- 1Biosciences Department, Centre for Geographic Medicine Research (Coast), Kenya Medical Research Institute-Welcome Trust Research Programme, Kilifi, Kenya
- 2College of Health Sciences, Makerere University, Kampala, Uganda
- 3Department of Modernising Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
- 4Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Controlled human infection studies offer a unique opportunity to study the efficacy of novel interventions, mechanisms of infection and disease, as well as determine correlates of protection that may underpin the development of novel interventions. Controlled human malaria infection (CHMI) studies supported the clinical development of the first malaria vaccines (i.e. RTSS/AS01 and R21/Matrix-M). The CHMI model accurately predicted efficacy of these vaccines and accelerated their clinical development. In addition to vaccine development, over the last decade CHMI studies have supported the advancement of drugs, monoclonal antibodies (mAbs) and been instrumental in characterising immunity to malaria by unravelling immunological and innate mechanisms that may mediate protection. Here, we briefly review the history and rationale of the available falciparum malaria CHMI models. We highlight key applications and lessons learned from CHMI studies conducted in naïve and endemic populations with respect to immunological advances, discoveries in therapeutic targets such as mAbs, and transferring of the models from high income to low- and middle-income settings.
Introduction
Malaria remains a global health concern as half the world’s population remains at risk of infection (1, 2). The World Health Organization (WHO) estimates ~263 million malaria cases with ~597–000 deaths occurred in 2023 (3) rising by ~11 million cases compared to 2022. The increasing reports of malaria resurgence in Africa (4), despite implementation of the available control tools, makes the historic approval of the first malaria vaccines (RTSS/AS01 in 2021 and R21/Matrix-M in 2023) highly welcomed (5, 6). However, the level of vaccine protection offered by these vaccines is incomplete (7). Therefore, continued investment in research and development is important including other pre-erythrocytic vaccines (e.g. whole sporozoite vaccines), efforts to develop and implement either multistage vaccines (e.g. including erythrocytic or transmission blocking components) or therapeutic interventions such as monoclonal antibodies (mAbs).
Malaria elimination will likely require multiple interventions, including currently established approaches (vector control strategies – such as indoor residual spraying with effective insecticides, and the use of long-lasting insecticide treated bed nets, plus the accurate diagnosis and treatment with appropriate antimalarial drugs (8, 9)) and additional tools such as the vaccines R21/Matrix -M and RTS,S/AS01, that are currently being deployed in a number of African countries, specifically in areas of high malaria transmission (7). Emerging interventions such as fast acting mAbs and long-acting drugs may also contribute to future malaria control (10). Controlled human infection studies provide considerable utility for the clinical development and licensing of new interventions (11, 12). Challenge studies may be used to derisk costly phase III trials (13). For example, the clinical development and licensure of Vaxchora (for cholera) (14) and Vi-tetanus toxoid conjugate vaccine (for Salmonella typhi) (15) benefited from controlled human infection studies. In addition, challenge studies provide a platform to study disease pathogenesis, aetiology, natural history, acquired immunity, and immune correlates/surrogates of protection that may further guide intervention design (11, 13).
Human infection studies involve the experimental exposure of an individual (mainly healthy adults) to a disease- and/or infection-causing microbe (parasite, virus or bacteria) in a highly controlled setting for scientific merit (13). Since their practice in the Middle East during the early modern era (and likely earlier) and further uptake by Edward Jenner (while studying smallpox) in the early 18th century, important scientific lessons have been gained across the >30 disease models developed to date (16). For malaria, the controlled human malaria infection studies (CHMI) have played key roles in vaccine and drug development such as the advance of the now approved RTS, S/AS01 and R21/Matrix M malaria vaccines (17, 18) and drugs such as atovaquone–proguanil (19) and Artefenomel (20). CHMI studies have also been a source of discovery and development for mAbs (21, 22), genetically attenuated parasites (GAPs) (23–25) and chemoprophylaxis vaccination with sporozoites (CVac) using chloroquine (late arresting parasites for improved liver stage T cell immunity) (26–28).
Despite the burden of malaria being greatest in Africa, the majority of CHMI studies have been conducted in malaria naïve volunteers in high-income countries (29). The reasons for this include: (a) lack of availability of challenge agents suitable for delivery in endemic settings; (b) hesitance to conduct research on apparently “vulnerable” populations in LMICs; (c) lower research infrastructure and capacity; (d) funding availability; and (e) ethical and regulatory challenges. Despite these, the first modern CHMI (well-designed and ethical) conducted in Africa occurred in 2012 in Tanzania followed by a study in Kenya the following year using the then newly developed, aseptic, purified, cryopreserved Plasmodium falciparum sporozoite product, Sanaria® PfSPZ Challenge (NF54) (30, 31). Since then, multiple studies have occurred, and several lessons and insights have been gained thus changing the global landscape and capacity for the conduct of CHMI studies (Figure 1).

Figure 1. Global status of CHMI studies. Map of countries where CHMI studies have been conducted in both the global north and global south. Red represents where more than five CHMI study protocols have been undertaken, and blue represents countries where five or less CHMI protocols have been undertaken. This represents studies of all malaria parasite strains including falciparum (Australia, Belgium, Equatorial Guinea, Gabon, Gambia, Germany, Kenya, Mali, Spain, Tanzania, The Netherlands, UK, USA); malariae (Australia) and vivax (Australia, Colombia, Thailand, and USA).
Here, we briefly review the history and rationale of the different CHMI models. We then summarise the immunological and ethical lessons learned from CHMI studies conducted among semi-immune individuals in Africa.
Design of controlled human malaria infection models
The discovery of mosquitoes as the vector of the Plasmodium parasite by Grassi, Bignami and Ross may be considered the beginning of CHMI studies (32–34). They fed mosquitoes on hospitalized malaria patients, then after allowing the mosquitoes sufficient time to become infectious, they used these mosquitoes to infect healthy volunteers, thus confirming mosquitoes as the malaria transmission vector (35). Deliberate human infections with malaria then grew in prominence with the development of malariotherapy (the use of malaria-induced fever for the treatment of neurosyphilis) which earned Julius Wagner-Jauregg the Nobel prize in 1927 (36–38). Malariotherapy strengthened our understanding and later acceptance of controlled human malaria infection until its use waned after the discovery of antibiotics in the 1940s (36, 37).
The first modern-day controlled human malaria infection (CHMI) experiment in healthy volunteers was conducted by researchers of the Walter Reed Army Institute and the Naval Medical Research Institute in the USA in 1986. Since then, >80 CHMI studies have been conducted. Modern CHMI involves the intentional infection of healthy volunteers (adults) with malaria parasites prepared under highly regulated Good Manufacturing Practice (GMP) guidelines. In the modern era, volunteers only participate after appropriate screening and full informed consent. Individuals are followed daily to a pre-determined density of blood-stage parasites, or a clinical symptomology define endpoint where curative doses of appropriate anti-malarial drugs are provided (39, 40). The monitoring of blood parasitemia was initially carried out by thick blood smear microscopy, however in recent times the more sensitive 18s ribosomal quantitative polymerase chain reaction (qPCR) assays are preferred (41–43). Among CHMI studies conducted to date no severe malaria symptoms have been reported, although acute myocarditis with full recovery probably linked to PFSPZ infection by mosquito bite under chloroquine chemoprophylaxis has been described in two volunteers (44, 45). Importantly, no deaths or lasting disabilities have ever occurred as a result of CHMI.
Controlled human malaria infection reagents, models and applications
In summary, the malaria life cycle begins with anopheline mosquitoes injecting sporozoites into the skin or directly into blood vessels during blood feeding. Sporozoites then invade liver cells and develop into merozoites that are released into blood. Merozoites invade blood cells to form trophozoites, and a continuous cycle of asexual replication leads to successive generations of merozoites that re-invade red blood cells, potentially leading to exponential parasite growth. It is at this point that disease symptoms begin to present. A subset of merozoites differentiate into sexual forms (gametocytes) that infect mosquitoes for onward transmission (Figure 2).
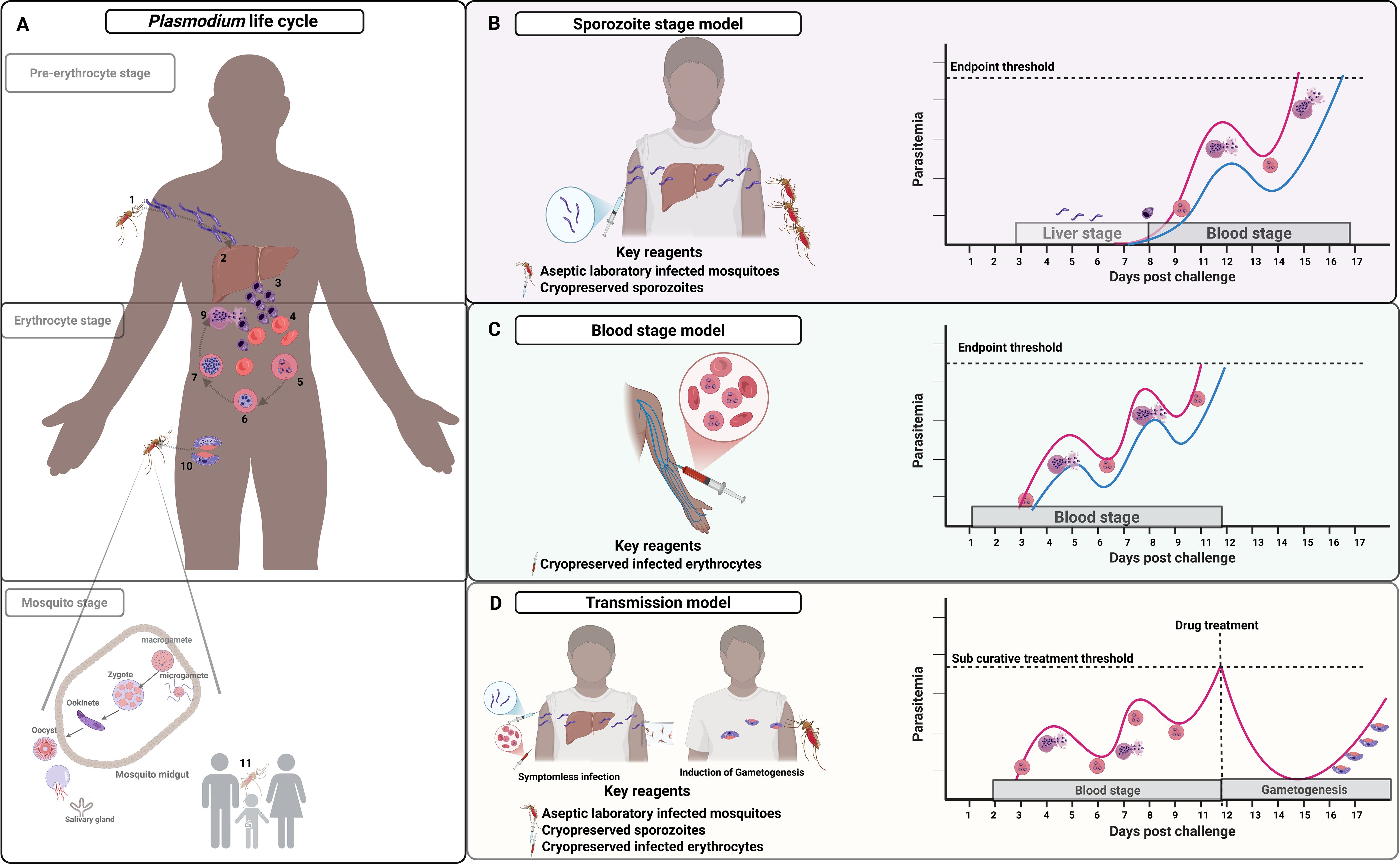
Figure 2. Plasmodium falciparum challenge models in relation to life cycle stages. CHMI models are currently set up to cover and replicate the pre-erythrocytic, erythrocytic, and mosquito stages life cycle stages. (A) shows the natural life cycle involving: mosquito injection of sporozoites (1); liver hepatocyte development into liver stage schizonts which release merozoites into blood circulation (2); merozoites invade red blood cells initiating the erythrocytic stage (3-4); continuous ring stage to trophozoite differentiation and schizont stage which ends with red cell rupture releasing newly formed erythrocytic merozoites (5-9); leading to sexual gametocytes and uptake by mosquitoes (10); and male and female gametocyte fertilization and onward transmission of sporozoites (11). (B) shows the sporozoite model with injection of sporozoites (either aseptic laboratory infected mosquitoes or cryopreserved sporozoites) then parasite monitoring from day 6 post-infection (red line for naïve individuals and blue line semi-immune). (C) shows a blood-stage model with injection of infected erythrocytes and parasite monitoring typically from day 2 post-infection; and (D) shows the transmission model involving sub-curative drug treatment following infection (either sporozoite or blood-stage) at pre-defined parasitaemia threshold to promote gametocyte development with transmission experiments either ex vivo or direct skin mosquito feeding. In all the models, the endpoint is pre-specified: symptoms and/or set parasitaemia threshold (e.g. 500 parasites/μl).
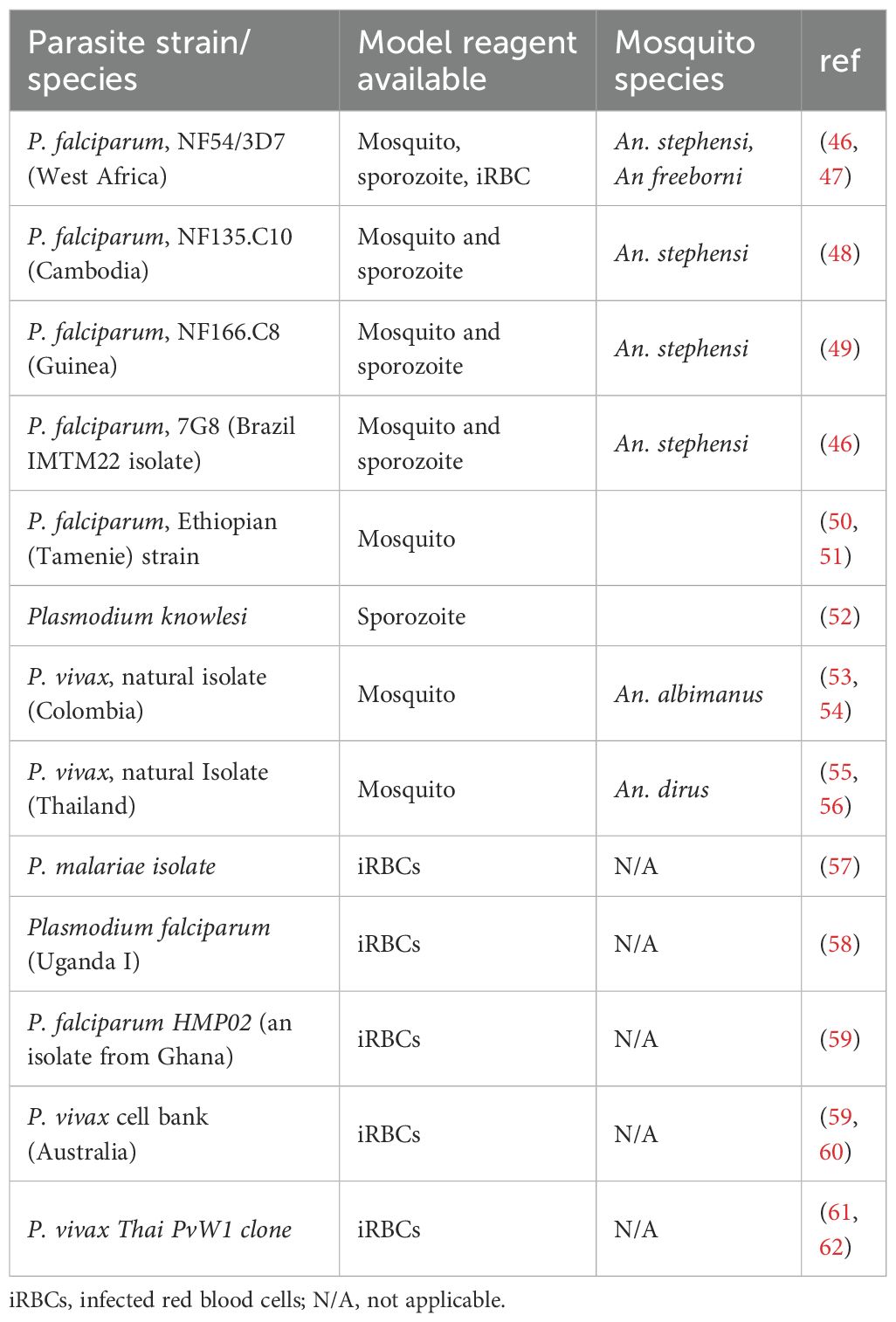
To achieve safe, ethical and reproducible infection of volunteers, initially high quality and then primary GMP level reagents were developed. These included non-aseptic and aseptic laboratory-reared Plasmodium infected mosquitoes; and aseptic, purified, cryopreserved P. falciparum sporozoites (PfSPZ Challenge), that underpin the sporozoite model. Aseptic Plasmodium infected erythrocytes have been developed to support the blood stage models. (Table 1).
Controlled human infection reagents
Malaria challenge experiments can be conducted with a range of challenge agents:
1. Laboratory-reared Plasmodium infected mosquitoes: Early studies relied on mosquitoes fed on malaria infected volunteers or their collected blood samples. This approach had safety, reproducibility, and efficiency concerns (63). Further, the availability of gametocytemic P. falciparum-infected people with which to infect mosquitoes is a limiting resource. Consequently, to facilitate safer volunteer infections, methods for rearing, aseptic P. falciparum sporozoite-infected mosquitoes under GMP were developed (64, 65). This involved the methods for the determination of sporozoite loads, confirmation of malaria transmission, and the avoidance of mosquito co-infections (64, 65). An important development was the growth of continuous in-vitro culture methods of P. falciparum (66, 67), and blood feeding of laboratory grown mosquitoes (68). These eliminated the need for blood from infected patients and increased access to infected mosquitoes. This approach became the mainstay approach for malaria infection and multiple parasite strains were developed and utilized for human infection using these methods (Table 1). All such studies have been done with non-aseptic mosquitoes except for two trials with aseptic A. stephensi mosquitoes (39, 64).
2. Aseptic, purified, cryopreserved sporozoites: Scientists at Sanaria Inc in Maryland USA advanced methods to produce aseptic, purified, cryopreserved viable PfSPZ (69). This effort was motivated by developing whole sporozoite based vaccines, but in addition has significantly increased the potential for CHMI studies as aseptic, purified, cryopreserved sporozoites could be stored and transported across sites with relative ease, and can be injected with needle and syringe without the requirement for mosquito containment facilities. This has particularly been important for facilitating the first CHMI studies in Africa, beginning in Tanzania (70), and Kenya (71) only two years after the first CHMI study with PfSPZ Challenge (NF54) in the Netherlands (31). To date CHMI studies have been conducted in Mali (72), Gabon (73), Gambia (74) and Equatorial Guinea (75).
3. Aseptic Plasmodium infected erythrocytes: Early accounts of direct blood infection studies have been reported and suffer ethical and safety concerns (76, 77). However, in the modern era GMP grade aseptic Plasmodium infected erythrocytes stocks were generated by researchers in Australia at the Queensland Institute of Medical Research (QIMR) (78). Two donors were deliberately infected with P. falciparum 3D7 via mosquito bite and blood aliquots of cryopreserved erythrocytes prepared. Blood samples were extensively tested to ensure the safety to potential volunteers (78). Direct infection using blood stage parasites hinges on the availability of blood stocks collected from suitable malaria-infected “universal” bloodxgroup O Rh D-negative blood doners (79) which may be a finite resource. The alternative approach is to generate stocks from laboratory culturing of large volumes of defined P. falciparum isolates in blood group O Rh D-negative blood at GMP levels facilities. This is followed by characterization cryopreservation in appropriate aliquots (46).
The mosquito bite model
Aseptic laboratory reared mosquitoes infected by membrane feeding approaches using gametocyte-stage parasites from laboratory culture (standard membrane feeding assays (SMFAs)), are used to challenge consenting volunteers (68). Typically, three-five infected mosquitoes are allowed to feed on volunteers for 5 mins (63, 80, 81). Parasitaemia is then monitored either by microscopy or qPCR to study endpoints as described above (Figure 2B). For P. vivax challenge studies however, continuous in vitro culture is not yet established, therefore the P. vivax model is limited to the use of clinically infected individuals for direct mosquito infection or to provide blood aliquots that can be used to feed mosquitoes, as was previously the case for falciparum. On the other hand, gametocytemia is more frequent and earlier to develop in P. vivax infection, and so this requirement is somewhat less limiting than was historically the case for falciparum (82). The mosquito challenge model has been the hallmark of CHMI studies and supported the majority of CHMI studies (Table 2). Using this approach >2,000 human volunteers have been challenged safely.
Cryopreserved sporozoites
In this model, aseptic purified and cryopreserved sporozoites can be thawed and injected into human volunteers via intravenous, intramuscular or subcutaneous injections at different doses of choice (117, 118) (Figure 2B). The mosquito bite challenge closely recapitulates the natural course of infection compared to the PfSPZ infection model. In general, it has taken exposure to the bites of five non-aseptic, infected mosquitoes inoculating an estimated 15 to 500 Pf sporozoites to achieve 100% infection of non-immune recipients (119). In contrast it takes administration of 3,200 aseptic, purified PfSPZ via direct venous inoculation to achieve 100% infection of non-immune recipients (118). This is due to the loss of infectivity associated with cryopreservation of PfSPZ. However, it has provided considerable utility in the study of vaccine candidates, drugs and biologics and acquired immunity.
CHMI of PfSPZ with needle and syringe provides an exact quantifiable dose of inoculum and allows good control, quality assurance of CHMI that do not depend on infection, rearing and biting of infected mosquitoes that are needed for direct mosquito infections. Furthermore, mosquito bite challenge currently depends on the vector Anopheles stephensii, which has become an invasive vector in some parts of Africa and so would be problematic to import and implement (120).
Blood stage model
The blood-stage challenge model involves the intravenous administration of aseptic Plasmodium infected erythrocytes, leading to a blood stage infection in the absence of pre-erythrocytic stages (78, 79, 121). This is typically a few hundred to 2,500, parasites. Blood-stage parasite multiplication is then monitored by microscopy or qPCR (41). This continues until a defined parasite threshold or patient symptomology when curative doses of antimalarial drugs are administered (Figure 2C). This model skips the liver stages of disease and would not allow study of pre-erythrocytic immunity such as the effect of circumsporozoite antibodies. It does however offer control over the starting blood stage parasite burden therefore enabling comparisons and study of parasite growth rate, whereas the sporozoite based challenge models result in greater variability in the number of merozoites exiting the liver stage, which may complicate precision in studying growth rates (122). Another advantage of the blood-stage challenge model is that it allows longer follow-up periods, due to low inoculation dose compared to the number of merozoites released from an infected hepatocyte(s).
Transmission-blocking model
This model was developed to study the transmission of gametocytes from an infected individual to mosquitoes (123, 124). The premise is the need to understand transmission blocking interventions such as vaccines, drugs and monoclonal antibodies (mAbs) that may be important for malaria elimination particularly to enable control of residual transmission on the path to elimination (125).
First, symptomless malaria infection of a volunteer is induced. This can be achieved by using any of the infection models (i.e mosquito bites, cryopreserved sporozoites or infected blood cells), and providing antimalarial drugs in non-curative doses to suppress parasitemia while allowing the development of sexual stage parasites (Figure 2D). In P. falciparum infections, sexual parasites appear later in the time course of infections. Sub curative doses of antimalarials drugs (such as sulfadoxine-pyremethamine and or piperaquine), may stimulate the appearance of gametocytes in the peripheral circulation (123, 126). Finally, transmission is determined by feeding mosquitoes through membrane feeding devise or directly on skin. Fed mosquitoes are dissected and oocysts quantified to measure transmission potential (11, 127).
Applications for controlled human malaria infection studies
Controlled human infections have been utilized for antimalarial drug assessment, vaccine and mAb efficacy estimation, and study of naturally acquired immunity and innate resistance to malaria (11). Other uses of the model include evaluating diagnostic tools and biomarkers, studying immune correlates and disease pathogenesis.
Antimalarial drug assessment: Drug resistance to commonly used antimalarial drugs is on the rise and is a major concern (3, 128). The development of new, long-acting and single dose treatments is therefore a research and public health priority. Following pre-clinical development and safety studies, CHMIs offers the opportunity to quickly evaluate the therapeutic activity, pharmacokinetic and pharmacodynamics properties of new drugs (19, 20). The highly controlled design of these studies allows appropriate drug efficacy reporting in non-immune participants without the potential overestimations of efficacy that may be seen in semi-immune adults, or the risks of first testing efficacy in children. Both mosquito bite- and blood-stage-initiated infections have been used to study antimalarial drugs (46). However, the blood stage approach may be favored as it allows control of the initial parasite burden.
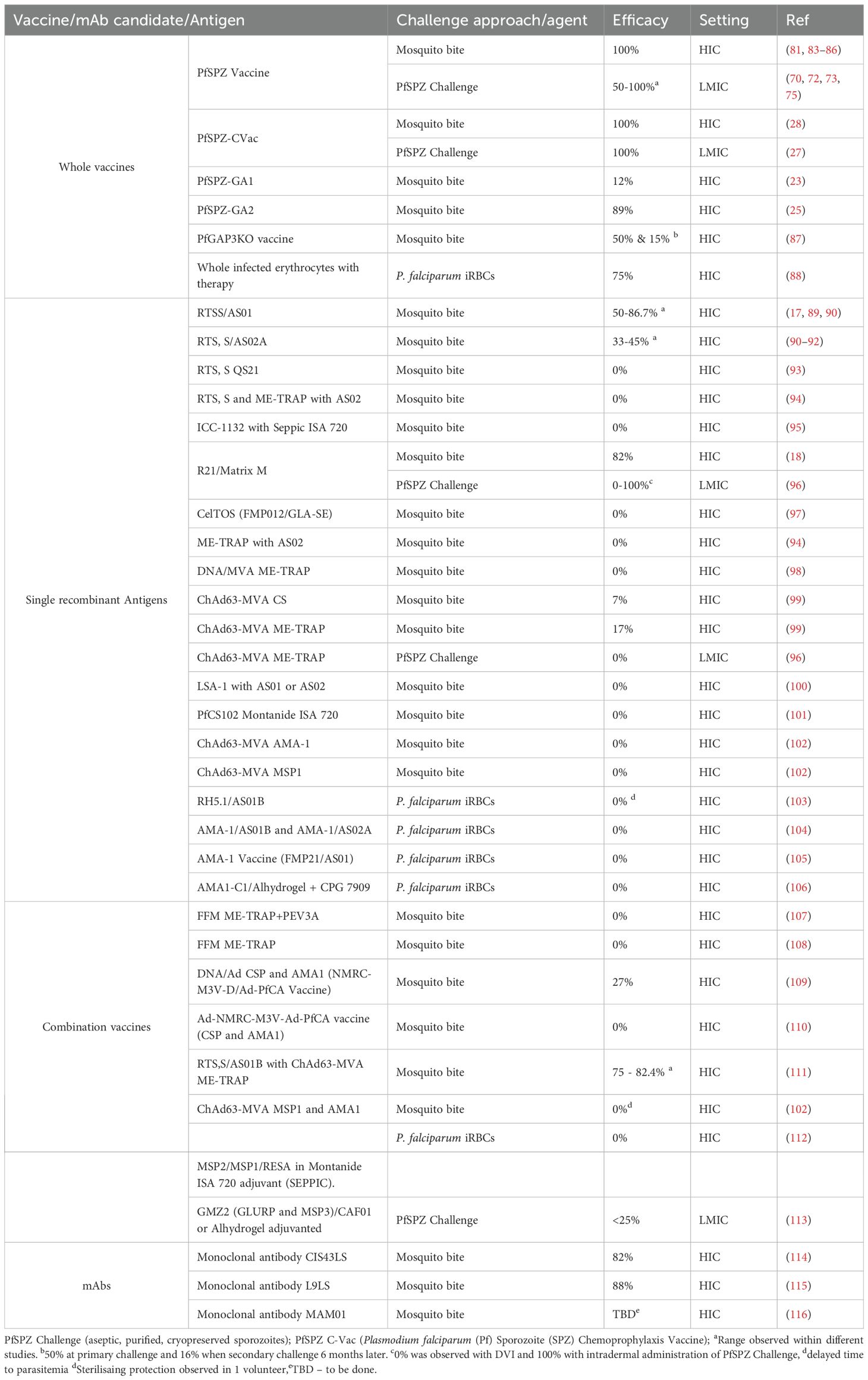
Vaccine and monoclonal antibody efficacy estimation: RTS, S for example underwent a lengthy developmental phase spanning over 30 years, supported by efficacy data from CHMI studies (10). CHMI models allowed the optimization of vaccine dose, route, schedule, and adjuvant formulation prior to (or in some cases alongside) field trials. Similarly, R21/Matrix-M demonstrated early efficacy within CHMI studies that supported its rapid clinical development. Other recombinant vaccines candidates, DNA/viral vector-based vaccines and whole sporozoite vaccines have been evaluated in CHMI models providing important proof-of-concept efficacy data for go/no go development decision making (Table 2). Initial concerns were that CHMI studies may be too stringent for vaccine candidate evaluations and would not correlate with actual field efficacies. A reduction in efficacy within the field that probably relates to immunogenicity reductions in malaria-exposed children, waning of antibody levels, and heterogeneity in circulating parasites stains has been observed. While efficacy studies using CHMI models in naïve adults conducted in the US and UK for subunit vaccines have often translated to field efficacy it has not necessarily been the case for whole parasite approaches such as whole PfSPZ vaccine studies (129). It is therefore important to test vaccine candidates using CHMI models within malaria endemic populations. Hence two key considerations that may improve the accuracy of CHMI models in predicting efficacy are: a) conducting CHMI in endemic areas, therefore capturing the impact of potential reductions in immunogenicity with some vaccinations; b) developing new parasite strains to use as challenge agents in CHMI to capture the potential impact of diverse genotypes in the field.
More recently, monoclonal antibodies (mAbs) are undergoing clinical development as potential tools for malaria control. The highly potent and protective CIS43LS (21) and L9LS (22) mAbs were discovered in samples from individuals vaccinated by PfSPZ vaccine who were protected in sporozoite challenge studies. The efficacies of these mAbs were later tested within CHMI models and were shown to be highly protective, and efficacy for one mAb was recapitulated within clinical field trials in malaria endemic areas. Currently, field efficacy trials are ongoing to evaluate their utility in light of cost, production and implementation constraints (115, 130).
Whole sporozoites for vaccination: Here the immune system can be exposed to the entire array of parasite antigens as opposed to a single recombinant protein. Radiation attenuated, chemically and genetically attenuated parasites that arrest parasite growth are in different stages of clinical development (131). Radiation attenuated PfSPZ are most advanced and have demonstrated sterile protection (132, 133). Administration of PfSPZ under chemoprophylaxis (PfSPZ-CVac) involves infection through mosquito-bite or direct venous sporozoite induced infections under chemoprophylaxis. This has been conducted using anti-malarial drugs such as chloroquine, mefloquine and pyrimethamine, that prevent blood stage infection while allowing the completion of liver stage parasite development. Sterile protection against heterologous CHMI for 3 months has been observed with this approach. Genetically attenuated parasites that arrest development are also in development (PfSPZ-GA1 (23); PfSPZ-GA2 (25); PfGAP3KO vaccine (87)) with varying levels of efficacy. These parasite lines may be utilized as tools to study immunity (134, 135).
Whole blood stage vaccines have also been evaluated. Here parasitized red blood cells and are administered with each infection controlled with Malarone (atovaquone-proguanil) prior to patency (88). Protection was observed in 3 out of 4 volunteers, however residual anti-malarial drugs that were not seen in the control arm because of their lack of prior exposure may have been a significant confounder of the protection data (136).
Diagnostic development and evaluation: Finally, the fast, accurate, and cost-effective diagnosis of malaria using dipstick rapid diagnostic tools that target the Histidine rich protein-2 (HRP2) transformed diagnosis and treatment. However, the concerning emergence of HRP2 deleted parasites that render RDTs ineffective is worrying (137, 138). Although the parasite lactate dehydrogenase (pLDH) based test kits are available, the development of more sensitive, cheap, non-invasive tools is important (139, 140). CHMI provides a platform to test different diagnostic tools providing specimens with controlled timing of infection and monitoring. Examples of the utilization of CHMI for diagnostic development include, the evaluation of breath specimens for Plasmodium falciparum biomarkers and the development of Plasmodium falciparum HRP-2 antigen a rapid dipstick antigen-capture assay (141).
Study of acquired immunity: Early studies to understand immunity utilized post-hoc analysis of patients receiving malariotherapy and highlighted its slow acquisition over repeated exposures and its strain-dependent nature (36–38, 142, 143). More recently homologous repeat challenge experiments among naïve adults have been conducted to evaluate acquired immunity (144, 145). One study used a mosquito bite challenge model to induce four consecutive repeat malaria episodes with a homologous strain (NF54), where a delay in patent parasitemia was observed with each iterative infection (144). A CHMI study among Kenyan adults with different levels of malaria exposure showed differing clinical phenotypes and parasite growth patterns. A proportion of individuals from areas with higher exposure presented with no parasites or with slower parasite growth, while those from areas of less exposure presented with exponential parasite growth with early development of clinical symptoms (71). These together confirm the slow acquisition of immunity over repeated exposures. Another study utilized a blood stage model to induce three consecutive infections; however no significant anti-parasitic blood-stage immunity was observed in the majority of re-challenges (145). Though careful interpretation is required this may suggest earlier acquisition or a lower protection threshold for liver stage responses compared to blood stage. The key immunological mechanisms of antimalarial immunity are reviewed in detail elsewhere (146–149).
Key lessons learned from CHMIs conducted in endemic populations
The earliest reports of deliberate human malaria infection in Africa occurred between 1954-1963. The first reported study was conducted in 1954 among Ugandan adults (58). This study showed the protective effect of sickle cell trait (SCT) against malaria. In the study, 30 volunteers (n=15 with SCT and n=15 controls) were infected by either parasitized red blood cells or exposure to infected mosquito bites. The individuals with SCT had lower parasite infection rates (2/15) and parasitemia compared with 14/15 in the control group (58). The same question was later addressed in modern CHMI studies using aseptic purified cryopreserved sporozoites administered by direct venous infection. This showed more nuanced findings with slower growth rates in individuals with SCT rather than complete protection as suggested in the 1954 study (73). In addition, modern CHMIs confirmed the protective effect of other blood genotypes in particular the Dantu blood group which had been seen to be protective in GWAS studies of severe malaria in children (150).
Another two early studies conducted in Nigeria in 1962 (151) and Liberia in 1963 (152) used infected red blood and infected mosquito bite respectively to induce malaria infection. Both clearly demonstrated acquired immunity showing the innate ability of malaria-exposed individuals to control parasitaemia and limit clinical symptoms (151, 152). These observations were reproduced in modern CHMI studies again using aseptic, purified and cryopreserved sporozoites in Africa. Furthermore, the administration of sporozoites to Kenyan (43, 71), Tanzanian (31) and Gabonese (73, 153) adults at doses that causes 100% infection among HIC volunteers resulted in a proportion of individuals with the ability to limit or completely suppress parasite growth.
Immunological studies confirm the relevance of antibodies to protection as seen in CHMI. The level of antibodies to whole parasites (anti-schizont (71), anti-merozoite (154), anti-ring stage (155)) is independently associated with protection from clinical disease. In addition, the ability of antibodies to induce Fc-mediated antibody effector functions (154–156) further explains protection in CHMI. The breadth of effector functions was shown important as the breadth of Fc function clearly distinguished clinical immune individuals from non-immune individuals (154). Understanding the full profile of antigen specific Fc mediated mechanisms of protection, their rate of acquisition and durability may be important to tailor vaccines with improved potency.
The prioritization of antigens for vaccine development has been challenging as Plasmodium parasites have a vast antigenic landscape with considerable diversity. A study conducted in Kenya used a custom protein microarray KILchip expressing >100 immunogenic merozoite surface antigens to screen responses among individuals with demonstrable clinical immunity post challenge (157). This study showed combinations of antigens that may be associated with sterile protection (manuscript under review). In parallel, another study explored anti-variant surface antigens (VSA) antibodies (158) and showed that the breadth of VSA was a stronger predictor of protection compared to a single VSA response. Identifying the minimum critical combination of responses needed to achieve sterile immunity by vaccination remains an important scientific goal. The fact that multiple responses are co-acquired over repeated exposures that may not necessary be critical for sterile immunity makes this challenging.
CHMI studies in Africa have also been used to test candidate vaccines, including the whole irradiated sporozoite vaccine evaluated in Tanzania (31) and Mali (72), GMZ2 in Gabon (113) and R21/Matrix-M in Kenya (96). CHMI in Africa in the modern era has relied on cryopreserved sporozoites, which were initially delivered by intramuscular or intradermal injection, but more recently by direct venous injection, which has been shown to be the most efficient method (159). In Kenya, comparisons have been made of R21/Matrix M protection against intradermal injection (ID) vs direct venous inoculation (DVI) of P. falciparum sporozoites (PfSPZ Challenge) (96). R21/Matrix-M was highly protective against intradermal inoculation of PfSPZ (i.e. 100%, 12 out of 12) but not protective against PfSPZ challenge by DVI (i.e. 0%, 0 out of 5) (96).
Ethical considerations learnt for CHMI in Africa
A key ethical consideration that has emerged from CHMI studies in Africa is the value of community engagement. This involves increased communication between communities and research teams during the planning and implementation of studies (160). Combining community involvement with informed consent greatly empowered the decision making of volunteers. A participatory approach acknowledges the cultural context and values of local populations, creating an ethical framework for research (161). Another important ethical consideration is the monetary compensation that is a fair reflection of the volunteers’ commitment to the study. This must be considered carefully to avoid undue inducement among different populations at different economic levels (127). Frameworks to engage with the community to determine adequate compensation have been developed (162, 163).
Improved understanding of CHMI studies among institutional review boards in different African countries has allowed improved regulation. Some African countries have developed specific regulatory guidelines for CHMI studies. This ensures that modern CHMI studies in many African countries are done according to international ethical standards (164). This may be supported by ethics training for researchers and local ethics committees to be able to handle the unique challenges of CHMI studies in Africa (165).
Conclusions
The use of human infection studies to guide vaccine development and understand pathophysiology of infection is expanding and diversifying to an increasing range of pathogens (11). The malaria parasite includes three main stages of its life cycle in humans which are targets of vaccination, and that are accessible to study through human infection studies, (i.e. controlled human malaria infection). Refinements to CHMI continue to be made with regards to the challenge agent and study conduct. As malaria vaccines are rolled out we predict that there will be increasing reliance on CHMI for the following reasons: a) the currently licensed vaccines will need multiple policy decisions regarding the timing of boosters, new regimens, and manufacturing process updates, and CHMI studies will be needed to confirm efficacy; b) it is likely that multi-stage vaccines will be developed by combining individual components targeting different life cycle stages, and similarly it will not be practical to design field trials that test the efficacy of each component at each stage of clinical development, whereas CHMI trials can be adapted to isolate efficacy for the different life cycle stages as well as optimize vaccine regimen; c) next generation vaccines will need to be compared against existing products (where replacement is proposed) or in addition to standard of care (where addition is proposed), and in either case field trials will become large. CHMI studies will de-risk these approaches; d) ongoing studies of correlates of protection and/or infection, either based on naturally acquired or vaccine induced immunity, will have greater power when the exposure is standardized in CHMI rather than heterogenous in field trials (71, 96). Continued investment in infrastructure, capacity, and the design of CHMI studies tailored for malaria endemic areas will be important for malaria elimination.
Author contributions
RO: Conceptualization, Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing. PB: Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. All authors are supported by a UKRI MRC grant (MR/V049976/1) for the conduct of an induced blood stage model and a core Wellcome award (203077/Z/16/Z).
Acknowledgments
We are grateful to Kelvias Keter, Stephen Hoffman, B Kim Lee Sim, Thomas Richie and Kevin Marsh for proof reading and providing advice on the manuscript. We are grateful to all the global teams who have conducted and continue to conduct CHMI studies in both the global north and south and particularly in Africa. This manuscript is published with permission/approval from KEMRI Director.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Van Voorhis WC, and Wells TNC. Malaria. Nat Rev Dis Primers. (2017) 3:17050. doi: 10.1038/nrdp.2017.50
4. Kamya MR, Nankabirwa JI, Arinaitwe E, Rek J, Zedi M, Maiteki-Sebuguzi C, et al. Dramatic resurgence of malaria after 7 years of intensive vector control interventions in Eastern Uganda. medRxiv. (2024). doi: 10.1101/2024.03.15.24304352
5. Adepoju P. Routine malaria vaccinations begin. Lancet. (2024) 403:423. doi: 10.1016/S0140-6736(24)00194-6
6. The L. Malaria vaccines: a test for global health. Lancet. (2024) 403:503. doi: 10.1016/S0140-6736(24)00235-6
9. Hemingway J, Shretta R, Wells TNC, Bell D, Djimdé AA, Achee N, et al. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PloS Biol. (2016) 14:e1002380. doi: 10.1371/journal.pbio.1002380
10. Ogwang R and Crawley J. Effective vaccination for malaria and wider implications for future global child health. Paediatrics Child Health. (2025) 35:79–87. doi: 10.1016/j.paed.2024.12.002
11. Choy RKM, Bourgeois AL, Ockenhouse CF, Walker RI, Sheets RL, and Flores J. Controlled human infection models to accelerate vaccine development. Clin Microbiol Rev. (2022) 35:e0000821. doi: 10.1128/cmr.00008-21
12. Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. (2009) 361:468–77. doi: 10.1056/NEJMoa0805832
13. Ramanathan R, Stibitz S, Pratt D, and Roberts J. Use of controlled human infection models (CHIMs) to support vaccine development: US regulatory considerations. Vaccine. (2019) 37:4256–61. doi: 10.1016/j.vaccine.2019.06.009
14. Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, et al. Single-dose live oral cholera vaccine CVD 103-hgR protects against human experimental infection withVibrio choleraeO1 El Tor. Clin Infect Diseases. (2016) 62:1329–35. doi: 10.1093/cid/ciw145
15. Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. (2017) 390:2472–80. doi: 10.1016/S0140-6736(17)32149-9
16. Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent). (2005) 18:21–5. doi: 10.1080/08998280.2005.11928028
17. Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: A phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. (2016) 214:762–71. doi: 10.1093/infdis/jiw237
18. Venkatraman N, Silman D, Bellamy D, Stockdale L, Bowyer G, Edwards NJ, et al. R21 in Matrix-M adjuvant in UK malaria-naive adult men and non-pregnant women aged 18–45 years: an open-label, partially blinded, phase 1-2a controlled human malaria infection study. Lancet Microbe. (2025) 6:100867. doi: 10.1016/S2666-5247(24)00083-1
19. Deye GA, Miller RS, Miller L, Salas CJ, Tosh D, Macareo L, et al. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of plasmodium falciparum malaria in a human challenge model. Clin Infect Diseases. (2012) 54:232–9. doi: 10.1093/cid/cir770
20. McCarthy JS, Baker M, O’Rourke P, Marquart L, Griffin P, Hooft van Huijsduijnen R, et al. Efficacy of OZ439 (artefenomel) against earlyPlasmodium falciparumblood-stage malaria infection in healthy volunteers. J Antimicrobial Chemother. (2016) 71:2620–7. doi: 10.1093/jac/dkw174
21. Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med. (2018) 24:408–16. doi: 10.1038/nm.4512
22. Wang LT, Pereira LS, Flores-Garcia Y, O’Connor J, Flynn BJ, Schon A, et al. A potent anti-malarial human monoclonal antibody targets circumsporozoite protein minor repeats and neutralizes sporozoites in the liver. Immunity. (2020) 53:733–44.e8. doi: 10.1016/j.immuni.2020.08.014
23. Roestenberg M, Walk J, van der Boor SC, Langenberg MCC, Hoogerwerf MA, Janse JJ, et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med. (2020) 12(544):eaaz5629. doi: 10.1126/scitranslmed.aaz5629
24. van Schaijk BCL, Ploemen IHJ, Annoura T, Vos MW, Foquet L, van Gemert G-J, et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. eLife. (2014) 3:e03582. doi: 10.7554/eLife.03582
25. Lamers OAC, Franke-Fayard BMD, Koopman JPR, Roozen GVT, Janse JJ, Chevalley-Maurel SC, et al. Safety and efficacy of immunization with a late-liver-stage attenuated malaria parasite. New Engl J Med. (2024) 391:1913–23. doi: 10.1056/NEJMoa2313892
26. Bijker EM, Borrmann S, Kappe SH, Mordmüller B, Sack BK, and Khan SM. Novel approaches to whole sporozoite vaccination against malaria. Vaccine. (2015) 33:7462–8. doi: 10.1016/j.vaccine.2015.09.095
27. Coulibaly D, Kone AK, Traore K, Niangaly A, Kouriba B, Arama C, et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: A randomized, controlled phase 1 trial. eClinicalMedicine. (2022) 52:101579. doi: 10.1016/j.eclinm.2022.101579
28. Mwakingwe-Omari A, Healy SA, Lane J, Cook DM, Kalhori S, Wyatt C, et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature. (2021) 595:289–94. doi: 10.1038/s41586-021-03684-z
29. Kibwana E, Kapulu M, and Bejon P. Controlled human malaria infection studies in africa-past, present, and future. Curr Top Microbiol Immunol. (2024) 445:337–65. doi: 10.1007/82_2022_256
30. Hodgson SH, Juma E, Salim A, Magiri C, Kimani D, Njenga D, et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol. (2014) 5:686. doi: 10.3389/fmicb.2014.00686
31. Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. (2014) 91:471–80. doi: 10.4269/ajtmh.14-0119
32. Laveran A. Note sur un nouveau parasite trouvé dans le sang de plusieurs malades atteints de fièvre palustre. Bull Acad Med. (1880) 9:1235–6.
33. Ross R. On some peculiar pigmented cells found in two mosquitos fed on malarial blood. Br Med J. (1897) 2:1786–8. doi: 10.1136/bmj.2.1929.1786
34. Grassi B, Bignami A, and Bastianelli G. Ulteriore ricerche sul ciclo dei parassiti malarici umani sul corpo del zanzarone (Translated: Further research on the cycle of human malaria parasites on the mosquito body). Atti Reale Accad Lincei. (1899) 8:21–8.
35. Cox FEG. History of the discovery of the malaria parasites and their vectors. Parasites Vectors. (2010) 3:5. doi: 10.1186/1756-3305-3-5
36. James SP. Some general results of a study of induced malaria in England. Trans R Soc Trop Med Hygiene. (1931) 24:477–525. doi: 10.1016/S0035-9203(31)90068-0
37. Mayne B. The injection of mosquito sporozoites in malaria therapy. Public Health Rep (1896-1970). (1933) 48:909–16. doi: 10.2307/4580870
38. Brown EM. Why Wagner-Jauregg won the Nobel Prize for discovering malaria therapy for general paresis of the insane. History Psychiatry. (2000) 11:371–82. doi: 10.1177/0957154X0001104403
39. Laurens MB, Duncan CJ, Epstein JE, Hill AV, Komisar JL, Lyke KE, et al. A consultation on the optimization of controlled human malaria infection by mosquito bite for evaluation of candidate malaria vaccines. Vaccine. (2012) 30:5302–4. doi: 10.1016/j.vaccine.2012.04.088
40. Moorthy VS, Diggs C, Ferro S, Good MF, Herrera S, Hill AV, et al. Report of a consultation on the optimization of clinical challenge trials for evaluation of candidate blood stage malaria vaccines, 18–19 March 2009, Bethesda, MD, USA. Vaccine. (2009) 27:5719–25. doi: 10.1016/j.vaccine.2009.07.049
41. Murphy SC, Prentice JL, Williamson K, Wallis CK, Fang FC, Fried M, et al. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg. (2012) 86:383–94. doi: 10.4269/ajtmh.2012.10-0658
42. Kibwana E, Kimani D, Edwards NJ, Keter K, Mutiso A, Nyamako L, et al. Quantification of Plasmodium falciparum: validation of quantitative polymerase chain reaction assays for detection of parasites in controlled human malaria infection studies. Front Malaria. (2025) 3. doi: 10.3389/fmala.2025.1497613
43. Kapulu MC, Njuguna P, Hamaluba M, Kimani D, Ngoi JM, Musembi J, et al. Safety and PCR monitoring in 161 semi-immune Kenyan adults following controlled human malaria infection. JCI Insight. (2021) 6(17):e146443. doi: 10.1172/jci.insight.146443
44. van Meer MP, Bastiaens GJ, Boulaksil M, de Mast Q, Gunasekera A, Hoffman SL, et al. Idiopathic acute myocarditis during treatment for controlled human malaria infection: a case report. Malar J. (2014) 13:38. doi: 10.1186/1475-2875-13-38
45. Nieman AE, de Mast Q, Roestenberg M, Wiersma J, Pop G, Stalenhoef A, et al. Cardiac complication after experimental human malaria infection: a case report. Malar J. (2009) 8:277. doi: 10.1186/1475-2875-8-277
46. Stanisic DI, Liu XQ, De SL, Batzloff MR, Forbes T, Davis CB, et al. Development of cultured Plasmodium falciparum blood-stage malaria cell banks for early phase in vivo clinical trial assessment of anti-malaria drugs and vaccines. Malar J. (2015) 14:143. doi: 10.1186/s12936-015-0663-x
47. Delemarre BJ and van der Kaay HJ. Tropical malaria contracted the natural way in the Netherlands. Ned Tijdschr Geneeskd. (1979) 123:1981–2.
48. Teirlinck AC, Roestenberg M, van de Vegte-Bolmer M, Scholzen A, Heinrichs MJ, Siebelink-Stoter R, et al. NF135.C10: a new Plasmodium falciparum clone for controlled human malaria infections. J Infect Dis. (2013) 207:656–60. doi: 10.1093/infdis/jis725
49. Langenberg MCC, Wammes LJ, McCall MBB, Bijker EM, van Gemert G-J, Graumans W, et al. Controlled human malaria infection with graded numbers of plasmodium falciparum NF135.C10- or NF166.C8-infected mosquitoes. Am J Trop Med Hygiene. (2018) 99:709–12. doi: 10.4269/ajtmh.18-0194
50. Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, and Sell KW. Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hygiene. (1974) 68:258–9. doi: 10.1016/0035-9203(74)90129-1
51. Rieckmann KH, Beaudoin RL, Cassells JS, and Sell KW. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull World Health Organ. (1979) 57 Suppl 1:261–5.
52. Knowles R and Gupta BMD. A study of monkey-malaria, and its experimental transmission to man. Ind Med Gaz. (1932) 67:301–20.
53. Herrera S, Palacios R, Jordán-Villegas A, Echavarría JF, Richie TL, Solarte Y, et al. Consistent safety and infectivity in sporozoite challenge model of plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hygiene. (2011) 84:4–11. doi: 10.4269/ajtmh.2011.09-0498
54. Herrera Sc, Fernández O, Manzano M, Murrain B, Vergara J, Blanco P, et al. Successful sporozoite challenge model in human volunteers with plasmodium vivax strain derived from human donors. Am J Trop Med Hygiene. (2009) 81:740–6. doi: 10.4269/ajtmh.2009.09-0194
55. Sinnis P, Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, et al. Phase 1/2a trial of plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Diseases. (2016) 10:e0004423. doi: 10.1371/journal.pntd.0004423
56. Kamau E, Bennett JW, and Yadava A. Safety and tolerability of mosquito bite-induced controlled human infection with plasmodium vivax in malaria-naive study participants-clinical profile and utility of molecular diagnostic methods. J Infect Dis. (2022) 225:146–56. doi: 10.1093/infdis/jiab332
57. Woodford J, Collins KA, Odedra A, Wang C, Jang IK, Domingo GJ, et al. An experimental human blood-stage model for studying plasmodium malariae infection. J Infect Diseases. (2019) 221:948–55. doi: 10.1093/infdis/jiz102
58. Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. BMJ. (1954) 1:290–4. doi: 10.1136/bmj.1.4857.290
59. McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. (2013) 208:1688–94. doi: 10.1093/infdis/jit394
60. Sinnis P, Griffin P, Pasay C, Elliott S, Sekuloski S, Sikulu M, et al. Safety and reproducibility of a clinical trial system using induced blood stage plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Negl Trop Diseases. (2016) 10:e0005139. doi: 10.1371/journal.pntd.0005139
61. Minassian AM, Themistocleous Y, Silk SE, Barrett JR, Kemp A, Quinkert D, et al. Controlled human malaria infection with a clone of Plasmodium vivax with high-quality genome assembly. JCI Insight. (2021) 6(23):e152465. doi: 10.1172/jci.insight.152465
62. Hou MM, Barrett JR, Themistocleous Y, Rawlinson TA, Diouf A, Martinez FJ, et al. Vaccination with Plasmodium vivax Duffy-binding protein inhibits parasite growth during controlled human malaria infection. Sci Trans Med. (2023) 15:eadf1782. doi: 10.1126/scitranslmed.adf1782
63. Epstein Judith E, Rao S, Williams F, Freilich D, Luke T, Sedegah M, et al. Safety and Clinical Outcome of Experimental Challenge of Human Volunteers withPlasmodium falciparum–Infected Mosquitoes: An Update. J Infect Diseases. (2007) 196:145–54. doi: 10.1086/518510
64. Rénia L, Lyke KE, Laurens M, Adams M, Billingsley PF, Richman A, et al. Plasmodium falciparum Malaria Challenge by the Bite of Aseptic Anopheles stephensi Mosquitoes: Results of a Randomized Infectivity Trial. PLoS One. (2010) 5:e13490. doi: 10.1371/journal.pone.0013490
65. Hoffman Stephen L, Goh Lucy ML, Luke Thomas C, Schneider I, Le Thong P, Doolan Denise L, et al. Protection of Humans against Malaria by Immunization with Radiation-AttenuatedPlasmodium falciparumSporozoites. J Infect Diseases. (2002) 185:1155–64. doi: 10.1086/339409
66. Trager W and Jensen JB. Human malaria parasites in continuous culture. Science. (1976) 193:673–5. doi: 10.1126/science.781840
67. Grun JL and Weidanz WP. Cultivation of Plasmodium falciparum in commercially available sera depleted of natural antibodies reactive with human erythrocytes. J Parasitol. (1987) 73:384–8. doi: 10.2307/3282094
68. Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, et al. Malaria transmitted to humans by mosquitoes infected from cultured plasmodium falciparum. Am J Trop Med Hygiene. (1986) 35:66–8. doi: 10.4269/ajtmh.1986.35.66
69. Chakravarty S, Shears MJ, James ER, Rai U, Kc N, Conteh S, et al. Efficient infection of non-human primates with purified, cryopreserved Plasmodium knowlesi sporozoites. Malaria J. (2022) 21:247. doi: 10.1186/s12936-022-04261-z
70. Jongo SA, Shekalaghe SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am J Trop Med Hyg. (2018) 99:338–49. doi: 10.4269/ajtmh.17-1014
71. Kapulu MC, Kimani D, Njuguna P, Hamaluba M, Otieno E, Kimathi R, et al. Controlled human malaria infection (CHMI) outcomes in Kenyan adults is associated with prior history of malaria exposure and anti-schizont antibody response. BMC Infect Dis. (2022) 22:86. doi: 10.1186/s12879-022-07044-8
72. Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. (2022) 22:377–89. doi: 10.1016/S1473-3099(21)00332-7
73. Lell B, Mordmuller B, Dejon Agobe JC, Honkpehedji J, Zinsou J, Mengue JB, et al. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg. (2018) 98:508–15. doi: 10.4269/ajtmh.17-0343
74. D’Alessandro U, Bousema T, Drakeley C, Hoffman SL, Sim BKL, Billingsley PF, et al. Serologic markers of previous malaria exposure and functional antibodies inhibiting parasite growth are associated with parasite kinetics following a plasmodium falciparum controlled human infection. Clin Infect Diseases. (2020) 70:2544–52. doi: 10.1093/cid/ciz740
75. Jongo SA, Church LWP, Nchama V, Hamad A, Chuquiyauri R, Kassim KR, et al. Multi-dose priming regimens of PfSPZ vaccine: safety and efficacy against controlled human malaria infection in equatoGuinean adults. Am J Trop Med Hyg. (2022) 106:1215–26. doi: 10.4269/ajtmh.21-0942
76. Martin D. Studies on an East African strain of Plasmodium falciparum*1. Trans R Soc Trop Med Hygiene. (1967) 61:331–9. doi: 10.1016/0035-9203(67)90005-3
77. Martin DC, Arnold JD, Clyde DF, Ibrahim MA, Carson PE, Rieckmann KH, et al. A quinoline methanol (WR 30090) for treatment of acute malaria. Antimicrobial Agents Chemother. (1973) 3:214–9. doi: 10.1128/AAC.3.2.214
78. von Seidlein L, McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, et al. A pilot randomised trial of induced blood-stage plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One. (2011) 6:e21914. doi: 10.1371/journal.pone.0021914
79. Engwerda CR, Minigo G, Amante FH, and McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. (2012) 28:515–21. doi: 10.1016/j.pt.2012.09.001
80. Spring M, Polhemus M, and Ockenhouse C. Controlled human malaria infection. J Infect Dis. (2014) 209 Suppl 2:S40–5. doi: 10.1093/infdis/jiu063
81. Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. (2017) 2:e89154. doi: 10.1172/jci.insight.89154
82. Roobsoong W, Yadava A, Draper SJ, Minassian AM, and Sattabongkot J. The challenges of Plasmodium vivax human malaria infection models for vaccine development. Front Immunol. (2022) 13:1006954. doi: 10.3389/fimmu.2022.1006954
83. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. (2013) 341:1359–65. doi: 10.1126/science.1241800
84. Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M, Vallejo AF, Amado-Garavito AB, Céspedes N, et al. Protective Efficacy of Plasmodium vivax Radiation-Attenuated Sporozoites in Colombian Volunteers: A Randomized Controlled Trial. PLoS Negl Trop Dis. (2016) 10:e0005070. doi: 10.1371/journal.pntd.0005070
85. Rieckmann KH. Human immunization with attenuated sporozoites. Bull World Health Organ. (1990) 68 Suppl:13–6.
86. Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci. (2017) 114:2711–6. doi: 10.1073/pnas.1615324114
87. Murphy SC, Vaughan AM, Kublin JG, Fishbauger M, Seilie AM, Cruz KP, et al. A genetically engineered Plasmodium falciparum parasite vaccine provides protection from controlled human malaria infection. Sci Transl Med. (2022) 14:eabn9709. doi: 10.1126/scitranslmed.abn9709
88. Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. (2002) 360:610–7. doi: 10.1016/S0140-6736(02)09784-2
89. Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. (1997) 336:86–91. doi: 10.1056/NEJM199701093360202
90. Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. (2009) 200:337–46. doi: 10.1086/600120
91. Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, et al. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine. (2006) 24:2850–9. doi: 10.1016/j.vaccine.2005.12.041
92. Kester KE, Cummings JF, Ockenhouse CF, Nielsen R, Hall BT, Gordon DM, et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. (2008) 26:2191–202. doi: 10.1016/j.vaccine.2008.02.048
93. Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Diseases. (1998) 178:1139–44. doi: 10.1086/515657
94. Kester KE, Gray Heppner D Jr., Moris P, Ofori-Anyinam O, Krzych U, Tornieporth N, et al. Sequential Phase 1 and Phase 2 randomized, controlled trials of the safety, immunogenicity and efficacy of combined pre-erythrocytic vaccine antigens RTS,S and TRAP formulated with AS02 Adjuvant System in healthy, malaria naive adults. Vaccine. (2014) 32:6683–91. doi: 10.1016/j.vaccine.2014.06.033
95. Walther M, Dunachie S, Keating S, Vuola j M, Berthoud T, Schmidt A, et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. (2005) 23:857–64. doi: 10.1016/j.vaccine.2004.08.020
96. Kapulu MC, Orenge F, Kimani D, Kibwana E, Kibet H, Mutahi M, et al. R21 malaria vaccine is protective against intradermal but not intravenous Plasmodium falciparum sporozoites in a randomized controlled human malaria infection study in Kenyan adults. medRxiv. (2025).
97. Phase 1 study with the vaccine candidate plasmodium falciparum malaria protein (FMP012), an E.Coli-expressed cell-traversal protein, administered intramuscularly in healthy malaria-naive adults(2012). Available online at: https://clinicaltrials.gov/study/NCT01540474 (Accessed April 10, 2025).
98. McConkey SJ, Reece WHH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. (2003) 9:729–35. doi: 10.1038/nm881
99. Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. Evaluation of the efficacy of chAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Diseases. (2015) 211:1076–86. doi: 10.1093/infdis/jiu579
100. Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-γ/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. (2010) 28:5135–44. doi: 10.1016/j.vaccine.2009.08.046
101. Genton B, D’Acremont V, Lurati-Ruiz F, Verhage D, Audran R, Hermsen C, et al. Randomized double-blind controlled Phase I/IIa trial to assess the efficacy of malaria vaccine PfCS102 to protect against challenge with P. falciparum. Vaccine. (2010) 28:6573–80. doi: 10.1016/j.vaccine.2010.07.067
102. Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. (2012) 20:2355–68. doi: 10.1038/mt.2012.223
103. Minassian AM, Silk SE, Barrett JR, Nielsen CM, Miura K, Diouf A, et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med. (2021) 2:701–19.e19. doi: 10.1016/j.medj.2021.03.014
104. Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PloS One. (2009) 4:e5254. doi: 10.1371/journal.pone.0005254
105. Payne RO, Milne KH, Elias SC, Edwards NJ, Douglas AD, Brown RE, et al. Demonstration of the Blood-Stage Plasmodium falciparum Controlled Human Malaria Infection Model to Assess Efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01. J Infect Dis. (2016) 213:1743–51. doi: 10.1093/infdis/jiw039
106. Gregson A, Duncan CJA, Sheehy SH, Ewer KJ, Douglas AD, Collins KA, et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/alhydrogel+CPG 7909. PloS One. (2011) 6:e22271. doi: 10.1371/journal.pone.0022271
107. Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PloS One. (2008) 3:e1493. doi: 10.1371/journal.pone.0001493
108. Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci. (2005) 102:4836–41. doi: 10.1073/pnas.0406381102
109. Ellis RD, Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, et al. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PloS One. (2013) 8:e55571.
110. Tamminga C, Sedegah M, Maiolatesi S, Fedders C, Reyes S, Reyes A, et al. Human adenovirus 5-vectoredPlasmodium falciparumNMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum Vaccines Immunotherapeutics. (2014) 9:2165–77. doi: 10.4161/hv.24941
111. Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia ankara vectored vaccines expressing ME-TRAP. J Infect Dis. (2016) 214:772–81. doi: 10.1093/infdis/jiw244
112. Lawrence G, Cheng QQ, Reed C, Taylor D, Stowers A, Cloonan N, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. (2000) 18:1925–31. doi: 10.1016/S0264-410X(99)00444-2
113. Dejon-Agobe JC, Ateba-Ngoa U, Lalremruata A, Homoet A, Engelhorn J, Nouatin OP, et al. Controlled human malaria infection of healthy adults with lifelong malaria exposure to assess safety, immunogenicity, and efficacy of the asexual blood stage malaria vaccine candidate GMZ2. Clin Infect Diseases. (2019) 69:1377–84. doi: 10.1093/cid/ciy1087
114. Lyke KE, Berry AA, Mason K, Idris AH, O’Callahan M, Happe M, et al. Low-dose intravenous and subcutaneous CIS43LS monoclonal antibody for protection against malaria (VRC 612 Part C): a phase 1, adaptive trial. Lancet Infect Diseases. (2023) 23:578–88. doi: 10.1016/S1473-3099(22)00793-9
115. Wu RL, Idris AH, Berkowitz NM, Happe M, Gaudinski MR, Buettner C, et al. Low-dose subcutaneous or intravenous monoclonal antibody to prevent malaria. New Engl J Med. (2022) 387:397–407. doi: 10.1056/NEJMoa2203067
116. A phase 1, dose escalation, double blind, placebo controlled clinical trial with controlled human malaria infections (CHMI) to evaluate safety, tolerability, pharmacokinetics, and protective efficacy of an anti-malaria human monoclonal antibody, MAM01, in healthy, malaria-naive adults(2023). Available online at: https://clinicaltrials.gov/study/NCT05891236 (Accessed April 10, 2025).
117. Gómez-Pérez GP, Legarda A, Muñoz J, Sim BKL, Ballester MR, Dobaño C, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naïve volunteers: effect of injection volume and dose on infectivity rates. Malaria J. (2015) 14:306. doi: 10.1186/s12936-015-0817-x
118. Mordmuller B, Supan C, Sim KL, Gomez-Perez GP, Ospina Salazar CL, Held J, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J. (2015) 14:117. doi: 10.1186/s12936-015-0628-0
119. Rickman LS, Jones TR, Long GW, Paparello S, Schneider I, Paul CF, et al. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am J Trop Med Hyg. (1990) 43:441–5. doi: 10.4269/ajtmh.1990.43.441
120. Ochomo EO, Milanoi S, Abong’o B, Onyango B, Muchoki M, Omoke D, et al. Detection of anopheles stephensi mosquitoes by molecular surveillance, Kenya. Emerg Infect Dis. (2023) 29:2498–508. doi: 10.3201/eid2912.230637
121. Sanderson F, Andrews L, Douglas AD, Hunt-Cooke A, Bejon P, and Hill AVS. Blood-stage challenge for malaria vaccine efficacy trials: A pilot study with discussion of safety and potential value. Am J Trop Med Hygiene. (2008) 78:878–83. doi: 10.4269/ajtmh.2008.78.878
122. Bejon P, Andrews L, Andersen Rikke F, Dunachie S, Webster D, Walther M, et al. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Diseases. (2005) 191:619–26. doi: 10.1086/427243
123. Collins KA, Wang CYT, Adams M, Mitchell H, Rampton M, Elliott S, et al. A controlled human malaria infection model enabling evaluation of transmission-blocking interventions. J Clin Invest. (2018) 128:1551–62. doi: 10.1172/JCI98012
124. Alkema M, Reuling IJ, de Jong GM, Lanke K, Coffeng LE, van Gemert G-J, et al. A randomized clinical trial to compare plasmodium falciparum gametocytemia and infectivity after blood-stage or mosquito bite–induced controlled malaria infection. J Infect Diseases. (2021) 224:1257–65. doi: 10.1093/infdis/jiaa157
125. Sauerwein RW and Bousema T. Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine. (2015) 33:7476–82. doi: 10.1016/j.vaccine.2015.08.073
126. Reuling IJ, van de Schans LA, Coffeng LE, Lanke K, Meerstein-Kessel L, Graumans W, et al. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. Elife. (2018) 7:e31549. doi: 10.7554/eLife.31549
127. Kessy EJ and Olotu AI. Controlled human malaria infection: overview and potential application in the evaluation of transmission-blocking interventions in malaria-endemic areas. Malaria J. (2025) 24:33. doi: 10.1186/s12936-025-05277-x
128. Ogwang R, Osoti V, Wamae K, Ndwiga L, Muteru K, Ningwa A, et al. A retrospective analysis of P. falciparum drug resistance markers detects an early (2016/17) high prevalence of the k13 C469Y mutation in asymptomatic infections in Northern Uganda. Antimicrob Agents Chemother. (2024) 68:e0157623. doi: 10.1128/aac.01576-23
129. Abuelazm MT, Elzeftawy MA, Kamal MA, Badr H, Gamal M, Aboulgheit M, et al. Protective efficacy and safety of radiation-attenuated and chemo-attenuated Plasmodium Falciparum sporozoite vaccines against controlled and natural malaria infection: a systematic review and meta-analysis of randomized controlled trials. Infection. (2024) 52:707–22. doi: 10.1007/s15010-024-02174-4
130. Kayentao K, Ongoiba A, Preston AC, Healy SA, Hu Z, Skinner J, et al. Subcutaneous administration of a monoclonal antibody to prevent malaria. N Engl J Med. (2024) 390:1549–59. doi: 10.1056/NEJMoa2312775
131. Nunes-Cabaco H, Moita D, and Prudencio M. Five decades of clinical assessment of whole-sporozoite malaria vaccines. Front Immunol. (2022) 13:977472. doi: 10.3389/fimmu.2022.977472
132. Oneko M, Steinhardt LC, Yego R, Wiegand RE, Swanson PA, Kc N, et al. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med. (2021) 27:1636–45. doi: 10.1038/s41591-021-01470-y
133. Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. (2017) 542:445–9. doi: 10.1038/nature21060
134. Roozen GVT, van Schuijlenburg R, Hensen ADO, Koopman JPR, Lamers OAC, Geurten FJA, et al. Single immunization with genetically attenuated PfΔmei2 (GA2) parasites by mosquito bite in controlled human malaria infection: a placebo-controlled randomized trial. Nat Med. (2025) 31:218–22. doi: 10.1038/s41591-024-03347-2
135. Colstrup E, Nakajima R, Krol JMM, Lamers OAC, de Assis RR, Jain A, et al. Correlative humoral and cellular immunity to genetically attenuated malaria parasites in humans. iScience. (2025) 28:112589. doi: 10.1016/j.isci.2025.112589
136. Edstein MD, Kotecka BM, Anderson KL, Pombo DJ, Kyle DE, Rieckmann KH, et al. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrobial Agents Chemother. (2005) 49:4421–2. doi: 10.1128/AAC.49.10.4421-4422.2005
137. Verma AK, Bharti PK, and Das A. HRP-2 deletion: a hole in the ship of malaria elimination. Lancet Infect Diseases. (2018) 18:826–7. doi: 10.1016/S1473-3099(18)30420-1
138. Poti KE, Sullivan DJ, Dondorp AM, and Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. (2020) 36:112–26. doi: 10.1016/j.pt.2019.12.004
139. Jang IK, Tyler A, Lyman C, Rek JC, Arinaitwe E, Adrama H, et al. Multiplex human malaria array: quantifying antigens for malaria rapid diagnostics. Am J Trop Med Hyg. (2020) 102:1366–9. doi: 10.4269/ajtmh.19-0763
140. Barney R, Velasco M, Cooper CA, Rashid A, Kyle DE, Moon RW, et al. Diagnostic characteristics of lactate dehydrogenase on a multiplex assay for malaria detection including the zoonotic parasite plasmodium knowlesi. Am J Trop Med Hygiene. (2022) 106:275–82. doi: 10.4269/ajtmh.21-0532
141. Beadle C, Long GW, McElroy PD, Hoffman SL, Long GW, Weiss WR, et al. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. (1994) 343:564–8. doi: 10.1016/S0140-6736(94)91520-2
142. Covell G and Nicol WD. Clinical, chemotherapeutic and immunological studies on induced malaria. Br Med Bulletin. (1951) 8:51–5. doi: 10.1093/oxfordjournals.bmb.a074054
143. Collins WE and Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with plasmodium falciparum in patients previously infected with heterologous species of plasmodium: effect on development of parasitologic and clinical immunity. Am Soc Trop Med Hygiene. (1999) 61:36–43. doi: 10.4269/tropmed.1999.61-036
144. Ferrer P, Berry AA, Bucsan AN, Prajapati SK, Krishnan K, Barbeau MC, et al. Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms. Nat Commun. (2024) 15:5194. doi: 10.1038/s41467-024-49041-2
145. Salkeld J, Themistocleous Y, Barrett JR, Mitton CH, Rawlinson TA, Payne RO, et al. Repeat controlled human malaria infection of healthy UK adults with blood-stage Plasmodium falciparum: Safety and parasite growth dynamics. Front Immunol. (2022) 13:984323. doi: 10.3389/fimmu.2022.984323
146. Opi DH, Kurtovic L, Chan JA, Horton JL, Feng G, and Beeson JG. Multi-functional antibody profiling for malaria vaccine development and evaluation. Expert Rev Vaccines. (2021) 20:1257–72. doi: 10.1080/14760584.2021.1981864
147. Pohl K and Cockburn IA. Innate immunity to malaria: The good, the bad and the unknown. Front Immunol. (2022) 13:914598. doi: 10.3389/fimmu.2022.914598
148. Julien JP and Wardemann H. Antibodies against Plasmodium falciparum malaria at the molecular level. Nat Rev Immunol. (2019) 19:761–75. doi: 10.1038/s41577-019-0209-5
149. Langhorne J, Ndungu FM, Sponaas A-M, and Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. (2008) 9:725–32. doi: 10.1038/ni.f.205
150. Kariuki SN, Macharia AW, Makale J, Nyamu W, Hoffman SL, Kapulu MC, et al. The Dantu blood group prevents parasite growth in vivo: Evidence from a controlled human malaria infection study. eLife. (2023) 12:e83874. doi: 10.7554/eLife.83874.sa2
151. Bruce-Chwatt LJ and World Health O. A longitudinal survey of natural malaria infection in a group of West African adults/by L. Bruce-Chwatt J, editor. Geneva: World Health Organization (1962).
152. Bray RS, Gunders AE, Burgess RW, Freeman JB, Etzel E, Guttuso C, et al. The inoculation of semi-immune Africans with sporozoites of Laverania falcipara (Plasmodium falciparum) in Liberia. Riv Malariol. (1962) 41:199–210.
153. Manurung MD, de Jong SE, Kruize Y, Mouwenda YD, Ongwe MEB, Honkpehedji YJ, et al. Immunological profiles associated with distinct parasitemic states in volunteers undergoing malaria challenge in Gabon. Sci Rep. (2022) 12:13303. doi: 10.1038/s41598-022-17725-8
154. Nkumama IN, Ogwang R, Odera D, Musasia F, Mwai K, Nyamako L, et al. Breadth of Fc-mediated effector function correlates with clinical immunity following human malaria challenge. Immunity. (2024) 57:1215–24.e6. doi: 10.1016/j.immuni.2024.05.001
155. Musasia FK, Nkumama IN, Frank R, Kipkemboi V, Schneider M, Mwai K, et al. Phagocytosis of Plasmodium falciparum ring-stage parasites predicts protection against malaria. Nat Commun. (2022) 13:4098. doi: 10.1038/s41467-022-31640-6
156. Odera DO, Tuju J, Mwai K, Nkumama IN, Furle K, Chege T, et al. Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria. Sci Transl Med. (2023) 15:eabn5993. doi: 10.1126/scitranslmed.abn5993
157. Kamuyu G, Tuju J, Kimathi R, Mwai K, Mburu J, Kibinge N, et al. KILchip v1.0: A Novel Plasmodium falciparum Merozoite Protein Microarray to Facilitate Malaria Vaccine Candidate Prioritization. Front Immunol. (2018) 9:2866. doi: 10.3389/fimmu.2018.02866
158. Kimingi HW, Kinyua AW, Achieng NA, Wambui KM, Mwangi S, Nguti R, et al. Breadth of antibodies to plasmodium falciparum variant surface antigens is associated with immunity in a controlled human malaria infection study. Front Immunol. (2022) 13:894770. doi: 10.3389/fimmu.2022.894770
159. Richie TL, Church LWP, Murshedkar T, Billingsley PF, James ER, Chen MC, et al. Sporozoite immunization: innovative translational science to support the fight against malaria. Expert Rev Vaccines. (2023) 22:964–1007. doi: 10.1080/14760584.2023.2245890
160. Mumba N, Njuguna P, Chi P, Marsh V, Awuor E, Hamaluba M, et al. Undertaking community engagement for a controlled human malaria infection study in Kenya: approaches and lessons learnt. Front Public Health. (2022) 10:793913. doi: 10.3389/fpubh.2022.793913
161. Tindana PO, Singh JA, Tracy CS, Upshur RE, Daar AS, Singer PA, et al. Grand challenges in global health: community engagement in research in developing countries. PLoS Med. (2007) 4:e273. doi: 10.1371/journal.pmed.0040273
162. Chi PC, Owino EA, Jao I, Olewe F, Ogutu B, Bejon P, et al. Understanding the benefits and burdens associated with a malaria human infection study in Kenya: experiences of study volunteers and other stakeholders. Trials. (2021) 22:494. doi: 10.1186/s13063-021-05455-7
163. Dabira ED, Fehr A, Beloum N, Van geertruyden J-P, Achan J, Erhart A, et al. Perceptions and acceptability of the controlled human malaria infection (CHMI) model in The Gambia: a qualitative study. Sci Rep. (2023) 13:8708. doi: 10.1038/s41598-023-35752-x
164. Eberts JD, Eyal N, and Banerjee S. Ethics & utility of controlled human infection studies (CHIS) in low- & middle-income countries. Indian J Med Res. (2024) 160:262–6. doi: 10.25259/IJMR_985_2024
Keywords: human infection studies, Plasmodium falciparum, vaccines, monoclonal antibodies, immunity, correlate of protection, Africa
Citation: Ogwang R, Adan M, Bejon P and Kapulu MC (2025) Controlled human malaria infection studies: insights into recent advances and key immunological and ethical implementation lessons. Front. Immunol. 16:1672945. doi: 10.3389/fimmu.2025.1672945
Received: 25 July 2025; Accepted: 27 August 2025;
Published: 18 September 2025.
Edited by:
Xinming Tang, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Nirianne Querijero Palacpac, Osaka University, JapanKazutoyo Miura, National Institute of Allergy and Infectious Diseases (NIH), United States
Copyright © 2025 Ogwang, Adan, Bejon and Kapulu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodney Ogwang, cm9nd2FuZ0BrZW1yaS13ZWxsY29tZS5vcmc=; Melissa C. Kapulu, bWthcHVsdUBrZW1yaS13ZWxsY29tZS5vcmc=
 Rodney Ogwang
Rodney Ogwang Mohamed Adan1
Mohamed Adan1 Philip Bejon
Philip Bejon Melissa C. Kapulu
Melissa C. Kapulu