- 1Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 2Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 3Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins Medical Institutions, Baltimore, MD, United States
Ovarian cancer (OC) remains one of the most aggressive gynecological malignancies, with a five-year survival rate below 45% despite the recent advances in the introduction of targeted therapy. Moreover, immunotherapy, such as immune checkpoint inhibitors, does not improve the survival of OC patients. Lack of sufficient knowledge in understanding the complexity of the tumor microenvironment likely confers the treatment ineffectiveness. Recently, tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) have garnered research attention as they shape the tumor immune microenvironment, which plays a crucial role in disease progression and treatment response. This article reviews the complex roles of these innate immune cells in OC progression. TAMs represent a significant component of the immune infiltrate in OC, exhibiting considerable functional plasticity and can shift between anti-tumoral (M1) and pro-tumoral (M2) phenotypes. M2-like TAMs typically predominate in the tumor microenvironment, which aids in the development of immune suppression and disease progression. They also contribute to chemoresistance and metastasis; hence, their presence in tumors is associated with a worse prognosis. TANs, like TAMs, exhibit N1/N2 polarization and influence tumor progression through the formation of neutrophil extracellular traps. Understanding the biological interactions between various immune cells and cancer cells may offer new therapeutic opportunities. This review sheds light on the dynamic ecological transformation of the OC tumor microenvironment and highlights the potential of targeting TAM/TAN-mediated processes to improve OC treatment outcomes.
1 Introduction
Ovarian cancer (OC) ranks as the eighth most prevalent cancer worldwide, with approximately 313,959 new cases and 207,252 deaths reported each year (1, 2). The five-year cause-specific survival rate for OC varies significantly by stage, ranging from 90% in stage I and 70% in stage II, to 40% in stage III and as low as 20% in stage IV (3). In addition to the lack of early detection methods, late diagnosis often resulted in poor disease outcomes, including resistance to treatment and rapid disease progression. In fact, recurrence occurs in approximately 80% of OC patients (4). Because of the high relapse rates, subsequent treatments tend to be more toxic, significantly impacting patients’ quality of life and incurring substantial financial burdens (5).
OC displays significant heterogeneity, with diverse histological subtypes originating from different cell types within the ovary. These subtypes vary not only in their morphological and molecular characteristics but also in their behavior, prognosis, and response to treatment. OC can arise from different ovarian tissues, including epithelial, mesenchymal, sex cord stromal, and germ cells. Epithelial tumors account for over 95% of all ovarian malignancies, while stromal and germ cell tumors collectively make up the remaining 5% (6). Among the epithelial tumors, approximately 80% are high-grade serous carcinoma (HGSC), with 75% of these cases diagnosed at FIGO stages III and IV. The remaining 20% includes low-grade serous carcinoma (LGSC), endometrioid, mucinous, clear cell, as well as mixed and undifferentiated carcinomas (7, 8). These OC subtypes can be broadly categorized into two groups based on genetic, molecular, and pathological characteristics (Figure 1).
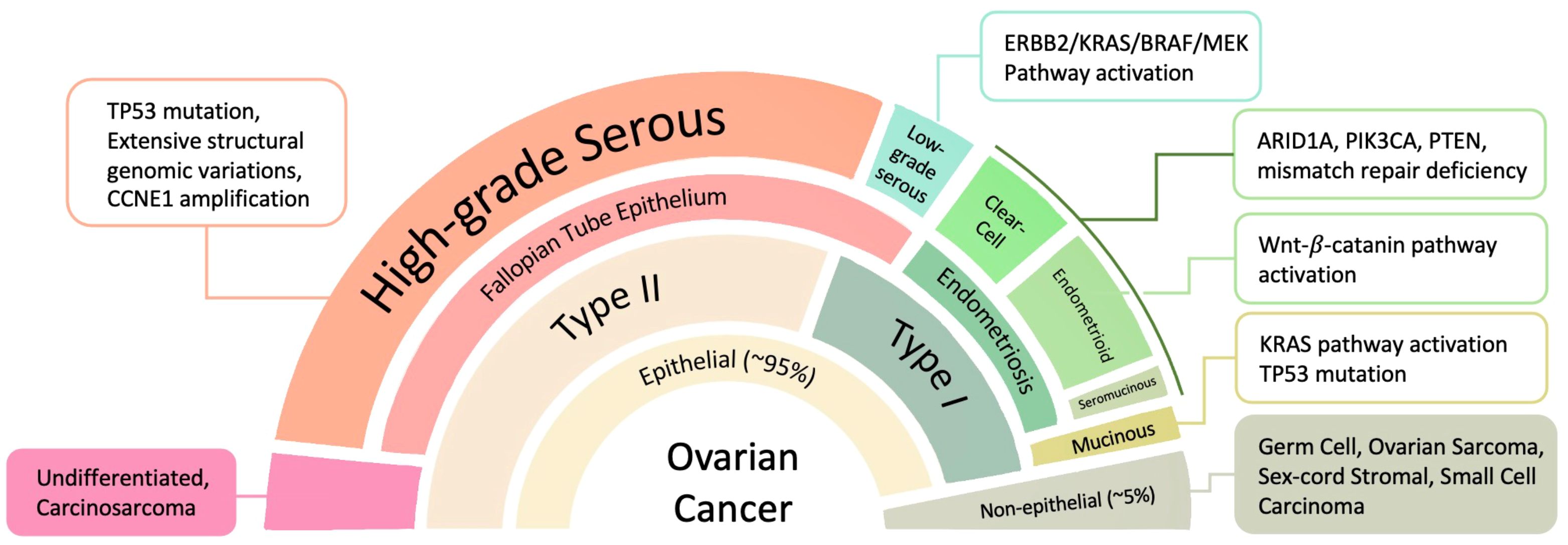
Figure 1. OC subtypes originate from different tissues and feature significant molecular pathway changes. ARID1A, AT-rich interactive domain-containing protein 1A; CCNE1, G1/S-specific cyclin-E1; ErbB, extracellular region binding protein; MEK (alias mitogen-activated protein kinase, MAPK); PIK3CA, phosphatidylinositol 3-kinase catalytic subunit α; PTEN, phosphatase and tensin homologue.
Type I OC includes several distinct histological subtypes: (1) endometrioid, clear cell, and seromucinous carcinomas; (2) low-grade serous carcinomas; and (3) mucinous carcinomas along with malignant Brenner tumors. These malignancies typically originate from benign extraovarian lesions and are characterized by relative genetic stability. They are often diagnosed at early clinical stages and are associated with a comparatively low mortality rate of approximately 10%. Common genetic mutations found in Type I tumors include PTEN (phosphatase and tensin homolog), ERK (extracellular signal-regulated kinase), ARID1A (AT-rich interactive domain-containing protein 1A), BRAF (B-Raf proto-oncogene, serine/threonine kinase), MAPK (mitogen-activated protein kinase), PIK3CA (phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α), and KRAS (Kirsten rat sarcoma viral oncogene homolog) (9).
In contrast, Type II OC is mostly high-grade serous carcinoma, markedly more aggressive, and carries a significantly worse prognosis, primarily due to its tendency to be diagnosed at advanced stages. Type II tumors have high chromosome abnormalities and mutations or DNA copy number variations in key regulatory genes such as TP53 (tumor protein p53), RB1 (retinoblastoma 1), FOXM1 (forkhead box M1), genes encoding CCNE1 (cyclin E1), and NOTCH3 (10).
Despite the recent emergence of innovative targeted medications, treatment resistance and the lack of improvement in overall survival rates in OC demand a thorough study of these challenges to develop new strategies. Understanding the immune landscape of OCs represents an emerging research direction. Two of the promising emerging immunotherapeutic approaches are immune checkpoint inhibitors (ICIs) and CAR (Chimeric Antigen Receptor)-T therapy. ICIs work by blocking inhibitory checkpoint ligands on the T cells or tumor cells, effectively lifting the “brakes” on the immune response and reactivating T cell-mediated anti-tumor activity. While ICIs have demonstrated remarkable success in malignancies such as melanoma and endometrial cancer (11), particularly in cases with DNA mismatch repair (MMR) deficiency (12), their efficacy in OC has been limited, with response rates ranging from 10% to 15%. This limited effect is largely attributed to OC’s immunologically “cold” tumor microenvironment, which suppresses effector T cell activation and infiltration (13). Another emerging technique is CAR-T therapy, which provides a precise, individualized approach for each patient by collecting T cells from the patient and re-engineering them to produce CARs on the surface of T cells, which detect cancer cells’ surface antigens and effectively destroy cancer cells. Despite its potential, the use of CAR-T approach faces several obstacles, including difficulty penetrating solid tumor masses, an immunosuppressive tumor microenvironment, and T cell exhaustion (14). Moreover, efforts to identify antigens present on the surfaces of solid tumors but not on healthy cells have largely been unsuccessful.
To overcome these limitations, researchers are exploring other strategies to circumvent the immune-suppressive or “immune-cold” milieu associated with many solid tumors, including OC. The tumor microenvironment (TME) comprises tumor cells, immune cells (such as lymphocytes, dendritic cells, macrophages, and neutrophils), endothelial cells, fibroblasts, and extracellular matrix components, including hyaluronic acid, fibronectin, laminin, and collagen (15). As OC recurs, the tumor undergoes dynamic changes, leading to a more complex and often suppressive immune milieu, which significantly influences treatment outcomes (16).
Given the complexities and evolving nature of the TME, researchers are increasingly focused on understanding the involvement of distinct immune cell populations in disease progression and therapeutic resistance. Among these, tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) have emerged as important regulators of tumor behavior. TAMs and TANs, which are significant components of the innate immune system inside TME, are highly plastic and can adopt diverse phenotypes that either promote or inhibit tumor progression depending on environmental cues (17, 18). In many solid tumors, including OC, TAMs and TANs are often polarized toward pro-tumoral states, contributing to immunosuppression, angiogenesis, metastasis, and therapeutic resistance (19–21). Therapeutic reprogramming of TAMs and TANs is now considered a cutting-edge area of research, with several new medicines entering preclinical and early-phase clinical trials (22, 23). Therefore, this review aims to focus on the emerging and critical roles of TAMs and TANs in OC. We will discuss their origins, phenotypic plasticity, functional heterogeneity, contributions to disease progression, and therapeutic strategies. We seek to highlight the potential of innate immune-targeted therapies to overcome the immune-suppressive obstacles that have hampered the success of traditional immunotherapies in OC.
2 The OC TME: origin, composition, and immune landscape
OC, particularly HGSOC, is characterized by a distinct peritoneal TME that coordinates the intricate interactions between tumor cells, resident cells in the peritoneal cavity, and various host immune cells (Figure 2). Like many malignancies, OC maintains a chronic inflammatory environment with high amounts of growth hormones, cytokines, chemokines, and reactive oxygen species (ROS), similar to damaged tissues and unhealing wounds (24).
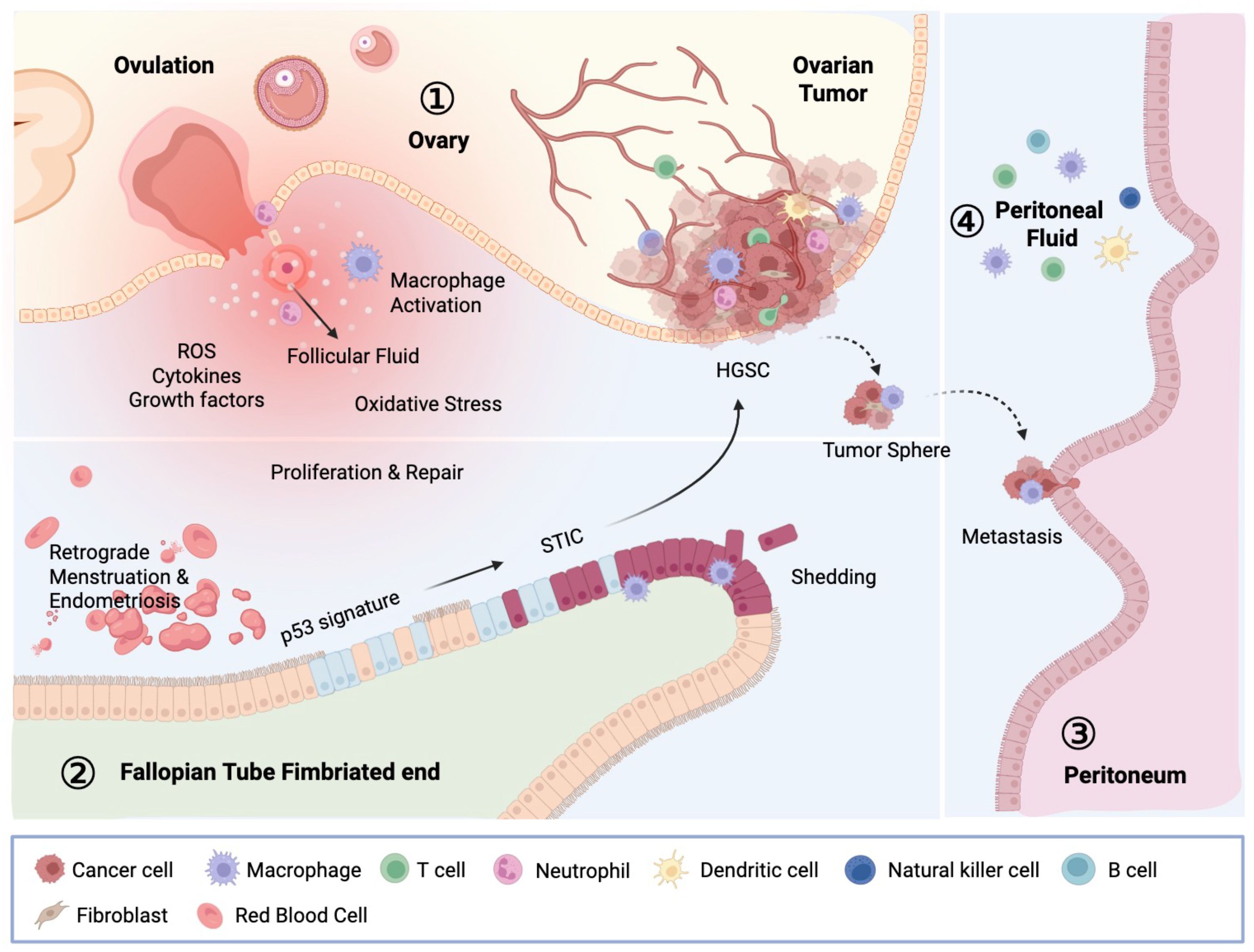
Figure 2. Carcinogenesis of OC and the OC TME. The OC microenvironment comprises the ovary, fallopian tube, peritoneum, and peritoneal fluid, collectively shaping the milieu in which OC develops and progresses. STIC, located at the fimbriated end, is the immediate precursor of HGSC. STIC cells first acquire invasive potential within the fallopian tube, and after detachment, spread across peritoneal surfaces. Those malignant cells encapsulate organs such as the ovary, bowel, peritoneal wall, and omentum. Within the peritoneal cavity, emigrated STIC cells adapt to specific tissue-environmental niches, forming tumor nodules and contributing to the accumulation of tumor ascites. This microenvironment, influenced by ovulation-related damage, infections, and inflammatory conditions, supports tumor progression, metastasis, and the development of chemotherapy resistance. This intricate interplay between tumor cells and immune cells exhibits their crucial role in tumor regulation, therefore affecting patients’ response to therapy. STIC, serous tubal intraepithelial carcinoma; HGSC, high-grade serous carcinoma.
Serous tubal intraepithelial carcinomas (STICs), commonly regarded as precursor lesions of HGSC, are primarily detected in the fimbriae, the distal region of the fallopian tube in close proximity to the ovary (25, 26). The exposure of fallopian tube epithelium, particularly at the fimbriated ends, to follicular fluids during ovulation is hypothesized to be a carcinogenic mechanism that converts fallopian tube epithelial cells to STIC lesions. This is because follicular fluid contains a high concentration of ROS and cytokines, which can directly damage epithelial cell DNA and may cause persistent inflammation. Moreover, tissue damages related to monthly ovulation may also contribute to the inflammatory milieu in the fallopian tubes which add to the malignant alteration of tubal epithelium (24). As a result, incessant ovulation is the highest risk factor of ovarian cancer and reducing ovulation via taking oral contraceptives, surgical removal of ovaries, and pregnancy/breast feeding have been found to reduce OC risks (27).
As the tumor advances, TME becomes increasingly complex and immunosuppressive (28). Innate and adaptive immune cells infiltrate the OC TME and actively shape the tumor immune landscape, which affects treatment response and disease outcome. The TME immune cells include intraepithelial tumor-infiltrating lymphocytes (TILs), natural killer (NK) cells, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and tumor-associated neutrophils (TANs) (29).
TAMs and TANs are the largest components of innate immune cell populations infiltrating OC. These myeloid-derived cells have garnered increased attention due to their plasticity and significant impact on tumor biology. They not only modulate inflammatory responses and angiogenesis but also influence tumor expansion, invasion, and metastasis. In the following sections, we will provide a comprehensive overview of the functional roles and molecular contributions of TAMs and TANs within the OC microenvironment, emphasizing their potential as therapeutic targets and prognostic markers.
3 Macrophages in OC
Macrophages are pivotal components of the innate immune system, possessing phagocytic, antigen-presenting, and hemostatic functions. They protect the host from infection and injury by engulfing and digesting foreign substances and pathogens (30). Upon phagocytizing pathogens, macrophages present antigens via MHC class II molecules to CD4+ T cells, which amplifies the immune response. Furthermore, macrophages play a critical role in tissue repair by recognizing damage-associated molecular patterns (DAMPs) released by tumorigenic cells using toll-like receptors (TLRs) and leads to downstream direct and indirect anti-cancer cellular responds such as T cell activation and TME modification (31).
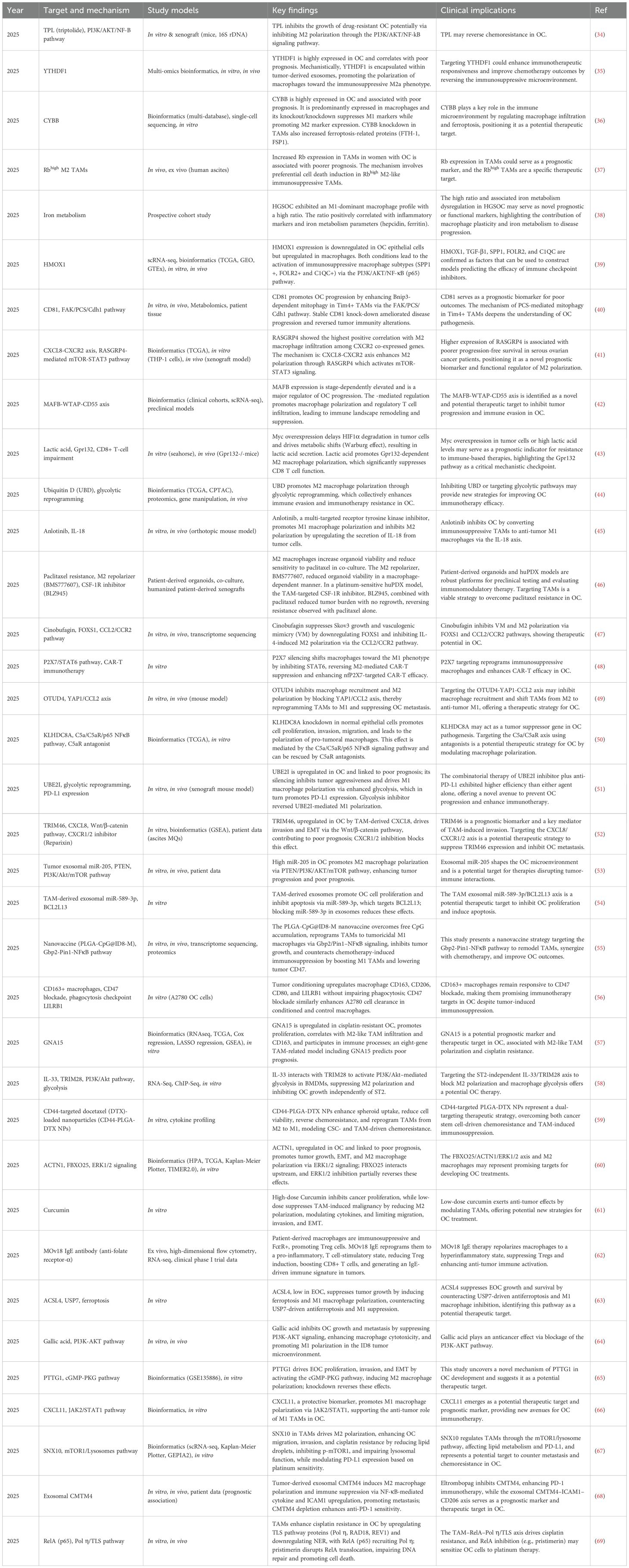
Macrophages account for approximately 10% of all hematopoietic cells and represent the most abundant immune population in the OC TME, comprising 39% of immune cells, followed by CD4+ T cells at 12% (32). Their presence in tumor tissues is commonly enriched and is dynamic, heterogeneous, and highly plastic. Depending on their state of polarization, TAMs can exert pro-tumor or anti-tumor actions. Research has demonstrated that TAMs contribute to tumor progression through mechanisms such as angiogenesis, ECM remodeling, metastasis, and the establishment of an immunosuppressive TME, which correlates with poor patient outcomes (33). Moreover, TAMs are critically involved in the development of chemoresistance, significantly impacting the prognosis of cancer patients (Figure 3). Recently, TAMs have received considerable attention, and studies in OC have expanded our understanding of their potential as therapeutic targets (Table 1).
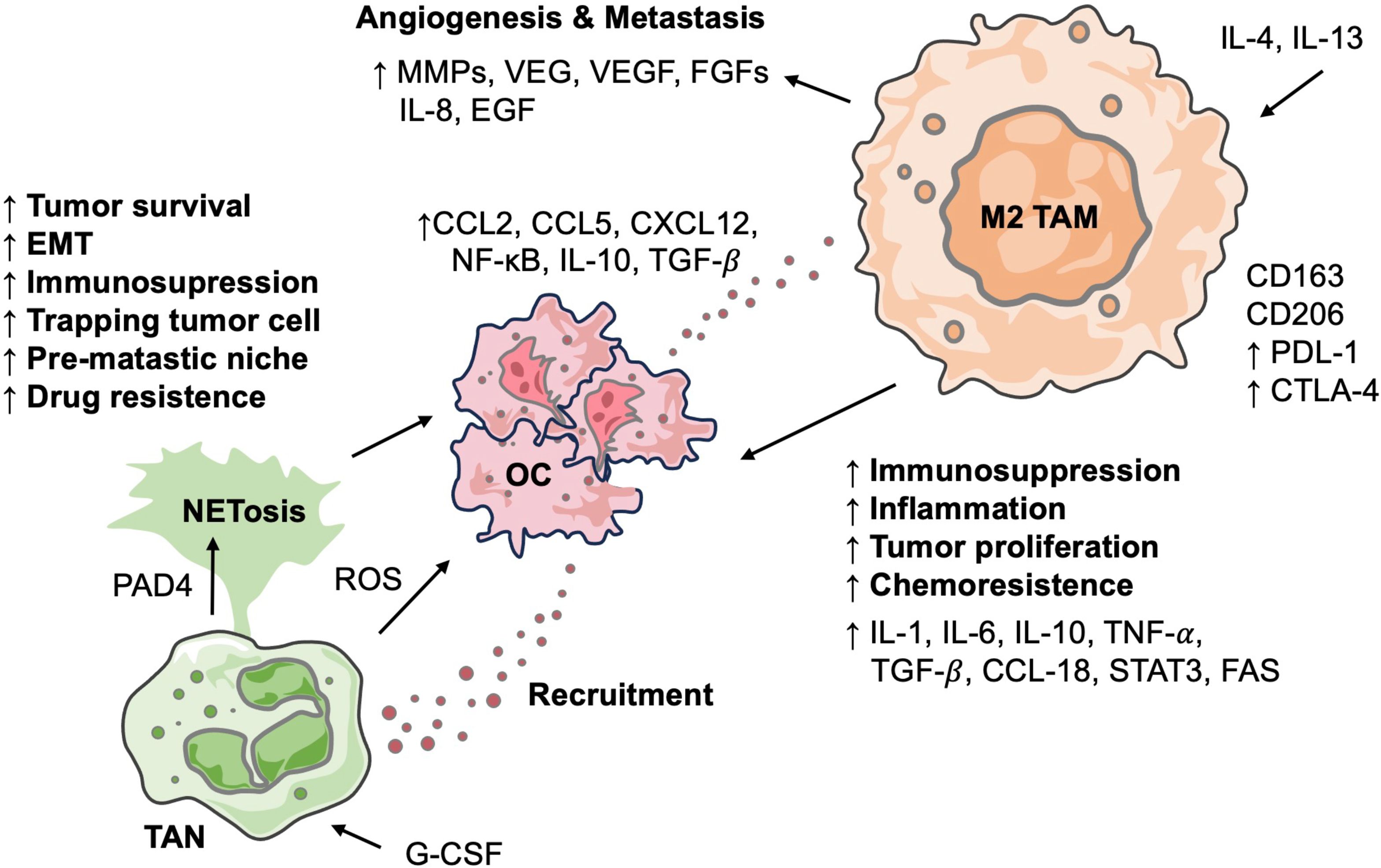
Figure 3. Interactions between neutrophils and macrophages in OC TME. Diagram outlines how TANs and polarized M2 TAMs collaboratively drive OC progression. TANs promote OC progression through NETosis and the release of factors. OC recruit and polarize monocytes into M2 TAMs, which, in turn, are central promoters of angiogenesis, metastasis, inflammation, and chemoresistance by secreting various cytokines. These interactions highlight a crucial immune axis that facilitates tumor malignancy.
3.1 The M1/M2 dichotomy and spectrum of TAMs
The heterogeneous population of TAMs has been broadly divided into the M1/M2 dichotomy based on their metabolic profiles, immunological responses, and activation states (70). Traditionally, M1 phenotype macrophages, also known as classically activated macrophages, exhibit anti-tumorigenic behavior by producing angiostatic factors, such as IL-12, IL-23, and CXCL10, when activated by bacterial products like lipopolysaccharide and pro-inflammatory cytokines (71). Due to the accumulation of Kreb cycle metabolites, these macrophages exhibit enhanced antigen-presenting capabilities, marked by increased expression of MHC class II, CD80, and CD86, and elevated production of NO, reactive oxygen intermediates (ROIs), and prostaglandins, collectively reinforcing their pro-inflammatory phenotype (72, 73).
In contrast, M2 phenotype macrophages, also known as alternatively activated macrophages, are pro-tumorigenic cells stimulated by Th2-related cytokines, including IL-4, IL-10, IL-13, and TGF-β. This stimulation leads to increased expression of dectin-1, C-type lectin DC-SIGN, mannose receptor, scavenger receptor A (SR-A), scavenger receptor B-1 (SR-B1), CD163, CD68, CCR2, CXCR1, CXCR2, VEGF-A, and MGL-1 (70). Furthermore, T-cells finely tune macrophage polarization via the CD40-CD40L interaction, where specific ligand residues encode distinct messages (74). Unlike M1 macrophages, M2 macrophages utilize arginine metabolism for ornithine production and generate substrates for fatty acid oxidation (FAO), a critical energy source (75). Elevated serum ornithine levels have been found in many cancer patients. In addition to serving as an energy source, lipid metabolism plays a crucial role in TAM functionality. Studies have demonstrated that FAO is critical for maintaining an immunosuppressive TME and modulating the antigen-presenting capacity of immune cells (76–78). M2 macrophages are implicated in promoting tumor growth, facilitating invasion and metastasis, and fostering an immunosuppressive TME. A low M1/M2 ratio is correlated with poor prognosis, while a high M1/M2 ratio indicates the opposite; this phenomenon is observed in many cancers, including OC (79).
Of all the TAMs in OC, >50% exhibit an M2 phenotype, while M0 and M1 phenotypes account for the remaining populations (80). However, recent studies challenge the classical M1/M2 dichotomy, the TAM population consists of a spectrum of phenotypes with overlapping functions (81). With technology advances, we are now discovering TAMs that do not fit into current categories, and TAM subtypes can differ significantly cancer type, stage, and histological landscape, necessitating more investigation and characterization (82). Some researchers classified the more complex M2 phenotype into 4 subtypes: M2a [alternatively activated, M(IL-4)], M2b (Type 2 macrophages, M(Ic)), M2c (Deactivated macrophages, further separated into M[IL-10), M(GC), and M(GC+TGFβ)], and M2d (83). Beyond this, single-cell RNA sequencing has been employed to investigate cellular diversity in several malignancies, and up to seven subtypes of TAMs have been identified based on expressed genes, pathways, and functions (84, 85). Even so, the M1/M2 structure is still widely used because of the extensive experimental data accumulated.
3.2 TAMs in OC progression
TAMs represent a major component of the TME in OC and play a critical role in disease progression. They often skewed toward an M2-like phenotype, contributing to multiple oncogenic processes through complex interactions with cancer cells and stromal elements. Understanding the multifaceted roles of TAMs in the progression of OC is essential for identifying new therapeutic targets and improving patient outcomes.
3.2.1 Chemoresistance
TAMs significantly contribute to chemoresistance in OC through mechanisms involving immune modulation, cytokine secretion, and metabolic reprogramming.
Co-culture studies have demonstrated that interactions between TAMs and OC cells lead to the upregulation of PD-L1 in both cell types (86). This upregulation is associated with increased expression of IL-6, IL-10, VEGF, STAT3, B-cell lymphoma 2 (BCL2), and multidrug resistance protein 1 (MDR1) in OC cells, thereby promoting proliferation, migration, and resistance to carboplatin (86). Crucially, silencing PD-L1 restores carboplatin sensitivity. Recently, this PD-L1-mediated resistance is supported by several molecular pathways that promote M2 polarization. For example, UBE2I upregulation in OC drives M1 macrophage polarization via enhanced glycolysis, which promotes PD-L1 expression, with UBE2I inhibitors synergizing with anti-PD-L1 therapy to enhance efficacy (51). Similarly, the TAM protein SNX10 drives M2 polarization, enhances cisplatin resistance, and modulates PD-L1 expression by inhibiting the mTOR1/lysosome pathway and disrupting lipid metabolism, presenting a target for anti-metastasis and chemosensitization (67). Furthermore, tumor-derived exosomal CMTM4 induces M2 macrophage polarization and immune suppression via the NF-B pathway and ICAM1 upregulation, promoting metastasis and attenuating anti-PD-1 sensitivity; its inhibition with Eltrombopag can enhance immunotherapy (68).
The CCL2/CCR2 axis recruits monocytes to the OC TME, where CCL2 drives their M2-like TAM differentiation, promoting tumor growth and chemoresistance. Paclitaxel-resistant OC cells drive chemoresistance by secreting CCL2, which recruits and polarizes macrophages into M2-like TAMs that reinforce resistance by secreting IL-6 and IL-10; consequently, inhibition of the CCL2/CCR2 signaling pathway restores paclitaxel sensitivity (87). Recent studies shows, cinobufungin inhibited IL-4 induced M2 polarization via the CCL2/CCR2 pathway (47), and OTUD4 inhibits macrophage recruitment and M2 polarization by blocking Y.
AP1/CCL2 axis (49). Furthermore, UBR5 (Ubiquitin protein ligase E3 component n-recognin 5) is implicated in recruiting TAMs to OC cells via the CCL20-CCR6 axis (88), potentially contributing to increased metastasis and paclitaxel resistance (89). Compounding this, platinum treatment may activate the STAT3 pathway by increasing IL-6, IL-10, and PGE2 production, leading to M2 polarization and tumor progression (90).
Metabolic reprogramming of TAMs is another mechanism contributing to chemoresistance. Gaude et al. (2018) identified metabolic heterogeneity in OC, defining low- and high-OXPHOS subtypes. High-OXPHOS tumors depend on the PML–PGC-1α axis to sustain oxidative metabolism and exhibit enhanced chemosensitivity driven by oxidative stress and ferroptosis (91). Additionally, enhanced fatty acid (FA) uptake and metabolism are another key feature of metabolic reprogramming in OC. The primary tumor and omental metastatic sites are enriched in FAs due to ascitic fluid accumulation and adipocyte-derived secretions (92). Moreover, OC induce cholesterol efflux from TAMs through ATP-binding cassette (ABC) transporters, depleting of lipid rafts and promoting IL-4-mediated M2 polarization. This reprogramming suppresses IFN-γ-induced gene expression, facilitating an immunosuppressive TME. Genetic deletion of ABC transporters in TAMs reverses these effects (93).
3.2.2 Immunosuppression and inflammation
TAMs play a pivotal role in establishing an immunosuppressive TME, facilitating tumor progression and immune evasion. TAMs secrete immunosuppressive cytokines such as IL-10 and TGF-β, which inhibit the function of effector T cells and NK cells, thereby dampening anti-tumor immune responses. Specifically, TGF-β impairs mitochondrial respiration in CD4+ T cells, leading to reduced production of IFN-γ and granzyme B, crucial components of cytotoxic activity (94, 95). The immunosuppressive milieu orchestrated by TAMs is further compounded by their interactions with other immune cells and factors within the TME. TAMs have been implicated in the suppression of dendritic cell maturation and function, as well as the inhibition of NK cell cytotoxicity. Moreover, the expression of immune checkpoint molecules such as PD-L1 and CTLA-4 on TAMs contributes to the attenuation of T cell activation and proliferation (96).
In addition to their immunosuppressive functions, TAMs promote an inflammatory environment that promotes tumor progression. They secrete pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, and CCL18, which facilitate tumor cell proliferation, angiogenesis, and metastasis. The dual role of TAMs in mediating immunosuppression and inflammation underscores their significance in the pathophysiology of OC and highlights the potential of targeting TAMs as a therapeutic strategy (32).
3.2.3 Angiogenesis and metastasis
Angiogenesis is essential for tumor growth beyond a certain size, as the expanded vasculature supplies oxygen and nutrients while providing routes for metastasis. M2 TAMs are key derivers of this process, secreting pro-angiogenic factors such as matrix metalloproteinases (MMPs), fibroblast growth factors (FGFs), and vascular endothelial growth factor (VEGF). Affymetrix gene profiling of TAMs isolated from OC expressed genes associated with extracellular matrix remodeling, including high levels of cathepsins (L, C, Z, and B), urokinase-type plasminogen activator (uPA), lysosomal enzymes, ADAM proteases, and MMPs (1, 9, 12, and 14), which facilitate ECM degradation and enable vessel sprouting and remodeling (97). TAM-derived MMPs remodel the ECM, promoting endothelial cell migration during angiogenesis, while FGFs and cytokines further stimulate endothelial proliferation and blood vessel formation (98). Additionally, TIE2+ TAMs are abundant in OC lesions, ascites, and patient’s blood, correlating positively with microvessel density (99). Ang2, the TIE2 ligand, promotes TIE2+ TAM recruitment to the TME, enhancing angiogenesis through IGF-1 signaling (99).
VEGF, produced by TAMs, binds to endothelial cell receptors and stimulates the formation of new blood vessels to supply the tumor with nutrients and oxygen, hence boosting tumor growth and survival. Preclinical studies have shown that overexpression of VEGF can transform normal ovarian epithelium into ascites-producing, neoplastic tissue (100). Additionally, VEGF may suppress T cell activation and proliferation, contributing to immune evasion (101). High levels of VEGF have been observed in both primary OC and ascitic fluid, and their expression is strongly correlated with poor patient survival (102, 103). Pre-operative plasma VEGF-C levels were highly associated with recurrence and poor prognosis in OC patients (104). Additionally, VEGF expression is higher in OC-induced ascites than in ascitic fluids of nonmalignant origin (105). Recent evidence from Zhou et al. demonstrates that VEGF/VEGFR inhibitors significantly improve progression-free and overall survival in patients with platinum-resistant OC, while maintaining a manageable safety profile (106). Notably, macrophage depletion alone has been shown to reduce VEGF levels, thereby limiting the accumulation of ascites and metastatic dissemination (107).
TAMs play a critical role in facilitating tumor metastasis. In the peritoneal cavity, TAMs contribute to the formation of multicellular spheroids with tumor cells, providing a protective environment that enhances tumor survival and facilitates peritoneal dissemination. Also, studies reveal that M2 macrophage–derived CCL4 activates the CCR5/PI3K pathway in mesothelial cells, inducing P-selectin expression. This facilitates CD24-mediated tumor–mesothelial adhesion in vitro and in vivo(108). TAMs within spheroids can secrete epidermal growth factor (EGF), resulting in the downstream upregulation of EGFR and VEGF signaling that promote tumor cell proliferation and migration. In mouse OC models treated with erlotinib, an EGFR inhibitor, exhibited reduced spheroid formation and metastatic progression, underscoring the important role of TAMs in disease progression (32). Moreover, EGF upregulates αMβ2 integrin on TAMs and ICAM-1 on tumor cells. Therefore, blocking EGFR signaling or neutralizing ICAM-1 reduced spheroid formation and cancer progression in mouse models (109, 110).
TAMs also play a pivotal role in promoting tumor cell dissemination by inducing epithelial-mesenchymal transition (EMT)—a process in which epithelial cells acquire mesenchymal traits, thereby enhancing their migratory and invasive capabilities. TAM-derived cytokines such as IL-6 and TNF-α activate signaling pathways like STAT3 and NF-κB in tumor cells, inducing EMT and increasing metastatic potential (111). Building on these findings, Li et al. demonstrated that TAM-derived CXCL8 promotes OC cell invasion by upregulating TRIM46 expression, which activates the Wnt/β-catenin signaling pathway and induces EMT (52). Furthermore, TAMs secrete MMPs that degrade the extracellular matrix, allowing tumor cells to invade surrounding tissues and enter the circulation (112).
4 Neutrophil in OC
Neutrophils are the most abundant circulating leukocytes and play a critical role in bridging innate and adaptive immunity. They are among the first immune cells recruited to areas of inflammation or malignancies and can influence the activity of other immune cells, including those of the adaptive immune system. Neutrophils have a short lifespan of approximately 7–10 hours in both humans and mice. However, cytokines secreted by tumor cells, such as G-CSF, IL-1β, IL-6, and TNF, can extend their longevity (113–115). Indeed, neutrophils are now recognized to be much longer-lived than previously thought, surviving for 5 days or more in the circulation (116), and they may even be able to survive for weeks in tissues.
In various malignancies—including lung, breast, and gastric cancers—neutrophils constitute a substantial portion of immune cells infiltrating primary tumors, and their presence has been consistently associated with reduced overall survival and recurrence-free survival (117, 118). Extensive evidence supports a pro-tumor role for neutrophils in cancer progression. For instance, Bekes et al. demonstrated that neutrophils produce MMP9 within the TME, promoting angiogenesis, tumor growth, and metastasis in mouse transplantation models (119). Similarly, Yang et al. reported that elevated infiltration of TANs in epithelial OC impairs CD8+ T cell cytotoxicity, thereby fostering an immune-tolerant microenvironment and increasing the risk of recurrence (120). In HGSOC, TANs have also been shown to express high levels of immunosuppressive markers such as PD-L1 and CD14, with their presence correlating with diminished T cell function (121). Despite the growing recognition of NETs in cancer biology, their roles in OC have only begun to be explored (Table 2). The following subsections provide a detailed description of TANs.
4.1 TANs polarization
Based on animal studies, in 2009 Fridlender et al. proposed a hypothesis that TANs, like TAMs, can be polarized into anti-tumor (N1 type) and pro-tumor (N2 type) phenotypes (132). However, it is largely unknown whether the N1/N2 profile observed in mouse models applies to human TANs. Both polarization pathways are orchestrated by cytokines within the TME (133). This polarization is reversible; for example, blocking TGF-β can repolarize TANs from the N2 state back to the N1 state (132).
N1 TANs exhibit anti-tumor properties, including enhanced production of immunostimulatory cytokines and chemokines, reduced expression of arginase, and increased cytotoxicity against tumor cells in vitro. Moreover, neutrophil-derived oxidants, cytokines, and enzymes contribute to tumor suppression. For example, ROS generated by neutrophils activate an H2O2-dependent calcium channel in cancer cells, leading to calcium influx and subsequent cell death (134, 135). Furthermore, neutrophils have the capacity to produce TNF-related apoptosis-inducing ligand (TRAIL), which induces apoptosis in cancer cells. The efficacy of this pathway is further enhanced by stimulating neutrophils with IFN-γ (136). In contrast, neutrophils with an N2-like phenotype promote invasion and metastasis in OC by upregulating MAPK signaling (137). Upregulation of ARG1 is also associated with N2’s tumor-supportive, T cell inhibitory phenotype (138).
Despite these findings, the precise phenotypic classification of TANs remains controversial. While it is well-established that neutrophils express diverse surface markers and receptors that may influence tumor progression and clinical outcomes, the existence and functional relevance of distinct pro-tumor and anti-tumor TAN subsets in human cancers require further investigation.
4.2 Tumor-induced NETosis
The intricate connection between OC development and neutrophil extracellular traps (NETs) has been the subject of recent research. NETs formation was first recognized as a mechanism by which neutrophils ensnare and destroy microorganisms (139). NETs, released by neutrophils in response to external pathogens, are primarily composed of fibrous decondensed chromatin bound with histones, myeloperoxidase (MPO), and various cytoplasmic proteins such as neutrophil elastase, cathepsin G, and lactoferrin (140, 141).
NET release, also known as ‘NETosis’, was identified in biopsy samples from two out of eight pediatric patients with Ewing sarcoma (142). In the context of cancer, NETs have been implicated in promoting thrombosis, systemic inflammation, and multi-organ failure (143). NETs also play a role in tumor survival, pre-metastatic niche development, and resistance to treatments (144). In OC, neutrophils are drawn to the omental niche by tumor-derived cytokines such as IL-8, growth-regulated oncogenes α/β (GROα/β), G-CSF, and monocyte chemoattractant protein-1 (MCP-1) (145). Infiltrating neutrophils can produce NETs, resulting in a pro-metastatic milieu that favors tumor implantation and progression, a phenomenon called “neutrophil-assisted soil preparation in metastasis” (145).
Supporting these findings, Singel et al. (2019) demonstrated that ascitic fluid from OC patients chemoattracted neutrophils and induced NET release in vitro, an effect attenuated by DNase treatment. Studies on human samples have shown that DNase I treatment of ascites supernatants inhibits NET release by depleting both genomic DNA (gDNA) and mitochondrial DNA (mtDNA) (146). Importantly, exposure to ascites reprogrammed neutrophils toward an immunosuppressive phenotype that inhibited T cell proliferation, suggesting a role for NETosis in tumor-induced immune evasion. In clinical studies, high levels of mitochondrial DNA (mtDNA) and neutrophil elastase—markers of NETosis—in ascites were associated with significantly shorter progression-free survival, indicating that tumor-derived components such as mtDNA can trigger NET formation, platelet activation, and subsequent metastatic spread (146).
4.2.1 NETs as diagnostic and prognostic markers
In addition to their role in OC TME, several studies had proposed the possible diagnostic and prognostic significance of NET markers. In a study of ascites samples, high mtDNA levels correlated with shorter progression-free survival and enhanced NET and platelet activation, suggesting that mtDNA may serve as a prognostic marker and therapeutic target (146). Similarly, Montes et al. (2023) reported that elevated levels of NETosis biomarkers—including cell-free DNA (cfDNA), CitH3, calprotectin, and MPO—were detected compared to controls, suggesting their roles in minimally invasive surrogate biomarkers for HGSOC (128). Furthermore, a prospective two-center study involving 188 patients with newly diagnosed EOC, high pretreatment serum levels of genomic DNA, myeloperoxidase (MPO), and citrullinated histone H3 (CitH3)—markers of neutrophil activation and NET formation—were independently associated with worse overall survival (124). However, in contrast to these findings, Dobilas et al. analyzed plasma samples from 199 women with adnexal masses found no significant differences in circulating NETs markers (H3Cit-DNA and dsDNA) between benign, borderline, and malignant groups, suggesting limited diagnostic value in this context (129). Collectively, these findings suggest that while NET markers hold promise for prognostication in OC, their diagnostic utility remains context-dependent and warrants further investigation.
4.2.2 NETs in OC progression
NETs have been shown to promote tumor progression through direct interactions with cancer cells. In lung carcinoma models, NETs were found to physically bind tumor cells, and this interaction was abolished by DNase or neutrophil elastase inhibitors, suggesting a functional role of NETs in tumor adhesion and spread (147). In OC, the relationship between NETs and disease progression appears complex and, at times, contradictory. Yamamoto et al. reported that OC-induced neutrophilia and elevated G-CSF levels contribute to NET formation, potentially promoting cancer dissemination (148). Similarly, Lee et al. demonstrated that the metastatic tropism of OC is facilitated by NET formation in the premetastatic omental niche, which traps circulating tumor cells and enhances their seeding efficiency (145). However, Muqaku et al. observed a paradoxical association between NET formation and improved overall survival in patients with HGSOC (131).
4.2.3 NETs in OC chemoresistance and metastasis
Recurrence and chemoresistance are the primary causes of mortality in OC. An emerging concept is that NETs may directly impair the efficacy of chemotherapy. Tamura et al. (2022) demonstrated that neutrophils stimulated with PMA or LPS release NETs that physically bind chemotherapy agents, such as doxorubicin (DOX). In 3D culture models of OC, the presence of NETs markedly reduced DOX-induced apoptosis in cancer cells. Mechanistically, DOX was captured by the NET fibers, limiting its bioavailability. Importantly, co-treatment with DNase I dismantled the NET structures and restored DOX cytotoxicity, indicating that NETs may act as drug-absorbing scaffolds within the TME (130). These findings suggest that targeting NETs may represent a promising therapeutic strategy to enhance the efficacy of chemotherapy, particularly in malignancies with NET-rich microenvironments.
Moreover, NETs have been shown to facilitate tumor invasion and metastasis in the TME by inducing tumor‐related inflammatory reactions (149), accelerating EMT, trapping circulating tumor cells, and increasing vascular permeability (150). NETs, stimulated by inflammatory factors secreted by OC, play a critical role in establishing the pre-metastatic omental niche. In murine models, omental colonization was significantly reduced in mice with neutrophil-specific deletion of peptidyl arginine deiminase 4 (PAD4), an enzyme essential for NET formation (145). Similarly, pharmacological inhibition of PAD4 suppressed NET formation and diminished metastatic implantation (145). In a recent study (2025), the same research group further demonstrated that neutrophils infiltrating the omentum in early-stage OC undergo NETosis, depositing NETs that contribute to the recruitment of IL-10–producing innate B cells via NET-induced CXCL13 expression. These B cells subsequently expand local Treg populations through the secretion of IL-10, establishing an immunosuppressive niche that supports tumor cell implantation and proliferation (123). Moreover, a novel study pointed out that upregulating miR142 can dismiss the recruitment of neutrophil with in TME by the expression of CXCL1 regulated by miR-146a, which shed light on a potential therapeutic strategy (151).
Neutropenia, a frequent adverse effect of platinum-taxane chemotherapy, affects approximately 60% of OC patients (152). To mitigate this, G-CSF is commonly administered. However, G-CSF has also been implicated in promoting NET formation, raising concerns about its potential to exacerbate metastasis (145). Notably, G-CSF has been shown to promote N2-type neutrophil polarization in breast cancer (153), further suggesting its potential role in immunosuppressive and tumor-supportive processes. These findings underscore the need for careful evaluation of G-CSF use in patients with a high risk of metastatic spread.
Recent studies have revealed that NETs may reactivate dormant tumor cells, contributing to cancer recurrence and metastasis (154, 155). This process is mediated by NET-associated proteases that remodel the extracellular matrix (ECM), particularly laminin. Laminin degradation exposes new epitopes that activate integrin signaling in dormant cancer cells, triggering their proliferation (155). This highlights a novel and concerning mechanism by which NETs promote tumor relapse.
5 TAMs and TANs crosstalk in the TME
Originating from a shared myeloid progenitor lineage, TAMs and TANs play diverse and complementary roles in nearly all stages of tumor development and metastatic progression. Although extensive research effort has been done on neutrophil-lymphoid interactions, fewer studies have examined neutrophil-myeloid cell crosstalk in the OC TME context. Activated neutrophils release IL-8 and TNF-α, which recruit macrophages to the site of inflammation (156). Neutrophils release chemokines such CCL2, CCL3, and CCL4, which draw monocytes and dendritic cells and aid in the recruitment of more myeloid cells into the TME (157). Research has demonstrated that TANs isolated from HCC patients release significant amounts of CCL2 and CCL17, which promote the migration and in vitro activation of macrophages and Treg cells in HCC (158, 159).
Expanding on these interactions, Kumar et al. demonstrated that in multiple mouse tumor models, pharmacological inhibition or antibody-mediated neutralization of colony-stimulating factor 1 receptor (CSF1R) led to a compensatory increase in the infiltration of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs; CD11b+ Ly6Clo Ly6G+) (160). These PMN-MDSCs, recruited by carcinoma-associated fibroblasts, ultimately undermined the anticipated therapeutic efficacy of CSF1R blockade (160). Later, in a study by Wang et al., a panel of eight mouse triple-negative breast cancer models was used to demonstrate that tumors did not uniformly recruit TANs and TAMs (161). Despite sharing the same breast cancer subtype, these tumors could be further immunologically subtyped into two distinct subtypes: neutrophil-enriched subtypes (NES, characterized by CD11b+ Ly6Cmid Ly6G+ cells) and macrophage-enriched subtypes (MES, characterized by CD11b+ Ly6G- Ly6C- F4/80+ cells). A mutual exclusivity was observed between TANs and TAMs, whereby the depletion of one population led to the upregulation of the other (161). This reciprocal regulation suggests a complex interplay and potential compensatory dynamics between TANs and TAMs that may influence tumor progression and therapeutic response. Translating these insights to OC, it becomes imperative to further elucidate the mechanisms governing TAN-TAM crosstalk to develop more effective combinatorial therapeutic strategies.
The investigations collectively demonstrated intricate crosstalk between TANs, TAMs, and other components of the TME, highlighting the necessity for integrated therapeutic approaches that consider the plasticity and compensatory pathways within the myeloid cell network.
6 Treatment targeting macrophages and neutrophils in OC
OC has a poor response to immune checkpoint inhibitors, which is largely due to robust immunosuppressive TME and poor T cell immunity. The immunosuppressive TME in OC is predominantly driven by TAMs with tumor-promoting properties. The heterogeneity of immune cell populations within the TME poses significant challenges for developing effective therapies for OC. To overcome these challenges, current treatment strategies increasingly focus on personalized approaches that target the unique immune landscape of each tumor.
Growing information from preclinical and clinical investigations has deepened our understanding of the critical role TAMs play in driving tumor progression and resistance to therapies. As a result, TAMs have emerged as a key target in the development of novel cancer therapies aiming at improving outcomes for patients with OC. The most extensively studied strategies for targeting TAMs are: (1) inhibiting recruitment to the TME, (2) depleting TAM populations or disrupting their survival, (3) reprogramming or repolarizing toward an anti-tumor phenotype, (4) restoring their innate tumor-suppressive functions, (5) suppressing tumor-promoting activities, and (6) CAR-macrophages (CAR-Ms) (32, 162). However, TAM-targeting therapies face challenges due to TAMs’ high plasticity and heterogeneity, with their diverse phenotypes varying by tumor type and location within the same tumor (163). Recently, Klichinsky et al. pioneered the generation of CAR-Ms, macrophages that show antigen-specific phagocytosis and tumor clearance in vitro, demonstrating their ability to target tumor cells and activate adaptive immunity in humanized mice (164). CAR-Ms targeting HER2 and CD47 displayed antigen-specific phagocytosis of OC cells in vitro and the ability to activate CD8+ cytotoxic T lymphocytes (165). On the other hand, selective elimination of FRβ+ TAMs (M2-like) via CAR-T cells reshapes the TME, leading to improved antitumor immunity and tumor-directed CAR-T therapies (166).
Targeting NETs represents a promising strategy for boosting the immune response against tumors and improving the efficacy of existing cancer treatments; nevertheless, this concept shall be assessed rigorously in clinical studies (167). Currently, most therapeutic studies were performed on animal models (167). For example, treatment with NET-inhibiting agents reduced omental colonization in NET-competent mice without affecting neutrophil influx. In neutrophil-depleted mice, omental metastasis was inhibited by about 70%, indicating that NET formation plays a key role in tumor development (145). Biomarkers associated with NETs formation, such as H3Cit and MPO-DNA, may have prognostic significance for cancer patients (168). Understanding the role of NETs in the TME of OC is critical for developing targeted therapies, ultimately improving patient outcomes and facilitating personalized treatment approaches.
7 Conclusion
OC is shaped by a profoundly immunosuppressive tumor microenvironment, in which TAMs and TANs exert decisive, yet dynamic, influences on disease progression. Both cell types demonstrate functional plasticity, contributing to immune evasion, angiogenesis, and resistance to therapy. The development of NETs provides an additional layer of immunomodulation and metastatic potential. Recent research on the reciprocal regulation of TAMs and TANs has revealed compensatory mechanisms that may undermine the efficacy of monotherapy targeting either cell population alone. These findings underscore the need for combinatorial strategies that consider the broader myeloid landscape. Novel approaches, such as CAR-engineered macrophages and NET inhibition, offer promise but require further validation in clinical settings. A deeper understanding of the spatial and functional dynamics of innate immune cells in the OC TME is essential. Future efforts should focus on identifying predictive biomarkers and developing rational, immune-targeted therapies that exploit the full potential of myeloid modulation to improve patient outcomes.
Author contributions
K-CC: Conceptualization, Data curation, Formal Analysis, Resources, Visualization, Writing – original draft, Writing – review & editing. Y-HL: Conceptualization, Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. D-SW: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. I-MS: Funding acquisition, Resources, Visualization, Writing – review & editing. T-LW: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We appreciate the funding support by NIH/NCI grants P50CA228991, R01CA260628, and U2CCA271891, and DoD grant W81XWH-22-1-0852.
Acknowledgments
We deeply appreciate all who contributed, directly or indirectly, to the TAM and TAN research areas.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Zsiros E, Lynam S, Attwood KM, Wang C, Chilakapati S, Gomez EC, et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: A phase 2 nonrandomized clinical trial. JAMA Oncol. (2021) 7:78–85. doi: 10.1001/jamaoncol.2020.5945
4. Shender VO, Anufrieva KS, Shnaider PV, Arapidi GP, Pavlyukov MS, Ivanova OM, et al. Therapy-induced secretion of spliceosomal components mediates pro-survival crosstalk between ovarian cancer cells. Nat Commun. (2024) 15. doi: 10.1038/s41467-024-49512-6
5. Chandra A, Pius C, Nabeel M, Nair M, Vishwanatha JK, Ahmad S, et al. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. (2019) 8:7018–31. doi: 10.1002/cam4.2560
6. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
7. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. (2011) 474:609–15. doi: 10.1038/nature10166
8. Pankowska KA, Będkowska GE, Chociej-Stypułkowska J, Rusak M, Dąbrowska M, and Osada J. Crosstalk of immune cells and platelets in an ovarian cancer microenvironment and their prognostic significance. Int J Mol Sci. (2023) 24:9279. doi: 10.3390/ijms24119279
9. Kurman RJ and Shih I-M. The dualistic model of ovarian carcinogenesis. Am J Pathol. (2016) 186:733–47. doi: 10.1016/j.ajpath.2015.11.011
10. Ordulu Z, Watkins J, and Ritterhouse LL. Molecular pathology of ovarian epithelial neoplasms: predictive, prognostic, and emerging biomarkers. Clin Lab Med. (2024) 44:199–219. doi: 10.1016/j.cll.2023.08.004
11. Carlino MS, Larkin J, and Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
12. O'Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jesus Acosta A, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. (2022) 40:752–61. doi: 10.1200/JCO.21.01874
13. Galluzzi L, Vacchelli E, Pedro J-MB-S, Buqué A, Senovilla L, Baracco EE, et al. Classification of current anticancer immunotherapies. Oncotarget. (2014) 5:12472–508. doi: 10.18632/oncotarget.2998
14. Xin Q, Chen Y, Sun X, Li R, Wu Y, and Huang X. CAR-T therapy for ovarian cancer: Recent advances and future directions. Biochem Pharmacol. (2024) 226:116349. doi: 10.1016/j.bcp.2024.116349
15. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. (2020) 18:59. doi: 10.1186/s12964-020-0530-4
16. Li YR, Ochoa CJ, Zhu Y, Kramer A, Wilson M, Fang Y, et al. Profiling ovarian cancer tumor and microenvironment during disease progression for cell-based immunotherapy design. iScience. (2023) 26:107952. doi: 10.1016/j.isci.2023.107952
17. Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
18. Wu L, Saxena S, Awaji M, and Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers (Basel). (2019) 11. doi: 10.3390/cancers11040564
19. Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. (2019) 178:346–60.e24. doi: 10.1016/j.cell.2019.05.047
20. Powell DR and Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. (2016) 37:41–52. doi: 10.1016/j.it.2015.11.008
21. Cassetta L and Kitamura T. Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol. (2018) 6:38. doi: 10.3389/fcell.2018.00038
22. De Palma M, Biziato D, and Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. (2017) 17:457–74. doi: 10.1038/nrc.2017.51
23. Hagerling C, Casbon AJ, and Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol. (2015) 25:214–20. doi: 10.1016/j.tcb.2014.11.001
24. Savant SS, Sriramkumar S, and O'Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10080251
25. Seidman JD. Serous tubal intraepithelial carcinoma localizes to the tubal-peritoneal junction: a pivotal clue to the site of origin of extrauterine high-grade serous carcinoma (ovarian cancer). Int J Gynecol Pathol. (2015) 34:112–20. doi: 10.1097/PGP.0000000000000123
26. Gross AL, Kurman RJ, Vang R, Shih Ie M, and Visvanathan K. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol. (2010) 2010:126295. doi: 10.1155/2010/126295
27. Fu Z, Brooks MM, Irvin S, Jordan S, Aben KKH, Anton-Culver H, et al. Lifetime ovulatory years and risk of epithelial ovarian cancer: a multinational pooled analysis. J Natl Cancer Inst. (2023) 115:539–51. doi: 10.1093/jnci/djad011
28. Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009
29. Baci D, Bosi A, Gallazzi M, Rizzi M, Noonan DM, Poggi A, et al. The ovarian cancer tumor immune microenvironment (TIME) as target for therapy: A focus on innate immunity cells as therapeutic effectors. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21093125
30. Watanabe S, Alexander M, Misharin AV, and Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. (2019) 129:2619–28. doi: 10.1172/JCI124615
31. Patidar A, Selvaraj S, Sarode A, Chauhan P, Chattopadhyay D, and Saha B. DAMP-TLR-cytokine axis dictates the fate of tumor. Cytokine. (2018) 104:114–23. doi: 10.1016/j.cyto.2017.10.004
32. Schweer D, McAtee A, Neupane K, Richards C, Ueland F, and Kolesar J. Tumor-associated macrophages and ovarian cancer: implications for therapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14092220
33. Chen Y, Song Y, Du W, Gong L, Chang H, and Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. (2019) 26. doi: 10.1186/s12929-018-0492-7
34. Le F, Yang L, Han Y, Zhong Y, Zhan F, Feng Y, et al. Correction: TPL inhibits the invasion and migration of drug-resistant ovarian cancer by targeting the PI3K/AKT/NF-κB-signaling pathway to inhibit the polarization of M2 TAMs. Front Oncol. (2025) 15:1641399. doi: 10.3389/fonc.2025.1641399
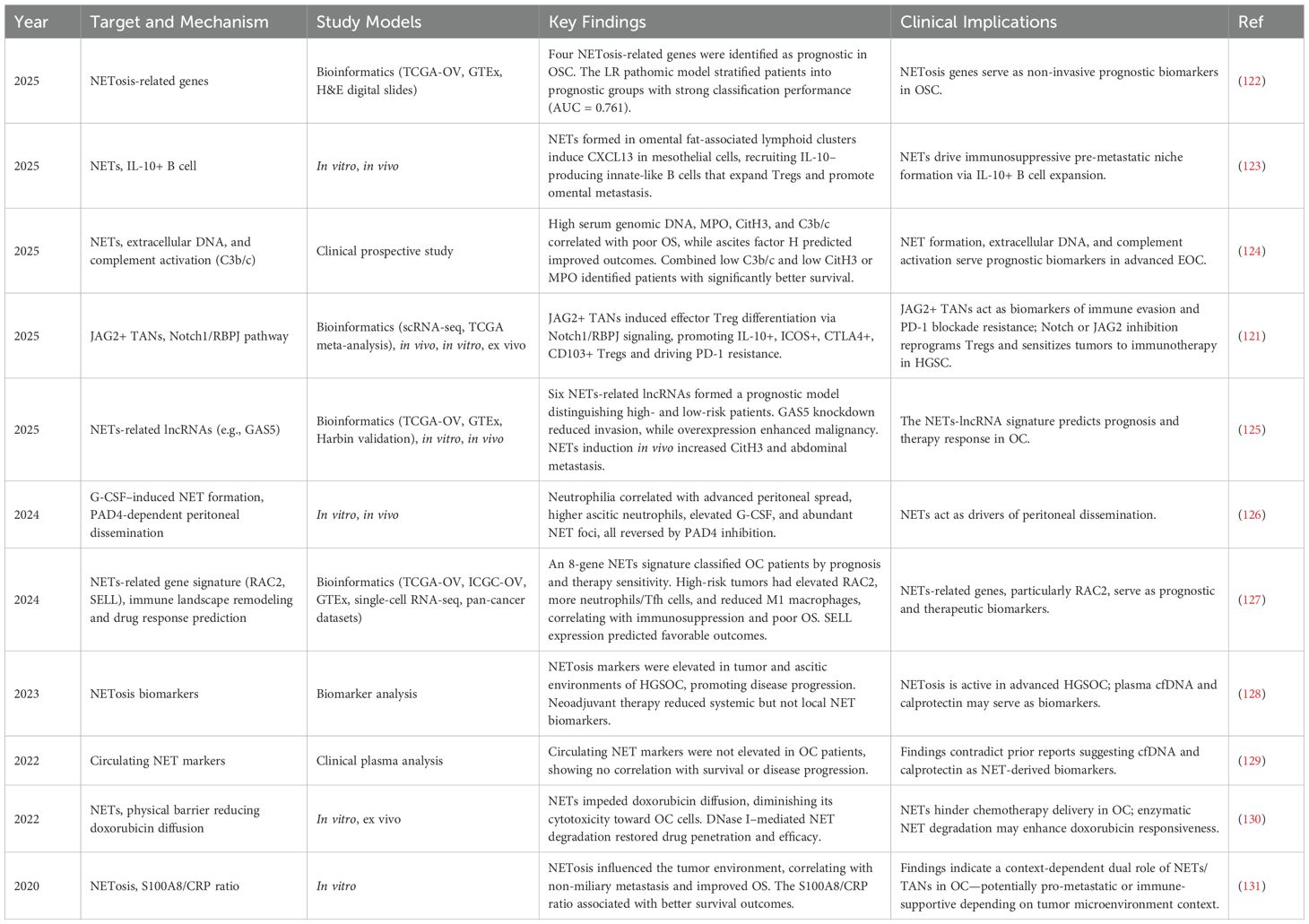
35. Yin B and Zhou H. Investigating the oncogenic and immunological implications of YTHDF1 in ovarian cancer. Biologics. (2025) 19:443–62. doi: 10.3389/fonc.2025.1641399
36. Ye TY, Wu SF, and Wang XY. CYBB as a potential therapeutic target through influencing ferroptosis and macrophage in ovarian cancer. Discov Oncol. (2025) 16:1513. doi: 10.1007/s12672-025-02891-8
37. Tcyganov EN, Kwak T, Yang X, Poli ANR, Hart C, Bhuniya A, et al. Targeting LxCxE cleft pocket of retinoblastoma protein in immunosuppressive macrophages inhibits ovarian cancer progression. Cancer Immunol Res. (2025) 111:6725–42. doi: 10.1158/2326-6066.CIR-24-0440
38. Neri M, Sanna E, Ferrari PA, Madeddu C, Lai E, Vallerino V, et al. Divergent immune-metabolic profiles in endometriosis and ovarian cancer: A cross-sectional analysis. Cancers (Basel). (2025) 17. doi: 10.3390/cancers17142325
39. Liu Y, Jiang LJ, Liu HF, Chen L, Guo L, Ge J, et al. Distinct roles of HMOX1 on tumor epithelium and macrophage for regulation of immune microenvironment in ovarian cancer. Int J Surg. (2025) 111:6725–42. doi: 10.1097/JS9.0000000000002829
40. Ni J, Li X, Wu Y, Tu X, Zhang X, Wang L, et al. CD81 Aggravates Ovarian Cancer Progression via p-Cresyl Sulfate-Mediated Mitophagy in Tim4(+) Tumour-Associated Macrophages. J Cell Mol Med. (2025) 29:e70701. doi: 10.1111/jcmm.70701
41. Ren M, Chen LL, Jiang LY, Yu HH, and Ji HZ. The CXCL8-CXCR2 axis promotes M2 macrophage polarization in ovarian cancer via RASGRP4-mediated mTOR-STAT3 signaling. Apoptosis. (2025) 30:1839–51. doi: 10.1007/s10495-025-02128-7
42. Li Q, Zhang S, Wang M, Yi Q, Xu H, Wang J, et al. Activated MAFB in ovarian cancer promotes cytoskeletal remodeling and immune microenvironment suppression by interfering with m6A modifications through WTAP competition. Oncogene. (2025) 44:3799–815. doi: 10.1038/s41388-025-03522-w
43. Liu X, Wang X, Zhang J, Tian T, Ning Y, Chen Y, et al. Myc-mediated inhibition of HIF1a degradation promotes M2 macrophage polarization and impairs CD8 T cell function through lactic acid secretion in ovarian cancer. Int Immunopharmacol. (2024) 141:112876. doi: 10.1016/j.intimp.2024.112876
44. Zhang N, Zhao F, Chen H, Wang J, and Li H. UBD-mediated glycolytic reprogramming promotes M2 macrophage polarization in ovarian cancer immune evasion. J Cell Commun Signal. (2025) 19:e70034. doi: 10.1002/ccs3.70034
45. Zhang W, Li X, Ji Y, Qi Y, Yao Q, Liu S, et al. Promoting tumor cell secretion of IL18 to induce reprogramming of tumor-associated macrophages - The novel anticancer mechanism of anlotinib in ovarian cancer. Biochem Pharmacol. (2025) 241:117170. doi: 10.1016/j.bcp.2025.117170
46. Nikeghbal P, Burke D, Armijo D, Aldarondo-Quiñones S, Lidke DS, and Steinkamp MP. Patient-derived ovarian cancer models demonstrate the influence of tumor-associated macrophages on therapeutic response. Oncoimmunology. (2025) 14:2537710. doi: 10.1080/2162402X.2025.2537710
47. Wang N, Yang Y, Wang H, Li Y, Wang M, and Li Q. Cinobufagin modulates vasculogenic mimicry and tumor-associated macrophages to inhibit ovarian cancer progression. Eur J Pharmacol. (2025) 987:177157. doi: 10.1016/j.ejphar.2024.177157
48. Si Q, Yang L, Liu J, Liu H, Bu R, and Cui N. Nucleotide receptor P2X7/STAT6 pathway regulates macrophage M2 polarization and its application in CAR-T immunotherapy. Immunobiology. (2025) 230:152863. doi: 10.1016/j.imbio.2024.152863
49. Li M, Tian Y, Si L, Fu H, Lai T, and Guo R. OTUD4-mediated inhibition of YAP1 signaling pathway in ovarian cancer: Implications for macrophage polarization and recruitment. Int Immunopharmacol. (2025) 147:114011. doi: 10.1016/j.intimp.2024.114011
50. Fang J, Wang J, Zhao X, Yang Y, and Xiao Y. KLHDC8A knockdown in normal ovarian epithelial cells promoted the polarization of pro-tumoral macrophages via the C5a/C5aR/p65 NFκB signaling pathway. Cell Immunol. (2025) 409-410:104913. doi: 10.1016/j.cellimm.2024.104913
51. Zhao L, Zhang Y, Wang J, Li D, and Hao X. UBE2I depletion regulated tumor-associated macrophage polarization into M1 type through reprogramming glycolysis and increases immunotherapy efficacy of anti-PD-L1 in ovarian cancer. Mol Immunol. (2025) 179:29–41. doi: 10.1016/j.molimm.2025.01.007
52. Wang YY, Choi MJ, Kim JH, and Choi JH. Enhanced expression of TRIM46 in ovarian cancer cells induced by tumor-associated macrophages promotes invasion via the wnt/β-catenin pathway. Cells. (2025) 14. doi: 10.3390/cells14030214
53. He L, Chen Q, and Wu X. Tumour-derived exosomal miR-205 promotes ovarian cancer cell progression through M2 macrophage polarization via the PI3K/Akt/mTOR pathway. J Ovarian Res. (2025) 18:28. doi: 10.1186/s13048-025-01616-3
54. Wang J, Zhu Y, He Y, and Shao W. TAM-derived exosomal miR-589-3p accelerates ovarian cancer progression through BCL2L13. J Ovarian Res. (2025) 18:36. doi: 10.1186/s13048-025-01618-1
55. Xiong J, Huang J, Xu H, Wu Q, Zhao J, Chen Y, et al. CpG-based nanovaccines enhance ovarian cancer immune response by gbp2-mediated remodeling of tumor-associated macrophages. Advanced Sci. (2025) 12:2412881. doi: 10.1002/advs.202412881
56. Antonsen KW, Jensen AG, Sorensen BS, Etzerodt A, Moestrup SK, and Møller HJ. In vitro ovarian tumor-conditioned CD163+ human macrophages retain phagocytic response to CD47 blockade. Cell Immunol. (2025) 409-410:409–10, 104932. doi: 10.1016/j.cellimm.2025.104932
57. Liu Q, Sun Y, Zhang T, Lin W, Zhang J, Zhang H, et al. GNA15 predicts poor outcomes as a novel biomarker related to M2 macrophage infiltration in ovarian cancer. Front Immunol. (2025) 16:1512086. doi: 10.3389/fimmu.2025.1512086
58. Zhao Y, Xu H, Liu Q, Yuan Y, Li R, Li D, et al. The interaction between IL-33 and TRIM28 in the regulation of macrophage polarization in an ST2-independent manner. Int Immunopharmacol. (2025) 152:114318. doi: 10.1016/j.intimp.2025.114318
59. Shrestha S, Giri A, Shrestha P, Kweon S, Hong I-S, Kwon TK, et al. CD44-targeted nanoparticles for remodeling the tumor microenvironment in a 3D macrophage-embedded ovarian cancer model with stem cell-like features. Int J Pharm. (2025) 674:125483. doi: 10.1016/j.ijpharm.2025.125483
60. Sun Z, Zhang Z, Zhang J, Yang Z, Pan S, Li X, et al. F-box only protein 25-mediated α-actinin 1 upregulation drives ovarian cancer progression via ERK1/2 signaling in tumor cells and macrophage M2 polarization. Int Immunopharmacol. (2025) 153:114479. doi: 10.1016/j.intimp.2025.114479
61. Li X, Su L, Qian C, Qiu W, Tao L, Guo Z, et al. Curcumin suppresses Malignant behaviors of ovarian cancer through regulation of tumor-associated macrophages. Med Oncol. (2025) 42:151. doi: 10.1007/s12032-025-02682-9
62. Osborn G, López-Abente J, Adams R, Laddach R, Grandits M, Bax HJ, et al. Hyperinflammatory repolarisation of ovarian cancer patient macrophages by anti-tumour IgE antibody, MOv18, restricts an immunosuppressive macrophage:Treg cell interaction. Nat Commun. (2025) 16:2903. doi: 10.1038/s41467-025-57870-y
63. Qi Y, Li Q, Chen L, Zhao S, Nie J, and Liu G. A new perspective: Acyl-CoA synthetase long-chain family member 4 inhibits ubiquitin-specific protease 7-induced epithelial ovarian cancer progression by inducing ferroptosis and M1 macrophage polarization. Cytojournal. (2025) 22:28. doi: 10.25259/Cytojournal_241_2024
64. Meng R and Zhang Z. Gallic acid inhibits the proliferation and migration of ovarian cancer cells via inhibition of the PI3K-AKT pathway and promoting M1-like macrophage polarization. Anal Cell Pathol (Amst). (2025) 2025:3880719. doi: 10.1155/ancp/3880719
65. Tian L, Liu L, Wang C, Kong Y, Miao Z, Yao Q, et al. PTTG1 promotes M2 macrophage polarization via the cGMP-PKG signaling pathway and facilitates EMT progression in human epithelial ovarian cancer cells. Discov Oncol. (2025) 16:730. doi: 10.1007/s12672-025-02512-4
66. Ye Y, Liu T, Xu F, Shen J, and Xu S. Integrated analyses reveal CXCL11 as an inhibitor in ovarian cancer and its facilitation of an M1 macrophage switch via the JAK2/STAT1 pathway. Int Immunopharmacol. (2025) 159:114900. doi: 10.1016/j.intimp.2025.114900
67. Chai R, Zheng K, Xu T, Wang H, Cheng X, Lu C, et al. SNX10 is involved in ovarian cancer cell metastasis by repolarizing tumor-associated macrophages through mTOR1/lysosomes pathway. Biomedicines. (2025) 13. doi: 10.3390/biomedicines13051021
68. Yin B, Ding J, Liu J, Hu H, Zhu Y, Yang M, et al. Exosomal CMTM4 induces immunosuppressive macrophages to promote ovarian cancer progression and attenuate anti-PD-1 immunotherapy. Adv Sci (Weinh). (2025) 12:e04436. doi: 10.1002/advs.202504436
69. Chatterjee B, Sarkar M, Ghosh D, Mishra S, Bose S, Khan MMA, et al. Tumor-associated macrophages contribute to cisplatin resistance via regulating Pol η-mediated translesion DNA synthesis in ovarian cancer. Cell Mol Life Sci. (2025) 82:220. doi: 10.1007/s00018-025-05731-8
70. Wang N, Liang H, and Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol. (2014) 5. doi: 10.3389/fimmu.2014.00614
71. Okła K, Czerwonka A, Wawruszak A, Bobiński M, Bilska M, Tarkowski R, et al. Clinical relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor cells (MDSCs) in epithelial ovarian cancer. Front Immunol. (2019) 10:691. doi: 10.3389/fimmu.2019.00691
72. Verreck FAW, De Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci. (2004) 101:4560–5. doi: 10.1073/pnas.0400983101
73. O’Neill LAJ and Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. (2016) 213:15–23. doi: 10.1084/jem.20151570
74. Sarode AY, Jha MK, Zutshi S, Ghosh SK, Mahor H, Sarma U, et al. Residue-specific message encoding in CD40-ligand. iScience. (2020) 23:101441. doi: 10.1016/j.isci.2020.101441
75. Silveira LS, Antunes Bde M, Minari AL, Dos Santos RV, Neto JC, and Lira FS. Macrophage polarization: implications on metabolic diseases and the role of exercise. Crit Rev Eukaryot Gene Expr. (2016) 26:115–32. doi: 10.1615/CritRevEukaryotGeneExpr.2016015920
76. Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. (2020) 80:1438–50. doi: 10.1158/0008-5472.CAN-19-2994
77. Vassiliou E and Farias-Pereira R. Impact of lipid metabolism on macrophage polarization: implications for inflammation and tumor immunity. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241512032
78. Di Conza G, Tsai C-H, Gallart-Ayala H, Yu Y-R, Franco F, Zaffalon L, et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat Immunol. (2021) 22:1403–15. doi: 10.1038/s41590-021-01047-4
79. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. (2014) 7:19. doi: 10.1186/1757-2215-7-19
80. El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M, et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal. (2020) 68:109539. doi: 10.1016/j.cellsig.2020.109539
81. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.01731
82. Li T, Akhtarkhavari S, Qi S, Fan J, Chang TY, Shen YA, et al. Inhibition of glutamine metabolism attenuates tumor progression through remodeling of the macrophage immune microenvironment. Adv Biol (Weinh). (2025) 9:e00738. doi: 10.1002/adbi.202400738
83. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflammation. (2015) 2015:1–16. doi: 10.1155/2015/816460
84. Ma RY, Black A, and Qian BZ. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. (2022) 43:546–63. doi: 10.1016/j.it.2022.04.008
85. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathology: Mech Disease. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
86. Jang YS, Kim TW, Ryu JS, Kong HJ, Jang SH, Nam GH, et al. Upregulation of programmed death ligand-1 in tumor-associated macrophages affects chemotherapeutic response in ovarian cancer cells. PloS One. (2023) 18:e0277285. doi: 10.1371/journal.pone.0277285
87. Yang YI, Wang YY, Ahn JH, Kim BH, and Choi JH. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. BioMed Pharmacother. (2022) 153:113474. doi: 10.1016/j.biopha.2022.113474
88. Esplen HP, Yang RK, Kalia A, Tang Z, Tang G, Medeiros LJ, et al. Recurrent somatic copy number alterations and their association with oncogene expression levels in high-grade ovarian serous carcinoma. Life (Basel). (2023) 13. doi: 10.3390/life13112192
89. Mlynska A, Povilaityte E, Zemleckaite I, Zilionyte K, Strioga M, Krasko J, et al. Platinum sensitivity of ovarian cancer cells does not influence their ability to induce M2-type macrophage polarization. Am J Reprod Immunol. (2018) 80:e12996. doi: 10.1111/aji.12996
90. Wang T, Zhou Y, Zhou Z, Zhang P, Yan R, Sun L, et al. Secreted protease PRSS35 suppresses hepatocellular carcinoma by disabling CXCL2-mediated neutrophil extracellular traps. Nat Commun. (2023) 14:1513. doi: 10.1038/s41467-023-37227-z
91. Gentric G, Kieffer Y, Mieulet V, Goundiam O, Bonneau C, Nemati F, et al. PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab. (2019) 29:156–73.e10. doi: 10.1016/j.cmet.2018.09.002
92. Motohara T, Masuda K, Morotti M, Zheng Y, El-Sahhar S, Chong KY, et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. (2019) 38:2885–98. doi: 10.1038/s41388-018-0637-x
93. Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. (2019) 29:1376–89.e4. doi: 10.1016/j.cmet.2019.02.016
94. Xu T, Yu S, Zhang J, and Wu S. Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers. J Hematol Oncol. (2021) 14:181. doi: 10.1186/s13045-021-01198-9
95. Dimeloe S, Gubser P, Loeliger J, Frick C, Develioglu L, Fischer M, et al. Tumor-derived TGF-β inhibits mitochondrial respiration to suppress IFN-γ production by human CD4(+) T cells. Sci Signal. (2019) 12. doi: 10.1126/scisignal.aav3334
96. Yang Y, Yang Y, Yang J, Zhao X, and Wei X. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol. (2020) 8:758. doi: 10.3389/fcell.2020.00758
97. Liguori M, Solinas G, Germano G, Mantovani A, and Allavena P. Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers (Basel). (2011) 3:3740–61. doi: 10.3390/cancers3043740
98. Yu S, Wang S, Wang X, and Xu X. The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis. Cancer Cell Int. (2024) 24:335. doi: 10.1186/s12935-024-03518-8
99. Wang X, Zhu Q, Lin Y, Wu L, Wu X, Wang K, et al. Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer. (2017) 117:1371–82. doi: 10.1038/bjc.2017.297
100. Schumacher JJ, Dings RP, Cosin J, Subramanian IV, Auersperg N, and Ramakrishnan S. Modulation of angiogenic phenotype alters tumorigenicity in rat ovarian epithelial cells. Cancer Res. (2007) 67:3683–90. doi: 10.1158/0008-5472.CAN-06-3608
101. Rakina M, Kazakova A, Villert A, Kolomiets L, and Larionova I. Spheroid formation and peritoneal metastasis in ovarian cancer: the role of stromal and immune components. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23116215
102. Rudlowski C, Pickart AK, Fuhljahn C, Friepoertner T, Schlehe B, Biesterfeld S, et al. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer. (2006) 16 Suppl 1:183–9. doi: 10.1111/j.1525-1438.2006.00307.x
103. Kassim SK, El-Salahy EM, Fayed ST, Helal SA, Helal T, Azzam Eel D, et al. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem. (2004) 37:363–9. doi: 10.1016/j.clinbiochem.2004.01.014
104. Bhaskari J, Bhagat R, Shilpa V, Premalata CS, and Krishnamoorthy L. Pre-operative plasma VEGF-C levels portend recurrence in epithelial ovarian cancer patients and is a bankable prognostic marker even in the initial assessment of a patient. J Ovarian Res. (2024) 17:77. doi: 10.1186/s13048-024-01398-0
105. Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, and Ellis LM. Markedly elevated levels of vascular endothelial growth factor in Malignant ascites. Ann Surg Oncol. (1999) 6:373–8. doi: 10.1007/s10434-999-0373-0
106. Huang D, Ke L, Cui H, Li S, and Sun F. Efficacy and safety of VEGF/VEGFR inhibitors for platinum-resistant ovarian cancer: a systematic review and meta-analysis of randomized controlled trials. BMC Womens Health. (2024) 24:34. doi: 10.1186/s12905-023-02879-y
107. Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. (2007) 67:5708–16. doi: 10.1158/0008-5472.CAN-06-4375
108. Carroll MJ, Fogg KC, Patel HA, Krause HB, Mancha AS, Patankar MS, et al. Alternatively-activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res. (2018) 78:3560–73. doi: 10.1158/0008-5472.CAN-17-3341
109. Gao Q, Yang Z, Xu S, Li X, Yang X, Jin P, et al. Correction: Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J Exp Med. (2019) 216:2448. doi: 10.1084/jem.2018076508222019c
110. Zeng X-Y, Xie H, Yuan J, Jiang X-Y, Yong J-H, Zeng D, et al. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol Ther. (2019) 20:956–66. doi: 10.1080/15384047.2018.1564567
111. Amer H, Kampan NC, Itsiopoulos C, Flanagan KL, Scott CL, Kartikasari AER, et al. Interleukin-6 modulation in ovarian cancer necessitates a targeted strategy: from the approved to emerging therapies. Cancers. (2024) 16:4187. doi: 10.3390/cancers16244187
112. Ghoneum A, Afify H, Salih Z, Kelly M, and Said N. Role of tumor microenvironment in the pathobiology of ovarian cancer: Insights and therapeutic opportunities. Cancer Med. (2018) 7:5047–56. doi: 10.1002/cam4.1741
113. Coffelt SB, Wellenstein MD, and de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
114. Colotta F, Re F, Polentarutti N, Sozzani S, and Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. (1992) 80:2012–20. doi: 10.1182/blood.V80.8.2012.2012
115. van Raam BJ, Drewniak A, Groenewold V, van den Berg TK, and Kuijpers TW. Granulocyte colony-stimulating factor delays neutrophil apoptosis by inhibition of calpains upstream of caspase-3. Blood. (2008) 112:2046–54. doi: 10.1182/blood-2008-04-149575
116. Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5. 4 days Blood. (2010) 116:625–7. doi: 10.1182/blood-2010-01-259028
117. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. (2015) 21:938–45. doi: 10.1038/nm.3909
118. Shaul ME and Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. (2019) 16:601–20. doi: 10.1038/s41571-019-0222-4
119. Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of Malignant cell intravasation. Am J Pathol. (2011) 179:1455–70. doi: 10.1016/j.ajpath.2011.05.031
120. Yang M, Zhang G, Wang Y, He M, Xu Q, Lu J, et al. Tumour-associated neutrophils orchestrate intratumoural IL-8-driven immune evasion through Jagged2 activation in ovarian cancer. Br J Cancer. (2020) 123:1404–16. doi: 10.1038/s41416-020-1026-0
121. Wang C, Yang M, Zhong Y, Cao K, Wang X, Zhang C, et al. Immunosuppressive JAG2(+) tumor-associated neutrophils hamper PD-1 blockade response in ovarian cancer by mediating the differentiation of effector regulatory T cells. Cancer Commun (Lond). (2025) 45:747–73. doi: 10.1002/cac2.70021
122. Zhan F, Guo Y, and He L. NETosis genes and pathomic signature: A novel prognostic marker for ovarian serous cystadenocarcinoma. J Imaging Inform Med. (2025) 38:2412–27. doi: 10.1007/s10278-024-01366-6
123. Lee W, Ko SY, Akasaka H, Weigert M, Lengyel E, and Naora H. Neutrophil extracellular traps promote pre-metastatic niche formation in the omentum by expanding innate-like B cells that express IL-10. Cancer Cell. (2025) 43:69–85.e11. doi: 10.1016/j.ccell.2024.12.004
124. Ricciuti J, Liu Q, Khan A, Joseph JM, Veuskens B, Giridharan T, et al. Prognostic significance of serum complement activation, neutrophil extracellular traps and extracellular DNA in newly diagnosed epithelial ovarian cancer. Gynecol Oncol. (2025) 193:49–57. doi: 10.1016/j.ygyno.2024.12.006
125. Wang J, Liang Y, Meng Y, Chen J, Fang L, Yang H, et al. Assessment of lncRNA biomarkers based on NETs for prognosis and therapeutic response in ovarian cancer. Sci Rep. (2025) 15:13042. doi: 10.1038/s41598-025-97548-5
126. Bun M, Kawano M, Yamamoto G, Sakata M, Shimura K, Toda A, et al. G-CSF induces neutrophil extracellular traps formation and promotes ovarian cancer peritoneal dissemination. J Leukoc Biol. (2024) 116:1157–68. doi: 10.1093/jleuko/qiae166
127. Zhang Y, Wang C, Cheng S, Xu Y, Gu S, Zhao Y, et al. A neutrophil extracellular traps-related signature predicts clinical outcomes and identifies immune landscape in ovarian cancer. J Cell Mol Med. (2024) 28:e70302. doi: 10.1111/jcmm.70302
128. Tomás-Pérez S, Oto J, Aghababyan C, Herranz R, Cuadros-Lozano A, González-Cantó E, et al. Increased levels of NETosis biomarkers in high-grade serous ovarian cancer patients' biofluids: Potential role in disease diagnosis and management. Front Immunol. (2023) 14:1111344. doi: 10.3389/fimmu.2023.1111344
129. Dobilas A, Thalin C, Wallen H, and Borgfeldt C. Circulating markers of neutrophil extracellular traps (NETs) in patients with ovarian tumors. Anticancer Res. (2022) 42:965–71. doi: 10.21873/anticanres.15556
130. Tamura K, Miyato H, Kanamaru R, Sadatomo A, Takahashi K, Ohzawa H, et al. Neutrophil extracellular traps (NETs) reduce the diffusion of doxorubicin which may attenuate its ability to induce apoptosis of ovarian cancer cells. Heliyon. (2022) 8:e09730. doi: 10.1016/j.heliyon.2022.e09730
131. Muqaku B, Pils D, Mader JC, Aust S, Mangold A, Muqaku L, et al. Neutrophil extracellular trap formation correlates with favorable overall survival in high grade ovarian cancer. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12020505
132. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
133. Sionov RV, Fridlender ZG, and Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. (2015) 8:125–58. doi: 10.1007/s12307-014-0147-5
134. Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. (2018) 78:2680–90. doi: 10.1158/0008-5472.CAN-17-3614
135. Giese MA, Hind LE, and Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. (2019) 133:2159–67. doi: 10.1182/blood-2018-11-844548
136. Koga Y, Matsuzaki A, Suminoe A, Hattori H, and Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. (2004) 64:1037–43. doi: 10.1158/0008-5472.CAN-03-1808
137. Li C, Zhou Y, Men C, Yang W, Liu Q, and Cheng Z. N2-neutrophils promote invasion and metastasis of ovarian cancer by upregulating MAPK signaling. Res Square. (2022). doi: 10.21203/rs.3.rs-59568/v2
138. Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. (2006) 108:1627–34. doi: 10.1182/blood-2006-11-010389
139. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
140. Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. (2012) 109:13076–81. doi: 10.1073/pnas.1200419109
141. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. (2016) 76:1367–80. doi: 10.1158/0008-5472.CAN-15-1591
142. Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. (2013) 4:48. doi: 10.3389/fimmu.2013.00048
143. Olsson A and Cedervall J. NETosis in cancer – platelet–neutrophil crosstalk promotes tumor-associated pathology. Front Immunol. (2016) 7. doi: 10.3389/fimmu.2016.00373
144. Castaño M, Tomás-Pérez S, González-Cantó E, Aghababyan C, Mascarós-Martínez A, Santonja N, et al. Neutrophil extracellular traps and cancer: trapping our attention with their involvement in ovarian cancer. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24065995
145. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, and Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. (2019) 216:176–94. doi: 10.1084/jem.20181170
146. Singel KL, Grzankowski KS, Khan A, Grimm MJ, D'Auria AC, Morrell K, et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br J Cancer. (2019) 120:207–17. doi: 10.1038/s41416-018-0339-8
147. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. (2013) 123:3446–58. doi: 10.1172/JCI67484
148. Yamamoto G, Kawano M, Bun M, Shimura K, Toda A, Nakamura K, et al. Ovarian cancer predisposes neutrophils to form neutrophil extracellular traps (NETs). Cancer Res. (2024) 84:5329. doi: 10.1158/1538-7445.AM2024-5329
149. Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. (2020) 13:3. doi: 10.1186/s13045-019-0836-0
150. De Meo M and Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. (2022) 57:101595. doi: 10.1016/j.smim.2022.101595
151. Awasthi D and Sarode A. Neutrophils at the crossroads: unraveling the multifaceted role in the tumor microenvironment. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25052929
152. Hashiguchi Y, Kasai M, Fukuda T, Ichimura T, Yasui T, and Sumi T. Chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic Malignancy. Anticancer Drugs. (2015) 26:1054–60. doi: 10.1097/CAD.0000000000000279
153. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. (2015) 522:345–8. doi: 10.1038/nature14282
154. Bordon Y. NETs awaken sleeping cancer cells. Nat Rev Immunol. (2018) 18:665 –. doi: 10.1038/s41577-018-0081-8
155. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. (2018) 361. doi: 10.1126/science.aao4227
156. Kumar V and Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. (2010) 10:1325–34. doi: 10.1016/j.intimp.2010.08.012
157. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, and Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
158. Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. (2016) 150:1646–58.e17. doi: 10.1053/j.gastro.2016.02.040
159. Wouters MCA and Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. (2018) 24:6125–35. doi: 10.1158/1078-0432.CCR-18-1481
160. Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. (2017) 32:654–68.e5. doi: 10.1016/j.ccell.2017.10.005
161. Kim IS, Gao Y, Welte T, Wang H, Liu J, Janghorban M, et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. (2019) 21:1113–26. doi: 10.1038/s41556-019-0373-7
162. Song J, Xiao T, Li M, and Jia Q. Tumor-associated macrophages: Potential therapeutic targets and diagnostic markers in cancer. Pathol Res Pract. (2023) 249:154739. doi: 10.1016/j.prp.2023.154739
163. Truxova I, Cibula D, Spisek R, and Fucikova J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2022-005968
164. Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. (2020) 38:947–53. doi: 10.1038/s41587-020-0462-y
165. Chen Y, Zhu X, Liu H, Wang C, Chen Y, Wang H, et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J Transl Med. (2023) 21:654. doi: 10.1186/s12967-023-04479-8
166. Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O'Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. (2021) 12:877. doi: 10.1038/s41467-021-20893-2
167. Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. (2015) 75:2653–62. doi: 10.1158/0008-5472.CAN-14-3299
Keywords: tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), ovarian cancer, tumor microenvironment, immunotherapy
Citation: Cheng K-C, Lin Y-H, Wu D-S, Shih I-M and Wang T-L (2025) Macrophages and neutrophils in ovarian cancer microenvironment. Front. Immunol. 16:1677441. doi: 10.3389/fimmu.2025.1677441
Received: 31 July 2025; Accepted: 23 October 2025;
Published: 06 November 2025.
Edited by:
Noha Mousaad Elemam, University of Sharjah, United Arab EmiratesReviewed by:
Aditya Yashwant Sarode, Columbia University, United StatesDeepika Awasthi, NewYork-Presbyterian, United States
Copyright © 2025 Cheng, Lin, Wu, Shih and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-Li Wang, dGx3QGpobWkuZWR1
†These authors have contributed equally to this work and share first authorship
 Kuang-Chao Cheng
Kuang-Chao Cheng Yu-Hsin Lin
Yu-Hsin Lin Dao-Sian Wu
Dao-Sian Wu Ie-Ming Shih
Ie-Ming Shih Tian-Li Wang
Tian-Li Wang