- 1Department of Hematology and Oncology, Geriatric Hospital of Nanjing Medical University, Jiangsu Province Geriatric Institute, Jiangsu Province Official Hospital, Nanjing, Jiangsu, China
- 2College of Pharmaceutical Science, Soochow University, Suzhou, Jiangsu, China
- 3Clinical Pharmacology Research Lab, Department of Pharmacy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 4Institute of Neuroscience & Jiangsu Key Laboratory of Neuropsychiatric Diseases, Soochow University, Suzhou, Jiangsu, China
- 5Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, Suzhou, Jiangsu, China
Cancer-associated fibroblasts (CAFs) are significant contributors to the establishment of the immunosuppressive tumor microenvironment (TME) and pose a significant challenge to the effectiveness of successful immunotherapy. CAFs can secrete cytokines, chemokines, and extracellular matrix components; inhibit the invasion of immune cells; promote regulatory cell populations; and induce T cell exclusion phenotypes, thereby lowering the effectiveness of immune checkpoint inhibitors (ICIs). With the development of the field of nanotechnology, increasing studies have paid attention to employing nano-strategies to specifically control and target CAFs. These nanoplatforms can transport therapeutic cargos, e.g., CAF-toxic chemicals, signal regulators, or phenotype-modifying agents, precisely to CAFs, respectively, lowering systemic toxicity. Furthermore, the combination therapy of CAF-targeting nanoparticles and immune checkpoint inhibitors had, in preclinical scenarios, the synergistic effect of promoting T cell infiltration, antigen presentation, and cytotoxicity. However, heterotypic CAF subpopulations, inconsistency of different cancer models, inefficient cargo delivery, and translatability constraints in the clinic are serious challenges. Development of multifunctional and stimulus-active nanomedicine has great potential to overcome these challenges. Initial clinical trials, including fibroblast activation protein (FAP)-targeted CAR-T cells and antibody-drug conjugates, highlight the increasing translational potential of CAF-targeted nano-immunotherapy. This review summarizes the current progress in CAF-targeted nano-immunotherapy, emphasizing that a comprehensive molecular understanding and thorough clinical validation are essential for facilitating its clinical application in the treatment of solid malignancies.
1 Introduction
Immunotherapy has transformed cancer therapy, providing durable tumour response and greatly enhancing survival in some patients (1, 2). Nonetheless, it becomes ineffective as a result of the immunosuppressive tumor immune microenvironment (TIME) as well as complicated immune evasion methods, for various persons (3–6). Immunosuppression by cytokine production, extracellular matrix remodeling, and physical expulsion of immune cells by CAFs, the most dominant stromal element of TIME (7, 8). CAFs obstruct immune cell infiltration, inhibit T cell function, and enhance the expression of immunological checkpoints, thereby diminishing therapeutic responses and encouraging treatment resistance. Targeting CAFs has consequently emerged as a possible technique to enhance the efficacy of immune therapy. Targeting CAFs has thus emerged as a promising strategy to augment immunotherapy efficacy (9–12). Nanotechnology demonstrates advantages in targeting CAFs, facilitating the accurate delivery and targeted modulation of CAFs to enhance therapeutic drug accumulation and specificity within the tumor microenvironment. Utilizing stimuli-responsive nanocarriers enables the targeted release of drugs at disease sites, thereby reducing off-target effects and strengthening the remodeling of the TIME, ultimately improving therapy outcomes. This review highlights recent progress in nanotechnology methods targeting CAFs, focusing on their contributions to enhancing immunotherapy, overcoming therapy resistance, and regulating the TME. Moreover, significant potential and challenges are discussed to provide insights for future translational research.
2 CAFs and their role in immunotherapy resistance
2.1 Origin and heterogeneity of CAFs
CAFs are a highly diverse and heterogeneous group of cells with complex and varied sources (Figure 1A). The majority of CAFs originate from tissue-resident fibroblasts, but there are also some CAFs arising from pericytes, mesenchymal stem cells, and other precursors when tumor-derived signals are activated (13–15). This cellular diversity endows CAFs with noticeable phenotypic and functional heterogeneity, complicating their roles in tumor progression and immune regulation. Classified by function, CAFs can be divided into subtypes such as immunosuppressive CAFs (iCAFs), matrix-generating CAFs (myCAFs), and antigen-presenting CAFs (apCAFs) (16, 17). It is worth noting that CAFs have phenotypic plasticity and can dynamically shift between states induced by cues from TME, thus playing diverse roles in tumor progression and immune modulation (18, 19).
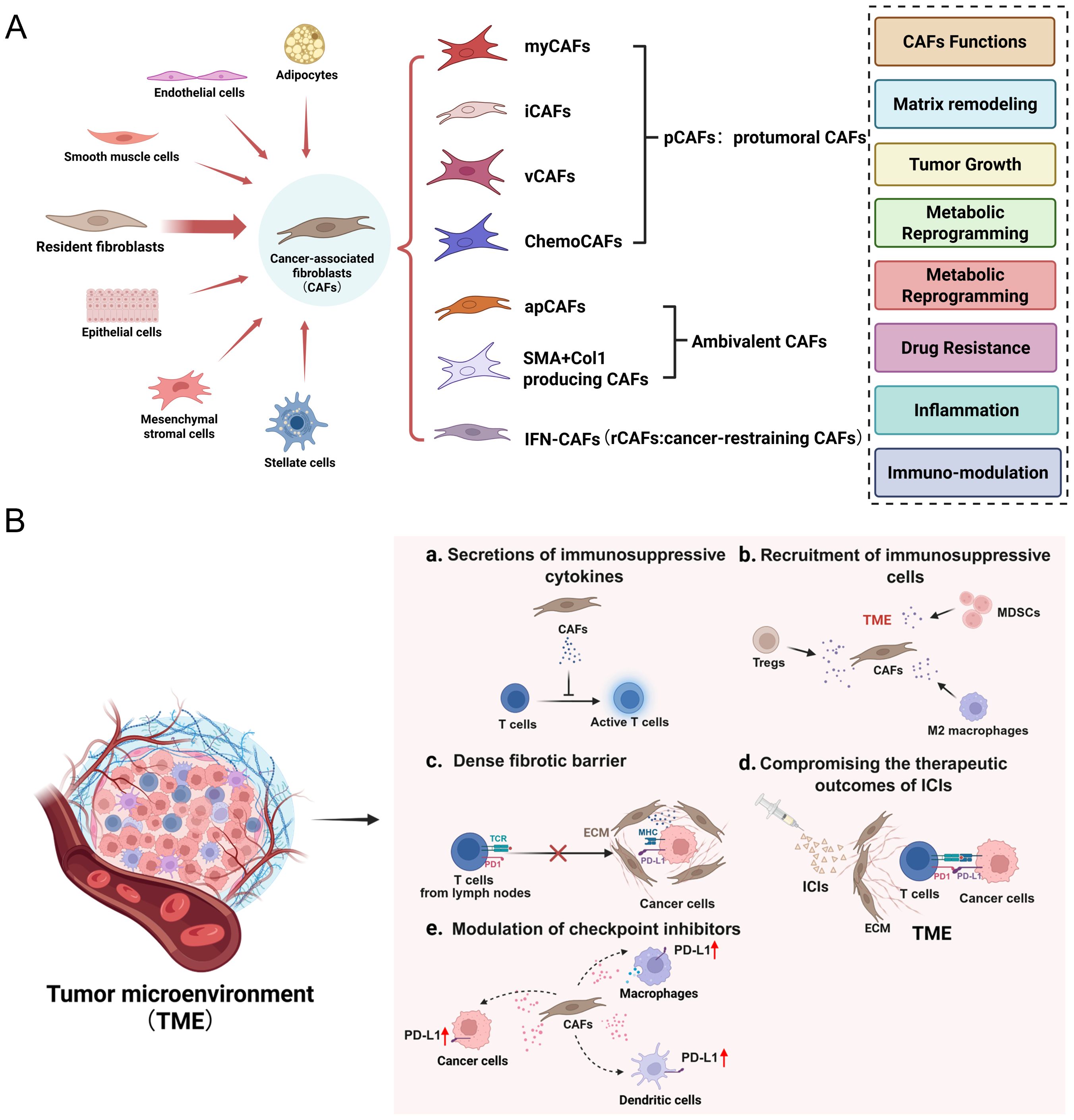
Figure 1. (A) The origin, subtypes, and functions of CAFs in TME. (B) CAFs induce immunosuppressive TME through distinct mechanisms.
2.2 CAF-Mediated Immune Suppression
CAFs contribute to immunosuppression in the TME by secreting immunomodulatory cytokines, remodeling the extracellular matrix, and limiting immune cell infiltration (Figure 1B). CAFs actively reshape the tumor immune microenvironment by secreting immunomodulatory cytokines, including TGF-β, IL-6, and CXCL12. These factors increase T cell exhaustion, promote the expansion of regulatory T cells (Tregs), and reduce effector T cell infiltration, collectively dampening antitumor immunity. In addition, CAF-derived chemokines, including CCL2 and CXCL12, recruit Tregs and myeloid-derived suppressor cells (MDSCs), thereby reinforcing the immunosuppressive milieu (20, 21). Concurrently, CAFs contribute to extracellular matrix (ECM) remodeling by depositing collagen and fibronectin, thereby generating a dense fibrotic barrier that physically restricts T cell access to tumor cores, thereby facilitating immune evasion (22–24).
Among the heterogeneous CAF subpopulations, iCAFs, myCAFs, and apCAFs contribute differently to the immunosuppressive tumor microenvironment. iCAFs (inflammatory CAFs) are characterized by high secretion of cytokines such as IL-6, CXCL12, and TGF-β, which promote the recruitment of immunosuppressive cells like Tregs and MDSCs, and inhibit effector T cell function. myCAFs (myofibroblastic CAFs) primarily produce extracellular matrix components including collagen and fibronectin, leading to physical exclusion of T cells from the tumor core and contributing to desmoplasia. In contrast, apCAFs (antigen-presenting CAFs) express MHC class II molecules and are capable of presenting antigens to CD4+ T cells, but lack co-stimulatory molecules, thereby inducing T cell anergy or tolerance rather than activation. Together, these distinct subtypes synergize to construct an immune-excluded, immunosuppressive microenvironment that impairs effective antitumor immunity.
2.3 CAFs and therapy resistance
CAFs play a crucial role in mediating resistance to immune checkpoint inhibitors, particularly PD-1/PD-L1 blockade. CAFs enhance immunosuppressive signaling by producing TGF-β and other suppressive cytokines, thereby suppressing T cell activation and limiting their infiltration into tumor regions (25–28). CAFs have been shown to upregulate PD-L1 expression on both tumor and immune cells, thereby diminishing the efficacy of PD-1/PD-L1 blockade therapy (29, 30). As a result, tumors enriched with CAFs tend to exhibit resistance to immune checkpoint inhibition. Moreover, CAF-induced ECM reconstruction and cytokine release result in a T-cell exclusion phenotype, in which effector T cells localize at the tumor edge but are unable to penetrate the tumor interior—a major contributor to immunotherapy failure (31).
3 Nanoparticle-based strategies for CAF modulation
3.1 Nano-strategies for targeting CAFs
3.1.1 Overview and classification of nanotechnology in CAF-targeted therapy
Nanotechnology has been created as a disruptive platform for the modulation of the tumor microenvironment and targeted drug delivery. In CAF-targeted therapy, nanoparticles have numerous advantages over traditional methods, including enhanced bioavailability, elongated circulation time, stimulus-responsive release, and spatial confinement within the fibrotic tumor stroma. A variety of nanoplatforms (liposomes, micelles, dendrimers, polymeric nanoparticles, and inorganic nanocarriers) have been designed to deliver small molecules, nucleic acids, or immunomodulatory agents. Active targeting has been applied on these nanosystems through the surface modification by CAF-specific ligands (e.g., FAP antibody, PDGFR-β-binding peptides), which enable selective accumulation in CAF-rich regions. Furthermore, stimulus-responsive nanocarriers (pH, redox potential, or enzyme-activatable) enable on-demand release in the tumor microenvironment. Nanotechnology is equally competent in offering combinatorial delivery of therapeutic agents (e.g., CAF-modulators and immune checkpoint inhibitors) in a single carrier, potentially elevating therapeutic synergy. Such attributes make nanotechnology a multifaceted and effective tool in overcoming CAFs’ biophysical and immunological barriers in solid tumors.
Nanoparticles, with sizes ranging from 1 to 100 nm, exhibit unique physicochemical properties that differ markedly from those of their bulk materials, which is mainly due to their high surface area-to-volume ratio. Owing to their unique physicochemical properties, these materials have been widespread used in fields such as biomedicine, electronics, energy, and environmental science (32, 33). Classification is typically based on composition, structure, or intended function. The following are several commonly used classification approaches.
Based on their composition, nanoparticles are generally divided into three categories: organic, inorganic, and those derived from biological cells (Figure 2A). The organic nanoparticles mainly include micelles, albumin nanoparticles, liposomes, polymeric nanoparticles, dendrimers, nanoemulsions, and nanogels. Inorganic nanoparticles mainly include metal nanoparticles (e.g., Au NPs, Ag NPs), metal oxide nanoparticles (e.g., Fe3O4 NPs, TiO2 NPs), silica nanoparticles (e.g., SiO2 NPs), carbon-based nanoparticles (e.g., carbon nanotubes, graphene, carbon quantum dots), and two-dimensional (2D) nanosheets (e.g., transition metal dichalcogenides such as XS2 and XSe2, and black phosphorus). Cell-derived nanoparticles are primarily composed of membrane vesicles originating from sources such as tumor cells, macrophages, red blood cells, and platelets, including exosomes secreted by animal or plant cells.
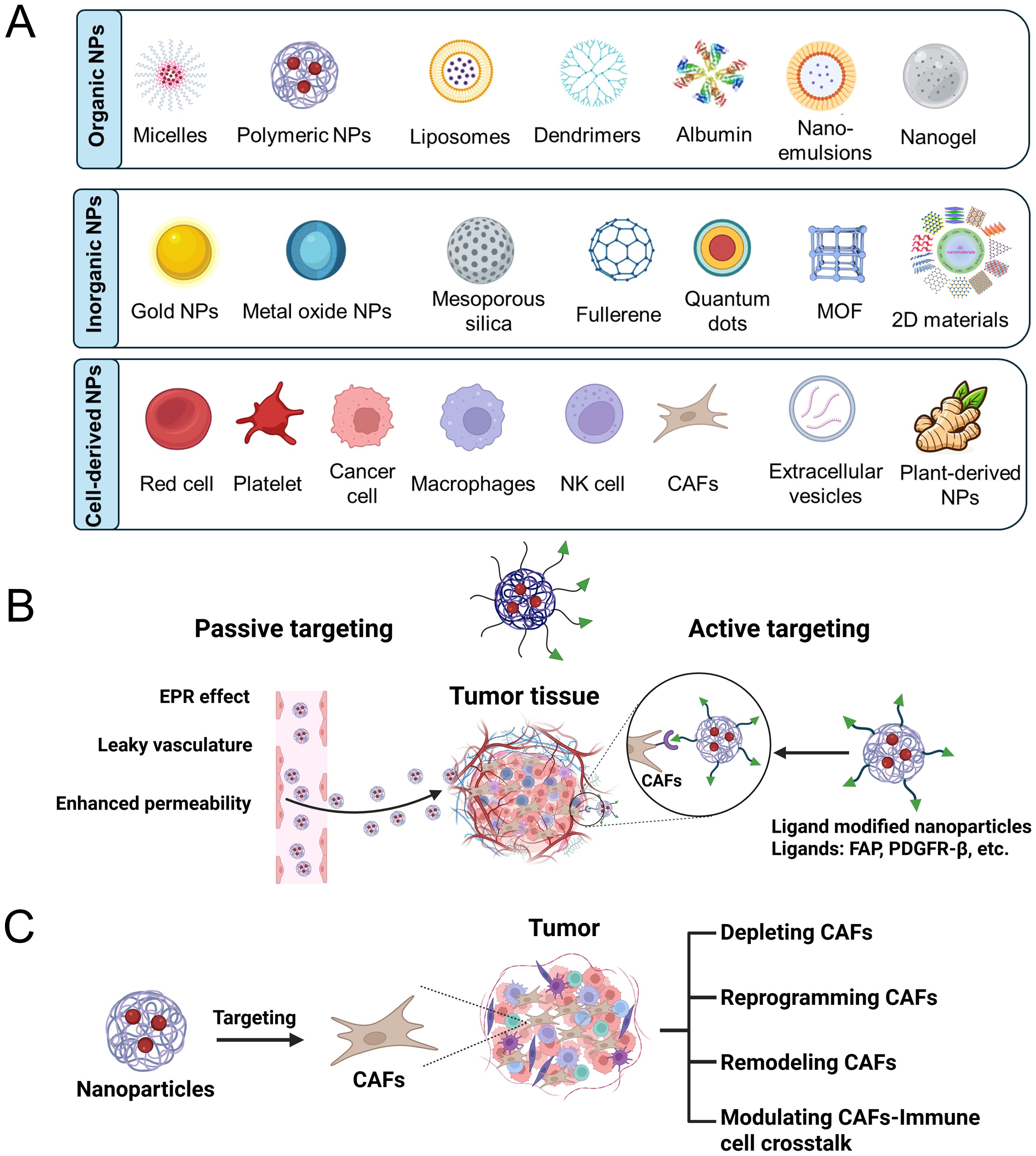
Figure 2. (A) Classification of nanoparticles by their compositional components. (B) Passive and active targeting CAFs by nano-strategies. (C) Modulation of CAFs by nanoparticles.
Based on their structures, nanoparticles can be classified into four types: zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D). 0D nanoparticles include quantum dots and nanodots. 1D nanoparticles include nanowires, nanorods, and nanotubes. 2D nanoparticles consist of materials such as graphene, MXenes, and black phosphorus nanosheets. 3D nanoparticles include porous nanostructures, core-shell structures, and nanogels.
Nanoparticles can be divided into various types based on their stimuli-responsive properties, such as pH-responsive, temperature-responsive, light-responsive, reactive oxygen species (ROS)-responsive, and enzyme-responsive nanoparticles.
3.1.2 Passive and active targeting of CAFs
Passive targeting of CAFs can be achieved with the aid of the enhanced permeability and retention (EPR) effect, which supports nanoparticles accumulating in tumor regions with hyperpermeable vasculature (Figure 2B) (34, 35). Due to their abundant presence in the tumor stroma, CAFs are more likely to encounter elevated concentrations of nanoparticles compared to other stromal cells. Such passive accumulation enables the possibility of regulating CAF activity and preferential delivery of therapeutic agents to the TME (36). However, the diverse and poor perfusion characteristics of the tumor stroma can limit the permeation of nanoparticles, thereby reducing delivery efficiency to CAFs and impairing therapeutic efficacy (37, 38).
Active targeting of CAFs advances beyond the EPR effect by introducing surface ligand modifications on nanoparticles to enhance targeting ability and retention within the tumor stroma (Figure 2C) (39, 40). As FAP, platelet-derived growth factor receptor β (PDGFRβ), and integrins are highly overexpressed on CAFs in various solid tumors, making it a promising target for selective drug delivery (41). Antibodies or peptide ligands with high affinity for these receptors can be used to decorate nanoparticles, thereby achieving selective binding to CAFs through EPR effects (42–44). This dual-targeting approach enhances cellular uptake and therapeutic precision towards CAFs, partially addressing the limitations of passive targeting delivery. Nevertheless, challenges still exist due to the heterogeneity of CAFs and side effects from possible off-target interactions with other stromal or normal fibroblastic cells.
3.2 CAF depletion strategies
CAF depletion nano-strategies often involve the delivery of cytotoxic agents or nucleic acid drugs (such as pDNA, siRNA, mRNA) selectively targeting CAF-associated markers such as FAP or alpha-smooth muscle actin (α-SMA) (45, 46). These strategies enable the specific removal of CAFs or the knockdown of CAF genes, thereby reducing their immunosuppressive effect and reshaping the TME to facilitate immune infiltration. These targeted approaches exert precise effects on CAFs, thus minimizing systemic toxicity and optimizing therapeutic outcomes. However, the total depletion of CAFs may pose potential risks due to their functional heterogeneity, as specific CAF subtypes may either restrain or promote tumor growth (47). Subtype-agnostic targeting strategies can disturb stromal homeostasis, which may lead to augmented tumor invasiveness and impaired therapeutic responses.
3.3 CAF-reprogramming approaches
CAF-reprogramming strategies aim to transform immunosuppressive CAFs into dormant or tumor-suppressing phenotypes by delivering agents such as TGF-β inhibitors, Hedgehog pathway inhibitors, or epigenetic modulators (48–50). Nano-strategies enable the site-specific and sustained delivery of these regulators within the TME, boosting effectiveness while reducing systemic side effects (51–53). This strategy offers a prospective approach to deplete CAFs by maintaining beneficial functions of stroma and alleviating tumorigenic signaling.
Nano-drug delivery systems can convert CAFs to a quiescent state by delivering modulatory molecules that inhibit their activation pathways. For example, TGF-β pathway inhibitors or microRNA-loaded drug delivery nano-systems restore a non-activated, stromal-supportive phenotype by inhibiting pro-fibrotic and immunosuppressive gene expression in CAFs (54–56). This reprogramming strategy attenuates CAF-mediated immune rejection and extracellular matrix (ECM) remodeling, thereby creating a more supportive TME for immunotherapy.
3.4 ECM remodeling and barrier disruption
The newly emerged nano-strategies to degrade ECM, such as collagenase-loaded nanoparticles, represent a potential approach to remodel the fibrotic stroma formed by CAFs. These nano-strategies contribute to the disruption of the physical barriers, which impede T-cell infiltration and restrict diffusion of modulatory agents into the tumor interior, by enzyme-mediated degradation of collagen and other ECM compositions (57, 58). This ECM-modulating approach can remarkably improve the efficacy of nano-immunotherapies by increasing their exposure to tumor cells. However, precise modulation is required to prevent exaggerated ECM degradation, which could potentially facilitate tumor invasion and metastatic progression (59).
3.5 Modulating CAFs-immune cell crosstalk
From the perspective of modulating CAF-immune cell crosstalk, targeting CAF-secreted factors, such as CXCL12, has been shown to alleviate T cell migration barriers and enhance immune cell infiltration within tumors (60). Building on this approach, various nano-delivery platforms have been developed to co-deliver CAF inhibitors and immune checkpoint blockers (ICBs), enabling synergistic effects through CAF reprogramming and immune activation, thereby improving the overall efficacy of immunotherapy.
3.6 Synergistic effects with immunotherapy
Combining CAF-targeting nanoplatforms with ICIs has emerged as a promising strategy to overcome the immunosuppressive tumor microenvironment and enhance therapeutic efficacy (61). By disrupting CAFs-mediated signaling, these nanoplatforms can enhance antigen presentation by dendritic cells and promote the activation and infiltration of cytotoxic T lymphocytes. In parallel, modulation of CAFs activity leads to a reduction in immunosuppressive cell populations such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), further relieving immune suppression. This synergistic approach reprograms both the stromal and immune components of the tumor microenvironment, paving the way for more durable and effective responses to ICIs (62, 63). Examples of combining CAF-targeting nanoplatforms and immunotherapy are shown in Table 1.
Preclinical studies have demonstrated that combining CAF-targeting strategies with ICIs significantly improves antitumor responses by enhancing T cell infiltration and reducing stromal barriers. For instance, blockade of the CXCL12/CXCR4 axis using nanocarriers has been shown to sensitize tumors to anti-PD-1 therapy in murine models of pancreatic and breast cancer (64, 65). Early-phase clinical trials are also exploring this synergy; one notable example is the combination of CXCR4 inhibitors with ICIs in patients with solid tumors, showing promising signs of improved immune activation and partial responses (66, 67). These findings support the translational potential of CAF-targeted approaches in amplifying the therapeutic benefits of current immunotherapies.
4 Translational potential and current challenges
Despite the promising synergistic outcomes of CAF-targeting nano-strategies combined with immunotherapy in preclinical settings, several translational challenges remain. First, significant disparities exist between murine models and human tumors, particularly in terms of CAF composition, immune cell profiles, and stromal architecture, which may lead to inconsistent therapeutic responses (77, 78). The intrinsic heterogeneity of CAFs further complicates effective targeting, as diverse CAF subpopulations (e.g., iCAFs, myCAFs, apCAFs) may play opposing roles in tumor progression and immune modulation (79).
Moreover, nanocarrier systems encounter biological barriers in vivo, including limited tumor penetration, off-target distribution, potential immunogenicity, and suboptimal pharmacokinetics. Ensuring the efficient and safe delivery of therapeutic agents to CAF-rich tumor sites remains a major hurdle (80). From a translational perspective, regulatory challenges and scalability issues further impede the clinical adoption of CAF-targeted nanomedicines. Concerns regarding long-term toxicity, manufacturing reproducibility, and quality control must be addressed to enable industrialization (81).
Nevertheless, several clinical trials are underway to evaluate CAF-targeted therapies in combination with immune checkpoint blockade. Notably, FAP-targeted nanoparticles and radiopharmaceuticals are currently being tested in solid tumors, providing early evidence of safety and potential immune modulation (82, 83). These trials mark an encouraging step toward validating CAF-targeted nano-strategies in the clinic.
5 Conclusion and outlook
CAFs play a central role in shaping the immunosuppressive tumor microenvironment and driving resistance to immunotherapy. Targeting CAFs has thus emerged as a promising strategy to overcome the limitations of current immunotherapeutic approaches. In recent years, nanotechnology-based platforms have demonstrated outstanding potential in modulating CAFs’ functions, offering precise delivery, controlled release, and multifunctional integration to reshape immune dynamics and enhance therapeutic responses. Particularly when combined with ICIs. These nano-strategies can simultaneously suppress CAF activity, promote T cell infiltration, and boost immune activation, leading to synergistic antitumor effects. However, the intrinsic heterogeneity and plasticity of CAFs pose significant challenges to targeted therapy, while issues associated with the in vivo delivery efficiency, biosafety, and immunogenicity of nanocarriers remain unresolved. Moving forward, deeper mechanistic insights into CAF-immune interactions and subtype-specific functions are urgently needed, along with the development of standardized translational and clinical evaluation frameworks. With continued innovation, CAF-targeted nano-immunotherapies hold great promise as customizable and translatable platforms, potentially transforming the treatment landscape for solid tumors.
Author contributions
JX: Writing – review & editing, Funding acquisition. ZC: Visualization, Writing – original draft. YQ: Investigation, Writing – review & editing. LT: Investigation, Writing – review & editing. SX: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work received support from Jiangsu Province Health Commission Scientific Research Project (No. M2024030), Jiangsu Funding Program for Excellent Postdoctoral Talent (No. 2024ZB683), the Postdoctoral Fellowship Program of CPSF (No. GZC20241189), and the National Natural Science Foundation of China (No. 82204523).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Y and Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
2. Hudson WH and Wieland A. Technology meets TILs: Deciphering T cell function in the-omics era. Cancer Cell. (2023) 41:41–57. doi: 10.1016/j.ccell.2022.09.011
3. Galassi C, Chan TA, Vitale I, and Galluzzi L. The hallmarks of cancer immune evasion. Cancer Cell. (2024) 42:1825–63. doi: 10.1016/j.ccell.2024.09.010
4. Roerden M and Spranger S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat Rev Immunol. (2025) 25:353–69. doi: 10.1038/s41577-024-01111-8
5. Chen DS and Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. (2017) 541:321–30. doi: 10.1038/nature21349
6. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
7. Forsthuber A, Aschenbrenner B, Korosec A, Jacob T, Annusver K, Krajic N, et al. Cancer-associated fibroblast subtypes modulate the tumor-immune microenvironment and are associated with skin cancer Malignancy. Nat Commun. (2024) 15:9678. doi: 10.1038/s41467-024-53908-9
8. Kay EJ and Zanivan S. The tumor microenvironment is an ecosystem sustained by metabolic interactions. Cell Rep. (2025) 44:115432. doi: 10.1016/j.celrep.2025.115432
9. Arpinati L, Carradori G, and Scherz-Shouval R. CAF-induced physical constraints controlling T cell state and localization in solid tumours. Nat Rev Cancer. (2024) 24:676–93. doi: 10.1038/s41568-024-00740-4
10. Lakins MA, Ghorani E, Munir H, Martins CP, and Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Nat Commun. (2018) 9:948. doi: 10.1038/s41467-018-03347-0
11. Song J, Wei R, Liu C, Zhao Z, Liu X, Wang Y, et al. Antigen-presenting cancer associated fibroblasts enhance antitumor immunity and predict immunotherapy response. Nat Commun. (2025) 16:2175. doi: 10.1038/s41467-025-57465-7
12. Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U.S.A. (2013) 110:20212–7. doi: 10.1073/pnas.1320318110
13. Yang D, Liu J, Qian H, and Zhuang Q. Cancer-associated fibroblasts: from basic science to anticancer therapy. Exp Mol Med. (2023) 55:1322–32. doi: 10.1038/s12276-023-01013-0
14. Luo H, Tu G, Liu Z, and Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. (2015) 361:155–63. doi: 10.1016/j.canlet.2015.02.018
15. LeBleu VS and Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. (2018) 11:dmm029447. doi: 10.1242/dmm.029447
16. Cheng PS, Zaccaria M, and Biffi G. Functional heterogeneity of fibroblasts in primary tumors and metastases. Trends Cancer. (2025) 11:135–53. doi: 10.1016/j.trecan.2024.11.005
17. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. (2019) 9:1102–23. doi: 10.1158/2159-8290.CD-19-0094
18. Chhabra Y and Weeraratna AT. Fibroblasts in cancer: unity in heterogeneity. Cell. (2023) 186:1580–609. doi: 10.1016/j.cell.2023.03.016
19. Luo H, Xia X, Huang L-B, An H, Cao M, Kim GD, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. (2022) 13:6619. doi: 10.1038/s41467-022-34395-2
20. Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduction Target Ther. (2024) 9:176. doi: 10.1038/s41392-024-01868-3
21. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16:582–98. doi: 10.1038/nrc.2016.73
22. De Jaeghere EA, Denys HG, and De Wever O. Fibroblasts fuel immune escape in the tumor microenvironment. Trends Cancer. (2019) 5:704–23. doi: 10.1016/j.trecan.2019.09.009
23. Li X, Yong T, Wei Z, Bie N, Zhang X, Zhan G, et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat Commun. (2022) 13:2794. doi: 10.1038/s41467-022-30306-7
24. Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. (2014) 25:719–34. doi: 10.1016/j.ccr.2014.04.005
25. Zhou M, Wang J, Pan J, Wang H, Huang L, Hou B, et al. Nanovesicles loaded with a TGF-β receptor 1 inhibitor overcome immune resistance to potentiate cancer immunotherapy. Nat Commun. (2023) 14:3593. doi: 10.1038/s41467-023-39035-x
26. Derynck R, Turley SJ, and Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. (2021) 18:9–34. doi: 10.1038/s41571-020-0403-1
27. Pei L, Liu Y, Liu L, Gao S, Gao X, Feng Y, et al. Roles of cancer-associated fibroblasts (CAFs) in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. (2023) 22:29. doi: 10.1186/s12943-023-01731-z
28. Hou W. Role of TGFβ-activated cancer-associated fibroblasts in the resistance to checkpoint blockade immunotherapy. Front Oncol. (2025) 15:1602452. doi: 10.3389/fonc.2025.1602452
29. Li Z, Zhou J, Zhang J, Li S, Wang H, and Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. (2019) 145:1946–57. doi: 10.1002/ijc.32278
30. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. (2018) 554:544–8. doi: 10.1038/nature25501
31. Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. (2012) 122:899–910. doi: 10.1172/JCI45817
32. Xu S, Zhang P, Heing-Becker I, Zhang J, Tang P, Bej R, et al. Dual tumor-and subcellular-targeted photodynamic therapy using glucose-functionalized MoS2 nanoflakes for multidrug-resistant tumor ablation. Biomaterials. (2022) 290:121844. doi: 10.1016/j.biomaterials.2022.121844
33. Xu S, Bhatia S, Fan X, Nickl P, and Haag R. Glycosylated MoS2 sheets for capturing and deactivating E. coli bacteria: combined effects of multivalent binding and sheet size. Adv Mater Interfaces. (2022) 9:2102315. doi: 10.1002/admi.202102315
34. Fang J, Nakamura H, and Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. (2011) 63:136–51. doi: 10.1016/j.addr.2010.04.009
35. Li X, Hu Y, Zhang X, Shi X, Parak WJ, and Pich A. Transvascular transport of nanocarriers for tumor delivery. Nat Commun. (2024) 15:8172. doi: 10.1038/s41467-024-52416-0
36. Jiang Y, Lyu Z, Ralahy B, Liu J, Roussel T, Ding L, et al. Dendrimer nanosystems for adaptive tumor-assisted drug delivery via extracellular vesicle hijacking. Proc Natl Acad Sci USA. (2023) 120:e2215308120. doi: 10.1073/pnas.2215308120
37. Jain RK and Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. (2010) 7:653–64. doi: 10.1038/nrclinonc.2010
38. Tan T, Hu H, Wang H, Li J, Wang Z, Wang J, et al. Bioinspired lipoproteins-mediated photothermia remodels tumor stroma to improve cancer cell accessibility of second nanoparticles. Nat Commun. (2019) 10:3322. doi: 10.1038/s41467-019-11235-4
39. Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. (2016) 244:108–21. doi: 10.1016/j.jconrel.2016.11.015
40. Liu J, Wang Y, Mu C, Li M, Li K, Li S, et al. Pancreatic tumor eradication via selective Pin1 inhibition in cancer-associated fibroblasts and T lymphocytes engagement. Nat Commun. (2022) 13:4308. doi: 10.1038/s41467-022-31928-7
41. Chen X and Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. (2019) 18:99–115. doi: 10.1038/s41573-018-0004-1
42. Lang J, Zhao X, Qi Y, Zhang Y, Han X, Ding Y, et al. Reshaping prostate tumor microenvironment to suppress metastasis via cancer-associated fibroblast inactivation with peptide-assembly-based nanosystem. ACS Nano. (2019) 13:12357–71. doi: 10.1021/acsnano.9b04857
43. Ruan Q, Feng J, Jiang Y, Zhang X, Duan X, Wang Q, et al. Preparation and bioevaluation of 99mTc-labeled FAP inhibitors as tumor radiotracers to target the fibroblast activation protein. Mol Pharm. (2022) 19:160–71. doi: 10.1021/acs.molpharmaceut.1c00712
44. Kaps L and Schuppan D. Targeting cancer associated fibroblasts in liver fibrosis and liver cancer using nanocarriers. Cells. (2020) 9:2027. doi: 10.3390/cells9092027
45. Shen W, Yao P-a, Li W, Gu C, Gao T, Cao Y, et al. Cancer-associated fibroblast-targeted nanodrugs reshape colorectal tumor microenvironments to suppress tumor proliferation, metastasis and improve drug penetration. J Mater Chem B. (2023) 11:1871–80. doi: 10.1039/d2tb02253b
46. Akai M, Noma K, Kato T, Nishimura S, Matsumoto H, Kawasaki K, et al. Fibroblast activation protein-targeted near-infrared photoimmunotherapy depletes immunosuppressive cancer-associated fibroblasts and remodels local tumor immunity. Br J Cancer. (2024) 130:1647–58. doi: 10.1038/s41416-024-02639-1
47. Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. (2019) 79:5367–81. doi: 10.1158/0008-5472
48. Sleeboom JJ, van Tienderen GS, Schenke-Layland K, van der Laan LJ, Khalil AA, and Verstegen MM. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci Transl Med. (2024) 16:eadg3840. doi: 10.1126/scitranslmed.adg3840
49. Panagi M, Voutouri C, Mpekris F, Papageorgis P, Martin MR, Martin JD, et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics. (2020) 10:1910. doi: 10.7150/thno.36936
50. Kehrberg RJ, Bhyravbhatla N, Batra SK, and Kumar S. Epigenetic regulation of cancer-associated fibroblast heterogeneity. Biochim Biophys Acta Rev Cancer. (2023) 1878:188901. doi: 10.1016/j.bbcan.2023.188901
51. Yu Q, Qiu Y, Li J, Tang X, Wang X, Cun X, et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Control Release. (2020) 321:564–75. doi: 10.1016/j.jconrel.2020.02.040
52. Chen D, Zhang G, Li R, Guan M, Wang X, Zou T, et al. Biodegradable, hydrogen peroxide, and glutathione dual responsive nanoparticles for potential programmable paclitaxel release. J Am Chem Soc. (2018) 140:7373–6. doi: 10.1021/jacs.7b12025
53. Panagi M, Mpekris F, Chen P, Voutouri C, Nakagawa Y, Martin JD, et al. Polymeric micelles effectively reprogram the tumor microenvironment to potentiate nano-immunotherapy in mouse breast cancer models. Nat Commun. (2022) 13:7165. doi: 10.1038/s41467-022-34744-1
54. Yang M, Qin C, Tao L, Cheng G, Li J, Lv F, et al. Synchronous targeted delivery of TGF-β siRNA to stromal and tumor cells elicits robust antitumor immunity against triple-negative breast cancer by comprehensively remodeling the tumor microenvironment. Biomaterials. (2023) 301:122253. doi: 10.1016/j.biomaterials.2023.122253
55. Kim J, Kim M, Yong S-B, Han H, Kang S, Lahiji SF, et al. Engineering TGF-β inhibitor-encapsulated macrophage-inspired multi-functional nanoparticles for combination cancer immunotherapy. Biomater Res. (2023) 27:136. doi: 10.1186/s40824-023-00470-y
56. Corvigno S, Liu Y, Bayraktar E, Stur E, Bayram NN, Ahumada AL, et al. Enhanced plant-derived vesicles for nucleotide delivery for cancer therapy. NPJ Precis Oncol. (2024) 8:86. doi: 10.1038/s41698-024-00556-3
57. Guan X, Chen J, Hu Y, Lin L, Sun P, Tian H, et al. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials. (2018) 171:198–206. doi: 10.1016/j.biomaterials.2018.04.039
58. Li Y, Chen W, Kang Y, Zhen X, Zhou Z, Liu C, et al. Nanosensitizer-mediated augmentation of sonodynamic therapy efficacy and antitumor immunity. Nat Commun. (2023) 14:6973. doi: 10.1038/s41467-023-42509-7
59. He X, Yang Y, Han Y, Cao C, Zhang Z, Li L, et al. Extracellular matrix physical properties govern the diffusion of nanoparticles in tumor microenvironment. Proc Natl Acad Sci U.S.A. (2023) 120:e2209260120. doi: 10.1073/pnas.2209260120
60. Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U.S.A. (2019) 116:4558–66. doi: 10.1073/pnas.1815515116
61. Liu L, Zhang B, Wu X, Cheng G, Han X, Xin X, et al. Bioresponsive nanocomplex integrating cancer-associated fibroblast deactivation and immunogenic chemotherapy for rebuilding immune-excluded tumors. Nanomedicine. (2024) 58:102743. doi: 10.1016/j.nano.2024.102743
62. Barrett RL and Puré E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife. (2020) 9:e57243. doi: 10.7554/eLife.57243
63. Tang T, Huang X, Zhang G, Hong Z, Bai X, and Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduction Target Ther. (2021) 6:72. doi: 10.1038/s41392-020-00449-4
64. Rueda A, Serna N, Mangues R, Villaverde A, and Unzueta U. Targeting the chemokine receptor CXCR4 for cancer therapies. biomark Res. (2025) 13:68. doi: 10.1186/s40364-025-00778-y
65. Sledge GW. Targeting CXCR4-induced desmoplasia to improve checkpoint inhibition in breast cancer. Proc Natl Acad Sci U.S.A. (2019) 116:4769–71. doi: 10.1073/pnas.1900368116
66. Ciavattone NG, Bevoor A, Farfel A, Rehman A, Ho KK, Rock EC, et al. Inhibiting CXCR4 reduces immunosuppressive effects of myeloid cells in breast cancer immunotherapy. Sci Rep. (2025) 15:5204. doi: 10.1038/s41598-025-89882-5
67. Biasci D, Smoragiewicz M, Connell CM, Wang Z, Gao Y, Thaventhiran JE, et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci U.S.A. (2020) 117:28960–70. doi: 10.1073/pnas.2013644117
68. Qi F, Fu D, Cai H, Zheng Y, Wang N, and Xu Z. Metabolic reprogramming of cancer-associated fibroblasts: transforming tumor accomplices into immunotherapeutic allies. Adv Funct Mater. (2025) 35:2418240. doi: 10.1002/adfm.202418240
69. Qiu ZW, Zhong YT, Lu ZM, Yan N, Kong RJ, Huang JQ, et al. Breaking physical barrier of fibrotic breast cancer for photodynamic immunotherapy by remodeling tumor extracellular matrix and reprogramming cancer-associated fibroblasts. ACS Nano. (2024) 8:9713–35. doi: 10.1021/acsnano.4c01499
70. Liu H, Yong T, Zhang X, Wei Z, Bie N, Xu S, et al. Spatial regulation of cancer-associated fibroblasts and tumor cells via pH-responsive bispecific antibody delivery for enhanced chemo-immunotherapy synergy. ACS Nano. (2025) 19:11756–73. doi: 10.1021/acsnano.4c13277
71. Guo D, Ji X, Xie H, Ma J, Xu C, Zhou Y, et al. Targeted reprogramming of vitamin B(3) metabolism as a nanotherapeutic strategy towards chemoresistant cancers. Adv Mater. (2023) 35:e2301257. doi: 10.1002/adma.202301257
72. Zhang Y, Fang Z, Pan D, Li Y, Zhou J, Chen H, et al. Dendritic polymer-based nanomedicines remodel the tumor stroma: improve drug penetration and enhance antitumor immune response. Adv Mater. (2024) 36:e2401304. doi: 10.1002/adma.202401304
73. Geng S, Xiang T, Zhang Y, Guo P, Zhang H, Zhang Z, et al. Safe engineering of cancer-associated fibroblasts enhances checkpoint blockade immunotherapy. J Control Release. (2023) 356:272–87. doi: 10.1016/j.jconrel.2023.02.041
74. Panagi M, Mpekris F, Chen P, Voutouri C, Nakagawa Y, Martin JD, et al. Polymeric micelles effectively reprogram the tumor microenvironment to potentiate nano-immunotherapy in mouse breast cancer models. Nat Commun. (2022) 13:7165. doi: 10.1038/s41467-022-34744-1
75. Li X, Yong T, Wei Z, Bie N, Zhang X, Zhan G, et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat Commun. (2022) 13:2794. doi: 10.1038/s41467-022-30306-7
76. Freag MS, Mohammed MT, Kulkarni A, Emam HE, Maremanda KP, and Elzoghby AO. Modulating tumoral exosomes and fibroblast phenotype using nanoliposomes augments cancer immunotherapy. Sci Adv. (2024) 10:eadk3074. doi: 10.1126/sciadv.adk3074
77. Chen Y, McAndrews KM, and Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. (2021) 18:792–804. doi: 10.1038/s41571-021-00546-5
78. Tian C, Clauser KR, Öhlund D, Rickelt S, Huang Y, Gupta M, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci USA. (2019) 116:19609–18. doi: 10.1073/pnas.1908626116
79. Wan Y, Hu Q, Sun K, Shi J, Liu L, Zhang X, et al. Heterogenous cancer-associated fibroblasts related tumor microenvironment marked by CD10/KLF4/TIAM1 were identified in pancreatic adenocarcinoma by integrated transcriptomics. Front Immunol. (2025) 16:1557698. doi: 10.3389/fimmu.2025.1557698
80. Shen X, Pan D, Gong Q, Gu Z, and Luo K. Enhancing drug penetration in solid tumors via nanomedicine: Evaluation models, strategies and perspectives. Bioact Mater. (2024) 32:445–72. doi: 10.1016/j.bioactmat.2023.10.017
81. Wang B, Hu S, Teng Y, Chen J, Wang H, Xu Y, et al. Current advance of nanotechnology in diagnosis and treatment for Malignant tumors. Signal Transduction Target Ther. (2024) 9:200. doi: 10.1038/s41392-024-01889-y
82. Wang R, Huang M, Wang W, Li M, Wang Y, and Tian R. Preclinical evaluation of 68Ga/177Lu-labeled FAP-targeted peptide for tumor radiopharmaceutical imaging and therapy. J Nucl Med. (2025) 66:250–6. doi: 10.2967/jnumed.124.268689
Keywords: cancer-associated fibroblasts (CAFs), tumor microenvironment (TME), immunotherapy resistance, nanoparticle delivery systems, CAFs-targeted immunomodulation
Citation: Xu J, Chen Z, Qin Y, Tan L and Xu S (2025) Nano-strategies targeting cancer-associated fibroblasts to enhance immunotherapy and reverse resistance. Front. Immunol. 16:1668199. doi: 10.3389/fimmu.2025.1668199
Received: 17 July 2025; Accepted: 13 August 2025;
Published: 09 September 2025.
Edited by:
Qiong Lu, Central South University, ChinaReviewed by:
Lei Wang, Anhui University of Chinese Medicine, ChinaCopyright © 2025 Xu, Chen, Qin, Tan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juqing Xu, anVxaW5neHUxOTg2QG5qbXUuZWR1LmNu; Shaohui Xu, c2hhb2h1aS54dTE4QGdtYWlsLmNvbQ==
 Juqing Xu
Juqing Xu Ze Chen2
Ze Chen2 Shaohui Xu
Shaohui Xu