- 1Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Cancer Epidemiology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
Background: Immunotherapy has demonstrated unique advantages in MSI-H/dMMR colorectal cancer (CRC) for its later-line, first-line in metastatic status, and neoadjuvant therapy. However, evidence regarding its value in postoperative adjuvant therapy remains limited.
Methods: We retrospectively analyzed 261 stage II/III MSI-H/dMMR CRC patients who underwent radical resection with over 2 years of follow-up. Disease-free survival (DFS) curves were used to compare prognoses under different postoperative strategies, and factors associated with recurrence were investigated.
Results: The patients cohort (mean age 55.3, range 19-86 years and male for 56.3%) had a median follow-up of 30 (range 24-45) months. Recurrence occurred in 18 patients (6.9%), with an overall DFS rate of 93.1% during follow-up period. Overall, postoperative immunotherapy showed non-significant DFS advantage over watchful waiting (WW) (HR = 0.19, 95%CI: 0.03-1.39, P = 0.101), but it demonstrated statistically superior DFS compared to chemotherapy (HR = 0.26, 95%CI: 0.08-0.89, P = 0.033). Subgroup analyses revealed: 1) For patients achieving pathologic complete response after neoadjuvant therapy, postoperative WW and immunotherapy were equivalent (both DFS 100%); 2) For Stage II, WW and immunotherapy showed comparable DFS (HR = 0.21, 95%CI: 0.003-13.04, P = 0.463). 3) For Stage III, immunotherapy showed a trend toward superior DFS versus chemotherapy, though statistical significance was not reached (HR = 0.28, 95%CI: 0.04-1.96, P = 0.204), and both outperformed WW (HR = 0.05, 95%CI: 0.004-0.54, P = 0.014 and HR = 0.34, 95%CI: 0.05-2.17, P = 0.113, respectively). Factors significantly associated with recurrence included Lynch-negative (P = 0.02) and perineural invasion (P = 0.014).
Conclusions: MSI-H/dMMR CRC exhibits excellent prognosis after radical surgery. Postoperative WW remains the preferred strategy for Stage II patients, While patients with stage III requires intensive adjuvant therapy, and immunotherapy may surpass conventional chemotherapy recommended by the current guidelines.
1 Introduction
Colorectal cancer (CRC) characterized by high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR) arises from defective DNA repair machinery, leading to hypermutation and abundant neoantigen generation, which fosters a highly immunogenic microenvironment enriched with tumor-infiltrating lymphocytes and elevated expression of immune checkpoints (e.g., PD-1/PD-L1) (1). This biological uniqueness underpins their exceptional responsiveness to immune checkpoint inhibitors (ICIs) (2, 3). In 2015, Le DT et al. first discovered that MSI-H/dMMR metastatic CRC (mCRC) could significantly benefit from immunotherapy with pembrolizumab, an ICI named PD-1 monoclonal antibody. Thereafter, immunotherapy enters the later-line treatment of mCRC (4, 5). Landmark trials (KEYNOTE-177, ChecKMate-142) have established immunotherapy as first-line standard for MSI-H/dMMR mCRC, achieving objective response rates (ORR) of 45-69% and durable survival benefits (5–9). Compared to chemotherapy, the CheckMate 8HW trial recently established a huge superiority of progression-free survival in nivolumab plus ipilimumab group in MSI-H/dMMR mCRC (HR 0.21, 95% CI 0.14–0.31) (10). More recently, neoadjuvant immunotherapy has demonstrated unprecedented efficacy in both locally advanced MSI-H/dMMR rectal (11–13) and colon cancer (14, 15), potentially enabling organ preservation. The success of ICIs in advanced stages has ignited interest in extending their application to the adjuvant setting.
ICI has been reported as postoperative adjuvant therapy for melanoma (16), non-small cell lung cancer (17), and clear cell renal carcinoma (18). However, the role of postoperative immunotherapy following radical resection for stage II/III MSI-H/dMMR CRC remains poorly defined due to the persistent scarcity of clinical evidence, creating a research gap in this domain. Recently, the ATOMIC trial presented at ASCO 2025 has decisively complemented this knowledge gap. By adding atezolizumab to mFOLFOX6, it achieved an unprecedented 50% reduction in recurrence/death risk and nearly 10% increase of 3-year disease-free survival (DFS) rate from 76.6% to 86.4%, establishing the first ICI-based adjuvant standard in stage III dMMR colon cancer (19). However, this breakthrough excluded adjuvant ICI monotherapy, rectal patients, and stage II patients, leaving 50-80% of real-world MSI-H/dMMR CRC patients without evidence-based guidance.
These unresolved challenges highlight the urgent need for precision-oriented postoperative strategies beyond ATOMIC’s one-size-fits-all approach. Specifically, several questions remain unanswered: First, can WW remain the standard for stage II MSI-H/dMMR CRC given its favorable prognosis (20), or do subsets with high-risk features require escalation? Second, is chemotherapy mandatory for all stage III patients, or can ICI monotherapy suffice for low-risk subgroups (e.g., T3N1) to mitigate chemotoxicity? Third, whether to continue adjuvant therapy postoperatively in patients who achieve pathologic complete response (pCR) after neoadjuvant ICI, a fast-growing cohort excluded from the ATOMIC trial? Fourth, what biomarkers can predict recurrence risk in ICI-treated patients to facilitate precise risk stratification?
To bridge these gaps, we retrospectively conducted a real-world cohort study of 261 stage II/III MSI-H/dMMR CRC patients who underwent radical resection with over 2 years of postoperative follow-up to compare DFS of different postoperative strategies and analyze factors associated with postoperative recurrence, with the aim of complementing the clinical evidence for postoperative strategies for MSI-H/dMMR CRC.
2 Methods
2.1 Patients cohort
We conducted a real-world retrospective analysis of stage II/III MSI-H/dMMR CRC patients undergoing radical resection in the Department of Colorectal Surgery, Gastrointestinal Surgery, Gastroenterology, Oncology etc. at the First Affiliated Hospital of Zhengzhou University and Henan Cancer Hospital from January 2021 to December 2022. Inclusion criteria required: 1) CRC patients received radical resection surgery; 2) Confirmed MSI-H by PCR or next-generation sequencing, or dMMR confirmed by immunohistochemistry; 3) Postoperative pathologic stage suggestive of T3/4 with any N or N+ with any T stage (consistent pre-neoadjuvant imaging stage); 4) Over 2 years follow-up. Exclusion criteria included: 1. Stage IV (M1); 2) Death due to non-disease-related accidents during follow-up; 3) Lost to follow-up; 4) Incomplete acquisition of clinicopathological information; 5) Recurrence of the surgical area within 1 month after surgery; 6) refusal of informed consent. All patients who met the inclusion criteria were recruited strictly and consecutively to minimize selection bias. This study utilized existing clinical data without imposing additional procedures, financial burdens, or altering treatment decisions. All participants received informed consent through follow-up, with patient identities anonymized to protect privacy. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No.2023KY0552002). Detailed in Figure 1.
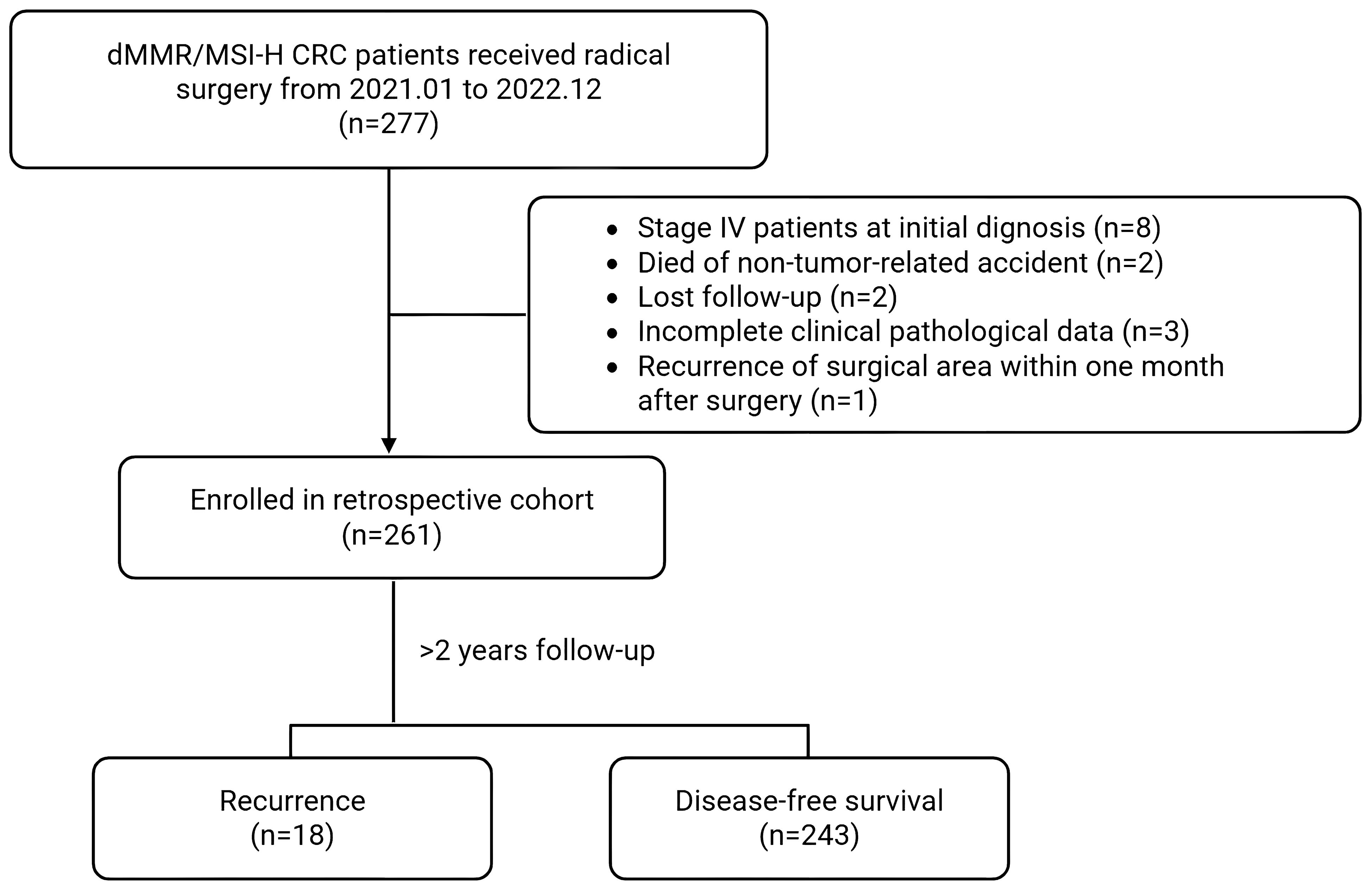
Figure 1. Flowchart of a study on dMMR/MSI-H CRC patients who received radical surgery from January 2021 to December 2022. Out of 277 patients, 261 were enrolled in a retrospective cohort after exclusions for various reasons. Following more than two years of follow-up, 18 experienced recurrence, while 243 achieved disease-free survival.
2.2 Data collection
Complete demographic and clinicopathological information of patients cohort were collected, including gender, age, diagnosis, tumor location, histological type, clinical and pathological stage, TNM stage, whether Lynch syndrome, whether preoperative neoadjuvant therapy, postoperative pathology, postoperative strategies etc. Morning fasting blood samples collected within 24 hours of admission included: serum carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA-199), complete blood count (white blood cells [WBC], hemoglobin, neutrophils, lymphocytes, biochemical panel (albumin, pre-albumin, alanine aminotransferase [ALT], aspartate transaminase [AST], serum creatinine [Scr], and blood urea nitrogen [BUN]). We also calculated inflammatory index named CRP-albumin-lymphocyte index(CALLY) and log odds of positive nodes (LODDS), a lymph node marker calculated as log of the ratio between the number of positive nodes and the number of negative nodes, both of which were reported as prognostic predictors previously (21, 22).
2.3 Follow-up data
All patients were followed up after surgery according to the guidelines (23, 24), and the frequency of re-examination was once every 3 months (within 2 years after surgery) and once every 6 months (2-5 years after surgery), including tumor biomarkers, contrast-enhanced CT of chest and abdomen, contrast-enhanced MRI, and ctDNA, PET-CT, colonoscopy if necessary. DFS records the time from surgery date to recurrence or the end of follow-up. DFS curves are plotted for different patient cohorts for comparison.
2.4 Postoperative adjuvant therapy
Adjuvant therapy is initiated 2 to 4 weeks after surgery, with a treatment window of about 6 months. The adjuvant strategies generally follow clinical guidelines: watchful waiting (WW) for stage II, while chemotherapy based on 5-fluorouracil for stage III, unless some special circumstances, such as high-risk factors or participation in clinical trials.
The specific adjuvant therapy groups are as follows:
1. WW group (76 cases): 67 cases of stage II, while 9 cases of stage III who opted for WW due to being over 80 years old, combined with other illness including severe malnutrition, myelosuppression, and/or liver/kidney dysfunction.
2. Immunotherapy group (46 cases): 15 cases underwent ICI monotherapy, and 31 cases received ICI+chemotherapy, mainly participating in different clinical trial projects. Sintilimab and Pembrolizumab were the main used ICI drugs and have been officially approved for free use in clinical trials.
3. Chemotherapy group (139 cases): including 97 cases of stage II (combined with other risk factors and/or continuation of preoperative neoadjuvant chemotherapy) and 42 cases of stage III patients. The adjuvant chemotherapy regimen is oxaliplatin plus 5-fluorouracil, while 7 cases received 5-fluorouracil monotherapy.
2.5 dMMR/MSI-H testing
Immunohistochemistry (IHC) for MMR proteins (MLH1, PMS2, MSH2, MSH6), next generation sequencing (NGS), and/or PCR-based MSI testing were performed on tumor tissue samples obtained from preoperative colonoscopies or surgical resections to determine the dMMR/MSI status, and all individuals underwent at least one testing method. Among them, 232 cases underwent IHC testing, 10 cases underwent NGS, and 172 cases underwent PCR testing. 153 patients received both IHC and PCR testing, with a concordance rate of 92.2% (141 cases).
2.6 Statistical methods
Continuous numerical variables were presented as medians and ranges. Normally distributed continuous variables were compared using analysis of variance, non-normally distributed variables were analyzed with Mann-Whitney U test, then pairwise comparisons were conducted in multiple groups. Categorical variables were presented as numbers and percentages, and compared using the Chi-square test or Fisher’s exact test, pairwise comparisons were conducted as well. Cumulative DFS were presented using Kaplan-Meier curves, and different groups were compared using the log-rank test. Numerical and categorical variables were analyzed by independent samples t-test and chi-square test to analyze the correlation between variables and postoperative recurrence, respectively. All statistical steps were performed using SPSS 20.0 (Chicago, IL). P value < 0.05 was considered statistically significant.
3 Results
3.1 Clinicopathologic information of patients cohort
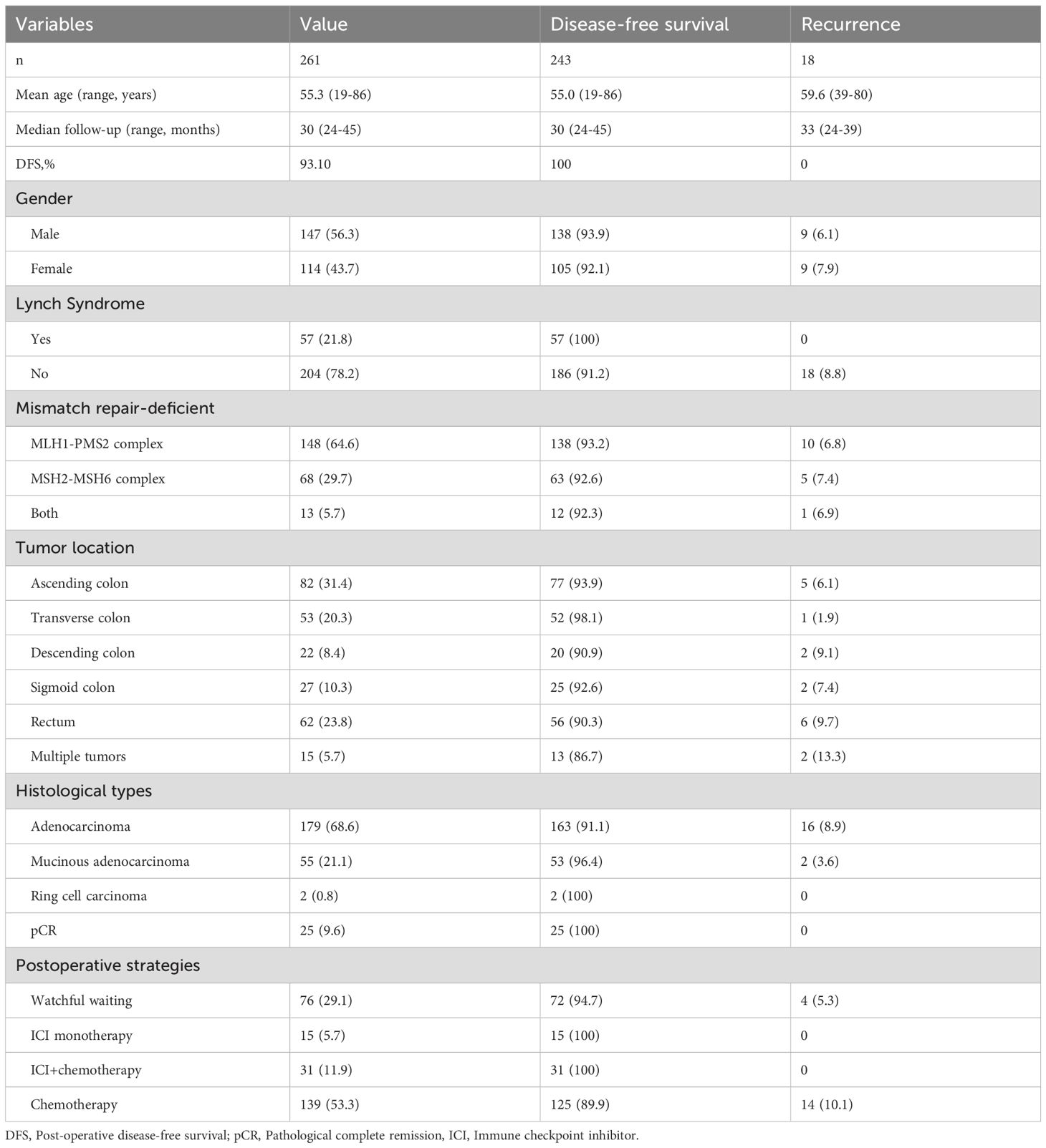
Among 261 patients included in the retrospective analysis, 147 (56.3%) were male and 114 (43.7%) female. The mean age was 55.3 range from 19 to 86 years old. The median follow-up time was 30 range from 24 to 45 months. During the follow-up period, no disease-related deaths occurred, and 18 patients (6.9%) experienced recurrence, with an overall DFS rate of 93.1% and the median DFS not reached. According to revised Bethesda criteria and germline mutation confirmation (25), 57 patients (21.8%) were Lynch syndrome, and dMMR occurred in 148 patients (64.6%) in MLH1-PMS2 complex, 68 (29.7%) in MSH2-MSH6 complex and 13 (5.7%) in both. Postoperative WW, ICI monotherapy, ICI+chemotherapy, and conventional chemotherapy patients are 76 (29.1%), 15 (5.7%), 31 (11.9%), 139 (53.3%) respectively. All the 18 patients with postoperative recurrence were Lynch-negative patients. See Table 1 for details.
The DFS rates of postoperative WW, immunotherapy (with or without chemotherapy) and chemotherapy groups were 94.74%, 100.00% and 89.93%, respectively. Except for pathological type and clinical stage, there were no significant differences in gender, age, tumor markers, tumor location, dMMR type and pathological high factors among the three groups. Detailed in Table 2.
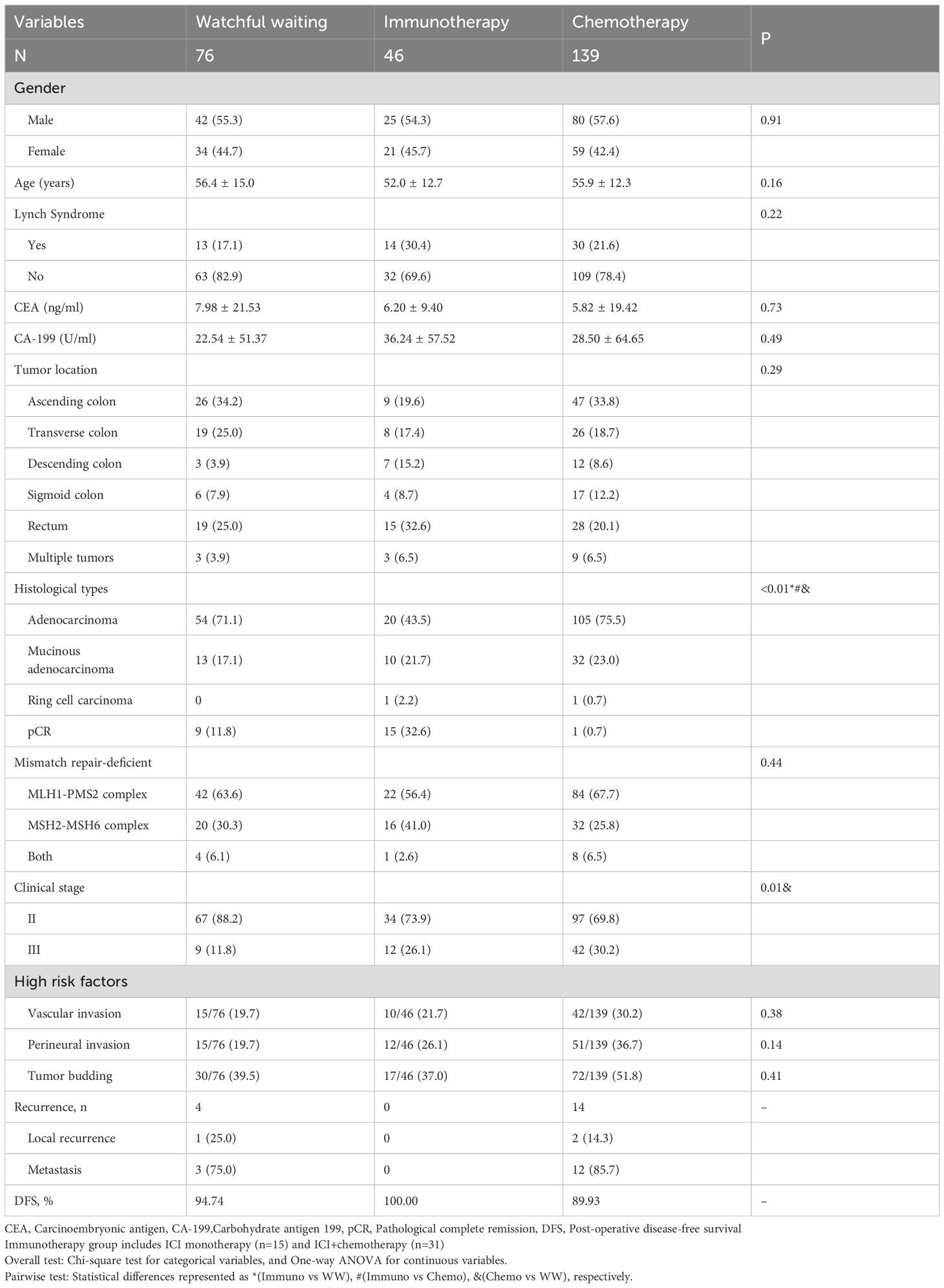
Table 2. Clinicopathological characteristics of patients grouped by different postoperative strategies(%).
Total of 25 of 45 patients received neoadjuvant therapy achieved pCR (22/25 in neoadjuvant immunotherapy and 3/20 in neoadjuvant chemotherapy). Not shown in table.
3.2 Prognostic analysis of different postoperative strategies for MSI-H/dMMR colorectal cancer
All the MSI-H/dMMR CRC patients survived during the follow-up period, and the median OS and DFS were not reached. We then performed pairwise comparisons among three different postoperative strategies to identify specific differences.
3.2.1 Watchful waiting vs. immunotherapy
Postoperative immunotherapy showed superiority over WW on the DFS curve, but did not reach a statistical difference (HR = 0.19, 95%CI: 0.03-1.39, P = 0.101, Figure 2A). There is still a lack of clinical evidence as to whether pCR patients after neoadjuvant therapy need to continue postoperative consolidation treatment. Our results showed no postoperative recurrence of WW or immunotherapy, suggesting that WW may be the better choice for pCR patients (Figure 2B). According to current guidelines, MSI-H/dMMR CRC patients are recommended WW for stage II and adjuvant treatment for stage III after surgery. Subgroup analysis showed a significant DFS advantage of immunotherapy over the WW in stage III (HR = 0.05, 95%CI: 0.004-0.54, P = 0.014, Figure 2D) and no difference in stage II (HR = 0.21, 95%CI: 0.003-13.04, P = 0.463, Figure 2C) reaffirming the guideline recommendation.
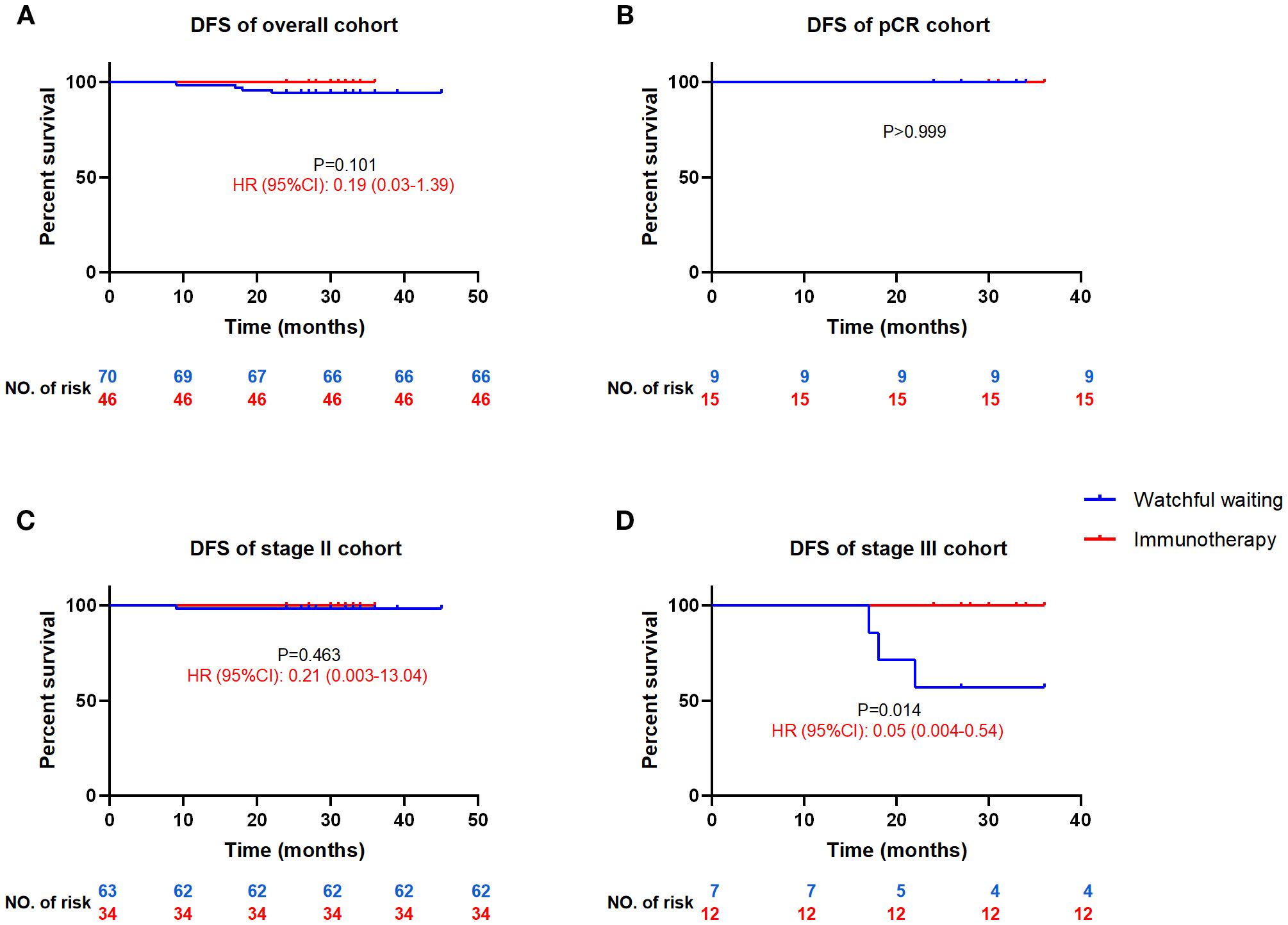
Figure 2. Comparison of DFS between the postoperative watchful waiting and immunotherapy for MSI-H/dMMR colorectal cancer patients DFS, disease-free survival; HR, hazard ratio (watchful waiting as reference), CI, confidence interval. DFS curves for all cohort patients (A), patients achieved pCR (B), stage II patients (C) and stage III patients (D).
3.2.2 Immunotherapy vs. chemotherapy
There is a lack of evidence for adjuvant immunotherapy after surgery for MSI-H/dMMR CRC, and guidelines only recommend conventional chemotherapy stage III. While the overall cohort demonstrated a statistically significant DFS advantage of immunotherapy over chemotherapy (HR = 0.26, 95%CI: 0.08-0.89, P = 0.033, Figure 3A), the stage III subgroup analysis showed a consistent magnitude of benefit (HR = 0.28, 95%CI:0.04-1.96) that did not reach statistical significance due to reduced sample size (P = 0.204, Figure 3B). There was no difference in DFS between ICI with and without chemotherapy, but both showed a DFS advantage over chemotherapy alone (0.29<all HR<0.34), although the statistical difference was lost due to reduced sample size (0.07<all P<0.52, Figures 3C, D).
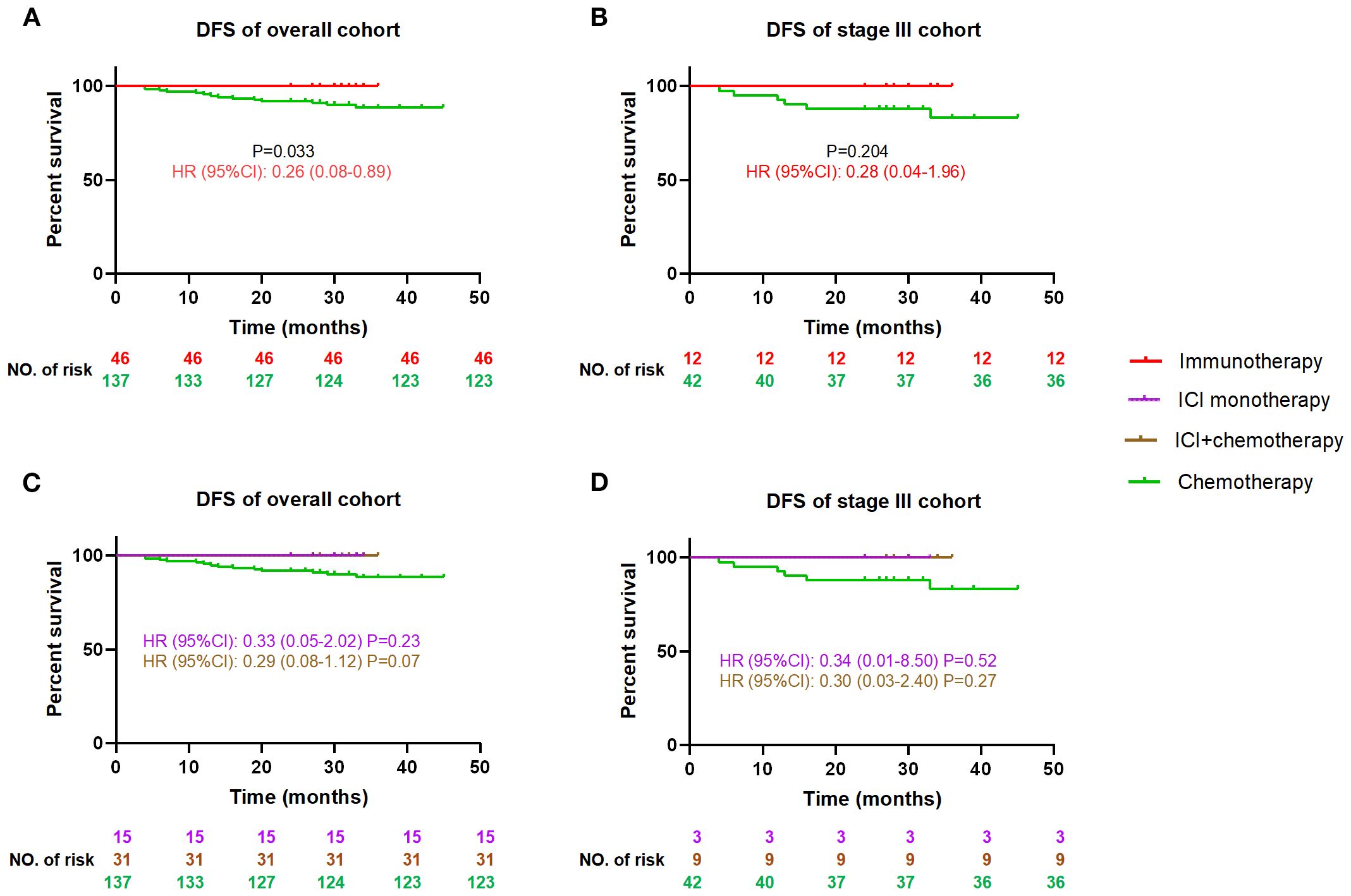
Figure 3. Comparison of DFS between the postoperative immunotherapy and chemotherapy for MSI-H/dMMR colorectal cancer patients DFS, disease-free survival; HR, hazard ratio (chemotherapy as reference), CI, confidence interval, ICI, immune checkpoint inhibitor. DFS curves between immunotherapy and chemotherapy for all cohort patients (A), and stage III patients (B); DFS curves among ICI monotherapy, ICI+chemotherapy and chemotherapy for all cohort patients (C), and stage III patients (D).
3.2.3 Chemotherapy vs. watchful waiting
Overall, MSI-H/dMMR CRC presents favorable prognosis. Only 9 patients with stage III in our patients cohort opted for WW instead of guideline-recommended adjuvant chemotherapy. Subgroup analysis of stage III patients revealed that, although the chemotherapy approach showed no statistically significant difference compared to WW due to small sample size (HR = 0.34, 95%CI: 0.05-2.17, P = 0.113), it demonstrated a better trend in the DFS curve (Supplementary Figure 1).
3.3 Factors associated with postoperative recurrence in MSI-H/dMMR CRC patients
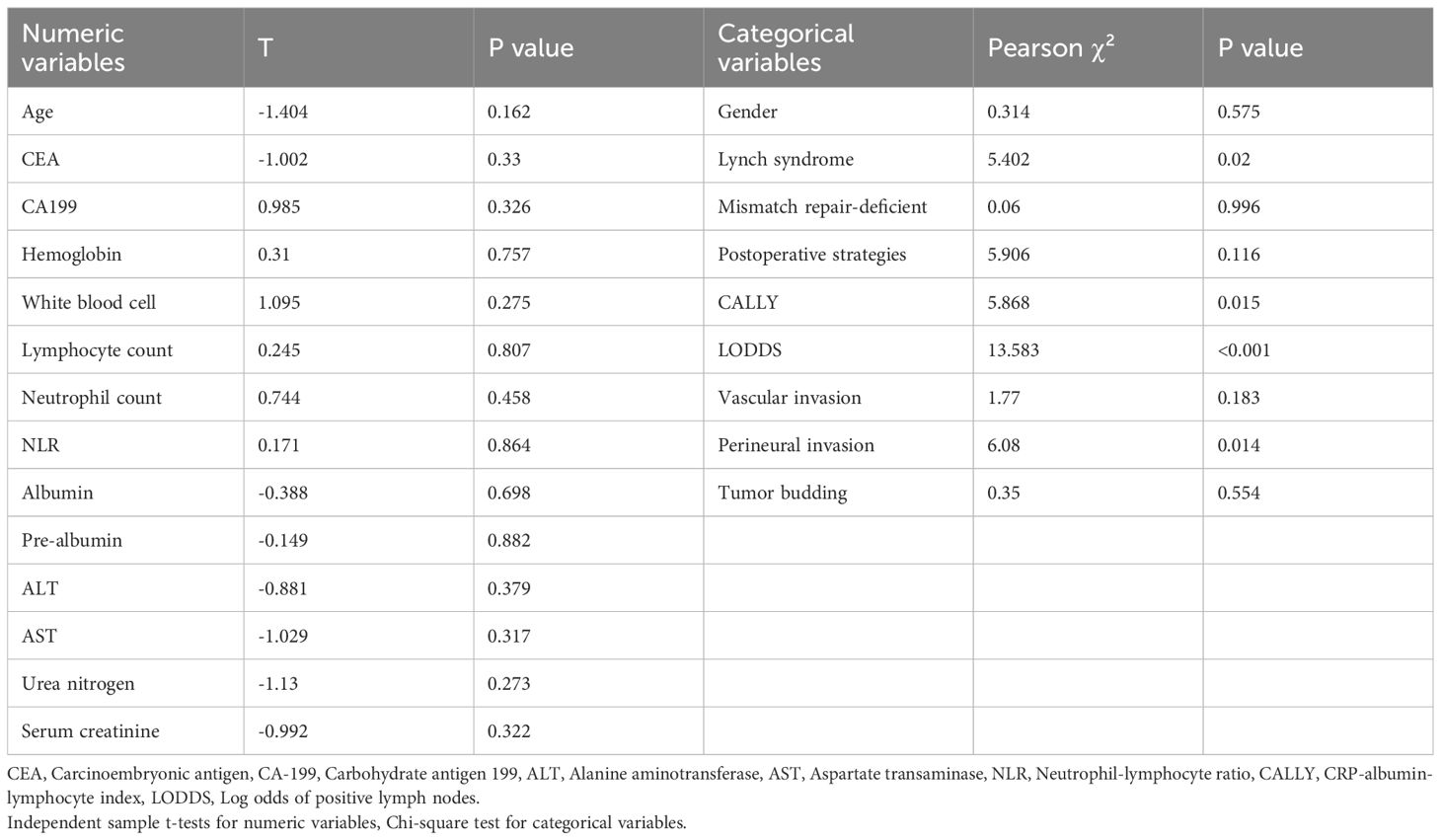
To facilitate prognostic risk stratification for MSI-H/dMMR CRC patients, we analyzed the correlation between multiple clinicopathological factors and postoperative recurrence. The results demonstrated that Lynch-negative (P = 0.02) and perineural invasion (P = 0.014) were significantly correlated with recurrence, whereas no other factors showed statistical significance, as detailed in Table 3.
4 Discussion
Our real-world study provides evidence redefining postoperative management for MSI-H/dMMR CRC. The 93.1% overall DFS rate at median 30-month follow-up corroborates the exceptional prognosis of this subtype, challenging conventional adjuvant paradigms. Most notably, we established that WW remains appropriate for stage II patients (95.5% DFS), while revealing immunotherapy’s potential superiority over chemotherapy in stage III disease (HR = 0.28, 95%CI: 0.04-1.96).These results support the latest guidelines that explicitly advise WW for stage II, but compel reconsideration of guidelines recommending blanket chemotherapy for stage III CRC regardless of MMR status (23, 26, 27) given chemotherapy’s established neurotoxicity (28) and marginal benefit in dMMR tumors (29, 30). Another finding is that patients achieving pCR after neoadjuvant ICIs had pretty good DFS (100%) in our cohort. However, given the retrospective nature and limited sample size, we still need to be cautious about the organ preservation strategy for those cCR acguevers after neoadjuvant therapy (31, 32).
The contrast between immunotherapy and chemotherapy was the main finding. While the overall cohort demonstrated a statistically significant DFS advantage of immunotherapy over chemotherapy (HR = 0.26, 95% CI 0.08–0.89; P = 0.033), the stage III subgroup analysis showed a consistent magnitude of benefit (HR = 0.28, 95% CI 0.04–1.96) but lost statistical significance (P = 0.204). This apparent discrepancy primarily stems from limited statistical power in the stage III immunotherapy cohort (n=12), reflecting the real-world constraint of off-guideline treatment adoption. Despite the small sample size, the clinically meaningful separation of Kaplan-Meier curves (Figure 3B) aligns with the significant effect observed in the overall analysis (Figure 3A), suggesting that the lack of statistical significance likely represents a type II error rather than a true absence of treatment effect.
While the practice-changing ATOMIC trial would soon establish ICI+chemotherapy as adjuvant standard for stage III dMMR colon cancer, our findings well complement its critical limitations: First, by validating WW for stage II patients (excluded in ATOMIC), we provide evidence for about 40% of real-world dMMR CRC population currently without guidance. Second, the same efficiency of ICI monotherapy and ICI+chemotherapy suggests chemotherapy may be safely omitted in select patients, particularly considering the 43.1% incidence of grade ≥3 adverse events in ATOMIC (19). This suggests that the benefit of ICI+chemotherapy in the ATOMIC trial may be primarily derived from ICI, and we call for future large prospective studies of postoperative ICI monotherapy to support treatment intensity de-escalation.
Previous reports have confirmed the advantages of immunotherapy over chemotherapy in dMMR/MSI-H CRC. For dMMR/MSI-H mCRC, the Keynote-177 study reported that ICI monotherapy significantly outperformed chemotherapy in terms of PFS, OS, and adverse events (8), while the CheckMate-8HW study found that dual ICIs was even more effective. Our comparative data focuses on non-metastatic dMMR/MSI-H CRC patients who have undergone radical surgery (10), and we draw similar conclusions of immunotherapy’s superiority over chemotherapy in the phase III subgroup. As for non-metastatic dMMR/MSI-H CRC, the NICHE series of studies has broadened the applicable scenarios for neoadjuvant immunotherapy, although there are no direct comparisons to chemotherapy (14, 15), its extremely high pCR (68%) is far superior to conventional neoadjuvant chemotherapy (<30% pCR) (33–35). Our study will provide evidence to continue to broaden the applicable scenarios for postoperative adjuvant immunotherapy.
In China, ICIs and chemotherapeutic agents are almost entirely covered by the national medical insurance system, rendering the economic burden of immunotherapy comparable to that of chemotherapy but substantially lower than targeted therapy. Although ICIs may be associated with severe immune-related adverse events (e.g., myocarditis), their overall incidence remains lower compared to adverse events induced by chemotherapy (36–39). Furthermore, immunotherapy shows better DFS and reduced neurotoxicity. Ultimately, immunotherapy demonstrates superior long-term cost-effectiveness and quality-of-life outcomes relative to chemotherapy. Formal cost-effectiveness analysis is warranted.
Our biomarker analysis reveals actionable stratification tools. Lynch syndrome has been reported to not only show a better prognosis (40, 41) but also a better response to immunotherapy (NICHE 2 trial) (14) compared with sporadic MSI-H/dMMR CRC, but negative results have also been reported (42). Despite the controversy, our results suggest that Lynch syndrome is a strong protective factor in reducing postoperative recurrence (0% vs 8.8% in sporadic cases). The possible mechanism is that Lynch-associated tumors exhibit higher neoantigen burden and T-cell infiltration (43). Current guidelines define MSI-H/dMMR as a low-risk factor regardless of the results of traditional high-risk factors, such as vascular and perineural invasion (23, 24, 27). Our results suggest that perineural invasion should still be considered as a prognostic stratification and treatment guidance factor in MSI-H/dMMR CRC patients. Lynch-negative and perineural invasion together identify patients in need of intensive therapy, while patients without high-risk markers can be considered for treatment de-escalation, such as Lynch syndrome.
This is the first real-world study comparing immunotherapy and chemotherapy as postoperative adjuvant therapy for MSI-H/dMMR CRC. However, study have limitations include retrospective design and modest recurrence events (n=18) constraining multivariate analysis. The chemotherapy cohort’s higher recurrence (10.07% vs immunotherapy’s 0%) may reflect residual confounding by high-risk feature clustering. Ongoing clinical trials will prospectively validate our framework, such as PACE trial (NCT05236972) for ICI monotherapy (44). Future research must define optimal ICI duration and integrate emerging biomarkers like ctDNA clearance, particularly for pCR patients where adjuvant omission appears safe. Which is better, preoperative neoadjuvant immunotherapy or postoperative adjuvant immunotherapy? Moreover, cost-effectiveness analyses should evaluate WW/immunotherapy against chemotherapy in resource-limited settings.
Our results establish a strategic framework for postoperative management of stage II/III MSI-H/dMMR CRC (Figure 4). For stage II patients, WW demonstrates non-inferior DFS to immunotherapy, validating current guideline endorsements for WW strategy in this subgroup. Conversely, in stage III patients, immunotherapy may outperform guideline-recommended chemotherapy, emerging as a superior alternative for DFS optimization. We further emphasize the need to individualize treatment based on the strategic framework, by using postoperative recurrence risk factors (Lynch-negative, perineural invasion) to facilitate precise risk stratification and minimize overtreatment.
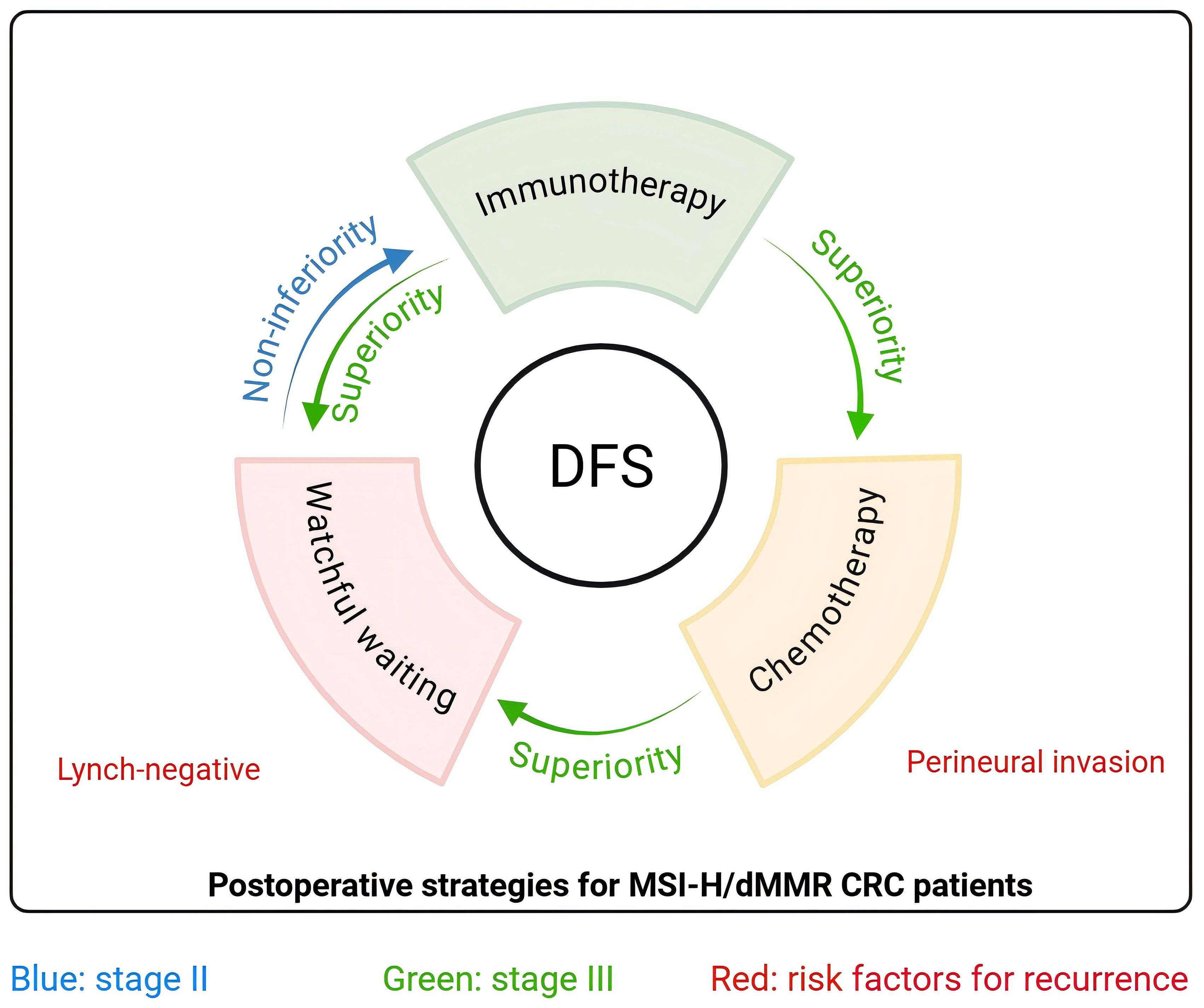
Figure 4. Graphical abstract Strategic framework for postoperative management of MSI-H/dMMR CRC patients. For stage II (blue arrow), watchful waiting is non-inferior to immunotherapy as the preferred strategy postoperatively. For stage III (green arrow), immunotherapy is superior to chemotherapy and watchful waiting as the preferred strategy postoperatively. Lynch-negative and perineural invasion are risk factors (red) for postoperative recurrence. DFS, disease-free survival; CRC, colorectal cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Writing – original draft. PL: Data curation, Writing – original draft. SY: Data curation, Writing – review & editing. FW: Data curation, Writing – review & editing. SZ: Data curation, Writing – review & editing. MZ: Conceptualization, Writing – review & editing. WY: Conceptualization, Writing – review & editing. QZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. Henan Provincial Natural Science Foundation Youth Project (242300421487), Postdoctoral Research Funding Project of Henan Province (HN2022094), Henan Provincial Medical Science and Technology Research Program Joint Construction Project (LHGJ20200276).
Acknowledgments
We would like to thank the colorectal tumor MDT team of the First Affiliated Hospital of Zhengzhou University, and the clinical trials and participants related to colorectal immunotherapy carried out by the team, which provided valuable data for our research beyond the current guideline treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1664684/full#supplementary-material
Supplementary Figure 1 | Comparison of DFS between the postoperative watchful waiting and chemotherapy for stage III MSI-H/dMMR colorectal cancer patients DFS, disease-free survival; HR, hazard ratio (chemotherapy as reference), CI, confidence interval.
Abbreviations
ALT, alanine aminotransferase; IHC, immunohistochemistry; AST, aspartate transaminase; LODDS, log odds of positive nodes; AUC, area under curve; mCRC, metastatic CRC; BUN, blood urea nitrogen; MSI-H, high microsatellite instability; CA-199, carbohydrate antigen 199; NGS, next generation sequencing; CALLY, CRP-albumin-lymphocyte index; ORR, objective response rate; CEA, serum carcinoembryonic antigen; pCR, pathologic complete response; CRC, Colorectal cancer; ROC, receiver operating characteristic; DFS, disease-free survival; Scr, serum creatinine; dMMR, deficient mismatch repair; WBC, white blood cells; HR, HR, hazard ratio; WW, watchful waiting; ICI, immune checkpoint inhibitor; 95%CI: 95% confidence interval.
References
1. Wankhede D, Yuan T, Kloor M, Halama N, Brenner H, and Hoffmeister M. Clinical significance of combined tumour-infiltrating lymphocytes and microsatellite instability status in colorectal cancer: a systematic review and network meta-analysis. Lancet Gastroenterol hepatology. (2024) 9:609–19. doi: 10.1016/s2468-1253(24)00091-8
2. Bari S, Matejcic M, Kim RD, Xie H, Sahin IH, Powers BD, et al. Practice patterns and survival outcomes of immunotherapy for metastatic colorectal cancer. JAMA network Open. (2025) 8:e251186. doi: 10.1001/jamanetworkopen.2025.1186
3. Zheng C and Zhang Z. New era of cancer immunology driven by big data. Medical review. (2021). 3(6):449–51. doi: 10.1515/mr-2023-0070
4. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
5. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2023) 34:10–32. doi: 10.1016/j.annonc.2022.10.003
6. Diaz LA, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. (2022) 23:659–70. doi: 10.1016/s1470-2045(22)00197-8
7. Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkMate 142 study. J Clin Oncol Off J Am Soc Clin Oncol. (2022) 40:161–70. doi: 10.1200/jco.21.01015
8. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. New Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
9. Andre T, Elez E, Van Cutsem E, Jensen LH, Bennouna J, Mendez G, et al. Nivolumab plus ipilimumab in microsatellite-instability-high metastatic colorectal cancer. New Engl J Med. (2024) 391:2014–26. doi: 10.1056/NEJMoa2402141
10. André T, Elez E, Lenz HJ, Jensen LH, Touchefeu Y, Van Cutsem E, et al. Nivolumab plus ipilimumab versus nivolumab in microsatellite instability-high metastatic colorectal cancer (CheckMate 8HW): a randomised, open-label, phase 3 trial. Lancet (London England). (2025) 405:383–95. doi: 10.1016/s0140-6736(24)02848-4
11. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. New Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
12. Li Y, Tan L, Chen N, Liu X, Liang F, Yao Y, et al. Neoadjuvant Immunotherapy Alone for Patients With Locally Advanced and Resecta ble Metastatic Colorectal Cancer of dMMR/MSI-H Status. Dis colon rectum. (2024) 67:1413–22. doi: 10.1097/dcr.0000000000003290
13. Deng Z, Luo Y, Chen X, Pan T, Rui Y, Hu H, et al. Pathological response following neoadjuvant immunotherapy and imaging characteristics in dMMR/MSI-H locally advanced colorectal cancer. Front Immunol. (2024) 15:1466497. doi: 10.3389/fimmu.2024.1466497
14. Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, et al. Neoadjuvant immunotherapy in locally advanced mismatch repair-deficient colon cancer. New Engl J Med. (2024) 390:1949–58. doi: 10.1056/NEJMoa2400634
15. de Gooyer PGM, Verschoor YL, van den Dungen LDW, Balduzzi S, Marsman HA, Geukes Foppen MH, et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial. Nat Med. (2024) 30:3284–90. doi: 10.1038/s41591-024-03250-w
16. Luke JJ, Ascierto PA, Khattak MA, de la Cruz Merino L, Del Vecchio M, Rutkowski P, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma: final analysis of distant metastasis-free survival in the phase III KEYNOTE-716 study. J Clin Oncol Off J Am Soc Clin Oncol. (2024) 42:1619–24. doi: 10.1200/jco.23.02355
17. O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/s1470-2045(22)00518-6
18. Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:1133–44. doi: 10.1016/s1470-2045(22)00487-9
19. Sinicrope FA. LBA1: Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III deficient DNA mismatch repair(dMMR) colon cancer (Alliance A021502; ATOMIC). Chicago: ASCO (2025).
20. Taieb J, Shi Q, Pederson L, Alberts S, Wolmark N, Van Cutsem E, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol Off J Eur Soc Med Oncol. (2019) 30:1466–71. doi: 10.1093/annonc/mdz208
21. Yang M, Lin SQ, Liu XY, Tang M, Hu CL, Wang ZW, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol. (2023) 14:1131496. doi: 10.3389/fimmu.2023.1131496
22. Huang B, Chen C, Ni M, Mo S, Cai G, Cai S, et al. Log odds of positive lymph nodes is a superior prognostic indicator in stage III rectal cancer patients: A retrospective analysis of 17,632 patients in the SEER database. Int J Surg (London England). (2016) 32:24–30. doi: 10.1016/j.ijsu.2016.06.002
23. Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, et al. Colon cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. (2024) 32:24–30. doi: 10.6004/jnccn.2024.0029
24. Benson AB, Venook AP, Adam M, Chang G, and Chen YJ. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
25. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Institute. (2004) 96:261–8. doi: 10.1093/jnci/djh034
26. Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2020) 31:1291–305. doi: 10.1016/j.annonc.2020.06.022
27. Hofheinz RD, Fokas E, Benhaim L, Price TJ, Arnold D, Beets-Tan R, et al. Localised rectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2025) 31(10):1291–305. doi: 10.1016/j.annonc.2025.05.528
28. Teng C, Blinman PL, and Vardy JL. Patterns of patient-reported chemotherapy-induced peripheral neuropathy in colorectal cancer survivors. J Natl Compr Cancer Network: JNCCN. (2022) 20:1308–15. doi: 10.6004/jnccn.2022.7050
29. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. New Engl J Med. (2003) 349:247–57. doi: 10.1056/NEJMoa022289
30. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2010) 28:3219–26. doi: 10.1200/jco.2009.27.1825
31. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol hepatology. (2023) 8:422–31. doi: 10.1016/s2468-1253(22)00439-3
32. Yu JH, Liao LE, Xiao BY, Zhang X, Wu AW, Cheng Y, et al. Long-term outcomes of dMMR/MSI-H rectal cancer treated with anti-PD-1-based immunotherapy as curative-intent treatment. J Natl Compr Cancer Network: JNCCN. (2024) 8(5):422–31. doi: 10.6004/jnccn.2023.7096
33. Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. (2023) 41:1541–52. doi: 10.1200/jco.22.00046
34. Zhang J, Chi P, Shi L, Cui L, Gao J, Li W, et al. Neoadjuvant modified infusional fluorouracil, leucovorin, and oxaliplatin with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: updated results of the FOWARC study after a median follow-up of 10 years. J Clin Oncol Off J Am Soc Clin Oncol. (2025) 43:633–40. doi: 10.1200/jco-24-01676
35. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:702–15. doi: 10.1016/s1470-2045(21)00079-6
36. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. New Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
37. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
38. Sullivan RJ and Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. (2022) 21:495–508. doi: 10.1038/s41573-021-00259-5
39. Dougan M, Luoma AM, Dougan SK, and Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. (2021) 184:1575–88. doi: 10.1016/j.cell.2021.02.011
40. Xu Y, Li C, Zheng CZ, Zhang YQ, Guo TA, Liu FQ, et al. Comparison of long-term outcomes between Lynch sydrome and sporadic colorectal cancer: a propensity score matching analysis. BMC cancer. (2021) 21:45. doi: 10.1186/s12885-020-07771-8
41. Colle R, Lonardi S, Cachanado M, Overman MJ, Elez E, Fakih M, et al. BRAF V600E/RAS mutations and lynch syndrome in patients with MSI-H/dMMR metastatic colorectal cancer treated with immune checkpoint inhibitors. oncologist. (2023) 28:771–9. doi: 10.1093/oncolo/oyad082
42. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Sci (New York NY). (2017) 357:409–13. doi: 10.1126/science.aan6733
43. Gebert J, Gelincik O, Oezcan-Wahlbrink M, Marshall JD, Hernandez-Sanchez A, Urban K, et al. Recurrent frameshift neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a lynch syndrome mouse model. Gastroenterology. (2021) 161:1288–302.e13. doi: 10.1053/j.gastro.2021.06.073
Keywords: colorectal cancer, microsatellite instability high, mismatch repair deficient, immunotherapy, adjuvant therapy
Citation: Sun H, Lyu P, Yang S, Wang F, Zhai S, Zhao M, Yuan W and Zhou Q (2025) Immunotherapy versus chemotherapy as adjuvant therapy for resected MSI-H/dMMR colorectal cancer: real-world evidence informing precision strategies. Front. Immunol. 16:1664684. doi: 10.3389/fimmu.2025.1664684
Received: 12 July 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Zhaoxu Zheng, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Samantha Sharma, Indiana University Bloomington, United StatesJuan Manuel Oconnor, Alexander Fleming Specialized Medical Institute, Argentina
Copyright © 2025 Sun, Lyu, Yang, Wang, Zhai, Zhao, Yuan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weitang Yuan, eXVhbndlaXRhbmdAenp1LmVkdS5jbg==; Mengfei Zhao, emhhb21mbmp1QDE2My5jb20=; Quanbo Zhou, ZmNjemhvdXFiQHp6dS5lZHUuY24=
†These authors have contributed equally to this work
 Haifeng Sun
Haifeng Sun Pin Lyu1†
Pin Lyu1† Shuaixi Yang
Shuaixi Yang