- Department of Gynecological Oncology, The First Hospital of Jilin University, Changchun, China
Ovarian cancer remains the deadliest gynecologic malignancy, with its aggressive progression and therapeutic resistance heavily influenced by the tumor microenvironment (TME). Tumor-associated macrophages (TAMs), the predominant immune infiltrates in OC, play pivotal roles in metastasis, immunosuppression, and chemoresistance by adopting a pro-tumoral M2 phenotype. Despite promising preclinical results, clinical translation faces challenges, such as on-target toxicity and incomplete understanding of TAM ontogeny in humans. This review summarizes the origins, heterogeneity, and functional plasticity of TAMs, emphasizing their mechanistic contributions to OC progression through epithelial-mesenchymal transition (EMT), angiogenesis, and immune evasion. We outline the emerging evidence that TAMs drive platinum resistance via exosomal signaling and metabolic reprogramming, underscoring TAMs as central mediators of OC pathogenesis and treatment paradigms.
1 Introduction
Ovarian cancer remains the most lethal gynecologic malignancy worldwide, with its pathogenesis and progression intricately shaped by the tumor microenvironment (TME)—a dynamic ecosystem comprising not only malignant epithelial cells, but also adipocytes, vasculature, stromal fibroblasts, lymphocytes, dendritic cells, cancer-associated fibroblasts, and tumor-associated macrophages (TAMs) (1, 2). Through continuous bidirectional communication with both cellular and acellular components, tumor cells operate as adaptive entities that integrate cues from immune, endocrine, and nervous systems to construct a self-sustaining niche promoting oncogenesis, metastasis, and therapeutic resistance (3). The ovarian cancer TME is notably immunosuppressive, fostering unchecked tumor expansion and evasion of host surveillance (4, 5).
Among immune components of the ovarian cancer TME, macrophages constitute the predominant infiltrating population (6, 7). These cells contribute to multiple hallmarks of malignancy, including facilitating intravasation of tumor cells into the circulation and suppressing anti-tumor immunity. Fibroblasts represent another critical population, supporting migration of tumor cells from the primary site, aiding systemic dissemination, and guiding endothelial cells during tumor angiogenesis (8). Increasing evidence highlights TAMs as central mediators of bidirectional crosstalk between tumor cells and the TME. In ovarian cancer, TAMs predominantly exhibit an immunosuppressive M2 phenotype that promotes tumor growth, invasion, angiogenesis, immune evasion, and metastatic competence (9). Given these multifaceted roles, TAMs have emerged as key targets for therapeutic intervention. This review summarizes the mechanistic roles of TAMs in ovarian cancer progression and explores strategies to modulate TAM function, with the aim of identifying innovative approaches to improve outcomes for patients with ovarian cancer.
2 Origins of TAMs and emerging concepts
Macrophages were once thought to arise exclusively from circulating monocytes (10). However, lineage-tracing studies have challenged this notion, revealing that while many macrophages originate from bone marrow and splenic progenitors, a considerable proportion are established during embryogenesis and persist as self-renewing tissue-resident macrophages (TRMs) (11, 12). During development, macrophages derived from the yolk sac and fetal liver seed peripheral tissues and are later complemented by bone marrow–derived monocytes in response to injury, infection, or inflammation (13). These insights have reshaped the traditional M1/M2 polarization paradigm. For instance, TAMs expressing CD163 or CD206—markers typically aligned with M2 phenotypes—can exhibit M1-like, T cell–activating properties in gastrointestinal cancers and ovarian cancer ascites (12). M1/M2 dichotomy oversimplifies the functional continuum of macrophages in the TME (14). High-dimensional analyses, including single-cell RNA sequencing, have revealed remarkable heterogeneity that transcends classical classifications. In tumors, TAMs predominantly exhibit M2-like features, though a minority display M1-like traits, contributing to tumor initiation, angiogenesis, and metastasis. Human TRMs lack definitive lineage markers, leaving their developmental origins and specialized roles insufficiently characterized (12). This underscores an urgent need to clarify macrophage heterogeneity and lineage diversity in human tumors to advance precision immunotherapy.
3 Roles of TAMs in ovarian cancer
3.1 TAMs facilitate ovarian cancer metastasis
TAMs, the predominant immune cell population within the ovarian tumor microenvironment (TME), are critical mediators of tumor progression and metastatic dissemination (15). These cells facilitate tumor cell proliferation, invasion, and the establishment of peritoneal metastases, processes closely linked to malignant ascites, recurrence, and poor prognosis. Within ascitic fluid, tumor cells frequently aggregate into multicellular spheroids that adhere to peritoneal mesothelium, initiating secondary lesions (16). Tos et al (17). revealed that highly metastatic ovarian cancer cells, upon intrabursal injection in mice, produce persistent peritoneal dissemination, whereas non-metastatic lines fail to spread. Mechanistically, β-catenin signaling, which underpins tumor growth and invasion, plays a key role; its silencing reduces omental metastases and metastatic nodules, with concurrent depletion of CD68+ and CD163+ TAMs (7, 18). β-catenin activation in tumor cells upregulates EMT-promoting transcription factors such as ZEB1 and Snail, as well as chemokines including CCL2 and CCL3, which recruit monocytes and polarize them into M2-like TAMs (17, 19–22). These recruited TAMs secrete high levels of CCL2 and IL-6, which act on tumor cells via CCR2 and IL-6R, respectively (23, 24). IL-6 binding activates the JAK/STAT3 axis, driving expression of EMT-related genes and enhancing tumor motility (25). Simultaneously, CCL2–CCR2 signaling stimulates NF-κB activity, which synergizes with β-catenin to reinforce EMT programs (26). This cytokine-driven positive feedback loop sustains mesenchymal states, promotes invasion and peritoneal dissemination, and perpetuates TAM recruitment and polarization. The EMT program further amplifies CCL2 production, fostering continuous macrophage influx and establishing a self-reinforcing TAM–tumor interaction that accelerates migration, invasion, and metastasis (27).
3.2 TAMs promote chemoresistance in ovarian cancer
Cytoreductive surgery followed by platinum-based chemotherapy remains the standard treatment for ovarian cancer. However, resistance to platinum compounds—whether intrinsic or acquired—remains a central challenge in disease management, often driven by pre-existing resistant clones or selective pressure from repeated treatments (28). Accumulating evidence implicates the TME as a major contributor to relapse and drug resistance (29). TAMs are predominant and contribute to angiogenesis, immune evasion, metastasis, and particularly chemoresistance (30). Although initially characterized in breast cancer, TAM-driven resistance is increasingly recognized in ovarian cancer (31). Notably, the M2-polarized subset of TAMs is closely associated with tumor progression, immune evasion, and drug resistance (32). Li et al (30). reported elevated serum circITGB6 in patients with platinum-resistant ovarian cancer compared to platinum-sensitive cases, which was accompanied by an expansion of M2 macrophages, suggesting circITGB6-driven M2 polarization as a mechanism of resistance (30). Similarly, Jang et al (33). reported that co-culture of ovarian cancer cells with macrophages reduced carboplatin sensitivity in a dose-dependent manner, coinciding with a shift toward an M2-like phenotype. Importantly, emerging preclinical evidence supports the therapeutic potential of inhibiting exosome secretion to counter TAM-mediated chemoresistance (34, 35). For example, the pharmacological inhibitor GW4869, which blocks neutral sphingomyelinase and thereby suppresses exosome biogenesis and release, has been shown to reduce exosomal miR-223 levels, restore PTEN expression, and enhance cisplatin sensitivity in ovarian cancer cells co-cultured with TAMs (35–37). In vivo, GW4869 administration attenuates tumor growth and enhances chemotherapy efficacy, further validating exosome-targeted interventions as a promising strategy to disrupt TAM–tumor crosstalk and overcome drug resistance in ovarian cancer (38). Zhu et al (37). further demonstrated that hypoxic ovarian cancer cells recruit macrophages and polarize them into a TAM-like phenotype, whose exosomal miR-223 confers chemoresistance in vitro and in vivo through the PTEN–PI3K/AKT pathway.
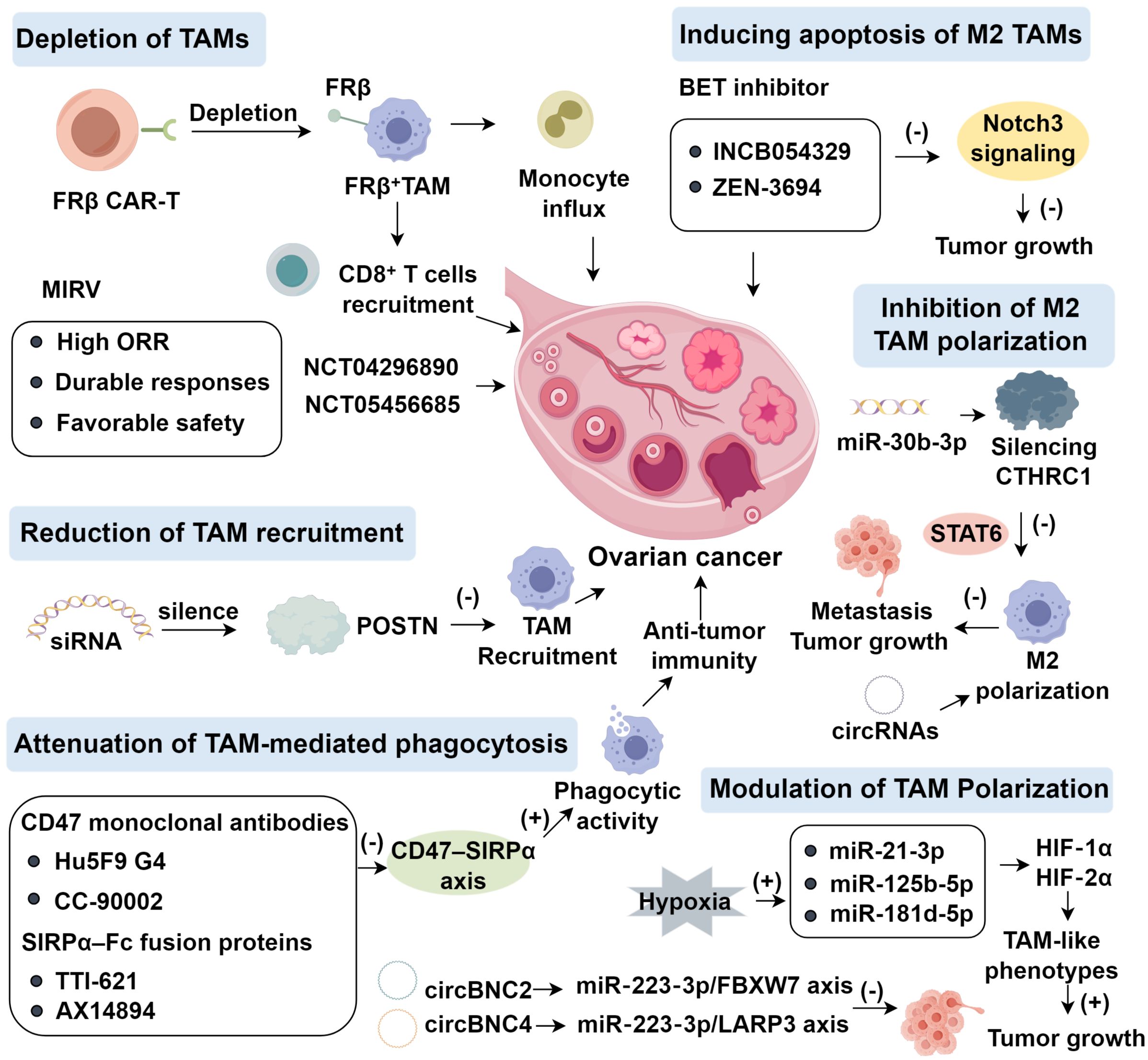
4 Therapeutic targeting of TAMs in ovarian cancer
4.1 Depletion of TAMs
The folate receptor beta (FRβ) is specifically overexpressed on M2-polarized TAMs in various epithelial malignancies, including ovarian cancer, making it a promising immunotherapeutic target for modulating the tumor microenvironment (39). Unlike FRα, which is mainly expressed on tumor cells, FRβ localizes predominantly on immune cells within the TME, particularly immunosuppressive macrophages (40, 41). In syngeneic mouse models, chimeric antigen receptor (CAR) T cells engineered to recognize FRβ have demonstrated the ability to selectively eradicate FRβ+ TAMs (42). This approach effectively reshapes the immunosuppressive TME into a pro-inflammatory milieu, enhancing monocyte influx, endogenous CD8+ T cell recruitment, delaying tumor progression, and prolonging survival (43). In syngeneic mouse models, chimeric antigen receptor (CAR) T cells directed against FRβ selectively eradicated FRβ+ TAMs, thereby reshaping the tumor microenvironment toward a pro-inflammatory state, with enhanced monocyte influx, recruitment of endogenous CD8+ T cells, delayed tumor progression, and prolonged survival. Rodriguez-Garcia et al (42). further demonstrated that murine FRβ CAR-T treatment led to transient weight loss but specific depletion of FRβ+ TAMs and conferred a significant survival advantage.
In contrast, the folate receptor alpha (FRα) is overexpressed directly on tumor cells in epithelial ovarian cancer, and its selective expression pattern has enabled the development of antibody–drug conjugates (ADCs) for targeted tumor cell elimination (44, 45). Mirvetuximab soravtansine (MIRV) is an FRα-targeting ADC that has demonstrated clinical efficacy in platinum-resistant epithelial ovarian cancer (46). In the phase II SORAYA trial, Matulonis et al (44). evaluated MIRV in FRα-high, platinum-resistant epithelial ovarian cancer previously treated with bevacizumab (48% ≥3 prior lines, 13% prior PARP inhibitors). MIRV monotherapy yielded a high objective response rate (ORR) with durable responses and a favorable safety profile, irrespective of prior therapies. Subsequently, Moore et al (47). in a phase III trial compared MIRV to standard chemotherapy and reported lower rates of grade ≥3 adverse events, fewer dose reductions, and discontinuations. In patients with high FRα expression, MIRV surpassed chemotherapy on secondary endpoints, showing higher ORR, greater CA125 responses, and improved patient-reported outcomes, although progression-free survival did not differ significantly. MIRV has now been approved for FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers after one to three prior regimens (48). The ADC therapeutic field is rapidly expanding, with >20 ongoing trials across gynecologic malignancies, including phase III (NCT04296890) of MIRV in FRα-high platinum-resistant ovarian cancers (49, 50).
4.2 Reduction of TAM recruitment
Periostin (POSTN), a secreted matricellular protein, is implicated in tumor progression and poor prognosis across diverse malignancies, including ovarian cancer (51). POSTN overexpression enhances migration, chemoresistance, and macrophage recruitment (52). Tang et al (53). demonstrated that siRNA-mediated POSTN silencing in A2780 ovarian cancer cells markedly diminished chemotactic recruitment of THP-1–derived or M-CSF–induced macrophages toward A2780-conditioned medium (CM). Similarly, Zeng et al (54). showed that POSTN depletion from intrahepatic cholangiocarcinoma CM suppressed macrophage migration, whereas POSTN supplementation restored monocyte invasion. POSTN strongly attracts macrophages and drives M2 polarization, identifying it as both a prognostic biomarker and therapeutic target. Lin et al (51). reported that POSTN enrichment in invasive ovarian cancer correlates with increased migration, invasion, and metastasis, whereas knockdown reduced tumor growth in vivo. Mechanistically, POSTN activates integrin–FAK/NF-κB signaling, inducing cytokines (MIP-1β, MCP-1, TNF-α, RANTES), thereby enhancing monocyte chemotaxis and M2 polarization (55); metastases from POSTN-overexpressing SKOV3 cells were enriched in cancer-associated fibroblasts (CAFs) (51). DDR2-expressing CAFs regulate POSTN via ITGB1 to activate PI3K/AKT and Src pathways (56). Furthermore, LINC00520 upregulates POSTN by sponging miR-577, triggering ILK/Akt/mTOR activation; POSTN knockdown or ILK/Akt/mTOR inhibition (OSU-T315) abrogates these effects (57). Beyond oncology, POSTN deficiency exacerbates alcohol-associated liver disease in mice, whereas hepatic POSTN restoration is protective (58). Despite its central role, no clinical study has yet targeted POSTN directly (Figure 1).
4.3 Attenuation of TAM-mediated phagocytosis
CD47 is a widely expressed glycoprotein that transmits a “don’t eat me” signal through interaction with signal regulatory protein α (SIRPα) on macrophages, a mechanism exploited by tumor cells to evade immune surveillance (59). Blocking CD47–SIRPα interactions restores phagocytic activity and has emerged as a key immunotherapeutic strategy. Inhibiting CD47–SIRPα signaling reinstates macrophage phagocytic function and represents a promising immunotherapeutic approach (60, 61). Therapeutic candidates include anti-CD47 monoclonal antibodies Hu5F9 G4 and CC-90002, and SIRPα–Fc fusion proteins such as TTI-621 and ALX14894 (62–64). In a phase Ib trial (NCT02953782), Hu5F9-G4 combined with cetuximab yielded encouraging responses in advanced solid tumors, including late-stage ovarian cancer (65). In Sézary syndrome, CD47 expression is upregulated by interleukins 4 (66). Blocking CD47–SIRPα with the decoy receptor TTI-621 enhances macrophage phagocytosis and reduces tumor burden. A phase I trial reported by Ansell et al. (67) confirmed the safety and clinical responses of TTI-621 monotherapy in various hematologic malignancies, including B- and T-cell lymphomas. Mechanistically, TTI-621 not only augments macrophage function but also enhances CD8+ T cell cytotoxicity and promotes M1 polarization in synergy with anti–PD-L1, effectively suppressing lymphoma growth in vitro (68). Beyond enhancing phagocytosis, ALX148 activates dendritic cells and reprograms TAMs toward an inflammatory phenotype, thereby stimulating innate antitumor immunity. Evorpacept (ALX78), a next-generation fusion protein comprising a modified SIRPα D1 domain linked to an inactive human IgG1 Fc fragment with half the molecular weight of a conventional antibody, represents another CD47-targeted approach (69). Lakhani et al. (41) demonstrated that evorpacept is hematologically safe and, in preclinical models, synergizes with anti–PD-1/PD-L1 antibodies to enhance phagocytosis, pro-inflammatory polarization, dendritic cell activation, and cytotoxic immune responses. A phase II study (NCT05467670) is testing ALX148 with liposomal doxorubicin and pembrolizumab in platinum-resistant ovarian cancer. Ligufalimab (AK117), a novel humanized IgG4 anti-CD47 antibody, binds CD47 with high affinity while avoiding hemagglutination (70).
4.4 Inducing apoptosis of M2 TAMs and reprogramming toward an M1-like phenotype
Bromodomain and extraterminal domain inhibitors (BETi), which regulate epigenetic transcription by binding bromodomains, have emerged as promising modulators of TAMs. In ovarian cancer, Wilson et al (71). reported that the BET inhibitor INCB054329 impairs homologous recombination and augments the efficacy of poly (ADP-ribose) polymerase inhibitors (PARPis), whose clinical benefit is limited by resistance and toxicity. Novel delivery platforms, including JQ1-loaded nanocarriers, further improve outcomes. In ovarian and breast cancer models, Juan et al (72). showed that JQ1-loaded formulations enhanced antiproliferative effects and synergized with olaparib, while Villar-Prados et al (73). demonstrated that BET inhibition suppresses Notch3 signaling and reduces tumor growth in situ. ZEN-3694, a BET inhibitor, is currently under clinical evaluation in combination regimens for solid tumors, including recurrent ovarian cancer (NCT05422794, NCT05327010, NCT03901469, NCT04986423, NCT04471974, NCT05071937) (74).
Beyond direct anti-tumor effects, recent evidence suggests that BET inhibitors also modulate the tumor immune microenvironment by reprogramming tumor-associated macrophages (75). BET inhibition downregulates M2-polarizing transcription factors such as IRF4 and STAT6, while simultaneously enhancing NF-κB–dependent pro-inflammatory gene expression, thereby promoting a phenotypic switch from immunosuppressive M2 macrophages to antitumor M1 macrophages (76, 77). This reprogramming leads to increased secretion of cytokines like IL-12 and TNF-α, enhanced antigen presentation, and improved cytotoxic T cell recruitment (78, 79). Notably, in breast and ovarian cancer models, JQ1 treatment reduced macrophage infiltration and upregulated MHC II and iNOS expression in macrophages, supporting M1 polarization and fostering an inflamed TME conducive to immune-mediated tumor clearance (80, 81). In addition, BETi-mediated epigenetic remodeling suppresses the expression of immune checkpoint molecules such as PD-L1 on both tumor cells and TAMs, potentially enhancing the efficacy of checkpoint inhibitors (82–84). These dual effects on tumor cells and immune components underscore the therapeutic promise of BET inhibitors as both direct anti-cancer agents and immunomodulators. In parallel, M1 macrophage-derived extracellular vesicles (M1 MEVs) have been proposed to reprogram TAMs. Schweer et al (85). demonstrated that human M1 MEVs robustly induce M2-to-M1 repolarization both in isolated macrophages and in co-culture with ovarian cancer cells, and can target tumor xenografts, although clinical translation remains unproven.
4.5 Inhibition of M2 TAM polarization
M2-polarized TAMs, as dominant components of the TME, critically drive migration, invasion, immune evasion, and therapeutic resistance in ovarian cancer. In epithelial ovarian cancer, overexpression of CTHRC1 promotes EMT, thereby enhancing tumor invasion and metastasis (86), a mechanism also implicated in lung, gastrointestinal, breast, and pancreatic cancers (87). Ovarian cancer cells secrete CTHRC1, which activates STAT6 signaling in TAMs, inducing their M2 polarization. These M2 TAMs, in turn, further stimulate tumor migration and invasion, forming a positive feedback loop. Silencing CTHRC1 abrogates STAT6-mediated M2 polarization, suppresses metastasis, and delays disease progression, highlighting CTHRC1 as a potential therapeutic target. Additionally, miR-30b-3p, downregulated in ovarian cancer R3 cells, suppresses proliferation, promotes apoptosis, slows cell cycle progression, and inhibits migration and invasion upon overexpression; it directly targets CTHRC1, thereby linking it to EMT and suggesting its potential as a biomarker and therapeutic candidate (88). Circular RNAs (circRNAs) provide an additional regulatory layer. circITGB6 interacts with IGF2BP2 and FGF9 mRNA to stabilize FGF9 transcripts, induce M2 polarization, and confer cisplatin resistance (14). Combined cisplatin and antisense oligonucleotide (ASO) targeting circITGB6 markedly suppress tumor growth and improve survival.
4.6 Modulation of TAM polarization
Hypoxia, a hallmark of solid tumors, profoundly shapes ovarian cancer progression (51). In ascitic fluid, exosomal miR-940 is transferred to macrophages, reprogramming them toward an M2 phenotype that promotes ovarian cancer cell proliferation and migration (89). Thus, miR-940 functions as a tumor-promoting regulator through TAM polarization. In parallel, hypoxic stress elevates the levels of miR-21-3p, miR-125b-5p, and miR-181d-5p in ovarian cancer–derived exosomes. Uptake of these vesicles by macrophages which are mediated via HIF-1α and HIF-2α induces TAM-like phenotypes that further enhance tumor growth and metastatic potential (90). Importantly, inhibition of miR-223 partially attenuates TAM-derived exosome–induced chemoresistance, indicating that additional exosomal cargos, including proteins and other miRNAs, contribute to drug resistance (37). Among these, the miR-223/PTEN/PI3K/AKT axis has been identified as a major driver of chemoresistance in ovarian cancer cells, underscoring exosomes as potential therapeutic targets to restore chemosensitivity. Recent findings demonstrated that circ-BNC2 inhibits ovarian cancer progression via the miR-223-3p/FBXW7 axis (91). FBXW7, a recognized tumor suppressor, inhibits EMT in oral squamous cell carcinoma through PI3K/AKT signaling (92) and regulates proliferation and apoptosis in colorectal cancer via Notch and Akt/mTOR pathways (93). In ovarian cancer, FBXW7 expression is reduced and inversely associated with miR-223-3p while positively correlating with circ-BNC2, and it functionally suppresses invasion and migration (94). Moreover, circ-BNC4/miR-223-3p/LARP3 axis was identified with similar regulatory implications (95).
5 Conclusion
The immunosuppressive tumor microenvironment (TME) in ovarian cancer fosters immune evasion, metastasis, and chemoresistance. Tumor-associated macrophages (TAMs), predominantly M2-polarized, play central roles in these processes. Accordingly, therapeutic strategies targeting TAMs—through depletion, recruitment blockade, phagocytosis restoration, apoptosis induction, or phenotype reprogramming—offer promising avenues. However, translational barriers remain, including TAM heterogeneity, lack of specific markers, incomplete understanding of macrophage ontogeny in humans, and potential on-target toxicity. Most TAM-targeted therapies are still in early-phase trials without definitive clinical validation.
To bridge these gaps, future studies should employ single-cell and spatial transcriptomics to define TAM subsets, develop humanized models that recapitulate the TME, and design rational combination therapies. Biomarker-guided clinical trials are essential to optimize patient selection and therapeutic efficacy. In sum, a deeper mechanistic understanding of TAM plasticity and intercellular networks will be key to advancing TAM-directed interventions toward clinical translation in ovarian cancer.
Author contributions
ML: Writing – original draft. YM: Writing – original draft. TD: Writing – original draft. YW: Writing – original draft. YY: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that no competing financial interests or commercial relationships have influenced the research presented herein.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yin B, Ding J, Liu J, Hu H, Zhu Y, Yang M, et al. Exosomal CMTM4 induces immunosuppressive macrophages to promote ovarian cancer progression and attenuate anti-PD-1 immunotherapy. Adv Sci (Weinh). (2025) 12:e04436. doi: 10.1002/advs.202504436
2. Kader T, Lin JR, Hug CB, Coy S, Chen YA, de Bruijn I, et al. Multimodal spatial profiling reveals immune suppression and microenvironment remodeling in fallopian tube precursors to high-grade serous ovarian carcinoma. Cancer Discov. (2025) 15:1180–202. doi: 10.1158/2159-8290.CD-24-1366
3. Chen X and Song E. The theory of tumor ecosystem. Cancer Commun (Lond). (2022) 42:587–608. doi: 10.1002/cac2.12316
4. Cui M, Liu Y, Cheng L, Li T, Deng Y, and Liu D. Research progress on anti-ovarian cancer mechanism of miRNA regulating tumor microenvironment. Front Immunol. (2022) 13:1050917. doi: 10.3389/fimmu.2022.1050917
5. Shi J, Xiao W, Liu Y, Fu X, and Peng M. Tumor-associated macrophages and platelets in tumor microenvironment and its potential therapeutic role in ovarian cancer. Clin Transl Oncol. (2025). doi: 10.1007/s12094-025-03987-x
6. Zhong Y, Wang Y, Wang C, Cao K, Wang X, Xu X, et al. Targeting mesothelin-CD24 axis repolarizes tumor-associated macrophages to potentiate PD-1 blockade therapy in high-grade serous ovarian cancer. J Immunother Cancer. (2025) 13:e011230. doi: 10.1136/jitc-2024-011230
7. Xu C, Chen J, Tan M, and Tan Q. The role of macrophage polarization in ovarian cancer: from molecular mechanism to therapeutic potentials. Front Immunol. (2025) 16:1543096. doi: 10.3389/fimmu.2025.1543096
8. Anderson NM and Simon MC. The tumor microenvironment. Current biology (2020) 30:R921–5. doi: 10.1016/j.cub.2020.06.081
9. Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. (2020) 11:6298. doi: 10.1038/s41467-020-20140-0
10. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
11. Ginhoux F and Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. (2016) 44:439–49. doi: 10.1016/j.immuni.2016.02.024
12. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. (2020) 11:1731. doi: 10.3389/fimmu.2020.01731
13. Zhao Y, Zou W, Du J, and Zhao Y. The origins and homeostasis of monocytes and tissue-resident macrophages in physiological situation. J Cell Physiol. (2018) 233:6425–39. doi: 10.1002/jcp.26461
14. Xiang X, Wang J, Lu D, and Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. (2021) 6:75. doi: 10.1038/s41392-021-00484-9
15. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. (2019) 18:64. doi: 10.1186/s12943-019-0976-4
16. Rakina M, Kazakova A, Villert A, Kolomiets L, and Larionova I. Spheroid formation and peritoneal metastasis in ovarian cancer: the role of stromal and immune components. Int J Mol Sci (2022) 23:6215. doi: 10.3390/ijms23116215
17. To SKY, Tang MKS, Tong Y, Zhang J, Chan KKL, Ip PPC, et al. A selective β-catenin-metadherin/CEACAM1-CCL3 axis mediates metastatic heterogeneity upon tumor-macrophage interaction. Adv Sci (Weinh). (2022) 9:e2103230. doi: 10.1002/advs.202103230
18. Sharma N, Panigrahi R, Pradhan P, Parida S, and Sahoo SR. Expression of CD68+ Tumor associated macrophages in relation to β-catenin in carcinoma stomach. Indian J Pathol Microbiol. (2024) 67:15–20. doi: 10.4103/ijpm.ijpm_535_22
19. Hu C, Dong T, Li R, Lu J, Wei X, and Liu P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3β/β-catenin/ZEB1 signaling pathway. Oncol Rep. (2016) 35:2027–34. doi: 10.3892/or.2016.4591
20. Bu S, Wang Q, Sun J, Li X, Gu T, and Lai D. Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. (2020) 11:644. doi: 10.1038/s41419-020-02906-y
21. Aretz P, Maciaczyk D, Yusuf S, Sorg RV, Hänggi D, Liu H, et al. Crosstalk between β-catenin and CCL2 drives migration of monocytes towards glioblastoma cells. Int J Mol Sci. (2022) 23:4562. doi: 10.3390/ijms23094562
22. Wang M, Sun Y, Gu R, Tang Y, Han G, and Zhao S. Shikonin reduces M2 macrophage population in ovarian cancer by repressing exosome production and the exosomal galectin 3-mediated β-catenin activation. J Ovarian Res. (2024) 17:101. doi: 10.1186/s13048-024-01430-3
23. Wang N, Yang Y, Wang H, Li Y, Wang M, and Li Q. Cinobufagin modulates vasculogenic mimicry and tumor-associated macrophages to inhibit ovarian cancer progression. Eur J Pharmacol. (2025) 987:177157. doi: 10.1016/j.ejphar.2024.177157
24. Wu X, Lu W, Xu C, Jiang C, Zhuo Z, Wang R, et al. Macrophages phenotype regulated by IL-6 are associated with the prognosis of platinum-resistant serous ovarian cancer: integrated analysis of clinical trial and omics. J Immunol Res. (2023) 2023:6455704. doi: 10.1155/2023/6455704
25. Wang R, Ye H, Yang B, Ao M, Yu X, Wu Y, et al. m6A-modified circNFIX promotes ovarian cancer progression and immune escape via activating IL-6R/JAK1/STAT3 signaling by sponging miR-647. Int Immunopharmacol. (2023) 124:110879. doi: 10.1016/j.intimp.2023.110879
26. Yang YI, Wang YY, Ahn JH, Kim BH, and Choi JH. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. BioMed Pharmacother. (2022) 153:113474. doi: 10.1016/j.biopha.2022.113474
27. Chen W, Chen M, Hong L, Xiahenazi A, Huang M, Tang N, et al. M2-like tumor-associated macrophage-secreted CCL2 facilitates gallbladder cancer stemness and metastasis. Exp Hematol Oncol. (2024) 13:83. doi: 10.1186/s40164-024-00550-2
28. Nowak M and Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. (2020) 9:1299. doi: 10.3390/cells9051299
29. Xiong Z, Huang Y, Cao S, Huang X, and Zhang H. A new strategy for the treatment of advanced ovarian cancer: utilizing nanotechnology to regulate the tumor microenvironment. Front Immunol. (2025) 16:1542326. doi: 10.3389/fimmu.2025.1542326
30. Li H, Luo F, Jiang X, Zhang W, Xiang T, Pan Q, et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J Immunother Cancer. (2022) 10:e004029. doi: 10.1136/jitc-2021-004029
31. Cheng H, Wang Z, Fu L, and Xu T. Macrophage polarization in the development and progression of ovarian cancers: an overview. Front Oncol. (2019) 9:421. doi: 10.3389/fonc.2019.00421
32. Le F, Yang L, Han Y, Zhong Y, Zhan F, Feng Y, et al. TPL inhibits the invasion and migration of drug-resistant ovarian cancer by targeting the PI3K/AKT/NF-κB-signaling pathway to inhibit the polarization of M2 TAMs. Front Oncol. (2021) 11:704001. doi: 10.3389/fonc.2021.704001
33. Jang YS, Kim TW, Ryu JS, Kong HJ, Jang SH, Nam GH, et al. Upregulation of programmed death ligand-1 in tumor-associated macrophages affects chemotherapeutic response in ovarian cancer cells. PLoS One (2023) 18:e0277285. doi: 10.1371/journal.pone.0277285
34. Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer Res. (2021) 19:1583–95. doi: 10.1158/1541-7786.MCR-20-0956
35. Shen J, Zhu X, Fei J, Shi P, Yu S, and Zhou J. Advances of exosome in the development of ovarian cancer and its diagnostic and therapeutic prospect. Onco Targets Ther. (2018) 11:2831–41. doi: 10.2147/OTT.S159829
36. Orsini M, Chateauvieux S, Rhim J, Gaigneaux A, Cheillan D, Christov C, et al. Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells. Cell Death Differ. (2019) 26:1796–812. doi: 10.1038/s41418-018-0245-x
37. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. (2019) 38:81. doi: 10.1186/s13046-019-1095-1
38. Gurunathan S and Kim JH. Graphene oxide enhances biogenesis and release of exosomes in human ovarian cancer cells. Int J Nanomed. (2022) 17:5697–731. doi: 10.2147/IJN.S385113
39. Roy AG, Robinson JM, Sharma P, Rodriguez-Garcia A, Poussin MA, Nickerson-Nutter C, et al. Folate receptor beta as a direct and indirect target for antibody-based cancer immunotherapy. Int J Mol Sci. (2021) 22:5572. doi: 10.3390/ijms22115572
40. Zhang F, Huang B, Utturkar SM, Luo W, Cresswell G, Herr SA, et al. Tumor-specific activation of folate receptor beta enables reprogramming of immune cells in the tumor microenvironment. Front Immunol. (2024) 15:1354735. doi: 10.3389/fimmu.2024.1354735
41. Siddiqui B, Ur Rehman A, Gul R, Chaudhery I, Shah KU, and Ahmed N. Folate decorated chitosan-chondroitin sulfate nanoparticles loaded hydrogel for targeting macrophages against rheumatoid arthritis. Carbohydr Polym. (2024) 327:121683. doi: 10.1016/j.carbpol.2023.121683
42. Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O’Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. (2021) 12:877. doi: 10.1038/s41467-021-20893-2
43. Cresswell GM, Wang B, Kischuk EM, Broman MM, Alfar RA, Vickman RE, et al. Folate receptor beta designates immunosuppressive tumor-associated myeloid cells that can be reprogrammed with folate-targeted drugs. Cancer Res. (2021) 81:671–84. doi: 10.1158/0008-5472.CAN-20-1414
44. Matulonis UA, Lorusso D, Oaknin A, Pignata S, Dean A, Denys H, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. (2023) 41:2436–45. doi: 10.1200/JCO.22.01900
45. Moore KN, Angelergues A, Konecny GE, García Y, Banerjee S, Lorusso D, et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl J Med. (2023) 389:2162–74. doi: 10.1056/NEJMoa2309169
46. Van Gorp T, Moore KN, Konecny GE, Leary A, García-García Y, Banerjee S, et al. Patient-reported outcomes from the MIRASOL trial evaluating mirvetuximab soravtansine versus chemotherapy in patients with folate receptor α-positive, platinum-resistant ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. (2025) 26:503–15. doi: 10.1016/S1470-2045(25)00021-X
47. Moore KN, Oza AM, Colombo N, Oaknin A, Scambia G, Lorusso D, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. (2021) 32:757–65. doi: 10.1016/j.annonc.2021.02.017
48. Chelariu-Raicu A, Mahner S, Moore KN, Lorusso D, and Coleman RL. Integrating antibody drug conjugates in the management of gynecologic cancers. Int J Gynecol Cancer. (2023) 33:420–9. doi: 10.1136/ijgc-2022-003701
49. Moore KN, Lorusso D, Oaknin A, Oza A, Colombo N, Van Gorp T, et al. Safety and tolerability of mirvetuximab soravtansine monotherapy for folate receptor alpha-expressing recurrent ovarian cancer: An integrated safety summary. Gynecol Oncol. (2024) 191:249–58. doi: 10.1016/j.ygyno.2024.10.013
50. Coleman RL, Lorusso D, Oaknin A, Cecere SC, Denys H, Colombo N, et al. Mirvetuximab soravtansine in folate receptor alpha (FRα)-high platinum-resistant ovarian cancer: final overall survival and post hoc sequence of therapy subgroup results from the SORAYA trial. Int J Gynecol Cancer. (2024) 34:1119–25. doi: 10.1136/ijgc-2024-005401
51. Lin SC, Liao YC, Chen PM, Yang YY, Wang YH, Tung SL, et al. Periostin promotes ovarian cancer metastasis by enhancing M2 macrophages and cancer-associated fibroblasts via integrin-mediated NF-κB and TGF-β2 signaling. J BioMed Sci. (2022) 29:109. doi: 10.1186/s12929-022-00888-x
52. Moniuszko T, Wincewicz A, Koda M, Domysławska I, and Sulkowski S. Role of periostin in esophageal, gastric and colon cancer. Oncol Lett. (2016) 12:783–7. doi: 10.3892/ol.2016.4692
53. Tang M, Liu B, Bu X, and Zhao P. Cross-talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci. (2018) 109:1309–18. doi: 10.1111/cas.13567
54. Zeng J, Liu Z, Sun S, Xie J, Cao L, Lv P, et al. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncol Lett. (2018) 15:8681–6. doi: 10.3892/ol.2018.8372
55. Jin X, Deng Q, Ye S, Liu S, Fu Y, Liu Y, et al. Cancer-associated fibroblast-derived periostin promotes papillary thyroid tumor growth through integrin-FAK-STAT3 signaling. Theranostics. (2024) 14:3014–28. doi: 10.7150/thno.94207
56. Akinjiyan FA, Dave RM, Alpert E, Longmore GD, and Fuh KC. DDR2 expression in cancer-associated fibroblasts promotes ovarian cancer tumor invasion and metastasis through periostin-ITGB1. Cancers (Basel). (2022) 14:3482. doi: 10.3390/cancers14143482
57. Guo Y and Feng L. N6-methyladenosine-mediated upregulation of LINC00520 accelerates breast cancer progression via regulating miR-577/POSTN axis and downstream ILK/AKT/mTOR signaling pathway. Arch Biochem Biophys. (2022) 729:109381. doi: 10.1016/j.abb.2022.109381
58. Zhang Y, Jin J, Wu H, Huang J, Ye S, Qiu J, et al. Periostin protects against alcohol-related liver disease by activating autophagy by interacting with protein disulfide isomerase. Cell Mol Gastroenterol Hepatol. (2023) 15:1475–504. doi: 10.1016/j.jcmgh.2023.02.005
59. Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. (2021) 17:3281–7. doi: 10.7150/ijbs.60782
60. Wang H, Sun Y, Zhou X, Chen C, Jiao L, Li W, et al. CD47/SIRPα blocking peptide identification and synergistic effect with irradiation for cancer immunotherapy. J Immunother Cancer. (2020) 8:e000905. doi: 10.1136/jitc-2020-000905
61. Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, and Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. (2019) 19:568–86. doi: 10.1038/s41568-019-0183-z
62. Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα Axis. Front Immunol. (2020) 11:18. doi: 10.3389/fimmu.2020.00018
63. Zeidan AM, DeAngelo DJ, Palmer J, Seet CS, Tallman MS, Wei X, et al. Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann Hematol. (2022) 101:557–69. doi: 10.1007/s00277-021-04734-2
64. Narla RK, Modi H, Bauer D, Abbasian M, Leisten J, Piccotti JR, et al. Immunotherapy: Modulation of CD47-SIRPα innate immune checkpoint axis with Fc-function detuned anti-CD47 therapeutic antibody. Cancer Immunol Immunother (2022) 71:473–89. doi: 10.1007/s00262-021-03010-6
65. Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 blockade by hu5F9-G4 and rituximab in non-hodgkin’s lymphoma. N Engl J Med. (2018) 379:1711–21. doi: 10.1056/NEJMoa1807315
66. Johnson LD, Banerjee S, Kruglov O, Viller NN, Horwitz SM, Lesokhin A, et al. Targeting CD47 in sézary syndrome with SIRPαFc. Blood Adv (2019) 3:1145–53. doi: 10.1182/bloodadvances.2018030577
67. Han Z, Wu X, Qin H, Yuan YC, Zain J, Smith DL, et al. Blockade of the immune checkpoint CD47 by TTI-621 potentiates the response to anti-PD-L1 in cutaneous T-cell lymphoma. J Invest Dermatol. (2023) 143:1569–1578.e1565. doi: 10.1016/j.jid.2023.02.017
68. Kauder SE, Kuo TC, Harrabi O, Chen A, Sangalang E, Doyle L, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PloS One. (2018) 13:e0201832. doi: 10.1371/journal.pone.0201832
69. Lakhani NJ, Chow LQM, Gainor JF, LoRusso P, Lee KW, Chung HC, et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. (2021) 22:1740–51. doi: 10.1016/S1470-2045(21)00584-2
70. Qu T, Zhong T, Pang X, Huang Z, Jin C, Wang ZM, et al. Ligufalimab, a novel anti-CD47 antibody with no hemagglutination demonstrates both monotherapy and combo antitumor activity. J Immunother Cancer (2022) 10:e005517. doi: 10.1136/jitc-2022-005517
71. Wilson AJ, Stubbs M, Liu P, Ruggeri B, and Khabele D. The BET inhibitor INCB054329 reduces homologous recombination efficiency and augments PARP inhibitor activity in ovarian cancer. Gynecol Oncol. (2018) 149:575–84. doi: 10.1016/j.ygyno.2018.03.049
72. Juan A, Noblejas-López MDM, Bravo I, Arenas-Moreira M, Blasco-Navarro C, Clemente-Casares P, et al. Enhanced antitumoral activity of encapsulated BET inhibitors when combined with PARP inhibitors for the treatment of triple-negative breast and ovarian cancers. Cancers (Basel). (2022) 14:4474. doi: 10.3390/cancers14184474
73. Villar-Prados A, Wu SY, Court KA, Ma S, LaFargue C, Chowdhury MA, et al. Predicting novel therapies and targets: regulation of notch3 by the bromodomain protein BRD4. Mol Cancer Ther. (2019) 18:421–36. doi: 10.1158/1535-7163.MCT-18-0365
74. Aggarwal RR, Schweizer MT, Nanus DM, Pantuck AJ, Heath EI, Campeau E, et al. A phase ib/IIa study of the pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. (2020) 26:5338–47. doi: 10.1158/1078-0432.CCR-20-1707
75. Wu Y, Jennings NB, Sun Y, Dasari SK, Bayraktar E, Corvigno S, et al. Targeting CCR2(+) macrophages with BET inhibitor overcomes adaptive resistance to anti-VEGF therapy in ovarian cancer. J Cancer Res Clin Oncol. (2022) 148:803–21. doi: 10.1007/s00432-021-03885-z
76. Welsh SJ, Barwick BG, Meermeier EW, Riggs DL, Shi CX, Zhu YX, et al. Transcriptional heterogeneity overcomes super-enhancer disrupting drug combinations in multiple myeloma. Blood Cancer Discov. (2024) 5:34–55. doi: 10.1158/2643-3230.BCD-23-0062
77. Palomés-Borrajo G, Navarro X, and Penas C. BET protein inhibition in macrophages enhances dorsal root ganglion neurite outgrowth in female mice. J Neurosci Res. (2022) 100:1331–46. doi: 10.1002/jnr.25036
78. Wellinger LC, Hogg SJ, Newman DM, Friess T, Geiss D, Michie J, et al. BET inhibition enhances TNF-mediated antitumor immunity. Cancer Immunol Res. (2022) 10:87–107. doi: 10.1158/2326-6066.CIR-21-0224
79. Xu J, Ding L, Mei J, Hu Y, Kong X, Dai S, et al. Dual roles and therapeutic targeting of tumor-associated macrophages in tumor microenvironments. Signal Transduct Target Ther. (2025) 10:268. doi: 10.1038/s41392-025-02325-5
80. Chen X, Jiang Q, Ren L, Ren H, Xu H, Wang J, et al. BET proteins inhibitor JQ1 impairs GM-CSF-promoted peritoneal macrophage self-renewal and IL-4-induced alternative polarization. Int Immunopharmacol. (2023) 124:110942. doi: 10.1016/j.intimp.2023.110942
81. Schuetze KB, Stratton MS, Bagchi RA, Hobby ARH, Felisbino MB, Rubino M, et al. BRD4 inhibition rewires cardiac macrophages toward a protective phenotype marked by low MHC class II expression. Am J Physiol Heart Circ Physiol. (2025) 328:H294–h309. doi: 10.1152/ajpheart.00438.2024
82. Marbach D, Brouer-Visser J, Brennan L, Wilson S, Davydov II, Staedler N, et al. Immune modulation in solid tumors: a phase 1b study of RO6870810 (BET inhibitor) and atezolizumab (PD-L1 inhibitor). BMC Cancer. (2025) 25:500. doi: 10.1186/s12885-025-13851-4
83. Qiao J, Chen Y, Mi Y, Jin H, Wang L, Huang T, et al. Macrophages confer resistance to BET inhibition in triple-negative breast cancer by upregulating IKBKE. Biochem Pharmacol. (2020) 180:114126. doi: 10.1016/j.bcp.2020.114126
84. Chen X, Pan X, Zhang W, Guo H, Cheng S, He Q, et al. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. (2020) 10:723–33. doi: 10.1016/j.apsb.2019.09.006
85. Schweer D, Anand N, Anderson A, McCorkle JR, Neupane K, Nail AN, et al. Human macrophage-engineered vesicles for utilization in ovarian cancer treatment. Front Oncol. (2022) 12:1042730. doi: 10.3389/fonc.2022.1042730
86. Mei D, Zhu Y, Zhang L, and Wei W. The role of CTHRC1 in regulation of multiple signaling and tumor progression and metastasis. Mediators Inflammation. (2020) 2020:9578701. doi: 10.1155/2020/9578701
87. Bai Y, Yin K, Su T, Ji F, and Zhang S. CTHRC1 in ovarian cancer promotes M2-like polarization of tumor-associated macrophages via regulation of the STAT6 signaling pathway. Onco Targets Ther. (2020) 13:5743–53. doi: 10.2147/OTT.S250520
88. Li Y, Zhou J, Wang J, Chen X, Zhu Y, and Chen Y. Mir-30b-3p affects the migration and invasion function of ovarian cancer cells by targeting the CTHRC1 gene. Biol Res. (2020) 53:10. doi: 10.1186/s40659-020-00277-4
89. Chen X, Ying X, Wang X, Wu X, Zhu Q, and Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. (2017) 38:522–8. doi: 10.3892/or.2017.5697
90. Chen X, Zhou J, Li X, Wang X, Lin Y, and Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. (2018) 435:80–91. doi: 10.1016/j.canlet.2018.08.001
91. Liu T, Yuan L, and Zou X. Circular RNA circ-BNC2 (hsa_circ_0008732) inhibits the progression of ovarian cancer through microRNA-223-3p/FBXW7 axis. J Ovarian Res. (2022) 15:95. doi: 10.1186/s13048-022-01025-w
92. Li C, Lin XF, Wang JN, and Ren XS. FBXW7 inhibited cell proliferation and invasion regulated by miR-27a through PI3K/AKT signaling pathway and epithelial-to-mesenchymal transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. (2020) 24:3701–9. doi: 10.26355/eurrev_202004_20833
93. Liu Z, Ma T, Duan J, Liu X, and Liu L. MicroRNA−223−induced inhibition of the FBXW7 gene affects the proliferation and apoptosis of colorectal cancer cells via the Notch and Akt/mTOR pathways. Mol Med Rep. (2021) 23:154. doi: 10.3892/mmr.2020.11793
94. Zhong L, Pan Y, and Shen J. FBXW7 inhibits invasion, migration and angiogenesis in ovarian cancer cells by suppressing VEGF expression through inactivation of β-catenin signaling. Exp Ther Med. (2021) 21:514. doi: 10.3892/etm.2021.9945
Keywords: ovarian cancer, tumor-associated macrophages, tumor microenvironment, epithelial-mesenchymal transition, chemoresistance, TAM polarization
Citation: Li M, Ma Y, Dai T, Wang Y and Yue Y (2025) Advance in therapies targeting tumor-associated macrophages in ovarian cancer. Front. Immunol. 16:1677839. doi: 10.3389/fimmu.2025.1677839
Received: 01 August 2025; Accepted: 01 September 2025;
Published: 11 September 2025.
Edited by:
Lilong Zhang, Renmin Hospital of Wuhan University, ChinaReviewed by:
Yunfei Liu, Central South University, ChinaCopyright © 2025 Li, Ma, Dai, Wang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Yue, eXVleUBqbHUuZWR1LmNu
 Man Li
Man Li Yongxin Wang
Yongxin Wang Ying Yue
Ying Yue