- 1Department of Pharmacy, Guangxi Hospital Division of The First Affiliated Hospital, Sun Yat-Sen University, Nanning, Guangxi, China
- 2School of Pharmaceutical Science, Guangxi Medical University, Nanning, Guangxi, China
- 3Department of Pharmacy, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 4State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu, China
- 5School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: Immune checkpoint inhibitor (ICI) and targeted therapy (TT) combinations have emerged as promising first-line treatments for unresectable or advanced hepatocellular carcinoma (u/aHCC), leveraging synergistic anti-tumor effects. However, the comparative efficacy and safety of ICI-TT regimens versus sorafenib or lenvatinib (S/L) monotherapy require further elucidation across larger patient populations. This meta-analysis synthesizes data from phase 3 trials to evaluate the clinical benefits and risks of first-line ICI-TT combination therapy in u/aHCC.
Methods: We conducted systematic searches in PubMed and major oncology conference proceedings up to June 10, 2025. Eligible studies were randomized phase 3 trials comparing first-line ICI-TT versus S/L monotherapy in u/aHCC. Efficacy outcomes included progression-free survival (PFS), overall survival (OS) (summarized as hazard ratios [HRs] with 95% confidence intervals [CIs]), and objective response rate (ORR) (evaluated using odds ratios [ORs]). Safety outcomes assessed grade 3–5 treatment-related adverse events (TRAEs) and serious TRAEs, reported as relative risks (RRs).
Results: Eight phase 3 trials (IMbrave150, ORIENT-32, COSMIC-312, CARES-310, LEAP-002, SCT-I10A-C301, HEPATORCH, APOLLO) involving 4,379 patients were included. Compared with S/L monotherapy, ICI-TT combination therapy demonstrated significantly improved ORR (OR 3.93; 95% CI 2.64–5.85), PFS (HR 0.62; 95% CI 0.54–0.71), and OS (HR 0.71; 95% CI 0.62–0.82). The risk of grade 3–5 TRAEs was not significantly increased with combination therapy (RR 1.13; 95% CI 0.96–1.33). However, combination therapy was associated with a significantly higher risk of serious TRAEs (RR 1.97; 95% CI 1.50–2.60).
Conclusion: First-line ICI-plus-TT combination therapy demonstrates superior efficacy in ORR, PFS, and OS compared to S/L monotherapy for u/aHCC, without a significant increase in grade 3–5 TRAEs. Clinicians should be aware of the elevated risk of serious TRAEs associated with combination regimens. These findings support ICI-TT as a preferred first-line strategy for eligible patients, although individualized risk-benefit assessment remains crucial.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251053588.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and ranks among the leading causes of cancer-related mortality worldwide (1, 2). It typically arises in the setting of chronic liver disease, including hepatitis B or C virus (HBV/HCV) infection, alcoholic liver disease, and non-alcoholic fatty liver disease, frequently on a background of cirrhosis (1). Despite advances in early detection and the development of locoregional therapies, a substantial proportion of patients are diagnosed at an unresectable or advanced stage, for which systemic therapy remains the mainstay of treatment (1, 3–5).
Historically, the multi-kinase inhibitors sorafenib and lenvatinib constituted the standard first-line therapies for unresectable or advanced HCC (u/aHCC), providing modest survival benefits with median overall survival (OS) ranging from 12 to 15 months (6, 7). However, prognosis remained poor and durable responses were uncommon.
A major paradigm shift occurred with the advent of immune checkpoint inhibitor (ICI)-based combination therapies, particularly those co-targeting the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) axis and vascular endothelial growth factor (VEGF) signaling. The landmark IMbrave150 trial was the first to demonstrate a significant OS benefit with the combination of atezolizumab and bevacizumab compared to sorafenib, achieving a median OS of 19.2 months and a 34% reduction in the risk of death (8, 9). This established a new standard of care and catalyzed the development of various ICI-based therapeutic strategies.
Subsequent phase 3 trials, however, have yielded mixed results. Studies such as ORIENT-32 (sintilimab plus bevacizumab biosimilar) (10), CARES-310 (camrelizumab plus apatinib) (11), SCT-I10A-C301 (Finotonlimab plus bevacizumab biosimilar) (12, 13), APOLLO (penpulimab plus anlotinib) (14), and HEPATORCH (toripalimab plus bevacizumab) (15) have all reported significant survival benefits, reinforcing the potential of dual ICI and VEGF blockade. Conversely, trials such as COSMIC-312 (atezolizumab plus cabozantinib) and LEAP-002 (pembrolizumab plus lenvatinib) failed to meet their primary endpoints for progression-free survival (PFS) and/or OS (16, 17). Additionally, many of these individual trials were not sufficiently powered to evaluate treatment effects in clinically relevant subgroups.
Several systematic reviews and meta-analyses have previously addressed the efficacy of ICI-based combinations in advanced HCC (18–23). These studies reinforced the paradigm shift initiated by IMbrave150, but several important gaps remain. None of the existing meta-analyses integrated the most up-to-date phase 3 trials (e.g., APOLLO, HEPATORCH, SCT-I10A-C301), nor did they provide comprehensive subgroup analyses across sex, etiology, tumor burden, AFP, ECOG status, and prior local therapy.
To address these gaps, we conducted the largest and most current meta-analysis to date, synthesizing phase 3 randomized controlled trials (RCTs) comparing ICI plus targeted therapy (TT) combinations versus sorafenib or lenvatinib (S/L) monotherapy in patients with u/aHCC. The primary aims of this study were to provide an updated comprehensive evaluation of the efficacy and safety of first-line ICI-TT combination therapy and to quantify the magnitude of benefit across different clinically relevant subpopulations, thereby informing clinical decision-making and guiding future therapeutic development.
Methods
Protocol and reporting guidelines
This research protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD420251053588) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (24).
Information sources and search strategy
A systematic search of PubMed and major oncology conference proceedings was conducted to comprehensively identify all relevant phase 3 trials published up to June 10, 2025. Search terms included 'immune checkpoint inhibitors,' 'targeted therapy,' 'hepatocellular carcinoma,' as well as the names of specific therapeutic agents. The complete search strategies are detailed in Supplementary Table 1.
Selection criteria
To be included in the meta-analysis, studies were required to meet the following criteria: (i) phase 3 RCTs comparing a combination of ICI and TT with S/L monotherapy; (ii) enrollment of patients with previously untreated u/aHCC; and (iii) availability of survival outcome data, including hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Studies were excluded if they: (i) were not phase 3 RCTs; (ii) did not use S/L as the control arm; (iii) included ICI monotherapy or did not incorporate an ICI in the experimental arm; or (iv) were ongoing trials without published results at the time of the literature search. Only studies that met all inclusion criteria were incorporated into the meta-analysis.
Data collection and assessment of risk of bias
Data from all included studies were extracted and summarized by one investigator and independently verified by a second reviewer. Where available, the following information was collected: trial name, year of publication, sample size, treatment regimens, HRs with corresponding 95% CIs for PFS and OS, the number of patients who achieved complete and partial responses, and the incidence of grade 3–5 and severe treatment-related adverse events (TRAEs). Grade 3–5 TRAEs refer to the severity of adverse events as graded by the Common Terminology Criteria for Adverse Events (CTCAE). Grade 3 events are severe, Grade 4 are life-threatening, and Grade 5 are fatal. Serious TRAEs refer to the medical significance of the event, regardless of its CTCAE grade. A serious TRAE is defined by regulatory criteria as any event that results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect. In addition, study design characteristics were collected to assess the risk of bias for each included trial. The risk of bias was evaluated in accordance with the Cochrane Risk of Bias Assessment Tool (25).
Statistical analysis
Pooled estimates were generated using either fixed-effects or random-effects models, depending on the degree of heterogeneity observed. Statistical heterogeneity was assessed using the I² statistic and Cochrane’s Q test, with heterogeneity considered significant when I² exceeded 50% and the Q test p-value was less than 0.1. Specifically, DerSimonian-Laird random-effects models were employed for outcomes with substantial variability, whereas Mantel-Haenszel fixed-effects models were applied when homogeneity was strongly supported by the data.
To evaluate therapeutic efficacy, pooled HRs with corresponding 95% CIs were calculated for PFS and OS, while odds ratios (ORs) with 95% CIs were computed for objective response rate (ORR). For safety assessments, relative risks (RRs) with 95% CIs for AEs were determined on a per-study basis to provide a comprehensive evaluation. Given the limited statistical power to definitively rule out heterogeneity in subgroup analyses, random-effects models were applied in these analyses.
Potential publication bias was examined using funnel plots and Egger’s test. Sensitivity analyses were performed using a leave-one-out approach to evaluate the robustness of the pooled estimates. All statistical analyses were conducted using R software (version 4.5.1), with a two-tailed p-value of < 0.05 considered indicative of statistical significance.
Results
Eight phase 3 trials with 4,379 patients were included
The literature search identified 64 records, of which eight trials met the eligibility criteria and were included in the final analysis (8–12, 14–17, 26). The PRISMA flow diagram illustrating the study selection process is presented in Supplementary Figure 1.
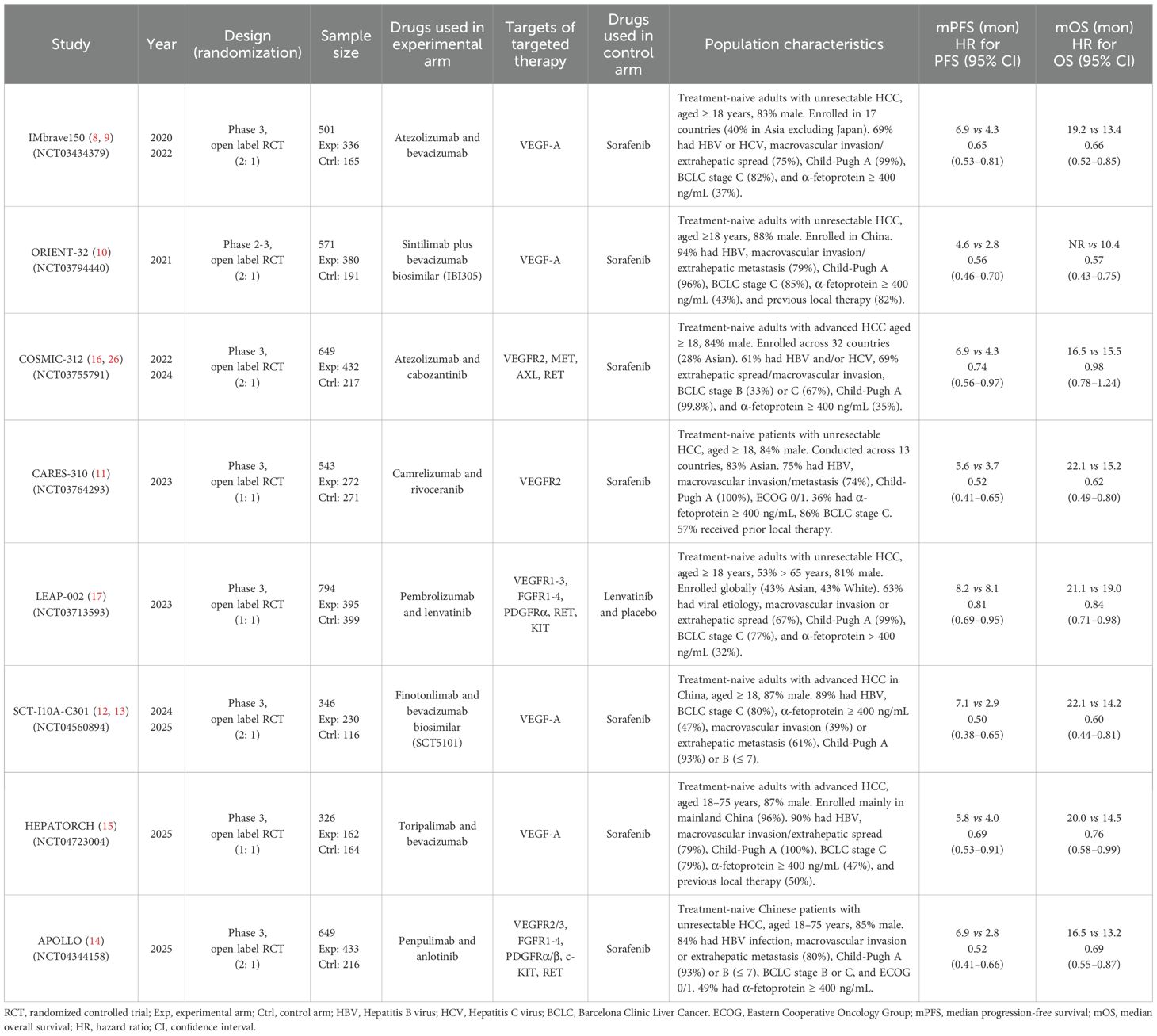
Among the eight included trials, all employed an open-label design. A total of 4,379 patients with u/aHCC were enrolled, of whom 2,640 (60.3%) received a combination of ICIs and TT, while 1,739 (39.7%) received S/L monotherapy. The ICI used in the experimental arms included atezolizumab (anti–PD-L1), sintilimab, camrelizumab, pembrolizumab, SCT-I10A, toripalimab, and penpulimab (all anti–PD-1 agents). The targeted therapies administered in the combination regimens comprised bevacizumab, bevacizumab biosimilars (IBI305 and SCT5101), cabozantinib, rivoceranib, lenvatinib, and anlotinib. In all trials, the control arms consisted of S/L monotherapy. Detailed characteristics of each trial are summarized in Table 1. Thus, the analysis included a substantial cohort of patients from globally conducted trials, providing a robust foundation for the subsequent efficacy and safety evaluations.
ICI-TT combination therapy significantly improved ORR, PFS, and OS
ORR data, derived from 4,328 patients, showed a nearly fourfold increase in response with ICI-TT compared to S/L monotherapy (OR, 3.93; 95% CI, 2.64–5.85; Figure 1). These data indicate that ICI-TT combination therapy markedly enhances the tumor response rate compared to S/L monotherapy. PFS and OS data were available from all eight trials, encompassing a total of 4,379 patients. The pooled HR for PFS demonstrated a significant benefit with ICI-TT compared to S/L monotherapy (HR, 0.62; 95% CI, 0.54–0.71; Figure 2), corresponding to a 38% relative reduction in the risk of disease progression. This represents a statistically significant and clinically meaningful reduction in the risk of disease progression. For OS, the combined HR indicated a 29% reduction in the risk of death with ICI-TT (HR, 0.71; 95% CI, 0.62–0.82; Figure 3). The consistency in the direction and magnitude of benefit across response and survival endpoints strongly supports the superior efficacy of ICI-TT combinations. Collectively, these results demonstrate that first-line ICI-TT therapy provides substantial and consistent improvements in both tumor control and overall survival for patients with u/aHCC.
![Forest plot displaying odds ratios for eight studies comparing ICI plus TT versus S/L. Each study has odds ratios with 95% confidence intervals and weights. Combined odds ratio is 3.93 with a confidence interval of [2.64; 5.85]. Random effects model shows significant heterogeneity (I squared = 75.1%, p = 0.0002).](https://www.frontiersin.org/files/Articles/1667793/fimmu-16-1667793-HTML/image_m/fimmu-16-1667793-g001.jpg)
Figure 1. Forest plot of objective response rate comparing immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy in patients with hepatocellular carcinoma.
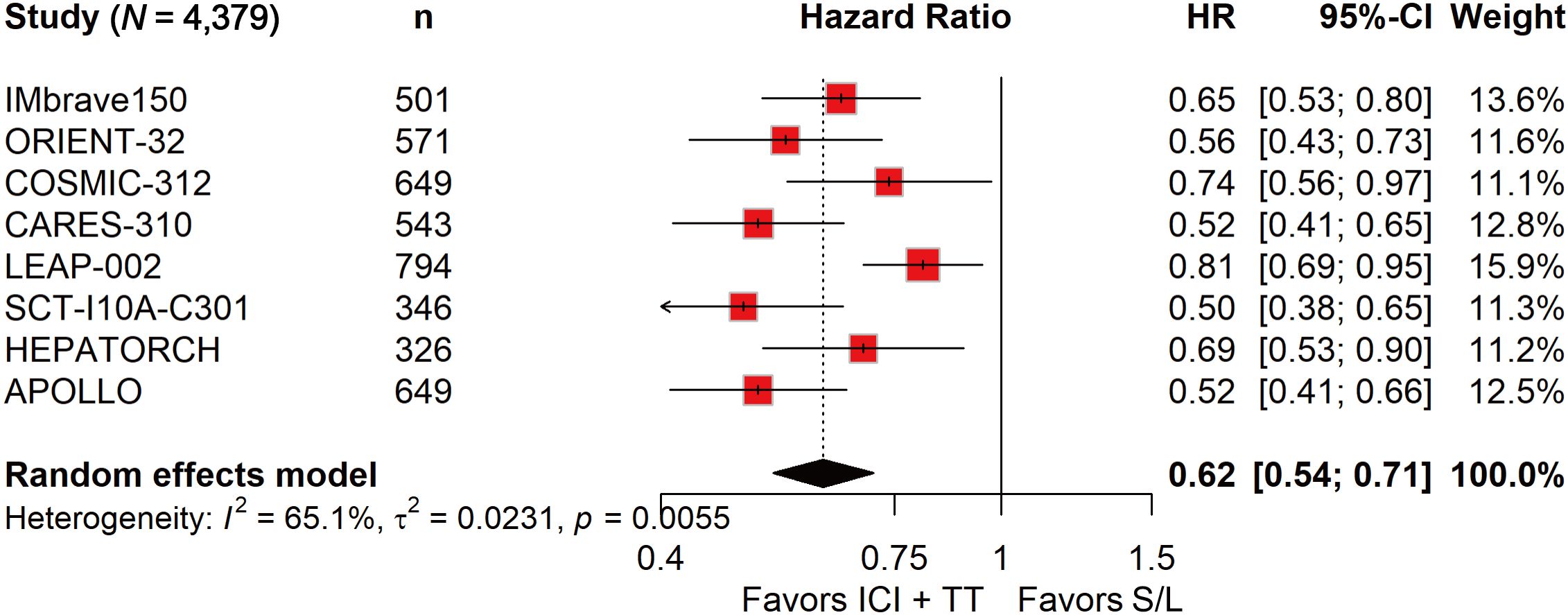
Figure 2. Forest plot of progression-free survival comparing immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy in patients with hepatocellular carcinoma.
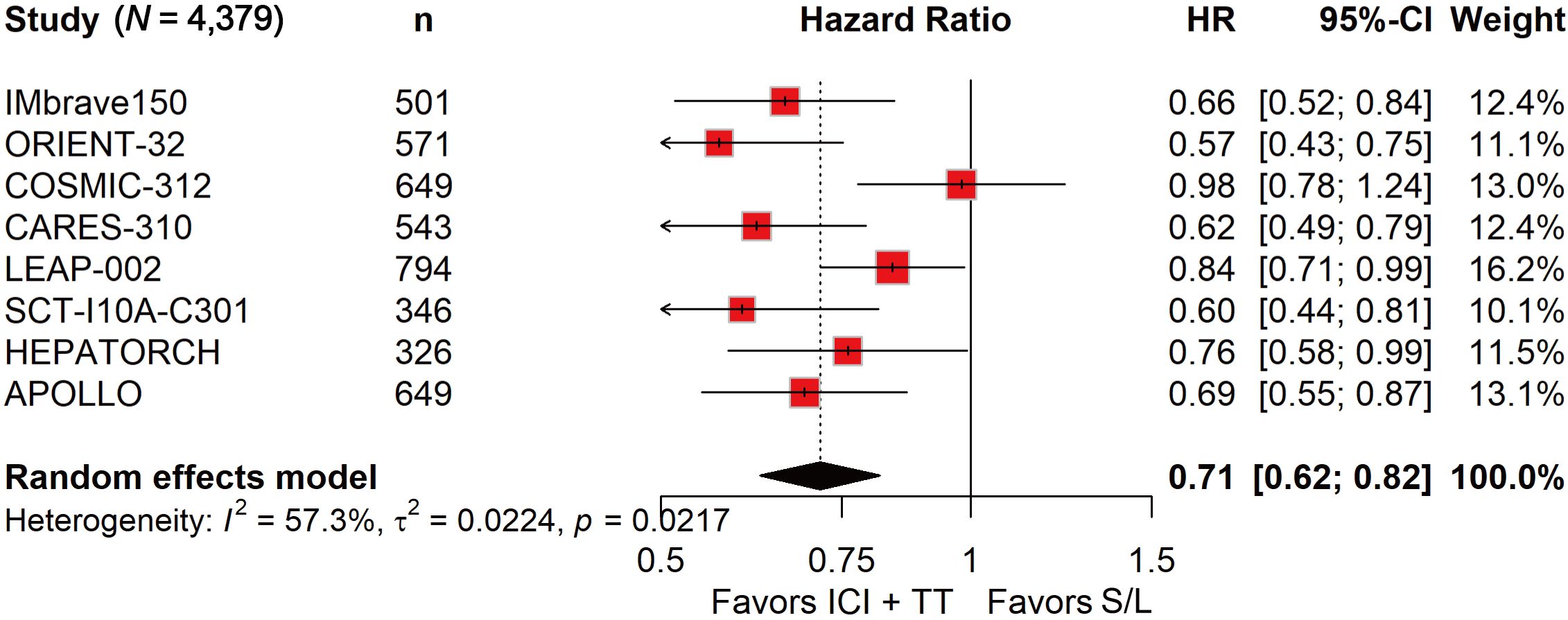
Figure 3. Forest plot of overall survival comparing immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy in patients with hepatocellular carcinoma.
The risk of serious TRAEs was higher with ICI-TT, while grade 3–5 TRAEs were comparable
Among the 2,629 patients who received ICI in combination with TT, 1,349 (51.3%) experienced grade 3–5 TRAEs, compared to 798 of 1,703 patients (46.9%) treated with S/L monotherapy. The pooled RR indicated that the ICI-TT combination did not significantly increase the risk of grade 3–5 AEs (RR, 1.13; 95% CI, 0.96–1.33; Figure 4). Safety data on serious TRAEs were available from all eight trials. The incidence of serious TRAEs was 21.1% (556/2,629) in the ICI-TT group versus 11.5% (196/1,703) in the S/L monotherapy group. Pooled analysis revealed a significantly higher risk of serious TRAEs in patients receiving ICI-TT compared to those on S/L monotherapy (RR, 1.97; 95% CI, 1.50–2.60; Figure 5). In summary, while the risk of grade 3–5 TRAEs was comparable between groups, combination therapy was associated with a significantly higher incidence of serious TRAEs. This underscores the necessity of vigilant monitoring and proactive management when administering ICI-TT regimens, particularly in patients with borderline organ function.
![Forest plot showing results from eight studies comparing ICI plus TT against S/L in terms of risk ratio and percentages. COSMIC-312 shows the highest risk ratio at 1.66. The overall risk ratio is 1.13 with a ninety-five percent confidence interval of [0.96; 1.33]. Heterogeneity is high (I-squared = 84.6%). Red squares represent study data; the diamond indicates the overall effect estimate.](https://www.frontiersin.org/files/Articles/1667793/fimmu-16-1667793-HTML/image_m/fimmu-16-1667793-g004.jpg)
Figure 4. Forest plot of grade 3–5 treatment-related adverse events comparing immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy in patients with hepatocellular carcinoma.
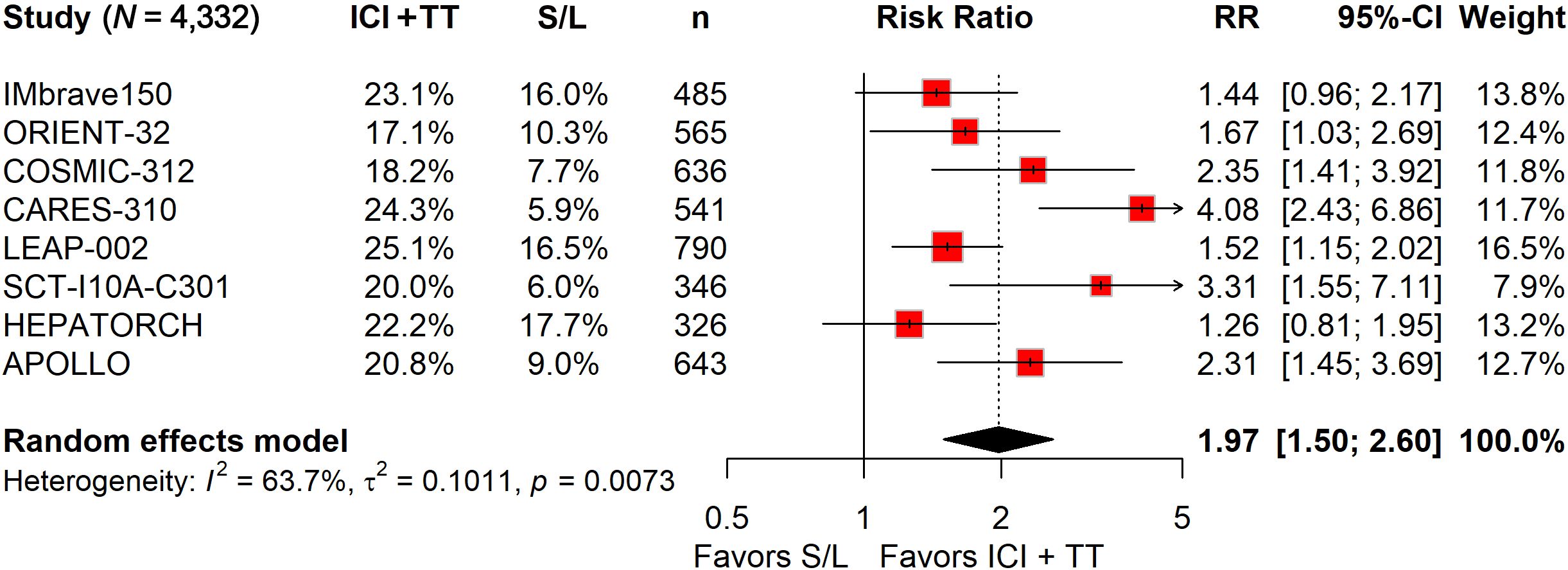
Figure 5. Forest plot of serious treatment-related adverse events comparing immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy in patients with hepatocellular carcinoma.
Subgroup analyses suggest heterogeneous benefits across sex, etiology, and disease burden
Given the limited power of individual studies to evaluate diverse clinically relevant subgroups, we conducted a series of subgroup analyses to better characterize the efficacy of ICI combined with TT in specific patient populations and to inform individualized precision treatment. The specific details of these subgroup analyses, including efficacy outcomes in different patient populations, are presented in Table 2.
Age-stratified subgroup analysis showed that ICI plus TT significantly prolonged PFS and OS in both patients younger than 65 years (PFS: HR, 0.63, 95% CI, 0.52–0.76; OS: HR, 0.80, 95% CI, 0.66–0.97) and those aged ≥65 years (PFS: HR, 0.61, 95% CI, 0.48–0.76; OS: HR, 0.74, 95% CI, 0.62–0.89) (Supplementary Figure 2).
Stratification by sex revealed that male patients derived significant benefits in both PFS (HR, 0.60; 95% CI, 0.53–0.67) and OS (HR, 0.74; 95% CI, 0.64–0.86) from ICI plus TT. For female patients, only PFS (HR, 0.69; 95% CI, 0.50–0.96) improvement was observed (Supplementary Figure 3).
Analysis by Eastern Cooperative Oncology Group (ECOG) status showed consistent efficacy regardless of performance status. For PFS: ECOG 0 (HR 0.63, 95% CI 0.55–0.72) and ECOG 1 (HR 0.59, 95% CI 0.49–0.70); for OS: ECOG 0 (HR 0.79, 95% CI 0.66–0.93) and ECOG 1 (HR 0.67, 95% CI 0.58–0.76) (Supplementary Figure 4).
Stratification by α-fetoprotein (AFP) level indicated significant PFS (AFP <400 ng/mL: HR 0.60, 95% CI 0.51–0.72; AFP ≥400 ng/mL: HR 0.57, 95% CI 0.44–0.72) and OS (AFP <400 ng/mL: HR 0.71, 95% CI 0.58–0.87; AFP ≥400 ng/mL: HR 0.70, 95% CI 0.60–0.81) benefits across all AFP levels (Supplementary Figure 5).
Patients with Barcelona Clinic Liver Cancer (BCLC) stage B showed significant PFS benefit from ICI plus TT (HR, 0.70; 95% CI, 0.54–0.90), whereas no significant OS improvement was detected. For stage C, significant improvements were observed in both PFS (HR, 0.58; 95% CI, 0.51–0.65) and OS (HR, 0.72; 95% CI, 0.64–0.81) (Supplementary Figure 6).
Among patients with HBV-related HCC, ICI plus TT significantly prolonged both PFS (HR, 0.55; 95% CI, 0.49–0.61) and OS (HR, 0.66; 95% CI, 0.59–0.74). For HCV-related cases, only PFS (HR, 0.63; 95% CI, 0.42–0.95) was significantly improved, with no OS benefit observed. Patients with non-viral etiology showed no significant improvements in either endpoint (Supplementary Figure 7).
Macrovascular invasion (MVI) status did not affect efficacy, with significant PFS (no MVI: HR, 0.64; 95% CI, 0.56–0.74; MVI: HR, 0.53; 95% CI 0.44–0.64) and OS (no MVI: HR, 0.78; 95% CI, 0.66–0.91; MVI: HR, 0.65; 95% CI, 0.56–0.76) benefits observed regardless of MVI presence (Supplementary Figure 8).
Subgroup analysis of extrahepatic spread (EHS) confirmed that ICI plus TT significantly improves both PFS and OS regardless of EHS status: for PFS, benefits were observed in patients without EHS (HR, 0.65, 95% CI, 0.55–0.78) and those with EHS (HR, 0.58, 95% CI, 0.51–0.66); for OS, significant benefits were also seen in patients without EHS (HR, 0.79, 95% CI, 0.64–0.97) and with EHS (HR, 0.71, 95% CI, 0.62–0.82) (Supplementary Figure 9).
Combined MVI/EHS status analysis revealed significant PFS benefit in patients without these features (HR, 0.72; 95% CI; 0.57–0.90) and with either/both features (HR, 0.57; 95% CI, 0.51–0.64). OS benefit was significant only in patients with either/both features (HR, 0.70; 95% CI, 0.63–0.79) (Supplementary Figure 10).
Prior local therapy history did not influence outcomes, with significant PFS (no prior: HR, 0.59; 95% CI, 0.48–0.73; prior: HR, 0.59; 95% CI, 0.49–0.71) and OS (no prior: HR, 0.65; 95% CI, 0.54–0.78; prior: HR, 0.66; 95% CI, 0.49–0.90) benefits observed irrespective of treatment history (Supplementary Figure 11).
Taken together, subgroup findings suggest potential heterogeneity of benefit by etiology, sex, and tumor burden. However, these analyses were exploratory and underpowered, and therefore should be interpreted as hypothesis-generating rather than definitive.
Risk of bias and sensitivity analysis
The risk of bias across the included trials is graphically summarized in Supplementary Figures 12, Supplementary Figures 13. All eight studies were randomized, open-label trials with investigator-assessed outcomes and pre-specified analysis strategies. As a result, all studies were deemed to have a low risk of selection, detection, attrition, and reporting bias, but a high risk of performance bias due to the open-label design. Funnel plots and Egger’s tests revealed no evidence of publication bias (Supplementary Figure 14). Sensitivity analyses confirmed the robustness of the pooled results (Supplementary Figure 15). Overall, the included studies demonstrated a low risk of bias in key domains, and the primary findings were robust upon sensitivity analysis. The main limitation, consistent across all trials, was the high risk of performance bias inherent to the open-label design.
Discussion
This comprehensive meta-analysis of eight phase 3 RCTs, encompassing 4,379 patients with u/aHCC, provides robust evidence supporting the superiority of first-line ICI plus TT combinations over S/L monotherapy. The key findings demonstrate significant improvements in ORR (OR 3.93), PFS (HR 0.62), and OS (HR 0.71) with combination therapy. While the risk of grade 3–5 TRAEs was not significantly elevated, the 97% increase in serious TRAEs (RR 1.97) underscores the need for careful patient selection and vigilant monitoring. These findings consolidate the evolving role of ICI-based regimens as the cornerstone of systemic therapy in u/aHCC and highlight the need for thoughtful patient selection to balance efficacy with tolerability.
Our findings are broadly consistent with previous meta-analyses that established the superiority of ICI-TT combinations over sorafenib or lenvatinib. Nonetheless, our study provides incremental value in three key aspects. First, it incorporates the most comprehensive dataset to date, pooling eight phase 3 RCTs with 4,379 patients, whereas earlier reviews included only four to six trials. Second, it integrates the latest evidence from recently reported global and regional phase 3 trials (HEPATORCH, APOLLO, SCT-I10A-C301), which were not available in prior analyses and substantially expand the evidence base. Third, it offers the most detailed subgroup evaluation to date, spanning etiology, sex, AFP levels, ECOG status, BCLC stage, and prior therapy. By critically situating our work within the existing literature, we highlight how our analysis extends beyond confirmatory findings to generate clinically relevant, hypothesis-generating insights.
Compared with prior studies, Li et al. synthesized four phase 3 RCTs and confirmed that PD-1/PD-L1 inhibitors plus antiangiogenic agents improved OS and PFS versus sorafenib, but their work was limited by smaller sample size and fewer subgroup data (20). Zhou et al. performed a network meta-analysis comparing anti-PD-1/L1 plus VEGF antibody with anti-PD-1/L1 plus VEGFR-TKI, but they did not focus on pooled head-to-head evidence against sorafenib or lenvatinib (21). Fulgenzi et al. also compared novel first-line strategies, identifying atezolizumab plus bevacizumab as a benchmark, but their dataset was restricted to studies published before early 2022 (18). Zhu et al. included five RCTs and confirmed improved OS and PFS with PD-1/PD-L1 plus anti-angiogenic therapy, although their analysis lacked the most recently completed phase 3 trials (23). Li et al. conducted a large-scale network meta-analysis involving 17 trials, but their scope extended beyond immunotherapy-based regimens and incorporated HAIC-FO and TACE-based strategies, limiting the specificity of conclusions for ICI-TT (19). She et al. addressed a similar question, but their work did not integrate the newest phase 3 evidence (22).
The observed 38% reduction in progression risk and 29% reduction in mortality risk establish ICI-TT regimens as the new therapeutic benchmark, surpassing historical outcomes achieved with tyrosine kinase inhibitor (TKI) monotherapy. The near-quadrupling of objective response rates holds particular clinical relevance for patients requiring rapid symptom control or tumor downstaging. Importantly, the absence of a significant increase in grade 3–5 TRAEs suggests manageable toxicity profiles in experienced centers. However, the substantially elevated risk of serious TRAEs necessitates rigorous patient assessment, especially for those with compromised liver function (Child-Pugh B), significant portal hypertension, or autoimmune comorbidities. This safety-efficacy balance forms the cornerstone of clinical decision-making when considering combination therapy.
The biological rationale for successful ICI-TT synergy centers on reversing the characteristically immunosuppressive tumor microenvironment of HCC (27, 28). VEGF blockade normalizes the aberrant tumor vasculature, enhancing T-cell infiltration while simultaneously reducing immunosuppressive regulatory T cells and myeloid-derived suppressor cells (29). PD-1/PD-L1 inhibition then reinvigorates exhausted CD8+ T-cells and enhances antigen presentation (30).
Subgroup analyses revealed heterogeneous responses across patient populations, offering critical guidance for personalized treatment. PFS benefits were consistent across age groups, with significant improvements observed in both patients <65 years (HR 0.63) and those ≥65 years (HR 0.61). For OS, significant benefits were also demonstrated in both younger (HR 0.80) and older (HR 0.74) patients, supporting the efficacy of combination therapy across the adult age spectrum, provided liver function and performance status are adequate. Male patients derived significant benefits in both PFS (HR 0.60) and OS (HR 0.74). In contrast, female patients showed a significant improvement in PFS (HR 0.69) but not in OS. These findings warrant cautious interpretation given the smaller female sample size and possible sex-related immunological differences, underscoring the need for further exploration of sex-specific tumor-immune dynamics. Consistent efficacy across ECOG 0 and 1 patients reinforces the generalizability of ICI-TT benefits across a broad spectrum of functional status. Furthermore, patients with high AFP (≥400 ng/mL) demonstrated significant PFS and OS benefits comparable to those with low AFP, challenging the notion that AFP is a negative prognostic marker for immunotherapy (31).
Etiology-specific analyses yielded clinically relevant patterns. Patients with HBV-related HCC experienced robust improvements in both PFS (HR 0.55) and OS (HR 0.66), solidifying ICI-TT as the preferred first-line approach for this population. For HCV-related HCC, a significant PFS benefit (HR 0.63) was observed, but this did not translate into a significant OS improvement. Most notably, patients with non-viral HCC (predominantly NAFLD/NASH etiology) showed no significant improvements in either PFS or OS. This suggests distinct tumor biology in metabolic syndrome-driven hepatocarcinogenesis, characterized by profoundly immunosuppressive features including CD8+ T-cell exhaustion, altered gut microbiota, and fibrotic stroma (32, 33).
Tumor burden and aggressiveness features further refined benefit predictions. Patients with BCLC stage B disease derived a significant PFS benefit (HR 0.70) but not a significant OS improvement, possibly due to effective crossover to second-line therapies after progression. Conversely, those with stage C disease demonstrated significant improvements in both PFS (HR 0.58) and OS (HR 0.72), highlighting the particular value of combination therapy in biologically aggressive disease. The presence of MVI or EHS were significant determinants of therapeutic benefit. Significant OS improvement was confined to patients with MVI (HR 0.65) and/or EHS (HR 0.71)—populations with historically dismal prognoses—supporting early, aggressive combination therapy in these high-risk subgroups. The analysis of prior local therapy revealed consistent PFS and OS benefits regardless of treatment history, suggesting ICI-TT combinations can overcome resistance to prior interventions, potentially by reversing therapy-induced immunosuppression.
Our findings have significant clinical implications. First, they reinforce ICI-TT combinations as the preferred first-line treatment option for eligible patients with u/aHCC, aligning with recent guideline updates. Second, the identification of subgroups with differential benefit supports a more nuanced, precision medicine approach, emphasizing the need for biomarkers predictive of response and toxicity. Third, the elevated risk of serious AEs calls for the development of standardized management protocols and patient education to optimize safety.
Despite the strengths of this meta-analysis, including the large pooled sample size and rigorous methodology, several limitations merit discussion. First, the heterogeneity among included regimens–encompassing diverse combinations of PD-1/PD-L1 inhibitors with VEGF monoclonal antibodies or TKIs–inherently assumes class effects that may not uniformly hold across specific agents. Furthermore, all included trials employed open-label designs, introducing potential performance and reporting bias despite pre-specified outcome assessment protocols. Moreover, subgroup analyses, while informative, were statistically underpowered for smaller populations such as non-viral HCC patients or females Additionally, variable follow-up durations across trials, with mature OS data unavailable for newer studies like APOLLO and HEPATORCH, may affect long-term outcome assessments. Finally, systematic exclusion of Child-Pugh B patients from most of the included trials limits generalizability to this clinically important population with compromised hepatic function.
Future research directions should address these knowledge gaps while building upon current successes. Biomarker development remains paramount, as PD-L1 expression demonstrates inconsistent predictive value in HCC. Emerging candidates including tumor mutational burden, inflammatory gene signatures, and gut microbiome profiles require rigorous validation. Early on-treatment biomarkers such as AFP response (defined as ≥20% decline at week 6) show promise as early efficacy indicators (34, 35). Novel agents warrant exploration, particularly bispecific antibodies (e.g., anti-PD-1/VEGF inhibitors). For the challenging non-viral HCC subgroup, TGF-β inhibitors merit investigation to counteract the profound stromal immunosuppression characteristic of these tumors. Sequencing strategies post-progression represents another critical knowledge gap, requiring prospective evaluation of optimal therapeutic approaches after ICI-TT failure. Finally, given the substantial costs of combination immunotherapies, rigorous cost-effectiveness analyses are essential to ensure global accessibility and sustainability of these advances.
Conclusion
This meta-analysis solidifies ICI-TT combinations as the cornerstone of first-line therapy for u/aHCC, demonstrating unequivocal superiority over S/L monotherapy across survival and response endpoints. The consistent benefit observed across most clinically relevant subgroups–particularly those with high-risk features including MVI, EHS, and HBV etiology–supports broad applicability in routine practice. However, the significantly increased risk of serious TRAEs necessitates stringent patient selection criteria. Future efforts must prioritize biomarker-driven personalization, novel combination strategies for non-viral HCC subtypes, and interventions to enhance safety without compromising efficacy. As the therapeutic landscape continues to evolve, this comprehensive analysis provides a robust foundation for evidence-based clinical decision-making while underscoring the transformative impact of immunotherapy integration in HCC management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YXL: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. YHL: Data curation, Formal Analysis, Writing – original draft. BL: Data curation, Formal Analysis, Writing – original draft. JS: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors extended their appreciation to the databases that provided the invaluable data resources for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to polish the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1667793/full#supplementary-material
References
1. Singal AG, Kanwal F, and Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2023) 20:203–22. doi: 10.1038/s41575-022-00704-9
4. Sangro B, Kudo M, Erinjeri JP, Qin S, Ren Z, Chan SL, et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): a multiregional, randomised, double-blind, placebo-controlled, phase 3 study. Lancet. (2025) 405:216–32. doi: 10.1016/S0140-6736(24)02551-0
5. Kudo M, Ren Z, Guo Y, Han G, Lin H, Zheng J, et al. Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): a multicentre, randomised, double-blind, phase 3 study. Lancet. (2025) 405:203–15. doi: 10.1016/S0140-6736(24)02575-3
6. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
7. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
8. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
9. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
10. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
11. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
12. Xu J, Zhang Y, Wang G, Chen W, Zhuang L, Gu S, et al. SCT-I10A combined with a bevacizumab biosimilar (SCT510) versus sorafenib in the first-line treatment of advanced hepatocellular carcinoma: A randomized phase 3 trial. J Clin Oncol. (2024) 42:4092. doi: 10.1200/JCO.2024.42.16_suppl.4092
13. Zhao C, Zhang Y, Wang G, Zheng J, Chen W, Lu Z, et al. Finotonlimab (PD-1 inhibitor) plus bevacizumab (bevacizumab biosimilar) as first-tier therapy for late-stage hepatocellular carcinoma: a randomized phase 2/3 trial. Signal Transduct Target Ther. (2025) 10:249. doi: 10.1038/s41392-025-02333-5
14. Zhou J, Bai L, Luo J, Bai Y, Pan Y, Yang X, et al. Anlotinib plus penpulimab versus sorafenib in the first-line treatment of unresectable hepatocellular carcinoma (APOLLO): a randomised, controlled, phase 3 trial. Lancet Oncol. (2025) 26:719–31. doi: 10.1016/S1470-2045(25)00190-1
15. Shi Y, Han G, Zhou J, Shi X, Jia W, Cheng Y, et al. Toripalimab plus bevacizumab versus sorafenib as first-line treatment for advanced hepatocellular carcinoma (HEPATORCH): a randomised, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. (2025) 10:658–70. doi: 10.1016/S2468-1253(25)00059-7
16. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2022) 23:995–1008. doi: 10.1016/S1470-2045(22)00326-6
17. Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1399–410. doi: 10.1016/S1470-2045(23)00469-2
18. Fulgenzi CAM, D'Alessio A, Airoldi C, Scotti L, Demirtas CO, Gennari A, et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: A network metanalysis of phase III trials. Eur J Cancer. (2022) 174:57–67. doi: 10.1016/j.ejca.2022.06.058
19. Li J, Yang B, Teng Z, Liu Y, Li D, and Qu X. Efficacy and safety of first-line treatments for advanced hepatocellular carcinoma patients: a systematic review and network meta-analysis. Front Immunol. (2024) 15:1430196. doi: 10.3389/fimmu.2024.1430196
20. Li J, Liao C, Liu Z, Xiong H, Cai J, and Liu T. PD-1/PD-L1 inhibitors plus antiangiogenic drugs versus sorafenib as the first line treatment for advanced hepatocellular carcinoma: A phase 3 RCTs based meta-analysis. Technol Cancer Res Treat. (2024) 23:15330338241305700. doi: 10.1177/15330338241305700
21. Zhou Y, Li J, and Ying J. Anti-PD-1/L1 antibody plus anti-VEGF antibody vs. plus VEGFR-targeted TKI as first-line therapy for unresectable hepatocellular carcinoma: a network meta-analysis. Explor Target Antitumor Ther. (2024) 5:568–80. doi: 10.37349/etat.2024.00236
22. She M, Wu Y, Cheng M, Feng S, Li G, and Rong H. Efficacy and safety of PD-1/PD-L1 inhibitor-based immune combination therapy versus sorafenib in the treatment of advanced hepatocellular carcinoma: a meta-analysis. Front Med (Lausanne). (2024) 11:1401139. doi: 10.3389/fmed.2024.1401139
23. Zhu H, Zhao W, Chen H, Zhu X, You J, and Jin C. Evaluation of the effectiveness and safety of combining PD-1/PD-L1 inhibitors with anti-angiogenic agents in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Immunol. (2024) 15:1468440. doi: 10.3389/fimmu.2024.1468440
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Yau T, Kaseb A, Cheng AL, Qin S, Zhu AX, Chan SL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): final results of a randomised phase 3 study. Lancet Gastroenterol Hepatol. (2024) 9:310–22. doi: 10.1016/S2468-1253(23)00454-5
27. Chen C, Wang Z, Ding Y, and Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. (2023) 14:1133308. doi: 10.3389/fimmu.2023.1133308
28. Donne R and Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. (2023) 77:1773–96. doi: 10.1002/hep.32740
29. Yang J, Yan J, and Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. (2018) 9:978. doi: 10.3389/fimmu.2018.00978
30. Peña-Asensio J, Calvo H, Torralba M, Miquel J, Sanz-de-Villalobos E, and Larrubia JR. Anti-PD-1/PD-L1 based combination immunotherapy to boost antigen-specific CD8(+) T cell response in hepatocellular carcinoma. Cancers (Basel). (2021) 13:1922. doi: 10.3390/cancers13081922
31. Yeo YH, Lee YT, Tseng HR, Zhu Y, You S, Agopian VG, et al. Alpha-fetoprotein: Past, present, and future. Hepatol Commun. (2024) 8:e0422. doi: 10.1097/HC9.0000000000000422
32. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. (2021) 592:450–6. doi: 10.1038/s41586-021-03362-0
33. Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. (2023) 20:487–503. doi: 10.1038/s41575-023-00754-7
34. Yang Z, Fu Y, Wang Q, Pan Y, Wang J, Chen J, et al. Dynamic changes of serum α-fetoprotein predict the prognosis of bevacizumab plus immunotherapy in hepatocellular carcinoma. Int J Surg. (2025) 111:751–60. doi: 10.1097/JS9.0000000000001860
35. Tian BW, Yan LJ, Ding ZN, Liu H, Meng GX, Xue JS, et al. Early alpha-fetoprotein response predicts prognosis of immune checkpoint inhibitor and targeted therapy for hepatocellular carcinoma: a systematic review with meta-analysis. Expert Rev Gastroenterol Hepatol. (2023) 17:73–83. doi: 10.1080/17474124.2022.2156859
Keywords: immune checkpoint inhibitors, targeted therapy, sorafenib, lenvatinib, hepatocellular carcinoma, meta-analysis
Citation: Lin Y, Liao Y, Luo B and Shen J (2025) First-line immune checkpoint inhibitors plus targeted therapy versus sorafenib or lenvatinib monotherapy for unresectable or advanced hepatocellular carcinoma: a meta-analysis of phase 3 trials. Front. Immunol. 16:1667793. doi: 10.3389/fimmu.2025.1667793
Received: 17 July 2025; Accepted: 30 September 2025;
Published: 15 October 2025.
Edited by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceReviewed by:
Sagnik Giri, University of Arizona, United StatesLiang Shan, Anhui Medical University, China
Copyright © 2025 Lin, Liao, Luo and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhai Shen, c2hlbmpoX3BoYXJtQDEyNi5jb20=
†ORCID: Jinhai Shen, orcid.org/0000-0001-8673-087X
 Yuxuan Lin
Yuxuan Lin Yonghe Liao
Yonghe Liao Bo Luo4
Bo Luo4 Jinhai Shen
Jinhai Shen