- 1Department of Oncology, The First Affiliated Hospital with Nanjing Medical University, Nanjing Medical University, Nanjing, Jiangsu, China
- 2Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China
- 3Department of Oncology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, China
Thymic squamous cell carcinoma (TSCC) is a rare malignancy with an annual incidence of 0.15–0.32 per 100,000 population and exhibits aggressive behavior including early metastasis. Anti-PD-1 immunotherapy combined with chemotherapy has emerged as a potential strategy for TSCC. We report a 60-year-old male with a 1-month history of persistent dry cough and generalized fatigue. Diagnostic evaluation revealed advanced TSCC (cT3N2M1b), with bilateral lung, right pleural, left adrenal, mediastinal and right hilar lymph nodes metastases. Significantly elevated CA125 and CYFRA211 levels were observed. Initial first-line carboplatin plus polymeric micellar paclitaxel (PM-PTX) stabilized the disease. Subsequent adjustments to the immunotherapy regimen led to a two-phase approach combining tislelizumab, lenvatinib, PM-PTX, with or without carboplatin. This strategy achieved sustained tumor burden reduction with improved quality of life and nutritional status throughout treatment, without significant immune-related adverse events (irAEs). Additionally, we discuss the mechanisms of combination therapy, immunotherapy toxicities in thymic malignancies, and propose personalized combination strategies to guide clinicians in selecting treatment options.
Introduction
Thymic carcinoma is a rare anterior mediastinal malignancy with an annual incidence of 0.07 to 0.38/100,000 and a 55% 5-year survival rate (1, 2). Thymic squamous cell carcinoma (TSCC), its most common histological subtype, arises from thymic epithelial cells with aggressive behavior (1). TSCC typically expresses CD5, CK5, p40, and p63 by immunohistochemistry (IHC) (3), and frequently invades adjacent structures such as pericardium, lungs, phrenic nerve, and major blood vessels, or metastasizes to regional lymph nodes and extrathoracic sites (2). While localized TSCC is managed surgically alone or in combination with radiotherapy and chemotherapy, approximately 68% present with metastasis at the time of diagnosis. Platinum-based chemotherapy remains standard for unresectable disease (3, 4).
Immune checkpoint inhibitors targeting PD-1/PD-L1 have shown promise in thymic carcinoma. Giaccone et al. reported pembrolizumab’s efficacy as second-line therapy, albeit with higher incidence of severe immune-related adverse events (irAEs, 15%) compared to other tumor types (5). Lenvatinib, a multi-target inhibitor of VEGFR, FGFR, RET, c-Kit, and other kinases, also demonstrates a positive effect on advanced cases (6). Optimal immune-combination strategies for advanced TSCC require further exploration.
Here, we describe an advanced metastatic TSCC patient successfully treated with tislelizumab (anti-PD-1), lenvatinib, and polymeric micellar paclitaxel (PM-PTX), achieving tumor reduction while preserving quality of life through regimen adjustment. We also review the mechanisms underlying the enhanced anti-tumor efficacy of combination therapy, analyze patient selection criteria suitable for immunotherapy, and propose personalized combination strategies.
Case presentation
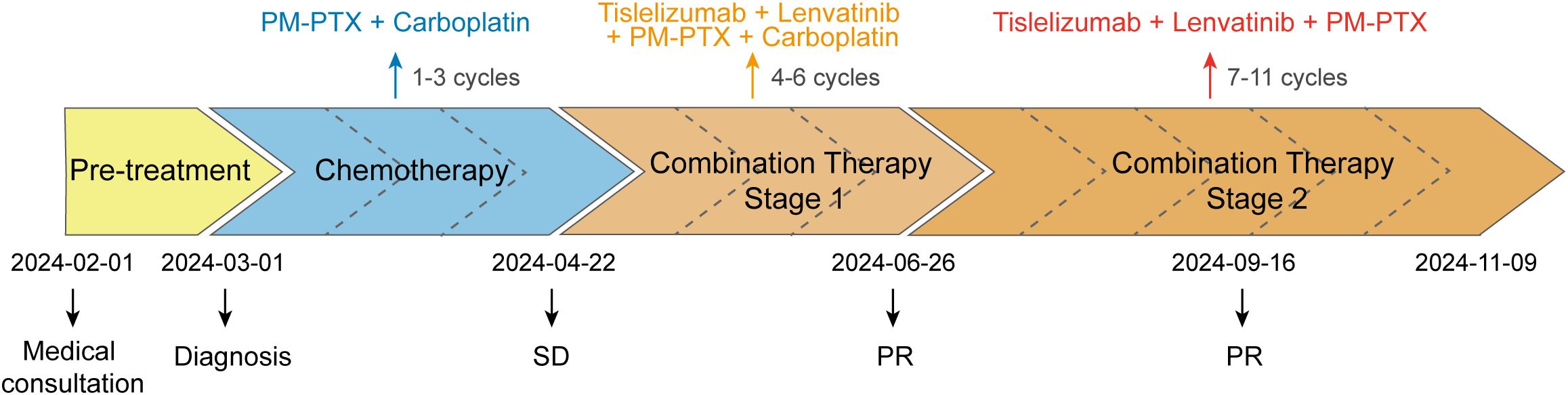
In February 2024, a 60-year-old male patient presented with a 1-month history of a dry cough and fatigue without an apparent cause, which prompted a visit to a local hospital. A chest computed tomography (CT) scan revealed a right lung mass. For further evaluation and treatment, the patient was admitted to our oncology department on March 1, 2024. Enhanced chest and whole-abdomen CT scan showed a space-occupying lesion in the right middle lobe (RML) of the lung, multiple solid nodules in both lungs, multiple soft tissue density shadows in the right pleura, an inhomogeneous enhancing lesion in the left adrenal gland, a mass-like soft tissue density shadow in the anterior mediastinum, and multiple enlarged lymph nodes in the mediastinum and right hilum (Figure 1). On March 5, 2024, CT-guided right lung mass biopsy indicated invasive carcinoma. IHC showed CK5/6(+), P40(+), P63(+), TTF-1(-), Napsin A(-), CD5(+), and CD117(+), with a Ki-67 ~35% (Figure 2). Multidisciplinary diagnosis confirmed advanced thymic squamous cell carcinoma, with metastasis to lung, right pleura, left adrenal gland, and mediastinal and right hilar lymph nodes (cT3N2M1b, Stage IVB). Biomarker and NGS analysis revealed PD-L1 negativity (TPS<1%, details in Supplementary Table 1). Tumor markers abnormally elevated: CA125–274 ng/mL (ref: 0-24.00), CYFRA211 26.89 ng/mL (ref: 0-3.3) (Figure 3).
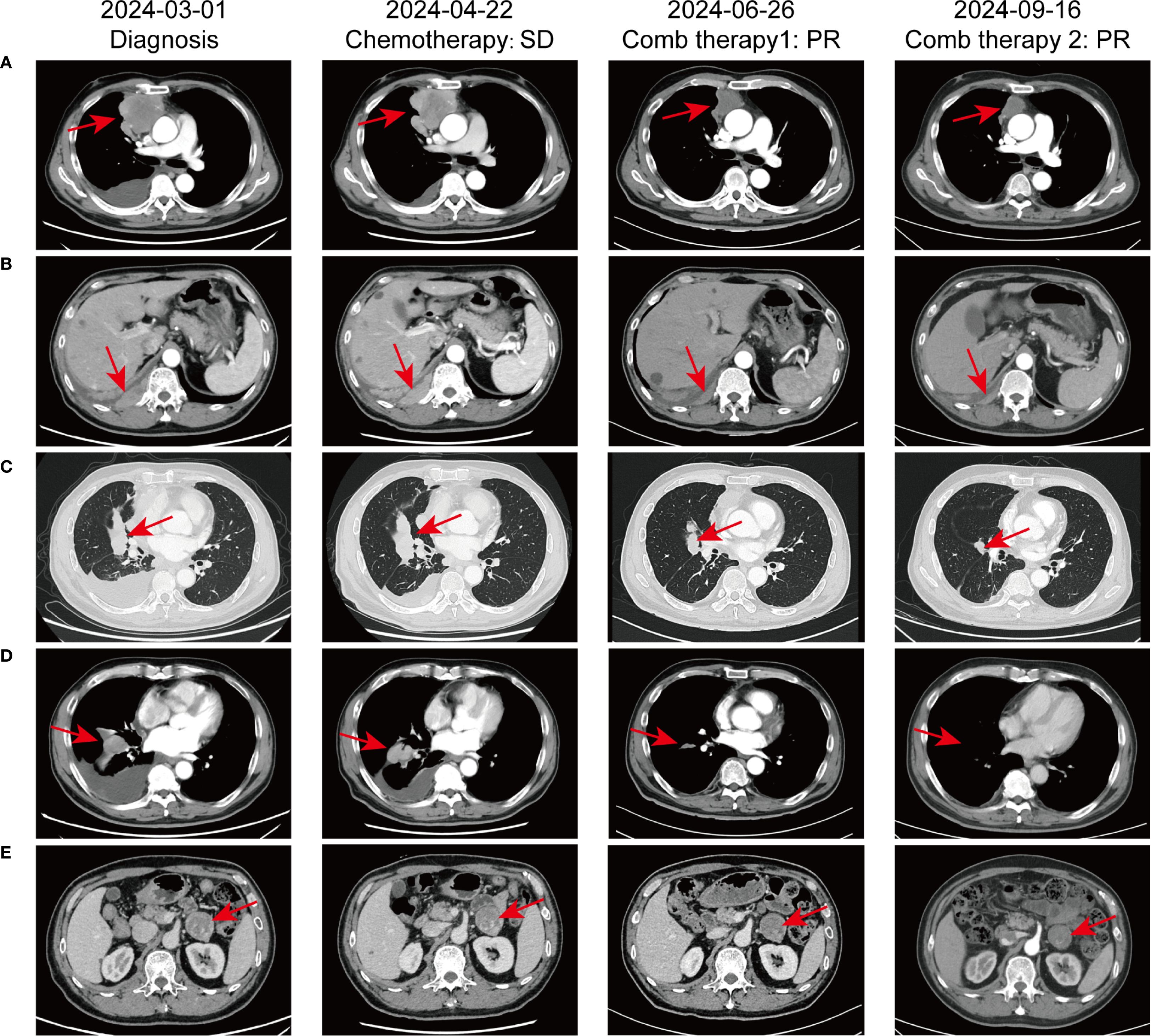
Figure 1. Comparative chest CT scans illustrating the progression of pulmonary lesions throughout the patient’s treatment course. (A) Irregular mass-like soft tissue shadow in the anterior superior mediastinum; (B) Mass-like soft tissue shadow in the right pleura; (C) Mass in the right middle lobe of the lung; (D) Multiple lymph node metastases in the mediastinum and right hilar lymph nodes; (E) Metastasis to the left adrenal gland.
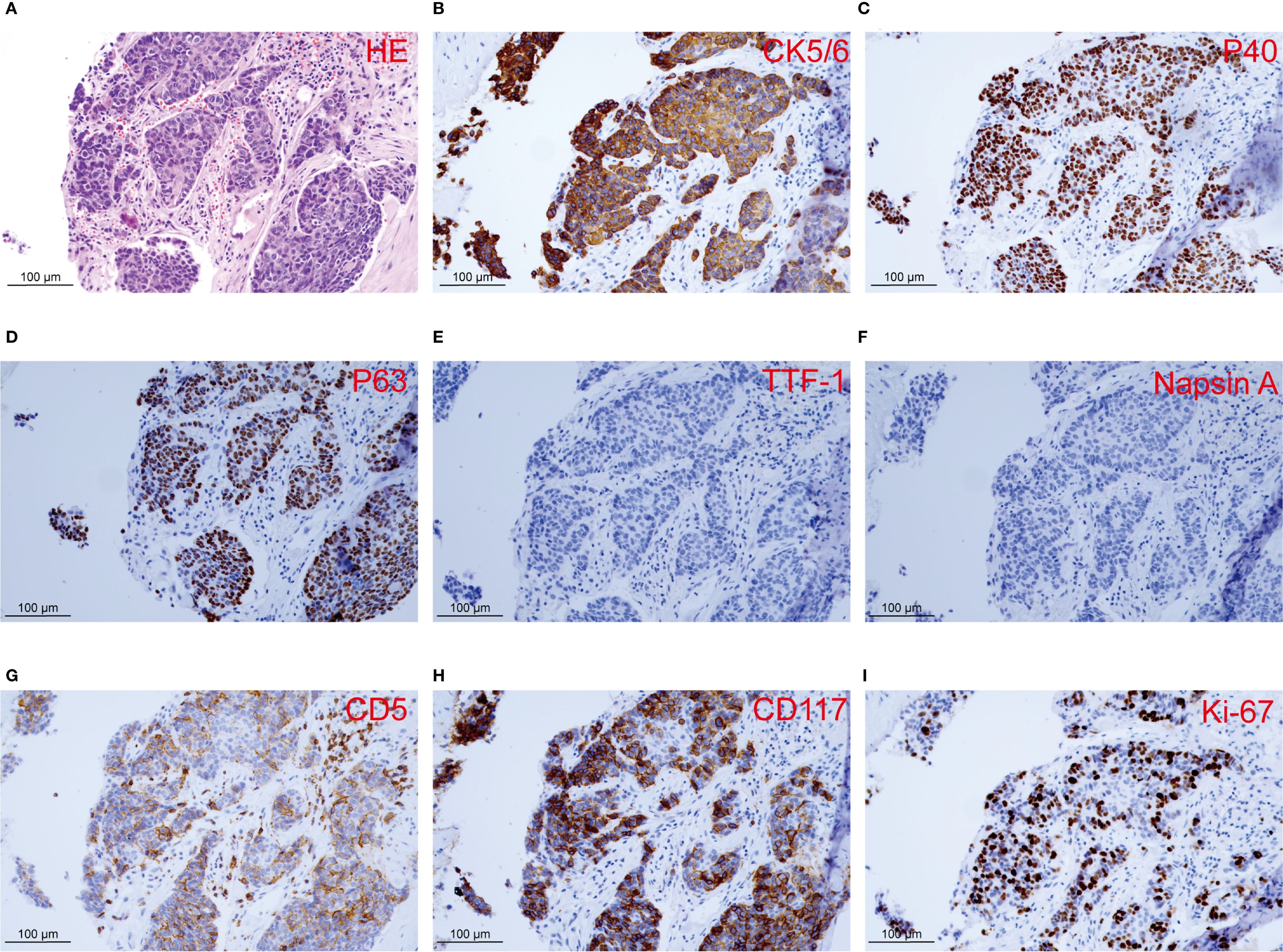
Figure 2. Histopathology and immunohistochemistry of the right lung lesion observed in the patient. (A) Histological section with HE staining reveals invasive carcinoma. (B-I) IHC staining of CK5/6, P40, P63, TTF-1, Napsin A, CD5, CD117, and Ki-67.
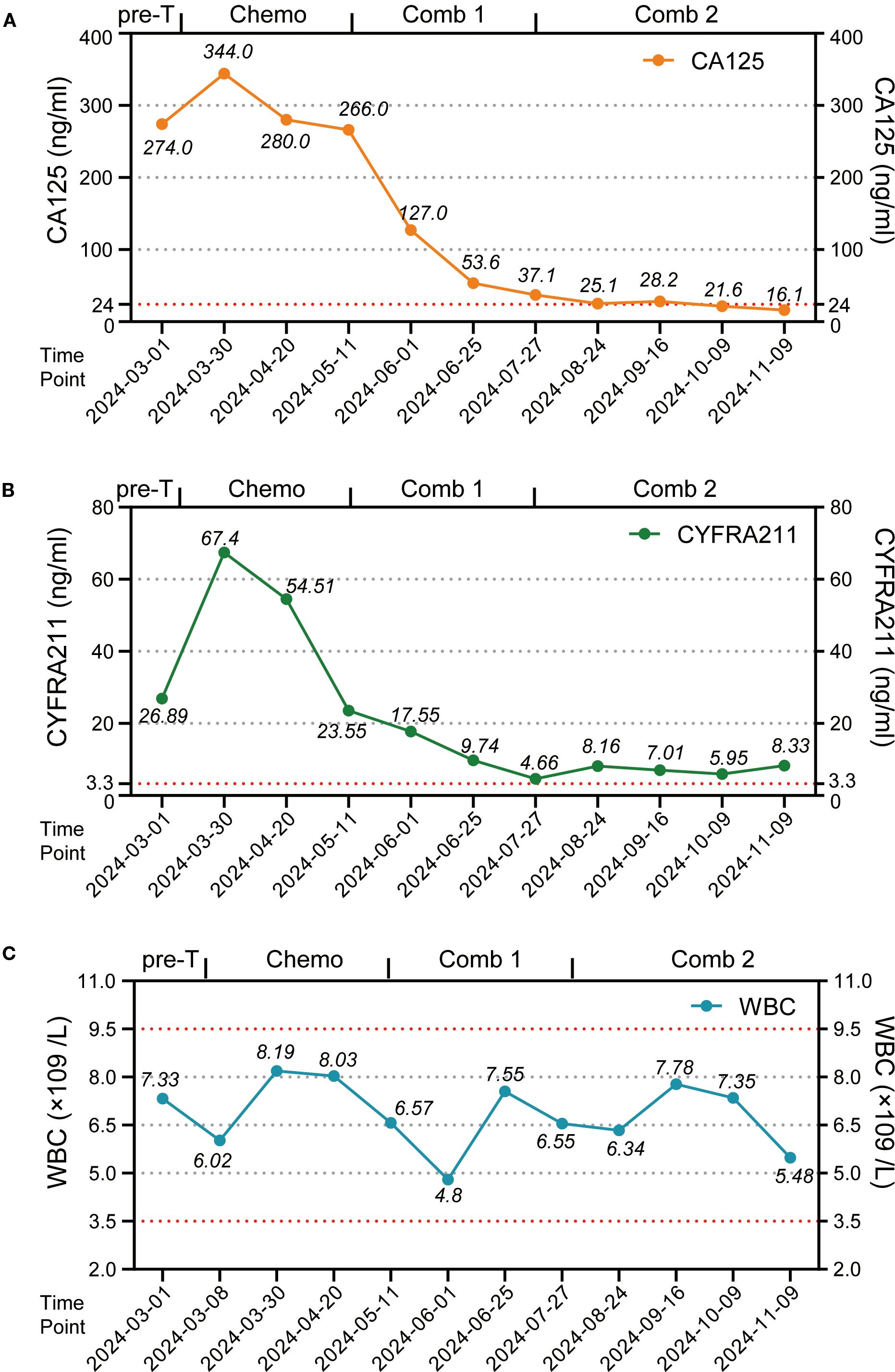
Figure 3. The variations in tumor markers and leukocyte counts during patient treatment. (A) Point-line plot of tumor marker CA125 levels. (B) Point-line plot of tumor marker CYFRA 21–1 levels. (C) Point-line plot of WBC counts. Pre-T, pre-treatment; Chemo, chemotherapy; Comb 1, combination therapy stage 1; Comb 2, combination therapy stage 2; CA125, Cancer Antigen 125; CYFRA211, Cytokeratin 19 fragment antigen 21-1.
For unresectable disease, the patient underwent three cycles of first-line chemotherapy from March 7 to April 20, 2024: PM-PTX 360 mg D1 q3w + carboplatin 450 mg D1 q3w. A follow-up chest CT on April 22 showed largely unchanged lesions after treatment, resulting in an efficacy evaluation of stable disease (SD) (Figure 1). CA125 and CYFRA211 remained elevated (Figure 3). The patient experienced tolerable chemotherapy side effects, including decreased appetite and slight weight loss.
To reduce tumor burden, we evaluated the feasibility of immunotherapy and oral anti-angiogenic agents. Based on the REMORA study, lenvatinib has been shown to provide survival benefits for thymic carcinoma patients who have undergone first-line platinum-based chemotherapy (7). Consequently, from May 11 to June 25, 2024, the patient received three cycles of PM-PTX 360 mg D1 q3w + carboplatin 450 mg D1 q3w + tislelizumab 200 mg D1 q3w + lenvatinib 8 mg qd. CT on June 26, 2024, showed reduction of both primary and metastatic lesions. The efficacy evaluation indicated a partial response (PR), confirming the initial effectiveness of the added immunotherapy. CA125 significantly decreased to 53.6 ng/mL, CYFRA211 to 9.74 ng/mL; WBC level remained within the normal range (Figure 3). Throughout the treatment period, the patient maintained a positive mental state, experienced reduced toxic side effects from the medications, and no adverse reactions to the immunotherapy were observed.
Given the cumulative toxicity resulting from the long-term use of chemotherapeutic drugs, carboplatin was discontinued. From July 27 to November 9, 2024, the patient underwent five cycles of PM-PTX 360mg D1 q3w + tislelizumab 200mg D1 q3w + lenvatinib 8mg qd. After three cycles of combination therapy, CT on September 16 revealed further shrinkage of the primary and metastatic lesions compared to June 25, with minor chronic inflammation observed in the right lung and the lower lobe of the left lung. The efficacy was evaluated as PR, confirming that the combination therapy has effectively controlled the patient’s condition. CA125 and CYFRA211 neared normal, and WBC remains within the normal range (Figure 3). During the five cycles of combined treatment, the patient demonstrated an improved mental state, further alleviation of clinical symptoms, and steady weight gain. The toxic side effects of anti-tumor treatments, particularly immunotherapy, can significantly impact the patient’s quality of life and adherence to treatment (8). However, over total eight immunotherapy cycles administered in two phases, the patient experienced no grade ≥3 irAEs. Only grade 1–2 toxicities were observed, including forearm erythema, abdominal discomfort, and mildly elevated transaminases (Supplementary Table 2). Tumor condition improved significantly with preserved quality of life. Treatment schedule is illustrated in Figure 4.
Discussion
This case highlights the critical balance between efficacy and toxicity management in advanced TSCC. Our patient achieved sustained tumor regression through a two-phase regimen of tislelizumab, lenvatinib, PM-PTX, with or without carboplatin. Notably, the patient reported high satisfaction with treatment outcomes, citing effective tumor control without significant decline in quality of life or physical discomfort throughout the therapeutic course. This outcome underscores the potential of personalized chemo-immunotherapy combinations for aggressive malignancies.
PD-1 blockade reshapes the tumor microenvironment (TME) by disrupting PD-1/PD-L1 interactions. This mechanism enhances anti-tumor immunity through dual pathways: activating effector T cells, B cells, and NK cells while simultaneously depleting immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (9, 10). These coordinated actions increase tumor-infiltrating lymphocytes and improve antigen presentation, ultimately driving robust anti-tumor responses (11).
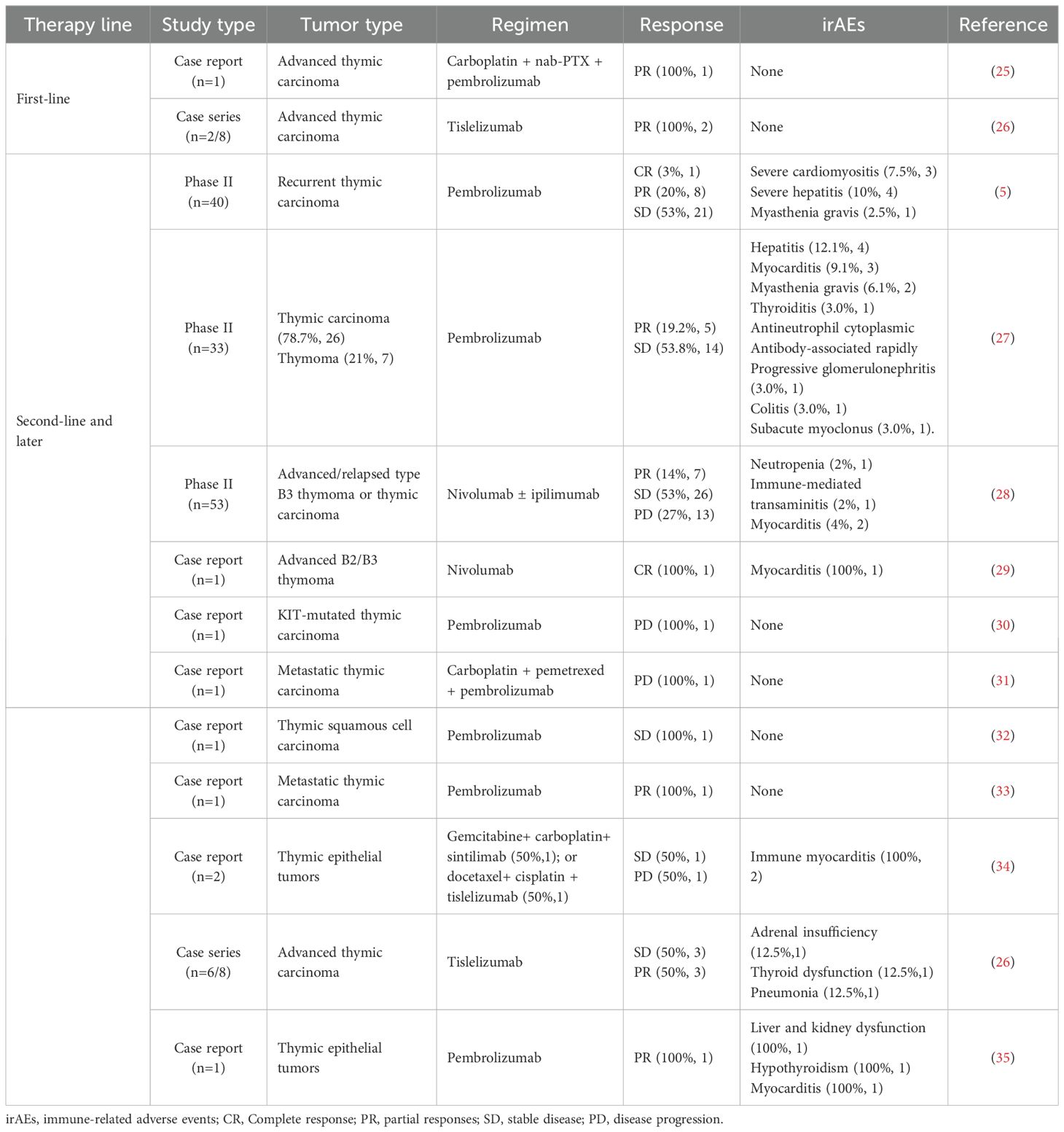
Our analysis of published case reports/series and clinical trials of immunotherapy in thymic tumors (both first-line and later-line settings) reveals a complex efficacy-safety profile (Table 1). While immunotherapy demonstrates clinical activity across treatment lines, thymic malignancies exhibit particularly high rates of severe multi-organ irAEs. Notably, a phase II clinical trial demonstrated that pembrolizumab monotherapy had a 15% incidence of Grade 3–4 irAEs, including immune-related hepatitis, myocarditis, thyroiditis, colitis, conjunctivitis, and nephritis (5). These irAEs increase complexity and cost while compromising survival benefits, quality of life and treatment adherence.
For unresectable or recurrent/metastatic thymic carcinoma beyond first-line therapy, identifying irAE-prone populations is critical. TSCC patients with chronic obstructive pulmonary disease (COPD), autoimmune diseases, or long-term immunosuppressive therapy exhibit heightened susceptibility to irAEs such as pneumonitis (12). Furthermore, combined targeted therapy may further increase irAE incidence despite enhancing anti-tumor immunity. These factors enable pretreatment risk assessment and preventive strategy implementation.
Chemotherapy exerts dual effects on immunity. While primarily inhibiting tumor proliferation via DNA disruption, its non-selective cytotoxicity significantly suppresses immune function by depleting rapidly dividing lymphocytes, particularly effector T cells, B cells, and NK cells. For instance, peripheral T cells of cancer patients can decrease by up to 75% following cyclophosphamide chemotherapy (13). Paradoxically, this systemic immunosuppression may mitigate excessive immune activation induced by immunotherapy, thereby reducing irAE risk. Simultaneously, chemotherapy enhances tumor-specific immunity through immunogenic cell death (ICD). Agents like oxaliplatin release tumor antigens and damage-associated molecular patterns (DAMPs) that activate CD8+ T cells, while selectively eliminating immunosuppressive MDSCs via ROS-induced apoptosis (14, 15). In this case report, the patient with TSCC received three cycles of chemotherapy before initiating immunotherapy, which aids in activating the immune system. Therefore, the balance of systemic suppression and localized activation within tumors coordinates therapeutic effects.
Critically, the combination of chemotherapy with immunotherapy has demonstrated synergistic efficacy, facilitates the formation of immune memory, and is associated with reduced irAEs versus monotherapy (16). KEYNOTE trials demonstrate PD-1 inhibitors combined with chemotherapy reduce irAE incidence versus PD-1 inhibitors monotherapy (17, 18). In our case, chemotherapy’s lymphocyte depletion likely counterbalanced immunotherapy-induced hyperactivation, explaining the absence of severe irAEs despite prolonged therapy.
Notably, Lenvatinib remodels TME through dual mechanisms. It normalizes tumor vasculature by inhibiting VEGFR/FGFR signaling, reducing vascular endothelial-cadherin density and enhancing T-cell infiltration. Concurrently, it depletes immunosuppressive cells (e.g., tumor-associated macrophages and MDSCs) and promotes M1 macrophage polarization (19, 20). Critically, Lenvatinib downregulates exhaustion markers (PD-1, TIM-3) on cytotoxic T lymphocytes by restoring IFNγ-mediated JAK/STAT signaling through FGFR suppression (21). These immunomodulatory effects not only augment anti-PD-1 efficacy but also mitigate irAEs by tempering macrophage-derived excessive inflammation.
Lenvatinib’s role in this regimen is supported by the single-arm phase 2 REMORA trial and the recent long-term follow up, which reported a 38% ORR (90% CI 25.6-52.0) and 28.3-month median OS (95% CI 17.1-34.0) in platinum-pretreated unresectable advanced or metastatic thymic carcinoma patients receiving lenvatinib monotherapy (24 mg/day), though with 64% incidence of grade ≥3 hypertension (6, 7). Our modified dosing (8 mg/day) with close monitoring likely contributed to maintained efficacy while avoiding significant hypertension. This contrast underscores the advantage of combination regimens in optimizing safety while maintaining efficacy.
Additionally, our triple combination finds support in translational and clinical precedents. The PECATI trial demonstrated that lenvatinib plus pembrolizumab shows promising activity in pretreated thymic tumors, while studies in hepatocellular carcinoma have established the efficacy and safety of tislelizumab plus lenvatinib combination therapy (22, 23). These findings provide translational rationale for our triple combination regimen.
The strategy of balancing chemo-immunotherapy involves several key aspects. Firstly, baseline differences such as nutritional status and mental state can significantly influence their response and tolerance of treatment. Therefore, personalized strategy should be formulated based on various factors, including the patient’s PS score, pathological type, TNM stage, and PD-L1 expression level. Secondly, the sequence of administration in combination therapy is crucial. Administering chemotherapy drugs prior to immunotherapy may yield favorable therapeutic effects by prematurely activating the immune system. Thirdly, the selection of chemotherapy and immunotherapy drugs is critical, as opting for multi-target chemotherapy agents can enhance the efficacy of immunotherapy. Fourthly, the incidence and severity of adverse reactions may differ among various immunotherapy agents, making it essential to promptly transition to those that provide improved safety and tolerability for patients. In suitable cases, de-escalation of immunotherapy may be considered, such as shifting from a dual-target CTLA-4 + PD-1 bispecific antibody to a PD-1 monoclonal antibody (24). Fifthly, close monitoring and management during the treatment process may help promptly identify and address potential irAEs. Finally, it is important to emphasize the timely and individualized adjustment of chemotherapy drug dosages. Low doses of specific chemotherapeutic agents can modify TME thereby enhancing the efficacy of immunotherapy while minimizing toxicity.
In this case, during the second phase of immunotherapy, carboplatin was omitted, and only PM-PTX were administered. This strategy not only decreased the cumulative toxicity associated with carboplatin but also maintained a balance between chemotherapy and immunotherapy, thereby sustaining the antitumor effect and facilitating a continuous reduction of tumor burden.
Conclusion
This case supports tislelizumab + lenvatinib + PM-PTX as an effective regimen for advanced TSCC after platinum stabilization, providing tumor control with preserved quality of life. Consider this approach when first-line response is suboptimal, with vigilant toxicity monitoring and regimen tailoring to maintain safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Investigation, Funding acquisition, Resources, Writing – original draft. YW: Writing – original draft, Formal analysis. MS: Writing – original draft, Data curation, Methodology. JQ: Writing – original draft, Data curation, Methodology. LM: Data curation, Conceptualization, Writing – review & editing. YS: Funding acquisition, Resources, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no.82172889), Jiangsu Provincial Medical Innovation Center (grant no. CXZX202204), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant no. KYCX24_2037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1677723/full#supplementary-material
Abbreviations
TSCC, thymic squamous cell carcinoma; PM-PTX, polymeric micellar paclitaxel; irAEs, immune-related adverse events; IHC, immunohistochemistry; CT, computed tomography; RML, right middle lobe; SD, stable disease; PR, partial response; WBC, white blood cell; Tregs, regulatory T cells; MDSCs, myeloid-derived suppressor cells; ICD, immunogenic cell death; DAMPs, damage associated molecular patterns; COPD, chronic obstructive pulmonary disease; PS, performance status; NGS, Next-generation sequencing.
References
1. Falkson CB, Vella ET, Ellis PM, Maziak DE, Ung YC, and Yu E. Surgical, radiation, and systemic treatments of patients with thymic epithelial tumors: A systematic review. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2023) 18:299–312. doi: 10.1016/j.jtho.2022.10.016
2. Basse C and Girard N. Thymic tumours and their special features. Eur Respir Rev Off J Eur Respir Soc. (2021) 30:200394. doi: 10.1183/16000617.0394-2020
3. Roden AC, Ahmad U, Cardillo G, Girard N, Jain D, Marom EM, et al. Thymic carcinomas-A concise multidisciplinary update on recent developments from the thymic carcinoma working group of the international thymic Malignancy interest group. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2022) 17:637–50. doi: 10.1016/j.jtho.2022.01.021
4. Serpico D, Trama A, Haspinger ER, Agustoni F, Botta L, Berardi R, et al. Available evidence and new biological perspectives on medical treatment of advanced thymic epithelial tumors. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:838–47. doi: 10.1093/annonc/mdu527
5. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. (2018) 19:347–55. doi: 10.1016/S1470-2045(18)30062-7
6. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. (2020) 21:843–50. doi: 10.1016/S1470-2045(20)30162-5
7. Niho S, Sato J, Satouchi M, Itoh S, Okuma Y, Mizugaki H, et al. Long-term follow-up and exploratory analysis of lenvatinib in patients with metastatic or recurrent thymic carcinoma: Results from the multicenter, phase 2 REMORA trial. Lung Cancer Amst Neth. (2024) 191:107557. doi: 10.1016/j.lungcan.2024.107557
8. Dougan M, Luoma AM, Dougan SK, and Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. (2021) 184:1575–88. doi: 10.1016/j.cell.2021.02.011
9. Chu X, Tian W, Wang Z, Zhang J, and Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials. Mol Cancer. (2023) 22:93. doi: 10.1186/s12943-023-01800-3
10. Pang K, Shi Z-D, Wei L-Y, Dong Y, Ma Y-Y, Wang W, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist Update Rev Comment Antimicrob Anticancer Chemother. (2023) 66:100907. doi: 10.1016/j.drup.2022.100907
11. Anderson HG, Takacs GP, Harris DC, Kuang Y, Harrison JK, and Stepien TL. Global stability and parameter analysis reinforce therapeutic targets of PD-L1-PD-1 and MDSCs for glioblastoma. J Math Biol. (2023) 88:10. doi: 10.1007/s00285-023-02027-y
12. Whittaker H, Rubino A, Müllerová H, Morris T, Varghese P, Xu Y, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: A UK routine health care data study. Int J Chron Obstruct Pulmon Dis. (2022) 17:427–37. doi: 10.2147/COPD.S346591
13. Cheng Y, Xu S, Pei R, Chen D, Du X, Li S, et al. Etoposide + cytarabine + pegfilgrastim versus cyclophosphamide + G-CSF for stem cell mobilization in patients with poorly mobilized multiple myeloma and lymphoma. Transfus Apher Sci Off J World Apher Assoc Off J Eur Soc Haemapheresis. (2025) 64:104096. doi: 10.1016/j.transci.2025.104096
14. Smith TM, Butler SE, Wang X-YS, and Manjili MH. Low-dose chemotherapy induces immunogenic tumor dormancy in mouse mammary carcinoma cells. J Immunol. (2017) 198:204. doi: 10.4049/jimmunol.198.Supp.204.14
15. Li P, Wang W, Wang S, Cao G, Pan T, Huang Y, et al. PTPRC promoted CD8+ T cell mediated tumor immunity and drug sensitivity in breast cancer: based on pan-cancer analysis and artificial intelligence modeling of immunogenic cell death-based drug sensitivity stratification. Front Immunol. (2023) 14:1145481. doi: 10.3389/fimmu.2023.1145481
16. Li H, Liu Q, Li B, Chen Y, Lin J, Meng Y, et al. Comparison of short-term efficacy of neoadjuvant immunotherapy combined with chemotherapy and surgery alone for locally advanced resectable Non-small cell lung cancer. Zhongguo Fei Ai Za Zhi Chin J Lung Cancer. (2024) 27:421–30. doi: 10.3779/j.issn.1009-3419.2024.102.26
17. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/jco.21.00174
18. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/jco.22.01989
19. Chen Y, Dai S, Cheng CS, and Chen L. Lenvatinib and immune-checkpoint inhibitors in hepatocellular carcinoma: mechanistic insights, clinical efficacy, and future perspectives. J Hematol Oncol. (2024) 17:130. doi: 10.1186/s13045-024-01647-1
20. Sun Q, Shen M, Zhu S, Liao Y, Zhang D, Sun J, et al. Targeting NAD(+) metabolism of hepatocellular carcinoma cells by lenvatinib promotes M2 macrophages reverse polarization, suppressing the HCC progression. Hepatol Int. (2023) 17:1444–60. doi: 10.1007/s12072-023-10544-7
21. Zhu J, Fang P, Wang C, Gu M, Pan B, Guo W, et al. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. (2021) 10:7977–87. doi: 10.1002/cam4.4312
22. Remon J, Girard N, Novello S, de Castro J, Bigay-Game L, Bernabé R, et al. PECATI: A multicentric, open-label, single-arm phase II study to evaluate the efficacy and safety of pembrolizumab and lenvatinib in pretreated B3-thymoma and thymic carcinoma patients. Clin Lung Cancer. (2022) 23:e243–6. doi: 10.1016/j.cllc.2021.07.008
23. Xu L, Chen J, Liu C, Song X, Zhang Y, Zhao H, et al. Efficacy and safety of tislelizumab plus lenvatinib as first-line treatment in patients with unresectable hepatocellular carcinoma: a multicenter, single-arm, phase 2 trial. BMC Med. (2024) 22:172. doi: 10.1186/s12916-024-03356-5
24. Milberg O, Gong C, Jafarnejad M, Bartelink IH, Wang B, Vicini P, et al. A QSP model for predicting clinical responses to monotherapy, combination and sequential therapy following CTLA-4, PD-1, and PD-L1 checkpoint blockade. Sci Rep. (2019) 9:11286. doi: 10.1038/s41598-019-47802-4
25. Nishii Y, Furuhashi K, Ito K, Sakaguchi T, Suzuki Y, Fujiwara K, et al. Good response of advanced thymic carcinoma with low PD-L1 expression to chemotherapy plus pembrolizumab as first-line therapy and to pembrolizumab as maintenance therapy: A case report. Pharm (Basel). (2022) 15:889. doi: 10.3390/ph15070889
26. Zhang L, Zhang Y, Li S, Wang Y, Yu Y, He J, et al. Case Report: Robust and durable response to the combination of tislelizumab and chemotherapy in advanced thymic epithelial tumors: a case series. Front Immunol. (2025) 16:1516297. doi: 10.3389/fimmu.2025.1516297
27. Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. (2019) 37:2162–70. doi: 10.1200/jco.2017.77.3184
28. Girard N, Ponce Aix S, Cedres S, Berghmans T, Burgers S, Toffart AC, et al. Efficacy and safety of nivolumab for patients with pre-treated type B3 thymoma and thymic carcinoma: results from the EORTC-ETOP NIVOTHYM phase II trial. ESMO Open. (2023) 8:101576. doi: 10.1016/j.esmoop.2023.101576
29. Luciano A, Pietroluongo E, Ottaviano M, Grieco A, Peddio A, De Placido P, et al. Case report: Potential role of immunotherapy in thymic Malignancies: a unique case of a durable and complete response upon an immune checkpoint inhibitor. Front Immunol. (2024) 15:1423800. doi: 10.3389/fimmu.2024.1423800
30. De Pas TM, Giaccone G, Catania C, Conforti F, Pala L, Mitsakis P, et al. First report of pembrolizumab activity in KIT-mutated thymic carcinoma. Curr Oncol. (2025) 32:68. doi: 10.3390/curroncol32020068
31. Thomas QD, Basse C, Luporsi M, and Girard N. Pembrolizumab plus chemotherapy in metastatic thymic carcinoma: A case report. Front Oncol. (2021) 11:814544. doi: 10.3389/fonc.2021.814544
32. Cafaro A, Bongiovanni A, Di Iorio V, Oboldi D, Masini C, and Ibrahim T. Pembrolizumab in a patient with heavily pre-treated squamous cell thymic carcinoma and cardiac impairment: A case report and literature review. Front Oncol. (2020) 10:1478. doi: 10.3389/fonc.2020.01478
33. Sakamori Y, Hamada K, Kawachi H, Yamoto M, Fukao A, Terashita S, et al. Successful treatment with pembrolizumab for microsatellite instability-high thymic carcinoma: A case report. Respir Med Case Rep. (2025) 57:102272. doi: 10.1016/j.rmcr.2025.102272
34. Liu S, Ma G, Wang H, Yu G, Chen J, and Song W. Severe cardiotoxicity in 2 patients with thymoma receiving immune checkpoint inhibitor therapy: A case report. Med (Baltimore). (2022) 101:e31873. doi: 10.1097/md.0000000000031873
Keywords: advanced thymic squamous cell carcinoma, immunotherapy, irAEs, case report, literature review
Citation: Chen J, Wang Y, Shou M, Qian J, Ma L and Shu Y (2025) Case report and literature review: tislelizumab combined with lenvatinib and polymeric micellar paclitaxel for thymic squamous cell carcinoma. Front. Immunol. 16:1677723. doi: 10.3389/fimmu.2025.1677723
Received: 01 August 2025; Accepted: 03 September 2025;
Published: 18 September 2025.
Edited by:
Cleber Machado-Souza, Pelé Pequeno Príncipe Research Institute, BrazilReviewed by:
Zhuo Liu, Dalian Medical University, ChinaNahed Damaj, Saint Joseph University, Lebanon
Copyright © 2025 Chen, Wang, Shou, Qian, Ma and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongqian Shu, c2h1eW9uZ3FpYW5AY3Njby5vcmcuY24=
†These authors have contributed equally to this work
 Jingwen Chen
Jingwen Chen Yihan Wang
Yihan Wang Minyue Shou
Minyue Shou Jieyi Qian
Jieyi Qian Ling Ma
Ling Ma Yongqian Shu
Yongqian Shu