- 1Division of Molecular Immunology, Medical Mycology Research Center, Chiba University, Chiba, Japan
- 2School of Pharmaceutical Sciences, Department of Clinical e Toxicological Analysis, University of São Paulo, São Paulo, Brazil
Introduction: The emerging fungal pathogen Sporothrix brasiliensis has been responsible for epidemic outbursts of sporotrichosis in Latin America, particularly Brazil, in recent years. The higher aggressiveness of the infection and its zoonotic nature are hallmarks of the pathogen, but the immunological markers of protection are not fully characterized. The C-type lectin receptors – dectin-1 and dectin-2 – drive key antifungal responses, and here we aimed to uncover their contribution against S. brasiliensis in a murine model of disseminated sporotrichosis.
Methods: Wild-type, Dectin-1 and/or Dectin-2 knockout, and IL-17A/F knockout C57BL/6J mice were challenged with S. brasiliensis in a model of systemic infection. Animals were monitored for parameters as survival and body weight loss. Immunological analyses as assessment of cytokines and immune cell profiling were conducted in the livers.
Results: We showed that the receptors are essential for host survival, necessary to limit the fungal dissemination, and that their main effector functions can be related to shaping the T cell response, notably the cytotoxic CD8+ and Treg cell populations, instead of a conventional TH17 profile. While we also observed a contribution of IL-17 in the host defense, the cytokine is not involved in the restriction of the fungal growth.
Discussion: Our results uncover dectin-1 and dectin-2 as novel determinants of protection against S. brasiliensis, but their effector function is not linked to the induction of IL-17 responses. Our fundings help to expand the understanding of the pathophysiology of this infection.
Introduction
Sporothrix brasiliensis is an emerging fungal pathogen associated with epidemic outbreaks of a more aggressive form of the mycosis sporotrichosis (1). Belonging to the Sporothrix schenckii complex, Sporothrix spp. have always caused concern among the scientific community (2). However, the higher severity of S. brasiliensis infections linked to its strong epidemic potential has put this pathogen in the spotlight in recent years (3). Currently, S. brasiliensis has surpassed the other members of the S. schenckii complex as the main causative agent of sporotrichosis in Brazil, and unfortunately, it is spreading across several countries in Latin America (4), with isolated cases already being reported in Europe and North America (5).
Infections by Sporothrix spp. are primarily associated with contact with contaminated soil, plants, or organic matter (hence, the alias as the “gardener’s disease”) (6); however, for S. brasiliensis, zoonotic sporotrichosis and animal-to-human transmissions have been recognized as the main sources of contamination (1, 4). For instance, the ability of S. brasiliensis to infect animals, particularly stray cats, helps to explain its rapid dissemination and poses a great challenge for control by public health measures (4, 7). In addition, despite the subcutaneous nature of the mycosis, atypical (extra-cutaneous) presentations with invasive commitment linked to S. brasiliensis are more common and are on the rise (7, 8).
The higher virulence of S. brasiliensis is not fully understood and cannot be traced to a single trait. The most important features include: i) higher thermotolerance and thermodimorphic behavior (whereas the mycelial form is considered saprophytic and the yeast phase parasitic) (7)—but for S. brasiliensis, the zoonotic transmission occurs directly by yeast inoculation; ii) the ability to form biofilms (9); and iii) the production of enzymes, adhesion molecules, and melanin (10). Nevertheless, the interaction of these attributes with the host system is what determines the infection outcome.
From the host’s perspective, an even larger gap exists about the immune response triggered against S. brasiliensis, and only in recent years have some advances in addressing those questions been observed. In this context, innate immunity is our first layer of defense, and its operation requires pathogen detection mediated by pattern recognition receptors (11). The prototypical innate molecules Toll-like receptor 2 (TLR2) and TLR4 have been shown to be important for host defense in murine models of S. brasiliensis infection, mainly by regulating the effector function of phagocytes and the inflammatory response, although their deficiency did not compromise animal survival upon the fungal challenge (12, 13). The complement protein C3 and the surface molecule CD11b have also been shown to be important for the interaction between S. brasiliensis and macrophages (14).
The main sensors involved in fungal recognition, however, belong to the family of the C-type lectin receptors (CLRs). Dectin-1 (CLEC7A in humans and Clec7a in mice) and dectin-2 (CLEC6A/Clec4n) are the most studied CLRs, and we and others have reported their roles in the defense against a plethora of fungal pathogens (15–20). The induction of T helper 17 (TH17) responses is considered their canonical, but not solely, effector function (21).
Independent groups have suggested a marginal contribution of dectin-1 in the interaction between S. brasiliensis and phagocytes (22, 23), but these findings are limited to in vitro settings. Thus, the in vivo relevance of CLRs in anti-S. brasiliensis response is still an open question. Here, we proposed to evaluate the relevance of dectin-1/dectin-2 in the host response to S. brasiliensis in an experimental model of disseminated disease. We observed that the lack of these receptors severely compromised the ability to resist the fungal challenge. Curiously, the defective antifungal response in the absence of CLRs was not directly linked to an interleukin 17 (IL-17) response, but rather to a dysbalanced profile of cytotoxic CD8+ T cells and regulatory T cells (Tregs), which could be the result of a poor ability to activate dendritic cells. Our findings underscore dectin-1/dectin-2 as key determinants for an efficient host defense against S. brasiliensis.
Materials and methods
Mice
Female mice in C57BL/6J genetic background deficient for dectin-1 (Clec7a–/–), dectin-2 (Clec4n–/–), dectin-1/dectin-2 (Clec7a–/––Clec4n–/–), IL-17A/IL-17F (Il17a–/––Il17f–/–), and rag2 (Rag2–/–) were used in this study (15). C57BL/6J wild-type (WT) mice were acquired from CLEA Japan (Tokyo, Japan) and co-housed with the knockout animals for at least 1 week prior to the experiments. All mice were maintained under specific pathogen-free conditions with a gamma ray-sterilized diet and acidified tap water (0.002 N HCl) ad libitum.
All experiments were conducted following the “Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the Jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology” (Ministry of Education, Culture, Sports, Science and Technology, Japan, 2006). The Institutional Animal Care and Use Committee from Chiba University approved the protocols reported in this paper under process number A7-198.
Fungal strain and inoculum preparation
The reference strain S. brasiliensis 5110 (Sporothrix brasiliensis Marimon MYA-4823; American Type Culture Collection, Manassas, VA, USA) was used throughout this study. The fungus was maintained in brain heart infusion (BHI) agar (BD, Franklin Lakes, NJ, USA) at 37°C with biweekly subcultures.
For inoculum preparation, the fungus was seeded in BHI agar plates and incubated for 5 days at 37°C. Colonies were harvested and washed in 0.1% Tween-80/PBS (phosphate-buffered saline) solution, resuspended in saline solution (0.9% NaCl), and kept at 4°C until use.
In vivo infections
For in vivo infections, the animals were inoculated through the intravenous route (lateral caudal vein) with 5 × 106 yeast cells in 100 μl of saline solution. Animal weight and survival were monitored daily for up to 27 days post-infection (dpi).
Fungal burden analysis
On the indicated dpi, the animals were euthanized by cervical dislocation, and the organs were perfused with ice-cold PBS before surgical removal. After being weighed, the organs were macerated in PBS through mesh sieves. Dilutions of the macerates were plated on PDA plates (Eiken Chemical, Tokyo, Japan), incubated at 30°C for 4 days, and the recovered colony-forming units (CFU) counted. Fungal burden was expressed as CFU per gram of organ. The macerates were centrifuged at 14,000 × g for 5 min, and the supernatants were collected and stored at −80°C for cytokine analysis (see below).
Histopathological analyses
On the indicated dpi, the livers were perfused with PBS and fixed overnight in commercial formalin solution (Fujifilm Wako, Osaka, Japan). Samples were embedded in paraffin, and sections were stained with routine hematoxylin–eosin, Grocott’s methenamine silver, or Masson’s trichrome staining method.
Isolation of liver leukocytes and flow cytometry analysis
Liver leukocytes were isolated from the sample macerates by Percoll centrifugation as described by Prosser et al. (24). The recovered cells were submitted to surface and intracellular staining for flow cytometry evaluation. For cell permeabilization, the commercial kits “Foxp3 Staining Buffer Set” (for CD4+ T-cell evaluation) and “Fixation & Permeabilization Buffer Set” (CD8+ T cells) (eBioscience, San Diego, CA, USA) were used according to the manufacturer’s instructions. Data were acquired with a FACSVerse flow cytometer (eight-color; BD, Franklin Lakes, NJ, USA) and analyzed using FlowJo (v.10.7.1 for Mac OS X; BD, Franklin Lakes, NJ, USA). The list of antibodies used for the analysis is provided in Supplementary Table S1, and representative gating strategies are shown in Supplementary Figure S1.
Bone marrow-derived dendritic cells and in vitro infections
Bone marrow-derived dendritic cells (BMDCs) were generated from bone marrow cells harvested from the femur and tibia of the WT and Clec7a–/––Clec4n–/– mice by granulocyte–macrophage colony-stimulating factor (GM-CSF) differentiation protocol as previously described (15). On the day of the assay, 1 × 106 BMDCs were stimulated with freshly harvested S. brasiliensis yeast cells (multiplicity of infection, 1:1) or 100 ng/ml of lipopolysaccharide (LPS) (from Escherichia coli O111:B4; Sigma-Aldrich, St. Louis, MO, USA) for 24h at 37°C and 5% CO2. The supernatants were harvested for cytokine measurements (see below), and the cells were stained with antibodies (Supplementary Table S1) for the flow cytometry analysis.
Cytokine measurements
Cytokines [except for IL-10 and transforming growth factor beta (TGF-β)] were quantified using the BD Cytometric Bead Array assay according to the manufacturer’s instructions. Data were acquired using FACSVerse and analyzed with the FCAP Array software (v.3.0.1; BD, Franklin Lakes, NJ, USA). The detection limits were as follows: IL-1β = 1.9 pg/ml, IL-6 = 1.4 pg/ml, tumor necrosis factor (TNF) = 2.8 pg/ml, interferon gamma (IFN-γ) = 0.5 pg/ml, IL-4 = 0.3 pg/ml, IL-17A = 0.95 pg/ml, and IL-17F = 0.81 pg/ml.
The levels of IL-10 and TGF-β were quantified by sandwich ELISA using commercially available kits (DuoSet™ ELISA Development System, BioTechne/R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The adopted measurement range was 2,000–31.2 pg/ml.
Statistical analysis
Statistical analyses were performed using the software GraphPad Prism (v.10 for OSX; GraphPad Inc., La Jolla, CA, USA). Data were screened for the detection of outliers using the ROUT method. The statistical test employed for each analysis, the sample size, and the number of replicates in each experiment are described in the figure legends. A p-value <0.05 was considered statistically significant.
Results
Dectin-1/dectin-2 are essential for protection against S. brasiliensis infection
Systemic sporotrichosis is the most studied experimental model for investigating host–pathogen interactions and the immune response (25). We established a model of disseminated disease by administering S. brasiliensis yeast cells through the intravenous route and analyzed the outcome of the infection among WT and dectin-1 and/or dectin-2 knockout mice (Figure 1).
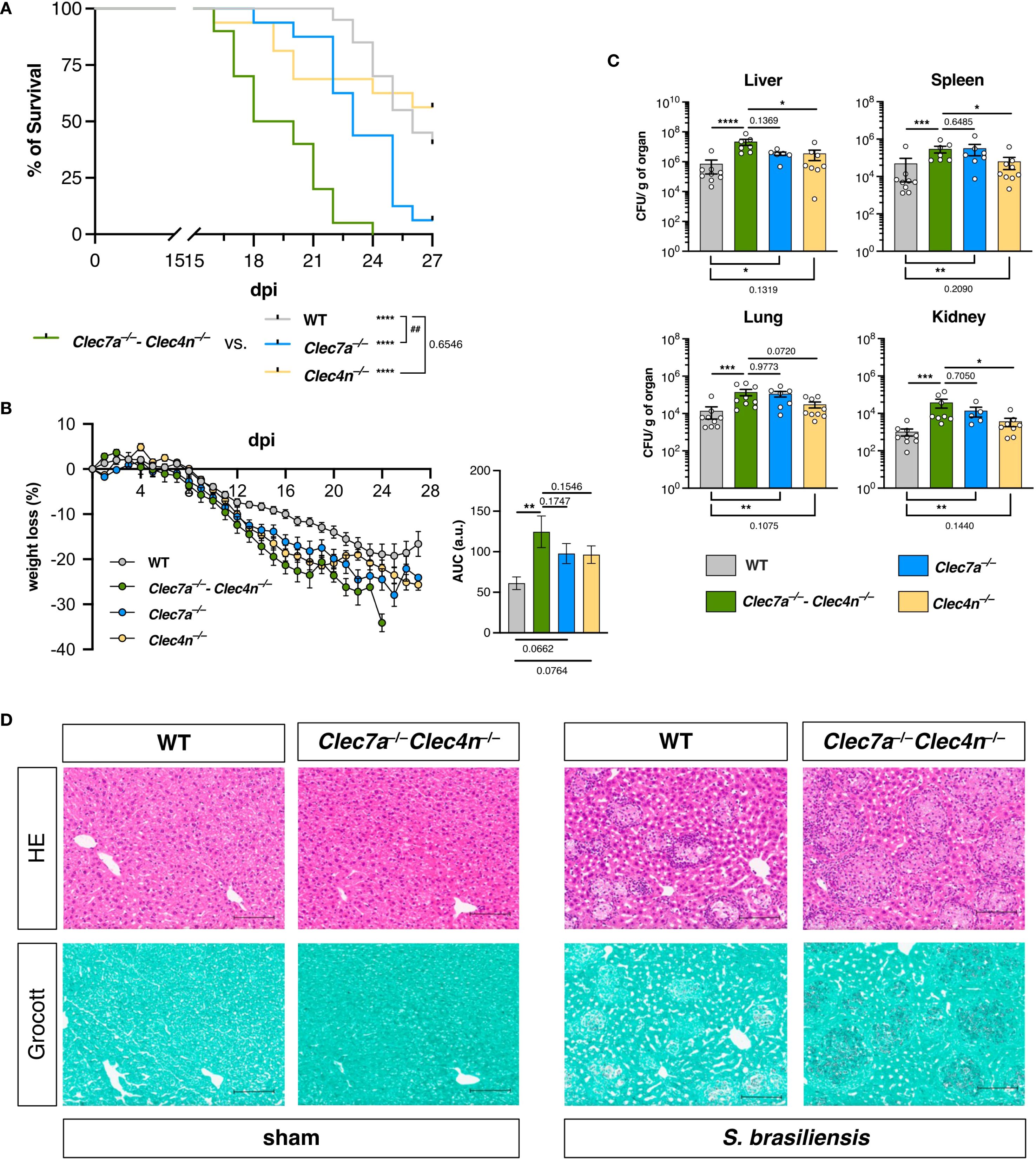
Figure 1. Dectin-1 and dectin-2 are essential for resistance against Sporothrix brasiliensis infection. (A, B) Wild type (WT), Clec7a−/−, Clec4n−/−, and Clec7a−/−–Clec4n−/− mice were infected intravenously with 5 × 106 yeast cells, and the survival (A) and body weight loss (B) were monitored for up to 27 days post-infection (dpi). n = 16–20 mice per group, pooled from two independent experiments. (A) Survival curves compared by log-rank (Mantel–Cox) test: ## p < 0.01 (vs. WT); ****p < 0.0001 (vs. Clec7a−/− and Clec4n−/−). (B) Body weight loss plots and area under the curve (AUC) bars shown as the mean ± SEM. One-way ANOVA and Fisher’s least significant difference (LSD) posttest: **p < 0.001. (C) Fungal burden in the organs harvested at 14 dpi. Data shown as colony-forming units (CFU) per gram of organ. n = 7–9 mice per group, pooled from two independent experiments. Each dot represents one mouse, and bars indicate the mean ± SEM. Kruskal–Wallis and Dunn’s posttest: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (D) Micrographs of the liver sections stained with hematoxylin–eosin (HE, first row) or Grocott’s methenamine silver (second row) methods. Scale bars represent 100 μm. Data representative of two (sham)–four (infected) mice per group from two independent experiments.
In agreement with our initial expectations, animals lacking dectin-1/dectin-2 were remarkably susceptible to S. brasiliensis infection (Figure 1). In addition to the enhanced mortality observed in the Clec7a−/−–Clec4n−/− animals (Figure 1A), the infection caused a more intense weight loss during the course of the experiment compared with their WT counterparts (Figure 1B), indicating a more aggressive disease in the absence of the receptors. In line with this, the knockouts also presented higher fungal burdens in the liver, spleen, lungs, and kidneys (measured at 14 dpi), pointing to a systemic inability to restrain the pathogen dissemination (Figure 1C).
Interestingly, when the individual contribution of each receptor was analyzed using single knockout animals, dectin-1 appeared to play the dominant role, while the lack of dectin-2 alone did not remarkably alter the analyzed parameters (Figure 1). Nevertheless, it must be highlighted that the Clec7a−/− mice could not fully recapitulate the double-knockout profile, as the Clec7a−/−–Clec4n−/− animals remained the most sensitive group. This suggests that dectin-2 is still involved in the protective response, but it may act by potentiating the functionality of dectin-1. Thus, to better characterize the host mechanisms involved, we followed the subsequent analyses with double-knockout animals.
These initial results indicate that dectin-1/dectin-2 are essential players in the host defense against S. brasiliensis, required for fungal restriction and maintenance of the host fitness.
Lack of dectin-1/dectin-2 does not enhance tissue inflammation
The results from the fungal burden analysis pointed to the liver as the most compromised organ (Figure 1C), and it was chosen as a proxy for the response characterization. Initially, we confirmed the fungal colonization in the livers by histopathological analysis (Figure 1D). Interestingly, S. brasiliensis infection led to the development of diffuse, granuloma-like inflammatory foci around the fungal structures both in the WTs and the knockouts (Figure 1D, top row). However, as expected, in the Clec7a−/−–Clec4n−/− mice, a massive fungal burden could be detected, as observed in the silver staining images (Figure 1D, bottom row). Thus, we next measured the levels of the cytokines classically involved in the inflammatory response and host defense to fungal pathogens (26) (Figure 2).
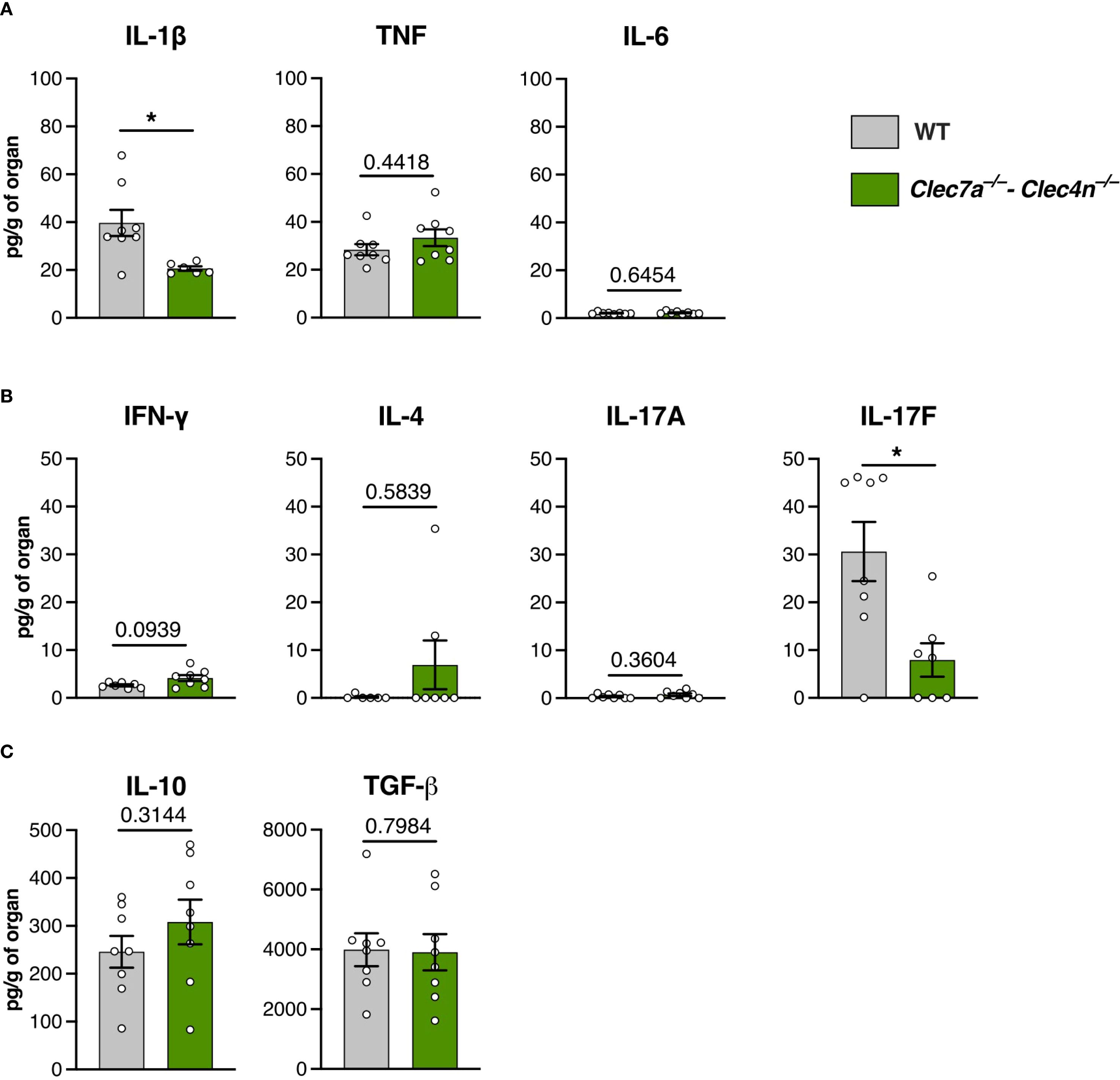
Figure 2. Cytokine profile in the liver macerates of Sporothrix brasiliensis-infected mice. Wild-type (WT) and Clec7a−/−–Clec4n−/− mice were infected intravenously with 5 × 106 yeast cells, and livers were harvested at 14 days post-infection (dpi). (A) Levels of IL-1β, TNF, and IL-6. (B) Levels of IFN-γ, IL-4, IL-17A, and IL-17F. (C) Levels of IL-10 and TGF-β. Data shown as picograms of cytokine per gram of organ. n = 8 mice per group, pooled from two independent experiments. Each dot represents one mouse, and bars indicate the mean ± SEM. Mann–Whitney U test: *p < 0.05.
Curiously, despite the massive fungal colonization, the inflammatory cytokines were not proportionally augmented (Figure 2A). While TNF and IL-6 were not altered by the lack of dectin-1/dectin-2, lower levels of IL-1β were found in the knockouts. Interestingly, with regard to the cytokines associated with adaptive immunity, we found a predominance of the IL-17 response, particularly IL-17F, whose levels were compromised by the deficiency of the receptors (Figure 2B). Concurrently, we did not detect differences in the levels of the anti-inflammatory cytokines, i.e., IL-10 and TGF-β (Figure 2C).
Thus, instead of an overt inflammation driven by an unrestrained fungal growth, the lower levels of IL-1β and IL-17F would argue in favor of a hypothesis of dectin-1/dectin-2 promoting protection against S. brasiliensis through the induction of a prototypical type 3 (IL-17–driven) response, which is the paradigmatic branch of the adaptive immunity linked to resistance against fungal infections (27).
IL-17A/F are required for protection, but they do not regulate the fungal containment
To validate the importance of IL-17 in our model, we challenged the Il17a−/−–Il17f−/− mice with S. brasiliensis and compared their infection outcome to those of the WT and Clec7a−/−–Clec4n−/− groups (Figures 3A, B). As expected, IL-17 deficiency did compromise the host defense, leading to higher mortality and weight loss compared with the WT. Nonetheless, the Clec7a−/−–Clec4n−/− mice were still more susceptible than their Il17a−/−–Il17f−/− counterparts, indicating the involvement of additional mechanisms.
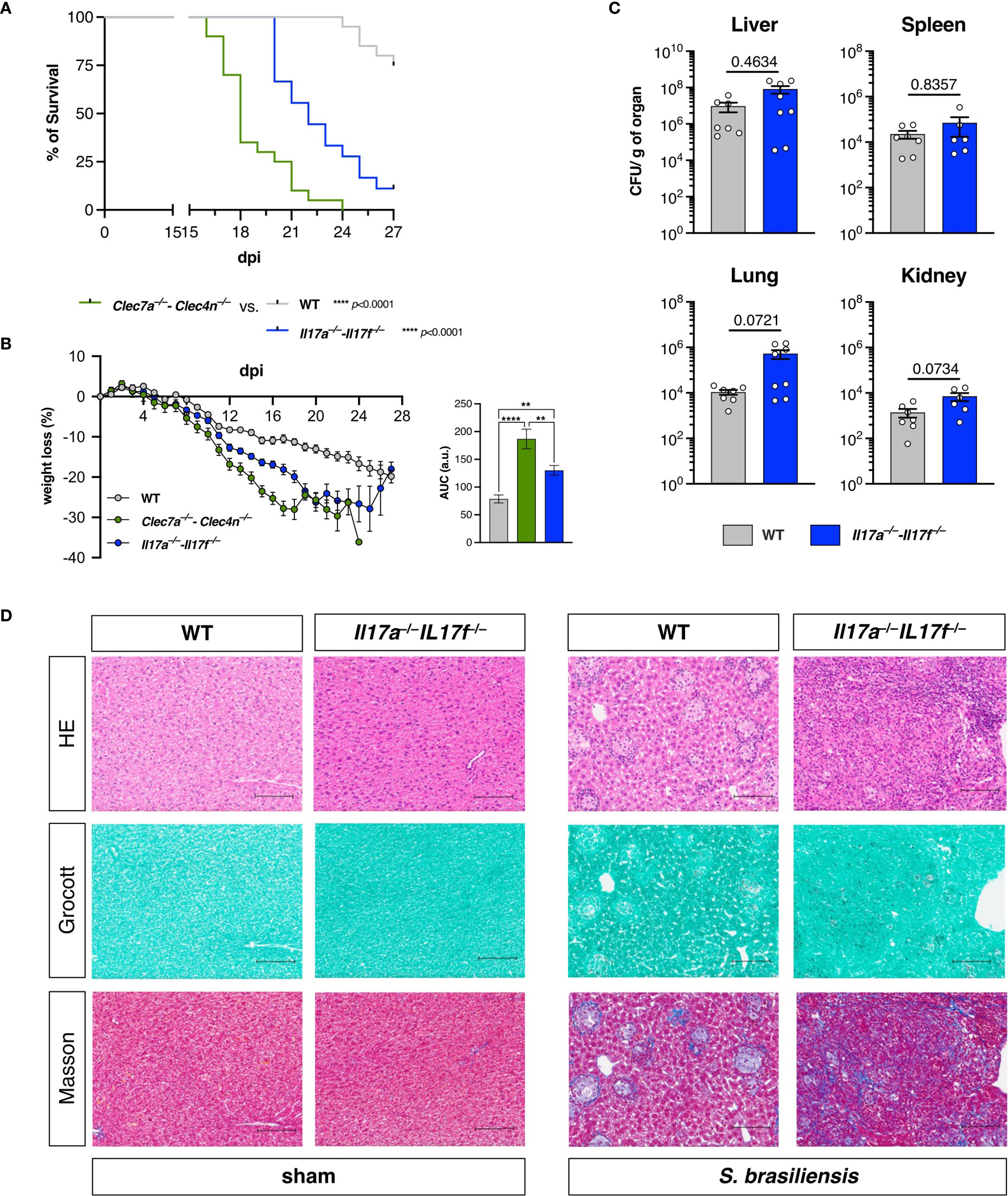
Figure 3. IL-17A/F promote resistance against Sporothrix brasiliensis infection, but do not alter fungal restriction. (A, B) Wild-type (WT), Clec7a−/−–Clec4n−/−, and Il17a−/−–Il17f−/− mice were infected intravenously with 5 × 106 yeast cells, and the survival (A) and body weight (B) loss were monitored for up to 27 days post-infection (dpi). n = 18–20 mice per group, pooled from two independent experiments. (A) Survival curves compared by log-rank (Mantel–Cox) test: ****p < 0.0001. (B) Body weight loss plots and area under the curve (AUC) bars shown as the mean ± SEM. One-way ANOVA and Fisher’s least significant difference (LSD) posttest: **p < 0.001, ****p < 0.0001. (C) Fungal burden in the organs harvested at 18 dpi. Data shown as colony-forming units (CFU) per gram of organ. n = 8 mice per group, pooled from two independent experiments. Each dot represents one mouse, and bars indicate the mean ± SEM. Mann–Whitney U test: no significance detected. (D) Micrographs of the liver sections stained with hematoxylin–eosin (HE, first row), Grocott’s methenamine silver (second row), or Masson’s trichrome (third row) method. Scale bars represent 100 μm. Data representative of two (sham)–four (infected) mice per group from two independent experiments.
Astoundingly, despite the higher susceptibility of the IL-17 knockouts to the fungal challenge, the absence of the cytokines did not affect the fungal burden in any of the assessed organs (Figure 3C) as observed for dectin-1/dectin-2 deficiency (Figure 1C). Rather than uncontrolled fungal dissemination, the Il17a−/−–Il17f−/− mice were colonized to the same levels as the WTs.
To obtain further insight into this observation, we also performed histopathological analysis of the livers from these animals (Figure 3D). Intriguingly, in contrast to the diffuse inflammatory foci observed, as shown in Figure 1D, deficiency in IL-17 led to a disorganized tissue structure, characterized by massive fibrosis, as revealed by Masson’s trichrome staining. It should also be noted that these features were not accompanied by widespread fungal growth, in agreement with the fungal burden data (Figure 3C), unlike what was observed in animals deficient in dectin-1/dectin-2 (Figure 1). These results suggest that the maintenance of host fitness against S. brasiliensis does not exclusively involve pathogen containment.
Dectin-1/dectin-2 do not shape the local T-helper cell profile
The disconnection between dectin-1/dectin-2 and IL-17 in the control of the fungal dissemination prompted us to re-evaluate whether type 3 immunity is the major response induced by the CLRs.
Initially, we aimed to confirm the requirement of lymphocytes for host resistance in our model by using animals knockout for Rag2 (15) and comparing their performance upon S. brasiliensis challenge (Figures 4A, B). Indeed, the lack of lymphocytes severely compromised the survival of the mice and led to a marked weight loss during the experiment. More importantly, their phenotype was virtually identical to Clec7a−/−–Clec4n−/− mice, strongly implying that the lymphocyte response could be the primary effector function of dectin-1/dectin-2.
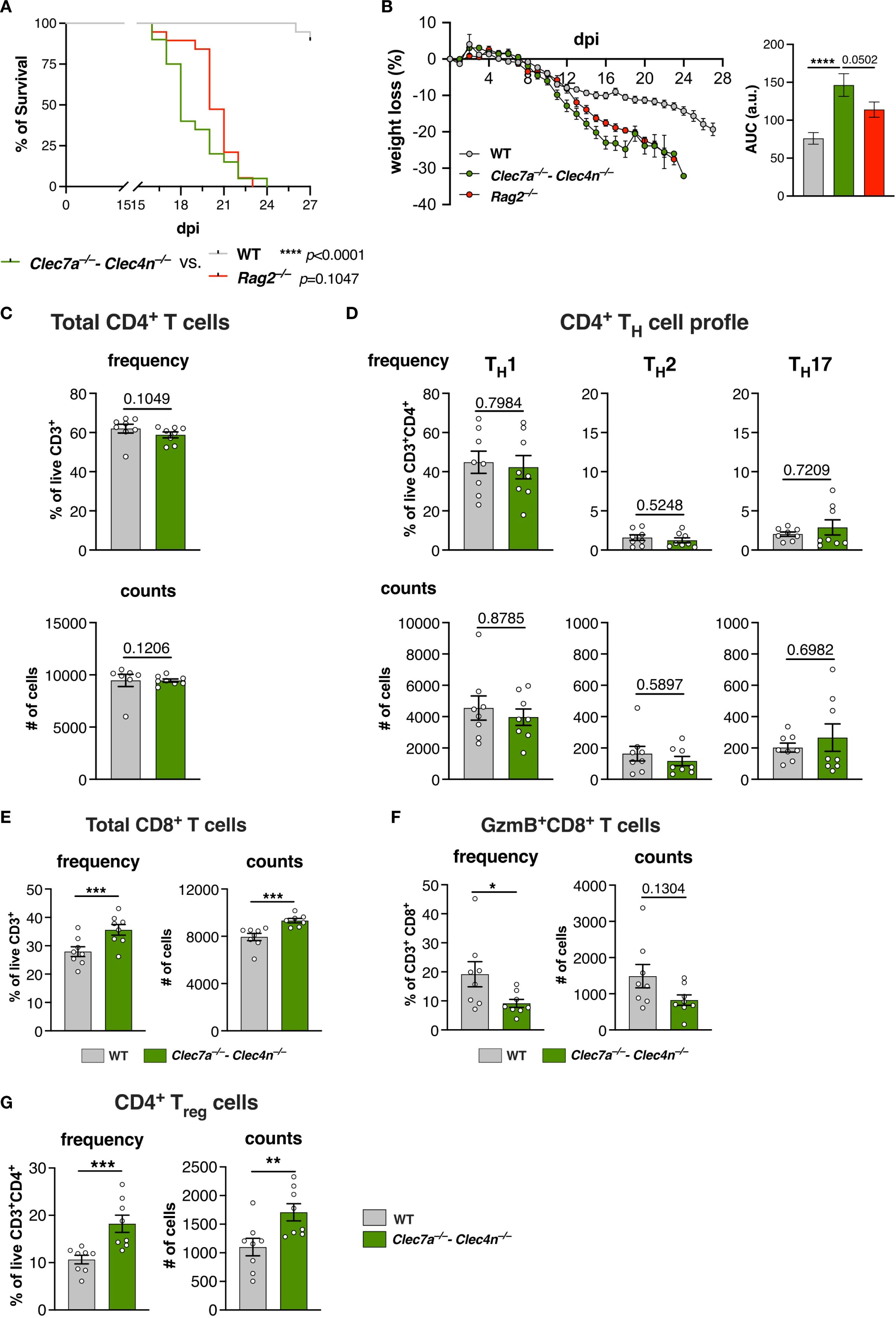
Figure 4. The T-cell profile is affected by the lack of dectin-1/dectin-2. (A, B) Wild type (WT), Clec7a−/−–Clec4n−/−, and Rag2−/− mice were infected intravenously with 5 × 106 yeast cells, and the survival (A) and body weight loss (B) were monitored for up to 27 days post-infection (dpi). n = 19–20 mice per group, pooled from two independent experiments. (A) Survival curves compared by log-rank (Mantel–Cox) test: ****p < 0.0001. (B) Body weight loss plots and area under the curve (AUC) bars shown as the mean ± SEM. One-way ANOVA and Fisher’s least significant difference (LSD) posttest: ****p < 0.0001. (C–G) T-cell profile in the livers of infected mice harvested at 14 dpi. Frequency and counts of the total CD4 T cells (C); TH1, TH2, and TH17 cells (D); total CD8 T cells (E); granzyme B (GzmB)-expressing CD8 T cells (F); and regulatory T cells (Tregs) (G). n = 8 mice per group, pooled from two independent experiments. Each dot represents one mouse, and bars indicate the mean ± SEM. Mann–Whitney U test: *p < 0.05, ***p < 0.001.
Subsequently, we aimed to identify the dominant adaptive response induced by S. brasiliensis. Firstly, we harvested splenocytes from infected mice, re-stimulated them with the pathogen yeast cells, and measured the hallmark cytokines in the culture supernatants (Supplementary Figure S2). Interestingly, IFN-γ was the predominant cytokine observed, whereas IL-4, IL-17A, and IL-17F were barely detected. Furthermore, the levels of IFN-γ were compromised by the lack of dectin-1/dectin-2. These results suggest that S. brasiliensis infection polarizes toward a type 1/TH1, not type 3/TH17, profile and that the process is instructed by dectin-1/dectin-2.
Thus, we next evaluated the profile of the CD4+ T-cell population in the livers of the infected animals (Figures 4C, D), which revealed no alterations in the population of total CD4+ T cells (Figure 4C). Furthermore, we characterized the subpopulations of TH cells based on the expression of the classical transcription factors, i.e., T-bet (TH1), GATA3 (TH2), and RORγt (TH17) (28). In agreement with the splenocyte results (Supplementary Figure S2), the major subset was composed of TH1 cells, whereas the other subtypes were detected at lower levels (Figure 4D). Unexpectedly, no changes in their proportions were observed due to the lack of CLRs.
Therefore, even though dectin-1/dectin-2 might be needed to shape the T-cell response in secondary lymphoid organs, such as the spleen, this does not necessarily reflect in the cell profile at peripheral organs.
Deficiency of dectin-1/dectin-2 favors an immunosuppressed T-cell environment
The weak influence of dectin-1/dectin-2 over the TH-cell population prompted us to investigate other branches of the T-cell response, particularly CD8+ T cells and Tregs (Figures 4E–G).
Curiously, there was a pronounced influx of CD8+ T cells in the knockout group (Figure 4E). However, these cells showed lower levels of granzyme B (GzmB) compared with their WT counterparts (Figure 4F). Therefore, despite the higher presence of CD8+ lymphocytes, they displayed a dampened cytotoxic profile in the absence of dectin-1/dectin-2.
Remarkably, we could also detect a significant population of Tregs that was further increased in the Clec7a−/−−Clec4n−/− mice (Figure 4G). Together with the impaired presence of cytotoxic CD8+ T cells, these results indicate that the lack of dectin-1/dectin-2 favors an immunosuppressed environment that might be less able to counter the fungal growth.
S. brasiliensis is a poor activator of dendritic cells
Our results indicate that dectin-1/dectin-2 are required for the balance of the lymphocyte response against S. brasiliensis. However, notwithstanding the profile of T cells that the receptors might enforce, rather than working directly on lymphocytes, CLRs act by shaping the profile of antigen-presenting cells, particularly dendritic cells (28).
To conciliate our findings, we analyzed the response of BMDCs stimulated with S. brasiliensis (Figure 5). Interestingly, we observed that the pathogen is a very weak BMDC activator. In contrast to the positive control LPS, S. brasiliensis triggered almost no cytokine production (Figure 5A) or expression of the co-stimulatory molecule CD86 (Figure 5B). We only observed a dectin-1/dectin-2-dependent production of TNF (Figure 5A), suggesting that these CLRs are still needed for a minimal level of cell activation.
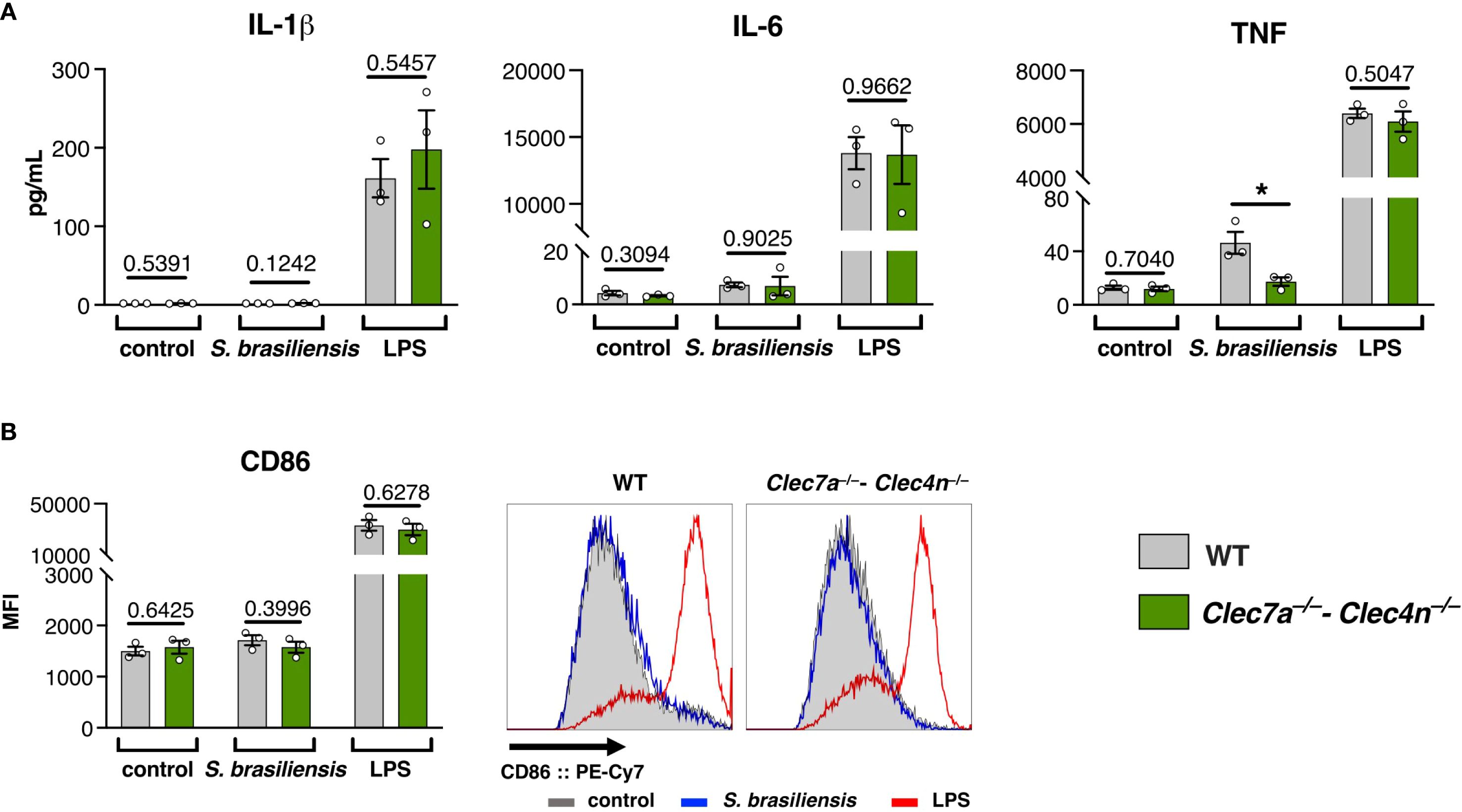
Figure 5. Sporothrix brasiliensis is a poor activator of bone marrow-derived dendritic cells (BMDCs). BMDCs were stimulated with S. brasiliensis or lipopolysaccharides (LPS) for 24h, and the activation markers were analyzed. (A) Levels of IL-1β, IL-6, and TNF in the culture supernatants. (B) Expression of CD86 on the BMDC surface (representative histograms on the right side). Data shown as the mean ± SEM, pooled from three independent experiments. Unpaired t-test: *p < 0.05.
The poor response of BMDCs could be indicative of a polarization toward a tolerogenic profile (29), and dendritic cells lacking dectin-1/dectin-2 show an even less activated phenotype that might reflect in potentiation of Treg differentiation, leading to a poorer cytotoxic environment that favors fungal proliferation.
Discussion
The world is witnessing a mounting rise in the cases of fungal infections in recent years, partially driven by the emergence of novel, more aggressive pathogens such as S. brasiliensis, Candida auris (30), and Trichophyton indotineae (31). The understanding of the immunology of these infections is an urgent requirement for counteractions. Here, we showed that dectin-1 and dectin-2 are key receptors for host resistance against S. brasiliensis, but the infection itself displays features of dampened inflammation, which can sustain the chronic evolution of the disease.
The poor activation of BMDCs argues in favor of this hypothesis. In agreement with our data, human dendritic cells and granulocytes were also shown to be less responsive to S. brasiliensis stimulation (22, 23), while human macrophages were more sensitive (23). Interestingly, most of the immunogenicity of S. brasiliensis is suggested to be carried by extracellular vesicles secreted by the fungus instead of the fungal cell per se (32, 33). Antigen masking could be a possible strategy to escaping host detection, as S. brasiliensis has been shown to have a thicker cell wall with less antigen exposure (34, 35). Along this line, while García-Carnero et al. reported low S. brasiliensis-driven cytokine responses by human peripheral blood mononuclear cells (PBMCs) (36), Kischkel et al. detected high responsiveness in equivalent PBMC samples (37); however, the latter employed heat-killed yeast cells instead of native cells as the former, and the heat treatment might have enhanced the immunogenicity of the material. Alternatively, interspecies variables have to be taken into consideration, as human and murine immune cells may display distinct recognition patterns, as observed for Candida albicans (38), which might affect the interpretation of the profiles and limit direct extrapolations.
Although the definitive contribution of each phagocyte type to host defense needs to be addressed in the future, the overall poor inflammatory potential of S. brasiliensis in vivo might in fact contribute to the aggressiveness of the infection as the host response is moved toward an environment highly permissive to fungal dissemination and persistence. In this scenario, dectin-1/dectin-2 act by limiting the polarization of Tregs and favoring the cytotoxic activity of CD8 T cells.
In parallel, the finding that IL-17 is involved in host survival, but not due to a presumed antifungal activity, was unexpected and intriguing. In addition to the well-known roles of IL-17 cytokines in driving inflammatory responses, the cytokines are also involved in tissue maintenance and repair (39). In support of this idea, our histopathological analysis showed that the IL-17 knockouts did not exhibit overwhelming fungal dissemination, as observed in dectin-1/dectin-2 knockouts, but presented compromised, fibrotic livers, which may have contributed to the demise of the animals. The role of IL-17 in organ fibrosis remains a matter of debate, as this cytokine can exert either anti- or profibrotic effects according to the context of the underlying disease (40). Interestingly, it has been reported that IL-17A neutralization reduced the extent of granuloma formation in a model of infection with the parasite Schistosoma japonicum (41), and a similar mechanism might be occurring in S. brasiliensis infection, where IL-17 helps to limit the spread of fungal colonization. Hence, it is tempting to speculate that the primary role of IL-17 here might be containment of the tissue damage linked to the infection rather than driving a direct antifungal response.
The lower levels of IL-17F found in the liver of the Clec7a−/−–Clec4n−/− animals suggest that dectin-1/dectin-2 can also regulate the local production of the cytokine; however, this feature might be playing a coadjutant role. Our results also hint that the cytokine might not come from a conventional TH17 cell and that alternative sources could include the local population of γδ T cells or group 3 innate lymphoid cells (42). Nonetheless, considering the decoupling in the phenotypes between the dectin-1/dectin-2 and IL-17 knockouts for fungal restriction, assessment of the roles of these cytokines requires an independent evaluation beyond the scope of this manuscript.
In contrast to our results, Batista-Duharte et al. reported a mixed IFN-γ/IL-17 (TH1/TH17) profile in their infected WT mice (43). However, they based their interpretations on phorbol myristate acetate (PMA)/ionomycin-stimulated cells, while we employed S. brasiliensis yeast cells (antigen-specific stimulation), which could explain the discrepancy in the results. In addition, they did not employ immunodeficient animals or pharmacological blockers to confirm the relevance of these cells/cytokines in their model, hindering comparisons about functionality between their study and ours. Nonetheless, they observed that S. brasiliensis induced a weaker inflammatory response compared with S. schenckii-infected animals, which was associated with the induction of Tregs. The same authors have also shown that Tregs are actively repressing the clearance of S. schenckii (44), indicating that this might be a common denominator of S. brasiliensis-driven pathogenesis.
Finally, we acknowledge that we did not investigate the contribution of B cells and antibodies here. However, it also needs to be recognized that the relationship between humoral immunity and the pathogenesis of fungal infections in general is still a poorly explored territory. In the sporotrichosis field, the glycoprotein gp70 is well known as the main virulence factor and antigenic component of Sporothrix spp (45). Although anti-gp70 antibodies can ameliorate the infection severity (46), most of the studies have focused on their use as biomarkers for diagnosis (47) or vaccine targets for therapy (48) rather than on their role in the immunopathogenesis of the infection. Far more obscure is the connection between dectin-1/dectin-2 and antibody production; however, it is suggested that β-glucan-driven dectin-1 activation might help in the production of IgG1 antibodies by B cells (49). Nevertheless, the humoral immunity is a field worth exploring in future works.
In summary, we showed here that dectin-1 and dectin-2 are key determinants of host protection against S. brasiliensis infection. However, rather than shaping a classical TH17 response, they are involved in counterbalancing the immunosuppressed environment driven by the fungal pathogen. Our work paves the way for the exploration of these receptors and their associated signaling pathways as key targets to uncover new therapeutic strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of Chiba University (Approval number: A7-198). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FY: Investigation, Conceptualization, Writing – original draft. SRA: Conceptualization, Resources, Writing – review & editing. SS: Conceptualization, Resources, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI grant numbers 24K18433 (FSYY) and 25K10398 (SS), Joint Usage/Research Program of Medical Mycology Research Center, Chiba University grant number 25-05 (SRA), and FAPESP grant number 22/11944-9 (SRA). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Acknowledgments
We thank Junko Minakuchi for technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1668445/full#supplementary-material
References
1. Gremião IDF, Miranda LHM, Reis EG, Rodrigues AM, and Pereira SA. Zoonotic epidemic of Sporotrichosis: cat to human transmission. PLoS Pathog. (2017) 13:e1006077. doi: 10.1371/journal.ppat.1006077
2. López-Romero E, Reyes-Montes M del R, Pérez-Torres A, Ruiz-Baca E, Villagómez-Castro JC, Mora-Montes HM, et al. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. (2011) 6:85–102. doi: 10.2217/fmb.10.157
3. Rodrigues AM, Gonçalves SS, de Carvalho JA, Borba-Santos LP, Rozental S, and de Camargo ZP. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi (Basel). (2022) 8:776. doi: 10.3390/jof8080776
4. Santos MT, Nascimento LF de J, Barbosa AAT, Martins MP, Tunon GIL, Santos POM, et al. The rising incidence of feline and cat-transmitted sporotrichosis in Latin America. Zoonoses Public Health. (2024) 71:609–19. doi: 10.1111/zph.13169
5. Xavier MO, Poester VR, Trápaga MR, and Stevens DA. Sporothrix brasiliensis: epidemiology, therapy, and recent developments. J Fungi (Basel). (2023) 9:921. doi: 10.3390/jof9090921
6. Queiroz-Telles F, Bonifaz A, Rossow J, and Chindamporn A. Sporothrix and sporotrichosis. In: Rezaei N, editor. Encyclopedia of Infection and Immunity. Elsevier, Oxford (2022). p. 376–96. doi: 10.1016/B978-0-12-818731-9.00046-X
7. Rossow JA, Queiroz-Telles F, Caceres DH, Beer KD, Jackson BR, Pereira JG, et al. A one health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi (Basel). (2020) 6:247. doi: 10.3390/jof6040247
8. Poester VR, Xavier MO, Munhoz LS, Basso RP, Zancopé-Oliveira RM, Freitas DFS, et al. Sporothrix brasiliensis causing atypical Sporotrichosis in Brazil: A systematic review. J Fungi (Basel). (2024) 10:287. doi: 10.3390/jof10040287
9. Dos Santos GMP, Borba-Santos LP, Vila T, Ferreira Gremião ID, Pereira SA, De Souza W, et al. Sporothrix spp. Biofilms impact in the zoonotic transmission route: feline claws associated biofilms, itraconazole tolerance, and potential repurposing for miltefosine. Pathogens. (2022) 11:206. doi: 10.3390/pathogens11020206
10. Gómez-Gaviria M, Martínez-Álvarez JA, and Mora-Montes HM. Current progress in Sporothrix brasiliensis basic aspects. J Fungi (Basel). (2023) 9:533. doi: 10.3390/jof9050533
11. Brubaker SW, Bonham KS, Zanoni I, and Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. (2015) 33:257–90. doi: 10.1146/annurev-immunol-032414-112240
12. Rossato L, Silvana Dos Santos S, Ferreira LG, and Rogério de Almeida S. The impact of the absence of Toll-like receptor-2 during Sporothrix brasiliensis infection. J Med Microbiol. (2019) 68:87–94. doi: 10.1099/jmm.0.000876
13. Rossato L, Santos SSD, Ferreira LG, and de Almeida SR. The importance of Toll-like receptor 4 during experimental Sporothrix brasiliensis infection. Med Mycol. (2019) 57:489–95. doi: 10.1093/mmy/myy048
14. Neves GWP, Wong SSW, Aimanianda V, Simenel C, Guijarro JI, Walls C, et al. Complement-mediated differential immune response of human macrophages to Sporothrix species through interaction with their cell wall peptidorhamnomannans. Front Immunol. (2021) 12:749074. doi: 10.3389/fimmu.2021.749074
15. Yoshikawa FS, Yabe R, Iwakura Y, de Almeida SR, and Saijo S. Dectin-1 and Dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis. Innate Immun. (2016) 22:316–24. doi: 10.1177/1753425916645392
16. Yoshikawa FSY, Wakatsuki M, Yoshida K, Yabe R, Torigoe S, Yamasaki S, et al. Dectin-1/IL-15 Pathway Affords Protection against Extrapulmonary Aspergillus fumigatus Infection by Regulating Natural Killer Cell Survival. J Innate Immun. (2023) 15:397–411. doi: 10.1159/000527188
17. Chen SM, Shen H, Zhang T, Huang X, Liu XQ, Guo SY, et al. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence. (2017) 8:1643–56. doi: 10.1080/21505594.2017.1346756
18. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 Recognition of α-Mannans and Induction of Th17 Cell Differentiation Is Essential for Host Defense against Candida albicans. Immunity. (2010) 32:681–91. doi: 10.1016/j.immuni.2010.05.001
19. Dutta O, Espinosa V, Wang K, Avina S, and Rivera A. Dectin-1 promotes type I and III interferon expression to support optimal antifungal immunity in the lung. Front Cell Infect Microbiol. (2020) 10:321. doi: 10.3389/fcimb.2020.00321
20. Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, et al. Identification of distinct ligands for the C-type lectin receptors mincle and dectin-2 in the pathogenic fungus malassezia. Cell Host Microbe. (2013) 13:477–88. doi: 10.1016/j.chom.2013.03.008
21. Saijo S and Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol. (2011) 23:467–72. doi: 10.1093/intimm/dxr046
22. Galván-Hernández AK, Gómez-Gaviria M, Martínez-Duncker I, Martínez-Álvarez JA, and Mora-Montes HM. Differential recognition of clinically relevant sporothrix species by human granulocytes. J Fungi (Basel). (2023) 9:986. doi: 10.3390/jof9100986
23. Gómez-Gaviria M, Martínez-Duncker I, García-Carnero LC, and Mora-Montes HM. Differential Recognition of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by Human Monocyte-Derived Macrophages and Dendritic Cells. Infect Drug Resist. (2023) 16:4817–34. doi: 10.2147/IDR.S419629
24. Prosser A, Dart S, Larma-Cornwall I, and Lucas M. Flow cytometric characterization of tissue-resident lymphocytes after murine liver and heart transplantation. STAR Protoc. (2021) 2:100810. doi: 10.1016/j.xpro.2021.100810
25. Vargas-Macías AP, Gómez-Gaviria M, García-Carnero LC, and Mora-Montes HM. Current models to study the sporothrix-host interaction. Front Fungal Biol. (2022) 3:833111. doi: 10.3389/ffunb.2022.833111
26. Vinh DC. Human immunity to fungal infections. J Exp Med. (2025) 222:e20241215. doi: 10.1084/jem.20241215
27. Conti HR and Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. (2015) 195:780–8. doi: 10.4049/jimmunol.1500909
28. Geijtenbeek TBH and Gringhuis SI. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol. (2016) 16:433–48. doi: 10.1038/nri.2016.55
29. Hasegawa H and Matsumoto T. Mechanisms of tolerance induction by dendritic cells in vivo. Front Immunol. (2018) 9:350. doi: 10.3389/fimmu.2018.00350
30. Coste AT, Imbert C, and Hennequin C. Candida auris, an emerging and disturbing yeast. J Mycol Med. (2019) 29:105–6. doi: 10.1016/j.mycmed.2019.05.002
31. Uhrlaß S, Verma SB, Gräser Y, Rezaei-Matehkolaei A, Hatami M, Schaller M, et al. Trichophyton indotineae-an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide-A multidimensional perspective. J Fungi (Basel). (2022) 8:757. doi: 10.3390/jof8070757
32. Ikeda MAK, de Almeida JRF, Jannuzzi GP, Cronemberger-Andrade A, Torrecilhas ACT, Moretti NS, et al. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front Microbiol. (2018) 9:2286. doi: 10.3389/fmicb.2018.02286
33. Campos RMS, Jannuzzi GP, Ikeda MAK, de Almeida SR, and Ferreira KS. Extracellular vesicles from Sporothrix brasiliensis yeast cells increases fungicidal activity in macrophages. Mycopathologia. (2021) 186:807–18. doi: 10.1007/s11046-021-00585-7
34. Castro RA, Kubitschek-Barreira PH, Teixeira PAC, Sanches GF, Teixeira MM, Quintella LP, et al. Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS One. (2013) 8:e75656. doi: 10.1371/journal.pone.0075656
35. Lopes-Bezerra LM, Walker LA, Niño-Vega G, Mora-Montes HM, Neves GWP, Villalobos-Duno H, et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. PLoS Negl Trop Dis. (2018) 12:e0006169. doi: 10.1371/journal.pntd.0006169
36. García-Carnero LC, Martínez-Duncker I, Gómez-Gaviria M, and Mora-Montes HM. Differential recognition of clinically relevant sporothrix species by human mononuclear cells. J Fungi (Basel). (2023) 9:448. doi: 10.3390/jof9040448
37. Kischkel B, Lopes-Bezerra L, Taborda CP, Joosten LAB, Dos Santos JC, and Netea MG. Differential recognition and cytokine induction by the peptidorhamnomannan from Sporothrix brasiliensis and S. Schenckii. Cell Immunol. (2022) 378:104555. doi: 10.1016/j.cellimm.2022.104555
38. Gow NAR, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. (2007) 196:1565–71. doi: 10.1086/523110
39. Adamopoulos IE and Kuchroo V. IL-17A and IL-17F in tissue homeostasis, inflammation and regeneration. Nat Rev Rheumatol. (2023) 19:535–6. doi: 10.1038/s41584-023-01004-5
40. Ramani K and Biswas PS. Interleukin-17: Friend or foe in organ fibrosis. Cytokine. (2019) 120:282–8. doi: 10.1016/j.cyto.2018.11.003
41. Zhang Y, Chen L, Gao W, Hou X, Gu Y, Gui L, et al. IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur J Immunol. (2012) 42:1523–35. doi: 10.1002/eji.201141933
42. Cua DJ and Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. (2010) 10:479–89. doi: 10.1038/nri2800
43. Batista-Duharte A, Téllez-Martínez D, Roberto de Andrade C, Portuondo DL, Jellmayer JA, Polesi MC, et al. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. (2018) 122:1163–70. doi: 10.1016/j.funbio.2018.08.004
44. Batista-Duharte A, Téllez-Martínez D, de Andrade CR, Polesi MC, Portuondo DL, and Carlos IZ. Transient Foxp3(+) regulatory T-cell depletion enhances protective Th1/Th17 immune response in murine sporotrichosis caused by Sporothrix schenckii. Immunobiology. (2020) 225:151993. doi: 10.1016/j.imbio.2020.151993
45. Padró-Villegas L, Gómez-Gaviria M, Martínez-Duncker I, López-Ramírez LA, Martínez-Álvarez JA, Niño-Vega GA, et al. Sporothrix brasiliensis Gp70 is a cell wall protein required for adhesion, proper interaction with innate immune cells, and virulence. Cell Surf. (2025) 13:100139. doi: 10.1016/j.tcsw.2024.100139
46. de Almeida JRF, Kaihami GH, Jannuzzi GP, and de Almeida SR. Therapeutic vaccine using a monoclonal antibody against a 70-kDa glycoprotein in mice infected with highly virulent Sporothrix schenckii and Sporothrix brasiliensis. Med Mycol. (2015) 53:42–50. doi: 10.1093/mmy/myu049
47. Rodrigues AM, Fernandes GF, Araujo LM, Della Terra PP, dos Santos PO, Pereira SA, et al. Proteomics-based characterization of the humoral immune response in sporotrichosis: toward discovery of potential diagnostic and vaccine antigens. PLoS Negl Trop Dis. (2015) 9:e0004016. doi: 10.1371/journal.pntd.0004016
48. de Almeida JRF, Jannuzzi GP, Kaihami GH, Breda LCD, Ferreira KS, and de Almeida SR. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci Rep. (2018) 8:4192. doi: 10.1038/s41598-018-22709-8
Keywords: Sporothrix brasiliensis, Dectin-1, Dectin-2, IL-17, Treg
Citation: Yoshikawa FSY, de Almeida SR and Saijo S (2025) Dectin-1 and dectin-2 drive protection against Sporothrix brasiliensis in experimental sporotrichosis. Front. Immunol. 16:1668445. doi: 10.3389/fimmu.2025.1668445
Received: 18 July 2025; Accepted: 08 September 2025;
Published: 25 September 2025.
Edited by:
Hector Mora Montes, University of Guanajuato, MexicoReviewed by:
Aldo Henrique Tavares, University of Brasilia, BrazilLaura Garcia-Carnero, University of São Paulo, Ribeirão Preto, Brazil
Copyright © 2025 Yoshikawa, de Almeida and Saijo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinobu Saijo, c2Fpam9AZmFjdWx0eS5jaGliYS11Lmpw
 Fabio Seiti Yamada Yoshikawa
Fabio Seiti Yamada Yoshikawa Sandro Rogerio de Almeida
Sandro Rogerio de Almeida Shinobu Saijo
Shinobu Saijo