- 1Department of Molecular Bioscience, College of Biomedical Science, Kangwon National University, Chuncheon, Republic of Korea
- 2Division of Biomedical Convergence, College of Biomedical Science, Kangwon National University, Chuncheon, Republic of Korea
- 3Multidimensional Genomics Research Center, Kangwon National University, Chuncheon, Republic of Korea
Adoptive cell therapy has progressed rapidly following the success of CAR-T therapy, with NK cells emerging as strong candidates for next-generation treatments. NK cells naturally recognize abnormal cells without genetic modification and do not cause graft-versus-host disease, making them suitable for mass production from donor blood. Traditional NK cell expansion often relies on cancer-derived feeder cells, posing safety and quality concerns. This review examines both feeder cell-based and feeder-free NK cell culture methods. We analyze the limitations of feeder-based approaches and categorize feeder-free strategies, including cytokine combinations, antibody stimulation, blood components, nanoparticles, and hydrogels. Among these, nanoparticle-based methods show strong potential for enabling safe, scalable, and standardized NK cell expansion without the risks associated with feeder cells. In this paper, we review recent advancements in NK cell culture techniques and propose the potential of nanoparticle technology in developing feeder-free NK cell culture methods.
1 Introduction
Cancer immunotherapy, represented by immune checkpoint inhibitors (ICIs), has revolutionized cancer treatment by showing the potential for durable responses with relatively low toxicity (1). ICIs led some patients to complete remission, triggering diverse research about the curability of cancer through immune-based approaches. However, most patients fail to achieve long-term remission, prompting intense research into predictive biomarkers and combination strategies (2, 3). In this context, chimeric antigen receptor (CAR) T cell therapy has emerged as a breakthrough, particularly in hematological malignancies, demonstrating outstanding efficacy in patients refractory to conventional treatments (4). The therapeutic concept of expanding and infusing tumor-targeting cytotoxic lymphocytes has validated the potential of gene and cell therapies. Despite initial concerns about cytokine release syndrome and neurotoxicity, improved clinical management has rendered these adverse effects more controllable, shifting the focus toward broader accessibility and standardization of CAR therapies (5).
A major limitation of current CAR-T cell therapy lies in its autologous nature, requiring personalized manufacturing from patient-derived T cells (6). This process is time-consuming and often suboptimal in patients with advanced disease. As a solution, allogeneic cell therapy—leveraging immune cells from healthy donors—has gained attention as a promising strategy for off-the-shelf cellular products (7). Among candidate effector cells for allogeneic therapy, natural killer (NK) cells have advantages. Unlike T cells, NK cells do not induce graft-versus-host disease (GvHD) and are abundantly present in peripheral blood, enabling scalable isolation (8). Numerous studies have explored NK cells as next-generation therapeutic platforms, and recent clinical data have shown that CAR-NK cells can achieve antitumor efficacy comparable to CAR-T cells, highlighting the translational potential of NK cell-based immunotherapy (9).
A critical requirement for the clinical success of NK cell therapy is the development of efficient and safe expansion methods. Enhancing the fold expansion of NK cells not only improves therapeutic efficacy but also contributes to cost reduction and quality assurance (10). Compared with T cells, the ex vivo expansion of NK cells is generally more challenging. While T cells can be robustly activated and expanded using CD3/CD28 stimulation alone (11), NK cell activation requires the integration of multiple activating and inhibitory signals (12). This complexity makes it difficult to identify a single molecular combination equivalent to CD3/CD28 for NK cells. Consequently, substantial efforts continue to be directed toward the development of advanced NK cell culture methods that can achieve robust expansion while meeting the requirements for clinical applicability (13). This review summarizes the current advances in feeder-free NK cell expansion technologies and discusses future directions toward standardized NK cell manufacturing for adoptive immunotherapy. Since the experimental conditions and methods of data presentation vary across studies, direct comparison is challenging. However, we summarized the reported results of NK cell expansion focusing on fold expansion, NK cell purity, and clinical compatibility, which is able to help in understanding the current trends in NK cell culture strategies. To this end, various strategies have been proposed to optimize NK cell culture systems.
2 Feeder cell-based NK cell expansion
Feeder cells for NK cell expansion are mostly treated with γ-ray irradiation to inhibit their proliferation and induce them to express stress ligands that can help to stimulate NK cells (Figure 1) (14). Source of feeder cells are diverse including PBMCs, tumor cell lines and EBV-transformed lymphoblastoid cells. The process of utilizing PBMC as feeder cells involves isolating NK cells from PBMC and subsequently subjecting the remaining non-NK cells to γ-ray irradiation for utilization. This approach, utilizing normal cells from the bloodstream as feeder cells, offers enhanced stability compared to employing cancer cell lines (15, 16). NK cell expansions using γ-ray irradiated PBMCs show fold expansion ranging from 794 ± 115.6 (15) on day 21 and from 80–419 on day 12 (16). K562 cells are the most widely used feeder cell for NK cell expansion and diverse genetically engineered K562 (GeK562) cells have been developed to improve NK cell expansion by expressing ligands that can help. GeK562 cells expressing 4-1BB ligand, membrane-bound IL-15 (mbIL-15) and membrane-bound IL-21 (mbIL-21) showed superior NK cell expansion as used feeder cells rather than K562 cells [fold expansion: 842-fold/purity: 91.5%/clinical suitability: not mentioned] (17). Additionally, GeK562 cells expressing molecules such as OX40 ligand [fold expansion: 578.9-2,959.5 fold/purity: 90%/clinical suitability: not mentioned] (18), membrane-bound IL-18 [fold expansion: 9,860-fold/purity: ≥98%/clinical suitability: not mentioned] (19, 20), and HLA-E [fold expansion: 1,632.43–12,748 fold/purity: 80%/clinical suitability: not mentioned] (21, 22) have been developed. Various GeK562 cells have been developed, and although there are differences in NK cell expansion observed in each study, it is difficult to determine which GeK562 is better based on fold expansion values. This difficulty arises not only from differences in feeder cells but also from variations in experimental conditions such as culture medium (23).

Figure 1. The feeder cell-based culture method for anticancer treatment using human blood-derived NK cells. Feeder cells, including cancer cells, PBMCs, and EBV-transformed lymphoblastoid cells, are treated with γ-ray irradiation to inhibit proliferation and induce the expression of stress ligands. Genetically engineered feeder cells expressing NK cell–stimulatory molecules have also been developed and are widely used to enhance expansion efficiency. Expanded NK cells can be obtained by co-culturing blood-derived mononuclear cells with γ-irradiated feeder cells. Created with BioRender.com.
Other tumor cell lines have been developed as feeder cells by genetic engineering for effective NK cell stimulation. PC-3 cell-line (prostate cancer) derived feeder cells having genetic engineering to express prostate stem cell antigen (PSCA) with IL-2, 4-1BBL and mbIL-15-mutDAP12 fusion protein on the cell membrane. An example of this is PC3PSCA -derived feeder cells, which induced the expression of high affinity IL-2 receptor through upregulation of DNAM-1 and NKG2C, resulting in fold expansion values of 86.2 ± 38.8 for PC3PSCA -IL-2-4-1BBL-mIL15d (24). Lymphoblastoid cell-lines transformed by Epstein–Barr virus (EBV) infection was used as feeder cells for NK cell expansion and showed 490 ± 260-fold expansion on day 21 with enhanced cytotoxicity functions (25, 26). HuT 78 cell-line (cutaneous T cell lymphoma) was systemically investigated to maximize the NK cell fold expansion and an engineered form of feeder cells to express 4-1BB ligand, membrane-bound TNF-α and mbIL-21 showed over 2,000-fold expansion on day 21 (27). Although early studies on genetically engineered feeder cells investigated the co-expression of mbIL-15 and mbIL-21, current feeder-based NK cell expansion most commonly employs soluble IL-15 supplementation with feeder cells expressing mbIL-21. IL-15 is required continuously to sustain NK cell proliferation, but feeder cells are rapidly cleared within a few days of culture, meaning mbIL-15 expression is less effective. In contrast, IL-21 primarily acts in the initial stage of NK cell culture, and its early signaling has been shown to exert long-lasting effects throughout the entire expansion process (18).
The feeder cell-based NK cell expansion is the most widely used methods for NK cell studies including clinical trials due to its high fold expansion rates. However, this method is largely affected by the feeder cell quality thus, culture or storage conditions of the feeder cells should be tightly managed. For this reason, development of new NK cell expansion methods that can expand NK cells and be stored easily is required to the quality control of expanded NK cells for clinical use.
3 Feeder-free NK cell expansion
3.1 Cytokine combination
Diverse investigations have been conducted to expand NK cells without using feeder cells, and the most fundamental approach was to identify the optimal cytokine combination. Depending on the treatment schedule, concentration, and combination, cytokines differently effect on NK cell expansion and functions. Purified NK cells cultured with treatment of IL-15 alone showed a significant increase in NK cell numbers during the initial 10 days, (all protocol with IL-15) whereas treatment with IL-21 alone failed to induce NK cell proliferation. NK cell culture using IL-15 treatment showed enhanced fold expansion under temporal IL-21 treatment at the beginning of NK cell culture (2- and 10- fold), it means that IL-21 alone is not sufficient to induce NK cell proliferation but can support to NK cell proliferation with treating other cytokines (28). Other cytokine combination including IL-2, IL-18, rIL-27 showed improved NK cell fold expansion (17.19 ± 4.85-fold) (29). Basically, IL-2 and IL-15 are essential cytokines showing mild NK cell proliferation when treated alone. IL-18, IL-21 and IL-27 are supported cytokines showing less NK cell proliferation when treated alone however, enhanced NK cell fold expansion when combined with essential cytokines. NK cells cultured with combination of essential with supported cytokines also showed increased expression of activation receptors and enhanced functions such as degranulation of cytotoxic vesicles and IFN-ɣ secretion under target cell stimulation.
3.2 Antibody stimulation
In T cell culture, effective proliferation can be induced using stimulation with anti-CD3/28 antibodies. Similarly, various studies have been conducted in NK cells to induce proliferation without feeder cells by utilizing combinations of agonist antibodies. Interestingly, the anti-CD3 agonist antibody clone OKT-3, commonly used for T cell proliferation, also exhibits efficacy in NK cell proliferation. This effect is attributed to the cytokines secreted by T cells in response to OKT-3 stimulation, which facilitate NK cell proliferation. NK cells cultured using OKT-3 stimulation from PBMC showed 700-fold expansion and 55% of NK cell purity (CD3-/CD56+) meaning OKT-3 alone is not ideal for replacing feeder cell-based NK cell culture (30). Antibody combination using minimal dose of OKT-3 (0.001 ~ 0.01 µg/mL) and 20 µg/mL anti-CD52 antibody showed improved NK cell expansion with around 60% of NK cell purity [fold expansion: ~1,000-fold/purity: ~60%/clinical suitability: possible] (31). Another antibody combination using OKT-3 with anti-CD16 agonistic monoclonal antibody increase the NK cell purity to 75.2 ± 16.3%, while T cells (CD3+/CD56-) decreased from 66.5 ± 7.8% to 16.5 ± 6.2% after expansion [fold expansion: 352 ± 344-fold/purity: 75.2 ± 16.3%/clinical suitability: possible] (32). However, anti-CD16 stimulation showed wide range of donor variations (NK cell purity range 33.9 ~ 93.2%) and the variations was maintained regardless of anti-CD16 antibody clones (33). NK cell culture methods using OKT-3 have limited NK cell purity due to residual T cells, thus antibody combinations excluding OKT-3 have been studied for NK cell culture from T cell-depleted PBMCs. Stimulation of T cell-depleted PBMCs with anti-NKp46 and anti-CD16 antibody combination resulted in over 98% of NK cell purity with 1,000-fold expansion (34). Both NK cell purity and fold expansion are superior compared to other antibody combinations, but differences in culture reagents, such as the type of culture media and cytokine combinations, also influence the results.
3.3 Human blood derived components
The use of serum, human serum (HS), or fetal bovine serum (FBS), in cell culture carries the risk of allergic reactions, and its composition is not well characterized, making it difficult to maintain consistency in culture conditions and performance during manufacturing processes. Autologous plasma as a culture supplement is best to Xeno-free culture media and it showed prominent NK cell expansion [fold expansion: 496.5 ± 55.0-fold/purity: 99.0 ± 0.6%/clinical suitability: possible] (35). However, securing enough amount of plasma does not come easy thus, investigation of replacement for the serum component is needed. In the study of stem cell-derived NK cell culture, human platelet lysate (hPL) was used instead of human serum. After NK cell differentiation and expansion from CD34+ stem cells in hPL- or HS-containing media, both media were capable of producing mature NK cells. However, hPL was found to promote greater NK cell proliferation and expression of activation receptors, such as CD16 and NKp46, indicating that hPL may aid in the generation of mature and cytotoxic NK cells (36). In other study showed that hPL containing media with Cloudz which is a stimulator of NK cells decorated with anti-CD2 and anti-NKp46 agonist antibodies can be used for NK cell expansion from PBMCs [fold expansion: 387 ± 100-fold/purity: 71% ± 15%/clinical suitability: possible] (37). These results mean that serum contained in NK cell culture media can be replaced to hPL for the Xeno-free protocol of NK cell expansion.
3.4 Nanoparticle
Nanomaterials are substances having nano sizes ranging from 1 nm to 200 nm, and they have been widely investigated for various research such as drug and gene delivery (38). Nanomaterials possess own unique properties following 1) the ability to have multivalent molecules on their surface, 2) the ease of modifying their functionality through chemical conjugation, 3) effectively uptake into targeted cells through the receptor-mediated endocytosis pathway (38–40). Recently, nanomaterial usages for NK cell expansion have been investigated to utilize their unique advantages (Figure 2).
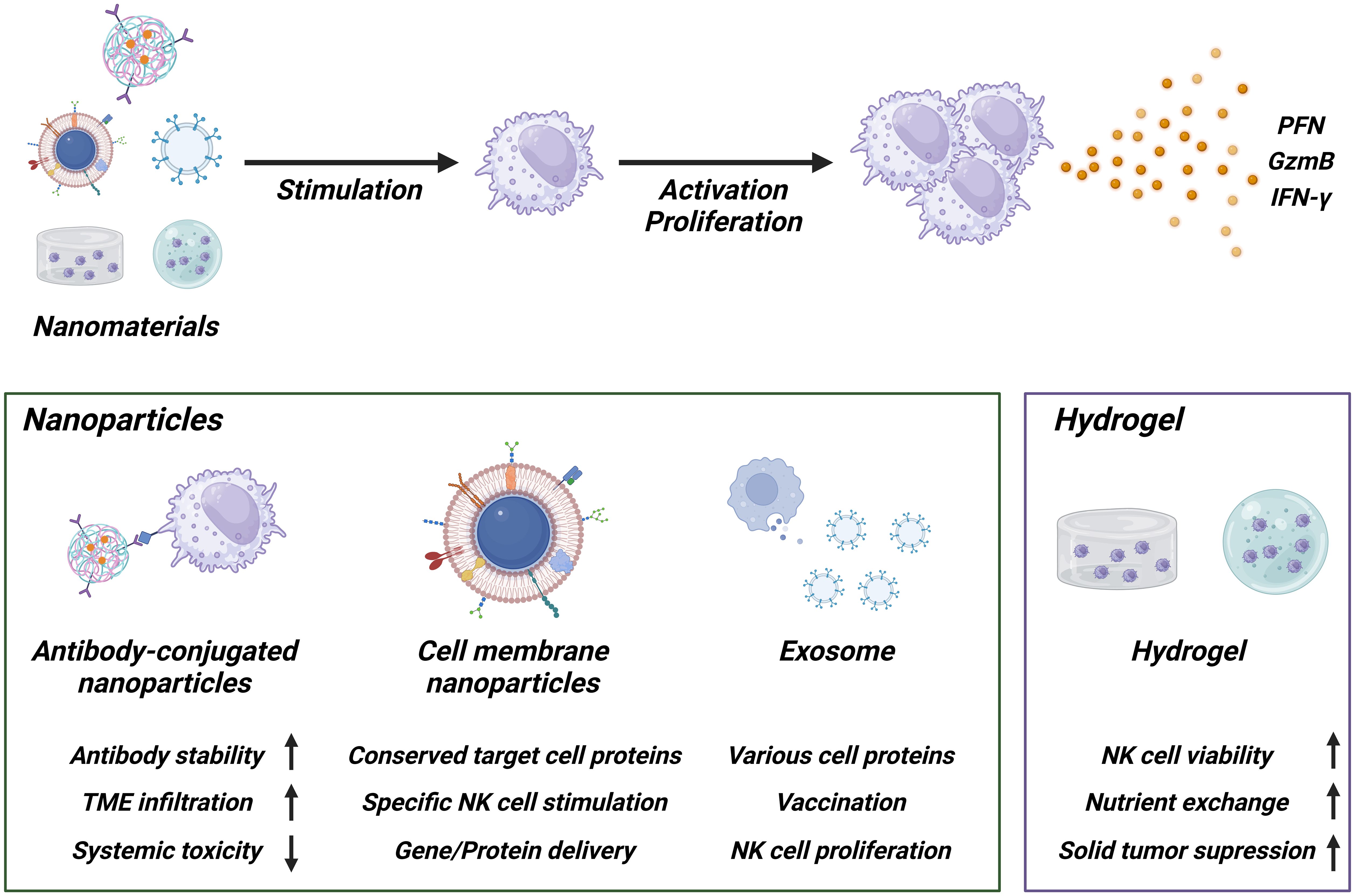
Figure 2. Schematic of NK cell activation using nanomaterials. Antibody-conjugated nanoparticles enhance the stability of antibodies, improve tumor microenvironment (TME) penetration, and reduce systemic toxicity. Cell membrane nanoparticles and exosomes maintain the protein expression profile of the originating cells, demonstrating high functionality and suitability for gene or protein delivery. Hydrogels help maintain NK cell functionality and survival within the TME condition, enhancing therapeutic efficacy against solid tumor. Created with BioRender.com.
One of the strategies is that fabrication of protein-conjugated nanogels. For instance, nanogels incorporating IL-21 and CD45 (ILNPs) were developed and shown to “hitchhike” NK cell. Incorporation of IL-21 allowed controlled activation in the tumor microenvironment, thereby reducing systemic toxicity and inducing IL-12-mediated innate immune activation (41). Beyond the chemical conjugation methods, NK cell expansion with cell membrane-derived nanomaterial has been attracted as next generation methods because it contains whole membrane protein profiles that are consistent with profiles of source cells, supporting recognition of target cell proteins (42, 43). The Park group developed cancer cell membrane-expressed magnetic nanoparticles that specifically stimulate NK cells and also functioned as gene delivery carriers to induce EGFR-CAR expression. Multifunctional nanoparticles (MF-NPs) coated with caffeic acid, polydopamine, and polyethyleneimine show reduced toxicity once they are effectively internalized into NK cells. They then induce the expression of EGFR-CAR on the NK cell surface due to their gene delivery carrier ability (44).
Feeder cell-mimicking strategies have also been reported. The Copik group demonstrated that feeder cell membrane-derived particles can be used to replace common feeder cell-based NK cell expansion methods. When plasma membrane particles (PM-particles) were used in place of live feeder cells, the results showed that NK cell expansion and NK cell phenotype were comparable (45). Similarly, the Wang group showed another application for nanomaterials-based NK cell expansion by developing cell membrane-encapsulated magnetic nanoparticles for activating NK cells and enhancing their antitumoral efficacy (46). Extracellular vesicles (EVs), another type of nanomaterial secreted from cells, contain various specific proteins from the original cells and have been investigated for use in vaccines, immune regulation, and other applications (40, 47). The Chaput group demonstrated that dendritic cell-derived exosomes (Dex) possessing functional MHC/peptide complexes act as promoters of T cell-dependent tumor rejection and supported NK cell proliferation and activation via IL-15Rα and NKG2D signaling (48).
Taken together, nanomaterials represent customizable feeder-free platforms that mimic cell-to-cell interactions and can enhance NK cell expansion while reducing toxicity. In addition, because they can be produced in a well-defined manner and stored long-term under GMP-compatible conditions, they hold promise for clinical translation, although further evaluation of safety, reproducibility, and cost is required.
3.5 Hydrogel
Hydrogel-based adoptive cell therapy using NK cells has also been explored, particularly against solid tumors. Hydrogels provide three-dimensional, biocompatible scaffolds that can encapsulate the immune cells with supportive factors, thereby preserving their viability and anti-tumor activity in the immunosuppressive tumor microenvironment (TME). While many studies have employed NK-92 cells as a model system, these approaches are broadly applicable to NK cell-based therapies.
For example, NK-92 cells encapsulated in sodium alginate/gelatin hydrogels (ALG/GEL-2) showed higher viability and cytotoxic function compared with cells cultured without hydrogels (49). Microspheres fabricated using PEO/ALG showed high permeability and efficient nutrient exchange, enabling NK-92MI cell survival rates of over 85% after 72 hours of culture, with viability maintained for up to 14 days. Encapsulated NK-92MI cells effectively destroyed tumor cells through secretion of perforin and granzyme and porous hydrogel microspheres further protected NK-92MI cells from the immune rejection response, supporting their survival and proliferation (50). Another study created NK-CellDex by encapsulating NK-92 cells engineered with CellDex into GelMA bioink-based hydrogels. These hydrogel capsules supported cell proliferation, increased viability, and maintained cell morphology similar to 2D culture, indicating that hydrogels can support NK cell expansion even after encapsulation (51).
Mechanistically, hydrogels help protect NK cells from TME-derived inhibitory signals, while supporting sustained nutrient exchange. They can also be engineered to deliver stimulatory molecules that enhance NK cell activity. Importantly, beyond serving as delivery vehicles, hydrogels provide a microenvironment that may promote NK cell proliferation in vivo, thereby offering the potential for feeder-free expansion strategies. Moreover, localized and sustained NK cell delivery could reduce systemic toxicity and enhance therapeutic efficacy.
Overall, hydrogel-based NK cell delivery systems represent promising and translatable feeder-free approaches. They offer advantages such as biocompatibility, modular design, and scalability, while also holding potential to contribute to NK cell expansion. Nonetheless, challenges such as regulatory approval and reproducibility remain to be addressed before clinical applications.
4 Conclusions
In summary, feeder-free strategies using cytokines, antibodies, and blood-derived components have advanced NK cell expansion but remain limited by inconsistent proliferation and reproducibility. Although defined cytokine- or antibody-based systems would be more suitable for clinical use, their development is made difficult by the complex signaling requirements of NK cells. As a result, feeder cell–based systems are still widely used, though their clinical translation raises safety and standardization concerns. Emerging nanomaterial- and hydrogel-based platforms provide GMP-compatible, storable alternatives that can deliver stimulatory cues, protect cells from immunosuppression, and sustain viability. These approaches represent new possibilities for overcoming current limitations and advancing NK cell therapies.
Author contributions
JL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. HJ: Writing – original draft. IN: Writing – original draft. JP: Conceptualization, Funding acquisition, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by multiple research grants. The following three grants were awarded to Park and contributed to the overall progress of this study as well as to NK cell culture research: 2024 Research Grant from Kangwon National University; the G-LAMP Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00301850); and the Basic Science Research Program through the NRF funded by the Ministry of Education (2022R1I1A1A01063081). In addition, this work was supported by an NRF grant funded by the Korea government (MSIT) (RS-2024-00453805) awarded to IN, which contributed to nanotechnology-convergence research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Topalian SL, Drake CG, and Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. (2015) 27:450–61. doi: 10.1016/j.ccell.2015.03.001
2. Marin-Acevedo JA, Kimbrough EO, and Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. (2021) 14:45. doi: 10.1186/s13045-021-01056-8
3. Cook AM, Lesterhuis WJ, Nowak AK, and Lake RA. Chemotherapy and immunotherapy: mapping the road ahead. Curr Opin Immunol. (2016) 39:23–9. doi: 10.1016/j.coi.2015.12.003
4. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
5. Chohan KL, Siegler EL, and Kenderian SS. CAR-T cell therapy: the efficacy and toxicity balance. Curr Hematol Malig Rep. (2023) 18:9–18. doi: 10.1007/s11899-023-00687-7
6. Moradi V, Omidkhoda A, and Ahmadbeigi N. The paths and challenges of “off-the-shelf” CAR-T cell therapy: An overview of clinical trials. BioMed Pharmacothe. (2023) 169:115888. doi: 10.1016/j.biopha.2023.115888
7. Jallouk AP, Sengsayadeth S, Savani BN, Dholaria B, and Oluwole O. Allogeneic and other innovative chimeric antigen receptor platforms. Clin Hematol Int. (2024) 6:61–72. doi: 10.46989/001c.121404
8. Berrien-Elliott MM, Jacobs MT, and Fehniger TA. Allogeneic natural killer cell therapy. Blood. (2023) 141:856–68. doi: 10.1182/blood.2022016200
9. Zhang L, Meng Y, Feng X, and Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. biomark Res. (2022) 10:12. doi: 10.1186/s40364-022-00364-6
10. Ojo EO, Sharma AA, Liu R, Moreton S, Checkley-Luttge MA, Gupta K, et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep. (2019) 9:14916. doi: 10.1038/s41598-019-51287-6
11. Chen Y, Zhao R, Fan Q, Liu M, Huang Y, and Shi G. Enhancing the activation of T cells through anti-CD3/CD28 magnetic beads by adjusting the antibody ratio. IUBMB Life. (2024) 76:1175–85. doi: 10.1002/iub.2898
12. Maia A, Tarannum M, Lérias JR, Piccinelli S, Borrego LM, Maeurer M, et al. Building a better defense: expanding and improving natural killer cells for adoptive cell therapy. Cells. (2024) 13:451. doi: 10.3390/cells13050451
13. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, and Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. (2021) 14:7. doi: 10.1186/s13045-020-01014-w
14. Llames S, Garcia-Perez E, Meana A, Larcher F, and del Río M. Feeder layer cell actions and applications. Tissue Eng Part T Rev. (2015) 21:345–53. doi: 10.1089/ten.TEB.2014.0547
15. Lee HR, Son CH, Koh EK, Bae JH, Kang CD, Yang K, et al. Expansion of cytotoxic natural killer cells using irradiated autologous peripheral blood mononuclear cells and anti-CD16 antibody. Sci Rep. (2017) 7:11075. doi: 10.1038/s41598-017-09259-1
16. Delso-Vallejo M, Kollet J, Koehl U, and Huppert V. Influence of irradiated peripheral blood mononuclear cells on both ex vivo proliferation of human natural killer cells and change in cellular property. Front Immunol. (2017) 8:854. doi: 10.3389/fimmu.2017.00854
17. Oberoi P, Kamenjarin K, Ossa JFV, Uherek B, Bönig H, and Wels WS. Directed differentiation of mobilized hematopoietic stem and progenitor cells into functional NK cells with enhanced antitumor activity. Cells (Basel). (2020) 9:811. doi: 10.3390/cells9040811
18. Kweon S, Phan MT, Chun S, Yu H, Kim J, Kim S, et al. Expansion of human NK cells using K562 cells expressing OX40 ligand and short exposure to IL-21. Front Immunol. (2019) 10:879. doi: 10.3389/fimmu.2019.00879
19. Thangaraj JL, Phan MT, Kweon S, Kim J, Lee JM, Hwang I, et al. Expansion of cytotoxic natural killer cells in multiple myeloma patients using K562 cells expressing OX40 ligand and membrane-bound IL-18 and IL-21. Cancer Immunol Immunother. (2022) 71:613–25. doi: 10.1007/s00262-021-02982-9
20. Senju H, Kumagai A, Nakamura Y, Yamaguchi H, Nakatomi K, Fukami S, et al. Effect of IL-18 on the expansion and phenotype of human natural killer cells: application to cancer immunotherapy. Int J Biol Sci. (2018) 14:331–40. doi: 10.7150/ijbs.22809
21. Phan MT, Kim J, Koh SK, Lim Y, Yu H, Lee M, et al. Selective expansion of NKG2C+ adaptive NK cells using K562 cells expressing HLA-E. Int J Mol Sci. (2022) 23:9426. doi: 10.3390/ijms23169426
22. Alekseeva NA, Streltsova MA, Vavilova JD, Ustiuzhanina MO, Palamarchuk AI, Boyko AA, et al. Obtaining gene-modified HLA-E-expressing feeder cells for stimulation of natural killer cells. Pharmaceutics. (2024) 16:133. doi: 10.3390/pharmaceutics16010133
23. Koh SK, Park J, Kim SE, Lim Y, Phan MT, Kim J, et al. Natural killer cell expansion and cytotoxicity differ depending on the culture medium used. Ann Lab Med. (2022) 42:638–49. doi: 10.3343/alm.2022.42.6.638
24. Michen S, Frosch J, Fussel M, Schackert G, Momburg F, and Temme A. Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self. Cytotherapy. (2020) 22:354–68. doi: 10.1016/j.jcyt.2020.02.004
25. Berg M, Lundqvist A, McCoy P Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. (2009) 11:341–55. doi: 10.1080/14653240902807034
26. Granzin M, Soltenborn S, Muller S, Kollet J, Berg M, Cerwenka A, et al. Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy. (2015) 17:621–32. doi: 10.1016/j.jcyt.2015.03.611
27. Min B, Yang B, Kim YS, Park GM, Kim H, Kim H, et al. Harnessing novel engineered feeder cells expressing activating molecules for optimal expansion of NK cells with potent antitumor activity. Cell Mol Immunol. (2022) 19:296–8. doi: 10.1038/s41423-021-00759-9
28. Wagner J, Pfannenstiel V, Waldmann A, Bergs JWJ, Brill B, Huenecke S, et al. A two-phase expansion protocol combining interleukin (IL)-15 and IL-21 improves natural killer cell proliferation and cytotoxicity against rhabdomyosarcoma. Front Immunol. (2017) 8:676. doi: 10.3389/fimmu.2017.00676
29. Choi YH, Lim EJ, Kim SW, Moon YW, Park KS, and An HJ. IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J Immunother Cancer. (2019) 7:1–12. doi: 10.1186/s40425-019-0652-7
30. Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. (2001) 62:1092–8. doi: 10.1016/s0198-8859(01)00313-5
31. Masuyama J, Murakami T, Iwamoto S, and Fujita S. Ex vivo expansion of natural killer cells from human peripheral blood mononuclear cells co-stimulated with anti-CD3 and anti-CD52 monoclonal antibodies. Cytotherapy. (2016) 18:80–90. doi: 10.1016/j.jcyt.2015.09.011
32. Chen M, Li Y, Wu Y, Xie S, Ma J, Yue J, et al. Anti-tumor activity of expanded PBMC-derived NK cells by feeder-free protocol in ovarian cancer. Cancers (Basel). (2021) 13:5866. doi: 10.3390/cancers13225866
33. Kim J, Phan MT, Hwang I, Park J, and Cho D. Comparison of the different anti-CD16 antibody clones in the activation and expansion of peripheral blood NK cells. Sci Rep. (2023) 13:9493. doi: 10.1038/s41598-023-36200-6
34. Nakazawa T, Maeoka R, Morimoto T, Matsuda R, Nakamura M, Nishimura F, et al. An efficient feeder-free and chemically-defined expansion strategy for highly purified natural killer cells derived from human cord blood. Regener Ther. (2023) 24:32–42. doi: 10.1016/j.reth.2023.05.006
35. Tanaka Y, Nakazawa T, Nakamura M, Nishimura F, Matsuda R, Omoto K, et al. Ex vivo-expanded highly purified natural killer cells in combination with temozolomide induce antitumor effects in human glioblastoma cells in vitro. PloS One. (2019) 14:e0212455. doi: 10.1371/journal.pone.0212455
36. Li C, Zhu H, Zhang L, Liu X, Ji Y, Zhang H, et al. Human platelet lysate as a substitute for serum in natural killer cell generation and expansion. Life Med. (2023) 2:lnad011. doi: 10.1093/lifemedi/lnad011
37. Johnson CDL, Zale NE, Frary ED, and Lomakin JA. Feeder-cell-free and serum-free expansion of natural killer cells using Cloudz microspheres, G-Rex6M, and human platelet lysate. Front Immunol. (2022) 13:803380. doi: 10.3389/fimmu.2022.803380
38. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, and Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. (2021) 20:101–24. doi: 10.1038/s41573-020-0090-8
39. Weissleder R, Kelly K, Sun EY, Shtatland T, and Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. (2005) 23:1418–23. doi: 10.1038/nbt1159
40. Noh I, Guo ZY, Zhou JR, Gao W, Fang RH, and Zhang L. Cellular nanodiscs made from bacterial outer membrane as a platform for antibacterial vaccination. ACS Nano. (2023) 17:1120–7. doi: 10.1021/acsnano.2c08360
41. Meng DD, Pan H, He W, Jiang X, Liang Z, Zhang X, et al. In situ activated NK cell as bio-orthogonal targeted live-cell nanocarrier augmented solid tumor immunotherapy. Adv Funct Mater. (2022) 32:2202603. doi: 10.1002/adfm.202202603
42. Fang RH, Kroll AV, Gao W, and Zhang L. Cell membrane coating nanotechnology. Adv Mater. (2018) 30:e1706759. doi: 10.1002/adma.201706759
43. Guo Z, Noh I, Zhu AT, Yu Y, Gao W, Fang RH, et al. Cancer cell membrane nanodiscs for antitumor vaccination. Nano Lett. (2023) 23:7941–9. doi: 10.1021/acs.nanolett.3c01775
44. Kim KS, Han JH, Park JH, Kim HK, Choi SH, Kim GR, et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials. (2019) 221:119418. doi: 10.1016/j.biomaterials.2019.119418
45. Oyer JL, Igarashi RY, Kulikowski AR, Colosimo DA, Solh MM, Zakari A, et al. Generation of highly cytotoxic natural killer cells for treatment of acute myelogenous leukemia using a feeder-free, particle-based approach. Biol Blood Marrow Transpl. (2015) 21:632–9. doi: 10.1016/j.bbmt.2014.12.037
46. Wu D, Shou X, Zhang Y, Li Z, Wu G, Wu D, et al. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomedicine. (2021) 32:102333. doi: 10.1016/j.nano.2020.102333
47. Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. (2023) 23:236–50. doi: 10.1038/s41577-022-00763-8
48. Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PloS One. (2009) 4:e4942. doi: 10.1371/journal.pone.0004942
49. Kim D, Jo S, Lee D, Kim SM, Seok JM, Yeo SJ, et al. NK cells encapsulated in micro/macropore-forming hydrogels via 3D bioprinting for tumor immunotherapy. Biomater Res. (2023) 27:60. doi: 10.1186/s40824-023-00403-9
50. Wu D, Yu Y, Zhao C, Shou X, Piao Y, Zhao X, et al. NK-cell-encapsulated porous microspheres via microfluidic electrospray for tumor immunotherapy. ACS Appl Mater Interf. (2019) 11:33716–24. doi: 10.1021/acsami.9b12816
Keywords: NK cell expansion, feeder-free, nanoparticle, adoptive cell therapy, allogenic cell therapy
Citation: Lee J, Kim M, Jeong H, Noh I and Park J (2025) Recent advances in feeder-free NK cell expansion as a future direction of adoptive cell therapy. Front. Immunol. 16:1675353. doi: 10.3389/fimmu.2025.1675353
Received: 29 July 2025; Accepted: 16 September 2025;
Published: 29 September 2025.
Edited by:
Youwei Wang, Tianjin University, ChinaReviewed by:
Joo Dong Park, Sungkyunkwan University Suwon, Republic of KoreaCopyright © 2025 Lee, Kim, Jeong, Noh and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeehun Park, amVlaHVuQGthbmd3b24uYWMua3I=
†These authors have contributed equally to this work
 Jimin Lee1†
Jimin Lee1† Jeehun Park
Jeehun Park