- 1Department of Pediatric Neurology, Tianjin Children’s Hospital/Children’s Hospital, Tianjin University, Tianjin, China
- 2Department of Neuroelectrophysiology, Pediatric Neurology, Tianjin Children’s Hospital/Children’s Hospital, Tianjin University, Tianjin, China
Influenza A (H1N1) virus infection has been associated with various immune-mediated neurological disorders. Previous studies have reported cases of stiff person spectrum disorder (SPSD) subsequent to viral infections. We report a case of a 4-year-old boy who developed SPSD following H1N1 infection. The patient primarily presented with axial and limb muscle rigidity, painful spasms, and exaggerated startle responses. Electromyography (EMG) revealed continuous synchronous discharges in agonist and antagonist muscles, consistent with the characteristic manifestations of stiff person syndrome (SPS), meanwhile, repetitive compound muscle action potentials (R-CMAPs) following stimulation of the median, common peroneal and tibial nerves were observed on electroneurography (ENG). Symptoms markedly improved following benzodiazepine therapy, and complete remission was achieved after combined treatment with IVIG and glucocorticoids.
1 Introduction
Stiff person spectrum disorder (SPSD) is a rare group of neuroimmunological disorders characterized by progressive rigidity and triggered painful spasms of the axial musculature. The clinical spectrum varies from classical stiff person syndrome (SPS), stiff limb (or leg) syndrome (SLS), to progressive encephalomyelitis with rigidity and myoclonus (PERM) (1, 2). Antibodies associated with SPSD include anti-GAD65 antibodies usually found in classic SPS, anti-glycine receptor (anti-GlyR) antibodies commonly found in PERM, antibodies against amphiphysin/dipeptidyl-peptidase-like protein(DPPX) in subsets, including paraneoplastic, etc. H1N1 virus infection is associated with seizures, influenza-associated encephalopathy or encephalitis (IAE) (3), acute disseminated encephalomyelitis (ADEM), Guillain-Barre syndrome (4). We report a case of a 4-year-old boy who developed SPSD following H1N1 infection. The patient primarily presented with axial and limb muscle rigidity, painful spasms, and exaggerated startle responses. Electromyography (EMG) revealed continuous synchronous discharges in agonist and antagonist muscles, consistent with the characteristic manifestations of stiff person syndrome (SPS). Symptoms remission was achieved after treatment with Intravenous Immunoglobulin (IVIG), glucocorticoids and benzodiazepine. SPSD is exceedingly rare in the pediatric population, early immunomodulatory treatment is critical for improving long-term neurological outcomes.
2 Case presentation
A 4-year-old boy was admitted to our hospital during the local H1N1 epidemic with a 7-day history of intermittent fever and 1-day history of pain in the right lower extremity, accompanied by abnormal gait. On the second day of admission, muscular hypertonia was observed in the extremities, neck muscles, and abdominal muscles, resulting in a posture characterized by flexion of the upper limbs and extension of the lower limbs. The patient demonstrated difficulty in sitting up, reduced facial expression, and a tense, restless mental state. External stimuli exacerbated rigidity, and somatosensory stimulation could trigger a startle-like response. However, the pharyngeal and laryngeal muscle groups were not involved, as evidenced by preserved speech and swallowing functions. The symptoms showed mild alleviation during sleep. Partial improvement was observed following diazepam administration.
The patient was the first child of non-consanguineous Chinese parents, born at full term with normal birth parameters. Age-appropriate psychomotor development was noted. He has no previous history of other illnesses or medication use. No family history of genetic disorders was reported.
During examination, the patient was alert and anxious, presented with a forced head position but was fully conscious and articulate. The patient displayed hyperreflexia and exaggerated startle response to noise, tactile (such as light touch), accompanied by pain. Repeated stimuli consistently elicited these startle reactions. General responsiveness was preserved, with no observed rash, cyanosis, jaundice, or herpetic lesions. Cranial nerve examination revealed no abnormalities. Lead-pipe rigidity was noted in both lower extremities, while the upper limbs exhibited a persistent flexed and rigid posture. Abdominal muscles rigidity was evident, presenting as a board-like rigidity with intact abdominal wall reflexes (+). Babinski’s sign was negative bilaterally. Though difficult to test, coordination appeared to be normal and a slight decline in muscle strength was detected. Nuchal rigidity was observed due to the markedly increased neck muscle tone.
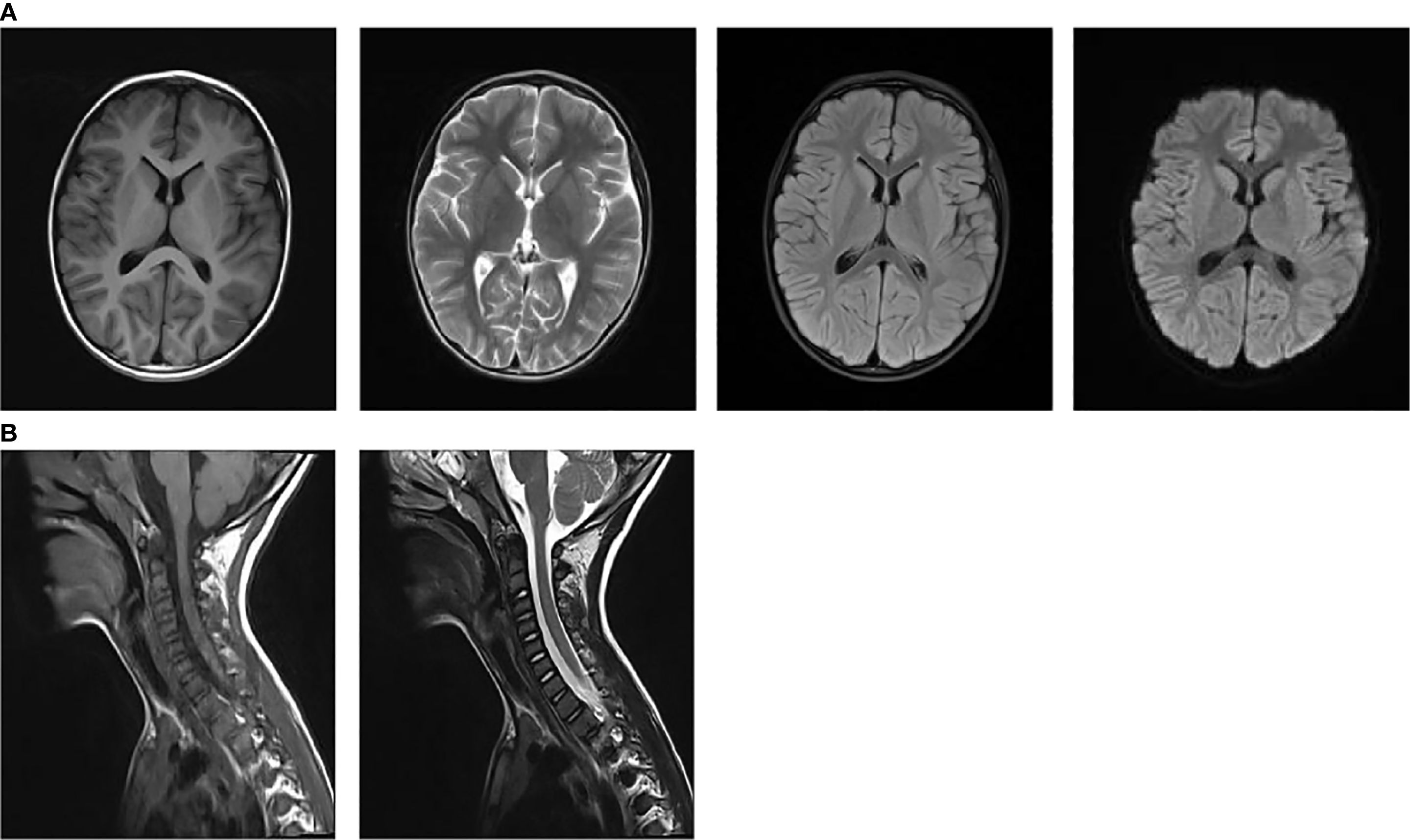
On admission, nasopharyngeal swab test was positive for H1N1. Routine laboratory exams showed mild neutrophilic leukocytosis and raised inflammatory markers (PCT 0.25 ng/ml, n.v. <0.05; interleukin 6 109.05 pg/ml, n.v. <7.1; interleukin 10 49.1pg/ml, n.v. <10.6). Chest radiography revealed increased and disorganized bilateral lung markings. Routine and biochemical cerebrospinal fluid (CSF) analysis were normal. CSF microbiological analyses were negative. Diagnostic work-up for malignancy with abdominal and testicular ultrasonography was unremarkable. Antinuclear antibody (ANA) and anti-extractable nuclear antigen antibody (ENA) testing yielded negative results. Thyroid function and thyroid ultrasonography showed no abnormalities.1.5 T magnetic resonance imaging (MRI) of the brain and cervical spinal cord were unremarkable at 1 week after symptoms onset (Figures 1A, B). Electroencephalogram (EEG) with polygraphy excluded a cortical origin of myoclonus. Needle EMG showed continuous motor unit activity (CMUA) and co-activation of agonist and antagonist muscles with normal-appearing motor unit potentials (MUPs), particularly in the lower extremities (Figure 2A). R-CMAPs were recorded by stimulating the median, tibial, and common peroneal nerves for approximately 20–50 milliseconds (Figure 2B).
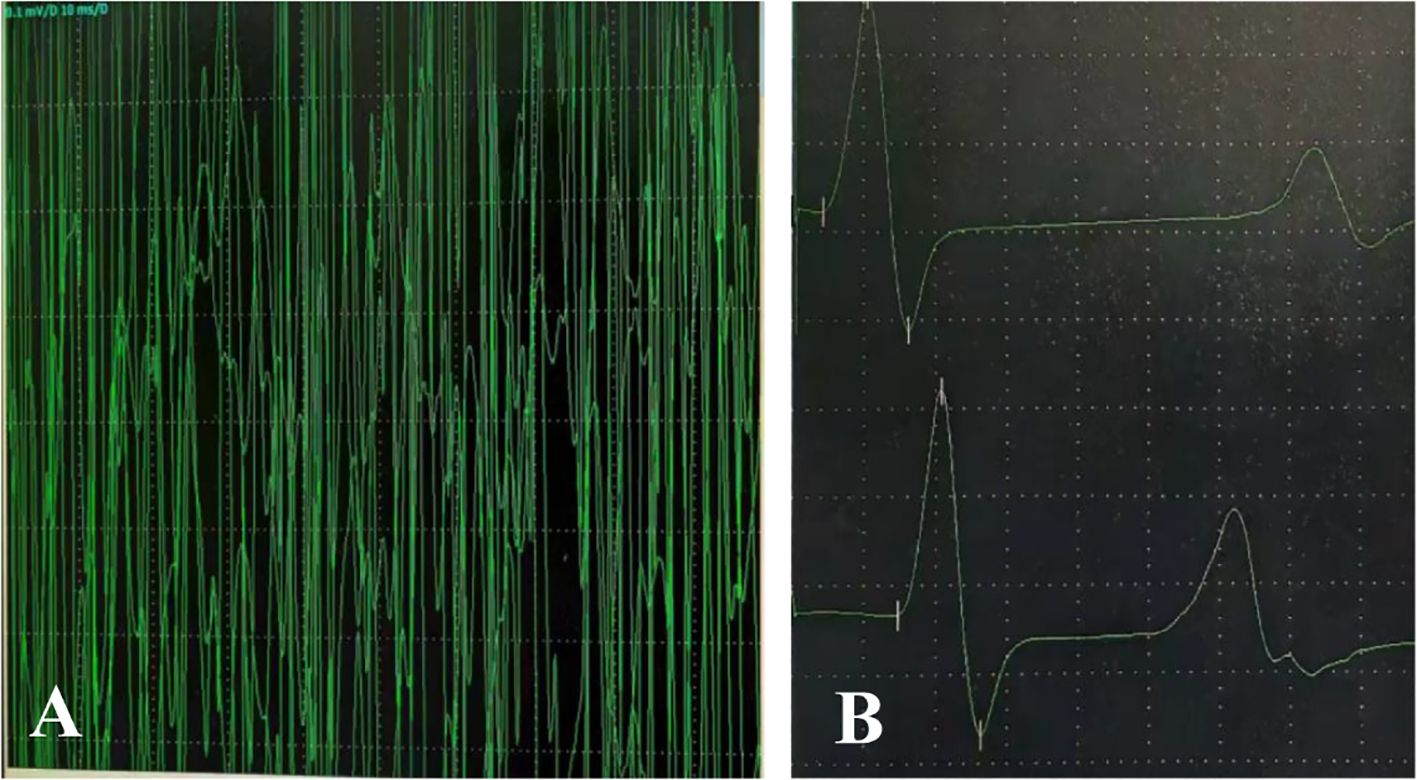
Figure 2. Continuous discharges were recorded in the gastrocnemius muscle (antagonist) during contraction of the tibialis anterior muscle (agonist) (C). Upon electrical stimulation of the tibial nerve, repetitive compound muscle action potentials (R-CMAPs) with slightly reduced amplitude were observed after 20–50 milliseconds (D).
Antibodies were detected by indirect immunofluorescence test (IIFT), yielded negative results in both serum and CSF(NMDAR, AMPAR, LGI1, CASPR2, GABAAR, GABABR, IgLON5, DPPX, GlyRα1, mGluR5, D2R, Neurexin-3α, GAD65, KLHL11, AK5), and also paraneoplastic antibody panels(Hu, Yo, Ri, MA2, CV2, Amphiphysin, ANNA-3, Tr, PCA-2) in serum. The negative antibody results in this case may be attributable to several factors: undetected known antibodies, unidentified novel antibodies, cell-mediated immune mechanisms, or low-titer antibodies.
Differential diagnosis was systematically performed. The patient had no history of skin trauma and did not exhibit typical tetanus manifestations such as trismus or hydrophobia, and the effectiveness of benzodiazepine therapy further ruled out tetanus. Neuromyotonia is electrophysiologically characterized by spontaneous, continuous motor unit discharges of high firing frequencies (150–300 Hz), which were not observed in this case.
The patient presented with stiffness (axial or limb), episodic spasms triggered by noises, tactile stimuli, emotional stress, increased muscle tone (axial or limb), concurrent stiffness of abdominal muscles, co-contraction of agonist/antagonist muscles by EMG, after exclusion of other differential diagnoses, he met the diagnostic criteria for probable SPSD, with benzodiazepine responsiveness as supportive (5).
On the 3rd day of admission, the patient was treated with IVIG at a total dose of 2 g/kg in 3 days as well as corticosteroids (5 mg/kg/day intravenous methylprednisolone, followed by a tapered reduction) for 10 days with significant efficacy. Oral diazepam was administered (2.5mg/d). Oseltamivir for influenza virus infection was implemented. The patient had no neurological sequel at the time of discharge (Figure 3). Given the negative antibody results during the initial phase and complete clinical resolution at follow-up, antibody re-testing was not performed. All medications were discontinued one-month post-discharge, with the patient exhibiting normal motor function and attending regular kindergarten without restrictions.
3 Discussion
Chia et al. (5) in 2023 proposed possible diagnostic criteria for SPSD while highlighting the particular importance of electrophysiological diagnostic testing for the minority of seronegative SPSD cases. EMG is highly specific for the hallmark findings of sustained co-contraction of agonist and antagonist muscles, as well as continuous motor unit activity (CMUA), regardless of body region tested (6, 7). This phenomenon stems from the dysfunction of normal physiological GABAergic inhibitory mechanisms, resulting in continuous firing of gamma motor neurons due to deficiency of inhibitory signals, which leads to overstimulation of muscle spindles, clinically expressed as stiffness (8, 9). Following treatment with benzodiazepines, the motor unit potential may decrease or even disappear. In this patient, CMUA were observed in the antagonist muscle during agonist contraction (Supplementary Video 1). Unfortunately, simultaneous recording from the agonist muscle was not performed, and diazepam was not administered during the examination. However, subsequent administration of the medication in the ward showed marked alleviation. Future studies on similar cases should prioritize the use of dual-channel synchronous recording techniques to obtain more definitive evidence. Given the practical challenges of performing paraspinal EMG in pediatric patients, future cases could attempt to record paraspinal muscle activity using surface electrodes during electroencephalography (EEG) monitoring. According to previous studies, nearly all SPS patients exhibit spasmodic reflex myoclonus, manifested on EMG as reflexive repetitive electrical activity occurring 50-80ms after median or tibial nerve stimulation and lasting several seconds (10). This phenomenon further indicates motor unit hyperexcitability. Notably, ENG revealed repetitive compound muscle action potentials (R-CMAPs) after stimulation of the common peroneal and tibial nerves in this patient (Figure 2B).
Specific triggers for SPSD are still unknown, except for paraneoplastic associations. Although virus infections can trigger several autoimmune diseases, there is currently bare evidence supporting their involvement in SPSD. A case of GAD antibody-positive SPS was described following West Nile virus (WNV) infection (11). Dalakas et al. (12) reported a 39-year-old male patient who initially presented with mild stiff-leg syndrome (SLS) progressed to severe generalized SPS one week after the onset of COVID-19. Neo and colleagues (13)reported a case of SPS with cerebellar features in a 47-year-old female with SARS-CoV-2 infection. Furthermore, a case of PERM associated with GlyR antibodies has been described after SARS-CoV-2 infection (14). In a separate case, PERM manifestation occurred after varicella zoster virus (VZV) recurrence, accompanied by positive serum anti-GlyR antibodies. In our department, another pediatric case was observed, in whom symptoms of SPS emerged following mycoplasma infection, with a transiently positive anti-GlyR antibody in CSF, suggesting a potential post-infectious immune-mediated mechanism.
To our knowledge, this is the first H1N1-associated SPSD. Although the observed association does not necessarily imply causation, the temporal onset of SPSD symptoms, approximately one week after H1N1 infection, strongly suggests a virally triggered autoimmune or post-viral inflammatory process. The patient’s sustained response to IVIG further supports the hypothesis of H1N1-induced immune dysregulation. Unfortunately, convalescent serum samples were not collected. Consequently, it was not possible to provide more direct serological evidence to support the hypothesis that H1N1 infection triggers an autoimmune mechanism. This is one of the limitations of the study. In addition, anti-thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TGAb) should be tested despite normal thyroid function. The short interval between infection and neurological symptom onset may be attributed to a virus-triggered underlying autoimmune reaction, which could induce disease. This phenomenon has been documented in various autoimmune disorders following H1N1 infection, including immediately post-infectious GBS (15, 16), ADEM (17, 18), or acute necrotizing encephalopathy (ANE) (19).
We hypothesize this patient developed a post-infectious immune-mediated process predominantly affecting GABAergic neurons following acute H1N1 infection. The sequence or structural similarity between microbial antigenic epitopes may trigger an immune response, potentially leading to autoimmune diseases. This cross-reactivity and molecular mimicry can amplify specific immune responses, this possibility has been previously proposed in a case of SPS manifested after WNV infection, based on partial amino acid sequence homology between GAD65 and WNV (11). Concurrently, H1N1 viral infection may disrupt the blood-brain barrier (BBB), facilitating the entry of circulating autoantibodies or inflammatory cells into the central nervous system (CNS) (20). An infection-triggered immune response was considered a potential contributing factor, due to a favorable response to IVIG and glucocorticoid therapy, with significant symptom alleviation within a short period, confirming the presence of an immune-mediated mechanism in this case. However, further clinical observations and laboratory data are required to substantiate this hypothesis.
Currently, there is no specific treatment plan for SPSD. Among the etiological treatments, IVIG and steroid therapy are frequently attempted in clinical practice. Plasma exchange and rituximab are also commonly used. Symptomatic treatment aims to overcome the impairment of GABAergic neurotransmission to alleviate stiffness and spasms in patients. This may include benzodiazepines (such as diazepam or clonazepam), baclofen, as well as antiepileptic drugs that enhance GABA synthesis or facilitate GABAergic transmission. Clonidine has also been reported in the literature. A recent study described the successful use of clonidine in a patient with refractory stiff-person syndrome who presented with severe rigidity and painful spasms and did not respond to conventional symptomatic medications (21).
The natural course of SPSD typically exhibits slow progression. In this case, rapid clinical improvement was achieved following immunotherapy combined with diazepam, and no relapse was observed during the eight-month follow-up period. This favorable outcome may be attributed to early diagnosis and prompt immunotherapy. However, the possibility remains that the pathophysiology in such patients may differ from that of classical SPSD. Further clinical data and long-term follow-up studies are required to validate this hypothesis. For such patients, heightened vigilance must be maintained at the emergence of symptoms resembling SPSD following viral infection. Clinical management should prioritize early administration of antiviral agents and appropriate immunomodulators, accompanied by prompt EMG examinations, MRI, and antibody screening. It is particularly noteworthy that, given the prolonged duration required for autoantibody detection, empirical immunotherapy should be initiated as early as possible based on clinical manifestations after excluding other etiologies, with the aim of improving long-term prognosis in patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Tianjin Children’s Hospital/Children’s Hospital, Tianjin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL: Visualization, Writing – original draft, Data curation, Writing – review & editing. ML: Writing – original draft, Writing – review & editing, Formal Analysis, Data curation. MT: Data curation, Writing – review & editing. YZ: Writing – review & editing, Investigation. XY: Writing – review & editing, Supervision, Conceptualization. DL: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank our patient for consenting to the publication of this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1673538/full#supplementary-material
Supplementary Video 1 | Insertional activity was observed during the initial 1–4 seconds of needle electromyography (EMG) recording. From 5 to 15 seconds, continuous motor unit activity was recorded in the gastrocnemius muscle (antagonist) during voluntary contraction of the tibialis anterior muscle (agonist). After 16 seconds, while in the resting state, EMG activity with normal MUP morphology was still observed.
References
1. Martinez-Hernandez E, Ariño H, McKeon A, Iizuka T, Titulaer MJ, Simabukuro MM, et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol. (2016) 73:714–20. doi: 10.1001/jamaneurol.2016.0133
2. Newsome SD and Johnson T. Stiff person syndrome spectrum disorders; more than meets the eye. J Neuroimmunol. (2022) 369:577915. doi: 10.1016/j.jneuroim.2022.577915
3. Steininger C, Popow-Kraupp T, Laferl H, Seiser A, Gödl I, Djamshidian S, et al. Acute encephalopathy associated with influenza A virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. (2003) 36:567–74. doi: 10.1086/367623
4. Yamana M, Kuwahara M, Fukumoto Y, Yoshikawa K, Takada K, and Kusunoki S. Guillain-Barré syndrome and related diseases after influenza virus infection. Neurol Neuroimmunol Neuroinflammation. (2019) 6:e575. doi: 10.1212/NXI.0000000000000575
5. Chia NH, McKeon A, Dalakas MC, Flanagan EP, Bower JH, Klassen BT, et al. Stiff person spectrum disorder diagnosis, misdiagnosis, and suggested diagnostic criteria. Ann Clin Transl Neurol. (2023) 10:1083–94. doi: 10.1002/acn3.51791
6. Roy S, Huang Y, Hu C, Fitzgerald KC, Wang Y, and Newsome SD. Core diagnostic features of stiff person syndrome: insights from a case-control study. J Neurol. (2025) 272:377. doi: 10.1007/s00415-025-13103-2
7. Moura J, Rocchi L, Zandi M, Balint B, Bhatia KP, and Latorre A. Neurophysiological insights into the pathophysiology of stiff-person spectrum disorders. Mov Disord Clin Pract. (2025) 12:409–17. doi: 10.1002/mdc3.14328
8. Dalakas MC. Stiff-person syndrome and GAD antibody-spectrum disorders: GABAergic neuronal excitability, immunopathogenesis and update on antibody therapies. Neurother J Am Soc Exp Neurother. (2022) 19:832–47. doi: 10.1007/s13311-022-01188-w
9. Sandbrink F, Syed NA, Fujii MD, Dalakas MC, and Floeter MK. Motor cortex excitability in stiff-person syndrome. Brain J Neurol. (2000) 123:2231–9. doi: 10.1093/brain/123.11.2231
11. Hassin-Baer S, Kirson ED, Shulman L, Buchman AS, Bin H, Hindiyeh M, et al. Stiff-person syndrome following West Nile fever. Arch Neurol. (2004) 61:938–41. doi: 10.1001/archneur.61.6.938
12. Dalakas MC. Severe stiff-person syndrome after COVID: the first video-documented COVID exacerbation and viral implications. Neurol Neuroimmunol Neuroinflammation. (2024) 11:e200192. doi: 10.1212/NXI.0000000000200192
13. Neo S, Lunn MP, and Bhatia KP. Infections and stiff-person spectrum disorders. Mov Disord Clin Pract. (2024) 11:590–1. doi: 10.1002/mdc3.14004
14. Giacomozzi S, Barone V, Merli E, Contardi S, Ricciardiello F, Giannoccaro MP, et al. Glycine receptor antibody-associated progressive encephalomyelitis with rigidity and myoclonus (PERM) during SARS-CoV-2 infection: a video-case report. Mov Disord Clin Pract. (2023) 10:824–6. doi: 10.1002/mdc3.13712
15. Sivadon-Tardy V, Orlikowski D, Porcher R, Sharshar T, Durand MC, Enouf V, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. (2009) 48:48–56. doi: 10.1086/594124
16. Grisanti SG, Franciotta D, Garnero M, Zuppa A, Massa F, Mobilia EM, et al. A case series of parainfectious Guillain-Barré syndrome linked to influenza A (H1N1) virus infection. J Neuroimmunol. (2021) 357:577605. doi: 10.1016/j.jneuroim.2021.577605
17. Ozkale Y, Erol I, Ozkale M, Demir S, and Alehan F. Acute disseminated encephalomyelitis associated with influenza A H1N1 infection. Pediatr Neurol. (2012) 47(1):62–64. doi: 10.1016/j.pediatrneurol.2012.03.019
18. Athauda D, Andrews TC, Holmes PA, and Howard RS. Multiphasic acute disseminated encephalomyelitis (ADEM) following influenza type A (swine specific H1N1). J Neurol. (2012) 259:775–8. doi: 10.1007/s00415-011-6258-8
19. Bartolini L, Ricci S, Azzari C, Moriondo M, Nieddu F, L’Erario M, et al. Severe A(H1N1)pdm09 influenza acute encephalopathy outbreak in children in Tuscany, Italy, December 2023 to January 2024. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. (2024) 29:2400199. doi: 10.2807/1560-7917.ES.2024.29.17.2400199
20. Ichiyama T, Morishima T, Isumi H, Matsufuji H, Matsubara T, and Furukawa S. Analysis of cytokine levels and NF-kappaB activation in peripheral blood mononuclear cells in influenza virus-associated encephalopathy. Cytokine. (2004) 27:31–7. doi: 10.1016/j.cyto.2004.03.012
Keywords: stiff person spectrum disorder, influenza A, autoimmunity, electromyography, immunotherapy
Citation: Liu X, Lei M, Tian M, Zhang Y, Yu X and Li D (2025) Case Report: A case of influenza A infection-associated stiff person spectrum disorder with favorable outcome. Front. Immunol. 16:1673538. doi: 10.3389/fimmu.2025.1673538
Received: 26 July 2025; Accepted: 29 September 2025;
Published: 14 October 2025.
Edited by:
Vincenzo Di Stefano, University of Palermo, ItalyReviewed by:
Umberto Quartetti, University of Palermo, ItalySalvatore Maria Lima, University of Palermo, Italy
Copyright © 2025 Liu, Lei, Tian, Zhang, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Yu, eXhsbml1QDE2My5jb20=; Dong Li, dGpla3NqQDE2My5jb20=
†These authors have contributed equally to this work
 Xiaoqian Liu
Xiaoqian Liu Meifang Lei
Meifang Lei Mengna Tian1
Mengna Tian1 Xiaoli Yu
Xiaoli Yu