- 1Eye Center, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 2Frontier Science Center for Immunology and Metabolism, Medical Research Institute, Wuhan University, Wuhan, Hubei, China
Retinal gene therapy has advanced significantly, offering potential treatments for inherited retinal diseases (IRDs) such as retinitis pigmentosa, which previously lacked effective interventions. Central to this progress are adeno-associated virus (AAV)-based delivery systems, which have become the primary platform for ocular gene therapy due to their favorable safety profile, ability to target specific retinal cell types, and long-lasting therapeutic effects. However, accumulating evidence reveals that even “immune-privileged” retinal microenvironments are not exempt from immune challenges, affecting both the safety and efficacy of these therapies. Both innate immune pathways and adaptive responses can induce intraocular inflammation, leading to reduced transgene expression and compromised treatment. Understanding how these immune mechanisms interact with therapeutic outcomes is crucial for developing effective intervention strategies. This review examines evidence from both animal models and human trials to explore how immune activation affects treatment efficacy across various delivery methods and vector designs. We also assess emerging strategies aimed at protecting retinal function while reducing systemic toxicity.
1 Introduction
Inherited retinal diseases (IRDs) are a diverse group of genetic disorders that cause progressive retinal degeneration and irreversible vision loss. Affecting an estimated 5 to 10 million people worldwide, with a prevalence of 0.06% to 0.2%, IRDs pose a significant public health challenge (1). While no curative treatments currently exist, gene therapy has emerged as a promising approach to preserving or restoring vision by delivering functional genes to affected retinal cells. Among various gene delivery methods, adeno-associated virus (AAV) vectors have gained prominence due to their ability to efficiently deliver genes, sustain long-term expression, and maintain a strong safety profile with minimal risk of integrating into the host genome (2). However, immune responses to AAV vectors remain a major challenge. Pre-existing neutralizing Antibodies (NAbs), along with innate and adaptive immune activation following vector administration, can reduce transduction efficiency, trigger inflammation, and ultimately affect treatment success.
Although the retina is traditionally considered an immune-privileged site, IRDs alter its immune microenvironment in ways that may influence vector-mediated immune responses. Figures 1 and 2 illustrate these changes, highlighting the immune landscape in IRDs and the impact of gene therapy. This review provides a comprehensive analysis of immune responses in retinal gene therapy and explores emerging strategies to overcome these immune related barriers.
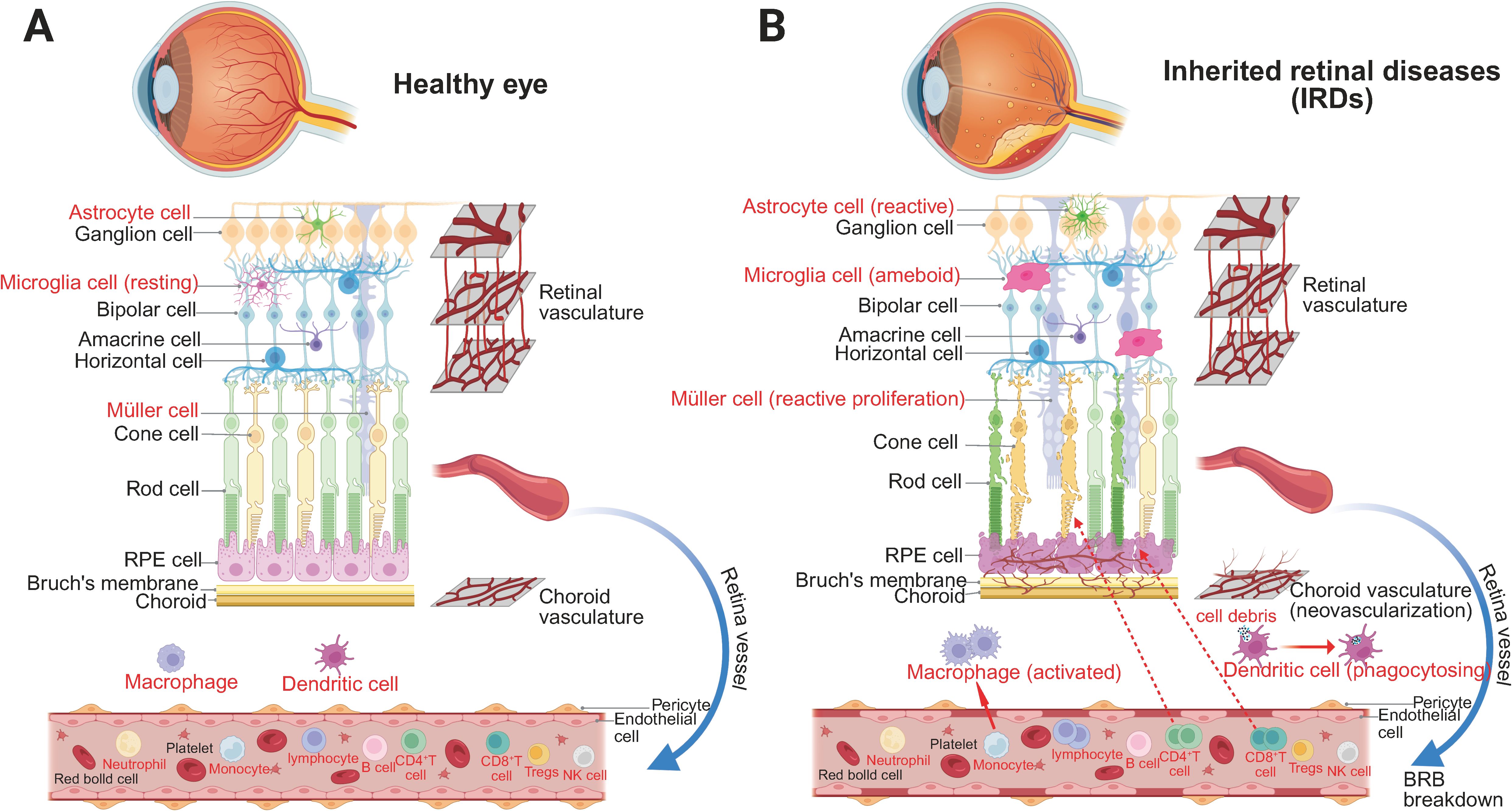
Figure 1. The immune microenvironment of the retina in two different conditions. (A) Homeostatic retinal immune microenvironment. Under physiological conditions, the retinal structure is well-preserved and tightly regulated. Astrocytes, resting microglia, and Müller cells maintain homeostasis by supporting the BRB, monitoring neural integrity, and regulating the ionic microenvironment. The BRB effectively restricts the entry of systemic immune cells and inflammatory mediators, ensuring immune quiescence and retinal stability. (B) Retinal immune microenvironment in IRDs. In IRDs, progressive degeneration of photoreceptors, bipolar cells, and ganglion cells disrupts retinal architecture, accompanied by RPE dysfunction and gliosis. Astrocytes become reactive, microglia transition to an ameboid morphology, and Müller cells undergo reactive gliosis and proliferation. Breakdown of the BRB, due to pericyte and endothelial cell loss, leads to vascular leakage and pathological neovascularization. Macrophages and dendritic cells actively phagocytose cellular debris, while infiltrating CD4+ and CD8+ T cells contribute to local immune activation, exacerbating retinal inflammation and degeneration.
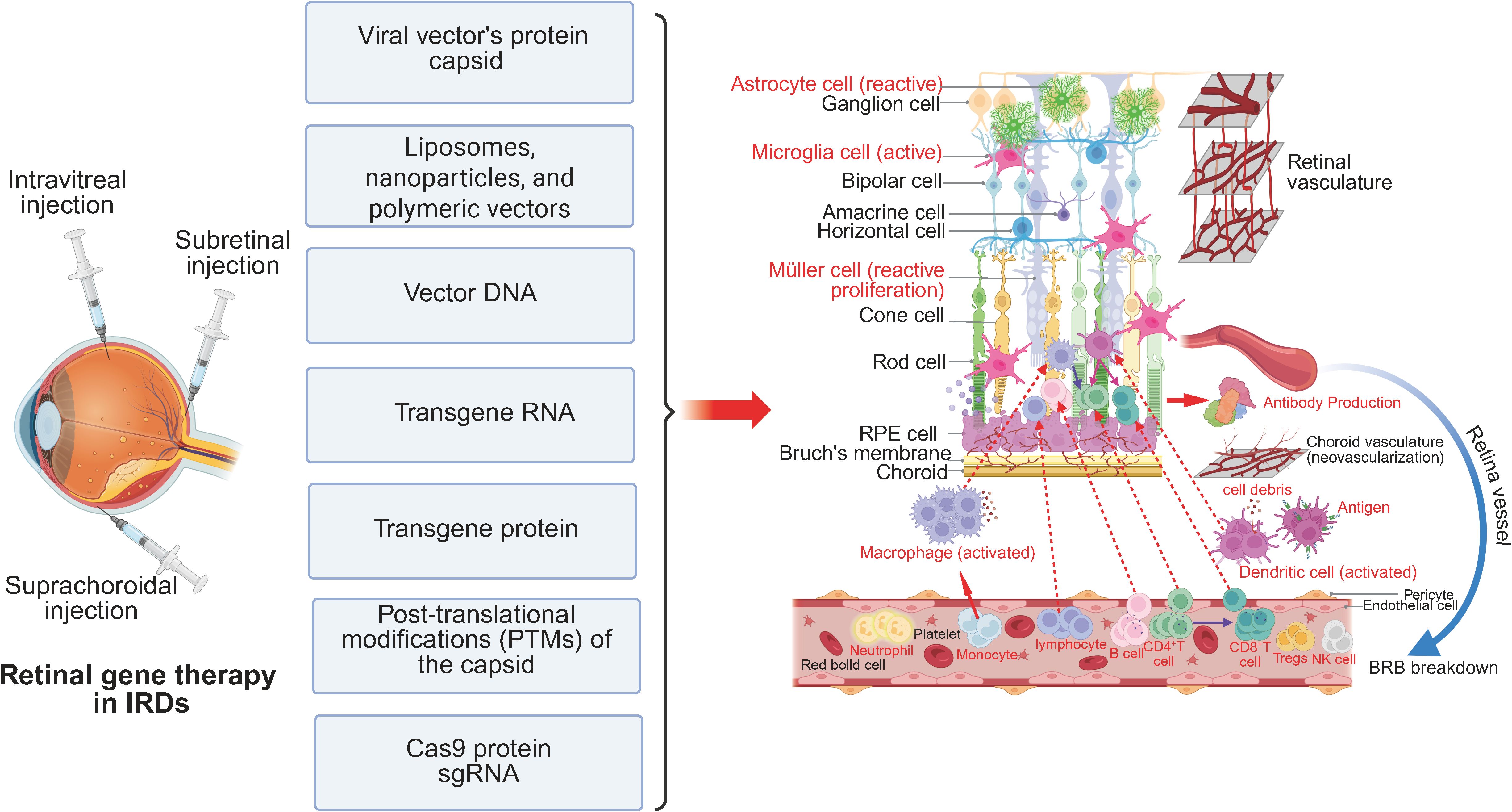
Figure 2. Retinal immune microenvironment in IRDs following gene therapy. Retinal gene therapy is commonly administered via intravitreal, subretinal, or suprachoroidal injection. These delivery methods introduce immunogenic components, including viral capsid proteins, liposomes, nanoparticles, polymeric vectors, vector DNA, transgene RNA, transgene-derived proteins, PTMs of the capsid, and CRISPR-associated proteins such as Cas9 and sgRNA. While therapeutic transgene expression may facilitate partial restoration of retinal function, it can also exacerbate immune activation. Astrocytes, microglia, and Müller cells undergo heightened reactive gliosis and proliferation. Additionally, increased infiltration and activation of peripheral immune cells, including macrophages, dendritic cells, and T lymphocytes, alter the local immune microenvironment, potentially impacting therapeutic efficacy and long-term retinal homeostasis.
To ensure appropriate scope and depth, we conducted a structured search across PubMed, Web of Science Core Collection, and Scopus focusing on IRDs, AAV mediated gene therapy, and immune responses, complemented by targeted screening of ClinicalTrials.gov and backward citation chaining. Queries combined controlled vocabulary and free text terms, including retinal dystrophy and retinal degeneration, IRDs, AAV, gene therapy, NAbs, innate immunity, adaptive immunity, intravitreal delivery, and subretinal delivery, covering January 1990 to June 2025. We included peer reviewed original studies, methods papers, reviews, and clinical trials that directly addressed the topic, and we excluded conference abstracts, duplicates, studies lacking primary data, and studies with low topical relevance.
2 Current status of AAV-based retinal gene therapy clinical trials
2.1 Ongoing clinical trials
AAV-mediated gene therapy has made significant strides in treating IRDs and age-related retinal degenerative disorders. Currently, most clinical trials are in phases I and II, with fewer advancing to phase III. The ongoing clinical trials, summarized in Table 1, use various AAV serotypes (AAV2, AAV5, AAV8, AAV2/4, AAV2/5, AAV2(tYF)), target different genes (e.g., RPE65, REP1, RPGR, sFLT1, VEGF, RS1), and employ diverse delivery methods, including subretinal injection (SRI) and intravitreal injection (IVT). A significant challenge in these trials is the immune response triggered against both the AAV capsid and the transgene product, which can affect the efficacy and safety of the therapy. While the overall incidence of immune-related adverse events is relatively low, most cases are mild. The majority of inflammatory cases involve the entire eye, including both the anterior and posterior segments, while a smaller proportion are limited to the anterior segment. Overall, Immune responses to retinal diseases are variable, depending on the disease type, the patient’s genetic background, and the specific gene targeted.
2.2 Influence of dose, serotype, administration, and immunosuppression on immune responses in AAV clinical trials
AAV retinal gene therapy has demonstrated promising efficacy across various indications. However, factors such as dose, serotype, and administration route significantly influence both gene expression and the immune response. As the dose of AAV vectors increases, the incidence and severity of immune responses also rise. When the AAV dose exceeds 1×10¹¹ vg/eye, ocular immune responses and severe complications significantly increase (3). In human IVT trials for X-linked retinoschisis (AAV8-RS1), dose-related ocular inflammation was observed, with all subjects at 1×10¹¹–3×10¹¹ vg/eye developing intraocular inflammation in one study (4), whereas another report noted that doses up to 1×10¹¹ vg/eye were generally tolerated but still showed dose related ocular events (5).
Clinical data also indicate serotype dependent differences in immune responses. AAV2, including engineered intravitreal variants such as 2.7m8, has shown more pronounced inflammatory signals in humans. In a diabetic macular edema program employing anti VEGF expression, severe inflammation and ocular hypotony led to early termination, whereas cohorts with neovascular age related macular degeneration exhibited milder steroid responsive inflammation over longer term follow up (6). AAV8 in human XLRS (AAV8-RS1) produced inflammatory events that were generally controllable (5). In patients with X linked retinitis pigmentosa, AAV5 hRKp.RPGR (Botaretigene Sparoparvovec) has predominantly shown manageable inflammation alongside functional signals in phase I and II studies (7).
SRI is the most common route for retinal gene therapy, carrying a lower risk of systemic immune responses than IVT. However, it can still cause localized retinal inflammation, typically mild anterior chamber reactions such as uveitis, and a temporary rise in anti-AAV NAbs. In contrast, IVT elicits a stronger immune response, especially at higher doses. In the NCT01024998 trial, 12 of 19 patients developed anti-AAV2 antibodies, potentially reducing treatment efficacy (8). This highlights the greater immune challenge of IVT and the need for stronger immunosuppressive strategies. Additionally, IVT induces higher NAb levels in serum and ocular fluids, which may impact second-eye treatment success (9).
Additionally, immune management and long-term monitoring are crucial considerations. Most patients exhibit mild immune responses, typically resolved over time either with corticosteroid (7) or even without intervention. Most treatment protocols involve the use of corticosteroids during the first two months following retinal gene therapy. While corticosteroids are effective in controlling inflammation, their long-term use can result in complications such as increased intraocular pressure (IOP), cataracts, and other immune suppression-related issues (10). Therefore, it is crucial to closely monitor patients for these potential side effects to ensure treatment safety. Given the limitations associated with corticosteroid use, future research should focus on developing more targeted and less invasive immunomodulatory strategies.
Many current clinical trials have short follow-up periods, leaving the long-term immune response and durability of treatment effects unclear. Continuous monitoring of immune responses, the persistence of AAV vectors, and overall efficacy is therefore essential to ensure the long-term safety and effectiveness of gene therapy. Some clinical trials have not provided detailed immune response data and treatment effectiveness. Future studies should combine immune response with clinical outcomes, vision improvement and retinal function recovery, to comprehensively evaluate the success of gene therapy. The impact of repeated dosing in the same eye on immune responses is still uncertain and requires further investigation. Additionally, optimizing immunosuppressive protocols, such as adjusting corticosteroid dosing and duration, should be a priority. Exploring the use of low-immunogenic AAV vectors will also be crucial to minimizing the impact of immune responses on the effectiveness of gene therapy.
3 Current challenges in retinal gene therapy
3.1 Immunogenic component
3.1.1 Viral vector capsid
The variation in the capsid structure of viral vectors, especially AAV vectors, has a significant impact on immune responses. Different serotypes of AAV have unique capsid structures, which determine their ability to target specific tissues by binding to specific receptors on host cell surfaces. These structural variations affect antigenic properties, viral entry mechanisms, targeting specificity, and transduction efficiency. AAV2 is the earliest and most widely used AAV serotype. It enters cells by binding to heparan sulfate, a type of glycosaminoglycan expressed on the surface of host cells, effectively transducing RPE cells (11). AAV5 and AAV8 serotypes are more effective in transducing retinal photoreceptor cells (12).
After AAV vectors enter the body, the host immune system recognizes specific antigenic epitopes on their capsid proteins such as the major structural proteins VP1, VP2, and VP3 of AAV (13). B cells produce specific antibodies (mainly IgG) against these antigens. These antibodies can bind to AAV vectors, forming antigen-antibody immune complexes, which then trigger the activation of the complement system. Complement activation by AAV IgG immune complexes has been demonstrated in human sera, including with AAV9, generating C3a and C5a and highlighting a potential risk of inflammatory injury at high vector doses (14).The deposition of C3b enhances the clearance of immune complexes and may also form the membrane attack complex (MAC), causing direct damage to the AAV vector and the transduced cells (15). In terms of cellular immunity, variations in the AAV capsid can affect the activation of CD4+ T cells and CD8+ T cells. The activation of these T cells can trigger more severe inflammatory responses and immune-mediated tissue damage. In murine intravitreal studies, administration of AAV2 provokes clinically evident vitritis within about one week and this largely resolves by about one month, whereas subclinical CD45+ infiltration, predominantly T cells, persists; prior exposure to AAV accelerates these adaptive responses (16). In particular, the cytotoxic responses mediated by CD8+ T cells may directly damage transduced cells, affecting treatment outcomes (17). Evidence from non-ocular clinical studies shows expansion of capsid specific CD8+ T cells after AAV exposure and loss of transduced cells in a hemophilia B trial, directly linking capsid antigen presentation to T cell mediated cytotoxic clearance (18).
3.1.2 Vector DNA
The structure and sequence of rAAV genomes critically shape innate sensing and downstream adaptive responses. Across mouse hepatic and pulmonary models, self-complementary genomes expose CpG motifs more efficiently than single stranded genomes and consequently elicit stronger endosomal TLR9 dependent MyD88 signaling, type I interferon (IFN) responses, and capsid specific CD8+T cell priming; these effects are markedly blunted in Tlr9 deficient or Myd88 deficient mice (19–21). The GC-rich palindromic inverted terminal repeat (ITR) hairpins, while indispensable for replication and packaging, also harbor CpG motifs recognized by TLR9. Genome engineering strategies, including CpG depletion within the expression cassette and ITRs and the insertion of short TLR9 inhibitory oligomers, reduce inflammatory readouts in mouse liver, muscle, and retina, and after subretinal delivery in pigs. By contrast, in macaques, intravitreal dosing delayed but did not prevent uveitis, underscoring route and species dependent constraints (22, 23).
Beyond TLR9, interspecies differences in cytosolic DNA sensing complicate translation. Murine cells depend mainly on the cGAS-STING pathway, whereas human cells also elicit a strong STING independent response mediated by DNA dependent protein kinase, a kinase that can sense DNA ends and initiate innate signaling (24). Finally, promoter and transgene features, including length, GC content and CpG content, and predicted secondary structure, modulate both expression and engagement of TLR9. CpG depleted cassettes dampen TLR9 dependent cross priming without compromising expression in muscle and liver. CpG depleted ITRs have retained vector activity in vivo (20, 25). Consistent with these model data, human whole blood assays indicate that plasmacytoid dendritic cell TLR9 dependent type I IFN responses to AAV9 require pre-existing anti AAV9 antibodies, highlighting the influence of host humoral background on genome sensing (26).
3.1.3 Transgene RNA
Beyond the capsid and vector DNA, vector encoded transcripts are not immunological bystanders. Engagement of pattern recognition receptors (PRRs) and the magnitude of downstream interferon programs are determined by intrinsic RNA features, including length, sequence composition such as GU richness, chemical modifications, and higher order structure. Within endosomes, TLR3 senses long double stranded RNA, as shown in Tlr3 deficient mice and in polyinosinic polycytidylic acid challenge systems, whereas TLR7 and TLR8 detect GU rich single stranded RNA with pronounced species asymmetry. In mice, responses are dominated by TLR7 and TLR8 activity is weak or non-canonical. In humans, TLR8 is strongly responsive to single stranded RNA in primary monocytes, myeloid dendritic cells, and peripheral blood mononuclear cells (27–29).
In the cytosol, RIG I preferentially recognizes short RNAs bearing a5′-triphosphorylate, including double stranded and single stranded species, whereas MDA5 senses long double stranded RNA, and both receptors converge on MAVS. These assignments are supported by studies in knockout mice, human primary cells, and biochemical mapping of structure and length determinants (30, 31). Relevant to AAV, sustained transduction can generate antisense or minus-strand transcripts from the vector template, enabling sense–antisense pairing and dsRNA biogenesis. Across transformed cell lines (HeLa, HEK293, HepG2, Huh7), primary human hepatocytes (about 10–12 donors), and mice bearing human hepatocyte xenografts, dsRNA engages MDA5, signals through MAVS, and induces IFN-β, thereby curtailing transgene expression. siRNA knockdown of MDA5 or MAVS restores expression in vitro, directly linking transgene derived double stranded RNA to innate activation in human cells and in humanized mice (32). Collectively, these findings establish transgene RNA quality, not capsid alone, as a tunable driver of AAV immunogenicity and durability, and highlight the requirement to factor specie specific TLR7 and 8 biology into translational inference.
3.1.4 Transgene protein
Transgenic proteins, as the final expression product of gene therapy, may be recognized as antigens by the host immune system, triggering a specific immune response. The strength and nature of this response depend on several factors related to the transgenic protein, including its structure, function, stability, and folding state (33). In particular, the glycosylation patterns and folding quality of the transgenic protein play an important role in its immunogenicity. For example, abnormal glycosylation patterns may cause the transgenic protein to be identified as a foreign antigen, while a protein that is not fully folded may expose atypical epitopes that can be recognized by the immune system. Detection techniques, such as proteomics (34) and ELISA, can be used in laboratory settings to assess the immunogenicity of proteins.
3.1.5 Post-translational modifications of the protein capsid
Viral vector capsids can undergo various PTMs, such as phosphorylation, glycosylation, and ubiquitination (35). These modifications can significantly alter the capsid’s three-dimensional structure, affecting its antigenicity and immunogenicity (36). Different types of PTMs may lead to changes in the immunogenicity of the viral vector in the body, which can impact both the safety and therapeutic effectiveness of gene therapy (37).
3.2 Pre-existing immunity against AAV
In AAV mediated gene therapy, pre-existing antibodies against AAV capsid proteins pose a significant challenge. Many individuals have anti-AAV antibodies from previous infections, which reduces the effectiveness of gene therapy by neutralizing viral vectors (38). Because AAV capsid proteins have highly conserved amino acid sequences, NAbs may cross-react with multiple serotypes (39). Vectors containing exons and regulatory sequences from non-human sources can be recognized as foreign by the immune system (40). Furthermore, degradation products of AAV vectors and residual materials from vector production can provoke immune responses (41).
3.3 Immune responses within the eye
The eye is considered an immune-privileged site, with mechanisms such as the blood-retina barrier (BRB) and local immunosuppressive factors that protect against excessive immune responses (42, 43). However, this unique immune environment can pose challenges in retinal gene therapy, especially when using AAV vectors. While the eye generally exhibits a tempered immune response, AAV vectors can still trigger both humoral and cellular immune responses, particularly in patients with pre-existing immunity, leading to the neutralization of the vector and reduced therapeutic efficacy. Additionally, local inflammatory responses may cause retinal damage, complicating the control of immune reactions and affecting the long-term success of gene therapy.
3.3.1 Innate immune response
The innate immune system is the first line of defense against foreign pathogens, and in gene therapy, both viral vectors and gene editing tools like CRISPR/Cas9 and RNA interference (RNAi) can activate innate immune responses. Components of viral vectors, such as the protein capsid (44), vector DNA/RNA sequences (45), Cas proteins, associated small guide RNA sequences (sgRNA), and non-specific RNA degradation products in RNAi technology can be recognized by the host’s PRRs, triggering innate immune responses (46). PRRs are expressed on the cell membrane and in the cytoplasm of various cells, including innate immune cells (e.g., macrophages, monocytes, neutrophils, natural killer cells, and dendritic cells) (47), adaptive immune cells (such as T cells and B cells), and non-immune cells (such as epithelial cells, endothelial cells, and fibroblasts) (48). In the retina, PRRs are predominantly found in retinal pigment epithelial cells (RPE cells) (49), microglial cells (50), retinal ganglion cells (RGCs) (51), retinal astrocytes (52), and photoreceptor cells (53).
Immune responses triggered by AAV vectors involve multiple PRR pathways. TLR2 recognizes the protein capsid of AAV, activating the NF-κB pathway and inducing the production of inflammatory cytokines such as TNF-α and IL-1β (39). Unmethylated CpG motifs within AAV genomes are detected by TLR9, particularly in mouse models where genetic ablation of Tlr9 or Myd88 blunts type-I IFN induction and downstream adaptive immunity (19). In the cytoplasm, RIG-I and MDA5 detect double-stranded RNA produced after AAV infection, which activates type I IFN and NF-κB signaling pathways (32). Additionally, the NLRP3 inflammasome responds to AAV-induced cellular stress and damage by activating caspase-1, leading to the release of IL-1β and IL-18. AIM2 detects double-stranded DNA in the cytoplasm, triggering inflammasome activation (54).
Moreover, cGAS recognizes AAV genomic DNA and produces cGAMP, which activates STING. Upon activation, STING induces the production of type I IFNs and inflammatory cytokines. Mouse retina shows cGAS–STING activation in vivo (e.g., diabetic retinopathy), supporting the pathway’s relevance to ocular inflammation and its potential contribution to innate responses triggered by vector (55, 56). Figure 3 shows the AAV transduction process and the innate immune responses triggered in retinal gene therapy. These molecular pathways together form a complex immune response network triggered by various gene therapy methods.
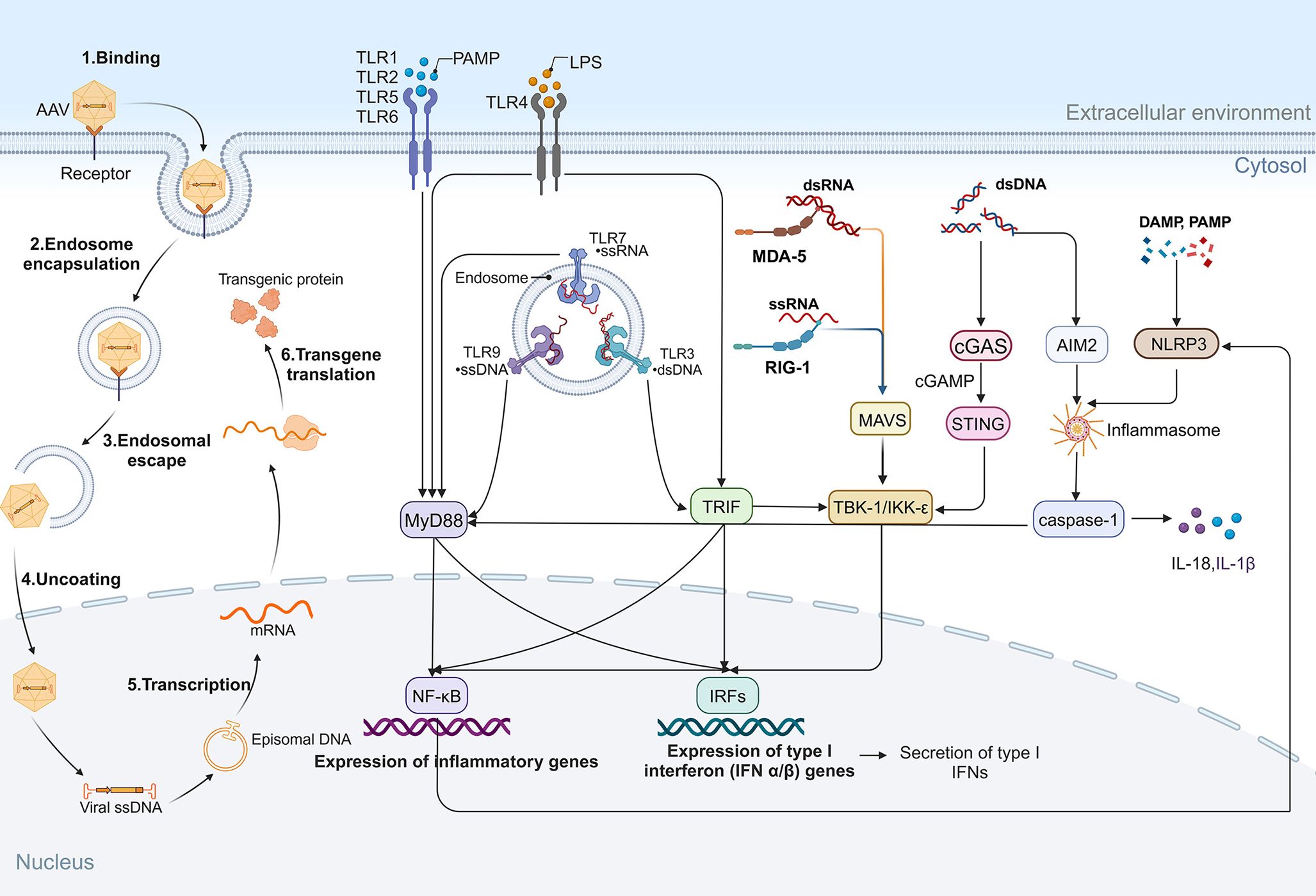
Figure 3. AAV transduction and innate immune responses in retinal gene therapy. Following administration, AAV transduction occurs through sequential steps, including cell surface binding, endocytosis, endosomal escape, capsid uncoating, transcription, and transgene translation. During this process, PRRs detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), initiating innate immune responses. TLRs play a key role in immune sensing: TLR1, TLR2, TLR5, and TLR6 recognize PAMPs and activate MyD88-dependent pathways, leading to NF-κB activation and pro-inflammatory gene expression. TLR4, upon recognizing LPS, signals through MyD88 and TRIF, triggering NF-κB and IRFs, resulting in the secretion of inflammatory cytokines and type I IFNs. Additionally, cytoplasmic RNA sensors contribute to immune activation: MDA5 binds dsRNA, while RIG-I binds ssRNA, both of which activate MAVS and TBK1/IKKϵ, promoting NF-κB and IRF signaling. Cytosolic DNA, via the cGAS/STING pathway, activates TBK1/IKKϵ, while also stimulating the AIM2 inflammasome and caspase-1, further amplifying inflammatory cascades. NLRP3 inflammasome activation, triggered by cytoplasmic DAMPs and PAMPs, reinforces inflammatory responses.
3.3.2 Adaptive immune response
The adaptive immune system (T cells and B cells) is gradually activated within days to weeks after gene therapy, potentially leading to vector clearance, loss of gene expression, and even cytotoxic reactions. In retinal gene therapy, adaptive immune responses mediated by T cells are key to treatment efficacy. CD4+ helper T cells activate and regulate immune responses through cytokine secretion, while CD8+ cytotoxic T cells directly kill infected cells. Both T cell types are essential for recognizing AAV vector components and transgene products (57).
Antigen-presenting cells (APCs), such as dendritic cells and macrophages, process and present antigenic peptide fragments from viral vectors or transgenes to T cells, triggering T cell activation. After activation, CD8+ cytotoxic T lymphocytes (CTLs) proliferate and differentiate, releasing perforins and granzymes to induce apoptosis and kill infected cells (58). During antigen presentation, CD4+ T cells recognize viral vector or transgene antigens through MHC II molecules. Upon activation, CD4+ T cells differentiate into various subsets, including T helper 1 (Th1), Th2, Th17, and T regulatory cells (Tregs). Each subset plays a distinct role in the immune response (59). TH1 cells secrete IFN-γ, which promotes CTL activation and enhances macrophage activity, driving cell-mediated immunity (60). In contrast, Th2 cells produce interleukins such as IL-4, IL-5, and IL-13, which are key for promoting B cell-mediated antibody production and humoral immunity (61). Th17 cells secrete IL-17 and IL-22, involved in inflammatory responses and tissue remodeling processes (62). Tregs secrete immunosuppressive cytokines (IL-10 and TGF-β) to suppress excessive immune responses, prevent uncontrollable inflammation, and maintain immune homeostasis (63).
CD8+ T cells identify and eliminate target cells expressing viral vector or transgene antigens via MHC I molecules. This process is essential for clearing infected cells and regulating transgene expression (58). Following antigen clearance, some T cells differentiate into memory T cells. Central memory T cells (TCM) and effector memory T cells (TEM) can rapidly respond to subsequent exposures, enhancing immune protection in future gene therapy treatments. B cells play a pivotal role in humoral immunity by recognizing antigens, producing specific antibodies through plasma cell differentiation, and forming memory B cells for long-term immune memory (64). Antibodies neutralize viruses by blocking binding to host cell receptors or by interfering with viral entry into the host cell membrane. This neutralization diminishes the virus’s ability to infect and reduces the transduction efficiency of viral vectors. Furthermore, antibodies can bind to viral antigens on the surface of infected cells, engaging Fc receptors on effector cells such as NK cells, thereby triggering antibody-dependent cellular cytotoxicity (ADCC). Antibodies can also activate the complement system, initiating a cascade that leads to viral lysis and phagocytosis. Figure 4 illustrates the cellular and humoral immune responses during retinal gene therapy.
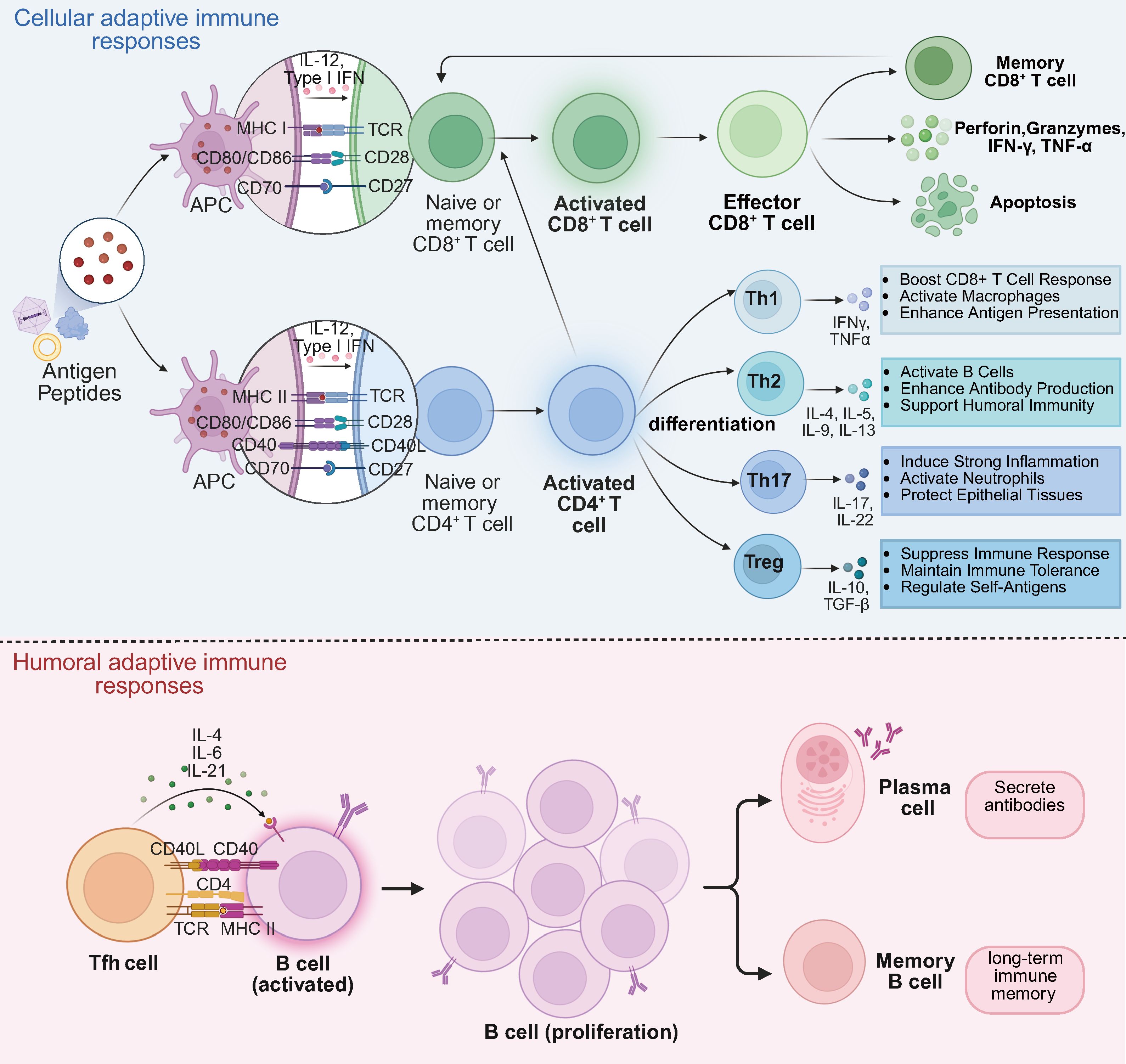
Figure 4. Cellular and humoral adaptive immune responses in retinal gene therapy. Cellular adaptive immune responses. Antigenic peptides derived from gene therapy are presented to naïve and memory CD8+ and CD4+ T cells via MHC molecules. Naïve and memory CD8+ T cells differentiate into activated CD8+ T cells, which further develop into effector CD8+ T cells. Effector CD8+ T cells mediate cytotoxicity by secreting perforin, granzymes, IFN-γ, and TNF-α, leading to apoptosis of target cells. Upon antigen re-exposure, memory CD8+ T cells rapidly differentiate into effector or memory subsets, enabling a rapid immune response. Naïve and memory CD4+ T cells, upon antigen recognition, differentiate into activated CD4+ T cells, which further polarize into Th1, Th2, Th17, and Treg subsets, each secreting distinct cytokines to modulate immune responses. Additionally, activated CD4+ T cells enhance CD8+ T cell activation, reinforcing cellular immunity. Humoral adaptive immune responses. Tfh cells promote B cell activation, leading to the differentiation of B cells into plasma cells and memory B cells. Plasma cells secrete antigen-specific antibodies, contributing to long-term immune protection, while memory B cells provide rapid recall responses upon re-exposure to the same antigen.
3.4 Challenges in redosing
Currently, AAV-mediated gene therapy for ocular diseases in clinical settings typically employs a unilateral injection strategy. This approach is primarily driven by safety considerations and the goal of preserving the patient’s vision to the greatest extent possible. However, following a single intravitreal injection of AAV, transgene expression often declines over time due to local or systemic immune-mediated clearance, vector off-target distribution, or suboptimal initial transduction efficiency, making it challenging to achieve durable therapeutic effects. Therefore, once efficacy is confirmed in one eye, safely and effectively redosing AAV in the other eye or the same eye to sustain transgene expression for bilateral or repeated treatment remains a major challenge in ocular gene therapy.
The success of AAV redosing depends on factors such as administration route, vector dose, dosing interval, and recipient age, all of which significantly influence the host immune response and thus determine the outcome of repeated gene delivery. It has been reported that intravitreal injection of AAV induces a humoral immune response against the capsid, which impairs transgene expression upon re-administration via the same intravitreal route, but does not affect transduction following SRI in the contralateral eye. In contrast, subretinal administration does not elicit a humoral immune response and does not interfere with subsequent AAV delivery, whether intravitreal or subretinal (65). Lindsey Weed and colleagues demonstrated in non-human primates (NHPs) that AAV2-hRPE65v2 can be safely and effectively readministered subretinally in the same eye, even in the presence of NAbs, with good local and systemic tolerability (66).
Strategies to overcome humoral immunity and enable effective AAV redosing are actively being explored, building on insights from ocular studies. For example, the IgG-degrading endopeptidase Imlifidase (IdeS) reduces anti-AAV antibody levels, facilitating efficient liver gene transfer in mice and enhancing transduction in NHPs, even after vector re-administration (67). Alternatively, tolerogenic nano-adjuvants (RICP), combining rapamycin (RAPA) and itaconate (ITA), disrupt the T follicular helper cell (Tfh cell) and germinal center B cell axis via metabolic modulation while inducing Tregs, significantly improving hepatic transgene expression upon redosing (68). B cell–targeted approaches also show promise: Anti-CD19 CAR-T therapy depletes peripheral and tissue-resident B cells and plasma cells, eradicating high-titer AAV8 NAbs and permitting successful transgene expression upon systemic AAV8 re-administration in mice (69). Similarly, plasmapheresis offers a clinically feasible method to lower anti-AAV antibodies, particularly for repeat dosing (70). Capsid engineering further expands redosing options. Recent studies demonstrate that modified AAV9 vectors can cross the blood-brain barrier, enabling efficient brain endothelial transduction in mice and NHPs after multiple doses. Although species-specific differences exist, this approach allows serotype switching to circumvent pre-existing immunity during redosing (71).
3.5 Personalized immune management
Immune responses can vary significantly between individuals due to factors such as sex, age (72), genetic differences, personal immune history, lifestyle, diet, environmental exposures, immune system variations, and physiological conditions. Personalized immune management is especially important in gene therapy for inherited retinal diseases. By creating personalized treatment plans based on each patient’s genomic data and immune history, genetic variants related to therapeutic responses can be precisely identified, optimizing treatment strategies. The challenge lies in effectively integrating real-time immune monitoring technologies and making timely adjustments to treatment plans based on monitoring results to enhance safety and efficacy. By integrating genomic data and immune biomarkers, more precise treatment plans can be developed. Additionally, a validated IFN-γ ELISpot method facilitate adjustments to treatment based on changes in immune status during clinical trials, thereby improving treatment outcomes and reducing side effects (73).
4 Strategies to harness the immune system
4.1 Pharmacological and therapeutic interventions
4.1.1 Immunosuppressive drugs
In retinal gene therapy, immunosuppressive drugs play a pivotal role in managing post-treatment inflammation and enhancing safety. Corticosteroids such as dexamethasone and prednisone are commonly used to suppress the release of inflammatory mediators, significantly reducing retinal tissue inflammation. Dexamethasone, well-known for its strong anti-inflammatory effects, inhibits cytokine and chemokine release, thus controlling immune responses to gene therapy (74). Additionally, it induces dendritic cells to exhibit tolerogenic properties, reducing the expression of co-stimulatory molecules and limiting T cell activation (75). Prednisone reduces inflammation by blocking the NF-κB pathway and decreasing inflammatory gene expression (76), offering protection to the retinal microenvironment. Clinical trials also indicate that prednisone helps mitigate chronic inflammation and retinal degeneration over prolonged treatment periods (6). Despite their therapeutic benefits, corticosteroids are not a panacea and present significant limitations. Local administration carries substantial risks, with 20-30% of long-term users developing corticosteroid-induced glaucoma and cataract formation. Systemic administration (oral/intravenous routes) may lead to more extensive complications, including immunosuppression and metabolic disturbances with prolonged use (77).
Beyond steroids, calcineurin inhibitors modulate T cell activation. Cyclosporine A mitigates disease in rat experimental autoimmune uveoretinitis (EAU), supporting a T cell targeted mechanism in vivo (78). Tacrolimus (FK506) similarly inhibits calcineurin and reduces ocular inflammation in rabbit and rat EAU after intravitreal delivery, with preservation of retinal structure and electroretinography (ERG) function (79, 80). Although ocular AAV specific tacrolimus data are limited, systemic tacrolimus prolonged rAAV8 and rAAV9 transgene expression in NHPs after skeletal and muscle delivery, indicating translatable T cell control across species and tissues (81, 82). Other agents are being explored. Mycophenolate mofetil inhibits lymphocyte proliferation. However, in mouse liver directed models, it lessens single stranded AAV transduction and may thereby limit overall efficiency (83). These challenges mirror broader findings in AAV gene therapy, where a systematic review of 73 clinical and real-world studies identified immunosuppression optimization as a critical determinant of treatment safety and efficacy (84).
4.1.2 Immune modulation therapy
Immune modulation is essential in controlling immune responses during retinal gene therapy. Immunoglobulin (IVIG), a key immunomodulator, neutralizes pathogenic antibodies and regulates immune functions, significantly reducing immune-mediated adverse reactions. IVIG is commonly used in clinical settings to manage acute immune reactions triggered by AAV vectors by binding to immune molecules, reducing inflammation, and improving treatment outcomes.
Plasmapheresis, though rarely used, can effectively reduce inflammation by removing pathogenic antibodies and immune complexes from the bloodstream, alleviating retinal inflammation (70, 85). In NHPs with pre-existing AAVrh74 immunity, two to three exchanges lowered neutralizing titers and enabled redosing, capsid based immunoadsorption columns also deplete anti-AAV IgG ex vivo and in animal models (70).
Rituximab (anti-CD20) depletes B cells, thereby reducing antibody mediated responses. In mouse and NHP models, B cell depletion, frequently paired with sirolimus, mitigated anti-capsid immunity and permitted AAV redosing. In clinical practice, rituximab is also employed for refractory ocular inflammation (86, 87). mTOR inhibition with rapamycin or sirolimus suppresses T and B cell activity. Phase III trials demonstrated that intravitreal sirolimus improves non-infectious uveitis. In mice and NHPs, rapamycin loaded tolerogenic nanoparticles (ImmTOR) attenuated anti-AAV immunity and enabled vector redosing (88, 89). JAK inhibition similarly reduces inflammatory signaling. Tofacitinib suppresses experimental autoimmune uveitis in mice, and ruxolitinib alleviates endotoxin-induced uveitis in rats, supporting pathway relevance to ocular inflammation even if direct AAV data are preclinical (90, 91). Moreover, immunoenzymes that transiently debulk antibodies are particularly promising in AAV settings. IgG-degrading endopeptidases (IdeS/IdeZ) restore AAV transduction in mice passively immunized with human Ig and permit in vivo gene transfer in NHPs in the face of NAbs. IceMG, a dual protease engineered to cleave IgM and IgG, rapidly clears both isotypes in rhesus macaques, reinstates AAV transduction in mice carrying human antisera, and reduces complement activation (67, 92, 93).
4.1.3 Cytokine and signaling pathway modulation
In retinal gene therapy, cytokine modulation plays a key role in managing inflammation. AAV vector injections activate Müller glial cells and microglia, triggering the release of pro-inflammatory cytokines including TNF-α and IL-1β (94). These glia–cytokine dynamics are consistent with broader microglia and Müller biology in mouse retina.
Although not specific to gene therapy, clinical experience in humans with non-infectious uveitis supports targeting these cytokines when intraocular inflammation threatens structure or function. Anti-TNF-α agents (e.g., infliximab) achieve corticosteroid sparing control in refractory uveitis (adult and pediatric cohorts), while anti-IL-6R (tocilizumab) is effective for uveitic cystoid macular edema and in juvenile idiopathic arthritis associated uveitis refractory to anti-TNF therapy. In ophthalmic practice they are generally systemic and used off label to suppress ocular inflammation (95–98).
Targeting the NF-κB and PI3K/Akt signaling pathways can also reduce immune overactivation, providing an additional strategy for immune response modulation. In mouse models of retinal and choroidal inflammation and neovascularization, microglial NF-κB activation is a driver of lesion development. Pharmacologic or genetic dampening of this axis reduces inflammatory infiltration and pathology. In parallel, PI3K/Akt signaling in retinal microglia governs activation and cytokine release, and blocking PI3K (e.g., LY294002) reduces retinal inflammatory programs in the oxygen-induced retinopathy model while dampening BV2 activation. Microglia-focused PI3K regulation (e.g., PIK3IP1) further links this pathway to retinal inflammatory angiogenesis in mouse models. Together, these data support NF-κB and PI3K/Akt as tractable nodes for adjunctive immunomodulation around ocular gene transfer, while acknowledging translation to AAV specific settings remains to be fully defined (99–101).
4.2 Vector design and optimization
4.2.1 AAV vector engineering
Modifications to AAV vector capsids are critical in reducing immunogenicity and improving therapeutic efficacy. Glycosylation of the capsid helps mask antigenic epitopes, making the vector less recognizable by the immune system (102). Techniques such as directed evolution and synthetic biology enable the development of AAV variants with lower immunogenicity. Mutations in capsid proteins, sequence recombination, and structural optimization can reduce immune responses while maintaining high transduction efficiency (103, 104). CRISPR/Cas9 technology also facilitates precise modifications to existing AAV vectors, further enhancing their properties (105). Non-viral vectors, such as liposomes and nanoparticles, also hold potential in retinal gene therapy, with modifications in size, surface charge, and composition improving their ability to evade the immune system and deliver genes more efficiently (106, 107). The use of AAV serotypes with low immunogenicity, such as AAV8 and AAV9, is an established strategy to enhance treatment outcomes. These serotypes exhibit high transduction efficiency and reduced immunogenicity in certain tissues (108).
4.2.2 Immune evasion
Polyethylene glycol (PEG) modification of AAV vectors can reduce immune recognition, extending their circulation time in the body (109). Immunomodulatory coatings, such as the regulatory protein CTLA-4, can also be applied to the surface of AAV vectors to reduce immune responses (110). Decoy capsid technology, where immune cells bind to non-therapeutic capsids, can further reduce immune activation against the therapeutic vector (111). Epitope masking, which conceals antigenic epitopes, is another promising approach to reducing immune recognition (112).
5 Future directions and clinical implications
5.1 Next-generation gene therapy approaches
Emerging non-viral vectors, including lipid nanoparticles (LNPs), polymeric nanoparticles, and mRNA delivery systems, are opening new options for retinal gene therapy across multiple preclinical systems. For example, PEG variant LNPs achieved robust genome editing after SRI in mice (RPE-restricted editing with some Müller transduction), and certain surface chemistries unexpectedly enabled photoreceptor transfection, highlighting tunable tropism by formulation choice (113). Peptide guided LNPs have likewise expanded neural retina mRNA delivery in mice, while separate studies show mouse and human retinal tissues can be transfected with LNP-mRNA without marked inflammation, supporting translational potential (106, 114). In parallel, compacted DNA nanoparticles (CK30-PEG) demonstrated high level photoreceptor and RPE expression in mice and favorable ocular safety in vivo, offering a non-viral plasmid platform with large cargo capacity (115).
Another active area is precise regulation of transgene expression. Doxycycline inducible Cre in RPE and tamoxifen-inducible CreERT2 lines targeting RGCs or RPE constitute robust conditional platforms in the mouse retina, enabling spatiotemporal regulation while underscoring practical issues, including tamoxifen dosing and toxicity (116–118). Optogenetic control provides a complementary route. A clinical proof of concept in degenerative blindness demonstrated partial vision recovery with an intravitreal vector combined with light stimulating goggles, and concordant studies in NHPs using the same regimen provided supporting evidence (119).
The application of single-cell sequencing technologies is enabling deeper insights into the retinal immune microenvironment (120, 121). This approach allows researchers to better understand the complex interactions between retinal cells and the immune system, identifying potential therapeutic targets and informing the design of more effective treatments. Finally, AI-driven design is beginning to optimize vector components to improve targeting and, by enabling lower and more selective dosing, may help mitigate immune risks. Recent Machine Learning (ML) models trained on mouse in vivo and human in vitro screens accurately predict macaque AAV capsid performance, evidencing cross-species generalization, and reviews describe their integration into retinal gene delivery pipelines (122, 123).
5.2 Emerging strategies for immune tolerance
Cell-based Tregs therapies, encompassing polyclonal and antigen-specific Tregs and exploratory CAR-Tregs, have shown feasibility and early safety in transplantation and autoimmunity, yet remain preclinical or at best early phase for controlling AAV immunogenicity (124, 125). For retinal translation, several priorities stand out. First, generate lineage-stable FOXP3+ Tregs with a demethylated TSDR that are resistant to pro-inflammatory cytokine signaling (126). Second, confer specificity for the AAV capsid and the therapeutic transgene to limit bystander suppression (125). Third, achieve ocular tropism and residence, choose the optimal route (systemic, periocular, intravitreal, or subretinal), and set dose levels with a primary emphasis on safety. Finally, incorporate safety circuits such as suicide switches, and adopt standardized immune and ocular monitoring, including binding and NAbs assays, T cell readouts, inflammation grading, and optical coherence tomography (OCT) or ERG, to link immunomodulation with clinical benefit. Given the added manufacturing and clinical complexity, cell-based Treg therapy is best positioned as a mid to long-term strategy pending AAV retina specific evidence of efficacy and durability.
As efforts to translate Treg therapies continue, attention is also turning to Mesenchymal stromal cells (MSCs) derived modalities that may broaden the immunomodulatory toolkit for retinal gene therapy. MSCs and their extracellular vesicles (EVs) attenuate Th1/Th17 responses, expand Tregs, and confer neuroprotection in ocular inflammatory and degenerative models, largely via paracrine mediators such as IL-10, TGF-β, and PGE2 (127–129). EVs and exosomes recapitulate much of this immunomodulatory and neuroprotective activity in experimental autoimmune uveitis and retinal injury models (129, 130). Therefore, direct evidence that MSCs mitigate anti-AAV ocular immunity remains limited.
5.3 Translational challenges and regulatory considerations
During treatment, regular immune monitoring is essential, including the use of flow cytometry to analyze changes in T cell subsets in peripheral blood (131). Researchers have also optimized IFN-γ and IL-2-based ELISpot assays to assess T cell responses to AAV peptide antigens. Additionally, a chemiluminescence based ELISA has been developed to measure antibody levels against AAV8 in human serum, offering an alternative to traditional NAb assays. This method, owing to its simplicity and operational ease, is a valuable tool for pre-dose baseline screening (132).
Moreover, all clinical trials must adhere to the regulatory guidelines of the respective country, such as FDA regulations in the United States or EMA standards in Europe. Trial designs and operations must also undergo review by ethics committees to ensure compliance with ethical standards and protect participants’ rights. Any adverse events related to immune responses must be thoroughly documented and reported to allow for timely adjustments to treatment protocols, thereby improving future research. To mitigate immune risks, researchers have suggested strategies such as adjusting the dose and frequency of gene therapy administration to reduce the immune system’s burden (133, 134). Figure 5 illustrates strategies to address immune responses in retinal gene therapy.
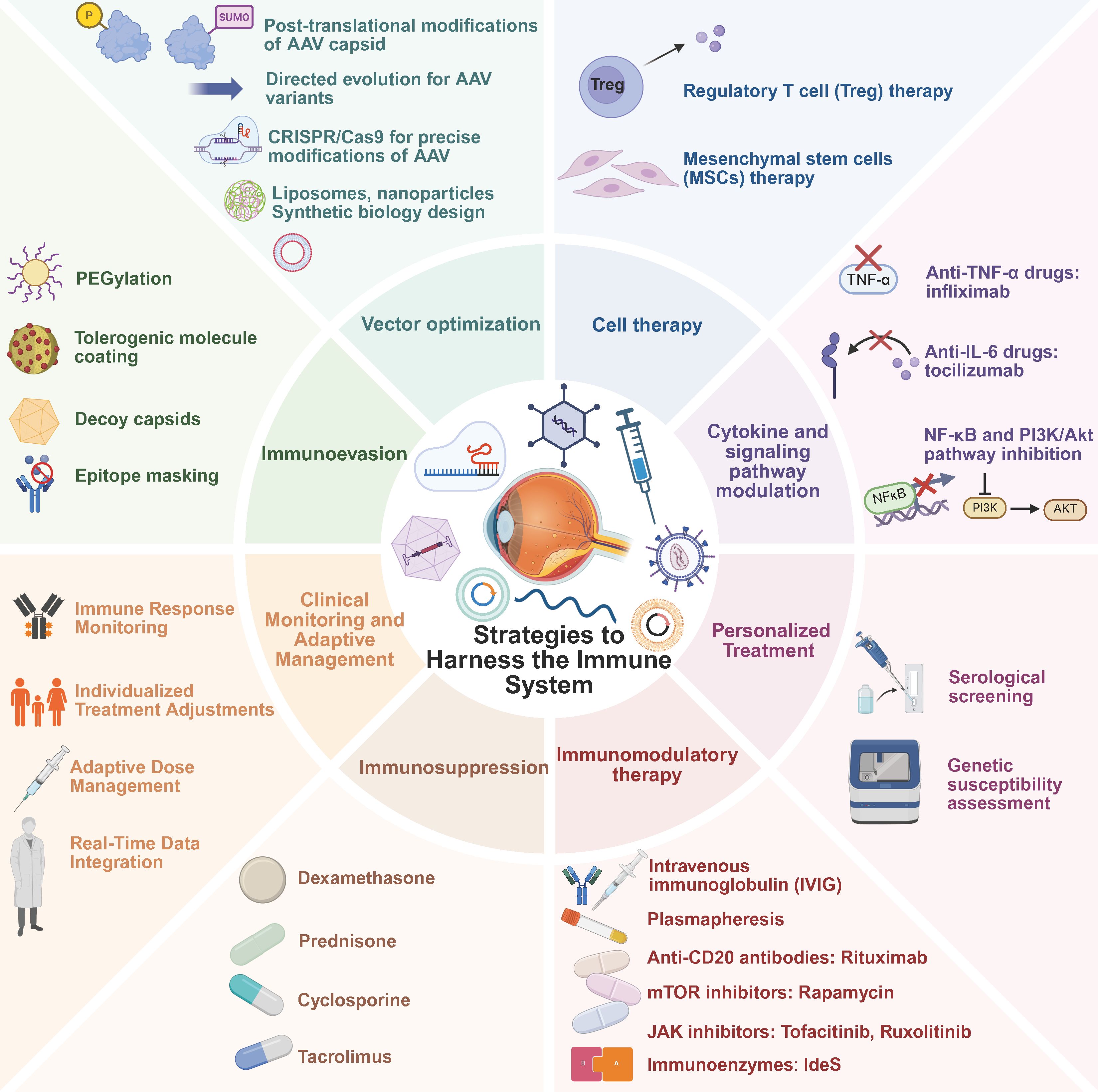
Figure 5. Strategies to harness immune responses in retinal gene therapy. Various strategies are currently employed to prevent and mitigate immune responses in retinal gene therapy. Personalized Treatment includes (1) serological screening and (2) genetic susceptibility assessment. Immunomodulatory Therapy encompasses (1) IVIG, (2) plasmapheresis, (3) anti-CD20 antibodies such as Rituximab, (4) mTOR inhibitors like Rapamycin, (5) JAK inhibitors such as Tofacitinib and Ruxolitinib, and (6) immunoenzymes like IdeS. Immunosuppression involves (1) dexamethasone, (2) prednisone, (3) cyclosporine, and (4) tacrolimus. Clinical Monitoring and Adaptive Management include (1) immune response monitoring, (2) individualized treatment adjustments, (3) adaptive dose management, and (4) real-time data integration. Immunoevasion strategies involve (1) PEGylation, (2) tolerogenic molecule coating, (3) decoy capsids, and (4) epitope masking. Vector Optimization includes (1) post-translational modifications of AAV capsid, (2) directed evolution for AAV variants, (3) CRISPR/Cas9 for precise modifications of AAV, (4) liposomes and nanoparticles, and (5) synthetic biology design. Cell Therapy consists of (1) Treg therapy and (2) MSCs therapy. Cytokine and Signaling Pathway Modulation involves (1) anti-TNF-α drugs such as Infliximab, (2) anti-IL-6 drugs like Tocilizumab, and (3) NF-κB and PI3K/Akt pathway inhibition.
6 Conclusion
Retinal gene therapy has made substantial progress in treating IRDs. However, immune responses remain a significant challenge, impacting the safety and long-term efficacy of these therapies. Despite the retina’s immune-privileged status, AAV vectors can trigger immune reactions, leading to inflammation, uveitis, or other adverse effects. While some immune responses are inevitable, strategies such as immunosuppressive drugs, immune modulation, and vector engineering have shown promise in managing these responses. Furthermore, personalized treatment approaches, immune monitoring, and adjunctive therapies like cell therapy hold significant potential for enhancing treatment success. Future research should focus on optimizing vector design, refining immunomodulatory therapies, and improving immune monitoring techniques to ensure the safety and long-term effectiveness of retinal gene therapies. Collaboration between clinicians, immunologists, and gene therapy researchers will be essential in overcoming the immune-related barriers and realizing the full potential of gene therapy for retinal diseases.
Author contributions
YM: Conceptualization, Writing – original draft. YS: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (NSFC No. 82471086), the Hubei Provincial Health and Health Technology Project (WJ2025Z003).
Acknowledgments
The authors appreciate the publications included in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fenner BJ, Tan TE, Barathi AV, Tun SBB, Yeo SW, Tsai ASH, et al. Gene-based therapeutics for inherited retinal diseases. Front Genet. (2021) 12:794805. doi: 10.3389/fgene.2021.794805
2. Botto C, Rucli M, Tekinsoy MD, Pulman J, Sahel JA, and Dalkara D. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog Retin Eye Res. (2022) 86:100975. doi: 10.1016/j.preteyeres.2021.100975
3. Dufour VL, Cideciyan AV, Ye GJ, Song C, Timmers A, Habecker PL, et al. Toxicity and efficacy evaluation of an adeno-associated virus vector expressing codon-optimized RPGR delivered by subretinal injection in a canine model of X-linked retinitis pigmentosa. Hum Gene Ther. (2020) 31:253–67. doi: 10.1089/hum.2019.297
4. Mishra A, Vijayasarathy C, Cukras CA, Wiley HE, Sen HN, Zeng Y, et al. Immune function in X-linked retinoschisis subjects in an AAV8-RS1 phase I/IIa gene therapy trial. Mol Ther. (2021) 29:2030–40. doi: 10.1016/j.ymthe.2021.02.013
5. Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. (2018) 26:2282–94. doi: 10.1016/j.ymthe.2018.05.025
6. Khanani AM, Boyer DS, Wykoff CC, Regillo CD, Busbee BG, Pieramici D, et al. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): a prospective, two-year, multicentre phase 1 study. EClinicalMedicine. (2024) 67:102394. doi: 10.1016/j.eclinm.2023.102394
7. Michaelides M, Besirli CG, Yang Y, TAC DEG, Wong SC, Huckfeldt RM, et al. Phase 1/2 AAV5-hRKp.RPGR (Botaretigene sparoparvovec) gene therapy: safety and efficacy in RPGR-associated X-linked retinitis pigmentosa. Am J Ophthalmol. (2024) 267:122–34. doi: 10.1016/j.ajo.2024.05.034
8. Heier JS, Kherani S, Desai S, Dugel P, Kaushal S, Cheng SH, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. (2017) 390:50–61. doi: 10.1016/S0140-6736(17)30979-0
9. Reichel FF, Peters T, Wilhelm B, Biel M, Ueffing M, Wissinger B, et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. (2018) 59:1910–5. doi: 10.1167/iovs.17-22494
10. Liu C, Wang X, and Cao X. Ophthalmic corticosteroids-related adverse events: the FDA adverse event reporting system (FAERS) database pharmacovigilance study. Front Pharmacol. (2024) 15:1502047. doi: 10.3389/fphar.2024.1502047
11. Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. (2001) 28:92–5. doi: 10.1038/ng0501-92
12. Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. (2011) 3:88ra54. doi: 10.1126/scitranslmed.3002103
13. Gurda BL, DiMattia MA, Miller EB, Bennett A, McKenna R, Weichert WS, et al. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J Virol. (2013) 87:9111–24. doi: 10.1128/JVI.00622-13
14. West C, Federspiel JD, Rogers K, Khatri A, Rao-Dayton S, Ocana MF, et al. Complement activation by adeno-associated virus-neutralizing antibody complexes. Hum Gene Ther. (2023) 34:554–66. doi: 10.1089/hum.2023.018
15. Dunkelberger JR and Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. (2010) 20:34–50. doi: 10.1038/cr.2009.139
16. Tummala G, Crain A, Rowlan J, and Pepple KL. Characterization of gene therapy associated uveitis following intravitreal adeno-associated virus injection in mice. Invest Ophthalmol Vis Sci. (2021) 62:41. doi: 10.1167/iovs.62.2.41
17. Hui DJ, Edmonson SC, Podsakoff GM, Pien GC, Ivanciu L, Camire RM, et al. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol Ther Methods Clin Dev. (2015) 2:15029. doi: 10.1038/mtm.2015.29
18. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. (2006) 12:342–7. doi: 10.1038/nm1358
19. Zhu J, Huang X, and Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. (2009) 119:2388–98. doi: 10.1172/JCI37607
20. Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. (2013) 123:2994–3001. doi: 10.1172/JCI68205
21. Ashley SN, Somanathan S, Giles AR, and Wilson JM. TLR9 signaling mediates adaptive immunity following systemic AAV gene therapy. Cell Immunol. (2019) 346:103997. doi: 10.1016/j.cellimm.2019.103997
22. Bertolini TB, Shirley JL, Zolotukhin I, Li X, Kaisho T, Xiao W, et al. Effect of cpG depletion of vector genome on CD8(+) T cell responses in AAV gene therapy. Front Immunol. (2021) 12:672449. doi: 10.3389/fimmu.2021.672449
23. Chan YK, Wang SK, Chu CJ, Copland DA, Letizia AJ, Costa Verdera H, et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med. (2021) 13. doi: 10.1126/scitranslmed.abd3438
24. Burleigh K, Maltbaek JH, Cambier S, Green R, Gale M Jr., James RC, et al. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci Immunol. (2020) 5. doi: 10.1126/sciimmunol.aba4219
25. Pan X, Yue Y, Boftsi M, Wasala LP, Tran NT, Zhang K, et al. Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. (2022) 29:333–45. doi: 10.1038/s41434-021-00296-0
26. Alakhras NS, Moreland CA, Wong LC, Raut P, Kamalakaran S, Wen Y, et al. Essential role of pre-existing humoral immunity in TLR9-mediated type I IFN response to recombinant AAV vectors in human whole blood. Front Immunol. (2024) 15:1354055. doi: 10.3389/fimmu.2024.1354055
27. Alexopoulou L, Holt AC, Medzhitov R, and Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. (2001) 413:732–8. doi: 10.1038/35099560
28. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. (2004) 303:1526–9. doi: 10.1126/science.1093620
29. Guiducci C, Gong M, Cepika AM, Xu Z, Tripodo C, Bennett L, et al. RNA recognition by human TLR8 can lead to autoimmune inflammation. J Exp Med. (2013) 210:2903–19. doi: 10.1084/jem.20131044
30. Rehwinkel J and Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. (2020) 20:537–51. doi: 10.1038/s41577-020-0288-3
31. Lu C, Xu H, Ranjith-Kumar CT, Brooks MT, Hou TY, Hu F, et al. The structural basis of 5’ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure. (2010) 18:1032–43. doi: 10.1016/j.str.2010.05.007
32. Shao W, Earley LF, Chai Z, Chen X, Sun J, He T, et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. (2018) 3. doi: 10.1172/jci.insight.120474
33. Kumar SRP, Hoffman BE, Terhorst C, de Jong YP, and Herzog RW. The balance between CD8(+) T cell-mediated clearance of AAV-encoded antigen in the liver and tolerance is dependent on the vector dose. Mol Ther: J Am Soc Gene Ther. (2017) 25:880–91. doi: 10.1016/j.ymthe.2017.02.014
34. Urbiola-Salvador V, Miroszewska D, Jabłońska A, Qureshi T, and Chen Z. Proteomics approaches to characterize the immune responses in cancer. Biochim Biophys Acta Mol Cell Res. (2022) 1869:119266. doi: 10.1016/j.bbamcr.2022.119266
35. Mary B, Maurya S, Arumugam S, Kumar V, and Jayandharan GR. Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. (2019) 286:4964–81. doi: 10.1111/febs.15013
36. Maurya S and Jayandharan GR. Gene therapy: contest between adeno-associated virus and host cells and the impact of UFMylation. Mol Pharm. (2020) 17:3649–53. doi: 10.1021/acs.molpharmaceut.0c00512
37. Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. (2010) 18:135–42. doi: 10.1038/mt.2009.257
38. Louis Jeune V, Joergensen JA, Hajjar RJ, and Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. (2013) 24:59–67. doi: 10.1089/hgtb.2012.243
39. Hösel M, Broxtermann M, Janicki H, Esser K, Arzberger S, Hartmann P, et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatol (Baltimore Md). (2012) 55:287–97. doi: 10.1002/hep.24625
40. Chandler LC, Barnard AR, Caddy SL, Patrício MI, McClements ME, Fu H, et al. Enhancement of adeno-associated virus-mediated gene therapy using hydroxychloroquine in murine and human tissues. Mol Ther Methods Clin Dev. (2019) 14:77–89. doi: 10.1016/j.omtm.2019.05.012
41. Liu YF, Huang S, Ng TK, Liang JJ, Xu Y, Chen SL, et al. Longitudinal evaluation of immediate inflammatory responses after intravitreal AAV2 injection in rats by optical coherence tomography. Exp Eye Res. (2020) 193:107955. doi: 10.1016/j.exer.2020.107955
42. Taylor AW. Ocular immune privilege and transplantation. Front Immunol. (2016) 7:37. doi: 10.3389/fimmu.2016.00037
43. Mochizuki M, Sugita S, and Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res. (2013) 33:10–27. doi: 10.1016/j.preteyeres.2012.10.002
44. Wang Y and Shao W. Innate immune response to viral vectors in gene therapy. Viruses. (2023) 15. doi: 10.3390/v15091801
45. Li C and Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. (2020) 21:255–72. doi: 10.1038/s41576-019-0205-4
46. Toral MA, Charlesworth CT, Ng B, Chemudupati T, Homma S, Nakauchi H, et al. Investigation of Cas9 antibodies in the human eye. Nat Commun. (2022) 13:1053. doi: 10.1038/s41467-022-28674-1
47. Taguchi T and Mukai K. Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol. (2019) 59:1–7. doi: 10.1016/j.ceb.2019.02.002
48. Todd L, Palazzo I, Suarez L, Liu X, Volkov L, Hoang TV, et al. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J Neuroinflammation. (2019) 16:118. doi: 10.1186/s12974-019-1505-5
49. Kumar MV, Nagineni CN, Chin MS, Hooks JJ, and Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. (2004) 153:7–15. doi: 10.1016/j.jneuroim.2004.04.018
50. Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, and Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retinal Eye Res. (2015) 45:30–57. doi: 10.1016/j.preteyeres.2014.11.004
51. Coyle S, Khan MN, Chemaly M, Callaghan B, Doyle C, Willoughby CE, et al. Targeting the NLRP3 inflammasome in glaucoma. Biomolecules. (2021) 11. doi: 10.3390/biom11081239
52. Jiang G, Ke Y, Sun D, Wang Y, Kaplan HJ, and Shao H. Regulatory role of TLR ligands on the activation of autoreactive T cells by retinal astrocytes. Invest Ophthalmol Vis Sci. (2009) 50:4769–76. doi: 10.1167/iovs.08-3303
53. Böhm MR, Schallenberg M, Brockhaus K, Melkonyan H, and Thanos S. The pro-inflammatory role of high-mobility group box 1 protein (HMGB-1) in photoreceptors and retinal explants exposed to elevated pressure. Lab Invest. (2016) 96:409–27. doi: 10.1038/labinvest.2015.156
54. Reichel FF, Dauletbekov DL, Klein R, Peters T, Ochakovski GA, Seitz IP, et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol Ther: J Am Soc Gene Ther. (2017) 25:2648–60. doi: 10.1016/j.ymthe.2017.08.018
55. Hu B, Ma JX, and Duerfeldt AS. The cGAS-STING pathway in diabetic retinopathy and age-related macular degeneration. Future Med Chem. (2023) 15:717–29. doi: 10.4155/fmc-2022-0301
56. Liu H, Ghosh S, Vaidya T, Bammidi S, Huang C, Shang P, et al. Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.168945
57. Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M, et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol (Baltimore Md: 1950). (2009) 182:6051–60. doi: 10.4049/jimmunol.0803965
58. Ren D, Fisson S, Dalkara D, and Ail D. Immune responses to gene editing by viral and non-viral delivery vectors used in retinal gene therapy. Pharmaceutics. (2022) 14. doi: 10.3390/pharmaceutics14091973
59. Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. (2017) 546:421–5. doi: 10.1038/nature22360
60. Noël G, Fontsa ML, Garaud S, De Silva P, de Wind A, Van den Eynden GG, et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J Clin Invest. (2021) 131. doi: 10.1172/JCI139905
61. Jarnicki AG and Fallon PG. T helper type-2 cytokine responses: potential therapeutic targets. Curr Opin Pharmacol. (2003) 3:449–55. doi: 10.1016/S1471-4892(03)00077-8
62. Ghilardi N and Ouyang W. Targeting the development and effector functions of TH17 cells. Semin Immunol. (2007) 19:383–93. doi: 10.1016/j.smim.2007.10.016
63. Keino H, Horie S, and Sugita S. Immune privilege and eye-derived T-regulatory cells. J Immunol Res. (2018) 2018:1679197. doi: 10.1155/2018/1679197
64. Ordovas-Montanes J, Beyaz S, Rakoff-Nahoum S, and Shalek AK. Distribution and storage of inflammatory memory in barrier tissues. Nat Rev Immunol. (2020) 20:308–20. doi: 10.1038/s41577-019-0263-z
65. Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. (2008) 14:1760–9.
66. Weed L, Ammar MJ, Zhou S, Wei Z, Serrano LW, Sun J, et al. Safety of same-eye subretinal sequential readministration of AAV2-hRPE65v2 in non-human primates. Mol Ther Methods Clin Dev. (2019) 15:133–48. doi: 10.1016/j.omtm.2019.08.011
67. Leborgne C, Barbon E, Alexander JM, Hanby H, Delignat S, Cohen DM, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. (2020) 26:1096–101. doi: 10.1038/s41591-020-0911-7
68. Liu Y, Liang F, Zhang R, Tang C, Li X, Wang Y, et al. Nanotherapeutics-mediated tolerogenic induction for enabling adeno-associated virus vector gene therapy re-administration by overcoming anti-drug antibodies. Mater Horiz. (2025) 12(17):6751–64. doi: 10.1039/D5MH00203F
69. Doshi BS, Markmann CA, Novak N, Juarez Rojas S, Davidson R, Chau JQ, et al. Use of CD19-targeted immune modulation to eradicate AAV-neutralizing antibodies. Mol Ther. (2025) 33:3073–85. doi: 10.1016/j.ymthe.2025.03.003
70. Potter RA, Peterson EL, Griffin D, Cooper Olson G, Lewis S, Cochran K, et al. Use of plasmapheresis to lower anti-AAV antibodies in nonhuman primates with pre-existing immunity to AAVrh74. Mol Ther Methods Clin Dev. (2024) 32:101195. doi: 10.1016/j.omtm.2024.101195
71. Chen X, Wolfe DA, Bindu DS, Zhang M, Taskin N, Goertsen D, et al. Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nat Commun. (2023) 14:3345. doi: 10.1038/s41467-023-38582-7
72. Clare AJ, Langer PM, Ward A, Chan YK, Dick AD, and Copland DA. Characterization of the ocular inflammatory response to AAV reveals divergence by sex and age. Mol Ther. (2025) 33:1246–63. doi: 10.1016/j.ymthe.2025.01.028
73. Patton KS, Harrison MT, Long BR, Lau K, Holcomb J, Owen R, et al. Monitoring cell-mediated immune responses in AAV gene therapy clinical trials using a validated IFN-γ ELISpot method. Mol Ther Methods Clin Dev. (2021) 22:183–95. doi: 10.1016/j.omtm.2021.05.012
74. Schiffmann N, Liang Y, Nemcovsky CE, Almogy M, Halperin-Sternfeld M, Gianneschi NC, et al. Enzyme-responsive nanoparticles for dexamethasone targeted delivery to treat inflammation in diabetes. Adv Healthc Mater. (2023) 12:e2301053. doi: 10.1002/adhm.202301053
75. Li V, Binder MD, and Kilpatrick TJ. The tolerogenic influence of dexamethasone on dendritic cells is accompanied by the induction of efferocytosis, promoted by MERTK. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242115903
76. Cain DW and Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. (2017) 17:233–47. doi: 10.1038/nri.2017.1
77. Gaballa SA, Kompella UB, Elgarhy O, Alqahtani AM, Pierscionek B, Alany RG, et al. Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Delivery Transl Res. (2021) 11:866–93. doi: 10.1007/s13346-020-00843-z
78. Mochizuki M and Kawashima H. Effects of FK506, 15-deoxyspergualin, and cyclosporine on experimental autoimmune uveoretinitis in the rat. Autoimmunity. (1990) 8:37–41. doi: 10.3109/08916939008998430
79. Zhang R, He R, Qian J, Guo J, Xue K, and Yuan YF. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of tacrolimus (FK506) encapsulated in liposomes. Invest Ophthalmol Vis Sci. (2010) 51:3575–82. doi: 10.1167/iovs.09-4373
80. Ishikawa T, Hokama H, Katagiri Y, Goto H, and Usui M. Effects of intravitreal injection of tacrolimus (FK506) in experimental uveitis. Curr Eye Res. (2005) 30:93–101. doi: 10.1080/02713680490904368
81. Ishii A, Okada H, Hayashita-Kinoh H, Shin JH, Tamaoka A, Okada T, et al. rAAV8 and rAAV9-mediated long-term muscle transduction with tacrolimus (FK506) in non-human primates. Mol Ther Methods Clin Dev. (2020) 18:44–9. doi: 10.1016/j.omtm.2020.05.012
82. Chu WS and Ng J. Immunomodulation in administration of rAAV: preclinical and clinical adjuvant pharmacotherapies. Front Immunol. (2021) 12:658038. doi: 10.3389/fimmu.2021.658038
83. Montenegro-Miranda PS, ten Bloemendaal L, Kunne C, de Waart DR, and Bosma PJ. Mycophenolate mofetil impairs transduction of single-stranded adeno-associated viral vectors. Hum Gene Ther. (2011) 22:605–12. doi: 10.1089/hum.2010.222
84. Vrellaku B, Sethw Hassan I, Howitt R, Webster CP, Harriss E, McBlane F, et al. A systematic review of immunosuppressive protocols used in AAV gene therapy for monogenic disorders. Mol Ther. (2024) 32:3220–59. doi: 10.1016/j.ymthe.2024.07.016
85. Orlowski A, Katz MG, Gubara SM, Fargnoli AS, Fish KM, and Weber T. Successful transduction with AAV vectors after selective depletion of anti-AAV antibodies by immunoadsorption. Mol Ther Methods Clin Dev. (2020) 16:192–203. doi: 10.1016/j.omtm.2020.01.004
86. Corti M, Elder M, Falk D, Lawson L, Smith B, Nayak S, et al. B-cell depletion is protective against anti-AAV capsid immune response: A human subject case study. Mol Ther Methods Clin Dev. (2014) 1:14033–. doi: 10.1038/mtm.2014.33
87. Corti M, Cleaver B, Clément N, Conlon TJ, Faris KJ, Wang G, et al. Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in pompe disease: preclinical to clinical planning. Hum Gene Ther Clin Dev. (2015) 26:185–93. doi: 10.1089/humc.2015.068
88. Nguyen QD, Merrill PT, Clark WL, Banker AS, Fardeau C, Franco P, et al. Intravitreal sirolimus for noninfectious uveitis: A phase III sirolimus study assessing double-masKed uveitis TReAtment (SAKURA). Ophthalmology. (2016) 123:2413–23. doi: 10.1016/j.ophtha.2016.07.029
89. Ilyinskii PO, Michaud AM, Rizzo GL, Roy CJ, Leung SS, Elkins SL, et al. ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia. Mol Ther Methods Clin Dev. (2021) 22:279–92. doi: 10.1016/j.omtm.2021.06.015
90. Bing SJ, Lyu C, Xu B, Wandu WS, Hinshaw SJ, Furumoto Y, et al. Tofacitinib inhibits the development of experimental autoimmune uveitis and reduces the proportions of Th1 but not of Th17 cells. Mol Vis. (2020) 26:641–51.
91. Du L, Yip YWY, Ng HK, Ho BM, He JN, Chan SO, et al. Ruxolitinib alleviates uveitis caused by salmonella typhimurium endotoxin. Microorganisms. (2021) 9. doi: 10.3390/microorganisms9071481
92. Elmore ZC, Oh DK, Simon KE, Fanous MM, and Asokan A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight. (2020) 5. doi: 10.1172/jci.insight.139881
93. Smith TJ, Elmore ZC, Fusco RM, Hull JA, Rosales A, Martinez M, et al. Engineered IgM and IgG cleaving enzymes for mitigating antibody neutralization and complement activation in AAV gene transfer. Mol Ther. (2024) 32:2080–93. doi: 10.1016/j.ymthe.2024.05.004
94. Xiong W, Wu DM, Xue Y, Wang SK, Chung MJ, Ji X, et al. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc Natl Acad Sci U S A. (2019) 116:5785–94. doi: 10.1073/pnas.1821000116
95. Xiong A, Liu D, Chen H, Yang G, Xiong C, Shuai Y, et al. The efficacy and safety of infliximab in refractory noninfectious uveitis: A meta-analysis of observational studies. Front Pharmacol. (2021) 12:620340. doi: 10.3389/fphar.2021.620340
96. Nguyen BT, Hung JH, Thng ZX, El Feky D, Mobasserian A, Saengsirinavin AO, et al. Tocilizumab for cystoid macular edema secondary to immune recovery uveitis in a patient with contraindications to long-term systemic corticosteroid. Yale J Biol Med. (2024) 97:423–30. doi: 10.59249/NQRT7239
97. Leclercq M, Andrillon A, Maalouf G, Sève P, Bielefeld P, Gueudry J, et al. Anti-tumor necrosis factor α versus tocilizumab in the treatment of refractory uveitic macular edema: A multicenter study from the french uveitis network. Ophthalmology. (2022) 129:520–9. doi: 10.1016/j.ophtha.2021.11.013
98. Kreps EO, Epps SJ, Consejo A, Dick AD, Guly CM, and Ramanan AV. Infliximab in chronic non-infectious paediatric uveitis refractory to previous biologic therapy. Eye (Lond). (2024) 38:871–6. doi: 10.1038/s41433-023-02795-3
99. Hikage F, Lennikov A, Mukwaya A, Lachota M, Ida Y, Utheim TP, et al. NF-κB activation in retinal microglia is involved in the inflammatory and neovascularization signaling in laser-induced choroidal neovascularization in mice. Exp Cell Res. (2021) 403:112581. doi: 10.1016/j.yexcr.2021.112581
100. Di Y and Chen XL. Inhibition of LY294002 in retinal neovascularization via down-regulation the PI3K/AKT-VEGF pathway in vivo and in vitro. Int J Ophthalmol. (2018) 11:1284–9. doi: 10.18240/ijo.2018.08.06
101. Chen L, Cao Y, Shen Y, Li H, Ye R, and Yao J. Downregulation of PIK3IP1 in retinal microglia promotes retinal pathological neovascularization via PI3K-AKT pathway activation. Sci Rep. (2023) 13:12754. doi: 10.1038/s41598-023-39473-z
102. Mary B, Maurya S, Kumar M, Bammidi S, Kumar V, and Jayandharan GR. Molecular engineering of adeno-associated virus capsid improves its therapeutic gene transfer in murine models of hemophilia and retinal degeneration. Mol Pharm. (2019) 16:4738–50. doi: 10.1021/acs.molpharmaceut.9b00959
103. Tabebordbar M, Lagerborg KA, Stanton A, King EM, Ye S, Tellez L, et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. (2021) 184:4919–38.e22. doi: 10.1016/j.cell.2021.08.028
104. Lin R, Zhou Y, Yan T, Wang R, Li H, Wu Z, et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods. (2022) 19:976–85. doi: 10.1038/s41592-022-01547-7
105. Botto C, Dalkara D, and El-Amraoui A. Progress in gene editing tools and their potential for correcting mutations underlying hearing and vision loss. Front Genome Ed. (2021) 3:737632. doi: 10.3389/fgeed.2021.737632
106. Chambers CZ, Soo GL, Engel AL, Glass IA, Frassetto A, Martini PGV, et al. Lipid nanoparticle-mediated delivery of mRNA into the mouse and human retina and other ocular tissues. Transl Vis Sci Technol. (2024) 13:7. doi: 10.1167/tvst.13.7.7
107. Huang X and Chau Y. Investigating impacts of surface charge on intraocular distribution of intravitreal lipid nanoparticles. Exp Eye Res. (2019) 186:107711. doi: 10.1016/j.exer.2019.107711
108. Wiley LA, Boyce TM, Meyering EE, Ochoa D, Sheehan KM, Stone EM, et al. The degree of adeno-associated virus-induced retinal inflammation varies based on serotype and route of delivery: intravitreal, subretinal, or suprachoroidal. Hum Gene Ther. (2023) 34:530–9. doi: 10.1089/hum.2022.222
109. Yao T, Zhou X, Zhang C, Yu X, Tian Z, Zhang L, et al. Site-specific PEGylated adeno-associated viruses with increased serum stability and reduced immunogenicity. Molecules. (2017) 22. doi: 10.3390/molecules22071155
110. Adriouch S, Franck E, Drouot L, Bonneau C, Jolinon N, Salvetti A, et al. Improved immunological tolerance following combination therapy with CTLA-4/ig and AAV-mediated PD-L1/2 muscle gene transfer. Front Microbiol. (2011) 2:199. doi: 10.3389/fmicb.2011.00199
111. Mingozzi F, Anguela XM, Pavani G, Chen Y, Davidson RJ, Hui DJ, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. (2013) 5:194ra92. doi: 10.1126/scitranslmed.3005795
112. Emmanuel SN, Smith JK, Hsi J, Tseng YS, Kaplan M, Mietzsch M, et al. Structurally mapping antigenic epitopes of adeno-associated virus 9: development of antibody escape variants. J Virol. (2022) 96:e0125121. doi: 10.1128/JVI.01251-21
113. Gautam M, Jozic A, Su GL, Herrera-Barrera M, Curtis A, Arrizabalaga S, et al. Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat Commun. (2023) 14:6468. doi: 10.1038/s41467-023-42189-3
114. Herrera-Barrera M, Ryals RC, Gautam M, Jozic A, Landry M, Korzun T, et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci Adv. (2023) 9:eadd4623. doi: 10.1126/sciadv.add4623
115. Farjo R, Skaggs J, Quiambao AB, Cooper MJ, and Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PloS One. (2006) 1:e38. doi: 10.1371/journal.pone.0000038
116. Guo L, Xie X, Wang J, Xiao H, Li S, Xu M, et al. Inducible rbpms-creER(T2) mouse line for studying gene function in retinal ganglion cell physiology and disease. Cells. (2023) 12. doi: 10.3390/cells12151951
117. Choi EH, Suh S, Einstein DE, Leinonen H, Dong Z, Rao SR, et al. An inducible Cre mouse for studying roles of the RPE in retinal physiology and disease. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.146604
118. Le YZ. Conditional gene targeting: dissecting the cellular mechanisms of retinal degenerations. J Ophthalmol. (2011) 2011:806783. doi: 10.1155/2011/806783
119. Sahel JA, Boulanger-Scemama E, Pagot C, Arleo A, Galluppi F, Martel JN, et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med. (2021) 27:1223–9. doi: 10.1038/s41591-021-01351-4
120. Lu C, Mao X, and Yuan S. Decoding physiological and pathological roles of innate immune cells in eye diseases: the perspectives from single-cell RNA sequencing. Front Immunol. (2024) 15:1490719. doi: 10.3389/fimmu.2024.1490719
121. Xu H, Cao L, Chen Y, Zhou C, Xu J, Zhang Z, et al. Single-cell RNA sequencing reveals the heterogeneity and interactions of immune cells and Müller glia during zebrafish retina regeneration. Neural Regeneration Res. (2024) 20(12):3635–48. doi: 10.4103/NRR.NRR-D-23-02083
122. Eid FE, Chen AT, Chan KY, Huang Q, Zheng Q, Tobey IG, et al. Systematic multi-trait AAV capsid engineering for efficient gene delivery. Nat Commun. (2024) 15:6602. doi: 10.1038/s41467-024-50555-y
123. Nisanov AM, Rivera de Jesús JA, and Schaffer DV. Advances in AAV capsid engineering: Integrating rational design, directed evolution and machine learning. Mol Ther. (2025) 33:1937–45. doi: 10.1016/j.ymthe.2025.03.056
124. Amini L, Kaeda J, Fritsche E, Roemhild A, Kaiser D, and Reinke P. Clinical adoptive regulatory T Cell therapy: State of the art, challenges, and prospective. Front Cell Dev Biol. (2022) 10:1081644. doi: 10.3389/fcell.2022.1081644
125. Arjomandnejad M, Sylvia K, Blackwood M, Nixon T, Tang Q, Muhuri M, et al. Modulating immune responses to AAV by expanded polyclonal T-regs and capsid specific chimeric antigen receptor T-regulatory cells. Mol Ther Methods Clin Dev. (2021) 23:490–506. doi: 10.1016/j.omtm.2021.10.010
126. Kressler C, Gasparoni G, Nordström K, Hamo D, Salhab A, Dimitropoulos C, et al. Targeted de-methylation of the FOXP3-TSDR is sufficient to induce physiological FOXP3 expression but not a functional treg phenotype. Front Immunol. (2020) 11:609891. doi: 10.3389/fimmu.2020.609891
127. Chen X, Jiang Y, Duan Y, Zhang X, and Li X. Mesenchymal-stem-cell-based strategies for retinal diseases. Genes (Basel). (2022) 13. doi: 10.3390/genes13101901
128. Habibi A, Zarei-Behjani Z, Falamarzi K, Malekpour M, Ebrahimi F, Soleimani M, et al. Extracellular vesicles as a new horizon in the diagnosis and treatment of inflammatory eye diseases: A narrative review of the literature. Front Immunol. (2023) 14:1097456. doi: 10.3389/fimmu.2023.1097456
129. Li Y, Ren X, Zhang Z, Duan Y, Li H, Chen S, et al. Effect of small extracellular vesicles derived from IL-10-overexpressing mesenchymal stem cells on experimental autoimmune uveitis. Stem Cell Res Ther. (2022) 13:100. doi: 10.1186/s13287-022-02780-9
130. Zhang J, Li P, Zhao G, He S, Xu D, Jiang W, et al. Mesenchymal stem cell-derived extracellular vesicles protect retina in a mouse model of retinitis pigmentosa by anti-inflammation through miR-146a-Nr4a3 axis. Stem Cell Res Ther. (2022) 13:394. doi: 10.1186/s13287-022-03100-x
131. Chandler LC, McClements ME, Yusuf IH, Martinez-Fernandez de la Camara C, MacLaren RE, and Xue K. Characterizing the cellular immune response to subretinal AAV gene therapy in the murine retina. Mol Ther Methods Clin Dev. (2021) 22:52–65. doi: 10.1016/j.omtm.2021.05.011
132. Dai Y, Dong H, Gleason C, Mora J, Kolaitis G, Balasubramanian N, et al. Comparison of pre-existing anti-AAV8 total antibody screening and confirmatory assays with a cell-based neutralizing assay in normal human serum. AAPS J. (2023) 25:35. doi: 10.1208/s12248-023-00805-6
133. Pihlstrom BL and Barnett ML. Design, operation, and interpretation of clinical trials. J Dent Res. (2010) 89:759–72. doi: 10.1177/0022034510374737
Keywords: retinal gene therapy, immune responses, inherited retinal diseases, adeno-associated virus (AAV), immunomodulation
Citation: Ma Y and Shen Y (2025) Immune responses in retinal gene therapy: challenges, mechanisms, and future strategies. Front. Immunol. 16:1664968. doi: 10.3389/fimmu.2025.1664968
Received: 13 July 2025; Accepted: 01 October 2025;
Published: 15 October 2025.
Edited by:
Andrew W. Taylor, Boston University, United StatesReviewed by:
Joseph Larkin, University of Florida, United StatesManuel Soliño, University of Buenos Aires, Argentina
Copyright © 2025 Ma and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Shen, eWluc2hlbkB3aHUuZWR1LmNu
 Yawei Ma
Yawei Ma Yin Shen
Yin Shen