- 1Department of Radiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Key Laboratory of Anesthesiology (Shanghai Jiao Tong University), Ministry of Education, Shanghai, China
Aim: Reference data analysis and visualization methods were applied to identify current knowledge mapping methods in the neuroimmune modulation research field.
Methods: A comprehensive search of publications within the field of neuroimmune modulation in the Web of Science Core Collection database and PubMed from 2005–2024 was conducted. The reference type was restricted to articles and reviews in the WOSCC database and clinical trials in PubMed. The data were visualized and analyzed via visualization and bibliometric tools, including CiteSpace and VOSviewer.
Results: A total of 5,280 publications were included in the field of neuroimmune modulation. The United States ranked first as the most influential country in terms of both publication number and academic influence. Harvard University had the greatest impact on institutions. The leading scientists in the neuroimmune modulation field have focused on the mechanism at both the peripheral and central levels. Citation analysis and cocitation analysis revealed that acute kidney injury, neuropathic pain, and neuronal regulation are key focal points in neuroimmune research. Furthermore, keyword analysis revealed that “inflammation”, “neuroinflammation”, “microglia”, “cytokines”, “cholinergic anti-inflammatory pathway” and “neuroimmune” had more than 200 co-occurrences and represented research hotspots in this field. A total of 73 clinical trials were identified that targeted neuroimmune modulation to treat diseases. These studies highlighted the central role of the neuroimmune network in diseases, with a particular emphasis on innovative therapies that regulate inflammation through the cholinergic system. Further exploration is needed to develop precise intervention strategies targeting specific cytokines.
Conclusions: This analysis provides comprehensive knowledge mapping in the current research field of neuroimmune modulation. The trends in the field include mechanism studies combined with neuronal regulation. Neuroimmune crosstalk might provide new therapeutic targets for treating nervous system diseases.
1 Introduction
The interaction between the nervous and immune systems, referred to as neuroimmune modulation, is a bidirectional process facilitated by neurotransmitters, hormones, cytokines, and neural innervation (1, 2). Nerve-released neurotransmitters and neuromodulators interact with immune cell receptors, whereas endocrine-secreted hormones modulate immune responses. Cytokines influence the nervous system, and neural innervation of lymphoid organs directly affects immune cells, creating a reciprocal relationship between these systems.
This neuroimmune interaction is vital for sustaining both healthy and diseased states. During immune defense, it harmonizes responses to pathogens while curbing excessive inflammation (3). In autoimmune diseases, disruption of this neuroimmune interplay accelerates disease progression (4). Under neurodegenerative conditions, neuroinflammation intensifies neuronal injury (5). Notably, in mental health disorders, a functional link between the nervous and immune systems emerges, with chronic low-grade inflammation implicated in conditions such as depression. Consequently, unraveling the dynamics and mechanisms of this interaction is pivotal for developing novel therapies for diverse diseases.
In recent years, studies on neuroimmune regulation have proliferated significantly. Despite the vast and expanding literature in this domain, scientific approaches via bibliometric analyses capable of fully mapping research trends and elucidating knowledge structures remain scarce. While existing systematic reviews and meta-analyses offer valuable insights, they often focus on narrow aspects, limiting their capacity to provide a holistic perspective. Research indicates that comprehensive analyses of knowledge architecture and emerging research focal points are particularly advantageous for newcomers and early-stage investigators (6). Against this backdrop, bibliometric methodologies have gained traction as dual-purpose tools—facilitating both quantitative assessments of scientific output and qualitative evaluations of developmental trajectories. Moreover, advancements in information technology have propelled the adoption of visualization software such as CiteSpace and VOSviewer, which are now routinely employed across disciplines such as neuroscience, oncology, and pediatrics (7–9).
This study seeks to conduct a comprehensive bibliometric analysis of the scholarly literature on neuroimmune modulation spanning the past two decades (2005–2024). The analysis aims to map key contributors, assess the current research landscape, and outline potential avenues for future inquiry into the neuroimmune modulation research field.
2 Materials and methods
2.1 Data source and search strategy
We conducted a comprehensive search of the Web of Science Core Collection (WoSCC) database (10, 11), the Science Citation Index Expanded (SCI-E) edition and the PubMed (pubmed.ncbi.nlm.nih.gov) database. The retrieval strategy used in our study was as follows. The initial search phrase “neuroimmune modulation” was selected to align with the journal’s thematic focus. To ensure comprehensive coverage of relevant literature, we expanded our search by incorporating Medical Subject Headings (MeSH) terms associated with this concept (MeSH Unique ID: D015213). This approach, supported by bibliometric analyses, enabled the inclusion of a broader range of references pertinent to the research theme (12). The topic was set as “neuroimmunomodulation” or “neuro-immunomodulation” or “neuro immunomodulation” or “neuro-immune Interaction” or “neuro-immune Communication” or “neuro immune Interaction” or “neuro immune Communication” or “neuro-immune Axis” or “neuro immune Axis” or “neuroimmune Mechanism” or “neuroimmune Process” or “neuroimmune Interaction” or “neuroimmune Communication” or “neuroimmune Axis” or “cholinergic Anti-inflammatory Pathway” or “cholinergic Anti-inflammatory Pathway” or “vagal Anti-inflammatory Pathway” or “vagal Anti-inflammatory Pathway” or “vagal-immune Interaction” or “vagal immune Interactions”. The retrieval time ranged from January 2005 to December 2024, the literature types were “article” and “review”, and the language was “English” only. For the PubMed database, the topics and retrieval times were the same, and the literature types were restricted to “clinical trial” and “randomized controlled trial”. To minimize bias arising from frequent database updates, all literature retrieval and data downloads were completed on a single day, April 22, 2025. The retrieved references were independently screened by XH and YZ to assess their relevance to the topic. Discrepancies in relevance assessments between the researchers were resolved by XYG. References unrelated to the study topic were subsequently discarded (Figure 1).
2.2 Data processing
The dataset, including complete publication records and corresponding citations, was extracted for detailed examination. The analytical tools used included Microsoft Excel (version 2019), CiteSpace (version 6.2. R4), and VOSviewer (version 1.6.20).
Microsoft Excel was leveraged for its robust capabilities in data visualization (13). In this study, the tool facilitated the creation of charts and graphs to illustrate trends and insights, such as annual publication volumes, the prolific output of the top 10 contributing countries/regions, and a global map depicting research contributions by country/region on the basis of publication counts.
CiteSpace, a prominent bibliometric tool, was employed to map the research field’s evolutionary trends, identify emerging hotspots, and anticipate future trajectories (14). Specifically, it facilitated the visualization of collaborative networks among countries/regions, institutions, and authors while enabling cocitation analysis of references and burst detection to highlight sudden shifts in scholarly focus.
VOSviewer is a critical bibliometric tool developed by Professor van Eck and Waltman (15). In this study, it was used to visualize keyword co-occurrence networks, reveal thematic clusters, and analyze temporal patterns in keyword usage, thereby tracing evolving research focus areas.
These bibliometric tools were employed to comprehensively visualize and analyze data on neuroimmune modulation, enabling the exploration of research trends, core themes, and emerging hotspots. The goal was to derive actionable insights into the field’s trajectory, collaborative dynamics, and future priorities.
3 Results
3.1 Publication outputs
The total identified number of publications (5,280) serves as a key metric for tracking trends in neuroimmune modulation research from the WoSCC database. Of these, 3,454 were original articles, and 1,826 were reviews, reflecting robust scholarly engagement. Figure 2 illustrates the annual publication trajectory, which shows steady growth from 2005–2024, with a peak of 549 publications in 2021 (Figure 2). Despite minor fluctuations, this upward trend underscores the field’s expanding relevance and increasing interest over time.

Figure 2. The annual number of publications in the neuroimmune modulation research field from 2004–2024.
3.2 Basic knowledge structures of the neuroimmune modulation field
3.2.1 Analysis of the most productive countries/regions
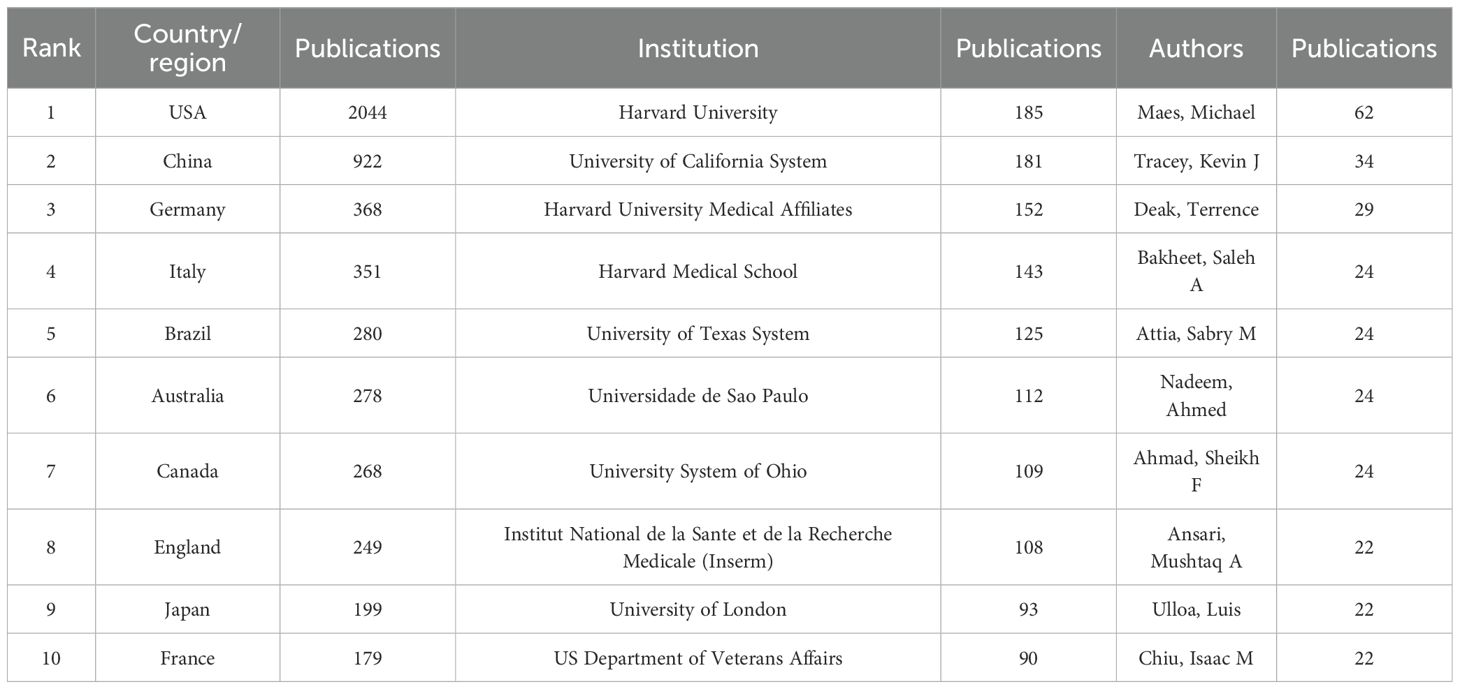
Figure 3A depicts the global distribution of publications via a color-coded world map, with North America, Western Europe, and East Asia dominating contributions. The United States topped the list with 2,044 documents, followed by P. R. China (922), Germany (368), Italy (351), and Brazil (280) (Table 1). Figure 3B leverages CiteSpace to visualize collaborative networks among 106 countries/regions connected by 835 links (density = 0.15), highlighting active international research partnerships.

Figure 3. (A) Publication counts of each country/region on a world map. A deeper color indicates a greater number of publications. (B) Visualized mapping of the collaboration analysis among countries/regions.
Betweenness centrality (BC) was applied to evaluate the network importance of countries, with values >0.1 indicating central hubs (purple-ring markers) (16). The United States (BC = 0.33), England (0.2), Italy (0.16), Brazil (0.13), Germany (0.12), and Canada (0.12) surpassed this threshold, highlighting their pivotal roles in connecting collaborative networks. While regional partnerships, especially in North America and Western Europe, are evident, global cooperation remains underdeveloped, underscoring the need for expanded transnational research collaboration.
3.2.2 Analysis of the most prolific institutions
The CiteSpace-generated institutional network comprised 703 nodes, which indicated institutions, and 2,816 links, which indicated collaborations (Figure 4A). The top five contributors to neuroimmune modulation research were all U.S. based, including Harvard University (185 publications), the University of California System (181), Harvard Medical Affiliates (152), Harvard Medical School (143), and the University of Texas System (125). (Table 1). All five are based in the United States, highlighting its leading role in this field. Harvard University has the highest publication volume and a broad range of research topics related to neuroimmune modulation. Institutions with high intermediary centrality, such as the University of California System (0.17), the Institut National de la Sante et de la Recherche Medicale (Inserm) (0.15), the University of London (0.13) and the Karolinska Institutet (0.1), were crucial hubs for fostering international collaboration. Despite these hubs, transnational institutional cooperation remains minimal, underscoring the need for strengthened global partnerships.

Figure 4. (A) Visualized mapping of the collaboration analysis among research institutions. (B) Visualized mapping of author collaboration analysis (via CiteSpace).
3.2.3 Analysis of influential authors
In neuroimmune modulation research, CiteSpace identified 7,062 authors and 22,177 collaborations, reflecting robust scholarly activity and frequent interdisciplinary partnerships (Figure 4B). Author productivity, as shown in Table 1, was dominated by Michael Maes (62 publications), a highly cited expert in neuropsychiatry with an H-index exceeding 120 and over 1,000 career publications. The work of Maes spans mental health biomarkers and neuroimmune disorders (17–21). Kevin J. Tracey ranked second, known for groundbreaking discoveries in inflammation, including HMGB1 mediators and bioelectronic medicine. As President of the Feinstein Institutes and Dean of the Elmezzi Graduate School, his research bridges immunology and neuroscience, with impacts on sepsis treatment and inflammatory reflex mechanisms (22–27). Terrence Deak, third in publication volume, focuses on neuroendocrine–immune interactions and early-life stress effects on brain function, using rodent models to dissect circuit-level changes linked to aging and social behavior. His recent work emphasized age-related social dysfunction mechanisms (28–31).
3.3 Overview of research hotspots and frontiers
3.3.1 Analysis of highly cited studies
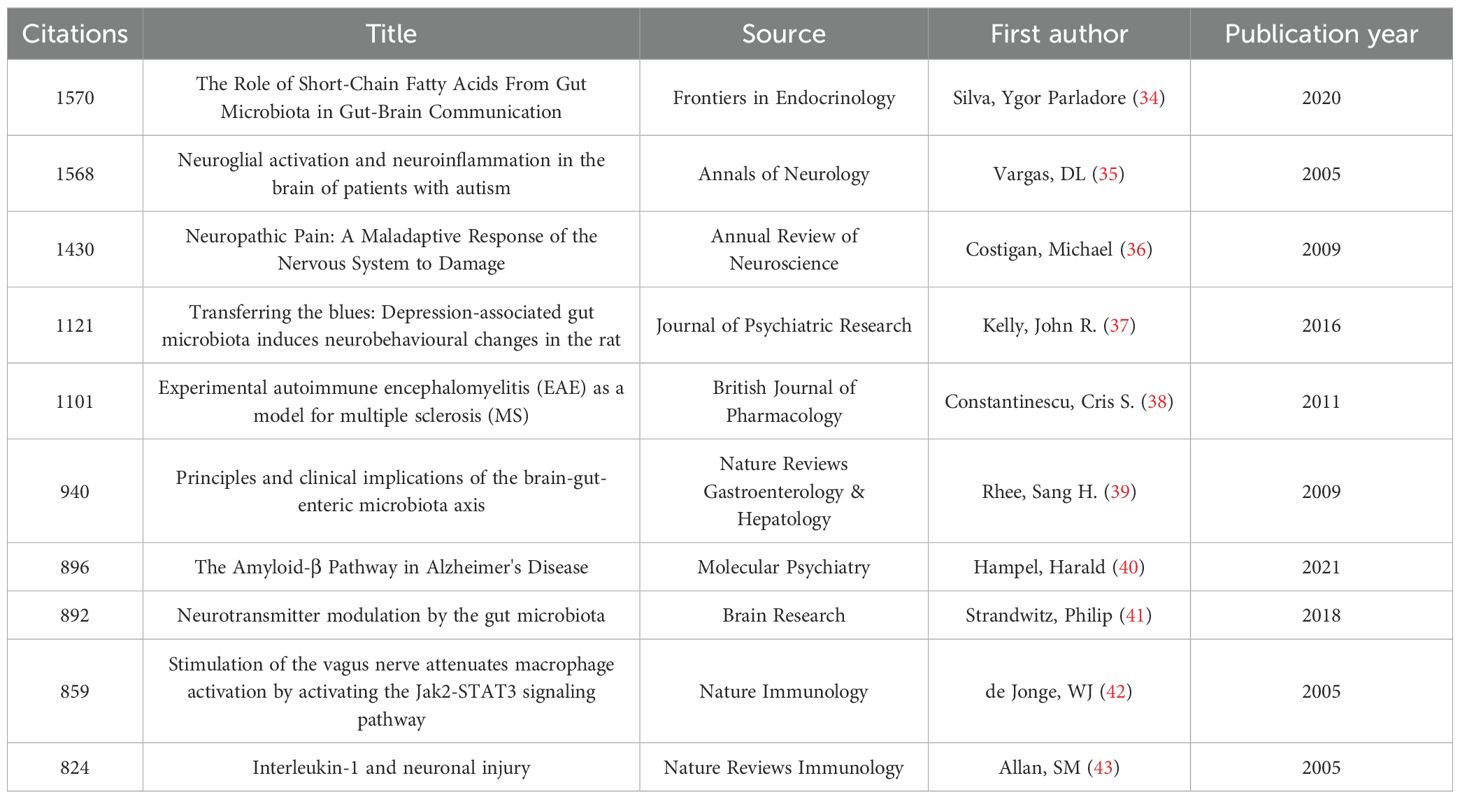
Citation analysis is fundamental to bibliometric studies, and despite some debate about its significance, citation counts are commonly viewed as indicators of a publication’s influence (32). Higher citation frequencies often reflect greater academic standing within a field (33). Table 2 highlights the seminal works that have garnered substantial attention, with each of the top 10 most-cited papers amassing over 820 citations (34–43).
The top-ranked paper, “The Role of Short-Chain Fatty Acids from the Gut Microbiota in Gut–Brain Communication”, by Ygor Parladore Silva et al., had 1520 citations (34). This review outlines the current knowledge about the involvement of short-chain fatty acids (SCFAs) in microbiota–gut–brain interactions and highlights how future treatments for central nervous system (CNS) disorders can utilize SCFAs to regulate neuro-immunoendocrine functions, such as their effects on microglial maturation, neurotransmitter levels, and the pathogenesis of various CNS and metabolic disorders. The second most-cited paper, titled “Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism” (35), investigated whether immune-mediated mechanisms are involved in autism. An examination of the brain tissues and cerebrospinal fluid of autistic patients revealed significant neuroglial activation and neuroinflammation, especially in the cerebellum. Unique cytokine expression profiles were detected. These findings suggest that innate immune responses play a role in autism pathogenesis, which might guide future therapies. The third-ranked title, “Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage,” by Michael Costigan, Joachim Scholz, and Clifford J. Woolf, examined the neurobiological mechanisms of neuropathic pain (36). A study revealed that neuropathic pain is a maladaptive response of the nervous system to damage involving multiple mechanisms, such as ectopic impulse generation, central sensitization, and neuroimmune interactions. Genetic factors also influence its development. Current treatments face challenges, and future research directions are proposed to better understand and manage these conditions.
3.3.2 References cocitation analysis
To investigate the foundational themes of neuroimmune modulation, we performed a reference co-occurrence analysis via CiteSpace. The resulting network comprises 965 nodes and 4198 links, with the top 10 most co-cited articles identified (Figure 5A). Notably, three of these articles have been co-cited more than 80 times, underscoring their importance in the field (27, 44–52). The most co-cited article, by Frieda A. Koopman et al. (2016), with 122 co-citations, focused on rheumatoid arthritis (RA) (44). This study aimed to determine whether vagus nerve stimulation inhibits TNF-α production and affects RA disease severity. It included epilepsy patients and two cohorts of RA patients. Vagus nerve stimulation was applied, and the study revealed that it inhibited TNF-α production and significantly attenuated RA disease severity. The second most cocited article with 91 co-citations was published by Mauricio Rosas-Ballina et al. (2011) (45). This research aimed to determine how the neural circuit of the inflammatory reflex terminates cholinergic signaling. Researchers have measured acetylcholine levels, studied the effect of vagus nerve stimulation in nude mice, and used ChAT(BAC)-EGFP mice to identify acetylcholine-producing T cells. The results showed that vagus nerve stimulation increases acetylcholine release in the spleen, that T cells are required for the inflammatory reflex, and that acetylcholine-producing memory T cells are integral to the inflammatory reflex. The third article, by Kevin J. Tracey (2007), received 81 co-citations (46). This review explores the physiology and immunology of the cholinergic anti-inflammatory pathway (CAP). Research has focused on how the nervous system, specifically via the vagus nerve’s inflammatory reflex, can regulate cytokine release to prevent tissue injury. The CAP, which is activated by vagus nerve stimulation or cholinergic agonists, can inhibit cytokine synthesis and protect against cytokine-mediated diseases in various experimental models. As seen from the wide-ranging research in this area, the regulation of cytokines and the role of the nervous system in inflammation have been important topics for researchers, and many studies have explored the potential therapeutic applications of modulating the CAP. The three highly co-cited publications focused on the interaction between the nervous system and the immune system. They explored how the vagus nerve and related neural mechanisms regulate cytokine production in diseases. One study revealed that vagus nerve stimulation benefits rheumatoid arthritis patients. Another study revealed the role of acetylcholine-synthesizing T cells in the vagus nerve circuit. The latter details the CAP. These findings suggest potential new therapies for inflammatory and autoimmune diseases.
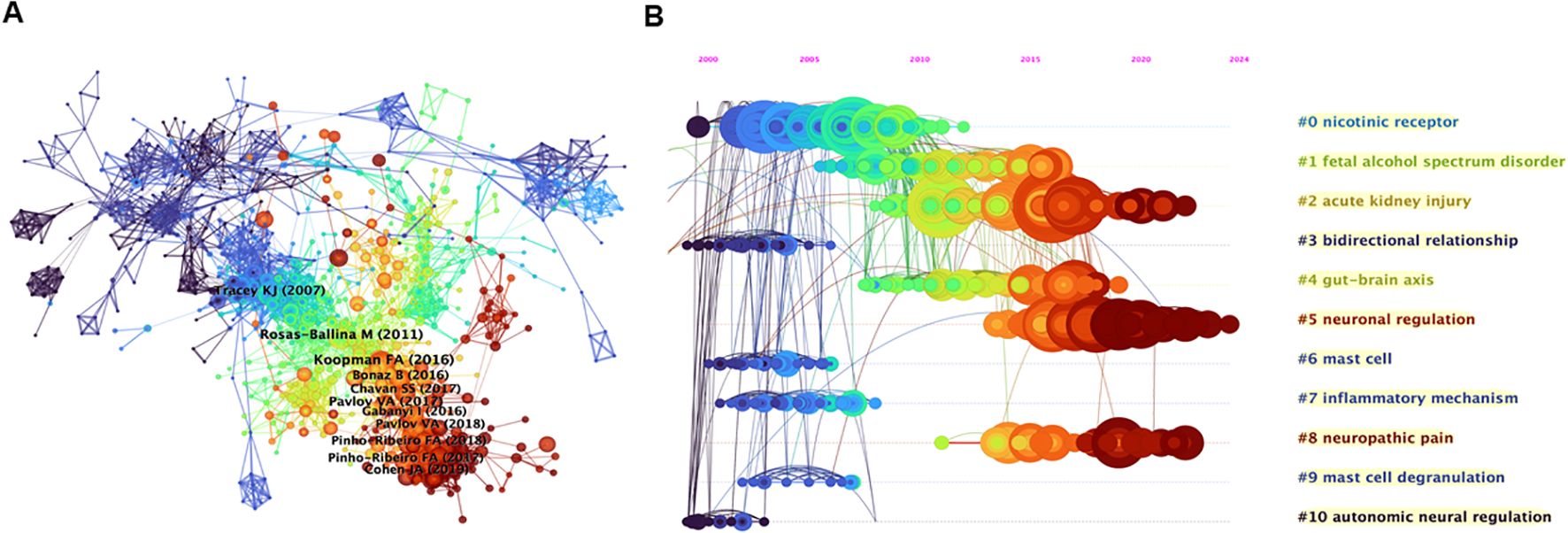
Figure 5. (A) References cocitation analysis generated by CiteSpace. (B) Clustering and timeline map of reference cocitation analysis.
Figure 5B presents a timeline illustrating temporal changes in nodes, links, and clusters postclustering. The references were organized into eleven primary clusters, ranked by size: nicotinic receptor, fetal alcohol spectrum disorder (FASD), acute kidney injury (AKI), bidirectional relationship, gut–brain axis, neuronal regulation, mast cell, inflammatory mechanism, neuropathic pain, mast cell degranulation, and autonomic neural regulation. As shown in Figure 4B, the two largest clusters, nicotinic receptor and FASD, dominated research before 2013 and 2016, respectively. In contrast, contemporary research has focused on AKI, neuronal regulation, and neuropathic pain, which are emerging as current hotspots.
3.3.3 Analysis of references with citation bursts
Burst detection, a method developed by Kleinberg, is widely recognized for identifying abrupt increases in the popularity of references or keywords within specific timeframes. This algorithm enables efficient tracking of concepts or topics that gain traction during defined periods. In our study, CiteSpace’s burst detection feature was employed to pinpoint pivotal references in neuroimmune modulation research. Figure 6 shows the top 25 references with the most substantial citation bursts, where the blue lines denote temporal spans and the red segments highlight periods of intense citation activity. Notably, the two references with the highest burst values—48.86 and 43.79—were previously discussed in the context of co-citation frequency (44, 45). While the majority of reference bursts have diminished, four references exhibit persistent bursts, signaling sustained scholarly interest in these topics (48, 51, 53, 54). The four papers collectively investigated the complex interplay between the nervous and immune systems. For example, “Molecular and Functional Neuroscience in Immunity” systematically examined the mechanisms by which neural pathways modulate immune responses and inflammation (48). This paper elucidates a bidirectional communication pathway: sensory neurons detect immune-related molecules and pathogens, transmitting signals to the central nervous system (CNS), which subsequently modulates immune activity via efferent autonomic neurons. Notably, the involvement of the vagus nerve in the inflammatory reflex and the concept of an immunological homunculus were highlighted as groundbreaking insights, offering therapeutic potential for inflammatory disorders. In parallel, “Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection” revealed that Streptococcus pyogenes exploits nociceptor neurons by releasing streptolysin S, triggering pain and inducing the release of calcitonin gene-related peptide (CGRP). This neuropeptide suppressed neutrophil recruitment and bactericidal function, identifying neural signaling as a critical target for combating invasive infection (51). Blocking this neuroimmune signaling with Botulinum neurotoxin A or a CGRP antagonist effectively treats infection, highlighting a new therapeutic approach. “Nociceptor sensory neurons suppress neutrophil and γδ T-cell responses in bacterial lung infections and lethal pneumonia” reveals that TRPV1+ nociceptors in the lung suppress protective immunity against Staphylococcus aureus pneumonia (53). Ablating these neurons improves survival, cytokine induction, and bacterial clearance by enhancing neutrophil and γδ T-cell responses. The neuropeptide CGRP, released by nociceptors, plays a crucial role in this immunosuppression. “Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens”, showing that allergens activate TRPV1+ sensory neurons, leading to Substance P release (54). This stimulates CD301b+ dendritic cells through MRGPRA1, promoting their migration to the draining lymph node and initiating the type-2 immune response. Sensory neurons thus act as primary allergen sensors crucial for allergic immune activation.
3.3.4 Analysis of keywords
Keywords are crucial indicators of the main topics and core content of a specific subject (55). In bibliometrics, keyword co-occurrence analysis is a common method for identifying research hotspots. This analysis assesses the relationships between keywords on the basis of their simultaneous appearance in documents (56). In our study, we extracted author keywords from 5,280 publications and analyzed them via VOSviewer. After the synonymous terms were consolidated, we identified 99 keywords that appeared at least 30 times. Table 3 lists the 20 most frequent keywords. “Inflammation” is the most frequently occurring keyword, and the keywords with more than 200 co-occurrences include “neuroinflammation”, “microglia”, “cytokines”, “cholinergic anti-inflammatory pathway” and “neuroimmune”. The VOSviewer co-occurrence map (Figure 7A) groups all keywords into four clusters depicted in different colors. Furthermore, VOSviewer color-coded all keywords on the basis of their average year of appearance (Figure 7B). Earlier keywords are shown in blue, whereas more recent keywords appear in red.

Figure 7. Visualized mapping of keyword co-occurrence analysis in clusters (A) or in years arranged (B, from 2004–2024) via VOSviewer.
3.4 Clinical practices of neuroimmune modulation
To further explore the current situation of targeting neuroimmune modulation for treating diseases clinically, we performed a literature search with the same topic and timeline in the PubMed database. A total of 73 published clinical trials were identified in the field of neuroimmune modulation (Figure 8A). On the basis of these trials, we categorized the diseases under investigation into four groups: nervous system disorders (such as multiple sclerosis (57, 58) and cognitive impairment (59, 60)), mental disorders (such as depression (61)), immune-inflammatory diseases (such as atopic dermatitis (62)), and other conditions (including breast cancer (63) and postoperative gastrointestinal dysfunction (64)) (Figure 8B). Among these trials, the inventions include neural modulation (such as transcutaneous vagus nerve stimulation (tVNS) to reduce cytokines (65)), drugs (such as vitamin D to evaluate IL-10 (57)) and comprehensive interventions (such as exercise to prevent migraine (66)) (Table 4). Most of the inventions were based on the mechanism of the CAP and the dynamic balance of cytokines, which was consistent with the above most frequent keywords. Although we did not apply a scale to comment on the trials we recruited, researchers should note that all these published trials had limitations such as relatively small sample sizes. Several trials had a short duration, which was finished by collecting the serum sample in one day.

Figure 8. The annual number (A) and categories (B) of published clinical trials on neuroimmune modulation.
4 Discussion
4.1 Primary findings
This study conducted visualized knowledge mapping on the basis of 5,280 publications and 73 clinical practices related to neuroimmune modulation over the past two decades. Since 2005, the number of publications related to neuroimmune modulation has steadily increased. The United States leads in both publication quantity and quality. Among institutions, Harvard University has the highest output. The most influential authors in this field include Maes, Michael, Kevin J. Tracey, and Terrence Deak. Acute kidney injury, neuronal regulation and neuropathic pain are current hotspots in the neuroimmune modulation field. “Inflammation”, together with “microglia”, “cytokines” and “cholinergic anti-inflammatory pathway”, were the most frequent author keywords. The findings from clinical practice highlight the potential of targeting neuroimmune modulation in the future.
4.2 Results of the study in context
4.2.1 The current academic landscape of neuroimmune modulation research
In the academic landscape of neuroimmune research, the United States dominates both the quantity and quality of publications. Additionally, institutions in the United States, such as Harvard University, reach the highest rank. The U.S. leading in the current research field reflects its powerful technological strength due to its economic volume. Additionally, such research in the neuroimmune field has provided novel topics and explored new research directions for further research. Notably, China has emerged as a significant contributor, likely owing to its economic growth and strategic scientific policies. However, academic silos remain prevalent. Cite space-derived betweenness centrality (BC) scores reveal low connectivity across countries, institutions, and researchers, reflecting limited collaboration. Addressing these barriers to foster global cooperation remains a critical challenge for the research community.
4.2.2 Acute kidney injury and neuropathic pain are key focuses of neuroimmune research
In AKI, the neuroimmune axis plays a crucial role in pathogenesis and progression, acting as a complex communication network between the nervous system and the immune system. Dysregulation of this crosstalk exacerbates kidney damage through inflammation, cell death, and impaired repair. On the one hand, overaction of the sympathetic nervous system leads to the release of catecholamines from sympathetic nerve endings and the adrenal medulla (67, 68). Then, catecholamines bind primarily to β-adrenergic receptors (β-ARs) on immune cells, which can stimulate the release of proinflammatory cytokines, increase neutrophil recruitment and activation, and modulate T-cell responses toward a more proinflammatory phenotype (67, 68). On the other hand, inflammatory signals can activate efferent vagus nerve fibers, and acetylcholine (ACh) is released from vagus nerve terminals in peripheral organs and potentially near renal nerves. The CAP is a major counterregulator of inflammation. Impairment of the CAP exacerbates AKI. Conversely, vagus nerve stimulation (VNS) or α7nAChR agonists are protective in experimental AKI models (69–71). In conclusion, stimulating the release of cytokines from both the sympathetic and parasympathetic nervous systems modulates immune cell function by releasing neurotransmitters that bind to specific receptors, with effects ranging from protective to deleterious. Balancing these autonomic pathways through pharmacological or nonpharmacological interventions or by targeting specific neurotransmitters or receptors requires further investigation to mitigate renal damage during AKI.
For neuropathic pain, neuroimmune crosstalk involves both the peripheral and central nervous systems. Nerve injury triggers neurotransmitter release, recruiting distinct immune cell populations, such as macrophages, neutrophils and mast cells. At the peripheral level, several cytokines, including TNF-α and IL-6, can directly activate nociceptive neurons (72–74). Moreover, infiltrating T cells can secrete IFN-γ to activate the STAT3 pathway in DRG neurons, leading to sustained hyperalgesia (75). At the central level, glial cells dominate central sensitization in the spinal cord and affect cortical and descending modulatory systems. In the spinal cord, microglia can receive signals such as CCL2 from the periphery, leading to the release of inflammatory mediators (76). Astrocytes can release IL-17 and S100β to persistently increase NMDA receptor activity and cause central sensitization (77, 78). Additionally, infiltrating T cells, such as CD4+ T cells, can secrete IL-17a to activate microglia/astrocytes and amplify inflammation, while Treg cell dysfunction leads to the loss of pain suppression (79, 80). In the brain, microglia release IL-1β to inhibit NE neurons, thus decreasing the degree of inhibition (81). Notably, sex disparities in pain responses underscore the complexity of this neuroimmune interplay (82). Targeting neural or immune components holds promise for novel therapeutic strategies against these recalcitrant conditions.
4.2.3 Four hotspots in neuroimmune studies and future directions
Notably, the cluster study by keyword co-occurrence map presented four colors, which indicated that the hotspots in neuroimmune studies could be categorized into four areas. We summarized these four areas as, first, the interaction between neuroimmune mechanisms and the gut microbiota and microbiome, as well as their effects on diseases such as asthma, atopic dermatitis, autism spectrum disorder, and inflammatory bowel disease. Second, the role and mechanism of the neuroimmune process in behaviors such as anxiety, depression and pain are influenced by factors such as aging and alcohol. Third, the associations between the acetylcholine–vagus nerve–immune–inflammatory axis and diseases as well as autonomic nerve function have been explored. Fourth, the role of neuroimmune-related mechanisms and their biomarkers in neurological diseases such as Alzheimer’s disease, autism, and Parkinson’s disease, as well as autoimmune diseases, should be explored.
The microbiota and microbiome have recently become popular topics in the academic field, and they are referred to as the second brain. However, how they affect the neuroimmune system in diseases remains largely unknown. Hong et al. revealed a beneficial shift in the gut microbiota composition in neuropathic pain treatment with reduced inflammatory cytokines (83). Li et al. reported that the gut microbiota may influence Alzheimer’s disease (AD) pathogenesis by modulating host homeostasis via a self-reinforcing mechanism involving immune signaling (84). Future works on how the microbiota forms a bidirectional regulatory network through the metabolite–immune–neural axis under different conditions might provide new insight for treating diseases.
With respect to behaviors such as anxiety, depression and pain, neuroimmune mechanisms are undoubtedly the research frontiers mentioned above. Recent studies have shown that peripheral immune-to-brain signaling can disrupt neural homeostasis via cytokines such as IL-6 and TNF-α and lead to depression (85). Moreover, inflammation-driven neuroendocrine-immune axis dysregulation, such as HPA axis hyperactivation and monoaminergic system suppression, can drive various abnormal behaviors (86–88). The development of BBB-penetrating anti-inflammatory drugs or the identification of personalized immune profiles for precision treatment might be considered in future studies.
In the context of the cholinergic axis, the CAP itself is a major counterregulator of inflammation. Targeting this axis, such as enhancing CAP, is expected to become a new direction for disease treatment, but organ-specific challenges in clinical translation need to be addressed. For neurological/autoimmune diseases, mechanisms and biomarkers are research frontiers. Peripheral-central immune crosstalk is undoubtedly the core mechanism of such diseases. For example, T cells can cross the BBB and attack myelin via MMP-9, together with B-cell-derived IL-6, leading to astrocyte reactivity and thus resulting in demyelination in multiple sclerosis (89). Several biomarkers, such as neurofilament light (NfL), can reflect the severity of axonal damage or be used to monitor treatment response in MS patients (90, 91). Multiomics integration, such as combining CSF proteomics and PET-MRI, could help to define a neuroimmune activity index for treating patients.
Taken together, these four areas encompass the majority of nervous system diseases, with neuroimmune mechanisms as the central theme. Unraveling the underlying molecular and cellular pathways governing these interactions could deepen our comprehension of neuroimmune dynamics, thereby facilitating the development of targeted therapeutic strategies for these conditions.
4.2.4 Clinical trials on neuroimmune modulation remain a challenge
We also performed clinical trials on neuroimmune modulation and reported that most current studies focus on conditions such as multiple sclerosis, depression, and chronic fatigue syndrome. Treatment approaches encompass neural regulation, pharmacological interventions and comprehensive therapy. These investigations collectively highlight the core mechanisms of neural–immune interactions, particularly the CAP and cytokine balance. However, several challenges, including small sample sizes in published trials, outcome measurement challenges and gaps between animal and human disconnection, remain. Contrary results were reported between trials and basic studies, such as transcutaneous vagus nerve stimulation (tVNS), which led to an increase in inflammatory cytokines in both young and elderly individuals (92). Although the authors account for the above phenomenon resulting from psychological stress, the work left the question of whether it is more complicated in the clinical situation. Furthermore, Tina et al. reported that tVNS has no anti-inflammatory effect on diabetes, which is known to be associated with chronic inflammation (93). All these trials raise the challenge that targeting neuroimmune modulation in different diseases requires further verification in the future.
Future trials may explore several avenues, including expanding the disease spectrum beyond typical neuroimmune disorders to include conditions such as Alzheimer’s disease and irritable bowel syndrome; innovating treatment methods such as precise neural regulation technologies, with noninvasive approaches such as transcranial magnetic stimulation warranting investigation; leveraging biomarkers for treatment efficacy assessment; addressing clinical translation challenges, including individual variability in treatment response and the need for personalized strategies incorporating genetic typing; overcoming limitations of predominantly short-term trials through longitudinal safety and efficacy data; and advancing interdisciplinary integration, as evidenced by combined psychological–neural interventions in cancer patients who simultaneously improve psychological and immune parameters, indicating a promising research direction.
4.3 Limitations of the current bibliometric study
The current study has several limitations. First, although the current study recruited only references from the WOSCC database and PubMed, the main bibliometric analysis was conducted on the basis of WOSCC data style. The main reason was that CiteSpace was built on the basis of WOS style. Second, references in the English language were included, which might undervalue the contributions from non-English publications. Third, we did not perform a sensitivity or specificity check for the search results. Fourth, the quality of the references was ranked by the software on the basis of the indexes and the time at which we conducted the analysis. For example, the number of citations of a certain reference could be updated rapidly, which might influence burst detection. Fifth, we recruited only published clinical trials from PubMed, which raises the risk of missing key studies from other clinical trial registry sources, such as ClinicalTrials.gov or the European Clinical Trials Registry. Sixth, we did not apply a scale to evaluate the quality of the clinical research, which might cause bias. Because our original purpose was to provide a visualized knowledge map of neuroimmune studies, future works evaluating neuroimmune trials could be carried out in the future.
5 Conclusions
This analysis offered knowledge mapping in the current research field of neuroimmune modulation. Mechanistic studies combined with neuronal regulation are currently popular in the field. Targeting neuroimmune crosstalk might provide new opportunities to treat nervous system diseases. The current analysis has provided valuable information on future directions within the field.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributions
XH: Formal analysis, Writing – original draft, Resources, Writing – review & editing, Funding acquisition, Visualization, Methodology, Data curation, Conceptualization, Software, Validation, Investigation. YZ: Funding acquisition, Writing – review & editing, Writing – original draft, Project administration, Validation, Visualization, Methodology, Supervision. XG: Visualization, Formal analysis, Project administration, Writing – original draft, Funding acquisition, Data curation, Validation, Methodology, Conceptualization, Investigation, Software, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Shanghai Natural Science Foundation (25ZR1401225), the Shanghai Engineering Research Center of Perioperative Organ Support and Function Preservation (20DZ2254200), the Renji Hospital Project (RJTJ25-QN-064, RJTJ23-RC-013, RJTJ25-MS-014, RJKY24-004), the National Natural Science Foundation of China (82171885), the Eastern Talent Plan Leading Project (LJ2023127), the Shanghai Science and Technology Committee Project, Explorer Project Funding (24TS1414800), and the Leading Talent Program of the Shanghai Municipal Health Commission (2022LJ023).
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stavrakis S and Po SS. Neuroimmunomodulation: A new frontier of treating cardiovascular diseases. Trends Cardiovasc Med. (2016) 26:12–3. doi: 10.1016/j.tcm.2015.04.007
2. Brooks WH, Cross RJ, Roszman TL, and Markesbery WR. Neuroimmunomodulation: neural anatomical basis for impairment and facilitation. Ann Neurol. (1982) 12:56–61. doi: 10.1002/ana.410120111
3. Gidron Y, Kupper N, Kwaijtaal M, Winter J, and Denollet J. Vagus-brain communication in atherosclerosis-related inflammation: a neuroimmunomodulation perspective of CAD. Atherosclerosis. (2007) 195:e1–9. doi: 10.1016/j.atherosclerosis.2006.10.009
4. Fehér E, Pongor É., Altdorfer K, Kóbori L, and Lengyel G. Neuroimmunomodulation in human autoimmune liver disease. Cell Tissue Res. (2013) 354:543–50. doi: 10.1007/s00441-013-1683-x
5. ThyagaRajan S, Hima L, Pratap UP, Priyanka HP, and Vasantharekha R. Estrogen-induced neuroimmunomodulation as facilitator of and barrier to reproductive aging in brain and lymphoid organs. J Chem Neuroanat. (2019) 95:6–12. doi: 10.1016/j.jchemneu.2018.02.008
6. Boyack KW and Klavans R. Co-citation analysis, bibliographic coupling, and direct citation: Which citation approach represents the research front most accurately? J Am Soc Inf Sci Technol. (2010) 61:2389–404. doi: 10.1002/asi.21419
7. van Eck NJ and Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
8. Chen C, Dubin R, and Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
9. Liu F, Chen N, Wang R, Zhang L, and Li Y. Visual analysis of allergic rhinitis in children based on web of science and CiteSpace software. Front Pediatr. (2022) 10:911293. doi: 10.3389/fped.2022.911293
10. Wang YX, Arora R, Choi Y, Chung HW, Egorov VI, Frahm J, et al. Implications of Web of Science journal impact factor for scientific output evaluation in 16 institutions and investigators’ opinion. Quant Imaging Med Surg. (2014) 4:453–61. doi: 10.3978/j.issn.2223-4292.2014.11.16
11. Yi F, Yang P, and Sheng H. Tracing the scientific outputs in the field of Ebola research based on publications in the Web of Science. BMC Res Notes. (2016) 9:221. doi: 10.1186/s13104-016-2026-2
12. Chen C and CiteSpace II. Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
13. Divisi D, Di Leonardo G, Zaccagna G, and Crisci R. Basic statistics with Microsoft Excel: a review. J Thorac Dis. (2017) 9:1734–40. doi: 10.21037/jtd.2017.05.81
14. Chen C and Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One. (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
15. Yeung AWK, Tzvetkov NT, Balacheva AA, Georgieva MG, Gan RY, Jozwik A, et al. Lignans: quantitative analysis of the research literature. Front Pharmacol. (2020) 11:37. doi: 10.3389/fphar.2020.00037
16. Zhang XL, Zheng Y, Xia ML, Wu YN, Liu XJ, Xie SK, et al. Knowledge domain and emerging trends in vinegar research: A bibliometric review of the literature from woSCC. Foods. (2020) 9:166. doi: 10.3390/foods9020166
17. Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, and Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. (2023) 28:564–78. doi: 10.1038/s41380-022-01836-9
18. Anderson G and Maes M. Melatonin: an overlooked factor in schizophrenia and in the inhibition of anti-psychotic side effects. Metab Brain Dis. (2012) 27:113–9. doi: 10.1007/s11011-012-9307-9
19. Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, et al. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in alzheimer’s disease. Curr Pharm Des. (2016) 22:6152–66. doi: 10.2174/1381612822666160907093807
20. Morris G, Berk M, Galecki P, Walder K, and Maes M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol. (2016) 53:1195–219. doi: 10.1007/s12035-015-9090-9
21. Morris G and Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab Brain Dis. (2013) 28:523–40. doi: 10.1007/s11011-012-9324-8
22. Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. (2007) 35:2762–8./ doi: 10.1097/01.CCM.0000288102.15975.BA
23. Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. (2009) 23:41–5. doi: 10.1016/j.bbi.2008.06.011
25. van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. (2006) 130:1822–30. doi: 10.1053/j.gastro.2006.02.022
26. Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U.S.A. (2008) 105:11008–13. doi: 10.1073/pnas.0803237105
27. Chavan SS, Pavlov VA, and Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. (2017) 46:927–42. doi: 10.1016/j.immuni.2017.06.008
28. Hueston CM and Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav. (2014) 124:77–91. doi: 10.1016/j.physbeh.2013.10.035
29. Doremus-Fitzwater TL, Gano A, Paniccia JE, and Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav. (2015) 148:131–44. doi: 10.1016/j.physbeh.2015.02.032
30. Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, and Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. (2015) 18:367–80. doi: 10.3109/10253890.2015.1053451
31. Gano A, Doremus-Fitzwater TL, and Deak T. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res. (2016) 1646:62–72. doi: 10.1016/j.brainres.2016.05.027
32. Fuster V. Impact factor: A curious and capricious metric. J Am Coll Cardiol. (2017) 70:1530–1. doi: 10.1016/j.jacc.2017.08.002
33. Huang SC, Chiu TM, Lee CY, Chang HC, Wu WJ, and Gau SY. Researching trends in pemphigoid diseases: A bibliometric study of the top 100 most cited publications. Front Med (Lausanne). (2022) 9:1088083. doi: 10.3389/fmed.2022.1088083
34. Silva YP, Bernardi A, and Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). (2020) 11:25. doi: 10.3389/fendo.2020.00025
35. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, and Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. (2005) 57:67–81. doi: 10.1002/ana.20315
36. Costigan M, Scholz J, and Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. (2009) 32:1–32. doi: 10.1146/annurev.neuro.051508.135531
37. Kelly JR, Borre Y, O'Brien C, Patterson E, Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
38. Constantinescu CS, Farooqi N, O’Brien K, and Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. (2011) 164:1079–106. doi: 10.1111/j.1476-5381.2011.01302.x
39. Rhee SH, Pothoulakis C, and Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
40. Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The amyloid-beta pathway in alzheimer’s disease. Mol Psychiatry. (2021) 26:5481–503. doi: 10.1038/s41380-021-01249-0
41. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. (2018) 1693:128–33. doi: 10.1016/j.brainres.2018.03.015
42. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. (2005) 6:844–51. doi: 10.1038/ni1229
43. Allan SM, Tyrrell PJ, and Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. (2005) 5:629–40. doi: 10.1038/nri1664
44. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U.S.A. (2016) 113:8284–9. doi: 10.1073/pnas.1605635113
45. Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. (2011) 334:98–101. doi: 10.1126/science.1209985
46. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. (2007) 117:289–96. doi: 10.1172/JCI30555
47. Pavlov VA and Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. (2017) 20:156–66. doi: 10.1038/nn.4477
48. Pavlov VA, Chavan SS, and Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol. (2018) 36:783–812. doi: 10.1146/annurev-immunol-042617-053158
49. Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. (2016) 28:948–53. doi: 10.1111/nmo.12792
50. Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, and Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. (2016) 164:378–91. doi: 10.1016/j.cell.2015.12.023
51. Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell. (2018) 173:1083–1097 e22. doi: 10.1016/j.cell.2018.04.006
52. Pinho-Ribeiro FA, Verri WA Jr., and Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. (2017) 38:5–19. doi: 10.1016/j.it.2016.10.001
53. Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, et al. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat Med. (2018) 24:417–26. doi: 10.1038/nm.4501
54. Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity. (2020) 53:1063–1077.e7. doi: 10.1016/j.immuni.2020.10.001
55. Qin Y, Chen S, Zhang Y, Liu W, Lin Y, Chi X, et al. A bibliometric analysis of endoscopic sedation research: 2001-2020. Front Med (Lausanne). (2021) 8:775495. doi: 10.3389/fmed.2021.775495
56. Wu H, Li Y, Tong L, Wang Y, and Sun Z. Worldwide research tendency and hotspots on hip fracture: a 20-year bibliometric analysis. Arch Osteoporos. (2021) 16:73. doi: 10.1007/s11657-021-00929-2
57. Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, and Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. (2015) 22:400–4. doi: 10.1159/000439278
58. Khalili M, Azimi A, Izadi V, Eghtesadi S, Mirshafiey A, Sahraian MA, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. (2014) 21:291–6. doi: 10.1159/000356145
59. Lewis JE, McDaniel HR, Agronin ME, Loewenstein DA, Riveros J, Mestre R, et al. The effect of an aloe polymannose multinutrient complex on cognitive and immune functioning in Alzheimer’s disease. J Alzheimers Dis. (2013) 33:393–406. doi: 10.3233/JAD-2012-121381
60. Yao S, Ding K, Liu S, Zhang Q, Li W, Tang L, et al. The managing cancer and living meaningfully (CALM) intervention alleviates chemotherapy-related cognitive impairment in patients with breast cancer by modulating pan-immune-inflammation values. Integr Cancer Ther. (2022) 21:15347354221140498. doi: 10.1177/15347354221140498
61. Zhang Y and Zhao X. Effects of the health belief model-based intervention on anxiety, depression, and quality of life in chronic obstructive pulmonary disease. Neuroimmunomodulation. (2021) 28:129–36. doi: 10.1159/000512993
62. Peters EM, Michenko A, Kupfer J, Kummer W, Wiegand S, Niemeier V, et al. Mental stress in atopic dermatitis–neuronal plasticity and the cholinergic system are affected in atopic dermatitis and in response to acute experimental mental stress in a randomized controlled pilot study. PloS One. (2014) 9:e113552. doi: 10.1371/journal.pone.0113552
63. Nunes DF, Rodriguez AL, da Silva Hoffmann F, Luz C, Braga Filho AP, Muller MC, et al. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J Psychosom Res. (2007) 63:647–55. doi: 10.1016/j.jpsychores.2007.07.004
64. Boeckxstaens G, Ayad S, Dukes G, Essandoh M, Gryder R, Kamble P, et al. A randomized phase 2 study of the 5-HT(4) receptor agonist felcisetrag for postoperative gastrointestinal dysfunction after bowel surgery. Am J Surg. (2024) 234:162–71. doi: 10.1016/j.amjsurg.2024.04.030
65. Aranow C, Atish-Fregoso Y, Lesser M, Mackay M, Anderson E, Chavan S, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. (2021) 80:203–8. doi: 10.1136/annrheumdis-2020-217872
66. Oliveira AB, Bachi ALL, Ribeiro RT, Mello MT, Vaisberg M, and Peres MFP. Exercise-induced change in plasma IL-12p70 is linked to migraine prevention and anxiolytic effects in treatment-naive women: A randomized controlled trial. Neuroimmunomodulation. (2017) 24:293–9. doi: 10.1159/000487141
67. Goldstein DS. Peripheral catecholamine systems: an evolutionary perspective. Am J Physiol Regul Integr Comp Physiol. (2025). doi: 10.1152/ajpregu.00169.2025
68. Zoccali C, Mallamaci F, Kanbay M, Tuttle KR, Kotanko P, De Caterina R, et al. The autonomic nervous system and inflammation in chronic kidney disease. Nephrol Dial Transplant. (2025) 40:1470–82. doi: 10.1093/ndt/gfaf020
69. Hilderman M and Bruchfeld A. The cholinergic anti-inflammatory pathway in chronic kidney disease-review and vagus nerve stimulation clinical pilot study. Nephrol Dial Transplant. (2020) 35:1840–52. doi: 10.1093/ndt/gfaa200
70. Wu T, Zhu W, Duan R, Sun J, Bao S, Chen K, et al. Magnetic vagus nerve stimulation ameliorates contrast-induced acute kidney injury by circulating plasma exosomal miR-365-3p. J Nanobiotechnol. (2024) 22:666. doi: 10.1186/s12951-024-02928-0
71. Goggins E, Mitani S, and Tanaka S. Clinical perspectives on vagus nerve stimulation: present and future. Clin Sci (Lond). (2022) 136:695–709. doi: 10.1042/CS20210507
72. Kalpachidou T, Kummer K, Handle V, Zimmermann D, Peteinareli M, Quarta S, et al. Context dependent role of miR-486 promoting neuroregeneration of primary sensory neurons downstream of interleukin-6 signal transducer. Mol Ther Nucleic Acids. (2025) 36:102670. doi: 10.1016/j.omtn.2025.102670
73. Sun S, Sun Y, Shen J, Mao Z, Zhang T, Gao Y, et al. CXCL13/CXCR5: a new target for pain treatment. Int J Surg. (2025) 111:6318–29. doi: 10.1097/JS9.0000000000002764
74. Le ACT, Fiuza-Fernandes J, Silva JM, Sampaio MT, Texeira-Castro A, Duarte-Silva S, et al. Pretreatment with dimethyl fumarate prevents chronic pain and its comorbidities via Nrf2 pathway in a rat model of neuropathic pain. Brain Behav Immun. (2025) 128:725–36. doi: 10.1016/j.bbi.2025.05.003
75. Ino Y, Maruyama M, Shimizu M, Morita R, Sakamoto A, Suzuki H, et al. TSLP in DRG neurons causes the development of neuropathic pain through T cells. J Neuroinflamm. (2023) 20:200. doi: 10.1186/s12974-023-02882-y
76. Chen Y, Liu S, Wu L, Liu Y, Du J, Luo Z, et al. Epigenetic regulation of chemokine (CC-motif) ligand 2 in inflammatory diseases. Cell Prolif. (2023) 56:e13428. doi: 10.1111/cpr.13428
77. Liu H, Lv X, Zhao X, Yi L, Lv N, Xu W, et al. Spinal astrocyte-derived interleukin-17A promotes pain hypersensitivity in bone cancer mice. Acta Pharm Sin B. (2024) 14:5249–66. doi: 10.1016/j.apsb.2024.09.016
78. Sun JL, Dai WJ, Shen XY, Lu N, and Zhang YQ. Interleukin-17 is involved in neuropathic pain and spinal synapse plasticity on mice. J Neuroimmunol. (2023) 377:578068. doi: 10.1016/j.jneuroim.2023.578068
79. Zhao Y, Shen L, and Huang Y. Low-dose IL-2 attenuates neuropathic pain via treg expansion in rats. J Pain Res. (2025) 18:5011–22. doi: 10.2147/JPR.S550012
80. Li W and Liu R. The causal relationship between immune cells and neuropathic pain: A two-sample mendelian randomization study based on genome-wide association analysis. J Pain Res. (2025) 18:1515–23. doi: 10.2147/JPR.S511182
81. Ma X, Chen W, Yang NN, Wang L, Hao XW, Tan CX, et al. Potential mechanisms of acupuncture for neuropathic pain based on somatosensory system. Front Neurosci. (2022) 16:940343. doi: 10.3389/fnins.2022.940343
82. Zhou WBS, Shi XQ, Zhang AP, Millecamps M, Mogil JS, and Zhang J. The impact of nerve injury on the immune system across the lifespan is sexually dimorphic. Neurobiol Pain. (2025) 18:100195. doi: 10.1016/j.ynpai.2025.100195
83. Hong SW, Piao L, Cho EH, Seo EH, and Kim SH. Effect of microglial activity on gut microbiota in rats with neuropathic pain. Int J Mol Sci. (2025) 26:5181. doi: 10.3390/ijms26115181
84. Li J, Yuan Z, Li J, Liu Z, Wang Y, Cui M, et al. Immune, blood-brain barrier, and metabolic biomarkers mediate gut-brain axis crosstalk in alzheimer’s disease. biomark Res. (2025) 13:137. doi: 10.1186/s40364-025-00851-6
85. Pinzi M, Fagiolini A, Koukouna D, Gualtieri G, Rescalli MB, Pierini C, et al. Inflammatory and immune biomarkers in mood disorders: from mechanistic pathways to clinical translation. Cells. (2025) 14:1558. doi: 10.3390/cells14191558
86. Rezaei S, Prevot TD, Vieira E, and Sibille E. LPS-induced inflammation reduces GABAergic interneuron markers and brain-derived neurotrophic factor in mouse prefrontal cortex and hippocampus. Brain Behav Immun Health. (2024) 38:100761. doi: 10.1016/j.bbih.2024.100761
87. Dai WB, Zhang X, Jiang XL, Zhang YZ, Chen LK, Tian WT, et al. The kynurenine pathway regulated by intestinal innate lymphoid cells mediates postoperative cognitive dysfunction. Mucosal Immunol. (2025) 18:53–65. doi: 10.1016/j.mucimm.2024.09.002
88. Md S, Hong SM, Lee JH, Park H, Chang KA, Kim HB, et al. Depression like-behavior and memory loss induced by methylglyoxal is associated with tryptophan depletion and oxidative stress: a new in vivo model of neurodegeneration. Biol Res. (2024) 57:87. doi: 10.1186/s40659-024-00572-4
89. Ibrahim I, Wassefy ME, Metwally SS, and Elmenshawi I. Matrix metalloproteases 9 rs3918242 gene polymorphism and serum vit D in MS Egyptian patients. Mult Scler Relat Disord. (2019) 32:103–6. doi: 10.1016/j.msard.2019.04.029
90. Civita E, Nicolella V, Fiorenza M, Cosimato V, Castaldo G, Morra VB, et al. Advancing clinical use of neurofilament light chain: translational insights from research to routine practice. biomark Insights. (2025) 20:11772719251364018. doi: 10.1177/11772719251364018
91. El Mahdaoui S, Kosa P, Komori M, Veiga Gonzalez JL, Chow HH, Ratzer R, et al. Profiling the cerebrospinal fluid proteome in progressive multiple sclerosis: treatment effects and associations with igM oligoclonal bands. J Neuroimmune Pharmacol. (2025) 20:98. doi: 10.1007/s11481-025-10263-w
92. Veiz E, Kieslich SK, Czesnik D, Herrmann-Lingen C, Meyer T, and Staab J. Increased concentrations of circulating interleukins following non-invasive vagus nerve stimulation: results from a randomized, sham-controlled, crossover study in healthy subjects. Neuroimmunomodulation. (2022) 29:450–9. doi: 10.1159/000524646
Keywords: neuroimmune modulation, crosstalk, web of science, pubmed, visualized knowledge mapping, citespace, VOSviewer, co-citation analysis
Citation: Han X, Zhou Y and Gu X (2025) Visualized knowledge mapping on the current research trends and emerging areas of neuroimmune modulation in the past two decades. Front. Immunol. 16:1671578. doi: 10.3389/fimmu.2025.1671578
Received: 23 July 2025; Accepted: 20 November 2025; Revised: 18 November 2025;
Published: 05 December 2025.
Edited by:
Bao-Chun Jiang, Zhejiang University, ChinaReviewed by:
Pankaj Gaur, Georgetown University, United StatesKumar Vaibhav, Augusta University, United States
Copyright © 2025 Han, Zhou and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Han, aGFueHVfeWdyaXR0ZUAxNjMuY29t; Yan Zhou, Y2xhcmUxNDc1QGhvdG1haWwuY29t; Xiyao Gu, Z3h5a2V2aW5AMTI2LmNvbQ==
 Xu Han
Xu Han Yan Zhou
Yan Zhou Xiyao Gu
Xiyao Gu