- 1Institute of Orthopaedics and Traumatology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, China
- 2The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Orthopaedics, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 4Department of Pharmacy, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 5School of Architecture, Department of Architecture, China Academy of Art, Hangzhou, China
Intervertebral disc degeneration (IVDD) is a major cause of low back pain, with nucleus pulposus (NP) cell pyroptosis—a highly inflammatory form of programmed cell death mediated by NLRP3 inflammasome—playing a crucial role in its pathogenesis by triggering inflammatory cascades and accelerating matrix degradation. Although ultraviolet B (UVB) irradiation has shown therapeutic potential in various inflammatory diseases, its effect on IVDD and underlying mechanisms remained unexplored. In this study, we established a lumbar spine instability (LSI)-induced IVDD mouse model and administered UVB irradiation (315 nm, ~1.0944 mW/cm²) for either 2 minutes (UVB+ group, ~0.13 J/cm²) or 4 minutes (UVB++ group, ~0.26 J/cm²) three times weekly over 8 weeks. UVB treatments effectively attenuated IVDD progression, as evidenced by improved behavioral outcomes, preserved disc height, and maintained structural integrity. Both UVB protocols enhanced extracellular matrix homeostasis, reduced cell apoptosis, and suppressed inflammatory responses, with the UVB+ regimen showing superior efficacy. Mechanistically, UVB significantly inhibited NLRP3-mediated pyroptosis by downregulating NLRP3, ASC, CASPASE1, and GSDMD expression through potent activation of the NRF2/KEAP1 antioxidant pathway. Our findings demonstrate that UVB irradiation effectively ameliorates IVDD by activating the NRF2/KEAP1 pathway and subsequently suppressing NLRP3-mediated pyroptosis, with the 2-minute protocol showing optimal therapeutic effects, establishing UVB irradiation as a promising non-invasive therapeutic strategy for IVDD treatment.
1 Introduction
Lower back pain (LBP) is one of the most prevalent musculoskeletal disorders worldwide. Its average prevalence rate among adults is approximately 12%, and the chronic lifelong rate is about 40% (1). Intervertebral disc (IVD) degeneration (IVDD) has been identified as a major pathological basis for LBP. Current therapeutic strategies for IVDD primarily consist of conservative treatments or surgical interventions. Conservative approaches often involve pain management regimens, including NSAIDs, muscle relaxants, opioids, and physical therapy, to alleviate symptoms. However, these methods are limited in their ability to reverse disc degeneration, while surgical procedures are associated with considerable risks and complications (2, 3). Therefore, developing effective and minimally invasive therapeutic approaches remains an urgent clinical need in the management of IVDD.
Ultraviolet (UV) light therapy, which emerged as a medical intervention in the early 20th century, encompasses three distinct spectral ranges: UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm) (4). Among these, UVB phototherapy has demonstrated remarkable therapeutic efficacy across various inflammatory and autoimmune conditions, including psoriasis, atopic dermatitis, vitiligo, and systemic lupus erythematosus (5–8). Particularly, narrowband UVB (NB-UVB) therapy has shown significant promise in ameliorating psoriasis symptoms through modulation of systemic inflammation via suppression of multiple inflammatory signaling pathways (9–11). Recent investigations have also revealed UVB’s potent antioxidant effects in conditions such as vitiligo, primarily through activation of endogenous antioxidant defense mechanisms (12). Furthermore, compelling evidence suggests that controlled UVB exposure at a specific wavelength of 316 nm can enhance bone formation, decrease bone resorption, and improve bone mass and mineral density without inducing adverse skin effects (13). These multifaceted therapeutic properties of UVB—particularly its anti-inflammatory and antioxidant effects—suggest its potential application in degenerative conditions beyond dermatological disorders.
The structure of a normal intervertebral disc consists of a central, highly viscous gel-like core, the nucleus pulposus, which is encapsulated by the peripheral annulus fibrosus—a fibrous ring primarily of type II collagen. The nucleus pulposus synthesizes glycosaminoglycans and proteoglycans, molecules critical for the disc's water-retention capacity. The pathophysiology of IVDD is characterized by a chronic degenerative process resulting in progressive structural deterioration, particularly disrupted ECM homeostasis, manifested as reduced AGGRECAN and Collagen 2 (COL2) expression, upregulated matrix-degrading enzymes (ADAMTS5 and MMP3), enhanced inflammatory responses, and accelerated cell apoptosis, ultimately leading to decreased disc height and impaired lumbar mechanical function (14). Recent evidence has highlighted that NP cell pyroptosis, a highly inflammatory form of programmed cell death, plays a crucial role in IVDD pathogenesis, which is primarily mediated by the NLRP3 inflammasome and requires CASPASE1 activation, leading to the release of pro-inflammatory cytokines such as IL-1β and IL-18. The resultant inflammatory microenvironment accelerates ECM homeostasis and further NP cell death, creating a detrimental feedback loop that expedites disc degeneration (15). Consequently, targeting NLRP3-mediated represents a promising therapeutic strategy to prevent IVDD progression.
Clinical evidence supporting UVB’s therapeutic potential in musculoskeletal conditions has emerged from a randomized single-blinded controlled trial involving fifty-two postmenopausal Korean women (older than 65 years) with osteoporosis, which demonstrated that low energy UVB exposure improved bone alkaline phosphatase (ALP) levels and bone formation in vitamin D insufficient group (16), while NB-UVB therapy significantly reduces the expression of inflammatory cytokines in various conditions, particularly IL-18 (17), IL-12, and IL-23 in psoriatic lesions, TNF-α, and IL-6 in PBMCs (18, 19), as well as IL-1β and Soluble interleukin 1 receptor type 1(sIL-1R1) in plasma (20) of patients with psoriasis, thereby suppressing inflammation and improving clinical symptoms. However, whether UVB could modulate NP cell pyroptosis-induced inflammation in the context of IVDD remains obscure.
Oxidative stress has been increasingly recognized as a key trigger for NLRP3-mediated pyroptosis during IVDD progression. The Nuclear factor erythroid 2-related factor 2 (NRF2)/Kelch-like ECH-associated protein 1 (KEAP1) pathway represents a master regulator of cellular antioxidant defense. Under physiological conditions, NRF2 is sequestered by KEAP1 in the cytoplasm. Upon activation, NRF2 translocates to the nucleus and induces the expression of antioxidant genes, including heme oxygenase-1 (HO-1) (21). Numerous studies have demonstrated that activation of the NRF2 pathway can effectively suppress NLRP3 inflammasome assembly and subsequent pyroptotic cell death in various inflammatory disease contexts (22–24). In addition, our latest findings demonstrated that enhancing NRF2-mediated antioxidant response might represent an effective approach to prevent IVDD progression (25, 26). Meanwhile, UVB radiation has demonstrated efficacy in ameliorating the progression of multiple skin disorders by modulating oxidative stress, particularly through targeting this same NRF2 antioxidant pathway (27, 28). Taken together, these studies support the hypothesis that UBV administration exhibits potential to attenuate NP cell pyroptosis and ameliorate the progression of IVDD through activation of the NRF2/KEAP1 antioxidant pathway.
In this study, we investigated the therapeutic effect of UVB against IVDD pathogenesis using a lumbar spine instability (LSI) surgery-induced IVDD mice. Mice underwent 2 or 4 minutes of UVB irradiation (three times per week) for 8 weeks post-surgery. Our findings demonstrate that UVB treatment significantly improved IVDD outcomes by preserving disc structure, maintaining ECM homeostasis, inhibiting apoptosis, and reducing inflammation. Notably, UVB suppressed NP pyroptosis by downregulating NLRP3, ASC, CASPASE1, and GSDMD expression, with mechanistic analysis revealing NRF2/KEAP1 pathway activation as the key regulatory mechanism underlying these therapeutic effects. Our in vivo findings provide further insights into the pharmacological properties of UVB concerning IVDD progression, highlighting its potential as a promising therapeutic modality for clinical IVDD management.
2 Materials and methods
2.1 Animals and experimental design
Male C57BL/6 J mice (4 weeks old, 14–16 g, n = 112) were obtained from the Animal Experimentation Center of Zhejiang Chinese Medical University (Grade SPF, SCXK Shanghai). Animals were housed in a specific pathogen-free (SPF) facility under controlled conditions (12-hour light/dark cycle, temperature 23 ± 2 °C, 55% humidity) with ad libtum access to water and standard laboratory chow. Polycarbonate cages without bedding or environmental enrichment SPF standards. All procedures adhered to the ARRIVE guidelines for animal research reporting and were approved by the Ethical Committee of Zhejiang Chinese Medical University (No. 20231030–03).
The mice were randomly divided into four groups (n = 28 per group): Sham (placebo surgery), Model (LSI surgery), UVB+ (LSI surgery + 2-min UVB exposure), and UVB++ (LSI surgery + 4-min UVB exposure). The LSI surgery-induced IVDD model was established as previously described (25). All mice except those in the Sham group, underwent IVDD modeling. Briefly, under isoflurane anesthesia, the L3-L5 spinous processes and associated ligaments were surgically exposed and removed. In the Sham group, only muscle exposure was performed without spinous process removal. Post-surgical care included wound closure and prophylactic gentamicin administration to prevent infection.
NB-UVB treatment commenced three days post-surgery using a fluorescent lamp (314 nm, ~1.0944 mW/cm²) positioned 20 cm above the dorsal region. To ensure consistent UVB delivery, the dorsal fur from the scapula to the sacrum was meticulously trimmed using electric clippers on a weekly basis. This procedure was performed 24 hours prior to the initial UVB irradiation session each week to minimize potential skin irritation and ensure uniform exposure. The UVB+ and UVB++ groups received irradiation three times weekly for 2 minutes (~0.13 J/cm²) and 4 (~0.26 J/cm²) minutes, respectively. Tissue samples (n = 7 per group) were collected at 1, 2, 4, and 8 weeks post-irradiation for subsequent analyses.
2.2 Behavioral assessment
Following a 7-day acclimation period, comprehensive behavioral assessments were conducted at 8 weeks post-irradiation by a single investigator under standardized conditions. On testing days, mice were allowed an additional 20-minute habituation period in the testing apparatus before assessment. The following behavioral parameters were evaluated:
2.2.1 Rearing behavior
Spontaneous vertical activity was quantified by recording the frequency of standing on hind limbs (with or without wall contact) over a 5-minute observation period (29).
2.2.2 Mechanical allodynia
Von Frey filament testing was performed using calibrated filaments (0.2-2.0g) applied to the plantar surface of hindpaws. The paw withdrawal threshold was determined as the lowest force eliciting withdrawal responses in at least three out of five trials. Values from both hindpaws were averaged for analysis (29).
2.2.3 Thermal nociception
The hargreaves test using a plantar test apparatus (Ugo Basile, model 55370). The latency to hindpaw withdrawal was measured, with results averaged from ten trials per mouse. A cut-off time of 20 seconds was implemented to prevent tissue damage (30).
2.2.4 Gait analysis
Following one week of pre-training, mice were assessed using the GaitScan system (CleverSys). Animals traversed a confined corridor (50 × 8 cm) with a glass floor under low-light conditions. Three complete runs were recorded per mouse and analyzed using TreadScan™ 2.0 software (29).
2.3 Micro-CT analysis
Lumbar vertebrae were scanned using a high-resolution micro-CT tomography system (Skyscan 1176, Bruker Micro-CT N.V., Kontich, Belgium) at a 90 kV/300 μA current with 9 μm resolution. Three-dimensional reconstructions were generated using CTVol v2.2 software. Lumbar (L) 3-L4 IVD height was calculated by averaging anterior, middle, and posterior measurements from coronal images.
2.4 Histological, and immunological analysis
Tissue specimens underwent sequential processing, including fixation (4% paraformaldehyde, 72 h), decalcification (14% EDTA, pH 7.4, 3 weeks), and paraffin embedding. Five-µm coronal sections were prepared for histological and immunological analyses. H&E staining was performed for general morphological assessment, with NP scores evaluated blindly according to Norcross criteria (31). For immunohistochemistry (IHC), sections were incubated overnight at 4°C with primary antibodies against matrix proteins (COL2, AGGRECAN, 1:300), degradative enzymes (ADAMTS5, MMP3, 1:300), apoptotic markers (CASPASE3, BAX, BCL2, 1:500), inflammatory factors (IL-6, TNF-α, IL-1β, IL-18, 1:500), and pyroptosis-related proteins (NLRP3, ASC, CASPASE1, GSDMD, 1:500). Detection was achieved using a horseradish peroxidase-conjugated streptavidin detection system (ZSGB-BIO, China) with hematoxylin counterstaining (Sigma-Aldrich, USA). For immunofluorescence (IF) analysis, sections were incubated with fluorescent-conjugated secondary antibodies for 1 hour at room temperature. Quantitative analysis was performed using Image-Pro Plus software (Version 6.0) by observers blinded to the experimental groups, with all experiments conducted in triplicate.
2.5 TUNEL assay
Apoptotic cells in IVD tissues were examined using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) by the TUNEL Bright Green Apoptosis Detection Kit (Vazyme Biotech, Nanjing, China) according to the manufacturer’s protocol. TUNEL-positive cells were quantified by examining three randomly selected fields from three independent sections per sample. The apoptotic rate was calculated as the ratio of TUNEL-positive cells to total DAPI-stained nuclei. All analyses were performed by investigators blinded to the experimental groups.
2.6 Statistical analysis
Data are presented as means ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 9.0 software. Normal distribution and variance homogeneity were assessed prior to applying one-way ANOVA with least significant difference (LSD) post-hoc testing for multiple comparisons. Statistical significance was defined as p < 0.05.
3 Results
3.1 UVB irradiation ameliorates behavioral function and maintains disc height in LSI surgery-induced IVDD mice
To evaluate the therapeutic potential of UVB on IVDD development, mice underwent LSI surgery followed by UVB irradiation (wavelength: 314 nm, power density: 1.0944 mW/cm²) for either 2 minutes or 4 minutes triweekly over 8 weeks. Comprehensive behavioral assessments revealed that IVDD mice exhibited significant motor impairments, evidenced by a 28% reduction in rearing frequency compared to Sham controls (33.33 ± 2.36 vs. 46.17 ± 2.61, p < 0.01). Both UVB treatments effectively improved rearing behavior, with frequencies restored to 38.33 ± 2.69 (UVB+) and 39.67 ± 4.15 (UVB++) (Figure 1A). Thermal nociception testing showed significantly increased latency time in IVDD mice (6.52 ± 0.57s vs. 2.38 ± 0.66s in Sham, p < 0.01), which was normalized by both UVB treatments (4.49 ± 1.30s in UVB+ and 3.91 ± 0.76s in UVB++) (Figure 1B). Similarly, mechanical pain threshold was elevated in IVDD mice (1.31 ± 0.42g vs. 0.89 ± 0.35g in Sham, p < 0.01) and normalized by UVB treatment (0.89 ± 0.30g in UVB+ and 1.17 ± 0.46g in UVB++) (Figure 1C). Gait analysis revealed reduced paw area in IVDD mice (0.38 ± 0.05 cm2 vs. 0.54 ± 0.09 cm2 in Sham, p < 0.01), which was significantly improved by both UVB treatments (0.54 ± 0.10 cm2 in UVB+ and 0.50 ± 0.08 cm2 in UVB++) (Figure 1D).
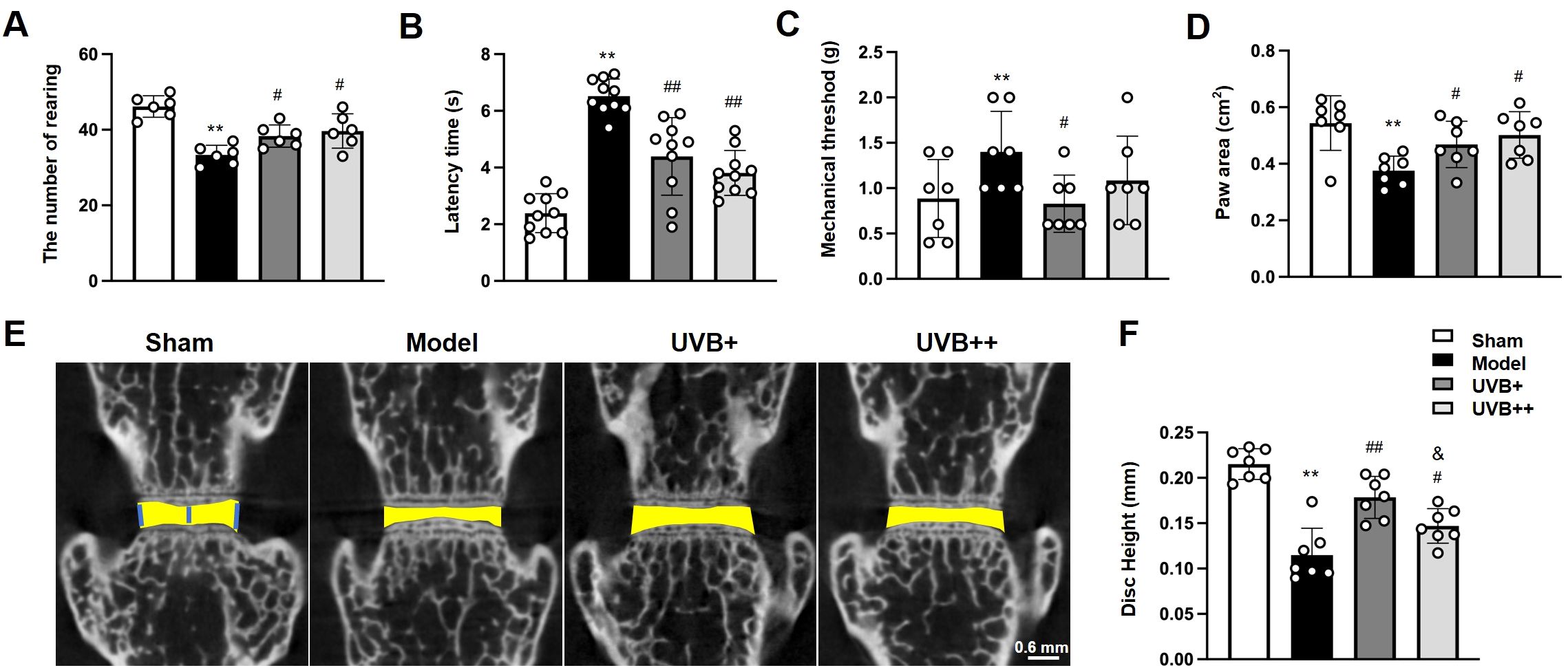
Figure 1. Middle-wavelength UVB irradiation ameliorates behavioral deficits and preserves disc height in LSI-induced IVDD mice. (A-D) Behavioral assessments after 8-week UVB treatment, including (A) rearing frequency test for spontaneous activity, (B) thermal nociceptive response in Hargreaves test, (C) mechanical pain threshold in Von-Frey test, and (D) paw area measurement in gait analysis. (E) Representative μCT images showing disc height changes in L4-L5 segments. (F) Quantitative analysis of disc height index. Data are presented as mean ± SD. **p < 0.01 vs Sham group; #p < 0.05, ##p < 0.01 vs Model group; & p < 0.05 vs UVB+ group. n = 6 per group.
Micro-CT analysis of the L4-L5 IVD revealed significant disc height reduction in IVDD mice (0.11 ± 0.01 mm vs. 0.22 ± 0.04 mm in Sham, representing a 48% decrease, p < 0.01). Both UVB treatments effectively preserved disc height, with UVB+ showing superior efficacy (0.18 ± 0.02 mm) compared to UVB++ (0.15 ± 0.02 mm) (Figures 1E, F).
3.2 UVB irradiation preserves structural integrity and ECM homeostasis in IVDD mice
To investigate the effects of UVB irradiation on IVD structure and ECM homeostasis, histopathological analysis of L4-L5 IVD revealed progressive degenerative changes in IVDD mice, with HE staining showing significant NP height reduction at week 4, which further deteriorated by week 8. The Model group also exhibited distinct morphological changes, including disorganized annulus fibrosus (AF) structure and notable cartilaginous endplate (CEP) calcification. Both UVB treatments effectively preserved disc height and tissue architecture, with maintained NP structure and reduced CEP ossification (Figure 2A). Quantitative histological scoring confirmed these observations, showing that the Model group scores decreased significantly (5.71 ± 1.11 vs. 13.43 ± 0.98 in Sham, p < 0.01). While both UVB treatments maintained significantly higher scores, UVB+ demonstrated superior protective effects (12.14 ± 1.34) compared to UVB++ (10.57 ± 1.13, p < 0.05 between UVB groups) (Figure 2B).
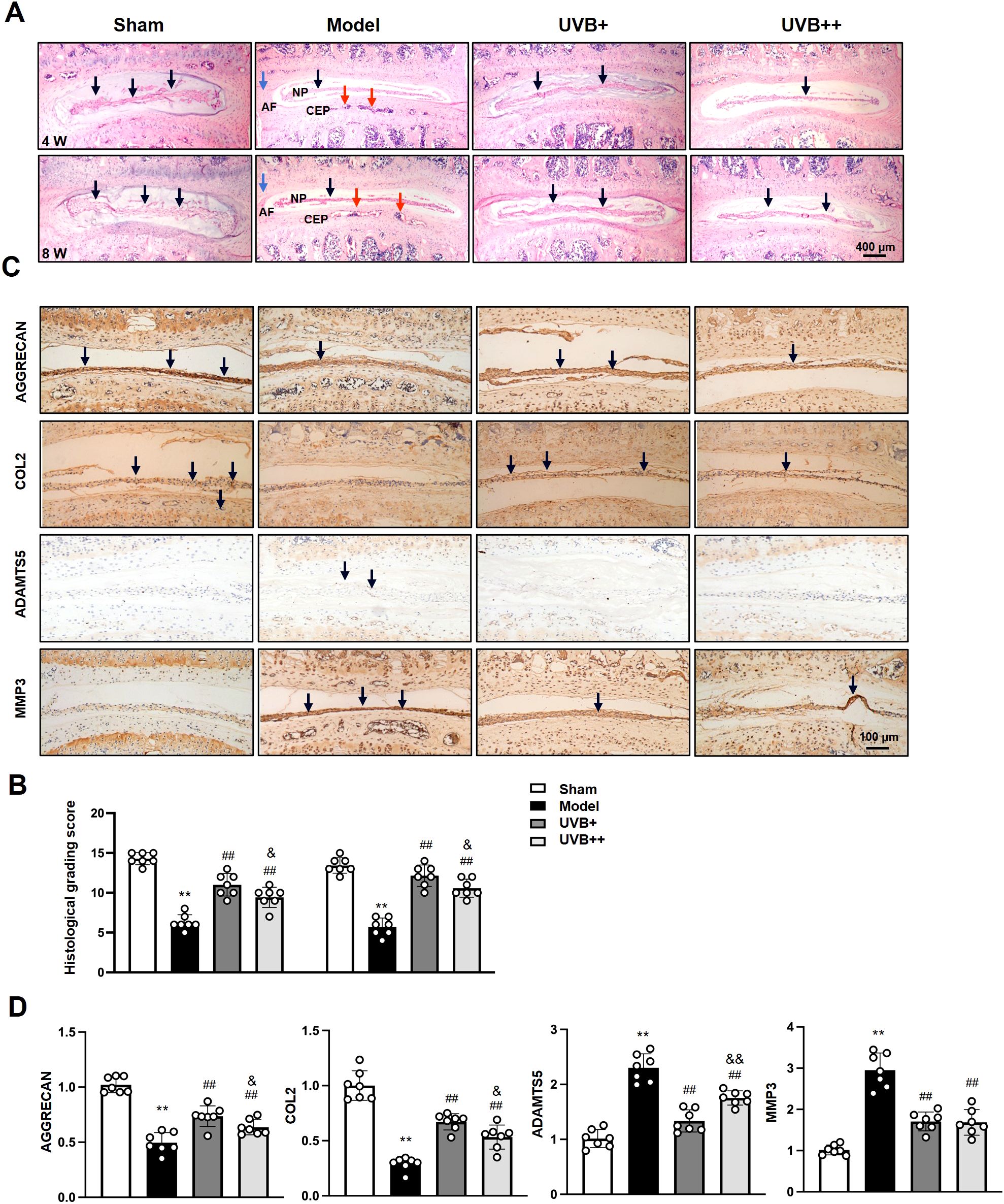
Figure 2. UVB treatment preserves disc structure and ECM homeostasis. (A) Representative H&E staining of L4-L5 IVDs at 4 and 8 weeks post-surgery. Black arrows indicate NP area, red arrows indicate CEP area, and blue arrows indicate AF area. Scale bar = 400 μm. (B) Histological grading scores. (C) Representative IHC images showing ECM-related protein expression. Scale bar = 100 μm. (D) Quantification of AGGRECAN, COL2, ADAMTS5, and MMP3 expression levels. Data are presented as mean ± SD. **p < 0.01 vs Sham group; ##p < 0.01 vs Model group; &p < 0.05 vs UVB+ group. n = 6 per group.
To assess the effect of UVB irradiation on ECM metabolism of IVDs, we determined the expression levels of major ECM components, AGGRECAN and COL2, and their corresponding degradases ADAMTS5 and MMP3, using IHC analysis. Substantial alterations in matrix homeostasis were observed following LSI surgery. AGGRECAN expression decreased by 50% (0.50 ± 0.10 vs. 1.01 ± 0.08 in Sham, p < 0.01), and COL2 levels reduced by 70% (0.30 ± 0.06 vs. 1.00 ± 0.14 in Sham, p < 0.01) in the Model group. Notably, while both UVB treatments improved ECM homeostasis, UVB+ exhibited superior efficacy in preserving AGGRECAN (0.74 ± 0.10 vs. 0.63 ± 0.07 in UVB++, p < 0.05) and COL2 levels (0.67 ± 0.07 vs. 0.52 ± 0.10 in UVB++, p < 0.05), as well as suppressing ADAMTS5 expression (1.32 ± 0.23 vs. 1.74 ± 0.14 in UVB++, p < 0.05). Both treatments demonstrated comparable inhibitory effects on MMP3 expression (1.70 ± 0.23 vs. 1.65 ± 0.31 in UVB+, p > 0.05) (Figures 2C, D).
3.3 UVB irradiation reduces NP cell apoptosis in IVDD mice
To identify the effect of UVB irradiation on the apoptosis of IVD cells, the expression of key apoptotic-related markers, BCL2, BAX, CASPASE3 were determined using IF analysis. The results showed significant dysregulation of apoptotic markers in IVDD mice. The model group showed a 60% decrease in anti-apoptotic BCL2 expression and 7.5-fold and 28.1-fold increases in pro-apoptotic markers including BAX and CASPASE3 in NP tissues, compared to the Sham mice. Notably, UVB+ demonstrated superior anti-apoptotic effects compared to UVB++, with higher BCL2 expression (1.29 ± 0.25 vs. 0.98 ± 0.17, p < 0.05) and lower BAX levels (1.69 ± 0.73 vs. 2.60 ± 0.58, p < 0.05), while both treatments showed comparable reduction in CASPASE3 expression (UVB+: 9.54 ± 3.93, UVB++: 8.01 ± 2.03) (Figures 3A-C). Similarly, the anti-apoptosis effect of UVB irradiation was further confirmed by TUNEL staining, showing significantly elevated apoptotic cells in the Model group (39.30 ± 1.94% vs. 0.96 ± 0.47% in Sham, p < 0.01), with UVB+ showing more potent anti-apoptotic effects (17.46 ± 2.36%) compared to UVB++ (22.24 ± 3.67%, p < 0.05) (Figure 3D).
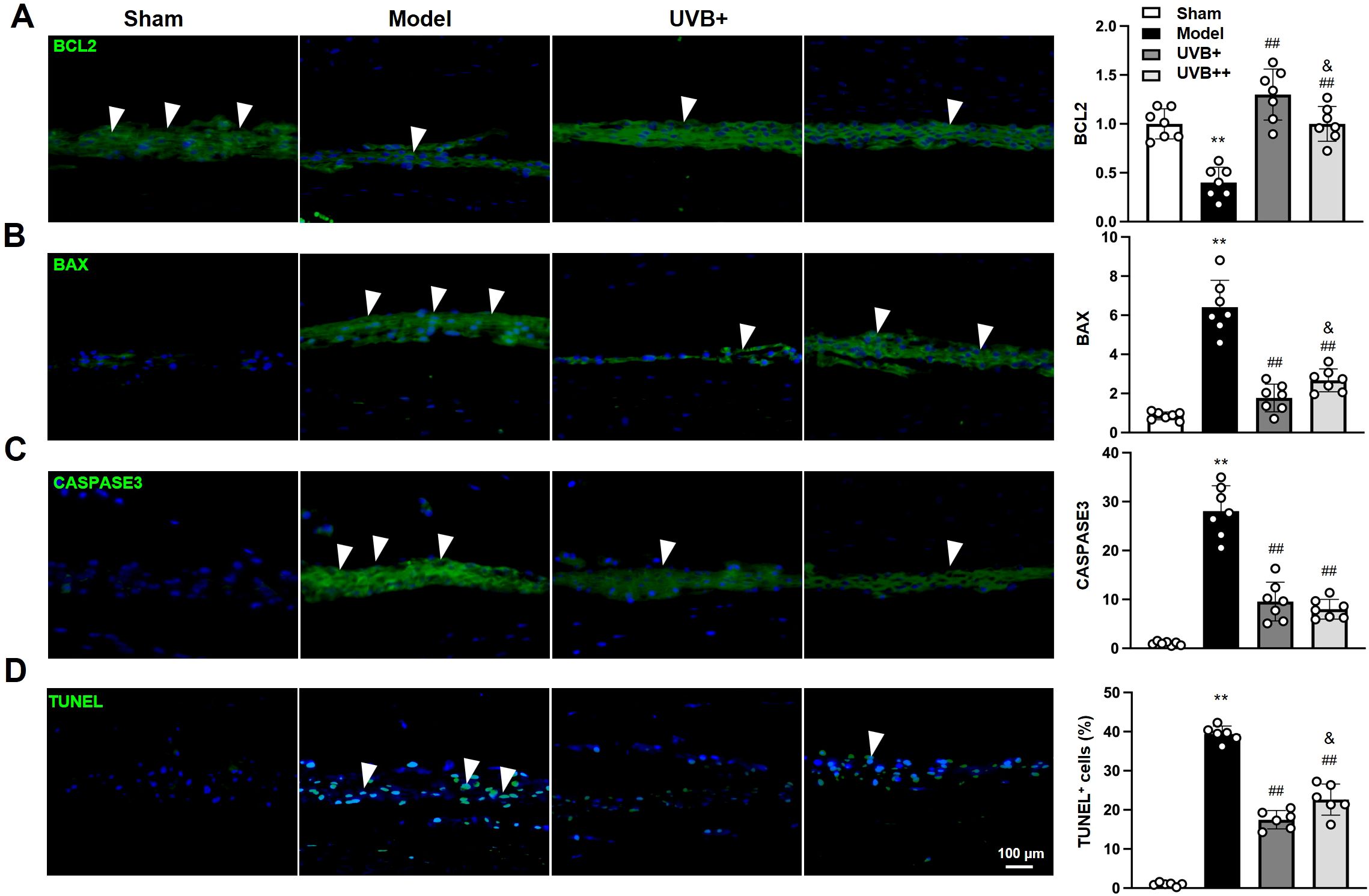
Figure 3. UVB irradiation suppresses NP cell apoptosis in IVDD mice. (A-D) Representative immunofluorescence images and quantification of (A) BCL2, (B) BAX, (C) Cleaved-CASPASE3, and (D) TUNEL-positive cells in NP tissues. Data are presented as mean ± SD. **p < 0.01 vs Sham group; ##p < 0.01 vs Model group; &p < 0.05 vs UVB+ group. n = 6 per group.
3.4 UVB irradiation inhibits inflammatory response in IVDD mice
Given the critical role of inflammation in IVDD pathogenesis, we next investigated whether UVB irradiation modulates inflammatory responses within the degenerative IVDs, the expressions of inflammatory mediators (IL-1β, IL-18, IL-6, and TNF-α) were examined using IF assay. As expected, LSI surgery significantly induced the upregulation of these inflammatory mediators in the NP tissues of IVDD mice, with the most pronounced increases observed in pyroptosis-associated cytokines IL-1β (11.13-fold) and IL-18 (12.2-fold) compared to Sham controls (p < 0.01). Other inflammatory markers also showed significant elevation, with IL-6 and TNF-α increasing by 6.7-fold and 4.8-fold, respectively (p < 0.01). Notably, both UVB treatments effectively suppressed these inflammatory markers, with UVB+ demonstrating superior anti-inflammatory effects compared to UVB++ in reducing IL-18 (UVB+: 3.39 ± 0.69, UVB++: 4.50 ± 0.83) and IL-6 (3.11 ± 0.52 vs. 4.11 ± 0.39, p < 0.05) levels. Meanwhile, both treatments showed comparable efficacy in suppressing IL-1β (UVB+: 3.17 ± 0.38, UVB++: 3.13 ± 0.61) and TNF-α (3.00 ± 0.62 vs. 3.27 ± 0.32, p < 0.05) expression (Figures 4A-D).
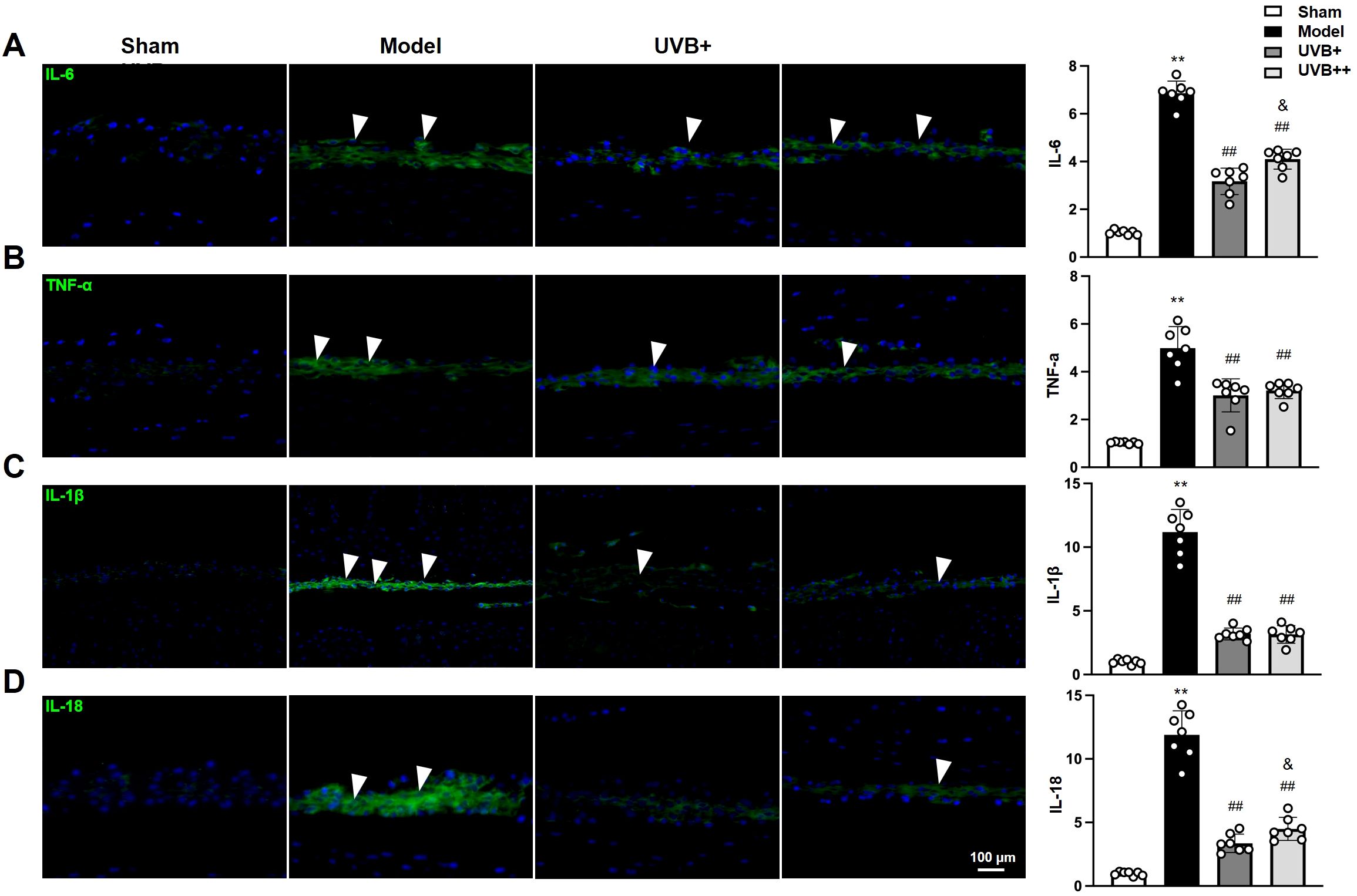
Figure 4. UVB treatment attenuates inflammatory responses in degenerative IVDs. (A-D) Representative immunofluorescence images and quantification of pro-inflammatory cytokines (A) IL-6, (B) TNF-α, (C) IL-1β, and (D) IL-18 expression in NP tissues. Data are presented as mean ± SD. **p < 0.01 vs Sham group; ##p < 0.01 vs Model group; &p < 0.05 vs UVB+ group. n = 6 per group.
3.5 UVB irradiation suppresses NLRP3-mediated pyroptosis in IVDD mice
Given the marked upregulation of pyroptosis-associated cytokines IL-1β and IL-18 in IVDD progression and their marked suppression following UVB irradiation treatment, we further investigated the role of NLRP3 inflammation pathway in UVB-mediated protection against IVDD by examining the expression of key pyroptosis-related proteins, including NLRP3, ASC, CASPASE-1, and GSDMD. IF analysis revealed significant activation of the pyroptotic cascade in the NP tissues of IVDD mice, characterized by significant increases in NLRP3 (4.7 ± 0.68-fold), ASC (3.8 ± 0.90-fold), CASPASE1 (10.3 ± 1.34-fold), and GSDMD (8.0 ± 0.7-fold) compared to Sham controls (all p < 0.01). Notably, UVB+ demonstrated superior efficacy in suppressing all pyroptotic markers compared to UVB++, as evidenced by lower expression levels of NLRP3 (1.71 ± 0.27 vs. 2.16 ± 0.47, p < 0.05), ASC (1.62 ± 0.36 vs. 2.78 ± 0.63, p < 0.05), and CASPASE-1 (2.29 ± 0.63 vs. 3.60 ± 0.80, p < 0.05). Although both treatments showed similar effectiveness in reducing GSDMD expression (UVB+: 2.15 ± 0.45, UVB++: 2.49 ± 0.86) (Figures 5A-D), the overall results indicate that UVB irradiation effectively inhibits NLRP3-mediated pyroptosis in IVDD mice, with UVB+ showing more potent suppressive effects.
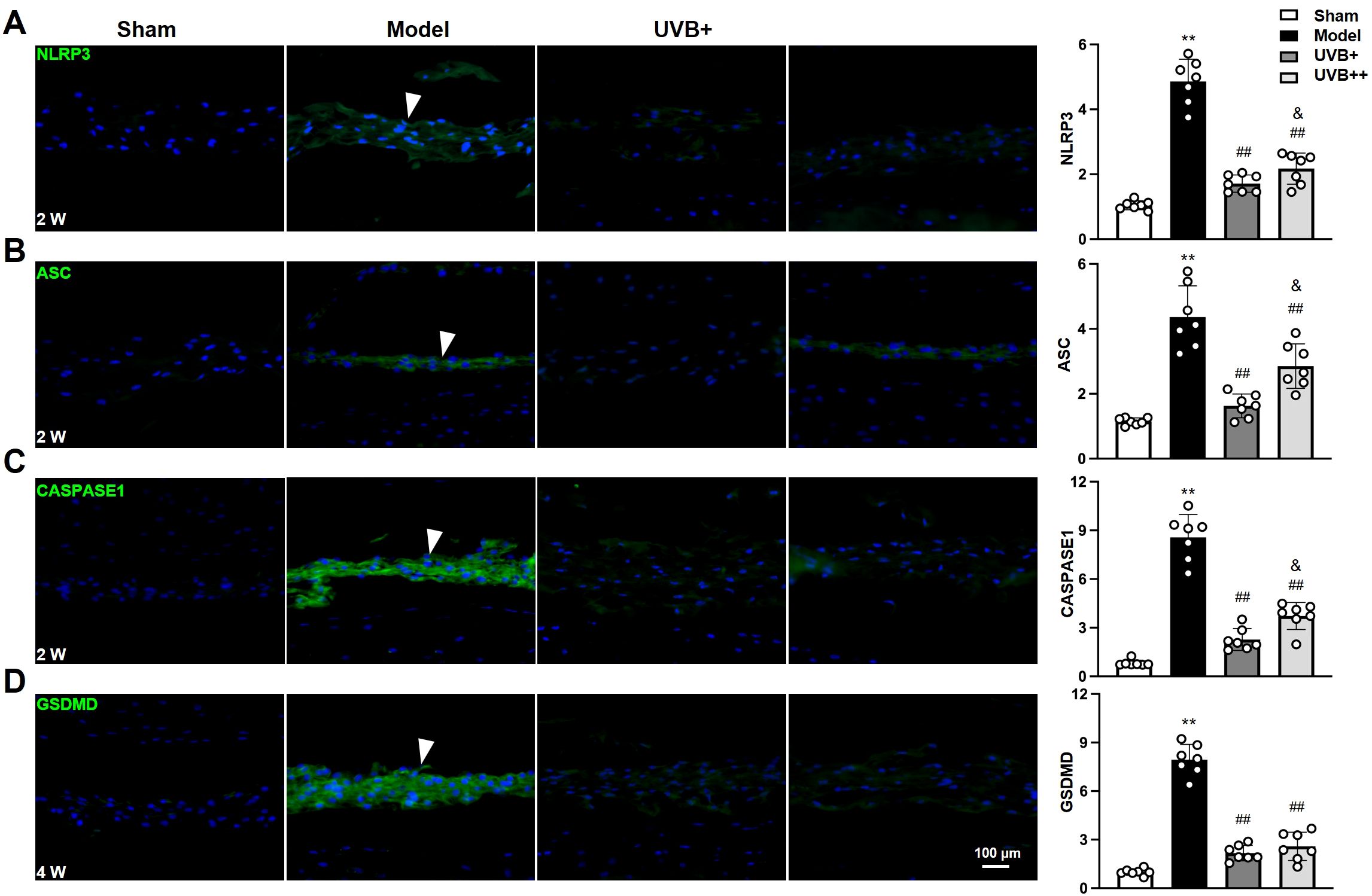
Figure 5. UVB exposure inhibits NLRP3-mediated pyroptosis in NP cells. (A-D) Representative immunofluorescence images and quantification of pyroptosis-related proteins (A) NLRP3, (B) ASC, (C) CASPASE1, and (D) GSDMD at indicated time points. Data are presented as mean ± SD. **p < 0.01 vs Sham group; ##p < 0.01 vs Model group; &p < 0.05 vs UVB+ group. n = 6 per group.
3.6 UVB irradiation activates the NRF2/KEAP1 antioxidant pathway in IVDD mice
To further elucidate the mechanisms underlying UVB’s anti-pyroptotic effects, we examined the NRF2/KEAP1 antioxidant pathway by analyzing the expression and phosphorylation levels of the antioxidant response protein NRF2, along with its negative regulator KEAP1 and downstream target HO-1. The results revealed significant suppression of this pathway in IVDD mice, manifested by marked reductions in p-NRF2 (0.44 ± 0.13 vs. 0.99 ± 0.16 in Sham, p < 0.01), total NRF2 (0.46 ± 0.21 vs. 0.99 ± 0.19 in Sham, p < 0.01), and HO-1 (0.56 ± 0.11 vs. 1.06 ± 0.23 in Sham, p < 0.01), accompanied by elevated KEAP1 expression (4.83 ± 0.72 vs. 1.03 ± 0.22 in Sham, p < 0.01). Both UVB treatments effectively restored pathway activity, with UVB+ showing superior effects in enhancing p-NRF2 expression (2.91 ± 0.33 vs. 2.29 ± 0.61 in UVB++, p < 0.05) and suppressing KEAP1 levels (1.31 ± 0.23 vs. 1.69 ± 0.22 in UVB++, p < 0.05). Interestingly, both treatments demonstrated comparable efficacy in upregulating total NRF2 (UVB+: 2.25 ± 0.41, UVB++: 2.45 ± 0.49) and HO-1 (UVB+: 2.49 ± 0.37, UVB++: 2.52 ± 0.37) (Figures 6A-D). These findings suggest that UVB irradiation protects against IVDD progression by activating the NRF2/KEAP1 antioxidant pathway, with UVB+ showing more potent effects on key regulatory components.
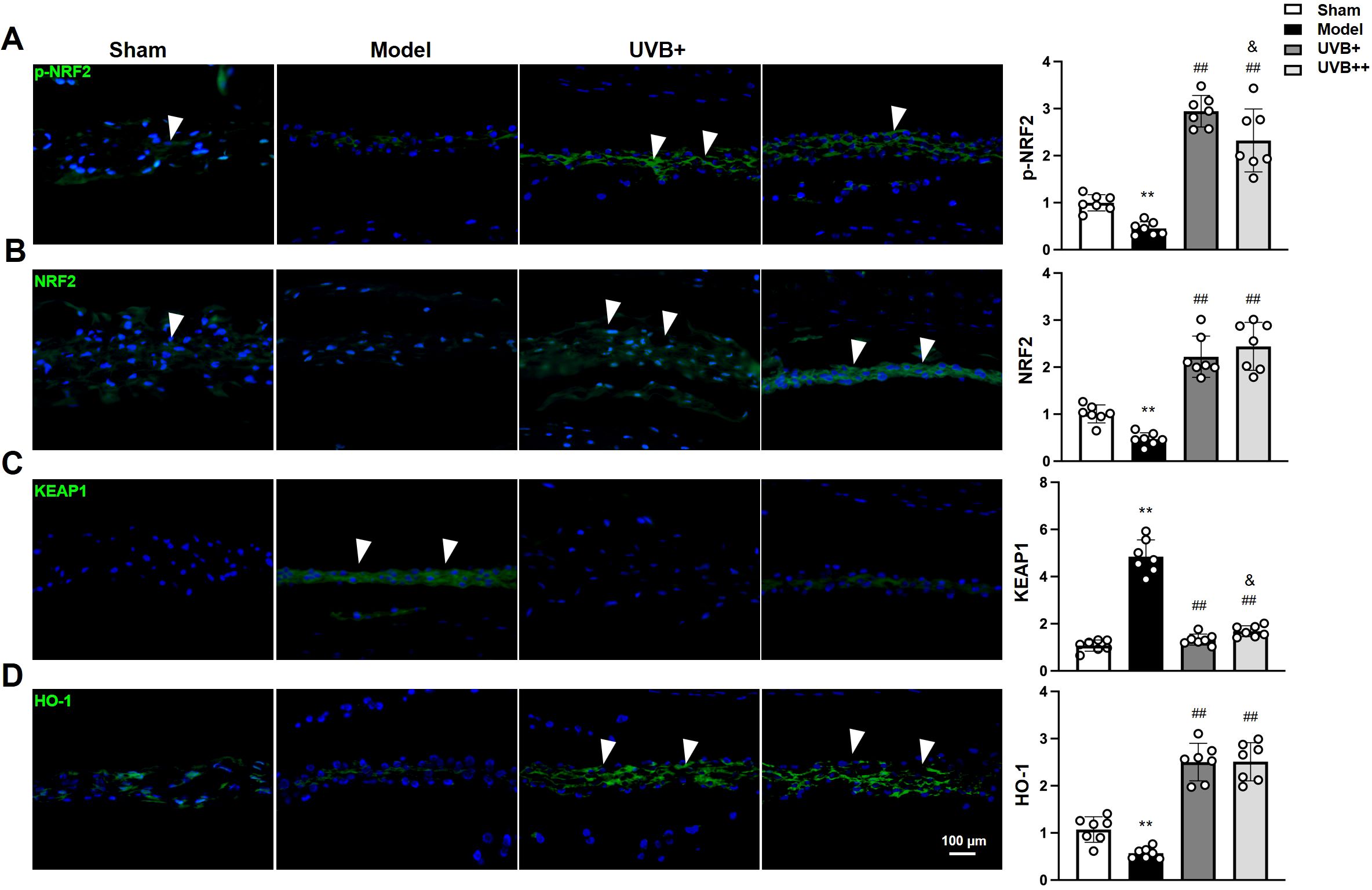
Figure 6. UVB activates NRF2/KEAP1 antioxidant pathway in degenerative discs. (A-D) Representative immunofluorescence images and quantification of (A) phosphorylated NRF2, (C) total NRF2, (E) KEAP1, and (G) HO-1 expression in NP tissues. Data are presented as mean ± SD. **p < 0.01 vs Sham group; ##p < 0.01 vs Model group; &p < 0.05 vs UVB+ group. n = 6 per group.
4 Discussion
IVDD is characterized by progressive structural deterioration and functional impairment, significantly impacting patients’ quality of life. Despite advances in understanding its pathogenesis, current treatments remain largely symptomatic, highlighting the urgent need for effective therapeutic strategies (32). The complex interplay between oxidative stress and inflammation in IVDD progression suggests that interventions targeting these pathways might offer promising therapeutic potential. UVB irradiation has shown remarkable therapeutic efficacy in various inflammatory conditions through its immunomodulatory and antioxidant properties (11, 33, 34); however, whether UVB could ameliorate IVDD and its underlying mechanisms remained unexplored. In the present study, we demonstrated that UVB irradiation effectively attenuated LSI surgery-induced IVDD progression, as evidenced by improved behavioral outcomes, preserved disc height, and maintained ECM homeostasis. Notably, the 2-minute UVB treatment showed superior therapeutic effects compared to the 4-minute regimen, particularly in preserving disc structure and ECM composition. Moreover, we identified that UVB treatment significantly suppressed NLRP3-mediated pyroptosis in NP cells, manifested by reduced expression of pyroptotic markers and associated inflammatory cytokines. Mechanistically, we uncovered that UVB treatment suppressed NLRP3-mediated pyroptosis in NP cells by downregulating key pyroptosis-related proteins, including NLRP3, ASC, GSDMD, and CASPASE1, likely through enhanced activation of NRF2/KEAP1 antioxidant pathway (Figures 7). These findings not only establish UVB irradiation as a promising therapeutic strategy for IVDD but also reveal novel regulatory mechanism underlying UVB-mediated disc protection.
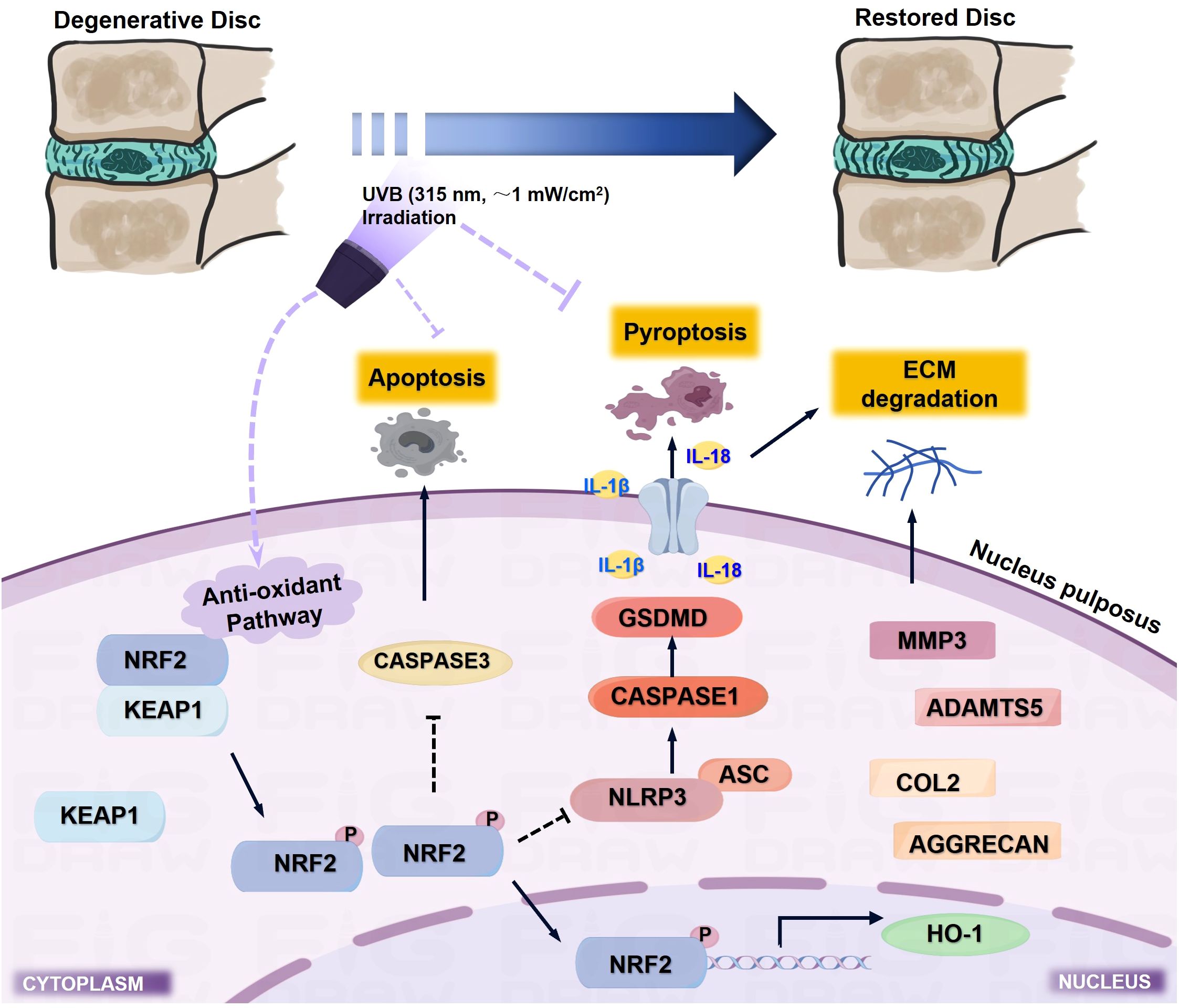
Figure 7. Schematic illustration of the molecular mechanism underlying UVB-mediated protection against IVDD progression. UVB irradiation (315 nm, ~1 mW/cm²) activates the NRF2/KEAP1 antioxidant pathway, which suppresses NLRP3-mediated pyroptosis and subsequent inflammatory cascade, while inhibiting apoptosis and ECM degradation, ultimately leading to preserved disc structure and function.
UVB phototherapy has been widely used in clinical practice for decades, particularly in treating inflammatory skin conditions such as psoriasis and atopic dermatitis, with well-established safety profiles and standardized protocols. For instance, in a case of generalized lichen nitidus, a twice-weekly regimen of 0.25 and 0.15 J/cm² NB-UVB irradiation over 8 to 10 weeks, resulting in cumulative doses of 5.36 and 3.05 J/cm², led to the complete clearance of the majority of lesions (35). Similarly, in a study involving five patients with moderate to severe atopic dermatitis, a total dose of 9.2 J/cm² delivered over 19 sessions significantly alleviated symptoms within three weeks (36). Additionally, ten patients with stable plaque psoriasis treated with NB-UVB at a cumulative dose of 9.8 J/cm² over 14 sessions showed a notable reduction in psoriasis area and severity index scores (37). In our study, the UVB emitter with an output of 1.09 mW/cm² delivered radiation doses of 0.13 J/cm² and 0.26 J/cm² for 2-min and 4-min exposures, respectively. Over an 8-week treatment period, this protocol resulted in cumulative doses of 3.12 J/cm² and 6.24 J/cm², demonstrating consistency with the therapeutic parameters reported in these clinical studies. Our findings extend UVB’s therapeutic potential to IVDD treatment, offering several unique advantages. First, UVB irradiation represents a non-invasive intervention that can be repeatedly administered without accumulative tissue damage. Second, the differential effects observed between UVB+ and UVB++ treatments suggest a critical therapeutic window, where the 2-minute protocol demonstrates optimal efficacy in preserving disc height and ECM homeostasis while potentially minimizing side effects. Moreover, the accessibility and cost-effectiveness of UVB therapy make it particularly attractive for long-term IVDD management. Although our current study utilized substantially lower UVB doses with shorter exposure durations, we recognize the potential risk of cutaneous damage in future studies involving chronic or escalated-dose protocols. To mitigate this risk, subsequent experiments will incorporate a combined approach of localized UVB irradiation with topical antioxidant administration as a protective measure against UV-induced genotoxicity.
The maintenance of ECM homeostasis is crucial for preserving disc function (15). Our results demonstrate that UVB treatment effectively prevents ECM degradation by simultaneously promoting matrix synthesis and inhibiting catabolic processes. The superior efficacy of UVB+ in maintaining AGGRECAN and COL2 levels while suppressing ADAMTS5 suggests a more balanced regulation of ECM metabolism compared to UVB ++. This optimal matrix homeostasis directly contributes to preserved disc height and improved behavioral outcomes, establishing a clear structure-function relationship in UVB-mediated IVDD protection.
Extensive studies have demonstrated that both apoptosis and pyroptosis contribute to IVDD progression, with pyroptosis uniquely capable of triggering inflammatory cytokine bursts while apoptosis does not (38, 39). Emerging evidence suggests UVB therapy can target and inhibit cell apoptosis while simultaneously suppressing the secretion of inflammatory factors including IL-18, IL-6, TNF-α, and IL-1R, thereby demonstrating therapeutic efficacy in the management of inflammatory dermatological conditions such as atopic dermatitis and psoriasis (17, 18, 34, 40), suggesting its potential therapeutic effects through modulation of both apoptotic and pyroptotic pathways. Indeed, our findings demonstrate that UVB effectively suppressed both cell death pathways in IVDD, as evidenced by restored BCL2/BAX balance and reduced CASPASE3 expression, along with significant inhibition of the pyroptotic cascade. The dramatic upregulation of IL-1β and IL-18 in the Model group, coupled with elevated NLRP3 inflammasome components, indicates the prominent role of pyroptosis in IVDD pathogenesis. UVB treatment, particularly the UVB+ protocol, effectively suppressed both the pyroptotic cascade and apoptotic pathway, with TUNEL staining confirming the comprehensive anti-cell death effects. This dual regulation supports our previous findings (25) that pyroptosis functions upstream of apoptosis in IVDD progression, where UVB primarily targets early pyroptotic pathways through NRF2/KEAP1 activation, subsequently reducing inflammatory cytokine-mediated secondary apoptosis, distinguishing it from conventional anti-inflammatory treatments.
The NRF2/KEAP1 signaling pathway serves as a master regulator of cellular antioxidant responses in IVDD progression by orchestrating the transcription of numerous cytoprotective genes, maintaining redox homeostasis, and suppressing inflammatory responses (41). Studies have demonstrated that extracellular nanovesicles, slowed the progression of IVDD by inhibiting nucleus pulposus cell ferroptosis via the NRF2/KEAP1 signaling pathway, and even by promoting m6A demethylation of Nrf2 (42, 43). Emerging evidence suggests that both pharmacological and physical interventions targeting NRF2 signaling exhibit therapeutic potential. For instance, chemical compounds such as senolytic agent quercetin (44), Verapamil (45) have been shown to enhance NRF2-mediated cytoprotective functions, thereby mitigating IVDD progression. Similarly, physical modalities have also shown significant therapeutic effects through NRF2 activation. Moderate mechanical stress has been found to alleviate osteoarthritis progression through NRF2 activation (46), and low-intensity pulsed ultrasound has demonstrated the ability to maintain alveolar bone homeostasis by modulating NRF2 signaling in periodontitis models (47). Moreover, inhibition of oxidative stress has been identified as a key mechanism in UVB therapy for various skin disorders (48–50), particularly through activation of the NRF2/KEAP1 antioxidant pathway (27, 28). The most intriguing finding of our study is the significant activation of the NRF2/KEAP1 pathway by UVB treatment. Notably, UVB not only restored but enhanced NRF2 pathway activity beyond physiological levels, while normalizing elevated KEAP1 expression. This unique activation pattern suggests that UVB might provide superior antioxidant protection compared to conventional NRF2 activators. The stronger activation of this pathway by UVB+ compared to UVB++ correlates well with its superior therapeutic effects, indicating that appropriate UVB dosing is crucial for optimal pathway modulation. However, the causal relationship between the NRF2/KEAP1 pathway and pyroptosis observed in our study requires further validation. Future investigations employing NRF2-specific inhibitors or genetic knockout models would be essential to establish a definitive mechanistic link.
Current evidence suggests that phototherapeutic modalities can directly target the human musculoskeletal system to achieve therapeutic effects, indicating the presence of photosensitive receptors capable of receiving and transmitting light signals in musculoskeletal tissues (51, 52). Although direct evidence of UVB acting on bone remains limited, studies have demonstrated that 408 nm visible light can penetrate up to 1 mm into the skin (53). In our experimental model, the murine epidermal thickness is approximately 0.4 mm, with vertebral bodies and IVDs located directly beneath the skin. Given that UVB wavelengths range between 290–320 nm, we selected NB-UVB with a peak emission at 315 nm, which is close to the most widely reported therapeutic wavelength (311 ± 2 nm). This choice was based on its enhanced penetration depth, improved energy concentration, and superior safety-profile compared to broadband UVB (6). However, whether UVB directly penetrates the epidermis to act on IVDs or exerts its effects indirectly through vitamin D-mediated pathways requires further experimental validation.
Several limitations of our study warrant further investigation. While the LSI surgery-induced IVDD model recapitulates key features of human disc degeneration, the acute nature of this model might not fully represent the chronic progression of human IVDD. Although we demonstrated that UVB irradiation significantly improved the local antioxidant status of IVDs, the precise regulatory mechanisms remain to be elucidated. UVB therapy has been shown to significantly affect serum 25-hydroxyvitamin D and total vitamin D levels (54), which has been recognized as crucial modulators of systemic antioxidant status (55), particularly through the NRF2/KEAP1 pathway (56), inflammatory responses (57, 58), and cellular pyroptosis (59–61). Recent studies have demonstrated that vitamin D receptor (VDR) activation can upregulate the NRF2/HO-1 signaling pathway to suppress ferroptosis and stimulate Nrf2/GPX4 signaling pathways (62, 63). Moreover, clinical studies on VDR polymorphisms (64) and experimental evidence from animal models (65, 66) have consistently demonstrated the protective role of vitamin D in IVDD through suppressing inflammatory responses (67, 68). Indeed, our preliminary data demonstrated that UVB treatment effectively restored the abnormally decreased serum 1,25-(OH)2-Vitamin D3 levels in ovariectomized-induced osteoporotic mice (data not shown), suggesting a potential systemic mechanism requiring further investigation. The therapeutic efficacy observed in our study may result from synergistic effects between direct NRF2/KEAP1 activation and vitamin D-mediated systemic responses, as recent evidence suggests potential mechanistic crosstalk between VDR signaling and NRF2 pathways that warrants further investigation. Although no adverse effects were observed in the current experimental setting, comprehensive safety assessments including detailed evaluation of skin integrity, DNA damage markers, long-term carcinogenic risks, bone integrity, surrounding tissues, and hematological parameters were not systematically performed. These assessments remain crucial for evaluating the long-term implications of UVB therapy and represent important considerations for future clinical translation and dose optimization protocols. Additionally, for clinical translation, several key aspects need to be addressed: (1) optimization of treatment parameters including radiation dose, frequency, and duration in human subjects; (2) establishment of standardized protocols for different patient populations; (3) evaluation of long-term safety and efficacy through well-designed clinical trials; and (4) development of personalized treatment strategies based on individual patient characteristics such as age, gender, and disease severity.
5 Conclusion
In conclusion, our study demonstrates that UVB irradiation, particularly with a 2-minute treatment protocol, effectively attenuates IVDD progression through potent activation of the NRF2/KEAP1 antioxidant pathway and subsequent suppression of NLRP3-mediated pyroptosis. These findings not only establish UVB irradiation as a promising therapeutic approach for IVDD treatment but also uncover its novel protective mechanism in degenerative disc disease. Future studies addressing the detailed molecular mechanisms, particularly the potential role of systemic vitamin D signaling, together with well-designed clinical trials will be essential for translating this therapeutic approach into clinical practice.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Zhejiang Chinese Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HLZ: Writing – original draft, Data curation, Formal analysis. XT: Data curation, Formal analysis, Writing – original draft. HQZ: Writing – review & editing, Data curation. CZ: Data curation, Writing – original draft. ZZ: Data curation, Writing – original draft. SS: Data curation, Writing – original draft. XF: Data curation, Writing – original draft. JD: Data curation, Writing – original draft. SC: Data curation, Writing – original draft. JZ: Validation, Writing – original draft, Funding acquisition. SX: Validation, Writing – original draft. ZC: Validation, Writing – original draft. FF: Funding acquisition, Writing – original draft. KT: Validation, Writing – original draft, Funding acquisition. HL: Conceptualization, Validation, Writing – original draft. JP: Conceptualization, Writing – original draft, Validation. CW: Conceptualization, Funding acquisition, Writing – original draft. HR: Conceptualization, Funding acquisition, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financially supported by the National Key Research and Development Program of China (No. 2024YFC3507402), the National Natural Science Foundation of China (No. U24A20780, 82174140, 82174401), the Key Research and Development Program of Zhejiang Province (No. 2024C03189), the Natural Science Foundation of Zhejiang Province (No. LQ23H270003), the Traditional Chinese Medical Administration of Zhejiang Province (No. 2025ZL066, 2023ZR019, 2022ZX005), the Zhejiang Medical and Health Science and Technology Project (No. 2025KY098, 2025KY1237, 2024KY1216, 2023RC194, 2023KY235), the Zhejiang Pharmaceutical Association Hospital Pharmacy Special Project (No. 2023ZYY11), the Research Project of Zhejiang Chinese Medical University (No. 2023JKZKTS40, 2021JKZDZC02), and the Research Project of Zhejiang Chinese Medical University Affiliated Hospital (No. 2024FSYYZY31, 2023FSYYZZ02, 2023JKZKTS27, 2023FSYYZY40).
Acknowledgments
We appreciate the technical support from the Medical Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chiarotto A and Koes BW. Nonspecific low back pain. N Engl J Med. (2022) 386:1732–40. doi: 10.1056/NEJMcp2032396
2. Xiang H, Zhao W, Jiang K, He J, Chen L, Cui W, et al. Progress in regulating inflammatory biomaterials for intervertebral disc regeneration. Bioact Mater. (2024) 33:506–31. doi: 10.1016/j.bioactmat.2023.11.021
3. Chen X, Zhang A, Zhao K, Gao H, Shi P, Chen Y, et al. The role of oxidative stress in intervertebral disc degeneration: Mechanisms and therapeutic implications. Ageing Res Rev. (2024) 98:102323. doi: 10.1016/j.arr.2024.102323
4. Cerutti P, Ghosh R, Oya Y, and Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ Health Perspect. (1994) 102 Suppl 10:123–9. doi: 10.1289/ehp.94102s10123
5. Hile GA, Coit P, Xu B, Victory AM, Gharaee-Kermani M, Estadt SN, et al. Regulation of photosensitivity by the hippo pathway in lupus skin. Arthritis Rheumatol. (2023) 75:1216–28. doi: 10.1002/art.42460
6. Musters AH, Mashayekhi S, Harvey J, Axon E, Lax SJ, Flohr C, et al. Phototherapy for atopic eczema. Cochrane Database Syst Rev. (2021) 10:CD013870. doi: 10.1002/14651858.CD013870.pub2
7. Seneschal J, Speeckaert R, Taïeb A, Wolkerstorfer A, Passeron T, Pandya AG, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: Position statement from the international Vitiligo Task Force-Part 2: Specific treatment recommendations. J Eur Acad Dermatol Venereol. (2023) 37:2185–95. doi: 10.1111/jdv.19450
8. van de Kerkhof PCM and de Gruijl FR. Phototherapy in the perspective of the chronicity of psoriasis. J Eur Acad Dermatol Venereol. (2020) 34:926–31. doi: 10.1111/jdv.16245
9. Romaní J, Caixàs A, Carrascosa JM, Ribera M, Rigla M, and Luelmo J. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. (2012) 166:1237–44. doi: 10.1111/j.1365-2133.2012.10883.x
10. Vacharanukrauh P, Meephansan J, Tangtanatakul P, Soonthornchai W, Wongpiyabovorn J, Serirat O, et al. High-throughput RNA sequencing reveals the effect of NB-UVB phototherapy on major inflammatory molecules of lesional psoriasis. Psoriasis (Auckl). (2021) 11:133–49. doi: 10.2147/PTT.S335913
11. Ye J, Huang H, Luo G, Yin L, Li B, Chen S, et al. NB-UVB irradiation attenuates inflammatory response in psoriasis. Dermatol Ther. (2020) 33:e13626. doi: 10.1111/dth.13626
12. Białczyk A, Wełniak A, Kamińska B, and Czajkowski R. Oxidative stress and potential antioxidant therapies in vitiligo: A narrative review. Mol Diagn Ther. (2023) 27:723–39. doi: 10.1007/s40291-023-00672-z
13. Ochiai S, Nishida Y, Higuchi Y, Morita D, Makida K, Seki T, et al. Short-range UV-LED irradiation in postmenopausal osteoporosis using ovariectomized mice. Sci Rep. (2021) 11:7875. doi: 10.1038/s41598-021-86730-0
14. Kang L, Zhang H, Jia C, Zhang R, and Shen C. Epigenetic modifications of inflammation in intervertebral disc degeneration. Ageing Res Rev. (2023) 87:101902. doi: 10.1016/j.arr.2023.101902
15. Ge Y, Chen Y, Guo C, Luo H, Fu F, Ji W, et al. Pyroptosis and intervertebral disc degeneration: mechanistic insights and therapeutic implications. J Inflammation Res. (2022) 15:5857–71. doi: 10.2147/JIR.S382069
16. Micić I, Jeon I-H, Park S-H, Hwa S-S, Chun J-M, and Stojiljković P. The effect of short-term low-energy ultraviolet B irradiation on bone mineral density and bone turnover markers in postmenopausal women with osteoporosis: a randomized single-blinded controlled clinical trial. Srp Arh Celok Lek. (2013) 141:615–22. doi: 10.2298/sarh1310615m
17. Piskin G, Tursen U, Sylva-Steenland RMR, Bos JD, and Teunissen MBM. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers – IL-12, IL-18 and IL-23. Exp Dermatol. (2004) 13:764–72. doi: 10.1111/j.0906-6705.2004.00246.x
18. Batycka-Baran A, Besgen P, Wolf R, Szepietowski JC, and Prinz JC. The effect of phototherapy on systemic inflammatory process in patients with plaque psoriasis. J Photochem Photobiol B. (2016) 161:396–401. doi: 10.1016/j.jphotobiol.2016.05.023
19. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J Eur Acad Dermatol Venereol. (2010) 24:1386–94. doi: 10.1111/j.1468-3083.2010.03647.x
20. Sigurdardottir G, Ekman A-K, Verma D, and Enerbäck C. Decreased systemic levels of endocan-1 and CXCL16 in psoriasis are restored following narrowband UVB treatment. Dermatology. (2018) 234:173–9. doi: 10.1159/000491819
21. Fu F, Luo H, Du Y, Chen Y, Tian K, Pan J, et al. AR/PCC herb pair inhibits osteoblast pyroptosis to alleviate diabetes-related osteoporosis by activating Nrf2/Keap1 pathway. J Cell Mol Med. (2023) 27:3601–13. doi: 10.1111/jcmm.17928
22. Song G, Wang J, Liu J, and Ruan Y. Dimethyl fumarate ameliorates erectile dysfunction in bilateral cavernous nerve injury rats by inhibiting oxidative stress and NLRP3 inflammasome-mediated pyroptosis of nerve via activation of Nrf2/HO-1 signaling pathway. Redox Biol. (2023) 68:102938. doi: 10.1016/j.redox.2023.102938
23. Lin Y, Luo T, Weng A, Huang X, Yao Y, Fu Z, et al. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing nrf2 signaling. Front Immunol. (2020) 11:580593. doi: 10.3389/fimmu.2020.580593
24. Kang J-Y, Xu M-M, Sun Y, Ding Z-X, Wei Y-Y, Zhang D-W, et al. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int Immunopharmacol. (2022) 109:108782. doi: 10.1016/j.intimp.2022.108782
25. Fu F, Bao R, Yao S, Zhou C, Luo H, Zhang Z, et al. Aberrant spinal mechanical loading stress triggers intervertebral disc degeneration by inducing pyroptosis and nerve ingrowth. Sci Rep. (2021) 11:772. doi: 10.1038/s41598-020-80756-6
26. Lao Z, Fang X, Shen S, Zhang Y, Chen X, Zhang H, et al. The onset of systemic lupus erythematosus triggers nucleus pulposus cell pyroptosis to exacerbate intervertebral disc degeneration. J Inflammation Res. (2024) 17:7705–19. doi: 10.2147/JIR.S486297
27. Addison R, Weatherhead SC, Pawitri A, Smith GR, Rider A, Grantham HJ, et al. Therapeutic wavelengths of ultraviolet B radiation activate apoptotic, circadian rhythm, redox signalling and key canonical pathways in psoriatic epidermis. Redox Biol. (2021) 41:101924. doi: 10.1016/j.redox.2021.101924
28. Bian Y, Yu H, Jin M, and Gao X. Repigmentation by combined narrow−band ultraviolet B/adipose−derived stem cell transplantation in the mouse model: Role of Nrf2/HO−1−mediated Ca2+ homeostasis. Mol Med Rep. (2022) 25. doi: 10.3892/mmr.2021.12522
29. Ge Y, Xie Y, Chai J, Ji W, Lou X, Tian K, et al. Augmented cornus officinalis levels in liuwei dihuang decoction inhibits nucleus pulposus cell pyroptosis to enhance therapeutic efficacy against intervertebral disc degeneration. J Inflammation Res. (2024) 17:4453–65. doi: 10.2147/JIR.S465690
30. Yoo JJ, Hayes M, Serafin EK, and Baccei ML. Early-life iron deficiency persistently alters nociception in developing mice. J Pain. (2023) 24:1321–36. doi: 10.1016/j.jpain.2023.03.012
31. Norcross JP, Lester GE, Weinhold P, and Dahners LE. An in vivo model of degenerative disc disease. J Orthop Res. (2003) 21:183–8. doi: 10.1016/S0736-0266(02)00098-0
32. Zhang H, Yang X, Huang Y, Li Y, Hu Q, Wei Q, et al. Reviving intervertebral discs: treating degeneration using advanced delivery systems. Mol Pharm. (2024) 21:373–92. doi: 10.1021/acs.molpharmaceut.3c00579
33. Elmelid A, Vandikas MS, Gillstedt M, Alsterholm M, and Osmancevic A. The effect of phototherapy on systemic inflammation measured with serum vitamin D-binding protein and hsCRP in patients with inflammatory skin disease. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25168632
34. Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. (2011) 128. doi: 10.1016/j.jaci.2011.05.042
35. Do M-O, Kim M-J, Kim S-H, Myung K-B, and Choi Y-W. Generalized lichen nitidus successfully treated with narrow-band UVB phototherapy: two cases report. J Korean Med Sci. (2007) 22:163–6. doi: 10.3346/jkms.2007.22.1.163
36. Grundmann-Kollmann M, Behrens S, Podda M, Peter RU, Kaufmann R, and Kerscher M. Phototherapy for atopic eczema with narrow-band UVB. J Am Acad Dermatol. (1999) 40:995–7. doi: 10.1016/s0190-9622(99)70090-3
37. Behrens S, Grundmann-Kollmann M, Schiener R, Peter RU, and Kerscher M. Combination phototherapy of psoriasis with narrow-band UVB irradiation and topical tazarotene gel. J Am Acad Dermatol. (2000) 42:493–5. doi: 10.1016/s0190-9622(00)90225-1
38. Lin J, Zheng X, Xiong Z, Xiang Q, Zhao Y, Jiang S, et al. DJ-1-mediated p62 degradation delays intervertebral disc degeneration by inhibiting apoptosis of nucleus pulposus cells. Apoptosis. (2023) 28:1357–71. doi: 10.1007/s10495-023-01862-0
39. Luo J, Yang Y, Wang X, Chang X, and Fu S. Role of pyroptosis in intervertebral disc degeneration and its therapeutic implications. Biomolecules. (2022) 12. doi: 10.3390/biom12121804
40. Yin L, Hu Y, Xu J, Guo J, Tu J, and Yin Z. Ultraviolet B inhibits IL-17A/TNF-α-stimulated activation of human dermal fibroblasts by decreasing the expression of IL-17RA and IL-17RC on fibroblasts. Front Immunol. (2017) 8:91. doi: 10.3389/fimmu.2017.00091
41. Xiang Q, Zhao Y, Lin J, Jiang S, and Li W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp Mol Med. (2022) 54:1067–75. doi: 10.1038/s12276-022-00829-6
42. Chen C, Wang X, Zhao Y, Duan X, Hu Z, et al. Exosomes inhibit ferroptosis to alleviate intervertebral disc degeneration via the p62-KEAP1-NRF2 pathway. Free Radic Biol Med. (2025)232:171–84. doi: 10.1016/j.freeradbiomed.2025.02.027
43. Gao X, Jia S, Gao L, Chen S, Zhang Y, Liang X, et al. MSC-derived exosomes alleviate oxidative stress-induced lysosomal membrane permeabilization damage in degenerated nucleus pulposus cells via promoting m6A demethylation of Nrf2. Free Radic Biol Med. (2025) 235:213–30. doi: 10.1016/j.freeradbiomed.2025.04.051
44. Shao Z, Wang B, Shi Y, Xie C, Huang C, Chen B, et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr Cartil. (2021) 29:413–22. doi: 10.1016/j.joca.2020.11.006
45. Chen Y, Cao X, Pan B, Du H, Li B, Yang X, et al. Verapamil attenuates intervertebral disc degeneration by suppressing ROS overproduction and pyroptosis via targeting the Nrf2/TXNIP/NLRP3 axis in four-week puncture-induced rat models both in vivo and in vitro. Int Immunopharmacol. (2023) 123:110789. doi: 10.1016/j.intimp.2023.110789
46. Han J, Zhan L-N, Huang Y, Guo S, Zhou X, Kapilevich L, et al. Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway. Sci Rep. (2024) 14:5078. doi: 10.1038/s41598-024-55629-x
47. Ying S, Tan M, Feng G, Kuang Y, Chen D, Li J, et al. Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress. Theranostics. (2020) 10:9789–807. doi: 10.7150/thno.42508
48. Darlenski R, Hristakieva E, Aydin U, Gancheva D, Gancheva T, Zheleva A, et al. Epidermal barrier and oxidative stress parameters improve during in 311 nm narrow band UVB phototherapy of plaque type psoriasis. J Dermatol Sci. (2018) 91:28–34. doi: 10.1016/j.jdermsci.2018.03.011
49. Karsli N, Akcali C, Ozgoztasi O, Kirtak N, and Inaloz S. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J Int Med Res. (2014) 42:799–805. doi: 10.1177/0300060513516294
50. Wacewicz M, Socha K, Soroczyńska J, Niczyporuk M, Aleksiejczuk P, Ostrowska J, et al. Concentration of selenium, zinc, copper, Cu/Zn ratio, total antioxidant status and c-reactive protein in the serum of patients with psoriasis treated by narrow-band ultraviolet B phototherapy: A case-control study. J Trace Elem Med Biol. (2017) 44:109–14. doi: 10.1016/j.jtemb.2017.06.008
51. Zhang Z, Wang R, Xue H, Knoedler S, Geng Y, Liao Y, et al. Phototherapy techniques for the management of musculoskeletal disorders: strategies and recent advances. Biomater Res. (2023) 27:123. doi: 10.1186/s40824-023-00458-8
52. Herrera-Arcos G, Song H, Yeon SH, Ghenand O, Gutierrez-Arango S, Sinha S, et al. Closed-loop optogenetic neuromodulation enables high-fidelity fatigue-resistant muscle control. Sci Robot. (2024) 9:eadi8995. doi: 10.1126/scirobotics.adi8995
53. Juzenas P, Juzeniene A, Kaalhus O, Iani V, and Moan J. Noninvasive fluorescence excitation spectroscopy during application of 5-aminolevulinic acid in vivo. Photochem Photobiol Sci. (2002) 1:745–8. doi: 10.1039/c5pp90043c
54. El-Hanbuli HM, Dawoud NM, and Mahmoud RH. Narrow-band UVB effects on cutaneous vitamin D receptor expression and serum 25-hydroxyvitamin D in generalized vitiligo. Photodermatol Photoimmunol Photomed. (2018) 34:175–83. doi: 10.1111/phpp.12362
55. Vázquez-Lorente H, Herrera-Quintana L, Jiménez-Sánchez L, Fernández-Perea B, and Plaza-Diaz J. Antioxidant functions of vitamin D and CYP11A1-derived vitamin D, tachysterol, and lumisterol metabolites: mechanisms, clinical implications, and future directions. Antioxid (Basel). (2024) 13. doi: 10.3390/antiox13080996
56. Qiao J, Ma H, Chen M, and Bai J. Vitamin D alleviates neuronal injury in cerebral ischemia-reperfusion via enhancing the Nrf2/HO-1 antioxidant pathway to counteract NLRP3-mediated pyroptosis. J Neuropathol Exp Neurol. (2023) 82:722–33. doi: 10.1093/jnen/nlad047
57. Almeida Moreira Leal LK, Lima LA, Alexandre de Aquino PE, Costa de Sousa JA, Jataí Gadelha CV, Felício Calou IB, et al. (VD3) antioxidative and anti-inflammatory activities: Peripheral and central effects. Eur J Pharmacol. (2020) 879:173099. doi: 10.1016/j.ejphar.2020.173099
58. Cojic M, Kocic R, Klisic A, and Kocic G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: A 6-month follow up randomized controlled study. Front Endocrinol (Lausanne). (2021) 12:610893. doi: 10.3389/fendo.2021.610893
59. Huang C, Zhang C, Yang P, Chao R, Yue Z, Li C, et al. Eldecalcitol inhibits LPS-induced NLRP3 inflammasome-dependent pyroptosis in human gingival fibroblasts by activating the nrf2/HO-1 signaling pathway. Drug Des Devel Ther. (2020) 14:4901–13. doi: 10.2147/DDDT.S269223
60. Shang L, Li J, Zhou F, Zhang M, Wang S, and Yang S. MiR-874-5p targets VDR/NLRP3 to reduce intestinal pyroptosis and improve intestinal barrier damage in sepsis. Int Immunopharmacol. (2023) 121:110424. doi: 10.1016/j.intimp.2023.110424
61. Tang L, Zhang D, Zhang Y, Peng Y, Li M, Song H, et al. Vitamin D3 alleviates lung fibrosis of type 2 diabetic rats via SIRT3 mediated suppression of pyroptosis. Apoptosis. (2023) 28:1618–27. doi: 10.1007/s10495-023-01878-6
62. Wang H, Yu X, Liu D, Qiao Y, Huo J, Pan S, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating nrf2/HO-1 signaling pathway in diabetic nephropathy. Adv Sci (Weinh). (2024) 11:e2305563. doi: 10.1002/advs.202305563
63. Xu P, Lin B, Deng X, Huang K, Zhang Y, and Wang N. VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic Biol Med. (2022) 193:720–35. doi: 10.1016/j.freeradbiomed.2022.11.013
64. Colombini A, Cauci S, Lombardi G, Lanteri P, Croiset S, Brayda-Bruno M, et al. Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J Steroid Biochem Mol Biol. (2013) 138:24–40. doi: 10.1016/j.jsbmb.2013.03.001
65. Huang H, Cheng S, Zheng T, Ye Y, Ye A, Zhu S, et al. Vitamin D retards intervertebral disc degeneration through inactivation of the NF-κB pathway in mice. Am J Transl Res. (2019) 11:2496–506.
66. Wang P, Yang C, Lu J, Ren Y, Goltzman D, and Miao D. Sirt1 protects against intervertebral disc degeneration induced by 1,25-dihydroxyvitamin D insufficiency in mice by inhibiting the NF-κB inflammatory pathway. J Orthop Translat. (2023) 40:13–26. doi: 10.1016/j.jot.2023.04.003
67. Colombini A, De Luca P, Cangelosi D, Perucca Orfei C, Ragni E, Viganò M, et al. High-throughput gene and protein analysis revealed the response of disc cells to vitamin D, depending on the VDR fokI variants. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22179603
Keywords: intervertebral disc degeneration, narrowband ultraviolet B, NLRP3-mediated pyroptosis, nucleus pulposus, NRF2/KEAP1 antioxidant pathway
Citation: Zhang H, Tang X, Zhou H, Zhou C, Zhang Z, Shen S, Fang X, Du J, Cheng S, Zhang J, Xing S, Chen Z, Fu F, Tian K, Luo H, Pan J, Wu C and Ruan H (2025) Narrowband ultraviolet B radiation attenuates nucleus pulposus pyroptosis to ameliorate intervertebral disc degeneration by activating NRF2/KEAP1 antioxidant pathway. Front. Immunol. 16:1663674. doi: 10.3389/fimmu.2025.1663674
Received: 10 July 2025; Accepted: 30 September 2025;
Published: 22 October 2025.
Edited by:
Yun Qian, Shanghai Jiao Tong University, ChinaReviewed by:
Shaghayegh Basatvat, Vitalant Research Institute, United StatesLiang Kang, First Affiliated Hospital of Anhui Medical University, China
Ankita Samanta, University of British Columbia, Canada
Sai Yao, Fudan University, China
Copyright © 2025 Zhang, Tang, Zhou, Zhou, Zhang, Shen, Fang, Du, Cheng, Zhang, Xing, Chen, Fu, Tian, Luo, Pan, Wu and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Pan, MDExMzAxNkBjYWEuZWR1LmNu; Chengliang Wu, d2NsQHpjbXUuZWR1LmNu; Hongfeng Ruan, cmhmQHpjbXUuZWR1LmNu
†These authors have contributed equally to this work
 Helou Zhang
Helou Zhang Xinyun Tang
Xinyun Tang Huiqing Zhou1,2†
Huiqing Zhou1,2† Chengcong Zhou
Chengcong Zhou Shuchao Shen
Shuchao Shen Xuliang Fang
Xuliang Fang Zikun Chen
Zikun Chen Fangda Fu
Fangda Fu Huan Luo
Huan Luo Chengliang Wu
Chengliang Wu Hongfeng Ruan
Hongfeng Ruan