- 1Department of Basic Medical Sciences, Qinghai University Medical College, Xining, Qinghai, China
- 2Department of Medical Laboratory, Qinghai Provincial People’s Hospital, Xining, Qinghai, China
- 3State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, Qinghai, China
- 4Research Center for High Altitude Medicine, Qinghai University, Xining, Qinghai, China
- 5Key Laboratory of the Ministry of High Altitude Medicine, Qinghai University, Xining, Qinghai, China
By Wang T, Sun J, Wang L, Lin Y, Wu Z, Jia Q, Zhang S, An J, Ma X, Wu Q, Su Z and Wang H (2025) Front. Immunol. 16:1529710. doi: 10.3389/fimmu.2025.1529710
There was a mistake in Figure 7F as published. The error was caused by the use of placeholder images during the typesetting process to maintain layout consistency; due to oversight, some placeholders were not replaced with the correct images. The corrected Figure 7F appears below.
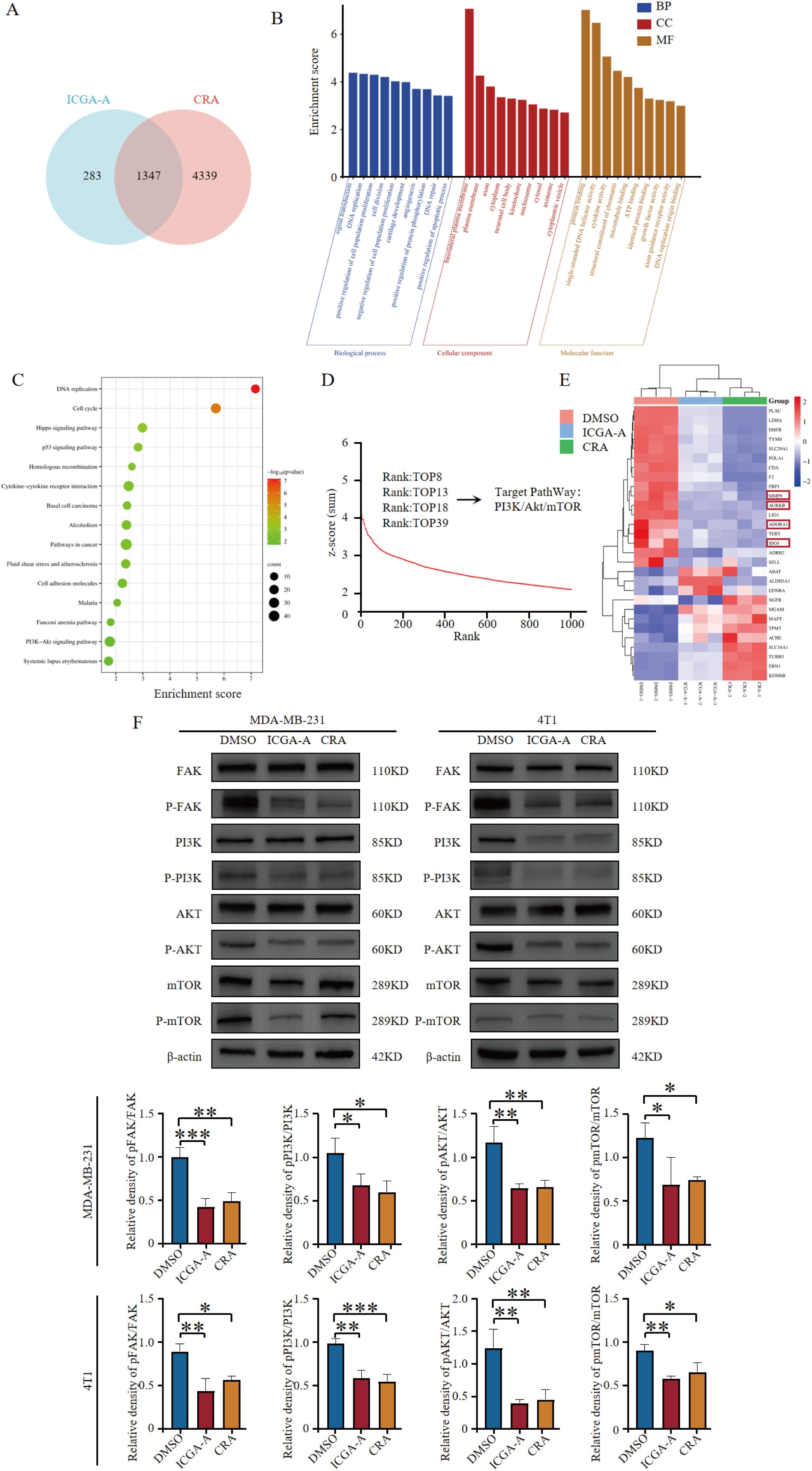
Figure 7. ICGA-A and CRA inhibited mRNA and protein expression levels of metabolic-associated proteins and pathways. (A) Venn diagram of DEGs in MDA-MB-231 cells treated with ICGA-A and CRA. (B) The top 10 GO terms in the BP, CC, and MF classifications of overlapping genes with MDA-MB-231. The x-axis represents the enriched terms, and the y-axis represents the enrichment score. (C) Top 20 KEGG pathways. KEGG pathway enrichment for the overlapping genes with MDA-MB-231. The x-axis represents the gene ratio (p < 0.05), and the y-axis represents the enriched terms. (D) The ranking of ICGA-A scored by HTS2 in a library of over 20,000 compounds. (E) Heatmap of the intersecting genes between RNA-seq and network pharmacology targets. (F) ICGA-A and CRA decreased the phosphorylation levels of FAK, PI3K, AKT, and mTOR. *p < 0.05, **p < 0.01, ***p < 0.001 vs. DMSO.
The original version of this article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: immune checkpoint blockade, Taraxacum officinale, PD-1/PD-L1 inhibitor 2, tumor microenvironments, triple-negative breast cancer
Citation: Wang T, Sun J, Wang L, Lin Y, Wu Z, Jia Q, Zhang S, An J, Ma X, Wu Q, Su Z and Wang H (2025) Correction: Therapeutic potential of isochlorogenic acid A from Taraxacum officinale in improving immune response and enhancing the efficacy of PD-1/PD-L1 blockade in triple-negative breast cancer. Front. Immunol. 16:1672972. doi: 10.3389/fimmu.2025.1672972
Received: 25 July 2025; Accepted: 27 August 2025;
Published: 02 September 2025.
Edited and reviewed by:
Hridayesh Prakash, Amity University, IndiaCopyright © 2025 Wang, Sun, Wang, Lin, Wu, Jia, Zhang, An, Ma, Wu, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Wang, d2FuZ2hhaXlhbkBxaHUuZWR1LmNu
 Tangyi Wang
Tangyi Wang Jingwei Sun
Jingwei Sun Li Wang1
Li Wang1 Yuxin Lin
Yuxin Lin Zhijing Wu
Zhijing Wu Qiangqiang Jia
Qiangqiang Jia Shoude Zhang
Shoude Zhang Juan An
Juan An Xueman Ma
Xueman Ma Qiong Wu
Qiong Wu Zhanhai Su
Zhanhai Su Haiyan Wang
Haiyan Wang