- 1School of Acupuncture and Tuina, Guizhou University of Traditional Chinese Medicine, Gui’yang, China
- 2Department of Rehabilitation Medicine, Ji’an Central People’s Hospital, Ji’an, China
Inflammatory bowel disease (IBD) is characterized by chronic intestinal inflammation, strongly influenced by gut microbiota dysbiosis, barrier dysfunction, and immune imbalance. Increasing evidence highlights natural polysaccharides as promising therapeutic agents due to their dual roles in microbiota modulation and barrier reinforcement. Polysaccharides promote the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium, enhance short-chain fatty acid (SCFA) production, and repair mucosal damage by upregulating goblet cells and tight junction proteins. These effects collectively restore microbial homeostasis and attenuate inflammation. Recent advances in polysaccharide-based nanocarriers, including chitosan, alginate, and hyaluronic acid, further enhance efficacy by enabling mucoadhesion, stimuli-responsive release, and targeted delivery within the inflamed colon. Such systems improve local drug retention, reshape the gut microenvironment, and amplify the therapeutic functions of polysaccharides. This review summarizes the pathological mechanisms of IBD, the regulatory effects of polysaccharides on gut microbiota, and the emerging role of nanotechnology in optimizing their delivery. Despite encouraging preclinical evidence, challenges remain regarding structural complexity, bioavailability, and clinical translation. Clarifying structure–activity relationships and developing multi-responsive nanocarriers represent future directions. Collectively, polysaccharides and their nanoformulations hold strong potential as safe and effective strategies for IBD therapy.
1 Introduction
Inflammatory bowel disease (IBD) comprises chronic inflammatory disorders of the gastrointestinal tract, primarily Crohn’s disease (CD) and ulcerative colitis (UC). Dysbiosis of the gut microbiota is now recognized as a critical factor in both the initiation and progression of IBD (1). The intestinal microbiome, which includes bacteria, viruses, fungi, and parasites, contains approximately 100 trillion microorganisms (2). Consequently, therapeutic strategies aimed at restoring microbial balance have emerged as promising approaches for IBD management (3).
5-Aminosalicylic acid (5-ASA) remains a cornerstone of IBD therapy (4, 5). However, its use is associated with a spectrum of adverse effects, including headaches, nausea, hepatotoxicity, pancreatitis, bone marrow suppression, and renal complications (6). Prolonged treatment may also result in diarrhea, alopecia, and myalgia. Similarly, corticosteroids, although effective, are linked to risks such as osteoporosis, hypertension, obesity, type 2 diabetes, and exacerbation of gastrointestinal ulcers (7–9). Some of these drug-induced adverse reactions may even be life-threatening (10). In addition, while antibiotics can temporarily reduce pathogenic bacteria, long-term use disrupts microbial homeostasis and promotes resistance (11).
Natural polysaccharides have demonstrated considerable benefits in IBD treatment, as they reduce drug-related side effects while enhancing therapeutic efficacy (12–14). This reduction in drug-related side effects is closely linked to their regulatory role in gut microbiota: Evidence suggests that polysaccharides stimulate the growth of beneficial bacteria, facilitate intestinal barrier repair, and regulate gut metabolism by promoting the production of SCFAs—for instance, SCFAs can alleviate 5-ASA-induced intestinal mucosal damage by enhancing epithelial barrier repair, and mitigate corticosteroid-associated oxidative stress via activating antioxidant pathways (15). Moreover, specific microbial enzymes degrade polysaccharides, converting them into SCFAs and other secondary metabolites with health-promoting properties (16). Such processes regulate microbial communities and support intestinal homeostasis. Recent findings further indicate that polysaccharides improve barrier integrity, modulate the gut microenvironment, and enhance metabolic functions in patients with IBD (17).
Additionally, polysaccharide-based nanoparticles have attracted attention for their ability to improve drug stability and sustain therapeutic activity (18, 19). Given their dual ability to regulate gut microbiota and reinforce intestinal barrier function, polysaccharides represent a highly promising strategy for IBD therapy (20). This review systematically discusses the mechanistic basis of polysaccharide interventions and highlights their potential in future therapeutic development.
2 The relationship between the intestinal microbiome and IBD
Numerous studies have documented significant alterations in the intestinal microbiota of patients with IBD compared with healthy individuals (21–23). In healthy hosts, a dynamic balance is maintained among beneficial, potentially harmful, and commensal microbial populations (24). However, reductions in microbial diversity, shifts in metabolite profiles, disruption of the mucosal barrier, or immune dysregulation—often caused by disease, pharmacotherapy (e.g., antibiotics, laxatives), or unhealthy diets—can collectively drive the onset and progression of IBD (3, 25–29).
For example, one study reported markedly reduced levels of anti-inflammatory taxa such as Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and other beneficial species in CD patients, alongside a significant increase in the pro-inflammatory species Ruminococcus gnavus (30). Similarly, murine models of colitis showed elevated levels of pathogenic genera including Shigella, Aeromonas, Clostridium, Sutterella, and Akkermansia muciniphila. These findings emphasize the contrasting roles of pathogenic and commensal bacteria in IBD pathophysiology (31, 32). Importantly, the impact of these bacteria is modulated by host immune status, environmental factors, and nutrient availability—conditions under which beneficial microbes may even exert neutral or deleterious effects, and vice versa.
Dynamic fluctuations in microbial populations critically influence gut diversity and ecosystem stability. In IBD, chronic inflammation disrupts this stability—pathogenic bacteria (e.g., Ruminococcus gnavus) proliferate rapidly, while beneficial taxa (e.g., Faecalibacterium prausnitzii) decline, leading to chaotic microbial fluctuations and reduced diversity (30, 32). Probiotic intervention can counteract such disruptive fluctuations: In a study of DSS-induced colitis, administration of Lactobacillus rhamnosus GG (LGG, 109 CFU/day) markedly improved microbiota diversity, reversing dysbiosis by enriching beneficial taxa such as Bifidobacterium, Olsenella, Paenibacillus, and butyrate-producing bacteria (33). Moreover, a 14-day LGG intervention suppressed pathogenic clusters, including Escherichia coli, Zhiphyllobacterium, Osteobacillus, and Desulphurobacteria, thereby alleviating colonic inflammation in UC mouse models (34).
Under physiological conditions, the mucus layer segregates luminal bacteria from intestinal epithelial cells (IECs), and the immune system maintains tolerance toward luminal antigens (26, 35). In IBD, barrier disruption increases permeability, enabling bacteria to contact IECs directly (36) and translocate into systemic circulation (37). This translocation triggers inflammatory cytokine expression and immune activation (38, 39). Barrier dysfunction is further characterized by reduced mucin content (40), diminished glucose-derived metabolites (41), impaired lipid-associated protective factors (42), and decreased secretion of pancreatic-derived defense molecules (43).
Metabolic studies further link microbial composition to functional pathways. Dysbiotic states are associated with reduced polysaccharide-degrading capacity and upregulated oxidative stress-related genes (44). Excess bacterial metabolites elevate ROS, thereby exacerbating epithelial injury and inflammation (45). Elevated Desulfovibrio spp. in IBD patients promote hydrogen sulfide overproduction, inducing oxidative stress, damaging IECs, and aggravating mucosal inflammation (46).
Additionally, dysbiosis frequently results in SCFA depletion, which worsens intestinal inflammation (47–49). As major products of polysaccharide fermentation, SCFAs suppress pathogen proliferation, enhance nutrient absorption, regulate immune responses, and reinforce the mucus barrier (50–53). They lower intestinal pH, facilitating the colonization of beneficial bacteria such as Lactobacillus and Bifidobacterium. These bacteria further ferment carbohydrates into SCFAs, strengthening mucosal immunity (54). Probiotics such as Bifidobacterium and Lactobacillus mitigate inflammation by modulating NF-κB signaling, enhancing epithelial adhesion, and inhibiting pathogens (55, 56). The dominant gut phyla, Bacteroidetes and Firmicutes, produce acetate and propionate (mainly Bacteroidetes) and butyrate (predominantly Firmicutes) (57). Butyrate, in particular, serves as the primary energy source for IECs and promotes epithelial proliferation, which is essential for mucosal repair (58).
3 Disruption of the intestinal upper barrier and imbalance of the microbiome
3.1 Interaction between the intestinal microbiome and the mucous layer
The intestinal surface is covered by a bi-layered mucus structure that plays a fundamental role in preserving the integrity of the upper intestinal barrier (59). The outer mucus layer directly interfaces with the gut microbiota and provides a nutrient source for commensal species. Certain bacteria, such as Akkermansia muciniphila and Bacteroides fragilis, secrete mucin-degrading enzymes that remodel this layer to facilitate colonization. A healthy microbiota also contributes to the maturation of gut-associated lymphoid tissue and modulates immune responses, thereby preventing pathogen invasion and endotoxin translocation. In addition, commensal microbes reinforce mucus barrier function by reducing luminal oxygen levels and stimulating host immune activity. Microbial metabolites act as key molecular mediators, directly regulating mucosal immune signaling, shaping host physiology, and maintaining immune homeostasis.
Johansson et al. demonstrated that Erysipelotrichia and Paenibacillus decreased mucus permeability, whereas Proteobacteria and Saccharibacteria increased it, highlighting that mucus properties are strongly influenced by microbiota composition (60). Probiotic supplementation can also upregulate mucin synthesis. For example, lymphocyte-associated pathways induce Mucin 2 expression in IECs, while colonization by rod-shaped bacteria restores mucus production (61). These findings underscore the impact of probiotics on mucus barrier integrity and intestinal health (62, 63).
Animal studies further revealed a marked reduction in goblet cells in germ-free mice (64). The mucus layer in these animals lacked critical immune molecules, such as regenerating islet-derived protein III, rendering them more vulnerable to bacterial infection. Under severe infection, the intestinal mucosa compensates by secreting large amounts of mucus to physically limit bacterial invasion (65). Moreover, SCFAs—key metabolites of the gut microbiota—regulate mucus dynamics, stimulating mucin secretion at low concentrations but potentially suppressing it at higher levels (66–68).
3.2 Influence of microbiota on IECs
The renewal and coordinated function of IECs are essential for maintaining barrier integrity. Together with gut microbes and the mucosal immune system, IECs constitute the first line of defense against luminal pathogens and antigens (69). Exposed directly to the intestinal lumen, IECs express a wide array of pattern recognition receptors that enable microbial sensing. Beneficial microbes form a protective biological barrier on the mucosal surface, outcompeting pathogens for adhesion sites, secreting antimicrobial compounds, stimulating mucus production, and strengthening tight junction complexes. Collectively, these interactions promote IEC growth, regeneration, proliferation, and repair, thereby preserving mucosal barrier function (70–73).
In contrast, pathogenic colonization disrupts commensal communities and adversely affects IEC structure and function (74). In IBD patients, elevated populations of sulfate-reducing bacteria produce hydrogen sulfide, which damages IECs and triggers mucosal inflammation (75). Notably, such pathogenic colonization also inhibits the growth of SCFA-producing bacteria (e.g., Firmicutes), reducing SCFA availability—a double blow to IEC homeostasis. Conversely, SCFAs, as key microbial metabolites, can reverse IEC damage: SCFAs interact with G-protein-coupled receptors (GPCR41 and GPCR43) on IECs to induce enteroendocrine hormone release, thereby modulating disease processes (76). Notably, butyrate provides a major energy source for IECs, regulates cell proliferation and differentiation, and stimulates Paneth cells to secrete antimicrobial peptides through GPCR43 signaling (77). Other microbial metabolites, including succinate and propionate, also contribute to IEC growth, differentiation, and colonic energy metabolism (78).
In addition, epithelial polysaccharides serve a decisive role in shaping gut microbiota by providing binding ligands and nutritional substrates, thereby influencing microbial composition and colonization. Recent evidence links IBD to altered O-glycan expression in the mucus layer, including increased levels of short-chain O-glycans and modified terminal structures. These changes impair mucus barrier function, disrupt lectin-sugar interactions, disturb host-microbe communication, and weaken mucosal immunity, collectively promoting IBD pathogenesis (79).
3.3 Effect of gut microbiota on intestinal permeability
The preceding discussion on microbial interactions with the mucus layer and IECs underscores how the gut microbiota governs multiple aspects of barrier function. To further clarify how specific bacterial species regulate barrier integrity—particularly mechanisms that alter intestinal permeability—additional analysis is warranted.
A prominent example is adherent-invasive Escherichia coli (AIEC), which has been closely associated with intestinal inflammation. Patients with IBD often exhibit elevated AIEC abundance. These bacteria compromise barrier function by directly increasing intestinal permeability, disrupting microbial diversity, and modulating the expression of inflammatory mediators (80). Enhanced colonization of epithelial-adherent pathogens such as AIEC exacerbates mucosal permeability, reshapes microbial community composition, and initiates inflammatory cascades via upregulation of pro-inflammatory genes, ultimately driving intestinal inflammation (81).
Conversely, certain probiotic strains protect against barrier disruption. Escherichia coli Nissle 1917 (EcN), a Gram-negative, non-lactic acid probiotic strain, can colonize the gut stably, interact with IECs and resident microbes, and exert protective effects. In T84 colonic epithelial cell models, Zyrek et al. demonstrated that EcN upregulates tight junction proteins zonula occludens-1 (ZO-1) and zonula occludens-2 (ZO-2), thereby preserving mucosal integrity and reducing permeability (82). In murine studies, oral administration of EcN (109 CFU/day) alleviated colitis symptoms, improved histopathological outcomes, protected intestinal permeability, reduced neutrophil and eosinophil infiltration, decreased chemokine and cytokine levels, and increased regulatory T cell populations within Peyer’s patches (83).
Mechanistically, EcN’s ability to preserve tight junction architecture is thought to involve the MLCK/MLC signaling pathway. Further investigations into EcN-mediated regulation of tight junction proteins—such as ZO-1, claudin-1, and occludin—through MLCK pathway modulation may provide deeper insights into molecular mechanisms underlying its barrier-protective effects.
4 intestinal microecology and intestinal immunity
The inflammatory response in IBD originates from the activation of innate immune cells—including macrophages, dendritic cells, neutrophils, natural killer cells, and innate lymphoid cells—which release cytokines, chemokines, and antimicrobial peptides. This innate activation subsequently triggers adaptive immunity, with T and B lymphocytes serving as central mediators of intestinal inflammation in IBD (84) (Figure 1).
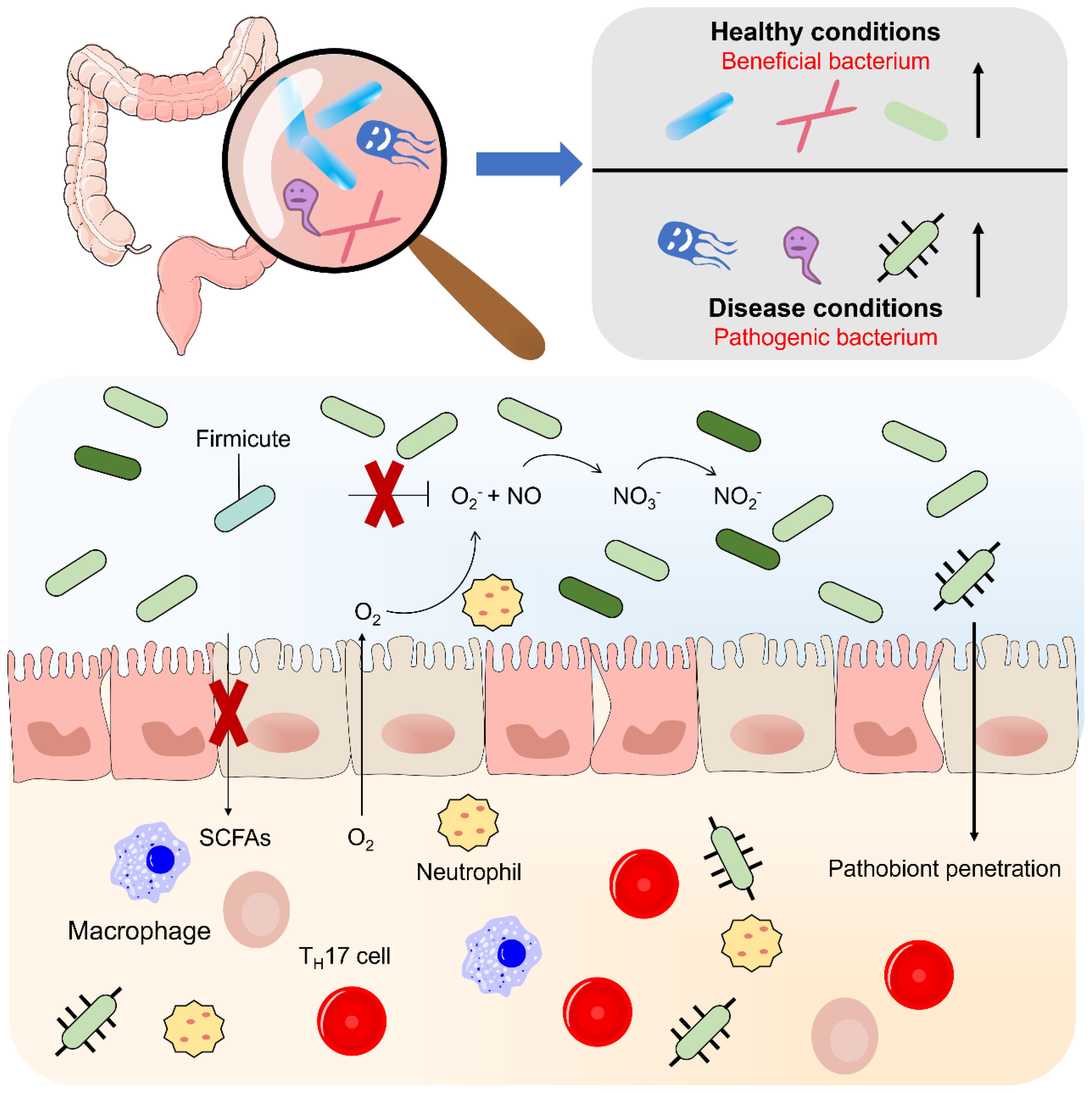
Figure 1. Flora disorders in inflammatory bowel disease. According to their relationship to the human body, normal intestinal bacteria can be divided into three categories: 1 symbiotic (beneficial) 2 conditional (neutral) 3 pathogenic (harmful). Inflammation leads to changes in bacterial clusters, such as the proliferation of deformed bacteria, caused by increased oxygen synthesis in the inflammatory intestinal environment, nitrate (NO-) and increased availability of host-generated oxygeon receptors and iron. The flora disorder is manifested by a general decrease in microbial diversity and a loss of beneficial bacteria, which may lead to increased mucous adhesion and transfer of the beneficial flora.
Accumulating evidence indicates that distinct microbial communities differentially regulate T cell-mediated immunity. For instance, Faecalibacterium prausnitzii alleviates chemically induced colitis in mice by enhancing regulatory T cell (Treg) activity (85). Similarly, Clostridium butyricum promotes the expansion of CD4+Foxp3+ Tregs in the intestinal lamina propria, thereby preventing colitis and attenuating hypersensitivity reactions (86).
Invariant natural killer T (iNKT) cells, which share features of both NK and T cell lineages, also contribute to intestinal immunoregulation. Germ-free mice display heightened susceptibility to iNKT-mediated colitis and asthma following oxazolone or ovalbumin challenge. This susceptibility arises because germ-free conditions lack microbial metabolites (e.g., SCFAs, bile acid derivatives) that suppress iNKT cell overactivation—neonatal exposure to commensal microbes promotes the production of these metabolites, thereby limiting iNKT-mediated inflammatory responses. Remarkably, neonatal exposure to commensal microbes is required to mitigate this susceptibility (87).
During programmed cell death, neutrophils release neutrophil extracellular traps (NETs)—web-like structures composed of chromatin, histones, proteases, granule proteins, and enzymes such as myeloperoxidase and neutrophil elastase. NETs restrict pathogen dissemination and exhibit bactericidal activity through associated proteases. However, their components can also disrupt immune homeostasis (88). NETs have been shown to influence B cell differentiation and function. In rheumatoid arthritis, for example, NET-immune cell interactions promote B cell proliferation and autoantibody production via B cell-activating factor, while synovial NETs provide citrullinated proteins that fuel anti-citrullinated protein antibody (ACPA) responses (89).
In IBD, depletion of SCFA-producing bacteria—particularly butyrate producers—correlates with increased neutrophil infiltration and NET formation, thereby accelerating disease progression (90). Microbial metabolites, especially SCFAs, also shape macrophage function: SCFAs suppress pro-inflammatory cytokine production by inhibiting histone deacetylases, while upregulating anti-inflammatory IL-10 (91). Butyrate, in particular, inhibits HDAC3, reduces mTOR activation and glycolysis, enhances macrophage bactericidal activity, and promotes an anti-inflammatory phenotype (92).
Considerable evidence supports a role for SCFAs in regulating CD4+ T cell subsets, particularly Tregs, which are critical for immune tolerance. SCFAs promote Treg differentiation by inhibiting HDAC activity; however, under strong anti-CD3 stimulation favoring Th1/Th17 polarization, this induction is attenuated (93). Although less studied, SCFAs also affect CD8+ T cells, which are essential for intracellular pathogen clearance and tumor surveillance. For example, systemic acetate elevation during bacterial infection enhances glycolysis and boosts memory CD8+ T cell recall responses (94).
Beneficial microbes such as Bifidobacterium and Lactobacillus further modulate intestinal Treg populations, highlighting their role in IBD pathogenesis (95). Treg-deficient mice spontaneously develop colitis, underscoring the indispensable role of Tregs in intestinal homeostasis (96). Moreover, LGG promotes B cell differentiation and IgA secretion in the intestinal lamina propria of piglets, thereby strengthening mucosal immunity (97).
Additional mechanisms involve microbial regulation of innate sensors and inflammatory pathways. For instance, Bacteroides fragilis suppresses NLRP3 inflammasome activation via SCFA production, thereby inhibiting M1 macrophage polarization and reducing pro-inflammatory cytokines such as IL-18 and IL-1β (98). Faecalibacterium prausnitzii exerts anti-inflammatory effects by downregulating IL-12 and IFN-α, while enhancing IL-10 secretion and inhibiting NF-κB signaling (85, 99).
5 Gut microbiota and oxidative stress
In IBD, excessive production of ROS—including superoxide anions, peroxynitrite, hypochlorite, and hydrogen peroxide—has been strongly implicated in disease progression. ROS directly damage IECs, activate mucosal immune responses, and trigger oxidative stress-related signaling pathways such as NF-κB and nuclear factor erythroid 2-related factor 2 (Nrf2), thereby aggravating barrier dysfunction and chronic inflammation (100, 101).
Metagenomic analyses of IBD patients have revealed downregulation of genes involved in carbohydrate and amino acid metabolism, coupled with upregulation of genes associated with oxidative stress responses. These findings suggest that altered microbial metabolic capacity may exacerbate IBD by enhancing oxidative stress and impairing epithelial integrity.
LGG exhibits potent antioxidant activity and supports intestinal barrier function. LGG reduces oxidative stress-induced damage by enhancing endogenous antioxidant enzyme production, including superoxide dismutase (SOD) and glutathione (GSH), while simultaneously suppressing ROS generation. These protective effects are mediated through activation of the Keap1/Nrf2 pathway and inhibition of ERK1/2 and NF-κB signaling (102). Further studies demonstrate that LGG reduces Giardia-induced colonization, enhances antioxidant defenses, and lowers lipid peroxidation, thereby maintaining epithelial integrity (103).
In a hydrogen peroxide-induced oxidative stress model using porcine IECs, extracellular polysaccharides from LGG accelerated ROS clearance by upregulating antioxidant enzyme expression and downregulating oxidative stress-related proteins. These effects collectively facilitated the repair of epithelial barrier damage (104).
Additional studies have investigated synergistic effects of natural polysaccharides with bioactive plant-derived compounds. For example, Lycium barbarum polysaccharides (LBPs), known for immunomodulatory properties, and capsaicin, an anti-inflammatory and antioxidant agent, were tested in a DSS-induced colitis rat model. LBPs alone decreased serum IL-6 and malondialdehyde (MDA, a lipid peroxidation marker), while enhancing catalase activity. Co-administration of LBPs and capsaicin further reduced IL-6 and colonic tumor necrosis factor-α (TNF-α), and significantly increased SOD activity. These results underscore the synergistic antioxidant and anti-inflammatory potential of combining natural polysaccharides with plant bioactives in ulcerative colitis therapy (105).
6 Polysaccharides regulate the gut microbiota
Natural polysaccharides are widespread biological macromolecules that function as structural components, energy reserves, and protective agents in diverse organisms. They are complex carbohydrates formed by the condensation of multiple monosaccharide units through glycosidic bonds, generally represented by the formula (C6H10O5)n (106). Polysaccharides can be derived from plants, algae, animals, or microorganisms (107, 108). Their physicochemical properties—including monosaccharide composition, chain length, branching degree, and substituents—profoundly influence their bioactivities. Hydrophilic groups such as hydroxyl, carboxyl, and amino groups confer high solubility and dispersibility, while additional functional groups permit chemical modification, enabling the formation of functionalized supramolecular structures (109) (Figure 2).
Figure 2. Polysaccharides modulate gut microbiota and enhance gut barrier function. The healthy gut barrier consists of a Tight layer of IECS, which are interconnected by Tight junctions that control the Permeability of matter. In good health, the intestinal immune system maintains the immune balance by secreting cytokines (such as IL-2, IL-5, IL-6, IL-9, TNF-α and IFN-γ) and activating immune cells (such as T cells, B cells and macrophages). Mucins, antimicrobial peptides and secretory IgA can form biochemical barrier and enhance intestinal protective function. Goblet cells secrete mucins that form a dense, sticky, and permeable gel that coats the intestinal mucosa, preventing erosion by microbes. Once the tight junctions are broken, a phenomenon known as Leaky gut is formed, which increases intestinal permeability and allows harmful substances and pathogens to enter the bloodstream.
However, due to variations in monosaccharide composition, degree of polymerization, and linkage patterns, extraction and purification of polysaccharides are inherently complex. For example, polysaccharides with high branching degrees require more precise ethanol concentration adjustments to avoid co-precipitation with impurities; high-molecular-weight polysaccharides (due to high polymerization degrees) easily clog membrane pores during separation, necessitating stricter pressure control; and different monosaccharide linkages affect the binding affinity to macroporous resins, complicating elution gradient design. In plant polysaccharide extraction, ethanol precipitation is commonly applied to remove proteins, lipids, nucleic acids, pigments, and other small molecules from crude extracts, followed by fractionation to obtain homogeneous polysaccharides. Frequently used purification strategies include macroporous adsorption resins for initial separation, ion-exchange chromatography for selective fractionation, and membrane separation techniques exploiting molecular weight cut-offs under controlled pressure (110, 111).
Polysaccharides are generally classified by source into animal-, plant-, microbial-, and marine-derived types (112). Animal polysaccharides—often early pharmaceutical candidates—are typically mucopolysaccharides with high water solubility (113, 114). Plant-derived polysaccharides, such as pectin, Angelica, LBP, rhubarb, and Bupleurum polysaccharides, are usually water-soluble and low in toxicity, making them suitable for precise dosing in experimental settings (115). In contrast, starch and cellulose are insoluble plant polysaccharides. Microbial polysaccharides are produced by bacteria and fungi, while marine polysaccharides, isolated from aquatic organisms, often possess unique biological activities (116, 117).
Functionally, polysaccharides act as fermentable carbon sources for probiotics, promoting their growth, reshaping microbial community structure, and suppressing pathogenic bacteria (118). For example, polysaccharides extracted from Polygonum multiflorum increase populations of Bifidobacterium and Lactobacillus while decreasing Helicobacter, thereby alleviating gut dysbiosis and contributing to IBD management (119). In juvenile Hucho taimen, dietary supplementation with lentinan enhanced beneficial genera such as Lactobacillus, Trichinella, and Ruminococcus, while reducing harmful taxa including Enterobacteriaceae, Fusobacteriaceae, and Flavobacteriaceae, thus improving microbial balance (120).
The structural features of polysaccharides also determine preferential fermentation by specific microbes (121). For instance, oat β-glucans selectively promote Bifidobacterium and Lactobacillus (122), while Bacteroides efficiently degrade fructans (123), and Prevotella bryantii utilizes xylan (124). In vitro fermentation studies with Fuzhuan brick tea polysaccharides (FBTPS-3) showed modulation of IBD patient microbiota toward a profile resembling that of healthy individuals, specifically by increasing Bacteroides and decreasing Escherichia/Shigella (125).
Beyond shaping microbial composition, polysaccharides reinforce the mucus barrier by enhancing thickness, adhesiveness, and protective capacity, thereby preventing pathogen invasion (126). Dietary polysaccharides also directly upregulate tight junction proteins such as occludin and ZO-1, strengthening epithelial barrier integrity. For example, interventions significantly enhanced occludin and claudin-1 expression while reducing pro-inflammatory cytokines including TNF-α and IL-1β (127).
Polysaccharides from natural sources have demonstrated protective effects against colitis. Gloiopeltis furcata polysaccharides safeguard colonic mucosa by modulating mucin-microbe interactions, promoting probiotic growth, and reducing epithelial injury (128). Dendrobium huoshanense polysaccharides increase goblet cell numbers and stimulate mucin secretion in both small and large intestines, reinforcing mucosal defenses (129). Functionalized fucoidan restores microbial balance and mucosal integrity after injury (130). In vitro studies reveal that glucomannan from Aloe vera gel maintains barrier function via the Nrf2-mitochondrial axis and alleviates anoikis induced by mitochondrial dysfunction (131).
Marine polysaccharides such as fucoidan exhibit strong anti-inflammatory and immunomodulatory activity by inhibiting NF-κB signaling and downregulating TNF-α, IL-6, and IL-8 (132). Fucoidan also promotes Treg differentiation, enhances tight junction proteins and IgA secretion, and reduces intestinal permeability (133). In murine models, fucoidan mitigates colitis by lowering nitric oxide, myeloperoxidase, and malondialdehyde levels, reducing immune cell infiltration, and preserving colon length (134, 135). Additionally, fucoidan may stimulate dendritic cell maturation via TNF-dependent pathways, strengthening host immunity.
Other plant polysaccharides also improve IEC structure. Yam polysaccharides maintain epithelial morphology, increase goblet cell density, and decrease inflammatory infiltration (136). High-molecular-weight fucoidan from Undaria pinnatifida and Sargassum fusiforme protects Caco-2 cells against ROS-induced injury, likely via antioxidant activity (137). Composite polysaccharides (e.g., yam plus inulin) modulate microbiota by reducing Proteus, Bacteroides, and Firmicutes, enhancing metabolism, and relieving oxidative stress, ultimately improving ulcerative colitis (138).
Polysaccharide-mediated modulation of SCFA production represents another therapeutic mechanism (139). Ganoderma lucidum polysaccharides significantly increase acetate, propionate, and butyrate levels (140, 141). Similarly, LBPs undergo fermentation to generate SCFAs while enriching Bifidobacterium and Lactobacillus (142). Longan polysaccharides enrich SCFA-producing taxa such as Bifidobacterium, Bacillus, and Bacteroides fragilis, boosting acetate, propionate, and butyrate synthesis (143). Seaweed polysaccharides also act as fermentation substrates, indirectly supporting probiotic growth (144–147).
Importantly, the structural features of polysaccharides dictate fermentation kinetics and SCFA profiles, with identical fermentation conditions producing variable SCFA yields (148). Thus, targeted research on specific polysaccharides is required to define their optimal application in colitis therapy (Table 1). Taken together, natural polysaccharides reshape gut ecology (beneficial taxa↑, SCFAs↑), reinforce the epithelial barrier (mucus/TJ proteins↑), rebalance mucosal immunity (Tregs↑, NF-κB↓), and mitigate oxidative stress (Nrf2 axis↑). However, these biological benefits are highly contingent on local concentration and residence time at inflamed colonic sites. Oral administration faces substantial hurdles—acidic gastric milieu, digestive enzymes, rapid mucus clearance, and heterogeneous lesion distribution—leading to suboptimal on-target exposure. This translational gap motivates an engineering solution: polysaccharide-based nanomedicines that exploit the inflammatory microenvironment (pH↓, ROS↑, bacterial glycosidases↑, receptor overexpression) to achieve spatiotemporally controlled delivery and thereby amplify the very mechanisms delineated above.
7 Nanotechnology delivers polysaccharides to treat IBD
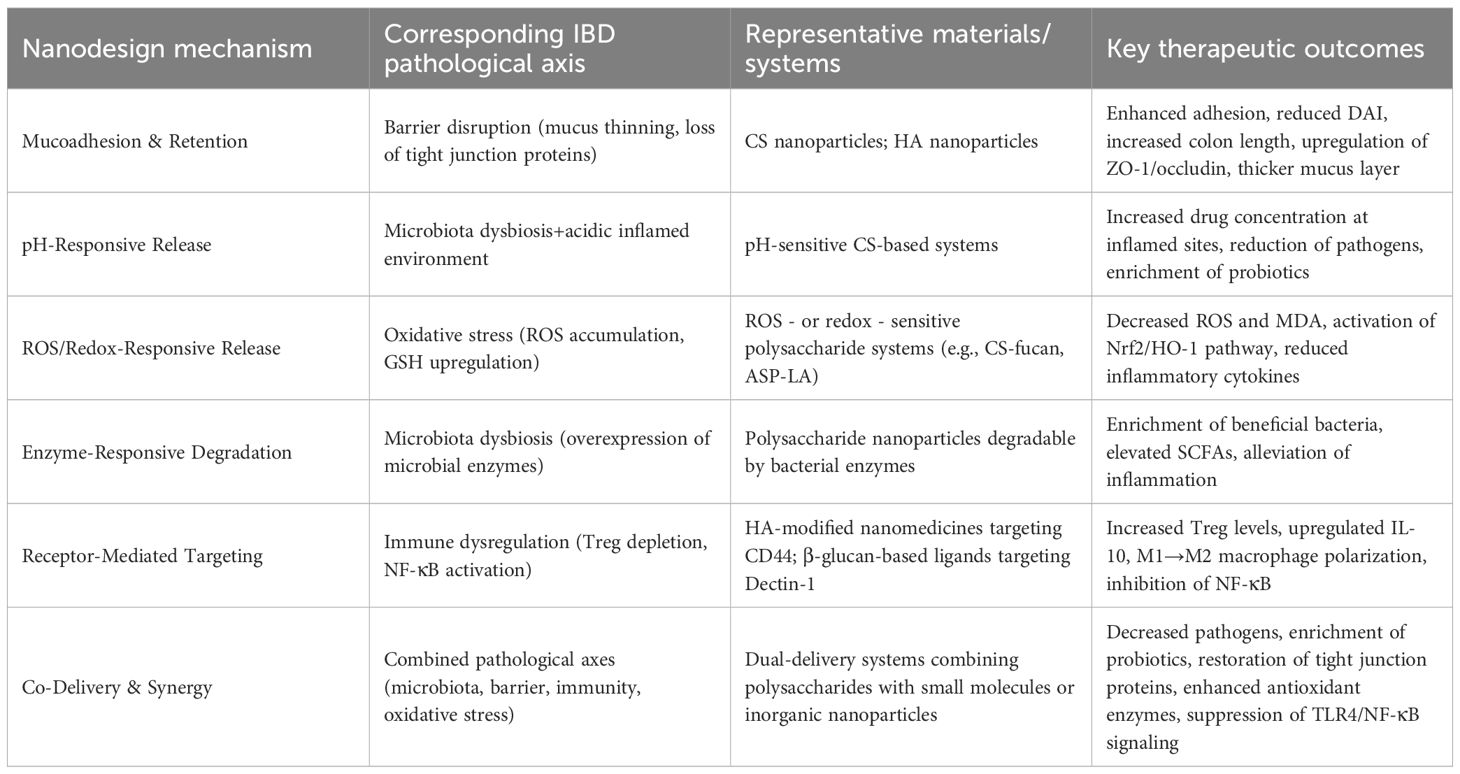
We therefore conceptualize polysaccharide nanomedicines as mechanism amplifiers: mucoadhesion and receptor targeting extend residence (boosting barrier repair); pH/ROS/enzyme responsiveness gates on-site release (boosting anti-inflammatory and antioxidant actions); and co-delivery strategies align metabolic support (SCFAs) with immune reprogramming (Tregs↑, M1→M2). The following subsections organize the evidence not by polymer name, but by pathophysiological lever addressed, creating a one-to-one mapping between IBD axes and nanodesign features. Natural polysaccharides are attractive candidates for nanocarrier development in IBD therapy due to their inherent bioactivity, pH responsiveness, gastric stability, susceptibility to colonic microbial degradation, and strong mucoadhesive properties (172–176). By modifying functional groups on their surfaces, polysaccharide-based nanocarriers can be engineered to encapsulate drugs, achieve sustained release, and selectively target specific gut microbial populations (177).
Nanomedicine delivery systems, owing to their nanoscale dimensions and unique structural properties, enhance drug accumulation and retention at target sites, thereby supporting localized therapy (178). The viscosity and intrinsic charge of polysaccharides further enable intimate interactions with the intestinal barrier, prolonging retention within the colon (13, 179). For instance, positively charged nanoparticles adhere to or penetrate negatively charged mucosal surfaces via electrostatic interactions, whereas negatively charged nanoparticles preferentially accumulate in positively charged inflamed tissues, thereby improving lesion targeting. Moreover, polysaccharide-based nanocarriers promote cellular uptake by IECs and immune cells through endocytosis and exocytosis (180). Collectively, these systems improve solubility, intestinal retention, and site-specific drug accumulation, resulting in enhanced therapeutic efficacy and reduced systemic side effects (172, 181).
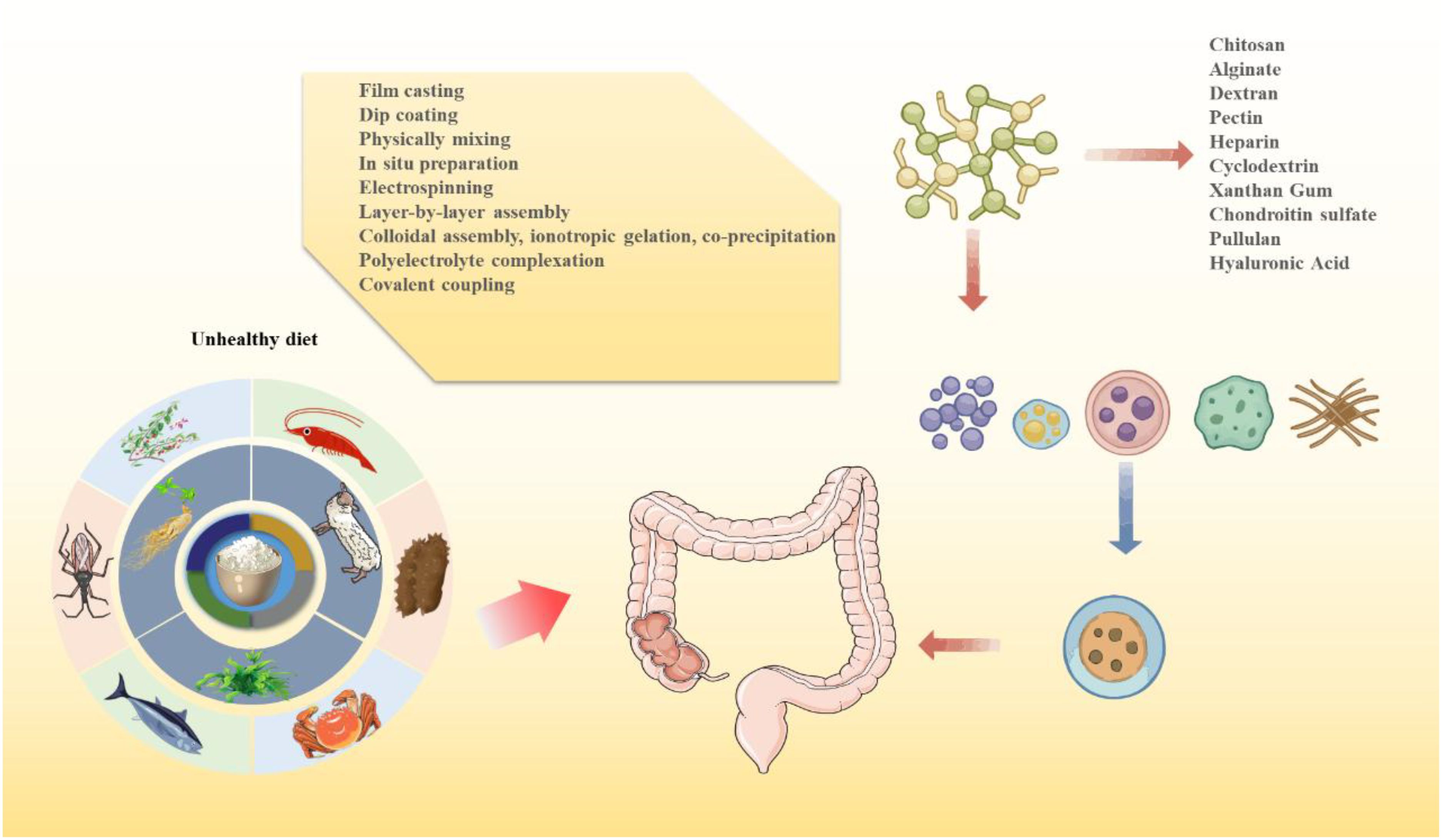
Currently, chitosan (CS), alginate (ALG), hyaluronic acid (HA), and Angelica sinensis polysaccharide (ASP) are among the most widely studied polysaccharide-based nanocarriers for IBD treatment, owing to their favorable biocompatibility and functionality (18, 19) (Figure 3). However, some polysaccharides exhibit high solubility and poor film-forming ability, leading to premature drug release and reduced colonic targeting. To address these limitations, composite nanocarrier systems combining multiple polysaccharides have been developed, effectively overcoming the weaknesses of single-component carriers (182, 183). Despite these advances, challenges remain, including instability in gastric acid and limited colon-targeting efficiency. Moreover, clinical data on dose-response relationships of polysaccharides in IBD remain scarce (131).
7.1 Properties of Cs and application of nano-carriers
CS is a cationic natural polysaccharide characterized by its positive charge, mucoadhesive properties, biocompatibility, non-toxicity, and biodegradability (136). Its cationic nature facilitates electrostatic interactions with the negatively charged mucus layer, thereby extending its retention time in the intestinal mucosa (184). As CS resists degradation in the upper gastrointestinal tract, orally administered CS can reach the colon intact, exerting localized therapeutic effects. In a DSS-induced colitis mouse model, CS reduced disease activity, ameliorated histopathological alterations, upregulated tight junction proteins, decreased TNF expression, and modulated gut microbial composition by increasing Lactobacillus and Blautia populations (185).
CS can also remodel gut microbial communities by increasing the abundance of probiotics such as Prevotella, Vibrio, and SCFA-producing taxa (186). Notably, the pH responsiveness of CS further supports its microbiota-regulating effect: Its amino groups impart pH responsiveness, enabling environmentally triggered drug release—it remains stable in the acidic gastric environment (pH 1.2) to avoid premature degradation, and only dissociates in the neutral-to-weakly alkaline colonic environment (pH 6.0-7.0) where it can directly interact with gut microbes and exert probiotic-enriching effects (187, 188). This pH-responsive feature, combined with targeted delivery, enhances therapeutic synergy: For instance, amphiphilic CS nanoparticles self-assembled with quercetin allow targeted intestinal delivery (189).CS hydrogels swell more under acidic than alkaline conditions, making them effective carriers for gastric nutrient release (190). A CS-pectin delivery system has also been developed to encapsulate anthocyanins, protecting them through the gastric environment and ensuring controlled release in the small intestine, thereby improving stability and bioavailability (191).
A dual-responsive nanodelivery system, RH-F/C-NPs, based on CS and fucan, exhibits pH/ROS sensitivity and strong mucoadhesion, making it suitable for ulcerative colitis therapy. This system significantly decreased pathogenic bacteria while increasing beneficial species such as Lactobacillus (192). The composite nanosystem achieves stable structure through electrostatic and hydrogen bonding interactions, ensuring efficient colon delivery and microbiota regulation (193).
Due to the strong adhesion between CS and mucins, CS-based nanoparticles persist in the colon, providing sustained drug release. They also restore gut microbial balance by inhibiting TLR4/NF-κB signaling, activating Nrf2/HO-1 antioxidant pathways, repairing epithelial barriers, and re-establishing gut homeostasis. Importantly, RH-F/C-NPs markedly upregulated barrier-associated proteins including occludin, claudin-1, and ZO-1, underscoring their therapeutic potential for epithelial injury repair (194–197).
7.2 Characteristics of alginic acid and application of nano-carriers
ALG is a naturally occurring polysaccharide rich in hydroxyl and carboxyl groups, enabling hydrogen bonding, gel formation, mucoadhesion, and enhanced transdermal penetration (198). Due to its excellent biocompatibility, biodegradability, and drug-loading capacity, ALG nanoparticles (NPs) are readily degraded in biological systems, thereby reducing the risk of long-term accumulation (198). Furthermore, ALG possesses intrinsic pH sensitivity, allowing structural modification for controlled release under specific gastrointestinal conditions.
For example, CS-calcium-ALG microparticles have been developed by crosslinking ALG with polymers and calcium through spray drying, encapsulating Lactobacillus casei together with inulin rich in fructooligosaccharides (199). This delivery system significantly improved mucosal integrity, promoted vasodilation and glandular development, and reduced inflammatory cell infiltration in colonic tissues. Moreover, Lactobacillus counts in treated rats returned to levels comparable to healthy controls (200).
Notably, shifts in the relative proportions of Lactobacillus and pathogenic bacteria such as Escherichia coli are closely associated with colonic inflammation (201). Enhancing the abundance of Lactobacillus may thus restore high-molecular-weight equilibrium and rebalance pro- and anti-inflammatory responses in the gut (202).
7.3 Properties of HA and application of nano-carriers
HA, a major component of synovial fluid and the extracellular matrix, exhibits notable immunomodulatory activities. It modulates macrophage function, stimulates antimicrobial peptide production, inhibits bacterial proliferation (203), and regulates CD4+ T cell responses. Studies have shown that HA protects the intestinal epithelium by reducing inflammation and permeability, thereby preserving barrier integrity. Chemically modified HA formulations, such as biphasic enema suspensions, significantly decreased inflammation and permeability while maintaining mucosal function in murine colitis models (204).
HA-based nanocarriers have been engineered for enhanced colonic targeting. For example, HA-functionalized polymer nanoparticles preferentially accumulate within inflamed intestinal epithelia compared with native HA, forming a protective barrier and strengthening tight junction signaling (204). Conjugation of HA to CS-modified nanoparticles via amide bonding improved targeting efficiency and cellular uptake, while CS-HA combinations synergistically attenuated colitis symptoms in mice. Amphiphilic HA-bilirubin conjugates have also been developed to form HA-bilirubin nanomedicine (HABN) (205). HABN preferentially accumulates in inflamed IECs, restores barrier integrity, and reshapes the gut microbiota, enriching Akkermansia muciniphila and Clostridium cluster XIV, both critical for gut homeostasis (206). A. muciniphila and its outer membrane protein Amuc alleviate inflammation by modulating host immune responses. HABN also increased Lactobacillus abundance (207), complementing the butyrate-mediated Treg activation by Clostridium cluster XIV (192).
Current evidence suggests that Lactobacillus exerts anti-inflammatory effects in various animal models of colitis (208–210) and in patients with IBD (211–213). Notably, treatment with broad-spectrum oral antibiotics partially diminished the protective efficacy of HABN against DSS-induced colitis, underscoring the role of microbiota in mediating these effects.
HA can also be metabolized by gut probiotics including Bacteroides, Lactobacillus, and Bifidobacterium, which degrade orally administered HA into unsaturated oligosaccharides. These metabolites are further converted into SCFAs, CO2, and H2 (214), providing nutrients for IECs and reinforcing epithelial defenses (215). SCFAs support epithelial turnover, mucosal growth, and immune regulation. In IBD models, HABN reduced tissue injury, immune infiltration, and peroxidase activity while enhancing colon length and antimicrobial peptide expression (216). Interestingly, the regulatory effects of HA within the gut depend on its molecular weight (217–221). High-molecular-weight HA stabilizes the intestinal mucosa and counteracts immune dysregulation, whereas low-molecular-weight HA enhances metabolic absorption and modulates innate immune responses. Specific HA fragment sizes also exhibit distinct biological activities, playing pivotal roles in inducing immune defense mechanisms within the intestinal epithelium (222, 223).
7.4 Characteristics of ASP and application of nano-carriers
Water-soluble polysaccharides can be transformed into amphiphilic polymers through partial dehydrogenation, enabling spontaneous self-assembly in aqueous environments. In such systems, hydrophobic moieties aggregate to form the core, while hydrophilic polysaccharide chains constitute the shell, yielding stable micellar structures (224). The hydrophobic core accommodates hydrophobic drugs via noncovalent interactions, whereas the hydrophilic shell can be chemically modified with responsive groups for controlled or targeted release (225).
ASP, owing to its high solubility, biocompatibility, biodegradability, abundant hydroxyl groups, and modifiability, is an ideal candidate for constructing amphiphilic polymeric micelles with therapeutic potential (226, 227). For instance, cystine dihydrochloride has been used as a crosslinker to synthesize ASP-based nanoparticles encapsulating proanthocyanidins for ulcerative colitis therapy. These nanoparticles were glutathione-sensitive, enabling efficient release in inflamed tissues. However, due to the complexity of the colonic microenvironment, single-responsive systems often fail to ensure precise delivery. To overcome this limitation, dual-responsive ASP nanocarriers, sensitive to both pH and redox conditions, were developed to deliver ginsenoside Rh2 selectively to inflamed colonic sites. This system significantly alleviated colitis symptoms and modulated gut microbial composition (228, 229).
ASP has also been chemically modified with allantoic acid to generate amphiphilic polymers that self-assemble into nanoparticles through carboxyl-mediated interactions. The imidazole group of allantoic acid confers pH sensitivity, promoting rapid degradation in acidic inflammatory sites (230). Similarly, ASP conjugation with α-lipoic acid introduces redox responsiveness via disulfide bonds, enabling degradation under glutathione-rich conditions (231). In vivo studies demonstrated that ASP-based nanocarriers enriched beneficial taxa such as Norank, Lactobacillus, and Lachnospiraceae, while reducing harmful genera including Bacteroides, Turicibacter, and Ruminococcus. These Rh2-loaded ASP nanoparticles enhanced SCFA production, particularly acetate, propionate, and butyrate, and upregulated ZO-1 expression in colonic tissues, thereby improving mucosal barrier homeostasis (232). The nanoparticles exhibited dual targeting: passive accumulation in inflamed tissues through the enhanced permeability and retention (EPR) effect, and active targeting via dual responsiveness. Their small particle size also facilitated cellular uptake by IECs and immune cells (e.g., neutrophils, macrophages, and M cells) through endocytosis (172). Collectively, ASP nanocarriers not only enhanced drug bioavailability but also increased anti-inflammatory efficacy in colitis therapy.
7.5 Characteristics of rhubarb polysaccharides and application of nano-carriers
Rhubarb polysaccharide (DHP), predominantly extracted from rhubarb, is characterized by its biodegradability, low immunogenicity, and minimal toxicity. Its abundant hydroxyl groups facilitate electrostatic and hydrogen-bond interactions, enabling co-assembly with berberine (BBR) to form BBR-DHP nanoparticles (BD). Studies using DSS-induced colitis models revealed that disease groups exhibited increased Proteobacteria and decreased Firmicutes. BD treatment restored microbial balance, reducing Proteobacteria and enriching Lactobacillus, a key probiotic genus within Firmicutes that promotes gut homeostasis (233).
Lactobacillus contributes to epithelial repair, mucosal defense, and immune regulation. It competitively inhibits pathogen adhesion to IECs, produces antimicrobial metabolites (e.g., lactic, acetic, and propionic acids, bacteriocins, ROS), and strengthens host defenses (234). Notably, Lactobacillus abundance was significantly reduced in both DSS and BBR-only groups, but maintained in the DHP and BD groups, consistent with genus- and phylum-level shifts. These findings suggest that the microbiota-modulating effect of BD is primarily attributable to DHP (233). Histological analyses further demonstrated that BD treatment ameliorated colonic injury, restoring crypt architecture, preserving goblet cells, and reducing muscular edema. BD also maintained colon length (~8 cm), comparable to healthy controls. Tight junction proteins occludin and ZO-1, markedly reduced in DSS groups, were restored by BD treatment. Deficient O-glycosylation compromises mucin production and disrupts the mucus barrier, thereby facilitating inflammasome activation (e.g., caspase-1, IL-1, IL-18) and exacerbating inflammation. High-performance liquid chromatography (HPLC) revealed that minor monosaccharides in DHP—such as mannose, xylose, and GalNAc—may promote glycosylation, reduce inflammation, and contribute to therapeutic efficacy (235, 236).
7.6 Properties of Phellodendron amurense polysaccharides and application of nano-carriers
Phellodendron amurense polysaccharide (PIP) has demonstrated the ability to improve the intestinal microenvironment by modulating gut microbiota and enhancing mucosal immunity (237). Given its potent anti-inflammatory and prebiotic properties, researchers developed a PIP-loaded CS-modified poly(lactic-co-glycolic acid) (PLGA) nanoparticle (CS-PIPP). Experimental results showed that CS-PIPP decreased pathogenic taxa while increasing beneficial bacteria such as Lactobacillus and Akkermansia muciniphila, underscoring its potential as a prebiotic agent (238). Further investigations revealed that both free PIP and CS-PIPP increased Lactobacillus and A. muciniphila populations. Notably, CS-PIPP more effectively reduced Escherichia coli and Shigella abundance, thereby limiting pathogen invasion of colonic mucosa and suppressing inflammatory responses. In addition, CS-PIPP enriched probiotic genera such as Alloprevotella while reducing harmful taxa including Romboutsia, often elevated in ulcerative colitis (239, 240).
CS-PIPP also exerted immunomodulatory effects. It enhanced IL-10 secretion, inhibited M1 macrophage polarization, and preserved tight junction proteins (ZO-1 and occludin), thereby maintaining barrier integrity. Regulation of SCFA production may represent an additional protective mechanism. Collectively, these findings highlight CS-PIPP as a synbiotic nanocarrier with multifaceted roles in protecting against IBD through reshaping the microbiota, strengthening the barrier, and modulating immune responses (241–243).
7.7 Characteristics of Eucommia ulmoides polysaccharide and application of nano-carriers
Eucommia ulmoides polysaccharide (EUP) refers to a group of sugars extracted from the leaves and roots of Eucommia ulmoides. Previous studies have shown that EUP possesses anti-inflammatory, antioxidant, and immunomodulatory properties (244, 245). Selenium nanoparticles (SeNPs) are known for their excellent biological activity in IBD therapy. In this context, EUP was used as a surface modifier to prepare EUP-SeNPs with an approximate size of 170 nm. Oral administration of EUP-SeNPs effectively counteracted DSS-induced reductions in beneficial bacteria such as Actinomycetes, DNA, Rikenellaceae, and Muribaculaceae. Concurrently, they decreased the abundances of pathogenic bacteria including Campylobacter, Escherichia coli, Vibrio, Desulfobacter, and Ruminococcus. These findings align with previous studies (246, 247), suggesting that EUP-SeNPs can mitigate colonic injury by modulating the gut microbiota, enhancing beneficial taxa, and suppressing harmful populations (248).
Mucins in the intestinal mucus layer act as a primary defense by preventing pathogen infiltration (249, 250). Studies revealed that EUP-SeNPs improve the expression of tight junction proteins by reducing inflammatory cell infiltration and intestinal permeability, increasing goblet cell numbers and mucin secretion, regulating IEC apoptosis and proliferation, and modulating inflammatory cytokines, collectively ameliorating DSS-induced colonic damage. Additionally, EUP-SeNPs inhibited activation of the TLR4/NF-κB signaling pathway. Maintaining redox balance is critical for overall health, with multiple indicators used to evaluate colonic oxidative status. Oral administration of EUP-SeNPs was found to significantly enhance colonic antioxidant capacity and attenuate the severity of DSS-induced colitis, underscoring their potential as a multifunctional therapeutic strategy for IBD (248).
7.8 Other polysaccharides
Polysaccharides from various natural sources, including Codonopsis pilosula, Dendrobium officinale, LBP, and Tremella fuciformis, have also shown therapeutic promise against IBD (Table 2).
Codonopsis pilosula polysaccharides (CPP) help maintain gut homeostasis by sustaining Lactobacillus abundance and reducing Escherichia-Shigella populations, thereby restoring microbial balance (251). Dendrobium officinale polysaccharides (DOP) significantly increased microbial diversity and improved the relative abundances of Firmicutes and Bacteroidetes in colitis models. DOP also suppressed harmful taxa such as Proteobacteria and reduced inflammation, oxidative stress, and apoptosis, ultimately enhancing barrier integrity by upregulating ZO-1 and occludin expression (252). LBPs improved microbial composition by elevating Lactobacillus and Bifidobacterium, restoring microbial diversity, and strengthening mucosal defenses. LBP supplementation increased SCFA levels, reduced colonic inflammation, and upregulated tight junction proteins, thereby ameliorating colitis pathology (253, 254). Tremella fuciformis polysaccharides (TFP) exhibited strong microbiota-regulating activity by increasing SCFA-producing bacteria and Lactobacillus, while simultaneously enhancing tight junction protein expression and reducing oxidative stress. These effects collectively protected epithelial integrity and alleviated colitis (255). In addition, selenium nanoparticles prepared with seaweed polysaccharides demonstrated anti-inflammatory efficacy by inhibiting NF-κB activation, preserving intestinal barrier integrity, and reducing inflammation in colitis models (256).
8 Challenges and prospects
This review has systematically analyzed the core pathological mechanisms underlying IBD, including gut microbiota dysbiosis, impaired intestinal barrier function, immune dysregulation, and oxidative stress. Natural polysaccharides, derived from diverse sources and exhibiting distinct structural characteristics, demonstrate strong therapeutic potential in modulating these pathways. Their dual mechanisms—microbiota regulation and barrier enhancement—include stimulation of beneficial bacteria such as Lactobacillus and Bifidobacterium, elevation of SCFA production (e.g., butyrate as an epithelial energy source, acetate for Treg differentiation), inhibition of TLR4/NF-κB inflammatory signaling, and activation of the Nrf2/HO-1 antioxidant pathway. Moreover, the context-dependent roles of key microbes, such as Akkermansia muciniphila, highlight their ability to maintain barrier integrity in health but exacerbate inflammation under pathological conditions.
Polysaccharide-based nanocarriers—including CS, ALG, HA, and ASP—further enhance therapeutic efficacy by enabling targeted, responsive, and sustained drug delivery. Smart designs, such as pH/ROS dual-sensitive RH-F/C-NPs and composite carriers like CS-PIPP, combine prebiotic activity, controlled release, and mucoadhesion, achieving superior probiotic enrichment and pathogen suppression compared with single-component systems. Recent advances have also underscored the importance of polysaccharide structure-activity relationships. For instance, low-molecular-weight fucoidan (2.56 kDa) and konjac glucomannan (KGM2, 7413 Da) exhibit enhanced anti-inflammatory activity due to improved microbial fermentation into SCFAs, while branched polysaccharides such as SHPS-1 exert effects via specific glycosidic linkages (257).
Despite these promising developments, challenges remain. Structural complexity, variability in extraction and purification, and difficulties in achieving reproducible formulations hinder translational application. Furthermore, polysaccharide bioactivity is strongly influenced by molecular weight, branching degree, and monosaccharide composition (258, 259). Although low-molecular-weight fractions often show superior bioactivity (260–262), results remain inconsistent. Future efforts should focus on clarifying structure-activity relationships, designing multi-responsive nanocarriers for precise release, and extending applications to biomacromolecule delivery systems such as vaccines and nucleic acids. In conclusion, polysaccharides and their nanoformulations represent highly promising therapeutic strategies for restoring microbial homeostasis, reinforcing mucosal barriers, and attenuating intestinal inflammation in IBD.
Author contributions
YJ: Writing – review & editing, Writing – original draft. JL: Writing – review & editing, Writing – original draft. QH: Writing – review & editing. JL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by the Guizhou Provincial Science and Technology Program, Project No. Qiankehe Basic Research-ZK[2024] General 384.
Acknowledgments
We would like to thank JL for providing valuable assistance with the guidance on the writing of this review., HQ and LJ for the data collection and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Forster SC, Clare S, Beresford-Jones BS, Harcourt K, Notley G, Stares MD, et al. Identification of gut microbial species linked with disease variability in a widely used mouse model of colitis. Nat Microbiol. (2022) 7:109. doi: 10.1038/s41564-022-01094-z
2. Liu C, Zhou N, Du MX, Sun YT, Wang K, Wang YJ, et al. The Mouse Gut Microbial Biobank expands the coverage of cultured bacteria. Nat Commun. (2020) 11:6143. doi: 10.1038/s41467-019-13836-5
3. Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. (2018) 24(1):5–20. doi: 10.3748/wjg.v24.i1.5
4. Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: Systematic review and meta-analysis. Am J Gastroenterol. (2011) 106(3):418–28. doi: 10.1038/ajg.2011.67
5. Perrotta C, Pellegrino P, Moroni E, De Palma C, Cervia D, Danelli P, et al. Five-aminosalicylic acid: An update for the reappraisal of an old drug. Gastroenterol Res Pract. (2015) 2015:456895. doi: 10.1155/2015/456895
6. Kaur R, Gulati M, and Singh SK. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int J Biol Macromol. (2017) 95:1183–92. doi: 10.1016/j.ijbiomac.2016.11.066
7. Ransford RAJ and Langman MJS. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. (2002) 51(4):536–41. doi: 10.1136/gut.51.4.536
8. Perez-Colon E, Dadlani GH, Wilmot I, and Miller M. Mesalamine-induced myocarditis and coronary vasculitis in a pediatric ulcerative colitis patient: A case report. Case Rep Pediatr. (2011) 2011:524364. doi: 10.1155/2011/524364
9. Deltenre P, Berson A, Marcellin P, Degott C, Biour M, and Pessayre D. Mesalazine (5-aminosalicylic acid) induced chronic hepatitis. Gut. (1999) 44(6):886–8. doi: 10.1136/gut.44.6.886
10. Ratner M. IL-17-targeting biologics aim to become standard of care in psoriasis. Nat Biotechnol. (2015) 33(1):3–4. doi: 10.1038/nbt0115-3
11. Turner D, Levine A, Kolho KL, Shaoul R, and Ledder O. Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: A preliminary report. J Crohns Colitis. (2014) 8(9):1184–90. doi: 10.1016/j.crohns.2014.05.010
12. Nie Y, Lin Q, and Luo F. Effects of non-starch polysaccharides on inflammatory bowel disease. Int J Mol Sci. (2017) 18(7):1372. doi: 10.3390/ijms18071372
13. Antonio E, dos Reis Antunes Junior O, Marcano RGDJV, Diedrich C, da Silva Santos J, MaChado CS, et al. Chitosan modified poly (lactic acid) nanoparticles increased the ursolic acid oral bioavailability. Int J Biol Macromol. (2021) 172:644–52. doi: 10.1016/j.ijbiomac.2021.01.041
14. Zhang S, Kang L, Hu S, Hu J, Fu Y, Hu Y, et al. Carboxymethyl chitosan microspheres loaded hyaluronic acid/gelatin hydrogels for controlled drug delivery and the treatment of inflammatory bowel disease. Int J Biol Macromol. (2021) 167:1127–35. doi: 10.1016/j.ijbiomac.2020.11.117
15. Kanwal S, Joseph TP, Aliya S, Song S, Saleem MZ, Nisar MA, et al. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J Funct Foods. (2020) 64:103641. doi: 10.1016/j.jff.2019.103641
16. Prabaharan M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromolecules. (2011) 49(1):1–12. doi: 10.1016/j.ijbiomac.2011.04.022
17. Cui M, Zhang M, Wu J, Han P, Lv M, Dong L, et al. Marine polysaccharides from Gelidium pacificum Okamura and Cereus sinensis reveal prebiotic functions. Int J Biol Macromol. (2020) 164:1773–82. doi: 10.1016/j.ijbiomac.2020.08.255
18. Liu Z, Jiao Y, Wang Y, Zhou C, and Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Rev. (2008) 60(15):1650–62. doi: 10.1016/j.addr.2008.09.001
19. An FF and Zhang XH. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics. (2017) 7(11):2640–56. doi: 10.7150/thno.19365
20. Florence AT. The oral absorption of micro- and nanoparticulates: Neither exceptional nor unusual. Pharm Res. (1997) 14(8):1059–66. doi: 10.1023/A:1012029517394
21. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species roseburia hominis and faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. (2014) 63(8):1275–83. doi: 10.1136/gutjnl-2013-305802
22. Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, et al. Precision editing of the gut microbiota ameliorates colitis. Nature. (2018) 553(7688):208–12. doi: 10.1038/nature25172
23. Wen X, Wang HG, Zhang MN, Zhang MH, Wang H, and Yang XZ. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J Gastroenterol. (2021) 27(21):2834–46. doi: 10.3748/wjg.v27.i21.2834
24. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, and Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. (2018) 11(1):1–12. doi: 10.1007/s12328-017-0813-5
25. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. (2008) 134(4):1165–80. doi: 10.1053/j.gastro.2007.11.059
26. Baümler AJ and Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. (2016) 535(7610):85–93. doi: 10.1038/nature18849
27. Zheng D, Liwinski T, and Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. (2020) 30(1):49–65. doi: 10.1038/s41422-020-0332-7
28. Okumura R and Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflammation Regeneration. (2018) 38(1):4. doi: 10.1186/s41232-018-0063-z
29. Baker S and The HC. Recent insights into Shigella. Curr Opin Infect Dis. (2018) 31(5):411–7. doi: 10.1097/QCO.0000000000000464
30. Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. (2011) 60(11):1505–12. doi: 10.1136/gut.2010.224407
31. Han Y, Song M, Gu M, Ren D, Zhu X, Cao X, et al. Dietary intake of whole strawberry inhibited colonic inflammation in dextran-sulfate-sodium-treated mice via restoring immune homeostasis and alleviating gut microbiota dysbiosis. J Agric Food Chem. (2019) 67(45):12691–700. doi: 10.1021/acs.jafc.8b05581
32. Wang YN, Meng XC, Dong YF, Zhao XH, Qian JM, Wang HY, et al. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin Med J (Engl). (2019) 132(19):2342–50. doi: 10.1097/CM9.0000000000000308
33. Yeo S, Park H, Seo E, Kim J, Kim BK, Choi IS, et al. Anti-inflammatory and gut microbiota modulatory effect of lactobacillus rhamnosus strain ldtm 7511 in a dextran sulfate sodium-induced colitis murine model. Microorganisms. (2020) 8(6):845. doi: 10.3390/microorganisms8060845
34. Tong L, Zhang X, Hao H, Liu Q, Zhou Z, Liang X, et al. Lactobacillus rhamnosus gg derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in dss-induced colitis mice. Nutrients. (2021) 13(10):3319. doi: 10.3390/nu13103319
35. Gkouskou KK, Deligianni C, Tsatsanis C, and Eliopoulos AG. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infection Microbiol. (2014) 4. doi: 10.3389/fcimb.2014.00028
36. Johansson MEV. Mucus layers in inflammatory bowel disease. Inflammation Bowel Dis. (2014) 20(1):270–8. doi: 10.1097/MIB.0000000000000117
37. Wang X, Ni J, You Y, Feng G, Zhang S, Bao W, et al. SNX10-mediated LPS sensing causes intestinal barrier dysfunction via a caspase-5-dependent signaling cascade. EMBO J. (2021) 40(12):e108080. doi: 10.15252/embj.2021108080
38. Merga Y, Campbell BJ, and Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: Possibilities for therapy. Digestive Diseases. (2014) 32(4):483–91. doi: 10.1159/000358156
39. Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. (2014) 63(3):411–9. doi: 10.1136/gutjnl-2012-303207
40. Johansson MEV, Holmén Larsson JM, and Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U.S.A. (2011) 108(42):17559–65. doi: 10.1073/pnas.1006451107
41. Theodoratou E, Campbell H, Ventham NT, Kolarich D, Pučić-Baković M, Zoldoš V, et al. The role of glycosylation in IBD. Nat Rev Gastroenterol hepatology. (2014) 11(11):679–91. doi: 10.1038/nrgastro.2014.78
42. Aamann L, Vestergaard EM, and Grønbæk H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol. (2014) 20(12):3223–32. doi: 10.3748/wjg.v20.i12.3223
43. Bandeira A, Mota-Santos T, Itohara S, Degermann S, Hausser C, Tonegawa S, et al. Localization of γ/δ T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. (1990) 172(1):239–48. doi: 10.1084/jem.172.1.239
44. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. (2012) 13(9):R79. doi: 10.1186/gb-2012-13-9-r79
45. Sun Y, Wang X, Li L, Zhong C, Zhang Y, Yang X, et al. The role of gut microbiota in intestinal disease: from an oxidative stress perspective. Front Microbiol. (2024) 15. doi: 10.3389/fmicb.2024.1328324
46. Gracie DJ, Hamlin PJ, and Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. (2019) 4(7):533–43. doi: 10.1016/S2468-1253(19)30089-5
47. Zhang Z, Zhang H, Chen T, Shi L, Wang D, and Tang D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Communication Signaling. (2022) 20(1):124. doi: 10.1186/s12964-022-00869-5
48. Zhang R, Yuan S, Ye J, Wang X, Zhang X, Shen J, et al. Polysaccharide from flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int J Biol Macromol. (2020) 149:1051–60. doi: 10.1016/j.ijbiomac.2020.02.044
49. Li L, Wei N, Min ZQ, Li Y, Zhang X, Wu Hy, et al. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol. (2020) 64(5):277–86. doi: 10.1111/1348-0421.12749
50. Macfarlane GT and Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. (2012) 95(2):300–11. doi: 10.5740/jaoacint.SGE_Macfarlane
51. Jung TH, Park JH, Jeon WM, and Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. (2015) 9(4):343–9. doi: 10.4162/nrp.2015.9.4.343
52. Peng L, Li ZR, Green RS, Holzman IR, and Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139(10):1944–50. doi: 10.3945/jn.109.104638
53. Federici M. Gut microbiome and microbial metabolites: a new system affecting metabolic disorders. J Endocrinological Invest. (2019) 42(11):1287–96. doi: 10.1007/s40618-019-01022-9
54. Cheng Y, Liu J, and Ling Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit Rev Food Sci Nutr. (022) 62(13):3466–84. doi: 10.1080/10408398.2021.1920884
55. Sharma D, Malik A, Guy CS, Karki R, Vogel P, and Kanneganti TD. Pyrin inflammasome regulates tight junction integrity to restrict colitis and tumorigenesis. Gastroenterology. (2018) 154(7):1954–68.e1957 . doi: 10.1053/j.gastro.2018.03.040
56. Zhou Y, Duan L, Zeng Y, Niu L, Pu Y, Jacobs JP, et al. The panda-derived lactobacillus plantarum G201683 alleviates the inflammatory response in DSS-induced panda microbiota-associated mice. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.747045
57. Louis P and Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. (2017) 19(11):4144–59. doi: 10.1111/1462-2920.13589
58. Liu Y, Liu G, and Fang J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J Nutr Biochem. (2024) 124:109505. doi: 10.1016/j.jnutbio.2023.109505
59. Martínez C, González-Castro A, Vicario M, and Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irrita ble bowel syndrome. Gut Liver. (2012) 6(1):1–18. doi: 10.5009/gnl.2012.6.1.1
60. Johansson MEV, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. (2015) 18(5):582–92. doi: 10.1016/j.chom.2015.10.007
61. Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. (2017) 117(8):1064–82. doi: 10.1017/S0007114516003760
62. Yuan JY and Zhou YH. Regulation of mucosal immunity and preventing infectious disease occurrence by intestinal microbiota. World Chin J Digestology. (2015) 23(30):4852–8. doi: 10.11569/wcjd.v23.i30.4852
63. Caballero-Flores G, Pickard JM, and Núñez G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol. (2023) 21(3):161–76. doi: 10.1038/s41579-022-00833-7
64. Lavelle A and Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17(2):120–34. doi: 10.1038/s41575-019-0258-z
65. Lindén SK, Florin THJ, and McGuckin MA. Mucin dynamics in intestinal bacterial infection. PloS One. (2008) 3(11):e3952. doi: 10.1371/journal.pone.0003952
66. Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. (2013) 5(4):1417–35. doi: 10.3390/nu5041417
67. Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, and Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. (2000) 46(2):218–24. doi: 10.1136/gut.46.2.218
68. Finnie IA, Dwarakanath AD, Taylor BA, and Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut. (1995) 36(1):93–8. doi: 10.1136/gut.36.1.93
69. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. (2013) 500(7461):232–6. doi: 10.1038/nature12331
70. Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol. (2015) 16(8):827–35. doi: 10.1038/ni.3233
71. Davison JM, Lickwar CR, Song L, Breton G, Crawford GE, and Rawls JF. Microbiota regulate intestinal epithelial gene expression by suppressing the transcription factor Hepatocyte nuclear factor 4 alpha. Genome Res. (2017) 27(1):91–102. doi: 10.1101/gr.220111.116
72. Chen X, Xu J, Shuai J, Chen J, Zhang Z, and Fang W. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int J Food Microbiol. (2007) 115(2):163–70(1):91–102. doi: 10.1016/j.ijfoodmicro.2006.11.007
73. Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A. (2010) 107(34):15064–9. doi: 10.1073/pnas.0913554107
74. Hopkins EGD, Roumeliotis TI, Mullineaux-Sanders C, Choudhary JS, and Frankel G. Intestinal epithelial cells and the microbiome undergo swift reprogramming at the inception of colonic citrobacter rodentium infection. mBio. (2019) 10(3):e00621–19. doi: 10.1128/mBio.00062-19
75. Rowan F, Docherty NG, Murphy M, Murphy B, Coffey JC, and O’Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. (2010) 53(9):1275–80. doi: 10.1007/DCR.0b013e3181f1e620
76. Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, and Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. (2008). doi: 10.21037/jpp.2008.04.08
77. O’Keefe SJD. Nutrition and colonic health: The critical role of the microbiota. Curr Opin Gastroenterol. (2008) 24(6):737–42. doi: 10.1097/MOG.0b013e3282f323f3
78. Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. (2018) 11(4):1054–64. doi: 10.1038/mi.2017.118
79. Kudelka MR, Stowell SR, Cummings RD, and Neish AS. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat Rev Gastroenterol Hepatol. (2020) 17(12):755–72. doi: 10.1038/s41575-020-0331-7
80. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. (2019) 4(2):293–305. doi: 10.1038/s41564-019-0442-5
81. Ahmed I, Roy BC, Khan SA, Septer S, and Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms. (2016) 4(2):20. doi: 10.3390/microorganisms4020020
82. Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, and Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. (2007) 9(1):160–72. doi: 10.1111/j.1462-5822.2006.00836.x
83. Souza ÉL, Elian SD, Paula LM, Garcia CC, Vieira AT, Teixeira MM, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J Med Microbiol. (2016) 65(10):1131–40. doi: 10.1099/jmm.0.000222
84. Lu Q, Yang MF, Liang YJ, Xu J, Xu HM, Nie YQ, et al. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflammation Res. (2022) 4377–98. doi: 10.2147/JIR.S353038
85. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U.S.A. (2008) 105(43):16731–6. doi: 10.1073/pnas.0804812105
86. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Sci (1979). (2011) 331(6015):337–41. doi: 10.1126/science.1198469
87. Hooper LV, Littman DR, and Macpherson AJ. Interactions between the microbiota and the immune system. Science. (2012) 336(6086):1268–73. doi: 10.1126/science.1223490
88. O’Neil LJ, Oliveira CB, Wang X, Navarrete M, Barrera-Vargas A, Merayo-Chalico J, et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann Rheum Dis. (2023) 82(5):814–23. doi: 10.1136/ard-2022-223568
89. Karmakar U and Vermeren S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology. (2021) 164(3):329–41. doi: 10.1111/imm.13412
90. Li G, Lin J, Zhang C, Gao H, Lu H, Gao X, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. (2021) 13(1):1–22. doi: 10.1080/19490976.2021.1968257
91. Kim JE, Li B, Fei L, Horne R, Lee D, Loe AK, et al. Gut microbiota promotes stem cell differentiation through macrophage and mesenchymal niches in early postnatal development. Immunity. (2022) 55(11):2020–35.e2026. doi: 10.1016/j.immuni.2022.11.003
92. Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. (2019) 50(1):110–22.e115. doi: 10.1016/j.immuni.2018.12.018
93. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. (2015) 8(4):800–12. doi: 10.1038/mi.2014.44
94. Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, et al. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity. (2016) 44(4):802–15. doi: 10.1016/j.immuni.2016.03.016
95. Yamada A, Arakaki R, Saito M, Tsunematsu T, Kudo Y, and Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. (2016) 22(7):2195–204. doi: 10.3748/wjg.v22.i7.2195
96. Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. (1998) 66(11):5224–31. doi: 10.1128/IAI.66.11.5224-5231.1998
97. Jin YB, Cao X, Shi CW, Feng B, Huang HB, Jiang YL, et al. Lactobacillus rhamnosus GG promotes early B lineage development and igA production in the lamina propria in piglets. J Immunol. (2021) 207(4):1044–54. doi: 10.4049/jimmunol.2100102
98. Shao X, Sun S, Zhou Y, Wang H, Yu Y, Hu T, et al. Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. (2021) 523:201–11. doi: 10.1016/j.canlet.2021.10.002
99. Satish Kumar CSV, Kondal Reddy K, Reddy AG, Vinoth A, Ch SRC, Boobalan G, et al. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int Immunopharmacol. (2015) 25(1):244–51. doi: 10.1016/j.intimp.2015.02.015
100. Bhattacharyya A, Chattopadhyay R, Mitra S, and Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. (2014) 94(4):1323–67. doi: 10.1152/physrev.00040.2012
101. Liu P, Li Y, Wang R, Ren F, and Wang X. Oxidative stress and antioxidant nanotherapeutic approaches for inflammatory bowel disease. Biomedicines. (2022) 10(4):864. doi: 10.3390/biomedicines10040864
102. Chivero ET, Sil S, Singh S, Thangaraj A, Gordon L, Evah-Nzoughe GB, et al. Protective role of lactobacillus rhamnosus probiotic in reversing cocaine-induced oxidative stress, glial activation and locomotion in mice. J Neuroimmune Pharmacol. (2022) 17(4):869–84. doi: 10.1007/s11481-021-10020-9
103. Goyal N, Rishi P, and Shukla G. Lactobacillus rhamnosus GG antagonizes Giardia intestinalis induced oxidative stress and intestinal disaccharidases: An experimental study. World J Microbiol Biotechnol. (2013) 29(10):1859–67. doi: 10.1007/s11274-013-1268-6
104. Li J, Li Q, Gao N, Wang Z, Li F, Li J, et al. Exopolysaccharides produced by: Lactobacillus rhamnosus GG alleviate hydrogen peroxide-induced intestinal oxidative damage and apoptosis through the Keap1/Nrf2 and Bax/Bcl-2 pathways in vitro. Food Funct. (2021) 12(11):5087–99. doi: 10.1039/D1FO00277E
105. Chen YS, Lian YZ, and Chao J. Protective Effect of Lycium barbarum Polysaccharides and Capsaicin in Rats With Dextran Sulfate Sodium-Induced Ulcerative Colitis via Anti-inflammation and Antioxidation. Curr Dev Nutr. (2021) 5(8):nzab037. doi: 10.1093/cdn/nzab037_016
106. Huang Q, Zhang Y, Chu Q, and Song H. The influence of polysaccharides on lipid metabolism: insights from gut microbiota. Mol Nutr Food Res. (2024) 68(3):e2300522. doi: 10.1002/mnfr.202300522
107. Alvarez-Lorenzo C, Blanco-Fernandez B, Puga AM, and Concheiro A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Advanced Drug Delivery Rev. (2013) 65(10):1261–85. doi: 10.1016/j.addr.2013.04.016
108. Jin W, Xiang L, Peng D, Liu G, He J, Cheng S, et al. Study on the coupling progress of thermo-induced anthocyanins degradation and polysaccharides gelation. Food Hydrocoll. (2020) 105:105822. doi: 10.1016/j.foodhyd.2020.105822
109. Luo Y, Wang Q, and Zhang Y. Biopolymer-based nanotechnology approaches to deliver bioactive compounds for food applications: A perspective on the past, present, and future. J Agric Food Chem. (2020) 68(14):3863–82. doi: 10.1021/acs.jafc.0c00277
110. Tang W, Peng QX, Yan YM, Mo ZX, Huang SH, Li X, et al. Study on purification of polysaccharides in root of Salvia miltiorrhiza by macroporous adsorption resin and ion-exchange resin methods. Zhong Yao Cai. (2010) 33:897–908. doi: 10.1016/j.ijbiomac.2022.08.156
111. Zhu YM, Pan LC, Zhang LJ, Yin Y, Zhu ZY, Sun HQ, et al. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int J Biol Macromol. (2020) 165:1470–8. doi: 10.1016/j.ijbiomac.2020.10.127
112. Benalaya I, Alves G, Lopes J, and Silva LR. A review of natural polysaccharides: sources, characteristics, properties, food, and pharmaceutical applications. Int J Mol Sci. (2024) 25(2):1322. doi: 10.3390/ijms25021322
113. Yang B, Yang C, Liu R, Sui W, Zhu Q, Jin Y, et al. The relationship between preparation and biological activities of animal-derived polysaccharides: A comprehensive review. Foods. (2024) 13(1):173. doi: 10.3390/foods13010173
114. Wang LC, Di LQ, Li JS, Hu LH, Cheng JM, and Wu H. Elaboration in type, primary structure, and bioactivity of polysaccharides derived from mollusks. Crit Rev Food Sci Nutr. (2019) 59(15):2489–508. doi: 10.1080/10408398.2017.1392289
115. Wang B, Yan L, Guo S, Wen L, Yu M, Feng L, et al. Structural elucidation, modification, and structure-activity relationship of polysaccharides in chinese herbs: A review. Front Nutr. (2022) 9. doi: 10.3389/fnut.2022.908175
116. Song Y, Li S, Gong H, Yip RCS, and Chen H. Biopharmaceutical applications of microbial polysaccharides as materials: A Review. Int J Biol Macromolecules. (2023) 239:124259. doi: 10.1016/j.ijbiomac.2023.124259
117. Guan X, Wang F, Zhou B, Sang X, and Zhao Q. The nutritional function of active polysaccharides from marine animals: A review. Food Biosci. (2024) 58:103693. doi: 10.1016/j.fbio.2024.103693
118. Jin M, Zhu Y, Shao D, Zhao K, Xu C, Li Q, et al. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol. (2017) 94:580–7. doi: 10.1016/j.ijbiomac.2016.09.099
119. He X, Liu J, Long G, Xia XH, and Liu M. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota. Biomedicine Pharmacotherapy. (2021) 137:111420. doi: 10.1016/j.biopha.2021.111420
120. Ren G, Xu L, Lu T, Zhang Y, Wang Y, and Yin J. Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen (Hucho taimen, Pallas). Int J Biol Macromol. (2019) 121:1110–7. doi: 10.1016/j.ijbiomac.2018.09.121
121. Xu X, Xu P, Ma C, Tang J, and Zhang X. Gut microbiota, host health, and polysaccharides. Biotechnol Adv. (2013) 31(6):848–59. doi: 10.1016/j.biotechadv.2012.12.009
122. Metzler-Zebeli BU, Zijlstra RT, Mosenthin R, and Gänzle MG. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol Ecol. (2011) 75(3):543–54. doi: 10.1111/j.1574-6941.2010.01017.x
123. Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. (2010) 141(7):1241–52. doi: 10.1016/j.cell.2010.05.005
124. Dodd D, Mackie RI, and Cann IKO. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol Microbiol. (2011) 79(4):919–34. doi: 10.1111/j.1365-2958.2010.07473.x
125. Li Y, Zhang X, Wang J, Chen L, Zhao H, Liu M, et al. Fuzhuan brick tea polysaccharides serve as a promising candidate for remodeling the gut microbiota from colitis subjects in vitro: Fermentation characteristic and anti-inflammatory activity. J Funct Foods. (2022) 95:105023. doi: 10.1016/j.jff.2022.105023
126. Ye HY, Shang ZZ, Zhang FY, Zha XQ, Li QM, and Luo JP. Dendrobium huoshanense stem polysaccharide ameliorates alcohol-induced gastric ulcer in rats through Nrf2-mediated strengthening of gastric mucosal barrier. Int J Biol Macromol. (2023) 236:124001. doi: 10.1016/j.ijbiomac.2023.124001
127. Huo J, Wu J, Huang M, and Sun B. A critical review on intestinal mucosal barrier protection effects of dietary polysaccharides. J Agric Food Chem. (2022) 70(14):4233–47:4233–47. doi: 10.1021/acs.jafc.1c05966
128. Pan L, Fu T, Cheng H, Mi J, Shang Q, and Yu G. Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans. Carbohydr Polym. (2022) 278:118921. doi: 10.1016/j.carbpol.2021.118921
129. Xie SZ, Liu B, Ye HY, Li QM, Pan LH, Zha XQ, et al. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polym. (2019) 206:244–53. doi: 10.1016/j.carbpol.2018.11.002
130. Li YF, Udayakumar V, Sathuvan M, Liu Y, Liu X, Zhang YQ, et al. Effects of laminarin zwitterionic carboxylate and sulfonate on the intestinal barrier function and gut microbiota. Carbohydr Polym. (2022) 278:118898. doi: 10.1016/j.carbpol.2021.118898
131. Kang S, Kim J, Park Y, Lee H, Jeong J, Kim M, et al. Fermentation supernatant of elderly feces with inulin and partially hydrolyzed guar gum maintains the barrier of inflammation-induced caco-2/HT29-MTX-E12 co-cultured cells. Nutrients. (2023) 15:378. doi: 10.3390/nu15020378
132. Guo X, Chen J, Yang J, He Q, Luo B, Lu Y, et al. Seaweed polysaccharide mitigates intestinal barrier dysfunction induced by enterotoxigenic Escherichia coli through NF-κB pathway suppression in porcine intestinal epithelial cells. J Anim Physiol Anim Nutr (Berl). (2021) 105(6):1227–38. doi: 10.1111/jpn.13540
133. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nat. (2010) 464(7285):59–65. doi: 10.1038/nature08821
134. Ahmad T, Ishaq M, Karpiniec S, Park A, Stringer D, Singh N, et al. Oral macrocystis pyrifera fucoidan administration exhibits anti-inflammatory and antioxidant properties and improves DSS-induced colitis in C57BL/6J mice. Pharmaceutics. (2022) 14(11):2383. doi: 10.3390/pharmaceutics14112383
135. Khil’chenko SR, Zaporozhets TS, Shevchenko NM, Zvyagintseva TN, Vogel U, Seeberger P, et al. Immunostimulatory activity of fucoidan from the brown alga Fucus evanescens: Role of sulfates and acetates. J Carbohydr Chem. (2011) 30(3–4):171–84. doi: 10.1080/07328303.2011.561448
136. Li P, Xiao N, Zeng L, Xiao J, Huang J, Xu Y, et al. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr Polym. (2020) 250:116958. doi: 10.1016/j.carbpol.2020.116958
137. Lim JM, Yoo HJ, and Lee KW. High molecular weight fucoidan restores intestinal integrity by regulating inflammation and tight junction loss induced by methylglyoxal-derived hydroimidazolone-1. Mar Drugs. (2022) 20(9):580. doi: 10.3390/md20090580
138. Cai Y, Liu W, Lin Y, Zhang S, Zou B, Xiao D, et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J Gastroenterol Hepatol (Australia). (2019) 34(10):1763–71. doi: 10.1111/jgh.14583
139. Tedelind S, Westberg F, Kjerrulf M, and Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J Gastroenterol. (2007) 13(20):2826–32. doi: 10.3748/wjg.v13.i20.2826
140. Ma G, Kimatu BM, Zhao L, Yang W, Pei F, and Hu Q. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. (2017) 8(8):2874–83. doi: 10.1039/C7FO00341B
141. Shao S, Wang D, Zheng W, Li X, Zhang H, Zhao D, et al. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int Immunopharmacol. (2019) 71:300–9. doi: 10.1016/j.intimp.2019.02.038
142. Xiao Z, Deng Q, Zhou W, and Zhang Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol Ther. (2022) 229:108284. doi: 10.1016/j.pharmthera.2022.108284
143. Zhang J, Yang G, Wen Y, Liu S, Li C, Yang R, et al. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol Nutr Food Res. (2017) 61(11):1700466. doi: 10.1002/mnfr.201700466
144. Rose DJ, Keshavarzian A, Patterson JA, Venkatachalam M, Gillevet P, and Hamaker BR. Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol Nutr Food Res. (2009) 53(1):121–32. doi: 10.1002/mnfr.200800033
145. Timm DA, Stewart ML, Hospattankar A, and Slavin JL. Wheat dextrin, psyllium, and inulin produce distinct fermentation patterns, gas volumes, and short-chain fatty acid profiles in vitro. J Med Food. (2010) 13(4):817–25. doi: 10.1089/jmf.2009.0153
146. Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. (2006) 72(12):7850–6. doi: 10.1128/AEM.01322-06
147. Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, De los Reyes-Gavilán CG, and Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. (2016) 7. doi: 10.3389/fmicb.2016.00185
148. Di T, Chen G, Sun Y, Ou S, Zeng X, and Ye H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J Funct Foods. (2018) 40:727–36. doi: 10.1016/j.jff.2017.10.040
149. Ji ZH, He S, Xie WY, Sen ZP, WZ R, Gao W, et al. Agaricus blazei polysaccharide alleviates DSS-induced colitis in mice by modulating intestinal barrier and remodeling metabolism. Nutrients. (2023) 15(23):4877. doi: 10.3390/nu15234877
150. Xiang XW, Wang R, Yao LW, Zhou YF, Sun PL, Zheng B, et al. Anti-inflammatory effects of mytilus coruscus polysaccharide on raw264.7 cells and dss-induced colitis in mice. Mar Drugs. (2021) 19(7):393. doi: 10.3390/md19070393
151. Huo Z, Li J, Li X, Xiao H, Lin Y, Ma Y, et al. Functional fractions of Astragalus polysaccharides as a potential prebiotic to alleviate ulcerative colitis. Int J Biol Macromol. (2024) 271:132580. doi: 10.1016/j.ijbiomac.2024.132580
152. Ma Y, Xie H, Xu N, Li M, Wang L, Ge H, et al. Large yellow tea polysaccharide alleviates HFD-induced intestinal homeostasis dysbiosis via modulating gut barrier integrity, immune responses, and the gut microbiome. J Agric Food Chem. (2024) 72(16):17004–16. doi: 10.1021/acs.jafc.4c00616
153. Wu M, Wang Q, Li X, Yu S, Zhao F, Wu X, et al. Gut microbiota-derived 5-hydroxyindoleacetic acid from pumpkin polysaccharides supplementation alleviates colitis via MAPKs-PPARγ/NF-κB inhibition. Int J Biol Macromol. (2024) 264:130385. doi: 10.1016/j.ijbiomac.2024.130385
154. Qin X, Nong K, Liu Z, Fang X, Zhang B, Chen W, et al. Regulation of the intestinal flora using polysaccharides from Callicarpa nudiflora Hook to alleviate ulcerative colitis and the molecular mechanisms involved. Int J Biol Macromol. (2024) 258:128887. doi: 10.1016/j.ijbiomac.2023.128887
155. Guo X, Liu L, Zhao W, Li X, Wang X, Ning A, et al. The protective effect of Schisandra chinensis (Turcz.) Baill. polysaccharide on DSS-induced ulcerative colitis in mice via the modulation of gut microbiota and inhibition of NF-κB activation. J Sci Food Agric. (2024) 104(8):2844–56. doi: 10.1002/jsfa.12974
156. Fu YP, Li CY, Peng X, Wangensteen H, Inngjerdingen KT, and Zou YF. Pectic polysaccharides from Aconitum carmichaelii leaves protects against DSS-induced ulcerative colitis in mice through modulations of metabolism and microbiota composition. Biomedicine Pharmacotherapy. (2022) 155:113767. doi: 10.1016/j.biopha.2022.113767
157. Li Q, Wu W, Fang X, Chen H, Han Y, Liu R, et al. Structural characterization of a polysaccharide from bamboo (Phyllostachys edulis) shoot and its prevention effect on colitis mouse. Food Chem. (2022) 387:132807. doi: 10.1016/j.foodchem.2022.132807
158. Zou MY, Wang YJ, Liu Y, Xiong SQ, Zhang L, and Wang JH. Huangshan floral mushroom polysaccharide ameliorates dextran sulfate sodium-induced colitis in mice by modulating th17/treg balance in a gut microbiota-dependent manner. Mol Nutr Food Res. (2023) 67(5):e2200408. doi: 10.1002/mnfr.202200408
159. Zhang Y, Yu S, Liu J, Liu Y, Luo J, and Liu Y. A polysaccharide from Allium tenuissimum L. flowers relieves ulcerative colitis by regulating the inflammatory signaling pathway and gut microbiota. Food Funct. (2023) 14(11):5279–92. doi: 10.1039/D3FO01434A
160. Fan L, Zhu X, Zhang D, Li D, and Zhang C. In vitro digestion properties of Laiyang pear residue polysaccharides and it counteracts DSS-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Int J Biol Macromol. (2024) 267:131482. doi: 10.1016/j.ijbiomac.2024.131482
161. Qi M, Chu S, Wang W, Fu X, Jiang C, Zhang L, et al. Safflower polysaccharide ameliorates acute ulcerative colitis by regulating STAT3/NF-κB signaling pathways and repairing intestinal barrier function. BioMed Pharmacother. (2024) 174:116553. doi: 10.1016/j.biopha.2024.116553
162. Peng S, Lu X, Lin F, Mao N, Yu L, Zhu T, et al. Rosa laevigata Polysaccharides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis of Beagles through Regulating Gut Microbiota. Chem Biodivers. (2024) 21(3):e2302102. doi: 10.1002/cbdv.202302102
163. Qin L, Xu H, Cao J, Wang K, Zhang L, Yao M, et al. Alleviative effects of sulfated polysaccharide from Ishige Okamurae against DSS-induced ulcerative colitis via inhibiting inflammation and modulating gut microbiota. Int J Biol Macromol. (2024) 268:131915. doi: 10.1016/j.ijbiomac.2024.131915
164. Feng Y, Chen S, Song Y, Liu S, Duan Y, Cai M, et al. A novel Sagittaria sagittifolia L. polysaccharides mitigate DSS-induced colitis via modulation of gut microbiota and MAPK/NF-κB signaling pathways. Int J Biol Macromol. (2024) 254:130745. doi: 10.1016/j.ijbiomac.2024.130745
165. Xu D, Wu Q, Liu W, Hu G, Meng H, and Wang J. Therapeutic efficacy and underlying mechanisms of Gastrodia elata polysaccharides on dextran sulfate sodium-induced inflammatory bowel disease in mice: Modulation of the gut microbiota and improvement of metabolic disorders. Int J Biol Macromol. (2023) 248:125919. doi: 10.1016/j.ijbiomac.2023.125919
166. Jiang K, Wang D, Su L, Liu X, Yue Q, Zhang S, et al. Tamarind seed polysaccharide hydrolysate ameliorates dextran sulfate sodium-induced ulcerative colitis via regulating the gut microbiota. Pharmaceuticals. (2023) 16(8):1133. doi: 10.3390/ph16081133
167. Ouyang Q, Li X, Liang Y, and Liu R. Sea buckthorn polysaccharide ameliorates colitis. Nutrients. (2024) 16(9):1280. doi: 10.3390/nu16091280
168. Lv H, Jia H, Cai W, Cao R, Xue C, and Dong N. Rehmannia glutinosa polysaccharides attenuates colitis via reshaping gut microbiota and short-chain fatty acid production. J Sci Food Agric. (2023) 103(11):5077–86. doi: 10.1002/jsfa.12326
169. Guo M, Xing D, Wang J, Zhang Y, Li Z, and Jiao X. Potent intestinal mucosal barrier enhancement of nostoc commune vaucher polysaccharide supplementation ameliorates acute ulcerative colitis in mice mediated by gut microbiota. Nutrients. (2023) 15(13):3054. doi: 10.3390/nu15133054
170. Cui L, He N, Yu S, Pang H, Zhang Z, Wang J, et al. Polysaccharides from paecilomyces hepiali prevent acute colitis in association with modulating gut microbiota and treg/th17 immune balance in mice. Molecules. (2023) 28(13):4984. doi: 10.3390/molecules28134984
171. Ma M, Fu T, Wang Y, Zhang A, Gao P, Shang Q, et al. Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Mar Drugs. (2022) 20(9):582. doi: 10.3390/md20090582
172. Zeeshan M, Ali H, Khan S, Khan SA, and Weigmann B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int J Pharmaceutics. (2019) 558:117814. doi: 10.1016/j.ijpharm.2018.12.074
173. Luo Y and Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromolecules. (2014) 64:267–76. doi: 10.1016/j.ijbiomac.2013.12.017
174. Joseph MM, Aravind SR, George SK, Varghese S, and Sreelekha TT. A galactomannan polysaccharide from Punica granatum imparts in vitro and in vivo anticancer activity. Carbohydr Polym. (2013) 98(2):1271–8. doi: 10.1016/j.carbpol.2013.07.023
175. Hu Q, Lu Y, and Luo Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr Polymers. (2021) 264:117999. doi: 10.1016/j.carbpol.2021.117999
176. Hu Q and Luo Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int J Biol Macromolecules. (2021) 179:637–53. doi: 10.1016/j.ijbiomac.2021.02.216
177. Ahmed HB. Recruitment of various biological macromolecules in fabrication of gold nanoparticles: Overview for preparation and applications. Int J Biol Macromolecules. (2019) 140:1189–206. doi: 10.1016/j.ijbiomac.2019.08.138
178. Hua S, Marks E, Schneider JJ, and Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine: Nanotechnology Biology Med. (2015) 11(10):1525–43. doi: 10.2217/nnm.15.29
179. Zhang S, Langer R, and Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. (2017) 16:118–36. doi: 10.1016/j.nantod.2017.08.006
180. dos Santos AM, Meneguin AB, Akhter DT, Fletcher N, Houston ZH, Bell C, et al. Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. Eur J Pharmaceutics Biopharmaceutics. (2021) 158:220–30. doi: 10.1016/j.ejpb.2020.12.004
181. Zu M, Ma Y, Cannup B, Xie D, Jung Y, Zhang J, et al. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Advanced Drug Delivery Rev. (2021) 176:113887. doi: 10.1016/j.addr.2021.113887
182. Song Q, Wang Y, Huang L, Shen M, Yu Y, Yu Q, et al. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res Int. (2021) 140:109858. doi: 10.1016/j.foodres.2020.109858
183. Kotla NG, Rana S, Sivaraman G, Sunnapu O, Vemula PK, Pandit A, et al. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Advanced Drug Delivery Rev. (2019) 146:297–325. doi: 10.1016/j.addr.2018.06.021
184. Moine L, Canali MM, Porporatto C, and Correa SG. Reviewing the biological activity of chitosan in the mucosa: Focus on intestinal immunity. Int J Biol Macromolecules. (2021) 189:816–30. doi: 10.1016/j.ijbiomac.2021.08.098
185. Wang X, Wang X, Jiang H, Cai C, Li G, Hao J, et al. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr Polymers. (2018) 195:496–508. doi: 10.1016/j.carbpol.2017.10.049
186. Tong AJ, Hu RK, Wu LX, Lv XC, Li X, Zhao LN, et al. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J Food Biochem. (2020) 44(10):e13421. doi: 10.1111/jfbc.13421
187. Hu L, Sun Y, and Wu Y. Advances in chitosan-based drug delivery vehicles. Nanoscale. (2013) 5(19):9223–37. doi: 10.1039/c3nr00338h
188. Garcia-Fuentes M and Alonso MJ. Chitosan-based drug nanocarriers: Where do we stand? J Controlled Release. (2012) 161(2):461–73. doi: 10.1016/j.jconrel.2012.03.015
189. Zhou P, Wang L, An S, Wang C, Jiang Q, and Li X. Fabrication of quercetin-loaded nanoparticles based on Hohenbuehelia serotina polysaccharides and their modulatory effects on intestinal function and gut microbiota in vivo. Innovative Food Sci Emerging Technologies. (2022) 78:102993. doi: 10.1016/j.ifset.2022.102993
190. Priya S, Batra U, Samshritha RN, Sharma S, Chaurasiya A, and Singhvi G. International Journal of Biological Macromolecules Polysaccharide-based nanofibers for pharmaceutical and biomedical applications : A review. Int J Biol Macromol. (2022) 218:1174–93. doi: 10.1016/j.ijbiomac.2022.08.021
191. Zhao X, Zhang X, Tie S, Hou S, Wang H, Song Y, et al. Facile synthesis of nano-nanocarriers from chitosan and pectin with improved stability and biocompatibility for anthocyanins delivery: An in vitro and in vivo study. Food Hydrocoll. (2020) 109:106114. doi: 10.1016/j.foodhyd.2020.106114
192. Dong L, Du H, Zhang M, Xu H, Pu X, Chen Q, et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytotherapy Res. (2022) 36:36(7):3044–57. doi: 10.1002/ptr.7429
193. Qi S, Luo R, Han X, Nie W, Ye N, Fu C, et al. pH/ROS dual-sensitive natural polysaccharide nanoparticles enhance ‘One stone four birds’ Effect of rhein on ulcerative colitis. ACS Appl Mater Interfaces. (2022) 14(45):50644–57. doi: 10.1021/acsami.2c17827
194. Tan C, Fan H, Ding J, Han C, Guan Y, Zhu F, et al. ROS-responsive nanoparticles for oral delivery of luteolin and targeted therapy of ulcerative colitis by regulating pathological microenvironment. Mater Today Bio. (2022) 14. doi: 10.1016/j.mtbio.2022.100246
195. Piotrowska M, Swierczynski M, Fichna J, and Piechota-Polanczyk A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol Res. (2021) 163:105243. doi: 10.1016/j.phrs.2020.105243
196. Zhuang S, Zhong J, Zhou Q, Zhong Y, Liu P, and Liu Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int Immunopharmacol. (2019) 71:310–8. doi: 10.1016/j.intimp.2019.03.030
197. Peng SN, Zeng HH, Fu AX, Chen XW, and Zhu QX. Effects of rhein on intestinal epithelial tight junction in IgA nephropathy. World J Gastroenterol. (2013) 19(26):4137–44. doi: 10.3748/wjg.v19.i26.4137
198. Rostami E. Recent achievements in sodium alginate-based nanoparticles for targeted drug delivery. Polymer Bull. (2022) 79:6885–904. doi: 10.1007/s00289-021-03781-z
199. Petreska Ivanovska T, Zhivikj Z, Mladenovska K, and Petrushevska-Tozi L. Influence of oligofructose-enriched inulin on survival of microencapsulated Lactobacillus casei 01 and adhesive properties of synbiotic microparticles. Macedonian Pharm Bulletin. (2015) 61(1):23–31. doi: 10.33392/mpb.2015.61.01.04
200. Ivanovska TP, Mladenovska K, Zhivikj Z, Pavlova MJ, Gjurovski I, Ristoski T, et al. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int J Pharm. (2017) 527(1-2):216–25. doi: 10.1016/j.ijpharm.2017.05.049
201. Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Diaz-Ropero MP, et al. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rats colitis. World J Gastroenterol. (2005) 11(46):7329–35. doi: 10.3748/wjg.v11.i46.7329
202. Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, and Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol Ther. (2015) 149:115–33. doi: 10.1016/j.pharmthera.2014.12.006
203. Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, and Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. (2015) 1(11):1071–80. doi: 10.1021/acsbiomaterials.5b00181
204. Kotla NG, Isa ILM, Rasala S, Demir S, Singh R, Baby BV, et al. Modulation of gut barrier functions in ulcerative colitis by hyaluronic acid system. Advanced Sci. (2022) 9(12):2103189. doi: 10.1002/advs.202103189
205. Wei F, Lang Y, Shen Q, Xu L, Cheng N, Chu Y, et al. Osteopontin-loaded PLGA nanoparticles enhance the intestinal mucosal barrier and alleviate inflammation via the NF-κB signaling pathway. Colloids Surf B Biointerfaces. (2020) 190:110952. doi: 10.1016/j.colsurfb.2020.110952
206. Lee Y, Sugihara K, Gillilland MG, Jon S, Kamada N, and Moon JJ. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. (2020) 19. doi: 10.1038/s41563-019-0462-9
207. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504(7480):446–50. doi: 10.1038/nature12721
208. Geier MS, Butler RN, Giffard PM, and Howarth GS. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int J Food Microbiol. (2007) 114(2):182–90. doi: 10.1016/j.ijfoodmicro.2006.09.018
209. Maldonado Galdeano C and Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. (2006) 13(2):219–26. doi: 10.1128/CVI.13.2.219-226.2006
210. Madsen KL, Doyle JS, Jewell LD, Tavernini MM, and Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. (1999) 116(4):944–53. doi: 10.1016/S0016-5085(99)70013-2
211. Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, et al. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther. (2012) 35(10):1232–40. doi: 10.1111/j.1365-2036.2011.04939.x
212. Zocco MA, Dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. (2006) 23(10):1465–72. doi: 10.1111/j.1365-2036.2006.02927.x
213. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology. (2004) 126(6):1620–33. doi: 10.1053/j.gastro.2004.03.024
214. Kawai K, Kamochi R, Oiki S, Murata K, and Hashimoto W. Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci Rep. (2018) 8(1):17278. doi: 10.1038/s41598-018-28886-w
215. Pan L, Ai X, Fu T, Ren L, Shang Q, Li G, et al. In vitro fermentation of hyaluronan by human gut microbiota: Changes in microbiota community and potential degradation mechanism. Carbohydr Polym. (2021) 269:118313. doi: 10.1016/j.carbpol.2021.118313
216. Hansberry DR, Shah K, Agarwal P, and Agarwal N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus. (2017). doi: 10.7759/cureus.1004
217. Zheng X, Wang B, Tang X, Mao B, Zhang Q, Zhang T, et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: A review. Carbohydr Polymers. (2023) 299:120153. doi: 10.1016/j.carbpol.2022.120153
218. Bosi A, Banfi D, Bistoletti M, Moretto P, Moro E, Crema F, et al. Hyaluronan: A neuroimmune modulator in the microbiota-gut axis. Cells. (2022) 11(20):3245. doi: 10.3390/cells11203245
219. Kim Y and de la Motte CA. The role of hyaluronan treatment in intestinal innate host defense. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.00569
220. Souza AB, de, Chaud MV, Alves TF, Souza JF, and de, Santana MHA. Hyaluronic acid in the intestinal tract: Influence of structure, rheology, and mucoadhesion on the intestinal uptake in rats. Biomolecules. (2020) 10(12):1664. doi: 10.3390/biom10121664
221. de Souza AB, Chaud MV, and Santana MHA. Hyaluronic acid behavior in oral administration and perspectives for nanotechnology-based formulations: A review. Carbohydr Polymers. (2019) 222:115001. doi: 10.1016/j.carbpol.2019.115001
222. Kessler SP, Obery DR, Nickerson KP, Petrey AC, McDonald C, and de la Motte CA. Multifunctional role of 35 kilodalton hyaluronan in promoting defense of the intestinal epithelium. Histochem Cytochem. (2018) 66(1):39–53. doi: 10.1369/0022155417746775
223. Hill DR, Kessler SP, Rho HK, Cowman MK, and de la Motte CA. Specific-sized hyaluronan fragments promote expression of human β-defensin 2 in intestinal epithelium. J Biol Chem. (2012) 287(45):38206–15. doi: 10.1074/jbc.M112.356238
224. Yarley OPN, Kojo AB, Zhou C, Yu X, Gideon A, Kwadwo HH, et al. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides0. Int J Biol Macromolecules. (2021) 183:1457–75. doi: 10.1016/j.ijbiomac.2021.06.072
225. Wu Z, Li H, Zhao X, Ye F, and Zhao G. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohydr Polymers. (2022) 284:119182. doi: 10.1016/j.carbpol.2022.119182
226. Zhang Y, Cui Z, Mei H, Xu J, Zhou T, Cheng F, et al. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr Polym. (2019) 219:447–56. doi: 10.1016/j.carbpol.2019.04.041
227. Zhang Y, Zhou T, Luo L, Cui Z, Wang N, Shu Y, et al. Pharmacokinetics, biodistribution and receptor mediated endocytosis of a natural Angelica sinensis polysaccharide. Artif Cells Nanomed Biotechnol. (2018) 46(8):1663–71. doi: 10.1080/21691401.2017.1421210
228. Dempsey E and Corr SC. Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.954328
229. Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, and Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.578386
230. Wang Y, Chen H, Liu Y, Wu J, Zhou P, Wang Y, et al. PH-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin A4 for the combination therapy against hepatocellular carcinoma. Biomaterials. (2013) 34(34):8741–52. doi: 10.1016/j.biomaterials.2013.05.081
231. Xu Y, Zhu BW, Li X, Li YF, Ye XM, and Hu JN. Glycogen-based pH and redox sensitive nanoparticles with ginsenoside Rh2 for effective treatment of ulcerative colitis. Biomaterials. (2022) 280:121077. doi: 10.1016/j.biomaterials.2021.121077
232. Xu Y, Zhu BW, Sun R, Li X, Wu D, and Hu JN. Colon-targeting angelica sinensis polysaccharide nanoparticles with dual responsiveness for alleviation of ulcerative colitis. ACS Appl Mater Interfaces. (2023) 15(11):15424–37. doi: 10.1021/acsami.3c02128
233. Feng Y, Wu C, Chen H, Zheng T, Ye H, Wang J, et al. Rhubarb polysaccharide and berberine co-assembled nanoparticles ameliorate ulcerative colitis by regulating the intestinal flora. Front Pharmacol. (2023) 14. doi: 10.3389/fphar.2023.1184183
234. Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vázquez U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. (2019) 9(1):41738. doi: 10.1038/s41598-019-41738-5
235. Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, et al. Defective intestinal mucin-type O-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology. (2016) 151(2):343–55.e345. doi: 10.1053/j.gastro.2016.03.039
236. Larsson JMH, Karlsson H, Crespo JG, Johansson MEV, Eklund L, Sjövall H, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflammation Bowel Dis. (2011) 17(11):2352–63. doi: 10.1002/ibd.21625
237. Liu Y, Wang C, Li J, Li T, Zhang Y, Liang Y, et al. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition. FASEB J. (2020) 34(10):13647–62. doi: 10.1096/fj.201901943RR
238. Bai X, Feng Z, Peng S, Zhu T, Jiao L, Mao N, et al. Chitosan-modified Phellinus igniarius polysaccharide PLGA nanoparticles ameliorated inflammatory bowel disease. Biomaterials Adv. (2022) 139:213002. doi: 10.1016/j.bioadv.2022.213002
239. Kim Y, West GA, Ray G, Kessler SP, Petrey AC, Fiocchi C, et al. Layilin is critical for mediating hyaluronan 35 kDa-induced intestinal epithelial tight junction protein ZO-1 in vitro and in vivo. Matrix Biol. (2018) 66:128–41. doi: 10.1016/j.matbio.2017.09.003
240. Niu YF, Wang HF, Fu J, Pan Y, and Liu ZH. Hyaluronic acids protects against gastric infection. Prog Biochem Biophysics. (2018) 45(4):411–8. doi: 10.13375/j.cnki.pbcs.2018.04.011
241. Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. (2019) 25(3):455–63. doi: 10.1038/s41591-018-0308-z
242. Mirsepasi-Lauridsen HC, Vallance BA, Krogfelt KA, and Petersen AM. Escherichia coli pathobionts associated with inflammatory bowel disease. Clin Microbiol Rev. (2019) 32(4):e00060–18. doi: 10.1128/CMR.00060-18
243. Zhang T, Ji X, Lu G, and Zhang F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl Microbiol Biotechnol. (2021) 105(13):5161–73. doi: 10.1007/s00253-021-11453-1
244. Deng Y, Ma F, Ruiz-Ortega LI, Peng Y, Tian Y, He W, et al. Fabrication of strontium Eucommia ulmoides polysaccharides and in vitro evaluation of their osteoimmunomodulatory property. Int J Biol Macromol. (2019) 140:1194–203. doi: 10.1016/j.ijbiomac.2019.08.145
245. Li Q, Feng Y, He W, Wang L, Wang R, Dong L, et al. Post-screening characterisation and in vivo evaluation of an anti-inflammatory polysaccharide fraction from Eucommia ulmoides. Carbohydr Polym. (2017) 169:427–35. doi: 10.1016/j.carbpol.2017.04.034
246. Zhao Y and Jiang Q. Roles of the polyphenol-gut microbiota interaction in alleviating colitis and preventing colitis-associated colorectal cancer. Adv Nutr. (2021) 12(3):898–917. doi: 10.1093/advances/nmaa104
247. Li C, Zhang W, Wu X, Cai Q, Tan Z, Hong Z, et al. Aromatic-turmerone ameliorates DSS-induced ulcerative colitis via modulating gut microbiota in mice. Inflammopharmacology. (2022) 30(3):877–91. doi: 10.1007/s10787-022-01007-w
248. Ye R, Guo Q, Huang J, Wang Z, Chen Y, and Dong Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J Nanobiotechnology. (2023) 21(1):196. doi: 10.1186/s12951-023-01965-5
249. Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. (2006) 131(6):1779–87. doi: 10.1053/j.gastro.2006.04.020
250. Yao D, Dai W, Dong M, Dai C, and Wu S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine. (2021) 74:103751. doi: 10.1016/j.ebiom.2021.103751
251. Mutalik S, Suthar NA, Managuli RS, Shetty PK, Avadhani K, Kalthur G, et al. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int J Biol Macromol. (2016) 86:724–33. doi: 10.1016/j.ijbiomac.2015.11.092
252. Bhol KC and Schechter PJ. Effects of nanocrystalline silver (NPI 32101) in a rat model of ulcerative colitis. Dig Dis Sci. (2007) 52(8):2081–8. doi: 10.1007/s10620-006-9738-4
253. Kaur G, Singh SK, Kumar R, Kumar B, Kumari Y, Gulati M, et al. Development of modified apple polysaccharide capped silver nanoparticles loaded with mesalamine for effective treatment of ulcerative colitis. J Drug Delivery Sci Technol. (2020) 60:101980. doi: 10.1016/j.jddst.2020.101980
254. Li Y, Fan L, Tang T, Tang Y, Xie M, Zeng X, et al. Modified apple polysaccharide prevents colitis through modulating IL-22 and IL-22BP expression. Int J Biol Macromol. (2017) 103:1123–32. doi: 10.1016/j.ijbiomac.2017.05.172
255. Ala M and Kheyri Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition. (2021) 85:111153. doi: 10.1016/j.nut.2021.111153
256. Zhu C, Zhang S, Song C, Zhang Y, Ling Q, Hoffmann PR, et al. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J Nanobiotechnology. (2017) 15(1):25. doi: 10.1186/s12951-017-0252-y
257. Hadji H and Bouchemal K. Advances in the treatment of inflammatory bowel disease: Focus on polysaccharide nanoparticulate drug delivery systems. Advanced Drug Delivery Rev. (2022) 181:114101. doi: 10.1016/j.addr.2021.114101
258. Mo X, Guo D, Jiang Y, Chen P, and Huang L. Isolation, structures and bioactivities of the polysaccharides from Radix Hedysari: A review. Int J Biol Macromolecules. (2022) 199:667–80. doi: 10.1016/j.ijbiomac.2021.12.095
259. Ji X, Peng B, Ding H, Cui B, Nie H, and Yan Y. Purification, structure and biological activity of pumpkin polysaccharides: A review. Food Rev Int. (2023) 39(4):677–704. doi: 10.1080/87559129.2021.1904973
260. Jiang F, Ding Y, Tian Y, Yang R, Quan M, Tong Z, et al. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomaterials Adv. (2022) 133:112637. doi: 10.1016/j.msec.2021.112637
261. Mao YH, Xu YX, Li YH, Cao J, Song FL, Zhao D, et al. Effects of konjac glucomannan with different molecular weights on gut microflora with antibiotic perturbance in in vitro fecal fermentation. Carbohydr Polym. (2021) 273:118546. doi: 10.1016/j.carbpol.2021.118546
Keywords: inflammatory bowel disease, gut microbiota, polysaccharides, nanoparticles, gut metabolites
Citation: Yang J, Huang Q, Long J and Li J (2025) Microbiota and inflammatory bowel disease: the dual effect mechanism of polysaccharide therapy. Front. Immunol. 16:1666866. doi: 10.3389/fimmu.2025.1666866
Received: 16 July 2025; Accepted: 06 October 2025;
Published: 31 October 2025.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Keyu Ren, The Affiliated Hospital of Qingdao University, ChinaYong Lai, Sichuan Academy of Traditional Chinese Medicine, China
Copyright © 2025 Yang, Huang, Long and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li, MjQ2MzMzNjZAcXEuY29t
 Jihao Yang1
Jihao Yang1