- 1School of Information Engineering, Henan University of Science and Technology, Luoyang, China
- 2Department of Ophthalmology, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 3Henan Key Laboratory of Cancer Epigenetic, Cancer Institute, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 4Department of Ophthalmology, Sanmenxia Central Hospital/Affiliated Sanmenxia Central Hospital of Henan University of Science and Technology, Sanmenxia Eye Hospital, International Joint Laboratory for Glaucoma Aqueous Humor Drainage in Henan Province, Engineering Technology Research Center for Minimally Invasive Eye Surgery in Henan Province, and Engineering Research Center for Minimally Invasive Ophthalmic Surgery in Henan Province, Sanmenxia, China
Retinoblastoma (RB) immunotherapy represents a paradigm shift in managing this aggressive pediatric eye cancer, overcoming limitations of conventional therapies. Recent breakthroughs reveal how circular RNAs (circRNAs) critically modulate the tumor-immune microenvironment: oncogenic circRNAs promote immune evasion by upregulating PD-L1 and suppressing T cell activity, while tumor-suppressive circRNAs such as circMKLN1 enhance antigen presentation and cytotoxic responses. The convergence of circRNA biology with immunotherapy has yielded innovative strategies, including circRNA-targeted immune checkpoint blockade to reverse T cell exhaustion, circRNA-engineered CAR-T cells with improved tumor homing and persistence, and circRNA-based oncolytic viruses that stimulate immunogenic cell death. Notably, exosomal circRNAs serve dual roles as both immune modulators and minimally invasive biomarkers for predicting immunotherapy response. While preclinical studies demonstrate remarkable synergy between circRNA inhibition and PD-1/CTLA-4 blockade in RB models, clinical translation requires optimization of delivery systems and combinatorial regimens. This review summarizes the latest evidence positioning circRNAs as central regulators of anti-tumor immunity and provides a strategic roadmap for the integration of circRNA-based approaches in precision immunotherapy for RB.
1 Introduction
Retinoblastoma (RB) is the most prevalent intraocular malignancy in children (1). It poses a severe threat to both vision and survival in infants and young children. Current therapeutic strategies, including chemotherapy, enucleation, laser photocoagulation, and cryotherapy, have improved outcomes but remain limited by non-specific toxicity, treatment resistance, and metastatic relapse (2, 3). To address these limitations, immunotherapy has emerged as a transformative approach in RB treatment, capable of enhancing tumor specificity, reducing systemic toxicity, and preventing recurrence through durable immune activation (3). Recent studies highlight the critical role of immune evasion in RB progression, with tumors often overexpressing PD-L1 and exhibiting T cell exhaustion signatures (4, 5). These findings have spurred interest in checkpoint inhibitors, CAR-T cells, and other immunomodulatory strategies tailored to RB (6).
In parallel, circular RNAs (circRNAs), a class of covalently closed-loop non-coding RNAs, have gained attention for their regulatory roles in tumor biology, including epigenetic control, cell proliferation, metastasis, and chemoresistance (7, 8). Notably, circRNAs influence immune responses by modulating antigen presentation, cytokine signaling, and immune checkpoint expression (9, 10). However, the immunological roles of circRNAs in RB remain underexplored. This review provides a comprehensive summary of the recent advances in circRNA research and immunotherapeutic strategies in the context of retinoblastoma, with the aim of highlighting novel insights into molecular mechanisms and translational application.
2 The regulatory role of circRNAs in RB progression
2.1 Overview of circular RNAs
Circular RNAs (circRNAs) are covalently closed-loop, non-coding RNAs that are widely expressed across diverse organisms. They primarily arise through back-splicing of precursor transcripts (11). This circular structure renders them resistant to exonuclease degradation, ensuring remarkable stability and prolonged half-lives, with approximately 80% localized in the cytoplasm (12). CircRNAs can be classified into intronic, exonic (ecircRNAs), and exon-intron forms, with ecircRNAs being the most prevalent (13, 14). Functionally, circRNAs act as microRNA (miRNA) sponges, sequestering miRNAs via the competing endogenous RNA (ceRNA) mechanism to modulate mRNA expression (15). Additionally, they interact with RNA-binding proteins, regulating RNA stability, transcription, and splicing, while also forming complexes with other RNAs that influence epigenetic and transcriptional processes (16, 17). Recent findings suggest that certain circRNAs possess coding potential, producing functional peptides (18). CircRNAs are increasingly recognized as pivotal regulators in tumorigenesis. For instance, circZNF566 promotes hepatocellular carcinoma progression by sponging miR-4738-3p, while circ-0052112 enhances breast cancer metastasis through targeting miR-125a-5p (19, 20). In RB, circRNAs play significant roles in oncogenesis, diagnosis, therapy response, and prognosis, positioning them as promising biomarkers and therapeutic targets (21).
2.2 Oncogenic circRNAs
2.2.1 circ-0000527 and circ-0000034
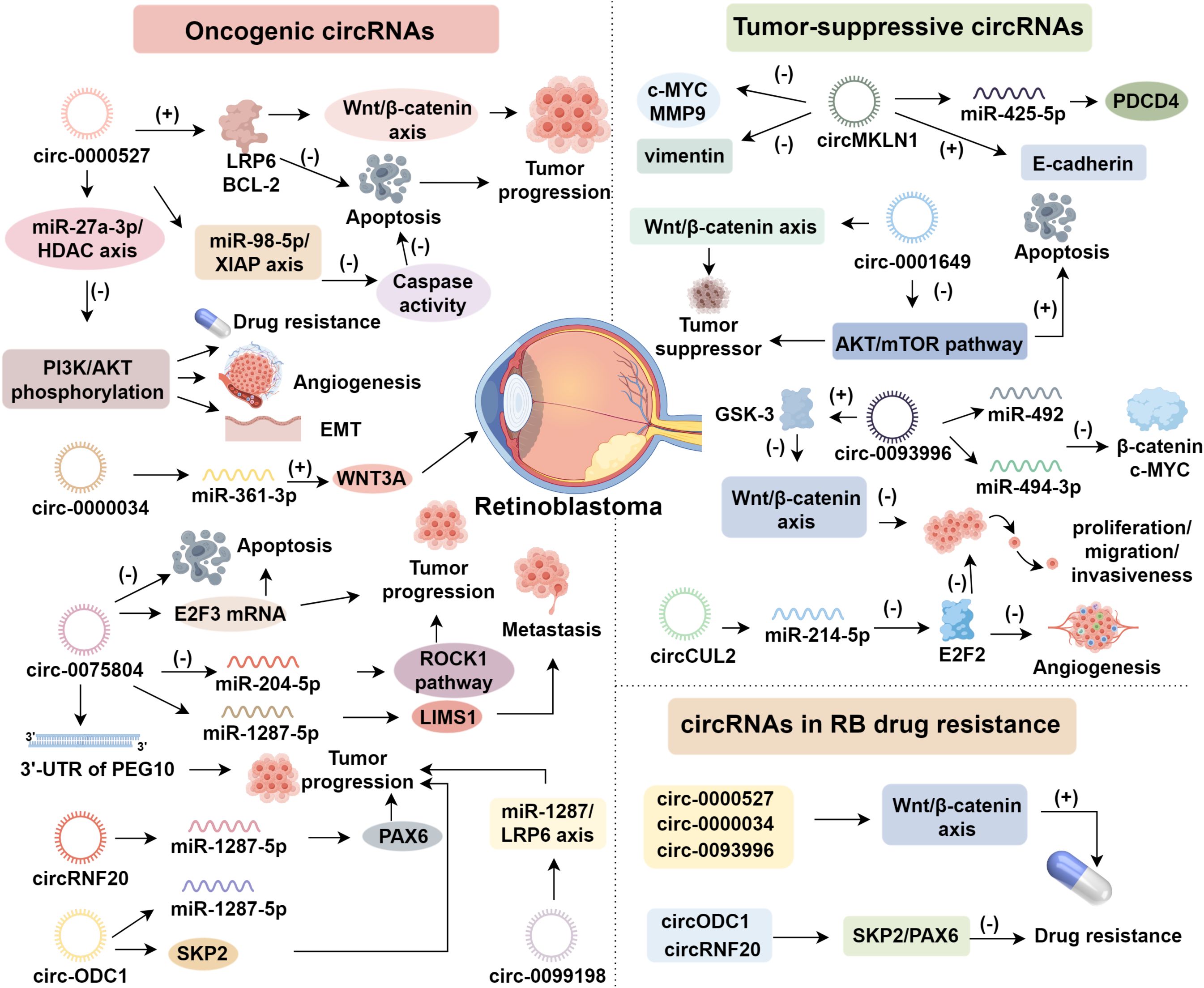
circ-0000527, also known as circ-FAM158A, is the sole circular RNA transcribed from the FAM158A gene and is notably overexpressed in RB (22). Functional investigations have demonstrated that silencing circ-0000527 inhibits RB cell proliferation, migration, invasion, angiogenesis, and induces apoptosis (22–24). Mechanistically, circ-0000527 functions as a molecular sponge for miR-646, leading to the upregulation of LRP6 and BCL-2. These molecules promote tumor progression via Wnt/β-catenin signaling and inhibit apoptosis, respectively (25, 26). Furthermore, circ-0000527 regulates the miR-27a-3p/HDAC axis, decreasing PI3K and AKT phosphorylation, which enhances epithelial–mesenchymal transition (EMT), drug resistance, and angiogenesis (27). Besides, circ-0000527 reduces caspase activity through the miR-98-5p/XIAP axis, further suppressing apoptosis (22). As a competing endogenous RNA (ceRNA) for miR-138-5p, it upregulates SLC7A5, facilitating amino acid uptake to satisfy the metabolic demands of tumor cells (24). These diverse mechanisms underscore circ-0000527 as a key oncogenic regulator in RB.
circ-0000034, also referred to as circ-001787 or circDHDDS, is derived from the DHDDS gene, mutations in which are linked to retinitis pigmentosa (28, 29). circ-0000034 has been shown to enhance RB cell viability, migration, invasion, autophagy, and EMT. Knockdown of circ-0000034 results in G0/G1 cell cycle arrest and induces apoptosis in RB cells (30, 31). To date, miR-361-3p is the only confirmed target of circ-0000034 in RB, acting as a tumor suppressor (32). As a ceRNA for miR-361-3p, circ-0000034 modulates downstream targets, including WNT3A and STX17 (31). By sponging miR-361-3p, circ-0000034 upregulates WNT3A, thereby promoting oncogenesis (29). Additionally, ADAM19, a disintegrin and metalloprotease involved in cell adhesion and proteolysis, is a downstream target of miR-361-3p, enhancing RB cell migration and invasiveness (28).
2.2.2 circ-0075804
circ-0075804, a circular RNA originating from the E2F3 gene located on chromosome 6, plays a pivotal role in the regulation of cell cycle-associated genes through its direct interaction with the RB protein (33). E2F3, as a transcription factor, regulates critical genes involved in the cell cycle and cellular proliferation, and its activity is tightly controlled by RB binding. In RB tissues and cell lines, an upregulation of circ-0075804 has been shown to promote cellular proliferation and malignant transformation, while simultaneously inhibiting apoptosis, a hallmark of cancer progression (34, 35). This effect is thought to be mediated by circ-0075804’s interaction with heterogeneous nuclear ribonucleoproteins, which stabilizes E2F3 mRNA, thereby enhancing its activity and contributing to oncogenic processes (35). ROCK1, a serine/threonine kinase of the AGC family encoded at 18q11.1, regulates various cellular processes, including proliferation, differentiation, adhesion, and cytoskeletal remodeling (36). circ-0075804 activates the ROCK1 pathway via miR-204-5p inhibition, thereby contributing to RB progression (36). Additionally, circ-0075804 serves as a sponge for miR-138-5p, which modulates the 3’-untranslated region (UTR) of the PEG10 gene. This interaction promotes tumor growth, underscoring its involvement in RB pathogenesis (37). Notably, circ-0075804 also targets miR-1287-5p, thereby upregulating LIMS1, a cytoskeletal scaffold protein that enhances cellular invasiveness and contributes to RB metastasis (38). These findings highlight circ-0075804 as a multifaceted regulator, influencing several key pathways in the progression and metastasis of RB.
2.2.3 Other oncogenic circRNAs
In addition to the aforementioned circRNAs, several others have emerged as significant players in the pathogenesis of RB. These include circRNF20, circ-ODC1, circ-0099198, circROBO1, and circ-0000989, all of which have been implicated in RB tumorigenesis (39, 40). PAX6 is indispensable for retinal and ocular development; its dysregulation has been linked to congenital aniridia, anterior segment dysgenesis, glaucoma, and RB (41). Notably, circRNF20 has been shown to promote RB progression by directly targeting the tumor-suppressive miR-132-3p, thereby indirectly upregulating PAX6 expression, which further contributes to tumorigenesis (39). Moreover, both the long non-coding RNA ZFPM2-AS1 and miR-130a-3p are involved in regulating RB development and chemoresistance, with a particular focus on their interaction with PAX6 (42, 43). The role of circ-ODC1 in RB progression is also of particular interest, as it encodes ODC1, a rate-limiting enzyme in polyamine biosynthesis, which has been recognized as an oncogene in a range of malignancies (44). Du et al. demonstrated that circ-ODC1 fosters RB progression by sponging miR-422a, resulting in the activation of SKP2 (40). SKP2 participates in ubiquitination, autophagy, cell cycle regulation, and signal transduction. Inhibiting SKP2 sensitizes non-small cell lung cancer and osteosarcoma cells to cisplatin both in vitro and in vivo, potentially overcoming drug resistance (45). Furthermore, circ-0099198 promotes the proliferation and metastasis of Y79 and SO-RB50 cells by regulating the miR-1287/LRP6 axis, accelerating cell cycle progression (46).
2.3 Tumor-suppressive circRNAs
2.3.1 circMKLN1
circMKLN1, a circular RNA derived from the MKLN1 gene, plays a critical role in the autophagic processes underlying diabetic retinopathy, where it has been shown to promote neovascularization (47). MKLN1 encodes a muscle protein that is pivotal in cell proliferation by mediating cytoskeletal organization and cellular motility (48). Notably, the upregulation of circMKLN1 facilitates its interaction with miR-425-5p, thereby regulating the expression of PDCD4, a key tumor suppressor and a downstream target of RB signaling (49). circMKLN1 promoted CDK8 expression through sponge adsorption of miR-26a/b, which regulates EMT (50). Notably, silencing of PDCD4 markedly attenuates the tumor-suppressive effects of circMKLN1 (49). Beyond these effects, PDCD4 constrains AP-1–dependent transcription and cap-dependent translation. Thus, the circMKLN1–miR-425-5p–PDCD4 axis plausibly couples transcriptional restraint (c-MYC programs) with suppression of invasion (MMP9, vimentin) and maintenance of epithelial identity (E-cadherin) (49, 51). Functionally, this positions circMKLN1 to counter EMT and metastatic competence while limiting proliferative signaling, providing a mechanistic counterweight to oncogenic circRNAs that amplify Wnt/β-catenin or PI3K/AKT activity (52).
2.3.2 circ-0001649
circ-0001649, a transcriptional product derived from the DNA repair gene SHPRH, as a tumor suppressor by negatively regulating tumorigenesis via the Wnt/β-catenin signaling pathway (53). This circRNA has been linked to several malignancies, including prostate, gastric, and hepatic cancers (54). circ-0001649 exerts its antitumor effects by inhibiting the AKT/mTOR pathway—a pivotal signaling axis that governs cellular processes such as proliferation, apoptosis, angiogenesis, and glucose metabolism in cancer cells. Both in vitro and in vivo studies have shown that overexpression of circ-0001649 results in reduced expression of AKT and mTOR, accompanied by diminished RB cell viability, inhibition of proliferation, and promotion of apoptosis (55). Furthermore, the PI3K/AKT/mTOR signaling pathway promotes forward regulation that stabilizes β-catenin and facilitates EMT. Inhibition of this pathway by circ-0001649 thus provides dual antitumor effects: attenuating anabolic growth processes while simultaneously suppressing β-catenin-mediated transcriptional activity (56, 57). In RB, where cell-cycle regulation intersects at the RB/E2F node, attenuation of the AKT/mTOR pathway by circ-0001649 is proposed to diminish cyclin-CDK activity, thereby reinforcing G1-phase arrest and sensitizing tumor cells to cytotoxic stress (58).
2.3.3 circ-0093996 and circCUL2
circ-0093996 is derived from the TET1 gene which functions as a pivotal tumor suppressor gene (59). The overexpression of circ-0093996 induces G0/G1 cell cycle arrest in Y79 and WERI-RB1 cells, effectively halting the progression of the cell cycle. This regulatory effect is mediated through the sequestration of miR-492 and miR-494-3p, which leads to the downregulation of key oncogenes, including β-catenin and c-MYC, while simultaneously upregulating GSK-3 (60, 61). GSK-3, a highly conserved serine/threonine kinase, inhibits β-catenin activity and prevents its cytoplasmic accumulation, thereby suppressing Wnt/β-catenin signaling. Ultimately, this cascade inhibits the proliferation, migration, and invasiveness of RB cells (61). circCUL2 arises from the back-splicing of CUL2 mRNA, which encodes an E3 ubiquitin ligase, and is involved in cell cycle regulation (62). E2F2, a member of the E2F transcription factor family, is an RB-regulated transcription factor that governs cell invasion and proliferation during cancer progression and erythroid maturation. circCUL2 acts as a ceRNA for miR-214-5p, thereby modulating the expression of E2F2 (62). In RB cells, circCUL2 is upregulated and indirectly represses E2F2 expression, resulting in inhibition of tumor angiogenesis and attenuation of RB cell proliferation and migration (63).
2.4 circRNAs in RB drug resistance
Drug resistance in RB involves complex molecular mechanisms, including circRNA-mediated regulation of oncogenic pathways. circ-0000527, circ-0000034, and circ-0093996 promote chemoresistance via the Wnt/β-catenin axis, while circODC1 and circRNF20 suppress resistance by targeting SKP2 and PAX6, respectively. In vivo, silencing circ-0000034 reduced RB tumor volume and weight, likely through downregulation of stemness markers CD133 and SOX2 (29). Mechanistically, circ-0000034 enhances autophagy by binding STX17, facilitating autophagosome–lysosome fusion. Depletion of circ-0000034 in Y79 and WERI-Rb1 cells leads to a marked reduction in the expression of LC3-II/LC3-I and Beclin-1, which impairs autophagic flux and diminishes drug resistance (31, 47). These findings suggest that circ-0000034–induced autophagy contributes to RB chemoresistance (64). Therefore, strategies targeting circRNAs may present a promising therapeutic approach to overcoming drug resistance and improving the prognosis of RB patients.
3 Prospects of circRNAs in the diagnosis and treatment of RB
3.1 circRNAs as potential diagnostic biomarkers for RB
Current clinical diagnosis of RB relies heavily on imaging techniques such as MRI, CT, and B-scan ultrasonography (65). However, the intraocular location of RB hinders tissue biopsy, and the young age of patients limits diagnostic compliance and accuracy (66). To overcome these challenges, non-invasive strategies like liquid biopsies based on molecular biomarkers have gained interest (67). circRNAs, characterized by high stability and tissue specificity, have emerged as promising diagnostic candidates. Lyu et al. (68) identified 550 downregulated circRNAs in RB tissues via RNA sequencing, with circ-0093996 most significantly reduced. Additional studies have reported differential expression of circRNAs such as circ-0000034, circ-0075804, circ-0000527, circ-0000989, and circ-0001649 in RB tumor tissues (34, 55, 69), suggesting their diagnostic potential. Nonetheless, most studies have focused on tumor samples and primary cell lines, limiting clinical translation. Recently, exosomal circRNAs have attracted attention due to their stability and presence in biofluids, enabling minimally invasive detection and real-time disease monitoring (70, 71). An et al. (39) demonstrated that serum-derived exosomes from RB patients contained elevated levels of circRNF20, especially in advanced TNM stages. Functionally, these exosomes enhanced viability and proliferation of RB cell lines, indicating that exosomal circRNF20 may contribute to RB progression and serve as a non-invasive biomarker.
3.2 circRNAs as therapeutic targets and prognostic biomarkers in RB
circRNAs have emerged as potential biomarkers in RB (72). Elevated levels of oncogenic circRNAs—such as circ-0119412, circRNF20, and circ-0000034—are associated with higher TNM stage, optic nerve invasion, and poor survival outcomes (28, 73). In contrast, reduced expression of tumor-suppressive circRNAs such as circ-0001649 predicts more aggressive disease, whereas circMKLN1 overexpression correlates with favorable prognosis (74, 75). Functional studies highlight the therapeutic relevance of circRNAs. Knockdown of circRNF20 via shRNA in RB cells reduced proliferation and colony formation by 50%, and xenograft models confirmed tumor volume reductions of similar magnitude (39). Likewise, circMKLN1 overexpression in Y79 and WERI-RB1 cells suppressed colony formation by over 60% and significantly reduced tumor burden in vivo (49). Additional circRNAs, including circ-0000034, circ-0075804, circ-ODC1, circ-0099198, and circ-0007534, exert growth-suppressive effects upon silencing, while overexpression of circ-0093996 yields similar outcomes. CircRNAs may also influence drug response. For instance, circ-0007534 overexpression diminishes the antitumor efficacy of osthole, reversing its inhibitory effects on cell viability and colony formation (76). Collectively, these findings underscore the dual role of circRNAs as both biomarkers and therapeutic targets, offering promising avenues to overcome chemoresistance and improve RB treatment outcomes (Figure 1).
4 Advances in immunotherapy
Recent advances in immunotherapy have transformed the therapeutic landscape of RB, offering strategies that harness and redirect immune responses against tumor cells. Immune checkpoint inhibitors (ICIs), such as PD-1, PD-L1, and CTLA-4 blockers, restore cytotoxic T cell–mediated surveillance by disrupting inhibitory signals within the tumor microenvironment (TME), and have shown promising efficacy in RB, where PD-L1 is upregulated and co-expression of PD-1/CTLA-4 correlates with poor prognosis (77, 78). Adoptive cell therapy (ACT), including tumor-infiltrating lymphocyte (TIL) and CAR-T approaches, enables personalized administration of antitumor lymphocytes (79). CAR-T cells targeting CD171 and GD2 effectively eliminate primary and metastatic RB cells and mitigate chemotherapy-associated toxicity (80, 81). Parallelly, inhibitors of cyclin-dependent kinases 4/6 (CDK4/6) such as palbociclib, ribociclib, and abemaciclib suppress Rb phosphorylation and cell cycle progression, while concurrently enhancing antitumor immunity by increasing antigen presentation and suppressing regulatory T cell (Treg) proliferation (82, 83). TFF1-overexpressing RB cells exhibit CDK6 downregulation, and exogenous TFF1 impairs the viability of RB cell lines, supporting the clinical utility of CDK4/6 inhibitors (84, 85). Anti-angiogenic therapy targeting VEGF, which is overexpressed in poorly differentiated RB, limits neovascularization and immunosuppression in the TME. Bevacizumab, an anti-VEGF monoclonal antibody, has shown efficacy in RB xenograft models, further validating VEGF as a viable target (86). Oncolytic viruses (OVs), particularly engineered adenoviruses, selectively replicate within and lyse tumor cells while stimulating systemic immune responses. Intra-tumoral delivery enhances local efficacy and minimizes systemic toxicity (87). Mouse models of RB treated with replication-competent and -deficient adenoviruses have demonstrated potent antitumor effects and synergism with chemo- or radiotherapy (88). Moreover, therapeutic cancer vaccines, especially DNA-based platforms, reawaken suppressed immunity by expanding tumor-reactive T cell pools. DNA vaccines offer multivalent, HLA-independent responses, favorable safety, and scalability (89). Racotumomab, an anti-idiotype vaccine targeting NeuGcGM3, has elicited strong immune responses in RB patients in clinical trials (90, 91).
Recent studies have begun to elucidate how circRNAs shape the immunological landscape of RB. Oncogenic circRNAs such as circ-0136666 and circ-0000512 promote immune escape by upregulating PD-L1 expression, either directly or via modulation of Wnt/β-catenin and ROCK1 pathways, which are known to suppress antigen presentation and impair CD8+ T cell cytotoxicity (92, 93). Conversely, tumor-suppressive circRNAs like circMKLN1 enhance anti-tumor immunity by upregulating PDCD4, a key regulator of antigen processing and presentation, thereby facilitating CD8+ T cell activation (49). Furthermore, circMKLN1 overexpression has been shown to elevate E-cadherin levels and reduce c-MYC and MMP9 expression, collectively fostering a TME more conducive to T cell infiltration and activation (52). Exosomal circRNAs such as circ-SPEF2 may also influence immune dynamics by promoting the expansion of immunosuppressive cell subsets, including Tregs (94), though direct evidence in RB remains limited. These findings highlight the dual roles of circRNAs as both intrinsic regulators of immune checkpoints and extrinsic communicators via exosomal signaling, underscoring their potential as targets for immunotherapy (95). Compared to conventional therapies, immunotherapy provides superior specificity, minimizes off-target toxicity, and potentially penetrates the blood–retinal and blood–brain barriers (96, 97). As the immunobiology of RB becomes clearer, and immune modulation strategies such as checkpoint blockade, ACT, CDK4/6 inhibition, anti-VEGF therapy, oncolytic virotherapy, and vaccination continue to evolve, these immunotherapeutic advances offer a compelling framework for improved RB management and expanded treatment paradigms (98).
5 Conclusion
Retinoblastoma therapy has entered a transformative era, driven by advances in circRNA biology and immunotherapeutic innovation. Circular RNAs (circRNAs), owing to their stability and tissue specificity, have emerged as critical regulators of tumorigenesis, chemoresistance, and metastatic progression. Moreover, their potential for non-invasive diagnostics, particularly through exosomal detection (circRNF20), offers new opportunities for early detection and monitoring. In parallel, immunotherapies including checkpoint inhibitors, CAR-T cells, and oncolytic viruses are overcoming the limitations of traditional therapies by harnessing immune-mediated tumor clearance and facilitating durable remission.
Despite these exciting developments, several knowledge gaps remain before clinical translation. Key challenges include optimizing in vivo delivery systems for circRNA-targeted therapeutics, identifying tumor-specific circRNA targets with minimal off-target effects, integrating circRNA modulation with immunotherapies to enhance efficacy and reduce resistance, and establishing exosomal circRNAs as biomarkers for treatment stratification and disease monitoring. Clinical validation requires robust preclinical RB models that mimic the human tumor microenvironment and prospective trials to assess safety, specificity, and long-term outcomes. The synergy between circRNA modulation and immune activation offers a strategy to overcome drug resistance and metastatic relapse. As the molecular landscape of RB is further understood, integrating circRNA diagnostics with precision immunotherapy promises personalized, low-toxicity therapies. Future efforts should focus on bridging preclinical findings to clinical applications, improving survival and quality of life for pediatric RB patients.
Author contributions
XW: Writing – original draft. JM: Writing – original draft. YD: Writing – original draft, Writing – review & editing. FL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Central Guidance Project of China for Developing Local Science and Technology (Grant No: Z20221341047), the International Science & Technology Cooperation Program of Henan (Grant No: 232102521033) and the Key R&D and Promotion Projects of Henan Province (Grant No: 232102310130).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li X, Chang Y, Zhao X, Liu X, Zhang Y, Wang X, et al. Global health inequities in retinoblastoma: a 1990–2021 analysis across socio-demographic index regions. Front Public Health. (2025) 13:1513526. doi: 10.3389/fpubh.2025.1513526
2. Fabian D, Abdallah E, Abdullahi SU, Abdulqader RA, Abdulrahaman AA, Abouelnaga S, et al. The Global Retinoblastoma Outcome Study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. (2022) 10:e1128–40. doi: 10.1016/S2214-109X(22)00250-9
3. Kritfuangfoo T and Rojanaporn D. Update on chemotherapy modalities for retinoblastoma: Progress and challenges. Asia Pac J Ophthalmol (Phila). (2024) 13:100061. doi: 10.1016/j.apjo.2024.100061
4. Shi H, Qin X, Jiang X, Jin X, Zhang J, Li H, et al. Electrically driven chimeric extracellular vesicles crossing the fundus barrier for retinoblastoma treatment. ACS Nano. (2025) 19:26200–16. doi: 10.1021/acsnano.5c08390
5. Jin X, Ding D, Yan Y, Li H, Wang B, Ma L, et al. Phosphorylated RB promotes cancer immunity by inhibiting NF-κB activation and PD-L1 expression. Mol Cell. (2019) 73:22–35.e26. doi: 10.1016/j.molcel.2018.10.034
6. Pascual-Pasto G, McIntyre B, Giudice AM, Alikarami F, Morrissey A, Matlaga S, et al. Targeting GPC2 on intraocular and CNS metastatic retinoblastomas with local and systemic delivery of CAR T cells. Clin Cancer Res. (2024) 30:3578–91. doi: 10.1158/1078-0432.CCR-24-0221
7. Ning Y, Qu X, Li X, Jin L, and Liu J. Molecular mechanism of RB progression and Circ_0082415 inhibits MKLN1 translation to suppress retinoblastoma progression: Changes in mRNA and protein levels. Int J Biol Macromol. (2025) 286:138524. doi: 10.1016/j.ijbiomac.2024.138524
8. Gong J, Song Z, Pan J, Huang X, Feng X, and Li Q. circSLC39A8 by sponging hsa-miR-11181-5p and direct binding to PIK3CA mRNA promotes retinoblastoma proliferation. J Cancer. (2025) 16:2434–48. doi: 10.7150/jca.99599
9. Li H, Peng K, Yang K, Ma W, Qi S, Yu X, et al. Circular RNA cancer vaccines drive immunity in hard-to-treat Malignancies. Theranostics. (2022) 12:6422–36. doi: 10.7150/thno.77350
10. Liu CX, Guo SK, Nan F, Xu YF, Yang L, and Chen LL. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol Cell. (2022) 82:420–34.e426. doi: 10.1016/j.molcel.2021.11.019
11. Tian H, Zhao L, Li H, Huang Y, and Wang Y. Circular RNA in retina: A potential biomarker and therapeutic target. Ophthalmic Res. (2023) 66:516–28. doi: 10.1159/000529207
12. Amaya L, Grigoryan L, Li Z, Lee A, Wender PA, Pulendran B, et al. Circular RNA vaccine induces potent T cell responses. Proc Natl Acad Sci U S A. (2023) 120:e2302191120. doi: 10.1073/pnas.2302191120
13. Long F, Li L, Xie C, Ma M, Wu Z, Lu Z, et al. Intergenic circRNA circ_0007379 inhibits colorectal cancer progression by modulating miR-320a biogenesis in a KSRP-Dependent manner. Int J Biol Sci. (2023) 19:3781–803. doi: 10.7150/ijbs.85063
14. Wang M, Xie F, Lin J, Zhao Y, Zhang Q, Liao Z, et al. Diagnostic and prognostic value of circulating circRNAs in cancer. Front Med (Lausanne). (2021) 8:649383. doi: 10.3389/fmed.2021.649383
15. Silenzi V, D’Ambra E, Santini T, D’Uva S, Setti A, Salvi N, et al. A tripartite circRNA/mRNA/miRNA interaction regulates glutamatergic signaling in the mouse brain. Cell Rep. (2024) 43:114766. doi: 10.1016/j.celrep.2024.114766
16. Li Q, Zhao YH, Xu C, Liang YL, Zhao Y, He QM, et al. Chemotherapy-Induced senescence reprogramming promotes nasopharyngeal carcinoma metastasis by circRNA-Mediated PKR activation. Adv Sci (Weinh). (2023) 10:e2205668. doi: 10.1002/advs.202205668
17. Margvelani G, Maquera KAA, Welden JR, Rodgers DW, and Stamm S. Translation of circular RNAs. Nucleic Acids Res. (2025) 53:1167. doi: 10.1093/nar/gkae1167
18. Yi Q, Feng J, Lan W, Shi H, Sun W, and Sun W. CircRNA and lncRNA-encoded peptide in diseases, an update review. Mol Cancer. (2024) 23:214. doi: 10.1186/s12943-024-02131-7
19. Li S, Weng J, Song F, Li L, Xiao C, Yang W, et al. Circular RNA circZNF566 promotes hepatocellular carcinoma progression by sponging miR-4738-3p and regulating TDO2 expression. Cell Death Dis. (2020) 11:452. doi: 10.1038/s41419-020-2616-8
20. Zhang HD, Jiang LH, Hou JC, Zhong SL, Zhou SY, Zhu LP, et al. Circular RNA hsa_circ_0052112 promotes cell migration and invasion by acting as sponge for miR-125a-5p in breast cancer. BioMed Pharmacother. (2018) 107:1342–53. doi: 10.1016/j.biopha.2018.08.030
21. Li F, Yin YK, Zhang JT, Gong HP, and Hao XD. Role of circular RNAs in retinoblastoma. Funct Integr Genomics. (2022) 23:13. doi: 10.1007/s10142-022-00942-9
22. Yu B, Zhao J, and Dong Y. Circ_0000527 Promotes Retinoblastoma Progression through Modulating miR-98-5p/XIAP Pathway. Curr Eye Res. (2021) 46:1414–23. doi: 10.1080/02713683.2021.1891255
23. Liang T, Fan M, Meng Z, Sun B, Mi S, and Gao X. Circ_0000527 drives retinoblastoma progression by regulating miR-1236-3p/SMAD2 pathway. Curr Eye Res. (2022) 47:624–33. doi: 10.1080/02713683.2021.2007535
24. Zheng T, Chen W, Wang X, Cai W, Wu F, and Lin C. Circular RNA circ-FAM158A promotes retinoblastoma progression by regulating miR-138-5p/SLC7A5 axis. Exp Eye Res. (2021) 211:108650. doi: 10.1016/j.exer.2021.108650
25. Singh L, Pushker N, Saini N, Sen S, Sharma A, Bakhshi S, et al. Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin Exp Ophthalmol. (2015) 43:259–67. doi: 10.1111/ceo.12397
26. Alrefaei AF and Abu-Elmagd M. LRP6 receptor plays essential functions in development and human diseases. Genes (Basel). (2022) 13:120. doi: 10.3390/genes13010120
27. Zuo X, Fu C, Xie J, Wang X, and Yan Z. Hsa_circ_0000527 downregulation suppresses the development of retinoblastoma by modulating the miR-27a-3p/HDAC9 pathway. Curr Eye Res. (2022) 47:115–26. doi: 10.1080/02713683.2021.1925697
28. Jiang Y, Xiao F, Wang L, Wang T, and Chen L. Correction to: Circular RNA hsa_circ_0000034 accelerates retinoblastoma advancement through the miR-361-3p/ADAM19 axis. Mol Cell Biochem. (2022) 477:1321. doi: 10.1007/s11010-021-04311-1
29. Wang H, Li M, Cui H, Song X, and Sha Q. CircDHDDS/miR-361-3p/WNT3A axis promotes the development of retinoblastoma by regulating proliferation, cell cycle, migration, and invasion of retinoblastoma cells. Neurochem Res. (2020) 45:2691–702. doi: 10.1007/s11064-020-03112-0
30. Jiang Y, Xiao F, Wang L, Wang T, and Chen L. Circular RNA has_circ_0000034 accelerates retinoblastoma advancement through the miR-361-3p/ADAM19 axis. Mol Cell Biochem. (2021) 476:69–80. doi: 10.1007/s11010-020-03886-5
31. Liu H, Yuan HF, Xu D, Chen KJ, Tan N, and Zheng QJ. Circular RNA circ_0000034 upregulates STX17 level to promote human retinoblastoma development via inhibiting miR-361-3p. Eur Rev Med Pharmacol Sci. (2020) 24:12080–92. doi: 10.26355/eurrev_202012_23997
32. Zhao D and Cui Z. MicroRNA-361-3p regulates retinoblastoma cell proliferation and stemness by targeting hedgehog signaling. Exp Ther Med. (2019) 17:1154–62. doi: 10.3892/etm.2018.7062
33. Tu J, Huo Z, Yu Y, Zhu D, Xu A, Huang MF, et al. Hereditary retinoblastoma iPSC model reveals aberrant spliceosome function driving bone Malignancies. Proc Natl Acad Sci U.S.A. (2022) 119:e2117857119. doi: 10.1073/pnas.2117857119
34. Wu W, Zhang Y, and Yang M. Emerging role of circular RNAs in the pathogenesis of retinoblastoma. Ophthalmic Res. (2024) 67:51–61. doi: 10.1159/000535329
35. Zhao W, Wang S, Qin T, and Wang W. Circular RNA (circ-0075804) promotes the proliferation of retinoblastoma via combining heterogeneous nuclear ribonucleoprotein K (HNRNPK) to improve the stability of E2F transcription factor 3 E2F3. J Cell Biochem. (2020) 121:3516–25. doi: 10.1002/jcb.29631
36. Huang Y, Xue B, Pan J, and Shen N. Circ-E2F3 acts as a ceRNA for miR-204-5p to promote proliferation, metastasis and apoptosis inhibition in retinoblastoma by regulating ROCK1 expression. Exp Mol Pathol. (2021) 120:104637. doi: 10.1016/j.yexmp.2021.104637
37. Zhang Y, Dou X, Kong Q, Li Y, and Zhou X. Circ_0075804 promotes the Malignant behaviors of retinoblastoma cells by binding to miR-138-5p to induce PEG10 expression. Int Ophthalmol. (2022) 42:509–23. doi: 10.1007/s10792-021-02067-7
38. Han Q, Ma L, Shao L, Wang H, and Feng M. Circ_0075804 regulates the expression of LASP1 by targeting miR-1287-5p and thus affects the biological process of retinoblastoma. Curr Eye Res. (2022) 47:1077–86. doi: 10.1080/02713683.2022.2053164
39. An D, Yang J, and Ma L. circRNF20 aggravates the Malignancy of retinoblastoma depending on the regulation of miR-132-3p/PAX6 axis. Open Med (Wars). (2022) 17:955–68. doi: 10.1515/med-2022-0483
40. Du S, Wang S, Zhang F, and Lv Y. SKP2, positively regulated by circ_ODC1/miR-422a axis, promotes the proliferation of retinoblastoma. J Cell Biochem. (2020) 121:322–31. doi: 10.1002/jcb.29177
41. Wu S, Li F, Mo K, Huang H, Yu Y, Huang Y, et al. IGF2BP2 maintains retinal pigment epithelium homeostasis by stabilizing PAX6 and OTX2. Invest Ophthalmol Vis Sci. (2024) 65:17. doi: 10.1167/iovs.65.6.17
42. Ni W, Li Z, and Ai K. lncRNA ZFPM2-AS1 promotes retinoblastoma progression by targeting microRNA miR-511-3p/paired box protein 6 (PAX6) axis. Bioengineered. (2022) 13:1637–49. doi: 10.1080/21655979.2021.2021346
43. Lu X, Tu H, Tang D, Huang X, and Sun F. miR-130a-3p enhances the chemosensitivity of Y79 retinoblastoma cells to vincristine by targeting PAX6 expression. Curr Eye Res. (2022) 47:418–25. doi: 10.1080/02713683.2021.1984537
44. Qiu L, Zhou R, Luo Z, Wu J, and Jiang H. CDC27-ODC1 axis promotes metastasis, accelerates ferroptosis and predicts poor prognosis in neuroblastoma. Front Oncol. (2022) 12:774458. doi: 10.3389/fonc.2022.774458
45. Niu J, Yan T, Guo W, Wang W, Ren T, Huang Y, et al. The COPS3-FOXO3 positive feedback loop regulates autophagy to promote cisplatin resistance in osteosarcoma. Autophagy. (2023) 19:1693–710. doi: 10.1080/15548627.2022.2150003
46. Jiang Y, Xiao F, Wang L, Wang T, and Chen L. Hsa_circ_0099198 facilitates the progression of retinoblastoma by regulating miR-1287/LRP6 axis. Exp Eye Res. (2021) 206:108529. doi: 10.1016/j.exer.2021.108529
47. Yang J, Tan C, Wang Y, Zong T, Xie T, Yang Q, et al. The circRNA MKLN1 regulates autophagy in the development of diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis. (2023) 1869:166839. doi: 10.1016/j.bbadis.2023.166839
48. Muhia M, YuanXiang P, Sedlacik J, Schwarz JR, Heisler FF, Gromova KV, et al. Muskelin regulates actin-dependent synaptic changes and intrinsic brain activity relevant to behavioral and cognitive processes. Commun Biol. (2022) 5:589. doi: 10.1038/s42003-022-03446-1
49. Xu L, Long H, Zhou B, Jiang H, and Cai M. CircMKLN1 suppresses the progression of human retinoblastoma by modulation of miR-425-5p/PDCD4 axis. Curr Eye Res. (2021) 46:1751–61. doi: 10.1080/02713683.2021.1927110
50. Zhu Y, Meng X, Zhu X, Zhang J, Lv H, Wang F, et al. Circular RNA MKLN1 promotes epithelial-mesenchymal transition in pulmonary fibrosis by regulating the miR-26a/b-5p/CDK8 axis in human alveolar epithelial cells and mice models. Arch Toxicol. (2024) 98:1399–413. doi: 10.1007/s00204-024-03700-x
51. Zai H, Wu X, Zhou Y, Hu Y, and Zhu Q. Lnc NBAT1 inhibits the proliferation and migration of liver cancer cells through the miR-21/PDCD4/AP-1 signaling axis. Appl Biochem Biotechnol. (2025) 197:1–18. doi: 10.1007/s12010-024-05008-z
52. Liu J, Dong Y, Ji Q, Wen Y, Ke G, Shi L, et al. Circ-MKLN1/miR-377-3p/CTGF axis regulates the TGF-β2-induced posterior capsular opacification in SRA01/04 cells. Curr Eye Res. (2022) 47:372–81. doi: 10.1080/02713683.2021.1988983
53. Xiong H, Huang G, Zhu Y, Chen R, Zuo L, and Liu H. Circ-SHPRH in human cancers: a systematic review and meta-analysis. Front Cell Dev Biol. (2023) 11:1182900. doi: 10.3389/fcell.2023.1182900
54. Sun H, Wang Q, Yuan G, Quan J, Dong D, Lun Y, et al. Hsa_circ_0001649 restrains gastric carcinoma growth and metastasis by downregulation of miR-20a. J Clin Lab Anal. (2020) 34:e23235. doi: 10.1002/jcla.23235
55. Xing L, Zhang L, Feng Y, Cui Z, and Ding L. Downregulation of circular RNA hsa_circ_0001649 indicates poor prognosis for retinoblastoma and regulates cell proliferation and apoptosis via AKT/mTOR signaling pathway. BioMed Pharmacother. (2018) 105:326–33. doi: 10.1016/j.biopha.2018.05.141
56. Wu L, Lin L, Yu M, Li H, Dang Y, Liang H, et al. Antitumor activity of USP7 inhibitor GNE-6776 in non-Small cell lung cancer involves regulation of epithelial-Mesenchymal transition, cell cycle, wnt/β-Catenin, and PI3K/AKT/mTOR pathways. Pharm (Basel). (2025) 18:245. doi: 10.3390/ph18020245
57. Yan X, Jia H, and Zhao J. LncRNA MEG3 attenuates the Malignancy of retinoblastoma cells through inactivating PI3K/Akt/mTOR signaling pathway. Exp Eye Res. (2023) 226:109340. doi: 10.1016/j.exer.2022.109340
58. Bonelli M, Terenziani R, Zoppi S, Fumarola C, La Monica S, Cretella D, et al. Dual inhibition of CDK4/6 and PI3K/AKT/mTOR signaling impairs energy metabolism in MPM cancer cells. Int J Mol Sci. (2020) 21:5165. doi: 10.3390/ijms21145165
59. Zhang X, Zhang Y, Wang C, and Wang X. TET (Ten-eleven translocation) family proteins: structure, biological functions and applications. Signal Transduct Target Ther. (2023) 8:297. doi: 10.1038/s41392-023-01537-x
60. Liu H, Chen L, Chen Y, Jin Y, Chen X, Ma N, et al. TCP1 promotes the progression of Malignant tumours by stabilizing c-Myc through the AKT/GSK-3β and ERK signalling pathways. Commun Biol. (2025) 8:563. doi: 10.1038/s42003-025-07867-6
61. Fu C, Wang S, Jin L, Zhang M, and Li M. CircTET1 Inhibits Retinoblastoma Progression via Targeting miR-492 and miR-494-3p through Wnt/β-catenin Signaling Pathway. Curr Eye Res. (2021) 46:978–87. doi: 10.1080/02713683.2020.1843685
62. Liu X, Zurlo G, and Zhang Q. The roles of cullin-2 E3 ubiquitin ligase complex in cancer. Adv Exp Med Biol. (2020) 1217:173–86. doi: 10.1007/978-981-15-1025-0_11
63. Zhang H, Qiu X, Song Z, Lan L, Ren X, and Ye B. CircCUL2 suppresses retinoblastoma cells by regulating miR-214-5p/E2F2 Axis. Anticancer Drugs. (2022) 33:e218–27. doi: 10.1097/CAD.0000000000001190
64. Hu X, Wen L, Li X, and Zhu C. Relationship between autophagy and drug resistance in tumors. Mini Rev Med Chem. (2023) 23:1072–8. doi: 10.2174/1389557522666220905090732
65. Rumboldt Z, Dodig D, Galluzzi P, Brumini I, Clarke R, Singh S, et al. Retinoblastoma and beyond: pediatric orbital mass lesions. Neuroradiology. (2025) 67:469–92. doi: 10.1007/s00234-024-03517-6
66. Raval V, Parulekar M, and Singh AD. Emerging new therapeutics for retinoblastoma. Ocul Oncol Pathol. (2022) 8:149–55. doi: 10.1159/000524919
67. Berry JL, Pike S, Shah R, Reid MW, Peng CC, Wang Y, et al. Aqueous humor liquid biopsy as a companion diagnostic for retinoblastoma: implications for diagnosis, prognosis, and therapeutic options: five years of progress. Am J Ophthalmol. (2024) 263:188–205. doi: 10.1016/j.ajo.2023.11.020
68. Lyu J, Wang Y, Zheng Q, Hua P, Zhu X, Li J, et al. Reduction of circular RNA expression associated with human retinoblastoma. Exp Eye Res. (2019) 184:278–85. doi: 10.1016/j.exer.2019.03.017
69. Chen NN, Chao DL, and Li XG. Circular RNA has_circ_0000527 participates in proliferation, invasion and migration of retinoblastoma cells via miR-646/BCL-2 axis. Cell Biochem Funct. (2020) 38:1036–46. doi: 10.1002/cbf.3535
70. Wang C, Xu M, Fan Q, Li C, and Zhou X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J Pharm Sci. (2023) 18:100772. doi: 10.1016/j.ajps.2022.100772
71. Wang J, Wang M, Jiang N, Ding S, Peng Q, and Zheng L. Emerging chemical engineering of exosomes as “bioscaffolds” in diagnostics and therapeutics. Genes Dis. (2023) 10:1494–512. doi: 10.1016/j.gendis.2022.10.020
72. Karami Fath M, Pourbagher Benam S, Kouhi Esfahani N, Shahkarami N, Shafa S, Bagheri H, et al. The functional role of circular RNAs in the pathogenesis of retinoblastoma: a new potential biomarker and therapeutic target? Clin Transl Oncol. (2023) 25:2350–64. doi: 10.1007/s12094-023-03144-2
73. Li X, Wang Y, Zhang B, Mao R, Wang Z, Jiang T, et al. Hsa_circ_0119412 contributes to development of retinoblastoma by targeting miR-186-5p/ELK4 axis. Mol Biotechnol. (2023) 65:1608–18. doi: 10.1007/s12033-023-00660-y
74. Sun N, Gong J, Zhang W, Yang X, and Liu J. Sevoflurane suppresses hepatocellular carcinoma cell progression via circ_0001649/miR-19a-3p/SGTB axis. Histol Histopathol. (2023) 38:537–47. doi: 10.14670/HH-18-484
75. Hansen EB, Fredsøe J, Okholm TLH, Ulhøi BP, Klingenberg S, Jensen JB, et al. The transcriptional landscape and biomarker potential of circular RNAs in prostate cancer. Genome Med. (2022) 14:8. doi: 10.1186/s13073-021-01009-3
76. Lv X, Yang H, Zhong H, He L, and Wang L. Osthole exhibits an antitumor effect in retinoblastoma through inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis. Pharm Biol. (2022) 60:417–26. doi: 10.1080/13880209.2022.2032206
77. Wang L, Li S, Mei J, and Ye L. Immunotherapies of retinoblastoma: Effective methods for preserving vision in the future. Front Oncol. (2022) 12:949193. doi: 10.3389/fonc.2022.949193
78. Davar D, Cavalcante L, Lakhani N, Moser J, Millward M, McKean M, et al. Correction: Phase I studies of davoceticept (ALPN-202), a PD-L1-dependent CD28 co-stimulator and dual PD-L1/CTLA-4 inhibitor, as monotherapy and in combination with pembrolizumab in advanced solid tumors (NEON-1 and NEON-2). J Immunother Cancer. (2024) 12:e009474. doi: 10.1136/jitc-2024-009474corr1
79. Zhang X, You W, Wang Y, Dejenie R, Wang C, Huang Y, et al. Prospects of anti-GD2 immunotherapy for retinoblastoma. Front Immunol. (2024) 15:1499700. doi: 10.3389/fimmu.2024.1499700
80. Qian H, Wang H, Guan X, Yi Z, and Ma F. Adoptive immunotherapy combined chemoradiotherapy for non-small-cell lung cancer: a meta-analysis. Anticancer Drugs. (2016) 27:433–8. doi: 10.1097/CAD.0000000000000346
81. Kondo T, Imura Y, Chikuma S, Hibino S, Omata-Mise S, Ando M, et al. Generation and application of human induced-stem cell memory T cells for adoptive immunotherapy. Cancer Sci. (2018) 109:2130–40. doi: 10.1111/cas.13648
82. Spring L, Bardia A, and Modi S. Targeting the cyclin D-cyclin-dependent kinase (CDK) 4/6-retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor-positive breast cancer: rationale, current status, and future directions. Discov Med. (2016) 21:65–74.
83. Cao J, Zhu Z, Wang H, Nichols TC, Lui GYL, Deng S, et al. Combining CDK4/6 inhibition with taxanes enhances anti-tumor efficacy by sustained impairment of pRB-E2F pathways in squamous cell lung cancer. Oncogene. (2019) 38:4125–41. doi: 10.1038/s41388-019-0708-7
84. Busch MA, Haase A, Alefeld E, Biewald E, Jabbarli L, and Dünker N. Trefoil family factor peptide 1-A new biomarker in liquid biopsies of retinoblastoma under therapy. Cancers (Basel). (2023) 15:4828. doi: 10.3390/cancers15194828
85. Weise A and Dünker N. High trefoil factor 1 (TFF1) expression in human retinoblastoma cells correlates with low growth kinetics, increased cyclin-dependent kinase (CDK) inhibitor levels and a selective down-regulation of CDK6. Histochem Cell Biol. (2013) 139:323–38. doi: 10.1007/s00418-012-1028-y
86. Liu L, Qin S, Zheng Y, Han L, Zhang M, Luo N, et al. Molecular targeting of VEGF/VEGFR signaling by the anti-VEGF monoclonal antibody BD0801 inhibits the growth and induces apoptosis of human hepatocellular carcinoma cells. Vitro vivo Cancer Biol Ther. (2017) 18:166–76. doi: 10.1080/15384047.2017.1282019
87. Koch J, Schober SJ, Hindupur SV, Schöning C, Klein FG, Mantwill K, et al. Targeting the Retinoblastoma/E2F repressive complex by CDK4/6 inhibitors amplifies oncolytic potency of an oncolytic adenovirus. Nat Commun. (2022) 13:4689. doi: 10.1038/s41467-022-32087-5
88. Ene CI, Fueyo J, and Lang FF. Delta-24 adenoviral therapy for glioblastoma: evolution from the bench to bedside and future considerations. Neurosurg Focus. (2021) 50:E6. doi: 10.3171/2020.11.FOCUS20853
89. Almeida AM, Queiroz JA, Sousa F, and Sousa Â. Cervical cancer and HPV infection: ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov Today. (2019) 24:2044–57. doi: 10.1016/j.drudis.2019.07.011
90. Cáceres-Lavernia HH, Nenínger-Vinageras E, Varona-Rodríguez LM, Olivares-Romero YA, Sánchez-Rojas I, Mazorra-Herrera Z, et al. Racotumomab in non-small cell lung cancer as maintenance and second-line treatment. MEDICC Rev. (2021) 23:21–8. doi: 10.37757/MR2021.V23.N3.5
91. Cacciavillano W, Sampor C, Venier C, Gabri MR, de Dávila MT, Galluzzo ML, et al. A phase I study of the anti-idiotype vaccine racotumomab in neuroblastoma and other pediatric refractory Malignancies. Pediatr Blood Cancer. (2015) 62:2120–4. doi: 10.1002/pbc.25631
92. Miao Z, Li J, Wang Y, Shi M, Gu X, Zhang X, et al. Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation. Mol Cancer. (2023) 22:205. doi: 10.1186/s12943-023-01883-y
93. Dong LF, Chen FF, Fan YF, Zhang K, and Chen HH. circ-0000512 inhibits PD-L1 ubiquitination through sponging miR-622/CMTM6 axis to promote triple-negative breast cancer and immune escape. J Immunother Cancer. (2023) 11:e005461. doi: 10.1136/jitc-2022-005461
94. Zhou J, Xu M, Chen Z, Huang L, Wu Z, Huang Z, et al. circ_SPEF2 regulates the balance of treg cells by regulating miR-16-5p/BACH2 in lymphoma and participates in the immune response. Tissue Eng Regener Med. (2023) 20:1145–59. doi: 10.1007/s13770-023-00585-2
95. Xu YJ, Zhao JM, Gao C, Ni XF, Wang W, Hu WW, et al. Hsa_circ_0136666 activates Treg-mediated immune escape of colorectal cancer via miR-497/PD-L1 pathway. Cell Signal. (2021) 86:110095. doi: 10.1016/j.cellsig.2021.110095
96. Kulbay M, Tuli N, Mazza M, Jaffer A, Juntipwong S, Marcotte E, et al. Oncolytic viruses and immunotherapy for the treatment of uveal melanoma and retinoblastoma: the current landscape and novel advances. Biomedicines. (2025) 13:108. doi: 10.3390/biomedicines13010108
97. Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. (2019) 80:101896. doi: 10.1016/j.ctrv.2019.101896
Keywords: retinoblastoma, circular RNAs, tumor metastasis, drug resistance, biomarkers, immunotherapy
Citation: Wang X, Ma J, Dang Y and Lei F (2025) Circular RNAs and immunotherapy in retinoblastoma: emerging biomarkers and precision therapeutic strategies. Front. Immunol. 16:1666606. doi: 10.3389/fimmu.2025.1666606
Received: 17 July 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Lin Zhang, Monash University, AustraliaReviewed by:
Zhijia Xia, Ludwig Maximilian University of Munich, GermanyCopyright © 2025 Wang, Ma, Dang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Lei, bGVpZmFuZzE2MThAMTI2LmNvbQ==; Yalong Dang, ZGFuZ3lhbG9uZ0BnbWFpbC5jb20=
 Xueting Wang
Xueting Wang Jiakang Ma
Jiakang Ma Yalong Dang4*
Yalong Dang4* Fang Lei
Fang Lei