:2195. doi: 10.1038/s41598-018-20634-4
17. Gupta KR, Arora G, Mattoo A, and Sajid A. Stringent response in mycobacteria: from biology to therapeutic potential. Pathog (Basel Switzerland). (2021) 10:1417. doi: 10.3390/pathogens10111417
18. Irving SE and Corrigan RM. Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiol (Reading England). (2018) 164:268–276. doi: 10.1099/mic.0.000621
19. Mechold U, Potrykus K, Murphy H, Murakami KS, and Cashel M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. (2013) 41:6175–6189. doi: 10.1093/nar/gkt302
20. Zhang Y, Zborníková E, Rejman D, and Gerdes K. Novel (p)ppGpp binding and metabolizing proteins of escherichia coli. mBio. (2018) 9:e02188. doi: 10.1128/mBio.02188-17
21. Yang J, Anderson BW, Turdiev A, Turdiev H, Stevenson DM, Amador-Noguez D, et al. The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p) ppGpp. Nat Commun. (2020) 11:5388. doi: 10.1038/s41467-020-19166-1
22. Wexselblatt E, Katzhendler J, Saleem-Batcha R, Hansen G, Hilgenfeld R, Glaser G, et al. ppGpp analogues inhibit synthetase activity of Rel proteins from Gram-negative and Gram-positive bacteria. Bioorganic Medicinal Chem. (2010) 18:4485–4497. doi: 10.1016/j.bmc.2010.04.064
23. Wexselblatt E, Oppenheimer-Shaanan Y, Kaspy I, London N, Schueler-Furman O, Yavin E, et al. Relacin, a novel antibacterial agent targeting the Stringent Response. PloS Pathog. (2012) 8:e1002925. doi: 10.1371/journal.ppat.1002925
24. Pokhrel A, Poudel A, Castro KB, Celestine MJ, Oludiran A, Rinehold AJ, et al. The (p)ppGpp synthetase RSH mediates stationary-phase onset and antibiotic stress survival in clostridioides difficile. J Bacteriology. (2020) 202:e00377–20. doi: 10.1128/JB.00377-20
25. Syal K, Flentie K, Bhardwaj N, Maiti K, Jayaraman N, Stallings CL, et al. Synthetic (p)ppGpp analogue is an inhibitor of stringent response in mycobacteria. Antimicrobial Agents Chemotherapy. (2017) 61:e00443–17. doi: 10.1128/AAC.00443-17
26. Dutta NK, Klinkenberg LG, Vazquez M-J, Segura-Carro D, Colmenarejo G, Ramon F, et al. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci Adv. (2019) 5:eaav2104. doi: 10.1126/sciadv.aav2104
27. Hall DC, Król JE, Cahill JP, Ji H-F, and Ehrlich GD. The development of a pipeline for the identification and validation of small-molecule relA inhibitors for use as anti-biofilm drugs. Microorganisms. (2020) 8:1310. doi: 10.3390/microorganisms8091310
28. Syal K, Bhardwaj N, and Chatterji D. Vitamin C targets (p)ppGpp synthesis leading to stalling of long-term survival and biofilm formation in Mycobacterium smegmatis. FEMS Microbiol Lett. (2017) 364:fnw282. doi: 10.1093/femsle/fnw282
29. Tkachenko AG, Kashevarova NM, Sidorov RY, Nesterova LY, Akhova AV, Tsyganov IV, et al. A synthetic diterpene analogue inhibits mycobacterial persistence and biofilm formation by targeting (p)ppGpp synthetases. Cell Chem Biol. (2021) 28:1420–1432.e9. doi: 10.1016/j.chembiol.2021.01.018
30. Sidorov RY and Tkachenko AG. DMNP, a synthetic analog of erogorgiaene, inhibits the ppGpp synthetase activity of the small alarmone synthetase relZ. Bio Web Conferences. (2023) 57:8002. doi: 10.1051/bioconf/20235708002
31. Sidorov RY and Tkachenko AG. The mechanism of inhibition of mycobacterial (p)ppGpp synthetases by a synthetic analog of erogorgiaene. Biochem Biokhimiia. (2024) 89:407–416. doi: 10.1134/S0006297924030027
32. Njire M, Wang N, Wang B, Tan Y, Cai X, Liu Y, et al. Pyrazinoic acid inhibits a bifunctional enzyme in mycobacterium tuberculosis. Antimicrobial Agents Chemotherapy. (2017) 61:e00070. doi: 10.1128/AAC.00070-17
33. de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, and Hancock REW. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PloS Pathog. (2014) 10:e1004152. doi: 10.1371/journal.ppat.1004152
34. de la Fuente-Núñez C and Hancock REW. Using anti-biofilm peptides to treat antibiotic-resistant bacterial infections. Postdoc Journal: A J Postdoctoral Res Postdoctoral Affairs. (2015) 3:1–8. doi: 10.14304/surya.jpr.v3n2.1
35. Andresen L, Tenson T, and Hauryliuk V. Cationic bactericidal peptide 1018 does not specifically target the stringent response alarmone (p)ppGpp. Sci Rep. (2016) 6:36549. doi: 10.1038/srep36549
36. Nowicki D, Maciąg-Dorszyńska M, Kobiela W, Herman-Antosiewicz A, Węgrzyn A, Szalewska-Pałasz A, et al. Phenethyl isothiocyanate inhibits shiga toxin production in enterohemorrhagic Escherichia coli by stringent response induction. Antimicrobial Agents Chemotherapy. (2014) 58:2304–2315. doi: 10.1128/AAC.02515-13
37. Nowicki D, Rodzik O, Herman-Antosiewicz A, and Szalewska-Pałasz A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: insight to the mode of action. Sci Rep. (2016) 6:22263. doi: 10.1038/srep22263
38. McGannon CM, Fuller CA, and Weiss AA. Different classes of antibiotics differentially influence shiga toxin production. Antimicrobial Agents Chemotherapy. (2010) 54:3790–3798. doi: 10.1128/aac.01783-09
39. Krause K, Pyrczak-Felczykowska A, Karczewska M, Narajczyk M, Herman-Antosiewicz A, Szalewska-Pałasz A, et al. Dietary isothiocyanates, sulforaphane and 2-phenethyl isothiocyanate, effectively impair vibrio cholerae virulence. Int J Mol Sci. (2021) 22:19. doi: 10.3390/ijms221910187
40. Karczewska M, Strzelecki P, Bogucka K, Potrykus K, Szalewska-Pałasz A, and Nowicki D. Increased Levels of (p)ppGpp Correlate with Virulence and Biofilm Formation, but Not with Growth, in Strains of Uropathogenic Escherichia coli. Int J Mol Sci. (2023) 24:3315. doi: 10.3390/ijms24043315
41. Karczewska M, Wang AY, Narajczyk M, Słomiński B, Szalewska-Pałasz A, and Nowicki D. Antibacterial activity of t-cinnamaldehyde: An approach to its mechanistic principle towards enterohemorrhagic Escherichia coli (EHEC). Phytomedicine: Int J Phytotherapy Phytopharmacology. (2024) 132:155845. doi: 10.1016/j.phymed.2024.155845
42. Prusa J, Zhu DX, and Stallings CL. The stringent response and Mycobacterium tuberculosis pathogenesis. Pathog Dis. (2018) 76:fty054. doi: 10.1093/femspd/fty054
43. Chuang Y-M, Dutta NK, Hung C-F, Wu T-C, Rubin H, and Karakousis PC. Stringent response factors PPX1 and PPK2 play an important role in mycobacterium tuberculosis metabolism, biofilm formation, and sensitivity to isoniazid in vivo. Antimicrobial Agents Chemotherapy. (2016) 60:6460–6470. doi: 10.1128/AAC.01139-16
44. Chuang Y-M, Dutta NK, Gordy JT, Campodónico VL, Pinn ML, Markham RB, et al. Antibiotic treatment shapes the antigenic environment during chronic TB infection, offering novel targets for therapeutic vaccination. Front Immunol. (2020) 11:680. doi: 10.3389/fimmu.2020.00680
45. Sinoliya P, Solanki PS, Niraj RRK, and Sharma V. In-silico approach to combat methicillin-resistant staphylococcus aureus: targeting relP protein with inhibitor peptide to mitigate drug resistance. Curr Drug Discov Technol. (2025) 22(4):e15701638337060. doi: 10.2174/0115701638337060250121154347
46. Beljantseva J, Kudrin P, Jimmy S, Ehn M, Pohl R, Varik V, et al. Molecular mutagenesis of ppGpp: turning a RelA activator into an inhibitor. Sci Rep. (2017) 7:41839. doi: 10.1038/srep41839
47. Hegde V, Raman AS, Patil PR, and Prakash B. Purification and preliminary characterization of four Rel homologues from pathogenic bacteria: Implications for species-specific inhibitor design. Protein Expression Purification. (2021) 177:105760. doi: 10.1016/j.pep.2020.105760
48. Murphy H and Cashel M. Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency. Methods enzymology. (2003) 371:596–601. doi: 10.1016/S0076-6879(03)71044-1
49. Potrykus K, Murphy H, Philippe N, and Cashel M. ppGpp is the major source of growth rate control in E. coli. Environ Microbiol. (2011) 13:563–575. doi: 10.1111/j.1462-2920.2010.02357.x
Keywords: stringent response, bacterial stress response, alarmone, (p)ppGpp, host-pathogen interaction, RelA/SpoT homologs (RSH)
Citation: Gąsior F, Bryszkowska K, Klasa W and Potrykus K (2025) Targeting the bacterial stringent response to combat human pathogens. Front. Immunol. 16:1663548. doi: 10.3389/fimmu.2025.1663548
Received: 10 July 2025; Accepted: 18 August 2025;
Published: 01 September 2025.
Edited by:
Paula Maria Tribelli, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Nancy López, IQUIBICEN-CONICET, ArgentinaCopyright © 2025 Gąsior, Bryszkowska, Klasa and Potrykus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filip Gąsior, ZmlsaXAuZ2FzaW9yQHVnLmVkdS5wbA==; Katarzyna Potrykus, a2F0YXJ6eW5hLnBvdHJ5a3VzQHVnLmVkdS5wbA==
 Filip Gąsior
Filip Gąsior Katarzyna Bryszkowska
Katarzyna Bryszkowska Wiktoria Klasa
Wiktoria Klasa Katarzyna Potrykus
Katarzyna Potrykus