- 1State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2State Key Laboratory of Integration and Innovation for Classic Formula and Modern Chinese Medicine, National Chinmedomics Research Center, Metabolomics Laboratory, Department of Pharmaceutical Analysis, Heilongjiang University of Chinese Medicine, Harbin, China
Gouty arthritis (GA), a condition characterized by monosodium urate (MSU) crystal deposition and NLRP3 inflammasome-driven inflammation, is a result of a complex interplay between hyperuricemia and immune dysregulation, which leads to systemic complications and joint damage. Current therapies for GA exhibit certain limitations, including cardiovascular risks, hepatotoxicity, low efficacy in special populations, and difficulty in dissolving tophi. Emerging evidence implicates fatty acid metabolism disorders as key pathogenic factors in GA. Elevated fatty acids (FAs) activate Toll-like receptors (TLRs) in macrophages, which act in synergy with MSU crystals to trigger NLRP3 inflammasome activation and pro-inflammatory cytokine release (e.g., IL-1β), thereby initiating the inflammatory cascade. Dysregulated FA metabolism promotes neutrophil recruitment through aberrant arachidonic acid (AA) metabolism and exacerbates hyperuricemia by increasing purine synthesis while inhibiting uric acid excretion. Consequently, future clinical practice may leverage the detection of FA signatures in GA patients to enable tailored therapeutic and dietary management, thereby maximizing treatment efficacy while minimizing adverse effects. The combined application of FA-modulating agents and anti-GA therapeutics synergistically enhances therapeutic efficacy, enabling comprehensive disease-modifying control over GA progression. This review systematically elucidates the mechanisms through which FA metabolism disorders drive the progression of GA, providing a scientific basis for the subsequent research on GA.
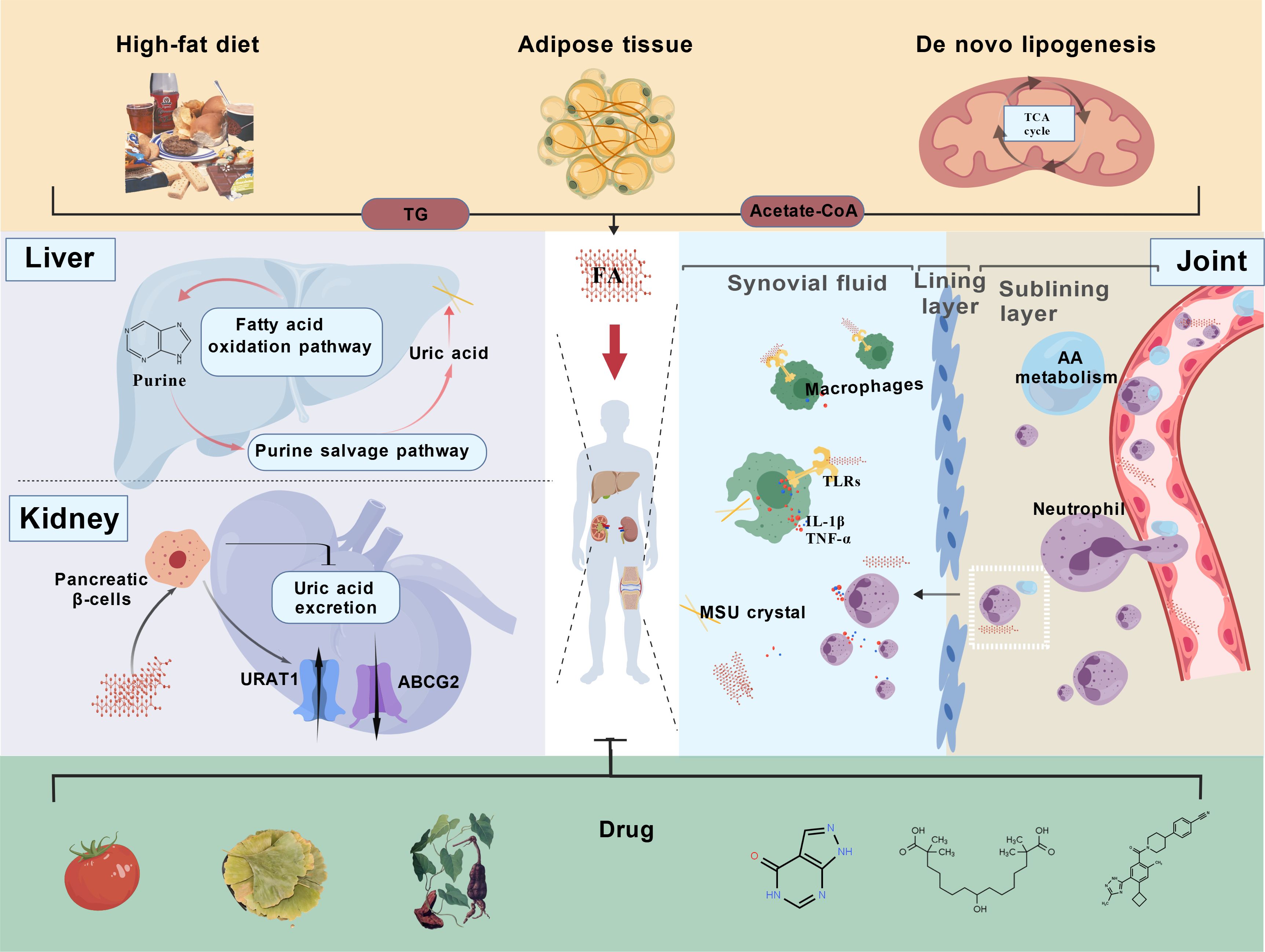
Graphical Abstract. FA, fatty acid; MSU, monosodium urate; TG, triglyceride; GLUT9, glucose transporter type 9; URAT1, urate transporter 1; AA, arachidonic acid; IL-1β, interleukin - 1 beta; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-alpha.
1 Introduction
Gouty arthritis (GA) is one of the most common types of inflammatory arthritis (1), characterized by the deposition of monosodium urate (MSU) crystals in joints and surrounding tissues under conditions of persistent hyperuricemia (2), which activates the innate immune system and triggers an inflammatory response. It is a disease caused by metabolic disorders (3). The global prevalence of GA ranges from 0.68% to 3.90%, increasing the burden on healthcare (4, 5). High uric acid levels alone are not a sufficient condition for the onset of GA: follow-up studies have shown that some individuals with low uric acid levels develop GA, while approximately 50% of individuals with high uric acid levels do not develop the condition during a 15-year follow-up period (6, 7). This contradictory clinical presentation poses a challenge for both clinicians and basic scientists.
MSU crystals were previously thought to be an endogenous danger signal for GA. However, most in vitro studies have identified MSU crystals as inflammasome activators needed to use lipopolysaccharide (LPS) or phorbol-12-myristate-13-acetate (PMA) to trigger the activation of the inflammasome in cells (8–11). Moreover, a comprehensive analysis of the data from 268,174 participants in the UK Biobank revealed that the plasma FA levels of 5,160 participants who developed GA were associated with the risk of GA onset (12). Another relevant study reported similar results: serum FA levels were significantly higher in patients with acute GA than in those with GA in remission, asymptomatic hyperuricemic patients, and normal controls (13). These findings suggest that FAs serve as important cofactors in MSU-induced inflammation. Other factors may act synergistically to trigger inflammation, and FA may be one of the key factors.
Further exploration revealed that during the course of inflammation, FA-related metabolic disorders promote inflammation by affecting the metabolism and microenvironment of macrophages and neutrophils (14–16). In addition, FA may aggravate hyperuricemia by promoting purine synthesis and inhibiting uric acid excretion (17–19). Traditional studies on GA and FAs, such as TAG, DAG, and PC, have focused primarily on lipid changes. However, this review uniquely bridges the knowledge gap in prior literature by elucidating the relationship between fatty acid metabolism and GA through a tripartite analysis of fatty acid synthesis, degradation pathways, and free FA levels. Moreover, fatty acid metabolism exerts distinct roles in GA, exemplified by its promotion of M1 macrophage polarization via fatty acid oxidation—a mechanism that operates conversely in other diseases. Given the strong associations between fatty acid metabolism and immune responses and elevated uric acid levels during GA attacks, the progression of GA under the mediation of fatty acid metabolic disorders was reviewed in this paper to provide a theoretical basis for exploring novel therapies for GA.
2 Comprehensive overview of fatty acids
FAs are organic acids that are defined mainly by the length and saturation of their aliphatic side chain. When classified according to the length of their side chain, FAs are divided into three types. Short-chain FAs (SCFAs), also known as volatile FAs, have 2 to 6 carbon atoms within their structures. FAs with chain lengths of 6 to 11 carbon atoms are called medium-chain fatty acids (MCFAs), whereas FAs with structures comprising alkyl chain lengths greater than 12 carbon atoms are considered long-chain fatty acids (LCFAs). LCFAs are usually present in vegetable oils, animal fats, or marine oils (20) and include palmitic acid (C16:0), palmitoleic acid (C16:1), arachidonic acid (20:4n-6), and docosahexaenoic acid (22:6n-3). C16-C18FAs are components of FA-derived signaling molecules. Long-chain polyunsaturated FAs such as arachidonic acid (AA) and docosahexaenoic acid serve as precursors of the major lipid signaling molecules prostaglandin (PEG2) and leukotriene (LT) (16). FAs also serve as important nutrients for the human body. The intake, transport, and metabolism of FAs in the body are complex and intricate processes.
2.1 Free fatty acid intake and transport
FA is ingested mainly from food in the form of triglycerides (TGs). TGs are formed by the combination of three FA molecules with one glycerol molecule and exist primarily in the form of dietary fat (about 95%) (21). TG is naturally hydrophobic. In the oral cavity, stomach, and intestinal cavity, TG is hydrolyzed by various lipases to produce two FA molecules and one monoacylglycerol (MAG) molecule. SCFAs (containing 2–6 carbon atoms) and MCFAs in the hydrolysis products directly enter the portal vein and are then transported to the liver through the bloodstream. The absorbed LCFA are resynthesized into TG in the small intestinal mucosal cells and form chylomicrons with apolipoproteins, cholesterol, etc (22). Subsequently, these chylomicrons are transported into the plasma through the lymphatic system, and most of the TG within these chylomicrons is absorbed by various tissues through the circulatory system (23).
2.2 Fatty acid metabolism
After cellular uptake, FAs are degraded mainly through mitochondrial FA β-oxidation, which is crucial for maintaining energy homeostasis in the human body (24). FAs bind to coenzyme A (CoA) in the cytoplasm to form acyl-CoA. Long-chain acyl-CoA then enters the mitochondria under the action of carnitine palmitoyltransferase 1 (CPT1), where it is converted to fatty acyl-carnitine (25). The β-oxidation process breaks down FAs into acetyl-CoA, which is then utilized in the mitochondrial tricarboxylic acid (TCA) cycle to produce adenosine triphosphate (ATP) for energy (26, 27). However, when FA levels exceed the energy requirements of the cells, these FAs are stored in the adipose tissue mainly in the form of TG. In periods of energy deficiency, TG hydrolysis occurs, producing FA and glycerol, and in this process of fat breakdown, energy is released for internal use. In addition, FAs enter the vascular system for use as an energy substrate in other organs (28). TG is hydrolyzed sequentially to form diacylglycerol (DAG) and then MAG, releasing FA at each stage. MAG is hydrolyzed to release the final FA and glycerol. The production of FA and glycerol from stored TG utilizes a series of highly coordinated enzymatic actions involving adipose triglyceride lipase (TGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL) (29).
In the condition of an imbalance of the production and degradation of FAs, the levels of circulating FAs in the body increase. This paper focuses on the role of elevated circulating FA levels in the pathogenesis of GA.
3 Fatty acid biomarkers for gout arthritis
A metabolomics study based on serum NMR spectroscopy of asymptomatic hyperuricemic and GA patients revealed significantly altered FA levels, which may serve as risk biomarkers for disease onset (30). A study that used lipidomics to distinguish early-onset hyperuricemia from GA revealed a systemic elevation in FA levels among patients with hyperuricemia and GA. Shen et al. identified dysregulated pathways and potential metabolic biomarkers for hyperuricemia and GA using serum metabolomics. The authors discovered that AA can be used as a marker to distinguish hyperuricemic patients from non-hyperuricemic individuals. Furthermore, the authors identified two fatty acids – AA and myristic acid – as potential biomarkers for differentiating GA patients from hyperuricemic patients (31). Moreover, Wang et al. reported that arachidic acid can be used as a metabolic marker to distinguish frequent GA episodes from infrequent GA episodes, thereby representing a potential biomarker. Furthermore, eicosapentaenoic acid levels are significantly lower in patients with frequent seizures than in those with infrequent seizures (32). A cohort study involving the integrated use of genetic susceptibility and metabolomics along with 1,708 GA cases and a follow-up period of 9.47 years to investigate the association between dietary polyunsaturated FAs and GA risk reported that PUFA, n-6 PUFA, n-3 PUFA, ALA, and EPA intake were negatively associated with gout risk, and AA intake was positively associated with gout risk (33). Wang et al. reported that stearic acid concentrations were reduced in acute GA patients and could potentially serve as a specific biomarker for acute GA (34). A comprehensive analysis of a population cohort and genetic data of 15,194 participants revealed that SFAs, MUFAs, n-3 PUFAs, and docosahexaenoic acid (DHA) were positively associated with GA events (P values < 0.0001), while PUFAs, n-6 PUFAs, and linoleic acid were negatively associated with GA incidence (all trend P values < 0.0001) (12). However, a study on dietary supplementation with n-3 PUFAs polyunsaturated FAs indicated that the intake of n-3 PUFAs, which are abundant in dietary fish, is associated with a lower risk of GA recurrence (35, 36). Therefore, the role of n-6 and n-3 PUFAs in the GA still requires further validation.
Clinical omics research has concluded that abnormal changes in FA levels play a pivotal role in the progression of gestational age (GA). However, the current studies on GA have not extensively investigated the role of unsaturated FAs in regulating the GA pathway, and the existing research remains incomplete. Pandey, S. proposed that the research on the treatment for immune-mediated diseases, such as rheumatoid arthritis, should attempt to achieve a more comprehensive understanding of various disease-related biomarkers, conducting large-scale high-throughput data analysis, and identifying and quantifying the disease metabolites (37). Therefore, subsequent research on GA can comprehensively analyze its lipid biomarkers through lipidomics, providing a basis for both fundamental research on GA and its precise clinical application.
4 Disorders of fatty acid metabolism promote the development of gouty arthritis
A diagram illustrating the relationship between gouty arthritis and immunity (Figure 1).
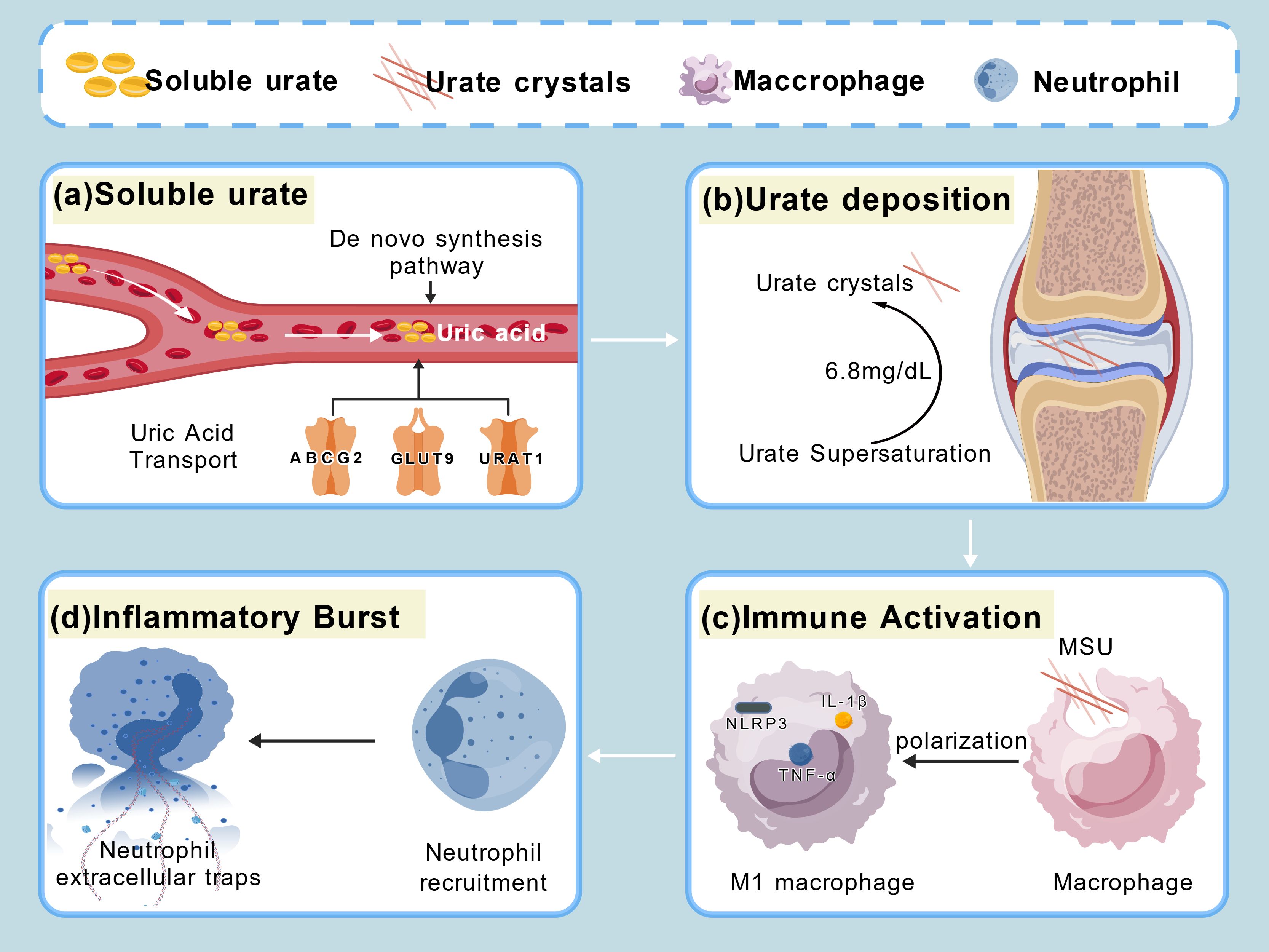
Figure 1. (a) Imbalance between uric acid production and transport leads to elevated uric acid levels. (b) Uric acid levels exceeding 6.8 mg/dL result in the precipitation of urate crystals. (c) Macrophages phagocytose MSU crystals to activate immune responses. (d) Macrophages are recruited to the site of injury and release neutrophil extracellular traps.
4.1 Macrophage fatty acid metabolic disorders potentiate MSU crystal-induced inflammation
Numerous studies have shown that the inflammatory factor interleukin-1 beta (IL-1β) is essential for MSU crystal-driven inflammation and is released, in place of infiltrating neutrophils or monocytes, from the resident macrophages in the initial stage of GA (38, 39). However, the production of IL-1β during the GA process is puzzling, with studies reporting that MSU crystals alone do not participate in the transcription of IL-1β but only in the regulation of the conversion of inactive IL-1β to an activated state (10, 11). Post-transcriptional IL-1β exists solely in the inactive Pro-IL-1β form, which has to be cleaved by the NLRP3 inflammasome-activated effector protein caspase-1 for activation, and this activated form then exerts the proinflammatory effects (40).
Joosten et al. conducted an in-depth study and reported that FAs participate in the transcription of NLRP3 and Pro-IL-1β, promoting the onset of GA inflammation by activating the Toll-like receptor (TLR) pathway in conjunction with MSU (41). Mechanistically, the TLR2/TLR4 proteins, which are members of the TLR family and are highly expressed in the synovial macrophages of GA patients, are first activated through the recognition of FAs. TLRs are pattern recognition receptors (PRRs) involved in the innate immune system and can recognize pathogen-associated molecular patterns (PAMPs) and endogenous ligands (41–44). Another perspective is that fatty acid synthesis may promote inflammation by facilitating TLR activation (45). The palmitate produced through the FASN pathway can be reversibly conjugated to the cysteine residues of target proteins in a process known as palmitoylation (S-acylation) (46). The palmitoylation process affects TLR/MYD88 signaling, and FASN supplies the endogenous FAs required for MYD88 palmitoylation, with cysteine 113 serving as a critical site for MYD88 palmitoylation. This process stabilizes the intermediate domain, which ensures the binding of MYD88 to interleukin-1 receptor-associated kinase 4 (IRAK4). IRAK4 is a pivotal molecule linking the TLR receptors to downstream inflammatory signaling activation, thereby initiating the TLR downstream signaling cascade (45). Studies have indicated that fatty acid synthesis enzyme (FASN) influences subsequent inflammatory development by regulating the TLR signaling pathways. FASN is a multidomain enzyme with a ketosynthetase domain that processes acetoacetyl-CoA, an intermediate metabolite that serves as a key substrate for lipid rafts. The activation of FASN increases the production of acetoacetyl-CoA, significantly altering lipid raft composition (47). Inhibition of FASN or acetoacetyl-CoA effectively reduces the affinity between the lipid rafts and TLR4, thereby diminishing the migration of TLR4 toward the lipid rafts, preventing its specific recognition function (47) Therefore, activated FASN may significantly increase the ability of TLR4 to recognize ligands.
Thus, TLR2/TLR4 activation promotes the dissociation of the inhibitor of nuclear factor kappa-B (I-κB)/NF-κB complex through a myeloid differentiation primary response 88-dependent (MyD88) signaling pathway, mediating the nuclear translocation of nuclear factor kappaB (NF-κB) and specifically acting on the NLRP3 and IL-1B gene promoter regions. This leads to the mediation of the transcription of NLRP3 and pro-IL-1β, resulting in the production of many inactive inflammatory mediators, leading to subsequent protease cleavage and activation (48–51). TLR4 can also form a TLR4/myeloid differentiation factor 2 (MD2) complex to further mediate the signal transduction (52). Finally, inactive NLRP3 and pro-IL-1β mediate the initiation of inflammation upon stimulation by MSU crystals. After the macrophages ingest MSU through the phagocytic pathway, NLRP3 inflammasome assembly and activation are triggered, and the generated complex consists of NLRP3, the adaptor protein apoptosis-related cyclin-like protein (ASC), and caspase-1 (53). The activation of this complex induces the self-cleavage of pro-caspase-1 into active caspase-1, which then undergoes a site-specific hydrolysis of pro-IL-1β to generate mature IL-1β, thereby activating the related signaling pathways to mediate the inflammatory cascades (40, 54). Moreover, Leishman et al. proposed that fatty acid synthesis pathways play crucial roles in NLRP3 inflammasome assembly and activation by palmitoylating NLRP3, during the initiation step, at the Cys898 site of NLRP3 (55). Subsequently, palmitoylation mediates the process of NLRP3 accumulation within the endosome, facilitating its binding to the dispersed trans-Golgi network vesicles for their complete assembly (55, 56). On the basis of the above mechanism, it can be inferred that fatty acid metabolism may play an important role in the initiation of inflammation in GA macrophages. Single MSU crystal phagocytosis has a limited effect on the production of large amounts of inflammatory factors, and the FA-mediated TLR/MyD88 pathway plays a key role in this process.
FA can also induce the direct activation of the NLRP3 inflammasome in macrophages. Palmitate can activate the NLRP3 inflammasome through lysosomal instability in macrophages (57). In addition, palmitate inhibits the phosphorylation of adenosine 5denosinelation_ENREF_57” protein kinase (AMPK) and blocks autophagy, leading to increased ROS levels in macrophages, which in turn activate the NLRP3 inflammasome and IL-1β secretion (58).
On the other hand, macrophages respond to various stimuli in the microenvironment and are reprogrammable into different functional subtypes after activation, usually converting in two directions: pro-inflammatory and anti-inflammatory subtypes, which are not fixed states but are mutually convertible for suitability to the changing needs and environments (59). In a gout zebrafish model within a complete microenvironment, macrophage-dependent fatty acid oxidation (FAO) promoted the conversion of the proinflammatory phenotype (60). FAO-driven mitochondrial-derived ROS (mROS) depend on the expression of immune-responsive gene 1 (irg1) (61), which has been identified as one of the highly overexpressed genes in acute GA macrophages (62). MSU crystal-activated irg1 drives mROS to promote macrophage IL-1β and tumor necrosis factor-alpha (TNF-α) expression. Many previous studies have suggested that FA is the main metabolic pattern of anti-inflammatory macrophages, whereas aerobic glycolysis is considered the driving factor of pro-inflammatory states (27). However, most studies on macrophage metabolic reprogramming have remained limited to the use of in vitro techniques utilizing few inflammatory stimuli (mainly LPS) or non-GA models, and are, therefore, inadequate for explaining macrophage polarization in GA (63). Jiang et al. explored this topic and reported that inhibiting FAO can suppress the inflammatory response of macrophages in MSU crystal-induced GA models, providing evidence supporting the above view (64). Liu et al. reported that suppressing AA metabolism by using COX-2, 5-LOX, and CYP4A in macrophages under GA conditions shifted the macrophages away from the M1 phenotype (65). On the basis of the above explanation, it is possible that the metabolic pattern of macrophages relies highly on changes in the intact microenvironment and that FAO in the GA model drives macrophages toward a proinflammatory phenotype.
In summary, a large body of research evidence shows that FA synergizes with MSU crystals to induce the initiation of inflammation in GA: FA induces macrophage activation by activating TLR receptors, lysosomal instability/NLRP3, and AMPK/ROS/NLRP3 pathways, while more active FAO promotes the conversion of macrophages to a pro-inflammatory phenotype (Figure 2).
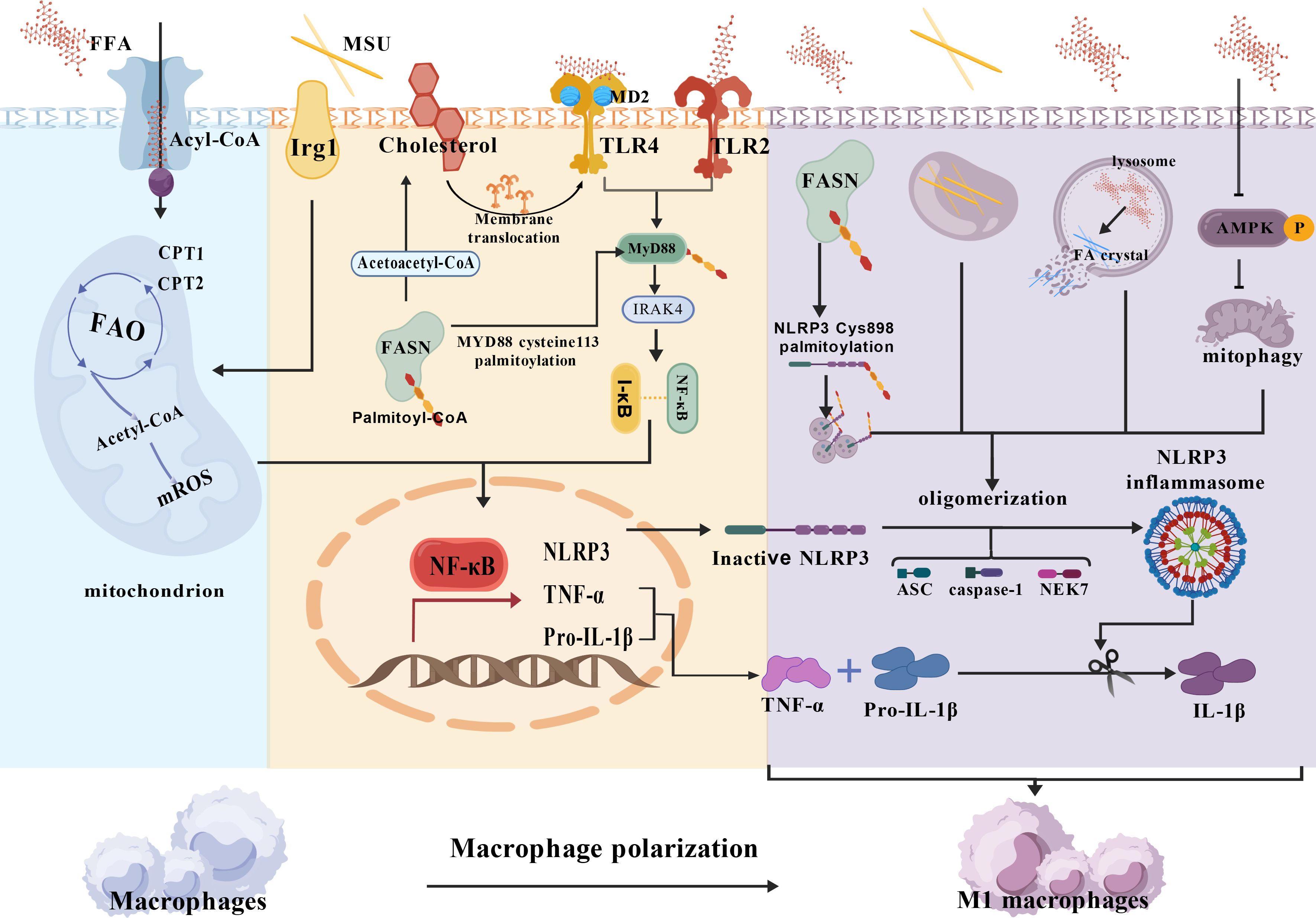
Figure 2. Macrophage fatty acid metabolic disorder potentiates MSU crystal-induced inflammation. [Created with BioGDP.com (244)] NLRP3, Nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3; ASC, adaptor protein apoptosis-related cyclin-like protein; FAO, Fatty acid oxidation; CPT1,2, Carnitine palmitoyltransferase 1,2; Irg1, Immune-responsive gene 1; MyD88, Myeloid differentiation primary response 88; I-κB, inhibitor of nuclear factor kappa-B; MD2, Myeloid differentiation factor 2;AMPK, adenosine 5′-monophosphate-activated protein kinase; NF-κB, Nuclear factor kappaB; FASN, Fatty Acid Synthase; (1) FFA promotes Pro-IL-1β, TNF-α, and NLRP3 transcription via FAO, FFA/TLRs; (2) FFA synergizes with MSU crystals to activate NLRP3 inflammatory vesicles via AMPK/ROS/NLRP3, lysosomal instability/NLRP3 pathways, promotes IL-1β release, and promotes macrophage M2 polarization.3.2 Disorders of fatty acid metabolism: promotion of neutrophil recruitment.
4.2 Fatty acid metabolic dysregulation promotes neutrophil recruitment in gouty arthritis
Neutrophils are the most abundant white blood cells in human peripheral blood. These cells are rapidly recruited from the blood to the inflamed tissues under the action of chemokines that signal danger (66). Acute inflammation in GA is accompanied by neutrophil infiltration, which can promote this process by stimulating the macrophages to produce inflammatory factors and chemokines (67, 68). Furthermore, studies have shown that the FA metabolites in neutrophils play a chemotactic role in the infiltration of neutrophils into the inflammatory sites, with AA metabolism playing a dominant role (69, 70) Multiple clinical studies have revealed that patients with GA exhibit abnormal AA metabolism, significantly elevated 5-LOX transcription levels, and abnormal increases in LTB4 production, which acts as a neutrophil chemotactic factor (71–73). AA is broken down into different metabolites through three metabolic pathways: lipoxygenase, cytochrome P450 monooxygenase, and cyclooxygenase. LTB4 is produced through the lipoxygenase pathway and exerts its effects (74).
Rae et al. conducted clinical research and reported that MSU not only stimulates white blood cells to produce more LTB4 but also inhibits LTB4 metabolism, causing it to persist and recruit more neutrophils (75). Subsequent studies by other researchers investigated the treatment of GA by inhibiting the LTB4 production pathway, and the results further corroborated the above conclusion (61). Although the mechanism through which MSU crystals promote AA metabolism toward LTB4 production remains unclear to date, the mechanism through which MSU crystals recruit neutrophils to the sites of inflammation is relatively well understood. To elaborate, after responding to MSU crystal stimulation and producing primary chemotactic signals, neutrophils release LTB4 through autocrine and paracrine mechanisms and form chemotactic gradients to recruit cells from a distance, causing a significant increase in the range and persistence of detection (76–78). In the MSU-induced GA mouse model, complement C5a was secreted as a primary chemotactic signal for neutrophils (77), which could then bind to the G protein-coupled receptors to generate calcium ion flux (79), thereby mediating vesicle fusion with the cell membrane and promoting the secretion of exosomes coated with LTB4 (80). During exosome formation, MSU crystal-stimulated neutrophils promote the synthesis of LTB4: cytosolic phospholipase A2 alpha translocates to the nuclear membrane, where it releases AA from membrane-bound phospholipids, whereas 5-lipoxygenase (5-LOX) is mobilized to the nuclear membrane, where it binds to 5-LOX-activating protein (FLAP) and acts on AA to produce leukotriene A4 (LTA4). Proteomic and metabolomic studies revealed that the levels of phospholipase A2 in the synovial fluid of GA patients are significantly elevated and exhibit a strong interaction with the significantly increased metabolite PE (81). Simultaneously, 5-LOX is recruited to the nuclear envelope, where it binds to the 5-LOX-activating protein (FLAP) and acts on AA to generate leukotriene A4 (LTA4). LTA4 is ultimately converted, under the action of LTA4 hydrolase (LTA4H), into LTB4, which is encapsulated within exosomes for release (82).
The subsequently released LTB4 causes neutrophils to adhere to and become trapped within the blood vessels, leading to their extravasation and migration from the bloodstream into the inflamed tissue. The process involves multiple steps and fine regulation, with LTB4 signal transduction coordinating the dynamic redistribution of non-muscle myosin IIA (NMIIA) and β2-integrin (Itgb2), thereby promoting neutrophil arrest and extravasation. First, leukotriene B4 receptor 1 (BLT1) is a chemotactic G protein-coupled receptor expressed by leukocytes (83). LTB4 binds to BLT1 to activate leukocytes and prolong their survival time (84). The LTB4-BLT1 axis is essential for the sustained recruitment, stasis, and extravasation of neutrophils (85, 86). The LTB4-BLT1 axis subsequently promotes the redistribution of NMIIA from the cytoplasm to the cell cortex by regulating its kinetics, thereby providing the contractile force required for neutrophils to squeeze through the exudation site (85). Ultimately, the activated NMIIA continues to act on its downstream target, Itgb2, regulating its kinetics and localization on the plasma membrane (PM) to promote the stasis of rigid neutrophils (87). Abnormal AA metabolism disorders in GA promote the production of the neutrophil chemokine LTB4 and mediate the migration of neutrophils to the inflammatory sites through paracrine and autocrine mechanisms.
Additionally, the neutrophils arriving at the site of injury can trigger the release of inflammatory cytokines by releasing neutrophil extracellular traps (NETs), thereby promoting the inflammatory response (88). LTB4 acts as a key signaling molecule, coordinating neutrophil activation and guiding the formation of NETs (89). Research has demonstrated that saturated FAs promote the release of NETs through the TLR4-MD2/ROS signaling pathway (90) Neutrophil extracellular traps (NETs) are fibrous web-like structures that protrude from the membranes of activated neutrophils and are coated with histones, proteases, and granular cytosolic proteins (91). SFA induces the formation of the TLR4-MD2 complex, which further promotes NOX-derived ROS production. The generation of ROS serves as the basis for NET formation, thereby exacerbating NET release (90). Moreover, FAs activate NADPH oxidase to promote ROS production and increase the expression of p38, ERK, and JNK. The massive release of ROS and the activation of the JNK and ERK pathways induce the formation of NETs; however, the specific mechanism remains unclear (92).
In addition, studies have shown that dietary SFAs play a powerful role in promoting the transport of neutrophils from the bone marrow to the blood through the C-X-C motif chemokine ligand 2/C-X-C motif chemokine receptor 2 axis (14). The amplification of neutrophil purinergic activity during migration depends on Cpt1A-mediated FAO. Cpt1A is considered the rate-limiting enzyme in the LCFA mitochondrial metabolism, and its inhibition reportedly suppresses the purinergic transfer in neutrophils, reducing their chemotactic ability (93). Immature neutrophils also rely on mitochondrial FA β-oxidation for ATP. In experimental studies, when Mycobacterium tuberculosis (Mtb) infection induced neutrophil recruitment to the site of infection, immature neutrophils took in exogenous FA to obtain energy to reach the site of infection (94).
In summary, during acute GA attacks, the migration of neutrophils and the promotion of inflammatory processes are closely linked to FA metabolism (Figure 3).
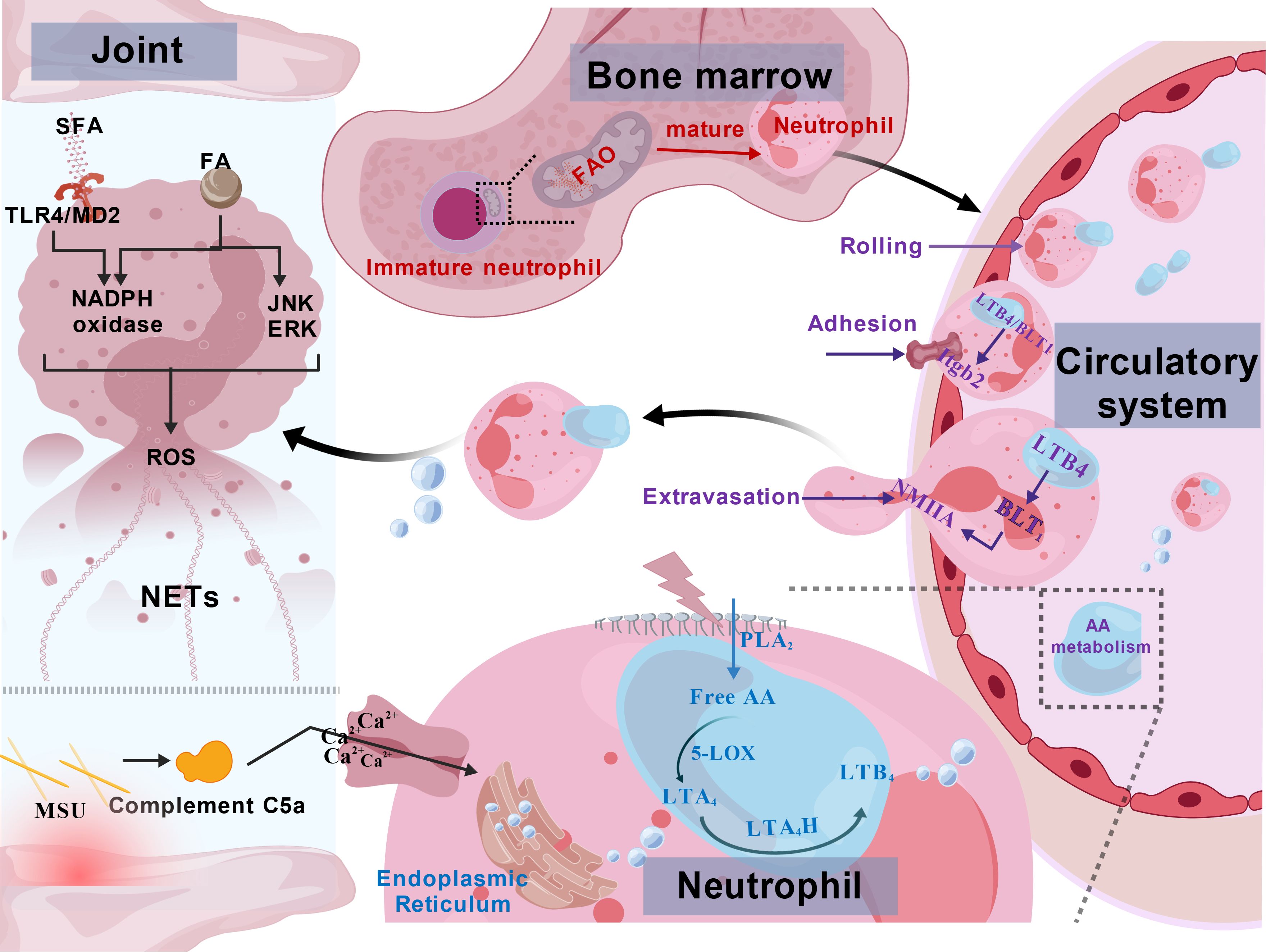
Figure 3. Fatty acid metabolic dysregulation promotes neutrophil recruitment in gouty arthritis. LTB4, Leukotriene B4; NMIIA, non-muscle myosin IIA; Itgb2, β2-integrin; LTA4, leukotriene A4; LTA4H, LTA4 hydrolase; PLA2, Phospholipase A2; 5-LOX, 5 – Lipoxygenase; NETs, Neutrophil Extracellular Traps; JNK, c-Jun N-terminal Kinase; ERK, Extracellular Signal-Regulated Kinase; SFA, Saturated Fatty Acids. (1) Immature neutrophils are dependent on FAO for energy supply; (2) the GA environment activates AA metabolism to produce LTB4 to form a chemotactic gradient that causes neutrophils to roll, adhere, extravasate, and recruit to the site of infection in the vasculature.
4.3 Fatty acid metabolic dysregulation induces hyperuricemia in gout pathogenesis
Hyperuricemia is an established modifiable risk factor for gouty arthritis (GA), which directly influences disease progression through dynamic fluctuations in serum urate levels (95). FAs derived from dietary fat intake, adipose tissue lipolysis, and de novo fatty acid synthesis (DNL) induce purine salvage synthesis pathways to promote excessive uric acid synthesis (18, 96–99). Most people with endogenous excess uric acid production appear to have a salvage pathway, and FA promotes uric acid synthesis through the purine salvage pathway by activating hypoxia-inducible factor-1α (HIF-1α) to upregulate the expression of purine metabolism-related enzymes (18, 100–102).
HIF-1α serves as the primary molecular mediator of the hypoxic response. The single-cell transcriptomic analysis of GA patients revealed that the encoding gene, HIF-1A, is significantly upregulated in the monocytes of GA patients (103). This upregulation occurs under hypoxic conditions, leading to its binding to HIF-1β within the cell nucleus, where HIF-1α interacts with hypoxia response elements (HREs) to induce the activation of numerous downstream genes (104).
FAs can increase the de novo synthesis of HIF-1α at the translational level through fatty acid-binding protein 5 (FABP5) (105), and in GA models, the FA-induced significant upregulation of acyl-CoA synthase long-chain family member 1 (ACSL1) and CPT1A-promoted FA β-oxidation together activate HIF-1α in the liver cells (106). Activated HIF-1α enters the cell nucleus, where it binds to the promoters of the xanthine dehydrogenase (XDH) and 5’-nucleotidase II (NT5C2) genes, thereby upregulating the expression of XDH and NT5C2. NT5C2 is the enzyme responsible for catalyzing the hydrolysis of inosine monophosphate (IMP) and guanosine monophosphate (GMP). After hydrolysis, IMP and GMP are converted, under the action of other enzymes, to hypoxanthine and xanthine, which serve as substrates for XDH, thereby increasing the consumption of hypoxanthine and the production of uric acid in the liver (107).
FAs may also increase uric acid levels by inhibiting the excretion process. Excessive FAs in the circulatory system promote insulin secretion from pancreatic beta cells, upregulate the expression of renal uric acid reabsorption proteins Glucose transporter type 9 (GLUT9) and urate transporter 1 (URAT1), and reduce the level of uric acid excretion protein ATP-binding cassette transporter G2 (ABCG2), resulting in elevated serum uric acid levels (16, 17, 19). Therefore, it may be inferred that fatty acid metabolism also plays a crucial role in promoting the increase in GA uric acid levels (Figure 4).
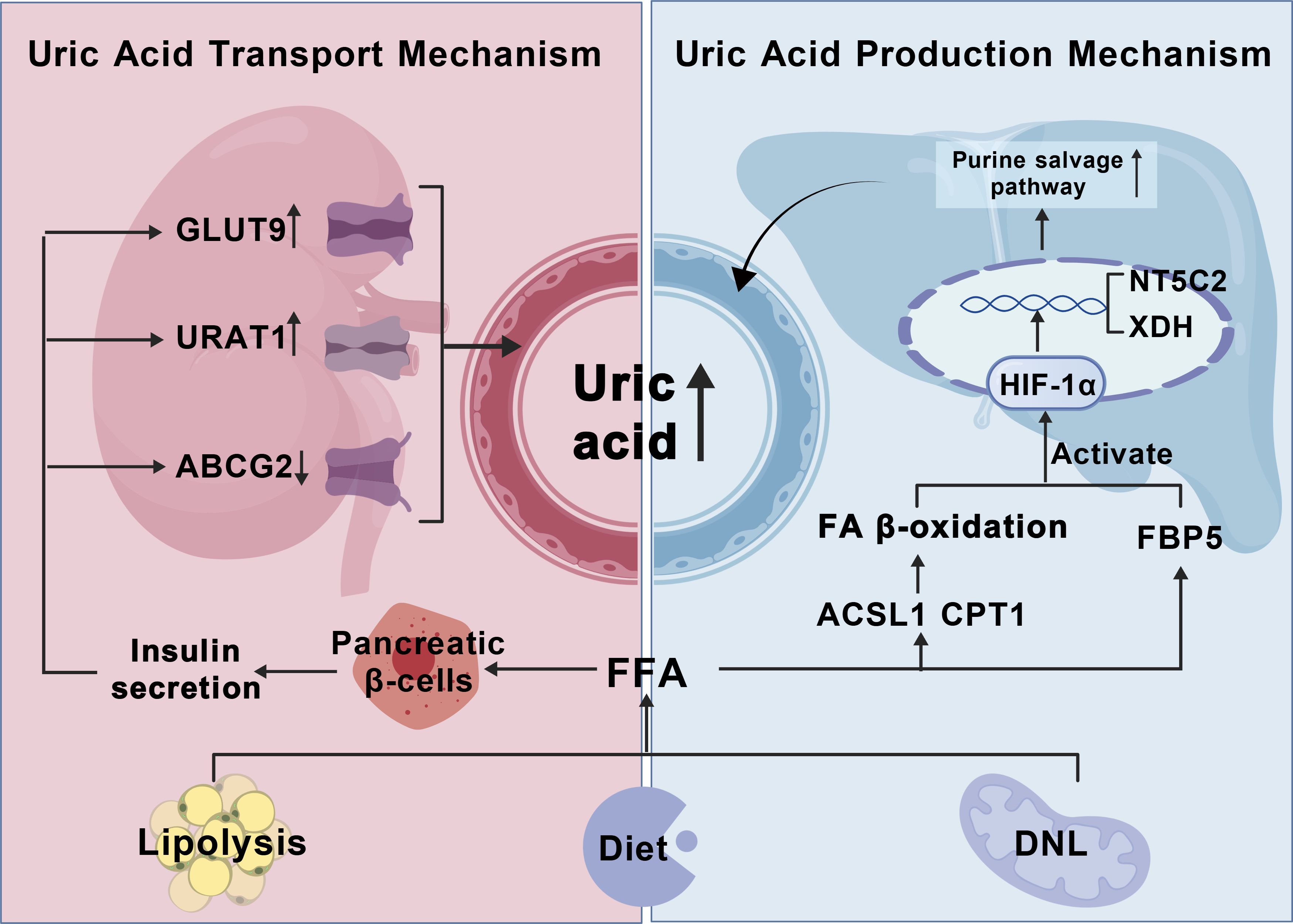
Figure 4. Fatty acid metabolic dysregulation induces hyperuricemia in gout pathogenesis. HIF-α, Hypoxia-inducible factor-1α; NT5C2, 5’-nucleotidase II; XDH, xanthine dehydrogenase; ACSL1, acyl - CoA synthetase long chain family member 1; CPT1, Carnitine Palmitoyltransferase 1; (1) FFA sources: high-fat diet, lipolysis of adipose tissue, ab initio fatty acid synthesis; (2) FAO induces HIF-α transcription, nuclear translocation, and promotes uric acid production by the purine salvage pathway (3) FFA promotes GLUT9,URAT1 uric acid reabsorption proteins through pancreatic islet β-cells, and inhibits ABCG2 uric acid excretory proteins to raise uric acid levels.
5 Bench to bedside: fatty acid metabolism modulation for gout therapy
5.1 Regulation of lipolysis and fat synthesis
5.1.1 PPARγ agonists
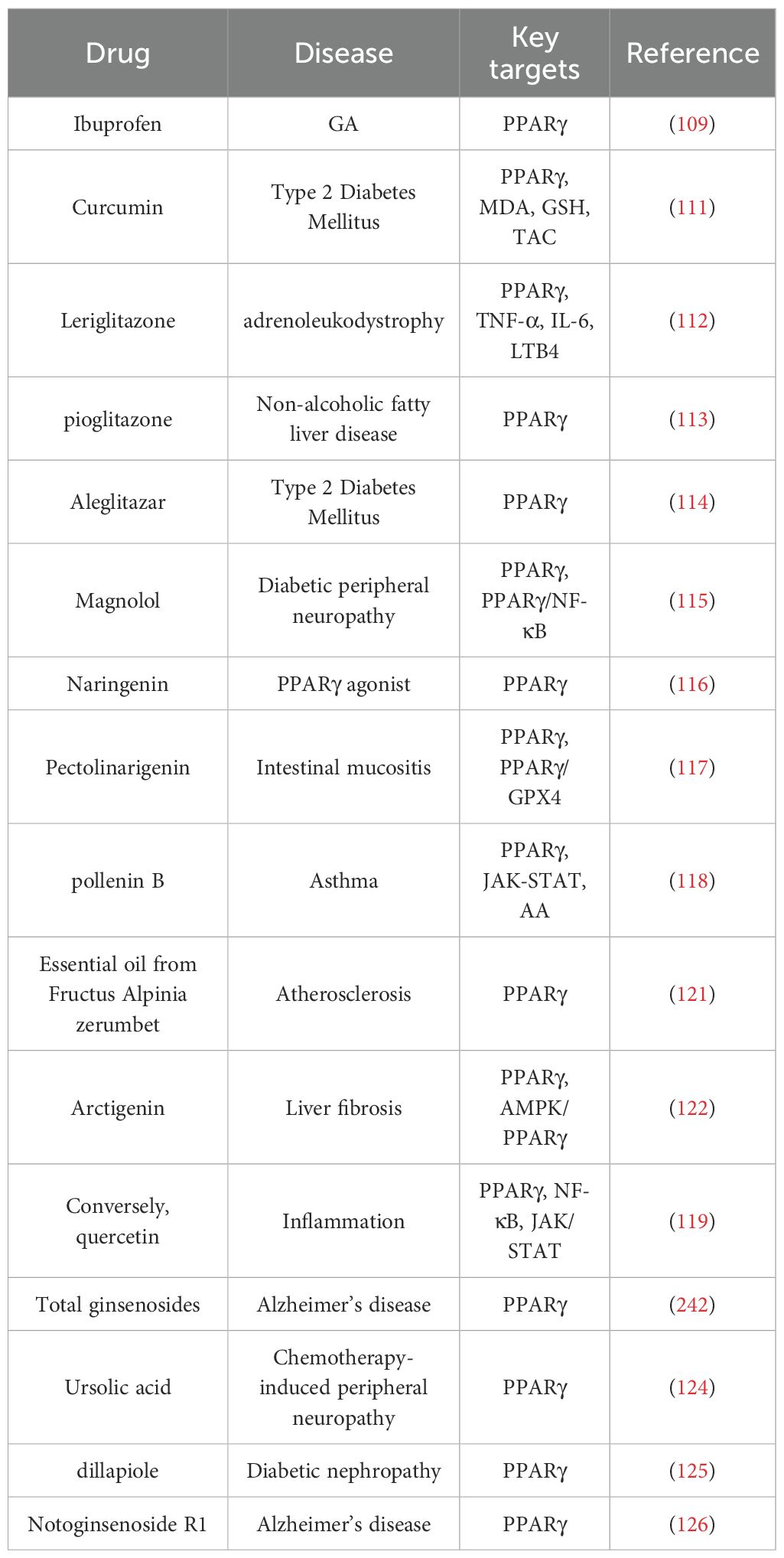
PPARγ utilizes both exogenous and endogenous FAs as substrates to promote lipogenesis and lipid synthesis. PPARγ is expressed in the white adipose tissue, the liver, skeletal muscle, the gut, and immune cells, and can effectively reduce the FA levels in the body (108). PPARγ has also been found to exert anti-inflammatory effects and potential protective effects in animal models of neurological, cardiovascular, and psychiatric disorders. Therefore, PPARγ agonists may serve as potential therapeutic agents for treating GA by regulating the FA levels.
Early clinical trials have revealed that ibuprofen can be used to treat acute GA, and no adverse reactions have been reported (109). Recent studies have indicated that ibuprofen may also function as a PPARγ agonist (110). Therefore, curcumin may be the most promising drug currently for the clinical treatment of GA episodes caused by excessively high FA levels. A clinical trial investigating the effects of curcumin on psychological status, inflammation, and oxidative damage markers in patients with type 2 diabetes and coronary heart disease (IRCT20170513033941N63) revealed reduced malondialdehyde (MDA) levels, increased total antioxidant capacity and glutathione (GSH) levels, and upregulated PPAR-γ gene expression in these patients (111). Leriglitazone can improve the pathological state of the disease through dual mechanisms of antioxidant action and agonist effects; indeed, it is an effective agonist of PPARγ. In all the clinical studies, it demonstrated overall good tolerability, with no significant safety findings reported. Lefiglitazone not only promotes FA storage by activating PPARγ but also exerts anti-inflammatory effects by modulating leukotriene synthesis and the expression of the proinflammatory factors TNF-α and IL-6 in patients (112); Pioglitazone is a PPARγ agonist. Post hoc analysis of its placebo-controlled trial in patients with non-alcoholic steatohepatitis (NCT00227110) demonstrated that pioglitazone reduces hepatic/visceral fat and improves necrosis and inflammation (113); Aleglitazar, a dual PPAR-α/γ agonist, not only regulates FA storage but has also been clinically demonstrated to have the ability to redistribute fat and reduce hepatic lipotoxicity (114). However, one of aleglitazar’ phase 3 trials (NCT01042769) was terminated because of cardiovascular issues.
Furthermore, numerous natural drug components that exert regulatory effects on PPARγ and can serve as PPARγ agonists have been identified in basic experimental studies. Magnolol is one such natural product derived from Magnolia officinalis, which acts as a PPARγ agonist and suppresses inflammation through the PPARγ/NF-κB signaling pathway. Naringenin exerts dual effects by regulating both FA levels and inflammation (115); Naringenin is a flavanone that is primarily found in citrus fruits and enhances adipogenesis through the activation of PPARγ (116); Pectolinarigenin (PEC), a bioactive compound isolated from the Chinese medicinal herb Dajitan, has anti-inflammatory, antioxidative, and anticancer properties, and it also activates PPARγ and enhances ferroptosis through the PPARγ/GPX4 signaling pathway (117); Pollenin B activates PPARγ and alleviates inflammatory responses by regulating the AA metabolism and the JAK-STAT signaling pathway through PPAR (118). Conversely, quercetin (Q), which is a naturally occurring flavonoid, has been demonstrated to have immunomodulatory functions. PPAR-α agonists inhibit NF-κB activation and suppress the JAK/STAT pathway to reduce the expression and release of IL-1β, IL-6, IL-8, and TNF-α, thereby exerting anti-inflammatory effects (119). Furthermore, during the process of cancer and tumorigenesis, IL - 6/JAK/STAT3 promotes fatty acid metabolic reprogramming, epigenetic dysregulation, and the occurrence of cancer and tumors through the activation of the IL - 6/JAK/STAT3 pathway (120); Essential oil from Fructus alpinia zerumbet reduces the ubiquitin-mediated degradation of PPARγ and directly binds to the PPARγ protein, thereby increasing its stability (121); Arctigenin, an indirect agonist of PPARγ, activates it through the AMPK/PPARγ pathway (122); Natural products such as asiatic acid (123), total ginsenosides, ursolic acid (124), dillapiole (125) and notoginsenoside R1 (126) can activate PPARγ and regulate FA levels, as detailed in Table 1.
5.1.2 Inhibits lipolysis
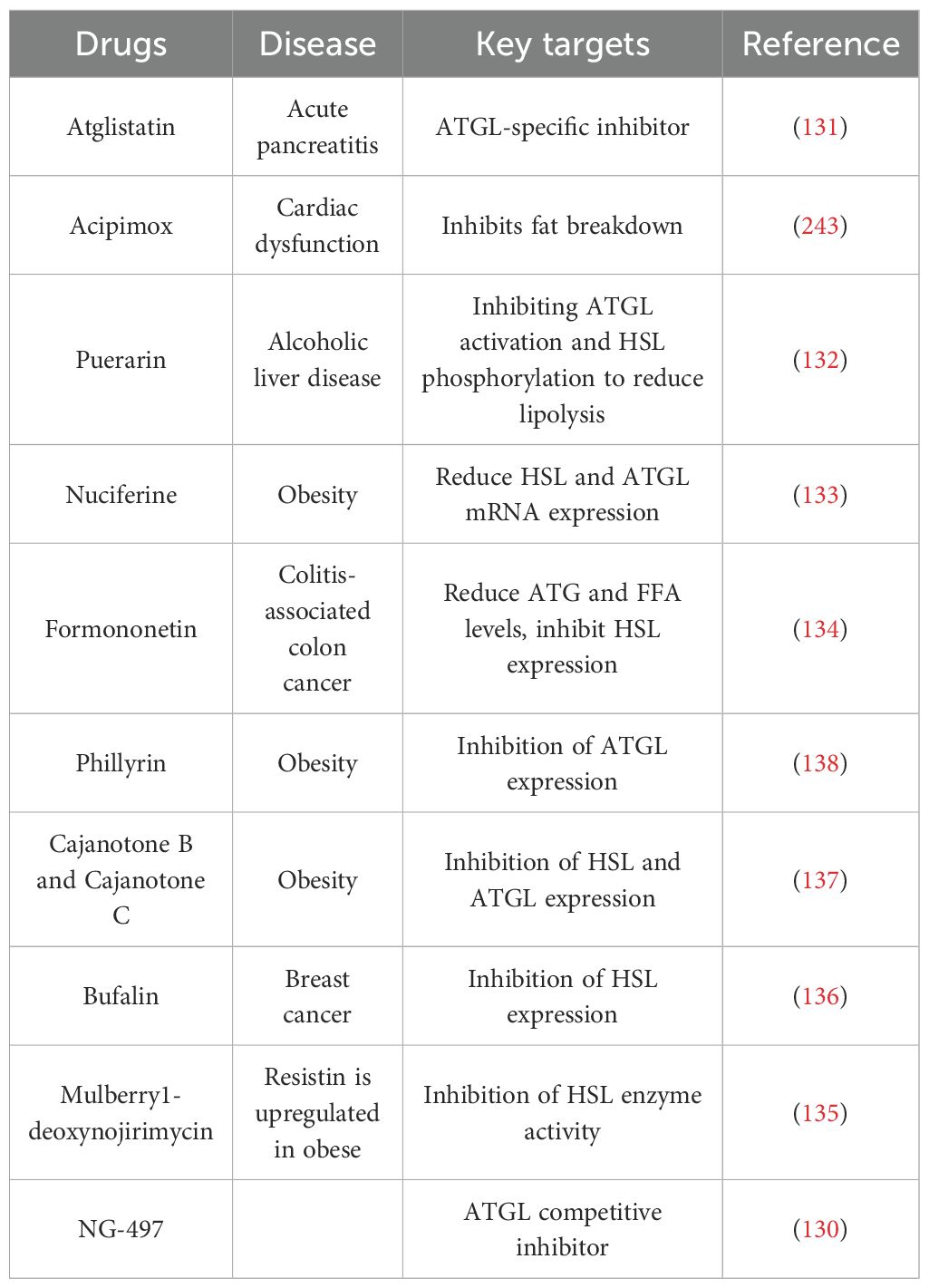
Inhibiting lipolysis is an effective way to reduce the level of serum FA. Adipose triglyceride lipase (ATGL) and HSL are two key enzymes that inhibit lipolysis. Allopurinol and febuxostat, as xanthine oxidase inhibitors, are medications used to decrease uric acid levels. However, recent studies utilizing serum metabolomics analysis in GA patients have revealed that allopurinol and febuxostat, while lowering uric acid levels, can also alleviate inflammatory responses in GA patients by reducing serum FA levels (97). Therefore, patients taking allopurinol and febuxostat treat GA through synergism: reducing FA levels to suppress inflammatory responses and inhibiting xanthine oxidase to lower uric acid levels. Patients in the intermission phase of GA may benefit from taking this medication to reduce recurrent episodes of GA, which holds significant clinical importance; Acipimox, an HSL inhibitor, significantly reduced FA levels in patients without affecting insulin-stimulated glucose uptake, as observed in a 6-month randomized placebo-controlled trial (NCT01488409) (127); Molecular docking and pharmacological validation revealed that phillyrin inhibits the enzymatic activity of ATGL and suppresses lipolysis (128); Masoprocol, a lipoxygenase inhibitor isolated from the creosote bush, has been shown to decrease adipose tissue lipolytic activity both in vivo and in vitro. The inhibition of HSL phosphorylation reduces HSL activity, thereby suppressing adipose tissue breakdown and FA levels (129). NG497 is the first human-specific ATGL small-molecule inhibitor that targets the patatin-like domain of human ATGL enzyme activity, eliminating the hormone-stimulated FA release in adipocytes (130).
Research has shown that drugs such as atglistatin (131), puerarin (132), nuciferine (133) and formononetin (134) improve the related diseases by inhibiting fat lipolysis through ATGL and HSL (135–138), as detailed in Table 2.
5.2 Inhibition of scratch fatty acid synthesis
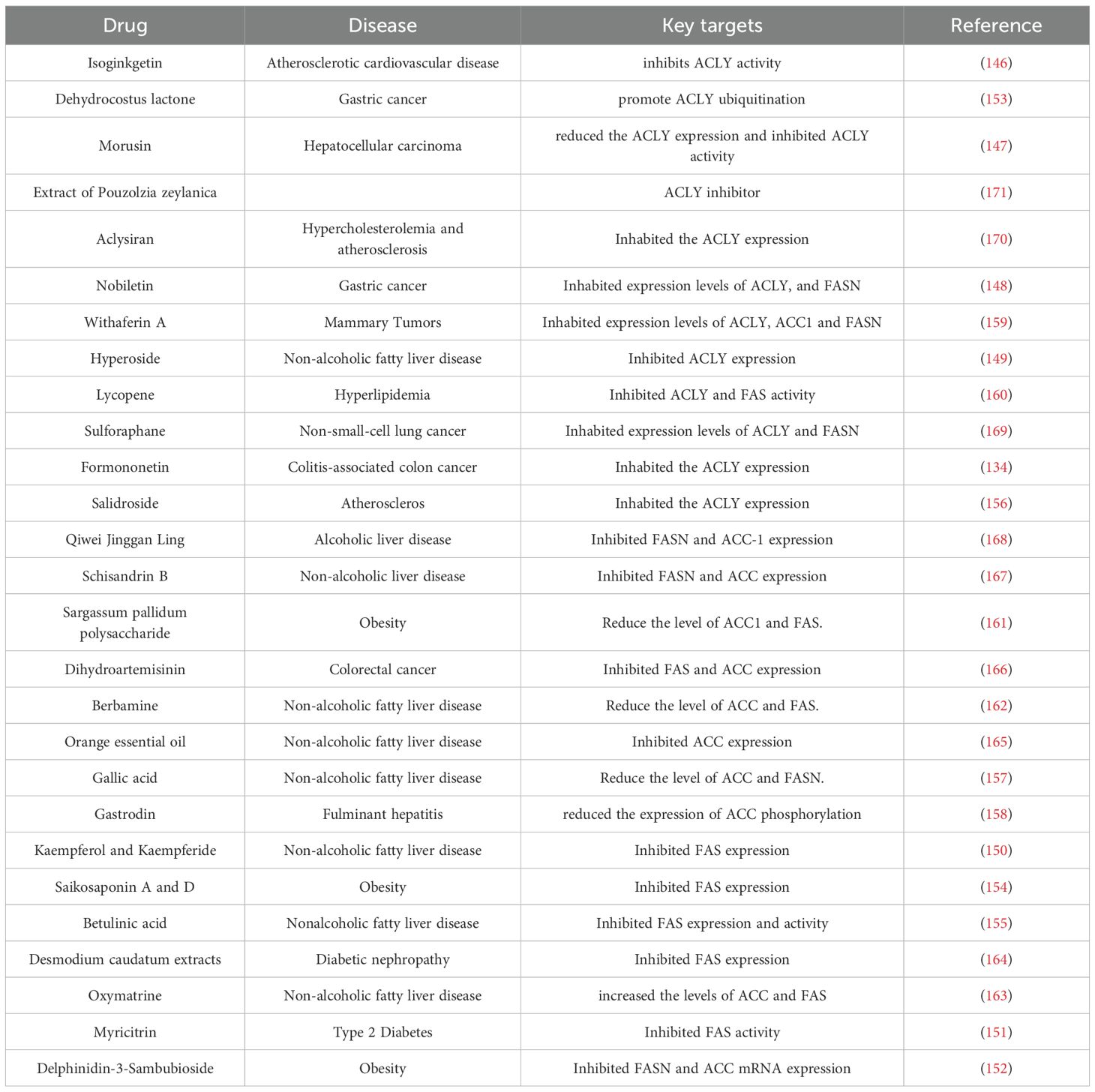
DNL is a process through which organisms synthesize fatty acids from scratch using non-fat precursors, such as acetyl-CoA, produced through carbohydrate metabolism. Carbohydrates undergo a series of enzymatic reactions and are converted to fatty acid synthesis substrates; first, they convert to acetyl coenzyme A in the presence of ATP-citrate lyase (ACL), which is the first step in endogenous fatty acid synthesis (139), and then, acetyl-CoA carboxylase (ACC) acts to produce malonyl-CoA, thereby initiating DNL. Malonyl-CoA is the main carbon source used for endogenous fatty acid synthesis (140). Fatty acid synthase (FAS) sequentially uses malonyl-CoA to extend the growing fatty acyl chain by two carbons to form the 16-carbon saturated fatty acid palmitate, which is the main product of fatty acid synthesis (141). ACL, ACC, and FAS are the key regulatory steps in endogenous fatty acid synthesis (142), and inhibition of the core enzymes of DNL to reduce FA levels is an attractive therapeutic strategy. Despite challenges in terms of efficacy, selectivity, and safety, several classes of novel synthetic DNL inhibitors are currently in the clinical stage of development and may form the basis of a novel class of therapies (142).
Bempedoic acid is a small-molecule first-in-class of inhibitors of ATP citrate lyase. The therapeutic effect is achieved by reducing FA and cholesterol synthesis through the inhibition of ACLY (143). Firsocostat and cilofexor are orally administered inhibitors that are used to target ACC in clinical drug development for the treatment of steatohepatitis associated with metabolic dysfunction (144). Denifanstat for the treatment of metabolic dysfunction-associated steatohepatitis has been tested in a multicenter, double-blind, randomized, placebo-controlled Phase 2b trial conducted at 100 clinical sites in the U.S., Canada, and Poland, and the results of this Phase 2b trial support the advancement of denifanstat into Phase 3 development (145). In addition, a large number of natural products, such as flavonoids isoginkgetin (146), Morusin (147), nobiletin (148), Hyperoside (149), Formononetin (134), Kaempferol, Kaempferide (150), Myricitrin (151), Delphinidin-3-sambubioside (152), Terpenoids Dehydrocostus lactone (153), Saikosaponin A and D (154), Betulinic acid (155), Phenols Salidroside (156), Gallic acid (157), Gastrodin (158), Sterols Withaferin A (159), Carotenoids lycopene (160) and natural polysaccharides Sargassum pallidum polysaccharide (161) and The alkaloids Berbamine (162), Oxymatrine (163) can also inhibit the synthesis of DNL for therapeutic purposes (164–171), and the specific drugs and their effects are shown in Table 3.
5.3 Inhibition of free fatty acid key target TLRs
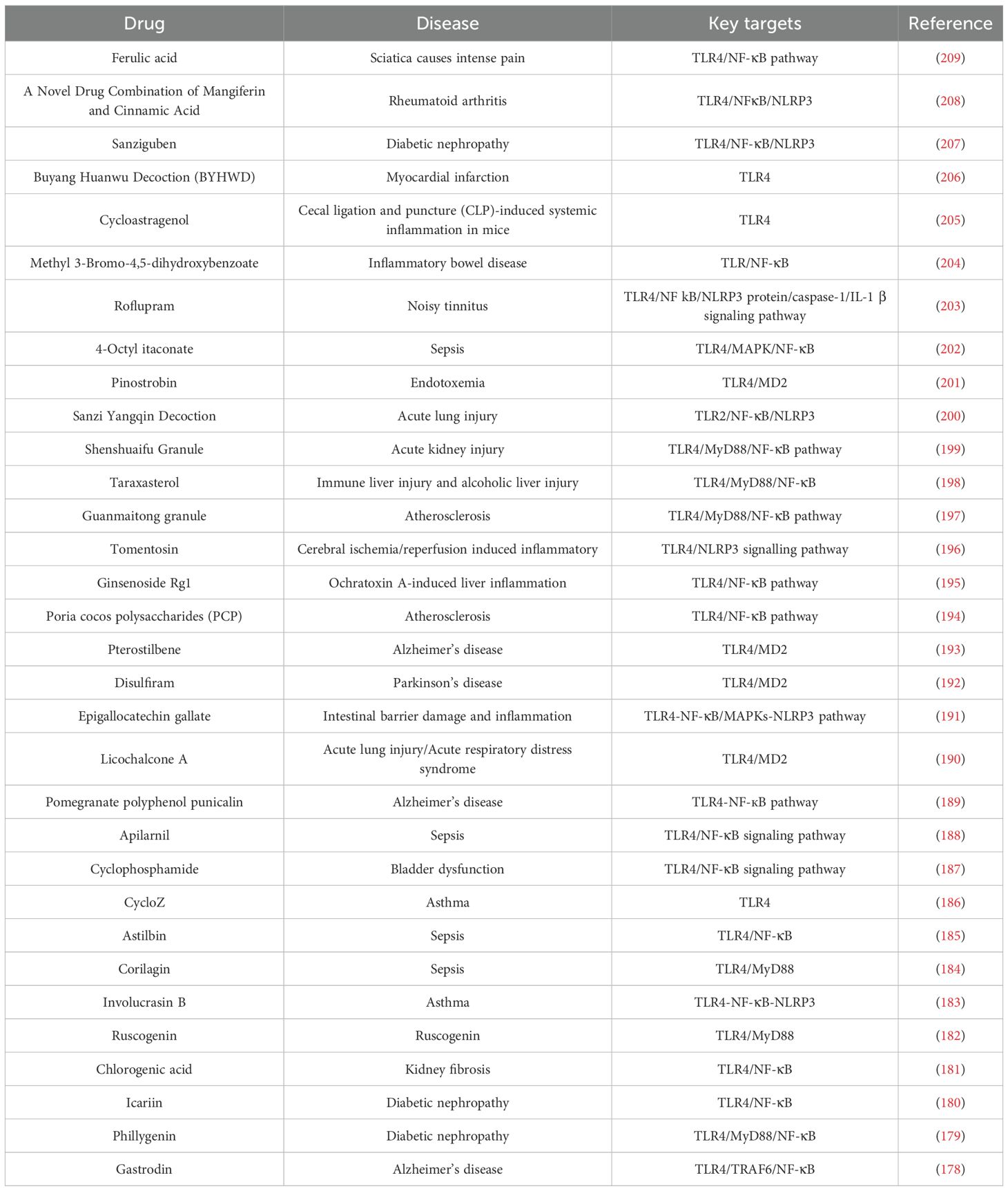
FAs promote the development of GA and trigger the initiation of inflammation, mainly by activating its downstream key targets TLR2/TLR4 (172, 173). Therefore, the development of drugs for the treatment of GA through the modulation of TLRs is important. For example, luteolin, a plant-derived flavonoid, downregulates the proinflammatory cytokines in macrophages and inhibits the production of nitric oxide and pro-inflammatory arachidonic acid by blocking the nuclear factor κB (NF-κB) signaling pathway (174). In the acute GA model, luteolin inhibited the expressions of TLR2 and TLR4 mRNAs, reduced their protein expression levels, and ameliorated acute inflammation in GA by downregulating the TLR/MyD88/NF-κB signaling pathway, which in turn inhibited the expression of the downstream inflammatory factors IL-1β, IL-6, and TNF-α (175). Ampelopsis grossedentata total flavonoids also exerted therapeutic effects on GA through the TLR4/MyD88/NF-κB inflammatory pathway in a combined GA model of hyperuricemia (176).
In addition to the drugs that ameliorate inflammation in GA by targeting TLRs, sparsolonin B inhibited FA-induced macrophage inflammation in an osteoarthritis model by selectively blocking the TLR4/MD-2/NF-κB axis (177). Modulation of TLR2/TLR4 by numerous other drugs, such as gastrodin, ferulic acid, sanziguben, cycloastragenol, and taraxasterol, to improve inflammation has been studied and experimentally validated (178–209). All of these factors can improve inflammation through TLR2/TLR4. Inflammation and specific drug information and details are provided in Table 4.
5.4 Regulation of arachidonic acid metabolism
Abnormal AA metabolism plays an important role in GA episodes by recruiting neutrophils to the inflammatory site and thereby promoting the inflammatory flare-ups and their persistence; therefore, exploring the development of drugs to improve GA by modulating AA metabolism is worthwhile.
5.4.1 COX-2 inhibitor
Etoricoxib is a selective cyclooxygenase-2 (COX-2) inhibitor, which was evaluated in a randomized, double-blind, active comparator-controlled trial for the treatment of acute GA. Research has shown that etoricoxib rapidly and selectively inhibits COX-2, and its efficacy in terms of treating various clinical manifestations of GA, including pain and inflammation, is comparable to that of indomethacin (210); Rofecoxib is a selective COX-2 inhibitor, which was evaluated in clinical trials for acute GA, and it was reported to reduce the expression of inflammatory mediators, such as IL-6, and alleviate patient pain by inhibiting COX-2-regulated AA metabolism (211); Celecoxib is a COX-2 inhibitor. The clinical trial for acute GA (NCT00549549), with a randomized, double-blind, double-dummy, active-controlled design, was conducted across 100 centers. The results indicated that monitoring the treatment response throughout the entire acute episode until complete resolution is clinically significant. The efficacy of high-dose celecoxib (800/400 mg) was comparable to that of indomethacin (50 mg tid). Furthermore, the duration of pain relief achieved using high-dose celecoxib appeared to be longer than that achieved with indomethacin (212); etodolac (a COX-2 inhibitor) demonstrated analgesic effects in clinical trials for general anesthesia (213).
Additionally, basic research has identified numerous drugs capable of inhibiting COX-2. Leonurine, for example, exerts its anti-GA effects by suppressing COX-2 and LOX-5 expression, thereby reducing the prostaglandin E2 (PGE2) and LTB4 levels in synovial macrophages and diminishing neutrophil recruitment (214); The whole plant extract of Fimbristylis aestivalis, which contains rosmarinic acid, catechin hydrate, and syringic acid, has inhibitory effects on COX-2 and effectively alleviates inflammatory responses (215); Thymol-1,5-disubstituted pyrazole hybrids and novel 5,6-diphenyl-1,2,4-triazine-3-thiol derivatives exhibit inhibitory potential against both COX-2 and 5-LOX enzymes and possess anti-inflammatory properties (216, 217); Resveratrol amide derivatives used as selective COX-2 inhibitors in molecular docking studies demonstrated partial entry into the 2°-pocket of the COX-2 active site and interaction with the amino acid residues responsible for COX-2 selectivity, which have orientation and binding interactions similar to Rofecoxib (218). Nimesulide (219), Vismodegib (220), New Pyrimidine-5-Carbonitriles (221), Sulfonamide-Pyrazole derivatives (222) and lariciresinol (223) exert regulatory effects on arachidonic acid metabolism by inhibiting COX-2, the specific drugs used are shown in Table 5.
5.4.2 LOX-5 inhibitor
The Chinese medicine Huzhen Tongfeng Formula (HZTF) is composed of four Chinese herbs: Polygoni Cuspidati Rhizoma et Radix (PCRR, the root and rhizome of Polygonum cuspidatum Sieb. et Zucc.), Ligustri Lucidi Fructus (LLF, the fruit of Ligustrum lucidum Ait.), Herba Plantaginis (HP, the dried whole grass of Plantago asiatica L.), and Nidus Vespae (NV, the honeycomb of Polistes olivaceus (De Geer), Polistes Japonicus Saussure, or Parapolybiavaria Fabricius) (224). In the GA model, HZTF attenuated leukocyte recruitment in the synovium by significantly inhibiting the lipoxygenase pathway of AA for the treatment of GA (224). Leucas zeylanica, which is used in traditional medicine for the treatment of GA, can also improve GA inflammation by reducing the metabolite levels through the inhibition of the AA lipoxygenase pathway (225).
In addition, many other drugs can improve inflammation by reducing neutrophil recruitment through the inhibition of this pathway (225). For example, zileuton (226), red-kerneled rice proanthocyanidin (227), malabaricone C (228), caffeic acid (89), lysionotin (229) and magnolin (230) inhibit AA lipoxygenase by inhibiting the expression of key enzymes or enzyme activity reduction to achieve the anti-inflammatory effects (231–236), the specific drugs used are shown in Table 6.
This paper systematically reviews the regulation of GA metabolism and immune responses in the hyperuricemia occurring due to FA metabolic disorders and identifies targets for personalized therapy based on the underlying mechanisms (210, 211). Therefore, the use of AA-related enzyme inhibitors to treat acute GA patients represents a reliable therapeutic strategy. Second, PPARγ agonists, the drugs that lower FA levels by promoting fat synthesis, are expressed in adipose tissue, the liver, skeletal muscle, and immune cells (108). This characteristic renders PPARγ agonists potentially applicable to patients with GA and hyperuricemia associated with cyclic FA disorders (30). However, the use of this medication may increase body weight and should be used with caution in patients with GA who are also obese or have insulin resistance. Third, HSL and ATGL inhibitors are used (127, 128), which reduce FA levels by suppressing lipolysis and do not selectively regulate specific types of FAs, and may, therefore, be applicable for the prevention and treatment of patients at risk of elevated circulating FA levels. Fourth, TLR inhibitors not only block fatty acid-induced inflammatory activation but also inhibit the TLR signaling associated with MSU crystals (45), thereby exerting a more comprehensive anti-inflammatory effect. These drugs may, during the course of GA, promote the resolution of inflammation and finally reduce the free FA levels by inhibiting key enzymes involved in fatty acid synthesis, such as FASN, ACC, and ACL. Moreover, palmitoylation during fatty acid synthesis influences the key inflammatory pathways, such as TLR/MyD88 (45) and NLRP3 inflammasome activation and assembly (55, 56). Therefore, such inhibitors may be more suitable for the resolution of GA-related inflammation.
Disordered FA metabolism further promotes inflammation, and elevation of the uric acid levels, primarily through high levels of FAs, activates the initiation of inflammation. The uric acid is taken up by cells as a substrate for FAO; moreover, abnormal AA metabolism contributes to the eruption and persistence of acute inflammation in GA. Therefore, by organizing and summarizing the drugs that modulate the above mechanisms, a basis for subsequent GA treatment and novel drug development is prepared.
6 Discussion and perspectives
GA is a metabolic-inflammatory disorder characterized by an intricate crosstalk between aberrant FA metabolism and immune dysregulation (237). Emerging evidence underscores the centrality of FA metabolism perturbations in driving disease progression, from hyperuricemia-triggered crystal deposition to sustained NLRP3 inflammasome activation, ultimately forming a self-perpetuating “metabolism-immunity” axis.
6.1 FA overload as a pathogenic catalyst
Elevated levels of circulating FAs, derived predominantly from adipose lipolysis, DNL, and excessive dietary intake (238), represent the critical pathogenic drivers of GA. Mechanistically, FAs exacerbate inflammation through three synergistic pathways. First, FAs synergize with monosodium urate (MSU) crystals to amplify macrophage activation through TLR2/MyD88/NF-κB and TLR4-MD2/MyD88/NF-κB signaling, leading to NLRP3 inflammasome assembly and IL-1β maturation (41). represent the critical pathogenic drivers of GA. Mechanistically, FAs exacerbate inflammation through three synergistic pathways. First, FAs synergize with MSU crystals to amplify macrophage activation through TLR2/MyD88/NF-κB and TLR4-MD2/MyD88/NF-κB signaling, leading to NLRP3 inflammasome assembly and IL-1β maturation (17, 57, 58). In addition, fatty acid metabolic reprogramming in GA drives distinct pathways: β-oxidation in hepatocytes and macrophages promotes urate synthesis and proinflammatory cytokine release (18, 64, 93), AA metabolism in neutrophils drives the LTB4-BLT1-mediated chemotaxis and NETosis, amplifying local inflammation (76–78).
6.2 Therapeutic implications and challenges
Despite the clinical correlations between FA dysmetabolism and GA severity, mechanistic insights remain fragmented, with most studies limited to biomarker associations rather than causal validation. This study identified three promising therapeutic strategies for targeting FA metabolism. First is the urate-lowering agents allopurinol and febuxostat, which are traditional clinical agents used to inhibit xanthine oxidase and reduce uric acid levels. Recent studies have revealed that these agents also exert a regulatory effect on FA levels in patients with FA-related GA. Although the precise mechanism remains unclear to date, these drugs hold potential as therapeutic options for GA patients with FA metabolism disorders (97). Second, metabolic enzyme inhibitors, which are the COX-2 inhibitor nonsteroidal anti-inflammatory drugs used in clinical trials to treat patients with GA, can target specific AA metabolic pathways as well as alleviate inflammation during acute GA episodes. These are, therefore, particularly suitable for patients identified through a lipidomic analysis as having AA metabolic dysfunction (210, 211). Third, the multitarget natural compounds curcumin, resveratrol, and berberine exhibit pleiotropic benefits by suppressing FA generation, blocking AA-derived eicosanoid synthesis, and enhancing insulin sensitivity, with favorable safety profiles (107, 146, 181).
However, clinical translation faces numerous challenges. First, there may be off-target effects from the systemic modulation of the FA pathway. For example, the nonsteroidal anti-inflammatory drug fenoprofen exerts its anti-inflammatory action by inhibiting prostaglandin (PG) biosynthesis through COX blockade. However, it also acts non-selectively on both COX-1 and COX-2 isoforms, leading to numerous side effects and off-target effects (239). Second, the tissue-specific differences in FA metabolism, where FA metabolism in various organs, such as the liver and kidneys, influences distinct stages of GA progression, and the varying tissue affinities exhibited by drugs, such as the PPARγ agonists pioglitazone (113) and aleglitazar (240), exhibit stronger affinity for the hepatic tissue, redistributing visceral fat to peripheral tissues. Whether this would induce adverse reactions in the pathological state of GA warrants further investigation. Additionally, the long-term safety concerns associated with single-pathway metabolic modulators must be considered. Drugs typically require multi-pathway regulation for the management of disease progression; however, the prolonged inhibition of a single pathway may lead to compensatory enhancement of other pathways. Therefore, the use of such drugs should be limited to short-term therapeutic interventions, and whether the side effects associated with long-term use are manageable must be carefully evaluated.
6.3 Future research directions
While this review synthesizes current evidence, critical knowledge gaps nonetheless persist. Neutrophil-FA dynamics: How FA metabolism regulates neutrophil plasticity (e.g., N1/N2 polarization) during GA flares remains unexplored. Spatiotemporal resolution: Advanced techniques (single-cell RNA-seq and spatial metabolomics) can be used to map FA flux heterogeneity across synovial cell populations. Preclinical models: Humanized GA models incorporating dietary/metabolic stressors are needed to recapitulate FA-immune interactions (241).
This study is, to the best of the author’s knowledge, the first systematic integration of FA metabolic derangements with GA pathogenesis and therapeutic development. This work, by delineating the “FA-AA-inflammasome” axis and cataloging the mechanism-based interventions, provides a framework for developing precision therapies that simultaneously address metabolic dysfunction and sterile inflammation in GA.
Author contributions
XPZ: Investigation, Writing – original draft, Writing – review & editing, Data curation, Supervision, Methodology, Software, Conceptualization, Resources, Visualization, Project administration, Formal analysis, Validation. YS: Validation, Conceptualization, Project administration, Data curation, Supervision, Methodology, Writing – review & editing, Investigation, Writing – original draft, Resources, Visualization, Formal analysis, Software. LY: Supervision, Formal analysis, Validation, Writing – review & editing, Conceptualization, Data curation, Investigation. HS (4th author): Visualization, Resources, Validation, Methodology, Writing – review & editing, Investigation. XYZ: Investigation, Writing – review & editing, Methodology, Validation. HS (6th author): Investigation, Project administration, Writing – review & editing, Conceptualization. GY: Validation, Conceptualization, Investigation, Writing – review & editing. XW: Validation, Supervision, Methodology, Conceptualization, Visualization, Investigation, Data curation, Funding acquisition, Software, Writing – review & editing, Formal analysis, Project administration, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (U23A20501, 82404816), the key research and development plan projects of Heilongjiang Province (2022ZX02C04), the Guangdong Basic and Applied Basic Research Found Project (2023A1515110703), the Traditional Chinese Medicine Bureau Of Guangdong Province (20254061), the State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ49, SZ2022KF16), the Second Affiliated Hospital of Guangzhou University of Chinese Medicine(YN2024GZRPY017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keller SF and Mandell BF. Management and cure of gouty arthritis. Rheum Dis Clin North Am. (2022) 48:479–92. doi: 10.1016/j.rdc.2022.03.001
2. Zhang S, Li D, Fan M, Yuan J, Xie C, Yuan H, et al. Mechanism of reactive oxygen species-guided immune responses in gouty arthritis and potential therapeutic targets. Biomolecules. (2024) 14:978. doi: 10.3390/biom14080978
3. Guo JW, Lin GQ, Tang XY, Yao JY, Feng CG, Zuo JP, et al. Therapeutic potential and pharmacological mechanisms of Traditional Chinese Medicine in gout treatment. Acta Pharmacol Sin. (2025) 46:1156–76. doi: 10.1038/s41401-024-01459-6
4. Han T, Chen W, Qiu X, and Wang W. Epidemiology of gout - Global burden of disease research from 1990 to 2019 and future trend predictions. Ther Adv Endocrinol Metab. (2024) 15:20420188241227295. doi: 10.1177/20420188241227295
5. He Q, Mok TN, Sin TH, Yin J, Li S, Yin Y, et al. Global, regional, and national prevalence of gout from 1990 to 2019: age-period-cohort analysis with future burden prediction. JMIR Public Health Surveill. (2023) 9:e45943. doi: 10.2196/45943
6. Dalbeth N, Aati O, Kalluru R, Gamble GD, Horne A, Doyle AJ, et al. Relationship between structural joint damage and urate deposition in gout: a plain radiography and dual-energy CT study. Ann Rheum Dis. (2015) 74:1030–6. doi: 10.1136/annrheumdis-2013-204273
7. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, and Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. (2018) 77:1048–52. doi: 10.1136/annrheumdis-2017-212288
8. Martin WJ, Walton M, and Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheumatol. (2009) 60:281–9. doi: 10.1002/art.24185
9. Liu L, Zhu L, Liu M, Zhao L, Yu Y, Xue Y, et al. Recent insights into the role of macrophages in acute gout. Front Immunol. (2022) 13:955806. doi: 10.3389/fimmu.2022.955806
10. Martinon F, Pétrilli V, Mayor A, Tardivel A, and Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. (2006) 440:237–41. doi: 10.1038/nature04516
11. Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. (2006) 116:2262–71. doi: 10.1172/JCI28075
12. Tao HW, Liu ZY, Jiang W, Miao MY, Lyu JQ, Zhao M, et al. Lower plasma linoleic acids as a risk factor for gout: an integrated analysis of population-based cohort and genetic data. Food Funct. (2024) 15:7567–76. doi: 10.1039/D4FO00987H
13. Pei L, Xie L, Wu J, Zhang H, and Zhang X. Study on the relationship between FFA and gout flare. Clin Rheumatol. (2020) 39:1251–5. doi: 10.1007/s10067-019-04903-9
14. Ortega-Gomez A, Lopez S, Varela LM, Jaramillo S, Muriana FJG, and Abia R. New evidence for dietary fatty acids in the neutrophil traffic between the bone marrow and the peripheral blood. Food Chem (Oxf). (2022) 5:100133. doi: 10.1016/j.fochms.2022.100133
15. Kanno T, Nakajima T, Miyako K, and Endo Y. Lipid metabolism in Th17 cell function. Pharmacol Ther. (2023) 245:108411. doi: 10.1016/j.pharmthera.2023.108411
16. Samovski D, Jacome-Sosa M, and Abumrad NA. Fatty acid transport and signaling: mechanisms and physiological implications. Annu Rev Physiol. (2023) 85:317–37. doi: 10.1146/annurev-physiol-032122-030352
17. Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol. (2017) 313:F826–f34. doi: 10.1152/ajprenal.00012.2017
18. Liang N, Yuan X, Zhang L, Shen X, Zhong S, Li L, et al. Fatty acid oxidation-induced HIF-1α activation facilitates hepatic urate synthesis through upregulating NT5C2 and XDH. Life Metab. (2024) 3:loae018. doi: 10.1093/lifemeta/loae018
19. Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. (2016) 64:925–32. doi: 10.1016/j.jhep.2015.11.022
20. Arellano H, Nardello-Rataj V, Szunerits S, Boukherroub R, and Fameau AL. Saturated long chain fatty acids as possible natural alternative antibacterial agents: Opportunities and challenges. Adv Colloid Interface Sci. (2023) 318:102952. doi: 10.1016/j.cis.2023.102952
21. Engin AB and Engin A. The checkpoints of intestinal fat absorption in obesity. Adv Exp Med Biol. (2024) 1460:73–95. doi: 10.1007/978-3-031-63657-8
22. Li X, Liu Q, Pan Y, Chen S, Zhao Y, and Hu Y. New insights into the role of dietary triglyceride absorption in obesity and metabolic diseases. Front Pharmacol. (2023) 14:1097835. doi: 10.3389/fphar.2023.1097835
23. Kozan DW, Derrick JT, Ludington WB, and Farber SA. From worms to humans: Understanding intestinal lipid metabolism via model organisms. Biochim Biophys Acta Mol Cell Biol Lipids. (2023) 1868:159290. doi: 10.1016/j.bbalip.2023.159290
24. Aqeel A, Akram A, Ali M, Iqbal M, Aslam M, Rukhma, et al. Mechanistic insights into impaired β-oxidation and its role in mitochondrial dysfunction: A comprehensive review. Diabetes Res Clin Pract. (2025) 223:112129. doi: 10.1016/j.diabres.2025.112129
25. Kim YA, Lee Y, and Kim MS. Carnitine shuttle and ferroptosis in cancer. Antioxid (Basel). (2025) 14:972. doi: 10.3390/antiox14080972
26. Vassiliou E and Farias-Pereira R. Impact of lipid metabolism on macrophage polarization: implications for inflammation and tumor immunity. Int J Mol Sci. (2023) 24:12032. doi: 10.3390/ijms241512032
27. Xiao S, Qi M, Zhou Q, Gong H, Wei D, Wang G, et al. Macrophage fatty acid oxidation in atherosclerosis. BioMed Pharmacother. (2024) 170:116092. doi: 10.1016/j.biopha.2023.116092
28. Cypess Aaron M. Reassessing human adipose tissue. New Engl J Med. (2022) 386:768–79. doi: 10.1056/NEJMra2032804
29. Cho CH, Patel S, and Rajbhandari P. Adipose tissue lipid metabolism: lipolysis. Curr Opin Genet Dev. (2023) 83:102114. doi: 10.1016/j.gde.2023.102114
30. Zhang Y, Zhang H, Chang D, Guo F, Pan H, and Yang Y. Metabolomics approach by (1)H NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res Ther. (2018) 20:111. doi: 10.1186/s13075-018-1600-5
31. Shen X, Wang C, Liang N, Liu Z, Li X, Zhu ZJ, et al. Serum metabolomics identifies dysregulated pathways and potential metabolic biomarkers for hyperuricemia and gout. Arthritis Rheumatol. (2021) 73:1738–48. doi: 10.1002/art.41733
32. Wang M, Li R, Qi H, Pang L, Cui L, Liu Z, et al. Metabolomics and machine learning identify metabolic differences and potential biomarkers for frequent versus infrequent gout flares. Arthritis Rheumatol. (2023) 75:2252–64. doi: 10.1002/art.42635
33. Chen L, Tan T, Wu Q, Cui F, Chen Y, Chen H, et al. Dietary polyunsaturated fatty acid and risk of gout: a cohort study integrating genetic predisposition and metabolomics. Eur J Epidemiol. (2025) 40:427–39. doi: 10.1007/s10654-025-01242-9
34. Wang W, Kou J, Zhang M, Wang T, Li W, Wang Y, et al. A metabonomic study to explore potential markers of asymptomatic hyperuricemia and acute gouty arthritis. J Orthop Surg Res. (2023) 18:96. doi: 10.1186/s13018-023-03585-z
35. Zhang M, Zhang Y, Terkeltaub R, Chen C, and Neogi T. Effect of dietary and supplemental omega-3 polyunsaturated fatty acids on risk of recurrent gout flares. Arthritis Rheumatol. (2019) 71:1580–6. doi: 10.1002/art.40896
37. Pandey S. Metabolomics for the identification of biomarkers in rheumatoid arthritis. Phenomics. (2025) 5:343–5. doi: 10.1007/s43657-025-00242-9
38. Ahn EY and So MW. The pathogenesis of gout. J Rheum Dis. (2025) 32:8–16. doi: 10.4078/jrd.2024.0054
39. Chen P, Luo Z, Lu C, Jian G, Qi X, and Xiong H. Gut-immunity-joint axis: a new therapeutic target for gouty arthritis. Front Pharmacol. (2024) 15:1353615. doi: 10.3389/fphar.2024.1353615
40. Liu YR, Wang JQ, and Li J. Role of NLRP3 in the pathogenesis and treatment of gout arthritis. Front Immunol. (2023) 14:1137822. doi: 10.3389/fimmu.2023.1137822
41. Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheumatol. (2010) 62:3237–48. doi: 10.1002/art.27667
42. Hu J, Wang H, Li X, Liu Y, Mi Y, Kong H, et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. (2020) 10:9702–20. doi: 10.7150/thno.44297
43. Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, et al. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. (2012) 61:1058–67. doi: 10.1136/gutjnl-2011-300269
44. Liu L, He S, Jia L, Yao H, Zhou D, Guo X, et al. Correlation analysis of serum TLR4 protein levels and TLR4 gene polymorphisms in gouty arthritis patients. PloS One. (2024) 19:e0300582. doi: 10.1371/journal.pone.0300582
45. Xiao Y, Gao Y, Hu Y, Zhang X, Wang L, Li H, et al. FASN contributes to the pathogenesis of lupus by promoting TLR-mediated activation of macrophages and dendritic cells. Int Immunopharmacol. (2024) 142:113136. doi: 10.1016/j.intimp.2024.113136
46. Zhang J, Wu S, Xu Y, Zhang L, Cong C, Zhang M, et al. Lipid overload meets S-palmitoylation: a metabolic signalling nexus driving cardiovascular and heart disease. Cell Commun Signal. (2025) 23:392. doi: 10.1186/s12964-025-02398-3
47. Carroll RG, Zasłona Z, Galván-Peña S, Koppe EL, Sévin DC, Angiari S, et al. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J Biol Chem. (2018) 293:5509–21. doi: 10.1074/jbc.RA118.001921
48. Luo X, Bao X, Weng X, Bai X, Feng Y, Huang J, et al. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci. (2022) 291:120064. doi: 10.1016/j.lfs.2021.120064
49. Li F, Yao JH, Li L, Nie Q, Cao JJ, and Ning XR. MiRNA-23a-5p is the biomarkers for gouty arthritis and promotes inflammation in rats of gouty arthritis via MyD88/NF-κB pathway by induction TLR2. Arch Rheumatol. (2022) 37:536–46. doi: 10.46497/ArchRheumatol.2022.9236
50. Yu C, Wang D, Yang Z, and Wang T. Pharmacological effects of polyphenol phytochemicals on the intestinal inflammation via targeting TLR4/NF-κB signaling pathway. Int J Mol Sci. (2022) 23:6939. doi: 10.3390/ijms23136939
51. Khan MZ, Li L, Wang T, Liu X, Chen W, Ma Q, et al. Bioactive compounds and probiotics mitigate mastitis by targeting NF-κB signaling pathway. Biomolecules. (2024) 14:1011. doi: 10.3390/biom14081011
52. Zhang Y, Liang X, Bao X, Xiao W, and Chen G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur J Med Chem. (2022) 235:114291. doi: 10.1016/j.ejmech.2022.114291
53. Lee JH, Kim HS, Lee JH, Yang G, and Kim HJ. Natural products as a novel therapeutic strategy for NLRP3 inflammasome-mediated gout. Front Pharmacol. (2022) 13:861399. doi: 10.3389/fphar.2022.861399
54. Cheng JJ, Ma XD, Ai GX, Yu QX, Chen XY, Yan F, et al. Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and nrf2 pathways. Drug Des Devel Ther. (2022) 16:2119–32. doi: 10.2147/DDDT.S356307
55. Leishman S, Aljadeed NM, Qian L, Cockcroft S, Behmoaras J, and Anand PK. Fatty acid synthesis promotes inflammasome activation through NLRP3 palmitoylation. Cell Rep. (2024) 43:114516. doi: 10.1016/j.celrep.2024.114516
56. Zhang Z, Venditti R, Ran L, Liu Z, Vivot K, Schürmann A, et al. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nat Immunol. (2023) 24:30–41. doi: 10.1038/s41590-022-01355-3
57. Karasawa T, Kawashima A, Usui-Kawanishi F, Watanabe S, Kimura H, Kamata R, et al. Saturated fatty acids undergo intracellular crystallization and activate the NLRP3 inflammasome in macrophages. Arterioscler Thromb Vasc Biol. (2018) 38:744–56. doi: 10.1161/ATVBAHA.117.310581
58. Anand PK. Lipids, inflammasomes, metabolism, and disease. Immunol Rev. (2020) 297:108–22. doi: 10.1111/imr.12891
59. Pérez S and Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: A redox perspective. Antioxid (Basel). (2022) 11:1394. doi: 10.3390/antiox11071394
60. Weiss JM, Palmieri EM, Gonzalez-Cotto M, Bettencourt IA, Megill EL, Snyder NW, et al. Itaconic acid underpins hepatocyte lipid metabolism in non-alcoholic fatty liver disease in male mice. Nat Metab. (2023) 5:981–95. doi: 10.1038/s42255-023-00801-2
61. Hall CJ, Boyle RH, Astin JW, Flores MV, Oehlers SH, Sanderson LE, et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. (2013) 18:265–78. doi: 10.1016/j.cmet.2013.06.018
62. Pessler F, Mayer CT, Jung SM, Behrens EM, Dai L, Menetski JP, et al. Identification of novel monosodium urate crystal regulated mRNAs by transcript profiling of dissected murine air pouch membranes. Arthritis Res Ther. (2008) 10:R64. doi: 10.1186/ar2435
63. Van den Bossche J, O’Neill LA, and Menon D. Macrophage immunometabolism: where are we (Going)? Trends Immunol. (2017) 38:395–406. doi: 10.1016/j.it.2017.03.001
64. Jiang H, Song D, Zhou X, Chen F, Yu Q, Ren L, et al. Maresin1 ameliorates MSU crystal-induced inflammation by upregulating Prdx5 expression. Mol Med. (2023) 29:158. doi: 10.1186/s10020-023-00756-w
65. Liu Y, Tang H, Liu X, Chen H, Feng N, Zhang J, et al. Frontline Science: Reprogramming COX-2, 5-LOX, and CYP4A-mediated arachidonic acid metabolism in macrophages by salidroside alleviates gouty arthritis. J Leukoc Biol. (2019) 105:11–24. doi: 10.1002/JLB.3HI0518-193R
66. Dahlgren C, Forsman H, Sundqvist M, Björkman L, and Mårtensson J. Signaling by neutrophil G protein-coupled receptors that regulate the release of superoxide anions. J Leukoc Biol. (2024) 116:1334–51. doi: 10.1093/jleuko/qiae165
67. Shin JH, Lee CM, and Song JJ. Transcutaneous auricular vagus nerve stimulation mitigates gouty inflammation by reducing neutrophil infiltration in BALB/c mice. Sci Rep. (2024) 14:25630. doi: 10.1038/s41598-024-77272-2
68. Chen C, Wang J, Guo Y, Li M, Yang K, Liu Y, et al. Monosodium urate crystal-induced pyroptotic cell death in neutrophil and macrophage facilitates the pathological progress of gout. Small. (2024) 20:e2308749. doi: 10.1002/smll.202308749
69. Van Bruggen S, Jarrot PA, Thomas E, Sheehy CE, Silva CMS, Hsu AY, et al. NLRP3 is essential for neutrophil polarization and chemotaxis in response to leukotriene B4 gradient. Proc Natl Acad Sci U S A. (2023) 120:e2303814120. doi: 10.1073/pnas.2303814120
70. Oster L, Schröder J, Rugi M, Schimmelpfennig S, Sargin S, Schwab A, et al. Extracellular pH controls chemotaxis of neutrophil granulocytes by regulating leukotriene B(4) production and cdc42 signaling. J Immunol. (2022) 209:136–44. doi: 10.4049/jimmunol.2100475
71. Gu H, Yu H, Qin L, Yu H, Song Y, Chen G, et al. MSU crystal deposition contributes to inflammation and immune responses in gout remission. Cell Rep. (2023) 42:113139. doi: 10.1016/j.celrep.2023.113139
72. Luo Y, Wang L, Peng A, and Liu JY. Metabolic profiling of human plasma reveals the activation of 5-lipoxygenase in the acute attack of gouty arthritis. Rheumatol (Oxford). (2019) 58:345–51. doi: 10.1093/rheumatology/key284
73. Huang Y, Xiao M, Ou J, Lv Q, Wei Q, Chen Z, et al. Identification of the urine and serum metabolomics signature of gout. Rheumatol (Oxford). (2020) 59:2960–9. doi: 10.1093/rheumatology/keaa018
74. Zhang Y, Liu Y, Sun J, Zhang W, Guo Z, and Ma Q. Arachidonic acid metabolism in health and disease. MedComm (2020). (2023) 4:e363. doi: 10.1002/mco2.363
75. Rae SA, Davidson EM, and Smith MJ. Leukotriene B4, an inflammatory mediator in gout. Lancet. (1982) 2:1122–4. doi: 10.1016/S0140-6736(82)92785-4
76. Majumdar R, Tavakoli Tameh A, Arya SB, and Parent CA. Exosomes mediate LTB4 release during neutrophil chemotaxis. PloS Biol. (2021) 19:e3001271. doi: 10.1371/journal.pbio.3001271
77. Cumpelik A, Ankli B, Zecher D, and Schifferli JA. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann Rheum Dis. (2016) 75:1236–45. doi: 10.1136/annrheumdis-2015-207338
78. Robinson E, Herbert JA, Palor M, Ren L, Larken I, Patel A, et al. Trans-epithelial migration is essential for neutrophil activation during RSV infection. J Leukoc Biol. (2023) 113:354–64. doi: 10.1093/jleuko/qiad011
79. Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. (1994) 12:593–633. doi: 10.1146/annurev.iy.12.040194.003113
80. Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. (2010) 11:495–502. doi: 10.1038/ni.1878
81. Fu W, Ge M, and Li J. Phospholipase A2 regulates autophagy in gouty arthritis: proteomic and metabolomic studies. J Transl Med. (2023) 21:261. doi: 10.1186/s12967-023-04114-6
82. Arya SB, Chen S, Jordan-Javed F, and Parent CA. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol. (2022) 24:1019–28. doi: 10.1038/s41556-022-00934-8
83. Luginina A, Gusach A, Lyapina E, Khorn P, Safronova N, Shevtsov M, et al. Structural diversity of leukotriene G-protein coupled receptors. J Biol Chem. (2023) 299:105247. doi: 10.1016/j.jbc.2023.105247
84. Yokomizo T, Nakamura M, and Shimizu T. Leukotriene receptors as potential therapeutic targets. J Clin Invest. (2018) 128:2691–701. doi: 10.1172/JCI97946
85. Subramanian BC, Melis N, Chen D, Wang W, Gallardo D, Weigert R, et al. The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J Cell Biol. (2020) 219(10):e201910215. doi: 10.1083/jcb.201910215
86. Shioda R, Jo-Watanabe A, Okuno T, Saeki K, Nakayama M, Suzuki Y, et al. The leukotriene B(4)/BLT1-dependent neutrophil accumulation exacerbates immune complex-mediated glomerulonephritis. FASEB J. (2023) 37:e22789. doi: 10.1096/fj.202201936R
87. Liew PX and Kubes P. The neutrophil’s role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
88. Wang H, Kim SJ, Lei Y, Wang S, Wang H, Huang H, et al. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct Target Ther. (2024) 9(1):235. doi: 10.1038/s41392-024-01933-x
89. Yu CM, Wang Y, Ren SC, Liu ZL, Zhu CL, Liu Q, et al. Caffeic acid modulates activation of neutrophils and attenuates sepsis-induced organ injury by inhibiting 5-LOX/LTB4 pathway. Int Immunopharmacol. (2023) 125:111143. doi: 10.1016/j.intimp.2023.111143
90. Xia Y, Lan J, Yang J, Yuan S, Xie X, Du Q, et al. Saturated fatty acid-induced neutrophil extracellular traps contribute to exacerbation and biologic therapy resistance in obesity-related psoriasis. Cell Mol Immunol. (2025) 22:597–611. doi: 10.1038/s41423-025-01278-7
91. Hidalgo A, Libby P, Soehnlein O, Aramburu IV, Papayannopoulos V, and Silvestre-Roig C. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc Res. (2022) 118:2737–53. doi: 10.1093/cvr/cvab329
92. Chen W, Chen H, Yang ZT, Mao EQ, Chen Y, and Chen EZ. Free fatty acids-induced neutrophil extracellular traps lead to dendritic cells activation and T cell differentiation in acute lung injury. Aging (Albany NY). (2021) 13:26148–60. doi: 10.18632/aging.203802
93. Pham L, Komalavilas P, Eddie AM, Thayer TE, Greenwood DL, Liu KH, et al. Neutrophil trafficking to the site of infection requires Cpt1a-dependent fatty acid β-oxidation. Commun Biol. (2022) 5:1366. doi: 10.1038/s42003-022-04339-z
94. Sankar P, Ramos RB, Corro J, Mishra LK, Nafiz TN, Bhargavi G, et al. Fatty acid metabolism in neutrophils promotes lung damage and bacterial replication during tuberculosis. PloS Pathog. (2024) 20:e1012188. doi: 10.1371/journal.ppat.1012188
95. Wang YB and Jin CZ. Roles of traditional Chinese medicine extracts in hyperuricemia and gout treatment: Mechanisms and clinical applications. World J Gastroenterol. (2024) 30:5076–80. doi: 10.3748/wjg.v30.i47.5076
96. Duwaerts CC and Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
97. Guma M, Dadpey B, Coras R, Mikuls TR, Hamilton B, Quehenberger O, et al. Xanthine oxidase inhibitor urate-lowering therapy titration to target decreases serum free fatty acids in gout and suppresses lipolysis by adipocytes. Arthritis Res Ther. (2022) 24:175. doi: 10.1186/s13075-022-02852-4
98. Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. (2006) 65:1312–24. doi: 10.1136/ard.2006.055269
99. Faller J and Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. (1982) 307:1598–602. doi: 10.1056/NEJM198212233072602
100. Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, and Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. (2004) 447:735–43. doi: 10.1007/s00424-003-1103-2
101. Mandal AK and Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
102. Pareek V, Tian H, Winograd N, and Benkovic SJ. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science. (2020) 368:283–90. doi: 10.1126/science.aaz6465
103. Alaswad A, Cabău G, Crişan TO, Zhou L, Zoodsma M, Botey-Bataller J, et al. Integrative analysis reveals the multilateral inflammatory mechanisms of CD14 monocytes in gout. Ann Rheum Dis. (2025) 84:1253–63. doi: 10.1016/j.ard.2025.01.046
104. Dai W, Guo R, Na X, Jiang S, Liang J, Guo C, et al. Hypoxia and the endometrium: An indispensable role for HIF-1α as therapeutic strategies. Redox Biol. (2024) 73:103205. doi: 10.1016/j.redox.2024.103205
105. Seo J, Jeong DW, Park JW, Lee KW, Fukuda J, and Chun YS. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun Biol. (2020) 3:638. doi: 10.1038/s42003-020-01367-5
106. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. (2001) 120:1183–92. doi: 10.1053/gast.2001.23256
107. Spychała J, Madrid-Marina V, and Fox IH. High Km soluble 5’-nucleotidase from human placenta. Properties and allosteric regulation by IMP and ATP. J Biol Chem. (1988) 263:18759–65.
108. Titus C, Hoque MT, and Bendayan R. PPAR agonists for the treatment of neuroinflammatory diseases. Trends Pharmacol Sci. (2024) 45:9–23. doi: 10.1016/j.tips.2023.11.004
109. Schweitz MC, Nashel DJ, and Alepa FP. Ibuprofen in the treatment of acute gouty arthritis. Jama. (1978) 239:34–5. doi: 10.1001/jama.1978.03280280034020
110. Cosgrove KT, Kuplicki R, Savitz J, Burrows K, Simmons WK, Khalsa SS, et al. Impact of ibuprofen and peroxisome proliferator-activated receptor gamma on emotion-related neural activation: A randomized, placebo-controlled trial. Brain Behav Immun. (2021) 96:135–42. doi: 10.1016/j.bbi.2021.05.023
111. Shafabakhsh R, Mobini M, Raygan F, Aghadavod E, Ostadmohammadi V, Amirani E, et al. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin Nutr ESPEN. (2020) 40:77–82. doi: 10.1016/j.clnesp.2020.09.029
112. Golse M, Weinhofer I, Blanco B, Barbier M, Yazbeck E, Huiban C, et al. Leriglitazone halts disease progression in adult patients with early cerebral adrenoleukodystrophy. Brain. (2024) 147(10):3344–51. doi: 10.1093/brain/awae169
113. Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, Belfort-DeAguiar R, et al. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. (2021) 41:2659–70. doi: 10.1111/liv.15005
114. Grobbee EJ, de Jong VD, Schrieks IC, Tushuizen ME, Holleboom AG, Tardif JC, et al. Improvement of non-invasive tests of liver steatosis and fibrosis as indicators for non-alcoholic fatty liver disease in type 2 diabetes mellitus patients with elevated cardiovascular risk profile using the PPAR-α/γ agonist aleglitazar. PloS One. (2022) 17:e0277706. doi: 10.1371/journal.pone.0277706
115. Yang J, Wei Y, Zhao T, Li X, Zhao X, Ouyang X, et al. Magnolol effectively ameliorates diabetic peripheral neuropathy in mice. Phytomedicine. (2022) 107:154434. doi: 10.1016/j.phymed.2022.154434
116. Bae J, Yang Y, Xu X, Flaherty J, Overby H, Hildreth K, et al. Naringenin, a citrus flavanone, enhances browning and brown adipogenesis: Role of peroxisome proliferator-activated receptor gamma. Front Nutr. (2022) 9:1036655. doi: 10.3389/fnut.2022.1036655
117. Ge X, Fan YZ, Deng Y, Chen Y, Gao J, Zhu G, et al. Pectolinarigenin mitigates 5-FU-induced intestinal mucositis via suppressing ferroptosis through activating PPARγ/GPX4 signaling. Phytomedicine. (2025) 143:156843. doi: 10.1016/j.phymed.2025.156843
118. Wu Y, Zeng M, Jiao X, Ma X, Wang H, Chen Y, et al. Protective effects of pollenin B in asthma: PPAR-γ-mediated regulation of inflammatory pathways and arachidonic acid metabolism. Phytomedicine. (2025) 145:156975. doi: 10.1016/j.phymed.2025.156975
119. Das D, Banerjee A, Mukherjee S, and Maji BK. Quercetin inhibits NF-kB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: an in vitro approach. Mol Biol Rep. (2024) 51:277. doi: 10.1007/s11033-024-09245-7
120. Rodrigues JA, Luo J, Sabel AR, Chen Z, Tang X, Kuo F, et al. Abstract 4366: Loss of EP300 triggers IL-1α signaling and subsequent activation of the IL-6/JAK/STAT3 axis to drive oncogenesis in bladder cancer. Cancer Res. (2024) 84:4366–. doi: 10.1158/1538-7445.AM2024-4366
121. Wang SQ, Xiang J, Zhang GQ, Fu LY, Xu YN, Chen Y, et al. Essential oil from Fructus Alpinia zerumbet ameliorates atherosclerosis by activating PPARγ-LXRα-ABCA1/G1 signaling pathway. Phytomedicine. (2024) 123:155227. doi: 10.1016/j.phymed.2023.155227
122. Lv M, Chen S, Shan M, Si Y, Huang C, Chen J, et al. Arctigenin induces activated HSCs quiescence via AMPK-PPARγ pathway to ameliorate liver fibrosis in mice. Eur J Pharmacol. (2024) 974:176629. doi: 10.1016/j.ejphar.2024.176629
123. Niu K, Bai P, Yang B, Feng X, and Qiu F. Asiatic acid alleviates metabolism disorders in ob/ob mice: mechanistic insights. Food Funct. (2022) 13:6934–46. doi: 10.1039/D2FO01069K
124. Yang Y, He Z, and Wu S. Ursolic acid alleviates paclitaxel-induced peripheral neuropathy through PPARγ activation. Toxicol Appl Pharmacol. (2024) 484:116883. doi: 10.1016/j.taap.2024.116883
125. Shafi S, Khurana N, and Gupta J. PPAR gamma agonistic activity of dillapiole: protective effects against diabetic nephropathy. Nat Prod Res. (2025) 39:3581–6. doi: 10.1080/14786419.2024.2334323
126. Li Z, Zhang Y, Su R, Yang J, Chen F, Chen L, et al. Notoginsenoside R1, a novel natural PPARγ Agonist, attenuates cognitive deficits in a mouse model of diabetic alzheimer’s disease through enhancing GLUT4-dependent neuronal glucose uptake. Phytother Res. (2025). doi: 10.1002/ptr.70006
127. Makimura H, Stanley TL, Suresh C, De Sousa-Coelho AL, Frontera WR, Syu S, et al. Metabolic effects of long-term reduction in free fatty acids with acipimox in obesity: A randomized trial. J Clin Endocrinol Metab. (2016) 101:1123–33. doi: 10.1210/jc.2015-3696
128. Zhou C, Yan L, Xu J, Hamezah HS, Wang T, Du F, et al. Phillyrin: an adipose triglyceride lipase inhibitor supported by molecular docking, dynamics simulation, and pharmacological validation. J Mol Model. (2024) 30:68. doi: 10.1007/s00894-024-05875-7
129. Gowri MS, Azhar RK, Kraemer FB, Reaven GM, and Azhar S. Masoprocol decreases rat lipolytic activity by decreasing the phosphorylation of HSL. Am J Physiol Endocrinol Metab. (2000) 279:E593–600. doi: 10.1152/ajpendo.2000.279.3.E593
130. Grabner GF, Guttenberger N, Mayer N, Migglautsch-Sulzer AK, Lembacher-Fadum C, Fawzy N, et al. Small-molecule inhibitors targeting lipolysis in human adipocytes. J Am Chem Soc. (2022) 144:6237–50. doi: 10.1021/jacs.1c10836
131. Xie X, Liu Y, Yang Q, Ma X, Lu Y, Hu Y, et al. Adipose triglyceride lipase–mediated adipocyte lipolysis exacerbates acute pancreatitis severity in mouse models and patients. Am J Pathol. (2024) 194:1494–510. doi: 10.1016/j.ajpath.2024.03.014
132. Zheng K, Yang L, Zhang RS, Qian YH, Zhou YG, Huang WF, et al. Puerarin alleviates alcoholic liver disease via suppressing lipolysis induced by sympathetic outflow. Am J Chin Med. (2025) 53:863–88. doi: 10.1142/S0192415X25500326
133. Xu H, Wang L, Yan K, Zhu H, Pan H, Yang H, et al. Nuciferine inhibited the differentiation and lipid accumulation of 3T3-L1 preadipocytes by regulating the expression of lipogenic genes and adipokines. Front Pharmacol. (2021) 12:632236. doi: 10.3389/fphar.2021.632236
134. Liu M, Yang C, Peng X, Zheng S, He H, Wang W, et al. Formononetin suppresses colitis-associated colon cancer by targeting lipid synthesis and mTORC2/Akt signaling. Phytomedicine. (2025) 142:156665. doi: 10.1016/j.phymed.2025.156665
135. Wen F, Dai P, Song Z, Jin C, Ji X, Hou J, et al. Alleviating effect of mulberry leaf 1-deoxynojirimycin on resistin-induced hepatic steatosis and insulin resistance in mice. J Physiol Pharmacol. (2022) 73(6). doi: 10.26402/jpp.2022.6.07
136. Wu S, Wu X, Wang Q, Chen Z, Li L, Chen H, et al. Bufalin induces ferroptosis by modulating the 2,4-dienoyl-CoA reductase (DECR1)-SLC7A11 axis in breast cancer. Phytomedicine. (2024) 135:156130. doi: 10.1016/j.phymed.2024.156130
137. Yao L, Zhao L, Liu F, Al-BukHaiti WQ, Huang X, Lin T, et al. New stilbenes from Cajanus cajan inhibit adipogenesis in 3T3-L1 adipocytes through down-regulation of PPARγ. Bioorg Chem. (2024) 153:107851. doi: 10.1016/j.bioorg.2024.107851
138. Fang Z, Wei L, Lv Y, Wang T, Hamezah HS, Han R, et al. Phillyrin restores metabolic disorders in mice fed with high-fat diet through inhibition of interleukin-6-mediated basal lipolysis. Front Nutr. (2022) 9:956218. doi: 10.3389/fnut.2022.956218
139. Duarte Lau F and Giugliano RP. Adenosine triphosphate citrate lyase and fatty acid synthesis inhibition: A narrative review. JAMA Cardiol. (2023) 8:879–87. doi: 10.1001/jamacardio.2023.2402
140. Liu M, Zhang Z, Chen Y, Feng T, Zhou Q, and Tian X. Circadian clock and lipid metabolism disorders: a potential therapeutic strategy for cancer. Front Endocrinol (Lausanne). (2023) 14:1292011. doi: 10.3389/fendo.2023.1292011
141. Günenc AN, Graf B, Stark H, and Chari A. Fatty acid synthase: structure, function, and regulation. Subcell Biochem. (2022) 99:1–33. doi: 10.1007/978-3-031-00793-4_1
142. Batchuluun B, Pinkosky SL, and Steinberg GR. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat Rev Drug Discov. (2022) 21:283–305. doi: 10.1038/s41573-021-00367-2
143. Ruscica M, Sirtori CR, Carugo S, Banach M, and Corsini A. Bempedoic acid: for whom and when. Curr Atheroscler Rep. (2022) 24:791–801. doi: 10.1007/s11883-022-01054-2
144. Weber EJ, Younis IR, Nelson C, Qin AR, Watkins TR, and Othman AA. Evaluation of the potential for cytochrome P450 and transporter-mediated drug-drug interactions for firsocostat, a liver-targeted inhibitor of acetyl-coA carboxylase. Clin Pharmacokinet. (2024) 63:1423–34. doi: 10.1007/s40262-024-01420-0
145. Loomba R, Bedossa P, Grimmer K, Kemble G, Bruno Martins E, McCulloch W, et al. Denifanstat for the treatment of metabolic dysfunction-associated steatohepatitis: a multicentre, double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. (2024) 9:1090–100. doi: 10.1016/S2468-1253(24)00246-2
146. Zhang Z, Chen M, Xu Y, Wang Z, Liu Z, He C, et al. A natural small molecule isoginkgetin alleviates hypercholesterolemia and atherosclerosis by targeting ACLY. Theranostics. (2025) 15:4325–44. doi: 10.7150/thno.105782
147. Li D, Yuan X, Ma J, Lu T, Zhang J, Liu H, et al. Morusin, a novel inhibitor of ACLY, induces mitochondrial apoptosis in hepatocellular carcinoma cells through ROS-mediated mitophagy. BioMed Pharmacother. (2024) 180:117510. doi: 10.1016/j.biopha.2024.117510
148. Chen M, Zhang R, Chen Y, Chen X, Li Y, Shen J, et al. Nobiletin inhibits de novo FA synthesis to alleviate gastric cancer progression by regulating endoplasmic reticulum stress. Phytomedicine. (2023) 116:154902. doi: 10.1016/j.phymed.2023.154902
149. Wang S, Sheng F, Zou L, Xiao J, and Li P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J Adv Res. (2021) 34:109–22. doi: 10.1016/j.jare.2021.06.001
150. Tie F, Ding J, Hu N, Dong Q, Chen Z, and Wang H. Kaempferol and kaempferide attenuate oleic acid-induced lipid accumulation and oxidative stress in hepG2 cells. Int J Mol Sci. (2021) 22:8847. doi: 10.3390/ijms22168847
151. Kim SR, Kim YJ, Kim H, Park S, and Jung UJ. Therapeutic potential of myricitrin in a db/db mouse model of type 2 diabetes. Molecules. (2025) 30:1460. doi: 10.3390/molecules30071460
152. Long Q, Chen H, Yang W, Yang L, and Zhang L. Delphinidin-3-sambubioside from Hibiscus sabdariffa. L attenuates hyperlipidemia in high fat diet-induced obese rats and oleic acid-induced steatosis in HepG2 cells. Bioengineered. (2021) 12:3837–49. doi: 10.1080/21655979.2021.1950259
153. Chen Y, Shen J, Yuan M, Li H, Li Y, Zheng S, et al. Dehydrocostus lactone suppresses gastric cancer progression by targeting ACLY to inhibit fatty acid synthesis and autophagic flux. J Adv Res. (2025) 67:331–48. doi: 10.1016/j.jare.2024.01.028
154. Lim SH, Lee HS, Han HK, and Choi CI. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int J Mol Sci. (2021) 22:11409. doi: 10.3390/ijms222111409
155. Mu Q, Wang H, Tong L, Fang Q, Xiang M, Han L, et al. Betulinic acid improves nonalcoholic fatty liver disease through YY1/FAS signaling pathway. FASEB J. (2020) 34:13033–48. doi: 10.1096/fj.202000546R
156. Song T, Wang P, Li C, Jia L, Liang Q, Cao Y, et al. Salidroside simultaneously reduces de novo lipogenesis and cholesterol biosynthesis to attenuate atherosclerosis in mice. BioMed Pharmacother. (2021) 134:111137. doi: 10.1016/j.biopha.2020.111137
157. Lu Y, Zhang C, Song Y, Chen L, Chen X, Zheng G, et al. Gallic acid impairs fructose-driven de novo lipogenesis and ameliorates hepatic steatosis via AMPK-dependent suppression of SREBP-1/ACC/FASN cascade. Eur J Pharmacol. (2023) 940:175457. doi: 10.1016/j.ejphar.2022.175457
158. Lv H, Liu Y, Zhang B, Zheng Y, Ji H, and Li S. The improvement effect of gastrodin on LPS/GalN-induced fulminant hepatitis via inhibiting inflammation and apoptosis and restoring autophagy. Int Immunopharmacol. (2020) 85:106627. doi: 10.1016/j.intimp.2020.106627
159. Singh KB, Hahm ER, Kim SH, and Singh SV. Withaferin A inhibits fatty acid synthesis in rat mammary tumors. Cancer Prev Res (Phila). (2023) 16:5–16. doi: 10.1158/1940-6207.CAPR-22-0193
160. Elseweidy MM, Elawady AS, Sobh MS, and Elnagar GM. Lycopene ameliorates hyperlipidemia via potentiation of AMP-activated protein kinase and inhibition of ATP-citrate lyase in diabetic hyperlipidemic rat model. Life Sci. (2022) 308:120934. doi: 10.1016/j.lfs.2022.120934
161. Yuan D, Huang Q, Li C, and Fu X. A polysaccharide from Sargassum pallidum reduces obesity in high-fat diet-induced obese mice by modulating glycolipid metabolism. Food Funct. (2022) 13:7181–91. doi: 10.1039/D2FO00890D
162. Sharma A, Anand SK, Singh N, Dwarkanath A, Dwivedi UN, and Kakkar P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. (2021) 12:892–909. doi: 10.1039/D0FO02501A
163. Zhang H, Yang L, Wang Y, Huang W, Li Y, Chen S, et al. Oxymatrine alleviated hepatic lipid metabolism via regulating miR-182 in non-alcoholic fatty liver disease. Life Sci. (2020) 257:118090. doi: 10.1016/j.lfs.2020.118090
164. Lin HH, Tseng CY, Yu PR, Ho HY, Hsu CC, and Chen JH. Therapeutic effect of desmodium caudatum extracts on alleviating diabetic nephropathy mice. Plant Foods Hum Nutr. (2024) 79:374–80. doi: 10.1007/s11130-024-01192-9
165. Wang QS, Li M, Li X, Zhang NW, Hu HY, Zhang LL, et al. Protective effect of orange essential oil on the formation of non-alcoholic fatty liver disease caused by high-fat diet. Food Funct. (2022) 13:933–43. doi: 10.1039/D1FO03793E
166. Hu X, Fatima S, Chen M, Huang T, Chen YW, Gong R, et al. Dihydroartemisinin is potential therapeutics for treating late-stage CRC by targeting the elevated c-Myc level. Cell Death Dis. (2021) 12:1053. doi: 10.1038/s41419-021-04247-w
167. Ma R, Zhan Y, Zhang Y, Wu L, Wang X, and Guo M. Schisandrin B ameliorates non-alcoholic liver disease through anti-inflammation activation in diabetic mice. Drug Dev Res. (2022) 83:735–44. doi: 10.1002/ddr.21905
168. Wan W, Wei R, Xu B, Cao H, Zhi Y, Guo F, et al. Qiwei Jinggan Ling regulates oxidative stress and lipid metabolism in alcoholic liver disease by activating AMPK. Phytomedicine. (2024) 135:156125. doi: 10.1016/j.phymed.2024.156125
169. Yan Y, Zhou Y, Li J, Zheng Z, Hu Y, Li L, et al. Sulforaphane downregulated fatty acid synthase and inhibited microtubule-mediated mitophagy leading to apoptosis. Cell Death Dis. (2021) 12:917. doi: 10.1038/s41419-021-04198-2
170. Chen M, Little PJ, Kong S, Banach M, Weng J, and Xu S. Aclysiran, an RNAi therapeutic agent targeting ACLY, treats hypercholesterolemia and atherosclerosis in ApoE (-/-) mice. Acta Pharm Sin B. (2024) 14:5505–8. doi: 10.1016/j.apsb.2024.10.008
171. Li JL, Jiang JY, Gu SS, Zhang L, Sun Y, Wang XH, et al. Identification of lignans as ATP citrate lyase (ACLY) inhibitors from the roots of Pouzolzia zeylanica. Nat Prod Res. (2024) 38:1719–26. doi: 10.1080/14786419.2023.2219816
172. Tzeng HT, Chyuan IT, and Chen WY. Shaping of innate immune response by fatty acid metabolite palmitate. Cells. (2019) 8:1633. doi: 10.3390/cells8121633
173. Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. (2012) 53:2002–13. doi: 10.1194/jlr.D029546
174. Zhu M, Sun Y, Su Y, Guan W, Wang Y, Han J, et al. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother Res. (2024) 38:3417–43. doi: 10.1002/ptr.8217
175. Shen R, Ma L, and Zheng Y. Anti-inflammatory effects of luteolin on acute gouty arthritis rats via TLR/MyD88/NF-κB pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2020) 45:115–22. doi: 10.11817/j.issn.1672-7347.2020.190566
176. Li JC, Li SY, Song Q, Ma EX, and Aimaijiang MK. Mechanism of total flavonoids from Ampelopsis grossedentata against gouty arthritis based on multi-level interactive network and in vivo experimental validation. Zhongguo Zhong Yao Za Zhi. (2022) 47:4733–43. doi: 10.19540/j.cnki.cjcmm.20220223.701
177. Ma H, Xie C, He G, Chen Z, Lu H, Wu H, et al. Sparstolonin B suppresses free fatty acid palmitate-induced chondrocyte inflammation and mitigates post-traumatic arthritis in obese mice. J Cell Mol Med. (2022) 26:725–35. doi: 10.1111/jcmm.17099
178. Wang W, Wang Y, Wang F, Xie G, Liu S, Li Z, et al. Gastrodin regulates the TLR4/TRAF6/NF-κB pathway to reduce neuroinflammation and microglial activation in an AD model. Phytomedicine. (2024) 128:155518. doi: 10.1016/j.phymed.2024.155518
179. Feng Q, Yu X, Xie J, Liu F, Zhang X, Li S, et al. Phillygenin improves diabetic nephropathy by inhibiting inflammation and apoptosis via regulating TLR4/MyD88/NF-κB and PI3K/AKT/GSK3β signaling pathways. Phytomedicine. (2025) 136:156314. doi: 10.1016/j.phymed.2024.156314
180. Qi MY, He YH, Cheng Y, Fang Q, Ma RY, Zhou SJ, et al. Icariin ameliorates streptozocin-induced diabetic nephropathy through suppressing the TLR4/NF-κB signal pathway. Food Funct. (2021) 12:1241–51. doi: 10.1039/D0FO02335C
181. Jiao H, Zhang M, Xu W, Pan T, Luan J, Zhao Y, et al. Chlorogenic acid alleviate kidney fibrosis through regulating TLR4/NF-κB mediated oxidative stress and inflammation. J Ethnopharmacol. (2024) 335:118693. doi: 10.1016/j.jep.2024.118693
182. Wang YW, Wu YH, Zhang JZ, Tang JH, Fan RP, Li F, et al. Ruscogenin attenuates particulate matter-induced acute lung injury in mice via protecting pulmonary endothelial barrier and inhibiting TLR4 signaling pathway. Acta Pharmacol Sin. (2021) 42:726–34. doi: 10.1038/s41401-020-00502-6
183. Yang Z, Li X, Wei L, Bao L, Hu H, Liu L, et al. Involucrasin B suppresses airway inflammation in obese asthma by inhibiting the TLR4-NF-κB-NLRP3 pathway. Phytomedicine. (2024) 132:155850. doi: 10.1016/j.phymed.2024.155850
184. Wu S, Liao J, Hu G, Yan L, Su X, Ye J, et al. Corilagin alleviates LPS-induced sepsis through inhibiting pyroptosis via targeting TIR domain of MyD88 and binding CARD of ASC in macrophages. Biochem Pharmacol. (2023) 217:115806. doi: 10.1016/j.bcp.2023.115806
185. Fang Z, Wang G, Huang R, Liu C, Yushanjiang F, Mao T, et al. Astilbin protects from sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-κB pathway. Phytother Res. (2024) 38:1044–58. doi: 10.1002/ptr.8093
186. Lee D, Jeon J, Baek S, Park O, Kim AR, Do MS, et al. CycloZ suppresses TLR4-driven inflammation to reduce asthma-like responses in HDM-exposed mouse models. Cells. (2024) 13:2034. doi: 10.3390/cells13232034
187. Engin S, Barut EN, Yaşar YK, Soysal A, Arıcı T, Kerimoğlu G, et al. Trimetazidine attenuates cyclophosphamide-induced cystitis by inhibiting TLR4-mediated NFκB signaling in mice. Life Sci. (2022) 301:120590. doi: 10.1016/j.lfs.2022.120590
188. Inandiklioglu N, Doganyigit Z, Okan A, Kaymak E, and Silici S. Nephroprotective effect of apilarnil in lipopolysaccharide-induced sepsis through TLR4/NF-κB signaling pathway. Life Sci. (2021) 284:119875. doi: 10.1016/j.lfs.2021.119875
189. Chen P, Guo Z, Lei J, and Wang Y. Pomegranate polyphenol punicalin ameliorates lipopolysaccharide-induced memory impairment, behavioral disorders, oxidative stress, and neuroinflammation via inhibition of TLR4-NF-кB pathway. Phytother Res. (2024) 38:3489–508. doi: 10.1002/ptr.8219
190. Zhu W, Wang M, Jin L, Yang B, Bai B, Mutsinze RN, et al. Licochalcone A protects against LPS-induced inflammation and acute lung injury by directly binding with myeloid differentiation factor 2 (MD2). Br J Pharmacol. (2023) 180:1114–31. doi: 10.1111/bph.15999
191. Liu C, Liu J, Wang W, Yang M, Chi K, Xu Y, et al. Epigallocatechin gallate alleviates staphylococcal enterotoxin A-induced intestinal barrier damage by regulating gut microbiota and inhibiting the TLR4-NF-κB/MAPKs-NLRP3 inflammatory cascade. J Agric Food Chem. (2023) 71:16286–302. doi: 10.1021/acs.jafc.3c04526
192. Bai Y, Min R, Chen P, Mei S, Deng F, Zheng Z, et al. Disulfiram blocks inflammatory TLR4 signaling by targeting MD-2. Proc Natl Acad Sci U S A. (2023) 120:e2306399120. doi: 10.1073/pnas.2306399120
193. Xu J, Liu J, Li Q, Li G, Zhang G, Mi Y, et al. Pterostilbene participates in TLR4- mediated inflammatory response and autophagy-dependent Aβ(1-42) endocytosis in Alzheimer’s disease. Phytomedicine. (2023) 119:155011. doi: 10.1016/j.phymed.2023.155011
194. Li W, Yu J, Zhao J, Xiao X, Li W, Zang L, et al. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE(-/-) mice by inhibiting inflammation. Phytother Res. (2021) 35:2220–9. doi: 10.1002/ptr.6980
195. Zhai S, Peng X, Liu C, Zhang R, Jin C, Jiang X, et al. Ginsenoside Rg1 alleviates ochratoxin A-induced liver inflammation in ducklings: Involvement of intestinal microbiota modulation and the TLR4/NF-κB pathway inhibition. Ecotoxicol Environ Saf. (2025) 296:118186. doi: 10.1016/j.ecoenv.2025.118186
196. He J, Wu H, Zhou Y, and Zheng C. Tomentosin inhibit cerebral ischemia/reperfusion induced inflammatory response via TLR4/NLRP3 signalling pathway - in vivo and in vitro studies. BioMed Pharmacother. (2020) 131:110697. doi: 10.1016/j.biopha.2020.110697
197. Yang M, Jiao H, Li Y, Zhang L, Zhang J, Zhong X, et al. Guanmaitong granule attenuates atherosclerosis by inhibiting inflammatory immune response in apoE(-/-) mice fed high-fat diet. Drug Des Devel Ther. (2022) 16:3145–68. doi: 10.2147/DDDT.S372143
198. Li Z, Lian Y, Wei R, Jin L, Cao H, Zhao T, et al. Effects of taraxasterol against ethanol and high-fat diet-induced liver injury by regulating TLR4/MyD88/NF-κB and Nrf2/HO-1 signaling pathways. Life Sci. (2020) 262:118546. doi: 10.1016/j.lfs.2020.118546
199. Jin X, He R, Liu J, Wang Y, Li Z, Jiang B, et al. An herbal formulation “Shenshuaifu Granule” alleviates cisplatin-induced nephrotoxicity by suppressing inflammation and apoptosis through inhibition of the TLR4/MyD88/NF-κB pathway. J Ethnopharmacol. (2023) 306:116168. doi: 10.1016/j.jep.2023.116168
200. Gan A, Chen H, Lin F, Wang R, Wu B, Yan T, et al. Sanzi Yangqin Decoction improved acute lung injury by regulating the TLR2-mediated NF-κB/NLRP3 signaling pathway and inhibiting the activation of NLRP3 inflammasome. Phytomedicine. (2025) 139:156438. doi: 10.1016/j.phymed.2025.156438
201. Athapaththu A, Lee KT, Kavinda MHD, Lee S, Kang S, Lee MH, et al. Pinostrobin ameliorates lipopolysaccharide (LPS)-induced inflammation and endotoxemia by inhibiting LPS binding to the TLR4/MD2 complex. BioMed Pharmacother. (2022) 156:113874. doi: 10.1016/j.biopha.2022.113874
202. Li R, Ma Y, Wu H, Zhang X, Ding N, Li Z, et al. 4-Octyl itaconate alleviates endothelial cell inflammation and barrier dysfunction in LPS-induced sepsis via modulating TLR4/MAPK/NF-κB signaling: 4-Octyl itaconate alleviates endothelial dysfunction. Mol Med. (2025) 31:240. doi: 10.1186/s10020-025-01160-2
203. Luo L, Lin S, Hu G, Wu J, Hu Y, Nong F, et al. Molecular mechanism of Rolupram reducing neuroinflammation in noise induced tinnitus mice through TLR4/NF kB/NLRP3 protein/caspase-1/IL-1 β signaling pathway. Int J Biol Macromol. (2024) 278:134987. doi: 10.1016/j.ijbiomac.2024.134987
204. Huang J, Li L, Xu L, Feng L, Wang Y, Sik AG, et al. Methyl 3-bromo-4,5-dihydroxybenzoate attenuates inflammatory bowel disease by regulating TLR/NF-κB pathways. Mar Drugs. (2025) 23:401. doi: 10.3390/md23010047
205. Chen G, Wang W, Guan B, Zhang G, Zhang Z, Lin L, et al. Cycloastragenol reduces inflammation in CLP-induced septic MICE by suppressing TLR4 signaling pathways. Phytomedicine. (2025) 142:156645. doi: 10.1016/j.phymed.2025.156645
206. Zhang G, Han X, Xu T, Liu M, Chen G, Xie L, et al. Buyang Huanwu Decoction suppresses cardiac inflammation and fibrosis in mice after myocardial infarction through inhibition of the TLR4 signalling pathway. J Ethnopharmacol. (2024) 320:117388. doi: 10.1016/j.jep.2023.117388
207. Wang F, Liu C, Ren L, Li Y, Yang H, Yu Y, et al. Sanziguben polysaccharides improve diabetic nephropathy in mice by regulating gut microbiota to inhibit the TLR4/NF-κB/NLRP3 signalling pathway. Pharm Biol. (2023) 61:427–36. doi: 10.1080/13880209.2023.2174145
208. Li W, Wang K, Liu Y, Wu H, He Y, Li C, et al. A novel drug combination of mangiferin and cinnamic acid alleviates rheumatoid arthritis by inhibiting TLR4/NFκB/NLRP3 activation-induced pyroptosis. Front Immunol. (2022) 13:912933. doi: 10.3389/fimmu.2022.912933
209. Zhang D, Jing B, Chen ZN, Li X, Shi HM, Zheng YC, et al. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci Ther. (2023) 29:1000–11. doi: 10.1111/cns.14060
210. Schumacher HR Jr., Boice JA, Daikh DI, Mukhopadhyay S, Malmstrom K, Ng J, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. Bmj. (2002) 324:1488–92. doi: 10.1136/bmj.324.7352.1488
211. Wang XM, Wu TX, Hamza M, Ramsay ES, Wahl SM, and Dionne RA. Rofecoxib modulates multiple gene expression pathways in a clinical model of acute inflammatory pain. Pain. (2007) 128:136–47. doi: 10.1016/j.pain.2006.09.011
212. Schumacher HR, Berger MF, Li-Yu J, Perez-Ruiz F, Burgos-Vargas R, and Li C. Efficacy and tolerability of celecoxib in the treatment of acute gouty arthritis: a randomized controlled trial. J Rheumatol. (2012) 39:1859–66. doi: 10.3899/jrheum.110916
213. Mizraji M. Clinical response to etodolac in the management of pain. Eur J Rheumatol Inflamm. (1990) 10:35–43.
214. Liu Y, Duan C, Chen H, Wang C, Liu X, Qiu M, et al. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol. (2018) 351:1–11. doi: 10.1016/j.taap.2018.05.010
215. Talukder S, Ahmed KS, Hossain H, Hasan T, Liya IJ, Amanat M, et al. Fimbristylis aestivalis Vahl: a potential source of cyclooxygenase-2 (COX-2) inhibitors. Inflammopharmacology. (2022) 30:2301–15. doi: 10.1007/s10787-022-01057-0
216. El-Miligy MMM, Al-Kubeisi AK, Bekhit MG, El-Zemity SR, Nassra RA, and Hazzaa AA. Towards safer anti-inflammatory therapy: synthesis of new thymol-pyrazole hybrids as dual COX-2/5-LOX inhibitors. J Enzyme Inhib Med Chem. (2023) 38:294–308. doi: 10.1080/14756366.2022.2147164
217. Saraf P, Nath Tripathi P, Kumar Tripathi M, Tripathi A, Verma H, Kumar Waiker D, et al. Novel 5,6-diphenyl-1,2,4-triazine-3-thiol derivatives as dual COX-2/5-LOX inhibitors devoid of cardiotoxicity. Bioorg Chem. (2022) 129:106147. doi: 10.1016/j.bioorg.2022.106147
218. Xin M, Wu H, Du Y, Liu S, Zhao F, and Mou X. Synthesis and biological evaluation of resveratrol amide derivatives as selective COX-2 inhibitors. Chem Biol Interact. (2023) 380:110522. doi: 10.1016/j.cbi.2023.110522
219. Vunnam N, Young MC, Liao EE, Lo CH, Huber E, Been M, et al. Nimesulide, a COX-2 inhibitor, sensitizes pancreatic cancer cells to TRAIL-induced apoptosis by promoting DR5 clustering †. Cancer Biol Ther. (2023) 24:2176692. doi: 10.1080/15384047.2023.2176692
220. Yasir M, Park J, Han ET, Park WS, Han JH, Kwon YS, et al. Vismodegib identified as a novel COX-2 inhibitor via deep-learning-based drug repositioning and molecular docking analysis. ACS Omega. (2023) 8:34160–70. doi: 10.1021/acsomega.3c05425
221. Al-Ghulikah HA, El-Sebaey SA, Bass AKA, and El-Zoghbi MS. New pyrimidine-5-carbonitriles as COX-2 inhibitors: design, synthesis, anticancer screening, molecular docking, and in silico ADME profile studies. Molecules. (2022) 27:7485. doi: 10.3390/molecules27217485
222. Elgohary MK, Elkotamy MS, Alkabbani MA, El Hassab MA, Al-Rashood ST, Binjubair FA, et al. Sulfonamide-Pyrazole derivatives as next-generation Cyclooxygenase-2 enzyme inhibitors: From molecular design to in vivo efficacy. Int J Biol Macromol. (2025) 293:139170. doi: 10.1016/j.ijbiomac.2024.139170
223. Hou X, Chen R, Wu J, Huang Y, Zhang X, Xu S, et al. Selective COX-2 inhibitors from natural sources: Insights from the analysis of components absorbed into blood from Isatidis Radix Lignans. J Ethnopharmacol. (2025) 349:119930. doi: 10.1016/j.jep.2025.119930
224. Deng J, Wu Z, Chen C, Zhao Z, Li Y, Su Z, et al. Chinese medicine huzhen tongfeng formula effectively attenuates gouty arthritis by inhibiting arachidonic acid metabolism and inflammatory mediators. Mediators Inflamm. (2020) 2020:6950206. doi: 10.1155/2020/6950206
225. Napagoda M, Gerstmeier J, Butschek H, Lorenz S, Kanatiwela D, Qader M, et al. Lipophilic extracts of Leucas zeylanica, a multi-purpose medicinal plant in the tropics, inhibit key enzymes involved in inflammation and gout. J Ethnopharmacol. (2018) 224:474–81. doi: 10.1016/j.jep.2018.04.042
226. Liu L, Zhang P, Zhang Z, Liang Y, Chen H, He Z, et al. 5-Lipoxygenase inhibition reduces inflammation and neuronal apoptosis via AKT signaling after subarachnoid hemorrhage in rats. Aging (Albany NY). (2021) 13:11752–61. doi: 10.18632/aging.202869
227. Toda K, Tsukayama I, Nagasaki Y, Konoike Y, Tamenobu A, Ganeko N, et al. Red-kerneled rice proanthocyanidin inhibits arachidonate 5-lipoxygenase and decreases psoriasis-like skin inflammation. Arch Biochem Biophys. (2020) 689:108307. doi: 10.1016/j.abb.2020.108307
228. Tsukayama I, Kawakami Y, Tamenobu A, Toda K, Maruoka S, Nagasaki Y, et al. Malabaricone C derived from nutmeg inhibits arachidonate 5-lipoxygenase activity and ameliorates psoriasis-like skin inflammation in mice. Free Radic Biol Med. (2022) 193:1–8. doi: 10.1016/j.freeradbiomed.2022.09.028
229. Shao XX, Chen C, Liu J, Li QJ, He S, Qi XH, et al. Biological evaluation of lysionotin: a novel inhibitor of 5-lipoxygenase for anti-glioma. Chin J Integr Med. (2024) 30:826–34. doi: 10.1007/s11655-024-3763-z
230. Yao T, Yao YY, Wang JZ, Jiang SM, and Li LJ. Magnolin alleviated DSS-induced colitis by inhibiting ALOX5-mediated ferroptosis. Kaohsiung J Med Sci. (2024) 40:360–73. doi: 10.1002/kjm2.12806
231. Liang XY, Hong FF, and Yang SL. Astragaloside IV alleviates liver inflammation, oxidative stress and apoptosis to protect against experimental non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. (2021) 14:1871–83. doi: 10.2147/DMSO.S304817
232. Wenjing X, Pei W, Yueshi M, Yong L, Weiqin Z, and Liping G. Compound dahuang baiji spray improves acute radiodermatitis by down-regulating the expression of ALOX5. Altern Ther Health Med. (2024) 30:214–21.
233. Villar A, Silva-Fuentes F, Mulà A, and Zangara A. Anti-inflammatory potential of pygeum africanum bark extract: an in vitro study of cytokine release by lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Int J Mol Sci. (2024) 2(2-3):123–34. doi: 10.3390/ijms25158298
234. Ullah R, Ali G, Rasheed A, Subhan F, Khan A, Ahsan Halim S, et al. The 7-Hydroxyflavone attenuates chemotherapy-induced neuropathic pain by targeting inflammatory pathway. Int Immunopharmacol. (2022) 107:108674. doi: 10.1016/j.intimp.2022.108674
235. Park NY, Im S, and Jiang Q. Different forms of vitamin E and metabolite 13’-carboxychromanols inhibit cyclooxygenase-1 and its catalyzed thromboxane in platelets, and tocotrienols and 13’-carboxychromanols are competitive inhibitors of 5-lipoxygenase. J Nutr Biochem. (2022) 100:108884. doi: 10.1016/j.jnutbio.2021.108884
236. Cerchia C, Küfner L, Werz O, and Lavecchia A. Identification of selective 5-LOX and FLAP inhibitors as novel anti-inflammatory agents by ligand-based virtual screening. Eur J Med Chem. (2024) 263:115932. doi: 10.1016/j.ejmech.2023.115932
237. Pillinger MH and Mandell BF. Therapeutic approaches in the treatment of gout. Semin Arthritis Rheum. (2020) 50:S24–s30. doi: 10.1016/j.semarthrit.2020.04.010
239. Useini L, Mojić M, Laube M, Lönnecke P, Mijatović S, Maksimović-Ivanić D, et al. Carborane analogues of fenoprofen exhibit improved antitumor activity. ChemMedChem. (2023) 18:e202200583. doi: 10.1002/cmdc.202200583
240. Lincoff AM, Tardif JC, Schwartz GG, Nicholls SJ, Rydén L, Neal B, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. Jama. (2014) 311:1515–25. doi: 10.1001/jama.2014.3321
241. Luo J and Solit DB. Leveraging real-world data to advance biomarker discovery and precision oncology. Cancer Cell. (2025) 43:606–10. doi: 10.1016/j.ccell.2025.03.012
242. He S, Shi J, Ma L, Pei H, Zhang P, Shi D, et al. Total ginsenosides decrease Aβ production through activating PPARγ. BioMed Pharmacother. (2024) 174:116577. doi: 10.1016/j.biopha.2024.116577
243. Espinoza-Derout J, Arambulo JML, Ramirez-Trillo W, Rivera JC, Hasan KM, Lao CJ, et al. The lipolysis inhibitor acipimox reverses the cardiac phenotype induced by electronic cigarettes. Sci Rep. (2023) 13:18239. doi: 10.1038/s41598-023-44082-x
Keywords: macrophages, neutrophil, fatty acid, gouty arthritis, monosodium urate, fatty acid metabolism
Citation: Zhao X, Sun Y, Yang L, Sun H, Zhang X, Sun H, Yan G and Wang X (2025) Fatty acid metabolism in gouty arthritis: mechanisms to therapeutic targeting. Front. Immunol. 16:1671548. doi: 10.3389/fimmu.2025.1671548
Received: 23 July 2025; Accepted: 29 September 2025;
Published: 16 October 2025.
Edited by:
Yoshiro Kobayashi, Toho University, JapanReviewed by:
Swarnima Pandey, University of Maryland, United StatesJiaqian Luo, Fudan University, China
Copyright © 2025 Zhao, Sun, Yang, Sun, Zhang, Sun, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijun Wang, eGlqdW53QHNpbmEuY29t
†These authors share first authorship
 Xueping Zhao
Xueping Zhao Ye Sun1†
Ye Sun1† Le Yang
Le Yang Xinya Zhang
Xinya Zhang Hui Sun
Hui Sun Xijun Wang
Xijun Wang